Abstract
Background
The D3-creatine (D3Cr) dilution method provides a direct measure of skeletal muscle. The aim of this study was to compare the association of D3Cr muscle mass with lean body mass (LBM) measured by dual-energy x-ray absorptiometry (DXA) and examine its relation with physical function in postmenopausal women.
Methods
Seventy-four community-dwelling women (mean age 82.3 ± 5.4) participated in this pilot study from the Buffalo, New York clinical site of the Women’s Health Initiative (WHI). Participants attended a clinic visit which included anthropometric measures, blood draw, DXA scan, measures of physical function, and initiated the D3Cr protocol. Physical function was evaluated using hand grip strength, short physical performance battery (SPPB), and RAND-36 physical function scale. Descriptive statistics and logistic regression models were used to examine the associations of D3Cr muscle mass with functional outcomes.
Results
D3-creatine muscle mass was moderately correlated with DXA LBM (r = 0.50) and DXA appendicular lean mass (ALM) (r = 0.50). Individuals with high D3Cr muscle mass (%) had higher physical function compared to individuals with low muscle mass (%), indicated by high scores on SPPB (odds ratio [OR] = 5.24; 95% confidence interval [CI]: 1.40, 19.58). We observed stronger relationships between high D3Cr and physical function than either DXA LBM (OR = 3.40; 95% CI: 0.88, 13.11) or DXA ALM (OR = 4.15; 95% CI: 1.10, 15.68) and physical function.
Conclusions
Our findings provide strong preliminary data for the associations of D3Cr muscle mass with measures of physical function in older women. These findings support and extend prior work on D3Cr muscle mass in older men.
Keywords: ALM, DXA, Lean body mass, Physical performance, Sarcopenia
Background
Sarcopenia is a geriatric syndrome characterized by low lean body mass (LBM), low muscle strength, and physical functioning (1–4). In older adults, there is an equivocal relationship between LBM, often measured by dual-energy x-ray absorptiometry (DXA), and health-related outcomes. The authors of the Foundation for the National Institutes of Health sarcopenia project concluded that low lean mass, by itself, is a poor predictor of physical functional impairment when compared to low strength (3).
A potential explanation for the inconsistent relation between DXA LBM and health outcomes relates to the measurement of lean mass (5). Lean body mass from DXA is calculated by subtraction; representing the nonbone, nonfat component of total body composition and includes body water, viscera, and connective tissue (6). Dual-energy x-ray absorptiometry provides accurate measurement of bone, fat, and LBM, but LBM is not equivalent to skeletal muscle mass (7,8). Skeletal muscle mass is a component of lean mass, but it is not the only component (9).
Recently, Evans and colleagues developed the D3-creatine (D3Cr) dilution method to directly measure skeletal muscle mass (10). This method uses a single enteral dose of D3Cr, which is digested, absorbed, and transported to all muscle cells. Intramyocellular creatine is turned over through the nonenzymatic conversion of creatine to creatinine, which is rapidly excreted. A single spot urine sample is analyzed to determine the enrichment of D3-creatinine, which in turn gives a direct measurement of the total body creatine pool. This method is supported by several well-known aspects of creatine biology and metabolism (11) and has been validated in rodents (12) and humans (7,13). The D3Cr method has also been used in a large cohort of community-dwelling older men (5,9,14–17).
There has been limited investigation of D3Cr in older women (13,18). In this study, our primary objectives are to compare skeletal muscle mass measured by D3Cr with DXA LBM and appendicular lean mass (ALM) and examine the cross-sectional association of D3Cr, DXA LBM, and DXA ALM with measures of physical function. Muscle mass may differ between men and women, in terms of quantity and function; thus, it is important to examine these relationships in a sample of postmenopausal women.
Method
Study Population
We enrolled 74 community-dwelling postmenopausal women from the Buffalo, New York clinical site of the Women’s Health Initiative (WHI). Details of the WHI have been described previously (19). For this pilot study, women were recruited from a simple random sample of WHI participants living within 50 miles of the WHI Buffalo site. There were no additional eligibility criteria. Reasons for not consenting were related to health (22%; health too poor to attend in-clinic visit, caregiving responsibilities), scheduling (12%; not available during 4-week pilot period but interested in the study), travel/transportation concerns (11% does not drive, lives too far). Of those contacted, only 3% (n = 5) chose not to participate because they did not want to take the D3Cr pill. Of the 74 participants, 99% (n = 73) completed the D3Cr protocol; 1 participant had a family emergency and was not able to return her urine sample.
Study Design
Participants were mailed a recruitment package to invite them to join in the study. The mailing included a recruitment letter, informed consent form, and two questionnaires (physical activity and activities of daily living). Women were subsequently contacted by telephone and those who agreed to participate were invited to a clinic visit at the WHI Buffalo study site. The clinic visit followed a standard protocol and was approximately 1 hour in length, including: (i) anthropometric measures, (ii) blood pressure measurement, (iii) a blood draw, (iv) a whole body DXA scan (Hologic QDR-4500), (v) measures of physical function, and (vi) D3Cr pill consumption. The study was approved by the Institutional Review Board at the University at Buffalo. Specific components of the study visits are described in detail below; additional information is included in Supplementary Appendix 1.
Measures of physical function
Trained examiners administered the short physical performance battery (SPPB) (20). The SPPB consists of three tests: balance, gait speed, and chair stand (20). Short physical performance battery scores range from 0 to 12, with higher scores indicating better physical function. Participants also self-rated their physical function using a subscale of the RAND-36 scale (21). The physical function subscale is commonly used in large-scale epidemiological studies, including the WHI (22). The cumulative scores on this scale range from 0 to 100, with higher scores indicating better physical function (23). Two trials of grip strength (kg) for each hand were assessed using a handheld dynamometer (Jamar hand dynamometer; Lafayette Instruments, Lafayette, IN). The participant was instructed to squeeze the handle of the dynamometer as hard as she could. The higher score of the dominant hand was used in this analysis.
D3Cr dilution method
At the clinic visit, participants consumed an oral dose of 30 mg D3Cr (Cambridge Isotope Laboratories, Inc., Tewksbury, MA; encapsulated by Valor Compounding Pharmacy, Berkeley, CA) and were sent home with a urine sample collection kit, collection instructions, and return mailing instructions. The urine collection kit included a dipstick, urine collection cup, styrofoam return mailer, ice pack, and prefilled FedEx mailing label. A research team member recorded the date and time the pill was consumed. Participants were asked to provide a fasting morning urine sample on a specific date between 72 and 144 hours after their clinic visit. After sample collection, they were asked to pack the dipstick containing their urine sample with an ice pack in the styrofoam mailer and call FedEx for a same-day at-home pickup. Samples were sent via overnight shipping and arrived at the WHI Buffalo study center less than 24 hours after sample collection. The D3Cr protocol has been described previously (5,10).
Once the samples were received, they were stored in freezers (−20°C) in the Biospecimen Bank in the Department of Epidemiology and Environmental Health at the University at Buffalo. Samples were sent in two batches by FedEx overnight shipping to the University of California, Berkeley. Urinary creatine, creatinine, and D3-creatinine were measured by liquid chromatography-tandem mass spectrometry, and skeletal muscle mass was estimated by a validated algorithm (24).
Measures
Exposure
We examined both absolute and relative measures of D3Cr muscle mass, LBM, and ALM. Appendicular lean mass is calculated as the sum of the lean mass of both arms and legs (5). Absolute values refer to D3Cr, LBM, and ALM values in kilograms (kg). Relative measures are reported as a percentage (%), indicating D3Cr, LBM, or ALM values scaled to account for body size. To scale D3Cr, LBM, and ALM values, we divided the absolute value by body weight (kg), height squared (m2), or body mass index (BMI) (kg/m2). Absolute and relative muscle mass were examined as continuous variables and also categorized as low and high by median split. In Supplementary Appendix 2 we describe additional information on these exposure variables, including minimum, maximum, median, mean, standard deviation, and 25th and 75th percentiles.
Outcome
Physical function was measured via in-person SPPB measurement and RAND-36 questionnaire. Consistent with prior literature, low function is defined as SPPB scores of 0–9 and high function is defined as 10–12. RAND-36 scores less than 78 indicated low function and scores over 78 indicated high function (23). We present results from these dichotomous outcome variables in the main text; see Supplementary Appendix 3 (Table 3a) for additional analyses using a continuous form of these measures.
Covariates
Anthropometric measures (height, weight, hip and waist circumference) were obtained at the clinic visit by trained WHI clinic staff using standardized protocols. Body mass index was calculated as weight divided by height squared (kg/m2). Systolic and diastolic blood pressure were measured after 5 minutes of quiet sitting with legs uncrossed using a manual sphygmomanometer. Hypertension was defined as a systolic pressure ≥140 mm Hg or a diastolic pressure ≥90 mm Hg. Physical activity was measured by the validated WHI physical activity questionnaire, used to calculate weekly physical activity in metabolic equivalent-hours per week (MET-h/wk) (25). A fasting blood sample was drawn by venipuncture by a trained phlebotomist. Vials were processed immediately and frozen onsite. Sample were analyzed by an external laboratory (Kaleida Health, Buffalo, NY) and tests included a comprehensive metabolic panel, hemoglobin A1c (HbA1c), insulin, lipid panel, and complete blood count. Blood biomarkers relevant as covariates in this analysis include HbA1c and blood lipids (triglycerides, total cholesterol, HDL cholesterol, LDL cholesterol).
Basic demographic characteristics (eg, age, race) were extracted from WHI study database.
Statistical Analyses
To compare skeletal muscle mass measured by D3Cr with DXA LBM and ALM we examined scatterplots and Pearson correlation coefficients. We also fitted a quadratic prediction line in each scatterplot. We used logistic regression models to examine the association of low and high D3Cr muscle mass, LBM, and ALM with SPPB and RAND-36 physical function scores. Logistic regression results are presented with exposure categories defined by median split (high/low) and a dichotomous outcome variable. This analytic strategy was used to enhance the comparability of our study results with prior research on D3Cr muscle mass. In Supplementary Appendix 3 (Table 3a) we present corresponding linear regression results with a continuous outcome variable for SPPB and RAND-36. We present crude (unadjusted) logistic regression models with relative D3Cr, DXA ALM and LBM scaled to kg, BMI, and height squared as well as a series of sequentially adjusted multivariable models: adjusted for age (Models 2 and 6), then additionally adjusted for race/ethnicity, HbA1c, hypertension, triglycerides, and cholesterol (Models 3 and 7), and finally, additionally adjusted for physical activity (Models 4 and 8). We further present crude and adjusted logistic regression models with continuous D3Cr muscle mass as the exposure and dichotomous outcome variables. Additional logistic regression analyses with the dichotomous absolute values of the exposure variable (D3Cr, DXA LBM, and DXA ALM) are presented in Supplementary Appendix 3 (Table 3b). Analyses were completed in SAS 9.4 (Cary, NC).
Results
We received 73 out of 74 (99%) urine samples from study participants in good condition. Demographic characteristics of the total study population are presented in Table 1, overall and stratified by muscle mass category (n = 37 with high muscle mass and 36 women with low muscle mass, categorized by median = 27.2%). The mean age of study participants was 82.3 ± 5.4 years (range: 74–96) and 93% were non-Hispanic White (n = 68). Mean absolute D3Cr muscle mass (kg), and relative D3Cr muscle mass (%) values were: 18.0 ± 3.5 (range: 12.3–30.1) and 28.0 ± 5.9 (range: 17.3–45.9).
Table 1.
Characteristics of Pilot Study Participants in the Total Study Population and Stratified by Low and High D3Cr Muscle Mass Per Kilogram Body Weight
| Characteristics | Relative D3Cr Muscle Mass/kg | Total (n = 73) | |
|---|---|---|---|
| Low (n = 36) | High (n = 37) | ||
| Age, mean (SD) | 82.78 (5.75) | 81.92 (5.10) | 82.34 (5.37) |
| Race/ethnicity, n (%) | |||
| Non-White | 2 (5.6) | 3 (8.1) | 5 (6.8) |
| White | 34 (94.4) | 34 (91.9) | 68 (93.2) |
| BMI (kg/m2), mean (SD) | 27.68 (4.79) | 24.4 (3.39) | 25.95 (4.44) |
| 18.5–24.9, n (%) | 12 (33.3) | 26 (70.2) | 38 (52.1) |
| 25–29.9, n (%) | 14 (38.9) | 7 (18.9) | 21 (28.8) |
| ≥30, n (%) | 10 (27.8) | 4 (10.8) | 14 (19.2) |
| Waist circumference (cm), mean (SD) | 90.94 (13.77) | 82.82 (10.39) | 86.75 (12.69) |
| Hip circumference (cm), mean (SD) | 105.87 (9.34) | 98.55 (6.08) | 102.08 (8.60) |
| Waist-to-hip ratio, mean (SD) | 0.86 (0.10) | 0.84 (0.08) | 0.85 (0.09) |
| Physical activity (MET-h/wk), mean (SD) | 12.74 (12.18) | 18.7 (13.20) | 16.61 (14.80) |
| Relative DXA LBM/kg, mean (SD) | 0.59 (0.05) | 0.64 (0.05) | 0.61 (0.05) |
| Relative DXA ALM/kg, n (%) | 0.25 (0.02) | 0.27 (0.03) | 0.26 (0.03) |
| Grip strength, mean (SD) | 21.1 (5.02) | 21.6 (5.08) | 21.3 (5.02) |
| Balance (%) | |||
| Side by side | 94.6 | 100.0 | 97.3 |
| Semi-tandem | 59.5 | 75.0 | 67.1 |
| Tandem | 91.9 | 97.2 | 95.9 |
| Gait speed (%) | |||
| <4.82 s | 29.7 | 44.4 | 36.95 |
| >4.82 & <6.20 s | 51.4 | 38.9 | 45.2 |
| >6.20 & <8.70 s | 13.5 | 13.9 | 13.7 |
| >8.70 s | 5.4 | 2.78 | 4.1 |
| Chair stand (%) | |||
| >14 s | 62.2 | 36.1 | 49.3 |
| <14 s | 37.8 | 63.9 | 50.7 |
| SPPB score, mean (SD) | 8.02 (2.40) | 9.51 (2.17) | 8.78 (2.39) |
| Physical function score, mean (SD) | 69.6 (24.4) | 82.8 (21.4) | 76.30 (23.71) |
| Self-report limitation (%) | |||
| Moderate physical activity | 32.4 | 19.5 | 26.0 |
| Lifting groceries | 16.2 | 16.6 | 16.4 |
| Climbing 1 flight of stairs | 32.4 | 8.3 | 20.6 |
| Bending, kneeling | 48.7 | 25.0 | 37.0 |
| Walking >1 mile | 64.8 | 41.5 | 53.4 |
| Walking 1 block | 24.3 | 8.3 | 16.4 |
| Bathing or dressing | 5.41 | 2.8 | 4.1 |
| Self-rated health, mean (SD) | 2.37 (0.79) | 2.5 (1.25) | 2.43 (1.04) |
Notes: ALM = appendicular lean mass; BMI = body mass index; D3Cr = D3-creatine; DXA = dual-energy x-ray absorptiometry; LBM = lean body mass; SD = standard deviation; SPPB = short physical performance battery.
Mean BMI in the high muscle mass group was 3.28 kg/m2 lower than in the low muscle mass group. This trend was also evident when comparing across BMI categories; in the high muscle mass group, 70.2% had BMI 18.5–24.9 kg/m2 and 10.8% had BMI ≥ 30 kg/m2, whereas in the low muscle mass group, 33.3% had BMI 18.5–24.9 kg/m2 and 27.8% had BMI ≥ 30 kg/m2. Waist circumference, hip circumference, and waist-to-hip ratio were all higher in the low muscle mass group. Grip strength was nearly identical in both groups (21.1 vs 21.6). Women with high muscle mass: (i) were more likely to complete side by side, semi-tandem, and fully tandem balance tests, (ii) had faster walking speeds, and (iii) were able to complete the chair stand test quicker than women with low muscle mass. These differences are reflected in a higher average SPPB score in women with high relative D3Cr muscle mass (9.1) compared to low relative D3Cr muscle mass (7.4). Women with high D3Cr muscle mass also reported higher overall physical function, whereas low muscle mass was associated with physical limitations. For instance, women with low relative D3Cr muscle mass were 24% more likely to report difficulty climbing 1 flight of stairs compared to women with high relative D3Cr muscle mass. Women with low relative D3Cr muscle mass were nearly twice as likely to report that their health limited their ability to bathe or dress themselves (5.4%) compared to women with high relative D3Cr muscle mass (2.8%).
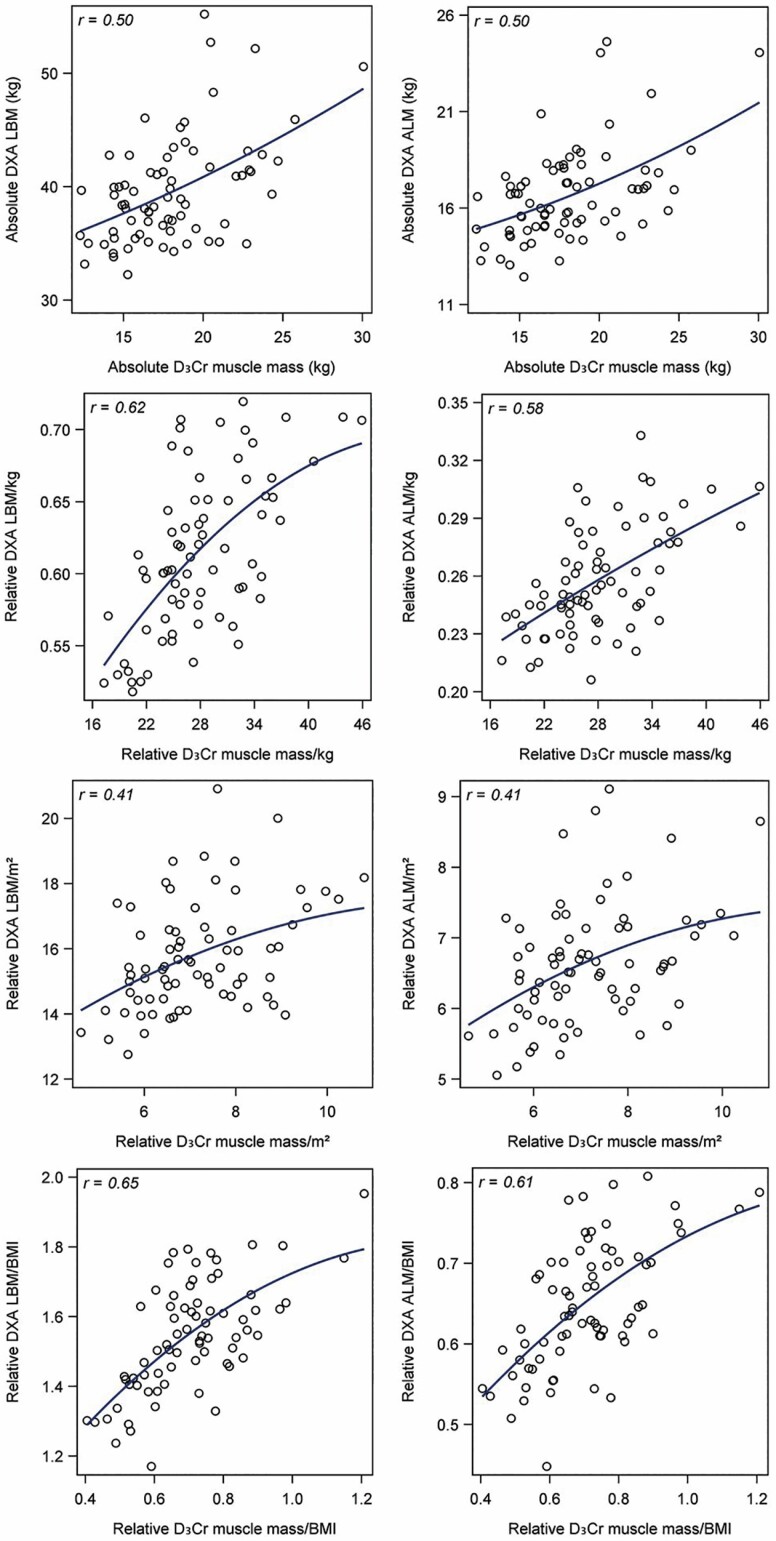
There was a moderate positive correlation between absolute D3Cr muscle mass with LBM (r = 0.50) and ALM (r = 0.50) as shown in Figure 1. The correlation was slightly stronger when we scaled D3Cr muscle mass and LBM to weight (r = 0.62) or BMI (r = 0.65), but weaker if scaled to height squared (r = 0.41). The correlations between D3Cr muscle mass and ALM scaled to weight (r = 0.58), height squared (r = 0.41), or BMI (r = 0.61) were similar. Age-adjusted correlations between these variables remained positive and moderate; partial correlations were also stronger for D3Cr, ALM, and LBM scaled to weight or BMI, but weaker if scaled to height squared (see Supplementary Appendix 3 (Table 3c)).
Figure 1.
Correlations between D3Cr muscle mass and DXA LBM or DXA ALM, with absolute values and values scaled to weight, height2, and BMI. ALM = appendicular lean mass; BMI = body mass index; D3Cr = D3-creatine; DXA = dual-energy x-ray absorptiometry; LBM = lean body mass. The curved line represents a quadratic prediction line.
Primary study results are presented in Table 2. High relative muscle mass (D3Cr/kg and D3Cr/BMI) was associated with physical functioning in crude and adjusted models. Odds ratios were slightly stronger in models adjusted for age, race/ethnicity, hypertension, HbA1c, triglycerides, and cholesterol, but attenuated when additionally adjusted for physical activity. Table 2 also includes results for the association of LBM and ALM with physical function outcomes. Lean body mass and ALM were positively associated with physical function, but odds ratios were uniformly lower for DXA measures compared to D3Cr measures. In D3Cr models, odds ratios were higher in the models when the exposure was scaled to BMI (eg, D3Cr/BMI), whereas in nearly all DXA models (eg, LBM/BMI and ALM/BMI) associations were weaker in models scaled to BMI. Table 3 presents results from logistic regression models with D3Cr muscle mass parameterized as a continuous exposure variable. These results demonstrate a positive relationship between increasing muscle mass and odds of high SPPB or RAND-36 score, and are consistent with results presented in Table 2. Additional information on the relation between D3Cr and DXA measures with continuous measures from gait speed (meters/second) and chair rise tests (seconds) is included in Supplementary Appendix 4.
Table 2.
Associations of Physical Performance and Physical Function With D3Cr Muscle Mass, DXA LBM or DXA ALM Using Logistic Regression Models [OR (95% CI)]
| Physical Performance* | Physical Function+ | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1† | Model 2‡ | Model 3§ | Model 4|| | Model 5† | Model 6‡ | Model 7§ | Model 8|| | |
| Muscle mass (D3Cr) | ||||||||
| Relative D3Cr muscle mass/kg | ||||||||
| Low (n = 36) | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- |
| High (n = 37) | 4.93 (1.80, 13.47) | 5.93 (1.91, 18.43) | 5.24 (1.40, 19.58) | 4.68 (1.16, 18.88) | 3.24 (1.17, 9.00) | 3.15 (1.13, 8.84) | 2.25 (0.65, 7.84) | 1.77 (0.48, 6.58) |
| Relative D3Cr muscle mass/BMI | ||||||||
| Low (n = 36) | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- |
| High (n = 37) | 8.63 (2.95, 25.28) | 8.20 (2.56, 26.29) | 8.13 (2.02, 32.70) | 7.75 (1.75, 34.30) | 4.29 (1.50, 12.25) | 3.93 (1.34, 11.51) | 2.82 (0.77, 10.31) | 2.28 (0.58, 9.02) |
| Relative D3Cr muscle mass/m2 | ||||||||
| Low (n = 36) | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- |
| High (n = 37) | 1.87 (0.73, 4.77) | 1.44 (0.52, 3.99) | 0.99 (0.29, 3.39) | 1.00 (0.27, 3.79) | 0.72 (0.27, 1.91) | 0.62 (0.23, 1.71) | 0.18 (0.04, 0.88) | 0.10 (0.02, 0.71) |
| DXA LBM | ||||||||
| Relative DXA LBM/kg | ||||||||
| Low (n = 37) | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- |
| High (n = 37) | 1.73 (0.69, 4.38) | 3.65 (1.17, 11.38) | 3.40 (0.88, 13.11) | 2.55 (0.61, 10.71) | 1.84 (0.69, 4.90) | 2.47 (0.85, 7.18) | 1.53 (0.42, 5.60) | 1.08 (0.26, 4.54) |
| Relative DXA LBM/BMI | ||||||||
| Low (n = 37) | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- |
| High (n = 37) | 2.17 (0.85, 5.53) | 2.47 (0.88, 6.93) | 2.90 (0.78, 10.77) | 2.43 (0.59, 9.98) | 2.37 (0.88, 6.40) | 2.38 (0.87, 6.52) | 4.59 (1.00, 21.00) | 4.59 (1.00, 21.00) |
| Relative DXA LBM /m2 | ||||||||
| Low (n = 37) | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- |
| High (n = 37) | 0.58 (0.23, 1.46) | 0.47 (0.17, 1.32) | 0.53 (0.15, 1.89) | 0.52 (0.12, 2.28) | 0.33 (0.12, 0.90) | 0.30 (0.11, 0.86) | 0.23 (0.05, 0.99) | 0.21 (0.04, 1.08) |
| DXA ALM | ||||||||
| Relative DXA ALM/kg | ||||||||
| Low (n = 37) | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- |
| High (n = 37) | 2.73 (1.06, 7.05) | 4.20 (1.39, 12.67) | 4.15 (1.10, 15.68) | 2.71 (0.66, 11.15) | 2.37 (0.88, 6.4) | 2.64 (0.95, 7.36) | 2.80 (0.75, 10.50) | 2.03 (0.49, 8.53) |
| Relative DXA ALM/BMI | ||||||||
| Low (n = 37) | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- |
| High (n = 37) | 1.12 (0.45, 2.79) | 1.24 (0.46, 3.39) | 0.97 (0.29, 3.21) | 0.79 (0.21, 2.98) | 1.13 (0.43, 2.96) | 1.15 (0.43, 3.06) | 0.91 (0.25, 3.32) | 0.74 (0.19, 2.97) |
| Relative DXA ALM /m2 | ||||||||
| Low (n = 37) | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- | -ref- |
| High (n = 37) | 0.90 (0.36, 2.24) | 0.62 (0.22, 1.72) | 0.76 (0.20, 2.86) | 0.71 (0.15, 3.78) | 0.42 (0.16, 1.14) | 0.34 (0.12, 0.97) | 0.20 (0.04, 0.96) | 0.17 (0.03, 0.94) |
Notes: ALM = appendicular lean mass; BMI = body mass index; CI = confidence interval; D3Cr = D3-creatine; DXA = dual-energy x-ray absorptiometry; HbA1c = hemoglobin A1c; HDL = high-density lipoprotein cholesterol; LBM = lean body mass; LDL = low-density lipoprotein cholesterol; OR = odds ratio; SPPB = short physical performance battery.
*SPPB used as a dichotomous outcome, comparing individuals with SPPB score 0–9 with 10–12.
+ RAND-36 used as a dichotomous outcome, comparing individuals with RAND-36 score 0–77 with >78.
†Crude model.
‡Adjusted for age (continuous).
§Adjusted for age (continuous), race/ethnicity (non-White and White), HbA1c (<5.7% and ≥5.7%), hypertension (≥140/90 mm Hg), triglyceride (<150 and ≥150 mg/dL), total cholesterol (<200 and ≥200 mg/dL), HDL cholesterol (<60 and ≥60 mg/dL), and LDL cholesterol (<130 and ≥130 mg/dL).
||Further adjusted for physical activity (continuous).
Table 3.
Odds Ratios (ORs) and 95% Confidence Intervals (CIs) for Continuous D3Cr Muscle Mass as an Exposure Variable With Physical Performance and Physical Function, Using Logistic Regression Models [OR (95% CI)]
| Physical Performance* (SPPB) | Physical Function+ (RAND-36) | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1† | Model 2‡ | Model 3§ | Model 4|| | Model 5† | Model 6‡ | Model 7§ | Model 8|| | |
| Absolute D3Cr muscle mass (kg) | ||||||||
| Per 1 kg increase | 1.12 (0.97, 1.28) | 1.02 (0.88, 1.19) | 0.99 (0.83, 1.20) | 1.03 (0.85, 1.25) | 0.99 (0.86, 1.14) | 1.04 (0.89, 1.21) | 1.09 (0.90, 1.33) | 1.09 (0.89, 1.34) |
| Relative D3Cr muscle mass/kg | ||||||||
| Per 1% increase | 1.15 (1.05, 1.27) | 1.15 (1.04, 1.28) | 1.14 (1.01, 1.29) | 1.12 (0.98, 1.27) | 0.92 (0.84, 1.01) | 0.92 (0.84, 1.01) | 0.98 (0.86, 1.11) | 1.01 (0.89, 1.16) |
| Relative D3Cr muscle mass/BMI | ||||||||
| Per 0.1 kg/(kg/ m2) increase | 1.75 (1.19, 2.57) | 1.65 (1.11, 2.48) | 1.59 (0.97, 2.6) | 1.5 (0.92, 2.45) | 0.72 (0.50, 1.04) | 0.75 (0.52, 1.08) | 0.87 (0.54, 1.42) | 0.97 (0.59, 1.60) |
| Relative D3Cr muscle mass/m2 | ||||||||
| Per 1 kg/m2 increase | 1.35 (0.93, 1.95) | 1.14 (0.76, 1.71) | 1.08 (0.67, 1.75) | 1.13 (0.68, 1.86) | 0.96 (0.66, 1.4) | 1.05 (0.71, 1.57) | 1.32 (0.79, 2.22) | 1.38 (0.80, 2.39) |
Notes: BMI = body mass index; D3Cr = D3-creatine; HbA1c = hemoglobin A1c; HDL = high-density lipoprotein cholesterol; LDL = low-density lipoprotein cholesterol; SPPB = short physical performance battery.
*SPPB used as a dichotomous outcome, comparing individuals with SPPB score 0–9 with 10–12.
+ RAND-36 used as a dichotomous outcome, comparing individuals with RAND-36 score 0–77 with >78.
†Crude model.
‡Adjusted for age (continuous).
§Adjusted for age (continuous), race/ethnicity (non-White and White), HbA1c (<5.7% and ≥5.7%), hypertension (≥140/90 mm Hg), triglyceride (<150 and ≥150 mg/dL), total cholesterol (<200 and ≥200 mg/dL), HDL cholesterol (<60 and ≥60 mg/dL), and LDL cholesterol (<130 and ≥130 mg/dL).
||Further adjusted for physical activity (continuous).
Discussion
The current study demonstrates a clear relationship between muscle mass, measured by D3Cr, and physical function in community-dwelling postmenopausal women. Women with high muscle mass had higher scores on all domains of the SPPB (balance, gait speed, and chair stand) and were less likely to report any prevalent physical limitations (physical activity, climbing stairs, bending/kneeling, walking, bathing/dressing). Associations of D3Cr with physical function were stronger than DXA-defined LBM or ALM, lending support to the hypothesis that D3Cr is a more accurate measure of muscle mass than measures from DXA. In a commentary on the use of the D3Cr method, Schaap succinctly describes one of the key benefits of this approach: “By isolating contractile muscle mass from non-contractile components including fat, the D3-creatine assessment is not only an accurate method to assess muscle mass but is less biased by obesity and aging than DXA [appendicular lean mass] (p.842)” (17).
Our findings are broadly consistent with measures of D3Cr and physical performance in older men in the Osteoporotic Fractures in Men (MrOS) study (5). Cawthon and colleagues reported older men in MrOs with low D3Cr muscle mass had worse physical function, higher incident disability (ie, difficulty completing activities of daily living and instrumental activities of daily living and mobility disability), and mortality risk (5,14). The MrOs results are similar to our results in postmenopausal women; both studies report strong and consistent associations between muscle mass, SPPB, and functional limitation and weaker associations were observed for the relation of DXA ALM and physical function (5). Women in this study had lower absolute values of D3Cr muscle mass than men in the MrOS study, as well as lower physical function scores than men (eg, grip strength, SPPB) (5). These results demonstrate that although the association between D3Cr functional outcomes is similar in older men and women, we observe notable sex differences. These results echo previous results on body composition in older adults using DXA. The Copenhagen Sarcopenia Study highlighted differences in DXA total body lean mass in 1305 older men and women: total body lean mass was 57.6 kg in men aged 60–69, 53.6 kg in men aged 70–79, and 51.3 kg in men >80 years compared to 40.8 kg, 39.4 kg, and 36.9 kg among women in the same age groups (26). Given known differences in body composition in older men and women, there is a clear scientific need for further research examining muscle mass in a large sample of older women. The D3Cr method presents a tremendous opportunity for researchers interested in aging and body composition. There are many potential future directions for research using the D3Cr method, including analyses comparing older men and women, exploration of the dynamic relationship between muscle mass and fat mass in older adults, and the relation of D3Cr with risk of incident disease and morbidity.
This study was designed as a pilot study to examine the use of D3Cr in older women and has limitations similar to most pilot studies. Given the small sample size, we may be underpowered to detect the main effects of interest. Additional research is needed in a larger group of women to confirm the relationships between muscle mass, physical function, and functional limitation. A larger sample size would also generate a more precise estimates of the association between D3Cr and physical function outcomes. Given the local recruitment and predominantly White study participants, our findings might not be generalizable to a larger sample of women in the WHI or older women in the general U.S. population. Our results also demonstrated that all odds ratios adjusted for physical activity were attenuated, which may be due to physical activity being a mediator of the relationship between muscle mass and physical functioning (27). Additional analyses, including a causal mediation analysis in a larger sample, would be valuable to disentangle the complex relationship between muscle mass and physical activity (28). Despite these limitations, this study represents an important step forward for our understanding of muscle mass in a community-dwelling sample of postmenopausal women. At present, the largest study examining the relation of D3Cr muscle mass with outcomes includes older men only. The results of this pilot study make an important scientific and clinical contribution to our understanding of muscle mass in older women and clearly highlight the need for future research in a larger sample of older women.
Our study contributes to an emerging body of literature on the relation of D3Cr and age-related outcomes. This study presents preliminary cross-sectional data on muscle mass and functional outcomes in postmenopausal women. Longitudinal research is urgently needed in a large, diverse sample of older adults. As women age, the risk of falls, fractures, and frailty markedly increases (29,30). Studying the relation between muscle mass and functional outcomes is an important first step toward developing intervention strategies to reduce morbidity in this high-risk age group.
Funding
This work was supported by a pilot grant by the University at Buffalo Center for Successful Aging (H. R. Banack). The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts, HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C; H. R. Banack: Canadian Institute of Health Research (CIHR) Banting Postdoctoral Fellowships Program; B. R. Troen: National Institute on Aging (AG060266), Veterans Affairs (RX002902, BX004369), New York State Department of Health, Indian Trail Foundation.
Supplementary Material
Acknowledgments
WHI Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller. WHI Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, Washington) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg. WHI Investigators and Academic Centers: (Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, District of Columbia) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, California) Marcia L. Stefanick; (The Ohio State University, Columbus, Ohio) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, Arizona) Cynthia A. Thomson; (University at Buffalo, Buffalo, New York) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, Florida) Marian Limacher; (University of Iowa, Iowa City/Davenport, Iowa) Jennifer Robinson; (University of Pittsburgh, Pittsburgh, Pennsylvania) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, North Carolina) Sally Shumaker; (University of Nevada, Reno, Nevada) Robert Brunner; (University of Minnesota, Minneapolis, Minnesota) Karen L. Margolis. WHI Memory Study: (Wake Forest University School of Medicine, Winston-Salem, North Carolina) Mark Espeland.
Conflict of Interest
None declared.
References
- 1. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. ; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2 . Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dennison EM, Sayer AA, Cooper C. Epidemiology of sarcopenia and insight into possible therapeutic targets. Nat Rev Rheumatol. 2017;13:340–347. doi: 10.1038/nrrheum.2017.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Studenski SA, Peters KW, Alley DE, et al. . The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547–558. doi: 10.1093/gerona/glu010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fielding RA, Vellas B, Evans WJ, et al. . Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cawthon PM, Orwoll ES, Peters KE, et al. . Strong relation between muscle mass determined by D3-creatine dilution, physical performance and incidence of falls and mobility limitations in a prospective cohort of older men. J Gerontol A Biol Sci Med Sci. 2019;74:844–852. doi: 10.1093/gerona/gly129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Withers RT, LaForgia J, Pillans RK, et al. . Comparisons of two-, three-, and four-compartment models of body composition analysis in men and women. J Appl Physiol (1985). 1998;85:238–245. doi: 10.1152/jappl.1998.85.1.238 [DOI] [PubMed] [Google Scholar]
- 7. Clark RV, Walker AC, Miller RR, O’Connor-Semmes RL, Ravussin E, Cefalu WT. Creatine (methyl-d3) dilution in urine for estimation of total body skeletal muscle mass: accuracy and variability vs. MRI and DXA. J Appl Physiol (1985). 2018;124:1–9. doi: 10.1152/japplphysiol.00455.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scafoglieri A, Clarys JP. Dual energy X-ray absorptiometry: gold standard for muscle mass? J Cachexia Sarcopenia Muscle. 2018;9:786–787. doi: 10.1002/jcsm.12308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Orwoll ES, Peters KE, Hellerstein M, Cummings SR, Evans WJ, Cawthon PM. The importance of muscle versus fat mass in sarcopenic obesity: a re-evaluation using D3-creatine muscle mass versus DXA lean mass measurements. J Gerontol A Biol Sci Med Sci. 2020;75:1362–1368. doi: 10.1093/gerona/glaa064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Evans WJ, Hellerstein M, Orwoll E, Cummings S, Cawthon PM. D3-creatine dilution and the importance of accuracy in the assessment of skeletal muscle mass. J Cachexia Sarcopenia Muscle. 2019;10:14–21. doi: 10.1002/jcsm.12390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80:1107–1213. doi: 10.1152/physrev.2000.80.3.1107 [DOI] [PubMed] [Google Scholar]
- 12. Stimpson SA, Turner SM, Clifton LG, et al. . Total-body creatine pool size and skeletal muscle mass determination by creatine-(methyl-D3) dilution in rats. J Appl Physiol (1985). 2012;112:1940–1948. doi: 10.1152/japplphysiol.00122.2012 [DOI] [PubMed] [Google Scholar]
- 13. Clark RV, Walker AC, O’Connor-Semmes RL, et al. . Total body skeletal muscle mass: estimation by creatine (methyl-d3) dilution in humans. J Appl Physiol (1985). 2014;116:1605–1613. doi: 10.1152/japplphysiol.00045.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cawthon PM, Blackwell T, Cummings SR, et al. . Muscle mass assessed by the D3-creatine dilution method and incident self-reported disability and mortality in a prospective observational study of community-dwelling older men. J Gerontol A Biol Sci Med Sci. 2021;76:123–130. doi: 10.1093/gerona/glaa111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Duchowny KA, Peters KE, Cummings SR, et al. ; Osteoporotic Fractures in Men (MrOS) Study Research Group . Association of change in muscle mass assessed by D3-creatine dilution with changes in grip strength and walking speed. J Cachexia Sarcopenia Muscle. 2020;11:55–61. doi: 10.1002/jcsm.12494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rogers-Soeder TS, Peters KE, Lane NE, et al. . Dietary intake, D3Cr muscle mass, and appendicular lean mass in a cohort of older men. J Gerontol A Biol Sci Med Sci. 2020;75:1353–1361. doi: 10.1093/gerona/glz145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schaap LA. D3-creatine dilution to assess muscle mass. J Gerontol A Biol Sci Med Sci. 2018;74:842–843. doi: 10.1093/gerona/gly180 [DOI] [PubMed] [Google Scholar]
- 18. Buehring B, Siglinsky E, Krueger D, et al. . Comparison of muscle/lean mass measurement methods: correlation with functional and biochemical testing. Osteoporos Int. 2018;29:675–683. doi: 10.1007/s00198-017-4315-6 [DOI] [PubMed] [Google Scholar]
- 19. Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women’s Health Initiative observational study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13(9 suppl):S107–S121. doi: 10.1016/s1047-2797(03)00047-4 [DOI] [PubMed] [Google Scholar]
- 20. Guralnik JM, Simonsick EM, Ferrucci L, et al. . A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85 [DOI] [PubMed] [Google Scholar]
- 21. Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item Health Survey 1.0. Health Econ. 1993;2:217–227. doi: 10.1002/hec.4730020305 [DOI] [PubMed] [Google Scholar]
- 22. Beasley JM, LaCroix AZ, Neuhouser ML, et al. . Protein intake and incident frailty in the Women’s Health Initiative observational study. J Am Geriatr Soc. 2010;58:1063–1071. doi: 10.1111/j.1532-5415.2010.02866.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zaslavsky O, Zelber-Sagi S, Gray SL, et al. . Comparison of frailty phenotypes for prediction of mortality, incident falls, and hip fracture in older women. J Am Geriatr Soc. 2016;64:1858–1862. doi: 10.1111/jgs.14233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shankaran M, Czerwieniec G, Fessler C, et al. . Dilution of oral D3-creatine to measure creatine pool size and estimate skeletal muscle mass: development of a correction algorithm. J Cachexia Sarcopenia Muscle. 2018;9:540–546. doi: 10.1002/jcsm.12278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meyer AM, Evenson KR, Morimoto L, Siscovick D, White E. Test-retest reliability of the Women’s Health Initiative physical activity questionnaire. Med Sci Sports Exerc. 2009;41:530–538. doi: 10.1249/MSS.0b013e31818ace55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Suetta C, Haddock B, Alcazar J, et al. . The Copenhagen Sarcopenia Study: lean mass, strength, power, and physical function in a Danish cohort aged 20-93 years. J Cachexia Sarcopenia Muscle. 2019;10:1316–1329. doi: 10.1002/jcsm.12477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15:615–625. doi: 10.1097/01.ede.0000135174.63482.43 [DOI] [PubMed] [Google Scholar]
- 28. Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18:137–150. doi: 10.1037/a0031034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ensrud KE, Ewing SK, Taylor BC, et al. ; Study of Osteoporotic Fractures Research Group . Frailty and risk of falls, fracture, and mortality in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2007;62:744–751. doi: 10.1093/gerona/62.7.744 [DOI] [PubMed] [Google Scholar]
- 30. Phelan EA, Rillamas-Sun E, Johnson L, et al. . Determinants, circumstances and consequences of injurious falls among older women living in the community. Inj Prev. 2021;27(1):34–41. doi: 10.1136/injuryprev-2019-043499 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.