Abstract
Chronic kidney disease (CKD) is a leading cause of global morbidity and mortality and is independently associated with cardiovascular disease. The mainstay of treatment for CKD is blockade of the renin–angiotensin–aldosterone system (RAAS), which reduces blood pressure and proteinuria and slows kidney function decline. Despite this treatment, many patients progress to kidney failure, which requires dialysis or kidney transplantation, and/or die as a result of cardiovascular disease. The apelin system is an endogenous physiological regulator that is emerging as a potential therapeutic target for many diseases. This system comprises the apelin receptor and its two families of endogenous ligands, apelin and elabela/toddler. Preclinical and clinical studies show that apelin receptor ligands are endothelium-dependent vasodilators and potent inotropes, and the apelin system has a reciprocal relationship with the RAAS. In preclinical studies, apelin regulates glomerular haemodynamics and acts on the tubule to promote aquaresis. In addition, apelin is protective in several kidney injury models. Although the apelin system has not yet been studied in patients with CKD, the available data suggest that apelin is a promising potential therapeutic target for kidney disease.
Subject terms: Kidney, Chronic kidney disease, Glomerulus, Cardiovascular diseases
The apelin system is a broad regulator of physiology that has beneficial cardiovascular and renal effects. This Review focuses on the role of this system in kidney and cardiovascular health and disease and its potential as a therapeutic target.
Key points
Chronic kidney disease (CKD) is common worldwide and is associated with high cardiovascular morbidity and mortality; however, treatment options are limited.
The apelin system is a broad regulator of physiology that opposes the renin–angiotensin–aldosterone system (RAAS) and has beneficial cardiovascular effects in health and disease.
Increasing evidence indicates that apelin has favourable effects on renal physiology, including roles in the regulation of fluid balance and glomerular haemodynamics.
Preclinical models demonstrate direct anti-inflammatory and anti-fibrotic actions of the apelin system in models of kidney injury; targeting the apelin receptor in combination with RAAS blockers might offer synergistic benefits.
The apelin system is an attractive potential therapeutic target for CKD, offering direct renal protection in addition to targeting the associated cardiovascular complications.
Introduction
Chronic kidney disease (CKD) is an increasingly common public health concern with a global prevalence of ~10%1. This disease now ranks as the 12th leading cause of death worldwide1. CKD results from a heterogeneous group of conditions that lead to a progressive and irreversible impairment in kidney function. It is defined as a reduction in estimated glomerular filtration rate (eGFR) to <60 ml/min/1.73 m2 and/or the presence of markers of kidney damage on at least two occasions at least 3 months apart2.
CKD is independently associated with cardiovascular disease3. As eGFR decreases, the risks of major cardiovascular events, cardiovascular mortality and all-cause mortality increase4. Importantly, patients with stage 1–3 CKD (eGFR >30 ml/min/1.73 m2) are more likely to die from cardiovascular disease than they are to reach kidney failure4,5 and around 50% of patients with kidney failure die from cardiovascular causes6. Not only are cardiovascular events more common in patients with CKD, but outcomes following such events are worse than in the general population7. Almost 8% of global cardiovascular deaths in 2017 were attributable to CKD1.
Hypertension is both a cause and a consequence of CKD. As kidney function declines, blood pressure rises, and more than 85% of patients with CKD are hypertensive8. Thus, reducing blood pressure in CKD is a key therapeutic strategy that not only slows the progression to kidney failure but also reduces cardiovascular risk9. However, more than 30% of patients with CKD require four or more antihypertensive agents to achieve adequate blood pressure control and up to 50% never reach their target blood pressure8. Uncontrolled hypertension promotes the development of left ventricular hypertrophy (LVH). The prevalence of LVH increases as kidney function declines and it is present in ~50% of patients with an eGFR of <25 ml/min/1.73 m2 (ref.10). Alongside hypertension and LVH, arterial stiffness, endothelial dysfunction and proteinuria are characteristic features of CKD and important independent predictors of cardiovascular disease4,11–14.
Current evidence-based management of CKD is limited to blockers of the renin–angiotensin–aldosterone system (RAAS) that slow CKD progression4,8. Sodium–glucose co-transporter 2 (SGLT2) inhibitors, which were originally developed for the treatment of type 2 diabetes mellitus (T2DM), have also now been shown to improve kidney and cardiovascular end points in patients with and without T2DM15,16. However, an urgent unmet need remains for novel treatments17. The ideal therapy would provide direct renoprotection and reduce proteinuria, while also offering broad cardiovascular protection. The apelin system has exciting therapeutic potential in this regard. In this Review, we focus on current understanding of the apelin system, its cardiovascular and renal benefits, and the next steps required to explore its utility as a potential therapy for CKD.
Biology of the apelin system
The apelin system comprises the apelin receptor and its two endogenous ligands, apelin and elabela/toddler (ELA; also known as apelin receptor early endogenous ligand).
Apelin receptor
The APLNR gene (also known as APJ) was identified and cloned18 in 1993. This gene was found to encode the apelin receptor, a novel G protein-coupled receptor (GPCR) that has ~50% homology with the type 1 angiotensin II (AT1) receptor. The apelin receptor system opposes the actions of angiotensin II (Ang II) agonism at the AT1 receptor in vitro and in vivo19–27. In the cardiovascular system, apelin and AT1 receptors are co-expressed. The apelin receptor is highly conserved between species with ~90% sequence similarity between mouse, rat and human proteins28. Only one apelin receptor has been identified in mammals, although two are expressed in fish and amphibians29–31.
Apelin receptor ligands
The two endogenous ligands of the apelin receptor are products of distinct genes, which is unusual for a peptide–ligand GPCR. The first to be discovered, apelin (encoded by APLN), was identified in 1998 and the second, ELA (encoded by APELA), was identified in 2013 (refs32–35).
Apelin
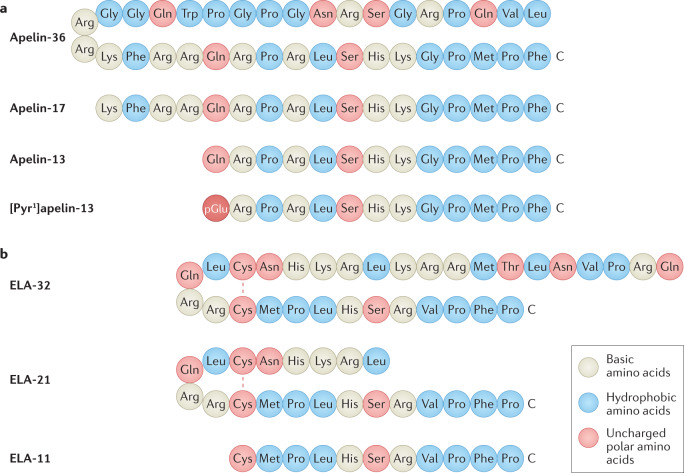
Apelin peptides are produced by C-terminal cleavage of a 77-amino-acid precursor, pre-pro-apelin. Peptide fragments of varying lengths circulate in vivo; the major isoforms are apelin-36, apelin-17 and apelin-13 (ref.28) (Fig. 1a). The pyroglutamated form of apelin-13, [Pyr1]apelin-13, is structurally more resistant to metabolism by aminopeptidases than apelin-13 and is the most abundant apelin isoform in the cardiovascular system and human plasma36,37.
Fig. 1. The peptide sequences of apelin and ELA isoforms.
a | The amino acid sequences of apelin-36, apelin-17, apelin-13 and [Pyr1]apelin-13 aligned by the C terminus, which is crucial for receptor binding and biological activity. The N-terminal Gln residue of apelin-13 undergoes translational modification to pyroglutamate, which increases resistance to metabolism by aminopeptidases. The resulting pyroglutamated form of apelin-13, [Pyr1]apelin-13, is the most abundant apelin isoform in humans34,35. b | The predicted amino acid sequences of ELA-32, ELA-21 and ELA-11 isoforms23,33. The N-terminal Gln of ELA-32 is predicted to undergo transformation to pyroglutamate. ELA-32 and ELA-21 form bridges between Cys residues. The occurrence and relative abundance of ELA isoforms have not yet been confirmed experimentally. Apelin and ELA isoforms have similar physicochemical properties, particularly within the C terminus.
The mechanisms of apelin peptide metabolism are not yet fully defined. Experimental evidence suggests that furin (also known as PCSK3), plasma kallikrein and neprilysin cleave apelin peptides, but only neprilysin has been shown to completely inactivate these peptides27,38–41. This finding is of interest when considering the cardioprotective effects of neprilysin inhibition in heart failure42. The mechanisms that underlie these effects remain undefined but prevention of the neprilysin-mediated degradation of apelin peptides could potentially contribute.
The carboxypeptidase angiotensin-converting enzyme 2 (ACE2) has been clearly demonstrated to cleave apelin isoforms both in vitro and in vivo, resulting in the removal of the common C-terminal phenylalanine43–45. This enzyme is highly expressed on the surface of lung alveolar epithelial cells and enterocytes and is expressed at lower levels in most organs including kidney and heart and on arterial and venous endothelial cells45–47. ACE2 promotes vasodilatation by converting Ang II into angiotensin-1-7 (Ang(1-7))43. In addition, ACE2 acts as a functional cellular entry receptor for severe acute respiratory syndrome coronaviruses, including SARS-CoV-2, which causes COVID-19 (refs48–50). ACE2 was thought to inactivate [Pyr1]apelin-13 (ref.44); however the [Pyr1]apelin-13 metabolite, [Pyr1]apelin-13(1–12), is detectable in human tissues and retains biological activity in vitro and in vivo although it is less active than [Pyr1]apelin-13 (refs44,45). In healthy volunteers who received [Pyr1]apelin-13 infusions, the major metabolite generated in the plasma was [Pyr1]apelin-13(1–12); [Pyr1]apelin-13(1–10) and [Pyr1]apelin-13(1–6) were also identified51.
Elabela
ELA is a 54-amino-acid peptide that is predicted to be processed to form mature peptides including ELA-32, ELA-21 and ELA-11 (Fig. 1b). The discovery of ELA as the second endogenous ligand for the apelin receptor provided an explanation for the unexpected and marked discrepancy between the phenotypes of apelin receptor-knockout mice, which were not born in expected Mendelian ratios and had substantial cardiovascular developmental defects, and apelin-knockout mice, which developed normally52.
In zebrafish, most ELA mutants die early in development as a result of cardiovascular abnormalities, including poorly developed or absent hearts. This phenotype could be rescued by injection of ELA, and the cardiovascular abnormalities could be recapitulated by knocking out the apelin receptor but not by knocking out apelin30,34,35,53.
Mice that lack ELA show low-penetrance embryonic lethality and cardiovascular abnormalities; however, their phenotype differs from that of apelin receptor knockouts54. Loss of ELA leads to yolk sac vascular remodelling defects with cardiac abnormalities and disrupted fetal circulation, whereas mice with loss of the apelin receptor have a lower incidence of such defects and some embryos show abnormal tail bending. The abnormalities in coronary vascularization in ELA knockouts and apelin receptor knockouts may be overcome by alternative progenitor cell pathways as development progresses, perhaps explaining the low-penetrance lethality55. ELA is detectable in human plasma but its specific tissue isoform expression has not yet been explored56.
Expression of the apelin system
Apelin and the apelin receptor are widely expressed within the central nervous system and peripheral organs. In rodents and humans, apelin receptor mRNA is detected in many tissues, particularly in brain, spinal cord, placenta, lung, heart, kidney, adipose tissue and skeletal muscle57–60. Apelin receptor protein has been identified in the brain, spinal cord, heart, kidney and lung of rats and humans59,61,62. In human heart, apelin receptor is expressed by cardiomyocytes, endothelial cells of the endocardium and intramyocardial blood vessel, endothelial cells and smooth muscle of conduit artery and vein59,61,62. Within the kidney, apelin receptor protein localizes to the cortex and vasculature61,62.
In humans and rats, mRNA encoding apelin is widely expressed throughout the brain, spinal cord, lung, kidney, heart and stomach with lower expression in other tissues33,58,59,63. In the periphery, apelin protein localizes predominantly to vascular endothelial cells, for example, in human heart, kidney and adrenal gland and to the endocardial endothelial cells that line the atria and ventricle64. Apelin is also an adipokine65 that localizes to adipose tissue and is produced by and secreted from human and rodent adipocytes60,66. However, apelin does not seem to be a major circulating hormone, and plasma concentrations are lower than tissue levels36,67. Given this finding and the similar locations of apelin peptide and apelin receptor expression, it seems probable that the apelin system has a predominantly autocrine or paracrine mechanism of action. In contrast to apelin, ELA was initially reported to show kidney-specific expression in adult rat, largely confined to the renal tubules, but was subsequently identified in rodent heart68–70. To date, human studies have detected ELA expression only on the vascular endothelium and within the kidney56,71.
Apelin receptor binding and signalling
The endogenous apelin isoforms bind with nanomolar affinity to their cognate receptor in cell expression systems and in rat and human cardiovascular tissues45,57,59,61,67,72,73. However, structure–activity studies suggest that the longer isoforms, such as apelin-36, and particularly apelin-17, have somewhat higher affinity than the shorter isoforms such as [Pyr1]apelin-13 (refs45,74,75). The N-terminal RPRL sequence of [Pyr1]apelin-13, which is present in all longer apelin isoforms, is required for receptor binding with some contribution from other residues such as Pro12 (refs76–78) (Fig. 1a). The smallest apelin fragment to retain biological activity, although with reduced affinity, is the 10-amino-acid apelin-13(2–11)45,57,79.
To date, only one crystal structure has been reported for the apelin receptor in complex with a 17-amino-acid non-endogenous apelin agonist80. Substantial mutation was required for successful crystallization of the receptor. However, using this structure in combination with molecular dynamic simulations and molecular modelling, the study demonstrated a two-site peptide–ligand binding mode. This ‘message–address’ concept of peptide binding was also suggested by earlier studies, including one in which amino acids in the N-terminal tail and first extracellular loop were shown to be important both for folding of the receptor protein and binding of apelin peptides81. In another structural study, the upper face of the apelin receptor binding site was visualized using 3D homology models, and the interactions of basic residues in the N-terminal RPRL of [Pyr1]apelin-13 with acidic residues in extracellular loops I and II as well as the interface between extracellular loop III and transmembrane VII were confirmed using site-directed mutagenesis82.
The ELA peptides show little sequence similarity to apelin although the predicted isoforms ELA-32, ELA-21 and ELA-11 bind to the apelin receptor in human heart; ELA-32 bound with subnanomolar affinity, ELA-21 with nanomolar affinity (comparable to that of [Pyr1]apelin-13) and the shortest isoform, ELA-11, bound with affinity an order of magnitude lower than that of the other peptides56. An alanine scan and mutagenesis analysis suggested that apelin and ELA peptides engage with different residues in rat and human apelin receptors83.
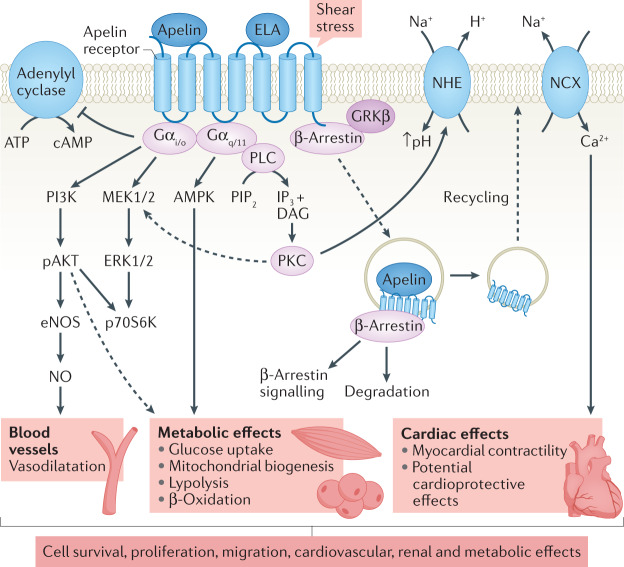
The apelin receptor couples to pertussis toxin-sensitive inhibitory G proteins (Gαi/o) with subsequent activation of extracellular-regulated kinases (ERKs) and phosphoinositide 3-kinase (PI3K)–AKT (also known as protein kinase B (PKB)) signalling cascades57,84–87 (Fig. 2). These cascades lead to a broad range of physiological effects that are dependent on the type of cell that is activated. All putative apelin and ELA isoforms elicit canonical Gi-mediated inhibition of adenylate cyclase, which results in inhibition of cAMP45,56,67,72,84. Similar to other GPCRs, the apelin receptor can engage with other heterotrimeric G proteins, particularly Gq, resulting in downstream stimulation of phospholipase C (PLC) and AMP-activated protein kinase (AMPK) pathways88,89.
Fig. 2. Apelin receptor activation leads to a broad range of physiological actions that are mediated by several signalling pathways.
In vascular endothelial cells, binding of apelin or elabela/toddler (ELA) to the apelin receptor results in Gαi-mediated inhibition of cAMP production and activation of phosphoinositide 3-kinase (PI3K)–AKT signalling cascades, leading to vasodilatation. In cardiomyocytes, activation of the apelin receptor leads to a Gαq-mediated increase in cardiac contractility and cardiac output through activation of the phospholipase C (PLC)–protein kinase C (PKC) pathway with subsequent enhanced activity of the Na+/H+ exchanger (NHE) and increased intracellular pH. The increase in intracellular Na+ activates the Na+/Ca2+ exchanger (NCX), leading to a rise in intracellular calcium and increased myocardial contractility. Apelin-13 is thought to promote cardiomyocyte hypertrophy via PI3K–AKT–ERK1/ERK2–p70S6K and PI3K-induced autophagy (not shown). The metabolic effects of apelin receptor activation have been proposed to be mediated via Gαi and Gαq. Signalling via β-arrestin leads to apelin receptor desensitization and internalization. The internalized receptor is targeted for degradation or recycled to the cell surface. β-Arrestin-mediated signalling may also contribute to the actions of apelin. DAG, diacylglycerol; eNOS, endothelial nitric oxide synthase; ERK1, extracellular signal-regulated kinase 1; GRKβ: G protein coupled receptor kinase-β; IP3, inositol tris-phosphate; MEK1, mitogen-activated protein kinase 1; NO, nitric oxide; pAKT, phosphorylated AKT; PIP2, phosphatidylinositol 4,5-bisphosphate; p70S6K, p70 ribosomal S6 kinase.
Following activation, GPCRs can be uncoupled from their G proteins and internalized via recruitment of β-arrestins to the receptor. The extent and kinetics of apelin receptor-mediated internalization may be isoform specific, at least in vitro, with apelin-36 suggested to produce more prolonged internalization than [Pyr1]apelin-13 (refs72,90–92). For successful translation of apelin receptor agonists to the clinical setting, the finding that GPCR ligands may preferentially activate a subset of the signalling repertoire of a GPCR or indeed stimulate some signalling pathways and inhibit others, so called ‘biased signalling’, is an important discovery93. Compared with [Pyr1]apelin-13, longer apelin ligand isoforms such as apelin-36, apelin-17, ELA-32 and ELA-21 have been shown to exhibit some bias towards β-arrestin recruitment45,56,73. The in vivo relevance of these observations to apelin receptor physiology or pathophysiology remains to be determined.
Ligand-independent signalling through the apelin receptor has also been reported19,94 and may be pathologically important. Phosphorylation of ERK1 and ERK2 downstream of Ang II-mediated activation of AT1 receptor was inhibited in cells that co-expressed the apelin receptor and this inhibition was abolished by apelin in a pertussis toxin-sensitive manner19. Subsequently, genetic loss of the apelin receptor was demonstrated to protect against cardiac hypertrophy and heart failure in a chronic pressure overload mouse model, whereas loss of apelin had no effect94. This discrepancy could potentially be explained by the later discovery of ELA. However, isolated cardiomyocytes from apelin receptor-knockout mice exhibited a reduced response to stretch, implying that the apelin receptor can act as a mechanosensor even in the absence of ligand. This stretch-mediated response was abrogated by knockdown of β-arrestins and blunted by the addition of apelin94. The apelin receptor might therefore have pro-hypertrophic effects via β-arrestin signalling in the absence of ligand and anti-hypertrophic effects via β-arrestin-independent pathways when activated by apelin.
Clinical targeting of the apelin system
As apelin peptides have a half-life of only a few minutes in humans95–97, and rapid receptor desensitization via coupling to β-arrestins has been reported98, clinical studies of the apelin system are challenging. These issues have led to efforts to produce apelin receptor agonists and antagonists that may be used as pharmacological probes to explore the role of the system in health and disease. Clear understanding of the relevant signalling pathways is essential to enable the development of appropriately targeted therapeutic agents.
Apelin analogues with enhanced biological activity compared with the endogenous peptides and resistance to degradation have now been developed99,100. In a human forearm blood flow study, a cyclic apelin peptide mimetic, MM07, which has an extended half-life, showed biased agonism to beneficial G protein pathways and enhanced vasodilatation compared with [Pyr1]apelin-13 (ref.97). Similarly, another apelin mimetic peptide bound to anti-serum albumin domain antibodies to extend its half-life had in vivo potency, lowering blood pressure and increasing cardiac contractility, stroke volume, heart rate and cardiac output in rodents75.
From a clinical perspective, the ideal agent would likely be a small-molecule compound biased towards G protein signalling that does not effectively recruit β-arrestins and, therefore, limits receptor desensitization and subsequent downregulation with continued clinical use97,101. One such small molecule, CMF-019, has been developed. This molecule exhibits high affinity for the apelin receptor and has in vivo functional activity with a bias towards G protein signalling101. However, its use as an oral agent may be limited by its poor solubility.
Two small-molecule agonists of the apelin receptor, AMG-986 and AMG-8123, with extended plasma half-lives (>2–4 h in rats and dogs) have also been developed. These molecules have a high affinity for the apelin receptor and were shown to be functional in vivo and in animal models of heart failure102. Other small-molecule apelin agonists are also in development28.
Although several compounds have been suggested to act as apelin antagonists, contradictory studies suggest that some also have agonist activity. The field would benefit from the identification of well-characterized antagonists. If successful, the development of apelin agonists and antagonists could lead to a new era of research and therapeutics based on the apelin system28.
Apelin in cardiovascular physiology
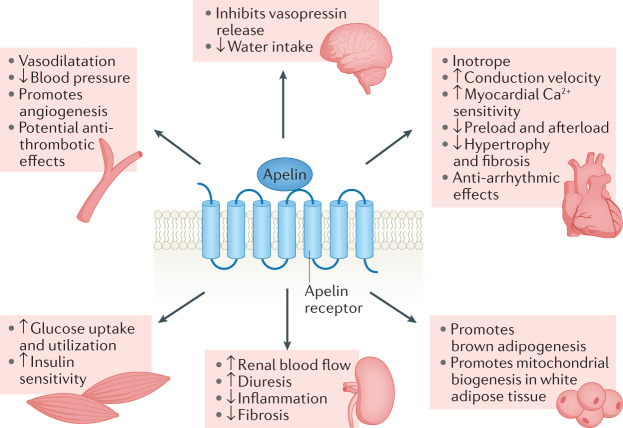
The apelin system is a broad regulator of physiology (Fig. 3). In the cardiovascular system, apelin has roles in control of vascular tone, blood pressure, inotropy, angiogenesis and haemostasis.
Fig. 3. Physiological actions of the apelin system in different organs and tissues.
Activation of the apelin receptor results in a broad range of physiological effects in central and peripheral tissues. Within the vasculature, the apelin system promotes vasodilatation, subsequent lowering of blood pressure and angiogenesis and might also have antithrombotic effects. Apelin is a potent inotrope that increases myocardial Ca2+ sensitivity and reduces cardiac preload and afterload. It also increases the conduction velocity within cardiomyocytes, has anti-arrhythmic effects and reduces myocardial hypertrophy and fibrosis. In the kidney, activation of the apelin system increases renal blood flow and diuresis and reduces inflammation and fibrosis. The apelin system also has a central role in the regulation of fluid homeostasis: apelin inhibits hypothalamic vasopressin release and reduces water intake. Finally, the apelin system has a range of metabolic effects. It promotes brown adipogenesis and mitochondrial biogenesis in white adipose tissue, increases muscle glucose uptake and utilization and increases insulin sensitivity.
Vascular function and blood pressure
Apelin has well-described effects on the vasculature. Preclinical studies established that in healthy vessels, apelin acts on the endothelium to promote vasodilatation through an increase in nitric oxide (NO)21,103 (Fig. 2). By contrast, in endothelium-denuded vessels, apelin acts directly on vascular smooth muscle cells to promote vasoconstriction36,45. Various animal models have demonstrated that multiple apelin peptides lead to a rapid, transient and dose-dependent reduction in blood pressure, with variable effects on heart rate33,45,72,104,105. The blood pressure-lowering action of apelin in vivo is also NO dependent21,105.
In humans, infusions of [Pyr1]apelin-13, [Pyr1]apelin-13(1–12) and apelin-36 led to reproducible, dose-dependent and NO-dependent arteriolar vasodilatation in the forearm45,95,97. In keeping with in vitro data that show slower dissociation of apelin-36 than [Pyr1]apelin-13 from the apelin receptor, this peptide has a more prolonged action in humans57,95. Systemic studies in healthy volunteers demonstrated that apelin reduces blood pressure; [Pyr1]apelin-13 led to a 5–10% fall in blood pressure, reduced peripheral vascular resistance and caused a small increase in heart rate that was most likely compensatory96. The effects of apelin on venous tone are less consistent. One study found that [Pyr1]apelin-13 reversed noradrenaline-induced pre-constriction of dorsal hand veins, whereas another showed no effect95,97. However, this discrepancy may be explained by the ~30-fold difference in peptide dose in these studies.
ELA also promotes vasodilatation, although its mechanism of action may differ from that of apelin. In preclinical studies, ELA relaxed pre-constricted mouse aortic rings in a dose-dependent manner and induced carotid artery vasodilatation in the rat70,71. In vivo data confirm that systemic infusions of both ELA and a bioactive analogue, ELA(19–32), reduce blood pressure in rodents56,99. In contrast to apelin, ELA-induced vasodilatation is NO independent and only partly dependent on the endothelium71. The effects of ELA have not yet been investigated in humans.
Inotropy and conduction
Apelin acts via several signalling pathways and is the most potent endogenous inotrope discovered to date36,88,106,107 (Fig. 2). Mice with knockout of apelin develop a progressive reduction in cardiac contractility that can be restored by apelin infusion108. Apelin administration accentuated sarcomere shortening in healthy rat cardiomyocytes and resulted in a dose-dependent increase in contractility in isolated perfused rat hearts88,106,109. Consistent with this finding, chronic [Pyr1]apelin-13 infusion increased cardiac output in vivo in mice110.
A number of apelin peptides have been shown to promote inotropy with subnanomolar potency in human paced atrial strips36,45. In a clinical study in healthy volunteers, intracoronary boluses of apelin-36 and systemic infusions of [Pyr1]apelin-13 and apelin-36 increased cardiac output36,45,96. This effect was maintained in the setting of renin–angiotensin system (RAS) activation owing to either salt depletion or Ang II co-infusion24.
Clear evidence exists of antagonism between the apelin system and the RAAS. In addition to impaired contractility, apelin-deficient mice have reduced ACE2 expression, and inhibition of AT1 receptors or infusion of Ang(1-7) rescued cardiac function and restored ACE2 expression in these mice111. In addition, Ang II-induced cardiac dysfunction is enhanced by apelin deficiency112. Many inotropic agents result in LVH and subsequently increased mortality; however, apelin does not cause cellular hypertrophy in animal studies110. This difference is likely a consequence of the systemic haemodynamic effects of apelin, which are in contrast to the effects of other inotropic agents and increase its appeal as a therapeutic agent.
ELA is also a positive inotrope in preclinical models. In isolated adult rat hearts, ELA-32 increased cardiac contractility70. This was replicated in a second study that also showed ELA(19–32) to be inotropic99. Further in vivo studies confirmed that in rats, ELA-32 increases cardiac contractility, ejection fraction and cardiac output56.
The apelin system has also been shown to influence cardiac conduction. Preclinical studies demonstrate that apelin increases conduction velocity within cardiomyocytes and influences the cardiac action potential109,113. For example, apelin shortens the action potential in atrial myocytes via effects on multiple ion currents113.
Angiogenesis
Apelin is necessary for normal vascular development during embryogenesis114,115. Embryos with knockout of apelin initially show reduced-calibre blood vessels115. Other factors seem to be able to ‘rescue’ these vessels later in development, although these remain undefined. However, studies of ocular blood vessel formation show that apelin-knockout mice have impaired retinal vascularization and reduced responsiveness to pro-angiogenic factors such as vascular endothelial growth factor (VEGF) and fibroblast growth factor 2 (ref.116). Preclinical studies confirm that ELA also promotes angiogenesis by acting on the apelin receptor55,71.
Apelin expression is induced by hypoxia both in vitro and in vivo, and hypoxia-inducible factor 1α (HIF1α) promotes apelin gene transcription114,117,118. Interestingly, hypoxia-stimulated endothelial cell proliferation is prevented by inhibition of the apelin pathway, suggesting that the apelin system contributes to physiological and pathophysiological regulation of blood vessel growth117.
In addition to its physiological role in vascular development, apelin might have a pathological role in promoting angiogenesis in various cancers (Box 1).
Box 1 Apelin in cancer.
Apelin is overexpressed in one-third of human solid organ malignancies and may act in a paracrine or autocrine manner to promote angiogenesis179,180. Hypoxia has been shown to upregulate apelin expression in tumour cell lines179,181. However, cancer biology is unique to the type of malignant cell. Studies in colorectal cancer cell lines found that apelin was anti-apoptotic, and the apelin receptor antagonist F13A reduced cell proliferation180. Genetic overexpression of apelin in mouse non-small cell lung cancer (NSCLC) cells promoted tumour growth, and loss of apelin in models of breast and lung cancer reduced tumour growth and angiogenesis and increased survival182,183. In addition, loss of apelin or treatment with the biased apelin receptor ligand MM54 had synergistic antitumour effects with the tyrosine kinase inhibitor sunitinib in the MMTV-NeuT mouse model of mammary cancer183. Although previously considered to be an apelin receptor antagonist, MM54 has been shown to act as a partial agonist and to promote G protein signalling pathways184.
Studies in models of glioblastoma, the most common primary adult brain tumour, suggest that endothelium-derived apelin sustains glioblastoma stem-like cells, which have been implicated in initiation and facilitation of tumour growth as well as treatment resistance. MM54 inhibited tumour growth and prolonged survival in intracranially xenografted mice185. Interestingly, bevacizumab, a monoclonal antibody to vascular endothelial growth factor (VEGF), can reduce apelin expression in biopsy samples from patients with glioblastoma and is known to increase cell invasion186. F13A reduced cell invasion and tumour angiogenesis and showed synergistic anti-angiogenic effects when combined with VEGF inhibition in a mouse model of glioblastoma186.
Elabela/toddler (ELA) is downregulated in human renal cell carcinoma (RCC), and activation of the apelin receptor by ELA reduced tumour cell survival, proliferation and motility in cultured renal cancer cells and in mouse models of RCC. Overexpression of ELA in renal cancer cells induced apelin receptor expression, and the tumour-suppressing effect of ELA was enhanced in vivo in the presence of sunitinib187.
Intriguingly, a functional apelin receptor is necessary for tumour T cell effector function and successful cancer immunotherapy; however, this effect may be ligand independent188. In RCC, apelin receptor expression is inversely associated with tumour grade and the presence of metastases, and lower apelin receptor expression is an independent predictor of shorter cancer-specific and overall survival189. In NSCLC models, elevated levels of apelin protein are also independently associated with poorer survival182. Overall, the available data suggest that targeting the apelin system may offer therapeutic promise in several cancers.
Haemostasis
Both apelin and the apelin receptor are expressed on human platelets. Apelin-13 inhibits platelet aggregation induced by low (but not high) concentrations of thrombin or collagen in vitro but does not seem to influence platelet activation by other stimuli such as thromboxane A2 or ADP119. The effects of apelin on platelets are mediated through inhibition of calcium mobilization as well as the generation of NO119, which promotes platelet production of cGMP. The NO–cGMP pathway has a biphasic effect with low concentrations activating platelets and high concentrations being inhibitory120. Thrombin increases cGMP levels within platelets, promoting their activation. The addition of apelin further increases cGMP levels, leading to inhibition of platelet activity119.
Animal data confirm the antithrombotic effect of apelin in vivo. Apelin-deficient mice are pro-thrombotic, with reduced tail bleeding time, enhanced platelet aggregation and faster thrombotic occlusion of venules compared with wild-type controls. Infusion of apelin-13 prolongs bleeding time in both wild-type and apelin-deficient mice119. Whether apelin also has an antithrombotic effect in humans has not yet been investigated. Current antiplatelet therapies focus on inhibition of cyclooxygenase 1 (using aspirin) or antagonism of the ADP P2Y12 receptor (using clopidogrel, prasugrel or ticagrelor). Thrombotic events can still occur in patients who are receiving antithrombotic combination therapies, and an agent that provides broader cardiovascular protection while reducing the risk of thrombo-occlusive events would be very desirable.
Apelin in cardiovascular disease
The apelin system is dysregulated in cardiovascular disease, and beneficial effects of its activation have been shown in disease models. The apelin receptor is therefore an appealing target for pharmacological intervention.
Vascular actions of apelin in disease
CKD is characterized by medial vascular calcification and the development of arterial stiffness. This change in vascular structure alongside endothelial injury leads to endothelial dysfunction. Importantly, preclinical and clinical studies show that the vasodilatory effect of apelin persists even in states of endothelial dysfunction, and this effect seems to be independent of NO36,103. In this setting, apelin might act via a prostanoid-dependent mechanism. However, the available data are inconclusive, perhaps owing to the use of different cyclooxygenase inhibitors and their differential effects on downstream signalling36,103.
In clinical studies, patients with heart failure and healthy volunteers had equivalent dose-dependent vasodilatation of forearm vessels in response to [Pyr1]apelin-13 (ref.96). In a mouse model of immune-mediated vascular injury after heart transplantation, apelin promoted vascular repair and endothelial cell differentiation and reduced immune cell adhesion121. This finding could have exciting therapeutic potential for similar immune-mediated vascular injury following kidney transplantation.
The vascular endothelium is an important regulator of coagulation. Endothelial release of tissue plasminogen activator promotes fibrinolysis, and its activity is regulated by plasminogen activator inhibitor 1 (PAI1). In states of endothelial dysfunction, fibrinolysis may be impaired. A reciprocal relationship exists between expression of the apelin system, the AT1 receptor and PAI1 in patients with atrial fibrillation and thrombosis122. In left atrial appendages from these patients, expression of apelin and the apelin receptor was reduced and expression of AT1 receptors and PAI1 was increased compared with those from patients with sinus rhythm or with atrial fibrillation without thrombosis. In healthy mice, 3 weeks of treatment with Ang II promotes PAI1 gene expression, whereas similar exposure to apelin downregulates PAI1 gene expression in preclinical models23. This finding may provide an additional mechanism by which apelin regulates haemostasis.
Atherosclerosis
The apolipoprotein E (Apoe)-knockout mouse is a widely used model of atherosclerosis. Mice that are deficient in both ApoE and apelin develop more atheromatous lesions than Apoe-knockout mice, which in turn develop more atheromatous lesions than Apoe and apelin receptor-double-knockout mice123,124. The reasons for these contrasting data are unclear but it is noteworthy that mice that lack the apelin receptor show increased prenatal death, and those that do survive have cardiovascular malformations that likely contribute to accelerated atherosclerosis. The apelin receptor might have ligand-independent effects on the development of atherosclerosis, but such effects have not been noted in studies using apelin receptor-knockout mice. In the Apoe-knockout mouse, treatment with apelin alone had no effect on the development of atheromatous lesions123. However, administration of Ang II resulted in an increase in atherosclerotic lesions that was abrogated by coadministration of apelin, providing further evidence of functional interaction of the RAS and the apelin system in vivo123.
Heart failure
The available data suggest that the apelin system is upregulated early in heart failure and downregulated as the disease progresses125. Plasma apelin concentrations show a similar pattern22,125–128. Evidence exists of functional crosstalk between the apelin system and the RAS in heart failure. In animal models, Ang II infusion results in downregulation of cardiac apelin mRNA and this effect can be inhibited by an angiotensin receptor blocker (ARB). Similarly, in animals with heart failure, downregulation of the apelin system can be restored by an ARB22,129. Administration of l-NG-monomethylarginine (l-NAME), an inhibitor of NO synthesis, abrogated the beneficial effects of an ARB (olmesartan) on cardiac function and the apelin system in a heart failure model129. These findings together with the inotropic effect of apelin have led to sustained enthusiasm regarding the potential therapeutic role of apelin in heart failure.
Preclinical studies have demonstrated that apelin has inotropic effects in models of heart failure109,130. Moreover, systemic infusion of [Pyr1]apelin-13 resulted in a modest fall in blood pressure and systemic vascular resistance and a ~10% increase in cardiac index in patients with stable New York Heart Association Class II–IV heart failure symptoms and a left ventricular ejection fraction of <40% (or shortening fraction <20%) who were receiving optimized treatment96. Further studies found this improvement in cardiac output to be sustained during prolonged apelin infusion (6 h), with an associated increase in ejection fraction of ~10%24. At present, the utility of apelin in heart failure is limited by the lack of long-acting preparations.
Myocardial infarction
Studies in several preclinical models have demonstrated that apelin protects against ischaemic myocardial injury. Apelin-knockout mice show enhanced susceptibility to injury, with larger infarct size, increased ventricular dilatation and increased mortality compared with wild-type controls100. Administration of apelin or an apelin analogue had protective effects in models of myocardial infarction, including improved functional recovery and a reduction in infarct size. These effects were most likely due to promotion of NO production and angiogenesis100,131.
Following any ischaemic injury, angiogenesis is stimulated to promote oxygen delivery to tissues. In a mouse model of myocardial infarction, overexpression of HIF1α reduced infarct size and the development of heart failure132. Apelin signalling is critically important in angiogenesis, APLN may be upregulated by HIF1α, and apelin-deficient animals have impaired angiogenic responses to ischaemia–reperfusion injury (IRI)100,118. Currently, following myocardial infarction, patients are treated with RAS blockers to reduce the detrimental effects of Ang II. Importantly, a preclinical study showed that combination treatment with apelin and the ARB losartan reduced infarct size by ~50%, compared with a ~30% reduction when either treatment was used individually133. This finding holds promise for future clinical studies in this area.
Arrhythmias
Clinical studies have shown that plasma apelin levels are reduced in patients with supraventricular tachycardias, including atrial fibrillation134,135. Moreover, apelin levels predict the recurrence of atrial arrhythmias136. Patients with atrial fibrillation have reduced atrial apelin levels, and apelin administration completely prevented the induction of atrial arrhythmias in mice with increased susceptibility owing to chronic intense exercise137. Atrial fibrillation is associated with a high risk of ischaemic stroke and is common in patients with CKD. The prevalence of atrial fibrillation increases as kidney function declines, reaching ~20% in patients not requiring kidney replacement therapy138,139. Decisions on anticoagulation in this patient group are complex as bleeding risk also increases as kidney function declines140. If targeting of the apelin system is able to protect against arrhythmia, this strategy could be particularly advantageous for patients with CKD.
Apelin in kidney physiology
Although widely expressed in human kidney, studies of the role of apelin in human kidney physiology are lacking. Animal studies suggest that apelin has direct actions on the kidney that contribute to the regulation of renal haemodynamics and fluid homeostasis.
Glomerular haemodynamics
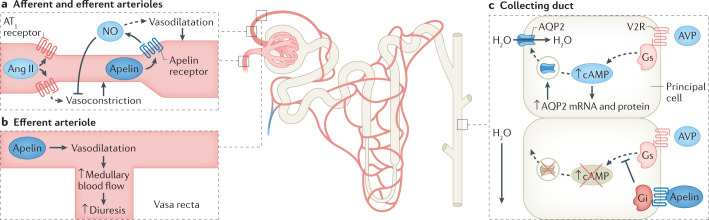
Glomerular blood flow is under the tight control of the RAS, which regulates both glomerular perfusion and GFR, and crosstalk exists between the RAS and the apelin system (Fig. 4a). By increasing intracellular calcium levels, Ang II vasoconstricts both the afferent and efferent arterioles, with a preferential effect on the efferent arteriole. In rats, apelin reverses Ang II-induced vasoconstriction of the afferent and efferent glomerular arterioles and causes a rapid fall in intracellular calcium levels. This vasodilatory action of apelin is dependent on an intact endothelium and NO production141. As the vasa recta are supplied by the efferent arteriole, apelin-induced vasodilatation might promote diuresis through an increase in renal medullary blood flow (Fig. 4b).
Fig. 4. The actions of apelin in the nephron.
a | Apelin acts at the afferent and efferent arterioles to promote vasodilatation via production of nitric oxide (NO), opposing the action of angiotensin II (Ang II). b | Increased vasodilatation at the efferent arteriole directly increases blood flow through the vasa recta, leading to increased medullary blood flow and promoting diuresis. c | The action of apelin counteracts vasopressin signalling in the kidney tubules. In the principal cells of the collecting duct, apelin prevents vasopressin-induced translocation of aquaporin 2 (AQP2) channels to the apical membrane and therefore prevents water reabsorption. AT1 receptor, type 1 angiotensin II receptor; AVP, arginine vasopressin; Gi, inhibitory G protein α-subunit; Gs, stimulatory G protein α-subunit; V2R, vasopressin v2 receptor.
Tubular function
Apelin contributes to fluid homeostasis by opposing the actions of vasopressin, with crosstalk between the systems. Apelin, the apelin receptor and vasopressin co-localize within the magnocellular neurons of the supraoptic and paraventricular nuclei of the hypothalamus, and clear reciprocal regulation of the peptides exists142–144. Clinical studies in healthy volunteers support these findings, with changes in plasma osmolality accompanied by parallel reciprocal changes in vasopressin and apelin levels145.
Both central and peripheral injections of apelin peptides and ELA promote diuresis in animals68,141,143,144,146. In addition, apelin acts directly on the renal tubule to promote a dose-dependent aquaresis without affecting renal sodium or potassium handling141,146. In vitro and in vivo studies suggest that this aquaresis is due to inhibition of aquaporin 2 channel insertion into the apical plasma membrane of the collecting duct146,147 (Fig. 4c). Within the rodent kidney, ELA is more abundantly expressed than apelin and predominates in the medullary collecting ducts69. The specific mechanism of ELA-induced diuresis has not yet been defined. However, given that expression of ELA is restricted to the vascular endothelium and kidney in adults, a direct renal action would be expected.
Apelin in kidney disease
Several in vitro and in vivo studies have investigated the effects of the apelin system in kidney pathology. Apelin is protective in a broad range of diseases owing to its anti-inflammatory and anti-fibrotic effects and might also have important roles in certain kidney diseases.
Acute kidney injury
Acute kidney injury (AKI) affects 8–17% of hospitalized patients and >50% of those who are admitted to intensive care148,149. Moreover, AKI is strongly associated with adverse outcomes including the development of CKD, kidney failure and death, although the mechanisms are poorly defined150. Studies in models of AKI suggest early upregulation of the apelin system that is followed by downregulation as injury progresses151,152. This dynamic change is similar to that seen in heart failure.
Both in vitro and in vivo data support a direct protective effect of apelin treatment in kidney IRI, which is a widely used model of AKI151,153. Following IRI in rats, twice-daily administration of apelin-13 for 3 days protected against tubular injury, the hallmark of AKI, and also limited functional kidney impairment151. Apelin-13 also influenced injury-induced alterations in gene transcription both in vitro and in vivo, preventing downregulation of apelin mRNA and upregulation of HIF1α and transforming growth factor β (TGFβ), which are important mediators of progressive injury. Upregulation of inflammatory markers such as intracellular adhesion molecule 1 and monocyte chemokine protein 1 was also reduced, as was apoptosis151. Importantly, apelin was protective when administered before IRI153, suggesting potential clinical applications in settings where AKI might be anticipated, including kidney transplantation or cardiothoracic surgery. Renoprotective effects of apelin have also been reported in other models of AKI, such as cyclosporin A-induced tubular injury, suggesting that apelin might protect against various renal insults, including drug-induced injury154.
ELA also protects against kidney IRI. Treatment with either ELA-32 or ELA-11 inhibited IRI-induced inflammation, apoptosis and fibrosis and preserved kidney function in a mouse model of unilateral ureteric obstruction155. Like apelin, specific ELA fragments have different signalling effects, with the shorter fragment, ELA-11, being more protective than ELA-32 both in vitro and in vivo. The mechanism of action is not clear, as doses of peptide that conferred protection did not induce a change in intracellular cAMP, which would be expected with apelin receptor activation155. Apelin-13 and ELA therefore seem to have different mechanisms of action and a synergistic effect has been suggested, with combination treatment offering greater protection against in vitro cell death155. Whether this finding could translate to synergy in humans and greater protection against AKI is unclear, but should be explored in clinical studies.
Diabetic kidney disease
Diabetic kidney disease (DKD) is the most common cause of kidney failure worldwide156. Hyperglycaemia alters glomerular haemodynamics, leading to glomerular hypertrophy, damage to the filtration barrier and the development of albuminuria. Preclinical studies suggest that expression of the apelin system is altered in DKD. Cultured mouse podocytes have been shown to downregulate APLNR mRNA157 but upregulate apelin receptor expression158 in response to a high-glucose environment. Ang II administration further increased APLNR mRNA downregulation in these cells157. Some studies in animal models of T1DM and T2DM have shown reduced kidney expression of the apelin receptor and pre-pro-apelin, whereas others have shown the opposite157,159,160.
Definition of the role of the apelin system in DKD is limited by a lack of clinical studies and conflicting animal data161. In cultured mouse podocytes, apelin-13 had anti-apoptotic effects157. In the Akita mouse model of T1DM, apelin-13 preserved glomerular architecture, reduced proteinuria and inhibited renal inflammation162. The researchers found that this protection was due, in part, to an inhibitory effect of apelin on diabetes-induced histone hyperacetylation. Similar protection was observed in Ove26 mice with T1DM in which apelin-13 was found to have antioxidant effects and prevent the loss of megalin expression in the proximal tubule, resulting in maintenance of albumin resorption159.
Conversely, in the KK-Ay mouse model of T2DM, apelin contributed to disease progression, worsening of albuminuria and creatinine clearance via detrimental effects on renal blood flow, podocyte apoptosis and autophagy158,160,163,164. In this model, F13A conferred protection, which was purported to reflect antagonism of the apelin receptor. However, whether this compound is a true antagonist is unclear. Overall, the available preclinical data suggest that apelin is protective in DKD. However, systemic application of apelin in patients with diabetes could potentially be limited by adverse effects such as the promotion of diabetic retinopathy (Box 2).
Box 2 Apelin in diabetic retinopathy.
Approximately 7% of patients with diabetes mellitus have proliferative diabetic retinopathy190. Preclinical studies have shown that apelin and vascular endothelial growth factor (VEGF) are upregulated in diabetic retina, and a high-glucose environment upregulates apelin in human retinal pigment epithelial cells191,192. Vitreous apelin levels are also increased in patients with proliferative diabetic retinopathy, although data regarding circulating apelin levels are conflicting193,194.
Intravitreal administration of the apelin receptor antagonist F13A in diabetic rats reduced retinal gliosis and restored VEGF expression191. This finding should be interpreted with caution as F13A is reported to also have agonist activity at the apelin receptor. However, apelin knockdown using a small interfering RNA approach suppressed pathological angiogenesis in a model of ischaemic retinopathy195. Overall, apelin seems to promote neovascularization in diabetic retinopathy. Further studies are needed to investigate whether apelin receptor antagonism could be clinically useful in these patients.
Disorders of fluid homeostasis
The relationship between the apelin system and vasopressin is disrupted in disorders of fluid homeostasis. Syndrome of inappropriate antidiuretic hormone (SIADH) results in water retention due to vasopressin excess. Plasma copeptin is a reliable marker of vasopressin secretion and the apelin to copeptin ratio indicates relative activation of these systems. Patients with SIADH have substantially elevated plasma vasopressin levels and modest increases in plasma apelin. The apelin to copeptin ratio is lower than anticipated in ~90% of these patients165.
Disruption of apelin–vasopressin balance is also seen in patients with polyuria and polydipsia syndromes. Parallel decreases in plasma apelin and copeptin levels occur in primary polydipsia and cranial diabetes insipidus, whereas parallel increases in these levels occur in nephrogenic diabetes insipidus, resulting in alterations in the apelin to copeptin ratio166. Alterations in circulating apelin are suggested to be adaptations to restore the balance of diuresis and antidiuresis; however, they seem to be unable to fully compensate. Kidney function may be impaired in disorders of fluid homeostasis as a direct consequence of the cause of the disorder (such as lithium toxicity or nephrogenic diabetes insipidus) or as a consequence of hypovolaemia. The impact of reduced kidney function on circulating apelin levels is currently unclear.
Polycystic kidney disease
Autosomal dominant polycystic kidney disease (ADPKD) is the most common human monogenic disease. Vasopressin has an important role in the pathogenesis of ADPKD, and vasopressin antagonists are the first licensed treatment for this disease. Patients with ADPKD have higher plasma copeptin and lower plasma apelin concentrations than healthy individuals167. Moreover, in ADPKD, plasma apelin concentration falls as kidney function declines and correlates negatively with TGFβ1, which is a marker of kidney fibrosis167,168. Although noteworthy, this finding does not enable conclusions to be drawn more broadly regarding apelin levels in CKD given the close relationship between apelin and vasopressin. Little is known about the renal expression of the apelin system in ADPKD. However, the inhibitory effect of apelin on aquaporin 2 channel insertion in the collecting duct146,147 suggests that treatment with apelin might be a potential alternative approach to use of the vasopressin 2 receptor antagonist tolvaptan. An aquaretic with direct anti-fibrotic effects on the kidney and beneficial cardiovascular effects would be a very attractive treatment option for this at-risk patient group.
Kidney fibrosis
The development of glomerulosclerosis and interstitial fibrosis is the final common end point of many CKDs. In the early stages of kidney injury, upregulation of inflammatory and pro-fibrotic mediators occurs. Left unchecked, these mediators promote progressive fibrosis and a reduction in GFR. The RAS promotes kidney fibrosis through the action of Ang II on the AT1 receptor, and blockade of the system is beneficial in CKD169–171.
In vitro studies demonstrate that expression of the intrarenal RAS is regulated by the apelin system. For example, in collecting duct-derived M1 cells, ELA-32 reduced expression of the (pro)renin receptor, prorenin and renin172. In mice, knockout of the (pro)renin receptor led to increased levels of apelin and ELA mRNA172. The impact of (pro)renin receptor knockout on the apelin receptor was dependent on the location of the knockout: nephron-specific knockout resulted in reduced medullary apelin receptor mRNA and protein, whereas collecting duct-specific knockout had no effect. Animal models confirm reciprocal regulation of the RAS and apelin systems. For example, in Dahl salt-sensitive rats, ELA-32 infusion prevented high-salt diet-induced upregulation of renin, AT1 receptor and AT2 receptor mRNA172.
Apelin has anti-fibrotic effects173, and ELA overexpression or ELA-32 peptide administration reduced renal fibrosis and inflammation in salt-sensitive rats172,174. In the unilateral ureteric obstruction model of kidney fibrosis, the apelin system is upregulated in the obstructed kidney with evidence of activation of the AKT–endothelial nitric oxide synthase (eNOS) pathway, which is stimulated by apelin175. In this model, losartan, which blocks AT1 receptors, reduced kidney fibrosis and promoted activation of the AKT–eNOS pathways and expression of apelin mRNA. Intriguingly, inhibition of the apelin system using either an apelin receptor antagonist or l-NAME abrogated the anti-fibrotic effect of losartan. Administration of l-NAME together with losartan increased fibrosis compared with controls175. In addition, the AT1 receptor blocker, telmisartan, restored apelin receptor and pre-pro-apelin mRNA expression in a mouse model of T2DM157. These observations suggest that the anti-fibrotic effects of ARBs are partly mediated through crosstalk with the apelin system, probably through NO generation. It would be of great interest to investigate whether combined treatment with an ARB and apelin can act synergistically in the kidney, as has been seen in in a model of myocardial IRI133. If translated to clinical studies, such synergistic benefits would represent a remarkable therapeutic advance for patients with CKD.
Conclusions
CKD is a global health problem and new treatments that delay progression to kidney failure are urgently needed. Targeting the apelin system is a promising strategy given its beneficial renal and cardiovascular effects. In addition, apelin positively affects lipid metabolism and improves glycaemic control by enhancing insulin sensitivity and promoting glucose utilization176,177. These protective effects on metabolic health are particularly attractive for patients with DKD. However, many unanswered questions remain. At present, no clinical studies exist of the effects of apelin on renal physiology in either health or disease. In addition, the blood pressure-lowering and inotropic effects of apelin have been robustly demonstrated in healthy individuals and in patients with heart failure, but whether apelin has similar effects in patients with CKD who commonly have multiple comorbidities is unclear. Studies are now underway to answer these questions178. Moreover, current therapeutic use of apelin in CKD would require systemic administration. The endogenous apelin system appears to have tissue-specific expression with paracrine or autocrine signalling. Systemic application might have undesirable off-target effects, such as promotion of pathological angiogenesis, which is an important consideration for patients with multimorbidity. Overall, the apelin system clearly warrants further investigation and has exciting potential as a therapeutic target that could offer multi-system protection to improve outcomes for patients with kidney disease.
Acknowledgements
F.A.C. is supported by a Kidney Research UK Training Fellowship (TF_006_20171124). D.N., J.J.M. and A.P.D. are supported in whole or part by the Wellcome Trust (WT203814/Z/16/A for D.N.; WT107715/Z/15/Z for A.P.D. and J.J.M.). D.E.N. is supported by the British Heart Foundation (FS/06/064, FS/09/019, CH09/002, RG/16/10/32375, RE/18/5/34216) and Wellcome Trust (WT103782AIA). N.D. is supported by a Senior Clinical Research Fellowship from the Chief Scientist Office (SCAF/19/02).
Glossary
- ‘Message–address’ concept of peptide binding
The concept that agonists contain two distinct parts: one determines receptor efficacy and activation (the ‘message’); the other influences receptor selectivity (the ‘address’).
- Inotrope
A substance that alters the force of contraction of a muscle. This term is normally used to refer to effects on the myocardium and, unless otherwise specified, implies increased contractility.
Author contributions
All authors contributed to researching the data, discussing the content, writing the text and reviewing or editing the manuscript before submission.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information
Nature Reviews Nephrology thanks Mirjam Christ-Crain, who co-reviewed with Sophie Monnerat, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bikbov B, et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann. Intern. Med. 2013;158:825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 3.Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. 2017;389:1238–1252. doi: 10.1016/S0140-6736(16)32064-5. [DOI] [PubMed] [Google Scholar]
- 4.Gansevoort RT, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382:339–352. doi: 10.1016/S0140-6736(13)60595-4. [DOI] [PubMed] [Google Scholar]
- 5.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organisation. Arch. Intern. Med. 2004;164:659–663. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- 6.Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation. 2007;116:85–97. doi: 10.1161/CIRCULATIONAHA.106.678342. [DOI] [PubMed] [Google Scholar]
- 7.Anavekar NS, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N. Engl. J. Med. 2004;351:1285–1295. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 8.Muntner P, et al. Hypertension awareness, treatment, and control in adults with CKD: results from the Chronic Renal Insufficiency Cohort (CRIC) study. Am. J. Kidney Dis. 2010;55:441–451. doi: 10.1053/j.ajkd.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung AK, et al. Effects of intensive BP control in CKD. J. Am. Soc. Nephrol. 2017;28:2812–2823. doi: 10.1681/ASN.2017020148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levin A, Singer J, Thompson CR, Ross H, Lewis M. Prevalent left ventricular hypertrophy in the predialysis population: identifying opportunities for intervention. Am. J. Kidney Dis. 1996;27:347–354. doi: 10.1016/S0272-6386(96)90357-1. [DOI] [PubMed] [Google Scholar]
- 11.Townsend RR, et al. Aortic PWV in chronic kidney disease: a CRIC ancillary study. Am. J. Hypertens. 2010;23:282–289. doi: 10.1038/ajh.2009.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim ED, et al. Associations between kidney disease measures and regional pulse wave velocity in a large community-based cohort: the Atherosclerosis Risk in Communities (ARIC) study. Am. J. Kidney Dis. 2018;72:682–690. doi: 10.1053/j.ajkd.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 13.Dhaun N, Goddard J, Webb DJ. The endothelin system and its antagonism in chronic kidney disease. J. Am. Soc. Nephrol. 2006;17:943–955. doi: 10.1681/ASN.2005121256. [DOI] [PubMed] [Google Scholar]
- 14.Gerstein HC, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 15.Perkovic V, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N. Engl. J. Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 16.Heerspink HJL, et al. Dapagliflozin in patients with chronic kidney disease. N. Engl. J. Med. 2020;383:1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 17.Jafar TH, et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition. A patient-level meta-analysis. Ann. Intern. Med. 2003;139:244–252. doi: 10.7326/0003-4819-139-4-200308190-00006. [DOI] [PubMed] [Google Scholar]
- 18.O’Dowd BF, et al. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene. 1993;136:355–360. doi: 10.1016/0378-1119(93)90495-O. [DOI] [PubMed] [Google Scholar]
- 19.Sun X, et al. Non-activated APJ suppresses the angiotensin II type 1 receptor, whereas apelin-activated APJ acts conversely. Hypertens. Res. 2011;34:701–706. doi: 10.1038/hr.2011.19. [DOI] [PubMed] [Google Scholar]
- 20.Yang R, et al. Apelin/APJ axis improves angiotensin II-induced endothelial cell senescence through AMPK/SIRT1 signaling pathway. Arch. Med. Sci. 2018;14:725–734. doi: 10.5114/aoms.2017.70340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishida J, et al. Regulatory roles for APJ, a seven-transmembrane receptor related to angiotensin-type 1 receptor in blood pressure in vivo. J. Biol. Chem. 2004;279:26274–26279. doi: 10.1074/jbc.M404149200. [DOI] [PubMed] [Google Scholar]
- 22.Iwanaga Y, Kihara Y, Takenaka H, Kita T. Down-regulation of cardiac apelin system in hypertrophied and failing hearts: possible role of angiotensin II–angiotensin type 1 receptor system. J. Mol. Cell Cardiol. 2006;41:798–806. doi: 10.1016/j.yjmcc.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Siddiquee K, et al. Apelin protects against angiotensin II-induced cardiovascular fibrosis and decreases plasminogen activator inhibitor type-1 production. J. Hypertens. 2011;29:724–731. doi: 10.1097/HJH.0b013e32834347de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnes GD, et al. Sustained cardiovascular actions of APJ agonism during renin–angiotensin system activation and in patients with heart failure. Circ. Heart Fail. 2013;6:482–491. doi: 10.1161/CIRCHEARTFAILURE.111.000077. [DOI] [PubMed] [Google Scholar]
- 25.Sato T, et al. ELABELA–APJ axis protects from pressure overload heart failure and angiotensin II-induced cardiac damage. Cardiovasc. Res. 2017;113:760–769. doi: 10.1093/cvr/cvx061. [DOI] [PubMed] [Google Scholar]
- 26.Zhang ZZ, et al. Apelin is a negative regulator of angiotensin II-mediated adverse myocardial remodeling and dysfunction. Hypertension. 2017;70:1165–1175. doi: 10.1161/HYPERTENSIONAHA.117.10156. [DOI] [PubMed] [Google Scholar]
- 27.Wang W, et al. Apelin protects against abdominal aortic aneurysm and the therapeutic role of neutral endopeptidase resistant apelin analogs. Proc. Natl Acad. Sci. USA. 2019;116:13006–13015. doi: 10.1073/pnas.1900152116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Read C, et al. International Union of Basic and Clinical Pharmacology. CVII. Structure and pharmacology of the apelin receptor with a recommendation that elabela/toddler is a second endogenous peptide ligand. Pharmacol. Rev. 2019;71:467–502. doi: 10.1124/pr.119.017533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tucker B, et al. Zebrafish angiotensin II receptor-like 1a (agtrl1a) is expressed in migrating hypoblast, vasculature, and in multiple embryonic epithelia. Gene Expr. Patterns. 2007;7:258–265. doi: 10.1016/j.modgep.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Scott IC, et al. The G protein-coupled receptor agtrl1b regulates early development of myocardial progenitors. Dev. Cell. 2007;12:403–413. doi: 10.1016/j.devcel.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 31.Kalin RE, et al. Paracrine and autocrine mechanisms of apelin signaling govern embryonic and tumor angiogenesis. Dev. Biol. 2007;305:599–614. doi: 10.1016/j.ydbio.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Tatemoto K, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem. Biophys. Res. Commun. 1998;251:471–476. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- 33.Lee DK, et al. Characterization of apelin, the ligand for the APJ receptor. J. Neurochem. 2000;74:34–41. doi: 10.1046/j.1471-4159.2000.0740034.x. [DOI] [PubMed] [Google Scholar]
- 34.Chng SC, Ho L, Tian J, Reversade B. ELABELA: a hormone essential for heart development signals via the apelin receptor. Dev. Cell. 2013;27:672–680. doi: 10.1016/j.devcel.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Pauli A, et al. Toddler: an embryonic signal that promotes cell movement via Apelin receptors. Science. 2014;343:1248636. doi: 10.1126/science.1248636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maguire JJ, Kleinz MJ, Pitkin SL, Davenport AP. [Pyr1]apelin-13 identified as the predominant apelin isoform in the human heart: vasoactive mechanisms and inotropic action in disease. Hypertension. 2009;54:598–604. doi: 10.1161/HYPERTENSIONAHA.109.134619. [DOI] [PubMed] [Google Scholar]
- 37.Zhen EY, Higgs RE, Gutierrez JA. Pyroglutamyl apelin-13 identified as the major apelin isoform in human plasma. Anal. Biochem. 2013;442:1–9. doi: 10.1016/j.ab.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 38.Shin K, Pandey A, Liu XQ, Anini Y, Rainey JK. Preferential apelin-13 production by the proprotein convertase PCSK3 is implicated in obesity. FEBS Open. Bio. 2013;3:328–333. doi: 10.1016/j.fob.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fischer C, et al. Plasma kallikrein cleaves and inactivates apelin-17: palmitoyl- and PEG-extended apelin-17 analogs as metabolically stable blood pressure-lowering agents. Eur. J. Med. Chem. 2019;166:119–124. doi: 10.1016/j.ejmech.2019.01.040. [DOI] [PubMed] [Google Scholar]
- 40.Murza A, Belleville K, Longpre JM, Sarret P, Marsault E. Stability and degradation patterns of chemically modified analogs of apelin-13 in plasma and cerebrospinal fluid. Biopolymers. 2014;102:297–303. doi: 10.1002/bip.22498. [DOI] [PubMed] [Google Scholar]
- 41.McKinnie SM, et al. The metalloprotease neprilysin degrades and inactivates apelin peptides. Chembiochem. 2016;17:1495–1498. doi: 10.1002/cbic.201600244. [DOI] [PubMed] [Google Scholar]
- 42.Velazquez EJ, et al. Angiotensin–neprilysin inhibition in acute decompensated heart failure. N. Engl. J. Med. 2019;380:539–548. doi: 10.1056/NEJMoa1812851. [DOI] [PubMed] [Google Scholar]
- 43.Vickers C, et al. Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J. Biol. Chem. 2002;277:14838–14843. doi: 10.1074/jbc.M200581200. [DOI] [PubMed] [Google Scholar]
- 44.Wang W, et al. Angiotensin-converting enzyme 2 metabolizes and partially inactivates Pyr-Apelin-13 and Apelin-17: physiological effects in the cardiovascular system. Hypertension. 2016;68:365–377. doi: 10.1161/HYPERTENSIONAHA.115.06892. [DOI] [PubMed] [Google Scholar]
- 45.Yang P, et al. [Pyr(1)]Apelin-13(1–12) is a biologically active ACE2 metabolite of the endogenous cardiovascular peptide [Pyr(1)]Apelin-13. Front. Neurosci. 2017;11:92. doi: 10.3389/fnins.2017.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hamming I, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Donoghue M, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ. Res. 2000;87:E1–E9. doi: 10.1161/01.RES.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 48.Li W, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.To KF, Lo AW. Exploring the pathogenesis of severe acute respiratory syndrome (SARS): the tissue distribution of the coronavirus (SARS-CoV) and its putative receptor, angiotensin-converting enzyme 2 (ACE2) J. Pathol. 2004;203:740–743. doi: 10.1002/path.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benton DJ, et al. Receptor binding and priming of the spike protein of SARS-CoV-2 for membrane fusion. Nature. 2020;588:327–330. doi: 10.1038/s41586-020-2772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nyimanu D, et al. Development and validation of an LC–MS/MS method for detection and quantification of in vivo derived metabolites of [Pyr(1)]apelin-13 in humans. Sci. Rep. 2019;9:19934. doi: 10.1038/s41598-019-56157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Charo DN, et al. Endogenous regulation of cardiovascular function by apelin-APJ. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H1904–H1913. doi: 10.1152/ajpheart.00686.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeng XX, Wilm TP, Sepich DS, Solnica-Krezel L. Apelin and its receptor control heart field formation during zebrafish gastrulation. Dev. Cell. 2007;12:391–402. doi: 10.1016/j.devcel.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 54.Freyer L, et al. Loss of apela peptide in mice causes low penetrance embryonic lethality and defects in early mesodermal derivatives. Cell Rep. 2017;20:2116–2130. doi: 10.1016/j.celrep.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharma B, et al. Alternative progenitor cells compensate to rebuild the coronary vasculature in Elabela- and APJ-deficient hearts. Dev. Cell. 2017;42:655–666 e653. doi: 10.1016/j.devcel.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang P, et al. Elabela/toddler is an endogenous agonist of the apelin APJ receptor in the adult cardiovascular system, and exogenous administration of the peptide compensates for the downregulation of its expression in pulmonary arterial hypertension. Circulation. 2017;135:1160–1173. doi: 10.1161/CIRCULATIONAHA.116.023218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hosoya M, et al. Molecular and functional characteristics of APJ. Tissue distribution of mRNA and interaction with the endogenous ligand apelin. J. Biol. Chem. 2000;275:21061–21067. doi: 10.1074/jbc.M908417199. [DOI] [PubMed] [Google Scholar]
- 58.O’Carroll AM, Selby TL, Palkovits M, Lolait SJ. Distribution of mRNA encoding B78/apj, the rat homologue of the human APJ receptor, and its endogenous ligand apelin in brain and peripheral tissues. Biochim. Biophys. Acta. 2000;1492:72–80. doi: 10.1016/S0167-4781(00)00072-5. [DOI] [PubMed] [Google Scholar]
- 59.Medhurst AD, et al. Pharmacological and immunohistochemical characterization of the APJ receptor and its endogenous ligand apelin. J. Neurochem. 2003;84:1162–1172. doi: 10.1046/j.1471-4159.2003.01587.x. [DOI] [PubMed] [Google Scholar]
- 60.Dray C, et al. Apelin and APJ regulation in adipose tissue and skeletal muscle of type 2 diabetic mice and humans. Am. J. Physiol. Endocrinol. Metab. 2010;298:E1161–1169. doi: 10.1152/ajpendo.00598.2009. [DOI] [PubMed] [Google Scholar]
- 61.Katugampola SD, Maguire JJ, Matthewson SR, Davenport AP. [(125)I]-(Pyr(1))Apelin-13 is a novel radioligand for localizing the APJ orphan receptor in human and rat tissues with evidence for a vasoconstrictor role in man. Br. J. Pharmacol. 2001;132:1255–1260. doi: 10.1038/sj.bjp.0703939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kleinz MJ, Skepper JN, Davenport AP. Immunocytochemical localisation of the apelin receptor, APJ, to human cardiomyocytes, vascular smooth muscle and endothelial cells. Regul. Pept. 2005;126:233–240. doi: 10.1016/j.regpep.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 63.Wang G, et al. Apelin, a new enteric peptide: localization in the gastrointestinal tract, ontogeny, and stimulation of gastric cell proliferation and of cholecystokinin secretion. Endocrinology. 2004;145:1342–1348. doi: 10.1210/en.2003-1116. [DOI] [PubMed] [Google Scholar]
- 64.Kleinz MJ, Davenport AP. Immunocytochemical localization of the endogenous vasoactive peptide apelin to human vascular and endocardial endothelial cells. Regul. Pept. 2004;118:119–125. doi: 10.1016/j.regpep.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 65.Marsault E, et al. The apelinergic system: a perspective on challenges and opportunities in cardiovascular and metabolic disorders. Ann. N. Y. Acad. Sci. 2019;1455:12–33. doi: 10.1111/nyas.14123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boucher J, et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology. 2005;146:1764–1771. doi: 10.1210/en.2004-1427. [DOI] [PubMed] [Google Scholar]
- 67.Kawamata Y, et al. Molecular properties of apelin: tissue distribution and receptor binding. Biochim. Biophys. Acta. 2001;1538:162–171. doi: 10.1016/S0167-4889(00)00143-9. [DOI] [PubMed] [Google Scholar]
- 68.Deng C, Chen H, Yang N, Feng Y, Hsueh AJ. Apela regulates fluid homeostasis by binding to the APJ receptor to activate Gi signaling. J. Biol. Chem. 2015;290:18261–18268. doi: 10.1074/jbc.M115.648238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O’Carroll AM, et al. Expression and functional implications of the renal apelinergic system in rodents. PLoS ONE. 2017;12:e0183094. doi: 10.1371/journal.pone.0183094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Perjes A, et al. Characterization of apela, a novel endogenous ligand of apelin receptor, in the adult heart. Basic. Res. Cardiol. 2016;111:2. doi: 10.1007/s00395-015-0521-6. [DOI] [PubMed] [Google Scholar]
- 71.Wang Z, et al. Elabela–apelin receptor signaling pathway is functional in mammalian systems. Sci. Rep. 2015;5:8170. doi: 10.1038/srep08170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.El Messari S, et al. Functional dissociation of apelin receptor signaling and endocytosis: implications for the effects of apelin on arterial blood pressure. J. Neurochem. 2004;90:1290–1301. doi: 10.1111/j.1471-4159.2004.02591.x. [DOI] [PubMed] [Google Scholar]
- 73.Nyimanu D, et al. Apelin-36-[L28A] and Apelin-36-[L28C(30kDa-PEG)] peptides that improve diet induced obesity are G protein biased ligands at the apelin receptor. Peptides. 2019;121:170139. doi: 10.1016/j.peptides.2019.170139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McKinnie SMK, et al. Synthetic modification within the “RPRL” region of apelin peptides: impact on cardiovascular activity and stability to neprilysin and plasma degradation. J. Med. Chem. 2017;60:6408–6427. doi: 10.1021/acs.jmedchem.7b00723. [DOI] [PubMed] [Google Scholar]
- 75.Read C, et al. Apelin peptides linked to anti-serum albumin domain antibodies retain affinity in vitro and are efficacious receptor agonists in vivo. Basic. Clin. Pharmacol. Toxicol. 2020;126(Suppl 6):96–103. doi: 10.1111/bcpt.13227. [DOI] [PubMed] [Google Scholar]
- 76.Langelaan DN, Bebbington EM, Reddy T, Rainey JK. Structural insight into G-protein coupled receptor binding by apelin. Biochemistry. 2009;48:537–548. doi: 10.1021/bi801864b. [DOI] [PubMed] [Google Scholar]
- 77.Macaluso NJ, Pitkin SL, Maguire JJ, Davenport AP, Glen RC. Discovery of a competitive apelin receptor (APJ) antagonist. ChemMedChem. 2011;6:1017–1023. doi: 10.1002/cmdc.201100069. [DOI] [PubMed] [Google Scholar]
- 78.Murza A, et al. Elucidation of the structure–activity relationships of apelin: influence of unnatural amino acids on binding, signaling, and plasma stability. ChemMedChem. 2012;7:318–325. doi: 10.1002/cmdc.201100492. [DOI] [PubMed] [Google Scholar]
- 79.Zhang Y, et al. Identifying structural determinants of potency for analogs of apelin-13: integration of C-terminal truncation with structure–activity. Bioorg Med. Chem. 2014;22:2992–2997. doi: 10.1016/j.bmc.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 80.Ma Y, et al. Structural basis for apelin control of the human apelin receptor. Structure. 2017;25:858–866.e854. doi: 10.1016/j.str.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 81.Langelaan DN, et al. Structural features of the apelin receptor N-terminal tail and first transmembrane segment implicated in ligand binding and receptor trafficking. Biochim. Biophys. Acta. 2013;1828:1471–1483. doi: 10.1016/j.bbamem.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 82.Gerbier R, et al. New structural insights into the apelin receptor: identification of key residues for apelin binding. FASEB J. 2015;29:314–322. doi: 10.1096/fj.14-256339. [DOI] [PubMed] [Google Scholar]
- 83.Couvineau P, Llorens-Cortes C, Iturrioz X. Elabela/Toddler and apelin bind differently to the apelin receptor. Faseb j. 2020;34:7989–8000. doi: 10.1096/fj.201903029R. [DOI] [PubMed] [Google Scholar]
- 84.Habata Y, et al. Apelin, the natural ligand of the orphan receptor APJ, is abundantly secreted in the colostrum. Biochim. Biophys. Acta. 1999;1452:25–35. doi: 10.1016/S0167-4889(99)00114-7. [DOI] [PubMed] [Google Scholar]
- 85.Masri B, Lahlou H, Mazarguil H, Knibiehler B, Audigier Y. Apelin (65–77) activates extracellular signal-regulated kinases via a PTX-sensitive G protein. Biochem. Biophys. Res. Commun. 2002;290:539–545. doi: 10.1006/bbrc.2001.6230. [DOI] [PubMed] [Google Scholar]
- 86.Masri B, Morin N, Pedebernade L, Knibiehler B, Audigier Y. The apelin receptor is coupled to Gi1 or Gi2 protein and is differentially desensitized by apelin fragments. J. Biol. Chem. 2006;281:18317–18326. doi: 10.1074/jbc.M600606200. [DOI] [PubMed] [Google Scholar]
- 87.Hashimoto Y, et al. G protein-coupled APJ receptor signaling induces focal adhesion formation and cell motility. Int. J. Mol. Med. 2005;16:787–792. [PubMed] [Google Scholar]
- 88.Szokodi I, et al. Apelin, the novel endogenous ligand of the orphan receptor APJ, regulates cardiac contractility. Circ. Res. 2002;91:434–440. doi: 10.1161/01.RES.0000033522.37861.69. [DOI] [PubMed] [Google Scholar]
- 89.Dray C, et al. Apelin stimulates glucose utilization in normal and obese insulin-resistant mice. Cell Metab. 2008;8:437–445. doi: 10.1016/j.cmet.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 90.Evans NA, et al. Visualizing differences in ligand-induced beta-arrestin-GFP interactions and trafficking between three recently characterized G protein-coupled receptors. J. Neurochem. 2001;77:476–485. doi: 10.1046/j.1471-4159.2001.00269.x. [DOI] [PubMed] [Google Scholar]
- 91.Lee DK, Ferguson SS, George SR, O’Dowd BF. The fate of the internalized apelin receptor is determined by different isoforms of apelin mediating differential interaction with beta-arrestin. Biochem. Biophys. Res. Commun. 2010;395:185–189. doi: 10.1016/j.bbrc.2010.03.151. [DOI] [PubMed] [Google Scholar]
- 92.Zhou N, et al. Cell–cell fusion and internalization of the CNS-based, HIV-1 co-receptor, APJ. Virology. 2003;307:22–36. doi: 10.1016/S0042-6822(02)00021-1. [DOI] [PubMed] [Google Scholar]
- 93.Wootten D, Christopoulos A, Marti-Solano M, Babu MM, Sexton PM. Mechanisms of signalling and biased agonism in G protein-coupled receptors. Nat. Rev. Mol. Cell Biol. 2018;19:638–653. doi: 10.1038/s41580-018-0049-3. [DOI] [PubMed] [Google Scholar]
- 94.Scimia MC, et al. APJ acts as a dual receptor in cardiac hypertrophy. Nature. 2012;488:394–398. doi: 10.1038/nature11263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Japp AG, et al. Vascular effects of apelin in vivo in man. J. Am. Coll. Cardiol. 2008;52:908–913. doi: 10.1016/j.jacc.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 96.Japp AG, et al. Acute cardiovascular effects of apelin in humans: potential role in patients with chronic heart failure. Circulation. 2010;121:1818–1827. doi: 10.1161/CIRCULATIONAHA.109.911339. [DOI] [PubMed] [Google Scholar]
- 97.Brame AL, et al. Design, characterization, and first-in-human study of the vascular actions of a novel biased apelin receptor agonist. Hypertension. 2015;65:834–840. doi: 10.1161/HYPERTENSIONAHA.114.05099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pope GR, Tilve S, McArdle CA, Lolait SJ, O’Carroll AM. Agonist-induced internalization and desensitization of the apelin receptor. Mol. Cell Endocrinol. 2016;437:108–119. doi: 10.1016/j.mce.2016.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Murza A, et al. Discovery and structure–activity relationship of a bioactive fragment of ELABELA that modulates vascular and cardiac functions. J. Med. Chem. 2016;59:2962–2972. doi: 10.1021/acs.jmedchem.5b01549. [DOI] [PubMed] [Google Scholar]
- 100.Wang W, et al. Loss of Apelin exacerbates myocardial infarction adverse remodeling and ischemia–reperfusion injury: therapeutic potential of synthetic Apelin analogues. J. Am. Heart Assoc. 2013;2:e000249. doi: 10.1161/JAHA.113.000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Read C, et al. Cardiac action of the first G protein biased small molecule apelin agonist. Biochem. Pharmacol. 2016;116:63–72. doi: 10.1016/j.bcp.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ason B, et al. Cardiovascular response to small molecule APJ activation. JCI Insight. 2020;5:e132898. doi: 10.1172/jci.insight.132898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Salcedo A, et al. Apelin effects in human splanchnic arteries. Role of nitric oxide and prostanoids. Regul. Pept. 2007;144:50–55. doi: 10.1016/j.regpep.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 104.Lee DK, et al. Modification of the terminal residue of apelin-13 antagonizes its hypotensive action. Endocrinology. 2005;146:231–236. doi: 10.1210/en.2004-0359. [DOI] [PubMed] [Google Scholar]
- 105.Tatemoto K, et al. The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul. Pept. 2001;99:87–92. doi: 10.1016/S0167-0115(01)00236-1. [DOI] [PubMed] [Google Scholar]
- 106.Wang C, Du JF, Wu F, Wang HC. Apelin decreases the SR Ca2+ content but enhances the amplitude of [Ca2+]i transient and contractions during twitches in isolated rat cardiac myocytes. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H2540–H2546. doi: 10.1152/ajpheart.00046.2008. [DOI] [PubMed] [Google Scholar]
- 107.Perjes A, et al. Apelin increases cardiac contractility via protein kinase Cepsilon- and extracellular signal-regulated kinase-dependent mechanisms. PLoS ONE. 2014;9:e93473. doi: 10.1371/journal.pone.0093473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kuba K, et al. Impaired heart contractility in Apelin gene-deficient mice associated with aging and pressure overload. Circ. Res. 2007;101:e32–42. doi: 10.1161/CIRCRESAHA.107.158659. [DOI] [PubMed] [Google Scholar]
- 109.Farkasfalvi K, et al. Direct effects of apelin on cardiomyocyte contractility and electrophysiology. Biochem. Biophys. Res. Commun. 2007;357:889–895. doi: 10.1016/j.bbrc.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 110.Ashley EA, et al. The endogenous peptide apelin potently improves cardiac contractility and reduces cardiac loading in vivo. Cardiovasc. Res. 2005;65:73–82. doi: 10.1016/j.cardiores.2004.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sato T, et al. Apelin is a positive regulator of ACE2 in failing hearts. J. Clin. Invest. 2013;123:5203–5211. doi: 10.1172/JCI69608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sato T, et al. Loss of apelin augments angiotensin II-induced cardiac dysfunction and pathological remodeling. Int J Mol Sci. 2019;20:239. doi: 10.3390/ijms20020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cheng CC, et al. Apelin regulates the electrophysiological characteristics of atrial myocytes. Eur. J. Clin. Invest. 2013;43:34–40. doi: 10.1111/eci.12012. [DOI] [PubMed] [Google Scholar]
- 114.Cox CM, D’Agostino SL, Miller MK, Heimark RL, Krieg PA. Apelin, the ligand for the endothelial G-protein-coupled receptor, APJ, is a potent angiogenic factor required for normal vascular development of the frog embryo. Dev. Biol. 2006;296:177–189. doi: 10.1016/j.ydbio.2006.04.452. [DOI] [PubMed] [Google Scholar]
- 115.Kidoya H, et al. Spatial and temporal role of the apelin/APJ system in the caliber size regulation of blood vessels during angiogenesis. EMBO J. 2008;27:522–534. doi: 10.1038/sj.emboj.7601982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kasai A, et al. Retardation of retinal vascular development in apelin-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2008;28:1717–1722. doi: 10.1161/ATVBAHA.108.163402. [DOI] [PubMed] [Google Scholar]
- 117.Eyries M, et al. Hypoxia-induced apelin expression regulates endothelial cell proliferation and regenerative angiogenesis. Circ. Res. 2008;103:432–440. doi: 10.1161/CIRCRESAHA.108.179333. [DOI] [PubMed] [Google Scholar]
- 118.Ronkainen VP, et al. Hypoxia inducible factor regulates the cardiac expression and secretion of apelin. FASEB J. 2007;21:1821–1830. doi: 10.1096/fj.06-7294com. [DOI] [PubMed] [Google Scholar]
- 119.Adam F, et al. Apelin: an antithrombotic factor that inhibits platelet function. Blood. 2016;127:908–920. doi: 10.1182/blood-2014-05-578781. [DOI] [PubMed] [Google Scholar]
- 120.Zhang G, et al. Biphasic roles for soluble guanylyl cyclase (sGC) in platelet activation. Blood. 2011;118:3670–3679. doi: 10.1182/blood-2011-03-341107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Masoud AG, et al. Apelin directs endothelial cell differentiation and vascular repair following immune-mediated injury. J. Clin. Invest. 2020;130:94–107. doi: 10.1172/JCI128469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cheng H, et al. Involvement of apelin/APJ axis in thrombogenesis in valve heart disease patients with atrial fibrillation. Int. Heart J. 2019;60:145–150. doi: 10.1536/ihj.18-166. [DOI] [PubMed] [Google Scholar]
- 123.Chun HJ, et al. Apelin signaling antagonizes Ang II effects in mouse models of atherosclerosis. J. Clin. Invest. 2008;118:3343–3354. doi: 10.1172/JCI34871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hashimoto T, et al. Requirement of apelin–apelin receptor system for oxidative stress-linked atherosclerosis. Am. J. Pathol. 2007;171:1705–1712. doi: 10.2353/ajpath.2007.070471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Japp AG, Newby DE. The apelin–APJ system in heart failure: pathophysiologic relevance and therapeutic potential. Biochem. Pharmacol. 2008;75:1882–1892. doi: 10.1016/j.bcp.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 126.Földes G, et al. Circulating and cardiac levels of apelin, the novel ligand of the orphan receptor APJ, in patients with heart failure. Biochem. Biophys. Res. Commun. 2003;308:480–485. doi: 10.1016/S0006-291X(03)01424-4. [DOI] [PubMed] [Google Scholar]
- 127.Chong KS, Gardner RS, Morton JJ, Ashley EA, McDonagh TA. Plasma concentrations of the novel peptide apelin are decreased in patients with chronic heart failure. Eur. J. Heart Fail. 2006;8:355–360. doi: 10.1016/j.ejheart.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 128.Chen MM, et al. Novel role for the potent endogenous inotrope apelin in human cardiac dysfunction. Circulation. 2003;108:1432–1439. doi: 10.1161/01.CIR.0000091235.94914.75. [DOI] [PubMed] [Google Scholar]
- 129.Fukushima H, Kobayashi N, Takeshima H, Koguchi W, Ishimitsu T. Effects of olmesartan on apelin/APJ and Akt/endothelial nitric oxide synthase pathway in dahl rats with end-stage heart failure. J. Cardiovasc. Pharmacol. 2010;55:83–88. doi: 10.1097/FJC.0b013e3181c87a82. [DOI] [PubMed] [Google Scholar]
- 130.Berry MF, et al. Apelin has in vivo inotropic effects on normal and failing hearts. Circulation. 2004;110:II187–II193. doi: 10.1161/01.CIR.0000138382.57325.5c. [DOI] [PubMed] [Google Scholar]
- 131.Azizi Y, Faghihi M, Imani A, Roghani M, Nazari A. Post-infarct treatment with [Pyr1]-apelin-13 reduces myocardial damage through reduction of oxidative injury and nitric oxide enhancement in the rat model of myocardial infarction. Peptides. 2013;46:76–82. doi: 10.1016/j.peptides.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 132.Kido M, et al. Hypoxia-inducible factor 1-alpha reduces infarction and attenuates progression of cardiac dysfunction after myocardial infarction in the mouse. J. Am. Coll. Cardiol. 2005;46:2116–2124. doi: 10.1016/j.jacc.2005.08.045. [DOI] [PubMed] [Google Scholar]
- 133.Abbasloo E, Najafipour H, Vakili A. Chronic treatment with apelin, losartan and their combination reduces myocardial infarct size and improves cardiac mechanical function. Clin. Exp. Pharmacol. Physiol. 2020;47:393–402. doi: 10.1111/1440-1681.13195. [DOI] [PubMed] [Google Scholar]
- 134.Ellinor PT, Low AF, Macrae CA. Reduced apelin levels in lone atrial fibrillation. Eur. Heart J. 2006;27:222–226. doi: 10.1093/eurheartj/ehi648. [DOI] [PubMed] [Google Scholar]
- 135.Gurger M, et al. The association between apelin-12 levels and paroxysmal supraventricular tachycardia. J. Cardiovasc. Med. 2014;15:642–646. doi: 10.2459/JCM.0000000000000010. [DOI] [PubMed] [Google Scholar]
- 136.Falcone C, et al. Apelin plasma levels predict arrhythmia recurrence in patients with persistent atrial fibrillation. Int. J. Immunopathol. Pharmacol. 2010;23:917–925. doi: 10.1177/039463201002300328. [DOI] [PubMed] [Google Scholar]
- 137.Kim YM, et al. Apelin increases atrial conduction velocity, refractoriness, and prevents inducibility of atrial fibrillation. JCI Insight. 2020;5:e126525. doi: 10.1172/jci.insight.126525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Watanabe H, et al. Close bidirectional relationship between chronic kidney disease and atrial fibrillation: the Niigata preventive medicine study. Am. Heart J. 2009;158:629–636. doi: 10.1016/j.ahj.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 139.Ananthapanyasut W, et al. Prevalence of atrial fibrillation and its predictors in nondialysis patients with chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2010;5:173–181. doi: 10.2215/CJN.03170509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kumar S, et al. Anticoagulation in concomitant chronic kidney disease and atrial fibrillation: JACC review topic of the week. J. Am. Coll. Cardiol. 2019;74:2204–2215. doi: 10.1016/j.jacc.2019.08.1031. [DOI] [PubMed] [Google Scholar]
- 141.Hus-Citharel A, et al. Effect of apelin on glomerular hemodynamic function in the rat kidney. Kidney Int. 2008;74:486–494. doi: 10.1038/ki.2008.199. [DOI] [PubMed] [Google Scholar]
- 142.Reaux-Le Goazigo A, Morinville A, Burlet A, Llorens-Cortes C, Beaudet A. Dehydration-induced cross-regulation of apelin and vasopressin immunoreactivity levels in magnocellular hypothalamic neurons. Endocrinology. 2004;145:4392–4400. doi: 10.1210/en.2004-0384. [DOI] [PubMed] [Google Scholar]
- 143.Reaux A, et al. Physiological role of a novel neuropeptide, apelin, and its receptor in the rat brain. J. Neurochem. 2001;77:1085–1096. doi: 10.1046/j.1471-4159.2001.00320.x. [DOI] [PubMed] [Google Scholar]
- 144.De Mota N, et al. Apelin, a potent diuretic neuropeptide counteracting vasopressin actions through inhibition of vasopressin neuron activity and vasopressin release. Proc. Natl Acad. Sci. USA. 2004;101:10464–10469. doi: 10.1073/pnas.0403518101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Azizi M, et al. Reciprocal regulation of plasma apelin and vasopressin by osmotic stimuli. J. Am. Soc. Nephrol. 2008;19:1015–1024. doi: 10.1681/ASN.2007070816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hus-Citharel A, et al. Apelin counteracts vasopressin-induced water reabsorption via cross talk between apelin and vasopressin receptor signaling pathways in the rat collecting duct. Endocrinology. 2014;155:4483–4493. doi: 10.1210/en.2014-1257. [DOI] [PubMed] [Google Scholar]
- 147.Boulkeroua C, et al. Apelin-13 regulates vasopressin-induced aquaporin-2 expression and trafficking in kidney collecting duct cells. Cell Physiol. Biochem. 2019;53:687–700. doi: 10.33594/000000165. [DOI] [PubMed] [Google Scholar]
- 148.Sawhney S, et al. Intermediate and long-term outcomes of survivors of acute kidney injury episodes: a large population-based cohort study. Am. J. Kidney Dis. 2017;69:18–28. doi: 10.1053/j.ajkd.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hoste EA, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41:1411–1423. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 150.See EJ, et al. Long-term risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int. 2019;95:160–172. doi: 10.1016/j.kint.2018.08.036. [DOI] [PubMed] [Google Scholar]
- 151.Chen H, et al. Apelin protects against acute renal injury by inhibiting TGF-beta1. Biochim. Biophys. Acta. 2015;1852:1278–1287. doi: 10.1016/j.bbadis.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 152.Gholampour F, Bagheri A, Barati A, Masoudi R, Owji SM. Remote ischemic perconditioning modulates apelin expression after renal ischemia–reperfusion injury. J. Surg. Res. 2020;247:429–437. doi: 10.1016/j.jss.2019.09.063. [DOI] [PubMed] [Google Scholar]
- 153.Sagiroglu T, et al. Effects of apelin and leptin on renal functions following renal ischemia/reperfusion: an experimental study. Exp. Ther. Med. 2012;3:908–914. doi: 10.3892/etm.2012.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kim JS, et al. Protective role of apelin against cyclosporine-induced renal tubular injury in rats. Transpl. Proc. 2017;49:1499–1509. doi: 10.1016/j.transproceed.2017.03.080. [DOI] [PubMed] [Google Scholar]
- 155.Chen H, et al. ELABELA and an ELABELA fragment protect against AKI. J. Am. Soc. Nephrol. 2017;28:2694–2707. doi: 10.1681/ASN.2016111210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Couser WG, Remuzzi G, Mendis S, Tonelli M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011;80:1258–1270. doi: 10.1038/ki.2011.368. [DOI] [PubMed] [Google Scholar]
- 157.Muller T, et al. Apelinergic system in the kidney: implications for diabetic kidney disease. Physiol. Rep. 2018;6:e13939. doi: 10.14814/phy2.13939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Guo C, et al. Apelin promotes diabetic nephropathy by inducing podocyte dysfunction via inhibiting proteasome activities. J. Cell Mol. Med. 2015;19:2273–2285. doi: 10.1111/jcmm.12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Day RT, Cavaglieri RC, Feliers D. Apelin retards the progression of diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 2013;304:F788–F800. doi: 10.1152/ajprenal.00306.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang BH, Wang W, Wang H, Yin J, Zeng XJ. Promoting effects of the adipokine, apelin, on diabetic nephropathy. PLoS ONE. 2013;8:e60457. doi: 10.1371/journal.pone.0060457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hu H, He L, Li L, Chen L. Apelin/APJ system as a therapeutic target in diabetes and its complications. Mol. Genet. Metab. 2016;119:20–27. doi: 10.1016/j.ymgme.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 162.Chen H, et al. Apelin inhibits the development of diabetic nephropathy by regulating histone acetylation in Akita mouse. J. Physiol. 2014;592:505–521. doi: 10.1113/jphysiol.2013.266411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang J, Yin J, Wang Y, Li B, Zeng X. Apelin impairs myogenic response to induce diabetic nephropathy in mice. FASEB J. 2018;32:4315–4327. doi: 10.1096/fj.201701257R. [DOI] [PubMed] [Google Scholar]
- 164.Liu Y, Zhang J, Wang Y, Zeng X. Apelin involved in progression of diabetic nephropathy by inhibiting autophagy in podocytes. Cell Death Dis. 2017;8:e3006. doi: 10.1038/cddis.2017.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Blanchard A, et al. An abnormal apelin/vasopressin balance may contribute to water retention in patients with the syndrome of inappropriate antidiuretic hormone (SIADH) and heart failure. J. Clin. Endocrinol. Metab. 2013;98:2084–2089. doi: 10.1210/jc.2012-3794. [DOI] [PubMed] [Google Scholar]
- 166.Urwyler SA, et al. Plasma apelin concentrations in patients with polyuria–polydipsia syndrome. J. Clin. Endocrinol. Metab. 2016;101:1917–1923. doi: 10.1210/jc.2016-1158. [DOI] [PubMed] [Google Scholar]
- 167.Lacquaniti A, et al. Apelin and copeptin: two opposite biomarkers associated with kidney function decline and cyst growth in autosomal dominant polycystic kidney disease. Peptides. 2013;49:1–8. doi: 10.1016/j.peptides.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 168.Kocer D, Karakukcu C, Ozturk F, Eroglu E, Kocyigit I. Evaluation of fibrosis markers: apelin and transforming growth factor-beta1 in autosomal dominant polycystic kidney disease patients. Ther. Apher. Dial. 2016;20:517–522. doi: 10.1111/1744-9987.12412. [DOI] [PubMed] [Google Scholar]
- 169.Nogueira A, Pires MJ, Oliveira PA. Pathophysiological mechanisms of renal fibrosis: a review of animal models and therapeutic strategies. Vivo. 2017;31:1–22. doi: 10.21873/invivo.11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang Y, et al. beta-Arrestin-biased AT1R stimulation promotes extracellular matrix synthesis in renal fibrosis. Am. J. Physiol. Ren. Physiol. 2017;313:F1–F8. doi: 10.1152/ajprenal.00588.2016. [DOI] [PubMed] [Google Scholar]
- 171.Maschio G, et al. Effect of the angiotensin-converting-enzyme benazepril on the progression of chronic renal insufficiency. N. Engl. J. Med. 1996;334:939–945. doi: 10.1056/NEJM199604113341502. [DOI] [PubMed] [Google Scholar]
- 172.Xu C, et al. ELABELA antagonizes intrarenal renin–angiotensin system to lower blood pressure and protects against renal injury. Am. J. Physiol. Ren. Physiol. 2020;318:F1122–F1135. doi: 10.1152/ajprenal.00606.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang LY, et al. The regulatory peptide apelin: a novel inhibitor of renal interstitial fibrosis. Amino Acids. 2014;46:2693–2704. doi: 10.1007/s00726-014-1826-8. [DOI] [PubMed] [Google Scholar]
- 174.Schreiber CA, Holditch SJ, Generous A, Ikeda Y. Sustained ELABELA gene therapy in high-salt diet-induced hypertensive rats. Curr. Gene Ther. 2017;16:349–360. doi: 10.2174/1566523217666161121111906. [DOI] [PubMed] [Google Scholar]
- 175.Nishida M, et al. The role of apelin on the alleviative effect of angiotensin receptor blocker in unilateral ureteral obstruction-induced renal fibrosis. Nephron Extra. 2012;2:39–47. doi: 10.1159/000337091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Castan-Laurell I, Masri B, Valet P. The apelin/APJ system as a therapeutic target in metabolic diseases. Expert. Opin. Ther. Targets. 2019;23:215–225. doi: 10.1080/14728222.2019.1561871. [DOI] [PubMed] [Google Scholar]
- 177.Bertrand C, Valet P, Castan-Laurell I. Apelin and energy metabolism. Front. Physiol. 2015;6:115. doi: 10.3389/fphys.2015.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT03956576 (2018).
- 179.Sorli SC, Le Gonidec S, Knibiehler B, Audigier Y. Apelin is a potent activator of tumour neoangiogenesis. Oncogene. 2007;26:7692–7699. doi: 10.1038/sj.onc.1210573. [DOI] [PubMed] [Google Scholar]
- 180.Picault FX, et al. Tumour co-expression of apelin and its receptor is the basis of an autocrine loop involved in the growth of colon adenocarcinomas. Eur. J. Cancer. 2014;50:663–674. doi: 10.1016/j.ejca.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 181.Heo K, et al. Hypoxia-induced up-regulation of apelin is associated with a poor prognosis in oral squamous cell carcinoma patients. Oral. Oncol. 2012;48:500–506. doi: 10.1016/j.oraloncology.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 182.Berta, J. et al. Apelin expression in human non-small cell lung cancer. Role in angiogenesis and prognosis. J. Thorac. Oncol., 1120–1129 (2010). [DOI] [PubMed]
- 183.Uribesalgo I, et al. Apelin inhibition prevents resistance and metastasis associated with anti-angiogenic therapy. EMBO Mol. Med. 2019;11:e9266. doi: 10.15252/emmm.201809266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Davenport AP, et al. First in human study of a novel biased apelin receptor ligand, MM54, A G-alpha(i) agonist/beta-arrestin antagonist. Circ. Res. 2018;123:e69–e81. [Google Scholar]
- 185.Harford-Wright E, et al. Pharmacological targeting of apelin impairs glioblastoma growth. Brain. 2017;140:2939–2954. doi: 10.1093/brain/awx253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mastrella G, et al. Targeting APLN/APLNR improves antiangiogenic efficiency and blunts proinvasive side effects of VEGFA/VEGFR2 blockade in glioblastoma. Cancer Res. 2019;79:2298–2313. doi: 10.1158/0008-5472.CAN-18-0881. [DOI] [PubMed] [Google Scholar]
- 187.Soulet F, et al. ELA/APELA precursor cleaved by furin displays tumor suppressor function in renal cell carcinoma through mTORC1 activation. JCI Insight. 2020;5:2298. doi: 10.1172/jci.insight.129070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Patel SJ, et al. Identification of essential genes for cancer immunotherapy. Nature. 2017;548:537–542. doi: 10.1038/nature23477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tolkach Y, et al. Apelin and apelin receptor expression in renal cell carcinoma. Br. J. Cancer. 2019;120:633–639. doi: 10.1038/s41416-019-0396-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yau JWY, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lu Q, Feng J, Jiang YR. The role of apelin in the retina of diabetic rats. PLoS ONE. 2013;8:e69703. doi: 10.1371/journal.pone.0069703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Qin D, Zheng X-X, Jiang Y-R. Apelin-13 induces proliferation, migration, and collagen I mRNA in human RPE cells via PI3K/Akt and MEK/Erk signalling pathways. Mol. Vis. 2013;2013:2227–2236. [PMC free article] [PubMed] [Google Scholar]
- 193.Tao Y, et al. Apelin in plasma and vitreous and in fibrovascular retinal membranes of patients with proliferative diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 2010;51:4237–4242. doi: 10.1167/iovs.09-4466. [DOI] [PubMed] [Google Scholar]
- 194.Du JH, et al. Elevation of serum apelin-13 associated with proliferative diabetic retinopathy in type 2 diabetic patients. Int. J. Ophthalmol. 2014;7:968–973. doi: 10.3980/j.issn.2222-3959.2014.06.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kasai A, et al. Inhibition of apelin expression switches endothelial cells from proliferative to mature state in pathological retinal angiogenesis. Angiogenesis. 2013;16:723–734. doi: 10.1007/s10456-013-9349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]