Abstract
As NASA prepares for longer space missions aiming for the Moon and Mars, astronauts’ health and performance are becoming a central concern due to the threats associated with galactic cosmic radiation, unnatural gravity fields, and life in extreme environments. In space, the human brain undergoes functional and structural changes related to fluid shift and intracranial pressure. Behavioral abnormalities, such as cognitive deficits, sleep disruption, and visuomotor difficulties, as well as psychological effects, are also a concern. We discuss opportunities and challenges of noninvasive brain stimulation (NiBS) methods — including transcranial magnetic stimulation (TMS) and transcranial electrical stimulation (tES) — to support space exploration in several ways. NiBS includes safe and portable techniques already applied in a wide range of cognitive and motor domains, as well as therapeutically. NiBS could be used to enhance in-flight performance, supporting astronauts during pre-flight Earth-based training, as well as to identify biomarkers of post-flight brain changes for optimization of rehabilitation/compensatory strategies. We review these NiBS techniques and their effects on brain physiology, psychology, and cognition.
Keywords: Brain stimulation, spaceflight, cosmic radiation, TMS, tDCS, EEG
1). INTRODUCTION
Space travel presents significant challenges to human physiology. The average length of spaceflights has increased from few days up to several months, with the forthcoming first Mars landing (scheduled by NASA for year ~2034) expected to require approximately 2 years of travel. Even shorter missions, such as the ~30/60 days Moon landing in ~2024 will present challenges, ultimately leading to the complexity of building long-lasting space installations on Lunar and Martian surfaces. Space exploration involves multiple classes of stressors, ranging from microgravity and cosmic radiations affecting human physiology to those related to living in a confined and isolated environment impacting mood and cognition. Therefore, acute and long-lasting health consequences need to be evaluated and new potential countermeasures investigated, both during lunar missions (as an analog to Mars missions) and ground-based studies. For astronauts, the need to optimize cognitive performance in response to unanticipated situations is pivotal to mission success. In the last two decades, different cognitive tasks and relevant brain regions have been investigated for their response to radiation exposure in animal models, with deficits mostly involving the medial prefrontal cortex (mPFC) and the hippocampus, with impairments in episodic and spatial memory retention (Britten et al., 2014; Lonart et al., 2012; Parihar et al., 2015; Tseng et al., 2014). Specifically, cognitive deficits seem to result from a reduction of dendritic complexity and spine density due to exposure to space-relevant flows of charged particles (Parihar et al., 2015). On the other hand, microgravity seems to be associated with motor impairment as a consequence of the cortical reorganization of motor cortices (Demertzi et al., 2016). The prolonged stress caused by living in an Isolated, Confined, and Extreme (ICE) environment, can also induce depressive states and anxiety and associated with abnormal sleep patterns and loss of appetite, as seen in animals (Barger et al., 2014; Dunn et al., 2004). Furthermore, cognitive and brain adaptation appears as a result of CO2 increase in the space cabin (Law et al., 2014; NASA/TP–2010– 216126). Currently proposed solutions are mainly directed at the spacecraft design level to mitigate some stressors: thick shields incorporated into the walls of the spacecraft habitat to minimize radiation exposure, and rotating habitats designed to produce an artificial gravitational field to mitigate effects of microgravity (Durante, 2014). Although these ideas can be effective, additional solutions directly targeting and interacting with human physiology could ensure more protection of astronauts’ health (Sprugnoli et al., 2019).
Noninvasive brain stimulation (NiBS) encompasses techniques able to modify brain activity employing controlled, high-resolution transcranial delivery of electric field stimuli (Ridding and Rothwell, 2007; Rossini and Rossi, 2007; Valero-Cabré et al., 2017). Among various NiBS techniques, transcranial magnetic stimulation (TMS) and transcranial electrical stimulation (tES) might represent a valid set of potential countermeasures to target a wide range of spaceflight risks (Figure 1) recognized by the Human Research Program (HRP) roadmap (NASA IG-18-021). TMS safety and efficacy have enabled its approval by the Food and Drug Administration (FDA) for the treatment of pharmacoresistant depression, obsessive-compulsive disorder, and migraine. TMS generates a high electric field (~100–200 V/m) through a time-varying magnetic field induced by a coil and can focally produce neuronal spiking (Rossi et al., 2009). Albeit TMS application may be unpractical during actual spaceflights due to its weight and size, its use before and after missions could help to collect data on cortical excitability, plasticity and connectivity levels in the brain (Bestmann, 2008; Ferreri and Rossini, 2013). TMS can thus constitute a valuable tool to uncover the (patho)-physiological brain response to space missions and reveal specific biomarkers of astronaut brain adaptation to spaceflight. Differently, tES modulates neuronal populations through a weak (usually below 2mA) electric current delivered via scalp electrodes, generating cortical electric fields insufficient to trigger an action potential but strong enough to modulate membrane excitability (Paulus, 2011). Specifically, tES can entrain brain oscillatory activity and change cortical excitability (Paulus, 2011). Various tES studies have already been implemented in a wide range of cognitive tasks, leading to an enhancement of performance in many cognitive functions, such as alertness, multitasking, language, visuomotor coordination, visual acuity, and working memory span (Brunoni and Vanderhasselt, 2014; McKinley et al., 2013; Santarnecchi et al., 2017), as well as higher cognitive domains such as abstract reasoning, fluid intelligence, and insight (Dockery et al., 2009; Santarnecchi et al., 2013; Santarnecchi et al., 2019). Due to tES safety profile and portability, its application in clinical and research domains is particularly appealing (Antal et al., 2017). Studies have shown an improvement in cognitive and motor performances with results translating outside the laboratory walls, also in terms of performance enhancement on professional athletes, soldiers, surgeons, and air force pilots (Ciechanski et al., 2018, 2017; McKinley et al., 2013; Okano et al., 2015). Considering the promising results obtained on “ground-based” populations, tES could be a useful tool to enhance visuomotor and cognitive skills of astronauts and cosmonauts, accelerating training efficacy and possibly preventing detrimental effects of spaceflight. This application could also be valuable for mission control operations to facilitate the training and performance of Earth-based crew personnel during missions.
Figure 1. Areas pivotal for space exploration that could potentially benefit from NiBS applications.
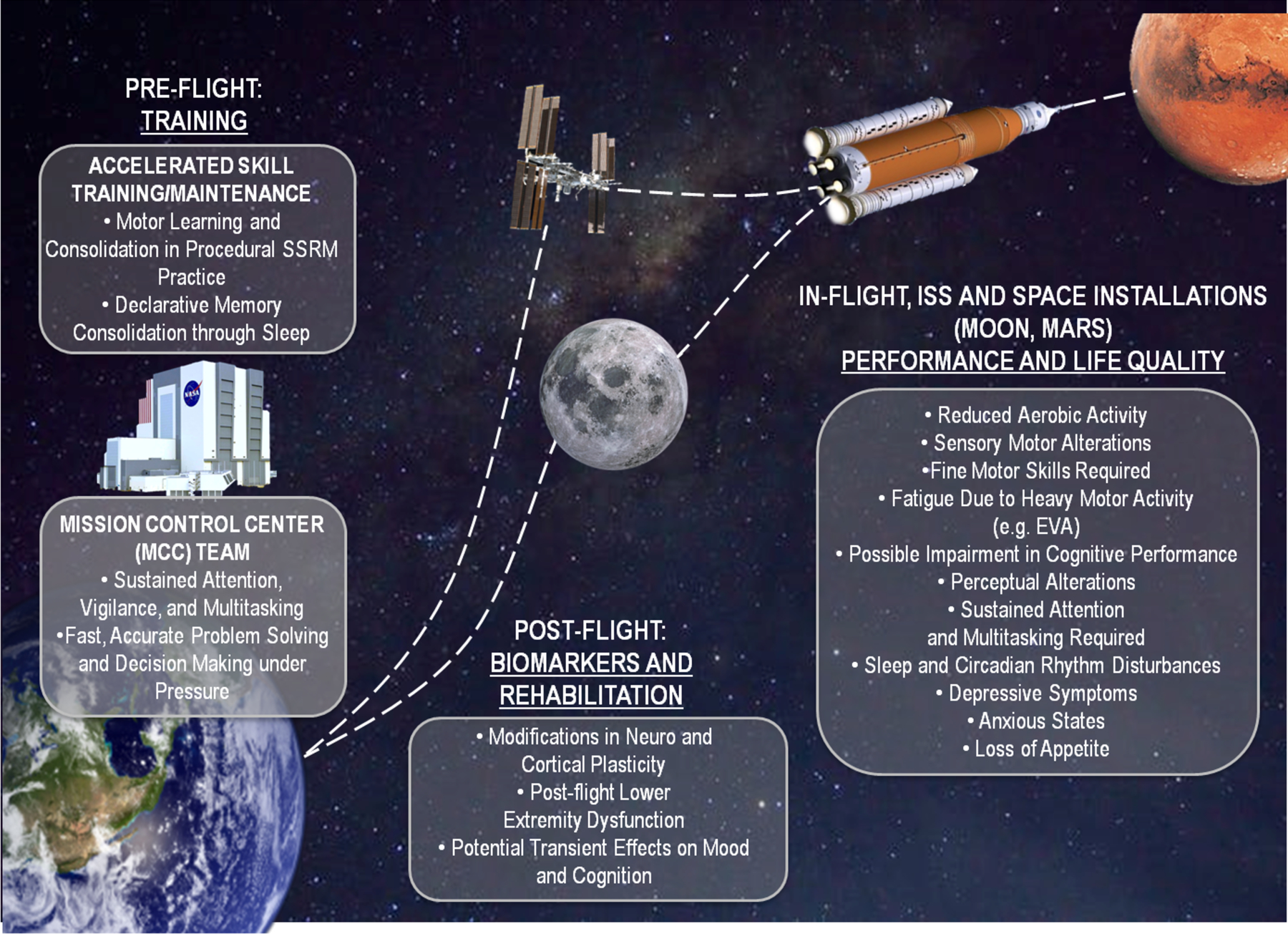
NiBS could be adapted to (i) improve astronaut physical and psychomotor training, cognitive performance, and adaptation to space-related stressors on Earth, as well as enhance and support the performance of the team at the Mission Control Centre during operations. NiBS could also (ii) mitigate the impact of ICE, microgravity, and cosmic radiation during spaceflight or long-duration missions on the ISS, and (iii) further tackle these issues in a habitat on Moon and Mars. Finally, NiBS-based measures of cortical excitability, plasticity, and excitation-inhibition balance could be used to investigate brain changes and support the post-flight return to baseline/recovery.
The present review offers an overview of the opportunities and challenges in NiBS applications for space exploration. We firstly present currently available TMS and tES protocols, discussing their safety and feasibility, as well as more advanced methods including the optimization and combination of these techniques (Antal et al., 2017). We then summarize the main space-related stress factors such as gravity changes, cosmic radiation, and ICE-related consequences. Considering the reported detrimental effects of space missions on the central nervous system (CNS; Acharya et al., 2019), we suggest NiBS as a potential tool to enhance crew performance and well-being in a wide range of cognitive, motor, and psychological domains. Such an approach could be particularly helpful for a long-duration stay in space installations on Lunar and Martian surfaces, as well as on the International Space Station (ISS). Applications also include facilitation of pre-flight Earth-based training, to consolidate and potentially accelerate procedural learning. We also suggest NiBS implementation to enhance performance in Earth-based mission control personals during active shifts and for the measurement of perturbation biomarkers pre and post-flight. We finally address open issues and technical challenges for NiBS implementation during space missions, proposing possible tests, and further research.
2). SPACEFLIGHT RELATED STRESSORS
Many factors can play a role in threat or maintain astronaut wellbeing. These can be divided in environmental stressors, including gravity variance, cosmic radiation, pressure, extreme temperature, and changes in light/dark cycles; spacecraft stressors, such as vibration, noise, internal temperature, light, life support systems, and habitable design; psychological stressors, like isolation, danger, monotony, workload, and teamwork (Kanas and Manzey, 2008). Several gaps identified by NASA HRP (Supplementary Figure 1) open potential implementations compatible with the capabilities of NiBS. Below we introduce three classes of stressors and their consequences on human physiology, such as weightlessness (Microgravity), Galactic Cosmic Radiation (GCR), and life in Isolated, Confined, and Extreme confined environment (ICE).
2.1). Weightlessness (Microgravity)
Future space missions will consist not only in longer time spent in spacecraft but also longer periods of exposure to weightlessness with several transitions between different gravity levels, from 1g on Earth to 0.16g on the Moon, to 0.38g on Mars. Gravity changes introduce physiological deconditioning, involving for example hydrostatic shift and neuro vestibular adaption, and, ultimately, potential modifications in brain anatomy and neurophysiology (Demertzi et al., 2016). For instance, investigations conducted on actual spaceflight reported an increase in α frequency (8–12 Hz) in parieto-occipital and sensorimotor areas, possibly related to decreased gravitational input (Cheron et al., 2006). Because of the inverse relationship between α power amplitude and BOLD signal (Feige et al., 2005), the increase in α oscillations during permanence on the ISS has been associated with a general decrease of cerebral blood oxygenation (Schneider et al., 2008), further underlined stronger α power desynchronization (event-related desynchronization: ERD) from occipital-parietal (α ERD) to central areas (μ ERD). Interestingly, authors also found a significant contribution to this α rhythm of the cerebellum and vestibular network in microgravity (Cebolla et al., 2016), possibly due to increased processing effort and demand necessary for postural stabilization to integrate incongruent vestibular information.
To date, only two studies reported functional MRI (fMRI) results related to spaceflight (Demertzi et al., 2016). The fMRI protocol was applied twice on a 44yo male cosmonaut during his first long-duration mission (169 days) on the ISS, i.e., 30 days before launch and 9 days after earth re-entry. The authors reported a decrease of resting-state functional connectivity of the right insula as well as between the left cerebellum and right motor cortex. The functional modification detected in the insula can be explained by its role in the integration of neurosensory input (i.e., vestibular, visual and proprioceptive) and its functions in the processing of self-motion, spatial orientation (Brandt et al., 2005), perception of vertical (Lopez et al., 2007), and visual processing related to gravitational cues (Lopez et al., 2009). Motor cortex appeared less connected during resting-state and it was activated more during a motor imagery task (e.g. playing tennis or walking) probably due to compensatory and adaptive response to a microgravity environment. Regions showing adaptation to microgravity, such as the precentral/postcentral gyri and cerebellum, are associated with voluntary motor initiation, proprioception, and motor coordination (Demertzi et al., 2016). Functional alterations in these brain areas due to weightlessness, are followed by decreased speed and accuracy of fine goal-oriented movements, somatosensory difficulties, and movement-timing impairment (De la Torre, 2014). A second study explored task-based functional connectivity alterations of 11 astronauts after long-term mission respect to healthy controls not involved in space missions (Pechenkova et al., 2019). A plantar stimulation was applied with an on-off paradigm to elicit the mechanoreceptors responsible for the postural and locomotor control that are strongly impaired during the spaceflight as well as upon return to Earth. The investigators found a post-flight increase in connectivity in the right posterior supramarginal gyrus (involved in vestibular input processing and perception of upright position) as well as a decrease between the vestibular nuclei, right inferior parietal cortex, cerebellum and motor, visual, vestibular, and proprioception areas. Furthermore, the post-flight to the pre-flight difference in connectivity between the right supramarginal gyrus and the left anterior insula was found to be positively correlated with the severity of space motion sickness symptoms (Pechenkova et al., 2019). Authors referred the functional connectivity alterations to the long-term microgravity exposure that cause an important sensory deprivation, while on the other hand, the absence of differences in pure brain activation due to plantar stimulation might be attributed to the fast recovery upon returning to Earth (Pechenkova et al., 2019). In any case, microgravity seems to induce changes in the multisensory and locomotor brain domains, that however require long-term investigations and larger samples to appropriately disentangle its (patho)physiological consequences (Pechenkova et al., 2019).
It is pivotal also to discuss a recognized ophthalmological health risk often evolved during long-duration spaceflight, the Spaceflight Associated Neuro-ocular Syndrome (SANS), previously called Vision Impairment and Intracranial Pressure (VIIP). This syndrome affects two-thirds of US crew members who flew on ISS (NASA, Human Exploration Research Opportunities: HERO, 2015), consisting of visual performance decrements and ocular structural changes (Kramer et al., 2012; Mader et al., 2011). Following their return to Earth, some astronauts showed partially reversed modifications to previous conditions, while changes persisted in others (Mader et al., 2011). SANS seems to be triggered by a cephalic fluid shift due to microgravity, disrupting the balance between hydrostatic and local tissue pressures. An impaired cerebrospinal fluid (CSF) absorption would then increase intracranial pressure, directly swelling choroid, and affecting eyes (Hargens and Richardson, 2009; Herault et al., 2000).
Ground-based Analogues.
Space neuroscience includes different ground-based analogs aimed at reproducing the impact of microgravity on the human body on Earth, such as dry immersion, head-down bed rest (HDBR), and parabolic flights. Since 1986 HDBR has been one of the most implemented space analogs, enabling insight on bodily and mental changes induced by immobilization, isolation, and monotony of activities. During HDBR, the subjects lie in an inclined bed with the head down (−6 or −12 degree in most cases) for a period ranging from a few hours to several weeks. HDBR causes a cephalic fluid shift thought to be responsible for SASN, alterations in cerebral oxygenation, and changes in cerebral perfusion (Pavy-Le Traon et al., 2007). However, this solution does not provide any gravitational and vestibular modifications (Pavy-Le Traon et al., 2007). Instead, during dry immersion subjects are immersed in thermoneutral water while covered in an elastic waterproof fabric to keep them dry, avoiding direct contact with water. Immersion is an adequate alternative since it mimics several spaceflight features, such as lack of a supporting structure against the body, centralization of bodily fluids, confinement, immobilization, and hypokinesia (Navasiolava et al., 2011). Finally, during parabolic flights, a specific flight trajectory is carried out by an airplane, so that normo-, hyper-, and micro-gravity phases are experienced by subjects on board. The plane can modify the parabolas trajectory to simulate Martian gravity (0.38 g) and lunar gravity (0.16 g). Albeit a typical duration of microgravity onboard of parabolic flights is 25–30s, aircraft are usually able to perform around 30 parabolas during one mission (Karmali and Shelhamer, 2008).
Analog-based studies only partially corroborated findings of actual spaceflight research, underlining the limitations of such space analogs. A recent dry immersion study reported decreases (instead of a general increase and desynchronization) in α power and a widespread increase in θ (4–7 Hz) power (Kuznetsova et al., 2015). Parabolic flight studies showed a decrease in β (15–30 Hz) power, possibly related to different factors such as emotional reaction to weightlessness (Schneider et al., 2008), baroreceptor stimulation (Lipnicki, 2009), or lower arousal levels (Wiedemann et al., 2011). The first study using low-resolution brain electromagnetic tomography (LORETA) in low gravity demonstrated that microgravity phases of parabolic flights result in a considerable increase in the spectral power of β activity (18–35 Hz) specifically in the right superior frontal gyrus (Schneider et al., 2008), possibly explaining part of the modifications in performance in cognitive tasks and emotional processing (Faw, 2003; Miller and Cohen, 2001). HDBR studies corroborated the increase in α power as seen during actual spaceflight, but have also reported contrasting findings, probably due to the constant gravitational input still present in these settings (Han et al., 2001).
As for MRI, a study on parabolic flight showed a decreased intrinsic connectivity strength in the right angular gyrus, known to be involved in multisensory integration, as well as in cognitive and spatial tasks (Van Ombergen et al., 2016). Many different MRI-based studies have been run on subjects in HDBR for short or longer experimental time (Van Ombergen et al., 2017), reporting alterations in fine motor skills (Liao et al., 2015), executive function (Liao et al., 2012), and spatial working memory (Cassady et al., 2016). No MRI-based studies of dry immersion have been performed so far.
2.2). Cosmic Radiation
Cosmic radiation (CR) is composed of high-energy particles of GCRs and solar particle events, including protons, helium nuclei, and HZE ions. Humans on Earth and in low Earth orbit (LEO) are protected from space radiation by Earth’s magnetosphere, which deflects these high-energy particles. However, humans beyond LEO and outside the bounds of Van Allen Belt have no such protection, suffering from direct and indirect damage due to radiation exposure. ISS is still also partially protected by the magnetosphere, while the journey to Moon and Mars will involve a heavier and longer exposition to radiations. Data collected by the Curiosity Rover roaming on Mars surface till February 2019, found unusually high levels of space radiations on the Martian surface (Zeitlin et al., 2013). Apart from the well-known lifetime increase in cancer risk, space radiations are linked to acute and late brain effects. Acute CNS risks include altered cognitive function, reduced motor function, and behavioral changes, all of which may affect performance and health. Cognitive deficits include short-term memory, learning, spatial orientation, motor function, emotion recognition, risk decision making, vigilance, reaction time, processing speed, circadian regulation, and fatigue (NASA SP-2009-3405, Strangman et al., 2014). Late CNS risks may include brain atrophy and accumulation of amyloid-β, possibly leading to neurological disorders such as Alzheimer’s disease (AD) and premature aging (NASA SP-2009-3405). A lack of human epidemiology data on CNS risk complicates research for countermeasures. Possible observation of CNS effects in astronauts participating in past NASA missions is highly unlikely because lengths of past missions were relatively short and small sample sizes, as well as because astronauts were partially protected by Earth’s magnetic field and LEO, which together reduce the GCR dose-rate. To characterize radiation effects on the CNS, radiotherapy patients (Greene-Schloesser et al., 2012) and ground-based studies in animals have been conducted for more than two decades using charged particle accelerators (Cucinotta, 2015) delivering doses of charged particles similar to those expected during a mission to Mars (Britten et al., 2016, 2014, 2012). These models confirmed not only direct and indirect damage to DNA and proteins (for a review see Barcellos-Hoff et al., 2015) but also an impact of HZE nuclei on neurogenesis and (possibly consequent) cognitive impairment. HZE nuclei are capable of producing a column of heavily damaged cells along their path through tissues, described as “microlesions” (Todd, 1989), responsible of detrimental consequences on CNS function. Investigation on irradiated animal models showed different mechanisms behind consequences of GCR on CNS that have been reviewed elsewhere (NASA SP-2009-3405).
Incorporating animal research into actual space missions, other than reproduce similar conditions on Earth, is vitally important to understanding the biological impacts of deep space. A relevant new metabolic control technology seems to give great advantages in deep space transition. Synthetic torpor consists of artificially inducing a regulated, reversible depressed metabolic states of experimental animals (Cerri et al., 2016). Compared to active metabolic states, the advantages include reduced mass, volume, and power life support within the spacecraft and mitigated negative health effects induced from radiation and microgravity (Cerri et al., 2013; Gemignani et al., 2015; Tupone et al., 2013), (for a comprehensive review see Cerri et al., 2016). Synthetic torpor-inducing systems may also start as preliminary tests for hibernating systems to maintain human crewmembers in similar metabolic states on long-duration missions. Below we review the main symptomatology associated with exposure to GCR.
Cognitive Deficits.
Recent neuronal morphometry investigations using Golgi silver stain in mice and rats demonstrated that γ-rays, protons, and 56Fe radiation cause reductions in hippocampal neuron arborization (>50% at 30 days) as well as the loss of dendritic spines, each of which can limit the complexity of signal processing (Chakraborti et al., 2012; Parihar and Limoli, 2013; Quasem et al. 2007). Notably, spine density positively correlates with cognitive performance using a novel object in place paradigms (Parihar et al., 2015). Denisova and coworkers (2002) exposed rats to moderate doses of 56Fe particles and tested their spatial memory in an eight-arm radial maze. Cognitive behavior deficits were observed, specifically exposed rats committed more errors than control rats. The former was, in fact, unable to adopt a spatial strategy to solve the maze (Denisova et al., 2002). Britten and colleagues (Britten et al., 2016, 2012; Lonart et al., 2012) considered that neurocognitive tasks regulated by the prefrontal cortex could be impaired after exposure to low doses of HZE-particles, which would prevent astronauts from performing complex executive functions. The authors used rats receiving either sham or real irradiation treatment and tested their ability to perform attentional set-shifting 3 months later. Rats that received low doses of 56Fe particles showed significant impairments in their ability to complete the test, with only 17% of irradiated rats completing all stages as opposed to 78% of control rats. These observations suggest that exposure to mission-relevant doses of 56Fe particles results in the loss of functionality in the prefrontal cortex (Lonart et al., 2012). More recently, a new experimental protocol on mice simulating exposures from GCR during a prolonged mission in space (mixed field of neutrons and photons for 6 months with a dose rate of 192 mGy/day), showed a decrease in hippocampal neuronal excitability and disrupted cortical LTP (Acharya et al., 2019). Moreover, mice developed social avoidance, anxiety, impaired fear extinction memory, and difficulties in recognize location and object novelty, all features that can threaten the crew and that the authors estimated will be developed by at least one astronaut during the trip to Mars (Acharya et al., 2019).
Anxiety.
Anxiety can be measured in rodents by an aversion to enter and/or remain in open, often brightly lit areas (Walf and Frye, 2007). Anxiety-like phenotypes have been reported in rodents chronically after GCR exposure and up to one-year post helium exposure alone, suggesting a link between anxiety-like states and GCR exposure (Acharya et al., 2019; Walf and Frye, 2007).
Conditioned Taste Aversion.
The conditioned taste aversion (CTA) test assesses avoidance behavior when the ingestion of a normally acceptable food item is associated with illness (Riley and Tuck, 1985). Deficits in CTA seem to be partially induced by very low doses of heavy ions (Hunt et al., 1989; Rabin et al., 2000, 1994, 1991, 1989).
Terrestrial Human Data.
Data on radiotherapy patients confirmed the deleterious effects of ionizing radiation on CNS (Greene-Schloesser et al., 2012). Behavioral changes, such as chronic fatigue and depression, occur in many patients undergoing irradiation for cancer therapy. Neurocognitive effects are observed at lower doses, especially in children (BEIR, 1990; Schultheiss et al., 1995). Radiotherapy treatment in oncology for several tumors found impairments in cognitive functioning, language acquisition, visual-spatial ability, memory, and executive functioning, as well as changes in social behaviors. Similar effects did not appear in patients treated with chemotherapy (Goldberg et al., 1982; Keime-Guibert et al., 1998). Atomic bombing and Chernobyl accident victims, receiving low to moderate doses of radiation, showed evidence of memory and cognitive impairments (Bromet et al., 2011; Loganovsky and Yuryev, 2001; Loganovsky and Loganovskaja, 2000; Yamada et al., 2009).
2.3). Isolated, Confined, and Extreme (ICE) Environments.
Manned space missions entail unusual conditions that astronauts must adapt to. They include not only life-threatening conditions such as microgravity and cosmic radiations but a wide range of stressing factors such as isolation from family and friends, confinement in cramped spaces, and coping with extreme working conditions. Spacecrafts are artificial areas with a preset of environmental conditions including Environmental Control and Life Support System (ECLSS), limited habitable volume, and living conditions. Spacecraft’s normal life is stressing also because of monotony, restricted consumables, and non-24h light-dark cycles. Longer distances from Earth and delays in communication increase the sense of isolation, requiring the crew to work more independently without assistance from NASA’s Mission Control. Some of these factors induce more risk to the health of crew members such as increasing levels of CO2 in the cabin at all times or light conditions causing the change in the circadian rhythm of the crew (Law et al., 2014; NASA/TP–2010– 216126). To better understand potential consequences, studies on Earth-based analog ICE environments have been performed. At these facilities, crewmembers spend months in isolation and harsh weather conditions, performing a variety of tasks and procedures like those carried out in space missions. Some examples include Antarctica that is perhaps the best known and most commonly studied analog environment (Lugg, 2005); Aquarius, a submarine installation with atmospheric control capability; remote locations in the desert (NASA’s Desert Research and Technology Studies or DRATS); and mission control based (NASA JSC Human Exploration Research Analog, HERA).
Reactions to ICE.
ICE environmental characteristics contribute to creating a state of anxiety, lack of motivation, irritability, and apathy. Going further, isolation and high prolonged alertness are some of the stressing factors that could trigger anxiety and high levels of cortisol (also given by shift in sleep-wake cycles) impacting upon appetite-regulating hormones, immune system, and hypothalamic-pituitary-gonadal axis, which plays a critical part in reproductive and immune system regulation (Dunn et al., 2004). Impaired physical and social interactions may impact teamwork, especially in long-duration missions where the crew has to work together and without another human contact for a long time. These issues may result in several potentially hazardous conditions, such as lower performance, mood disorders, and other psychological conditions (Van Baarsen et al., 2009).
Because behavioral, mood and cognitive impairment may put the crew members and the missions at risk, new potential solutions need to be explored. Noninvasive brain stimulation (NiBS) includes safe and portable techniques that have been widely applied therapeutically in cognitive and sensory domains.
3). NONINVASIVE BRAIN STIMULATION TECHNIQUES
NiBS techniques rely on electromagnetic principles to noninvasively influence neural activity through the generation of cortical electrical fields. They have been extensively used to investigate the neural basis of many cognitive and sensory-motor domains, as well as potential therapeutic interventions to restore physiological brain activity in psychiatric and neurological diseases (Ridding and Rothwell, 2007; Rossini and Rossi, 2007; Valero-Cabré et al., 2017). Specifically, two main classes of NiBS are currently applied for clinical and research purposes, that is TMS and tES (Figure 2). Here, we offer an overview of both techniques, covering their underlying mechanisms of action and the most validated protocols. We will also review the safety and feasibility of NiBS protocols, discussing the advantages and limitations of these techniques over other forms of stimulation.
Figure 2. Noninvasive Brain Stimulation Techniques.
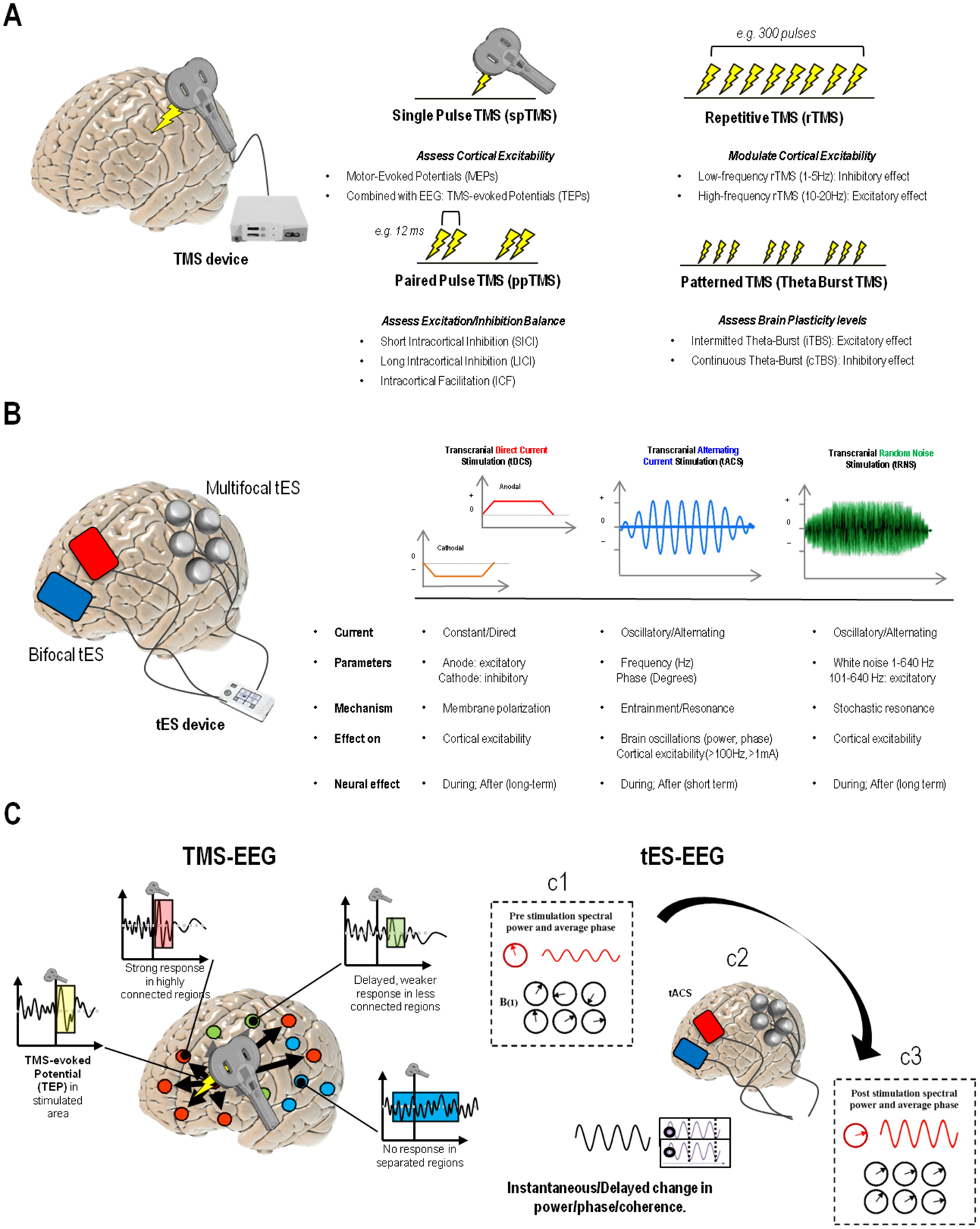
(Panel A) Transcranial Magnetic Stimulation (TMS) relies on the electrical current going through a conductive coil, inducing a time-varying focal high magnetic field (~2T) that generates a strong electric field (>100 V/m) directly causing neuronal spiking with a high spatial resolution (~0.53 cm of activated brain tissue) (down, left). Different TMS protocols can be implemented by delivering trains of TMS pulses (repetitive TMS- rTMS) or by pairing pulses, inducing various effects including increase/decrease of corticospinal excitability, increase/decrease in cortical plasticity, modulation of brain excitatory/inhibitory balance, and changes in local connectivity and blood flow/perfusion. (Panel B) Transcranial Electrical Stimulation (tES) requires at least two electrodes (red=anode, blue=cathode), with at least one applied directly on the scalp (up, left). More channels can be added to extensively record EEG and/or perform high-resolution “multifocal” tES. Available protocols include transcranial direct current stimulation (tDCS), transcranial alternating current stimulation (tACS), and transcranial random noise stimulation (tRNS). Stimulation protocols differ from one another and allow to induce changes in cortical excitability, as well as more specific effects on brain oscillations, e.g., the α or γ rhythms. (Panel C)Stimulation techniques can be also combined with electrophysiology, as in the case of simultaneous TMS-EEG (panel and tES-EEG recording. In the case of TMS, a focal magnetic pulse is delivered to a specific brain region using a neuronavigation system (based on individual’s MRI) which allows for precise anatomical targeting of cortical areas at 1-millimeter resolution. The activity elicited by the pulse is mostly local, with distant effects usually observed for regions structurally or functionally connected to the stimulation site. Both local reactivity and short-long range connectivity can be evaluated, either in terms of TEPs or time-frequency analysis. Distant or out-of-network regions might show delayed or even no responses. While TMS provides higher spatial resolution, when applied using alternating current, tES allows for frequency-specific modulation of brain electrical activity by supposedly tuning neuronal populations towards an externally induced oscillatory pattern. The response to tACS can be expressed in terms of spectral power changes investigated pre (c1), during (c2) and/or after stimulation (c3), as well as phase-coherence and other connectivity metrics, with the effects being measurable both at the stimulated area as well as other distant, resonant regions.
3.1). Transcranial Magnetic Stimulation
TMS is based on Faraday’s principle of electromagnetic induction: a pulse of electrical current flows through loops of wire (forming the coil) and generates a time-varying magnetic field that in turn creates an electric field. The induced electric field alters ions disposition and depolarizes neurons to the point of triggering an action potential. Different electric field strength and forms can be generated by TMS through the modification of physical and biological parameters, such as magnetic pulse waveform, coil shape, and orientation, intensity, frequency, and patterns of stimulation (Rossi et al., 2009a).
When single-pulse TMS (spTMS) is applied to the motor cortex (M1), the stimulation activates the corticospinal descending pathways triggering the motor evoked potentials (MEPs). MEPs can be recorded through electromyographic recordings from contralateral muscles from the stimulation (Rossi et al., 2009). RMT refers to the lowest TMS intensity necessary to evoke an MEP in a target muscle with an amplitude of 50μV with at least a 50% probability (Rossi et al., 2009). RMT reflects membrane excitability of corticospinal neurons and interneurons projecting onto the M1, as well as the excitability of motor neurons in the spinal cord and neuromuscular junctions (Rossi et al., 2009).
Paired-pulse TMS (ppTMS) protocols consist of 2 successive pulses with an inter-stimulus interval (ISI) ranging from few milliseconds to hundreds of milliseconds. Both pulses are generally applied over the M1 of the dominant hemisphere. This method is used to explore intracortical networks depending on the intensity and ISI used (Kujirai et al., 1993; Valls-Solé et al., 1992). ISI of few milliseconds is generally used to investigate short intra-cortical inhibition (SICI) mechanisms that are thought to reflect GABAergic interneurons activity (Rossi et al., 2009). ISIs between 7–20ms is chosen instead for intra-cortical facilitation (ICF) mechanisms, primarily reflecting glutamatergic interneurons activity (Kujirai et al., 1993). TMS pulses delivered over the M1 of both hemispheres can be useful to explore inter-hemispheric inhibition (e.g., transcallosal inhibition; Ferbert et al., 1992).
Contrary to spTMS and ppTMS, repetitive TMS (rTMS) can change and modulate cortical activity beyond the stimulation period. The physiological bases of rTMS after-effects have not yet been identified, but the main underlying mechanisms seem to involve long-term potentiation (LTP) and long-term depression (LTD). LTP is defined as an increase in synaptic strength, whereas LTD reflects a decrease (Klomjai et al., 2015). Accordingly, rTMS protocols affect the excitability and neuroplasticity of a stimulated area outlasting TMS duration per se, depending on inter-individual variability (Maeda et al., 2000) as well as on the stimulation parameters, leading to a decreased cortical excitability when low-frequency rTMS (≤1 Hz) is applied, whereas an increase is seen following high-frequency rTMS (≥5 Hz) protocols (Ni and Chen, 2015, Rossi et al., 2009). rTMS applications have been mainly used to study cognition, brain-behavior relations, and the pathophysiology of various neurologic and psychiatric disorders (Ridding and Rothwell, 2007; Rossi et al., 2009; Rossini and Rossi, 2007). New rTMS approaches involve the application of high-frequency bursts of stimuli at theta frequencies, known as theta-burst stimulation (TBS). Stimulus intensity required for TBS is lower compared to other rTMS protocols (Huang et al., 2005) and can be applied in a continuous (cTBS) or intermittent (iTBS) fashion depending on the purpose: cTBS tends to depress excitability of the M1, while iTBS has the opposite effect (Huang et al., 2005).
While stimulating the scalp, the resulting electrical field produced current density distributions that are asymmetrical in magnitude and direction. Because TMS has a spatial resolution of approximately 0.5–1cm (Thielscher and Kammer, 2002; Toschi et al., 2008), precise targeting of the area to stimulate is pivotal for the outcome. To finely target areas, especially when a rapid and objective outcome measure cannot be revealed such as for M1 with MEP and MT, navigated brain stimulation (NBS) has been developed. NBS devices consist of an infrared camera detecting trackers placed on a headband worn by the subject and on the coil. Using Magnetic Resonance Imaging (MRI) brain data, NBS can rebuild the subject’s head in 3-D and record coil position, ensuring better accuracy in targeting the chosen areas and as well as to have a finer estimation of the strength and direction of TMS-induced electrical field.
3.2). Transcranial Electrical Stimulation
tES applies low transcranial electrical currents (0.5–2mA) that generate weak electric fields to target specific brain areas, allowing for the sub-threshold modulation of firing properties of cortical neurons and ongoing rhythmic brain activity. Various tES protocols can be implemented through different stimulation parameters, such as shape, position, and numbers of electrodes, current waveform, frequency, and duration of stimulation. The electrical current is delivered through two or more surface electrodes placed on the scalp and connected to a current waveform generator. Transcranial direct current stimulation (tDCS), transcranial alternating current stimulation (tACS), and transcranial random noise stimulation (tRNS) are the most common protocols (Paulus, 2011).
tDCS.
tDCS induces a low-amplitude (0.5–2mA) direct current that modulates brain excitability eliciting neuronal membrane modifications depending on the direction of the generated electric field (Ruffini et al., 2013). Current flows from the anodal electrode to the cathode, creating intracranial electric fields that alter cell membrane depolarization (i.e., increase of excitability) underneath the anode, and hyperpolarization (i.e., decrease of excitability) in the cathode (Nitsche et al., 2005), with plastic effects. Anodal tDCS over M1 has indeed been shown to increase MEP amplitude, whereas cathodal tDCS decreases it (Paulus, 2011). In particular, tDCS brings the underlying neurons closer or further from their firing threshold (Bikson et al., 2004), leading to an increase in glutamine and glutamate levels (Hunter et al., 2009) and/or decreasing γ-aminobutyric acid (GABA) concentrations (Bachtiar et al., 2015; Stagg et al., 2011). N-methyl-D-aspartate (NMDA) receptors-dependent mechanisms as well as and brain-derived neurotrophic factors (BDNF, Fritsch et al., 2010) also play a key role (Liebetanz et al., 2002; Nitsche et al., 2003). Although the increased duration and/or intensity of stimulation might be assumed to maximize tES effects, studies have suggested that the dose-response relationship is nonlinear (Batsikadze et al., 2013; Kidgell et al., 2013; Monte-Silva et al., 2013). Jamil and coworkers (2017) have recently investigated tDCS dose-dependency by systematically measuring neuroplastic responses to stimulation. Healthy participants received anodal or cathodal tDCS applied for 15 min to left M1 at different intensities: sham, 0.5, 1.0, 1.5, and 2.0 mA. MEPs elicited by single-pulse TMS were recorded before and at multiple time points up to 2h following stimulation to quantify M1 plasticity. Results indicated a nonlinear relationship between stimulation intensity and the induced neuroplastic response, for both anodal or cathodal tDCS. Consistent with previous research (Labruna et al., 2016), they found that the facilitatory response is seen after 1.0 mA anodal tDCS was greater in participants with higher sensitivity to TMS (i.e., those requiring a lower TMS intensity to elicit MEPs). This relationship is currently assumed to reflect inter‐individual differences in anatomical (e.g. skull thickness, cortical morphology) and physiological (e.g. neurotransmitter availability and receptors distribution) factors, that similarly affect NiBS techniques both online (Labruna et al., 2016) and after stimulation (Ridding and Ziemann, 2010). Modifications in synaptic strength are also enabled/hampered by a mechanism of homeostatic plasticity that may cause further variability in efficacy. Synaptic connection is, in fact, facilitated/suppressed depending on the previous amount of network activity. As an example, Monte-Silva and coworkers (2010) found that the efficacy of inhibitory cathodal tDCS could be enhanced when followed by a second session of stimulation during the aftereffects of the first one. On the other hand, the effect was significantly reduced if the second stimulation was applied when the aftereffects of the first session were dissipated (Montesilva et al., 2010). This will furthermore require particular attention when planning multiple sessions of stimulations and testing tDCS efficacy.
tACS.
Most aspects of human cognition show corresponding patterns of brain oscillatory activity, determined by the synchronous neuronal firing across different spatial and temporal scales. Therefore, the chance to engage oscillatory activity, rather than simply increase or decrease activity in a target region as with tDCS, could lead to a more powerful, finer control of brain activity and corresponding behavior. In this regard, tACS has been suggested as the most promising technique to safely and noninvasively modulate brain rhythms. tACS delivers alternating current that continuously shifts between positive and negative electric fields (Tavakoli and Yun, 2017), thus inducing periodic shifts in the transmembrane potential, alternating depolarizing and hyperpolarizing effects, enabling the entrainment of intrinsic brain oscillations due to its sinusoidal waveform (Antal and Paulus, 2013; Paulus, 2011). In particular, tACS drives cortical populations to oscillate at the same natural frequency as the one delivered by the stimulation itself, with a greater amplitude as per the resonance phenomenon (Paulus, 2011). As with tDCS, it also allows stimulation over multiple brain regions at the same time, i.e. favoring synchronization or tACS can be applied over a wide frequency range, from 0.75Hz during NREM sleep to enhance declarative memories (Ketz et al., 2018), to γ frequency (40–80Hz) to modulate fluid intelligence (Santarnecchi et al., 2013), problem-solving ability (Santarnecchi et al., 2019), and visuospatial coordination (Santarnecchi et al., 2017). Behavioral effects have been demonstrated at the level of sensorimotor (Feurra et al., 2011, 2013; Santarnecchi et al., 2017), visual (Kanai et al., 2008), somatosensory (Feurra et al., 2011b), and higher-order cognitive domains (Polania et al., 2012; Santarnecchi et al., 2013).
tRNS.
Differently, tRNS elicits an increase of cortical excitability with stimulation delivered in the form of random noise, produced through electrical patterns at a random frequency over a broad spectrum (0.1–640Hz; Terney et al., 2008). tRNS can increase or decrease the excitability at different intensity range (Moliadze et al., 2012). This type of stimulation is thought to induce long-term potentiation of cortical plasticity through different mechanisms, for example through the repeated opening of sodium channels (Terney et al., 2008). Indeed, in a pilot study on tRNS application over the M1, MEPs were inhibited by carbamazepine, a Sodium (Na+) channel blocker (Chaieb et al., 2015). As second hypothesized mechanisms of action, tRNS might act using stochastic resonance (Miniussi et al., 2013), according to which weak signals can be amplified by the addition of noise (McDonnell and Abbott, 2009). In this sense, random noise added to subthreshold neural oscillations in the brain would result in a summation of the two currents strong enough to exceed the threshold. To date, few studies have investigated the effects of tRNS in modifying EEG features, mostly focusing on a simple motor or sensory tasks (Fertonani et al., 2011; Terney et al., 2008). As demonstrated via spTMS, tRNS seems to be the most effective tES technique to increase cortical excitability of the M1 Although the potential applications of tES protocols to modulate cognitive and motor abilities are well-known and replicated, they also require particular attention to factors that may affect the results of stimulation (for a comprehensive review see Krause and Kadosh, 2014). Different studies over the last decade have shown varying results between individuals due to the differences in cortical activity (Krause et al., 2013), tissue composition under the stimulating electrodes (Russell et al., 2013), age (Leach et al., 2019), and gender (Russell et al., 2017). Moreover, the proportion of individuals fail to respond to stimulation altogether (López-Alonso et al., 2014). Besides, the electric field resulted by tES protocol can be significantly altered by various parameters of methodological decisions, such as current intensity (Bastani and Jaberzadeh, 2013), electrode placement (Moliadze et al., 2010), and current phase and frequency in the case of tACS and tRNS (Nakazono et al., 2016).
3.3). Biophysical Modeling for Precise Targeting in NiBS
Under the exponentially increasing demand for tES solutions to be implemented in clinical trials, the field of biophysical modeling of tES and TMS has significantly grown over the last decade, reaching unprecedented accuracy. The principal interaction mechanism in tES is thought to arise from the coupling of electric fields and populations of ordered, elongated neurons in the cortex, especially pyramidal cells. The role of other types of neurons (e.g., interneurons, such as basket cells) or other brain cells, such as glia, is less well understood. The external electric field forces the displacement of intracellular ions (which move in response to the change in the intracellular field), inverting the neurons’ internal charge distribution and resulting in a transmembrane potential difference. For a long fiber with space constant λ in a homogeneous electric field (E), the transmembrane potential difference is largest at the fiber termination, with a value that can be approximated by λ E · n, where n is the unit vector defining the fiber axis.
This is essentially a first-order approximation, with a spatial scale provided by the membrane space constant and directions by field and fiber orientation (Ruffini et al., 2013). As a consequence, a necessary first step in understanding the effects of tES is to determine the spatial distribution of the generated electric vector field in the brain. For this reason, software models have been developed using finite element modeling to predict the distribution of electric fields produced by bipolar or multichannel stimulation montages (Miranda et al., 2013).
Such realistic computational models of the head can predict electric field distribution in the human brain with reasonable accuracy, as the availability of recent measurements in-vivo confirms (Huang et al., 2017; Opitz et al., 2016). The key element of these models is a description of the geometry of the head as a volume conductor and the electrical properties of tissues in the model. Although the use of MRI provides very valuable input for the creation of a model, there is still a need for better estimates of tissue conductivities, as there are discrepancies in the literature about their values and individual variability (Figure 3, Panel A and B).
Figure 3. Biophysical modeling and Network Targeting.
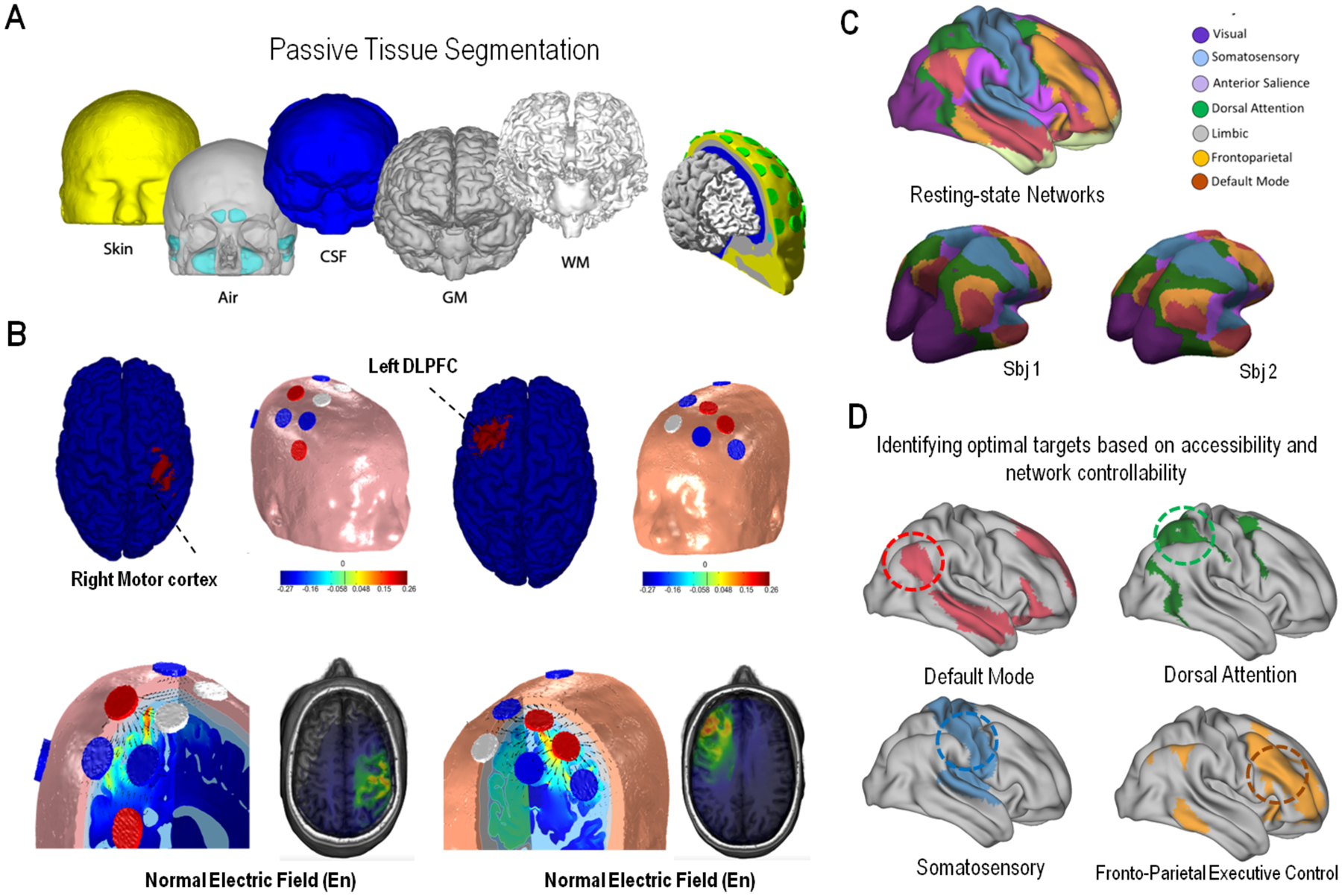
(Panel A) Starting from the MRI of a subject, the head is segmented into several layers to create a finite element model for the analysis of the electric field and current distribution. (Panel B) A target is specified, e.g. the right motor cortex or left dorsolateral prefrontal cortex, and a genetic algorithm is used to find the optimal location and currents of a subset of electrodes to maximize stimulation over the target while minimizing it over the rest of the brain (left, right motor cortex, right: left motor cortex target). At the bottom, we provide a visualization of the electric vector field (arrows), and in color scale the amplitude of the normal component of the electric field (En) to the cortical surface for two example montages. (Panel C) Network mapping can help to identify the topography of known brain networks using structural and functional MRI data, to then (Panel D) further identify optimal stimulation site(s) for each network/individual based on criteria including targetability (i.e. location of target area) and propagation ability within the target network.
The first calculations of the electric field in the brain during tDCS (based on a realistic head model) were published almost ten years ago (Parazzini et al., 2011, Salvador et al., 2010). Today, such models are routinely developed for research applications. The systematic use of individual models should enhance the rigor and reproducibility of tES research (Miranda et al., 2018), as it provides better control on what is believed to be a crucial mechanistic element in tES, the electric field. Such analysis showed that the use of large sponges with electrode positioning is a poor way to control the location and distribution of the electric field, and hence the effects of tES. For this reason, modern solutions employ multichannel systems and small electrodes. The combination of such technologies with more sophisticated modeling algorithms results in more robust, adaptable, and effective solutions. The next generation of models, however, will need to combine representations of both physical and physiological elements involved, such as the “hybrid brain models” (Sanchez-Todo et al., 2018). These models represent the framework for next-generation optimization algorithms that can assimilate physiological data such as EEG or TMS-EEG.
Based on this rapid electric field simulator, Ruffini and coworkers (2014), for example, developed an optimization system called Stimweaver, where optimal montages problems can be defined and solved. In short, the Stimweaver algorithm adopts a fast calculation of multifocal tDCS electric fields (including components normal to the cortical surface) using an MRI derived finite element realistic head model with 6 segmented tissues. Under the assumption that the effects of current stimulation are to first-order due to the interaction of the electric fields with populations of pyramidal cortical neurons, the optimization problem for tDCS stimulation can be defined in terms of the component of the electric field normal to the cortical surface. Solutions are found using constrained least-squares comparing weighted target and normal electric field cortical maps to optimize current intensities. Electrode number and their locations are selected using a genetic algorithm that searches in montage configuration space. The population in the genetic algorithm thus consists of individuals that encode for a particular montage and the optimal currents associated with it. Rules for genetic cross-over and mutation are described in Ruffini et al., (2014). This algorithm has been used by several groups (e.g., Dagan et al., 2018; Fischer et al., 2017; Sprugnoli et al., 2019).
3.4). Network Targeting with NiBS
The same approach can be used to target brain functional networks. Brain activity is organized in a set of resting-state functional networks (RSNs) whose synchronized activity span between spatially distinct interconnected regions (Figure 3, Panel C). Functional connectivity (FC) within the networks can be measured via fMRI. RSNs involve areas responsible for both sensory processing as well as for high order cognition. The most studied networks include the Default Mode Network (DMN), Frontoparietal Control Network (FPCN), Sensorimotor (SM), Ventral Attention Network (VAN), Anterior Salience Network (AS) (Damoiseaux et al., 2006; Heuvel et al., 2009; Zuo et al., 2010). The DMN is particularly relevant due to its pivotal role in the spontaneous activity of the human brain and because of its vulnerability in modifications due to deep space missions.
Although studies have administered stimulation over singular specific derivations (Figure 3, Panel D), new devices enable multisite stimulation of neural networks, engaging interconnected cortical areas as well as entire networks (Dmochowski et al., 2011; Miranda et al., 2013; Ruffini et al., 2014). To do that, multifocal stimulation using several relatively small electrodes has been used to achieve more focal stimulation of specific cortical targets (Ruffini et al., 2014). This allows a spatially specific protocol that can simultaneously stimulate different areas belonging to the same (e.g. DMN) or different networks. Modulating a whole network associated with brain function may also imitate a more natural cortical activation, therefore offering a more efficient stimulation.
3.5). Framework and Targets for NiBS application
Considering the safety and feasibility of NiBS, and the wide range of aforementioned studies on its efficacy on different cognitive domains, NiBS may be a useful tool in space exploration. Potential implementations may include protocols to enable visuomotor and cognitive skills during pre-flight training, enhancement of in-flight performance, prevention/treatment of anxiety/mood disorders, prevention of detrimental effects of microgravity and cosmic radiations, as well as post-flight rehabilitation.
NiBS protocols are frequently administered in “online” sessions, meaning stimulation is applied while participants are involved in tasks or other activities. Especially in the case of tES, brain stimulation can represent a versatile tool applicable at multiple stages in the astronaut lifetime, ranging from cognitive and motor training on Earth in preparation for space missions, to actual spaceflight (Figure 4). Online stimulation is not always possible during spaceflights (e.g. EVA or cognitive training in some ICE environments). In those situations, stimulation can be performed before the task, triggering different forms of cortical plasticity that would potentially boost performance/learning. For instance, tDCS specifically induces online and offline effects (Labruna et al., 2016; Ridding and Ziemann, 2010), as reported in multiple TMS studies on M1 cortical excitability (e.g. Jamil et al., 2017). Additionally, both of these effects have been proven to outlast the stimulation period and be even more robust offline than during stimulation(Santarnecchi et al., 2014). Cathodal tDCS on M1 leads to an offline long-lasting inhibition in cortical excitability, as measured by a drop in MEP amplitude up to 90 minutes after stimulation (Nitsche and Paulus, 2001, 2000). Similar after-stimulation effects of tDCS have been shown also on different areas, such as somatosensory cortices (Matsunaga et al., 2004), DLPFC (Keeser et al., 2011), and cerebellum (Grimaldi et al., 2016). In various contexts, it is also possible to perform stimulation after a training session to enable knowledge/learning consolidation, or in-between cognitive and motor tasks to prime/consolidate memory traces (Rumpf et al., 2017). Moreover, tES can also be used during sleep, modulating deep sleep stages involved in the consolidation of declarative memory (Jones et al., 2011; Ketz et al., 2018; Marshall et al., 2006, 2004).
Figure 4. Framework for NiBS application.
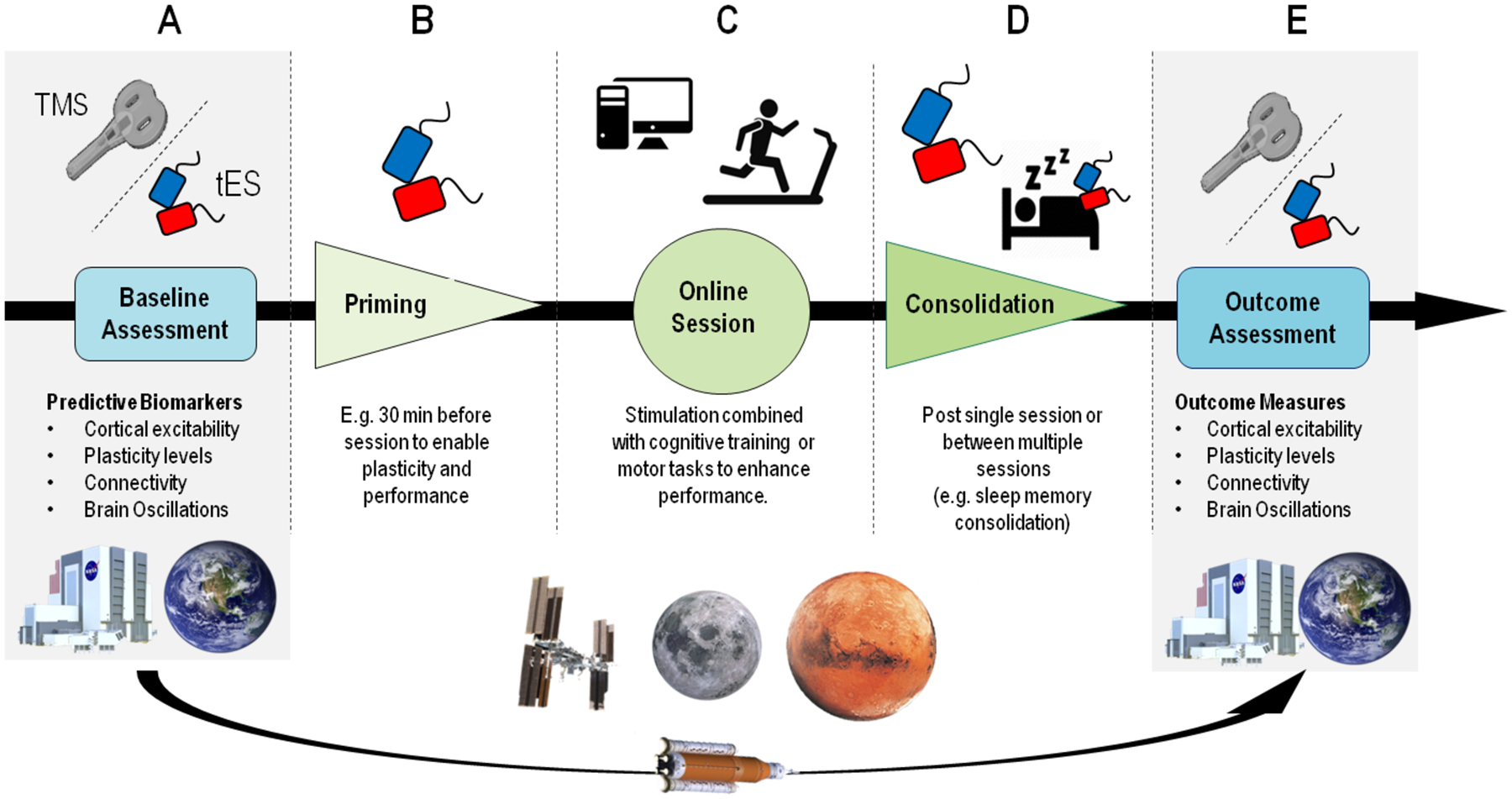
NiBS can be applied with different outcomes depending on the specific goal and chosen time of administration. Both TMS and tES could be implemented for Earth-based assessment to investigate biomarkers and modifications in cortical parameters, such as excitability and brain oscillations, testing astronauts before and after spaceflights (panel A and E). On the ISS, in space installations, as well as on Earth, tES could be applied to enhance performance in different domains. In the case of activities that do not allow concurrent tES application (e.g., EVA), stimulation could be performed before a specific task to enable plasticity (panel B). The most common paradigm involves “online” stimulation, i.e. tES/TMS performed concurrently to a subject performing a motor or cognitive task (panel C). Stimulation can also be administered after a task/training, to consolidate learned abilities and memory traces, also during sleep (panel D). Finally, the same biomarkers collected at baseline could be used to assess the impact of long-term spaceflight on brain structure and function (panel E).
TMS and tES are also powerful tools to investigate biomarkers of adaptation to spaceflight. By controlling the input sent to cortical areas by TMS or tES, it is possible to quantify local responses, speed of signal propagation across networks, and network resilience to perturbation. In astronauts, TMS and tES assessments combined with EEG and neuroimaging before and after a mission could help identify potential biomarkers of local plasticity, cortical excitability, connectivity, and changes in brain oscillations (Bestmann, 2008; Ferreri and Rossini, 2013) previously associated with, e.g., depression and neurodegeneration. More details on specific NiBS applications are available in the dedicated sections below.
4). PRE-FLIGHT NiBS APPLICATIONS: TRAINING
Before facing the challenging environment of deep space, future astronauts are carefully trained on Earth. This includes medical tests, physical and EVA training, procedure knowledge test, as well as a preparation for experiments that the crew will accomplish during their mission. Pre-flight training usually lasts 2–4 years, and it is geared to special conditions and environments astronauts will be confronted with during launch, in space, and during landing. For instance, motor learning and consolidation is particularly relevant to operate the Space Station Remote Manipulator System (SSRMS) on ISS. The appropriate manipulation of this robotic arm is essential to perform advanced reparation at modules and on ISS itself, thus requiring 4 years of training of preplanned motor patterns and sequences on different simulators. Astronauts need to maintain a high level of visuomotor coordination, continuously checked and corrected by leveraging multitasking ability and sustained attention. In this context, tDCS protocols have been administered to enhance the consolidation of motor processes and procedural learning (Buch et al., 2017). During years-long training, astronauts are also required to learn a great amount of procedure and high-detailed knowledge of a wide range of domains, from space physiology to geology and spacecraft engineering. tES has been applied during sleep to improve declarative memory learning, consolidation, and recall (Marshall et al., 2004, 2006). Below we provide an overview of domains where NiBS could be implemented in pre-flight operations.
4.1). TMS to Induce Neuroplasticity and Motor Learning
Changes in synaptic strength (i.e. neuroplasticity) are governed by various mechanisms, such as LTP and LTD (Malenka and Bear, 2004). LTP can be naturally induced following learning (Rioult-Pedotti et al., 2000), but can also be experimentally triggered by external stimulation delivered at certain patterns to mimic natural brain rhythms. Theta Burst Stimulation (TBS) is a TMS protocol widely known for its plasticity-inducing ability (from Larson et al., 1986; for a comprehensive review see Larson and Munkácsy, 2015). As aforementioned, early reports suggested that an intermittent pattern of stimulation (iTBS) resulted in increased cortical excitability (similar to LTP) (Huang et al., 2005). These findings have been widely replicated (for reviews see Huang et al., 2017; Suppa et al., 2016). iTBS could, therefore, be used to induce neuroplasticity and enable performance in learning tasks, with a focus on motor and visuomotor abilities.
Intermittent TBS provides a potential tool to enhance performance specifically in the early phase of motor learning (Honda et al., 1998; Iezzi et al., 2010). As an example, excitatory iTBS over M1 administered 10 minutes before a motor task enhanced the learning of ballistic movements (Agostino et al., 2008; Teo et al., 2011). Koch and coworkers (2020) administered cerebellar iTBS to accelerate the adaptation in a visuomotor adaptation task (VAT) (Krakauer, 2009), a specific form of motor learning task which evaluates errors in response to a novel perturbation. iTBS accelerated the error reduction slope in response to new perturbation (Koch et al., 2020). This may be a relevant application in speeding adaptation of Earth-based learning motor tasks.
Interestingly, TBS protocol may also help in the training of non-dominant hand while engaging in tasks such as operating the SSRMS, Moon and Mars landing, and EVA operations. The performance of the non-dominant hand after intense motor training can outperform the dominant one (Platz et al., 2012b, 2012a; Ridding and Flavel, 2006). Platz and colleagues (2018) stimulated healthy right-handed subjects performing an arm ability training (AAT) for one week, administering iTBS over either S1 or M1 contralateral to the trained left arm. The authors reported that the excitatory priming of S1 or M1 directly before a daily training session enhanced sensorimotor learning (Platz et al., 2018). Participants not only showed better performance at the AAT task when stimulated with iTBS, but they also saw an improvement in the generalization task for the trained left hand. iTBS could be used to enhance motor learning across different sensorimotor abilities (Platz et al., 2018).
4.2). tES, Motor Learning, and Motor Memory Consolidation.
In the context of motor learning, tDCS has also been shown to modulate both overall motor performance as well as specific processes involved in learning and consolidation (Kang and Paik, 2011; Krause et al., 2016; Orban de Xivry and Shadmehr, 2014). As aforementioned, M1 is the most involved in early learning phases, while motor learning is mediated by different areas as premotor and parietal association areas (Honda et al., 1998). Different studies applied excitatory-anodal tDCS over M1 during or immediately after a motor sequence learning task, showing facilitation in early consolidation of procedural learning (Antal et al., 2004; Nitsche et al., 2003; Tecchio et al., 2010), although this has not been reproduced in all available studies (Ambrus et al., 2015; Ehsani et al., 2016). Stagg and coworkers have shown polarity specific effects of tDCS during explicit motor learning, with improvement in performance observed only with anodal stimulation and when stimulation was performed concurrently with the task (Stagg et al., 2011). Along the same line, implicit motor learning was enhanced with tDCS applied concurrently with training by positioning the anode over M1, while stimulation of frontal and prefrontal areas did not affect performance (Antal et al., 2004; Nitsche et al., 2003). In the context of motor adaptation (i.e., modify movements in response to different sensory inputs or motor outputs, leading to a reduction in errors introduced by the altered conditions), Hunter and coworkers (2009) tested the motor performance of reaching a target with the arm experiencing different physical environments such as robot-induced velocity-dependent force fields. The authors administered active or sham tDCS over the M1 during the adaptation phase of the movement when the velocity-dependent force field was applied. Although the global error in arm reaching was similar in both conditions, real 1mA anodal tDCS induced a significantly better global reaching performance. These results suggested that tDCS enhances the development of an internal representation of a novel adapted movement. Importantly, tDCS effects could persist for months when applied in multiday protocols, due to its ability to modify brain plasticity. Reis and coworkers (2009) showed that tDCS delivered over 5 consecutive days combined with a motor learning protocol improved performance, not only during the experimental paradigm but for at least 3 months after training. Similarly to tDCS, tRNS applied over the M1 for 10 minutes improved implicit motor sequence learning and caused excitatory aftereffects lasting up to 1.5 hr (Terney et al., 2008). In the context of motor adaptation (i.e., modify movements in response to different sensory inputs or motor outputs, leading to a reduction in errors introduced by the altered conditions; Tanaka et al., 2011), two studies have shown cerebellar tDCS being more effective for M1 stimulation (Galea et al., 2011; Herzfeld et al., 2014).
In the case of fine motor learning, such as surgical training, tDCS applied on M1 in non-surgeon participants performing a virtual neurosurgery session was able to enhance the performance of skilled subjects in comparison to placebo stimulation (Ciechanski et al., 2017) with long-lasting effects detected at 6-weeks follow-up visit. Additionally, in another experiment, participants stimulated with tDCS on M1 during training with a virtual simulator for laparoscopic surgery improved their performance to greater extent respect to the sham group (Ciechanski et al., 2018). It worth notice that training of surgeon involving microscope or minimally invasive tools (as in laparoscopic surgery) requires intensive and dedicated training for the intrinsic difficulties related to the lack of tactile sensation, depth perception and alteration of hand-eye coordination (Ding et al., 2014, Maciel et al., 2008), similar to what experienced by the astronauts handling the SSRMS robotic arm.
While tES applied during tasks (i.e. online) seems to be the best protocol to enhance motor memory learning, sleep modulation may promote memory consolidation of previously learned motor sequences. Lustenberger and colleagues (2016) applied the EEG-feedback-controlled approach that restricts the application of tACS at 12Hz to an NREM sleep spindle detection. This targeted modulation increased motor memory consolidation tested by a motor sequence tapping task. Differently, Lafon and coworkers, (2017) reported an unsuccessful attempt to entrain sleep spindles while applying low-frequency tACS in healthy subjects. The authors measured endogenous spindle power intracranially during NREM sleep using invasive pre-surgical electrocorticography monitoring in 13 patients with epilepsy, finding no stable evidence of entrainment. Even though the main reason for the failure could be attributed to the underlying epileptic activity, it should also be noted that stimulation intensity varied across the two studies, with the latter applying significantly weaker currents (<0.05 V/m).
4.3). Declarative Memory Consolidation through Sleep.
The modulation and enhanced consolidation through tES are also possible for strengthening declarative memories. Marshall and coworkers (2006, 2004) were the first to modulate sleep to increase recall in declarative memories with electrical stimulation. They investigated the effects of bifrontal 0.75 Hz oscillating tDCS or tDCS (F3, F4) during slow-wave sleep (SWS) in young adults. Subjects performed a declarative memory task before and again immediately after sleep. For the first time, the authors demonstrated a link between the boost of slow oscillations during N3 and the improvement in declarative memory consolidation (Marshall et al., 2006, 2004). Marshall’s group also demonstrated the effect of this protocol of otDCS on cognitive functions during NREM sleep investigating cortical activity in a rodent model (Binder et al., 2014). Accordingly, more recent studies found better performance in declarative memory task after the application of sinusoidal wave at a frequency of 0.5–1.2Hz within a closed-loop tACS-EEG device overnight, enhancing δ power and an increase of γ and sigma waves (Jones et al., 2018; Ketz et al., 2018). However, in successive studies applying similar experimental paradigms to replicate the declarative memory consolidation in a different cohort of healthy elderly did not found beneficial effects (Eggert et al., 2013; Paßmann et al., 2016).
Applying slow oscillations through different protocols of tES (either tACS or oscillatory tDCS) to older adults during afternoon naps also was able to manipulate slow-wave activity (SWA) and enhance memory consolidation. Westerberg and colleagues heightened SWA with the following improvement in word-pair performance (Westerberg et al., 2015), whereas Ladenbauer and coworkers enhanced slow oscillations and fast sleep spindle power leading to a benefit in a visual memory task (Ladenbauer et al., 2016).
5). MISSION CONTROL, SPACECRAFT, SPACE INSTALLATIONS, AND IN-FLIGHT/ IN-TRANSIT NiBS APPLICATIONS: PERFORMANCE AND LIFE QUALITY
Any space mission occurs in an extreme environment that has unique stressors. Even with careful selection methods and after a detailed training for potential behavioral problems among spaceflight crews, the mission success remains threatened. NASA Human Research Program (HRP) underlined three behavioral and cognitive main risks happening during flights and on space installations: (1) risk of performance decrements and adverse health outcomes resulting from sleep loss, circadian desynchronization, and work overload; (2) risk of performance and behavioral health decrements due to psychological stress, inadequate cooperation, coordination, communication, and psychosocial adaptation within a team; and (3) risk of adverse cognitive or behavioral conditions and psychiatric disorders (NASA SP-2009-3405). We discuss performance impairment on different domains typically seen in astronauts, such as cognition, motor, and sensorimotor coordination, sleep loss and shift, and altered psychological states (for a comprehensive scheme of NiBS applications in space see Figure 5). Because NiBS, specifically tES, have been extensively applied to overcome similar impairments in healthy participants and patients, their applications to tackle detrimental space effects on CNS should be taken into consideration.
Figure 5. Major Stressors and Possible In-Flight NiBS countermeasures.
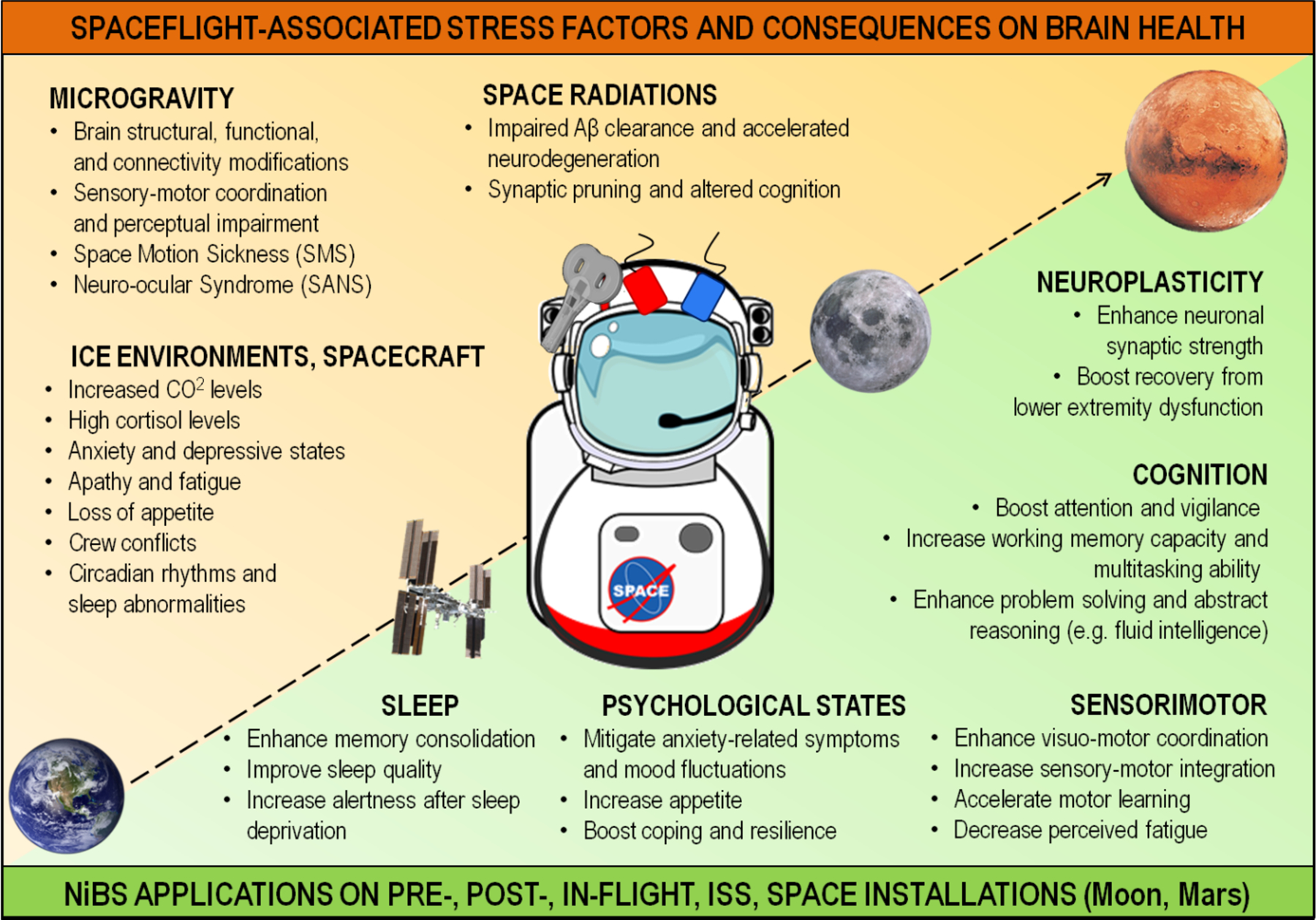
In green, domains that can be targeted by NiBS during space missions. The second panel shows major stress factors due to the space environment that can trigger adverse reactions and threaten crew health.
During space missions, NiBS could also be applied to ensure the performance and results of Earth-based flight control personnel. The Mission Control Center (MCC) is responsible for the safety and planning of ISS and spacecraft, facing problems that often arise during deep space missions and may pose a threat to the health and well-being of the flight crew. MCC personnel work in a stressful environment due to complex situations, such as malfunctioning equipment on the spacecraft, sickness, or injury of a crewmember, or novel incidents, that often require effective decision-making, problem-solving, and multitasking skills, as well as ability to maintain vigilance and attention for long hours (for a comprehensive review see Fiore et al., 2014). Therefore, the MCC team could also take advantage of a NiBS implementation, similar to astronauts in pre-flight training.
5.1). Visual System
The brain, as a control function of the visual system, is a critical pathway even for ocular health. Two tES protocols may help mitigate SANS-related cortical modifications. Particularly, a stimulation on α frequency (10Hz) may be chosen due to its dominant role in visual system oscillatory behavior and its relevance for attentional processes and visual processing. Even though the following hypothesis should be considered purely speculative, external perturbation of visual regions via tACS in the α band may help contrasting modifications in cortical activity due to SANS. Similarly, anodal tDCS over the same area could also be beneficial in treating visual impairments.
5.2). Motor System
Even though the plasticity of the human central nervous system allows individuals to adapt to altered stimulus conditions encountered in a microgravity environment, the integration of signals from all sensory and motor systems is drastically modified in an environment lacking gravity force. The magnitude of sensory, sensory-motor, and perceptual disturbances, as the time needed to recover from them, tend to vary as a function of mission duration and space travelers prior experience with stimulus rearrangement of spaceflight. These alterations trigger motion control disturbances, altered eye-hand coordination, unstable vision, and illusory motion of self and visual scene. Furthermore, approximately 70% of astronauts experience Space Motion Sickness (SMS) during the first week of the mission (Clément and Reschke, 2008). Astronauts become easily disoriented when sensory input received from his or her eyes, muscles and joints, or vestibular organs conflicts with one another, and this can produce this syndrome causing nausea and vertigo. Going further, the transition from different gravity fields back and forth (earth: 1g, ISS: partial gravity; Mars: 0,38g) affects also manual manipulation of objects and tools. Accordingly, MRI-based HDBR studies have reported alterations related to motor-related tasks, such as fine motor control (Liao et al., 2015).
Another pivotal activity, requiring specific experience, is represented by the EVA, term applied for a spacewalk outside the ISS, on lunar surface exploration, and hopefully Mars exploration. EVA is not only a delicate and dangerous action but also an extremely fatiguing activity. The suit is pressurized as a countermeasure for microgravity and dangerous space conditions outside earth crafted vehicles. Therefore, due to stiffness of the whole spacesuit, the force applied to make simple movements requires a lot of energy as well as extreme accuracy to maintain high performance, while also causing high fatigue (NASA IG-18-021).
5.2.1). Sensory Motor Integration and Visuo-Motor Coordination.
tACS has been used to investigate motor enhancement and sensory-motor integration. Wach and coworkers (2013) investigated the effect of 10 and 20 Hz tACS for 10 min over left M1 while assessing movement speed and accuracy of the right hand. While 10 Hz tACS particularly increased movement variability, especially in tasks requiring internal pacing, 20 Hz tACS resulted in movement slowing. A few years later, the γ band entrainment of M1 proved to enhance movement acceleration and velocity in visually triggered movements (Moisa et al., 2016). During the same year, Guerra and colleagues (2016) administered different protocols of TMS during tACS at motor and nonmotor resonance frequency over M1 and showed promotion of sensorimotor integration by β band stimulation (i.e. 20Hz tACS). Replication studies suggest that the direction of the effect of a NiBS protocol may not always be easily predictable. For example, different cortical interneuronal populations are differentially modulated by the phase and frequency of tACS-imposed oscillations (Guerra et al., 2016). NiBS protocol may produce a mixture of inhibitory and facilitatory effects, and the measured effect would be the added-effect of both (Huang et al., 2011).
Finally, Santarnecchi et al. (2017) applied tACS at a different frequency (5Hz, 20 Hz, 60 Hz, and 80 Hz) over dominant M1 during a visuomotor coordination task reporting that, while 20Hz administration slowed participants, high γ tACS (80 Hz) increased performance, promoting visuomotor coordination. Therefore, tES could enhance sensory-motor integration and visuomotor coordination, helping astronauts to tackle difficulties due to motion control disturbances and altered eye-hand coordination during spaceflights.
5.2.2). Motor Performance and Fatigue
Different comprehensive reviews underlined how tES can affect non-motor aspects relevant during long and intensive fine motor procedures, as well as enhance physical performance in professional athletes and amateurs (Angius et al., 2018; Colzato et al., 2017). Stimulation over M1, specifically, seems able to improve the isolated groups of muscles and endurance. Vines and coworkers (2006) showed that anodal tDCS stimulation on left M1 improves right-hand finger sequence performance, whereas cathodal stimulation of the same area improved performance of left hand (Vines et al., 2006). Moreover, the same group reported that concurrent left M1 cathodal tDCS for 20 min at 1mA and right M1 anodal stimulation improved motor performance of left hand compared to anodal stimulation of the right M1 alone (Vines et al., 2008). During the last few years, other positive reports of tDCS effects have been shown with the anode over M1 on isolated muscle groups, such as the elbow flexor (Abdelmoula et al., 2016; Cogiamanian et al., 2007). Flood and coworkers (2017) changed the target area also investigating different parameters of motor performance. They noted that tDCS over the sensorimotor cortex reduced the perception of pain during fatiguing lower limb exercise in 12 subjects (Flood et al., 2017). Even though they found no significant effect on muscle endurance or maximal production of force, other studies reported an enhancement of muscle endurance due to tES (Williams et al., 2013). The authors examined sustained submaximal contractions of the elbow flexion in 18 healthy participants during fatigue task performance. In the meantime, either anodal or sham stimulation was administered to M1 for up to 20 min. These results indicate that anodal stimulation increased time to task failure and the amount of muscle fatigue, suggesting that tDCS administration can enhance the capability to exercise under challenging conditions (Williams et al., 2013). It is relevant to point that the effect induced in the M1 by NiBS may be vulnerable to voluntary muscle activity (Huang, 2016), particularly when this activity happens simultaneously with stimulation. As an example, tonic contraction immediately after tDCS tended to eliminate the aftereffects of both anodal and cathodal stimulation in one study (Thirugnanasambandam et al., 2011). Because of the relevance of brain state during and after stimulation, more systematic studies are needed to explore this dynamic interaction to maximize NiBS’ ability to enable behavioral learning and performance.
More interestingly, noninvasive brain stimulation might be able to enhance physical performance, as reported in studies on professional athletes. Okano and coworkers studied effects of 20 min tDCS with the anode over the left temporal cortex on trained cyclists during an incremental cycling test. They found significantly improved peak power, as well as reduced heart rate and perception of effort at submaximal workloads (Okano et al., 2015). Angius and colleagues (2017), likewise, reported reduced perception of effort and increased endurance in 9 cyclists following anodal stimulation over M1 when cathode was placed on the contralateral shoulder but not when placed over the prefrontal region (Angius et al., 2017). Similarly, Vitor-Costa and colleagues (2015) investigated the enhancing effect of tDCS over M1 on muscle fatigue and exercise tolerance in 11 cyclists. Anodal tDCS enhanced the time to exhaustion at 80% of peak power output. Interestingly, no significant effect was found on perceived exertion and heart rate. Therefore, anodal tDCS selectively enhanced performance without affecting physiological and perceptual variables (Vitor-Costa et al., 2015). The beneficial effects of tDCS on motor performance during physical exercise in athletes and healthy individuals may be explained by tES interaction on perceived fatigue. Various authors suggested that the improvement in workload was achieved by a significant reduction in perception of effort (Lattari et al., 2018, 2016). This may be due to a reduction in excitatory inputs from the supplementary motor area (SMA) and other brain regions (Morree et al., 2012; Zénon et al., 2015). Other authors linked improvement in endurance performance following anodal tDCS over M1 in increased neural drive and reduction in central fatigue (Oki et al., 2016; Vitor-Costa et al., 2015). Accordingly, fatigue reported in different pathologies seems to benefit of rTMS and tDCS application, such as Multiple Sclerosis, Parkinson’s Disease, and Fibromyalgia (for a review see Lefaucheur et al., 2017).
In conclusion, tDCS seems to be a promising tool to enhance muscular strength, muscle endurance, and fatigue which are all crucial not only for professional athletes but potentially also for astronauts and cosmonauts. Although, before its implementation on the in-flight crew, research needs to clarify whether tDCS might be useful in highly trained individuals. One study, indeed, failed to further improve maximum performance in fine motor control in elite pianists (Furuya et al., 2013).
5.3). Cognition
An even bigger threat to the success of space missions is an inadequate and ineffective crew cognitive performance (Clément and Reschke, 2008). Results from Earth-based research highlighted the importance of studying the effects of stress on cognitive performance. Right now, cognitive measurements are routinely performed by astronauts aboard ISS before or after their periodic health status test. Some outdated cognitive tests and batteries are sometime still administered, such as MINICOG (Shephard and Kosslyn, 2005), AGARD (Draycott et al., 1996), and Spaceflight Cognitive Assessment Tool for Windows (WinSCAT; Kane et al., 2005). Cognition (Basner et al., 2015) is a time-constrained test that covers main cognitive domains, such as executive, episodic memory, complex cognition, social cognition, and sensorimotor speed, now the current standard for check-ups in space operations.
Evidence of the effects of spaceflight on cognitive functioning is controversial. Strangman (2014) examined attention, memory, learning, executive or higher-order functioning, emotion processing, and social processing in his extensive review of cognition in spaceflight and other ICE environments. While empirical results reviewed failed to find significant objective decrements in cognitive functioning during spaceflight, the study highlighted a high prevalence of anecdotal reports of difficulties attending to tasks, complaints of cognitive slowing, and memory problems while on orbit. As aforementioned, various animal studies proved cognitive deficits due to a synaptic pruning for cosmic radiation exposure (Britten et al., 2016, 2012; Chakraborti et al., 2012; Denisova et al., 2002; Lonart et al., 2012; Parihar et al., 2018). This makes it difficult to conclude that there is no significant cognitive decrement occurring. A possible explanation involves the concept of “reserve capacity”. Higher functioning individuals are postulated to possess a reserve factor that moderates the expression of impairments in cognitive functioning in the face of brain pathology (Jones et al., 2011). Reserve capacity is further conceptualized in terms of two models: brain and cognitive reserve. Brain reserve refers to structural aspects of the brain (e.g., size, number of neurons, synapses), whereas cognitive reserve involves aspects of complex cognitive processes (efficiency, capacity, or flexibility; Barulli and Stern, 2013). More intelligent or better-educated individuals are thought to possess a greater level of cognitive reserve and during the brain, pathology will manifest lower amounts of cognitive impairment than those with lower amounts of cognitive reserve (lower educated or IQ individuals). Before starting the challenging Earth-based training, future astronauts are carefully chosen from a big pool of candidates, usually holding a high-level degree (M.D. or Ph.D. in astrophysics/engineering) or involved in a job requiring high and fine skills (Air-Force pilots, test pilots, military). Subjects of this profile are probably candidates with a high cognitive reserve that would help them to countermeasure detrimental cognitive performance in space.
An interesting window of observation is also offered by studies involving highly specialized professional figures, where a “ceiling effect” in terms of behavioral improvement after any intervention is expected. These individuals have reached their capacity via standardized training, making it hard to enhance their abilities in general. However, targeted, personalized CNS changes induced by NiBS can offer an advantage over other enhancement interventions (e.g. drugs, exercise, diet, supplements), allowing to accelerate learning or boost performance in individuals otherwise performing at their physiological peak.
Even though cognitive impairment may not always be tested and visible among crew members, astronaut’s performance should be constantly monitored and possibly enhanced to allow better outcomes of space missions. During space missions, astronauts are daily required to keep sustained attention, be able to perform in a hostile environment, under stressful, and time pressing situations. Other than attentional ability and working memory, human multitasking capability is a crucial key interest. Spaceflights, and even normal standard situations on ISS, require a human operator to monitor and respond to multiple events simultaneously over a long period, risking a decline in performance as a result of information overload (Cheshire, 2015). Perceptual problems are also related to microgravity environment characteristics that make astronauts see objects in non-customary orientations. The proper perception of objects might be therefore negatively affected. One well-known example is the impairment in the perception of faces during spaceflights (Kanas and Manzey, 2008). Among the thousands of potentially threatening situations that astronauts and cosmonauts face, some of them require more than just apply standard protocols but also find new and creative solutions, such as the example of the famous fix during the mission Apollo 13 (King, 1997).
Different protocols of tES proved to be able to entrain and modulate cognition in various domains (for a comprehensive review see Kuo and Nitsche, 2012). We review NiBS applications in cognitive domains, selecting pivotal studies from the multitude conducted during the last years.
5.3.1). Attention and Vigilance.
Bolognini and coworkers (2010) coupled a 30 min multisensory visual field exploration training with anodal-excitatory tDCS over the posterior parietal cortex (PPC) with right and left hemisphere stimulated in different experiments. They tested the performance in visual exploration speed, visual scanning, and visuospatial orienting. tDCS applied to the right PPC increased training-induced behavioral improvement of visual exploration. Results also proved that tDCS applied on the right parietal lobe can enhance visual search even when not associated with training (Bolognini et al., 2010).
Using α and γ tACS during two spatial cueing tasks, Hopfinger and Parsons (2016) investigated modulation on endogenous and exogenous attention. Because γ tACS (40Hz) significantly facilitated endogenous attention with no difference in exogenous one, authors suggested that γ waves play a dominant role in attentional disengagement and reorientation, further proving a way to enhance it.
In a study supported by the U.S. Air Force, tDCS improved vigilance (sustained attention) and target detection of pilots. Twenty‐seven Air Force pilots received 90 minutes of preparatory training for “synthetic aperture radar target learning task” during a tDCS session (2 mA for 30 minutes, the anode in area F10- forehead side) or sham. The task consists of a radar simulator with circular patterns in which participants had to identify target stimuli (for example, vehicles or missiles), which could appear in different points of space. Anodal tDCS during training produced improvement in the accuracy of visual search during the task by about 25% compared to the sham group (McKinley et al., 2013b). This application might specifically be valuable for Earth-based mission control operations.
5.3.2). Multitasking and Working Memory.
tDCS application on working memory (WM) has been proved in different studies, with a focus mostly on the dorsolateral prefrontal cortex (DLPFC) stimulation. Fregni and colleagues (2005) administered anodal, sham, or cathodal tDCS during a sequential-letter working memory task (3-back letter task). Results indicated that anodal stimulation over left DLPFC was able to increase accuracy by enhancing working memory capacity. Zaehle and coworkers (2011) described similar positive effects of anodal tDCS on response accuracy in a 2-back WM task, also increasing α and θ power, while cathodal tDCS impaired performance triggering a drop of these frequencies. A similar paradigm has been applied to study the time-dependent effect of stimulation. Ohn and coworkers (2008) administered 20 min of 1mA anodal tDCS during the 3-back verbal task. Beneficial effects of anodal tDCS on performance accuracy were not only evident during online stimulation but also maintained up to 30 minutes after the end of the session (Ohn et al., 2008b). Similarly, Hoy and colleagues (2013) found that anodal tDCS applied to left DLFPC generated significant improvements in accuracy during a 2-back test. In fact, in most studies on tDCS and WM, the anode was placed over left DLPFC rather than the right (for a meta-analysis, see Brunoni and Vanderhasselt, 2014). The same research group that showed tDCS ability to improve spatial recognition accuracy in Air Force pilots, applied anodal tDCS over DLPFC in 20 Wright-Patterson Air Force participants to enhance multitasking capability (Nelson et al., 2016). The stimulation significantly improved information processing capability resulting in improved performance compared to placebo protocol.
Although tDCS ability in modulating behavioral performance seems to be widely replicated, a 2015 review of tDCS studies found no statistical evidence to support strong changes in cognitive performance after a single session tDCS in healthy subjects (Horvath et al., 2015a). A second study also found a small statistically reliable impact of tDCS on various neurophysiological outcomes (Horvath et al., 2015b). Ambiguous outcomes in the case of tDCS sessions on cognitive enhancement may be explained by individual differences, considering how the effect induced by external stimulating such as low current stimulation is highly influenced by the state of the brain (Hsu et al., 2016). Moreover, the reviews explore cognitive measures collected during or following one single tDCS session. Many studies investigated multi-day stimulation paradigms (Antonenko et al., 2019; Talsma et al., 2016) reporting successful effects on cognition. In this context, future studies should consider, overnight consolidation as a crucial component supporting NiBS effects (Marshall et al., 2006).
On the other hand, tACS has been proven effective in inducing a frequency-specific effect on short-term memory capacity (Feurra et al., 2016). Oscillatory stimulation in θ (5Hz), α (10Hz), β (20Hz) and γ (40Hz) range were delivered during a digit span task over the left posterior parietal cortex, a cortical region thought to sustain maintenance processes in short-term memory through oscillatory waves in β range. As expected, Feurra and coworkers found the β-tACS robustly increased the forward memory span (Feurra et al., 2016).
5.3.3). Problem Solving, Insight, Fluid Intelligence, and Creativity.
Dockery and coworkers (2009) described a phase-specific effect of tDCS over left DLPFC on performance in the Tower of London task, which involves strategic planning. Cathodal tDCS improved performance during the early acquisition phase of task performance, probably due to its reducing effect on distractive cortical noise, whereas anodal stimulation improved performance in later stages of task performance, presumably via its activity-enhancing effect on task-related neuronal activity (Dockery et al., 2009b).
Santarnecchi and colleagues (2019) administered tACS to modulate insight ability, a particular form of problem-solving consisting of a sudden realization of a solution for the problem presented (Santarnecchi et al., 2019). 10Hz tACS and 40Hz tACS were administered during two insight tasks on parietal (P4) or temporal (T8) area respectively, along with sham stimulation, according to neurophysiological pieces of evidence of α and γ band activity raise during an insight moment (Jung-Beeman et al., 2004). As expected, γ-tACS applied on the right temporal lobe increased accuracy, correlating also with an increase in γ spectral power over bilateral temporal poles.
Fluid intelligence (Gf) represents the capacity to reason and solve novel problems independently from previously acquired knowledge (e.g. logical problems). This higher cognitive domain has also been modulated in a single session tACS intervention. Santarnecchi and colleagues (2013) administered tACS over left middle frontal gyrus on 20 tACS-naive, right-handed, healthy volunteers while performing an extended version of Raven’s matrices, a classic task to assess Gf (Matzen et al., 2010). Experimental paradigm compared the accuracy and timely responses in performance during four tACS conditions: 5 Hz (θ), 10 Hz (α), 20 Hz (β), and 40 Hz (γ), and placebo stimulation. Gamma-band stimulation resulted in a shorter time required to find the right solution during the task. The performance was enhanced only during this particular frequency stimulation and in complex trials involving conditional/logical reasoning (Santarnecchi et al., 2013).
While γ tACS can enhance abstract reasoning, α stimulation over the frontal cortex (10Hz tACS) has been shown to correlate with another high order cognitive function: creativity, as the ability to produce innovative ideas. In a randomized, balanced cross-over design on 20 healthy participants, Lustenberger and coworkers (2015) reported a frequency-specific significant improvement of 7.4% in the Creativity Index during stimulation at 10Hz.
5.4). Sleep
Sleep loss, fatigue, and poor sleep quality, with various consequences, have been reported and replicated on numerous space missions (Barger et al., 2014; Stampi, 1994). The causing factors are related to modifications of classical Earth environmental cues, such as altered light-dark cycles, light exposure and low light intensity, sleep-disrupting noise levels in the spacecraft during rest which produces disturbances on the circadian rhythm and consequent psycho-physiological effects (Dijk et al., 2001). Particularly, noise levels 4–24dB higher than normal limits have been registered on ISS, which sometimes calls for extra measures in the form of anti-noise earplugs and hearing protection devices. Although in some studies sleep patterns seem to differ minimally from Earth-based sleep (Dijk et al., 2001; Pavy-Le Traon and Taillard, 2010), a reduction of total sleep to 5–6h sleep per night has been registered along with a clear alteration of circadian sleep (Dijk et al., 2001; Frost et al., 1976). Monk and colleagues (1998) analyzed the sleep of four astronauts finding no alterations of REM sleep, but a general decrease of slow-wave activity indicating how sleep was shallower in space compared to Earth. Other studies found a considerable increase in REM sleep total time and a reduction of REM latency during the first week after return to Earth (Dijk et al., 2001; Frost et al., 1976). On long-duration missions, major sleep disruptions problems related to this may appear and compromise performance levels, leading to more mistakes, depression, anxiety, and higher cortisol levels (Buckey, 2006). A reduction in melatonin has also been reported in microgravity (Holley et al., 1991). Although the use of drugs is usually not indicated due to their side and sedative effects, some sleep medications have been used in long-duration missions upon the approval of the medical team.
5.4.1). Sleep Quality and Sleepiness.
Subjective sleep quality is positively correlated with time spent in slow-wave sleep (SWS) itself (Akerstedt et al., 2009). Therefore, the researcher investigated how to increase sleep quality targeting SWS through tACS. During the experiment run by Ketz and colleagues (2018) with the purpose of memory consolidation, the team applied closed-loop tACS matching SWS in NREM stages, while collecting different data on sleep quality such as sleep efficiency, time spent in NREM stages, SWS, and REM sleep time. The authors underlined a significant raise in SWS, clear signs of improved sleep quality (Robinson et al., 2018). This result is not only relevant for healthy participants but also subjects with more severe sleep disturbances. Saebipour and coworkers (2015) applied 5-min blocks of 0.75Hz otDCS during SWS in a cohort of patients affected by primary insomnia, reaching a sleep deepening effect (i.e. an increase of NREM3), a decrease of time spent in NREM1 and a drop of wake time after sleep onset (WASO). To date, only one study applied tES with the precise goal to promote sleepiness in awake participants, hopefully, to induce sleep in the future. The authors applied a particular protocol of otDCS at 5 Hz in the frontal area, resulting in an effective change of both subjective sleepiness and spontaneous low-frequency EEG activity (Atri et al., 2016).
5.4.2). Sleep Deprivation and Alertness.
tDCS effects on vigilance have been observed in sleep-deprived research participants. Using the same tDCS paradigm McKinley and colleagues used to enhance vigilance and multitasking capability, McIntire and coworkers (2014) demonstrated that tDCS mitigated vigilance decrement due to sleep deprivation for at least 6 hours, 3 times longer than the effect of caffeine. Additionally, both tDCS and caffeine led to improvements in reaction time and fewer lapses on a simple reaction time task, both of which are highly sensitive to fatigue. Subjective reports revealed that participants receiving tDCS experienced also less fatigue and/or drowsiness and more energy following stimulation as compared to subjects who received sham tDCS. The same research group examined the effects when tDCS was applied 10 hours earlier in the sleep deprivation period (McIntire et al., 2017). The study confirmed that tDCS prevents declines in vigilance performance for approximately 6 hours post-stimulation. Moreover, the effects on arousal and mood were found to persist much longer, at least 24 hours post-tDCS. These findings suggest that it may be possible to administer tDCS before the start of the shift on ISS or on Earth-based mission control to provide performance benefits that last until the end of the workday.
5.5). Psychological States
Stressing situations (such as hypoxia and cosmic radiation) causing dysfunction of the prefrontal area can not only lead to mistakes in cognitive performance and sleep disruptions, but also dramatic modifications in emotional processing. ICE environments, as aforementioned, are usually triggers of stressing reactions. Astronauts are required to adapt effectively to ICE situations they encounter. It is well known that people differ in how quickly they adapt to spaceflight and other extreme conditions, such as analogous situations on Earth (Kanas et al., 2010; Sandal et al., 1995).
Clinical conditions such as a following severe depressive state, even in only one crew member, can interfere with performance and, unfortunately, terminate a space mission, as happened with Salyut 7 in 1985. Anxiety is also a common problem in the dangerous and stressful environment, and it has been detected in crewmembers on Antarctic missions. It is, however, not common in space missions, even though it may appear, as indicated by some MIR missions (Kanas and Manzey, 2008; Linenger et al., 2000). In summary, although reports of depressive and anxious states are often vague or subjective, they are encountered in astronauts (Barger et al., 2014).
As aforementioned, high levels of cortisol due to dangerous and risky situations reduce appetite-regulating hormones. Loss of appetite is common in astronauts, and it has been documented from Project Mercury in 1961–1963 (Carpentier et al., 2018). Cosmic radiations are also responsible for anorexic behavior, as seen in animal models (Fajardo et al., 2001; Hunt et al., 1989; Rabin et al., 2000, 1994, 1991, 1989).
5.5.1). Depressive States and Anxiety.
Various protocols of tES have been suggested and applied to mitigate clinical symptoms of patients affected by different neuropsychiatric disorders, such as depression, schizophrenia, anxiety, autism, and craving, with good results (for a review see (Kuo et al., 2017, 2014; Philip et al., 2017). Depression represents the more treated and investigated psychiatric condition in efficacy studies with tES. The classical tDCS protocol typically involves an anode placed over the left DLPFC. Although initial tDCS studies generated mixed results regarding potential efficacy (Boggio et al., 2012; Fregni et al., 2005; Loo et al., 2010), the latest and more extensive trials showed positive effects. To date, the largest randomized study (n=120) with tDCS administered twelve 30-minute sessions of 2 mA tDCS: 10 consecutive workday sessions followed by a single session delivered every other week comparing the outcome with sertraline in a 2×2 factorial design. Albeit a greater drop in depressive scores was seen in tDCS and sertraline combined therapy group, active tDCS monotherapy was superior not only to sham tDCS but also to sertraline monotherapy (Brunoni et al., 2013). In 2016, a European meta-analysis attributed level B evidence (probable efficacy) of tDCS for depression (Lefaucheur et al., 2017a).
What could be used for a clinical population may also be generalized for healthy subjects, as seen in different studies. In various experimental paradigms focused on investigating other domains, mood improvements have also been found as a collateral outcome. Marshall and coworkers (2004), found also that anodal tDCS on bilateral DLPFC triggered an improvement in mood measured with Eigenschaftswörterliste (EWL), an adjective checklist describing the subject’s mood, while the main aim was to enhance memory consolidation overnight. Similarly, as aforementioned before, McIntire and colleagues (2017) tested healthy participants with a 15-items Likert Scale Mood Questionnaire and the Profile of Mood States (POMS), a 65-item questionnaire that measures mood using 6 categories: tension-anxiety, depression-dejection, anger-hostility, vigor-activity, fatigue-inertia, and confusion-bewilderment while studying the ability of tDCS on DLPFC to countermeasure fatigue due to sleep deprivation similarly to the caffeine effect. Researchers reported a drop in depressive scores. Participants receiving early intervention tDCS also reported feeling more able to do the task and less bored (McIntire et al., 2017). tDCS efficacy to alter mood state in healthy subjects could be explained by its ability to mitigate the negative effects of stressing factors such as fatigue.
5.5.2). Loss of Appetite.
Although tDCS research mostly focused on food cravings (Boggiano et al., 2017; Goldman et al., 2011; Montenegro et al., 2012), this stimulation protocol seems to be a valuable countermeasure also for loss of appetite. Pieces of evidence have been provided that food craving is reduced when excitatory (anodal) tDCS is applied over the right DLPFC as opposite as effect on anorexia nervosa (AN) where excitatory stimulation is applied over left DLPFC. For example, Fregni and coworkers (2008) compared both tDCS protocols, anode right/cathode left and anode left/cathode right, to sham stimulation, and found that food craving was reduced, remained stable and increased in these conditions, respectively. tES has been also proven to reduce AN-related behaviors and thoughts (Lee et al., 2018; McClelland et al., 2013). Finally, tDCS applied bilaterally on DLPFC in a cohort of AN patients was able to significantly increase appetite, resulting in a higher Body Mass Index persisting also 1 month later, compared to the standard Family-Based Intervention (Costanzo et al., 2018).
6). POST-FLIGHT NiBS APPLICATIONS: BIOMARKERS AND SUPPORT
Even after the space mission, astronauts are not yet spared from potential effects on their health, cognitive, and psychological states. If on one hand, a long-duration mission can cause a direct modification of the brain anatomy-physiology, on the other it can also trigger long-term adaptation mechanisms that, upon return to Earth, are no longer beneficial but may be harmful since the abolition of the space-related stressors. NiBS applications can be a powerful tool to investigate space-induced modifications in neurophysiology, particularly regarding the neuroplasticity and cortical excitability field via TMS and tACS assessment before and after the flight.
Astronauts also shift from working for months on spacecraft to readjusting on Earth-based normal life. Cognitive deficit and depressive states are two of the possible consequences of this stressful transition. NiBS, as seen in in-flight applications, may support cognitive and psychological rehabilitation of crewmembers after the end of the mission.
6.1). TMS to Assess Neuroplasticity and Cortical Excitability
Neuroplasticity is pivotal for functional recovery after injury and has been suggested as a potential resource to counteract the effect of microgravity (Ombergen et al., 2017). TMS has been frequently used to investigate cortical plasticity in healthy participants and patients (for a review on TMS in neuroplasticity studies see Bestmann, 2008; Ferreri and Rossini, 2013) as well as investigate corticospinal excitability to hyper- and micro-gravity. In a preliminary investigation, Davey and coworkers (2004) administered TMS over M1 on 3 healthy subjects during 10 parabolas to produce MEP and recording in the deltoid and left and right erector spinae (ES) muscles. Data showed a similar pattern in all participants, revealing the facilitation of MEP responses in left and right ES muscles in periods of 0g. MEPs increase suggested that microgravity produced activation of ES muscles through an increase in corticospinal excitability (Davey et al., 2004).
Concerning ground-based analog, Roberts and colleagues (2010) combined TMS and fMRI to investigate whether simulated gravity through 90 days of HDBR induced changes in corticospinal tract excitability. They found reduced cortical activity in motor areas (specifically the leg cortical representation) and a decrease in corticospinal excitability after HDBR. According to the authors, these reductions in cortical motor function could underlie motor-related difficulties in astronauts (Roberts et al., 2010). Additionally, in the post-HDBR period, they reported an increase in corticospinal excitability that inversely correlated with functional mobility impairment, leading them to assume that TMS could be used as a possible countermeasure against lower extremity dysfunction. The authors suggest TMS to become part of a countermeasure regime for astronauts on long-duration space missions to counteract lower extremity dysfunction, for example before operations on a planetary surface (Roberts et al., 2010).
6.2). Cognitive and Mood Enhancement
A recent study investigated the consequences of prolonged space exposure on two identical twins, one who spent a year on ISS and one on Earth. Cognitive speed decreased for all tests of Cognition except for the digit symbol substitution task, and accuracy decreased for all domains except for spatial orientation post-flight. The decline was significantly greater in siblings who went on space post-flight and persisted up to 6 months after the end of the mission in both speed and accuracy domains (Garrett-Bakelman et al., 2019). Some explanations about this persisted cognitive deficit seem to involve a depressive state due to being back on Earth after spaceflight. Therefore, every in-flight application presented here may be also implemented as a support for a cognitive and affective readjustment of crew members on their return on Earth. Specifically, tES application to mitigate depressive states has been suggested, considering its good outcomes on depressive states, whether alone or in combination with antidepressant drugs (Brunoni et al., 2013b). More important, during a space mission, tES could be the only NiBS technique able to be implemented in-flight, while astronauts on Earth-based post-flight rehabilitation can be successfully treated with TMS. Prefrontal rTMS therapy repeated daily over 4–6 weeks (20–30 sessions) is FDA approved for the treatment of Major Depressive Disorder (MDD) in adults who have not responded to prior antidepressant medications (for a review see Perera et al., 2016). Depression involves a distributed network of cortical and limbic regions including left DLPFC, hippocampus, and subgenual cingulate among others (Pascual-Leone et al., 1996) and TMS studies have focused on left DLPFC as one accessible node of this depression network. The efficacy and safety of TMS using a specific, defined treatment protocol of high-frequency, left prefrontal TMS was confirmed in two large, multisite, randomized controlled trials (George et al., 2010; O’Reardon et al., 2007) and one large, multicentre trial that used a form of more focalized TMS (Deep TMS; Levkovitz et al., 2015). In conclusion, rTMS may be implemented as well with astronauts on an Earth-based treatment in case of post-flight depressive episodes.
6.3). Perturbational-based Biomarkers
Combined neuroimaging/neuromodulation solutions could provide extensive and comprehensive information about cortical functional dynamics modified by longer space missions. TMS and tES combined with electrophysiology or neuroimaging techniques allow to determine (i) where exactly to stimulate, by adapting the standard target regions onto individual subject anatomy (e.g. using anatomical MRI) or to individual functional activity (e.g. functional MRI), (ii) when delivering stimulation (by using EEG data that display greater temporal resolution respect to neuroimaging acquisition), and (iii) how to set the stimulation parameters (i.e., stimulation intensity). Additionally, neuroimaging and neurophysiology represent useful readouts of changes induced by NiBS, both during stimulation as well as after the intervention (or at follow-up sessions) Santarnecchi et al., 2018). In general, neuroimaging data are usually considered to optimize the targeting of specific regions, with the possibility of using anatomical scans (i.e. T1- or T2-weighted images), functional data (e.g. fMRI or perfusion MRI data), and metabolic information (Positron Emission Tomography – PET or Magnetic Resonance Spectroscopy), depending on the specific aim of the intervention. Also, neuroimaging has been pivotal in revealing some crucial effects of NiBS, for example, TMS effects on neurotransmitters via ligand-PET (Strafella et al., 2001), and the modifications of neuronal activity in distant sites respect to the stimulated target (Bestmann et al., 2005).
One of the most promising multimodal techniques is concurrent TMS and EEG recording (TMS-EEG). When a single TMS pulse is applied over a cortical region, the transmission of generated activity can be discerned by space-temporal analysis of TMS-evoked potentials (TEPs; Ilmoniemi et al., 1997). TMS-EEG is particularly interesting to study causal communication between brain connections with a high temporal resolution, providing insights into mechanisms of effective connectivity (Friston et al., 1993; Massimini et al., 2005). TMS-EEG can provide information about cortical inhibition and excitation, plasticity, and connectivity in clinical and healthy populations (for a comprehensive review see Tremblay et al., 2019).
Similarly, tES and EEG protocols can be combined to enhance experimental inference(Boyle et al., 2013; Roh et al., 2014; tES-EEG). EEG is used to probe the state of the cortical area affected by tES, which could be the stimulation site or an interconnected region, and to evaluate connectivity and excitability modifications within a functional network (Miniussi et al., 2012). EEG recording allows the study of Event-Related Potential (ERPs) or EEG deflections time-locked to specific external or internal signals (Buzsáki, 2009). Thus, amplitude and latency of the ERPs (generated in response to somatosensory, visual, or auditory stimuli) may provide information regarding activity modulation induced by tES in specific cortical regions (Miniussi et al., 2012). Furthermore, t combined tACS-EEG can be used to evaluate specific changes in brain oscillations in a specific area or the entire brain.
More specifically, in the case of deep space flights, astronauts change ICP and fluid shift that might affect brain function and induce SANS. Increased ICP can also trigger changes in topology and organization of brain networks, such as changes in modularity or resilience, which can be investigated by TMS-EEG (Massimini et al., 2012; Ozdemir et al., 2020). Similarly, it is relevant to test changes in brain efficiency as measured by the propagation of electrical activity injected by TMS. TACS-EEG, on the other hand, may help in studying long-distance synchronization and modifications in oscillatory activity, while offering the crucial element of portability.
7). MONITORING AND MODULATING FUNCTIONAL CONNECTIVITY
As mentioned earlier, Demertzi and colleagues showed significant differences in post-flight FC between the motor cortex and cerebellum, as well as changes within the Default Mode Network (DMN) on a single cosmonaut (Demertzi et al., 2016, see paragraph 2.1). Most importantly, FC changes were still present 9 days after landing on Earth. This may pose a new potential threat for Mars missions, where MCC assistance could not be provided.
DMN alterations in- and post-flight may be triggered by various factors. Firstly, DMN may play a role in initiating the adaptation to microgravity occurring during long-duration spaceflights. Otsuka and coworkers suggested that exposure to magnetic variation in space, via the retina, or the brain directly, may activate the dynamics of large-scale brain networks, and this process would be initiated by DMN (Otsuka et al., 2019). DMN alterations may also be enabled by frequent sleep disruptions, typical on space missions (Barger et al., 2014; Dijk et al., 2001; Stampi, 1994). On Earth-based studies in healthy participants, sleep alterations have generally been associated with alterations in FC of the DMN and VAN (De Havas et al., 2012; Scullin, 2017). Even lack of one single nighttime sleep has been associated with a deficit in the FC within the DMN the following morning (Kaufmann et al., 2016). Interestingly, Santarnecchi and colleagues (2018) reported how patients with chronic insomnia present a weaker connection between DMN and the supplemental motor area. Furthermore, the authors found that earlier age of insomnia onset positively correlated with FC within DMN (Santarnecchi et al., 2018). Addressing insomnia-related symptoms and sleep alterations may prevent long-lasting DMN connectivity reshaping. Importantly, DMN modifications may also be correlated with depressive symptoms. Among other RNSs alterations, abnormally increased DMN connectivity has been widely reported in depression (Anand et al., 2005; Greicius et al., 2007; Kaiser et al., 2015; Liston et al., 2014; Sheline et al., 2010).
DMN plays a pivotal role in memory consolidation, mental imagery, internal dialogue and maintenance of long-term memory (Buckner et al., 2008; Uddin et al., 2009), and has been promoted as a marker of healthy cognitive functioning and healthy aging (Santarnecchi et al., 2017; Spreng et al., 2016). Thus, its alterations may enable cognitive difficulties and depression vulnerability. Moreover, recent studies investigated the brain correlates of coping strategies, a combination of mental and behavioral abilities aimed to minimize stress due to dangerous, conflictual, or anxious situations. Santarnecchi and coworkers (2018) analyzed resting-state fMRI data from 102 healthy adults and their psychometric scores of coping abilities. Specific cortical networks, such as DMN and AS, were found to be involved in the propensity to adopt different coping styles in stressful situations (Santarnecchi et al., 2018b). Although there are no data or trial specifically targeting coping styles, the possibility to increase coping abilities by stimulating nodes of DMN with NiBS may be promising.
8). PRACTICAL SUGGESTIONS: TECHNICAL CHALLENGES AND CAVEATS
Although NiBS techniques showed high safety and feasibility in healthy and clinical populations (Antal et al., 2017; Rossi et al., 2009), their applications in space missions require some considerations. In the last years, there has been a growing interest in offering TMS treatment outside the experimental environment, and in the absence of trained operators. A recent study of an individually-tailored TMS helmet applied over M1 suggested it as a feasible and reliable alternative to traditional laboratory settings (Badran et al., 2020). This opens the opportunity to administer TMS in non-laboratory settings with variable gravity and minimal training.
Furthermore, space environments, such as ISS, spacecraft, Moon, and Mars call for more innovative solutions than earth-based non-laboratory settings. They differ from Earth for many features, such as temperature and pressure (NASA IG-18-021). These could potentially harm stimulation devices. In-flight NiBS applications, specifically, should carefully be tested. Below we address the feasibility of NiBS techniques as well as characteristics of new environments that will need to be evaluated to meet the technological requirements of TMS/tES devices. We also discuss how stimulating the brain through electrical currents in astronauts may open a new challenge, due to cerebrospinal fluid shift in microgravity conditions.
8.1). Feasibility of NiBS
Even though TMS application may seem impractical on ISS or spaceflights due to weight and interference in the magnetic field, it may still be a useful tool to investigate differences in cortical excitability before and after missions (i.e. perturbation-based biomarkers), or during experimental paradigms in space analog environments. As aforementioned, a protocol of test-retest paradigm on astronauts upon returning from a mission or after a simulation could be easily implemented as part of routine health screenings and follow-up visits performed to investigate possible response changes to external perturbation due to microgravity/cosmic radiation. tES, on the other hand, is a more practical technique during actual spaceflights. A tES session is usually not longer than 30 minutes and since the stimulation is noninvasive and it does not trigger any sensations, the subject can move, talk, and perform tasks.
8.2). Environmental Challenges
The environment can be categorized into three timeframes:
On Planetary Surface.
On Earth, NiBS technology is designed for applications in clinical settings via a trained specialist with a medical background and unlimited access to technical support. On other planets, like Mars or the Moon, the environment is not as controlled. Assuming the hardware would not be damaged during the spaceflight, the equipment will face extreme temperature swings and possible radiation. Furthermore, the device has to be used by crew members with limited training. The interaction with ground control would be very limited and in the case of Mars with at least 20 min delay. Therefore, testing both hardware and software of the existing technologies for this environment is crucial (Seyedmadani, 2019).
During launch or recovery.
Due to the limitation of volume and cost associated with the mass, small profile, and lightweight devices are highly desired. Payloads usually launch on cargo systems that experience a variety of profiles of gravity, temperature changes, and extreme vibration. Therefore, the chosen technology for each mission must tolerate such impacts (Duncan, 2007).
On-Board of Spacecraft.
On-board of spaceflight, the environment is under control, meaning the temperature, humidity, and oxygen level are carefully defined. Temperature and humidity conditions required to administer tES need to match with those experienced-on board. For example, atmospheric pressure is maintained on ISS thanks to a module called ECLSS (Environmental Control and Life Support System) at 101.3 kPa (NASA/TP-2015–218570), right above the limit of the influence of the device (700–1.000hPa). The effect of weightlessness also impacts hardware performance, including lack of heat rejection. Besides, radiation consequences should be further investigated.
8.3). Technological requirements
Beyond environmental factors and launch limitations, any existent technology resource on board of the craft should be addressed and carefully selected for the mission. NASA Standard SSP 30423 and MIL-STD 810-G are the guidelines followed by NASA Research Operation and Integration Group for use on the board of ISS of off-the-shelf hardware. Factors to be addressed include material compatibility in a closed system, thermal exchange, limited or no resupply possibility, no lithium battery allowed to reduce the chance of combustion, the necessity to control electromagnetic noise generated (EMI), radiation impact, toxicology, and training time.
Albeit the tES device is feasible and simple to use, some of the above standards need to be addressed. A theoretically simple concern could also be the supply of gel electrode and EMI limitation during the sessions. On the other side, the risk of increase or decrease current during the protocol due to solar particle events represents the main concern for the tES application. Going further, tES software needs to be checked with load capacity and technology on board of the craft. EEG data also requires space and time to be downloaded. Finally, crew training is pivotal to manage protocols and to modify protocols and parameters through the interface. Interaction between software and hardware over WiFi or Bluetooth goes back to craft capabilities. Unfortunately, radiation and particle events could directly affect the electrical board of the system and cause a false positive or negative. Considering NiBS applications and possible outcomes of such an event, a full radiation test has to be performed on the device.
8.4). Limitation on Analog and Simulators
Beyond technological and environmental factors of spacecraft, performance tES feasibility in deep space and extreme conditions should be addressed. A major shift in CSF during spaceflights, as proved by the aforementioned SANS, could interfere with electrical current administration. Modifications in pressure and CSF distribution would require different biophysical modeling because they could create an uneven flow of electrical current, triggering different activation of neural networks and brain areas compared to normal gravity conditions. For this purpose, parametric models of anatomy can be created and techniques such as electrical impedance tomography (EIT, Gonsalvez et al., 2016) can be applied to estimate tissue conductivities. Therefore, developing simulators able to mimic neurophysiological changes due to space mission is a key for tES feasibility.
9). CONCLUSIONS
NiBS techniques have been shown to enhance performance in different domains, many of which could be relevant for astronauts and flight controllers. Earth-based specific NiBS protocols could be used to accelerate/consolidate training before missions, as well as identify post-flight biomarkers of brain changes/adaptation and guide rehabilitation. In-flight NiBS could also be implemented to enhance crew performance and psychological well-being, but further validation of mission-specific protocols is needed.
Supplementary Material
Acknowledgments
Dr. Santarnecchi is partially supported by the Office of the Director of National Intelligence (ODNI), Intelligence Advanced Research Projects Activity (IARPA), via 2014-13121700007. The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies or endorsements, either expressed or implied, of the ODNI, IARPA, or the U.S. Government. Dr. Santarnecchi is supported by the Beth Israel Deaconess Medical Center (BIDMC) via the Chief Academic Officer (CAO) Award 2017, the Defence Advanced Research Projects Agency (DARPA) via HR001117S0030, and the NIH (P01 AG031720-06A1, R01 MH117063-01, R01 AG060981-01). The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of Harvard University and its affiliated academic health care centers, or the National Institutes of Health. Giulio Ruffini is partially supported by the Fet Open Luminous project (European Union’s Horizon 2020 research and innovation program under grant agreement No 686764). Giulio Ruffini is a shareholder and works for Neuroelectrics, a company developing medical devices for non-invasive brain stimulation. Simone Rossi is consultant for Neurocare Group Italy. Sara Romanella, Giulia Sprugnoli, and Kimia Seyedmadani have no conflict of interest nor commercial association
LIST OF ABBREVIATIONS
- AAT
Arm ability training
- AD
Alzheimer’s disease
- APP
Amyloid precursor protein
- AS
Anterior salience network
- BDNF
Brain-derived neurotrophic factor
- CNS
Central nervous system
- CR
Cosmic Radiation
- CSF
Cerebrospinal fluid
- DLPFC
Dorsolateral prefrontal cortex
- DMN
Default mode network
- ECLSS
Environmental Control and Life Support System
- EEG
Electroencephalography
- EIT
Electrical impedance tomography
- EMI
Electromagnetic noise generated
- ERD
Event-related desynchronization
- ERPs
Event-Related Potential
- ES
Erector spinae
- EVA
Extravehicular activity
- FC
Functional connectivity
- FDA
Food and Drug Administration
- fEPSP
Field excitatory postsynaptic potentials
- fMRI
Functional magnetic resonance imaging
- FUS
Focused ultrasound stimulation
- GCR
Galactic Cosmic Radiation
- HDBR
head-down bed rest
- HRP
Human Research Program
- ICE
Isolated, Confined, and Extreme
- ICF
Intra-cortical facilitation
- IFCN
International Federation of Clinical Neurophysiology
- ISI
inter-stimulus interval
- ISS
International Space Station
- LEO
Low Earth orbit
- LORETA
Low-resolution brain electromagnetic tomography
- LTP
Long-term potentiation
- LTD
Long-term depression
- M1
Motor cortex
- MCC
Mission Control Center
- MDD
Major Depressive Disorder
- MEPs
Motor evoked potentials
- MRI
Magnetic resonance imaging
- NBS
Navigated brain stimulation
- NiBS
Noninvasive brain stimulation
- NMDA
N-methyl-D-aspartate
- otDCS
Oscillatory transcranial direct current stimulation
- PBM
Photobiomodulation
- PET
Positron emission tomography
- PPC
Posterior parietal cortex
- ppTMS
paired-pulse TMS
- RMT
Resting motor threshold
- rTMS
Repetitive TMS
- SANS
Spaceflight Associated Neuro-ocular Syndrome
- SM
Sensorimotor network
- SMA
Supplementary motor area
- SMS
Space Motion Sickness
- spTMS
Single-pulse TMS
- SSRMS
Space Station Remote Manipulator System
- SWA
Slow-wave activity
- SWS
Slow-wave sleep
- tACS
Transcranial alternating current stimulation
- TBS
Theta-burst stimulation
- tDCS
Transcranial direct current stimulation
- tES
Transcranial electrical stimulation
- TMS
Transcranial magnetic stimulation
- tRNS
Transcranial random noise stimulation
- VAN
Ventral attention network
- VAT
Visuomotor adaptation task
- VIIP
Vision Impairment and Intracranial Pressure
- WM
Working memory
Footnotes
Financial disclosures: All authors report no conflict of interest.
REFERENCES
- Abdelmoula A, Baudry S, Duchateau J, 2016. Anodal transcranial direct current stimulation enhances time to task failure of a submaximal contraction of elbow flexors without changing corticospinal excitability. Neuroscience 322, 94–103. 10.1016/j.neuroscience.2016.02.025 [DOI] [PubMed] [Google Scholar]
- Acharya MM, Baulch JE, Klein PM, Baddour AAD, Apodaca LA, Kramár EA, Alikhani L, Garcia C, Angulo MC, Batra RS, Fallgren CM, Borak TB, Stark CEL, Wood MA, Britten RA, Soltesz I, Limoli CL, 2019a. New Concerns for Neurocognitive Function during Deep Space Exposures to Chronic, Low Dose-Rate, Neutron Radiation. eNeuro 6. 10.1523/ENEURO.0094-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostino R, Iezzi E, Dinapoli L, Suppa A, Conte A, Berardelli A, 2008. Effects of intermittent theta-burst stimulation on practice-related changes in fast finger movements in healthy subjects. European Journal of Neuroscience 28, 822–828. 10.1111/j.1460-9568.2008.06373.x [DOI] [PubMed] [Google Scholar]
- Akerstedt T, Kecklund G, Ingre M, Lekander M, Axelsson J, 2009. Sleep homeostasis during repeated sleep restriction and recovery: support from EEG dynamics. Sleep February32, 217–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrus GG, Pisoni A, Primaßin A, Turi Z, Paulus W, Antal A, 2015. Bi-frontal transcranial alternating current stimulation in the ripple range reduced overnight forgetting. Frontiers in Cellular Neuroscience 9. 10.3389/fncel.2015.00374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, Mathews VP, Kalnin A, Lowe MJ, 2005. Activity and Connectivity of Brain Mood Regulating Circuit in Depression: A Functional Magnetic Resonance Study. Biological Psychiatry 57, 1079–1088. 10.1016/j.biopsych.2005.02.021 [DOI] [PubMed] [Google Scholar]
- Andrews SC, Hoy KE, Enticott PG, Daskalakis ZJ, Fitzgerald PB, 2011. Improving working memory: the effect of combining cognitive activity and anodal transcranial direct current stimulation to the left dorsolateral prefrontal cortex. Brain Stimul 4, 84–89. 10.1016/j.brs.2010.06.004 [DOI] [PubMed] [Google Scholar]
- Ang KK, Guan C, Phua KS, Wang C, Zhao L, Teo WP, Chen C, Ng YS, Chew E, 2015. Facilitating effects of transcranial direct current stimulation on motor imagery brain-computer interface with robotic feedback for stroke rehabilitation. Arch Phys Med Rehabil 96, S79–87. 10.1016/j.apmr.2014.08.008 [DOI] [PubMed] [Google Scholar]
- Angius L, Hopker J, Mauger AR, 2017. The Ergogenic Effects of Transcranial Direct Current Stimulation on Exercise Performance. Front Physiol 8, 90. 10.3389/fphys.2017.00090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angius L, Pascual-Leone A, Santarnecchi E, 2018. Brain stimulation and physical performance, in: Progress in Brain Research. Elsevier, pp. 317–339. 10.1016/bs.pbr.2018.07.010 [DOI] [PubMed] [Google Scholar]
- Antal A, Alekseichuk I, Bikson M, Brockmöller J, Brunoni AR, Chen R, Cohen LG, Dowthwaite G, Ellrich J, Flöel A, Fregni F, George MS, Hamilton R, Haueisen J, Herrmann CS, Hummel FC, Lefaucheur JP, Liebetanz D, Loo CK, McCaig CD, Miniussi C, Miranda PC, Moliadze V, Nitsche MA, Nowak R, Padberg F, Pascual-Leone A, Poppendieck W, Priori A, Rossi S, Rossini PM, Rothwell J, Rueger MA, Ruffini G, Schellhorn K, Siebner HR, Ugawa Y, Wexler A, Ziemann U, Hallett M, Paulus W, 2017. Low intensity transcranial electric stimulation: Safety, ethical, legal regulatory and application guidelines. Clin Neurophysiol 128, 1774–1809. 10.1016/j.clinph.2017.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal A, Kincses TZ, Nitsche MA, Bartfai O, Paulus W, 2004. Excitability changes induced in the human primary visual cortex by transcranial direct current stimulation: direct electrophysiological evidence. Invest. Ophthalmol. Vis. Sci 45, 702–707. [DOI] [PubMed] [Google Scholar]
- Antonenko D, Thams F, Uhrich J, Dix A, Thurm F, Li S-C, Grittner U, Flöel A, 2019. Effects of a Multi-Session Cognitive Training Combined With Brain Stimulation (TrainStim-Cog) on Age-Associated Cognitive Decline – Study Protocol for a Randomized Controlled Phase IIb (Monocenter) Trial. Front. Aging Neurosci 11. 10.3389/fnagi.2019.00200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atri A, Simoni E, Gorgoni M, Ferrara M, Ferlazzo F, Rossini P, Gennaro L, 2016. D’ De DeElectrical stimulation of the frontal cortex enhances slow-frequency EEG activity and sleepiness. Neuroscience June2324SRC-BaiduScholar, 119–30. [DOI] [PubMed] [Google Scholar]
- Ayaz H, Shewokis PA, Bunce S, Izzetoglu K, Willems B, Onaral B, 2012. Optical brain monitoring for operator training and mental workload assessment. Neuroimage 59, 36–47. 10.1016/j.neuroimage.2011.06.023 [DOI] [PubMed] [Google Scholar]
- Badran BW, Caulfield KA, Lopez JW, Cox C, Stomberg-Firestein S, DeVries WH, McTeague LM, George MS, Roberts D, 2020. Personalized TMS helmets for quick and reliable TMS administration outside of a laboratory setting. Brain Stimulation: Basic, Translational, and Clinical Research in Neuromodulation 13, 551–553. 10.1016/j.brs.2020.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger LK, Flynn-Evans EE, Kubey A, Walsh L, Ronda JM, Wang W, Wright KP, Czeisler CA, 2014. Prevalence of Sleep Deficiency and Hypnotic Use Among Astronauts Before, During and After Spaceflight: An Observational Study. Lancet Neurol 13, 904–912. 10.1016/S1474-4422(14)70122-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett DW, Gonzalez-Lima F, 2013. Transcranial infrared laser stimulation produces beneficial cognitive and emotional effects in humans. Neuroscience 230, 13–23. 10.1016/j.neuroscience.2012.11.016 [DOI] [PubMed] [Google Scholar]
- Barulli D, Stern Y, 2013. Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends Cogn. Sci. (Regul. Ed.) 17, 502–509. 10.1016/j.tics.2013.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basner M, Savitt A, Moore TM, Port AM, McGuire S, Ecker AJ, Nasrini J, Mollicone DJ, Mott CM, McCann T, Dinges DF, Gur RC, 2015. Development and Validation of the Cognition Test Battery for Spaceflight. Aerosp Med Hum Perform 86, 942–952. 10.3357/AMHP.4343.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassolino M, Franza M, Bello Ruiz J, Pinardi M, Schmidlin T, Stephan MA, Solcà M, Serino A, Blanke O, 2018. Non-invasive brain stimulation of motor cortex induces embodiment when integrated with virtual reality feedback. Eur. J. Neurosci 47, 790–799. 10.1111/ejn.13871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastani A, Jaberzadeh S, 2013. Differential modulation of corticospinal excitability by different current densities of anodal transcranial direct current stimulation. PLoS ONE 8, e72254. 10.1371/journal.pone.0072254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batsikadze G, Moliadze V, Paulus W, Kuo M-F, Nitsche MA, 2013. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J Physiol 591, 1987–2000. 10.1113/jphysiol.2012.249730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman MH, Halper JP, Nichols TW, Jarrett H, Lundy A, Huang JH, 2017. Photobiomodulation with Near Infrared Light Helmet in a Pilot, Placebo Controlled Clinical Trial in Dementia Patients Testing Memory and Cognition. J Neurol Neurosci 8. 10.21767/2171-6625.1000176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestmann S, 2008. The physiological basis of transcranial magnetic stimulation. Trends Cogn. Sci. (Regul. Ed.) 12, 81–83. 10.1016/j.tics.2007.12.002 [DOI] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J, 2005. BOLD MRI responses to repetitive TMS over human dorsal premotor cortex. Neuroimage 28, 22–29. 10.1016/j.neuroimage.2005.05.027 [DOI] [PubMed] [Google Scholar]
- Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, Mourdoukoutas AP, Kronberg G, Truong D, Boggio P, Brunoni AR, Charvet L, Fregni F, Fritsch B, Gillick B, Hamilton RH, Hampstead BM, Jankord R, Kirton A, Knotkova H, Liebetanz D, Liu A, Loo C, Nitsche MA, Reis J, Richardson JD, Rotenberg A, Turkeltaub PE, Woods AJ, 2016. Safety of Transcranial Direct Current Stimulation: Evidence Based Update 2016. Brain Stimul 9, 641–661. 10.1016/j.brs.2016.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M, Inoue M, Akiyama H, Deans JK, Fox JE, Miyakawa H, Jefferys JGR, 2004. Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro. J. Physiol. (Lond.) 557, 175–190. 10.1113/jphysiol.2003.055772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore J, Shrivastava S, Sallet J, Butler CR, Cleveland RO, 2019. Ultrasound Neuromodulation: A Review of Results, Mechanisms and Safety. Ultrasound in Medicine & Biology 45, 1509–1536. 10.1016/j.ultrasmedbio.2018.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco NJ, Maddox WT, Gonzalez-Lima F, 2017a. Improving executive function using transcranial infrared laser stimulation. J Neuropsychol 11, 14–25. 10.1111/jnp.12074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco NJ, Saucedo CL, Gonzalez-Lima F, 2017b. Transcranial infrared laser stimulation improves rule-based, but not information-integration, category learning in humans. Neurobiol Learn Mem 139, 69–75. 10.1016/j.nlm.2016.12.016 [DOI] [PubMed] [Google Scholar]
- Boggiano MM, Wenger LE, Burgess EE, Tatum MM, Sylvester MD, Morgan PR, Morse KE, 2017. Eating tasty foods to cope, enhance reward, socialize or conform: What other psychological characteristics describe each of these motives? J Health Psychol 22, 280–289. 10.1177/1359105315600240 [DOI] [PubMed] [Google Scholar]
- Boggio P, Ferrucci R, Mameli F, Martins D, Martins O, Vergari M, Tadini L, Scarpini E, Fregni F, Priori A, 2012a. Prolonged visual memory enhancement after direct current stimulation in Alzheimer’s disease. Brain Stimul Jul 5, 223–230. [DOI] [PubMed] [Google Scholar]
- Boggio P, Ferrucci R, Mameli F, Martins D, Martins O, Vergari M, Tadini L, Scarpini E, Fregni F, Priori A, 2012b. Prolonged visual memory enhancement after direct current stimulation in Alzheimer’s disease. Brain Stimul Jul 5, 223–230. [DOI] [PubMed] [Google Scholar]
- Boggio P, Khoury L, Martins D, Martins O, de Macedo E, Fregni F, J, 2009. Temporal cortex direct current stimulation enhances performance on a visual recognition memory task in Alzheimer disease. Neurosurg Psychiatry Apr 80, 444–7. [DOI] [PubMed] [Google Scholar]
- Bolognini N, Fregni F, Casati C, Olgiati E, Vallar G, 2010. Brain polarization of parietal cortex augments training-induced improvement of visual exploratory and attentional skills. Brain Res. 1349, 76–89. 10.1016/j.brainres.2010.06.053 [DOI] [PubMed] [Google Scholar]
- Brem A-K, Almquist JN-F, Mansfield K, Plessow F, Sella F, Santarnecchi E, Orhan U, McKanna J, Pavel M, Mathan S, Yeung N, Pascual-Leone A, Kadosh RC, Honeywell SHARP Team authors, 2018. Modulating fluid intelligence performance through combined cognitive training and brain stimulation. Neuropsychologia 118, 107–114. 10.1016/j.neuropsychologia.2018.04.008 [DOI] [PubMed] [Google Scholar]
- Britten RA, Davis LK, Jewell JS, Miller VD, Hadley MM, Sanford LD, Machida M, Lonart G, 2014. Exposure to Mission Relevant Doses of 1 GeV/Nucleon 56Fe Particles Leads to Impairment of Attentional Set-Shifting Performance in Socially Mature Rats. Radiat Res 182, 292–298. 10.1667/RR3766.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten RA, Davis LK, Johnson AM, Keeney S, Siegel A, Sanford LD, Singletary SJ, Lonart G, 2012. Low (20 cGy) doses of 1 GeV/u (56)Fe--particle radiation lead to a persistent reduction in the spatial learning ability of rats. Radiat. Res 177, 146–151. [DOI] [PubMed] [Google Scholar]
- Britten RA, Jewell JS, Miller VD, Davis LK, Hadley MM, Wyrobek AJ, 2016. Impaired Spatial Memory Performance in Adult Wistar Rats Exposed to Low (5–20 cGy) Doses of 1 GeV/n (56)Fe Particles. Radiat. Res 185, 332–337. 10.1667/RR14120.1 [DOI] [PubMed] [Google Scholar]
- Bromet EJ, Havenaar JM, Guey LT, 2011. A 25 Year Retrospective Review of the Psychological Consequences of the Chernobyl Accident. Clinical Oncology, The Radiobiological Consequences of the Chernobyl Accident 25 Years On- April 2011 23, 297–305. 10.1016/j.clon.2011.01.501 [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Valiengo L, Baccaro A, Zanão TA, de Oliveira JF, Goulart A, Boggio PS, Lotufo PA, Benseñor IM, Fregni F, 2013a. The sertraline vs. electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA Psychiatry 70, 383–391. 10.1001/2013.jamapsychiatry.32 [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Valiengo L, Baccaro A, Zanão TA, de Oliveira JF, Goulart A, Boggio PS, Lotufo PA, Benseñor IM, Fregni F, 2013b. The sertraline vs. electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA Psychiatry 70, 383–391. 10.1001/2013.jamapsychiatry.32 [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Vanderhasselt M-A, 2014. Working memory improvement with non-invasive brain stimulation of the dorsolateral prefrontal cortex: a systematic review and meta-analysis. Brain Cogn 86, 1–9. 10.1016/j.bandc.2014.01.008 [DOI] [PubMed] [Google Scholar]
- Buch ER, Santarnecchi E, Antal A, Born J, Celnik PA, Classen J, Gerloff C, Hallett M, Hummel FC, Nitsche MA, Pascual-Leone A, Paulus WJ, Reis J, Robertson EM, Rothwell JC, Sandrini M, Schambra HM, Wassermann EM, Ziemann U, Cohen LG, 2017. Effects of tDCS on motor learning and memory formation: A consensus and critical position paper. Clinical Neurophysiology 128, 589–603. 10.1016/j.clinph.2017.01.004 [DOI] [PubMed] [Google Scholar]
- Buckey JC, 2006. Space Physiology. Oxford University Press, Oxford, New York. [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL, 2008. The Brain’s Default Network. Annals of the New York Academy of Sciences 1124, 1–38. 10.1196/annals.1440.011 [DOI] [PubMed] [Google Scholar]
- Buzsáki G, 2009. Rhythms of The Brain, in: Rhythms of the Brain. pp. xiv, 448 p. 10.1093/acprof:oso/9780195301069.001.0001 [DOI] [Google Scholar]
- Cappelletti M, Pikkat H, Upstill E, Speekenbrink M, Walsh V, 2015. Learning to integrate versus inhibiting information is modulated by age. J. Neurosci 35, 2213–2225. 10.1523/JNEUROSCI.1018-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier WR, Charles JB, Shelhamer M, Hackler AS, Johnson TL, Domingo CMM, Sutton JP, Scott GBI, Wotring VE, 2018. Biomedical findings from NASA’s Project Mercury: a case series. NPJ Microgravity 4. 10.1038/s41526-018-0040-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casali AG, Gosseries O, Rosanova M, Boly M, Sarasso S, Casali KR, Casarotto S, Bruno M-A, Laureys S, Tononi G, Massimini M, 2013. A theoretically based index of consciousness independent of sensory processing and behavior. Sci Transl Med 5, 198ra105. 10.1126/scitranslmed.3006294 [DOI] [PubMed] [Google Scholar]
- Cassano P, Cusin C, Mischoulon D, Hamblin MR, De Taboada L, Pisoni A, Chang T, Yeung A, Ionescu DF, Petrie SR, Nierenberg AA, Fava M, Iosifescu DV, 2015. Near-Infrared Transcranial Radiation for Major Depressive Disorder: Proof of Concept Study. Psychiatry J 2015, 352979. 10.1155/2015/352979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerri M, Mastrotto M, Tupone D, Martelli D, Luppi M, Perez E, Zamboni G, Amici R, 2013. The Inhibition of Neurons in the Central Nervous Pathways for Thermoregulatory Cold Defense Induces a Suspended Animation State in the Rat. J. Neurosci 33, 2984–2993. 10.1523/JNEUROSCI.3596-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerri M, Tinganelli W, Negrini M, Helm A, Scifoni E, Tommasino F, Sioli M, Zoccoli A, Durante M, 2016. Hibernation for space travel: Impact on radioprotection. Life Sciences in Space Research 11, 1–9. 10.1016/j.lssr.2016.09.001 [DOI] [PubMed] [Google Scholar]
- Chaieb L, Antal A, Paulus W, 2015. Transcranial random noise stimulation-induced plasticity is NMDA-receptor independent but sodium-channel blocker and benzodiazepines sensitive. Front Neurosci 9, 125. 10.3389/fnins.2015.00125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborti A, Allen A, Allen B, Rosi S, Fike JR, 2012. Cranial irradiation alters dendritic spine density and morphology in the hippocampus. PLoS ONE 7, e40844. 10.1371/journal.pone.0040844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheron G, Leroy A, De Saedeleer C, Bengoetxea A, Lipshits M, Cebolla A, Servais L, Dan B, Berthoz A, McIntyre J, 2006. Effect of gravity on human spontaneous 10-Hz electroencephalographic oscillations during the arrest reaction. Brain Res. 1121, 104–116. 10.1016/j.brainres.2006.08.098 [DOI] [PubMed] [Google Scholar]
- Cheshire W, 2015. Multitasking and the neuroethics of distraction. Ethics & medicine : a Christian perspective on issues in bioethics 31, 19–25. [Google Scholar]
- Ciechanski P, Cheng A, Damji O, Lopushinsky S, Hecker K, Jadavji Z, Kirton A, 2018. Effects of transcranial direct-current stimulation on laparoscopic surgical skill acquisition. BJS Open 2, 70–78. 10.1002/bjs5.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanski P, Cheng A, Lopushinsky S, Hecker K, Gan LS, Lang S, Zareinia K, Kirton A, 2017. Effects of Transcranial Direct-Current Stimulation on Neurosurgical Skill Acquisition: A Randomized Controlled Trial. World Neurosurg 108, 876–884.e4. 10.1016/j.wneu.2017.08.123 [DOI] [PubMed] [Google Scholar]
- Clément G, Reschke MF, 2008. Space Neuroscience: What Is It?, in: Clément G, Reschke MF (Eds.), Neuroscience in Space. Springer; New York, New York, NY, pp. 1–32. 10.1007/978-0-387-78950-7_1 [DOI] [Google Scholar]
- Cogiamanian F, Marceglia S, Ardolino G, Barbieri S, Priori A, 2007. Improved isometric force endurance after transcranial direct current stimulation over the human motor cortical areas. Eur. J. Neurosci 26, 242–249. 10.1111/j.1460-9568.2007.05633.x [DOI] [PubMed] [Google Scholar]
- Colzato LS, Nitsche MA, Kibele A, 2017. Noninvasive Brain Stimulation and Neural Entrainment Enhance Athletic Performance—a Review. J Cogn Enhanc 1, 73–79. 10.1007/s41465-016-0003-2 [DOI] [Google Scholar]
- Costanzo F, Menghini D, Maritato A, Castiglioni MC, Mereu A, Varuzza C, Zanna V, Vicari S, 2018. New Treatment Perspectives in Adolescents With Anorexia Nervosa: The Efficacy of Non-invasive Brain-Directed Treatment. Front Behav Neurosci 12. 10.3389/fnbeh.2018.00133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté S, Bouchard S, 2005. Documenting the efficacy of virtual reality exposure with psychophysiological and information processing measures. Appl Psychophysiol Biofeedback 30, 217–232. 10.1007/s10484-005-6379-x [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts S. a. R.B., Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF, 2006. Consistent resting-state networks across healthy subjects. PNAS 103, 13848–13853. 10.1073/pnas.0601417103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey NJ, Rawlinson SR, Nowicky AV, McGregor AH, Dubois K, Strutton PH, Schroter RC, 2004. Human corticospinal excitability in microgravity and hypergravity during parabolic flight. Aviat Space Environ Med 75, 359–363. [PubMed] [Google Scholar]
- De Havas JA, Parimal S, Soon CS, Chee MWL, 2012. Sleep deprivation reduces default mode network connectivity and anti-correlation during rest and task performance. NeuroImage 59, 1745–1751. 10.1016/j.neuroimage.2011.08.026 [DOI] [PubMed] [Google Scholar]
- Demertzi A, Van Ombergen A, Tomilovskaya E, Jeurissen B, Pechenkova E, Di Perri C, Litvinova L, Amico E, Rumshiskaya A, Rukavishnikov I, Sijbers J, Sinitsyn V, Kozlovskaya IB, Sunaert S, Parizel PM, Van de Heyning PH, Laureys S, Wuyts FL, 2016. Cortical reorganization in an astronaut’s brain after long-duration spaceflight. Brain Struct Funct 221, 2873–2876. 10.1007/s00429-015-1054-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denisova NA, Shukitt-Hale B, Rabin BM, Joseph JA, 2002. Brain Signaling and Behavioral Responses Induced by Exposure to 56Fe-Particle Radiation. Radiation Research 158, 725–734. 10.1667/0033-7587(2002)158[0725:BSABRI]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Deppermann S, Notzon S, Kroczek A, Rosenbaum D, Haeussinger FB, Diemer J, Domschke K, Fallgatter AJ, Ehlis A-C, Zwanzger P, 2016. Functional co-activation within the prefrontal cortex supports the maintenance of behavioural performance in fear-relevant situations before an iTBS modulated virtual reality challenge in participants with spider phobia. Behav. Brain Res 307, 208–217. 10.1016/j.bbr.2016.03.028 [DOI] [PubMed] [Google Scholar]
- Dijk D-J, Neri DF, Wyatt JK, Ronda JM, Riel E, Ritz-De Cecco A, Hughes RJ, Elliott AR, Prisk GK, West JB, Czeisler CA, 2001. Sleep, performance, circadian rhythms, and light-dark cycles during two space shuttle flights. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 281, R1647–R1664. 10.1152/ajpregu.2001.281.5.R1647 [DOI] [PubMed] [Google Scholar]
- Disner SG, Beevers CG, Gonzalez-Lima F, 2016. Transcranial Laser Stimulation as Neuroenhancement for Attention Bias Modification in Adults with Elevated Depression Symptoms. Brain Stimul 9, 780–787. 10.1016/j.brs.2016.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditye T, Jacobson L, Walsh V, Lavidor M, 2012. Modulating behavioral inhibition by tDCS combined with cognitive training. Exp Brain Res 219, 363–368. 10.1007/s00221-012-3098-4 [DOI] [PubMed] [Google Scholar]
- Dmochowski JP, Datta A, Bikson M, Su Y, Parra LC, 2011. Optimized multi-electrode stimulation increases focality and intensity at target. J. Neural Eng 8, 046011. 10.1088/1741-2560/8/4/046011 [DOI] [PubMed] [Google Scholar]
- Dockery CA, Hueckel-Weng R, Birbaumer N, Plewnia C, 2009a. Enhancement of planning ability by transcranial direct current stimulation. J. Neurosci 29, 7271–7277. 10.1523/JNEUROSCI.0065-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockery CA, Hueckel-Weng R, Birbaumer N, Plewnia C, 2009b. Enhancement of planning ability by transcranial direct current stimulation. J. Neurosci 29, 7271–7277. 10.1523/JNEUROSCI.0065-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draycott SG, Corporation TP, Kline P, 1996. Validation of the AGARD STRES battery of performance tests. Human Factors. [Google Scholar]
- Duncan JMB, 2007. Organization and Management of the International Space Station (ISS) Multilateral Medical Operations. Presented at the16th IAA Humans in Space Symposium, Beijing, China. [Google Scholar]
- Durante M, 2014. Space radiation protection: Destination Mars. Life Sci Space Res (Amst) 1, 2–9. 10.1016/j.lssr.2014.01.002 [DOI] [PubMed] [Google Scholar]
- Ehsani F, Bakhtiary AH, Jaberzadeh S, Talimkhani A, Hajihasani A, 2016. Differential effects of primary motor cortex and cerebellar transcranial direct current stimulation on motor learning in healthy individuals: A randomized double-blind sham-controlled study. Neuroscience Research 112, 10–19. 10.1016/j.neures.2016.06.003 [DOI] [PubMed] [Google Scholar]
- Esculier J-F, Vaudrin J, Bériault P, Gagnon K, Tremblay LE, 2012. Home-based balance training programme using Wii Fit with balance board for Parkinsons’s disease: a pilot study. J Rehabil Med 44, 144–150. 10.2340/16501977-0922 [DOI] [PubMed] [Google Scholar]
- Fang T-Y, Wang P-C, Liu C-H, Su M-C, Yeh S-C, 2014. Evaluation of a haptics-based virtual reality temporal bone simulator for anatomy and surgery training. Comput Methods Programs Biomed 113, 674–681. 10.1016/j.cmpb.2013.11.005 [DOI] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD, 1992. Interhemispheric inhibition of the human motor cortex. J. Physiol. (Lond.) 453, 525–546. 10.1113/jphysiol.1992.sp019243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreri F, Rossini PM, 2013. TMS and TMS-EEG techniques in the study of the excitability, connectivity, and plasticity of the human motor cortex. Rev Neurosci 24, 431–442. 10.1515/revneuro-2013-0019 [DOI] [PubMed] [Google Scholar]
- Ferrucci R, Mameli F, Guidi I, Mrakic-Sposta S, Vergari M, Marceglia S, Cogiamanian F, Barbieri S, Scarpini E, Priori A, 2008. Transcranial direct current stimulation improves recognition memory in Alzheimer disease. Neurology Aug 1271, 493–8. [DOI] [PubMed] [Google Scholar]
- Fertonani A, Ferrari C, Miniussi C, 2015. What do you feel if I apply transcranial electric stimulation? Safety, sensations and secondary induced effects. Clin Neurophysiol 126, 2181–2188. 10.1016/j.clinph.2015.03.015 [DOI] [PubMed] [Google Scholar]
- Feurra M, Galli G, Pavone EF, Rossi A, Rossi S, 2016. Frequency-specific insight into short-term memory capacity. Journal of Neurophysiology 116, 153–158. 10.1152/jn.01080.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore SM, Wiltshire TJ, Oglesby JM, O’Keefe WS, Salas E, 2014a. Complex Collaborative Problem-Solving Processes in Mission Control. aviat space environ med 85, 456–461. 10.3357/ASEM.3819.2014 [DOI] [PubMed] [Google Scholar]
- Fiore SM, Wiltshire TJ, Oglesby JM, O’Keefe WS, Salas E, 2014b. Complex Collaborative Problem-Solving Processes in Mission Control. aviat space environ med 85, 456–461. 10.3357/ASEM.3819.2014 [DOI] [PubMed] [Google Scholar]
- Fischer DB, Fried PJ, Ruffini G, Ripolles O, Salvador R, Banus J, Ketchabaw WT, Santarnecchi E, Pascual-Leone A, Fox MD, 2017. Multifocal tDCS targeting the resting state motor network increases cortical excitability beyond traditional tDCS targeting unilateral motor cortex. NeuroImage 157, 34–44. 10.1016/j.neuroimage.2017.05.060 [DOI] [PubMed] [Google Scholar]
- Flood A, Waddington G, Keegan RJ, Thompson KG, Cathcart S, 2017. The effects of elevated pain inhibition on endurance exercise performance. PeerJ 5, e3028. 10.7717/peerj.3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fomenko A, Neudorfer C, Dallapiazza RF, Kalia SK, Lozano AM, 2018. Low-intensity ultrasound neuromodulation: An overview of mechanisms and emerging human applications. Brain Stimulation 11, 1209–1217. 10.1016/j.brs.2018.08.013 [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Nitsche M, Bermpohl F, Antal A, Feredoes E, Marcolin MA, Rigonatti SP, Silva MTA, Paulus W, Pascual-Leone A, 2005. Anodal transcranial direct current stimulation of prefrontal cortex enhances working memory. Exp Brain Res 166, 23–30. 10.1007/s00221-005-2334-6 [DOI] [PubMed] [Google Scholar]
- Fregni F, Orsati F, Pedrosa W, Fecteau S, Tome FAM, Nitsche MA, Mecca T, Macedo EC, Pascual-Leone A, Boggio PS, 2008. Transcranial direct current stimulation of the prefrontal cortex modulates the desire for specific foods. Appetite 51, 34–41. 10.1016/j.appet.2007.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J, Richardson JD, Baker JM, Rorden C, 2011. Transcranial direct current stimulation improves naming reaction time in fluent aphasia: a double-blind, sham-controlled study. Stroke 42, 819–821. 10.1161/STROKEAHA.110.600288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, Lu B, 2010. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron 66, 198–204. 10.1016/j.neuron.2010.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost JD, Shumate WH, Salamy JG, Booher CR, 1976. Sleep monitoring: the second manned Skylab mission. Aviat Space Environ Med 47, 372–382. [PubMed] [Google Scholar]
- Furuya S, Nitsche MA, Paulus W, Altenmüller E, 2013. Early optimization in finger dexterity of skilled pianists: implication of transcranial stimulation. BMC Neurosci 14, 35. 10.1186/1471-2202-14-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett-Bakelman FE, Darshi M, Green SJ, Gur RC, Lin L, Macias BR, McKenna MJ, Meydan C, Mishra T, Nasrini J, Piening BD, Rizzardi LF, Sharma K, Siamwala JH, Taylor L, Vitaterna MH, Afkarian M, Afshinnekoo E, Ahadi S, Ambati A, Arya M, Bezdan D, Callahan CM, Chen S, Choi AMK, Chlipala GE, Contrepois K, Covington M, Crucian BE, Vivo ID, Dinges DF, Ebert DJ, Feinberg JI, Gandara JA, George KA, Goutsias J, Grills GS, Hargens AR, Heer M, Hillary RP, Hoofnagle AN, Hook VYH, Jenkinson G, Jiang P, Keshavarzian A, Laurie SS, Lee-McMullen B, Lumpkins SB, MacKay M, Maienschein-Cline MG, Melnick AM, Moore TM, Nakahira K, Patel HH, Pietrzyk R, Rao V, Saito R, Salins DN, Schilling JM, Sears DD, Sheridan CK, Stenger MB, Tryggvadottir R, Urban AE, Vaisar T, Espen BV, Zhang J, Ziegler MG, Zwart SR, Charles JB, Kundrot CE, Scott GBI, Bailey SM, Basner M, Feinberg AP, Lee SMC, Mason CE, Mignot E, Rana BK, Smith SM, Snyder MP, Turek FW, 2019. The NASA Twins Study: A multidimensional analysis of a year-long human spaceflight. Science 364, eaau8650. 10.1126/science.aau8650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemignani J, Gheysens T, Summerer L, 2015. Beyond astronaut’s capabilities: The current state of the art. Presented at the Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, pp. 3615–3618. 10.1109/EMBC.2015.7319175 [DOI] [PubMed] [Google Scholar]
- George MS, Lisanby SH, Avery D, McDonald WM, Durkalski V, Pavlicova M, Anderson B, Nahas Z, Bulow P, Zarkowski P, Holtzheimer PE, Schwartz T, Sackeim HA, 2010. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch. Gen. Psychiatry 67, 507–516. 10.1001/archgenpsychiatry.2010.46 [DOI] [PubMed] [Google Scholar]
- Goldberg ID, Bloomer WD, Dawson DM, 1982. Nervous System Toxic Effects of Cancer Therapy. JAMA 247, 1437–1441. 10.1001/jama.1982.03320350041026 [DOI] [PubMed] [Google Scholar]
- Goldman RL, Borckardt JJ, Frohman HA, O’Neil PM, Madan A, Campbell LK, Budak A, George MS, 2011. Prefrontal cortex transcranial direct current stimulation (tDCS) temporarily reduces food cravings and increases the self-reported ability to resist food in adults with frequent food craving. Appetite 56, 741–746. 10.1016/j.appet.2011.02.013 [DOI] [PubMed] [Google Scholar]
- Gonsalvez I, Baror R, Fried P, Santarnecchi E, Pascual-Leone A, 2016. Therapeutic Noninvasive Brain Stimulation in Alzheimer’s Disease. Current Alzheimer Research 13, 1–1. 10.2174/1567205013666160930113907 [DOI] [PubMed] [Google Scholar]
- Goutagny R, Gu N, Cavanagh C, Jackson J, Chabot J, Quirion R, Krantic S, Williams S, J, 2013. G, Alterations in hippocampal network oscillations and theta-gamma coupling arise before Aβ overproduction in a mouse model of Alzheimer’s disease. Eur 37SRC-BaiduScholar, 1896–1902. [DOI] [PubMed] [Google Scholar]
- Greene-Schloesser D, Robbins ME, Peiffer AM, Shaw EG, Wheeler KT, Chan MD, 2012. Radiation-induced brain injury: A review. Front Oncol 2, 73. 10.3389/fonc.2012.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF, 2007. Resting-State Functional Connectivity in Major Depression: Abnormally Increased Contributions from Subgenual Cingulate Cortex and Thalamus. Biological Psychiatry 62, 429–437. 10.1016/j.biopsych.2006.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi G, Argyropoulos GP, Bastian A, Cortes M, Davis NJ, Edwards DJ, Ferrucci R, Fregni F, Galea JM, Hamada M, Manto M, Miall RC, Morales-Quezada L, Pope PA, Priori A, Rothwell J, Tomlinson SP, Celnik P, 2016. Cerebellar Transcranial Direct Current Stimulation (ctDCS). Neuroscientist 22, 83–97. 10.1177/1073858414559409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynszpan O, Perbal S, Pelissolo A, Fossati P, Jouvent R, Dubal S, Perez-Diaz F, 2011. Efficacy and specificity of computer-assisted cognitive remediation in schizophrenia: a meta-analytical study. Psychol Med 41, 163–173. 10.1017/S0033291710000607 [DOI] [PubMed] [Google Scholar]
- Hargens AR, Richardson S, 2009. Cardiovascular adaptations, fluid shifts, and countermeasures related to space flight. Respir Physiol Neurobiol 169 Suppl 1, S30–33. 10.1016/j.resp.2009.07.005 [DOI] [PubMed] [Google Scholar]
- Herault S, Fomina G, Alferova I, Kotovskaya A, Poliakov V, Arbeille P, 2000. Cardiac, arterial and venous adaptation to weightlessness during 6-month MIR spaceflights with and without thigh cuffs (bracelets). Eur. J. Appl. Physiol 81, 384–390. 10.1007/s004210050058 [DOI] [PubMed] [Google Scholar]
- Herpich F, Melnick MD, Agosta S, Huxlin KR, Tadin D, Battelli L, 2019. Boosting Learning Efficacy with Noninvasive Brain Stimulation in Intact and Brain-Damaged Humans. J. Neurosci 39, 5551–5561. 10.1523/JNEUROSCI.3248-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Mandl RCW, Kahn RS, Pol HEH, 2009. Functionally linked resting‐state networks reflect the underlying structural connectivity architecture of the human brain. Human Brain Mapping 30, 3127–3141. 10.1002/hbm.20737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley DC, Soliman MR, Kaddis F, Markley CL, Krasnov I, 1991. Pineal physiology in microgravity: relation to rat gonadal function aboard Cosmos 1887. Aviat Space Environ Med 62, 953–958. [PubMed] [Google Scholar]
- Honda M, Deiber MP, Ibáñez V, Pascual-Leone A, Zhuang P, Hallett M, 1998. Dynamic cortical involvement in implicit and explicit motor sequence learning. A PET study. Brain 121 (Pt 11), 2159–2173. [DOI] [PubMed] [Google Scholar]
- Hopfinger J, Parsons J, 2016. Fröhlich F. Differential effects of 10Hz and 40Hz transcranial alternating current stimulation tACS on endogenous versus exogenous attention Cogn Neurosci 8SRC-BaiduScholar, 102–111. [DOI] [PubMed] [Google Scholar]
- Horvath JC, Forte JD, Carter O, 2015a. Quantitative Review Finds No Evidence of Cognitive Effects in Healthy Populations From Single-session Transcranial Direct Current Stimulation (tDCS). Brain Stimul 8, 535–550. 10.1016/j.brs.2015.01.400 [DOI] [PubMed] [Google Scholar]
- Horvath JC, Forte JD, Carter O, 2015b. Evidence that transcranial direct current stimulation (tDCS) generates little-to-no reliable neurophysiologic effect beyond MEP amplitude modulation in healthy human subjects: A systematic review. Neuropsychologia 66, 213–236. 10.1016/j.neuropsychologia.2014.11.021 [DOI] [PubMed] [Google Scholar]
- Hoy KE, Emonson MRL, Arnold SL, Thomson RH, Daskalakis ZJ, Fitzgerald PB, 2013. Testing the limits: Investigating the effect of tDCS dose on working memory enhancement in healthy controls. Neuropsychologia 51, 1777–1784. 10.1016/j.neuropsychologia.2013.05.018 [DOI] [PubMed] [Google Scholar]
- Hsu T-Y, Juan C-H, Tseng P, 2016. Individual Differences and State-Dependent Responses in Transcranial Direct Current Stimulation. Front Hum Neurosci 10. 10.3389/fnhum.2016.00643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y-Z, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC, 2005. Theta burst stimulation of the human motor cortex. Neuron 45, 201–206. 10.1016/j.neuron.2004.12.033 [DOI] [PubMed] [Google Scholar]
- Huang Y-Z, Lu M-K, Antal A, Classen J, Nitsche M, Ziemann U, Ridding M, Hamada M, Ugawa Y, Jaberzadeh S, Suppa A, Paulus W, Rothwell J, 2017. Plasticity induced by non-invasive transcranial brain stimulation: A position paper. Clin Neurophysiol 128, 2318–2329. 10.1016/j.clinph.2017.09.007 [DOI] [PubMed] [Google Scholar]
- Huang Y-Z, Rothwell JC, Lu C-S, Chuang W-L, Chen R-S, 2011. Abnormal bidirectional plasticity-like effects in Parkinson’s disease. Brain 134, 2312–2320. 10.1093/brain/awr158 [DOI] [PubMed] [Google Scholar]
- Hunt WA, Joseph JA, Rabin BM, 1989. Behavioral and neurochemical abnormalities after exposure to low doses of high-energy iron particles. Advances in Space Research 9, 333–336. 10.1016/0273-1177(89)90456-0 [DOI] [PubMed] [Google Scholar]
- Hunter T, Sacco P, Nitsche MA, Turner DL, 2009. Modulation of internal model formation during force field-induced motor learning by anodal transcranial direct current stimulation of primary motor cortex. J. Physiol. (Lond.) 587, 2949–2961. 10.1113/jphysiol.2009.169284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J, Castelli DM, Gonzalez-Lima F, 2016. Cognitive enhancement by transcranial laser stimulation and acute aerobic exercise. Lasers Med Sci 31, 1151–1160. 10.1007/s10103-016-1962-3 [DOI] [PubMed] [Google Scholar]
- Iaccarino H, Singer A, Martorell A, Rudenko A, Gao F, Gillingham T, Mathys H, Seo J, Kritskiy O, Abdurrob F, Adaikkan C, Canter R, Rueda R, Brown E, Boyden E, Tsai LH, 2016. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 540SRC-BaiduScholar, 230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iezzi E, Suppa A, Conte A, Agostino R, Nardella A, Berardelli A, 2010. Theta-burst stimulation over primary motor cortex degrades early motor learning. Eur. J. Neurosci 31, 585–592. 10.1111/j.1460-9568.2010.07090.x [DOI] [PubMed] [Google Scholar]
- Jamil A, Batsikadze G, Kuo H, Labruna L, Hasan A, Paulus W, Nitsche MA, 2017. Systematic evaluation of the impact of stimulation intensity on neuroplastic after‐effects induced by transcranial direct current stimulation. J Physiol 595, 1273–1288. 10.1113/JP272738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NN, Carey J, Edelman BJ, Doud A, Grande A, Lakshminarayan K, He B, 2018. Combined rTMS and virtual reality brain-computer interface training for motor recovery after stroke. J Neural Eng 15, 016009. 10.1088/1741-2552/aa8ce3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone DM, Coleman K, Moro C, Torres N, Eells JT, Baker GE, Ashkan K, Stone J, Benabid A-L, Mitrofanis J, 2014. The potential of light therapy in Parkinson's disease [WWW Document]. ChronoPhysiology and Therapy. 10.2147/CPT.S57180 [DOI] [Google Scholar]
- Jones AP, Choe J, Bryant NB, Robinson CSH, Ketz NA, Skorheim SW, Combs A, Lamphere ML, Robert B, Gill HA, Heinrich MD, Howard MD, Clark VP, Pilly PK, 2018. Dose-Dependent Effects of Closed-Loop tACS Delivered During Slow-Wave Oscillations on Memory Consolidation. Frontiers in Neuroscience 12. 10.3389/fnins.2018.00867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RN, Manly J, Glymour MM, Rentz DM, Jefferson AL, Stern Y, 2011. Conceptual and measurement challenges in research on cognitive reserve. J Int Neuropsychol Soc 17, 593–601. 10.1017/S1355617710001748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung E-Y, Park DK, Lee YH, Jo HS, Lim YS, Park RW, 2012. Evaluation of practical exercises using an intravenous simulator incorporating virtual reality and haptics device technologies. Nurse Educ Today 32, 458–463. 10.1016/j.nedt.2011.05.012 [DOI] [PubMed] [Google Scholar]
- Jung-Beeman M, Bowden EM, Haberman J, Frymiare JL, Arambel-Liu S, Greenblatt R, Reber PJ, Kounios J, 2004. Neural Activity When People Solve Verbal Problems with Insight. PLOS Biology 2, e97. 10.1371/journal.pbio.0020097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA, 2015. Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-analysis of Resting-State Functional Connectivity. JAMA Psychiatry 72, 603–611. 10.1001/jamapsychiatry.2015.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanas N, Manzey D, 2008. Space psychology and psychiatry, 2. ed. ed, Space technology library. Springer, El Segundo, Calif.: Microcosm Press. [Google Scholar]
- Kandalaft MR, Didehbani N, Krawczyk DC, Allen TT, Chapman SB, 2013. Virtual reality social cognition training for young adults with high-functioning autism. J Autism Dev Disord 43, 34–44. 10.1007/s10803-012-1544-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane RL, Short P, Sipes W, Flynn CF, 2005. Development and validation of the spaceflight cognitive assessment tool for windows (WinSCAT). Aviat Space Environ Med 76, B183–191. [PubMed] [Google Scholar]
- Kang EK, Paik N-J, 2011. Effect of a tDCS electrode montage on implicit motor sequence learning in healthy subjects. Exp Transl Stroke Med 3, 4. 10.1186/2040-7378-3-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann T, Elvsåshagen T, Alnæs D, Zak N, Pedersen PØ, Norbom LB, Quraishi SH, Tagliazucchi E, Laufs H, Bjørnerud A, Malt UF, Andreassen OA, Roussos E, Duff EP, Smith SM, Groote IR, Westlye LT, 2016. The brain functional connectome is robustly altered by lack of sleep. NeuroImage 127, 324–332. 10.1016/j.neuroimage.2015.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeser D, Padberg F, Reisinger E, Pogarell O, Kirsch V, Palm U, Karch S, Möller H-J, Nitsche MA, Mulert C, 2011. Prefrontal direct current stimulation modulates resting EEG and event-related potentials in healthy subjects: a standardized low resolution tomography (sLORETA) study. Neuroimage 55, 644–657. 10.1016/j.neuroimage.2010.12.004 [DOI] [PubMed] [Google Scholar]
- Keime-Guibert F, Napolitano M, Delattre J-Y, 1998. Neurological complications of radiotherapy and chemotherapy. J Neurol 245, 695–708. 10.1007/s004150050271 [DOI] [PubMed] [Google Scholar]
- Ketz N, Jones AP, Bryant NB, Clark VP, Pilly PK, 2018a. Closed-Loop Slow-Wave tACS Improves Sleep-Dependent Long-Term Memory Generalization by Modulating Endogenous Oscillations. The Journal of Neuroscience 38, 7314–7326. 10.1523/JNEUROSCI.0273-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketz N, Jones AP, Bryant NB, Clark VP, Pilly PK, 2018b. Closed-Loop Slow-Wave tACS Improves Sleep-Dependent Long-Term Memory Generalization by Modulating Endogenous Oscillations. The Journal of Neuroscience 38, 7314–7326. 10.1523/JNEUROSCI.0273-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khraiche ML, Phillips WB, Jackson N, Muthuswamy J, 2008. Ultrasound induced increase in excitability of single neurons, in: 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. Presented at the2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, pp. 4246–4249. 10.1109/IEMBS.2008.4650147 [DOI] [PubMed] [Google Scholar]
- Kidgell DJ, Daly RM, Young K, Lum J, Tooley G, Jaberzadeh S, Zoghi M, Pearce AJ, 2013. Different Current Intensities of Anodal Transcranial Direct Current Stimulation Do Not Differentially Modulate Motor Cortex Plasticity. Neural Plast 2013. 10.1155/2013/603502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BR, Chun MH, Kim LS, Park JY, 2011. Effect of virtual reality on cognition in stroke patients. Ann Rehabil Med 35, 450–459. 10.5535/arm.2011.35.4.450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Ku J, Cho S, Kim HJ, Cho YK, Lim T, Kang YJ, 2014. Facilitation of corticospinal excitability by virtual reality exercise following anodal transcranial direct current stimulation in healthy volunteers and subacute stroke subjects. J Neuroeng Rehabil 11, 124. 10.1186/1743-0003-11-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MJ, 1997. Apollo 13 creativity: in-the-box innovation. J Creat Behav 31, 299–308. [DOI] [PubMed] [Google Scholar]
- Klinger E, Légeron P, Roy S, Chemin I, Lauer F, Nugues P, 2004. Virtual reality exposure in the treatment of social phobia. Stud Health Technol Inform 99, 91–119. [PubMed] [Google Scholar]
- Klomjai W, Katz R, Lackmy-Vallée A, 2015. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Annals of Physical and Rehabilitation Medicine, Neuromodulation / Coordinated by Bernard Bussel, Djamel Ben Bensmail and Nicolas Roche 58, 208–213. 10.1016/j.rehab.2015.05.005 [DOI] [PubMed] [Google Scholar]
- Koch G, Esposito R, Motta C, Casula EP, Di Lorenzo F, Bonnì S, Cinnera AM, Ponzo V, Maiella M, Picazio S, Assogna M, Sallustio F, Caltagirone C, Pellicciari MC, 2020. Improving visuo-motor learning with cerebellar theta burst stimulation: Behavioral and neurophysiological evidence. Neuroimage 208, 116424. 10.1016/j.neuroimage.2019.116424 [DOI] [PubMed] [Google Scholar]
- Krakauer JW, 2009. Motor Learning and Consolidation: The Case of Visuomotor Rotation, in: Sternad D (Ed.), Progress in Motor Control: A Multidisciplinary Perspective, Advances in Experimental Medicine and Biology. Springer; US, Boston, MA, pp. 405–421. 10.1007/978-0-387-77064-2_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer LA, Sargsyan AE, Hasan KM, Polk JD, Hamilton DR, 2012. Orbital and intracranial effects of microgravity: findings at 3-T MR imaging. Radiology 263, 819–827. 10.1148/radiol.12111986 [DOI] [PubMed] [Google Scholar]
- Krause B, Cohen Kadosh R, 2014. Not all brains are created equal: the relevance of individual differences in responsiveness to transcranial electrical stimulation. Front Syst Neurosci 8, 25. 10.3389/fnsys.2014.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause B, Márquez-Ruiz J, Cohen Kadosh R, 2013. The effect of transcranial direct current stimulation: a role for cortical excitation/inhibition balance? Front Hum Neurosci 7, 602. 10.3389/fnhum.2013.00602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause V, Meier A, Dinkelbach L, Pollok B, 2016. Beta Band Transcranial Alternating (tACS) and Direct Current Stimulation (tDCS) Applied After Initial Learning Facilitate Retrieval of a Motor Sequence. Front Behav Neurosci 10, 4. 10.3389/fnbeh.2016.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T, Sato M, Rothwell JC, Cohen LG, 1993a. The effect of transcranial magnetic stimulation on median nerve somatosensory evoked potentials. Electroencephalogr Clin Neurophysiol 89, 227–234. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Sato M, Rothwell JC, Cohen LG, 1993b. The effect of transcranial magnetic stimulation on median nerve somatosensory evoked potentials. Electroencephalogr Clin Neurophysiol 89, 227–234. [DOI] [PubMed] [Google Scholar]
- Kuo M-F, Chen P-S, Nitsche MA, 2017. The application of tDCS for the treatment of psychiatric diseases. Int Rev Psychiatry 29, 146–167. 10.1080/09540261.2017.1286299 [DOI] [PubMed] [Google Scholar]
- Kuo M-F, Nitsche MA, 2012. Effects of transcranial electrical stimulation on cognition. Clin EEG Neurosci 43, 192–199. 10.1177/1550059412444975 [DOI] [PubMed] [Google Scholar]
- Kuo M-F, Paulus W, Nitsche MA, 2014. Therapeutic effects of non-invasive brain stimulation with direct currents (tDCS) in neuropsychiatric diseases. NeuroImage, Neuro-enhancement 85, 948–960. 10.1016/j.neuroimage.2013.05.117 [DOI] [PubMed] [Google Scholar]
- Labruna L, Jamil A, Fresnoza S, Batsikadze G, Kuo M-F, Vanderschelden B, Ivry RB, Nitsche MA, 2016. Efficacy of Anodal Transcranial Direct Current Stimulation is related to Sensitivity to Transcranial Magnetic Stimulation. Brain Stimul 9, 8–15. 10.1016/j.brs.2015.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafon B, Henin S, Huang Y, Friedman D, Melloni L, Thesen T, Doyle W, Buzsáki G, Devinsky O, Parra LC, Liu AA, 2017. Low frequency transcranial electrical stimulation does not entrain sleep rhythms measured by human intracranial recordings. Nature Communications 8. 10.1038/s41467-017-01045-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson J, Munkácsy E, 2015. Theta-Burst LTP. Brain Res 1621, 38–50. 10.1016/j.brainres.2014.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson J, Wong D, Lynch G, 1986. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res. 368, 347–350. 10.1016/0006-8993(86)90579-2 [DOI] [PubMed] [Google Scholar]
- Lattari E, Andrade M, Filho A, Moura A, Neto G, Silva J, Rocha N, Yuan T-F, Arias-Carrión O, Machado S, 2016. Can Transcranial Direct Current Stimulation Improve the Resistance Strength and Decrease the Rating Perceived Scale in Recreational Weight-Training Experience? Journal of Strength and Conditioning Research 30, 3381–3387. 10.1519/JSC.0000000000001457 [DOI] [PubMed] [Google Scholar]
- Lattari E, Filho BR, Junior SF, Murillo-Rodriguez E, Rocha N, Machado S, Neto GM, 2018. Effects on Volume Load and Ratings of Perceived Exertion in Individuals Advanced Weight-Training After Transcranial Direct Current Stimulation. Journal of Strength and Conditioning Research Publish Ahead of Print. 10.1519/JSC.0000000000002434 [DOI] [PubMed] [Google Scholar]
- Laver K, George S, Thomas S, Deutsch JE, Crotty M, 2015. Virtual reality for stroke rehabilitation: an abridged version of a Cochrane review. Eur J Phys Rehabil Med 51, 497–506. [PubMed] [Google Scholar]
- Law J, Van Baalen M, Foy M, Mason SS, Mendez C, Wear ML, Meyers VE, Alexander D, 2014. Relationship Between Carbon Dioxide Levels and Reported Headaches on the International Space Station. Journal of Occupational and Environmental Medicine 56, 477. 10.1097/JOM.0000000000000158 [DOI] [PubMed] [Google Scholar]
- Leach RC, McCurdy MP, Trumbo MC, Matzen LE, Leshikar ED, 2019. Differential Age Effects of Transcranial Direct Current Stimulation on Associative Memory. J Gerontol B Psychol Sci Soc Sci 74, 1163–1173. 10.1093/geronb/gby003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DJ, Elias GJB, Lozano AM, 2018. Neuromodulation for the treatment of eating disorders and obesity. Ther Adv Psychopharmacol 8, 73–92. 10.1177/2045125317743435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Chun MH, 2014. Combination transcranial direct current stimulation and virtual reality therapy for upper extremity training in patients with subacute stroke. Arch Phys Med Rehabil 95, 431–438. 10.1016/j.apmr.2013.10.027 [DOI] [PubMed] [Google Scholar]
- Lefaucheur J-P, Antal A, Ayache SS, Benninger DH, Brunelin J, Cogiamanian F, Cotelli M, De Ridder D, Ferrucci R, Langguth B, Marangolo P, Mylius V, Nitsche MA, Padberg F, Palm U, Poulet E, Priori A, Rossi S, Schecklmann M, Vanneste S, Ziemann U, Garcia-Larrea L, Paulus W, 2017a. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol 128, 56–92. 10.1016/j.clinph.2016.10.087 [DOI] [PubMed] [Google Scholar]
- Lefaucheur J-P, Chalah MA, Mhalla A, Palm U, Ayache SS, Mylius V, 2017b. The treatment of fatigue by non-invasive brain stimulation. Neurophysiologie Clinique/Clinical Neurophysiology 47, 173–184. 10.1016/j.neucli.2017.03.003 [DOI] [PubMed] [Google Scholar]
- Levkovitz Y, Isserles M, Padberg F, Lisanby SH, Bystritsky A, Xia G, Tendler A, Daskalakis ZJ, Winston JL, Dannon P, Hafez HM, Reti IM, Morales OG, Schlaepfer TE, Hollander E, Berman JA, Husain MM, Sofer U, Stein A, Adler S, Deutsch L, Deutsch F, Roth Y, George MS, Zangen A, 2015. Efficacy and safety of deep transcranial magnetic stimulation for major depression: a prospective multicenter randomized controlled trial. World Psychiatry 14, 64–73. 10.1002/wps.20199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linde AS, Kunkler K, 2016. The Evolution of Medical Training Simulation in the U.S. Military. Stud Health Technol Inform 220, 209–214. [PubMed] [Google Scholar]
- Lindenberg R, Renga V, Zhu LL, Nair D, Schlaug G, 2010. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology 75, 2176–2184. 10.1212/WNL.0b013e318202013a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Chen AC, Zebley BD, Drysdale AT, Gordon R, Leuchter B, Voss HU, Casey BJ, Etkin A, Dubin MJ, 2014. Default Mode Network Mechanisms of Transcranial Magnetic Stimulation in Depression. Biological Psychiatry 76, 517–526. 10.1016/j.biopsych.2014.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loganovsky K, Yuryev L, K., 2001. EEG Patterns in Persons Exposed to Ionizing Radiation as a Result of the Chernobyl Accident: Part 1: Conventional EEG Analysis. The Journal of neuropsychiatry and clinical neurosciences 13, 441–58. 10.1176/appi.neuropsych.13.4.441 [DOI] [PubMed] [Google Scholar]
- Loganovsky KN, Loganovskaja TK, 2000. Schizophrenia spectrum disorders in persons exposed to ionizing radiation as a result of the Chernobyl accident. Schizophr Bull 26, 751–773. 10.1093/oxfordjournals.schbul.a033492 [DOI] [PubMed] [Google Scholar]
- Lonart G, Parris B, Johnson AM, Miles S, Sanford LD, Singletary SJ, Britten RA, 2012. Executive function in rats is impaired by low (20 cGy) doses of 1 GeV/u (56)Fe particles. Radiat. Res 178, 289–294. [DOI] [PubMed] [Google Scholar]
- Loo CK, McFarquhar TF, Mitchell PB, 2008. A review of the safety of repetitive transcranial magnetic stimulation as a clinical treatment for depression. Int. J. Neuropsychopharmacol 11, 131–147. 10.1017/S1461145707007717 [DOI] [PubMed] [Google Scholar]
- Loo CK, Sachdev P, Martin D, Pigot M, Alonzo A, Malhi GS, Lagopoulos J, Mitchell P, 2010. A double-blind, sham-controlled trial of transcranial direct current stimulation for the treatment of depression. Int. J. Neuropsychopharmacol 13, 61–69. 10.1017/S1461145709990411 [DOI] [PubMed] [Google Scholar]
- López-Alonso V, Cheeran B, Río-Rodríguez D, Fernández-Del-Olmo M, 2014. Inter-individual variability in response to non-invasive brain stimulation paradigms. Brain Stimul 7, 372–380. 10.1016/j.brs.2014.02.004 [DOI] [PubMed] [Google Scholar]
- Lugg DJ, 2005. Behavioral health in Antarctica: implications for long-duration space missions. Aviat Space Environ Med 76, B74–77. [PubMed] [Google Scholar]
- Lustenberger C, Boyle MR, Foulser AA, Mellin JM, Fröhlich F, 2015. Functional role of frontal alpha oscillations in creativity. Cortex 67, 74–82. 10.1016/j.cortex.2015.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mader TH, Gibson CR, Pass AF, Kramer LA, Lee AG, Fogarty J, Tarver WJ, Dervay JP, Hamilton DR, Sargsyan A, Phillips JL, Tran D, Lipsky W, Choi J, Stern C, Kuyumjian R, Polk JD, 2011. Optic Disc Edema, Globe Flattening, Choroidal Folds, and Hyperopic Shifts Observed in Astronauts after Long-duration Space Flight. Ophthalmology 118, 2058–2069. 10.1016/j.ophtha.2011.06.021 [DOI] [PubMed] [Google Scholar]
- Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A, 2000. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp Brain Res 133, 425–430. [DOI] [PubMed] [Google Scholar]
- Maksimovich IV, 2015. Dementia and Cognitive Impairment Reduction after Laser Transcatheter Treatment of Alzheimer’s Disease. World Journal of Neuroscience 5, 720–726. 10.4236/wjns.2015.53021 [DOI] [Google Scholar]
- Malenka RC, Bear MF, 2004. LTP and LTD: an embarrassment of riches. Neuron 44, 5–21. 10.1016/j.neuron.2004.09.012 [DOI] [PubMed] [Google Scholar]
- Marshall L, Hallschmid M, Born J, J, 2004a. Mölle M, Transcranial direct current stimulation during sleep improves declarative memory. November324, 9985–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Hallschmid M, Born J, J, 2004b. Mölle M, Transcranial direct current stimulation during sleep improves declarative memory. November324, 9985–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Helgadóttir H, Mölle M, Born J, 2006. Boosting slow oscillations during sleep potentiates memory. Nature 444, 610–613. 10.1038/nature05278 [DOI] [PubMed] [Google Scholar]
- Martin DM, Liu R, Alonzo A, Green M, Player MJ, Sachdev P, Loo CK, 2013. Can transcranial direct current stimulation enhance outcomes from cognitive training? A randomized controlled trial in healthy participants. Int. J. Neuropsychopharmacol 16, 1927–1936. 10.1017/S1461145713000539 [DOI] [PubMed] [Google Scholar]
- Matzen LE, Benz ZO, Dixon KR, Posey J, Kroger JK, Speed AE, 2010. Recreating Raven’s: Software for systematically generating large numbers of Raven-like matrix problems with normed properties. Behavior Research Methods 42, 525–541. 10.3758/BRM.42.2.525 [DOI] [PubMed] [Google Scholar]
- McClelland J, Bozhilova N, Campbell I, Schmidt U, 2013. A systematic review of the effects of neuromodulation on eating and body weight: evidence from human and animal studies. Eur Eat Disord Rev 21, 436–455. 10.1002/erv.2256 [DOI] [PubMed] [Google Scholar]
- McDonnell MD, Abbott D, 2009. What Is Stochastic Resonance? Definitions, Misconceptions, Debates, and Its Relevance to Biology. PLoS Comput Biol 5. 10.1371/journal.pcbi.1000348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire LK, McKinley RA, Goodyear C, Nelson J, 2014. A comparison of the effects of transcranial direct current stimulation and caffeine on vigilance and cognitive performance during extended wakefulness. Brain Stimul 7, 499–507. 10.1016/j.brs.2014.04.008 [DOI] [PubMed] [Google Scholar]
- McIntire LK, McKinley RA, Nelson JM, Goodyear C, 2017. Transcranial direct current stimulation versus caffeine as a fatigue countermeasure. Brain Stimul 10, 1070–1078. 10.1016/j.brs.2017.08.005 [DOI] [PubMed] [Google Scholar]
- McKendrick R, Ayaz H, Olmstead R, Parasuraman R, 2014. Enhancing dual-task performance with verbal and spatial working memory training: continuous monitoring of cerebral hemodynamics with NIRS. Neuroimage 85Pt 3, 1014–1026. 10.1016/j.neuroimage.2013.05.103 [DOI] [PubMed] [Google Scholar]
- McKinley RA, McIntire L, Bridges N, Goodyear C, Bangera NB, Weisend MP, 2013a. Acceleration of image analyst training with transcranial direct current stimulation. Behav. Neurosci 127, 936–946. 10.1037/a0034975 [DOI] [PubMed] [Google Scholar]
- McKinley RA, McIntire L, Bridges N, Goodyear C, Bangera NB, Weisend MP, 2013b. Acceleration of image analyst training with transcranial direct current stimulation. Behav. Neurosci 127, 936–946. 10.1037/a0034975 [DOI] [PubMed] [Google Scholar]
- Melby-Lervåg M, Redick TS, Hulme C, 2016. Working Memory Training Does Not Improve Performance on Measures of Intelligence or Other Measures of “Far Transfer”: Evidence From a Meta-Analytic Review. Perspect Psychol Sci 11, 512–534. 10.1177/1745691616635612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miniussi C, Brignani D, Pellicciari MC, 2012. Combining transcranial electrical stimulation with electroencephalography: a multimodal approach. Clin EEG Neurosci 43, 184–191. 10.1177/1550059412444976 [DOI] [PubMed] [Google Scholar]
- Miniussi C, Harris JA, Ruzzoli M, 2013. Modelling non-invasive brain stimulation in cognitive neuroscience. Neurosci Biobehav Rev 37, 1702–1712. 10.1016/j.neubiorev.2013.06.014 [DOI] [PubMed] [Google Scholar]
- Miniussi C, Thut G, 2010. Combining TMS and EEG offers new prospects in cognitive neuroscience. Brain Topogr 22, 249–256. 10.1007/s10548-009-0083-8 [DOI] [PubMed] [Google Scholar]
- Miranda PC, Mekonnen A, Salvador R, Ruffini G, 2013. The electric field in the cortex during transcranial current stimulation. Neuroimage 70, 48–58. 10.1016/j.neuroimage.2012.12.034 [DOI] [PubMed] [Google Scholar]
- Mirelman A, Maidan I, Herman T, Deutsch JE, Giladi N, Hausdorff JM, 2011. Virtual reality for gait training: can it induce motor learning to enhance complex walking and reduce fall risk in patients with Parkinson’s disease? J. Gerontol. A Biol. Sci. Med. Sci 66, 234–240. 10.1093/gerona/glq201 [DOI] [PubMed] [Google Scholar]
- Moliadze V, Antal A, Paulus W, 2010. Boosting brain excitability by transcranial high frequency stimulation in the ripple range. J. Physiol. (Lond.) 588, 4891–4904. 10.1113/jphysiol.2010.196998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momi D, Smeralda C, Sprugnoli G, Neri F, Rossi S, Rossi A, Di Lorenzo G, Santarnecchi E, 2019. Thalamic morphometric changes induced by first-person action videogame training. Eur. J. Neurosci 49, 1180–1195. 10.1111/ejn.14272 [DOI] [PubMed] [Google Scholar]
- Monk TH, Buysse DJ, Billy BD, Kennedy KS, Willrich LM, 1998. Sleep and circadian rhythms in four orbiting astronauts. J. Biol. Rhythms 13, 188–201. 10.1177/074873098129000039 [DOI] [PubMed] [Google Scholar]
- Montenegro RA, Okano AH, Cunha FA, Gurgel JL, Fontes EB, Farinatti PTV, 2012. Prefrontal cortex transcranial direct current stimulation associated with aerobic exercise change aspects of appetite sensation in overweight adults. Appetite 58, 333–338. 10.1016/j.appet.2011.11.008 [DOI] [PubMed] [Google Scholar]
- Monte-Silva K, Kuo M-F, Hessenthaler S, Fresnoza S, Liebetanz D, Paulus W, Nitsche MA, 2013. Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimul 6, 424–432. 10.1016/j.brs.2012.04.011 [DOI] [PubMed] [Google Scholar]
- de Morree HM, Klein C, Marcora SM, 2012. Perception of effort reflects central motor command during movement execution. Psychophysiology 49, 1242–1253. 10.1111/j.1469-8986.2012.01399.x [DOI] [PubMed] [Google Scholar]
- Nakazono H, Ogata K, Kuroda T, Tobimatsu S, 2016. Phase and Frequency-Dependent Effects of Transcranial Alternating Current Stimulation on Motor Cortical Excitability. PLoS ONE 11, e0162521. 10.1371/journal.pone.0162521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NASA IG-18-021, n.d.
- NASA/TP–2010– 216126, n.d.
- NASA/TP-2015–218570, n.d.
- Nelson J, McKinley RA, Phillips C, McIntire L, Goodyear C, Kreiner A, Monforton L, 2016. The Effects of Transcranial Direct Current Stimulation (tDCS) on Multitasking Throughput Capacity. Front Hum Neurosci 10. 10.3389/fnhum.2016.00589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuijsen ER, Zemper E, Miner KR, Epstein M, 2006. Health behavior change models and theories: contributions to rehabilitation. Disabil Rehabil 28, 245–256. 10.1080/09638280500197743 [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W, 2001. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 57, 1899–1901. 10.1212/wnl.57.10.1899 [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W, 2000. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. (Lond.) 527Pt 3, 633–639. 10.1111/j.1469-7793.2000.t01-1-00633.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Schauenburg A, Lang N, Liebetanz D, Exner C, Paulus W, Tergau F, 2003. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J Cogn Neurosci 15, 619–626. 10.1162/089892903321662994 [DOI] [PubMed] [Google Scholar]
- Notzon S, Deppermann S, Fallgatter A, Diemer J, Kroczek A, Domschke K, Zwanzger P, Ehlis A-C, 2015. Psychophysiological effects of an iTBS modulated virtual reality challenge including participants with spider phobia. Biol Psychol 112, 66–76. 10.1016/j.biopsycho.2015.10.003 [DOI] [PubMed] [Google Scholar]
- Ohn SH, Park C-I, Yoo W-K, Ko M-H, Choi KP, Kim G-M, Lee YT, Kim Y-H, 2008a. Time-dependent effect of transcranial direct current stimulation on the enhancement of working memory. Neuroreport 19, 43–47. 10.1097/WNR.0b013e3282f2adfd [DOI] [PubMed] [Google Scholar]
- Ohn SH, Park C-I, Yoo W-K, Ko M-H, Choi KP, Kim G-M, Lee YT, Kim Y-H, 2008b. Time-dependent effect of transcranial direct current stimulation on the enhancement of working memory. Neuroreport 19, 43–47. 10.1097/WNR.0b013e3282f2adfd [DOI] [PubMed] [Google Scholar]
- Okano AH, Fontes EB, Montenegro RA, de T.V. Farinatti P, Cyrino ES, Li LM, Bikson M, Noakes TD, 2015. Brain stimulation modulates the autonomic nervous system, rating of perceived exertion and performance during maximal exercise. Br J Sports Med 49, 1213–1218. 10.1136/bjsports-2012-091658 [DOI] [PubMed] [Google Scholar]
- Oki K, Mahato NK, Nakazawa M, Amano S, France CR, Russ DW, Clark BC, 2016. Preliminary Evidence That Excitatory Transcranial Direct Current Stimulation Extends Time to Task Failure of a Sustained, Submaximal Muscular Contraction in Older Adults. J Gerontol A Biol Sci Med Sci 71, 1109–1112. 10.1093/gerona/glw011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ombergen AV, Laureys S, Sunaert S, Tomilovskaya E, Parizel PM, Wuyts FL, 2017. Spaceflight-induced neuroplasticity in humans as measured by MRI: what do we know so far? npj Microgravity 3, 2. 10.1038/s41526-016-0010-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban de Xivry J-J, Shadmehr R, 2014. Electrifying the motor engram: effects of tDCS on motor learning and control. Exp Brain Res 232, 3379–3395. 10.1007/s00221-014-4087-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, McDonald WM, Avery D, Fitzgerald PB, Loo C, Demitrack MA, George MS, Sackeim HA, 2007. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol. Psychiatry 62, 1208–1216. 10.1016/j.biopsych.2007.01.018 [DOI] [PubMed] [Google Scholar]
- Otsuka K, Cornelissen G, Kubo Y, Shibata K, Mizuno K, Ohshima H, Furukawa S, Mukai C, 2019. Anti-aging effects of long-term space missions, estimated by heart rate variability. Sci Rep 9, 8995. 10.1038/s41598-019-45387-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallavicini F, Argenton L, Toniazzi N, Aceti L, Mantovani F, 2016. Virtual Reality Applications for Stress Management Training in the Military. Aerosp Med Hum Perform 87, 1021–1030. 10.3357/AMHP.4596.2016 [DOI] [PubMed] [Google Scholar]
- Parazzini M, Fiocchi S, Rossi E, Paglialonga A, Ravazzani P, 2011. Transcranial direct current stimulation: estimation of the electric field and of the current density in an anatomical human head model. IEEE Trans Biomed Eng 58, 1773–1780. 10.1109/TBME.2011.2116019 [DOI] [PubMed] [Google Scholar]
- Parihar VK, Limoli CL, 2013. Cranial irradiation compromises neuronal architecture in the hippocampus. PNAS 110, 12822–12827. 10.1073/pnas.1307301110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar VK, Maroso M, Syage A, Allen BD, Angulo MC, Soltesz I, Limoli CL, 2018. Persistent nature of alterations in cognition and neuronal circuit excitability after exposure to simulated cosmic radiation in mice. Exp. Neurol 305, 44–55. 10.1016/j.expneurol.2018.03.009 [DOI] [PubMed] [Google Scholar]
- Parihar VK, Pasha J, Tran KK, Craver BM, Acharya MM, Limoli CL, 2015. Persistent changes in neuronal structure and synaptic plasticity caused by proton irradiation. Brain Struct Funct 220, 1161–1171. 10.1007/s00429-014-0709-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K-M, Ku J, Choi S-H, Jang H-J, Park J-Y, Kim SI, Kim J-J, 2011. A virtual reality application in role-plays of social skills training for schizophrenia: a randomized, controlled trial. Psychiatry Res 189, 166–172. 10.1016/j.psychres.2011.04.003 [DOI] [PubMed] [Google Scholar]
- Park S-H, Seo J-H, Kim Y-H, Ko M-H, 2014. Long-term effects of transcranial direct current stimulation combined with computer-assisted cognitive training in healthy older adults. Neuroreport 25, 122–126. 10.1097/WNR.0000000000000080 [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Rubio B, Pallardó F, Catalá MD, 1996. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet 348, 233–237. 10.1016/s0140-6736(96)01219-6 [DOI] [PubMed] [Google Scholar]
- Paulus W, 2011. Transcranial electrical stimulation (tES-tDCS; tRNS, tACS) methods. Neuropsychol. Rehabil 21SRC-BaiduScholar, 602–617. [DOI] [PubMed] [Google Scholar]
- Pavy-Le Traon A, Taillard J, 2010. Sommeil et vols spatiaux. Médecine du Sommeil 7, 8–14. 10.1016/j.msom.2009.10.014 [DOI] [Google Scholar]
- Pechenkova E, Nosikova I, Rumshiskaya A, Litvinova L, Rukavishnikov I, Mershina E, Sinitsyn V, Van Ombergen A, Jeurissen B, Jillings S, Laureys S, Sijbers J, Grishin A, Chernikova L, Naumov I, Kornilova L, Wuyts FL, Tomilovskaya E, Kozlovskaya I, 2019. Alterations of Functional Brain Connectivity After Long-Duration Spaceflight as Revealed by fMRI. Front. Physiol 10. 10.3389/fphys.2019.00761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera T, George MS, Grammer G, Janicak PG, Pascual-Leone A, Wiercki TS, 2016. The Clinical TMS Society Consensus Review and Treatment Recommendations for TMS Therapy for Major Depressive Disorder. Brain Stimul 9, 336–346. 10.1016/j.brs.2016.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip NS, Nelson BG, Frohlich F, Lim KO, Widge AS, Carpenter LL, 2017. Low-Intensity Transcranial Current Stimulation in Psychiatry. Am J Psychiatry 174, 628–639. 10.1176/appi.ajp.2017.16090996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platz T, Adler-Wiebe M, Roschka S, Lotze M, 2018. Enhancement of motor learning by focal intermittent theta burst stimulation (iTBS) of either the primary motor (M1) or somatosensory area (S1) in healthy human subjects. Restor. Neurol. Neurosci 36, 117–130. 10.3233/RNN-170774 [DOI] [PubMed] [Google Scholar]
- Poreisz C, Boros K, Antal A, Paulus W, 2007. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res Bull 72, 208–214. [DOI] [PubMed] [Google Scholar]
- pubmeddev, al, van ‘t W.-F. M, et, n.d.Combined transcranial direct current stimulation with virtual reality exposure for posttraumatic stress disorder: Feasibility and pilot results.- PubMed- NCBI [WWW Document]. URL https://www.ncbi.nlm.nih.gov/pubmed/30266416 (accessed 4.6.20). [DOI] [PMC free article] [PubMed]
- Rabin BM, Hunt WA, Joseph JA, 1989. An Assessment of the Behavioral Toxicity of High-Energy Iron Particles Compared to Other Qualities of Radiation. Radiation Research 119, 113–122. 10.2307/3577371 [DOI] [PubMed] [Google Scholar]
- Rabin BM, Hunt WA, Joseph JA, Dalton TK, Kandasamy SB, 1991. Relationship between Linear Energy Transfer and Behavioral Toxicity in Rats Following Exposure to Protons and Heavy Particles. Radiation Research 128, 216–221. 10.2307/3578141 [DOI] [PubMed] [Google Scholar]
- Rabin BM, Joseph JA, Hunt WA, Dalton TB, Kandasamy SB, Harris AH, Ludewig B, 1994. Behavioral endpoints for radiation injury. Advances in Space Research 14, 457–466. 10.1016/0273-1177(94)90500-2 [DOI] [PubMed] [Google Scholar]
- Rabin BM, Joseph JA, Shukitt-Hale B, McEwen J, 2000. Effects of exposure to heavy particles on a behavior mediated by the dopaminergic system. Advances in Space Research, Life Sciences: Microgravity and Space Radiation Effects 25, 2065–2074. 10.1016/S0273-1177(99)01014-5 [DOI] [PubMed] [Google Scholar]
- Reid D, 2004. The influence of virtual reality on playfulness in children with cerebral palsy: a pilot study. Occup Ther Int 11, 131–144. 10.1002/oti.202 [DOI] [PubMed] [Google Scholar]
- Reid DT, 2002. Benefits of a virtual play rehabilitation environment for children with cerebral palsy on perceptions of self-efficacy: a pilot study. Pediatr Rehabil 5, 141–148. 10.1080/1363849021000039344 [DOI] [PubMed] [Google Scholar]
- Reinhart RMG, Nguyen JA, 2019. Working memory revived in older adults by synchronizing rhythmic brain circuits. Nature Neuroscience 22, 820. 10.1038/s41593-019-0371-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, Celnik PA, Krakauer JW, 2009. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. PNAS 106, 1590–1595. 10.1073/pnas.0805413106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridding MC, Flavel SC, 2006. Induction of plasticity in the dominant and non-dominant motor cortices of humans. Exp Brain Res 171, 551–557. 10.1007/s00221-005-0309-2 [DOI] [PubMed] [Google Scholar]
- Ridding MC, Ziemann U, 2010. Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J Physiol 588, 2291–2304. 10.1113/jphysiol.2010.190314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley AL, Tuck DL, 1985. Conditioned Taste Aversions: A Behavioral Index of Toxicity. Annals of the New York Academy of Sciences 443, 272–292. 10.1111/j.1749-6632.1985.tb27079.x [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Donoghue JP, 2000. Learning-induced LTP in neocortex. Science 290, 533–536. 10.1126/science.290.5491.533 [DOI] [PubMed] [Google Scholar]
- Roberts DR, Ramsey D, Johnson K, Kola J, Ricci R, Hicks C, Borckardt JJ, Bloomberg JJ, Epstein C, George MS, 2010. Cerebral Cortex Plasticity After 90 Days of Bed Rest: Data from TMS and fMRI. Aviat Space Environ Med 81, 30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson C, Bryant N, Maxwell J, Jones A, Robert B, Lamphere M, Combs A, Al Azzawi H, Gibson B, Sanguinetti J, Ketz N, Pilly P, Clark V, 2018. The Benefits of Closed-Loop Transcranial Alternating Current Stimulation on Subjective Sleep Quality. Brain Sciences 8, 204. 10.3390/brainsci8120204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A, Safety of TMS Consensus Group, 2009a. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 120, 2008–2039. 10.1016/j.clinph.2009.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A, Safety of TMS Consensus Group, 2009b. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 120, 2008–2039. 10.1016/j.clinph.2009.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffini G, Fox MD, Ripolles O, Miranda PC, Pascual-Leone A, 2014. Optimization of multifocal transcranial current stimulation for weighted cortical pattern targeting from realistic modeling of electric fields. Neuroimage 89, 216–225. 10.1016/j.neuroimage.2013.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffini G, Wendling F, Merlet I, Molaee-Ardekani B, Mekonnen A, Salvador R, Soria-Frisch A, Grau C, Dunne S, Miranda PC, 2013. Transcranial Current Brain Stimulation (tCS): Models and Technologies. IEEE Transactions on Neural Systems and Rehabilitation Engineering 21, 333–345. 10.1109/TNSRE.2012.2200046 [DOI] [PubMed] [Google Scholar]
- Rumpf J-J, Wegscheider M, Hinselmann K, Fricke C, King BR, Weise D, Klann J, Binkofski F, Buccino G, Karni A, Doyon J, Classen J, 2017. Enhancement of motor consolidation by post-training transcranial direct current stimulation in older people. Neurobiol. Aging 49, 1–8. 10.1016/j.neurobiolaging.2016.09.003 [DOI] [PubMed] [Google Scholar]
- Russell MJ, Goodman T, Pierson R, Shepherd S, Wang Q, Groshong B, Wiley DF, 2013. Individual differences in transcranial electrical stimulation current density. J Biomed Res 27, 495–508. 10.7555/JBR.27.20130074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell MJ, Goodman TA, Visse JM, Beckett L, Saito N, Lyeth BG, Recanzone GH, 2017. Sex and Electrode Configuration in Transcranial Electrical Stimulation. Front Psychiatry 8, 147. 10.3389/fpsyt.2017.00147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saebipour MR, Joghataei MT, Yoonessi A, Sadeghniiat-Haghighi K, Khalighinejad N, Khademi S, 2015. Slow oscillating transcranial direct current stimulation during sleep has a sleep-stabilizing effect in chronic insomnia: a pilot study. Journal of Sleep Research 24, 518–525. 10.1111/jsr.12301 [DOI] [PubMed] [Google Scholar]
- Salehpour F, Mahmoudi J, Kamari F, Sadigh-Eteghad S, Rasta SH, Hamblin MR, 2018. Brain Photobiomodulation Therapy: a Narrative Review. Mol Neurobiol 55, 6601–6636. 10.1007/s12035-017-0852-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandal GM, Vaernes R, Ursin H, 1995. Interpersonal relations during simulated space missions. Aviat Space Environ Med 66, 617–624. [PubMed] [Google Scholar]
- Santarnecchi E, Bianco C, Sicilia I, Momi D, Lorenzo G, Ferrone S, Sprugnoli G, Rossi S, Rossi A, 2018. Del DiAge of Insomnia Onset Correlates with a Reversal of Default Mode Network and Supplementary Motor Cortex Connectivity. Neural Plast April136785342018SRC-BaiduScholar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarnecchi E, Emmendorfer A, Tadayon S, Rossi S, Rossi A, Pascual-Leone A, 2017. Network connectivity correlates of variability in fluid intelligence performance. Intelligence 65, 35–47. 10.1016/j.intell.2017.10.002 [DOI] [Google Scholar]
- Santarnecchi Emiliano, Momi D, Sprugnoli G, Neri F, Pascual-Leone A, Rossi A, Rossi S, 2018a. Modulation of network-to-network connectivity via spike-timing-dependent noninvasive brain stimulation. Human Brain Mapping 39. 10.1002/hbm.24329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarnecchi E, Polizzotto N, Godone M, Giovannelli F, Feurra M, Matzen L, Rossi A, Rossi S, 2013. Frequency-dependent enhancement of fluid intelligence induced by transcranial oscillatory potentials. Curr Biol 23SRC-BaiduScholar, 1449–1453. [DOI] [PubMed] [Google Scholar]
- Santarnecchi E, Sprugnoli G, Bricolo E, Costantini G, Liew S-L, Musaeus CS, Salvi C, Pascual-Leone A, Rossi A, Rossi S, 2019. Gamma tACS over the temporal lobe increases the occurrence of Eureka! moments. Sci Rep 9, 5778. 10.1038/s41598-019-42192-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarnecchi Emiliano, Sprugnoli G, Tatti E, Mencarelli L, Neri F, Momi D, Di Lorenzo G, Pascual-Leone A, Rossi S, Rossi A, 2018b. Brain functional connectivity correlates of coping styles. Cogn Affect Behav Neurosci 18, 495–508. 10.3758/s13415-018-0583-7 [DOI] [PubMed] [Google Scholar]
- Schiffer F, Johnston AL, Ravichandran C, Polcari A, Teicher MH, Webb RH, Hamblin MR, 2009. Psychological benefits 2 and 4 weeks after a single treatment with near infrared light to the forehead: a pilot study of 10 patients with major depression and anxiety. Behav Brain Funct 5, 46. 10.1186/1744-9081-5-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scullin MK, 2017. Do Older Adults Need Sleep? A Review of Neuroimaging, Sleep, and Aging Studies. Curr Sleep Medicine Rep 3, 204–214. 10.1007/s40675-017-0086-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyedmadani KG, 2019. Process for Development of Space Flight Biomedical and Health Hardware According to the Requirements Needs of Users in Medical Device and Diagnostic Industry.
- Sheline YI, Morris JC, Snyder AZ, Price JL, Yan Z, D’Angelo G, Liu C, Dixit S, Benzinger T, Fagan A, Goate A, Mintun MA, 2010. APOE4 Allele Disrupts Resting State fMRI Connectivity in the Absence of Amyloid Plaques or Decreased CSF Aβ42. J. Neurosci 30, 17035–17040. 10.1523/JNEUROSCI.3987-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard JM, Kosslyn SM, 2005. The minicog rapid assessment battery: developing a “blood pressure cuff for the mind”. Aviat Space Environ Med 76, B192–197. [PubMed] [Google Scholar]
- Siu K-C, Best BJ, Kim JW, Oleynikov D, Ritter FE, 2016. Adaptive Virtual Reality Training to Optimize Military Medical Skills Acquisition and Retention. Mil Med 181, 214–220. 10.7205/MILMED-D-15-00164 [DOI] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Viviano JD, Schacter DL, 2016. Attenuated anticorrelation between the default and dorsal attention networks with aging: evidence from task and rest. Neurobiology of Aging 45, 149–160. 10.1016/j.neurobiolaging.2016.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprugnoli G, Cagle YD, Santarnecchi E, 2019. Microgravity and Cosmic Radiations During Space Exploration as a Window Into Neurodegeneration on Earth. JAMA Neurol. 10.1001/jamaneurol.2019.4003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Bachtiar V, Johansen-Berg H, 2011. The role of GABA in human motor learning. Curr. Biol 21, 480–484. 10.1016/j.cub.2011.01.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampi C, 1994. Sleep and circadian rhythms in space. J Clin Pharmacol 34, 518–534. [DOI] [PubMed] [Google Scholar]
- Strafella AP, Paus T, Barrett J, Dagher A, 2001. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J. Neurosci 21, RC157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strangman GE, Sipes W, Beven G, 2014. Human cognitive performance in spaceflight and analogue environments. Aviat Space Environ Med 85, 1033–1048. 10.3357/ASEM.3961.2014 [DOI] [PubMed] [Google Scholar]
- Suppa A, Huang Y-Z, Funke K, Ridding MC, Cheeran B, Di Lazzaro V, Ziemann U, Rothwell JC, 2016. Ten Years of Theta Burst Stimulation in Humans: Established Knowledge, Unknowns and Prospects. Brain Stimul 9, 323–335. 10.1016/j.brs.2016.01.006 [DOI] [PubMed] [Google Scholar]
- Talsma LJ, Kroese HA, Slagter HA, 2016. Boosting Cognition: Effects of Multiple-Session Transcranial Direct Current Stimulation on Working Memory. Journal of Cognitive Neuroscience 29, 755–768. 10.1162/jocn_a_01077 [DOI] [PubMed] [Google Scholar]
- Tavakoli AV, Yun K, 2017. Transcranial Alternating Current Stimulation (tACS) Mechanisms and Protocols. Frontiers in Cellular Neuroscience 11. 10.3389/fncel.2017.00214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecchio F, Zappasodi F, Assenza G, Tombini M, Vollaro S, Barbati G, Rossini PM, 2010. Anodal transcranial direct current stimulation enhances procedural consolidation. J. Neurophysiol 104, 1134–1140. 10.1152/jn.00661.2009 [DOI] [PubMed] [Google Scholar]
- Teo JTH, Swayne OBC, Cheeran B, Greenwood RJ, Rothwell JC, 2011. Human θ burst stimulation enhances subsequent motor learning and increases performance variability. Cereb. Cortex 21, 1627–1638. 10.1093/cercor/bhq231 [DOI] [PubMed] [Google Scholar]
- Teo W-P, Muthalib M, Yamin S, Hendy AM, Bramstedt K, Kotsopoulos E, Perrey S, Ayaz H, 2016. Does a Combination of Virtual Reality, Neuromodulation and Neuroimaging Provide a Comprehensive Platform for Neurorehabilitation? – A Narrative Review of the Literature. Front Hum Neurosci 10. 10.3389/fnhum.2016.00284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thielscher A, Kammer T, 2002. Linking physics with physiology in TMS: a sphere field model to determine the cortical stimulation site in TMS. Neuroimage 17, 1117–1130. 10.1006/nimg.2002.1282 [DOI] [PubMed] [Google Scholar]
- Thirugnanasambandam N, Sparing R, Dafotakis M, Meister IG, Paulus W, Nitsche MA, Fink GR, 2011. Isometric contraction interferes with transcranial direct current stimulation (tDCS) induced plasticity – evidence of state-dependent neuromodulation in human motor cortex. Restorative Neurology and Neuroscience 29, 311–320. 10.3233/RNN-2011-0601 [DOI] [PubMed] [Google Scholar]
- Thomson H, 2018. How flashing lights and pink noise might banish Alzheimer’s, improve memory and more. Nature Mar 1555, 20–22. [DOI] [PubMed] [Google Scholar]
- Thomson K, Pollock A, Bugge C, Brady M, 2014. Commercial gaming devices for stroke upper limb rehabilitation: a systematic review. Int J Stroke 9, 479–488. 10.1111/ijs.12263 [DOI] [PubMed] [Google Scholar]
- Thut G, Miniussi C, 2009. New insights into rhythmic brain activity from TMS-EEG studies. Trends Cogn. Sci. (Regul. Ed.) 13, 182–189. 10.1016/j.tics.2009.01.004 [DOI] [PubMed] [Google Scholar]
- Todd P, 1989. Stochastics of HZE-induced microlesions. Adv Space Res 9, 31–34. [DOI] [PubMed] [Google Scholar]
- Toschi N, Welt T, Guerrisi M, Keck ME, 2008. A reconstruction of the conductive phenomena elicited by transcranial magnetic stimulation in heterogeneous brain tissue. Phys Med 24, 80–86. 10.1016/j.ejmp.2008.01.005 [DOI] [PubMed] [Google Scholar]
- Tremblay S, Rogasch NC, Premoli I, Blumberger DM, Casarotto S, Chen R, Di Lazzaro V, Farzan F, Ferrarelli F, Fitzgerald PB, Hui J, Ilmoniemi RJ, Kimiskidis VK, Kugiumtzis D, Lioumis P, Pascual-Leone A, Pellicciari MC, Rajji T, Thut G, Zomorrodi R, Ziemann U, Daskalakis ZJ, 2019. Clinical utility and prospective of TMS-EEG. Clin Neurophysiol 130, 802–844. 10.1016/j.clinph.2019.01.001 [DOI] [PubMed] [Google Scholar]
- Tseng BP, Giedzinski E, Izadi A, Suarez T, Lan ML, Tran KK, Acharya MM, Nelson GA, Raber J, Parihar VK, Limoli CL, 2014. Functional consequences of radiation-induced oxidative stress in cultured neural stem cells and the brain exposed to charged particle irradiation. Antioxid. Redox Signal 20, 1410–1422. 10.1089/ars.2012.5134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufail Y, Matyushov A, Baldwin N, Tauchmann ML, Georges J, Yoshihiro A, Tillery SIH, Tyler WJ, 2010. Transcranial Pulsed Ultrasound Stimulates Intact Brain Circuits. Neuron 66, 681–694. 10.1016/j.neuron.2010.05.008 [DOI] [PubMed] [Google Scholar]
- Tupone D, Madden CJ, Morrison SF, 2013. Central Activation of the A1 Adenosine Receptor (A1AR) Induces a Hypothermic, Torpor-Like State in the Rat. J. Neurosci 33, 14512–14525. 10.1523/JNEUROSCI.1980-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AMC, Biswal BB, Castellanos FX, Milham MP, 2009. Functional connectivity of default mode network components: Correlation, anticorrelation, and causality. Human Brain Mapping 30, 625–637. 10.1002/hbm.20531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls-Solé J, Pascual-Leone A, Wassermann EM, Hallett M, 1992. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol 85, 355–364. [DOI] [PubMed] [Google Scholar]
- Van Baarsen B, Ferlazzo F, Ferravante D, Di Nocera F, Jörgensen J, Smit J, Duijn M, Giannini A. maria, Kuipers A, Van Der Pligt J, 2009. Digging into space psychology and isolation: The Mars520 lodgead study: Preliminary results of the Mars105 pilot study. 60th International Astronautical Congress 2009, IAC 2009 1, 76–79. [Google Scholar]
- Vines BW, Cerruti C, Schlaug G, 2008. Dual-hemisphere tDCS facilitates greater improvements for healthy subjects’ non-dominant hand compared to uni-hemisphere stimulation. BMC Neurosci 9, 103. 10.1186/1471-2202-9-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitor-Costa M, Okuno NM, Bortolotti H, Bertollo M, Boggio PS, Fregni F, Altimari LR, 2015. Improving Cycling Performance: Transcranial Direct Current Stimulation Increases Time to Exhaustion in Cycling. PLOS ONE 10, e0144916. 10.1371/journal.pone.0144916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlkolinsky R, Titova E, Krucker T, Chi BB, Staufenbiel M, Nelson GA, Obenaus A, 2010. Exposure to 56Fe-particle radiation accelerates electrophysiological alterations in the hippocampus of APP23 transgenic mice. Radiat. Res 173, 342–352. 10.1667/RR1825.1 [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA, 2007. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc 2, 322–328. 10.1038/nprot.2007.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, 2012. [Research and application of a haptics-based virtual-reality training system for craniomaxillofacial surgery]. Zhonghua Kou Qiang Yi Xue Za Zhi 47, 458–462. 10.3760/cma.j.issn.1002-0098.2012.08.003 [DOI] [PubMed] [Google Scholar]
- Whelan HT, Smits RL, Buchman EV, Whelan NT, Turner SG, Margolis DA, Cevenini V, Stinson H, Ignatius R, Martin T, Cwiklinski J, Philippi AF, Graf WR, Hodgson B, Gould L, Kane M, Chen G, Caviness J, 2001. Effect of NASA light-emitting diode irradiation on wound healing. J Clin Laser Med Surg 19, 305–314. 10.1089/104454701753342758 [DOI] [PubMed] [Google Scholar]
- Williams PS, Hoffman RL, Clark BC, 2013. Preliminary Evidence That Anodal Transcranial Direct Current Stimulation Enhances Time to Task Failure of a Sustained Submaximal Contraction. PLOS ONE 8, e81418. 10.1371/journal.pone.0081418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Kasagi F, Mimori Y, Miyachi T, Ohshita T, Sasaki H, 2009. Incidence of dementia among atomic-bomb survivors — Radiation Effects Research Foundation Adult Health Study. Journal of the Neurological Sciences 281, 11–14. 10.1016/j.jns.2009.03.003 [DOI] [PubMed] [Google Scholar]
- Yeh S-C, Lee S-H, Chan R-C, Chen S, Rizzo A, 2014. A virtual reality system integrated with robot-assisted haptics to simulate pinch-grip task: Motor ingredients for the assessment in chronic stroke. NeuroRehabilitation 35, 435–449. 10.3233/NRE-141134 [DOI] [PubMed] [Google Scholar]
- Zaehle T, Sandmann P, Thorne JD, Jäncke L, Herrmann CS, 2011. Transcranial direct current stimulation of the prefrontal cortex modulates working memory performance: combined behavioural and electrophysiological evidence. BMC Neurosci 12, 2. 10.1186/1471-2202-12-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahiri M, Booton R, Nelson CA, Oleynikov D, Siu K-C, 2018. Virtual Reality Training System for Anytime/Anywhere Acquisition of Surgical Skills: A Pilot Study. Mil Med 183, 86–91. 10.1093/milmed/usx138 [DOI] [PubMed] [Google Scholar]
- Zénon A, Sidibé M, Olivier E, 2015. Disrupting the Supplementary Motor Area Makes Physical Effort Appear Less Effortful. J. Neurosci 35, 8737–8744. 10.1523/JNEUROSCI.3789-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C-J, Liao W-J, Xia W-G, 2015. Effect of combined low-frequency repetitive transcranial magnetic stimulation and virtual reality training on upper limb function in subacute stroke: a double-blind randomized controlled trail. J. Huazhong Univ. Sci. Technol. Med. Sci 35, 248–254. 10.1007/s11596-015-1419-0 [DOI] [PubMed] [Google Scholar]
- Zuo X-N, Kelly C, Adelstein JS, Klein DF, Castellanos FX, Milham MP, 2010. Reliable intrinsic connectivity networks: Test–retest evaluation using ICA and dual regression approach. NeuroImage 49, 2163–2177. 10.1016/j.neuroimage.2009.10.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


