Abstract
Background
Allergy is a global disease with overall frequencies of >20%. Symptoms vary from irritating local itching to life‐threatening systemic anaphylaxis. Even though allergies are allergen‐specific, there is a wide range of cross‐reactivities (eg apple and latex) that remain largely unexplained. Given the abilities of low‐affinity IgG antibodies to inhibit mast cells activation, here we elucidate the minimal affinity of IgE antibodies to induce type I hypersensitivity.
Methods
Three mature (high‐affinity) IgE antibodies recognizing three distinct epitopes on Fel d 1, the major cat allergen, were back‐mutated to germline conformation, resulting in binding to Fel d 1 with low affinity. The ability of these IgE antibodies to activate mast cells in vitro and in vivo was tested.
Results
We demonstrate that affinities as low as 10−7 M are sufficient to activate mast cells in vitro and drive allergic reactions in vivo. Low‐affinity IgE antibodies are able to do so, since they bind allergens bivalently on the surface of mast cells, leading to high‐avidity interactions.
Conclusions
These results suggest that the underlying mechanism of allergen cross‐reactivity may be low‐affinity but high‐avidity binding between IgE antibodies and cross‐reactive allergen.
Keywords: affinity, allergen cross‐reactivity, allergy, avidity, IgE antibody, specificity
Germline IgE antibodies were of low affinity to allergen. Low‐affinity IgE antibodies are able to activate mast cells in vitro and in vivo via high‐avidity binding. Allergen A induces specific high‐affinity IgE antibodies, but with low affinity to allergen B, which could activate mast cells by high‐avidity binding to IgE antibodies. Abbreviations: OD595, optical density 595 nm; PBS, phosphate‐buffered saline.
Abbreviations
- OD595
optical density 595 nm
- PBS
phosphate‐buffered saline
1. INTRODUCTION
Type I allergies are mediated by IgE and have reached epidemic proportions. Indeed, allergic rhino‐conjunctivitis and asthma affect now about one third of the population in developed countries.1, 2 There are two distinct receptors for IgE, the high‐affinity IgE receptor FcεRI and the low‐affinity IgE receptor CD23. The principal IgE receptor for type I allergies is FcεRI, while CD23 is more important for the regulation of IgE production and elimination as well as antigen presentation.3, 4, 5, 6, 7 FcεRI is expressed by a number of cells, including mast cells and basophils. In contrast to CD23 and receptors for IgG, IgE binds to FcεRI with high affinity in free form and stimulates activation of these cells upon cross‐linking by allergens, causing release of mediators (histamines and others) that cause allergic symptoms.8, 9
IgE‐mediated diseases are treated in a number of ways, mostly symptomatically using histamine blockers and steroids. Allergen‐specific immunotherapy (SIT) is the only disease‐modifying therapy and consists of multiple administration of low doses of environmental allergen, resulting in increased tolerance of the allergen upon natural exposure.10, 11 Induction of allergen‐specific IgG and a shift away from type 2 Th cells may be responsible for better allergen tolerance. Even though there is an ongoing discussion about the most critical effector mechanism(s) responsible for this state of enhanced ‘tolerance’, it is clear that induction of allergen‐specific IgG correlates with reduced symptoms and is taken as evidence for successful therapy.12, 13, 14 Furthermore, passive transfer of allergen‐specific IgG antibodies results in protection against allergic reactions both in preclinical and clinical settings.14, 15, 16, 17, 18 In support of this concept, we have recently shown that IgG antibodies with even surprisingly low affinity for the allergen are able to block mast cell activation by engaging the inhibitory FcγRIIb.19 Furthermore, murine models demonstrated that polyclonal18 and monoclonal17 antibodies specific for a single allergen of peanut (Ara h 2) were able to block allergic symptoms mediated by the whole extract.
With the understanding that low‐affinity IgG was sufficient to inhibit allergy, it remained interesting to reveal minimal antibody affinities for mediating IgE‐dependent type I allergy. Previously, it has been reported that increased affinity and clonality of IgE antibodies with different specificities might promote human basophil sensitivity in vitro.20 Here, we systematically analysed the influence of the affinity of IgE antibodies recognizing three distinct epitopes on Fel d 1, the major cat allergen, by back‐mutating high‐affinity, mature antibodies to their germline conformation, resulting in low‐affinity versions of the original antibodies but sharing their specificity. As expected, the germline IgE antibodies recognized Fel d 1 with low affinity (10−7 M). Nevertheless, these low‐affinity antibodies could induce mast cells activation in vitro as well as in vivo. Furthermore, we found that high‐avidity binding of low‐affinity IgE antibodies to allergen was driving allergen recognition and mast cell activation. These results support the hypothesis that high‐avidity but low‐affinity binding of IgE antibody may allow binding to surprisingly unrelated allergens and contributes to cross‐reactivity.
2. METHODS
2.1. Cloning and expressing germline IgE antibodies
Germline IgE antibodies A044 and F127 were accomplished by exchanging the heavy chain constant region of germline IgG (previously described in ref. [19]) from gamma chain to epsilon chain.19 The third antibody G078 was screened when B cells were blocked with A044 and F127 antibodies. The germline G078 configuration was obtained by aligning the G078 variable region sequence with germline genomic database available at http://www.imgt.org. Then, the whole heavy and light chain of germline G078 IgE were synthesized by Integrated DNA technologies (IDT), with addition of NheI and PmeI, AscI and PacI restriction sites in the end, respectively. To clone heavy and light chain of germline G078 IgE into expression plasmid pCB15, the plasmids were digested by NheI and PmeI to assemble heavy chain, followed by digestion of AscI and PacI to ligate light chain to pCB15 with heavy chain. In the end, the Germline‐G078 IgE construct (pCB15‐GermlineG078) contained heavy and light chain under individual pCMV promoter.
To get germline IgE antibodies, the final construct plasmids were transfected into HEK293T cells with polyethylenimine (PEI). Briefly, cells were cultured in complete DMEM media (catalog 11965084; Gibco, Carlsbad, Calif, supplemented with 10% FBS and 1% penicillin and streptomycin) until 70% confluent in T75 flask, when the complete DMEM media were changed to DMEM media (serum free). Plasmid DNA (15 μg) and PEI (45 μl, 1 μg/μl) were incubated at room temperature for 15 min and subsequently dropped into cell culture. The transfection reagents were discarded after 6 h incubation, and fresh complete DMEM media were added to cells. Supernatant media containing germline IgE antibodies were collected 12 h, 48 h, 3 d and 5 d after transfection. IgE antibodies were purified by loading supernatant media to HiTrapTM Protein L column (catalog 17‐5478‐51, GE Healthcare) in Äkta Pure protein purification system (GE Healthcare).
2.2. Binding of germline and mature IgE antibodies to Fel d 1 by ELISA.
Recombinant Fel d 1 was produced as described,12 and monomer was separated from dimer via size exclusion column (HiLoad 26/600 Superdex 75pg, catalog 28‐9893‐34, GE Healthcare). Firstly, Fel d 1 monomer or dimer (1 μg/ml or 0.1 μg/ml) was coated on half‐well ELISA plates at 4°C overnight. Afterwards, germline or mature IgE antibodies were serially diluted in wells and incubated at room temperature for 1 h after plates were blocked with PBS/0.5% casein. Then, the binding of IgE antibodies on plates was detected with horseradish peroxidase conjugated goat anti‐mouse IgE (catalog STAR110P, Bio Rad). Finally, the reaction was developed by TMB substrate and stopped by 1 mol/L sulphuric acid. Plates were read at OD450nm in a standard ELISA reader (BioTek Microplate Readers; BioTek, Winooski, Vt).
The competitive ELISA was performed as described above with some modification according to previous publication21. Plates were coated with 1 µg/ml Fel d 1 dimer overnight at 4˚C. Instead of antibodies alone, mature or germline IgE antibodies (5 µg/ml) were incubated with Fel d 1 (100, 10, 1, 0.1, 0.01, 0 µg/ml) at room temperature for 1h, and then, the immune complex solution was added to plates after blocking. Afterwards, the binding of free IgE antibodies to coated Fel d 1 was determined by adding horseradish peroxidase conjugated goat anti‐mouse IgE. Triplicates were performed in parallel.
2.3. Binding affinity of germline and mature IgG2a antibodies to Fel d 1 using surface plasmon resonance
Affinity constants of germline and mature IgG2a A044 and F127 antibodies were published,19 and the same procedure was performed for IgG2a G078. Shortly, CM5 chip was immobilized with 2500 RU Protein A/G, and then, capture antibody mature IgG2a G078 (50 nM) or germline‐IgG2a G078 (200 nM) was flowed, followed by adding Fel d 1 dimer or monomer. Affinity constants were calculated based on the on‐rate (Ka) and off‐rate (Kd) given by BIA evaluation software.
2.4. Bone marrow derived mast cells (BMMCs) maturation
Bone marrow derived mast cells were created as described previously.19 Basically, tibia and femur of BALB/c mouse were collected and both ends were cut to flush bone marrow cells out. Then, cells were incubated in ACK buffer (0.15M NH4Cl, 0.01M KHCO3) on ice for 5 min to lyse red blood cells, after which RPMI 1640 medium was added to stop the reaction. Eventually, bone marrow cells were seeded at 5 × 105 cells/ml in complete BMMC medium, which consisted of RPMI 1640 medium supplemented with 10% FBS, 1 mM sodium pyruvate, 30 ng/ml recombinant murine IL‐3 (catalog 213‐13, PEPROTECH), 50ng/ml recombinant murine SCF (catalog 250‐03, PEPROTECH), 2.5 μg/ml Amphotericin B and 1mM Pen/Strep), and cultured for 4 weeks, during which fresh complete BMMC medium was added. The maturation was defined by expression of c‐kit (CD117) and FcεRI using flow cytometry.
2.5. Labelling Fel d 1 with Alexa Fluor 647 (AF647).
To test the binding of Fel d 1 to IgE bound on BMMCs, Fel d 1 protein was labelled with fluorophore Alexa Fluor 647 (catalog A20006, Invitrogen) as manual. Simply, reconstituted Alexa Fluor 647 dye was mixed with Fel d 1 and incubated at room temperature, 450 rpm shaking for 1 h. Then, the excess dye was removed by passing reaction mixture through the Zeba Spin Desalting column (catalog 89882, Thermo Scientific). Since the recombinant Fel d 1 tends to form dimer, AF647‐labelled Fel d 1 monomer was separated by HiLoad 26/600 Superdex 75pg column (catalog GE28‐9893‐34, GE Healthcare) from dimer.
2.6. Binding and activation of BMMCs mediated by Fel d 1 bound on IgE antibodies
2.6.1. Binding and activation assays by Fel d 1 dimer
Mature BMMCs were used to investigate the binding of Fel d 1 to mature or germline IgE antibodies in vitro. Essentially, 10,000 BMMCs were incubated with 1 μg/ml germline or mature IgE antibodies overnight at 37°C, 5% CO2. Then, the unbound antibodies were washed away with FACS buffer (PBS supplemented with 2% FBS), followed by incubating cells in complete BMMC medium containing AF647‐labelled Feld 1 dimer (10,000, 1000, 100, 10, 1, 0.1, 0.01 ng/ml) at 37°C for 30 min. Lastly, cells were stained with anti‐mouse CD63‐VioBright FITC (catalog 130‐108‐927, Miltenyi Biotec) and analysed by flow cytometry (Guava easyCyte Flow Cytometer, Merck Millpore). The mean fluorescence intensity (MFI) in APC channel represented binding of Fel d 1 and in FITC channel stood for activation status of BMMCs.
2.6.2. Mobility assays
IgE antibody bound on cell surface could aggregate together concerning the mobility of cell membrane to interact upcoming Fel d 1 in avidity manner. Therefore, BMMCs were incubated with Fel d 1 on ice (4°C) or 37°C for 30 min after germline IgE antibody binding overnight at 37°C. The binding of Fel d 1 on frozen cell membrane was measured as described above, and the activation was tested after transferring cells back to 37°C.
2.6.3. Binding and activation assays by Fel d 1 monomer
To explore the role of receptor crosslink mediated by germline IgE antibody on mast cell activation, Fel d 1 monomer (1000 ng/ml) was applied to BMMCs, which were incubated with single or two different germline IgE antibodies (1μg/ml) overnight beforehand. Similarly, the binding and activation were tested as above.
2.7. Image stream
To illustrate the binding of IgE antibody and Fel d 1 on cells, BMMCs were captured by Image Stream‐X MK II (Amnis) after treated as described in 2.6.1, namely, AF647‐labelled Fel d 1 dimer (1000 ng/ml) was added to cells after mature or germline IgE incubation. Then, cells were stained with anti‐mouse FcεRI PE (catalog 12‐5898‐82, Invitrogen), followed by analysing cells (40 × amplification) in PE and APC channels according to manual.
2.8. Ear prick test
BALB/cOlaHsd female mice were purchased from Envigo (Horst, The Netherlands) as 7 weeks old and kept in specific pathogen‐free BLS2 animal facility according to Cantonal Veterinary guidelines, and all experiments were performed in accordance with ethical principles and guidelines of the Cantonal Veterinary Office Bern, Switzerland. Mice (8–12 weeks) were injected intravenously with 20 μg mature or germline IgE antibody (A044, F127, and G078), and 200 μl Evans Blue dye (0.5% PBS) was administrated in the same way after 24 h. Then, one drop of Fel d 1 dimer (200 μg/ml) was located on the pricked ear after the mice were anaesthetized. The allergic reaction was quantified by the amount of Evans Blue congregated to the pricked ear. Briefly, mice were sacrificed 45 min after Fel d 1 drop application and pricked ear was cut and digested in 150 μl KOH (1 mol/L) overnight at 37°C, 450 rpm shaking. Then, the dye was extracted in 150 μl 5 % H3PO4 in acetone, followed by 15,000 g, 10 min centrifuge. Finally, the supernatant was read at OD595nm. For mature and germline G078 and PBS groups, 5 mice were analysed, 4 mice in mature A044 group and 3 mice in mature and germline F127 groups and in germline A044 group.
For dose‐response assays, BALB/c mice were sensitized with 20 µg mature or germline IgE antibodies by intravenous injection. Mouse was pricked with in one ear with 200 µg/ml Fel d 1 drop and in another with 63 µg/ml, 20 µg/ml or 6.7 µg/ml after Evans Blue application. Alternatively, area of the spot was measured to reflect the severity of allergic reaction (Figure S1). Five mice per group were checked and plotted except in following groups: mature F127 200 µg/ml and 20 µg/ml, germline F127 20 µg/ml, and mature G078 200 µg/ml and 20 µg/ml where less mice were plotted because of failure of i.v. injection.
Regarding the desensitization assay, mice were firstly intravenously injected with or without (control group) 200 μg germline F127 IgE or 200 μg anti‐Ara h 2 IgE (peanut allergen, non‐specific, control antibody), 5 h after mice were sensitized with 20 μg mature F127 IgE antibody and challenged on ears with 6.7 μg/ml Fel d 1 24 h later.
2.9. Passive systemic anaphylaxis
BALB/c mice (8–12 weeks) were sensitized with 20 μg mature or germline IgE antibody (A044, F127 and G078) as well as PBS by intravenous injection. Twenty‐four hours later, mice were challenged intravenously with 20 μg Fel d 1 dimer, followed by body core temperature measurement every 10 min. Mice were euthanized if body temperature dropped below 32°C. Results for 5 mice per group were plotted, except 6 mice in the PBS group.
2.10. Statistical analysis
The significance analysis was performed in GraphPad PRISM 6.0 (GraphPad Software, Inc. La Jolla, CA, USA). And p value from unpaired t test was indicated as ≤0.05 (*), ≤0.01 (**), ≤0.001 (***), ≤0.0001 (****). All error bars were displayed as mean ± SEM.
3. RESULTS
3.1. Germline IgE antibodies bind to Fel d 1 with low affinity
We have previously generated a set of 3 monoclonal antibodies recognizing 3 distinct epitopes of Fel d 1.12 More recently, we back‐mutated 2 of these antibodies to germline, resulting in low‐affinity germline antibodies of the same epitope specificity.19 Here, we performed the same procedure for the third antibody (Table 1), resulting in an additional low‐affinity IgG antibody (Table 2) and expressed all 6 high‐affinity and low‐affinity antibodies in an IgE format. These germline antibodies bound monomeric and dimeric Fel d 1 with an affinity in the range of 10−7 M, while the mature antibodies showed affinities in the range 10−9 to 10−10 M for both monomeric and dimeric Fel d 1 when measured by surface plasmon resonance (Biacore, Cytiva, GE Healthcare) (Table 2).
TABLE 1.
| Germline, light chain | Mutations in light chain | Germline, heavy chain | Mutations in heavy chain | Total mutations | |||
|---|---|---|---|---|---|---|---|
| FR | CDR | FR | CDR | ||||
| A044a | V: IGKV9‐120*01 | 8 | V: IGHV1‐7*01 | 14 | 22 | ||
| J: IGKJ2*01 | J: IGHJ2*01 | ||||||
| 6 | 2 | D: IGHD1‐1*01 | 9 | 5 | |||
| F127a | V: IGKV3‐4*01 | 7 | V: IGHV1‐82*01F | 22 | 29 | ||
| J: IGKJ4*01 | J: IGHJ3*01F | ||||||
| 6 | 1 | D: IGHD2‐2*01F | 19 | 3 | |||
| G078 | V: IGKV6‐25*01F | 19 | V: IGHV3‐6*01F | 17 | 36 | ||
| J: IGKJ2*01F | J: IGHJ3*01F | 1 | |||||
| 14 | 5 | D: IGHD1‐1*02F | 13 | 3 | |||
Mutations were published.19
TABLE 2.
Affinity of mature and germline IgG2a antibodies to Fel d 1
| Antibodies | Ka (M−1 s−1) | Kd (s−1) | KD (M) |
|---|---|---|---|
| Fel d 1 monomer | |||
| Mature IgG2a G078 | 5.7 × 105 | 3.1 × 10−4 | 5.5 × 10−10 |
| Germline IgG2a G078 | 1.7 × 104 | 6.3 × 10−3 | 3.6 × 10−7 |
| Mature IgG2a A044a | 2.3 × 105 | 9.8 × 10−4 | 4.3 × 10−9 |
| Germline IgG2a A044a | ND | ND | 2.88 × 10−7 |
| Mature IgG2a F127a | 3.0 × 105 | 3.9 × 10−4 | 1.3 × 10−9 |
| Germline IgG2a F127a | 9.1 × 103 | 7.9 × 10−3 | 8.6 × 10−7 |
| Fel d 1 dimer | |||
| Mature IgG2a G078 | 1.1 × 106 | 1.7 × 10−4 | 1.5 × 10−10 |
| Germline IgG2a G078 | 1.3 × 105 | 9.5 × 10−3 | 7.4 × 10−8 |
| Mature IgG2a A044a | 3.5 × 105 | 3.8 × 10−4 | 1.1 × 10−9 |
| Germline IgG2a A044a | ND | ND | 2.8 × 10−7 |
| Mature IgG2a F127a | 3.7 × 105 | 2.9 × 10−4 | 7.8 × 10−10 |
| Germline IgG2a F127a | 1.8 × 104 | 3.7 × 10−3 | 2.0 × 10−7 |
Kinetic constants were published.19
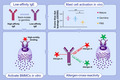
In ELISA experiments, however, the germline antibodies bound Fel d 1 almost as well as the high‐affinity mature antibodies when 1 μg/ml dimeric or monomeric Fel d 1 was coated (Figure 1A,B). Antibodies often bind to densely coated plastic‐bound antigens in a bivalent fashion, allowing high‐avidity interactions despite low affinity. Lowering coating densities usually prevents bivalent binding, and ELISA responses better reflect antibody affinities. Indeed, lowering the coating density by 10‐fold essentially abrogated binding of the low‐affinity germline antibodies while binding of the high‐affinity antibodies was not affected (Figure 1C,D). Interestingly, the binding of the germline antibodies was equally low for the Fel d 1 monomer as for the dimer, indicating that antibodies bind monovalently to the dimer. Consistent with the Biacore data, these results together elucidated the germline antibodies have low affinity for Fel d 1.
FIGURE 1.
Binding of mature and germline IgE antibodies to Fel d 1 by ELISA. Plates were coated with 1 μg/ml Fel d 1 dimer (A), 1 μg/ml Fel d 1 dimer (B), 0.1 μg/ml Fel d 1 dimer (C) and 0.1 μg/ml Fel d 1 monomer (D). Competitive Fel d 1‐IgE binding assay. The IC50 corresponds to the concentration of Fel d 1 dimer at which 50% inhibition of IgE binding to immobilized Fel d 1 is observed (E)
To further elucidate the binding capacity, competition ELISAs were performed which confirmed a higher avidity to Fel d 1 than their affinity would indicate. Even though avidity binding was roughly 10‐time lower than avidity binding of mature antibodies, the avidity binding of germline antibodies was 100‐times higher than their affinity binding, confirming the high‐avidity binding of germline antibodies in the 10−9 M range (Figure 1E).
3.2. Fel d 1 interacts bivalently with low‐affinity IgE antibodies on mast cell surfaces, resulting in high‐avidity binding
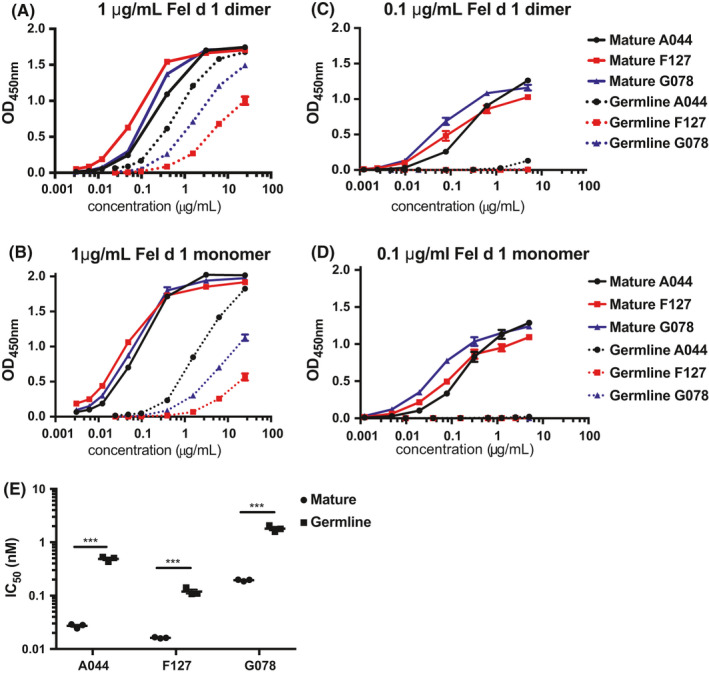
In comparison with plastic surfaces, the situation may be different for cell surface bound IgE, where the receptor‐bound IgE molecules can move laterally and two antibodies may bind dimeric Fel d 1 simultaneously, stabilizing allergen binding. Indeed, dimeric Fel d 1 bound very well to low‐affinity germline antibodies bound to FcεRI on mast cells (Figure 2A,E). In contrast, monomeric Fel d 1, which cannot bind simultaneously to two IgE molecules of the same specificity, may not be able to stabilize the binding (Figure 2C). Indeed, monomeric Fel d 1 failed to efficiently bind to mast cells pulsed with single low‐affinity germline IgE. In contrast, monomeric Fel d 1 bound well to high‐affinity antibodies on mast cells. Furthermore, stabilization of monomeric Fel d 1 binding may be possible for mast cells pulsed with 2 different low‐affinity germline IgE antibodies. Indeed, monomeric Fel d 1 binds well under these conditions (Figure 2D). In addition, Image Stream analysis demonstrates good co‐localization between germline IgE bound to mast cells and cross‐linked with dimeric Fel d 1, indicating that dimeric Fel d 1 binds to germline IgE molecules and cross‐links them on cell surfaces (Figure 2E).
FIGURE 2.
Binding of mature and germline IgE antibodies to Fel d 1 in vitro. Mean fluorescent intensities of Fel d 1‐AF647 were analysed after BMMCs were incubated with IgE antibodies overnight and followed by 10,000, 1000, 100, 10, 1, 0.1 and 0.01 ng/ml Fel d 1 dimer (A); the same measurement was performed to determine the binding of Fel d 1 dimer to germline IgE antibodies bound on BMMCs surface at 4°C and 37°C (B); the binding was checked as with 1000 ng/ml Fel d 1 dimer or monomer added to BMMCs after germline IgE antibodies overnight incubation (C); binding of Fel d 1 monomer on BMMCs with combinations of different germline IgE antibodies overnight was shown in (D); (E) Image stream analysis was used to illustrate the binding and co‐localization of Fel d 1 dimer (1000 ng/ml) with IgE on surface of BMMCs
Stabilization of Fel d 1 requires lateral movement of IgE molecules in the cell membrane. This is why we preformed the experiments at 37°C. Membranes freeze at temperatures below the Krafft temperature22 and no lateral movement is possible under these conditions. To explore the binding of Fel d 1 dimer to germline IgE bound on cell surface without lateral mobility, we performed in vitro binding assay at 4°C and for control purpose as well at 37°C. Indeed, dimeric Fel d 1 failed to bind to cell bound low‐affinity germline IgE at 4°C (Figure 2B), further confirming that bivalent binding was essential, as exemplified in Figure 3E.
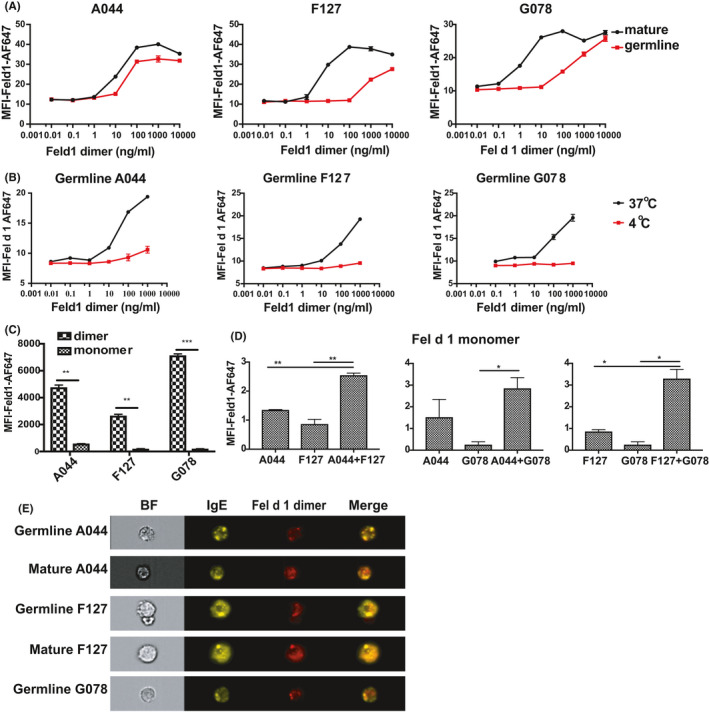
FIGURE 3.
Activation of BMMCs after Fel d 1 binding to mature and germline IgE antibodies in vitro. Mean fluorescent intensities of anti‐CD63‐FITC were analysed after BMMCs were incubated with IgE antibodies overnight and followed by 10000, 1000, 100, 10, 1, 0.1 and 0.01 ng/ml Fel d 1 dimer (A); the same measurement was performed to determine the activation of BMMCs after germline IgE incubation at 4°C and 37°C (B); (C) illustrates the activation of BMMCs by Fel d 1 dimer and monomer (1000 ng/ml) after mature and germline IgE antibodies incubation overnight; activation of BMMCs upon Fel d 1 monomer (1000 ng/ml) binding to different germline IgE antibody combinations overnight was shown in (D); (E) illustrates the binding pattern of Fel d 1 dimer to germline IgE antibodies with and without lateral movements; (F) summarizes different Fel d 1 molecules binding to germline IgE antibodies on cell surface and the activation status
3.3. Low‐affinity IgE antibodies were capable to activate mast cells upon stimulation with dimeric fel d 1 in vitro
Having demonstrated that Fel d 1 can bind to mast cells via low‐affinity IgE antibodies at 37°C, we next wanted to assess whether the allergen could activate mast cells in the form of dimer and monomer. Activation of mast cells mirrored exactly the binding of Fel d (Figure 3A, F). Indeed, dimeric Fel d 1 efficiently activated mast cells loaded with single low‐affinity germline antibodies while monomeric Fel d 1 failed to do so (Figure 3C, D, F). In contrast, however, monomeric Fel d 1 was similarly efficient at activating mast cells pulsed with 2 different low‐affinity germline IgEs again reflecting allergen binding (Figure 3D, F). In line with this, mast cells loaded with germline IgE antibodies and pulsed with dimeric Fel d 1 at 4°C failed to be activated when transferred to 37°C (Figure 3B). Thus, high‐avidity binding of Fel d 1 to low‐affinity antibodies tightly correlated with mast cell activation.
3.4. Low‐affinity IgE antibodies induce allergic reactions in vivo
Finally, we assessed whether low‐affinity IgE antibodies may be able to cause allergic reaction in vivo. To this end, we sensitized mice with either mature or germline IgE antibodies for 24 hours and then monitored local and systemic allergic reactions. The findings obtained in vitro translated to the in vivo situation, as mice primed with low‐affinity germline antibodies reacted with local allergic reactions (skin prick test) in a similar manner as mice primed with high‐affinity IgE antibodies if challenged with dimeric Fel d 1 (Figure 4A). This demonstrated that low‐affinity germline IgE antibodies are functionally active in vivo.
FIGURE 4.
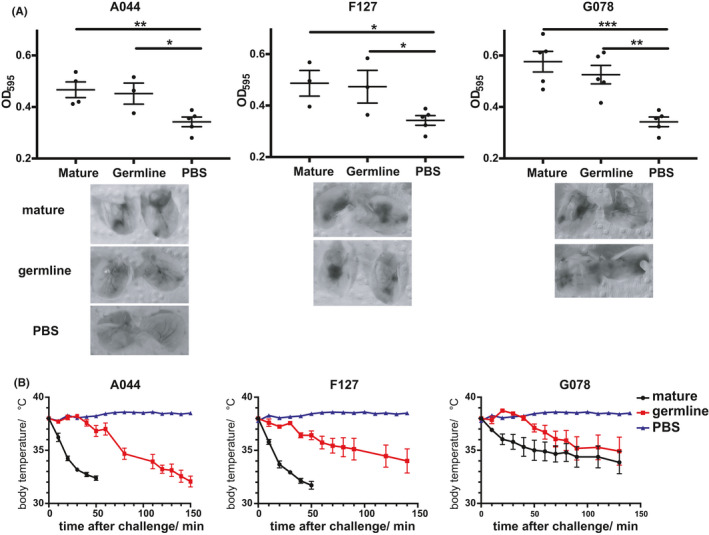
Allergic reactions induced by mature and germline IgE antibodies in vivo. Local allergic reaction (A) quantification of Evans blue dye extracted from ears by OD595 nm indicates the severity of allergy; systemic allergy (B) core body temperature changes were displayed with mature and germline IgE antibodies (A044, F127 and G078) sensitization
Systemic allergic reaction was recorded by means of body core temperature changes after Fel d 1 challenge (Figure 4B). Overall, germline IgE antibodies caused anaphylaxis in a manner similar to their mature counterparts, verifying the low‐affinity IgE promoted allergic reaction in vivo. Interestingly, the systemic reaction raised by germline IgE antibodies was delayed compared to mature antibodies, which may reflect a relatively mild allergic reaction.
To determine the allergen dose which induces allergy by mature but not by germline IgE antibody, different allergen concentrations were examined in sensitized BALB/c mice (Figure S1A). Mice sensitized with either mature or germline F127 IgE showed similar allergic reactions when 200 µg/ml and 63 µg/ml Fel d 1 were applied. However, when lower doses of Fel d 1 ranging from 20 to 6.7 µg/ml were used for pricking, the germline F127 IgE sensitized mice showed significantly less allergic reaction than with the corresponding allergen concentrations in the groups sensitized with mature F127. The same experiments were also performed for A044 and G078 IgE antibodies with 200 and 20 µg/ml of Fel d 1, which resulted in similar reduced allergic reactions for germline IgE antibodies at a concentration of 20 µg/ml of Fel d 1.
To test the hypothesis that low‐affinity IgE antibodies could block high‐affinity antibodies, we assessed the ability of germline IgE antibody to desensitize allergic mice.23 As shown in Figure S1B, germline IgE antibody significantly reduced the allergic reaction in mice which were sensitized with mature F127 IgE, suggesting its potential to block the allergic function of mature IgE. To rule out that desensitization might occur through blocking of non‐specific IgE binding, unrelated IgE (anti‐Ara h 2, peanut allergen) was used as a control, which exhibited similar blocking function in mature F127 IgE sensitized mice (Figure S1C). In conclusion, germline IgE antibody could not desensitize mice by means of antagonism.
4. DISCUSSION
It is well accepted that IgE plays a critical part in type I hypersensitivity. However, the role of IgE affinity for specific allergen has never been clearly addressed. Because polyclonal IgE antibodies with variable affinity may preclude a systematic investigation, we utilized monoclonal antibodies recognizing distinct epitopes on Fel d 1 and engineered them back to germline configuration, resulting in low‐affinity antibodies binding to the exact same epitopes on Fel d 1 as the corresponding mature ones. A set of complementary binding assays elucidated that low‐affinity antibodies may still bind well to allergens due to bivalent interactions, resulting in high‐avidity interactions. Thus, based on these high‐avidity interactions, low‐affinity IgE antibodies cross‐linked by Fel d 1 resulted in allergic responses both in vitro and in vivo.
The capacity of low‐affinity IgE to activate mast cells in vitro and in vivo may offer a simple explanation for unexpected cross‐reactivities seen in clinical allergology. Indeed, we show here (cross‐reactive) affinities as little as 10−7 M are sufficient to trigger type I allergic reactions. It is noteworthy that antibody binding with an affinity of 10−7 M may not even be measurable, in particular if allergen extracts are to be coated in ELISA experiments, as individual allergens tend to be of limited concentration within extracts (forcing monovalent binding of potentially cross‐reactive antibodies). As shown in Figure 1, such low coating densities may result in failure of the low‐affinity antibodies to recognize the antigen. Similar to cross‐reactivities found in clinic, low‐affinity IgE antibodies are able to cause allergic atopy with cross‐reactive allergen even undetectable in skin prick test (SPT).24
Graphical abstract combines the current findings and extrapolates our insights for low‐affinity antibodies to a defined original antigen (Fel d 1) to potential low‐affinity antibodies against cross‐reactive allergen. The figure indicates that cross‐reactive IgE antibody bind to the allergy‐inducing allergen with high affinity and only with low affinity to cross‐reactive allergen. Upon exposure to the cross‐reactive allergen, low‐affinity IgE antibodies will be stabilized on the mast cell surface by the cross‐reactive allergen and consequently may activate mast cells despite low affinity.
Taken together, we demonstrate here that IgE antibodies with low affinity can bind allergens with high avidity by interacting with multiple epitopes on the allergen. Thus, avidity rather than affinity drives IgE‐mediated allergic reactions for low‐affinity IgE antibodies. These findings may offer an explanation for the unexpected cross‐reactivity between different allergens. Therefore, allergen cross‐reactivity may be driven by low affinity but high‐avidity interaction.
CONFLICT OF INTEREST
M. F. Bachmann declares to be involved in several companies developing vaccines for allergic diseases. The other authors declare no further conflict of interests.
AUTHOR CONTRIBUTIONS
Xinyue Chang and Alexandra Wallimann conducted the assays. Pascal Krenger and Xuelan Liu helped with data analysis. Mona Mohsen was involved in drawing diagrams. Monique Vogel, Lisha Zha and Martin Bachmann contributed to designing the work. Xinyue Chang, Monique Vogel, Lisha Zha and Martin Bachmann participated in manuscript writing and revising.
Supporting information
Supplementary Material
Fig S1
Fig S2
ACKNOWLEDGEMENTS
We thank Marianne Zwicker for helping germline IgE antibodies purification and Franziska Thoms for kindly providing mature IgE antibodies. We are truly grateful to Dr. Denis Grandgirard for all the kind help with Bio‐Plex Pro assay.
Chang X, Zha L, Wallimann A, et al. Low‐affinity but high‐avidity interactions may offer an explanation for IgE‐mediated allergen cross‐reactivity. Allergy. 2021;76:2565–2574. 10.1111/all.14864
Funding information
This work is supported by Swiss National Foundation grant to Martin Bachmann (SNF Nr. 310030 185114), to Monique Vogel (SNF Nr. 310030 179165), and a PhD fellowship from China Scholarship Council (CSC Nr. 201706740091, to Xinyue Chang)
Contributor Information
Lisha Zha, Email: zhalisha@ahau.edu.cn.
Martin F. Bachmann, Email: martin.bachmann@me.com.
REFERENCES
- 1.Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol. 2008;8:205‐217. [DOI] [PubMed] [Google Scholar]
- 2.Upton MN, McConnachie A, McSharry C, et al. Intergenerational 20 year trends in the prevalence of asthma and hay fever in adults: the Midspan family study surveys of parents and offspring. BMJ 2000;321:88‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acharya M, Borland G, Edkins AL, et al. CD23/FcεRII: molecular multi‐tasking. Clin Exp Immunol. 2010;162:12‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conrad DH, Ford JW, Sturgill JL, Gibb DR. CD23: an overlooked regulator of allergic disease. Curr Allergy Asthma Rep. 2007;7:331‐337. [DOI] [PubMed] [Google Scholar]
- 5.Fellmann M, Buschor P, Röthlisberger S, Zellweger F, Vogel M. High affinity targeting of CD23 inhibits IgE synthesis in human B cells. Immun Inflamm Dis. 2015;3:339‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balbino B, Conde E, Marichal T, Starkl P, Reber LL. Approaches to target IgE antibodies in allergic diseases. Pharmacol Ther. 2018;191:50‐64. [DOI] [PubMed] [Google Scholar]
- 7.Yu P, Kosco‐Vilbois M, Richards M, Köhler G, Lamers MC. Negative feedback regulation of IgE synthesis by murine CD23. Nature 1994;369:753‐756. [DOI] [PubMed] [Google Scholar]
- 8.Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18:693‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metcalfe DD, Peavy RD, Gilfillan AM. Mechanisms of mast cell signaling in anaphylaxis. J Allergy Clin Immunol. 2009;124:639‐646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larché M, Akdis CA, Valenta R. Immunological mechanisms of allergen‐specific immunotherapy. Nat Rev Immunol. 2006;6:761‐771. [DOI] [PubMed] [Google Scholar]
- 11.Konradsen J, Arvidsson M. Allergen‐specific immunotherapy provides long‐lasting symptom relief. Lakartidningen 2016;113:DW74. [PubMed] [Google Scholar]
- 12.Uermösi C, Beerli RR, Bauer M, et al. Mechanisms of allergen‐specific desensitization. J Allergy Clin Immunol. 2010;126:375‐383. [DOI] [PubMed] [Google Scholar]
- 13.Yukselen A, Kendirli SG. Role of immunotherapy in the treatment of allergic asthma. World J Clin Cases. 2014;2:859‐865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strait RT, Morris SC, Finkelman FD. IgG‐blocking antibodies inhibit IgE‐mediated anaphylaxis in vivo through both antigen interception and Fc gamma RIIb cross‐linking. J Clin Invest. 2006;116:833‐841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uermösi C, Zabel F, Manolova V, et al. IgG‐mediated down‐regulation of IgE bound to mast cells: a potential novel mechanism of allergen‐specific desensitization. Allergy 2014;69:338‐347. [DOI] [PubMed] [Google Scholar]
- 16.Orengo JM, Radin AR, Kamat V, et al. Treating cat allergy with monoclonal IgG antibodies that bind allergen and prevent IgE engagement. Nat Commun. 2018;9:1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Storni F, Cabral‐Miranda G, Roesti E, et al. A single monoclonal antibody against the peanut allergen Ara h 2 protects against systemic and local peanut allergy. Int Arch Allergy Immunol. 2020;181:334‐341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Storni F, Zeltins A, Balke I, et al. Vaccine against peanut allergy based on engineered virus‐like particles displaying single major peanut allergens. J Allergy Clin Immunol. 2020;145:1240‐1253. [DOI] [PubMed] [Google Scholar]
- 19.Zha L, Leoratti FMS, He L, et al. An unexpected protective role of low‐affinity allergen‐specific IgG through the inhibitory receptor FcγRIIb. J Allergy Clin Immunol. 2018;142:1529‐1536. [DOI] [PubMed] [Google Scholar]
- 20.Christensen LH, Holm J, Lund G, Riise E, Lund K. Several distinct properties of the IgE repertoire determine effector cell degranulation in response to allergen challenge. J Allergy Clin Immunol. 2008;122:298‐304. [DOI] [PubMed] [Google Scholar]
- 21.Irahara M, Shinahara W, Sugimoto M, et al. Trajectories of class‐switching‐related egg and cow's milk allergen‐specific immunoglobulin isotype formation and its modification by eczema with low‐ and high‐affinity immunoglobulin E during early infancy. Immun Inflamm Dis. 2019;7:74‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kandušer M, Šentjurc M, Miklavčič D. The temperature effect during pulse application on cell membrane fluidity and permeabilization. Bioelectrochemistry 2008;74:52‐57. [DOI] [PubMed] [Google Scholar]
- 23.Torigoe C, Inman JK, Metzger H. An unusual mechanism for ligand antagonism. Science 1998;281:568‐572. [DOI] [PubMed] [Google Scholar]
- 24.Pierson‐Mullany LK, Jackola DR, Blumenthal MN, Rosenberg A. Evidence of an affinity threshold for IgE‐allergen binding in the percutaneous skin test reaction. Clin Exp Allergy. 2002;32:107‐116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Fig S1
Fig S2


