Summary
In Europe, swine represent economically important farm animals and furthermore have become a preferred preclinical large animal model for biomedical studies, transplantation and regenerative medicine research. The need for typing of the swine leukocyte antigen (SLA) is increasing with the expanded use of pigs as models for human diseases and organ‐transplantation experiments and their use in infection studies and for design of veterinary vaccines. In this study, we characterised the SLA class I (SLA‐1, SLA‐2, SLA‐3) and class II (DRB1, DQB1, DQA) genes of 549 farmed pigs representing nine commercial pig lines by low‐resolution (Lr) SLA haplotyping. In total, 50 class I and 37 class II haplotypes were identified in the studied cohort. The most common SLA class I haplotypes Lr‐04.0 (SLA‐1*04XX‐SLA‐3*04XX(04:04)‐SLA‐2*04XX) and Lr‐32.0 (SLA‐1*07XX‐SLA‐3*04XX(04:04)‐SLA‐2*02XX) occurred at frequencies of 11.02 and 8.20% respectively. For SLA class II, the most prevalent haplotypes Lr‐0.15b (DRB1*04XX(04:05/04:06)‐DQB1*02XX(02:02)‐DQA*02XX) and Lr‐0.12 (DRB1*06XX‐DQB1*07XX‐DQA*01XX) occurred at frequencies of 14.37 and 12.46% respectively. Meanwhile, our laboratory has contributed to several vaccine correlation studies (e.g. Porcine Reproductive and Respiratory Syndrome Virus, Classical Swine Fever Virus, Foot‐and‐Mouth Disease Virus and Swine Influenza A Virus) elucidating the immunodominance in the T‐cell response with antigen specificity dependent on certain SLA‐I and SLA‐II haplotypes. Moreover, these SLA–immune response correlations could facilitate tailored vaccine development, as SLA‐I Lr‐04.0 and Lr‐32.0 as well as SLA‐II Lr‐0.15b and Lr‐0.12 are highly abundant haplotypes in European farmed pigs.
Keywords: polymorphism, sequence‐specific primers PCR, Sus scrofa, swine leukocyte antigen
The porcine major histocompatibility complex (MHC) harbours the highly polymorphic swine leukocyte antigen (SLA) class I and II gene clusters encoding glycoproteins which present antigenic peptides to T cells that are required to stimulate the adaptive immune response (Lunney et al. 2009; Hammer et al. 2020; Kamal et al. 2020). As pathogen effects on SLA gene expression drive swine immune responses, the SLA complex plays a key role for swine models in biomedical research (reviewed in Hammer et al. 2020). Associations of SLA class I and/or class II genes or haplotypes with differences in swine vaccine and disease responses are well documented (reviewed in Lunney et al. 2009). In vaccine research, either genetically defined pig lines (e.g., Babraham pigs) or outbred pig lines are used (Tungatt et al. 2018; De León et al. 2020). As well as using SLA‐typed animals in vaccine research, pigs are often used to develop disease models and for basic research studying allogeneic and xenogeneic transplantation (reviewed in Ladowski et al. 2019; Hammer et al. 2020; Ladowski et al. 2021). To understand and control SLA complexity, mainly miniature swine models are used to establish SLA‐inbred/‐defined pig lines (reviewed in Hammer et al. 2020; Ladowski et al. 2021). In contrast, in vascularised composite allograft transplantation or for end‐stage renal disease, porcine transplantation models have been established with SLA‐mismatched outbred pigs (I. Arenas Hoyos et al. and M. Jensen‐Waern et al. unpublished data).
Here we propose two underlying rationales for conducting SLA haplotyping‐assisted animal trials in vaccine and transplantation research: (i) SLA typing of the resource population enables directed mating of founder animals based on their SLA‐background (Fig. S1): and (ii) the designation of SLA‐defined study groups achieves an experimental advantage of pre‐selecting animals expressing certain SLA phenotypes and thus enhancing the understanding of experimental outcomes (Fig. S1). As a prerequisite for transplantation and vaccine research, our laboratory provides information about the MHC background usingy high‐throughput low‐resolution (Lr) SLA haplotyping in swine specifying SLA gene‐specific allele groups (reviewed in Hammer et al. 2020).
We have contributed to several correlation studies addressing vaccine design against Porcine Reproductive and Respiratory Syndrome Virus (PRRSV), Classical Swine Fever Virus, Foot‐and‐Mouth Disease Virus (FMDV) and Swine Influenza A Virus (FLUAVsw) by SLA haplotyping outbred pigs. Furthermore, our laboratory is involved in studies with minipigs for various purposes in transplantation research (Fig. 1, Table S1). In this study, we present comprehensive data about SLA alleles and low‐resolution haplotypes and their prevalence in nine commercial European pig populations.
Figure 1.
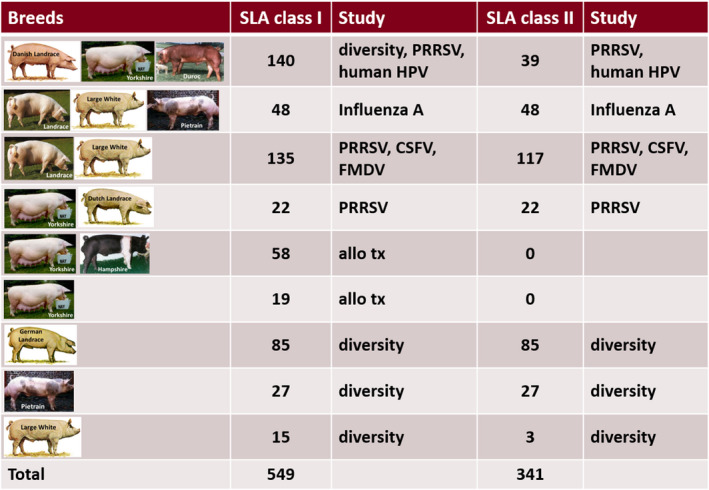
List of European farmed pigs incorporated in the present study. CSFV, Classical Swine Fever Virus; FMDV, Foot‐and‐Mouth Disease Virus; HPV, Human Papilloma Virus; PRRSV, Porcine Respiratory and Reproductive Syndrome Virus; tx, transplantation.
A total of 549 farmed pigs (Fig. 1, Table S1) representing nine commercial pig lines were genotyped for their SLA class I and II haplotypes by running low‐resolution PCR screening assays on Peripheral blood mononuclear cell (PBMC)‐ or whole blood‐derived genomic DNA. Therefore, genomic DNA was isolated from 5 × 106 porcine PBMCs or 200 µl whole blood using commercial kits following the manufacturer’s instructions (DNeasy Blood and Tissue Kit, Qiagen; E.Z.N.A.® Blood and Tissue DNA Kit, Omega Bio‐tek, Inc.). SLA class I (SLA‐I) and SLA class II (SLA‐II) low‐resolution haplotypes (Lr‐Hp) were identified by a PCR‐based typing assay to define the animals’ MHC backgrounds on the allele‐group level. SLA typing was performed by PCR with the complete set of typing primers specific for the allele groups of three SLA‐I loci (SLA‐1, SLA‐2 and SLA‐3) and three SLA‐II loci (DRB1, DQB1 and DQA) (Table S2; Ho et al. 2009a, 2010; Essler et al. 2013; Gimsa et al. 2017). The criteria and nomenclature used for SLA‐I and SLA‐II haplotyping were based on those proposed by the SLA Nomenclature Committee (Ho et al. 2009b and reviewed in Hammer et al. 2020). Interpretation of the results was deduced from the presence of allele‐specific PCR products of the expected size in each lane. Low‐resolution SLA‐I and ‐II haplotypes were assigned based on comparison with previously published haplotypes (Ho et al. 2009a, 2010; Gao et al. 2017, reviewed in Hammer et al. 2020) and unpublished breed‐ or farm‐specific haplotypes (C.‐S. Ho et al. unpublished data).
The studied cohort of 549 farmed pigs representing nine commercial pig lines comprised 50 SLA‐I Lr‐Hp, including three potential novel allele‐group combinations (Lr‐01.0/04.0, Lr‐V.0, Lr‐Y1.0) (Table 1). Eight haplotypes (Lr‐04.0, Lr‐32.0, Lr‐22.0, Lr‐01.0, Lr‐59.0, Lr‐24.0, Lr‐37.0 and Lr‐43.0) explained 51.37% of the SLA‐I diversity (Figs S2a & S3a). The two most abundant SLA‐I haplotypes – Lr‐04.0 (SLA‐1*04XX‐SLA‐3*04XX(04:04)‐SLA‐2*04XX) and Lr‐32.0 (SLA‐1*07XX‐SLA‐3*04XX(04:04)‐SLA‐2*02XX) – occurred at frequencies of 11.02 and 8.20% respectively (Figs. S2a & S3a). Note: ‘XX’ indicates SLA gene‐specific allele groups. Comparing these findings with previously published SLA‐typing studies, Lr‐04.0 was also found in the pig populations (i) of studies from the Kansas State University (KSU, PRRSV study, unknown breed raised in the USA), (ii) of studies with Porcine Circo Virus (PCV, pigs with susceptibility to subgroups of PCV type 2, unknown breed raised in the USA), (iii) of the Big Pig group (Large White/Landrace crosses raised in the USA) and (iv) of Yorkshire pigs of Canadian origin (Ho et al. 2009a; Gao et al. 2017). In contrast, Lr‐32.0 was observed only in the pig groups Big Pig and Landrace of Canadian origin (Ho et al. 2009a; Gao et al. 2017). Lr‐22.0 and Lr‐01.0 were shared with KSU, PCV and Big Pig, and the latter with the Yorkshire only (Ho et al. 2009a; Gao et al. 2017). Lr‐59.0 was only found within the PCV group, Lr‐43.0 was found in the KSU group and Lr‐37.0 was shared in Yorkshire pigs, but Lr‐24.0 did not occur in any of these five studied cohorts (Ho et al. 2009a; Gao et al. 2017).
Table 1.
Swine leukocyte antigen (SLA) class I low‐resolution haplotypes characterised in nine European commercial pig populations by PCR screening assays
| Low resolution haplotype | Allele specificity1 | Haplotype frequency (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SLA‐1 | SLA‐3 | SLA‐2 | LRYS/D | LWLR/P | LR × LW | YS × NL LR | YS × Ham | YS | GER LR | P | LW | Combined | |
| 140 | 48 | 135 | 22 | 58 | 19 | 85 | 27 | 15 | 549 | ||||
| 01.0/04.02 | 04XX | 01XX | 01XX | 1.11 | 13.64 | 0.82 | |||||||
| 01.0 | 01XX | 01XX | 01XX | 3.57 | 10.42 | 13.70 | 15.91 | 0.59 | 5.56 | 6.28 | |||
| 02.0 | 02XX,07XX | 04XX3 | 02XX | 8.57 | 0.59 | 2.28 | |||||||
| 04.0 | 04XX | 04XX (04:04) | 04XX | 18.93 | 7.29 | 8.89 | 6.82 | 15.79 | 21.55 | 1.76 | 11.02 | ||
| 05.0 | 04XX | 05XX | 08XX | 1.79 | 3.13 | 6.67 | 0.91 | ||||||
| 06.0 | 08XX | 06XX (06:01) | 05XX | 1.79 | 2.08 | 2.22 | 11.76 | 3.01 | |||||
| 07.0 | 08XX | 07XX | 05XX | 7.14 | 2.08 | 1.11 | 1.76 | 2.55 | |||||
| 08.0 | 02XX,04XX | 03XX | 07XX | 1.04 | 0.09 | ||||||||
| 11.0 | 01XX,09XX | 07XX | 05XX | 0.37 | 1.85 | 0.18 | |||||||
| 16.0 mod4 | 11XX | 06XX | 09XX | 0.74 | 0.18 | ||||||||
| 18.0 | 04XX | 03XX | 06XX | 1.04 | 9.09 | 0.46 | |||||||
| 21.0 | 07:03 | 06XX (06:01) | 05XX | 0.74 | 2.63 | 4.31 | 0.73 | ||||||
| 22.0 | 08XX | 06XX (06:01) | 12XX | 8.21 | 1.04 | 17.04 | 2.27 | 2.59 | 6.74 | ||||
| 23.0 | 12XX | 03XX | Blank | 2.08 | 0.18 | ||||||||
| 24.0 | Blank5 | 04XX (04:04) | 06XX | 2.14 | 11.46 | 1.48 | 11.36 | 2.63 | 5.17 | 1.76 | 16.67 | 33.33 | 5.01 |
| 25.0 | 11XX | 03XX | 07XX | 1.07 | 4.17 | 0.37 | 5.88 | 7.41 | 2.00 | ||||
| 26.0 | 08XX | 05XX | 10XX | 3.57 | 2.08 | 2.63 | 2.59 | 0.59 | 9.26 | 2.00 | |||
| 27.0 | 06XX,08XX | 01XX | 01XX | 3.33 | 0.09 | ||||||||
| 28.0 | 09XX,15XX | 07XX | 05XX | 13.54 | 1.11 | 1.18 | 3.70 | 1.82 | |||||
| 29.0 | Blank | 05XX | 09XX | 1.43 | 1.04 | 1.85 | 1.85 | 1.00 | |||||
| 32.0 | 07XX | 04XX (04:04) | 02XX | 15.36 | 7.29 | 0.74 | 2.63 | 21.55 | 6.47 | 1.85 | 8.20 | ||
| 33.0 | Blank5 | 05XX | 06XX | 0.37 | 1.18 | 0.27 | |||||||
| 34.0 | Blank | 04XX (04:04) | 05XX | 0.36 | 1.04 | 1.85 | 6.82 | 11.18 | 2.64 | ||||
| 35.0 | 12XX,13XX (13:01) | 05XX | 10XX | 0.71 | 3.13 | 3.33 | 11.36 | 2.63 | 5.17 | 4.71 | 7.41 | 3.46 | |
| 36.0 | 02XX | 01XX | 11XX | 1.04 | 0.74 | 7.89 | 8.62 | 1.46 | |||||
| 37.0 | 07XX | 05XX | 09XX | 15.56 | 6.82 | 4.10 | |||||||
| 38.0 | 15XX | 04XX (04:04) | 12XX + 11:04 | 1.07 | 3.13 | 2.59 | 13.16 | 1.76 | 1.91 | ||||
| 39.0 | Blank | 05XX | 10XX | 2.86 | 1.04 | 0.37 | 2.63 | 4.31 | 3.53 | 1.85 | 13.33 | 2.46 | |
| 40.0 | 16XX | 05XX | 10XX | 0.71 | 1.04 | 1.85 | 0.36 | ||||||
| 42.0 | 08XX | 06XX (06:02) | 09XX | 0.36 | 0.09 | ||||||||
| 43.0 | 11XX | 04XX (04:04) | 04XX | 8.33 | 0.37 | 9.09 | 13.16 | 5.29 | 20.37 | 13.33 | 3.83 | ||
| 45.0 | 08XX + 17:01 | 07XX | 08XX + 10XX | 6.07 | 1.04 | 1.85 | 4.12 | 2.73 | |||||
| 46.0 | 12XX | 04XX (04:04) | 06XX | 1.04 | 0.37 | 0.59 | 0.27 | ||||||
| 47.0 | Blank | 06XX (06:01) | 05XX | 1.79 | 0.37 | 1.76 | 0.82 | ||||||
| 49.0 | 08XX | 05XX | Blank | 3.57 | 4.12 | 1.55 | |||||||
| 52.0 | Blank | 07XX | 03XX/11:04 | 10.00 | 1.55 | ||||||||
| 53.0 | Blank | 08XX | 11:04/15XX | 0.37 | 0.09 | ||||||||
| 55.0 | 15XX | 04XX (04:04) | 11:04 | 1.85 | 11.11 | 23.33 | 1.73 | ||||||
| 56.0 | 11XX | 03XX | 15XX | 1.18 | 0.18 | ||||||||
| 57.0 | 02XX | 01XX | 11XX | 3.21 | 0.74 | 1.00 | |||||||
| 58.0 | 08XX | 03XX (03:06) | 09XX (09:03) | 0.37 | 0.09 | ||||||||
| 59.0 | 11XX (11:03) | 05XX | 16:02 | 3.13 | 14.44 | 2.27 | 26.32 | 11.21 | 1.18 | 6.19 | |||
| 61.0 | 07:05 | 03XX | 06XX | 5.00 | 4.12 | 1.91 | |||||||
| 62.0 | 14XX | 04:04 | 06XX | 0.71 | 0.74 | 2.27 | 11.76 | 2.28 | |||||
| 64.0 | 14XX | 05XX | 10XX | 7.41 | 0.36 | ||||||||
| 66.0 | 15XX | 04XX (04:04) | 04XX | 3.13 | 2.27 | 0.36 | |||||||
| 67.0 | 15XX | 05XX | 10XX | 2.08 | 1.85 | 0.27 | |||||||
| 31.0/63.0 | 15XX | 07XX | 16XX | 1.85 | 0.46 | ||||||||
| V.04 | Blank | 07XX | 08XX + 16XX | 8.62 | 0.91 | ||||||||
| Y1.04 | 08XX | 04XX (04:04) or blank | 09XX | 2.63 | 4.31 | 0.55 | |||||||
| n.d. | XXXX | XXXX | XXXX | 1.04 | 0.37 | 5.26 | 1.18 | 6.67 | 0.55 | ||||
| No of Lr‐Hp | 24 | 28 | 33 | 13 | 13 | 12 | 26 | 16 | 7 | 51 | |||
D, Duroc; GER LR, German Landrace; Ham, Hampshire; LR, Landrace; LW, Large White; NL LR, Dutch Landrace; P, Pietrain; YS, Yorkshire; n.d., not defined.
Allele designations in parentheses indicates medium‐ or high‐resolution specificities.
Not yet confirmed haplotype.
Probably owing to the presence of SLA‐3*04XX‐like pseudogenes as this haplotype did not appear to possess an expressed SLA‐3 gene (Ho et al. 2009a).
Ambiguity could not be resolved owing to the detection of this haplotype in only one heterozygous animal.
Untyped SLA class I locus.
With respect to the allele groups discovered, SLA‐1 was more polymorphic than SLA‐2 followed by SLA‐3 (Fig. 2). For SLA‐1, we found 23 allele groups, and three of them explained 46.27% of the diversity. In detail, SLA‐1*08XX, SLA‐1*07XX and SLA‐1*blank represented frequencies of 16.58, 15.03 and 14.66% (Fig. 2). Note: ‘Blank’ indicates alleles that cannot be detected with the primer sets utilised in the current study. For SLA‐2, three out of 22 detected allele groups were responsible for 47.63% of the diversity. More precisely, SLA‐2*04XX, SLA‐2*05XX and SLA‐2*02XX showed frequencies of 15.21, 11.75 and 10.84% (Fig. 2). The lesser polymorphic locus, SLA‐3, was characterised by 10 allele groups, and among them, SLA‐3*04XX (39.80%) and SLA‐3*05XX (22.77%) explained 62.57% of the diversity (Fig. 2).
Figure 2.
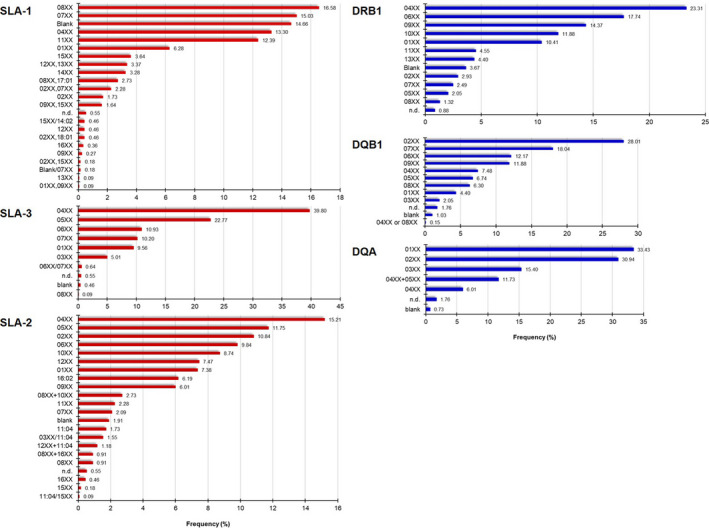
Frequencies (x‐axis) of swine leukocyte antigen (SLA) class I (SLA‐1, SLA‐3 and SLA‐2) and class II (DRB1, DQB1 and DQA) allele groups (y‐axis) identified in the studied European farmed pigs. ‘Blank’ indicates alleles that cannot be detected with the primer sets utilised in the current study. n.d., Not determined.
For SLA‐II, 37 haplotypes were found, including seven potential novel allele‐group combinations (Lr‐YDLR‐0.1, Lr‐YDLR‐0.2, Lr‐PIE‐0.1, Lr‐PIE‐0.2, Lr‐LWLR‐0.1, Lr‐LRYD‐0.1 and Lr‐NN; Table 2). The four haplotypes Lr‐0.15b, Lr‐0.12, Lr‐0.23 and Lr‐0.21 explained 44.43% of the SLA‐II diversity (Figs S2b & S3b).
Table 2.
Swine leukocyte antigen class II low‐resolution haplotypes characterised in seven European commercial pig populations by PCR screening assays
| Low resolution haplotype | Allele specificity1 | Haplotype frequency (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DRB1 | DQB1 | DQA | LRYS/D | LWLR/P | LR × LW | YS × NL LR | GER LR | P | LW | Combined | |
| 39 | 48 | 117 | 22 | 85 | 27 | 3 | 341 | ||||
| 0.01 | 01XX | 01XX | 01XX | 3.85 | 3.13 | 7.69 | 9.09 | 5.56 | 4.55 | ||
| 0.02 | 02XX | 02XX | 02XX | 5.13 | 0.85 | 4.55 | 33.33 | 1.47 | |||
| 0.04 | 02XX | 04XX | 02XX | 2.56 | 0.88 | ||||||
| 0.05 | 05XX | 02XX | 02XX | 1.28 | 2.14 | 0.88 | |||||
| 0.06 | 05XX | 08XX | 01XX | 1.04 | 2.14 | 2.27 | 1.85 | 1.17 | |||
| 0.07 | 06XX | 06XX | 01XX | 0.43 | 4.55 | 0.44 | |||||
| 0.08b2 | 08XX | 02XX (02:03) | 02XX | 2.56 | 3.13 | 2.35 | 1.32 | ||||
| 0.09 | 02XX | 04XX | 03XX | 4.17 | 0.59 | ||||||
| 0.10 | 04XX | 08XX | 03XX | 1.04 | 0.15 | ||||||
| 0.11 | 09XX/09:06 | 04XX | 03XX | 1.28 | 11.46 | 2.56 | 4.55 | 2.94 | 3.67 | ||
| 0.12 | 06XX | 07XX | 01XX | 2.56 | 3.42 | 9.09 | 39.41 | 7.41 | 12.46 | ||
| 0.13 | 04XX (04:03) | 03XX (03:03) | 02XX | 0.43 | 7.41 | 0.73 | |||||
| 0.14 | 09XX (09:06) | 08XX | 03XX | 1.28 | 1.04 | 13.64 | 6.47 | 25.93 | 16.67 | 4.99 | |
| 0.15a2 | 04XX (04:01) | 02XX | 02XX | 3.85 | 3.85 | 0.59 | 5.56 | 2.35 | |||
| 0.15b2 | 04XX (04:05/04:06) | 02XX(02:02) | 02XX | 7.69 | 19.79 | 31.20 | 14.37 | ||||
| 0.19a2 | 04XX (04:03/04:04) | 07XX | 03XX | 8.97 | 4.17 | 0.43 | 3.53 | 16.67 | 3.96 | ||
| 0.19b2 | 04XX (04:05/04:06) | 07XX | 03XX | 2.27 | 5.88 | 1.61 | |||||
| 0.20 | 06XX | 03XX | 01XX | 6.25 | 3.70 | 1.17 | |||||
| 0.21 | 01XX | 05XX | 04XX+05XX3 | 2.56 | 3.85 | 18.18 | 12.94 | 6.01 | |||
| 0.22 | 06XX | 02XX (02:04) | 02XX | 1.04 | 1.28 | 2.27 | 0.73 | ||||
| 0.23 | 10XX (10:06) | 06XX (06:03) | 01XX | 10.26 | 25.00 | 7.26 | 18.18 | 8.82 | 9.26 | 33.33 | 11.58 |
| 0.24 | 07XX | 02XX | 02XX | 6.41 | 1.04 | 4.27 | 1.85 | 2.49 | |||
| 0.25 | 13XX | 09XX | 04XX + 05XX3 | 12.82 | 3.13 | 2.14 | 5.88 | 11.11 | 4.99 | ||
| 0.26 | 11XX | 04XX | 02XX | 3.13 | 1.71 | 4.55 | 4.12 | 2.35 | |||
| 0.27 | 09XX/09:06 | 09XX | 04XX + 05XX3 | 16.24 | 16.67 | 5.72 | |||||
| 0.29 | Blank4 | 09XX | 04XX + 05XX3 | 1.04 | 5.29 | 1.47 | |||||
| 0.30 | 11XX (11:01) | 05XX | 02XX | 0.85 | 4.55 | 0.59 | 0.73 | ||||
| 0.32 | 06XX | Blank | 02XX | 5.21 | 0.85 | 1.03 | |||||
| 0.33 | 11XX (11:01/11:03) | 02XX (02:06) | 02XX | 5.13 | 4.17 | 0.85 | 1.47 | ||||
| 0.35 | 01XX (01:04) | 04XX | 02XX | 1.04 | 0.15 | ||||||
| YDLR‐0.15 | 06XX | 05XX | 03XX | 2.27 | 0.15 | ||||||
| YDLR‐0.25 | 06XX | 02:02/02:04 | 03XX | 0.43 | 0.15 | ||||||
| PIE‐0.15 | 01XX | 05XX | Blank | 1.85 | 0.15 | ||||||
| PIE‐0.25 | 06XX | 03XX | 03XX | 1.85 | 0.15 | ||||||
| LWLR‐0.15 | Blank | 02XX | 02XX | 0.85 | 0.29 | ||||||
| LRYD‐0.15 | 06XX | 02XX | 01XX | 12.82 | 1.47 | ||||||
| NN5 | Blank | 02XX | 02XX | 11.54 | 1.32 | ||||||
| n.d. | XXXX | XXXX | XXXX | 1.71 | 1.18 | 0.88 | |||||
| No of Lr‐Hp | 17 | 19 | 25 | 14 | 14 | 13 | 4 | 38 | |||
D, Duroc; GER LR, German Landrace; Ham, Hampshire; LR, Landrace; LRYD, Landrace/Yorkshire/Duroc crosses; LW, Large White; LWLR, Large White/Landrace crosses; NL LR, Dutch Landrace; P, Pietrain; PIE, Pietrain (Austria); YDLR, Yorkshire/Dutch Landrace crosses; YS, Yorkshire; n.d., not defined.
Allele designations in parentheses indicates medium‐ or high‐resolution specificities.
The alphabetical suffix in haplotype designations was used to differentiate between closely related haplotypes (i.e. haplotypes with identical low‐resolution group specificities, but different allele specificities).
Positive with both DQA*04XX primer sets in lanes D12 and C12 (Table S2b).
Untyped swine leukocyte antigen class II locus.
Not yet confirmed haplotype.
The two most abundant SLA‐II haplotypes, Lr‐0.15b (DRB1*04XX(04:05/04:06)‐DQB1*02XX(02:02)‐DQA*02XX) and Lr‐0.12 (DRB1*06XX‐DQB1*07XX‐DQA*01XX), occurred at frequencies of 14.37 and 12.46% respectively (Figs S2b & S3b). With respect to previous studies, Lr‐0.15b was also found in the pig populations KSU, PCV, Big Pig and Yorkshire (Ho et al. 2010; Gao et al. 2017). Lr‐0.12 and Lr‐0.23 were shared in Big Pig and Landrace together with PCV (Lr‐0.12) and Yorkshire (Lr‐0.23) (Ho et al. 2010; Gao et al. 2017). In contrast, Lr‐0.21 was observed only in the pig groups KSU and PCV (Ho et al. 2010).
As expected, regarding the detected number of SLA‐II allele groups, DRB1 was more polymorphic than DQB1 followed by DQA (Fig. 2). For DRB1, we found 13 allele groups, and two of them explained 41.06% of the diversity. Specifically, DRB1*04XX and DRB1*06XX represented frequencies of 23.31 and 17.74% respectively (Fig. 2). For DQB1, two out of 12 detected allele groups were responsible for 46.04% of the diversity with DQB1*02XX and DQB1*07XX showing frequencies of 28.01 and 18.04% (Fig. 2). The lesser polymorphic locus, DQA, was characterised by seven allele groups and among them DQA*02XX (33.43%) and DQA*07XX (30.94%) explained 64.37% of the diversity (Fig. 2).
In veterinary vaccine design, the characterisation of the peptide‐binding specificity of SLA‐I and SLA‐II molecules is pivotal to understanding adaptive immune responses of swine towards infectious pathogens (reviewed in Hammer et al. 2020). Herein we briefly discuss key findings on the correlation of SLA haplotypes and immune responses for the animals enrolled in this study. Immunity against the PRRSV is not well understood, although there is evidence suggesting that virus‐specific T‐cell IFN‐γ responses play an important role. It was demonstrated that PRRSV‐vaccinated and challenged pigs carrying SLA‐I haplotype Lr‐01.0/04.0 or Lr‐59.0 and SLA‐II haplotype Lr‐0.27 showed significant IFN‐γ responses, pointing towards a positive correlation of SLA haplotype and T‐cell response (Burgara‐Estrella et al. 2013). Another PRRSV study suggested that the antigenic region NSP5156–167 could be restricted by the SLA‐I haplotype Lr‐22.0, meaning that a T cell will only respond to this particular antigen when it is bound to either SLA‐1*08XX, SLA‐3*06:01 or SLA‐2*12XX. Additionally, pigs demonstrating CD4+ T cell responses to the antigenic peptide M29–43 were haploidentical, sharing both SLA‐II haplotypes Lr‐0.01 and Lr‐0.15b. This combination appearing exclusively in these animals suggests restriction by one of these two haplotypes (Mokhtar et al. 2014, 2016).
A proteome‐wide screening revealed immunodominance in the CD8 T‐cell response against Classical Swine Fever Virus with antigen specificity dependent on SLA‐I haplotypes. The variability in the antigen‐specificity of these immunodominant CD8 T‐cell responses was confirmed to be associated with the expression of distinct SLA‐I haplotypes. Moreover, recognition of NS21223–1230 STVTGIFL (Lr‐22.0) and NS31902–1912 VEYSFIFLDEY (Lr‐01.0) by a larger group of C‐strain vaccinated animals showed that these peptides could be restricted by additional haplotypes (Franzoni et al. 2013).
In the analysis of FLUAVsw, the porcine T‐cell response has been poorly characterised to date. In a cohort of 40 outbred pigs, Talker and co‐workers showed that animals with a strong expansion of Ki‐67+CD8β+ T cells and the highest frequencies of FLUAVsw‐specific cytokine‐producing CD4+ T cells were homozygous for the SLA‐I haplotype Lr‐01.0 and for the SLA‐DQA locus (DQA*02XX) (Talker et al. 2015, 2016). In 2018, Schwartz and co‐workers fully characterised the SLA background of the inbred Babraham pigs at a high‐resolution level: SLA‐1*14:02‐SLA‐3*04XX‐SLA‐2*11:04 and DRB1*05:01‐DQB1*08:01/02‐DQA*01:03. Based on this SLA‐defined pig model, it was then possible to develop a toolset that included the identification of novel immunodominant FLUAVsw‐derived T‐cell epitopes (Schwartz et al. 2018; Tungatt et al. 2018).
Previous studies showed the promising potential of dendrimer peptides as vaccine candidates against FMDV. Several B‐cell epitope dendrimers, harbouring a major FMDV antigenic B‐cell site in VP1 protein that is covalently linked to heterotypic T‐cell epitopes from 3A and/or 3D proteins, elicited consistent levels of neutralising antibodies and IFN‐γ‐producing cells in pigs (De León et al. 2020). Robust correlations of certain SLA haplotypes (Lr‐22.0, Lr‐59.0, Lr‐0.15b, Lr‐0.24 and Lr‐0.27) with antibody titres and IFN‐γ‐producing cells support the contribution of SLA class‐II restricted T‐cells to the magnitude of the T‐cell response and to the antibody response evoked by the B2T dendrimers, being of potential value for peptide vaccine design against FMDV (De León et al. 2020). In addition, Patch and colleagues used inbred minipigs to show that FMDV infection results in induction of cytotoxic T cell responses that are classically antigen specific and MHC restricted (Patch et al. 2014). Following on, these investigators used SLA‐1*04:01 and SLA‐2*04:01 class I tetramers to show that, upon vaccination with replication defective adenovirus 5 vectors expressing the FMDV P1 protein, T cell specificities expand with each vaccine boost (Pedersen et al. 2016).
In conclusion, these correlations could carry potential for veterinary vaccine design, as SLA‐I Lr‐01.0 (6.28%), Lr‐04.0 (11.02%), Lr‐22.0 (6.74%) and Lr‐59.0 (6.19%) and SLA‐II Lr‐0.01 (4.55%), Lr‐0.15b (14.37%) and Lr‐0.27 (5.72%) are highly abundant haplotypes in European farmed pigs (Tables 1 & 2). On the other hand, targeting common haplotypes may reduce diversity over time, leading to susceptibility to other diseases and a lack of vaccine efficacy. Hence, a vaccine that works across a wide range of haplotypes potentially could be a safer strategy.
Conflict of interest
The authors declare no known conflicts of interest associated with this publication.
Supporting information
Table S1 Detailed list of European farmed pigs incorporated in the present study.
Table S2 Plate layout of the PCR primer panel for genotyping swine leukocyte antigen class I (a) and class II (b) alleles.
Figure S1 Two basics concepts for swine leukocyte antigen haplotyping‐assisted animal trials in vaccine and transplantation research.
Figure S2 Frequency of swine leukocyte antigen class I (a) and class II (b) low‐resolution haplotypes identified in 549 and 341 European farmed pigs by PCR screening assays respectively.
Figure S3 Swine leukocyte antigen class I (a) and class II (b) low‐resolution haplotype diversity in nine and seven European commercial pig populations respectively.
Acknowledgements
For the collaboration by providing samples for this study, we are indebted to Christiane Jansen (Research Department of Animal Sciences, Wageningen University), Artur Summerfield (Institute of Virology and Immunology, Vetsuisse‐Faculty, University of Bern) and Simon Graham (The Pirbright Institute). This work is part of a startup project financially supported by Profile Line 2 ‘Infection and prevention’ from the University of Veterinary Medicine Vienna, Austria.
Data availability statement
Further information about data and reagents used is available by request to the corresponding author. Minipig‐derived SLA typing data are confidential because of a non‐disclosure agreement.
References
- Burgara‐Estrella A., Díaz I., Rodríguez‐Gómez I., Essler S.E., Hernández J. & Mateu E. (2013) Predicted peptides from non‐structural proteins of porcine reproductive and respiratory syndrome virus are able to induce IFN‐γ and IL‐10. Viruses 5, 663–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De León P., Cañas‐Arranz R., Saez Y.et al. (2020) Association of porcine Swine Leukocyte Antigen (SLA) haplotypes with B‐ and T‐cell immune response to foot‐and‐mouth disease virus (FMDV) peptides. Special issue: Evaluation of Vaccine Immunogenicity. Vaccines 8, E513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essler S.E., Ertl W., Deutsch J., Ruetgen B.C., Groiss S., Stadler M., Wysoudil B., Gerner W., Ho C.‐S. & Saalmüller A. (2013) Molecular characterization of swine leukocyte antigen gene diversity in purebred Pietrain pigs. Animal Genetics 44, 202–5. [DOI] [PubMed] [Google Scholar]
- Franzoni G., Kurkure N.V., Essler S.E., Everett H.E., Bodman‐Smith K., Crooke H.R. & Graham S.P. (2013) Proteome‐wide screening reveals immunodominance in the CD8 T‐cell response against classical swine fever virus with antigen‐specificity dependent on MHC class I haplotype. PLoS One 8, e84246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C., Quan J., Jiang X., Li C., Lu X. & Chen H. (2017) Swine leukocyte antigen diversity in Canadian specific pathogen‐free Yorkshire and landrace pigs. Frontiers in Immunology 8, 282. eCollection 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimsa U., Ho C.‐S. & Hammer S.E. (2017) Preferred SLA class I/ class II haplotype combinations in German Landrace pigs. Immunogenetics 69, 39–47. [DOI] [PubMed] [Google Scholar]
- Hammer S.E., Ho C.‐S., Ando A., Rogel‐Gaillard C., Charles M., Tector M., Tector A.J. & Lunney J.K. (2020) Importance of the MHC (SLA) in swine health and biomedical research. Annual Review in Animal Biosciences 8, 171–98. [DOI] [PubMed] [Google Scholar]
- Ho C.‐S., Lunney J.K., Ando A., Rogel‐Gaillard C., Lee J.H., Schook L.B. & Smith D.M. (2009b) Nomenclature for factors of the SLA system, update 2008. Tissue Antigens 73, 307–15. [DOI] [PubMed] [Google Scholar]
- Ho C.‐S., Lunney J.K., Franzo‐Romain M.H., Martens G.W., Lee Y.J., Lee J.H., Wysocki M., Rowland R.R. & Smith D.M. (2009a) Molecular characterization of swine leucocyte antigen class I genes in outbred pig populations. Animal Genetics 40, 468–78. [DOI] [PubMed] [Google Scholar]
- Ho C.‐S., Lunney J.K., Lee J.H., Franzo‐Romain M.H., Martens G.W., Rowland R.R.R. & Smith D.M. (2010) Molecular characterization of swine leucocyte antigen class II genes in outbred pig populations. Animal Genetics 41, 428–32. [DOI] [PubMed] [Google Scholar]
- Kamal S., Kerndt C.C. & Lappin S.L. (2020) Genetics, histocompatibility antigen. In: StatPearls [Internet]. StatPearls Publishing, Treasure Island, FL. 2021 Jan. PMID: 31082067. [PubMed] [Google Scholar]
- Ladowski J.M., Hara H. & Cooper D.K.C. (2021) The role of SLAs in xenotransplantation. Transplantation 105, 300–7. [DOI] [PubMed] [Google Scholar]
- Ladowski J., Martens G., Estrada J., Tector M. & Tector J. (2019) The desirable donor pig to eliminate all xenoreactive antigens. Xenotransplantation 26, e12504. [DOI] [PubMed] [Google Scholar]
- Lunney J.K., Ho C.‐S., Wysocki M. & Smith D.M. (2009) Molecular genetics of the swine major histocompatibility complex, the SLA complex. Developmental and Comparative Immunology 33, 362–74. [DOI] [PubMed] [Google Scholar]
- Mokhtar H., Eck M., Morgan S.B., Essler S.E., Frossard J.P., Ruggli N. & Graham S.P. (2014) Proteome‐wide screening of the European porcine reproductive and respiratory syndrome virus reveals a broad range of T cell antigen reactivity. Vaccine 32, 6828–37. [DOI] [PubMed] [Google Scholar]
- Mokhtar H., Pedrera M., Frossard J.P.et al. (2016) The non‐structural protein 5 and matrix protein are major antigenic targets of T cell immunity to porcine reproductive and respiratory syndrome virus. Frontiers in Immunology 7, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patch J.R., Dar P., Waters R., Toka F.N., Barerra J., Schutta C., Kondabattula G. & Golde W.T. (2014) Infection with foot‐and‐mouth disease virus (FMDV) induces a natural killer (NK) cell response in cattle that is lacking following vaccination. Comparative Immunology Microbiology and Infectious Diseases 37, 249–57. [DOI] [PubMed] [Google Scholar]
- Pedersen L.E., Patch J.R., Kenney M.A., Glabman R.A., Nielsen M., Jungersen G., Buus S. & Golde W.T. (2016) Expanding specificity of CD8 T cells for viral epitopes following multiple inoculations of swine with adeno‐FMDV vaccine. Veterinary Immunology and Immunopathology 181, 59–67. [DOI] [PubMed] [Google Scholar]
- Schwartz J.C., Hemmink J.D., Graham S.P., Tchilian E., Charleston B., Hammer S.E., Ho C.‐S. & Hammond J.A. (2018) The major histocompatibility complex homozygous inbred Babraham pig as a resource for veterinary and translational medicine. HLA 92, 40–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talker S.C., Koinig H., Stadler M.et al. (2015) Magnitude and kinetics of multifunctional CD4+ and CD8+ T cells in pigs infected with swine influenza A virus. Veterinary Research 46, 52–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talker S.C., Stadler M., Koinig H.C.et al. (2016) Influenza A virus infection in pigs attracts multifunctional and cross‐reactive T cells to the lung. Journal of Virology 90, 9364–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tungatt K., Dolton G., Morgan S.B.et al. (2018) Induction of influenza‐specific local CD8 T‐cells in the respiratory tract after aerosol delivery of vaccine antigen or virus in the Babraham inbred pig. PLoS Pathogens 14, e1007017. eCollection 2018 May. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Detailed list of European farmed pigs incorporated in the present study.
Table S2 Plate layout of the PCR primer panel for genotyping swine leukocyte antigen class I (a) and class II (b) alleles.
Figure S1 Two basics concepts for swine leukocyte antigen haplotyping‐assisted animal trials in vaccine and transplantation research.
Figure S2 Frequency of swine leukocyte antigen class I (a) and class II (b) low‐resolution haplotypes identified in 549 and 341 European farmed pigs by PCR screening assays respectively.
Figure S3 Swine leukocyte antigen class I (a) and class II (b) low‐resolution haplotype diversity in nine and seven European commercial pig populations respectively.
Data Availability Statement
Further information about data and reagents used is available by request to the corresponding author. Minipig‐derived SLA typing data are confidential because of a non‐disclosure agreement.


