Abstract
Co-use of alcohol and medication can have serious negative health effects (e.g., overdose risk, liver damage). Research has primarily focused on older adults or the pharmacokinetics of specific medication-alcohol combinations. Little work has been done on the subjective experience of persons who take alcohol-interactive (AI) medications and also drink alcohol, particularly in psychiatric samples at high risk for problematic alcohol use and high rates of prescription medication use, such as individuals with borderline personality disorder (BPD). Data from a larger ecological momentary assessment (EMA) study of alcohol use in 52 persons diagnosed with BPD (83% women; Mage=26 years) were used to examine the influence of alcohol intoxication (i.e., estimated blood alcohol concentration; eBAC) and medication co-use on momentary subjective experience while drinking. Participants reported AI medication use at baseline and completed multiple EMA reports per day over 21 days, which included reports of alcohol use, subjective effects of alcohol (e.g., pleasure, feeling worse), and negative and positive affect. AI medications significantly moderated the association between eBAC and pleasurable effects of alcohol, such that at higher levels of eBAC, those taking AI medications experienced blunted subjective pleasure compared to those not taking AI medications. AI medications did not moderate the associations between eBAC and subjective relief, feeling worse, positive affect, or negative affect. Attenuated pleasure during drinking could lead to increased drinking in an attempt to achieve a desirable state among individuals who co-use psychiatric medications and alcohol, and therefore may represent a useful target for prevention and intervention.
Keywords: alcohol use, alcohol-medication interactions, borderline personality disorder, ecological momentary assessment, subjective effects of alcohol
Introduction
Epidemiological studies indicate that nearly half of current adult drinkers in the United States reported taking at least one medication for which alcohol use is cautioned against (Breslow et al., 2015). This can be problematic because of known interaction effects between alcohol and certain medications, including over-the-counter medications, medications for acute or chronic medical conditions, and psychotropic medications (National Institutes of Health, 2014). Alcohol-medication interaction effects can be extremely dangerous, as they may cause substantial central nervous system impairment (Weathermon & Crabb, 1999), and can contribute to overdose (Jones et al., 2014), gastrointestinal bleeding (Moore et al., 2015), and liver damage (Food and Drug Administration, 2009). Of individuals taking medications that interact with alcohol, approximately 5% were also using alcohol in a way (3+ drinks on occasion) that put them at high risk for adverse events (Jalbert et al., 2008). Furthermore, alcohol-medication interactions contribute to emergency department visits with more serious patient outcomes compared to adverse drug reactions not involving alcohol (Castle et al., 2016), and increase the likelihood of admission to inpatient treatment following emergency department visits (White et al., 2018).
In addition to the epidemiological studies describing prevalence of co-use, most prior work (e.g., Weathermon & Crabb, 1999) has focused on variables such as physiological effects of co-use, or the therapeutic impact of medications for reducing drinking (e.g., effect of topiramate on alcohol craving; Miranda et al., 2016), rather than subjective effects of alcohol consumption while taking AI medications. Additionally, although prior work has examined the prevalence and risk of AI medication and alcohol co-use, this literature tends to focus on adults over 60 or 65 years in age (e.g., Holton et al., 2017). This particular subgroup is important to study given the age-associated changes in how alcohol and/or medications are processed. However, additional research is needed with younger adults, and with psychiatric clinical samples, especially diagnostic groups or subpopulations in which prescription medication and alcohol use are common.
One such condition is borderline personality disorder (BPD), which is characterized by pervasive dysregulation in emotions, behaviors, relationships, and identity (American Psychiatric Association, 2013). Prescription medication use, including long-term use, is common among persons with BPD (Stoffers-Winterling et al., 2018; Tomko et al., 2014). Estimates indicate that over 80% of persons diagnosed with BPD are on standing medications, most commonly antidepressants (i.e., SSRIs), and often take multiple prescribed psychiatric medications concurrently (Zanarini et al., 2004; Zanarini et al., 2015). Highly relevant to the current investigation is the association between BPD and alcohol use and alcohol use disorder (AUD). Notably, BPD and AUD are highly comorbid, with close to 50% of individuals with BPD also meeting criteria for AUD (Carpenter et al., 2016; Trull et al., 2018). Further, BPD features predict later alcohol problems and development of AUD (Stepp et al., 2005). Finally, daily-life studies demonstrate that individuals with BPD experience greater affective instability on days that they drink alcohol (Jahng et al., 2011), consume alcohol at a faster rate than community control individuals (Carpenter et al., 2017), and crave alcohol across a broader set of contexts, including less socially-normative contexts (e.g., weekdays), than do community control individuals (Lane et al., 2016). Taken together, this suggests that individuals with BPD are particularly prone to problematic patterns of alcohol use. Younger individuals with BPD tend to have more BPD symptoms (i.e., impulsivity, suicidality) and symptom expression tends to decrease with age (e.g., Stepp & Pilkonis, 2008), thus providing further rationale for focusing on younger adults with BPD as opposed to older adult samples (in which most alcohol-medication co-use research is focused). Given high levels of impulsivity, risky alcohol use, and high rates of prescribed medications (e.g., Stoffers-Winterling et al., 2018), adults with BPD represent an important population in which to study the effects of medication use on drinking experiences in daily life.
Given the potential serious negative consequences of combining medications and alcohol (i.e., “co-use”), it is important to understand the motivations and consequences associated with co-use. Studying patients’ subjective experiences while drinking alcohol (i.e., negative or positive effects) may help improve prevention and intervention efforts for providers who prescribe medications to patients who also consume alcohol. Here, consistent with the literature, we use the term alcohol-interactive (AI) medications to represent medications that have known interactive effects with alcohol, such that alcohol use with these medications is either medically contraindicated or should be avoided.1
Ecological momentary assessment (EMA) methods are uniquely capable of accurately and validly assessing substance use in daily life given its variable and episodic nature (Shiffman, 2009). Moreover, daily-life assessments of use, subjective effects, and affect are more accurate than retrospective recall (e.g., Ben-Zeev & Young, 2010). Although EMA has been used to investigate the co-use of alcohol and other substances (e.g., tobacco, cannabis, illicit substances), only a handful of studies (e.g., Linden-Carmichael et al., 2020; Piasecki et al., 2011; Piasecki et al., 2012) focused on subjective experiences of co-use in daily life. To date, no work has examined daily-life affect and subjective effects among those co-using alcohol and AI medications.
Current Study
This study is a secondary data analysis from a larger project that examined alcohol use and affect in daily life, among individuals with and without a BPD diagnosis (Carpenter et al., 2017; Lane et al., 2016). The current study used EMA to examine alcohol and AI medication co-use in a community-based sample of individuals who were receiving outpatient treatment and had a BPD diagnosis. The present study operationalizes “co-use” as the endorsement of medication use at baseline and alcohol use in daily life (EMA). Co-use in this study encompasses situations in which participants may have taken the medication and consumed alcohol around the same time (commonly termed simultaneous use in the literature on polysubstance use, e.g., Yurasek et al., 2017), or medication and alcohol use across the study period, but not necessarily at the same time (commonly termed concurrent use in the literature). Given the half-life of medication and scheduled intervals of many medications (e.g., antidepressants are often prescribed for once daily use), it is likely that medication effects and alcohol effects overlapped across the study period for those individuals who reported baseline medication use and daily-life alcohol use.
We had two primary aims: 1) Examine whether AI medications (e.g., sertraline, trazodone) moderate the association between level of intoxication (i.e., estimated BAC [eBAC]2) and momentary subjective effects of alcohol (i.e., experiencing pleasure, relief, or feeling worse). 2) Examine whether AI medications moderate the association between eBAC and momentary affect while drinking. The classes of medications that are likely prevalent in a psychiatric sample such as ours (e.g., antidepressants, benzodiazepines, anticonvulsants) should interact with alcohol to lead to unpleasant states such as drowsiness, lightheadedness, and sedation (Weathermon & Crabb, 1999). Therefore, we hypothesized for both aims that, compared to the effect of eBAC alone (i.e., for individuals not taking AI medications), the interaction of AI medications and eBAC would lead to attenuated positive subjective effects and affect, and greater negative subjective effects and affect.
Method
Participants
This sample included 56 individuals with a BPD diagnosis (subset from a larger study that also included a group of community control participants; Lane et al., 2016). The community control group was not included in the present study given the body of literature concluding that individuals diagnosed with BPD have higher likelihood of risky alcohol use and higher rates of prescription medication. Additionally, individuals in the community control group had relatively low endorsement of prescription medication use, thus, we would be unable to adequately assess the association between alcohol use and AI medication use in reference to subjective effects of alcohol and affect. Original study eligibility criteria included 1) aged 18-45, 2) report drinking at least once/week, 3) no psychosis, intellectual disability, and significant head trauma resulting in neurological dysfunction, 4) not currently in or seeking alcohol use treatment, report no past-year unsuccessful attempts to cut down or stop drinking, and report no severe withdrawal symptoms, and 5) not pregnant or planning to become pregnant. Individuals included in the current sample also met DSM-IV criteria for BPD, endorsed the affective instability criterion for BPD, and were enrolled in outpatient psychiatric/psychological treatment for BPD/emotion regulation difficulties. Four participants were removed from the present analyses: two for reporting no drinking over the study period, and two for not providing data on subjective effects of alcohol. The final sample (N = 52) had a mean age of 26.23 years (SD = 7.34, range = 18-45). Participants were 82.69% women, 82.69% Caucasian, 5.77% African American, 1.92% Hispanic, 1.92% Asian American, and 7.69% identified as “Other race/ethnicity.” Forty-four participants (84.62%) reported using at least one medication at the time of the study, with 36 participants (69.23%) reporting using at least one medication for psychiatric reasons. Thirty-six participants (69.23%) reported use of at least one AI medication. The study had institutional review board approval (University of Missouri, #1133597) and all participants provided written informed consent following description of study procedures.
Procedure
Participants were recruited through flyers posted in local psychiatric clinics and advertisements in the general community. If eligible, participants came to the lab for an orientation session, which included self-report questionnaires and EMA instructions. Participants reported whether they were currently taking any medications and, if applicable, the name of and reason for taking the medications. Participants were asked to keep a paper log of any prescription medication changes over the duration of the study; no participants reported such changes.
Participants were provided with an electronic diary (Palm Tungsten E2 handheld computer) programmed with customized software. The EMA period lasted approximately 21 days (M = 21.65; SD = 3.21; range = 7-29). During this time, participants completed weekly lab visits wherein the study team downloaded data and provided payment. Participants were compensated $10 for the orientation session and $50 for at least 80% compliance per EMA week. For every 10% drop below 80% compliance, there was a $10 reduction in pay. Participants also completed a battery of self-report questionnaires at their final lab visit, for which they were compensated $10.
Participants completed seven types of prompts during the EMA period (described in Lane et al., 2016). Because the present study aimed to assess the interactive effects of alcohol and AI medications, we retained data collected from user-initiated initial drink reports and drinking follow-ups where participants reported consuming alcohol,3 resulting in a final dataset of 705 prompts. For completeness, additional prompt types are described here, even if not included in the present study analyses. Random prompts were scheduled at least 60 minutes apart, and were sent approximately 6 times per day on average. On random prompts, participants were asked to indicate whether they had consumed alcohol since the previous prompt, but were not asked about subjective effects of alcohol. Participants were instructed to self-initiate an initial drink report at the completion of their first alcoholic beverage in a drinking episode. Drinking follow-ups were then triggered to occur at 30, 60, 120, and 180 minutes after the initial drink report or after drinking was reported in random prompts. If participants reported additional drinking in a follow-up prompt, an additional follow-up assessment was added to cover an additional 60 minutes. Participants were asked to rate the subjective effects of alcohol when they reported drinking in initial drink or drinking follow-up prompts.
Random-prompt compliance in the present sample was 88.50%. Although random prompts were not included in the present analyses, this compliance estimate serves as a general indication of how well the participants in the present sample adhered to the study procedures. Participants completed an average of 4.47 (SD = 3.17, range = 1-12) user-initiated initial drink prompts and an average of 18.98 (SD = 14.06, range = 1-60) drinking follow-up prompts per person. Per drinking episode, participants completed an average of 3.03 (SD = 0.78, range = 1-5) follow-up prompts, of which an average of 1.97 (SD = 0.79, range = 1-3.67) contained additional reported drinking. In total, an average of 5.62 (SD = 3.79, range = 1-16) drinking episodes per person were included in these analyses.
Measures
BPD Diagnostic Interview
Only individuals with BPD were included in this sample. Therefore, the Structured Interview for DSM-IV Personality (SIDP-IV; Pfohl et al., 1994) was used to establish BPD diagnosis. Twenty participants were randomly selected from the total sample to have a second interviewer rate video recordings of the diagnostic interviews. Inter-rater agreement was excellent for the diagnosis of BPD (κ = .88).4
Alcohol-Interactive Medications
Medication drug classes (e.g., antidepressant, anticonvulsant) and related medical guidance (i.e., alcohol use should be avoided) were determined using Drugs.com, a reference site that compiles information from professional databases (including Cerner Multum Information Services, Micromedex, Physicians’ Desk Reference, and Wolters Kluwer Health), pharmaceutical companies, the Federal Drug Administration (FDA) and other relevant agencies. Information is compiled and reviewed by expert physicians and pharmacists and is updated at least quarterly. References are provided for each interaction reported on the site. Previous studies of AI and alcohol co-use have utilized Drugs.com as a primary reference for classifying AI medications (e.g., Breslow, Dong & White, 2015), or have used one of the contributing databases as a primary reference (e.g., Pringle et al., 2005; Qato, Manzoor & Lee, 2015).
If alcohol was listed as a major or moderate possible interaction,5 the medication was coded as an AI medication; thus, all AI medications identified in this sample reflect those in which alcohol use is contraindicated or has an avoidance advisory. A dichotomous variable represents yes/no AI medication use (medications endorsed by participants are listed in Supplemental Materials, ST1). AI medications in this study included a large proportion of antidepressants (e.g., 58% were taking SSRI/SNRIs) and other psychiatric medication classes (e.g., 29% were taking anticonvulsants such as lamotrigine, and 15% were taking anxiolytics, such as clonazepam).
Estimated BAC
Prior to limiting the dataset to prompts used in the current study analyses (i.e., initial drink and drinking follow-up prompts where drinking occurred; see Procedure), momentary eBAC was computed using procedures detailed in Carpenter et al. (2017). For each prompt, gender, height, weight, average population rate for metabolizing alcohol, time elapsed, and number of drinks were input into the Matthews and Miller (1979) formula for eBAC. Because we did not assess the time it took for participants to consume their first drink in an episode, the time elapsed for the consumption of the initial drink was set at 20 minutes for all participants, as opposed to assuming immediate absorption. This changed the scale of momentary eBAC but did not affect the rank ordering of eBAC data or the correlations between eBAC and other variables (Carpenter et al., 2017). Drinking episodes were defined as the initial drink reported by participants, through three hours after the last reported drink (or until participant self-initiated a bedtime report or failed to complete the last scheduled follow-up), or through 5.5 hours after initial drink for longer episodes.6 Additionally, if eBAC reached 0.000% and a participant reported a new “initial drink”, this was considered a new episode. For persons with more than one drinking episode per day (n = 16; 20 total days), only the first drinking episode was included to ensure that eBAC started at 0.000g%. For the few prompts which produced unlikely eBAC values (i.e., values associated with coma and death), momentary eBAC was winsorized to 0.250g% (Carpenter et al., 2017).
Momentary Subjective Effects of Alcohol
When participants reported drinking, they were asked to rate the degree to which the drink was “pleasurable,” “relieved an unpleasant feeling or symptom,” or made them “feel worse” on a scale of 1 (“not at all”) to 5 (“extremely”). These items have been used in previous EMA studies that assessed momentary subjective effects of drinking (e.g., Piasecki et al., 2011).
Momentary Affect
Momentary affect was assessed using items from the Positive and Negative Affect Schedule-Extended version (PANAS-X; Watson & Clark, 1999). Participants were instructed to rate the degree to which they felt each item in the past 15 minutes on a scale of 1 (“very slightly or not at all”) to 5 (“extremely”). General negative affect (21 items) and positive affect (10 items) subscales (i.e., mean of items) were assessed in the present study. These items were assessed independent of drinking (i.e., participants were not asked to rate how the drink impacted their present mood state).
Analytic Approach
The dataset used for the present analyses included momentary reports (n = 705), nested within days (n = 292), nested within people (n = 52). Hierarchical linear modeling was conducted using SAS PROC MIXED (SAS 9.4; 2014). We tested five models, one for each momentary-level dependent variable (DV): subjective pleasure, relief, and feeling worse from drinking; and negative and positive affect. Predictors in each model were: momentary-, day-, and person-level eBAC; person-level presence/absence of AI medications; interaction between momentary eBAC and AI medications; and covariates (sex, sample-centered age, day of the week, hour after wake, and day- and person-level eBAC and their interactions with AI medications). Momentary-level eBAC was centered on the average of all eBAC moments within that day, day-level eBAC was centered on the person-mean of eBAC, and person-level eBAC was centered on the sample-mean of eBAC. The primary effect of interest in each model was the interaction between momentary-level eBAC and AI medications. Thus, day- and person-level eBAC and their interactions with AI medications were included to disaggregate the three levels of analysis (Curran & Bauer, 2011) and can be considered covariates. Given our five effects of interest, a Bonferroni correction was used to address Type I Error, resulting in an adjusted alpha of 0.01. All analyses included random intercepts at the momentary and day levels. Models also included random slopes, which were retained if the model converged and if they were significant (p < 0.05).
Results
Table 1 includes descriptive statistics for the primary variables of interest in the study.
Table 1.
EMA Aggregate (person-level) Descriptive Statistics for Primary Variables
| M | SD | Range | Possible Range | |
|---|---|---|---|---|
| Alcohol Use | ||||
| Estimated BAC (eBAC) | 0.05 | 0.03 | 0.01 - 0.14 | 0.00 - 0.25a |
| Subjective Effects | ||||
| Pleasurable | 4.01 | 0.64 | 2.00 - 5.00 | 1.00 - 5.00 |
| Relieved | 2.66 | 1.09 | 1.00 - 5.00 | 1.00 - 5.00 |
| Worse | 1.23 | 0.38 | 1.00 - 2.50 | 1.00 - 5.00 |
| Affect | ||||
| Negative affect | 1.41 | 0.56 | 1.00 - 4.23 | 1.00 - 5.00 |
| Positive affect | 2.50 | 0.69 | 1.45 - 4.24 | 1.00 - 5.00 |
| N | % | – | – | |
| Medication | – | – | ||
| Participants taking AI medicationsb | 36 | 69.23% | – | – |
Note. N = 52.
Possible range could encompass values greater than 0.250g%; however, variable was winsorized to account for unlikely values (see Method section). Mean eBAC is for momentary prompts included in the present analyses only (i.e., initial drink reports and drinking follow-up prompts where drinking was reported).
The mean number of AI medications per participant was 1.65 (SD = 1.49), with a range of 0 to 6 AI medications.
Subjective Effects of Alcohol (Table 2)
Table 2.
Proc MIXED: Effects of AI Medications on Affect and Subjective Effects while Drinking
| Momentary Subjective Effects of Alcohol |
Momentary Affect |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pleasurablea | Relievedb | Worsec | Positived | Negativeb | ||||||
|
|
|
|
|
|
|
|||||
| Fixed Effects | Est. | SE | Est. | SE | Est. | SE | Est. | SE | Est. | SE |
|
|
|
|||||||||
| intercept | 4.22 ** | 0.26 | 2.33 ** | 0.39 | 1.26 ** | 0.14 | 2.73 ** | 0.23 | 1.36 ** | 0.18 |
| Momentary level | ||||||||||
| eBAC | 4.09 | 1.91 | 3.10 | 1.56 | 0.66 | 0.96 | 2.18 * | 0.84 | −0.51 | 0.54 |
| eBAC*medication | −5.98 * | 2.24 | −0.22 | 1.82 | 0.23 | 1.14 | −0.57 | 0.97 | −0.51 | 0.54 |
| Day level | ||||||||||
| eBAC | 3.20 | 3.24 | 1.53 | 3.95 | 1.70 | 1.47 | 3.18 | 2.32 | 1.20 | 1.63 |
| eBAC*medication | −1.00 | 3.64 | 1.84 | 4.44 | −0.55 | 1.66 | −0.82 | 2.60 | −1.49 | 1.83 |
| Person level | ||||||||||
| eBAC | 4.20 | 5.22 | 12.92 | 9.33 | 0.26 | 2.95 | 10.33 | 5.78 | −0.22 | 4.77 |
| medication | −0.12 | 0.19 | −0.04 | 0.34 | −0.08 | 0.11 | −0.17 | 0.21 | 0.02 | 0.18 |
| eBAC*medication | −3.69 | 5.77 | −12.02 | 10.45 | −1.02 | 3.30 | −13.00 | 6.49 | −3.75 | 5.37 |
| Covariates | ||||||||||
| age | 0.00 | 0.01 | 0.00 | 0.02 | 0.00 | 0.01 | 0.01 | 0.01 | 0.00 | 0.01 |
| gender (ref = fem.) | −0.14 | 0.23 | −0.22 | 0.43 | −0.09 | 0.13 | 0.11 | 0.26 | −0.04 | 0.22 |
| hour after wake | −0.01 | 0.02 | 0.01 | 0.02 | 0.00 | 0.01 | −0.01 | 0.01 | 0.00 | 0.01 |
| day (ref = Sat.) | ||||||||||
| Sunday | 0.05 | 0.15 | −0.08 | 0.17 | −0.03 | 0.08 | −0.07 | 0.10 | 0.05 | 0.06 |
| Monday | −0.05 | 0.16 | 0.37 | 0.19 | 0.16 | 0.08 | 0.05 | 0.11 | 0.14 | 0.08 |
| Tuesday | −0.12 | 0.18 | 0.67 * | 0.22 | 0.04 | 0.09 | −0.03 | 0.13 | 0.09 | 0.09 |
| Wednesday | 0.15 | 0.16 | 0.50 | 0.19 | 0.05 | 0.08 | −0.06 | 0.11 | 0.06 | 0.08 |
| Thursday | −0.02 | 0.15 | 0.54 * | 0.17 | 0.01 | 0.07 | 0.02 | 0.10 | 0.02 | 0.07 |
| Friday | 0.13 | 0.14 | 0.25 | 0.16 | 0.03 | 0.07 | 0.00 | 0.09 | −0.03 | 0.06 |
Note.
p < 0.01
p < 0.001. Medication is a person-level variable only and is included as interaction with eBAC at each level (e.g., moment, day). Main effect for medication is only represented in “Person level” section of table.
All continuous predictors are centered. Momentary-level variables are centered within day; day-level variables are centered within person; person-level variables are centered within sample. All models include random intercepts for eBAC (moment- and day-level).
Random slope for moment-level eBAC was significant and retained. Random slope for day-level eBAC not retained due to convergence.
Random slopes not retained due to nonsignificance (moment-level eBAC) and/or convergence (day-level eBAC).
Random slopes for eBAC (moment- and day-level) not retained due to convergence.
Random slopes for eBAC (moment- and day-level) not retained due to nonsignificance.
Feeling Pleasure
There were no significant main effects of momentary-level eBAC or AI medications in the prediction of momentary-level subjective pleasurable effects of alcohol. However, the interaction between momentary-level eBAC and AI medications was significant. This interaction suggests a positive association between eBAC and pleasurable subjective effects for individuals not using AI medications, but a negative association between eBAC and pleasurable effects for those reporting AI medication use. However, the simple slopes were not significant (no AI meds: Est = 4.09, SE = 1.91, t(56.7) = 2.14, p = 0.037; AI meds: Est = −1.89, SE = 1.26, t(69.6) = −1.51, p = 0.137), limiting our interpretation.7
Feeling Relief
There were no significant main effects of AI medications, momentary-level eBAC, nor the interaction between momentary-level eBAC and AI medications in the prediction of momentary subjective feelings of relief when drinking.
Feeling Worse
The effects of AI medications, momentary-level eBAC, and the interaction between eBAC and AI medications were not significant predictors of momentary endorsement of feeling worse from drinking.
Affect (Table 2)
Positive Affect
There was a significant, positive main effect of momentary-level eBAC on momentary positive affect while drinking. The main effect of AI medication and the interaction between eBAC and AI medication were not significant predictors of momentary positive affect.
Negative Affect
There were no significant main or interaction effect of momentary-level eBAC and AI medications predicting momentary negative affect while drinking.
Discussion
We sought to characterize the effects of taking AI medications on subjective effects of alcohol and affect during daily-life drinking episodes in a sample of individuals with BPD, a population with high rates of prescription psychiatric medication use and high prevalence of AUD. This study is the first to examine the daily-life subjective effects of the co-use of alcohol and AI medications, which addresses an important gap in the literature given the harmful effects of co-using alcohol and AI medications. Our results suggest that individuals with BPD who take AI medications (as reported at baseline) and use alcohol tend to experience blunted positive responses to alcohol intoxication, particularly as level of intoxication increases (i.e., as eBAC increases relative to one’s average eBAC). Specifically, participants taking AI medications who also reached a higher relative eBAC reported experiencing significantly less pleasurable effects of alcohol intoxication than those not taking AI medications. Person-level AI medications did not interact with EMA-assessed estimated BAC in the prediction of other subjective effects or affect. Taken together, the results of this study, including the null effects, should be interpreted with caution given the limited sample size, nature of AI medication assessment (at baseline, rather than in EMA prompts), and novel nature of the research questions. This study provides a strong foundation for future investigations of these associations to elucidate the association of alcohol and medication use and its impact on alcohol-related effects while drinking.
Our findings indicate that use of AI medications with alcohol does not lead to increased momentary aversive effects. It also does not seem to decrease negative mood/aversive subjective experiences (e.g., feeling worse), or improve or increase positive mood or experiences (e.g., feeling relieved). Rather it is associated with diminished positive effects of alcohol. The primary implication of this finding is that individuals who are advised to not drink while taking certain medications may not necessarily experience immediate negative or punishing effects from this combination, which may indirectly contribute to continued co-use. Patients may consequently discount possible medication-alcohol interactions and ultimately not comply with medical advice. This process could help explain substantial rates of alcohol use while also taking AI medications (e.g., Breslow et al., 2015; Pringle et al., 2005).
Past drinking experiences and subsequent expectations for alcohol’s effects may also be influential. Individuals using AI medications may be motivated to drink more alcohol or drink more rapidly in an attempt to achieve the same rewarding positive effects of alcohol as those experienced without such medications. Binge drinking is increasingly a topic of empirical investigation and some efforts have found long-term associations between medical prescription use and binge drinking (e.g., McCabe & West, 2013). Future studies should investigate these associations more closely (e.g., use of medication during high intensity drinking). It is also possible that emotional blunting associated with antidepressant use (e.g., Goodwin, Price, De Bodinat, & Laredo, 2017; Price, Cole, & Goodwin, 2008; Read & Williams, 2018) could partially explain the experience of diminished pleasure among individuals in our sample who were taking AI mediations. Although we did not find a main effect of AI medications on subjective pleasure during drinking in general, emotional blunting could certainly become more apparent at higher levels of eBAC when increased pleasure is expected. Future work would benefit from measuring emotional blunting and considering this in analyses of medication and alcohol interactions.
Understanding why individuals consume alcohol while taking AI medications may highlight paths for intervention. Within BPD or other psychiatric samples in particular, alcohol use may be motivated by a desire to cope with negative affect (Baker et al., 2004; Cooper et al., 2016). In a similar vein, medication use itself may serve as a proxy for another particularly salient variable, such as severity of psychopathology, negative affectivity, or impulsivity – all variables common to both alcohol use and psychiatric medication use. Parsing these associations and commonalities is important going forward. Further, the importance of not mixing alcohol and medications may not be salient when individuals are not experiencing immediate and/or noticeable negative effects. If medical providers discuss co-use with patients, they may choose to discuss blunted positive experiences as a possible immediate effect, or highlight longer-term consequences that may be more covert, such as liver damage. Theories of addiction should also be considered regarding the experience of blunted pleasure during drinking. As addiction progresses, individuals experience less positive affective responses to alcohol use (Cho et al., 2019; Koob & LeMoal, 2001; Koob & Volkow, 2010), potentially leading to increased use of alcohol in an attempt to achieve the desired positive affective/pleasurable state. Providing patient education regarding such changes in reinforcement along the addiction cycle, as well as possible changes in reinforcement due to medication-alcohol interactions for individuals with BPD, may be a point of discussion during the course of treatment planning and delivery. Future research should focus on the best method and content for dissemination to patients and investigate the outcomes of such in-session interventions.
Limitations and Future Directions
This study had limitations that merit discussion. First, medications were only assessed at baseline, rendering us unable to determine how within-person factors such as dosage or medication compliance might have affected results. Our study marks an important first step in showing the utility of examining AI medication co-use with alcohol in individuals’ daily lives, but future studies should extend this line of work to measure momentary- or day-level medication use including dosage, whether participants took their medications as prescribed, timing of use, etc. Additional detail regarding individuals’ medication use (e.g., dosage, additive effects of multiple medications) could expand our understanding of how exactly AI prescription medications influence individuals’ subjective experiences while consuming alcohol. Further, this would expand our understanding of how simultaneous alcohol and medication use may differ from concurrent use, and/or how regularly scheduled use may be similar or different from as-needed (PRN) medications. At the same time, person-level information about medication use is useful in that many commonly endorsed medications (e.g., antidepressants) are prescribed for daily use; thus, person-level data would be similar to EMA-based data. Regardless, future research assessing both medication and alcohol use at the same levels of EMA (i.e., momentary) would be beneficial to pick up on more nuanced effects of certain medications, use of PRN medications, and/or interactions based on time of day or context.
Second, this study had a limited sample size and, of the individuals included, there were an average of five drinking episodes per person. Although five drinking episodes over a period of 21 days (on average) is not inconsequential, power to detect significant effects may be limited. Therefore, the null results in this study should be interpreted cautiously and future research should focus on replication.
Third, the current study focused on individuals with BPD, and it is unclear whether results would generalize to the broader set of individuals who consume alcohol while taking AI medications. Given the sample (i.e., BPD) and composition of AI medications that are unique to this sample, we are unable to generalize to other diagnostic groups and other combinations of AI medications. Similarly, the sample consisted predominantly of white females, limiting the generalizability of the findings. Future work should investigate the association between alcohol use and medication on subjective effects and affect in among other demographics and taking other considerations into account (e.g., health disparities, access to medications).
Finally, it is unclear to what extent individuals in our study may have intentionally mixed AI medications and alcohol. Were participants aware of contraindications and chose to co-use medications and alcohol anyway? Further, participants’ experience of side effects (e.g., sedation) may vary, such that some individuals may experience some effects as ‘positive’ whereas others may consider it ‘negative’. Future studies might benefit from assessing participants’ awareness of and opinions about contraindications, and motives for and expectancies of co-use. Collectively, this information could provide insights into risk factors for co-using AI medications and alcohol and, in turn, lead to better recommendations for physicians who prescribe medications and therapists who treat individuals with AUD and comorbid mental illness, particularly BPD. Despite these limitations, this study is the first to examine participants’ ratings of subjective effects of alcohol in their daily lives while also using AI medications. Our findings identify experiences (e.g., blunted positive affect) among individuals with BPD who take AI medications and also drink alcohol and suggest possible points of intervention for treatment providers.
Supplementary Material
Figure 1. Simple Slopes of Interaction Term (AI Medications*eBAC) Predicting Pleasurable Effects of Alcohol.
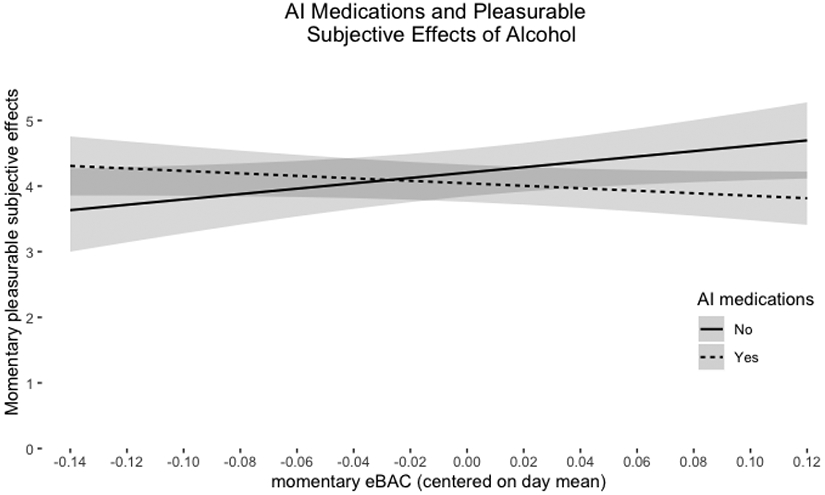
Note: The shaded bands around each line represent 95% Confidence Intervals.
Acknowledgments
This research was supported by the National Institutes of Health Grants T32 AA013526 (PI: Sher) to Fleming, Freeman, Griffin, Helle, Vebares; F31 AA027958 to Wycoff; R25 AA023687 (PI: Sher) to Rodriguez, Zapata; P60 AA11998 (PI: Heath) to Trull. The funding sources had no role other than financial support. Dr. Trull is a Scientific Advisor for Boehringer Ingelheim Pharma. There are no other potential conflicts of interest for Dr. Trull or other authors.
Footnotes
AI medications specific to the current study are listed in the Supplemental Materials.
Estimated BAC has been identified as a valid indicator of BAC evaluated by breath (e.g., Hustad & Carey, 2005).
Drinking reported on drinking follow-up prompts that were included in the present study may have been follow-ups resulting from drinking reported in initial drink reports and/or random prompts, although the random prompt data themselves were not included in the present study analyses (given that subjective effects were not assessed in random prompts).
Kappa for BPD diagnosis was for the entire original sample, which included BPD and community groups.
Interactions included a range of effects (e.g., exacerbation of effects of alcohol, physical health consequences).
Consistent with Carpenter et al. (2017), data were censored after 350 minutes (5.5 hours plus 20 minutes for initial drink) given that few assessments occurred after a sixth follow-up prompt.
In addition to the dichotomous representation of AI medications, the number of AI medications significantly moderated the association between eBAC and feelings of pleasure when drinking, demonstrating a similar pattern (p < 0.05). Results are presented in Supplemental Materials, Table 2 (ST2).
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM-5®). Washington, DC: American Psychiatric Association. [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, & Fiore MC (2004). Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychological review, 111(1), 33–51. [DOI] [PubMed] [Google Scholar]
- Ben-Zeev D, & Young MA (2010). Accuracy of hospitalized depressed patients' and healthy controls' retrospective symptom reports: an experience sampling study. The Journal of nervous and mental disease, 198(4), 280–285. [DOI] [PubMed] [Google Scholar]
- Breslow RA, Dong C, & White A (2015). Prevalence of alcohol-interactive prescription medication use among current drinkers: United States, 1999 to 2010. Alcoholism: Clinical and Experimental Research, 39(2), 371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter RW, Trela CJ, Lane SP, Wood PK, Piasecki TM, & Trull TJ (2017). Elevated rate of alcohol consumption in borderline personality disorder patients in daily life. Psychopharmacology, 234(22), 3395–3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter RW, Wood PK, & Trull TJ (2016). Comorbidity of borderline personality disorder and lifetime substance use disorders in a nationally representative sample. Journal of Personality Disorders, 30(3), 336–350. [DOI] [PubMed] [Google Scholar]
- Castle IJP, Dong C, Haughwout SP, & White AM (2016). Emergency department visits for adverse drug reactions involving alcohol: United States, 2005 to 2011. Alcoholism: Clinical and Experimental Research, 40(9), 1913–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SB, Su J, Kuo SI, Bucholz KK, Chan G, Edenberg HJ, … Dick DM (2019). Positive and negative reinforcement are differentially associated with alcohol consumption as a function of alcohol dependence. Psychology of Addictive Behaviors, 33, 58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ML, Kuntsche E, Levitt A, Barber LL, & Wolf S (2016). Motivational models of substance use: a review of theory and research on motives for using alcohol, marijuana, and tobacco (pp. 375–421). In Sher KJ (Ed)., The Oxford handbook of substance use disorders (Vol 1). New York, Oxford University Press. [Google Scholar]
- Curran PJ, & Bauer DJ (2011). The disaggregation of within-person and between-person effects in longitudinal models of change. Annual review of psychology, 62, 583–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration, HHS. (2009). Organ-specific warnings; internal analgesic, antipyretic, and antirheumatic drug products for over-the-counter human use; final monograph. Final rule. Federal register, 74(81), 19385. [PubMed] [Google Scholar]
- Gorka SM, Hedeker D, Piasecki TM, & Mermelstein R (2017). Impact of alcohol use motives and internalizing symptoms on mood changes in response to drinking: An ecological momentary assessment investigation. Drug and alcohol dependence, 173, 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holton AE, Gallagher P, Fahey T, & Cousins G (2017). Concurrent use of alcohol interactive medications and alcohol in older adults: a systematic review of prevalence and associated adverse outcomes. BMC geriatrics, 17(1), 148–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahng S, Solhan MB, Tomko RL, Wood PK, Piasecki TM, & Trull TJ (2011). Affect and alcohol use: An ecological momentary assessment study of outpatients with borderline personality disorder. Journal of abnormal psychology, 120(3), 572–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalbert JJ, Quilliam BJ, & Lapane KL (2008). A profile of concurrent alcohol and alcohol-interactive prescription drug use in the US population. Journal of general internal medicine, 23(9), 1318–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Paulozzi LJ, & Mack KA (2014). Alcohol involvement in opioid pain reliever and benzodiazepine drug abuse–related emergency department visits and drug-related deaths—United States, 2010. MMWR. Morbidity and mortality weekly report, 63(40), 881–885. [PMC free article] [PubMed] [Google Scholar]
- Koob GF, & Le Moal M (2001). Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology, 24(2), 97–129. [DOI] [PubMed] [Google Scholar]
- Koob GF, & Volkow ND, (2010). Neurocircuitry of addiction. Neuropsychopharmacology, 35, 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane SP, Carpenter RW, Sher KJ, & Trull TJ (2016). Alcohol craving and consumption in Borderline Personality Disorder: When, where, and with whom. Clinical Psychological Science, 4(5), 775–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden-Carmichael AN, Van Doren N, Masters LD, & Lanza ST (2020). Simultaneous alcohol and marijuana use in daily life: Implications for level of use, subjective intoxication, and positive and negative consequences. Psychology of addictive behaviors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DB, & Miller WR (1979). Estimating blood alcohol concentration: Two computer programs and their applications in therapy and research. Addictive behaviors, 4(1), 55–60. [DOI] [PubMed] [Google Scholar]
- McCabe SE, & West BT (2013). Medical and nonmedical use of prescription stimulants: results from a national multicohort study. Journal of the American Academy of Child & Adolescent Psychiatry, 52(12), 1272–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda R Jr, MacKillop J, Treloar H, Blanchard A, Tidey JW, Swift RM, … & Monti PM (2016). Biobehavioral mechanisms of topiramate's effects on alcohol use: an investigation pairing laboratory and ecological momentary assessments. Addiction biology, 21(1), 171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore N, Pollack C, & Butkerait P (2015). Adverse drug reactions and drug-drug interactions with over-the-counter NSAIDs. Therapeutics and clinical risk management, 11, 1061–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AA, Whiteman EJ, & Ward KT (2007). Risks of combined alcohol/medication use in older adults. The American journal of geriatric pharmacotherapy, 5(1), 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health (2014). National Institute on Alcohol Abuse and Alcoholism: Harmful interactions: Mixing alcohol with medicines. NIH Publication No. 13-5329. National Institutes of Health, US Department of Health and Human Services. Available at: https://www.niaaa.nih.gov/sites/default/files/publications/Harmful_Interactions.pdf [Google Scholar]
- Pfohl B, Blum N, & Zimmerman M (1994). Structured interview for DSM–IV personality disorders. Iowa City: University of Iowa Hospitals and Clinics. [Google Scholar]
- Piasecki TM, Cooper ML, Wood PK, Sher KJ, Shiffman S, & Heath AC (2014). Dispositional drinking motives: Associations with appraised alcohol effects and alcohol consumption in an ecological momentary assessment investigation. Psychological Assessment, 26(2), 363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM, Jahng S, Wood PK, Robertson BM, Epler AJ, Cronk NJ, … & Sher KJ (2011). The subjective effects of alcohol–tobacco co-use: An ecological momentary assessment investigation. Journal of abnormal psychology, 120(3), 557–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM, Wood PK, Shiffman S, Sher KJ, & Heath AC (2012). Responses to alcohol and cigarette use during ecologically assessed drinking episodes. Psychopharmacology, 223(3), 331–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle KE, Ahern FM, Heller DA, Gold CH, & Brown TV (2005). Potential for alcohol and prescription drug interactions in older people. Journal of the American Geriatrics Society, 53(11), 1930–1936. [DOI] [PubMed] [Google Scholar]
- Qato DM, Manzoor BS, & Lee TA (2015). Drug–alcohol interactions in older US adults. Journal of the American Geriatrics Society, 63(11), 2324–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S (2009). Ecological momentary assessment (EMA) in studies of substance use. Psychological assessment, 21(4), 486–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp SD, & Pilkonis PA (2008). Age-related differences in individual DSM criteria for borderline personality disorder. Journal of Personality Disorders, 22(4), 427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp SD, Trull TJ, & Sher KJ (2005). Borderline personality features predict alcohol use problems. Journal of Personality Disorders, 19(6), 711–722. [DOI] [PubMed] [Google Scholar]
- Stoffers-Winterling JM, Storebø OJ, Völlm BA, Mattivi JT, Nielsen SS, Kielsholm ML, … & Lieb K (2018). Pharmacological interventions for people with borderline personality disorder. Cochrane Database Syst Rev, 2, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomko RL, Trull TJ, Wood PK, & Sher KJ (2014). Characteristics of borderline personality disorder in a community sample: comorbidity, treatment utilization, and general functioning. Journal of personality disorders, 28(5), 734–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treloar H, Piasecki TM, McCarthy DM, Sher KJ, & Heath AC (2015). Ecological evidence that affect and perceptions of drink effects depend on alcohol expectancies. Addiction, 110(9), 1432–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trull TJ, Freeman LK, Vebares TJ, Choate AM, Helle AC, & Wycoff AM (2018). Borderline personality disorder and substance use disorders: an updated review. Borderline personality disorder and emotion dysregulation, 5(1), 15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D & Clark LA (1999). The PANAS-X: manual for the positive and negative affect schedule-expanded form. Unpublished manuscript, University of Iowa, Iowa City, IA. [Google Scholar]
- Weathermon R, & Crabb DW (1999). Alcohol and medication interactions. Alcohol Research & Health, 23(1), 40–51. [PMC free article] [PubMed] [Google Scholar]
- White AM, Slater ME, Ng G, Hingson R, & Breslow R (2018). Trends in alcohol-related emergency department visits in the United States: results from the Nationwide Emergency Department Sample, 2006 to 2014. Alcoholism: clinical and experimental research, 42(2), 352–359. [DOI] [PubMed] [Google Scholar]
- Yurasek AM, Aston ER, & Metrik J (2017). Co-use of alcohol and cannabis: A review. Current Addiction Reports, 4(2), 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanarini MC, Frankenburg FR, Hennen J, Reich DB, & Silk KR (2004). Axis I comorbidity in patients with borderline personality disorder: 6-year follow-up and prediction of time to remission. American Journal of Psychiatry, 161(11), 2108–2114. [DOI] [PubMed] [Google Scholar]
- Zanarini MC, Frankenburg FR, Reich DB, Harned AL, & Fitzmaurice GM (2015). Rates of psychotropic medication use reported by borderline patients and axis II comparison subjects over 16 years of prospective follow-up. Journal of clinical psychopharmacology, 35(1), 63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


