Abstract
Background
Mycosis fungoides (MF) is a cutaneous lymphoma; most patients present with early, skin-limited disease and are managed by dermatologists.
Objective
The purpose of this study was to systematically review and assess the evidence on topical treatments for early-stage (IA, IB, IIA) MF.
Methods
We performed a literature search via MEDLINE, Embase, Web of Science, and Cochrane databases. Grading Recommendations Assessment, Development and Evaluation (GRADE) criteria were used to assess the certainty of the data.
Results
Two searches yielded 1252 references; 26 met the inclusion criteria and included literature on nitrogen mustard, retinoids, corticosteroids, carmustine, fluorouracil, methotrexate-laurocapram, hexadecylphosphocholine, peldesine, ingenol mebutate, topical methotrexate with oxygen flow-assisted LP3 carrier, and resiquimod. Most studies were single intervention, observational series. Nitrogen mustard, with the most published reports, was effective with 12%-82% early-stage MF patients (total n > 1000) achieving complete remission (CR) (low certainty evidence). Clinical CR was achieved among 10%-60% treated with topical retinoids (low certainty evidence). Two moderate-sized retrospective case series on topical steroids had 18%-63% CR (low certainty evidence). Only single studies were available for the other therapies.
Conclusions
For most outcomes of interest, the GRADE certainty for topical therapies for early-stage MF was low. Further randomized controlled trials and inclusion of quality of life indicators are needed.
Key words: corticosteroids, GRADE, mycosis fungoides, nitrogen mustard, retinoids, topical treatments
Abbreviations used: 5FU, topical fluorouracil; BAD, British Association of Dermatologists; CR, complete remission; GRADE, Grading Recommendations Assessment, Development and Evaluation; MF, mycosis fungoides; NCCN, National Comprehensive Cancer Network; OFA-LP3, oxygen flow-assisted LP3 carrier; PR, partial remission; RCT, randomized, controlled, blinded trial; UK, United Kingdom; WHO-EORTC, World Health Organization-European Organisation for Research and Treatment of Cancer
Capsule Summary.
-
•
This systematic review incorporates the Grading Recommendations Assessment, Development, and Evaluation criteria to assess the evidence for topical therapies for early-stage mycosis fungoides.
-
•
To aid dermatologists in managing early mycosis fungoides, this article provides a comprehensive overview of options and estimates of effectiveness for topical nitrogen mustard, corticosteroids, retinoids, and other treatments.
Introduction
Mycosis fungoides (MF) is an uncommon skin lymphoma staged with the tumor-node-metastasis-blood system.1 Early-stage disease portends a positive prognosis with patients with stage IA disease experiencing a comparable overall survival to an age-adjusted population, whereas those with Stage IIB and above have an expected >50% mortality within 10 years.2 Most patients present with skin-only early-stage disease3 and are primarily managed by dermatologists.
Treatment for early-stage, patch/plaque disease (eg, stages IA, IB, IIA) is primarily skin-directed including local radiation, phototherapy, and topical medications.4, 5, 6, 7, 8, 9, 10 Within those treatment options for early-stage disease, few large, randomized, controlled studies exist, which also limits conventional systematic reviews and meta-analyses.11 Topical therapies are the most common first-line treatments,10,12,13 but the choice among topical steroids, retinoids, chemotherapy creams, or combination therapies may disproportionately depend on an individual dermatologist's experience and expert guidance, in addition to practical considerations such as cost and availability. To aid dermatologists in interpreting the evidence available for topical therapies for early-stage MF, we conducted a systematic review to assess the literature on their effectiveness and adverse effects.
Materials and methods
This systematic review was performed according to the Preferred Reporting Items for Systematic reviews and Meta-Analysis statement.14
Data sources
A search protocol was created and a literature review conducted with help from a librarian (P.B.). The electronic database search included MEDLINE, Embase, Web of Science, and the Cochrane library. Search terms included mycosis fungoides, cutaneous T-cell lymphoma, nitrogen mustard, topical bexarotene, imiquimod, topical steroids, tazarotene, and topical retinoids. The search strategy can be found in the Supplemental Material (available online via Mendeley at https://doi.org/10.17632/9jbmwpr433.1).
Study selection and data extraction
Covidence was used to import the references, screen the titles and abstracts, screen the full texts, and for data extraction. Independently, 3 authors (E.W., P.Q.R., and P.A.W.) selected the included studies based on the titles and abstracts and then screened the full text of each article. Data extraction was done independently by 5 authors (E.W., P.A.W., P.Q.R., G.E.M., and S.D.S.). Studies needed to contain information on ≥5 patients treated with topical therapies only for early-stage (patch/plaque skin-only disease), classic MF and contain ≥1 of our outcomes of interest. Further details on the study protocol can be found in the Supplemental Material (available online via Mendeley at https://doi.org/10.17632/9jbmwpr433.1).
Our primary outcomes of interest were the following: participant complete remission (CR; 100% clearance of disease as defined within each study); participant partial remission (PR; participants with >50% but <100% reduction of disease as defined within each study); investigator global assessment (0-6 scale with 0 being complete clearance); participants global assessment; and the mean reduction in lesion counts/surface area of involvement from baseline to assessment (absolute values or percentages). Secondary outcomes of interest were changes in quality of life (QOL) or pruritus from baseline and adverse effects of treatment in early-stage disease.
Consensus had to be reached in the event of conflicts (E.W., P.A.W.). In cases of missing information and data, the study authors were contacted for clarification. If no reply was obtained within 6 weeks and the missing information could not be otherwise mitigated, the study was excluded.
Quality assessment
Quality/certainty and risk of bias were assessed in Covidence for each study independently by 5 authors (E.W., P.A.W., P.Q.R., G.E.M., and S.D.S.) according to the Cochrane risk of bias tool15 and graded into high, low, and unknown. In case of conflict, an author not involved in that assessment would make the final decision.
Grading Recommendations Assessment, Development, and Evaluation (GRADE) criteria were used to assess the quality/certainty of the body of evidence. Four levels of certainty are specified in the GRADE approach. The highest certainty rating is for randomized trials, but the certainty of the evidence can be downgraded to moderate, low, and very low depending on the study design, risk of bias, result imprecision, and (in)directness of evidence. These 4 categories (high, moderate, low, and very low) were used as indicators of the quality for the body of evidence and as a basis for recommendations: strong versus conditional evidence for/against.16,17
Results
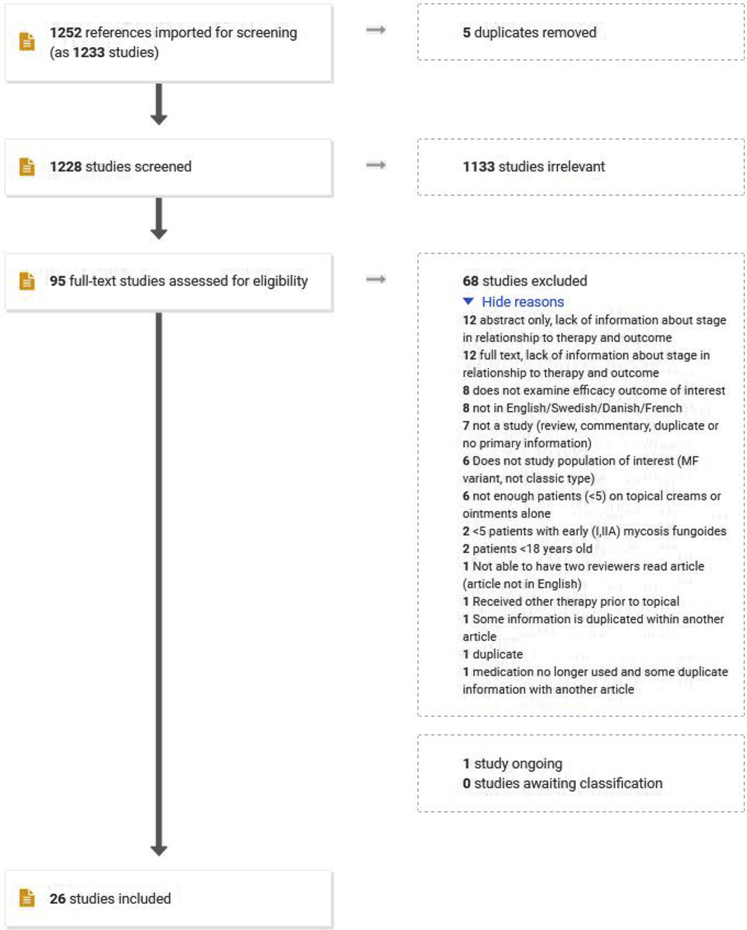
The initial search was conducted on March 20, 2019, and rerun on March 31, 2020. Of 1252 total identified references, 26 met the inclusion criteria (Fig 1). Several studies lacked information about the number of patients in each stage in relation to the therapy and outcome. One study, ClinicalTrials.gov Identifier: NCT02296164, met the inclusion criteria but the data on some end points such as QOL and therapeutic response rates have not yet been published.18
Fig 1.
PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) diagram of topical treatments for early-stage mycosis fungoides.
Among the 26 studies meeting our inclusion criteria, 11 studies were on nitrogen mustard, 4 on retinoids, 2 on corticosteroids, and 1 on each of the following: combination nitrogen mustard and corticosteroids, carmustine, fluorouracil, methotrexate-laurocapram, hexadecylphosphocholine, peldesine, ingenol mebutate, topical methotrexate with oxygen flow-assisted LP3 carrier (OFA-LP3), and resiquimod (Table I).
Table I.
Characteristics of the included studies of topical therapies for early-stage mycosis fungoides
| Author, Year published∗ | Therapy | Cohort description | Treatment regimen | Treatment adverse effects | Outcome(s) of interest |
|---|---|---|---|---|---|
| Ferreira 199019 | Nitrogen mustard | 8 patients; Stage IA = 4, IB = 2, IIA = 2 | Mechlorethamine applied to the entire skin surface, with the exception of the folds. Patients completed 18 cycles, with a median duration of 0.9 months of treatment per cycle | Contact dermatitis, hyperpigmentation | Good response (most lesions completely remitted) in Stage IA: 75%; Stage IB: 50%, Stage IIA: 100% |
| Foulc 200220 | Nitrogen mustard | 22 patients with Stage I | Mechlorethamine 0.2% diluted in 10 mL of solvent and 50 mL of water applied to lesions or the entire skin surface for 1 hour, then washed off with water | Allergic contact dermatitis, irritant contact dermatitis, mild pruritus, pigmentation, xerosis | CR = 59% with a mean treatment duration of 11.7 months |
| Hamminga 198221 | Nitrogen mustard | 17 patients; Stage IA = 8, IB = 6, IIA = 3 | mechlorethamine hydrochloride 10 mg in 40 mL tap water solution applied daily over the entire skin surface | Hyperpigmentation, irritant dermatitis, dry skin, pruritus, urticaria, telangiectasia, allergic contact dermatitis | CR = 82% in early-stage disease with 41-month median follow-up |
| Kim 200322,∗ | Nitrogen mustard | 195 patients; T1 = 107, T2 = 88 | 10 to 20 mg/100 mL solution applied to the entire skin surface daily until complete clinical clearance. Treatment was continued for 6-24 months as maintenance therapy | Irritant or allergic contact reactions, secondary cutaneous malignancies | T1 CR = 65% at median follow-up 73 months, range 5-269 months; T2 CR = 34% at median follow-up 55 months, range 5-242 months |
| Lamberg 198623,∗ | Nitrogen mustard | 88 patients; T1 = 41, T2 = 47 | mechlorethamine 10 mg daily applied to the entire skin surface; tapered frequency if the disease subsided | Not reported | T1 CR = 70% after mean 21 months; T2 CR = 60% after mean 33 months |
| Lessin 201324 | Nitrogen mustard | 260 total (130 in each group) patients with Stage IA, IB, or IIA | Mechlorethamine 0.02% gel or mechlorethamine 0.02% compounded in Aquaphor applied to all affected areas or total skin surface depending on T classification, once daily for 12 months | Skin irritation (18% patients using ointment, 20% gel); pruritus (20%, 25% gel); erythema (18%, 22% gel); contact dermatitis; (19% gel and ointment); skin hyperpigmentation (9%, 7% gel); folliculitis (5%, 7% gel) | CR of intention-to-treat population = 14% for gel and 12% CR for ointment; CR of the efficacy evaluable population = 19% for gel and 15% CR for ointment at mean 5.8 months; response rates gel 58.5% vs ointment 47.7% by CAILS; response rates gel 46.9% vs ointment 46.2% by mSWAT |
| Lindahl 201325 | Nitrogen mustard | 92 patients; T1 = 14 (includes 1 Stage IVA patient), T2 = 78 (includes 2 Stage IVA patients) | mechlorethamine hydrochloride 20 mg dissolved in 40 mL water applied with gauze to lesions or the entire skin surface daily for induction therapy for 14 days. Maintenance therapy of 2 treatments weekly for the first to second month until clearance or discontinued for side effects or progressive disease | Contact dermatitis, secondary cutaneous malignancies | T1 CR = 79%; T2 CR = 51% after median duration of treatment of 16.4 months |
| Ramsay 198826,∗ | Nitrogen mustard | 107 patients; Stage I = 63, Stage II = 44 (different staging system) | mechlorethamine 10 mg in 60 mL of tap water applied daily to the entire skin surface until complete remission. Thereafter tapered every 6 months to every other day application, twice weekly, weekly for 6 months, to stop | Not reported | Stage I CR = 59% after 12.2 months of treatment, 76% after 24 months of treatment; Stage II CR = 41% after 12.2 months of treatment, 45% after 24 months of treatment |
| Stone 200127 | Nitrogen mustard | 6 patients; Stage IA = 1, IB = 5 | Topical nitrogen mustard daily to the entire skin surface area | Not reported | Slight repigmentation of MF lesions in 66% of patients |
| Thomsen 197928 | Nitrogen mustard | 39 patients with histologically proven skin disease only (stage II) | mechlorethamine 20 mg dissolved in 40 mL water/m2 body surface applied daily to the entire skin surface for 0.5 months then weekly maintenance applications | Contact dermatitis | CR = 49% in early-stage skin-only disease |
| Vonderheid 198929,∗ | Nitrogen mustard | 201 patients; Stage IA = 89, Stage IB = 66, Stage IIA = 46 | mechlorethamine 10- 20 mg dissolved in 40-60 mL water applied daily to the entire skin surface except for the genital skin until a complete response was achieved. Once daily or every other day maintenance application for at least 36.5 months | Allergic contact dermatitis (12 patients); carcinogenicity (RR for SCC 7.8, 31 cases; RR BCC 1.8, 27 cases; colon cancer, Hodgkins disease, leukemia, stomach cancer, melanoma) | Stage IA CR = 80%; Stage IB CR = 68%; Stage IIA CR = 61% for mean duration of 53 months |
| de Quatrebarbes 200530 | Nitrogen mustard and corticosteroids | 64 patients; Stage IA = 33, IB = 26, IIA = 5 | mechlorethamine 0.02% aqueous solution twice weekly applications to the entire skin surface followed by an application of betamethasone cream for a 6-month period | 28% of patients had erythema, severe pruritus, burning sensation, or eczematous reaction after a mean of 3.4 ± 2.7 months | Overall CR for early-stage disease = 58% at mean 3.6 ± 2.5 months; Stage IA CR = 61% at mean 3.3 months; Stage IB CR = 58% at mean 3.8 months; Stage IIA CR = 40% at mean 3.0 months |
| Breneman 200231 | Retinoids (bexarotene) | 67 patients; Stage IA = 41; Stage IB = 20; Stage IIA = 5, Stage IIB = 1 | Bexarotene 0.1% gel applied to skin lesions starting daily and increasing up to 4 times daily or the maximal tolerated dose for a median treatment duration of 10.5 months | Rash in 73% of patients; pruritus in 33% of patients; pain in 24% of patients; headache in 6% of patients; vesiculobullous rash in 6% of patients | CR = 21% with median time to response of 4.7 months; IGA stage IA, IB, IIA: CR 21%, Stage IA, IB, IIA: PR 42% |
| Heald 200332 | Retinoids (bexarotene) | 50 patients; Stage IA = 25; Stage IB = 22; Stage IIA = 2; Stage IIB = 1 | Bexarotene gel 1% to all skin lesions 1-4 time daily for at least 3.7 months | 94% of patients experienced at least 1 treatment-related adverse event including irritant dermatitis; low CD4 counts; high glucose; granulocytopenia | PEC (primary end point classification for the study) as CCR (clinical complete response) = 10 % with median time to response of 4.7 months; BSA involvement median change from 9% at baseline to 4.5% at week 44; Investigator global improvement stage IA, IB, IIA, IIB: CR 2%, Stage IA, IB, IIA, IIB: PR 42%; Quality of life >75% moderately or much improved (5-point scale: much, moderately worse, about the same, moderately, much better by week 16); CAILS for stage IA, IB, IIA: CR 10%, stage IA, IB, IIA PR: 36% |
| Apisarnthanarax 200433 | Retinoids (tazarotene) | 19 patients with early-stage MF | Tazarotene 0.1% gel applied to skin lesions once daily for 5.6 months, could also use low-mid potency topical steroids | Skin irritation; erythema, burning, peeling in 84% of patients; fissuring of palms and soles in 11% of patients; transient nausea in 5% of patients; allergic dermatitis in 5% of patients | (all outcomes compared to baseline) BSA involvement mean change −22%; overall disease severity mean change −34%; pruritus difference −0.12%; plaque elevation difference −0.67%; scaling difference −0.70%; erythema difference −1.03%; lesional area mean change −37% after mean duration of treatment of 4.4 months |
| Besner-Morin 201634 | Retinoids (tazarotene) | 10 patients with stage IA-IIA MF | Tazarotene 0.1% cream applied to lesional skin on alternate days for the first 0.5 months and then increased to once daily application if tolerated for a total of 6 months on treatment | 70% experienced mild treatment-related side effects (pruritus, burning, erythema, desquamation) | CR = 60% with mean time to CR of 3.8 months; IGA stage IA, IB, IIA: CR 60%, Stage IA, IB, IIA: PR 0; AILDS 63% reduction of erythema, 86% reduction of scaling |
| Kartan 201935 | Corticosteroids | 31 patients; Stage IA = 22, IB = 9 | Twice daily application to lesional skin with class 1, 2, or 3 topical steroids including clobetasol propionate 0.05%, triamcinolone 0.1% (2/37, 5%) or mometasone 0.1% (1/37, 3%) ointments alone or in combination with a lower potency topical steroid, either desonide 0.05% or hydrocortisone 2.5% for intertriginous or facial skin | Not reported | Stage IA CR = 18%; Stage IB CR = 22% at median follow-up of 3 months; 65% decrease in BSA, 67% in mSWAT (however, decrease in BSA and mSWAT were for study patients of all stages initially receiving topical steroid monotherapy) |
| Zackheim 199836,∗ | Corticosteroids | 79 patients; Stage T1 = 51, T2 = 28 | Twice daily application to lesional skin with class 1, 2, or 3 topical steroids including 0.05% clobetasol propionate, 0.05% diflorasone diacetate, 0.05% halobetasol propionate, 0.05% fluocinonide, 0.1% triamcinolone acetonide, or 0.05% betamethasone valerate | Temporary minor irritation (2); Atrophy (1); Stretch marks (1); Temporary serum cortisol below the lower limit of normal (T1 = 4 patients, T2 = 6 patients) | Stage T1 CR = 63%; Stage T2 CR = 25% at a median follow-up of 9 months |
| Zackheim 199037,∗ | Carmustine | 109 patients; Stage IA = 49, Stage IB = 38, Stage IIA = 22 | Daily application of 10 mg/day to skin lesions. Most were treated for 1.6-3.3 months with a maximum of 4 months | Cutaneous adverse effects: erythema; telangiectasia; hyperpigmentation. Laboratory abnormalities: mild bone marrow depression; increase in aspartate aminotransferase | Stage IA CR = 86%, Stage IB CR = 47%, Stage IIA CR = 55% with median time to achieve CR of 2.7 months |
| Kannangara 201038 | 5-fluorouracil | 6 patients; Stage IA = 4; stage IB = 1, Stage IIA = 1 | 5-fluorouracil applied daily to skin lesions for 3-18 months | 2 patients experienced treatment-related adverse reactions that were mild and self-limited | CR = 67% for 3-18 months |
| Demierre 200339 | Methotrexate-laurocapram | 10 patients with stage IA or IB MF | Doses of 12.5 or 25 g/m2 applied to the entire skin surface every other day for 6 consecutive months | A total of 10 adverse events were reported in 7/10 patients. Pruritus 60%; rash 20%; dry skin 10%; contact dermatitis 10% | No CRs, slight-to-moderate response in 70% at 6 months |
| Dummer 199340 | Hexadecylphosphocholine | 10 patients; Stage IA = 6; Stage IB = 2; Stage IIA = 2 | Once daily application to a defined area during the first week, followed by twice daily application during the second week. Treated areas ranged between 200 and 3200 cm2 and duration of therapy ranged from 2 to 3 months | Slight erythema, fine scaling, and burning sensation within the first 10 minutes of application | CR = 20% at 2-3 month follow-up |
| Duvic 200141 | Peldesine (BCX-34) | 43 patients; Stage IA = 20; Stage IB = 23 | Twice daily applications to the entire skin surface for up to 6 months; randomized, double-blinded, placebo-controlled study | Pruritus 21%; rash 14% | Clinical CR = 2% at 6 months; overall response at study end not significantly different from placebo (P = .79); BSA involvement mean change 3% improvement (P = .84 compared with placebo) |
| Lebas 201742 | Ingenol mebutate | 9 patients with stage IB MF | 0.05% gel applied to target skin lesions in 2 applications spaced one week apart | All patients experienced treatment-related adverse events (burning sensations, oozing, and crusting) but did not stop treatment. 73% of lesions had mild-to-moderate hyperpigmentation | The mean overall CAILS score was reduced by 58.2%. The mean scores were improved by 73.6% for erythema, 93.9% for scaling, and 97.9% for plaque elevation at 2 month follow-up |
| Lebas 202043 | Topical methotrexate with topical drug carrier OFA-LP3 | 10 patients with stage IB MF | Four weekly treatments of OFA-LP3 (a proprietary microemulsion of oil-in-water with surface tension-breaking adjuvants carrying 3% methotrexate) on selected areas | Not reported | Mean modified CAILS scores were improved by 51.3% ± 33.6% for erythema, 53.2% ± 29.8% for scale, 49.6% ± 41.7% for plaque elevation, and 51.3% ± 32.2% for the total score at 2 month follow-up |
| Rook 201544 | Resiquimod | 12 patients; Stage IA = 1; Stage IB = 10; Stage IIA = 1 | Resiquimod 0.06% or 0.03% applied initially 3 or 5 times (respectively) weekly for 2 months, followed by 1 month rest, then another 2 months of treatment, followed by another month rest period | Skin irritation in 25% of patients; skin erosion in 33%; fever in 17%; headaches in 17%; myalgia in 8% of patients | CR = 17% at 6 months; CAILS score CR 33%, PR 42%; SWAT (evaluate overall disease burden & response = the overall CR) CR 17%, PR 75% |
AILDS, Assessment of Index Lesion Severity; BCC, basal cell carcinoma; BSA, body surface area; CAILS, composite assessment of index lesion severity; CCR, clinical complete response; CR, complete remission 100% clearance of disease (or as defined by each individual study); IGA, investigator global assessment; MF, mycosis fungoides; mSWAT, Modified Severity-Weighted Assessment Tool; OFA-LP3, oxygen flow-assisted LP3 carrier; PEC, primary end point classification for the study; PR, partial remission; RR, relative risk; SCC, squamous cell carcinoma; Stage IA, limited skin involvement (T1, N0, M0, B0 or B1); Stage IB, skin-only disease (T2, N0, M0, B0 or B1); Stage IIA, T1-2, N1-2, M0, B0 or B1; T1 Stage, patch/plaque disease <10% of the skin surface; T2 Stage, patch/plaque disease 10% or more of the skin surface.
When there were multiple studies on the same cohort, only the most recent study with information on our primary outcomes was cited.
If multiple studies were published on the same cohort, only the latest study with information on outcomes of interest was included. Not all studies were composed of patients with early-stage MF exclusively. If a study included participants with more advanced disease, we included only the results from patients with early-stage MF.19, 20, 21, 22, 23,25,26,29,35,37,40
Ten open-label case series and 1 randomized, controlled, observer-blinded study (RCT) of nitrogen mustard were included (n = 1035, Table II).19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29 A CR was observed in 12%-82% of early-stage MF patients with a durable response for 4-33 months. Seven studies (n = 633) included results on PR with 13%-45% of participants demonstrating PR. More advanced MF stages/skin involvement were associated with lower CR and PR rates. Contact dermatitis and skin carcinogenicity were the most frequently reported adverse events. Based on GRADE, the certainty of the evidence for nitrogen mustard was low for the reported outcomes because most studies were not randomized or blinded and there was a large range of estimated effect.
Table II.
Summary of GRADE findings for nitrogen mustard
| Outcomes | Estimated effect | Number of participants | Certainty of the evidence | Comments |
|---|---|---|---|---|
| Participant complete remission | 12%-82% (for all 11 studies) | 1035 (11 studies) | Low | Stage IA: 75%; Stage IB: 50%, Stage IIA: 100% (Ferreira 1990) Stage I: 59% (Foulc 2002) Stage I, IIA: 82% (Hamminga 1982) T1 disease: 65%, T2: 34% (Kim 2003∗) T1: 70%, T2: 60% (Lamberg 1986∗) Stage IA, IB, IIA: 14% for gel, 12% for ointment in ITT group (Lessin 2013) T1: 79%, T2: 51% (Lindahl 2013) Stage I: 76%, Stage II: 45% (Ramsay 1988∗) Slight repigmentation of MF lesions in 66% of patients (Stone 2001) 49% in early-stage skin-only disease (Thomsen 1979) Stage IA: 80%, Stage IB: 68%, Stage IIA: 61% (Vonderheid 1989∗) |
| Participant partial remission | 13%-45% (for 7 studies) | 633 (7 studies) | Low | Stage IA: 0%, IB: 13 %, IIA: 0% (Ferreira 1990) Stage IA/B: 23% (Foulc 2002) Stage IB, IIA: 18% (Hammiga 1982) T1 disease: 28%, T2: 38% (Kim 2003) Early-stage ITT group: gel: 45%, ointment: 36% (Lessin 2013) T1: 21%, T2: 40% (Lindahl 2013) 44% in early-stage skin-only disease (Thomsen 1979) |
| Mean reduction in lesion counts/body surface area (BSA) from baseline to assessment | Not estimable, lack of data | No studies addressed this outcome. | ||
| Investigator/physician global assessment | Not estimable, lack of data | No studies addressed this outcome. | ||
| Participants global assessment | Not estimable, lack of data | No studies addressed this outcome. | ||
| Adverse effect estimates | See comment | 834 (8 studies) | Low | Contact dermatitis (CD), hyperpigmentation (Ferreira 1990) Allergic contact dermatitis (ACD), irritant contact dermatitis (ICD), mild pruritus, pigmentation, xerosis (Foulc 2002) Hyperpigmentation, irritant dermatitis, dry skin, pruritus, urticaria, telangiectasia, ACD (Hammiga 1982) ICD or ACD, secondary cutaneous malignancies (Kim 2003) Skin irritation (18% patients using ointment, 20% using gel); pruritus (20%, 25% gel); erythema (18%, 22% gel); CD; (19% gel and ointment); (skin hyperpigmentation 9%, 7% gel); folliculitis (5%, 7% gel) (Lessin 2013) CD, secondary cutaneous malignancies (Lindahl 2013) CD (Thomsen 1979) ACD (12 patients); carcinogenicity (RR for SCC 7.8, 31 cases; RR BCC 1.8, 27 cases; colon cancer, Hodgkins disease, leukemia, stomach cancer, melanoma) (Vonderheid 1989) CD: 12.8%, pruritus: 9.7%, skin irritation 7.4%, erythema 5% (Kim 2020) |
ACD, Allergic contact dermatitis; BCC, basal cell carcinoma; CD, contact dermatitis; CR, complete remission defined as 100% clearance of disease or disappearance of all lesions; GRADE, Grading Recommendations Assessment, Development and Evaluation; ICD, irritant contact dermatitis; ITT, intention-to-treat; MF, mycosis fungoides; PR, partial remission defined as a minimum of 50% improvement and less than 100%; RR, relative risk; SCC, squamous cell carcinoma; Stage IA, limited skin involvement (T1, N0, M0, B0 or B1); Stage IB, skin-only disease (T2, N0, M0, B0 or B1); Stage IIA, T1-2, N1-2, M0, B0 or B1; T1 Stage, patch/plaque disease <10% of the skin surface; T2 Stage, patch/plaque disease 10% or more of the skin surface.
When there were multiple studies on the same cohort, only the most recent study with information on our primary outcomes was cited.
For topical retinoids in early-stage MF, 2 open-label studies of topical bexarotene (n = 117) were included, demonstrating CR rates of 10%-21% and a median time to response of 4.7 months. These 2 studies demonstrated PR rates of 42%-44% (Table III).24,32 Additionally, 1 small open-label pilot study (n = 10) showed a higher CR (60%) with tazarotene after a median of 3.8 months,34 and another small open-label study (n = 19) showed a mean reduction of 22% in body surface area involvement after a mean treatment duration of 4.4 months.33 The QOL life was moderately or much improved for 75% of participants after 16 weeks in an open-label study (n = 50). However, the majority of patients experienced cutaneous adverse events ranging from irritation and fissures to vesiculobullous rash, and for some, systemic adverse events including low CD4 count, high glucose levels, and granulocytopenia were noted. The certainty of the evidence for reported outcomes using retinoids was low also because of the lack of randomization, risk of bias, and wide range of estimated effects.
Table III.
Summary of GRADE findings for retinoids
| Outcomes | Estimated effect | Number of participants | Certainty of the evidence | Comments |
|---|---|---|---|---|
| Participant complete remission | 10%-60% | 127 (3 studies) | Low | Stage IA, IB, IIA, IIB: 21% (Breneman 2002) Stage IA, IB, IIA, IIB: 10% (Heald 2003) Stage IA, IB, IIA: 60% (Besner-Morin 2016) |
| Participant partial clearance/remission | 0-44% | 127 (3 studies) | Low | Stage IA, IB, IIA, IIB: 42% (Breneman 2002) Stage IA, IB, IIA, IIB: 44% (Heald 2003) Stage IA-IIA: 0% (Besner-Morin 2016) |
| Mean reduction in lesion counts/body surface area (BSA) from baseline to assessment | See comments | 69 (2 studies) | Low | BSA involvement mean change −22%; lesional area mean change −37% (Apisarnthanarax 2004) BSA involvement median change −4.5% at week 44 (Heald 2003) |
| Investigator/physician global assessment | See comments | 117 (2 studies) | Low | Stage IA, IB, IIA: CR 21%, Stage IA, IB, IIA: PR 42% (Breneman 2002) Stage IA, IB, IIA, IIB: CR 2%, Stage IA, IB, IIA, IIB: PR 42% (Heald 2003) Stage IA, IB, IIA: CR 60%, Stage IA, IB, IIA: PR 0% (Besner-Morin 2016) |
| Participants global assessment | Not estimable, lack of data. | No studies addressed this outcome. | ||
| Quality of life | >75% moderately or much improved by week 16 | 50 (1 study) | Low | Patients were asked to compare their general CTCL status on a 5-point scale: much, moderately worse, about the same, moderately, much better and at baseline and every 4 weeks until week 44 using the Spitzer questionnaire and CTCL-specific questionnaire (Heald 2003) |
| Adverse effect estimates | See comments | 146 (4 studies) | Low | Rash: 73%, pruritus: 33%, pain: 24%, headache: 6%, vesiculobullous rash: 6% (Breneman 2002) 94% of patients experienced at least 1 treatment-related adverse event including irritant dermatitis; low CD4 counts; high glucose; granulocytopenia (Heald 2003) Skin irritation; erythema, burning, peeling: 84%, fissuring of palms and soles: 11%, transient nausea: 5%, allergic dermatitis: 5% (Apisarnthanarax 2004) Mild treatment-related side effects: pruritus, burning, erythema, desquamation in 70% (Besner-Morin 2016) |
BSA, Body surface area; GRADE, Grading Recommendations Assessment, Development and Evaluation; CTCL, cutaneous T-cell lymphoma.
Two retrospective case series of moderate size (n = 110) were included in the assessment of topical corticosteroids, which revealed CR rates of 18%-63% for patients with stage IA and IB MF after a median of 3-9 months and PR rates of 31%-67%. Very few adverse events were noted35,36 (Table IV). Because of the lack of randomized, blinded studies and the wide range of estimated effect, the certainty of evidence for reported outcomes with the use of topical steroids was low.
Table IV.
Summary of GRADE findings for corticosteroids
| Outcomes | Estimated effect | Number of participants | Certainty of the evidence | Comments |
|---|---|---|---|---|
| Participant complete remission | 18%-63% (2 studies) | 110 (2 studies) | Low | Stage IA: 18%, IB: 22% (Kartan 2019) T1 disease: 63%, T2: 25% (Zackheim 1998) |
| Participant partial remission | 31%-67% (2 studies) | 110 (2 studies) | Low | Stage IA: 32%, IB 67% (Kartan 2019) T1: 31%, T2: 57% (Zackheim 1998) |
| Mean reduction in lesion counts/body surface area (BSA) from baseline to assessment | Not estimable, lack of data | No studies addressed this outcome. | ||
| Investigator/physician global assessment | Not estimable, lack of data | No studies addressed this outcome. | ||
| Participants global assessment | Not estimable, lack of data | No studies addressed this outcome. | ||
| Quality of life | Not estimable, lack of data | No studies addressed this outcome. | ||
| Adverse effect estimates | See comment | 79 (1 study) | Very low | Temporary minor irritation (2); Atrophy (1); Stretch marks (1); Temporary serum cortisol below lower limit of normal (T1 = 4 patients, T2 = 6 patients) (Zackheim 1998) |
BSA, Body surface area; GRADE, Grading Recommendations Assessment, Development and Evaluation.
An open-label prospective study (n = 64) of combined topical corticosteroids and mechlorethamine for stage IA, IB, or IIA MF met the inclusion criteria. This study demonstrated a CR rate of 58% and a PR rate of 8%. Adverse events were recorded among 28% of the patients and included erythema, severe pruritus, burning sensation, or eczematous reaction.30
One moderately sized retrospective cohort (n = 109) on carmustine for stage IA, IB, or IIA MF was included, which demonstrated CR rates of 47%-86% (Table I). Although cutaneous side effects such as rashes were common, there were also systemic side effects, most notably a 3.7% rate of leukopenia.37 Carmustine must be compounded for use in MF.
One small case series (n = 6) of topical fluorouracil (5FU) also met the inclusion criteria. The 5FU was applied daily to MF lesions for 3-18 months with 67% of patients achieving CR and 33% of patients achieving PR.38
Few studies had outcome information on investigator or participant global assessment distinct from CR and PR. Several studies based their rates of CR and PR on the investigator global assessment score.31,34 Fewer studies addressed QOL or associated symptoms such as pruritus changes with therapy (Table II, Table III, Table IV). More recent studies (post 2000) across therapies tended to incorporate clinical tools such as the Composite Assessment of Index Lesion Severity and/or Modified Severity-Weighted Assessment Tool scores as outcome measures.24,32,34,35,42, 43, 44
Several other studies on investigational therapies such as hexadecylphosphocholine,40 peldesine,41 ingenol mebutate,42 resiquimod,44 and combination therapies, such as methotrexate-laurocapram39 and methotrexate-OFA-LP3,43 were identified and included in the systematic review (Table I), but these therapies are not readily available for clinical use.
Discussion
MF is an uncommon disease, making it difficult to perform large-scale, RCTs on MF treatments. Most studies included in this systematic review were retrospective cohorts; only 2 were RCTs. No meta-analyses were performed because of the lack of comparable data.
Using the GRADE approach, we presented a comprehensive, systematic review and assessment of the existing evidence for topical treatments for early-stage MF. Based on the GRADE criteria, our assessment of the certainty of evidence ranges from low to very low for the various topical treatment outcomes. The majority of the included studies are considered to have a high risk of bias as they were not RCTs. The range of estimated effect was large and the sample sizes in many studies were small, contributing to further downgrading of the certainty of evidence.
The topical therapy with the greatest number of relevant studies spanning multiple countries and decades was nitrogen mustard. Ten open-label case series ranging from 8-201 participants and one RCT of 260 patients were included, but unfortunately the heterogeneity in study methods did not allow for meta-analysis. In general, the published case series tended to have higher estimates for CR and PR compared with the RCT. Overall, on balance of the efficacy estimates (12%-82% CR, 13%-45% PR) and adverse events (5%-25%, most commonly skin irritation), we would conditionally recommend topical nitrogen mustard as a treatment for early-stage MF.
In comparison, another commonly used medication for early-stage in MF,9 topical steroids, had only 2 studies meeting the inclusion criteria but fewer documented adverse events. Based on those moderately sized case series, topical steroids were reasonably effective for early-stage MF (estimated 18%-63% CR, 31%-67% PR) and had the advantage that most dermatologists are familiar with their use. Of note, a combination regimen of topical nitrogen mustard and steroids had similar rates of efficacy and adverse effects as either of those agents alone.30
The 4 included studies on topical retinoids showed 10%-60% CR rates, similar to the efficacy range of the other topical therapies. Although 1 study noted improved QOL, the majority of patients experienced cutaneous side effects ranging from irritation to vesiculobullous rash or fissures, and for some, systemic side effects were also noted, which on balance made this treatment less desirable.
Some of the therapies that were included in our review may be promising, but are not readily accessible for clinical use, such as methotrexate-laurocapram, hexadecylphosphocholine, ingenol mebutate, topical methotrexate-OFA-LP3, and resiquimod. Among them are therapies that were in development and others that are not on the market. Ingenol mebutate, for example, is being phased out worldwide as a potential increase in skin carcinogenesis is being investigated.45 Imiquimod was not included in the systematic review as the published studies did not have ≥5 patients with early-stage disease in a single study using topical therapy only.
This study was limited by the available literature meeting our inclusion criteria and the lack of RCTs and studies with comparable treatment regimens or outcome measures for meta-analysis. We were unable to include some studies as we were not able to obtain necessary data on stage, treatment, and outcome despite attempts to clarify or gain access to missing information. Likewise, we did not have access to unpublished materials.
Although there are several expert consensus panels and reviews regarding therapy for early-stage disease,9,46,47 this review is unique in its systematic nature, in-depth focus on topical therapies, and use of the GRADE framework to assess the quality/certainty of the evidence in the literature. In the United Kingdom (UK), the British Association of Dermatologists (BAD) and UK cutaneous lymphoma group have developed guidelines using the AGREE II framework for the management of primary cutaneous lymphomas.5 In Europe, clinical practice guidelines have been developed by the European Society for Medical Oncology6 using a framework based on the Infectious Diseases Society of America-United States Public Health Service. Additionally the WHO-EORTC (European Organisaton for Research and Treatment of Cancer) has published expert consensus-based recommendations for the treatment of MF.7 In North America, the National Comprehensive Cancer Network (NCCN) has published clinical practice guidelines based on expert consensus.8 From these entities, recommended topical therapies for early-stage MF are corticosteroids, nitrogen mustard, carmustine, retinoids, and imiquimod. These guidelines differ slightly among originating entities, for example imiquimod is listed as a potential therapy for limited/localized disease in the NCCN but not in the European guidelines. Some of the other topical therapies included in this systematic review (combination nitrogen mustard and corticosteroids, 5FU, methotrexate-laurocapram, hexadecylphosphocholine, peldesine, ingenol mebutate, methotrexate with OFA-LP3, resiquimod) were not mentioned in any of the expert recommendations.
The overall findings of this systematic review support and extend current expert consensus recommendations to use topical nitrogen mustard, corticosteroids, and retinoids. Suggestions to further our knowledge of the full effect of topical treatments for early-stage MF are to facilitate international data collaborations to collect more patients for rigorous RCTs, as well as including QOL as a primary end point in studies.
Conflict of interest
None disclosed.
Acknowledgments
AAD Cochrane Scholarship provided support for Dr Wu to attend the Cochrane Colloquium 2015. Various authors who were contacted provided additional information on their studies. UC Davis P30, NCI 5P30CA093373 provided career support for Dr Maverakis.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Olsen E., Vonderheid E., Pimpinelli N. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC) Blood. 2007;110(6):1713–1722. doi: 10.1182/blood-2007-03-055749. [DOI] [PubMed] [Google Scholar]
- 2.Kim Y., Liu H., Mraz-Gernhard S. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139(7):857–866. doi: 10.1001/archderm.139.7.857. [DOI] [PubMed] [Google Scholar]
- 3.Quaglino P., Pimpinelli N., Berti E. Time course, clinical pathways, and long-term hazards risk trends of disease progression in patients with classic mycosis fungoides: a multicenter, retrospective follow-up study from the Italian Group of Cutaneous Lymphomas. Cancer. 2012;118(23):5830–5839. doi: 10.1002/cncr.27627. [DOI] [PubMed] [Google Scholar]
- 4.Xia F., Ferket B., Huang V. Local radiation and phototherapy are the most cost-effective treatments for stage IA mycosis fungoides: a comparative decision analysis model in the United States. J Am Acad Dermatol. 2019;80(2):485–492. doi: 10.1016/j.jaad.2018.07.040. [DOI] [PubMed] [Google Scholar]
- 5.Gilson D., Whittaker S.J., Child F.J. British Association of Dermatologists and U.K. Cutaneous Lymphoma Group guidelines for the management of primary cutaneous lymphomas 2018. Br J Dermatol. 2019;180(3):496–526. doi: 10.1111/bjd.17240. [DOI] [PubMed] [Google Scholar]
- 6.Willemze R., Hodak E., Zinzani P.L. Primary cutaneous lymphomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv30–iv40. doi: 10.1093/annonc/mdy133. [DOI] [PubMed] [Google Scholar]
- 7.Willemze R., Jaffe E.S., Gn Burg. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105(10):3768–3785. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network Primary cutaneous lymphomas. National Comprehensive Cancer Network web site. 2020. https://www.nccn.org/professionals/physician_gls/pdf/primary_cutaneous.pdf Accessed March 2, 2021. Available at:
- 9.Jawed S.I., Myskowski P.L., Horwitz S. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sezary syndrome): part II. Prognosis, management, and future directions. J Am Acad Dermatol. 2014;70(2):223.e1–223.e17. doi: 10.1016/j.jaad.2013.08.033. ; quiz 40-2. [DOI] [PubMed] [Google Scholar]
- 10.Quaglino P., Prince H.M., Cowan R. Treatment of early-stage mycosis fungoides: results from the PROspective Cutaneous Lymphoma International Prognostic Index (PROCLIPI) study. Br J Dermatol. 2021;184(4):722–730. doi: 10.1111/bjd.19252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valipour A., Jäger M., Wu P. Interventions for mycosis fungoides. Cochrane Database Syst Rev. 2020;7(7):CD008946. doi: 10.1002/14651858.CD008946.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Agostino P., Kent A., Sharp E. Mycosis fungoides-type cutaneous T-cell lymphoma (MF-CTCL) epidemiology and treatment pathway in Spain: new insights for an accurate description. Drugs Context. 2020;2020(9):4–8. doi: 10.7573/dic.2020-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsang Y., Gu T., Sharma G. Healthcare resource utilization, costs of care, and treatment of mycosis fungoides cutaneous T-cell lymphoma patterns in a large managed care population: a retrospective US claims-based analysis. J Dermatolog Treat. 2018;29(8):747–753. doi: 10.1080/09546634.2018.1466026. [DOI] [PubMed] [Google Scholar]
- 14.Moher D., Shamseer L., Clarke M. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins J.P.T., Thomas J., Chandler J., editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.1. Cochrane; 2020. www.training.cochrane.org/handbook Accessed March 2, 2021. Available at: [Google Scholar]
- 16.Schünemann H., Brożek J., Guyatt G., Oxman A. The GRADE Working Group; 2013. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations.https://gdt.gradepro.org/app/handbook/handbook.html Accessed March 2, 2021. Available at: [Google Scholar]
- 17.Guyatt G.H., Oxman A.D., Vist G.E. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim E.J., Geskin L., Guitart J. Real-world experience with mechlorethamine gel in patients with mycosis fungoides-cutaneous lymphoma: preliminary findings from a prospective observational study. J Am Acad Dermatol. 2020;83(3):928–930. doi: 10.1016/j.jaad.2019.12.070. [DOI] [PubMed] [Google Scholar]
- 19.Ferreira L., Silva R., Brandao M. Mechlorethamine in the treatment of cutaneous T-cell lymphomas. Skin Cancer. 1990;5(2):79–82. [Google Scholar]
- 20.Foulc P., Evrard V., Dalac S. Evaluation of a 1-h exposure time to mechlorethamine in patients undergoing topical treatment. Br J Dermatol. 2002;147(5):926–930. doi: 10.1046/j.1365-2133.2002.04802.x. [DOI] [PubMed] [Google Scholar]
- 21.Hamminga B., Noordijk E.M., van Vloten W.A. Treatment of mycosis fungoides: total-skin electron-beam irradiation vs topical mechlorethamine therapy. Arch Dermatol. 1982;118(3):150–153. doi: 10.1001/archderm.118.3.150. [DOI] [PubMed] [Google Scholar]
- 22.Kim Y.H., Martinez G., Varghese A. Topical nitrogen mustard in the management of mycosis fungoides: update of the Stanford experience. Arch Dermatol. 2003;139(2):165–173. doi: 10.1001/archderm.139.2.165. [DOI] [PubMed] [Google Scholar]
- 23.Lamberg S.I. Retrospective analysis of electron-beam vs topical nitrogen-mustard treatment in 248 patients with mycosis fungoides. J Invest Dermatol. 1986;87(1):151. [Google Scholar]
- 24.Lessin S.R., Duvic M., Guitart J. Topical chemotherapy in cutaneous T-cell lymphoma: positive results of a randomized, controlled, multicenter trial testing the efficacy and safety of a novel mechlorethamine, 0.02%, gel in mycosis fungoides. JAMA Dermatol. 2013;149(1):25–32. doi: 10.1001/2013.jamadermatol.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindahl L.M., Fenger-Gron M., Iversen L. Topical nitrogen mustard therapy in patients with mycosis fungoides or parapsoriasis. J Eur Acad Dermatol Venereol. 2013;27(2):163–168. doi: 10.1111/j.1468-3083.2011.04433.x. [DOI] [PubMed] [Google Scholar]
- 26.Ramsay D.L., Halperin P.S., Zeleniuch-Jacquotte A. Topical mechlorethamine therapy for early stage mycosis fungoides. J Am Acad Dermatol. 1988;19(4):684–691. doi: 10.1016/s0190-9622(88)70223-6. [DOI] [PubMed] [Google Scholar]
- 27.Stone M.L., Styles A.R., Cockerell C.J. Hypopigmented mycosis fungoides: a report of 7 cases and review of the literature. Cutis. 2001;67(2):133–138. [PubMed] [Google Scholar]
- 28.Thomsen K. Scandinavian mycosis fungoides trial. Cancer Treat Rep. 1979;63(4):709–711. [PubMed] [Google Scholar]
- 29.Vonderheid E.C., Tan E.T., Kantor A.F. Long-term efficacy, curative potential, and carcinogenicity of topical mechlorethamine chemotherapy in cutaneous T cell lymphoma. J Am Acad Dermatol. 1989;20(3):416–428. doi: 10.1016/s0190-9622(89)70051-7. [DOI] [PubMed] [Google Scholar]
- 30.de Quatrebarbes J., Estève E., Bagot M. Treatment of early-stage mycosis fungoides with twice-weekly applications of mechlorethamine and topical corticosteroids: a prospective study. Arch Dermatol. 2005;141(9):1117–1120. doi: 10.1001/archderm.141.9.1117. [DOI] [PubMed] [Google Scholar]
- 31.Breneman D., Duvic M., Kuzel T. Phase 1 and 2 trial of bexarotene gel for skin-directed treatment of patients with cutaneous T-cell lymphoma. Arch Dermatol. 2002;138(3):325–332. doi: 10.1001/archderm.138.3.325. [DOI] [PubMed] [Google Scholar]
- 32.Heald P., Mehlmauer M., Martin A.G. Topical bexarotene therapy for patients with refractory or persistent early-stage cutaneous T-cell lymphoma: results of the phase III clinical trial. J Am Acad Dermatol. 2003;49(5):801–815. doi: 10.1016/s0190-9622(03)01475-0. [DOI] [PubMed] [Google Scholar]
- 33.Apisarnthanarax N., Talpur R., Ward S. Tazarotene 0.1% gel for refractory mycosis fungoides lesions: an open-label pilot study. J Am Acad Dermatol. 2004;50(4):600–607. doi: 10.1016/j.jaad.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 34.Besner Morin C., Roberge D., Turchin I. Tazarotene 0.1% cream as monotherapy for early-stage cutaneous T-cell lymphoma. J Cutan Med Surg. 2016;20(3):244–248. doi: 10.1177/1203475415626686. [DOI] [PubMed] [Google Scholar]
- 35.Kartan S., Shalabi D., O'Donnell M. Response to topical corticosteroid monotherapy in mycosis fungoides. J Am Acad Dermatol. 2021;84(3):615–623. doi: 10.1016/j.jaad.2020.05.043. [DOI] [PubMed] [Google Scholar]
- 36.Zackheim H.S., Kashani-Sabet M., Amin S. Topical corticosteroids for mycosis fungoides. Experience in 79 patients. Arch Dermatol. 1998;134(8):949–954. doi: 10.1001/archderm.134.8.949. [DOI] [PubMed] [Google Scholar]
- 37.Zackheim H.S., Epstein E.H., Jr., Crain W.R. Topical carmustine (BCNU) for cutaneous T cell lymphoma: a 15-year experience in 143 patients. J Am Acad Dermatol. 1990;22(5 Pt 1):802–810. doi: 10.1016/0190-9622(90)70112-u. [DOI] [PubMed] [Google Scholar]
- 38.Kannangara A.P., Levitan D., Fleischer A.B., Jr. Six patients with early-stage cutaneous T-cell lymphoma successfully treated with topical 5-fluorouracil. J Drugs Dermatol. 2010;9(8):1017–1018. [PubMed] [Google Scholar]
- 39.Demierre M.F., Vachon L., Ho V. Phase 1/2 pilot study of methotrexate-laurocapram topical gel for the treatment of patients with early-stage mycosis fungoides. Arch Dermatol. 2003;139(5):624–628. doi: 10.1001/archderm.139.5.624. [DOI] [PubMed] [Google Scholar]
- 40.Dummer R., Krasovec M., Röger J. Topical administration of hexadecylphosphocholine in patients with cutaneous lymphomas: results of a phase I/II study. J Am Acad Dermatol. 1993;29(6):963–970. doi: 10.1016/0190-9622(93)70275-x. [DOI] [PubMed] [Google Scholar]
- 41.Duvic M., Olsen E.A., Omura G.A. A phase III, randomized, double-blind, placebo-controlled study of peldesine (BCX-34) cream as topical therapy for cutaneous T-cell lymphoma. J Am Acad Dermatol. 2001;44(6):940–947. doi: 10.1067/mjd.2001.113478. [DOI] [PubMed] [Google Scholar]
- 42.Lebas E., Castronovo C., Arrese J.E. Prospective pilot evaluation of the efficacy and safety of topical ingenol mebutate gel for localized patch/plaque stage mycosis fungoides. Open Dermatol J. 2017;11:98–107. [Google Scholar]
- 43.Lebas E., Chapelier C., Quatresooz P. Exploratory assessment of oxygen flow-assisted cutaneous administration of methotrexate for superficial basal cell carcinoma, mycosis fungoides, and extramammary Paget disease. J Invest Dermatol. 2020;140(3):583–592. doi: 10.1016/j.jid.2019.08.443. [DOI] [PubMed] [Google Scholar]
- 44.Rook A.H., Gelfand J.M., Wysocka M. Topical resiquimod can induce disease regression and enhance T-cell effector functions in cutaneous T-cell lymphoma. Blood. 2015;126(12):1452–1461. doi: 10.1182/blood-2015-02-630335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Risks of Picato for actinic keratosis outweigh benefits. European Medicines Agency; 2020. [Google Scholar]
- 46.Tarabadkar E.S., Shinohara M.M. Skin directed therapy in cutaneous T-cell lymphoma. Front Oncol. 2019;9:260. doi: 10.3389/fonc.2019.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lovgren M.L., Scarisbrick J.J. Update on skin directed therapies in mycosis fungoides. Chin Clin Oncol. 2019;8(1):7. doi: 10.21037/cco.2018.11.03. [DOI] [PubMed] [Google Scholar]