Abstract
Exposure to discrimination or unfair treatment has emerged as an important risk factor for illness and disease that disproportionately affects racial and ethnic minorities. Discriminatory experiences may operate like other stressors in that they activate physiological responses that adversely affect the maintenance of homeostasis. Research suggests that inflammation plays a critical role in the pathophysiology of stress-related diseases. Recent findings on discrimination and inflammation are discussed. We highlight limitations in the current evidence and provide recommendations for future studies that seek to examine the association between discrimination and inflammation.
Keywords: Discrimination, Bias, Unfair treatment, Stress, Inflammation
1. Introduction
There is accumulating evidence that chronic exposure to unfair treatment compromises psychological well-being, and contributes to early mortality and a broad range of morbidity (Williams and Mohammed, 2009; Paradies et al., 2015; Lewis et al., 2015). Researchers have proposed that discrimination affects health through biological processes associated with the development of illness and disease. The experience of discrimination could trigger negative emotional states (e.g., anger, hostility, depression) that activate key regulatory physiological systems (Gibbons and Stock, 2018). Chronic alterations can lead to dysregulation of neuroendocrine and immune systems, partially characterized by increased levels of circulating inflammation (Liu et al., 2017). Inflammation is therefore a potential pathway by which discrimination affects health and contributes to health disparities. The purpose of this review is to provide an overview of recent findings related to discrimination and inflammation. We focus on what is known about the relationship between discrimination exposure and markers of inflammation, giving emphasis to major approaches, empirical findings, and methodological gaps that currently exist in the literature.
1.1. Discrimination as a stressor
1.1.1. Major discrimination vs. everyday discrimination
Discrimination is the differential treatment of individuals based on their membership to a social group (Williams et al., 2019). This review focuses on self-reported discrimination, which is discriminatory events that individuals report experiencing (Williams et al., 2019). Researchers use the terms “self-reported discrimination,” “perceived discrimination,” and “discrimination exposure,” synonymously and at times interchangeably. In an effort to reduce the inconsistency in connotations of pivotal concepts, we use the term “discrimination” throughout. Discrimination can be temporally segmented into two types of exposure: acute and chronic. Acute forms of discrimination are often referred to as “major experiences of discrimination” or “major lifetime discrimination” and are characterized as major life events, such as not being hired for a job or denied a bank loan because of a stigmatized identity (Kessler et al., 1999). In the discrimination literature, exposure to major discrimination is typically assessed by asking participants the number of times they have experienced certain acute discriminatory events. Major forms of discrimination, both racial and nonracial, have been linked to increased incidence of hypertension, (Dolezsar et al., 2014) poorer physical and cognitive functioning, (Shankar and Hinds, 2017) and greater chronic pain (Brown et al., 1982).
Chronic exposure to discrimination is referred to in the literature as “everyday discrimination” and is often characterized as being treated unfairly on a day-to-day basis in different social contexts (Williams et al., 1997). Everyday discrimination has also been associated with increased risk of obesity, diabetes, and cardiovascular disease (Lewis et al., 2015). Despite growing evidence linking both major and chronic forms of discrimination to physical health outcomes, to date, data on biological stress mechanisms remain unclear. Knowledge of how discrimination “gets under the skin” is crucial to informing health research and allocating and targeting resources for prevention and treatment (Adler and Rehkopf, 2008).
1.2. Discrimination and inflammation
Exposure to discrimination can activate three regulatory physiological systems—cardiovascular, neuroendocrine, and immune function (Ong et al., 2017). These interrelated systems play a critical role in responding to various external and internal changing demands and maintaining homeostasis (McEwen, 2008; Sterling, 2012). These physiological responses, however, are dependent on the person’s appraisal and affective reaction to discriminatory situations. Negative appraisal and affective reactions evoke changes in the function of organs and these physiological systems, such as the dilation of blood vessels, modulation of the immune and neuroendocrine systems, and changes in liver functions (Liu et al., 2017; Maydych, 2019). These modulations initiate expression of several pro-inflammatory cytokines (e.g., tumor necrosis factor α (TNF-α), interleukin-6 (IL-6)) and anti-inflammatory cytokines (e.g., IL-10, TNF-β) (Liu et al., 2017; Sorrells et al., 2009). The inflammatory responses are characterized by increased blood flow, capillary dilatation, leucocyte infiltration, and the localized production of a host of chemical mediators, which serve to initiate the elimination of toxic agents and repair damaged tissue (Liu et al., 2017). However, if inflammation becomes chronic, it can cause significant physical and mental health impairments (Ong et al., 2017). Chronic inflammation can also alter brain signal and neural circuitry (Eisenberger et al., 2017) and, as a result, heighten a person’s alertness and sensitivity to potential social threat. Therefore, a noxious cycle can ensue between discrimination and inflammation that consequently can increase the risk of poor health. Fig. 1 serves as a model to visualize the interactions between discrimination, physiological systems, and inflammation to influence mental and physical health risks.
Fig. 1.
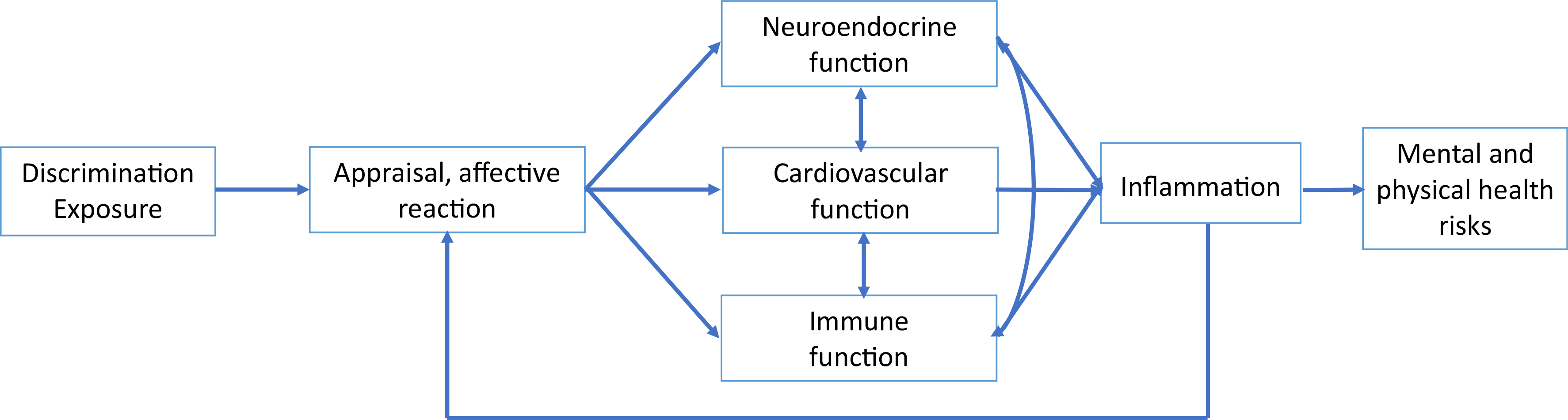
Potential pathway by which perceived discrimination may affect health. Adapted from Williams et al. (2019).
In the discrimination literature, blood pressure and cortisol have been the two primary biomarkers underlying the effects of discrimination on health (Dolezsar et al., 2014; Korous et al., 2017). However, inflammation has been implicated as a potential biomarker that may further explain the relationship between discrimination and health. To date, no study has evaluated the current state of empirical evidence linking experiences of discrimination to inflammatory markers.
1.3. Scope and organization of review
To gain greater insight into the role of discrimination in inflammation, the review is narrative rather than quantitative. Our goals in this review was to synthesize research that assessed the relationship between discrimination and inflammation. We use systematic methods and standardized procedures (Korous et al., 2017) for locating and evaluating the relevance of included studies. We discuss the role of behavioral and biological pathways. Finally, we highlight critical methodological challenges and suggest key directions for future research.
To provide greater detail than presented in the text, the review includes a table with lists of all cross-sectional, longitudinal, and experimental studies that were located in the literature review. Cross-sectional studies examined the association between discrimination and inflammation from a population, or a representative subgroup, at a specific point in time. Longitudinal studies examined whether levels of discrimination predicted future levels of inflammation across more extended periods. Experimental studies determined the moderating effects of self-reported discrimination on the relationship between laboratory stress and inflammatory reactivity.
2. Methods
Our protocol followed the Preferred Reporting Items for Systematic Reviews and Meta-analysis protocol for scoping reviews (PRISMA-ScR) (Tricco et al., 2018). All methods were uploaded on the Open Science Framework (www.osf.io/du4k7).
2.1. Data sources and searches
We assessed the current state of the empirical evidence from 2009 to December 2019 with attention to studies focusing on discrimination and systemic inflammation. PubMed, Scopus, and PsycINFO were searched for peer-reviewed studies published in English. To identify appropriate stress biomarker terms, we referred to three prominent reviews on stress and health, Schneiderman et al. (2005), Steptoe et al. (2007) and Juster et al. (2010). Keywords related to the HPA system, SNS, allostatic load, cytokines, cytokine levels, c-reactive protein, inflammation, inflammatory markers—including TNF-α, IFN-γ, IL-1β, IL-1ra, IL-2, IL-4, IL-6, IL-8, IL-10, and CRP, e-selectin, and fibrinogen. These words were matched with the discrimination-related keywords: discrimination [major headings: PubMed: majr; PsycInfo: mjsub], prejudice [major headings], racis*, stigma, sexis*, unfair treatment, ageis*, homophob* and xenophob*. The final search was run in December of 2019.
2.2. Eligibility criteria and study selection
Criteria for inclusion were full peer-reviewed, empirical journal articles in English language, which examined an inflammatory cytokine in relation to discrimination. A study was eligible for inclusion if it satisfied the following criteria: (a) empirically examined the association between self-reported discrimination (racial or non-racial) and an inflammatory marker, and (b) study was published between 2009 and 2019. Studies were excluded if they 1) focused on self-reported health, 2) were non-empirical (e.g., dissertations, commentaries, or theoretical papers), or 3) did not specifically focus on relationship between discrimination and at least one inflammatory biomarker. Fig. 2 shows details of the search and screening process.
Fig. 2.
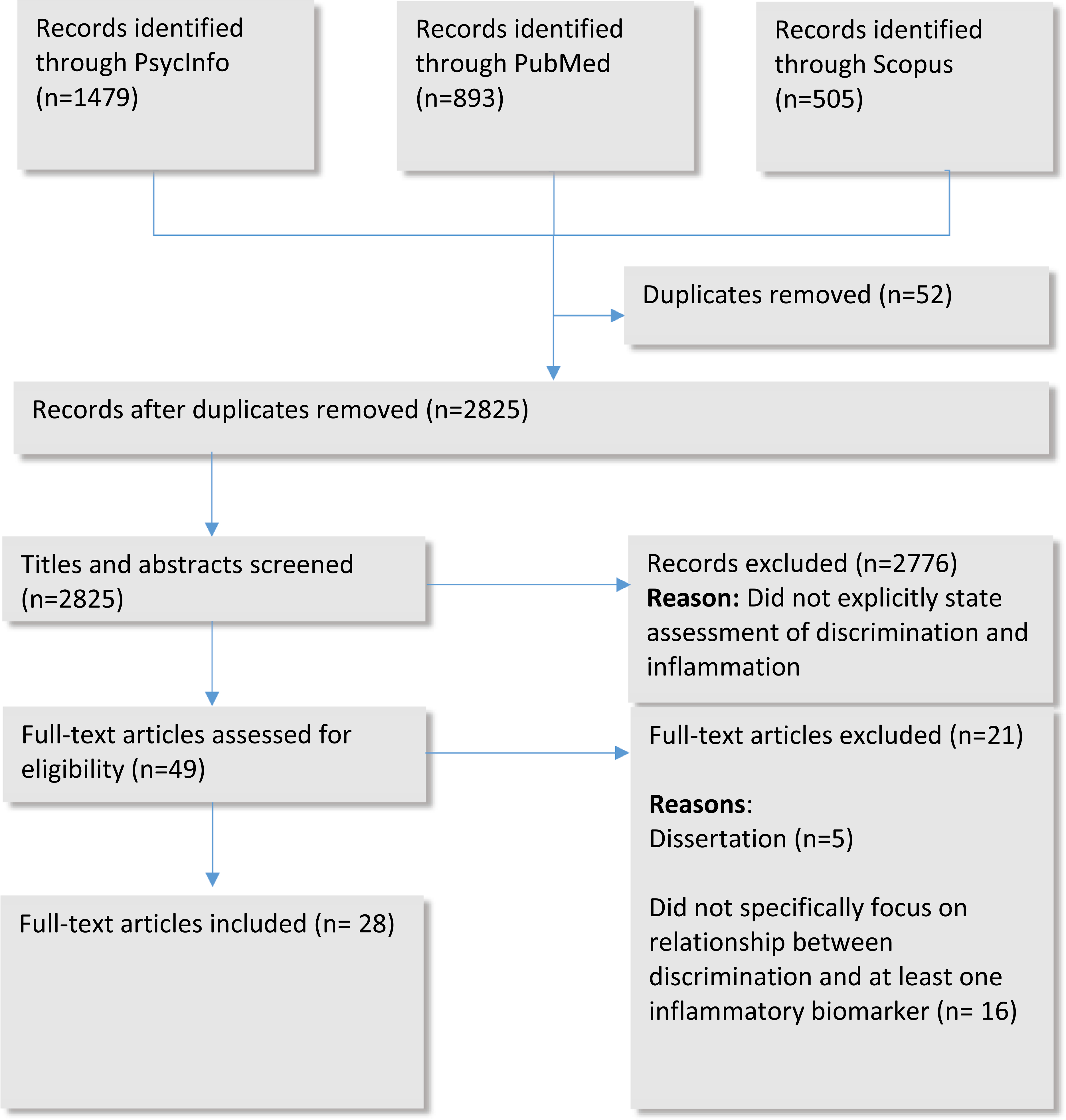
Flowchart of the Article Selection Process.
2.3. Data extraction and quality assessment
Two reviewers (TH, SWC) screened titles and abstracts of the articles followed by a full-text review to check inclusion criteria. Two other authors (AGC and KC) independently assessed risk of bias. Discrepancies were resolved by consensus.
3. Results
We conducted a search of 2877 abstracts in PubMed (893), Scopus (505) and PsycINFO (1479). From these articles, 48 articles were selected as potentially relevant after title and abstract screening. We then performed a full-text screening. Review articles were excluded, unless they were directly relevant to the themes of this review. Of the 48 citations, 28 articles were identified that met the search criteria.
3.1. Characteristics of included studies
The 28 studies recruited a total of 60,039 respondents. Among these participants, 27,755 identified as female and 18,402 were racial/ethnic minorities. Furthermore, the mean age of participants 55.26, with ages ranging between 11 and 86 years old. The majority of these studies were cross-sectional (n = 16, 57%), but studies with other designs were identified, including longitudinal (n = 9, 32%), and experimental (n = 3, 11%) studies. Descriptive details of the studies (i.e., study design, type of discrimination measure, inflammatory marker, participant description, covariates, and main study findings) are presented in Table 1.
Table 1.
Published research on discrimination and inflammation, 2009–2019.
| Author | Discrimination measure | Inflammation marker | Total number of participants | Relevant descriptive statistics | Covariates | Findings |
|---|---|---|---|---|---|---|
|
| ||||||
|
Cross-sectional
Allen et al. (2019) |
Experiences of discrimination | Inflammation measured by CRP and IL-6 | African American women residing in the San Francisco Bay area (n = 208) | Average age of 42 years old Approximately 20% were below the federal poverty threshold, 55% were employed, 30% were married, and 73% had health insurance. |
Age, education, educational attainment, poverty status, employment status, health insurance, marital/partner status smoking status, alcohol use, exercise, cardiovascular medication, diabetes medication, neuroticism | In the high education group, reporting high discrimination was associated with high inflammation compared to those who reporting moderate levels of education. |
| Cuevas et al. (2019) | Everyday discrimination and major lifetime discrimination | CRP | 882 Puerto Rican adults from the Boston metro area | Average age was 63.7 years old (ranged between 45 and 75). The majority of participants were women (73.1%). Approximately 52% of participants had a high school education (or General Education Development) or more. Approximately 12% were employed. Approximately 18% of participants smoked, 21% were moderate or heavy drinkers, and had and 18.9 depressive symptoms on average. |
Age, educational attainment, income-to-poverty ratio, marital status, language-based acculturation, years living in the mainland US, work history, current employment status, alcohol consumption, smoking status, physical activity, diet quality, insomnia symptoms | Greater everyday discrimination was associated with lower odds of having high CRP. Greater major lifetime discrimination was associated with higher odds of having high CRP. |
| Doyle and Molix (2014) | Everyday discrimination | Chronic inflammation measured by IL-6, E-selectin, CRP | 592 African American adults | The sample was 370 female and 222 male Average age was 51 years old |
Covariates not clearly stated. | Everyday discrimination was positively correlated with IL-6 and E-selectin. Everyday discrimination was indirectly associated with relationship strain through increased chronic inflammation. |
| Doyle and Molix (2016) | Everyday discrimination | IL-6 | 99 adults (80% white, 6% multiracial, 4% African Americans, 3% Asians, and 2% Hispanics) | The sample was 78 gay men and 21 lesbian women; average age and income were 35 years old and $60,000/year, respectively. | Race, age, alcohol consumption, medicine usage, BMI, alcohol consumption, prescription medications, BMI | Everyday discrimination was positively associated of higher IL-6 levels in gay men who engaged in less covering, but not for those who engaged in more covering. Everyday discrimination was associated with lower IL6 in lesbian women. |
| Goosby et al. (2015) | Everyday Discrimination | CRP | 58 African American youth | The sample was 79% young females with an average age of 12. Youths’ mothers had an average of some college education. All participants had income at 125% or greater the FPL, were born in the US. |
Age-adjusted BMI, waist circumference, sex, age, maternal education level | Everyday discrimination was positively associated with CRP levels among youth. |
| Giurgescu et al. (2016) | Experiences of discrimination | IL-1β, IL-2, IL-4, IL-6, IL-8 and IL-10 | 96 pregnant African American adult women from a midwifery practice | Participants were in the 2nd trimester of low-risk, singleton pregnancies. Average age was 24 years old and about 20 weeks pregnant. Most participants were multigravida, single, living with their child’s father, and unemployed. Approximately 30% of participants had clinically relevant depressive symptom scores. |
Education, employment status, smoking during pregnancy, BMI, gestational age at data collection, clinically relevant depressive symptoms | Experiences of discrimination was positively associated with IL-4 and IL-6 levels. |
| Kershaw et al. (2016) | Everyday discrimination, major lifetime discrimination due to any attribution, and major lifetime discrimination attributed to race/ethnicity | IL-6 and CRP | 6567 participants (38% White, 28% Black, 22% Hispanic, and 12% Chinese) | The sample was 3099 men and 3468 women Age ranged between 45 and 84 years old Participants were recruited from urban areas (New York City, Baltimore City/County, Forsyth County NC, Minneapolis, Chicago, LA County). |
Age, education, income, employment status, anti-inflammatory medicine usage, statin usage, hormone replacement therapy usage, recent infection, diabetes, hypertension, cholesterol, physical activity, smoking, BMI, and depressive symptoms | Everyday discrimination, lifetime discrimination due to any attribution, and lifetime discrimination attributed to race/ethnicity were positively associated with IL-6 in women. Everyday discrimination was inversely associated with IL-6 in men. Discrimination was not associated with CRP in all models for men and women. No race × discrimination interaction was found. |
| Lewis et al. (2010) | Everyday Discrimination | CRP | 296 African American adults | The sample was 71% female and 29% male. Average age was 73 years old. Participants had 14.5 years of education, a BMI of 29.4, 1.8 discrimination events, and 1.16 depressive symptoms on average. Approximately 7% of participants smoked, 21% had diabetes, and 71% had high blood pressure. |
Age, sex, education, depressive symptoms, smoking, chronic health conditions (i.e. heart disease, diabetes, hypertension, vascular disease), BMI | Everyday discrimination was positively associated with CRP levels. Association was attenuated when BMI was added as a covariate. No sex × discrimination interaction was found. |
| Ong et al. (2017) | Everyday Discrimination | Inflammation measured by CRP, IL-6, E-selectin, fibrinogen, ICAM-1 | 233 total African American adults | The sample was 64% women and 26% men. Age ranged between 37 and 85 years old with an average age of 53.6. About 55% had some college education or their bachelor’s degree. |
Age, gender, education, antihypertensives use, cholesterol lowering medication, steroids, antidepressants, smoking status, alcohol problems, depressive symptoms, major discrimination, and global perceived stress | Everyday discrimination was positively associated with inflammation. |
| Ong and Williams (2019) | Everyday discrimination and major lifetime discrimination | Inflammation burden measured by CRP, IL-6, fibrinogen, E-selectin, and ICAM-1 | 300 adults from Milwaukee | The ethnic representation was African American (77.7%), Hispanic (12.7%), Asian/Pacific Islander (5.6%), and Native American (4.0%) The average age was 53.9 years old (ranged between 36 and 85 years). Approximately 65% were female, 59% had some college education or at least a bachelor’s degree. Approximately 24% were smokers, 42% used antihypertensive medication, and 17.4 depressive symptoms on average |
Age, gender, educational attainment, use of antihypertensive, cholesterol lowering, steroid, antidepressant medications to lower clinical risk, smoking status, and the presence of alcohol problems |
Greater lifetime exposure to discrimination was associated with higher inflammation burden. Mediation analyses suggested that poor global sleep quality was a key mechanism underlying the link between lifetime discrimination and inflammation burden. No evidence of an association between everyday discrimination and inflammation burden. No sex × discrimination interaction was found. |
| Ratner et al. (2013) | Everyday discrimination | IL-6 | 41 total Black and Latina women (27 Blacks, 11 Latinas, 3 Black-Latinas) | Age ranged between 18 and 44 years, average age was 29 years old; Median income ranged from $20,000–30,000. |
Age, income, perceived stress | Everyday discrimination was positively associated with basal IL-6 levels. |
| Stepanikova et al. (2017) | Everyday and major lifetime discrimination | Fibrinogen, E-selectin, CRP, IL-6 | 1054 adults (884 Whites and 170 Blacks) | Sample was 57% women and 43% men. Average age was 54.56 years old. |
Age, gender, education, income, depressive symptoms, anxiety, stress reactivity, aggression, alienation, smoking status, physical activity, BMI, preventive aspirin, hypertension, diabetes, joint/bone diseases, persistent skin trouble, teeth problems, sleep problems | Major lifetime discrimination was positively associated with higher E-selection and IL-6 after controlling for SES and demographics and positively associated with fibrinogen in a fully adjusted model. |
| Sutin et al. (2014) | Everyday discrimination attributed to weight | CRP | 7394 total overweight/obese adults aged 50+ (85% White, 12% African American, and 3% other-race identifying) | The sample was 57% female. Average age was 67 years old. 9.4% of participants felt discriminated against based on their weight. Those with lower BMIs experienced weight discrimination with higher levels of inflammation. |
Age, sex, race, education, BMI | Everyday discrimination attributed to weight was positively associated with CRP levels at BMI between 25 and 30. |
| Thames et al. (2019) | Brief Perceived Ethnic Discrimination Questionnaire - Community Version | Conserved transcriptional response to adversity |
71 adults, including 37 HIV + persons and 34 HIV-persons | Approximately 67% of participants were Black and 23% were White. The average age was 52.9 years old. Approximately 29% were female and the average level of education was 13.8. Approximately 52% were HIV + and 29% were smokers |
Age, education, gender, HIV status, spouse’s education and occupation (for married participants), perceived stress, social status, alcohol use, tobacco use, BMI | Race was associated with higher pro-inflammatory transcription factor activity and discrimination explained more than 50% of total race-related difference in pro-inflammatory transcription factor activity. |
| Van Dyke et al. (2017) | Experiences of discrimination, adapted to measure SES discrimination | CRP | 401 adults (207 African Americans and 194 Whites) | The sample was 68% female, and 54% had a college education. | Age, gender, financial stress, general stress, BMI, smoking status, sleep quality | Signifiant race × education × SES discrimination interaction. SES discrimination was positively associated with CRP among higher educated African American participants, but not in lower educated African American participants or lower/higher educated White participants. |
| Zilioli et al. (2017) | Everyday discrimination | Inflammation measured by CRP, fibrinogen, IL-6, E-selectin, and ICAM-1. | 909 White adults | The sample was 54.13% female. Average age was 55.37 years old. Approximately 78% of participants had a chronic condition. Approximately 52% had just enough money for basic needs, 48% had a bachelor’s degree or higher, and 37.18% had incomes between 300 and 599% of the federal poverty level. |
Age, gender, education, family income, any chronic condition, current financial situation, money basic needs, difficulty paying bills, negative/positive affect, other forms of anger expression | Everyday discrimination was positively associated with inflammation. |
|
Longitudinal
Beatty Moody et al. (2014) |
Everyday Discrimination | CRP | 2,490 women (613 Black, 1,233 White, 221 Chinese, 178 Hispanic, 245 Japanese women) | Average age was 46 years; about 47% had a college degree or more. | Age, race, education, smoking, alcohol consumption, physical activity, SBP, cholesterol, BMI, cynicism, financial strain | Everyday discrimination was positively associated with CRP levels in non-obese women. No race × discrimination interaction effect was found. |
| Boen (2019) | Everyday discrimination and major lifetime discrimination | CRP | 7,280 adults (6,276 Whites and 1,004 Blacks) | Average age was 63 years; about 54% were female; about 87% completed high school. The average household income was $75,482. | Age, race, gender, education, marital status, wealth, and income | Everyday and major life discrimination were positively associated with CRP. Everyday and major life discrimination in the models reduced the racial differences in CRP by 2.3% and 7.2%, respectively. |
| Brody et al. (2014) | Schedule of Racist Events | CRP | 331 African American youth from the rural south age 16–18 | The sample was 53% female. Average age at first and second assessments were 11.2 and 20.2 years old, respectively. Approximately 78% of caregivers completed high school/have a GED; 46.3% of participants live below the FPL with a median household monthly income of $1,655. |
Cumulative SES risk, perceived stress, depressive symptoms, unhealthy behaviors (smoking, alcohol consumption, nutrition, exercise, marijuana use) | Racist events were positively associated with CRP levels. |
| Brody et al. (2015) | Schedule of Racist Events | Inflammation composite score: IL-1β, IL-6, IL-8, IL-10, TNF-α, and IFN-γ | 160 African American teens | The median household gross monthly income in Wave 1 was $2,016. Were 17 years old at the beginning of the study. In Wave 1, approximately 65% of participants had a single caregiver and 69% of caregivers completed high school/had their GED; 42% of families lived below the FPL. |
Gender, intervention, cumulative socioeconomic risk, life stress, depressive symptoms, racial identity, and BMI | Higher levels of racial discrimination was associated with higher cytokine levels. No gender × discrimination interaction. |
| Cunningham et al. (2012) | Experiences of Discrimination | CRP | 3336 adults (1515 Blacks and 1821 Whites) | The sample was 901 Black women, 614 Black men, 958 White women, and 863 White men. Black women: Average age was 31.64 years old Black men: Average age was 31.51 years old White women: Average age was 32.63 years old White men: Average age was 32.57 years old |
Age, gender, education, systolic blood pressure, total cholesterol and triglyceride levels, BMI, and insulin resistance. | Experiences of discrimination was positively associated with CRP levels. Black women reporting 1–2 experiences of discrimination had higher CRP levels compared to black women with no reported experiences of discrimination. Association was not significant among Black women who reported reporting 3+experiences of discrimination. White women who reported 3 + experiences of discrimination had higher CRP levels compared to White women who reported none. Experiences of discrimination and CRP levels was not significant among black and white men who reported 1–2 experiences. |
| Friedman et al. (2009) | Everyday and Major lifetime Discrimination | E-selectin | 804 adults (93% identified as white) | The sample was 365 men and 419 female. Ages ranged from 35 to 86 (average age for men was 59 and for women was 57.9) About 17% of participants had postsecondary education, 15.6% rated themselves as having poor/fair health |
Age, marital status, education, race, self-reported health status, BMI, antihypertensives, cholesterollowering steroids, or anti-depressants, smoking, chronic alcohol/caffeine use. | Major lifetime discrimination and everyday discrimination was positively associated with circulating levels of E-selectin in men. |
| Nguyen et al. (2019) | Healthcare discrimination using one item from everyday discrimination (receive poorer service or treatment than other people from doctors or hospitals). | CRP | 12,695 adults | The average age of participants was 65–67 years across waves, the majority were White (78–80%), and approximately 54–55% of the study sample were female. The average year of school was 13–13.6 across waves, the majority were unemployed (53–59%). |
Age, gender, race/ethnieity, educational attainment, year of interview, log-household size adjusted wealth, log-household size adjusted income, current employment status, marital status, health insurance, self-reported health status, BMI, physical activity, alcohol consumption, ever smoked, depressive symptoms, and personality traits. | Health care discrimination was associated with elevated CRP. Association was attenuated after adjusting for self-reported health behaviors, self-rated health, depressive symptoms, and personality. No race × discrimination interaction was found. No gender × discrimination was found. |
| Vadiveloo and Mattei (2017) | Everyday discrimination attributed to weight | Inflammation measured by CRP, fibrinogen, IL-6, E-selectin, and ICAM-1. | 986 adults (93% identified as white) | Sample was 57% female and 43% male. Average age was 57 years old. Approximately 41% of participants were obese, and 6% reported weight discrimination. Over 75% of participants exercise regularly, 15% were current smokers, and over 75% were either overweight or obese. |
Age, race, household income, education attainment, smoking status, and physical activity | Everyday discrimination attributed to weight was positively associated with inflammatory parameters. |
| Zahodne et al. (2019) | Everyday discrimination | CRP | 12,382 adults | The sample was 1,626 non-Hispanic Black, 1,117 Hispanic, and 9,639 non-Hispanic White adults. Average age for whole sample was 68.8 years old. Approximately 13% of participants were current smokers, and had 1.6 depressive symptoms on average. |
Baseline age and gender | Everyday discrimination did not directly predict change in CRP. Everyday discrimination mediated the relationship between race and CRP through psychological and behavioural factors. Black participants reported greater everyday discrimination compared to White participants. In turn, greater everyday discrimination predicted greater increase in CRP through greater external locus of control, and greater external locus of control. |
|
Experimental
John-Henderson et al. (2015) |
Social-Evaluative Threat | IL-6 | 190 adults (80 Asians, 69 Whites, 20 Latinx, 9 Middle Eastern, 8 African Americans, and 4 other-race identifying) | The sample was 66 male and 124 female. Ages ranged between 18 and 34, with an average age of 19.82 years. |
Ethnicity, gender, gender mismatch, self-reported sleep, BMI. | Lower subjective social class was positively associated with IL-6 levels at baseline. Lower subjective social class predicted a greater increase in IL-6 Speakers rated low in subjective social class responded to the stress test with higher levels of IL-6 regardless of their perception of the evaluator’s social class. Speakers of high class responded with high levels of IL-6 when the evaluator was perceived as having a high social class. |
| Lucas et al. (2017) | Everyday discrimination | CRP | 85 African American adults | The sample was 75% female and 25% male. Approximately 39% were between ages of 21–30 years old; 36% had incomes less than $15,000/year, and 46% completed high school/had a GED. Participants had good oral health, no pre-existing stress-related mental health conditions or endocrine disorders, and were not taking steroid anti-inflammatory medications or adrenergic agonists/antagonists. |
Sex, age, education, and income | Strong racial identity led to highly perceived discrimination that was positively associated with CRP levels during recovery after exposure to mild psychosocial stress. |
| Saban et al. (2018) | Everyday discrimination subscale of the Detroit Area Study Discrimination Scale | IL-6, CRP | 99 total postmenopausal women between ages 50 and 75 (50 African-American, 49 white) | Average age was 60 years old. Approximately 29% had annual incomes between $50,000 and $100,000; 45% completed some college/technical school; 47% were married; 64% were overweight; 68% had high blood pressure, and 43% were on an antihypertensive medication. |
Age, race, marital status, household income, BMI, statin use, childhood maltreatment, depressive symptoms, subjective social status, depressive symptoms | Perceived discrimination was significantly associated with salivary IL-6 prior to and during exposure to mild psychosocial stress. No race × discrimination interaction effect was found. |
3.2. Study design
Among the cross-sectional studies reviewed (n = 16), all reported a positive association between discrimination and inflammation. Despite a limited number of studies (n = 9), evidence from longitudinal studies suggests a positive association between discrimination and inflammation. Among the nine longitudinal studies (ranging from 15 months to 13 years), eight were consistent with theoretical predictions, (Beatty Moody et al., 2014; Boen, 2019; Brody et al., 2014, 2015; Cunningham et al., 2012; Friedman et al., 2009; Nguyen et al., 2019; Vadiveloo and Mattei, 2017) whereas one reported null findings (Zahodne et al., 2019). Very limited work evaluated whether role of discrimination and inflammatory in a laboratory context. Among the three experimental studies identified, (Saban et al., 2018; Lucas et al., 2017; John-Henderson et al., 2015) all found discrimination to predict inflammatory reactivity.
3.3. Inflammatory markers
A wide range of cytokines was examined, including CRP, fibrinogen, IL-6, E-selectin, and Intercellular Adhesion Molecule 1 (ICAM-1). IL-6 and CRP were the most assessed inflammatory marker. A total of 26 studies focused on either IL-6 or CRP or both. Two studies (Brody et al., 2015; Giurgescu et al., 2016) assessed a wider range of inflammatory markers, including IL-1β, IL-6, IL-8, IL-10, TNF-α, and IFN-γ. Blood samples were the most common method of data collection.
3.4. Discrimination and inflammation
The Everyday Discrimination Scale (Williams et al., 1997) was the most commonly assessed discrimination measure (n = 15). CRP and IL-6 were the two most common inflammatory markers assessed in studies of everyday discrimination. The second most common discrimination measure was the Experiences of Discrimination (EOD) scale (n = 4), which is a measure assessing the occurrence and frequency of acute forms of discrimination due to race/ethnicity over a lifetime, such as not getting hired or getting a job, not receiving medical care, or not receiving service in a store or restaurant (Krieger et al., 2005). Greater EOD was associated with higher levels of CRP, IL-4, and IL-6. Six studies (Boen, 2019; Cuevas et al., 2019; Friedman et al., 2009; Kershaw et al., 2016; Ong and Williams, 2019; Stepanikova et al., 2017) assessed acute forms of discrimination with the Major Experiences of Discrimination scale (Kessler et al., 1999). Greater lifetime exposure to major discrimination was associated with elevated levels of E-selectin, (Friedman et al., 2009; Stepanikova et al., 2017) IL-6, (Kershaw et al., 2016; Stepanikova et al., 2017) and CRP (Boen, 2019; Cuevas et al., 2019) and greater inflammation burden calculated as the sum of CRP, IL-6, fibrinogen, E-selectin, and ICAM-1 (Ong and Williams, 2019).
3.5. Characteristics of participants
Three studies examined the interaction effect of race/ethnicity and SES and discrimination on inflammation. Nguyen et al. (2019) and Kershaw et al. (2016) found no evidence that association between discrimination and inflammation varied by race/ethnicity. However, Van Dyke et al. (2017) found that SES discrimination was associated with higher CRP among higher educated African American participants but not for lower educated African American participants nor for lower and higher educated White participants. Five studies (Brody et al., 2015; Friedman et al., 2009; Kershaw et al., 2016; Ong and Williams, 2019; Lewis et al., 2010) examined the interaction effect of gender and discrimination on inflammation. Findings were mixed. Friedman et al. (2009) found a positive association between everyday discrimination and inflammation in men, but not women. Kershaw et al. (2016) found that everyday and lifetime discrimination were associated with higher IL-6 in women, but everyday discrimination was inversely related to IL-6 among men (Kershaw et al., 2016). Other studies did not find a significant gender by discrimination interaction effect on inflammation (Brody et al., 2015; Ong and Williams, 2019; Lewis et al., 2010).
4. Methodological challenges
This is the first systematic review to our knowledge that focuses on the association between discrimination and inflammation. Although the findings from the studies reviewed support a link, a significant number of included studies showed weak methodological quality. Of primary concern is the limited number of longitudinal studies and the reliability and validity of standard assessment tools for measuring inflammation and discrimination.
4.1. Longitudinal studies
Overall, perhaps one of the most striking findings is just how few studies have addressed issues related to the direction of association between discrimination and inflammation. Indeed, studies to date have largely been cross-sectional, making it difficult to infer the causal significance of associations. In addition to providing a more rigorous assessment of mechanistic pathways, prospective, multi-wave, longitudinal studies are critically important in advancing the science of discrimination and inflammation because they 1) allow for tests of theoretical models that assume stability of relations over time; 2) help address questions regarding duration of discrimination exposure and whether sustained or chronic exposure over time to discrimination is associated with inflammation above and beyond a single report; and 3) provide evidence against reverse-causality arguments, which posit that individuals who evidence inflammatory dysregulation may also report more discrimination. Overall, longitudinal studies addressing the reciprocal and long-term association between discrimination and inflammation are urgently needed.
4.2. Inflammation
Another key challenge concerns the measurement of inflammation. Inflammation is generally assessed through either saliva or blood collections. Each method provides its respective advantages and disadvantages. Saliva samples are less invasive and may be less burdensome to collect for both researchers and participants (Riis et al., 2015). Given these benefits, salivary values can provide integrated measurements for extended periods of time, making it appropriate for the study of chronic psychosocial stressors (Riis et al., 2015). Despite these advantages, at present there is no “gold standard” method of sample collection (e.g., time frame to collect and assess saliva samples) or clear criterion for the validity of salivary inflammation markers (Riis et al., 2015). Studies have shown that cytokines demonstrate modest correlations between blood and saliva (Slavish et al., 2015; Slavish and Szabo, 2019; Williamson et al., 2012). Bosch (2014) suggests that most proteins do not easily pass the multiple barriers between blood and saliva (e.g., the capillary wall, the interstitial space, the cytoplasm of the acinus or duct cell), which prevents many blood-derived markers of inflammation from accurately being detected with saliva samples. Unlike cortisol, cytokines and acute phase proteins are too large to enter saliva through diffusion or ultrafiltration and, thus, more likely to enter saliva through leaky patches such as through local sites of inflammation (Bosch, 2014). Therefore, saliva samples are more likely to capture inflammation related to local tissue damage or oral health, (Slavish et al., 2015; Bosch, 2014) suggesting that the inclusion of salivary biomarkers of inflammation in studies of discrimination may help to elucidate the relationship between racial discrimination and oral health (e.g., toothache and tooth loss) (Ben et al., 2014; Lawrence et al., 2016).
Likewise, blood remains the best body fluid for evaluating systemic inflammation (Williamson et al., 2012). However, blood collection is an invasive procedure for screening inflammatory risk and, thus, repeatedly collecting venous blood would be impractical. Dried blood spots (DBS) offer a promising alternative (Williams and McDade, 2009). DBS are drops of capillary whole blood from a simple prick of the finger collected on filter paper (Williams and McDade, 2009).. This procedure is a minimally invasive substitute to intravenous blood sampling and is a convenient method to store and transport inflammation data (Williams and McDade, 2009; McDade et al., 2004). Plasma DBS show high correlation in CRP measurements (Williams and McDade, 2009; Brindle et al., 2010). Given the validity and convenience of this method, it provides a viable alternative in community-based, epidemiologic studies to assess acute and chronic effects of discrimination. Dried blood spots, however, also have disadvantages. For instance, there is currently no universal protocol for the pre- and post-analytical phase of testing (e.g., clinical cut-offs, application of sample onto the filter paper, consistency in drying process, transportation and storage of the samples, an established reference interval), which can contribute to reproducibility issues (Zakaria et al., 2016; Lim et al., 2016). Except for CRP, validation findings for other biomarkers have been mixed or remains unexamined (Samuelsson et al., 2015). Standardizing the methods and techniques will be required as DBS become more widespread and begin to be used in discrimination research. For now, venipuncture sampling remains the gold standard for blood-based, diagnostic testing due to existing quality-assured procedures and diagnostic tests that help achieve reproducibility and replicability of results (Samuelsson et al., 2015).
CRP and IL-6 have been the primary biomarkers in the discrimination literature owing to the fact that they are sensitive to stress exposure and are heavily studied in cardiovascular health research (Fioranelli et al., 2018). However, CRP and IL-6 are two of many inflammatory markers that are induced by stress, as well as linked to cardiovascular disease (Segman and Stein, 2015; Kiecolt-Glaser et al., 2010). A few studies (Brody et al., 2015; Giurgescu et al., 2016; Ong and Williams, 2019; Zilioli et al., 2017) assessed the association between discrimination and a wider range of inflammatory markers, such as IL-1β, IL-6, IL-8, IL-10, TNF-α, and IFN-γ, and found support for their theoretical predictions. Future research should investigate understudied inflammatory cytokines to help validate previous findings and elucidate the inflammation pathway linking discrimination and health.
In addition, future research should go beyond blood cytokine proteins. Inflammation involves the activation of a highly coordinated genetic and molecular mechanisms (e.g., macrophages, lymphocytes, neutrophils, eicosanoids, resolvins) that operate in tandem to initiate and deactivate the inflammatory response and independently increase the risk of disease (Abdulkhaleq et al., 2018; Mietla et al., 2016; Natoli et al., 2011). The Conserved Transcriptional Response to Adversity (CTRA) provides a promising approach to understanding the inflammatory transcriptional mechanisms (both genetic and molecular processes) of discrimination exposure and disease. CTRA is characterized by increased pro-inflammatory gene expression and a suppression of anti-viral gene expression. Chronic upregulation of inflammation-related mediators (e.g., NF-κB and AP-1) can result in inflammation-related diseases, such as cardiovascular disease. Chronic downregulation of anti-viral gene expression (e.g., interferon response factors) can render individuals more susceptible to viral infections (Cole, 2019). Only one study (Thames et al., 2019) examined the role of discrimination on racial differences in inflammation-related gene expression profiles. Using CTRA, researchers found that Black participants had higher inflammatory signaling than Whites and racial discrimination explained more than 50% of total race-related difference in proinflammatory transcription factor activity (Thames et al., 2019). Integrating transcriptome data in discrimination studies can provide valuable insights into the molecular mechanisms related to inflammation and disease, the potential inflammatory cells related to discrimination, and the signaling pathways that are activated by transcription factors (Cole, 2019; Barrat et al., 2019; Fredrickson et al., 2015; Jura and Koj, 2011; Zak et al., 2014). Given its functional utility and clinical and public health relevance, CTRA provides a promising framework to understand the molecular patterns that underlie the association between discrimination and disease (Cole, 2019).
4.3. Discrimination measures
Beyond the measurement of inflammation, the measurement of discrimination is also an issue for the current review. To accurately understand the physiological effects of discrimination, measures of discrimination should capture both the level of severity and duration of discriminatory experiences. For instance, some acute discriminatory events may be longer in duration and more severe than other events (e.g., being harassed by police vs. being denied a bank loan). Given that the stressor chronicity, intensity, frequency, and modality of the stressor is a major factor in the overall response of physiological systems, (Herman et al., 2016) assessing these aspects of discrimination can aid in contextualizing the influence that discrimination may have on inflammation.
Future research should also explore how individuals appraise discrimination (e.g., threatening vs. challenging) and how appraisal processes promote or inhibit dysregulation of biomarkers (see Fig. 1). Challenge appraisals may play a protective role in buffering against the long-run psychological and physical health consequences of discrimination (Berjot and Gillet, 2011). In addition, most studies have focused on the frequency of exposure rather than the attribution of the type of discrimination experienced. Research suggests that exposure to discrimination, irrespective of which personal characteristic it is based, has negative consequences for health (Lewis et al., 2015; Cuevas and Williams et al., 2018; Grollman, 2014; Sutin et al., 2015). However, people with multiple marginalized identities due to race, gender, sexuality, or other attributes may be exposed to greater discrimination, (Seng et al., 1982. 2012,; Jackson et al., 2016) and therefore, at a greater risk of physiological dysregulation. Future research should examine the interdependence of discriminatory experiences based on multiple, co-occurring marginalized identities and how they combine to influence health (Cuevas and Williams et al., 2018).
Despite racial and nonracial discrimination having similar associations with health, it is unknown whether the underlying biological mechanisms are similar (Lewis et al., 2015). Discrimination attributed to an unmodifiable or unconcealable phenotype like race may induce different psychological and physiological responses compared to other modifiable or concealable phenotypes like weight and sexual orientation (Sumner et al., 2018). Racial discrimination, in particular, needs to be placed within a historical context as historical and contemporary experiences of racism (e.g., slavery, Jim Crow laws, mass incarceration) can be transmitted across generations (Colen et al., 2019; Krieger, 1999). For instance, racial discrimination is associated with higher inflammation in pregnant women and higher risk of preterm birth, which, can disrupt an infant’s normal biological and developmental trajectories (Giurgescu et al., 2016, 2013). Racial discrimination can also lead to distinct behavioral responses that may exacerbate the effect of discrimination on inflammation. For example, racial and ethnic minorities may mentally prepare for potential race-related discrimination through a process of “racism-related vigilance” (Williams and Mohammed, 2008). Racism-related vigilance is associated with sleep difficulty, obesity, and hypertension (Hicken et al., 1982; Hicken et al., 2014; Hicken et al., 2013). Future studies should continue to examine racial and nonracial forms of discrimination to understand their potentially distinct associations with inflammatory markers.
Discrimination should be understood as being part of a complex nexus that characterizes psychosocial stress. Psychosocial stress is a multidimensional construct that encompasses a wider range of stressors that disproportionately affect racial/ethnic minorities, including financial strain and relationship problems (Sternthal et al., 2011). Therefore, focusing solely on discrimination can lead to a biased estimate of the effects of stress on inflammation. Boen (2019) developed a cumulative stress burden index of multiple stressors—including stressful life events, financial strain, chronic stress, and everyday and major discrimination—and found that cumulative stress was associated with higher levels of CRP over time and explained a substantial proportion of Black-White differences in inflammation levels (Boen, 2019). Understanding how multiple stressors cluster at different stages of life could add valuable insight into the role of stress in contributing to inflammation.
5. Future research
Although there is grown support for an association between discrimination and inflammation, full understanding of the phenomenon is far from complete. Overall, the limitations in existing data provide important impetus for future work. Below, we highlight several critical yet unresolved issues for future research.
A need for a life course perspective. Inflammation levels vary by age-cohort, with levels increasing with age (Beydoun et al., 2018). Given that there is a natural decline in immune functioning as people age, the temporal nature of the association between discrimination and inflammation in cross-sectional studies remains unclear. As noted, while longitudinal studies have established a link between discrimination and elevated levels of inflammation, taking a life course perspective would allow researchers to better pinpoint the temporal order of discriminatory events as well as identifying critical periods that may contribute to long-term poor health outcomes. For instance, a study by Cuevas et al. (2019) found that initial exposure to racial discrimination during early childhood and adolescence was associated with greater odds of having any cardiovascular-related health condition later in life compared to individuals who reported no discrimination. Discrimination and inflammation can also have a cyclical relationship. Eisenberger et al. (2017) suggests that proinflammatory cytokines can signal the brain to heighten neural sensitivity to the social environment, leading to increases in sensitivity to social threat and other negative social experiences. Therefore, it is possible that discrimination can lead to greater inflammation, and, in turn, greater inflammation can lead to heightened vigilance or vigilant coping (i.e., the monitoring or modification of behavior to protect oneself from anticipated discrimination) (Himmelstein et al., 2015). Collecting life-course data at multiple time points would allow researchers to establish temporal causality and determine how changes in discrimination are coupled with changes in inflammation levels overtime.
5.1. Increasing generalizability
To date, most studies have focused predominantly on African American (or Black) and European American (or White) participants. Research should include samples that are more nationally representative and inclusive of other racial and ethnic minorities (e.g., Hispanics, Native Americans, Asians). Considering the heterogeneity of findings among minority racial groups, future studies should also look more closely at within-group differences, including ethnicity, nativity, and skin tone (Williams and Mohammed, 2008).
5.2. Discrimination, inflammation, and disparities
Few studies have examined the contribution that discrimination makes on racial/ethnic disparities in inflammation. Racial/ethnic minorities, particularly Black Americans, have increased levels of low-grade inflammation compared to Whites (Boen, 2019; Schmeer and Tarrence, 2018; Boen and Hummer, 2019). Boen and others have documented that discrimination exposure explains a substantial proportion of racial/ethnic disparities, above and beyond sociodemographic factors and other social determinants (Stepanikova et al., 2017; Boen, 2019). Given the indicative significance of discrimination contributing to racial/ethnic health disparities, (Williams et al., 2019) future research is needed to assess how acute and chronic discrimination affect racial/ethnic differences in inflammation.
5.3. Accounting for health behaviors
Medication, exercise, substances (e.g. caffeine, alcohol, contraception, tobacco), weight, and sleep can substantially alter circulating inflammation levels (Ong and Williams, 2019; Ehrlich et al., 2016; O’Connor et al., 2009). For instance, exercise is a known stress-coping behavior that also lowers inflammation by inhibiting TNF-α and promoting the release anti-inflammatory cytokines like IL-10 (Kiecolt-Glaser et al., 2010; Stults-Kolehmainen and Sinha, 2014). Alcohol consumption, is also a stress reliever, in that, alcohol is consumed to reduce stress-induced anxiety (Anthenelli and Grandison, 2012). However, chronic alcohol use can impair liver functions, altering the organ’s production of acute phase proteins and cytokines, and the containment of immune cells, and, in turn, can lead to chronic inflammation and liver damage (Robinson et al., 2016; Swain, 2000; Wang et al., 2010). Depending on the research question and inflammatory marker, researchers should be cognizant of the health behaviors that are included or excluded from the analyses.
Future research should also attend to assessment of potential confounders. Body mass index (BMI) and diet are major confounders. High BMI is strongly associated with chronic state of systemic low-grade inflammation (Ellulu et al., 2017; Forsythe et al., 2008; Visser et al., 1999) and is influenced by discrimination exposure (Hunte, 2011; Hunte and Williams, 2009). Dietary patterns (e.g., Mediterranean diet, high-fat diet) can modulate key pathways to inflammation, such as sympathetic activity and transcription factor activity levels (e.g., activation of NF-κB and production of proinflammatory cytokines. BMI rates and dietary patterns also varies by race/ethnicity and socioeconomic status—disproportionately affecting Blacks and Hispanics/Latinos and low-socioeconomic groups (Flegal et al., 2016; Ogden, 2017). Without considering the role of BMI and other health behaviors, researchers can mischaracterize the relationship between discrimination and inflammation. Establishing a robust examination of discrimination and inflammation would help determine whether potential confounders serve as potential mediators and therefore meaningful targets for intervention (see O’Connor et al., 2009).
5.4. Moving beyond inflammation
Analyzing one biomarker alone may not account for the full scope of physiological effects of discrimination, since biological systems often work in conjunction with one another. Allostatic load or multisystemic physiological dysregulation is characterized by elevated (or reduced) physiological activity across multiple regulatory systems, including the immune system, and cardiovascular and metabolic processes (McEwen, 2004). Exposure to discrimination can activate multiple physiological systems—that gradually place strain on the body’s ability to efficiently respond to stressors. Studies have found evidence of an association between discrimination and allostatic load. For instance, in a cross-sectional analysis of a sample of 233 African Americans using MIDUS data, Ong et al. (2017) found that everyday discrimination was associated with higher allostatic load, even when adjusting for sociodemographic characteristics, medication use, health behaviors, depressive symptoms, lifetime discrimination and global perceived stress. In a longitudinal study of 331 African American adolescents, Brody et al. (2014) found a significant positive correlation between high, stable levels of racial discrimination between the ages of 16 and 18 with allostatic load at age 20, even after adjusting for cumulative socioeconomic risk, perceived stress, and depressive symptoms and unhealthy behavior assessed at age 20. Therefore, assessing elevated (or reduced) physiological activity across multiple regulatory systems can comprehensively distinguish the relationship between discrimination and inflammation.
6. Conclusions
As more research continues to focus on the relationship between discrimination and health, there has been a growing interest in understanding the pathways through which discrimination operates. Emerging research indicates inflammation is one such pathway. The research reviewed here suggests that experiences of discrimination, both acute and chronic, can dysregulate immune function, characterized by elevated levels of inflammation. As research continues to examine inflammation and other potential pathways, addressing existing methodological and theoretical gaps in the extant literature can help inform prevention and intervention measures.
Funding
The development of the manuscript was partially supported by Cancer Disparities Research Network/Geographic Management Program (GMaP) Region 4 funded by 3 P30 CA006927-52S2 and CTSI Mentored Career Development Award (KL2 TR002545).
We thank Dr. Gregory Miller for his insightful comments and ideas during the early stages of the manuscript’s development. We thank Kasim Ortiz and Danielle DeNufrio Valerio for reviewing the manuscript.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbi.2020.07.017.
References
- Abdulkhaleq LA, Assi MA, Abdullah R, Zamri-Saad M, Taufiq-Yap YH, Hezmee MNM, 2018. The crucial roles of inflammatory mediators in inflammation: a review. Vet. World 11 (5), 627–635. 10.14202/vetworld.2018.627-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler NE, Rehkopf DHUS, 2008. Disparities in health: descriptions, causes, and mechanisms. Annu. Rev. Public Health 29 (1), 235–252. 10.1146/annurev.publhealth.29.020907.090852. [DOI] [PubMed] [Google Scholar]
- Allen AM, Thomas MD, Michaels EK, Reeves AN, Okoye U, Price MM, Hasson RE, Syme SL, Chae DH, 2019. Racial discrimination, educational attainment, and biological dysregulation among midlife African American women. Psychoneuroendocrinology 99, 225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthenelli R, Grandison L, 2012. Effects of stress on alcohol consumption. Alcohol Res. Curr. Rev. 34 (4), 381–382. [PMC free article] [PubMed] [Google Scholar]
- Barrat FJ, Crow MK, Ivashkiv LB, 2019. Interferon target-gene expression and epigenomic signatures in health and disease. Nat. Immunol. 20 (12), 1574–1583. 10.1038/s41590-019-0466-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty Moody DL, Brown C, Matthews KA, Bromberger JT, 2014. Everyday discrimination prospectively predicts inflammation across 7-years in racially diverse midlife women: study of women’s health across the nation: discrimination and C-reactive protein over time. J. Soc. Issues 70 (2), 298–314. 10.1111/josi.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben J, Paradies Y, Priest N, et al. , 2014. Self-reported racism and experience of toothache among pregnant Aboriginal Australians: the role of perceived stress, sense of control, and social support. J. Public Health Dent. 74 (4), 301–309. 10.1111/jphd.12059. [DOI] [PubMed] [Google Scholar]
- Berjot S, Gillet N, 2011. Stress and coping with discrimination and stigmatization. Front Psychol. 2. 10.3389/fpsyg.2011.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beydoun MA, Dore GA, Canas J-A, et al. , 2018. Systemic inflammation is associated with longitudinal changes in cognitive performance among urban adults. Front Aging Neurosci. 10. 10.3389/fnagi.2018.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boen C, 2019. Death by a thousand cuts: stress exposure and black-white disparities in physiological functioning in late life. J. Gerontol. Ser. B. 10.1093/geronb/gbz068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boen CE, Hummer RA, 2019. Longer—but harder—lives?: the hispanic health paradox and the social determinants of racial, ethnic, and immigrant-native health disparities from midlife through late life. J. Health Soc. Behav. 60 (4), 434–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch JA, 2014. The use of saliva markers in psychobiology: mechanisms and methods. Monogr. Oral Sci. 24, 99–108. 10.1159/000358864. [DOI] [PubMed] [Google Scholar]
- Brindle E, Fujita M, Shofer J, O’Connor KA, 2010. Serum, plasma, and dried blood spot high sensitivity C-reactive protein enzyme immunoassay for population research. J. Immunol. Methods 362 (1–2), 112–120. 10.1016/j.jim.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Lei M-K, Chae DH, Yu T, Kogan SM, Beach SRH, 2014. Perceived discrimination among African American Adolescents and allostatic load: a longitudinal analysis with buffering effects. Child Dev. 85 (3), 989–1002. 10.1111/cdev.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Yu T, Miller GE, Chen E, 2015. Discrimination, racial identity, and cytokine levels among African-American adolescents. J. Adolesc. Health 56 (5), 496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TT, Partanen J, Chuong L, Villaverde V, Chantal Griffin A, Mendelson A, 1982. Discrimination hurts: The effect of discrimination on the development of chronic pain. Soc. Sci. Med. 2018 (204), 1–8. 10.1016/j.socscimed.2018.03.015. [DOI] [PubMed] [Google Scholar]
- Cole SW, 2019. The conserved transcriptional response to adversity. Curr. Opin. Behav. Sci. 28, 31–37. 10.1016/j.cobeha.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colen CG, Li Q, Reczek C, Williams DR, 2019. The intergenerational transmission of discrimination: children’s experiences of unfair treatment and their mothers’ health at midlife. J. Health Soc. Behav. 60 (4), 474–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas AG, Rodgers J, DeNufrio D, Alley L, Allen J, Williams DR, 2019. Developmental timing of racial/ethnic discrimination exposure is associated with cardiovascular health conditions in adulthood. Ethn. Health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas AG, Wang K, Williams DR, Mattei J, Tucker KL, Falcon LM, 2019. The association between perceived discrimination and allostatic load in the Boston Puerto Rican Health Study. Psychosom. Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham TJ, Seeman TE, Kawachi I, et al. , 2012,. Racial/ethnic and gender differences in the association between self-reported experiences of racial/ethnic discrimination and inflammation in the CARDIA cohort of 4 US communities. Soc. Sci. Med. 1982 75 (5), 922–931. 10.1016/j.socscimed.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolezsar CM, McGrath JJ, Herzig AJM, Miller SB, 2014. Perceived racial discrimination and hypertension: a comprehensive systematic review. Health Psychol. Off. J. Div. Health Psychol. Am. Psychol. Assoc. 33 (1), 20–34. 10.1037/a0033718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle DM, Molix L, 2014. Perceived discrimination as a stressor for close relationships: Identifying psychological and physiological pathways. J. Behav. Med. 37 (6), 1134–1144. [DOI] [PubMed] [Google Scholar]
- Doyle DM, Molix L, 2016. Minority stress and inflammatory mediators: covering moderates associations between perceived discrimination and salivary interleukin-6 in gay men. J. Behav. Med. 39 (5), 782–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich KB, Miller GE, Rohleder N, Adam EK, 2016. Trajectories of relationship stress and inflammatory processes in adolescence. Dev. Psychopathol. 28 (1), 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Moieni M, Inagaki TK, Muscatell KA, Irwin MR, 2017. In sickness and in health: the co-regulation of inflammation and social behavior. Neuropsychopharmacology 42 (1), 242–253. 10.1038/npp.2016.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y, 2017. Obesity and inflammation: the linking mechanism and the complications. Arch. Med. Sci. AMS 13 (4), 851–863. 10.5114/aoms.2016.58928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioranelli M, Bottaccioli AG, Bottaccioli F, Bianchi M, Rovesti M, Roccia MG, 2018. Stress and inflammation in coronary artery disease: a review psychoneuroendocrineimmunology-based. Front. Immunol. 9, 2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL, 2016. Trends in obesity among adults in the United States, 2005 to 2014. JAMA 315 (21), 2284–2291. 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe LK, Wallace JM, Livingstone MBE, 2008. Obesity and inflammation: the effects of weight loss. Nutr. Res. Rev. 21 (2), 117–133. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL, Grewen KM, Algoe SB, et al. , 2015. Psychological well-being and the human conserved transcriptional response to adversity. PLOS ONE. 10 (3), e0121839. 10.1371/journal.pone.0121839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman EM, Williams DR, Singer BH, Ryff CD, 2009. Chronic discrimination predicts higher circulating levels of E-selectin in a national sample: the MIDUS study. Brain Behav. Immun. 23 (5), 684–692. 10.1016/j.bbi.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons FX, Stock ML, 2018. Perceived racial discrimination and health behavior: mediation and moderation. Oxf. Handb. Stigma Discrim. Health 355–377. [Google Scholar]
- Giurgescu C, Engeland CG, Zenk SN, Kavanaugh K, 2013. Stress, Inflammation and Preterm Birth in African American Women. Newborn Infant Nurs. Rev. 13 (4), 171–177. 10.1053/j.nainr.2013.09.004. [DOI] [Google Scholar]
- Giurgescu C, Engeland CG, Templin TN, Zenk SN, Koenig MD, Garfield L, 2016. Racial discrimination predicts greater systemic inflammation in pregnant African American women. Appl. Nurs. Res. ANR 32, 98–103. 10.1016/j.apnr.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grollman EA, 2014. Multiple disadvantaged statuses and health the role of multiple forms of discrimination. J. Health Soc. Behav. 55 (1), 3–19. 10.1177/0022146514521215. [DOI] [PubMed] [Google Scholar]
- Herman JP, McKlveen JM, Ghosal S, et al. , 2016. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr. Physiol. 6 (2), 603–621. 10.1002/cphy.c150015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicken MT, Lee H, Hing AK, 1982. The weight of racism: vigilance and racial inequalities in weight-related measures. Soc. Sci. Med. 2018 (199), 157–166. 10.1016/j.socscimed.2017.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicken MT, Lee H, Ailshire J, Burgard SA, Williams DR, 2013. “Every shut eye, ain’t sleep”: the role of racism-related vigilance in racial/ethnic disparities in sleep difficulty. Race Soc. Probl. 5 (2), 100–112. 10.1007/s12552-013-9095-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicken MT, Lee H, Morenoff J, House JS, Williams DR, 2014. Racial/ethnic disparities in hypertension prevalence: reconsidering the role of chronic stress. Am. J. Public Health 104 (1), 117–123. 10.2105/AJPH.2013.301395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmelstein MS, Young DM, Sanchez DT, Jackson JS, 2015. Vigilance in the discrimination-stress model for Black Americans. Psychol. Health 30 (3), 253–267. 10.1080/08870446.2014.966104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunte HER, 2011. Association between perceived interpersonal everyday discrimination and waist circumference over a 9-year period in the Midlife Development in the United States cohort study. Am. J. Epidemiol. 173 (11), 1232–1239. 10.1093/aje/kwq463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunte HER, Williams DR, 2009. The association between perceived discrimination and obesity in a population-based multiracial and multiethnic adult sample. Am J Public Health. 99 (7), 1285–1292. 10.2105/AJPH.2007.128090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JW, Williams DR, VanderWeele TJ, 2016. Disparities at the intersection of marginalized groups. Soc. Psychiatry Psychiatr. Epidemiol. 51 (10), 1349–1359. 10.1007/s00127-016-1276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John-Henderson NA, Stellar JE, Mendoza-Denton R, Francis DD, 2015. The role of interpersonal processes in shaping inflammatory responses to social-evaluative threat. Biol. Psychol. 110, 134–137. [DOI] [PubMed] [Google Scholar]
- Jura J, Koj A Regulatory mechanisms controlling inflammation and synthesis of acute phase proteins. Acute Phase Proteins – Regul Funct Acute Phase Proteins. 2011. doi: 10.5772/18207. [DOI] [Google Scholar]
- Juster R-P, McEwen BS, Lupien SJ, 2010. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci. Biobehav. Rev. 35 (1), 2–16. [DOI] [PubMed] [Google Scholar]
- Kershaw KN, Lewis TT, Roux AVD, et al. , 2016. Self-reported experiences of discrimination and inflammation among men and women: the multi-ethnic study of atherosclerosis. Health Psychol. 35 (4), 343–350. 10.1037/hea0000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Mickelson KD, Williams DR, 1999. The prevalence, distribution, and mental health correlates of perceived discrimination in the United States. J. Health Soc. Behav. 40 (3), 208–230. [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Christian L, Preston H, et al. , 2010. Stress, inflammation, and yoga practice. Psychosom. Med. 72 (2), 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korous KM, Causadias JM, Casper DM, 2017. Racial discrimination and cortisol output: a meta-analysis. Soc. Sci. Med. 193, 90–100. 10.1016/j.socscimed.2017.09.042. [DOI] [PubMed] [Google Scholar]
- Krieger N, 1999. Embodying inequality: a review of concepts, measures, and methods for studying health consequences of discrimination. Int. J. Health Serv. Plan. Adm. Eval. 29 (2), 295–352. 10.2190/M11W-VWXE-KQM9-G97Q. [DOI] [PubMed] [Google Scholar]
- Krieger N, Smith K, Naishadham D, Hartman C, Barbeau EM, 2005. Experiences of discrimination: validity and reliability of a self-report measure for population health research on racism and health. Soc. Sci. Med. 61 (7), 1576–1596. 10.1016/j.socscimed.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Lawrence HP, Cidro J, Isaac-Mann S, et al. , 2016. Racism and oral health outcomes among pregnant canadian aboriginal women. J. Health Care Poor Underserved 27 (1 Suppl), 178–206. 10.1353/hpu.2016.0030. [DOI] [PubMed] [Google Scholar]
- Lewis TT, Aiello AE, Leurgans S, Kelly J, Barnes LL, 2010. Self-reported experiences of everyday discrimination are associated with elevated C-reactive protein levels in older African-American adults. Brain Behav. Immun. 24 (3), 438–443. 10.1016/j.bbi.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TT, Cogburn CD, Williams DR, 2015. Self-reported experiences of discrimination and health: scientific advances, ongoing controversies, and emerging issues. Annu. Rev. Clin. Psychol. 11 (1), 407–440. 10.1146/annurev-clinpsy-032814-112728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim PW, Garssen J, Sandalova E, 2016. Potential Use of Salivary Markers for Longitudinal Monitoring of Inflammatory Immune Responses to Vaccination. Mediators Inflamm. 2016. 10.1155/2016/6958293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y-Z, Wang Y-X, Jiang C-L, 2017. Inflammation: the common pathway of stress-related diseases. Front. Hum. Neurosci. 11. 10.3389/fnhum.2017.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas T, Wegner R, Pierce J, Lumley MA, Laurent HK, Granger DA, 2017. Perceived discrimination, racial identity, and multisystem stress response to social evaluative threat among African American men and women. Psychosom. Med. 79 (3), 293–305. 10.1097/PSY.0000000000000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade TW, Burhop J, Dohnal J, 2004. High-sensitivity enzyme immunoassay for C-reactive protein in dried blood spots. Clin. Chem. 50 (3), 652–654. 10.1373/clinchem.2003.029488. [DOI] [PubMed] [Google Scholar]
- McEwen BS, 2004. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann. N Y Acad. Sci. 1032, 1–7. 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- McEwen BS, 2008. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 583 (2–3), 174–185. 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietla JA, Hoeferlin LA, Wijesinghe DS, Chalfant CE, 2016. Biochemical Mediators of Inflammation and Resolution. Published online. [Google Scholar]
- Natoli G, Ghisletti S, Barozzi I, 2011. The genomic landscapes of inflammation. Genes Dev. 25 (2), 101–106. 10.1101/gad.2018811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TT, Vable AM, Glymour MM, Allen AM, 2019. Discrimination in health care and biomarkers of cardiometabolic risk in US adults. SSM-Popul Health 7. [Google Scholar]
- O’Connor M-F, Bower JE, Cho HJ, et al. , 2009. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav. Immun. 23 (7), 887–897. 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, 2017. Prevalence of obesity among adults, by household income and education — United States, 2011–2014. MMWR Morb. Mortal Wkly. Rep 66. 10.15585/mmwr.mm6650a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong AD, Deshpande S, Williams DR, 2017. Biological Consequences of Unfair Treatment: A Theoretical and Empirical Review. In: The Handbook of Culture and Biology. John Wiley & Sons, Ltd; 279–315. doi: 10.1002/9781119181361.ch12. [DOI] [Google Scholar]
- Ong AD, Williams DR, Nwizu U, Gruenewald TL, 2017. Everyday unfair treatment and multisystem biological dysregulation in African American adults. Cultur. Divers. Ethnic Minor Psychol. 23, 27–35. 10.1037/cdp0000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong AD, Williams DR, 2019. Lifetime discrimination, global sleep quality, and inflammation burden in a multiethnic sample of middle-aged adults. Cultur. Divers Ethnic Minor Psychol. 25 (1), 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradies Y, Ben J, Denson N, et al. , 2015. Racism as a determinant of health: a systematic review and meta-analysis. PLoS One 10, e0138511. 10.1371/journal.pone.0138511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riis JL, Granger DA, DiPietro JA, Bandeen-Roche K, Johnson SB, 2015. Salivary cytokines as a minimally-invasive measure of immune functioning in young children: correlates of individual differences and sensitivity to laboratory stress. Dev. Psychobiol. 57 (2), 153–167. 10.1002/dev.21271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MW, Harmon C, O’Farrelly C, 2016. Liver immunology and its role in inflammation and homeostasis. Cell Mol. Immunol. 13 (3), 267–276. 10.1038/cmi.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas AG, Williams DR, 2018. Perceived discrimination and health: integrative findings. In: The Oxford Handbook of Integrative Health Science. [Google Scholar]
- Saban KL, Mathews HL, Bryant FB, et al. , 2018. Perceived discrimination is associated with the inflammatory response to acute laboratory stress in women at risk for cardiovascular disease. Brain Behav. Immun. 73, 625–632. 10.1016/j.bbi.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson LB, Hall MH, McLean S, et al. , 2015. Validation of biomarkers of CVD risk from dried blood spots in community-based research: Methodologies and study-specific serum equivalencies. Biodemography Soc. Biol. 61 (3), 285–297. 10.1080/19485565.2015.1068105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeer KK, Tarrence J, 2018. Racial-ethnic disparities in inflammation: evidence of weathering in childhood? J. Health Soc. Behav. 59 (3), 411–428. 10.1177/0022146518784592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman N, Ironson G, Siegel SD, 2005. Stress and health: psychological, behavioral, and biological determinants. Annu. Rev. Clin.. Psychol. 1 (1), 607–628. 10.1146/annurev.clinpsy.1.102803.144141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segman RH, Stein MB, 2015. C-reactive protein: a stress diathesis marker at the crossroads of maladaptive behavioral and cardiometabolic sequelae. Am. J. Psychiatry 172 (4), 307–309. 10.1176/appi.ajp.2015.15010063. [DOI] [PubMed] [Google Scholar]
- Seng JS, Lopez WD, Sperlich M, Hamama L, Meldrum CDR, 1982. 2012,,. Marginalized identities, discrimination burden, and mental health: Empirical exploration of an interpersonal-level approach to modeling intersectionality. Soc Sci Med 75 (12), 2437–2445. 10.1016/j.socscimed.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar A, Hinds P, 2017. Perceived discrimination: associations with physical and cognitive function in older adults. Health Psychol. 36 (12), 1126–1134. 10.1037/hea0000522. [DOI] [PubMed] [Google Scholar]
- Slavish DC, Szabo YZ, 2019. The effect of acute stress on salivary markers of inflammation: a systematic review protocol. Syst. Rev. 8 (1), 108. 10.1186/s13643-019-1026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavish DC, Graham-Engeland JE, Smyth JM, Engeland CG, 2015. Salivary markers of inflammation in response to acute stress. Brain Behav. Immun. 44, 253–269. 10.1016/j.bbi.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrells SF, Caso JR, Munhoz CD, Sapolsky RM, 2009. The Stressed CNS: When Glucocorticoids Aggravate Inflammation. Neuron. 64 (1), 33–39. 10.1016/j.neuron.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanikova I, Bateman LB, Oates GR, 2017. Systemic inflammation in midlife: race, socioeconomic status, and perceived discrimination. Am. J. Prev. Med. 52 (1 Suppl 1), S63–S76. 10.1016/j.amepre.2016.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y, 2007. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav. Immun. 21 (7), 901–912. [DOI] [PubMed] [Google Scholar]
- Sterling P, 2012. Allostasis: a model of predictive regulation. Physiol. Behav. 106 (1), 5–15. 10.1016/j.physbeh.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Sternthal MJ, Slopen N, Williams DR, 2011. Racial disparities in health: How much does stress really matter? Bois Rev. Soc. Sci. Res. Race 8 (01), 95–113. 10.1017/S1742058X11000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stults-Kolehmainen MA, Sinha R, 2014. The effects of stress on physical activity and exercise. Sports Med. Auckl. NZ 44 (1), 81–121. 10.1007/s40279-013-0090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner R, Burrow AL, Hill PL, 2018. The development of purpose in life among adolescents who experience marginalization: Potential opportunities and obstacles. Am Psychol. 73 (6), 740–752. 10.1037/amp0000249. [DOI] [PubMed] [Google Scholar]
- Sutin AR, Stephan Y, Luchetti M, Terracciano A, 2014. Perceived weight discrimination and C-reactive protein. Obesity 22 (9), 1959–1961. [DOI] [PubMed] [Google Scholar]
- Sutin AR, Stephan Y, Carretta H, Terracciano A, 2015. Perceived discrimination and physical, cognitive, and emotional health in older adulthood. Am. J. Geriatr. Psychiatry 23 (2), 171–179. 10.1016/j.jagp.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain MGI, 2000. Stress and hepatic inflammation. Am J Physiol-Gastrointest Liver Physiol. 279 (6), G1135–G1138. 10.1152/ajpgi.2000.279.6.G1135. [DOI] [PubMed] [Google Scholar]
- Thames AD, Irwin MR, Breen EC, Cole SW, 2019. Experienced discrimination and racial differences in leukocyte gene expression. Psychoneuroendocrinology 106, 277–283. 10.1016/j.psyneuen.2019.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maydych V, 2019. The interplay between stress, inflammation, and emotional attention: relevance for depression. Front Neurosci. 13. 10.3389/fnins.2019.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricco AC, Lillie E, Zarin W, et al. , 2018. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann. Intern. Med. 169 (7), 467–473. [DOI] [PubMed] [Google Scholar]
- Vadiveloo M, Mattei J, 2017. Perceived weight discrimination and 10-year risk of allostatic load among US Adults. Ann. Behav. Med. Publ. Soc.. Behav. Med. 51 (1), 94–104. 10.1007/s12160-016-9831-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke ME, Vaccarino V, Dunbar SB, et al. , 2017. Socioeconomic status discrimination and C-reactive protein in African-American and White adults. Psychoneuroendocrinology 82, 9–16. 10.1016/j.psyneuen.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB, 1999. Elevated C-reactive protein levels in overweight and obese adults. JAMA 282 (22), 2131–2135. [DOI] [PubMed] [Google Scholar]
- Wang HJ, Zakhari S, Jung MK, 2010. Alcohol, inflammation, and gut-liver-brain interactions in tissue damage and disease development. World J. Gastroenterol. WJG 16 (11), 1304–1313. 10.3748/wjg.v16.i11.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SR, McDade TW, 2009. The Use of Dried Blood Spot Sampling in the National Social Life, Health, and Aging Project. J Gerontol B Psychol Sci Soc Sci. 64B (Suppl 1), i131–i136. 10.1093/geronb/gbn022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Mohammed SA, 2008. Discrimination and racial disparities in health: evidence and needed research. J. Behav. Med. 32, 20–47. 10.1007/s10865-008-9185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Mohammed SA, 2009. Discrimination and racial disparities in health: evidence and needed research. J. Behav. Med. 32 (1), 20. 10.1007/s10865-008-9185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, Yan Y.u., Jackson JS, Anderson NB, 1997. Racial differences in physical and mental health: socio-economic status, stress and discrimination. J. Health Psychol. 2 (3), 335–351. 10.1177/135910539700200305. [DOI] [PubMed] [Google Scholar]
- Williams DR, Lawrence JA, Davis BA, Vu C, 2019. Understanding how discrimination can affect health. Health Serv. Res. 54, 1374–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson S, Munro C, Pickler R, Grap MJ, Elswick RK, 2012. Comparison of biomarkers in blood and saliva in healthy adults. Nurs. Res. Practice 2012, 1–4. 10.1155/2012/246178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Kraal AZ, Zaheed A, Farris P, Sol K, 2019. Longitudinal effects of race, ethnicity, and psychosocial disadvantage on systemic inflammation. SSM – Popul. Health 7. 10.1016/j.ssmph.2019.100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak DE, Tam VC, Aderem A, 2014. Systems-level analysis of innate immunity. Annu. Rev. Immunol. 32, 547–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakaria R, Allen KJ, Koplin JJ, Roche P, Greaves RF, 2016. Advantages and challenges of dried blood spot analysis by mass spectrometry across the total testing process. EJIFCC 27 (4), 288–317. [PMC free article] [PubMed] [Google Scholar]
- Zilioli S, Imami L, Ong AD, Lumley MA, Gruenewald T, 2017. Discrimination and anger control as pathways linking socioeconomic disadvantage to allostatic load in midlife. J. Psychosom. Res. 103, 83–90. 10.1016/j.jpsychores.2017.10.002. [DOI] [PubMed] [Google Scholar]


