Abstract
BACKGROUND
Spinal manipulation therapy (SMT) has been widely used worldwide to treat musculoskeletal diseases, but it can cause serious adverse events. Spinal epidural hematoma (SEH) caused by SMT is a rare emergency that can cause neurological dysfunction. We herein report three cases of SEH after SMT.
CASE SUMMARY
The first case was a 30-year-old woman who experienced neck pain and numbness in both upper limbs immediately after SMT. Her symptoms persisted after 3 d of conservative treatment, and she was admitted to our hospital. Magnetic resonance imaging (MRI) demonstrated an SEH, extending from C6 to C7. The second case was a 55-year-old man with sudden back pain 1 d after SMT, numbness in both lower limbs, an inability to stand or walk, and difficulty urinating. MRI revealed an SEH, extending from T1 to T3. The third case was a 28-year-old man who suddenly developed symptoms of numbness in both lower limbs 4 h after SMT. He was unable to stand or walk and experienced mild back pain. MRI revealed an SEH, extending from T1 to T2. All three patients underwent surgery after failed conservative treatment. The three cases recovered to ASIA grade E on day 5, 1 wk, and day 10 after surgery, respectively. All patients returned to normal after 3 mo of follow-up.
CONCLUSION
SEH caused by SMT is very rare, and the condition of each patient should be evaluated in full detail before operation. SEH should be diagnosed immediately and actively treated by surgery.
Keywords: Spinal epidural hematoma, Spinal manipulation therapy, Spinal cord injury, Magnetic resonance imaging, Surgery, Case report
Core Tip: Spinal manipulation therapy (SMT) has been widely used worldwide to treat musculoskeletal diseases, but it can cause serious adverse events. Spinal epidural hematoma (SEH) caused by SMT is a rare emergency that can cause neurological dysfunction. We herein report three cases of SEH after SMT, and review a series of literature. All three patients underwent surgery after failed conservative treatment and returned to normal after 3 mo of follow-up. We focused on early recognition and surgical treatment.
INTRODUCTION
Spinal manipulation therapy (SMT) has been widely used to treat musculoskeletal diseases. The most common indications are neck and back pain. SMT achieves better clinical results than acupuncture or medication[1-3], and complications are relatively rare. However, spinal epidural hematoma (SEH) is a serious complication after SMT, which can cause spinal neurological dysfunction and should therefore be treated. Here, we report three cases of SEH caused by SMT. One patient had a family history of hemangioma, whereas they all had no history of any coagulatory disease or anticoagulant medication. Platelets and coagulation were normal at the time of admission, and all patients underwent surgery after failed conservative treatment. All patients returned to normal after 3-mo follow-up.
CASE PRESENTATION
Chief complaints
Case 1: A 30-year-old woman was admitted for neck pain and numbness in both upper limbs for 3 d.
Case 2: A 55-year-old man was admitted to the emergency room due to back pain and numbness and weakness of both lower limbs for 1 d.
Case 3: A 28-year-old man suddenly developed numbness and weakness in both lower limbs 4 h after SMT.
History of present illness
Case 1: The patient developed symptoms immediately after SMT 3 d before admission. She took analgesic medications but her symptoms did not completely resolve.
Case 2: The patient experienced sudden back pain after SMT the day before admission, accompanied by numbness and weakness in both lower limbs, an inability to stand or walk, and difficulty urinating. Dehydration medication was given in the community hospital, and the symptoms were not significantly relieved, so he was transferred to our hospital.
Case 3: The patient suddenly developed numbness and weakness in both lower limbs 4 h after SMT. He was unable to stand or walk and had mild back pain, so he was rushed to our emergency room.
History of past illness
None of these three patients had obvious diseases in the past.
Personal and family history
Case three had a family history of hemangioma, and he also had a hemangioma about 1 cm in size, located on the T11. But they all had no history of any coagulatory disease or anticoagulant medication.
Physical examination
Case 1: Physical examination revealed reduced upper limb sensation and tendon reflexes, normal muscle strength, and grade D spinal cord injury.
Case 2: The sensations, tendon reflexes, and cremaster reflexes of the lower extremities were reduced on both sides. The patient’s muscle strength was grade IV, and his spinal cord injury was grade D.
Case 3: The skin sensation below the patient’s bilateral nipples was diminished, and muscle strength in the right lower extremity was grade III. The tendon reflex was weakened, the cremaster reflex was not elicited, the anal reflex was weakened, and the spinal cord injury was grade C.
Laboratory examinations
Platelets and coagulation were normal at the time of admission.
Imaging examinations
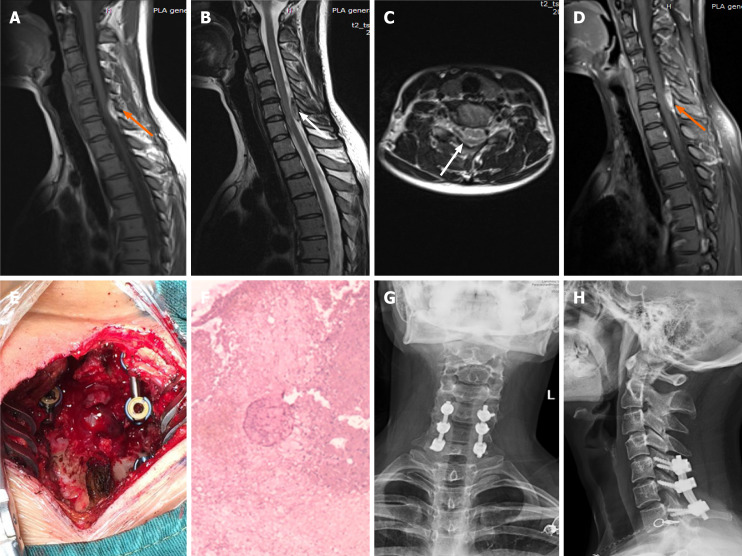
Case 1: Magnetic resonance imaging (MRI) after admission demonstrated an abnormal shadow in the cervical spinal canal, extending from C6 to C7, and compression of the spinal cord. A T1-weighted image showed high signal intensity, and a T2-weighted image showed low signal intensity that increased on enhanced MRI (Figure 1A-D).
Figure 1.
Imaging examinations of case 1. A: T1-weighted preoperative magnetic resonance imaging (MRI) image shows high signal intensity (orange arrow); B and C: Preoperative T2-weighted image shows low signal intensity, and an axial T2-weighted image demonstrates that the hematoma occurred in the posterior region (white arrow); D: Preoperative enhanced MRI suggests an enhanced hematoma signal (orange arrow); E: Intraoperative photograph shows that spinal cord compression has recovered; F: Postoperative pathology suggested a hematoma; G and H: X-ray at the 3-mo follow-up indicated intact internal fixation.
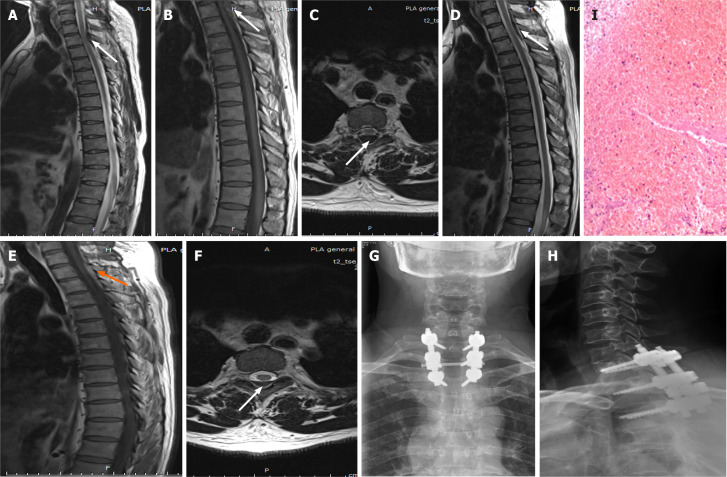
Case 2: Emergency MRI revealed a thoracic spinal canal epidural mass occupying T1 to T3, with spinal cord compression (Figure 2A-C).
Figure 2.
Imaging examinations of case 2. A-C: First emergency magnetic resonance imaging (MRI) images. T1- and T2-weighted images of the thoracic spine show low-intensity spinal epidural hematoma at T1–3; an axial T2-weighted image demonstrates that the hematoma was in the posterior region (white arrow); D-F: The hematoma still existed but was slightly reduced after conservative treatment compared with admission (arrow); G and H: Postoperative review showing good internal fixation; I: Postoperative pathology suggested a hematoma.
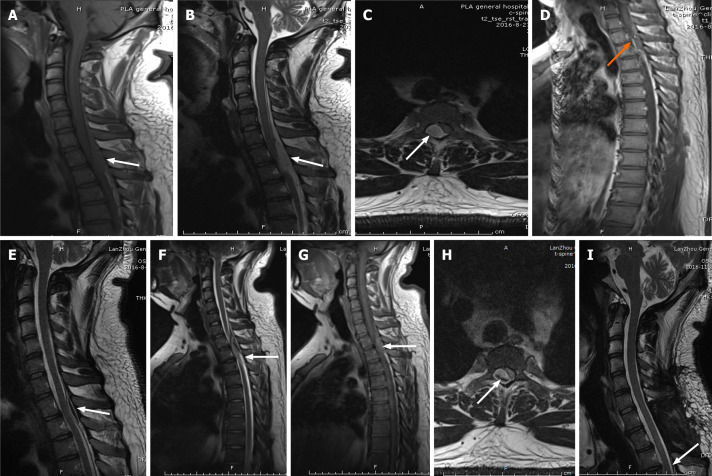
Case 3: Emergency MRI revealed a mass lesion in the thoracic spinal canal, extending from T1 to T2, with spinal cord compression (Figure 3A-C), and enhanced MRI indicated signal enhancement (Figure 3D).
Figure 3.
Imaging examinations of case 3. A: First emergency magnetic resonance imaging (MRI) scan. T1-weighted image of the thoracic spine shows isometric-intensity spinal epidural hematoma at T1–2 (white arrow); B and C: First emergency MRI scans. T2-weighted image shows high signal intensity; axial T2-weighted image shows that the hematoma was in the posterolateral region (white arrow); D: Preoperative MRI suggests an enhanced hematoma signal (orange arrow); E: The hematoma decreased in size after conservative treatment (white arrow); F-H: After 72 h of conservative treatment, MRI showed that the hematoma worsened again (white arrow); I: The hematoma completely disappeared at the 3-mo follow-up (white arrow).
FINAL DIAGNOSIS
All three patients were diagnosed with SEH. Blood clots were found during the operation in case 1 and case 2, and the postoperative pathology confirmed a hematoma. In case three, a vein ruptured during the operation, causing massive bleeding.
TREATMENT
Case 1: A laminectomy with internal fixation was performed immediately after admission and the hematoma was removed (Figure 1E). Blood clots were found during the operation, but no bleeding vessels were observed. The postoperative pathology confirmed a hematoma (Figure 1F).
Case 2: After admission, the patient refused surgery and requested conservative treatment, but his symptoms did not resolve. A review of the MRI scan obtained 72 h later revealed that the mass was still compressing the spinal cord (Figure 2D-F). Laminectomy and internal fixation were performed and the hematoma was removed (Figure 2G and H). Blood clots were found during the operation, but no bleeding vessels were observed. The pathological examination suggested a hematoma (Figure 2I).
Case 3: We recommended surgery after admission, but the patient wanted conservative treatment. A 66-h follow-up MRI scan showed a decrease in the mass (Figure 3E), so conservative treatment was continued. The patient suddenly developed paraplegia and complete loss of sensation in both legs after 72 h. A review of the MRI scan indicated fresh bleeding (Figure 3F-H), so an emergency laminectomy was performed and the hematoma was removed. A vein ruptured during the operation, causing massive bleeding; we used hemostatic material to tamp it. Due to the active bleeding during surgery, a specimen could not be obtained for pathological examination.
OUTCOME AND FOLLOW-UP
Case 1: The patient was discharged on postoperative day 5, and her neurological function had fully recovered to grade E. She returned to normal life after the 3-mo follow-up, and the internal fixation position was good (Figure 1G and H).
Case 2: The patient’s urine function was fully restored 4 d after surgery. His neurological function recovered to grade E 1 wk after surgery, and he returned to normal life after the 3-mo follow-up.
Case 3: The patient’s muscle strength returned to grade V on day 3 after surgery, but he still felt numbness in his feet and had difficulty urinating. The urinary function returned to normal on day 6. His neurological function was grade E on day 10 after surgery. He returned to normal life after 3-mo follow-up, and the hematoma completely disappeared (Figure 3I).
DISCUSSION
SMT is a manual technique for which there is currently no accepted definition, and it is usually performed with a high-speed, low-amplitude thrust that can quickly adjust the joint, often accompanied by a popping sound[4]. Moreover, due to its relatively low invasiveness, many medical and non-medical practitioners use SMT, such as chiropractors, osteopaths, and physical therapists[1]. Vertebral artery dissection, spinal cord injury, cauda equina syndrome, cervical subluxation, and cerebrovascular accident can occur even in the hands of experienced therapists, and may cause death in rare cases[5,6]. Patients with chronic coagulatory deficits, inflammatory spondylosis, osteoporosis, or aortic aneurysm or dissection, and those on long-term anticoagulant therapy are more likely to experience these adverse events[7].
Although many serious adverse events after SMT have been reported, the exact incidence has not been reported. Studies have reported serious adverse event rates of 1 in 20000 and 1 in 250000000 operations. Others reported rates of 1 in 400000 and 1 in 2 million operations[5,8]. Most reports assert that SEH is caused by SMT. We review 18 literature reports (Table 1). Three patients had a history of anticoagulant therapy, two had osteoporosis or spinal degeneration[9-11] , and three had a history of spinal degeneration[6,12,13]. There was also one case with an arteriovenous malformation[14]. Of our three cases, one had a family history of hemangioma, whereas they all had no history of coagulation disease or use of anticoagulant medication.
Table 1.
Summary of cases of spinal epidural hematoma secondary to spinal manipulation therapy
|
Ref.
|
Age (yr), sex
|
Symptoms
|
Interval to symptom onset
|
Level
|
Location of SEH
|
Treatment
|
Outcome
|
| Zupruk and Mehta[12], 1989 | 86, Male | Brown-Sequard Syndrome | 24 h | C2-7 | Posterolateral | Surgery | Recovery |
| Segal et al[28], 1996 | 33, Female | Left upper limb paresis | 15 min | C4-6 | Posterolateral | Surgery | Recovery |
| Ruelle et al[25], 1999 | 64, Female | Paraparesis | 2 h | T9-11 | Not reported | Surgery | Recovery |
| Tseng et al[13], 2002 | 67, Female | Brown-Sequard syndrome | Immediately | C3-5 | Posterolateral | Surgery | Recovery |
| Saxler and Barden[31], 2004 | 27, Female | Headache | 10 min | C1-S1 | Not reported | Conservative | Recovery |
| Whedon et al[9], 2006 | 79, Male | Lower extremity paralysis | Immediately | C4-5 | Posterolateral | Surgery | Recovery |
| Gouveia et al[26], 2007 | 34, Male | Tetraplegia | Few hours | C3-6 | Not reported | Surgery | Recovery |
| Domenicucci et al[16], 2017 | 52, Female | Brown-Sequard Syndrome | Immediately | C3-T1 | Posterolateral | Surgery | Recovery |
| Solheim et al[10], 2007 | 77, Male | Right lower extremity paresis | Immediately | L3-4 | Posterior | Surgery | Partial recovery |
| Heiner[32], 2009 | 38, Female | Neck and arms pain numbness | 4 h | C1-4 | Posterolateral | Conservative | Recovery |
| Lidder et al[21], 2010 | 64, Male | Hemiparesis | Immediately | C4-6 | Not reported | Surgery | Partial recovery |
| Lee et al[27], 2011 | 38, Female | Lower extremity paralysis | Immediately | T1-7 | Posterolateral | Surgery | Recovery |
| Huang et al[14], 2015 | 40, Male | Hemiparesis | Immediately | C2-T2 | Posterolateral | Surgery | Recovery |
| Ling et al[6], 2017 | 33, Male | Tetraplegia | Immediately | C4-7 | Posterolateral | Surgery | Die |
| Fattahi et al[30], 2017 | 44, Female | Tetraplegia | not reported | C1-4 | Anterior | Conservative | Recovery |
| Ryu et al[29], 2018 | 38, Male | Paraparesis | Immediately | C6-T1 | Anterior | Conservative | Recovery |
| Vanichkulbodee et al[18], 2019 | 20, Male | Paraparesis | 3 d | C6-T2 | Posterolateral | Surgery | not reported |
| Cooper et al[11], 2019 | 63, Male | Tetraplegia | 1 d | C6-7 | Posterior | Surgery | Partial recovery |
| Present case one | 30, Female | Neck and arms pain numbness | Immediately | C6-7 | Posterolateral | Surgery | Recovery |
| Present case two | 55, Male | Lower extremity paralysis | 1 d | T1-3 | Posterior | Surgery | Recovery |
| Present case three | 28, Male | Paraparesis | 4 h | T1-2 | Posterolateral | Surgery | Recovery |
SEH: Spinal epidural hematoma.
SEH is a rare emergency in which blood accumulates in the spinal epidural space; it begins with back and nerve root pain, and progresses to mild paralysis and urinary retention. In severe cases, SEH may develop into paraplegia or quadriplegia within minutes, hours, or days[15]. Common causes include trauma, lumbar puncture, drug-induced coagulopathy, and genetic coagulation disorders. A few idiopathic cases without a clear etiology have also been reported[16], in addition to clinical cases after minor trauma, such as SMT (Table 1). SEH has been characterized as “idiopathic, spontaneous, and secondary”[16,17]. Most patients develop limb numbness and weakness, severe back pain, and quadriplegia or dyspepsia soon after SMT (up to a few hours later; Table 1), but there have also been reports of delayed symptoms (after 1-3 d[11,18]). All of our cases developed symptoms immediately after SMT, but one case was only detected by examination 3 d later.
The causal relationship between SMT and SEH is often uncertain. Bleeding can occur from a vein or artery. Venous rupture is generally considered to be related to the intravertebral venous plexus[19]. The epidural space of the spinal cord is located in the spinal canal. It contains loose areolar tissue and an extensive internal venous plexus that receives projections from the vertebral bodies and spinal cord. This internal venous plexus communicates freely with the external venous plexus, which together form Batson’s veins. This low-pressure system without valves is particularly susceptible to sudden changes in pressure, such as when lifting, straining, coughing, or vomiting[15]. When performing SMT, a low-amplitude thrust will produce sufficient pressure in the spine for rupture of fragile veins, which in turn will leads to clinical symptoms of compression of the spinal cord. However, it has been posited that the hematoma originates from an artery[20]. Lidder et al[21] observed ruptured veins during the operation. We detected venous bleeding during surgery in a patient with a family history of hemangioma, but could not find the bleeding source. Hemostatic materials were used to stop the bleeding. Although the cause of bleeding was not clarified, timely diagnosis and early treatment are very important for SEH.
MRI has advantages for diagnosis of an SEH. The hematoma is usually isointense on T1-weighted images and hyperintense on T2-weighted images within 24 h of the onset of symptoms. The hematoma is often hyperintense on T1- and T2-weighted images after 24 h, while a chronic hematoma becomes hypointense on both T1- and T2-weighted images. The hematoma usually shows significant enhancement[22]. In both our first and third cases, MRI showed enhancement.
Most people believe that surgery is still the first choice for treating SEH. Liao et al[23] reported that surgery for SEH is safe and effective. When performed within 48 h after the initial attack, complete neurological dysfunction is recommended not to exceed 12 h[23]. Groen and van Alphen[24] reported that surgery was performed within 36 h in patients with complete neurological dysfunction, and within 48 h in those with incomplete neurological dysfunction[24]. The procedure involves decompressive laminectomy and evacuation of the hematoma. Most patients in the literature underwent surgery at the time of symptom onset or exacerbation, and postoperative recovery was good[12,14,25-27]. However, three patients underwent surgery after a delay of several days; their symptoms did not completely resolve[10,21,28] , and one patient died from complications[6]. Although the majority of studies reported good recovery after surgical excision of SEH, there have also been reports that conservative treatments can provide good results[29-32]. Fat tissue in the epidural space contains a huge network of capillaries that usually absorbs foreign substances; thus, the SEH should be absorbed over time. However, irreversible nerve damage can occur during absorption[33]. Conservative treatment was preferred in all of our cases, but the nerve injury symptoms did not completely disappear. Two patients were operated after conservative treatment proved ineffective; one patient developed sudden paraplegia during conservative treatment and was immediately operated in the emergency department. The symptoms disappeared after the operation.
Although surgery can achieve good results, clinical follow-up is needed to prevent the occurrence of complications. To avoid SEH after SMT, good planning and individualized assessment are needed, and risk analysis should be performed based on the results of multiple clinical studies[34].
CONCLUSION
In conclusion, before proceeding with SMT, each patient should be evaluated in detail and checked for risk factors. In cases where the physical condition changes rapidly, physicians should be alert to the danger and send the patient to the emergency department for a complete MRI examination. We recommend surgery if neurological symptoms appear.
Footnotes
Informed consent statement: Informed consent was obtained from the patients for the publication of this case report.
Conflict-of-interest statement: The authors declare that they have no conflict of interest to report.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Manuscript source: Unsolicited manuscript
Peer-review started: April 5, 2021
First decision: April 23, 2021
Article in press: May 17, 2021
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ferraioli G, Park SB S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Wang LYT
Contributor Information
Hua Liu, Department of Spine Surgery, The 940th Hospital of Joint Logistics Support Force of Chinese People’s Liberation Army, Lanzhou 730050, Gansu Province, China.
Tao Zhang, Department of Spine Surgery, The 940th Hospital of Joint Logistics Support Force of Chinese People’s Liberation Army, Lanzhou 730050, Gansu Province, China.
Tao Qu, Department of Spine Surgery, The 940th Hospital of Joint Logistics Support Force of Chinese People’s Liberation Army, Lanzhou 730050, Gansu Province, China.
Cheng-Wei Yang, Department of Spine Surgery, The 940th Hospital of Joint Logistics Support Force of Chinese People’s Liberation Army, Lanzhou 730050, Gansu Province, China.
Song-Kai Li, Department of Spine Surgery, The 940th Hospital of Joint Logistics Support Force of Chinese People’s Liberation Army, Lanzhou 730050, Gansu Province, China. lisongkai@gmail.com.
References
- 1.Hurwitz EL. Epidemiology: spinal manipulation utilization. J Electromyogr Kinesiol. 2012;22:648–654. doi: 10.1016/j.jelekin.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Giles LG, Muller R. Chronic spinal pain: a randomized clinical trial comparing medication, acupuncture, and spinal manipulation. Spine (Phila Pa 1976) 2003;28:1490–502; discussion 1502. doi: 10.1097/00007632-200307150-00003. [DOI] [PubMed] [Google Scholar]
- 3.Muller R, Giles LG. Long-term follow-up of a randomized clinical trial assessing the efficacy of medication, acupuncture, and spinal manipulation for chronic mechanical spinal pain syndromes. J Manipulative Physiol Ther. 2005;28:3–11. doi: 10.1016/j.jmpt.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Reggars JW. The manipulative crack. Frequency analysis. Australas Chiropr Osteopathy. 1996;5:39–44. [PMC free article] [PubMed] [Google Scholar]
- 5.Stevinson C, Ernst E. Risks associated with spinal manipulation. Am J Med. 2002;112:566–571. doi: 10.1016/s0002-9343(02)01068-9. [DOI] [PubMed] [Google Scholar]
- 6.Ling TH, Zakaria AF, Abdullah AT. Is neck massage safe? J Orthop Surg (Hong Kong) 2017;25:2309499017690459. doi: 10.1177/2309499017690459. [DOI] [PubMed] [Google Scholar]
- 7.Whedon JM, Mackenzie TA, Phillips RB, Lurie JD. Risk of traumatic injury associated with chiropractic spinal manipulation in Medicare Part B beneficiaries aged 66 to 99 years. Spine (Phila Pa 1976) 2015;40:264–270. doi: 10.1097/BRS.0000000000000725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nielsen SM, Tarp S, Christensen R, Bliddal H, Klokker L, Henriksen M. The risk associated with spinal manipulation: an overview of reviews. Syst Rev. 2017;6:64. doi: 10.1186/s13643-017-0458-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whedon JM, Quebada PB, Roberts DW, Radwan TA. Spinal epidural hematoma after spinal manipulative therapy in a patient undergoing anticoagulant therapy: a case report. J Manipulative Physiol Ther. 2006;29:582–585. doi: 10.1016/j.jmpt.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Solheim O, Jorgensen JV, Nygaard OP. Lumbar epidural hematoma after chiropractic manipulation for lower-back pain: case report. Neurosurgery. 2007;61:E170–1; discussion E171. doi: 10.1227/01.neu.0000279740.61048.e2. [DOI] [PubMed] [Google Scholar]
- 11.Cooper J, Battaglia P, Reiter T. Spinal epidural hematoma in a patient on chronic anticoagulation therapy performing self-neck manipulation: a case report. Chiropr Man Therap. 2019;27:41. doi: 10.1186/s12998-019-0264-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zupruk GM, Mehta Z. Brown-Séquard syndrome associated with posttraumatic cervical epidural hematoma: case report and review of the literature. Neurosurgery. 1989;25:278–280. [PubMed] [Google Scholar]
- 13.Tseng SH, Chen Y, Lin SM, Wang CH. Cervical epidural hematoma after spinal manipulation therapy: case report. J Trauma. 2002;52:585–586. doi: 10.1097/00005373-200203000-00033. [DOI] [PubMed] [Google Scholar]
- 14.Huang M, Barber SM, Moisi M, Powell S, Rivera A, Zwillman M, Rose J. Cervical Epidural Hematoma after Chiropractic Spinal Manipulation Therapy in a Patient with an Undiagnosed Cervical Spinal Arteriovenous Malformation. Cureus. 2015;7:e307. doi: 10.7759/cureus.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pear BL. Spinal epidural hematoma. Am J Roentgenol Radium Ther Nucl Med. 1972;115:155–164. doi: 10.2214/ajr.115.1.155. [DOI] [PubMed] [Google Scholar]
- 16.Domenicucci M, Mancarella C, Santoro G, Dugoni DE, Ramieri A, Arezzo MF, Missori P. Spinal epidural hematomas: personal experience and literature review of more than 1000 cases. J Neurosurg Spine. 2017;27:198–208. doi: 10.3171/2016.12.SPINE15475. [DOI] [PubMed] [Google Scholar]
- 17.Sarubbo S, Garofano F, Maida G, Fainardi E, Granieri E, Cavallo MA. Spontaneous and idiopathic chronic spinal epidural hematoma: two case reports and review of the literature. Eur Spine J. 2009;18:1055–1061. doi: 10.1007/s00586-009-1175-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vanichkulbodee A, Issaragrisil S, Inboriboon PC. Massage-induced spinal epidural hematoma presenting with delayed paraplegia. Am J Emerg Med. 2019;37:797.e1–797.e4. doi: 10.1016/j.ajem.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 19.Groen RJ, Grobbelaar M, Muller CJ, van Solinge G, Verhoof O, du Toit DF, Hoogland PV. Morphology of the human internal vertebral venous plexus: a cadaver study after latex injection in the 21-25-week fetus. Clin Anat. 2005;18:397–403. doi: 10.1002/ca.20153. [DOI] [PubMed] [Google Scholar]
- 20.Beatty RM, Winston KR. Spontaneous cervical epidural hematoma. A consideration of etiology. J Neurosurg. 1984;61:143–148. doi: 10.3171/jns.1984.61.1.0143. [DOI] [PubMed] [Google Scholar]
- 21.Lidder S, Lang KJ, Masterson S, Blagg S. Acute spinal epidural haematoma causing cord compression after chiropractic neck manipulation: an under-recognised serious hazard? J R Army Med Corps. 2010;156:255–257. doi: 10.1136/jramc-156-04-11. [DOI] [PubMed] [Google Scholar]
- 22.Figueroa J, DeVine JG. Spontaneous spinal epidural hematoma: literature review. J Spine Surg. 2017;3:58–63. doi: 10.21037/jss.2017.02.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao CC, Lee ST, Hsu WC, Chen LR, Lui TN, Lee SC. Experience in the surgical management of spontaneous spinal epidural hematoma. J Neurosurg. 2004;100:38–45. doi: 10.3171/spi.2004.100.1.0038. [DOI] [PubMed] [Google Scholar]
- 24.Groen RJ, van Alphen HA. Operative treatment of spontaneous spinal epidural hematomas: a study of the factors determining postoperative outcome. Neurosurgery. 1996;39:494–508; discussion 508. doi: 10.1097/00006123-199609000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Ruelle A, Datti R, Pisani R. Thoracic epidural hematoma after spinal manipulation therapy. J Spinal Disord. 1999;12:534–536. [PubMed] [Google Scholar]
- 26.Gouveia LO, Castanho P, Ferreira JJ, Guedes MM, Falcão F, e Melo TP. Chiropractic manipulation: reasons for concern? Clin Neurol Neurosurg. 2007;109:922–925. doi: 10.1016/j.clineuro.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Lee TH, Chen CF, Lee TC, Lee HL, Lu CH. Acute thoracic epidural hematoma following spinal manipulative therapy: case report and review of the literature. Clin Neurol Neurosurg. 2011;113:575–577. doi: 10.1016/j.clineuro.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Segal DH, Lidov MW, Camins MB. Cervical epidural hematoma after chiropractic manipulation in a healthy young woman: case report. Neurosurgery. 1996;39:1043–1045. doi: 10.1097/00006123-199611000-00034. [DOI] [PubMed] [Google Scholar]
- 29.Ryu JI, Han MH, Kim JM, Kim CH, Cheong JH. Cervical Epidural Hematoma That Induced Sudden Paraparesis After Cervical Spine Massage: Case Report and Literature Review. World Neurosurg. 2018;112:217–220. doi: 10.1016/j.wneu.2018.01.178. [DOI] [PubMed] [Google Scholar]
- 30.Fattahi A, Taheri M. Spontaneous resolved cervical spine epidural hematoma: A case report. Surg Neurol Int. 2017;8:183. doi: 10.4103/sni.sni_223_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saxler G, Barden B. [Extensive spinal epidural hematoma--an uncommon entity following cervical chiropractic manipulation] Z Orthop Ihre Grenzgeb. 2004;142:79–82. doi: 10.1055/s-2004-818032. [DOI] [PubMed] [Google Scholar]
- 32.Heiner JD. Cervical epidural hematoma after chiropractic spinal manipulation. Am J Emerg Med. 2009;27:1023.e1–1023.e2. doi: 10.1016/j.ajem.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 33.Pan G, Kulkarni M, MacDougall DJ, Miner ME. Traumatic epidural hematoma of the cervical spine: diagnosis with magnetic resonance imaging. Case report. J Neurosurg. 1988;68:798–801. doi: 10.3171/jns.1988.68.5.0798. [DOI] [PubMed] [Google Scholar]
- 34.Rushton A, Rivett D, Carlesso L, Flynn T, Hing W, Kerry R. International framework for examination of the cervical region for potential of Cervical Arterial Dysfunction prior to Orthopaedic Manual Therapy intervention. Man Ther. 2014;19:222–228. doi: 10.1016/j.math.2013.11.005. [DOI] [PubMed] [Google Scholar]