Abstract
Aims:
We sought to evaluate the association of angiotensin-converting-enzyme inhibitors (ACEI) or AT1 blockers (ARB) therapy with clinical outcomes in patients with coronavirus disease 2019 (COVID-19).
Methods and results:
Electronic databases were searched to identify published studies that reported clinical outcomes in patients with COVID-19 who were or were not taking an ACEI/ARB. We studied all-cause mortality and/or severe disease outcomes. Fully adjusted effect estimates from individual studies were pooled using a random-effects model. In total, 34 (31 cohort-based and three case–control) studies met our eligibility criteria. Due to the inherent differences between cohort and case–control studies, we did not combine results of these studies but used them to identify the consistency of their results. The 31 cohort studies provided outcome data for 87 951 patients with COVID-19, of whom 22 383/83 963 (26.7%) were on ACEI/ARB therapy. In pooled analysis, we found no association between the use of ACEI/ARB and all-cause mortality/severe disease [relative risk: 0.94, 95% confidence interval (CI): 0.86–1.03, I2 = 57%, P = 0.20] or occurrence of severe disease (relative risk: 0.93, 95% CI: 20.74–1.17, I2 = 56%, P = 0.55). Analysis of three population-based case–control studies identified no significant association between ACEI/ARB (pooled odds ratio: 1.00, 95% CI: 0.81–1.23, I2 = 0, P = 0.98) and all-cause mortality/severe disease. In 13 of the 31 cohort studies as well as in three case–control studies that reported outcomes separately for ACEI and ARB, there was no differential effect for mortality/severe disease outcomes.
Conclusion:
In patients with COVID-19, we found no association between ACEI/ARB treatment and mortality/severe disease. ACEI/ARB should not be discontinued, unless clinically indicated.
Keywords: coronavirus disease 2019, mortality, renin-angiotensin-system inhibitors, severe disease
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus causing coronavirus disease 2019 (COVID-19), uses the angiotensin-converting enzyme-2 (ACE2) receptors for entry into target cells. The same receptor plays an important mechanistic role in regulating the human renin–angiotensin–aldosterone system. Because angiotensin-converting-enzyme inhibitors (ACEI) and AT1 blockers (ARB) may upregulate ACE2 expression, there has been intense debate regarding whether use of ACEI or ARB (ACEI/ARB) is associated with clinical outcomes in patients with COVID-19 [1,2]. It is important to address this controversy because comorbidities such as hypertension, diabetes and cardiovascular disease are highly prevalent in patients with COVID-19 [3] and ACEI/ARB are one of the most common medications for management of these conditions. Many professional societies have recommended continuation of ACEI/ARB therapy unless cessation is clinically indicated but have called for additional research. Recently, several reports of the association between ACEI/ARB use and COVID-19 outcomes have been published, with conflicting results. To provide the most reliable clinical guidance, we conducted a comprehensive systemic review and meta-analysis of studies that have published relevant information on this topic and obtained additional data from a recently published population-based case–control study [4].
METHODS
The study protocol was registered (CRD42020185115) with the PROSPERO, the international database of prospectively registered systematic reviews (managed by the Center for Reviews and Dissemination). Our meta-analysis was performed in accordance with the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guidelines [5]. We conducted a systematic search of PubMed, Scopus and Google scholar literature from 2019 to 27 August 2020 using the following key words and Medical Subject Headings: ‘COVID-19,’ ‘coronavirus’ ‘SARS-CoV-2,’ ‘ACE inhibitors,’ ‘ARB,’ ‘angiotensin-convert-ing enzyme inhibitors,’ ‘angiotensin receptor blockers,’ ‘RAS inhibitors’ ‘renin–angiotensin system inhibitors’. No language restriction was employed (Supplement Table 1, http://links.lww.com/HJH/B554). In addition, we searched references from included studies to identify other reports that might meet our selection criteria.
Two of the authors (C.B. and F.H.M.) independently assessed search generated article titles and abstracts for potential eligibility and ultimately selected studies that reported data with regards to ACEI/ARBs use and all-cause mortality and/or severe disease outcomes in patients with COVID-19. To minimize the effect of confounding, we selected only those studies that reported adjusted effect estimates for ACEI/ARBs and outcomes. Our primary outcome was all-cause mortality/severe disease, but we also evaluated occurrence of severe disease as a secondary outcome. The outcome of all-cause mortality/severe disease includes either all-cause mortality or combined outcome of all-cause mortality or severe disease, if the study didn’t report mortality as a separate outcome. The outcome of severe disease includes admission to the ICU, need for mechanical ventilation or as defined in the individual study. Data were extracted using a standardized protocol and reporting form. The following information was obtained: study characteristics (study authors, publication year, country of origin, sample size, study design and follow-up duration), number of patients on ACEI/ARBs, main study outcomes (all-cause mortality, severe disease), reported adjusted effect estimates. We also extracted information about the variables used for adjustment in multivariable analysis from selected studies (Supplement Table 2, http://links.lww.com/HJH/B554). Study quality was assessed by use of the Newcastle-Ottawa scale with quality grades assigned based on the following three domains: selection of the study groups, comparability and assessment of outcomes.
Pooled analysis was performed using a random-effects model, which takes into account variance between and within studies. As reported by individual cohort studies, relative risk (RR), odds ratio (OR) or hazards ratio from multivariable or propensity-matched analysis were combined to estimate pooled RR. We transformed each study’s effect estimates and their confidence intervals (CIs) to natural logarithms to stabilize the variances [6]. Maximally adjusted risk estimates were used for the pooled analysis. Heterogeneity among the studies was assessed using the Higgins and Thompson I2 statistic. The I2 describes the proportion of total variation observed among the studies that is attributable to differences between studies rather than sampling error (chance), with I2 values corresponding to the following levels of heterogeneity: low (<25%), moderate (25–75%) and high (>75%). We performed sensitivity analyses using a more conservative Hartung–Knapp–Sidik–Jonkman model for random-effects meta-analysis [7]. Publication bias was tested using the Begg and Mazumdar’s rank correlation test and visual inspection of a funnel plot. The Duval and Tweedie nonparametric trim-and-fill method [8] was used to further assess the possible effect of publication bias in our meta-analysis.
The three case–control studies by Mancia et al. [4], Son et al. [9] and de Abajo et al. [10] was used to provide corresponding population-based information by comparing ACEI/ARB treatment in patients with COVID-19 who had mortality/severe disease outcome and population-based matched controls. Adjusted ORs for the association between ACEI/ARB, ACEI, and ARB and all-cause mortality/severe disease were used in this meta-analysis. Due to the inherent differences between cohort and case–control studies, we did not combine the results of these studies but used them to identify the consistency of their results. A two-tailed P less than 0.05 was considered statistically significant for all analyses. All analyses were performed using Stata statistical software, version 16 (StataCorp, College Station, Texas, USA).
RESULTS
Based on the selection criteria, 34 observational (31 cohort-based and three case–control) studies [4,9–41] were included in this systematic review (Table 1). The 31 cohort studies [11–41] included a total of 87 951 patients with COVID-19, 22 383/83 963 (26.7%) of whom were being treated with an ACEI/ARB. Twelve studies were conducted in Asia, 13 in Europe and six studies were from the North America continent. 25 studies reported all-cause mortality [11,12,14–18,20–22,24–30,32–37,39,40] while six studies [13,19,23,31,38,41] reported a combined outcome of all-cause mortality and severe disease. All the studies were of good to fair quality signifying a low-to-moderate risk for bias (Supplement Table 2, http://links.lww.com/HJH/B554).
TABLE 1.
Select characteristics of studies included in the meta-analysis
| Study | Study period | Region, location | Patients characteristics | Patients | ACEI or ARB, n | Age, male | Comorbidities | Outcomes |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Cohort studies | ||||||||
| Zhang et al. [11] | 31 December 2019 to 20 February 2020 | Wuhan, China | In-hospital patients with COVID-19 and hypertension | 1128 | 188 | 64, 53% | HTN 100% DM 21% CVD 12% CKD 3% | 28-Day mortality; severe disease |
| Reynolds et al. [13] | 1 March 2020 to 15 April 2020 | New York, USA | Patients with COVID-19 | 2211+ | 1110 | NR++ | NR++ | Combined end-point of in-hospital mortality or severe disease |
| Tedeschi et al. [12] | 22 February 2020 to 4 April 2020 | 10 Hospitals across Italy | In-hospital patients with COVID-19 and hypertension | 311 | 175 | 76, 72% | HTN 100% DM 24% CVD 42% | In-hospital mortality |
| Jung et al. [14] | Till 8 April 2020 | Nationwide database, South Korea | Patients with COVID-19 | 5179 | 762 | 45, 44% | HTN 22% DM 17% CVD 5% CKD 5% | In-hospital mortality |
| Cariou et al. [15] | 10 March 2020 to 10 April 2020 | 53 centers across France | In-hospital patients with COVID-19 and diabetes | 1317 | 752 | 70, 65% | HTN 77% DM 100% CVD 27% CKD 33% | 7-Day mortality |
| Zhou et al. [16] | 31 December 2019 to 21 April 2020 | Wuhan, China | In-hospital patients with COVID-19 | 2718+ | 906 | NR++ | NR++ | 28-Day mortality |
| Gao et al. [17] | 2 February 2020 to 15 March 2020 | Wuhan, China | In-hospital patients with COVID-19 | 710 | 527 | 64, 52% | DM 27% CVD 21% CKD 2% | In-hospital mortality |
| Felice et al. [18] | 9–31 March 2020 | Treviso, France | Patients with COVID-19 and hypertension referred to emergency department | 133 | 82 | 73, 65% | HTN 100% DM 26% CVD 60% | In-hospital mortality; severe disease |
| Liabeuf et al. [19] | 28 February 2020 to 30 March 2020 | Amiens, France | In-hospital patients with COVID-19 | 268 | 96 | 73, 58% | HTN 57% DM 21% CVD 12% CKD 7% | Combined endpoint of in-hospital mortality or severe disease; severe disease |
| Fosbol et al. [20] | 1 February 2020 to 4 May 2020 | Nationwide database, Denmark | Patients with COVID-19 | 4480 | 895 | 50–73a, 48% | HTN 19% DM 9% CVD 8% CKD 4% | In-hospital mortality; severe disease |
| Lopez-Otero et al. [21] | 10 March 2020 to 6 April 2020 | A Coruna, Spain | Patients with COVID-19 | 965 | 210 | 60, 44% | HTN 31% DM 13% CVD 4% | Mortality; severe disease |
| Selcuk et al. [22] | NR | Istanbul, Turkey | In-hospital patients with COVID-19 and hypertension | 113 | 74 | 64, 59% | HTN 100% DM 36% CVD 28% CKD 10% | In-hospital mortality |
| Bravi et al. [23] | Til 24 April 2020 | Province of Ferrara and Pescara, Italy | Patients with COVID-19 and hypertension | 543 | 450 | NR++ | NR++ | Combined endpoint of mortality or severe disease |
| Xu et al. [24] | 29 December 2019 to 15 February 2020 | Wuhan, China | In-hospital patients with COVID-19 and hypertension | 101 | 40 | 65, 52% | HTN 100% DM 19% CVD 13% CKD 2% | In-hospital mortality |
| Zhang et al. [25] | NR | Wuhan, China | In-hospital patients with COVID-19 and hypertension | 922b | 603 | 67, 51% | HTN 100% DM 36% CVD 43% CKD 6% | 28-Day mortality; severe disease |
| Dalan et al. [26] | Till 15 April 2020 | National Centre of Infectious diseases, Singapore | In-hospital patients with COVID-19 and hypertension | 139b | 90 | NR | NR | Mortality; severe disease |
| Shah et al. [27] | 2 March 2020 to 22 May 2020 | Albany, Georgia, USA | In-hospital African-American patients with COVID-19 | 531 | 207 | 60, 41% | HTN 80% DM 43% CVD 22% CKD 15% | In-hospital mortality; severe disease |
| Lam et al. [28] | 7 February 2020 to 23 May 2020 | New York, USA | In-hospital patients with COVID-19 and hypertension | 614 | 335 | 68–73a, 55% | HTN 100% DM 41% CVD 24% CKD 15% | In-hospital mortality; severe disease |
| Holt et al. [41] | 1 March 2020 to 1 April 2020 | Denmark | In-hospital patients with COVID-19 | 689 | 225 | 70, 58% | NR | Combined endpoint of in-hospital mortality or severe disease |
| Grasselli et al. [29] | 20 February 2020 to 22 April 2020 | Lombardy, Italy | Critically ill COVID-19 patients admitted to ICU | 3988 | NR | 63, 80% | HTN 41% DM 13% CVD 13% CKD 2% | In-hospital mortality |
| Kim et al. [30] | 18 February 2020 to 31 March 2020 | Daegu, South Korea | In-hospital patients with COVID-19 and diabetes | 235b | 70 | 68, 45% | HTN 63% DM 100% CVD 12% CKD 8% | In-hospital mortality |
| Cheung et al. [31] | 1 January 2020 to 27 April 2020 | Hong Kong Hospital Authority, Hong Kong | In-hospital patients with COVID-19 | 734 | 31 | NR | NR | Combined endpoint of mortality or severe disease |
| De Spiegeleer et al. [32] | 1 March 2020 to 16 April 2020 | Belgium | Nursing home residents with COVID-19 | 154 | 30 | 86, 33% | HTN 18% DM 25% | 14-Day mortality (in-hospital or nursing home) |
| Matsuzawa et al. [33] | 1 February 2020 to 1 May 2020 | Kanagawa Prefecture, Japan | In-hospital patients with COVID-19 | 151 | 22 | 60, 60% | HTN 26% DM 21% CVD 2% CKD 3% | In-hospital mortality; severe disease |
| Martínez-del Río et al. [34] | 1 March 2020 to 30 April 2020 | Ciudad Real, Spain | In-hospital patients with COVID-19 | 921 | 400 | 70, 54% | HTN 59% DM 21% CVD 16% | In-hospital mortality; severe disease |
| Lala et al. [35] | 27 February to 12 April 2020 | New York, USA | In-hospital patients with COVID-19 | 2736 | 601 | 66, 60% | HTN 39% DM 26% CVD 17% CKD 10% | In-hospital mortality |
| Imam et al. [36] | 1 March 2020 to 17 April 2020 | Michigan, USA | In-hospital patients with COVID-19 | 1305 | 565 | 61, 54% | HTN 56% DM 30% CVD 22% CKD 18% | In-hospital mortality |
| Ng et al. [37] | 1 March 2020 to 27 April 2020 | New York, USA | In-hospital patients with COVID-19 | 10 482 | 3012 | 66, 60% | HTN 61% DM 37% CVD 10% CKD 22% | In-hospital mortality |
| Gormez et al. [38] | 15 March 2020 to 15 April 2020 | Istanbul, Turkey | In-hospital patients with COVID-19 | 247 | 49 | 51, 38% | HTN 32% DM 40% CVD 10% CKD 4% | Combined endpoint of in-hospital mortality or severe disease |
| Díaz-Guardiola et al. [39] | NR | Madrid, Spain | In-hospital patients with COVID-19 | 1000 | 176 | 62, 55% | HTN 46% DM 19% CVD 16% CKD 8% | In-hospital mortality; severe disease |
| Trifiro et al. [40] | 21 February 2020 to 21 April 2020 | Lombardy, Veneto and the Reggio Emilia Local Health Unit, Italy | In-hospital patients with COVID-19 | 42 926 | 9522 | 69, 63 | HTN 13% DM 18% CVD 17% CKD 2% | In-hospital mortality |
| Case–control studiesc | ||||||||
| Mancia et al. [4] | 21 February 2020 to 11 March 2020 | Population-based case–control study in Lombardy region, Italy | Case: COVID-19 patients, Control: Residents ≥40 y who are beneficiaries of the Regional Health Service; Matching 1 : 5 by sex, age at index date, and municipality of residence | 451 cases, 2150 matched controls | 276 in cases, 1166 in controls | 68, 63% | Case: CVD 30% CKD 5% Control: CVD 22% CKD 3% | Mortality |
| de Abajo et al. [10] | 1–24 March 2020 | Seven hospitals in Madrid | Case: COVID-19 patients requiring hospital admission, Control: from a Spanish primary health-care database; Matching 1 : 10 by sex and age | 393 Cases, 3930 matched controls | 215 In cases, 1592 in controls | 69, 61% | Case: HTN 54% DM 27% CVD 11% CKD 8% Control: HTN 50% DM 20% CVD 8% CKD 5% | In-hospital mortality/severe disease |
| Son et al. [9] | Till 8 April 2020 | Population-based case–control study in South Korea | Case: COVID-19 hypertensive patients, Control: from Korean National Health Insurance System; Matching 1 : 2 based on age, sex, region and tested hospital | 38 Cases, 64 matched controls | 30 In cases, 47 in controls | 64, 51% | Case: DM 34% CVD 33% CKD 16% Control: DM 36% CVD 35% CKD 8% | Mortality |
ACEI, angiotensin-converting enzyme inhibitors; ARB, AT1 blockers; CKD, chronic kidney disease; COVID-19, coronavirus disease 2019; CVD, cardiovascular disease; DM, diabetes mellitus; HTN, hypertension; NR, not reported.
Sample size for propensity-matched analysis for ACEI/ARBs and mortality/severe disease.
Demographics and comorbidities specific to selected sample of COVID-19 patients used for the analysis was not available.
Median age in ACEI/ARB users and nonusers.
Sample size for ACEI/ARBs and mortality/severe disease as reported by individual study.
Age, sex and comorbidities data are with respect to overall sample of cases and control.
In a pooled analysis of the 31 cohort studies [11–41], there was no association between the use of ACEI/ARB and all-cause mortality/severe disease (RR: 0.94, 95% CI: 0.86–1.03, P = 0.20) (Fig. 1). Moderate heterogeneity was observed in the analysis (I2 = 57%). Sensitivity analysis using a conservative Hartung–Knapp–Sidik–Jonkman random-effects model yielded similar results. Meta-analysis of studies reporting RR or ORs and those reporting hazards ratios as effect estimates, also showed consistent results (Table 2). Subgroup analysis by sample size at least 1000 patients and by location of study (Asia, Europe, North America) also showed no association between ACEI/ARBs and all-cause mortality/severe disease. No publication bias was observed on visual inspection of funnel plot or using Egger’s regression test (P = 0.09) or Begg’s rank correlation test (P = 0.55). On recalculating pooled risk estimate using nonparametric Trim-and-Fill method and imputing seven studies, the overall RR (0.99, 95% CI: 0.90–1.09) remained nonsignificant (Supplement Fig. 1, http://links.lww.com/HJH/B554). Fifteen of the 31 cohort studies [11,18–21,24–28,30,32–34,39] reported on the association between ACEI/ARB use and occurrence of severe disease. We found no association between use of ACEI/ARBs and severe disease (pooled RR: 0.93, 95% CI: 0.74–1.17, P = 0.55, I2 = 56%) (Fig. 2).
FIGURE 1.
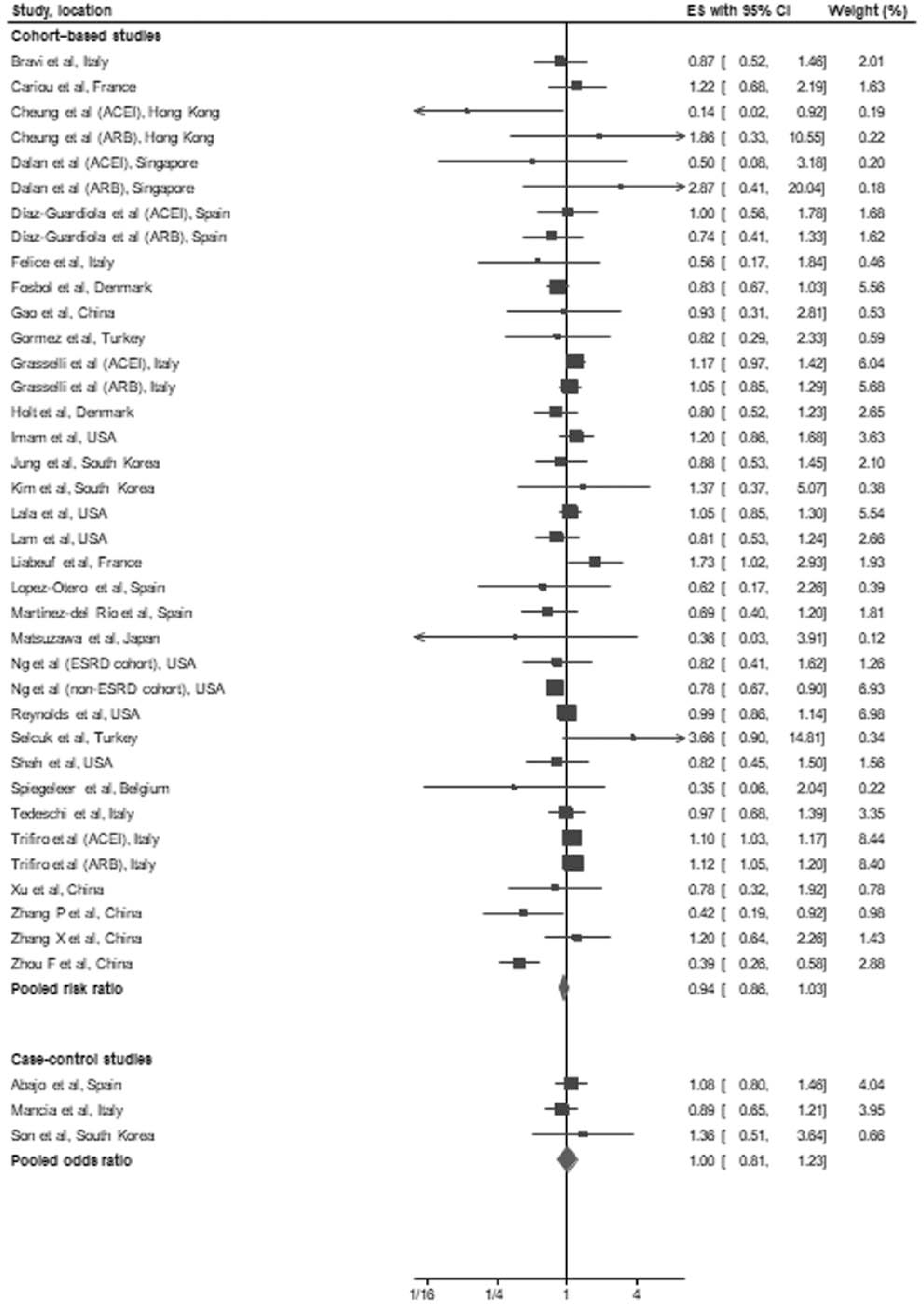
Forest plot of the association between treatment with an angiotensin-converting-enzyme inhibitors and/or AT1 blockers and all-cause mortality/severe disease in patients with coronavirus disease 2019. The figure shows effect estimates of outcomes (boxes) with 95% confidence limits (bars) for each study selected; pooled relative risk for cohort studies and pooled odds ratio for case–control studies is represented by a diamond in this forest plot. The confidence intervals for some studies may differ slightly due to log transformation of all effect estimates. ACEI, angiotensin-converting enzyme inhibitors; ARB; AT1 blockers; CI; confidence interval; ES; effect estimates.
TABLE 2.
Sensitivity analysis for association of angiotensin-converting enzyme inhibitor/AT1 blockers and primary endpoint of all-cause mortality/severe disease
| Analyses, no. of studies | Pooled relative risk (95% CI) |
|---|---|
|
| |
| Hartung–Knapp–Sidik–Jonkman model, n = 31 | 0.91 (0.78–1.07) |
| Studies from Asia, n = 12 | 0.74 (0.54–1.16), I2 = 51.1% |
| Studies from Europe, n = 13 | 1.05 (0.97–1.13), I2 = 30.9% |
| Studies from North America, n = 6 | 0.93 (0.82–1.06), I2 = 41.3% |
| Sample size < 1000, n = 18 | 0.92 (0.77–1.11), I2 = 11.5% |
| Sample size ≥ 1000, n = 13 | 0.95 (0.86–1.05), I2 = 73.3% |
| Studies reporting outcomes as odds ratio/relative risk, n = 23 | 0.91 (0.82–1.02), I2 = 14.3% |
| Studies reporting outcomes as hazards ratio, n = 8 | 0.98 (0.87–1.1), I2 = 76.8% |
CI, confidence interval.
FIGURE 2.
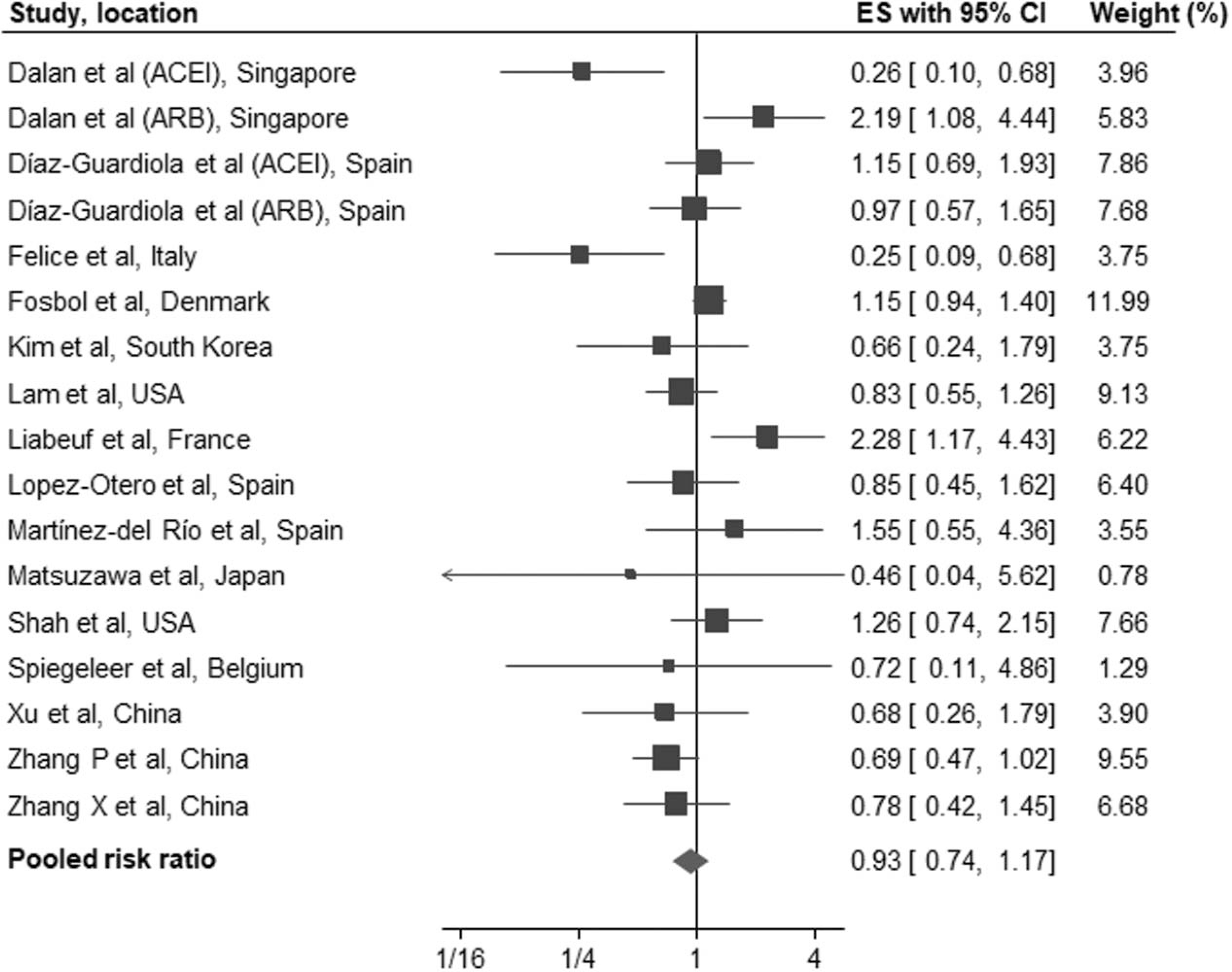
Forest plot of the association between treatment with an angiotensin-converting-enzyme inhibitors and/or AT1 blockers and severe disease in patients with coronavirus disease 2019. The figure shows effect estimates of outcomes (boxes) with 95% confidence limits (bars) for each study selected; pooled relative risk is represented by a diamond in this forest plot. ES, effect estimates.
We performed a separate analysis to evaluate whether there was any evidence of a differential effect of ACEI or ARB on the study outcomes. 13 of the 31 cohort studies [13,16,17,20,21,23,26,27,29,31,34,39,40] reported outcomes separately for ACEI and ARB. Eleven of these studies reported all-cause mortality, one while the other two studies [13,31] reported a combined outcome of all-cause mortality and severe disease. In pooled analysis, there was no association between ACEI (RR: 0.99, 95% CI: 0.87–1.12, I2 = 30%, P = 0.85) or ARB (RR: 0.88, 95% CI: 0.73–1.05, I2 = 64%, P = 0.16) for all-cause mortality/severe disease compared with controls (Figs. 3 and 4).
FIGURE 3.
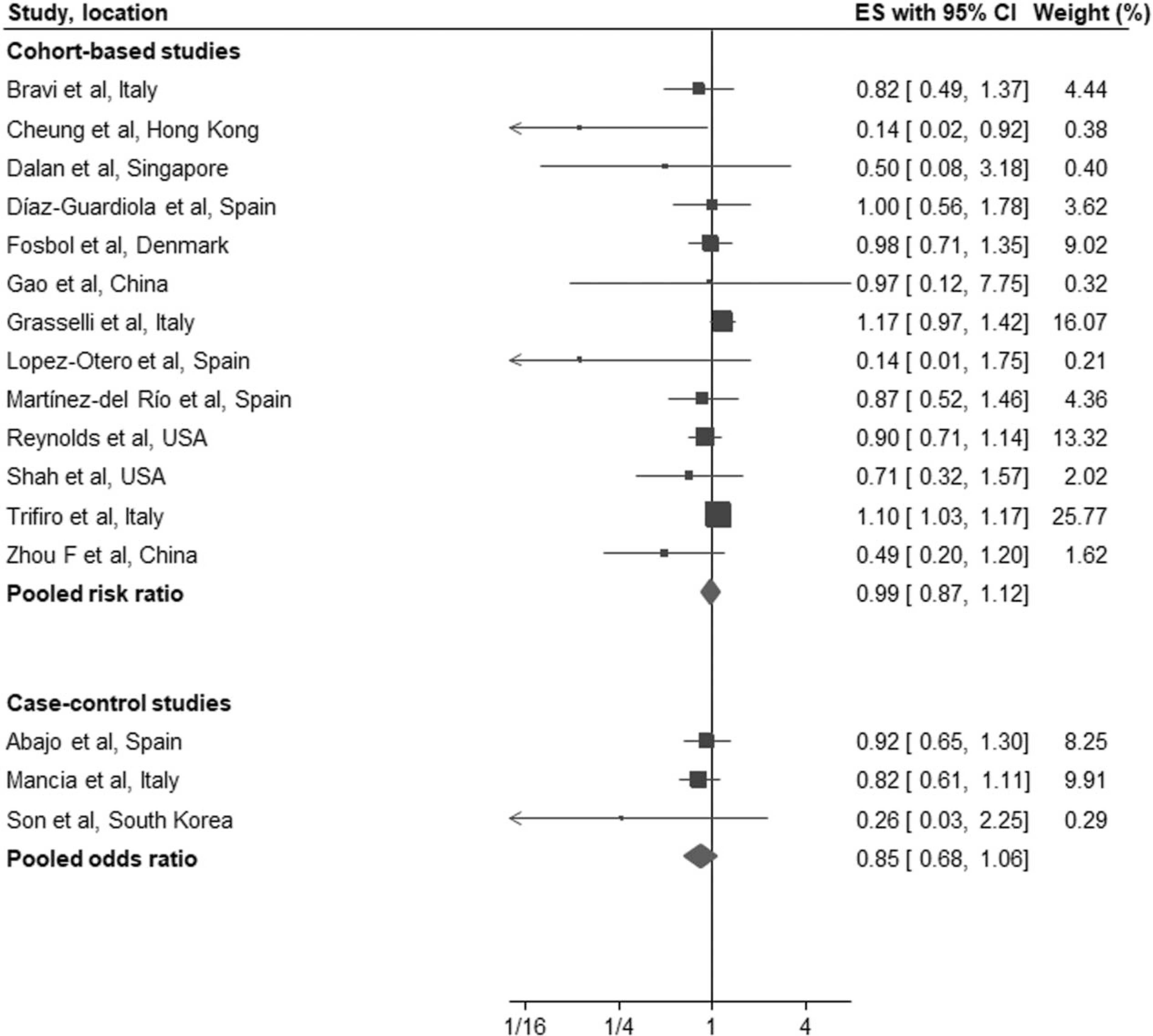
Forest plot of the association between treatment with an angiotensin-converting-enzyme inhibitors (a) or AT1 blockers (b) and all-cause mortality/severe disease in patients with coronavirus disease 2019. ACEI, angiotensin-converting enzyme inhibitors; ARB; AT1 blockers; CI; confidence interval; ES; effect estimates.
FIGURE 4.
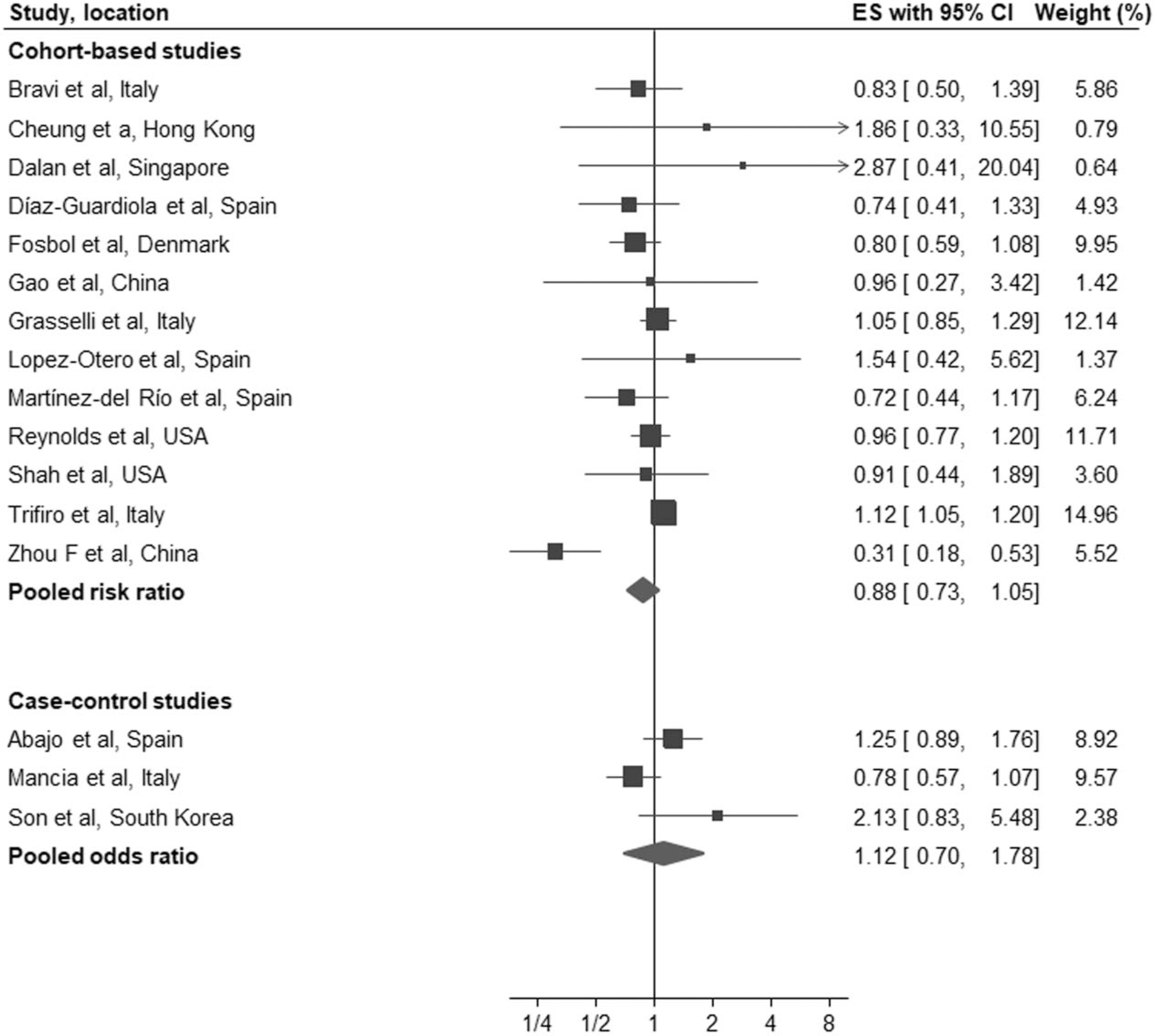
Forest plot of the association between treatment with an AT1 blockers and all-cause mortality/severe disease in patients with coronavirus disease 2019. ACEI, angiotensin-converting enzyme inhibitors; ARB; AT1 blockers; CI; confidence interval; ES; effect estimates.
Case–control studies
The three population-based case–control studies [4,9,10] compared 882 patients and 6144 matched controls for outcome of all-cause mortality/severe disease. Two studies [4,9] provided data on all-cause mortality while one study [10] reported combined endpoint of all-cause mortality/severe disease. The pooled adjusted ORs provided no evidence of a significant independent association between all-cause mortality/severe disease and treatment with ACEI/ARB (OR: 1.00, 95% CI: 0.81–1.23), or monotherapy with either ACEI (OR: 0.85, 95% CI: 0.68–1.06) or ARB (OR: 1.12, 95% CI: 0.70–1.78).
DISCUSSION
In this comprehensive systemic review and meta-analysis, we studied the relationship between ACEI and/or ARB and all-cause mortality as well as occurrence of severe disease in patients with COVID-19. We found no association for use of ACEI/ARBs and all-cause mortality/severe disease, overall or in the studies that also reported outcomes separately. The corresponding results in the three population-based case–control studies were consistent with those identified in the cohort meta-analysis.
Infection with SARS-CoV-2 is responsible for the ongoing COVID-19 pandemic. The virus enters human cells by attaching itself to the ACE2 receptor. Both animal and human studies have shown that treatment with an ACEI or ARB increases the expression of ACE2. Based on this, some researchers [42,43] have speculated that use of ACEI/ARB may not only predispose to an increased risk of COVID-19 but can be associated with worse outcomes in patients with COVID-19. In contrast, others [44,45] have postulated that the increased ACE2 expression enhances the degradation of angiotensin II and mitigates some of the risks associated with COVID-19. This concept is supported by findings from several observational studies [46–48] that have identified a reduction in the risk of influenza, pneumonia and pneumonia-related mortality in patients treated with an ACEI/ARB.
In the population-based case–control study by Mancia et al. [4], an overall multivariate adjusted analysis that compared treatment in 6272 cases and 30 759 matched controls, identified no significant association between use of either ACEI (OR: 0.96, 0.87–1.07) or ARB (OR: 0.95, 0.86–1.05) and COVID-19. This and other reports [13,49] suggest that there is no evidence of an independent relationship between renin–angiotensin–system inhibitors and susceptibility to COVID-19. Furthermore, our meta-analysis found no association between treatment with an ACEI or ARB and an increased likelihood of mortality or severe disease in patients with COVID-19.
The most common comorbidities in patients with COVID-19 are hypertension and diabetes. Patients with hypertension also appear to have increased risk for COVID-19 and risk for complications including mortality [50]. However, it is unclear if the increased risk is due to hypertension and its pathophysiologic effects or because patients with hypertension tend to be older and have increased burden of other comorbidities such as diabetes, cardiovascular disease, kidney diseases. Furthermore, ACEI/ARBs, commonly used in treatment of hypertension, also interact with ACE2 receptors, leading to a complex interplay between renin–angiotensin system, and COVID-19. Considering the controversy related to ACEI/ARB treatment in patients with COVID-19, with some researchers [42,43] even recommending stopping their use due to risk for harm, the results of our meta-analysis are reassuring. Our findings are consistent with the advice from several professional societies to continue ACEI/ARB therapy in patients with COVID-19 unless cessation is clinically indicated [1]. The current state of knowledge was summarized by Jarcho et al. [51] in an editorial that accompanied the publication of simultaneous studies [4,13] ‘Taken together, these three studies do not provide evidence to support the hypothesis that ACE inhibitor or ARB use is associated with the risk of SARS-CoV-2 infection, the risk of severe COVID-19 among those infected, or the risk of in-hospital death among those with a positive test’. However, the potential pleiotropic and salutary effects of ACEI/ARB treatment in patients with COVID-19 remains intriguing [2]. Several randomized controlled trials designed to study the effect of inhibitors of renin–angiotensin–aldosterone system in COVID-19 are in progress, although none of them are powered to assess an ACEI/ARB effect on mortality outcomes.
It is uncertain whether there is any differential effect of ACEI and ARBs on outcomes in COVID-19. In a laboratory study on Lewis rats, Ferrario et al. [52] experimentally documented the ARB losartan to almost triple cardiac ACE2 activity, whereas the ACEI lisinopril had no effect. Other animal models have shown mixed findings with respect to the effects of ACEIs or ARBs on ACE2 levels or activity in tissue. We found no difference in outcome between ACEI and ARBs in the 13 of the 31 cohort studies that reported outcomes separately. If there is a difference between ACEIs and ARBs in ACE2 upregulation, it seems to be of limited importance, if any with regard to COVID-19 infectivity or its clinical course.
Limitations
Our meta-analysis has several limitations. First, like any meta-analysis of observational reports, the limitations inherent to individual studies also apply to the overall analysis. Second, although we used only adjusted effect estimates in our meta-analysis, residual confounding cannot be excluded. Although, included studies adjusted for several covariates, the adjusted variables were not consistent across the studies and not all the studies adjusted for important covariates, and hence, combined results should be interpreted with caution. Third, lack of patient-level data precluded us in evaluating some important clinical variables such as duration of ACEI/ARBs use, dosage, medication adherence and changes after infection. Fourth, we combined different risk estimates across the studies which is not ideal and may introduce bias since OR can substantially overestimate the RR if the outcome is not rare. Fifth, observational studies evaluating the efficacy and safety of medications are susceptible to selection bias and immortal-time bias. The latter may have limited relevance for our results because we did not identify a significant treatment effect.
In conclusion, in our systematic review and meta-analysis including more than 87 000 patients, we found no association between treatment with an ACEI or ARB and risk for all-cause mortality and/or severe disease in patients with COVID-19. Similar findings were identified in population-based case–control studies. ACEI or ARB should not be discontinued, unless clinically indicated.
Supplementary Material
ACKNOWLEDGEMENTS
P.K.W. was supported by the Centers for Research Excellence grant from the National Institute of General Medical Sciences (P20GM109036).
Conflicts of interest
G.M. reports personal fees from Bayer, Boehringer Ingelheim, CVRx, Daiichi Sankyo, Ferrer, Medtronic, Menarini Int., Merck, Novartis, Recordati, Servier, outside the submitted work. G.C. reports grants from European Community, grants from Italian Medicines Agency (AIFA), Italian Ministry of Education, University and Research (MIUR), Novartis, GSK, other from Roche, AMGEN, BMS, outside the submitted work. F.H.M. reports consulting fees from Medtronic, Daiichi Sankyo and Menarini Int. C.B., and P.K.W. report no conflicts of interest.
Abbreviations:
- ACE2
angiotensin converting enzyme-2
- ACEI
angiotensin-converting-enzyme inhibitors
- ARB
AT1 blockers
- COVID-19
coronavirus disease 2019
- OR
odds ratio
- RR
relative risk
REFERENCES
- 1.Bavishi C, Maddox TM, Messerli FH. Coronavirus disease 2019 (COVID-19) infection and renin angiotensin system blockers. JAMA Cardiol 2020; 5:745–747. [DOI] [PubMed] [Google Scholar]
- 2.Messerli FH, Siontis GCM, Rexhaj E. COVID-19 and renin angiotensin blockers: current evidence and recommendations. Circulation 2020; 141:2042–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garg S, Kim L, Whitaker M, O’Halloran A, Cummings C, Holstein R, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 – COVID-NET, 14 states, March 1–30, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin–angiotensin–aldosterone system blockers and the risk of Covid-19. N Engl J Med 2020; 382:2431–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 6.Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration; 2011; Available from www.handbook.cochrane.org. [Accessed 9 May 2020]. [Google Scholar]
- 7.Cornell JE, Mulrow CD, Localio R, Stack CB, Meibohm AR, Guallar E, Goodman SN. Random-effects meta-analysis of inconsistent effects: a time for change. Ann Intern Med 2014; 160:267–270. [DOI] [PubMed] [Google Scholar]
- 8.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000; 56:455–463. [DOI] [PubMed] [Google Scholar]
- 9.Son M, Seo J, Yang S. Association between renin–angiotensin–aldosterone system inhibitors and COVID-19 infection in South Korea. Hypertension 2020; 76:742–749. [DOI] [PubMed] [Google Scholar]
- 10.de Abajo FJ, Rodriguez-Martin S, Lerma V, Mejiá-Abril G, Aguilar M, Garcia-Luque A, et al. Use of renin–angiotensin–aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study. Lancet 2020; 395:1705–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang P, Zhu L, Cai J, Lei F, Qin J Xie J AssociationJ,of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res 2020; 126:1671–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tedeschi S, Giannella M, Bartoletti M, Trapani F, Tadolini M, Borghi C, Viale P. Clinical impact of renin–angiotensin system inhibitors on in-hospital mortality of patients with hypertension hospitalized for COVID-19. Clin Infect Dis 2020; 71:899–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, et al. Renin–angiotensin–aldosterone system inhibitors and risk of Covid-19. N Engl J Med 2020; 382:2441–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung SY, Choi JC, You SH, Kim WY. Association of renin–angiotensin– aldosterone system inhibitors with COVID-19-related outcomes in Korea: a nationwide population-based cohort study. Clin Infect Dis 2020; 71:2121–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cariou B, Hadjadj S, Wargny M, Pichelin M, Al-Salameh A, Allix I, et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia 2020; 63:1500–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou F, Liu YM, Xie J, Li H, Lei F, Yang H, et al. Comparative impacts of ACE (angiotensin-converting enzyme) inhibitors versus angiotensin II receptor blockers on the risk of COVID-19 mortality. Hypertension 2020; 76:e15–e17. [DOI] [PubMed] [Google Scholar]
- 17.Gao C, Cai Y, Zhang K, Zhou L, Zhang Y, Zhang X, et al. Association of hypertension and antihypertensive treatment with COVID-19 mortality: a retrospective observational study. Eur Heart J 2020; 41:2058–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Felice C, Nardin C, Di Tanna GL, Grossi U, Bernardi E, Scaldaferri L, et al. Use of RAAS inhibitors and risk of clinical deterioration in COVID-19: results from an Italian cohort of 133 hypertensives. Am J Hypertens 2020; 33:944–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liabeuf S, Moragny J, Bennis Y, Batteux B, Brochot E, Schmit JL, et al. Association between renin–angiotensin system inhibitors and COVID-19 complications. Eur Heart J Cardiovasc Pharmacother 2020; [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fosbol EL, Butt JH, Ostergaard L, Andersson C, Selmer C, Kragholm K, et al. Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with COVID-19 diagnosis and mortality. JAMA 2020; 324:168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez-Otero D, Lopez-Pais J, Cacho-Antonio CE, Antúnez-Muiños PJ, González-Ferreiro T, Péerez-Poza M, et al. Impact of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on COVID-19 in a Western population. CARDIOVID registry. Rev Esp Cardiol (Engl Ed) 2020; S1885–5857:30224–30233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Selcuk M,Cinar T,Keskin M,Çiçek V, Kılıç, Kenan BET-AL>. Is the use of ACE inb/ARBs associated with higher in-hospital mortality in Covid-19 pneumonia patients? Clin Exp Hypertens 2020; 42:738–742. [DOI] [PubMed] [Google Scholar]
- 23.Bravi F, Flacco ME, Carradori T, Volta CA, Cosenza G, De Tog n et al. Predictors of severe or lethal COVID-19, including angiotensin converting enzyme inhibitors and angiotensin II receptor blockers, in a sample of infected Italian citizens. PLoS One 2020; 15:e0235248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu J, Huang C, Fan G, Liu Z, Shang L, Zhou F, et al. Use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers in context of COVID-19 outbreak: a retrospective analysis. Front Med 2020; 14:601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang XJ, Qin JJ, Cheng X, Shen L, Zhao YC, Yuan Y, et al. In-hospital use of statins is associated with a reduced risk of mortality among individuals with COVID-19. Cell Metab 2020; 32:176–187.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dalan R, Ang LW, Tan WYT, Fong SW, Tay WC, Chan YH, et al. The association of hypertension and diabetes pharmacotherapy with COVID-19 severity and immune signatures: an observational study. Eur Heart J Cardiovasc Pharmacother 2020; [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah P, Owens J, Franklin J, Jani Y, Kumar A, Doshi R. Baseline use of angiotensin-converting enzyme inhibitor/AT1 blocker and outcomes in hospitalized coronavirus disease 2019 African-American patients. J Hypertens 2020; 38:2537–2541. [DOI] [PubMed] [Google Scholar]
- 28.Lam KW, Chow KW, Vo J, Hou W, Li H, Richman PS, et al. Continued in-hospital ACE inhibitor and ARB use in hypertensive COVID-19 patients is associated with positive clinical outcomes. J Infect Dis 2020; 222:1256–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grasselli G, Greco M, Zanella A, Albano G, Antonelli M, Bellani G, et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med 2020; 180:1345–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim MK, Jeon JH, Kim SW, Moon JS, Cho NH, Han E, et al. The clinical characteristics and outcomes of patients with moderate-to-severe coronavirus disease 2019 infection and diabetes in Daegu, South Korea. Diabetes Metab J 2020; 44:602–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheung KS, Hung IFN, Leung WK. Association between angiotensin blockade and COVID-19 severity in Hong Kong. CMAJ 2020; 192:E635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Spiegeleer A, Bronselaer A, Teo JT, Byttebier G, De Tré G, Belmans L, et al. The effects of ARBs, ACEis, and statins on clinical outcomes of COVID-19 infection among nursing home residents. J Am Med Dir Assoc 2020; 21:909–914.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuzawa Y, Ogawa H, Kimura K, Konishi M, Kirigaya J, Fukui K, et al. Renin–angiotensin system inhibitors and the severity of coronavirus disease 2019 in Kanagawa, Japan: a retrospective cohort study. Hypertens Res 2020; 43:1257–1266. [DOI] [PubMed] [Google Scholar]
- 34.Martinez-Del Rio J, Piqueras-Flores J, Nieto-Sandoval Martin de la Sierra P, Negreira-Caamaño M,ģuila-GordoA D,Mateo-Gómez C,et al. Comparative analysis between the use of renin–angiotensin system antagonists and clinical outcomes of hospitalized patients with COVID-19 respiratory infection. Med Clin (Barc) 2020; 155:473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol 2020; 76:533–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Imam Z, Odish F, Gill I, O’Connor D, Armstrong J, Vanood A, et al. Older age and comorbidity are independent mortality predictors in a large cohort of 1305 COVID-19 patients in Michigan, United States. J Intern Med 2020; 288:469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ng JH, Hirsch JS, Wanchoo R, Sachdeva M, Sakhiya V, Hong S, et al. Outcomes of patients with end-stage kidney disease hospitalized with COVID-19. Kidney Int 2020; 98:1530–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gormez S, Ekicibasi E, Degirmencioglu A, Paudel A, Erdim R, Gumusel HK, et al. Association between renin–angiotensin–aldosterone system inhibitor treatment, neutrophil-lymphocyte ratio, D-dimer and clinical severity of COVID-19 in hospitalized patients: a multicenter, observational study. J Hum Hypertens 2020; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diaz-Guardiola P, Martin-Borge V, Garcia-Fernandez C, Ramirez-Prieto MT, Ramirez-Belmar MI, Garcia-Romero G, et al. Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with coronavirus disease 2019 severity and mortality. Am J Intern Med 2020; 8:204–210. [Google Scholar]
- 40.Trifiro G, Massari M, Da Cas R, Menniti Ippolito F, Sultana J, Crisafulli S, et al. Renin–angiotensin–aldosterone system inhibitors and risk of death in patients hospitalised with COVID-19: a retrospective Italian Cohort Study of 43,000 patients. Drug Saf 2020; 43:1297–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holt A, Mizrak I, Lamberts M, Lav Madsen P. Influence of inhibitors of the renin–angiotensin system on risk of acute respiratory distress syndrome in Danish hospitalized COVID-19 patients. J Hypertens 2020; 38:1612–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med 2020; 8:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aronson JK, Ferner RE. Drugs and the renin–angiotensin system in covid-19. BMJ 2020; 369:m1313. [DOI] [PubMed] [Google Scholar]
- 44.Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. N Engl J Med 2020; 382:1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Danser AHJ, Epstein M, Batlle D. Renin–angiotensin system blockers and the COVID-19 pandemic: at present there is no evidence to abandon renin–angiotensin system blockers. Hypertension 2020; 75:1382–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caldeira D, Alarcao J, Vaz-Carneiro A, Costa J. Risk of pneumonia associated with use of angiotensin converting enzyme inhibitors and angiotensin receptor blockers: systematic review and meta-analysis. BMJ 2012; 345:e4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chung SC, Providencia R, Sofat R. Association between angiotensin blockade and incidence of influenza in the United Kingdom. N Engl J Med 2020; 383:397–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kreutz R, Algharably EAE, Azizi M, Dobrowolski P, Guzik T, Janusze-wicz A, et al. Hypertension, the renin–angiotensin system, and the risk of lower respiratory tract infections and lung injury: implications for COVID-19. Cardiovasc Res 2020; 116:1688–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mehta N, Kalra A, Nowacki AS, Anjewierden S, Han Z, Bhat P, et al. Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020; 5:1020–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J 2020; 55:2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jarcho JA, Ingelfinger JR, Hamel MB, D’Agostino RB Sr, Harrington DP. Inhibitors of the renin–angiotensin–aldosterone system and Covid-19. N Engl J Med 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 2005; 111:2605–2610. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


