Abstract
Calcium (Ca2+) is a fundamental second messenger in all cell types and is required for numerous critical cellular functions including cardiac and skeletal muscle contraction. Intracellular free [Ca2+] is regulated primarily by ion channels, pumps (ATPases), exchangers and Ca2+ binding proteins. Defective regulation of [Ca2+] is found in a diverse spectrum of pathological states and human disorders affecting all the major organs. In the heart we and others have reported abnormalities in cytosolic [Ca2+]cyt and mitochondrial [Ca2+]mito regulation in heart failure (HF) and atrial fibrillation (AF), the two most common forms of heart disease and leading contributors to mortality and morbidity. This review is focused on mechanisms that regulate the major SR Ca2+ release channel in the heart, the ryanodine receptor type 2 (RyR2), how the channel becomes dysfunctional in HF and AF and is a potential therapeutic target. Inherited RyR2 mutations, and/or stress-induced phosphorylation and oxidation, destabilize the closed state of the channel resulting in a pathological SR Ca2+ leak that both triggers arrhythmias and impairs contractility. Recent high-resolution cryo-electron microscopy (cryo-EM) RyR structures (Figs. 4–6) have clarified the architecture of multiple functional states, ligand-binding sites and gating mechanisms, and informed as to mechanisms that go awry in HF and AF. Based on new understandings of SR Ca2+ leak as a common Ca2+-dependent pathological mechanism in HF and AF, a new class of drugs developed in our laboratory called Rycals, that stabilize RyR2 channels and prevent SR Ca2+ leak, are undergoing clinical trials.
Keywords: ryanodine receptor, calcium, calstabin, excitation contraction coupling, calcium channel, heart failure, atrial fibrillation, Rycal
Introduction
Heart failure (HF) and atrial fibrillation (AF) are leading causes of mortality and morbidity in developed countries. Globally, HF affects an estimated 26 million people and contributes to more than 1 million hospitalizations annually1. AF is the most common cardiac arrhythmia with a prevalence of 2.5–6.0 million in the United States, affecting 8% to 10% of individuals over the age of 802. HF and AF share common risk factors including hypertension, diabetes mellitus, and valvular heart disease which often occur together. The prevalence of AF in patients with symptomatic HF (NYHA class II-IV) is between 15 and 35% 3,4.
During AF the atria exhibit disorganized, rapid contractions, at rates of 400–500 beats per minute 5. This results in atria that fibrillate instead of contracting uniformly, which leads to ventricular underfilling and reduced cardiac output. The absence of regular cardiac contractions increases the risk of thrombosis within the left atrial appendage, which can embolize to the brain and cause ischemic strokes 6. AF can be managed by rate control or rhythm control. Rate control is achieved with medications that target conduction through the atrio-ventricular (AV) node, resulting in a reduction in the number of ventricular beats 5. With rhythm control, an effort is made to maintain sinus rhythm using anti-arrhythmic medications, electrical cardioversion, and/or catheter ablation.
Studies comparing rate control to rhythm control suggest at best clinical equivalence7 and even possible harm with aggressive rhythm control 8. Potential benefits from AF therapies are offset by limited efficacy of anti-arrhythmic medications and intolerable side effects which impair medication compliance. A recent study comparing radiofrequency catheter ablation to medical rate control suggested clinical benefit for patients receiving catheter ablation to maintain sinus rhythm, however this benefit was not observed with intention-to-treat analysis9. Survival benefit for rhythm control in AF is better established in patients with HF9,10.
Heart failure is a condition characterized by the inability to generate a cardiac output sufficient to meet the body’s metabolic needs. HF can be sub-classified as having either reduced ejection fraction (HFrEF) or preserved ejection fraction (HFpEF)11. Common causes of HFrEF are ischemia, hypertension, and valvular heart disease. Medical management of HF focuses on reducing the effect of elevated sympathetic tone using beta-blockers and/or blocking angiotensin 2 production, and reducing vascular tone and stress on the heart. Despite these interventions, many patients have disease progression requiring cardiac transplantation or mechanical assist devices12.
Although HF and AF are complex conditions with many contributing factors, alterations in intracellular Ca2+ handling in cardiac myocytes have been implicated in the pathogenesis of both conditions13. Ca2+ is a ubiquitous second messenger that controls numerous biological functions including muscle contraction, synapse transmission, hormone secretion, proliferation, and apoptosis14. Accumulating evidence indicates that defective cardiac Ca2+ signaling represents a central pathogenic mechanism underlying cardiac diseases, including HF and AF15–21. This review discusses Ca2+ dysregulation in HF and AF, and includes data from high-resolution cryo-EM structures of RyRs with a focus on clinical and therapeutic implications. Based on a recent pubmed search there have been >44,000 reviews written about HF and ~12,000 on AF. There is no need for another review with a broad comprehensive scope. Instead the present review is focused on just one part of the story: the role that dysregulation of Ca2+ homeostasis 21 in ventricular and atrial cardiomyocytes plays in HF and AF. As such we have not addressed some important molecular mechanisms that contribute to HF as well as structural considerations such as T-tubule remodeling 22; and those that contribute to AF as well as the roles of fibrosis and conduction defects, all of which are presented in detail in several excellent textbooks as well as reviews. Rather our intent is to focus on Ca2+ and in particular the role of leaky RyR2 channels. In so doing we do not address other equally important aspects of HF and AF. We have briefly summarized a few of the scientific disagreements in the field most of which have been addressed elsewhere 13,23. Our purpose herein is to highlight a common mechanism linking HF and AF that is also a therapeutic target: diastolic SR Ca2+ leak.
The mechanisms underlying AF and HF have been obscure for many decades and the current therapies are largely aimed at reducing symptoms, but are not disease modifying. This review makes the point that diastolic SR Ca2+ leak via dysfunctional RyR2 channels is a common underlying mechanism in both AF and HF and potentially can be treated with a new class of drugs called Rycals that fix leaky RyR2 channels. A key player in the etiology of the leak is the channel subunit calstabin (Calcium Channel Stabilizing subunit). We first showed that calstabin1 (FKBP12) is a subunit of RyR1 24 and that it is required to stabilize the closed state and prevent aberrant Ca2+ leak through the channel 25 and that calstabin2 (FKBP12.6) plays the same role in RyR2 26. Others have confirmed that calstabin2 plays an important physiological role in modulating RyR2 function 27–34.
The RyR2 dysfunction in AF and HF is due to oxidation and PKA hyperphosphorylation (defined as PKA phosphorylation of 3 or 4 of the 4 PKA sites on each homotetrameric RyR2 channel) of the RyR2 channel which cause leak by destabilizing the closed state of the channel associated with depletion of the stabilizing subunit calstabin2 from the RyR2 channel macromolecular complex 19,20,26,35–37. Rycals bind to RyR2 and prevent dissociation of calstabin2 via an allosteric mechanism. Of note a similar process occurs in skeletal muscle where leaky RyR1 channels cause impaired ECC and reduced exercise capacity (a major symptom in HF patients) 37–39. Rycals can also fix this leak and HF really should be thought of as a generalized myopathy involving both types of striated muscle, skeletal and cardiac 37–40. A rycal is now being tested in RyR1-myopathy patients in a clinical trial at the NIH (ClinicalTrials.gov Identifier: NCT04141670) that involves one month oral dosing of rycal and pre- and post- muscle biopsies to see whether the leaky RyR1 channels in this inherited form of muscular dystrophy caused by RyR1 mutations that make the channel leaky have been fixed.
1. Cardiac Excitation-contraction coupling
To understand Ca2+ signaling in the heart one must be familiar with cardiac excitation–contraction coupling (ECC). ECC which converts electrical signals into mechanical force, requires coordinated Ca2+ release and reuptake in cardiomyocytes. The Ca2+ release cycle is initiated by depolarization of the plasma membrane and the specialized invagination called the transverse tubule (T-tubule) 41. Voltage-gated L-type Ca2+ channels (LTCC) on the T-tubular membrane are activated by depolarization and allow a small inward Ca2+ current. This Ca2+ binds to and activates RyR2 channels, which release Ca2+ from the sarcoplasmic reticulum (SR) where it is stored at high concentration (high micromolar to low millimolar range).
The process by which Ca2+ activates RyR2 channels is known as Ca2+-induced-Ca2+-release (CICR) and was originally described by Fabiato and Fabiato 42,43. Ca2+ released into the cytoplasm raises [Ca2+]cyt ~10-fold (although local [Ca2+] is likely even higher) and binds to troponin C (and other Ca2+-sensitive proteins), allowing actin-myosin cross-bridging as the thick and thin filaments of the sarcomere slide past one another, shortening the sarcomere and causing muscle contraction (systole). Individual Ca2+ release events can be imaged as Ca2+ sparks 44 the frequency of which is a measure of SR Ca2+ release and/or leak which is increased in failing hearts 22. Cardiac relaxation, or diastole, is initiated when Ca2+ release is terminated and Ca2+ is pumped back into the SR by the sarco-endoplasmic reticulum Ca2+ ATPase 2a (SERCA2a), extruded by the Na+/Ca2+ exchanger (NCX1) (Fig. 1&3). Dysfunctional Ca2+ handling may occur at different stages of the ECC process including Ca2+ entry to the cardiomyocytes15, intracellular Ca2+ release45 and Ca2+ uptake and buffering46. This review will focus on the role of RyR2 dysfunction in Ca2+ dysregulation in HF and AF. Of note, RyR2 is also expressed in other organs including the brain47 and the pancreas48 and it is likely that RyR2 dysfunction in these and other organs also contributes to HF as a systemic disorder.
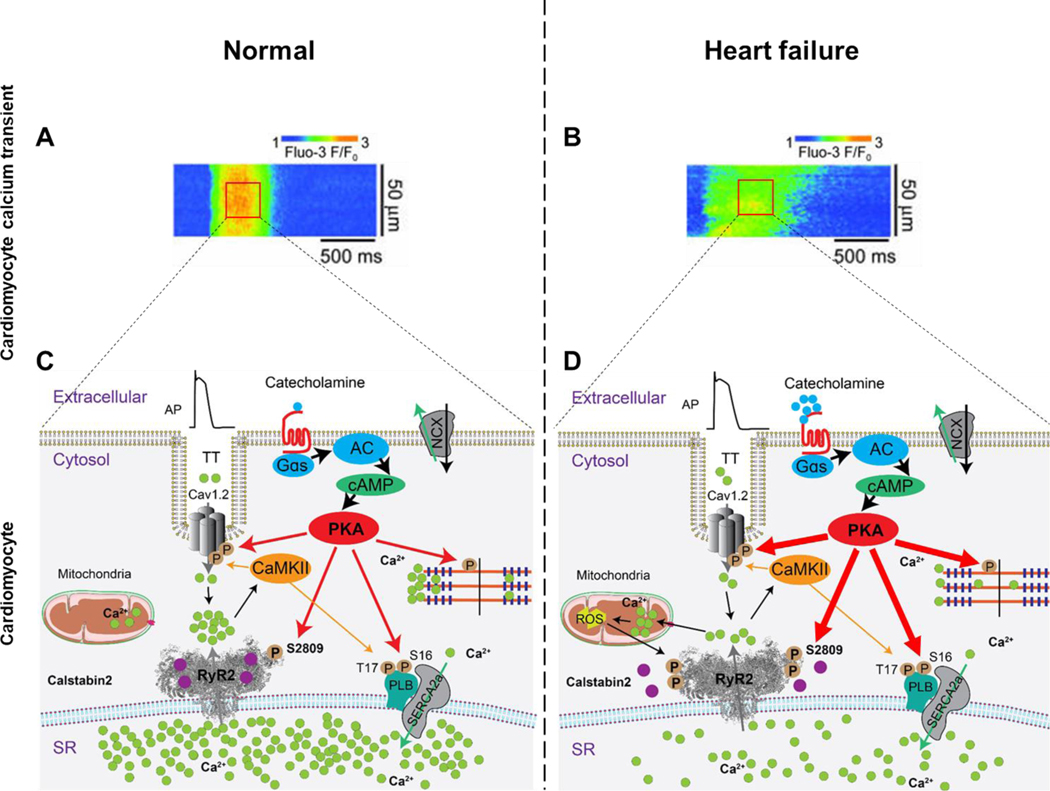
Figure 1. Diastolic SR Ca2+ leak in ventricular myocytes from failing hearts.
Representative line scan of Ca2+ sparks recorded in ventricular cardiomyocytes from normal (A) and failing murine hearts (B). Representative RyR2 single-channel tracings under conditions that simulate diastole when the cytosolic Ca2+ concentration is low (e.g. 100–150 nM), in normal (C) and heart failure (D) cardiomyocytes 243. Schematic representation of intracellular Ca2+ release in EC coupling in normal (E) and heart failure (F) cardiomyocytes. There is little or no diastolic SR Ca2+ leak in ventricular cardiomyocytes from normal non-failing hearts (panels A,C and E). In contrast, ventricular cardiomyocytes from failing hearts exhibit increased spontaneous Ca2+ sparks, increased RyR2 open probability, diastolic SR Ca2+ leak and reduced SR Ca2+ stores, all consistent with a pathologic leak of SR Ca2+via RyR2 remodeled (including PKA hyperphosphorylation, oxidation, nitrosylation and dissociation of calstabin2 from the RyR2 channel complex) resulting in RyR2 channels that do not close properly during diastole. (panels B,D and F). This results in reduced SR Ca2+ content. AP, action potential; TT, transverse tubule; cAMP, cyclic adenosine monophosphate; Cav1.2, L-type Ca2+ channel; NCX, Na+/Ca2+ exchanger; RyR2, ryanodine receptor type-2; SERCA2a, sarco/endoplasmic reticulum ATPase type-2a; PLB, phospholamban; PKA, protein kinase A; CaMKII, Ca2+/calmodulin-dependent protein kinase II; ROS, reactive oxygen species.
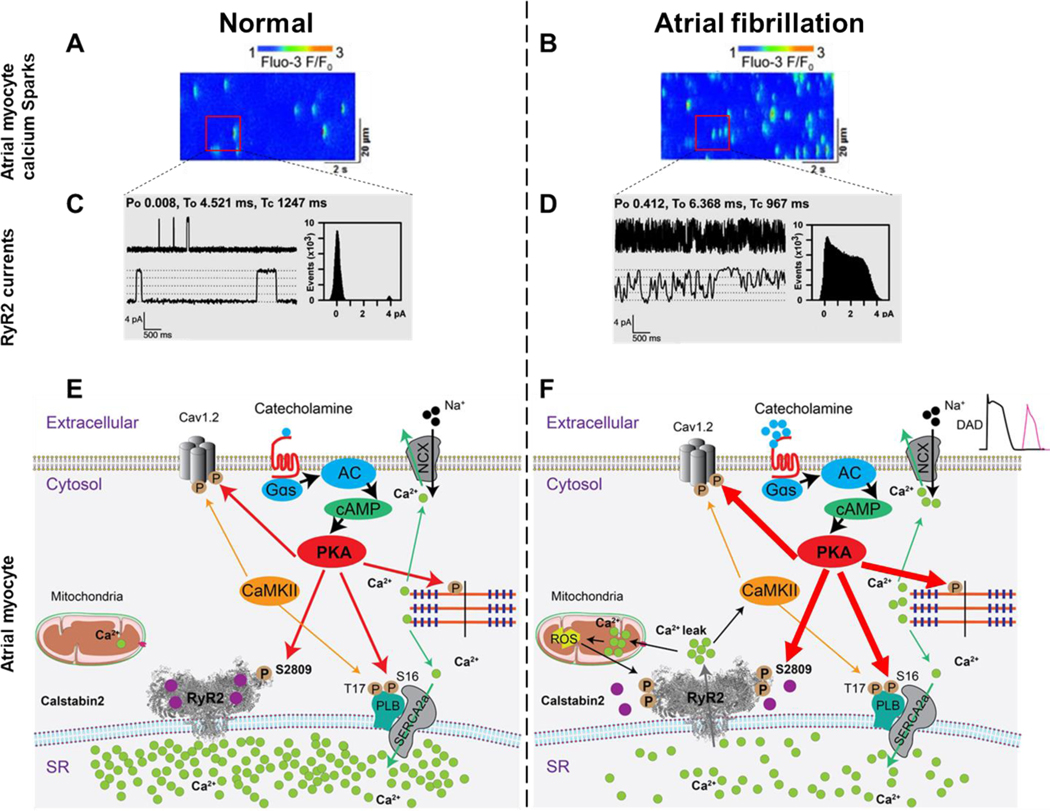
Figure 3. SR Ca2+ leak triggers AF in atrial myocytes.
Representative line scan of Ca2+ sparks recorded in atrial myocytes from normal (A) and atrial fibrillation (B)17. Representative RyR2 single-channel tracings recorded under conditions simulating diastole in atrial cardiomyocytes at a resting [Ca2+]cyt ≃ 150 nM, in normal (C) and atrial fibrillation cardiomyocytes (D)101. Schematic representation of Ca2+ signaling in normal (E) and atrial fibrillation (F) atrial myocytes. Atrial myocyte exhibit increased spontaneous Ca2+ sparks, increased RyR2 open probability, Ca2+ leak and reduced SR Ca2+ stores in AF.
2). Stress-induced RyR2-mediated diastolic SR Ca2+ leak
RyR2 is the largest known ion channel with a molecular weight in excess of 2 million Daltons. The channel is a homotetrameric, macromolecular complex comprised of four RyR2 565 kDa protomers49. RyR2 channel activity is regulated by kinases and phosphatases26, phosphodiesterase50, calmodulin51–57 and a stabilizing subunit protein FK506-binding protein 12.6 (FKBP12.6 or Calstabin2)25,26. Protein Kinase A (PKA) and Ca2+/calmodulin-dependent Kinase II (CaMKII) tether to RyR2 and phosphorylate the channel26,58. PKA is activated by the adrenergic receptor pathway59. Epinephrine and norepinephrine bind to the beta-adrenergic receptor in cardiomyocytes, activate adenylyl cyclase (AC) which produces cAMP resulting in downstream PKA activation that increases inotropy, lusitropy, and chronotropy60. CaMKII is initially activated by Ca2+/calmodulin, and then remains active due to autophosphorylation 58,61,62. We and others have identified Ser2808 26 and Ser2814 63,58 as the physiologic PKA and CaMKII phosphorylation sites, respectively. Both sites modulate RyR2 activity (Fig. 1–3).
Indicative of the importance of PKA phosphorylation of RyR2 is the finding that mice harboring an RyR2-S2808A mutation have blunted inotropic and chronotropic responses to catecholamines 19,20,35. On the other hand mice with a phosphomimetic mutation of the RyR2 PKA phosphorylation site (RyR2-Ser2808Asp) have leaky RyR2 channels and an age dependent cardiomyopathy and arrhythmias 20. Moreover, there is protection against HF progression in RyR2-Ser2808Ala knock-in mice 19,20,35–37. Another group generated an RyR2-S2808A mouse and used it to report that RyR2-Ser2808 plays no role in the cardiomyocyte response to β-adrenergic stimulation 64. These and other differences have been addressed in detail elsewhere 23.
Inherited mutations linked to exercise-induced sudden cardiac death (Catecholaminergic Polymorphic Ventricular Tachycardiac, CPVT) 59,65 as well as PKA phosphorylation, S-nitrosylation and Cys-oxidation of RyR2 can cause dissociation of calstabin2 from RyR220,36,37,66. Calstabin2 is tightly associated with RyR2 and modulates it function25,59,67. Similarly, Calstabin1 is bound to RyR1 and regulates EC coupling gain as shown by Dirksen and colleagues 68.
One molecule of calstabin2 binds to each protomer of RyR2 and stabilizes the channel closed state, increases mean open and closed times as well as facilitates coupled gating between neighboring RyR2 channels 69. Stress-induced calstabin2 dissociation leads to unstable channels that leak Ca2+ as the channel is unable to close properly25,26. Dysregulation of intracellular Ca2+ handling alters contractility and electrical stability. Chronic SR Ca2+ leak reduces SR Ca2+ stores as well as the Ca2+ wave amplitude necessary for optimal cardiomyocyte contraction during systole70. RyR2 Ca2+ leak can be observed in isolated cardiomyocytes as short unsynchronized SR Ca2+ release events during diastole71.
The physiologic role of calstabin2 binding to RyR2, and the stress-induced dissociation of calstabin2 binding from RyR2 have been challenged. For example, one group reported that only 10—20% of RyR2 have calstabin2 in the channel macromolecular complex, and that PKA phosphorylation of RyR2 did not affect calstabin2 binding 72. Another group overexpressed calstabin2 in cells expressing recombinant RyR2 with a phosphomimetic residue in the PKA site (Ser2809Asp) and did not observe a decrease in binding compared to WT RyR273. Our assessment is that these differences are due to variances in methods and data interpretation and have been addressed in detail elsewhere 23. Further supporting a role for PKA phosphorylation of RyR2 in dissociation of calstabin2, we observed that leaky RyR2-Ser2808Asp channels are depleted of calstabin2 and progressively oxidized both when heterologously expressed in HEK293 cells and in vivo in RyR20-Ser2808Asp mice, and that channel oxidation also contributes to the decrease in calstabin2 binding20.
Isoproterenol-induced dissociation of calstabin2 from RyR2 has been reported by others29 and overexpression of calstabin2 prevents SR Ca2+ leak31 and ventricular arrhythmias in mice exposed to isoproterenol and myocardial burst pacing27. In HF there is a reduction in the amount of calstabin2 bound to RyR274,75 and beta-blockers improve calstabin2 binding to RyR276 by preventing PKA phosphorylation of RyR2. The mechanisms by which PKA phosphorylation and oxidation/nitrosylation dissociate calstabin2 from RyR2 have been confirmed by a number of groups 27,31,67,74–77.
We first showed that PKA hyperphosphorylation of RyR2 depletes calstabin2 from the RyR2 complex 26. Years later we found that oxidation and nitrosylation of RyR2 also cause depletion of calstabin2 from the RyR2 complex: together all three processes (oxidation, nitrosylation and phosphorylation of Ser2808) fully deplete calstabin2 from the channel complex 19,20. RyR2-Ser2808Ala mice are protected from PKA phosphorylation-induced depletion of calstabin2 and RyR2-Ser2808Asp mice exhibit decreased levels of calstabin2 in the RyR2 complex 19,20 as confirmed by others 29,32.
Offering opposing views, Bers and colleagues, in disagreement with their own previous study which concluded that there is “an important SR-stabilizing effect of FKBP in intact rat ventricular myocytes” 78, reported that FKBP12.6 (calstabin2) does not play a significant role in cardiac physiology 72. Using a fluorescent-labeled calstabin2 with 1 nM affinity for RyR2 72 (compared to 160 nM reported by Fleischer 79) they estimated that ≤ 20% of the calstabin2 binding sites on RyR2 are occupied and that calstabin2 is not an important RyR2 regulator. Fleischer had reported that 83% of the calstabin2 sites on RyR2 are occupied 80.
Meissner showed that PKA phosphorylation of RyR2 does not cause calstabin dissociation from RyR2 using a molar excess of calstabin (see Figure 1B in 73). Indeed, other groups have reported that PKA phosphorylation does not remove castabin2 from RyR2 64,81,82,83–85 and/or that CaMKII is the more important kinase regulating the channel 86–90. Since RyR channels have > 30 exposed free cysteines in each monomer (>120 per tetrameric channel) 91, some disagreements about calstabin2 binding can be explained by RyR2 oxidation as it is customary, for example, to add antioxidants such as DTT to samples.
Valdivia and colleagues have challenged the roles of PKA hyperphosphorylation, calstabin2 depletion and diastolic SR Ca2+ leak via RyR2 channels in HF 85. We originally showed evidence for SR Ca2+ leak via HF RyR2 as manifested by increased open probability at diastolic [Ca2+]cyt (~150 nM). Valdivia used systolic [Ca2+]cyt (5 μM) and not surprisingly reported no leak since RyR2 is open at this activating [Ca2+]cyt and leak has no meaning when the channel is already open 85. Valdivia and colleagues further reported that HF RyR2 were not PKA hyperphosphorylated or depleted of calstabin, but did not normalize the amount of RyR2 protein (see Fig. 8A in 85) or co-immunoprecipitate RyR2 resulting in contamination of their RyR2 samples with calstabin1 80.
Chen has proposed an alternative mechanism for RyR2 leak which he refers to as store overload induced calcium release (SOICR) 92. In the Chen model SOICR depends on a Ca2+ sensor 92 that turns out to be cytoplasmic based on the channel structure 93,94.
CaMKII-dependent phosphorylation of RyR2 is increased in HF and alternative mechanisms involving CaMKIIδ have been proposed to explain SR Ca2+ leak in HF. 95 Mice expressing AC3-I, the CaMKII inhibitory peptide, are protected against HF 96, an RyR2-S2814A mutation that ablates CaMKII mediated RyR2 phosphorylation protects against HF after transverse aortic constriction (TAC) 88, but not after myocardial infarction 58. TAC is a hypertrophy model and CaMKII phosphorylation of RyR2 is associated with the hypertrophy. RyR2 CaMKII phosphorylation is not increased in a post-MI model but plays an important role in rate-related increase in contractility known as the Bowditch phenomenon 58.
Diastolic SR RyR2 Ca2+ leak promotes an inward depolarizing current via NCX1 antiporters which leads to delayed afterdepolarizations (DADs) and the initiation of triggered activity 97. At rest the leak is minimal, during adrenergic stress the cardiomyocyte becomes loaded with Ca2+ (PKA phosphorylation of DHPR, PLN and SERCA2a conspire to increase SR Ca2+ levels) and the leak is exacerbated, leading to arrhythmogenesis. RyR2-Ser2808 was the first residue identified to be phosphorylated in HF leading to calstabin2 dissociation, enhanced RyR2 open probability (P0), and diastolic Ca2+ leak26. Wehrens et al showed that preventing PKA hyper-phosphorylation of RyR2 inhibits progressive cardiac dysfunction in mice with ischemia-induced HF using genetically altered mice in which serine 2808 on RyR2 has been replaced by Ala (RyR2-Ser2808Ala mice)35. Thus, there is an important role for RyR2-Ser2808 in RyR2 regulation by the sympathetic nervous system (SNS). These findings were independently validated by Walweel et al 98 and are in line with the efficacy of beta-blockers in HF patients where the beta-antagonist reduces the incidence of cardiovascular events as well as the mortality in patients with chronic heart failure99. Indeed, because the RyR2 of RyR2-Ser2808Ala mice cannot be PKA-phosphorylated, calstabin2 binding to RyR2 is not reduced as seen in HF mice.
Multiple animal models have been used to support a role for PKA hyperphosphorylation of RyR2 in HF progression 19,20,26,35–37,39,50. As noted, genetically altered mice harboring RyR2 that cannot be PKA phosphorylated (RyR2-S2808A), are protected against calstabin2 depletion from the RyR2 complex and HF progression 4 week post-MI 35. Others reported that they could not reproduce these results, but published data in strong support of ours showing preserved cardiac function in RyR2-S2808A mice compared to WT mice (see their Supplemental Table 1, fractional shortening second line from the bottom in Reference 64) in a transverse aortic constriction (TAC) model of HF. These and other differences have been addressed in detail elsewhere 23.
Additionally, PDE4D3 deficient mice develop an age-dependent cardiomyopathy and arrhythmias, RyR2 PKA hyperphosphorylation and calstabin2 depletion. Crossing the PDE4D3 deficient mice with the RyR2-S2808A mice resulted in protection against cardiomyopathy 50. Genetically altered mice expressing mutant calstabin2-D37V, which binds to PKA phosphorylated RyR2 channels, are protected against post-MI HF 100. In summary, there is an important role for chronic PKA hyper-phosphorylation of RyR2 in HF progression as demonstrated using RyR2-S2808A mice.
Recently, high-resolution RyR cryo-EM structures have provided further details supporting phosphorylation-mediated diastolic Ca2+ leak. The RyR2-Ser2808 PKA phosphorylation site was found on the P2 rim of the helical domain where limited transient PKA phosphorylation may weaken its contact with SPRY3 of the neighboring protomer and contribute to a flexible conformation of RyR2 channels and enhanced open state probability (Fig. 4). More extensive PKA phosphorylation for longer periods of time might disrupt critical domain interactions resulting in destabilization of the channel closed state and diastolic SR Ca2+ leak however this possibility remains to be explored experimentally with new structures of PKA phosphorylated channels. We have also reported RyR2 PKA hyperphosphorylation at Ser2808 in a canine model of AF101. Aberrant diastolic SR Ca2+ release and DADs in atrial myocytes are capable of triggering arrhythmia giving arise to AF65.
Figure 4. RyR2 phosphorylation domain and CPVT-associated mutation hot spots.
CPVT-associated RyR2 mutations (http://www.uniprot.org/uniprot/Q92736) in the N-terminal domain (red spheres), bridging solenoid (blue spheres), core solenoid (purple spheres), transmembrane domain (orange spheres), and C-terminal domain (magenta), viewed from the cytosol (left) and from the plane of the SR membrane (right). Insets show the domain-domain interface between the NTD and the bridging solenoid at the ‘zipping’ point where DPc10 (black ribbon) is located, the newly revealed calmodulin binding site (green ribbon) and all 4 known ARVD-causing mutations (green spheres). On the right inset the interaction between CTD (cornflower blue ribbon) and core solenoid TAF motif also shows mutation clusters on both sides of this domain-domain interface. The location of the phosphorylation domain containing the PKA and CaMKII sites is shown.
The role of another potential RyR2 PKA phosphorylation site, Ser2030, in HF pathogenesis remains unclear. Ser2030 phosphorylation had no effect on RyR2 function (see their Fig. 7 in 102), and our data concur35. However, a recent publication of an RyR2Ser2030A mouse model reported a blunted response to catecholamines 81. Moreover, absence PKA phosphorylation of RyR2-Ser2808Ala in knock-in mice indicates that Ser2808 is the only RyR2 PKA phosphorylation site in-vivo 35,63.
A distinct phosphorylation site, RyR2-Ser2814, is phosphorylated by CaMKII63. However, mice engineered with channels that cannot be phosphorylated by CaMKII (RyR2-Ser2814Ala) are not protected in an ischemic model of HF58. Reactive oxygen species (ROS) induce CaMKII activation by direct oxidation of the enzyme’s regulatory domain103. Both oxidative stress and CaMKII have been linked to HF and AF. Oxidative stress is elevated in patients with AF104, and CaMKII-dependent phosphorylation of RyR2 has been reported to cause SR Ca2+ leak that may contribute to AF61.
In contrast we found that CaMKII phosphorylation of RyR2 at RyR2-Ser2814 is required for rate-dependent increase in cardiac contractility thus providing a molecular mechanism for this phenomenon first described almost 150 years ago by Henry Bowditch in 187158! This is a critical physiological response that enables the heart to maintain or increase cardiac output as heart rate increases with exercise. An impaired or blunted positive force-frequency relationship results in diminished cardiac output at higher heart rates which blunts the ability to respond to stress (fight-or-flight response). While the role of CaMKII phosphorylation of RyR2Ser2814 provides a mechanism for the Bowditch effect, the role of CaMKII phosphorylation of RyR2Ser2814 in HF and AF remains uncertain35. Although a link between adrenergic activation, the major trigger of cardiac arrhythmias, and CaMKII activation has been reported105.
Reduced phosphodiesterase activity50, causing impaired dephosphorylation of RyR2, contributes to SR Ca2+ leak in humans and animal models with cardiac disease106,107. Three major serine-threonine phosphatases including PP1, PP2A, and PP2B modulate RyR2 function. Our group has shown that PP1 and PP2 are tethered to RyR2 via the leucine-isoleucine zipper motif of their targeting proteins spinophilin and PR130, respectively108,109. In failing hearts, spinophilin is depleted from the RyR2 macromolecular complex resulting in reduced PP1, which promotes increased PKA hyper-phosphorylation of RyR2110. Decreased PP2A binding to RyR2 is also associated with RyR2 PKA hyper-phosphorylation26. PDE4D3 plays a critical role in regulating the local cAMP levels around the RyR250. Reduced PDE4D3 activity in HF or inhibition of PDE4D3 with the drug rolipram can promote RyR2 leak and cardiac dysfunction and arrhythmias50.
3). RyR2 oxidation and nitrosylation: role in HF and cardiac arrhythmias
Cardiac contractility is determined by the amplitude and kinetics of the Ca2+ transient. We showed that RyR2-mediated diastolic SR Ca2+ leak contributes to HF progression 26 and fatal ventricular arrhythmias 36,59,65,111. Before we reported the role of diastolic SR Ca2+ leak in HF, the dogma was that SR Ca2+ overload occurred in HF 112,113,114,115. Others have confirmed both SR Ca2+ depletion and diastolic SR Ca2+ leak in HF 27,31,32,116,117.
RyR2 is modified by superoxide anions, hydroxyl radicals and by reactive nitrogen species (RNS) such as nitric oxide (NO) and xanthine oxidase. Given the number of cysteine residues located in the same region as the Ser2808 phosphorylation site, it is plausible that excessive thiol modification in HF could potentiate RyR2 Ca2+ leak. HF is associated with increased ROS production from the uncoupled mitochondrial electron transport chain (ETC) as well as upregulation of nitric oxide synthase (NOS), xanthine oxidase (XO) and NADPH oxidase (NOX) resulting in increased generation of O2.- and decreased GSH/GSSH ratio118. In cardiac myocytes, mitochondria occupy 30–40% of the cellular volume and constitute the major source of intracellular ROS production. Up to 20% of the electrons in the ETC leak molecular oxygen to form superoxide anion and subsequently hydrogen peroxide (H2O2)119,120. Excessive mitochondrial ROS production has been implicated in the pathogenesis of HF and AF.
Moreover, electron microscopy studies have shown that mitochondria are in close proximity to the RyR2 with distances varying between 40 and 180 nm in rat myocardium121. Thus, mitochondrial ROS production modulates local as well as global RyR2-mediated Ca2+ release 122. NOX2 and NOX4 are the predominant isoforms of NADPH oxidase expressed in cardiac myocytes and are upregulated in patients with heart failure123,124. However, there are important differences between the two NOX isoforms with respect to their structure, localization, function, and pathophysiological significance125. NOX2 is located on the plasma membrane and is usually dormant. It is activated either mechanically (e.g. aortic banding) or hormonally (e.g. angiotensin II) increased myocardial afterload126. NOX4, on the other hand, is constitutively active and its precise localization (e.g. cytoplasm, mitochondria, SR/ER) remains controversial127,128.
The contribution of NOX to SR Ca2+ leak is also supported by the observation that addition of NOX to microsomes isolated from cardiac muscle significantly enhances S-glutathionylation of RyR2 and CICR129. Indeed, Sanchez. et al provided indirect evidence that NOX2 contributes to RyR2 glutathionylation during tachycardia129. We have recently demonstrated a physical interaction between NOX4 and the skeletal muscle isoform of the RyR (RyR1) during cancer-induced muscle weakness130. In this condition, the TGF-β pathway activates NOX4 in skeletal muscle causing RyR1 oxidation and increased intracellular Ca2+ leak, reducing SR Ca2+ and weakening muscle force production130. However, regulation of RyR2 by NOX still requires further investigation.
Oxidative stress during HF is associated with diastolic SR Ca2+ leak via enhanced RyR2 activity131,132. Cys-oxidation of RyR2 increases channel open probability causing diastolic Ca2+ leak, which leads to contractile dysfunction. In atrial myocytes this leak may trigger AF131. Importantly, reducing mitochondrial ROS production attenuates atrial diastolic SR Ca2+ leak and prevents AF. Combination of RyR2 PKA hyper-phosphorylation and Cys-oxidation contribute to calstabin2 dissociation, Ca2+ leak, and myocardial dysfunction 20. This synergy could be due to PKA phosphorylation exposing additional cysteine residues to oxidation and/or Ca2+ leak from PKA hyper-phosphorylation causing mitochondrial Ca2+ overload and ROS production which causes RyR2 oxidation, perpetuating the RyR2 Ca2+ leak.
4). RyR2 oxidation and nitrosylation: role in atrial fibrillation
Elevated levels of reactive oxygen species occur in persistent AF and AF recurrence after radio frequency catheter ablation in paroxysmal AF patients 104. The Framingham Study shows that HF is the strongest predictor for AF 133. HF results in RyR2 PKA hyperphosphorylation, oxidation, nitrosylation and calstabin2 dissociation from RyR2 molecular complex.
Despite intense interest and research for more than a century – much remains to be understood about the most common cardiac arrhythmia, atrial fibrillation 134,135, and treatment remains a challenge 136–141. At a gross, anatomical level of understanding, atrial enlargement and fibrosis play a role in AF maintenance 142. But what triggers AF?
Since AF occurs in structurally normal hearts atrial enlargement and fibrosis cannot be considered de facto requirements for AF. Indeed, individuals with leaky RyR2 channels but structurally normal hearts, who suffer from exercise-induced sudden cardiac death., known as catecholaminergic polymorphic ventricular tachycardia (CPVT) 143,144, also have AF 59,145,146. Diastolic SR Ca2+ leak via RyR2 is a feature of AF 61,101. Knock-in mice harboring leak-inducing RyR2 mutations are susceptible to AF 16,17,147. A striking example is a 2-year-old child with a CPVT linked RyR2 mutation and AF144
Oxidative stress is a feature of AF 148–152, and antioxidant drugs have some benefit 149,151,152. AF increases as does oxidative damage 153,154. and RyR2 undergoes stress-induced oxidation 146 in both ventricular and atrial myocytes 20,146,155–157,17. We have used murine models of human CPVT (e.g. RyR2-R2474S+/−, RyR2-R2386I+/−, and RyR2-L433P+/− mice) and mice expressing a phosphomimetic aspartic acid residue at position 2808 (RyR2-S2808D+/+) leading to constitutively leaky channels as ideal models to explore the mechanisms of AF because they have structurally normal hearts. We found that mitochondrial free radicals oxidize atrial RyR2, associated with depletion of calstabin2 from the RyR2 channel complex resulting in diastolic SR Ca2+ leak and pacing-induced AF that can be rescued by overexpressing human catalase targeted to mitochondria (mCAT mice) or the rycal S107 131.
The role of castabin2 binding to RyR2 in AF was first reported in myocytes from chronic AF patients 101 and Sood et al. reported pacing-induce AF in calstabin2 knockout mice 158. We showed that intra-esophageal pacing protocol induced AF in calstabin2 knockout mice that was not prevented by S107 in calstabin2 deficient mice showing the requirement for calstabin2 in the mechanism of action of the rycal. Interestingly, in contrast to ventricular arrhythmias that are induced by exercise and epinephrine, AF was induced without catecholamines was not inhibited by β-blocker treatment suggesting that catecholamines and PKA phosphorylation of RyR2 may not be as important in triggering atrial arrhythmias as it is in ventricular arrhythmias.
Chelu et al. reported CaMKII phosphorylation of RyR2 in mice with AF 159. The CaMKII inhibitor KN93 suppressed AF suggesting that CaMKII phosphorylation of Ca2+ cycling proteins including the L-type Ca2+ channel and phospholamban which modulates SERCA2a to regulate SR Ca2+ uptake may be involved as well as RyR2. We showed that RyR2-S2814A mice harboring RyR2 that cannot be CaMKII phosphorylated exhibit similar progression of heart failure after myocardial infarction compared to WT littermates 58and similar incidences of AF arguing that CaMKII phosphorylation of RyR2 does not play a major role in triggering AF in HF.
Rat atrial myocytes have higher SR Ca2+ uptake, ~3-fold higher SR Ca2+ load compared to ventricular myocytes 160 and increased Ca2+ spark frequencies in both WT and RyR2-R2474S+/− atrial myocytes compared to their ventricular counterparts and may lower the threshold for induction of atrial arrhythmias induced by burst atrial pacing. The baseline Ca2+ spark frequencies of WT atrial myocytes and RyR2-R2474S+/− ventricular myocytes were comparable (Figure 5, first and fourth bar), indicating comparable diastolic SR Ca2+ leak. This leak by itself is not sufficient to induce AF in vivo burst pacing stimulation in WT mice, or ventricular arrhythmias in RyR2-R2474S+/− mice. Clinically, VT is observed during exercise in patients with the RyR2-R2474S mutation indicating the importance of sympathetic activation of the SR Ca2+ uptake pathway and loading of the SR to increase the amplitude of the leak. The exact reasons for these differences are still not well understood. However, it is well known that unlike ventricular fibrillation which leads to sudden cardiac death, AF is typically not lethal in the absence of a by-pass tract. Therefore, there might be less evolutionary pressure to maintain a higher threshold for arrhythmias in the atria.
Figure 5. Architecture of the open (left) and closed (right) states of RyR2.
Side view (top panels) and top down views (bottom panels) are shown for the closed state (right) and the open state (left). In the closed state RyR2Glu4873 (red spheres) are in close contact with RyR2Arg4875 (blue spheres) of the adjacent protomer preventing Ca2+ flux through the pore, while in the open state (left) Arg4875 rotates out of the pore to open the channel. One of the four monomers forming the homotetrameric channel pore region is shown in cyan. Dashed lines indicate the transmembrane region.
Although re-entry and multiple wavelets are observed in AF, the molecular events initiating AF remain uncertain. In agreement with previous reports implicating abnormal Ca2+ handling in AF 61,161, we observed Ca2+ waves and indirectly Ca2+-activated inward current (DADs). In the setting of normal cardiac structure and function in CPVT mouse models, the diastolic SR Ca2+ leak via RyR2 leads to Ca2+ waves and DADs, wavelets and likely re-entry loops that trigger atrial tachycardia 17,101,131,162. Thus, we propose that atrial diastolic SR Ca2+ leak is an essential contributing factor in the pathogenesis of AF 16,17,147. Diastolic SR Ca2+ leak due to RyR2 dysfunction may be linked to multiple factors including phosphorylation, oxidation or pathological mutations of RyR2, all of which can result in channel dysfunction146.
Accumulating evidence suggest that oxidative stress 104 plays a pivotal role in the triggering and maintaining AF 148–150,163,164. Our findings strongly support others who have suggested that AF is associated with myocardial oxidative stress 148,163We have previously reported PKA hyperphosphorylation and calstabin2 dissociation from RyR2 in several models of chronic AF101. The importance of PKA phosphorylation in cardiac disease has been challenged by Valdivia’s group, who concluded that phosphorylation of Ser2808 plays no role in β-adrenergic cardiac response. The fact that our findings have been confirmed by multiple other groups is addressed in a recent review165.
Chronic PKA phosphorylation of ventricular RyR2 is associated with oxidation of the channel and the combination of PKA phosphorylation and oxidation of RyR2 results in significantly more calstabin2 dissociation from RyR2 compared to each post-translational modification alone 20. Furthermore, oxidation of atrial RyR2 has been shown to be a key contributor to diastolic SR Ca2+ leak and AF in animal models of CPVT17.
CaMKII is also a molecular target of oxidative stress, and oxidized CaMKII can phosphorylate RyR2 at Ser2814 inducing intracellular Ca2+ leak 166. KN-93 treatment reduced SR leak and arrhythmic activity in atrial cardiomyocytes 167. Inhibition of late sodium current and CaMKII in right atrial tissue decreased arrhythmic activity 168. Wehrens and colleagues showed that the phosphatase PP1 110 regulates RyR2 locally by dephosphorylating the CaMKII phosphorylation site in RyR2. Decreased local PP1 regulation of RyR2 contributes to RyR2 hyperactivity and promotes AF susceptibility 110.
There are multiple sources of ROS, including mitochondria, NADPH oxidases and NOS uncoupling that contribute to AF 138,141,169 and mitochondria are the major ROS source for long-term AF 153.
Mitochondria and the SR are co-localized in the ‘mitochondrial microdomain’ 170 and mitochondrial Ca2+ uptake via the mitochondrial Ca2+ uniporter is modulated by SR Ca2+ release 170–172. In atrial myocytes from RyR2-S2808D+/+ mice, leaky RyR2 channels were associated with an age-related increase in diastolic SR Ca2+ release without increase in systolic Ca2+ transient amplitudes. Pharmacological inhibition of RyR2 Ca2+ leak restored atrial mitochondrial morphology and function showing that mitochondrial Ca2+ overload plays a key role in AF pathophysiology 17,131.
RyR2 mediated intracellular Ca2+ leak caused by constitutive PKA phosphorylation or CPVT mutations triggers a vicious cycle in which atrial myocyte SR Ca2+ leak in causes mitochondrial dysfunction and increased ROS production promoting RyR2 oxidation and further Ca2+ leak. Pharmacological inhibition of leaky RyR2 channels or genetically inhibiting mitochondrial ROS production prevents AF thus leaky RyR2 could be new therapeutic targets for AF.
Increased persistent Na+ current due to gain-of-function NaV1.5 dysfunction leads to spontaneous and prolonged episodes of AF, left atrial enlargement and fibrosis 173. A murine model of gain-of-function NaV1.5 dysfunction is relevant to human AF. AF is increased in LQT3 patients who harbor SCN5A mutations altering NaV1.5 174. Na+ current is increased 26% in atrial appendages of patients with permanent AF 175. Ranolazine reduced atrial arrhythmias and new AF episodes 176,177: Increased persistent Na+ current is observed in heart failure, hypoxia, inflammation, oxidative stress 178.
5). Coupled gating of RyR2 channels: role in HF and cardiac arrhythmias
In cardiomyocytes, RyR2 channels are densely clustered on the surface of the SR179,180, with physical contact between the large cytosolic domains. RyR2 channels open and close in a concerted manner34,69, in a process known as coupled gating. Calstabin2 is required for coupled gating and this process contributes to the termination of Ca2+ release during ECC. Without Calstabin2, channels do not couple properly and exhibit instability and failure to close stably, manifested as sub-conductance states26,69. Decoupling and sub-conductance states are observed in RyR2 isolated from failing hearts181.
6). Ca2+ uptake, extrusion, sequestration, and buffering: role in HF and AF
Diastole begins with SR Ca2+ re-uptake, Ca2+ extrusion from the cytosol via the NCX1. The ability of SERCA2a to bind Ca2+ and pump it into the SR is governed by binding to phospholamban (PLB)182,183. AAV9 vector-mediated SERCA2a overexpression in a rabbit model of AF reduced diastolic cytosolic Ca2+ levels by increasing SR Ca2+ uptake and reversed pathologic electrical and structural remodeling184. SERCA2a levels are reduced in HF, resulting in SR Ca2+ depletion, lower Ca2+ transient amplitude, and reduced cardiac contractility. Accumulation of cytosolic Ca2+ increases Ca2+ extrusion via NCX1, promoting triggered activity185. Redox modification of SERCA2a by ROS has also been proposed to alter pump activity, impairing cardiomyocyte relaxation186.
Increasing SERCA2a activity in cardiomyocytes may improve contractility in HF, however it can also increase the incidence of arrhythmias187,188. An initial clinical trial testing viral-mediated SERCA2a overexpression in HF was not positive 189. Patients with lone AF (AF in the absence of known precipitating factors) exhibit increased phosphorylation of PLB, reducing SERCA2a inhibition. This may be a compensatory mechanism by the cell to increase Ca2+ uptake in the SR in order to reduce cytosolic Ca2+ overload161.
During phase 0 of the cardiac action potential, the inflow of Na+ through voltage-gated sodium channels causes NCX1 to transport Ca2+ into the cell. This improves the efficiency of CICR by priming RyR2190. During phases 2 and 3 of the action potential, NCX1 extrudes Ca2+ from the cytosol promoting membrane repolarization and Ca2+ transient decay 191,192. In HF and AF, Ca2+ leak from RyR2 activates NCX1, generating inward INCX, promoting action potential prolongation, reactivation of L-type channels and generation of DADs193–195. DAD-mediated triggered activity contributes to arrhythmogenesis. Na+ channel dysfunction manifested as maintained current has been linked to AF 162,173.
The heart is a high-energy consumption organ, with 95% of its ATP being supplied by oxidative metabolism. Mitochondria, which occupy 30% of cardiomyocytes by volume, are intimately connected to the SR196–198 and Ca2+ is essential for its optimal function through the stimulation of key enzymes in the tricarboxylic acid cycle (Krebs cycle) such as pyruvate dehydrogenase phosphatase, isocitrate dehydrogenase and α-ketoglutarate dehydrogenase199,200. Mitochondrial Ca2+ uptake increases ATP production in response to the energy requirements imposed by the ATPase in excitation contraction coupling201. However, excessive mitochondrial Ca2+ uptake contributes to cellular dysfunction, HF, and AF progression131,202. Ca2+ influx into the mitochondria occurs primarily via the mitochondrial calcium uniporter (MCU), and efflux occurs primarily via the mitochondrial Na+/Ca2+ exchanger (mNCX)203,204. Defective mitochondrial Ca2+ buffering has been implicated in mitochondrial dysfunction and increased ROS production in HF and AF131,205–208 but the main source of mitochondrial Ca2+ remained uncertain for several years. Since RyR2 is the main Ca2+ release channel in cardiac cells, it was postulated that RyR2 Ca2+ release during systole creates high local Ca2+ concentrations (~30μM) in the vicinity of the mitochondrial membrane, leading to mitochondrial Ca2+ uptake209–212. We recently demonstrated mitochondrial Ca2+ overload in HF202 coincided with RyR2 Ca2+ leak. Mice with constitutively leaky RyR2 channels exhibited altered mitochondrial structure, reduced ATP content, and increased mitochondrial ROS production202.
In contrast, eliminating type 2 inositol 1,4,5-trisphosphate (IP3R2) channels in cardiac muscle cells did not attenuate HF progression, alter Ca2+ sparks, SR Ca2+ load, mitochondrial Ca2+ level, or ROS production suggesting that these channels do not contribute to dysfunctional Ca2+ handling by the mitochondria in HF202.
As previously noted, excessive mitochondrial Ca2+ uptake increases mitochondrial ROS production and oxidative stress, a hallmark of HF and AF153. ROS react rapidly with lipids, nucleic acids, and proteins including ion channels such as the RyR. RyR2 is highly redox sensitive 91,55, with more than 90 cysteines residues per protomer, including 21 in the free thiol state and available for redox modifications91,213. Oxidation of the cysteine residues in RyR2 plays a role in calstabin2 dissociation from RyR2 and diastolic SR Ca2+ leak and contributes to HF progression 20 and AF 17,101,131. Recent work suggests that in the presence of H2O2, RyR undergoes covalent disulfide cross-linking between the N-terminal domain of the four neighboring subunits, adopting an open state conformation and generating SR Ca2+ leak214. Rebinding calstabin2 to RyR2 using Rycals such as S107 significantly reduces its open probability and the Ca2+ leak, despite the persistence of channel oxidation215. Reducing mitochondrial ROS production using antioxidants or transgenic mouse models attenuates RyR2 oxidation, prevents calstabin dissociation, diastolic SR Ca2+ leak, and prevents HF and AF131,202.
Thus, there is a feedback loop between the SR and mitochondria in HF and AF in which SR Ca2+ triggers mitochondrial dysfunction and ROS production, which in turn causes RyR2 oxidation and enhances intracellular Ca2+ leak, speeding the progress of HF and AF. Future studies will need to focus on the triggering events and identify which cysteine residues are central to the pathophysiology of HF and AF. Continuous advances in resolving the structure of RyR2 using single particle cryo-EM is a promising tool to answer this question.
7). Role of IP3R1 versus RyR2 in vascular smooth muscle during HF
IP3R1-mediated SR Ca2+ release regulates vascular smooth muscle (VSM) tone, impacting blood pressure control and afterload (force countering cardiac output)216–217. In contrast, RyR2-mediated Ca2+ release in VSM causes vasorelaxation218. This occurs via functional coupling of RyR2 with large, transient outward K+ (BK) channels such that a single Ca2+ spark generates a large, transient outward K+ current, which hyperpolarizes the plasma membrane, deactivating the voltage-dependent Ca2+ influx and causing VSM relaxation 219. Targeted VSM deletion of RyR2 eliminates Ca2+ sparks, leading to increased myogenic tone and higher systemic blood pressure220. The role of IP3R1 in determining afterload in HF has been proposed 216,221 and might provide additional novel therapeutic targets.
8). Structural bases for RyR2 pathology
Low resolution structures of RyRs using cryo-EM reconstructions originally provided images showing the beautiful four-fold symmetry of the channel 222. With the advent of direct electron detector cameras coupled with improved microscopes and software it rapidly became possible to resolve high-resolution RyR structures using cryo-EM49,223 which have revolutionized our understanding of the channel structure-function relationships (Figs. 4–6).
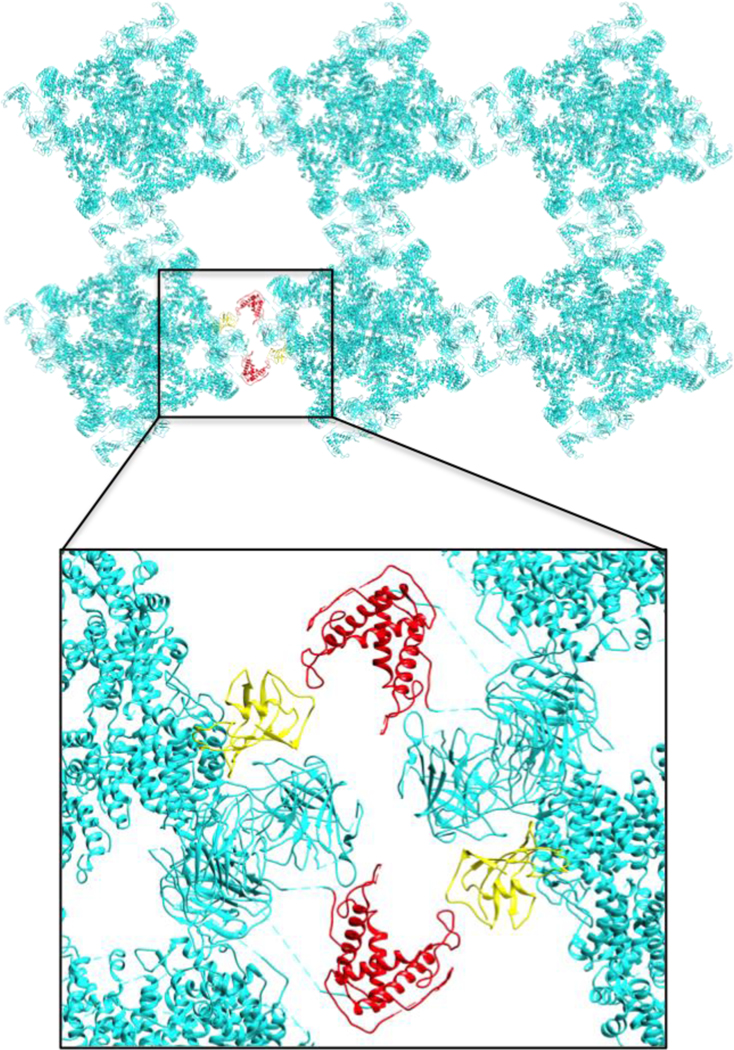
Figure 6. Contact sites formed by calstabin2 and the RY12 domain may promote coupled gating between neighboring RyR2 channels.
Possible domain-domain interactions between neighboring RyR2 channels in arrays as suggested by previous studies (Cryo-EM, 2D crystallography and freeze-fracture images of SR membranes). The interaction between RY12 domain (Red) and calstabin2 (yellow) of the neighboring RyR2 is a potential therapeutic target that may enhance coupled gating and prevent pathological SR Ca2+ leak.
In early 2015, 3D structures for the mammalian RyR1224–226 resolved the closed state of the channel (Fig. 5). The gating mechanism and activation of the channel by Ca2+ became clearer with structures of the open and the ‘primed’ states (an intermediate state between closed and open channels in which the channel is Ca2+ bound but not yet fully activated) of RyR1223,227,228 and RyR2229. Recent RyR2 structures provide high-resolution maps of RyR2 bound to apo- and Ca2+-calmodulin suggesting its modulation mechanism230. These structures revealed the exact location of most disease-causing mutations, providing clues to understanding the role of the Ca2+ leak in RyR2 in heart failure59, catecholaminergic polymorphic ventricular tachycardia (CPVT)231, and arrhythmogenic right ventricular dysplasia (ARVD) associated with RyR2 naturally accruing mutations and pathological post translation modifications.
The structure of RyR2 can be divided into three main regions: the dynamic cytosolic N-terminus and helical solenoid domain, the central core domain, and the transmembrane (TM) domain225. The cytosolic shell is the largest assembly, composed of the N-terminal domains NTD (residues 1–642), the three SPRY domains (643–1605), the first RYR motif (P1, 861–1066), and the large, helical solenoid domain (~35% of the sequence, 1642–3528) that contains the second RYR motif with the modulatory PKA and CaMKII phosphorylation sites (P2, 2701–2907) (Fig. 4). The cytoplasmic assembly is also the scaffold for many RyR2 modulatory proteins including calstabin2, calmodulin, kinases and phosphatases 26,39,50.
The NTD harbors a ‘hot spot’ of disease-causing mutations located at the interfaces between the four protomers232 (Fig 4). The NTD also forms an inter-domain interface with a second mutation hot-spot clustered on the helical solenoid domain located in close proximity to the calmodulin binding site (Fig 4). The dynamics between these two moving parts was shown to be coupled to the opening and closing of the pore223,229. Stabilizing these inter-domain contact sites is required to keep the ‘upward’ shell conformation of the closed state223,233. Disruption of this interface may destabilize the closed state, causing a diastolic SR Ca2+ leak. Interestingly, two mutation clusters are adjacent to the DPc10 peptide (Fig 4, black ribbon), a short peptide (Gly2460-Pro2495) that was shown to increase arrhythmia-triggering SR Ca2+ leak characteristic in CPVT 234. Intriguingly, all 4 known ARVD-causing mutations (Arg176Gln, Leu433Pro, Asn2386ILe, and Thr2504Met) are located in this interface between the two disease associated clusters (Figure 4 left inset, green spheres).
The second RYR motif repeat235,236, or P2 (Ry3&4 in RyR1), is located at the ‘top’ of the cytosolic assembly of the helical solenoid assembly. P2 is the PKA and CaMKII phosphorylation site (Ser2808 and Ser2814, respectively) (Fig. 4). It is a major channel modulatory site and was suggested as the cause for Ca2+ leak in chronic adrenergic stimulation-induced heart failure35,237. Given its location and that phosphorylation of this site increases open probability, addition of a phosphate group could promote the downward conformation of the cytosolic shell leading to a pathological SR Ca2+ leak, possibly by modifying and disrupting inter-domain interactions with the SPRY3 domain of the adjacent protomer, or by modifying protein-protein interactions with the voltage-gated Ca2+ channel in the T-tubule.
As with RyR1223, in the presence of activating ligands, the RyR2 outward rotation of the cytosolic shell is coupled to the dilation of the channel aperture229. The aperture dilation correlates with the degree of S6 helix bowing under the pressure created by the primed central solenoid U motif, or ‘thumb and forefinger’ (TaF) domain in RyR1, that engulfs the CTD. This interface between the central domain U motif and the CTD harbors the binding site for the activating Ca2+ and ATP and mediates the transmission of signals between the cytoplasmic assembly and the pore aperture. Multiple CPVT-causing mutations are located on both sides of this pivotal interaction site important to the channel gating apparatus. We can now speculate that disruption of this domain-domain interaction may contribute to diastolic SR Ca2+ leak.
Calstabin2 plays an important role in preventing Ca2+ leak and reducing HF progression65,69. The recent cryo-EM structures show that Ca2+ rigidifies the triangular interface between the helical solenoid domain, SPRY1, and SPRY2 similar to the role of calstabin1 in RyR149,223,238. Cryo-EM structures of RyR2 in the absence of calstabin suggest a greater level of flexibility of the helical solenoid evident from the lower local resolution in the P1 domain94,230 possibly due to a higher degree of freedom that calstabin2 prevents. Additionally, calstabin plays an important role in RyR2 and RyR1 coupled gating69,108. Freeze-fracture experiments239 and 2D crystallization in lipid membranes240 suggest that the P1 domain and the helical domain from the adjacent protomer form the contact sites between neighboring RyRs in the array. For RyR2 the interactions between channels in solution occur at the P1 domain and the calstabin2–SPRY1 interface241. In both cases calstabin plays a crucial role in stabilizing the “checkerboard” array of RyR channels (Fig. 6), either by directly interacting with the neighboring SPRY1 (Fig. 6) or by stabilizing the P1-helical domain interaction and promoting coupled gating between neighboring RyR channels 34,69. Coupled gating, which depends on calstabin binding to the channel, provides an additional mechanism for preventing channel leak because when one channel in the RyR array closes the channels in the array close due to protein-protein coupling with their neighbors as shown in Fig. 6. This pivotal contact site can control and modulate Ca2+ release and leak in RyR2 arrays and may be an important drug target for treatment of HF and AF.
It is important to note that the high resolution structures of RyR channels are based on single particle reconstruction and lack some regulatory proteins including Cav1.2, Casq2, junctin, and triadin242. Nevertheless, key insights are rapidly emerging based on new structures which have clearly opened the way for major advances in our understanding of cardiac contractility and arrhythmias.
9. Summary and conclusions
The notion that Ca2+ is important in the heart is nothing new. What has emerged in the past 20 years is a detailed understanding of the molecules that regulate cardiac Ca2+. This has made it feasible for the first time to discover basic mechanisms that cause HF and AF. However, there is still a lot of speculation about the causes of HF and AF. So students today are not significantly better off than they were 30 years ago in terms of understanding the underlying mechanisms of AF and HF. Why is that? Well, we are still uncovering the basic mechanisms of how the heart works. For example, 30 years ago it was not known that the ryanodine receptor was the SR Ca2+ release channel required for ECC. You could see RyRs – they were called “feet” bridging the gap between the terminal cisternae of the SR and the T-tubule. But they were not known to be Ca2+ channels. Cloning RyRs provided a big step forward, as it did for the other major Ca2+ handling proteins in the heart and skeletal muscle. The evolution of understanding of how the heart works pretty much paralleled the revolution in technology including advances in cloning, biophysics, generation of genetic animal models and imaging.
Today we are beginning to understand molecular signals that cause HF progression and trigger AF. We have the tools to advance these understandings at a rapid rate and no doubt the mechanisms that emerge will likely not be the ones we predict. Who would have thought 30 years ago that reduced systolic contractility in HF was due, at least in part, to a diastolic SR Ca2+ leak? Or that such a leak could be a therapeutic target for both HF and AF and might even improve exercise capacity in patients with heart failure?
The conclusion (slightly modified) from a review on HF written over 15 years ago 13. holds as well today: “The challenge remains, as always, to objectively assess new information as it comes forth in this complex field (Fig. 1–3) and to maintain a rigorous commitment to careful, controlled studies. Indeed, these requirements are not new in the field of medicine. The 12th century physician-philosopher Moses Maimonides wrote in his physician’s oath over 800 years ago, “Grant me the strength, time and opportunity always to correct what I have acquired, always to extend its domain; for knowledge is immense and the spirit of man can extend indefinitely to enrich itself daily with new requirements. Today he can discover his errors of yesterday and tomorrow he can obtain a new light on what he thinks himself sure of today.”
Figure 2. Reduced Ca2+ transients in ventricular myocytes from failing hearts.
Representative line scan showing Ca2+ waves (transients) recorded in ventricular cardiomyocytes from normal (A) and failing hearts (B)244. The Ca2+ transient amplitude is lower in HF ventricular cardiomyocytes primarily because of the reduced SR Ca2+ content due to RyR2 diastolic leak and reduced SERCA2a, and the Ca2+ transient duration is longer primarily due to slower re-uptake kinetics compared with normal ventricular cardiomyocytes resulting in impaired contractility in failing hearts. Schematic representation of Ca2+ signaling in normal (C) and heart failure (D) ventricular myocytes. Of note RyR1 in skeletal muscle are also leaky during HF. This contributes to impaired exercise capacity which is a common symptom in patients with advanced (Stage III and IV) HF 39.
Cardiac ryanodine receptors/calcium release channels are required for excitation-contraction coupling in heart.
Pathological oxidation and PKA-hyperphosphorylation of ryanodine receptors in heart failure, or due to inherited mutations, substantially contribute to SR Ca2+ leak, contributing to heart failure progression, atrial fibrillation, and ventricular arrhythmias.
Inhibiting ryanodine receptor mediated SR Ca2+ leak using drugs that stabilize the interactions between calstabins (FKBPs) and ryanodine receptors normalize can reduce heart failure progression and cardiac arrhythmias including atrial fibrillation.
Acknowledgements:
Supported by R01HL145473, R01DK118240, R01HL142903, R01HL140934, R01AR070194, T32 HL120826 (to ARM). Thanks to current and former members of the Marks laboratory for their contributions to the research discussed in this review.
Footnotes
Disclosure: ARM and Columbia University own shares in ARMGO Pharma, Inc. a biotechnology company developing RyR targeted drugs.
References
- 1.Ambrosy AP et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 63, 1123–1133, doi: 10.1016/j.jacc.2013.11.053 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Zimetbaum P. Atrial Fibrillation. Ann Intern Med 166, ITC33-ITC48, doi: 10.7326/AITC201703070 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Lee WC et al. Direct treatment cost of atrial fibrillation in the elderly American population: a Medicare perspective. J Med Econ 11, 281–298, doi: 10.3111/13696990802063425 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Lubitz SA, Benjamin EJ & Ellinor PT Atrial fibrillation in congestive heart failure. Heart Fail Clin 6, 187–200, doi: 10.1016/j.hfc.2009.11.001 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Writing Group, M. et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm 16, e66–e93, doi: 10.1016/j.hrthm.2019.01.024 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Chugh SS et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 129, 837–847, doi: 10.1161/CIRCULATIONAHA.113.005119 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagens VE et al. Effect of rate or rhythm control on quality of life in persistent atrial fibrillation. Results from the Rate Control Versus Electrical Cardioversion (RACE) Study. J Am Coll Cardiol 43, 241–247, doi: 10.1016/j.jacc.2003.08.037 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Wyse DG et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 347, 1825–1833, doi: 10.1056/NEJMoa021328 (2002). [DOI] [PubMed] [Google Scholar]
- 9.Packer DL et al. Effect of Catheter Ablation vs Antiarrhythmic Drug Therapy on Mortality, Stroke, Bleeding, and Cardiac Arrest Among Patients With Atrial Fibrillation: The CABANA Randomized Clinical Trial. JAMA 321, 1261–1274, doi: 10.1001/jama.2019.0693 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prabhu S. et al. Catheter Ablation Versus Medical Rate Control in Atrial Fibrillation and Systolic Dysfunction: The CAMERA-MRI Study. J Am Coll Cardiol 70, 1949–1961, doi: 10.1016/j.jacc.2017.08.041 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Writing Committee M. et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 128, e240–327, doi: 10.1161/CIR.0b013e31829e8776 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Levine A, Gupta CA & Gass A. Advanced Heart Failure Management and Transplantation. Cardiol Clin 37, 105–111, doi: 10.1016/j.ccl.2018.08.007 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Marks AR A guide for the perplexed: towards an understanding of the molecular basis of heart failure. Circulation 107, 1456–1459, doi: 10.1161/01.cir.0000059745.95643.83 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Berridge MJ, Lipp P. & Bootman MD The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1, 11–21, doi: 10.1038/35036035 (2000). [DOI] [PubMed] [Google Scholar]
- 15.Marks AR Calcium cycling proteins and heart failure: mechanisms and therapeutics. J Clin Invest 123, 46–52, doi: 10.1172/JCI62834 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie W. et al. Imaging atrial arrhythmic intracellular calcium in intact heart. Journal of molecular and cellular cardiology 64, 120–123, doi: 10.1016/j.yjmcc.2013.09.003 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shan J. et al. Calcium leak through ryanodine receptors leads to atrial fibrillation in 3 mouse models of catecholaminergic polymorphic ventricular tachycardia. Circulation research 111, 708–717, doi: 10.1161/CIRCRESAHA.112.273342 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fauconnier J. et al. Ryanodine receptor leak mediated by caspase-8 activation leads to left ventricular injury after myocardial ischemia-reperfusion. Proceedings of the National Academy of Sciences of the United States of America 108, 13258–13263, doi: 10.1073/pnas.1100286108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shan J. et al. Phosphorylation of the ryanodine receptor mediates the cardiac fight or flight response in mice. J Clin Invest 120, 4388–4398, doi: 10.1172/JCI32726 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shan J. et al. Role of chronic ryanodine receptor phosphorylation in heart failure and beta-adrenergic receptor blockade in mice. J Clin Invest 120, 4375–4387, doi: 10.1172/JCI3764937649 [pii] (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lompre AM et al. Ca2+ cycling and new therapeutic approaches for heart failure. Circulation 121, 822–830, doi: 10.1161/CIRCULATIONAHA.109.890954 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomez AM et al. Defective excitation-contraction coupling in experimental cardiac hypertrophy and heart failure. Science 276, 800–806, doi: 10.1126/science.276.5313.800 (1997). [DOI] [PubMed] [Google Scholar]
- 23.Marx SO & Marks AR Dysfunctional ryanodine receptors in the heart: new insights into complex cardiovascular diseases. Journal of molecular and cellular cardiology 58, 225–231, doi: 10.1016/j.yjmcc.2013.03.005 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jayaraman T. et al. FK506 Binding Protein Associated with the Calcium Release Channel (Ryanodine Receptor). J. Biol. Chem. 267, 9474–9477 (1992). [PubMed] [Google Scholar]
- 25.Brillantes AB et al. Stabilization of calcium release channel (ryanodine receptor) function by FK506-binding protein. Cell 77, 513–523, doi: 10.1016/0092-8674(94)90214-3 (1994). [DOI] [PubMed] [Google Scholar]
- 26.Marx SO et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 101, 365–376 (2000). [DOI] [PubMed] [Google Scholar]
- 27.Gellen B. et al. Conditional FKBP12.6 overexpression in mouse cardiac myocytes prevents triggered ventricular tachycardia through specific alterations in excitation-contraction coupling. Circulation 117, 1778–1786, doi:CIRCULATIONAHA.107.731893 [pii] 10.1161/CIRCULATIONAHA.107.731893 (2008). [DOI] [PubMed] [Google Scholar]
- 28.Chelu MG, Danila CI, Gilman CP & Hamilton SL Regulation of ryanodine receptors by FK506 binding proteins. Trends Cardiovasc Med 14, 227–234, doi: 10.1016/j.tcm.2004.06.003S1050-1738(04)00088-X [pii] (2004). [DOI] [PubMed] [Google Scholar]
- 29.George CH, Higgs GV & Lai FA Ryanodine receptor mutations associated with stress-induced ventricular tachycardia mediate increased calcium release in stimulated cardiomyocytes. Circ Res 93, 531–540, doi: 10.1161/01.RES.0000091335.07574.86 01.RES.0000091335.07574.86 [pii] (2003). [DOI] [PubMed] [Google Scholar]
- 30.Doi M. et al. Propranolol prevents the development of heart failure by restoring FKBP12.6-mediated stabilization of ryanodine receptor. Circulation 105, 1374–1379 (2002). [DOI] [PubMed] [Google Scholar]
- 31.Prestle J. et al. Overexpression of FK506-binding protein FKBP12.6 in cardiomyocytes reduces ryanodine receptor-mediated Ca(2+) leak from the sarcoplasmic reticulum and increases contractility. Circ Res 88, 188–194 (2001). [DOI] [PubMed] [Google Scholar]
- 32.Yano M. et al. Altered stoichiometry of FKBP12.6 versus ryanodine receptor as a cause of abnormal Ca(2+) leak through ryanodine receptor in heart failure. Circulation 102, 2131–2136 (2000). [DOI] [PubMed] [Google Scholar]
- 33.Ono K. et al. Altered interaction of FKBP12.6 with ryanodine receptor as a cause of abnormal Ca(2+) release in heart failure. Cardiovasc Res 48, 323–331, doi:S0008-6363(00)00191-7 [pii] (2000). [DOI] [PubMed] [Google Scholar]
- 34.Marx SO et al. Coupled gating between cardiac calcium release channels (ryanodine receptors). Circulation research 88, 1151–1158 (2001). [DOI] [PubMed] [Google Scholar]
- 35.Wehrens XH et al. Ryanodine receptor/calcium release channel PKA phosphorylation: a critical mediator of heart failure progression. Proceedings of the National Academy of Sciences of the United States of America 103, 511–518 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lehnart SE et al. Stabilization of cardiac ryanodine receptor prevents intracellular calcium leak and arrhythmias. Proceedings of the National Academy of Sciences of the United States of America 103, 7906–7910, doi: 10.1073/pnas.0602133103 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wehrens XH et al. Enhancing calstabin binding to ryanodine receptors improves cardiac and skeletal muscle function in heart failure. Proceedings of the National Academy of Sciences of the United States of America 102, 9607–9612, doi: 10.1073/pnas.0500353102 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andersson DC & Marks AR Fixing ryanodine receptor Ca leak - a novel therapeutic strategy for contractile failure in heart and skeletal muscle. Drug Discov Today Dis Mech 7, e151–e157, doi: 10.1016/j.ddmec.2010.09.009 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reiken S. et al. PKA phosphorylation activates the calcium release channel (ryanodine receptor) in skeletal muscle: defective regulation in heart failure. J Cell Biol 160, 919–928 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marks AR Novel therapy for heart failure and exercise-induced ventricular tachycardia based on ‘fixing’ the leak in ryanodine receptors. Novartis Found Symp 274, 132–147; discussion 147–155, 272–136 (2006). [PubMed] [Google Scholar]
- 41.Franzini-Armstrong C, Protasi F. & Tijskens P. The assembly of calcium release units in cardiac muscle. Ann N Y Acad Sci 1047, 76–85, doi: 10.1196/annals.1341.007 (2005). [DOI] [PubMed] [Google Scholar]
- 42.Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol 245, C1–14, doi: 10.1152/ajpcell.1983.245.1.C1 (1983). [DOI] [PubMed] [Google Scholar]
- 43.Fabiato A. & Fabiato F. Calcium-induced release of calcium from the sarcoplasmic reticulum of skinned cells from adult human, dog, cat, rabbit, rat, and frog hearts and from fetal and new-born rat ventricles. Ann N Y Acad Sci 307, 491–522, doi: 10.1111/j.1749-6632.1978.tb41979.x (1978). [DOI] [PubMed] [Google Scholar]
- 44.Cheng H, Lederer MR, Lederer WJ & Cannell MB Calcium sparks and [Ca2+]i waves in cardiac myocytes. Am J Physiol 270, C148–159, doi: 10.1152/ajpcell.1996.270.1.C148 (1996). [DOI] [PubMed] [Google Scholar]
- 45.Kushnir A. & Marks AR The ryanodine receptor in cardiac physiology and disease. Adv Pharmacol 59, 1–30, doi: 10.1016/S1054-3589(10)59001-X (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hobai IA & O’Rourke B. Decreased sarcoplasmic reticulum calcium content is responsible for defective excitation-contraction coupling in canine heart failure. Circulation 103, 1577–1584, doi: 10.1161/01.cir.103.11.1577 (2001). [DOI] [PubMed] [Google Scholar]
- 47.Liu X. et al. Role of leaky neuronal ryanodine receptors in stress-induced cognitive dysfunction. Cell 150, 1055–1067, doi: 10.1016/j.cell.2012.06.052 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Santulli G. et al. Calcium release channel RyR2 regulates insulin release and glucose homeostasis. J Clin Invest 125, 1968–1978, doi: 10.1172/JCI79273 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zalk R. et al. Structure of a mammalian ryanodine receptor. Nature 517, 44–49, doi: 10.1038/nature13950 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lehnart SE et al. Phosphodiesterase 4D deficiency in the ryanodine-receptor complex promotes heart failure and arrhythmias. Cell 123, 25–35, doi: 10.1016/j.cell.2005.07.030 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meissner G. & Henderson JS Rapid calcium release from cardiac sarcoplasmic reticulum vesicles is dependent on Ca2+ and is modulated by Mg2+, adenine nucleotide, and calmodulin. J Biol Chem 262, 3065–3073 (1987). [PubMed] [Google Scholar]
- 52.Xiong L, Zhang JZ, He R. & Hamilton SL A Ca2+-binding domain in RyR1 that interacts with the calmodulin binding site and modulates channel activity. Biophys J 90, 173–182, doi: 10.1529/biophysj.105.066092 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodney GG et al. Calcium binding to calmodulin leads to an N-terminal shift in its binding site on the ryanodine Receptor. J Biol Chem 276, 2069–2074, doi: 10.1074/jbc.M008891200 (2001). [DOI] [PubMed] [Google Scholar]
- 54.Hamilton SL & Reid MB RyR1 modulation by oxidation and calmodulin. Antioxid Redox Signal 2, 41–45, doi: 10.1089/ars.2000.2.1-41 (2000). [DOI] [PubMed] [Google Scholar]
- 55.Zhang JZ et al. Oxidation of the skeletal muscle Ca2+ release channel alters calmodulin binding. Am J Physiol 276, C46–53, doi: 10.1152/ajpcell.1999.276.1.c46 (1999). [DOI] [PubMed] [Google Scholar]
- 56.Porter Moore C, Zhang JZ & Hamilton SL A role for cysteine 3635 of RYR1 in redox modulation and calmodulin binding. J Biol Chem 274, 36831–36834, doi: 10.1074/jbc.274.52.36831 (1999). [DOI] [PubMed] [Google Scholar]
- 57.Moore CP et al. Apocalmodulin and Ca2+ calmodulin bind to the same region on the skeletal muscle Ca2+ release channel. Biochemistry 38, 8532–8537, doi: 10.1021/bi9907431 (1999). [DOI] [PubMed] [Google Scholar]
- 58.Kushnir A, Shan J, Betzenhauser MJ, Reiken S. & Marks AR Role of CaMKIIdelta phosphorylation of the cardiac ryanodine receptor in the force frequency relationship and heart failure. Proceedings of the National Academy of Sciences of the United States of America 107, 10274–10279, doi: 10.1073/pnas.1005843107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lehnart SE et al. Sudden death in familial polymorphic ventricular tachycardia associated with calcium release channel (ryanodine receptor) leak. Circulation 109, 3208–3214, doi: 10.1161/01.CIR.0000132472.98675.EC (2004). [DOI] [PubMed] [Google Scholar]
- 60.Bers DM Cardiac excitation-contraction coupling. Nature 415, 198–205, doi: 10.1038/415198a (2002). [DOI] [PubMed] [Google Scholar]
- 61.Neef S. et al. CaMKII-dependent diastolic SR Ca2+ leak and elevated diastolic Ca2+ levels in right atrial myocardium of patients with atrial fibrillation. Circ Res 106, 1134–1144, doi: 10.1161/CIRCRESAHA.109.203836 (2010). [DOI] [PubMed] [Google Scholar]
- 62.Danila CI & Hamilton SL Phosphorylation of ryanodine receptors. Biol Res 37, 521–525, doi: 10.4067/s0716-97602004000400005 (2004). [DOI] [PubMed] [Google Scholar]
- 63.Wehrens XH, Lehnart SE, Reiken SR & Marks AR Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res 94, e61–70, doi: 10.1161/01.RES.0000125626.33738.E2 (2004). [DOI] [PubMed] [Google Scholar]
- 64.Benkusky NA et al. Intact beta-adrenergic response and unmodified progression toward heart failure in mice with genetic ablation of a major protein kinase A phosphorylation site in the cardiac ryanodine receptor. Circ Res 101, 819–829, doi:CIRCRESAHA.107.153007 [pii] 10.1161/CIRCRESAHA.107.153007 (2007). [DOI] [PubMed] [Google Scholar]
- 65.Wehrens XH et al. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell 113, 829–840, doi: 10.1016/s0092-8674(03)00434-3 (2003). [DOI] [PubMed] [Google Scholar]
- 66.Lehnart SE, Wehrens XH & Marks AR Calstabin deficiency, ryanodine receptors, and sudden cardiac death. Biochem Biophys Res Commun 322, 1267–1279, doi: 10.1016/j.bbrc.2004.08.032 (2004). [DOI] [PubMed] [Google Scholar]
- 67.Yano M. et al. FKBP12.6-mediated stabilization of calcium-release channel (ryanodine receptor) as a novel therapeutic strategy against heart failure. Circulation 107, 477–484, doi: 10.1161/01.cir.0000044917.74408.be (2003). [DOI] [PubMed] [Google Scholar]
- 68.Avila G, Lee EH, Perez CF, Allen PD & Dirksen RT FKBP12 binding to RyR1 modulates excitation-contraction coupling in mouse skeletal myotubes. J Biol Chem 278, 22600–22608, doi: 10.1074/jbc.M205866200 (2003). [DOI] [PubMed] [Google Scholar]
- 69.Marx SO, Ondrias K. & Marks AR Coupled gating between individual skeletal muscle Ca2+ release channels (ryanodine receptors). Science 281, 818–821, doi: 10.1126/science.281.5378.818 (1998). [DOI] [PubMed] [Google Scholar]
- 70.Ruiz-Hurtado G. et al. Reconciling depressed Ca2+ sparks occurrence with enhanced RyR2 activity in failing mice cardiomyocytes. J Gen Physiol 146, 295–306, doi: 10.1085/jgp.201511366 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheng H. & Lederer WJ Calcium sparks. Physiol Rev 88, 1491–1545, doi: 10.1152/physrev.00030.2007 (2008). [DOI] [PubMed] [Google Scholar]
- 72.Guo T. et al. Kinetics of FKBP12.6 binding to ryanodine receptors in permeabilized cardiac myocytes and effects on Ca sparks. Circ Res 106, 1743–1752, doi:CIRCRESAHA.110.219816 [pii] 10.1161/CIRCRESAHA.110.219816 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stange M, Xu L, Balshaw D, Yamaguchi N. & Meissner G. Characterization of recombinant skeletal muscle (Ser-2843) and cardiac muscle (Ser-2809) ryanodine receptor phosphorylation mutants. J Biol Chem 278, 51693–51702, doi: 10.1074/jbc.M310406200M310406200 [pii] (2003). [DOI] [PubMed] [Google Scholar]
- 74.Yano M. et al. Altered stoichiometry of FKBP12.6 versus ryanodine receptor as a cause of abnormal Ca(2+) leak through ryanodine receptor in heart failure. Circulation 102, 2131–2136, doi: 10.1161/01.cir.102.17.2131 (2000). [DOI] [PubMed] [Google Scholar]
- 75.Ono K. et al. Altered interaction of FKBP12.6 with ryanodine receptor as a cause of abnormal Ca(2+) release in heart failure. Cardiovasc Res 48, 323–331, doi: 10.1016/s0008-6363(00)00191-7 (2000). [DOI] [PubMed] [Google Scholar]
- 76.Doi M. et al. Propranolol prevents the development of heart failure by restoring FKBP12.6-mediated stabilization of ryanodine receptor. Circulation 105, 1374–1379, doi: 10.1161/hc1102.105270 (2002). [DOI] [PubMed] [Google Scholar]
- 77.Yuan Q. et al. Calstabin 2: An important regulator for learning and memory in mice. Sci Rep 6, 21087, doi: 10.1038/srep21087 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McCall E. et al. Effects of FK-506 on contraction and Ca2+ transients in rat cardiac myocytes. Circ Res 79, 1110–1121 (1996). [DOI] [PubMed] [Google Scholar]
- 79.Jeyakumar LH et al. FKBP binding characteristics of cardiac microsomes from diverse vertebrates. Biochem Biophys Res Commun 281, 979–986, doi: 10.1006/bbrc.2001.4444S0006-291X(01)94444-4 [pii] (2001). [DOI] [PubMed] [Google Scholar]
- 80.Timerman AP et al. Selective binding of FKBP12.6 by the cardiac ryanodine receptor. J Biol Chem 271, 20385–20391 (1996). [DOI] [PubMed] [Google Scholar]
- 81.Potenza DM et al. Phosphorylation of the ryanodine receptor 2 at serine 2030 is required for a complete beta-adrenergic response. J Gen Physiol 151, 131–145, doi: 10.1085/jgp.201812155 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xiao B. et al. Ser-2030, but not Ser-2808, is the major phosphorylation site in cardiac ryanodine receptors responding to protein kinase A activation upon beta-adrenergic stimulation in normal and failing hearts. Biochem J 396, 7–16, doi: 10.1042/BJ20060116 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang H. et al. Hyperphosphorylation of the cardiac ryanodine receptor at serine 2808 is not involved in cardiac dysfunction after myocardial infarction. Circulation research 110, 831–840, doi: 10.1161/CIRCRESAHA.111.255158 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.MacDonnell SM et al. Adrenergic regulation of cardiac contractility does not involve phosphorylation of the cardiac ryanodine receptor at serine 2808. Circulation research 102, e65–72, doi: 10.1161/CIRCRESAHA.108.174722 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jiang MT et al. Abnormal Ca2+ release, but normal ryanodine receptors, in canine and human heart failure. Circ Res 91, 1015–1022 (2002). [DOI] [PubMed] [Google Scholar]
- 86.Uchinoumi H. et al. CaMKII-dependent phosphorylation of RyR2 promotes targetable pathological RyR2 conformational shift. Journal of molecular and cellular cardiology 98, 62–72, doi: 10.1016/j.yjmcc.2016.06.007 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sommese L. et al. Ryanodine receptor phosphorylation by CaMKII promotes spontaneous Ca(2+) release events in a rodent model of early stage diabetes: The arrhythmogenic substrate. International journal of cardiology 202, 394–406, doi: 10.1016/j.ijcard.2015.09.022 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Respress JL et al. Role of RyR2 Phosphorylation at S2814 During Heart Failure Progression. Circ Res 110, 1474–1483, doi:CIRCRESAHA.112.268094 [pii] 10.1161/CIRCRESAHA.112.268094 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu N. et al. Calmodulin kinase II inhibition prevents arrhythmias in RyR2(R4496C+/−) mice with catecholaminergic polymorphic ventricular tachycardia. Journal of molecular and cellular cardiology 50, 214–222, doi: 10.1016/j.yjmcc.2010.10.001 (2011). [DOI] [PubMed] [Google Scholar]
- 90.Ai X, Curran JW, Shannon TR, Bers DM & Pogwizd SM Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circulation research 97, 1314–1322, doi: 10.1161/01.RES.0000194329.41863.89 (2005). [DOI] [PubMed] [Google Scholar]
- 91.Xu L, Eu JP, Meissner G. & Stamler JS Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science 279, 234–237 (1998). [DOI] [PubMed] [Google Scholar]
- 92.Jiang D. et al. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR). Proceedings of the National Academy of Sciences of the United States of America 101, 13062–13067, doi: 10.1073/pnas.0402388101 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chi X. et al. Molecular basis for allosteric regulation of the type 2 ryanodine receptor channel gating by key modulators. Proceedings of the National Academy of Sciences of the United States of America 116, 25575–25582, doi: 10.1073/pnas.1914451116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peng W. et al. Structural basis for the gating mechanism of the type 2 ryanodine receptor RyR2. Science 354, doi: 10.1126/science.aah5324 (2016). [DOI] [PubMed] [Google Scholar]
- 95.Hoch B. et al. Differentiation-dependent expression of cardiac delta-CaMKII isoforms. J Cell Biochem 68, 259–268, doi: [pii] (1998). [DOI] [PubMed] [Google Scholar]
- 96.Ling H. et al. Requirement for Ca2+/calmodulin-dependent kinase II in the transition from pressure overload-induced cardiac hypertrophy to heart failure in mice. J Clin Invest 119, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zygmunt AC, Goodrow RJ & Weigel CM INaCa and ICl(Ca) contribute to isoproterenol-induced delayed after depolarizations in midmyocardial cells. Am J Physiol 275, H1979–1992, doi: 10.1152/ajpheart.1998.275.6.H1979 (1998). [DOI] [PubMed] [Google Scholar]
- 98.Walweel K. et al. Ryanodine receptor modification and regulation by intracellular Ca(2+) and Mg(2+) in healthy and failing human hearts. Journal of molecular and cellular cardiology 104, 53–62, doi: 10.1016/j.yjmcc.2017.01.016 (2017). [DOI] [PubMed] [Google Scholar]
- 99.Chia N, Fulcher J. & Keech A. Beta-blocker, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, nitrate-hydralazine, diuretics, aldosterone antagonist, ivabradine, devices and digoxin (BANDAID(2) ): an evidence-based mnemonic for the treatment of systolic heart failure. Intern Med J 46, 653–662, doi: 10.1111/imj.12839 (2016). [DOI] [PubMed] [Google Scholar]
- 100.Huang F, Shan J, Reiken S, Wehrens XH & Marks AR Analysis of calstabin2 (FKBP12.6)-ryanodine receptor interactions: rescue of heart failure by calstabin2 in mice. Proceedings of the National Academy of Sciences of the United States of America 103, 3456–3461, doi: 10.1073/pnas.0511282103 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vest JA et al. Defective cardiac ryanodine receptor regulation during atrial fibrillation. Circulation 111, 2025–2032, doi: 10.1161/01.CIR.0000162461.67140.4C (2005). [DOI] [PubMed] [Google Scholar]
- 102.Xiao B. et al. Characterization of a novel PKA phosphorylation site, serine-2030, reveals no PKA hyperphosphorylation of the cardiac ryanodine receptor in canine heart failure. Circ Res 96, 847–855, (2005). [DOI] [PubMed] [Google Scholar]
- 103.Erickson JR et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell 133, 462–474, doi: 10.1016/j.cell.2008.02.048 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shimano M. et al. Reactive oxidative metabolites are associated with atrial conduction disturbance in patients with atrial fibrillation. Heart Rhythm 6, 935–940,. [DOI] [PubMed] [Google Scholar]
- 105.Joseph P, Swedberg K, Leong DP & Yusuf S. The Evolution of beta-Blockers in Coronary Artery Disease and Heart Failure (Part 1/5). J Am Coll Cardiol 74, 672–682, doi: 10.1016/j.jacc.2019.04.067 (2019). [DOI] [PubMed] [Google Scholar]
- 106.Niggli E. et al. Posttranslational modifications of cardiac ryanodine receptors: Ca(2+) signaling and EC-coupling. Biochim Biophys Acta 1833, 866–875, doi: 10.1016/j.bbamcr.2012.08.016 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Terentyev D. & Hamilton S. Regulation of sarcoplasmic reticulum Ca(2+) release by serine-threonine phosphatases in the heart. Journal of molecular and cellular cardiology 101, 156–164, doi: 10.1016/j.yjmcc.2016.08.020 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marx SO et al. Phosphorylation-dependent regulation of ryanodine receptors: a novel role for leucine/isoleucine zippers. J Cell Biol 153, 699–708, doi: 10.1083/jcb.153.4.699 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Marks AR, Marx SO & Reiken S. Regulation of ryanodine receptors via macromolecular complexes: a novel role for leucine/isoleucine zippers. Trends Cardiovasc Med 12, 166–170 (2002). [DOI] [PubMed] [Google Scholar]
- 110.Chiang DY et al. Impaired local regulation of ryanodine receptor type 2 by protein phosphatase 1 promotes atrial fibrillation. Cardiovasc Res 103, 178–187, doi: 10.1093/cvr/cvu123 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wehrens XH et al. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science 304, 292–296, [DOI] [PubMed] [Google Scholar]
- 112.Lamers JM & Ruigrok TJ Diminished Na+/K+ and Ca2+ pump activities in the Ca2+ depleted heart: possible role in the development of Ca2+ overload during the Ca2+ paradox. Eur Heart J 4 Suppl H, 73–79, doi: 10.1093/eurheartj/4.suppl_h.73 (1983). [DOI] [PubMed] [Google Scholar]
- 113.Lee SL & Dhalla NS Subcellular calcium transport in failing hearts due to calcium deficiency and overload. The American journal of physiology 231, 1159–1165, doi: 10.1152/ajplegacy.1976.231.4.1159 (1976). [DOI] [PubMed] [Google Scholar]
- 114.Zimmerman AN & Hulsmann WC The calcium paradox: historical remarks. Eur Heart J 4 Suppl H, 3–4, doi: 10.1093/eurheartj/4.suppl_h.3 (1983). [DOI] [PubMed] [Google Scholar]
- 115.Schaffer SW, Roy RS & McMcord JM Possible role for calmodulin in calcium paradox-induced heart failure. Eur Heart J 4 Suppl H, 81–87, doi: 10.1093/eurheartj/4.suppl_h.81 (1983). [DOI] [PubMed] [Google Scholar]
- 116.Shannon TR, Pogwizd SM & Bers DM Elevated sarcoplasmic reticulum Ca2+ leak in intact ventricular myocytes from rabbits in heart failure. Circ Res 93, 592–594, [DOI] [PubMed] [Google Scholar]
- 117.Belevych A. et al. Enhanced ryanodine receptor-mediated calcium leak determines reduced sarcoplasmic reticulum calcium content in chronic canine heart failure. Biophys J 93, 4083– [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zima AV & Mazurek SR Functional Impact of Ryanodine Receptor Oxidation on Intracellular Calcium Regulation in the Heart. Rev Physiol Biochem Pharmacol 171, 39–62, doi: 10.1007/112_2016_2 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chance B, Sies H. & Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev 59, 527–605, doi: 10.1152/physrev.1979.59.3.527 (1979). [DOI] [PubMed] [Google Scholar]
- 120.Aon MA, Cortassa S, Marban E. & O’Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem 278, 44735–44744, doi: 10.1074/jbc.M302673200 (2003). [DOI] [PubMed] [Google Scholar]
- 121.Ramesh V, Sharma VK, Sheu SS & Franzini-Armstrong C. Structural proximity of mitochondria to calcium release units in rat ventricular myocardium may suggest a role in Ca2+ sequestration. Ann N Y Acad Sci 853, 341–344, doi: 10.1111/j.1749-6632.1998.tb08295.x (1998). [DOI] [PubMed] [Google Scholar]
- 122.Prosser BL, Ward CW & Lederer WJ X-ROS signaling: rapid mechano-chemo transduction in heart. Science 333, 1440–1445, doi: 10.1126/science.1202768 (2011). [DOI] [PubMed] [Google Scholar]
- 123.Heymes C. et al. Increased myocardial NADPH oxidase activity in human heart failure. J Am Coll Cardiol 41, 2164–2171, doi: 10.1016/s0735-1097(03)00471-6 (2003). [DOI] [PubMed] [Google Scholar]
- 124.Sirker A, Zhang M. & Shah AM NADPH oxidases in cardiovascular disease: insights from in vivo models and clinical studies. Basic Res Cardiol 106, 735–747, doi: 10.1007/s00395-011-0190-z (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Burgoyne JR, Mongue-Din H, Eaton P. & Shah AM Redox signaling in cardiac physiology and pathology. Circ Res 111, 1091–1106, doi: 10.1161/CIRCRESAHA.111.255216 (2012). [DOI] [PubMed] [Google Scholar]
- 126.Byrne JA et al. Contrasting roles of NADPH oxidase isoforms in pressure-overload versus angiotensin II-induced cardiac hypertrophy. Circ Res 93, 802–805, doi: 10.1161/01.RES.0000099504.30207.F5 (2003). [DOI] [PubMed] [Google Scholar]
- 127.Ago T. et al. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res 106, 1253–1264, doi: 10.1161/CIRCRESAHA.109.213116 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang M. et al. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proceedings of the National Academy of Sciences of the United States of America 107, 18121–18126, doi: 10.1073/pnas.1009700107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sanchez G, Pedrozo Z, Domenech RJ, Hidalgo C. & Donoso P. Tachycardia increases NADPH oxidase activity and RyR2 S-glutathionylation in ventricular muscle. Journal of molecular and cellular cardiology 39, 982–991, doi: 10.1016/j.yjmcc.2005.08.010 (2005). [DOI] [PubMed] [Google Scholar]
- 130.Waning DL et al. Excess TGF-beta mediates muscle weakness associated with bone metastases in mice. Nat Med 21, 1262–1271, doi: 10.1038/nm.3961 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xie W. et al. Mitochondrial oxidative stress promotes atrial fibrillation. Sci Rep 5, 11427, doi: 10.1038/srep11427 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nikolaienko R, Bovo E. & Zima AV Redox Dependent Modifications of Ryanodine Receptor: Basic Mechanisms and Implications in Heart Diseases. Front Physiol 9, 1775, doi: 10.3389/fphys.2018.01775 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Benjamin EJ et al. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA 271, 840–844 (1994). [PubMed] [Google Scholar]
- 134.Santulli G, Iaccarino G, De Luca N, Trimarco B. & Condorelli G. Atrial fibrillation and microRNAs. Frontiers in physiology 5, 15, doi: 10.3389/fphys.2014.00015 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Deedwania PC & Lardizabal JA Atrial fibrillation in heart failure: a comprehensive review. Am J Med 123, 198–204, [DOI] [PubMed] [Google Scholar]
- 136.Ganesan AN et al. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. Journal of the American Heart Association 2, e004549, doi: 10.1161/JAHA.112.004549 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.D’Ascia SL et al. Cardiac resynchronisation therapy response predicts occurrence of atrial fibrillation in non-ischaemic dilated cardiomyopathy. International journal of clinical practice 65, 1149–1155, doi: 10.1111/j.1742-1241.2011.02732.x (2011). [DOI] [PubMed] [Google Scholar]
- 138.Anderson EJ et al. Monoamine oxidase is a major determinant of redox balance in human atrial myocardium and is associated with postoperative atrial fibrillation. Journal of the American Heart Association 3, e000713, doi: 10.1161/JAHA.113.000713 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Santulli G, D’Ascia S L. & D’Ascia C. Development of atrial fibrillation in recipients of cardiac resynchronization therapy: the role of atrial reverse remodelling. The Canadian journal of cardiology 28, 245 e217, doi: 10.1016/j.cjca.2011.11.001 (2012). [DOI] [PubMed] [Google Scholar]
- 140.Aldhoon B, Melenovsky V, Peichl P. & Kautzner J. New insights into mechanisms of atrial fibrillation. Physiol Res 59, 1–12, doi:1651 [pii] (2010). [DOI] [PubMed] [Google Scholar]
- 141.Sovari AA & Dudley SC Jr. Reactive oxygen species-targeted therapeutic interventions for atrial fibrillation. Frontiers in physiology 3, 311, doi: 10.3389/fphys.2012.00311 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Burstein B. & Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol 51, 802–809,. [DOI] [PubMed] [Google Scholar]
- 143.Pizzale S, Gollob MH, Gow R. & Birnie DH Sudden death in a young man with catecholaminergic polymorphic ventricular tachycardia and paroxysmal atrial fibrillation. Journal of cardiovascular electrophysiology 19, 1319–1321, doi: 10.1111/j.1540-8167.2008.01211.x (2008). [DOI] [PubMed] [Google Scholar]
- 144.Di Pino A, Caruso E, Costanzo L. & Guccione P. A novel RyR2 mutation in a 2-year-old baby presenting with atrial fibrillation, atrial flutter, and atrial ectopic tachycardia. Heart Rhythm 11, 1480–1483, doi: 10.1016/j.hrthm.2014.04.037 (2014). [DOI] [PubMed] [Google Scholar]
- 145.Lehnart SE et al. Leaky Ca2+ release channel/ryanodine receptor 2 causes seizures and sudden cardiac death in mice. The Journal of clinical investigation 118, 2230–2245, doi: 10.1172/JCI35346 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Santulli G. & Marks AR Essential roles of intracellular calcium release channels in muscle, brain, metabolism, and aging. Current Molecular Pharmacology (2015). [DOI] [PubMed] [Google Scholar]
- 147.Zhang Y. et al. Acute atrial arrhythmogenicity and altered Ca(2+) homeostasis in murine RyR2-P2328S hearts. Cardiovasc Res 89, 794–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Korantzopoulos P, Kolettis TM, Galaris D. & Goudevenos JA The role of oxidative stress in the pathogenesis and perpetuation of atrial fibrillation. Int J Cardiol 115, 135–143, [DOI] [PubMed] [Google Scholar]
- 149.Huang CX, Liu Y, Xia WF, Tang YH & Huang H. Oxidative stress: a possible pathogenesis of atrial fibrillation. Med Hypotheses 72, 466–467. [DOI] [PubMed] [Google Scholar]
- 150.Mihm MJ et al. Impaired myofibrillar energetics and oxidative injury during human atrial fibrillation. Circulation 104, 174–180 (2001). [DOI] [PubMed] [Google Scholar]
- 151.Carnes CA et al. Ascorbate attenuates atrial pacing-induced peroxynitrite formation and electrical remodeling and decreases the incidence of postoperative atrial fibrillation. Circ Res 89, E32–38 (2001). [DOI] [PubMed] [Google Scholar]
- 152.Sakabe M. et al. Xanthine oxidase inhibition prevents atrial fibrillation in a canine model of atrial pacing-induced left ventricular dysfunction. J Cardiovasc Electrophysiol 23, 1130–1135, doi: 10.1111/j.1540-8167.2012.02356.x (2012). [DOI] [PubMed] [Google Scholar]
- 153.Dai DF & Rabinovitch PS Cardiac aging in mice and humans: the role of mitochondrial oxidative stress. Trends in cardiovascular medicine 19, 213–220, doi: 10.1016/j.tcm.2009.12.004 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Santulli G. & Iaccarino G. Pinpointing beta adrenergic receptor in ageing pathophysiology: victim or executioner? Evidence from crime scenes. Immunity & ageing : I & A 10, 10, doi: 10.1186/1742-4933-10-10 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cooper LL et al. Redox modification of ryanodine receptors by mitochondria-derived reactive oxygen species contributes to aberrant Ca2+ handling in ageing rabbit hearts. The Journal of physiology 591, 5895–5911, doi: 10.1113/jphysiol.2013.260521 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jiang X. et al. Polydatin protects cardiac function against burn injury by inhibiting sarcoplasmic reticulum Ca2+ leak by reducing oxidative modification of ryanodine receptors. Free Radic Biol Med 60, 292–299. [DOI] [PubMed] [Google Scholar]
- 157.Yano M. et al. Correction of defective interdomain interaction within ryanodine receptor by antioxidant is a new therapeutic strategy against heart failure. Circulation 112, 3633–3643. [DOI] [PubMed] [Google Scholar]
- 158.Sood S. et al. Intracellular calcium leak due to FKBP12.6 deficiency in mice facilitates the inducibility of atrial fibrillation. Heart Rhythm 5, 1047–1054, doi:DOI 10.1016/j.hrthm.2008.03.030 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chelu MG et al. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest 119, 1940–1951 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Walden AP, Dibb KM & Trafford AW Differences in intracellular calcium homeostasis between atrial and ventricular myocytes. J Mol Cell Cardiol 46, 463–473. [DOI] [PubMed] [Google Scholar]
- 161.Dai J. et al. Characterization of Ca+ handling proteins and contractile proteins in patients with lone atrial fibrillation. Int J Cardiol 202, 749–751, doi: 10.1016/j.ijcard.2015.10.010 (2016). [DOI] [PubMed] [Google Scholar]
- 162.Avula UMR et al. Heterogeneity of the action potential duration is required for sustained atrial fibrillation. JCI Insight 5, doi: 10.1172/jci.insight.128765 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Neuman RB et al. Oxidative stress markers are associated with persistent atrial fibrillation. Clin Chem 53, 1652–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bukowska A. et al. Mitochondrial dysfunction and redox signaling in atrial tachyarrhythmia. Experimental biology and medicine 233, 558–574, doi: 10.3181/0706-RM-155 (2008). [DOI] [PubMed] [Google Scholar]
- 165.Marx SO & Marks AR Dysfunctional ryanodine receptors in the heart: New insights into complex cardiovascular diseases. Journal of molecular and cellular cardiology, doi: 10.1016/j.yjmcc.2013.03.005 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Purohit A. et al. Oxidized Ca(2+)/calmodulin-dependent protein kinase II triggers atrial fibrillation. Circulation 128, 1748–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Guo X, Yuan S, Liu Z. & Fang Q. Oxidation- and CaMKII-mediated sarcoplasmic reticulum Ca(2+) leak triggers atrial fibrillation in aging. J Cardiovasc Electrophysiol 25, 645–652, doi: 10.1111/jce.12395 (2014). [DOI] [PubMed] [Google Scholar]
- 168.Liang F. et al. Inhibitions of late INa and CaMKII act synergistically to prevent ATX-II-induced atrial fibrillation in isolated rat right atria. Journal of molecular and cellular cardiology 94, 122–130, doi: 10.1016/j.yjmcc.2016.04.001 (2016). [DOI] [PubMed] [Google Scholar]
- 169.Wolke C, Bukowska A, Goette A. & Lendeckel U. Redox control of cardiac remodeling in atrial fibrillation. Biochimica et biophysica acta, doi: 10.1016/j.bbagen.2014.12.012 (2014). [DOI] [PubMed] [Google Scholar]
- 170.Kohlhaas M. & Maack C. Adverse bioenergetic consequences of Na+-Ca2+ exchanger-mediated Ca2+ influx in cardiac myocytes. Circulation 122, 2273–2280, [DOI] [PubMed] [Google Scholar]
- 171.Santulli G. et al. Calcium release channel RyR2 regulates insulin release and glucose homeostasis. Journal of Clinical Investigation (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Harbauer AB, Zahedi RP, Sickmann A, Pfanner N. & Meisinger C. The Protein Import Machinery of Mitochondria-A Regulatory Hub in Metabolism, Stress, and Disease. Cell metabolism 19, 357–372, doi: 10.1016/j.cmet.2014.01.010 (2014). [DOI] [PubMed] [Google Scholar]
- 173.Wan E. et al. Aberrant sodium influx causes cardiomyopathy and atrial fibrillation in mice. J Clin Invest 126, 112–122, doi: 10.1172/JCI84669 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Benito B. et al. A mutation in the sodium channel is responsible for the association of long QT syndrome and familial atrial fibrillation. Heart Rhythm 5, 1434–1440, doi: 10.1016/j.hrthm.2008.07.013 (2008). [DOI] [PubMed] [Google Scholar]
- 175.Sossalla S. et al. Altered Na(+) currents in atrial fibrillation effects of ranolazine on arrhythmias and contractility in human atrial myocardium. J Am Coll Cardiol 55, 2330–2342, doi: 10.1016/j.jacc.2009.12.055 (2010). [DOI] [PubMed] [Google Scholar]
- 176.Scirica BM et al. Effect of ranolazine, an antianginal agent with novel electrophysiological properties, on the incidence of arrhythmias in patients with non ST-segment elevation acute coronary syndrome: results from the Metabolic Efficiency With Ranolazine for Less Ischemia in Non ST-Elevation Acute Coronary Syndrome Thrombolysis in Myocardial Infarction 36 (MERLIN-TIMI 36) randomized controlled trial. Circulation 116, 1647–1652, doi: 10.1161/CIRCULATIONAHA.107.724880 (2007). [DOI] [PubMed] [Google Scholar]
- 177.Reiffel JA et al. The HARMONY Trial: Combined Ranolazine and Dronedarone in the Management of Paroxysmal Atrial Fibrillation: Mechanistic and Therapeutic Synergism. Circ Arrhythm Electrophysiol 8, 1048–1056, doi: 10.1161/CIRCEP.115.002856 (2015). [DOI] [PubMed] [Google Scholar]
- 178.Fischer TH et al. Ca2+/calmodulin-dependent protein kinase II and protein kinase A differentially regulate sarcoplasmic reticulum Ca2+ leak in human cardiac pathology. Circulation 128, 970–981, doi: 10.1161/CIRCULATIONAHA.113.001746 (2013). [DOI] [PubMed] [Google Scholar]
- 179.Franzini-Armstrong C, Protasi F. & Ramesh V. Shape, size, and distribution of Ca(2+) release units and couplons in skeletal and cardiac muscles. Biophys J 77, 1528–1539, doi: 10.1016/S0006-3495(99)77000-1 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Flucher BE & Franzini-Armstrong C. Formation of junctions involved in excitation-contraction coupling in skeletal and cardiac muscle. Proceedings of the National Academy of Sciences of the United States of America 93, 8101–8106, doi: 10.1073/pnas.93.15.8101 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Marx SO et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 101, 365–376 (2000). [DOI] [PubMed] [Google Scholar]
- 182.Xu A. & Narayanan N. Effects of aging on sarcoplasmic reticulum Ca2+-cycling proteins and their phosphorylation in rat myocardium. Am J Physiol 275, H2087–2094, doi: 10.1152/ajpheart.1998.275.6.H2087 (1998). [DOI] [PubMed] [Google Scholar]
- 183.Schmidt U. et al. Restoration of diastolic function in senescent rat hearts through adenoviral gene transfer of sarcoplasmic reticulum Ca(2+)-ATPase. Circulation 101, 790–796, doi: 10.1161/01.cir.101.7.790 (2000). [DOI] [PubMed] [Google Scholar]
- 184.Wang HL et al. Prevention of Atrial Fibrillation by Using Sarcoplasmic Reticulum Calcium ATPase Pump Overexpression in a Rabbit Model of Rapid Atrial Pacing. Med Sci Monit 23, 3952–3960, doi: 10.12659/msm.904824 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.O’Rourke B. et al. Mechanisms of altered excitation-contraction coupling in canine tachycardia-induced heart failure, I: experimental studies. Circ Res 84, 562–570, doi: 10.1161/01.res.84.5.562 (1999). [DOI] [PubMed] [Google Scholar]
- 186.Qin F. et al. Hydrogen peroxide-mediated SERCA cysteine 674 oxidation contributes to impaired cardiac myocyte relaxation in senescent mouse heart. J Am Heart Assoc 2, e000184, doi: 10.1161/JAHA.113.000184 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Baker DL et al. Targeted overexpression of the sarcoplasmic reticulum Ca2+-ATPase increases cardiac contractility in transgenic mouse hearts. Circ Res 83, 1205–1214, doi: 10.1161/01.res.83.12.1205 (1998). [DOI] [PubMed] [Google Scholar]
- 188.Chen Y. et al. Constitutive cardiac overexpression of sarcoplasmic/endoplasmic reticulum Ca2+-ATPase delays myocardial failure after myocardial infarction in rats at a cost of increased acute arrhythmias. Circulation 109, 1898–1903, doi: 10.1161/01.CIR.0000124230.60028.42 (2004). [DOI] [PubMed] [Google Scholar]
- 189.Greenberg B. et al. Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2): a randomised, multinational, double-blind, placebo-controlled, phase 2b trial. Lancet 387, 1178–1186, doi: 10.1016/S0140-6736(16)00082-9 (2016). [DOI] [PubMed] [Google Scholar]
- 190.Neco P. et al. Sodium-calcium exchange is essential for effective triggering of calcium release in mouse heart. Biophys J 99, 755–764, doi: 10.1016/j.bpj.2010.04.071 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Armoundas AA, Hobai IA, Tomaselli GF, Winslow RL & O’Rourke B. Role of sodium-calcium exchanger in modulating the action potential of ventricular myocytes from normal and failing hearts. Circulation research 93, 46–53, doi: 10.1161/01.RES.0000080932.98903.D8 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Blaustein MP & Lederer WJ Sodium/calcium exchange: its physiological implications. Physiol Rev 79, 763–854, doi: 10.1152/physrev.1999.79.3.763 (1999). [DOI] [PubMed] [Google Scholar]
- 193.Pogwizd SM, Qi M, Yuan W, Samarel AM & Bers DM Upregulation of Na(+)/Ca(2+) exchanger expression and function in an arrhythmogenic rabbit model of heart failure. Circ Res 85, 1009–1019, doi: 10.1161/01.res.85.11.1009 (1999). [DOI] [PubMed] [Google Scholar]
- 194.Despa S. & Bers DM Na(+) transport in the normal and failing heart - remember the balance. Journal of molecular and cellular cardiology 61, 2–10, doi: 10.1016/j.yjmcc.2013.04.011 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ottolia M, Torres N, Bridge JH, Philipson KD & Goldhaber JI Na/Ca exchange and contraction of the heart. Journal of molecular and cellular cardiology 61, 28–33, doi: 10.1016/j.yjmcc.2013.06.001 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Marchi S, Patergnani S. & Pinton P. The endoplasmic reticulum-mitochondria connection: one touch, multiple functions. Biochim Biophys Acta 1837, 461–469, doi: 10.1016/j.bbabio.2013.10.015 (2014). [DOI] [PubMed] [Google Scholar]
- 197.Rizzuto R. et al. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 280, 1763–1766, doi: 10.1126/science.280.5370.1763 (1998). [DOI] [PubMed] [Google Scholar]
- 198.Drago I, De Stefani D, Rizzuto R. & Pozzan T. Mitochondrial Ca2+ uptake contributes to buffering cytoplasmic Ca2+ peaks in cardiomyocytes. Proceedings of the National Academy of Sciences of the United States of America 109, 12986–12991, doi: 10.1073/pnas.1210718109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gong G, Liu X. & Wang W. Regulation of metabolism in individual mitochondria during excitation-contraction coupling. Journal of molecular and cellular cardiology 76, 235–246, doi: 10.1016/j.yjmcc.2014.09.012 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hoffman NE et al. Ca(2)(+) entry via Trpm2 is essential for cardiac myocyte bioenergetics maintenance. Am J Physiol Heart Circ Physiol 308, H637–650, doi: 10.1152/ajpheart.00720.2014 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Fink BD, Bai F, Yu L. & Sivitz WI Regulation of ATP production: dependence on calcium concentration and respiratory state. Am J Physiol Cell Physiol 313, C146–C153, doi: 10.1152/ajpcell.00086.2017 (2017). [DOI] [PubMed] [Google Scholar]
- 202.Santulli G, Xie W, Reiken SR & Marks AR Mitochondrial calcium overload is a key determinant in heart failure. Proceedings of the National Academy of Sciences of the United States of America 112, 11389–11394, doi: 10.1073/pnas.1513047112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Campanella M, Pinton P. & Rizzuto R. Mitochondrial Ca2+ homeostasis in health and disease. Biol Res 37, 653–660, doi: 10.4067/s0716-97602004000400022 (2004). [DOI] [PubMed] [Google Scholar]
- 204.Brown DA & O’Rourke B. Cardiac mitochondria and arrhythmias. Cardiovasc Res 88, 241–249, doi: 10.1093/cvr/cvq231 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Nickel A, Loffler J. & Maack C. Myocardial energetics in heart failure. Basic Res Cardiol 108, 358, doi: 10.1007/s00395-013-0358-9 (2013). [DOI] [PubMed] [Google Scholar]
- 206.Huss JM & Kelly DP Mitochondrial energy metabolism in heart failure: a question of balance. J Clin Invest 115, 547–555, doi: 10.1172/JCI24405 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Garcia-Rivas Gde J, Carvajal K, Correa F. & Zazueta C. Ru360, a specific mitochondrial calcium uptake inhibitor, improves cardiac post-ischaemic functional recovery in rats in vivo. Br J Pharmacol 149, 829–837, doi: 10.1038/sj.bjp.0706932 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Griffiths EJ, Balaska D. & Cheng WH The ups and downs of mitochondrial calcium signalling in the heart. Biochim Biophys Acta 1797, 856–864, doi: 10.1016/j.bbabio.2010.02.022 (2010). [DOI] [PubMed] [Google Scholar]
- 209.Szalai G, Csordas G, Hantash BM, Thomas AP & Hajnoczky G. Calcium signal transmission between ryanodine receptors and mitochondria. J Biol Chem 275, 15305–15313, doi: 10.1074/jbc.275.20.15305 (2000). [DOI] [PubMed] [Google Scholar]
- 210.Dedkova EN & Blatter LA Mitochondrial Ca2+ and the heart. Cell Calcium 44, 77–91, doi: 10.1016/j.ceca.2007.11.002 (2008). [DOI] [PubMed] [Google Scholar]
- 211.Dedkova EN, Seidlmayer LK & Blatter LA Mitochondria-mediated cardioprotection by trimetazidine in rabbit heart failure. Journal of molecular and cellular cardiology 59, 41–54, doi: 10.1016/j.yjmcc.2013.01.016 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Dorn GW 2nd, & Maack C. SR and mitochondria: calcium cross-talk between kissing cousins. Journal of molecular and cellular cardiology 55, 42–49, doi: 10.1016/j.yjmcc.2012.07.015 (2013). [DOI] [PubMed] [Google Scholar]
- 213.Donoso P, Sanchez G, Bull R. & Hidalgo C. Modulation of cardiac ryanodine receptor activity by ROS and RNS. Front Biosci (Landmark Ed) 16, 553–567, doi: 10.2741/3705 (2011). [DOI] [PubMed] [Google Scholar]
- 214.Mazurek SR, Bovo E. & Zima AV Regulation of sarcoplasmic reticulum Ca(2+) release by cytosolic glutathione in rabbit ventricular myocytes. Free Radic Biol Med 68, 159–167, doi: 10.1016/j.freeradbiomed.2013.12.003 (2014). [DOI] [PubMed] [Google Scholar]
- 215.Kushnir A. et al. Ryanodine Receptor Calcium Leak in Circulating B-Lymphocytes as a Biomarker in Heart Failure. Circulation 138, 1144–1154, doi: 10.1161/CIRCULATIONAHA.117.032703 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lin Q. et al. IP3 receptors regulate vascular smooth muscle contractility and hypertension. JCI Insight 1, e89402, doi: 10.1172/jci.insight.89402 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Gutstein DE & Marks AR Role of inositol 1,4,5-trisphosphate receptors in regulating apoptotic signaling and heart failure. Heart Vessels Suppl 12, 53–57 (1997). [PubMed] [Google Scholar]
- 218.Gollasch M. et al. Ontogeny of local sarcoplasmic reticulum Ca2+ signals in cerebral arteries: Ca2+ sparks as elementary physiological events. Circ Res 83, 1104–1114, doi: 10.1161/01.res.83.11.1104 (1998). [DOI] [PubMed] [Google Scholar]
- 219.Jaggar JH, Stevenson AS & Nelson MT Voltage dependence of Ca2+ sparks in intact cerebral arteries. Am J Physiol 274, C1755–1761, doi: 10.1152/ajpcell.1998.274.6.C1755 (1998). [DOI] [PubMed] [Google Scholar]
- 220.Kassmann M. et al. Role of Ryanodine Type 2 Receptors in Elementary Ca(2+) Signaling in Arteries and Vascular Adaptive Responses. J Am Heart Assoc 8, e010090, doi: 10.1161/JAHA.118.010090 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Yuan Q. et al. Maintenance of normal blood pressure is dependent on IP3R1-mediated regulation of eNOS. Proceedings of the National Academy of Sciences of the United States of America 113, 8532–8537, doi: 10.1073/pnas.1608859113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Radermacher M. et al. Cryo-electron microscopy and three-dimensional reconstruction of the calcium release channel/ryanodine receptor from skeletal muscle. J Cell Biol 127, 411–423 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.des Georges A. et al. Structural Basis for Gating and Activation of RyR1. Cell 167, 145–157 e117, doi: 10.1016/j.cell.2016.08.075 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zalk R. et al. Structure of a mammalian ryanodine receptor. Nature, doi: 10.1038/nature13950 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Yan Z. et al. Structure of the rabbit ryanodine receptor RyR1 at near-atomic resolution. Nature, doi: 10.1038/nature14063 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Efremov RG, Leitner A, Aebersold R. & Raunser S. Architecture and conformational switch mechanism of the ryanodine receptor. Nature, doi: 10.1038/nature13916 (2014). [DOI] [PubMed] [Google Scholar]
- 227.Wei R. et al. Structural insights into Ca(2+)-activated long-range allosteric channel gating of RyR1. Cell research 26, 977–994, doi: 10.1038/cr.2016.99 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Bai XC, Yan Z, Wu J, Li Z. & Yan N. The Central domain of RyR1 is the transducer for long-range allosteric gating of channel opening. Cell research 26, 995–1006, doi: 10.1038/cr.2016.89 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Peng W. et al. Structural basis for the gating mechanism of the type 2 ryanodine receptor RyR2. Science, doi: 10.1126/science.aah5324 (2016). [DOI] [PubMed] [Google Scholar]
- 230.Gong D. et al. Modulation of cardiac ryanodine receptor 2 by calmodulin. Nature 572, 347–351, doi: 10.1038/s41586-019-1377-y (2019). [DOI] [PubMed] [Google Scholar]
- 231.Priori SG et al. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation 103, 196–200 (2001). [DOI] [PubMed] [Google Scholar]
- 232.Tung CC, Lobo PA, Kimlicka L. & Van Petegem F. The amino-terminal disease hotspot of ryanodine receptors forms a cytoplasmic vestibule. Nature 468, 585–588. [DOI] [PubMed] [Google Scholar]
- 233.Kimlicka L, Lau K, Tung CC & Van Petegem F. Disease mutations in the ryanodine receptor N-terminal region couple to a mobile intersubunit interface. Nature communications 4, 1506, doi: 10.1038/ncomms2501 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Yang Z, Ikemoto N, Lamb GD & Steele DS The RyR2 central domain peptide DPc10 lowers the threshold for spontaneous Ca2+ release in permeabilized cardiomyocytes. Cardiovasc Res 70, 475–485 (2006). [DOI] [PubMed] [Google Scholar]
- 235.Yuchi Z, Lau K. & Van Petegem F. Disease mutations in the ryanodine receptor central region: crystal structures of a phosphorylation hot spot domain. Structure 20, 1201–1211, doi: 10.1016/j.str.2012.04.015 (2012). [DOI] [PubMed] [Google Scholar]
- 236.Sharma P. et al. Structural determination of the phosphorylation domain of the ryanodine receptor. The FEBS journal 279, 3952–3964, doi: 10.1111/j.1742-4658.2012.08755.x (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Reiken S. et al. beta-adrenergic receptor blockers restore cardiac calcium release channel (ryanodine receptor) structure and function in heart failure. Circulation 104, 2843–2848 (2001). [DOI] [PubMed] [Google Scholar]
- 238.Yan Z. et al. Structure of the rabbit ryanodine receptor RyR1 at near-atomic resolution. Nature 517, 50–55, doi: 10.1038/nature14063 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Block BA, Imagawa T, Campbell KP & Franzini-Armstrong C. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J Cell Biol 107, 2587–2600, doi: 10.1083/jcb.107.6.2587 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Yin CC, Blayney LM & Lai FA Physical coupling between ryanodine receptor-calcium release channels. J Mol Biol 349, 538–546, doi: 10.1016/j.jmb.2005.04.002 (2005). [DOI] [PubMed] [Google Scholar]
- 241.Cabra V, Murayama T. & Samso M. Ultrastructural Analysis of Self-Associated RyR2s. Biophys J 110, 2651–2662, doi: 10.1016/j.bpj.2016.05.013 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Chopra N. et al. Ablation of triadin causes loss of cardiac Ca2+ release units, impaired excitation-contraction coupling, and cardiac arrhythmias. Proceedings of the National Academy of Sciences of the United States of America 106, 7636–7641, doi: 10.1073/pnas.0902919106 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Reiken S. et al. Beta-blockers restore calcium release channel function and improve cardiac muscle performance in human heart failure. Circulation 107, 2459–2466, doi: 10.1161/01.CIR.0000068316.53218.49 (2003). [DOI] [PubMed] [Google Scholar]
- 244.Kubalova Z. et al. Abnormal intrastore calcium signaling in chronic heart failure. Proceedings of the National Academy of Sciences of the United States of America 102, 14104–14109, doi: 10.1073/pnas.0504298102 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]