Abstract
Despite decades of research on the physiological and psychological effects of the menstrual cycle, studies have not sufficiently adopted consistent methods for operationalizing the menstrual cycle. This has resulted in substantial confusion in the literature and limited possibilities to conduct systematic reviews and meta-analyses. In order to facilitate more rapid accumulation of knowledge on cycle effects, the present paper offers a set of integrative guidelines and standardized tools for studying the menstrual cycle as an independent variable. We begin with (1) an overview of the menstrual cycle and (2) premenstrual disorders, followed by (3) recommendations and tools regarding data collection in cycle studies. These recommendations address selecting the appropriate study design, sampling strategy, managing demand characteristics, identifying a sample of naturally-cycling individuals, measuring menstrual bleeding dates, ovarian hormones, and ovulation. We proceed with suggestions for (4) data preparation and coding of cycle day and phases, (5) data visualization, statistical modeling, and interpretation of menstrual cycle associations. We also provide (6) recommendations for using menses start day and ovulation testing to schedule visits in laboratory studies and end with a (7) comprehensive summary and conclusion. Regardless of whether the influence of the menstrual cycle is of central interest in a study or should be controlled to accurately assess the effects of another variable, the use of these recommendations and tools will help make study results more meaningful and replicable.
Keywords: menstrual cycle, estrogen, progesterone, methods, recommendations, review
1. Introduction
The monthly menstrual cycle causes normative changes in female1 physiological functioning (Schiller et al., 2016), and can cause severe changes in emotional, cognitive, and behavioral functioning for hormone-sensitive individuals, such as those with premenstrual dysphoric disorder (PMDD; Wei et al., 2018) and premenstrual exacerbation (PME) of underlying psychiatric disorders (Hartlage et al., 2004). However, despite decades of research, laboratories studying the menstrual cycle have not adopted consistent methods of operationalizing the menstrual cycle (Allen et al., 2016; Eisenlohr-Moul et al., 2017; Hampson, 2020). This leads to significant confusion in the literature and frustrates attempts at systematic reviews and meta-analysis. For example, in a recent meta-analysis on fluctuations of cardiac vagal activity across the natural menstrual cycle, previous inconsistencies in the literature could be partially resolved by applying a common definition of cycle phases to the 37 included studies (Schmalenberger et al., 2019).
The present work reviews current best practices in the field of menstrual cycle research and integrates and expands on existing recommendations to present a set of standardized tools and practical guidelines for studying the menstrual cycle. In the course of presenting this material, a uniform vocabulary will be presented which will allow future studies to specify their approaches in a more precise and standardized manner. Following these recommendations will help make study results more meaningful and replicable and, thus, facilitate more rapid accumulation of knowledge on the emotional, cognitive, and behavioral effects of the menstrual cycle. Critically, this standardization may also help to clarify why some individuals demonstrate large changes in function across the menstrual cycle, and others do not.
2. Overview of the Menstrual Cycle
The menstrual cycle is a natural process in the female reproductive system that repeats monthly from menarche (i.e., the first menstrual bleed during puberty) to menopause, allowing fertilization and pregnancy. Starting with the first day of menses and ending with the day before the subsequent bleeding onset, the average cycle length is 28 days. However, healthy cycles vary in length between 21 days (possible diagnosis of polymenorrhoea if shorter) and 37 days (possible diagnosis of oligomenorrhoea if longer; Long, 1990). The menstrual cycle phases are characterized by predictable fluctuations of ovarian hormones estradiol (E2) and progesterone (P4), as displayed in Figure 1 (for absolute levels see section 4.6). The follicular phase derives its name from the maturation of the ovarian follicles containing oocytes. It begins with the onset of menses (i.e., shedding of the proliferated endometrium) and lasts through the day of ovulation (i.e., oocyte release by the dominant follicle). While P4 levels remain consistently low, E2 rises gradually through the mid-follicular phase and then spikes dramatically just before ovulation. The luteal phase, defined as the day after ovulation through the day before menses, transforms the remains of the dominant follicle into the corpus luteum. The corpus luteum produces P4 and E2; thus, the luteal phase begins with gradually rising P4 and E2 levels. The mid-luteal phase is characterized by peaking P4 and a secondary peak in E2. If no fertilization of the oocyte occurs, the corpus luteum involutes, causing rapid perimenstrual withdrawal of E2 and P4 and triggering menstruation. With this subsequent menstrual onset, the cycle begins again.
Figure 1.
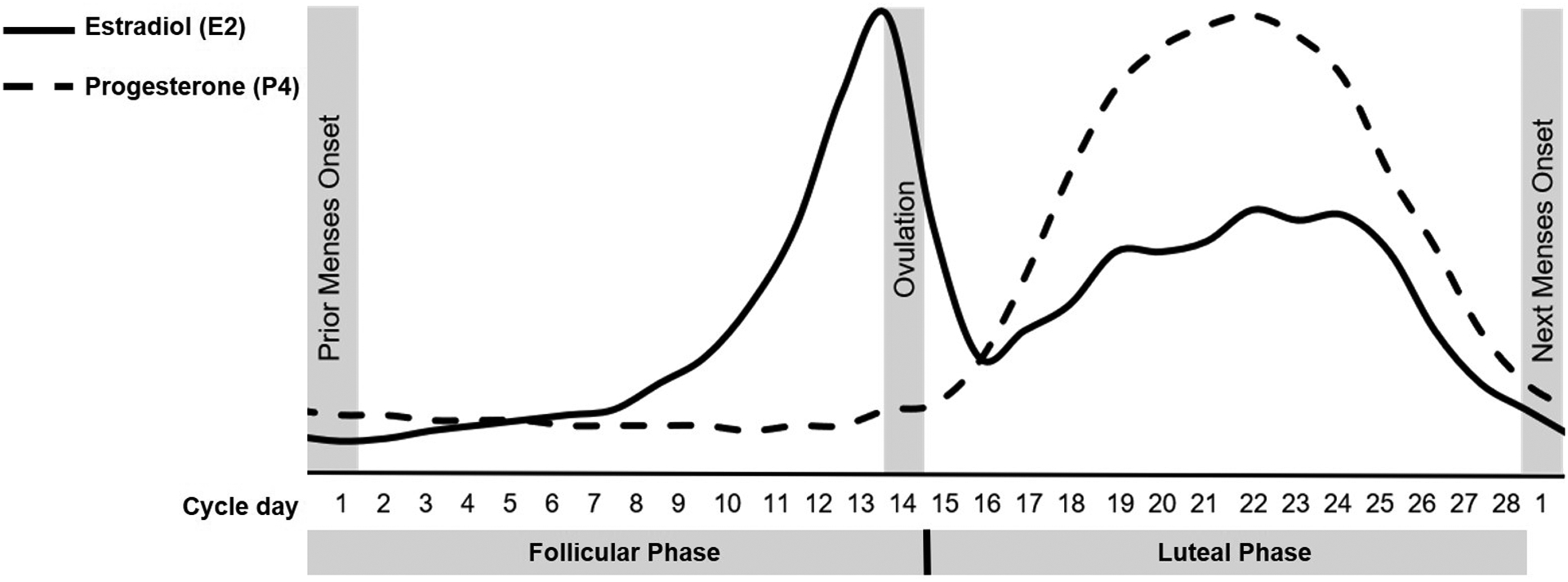
An idealized 28-day cycle with the characteristic fluctuations of the ovarian hormones estradiol (E2) and progesterone (P4) in the follicular and luteal menstrual cycle phase.
Due to the predictable lifespan of the corpus luteum, the luteal phase has a more consistent length than the follicular phase. The average length of the luteal phase is 13.3 days (SD = 2.1; 95% CI: 9–18 days), whereas the follicular phase generally lasts 15.7 days (SD = 3; 95% CI: 10–22 days; see Fehring et al., 2006, for meta-review). Although the differences may appear subtle, a study of 141 participants (1,060 cycles) found 69% of the variance in total cycle length could be attributed to variance in follicular phase length, whereas 3% of the variance was attributed to the luteal phase length.
3. Premenstrual Disorders
Rigorous longitudinal and experimental studies demonstrate that a subset of females have abnormal sensitivity to normal ovarian hormone change. This manifests as emotional, cognitive, and behavioral symptoms appearing primarily in the context of normative ovarian hormone changes during the midluteal and perimenstrual phases of the cycle (Schmidt et al., 1998; Wei et al., 2018). While females with PMDD (American Psychiatric Association, 2013) experience a severe luteal phase emergence of core emotional symptoms (i.e., affective lability, irritability, depressed mood, anxiety) that remit fully in the mid-follicular phase, females with PME of an underlying disorder suffer from cyclical worsening of a chronic disorder or symptom, such as depressive disorders (Hartlage et al., 2004) and borderline personality disorder (BPD; Eisenlohr-Moul et al., 2018; Peters et al., 2020).
It is crucial for research and healthcare to carefully identify hormone-sensitive individuals to (1) provide the right diagnosis and (2) investigate underlying psychopathology. Studies comparing retrospective and prospective premenstrual symptoms have found a remarkable bias toward false positive reports in retrospective self-report measures of premenstrual changes in affect (i.e., they do not converge better than chance with prospective daily ratings; Eisenlohr-Moul et al., 2017; Rubinow et al., 1984). Even more problematically, studies show that beliefs about premenstrual syndrome (PMS) may influence retrospective PMDD measures (Hart et al., 1987; Marván and Cortés-Iniestra, 2001). As a result, the Diagnostic and Statistical Manual of Mental Disorder (DSM-5) requires prospective daily monitoring of symptoms for at least two consecutive menstrual cycles for a PMDD diagnosis (American Psychiatric Association, 2013). We have developed a standardized system for diagnosing PMDD and PME based on daily symptom ratings, called the Carolina Premenstrual Assessment Scoring System (C-PASS; Eisenlohr-Moul et al., 2017). A paper worksheet, excel macro, R macro, and SAS macro for the C-PASS are available via the author’s website (www.cycledx.com). The C-PASS allows clinical and non-clinical researchers to screen samples for females experiencing a cyclical mood disorder, which may be a confounding variable.
4. Data Collection in Menstrual Cycle Studies
4.1. General Recommendations Regarding Study Design
Because females differ in their vulnerability to both “cyclical” changes and “non-cyclical” background symptoms (Wei et al., 2018), any effect of a between-subject cycle predictor variable will conflate the within-subject variance (i.e., variance attributable to changing hormone levels) with the between-subject variance (i.e., variance attributable to each woman’s baseline or “trait” levels of symptoms). Thus, the menstrual cycle is fundamentally a within-person process and should be treated as such in clinical assessment, experimental design, and statistical modeling. For this reason, repeated measures studies are the gold standard approach to cycle research, while treating the cycle or corresponding hormone levels as between-subject variables lacks validity. Therefore, daily or multi-daily (i.e., ecological momentary assessments; EMA) ratings of the outcome is the preferred method of data collection. For difficult-to-collect data such as psychophysiological or task-based outcomes, we recommend thoughtful selection of the number and timing of assessments after considering several factors, as follows. First, clearly state the purpose of the study and the hypotheses to be tested, and specify the required sampling structure across the cycle (and associated hormone levels or dynamics) required to test such a hypothesis. For example, researchers hypothesizing a positive association of E2 levels with performance on a computerized cognitive task may wish to sample at least in the mid-follicular phase (low and stable E2 and P4) and the periovulatory phase (peaking E2, low P4), while researchers hypothesizing an interaction between E2 and P4 in the prediction of heart rate variability (HRV) may wish to assess HRV in the mid-follicular phase (low and stable E2 and P4), the periovulatory phase (peaking E2, low P4), midluteal phase (elevated P4 and mildly elevated E2), and perimenstrual phases (falling E2 and P4; see section 5.2 for how to code menstrual cycle phase). Second, consider statistical approaches in advance, noting the number of observations that may be required to model variance appropriately. Given that the most reasonable basic statistical approach for analyzing menstrual cycle data are multilevel modeling (or random effects modeling) approaches which require at least three observations per person to estimate random effects of the cycle (see section 6 for detailed information), three repeated measures of the outcome across one cycle could be considered the minimal acceptable standard for estimating within-person effects of the menstrual cycle. However, for reliable estimation of between-person differences in within-person changes across the cycle (which we know to be substantial; Schmidt et al., 1998), three or more observations across two cycles allows for greater confidence in reliability of between-person differences (Kiesner, 2011). In general, it is best to have as many assessments as possible; however, when repeatedly assessing psychophysiological or task-based outcomes (e.g., cognitive tasks), possible order and training effects should be also be considered. We recommend avoiding them by counterbalancing the order of phase at first assessment.
Research on PMDD revealed that retrospective reporting on cycle changes of psychiatric symptoms is prone to biases (Eisenlohr-Moul et al., 2017; Rubinow et al., 1984), rendering cross-sectional study designs unsuitable for investigating the association between the cycle and psychiatric functioning. However, in studies in which the menstrual cycle is not the primary variable of interest (but its potential effects on primary variables should be controlled), a cross-sectional design is a defensible choice. In naturally-cycling participants, we recommend (1) scheduling the timing of samples to a constant cycle phase (rather than measuring and modeling menstrual cycle phase as a covariate or predictor) in combination with (2) screening participants to include only those with typical cycle lengths of 25–35 days. This enables proper phasing and reduces error variance related to the cycle. When visits are scheduled in the same phase for all participants, note that there is no true “baseline” phase of the cycle in which hormones would not affect an outcome; rather, the choice of phase should be made carefully after considering the specific research question at hand alongside the scientific knowledge about sex differences or cycle effects on the outcome. For example, as studies suggest that vagally-mediated HRV shows significant P4-related fluctuations across the natural menstrual cycle (Schmalenberger et al., 2020), future studies wishing to avoid the impact of P4 on HRV should consistently schedule all HRV assessments of their naturally-cycling participants to the mid-follicular phase (cycle days 5 to 10) which is characterized by low and stable P4 levels. In cases where this type of consistent phase scheduling is not possible, obtaining both forward- and backward- count (see section 5) cycle information can provide a covariate that is preferable to a lack of assessment.
4.2. Individual Differences in Cycle Effects and Sample Planning
Decades of research suggest naturally-cycling females differ greatly in their sensitivity to the cycle. We recommend three possible approaches to account for these between-person differences when sample planning: (1) Studies could identify hormone-sensitive females via prospective data collection of cycle-related psychiatric symptoms and compare to healthy controls. Carefully sampling hormone-sensitive females has been done in seminal experimental studies on PMDD (e.g.; Schmidt et al., 2017, 1998), postpartum depression (PPD; Bloch et al., 2000), and perimenopausal depression (Schmidt et al., 2015), thus contributing significantly to understanding their underlying psychopathology. (2) Alternatively, when sampling from the general population, the sample should be large enough to observe and statistically model between-person differences in hormone sensitivity (e.g., Gehlert et al., 2009); drawing from small convenience samples is unlikely to provide enough statistical power to investigate individual differences and yield clear conclusions. (3) In that context, it may also be useful to overrecruit based on factors shown to be associated with the severity of hormone sensitivity. Among those are historical exposure to extreme stress (i.e., history of trauma; Bertone-Johnson et al., 2014; Eisenlohr-Moul et al., 2016; Perkonigg et al., 2004; Pilver et al., 2011), recent stressful life events (Gordon et al., 2016), current stress (Gollenberg et al., 2010; Jahromi et al., 2011), impulsivity (Martel et al., 2017; Roberts et al., 2018), and BPD features (Eisenlohr-Moul et al., 2015).
4.3. Demand Characteristics in Menstrual Cycle Studies
Many menstrual cycle studies have been rightfully criticized for implying that all females experience a perimenstrual deterioration in function. We recommend that researchers take care to reduce demand characteristics of their studies, noting on recruitment materials that people with menstrual cycles can experience positive, negative, or no hormone effects.
4.4. Identifying a Sample of Naturally-Cycling Individuals
A simple Reproductive Status Questionnaire (RSQ; Appendix 1) can probe developmental state of the reproductive organs and general level of ovarian hormones in order to determine if an individual is “normally-cycling” and therefore suitable for study participation. Ideally, these questions would be repeated at the beginning and end of the study in order to detect any changes in reproductive status that may have occurred during the study.
4.4.1. Evaluating Gender and Sex
First, we strongly recommend that researchers take care to conceptually separate biological sex from gender identity. The first questions of the RSQ therefore inquire about gender identity (female/woman, male/man, nonbinary, genderfluid, other; question 1), preferred pronoun (he, she, other; question 2), biological sex assigned at birth (female, intersex, male; question 3), and which reproductive organs are present at the time of the assessment (question 4). We strongly encourage inclusivity of all gender identities, as many cycle studies receive valid criticism for overvaluing identification with female roles. Researchers can respect individual gender identity by applying recruitment and study materials which refer to “people” or “individuals” with menstrual cycles, rather than “females” or “women.” After the initial assessment of the participant’s gender identity, the individual should always be addressed using the indicated pronoun.
4.4.2. Puberty and Menopause
Menarche and menopause are the two bookend reproductive milestones for the fertile phase of life during which females typically experience regular and ovulatory menstrual cycles. However, the phases surrounding these milestones (i.e., peripubertal, perimenopausal) are characterized by irregular cycle lengths and increased anovulatory cycles (Fehring et al., 2006). Studies focusing on the regular cycle or ovarian hormones should therefore utilize caution when including participants near these two milestones.
With regard to potential peripubertal participants, RSQ question 6 collects the participant’s age of menarche. If the participant experienced menarche within the past 3 years, the cycle may not yet be regular enough to qualify for study participation. In addition to the RSQ, we recommend including the Pubertal Development Questionnaire (Carskadon and Acebo, 1993; Shirtcliff et al., 2009). Regarding potential perimenopausal participants, question 8 asks participants to identify typical symptoms of perimenopause. We suggest additionally using the Stages of Reproductive Aging Workshop (STRAW +10) staging system (Harlow et al., 2012) for participants above age 40 to measure menopausal status.
4.4.3. Pregnancy and Breastfeeding
Pregnancy and postpartum breast-feeding suppress fertility (and thus a regular menstrual cycle) for a variable time. The duration of breastfeeding-induced amenorrhea (i.e., absence of menstruation) shows great variability between and (in case of multiple children) within females. As breastfeeding declines, regular menstrual cycles resume; however, the first few menstrual cycles are generally unlikely to support a pregnancy due to low production of P4 by the corpus luteum (McNeilly, 2001). For this reason, menstrual cycle studies should exclude females who are currently pregnant or breast-feeding, (RSQ; question 9). We recommend participants experience three menstrual cycles post-breast feeding before participation in menstrual cycle research.
4.4.4. Hormonal Medications
Hormonal medications, while administered for various reproductive and non-reproductive reasons, may alter the natural cycle. Their use should be carefully assessed when recruiting potential study participants. Questions 10–14 of the RSQ determine whether study participation is possible for participants on hormonal medications.
Hormonal contraceptives (i.e., pill, patch, implant, injection, and vaginal ring) prevent ovulation-related hormone flux, thus altering the natural menstrual cycle (RSQ; question 10). Intrauterine devices (IUDs) need special consideration, as there are both hormone-containing IUDs (which, like other hormonal contraceptives, prevent ovulation) and copper IUDs (which do not alter the natural cycle; question 11). Depending on the research question, the use of one of these hormonal contraceptives might be exclusionary for study participation or should be covaried separately as a dichotomous medication variable in the analyses. If the study focus is the natural menstrual cycle, we recommend that at least two natural menstrual cycles have been experienced since the last use of the hormonal contraceptive.
Intake of other hormones should also be assessed for potential cycle-altering effects (question 12). For example, testosterone for gender confirmation treatment among female to male transgender participants can alter the cycle (Coleman et al., 2012). Also, any diagnosed gynecologic condition (i.e., polycystic ovarian syndrome) or any medical condition that affects a potential participant’s hormones or hormone cycling should be inquired (question 13). Finally, a complete list of medications should be obtained for all potential participants to determine potential impact on cyclical mood changes (i.e., selective serotonin reuptake inhibitors (SSRIs); see questions 14).
4.5. Measuring Menstrual Bleeding Dates
With low additional costs, researchers can approximate position within an individual’s menstrual cycle by using two dates: (1) the start date of the preceding cycle, and (2) the start date of the following cycle. With the widespread use of smartphone menstrual cycle tracking applications (Moglia et al., 2016), most individuals can easily access their exact start date. If a participant does not know the date, researchers can use the next menses start date to facilitate the preferred backward-count method. Participants should be notified during the consent process that they may be contacted 30 days after their study participation to report the start date of their next period. A brief daily survey for measuring menstrual bleeding days is provided in Appendix 2.
4.6. Measuring Ovarian Hormones and Associated Substances
In the following sections, reference ranges are provided for various hormones based on large samples reported in the literature; however, it is best practice to establish reference values within a given laboratory, or, when possible, within a given sample.
4.6.1. Luteinizing Hormone (LH) and Follicle-Stimulating Hormone (FSH)
During the menstrual cycle, FSH stimulates the growth of ovarian follicles, and LH triggers oocyte release by the dominant follicle (i.e., ovulation). Both FSH and LH levels show systematic variations across the natural cycle, including a singular pre-ovulatory peak. FSH serum concentrations are approximately 10 to 20 mIU/ml and peak around 40 mIU/ml, while LH concentrations are approximately 18 to 25 mIU/ml and peak around 60 mIU/mL (Barbieri, 2014). FSH and LH levels can be measured in samples of blood serum, plasma (via radioimmunoassay; RIA), or urine (via test stripes).
Given that ovulation typically occurs 10 to 12 hours after the LH surge (Park et al., 2002), LH-testing is traditionally used to determine if a menstrual cycle is ovulatory and when ovulation occurs. In research settings, commercially available home urinary LH-tests are frequently used. These tests have a sensitivity of 10–70 mIU/L and generate a positive result (i.e., indication of ovulation) once the sensitivity threshold is exceeded. In the case of LH-tests, where participants must compare the control line with the test line to obtain a result, researchers may increase reliability by requesting that participants electronically send photos of each test to the study personnel for verification. If the financial resources are available, for a standardized interpretation of the ovulation tests, we recommend the more costly tests delivered with electronic read-out. Anecdotally, LH tests without a computerized determination of the result lead to more participant confusion and allow greater room for error in interpretation of the result. For recommendations on how to use LH-testing to schedule laboratory visits see section 7.
In contrast, measuring basal FSH levels (on cycle day 3) in premenopausal women is traditionally used to evaluate ovarian reserve (Abdalla and Thum, 2004) which can clarify (in)fertility issues. Given that general FSH levels demonstrate a significant increase near perimenopause, FSH testing is informative on menopausal status as well (for an overview, see for example Burger et al., 1999).
4.6.2. Progesterone (P4) and its Metabolites
Across the natural menstrual cycle, P4 is mainly produced by the corpus luteum in the luteal phase. With generally low serum concentrations around 0.5 ng/ml, P4 levels start to rise within 12 hours of the initiation of the LH surge and peak mid-luteally around 11 ng/ml (Barbieri, 2014). Since anovulatory cycles show no change in P4 levels, a luteal increase of P4 validates the occurrence of ovulation and marks the start of the luteal phase of an ovulatory cycle.
P4 levels can be measured in samples of urine, saliva, blood plasma, or blood serum via RIA or gas-chromatography-mass spectrometry (GC-MS), each of which offers various sampling advantages and disadvantages (for an overview, see Tivis et al., 2005). In short, drawing blood for research requires high-cost preparations, such as highly trained personnel (e.g. phlebotomist, nurse), facilities capable of disposing of biohazard blood, and protocols for managing risk (e.g. fainting, bruising). In contrast, both saliva and urine sampling can be conveniently done in almost any research environment. However, saliva sampling does require potential disruptions to the participant’s daily routine (e.g., no smoking, no soft drinks). Those may be minimized by protocols that require higher participant compliance, such as having participants take saliva samples at home, store them in their freezer, and bring the samples to the laboratory at a later date. Assuming excellent compliance, studies suggest a high correlation between salivary and serum levels of P4 (Choe et al., 1983). Weighing relative advantages and disadvantages of each method is recommended for each study, but should remain consistent for all participants within a study.
An increasing body of research suggests some effects of P4 may be mediated by its neuroactive metabolites, among which pregnanolone and allopregnanolone (ALLO) are the most studied (Hantsoo and Epperson, 2020). These neuroactive steroid metabolites modulate the GABA system (i.e., the major inhibitory system of the adult central nervous system) by acting as a positive allosteric modulator of GABA-A receptors. While their common effect is inhibitory (e.g., anesthetic, sedative, anticonvulsant, anxiolytic), studies suggest that a small subgroup of naturally-cycling females have adverse effects (negative mood, anxiety, irritability or aggression) upon exposure (Andréen et al., 2009; Bäckström et al., 2011; Timby et al., 2016). Levels of neuroactive steroid metabolites like ALLO or pregnanolone can be measured in blood serum samples via RIA, GC-MS, or liquid chromatography‐mass spectrometry (LC-MS).
4.6.3. Estradiol (E2)
During the early follicular phase, serum concentrations of E2 start very low (around 40 pg/ml) then ascend due to additional E2 production by growing follicles. The rise of the dominant follicle results in the mid-to-late follicular spike of E2, during which serum E2 concentrations reach levels around 200 pg/ml (Barbieri, 2014). This E2 surge is followed in 24–36 hours by ovulation (Park et al., 2002) and is therefore well suited to determine if and when ovulation happens. The secondary mid-luteal E2 surge reaches concentrations around 120 pg/ml (Barbieri, 2014). Similar to P4, E2 levels can be measured in samples of urine, saliva, blood plasma, or blood serum via RIA or GC-MS. However, in contrast to P4, there is some evidence that serum and saliva E2 fail to correlate (Choe et al., 1983), while more recent studies suggest salivary E2 assessments to be an adequate estimate of serum E2 (Fiers et al, 2017).
Of note, neither E2 nor P4 levels show reliable between-person differences (for review, see Eisenlohr-Moul and Owens, 2016).
4.7. Measuring Cyclical Changes in Basal Body Temperature (BBT)
Across the natural menstrual cycle, P4’s effects on the hypothalamus cause predictable changes in BBT, or the body’s lowest temperature at rest. BBT is low across the follicular phase, demonstrates a dip (nadir) before ovulation, increases sharply at ovulation, and then remains high across the luteal phase. This temperature rise has typically been defined as BBT surpassing the threshold of 37°C (de Mouzon et al., 1984) or in relative terms, an increase from follicular levels by 0.2 to 0.3°C (Colombo et al., 2000; Ecochard et al., 2001). Ovulation can thus be approximated by two different definitions: (1) the BBT nadir or (2) the first day of the sustained temperature rise (de Mouzon et al., 1984; Ecochard et al., 2001; Martinez et al., 1992; Tenan et al., 2013). When compared against LH-testing methods, 210 biphasic assessments showed BBT nadirs occurring within one day of the urinary LH surge in 75% of cases and within two days in 90% of cases. Given that only a sustained temperature rise confirms the luteal phase, BBT is considered most accurate for retrospective identification of the periovulatory phase (Martinez et al., 1992). For research purposes, BBT should be collected every morning before arising across a complete menstrual cycle. Participants can utilize commercially-available oral or vaginal BBT thermometers and note their own results, or participants can continuously wear devices (e.g., a wristband, finger ring) with integrated thermometers that record BBT measures during the night and save them electronically.
Several factors should be considered when deciding whether to use BBT assessment or LH testing for determining ovulation in a given study. First, one must consider the focus and design of the study. For example, if it is necessary to immediately know about when the LH surge (ovulation) is happening so that the study team can schedule a periovulatory phase laboratory visit, LH-testing is required (see section 7 for detailed information on how to schedule visits) since BBT does not allow for confirmation of ovulation timing until much later in the cycle. However, if it is sufficient for the study’s purpose to retrospectively validate that the cycle was ovulatory, BBT assessments are a suitable choice. Second, cost should be considered alongside the available budget; historically, the use of a thermometer to capture BBT has been cheaper than the use of LH tests with computerized interpretation of results. However, this area of technology is rapidly evolving, and costs should be evaluated in light of currently available options and their evolving quality (e.g., LH tests with electronic read-out devices are highly accurate but more expensive than LH tests which are manually read out; BBT wristbands provide more accurate continuous monitoring of temperature but are a more expensive choice than oral BBT thermometers). Third, one must consider the burden that each approach puts on the participant (e.g., daily urine sampling for LH-testing vs. wearing a wristband for BBT monitoring vs. taking a temperature at the same time of day for BBT monitoring) and associated feasibility issues (e.g., daily urine sampling might be challenging for a highly distressed peripubertal clinical sample). In sum, given that BBT assessments and LH testing for determining ovulation are comparable in their ability to retrospectively confirm ovulation (Martinez et al., 1992), and given the rapidly evolving landscape of biomedical technologies, we do not recommend urine-based LH testing over BBT; rather, we recommend weighing timing considerations, cost, accuracy, feasibility, and burden of each method in each study in order to make a thoughtful selection that will facilitate the best possible science.
5. Data Preparation and Coding in Menstrual Cycle Studies
5.1. Coding Menstrual Cycle Day
Once two “bookend” menstrual cycle start dates are available (marking the beginning of two contiguous cycles), a cycle day variable can be calculated for each observation using a combination of forward-count and backward-count methods. First, count forward ten days from the prior period start date, where the first day of menstrual bleeding is day 1. If the observed date of interest is within this timeline from day 1 to 10, that observation’s cycle day is set as the forward-count value (e.g., +2). Next, for the same observation, count backward from the onset of the subsequent menses, where the day before the next menstrual bleeding is day −1. If the observation date is between −15 and −1 on that timeline, that observation’s cycle day is equal to the backward-count value (e.g., −10). If the observation date falls on both the forward- and backward-count timelines, the backward-count value is preferred. This creates a cycle day variable that ranges between −15 and +10. Step-by-step instructions for coding menstrual cycle day in any statistic software are available in Appendix 3. Of note, this raw variable creates an intuitive timeline relative to menses onset such that −1 is the day before menses onset, and +1 is the day of menses onset, but does not include 0; if continuous modeling methods (described in section 6) are used, days +1 through +10 should be recoded as days 0 to +9, in order to eliminate the single-day gap between days −1 and +1. Although the omission of day 0 on the timeline for cycle phase coding may cause confusion, it is preferred due to the strong and intuitive tradition of labeling the first day of menstrual bleeding as “day one” of the cycle.
5.2. Coding Menstrual Cycle Phase
The study of menstrual cycle phases aims to test hypotheses about the impact of discrete hormonal events (levels and/or acute changes) on outcomes of interest. However, methods used to determine these phase variables differ widely between studies. In this section, we propose several methods of cycle phase coding and verification. The most simple and straightforward is to categorize cycle day (see section 5.1) into phases based on forward- and backward-count day; LH-tests, BBT, and hormone levels can all be used in this process as well. Below, for commonly measured phases, we (a) describe the hormonal events that occur during that phase, (b) indicate best practices for coding and validating that phase using measures described above, and (c) indicate coding variations that could be used to test specific hypotheses. The proposed methods are divided into recommended phasing procedures (Table 1a), phasing procedures to use with caution (Table 1b), and phasing procedures to use with extreme caution (Table 1c). We additionally recommend phasing procedures for two exploratory menstrual cycle phases (Table 1b). A graphical overview of all menstrual cycle phases is provided by Figure 2.
Table 1a.
Overview of recommended procedures for cycle phasing.
| Recommended Phasing Procedures | ||||
|---|---|---|---|---|
| Mid-luteal | Perimenstrual | Mid-follicular | Periovulatory | |
| Hormonal Status and Definitions | High, stable E2/P4 | Falling E2/P4, low E2/P4 | Slight rise in E2, very low P4 | Strong rise and fall of E2, slight increase of P4 |
| Counting Methods | (2) day −9 to −5 before menstrual onset | (1) Day −3 before menstrual onset to Day 2 after onset | (2) Day +4 to +7 after menstrual onset | (2) day −15 to −12 before menstrual onset |
| LH-Test Definitions | (1) day +6 to +10 following positive test | N/A | (1) Day −7 to −3 before positive test | (1) Day −2 before positive test to day of and day +1 following positive test |
| BBT Definitions | (1) day +6 to +10 following nadir | N/A | (1) Day −7 to −3 before nadir | (1) Day −2 before nadir to day of and day +1 following nadir |
Table 1b.
Overview of phasing procedures for exploratory phases and commonly used phasing procedures to use with caution.
| Phasing Procedures for exploratory phases (Recommended) | Phasing Procedures to Use with Caution | |||
|---|---|---|---|---|
| Pre-ovulatory | Post-ovulatory | Menstrual week | Full premenstrual week | |
| Hormonal Status and Definitions | Strong E2 peak | Falling E2, rising P4 | End of withdrawal, low E2/P4 | High E2/P4, then abrupt falling E2/P4 |
| Counting Methods | N/A | N/A | (1) Day of menstrual onset to day +7 following menstrual onset | (1) Day −7 to day −1 before menstrual onset |
| LH-Test Definitions | (1) Day −2 to −1 before positive test | (1) day of and day +1 following positive test | N/A | (2) Day +8 to day +14 following positive test |
| BBT Definitions | (1) Day −2 to −1 before nadir | (1) day of and day +1 of nadir | N/A | (2) Day +8 to day +14 following nadir |
Table 1c.
Overview of phasing procedures for broad phases.
| Phasing Procedure for broad phases (Use with Extreme Caution) | ||
|---|---|---|
| Luteal | Follicular | |
| Hormonal Status and Definitions | Complex: rising and high E2/P4, withdrawal of E2/P4 | Complex: falling and low E2/P4, rising E2 |
| Counting Methods | Day −15 until day −1 before menstrual onset | Day 1 of menstrual onset until day−15 before menstrual onset |
| LH-Test Definitions | Day following positive test until menstrual onset | Day of menstrual onset until the day following positive test |
| BBT Definitions | Day following nadir until menstrual onset | Day of menstrual onset until the day following nadir |
Note. The numbering of the different methods in a cycle phase column ranks suitable methods for determining this cycle phase, with (1) being gold standard. See text for further explanation. N/A=not applicable.
Figure 2.
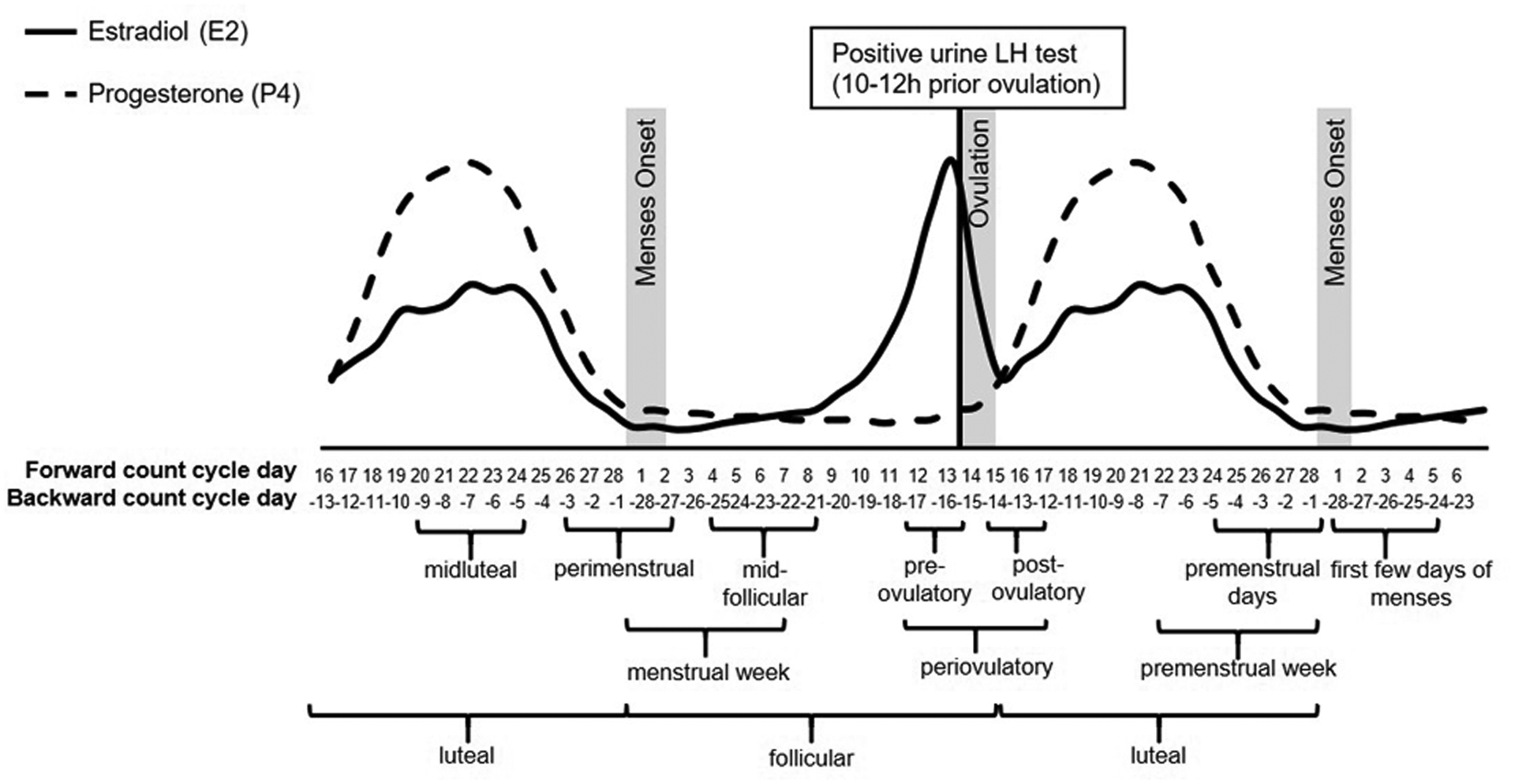
Graphical overview of the menstrual cycle and its phases.
5.2.1. Recommended Phasing Procedures
The following menstrual cycle phases are recommended for investigation in empirical studies as they are characterized by clearly delineated hormonal events rather than relative changes in hormone levels. Studies that determine these phases precisely produce more meaningful results regarding the association between specific hormonal events and outcomes in question. Step-by-step instructions for coding these cycle phases in a statistic software are available in Appendix 3.
Mid-luteal Phase.
The mid-luteal phase is hormonally characterized by a primary peak in P4 and secondary peak in E2 levels. Because the forward-count method does not allow for individual differences in cycle length, invariantly setting the mid-luteal phase as days 17 to 21 (which would only be correct in the case of a typical 28-day cycle), the precise determination of the mid-luteal phase cannot be achieved by the forward-count method alone. Therefore, to precisely determine the mid-luteal phase, it is highly recommended to first determine the individual’s day of ovulation (gold standard measure: LH-testing or BBT). With the day of ovulation set as day 0, the mid-luteal phase is defined as days +6 to +10 following the positive LH-test or the nadir in BBT. If no individual ovulatory information is available, the mid-luteal phase can also be determined with the backward-count method defining it as days −9 to −5 before the onset of the subsequent menses. However, the backward-count approach is less precise than the ovulation-based procedures, since it cannot ascertain whether ovulation occurred in this cycle. Anovulatory cycles, even among healthy young females, are not uncommon and usually go unnoticed since the following cycle still starts with bleeding.
Perimenstrual Phase.
The perimenstrual phase is hormonally defined as active withdrawal in E2 and P4 levels occurring approximately between premenstrual day −3 and menstrual bleeding day 3. In studies of PMDD, days −3 to +3 are consistently the most symptomatic days (Epperson et al., 2012; Hartlage et al., 2012), suggesting that for some females (Schmidt et al., 1998, 1991), active hormone withdrawal occurring during that timeframe may be critical for triggering psychiatric symptoms (Lovick et al., 2017). The recommended perimenstrual phasing procedure is a combination of backward- and forward-counting from cycle days −3 to +2; this captures the days in which perimenstrual symptoms are expected to be the highest, but also avoids progression into the follicular phase. In studies with laboratory appointments, researchers should determine a consistent rule for whether or not perimenstrual laboratory visits can be scheduled on day 1 of the participant’s menstrual cycle. Up to 50% of menstruating women experience some degree of dysmenorrhea (i.e., painful menstrual cramps) – especially in the first 24–36h after the onset of bleeding (Dawood, 2006). If, for example, psychological variables such as cognitive performance are to be examined in the laboratory appointment, it is possible that pain or discomfort could alter responses on the task.
Mid-follicular Phase.
The mid-follicular phase is hormonally defined as a slight rise in E2 levels and very low P4 levels. As mentioned, typical cycles range between 21 and 35 days, with a relatively robust 14-day luteal phase. This results in a follicular phase that starts at day 1 and ends between day 8 (in a 21-day cycle) and day 21 (in a 35-day cycle). Due to this large variance in the follicular phase length, we recommend determining the mid-follicular phase based on an individual’s date of ovulation and setting it to days −7 to −3 prior to the first positive LH-test or the nadir in BBT (gold standard). If no individual ovulatory information is available, the mid-follicular phase can also be determined using the forward-count method. As mentioned, the premenstrual E2 and P4 withdrawal roughly occurs between cycle days −3 to +2; thus hormone levels should be low and stable on cycle day +4. To account for inter- and intraindividual differences in cycle lengths, the mid-follicular phase is measured as days +4 to +7, as even individuals with short cycles (<21 days) are not expected to ovulate before day +8.
Periovulatory Phase.
Hormonally, the periovulatory phase is characterized by a steep increase, a primary peak, and a subsequent fall in E2 levels, while P4 levels start to slightly increase. We recommend individual measurement of ovulation via LH-testing or BBT assessment for periovulatory cycle phasing. This not only serves the purpose of knowing when ovulation took place, but also if it took place at all. As ovulation typically lasts 24–36 hours, the periovulatory phase is defined as the day of individually determined ovulation (i.e., day 0; positive LH-test or BBT nadir) and the following day. If ovulation testing is not available, the backward-count approach is the next best estimation of the periovulatory phase. Given the relatively robust 14-day luteal phase, the backward-count method defines the periovulatory phase as days −15 to −12 before the onset of the subsequent menses. Again, the backward-count method is less precise than ovulation-based methods, as it cannot ensure that ovulation took place in the cycle.
Pre-ovulatory and Post-ovulatory Phases.
In some cases, investigators have specific hypotheses regarding E2 surges (prior to ovulation) vs. high levels of E2 (the 24–36 hours of ovulation) vs. falling E2 (post-ovulation). In such cases, the use of either daily E2 levels or daily LH-testing is defensible to pick out two pre vs. post-ovulation days for comparison to other phases (e.g., the early-to-mid follicular phase, when E2 is low and stable) or each other. Roberts et al. (2018) found that among females with ADHD symptoms, symptoms increased in the post-ovulatory vs. pre-ovulatory days, which may indicate that falling E2 is deleterious for cognitive function among hormone-sensitive females. More work is needed to flesh out these methods, but they are reasonable when either LH-testing or E2 levels allow clear determination of the E2 peak.
5.2.2. Phasing Procedures to Use with Caution
In the following section, the premenstrual and menstrual weeks are assumed to be distinct cycle phases that can be studied. It is important to note that these time frames are defined by their relative position to menstruation, rather than with reference to a measurable hormonal change or status. Therefore, it is only defensible to use these phase designations if the study aims to understand events in relation to the premenstrual week or menstrual bleeding rather than in relation to hormonal events. However, since the study of cycle phases generally intends to test hypotheses about the association of discrete hormonal events with outcomes, it is usually advisable to code the phases surrounding the onset of menses such that they focus on hormone changes, as described above (i.e., mid-luteal, perimenstrual, mid-follicular phases). For this reason, we recommend the use of the following phases only in rare instances.
Full Menstrual Week.
From a hormonal perspective, the menstrual week is characterized by falling E2 and P4 levels during the first half and subsequent low and stable E2 and P4 levels during the second half, making this week heterogeneous both in hormonal state and potential mechanisms of effects. Furthermore, menstruation falls under the follicular phase, which accounts for the majority of cycle length variability; a typical menstrual flow can actually span 3–7 days, drawing into question the label “menstrual week”. The menstrual week can be determined via the forward-count method which defines it as days +1 (i.e., menses onset) to +7. This phase may be of interest to those studying dysmenorrhea or other phenomena directly related to menstrual bleeding. Importantly, if one is interested in studying actual menstrual bleeding (as opposed to the hormonal withdrawal that is signposted by menstrual bleeding onset), using daily reports of actual menses (with, for example, the survey in Appendix 2) and related outcomes of interest is the more appropriate choice.
Full Premenstrual Week.
The full premenstrual week is hormonally characterized by high E2 and P4 levels during the first half that show an abrupt fall in the second half, making this week also heterogeneous with respect to hormonal state and potential mechanisms of effects. A straightforward premenstrual week can be determined by counting backward 7 days from the onset of menses (i.e., days −1 to −7, where menses onset is day +1 and there is no day 0). Due to the relatively robust 14-day luteal phase, the premenstrual week can also be defined on the basis of the individually determined day of ovulation. The premenstrual week spans days +8 to +14 based on LH-testing or BBT, where day 0 is a positive LH-test or the BBT nadir, respectively.
5.2.3. Phasing Procedures to use with Extreme Caution
Systematic reviews of menstrual cycle research (e.g., Schmalenberger et al., 2019) show that many studies draw comparisons between the two halves of the cycle (i.e., follicular vs. luteal). However, many different hormonal events and levels characterize the follicular and the luteal phase of a menstrual cycle. The influence of a particular hormonal event on the outcome of interest cannot be accurately assessed by these two broad phases, and effects themselves may be washed out by dynamic changes within each phase; thus, this method of coding is not recommended for empirical studies. Nevertheless, due to its prevalence in current literature, we provide a brief discussion of these two possibilities.
Luteal Phase.
From a hormonal perspective, luteal phase P4 rises at the beginning, peaks in the middle, then abruptly drops. E2 drops at the beginning, subsequently rises, demonstrates a secondary peak halfway through the phase, and then abruptly drops with P4 in the final days of the luteal phase. Due to the relatively robust 14-day luteal phase, the backward-counting method sets the luteal phase from day −15 until day −1 before the subsequent menstrual onset. Ovulation-based methods define the luteal phase as the time span from the day +1 following the detected ovulation (i.e. day 0) until the subsequent onset of menses.
Follicular Phase.
The beginning of the follicular phase is characterized by an initial hormone withdrawal followed by stable and low E2 and P4 levels. E2 levels begin to rise mid-follicularly and peak towards the end of the phase right before ovulation occurs, while P4 levels remain consistently low throughout. A combination of forward- and backward-count methods defines the follicular phase as menstrual onset until day −15 before the subsequent onset of menses. According to ovulation-based methods, the follicular phase begins the day of menses onset and lasts until the day after the detected ovulation (day +1 where day 0 is a positive LH-test or the BBT nadir).
6. Visualization and Statistical Modeling of Menstrual Cycle Effects
6.1. Visualizing Menstrual Cycle Effects
Prior to exploratory or hypothesis-driven modeling, the effects of the cycle variable on (1) the raw outcome and (2) the person-centered outcome should be graphed for each person individually (e.g., spaghetti plots, see Singer and Willett, 2003), then for the group as a whole, in order to detect potential outliers or patterns relevant to later modeling strategies. The person-centered approach is based on generating an individual’s mean across all observations and subtracting that mean from each individual observation. This results in a repeated variable that represents how each observation stands in relation to the individual’s typical levels of that variable (i.e., how much lower than one’s mean, or higher than one’s mean, is this particular observation?; see Eisenlohr-Moul et al., 2015; Enders and Tofighi, 2007). Person-centering the outcome variable is recommended for visualizing menstrual cycle effects because it allows researchers to observe pure within-person variance across the cycle, which may be obscured by between-person variance in raw outcomes. For publication, we recommend presenting a graph of the person-centered outcome across the full span of the cycle day variable (usually from −15 to +10; see example in Figure 3). This ensures transparency with respect to all potential patterns in the data, allows other scientists to draw their own conclusions about the impact of the cycle on the outcome, and reduces the risk of reviewer bias in selecting cycle phase definitions that maximize the size or statistical significance of their findings. For the purposes of clearer visualization, some authors choose to apply a five-day rolling average transformation to their outcome data prior to person-centering and graphing; this may reduce the impact of idiosyncratic spikes in symptoms and produces a clearer graph of cyclical change (e.g., Klump et al., 2008).
Figure 3.
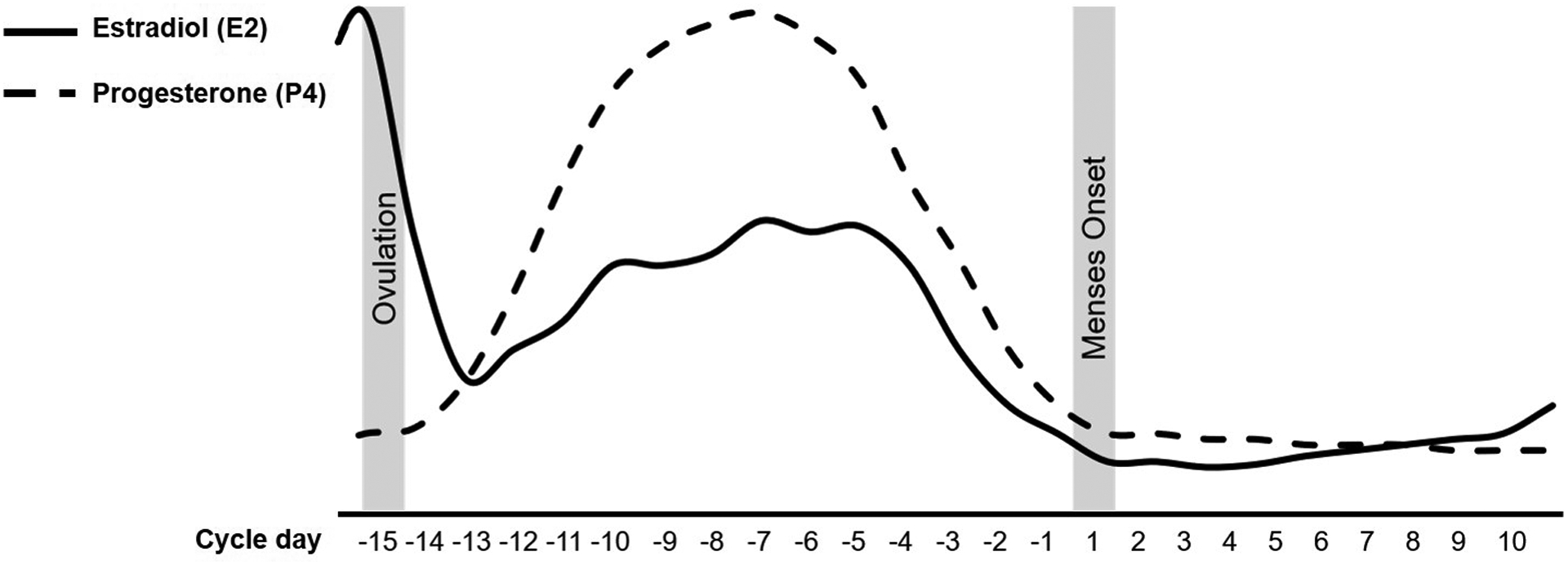
Recommended graphing method for visualizing menstrual cycle effects on person-centered outcomes across the full span of the cycle day variable (from −15 to +10, where menstrual onset is day +1 and there is no day 0).
6.2. Multilevel Modeling of Menstrual Cycle Effects
As noted above, repeated measures approaches are critical for effective modeling of menstrual cycle effects. Since there is ample experimental (Wei et al., 2018) and longitudinal (Gehlert et al., 2009) evidence for large between-person differences in response to the cycle, we recommend that models allow for random effects of within-person cycle phase or hormone levels. In other words, analyses should allow for the modeling of between-person differences in within-person change across the cycle. The most common method that meets these criteria is linear mixed modeling or multilevel modeling (MLM), which can be carried out in various statistical packages including SAS (PROC MIXED), HLM, STATA (xtmixed), R (lme4), mplus (TWOLEVEL), and SPSS’ multilevel modeling package (mixed). Because using repeated measures data will produce non-independent (i.e., clustered) observations, it is also methodologically critical to apply MLM to appropriately account for the nested structure of the data. Data should be prepared in a “long” format, where each daily/weekly (or otherwise repeated) observation has its own row in the data set, identified by a consistent between-subject identifier for each participant (ID number) and a time-varying cycle variable (day 1, 2, 3, etc).
One major benefit of MLM is that it allows for the estimation of both average cycle effects in the full sample (i.e., fixed effects) as well as individual differences in the size and direction of those effects (i.e., random effects); cross-level interaction terms (e.g., the interaction of trait impulsivity and cycle phase) can be used to identify which between-person characteristics might account for between-person differences in cyclical change. Modeling random effects of the cycle requires at least three observations. For each random effect in the model, one must specify an appropriate covariance structure for within-person error; model fit is generally used to compare the options and select the best-fitting structure. When daily repeated measures are used, one should specifically consider the appropriateness of an autoregressive error structure (usually day-1) to account for temporal correlations. Strategies for operationalizing and modeling within-person cycle effects, which can be categorical or continuous, are described below.
6.3. Categorical Cycle Phase Contrasts
The most straightforward method of testing for cycle effects is to examine within-person contrasts between cycle phases using MLM. In this approach, the within-person cycle phase variable is category coded such that a reference phase is specified, allowing pairwise comparisons between the means of each cycle phase for a given individual (Singer and Willett, 2003, pp. 194–195). In these cases, alternation of the reference group is necessary to observe all pairwise cycle phase comparisons. Random intercepts should be included to account for the nesting of each data point within a given individual and to model the degree of between-person variability in average values (i.e., intercept) of the outcome. Random effects of each cycle phase contrast should also be included, except where a likelihood ratio test indicates that their inclusion significantly worsens model fit (Singer and Willett, 2003); if the latter is true, this would suggest that the sample is homogeneous with respect to the cyclical effect.
6.4. Continuous Approaches
The menstrual cycle can also be conceptualized as a nonlinear function of time (Singer and Willett, 2003) and modeled using a continuous “cycle day” variable (see section 5.1); however, these approaches are more complex and can be difficult to correctly specify. The time axis can be specified as we have described above, with time ideally ranging from −15 to +10. If backward-count data are not available, one could specify time as a forward-count cycle day variable, although forward-count methods are prone to error due to the length variability of the follicular phase. Visualizations should be utilized to determine the appropriate shape of the time function. When a cycle day variable ranging from −15 to +9 (including zero) is used, a quadratic effect will generally capture the typical perimenstrual rise and postmenstrual fall of symptoms among hormone-sensitive individuals (Eisenlohr-Moul et al., 2019). When two cycles of data are available, a quartic function extends the quadratic effect to the second cycle (Kiesner, 2011). Once these units of analysis have been derived, various statistical tools can examine how cyclicity of different symptoms intercorrelate (e.g., cluster analysis, Kiesner, 2011; structural equation modeling, Kiesner and Granger, 2016). Finally, it should be noted that time series analysis techniques represent promising but as yet untapped options for characterizing the temporal dynamics of the association of hormones or cycle phases with symptoms.
6.5. Approaches to Modeling Individual Differences in Cyclical Change
Whether using continuous or categorical approaches, it is important to consider modeling between-person predictors of within-person cycle effects. Top-down, hypothesis-driven approaches can be tested using cross-level interactions in MLM, in which between-person characteristics (e.g., genotypes, early life stress exposure) are specified as moderators of within-person cycle effects (e.g., Eisenlohr-Moul et al., 2016; Roberts et al., 2018). Of note, some authors have utilized a bottom-up approach to derive unique groups of change across the cycle using cluster analysis of cosine trajectories across two cycles (Kiesner, 2011) and group-based trajectory modeling of quadratic or cubic trajectories across the perimenstrual frame (Eisenlohr-Moul et al., 2019).
6.6. Interpretation of Results
How should significant effects of the menstrual cycle be interpreted? In short, they should be regarded as correlations rather than evidence for causation. Only experimental manipulation of specific aspects of the cycle can demonstrate true effects of hormone levels or changes on outcomes (see Schmidt et al., 1991, for an example). It is tempting to assume that finding an association between an outcome and a particular cycle phase implies that the concurrent hormonal events drove the symptom change. However, at least with respect to symptoms of PMDD, studies suggest delayed effects of hormone change, such that the emergence of symptoms occurs in a different phase than the hormonal event. One study modeling longitudinal associations between hormones and PMDD symptoms found strong evidence for delayed rather than concurrent effects (Wang et al., 1996), and another demonstrated that hormone effects can be dependent on the derivative/direction of hormone change (McNamara et al., 2014). Even more importantly, experimental work in PMDD has demonstrated that periovulatory hormone changes provoke transient symptoms peaking roughly two weeks later before receding after about one month (Schmidt et al., 2017, 1998). Another study found that abrupt induction of hormone withdrawal in the midluteal phase did not impact the course of PMDD symptoms at all; the authors concluded that PMDD symptoms were therefore caused by (substantially) delayed impacts of periovulatory hormone change (Schmidt et al., 1991). In contrast, some studies provide evidence for concurrent (relatively immediate) effects of experimental hormone withdrawal on symptoms in menopause (Schmidt et al., 2015), around parturition (Bloch et al., 2000), and surrounding the perimenstrual phase (Eisenlohr-Moul et al., 2018) in individuals diagnosed with reproductive mood disorders.
Even when correlations with hormones and cycle phases are robust, observational studies should be extremely careful to not imply causation. Instead, interpretations of cycle correlations should emphasize the complex and often delayed nature of cycle pathways. When appropriate, studies should call for experimental tests to clarify the causal role of specific hormone changes in a given outcome.
7. Using Menses Start Day and Ovulation Testing to Schedule Visits in Laboratory Studies
Depending on the research question, studies can require participants to complete laboratory visits during certain cycle phases. The several methods for determining cycle phase discussed above each have a unique applicability for scheduling laboratory visits. First, analyzing hormone levels from blood or saliva is suitable only for retrospective validation of cycle phase, not for scheduling lab visits, due to the cost and resources required to analyze a sample. Second, regarding the periovulatory phase, the LH-surge method is the only suitable method to schedule a lab visit reliably. Given that ovulation happens 10–12 hours after the LH surge, participants should perform one LH-test per day several days before the expected ovulation. We recommend participants identify the shortest cycle of the previous six months, then subtract 14 days (i.e., the relatively robust length of the luteal phase) from this cycle length; this identifies the earliest cycle day on which the participant is likely to have ovulated in the past six months. Approximately four days before this cycle day, the participants should start daily ovulation testing which ensures that ovulation is not missed, even if the cycle in question is unexpectedly short, and familiarizes the participant with the testing routine early on. Ideally, the periovulatory lab visit should happen the day of the positive test or the following day. In contrast, the BBT method is best used for retrospectively identifying the periovulatory phase (Martinez et al., 1992) since only a sustained temperature rise indicates that ovulation has happened. Third, both the LH-surge or BBT method can be used to schedule mid-luteal, premenstrual, or perimenstrual lab visits by adding a corresponding number of cycle days to the detected day of ovulation (see section 5.2). Finally, for arranging menstrual and mid-follicular lab visits, a corresponding number of cycles days should be added to the reported day of menstrual onset (counting method).
8. Summary of Scientific and Reporting Recommendations
In this paper, we have provided recommendations for studying the effects of the human menstrual cycle on outcomes of interest. Here, we summarize the most important points:
Start with a hypothesized mechanism (usually ovarian hormones, neuroactive steroid metabolites) and select phases and design based on that (or use daily ratings)
Consider between-person differences in the sensitivity to the cycle (see section 3)
Measure the cycle as a within-person variable (see section 4.1)
Characterize your sample’s reproductive status (see section 4.4 and Appendix 1: Reproductive Status Questionnaire)
Select the best possible method for cycle phasing given your resources and questions of interest (see section 5)
Model the cycle as a within-person variable that differs in its effects between people (see section 6)
Visualize the full cycle using person-centered variables (see section 6.1)
10. Conclusion
Although the menstrual cycle and its effects on the physiological and psychological functioning of some individuals have been studied for years, we lack standardized guidelines regarding the questions of (1) how to select appropriate study designs, (2) how to collect and characterize study samples, (3) how to determine cycle phases (by means of measuring cycle days, testing LH-surge, assessing BBT, and analyzing hormone levels), and (4) how to statistically model the cycle for hypothesis testing. The present work fills these gaps in the literature by reviewing the current gold standards in the field of menstrual cycle research and by drawing recommendations for future studies. In addition, we present a uniform vocabulary to allow future studies to document their approach in a precise and standardized manner. Regardless of whether the influence of the cycle is of central interest in a study or should be controlled to accurately assess the effects of another variable, following these recommendations will help make study results more meaningful and replicable.
Supplementary Material
Highlights:
We provide guidelines and tools for standardizing menstrual cycle research.
Use a repeated measures design to study the menstrual cycle.
Select your sample of naturally-cycling individuals carefully.
Select the best cycle phasing method given your resources and research question.
Use statistical modeling techniques appropriate for within-person data.
Acknowledgment:
The authors wish to thank Dr. Crystal Schiller for helpful discussions during the early phases of writing this manuscript.
Funding:
This work was partially supported by the German Cusanuswerk, the United States National Institutes of Health [R00MH109667; RF1MH120843; F31-MH120965].
Footnotes
Preprint: This manuscript has been posted to the OSF Preprint server under the preprint doi 10.31219/osf.io/94jua. The preprint can be accessed at https://osf.io/94jua
Declarations of Interest: None.
Throughout the paper, the term female is used in a very limited sense to refer to individuals with at least one functioning ovary who experience a natural menstrual cycle. This is done with a full recognition that “female” refers to a much broader group, but because the focus is on the menstrual cycle, these are the relevant individuals.
References
- Abdalla H, Thum M, 2004. An elevated basal FSH reflects a quantitative rather than qualitative decline of the ovarian reserve. Hum. Reprod 19, 893–898. 10.1093/humrep/deh141. [DOI] [PubMed] [Google Scholar]
- Allen AM, McRae-Clark AL, Carlson S, Saladin ME, Gray KM, Wetherington CL, McKee SA, Allen SS, 2016. Determining menstrual phase in human biobehavioral research: A review with recommendations. Exp. Clin. Psychopharm 24, 1–11. 10.1037/pha0000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association, 2013. Diagnostic and statistical manual of mental disorders: DSM-5. Author, Arlington. 10.1590/s2317-17822013000200017. [DOI] [Google Scholar]
- Andréen L, Nyberg S, Turkmen S, van Wingen G, Fernández G, Bäckström T, 2009. Sex steroid induced negative mood may be explained by the paradoxical effect mediated by GABAA modulators. Psychoneuroendocrinology. 34, 1121–1132. 10.1016/j.psyneuen.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Barbieri RL, 2014. The endocrinology of the menstrual cycle, in: Rosenwaks Z, Wassarman PM (Eds.), Human Fertility: Methods and Protocols. Springer, New York, pp. 145–169. 10.1007/978-1-4939-0659-8. [DOI] [Google Scholar]
- Bertone-Johnson ER, Whitcomb BW, Missmer SA, Manson JE, Hankinson SE, Rich-Edwards JW, 2014. Early life emotional, physical, and sexual abuse and the development of premenstrual syndrome: a longitudinal study. J. Womens Health 23, 729–739. 10.1089/jwh.2013.4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR, 2000. Effects of gonadal steroids in women with a history of postpartum depression. Am. J. Psychiatry 157, 924–930. 10.1176/appi.ajp.157.6.924. [DOI] [PubMed] [Google Scholar]
- Burger HG, Dudley EC, Hopper JL, Groome N, Guthrie JR, Green A, Dennerstein L, 1999. Prospectively measured levels of serum follicle-stimulating hormone, estradiol, and the dimeric inhibins during the menopausal transition in a population-based cohort of women. J. Clin. Endocrinol. Metab 84, 4025–4030. 10.1210/jcem.84.11.6158. [DOI] [PubMed] [Google Scholar]
- Bäckström T, Haage D, Löfgren M, Johansson IM, Strömberg J, Nyberg S, Andréen L, Ossewaarde L, van Wingen GA, Turkmen S, Bengtsson SK, 2011. Paradoxical effects of GABA-A modulators may explain sex steroid induced negative mood symptoms in some persons. Neuroscience. 191, 46–54. 10.1016/j.neuroscience.2011.03.061. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C, 1993. A self-administered rating scale for pubertal development. J. Adolesc. Health 14, 190–195. 10.1016/1054-139X(93)90004-9. [DOI] [PubMed] [Google Scholar]
- Choe JK, Khan-Dawood FS, Yusoff-Dawood M, 1983. Progesterone and estradiol in the saliva and plasma during the menstrual cycle. Am. J. Obstet. Gynecol 147, 557–562. 10.1016/0002-9378(83)90016-9. [DOI] [PubMed] [Google Scholar]
- Coleman E, Bockting W, Botzer M, Cohen-Kettenis P, DeCuypere G, Feldman J, Fraser L, Green J, Knudson G, Meyer WJ, Monstrey S, Adler RK, Brown GR, Devor AH, Ehrbar R, Ettner R, Eyler E, Garofalo R, Karasic DH, Lev, Mayer G, Meyer-Bahlburg H, Hall BP, Pfaefflin F, Rachlin K, Robinson B, Schechter LS, Tangpricha V, van Trotsenburg M, Vitale A, Winter, Whittle S, Wylie KR, Zucker K, 2012. Standards of care for the health of transsexual, transgender, and gender-nonconforming people, version 7. Int.l J. Transgend 13, 165–232. 10.1080/15532739.2011.700873. [DOI] [Google Scholar]
- Colombo B, Masarotto G, 2000. Daily fecundability: first results from a new data base. Demogr. Res 3, 5. 10.4054/DemRes.2000.3.5. [DOI] [PubMed] [Google Scholar]
- Dawood MY, 2006. Primary dysmenorrhea: advances in pathogenesis and management. Obstet. Gynecol 108, 428–441. 10.1097/01.AOG.0000230214.26638.0c. [DOI] [PubMed] [Google Scholar]
- de Mouzon J, Testart J, Lefevre B, Pouly J-L, Frydman R, 1984. Time relationships between basal body temperature and ovulation or plasma progestins. Fertil. Steril 41, 254–259. 10.1016/S0015-0282(16)47600-4. [DOI] [PubMed] [Google Scholar]
- Ecochard R, Boehringer H, Rabilloud M, Marret H, 2001. Chronological aspects of ultrasonic, hormonal, and other indirect indices of ovulation. BJOG. 108, 822–829. 10.1111/j.1471-0528.2001.00194.x. [DOI] [PubMed] [Google Scholar]
- Eisenlohr-Moul TA, DeWall CN, Girdler SS, Segerstrom SC, 2015. Ovarian hormones and borderline personality disorder features: Preliminary evidence for interactive effects of estradiol and progesterone. Biol. Psychol, 109, 37–52. 10.1016/j.biopsycho.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenlohr-Moul TA, Girdler SS, Schmalenberger KM, Dawson DN, Surana P, Johnson JL, Rubinow DR, 2017. Toward the reliable diagnosis of DSM-5 premenstrual dysphoric disorder: the Carolina Premenstrual Assessment Scoring System (C-PASS). Am. J. Psychiatry 174, 51–59. 10.1176/appi.ajp.2016.15121510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenlohr-Moul TA, Kaiser G, Weise C, Schmalenberger KM, Kiesner J, Ditzen B, Kleinstäuber M, 2019. Are there temporal subtypes of premenstrual dysphoric disorder?: Using group-based trajectory modeling to identify individual differences in symptom change. Psychol. Med 50, 964–972. 10.1017/S0033291719000849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenlohr-Moul TA, Owens SA, 2016. Hormones and personality, in: Zeigler-Hill V, Shackelford TK (Eds.), Encyclopedia of Personality and Individual Differences. Springer, Cham, pp. 1–23. [Google Scholar]
- Eisenlohr-Moul TA, Rubinow DR, Schiller CE, Johnson JL, Leserman J, Girdler SS, 2016. Histories of abuse predict stronger within-person covariation of ovarian steroids and mood symptoms in women with menstrually related mood disorder. Psychoneuroendocrinology. 67, 142–152. 10.1016/j.psyneuen.2016.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenlohr-Moul TA, Schmalenberger KM, Owens SA, Peters JR, Dawson DN, Girdler SS, 2018. Perimenstrual exacerbation of symptoms in borderline personality disorder: evidence from multilevel models and the Carolina Premenstrual Assessment Scoring System. Psychol. Med 48, 2085–2095. 10.1017/S0033291718001253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders CK, Tofighi D, 2007. Centering predictor variables in cross-sectional multilevel models: a new look at an old issue. Psychol. Methods 12, 121–138. 10.1037/1082-989X.12.2.121. [DOI] [PubMed] [Google Scholar]
- Epperson CN, Steiner M, Hartlage SA, Eriksson E, Schmidt PJ, Jones I, Yonkers KA, 2012. Premenstrual dysphoric disorder: Evidence for a new category for DSM-5. Am. J. Psychiatry 169, 465–475. 10.1176/appi.ajp.2012.11081302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehring RJ, Schneider M, Raviele K, 2006. Variability in the phases of the menstrual cycle. J. Obstet. Gynecol. Neonatal Nurs 35, 376–384. 10.1111/j.1552-6909.2006.00051.x. [DOI] [PubMed] [Google Scholar]
- Fiers T, Dielen C, Somers S, Kaufman J-M, Gerris J, 2017. Salivary estradiol as a surrogate marker for serum estradiol in assisted reproduction treatment. Clin. Biochem 50, 145–149. 10.1016/j.clinbiochem.2016.09.016. [DOI] [PubMed] [Google Scholar]
- Gehlert S, Song I, Chang C-H, Hartlage S, 2009. The prevalence of premenstrual dysphoric disorder in a randomly selected group of urban and rural women. Psychol. Med 39, 129–136. 10.1017/S003329170800322X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollenberg AL, Hediger ML, Mumford SL, Whitcomb BW, Hovey KM, Wactawski-Wende J, Schisterman EF, 2010. Perceived stress and severity of perimenstrual symptoms: The BioCycle Study. J. Womens Health 19, 959–967. 10.1089/jwh.2009.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JL, Rubinow DR, Eisenlohr-Moul TA, Leserman J, Girdler SS (2016). Estradiol variability, stressful life events and the emergence of depressive symptomatology during the menopause transition. Menopause 23, 257–266. 10.1097/GME.0000000000000528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson E, 2020. A brief guide to the menstrual cycle and oral contraceptive use for researchers in behavioral endocrinology. Horm. Behav 119, 104655. 10.1016/j.yhbeh.2019.104655. [DOI] [PubMed] [Google Scholar]
- Hantsoo L, Epperson CN, 2020. Allopregnanolone in premenstrual dysphoric disorder (PMDD): Evidence for dysregulated sensitivity to GABA-A receptor modulating neuroactive steroids across the menstrual cycle. Neurobiol. Stress 12, 100213. 10.1016/j.ynstr.2020.100213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, Sherman S, Sluss PM, de Villiers TJ, STRAW + 10 Collaborative Group, 2012. Executive summary of the Stages of Reproductive Aging Workshop + 10: Addressing the unfinished agenda of staging reproductive aging. J. Clin. Endocrinol. Metab 97, 1159–1168. 10.1210/jc.2011-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart WG, Coleman GJ, Russell JW, 1987. Assessment of premenstrual symptomatology: A re-evaluation of the predictive validity of self-report. J. Psychosom. Res 31, 185–190. 10.1016/0022-3999(87)90075-4. [DOI] [PubMed] [Google Scholar]
- Hartlage SA, Brandenburg DL, Kravitz HM, 2004. Premenstrual exacerbation of depressive disorders in a community-based sample in the United States. Psychosom. Med 66, 698–706. 10.1097/01.psy.0000138131.92408.b9. [DOI] [PubMed] [Google Scholar]
- Hartlage SA, Freels S, Gotman N, Yonkers K, 2012. Criteria for premenstrual dysphoric disorder: secondary analyses of relevant data sets. Arch. Gen. Psychiatry 69, 300–305. 10.1001/archgenpsychiatry.2011.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahromi BN, Pakmehr S, Hagh-Shenas H, 2011. Work stress, premenstrual syndrome and dysphoric disorder: Are there any associations? Iran. Red Crescent Med. J 13, 199–202. [PMC free article] [PubMed] [Google Scholar]
- Kiesner J, 2011. One woman’s low is another woman’s high: Paradoxical effects of the menstrual cycle. Psychoneuroendocrinology. 36, 68–76. 10.1016/j.psyneuen.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Kiesner J, Granger DA, 2016. A lack of consistent evidence for cortisol dysregulation in premenstrual syndrome/premenstrual dysphoric disorder. Psychoneuroendocrinology, 65, 149–164. 10.1016/j.psyneuen.2015.12.009. [DOI] [PubMed] [Google Scholar]
- Klump K, Keel P, Culbert K, Edler C, 2008. Ovarian hormones and binge eating: Exploring associations in community samples. Psychol. Med 38, 1749–1757. 10.1017/S0033291708002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long WN, 1990. Abnormal vaginal bleeding, in: Walker HK, Hall WD, Hurst JW (Eds.), Clinical Methods: The History, Physical, and Laboratory Examinations, third ed. Butterworths, Boston, chapter 173. [PubMed] [Google Scholar]
- Lovick TA, Guapo VG, Anselmo-Franci JA, Loureiro CM, Faleiros MCM, Del Ben CM, Brandão ML, 2017. A specific profile of luteal phase progesterone is associated with the development of premenstrual symptoms. Psychoneuroendocrinology. 75, 83–90. 10.1016/j.psyneuen.2016.10.024. [DOI] [PubMed] [Google Scholar]
- Martel MM, Eisenlohr-Moul T, Roberts B, 2017. Interactive effects of ovarian steroid hormones on alcohol use and binge drinking across the menstrual cycle. J. Abnorm. Psychol 126, 1104–1113. 10.1037/abn0000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez AR, van Hooff MH, Schoute E, van der Meer M, Broekmans FJ, Hompes PG, 1992. The reliability, acceptability and applications of basal body temperature (BBT) records in the diagnosis and treatment of infertility. Eur. J. Obstet. Gynecol. Reprod. Biol 47, 121–127. 10.1016/0028-2243(92)90041-v. [DOI] [PubMed] [Google Scholar]
- Marván ML, Cortés-Iniestra S, 2001. Women’s beliefs about the prevalence of premenstrual syndrome and biases in recall of premenstrual changes. Health Psychol. 20, 276–280. 10.1037//0278-6133.20.4.276. [DOI] [PubMed] [Google Scholar]
- McNamara A, Moakes K, Aston P, Gavin C, Sterr A, 2014. The importance of the derivative in sex-hormone cycles: A reason why behavioural measures in sex-hormone studies are so mercurial. PloS One, 9, e111891. 10.1371/journal.pone.0111891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeilly AS, 2001. Lactational control of reproduction. Reprod. Fertil. Dev 13, 583–590. 10.1071/rd01056. [DOI] [PubMed] [Google Scholar]
- Moglia ML, Nguyen HV, Chyjek K, Chen KT, Castaño PM, 2016. Evaluation of smartphone menstrual cycle tracking applications using an adapted APPLICATIONS scoring system. Obstet. Gynecol 127, 1153–1160. 10.1097/AOG.0000000000001444. [DOI] [PubMed] [Google Scholar]
- Park SJ, Goldsmith LT, Weiss G, 2002. Age-related changes in the regulation of luteinizing hormone secretion by estrogen in women. Exp. Biol. Med 227, 455–464. 10.1177/153537020222700709. [DOI] [PubMed] [Google Scholar]
- Perkonigg A, Yonkers KA, Pfister H, Lieb R, Wittchen H-U, 2004. Risk factors for premenstrual dysphoric disorder in a community sample of young women: the role of traumatic events and posttraumatic stress disorder. J. Clin. Psychiatry 65, 1314–1322. 10.4088/jcp.v65n1004. [DOI] [PubMed] [Google Scholar]
- Peters JR, Owens SA, Schmalenberger KM, Eisenlohr‐Moul TA, 2020. Differential effects of the menstrual cycle on reactive and proactive aggression in borderline personality disorder. Aggress. Behav 46, 151–161. 10.1002/ab.21877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilver CE, Levy BR, Libby DJ, Desai RA, 2011. Posttraumatic stress disorder and trauma characteristics are correlates of premenstrual dysphoric disorder. Arch. Womens Ment. Health 14, 383–393. 10.1007/s00737-011-0232-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B, Eisenlohr-Moul T, Martel MM, 2018. Reproductive steroids and ADHD symptoms across the menstrual cycle. Psychoneuroendocrinology. 88, 105–114. 10.1016/j.psyneuen.2017.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinow DR, Roy-Byrne P, Hoban MC, Gold PW, Post RM, 1984. Prospective assessment of menstrually related mood disorders. Am. J. Psychiatry 141, 684–686. 10.1176/ajp.141.5.684. [DOI] [PubMed] [Google Scholar]
- Schiller CE, Johnson SL, Abate AC, Schmidt PJ, Rubinow DR, 2016. Reproductive steroid regulation of mood and behavior. Compr. Physiol 6, 1135–1160. 10.1002/cphy.c150014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalenberger KM, Eisenlohr-Moul TA, Jarczok MN, Eckstein M, Schneider E, Brenner IG, Duffy K, Schweizer S, Kiesner J, Thayer JF, Ditzen B (2020). Menstrual cycle changes in vagally-mediated heart rate variability are associated with progesterone: Evidence from two within-person studies. J. Clin. Med 9, 617. 10.3390/jcm9030617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalenberger KM, Eisenlohr-Moul TA, Würth L, Schneider E, Thayer JF, Ditzen B, Jarczok MN, 2019. A systematic review and meta-analysis of within-person changes in cardiac vagal activity across the menstrual cycle: implications for female health and future studies. J. Clin. Med 8, 1946. 10.3390/jcm8111946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PJ, Ben Dor R, Martinez PE, Guerrieri GM, Harsh VL, Thompson K, Koziol DE, Nieman LK, Rubinow DR, 2015. Effects of estradiol withdrawal on mood in women with past perimenopausal depression: A randomized clinical trial. JAMA Psychiatry. 72, 714–726. 10.1001/jamapsychiatry.2015.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PJ, Martinez PE, Nieman LK, Koziol DE, Thompson KD, Schenkel L, Wakim PG, Rubinow DR, 2017. Premenstrual dysphoric disorder symptoms following ovarian suppression: Triggered by change in ovarian steroid levels but not continuous stable levels. Am. J. Psychiatry 174, 980–989. 10.1176/appi.ajp.2017.16101113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR, 1998. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N. Engl. J. Med 338, 209–216. 10.1056/NEJM199801223380401. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Nieman LK, Grover GN, Muller KL, Merriam GR, Rubinow DR, 1991. Lack of effect of induced menses on symptoms in women with premenstrual syndrome. N. Engl. J. Med 324, 1174–1179. 10.1056/NEJM199104253241705. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Dahl RE, Pollak SD, 2009. Pubertal development: Correspondence between hormonal and physical development. Child Dev. 80, 327–337. 10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JD, Willett JB, 2003. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. Oxford University Press, New York. [Google Scholar]
- Tenan MS, Peng Y-L, Hackney AC, Griffin L, 2013. Menstrual cycle mediates vastus medialis and vastus medialis oblique muscle activity. Med. Sci. Sports Exerc 45, 2151–2157. 10.1249/MSS.0b013e318299a69d. [DOI] [PubMed] [Google Scholar]
- Timby E, Bäckström T, Nyberg S, Stenlund H, Wihlbäck A-CN, Bixo M, 2016. Women with premenstrual dysphoric disorder have altered sensitivity to allopregnanolone over the menstrual cycle compared to controls—a pilot study. Psychopharmacology. 233, 2109–2117. 10.1007/s00213-016-4258-1. [DOI] [PubMed] [Google Scholar]
- Tivis LJ, Richardson MD, Peddi E, Arjmandi B, 2005. Saliva versus serum estradiol: implications for research studies using postmenopausal women. Prog. Neuropsychopharmacol. Biol. Psychiatry 29, 727–732. 10.1016/j.pnpbp.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Wang M, Seippel L, Purdy RH, Bäckström T, 1996. Relationship between symptom severity and steroid variation in women with premenstrual syndrome: study on serum pregnenolone, pregnenolone sulfate, 5 alpha-pregnane-3, 20-dione and 3 alpha-hydroxy-5 alpha-pregnan-20-one. J. Clin. Endocrinol. Metab 81, 1076–1082. 10.1210/jcem.81.3.8772579. [DOI] [PubMed] [Google Scholar]
- Wei S-M, Schiller CE, Schmidt PJ, Rubinow DR, 2018. The role of ovarian steroids in affective disorders. Curr. Opin. Behav. Sci 23, 103–112. 10.1016/j.cobeha.2018.04.013. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


