Abstract
As we age, sleep patterns undergo severe modifications of their micro and macrostructure, with an overall lighter and more fragmented sleep structure. In general, interventions targeting sleep represent an excellent opportunity not only to maintain life quality in the healthy aging population, but also to enhance cognitive performance and, when pathology arises, to potentially prevent/slow down conversion from e.g. Mild Cognitive Impairment (MCI) to Alzheimer’s Disease (AD). Sleep abnormalities are, in fact, one of the earliest recognizable biomarkers of dementia, being also partially responsible for a cascade of cortical events that worsen dementia pathophysiology, including impaired clearance systems leading to build-up of extracellular amyloid-β (Aβ) peptide and intracellular hyperphosphorylated tau proteins. In this context, Noninvasive Brain Stimulation (NiBS) techniques, such as transcranial electrical stimulation (tES) and transcranial magnetic stimulation (TMS), may help investigate the neural substrates of sleep, identify sleep-related pathology biomarkers, and ultimately help patients and healthy elderly individuals to restore sleep quality and cognitive performance. However, brain stimulation applications during sleep have so far not been fully investigated in healthy elderly cohorts, nor tested in AD patients or other related dementias. The manuscript discusses the role of sleep in normal and pathological aging, reviewing available evidence of NiBS applications during both wakefulness and sleep in healthy elderly individuals as well as in MCI/AD patients. Rationale and details for potential future brain stimulation studies targeting sleep alterations in the aging brain are discussed, including enhancement of cognitive performance, overall quality of life as well as protein clearance.
1. INTRODUCTION
Growing older is a cause of both deficits in cognition and a reduced ability to initiate and maintain sleep (Ohayon et al., 2004). The most common aging-related sleep biomarkers include a pattern characterized by light and fragmented nighttime sleep, less slow cortical oscillations, and a drop of time spent in deeper sleep stages (N3; Ohayon et al., 2004). Sleep features like sleep spindles and K-complexes also demonstrate a dramatic fall in frequency and amplitude (Ohayon et al., 2004). This modified sleep pattern, involving a lack of deep sleep and disrupted circadian rhythms, is commonly recognized as an aging-related marker. Importantly, sleep disruptions have been identified as one of the earliest potential biomarkers of progression to dementia (Mander et al., 2016).
Alzheimer’s Disease (AD) is a progressive neurodegenerative disorder characterized by multi-domain cognitive impairment, ranging from short-term memory decline to speech difficulties, loss of behavioral control and loss of spatial and temporal orientation (Lane et al., 2018). Extracellular accumulation of amyloid-β (Aβ) peptide and intracellular aggregation of hyperphosphorylated tau proteins concur to AD neuro-pathogenesis and clinical symptoms (Ancoli-Israel et al., 2005; Liguori et al., 2014; Lim et al., 2013; Spira et al., 2013). Aβ and tau-proteins respectively clump in amyloid plaques and neurofibrillary tangles, which ultimately trigger a cascade of effects that leads to multidomain cognitive impairments (Lane et al., 2018). Nowadays, AD represents the most common form of dementia, affecting 3.9% of elderly individuals worldwide (Calderon-Garcidueñas and Duyckaerts, 2018), accounting for more than 72% of dementia cases (Reitz and Mayeux, 2014). Before a formal diagnosis, patients might present an intermediate stage called Mild Cognitive Impairment (MCI), associated with cognitive deficiencies that are however not severe enough to cause impairment in the Activities of Daily Living (ADL; Eshkoor et al., 2015). Interestingly, sleep abnormalities are considered early biomarkers of healthy elderly individuals at risk of developing MCI/AD. In fact, MCI/AD patients are more likely to show disrupted day–night activity patterns, even in early stages of the disease and/or before the clinical onset. For instance, a more accentuated drop in time spent in deeper stage of sleep (i.e. N3) is observed in early stage AD patients compared to healthy elderly individuals (Westerberg et al., 2012). Furthermore, AD patients also display additional sleep alterations, such as reduction of Rapid Eye Movement (REM) sleep (Prinz et al., 1982). Furthermore, a lack of deep sleep, together with fragmented and delayed circadian rhythms, slows down protein clearance mechanisms (Lim et al., 2013), favoring the consequent increase in Aβ levels that characterizes AD neuropathologic progression (Spira et al., 2013).
Therefore, early interventions targeting sleep disruptions may help avoiding, or at least slowing, conversion to dementia in vulnerable elderly individuals. Currently available experimental treatments for MCI/AD include pharmacological medications, diet, Bright Light Therapy, and Auditory Stimulation. Nevertheless, contrasting results have so far been reported. As such, there is a strong need for innovative treatment interventions.
Recently, a few studies have explored the possibility of treating healthy elderly and MCI/AD patients with Noninvasive Brain Stimulation (NiBS) techniques to restore sleep quality and preserve or enhance physiologically-declining cognitive functions in healthy older population. It has been theorized that the promotion of slow waves during NREM stage in the elderly population may also have a protective effect on the risk of developing AD by enhancing Aβ clearance(Xie et al., 2013). Furthermore, the up-regulation of slow waves may restore damage to proteins caused by oxidative stress, as demonstrated in animal models (Everson, 2014). Evidence highlights that the amplitude and duration of SWS during the NREM stage are both important for the long-term consolidation of newly acquired memories, suggesting that sleep enhancement would slow the decline in cognitive abilities in healthy elderly individuals.
In this review, we will first introduce general notion on sleep patterns in healthy elderly individuals as well as how these mechanisms undergo biological changes related to aging. Then, we discuss the bidirectional link between sleep modifications, protein accumulation, and AD pathology. We will follow by providing a detailed overview of the most common NiBS techniques and protocols. Particular interest will be paid to studies which have made use of NiBS during sleep in healthy and pathological aging population, either to quantify sleep quality or to manipulate brain oscillations to improve cognitive performance.
2. SLEEP MODIFICATIONS IN HEALTHY AGING
As we get older, substantial modifications of sleep macro and microstructure are part of the normal aging process. Sleep in the elderly population is worsened in both length and internal structure (Ohayon et al., 2004). The whole circadian rhythm decreases in amplitude and shows an early phase-shift (Monk, 2005). In fact, middle and older aged individuals present higher subjective sleepiness ratings during the late afternoon and early evening compared to younger subjects (Münch et al., 2005). Even so, the sleep-onset latency (time required to fall asleep) is almost twice as long as older than younger participants (Crowley, 2002), and after falling asleep elderly show a disrupted and shorter sleep pattern. Total Sleep Time (TST) lowers from around 7.4h in early life to 5.6h in older age (Crowley, 2002). As a consequence, regular diurnal napping increases, growing 25% after the 75th year, therefore worsening the cycle shift (Foley et al., 2007). During nighttime elderly individuals usually spend twice as much overnight time in unwanted wakefulness (Bliwise, 1993), drastically dropping sleep efficiency. Frequent arousals are a result of different concurrent factors. First, elderly individuals are more sensitive to the external environment, showing a lower arousal threshold to auditory stimuli (Zepelin et al., 1984). Furthermore, once sleep is interrupted, the transition from sleep to a fully awake state occurs more rapidly in older subjects without a subsequent increase of sleepiness, thereby delaying the start of sleep again (Dement et al., 1985).
The internal sleep structure is also disrupted. REM-NREM cycles are shorter and fewer, with a mean of 3.46 cycles per night in elderly individuals compared to the usual 4–5 in adults (Conte et al., 2014). Singularly, while time spent in the REM stage overnight seems to slightly decrease (Ohayon et al., 2004; Redline et al., 2004), this drop is not as noticeable as in N3 (also called slow-wave sleep or SWS) stage. In fact, the strongest age biomarker is a steady drop of time spent in N3 stage, as proved by a wide meta-analysis and different studies on great cohorts of participants (Bliwise, 1993; Cauter et al., 2000; Ohayon et al., 2004; Redline et al., 2004). N3 is then replaced by a greater amount in lighter phases, NREM1 (N1) and NREM2 (N2; Bliwise, 1993; Cauter et al., 2000; Ohayon et al., 2004; Redline et al., 2004).
The increase of time in lighter phases is greater in N1 than N2, but this latter undergoes more changes in its typical EEG features, with a decrement of sleep spindles (transient oscillations occurring at 12–15Hz playing a role in memory consolidation) and both spontaneous and elicited K-complexes (Kc, singular waveforms of about 0.5 seconds involved in preventing sleep fragmentation; Conte et al., 2014; Clawson et al., 2016; Halasz, 2005). Sleep spindles (SS) drop, in particular, is due to a substantial change in their form and occurrence. With advancing age, spindles undergo a constant decrement in density, amplitude, and duration (Carrier et al., 2011; Crowley, 2011, 2002). Disruptions were particularly marked during the last cycles (De Gennaro and Ferrara, 2003) and in anterior derivations, whereas duration changes were maximal in the posterior prominence (Martin et al., 2013), a sign of a differential propagation through the cortical network in the elderly. These alterations and SS role in sleep protection may explain difficulties experienced by elderly individuals in maintaining sleep during lighter NREM stages (Dang-Vu et al., 2011). Similarly, N3 modification is not only in its length but also in its EEG microstructure. A drop in total Slow Wave Activity (SWA; spectral power density from 0.5 to 4.5Hz; or delta activity), is a pivotal biomarker of aging and is used to test sleep quality and homeostatic sleep pressure (Bliwise, 1993; Cauter et al., 2000; Feinberg et al., 1967; Landolt et al., 1996). Similarly to SS, for elderly individuals, SWA fall is maximal over the prefrontal cortex (Carrier et al., 2011; Landolt et al., 1996).
To aggravate brain aging and consequent sleep difficulties, elderly individuals are also at higher risk of suffering from primary sleep disorders, such as insomnia and sleep-disordered breathing (SDB). Insomnia, diagnosed in case of disrupted sleep-wake cycle or difficulty falling asleep with a consequent impairment in daytime functioning (Diagnostic Classification Steering Committee TMJC; 2014), shows a prevalence that varies significantly between younger and older populations, reaching 40% in over 65 y/o individuals (Foley et al., 1995). The second most common sleep disorder in the older population is SDB, which includes a spectrum of respiratory disorders occurring during sleep with various severity, from benign snoring to obstructive sleep apnea (OSA). The latter is particularly insidious because of airflow cessation due to complete or partial collapse of upper airways. Similarly, to insomnia, SDB has a higher prevalence in the elderly. In a randomly selected cohort of 427 participants, 62% in the range age 65–95 showed an SDB (Ancoli-Israel et al., 1991). When diagnosed with SDB (e.g. OSA), elderly individuals also suffer significantly more than their younger counterparts with the same diagnosis. The influence of OSA on sleep microstructure is, in fact, more disruptive. Less delta activity is observed in EEG recordings of young OSA patients compared with older OSA patients, while the latter shows more theta, sigma, and alpha power (Lee et al., 2016).
3. AGE-RELATED BRAIN CHANGES LEADING TO SLEEP ALTERATIONS
Age-related sleep, as well as cognition, motor, and living skills, is modified by dramatic alterations at many different levels of the brain, including extracellular changes, cortical atrophy, and neuronal loss. Once the pattern of sleep changes, it creates a cascade of consequences, establishing a positive feedback cycle that often feeds itself. We address the main modifications that happen in the aging brain, linking them to specific sleep disruptions in elderly individuals.
One of the most characteristic biomarkers of the aging brain is the neuronal loss resulting in cortical volume fallout. It is marked and replicable (Courchesne et al., 2000; Pfefferbaum et al., 1994; Resnick et al., 2003; Thambisetty et al., 2010), measured through different parameters such as total volume of the brain, cerebrospinal fluid (CSF), gray and white matter, cortical thickness, surface area, cell loss, and neuronal shrinkage (for a comprehensive review see Sowell et al., 2004). The age-related deterioration in white matter is less marked than abnormalities in cell bodies. In older individuals (age 70–80) the former is decreased by 13%, versus 26% for the latter (Courchesne et al., 2000). Whereas white matter decrease is widespread among the whole brain, gray matter deterioration is significantly higher in the frontal and parietal lobes (Resnick et al., 2003; Thambisetty et al., 2010). White matter also starts to shrink considerably later. While gray matter starts to decrease in volume at the end of childhood, white matter does not reach its volume plateau until the 4th decade, and only after it starts declining in volume (Courchesne et al., 2000), as is demonstrated by studies using diffusion tensor imaging (DTI; Westlye et al., 2009).
Because SW requires a large number of neurons to propagate, SWA disruptions may be a result of shrinking in specific regions (Mander et al., 2016), such as lateral and medial prefrontal neuronal loss. Multiple studies showed how a decrement in SW amplitude is predicted by neuronal loss in the medial PFC and the middle frontal gyrus, while impairment in density are correlated with deterioration of the areas surrounding the lateral fissure (insula, superior temporal, parietal and middle frontal gyrus (Dube et al., 2015; Mander et al., 2013). The prefrontal area is also impaired in its functionality, with a lower resting metabolic rate in older individuals compared to younger ones (Kakimoto et al., 2016). A lack of cognitive activity in the elderly, and a subsequent modification in synaptic plasticity, is also a causal factor in SWA decline. SW requires not only large populations of healthy neurons able to produce oscillations but also efficient synaptic connections among them (Esser et al., 2007). Even a rapid increase or decrease in synaptic strength (in number or efficacy) during awake states will, therefore, reflect a parallel subsequent enhancement or fall in SWA. After wakefulness, slow oscillations renormalize neural activity in N3, promoting synaptic depression thanks to low levels of norepinephrine, serotonin, and acetylcholine (for a review see Hanlon et al., 2011). More synapses are potentiated during wakefulness due to learning and motor tasks, and greater SWA is required during subsequent sleep to balance plasticity. An age-related drop in brain plasticity and a lack of cognitive activity in the elderly will then require less SWA.
Circadian rhythms are regulated by a complicated network within the brain stem and hypothalamic nuclei, which is also affected by age on many levels (for a comprehensive review Rolls, 2012). Simplifying this intricate interrelationship the wake-promoting lateral hypothalamic area (LHA) and locus coeruleus (LC) help to maintain stable periods of wakefulness, while the preoptic area (POA) modulates LHA and LC function, sending inhibitory input to initiate and maintain sleep (Saper et al., 2010). The hypothalamic suprachiasmatic nucleus (SCN) is the endogenous clock promoting wakefulness during the day and permitting sleep (Mander et al., 2016; Wang et al., 2015). Age-related neuronal loss strikes these nuclei differently, disrupting the balance in sleep and wakefulness. The SCN age-related modifications involve alterations in neuronal network function, membrane properties, and modifications of components in cellular nuclei (for a complete review see Farajnia et al., 2014). Examinations post mortem showed a decrement in SCN volume and cell number in the elderly (Swaab et al., 1985; Zhou, 1995). Specific neuronal subpopulations are particularly affected by this shrinking, including those expressing vasoactive intestinal peptide (VIP), receiving direct light input from the retina, maintaining synchronicity between endogenous rhythms and the external light-dark cycle (Farajnia et al., 2014). Loss of VIP-ergic neurons would lead to a lower influence from light to internal rhythms, leading to reduced circadian control from SCN (Wang et al., 2015), and therefore advanced phase shift, greater nighttime movements, and an increased number of awakenings. Impairment in SCN function also reflects a desynchronized melatonin secretion cycle.
Melatonin cyclical concentrations are indeed controlled by the SCN via a multisynaptic pathway (for a comprehensive review see Benarroch, 2008). The peak in endogenous melatonin is 2 hours before habitual bedtime and is correlated with the onset of evening sleepiness. The aging process attenuates melatonin secretion at night in older individuals, while diurnal secretion is similar in elderly and young subjects (Cooke and Ancoli-Israel, 2011; Copinschi and Caufriez, 2013; Münch et al., 2005). Its overnight decrement is linked to nocturnal light exposure in the elderly, mediated by the SNC. In fact, light exposure during the night results in a dose-dependent suppression of melatonin secretion (Benarroch, 2008). By contrast, insufficient daytime light exposure, often seen in the elderly due to social isolation staying home-bound, elicits a desynchronization of the melatonin secretion cycle (Mishima et al., 2001), concurrent with a shift in the sleep-wake cycle.
The LHA is involved in wake-promoting thanks to neurons expressing hypocretin and orexin, with connections to other nuclei of the ascending brainstem arousal system. This structure, together with the LC, collectively maintains stable wake states (Saper et al., 2010). Both undergo a reduction of their neuronal subpopulations during the aging process. Elderly individuals show a 10% loss in the number of LHA hypocretin-orexin neurons (Hunt et al., 2015) and a significant neuronal reduction in LC volume (Sasaki et al., 2006). Deterioration in these two structures is partially responsible for fragmentation and fragility of sleep, higher frequency arousal, and greater sleepiness during awake states in the elderly. The POA is formed by cells expressing inhibitory neuropeptide galanin (Saper et al., 2010), which undergoes a significant decline in aging. This results in abnormalities in overnight sleep consolidation. In fact, the degree of cell loss in the POA predicts the severity of sleep fragmentation in older adults (Lim et al., 2014).
4. SLEEP AND COGNITION IN NON-PATHOLOGICAL AGING
As we age substantial changes also occur in various cognitive domains. Several studies tried to determine whether modifications in SWS, REM, and SS in aging directly lead to a general intellective decrease, or if changes in cognition and sleep share a common source, such as reduced brain volume, or cardiovascular, or neurochemical changes (for a review see Scullin and Bliwise, 2015). Many paradigms have studied how sleep deprivation influences subsequent cognitive performance in healthy elderly individuals. Surprisingly, after one or more nights of total sleep deprivation and sleep restriction, elderly people show less impaired cognitive performance during normal waking hours compared to younger adults (Bliese et al., 2007; Bonnet, 1989; Bonnet and Arand, 1989). The older group also presents faster reaction times and fewer lapses in tasks presented during normal sleeping hours (Duffy et al., 1998). Recent studies of experimentally altered circadian phase in subjects of different ages reported maintained reaction times in older versus younger individuals (Donnell et al., 2009; Silva et al., 2010). Therefore, even though sleep disruptions are far more marked in older adults than in younger adults, the former appears to be more resistant to the cognitive effects of sleep deprivation than younger controls. On the other hand, elderly people with self-reported insomnia presented a greater cognitive decline at a 3-year follow-up, underlining how long-term poor quality sleep is strongly associated with a greater risk of cognitive deficits (Cricco et al., 2001).
The main link between cognition and sleep has been provided by memory consolidation, the process that strengthens mnemonic traces. Sleep-dependent memory consolidation has been investigated by a wide range of learning protocols and results have been extensively reviewed (Diekelmann et al., 2009). NREM sleep plays the main role as the promoter of conversion of episodic representations from a labile hippocampal-dependent state to an increasingly hippocampal-independent one (Diekelmann et al., 2009). In fact, in young adults, SWS and delta power predict consolidation of episodic memories (Diekelmann et al., 2009). Reduction in NREM features due to aging would result in impoverished hippocampal memory consolidation, and therefore predict a subsequent decline in memory retention, as established in various studies, not only in healthy elderly people (Aly and Moscovitch, 2010; Backhaus et al., 2007; Giambra and Arenberg, 1993; Mary et al., 2013) but also in Mild Cognitive Impairment (MCI) patients (Westerberg et al., 2012). Due to aging, brain areas related to memory, such as the hippocampus and prefrontal cortex, undergo functional, neurochemical, and structural changes (Kalpouzos et al., 2009; Mander et al., 2013; Mueller and Weiner, 2009). Consequently, even with adequate sleep, memories would not be consolidated as efficiently in older adults (Harand et al., 2012; Pace-Schott and Spencer, 2011; Scullin, 2013; Scullin and Bliwise, 2015).
A study by Mander et al. (2015) demonstrated how age-related atrophy of mPFC predicts SW drop, as well as long-term episodic memory impairment (Mander et al., 2013). To complicate the situation, other studies report negative correlations between memory and SWS quantity (Feinberg et al., 1967; Scullin, 2013; Seeck-Hirschner et al., 2012) leaving the question open. Results may be cohesively explained by drawing a distinction between N3 quantity and N3 quality (measured by the density of SWA). The application of various paradigms of transcranial electrical stimulation (tES) has been applied as an innovative and efficient tool to investigate and manipulate this link between SWA and subsequent memory performance (see dedicated paragraph).
5. SLEEP DISRUPTIONS IN ALZHEIMER’S DISEASE
Typical age-associated sleep difficulties and disorders worsen life quality in the older population resulting in great distress for subjects and their caregivers. Drastic sleep disruptions are also a concurrent factor in accelerating and precipitate clinical symptoms and prognosis of a wide range of medical conditions, such as cardiovascular disease, arthritis, gastroesophageal reflux, and psychiatric comorbidities, including depression and anxiety. In particular, an abnormal sleep pattern is a major risk factor for developing Mild Cognitive Impairment (MCI), which may convert to Alzheimer’s Disease (AD). MCI identifies a spectrum of disease that describes an intermediate stage from normal cognitive function to dementia, involving cognitive complaints and deficits associated with minimal or no effect on complex daily activities (Roberts and Knopman, 2013), while AD is a chronic neurodegenerative disease characterized by extracellular accumulation of Aβ peptide and intracellular aggregation of hyperphosphorylated tau.
Drastic sleep modifications characterizing non-pathological aging represent a great risk factor for dementia. Studies on community-based populations have highlighted the link between delay and shrinkage of sleep-wake cycles (as measured by actigraphy) and the odds of developing AD (Tranah et al., 2011). General sleep fragmentation occurring with aging is associated with a 1.5-fold increased risk of developing dementia in a 6-year follow-up period (Lim et al., 2013). Excessive daytime sleepiness (EDS) itself has also been linked with twice the risk of developing dementia in a longitudinal cohort study (Foley et al., 2001). General sleep patterns are far more disrupted in AD patients compared to age-matched controls (Vitiello and Prinz, 1989). Specifically, AD patients show an increased number and duration of awakenings that lead to longer time spent in light sleep stages and daytime naps (Hatfield et al., 2004). NREM sleep keeps decreasing after the onset of AD and all along with the neurodegenerative progression (Loewenstein et al., 1982). Indeed, MCI patients already present a more accentuated decrease in the time spent in NREM related to age-matched controls (Westerberg et al., 2012). Decreased nighttime sleep efficiency in these patients is accompanied by marked daily EDS (Holth et al., 2017). Extreme sleep fragmentation is also observed in the sundown syndrome, associated with irritability, anxiety, agitation, and confusion happening from late afternoon to night time. Sleep pattern deteriorations similar to AD are also present in healthy elderly at higher biological risk for developing dementia, such as ApoE4 allele carriers (Hita-Yañez et al., 2013).
N3 sleep in AD is characterized by diminished SWA (Prinz et al., 1982). During N2 sleep, AD patients present a 40% reduction in Kc density (De Gennaro et al., 2017). While normal aging is associated with a decrease in both evoked and spontaneous Kc, AD patients show mainly a decrease of the latter over frontal sites (Montplaisir et al., 1995; Prinz et al., 1982). Kc drop is positively correlated with cognitive decline in AD, tested with the Mini Mental State Examination (MMSE; De Gennaro et al., 2017). SS also shows a significant drop in AD (Bliwise, 1993; Prinz et al., 1982), accentuated for fast parietal spindle density (Westerberg et al., 2012b). This modification has been linked with impaired immediate episodic recall in AD patients (Rauchs et al., 2008). The deterioration of sleep spindles seen in MCI patients (Gorgoni et al., 2016) may be an early biomarker of a preclinical stage of dementia.
REM sleep shows a significant decrease in AD patients compared to normal controls, worsening along with clinical progression (Prinz et al., 1982). This change is due to shorter REM episodes, though no effect is seen on the total number of episodes (Petit et al., 2004). Other REM features, like muscle atonia and phasic EMG activity, also remain unchanged (Montplaisir et al., 1995). REM sleep onset is generally delayed, and the rebound of REM sleep following selective deprivation is blunted (Prinz et al., 1982; Reynolds et al., 1990). Similar to NREM, REM sleep time is highly predictive of cognitive impairment in both MCI patients and healthy elderly controls, showing a strong link with individuals’ MMSE score (Liguori et al., 2014). Higher-frequency EEG components, such as alpha, beta, and gamma frequencies, have been observed to progressively shift toward slower frequency components proportionally with the severity of the disease (for a review Cassani et al., 2018; Huang et al., 2000). Overall EEG slowing appears imputable, at least in part, to the diffusion of SW and theta waves during awake states and REM stage (Bliwise, 1993b; Hassainia et al., 1997; Peter-Derex et al., 2015; Xie et al., 2013), mostly in temporoparietal and frontal regions (Petit et al., 2004).
Almost two-thirds of MCI/AD patients display symptoms of at least one sleep disorder, often Sleep Disordered Breathing (SDB) or Insomnia (Guarnieri et al., 2012). Successful treatment of sleep disorders might act in delaying AD conversion in MCI patients (Osorio et al., 2015) and even improve cognitive functions in severe stages of AD (Moraes et al., 2006). The incidence of obstructive sleep apnea syndrome (OSAS) increases with age, with higher risks for AD patients (Hoch et al., 1986; Reynolds et al., 1985). Those who present OSAS show more disrupted sleep patterns, characterized by a greater number of awakening episodes and less REM and SWS compared to AD patients without OSAS (Cooke et al., 2006). Some studies have also found a positive correlation between the severity of dementia and SDB, both showing progressive linear worsening (Ancoli-Israel et al., 1991).
6. SLEEP, AGING, AND PROTEIN CLEARANCE
Sleep abnormalities that occur very early in the prodromal phase of dementia accelerate AD pathogenesis through Aβ and tau proteins accumulation (Ju et al., 2014; Lucey et al., 2017; Lucey and Bateman, 2014). The potential link between sleep alterations, amyloid-β, and tau accumulation has been already extensively described in healthy elderly (for a review, see Holth et al., 2017). Other studies have stressed the correlation between different subjective and objective sleep measures and various typical AD features, such as cortical Aβ burden, cerebrospinal fluid (CSF) measures of Aβ, and phospohorylated tau (Ancoli-Israel et al., 2005; Liguori et al., 2014; Lim et al., 2013; Mander et al., 2015; Spira et al., 2013; Moraes et al., 2006). Brown and coworkers (2016) investigated Aβ burden by using Positron Emission Tomography (PET) with three different amyloid-binding ligands in 184 healthy elderly, together with measures of subjective sleep factors derived from the Pittsburgh Sleep Quality Index. Longer sleep latency was highly associated with higher neocortical Aβ levels, although sleep duration, sleep efficiency, and daytime sleepiness was not. With a similar paradigm, but in a smaller cohort (51 healthy elderly), Branger and coworkers (2016) supported Brown et al.’s findings linking Aβ levels in prefrontal regions with longer sleep latency, but not with other sleep quality factors. Aβ concentration in the angular gyrus, frontal medial orbital cortex, cingulate gyrus, and precuneus has also been shown to highly correlate with self-reported poor sleep quality and daytime sleepiness in healthy elderly (Sprecher et al., 2017). Studies, where NREM sleep was modulated in healthy, middle-aged participants, have confirmed that SWA suppression increases CSF Aβ levels in the subsequent morning (Ju et al., 2017). CSF levels of tau and Aβ protein predicted the degree of diminished SWA time in AD patients, together with the decrease in sleep efficiency and REM time (Liguori et al., 2014). Aβ effects on NREM neurophysiology have been described in terms of higher levels of Aβ correlating with deficient SWA and subsequent impairment in sleep-dependent memory (Mander et al., 2015; Varga et al., 2016). Gray matter atrophy in healthy elderly subjects was found to be correlated with age-related decline in SWA between a range of 1 to 4Hz (Mander et al., 2013). Differently, Aβ modifications in AD were mainly linked to changes in <1Hz SWA (Mander et al., 2015).
Animal studies also support the existence of a bidirectional link between sleep and AD, for which rodent AD models display increased sleep fragmentation and shorter NREM stages (Guarnieri et al., 2012). Experimentally increasing cortical Aβ has been reported to cause fragmentation of NREM sleep (Kang et al., 2009). Both the lack of sleep and pattern disruptions contribute to Aβ aggregation, which itself impairs SWS causing a vicious cycle of detrimental events (Ju et al., 2014). A few studies have suggested a major sleep-dependent role of the glymphatic system in dictating Aβ clearance (Xie et al., 2013). During NREM stages, glial cells shrink almost 60%, enabling CSF flow through the interstitial space. Clearance is therefore enhanced, removing extracellular toxins and metabolic components, including extracellular Aβ. Proteins are vacated twice as fast during SWS compared to wakefulness (Kang et al., 2009; Xie et al., 2013). In fact, because wakefulness requires a higher neurometabolic rate relative to NREM (Buchsbaum et al., 1989), longer time spent without sleep directly impacts Aβ clearance. During awake states, neurons consume greater levels of oxygen and Adenosine TriPhosphate (ATP). NREM not only requires less oxygen and glucose consumption, but it is also responsible for ATP replenishment (Dworak et al., 2010). Amplified neurometabolic activity during wakefulness results in increased amyloid precursor protein (APP) production, triggering Aβ accumulation due to oxidative stress (Misonou et al., 2000). NREM, on the other side, actively regulates oxidative stress and promotes cellular repair (Everson, 2014b; Villafuerte et al., 2015). Moreover, chronic sleep disruptions and OSAS increase blood vessel stiffness by triggering chronic hypertension (Kyrtsos and Baras, 2015). This, combined with cerebral amyloid-β angiopathy (Weller et al., 2009), reduces clearance efficiency, thereby favoring the accumulation of extracellular Aβ. Therefore, when wakeful metabolic distress is not balanced by sufficient sleep, a cascade of neurotoxic and oxidative events promotes AD pathophysiology. This has been proven in multiple animal trials (Everson, 2014; Villafuerte et al., 2015). Tabuchi (2015) has suggested that changes in neuronal excitability underlie the effects of sleep loss on AD pathogenesis. Aβ accumulation due to sleep deprivation was successfully blocked by actively decreasing intrinsic cortical excitability in a drosophila AD model. Conversely, increasing cortical excitability phenocopied the effects of sleep reduction on Aβ. Through action specific potassium (K+) currents, sleep loss exacerbates Aβ–induced hyperexcitability. Wakefulness neuronal activity also enable AD neurodegeneration due to wake-promoting peptide named orexin A/hypocretin-1 (Kang et al., 2009). Specifically, in transgenic mice that overexpress amyloid precursor protein (APP), Aβ level increases during wakefulness and after orexin-A infusion while it decreases during sleep and after infusion of an orexin-A receptor antagonist (Kang et al., 2009). In APP mice whose orexin gene is also knocked out, Aβ brain load is decreased and sleep time is increased (Roh et al., 2014). Moreover, sleep deprivation by the rescue of orexinergic neurons in APP mice increases the levels of cortical Aβ (Roh et al., 2014). Similarly, higher CSF orexin-A levels were observed in patients with AD compared with controls (Dauvilliers et al., 2014; Liguori et al., 2014; Wennström et al., 2012). CSF orexin-A levels are also correlated with tau protein levels and sleep-wake alterations in patients with AD (Liguori et al., 2014). Because orexin levels may directly influence amyloid clearance, Gabelle and coworkers (2019) collected PET images, Aβ load, CSF orexin-A level, and cognitive profile of 23 subjects older than 65 years with a diagnosis of narcolepsy type 1 (NT1). NT1 is a chronic disease, characterized by EDS and cataplexy, and caused by destruction of orexin neurons. Because it is usually diagnosed around 16–20 years of age, individuals without orexin influence on Aβ accumulation since young age, may be partially protected by AD risk. The study, in fact, showed lower cortical amyloid burden obtained with all subcortical and cortical reference regions (except cingulum) in NT1 subjects compared to age and sex matched controls. This delayed appearance of amyloid claque in narcoleptic patients proves orexin as one of the influencers in Aβ load.
In the last years, new studies are currently shifting the focus of AD research onto REM sleep. Indeed, MCI/AD patients, in contrast to healthy elderly patients, demonstrate REM abnormalities in overall time spent in this stage, quality, and EEG features (Petit et al., 2004; Prinz et al., 1982; Reynolds et al., 1990). A reduction in REM time is also positively correlated with Aβ levels (Kerbler et al., 2014), presumably mediated by degeneration of cholinergic projection neurons within the brainstem and basal forebrain (Montplaisir et al., 1998).
7. NETWORK MODIFICATIONS DURING SLEEP IN HEALTHY AND PATHOLOGICAL AGING
The human brain is organized in a set of functional networks whose synchronized activity span between spatially distinct interconnected areas (as measured via functional connectivity, FC, analysis using fMRI). These so-called resting-state networks (RSNs) include regions responsible for both sensory processing as well as for high order cognition, with the main networks being the default mode (DMN), executive control, sensorimotor, attention, salience, and visual network (Damoiseaux et al., 2006; Heuvel et al., 2009; Zuo et al., 2010). The DMN is particularly relevant due to its pivotal role in the spontaneous activity of the human brain. Anatomical regions belonging to the DMN include inferior parietal lobules, precuneus, posterior cingulate cortex, medial frontal cortices, lateral-medial temporal cortices, and the hippocampi (Kernbach et al., 2018). DMN plays a role in memory consolidation, mental imagery, internal dialogue and maintenance of long-term memory (Buckner et al., 2008; Uddin et al., 2009). Furthermore, its oscillatory activity is negatively correlated with other resting-state networks and has been promoted as a marker of healthy cognitive functioning and healthy aging (Santarnecchi et al., 2017; R. Nathan Spreng et al., 2016).
As for what concerns the relationship between network alterations and sleep, studies have revealed how FC between DMN regions increases in SWS and decreases in REM stages. This pattern is also similar to hippocampal activity, which is tightly related to long-term memory consolidation and retention (Diekelmann et al., 2011; Diekelmann and Born, 2010; Grosmark et al., 2012). DMN’s activity changes during the SWS periods have been found to be negatively correlated with those during the REM period (Watanabe et al., 2014). On the other hand, the frontoparietal network (FPN), which consists of regions engaged during attentional cognitive tasks (Dosenbach et al., 2006; Fair et al., 2009), shows the opposite pattern of modulation. Interestingly, local neural activity in frontoparietal areas is reduced during SWS periods (Huber et al., 2006, 2004; Nir et al., 2011).
Networks Modification Subsequent to Poor Sleep.
Sleep disruptions have generally been associated with alterations in FC of the DMN and attentional networks (De Havas et al., 2012; Scullin, 2017). A nighttime lack of sleep has been associated with a deficit in the FC within the DMN the following morning, as well as within other RSNs (Kaufmann et al., 2016). Interestingly, using resting-state fMRI in healthy controls under controlled sleep deprivation, studies have demonstrated an aberrant functional activity not only within the DMN but also between the DMN and its negatively correlated regions (De Havas et al., 2012; Verweij et al., 2014). Interestingly, patients with chronic sleep disorders (e.g. insomnia) show greater connectivity modifications during wakefulness. Santarnecchi and colleagues (2018) reported how patients with chronic insomnia present a weaker connection between DMN and the supplemental motor area. Furthermore, the authors found that earlier age of insomnia onset positively correlated with FC within DMN, suggesting the importance of addressing insomnia-related effects on brain connectivity as early as possible to possibly prevent long-lasting DMN connectivity reshaping. Additionally, further analysis highlighted a reduction of connectivity between the occipital lobe and two bilateral temporal clusters overlapping with the hippocampus (Santarnecchi et al., 2018), in line with the known role of temporal lobe structures in memory consolidation processes (Riemann et al., 2010; Prince and Abel, 2013; Havekes et al., 2016). These studies indicate the potential importance of sleep on the maintenance of DMN activity during wakefulness. This is particularly relevant when taking into consideration the tight relationship between memory deficit, cortical network modifications, and sleep disruptions in aging.
Networks Modifications in Aging.
Overall, the most consistent finding across all studies is that older adults have lower FC between regions of the DMN compared to younger adults (Dennis and Thompson, 2014; Ferreira and Busatto, 2013). Age-related differences in functional connectivity have also been observed in other brain networks such as the dorsal attention (DAN), salience, and sensorimotor networks (Allen et al., 2011; Onoda et al., 2012; Tomasi and Volkow, 2012). However, to date, none of these differences have been reported as strong and replicable as those observed in the DMN. New studies are now focusing on age-related differences in between-network FC in addition to just within-network FC. For example, Spreng and colleagues (2016) examined age-related FC differences within and between DMN and DAN. They found lower within-network functional connectivity and higher between-network functional connectivity in older adults compared to the younger controls (R.N. Spreng et al., 2016). Studies also found age-related FC changes in a context of selective attention. The Ventral Attention Network (VAN), specifically, underlies selective attention. Deslauriers and coworkers (2017) reported greater connectivity among posterior regions of the VAN in older adults when compared to a younger cohort, but weaker connectivity among anterior regions (right anterior insula, right medial superior frontal gyrus, and right middle frontal gyrus). Data suggest posterior regions increasing their engagement in older adults as a compensatory consequence of reduced connectivity within anterior regions, suggesting a reorganization of network FC as a potential biomarker of aging-related attention deficits (Deslauriers et al., 2017).
Networks Disruptions in AD.
Within the AD pathology, DMN posterior-ventral and anterior-dorsal progressive deterioration have been observed to closely mirror PET-detected amyloidosis and hippocampal atrophy, closely accounting for changes in cognition as well (Buckner et al., 2005; Jones et al., 2016). Furthermore, the carriers of the ApoE ε4 allele, which is the most potent risk factor for AD, also show DMN impairment similar to that of preclinical AD, even in the absence of Aβ deposition in the brain (Sheline et al., 2010). As the pathology progresses, the loss of connectivity within DMN posterior regions is accompanied by an increase in their interaction with ventral areas instead (Jones et al., 2016), reflecting a malignant hyperconnectivity that outbreaks DMN boundaries and rather starts to involve other brain networks. For instance, key areas of the Salience Network (SN), such as the cingulate cortex and ventral striatum, as well as areas belonging to the Executive Control Network, show increased connectivity in AD (Agosta et al., 2012; Zhou et al., 2010). Other studies have pointed out a progressive decrease in Dorsal Attention (DAN) and Sensorimotor network (SMN) connectivity to also accompany AD clinical progression (Brier et al., 2012), with a different profile of disaggregation differentiating different forms of dementia (Zhou et al., 2010).
In general, alterations of network dynamics rather than changes in regional activity seem to constitute better markers of disease, and potentially capture subtle changes characterizing transitions across disease stages. Interventions targeting sleep dynamics might have a beneficial impact on overall cognition and daytime performance in healthy and diseased individuals.
8. NONINVASIVE BRAIN STIMULATION (NiBS)
Sleep disorganization appears to be among the earliest observable symptoms of MCI/AD. The current concept is that sleep disruption accelerates AD pathogenesis by promoting Aβ and tau protein accumulation. Along this line, it has been suggested that early treatment of sleep dysfunctions in young elderly and MCI patients could prevent or slow down the progression to dementia (Roberts and Knopman, 2013). Traditional interventions on sleep abnormalities include Cognitive-behavioral Therapy, pharmacological medications, as well as Bright Light Therapy combined with melatonin administration (Dowling et al., 2008). Furthermore, auditory stimulation seems also able to enhance SWA and improve memory retention (Papalambros et al., 2017). Recent studies have explored the possibility of treating healthy elderly and MCI/AD patients with Noninvasive Brain Stimulation (NiBS) techniques to restore sleep quality and to preserve or enhance physiologically-declining cognitive functions. NiBS techniques include transcranial magnetic stimulation (TMS) and transcranial electrical stimulation (tES; Valero-Cabré et al., 2017). Both approaches have been applied as potential therapeutic interventions in psychiatry and neurology (Cappon et al., 2016; Lefaucheur et al., 2017; Rossini and Rossi, 2007). A brief description of the main TMS and tES techniques available to date for mapping and modulating brain functions are reported below (Figure 1).
Figure 1. Noninvasive Brain Stimulation Techniques.
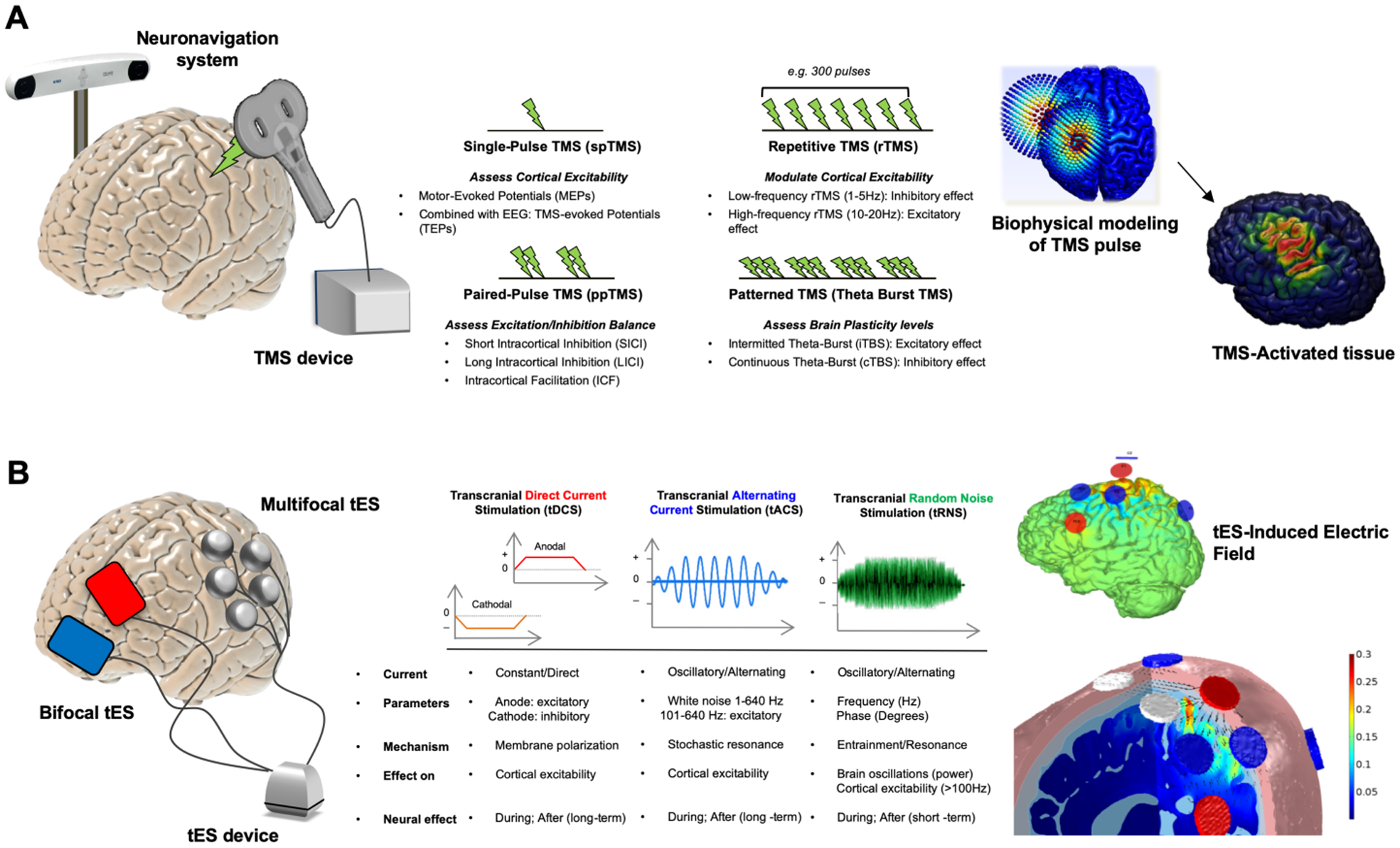
Panel A represents Transcranial Magnetic Stimulation (TMS). The coil induces a strong magnetic field exciting or inhibiting cortical activity (left). Different protocols can be implemented pairing more pulses with various effects (right). In panel B. we show the Transcranial Electrical Stimulation (tES). tES consists, at minimum, of two electrodes (red anode, blue cathode) applied directly on the scalp (left). More channels can be added to extensively record EEG or modulate brain activity via so-called multifocal/multielectrode protocols (Ruffini et al., 2013). Protocols include anodal (positive) and cathodal (negative) transcranial direct current stimulation (tDCS), transcranial alternating current stimulation (tACS), and transcranial random noise stimulation (tRNS). Stimulation protocols differ from one another on various parameters including frequency of stimulation and induction of after-effects (right).
Transcranial Magnetic Stimulation (TMS).
TMS is based on Faraday’s principle of electromagnetic induction: a pulse of electrical current flows through loops of wire (forming the magnetic coil) and generates a rapidly changing magnetic field perpendicular to coil plane, that induces an electric field parallel to the inner surface of the conductor (Rossi et al., 2009). TMS is able to modify intracortical excitability and activate distant cortical, subcortical, and spinal structures along with specific connections. Different strengths and forms of electrical fields can be generated by TMS, modifying physical and biological parameters, such as magnetic pulse waveform, coil shape, and orientation, intensity, frequency and pattern of stimulation. TMS has been applied by means of several protocols including single pulses (single-pulse TMS), pairs of stimuli separated by variable intervals (paired-pulse TMS), or trains of repetitive stimuli at various frequencies (repetitive TMS or rTMS). Below we offer a short review of the principal TMS-derived measures and protocols.
Single-pulse TMS is applied to the motor cortex at an appropriate stimulation intensity, eliciting motor evoked potentials (MEPs) which can be recorded by means of electromyography from the contralateral muscles. The activation of corticospinal descending pathways by means of TMS has been suggested to occur predominantly via interneurons in the superficial cortical layers (Di Lazzaro et al., 2007). MEPs amplitude reflects not only the integrity of corticospinal tracts and conduction properties along the peripheral motor pathways but also the degree of separation between successive pulses delivered through the same coil, with a possible inter-stimulus interval (ISI) lasting from a few milliseconds to hundreds of milliseconds. This method is used to explore inhibitory or excitatory intracortical networks depending on the intensity and ISI used (Kujirai et al., 1993; Valls-Solé et al., 1992). rTMS refers to the application of repeated stimuli over a single cortical site. Stimulation can be high-frequency (>1Hz) or low frequency (<1Hz; Rossi et al., 2009). rTMS can be used to study plasticity-like changes in healthy subjects, as the effects of stimulation on cortical excitability have been found to outlast the TMS duration, with differences depending on stimulation parameters and inter-individual variability (Maeda et al., 2000).
The combined use of TMS and EEG enables a deeper investigation of local reactivity and neural connectivity mechanisms. Indeed, TMS can be used to perturb neuronal networks, while EEG can enable the measurement of brain dynamics in response to perturbation (Figure 2). TMS pulse to a specific region is indeed known to evoke activation over secondary interconnected cortical areas. For this reason, TMS-EEG has become of particular interest to study causal communication connections between distant brain regions with a high temporal resolution, providing insights into mechanisms of effective connectivity (Friston et al., 1993; Massimini et al., 2005). When a single TMS pulse is applied over a specific cortical region, the transmission of generated activity can be detected by space-temporal analysis of TMS-evoked potentials (TEPs; Ilmoniemi et al., 1997). Furthermore, time-frequency analysis allow to measure TMS evoked cortical oscillations, highlighting how different cortical areas resonate at specific frequencies in response to perturbation (e.g. alpha in the occipital cortex, beta in the motor cortex), mirroring their dominant (so-called “natural”) spontaneous regional oscillatory activity (Rosanova et al., 2009).
Figure 2. Perturbation-Based Sleep Biomarkers via Combined TMS-EEG and tES-EEG Recording.
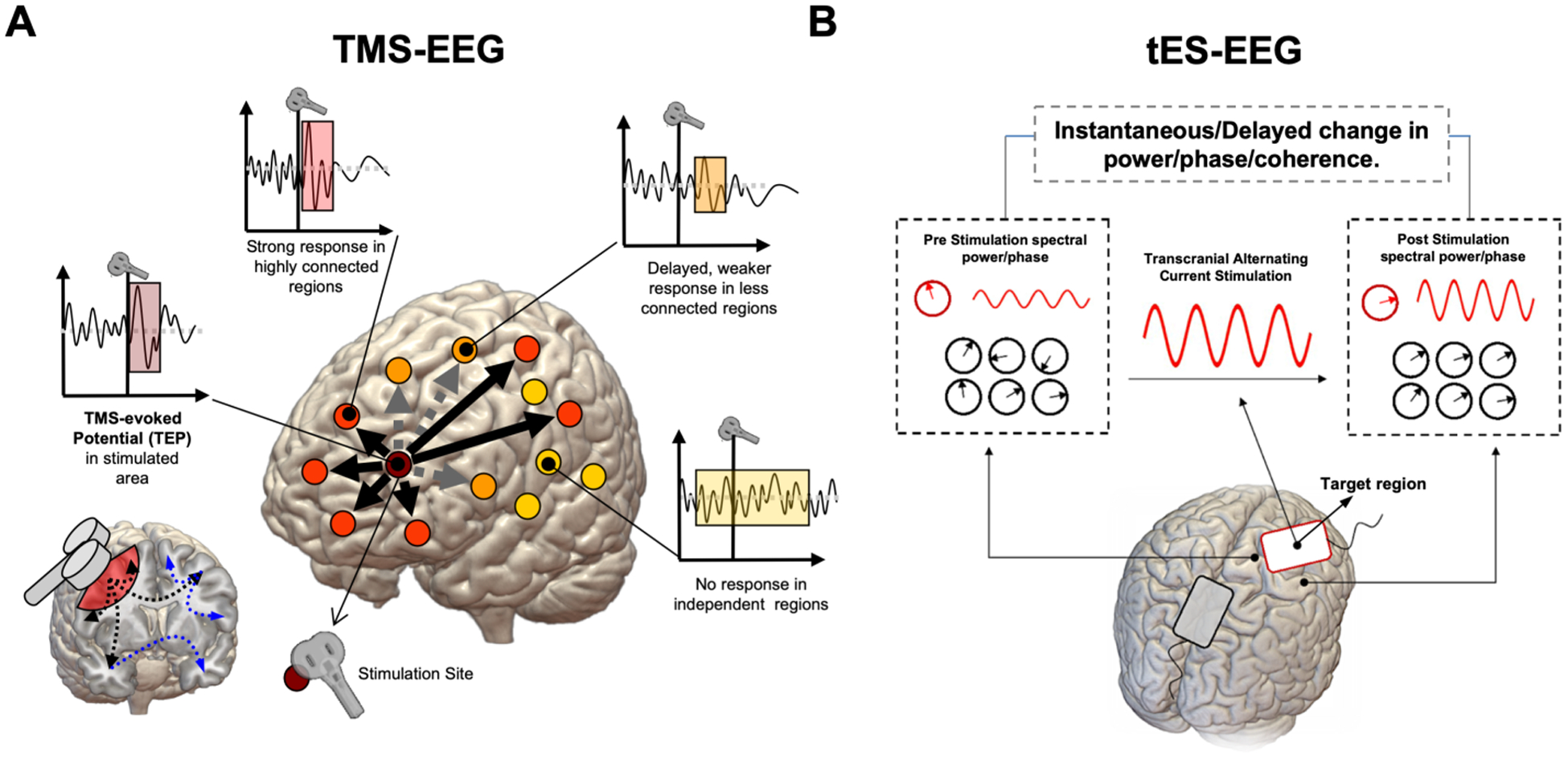
Brain Stimulation techniques can be combined with electrophysiology, as in the case of simultaneous TMS-EEG and tES-EEG recording. In the case of TMS, a focal magnetic pulse is delivered to a specific brain region using a neuronavigation system (based on individual’s MRI) which allows for precise anatomical targeting of cortical areas at 1-millimeter resolution (Panel A). The activity elicited by the pulse is mostly local, with distant effects usually observed for regions structurally or functionally connected to the stimulation site (i.e., orange dots). Both local reactivity and short-long range connectivity can be evaluated, either in terms of TEPs or time-frequency analysis. Indirect connections via third regions are also possible (blue and grey dashed lines), creating a complex topography. Individual response to TMS may differ in terms of the number of regions reacting to the TMS pulse, as well as for the timing of the response: strongly connected regions might show bigger and earlier responses to TMS, while distant or out-of-network regions might show delayed or even no responses. While TMS provides higher spatial resolution, when applied using alternating current, tES allows for frequency-specific modulation of brain electrical activity by supposedly tuning neuronal populations towards an externally induced oscillatory pattern (Panel B). The response to tACS can be expressed in terms of spectral power changes during and/or after stimulation, as well as phase-coherence and other connectivity metrics, with the effects being measurable both at the stimulated area as well as other distant, resonant regions.
Transcranial Electrical Stimulation (tES).
tES involves the transmission of weak electrical currents (1–2mA) through the scalp to modulate firing properties of cortical neurons and ongoing rhythmic brain activity (Paulus et al., 2013; Ruffini et al., 2013). tES requires at least two surface electrodes of different polarities, soaked with saline water or electroconductive gel, applied to the subject’s scalp and connected to a current-controlled waveform generator. Modern versions of tES employ multiple small electrodes and detailed, realistic modeling of the electric field generated by the montage in the individual brain. Various tES protocols can be implemented by changing dose parameters such as shape, position, and numbers of electrodes, current waveform, frequency, duration of stimulation and number and timing of sessions. Different techniques include transcranial direct current stimulation (tDCS), transcranial alternating current stimulation (tACS; Herrmann et al., 2013), and transcranial random noise stimulation (tRNS; Paulus, 2011; Ruffini et al., 2013). For the purpose of the review and their application in studies of sleep and aging, we below discuss only tDCS and tACS.
tDCS only modulates spontaneous neuronal activity and it does not cause direct neuronal firing. tDCS modulates brain excitability through the application of low-amplitude (0.5–2mA) electrical fields in the cortex (0.2–2V/m, Ruffini et al., 2018). In early studies, tDCS was combined with TMS to investigate the modification of primary motor cortex (M1) cortical excitability (Nitsche and Paulus, 2000). tDCS induces polarity specific changes in the targeted neural population, with anodal depolarization and cathodal hyperpolarization of the resting membrane (Nitsche et al., 2005). These modifications have been proved to outlast the stimulation time and they were even more robust offline than during current delivery. Moreover, it has been demonstrated that the effects can be altered by pharmacological interventions acting on the central nervous system (Stagg and Nitsche, 2011).
While tDCS induces a constant, non-oscillating current, tACS generates a current profile leading to alternating electric field directions (Herrmann et al., 2013; Tavakoli and Yun, 2017). tACS might be used to investigate and modulate neuronal oscillatory dynamics (Antal and Paulus, 2013; Paulus, 2011). When applied at one of conventional EEG frequencies (0.1–80Hz), tACS is able to interact with ongoing cortical rhythms, causing endogenous oscillations to become synchronized with outer stimuli. The efficacy of tACS can, therefore, be determined based on induced changes in EEG spectral power and phase-coherence induction on stimulated areas and networks (Boyle et al., 2013; T. Roh et al., 2014). To date, only “interleaved” EEG-tACS paradigms recording pre- and post-stimulus EEG (to avoid signal tACS artifacts on EEG) have been successfully demonstrated (see, e.g., Castellano et al., 2017) .
tES devices are portable and have an excellent safety profile (stimulation is generally well tolerated, relatively inexpensive, and readily available), therefore their application in clinical and research domains is particularly appealing. It has been widely used over the past decade and has made significant contributions in the field clinical psychology (for a review see Zhao et al., 2017) and cognitive processes (for a review see Kuo and Nitsche, 2012). Finally, personalized hybrid biophysical and physiological models of brain networks are poised to play a key role in the evolution of network-oriented transcranial stimulation. Current research is exploring the potential for model-driven optimization of tES to target both individual nodes or entire brain networks (Ruffini et al., 2018).
9. NiBS APPLICATIONS IN SLEEP
Historically, the application of an external current to induce sleep-like states started in early 1900. Robinovitch (1914) was the first one to introduce the concept of electrosleep therapy. Usually, electrosleep applied a pulsating direct current for up to 120 minutes, with electrodes attached to eyes and mastoids (for a comprehensive review see Guleyupoglu et al., 2013). Although most of the studies lack methodological rigor (e.g., no control with sham condition or no electrophysiological measures) this technique indeed induced sleep improvements (Brown, 1975; Sergeev, 1963; von Richthofen and Mellor, 1979) including inducing sleepiness, damping anxiety, fatigue, and depressive symptoms (Frankel, 1974). During the last decade, several TMS studies have also been conducted to investigate the physiology and pathophysiology of sleep structure in healthy and clinical populations. A growing number of studies exploited a combination of TMS paradigms (sTMS, ppTMS, rTMS, as well as TMS-EEG) to (1) gain insights into the mechanisms of sleep disorders to reveal patterns of abnormal cortical excitability; (2) assess the effects of specific pharmacological interventions; (3) treat patients with (e.g rTMS) in attempt to restore physiological excitability levels. We discuss below the successful combination of TMS with EEG to investigate cortical oscillations and their modifications during sleep.
TMS and TMS-EEG to Investigate Sleep Dynamics.
Causal relationships between sleep homeostasis, synaptic plasticity, consciousness, and slow waves have been investigated within the theoretical framework of the information integration theory of consciousness (IITC) proposed by Tononi (2004). In this context, consciousness is the ability of the brain to integrate information and effective communication between neuronal assemblies is crucial for conscious experience. The current concept is that efficient transfer of neuronal information is strictly dependent on effective brain connectivity, which refers to the opportunity for a group of neurons to causally regulate the behavior of other groups of neurons inside a network. In this context, the TMS-EEG approach has been adopted to understand if mechanisms of fading of consciousness (as during sleep) are related to a disruption of effective connectivity. In a series of experiments, TMS-EEG has been applied while participants kept their eyes closed on a reclining chair and proceeded from wakefulness to NREM sleep. Massimini and coworkers (2012, 2005) assessed the cortical reactivity during NREM sleep and compared it to a wakeful state. The authors found that TMS pulses during wakefulness generate a systematically organized propagation along with short- and long-distance cortico-cortical connections. By contrast, stimulation during deep stages of sleep resulted in a local cortical response that did not propagate. Moreover, TMS during wakefulness generated low amplitude high-frequency waves, while stimulation during sleep evoked high amplitude low-frequency waves. The authors argued that, while during wakefulness and REM sleep the brain is able to sustain long-range, complex sequences of activation, during NREM sleep, when consciousness dissolves, this ability is lost. During NREM stages, the neuronal system disintegrates into independent modules that produce the stereotypical SWA. Tononi and Cirelli (2003) have also proposed the synaptic homeostasis hypothesis, according to which SWA homeostasis reflects a cascade of functional events. These events may restore the level of energy required for synaptic functioning and may regulate the activity of cellular elements enabling learning. Therefore, if wakefulness is associated with synaptic potentiation resulting in an increase in synaptic strength, sleep is instead involved in triggering homeostatic regulation of synaptic weights. Consequently, the amplitude of TMS evoked potentials is expected to be higher at the end of a day of wakefulness and lower after a night of sleep (Tononi and Cirelli, 2006).
Because sleep deprivation sensitizes the brain to epileptogenic activity, it is considered a major risk factor for seizure precipitation in epileptic patients (Dinner, 2002). Different studies applied TMS to investigate the link between sleep deprivation and its ability to modify cortical and corticospinal excitability. De Gennaro and coworkers (2007) investigated those mechanisms by sleepiness assessment, EEG spectral power maps and several TMS measures, such as paired-pulse short intracortical facilitation and inhibition, and different motor thresholds (MTs), such as standard (ST), lower (LT), and upper threshold (UT). The cohort was composed of 33 normal subjects tested before and after 40 hours of sleep deprivation. Results showed that a night of sleep deprivation profoundly affects different measures of cortical activation. EEG slow frequencies increased in most cortical regions with a focus on midline frontal and central areas, accompanied by large increases in delta and theta activity. Standard MTs, LTs, and UTs, all increased as a consequence of sleep deprivation. Short interval intracortical facilitation (SICF) after nighttime wakefulness also displays a significant increase, but only in women. Overall neural deactivation was associated with an increase in subjective sleepiness, as well as a decrease of subjective alertness. Importantly, EEG slowing and loss of complexity are strong biomarkers of sleep deprivation, also observed in diseases like Alzheimer’s and Parkinson’s Disease (Abásolo et al., 2006; Dauwels et al., 2010; Ruffini et al., 2019). The study by De Gennaro et al (2007) partially confirms the synaptic homeostasis hypothesis by Tononi and Cirelli (2003) and it poses a first evidence of applying TMS to study sleep dynamics.
tES to Manipulate Sleep Oscillations.
While a unifying theoretical account of cortical SWA is still lacking, some lines of research have provided possible explanations of the underlying mechanisms and functional role of SWA and its link with faster oscillations. SW is caused by brief periods of hyperpolarization in cortical and thalamocortical neurons due to a firing rate reduction of ascending activating neuronal assemblies (down states; Llinás and Steriade, 2006). Experimental evidence has supported a strong link between SWA homeostatic regulation and learning mechanisms. According to the synaptic homeostasis hypothesis (Tononi and Cirelli, 2006), modifications in synaptic bounds during waking (e.g. due to learning) influence SWA formation and propagation. The up-regulation of SWA synchrony is functionally relevant in the consolidation of new memories during sleep. The functional interaction between SWA and spindles (SS) is one of the main factors that support this process. When firing rate reduction of ascending activating neuronal assemblies generates down-states, SS is suppressed. SWA and SS are, in fact, phase coupled during NREM and the spatio-temporal coupling between spindles and SWA is believed to trigger replay and strengthening of mnemonic traces (Diekelmann et al., 2009).
With the foregoing in mind, tES has been applied to enhance SWA during NREM, investigating the relationship between SWA and memory consolidation. Marshall and colleagues (2006, 2004) were the first to investigate the effects of anodal slow oscillation transcranial electrical current (otDCS, combination of tACS and tDCS) during sleep in young adults. Stimulation was applied during SWA in N2 at 0.75Hz on frontolateral areas (F3-F4). Participants performed a declarative episodic memory task before nighttime sleep and in the morning after awakening. The authors reported improved declarative memory linked with increased SWA (Marshall et al., 2006, 2004). Ten years after, Marshall’s group also demonstrated a positive effect on cognitive function in rodents, similarly triggered by tACS during NREM (Binder et al., 2014). During the following decade, these results were replicated, usually in young individuals. Typical experimental paradigms consisted of sinusoidal waves applied at frequencies of 0.5–1.2Hz over F3-F4 with an open or closed-loop tACS device. Enhanced SWA and delta power, triggering an increase in gamma and sigma waves followed by better performance in declarative memory tasks, has always been reported (Jones et al., 2018; Ketz et al., 2018).
Because subjective sleep quality positively correlates with SWA duration (Akerstedt et al., 2009), tACS or otDCS applied to enhance SWA and SWS time may also be of interest. Ketz and colleagues (2018) not only reported improved memory consolidation following a closed-loop tACS intervention designed to match the phase and frequency of endogenous SWA in NREM sleep (Robinson et al., 2018), but also significant improvements in sleep quality measures. Focusing on different memory domains and EEG features, Lustenberger and colleagues (2016) applied an EEG-controlled approach that restricted the application of 12Hz feedback-tACS (FB-tACS) to bilateral frontal lobes only when sleep spindles were detected, therefore stimulating only when the sigma power (11.5–15.5Hz) was prevalent. The authors reported increased motor memory consolidation after stimulation tested via a motor sequence tapping task. Differently, Lafon and coworkers, (2017) reported an unsuccessful attempt to entrain sleep spindles while applying low-frequency tACS in healthy subjects. The authors measured endogenous spindle power intracranially during NREM sleep using invasive pre-surgical electrocorticography monitoring in 13 patients with epilepsy, finding no stable evidence of entrainment. Even thought the main reason for the failure in entraining spindles activity could be attribute to the underlying epileptic activity, it should also be noted that stimulation intensity varied across the two studies, with the latter applying significantly weakers currents (<0.05 V/m).
Interestingly, an attempt has been made also at promoting sleepiness in awake participants using tES. In a recent study, authors administered 5Hz anodal oscillatory tDCS (a technique similar to tACS) on frontal areas, targeting spontaneous slow frequency EEG activity. As a result, the rising of slow oscillations seemed to increase subjective measures of sleepiness (Atri et al., 2016), opening the way to interesting applications in aging individuals. Finally, even though not immediately translatable to aging-related processes, one study has investigated the possibility of applying tES during REM sleep to modulate dreams. Voss and coworkers (2014) administered frontotemporal tACS following ~2min of uninterrupted arousal-free REM sleep with different frequencies. Participants were then awakened and asked to rate dream consciousness based on a validated scale. According to the authors, lower gamma band (40Hz) stimulation during REM sleep increased self-awareness of participants’ dreams (Voss et al., 2014), a potentially indirect marker of a more stable and organized REM cycle.
10. NIBS APPLICATIONS IN ALZHEIMER’S DISEASE DURING WAKEFULNESS
NiBS applications in healthy aging population during wakefulness can be successfully implemented with various positive outcomes, such as improving performance and quality of life. TMS and tES proved to be an excellent tool to treat cognitive functioning in the elderly, promoting plastic effects that could trigger beneficial compensatory activity. As for MCI/AD patients, to date, the Federal Drug Administration (FDA) has approved 5 drugs, mostly focusing on symptom management. Holistic and lifestyle modifications are recommended, including modifications to diet, exercise, and social environment, sometimes combined with computerized cognitive training. Because none of these existing therapies has a direct effect on the underlying neuropathology, NiBS methods may be a potentially crucial tool to slow MCI/AD conversion and progression (for a review see Gonsalvez et al., 2016). Therefore, we will give priority to discuss possible TMS and tES applications in MCI/AD patients, emphasizing outcomes and experimental paradigms. We refer elsewhere for a comprehensive review of NiBS applications in healthy aging population with the goal to preserve or enhance physiologically-declining cognitive functions (Tatti et al., 2016).
TMS in AD during Wakefulness.
TMS has been shown to enhance various cognitive skills, such as verbal memory, episodic memory, working memory, and executive functions (Manenti et al., 2012). Several clinical trials tested different rTMS protocols for cognitive improvement in AD patients with encouraging results (Cotelli et al., 2008, 2006, 2012; Devi et al., 2014; Eliasova et al., 2014; Koch et al., 2018). Recent meta-analytic work has highlighted cognitive improvements in patients with psychiatric and/or neurological disease and in healthy controls following high-frequency rTMS over the dorsolateral prefrontal cortex (DLPFC), suggesting this stimulation site as a gold standard (Guse et al., 2010). For example, rTMS over bilateral DLPFCs of 15 AD patients enhanced accuracy in an action naming task but not in object naming (Cotelli et al., 2006). These findings were replicated with a bigger sample size (24 patients) with varying AD severity (mild AD: >17/30 MMSE score; moderate to severe AD: <17/30 MMSE score). Again, rTMS over bilateral DLPFCs enhanced action but not object naming in the mild AD group. On the other hand, moderate to severe AD patients showed an improved accuracy for both classes of stimuli (Cotelli et al., 2008). In later years, the same research group also investigated rTMS-induced long-term cognitive effects (Cotelli et al., 2012). They divided 10 adults with AD in two groups on a multiple-baseline trial. One group was treated with high-frequency (20Hz) rTMS over left DLPFC, 5 times per week for 20 sessions. The second group received placebo rTMS for 2 weeks and then the same high-frequency rTMS for 2 weeks. After the first 10 sessions, participants who received real rTMS demonstrated significantly better performance than those receiving placebo. Moreover, both groups showed improved performance at 8 weeks follow up (Cotelli et al., 2012). Ahmed and coworkers (2012) applied high frequency rTMS in mild/moderate AD patients with positive effects on their MMSE, Instrumental Daily Living Activity Scale, and Geriatric Depression Scale scores. Results were found to be specific to both stimulation type and stage of illness, for which no effect was found either when low frequency stimulation was applied, nor when severe AD patients were chosen as the active group. Using slightly different stimulation sites, Eliasova and colleagues (2014) applied 10Hz rTMS over vertex and right inferior frontal gyrus (IFG) in patients with early AD. Significant improvement at Trail Making Test A and B was reported when IFG was the stimulation site. In a short term trial, 12 AD patients received 4 rTMS sessions at 10Hz over the bilateral DLPFC for 2 weeks (Devi et al., 2014). Overall, higher scores were obtained following administration of the Boston Diagnostic Aphasia Examination tests of verbal and non-verbal agility following TMS, accompanied by neuroimaging evidence of enhanced activation during performance of various cognitive tasks 4 weeks post-treatment (Devi et al., 2014). In a long randomized, double-blind, placebo-controlled trial, Zhao and coworkers (2017) stimulated 30 AD patients at different stages of illness. 20Hz rTMS was applied on P3/P4 and T5/T6 for 30 sessions for 6 weeks monitoring for performance levels at verbal memory, short term memory, ability to learn new information, memory on immediate recall and delayed recall, and long delayed recognition. After the rTMS treatment, a significant global cognitive improvement was found, whereby scores on the Assessment Scale-Cognitive Subscale (ADAS-cog), MMSE, Montreal Cognitive Assessment (MoCA), and World Health Organization University of California-Los Angeles Auditory Verbal Learning Test (WHO-UCLA AVLT) were significantly ameliorated. Furthermore, subgroup analysis showed that rTMS effects on memory and language performance were far superior in mild AD patients than in moderate AD. Koch and coworkers (2018) have also made use of 20Hz rTMS stimulation for 10 sessions over 2 weeks targeting the precuneus, a key area in AD memory impairment. By enhancing the activity of the precuneus and its connectivity with frontal areas, selective improvement in episodic memory was observed in 14 patients with early AD.
Finally, the combined efficacy of rTMS protocols and cognitive training has been addressed by several studies. Bentwich and colleagues (2011) treated 8 AD patients with daily cognitive rehabilitation and rTMS for 6 weeks, followed by 2 sessions per week for 3 months. High-frequency rTMS was delivered over 6 different brain regions, including Broca’s area, Wernicke’s area, bilateral DLPFC, and the right and left parietal somatosensory association cortices. Researchers found both a significant improvement in cognitive domain tested by means of the ADAS-Cog (baseline: 22.5, 6-weeks: 18.3, 18-weeks: 18.5), as well as amelioration of the symptomatology as assessed through the Clinical Global Impression of Change (CGIC; 6-weeks: 1.0, 18-weeks: 1.6 points). The MMSE, the Alzheimer Disease Assessment Scale Activities of Daily Living (ADAS-ADL), and the Hamilton Depression Scale also showed a trend toward improvement, though this did not reach statistical significance. Encouraging results have also been reported following shorter interventions (6 weeks; Brem et al., 2013; Nguyen et al., 2017), with aftereffects that persisted up to 3 months follow up (Rabey et al., 2013).
tES in AD during Wakefulness.
In parallel with findings from groups exploring TMS, various research groups have demonstrated tDCS efficacy in enhancing cognitive function in healthy subjects (for a review see Kuo and Nitsche, 2012). Additionally, a number of small trials have provided compelling evidence for its efficacy in AD patients. Ferrucci and coworkers (2008) tested performance in a word recognition task and in a visual attention task before and after a single tDCS application. They administered 1.5mA for 15 min to bilateral temporoparietal regions in 10 patients with a diagnosis of probable AD. When compared to sham, anodal stimulation improved word recognition accuracy, while cathodal stimulation worsened performance. No effect was reported for the visual attention task. Boggio and coworkers (2009) tested tDCS effects on recognition memory, working memory, and selective attention in AD patients. They demonstrated that a single session of anodal tDCS over the left DLPFC and temporal cortex was able to transiently improve visual recognition memory in AD patients. As in previous studies, results were task-specific. Long-lasting effects of tDCS in AD were also investigated by the same research group, whereby 2mA tDCS was delivered for 30min per 5 days through two scalp anodal electrodes placed over temporal regions and a reference electrode over the right deltoid muscle. Patients showed improved performance on a visual recognition memory task, which was maintained at 1 month follow up. However, visual attention and general cognitive performance did not benefit from stimulation (Boggio et al., 2012). In a similar study comparing the effects of anodal, cathodal or sham stimulation, 2 mA tDCS administered for 25 min/day per 10 days resulted in a significant increase in the MMSE score for both active conditions (Khedr et al., 2014).
Similar to studies of combined TMS and cognitive therapy, Cotelli and colleagues (2014) investigated the effects of tDCS combined with individualized computerized memory training. Thirty-six AD patients were randomly assigned to 3 groups: one group was treated with individualized computerized memory training and anodal tDCS on left DLPFC at 2mA for 25min over 5 consecutive days for 2 weeks; a second group underwent motor training and anodal tDCS with the same parameters; finally, a third group received placebo tDCS. Only the group receiving combined cognitive and electrical stimulation showed a significant improvement in a Face-Name Associations task (Cotelli et al., 2014).
11. tES DURING SLEEP IN ELDERLY INDIVIDUALS
NiBS applications include not only the investigation for pathological biomarker detection but also a potential tool to restore sleep quality and consequently preserve declining of cognitive functions in the elderly and AD. Since oscillatory activity characterizes human and animal brain activity, tACS may be the best methodology to study and find a possible therapy lied in the link between cognitive/motor/emotional functions and ongoing brain waves. Studies combining sleep and tES stimulation have mostly focused on the modulation of SWA during N3 state, testing the possibility of using these techniques to promote sleep. Below we provide an overview of the most relevant literature studies on tES application during sleep in healthy young and elderly subjects.
The same experimental paradigm implemented by Marshall and coworkers (Marshall et al., 2004) was tested in the elderly population by Eggert and colleagues. These authors, however, did not observe beneficial effects on memory consolidation in this older cohort, arguing that there might be crucial differences in memory consolidation processes between young and aged individuals (Eggert et al., 2013). Paßmann and colleagues also stimulated older adults by means of tACS over the frontal cortex during NREM. Authors found increased SWA and spindles activity, similar to previous results on young individuals, but again with no beneficial effects in the consolidation of visuospatial and verbal memories (Paßmann et al., 2016). In a double-blind, crossover design, Westerberg and coworkers (2015) investigated the effect of 0.75Hz slow oscillatory stimulation applied over F7-F8 during a 90-minute daytime nap in 19 elderly. They found increased SWA associated with an improvement in word-pair performance (Westerberg et al., 2015). A later study with a similar experimental paradigm on afternoon nap in older adults showed how enhancing SWA and fast sleep spindle power led to a benefit in visual memory tasks (Ladenbauer et al., 2016).
To our knowledge, only one clinical trial has been conducted where tES was applied during sleep in MCI/AD patients. Ladenbauer and coworkers (2017) enrolled 16 MCI patients in a crossover design to investigate SWA, sleep spindles, and memory consolidation in neurodegenerative disease. Participants (age range 50–81 with no history of sleep disorders) were tested on verbal memory, visuospatial declarative memory, and procedural memory prior to and after 90 minutes of sleep. Slow otDCS was applied at frontal locations F3-F4, with the anodal current oscillating sinusoidally at 0.75Hz (between 0 and 262μA), with a maximum current density of 0.5mA/cm2. Stimulation started after 4 minutes spent in the N2 stage. During each stimulation-free interval, online sleep scoring was performed, allowing the next stimulation block to start only when another N2 stage was detected. The synchronization and locking between SWA and fast spindle power were also measured and used to compute phase values for the estimation of a synchronization index. Results showed increased SWA and spindle power together with stronger synchronization during otDCS compared to sham stimulation. Furthermore, significant performance improvement in visual declarative memory was reported. This study suggests that individualized protocols may be most effective in sleep modulation. Although these results are encouraging, further clinical trials with a larger number of patients are needed. To our knowledge, no clinical trial has tested the effects of tES during sleep on MCI/AD protein clearance.
12. CHALLENGES AND OPPORTUNITIES
Past and possible future applications of tES during overnight sleep and awake states in healthy and pathological aging populations are presented in Figure 3 and Figure 4, delineating a complex and varied range of opportunities.
Figure 3. Temporal Framework for tES During Sleep in the Healthy and Pathological Aging Brain.
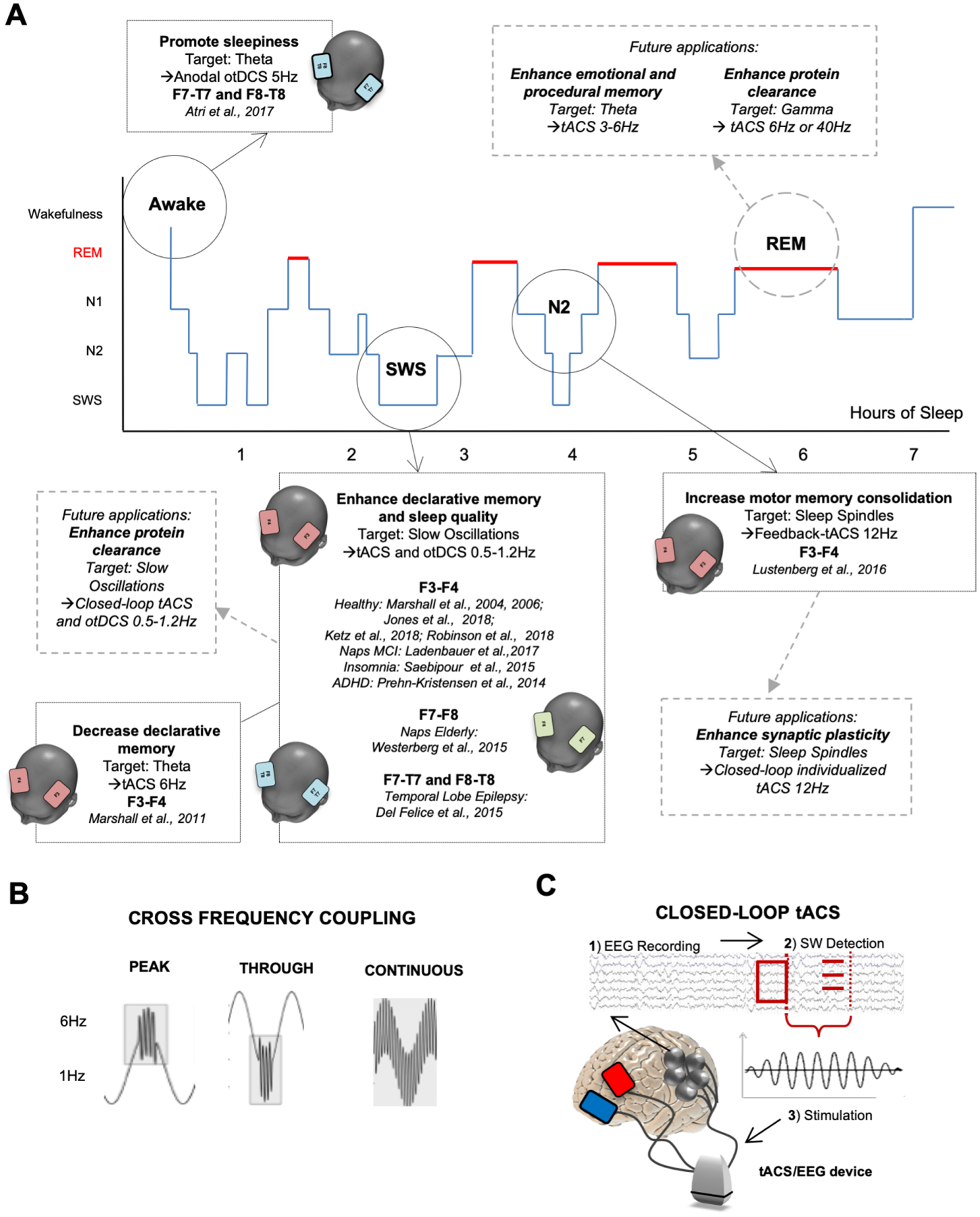
tES can be applied to manipulate various sleep stages, targeting specific cortical oscillations (panel A). Goals include improvement of cognitive and motor performance, as well as modulation of protein clearance and synaptic plasticity. More advanced methods can be applied, including cross-frequency coupling and closed-loop EEG-tES solutions (panel B-C). For instance, CFC allows to combine stimulation of slow frequency carrier activity (e.g. 0.75–1Hz) and faster oscillations in the theta, alpha, beta and gamma band (e.g. 6Hz or 40Hz), also allowing to couple the two oscillatory patterns with fine temporal precision (i.e. targeting peak or through of carrier frequency; panel B). In the case of closed-loop tES (panel C), stimulation is triggered by real-time EEG recording (C1) and detection of slow waves (C2), with semi-instantaneous delivery of tES matching individual SWS frequency (C3).
Figure 4. Tackling Network Alterations and Proteinopathy Overnight.
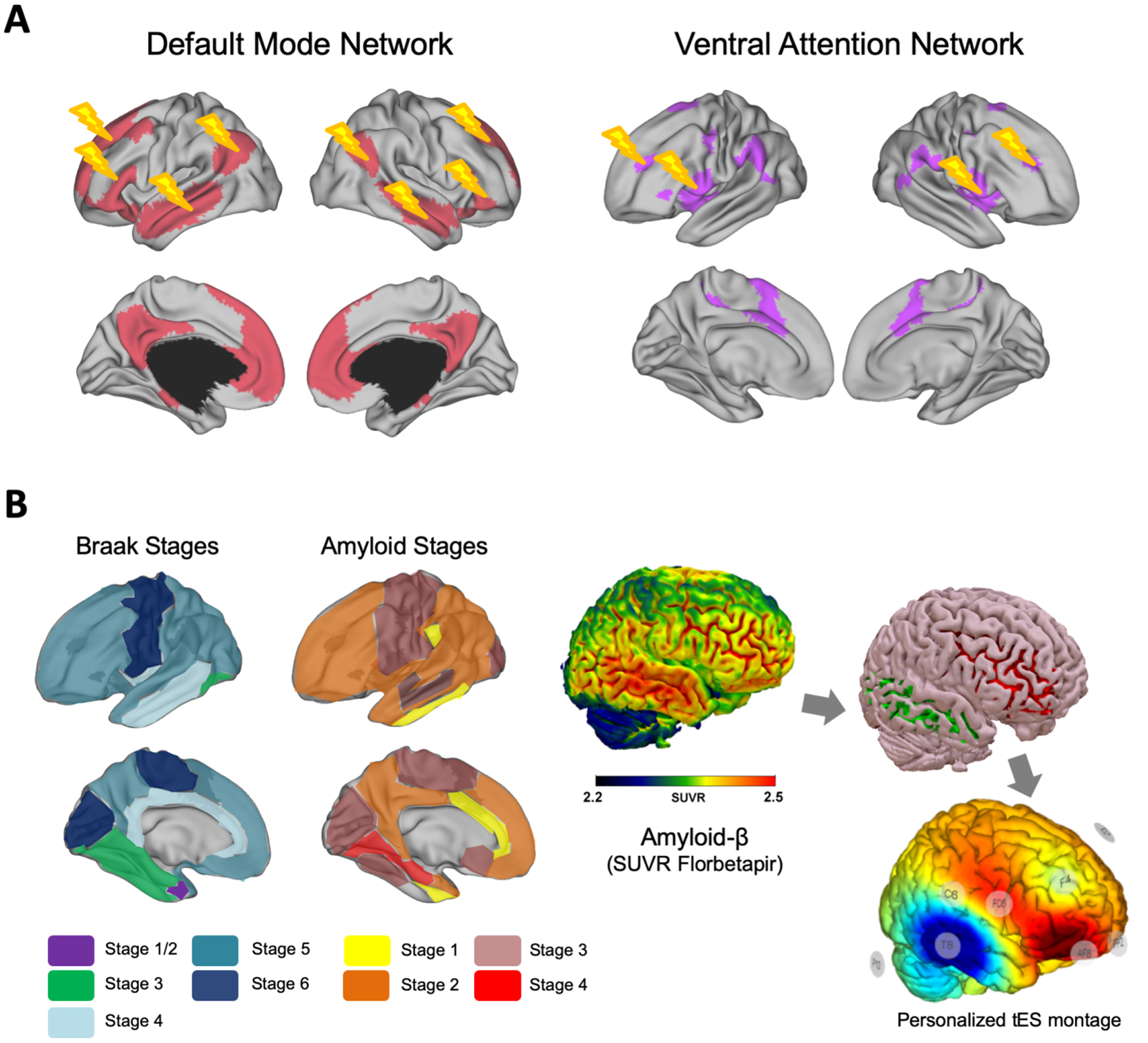
tES can be optimized to tackle alterations of spatio-temporal functional networks as those recorded via resting-state fMRI, with particular emphasis on networks previously shown as altered in healthy and pathological aging, e.g. the default mode and ventral attention networks (panel A). Given the relevance of protein clearance processes overnight, and the recent discovery of dominant oscillatory activity (i.e. theta activity) accompanying CSF clearance dynamics during sleep, tES could also be tailored to target individual tau and amyloid accumulation overnight by maximizing stimulation over regions with higher tracer uptake at PET imaging (panel B).
Temporal Framework for tES Delivery During Sleep (Figure 3).
tACS applications seem to be the preferred choices thanks to their effects on brain oscillations in both the healthy aging population and AD patients (Figure 3A). In different cohorts of individuals, young and old, prior work has already shown positive results for the use of tES during sleep on consolidation of procedural (Lustenberger et al., 2016) and declarative memory (Jones et al., 2018; Ketz et al., 2018; Marshall et al., 2006, 2004; Marshall and Born, 2011). Sleep interventions might offer the optimal timeframe to elicit SWA and modulate sleep spindles, with possible favorable outcomes in enhancing memory consolidation in healthy aging population and patients. Although some of these approaches have already been tested, numerous other scenarios remain to be explored. Indeed, sleep is one of the earliest and steadiest biomarkers used to recognize middle/late age individuals at risk of developing dementia. Given the strong relationship between amyloid clearance and some of the EEG features during sleep, manipulating SW sleep may tackle the progression of Alzheimer’s pathology in MCI patients and healthy individuals at risk. The rationale for such an intervention derives from the positive outcome of Ladenbauer’s study (2017). A clinical trial is now being run applying tACS during sleep with the purpose of modulating SWS, as well as clinical and cognitive symptoms in MCI patients (NCT03112902). Relative attenuation of gamma activity has also been found to characterize AD patients (Huang et al., 2000). Studies on rodent models of AD have been among the first to suggest new treatment approaches enhancing gamma power. Goutagny and coworkers (2013) underlined how deregulation of hippocampal theta/gamma coupling arises before the overproduction of Aβ, while Iaccarino (2016) found that exogenously-induced flickering lights at 40Hz were able to reduce tau concentrations in pre-symptomatic AD mice. Therefore, the modulation of gamma activity in MCI/AD patients may be a non-invasive and efficient treatment. Clinical trials with this aim are currently being run (Thomson, 2018). Further steps in this direction concern the use of tACS applied at 40Hz (gamma frequency) during sleep in MCI patients. The ultimate aim would be preventing, or at least slowing, neurodegenerative mechanisms by targeting gamma activity and sleep patterns concomitantly, with potentially positive effects in declarative memory consolidation, enhancement of sleep quality, and Aβ clearance.
Going further, new tES approaches allowing to more closely mimic physiological processes that can be implemented during sleep as well. For instance, cross-frequency coupling (CFC) implies stimulation combining multiple frequencies (Figure 3B; e.g. 1Hz and 6Hz). CFC provides a mechanism for synchronization between local and global processes across connected areas and cortical networks (Florin and Baillet, 2015). To date, during wakefulness, the most prominent example of CFC is between theta and gamma oscillatory activity during memory encoding/retrieval (Axmacher et al., 2010; Sauseng et al., 2009), but it may also play a role in memory consolidation during REM. Prominent REM theta rhythms coupled with gamma have been seen in rodents (Belluscio et al., 2012; Scheffzük et al., 2011) and monkeys (Takeuchi et al., 2015). Gamma seems to promote synaptic plasticity, supported by theta (Lisman and Buzsáki, 2008; Lisman and Jensen, 2013). This may be specific for phasic REM states, where the synchrony between theta and gamma is enhanced, suggesting that tonic REM phases support offline mnemonic processing while phasic bursts of activity may promote memory consolidation (Boyce et al., 2016; Montgomery and Woodall, 2008). Stimulating theta and gamma in CFC during REM may then help consolidate mnemonic traces as well as SWS stimulation. New studies could investigate this.
NiBS during sleep usually requires the experimenter to start the stimulation after 3–4 minutes of ongoing EEG typical of N2 or N3. A new experimental paradigm recently implemented a closed-loop algorithm able to independently start the stimulation when the subject is in N3 (Jones et al., 2018; Ketz et al., 2018b; Robinson et al., 2018). This algorithm for augmentation of slow-wave sleep first detects the presence of SW oscillations, computing the mean of the power spectra of SW (Figure 3C). The frequency of sinusoidal wave produced by tACS is then set to individualized SW frequency mean with the aim of matching the stimulation frequency and phase with the natural ongoing SW activity.
Targeting Network Alterations and Proteinopathy (Figure 4).
One of the biggest challenges in available tES studies in sleep concerns the electrodes montage and resulting cortical electric field. While most studies have administered stimulation over frontal and prefrontal derivations (F3-F4, rarely F7-F8), new devices enable multisite stimulation of neural networks, engaging interconnected cortical areas as well as entire networks (Figure 4A). As previously mentioned, the DMN possibly represents the cortical network whose FC is mostly altered by sleep deprivation and age-associated brain changes. Therefore, stimulation of DMN may enhance internal connectivity strength and act as an early intervention to counteract the disruption of resting-state brain connectivity patterns To do so, multifocal stimulation using several relatively small electrodes has been used to achieve more focal stimulation of specific cortical targets (Ruffini et al., 2014). This allows a spatially specific protocol that can simultaneously stimulate different areas belonging to the same (e.g. DMN) or different networks. Studies also found connectivity alterations in aging within anterior VAN regions (Deslauriers et al., 2017), making it a potential target to enhance selective attention in elderly subjects.
To target a specific individual network, the participant may undergo an fMRI able to identify the strongest and weakest areas included in the network allowing a more focal and individualized stimulation. Accurate modeling of the induced electrical currents and fields is now possible through the use of personalized brain models (Miranda et al., 2013). Ongoing trials are testing the feasibility of stimulating target regions with maximum amyloid levels using individualized multifocal montages (NCT03290326). Generally, this allows, in turn, the optimization of electrode montage based on individual neuroimaging data (Ruffini et al., 2014) such as amyloid-β PET (e.g. Florbetapir or PiB), CT and T1-weighted MRI data (Figure 4B).
13. CONCLUSIONS
Sleep represents an opportunity for the detection of relevant biomarkers in individuals at risk of developing dementia, as well as for treatment purposes aimed at improving cognitive functions and general quality of life. Although early results from NiBS application during sleep in healthy elderly individuals and patients with dementia are promising, further research is needed to improve our understanding of mechanisms, target definition and model-driven optimization of closed-loop protocols allowing the efficacious modulation of cortical activity.
ACKNOWLEDGEMENTS
ES is supported by the BROAD Institute at Harvard-MIT (Boston, MA, USA) via 2016P000351. ES is supported by Defense Advanced Research Projects Agency (DARPA) via HR001117S0030. ES is supported by the Beth Israel Deaconess Medical Center (BIDMC) via the Chief Academic Officer (CAO) grant 2017 and by NIH via R01 AG060981-01. ES is partially supported by R01 MH117063-01, P01 AG031720. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of Harvard University and its affiliated academic health care centers, the National Institutes of Health. GR is partially supported by the Luminous project (Future Emerging Technologies Open Luminous project (H2020-FETOPEN-2014-2015-RIA under agreement No. 686764) as part of the European Union’s Horizon 2020 research and training program 2014-2018.
REFERENCES
- Abásolo D, Hornero R, Gómez C, García M, López M, 2006. Analysis of EEG background activity in Alzheimer’s disease patients with Lempel-Ziv complexity and central tendency measure. Med Eng Phys 28, 315–322. 10.1016/j.medengphy.2005.07.004 [DOI] [PubMed] [Google Scholar]
- Agosta F, Pievani M, Geroldi C, Copetti M, Frisoni GB, Filippi M, 2012. Resting state fMRI in Alzheimer’s disease: beyond the default mode network. Neurobiol. Aging 33, 1564–1578. 10.1016/j.neurobiolaging.2011.06.007 [DOI] [PubMed] [Google Scholar]
- Ahmed M, Darwish E, Khedr E, Serogy Y, Ali AJ, 2012. El Effects of low versus high frequencies of repetitive transcranial magnetic stimulation on cognitive function and cortical excitability in Alzheimer’s dementia. 59, 83–92. [DOI] [PubMed] [Google Scholar]
- Akerstedt T, Kecklund G, Ingre M, Lekander M, Axelsson J, 2009. Sleep homeostasis during repeated sleep restriction and recovery: support from EEG dynamics. Sleep February32, 217–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen EA, Erhardt EB, Damaraju E, Gruner W, Segall JM, Silva RF, Havlicek M, Rachakonda S, Fries J, Kalyanam R, Michael AM, Caprihan A, Turner JA, Eichele T, Adelsheim S, Bryan AD, Bustillo J, Clark VP, Feldstein Ewing SW, Filbey F, Ford CC, Hutchison K, Jung RE, Kiehl KA, Kodituwakku P, Komesu YM, Mayer AR, Pearlson GD, Phillips JP, Sadek JR, Stevens M, Teuscher U, Thoma RJ, Calhoun VD, 2011. A Baseline for the Multivariate Comparison of Resting-State Networks. Front. Syst. Neurosci 5. 10.3389/fnsys.2011.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aly M, Moscovitch M, 2010. The effects of sleep on episodic memory in older and younger adults. Memory 18, 327–334. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Amatniek J, Ascher S, Sadik K, Ramaswamy K, 2005. Effects of Galantamine Versus Donepezil on Sleep in Patients With Mild to Moderate Alzheimer Disease and Their Caregivers: A Double-Blind, Head-to-Head, Randomized Pilot Study. Alzheimer Disease & Associated Disorders 19, 240–245. 10.1097/01.wad.0000189052.48688.36 [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Ancoli-Israel S, Kripke DF, Kripke DF, Klauber MR, Mason WJ, Mason WJ, Fell R, Fell R, Kaplan O, 1991. Sleep-Disordered Breathing in Community-Dwelling Elderly. Sleep 14, 486–495. 10.1093/sleep/14.6.486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal A, Paulus W, 2013. Transcranial alternating current stimulation (tACS). Front Hum Neurosci. 3177SRC-BaiduScholar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atri A, Simoni E, Gorgoni M, Ferrara M, Ferlazzo F, Rossini P, Gennaro L, 2016. D’ De De Electrical stimulation of the frontal cortex enhances slow-frequency EEG activity and sleepiness. Neuroscience June2324SRC-BaiduScholar, 119–30. [DOI] [PubMed] [Google Scholar]
- Axmacher N, Henseler MM, Jensen O, Weinreich I, Elger CE, Fell J, 2010. Cross-frequency coupling supports multi-item working memory in the human hippocampus. PNAS 107, 3228–3233. 10.1073/pnas.0911531107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhaus J, Born J, Hoeckesfeld R, Fokuhl S, Hohagen F, Junghanns K, 2007. Midlife decline in declarative memory consolidation is correlated with a decline in slow wave sleep. Learn Mem 14, 336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluscio MA, Mizuseki K, Schmidt R, Kempter R, Buzsáki G, 2012. Cross-frequency phase-phase coupling between θ and γ oscillations in the hippocampus. J. Neurosci 32, 423–435. 10.1523/JNEUROSCI.4122-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE, 2008. Suprachiasmatic nucleus and melatonin: Reciprocal interactions and clinical correlations. Neurology 71, 594–598. 10.1212/01.wnl.0000324283.57261.37 [DOI] [PubMed] [Google Scholar]
- Bentwich J, Dobronevsky E, Aichenbaum S, Shorer R, Peretz R, Khaigrekht M, Marton R, Rabey JJ, 2011. Beneficial effect of repetitive transcranial magnetic stimulation combined with cognitive training for the treatment of Alzheimer’s disease: a proof of concept study. Transm Vienna March118, 463–71. [DOI] [PubMed] [Google Scholar]
- Binder S, Berg K, Gasca F, Lafon B, Parra L, Born J, Marshall L, 2014. Transcranial slow oscillation stimulation during sleep enhances memory consolidation in rats. Brain Stimul JulAug7, 508–15. [DOI] [PubMed] [Google Scholar]
- Bliese P, McGurk D, Thomas J, Balkin T, Wesensten N, 2007. Discontinuous growth modeling of adaptation to sleep setting changes: individual differences and age. Aviat Space Environ Med May78, 485–92. [PubMed] [Google Scholar]
- Bliwise DL, 1993a. Sleep in Normal Aging and Dementia. Sleep 16, 40–81. 10.1093/sleep/16.1.40 [DOI] [PubMed] [Google Scholar]
- Bliwise DL, 1993b. Sleep in Normal Aging and Dementia. Sleep 16, 40–81. 10.1093/sleep/16.1.40 [DOI] [PubMed] [Google Scholar]
- Boggio P, Ferrucci R, Mameli F, Martins D, Martins O, Vergari M, Tadini L, Scarpini E, Fregni F, Priori A, 2012. Prolonged visual memory enhancement after direct current stimulation in Alzheimer’s disease. Brain Stimul July5, 223–230. [DOI] [PubMed] [Google Scholar]
- Boggio P, Khoury L, Martins D, Martins O, de Macedo E, Fregni FJ, 2009. Temporal cortex direct current stimulation enhances performance on a visual recognition memory task in Alzheimer disease. Neurosurg Psychiatry April80, 444–7. [DOI] [PubMed] [Google Scholar]
- Bonnet M, 1989. The effect of sleep fragmentation on sleep and performance in younger and older subjects. Neurobiol Aging JanFeb10, 21–5. [DOI] [PubMed] [Google Scholar]
- Bonnet M, Arand D, 1989. Sleep loss in aging. Clin Geriatr Med May5, 405–20. [PubMed] [Google Scholar]
- Boyce R, Glasgow SD, Williams S, Adamantidis A, 2016. Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science 352, 812–816. 10.1126/science.aad5252 [DOI] [PubMed] [Google Scholar]
- Boyle M, International I, Diego C, EMBS, 2013. Fröhlich F EEG feedbackcontrolled transcranial alternating current stimulation in 6th Annual on Neural Engineering San.
- Branger P, Arenaza-Urquijo EM, Tomadesso C, Mézenge F, André C, de Flores R, Mutlu J, de La Sayette V, Eustache F, Chételat G, Rauchs G, 2016. Relationships between sleep quality and brain volume, metabolism, and amyloid deposition in late adulthood. Neurobiology of Aging 41, 107–114. 10.1016/j.neurobiolaging.2016.02.009 [DOI] [PubMed] [Google Scholar]
- Brem A, Schilberg L, Freitas C, Atkinson N, Seligson E, Pascual-Leone A, 2013. K, Effects of cognitive training and rTMS in Alzheimer’s disease. Alzheimers Dement P664 9 SRC-BaiduScholar. [Google Scholar]
- Brier MR, Thomas JB, Snyder AZ, Benzinger TL, Zhang D, Raichle ME, Holtzman DM, Morris JC, Ances BM, 2012. Loss of intranetwork and internetwork resting state functional connections with Alzheimer’s disease progression. J. Neurosci 32, 8890–8899. 10.1523/JNEUROSCI.5698-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B, Rainey-Smith S, Villemagne V, Weinborn M, Bucks R, Sohrabi H, Laws S, Taddei K, Macaulay S, Ames D, Fowler C, Maruff P, Masters C, Rowe C, Martins R, AIBL, 2016. Group.The Relationship between Sleep Quality and Brain Amyloid Burden. Sleep May 139, 1063–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CC, 1975. Electroanesthesia and electrosleep. Am Psychol 30, 402–410. 10.1037//0003-066x.30.3.402 [DOI] [PubMed] [Google Scholar]
- Buchsbaum M, Gillin J, Wu J, Hazlett E, Sicotte N, Dupont RWE, 1989. Bunney Regional cerebral glucose metabolic rate in human sleep assessed by positron emission tomography. Life Sci 45, 1349–56. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL, 2008. The Brain’s Default Network. Annals of the New York Academy of Sciences 1124, 1–38. 10.1196/annals.1440.011 [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA, 2005. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J. Neurosci 25, 7709–7717. 10.1523/JNEUROSCI.2177-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon-Garcidueñas AL, Duyckaerts C, 2018. Alzheimer disease, in: Handbook of Clinical Neurology. Elsevier, pp. 325–337. 10.1016/B978-0-12-802395-2.00023-7 [DOI] [PubMed] [Google Scholar]
- Cappon D, Jahanshahi M, Bisiacchi P, 2016. Value and Efficacy of Transcranial Direct Current Stimulation in the Cognitive Rehabilitation: A Critical Review Since 2000. Front Neurosci 10, 157. 10.3389/fnins.2016.00157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier J, Viens I, Poirier G, Robillard R, Lafortune M, Vandewalle G, Martin N, Barakat M, Paquet J, Filipini D, 2011. Sleep slow wave changes during the middle years of life: Changes in slow waves with age. European Journal of Neuroscience 33, 758–766. 10.1111/j.1460-9568.2010.07543.x [DOI] [PubMed] [Google Scholar]
- Casali AG, Gosseries O, Rosanova M, Boly M, Sarasso S, Casali KR, Casarotto S, Bruno M-A, Laureys S, Tononi G, Massimini M, 2013. A theoretically based index of consciousness independent of sensory processing and behavior. Sci Transl Med 5, 198ra105. 10.1126/scitranslmed.3006294 [DOI] [PubMed] [Google Scholar]
- Cassani R, Estarellas M, San-Martin R, Fraga FJ, Falk TH, 2018. Systematic Review on Resting-State EEG for Alzheimer’s Disease Diagnosis and Progression Assessment. Disease Markers 2018, 1–26. 10.1155/2018/5174815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano M, Ibanez-Soria D, Kroupi E, Acedo J, Campolo M, Martinez X, Soria-Frisch A, Valls-Sole J, Verma A, Ruffini G, 2017. Occipital tACS bursts during a visual task impact ongoing neural oscillation power, coherence and LZW complexity. bioRxiv 198788. 10.1101/198788 [DOI] [Google Scholar]
- Cauter E, Leproult R, Plat L, 2000. Van Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA 284, 861–868. [DOI] [PubMed] [Google Scholar]
- Chang C-H, Lane H-Y, Lin C-H, 2018. Brain Stimulation in Alzheimer’s Disease. Frontiers in Psychiatry 9. 10.3389/fpsyt.2018.00201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte F, Arzilli C, Errico BM, Giganti F, Iovino D, Ficca G, 2014. Sleep Measures Expressing “Functional Uncertainty” in Elderlies’ Sleep.’ Gerontology 60, 448–457. 10.1159/000358083 [DOI] [PubMed] [Google Scholar]
- Cooke JR, Ancoli-Israel S, 2011. Normal and abnormal sleep in the elderly, in: Handbook of Clinical Neurology. Elsevier, pp. 653–665. 10.1016/B978-0-444-52006-7.00041-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke JR, Loredo JS, Liu L, Marler M, Corey-Bloom J, Fiorentino L, Harrison T, Ancoli-Israel S, 2006. Acetylcholinesterase Inhibitors and Sleep Architecture in Patients with Alzheimer’s Disease: Drugs & Aging 23, 503–511. 10.2165/00002512-200623060-00005 [DOI] [PubMed] [Google Scholar]
- Copinschi G, Caufriez A, 2013. Sleep and Hormonal Changes in Aging. Endocrinology and Metabolism Clinics of North America 42, 371–389. 10.1016/j.ecl.2013.02.009 [DOI] [PubMed] [Google Scholar]
- Cotelli M, Calabria M, Manenti R, Rosini S, Maioli C, Zanetti O, Miniussi C, 2012. Brain stimulation improves associative memory in an individual with amnestic mild cognitive impairment. Neurocase June18, 217–23. [DOI] [PubMed] [Google Scholar]
- Cotelli M, Manenti R, Brambilla M, Petesi M, Rosini S, Ferrari C, Zanetti O, Miniussi C, 2014. Anodal tDCS during face-name associations memory training in Alzheimer’s patients. Front Aging Neurosci March19386SRC-BaiduScholar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotelli M, Manenti R, Cappa S, Geroldi C, Zanetti O, Rossini P, Miniussi C, 2006. Effect of transcranial magnetic stimulation on action naming in patients with Alzheimer disease. Arch Neurol November63, 1602–4. [DOI] [PubMed] [Google Scholar]
- Cotelli M, Manenti R, Cappa S, Zanetti O, Miniussi CJ, 2008. Transcranial magnetic stimulation improves naming in Alzheimer disease patients at different stages of cognitive decline. Eur December15, 1286–92. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA, 2000. Normal Brain Development and Aging: Quantitative Analysis at in Vivo MR Imaging in Healthy Volunteers. Radiology 216, 672–682. 10.1148/radiology.216.3.r00au37672 [DOI] [PubMed] [Google Scholar]
- Cricco M, Simonsick E, Foley DJ, 2001. The impact of insomnia on cognitive functioning in older adults. Geriatr Soc September49, 1185–9. [DOI] [PubMed] [Google Scholar]
- Crowley K, 2011. Sleep and Sleep Disorders in Older Adults. Neuropsychology Review 21, 41–53. 10.1007/s11065-010-9154-6 [DOI] [PubMed] [Google Scholar]
- Crowley K, 2002. The effects of normal aging on sleep spindle and K-complex production. Clinical Neurophysiology 113, 1615–1622. 10.1016/S1388-2457(02)00237-7 [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts S. a. R.B., Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF, 2006. Consistent resting-state networks across healthy subjects. PNAS 103, 13848–13853. 10.1073/pnas.0601417103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang-Vu TT, Bonjean M, Schabus M, Boly M, Darsaud A, Desseilles M, Degueldre C, Balteau E, Phillips C, Luxen A, Sejnowski TJ, Maquet P, 2011. Interplay between spontaneous and induced brain activity during human non-rapid eye movement sleep. Proceedings of the National Academy of Sciences 108, 15438–15443. 10.1073/pnas.1112503108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauvilliers YA, Lehmann S, Jaussent I, Gabelle A, 2014. Hypocretin and brain β-amyloid peptide interactions in cognitive disorders and narcolepsy. Front Aging Neurosci 6, 119. 10.3389/fnagi.2014.00119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauwels J, Vialatte F, Musha T, Cichocki A, 2010. A comparative study of synchrony measures for the early diagnosis of Alzheimer’s disease based on EEG. NeuroImage 49, 668–693. 10.1016/j.neuroimage.2009.06.056 [DOI] [PubMed] [Google Scholar]
- De Gennaro L, Ferrara M, 2003a. Sleep spindles: an overview. Sleep Medicine Reviews 7, 423–440. 10.1053/smrv.2002.0252 [DOI] [PubMed] [Google Scholar]
- De Gennaro L, Ferrara M, 2003b. Sleep spindles: an overview. Sleep Medicine Reviews 7, 423–440. 10.1053/smrv.2002.0252 [DOI] [PubMed] [Google Scholar]
- De Gennaro L, Gorgoni M, Reda F, Lauri G, Truglia I, Cordone S, Scarpelli S, Mangiaruga A, D’atri A, Lacidogna G, Ferrara M, Marra C, Rossini PM, 2017. The Fall of Sleep K-Complex in Alzheimer Disease. Scientific Reports 7. 10.1038/srep39688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gennaro L, Marzano C, Veniero D, Moroni F, Fratello F, Curcio G, Ferrara M, Ferlazzo F, Novelli L, Concetta Pellicciari M, Bertini M, Rossini PM, 2007. Neurophysiological correlates of sleepiness: a combined TMS and EEG study. Neuroimage 36, 1277–1287. 10.1016/j.neuroimage.2007.04.013 [DOI] [PubMed] [Google Scholar]
- De Havas JA, Parimal S, Soon CS, Chee MWL, 2012. Sleep deprivation reduces default mode network connectivity and anti-correlation during rest and task performance. NeuroImage 59, 1745–1751. 10.1016/j.neuroimage.2011.08.026 [DOI] [PubMed] [Google Scholar]
- Dement W, Richardson G, Prinz P, Carskadon M, Kripke D, Czeisler C, In C, 1985. Changes of sleep and wakefulness with age. Finch and E L Schneider Eds Handbook of the biology of aging pp New York Van Nostrand Reinhold Company 692–717SRC-BaiduScholar. [Google Scholar]
- Dennis EL, Thompson PM, 2014. Functional Brain Connectivity Using fMRI in Aging and Alzheimer’s Disease. Neuropsychol Rev 24, 49–62. 10.1007/s11065-014-9249-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslauriers J, Ansado J, Marrelec G, Provost J-S, Joanette Y, 2017. Increase of posterior connectivity in aging within the Ventral Attention Network: A functional connectivity analysis using independent component analysis. Brain Res. 1657, 288–296. 10.1016/j.brainres.2016.12.017 [DOI] [PubMed] [Google Scholar]
- Devi G, Voss HU, Levine D, Abrassart D, Heier L, Halper J, Martin L, Lowe S, 2014. Open-label, short-term, repetitive transcranial magnetic stimulation in patients with Alzheimer’s disease with functional imaging correlates and literature review. Am J Alzheimers Dis Other Demen 29, 248–255. 10.1177/1533317513517047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Profice P, Ranieri F, Ricci V, Bria P, Tonali PA, Ziemann U, 2007. Segregating two inhibitory circuits in human motor cortex at the level of GABAA receptor subtypes: a TMS study. Clin Neurophysiol 118, 2207–2214. 10.1016/j.clinph.2007.07.005 [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Born J, 2010. The memory function of sleep. Nat Rev Neurosci 11, 114–126. 10.1038/nrn2762 [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Büchel C, Born J, Rasch B, 2011. Labile or stable: opposing consequences for memory when reactivated during waking and sleep. Nat Neurosci 14, 381–386. 10.1038/nn.2744 [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Wilhelm I, Born J, 2009a. The whats and whens of sleep-dependent memory consolidation. Sleep Med Rev October13, 309–21. [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Wilhelm I, Born J, 2009b. The whats and whens of sleep-dependent memory consolidation. Sleep Med Rev October13, 309–21. [DOI] [PubMed] [Google Scholar]
- Dinner DS, 2002. Effect of sleep on epilepsy. J Clin Neurophysiol 19, 504–513. 10.1097/00004691-200212000-00003 [DOI] [PubMed] [Google Scholar]
- Donnell D, Silva E, Ronda J, Wang W, Duffy JJ, 2009. O’ Münch M, Comparison of subjective and objective assessments of sleep in healthy older subjects without sleep complaints. Res June18, 254–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE, 2006. A Core System for the Implementation of Task Sets. Neuron 50, 799–812. 10.1016/j.neuron.2006.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling GA, Burr RL, Van Someren EJW, Hubbard EM, Luxenberg JS, Mastick J, Cooper BA, 2008. Melatonin and Bright-Light Treatment for Rest–Activity Disruption in Institutionalized Patients with Alzheimer’s Disease: MELATONIN AND LIGHT THERAPY IN ALZHEIMER’S DISEASE. Journal of the American Geriatrics Society 56, 239–246. 10.1111/j.1532-5415.2007.01543.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube J, Lafortune M, Bedetti C, Bouchard M, Gagnon JF, Doyon J, Evans AC, Lina J-M, Carrier J, 2015. Cortical Thinning Explains Changes in Sleep Slow Waves during Adulthood. Journal of Neuroscience 35, 7795–7807. 10.1523/JNEUROSCI.3956-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy J, Dijk D, Klerman E, Czeisler CJ, 1998. Later endogenous circadian temperature nadir relative to an earlier wake time in older people. Am November275, R1478–87. [DOI] [PubMed] [Google Scholar]
- Dworak M, McCarley RW, Kim T, Kalinchuk AV, Basheer R, 2010. Sleep and Brain Energy Levels: ATP Changes during Sleep. Journal of Neuroscience 30, 9007–9016. 10.1523/JNEUROSCI.1423-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert T, Dorn H, Sauter C, Nitsche MA, Bajbouj M, Danker-Hopfe H, 2013. No Effects of Slow Oscillatory Transcranial Direct Current Stimulation (tDCS) on Sleep-Dependent Memory Consolidation in Healthy Elderly Subjects. Brain Stimulation 6, 938–945. 10.1016/j.brs.2013.05.006 [DOI] [PubMed] [Google Scholar]
- Eliasova I, Anderkova L, Marecek R, Rektorova IJ, 2014. Non-invasive brain stimulation of the right inferior frontal gyrus may improve attention in early Alzheimer’s disease: a pilot study. Sci 346SRC-BaiduScholar, 318–22. [DOI] [PubMed] [Google Scholar]
- Eshkoor S, Hamid T, Mun C, Ng C, 2015. Mild cognitive impairment and its management in older people. Clin Interv Aging Apr 1010 SRC-BaiduScholar, 687–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser SK, Hill SL, Tononi G, 2007. Sleep Homeostasis and Cortical Synchronization: I. Modeling the Effects of Synaptic Strength on Sleep Slow Waves. Sleep 30, 1617–1630. 10.1093/sleep/30.12.1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everson C, 2014a. Cell injury and repair resulting from sleep loss and sleep recovery in laboratory rats. Sleep 37, 1929–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everson C, 2014b. Cell injury and repair resulting from sleep loss and sleep recovery in laboratory rats. Sleep 37, 1929–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NUF, Church JA, Miezin FM, Schlaggar BL, Petersen SE, 2009. Functional Brain Networks Develop from a “Local to Distributed” Organization. PLOS Computational Biology 5, e1000381. 10.1371/journal.pcbi.1000381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farajnia S, Deboer T, Rohling JHT, Meijer JH, Michel S, 2014. Aging of the Suprachiasmatic Clock. The Neuroscientist 20, 44–55. 10.1177/1073858413498936 [DOI] [PubMed] [Google Scholar]
- Feinberg I, Koresko RL, Heller N, 1967a. EEG sleep patterns as a function of normal and pathological aging in man. Journal of Psychiatric Research 5, 107–144. 10.1016/0022-3956(67)90027-1 [DOI] [PubMed] [Google Scholar]
- Feinberg I, Koresko RL, Heller N, 1967b. EEG sleep patterns as a function of normal and pathological aging in man. Journal of Psychiatric Research 5, 107–144. 10.1016/0022-3956(67)90027-1 [DOI] [PubMed] [Google Scholar]
- Ferreira LK, Busatto GF, 2013. Resting-state functional connectivity in normal brain aging. Neuroscience & Biobehavioral Reviews 37, 384–400. 10.1016/j.neubiorev.2013.01.017 [DOI] [PubMed] [Google Scholar]
- Ferrucci R, Mameli F, Guidi I, Mrakic-Sposta S, Vergari M, Marceglia S, Cogiamanian F, Barbieri S, Scarpini E, Priori A, 2008. Transcranial direct current stimulation improves recognition memory in Alzheimer disease. Neurology August1271, 493–8. [DOI] [PubMed] [Google Scholar]
- Florin E, Baillet S, 2015. The brain’s resting-state activity is shaped by synchronized cross-frequency coupling of neural oscillations. Neuroimage 111, 26–35. 10.1016/j.neuroimage.2015.01.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley D, Monjan A, Masaki K, Ross W, Havlik R, White LJ, 2001. Daytime sleepiness is associated with 3-year incident dementia and cognitive decline in older Japanese-American men. Geriatr Soc 49SRC-BaiduScholar, 1628–1632. [DOI] [PubMed] [Google Scholar]
- Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG, 1995. Sleep Complaints Among Elderly Persons: An Epidemiologic Study of Three Communities. Sleep 18, 425–432. 10.1093/sleep/18.6.425 [DOI] [PubMed] [Google Scholar]
- Foley DJ, Vitiello MV, Bliwise DL, Ancoli-Israel S, Monjan AA, Walsh JK, 2007. Frequent Napping Is Associated With Excessive Daytime Sleepiness, Depression, Pain, and Nocturia in Older Adults: Findings From the National Sleep Foundation ‘2003 Sleep in America’ Poll. The American Journal of Geriatric Psychiatry 15, 344–350. 10.1097/01.JGP.0000249385.50101.67 [DOI] [PubMed] [Google Scholar]
- Frankel BL, 1974. Research on cerebral electrotherapy (electrosleep): some suggestions. Am J Psychiatry 131, 95–98. 10.1176/ajp.131.1.95 [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Frackowiak RSJ, 1993. Time-dependent changes in effective connectivity measured with PET. Human Brain Mapping 1, 69–79. 10.1002/hbm.460010108 [DOI] [Google Scholar]
- Gabelle A, Jaussent I, Bouallègue FB, Lehmann S, Lopez R, Barateau L, Grasselli C, Pesenti C, Verbizier D. de, Béziat S, Mariano‐Goulart D, Carlander B, Dauvilliers Y, 2019. Reduced brain amyloid burden in elderly patients with narcolepsy type 1. Annals of Neurology 85, 74–83. 10.1002/ana.25373 [DOI] [PubMed] [Google Scholar]
- Giambra L, Arenberg D, 1993. Adult age differences in forgetting sentences. Psychol Aging Sep 8, 451–62. [DOI] [PubMed] [Google Scholar]
- Gonsalvez I, Baror R, Fried P, Santarnecchi E, Pascual-Leone A, 2016. Therapeutic Noninvasive Brain Stimulation in Alzheimer’s Disease. Current Alzheimer Research 13, 1–1. 10.2174/1567205013666160930113907 [DOI] [PubMed] [Google Scholar]
- Gorgoni M, Lauri G, Truglia I, Cordone S, Sarasso S, Scarpelli S, Mangiaruga A, Atri A, Tempesta D, Ferrara M, Marra C, Rossini P, Gennaro L, 2016. D’ De Parietal Fast Sleep Spindle Density Decrease in Alzheimer’s Disease and Amnesic Mild Cognitive Impairment. Neural Plast 83761082016 SRC-BaiduScholar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goutagny R, Gu N, Cavanagh C, Jackson J, Chabot J, Quirion R, Krantic S, Williams SJ, 2013. G, Alterations in hippocampal network oscillations and theta-gamma coupling arise before Aβ overproduction in a mouse model of Alzheimer’s disease. Eur 37SRC-BaiduScholar, 1896–1902. [DOI] [PubMed] [Google Scholar]
- Grosmark AD, Mizuseki K, Pastalkova E, Diba K, Buzsáki G, 2012. REM Sleep Reorganizes Hippocampal Excitability. Neuron 75, 1001–1007. 10.1016/j.neuron.2012.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarnieri B, Adorni F, Musicco M, Appollonio I, Bonanni E, Caffarra P, Caltagirone C, Cerroni G, Concari L, Cosentino F, Ferrara S, Fermi S, Ferri R, Gelosa G, Lombardi G, Mazzei D, Mearelli S, Morrone E, Murri L, Nobili F, Passero S, Perri R, Rocchi R, Sucapane P, Tognoni G, Zabberoni S, Sorbi S, 2012. Prevalence of sleep disturbances in mild cognitive impairment and dementing disorders: a multicenter Italian clinical cross-sectional study on 431 patients. Dement Geriatr Cogn Disord 33, 50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guleyupoglu B, Schestatsky P, Edwards D, Fregni F, Bikson M, 2013. Classification of methods in transcranial electrical stimulation (tES) and evolving strategy from historical approaches to contemporary innovations. J. Neurosci. Methods 219, 297–311. 10.1016/j.jneumeth.2013.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guse B, Falkai P, Wobrock T, 2010. Cognitive effects of high-frequency repetitive transcranial magnetic stimulation: a systematic review. J Neural Transm (Vienna) 117, 105–122. 10.1007/s00702-009-0333-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halasz P, 2005. K-complex, a reactive EEG graphoelement of NREM sleep: an old chap in a new garment. Sleep Med Rev 9, 391–412. [DOI] [PubMed] [Google Scholar]
- Hanlon E, Vyazovskiy V, Ugo F, Tononi G, Cirelli C, 2011. Synaptic potentiation and sleep need: clues from molecular and electrophysiological studies. Curr Top Med Chem 11, 2472–2482. [DOI] [PubMed] [Google Scholar]
- Harand C, Bertran F, Joie RL, Landeau B, Mézenge F, Desgranges B, Peigneux P, Eustache F, Rauchs G, 2012. The Hippocampus Remains Activated over the Long Term for the Retrieval of Truly Episodic Memories. PLOS ONE 7, e43495. 10.1371/journal.pone.0043495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassainia F, Petit D, Nielsen T, Gauthier S, Montplaisir J, Quantitative E, 1997. and statistical mapping of wakefulness and REMsleep in the evaluation of mild to moderate Alzheimer’s disease. Eur Neurol 37, 219–224. [DOI] [PubMed] [Google Scholar]
- Hatfield CF, Herbert J, van Someren EJW, Hodges JR, Hastings MH, 2004. Disrupted daily activity/rest cycles in relation to daily cortisol rhythms of home-dwelling patients with early Alzheimer’s dementia. Brain 127, 1061–1074. 10.1093/brain/awh129 [DOI] [PubMed] [Google Scholar]
- Havekes R, Park AJ, Tudor JC, Luczak VG, Hansen RT, Ferri SL, Bruinenberg VM, Poplawski SG, Day JP, Aton SJ, Radwańska K, Meerlo P, Houslay MD, Baillie GS, Abel T, 2016. Sleep deprivation causes memory deficits by negatively impacting neuronal connectivity in hippocampal area CA1. eLife 5, e13424. 10.7554/eLife.13424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann CS, Rach S, Neuling T, Strüber D, 2013. Transcranial alternating current stimulation: a review of the underlying mechanisms and modulation of cognitive processes. Frontiers in Human Neuroscience 7. 10.3389/fnhum.2013.00279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuvel MP van den, Mandl RCW, Kahn RS, Pol HEH, 2009. Functionally linked resting‐state networks reflect the underlying structural connectivity architecture of the human brain. Human Brain Mapping 30, 3127–3141. 10.1002/hbm.20737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hita-Yañez E, Atienza M, Cantero JL, 2013. Polysomnographic and Subjective Sleep Markers of Mild Cognitive Impairment. Sleep 36, 1327–1334. 10.5665/sleep.2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch C, Kupfer D, Houck P, Berman S, Stack JJ, 1986. Reynolds 3rd CF, Sleep-disordered breathing in normal and pathologic aging. Psychiatry 499e503 47SRC-BaiduScholar. [PubMed] [Google Scholar]
- Holth JK, Patel TK, Holtzman DM, 2017. Sleep in Alzheimer’s Disease–Beyond Amyloid. Neurobiology of Sleep and Circadian Rhythms 2, 4–14. 10.1016/j.nbscr.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Wahlund L, Dierks T, Julin P, Winblad B, Jelic V, 2000. Discrimination of Alzheimer’s disease and mild cognitive impairment by equivalent EEG sources: a cross-sectional and longitudinal study. Clin Neurophysiol 111, 1961–1967. [DOI] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Ferrarelli F, Riedner BA, Peterson MJ, Tononi G, 2006. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat Neurosci 9, 1169–1176. 10.1038/nn1758 [DOI] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Tononi G, 2004. Local sleep and learning. Nature 430, 78–81. 10.1038/nature02663 [DOI] [PubMed] [Google Scholar]
- Hunt NJ, Rodriguez ML, Waters KA, Machaalani R, 2015. Changes in orexin (hypocretin) neuronal expression with normal aging in the human hypothalamus. Neurobiology of Aging 36, 292–300. 10.1016/j.neurobiolaging.2014.08.010 [DOI] [PubMed] [Google Scholar]
- Iaccarino H, Singer A, Martorell A, Rudenko A, Gao F, Gillingham T, Mathys H, Seo J, Kritskiy O, Abdurrob F, Adaikkan C, Canter R, Rueda R, Brown E, Boyden E, Tsai L, 2016. H.Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 540SRC-BaiduScholar, 230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilmoniemi RJ, Virtanen J, Ruohonen J, Karhu J, Aronen HJ, Näätänen R, Katila T, 1997. Neuronal responses to magnetic stimulation reveal cortical reactivity and connectivity: NeuroReport 8, 3537–3540. 10.1097/00001756-199711100-00024 [DOI] [PubMed] [Google Scholar]
- Jones AP, Choe J, Bryant NB, Robinson CSH, Ketz NA, Skorheim SW, Combs A, Lamphere ML, Robert B, Gill HA, Heinrich MD, Howard MD, Clark VP, Pilly PK, 2018a. Dose-Dependent Effects of Closed-Loop tACS Delivered During Slow-Wave Oscillations on Memory Consolidation. Frontiers in Neuroscience 12. 10.3389/fnins.2018.00867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AP, Choe J, Bryant NB, Robinson CSH, Ketz NA, Skorheim SW, Combs A, Lamphere ML, Robert B, Gill HA, Heinrich MD, Howard MD, Clark VP, Pilly PK, 2018b. Dose-Dependent Effects of Closed-Loop tACS Delivered During Slow-Wave Oscillations on Memory Consolidation. Frontiers in Neuroscience 12. 10.3389/fnins.2018.00867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DT, Knopman DS, Gunter JL, Graff-Radford J, Vemuri P, Boeve BF, Petersen RC, Weiner MW, Jack CR, Alzheimer’s Disease Neuroimaging Initiative, 2016. Cascading network failure across the Alzheimer’s disease spectrum. Brain 139, 547–562. 10.1093/brain/awv338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju Y, Ooms S, Sutphen C, Macauley S, Zangrilli M, Jerome G, Fagan A, Mignot E, Zempel J, Claassen J, Holtzman D, 2017. Slow wave sleep disruption increases cerebrospinal fluid amyloid-β levels. Brain. August1;:. 140, 2104–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju Y-ES, Lucey BP, Holtzman DM, 2014a. Sleep and Alzheimer disease pathology—a bidirectional relationship. Nature Reviews Neurology 10, 115–119. 10.1038/nrneurol.2013.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju Y-ES, Lucey BP, Holtzman DM, 2014b. Sleep and Alzheimer disease pathology—a bidirectional relationship. Nature Reviews Neurology 10, 115–119. 10.1038/nrneurol.2013.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakimoto A, Ito S, Okada H, Nishizawa S, Minoshima S, Ouchi Y, 2016. Age-Related Sex-Specific Changes in Brain Metabolism and Morphology. Journal of Nuclear Medicine 57, 221–225. 10.2967/jnumed.115.166439 [DOI] [PubMed] [Google Scholar]
- Kalpouzos G, Chételat G, Baron J-C, Landeau B, Mevel K, Godeau C, Barré L, Constans J-M, Viader F, Eustache F, Desgranges B, 2009. Voxel-based mapping of brain gray matter volume and glucose metabolism profiles in normal aging. Neurobiol. Aging 30, 112–124. 10.1016/j.neurobiolaging.2007.05.019 [DOI] [PubMed] [Google Scholar]
- Kang J, Lim M, Bateman R, Lee J, Smyth L, Cirrito J, Fujiki N, Nishino S, Holtzman D, 2009. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science November13326, 1005–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann T, Elvsåshagen T, Alnæs D, Zak N, Pedersen PØ, Norbom LB, Quraishi SH, Tagliazucchi E, Laufs H, Bjørnerud A, Malt UF, Andreassen OA, Roussos E, Duff EP, Smith SM, Groote IR, Westlye LT, 2016. The brain functional connectome is robustly altered by lack of sleep. NeuroImage 127, 324–332. 10.1016/j.neuroimage.2015.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbler G, Fripp J, Rowe C, Villemagne V, Salvado O, Rose S, Coulson E, 2014. Alzheimer’s Disease Neuroimaging Initiative. Basal forebrain atrophy correlates with amyloid burden in Alzheimers disease Neuroimage Clin November277SRC-BaiduScholar, 105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernbach JM, Yeo BTT, Smallwood J, Margulies DS, Thiebaut de Schotten M, Walter H, Sabuncu MR, Holmes AJ, Gramfort A, Varoquaux G, Thirion B, Bzdok D, 2018. Subspecialization within default mode nodes characterized in 10,000 UK Biobank participants. Proc. Natl. Acad. Sci. U.S.A 115, 12295–12300. 10.1073/pnas.1804876115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketz N, Jones AP, Bryant NB, Clark VP, Pilly PK, 2018a. Closed-Loop Slow-Wave tACS Improves Sleep-Dependent Long-Term Memory Generalization by Modulating Endogenous Oscillations. The Journal of Neuroscience 38, 7314–7326. 10.1523/JNEUROSCI.0273-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketz N, Jones AP, Bryant NB, Clark VP, Pilly PK, 2018b. Closed-Loop Slow-Wave tACS Improves Sleep-Dependent Long-Term Memory Generalization by Modulating Endogenous Oscillations. The Journal of Neuroscience 38, 7314–7326. 10.1523/JNEUROSCI.0273-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedr EM, Gamal NFE, El-Fetoh NA, Khalifa H, Ahmed EM, Ali AM, Noaman M, El-Baki AA, Karim AA, 2014. A Double-Blind Randomized Clinical Trial on the Efficacy of Cortical Direct Current Stimulation for the Treatment of Alzheimer’s Disease. Front Aging Neurosci 6. 10.3389/fnagi.2014.00275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Bonnì S, Pellicciari MC, Casula EP, Mancini M, Esposito R, Ponzo V, Picazio S, Di Lorenzo F, Serra L, Motta C, Maiella M, Marra C, Cercignani M, Martorana A, Caltagirone C, Bozzali M, 2018. Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer’s disease. Neuroimage 169, 302–311. 10.1016/j.neuroimage.2017.12.048 [DOI] [PubMed] [Google Scholar]
- Kujirai T, Sato M, Rothwell JC, Cohen LG, 1993. The effect of transcranial magnetic stimulation on median nerve somatosensory evoked potentials. Electroencephalogr Clin Neurophysiol 89, 227–234. [DOI] [PubMed] [Google Scholar]
- Kuo M-F, Nitsche MA, 2012. Effects of transcranial electrical stimulation on cognition. Clin EEG Neurosci 43, 192–199. 10.1177/1550059412444975 [DOI] [PubMed] [Google Scholar]
- Kupfer D, Taska L, Hoch C, Sewitch D, Restifo KJ, 1985. Reynolds 3rd CF, Sleep apnea in Alzheimer’s dementia: correlation with mental deterioration. Psychiatry 257e6146SRC-BaiduScholar. [PubMed] [Google Scholar]
- Kyrtsos CR, Baras JS, 2015. Modeling the Role of the Glymphatic Pathway and Cerebral Blood Vessel Properties in Alzheimer’s Disease Pathogenesis. PLOS ONE 10, e0139574. 10.1371/journal.pone.0139574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladenbauer J, Külzow N, Passmann S, Antonenko D, Grittner U, Tamm S, Flöel A, 2016. Brain stimulation during an afternoon nap boosts slow oscillatory activity and memory consolidation in older adults. NeuroImage 142, 311–323. 10.1016/j.neuroimage.2016.06.057 [DOI] [PubMed] [Google Scholar]
- Ladenbauer Julia, Ladenbauer Josef, Külzow N, de Boor R, Avramova E, Grittner U, Flöel A, 2017. Promoting Sleep Oscillations and Their Functional Coupling by Transcranial Stimulation Enhances Memory Consolidation in Mild Cognitive Impairment. The Journal of Neuroscience 37, 7111–7124. 10.1523/JNEUROSCI.0260-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafon B, Henin S, Huang Y, Friedman D, Melloni L, Thesen T, Doyle W, Buzsáki G, Devinsky O, Parra LC, Liu AA, 2017. Low frequency transcranial electrical stimulation does not entrain sleep rhythms measured by human intracranial recordings. Nature Communications 8. 10.1038/s41467-017-01045-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolt H-P, Dijk D-J, Achermann P, Borbély AA, 1996. Effect of age on the sleep EEG: slow-wave activity and spindle frequency activity in young and middle-aged men. Brain Research 738, 205–212. 10.1016/S0006-8993(96)00770-6 [DOI] [PubMed] [Google Scholar]
- Lane CA, Hardy J, Schott JM, 2018. Alzheimer’s disease. Eur. J. Neurol 25, 59–70. 10.1111/ene.13439 [DOI] [PubMed] [Google Scholar]
- Lee Y, Kim J, Jeong D, EEG, 2016. Sleep in Young and Elderly Patients with Obstructive Sleep Apnea Syndrome. Psychiatry Investig March13, 217–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefaucheur J-P, Antal A, Ayache SS, Benninger DH, Brunelin J, Cogiamanian F, Cotelli M, De Ridder D, Ferrucci R, Langguth B, Marangolo P, Mylius V, Nitsche MA, Padberg F, Palm U, Poulet E, Priori A, Rossi S, Schecklmann M, Vanneste S, Ziemann U, Garcia-Larrea L, Paulus W, 2017. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol 128, 56–92. 10.1016/j.clinph.2016.10.087 [DOI] [PubMed] [Google Scholar]
- Liguori C, Romigi A, Nuccetelli M, Zannino S, Sancesario G, Martorana A, Albanese M, Mercuri N, Izzi F, Bernardini S, Nitti A, Sancesario GM, Sica F, Marciani M, Placidi F, JAMA, 2014. Orexinergic system dysregulation, sleep impairment, and cognitive decline in Alzheimer disease. December71, 1498–505. [DOI] [PubMed] [Google Scholar]
- Lim ASP, Ellison BA, Wang JL, Yu L, Schneider JA, Buchman AS, Bennett DA, Saper CB, 2014. Sleep is related to neuron numbers in the ventrolateral preoptic/intermediate nucleus in older adults with and without Alzheimer’s disease. Brain 137, 2847–2861. 10.1093/brain/awu222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim ASP, Kowgier M, Yu L, Buchman AS, Bennett DA, 2013a. Sleep Fragmentation and the Risk of Incident Alzheimer’s Disease and Cognitive Decline in Older Persons. Sleep 36, 1027–1032. 10.5665/sleep.2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim ASP, Kowgier M, Yu L, Buchman AS, Bennett DA, 2013b. Sleep Fragmentation and the Risk of Incident Alzheimer’s Disease and Cognitive Decline in Older Persons. Sleep 36, 1027–1032. 10.5665/sleep.2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y-C, Feng Y, Zhan S-Q, Li N, Ding Y, Hou Y, Wang L, Lin H, Sun Y, Huang Z-Y, Xue Q, Wang Y-P, 2015. Repetitive Transcranial Magnetic Stimulation for the Treatment of Restless Legs Syndrome. Chin Med J (Engl) 128, 1728–1731. 10.4103/0366-6999.159344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Buzsáki G, 2008. A neural coding scheme formed by the combined function of gamma and theta oscillations. Schizophr Bull 34, 974–980. 10.1093/schbul/sbn060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Jensen O, 2013. The θ-γ neural code. Neuron 77, 1002–1016. 10.1016/j.neuron.2013.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás RR, Steriade M, 2006. Bursting of thalamic neurons and states of vigilance. J. Neurophysiol 95, 3297–3308. 10.1152/jn.00166.2006 [DOI] [PubMed] [Google Scholar]
- Loewenstein R, Weingartner H, Gillin J, Kaye W, Ebert M, Mendelson W, 1982. Disturbances of sleep and cognitive functioning in patients with dementia. Neurobiol Aging 3SRC-BaiduScholar, 371–377. [DOI] [PubMed] [Google Scholar]
- Lucey B, Hicks T, McLeland J, Toedebusch C, Boyd J, Elbert D, Patterson B, Baty J, Morris J, Ovod V, Mawuenyega K, Bateman R, 2017. Effect of sleep on overnight CSF amyloid-β kinetics. Annals of Neurology :. 83, 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucey BP, Bateman RJ, 2014. Amyloid-β diurnal pattern: possible role of sleep in Alzheimer’s disease pathogenesis. Neurobiology of Aging 35, S29–S34. 10.1016/j.neurobiolaging.2014.03.035 [DOI] [PubMed] [Google Scholar]
- Lustenberger C, Boyle MR, Alagapan S, Mellin JM, Vaughn BV, Fröhlich F, 2016a. Feedback-Controlled Transcranial Alternating Current Stimulation Reveals a Functional Role of Sleep Spindles in Motor Memory Consolidation. Current Biology 26, 2127–2136. 10.1016/j.cub.2016.06.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustenberger C, Boyle MR, Alagapan S, Mellin JM, Vaughn BV, Fröhlich F, 2016b. Feedback-Controlled Transcranial Alternating Current Stimulation Reveals a Functional Role of Sleep Spindles in Motor Memory Consolidation. Current Biology 26, 2127–2136. 10.1016/j.cub.2016.06.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A, 2000. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp Brain Res 133, 425–430. [DOI] [PubMed] [Google Scholar]
- Mander B, Marks S, Vogel J, Rao V, Lu B, Saletin J, Ancoli-Israel S, Jagust W, Walker M, 2015. amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat Neurosci July18, 1051–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander BA, Rao V, Lu B, Saletin JM, Lindquist JR, Ancoli-Israel S, Jagust W, Walker MP, 2013. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nature Neuroscience 16, 357–364. 10.1038/nn.3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander BA, Winer JR, Jagust WJ, Walker MP, 2016. Sleep: A Novel Mechanistic Pathway, Biomarker, and Treatment Target in the Pathology of Alzheimer’s Disease? Trends in Neurosciences 39, 552–566. 10.1016/j.tins.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manenti R, Cotelli M, Robertson IH, Miniussi C, 2012. Transcranial brain stimulation studies of episodic memory in young adults, elderly adults and individuals with memory dysfunction: A review. Brain Stimulation 5, 103–109. 10.1016/j.brs.2012.03.004 [DOI] [PubMed] [Google Scholar]
- Marshall L, Born J, 2011. Brain Stimulation During Sleep. Sleep Medicine Clinics 6, 85–95. 10.1016/j.jsmc.2010.12.003 [DOI] [Google Scholar]
- Marshall L, Hallschmid M, Born JJ, 2004. Mölle M, Transcranial direct current stimulation during sleep improves declarative memory. Nov 324, 9985–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Helgadóttir H, Mölle M, Born J, 2006. Boosting slow oscillations during sleep potentiates memory. Nature 444, 610–613. 10.1038/nature05278 [DOI] [PubMed] [Google Scholar]
- Martin N, Lafortune M, Godbout J, Barakat M, Robillard R, Poirier G, Bastien C, Carrier J, 2013. Topography of age-related changes in sleep spindles. Neurobiology of Aging 34, 468–476. 10.1016/j.neurobiolaging.2012.05.020 [DOI] [PubMed] [Google Scholar]
- Mary A, Schreiner S, Peigneux P, 2013. Accelerated long-term forgetting in aging and intra-sleep awakenings. Front Psychol October167504SRC-BaiduScholar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimini M, Ferrarelli F, Esser SK, Riedner BA, Huber R, Murphy M, Peterson MJ, Tononi G, 2007. Triggering sleep slow waves by transcranial magnetic stimulation. Proceedings of the National Academy of Sciences 104, 8496–8501. 10.1073/pnas.0702495104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimini M, Ferrarelli F, Huber R, Esser SK, Singh H, Tononi G, 2005. Breakdown of cortical effective connectivity during sleep. Science 309, 2228–2232. 10.1126/science.1117256 [DOI] [PubMed] [Google Scholar]
- Massimini M, Ferrarelli F, Sarasso S, Tononi G, 2012. Cortical mechanisms of loss of consciousness: insight from TMS/EEG studies. Arch Ital Biol 150, 44–55. 10.4449/aib.v150i2.1361 [DOI] [PubMed] [Google Scholar]
- Miranda PC, Mekonnen A, Salvador R, Ruffini G, 2013. The electric field in the cortex during transcranial current stimulation. Neuroimage 70, 48–58. 10.1016/j.neuroimage.2012.12.034 [DOI] [PubMed] [Google Scholar]
- Mishima K, Okawa M, Shimizu TJ, 2001. Diminished melatonin secretion in the elderly caused by insufficient environmental illumination. Endocrinol Metab 12986SRC-BaiduScholar. [DOI] [PubMed] [Google Scholar]
- Misonou H, Morishima-Kawashima M, Ihara Y, 2000. Oxidative stress induces intracellular accumulation of amyloid beta-protein (Abeta) in human neuroblastoma cells. Biochemistry June1339, 6951–9. [DOI] [PubMed] [Google Scholar]
- Monk TH, 2005. Aging Human Circadian Rhythms: Conventional Wisdom May Not Always Be Right. Journal of Biological Rhythms 20, 366–374. 10.1177/0748730405277378 [DOI] [PubMed] [Google Scholar]
- Montgomery DC, Woodall WH, 2008. An Overview of Six Sigma. International Statistical Review 76, 329–346. 10.1111/j.1751-5823.2008.00061.x [DOI] [Google Scholar]
- Montplaisir J, Petit D, Gauthier S, Gaudreau H, 1998. Décary A Sleep disturbances and eeg slowing in alzheimers disease Sleep Res Online 1, 147–51. [PubMed] [Google Scholar]
- Montplaisir J, Petit D, Lorrain D, Gauthier S, Nielsen T, 1995. Sleep in Alzheimer’s disease: further considerations on the role of brainstem and forebrain cholinergic populations in sleep-wake mechanisms. Sleep April18, 145–8. [DOI] [PubMed] [Google Scholar]
- Mueller SG, Weiner MW, 2009. Selective effect of age, Apo e4, and Alzheimer’s disease on hippocampal subfields. Hippocampus 19, 558–564. 10.1002/hipo.20614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münch M, Knoblauch V, Blatter K, Schröder C, Schnitzler C, Kräuchi K, Wirz-Justice A, Cajochen C, 2005. Age-related attenuation of the evening circadian arousal signal in humans. Neurobiology of Aging 26, 1307–1319. 10.1016/j.neurobiolaging.2005.03.004 [DOI] [PubMed] [Google Scholar]
- Nardone R, Bergmann J, Kunz A, Christova M, Brigo F, Tezzon F, Trinka E, Golaszewski S, 2012. Cortical afferent inhibition is reduced in patients with idiopathic REM sleep behavior disorder and cognitive impairment: A TMS study. Sleep Medicine 13, 919–925. 10.1016/j.sleep.2012.03.009 [DOI] [PubMed] [Google Scholar]
- Nguyen J, Suarez A, Kemoun G, Meignier M, Saout E, Damier P, Nizard J, Lefaucheur J, 2017. Le Repetitive transcranial magnetic stimulation combined with cognitive training for the treatment of Alzheimer’s disease. Neurophysiol Clin February47, 47–53. [DOI] [PubMed] [Google Scholar]
- Nir Y, Staba RJ, Andrillon T, Vyazovskiy VV, Cirelli C, Fried I, Tononi G, 2011. Regional Slow Waves and Spindles in Human Sleep. Neuron 70, 153–169. 10.1016/j.neuron.2011.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche M, Seeber A, Frommann K, Klein C, Rochford C, Nitsche MS, Fricke K, Liebetanz D, Lang N, Antal A, Paulus W, Tergau F, 2005. a,. Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J Physiol 568, 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W, 2000. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. (Lond.) 527 Pt 3, 633–639. 10.1111/j.1469-7793.2000.t01-1-00633.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV, 2004a. Meta-Analysis of Quantitative Sleep Parameters From Childhood to Old Age in Healthy Individuals: Developing Normative Sleep Values Across the Human Lifespan. Sleep 27, 1255–1273. 10.1093/sleep/27.7.1255 [DOI] [PubMed] [Google Scholar]
- Onoda K, Ishihara M, Yamaguchi S, 2012. Decreased Functional Connectivity by Aging Is Associated with Cognitive Decline. Journal of Cognitive Neuroscience 24, 2186–2198. 10.1162/jocn_a_00269 [DOI] [PubMed] [Google Scholar]
- Osorio R, Gumb T, Pirraglia E, Varga A, Lu S, Lim J, Wohlleber M, Ducca E, Koushyk V, Glodzik L, Mosconi L, Ayappa I, Rapoport D, de Leon M, 2015. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology May1284, 1964–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott EF, Spencer RMC, 2011. Age-related changes in the cognitive function of sleep, in: Progress in Brain Research. Elsevier, pp. 75–89. 10.1016/B978-0-444-53752-2.00012-6 [DOI] [PubMed] [Google Scholar]
- Papalambros NA, Santostasi G, Malkani RG, Braun R, Weintraub S, Paller KA, Zee PC, 2017. Acoustic Enhancement of Sleep Slow Oscillations and Concomitant Memory Improvement in Older Adults. Frontiers in Human Neuroscience 11. 10.3389/fnhum.2017.00109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paßmann S, Külzow N, Ladenbauer J, Antonenko D, Grittner U, Tamm S, Flöel A, 2016. Boosting Slow Oscillatory Activity Using tDCS during Early Nocturnal Slow Wave Sleep Does Not Improve Memory Consolidation in Healthy Older Adults. Brain Stimulation 9, 730–739. 10.1016/j.brs.2016.04.016 [DOI] [PubMed] [Google Scholar]
- Paulus W, 2011a. Transcranial electrical stimulation (tES-tDCS; tRNS, tACS) methods. Neuropsychol. Rehabil. 21SRC-BaiduScholar, 602–617. [DOI] [PubMed] [Google Scholar]
- Paulus W, 2011b. Transcranial electrical stimulation (tES-tDCS; tRNS, tACS) methods. Neuropsychol. Rehabil. 21SRC-BaiduScholar, 602–617. [DOI] [PubMed] [Google Scholar]
- Paulus W, Antal A, Nitsche M, 2013. Physiological Basis and Methodological Aspects of Transcranial Electric Stimulation Transcranial alternating current stimulation (tACS). Front. Hum. Neurosci 317. 7SRC-BaiduScholar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter-Derex L, Yammine P, Bastuji H, Croisile B, 2015. Sleep and Alzheimer’s disease. Sleep Medicine Reviews 19, 29–38. 10.1016/j.smrv.2014.03.007 [DOI] [PubMed] [Google Scholar]
- Petit D, Gagnon J-F, Fantini ML, Ferini-Strambi L, Montplaisir J, 2004. Sleep and quantitative EEG in neurodegenerative disorders. Journal of Psychosomatic Research 56, 487–496. 10.1016/j.jpsychores.2004.02.001 [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO, 1994. A Quantitative Magnetic Resonance Imaging Study of Changes in Brain Morphology From Infancy to Late Adulthood. Archives of Neurology 51, 874–887. 10.1001/archneur.1994.00540210046012 [DOI] [PubMed] [Google Scholar]
- Prince T-M, Abel T, 2013. The impact of sleep loss on hippocampal function. Learn Mem 20, 558–569. 10.1101/lm.031674.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz PN, Vitaliano PP, Vitiello MV, Bokan J, Raskind M, Peskind E, Gerber C, 1982. Sleep, EEG and mental function changes in senile dementia of the Alzheimer’s type. Neurobiology of Aging 3, 361–370. 10.1016/0197-4580(82)90024-0 [DOI] [PubMed] [Google Scholar]
- Rabey J, Dobronevsky E, Aichenbaum S, Gonen O, Marton R, Khaigrekht MJ, 2013. Repetitive transcranial magnetic stimulation combined with cognitive training is a safe and effective modality for the treatment of Alzheimer’s disease: a randomized, double-blind study. Transm 120SRC-BaiduScholar, 813–819. [DOI] [PubMed] [Google Scholar]
- Rauchs G, Schabus M, Parapatics S, Bertran F, Clochon P, Hot P, Denise P, Desgranges B, Eustache F, Gruber G, Anderer P, 2008. Is there a link between sleep changes and memory in Alzheimer’s disease? Neuroreport. July16;:. 19, 1159–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redline S, Kirchner HL, Quan SF, Gottlieb DJ, Kapur V, Newman A, 2004. The Effects of Age, Sex, Ethnicity, and Sleep-Disordered Breathing on Sleep Architecture. Archives of Internal Medicine 164, 406. 10.1001/archinte.164.4.406 [DOI] [PubMed] [Google Scholar]
- Reitz C, Mayeux R, 2014. Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochem Pharmacol 88, 640–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C, 2003. Longitudinal Magnetic Resonance Imaging Studies of Older Adults: A Shrinking Brain. The Journal of Neuroscience 23, 3295–3301. 10.1523/JNEUROSCI.23-08-03295.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds C, Buysse D, Kupfer D, Hoch C, Houck P, Matzzie J, George C, 1990. rd,Rapid eye movement sleep deprivation as a probe in elderly subjects. Arch Gen Psychiatry December47, 1128–36. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Rothwell JC, 2007. Is there a future for therapeutic use of transcranial magnetic stimulation? Nat. Rev. Neurosci 8, 559–567. 10.1038/nrn2169 [DOI] [PubMed] [Google Scholar]
- Riemann D, Spiegelhalder K, Feige B, Voderholzer U, Berger M, Perlis M, Nissen C, 2010. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Medicine Reviews 19–31. [DOI] [PubMed] [Google Scholar]
- Rizzo V, Aricò I, Mastroeni C, Morgante F, Liotta G, Girlanda P, Silvestri R, Quartarone A, 2009. Dopamine agonists restore cortical plasticity in patients with idiopathic restless legs syndrome. Mov. Disord 24, 710–715. 10.1002/mds.22436 [DOI] [PubMed] [Google Scholar]
- Roberts R, Knopman DS, 2013. Classification and epidemiology of MCI. Clin. Geriatr. Med 29, 753–772. 10.1016/j.cger.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson C, Bryant N, Maxwell J, Jones A, Robert B, Lamphere M, Combs A, Al Azzawi H, Gibson B, Sanguinetti J, Ketz N, Pilly P, Clark V, 2018. The Benefits of Closed-Loop Transcranial Alternating Current Stimulation on Subjective Sleep Quality. Brain Sciences 8, 204. 10.3390/brainsci8120204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh JH, Jiang H, Finn MB, Stewart FR, Mahan TE, Cirrito JR, Heda A, Snider BJ, Li M, Yanagisawa M, de Lecea L, Holtzman DM, 2014. Potential role of orexin and sleep modulation in the pathogenesis of Alzheimer’s disease. J. Exp. Med 211, 2487–2496. 10.1084/jem.20141788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh T, Song K, Cho H, Shin D, Yoo H, IEEE, 2014. A wearable neuro-feedback system with EEG-based mental status monitoring and transcranial electrical stimulation. Biomed Circuits Syst 8SRC-BaiduScholar, 755–764. [DOI] [PubMed] [Google Scholar]
- Rolls A, 2012. Hypothalamic Control of Sleep in Aging. NeuroMolecular Medicine 14, 139–153. 10.1007/s12017-012-8175-0 [DOI] [PubMed] [Google Scholar]
- Rosanova M, Casali A, Bellina V, Resta F, Mariotti M, Massimini MJ, 2009. Natural frequencies of human corticothalamic circuits. Jun 1729, 7679–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A, Safety of TMS Consensus Group, 2009. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 120, 2008–2039. 10.1016/j.clinph.2009.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Rossi S, 2007. Transcranial magnetic stimulation: diagnostic, therapeutic, and research potential. Neurology 68, 484–488. 10.1212/01.wnl.0000250268.13789.b2 [DOI] [PubMed] [Google Scholar]
- Ruffini G, Fox MD, Ripolles O, Miranda PC, Pascual-Leone A, 2014. Optimization of multifocal transcranial current stimulation for weighted cortical pattern targeting from realistic modeling of electric fields. Neuroimage 89, 216–225. 10.1016/j.neuroimage.2013.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffini G, Ibañez D, Kroupi E, Gagnon J-F, Montplaisir J, Postuma RB, Castellano M, Soria-Frisch A, 2019. Algorithmic Complexity of EEG for Prognosis of Neurodegeneration in Idiopathic Rapid Eye Movement Behavior Disorder (RBD). Annals of Biomedical Engineering 47, 282–296. 10.1007/s10439-018-02112-0 [DOI] [PubMed] [Google Scholar]
- Ruffini G, Wendling F, Merlet I, Molaee-Ardekani B, Mekonnen A, Salvador R, Soria-Frisch A, Grau C, Dunne S, Miranda PC, 2013. Transcranial Current Brain Stimulation (tCS): Models and Technologies. IEEE Transactions on Neural Systems and Rehabilitation Engineering 21, 333–345. 10.1109/TNSRE.2012.2200046 [DOI] [PubMed] [Google Scholar]
- Ruffini G, Wendling F, Sanchez-Todo R, Santarnecchi E, 2018. Targeting brain networks with multichannel transcranial current stimulation (tCS). Current Opinion in Biomedical Engineering, Neural Engineering/ Novel Biomedical Technologies: Neuromodulation 8, 70–77. 10.1016/j.cobme.2018.11.001 [DOI] [Google Scholar]
- Santarnecchi E, Bianco C, Sicilia I, Momi D, Lorenzo G, Ferrone S, Sprugnoli G, Rossi S, Rossi A, 2018. Del Di Age of Insomnia Onset Correlates with a Reversal of Default Mode Network and Supplementary Motor Cortex Connectivity. Neural Plast April136785342018 SRC-BaiduScholar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarnecchi E, Emmendorfer A, Tadayon S, Rossi S, Rossi A, Pascual-Leone A, 2017. Network connectivity correlates of variability in fluid intelligence performance. Intelligence 65, 35–47. 10.1016/j.intell.2017.10.002 [DOI] [Google Scholar]
- Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE, 2010. Sleep State Switching. Neuron 68, 1023–1042. 10.1016/j.neuron.2010.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Tohyama K, Kanbara Y, Otsuka K, Ehara S, Sakai A, 2006. Shibata E1, Age-related changes in locus ceruleus on neuromelanin magnetic resonance imaging at 3 Tesla. Magn Reson Med Sci December5, 197–200. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Heise KF, Gruber WR, Holz E, Karim AA, Glennon M, Gerloff C, Birbaumer N, Hummel FC, 2009. Brain oscillatory substrates of visual short-term memory capacity. Curr. Biol 19, 1846–1852. 10.1016/j.cub.2009.08.062 [DOI] [PubMed] [Google Scholar]
- Scheffzük C, Kukushka VI, Vyssotski AL, Draguhn A, Tort ABL, Brankačk J, 2011. Selective Coupling between Theta Phase and Neocortical Fast Gamma Oscillations during REM-Sleep in Mice. PLOS ONE 6, e28489. 10.1371/journal.pone.0028489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scullin MK, 2017. Do Older Adults Need Sleep? A Review of Neuroimaging, Sleep, and Aging Studies. Curr Sleep Medicine Rep 3, 204–214. 10.1007/s40675-017-0086-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scullin MK, 2013. Sleep, memory, and aging: The link between slow-wave sleep and episodic memory changes from younger to older adults. Psychology and Aging 28, 105–114. 10.1037/a0028830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scullin MK, Bliwise DL, 2015. Sleep, Cognition, and Normal Aging: Integrating a Half Century of Multidisciplinary Research. Perspectives on Psychological Science 10, 97–137. 10.1177/1745691614556680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeck-Hirschner M, Baier P, Weinhold S, Dittmar M, Heiermann S, Aldenhoff J, Goder R, 2012. Declarative memory performance is associated with the number of sleep spindles in elderly women. American Journal of Geriatric Psychiatry 20SRC-BaiduScholar, 782–788. [DOI] [PubMed] [Google Scholar]
- Sergeev GV, 1963. ELECTROSLEEP AS A METHOD OF NEUROTROPIC THERAPY OF PATIENTS WITH HYPERTENSIVE DISEASE. Am. Heart J 66, 138–139. 10.1016/0002-8703(63)90081-4 [DOI] [PubMed] [Google Scholar]
- Sheline YI, Morris JC, Snyder AZ, Price JL, Yan Z, D’Angelo G, Liu C, Dixit S, Benzinger T, Fagan A, Goate A, Mintun MA, 2010. APOE4 Allele Disrupts Resting State fMRI Connectivity in the Absence of Amyloid Plaques or Decreased CSF Aβ42. J. Neurosci 30, 17035–17040. 10.1523/JNEUROSCI.3987-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva E, Wang W, Ronda J, Wyatt J, Duffy J, 2010. Circadian and wake-dependent influences on subjective sleepiness, cognitive throughput, and reaction time performance in older and young adults. Sleep April33, 481–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Toga AW, 2004. Mapping Changes in the Human Cortex throughout the Span of Life. The Neuroscientist 10, 372–392. 10.1177/1073858404263960 [DOI] [PubMed] [Google Scholar]
- Spira A, Gamaldo A, An Y, Wu M, Simonsick E, Bilgel M, Zhou Y, Wong D, Ferrucci L, Resnick S, JAMA, 2013a. Self-reported sleep and β-amyloid deposition in community-dwelling older adults. December70, 1537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira A, Gamaldo A, An Y, Wu M, Simonsick E, Bilgel M, Zhou Y, Wong D, Ferrucci L, Resnick S, JAMA, 2013b. Self-reported sleep and β-amyloid deposition in community-dwelling older adults. December70, 1537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprecher KE, Koscik RL, Carlsson CM, Zetterberg H, Blennow K, Okonkwo OC, Sager MA, Asthana S, Johnson SC, Benca RM, Bendlin BB, 2017. Poor sleep is associated with CSF biomarkers of amyloid pathology in cognitively normal adults. Neurology 89, 445–453. 10.1212/WNL.0000000000004171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng R Nathan, Stevens WD, Viviano JD, Schacter DL, 2016. Attenuated anticorrelation between the default and dorsal attention networks with aging: evidence from task and rest. Neurobiology of Aging 45, 149–160. 10.1016/j.neurobiolaging.2016.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Viviano JD, Schacter DL, 2016. Attenuated anticorrelation between the default and dorsal attention networks with aging: evidence from task and rest. Neurobiol. Aging 45, 149–160. 10.1016/j.neurobiolaging.2016.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaab DF, Fliers E, Partiman TS, 1985. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Research 342, 37–44. 10.1016/0006-8993(85)91350-2 [DOI] [PubMed] [Google Scholar]
- Tabuchi M, Lone S, Liu S, Liu Q, Zhang J, Spira A, Wu M, 2015. Sleep interacts with aβ to modulate intrinsic neuronal excitability. Curr Biol March1625, 702–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi S, Mima T, Murai R, Shimazu H, Isomura Y, Tsujimoto T, 2015. Gamma Oscillations and Their Cross-frequency Coupling in the Primate Hippocampus during Sleep. Sleep 38, 1085–1091. 10.5665/sleep.4818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavakoli AV, Yun K, 2017. Transcranial Alternating Current Stimulation (tACS) Mechanisms and Protocols. Frontiers in Cellular Neuroscience 11. 10.3389/fncel.2017.00214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thambisetty M, Wan J, Carass A, An Y, Prince JL, Resnick SM, 2010. Longitudinal changes in cortical thickness associated with normal aging. NeuroImage 52, 1215–1223. 10.1016/j.neuroimage.2010.04.258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson H, 2018. How flashing lights and pink noise might banish Alzheimer’s, improve memory and more. Nature March1555, 20–22. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND, 2012. Aging and functional brain networks. Mol Psychiatry 17, 549–558. 10.1038/mp.2011.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, 2004. An information integration theory of consciousness. BMC Neurosci 5, 42. 10.1186/1471-2202-5-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Cirelli C, 2006a. Sleep function and synaptic homeostasis. Sleep Medicine Reviews 10, 49–62. 10.1016/j.smrv.2005.05.002 [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C, 2006b. Sleep function and synaptic homeostasis. Sleep Medicine Reviews 10, 49–62. 10.1016/j.smrv.2005.05.002 [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C, 2003. Sleep and synaptic homeostasis: a hypothesis. Brain Res. Bull 62, 143–150. [DOI] [PubMed] [Google Scholar]
- Tranah G, Blackwell T, Stone K, Ancoli-Israel S, Paudel M, Ensrud K, 2011. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol 70, 722–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AMC, Biswal BB, Castellanos FX, Milham MP, 2009. Functional connectivity of default mode network components: Correlation, anticorrelation, and causality. Human Brain Mapping 30, 625–637. 10.1002/hbm.20531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero-Cabré A, Amengual JL, Stengel C, Pascual-Leone A, Coubard OA, 2017. Transcranial magnetic stimulation in basic and clinical neuroscience: A comprehensive review of fundamental principles and novel insights. Neuroscience & Biobehavioral Reviews 83, 381–404. 10.1016/j.neubiorev.2017.10.006 [DOI] [PubMed] [Google Scholar]
- Valls-Solé J, Pascual-Leone A, Wassermann EM, Hallett M, 1992. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol 85, 355–364. [DOI] [PubMed] [Google Scholar]
- Varga A, Ducca E, Kishi A, Fischer E, Parekh A, Koushyk V, Yau P, Gumb T, Leibert D, Wohlleber M, Burschtin O, Convit A, Rapoport D, Osorio R, Ayappa I, 2016. Effects of aging on slow-wave sleep dynamics and human spatial navigational memory consolidation. eurobiol Aging June42SRC-BaiduScholar, 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij IM, Romeijn N, Smit DJ, Piantoni G, Van Someren EJ, van der Werf YD, 2014. Sleep deprivation leads to a loss of functional connectivity in frontal brain regions. BMC Neuroscience 15, 88. 10.1186/1471-2202-15-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villafuerte G, Miguel-Puga A, Machado S, Manjarrez E, 2015. Rodríguez EM, Arias-Carrión O. Sleep deprivation and oxidative stress in animal models a systematic review Oxid Med Cell Longev 2349522015 SRC-BaiduScholar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello M, Prinz P, 1989. Alzheimer’s disease. Sleep and sleepwake patterns Clin Geriatr Med May5, 289–99. [PubMed] [Google Scholar]
- von Richthofen CL, Mellor CS, 1979. Cerebral electrotherapy: methodological problems in assessing its therapeutic effectiveness. Psychol Bull 86, 1264–1271. [PubMed] [Google Scholar]
- Voss U, Holzmann R, Hobson A, Paulus W, Koppehele-Gossel J, Klimke A, Nitsche MA, 2014a. Induction of self awareness in dreams through frontal low current stimulation of gamma activity. Nature Neuroscience 17, 810–812. 10.1038/nn.3719 [DOI] [PubMed] [Google Scholar]
- Voss U, Holzmann R, Hobson A, Paulus W, Koppehele-Gossel J, Klimke A, Nitsche MA, 2014b. Induction of self awareness in dreams through frontal low current stimulation of gamma activity. Nature Neuroscience 17, 810–812. 10.1038/nn.3719 [DOI] [PubMed] [Google Scholar]
- Wang JL, Lim AS, Chiang W-Y, Hsieh W-H, Lo M-T, Schneider JA, Buchman AS, Bennett DA, Hu K, Saper CB, 2015. Suprachiasmatic neuron numbers and rest-activity circadian rhythms in older humans: SCN and Rest-Activity Rhythms. Annals of Neurology 78, 317–322. 10.1002/ana.24432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Hanajima R, Shirota Y, Ohminami S, Tsutsumi R, Terao Y, Ugawa Y, Hirose S, Miyashita Y, Konishi S, Kunimatsu A, Ohtomo K, 2014. Bidirectional effects on interhemispheric resting-state functional connectivity induced by excitatory and inhibitory repetitive transcranial magnetic stimulation. Hum Brain Mapp May;35(5):1896–905. 10.1002/hbm.22300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wdos S, Poyares D, Guilleminault C, Ramos L, Bertolucci P, Tufik S, 2006. Moraes The effect of donepezil on sleep and REM sleep EEG in patients with Alzheimer disease: a double-blind placebo-controlled study. Sleep February29, 199–205. [DOI] [PubMed] [Google Scholar]
- Wei HG, Riel E, Czeisler CA, Dijk D-J, 1999. Attenuated amplitude of circadian and sleep-dependent modulation of electroencephalographic sleep spindle characteristics in elderly human subjects. Neuroscience Letters 260, 29–32. 10.1016/S0304-3940(98)00851-9 [DOI] [PubMed] [Google Scholar]
- Weller R, Preston S, Subash M, Carare R, 2009. Cerebral amyloid angiopathy in the aetiology and immunotherapy of Alzheimer disease. Alzheimers Res Ther October1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennström M, Londos E, Minthon L, Nielsen HM, 2012. Altered CSF orexin and α-synuclein levels in dementia patients. J. Alzheimers Dis 29, 125–132. 10.3233/JAD-2012-111655 [DOI] [PubMed] [Google Scholar]
- Westerberg CE, Florczak SM, Weintraub S, Mesulam M-M, Marshall L, Zee PC, Paller KA, 2015. Memory improvement via slow-oscillatory stimulation during sleep in older adults. Neurobiology of Aging 36, 2577–2586. 10.1016/j.neurobiolaging.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerberg CE, Mander BA, Florczak SM, Weintraub S, Mesulam M-M, Zee PC, Paller KA, 2012a. Concurrent Impairments in Sleep and Memory in Amnestic Mild Cognitive Impairment. Journal of the International Neuropsychological Society 18, 490–500. 10.1017/S135561771200001X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerberg CE, Mander BA, Florczak SM, Weintraub S, Mesulam M-M, Zee PC, Paller KA, 2012b. Concurrent Impairments in Sleep and Memory in Amnestic Mild Cognitive Impairment. Journal of the International Neuropsychological Society 18, 490–500. 10.1017/S135561771200001X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlye L, Walhovd K, Dale A, Bjornerud A, Due-Tonnessen P, Engvig A, Grydeland H, Tamnes C, Ostby Y, Fjell A, 2009. Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cereb Cortex 20, 2055–2068. [DOI] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M, 2013. Sleep Drives Metabolite Clearance from the Adult Brain. Science 342, 373–377. 10.1126/science.1241224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zepelin H, McDonald C, Zammit GJ, 1984. Effects of age on auditory awakening thresholds. 39SRC-BaiduScholar, 294–300. [DOI] [PubMed] [Google Scholar]
- Zhao H, Qiao L, Fan D, Zhang S, Turel O, Li Y, Li J, Xue G, Chen A, He Q, 2017. Modulation of Brain Activity with Noninvasive Transcranial Direct Current Stimulation (tDCS): Clinical Applications and Safety Concerns. Front Psychol 8, 685. 10.3389/fpsyg.2017.00685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, 1995. VIP neurons in the human SCN in relation to sex, age, and Alzheimer’s disease. Neurobiology of Aging 16, 571–576. 10.1016/0197-4580(95)00043-E [DOI] [PubMed] [Google Scholar]
- Zhou J, Greicius MD, Gennatas ED, Growdon ME, Jang JY, Rabinovici GD, Kramer JH, Weiner M, Miller BL, Seeley WW, 2010. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain 133, 1352–1367. 10.1093/brain/awq075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X-N, Kelly C, Adelstein JS, Klein DF, Castellanos FX, Milham MP, 2010. Reliable intrinsic connectivity networks: Test–retest evaluation using ICA and dual regression approach. NeuroImage 49, 2163–2177. 10.1016/j.neuroimage.2009.10.080 [DOI] [PMC free article] [PubMed] [Google Scholar]


