Abstract
Although reduced ambient lighting (~50 lux) does not increase the degree of form-deprivation myopia (FDM) in chickens or infant monkeys, it does reduce the probability that monkeys will recover from FDM and that the normal age-dependent reduction in hyperopia will occur in monkeys reared with unrestricted vision. These findings suggest that low ambient lighting levels affect the regulatory mechanism responsible for emmetropization. To study this issue, infant rhesus monkeys (age ~ 24 days) were reared under dim light (55 ± 9 lux) with monocular −3D (dim-light lens-induced myopia, DL-LIM, n = 8) or +3D spectacle lenses (dim-light lens-induced hyperopia, DL-LIH, n = 7) until approximately 150 days of age. Refractive errors, ocular parameters and sub-foveal choroidal thickness were measured periodically and compared with normal-light-reared, lens-control monkeys (NL-LIM, n = 16; NL-LIH, n = 7). Dim light rearing significantly attenuated the degree of compensatory anisometropias in both the DL-LIM (−0.63 ± 0.77D vs. −2.11 ± 1.10D in NL-LIM) and DL-LIH treatment groups (−0.18 ± 1.93D vs. +1.71 ± 0.39D in NL-LIH). These effects came about because the treated and fellow control eyes had a lower probability of responding appropriately to the eye’s effective refractive state. Vision-induced interocular differences in choroidal thickness were only observed in monkeys that exhibited compensating refractive changes, suggesting that failures in detecting the relative magnitude of optical errors underlay the abnormal refractive responses. Our findings suggest that low ambient lighting levels reduce the efficacy of the vision-dependent mechanisms that regulate refractive development.
Keywords: myopia, hyperopia, dim light, ambient lighting levels, lens compensation, emmetropization, lens-induced myopia
1. Introduction
During early postnatal growth, the eye’s optical components (i.e., cornea and crystalline lens) and vitreous chamber develop in a coordinated manner, such that the initial refractive errors in a given eye gradually decrease to near emmetropic levels and the inter-individual differences in refractive error are also reduced. This “emmetropization” process has been observed in humans and most common laboratory species (for a review, see Troilo et al., 2019).
Emmetropization is a vision-dependent process; in particular, the hallmark changes of emmetropization, such as the systematic reduction in refractive error and its inter-individual variability, are regulated by visual feedback associated with the eye’s effective refractive state. Such a regulatory feedback mechanism was first demonstrated by Schaeffel et al. (1988), who showed that chickens reared with negative- and positive-powered lenses exhibited relative myopic and hyperopic changes, respectively, that eliminated most of the lens-imposed optical errors (i.e., defocus). These compensating refractive changes were achieved by altering the axial elongation rate of the eye’s vitreous chamber. Qualitatively similar observations, or “lens compensations”, were later reported for mice (Barathi et al., 2008; Jiang et al., 2018; Pardue et al., 2013; Tkatchenko et al., 2010), tree shrews (Metlapally & McBrien, 2008; Siegwart Jr. & Norton, 2010), guinea pigs (Howlett & McFadden, 2009), marmosets (Troilo et al., 2009; Whatham & Judge, 2001), and macaques (Hung et al., 1995; Smith III & Hung, 1999), suggesting that a widely conserved feedback control mechanism operates to achieve and maintain the optimal refractive state via alterations in vitreous chamber growth.
It has been shown that other elements of visual experience, most notably absolute ambient lighting levels, can influence normal emmetropization and vision-induced alterations in refractive development. For example, elevated ambient lighting levels have been shown to protect a variety of animal species from form-deprivation myopia (FDM) (Ashby et al., 2009; Chen et al., 2017; Karouta & Ashby, 2015; Siegwart Jr. et al., 2012; Smith III et al., 2012). Elevated ambient lighting levels also slowed the reduction in hyperopia associated with normal emmetropization in chicks, resulting in relative hyperopic refractive errors (Cohen et al., 2011, 2012). With respect to lens-induced changes in refractive development, Ashby et al. showed that rearing monocularly defocused chicks under elevated ambient lighting (10,000 lux) slowed the compensation to monocular −7D lenses and accelerated the compensation to monocular +7D lenses in comparison to chickens reared under typical laboratory lighting levels (500 lux), but did not affect the final degree of axial ametropias (Ashby & Schaeffel, 2010). In a later study, Siegwart Jr. et al. also found that elevated ambient lighting (16,000 lux) slowed the myopic shifts produced by optically imposed hyperopic defocus in tree shrews and doubled the time required to achieve full compensation (Norton & Siegwart Jr., 2013). Similar results were also reported for guinea pigs reared with monocular −4D lenses (Li et al., 2014). These studies consistently showed that elevated ambient lighting could alter the rate of lens-induced refractive developments in a sign-dependent manner. However, the same elevated ambient lighting paradigms that protected infant rhesus monkeys from FDM (Smith III et al., 2012) did not have a significant effect on either emmetropization in the fellow control eyes or the myopic compensation to negative-lens-induced defocus (Smith III et al., 2013). The observations in macaque monkeys suggested that the ambient lighting parameters (i.e., intensity levels and/or length of exposures) required to affect form-deprivation myopia and lens-induced myopia for higher primates might not be identical.
The effects of elevated ambient lighting suggest that lower ambient lighting levels could conversely promote ocular growth and enhance relative myopic development. In this regard, Cohen et al., in the same studies noted above, showed that reducing ambient lighting levels to 50 lux during the normal daily light-on hours accelerated the reductions in hyperopia, producing absolute myopias and increasing the inter-individual variability in refractive error. These observations suggested that dim ambient lighting is a risk factor for myopia (Cohen et al., 2011, 2012). However, rearing infant monkeys with unrestricted vision under reduced ambient lighting (~55 lux) resulted in increases in hyperopia and in the overall variability of refractive errors between subjects (She et al., 2020). In contrast to the results from chickens, none of these monkeys developed myopia. In addition, neither chicks (Ashby et al., 2009) nor infant rhesus monkeys developed more FDM under reduced ambient lighting, suggesting that low lighting intensity, by itself, is not an environmental enhancer of vision-induced myopia (She et al., 2021). Interestingly, reduced ambient lighting interfered with the recovery from FDM and other emmetropizing responses that are normally observed in monkeys previously reared with diffusers (She et al., 2021). The observed failures of emmetropization and recovery from FDM suggest that low ambient lighting levels reduce the operational efficacy of mechanisms that are normally responsible for regulating refractive development, a speculation that has not been directly and longitudinally investigated. The present study was conducted to examine the effects of reduced ambient lighting on the phenomenon of lens compensation in rhesus monkeys.
2. Method
2.1. Animal subjects
The primary subjects were 15 infant rhesus monkeys (Macaca mulatta) acquired at approximately 2 weeks of age. Before the experimental rearing period, subjects were housed in a nursery room that was illuminated on a 12-hour-light/12-hour-dark cycle (7AM – 7PM) using “white” fluorescent lights (GE Ecolux® Starcoat® T8 F32T8/SP35/ECO, General Electric Co., Boston, MA). The mean ambient lighting intensity in the housing area was 504 ± 168 lux (“normal light” or NL. Correlated color temperature = 3170K). These pre-experiment rearing conditions were identical to those employed in many of our previous studies involving typical laboratory lighting conditions (She et al., 2020).
2.2. Experimental strategies
Starting at approximately 24 days of age, the subjects were reared under reduced ambient lighting with the treatment lenses that were randomly assigned to them (see below) until 153 ± 4 and 147 ± 10 days of age for the DL-LIM and DL-LIH groups, respectively.
2.2.1. Experimental “dim light”
The reduced ambient lighting levels (“dim light” or DL) employed in the present study were identical to those used in our previous studies (She et al., 2020; She et al., 2021). In brief, an aluminum-deposited, polyester film (Grafix™ Metalized Dura-Lar®, Silver, 0.05mm-thick; Grafix, Maple Heights, Ohio) was closely attached to the fluorescent lighting panels. This strategy maintained the same spectral energy emission profile of the fluorescent lighting, which was identical to that employed to illuminate the pre-experimental housing areas (correlated color temperature = 3170K. She et al., 2020). The average intensity level measured directly under the light panels at the level of the junction between the upper and lower cages was 55 ± 9 lux. The lighting intensity measured in the front of individual cages with the sensor facing horizontally to the outside of the cage ranged between 7–36 lux.
2.2.2. Treatment lenses
At the onset of dim-light rearing, the subjects were fitted with goggle-like helmets (Hung et al., 1995; Smith III & Hung, 1999) that held either a −3D (DL-LIM, n = 8) or +3D lens (DL-LIH, n = 7) in front of the treated eye and a zero-power (plano) lens for the fellow control eye. The helmets were custom fitted to each subject and were inspected and adjusted frequently during the daily light-on hours to ensure proper fit and cleanliness.
2.3. Control-group subjects
The primary control data were obtained from two groups of age-matched monkeys previously reared under normal ambient lighting levels with either monocular −3D (NL-LIM, n = 16) or +3D treatment lenses (NL-LIH, n = 7). The ambient lighting conditions in their rearing environments were similar to those experienced by the dim-light monkeys prior to the onset of the lens-rearing period. In addition, we also included data from infant monkeys previously reared without visual restrictions under either normal ambient lighting levels (“normal-light controls” or NL-controls, n = 41) or under identical dim ambient lighting levels (“dim-light controls” or DL-controls, n = 7) in order to better illustrate the effects of the dim ambient lighting levels on the refractive and ocular component changes produced in response to the imposed defocus. These datasets have been published and discussed previously (Hung et al., 2018, She et al., 2020).
2.4. Outcome measures and data collection
Refractive errors and ocular parameters were measured for both eyes of each animal at the onset (baseline) and periodically throughout the lens-rearing period. Refractive errors were measured using retinoscopy by two experienced examiners and were reported as the mean spherical equivalents of the spectacle-plane refractive corrections. Corneal powers were measured using a hand-held keratometer (Alcon Auto-keratometer: Alcon, Inc., St. Louis, MO, USA). The mean spherical equivalent of three independent measurements were reported. The measurement was performed using a corneal topographer (EyeSys 2000; EyeSys Vision, Inc. Houston, TX, USA.) in some of the baseline measurement sessions when the corneal powers of the infant monkeys were out of the measurement range of the keratometer (> 62 D, about 5% occurrence rate at ages corresponding to the onset of the experiment) (95% limits of inter-instrument agreement = + 0.49 to −0.37 D) (Kee et al., 2002). Ocular axial dimensions were measured with A-scan ultrasonography (OTI-Scan 1000, Ophthalmic Technologies Inc., Downsview, Ontario, Canada) using a 13 MHz transducer. The acoustic parameters of human eyes (cornea and lens: 1641 m/s, aqueous and vitreous: 1532 m/s) (Byrne & Green, 2002) were assumed for the calculation of axial separations, which were reported as the mean of ten independent readings that were obtained along the normal to the corneal apex.
Pupil diameters were measured from videography recordings taken in the front of an animal’s respective cage. The detailed method for obtaining pupil diameter and its rationale have been described previously (She et al., 2020).
Sub-foveal choroidal thicknesses were evaluated using spectral-domain, optical coherence tomography (SD-OCT; Spectralis, Heidelberg, Germany) to examine the presence of optical regulatory activity associated with lens compensation. The methodology has been described previously (Hung et al., 2018, She et al., 2021). In short, B-scan OCT images along the horizontal meridian that passed through the deepest point of the foveal depression were obtained using the “Enhanced-Depth Imaging” mode of the manufacturer’s operating software (Heidelberg Eye Explorer) and were segmented using a customized Matlab program (2019a, MathWorks, Natick, MA, USA). Choroidal thickness, defined as the distance between Bruch’s membrane and the outer border of the choroid, was measured perpendicular to the choroidal – retinal pigment epithelium interface. The average choroidal thicknesses of a 300-micron transverse region centered at the deepest point of the foveal depression are reported.
All rearing and experimental procedures followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were reviewed and approved by the University of Houston’s Institutional Animal Care and Use Committee.
2.5. Statistical methods
Unless otherwise indicated, data are presented as means ± SDs of the mean. Cross-sectional analyses were performed for data obtained at baseline and at the end of the experiment using unpaired (for between-group comparisons) and paired t-tests (for between-eye comparisons). For the LIM experiment, analysis of covariance (ANCOVA) was employed to examine the potential main effects of dim light due to a small, but significant, age-difference between the DL-LIM and NL-LIM monkeys at the end of the experiment. When parametric tests could not be applied, a Mann-Whitney rank-sum test (for between-group comparison) or a Wilcoxon signed-rank test (for between-eye comparisons) was employed. A two-tailed significance level of 0.05 was used.
For longitudinal data, mixed-effect model analyses were employed to compare the time-courses for refractive and corneal power development. Specifically, data were fitted as a 2nd order polynomial function of the length of the lens-rearing period to reflect the curvilinear nature of normal age-related changes in refractive error and corneal power. The syntax was specified in a way that dim-light effects on the rates of development in the early and late stages of the experiment were represented by the reported linear and quadratic coefficients, respectively (i.e., they represent the linear and quadratic components of dim-light effect on refractive development). This configuration is useful in identifying differences in rates of refractive development at different stages of the experiment (see section 3.3 and related discussion). Based on the mixed-effect method, we also examined whether there was a significant dim-light effect on the variability in refractive development using likelihood-ratio tests (Rabe-Hesketh & Skrondal, 2012).
Pearson correlation and linear regression were employed to examine the relationship between refractive error and axial elongation. To examine the axial nature of refractive error specifically, we used the vitreous chamber to corneal radius ratio (VC/CR ratio) to represent ocular axial dimension in these analyses. This unitless metric accounts for part of the potential influence of inter-individual differences in corneal power on ocular growth and significantly reduces the noise in correlational and regression analyses (She et al., 2020, Smith et al., 2020).
All statistical analyses were performed using STATA (MP14, STATACORP, College Station, TX, USA) at a significance level of 0.05.
3. Results
3.1. Baseline data
As summarized in Table 1, there were no statistically significant baseline differences in refraction or any ocular parameters between the DL- and the corresponding NL-groups (Table 1). In addition, the refractive errors and ocular parameters, except for anterior chamber depth for the NL-LIH group, were binocularly symmetrical in all groups (paired t-test, p > 0.05). All refractive error and ocular parameters in the lens-reared animals were also within the mean ± 2 SD range for NL-control values (Hung et al., 2018).
Table 1.
Baseline refractive error and ocular parameters for the LIM and LIH experiments. The significant difference in anterior chamber depth observed in the NL-LIH group likely reflects measurement noise.
| NL-LIM (24 ± 2 days) (n = 16) |
DL-LIM (23 ± 2 days) (n = 8) |
NL-LIH (24 ± 2 days) (n = 7) |
DL-LIH (25 ± 2 days) (n = 7) |
|||||
|---|---|---|---|---|---|---|---|---|
| Treated eye | Control eye | Treated eye | Control eye | Treated eye | Control eye | Treated eye | Control eye | |
| Refractive error (D) | +3.64 ± 1.05 | +3.69 ± 1.18 | +4.48 ± 0.99 | +4.47 ± 0.95 | +4.61 ± 1.2 | +4.66 ± 1.26 | +3.47 ± 1.61 | +3.40 ± 1.61 |
| Corneal power (D) | 61.19 ± 1.09 | 61.11 ± 1.16 | 60.18 ± 1.26 | 60.12 ± 1.30 | 62.18 ± 1.86 | 62.02 ± 1.59 | 61.39 ± 1.27 | 61.28 ± 1.42 |
| Anterior chamber depth (mm) | 2.49 ± 0.14 | 2.51 ± 0.15 | 2.48 ± 0.13 | 2.49 ± 0.13 | 2.43 ± 0.12 * | 2.59 ± 0.18 * | 2.43 ± 0.11 | 2.44 ± 0.10 |
| Lens Thickness (mm) | 3.67 ±0.17 | 3.67 ± 0.15 | 3.68 ± 0.33 | 3.39 ± 0.34 | 3.62 ± 0.22 | 3.60 ± 0.18 | 3.73 ± 0.11 | 3.74 ± 0.12 |
| Vitreous chamber depth (mm) | 8.62 ± 0.36 | 8.63 ± 0.35 | 8.62 ± 0.24 | 8.64 ± 0.23 | 8.41 ± 0.33 | 8.38 ± 0.33 | 8.52 ± 0.30 | 8.51 ± 0.30 |
Significant between-eye difference
3.2. Pupil diameters
Pupil diameters that are representative of the early and late lens-rearing periods were obtained from the DL-LIM and DL-LIH monkeys, respectively. At 36 ± 7 days of age, the pupil diameters were 5.13 ± 0.54 mm in the treated eyes and 5.15 ± 0.52 mm in the control eyes of the DL-LIM monkeys, both of which were similar to those of DL-controls in the early stage of dim-light rearing (4.98 ± 0.62 mm and 4.96 ± 0.74 mm for the right and left eyes, respectively), but larger than those obtained from normal-light-reared monkeys (3.58 ± 0.43 mm, p < 0.001; Hung et al., 2018). At 101 ± 9 days of age, the pupil diameters of the DL-LIH monkeys were 5.11 ± 0.24 mm and 5.27 ± 0.18 mm for the treated and control eyes, respectively, which were also larger than those obtained from normal-light-reared monkeys at older ages (132 ± 5 days of age; 3.90 ± 0.45 mm and 3.96 ± 0.45 mm for the right and left eyes, respectively; p < 0.001). Since pupil diameter increases with age in both normal-light-reared monkeys (Hung et al., 2018) and our DL-controls (She et al., 2020), it appears that the pupil diameters of our DL-subjects remained larger than the average, age-matched, normal-light-reared monkeys throughout the lens-rearing period.
3.3. Compensation to negative lenses in dim light
Dim light reduced the probability of compensation to negative lenses. Four of the DL-LIM monkeys did not develop obvious myopic anisometropias (Figure 1A – 1D); the refractive changes in the two eyes of these monkeys varied from small reductions in hyperopia (Figure 1A and 1B) to substantial binocular and symmetric myopic shifts (Figure 1C) and absolute myopias (Figure 1D). For the remainder of the DL-LIM subjects, myopic anisometropias (relative myopia in the treated eye in comparison to the fellow control eye) developed during the experiment, but these lens-induced anisometropias were not always sustained (Figure 1F).
Figure 1.
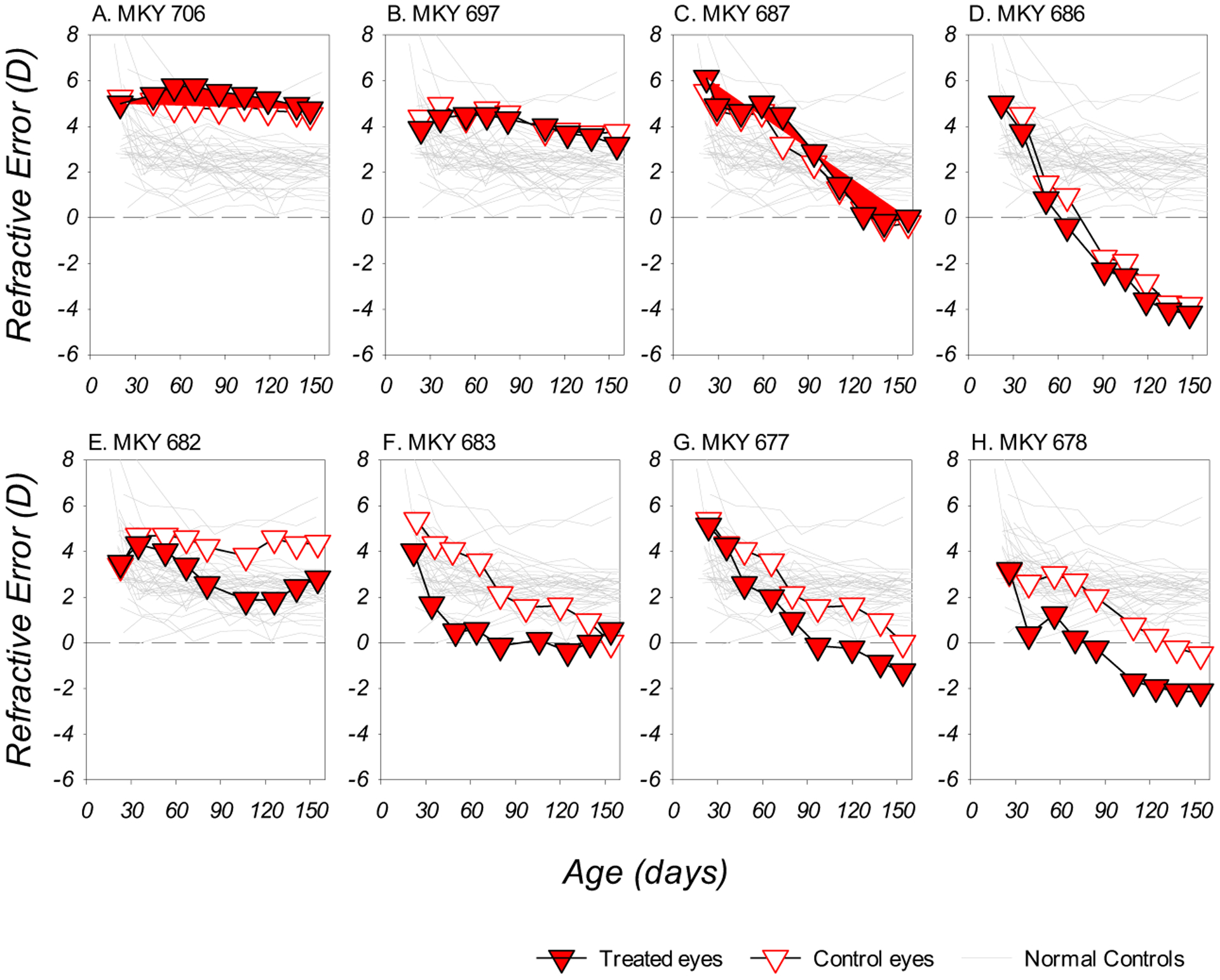
Refractive error plotted as a function of age for individual DL-LIM monkeys. The filled and open symbols represent data from the treated and control eyes, respectively. Data for the right eyes of the normal control monkeys were plotted as thin grey lines.
Due to the fact that many DL-LIM subjects failed to develop or maintain myopic anisometropia, the average degree of anisometropic compensation to monocular hyperopic defocus was significantly reduced. As illustrated in Figure 2A, all but one of the NL-LIM subjects successfully developed and maintained lens-induced myopic anisometropias. For the DL-LIM monkeys, however, the development of anisometropia was more variable (Figure 2B) and its pattern significantly differed from that observed in the NL-LIM monkeys (z = 3.13, p < 0.01). At the end of the lens-rearing period, the average degree of myopic anisometropia was significantly smaller in DL-LIM group than in the NL-LIM group (DL- vs. NL-LIM: −0.63 ± 0.77D vs. −2.11 ± 1.10D, t (22) = −3.41, p < 0.01).
Figure 2.

Panels A, B, D, E: Anisometropia (treated eye ametropia – control eye ametropia) plotted as a function of age for individual animals in the NL-LIM (panel A), DL-LIM (panel B), NL-LIH (panel E) and DL-LIH groups (panel E). The circular symbol with error bars in each panel represents the mean (±SD) anisometropia obtained at the end of the lens-rearing period. Panels C and F: Mean refractive changes plotted as a function of age for LIM (panel C) and LIH monkeys (panel F), respectively. To calculate the mean changes, the refractive data obtained at each measurement were linearly interpolated to the closest age-point in an equally spaced age sequence (range between 24–150 days, inclusive) and averaged. Error bars are not included for better clarity.
In dim light, negative-lens-induced refractive changes were more variable than in normal light (see Figure 1), and their magnitude exceeded that normally associated with successful compensation. Figure 2C illustrates the longitudinal changes in refractive error for the DL- and NL-LIM monkeys. On average, both DL- and NL-LIM monkeys exhibited reductions in hyperopia in their treated eyes, of which the initial rates were not statistically different (closed symbols). As the experiment continued, the course of treated-eye refractive development started to differ: whereas the reductions observed in the NL-LIM monkeys decelerated after ~70 days of age, there were no obvious signs of slowing for the DL-LIM monkeys until the later stages of the lens-rearing period (z = −2.17, p = 0.03 for the quadratic component). On the other hand, the control eyes of DL-LIM monkeys showed significantly greater reductions in hyperopia than those observed in the NL-LIM group soon after the onset of lens-rearing (z =3.49, p < 0.01 for the linear component). The rate of the reductions in control eye hyperopia in the DL-LIM group became greater than that observed in NL-LIM (open symbols) and in the NL-controls (shaded area), but they did not exceed the rate of change observed in the contralateral treated eyes of the DL-LIM animals (filled red symbols). For both the treated- and fellow-control eyes, the course of refractive development (likelihood-ratio test, p < 0.05) and the average refractive changes at the end of the treatment period (DL- vs. NL-LIM, treated eyes: −4.01 ± 3.28D vs. −2.80 ± 1.38D, z = 0.85, p = 0.39; control eyes: −3.38 ± 3.23D vs. −0.74 ± 1.15D, z = 1.69, p = 0.09) were significantly more variable in the DL-LIM group than those observed in the NL-LIM group. As a consequence, the differences in average refractive error between the DL-LIM and NL-LIM groups were not statistically significant (Table 2; Figure 3); however, both eyes of the DL-LIM monkeys were more myopic than those of the DL-controls (+3.66 ± 1.75D and +3.70 ± 1.36D for the right and left eyes, respectively; p < 0.05).
Table 2.
Refractive errors and ocular parameters obtained at the end of the lens-rearing period for NL- and DL-LIM monkeys. Statistically significant between-eye difference in refraction and ocular parameters were highlighted in bold. For the ANCOVA tests reported in the table, there was no evidence to reject the equal-slope assumption (i.e. there were no significant interactions between ambient lighting levels and length of treatment). The small, but significant, between-eye difference in lens thickness in the DL-LIM group likely reflected measurement noise.
| NL-LIM (144 ± 6 days) * | DL-LIM (153 ± 4 days) * | |||||||
|---|---|---|---|---|---|---|---|---|
| Treated eye | Control eye | Between-eye comparison | Treated eye | Compare to NL-LIM | Control eye | Compare to NL-LIM | Between-eye comparison | |
| Refractive error (D) | +0.84 ±1.89 | +2.95 ± 1.37 | t (15) = −7.67, p < 0.01 | + 0.47 ± 3.00 | F (1, 21) = 0.33, p = 0.57 | +1.09 ± 2.91 | z = 1.35, p = 0.18 | t (7) = −2.31, p = 0.06 |
| Corneal power (D) | 55.13 ± 1.49 | 55.01 ± 1.59 | t (15) = 0.99, p = 0.34 | 54.39 ± 1.4 | F (1, 21) = 2.75, p = 0.11 | 54.25 ± 1.61 | F (1, 21) = 2.28, p = 0.15 | t (7)= 0.69, p = 0.51 |
| Anterior chamber depth (mm) | 3.11 ± 0.15 | 3.10 ± 0.13 | t (15) = 1.03, p = 0.32 | 3.03 ± 0.11 | F (1, 21) = 0.25, p = 0.62 | 3.06 ± 1.21 | F (1, 21) = 0.04, p = 0.85 | t (7) = −2.02, p = 0.08 |
| Lens Thickness (mm) | 3.64 ± 0.12 | 3.65 ± 0.13 | t (15) = −0.58, p = 0.57 | 3.77 ± 0.22 | F (1, 21) = 1.28, p = 0.27 | 3.73 ± 0.22 | F (1, 21) = 0.98, p = 0.33 | t (7) = 2.50, p = 0.04 |
| Vitreous chamber depth (mm) | 10.40 ± 0.55 | 10.01 ± 0.49 | t (15) = 6.41, p < 0.01 | 10.64 ± 0.79 | F (1, 21) = 1.25, p = 0.28 | 10.42 ± 0.90 | z = −1.44, p = 0.15 | t (7) = 2.46, p = 0.04 |
Significant between-group difference.
Figure 3.

Refractive error (panel A) and vitreous chamber depth (panel B) obtained from the treated and control eyes of lens-rearing monkeys at the end of the treatment period and from both eyes of the control monkeys at ages correspond to the end of the lens-treatments. The asterisks on top of the box plots denote significant interocular difference (i.e., the successful development of compensating anisometropias).
3.4. Compensation to positive lenses in dim light
The development of LIH was severely curtailed by the dim-light rearing regimen. Figure 4 showed that only two DL-LIH monkeys developed obvious hyperopic anisometropias (Figure 4A and 4B). Three subjects did not develop (Figure 4C and 4E) or failed to maintain obvious hyperopic anisometropias throughout the experiment (Figure 4D). Most remarkably, two monkeys developed myopic anisometropias during lens-rearing (Figure 4F and 4G), which exacerbated, rather than reduced, the lens-imposed myopic defocus. In addition, for all DL-LIH subjects, the control eyes also showed attenuated or relatively normal reductions in hyperopia during the experiment.
Figure 4.
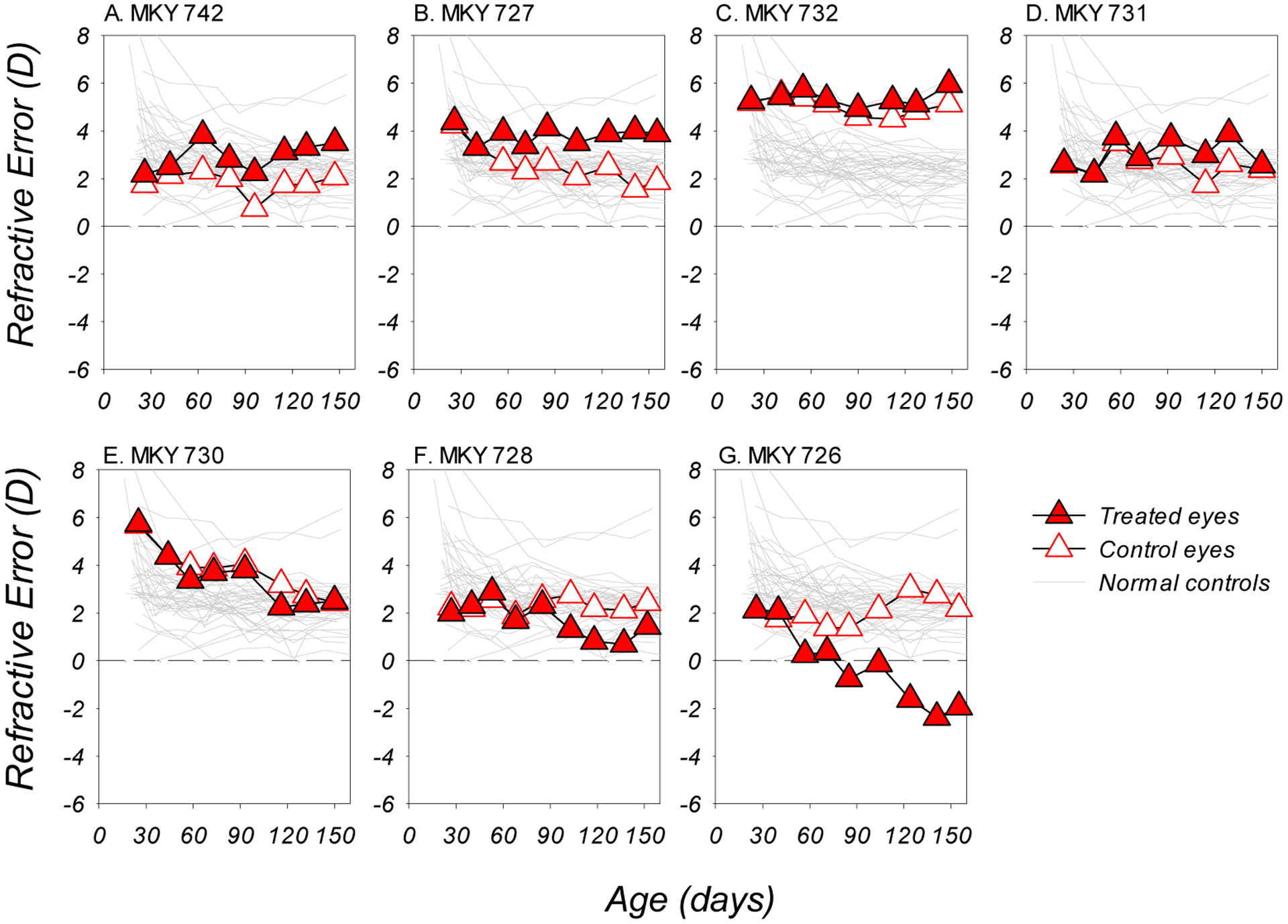
Refractive errors plotted as a function of age for the DL-LIH subjects. Red and white symbols represent data from the treated and control eyes, respectively; the grey thin lines in each plot represents the data from the right eyes of the normal control monkeys.
Due to the absence of expected and appropriate treated-eye refractive changes, the normally consistent development of compensating hyperopic anisometropias was disrupted in the DL-LIH monkeys. Figure 2D and 2E illustrate the longitudinal changes in anisometropia for the NL- and DL-LIH monkeys, respectively. In comparison to the NL-LIH monkeys, the DL-LIH monkeys showed substantially higher inter-individual variability in the degree and direction of anisometropic changes, thus the patterns of anisometropia-development were significantly different (z = −2.13, p = 0.03). At the end of the lens-rearing period, the DL-LIH monkeys were, on average, isometropic (−0.18 ± 1.93D vs. +1.71 ± 0.39D in the NL-LIH group; Mann-Whitney test, z = 2.56, p = 0.01; Figure 3) and the between-subject variability in anisometropia was significantly greater than that in the NL-LIH monkeys (variance ratio test, F = 0.04, p < 0.01).
Similar to that observed for LIM, the course of refractive development in response to monocular positive-lens wear was more variable in dim light (likelihood-ratio test, χ = 11.19, p = 0.004). Figure 2F illustrates the average longitudinal changes in refractive error for the NL-LIH and DL-LIH monkeys. For subjects reared under normal light, the monocular +3D lenses largely eliminated the normal age-related reductions in hyperopia in the treated eyes (filled black symbols), resulting in smaller overall changes in refractive error in comparison to their fellow control eyes and consistent compensating hyperopic anisometropias at the end of the lens-rearing period (Figure 3A). For the DL-LIH monkeys, the treated eyes, on average, exhibited significant myopic shifts in comparison to the NL-LIH monkeys (z = −2.48, p = 0.01 for the linear component); the time-course of these changes was similar to that observed in their fellow-control eyes (Figure 2F, red symbols), both of which were close to the upper 95% limit observed in the NL-controls. As a consequence, the typical developmental pattern of compensating anisometropia was not observed in the DL-LIH monkeys. At the end of the lens-rearing period, the treated eyes of the DL-LIH monkeys exhibited relative myopic changes that were comparable to those in their fellow control eyes (−0.92 ± 2.00 D and −0.67 ± 1.33 D for treated and control eyes, respectively); the refractive errors in both the treated and fellow control eyes of the DL-LIH monkeys did not differ significantly from those in the NL-LIH subjects (Figure 3A) and the DL-controls.
3.5. Ocular components and the axial nature of the observed refractive errors
We did not observe any significant differences in corneal powers, lens thicknesses, or anterior chamber depths that could account for the observed alterations in refractive error (Table 2 andTable 3). For corneal powers specifically, the time course of corneal power development and the corneal powers obtained at the end of the experiment were both similar in the DL- and the corresponding NL-groups, which is in agreement with our previous dim light investigations (She et al., 2020; She et al., 2021). Except for the DL-LIM group, which exhibited a significant, but negligible, interocular difference in lens thickness at the end of the lens-rearing period (Table 2), there were no significant between-eye differences in corneal power, anterior chamber depth, or lens thickness.
Table 3.
Refractive errors and ocular parameters obtained at the end of the lens-rearing period for NL- and DL-LIH monkeys. Statistical significance is highlighted in bold.
| NL-LIH (133 ± 14 days) | DL-LIH (147 ± 10 days) | |||||||
|---|---|---|---|---|---|---|---|---|
| Treated eye | Control eye | Between-eye comparison | Treated eye | Compare to NL-LIM | Control eye | Compare to NL-LIM | Between-eye comparison | |
| Refractive error (D) | +4.63 ± 0.91 | +2.92 ± 0.89 | t (6) = 11.73, p < 0.01 | + 2.55 ± 2.43 | t (12) = 1.19, p = 0.26 | +2.73 ± 1.07 | t (12) = 0.36, p = 0.73 | t (6) = −0.24, p = 0.82 |
| Corneal power (D) | 55.82 ± 1.65 | 55.61 ± 1.23 | t (6) = −1.48, p = 0.19 | 55.30 ± 1.25 | t (12) = −0.33, p = 0.75 | 55.33 ± 1.21 | t (12) = 0.06, p = 0.95 | t (6) = −0.16, p = 0.88 |
| Anterior chamber depth (mm) | 3.08 ± 0.08 | 3.05 ± 0.09 | t (6) = 3.67, p = 0.01 | 3.07 ± 0.19 | t (12) = 0.04, p = 0.97 | 3.10 ± 0.12 | t (12) = −2.25, p = 0.04 | t (6) = −0.53, p = 0.61 |
| Lens Thickness (mm) | 3.55 ± 0.14 | 3.56 ± 0.15 | t (6) = −1.21, p = 0.27 | 3.68 ± 0.17 | t (12) = −0.16, p = 0.88 | 3.67 ± 0.20 | t (12) = 0.41, p = 0.69 | t (6) = 2.50, p = 0.81 |
| Vitreous chamber depth (mm) | 9.51 ± 0.33 | 9.85 ± 0.33 | t (6) = −11.34, p < 0.01 | 10.07 ± 0.47 | t (12) = −2.18, p = 0.050 | 9.98 ± 0.34 | t (12) = −0.03, p = 0.97 | t (6) = 0.51, p = 0.63 |
Differences in vitreous chamber elongation were associated with the observed variability in refractive error and the development of compensating changes in response to imposed defocus. At the end of the lens-rearing period, the less hyperopic/more myopic eyes in both DL-groups also exhibited greater vitreous chamber depths at the end of the experiment (Table 2; Table 3; Figure 3B); the interocular differences in vitreous chamber depth (treated eye – fellow control eye) were smaller in magnitude in the DL-groups in comparison to those observed in the corresponding NL-groups (DL- vs. NL-LIM: +0.21 ± 0.25mm vs. +0.38 ± 0.24mm, t (22) = −1.61, p = 0.12; DL- vs. NL-LIH: +0.09 ± 0.47mm vs. −0.33 ± 0.08mm, t (12) = 2.36, p = 0.04). The observed changes in refractive error were significantly correlated with changes in vitreous chamber depth (r = −0.86, p < 0.01, Figure 5A). In addition, there was a significant correlation between refractive error and the vitreous chamber to corneal radius ratio (VC/CR ratio) (r = −0.89, p < 0.01). A linear regression analysis showed that variations in the VC/CR ratio accounted for 80% of the variability in refractive error (r2 = 0.80, p < 0.01; Figure 5B). Finally, the interocular differences in vitreous chamber depth observed at the end of the experiment were inversely correlated with the degree of anisometropia (r = −0.92, p < 0.01; Figure 5C). Thus, the refractive alterations observed in the present study showed the same axial nature as those observed under normal ambient lighting.
Figure 5.
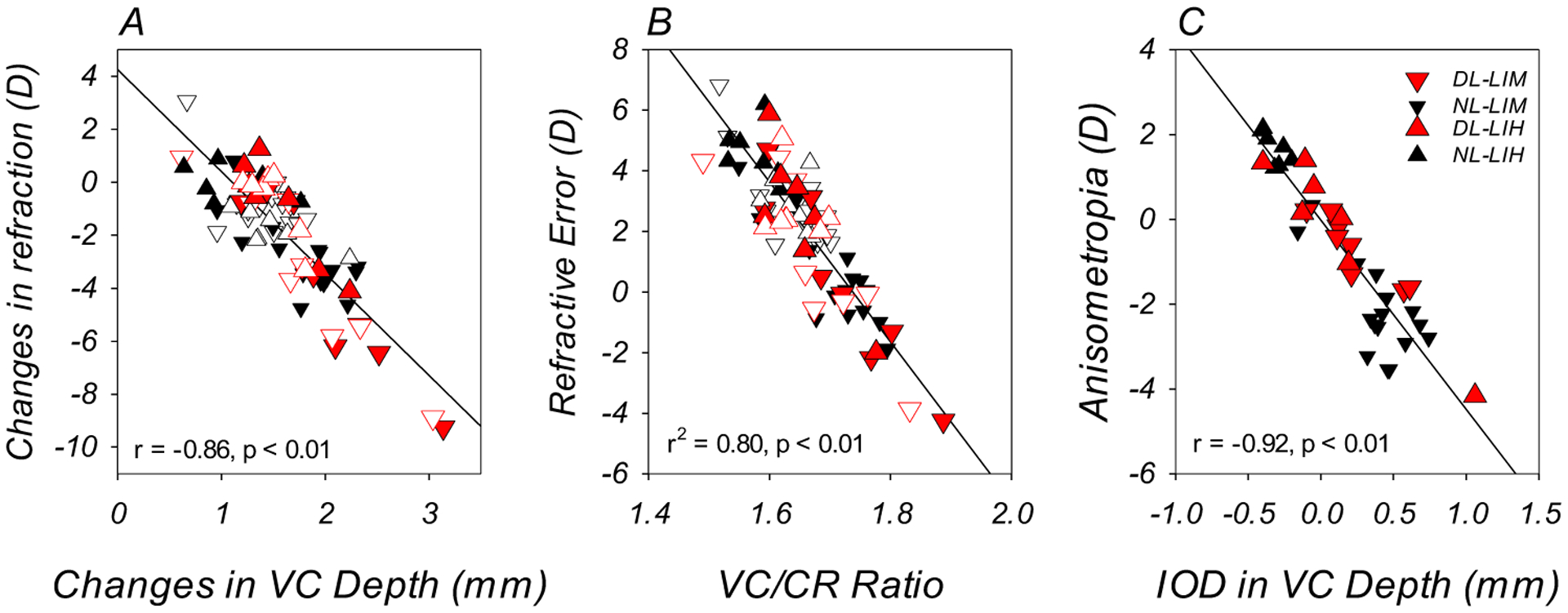
Panel A. Changes in refractive error plotted against the changes in vitreous chamber depth. The filled and open symbols represent the treated and control eyes of the DL- (red) and NL-subjects (black), respectively. Panel B. Refractive errors obtained at the end of the experiment plotted against the vitreous chamber to corneal radius ratios (VC/CR ratio). Panel C. Anisometropia obtained at the end of the experiment plotted against the interocular difference in vitreous chamber depth for individual NL and DL animals (treated eye – control eye).
3.6. Choroidal thickness changes
The observed failures to respond to imposed defocus were associated with an absence of the relative choroidal thickness changes that are normally induced by defocus. To examine the relationship between choroidal thickness change and refractive development for our DL-LIM monkeys, we disaggregated this group based on whether negative-lens compensation had occurred and then compared the longitudinal changes in choroidal thickness in these two subgroups. In comparison to the subgroup that did not show obvious compensating changes (the “isometropic subgroup”, Figure 6B), in which the choroidal thickness changes were similar in the treated and control eyes (Figure 6D), the subgroup that developed obvious compensating refractive changes (the “anisometropic subgroup”, Figure 6A) exhibited initial interocular differences in choroidal thickness that were in the appropriate direction in relation to the nature of the imposed defocus, i.e. relative choroidal thickening in the eyes that were more hyperopic/less myopic (Figure 6C). These interocular differences were due primarily to the abnormal age-related changes in the treated eye’s choroidal thickness. Specifically, the increases in treated-eye choroidal thickness in the anisometropic subgroup were much smaller in magnitude in comparison to those observed in their control eyes and those in the treated eyes of the isometropic subgroup. In contrast, the isometropic subgroups did not show any systematic interocular differences in choroidal thickness at any point throughout the experiment. At the end of the experiment, there were no significant interocular differences in choroidal thickness in either subgroup (treated vs. control eye, anisometropic subgroup: 40.37 ± 7.94 vs. 48.58 ± 14.3 μm, t (3) = −0.87, p = 0.45; isometropic subgroup: 19.01 ± 9.89 vs. 21.48 ± 9.22 μm, t (3) = −0.16, p = 0.88). Note that the anisometropic subgroup appeared to have thicker choroids than the isometropic subgroup near the end of the experiment. The cause of this difference is unclear. Speculatively, the axial growth and expansion of the posterior globe in the two isometropic monkeys that developed substantial myopia produced mechanical stretch that significantly offset the age-related increase in choroidal thickness observed under dim light (She et al., 2020).
Figure 6.

The degree of anisometropias (treated eye ametropia – control eye ametropia; panels A and B) and the mean (± SEM) changes in choroidal thickness (specified relative to the onset of the experiment) in the treated (filled symbol) and control eyes (open symbol) plotted as a function of age for DL-LIM monkeys that had developed compensatory anisometropia at any stage of the experiment (monkeys 682, 683, 677 and 678, left column) and those that remained isometropic throughout the experiment (monkeys, 706, 679, 687 and 688, right column). The shaded areas in panels A and B represent the 95% confidence range of interocular differences in refractive errors for DL-controls. The grey lines and symbols in Panels C and D represent the choroidal thickness in the two eyes of the DL controls.
Similar to the associations observed in the DL-LIM subjects, interocular differences in choroidal thickness change that were in the appropriate direction to compensate for the imposed defocus (i.e., relative thickening in the treated eye, illustrated as positive interocular differences) were observed early in the experiment in the subjects that developed obvious degrees of hyperopic anisometropia (monkeys 742, 727 and 731, Figure 7A – 7C). For the subjects that remained relatively isometropic (monkey 732, Figure 7D) or developed myopic anisometropia (monkeys 730, 728 and 726, Figure 7E – 7G), the expected relative increases in choroidal thickness in response to the imposed myopic were not observed in their treated eyes. On the contrary, there was a trend for the treated-eye choroids to be thinner in comparison to their fellow control eyes.
Figure 7.

Changes in the interocular differences (treated eye – control eye) in sub-foveal choroidal thickness (red symbols) and refractive error (white symbols) plotted as a function of age for individual DL-LIH monkeys. The dashed line in each panel represents zero interocular difference in the changes of refractive error and choroidal thickness. Analogous to that in the DL-LIM monkeys, choroidal thickness development in DL-LIH monkeys was appropriate for the direction of refractive changes (i.e., monkeys that did not successfully compensate for imposed myopic defocus exhibited relative choroidal thinning instead of thickening), at least in the early- and mid-stages of the lens-rearing period. Note that, unlike Figure 6 which illustrate the same association using averaged data, data of the DL-LIH group were presented individually because of the inter-subject variability in the direction of refractive changes the group averages were not representative of individual animals and obscured the association between refraction and choroidal thickness.
4. Discussion
We found that dim light rearing reduced the probability of refractive changes that compensate for lens-imposed defocus, increased the variability in refractive development between subjects, and reduced the average degree of compensating anisometropias. The failures to exhibit compensating refractive changes were associated with an absence of the relative choroidal thickness changes that are normally induced by defocus under normal ambient lighting. The inter-individual variability in refractive development and the inconsistent compensation that was observed in both groups of DL monkeys were axial in nature, like that normally associated with lens compensation under normal ambient lighting.
4.1. Effects of dim light on corneal power
We did not find any significant dim-light effects on corneal power development. This observation is in agreement with the findings from our previous investigations of emmetropization (She et al., 2020) and FDM in monkeys that were reared under similar dim ambient lighting (She et al., 2021). Together with the observations under elevated ambient lighting levels (Smith III et al., 2012, 2013), our data indicate that corneal power development in infant rhesus monkeys is largely unaffected by different ambient lighting levels. In contrast, both elevated and reduced lighting levels have been found to affect corneal power development in chicks (Cohen et al., 2011; 2012). In addition, negative-lens-imposed defocus has been reported to cause corneal steepening in mice reared under ambient lighting levels that were similar to the dim lighting levels employed in the present study (Landis et al., 2021). It appears that the biometrical nature of the refractive responses to ambient-lighting-level manipulations or the interaction between imposed defocus and reduced lighting levels might not be identical in avian and rodent species versus non-human primates.
4.2. Pupil dilation and its potential influence
Given that the effects of ambient illumination levels are presumably mediated by vision-related mechanisms, a more relevant measure for the environmental stimulus would be the illumination level on the retina (i.e. retinal illumination level. Thibos et al., 2018; Troland, 1917). In our experiment, the pupil dilation observed in DL-subjects (1.0 – 1.5mm larger in average diameter than NL-subjects, or 32% – 44% in percentage increases) resulted in 74% – 107% increases in pupil area in comparison to those in NL-subjects. As a numerical example of how this pupil size difference affects retinal illumination, an area on the white-painted walls (the brightest of all common visual targets in our dim-light housing) that subtends a 1° visual angle produces 1.51 × 10−4 photopic trolands of retinal illumination through the relatively dilated (5.14mm) pupil in a DL subject that is viewing along the normal to the wall at cage-level. In comparison to the retinal illumination produced by dim light through a non-dilated pupil (3.58mm, 7.32 × 10−5 photopic trolands), the larger pupils in the DL monkeys nearly doubled the effective retinal illumination. However, the resulting retinal illumination remained ~6 times lower than that in “normal” light (9.76 × 10−4 photopic trolands), thus the larger pupil size only partially offset the reductions in ambient illumination. Due to its unidirectional nature, this effect did not appear adequate to explain the high inter-individual variability, the absence of light-level-associated systematic refractive error alterations, and particularly the difference between DL-lens-reared subjects and the DL-controls.
The larger pupil diameters in our DL monkeys could also influence lens compensation by increasing the magnitude of their eyes’ higher-order monochromatic aberrations (HOAs). Specifically, higher HOAs at the onset of the experiment might reduce the efficacy of emmetropization (see section 4.3) through degradations in retinal image quality and increase the eye’s depth of field (DOF) (Charman, 2005; for a dim-light related discussion, see She et al., 2020). For animals that developed myopic changes in the early stages of lens-rearing, larger pupil size might have also exaggerated the influence of myopia-related increase in HOAs (Coletta et al., 2003, 2010; García De La Cera et al., 2006; Kisilak et al., 2006; Ramamirtham et al., 2007; Tian & Wildsoet, 2006) on retinal image quality, which might in-turn enhance the myopic changes. However, because other factors, particularly changes in axial dimensions, could also affect the nature (and probably the magnitude) of HOAs, the extent to which pupil-size-related alterations in HOAs contributed to the observed patterns of refractive development remains unclear. Given that emmetropization likely uses signals embedded in medium spatial frequencies, and the fact that some eyes with large experimental myopias and presumably increased HOAs recovered when treatment lenses were removed (Qiao-Grider et al., 2004; She et al., 2021), suggests that any contribution of HOAs, albeit probable, was small.
4.3. Dim-light effects on lens-induced refractive compensation
We found that, in comparison to typical laboratory lighting levels, dim ambient lighting significantly increased the between subject variability in refractive development. Similar observations have been reported for infant rhesus monkeys reared with unrestricted vision (She et al., 2020) and, in an analogous manner, for monkeys undergoing recovery from FDM (She et al., 2021). It has also been reported that unrestricted refractive development is more variable in chickens reared under low ambient lighting, whereas under typical or elevated ambient lighting levels there was less inter-individual variability around the eventual target refractive error (Cohen et al. 2011, 2012).
In comparison to that observed under typical laboratory lighting levels (Hung et al., 1995; Smith III & Hung, 1999), dim light reduced the likelihood of systematic compensating changes in refractive error in response to both imposed hyperopic and myopic defocus. This effect is illustrated in Figure 8, which plots the effective refractive error, defined as the through-the-lens refractive error for treated eyes of the lens-reared monkeys and the uncorrected refractive errors for controls and monkeys undergoing recovery from FDM, obtained at the onset and end of the relevant observation periods for individual monkeys reared under different lighting conditions. Under typical ambient lighting, eyes with a large range of initial effective refractive errors consistently emmetropized towards the target range of refractive errors for normal control monkeys (mean ± 2SD range of the NL-controls, Figure 8A, solid and dash lines). In contrast, many of our DL subjects either showed little change in effective refractive error or showed changes that were not in the appropriate directions (Figure 8B), despite the fact that their initial effective refractive errors were within the range of refractive errors that supported the expected responses in normal light. In particular, in dim light, eyes that presented with a significant dioptric stimulus for emmetropization (i.e., high degrees of hyperopia) often failed to exhibit emmetropization-associated reductions in hyperopia, whereas eyes that presented with optical “stop” signals (relative or absolute myopic defocus) frequently showed no compensating changes or exhibited inappropriate myopic changes. As a result, dim-light-rearing virtually eliminated the hallmark patterns in refractive development that are associated with normal active visual regulation (i.e., emmetropization, recovery from FDM, and lens-compensation), indicating that the efficacy of the feedback control mechanisms that regulates refractive development is reduced under dim lighting.
Figure 8.
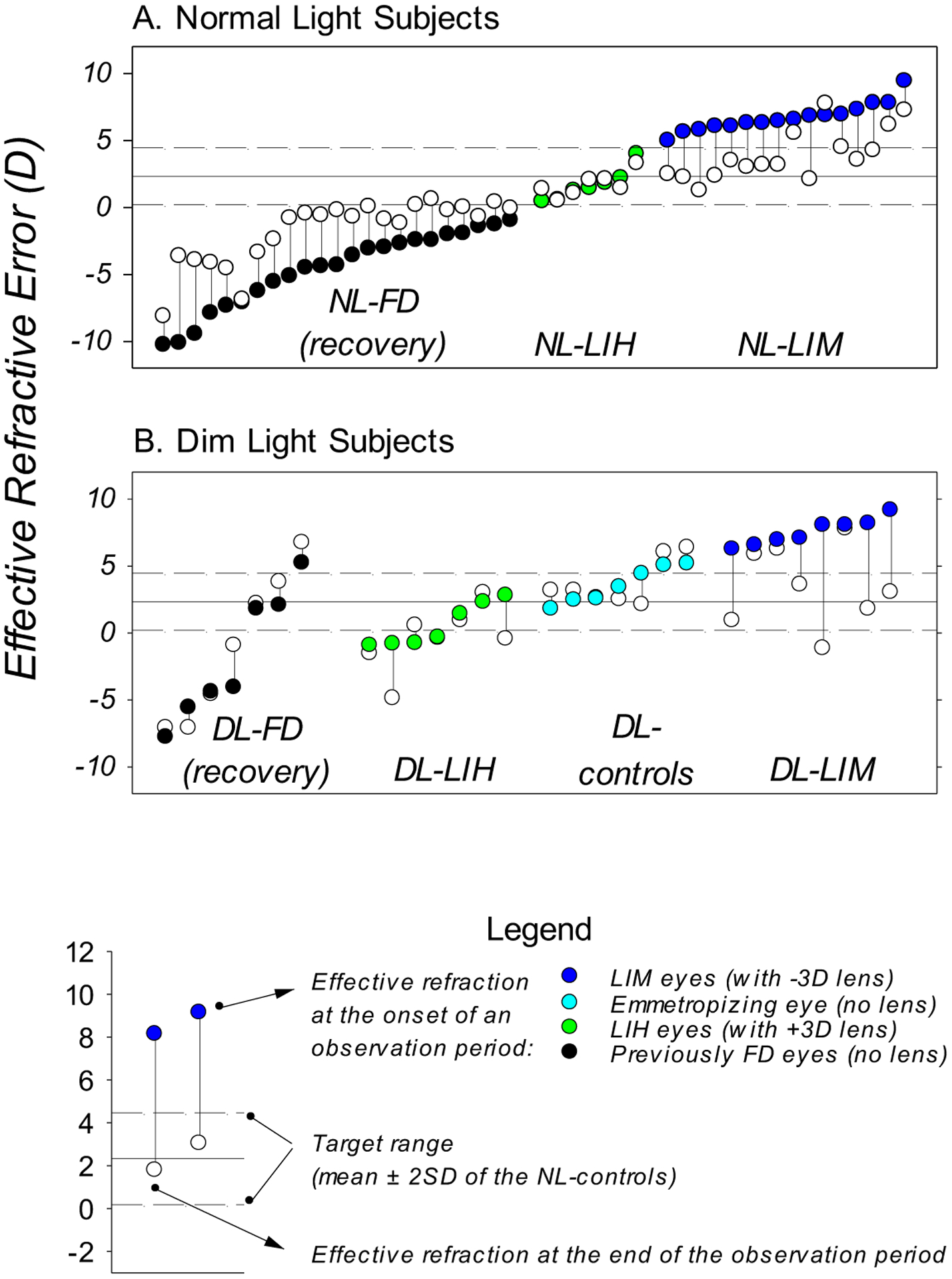
Effective refractive error obtained at the onset (color-coded symbols) and end (open symbols) of the observation period for individual monkeys. Panel A: In normal light, the effective refractive states of monkeys systematically changed towards a target range. Panel B: DL monkeys did not show systematic changes in refractive error analogous to that illustrated in panel A. Data are presented for eyes that were permitted visual experience that would support visual regulation under normal light, i.e., the treated eyes of lens-wearing monkeys, the right eyes of the control monkeys (She et al., 2020), and the diffuser-treated eyes of the FD monkeys at the time of removal of diffusers (i.e., the onset of recovery period. She et al., 2021). Color-coded symbols: treated eye of LIH (green) and LIM (blue) monkeys at the onset of lens treatment (approximately 24 days) and the right eyes of monkeys reared with unrestricted vision at ages that corresponded to the onset of lens-treatment (cyan); black symbols represent data of FD monkeys obtained at the onset of the “recovery” periods (approximately 150 days). Open symbols: data obtained from lens-treated monkeys at the end of the lens-rearing period (approximately 150 days for LIH monkeys and 135 days for and LIM monkeys), at ages that correspond to the end of lens-rearing period (for DL-controls), or at the end of the recovery period (300 and 350 days for NL- and DL-FD monkeys, respectively). These observation periods were within the age range in which refractive changes associated with visually regulated refractive development could be observed under normal ambient lightings. The vertical lines connect the data from the same eye to help identify the direction of refractive change.
Do the myopias observed in the treated eyes of some of our dim-light subjects, especially those in the DL-LIM monkeys, reflect a systematic myopiagenic effect? In the present study, we did not find evidence for an acceleration or deceleration in lens compensation analogous to the observations in chicks reared with imposed defocus under elevated ambient lighting (Ashby et al., 2012). In addition, neither the present investigation, nor our previous study on emmetropization, found signs of consistent, unidirectional increases in the age-related reductions in hyperopia that were analogous to those observed in dim-light-reared chickens (Cohen et al., 2011, 2012). Due to the absence of systematic alterations in the endpoint ametropia and the presence of large inter-individual variability, the substantial myopic changes observed in some of the lens-treated eyes are probably best explained by the reduced efficacy in the emmetropization process noted above. It is possible that the myopias developed because the dim light reduced the ability of regulatory mechanisms to encode or respond to optical signals that would normally reduce axial elongation. For example, for some DL-LIH monkeys, the age-related, “intrinsic” ocular growth could have dominated refractive development in the absence of optical “stop” signals that are normally associated with positive lens-wear (in a manner somewhat similar to that associated with FDM). For the DL-LIM monkeys, individual failures in detecting or processing the optical “stop” signals in their visual environment might have contributed to the sustained myopic changes observed in the later stage of the experiment. If this scenario is correct, the reduced responsiveness to absolute (She et al., 2021) and relative myopic defocus under dim ambient light could have significant consequence with respect to the development of myopia.
Dim ambient lighting increased the variability in refractive development in the control eyes of both DL-groups, which appeared to agree with a reduction in the efficacy of visual regulation. In addition, dim light also caused obvious myopic changes in the control eyes of the DL-LIM monkeys that developed myopic change in their treated eyes, a phenomenon that was not observed in the DL-controls nor in the control eyes of the NL-LIM monkeys. However, similar changes have been observed in the control eyes of form-deprived monkeys reared under dim light that developed myopia in their treated eyes (She et al., 2021). This between-study agreement suggests that the combination of dim light and vision-induced myopia in the treated eye can influence control eye refractive development (She et al., 2021). In relation to our speculations above, a reduction in the efficacy of the vision-dependent growth regulating mechanisms in the control eyes might increase the eyes’ susceptibility to the interocular factors associated with vision-induced myopia, i.e., a “contralateral eye influence” that causes myopic changes (Bradley et al., 1999; Raviola & Wiesel, 1985; Schmid & Wildsoet, 1996; Smith et al., 1987; Smith III & Hung, 2000; Wildsoet & Wallman, 1995).
Based on the above reasoning, we believe that reduced ambient lighting does not produce a systematic myopiagenic effect per se; instead, it reduces the efficacy of visual regulatory mechanisms that normally operate to optimize the existing refractive errors and to prevent myopia. For our subjects, this effect, particularly the absence of “stop” responses to relative myopic defocus, resulted in the increased occurrence of myopia.
4.4. Choroidal thickness changes and their implications
Vision-induced choroidal thickness changes in response to optical defocus (Wallman et al., 1995) are consistently observed in normal-light-reared animals (Howlett & McFadden, 2006; Hung et al., 2000; Siegwart Jr. & Norton, 1998; Troilo et al., 2000; Wallman et al., 1995). In the present study, the successful development of compensating anisometropias was associated with relative choroidal thickness changes that were in the appropriate direction for the sign of lens-imposed defocus. Similarly, in a previous study, sign-appropriate changes in choroidal thickness were only observed in monkeys successfully recovering from FDM in dim ambient lighting (She et al., 2020), which suggests that the absence of sign-appropriate choroidal thickness changes in the dim-light-reared monkeys that did not recover from FDM reflected a failure to respond to the existing defocus signals (She et al., 2021). In this respect, the observations of the present study also suggest that dim ambient lighting reduces the ability of the emmetropization process to detect and/or respond to the optically imposed interocular differences in refractive error, resulting in an absence of consistent lens-compensating responses. Thus, our choroidal thickness observations indicate that low intensity ambient lighting reduces the efficacy of retinal mechanisms responsible for emmetropization.
4.5. Possible explanations for the reduced efficacy of visual regulation
It is suggested that the visual cues required for emmetropization might be attenuated under dim light (Wallman et al., 1995). However, many parametric retinal image properties that are speculated to provide cues for emmetropization do not seem to change under reduced ambient lighting. For example, our ambient lighting paradigm preserved the relative spectral output profile across the visible spectrum (She et al., 2020), thereby maintaining the potential sign-of-defocus cues associated with the eye’s longitudinal chromatic aberration (Gawne & Norton, 2020). In addition, the filters that we used to reduce the ambient lighting levels produced uniform percentage reductions in energy irradiance across the visible spectrum (see She et al., 2020, Figure 1). As a consequence, common luminance contrast statistics (e.g. Weber’s contrast and Michelson contrast; see Rucker & Wallman, 2012), and probably the spatial frequency information, would remain unchanged. In this respect, many potential emmetropization cues, defined by common parametric image property statistics, are likely preserved under reduced ambient lighting.
Although the ambient lighting levels employed in our present and previous studies remained within the lower range of primate photopic vision, the resulting visual environment was perceptually dim for humans and probably infant monkeys. Thus, it is reasonable to assume that retinal adaptation in response to the reduced ambient lighting took place, and that some of these functional changes might have contributed to the speculated reduced efficacy of the emmetropization process. For example, low ambient lighting levels might increase the coupling of horizontal cell gap junctions and thus their receptive field size through light-level-associated changes in retinal dopamine production (i.e., events that are opposite to those that occur during light adaptation, see Baldridge, 2001; Dong & McReynolds, 1991; Weiler & Akopian, 1992; Zhang et al., 2011), thereby increasing the responsiveness to photic stimulation at the expense of spatial resolution. Although emmetropization does not necessarily rely on retinal mechanisms that are associated with high spatial resolution (Gawne & Norton, 2020; Rucker & Wallman, 2012; Schmid & Wildsoet, 2004), this example nonetheless demonstrates that some retinal functions (in this case, one that is important for emmetropization) are in a sub-optimal state under dim light. It is possible that dim-light produced the observed refractive effect by altering the activity level of retinal neuromodulators, most notably dopamine (Brainard & Morgan, 1987; Iuvone et al., 1978), thus altering the functional state of other retinal neurons and/or pathways. Dopamine is a probable candidate molecule in this respect due to its extensive involvement in the modulation of retinal function (Witkovsky & Dearry, 1991). Studies have shown that many retinal pathways that are subject to dopaminergic regulation (Chaffiol et al., 2017; Feigenspan & Bormann, 1994; Mazade et al., 2019; Mazade & Eggers, 2019; Qiao et al., 2016; Wellis & Werblin, 1995) play important roles in refractive development and in experimental myopia (for a review, see Zhou et al., 2017). If the dopaminergic system was indeed involved in the observed dim-light effects, the observed inter-individual variability in refraction might be attributed to the individual differences in the retinal dopaminergic system.
Finally, we noticed that some visual targets in the dim light housing were very poorly illuminated (e.g., the water outlet in the back of a lower cage), thus our subjects might occasionally encounter circumstances that require mesopic vision. We speculate that certain retinal physiological changes under such condition, e.g., rod activation and the co-mingling of rod signals into the cone pathway (particularly those via cone bipolar cells, i.e., the secondary and tertiary pathway, for reasons stated in the preceding paragraph), might also affect retinal functions that are important for emmetropization. The extent to which this condition contributed the observed refractive effect remains to be investigated.
4.6. Summary and implications
Rearing infant monkeys under reduced ambient lighting attenuated the compensation to negative lenses and severely disrupted the compensation to positive lenses. These effects came about because the eyes had a lower probability of responding appropriately to the presenting refractive state. In agreement with our previous observations that dim-light-rearing reduced the probability of successful emmetropization (She et al., 2020) and the recovery from FDM (She et al., 2021), the results of this study suggest that low intensity ambient lighting affects the defocus-driven mechanisms which normally regulate refractive development. In support of this view, we found that failures of lens compensation were associated with the absence of appropriate choroidal thickness changes. This finding was similar to that observed in dim-light-reared monkeys underdoing recovery from FDM, both suggesting that failure to detect and/or process optical signals was responsible for the failure to initiate compensating refractive changes in individual animals.
It should be noted that our animals did not have access to any higher ambient illuminations, a situation that rarely, if ever, occurs in real life (Ostrin, 2017). Given that animals emmetropize normally under ambient illuminations that are at or above typical laboratory lighting levels, any access to higher ambient lighting conditions would allow a period of time for the eyes to correctly detect and respond to the eyes’ refractive state, whereby overcoming, at least potentially, any adverse refractive consequences. In this respect, our long and consistent experimental paradigm might have exaggerated the refractive effect of low intensity ambient lighting on refractive development. Despite this limitation, our study clearly showed that extended exposure to low intensity ambient lighting could impair normal refractive development and might be myopiagenic for some young animals that are undergoing emmetropization. On the other hand, our findings suggest that the typical laboratory lighting levels that are commonly employed in animal experiments are sufficient to ensure normal emmetropization and to protect young animals from developing refractive anomalies such as those observed in the present study. In comparison to the elevated ambient lighting levels that have been shown to protect animals from FDM (2,500 – 40,000 lux, Chen et al., 2017; Karouta & Ashby, 2015; Siegwart Jr. et al., 2012; Smith III et al., 2012), the relative increase in ambient illuminance that appears to be protective against dim-light-associated refractive anomalies observed in this and our previous studies are clearly much smaller and can be more practically obtained. In this respect, a recent clinical study showed that elevating classroom desk/blackboard illumination levels from under 100 lux to just ~400 – 500 lux significantly reduced the onset of myopia and slowed axial myopic progression in school-age children (Hua et al., 2015). The anti-myopia effect associated with this modest improvement in classroom illumination might be associated, at least in part, to the “protective effect” of “typical” ambient lighting suggested by our dim-light observations.
Acknowledgements
This work was supported by National Institutes of Health Grants EY-03611 and EY-07551, funds from the Brien Holden Vision Institute, and the University of Houston Foundation.
The authors would like to thank Dr. Nimesh Patel for the development and maintenance of the Matlab program used in choroidal thickness analysis. We are also grateful to Rena McMahen, Angel Sausedo, Timothy Thomas, and Dr. David Brammer of the Animal Care Operation in University of Houston for their incessant dedications to the caring of research animals during hurricane Harvey in 2017.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: E. L. Smith III, (p) patents on optical and pharmaceutical treatment strategies for myopia, (C) consultant to Nevakar, SightGlass Vision, Treehouse Eyes, Acucela Inc. and Essilor; Z. She, None; L.-F. Hung, None; B. Arumugam, None; K. M. Beach, None.
Reference
- Ashby RS, Ohlendorf A, & Schaeffel F (2009). The effect of ambient illuminance on the development of deprivation myopia in chicks. Investigative Ophthalmology and Visual Science, 50(11), 5348–5354. 10.1167/iovs.09-3419 [DOI] [PubMed] [Google Scholar]
- Ashby RS, & Schaeffel F (2010). The effect of bright light on lens compensation in Chicks. Investigative Ophthalmology and Visual Science, 51(10), 5247–5253. 10.1167/iovs.09-4689 [DOI] [PubMed] [Google Scholar]
- Baldridge WH (2001). Triphasic adaptation of teleost horizontal cells. In Progress in Brain Research (Vol. 131, pp. 437–449). Elsevier. 10.1016/s0079-6123(01)31035-x [DOI] [PubMed] [Google Scholar]
- Barathi VA, Boopathi VG, Yap EPH, & Beuerman RW (2008). Two models of experimental myopia in the mouse. Vision Research, 48(7), 904–916. 10.1016/j.visres.2008.01.004 [DOI] [PubMed] [Google Scholar]
- Bradley DV, Fernandes A, Lynn M, Tigges M, & Boothe RG (1999). Emmetropization in the rhesus monkey (Macaca mulatta): Birth to young adulthood. Investigative Ophthalmology and Visual Science, 40(1), 214–229. [PubMed] [Google Scholar]
- Brainard GC, & Morgan WW (1987). Light-induced stimulation of retinal dopamine: a dose-response relationship. Brain Research, 424(1), 199–203. 10.1016/0006-8993(87)91211-X [DOI] [PubMed] [Google Scholar]
- Chaffiol A, Ishii M, Cao Y, & Mangel SC (2017). Dopamine Regulation of GABAA Receptors Contributes to Light/Dark Modulation of the ON-Cone Bipolar Cell Receptive Field Surround in the Retina. Current Biology, 27(17), 2600–2609.e4. 10.1016/j.cub.2017.07.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charman WN (2005). Aberrations and myopia. Ophthalmic and Physiological Optics, 25(4), 285–301. 10.1111/j.1475-1313.2005.00297.x [DOI] [PubMed] [Google Scholar]
- Chen S, Zhi Z, Ruan Q, Liu Q, Li F, Wan F, Reinach PS, Chen J, Qu J, & Zhou X (2017). Bright light suppresses form-deprivation myopia development with activation of dopamine d1 receptor signaling in the ON pathway in retina. Investigative Ophthalmology and Visual Science, 58(4), 2306–2316. 10.1167/iovs.16-20402 [DOI] [PubMed] [Google Scholar]
- Cohen Y, Belkin M, Yehezkel O, Solomon AS, & Polat U (2011). Dependency between light intensity and refractive development under light-dark cycles. Experimental Eye Research, 92(1), 40–46. 10.1016/j.exer.2010.10.012 [DOI] [PubMed] [Google Scholar]
- Cohen Y, Peleg E, Belkin M, Polat U, & Solomon AS (2012). Ambient illuminance, retinal dopamine release and refractive development in chicks. Experimental Eye Research, 103, 33–40. 10.1016/j.exer.2012.08.004 [DOI] [PubMed] [Google Scholar]
- Coletta NJ, Marcos S, & Troilo D (2010). Ocular wavefront aberrations in the common marmoset Callithrix jacchus: Effects of age and refractive error. Vision Research. 10.1016/j.visres.2010.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coletta NJ, Marcos S, Wildsoet CF, & Troilo D (2003). Double-pass measurement of retinal image quality in the chicken eye. Optometry and Vision Science. 10.1097/00006324-200301000-00008 [DOI] [PubMed] [Google Scholar]
- Dong CJ, & McReynolds JS (1991). The relationship between light, dopamine release and horizontal cell coupling in the mudpuppy retina. The Journal of Physiology, 440(1), 291–309. 10.1113/jphysiol.1991.sp018709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenspan A, & Bormann J (1994). Modulation of GABAC receptors in rat retinal bipolar cells by protein kinase C. The Journal of Physiology, 481(2), 325–330. 10.1113/jphysiol.1994.sp020442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García De La Cera E, Rodríguez G, & Marcos S (2006). Longitudinal changes of optical aberrations in normal and form-deprived myopic chick eyes. Vision Research. 10.1016/j.visres.2005.06.012 [DOI] [PubMed] [Google Scholar]
- Gawne TJ, & Norton TT (2020). An opponent dual-detector spectral drive model of emmetropization. Vision Research, 173, 7–20. 10.1016/j.visres.2020.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DG, Powers MK, & Banks MS (1980). Depth of focus, eye size and visual acuity. Vision Research, 20(10), 827–835. 10.1016/0042-6989(80)90063-2 [DOI] [PubMed] [Google Scholar]
- Howlett MHC, & McFadden SA (2006). Form-deprivation myopia in the guinea pig (Cavia porcellus). Vision Research, 46(1–2), 267–283. 10.1016/j.visres.2005.06.036 [DOI] [PubMed] [Google Scholar]
- Howlett MHC, & McFadden SA (2009). Spectacle lens compensation in the pigmented guinea pig. Vision Research, 49(2), 219–227. 10.1016/j.visres.2008.10.008 [DOI] [PubMed] [Google Scholar]
- Hua WJ, Jin JX, Wu XY, Yang JW, Jiang X, Gao GP, & Tao FB (2015). Elevated light levels in schools have a protective effect on myopia. Ophthalmic and Physiological Optics, 35(3), 252–262. 10.1111/opo.12207 [DOI] [PubMed] [Google Scholar]
- Hung LF, Arumugam B, Ostrin L, Patel N, Trier K, Jong M, & Smith III EL (2018). The adenosine receptor antagonist, 7-methylxanthine, alters emmetropizing responses in infant macaques. Investigative Ophthalmology and Visual Science, 59(1), 472–486. 10.1167/iovs.17-22337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung LF, Crawford MLJ, & Smith III EL (1995). Spectacle lenses alter eye growth and the refractive status of young monkeys. Nature Medicine, 1(8), 761–765. 10.1038/nm0895-761 [DOI] [PubMed] [Google Scholar]
- Hung LF, Wallman J, & Smith III EL (2000). Vision-dependent changes in the choroidal thickness of macaque monkeys. Investigative Ophthalmology and Visual Science, 41(6), 1259–1269. [PubMed] [Google Scholar]
- Iuvone MP, Galli CL, & Neff NH (1978). Retinal tyrosine hydroxylase: comparison of short-term and long-term stimulation by light. Mol Pharmacol, 14(6), 1212–1219. [PubMed] [Google Scholar]
- Jiang X, Kurihara T, Kunimi H, Miyauchi M, Ikeda SI, Mori K, Tsubota KK, Torii H, & Tsubota KK (2018). A highly efficient murine model of experimental myopia. Scientific Reports. 10.1038/s41598-018-20272-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karouta C, & Ashby RS (2015). Correlation between light levels and the development of deprivation myopia. Investigative Ophthalmology and Visual Science, 56(1), 299–309. 10.1167/iovs.14-15499 [DOI] [PubMed] [Google Scholar]
- Kisilak ML, Campbell MCW, Hunter JJ, Irving EL, & Huang L (2006). Aberrations of chick eyes during normal growth and lens induction of myopia. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology. 10.1007/s00359-006-0122-9 [DOI] [PubMed] [Google Scholar]
- Mazade RE, & Eggers ED (2019). Inhibitory components of retinal bipolar cell receptive fields are differentially modulated by dopamine D1 receptors. Visual Neuroscience, 37. 10.1017/S0952523819000129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazade RE, Flood MD, & Eggers ED (2019). Dopamine D1 receptor activation reduces local inner retinal inhibition to light-adapted levels. Journal of Neurophysiology, 121(4), 1232–1243. 10.1152/jn.00448.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metlapally S, & McBrien NA (2008). The effect of positive lens defocus on ocular growth and emmetropization in the tree shrew. Journal of Vision. 10.1167/8.3.1 [DOI] [PubMed] [Google Scholar]
- Norton TT, & Siegwart JT Jr. (2013). Light levels, refractive development, and myopia - A speculative review. Experimental Eye Research, 114(205), 48–57. 10.1016/j.exer.2013.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrin LA (2017). Objectively Measured Light Exposure in Emmetropic and Myopic Adults. Optometry and Vision Science. 10.1097/OPX.0000000000001013 [DOI] [PubMed] [Google Scholar]
- Pardue MT, Stone RA, & Iuvone PM (2013). Investigating mechanisms of myopia in mice. Experimental Eye Research, 114, 96–105. 10.1016/j.exer.2012.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao-Grider Y, Hung LF, Kee CS, Ramamirtham R, & Smith III EL (2004). Recovery from form-deprivation myopia in rhesus monkeys. Investigative Ophthalmology and Visual Science, 45(10), 3361–3372. 10.1167/iovs.04-0080 [DOI] [PubMed] [Google Scholar]
- Qiao SN, Zhang Z, Ribelayga CP, Zhong YM, & Zhang DQ (2016). Multiple cone pathways are involved in photic regulation of retinal dopamine. Scientific Reports, 6(1), 1–13. 10.1038/srep28916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe-Hesketh S, & Skrondal A (2012). Multilevel and Longitudinal Modeling Using Stata vol. II: Categorical Responses, Counts, and Survival In Stata Press. [Google Scholar]
- Ramamirtham R, Kee C. su, Hung LF, Qiao-Grider Y, Huang J, Roorda A, & Smith EL III (2007). Wave aberrations in rhesus monkeys with vision-induced ametropias. Vision Research. 10.1016/j.visres.2007.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviola E, & Wiesel TN (1985). An Animal Model of Myopia. New England Journal of Medicine, 312(25), 1609–1615. 10.1056/NEJM198506203122505 [DOI] [PubMed] [Google Scholar]
- Rucker FJ, & Wallman J (2012). Chicks use changes in luminance and chromatic contrast as indicators of the sign of defocus. Journal of Vision. 10.1167/12.6.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffel F, Glasser A, & Howland HC (1988). Accommodation, refractive error and eye growth in chickens. Vision Research, 28(5), 639–657. 10.1016/0042-6989(88)90113-7 [DOI] [PubMed] [Google Scholar]
- Schmid KL, & Wildsoet CF (1996). Effects on the compensatory responses to positive and negative lenses of intermittent lens wear and ciliary nerve section in chicks. Vision Research, 36(7), 1023–1036. 10.1016/0042-6989(95)00191-3 [DOI] [PubMed] [Google Scholar]
- Schmid KL, & Wildsoet CF (2004). Inhibitory Effects of Apomorphine and Atropine and Their Combination on Myopia in Chicks. Optometry and Vision Science. 10.1097/00006324-200402000-00012 [DOI] [PubMed] [Google Scholar]
- She Z, Hung LF, Arumugam B, Beach KM, & Smith III EL (2020). Effects of low intensity ambient lighting on refractive development in infant rhesus monkeys (Macaca mulatta). Vision Research, 176(July), 48–59. 10.1016/j.visres.2020.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegwart JT Jr., & Norton TT (1998). The susceptible period for deprivation-induced myopia in tree shrew. Vision Research, 38(22), 3505–3515. 10.1016/S0042-6989(98)00053-4 [DOI] [PubMed] [Google Scholar]
- Siegwart JT Jr., & Norton TT (2010). Binocular lens treatment in tree shrews: Effect of age and comparison of plus lens wear with recovery from minus lens-induced myopia. Experimental Eye Research, 91(5), 660–669. 10.1016/j.exer.2010.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegwart JT Jr., Ward AH, & Norton TT (2012). Moderately Elevated Fluorescent Light Levels Slow Form Deprivation and Minus Lens-Induced Myopia Development in Tree Shrews. Investigative Ophthalmology & Visual Science, 53(14), 3457. http://dx.doi.org/ [Google Scholar]
- Smith III EL, Arumugam B, Hung LF, She Z, Beach K, & Sankaridurg P (2020). Eccentricity-dependent effects of simultaneous competing defocus on emmetropization in infant rhesus monkeys. Vision Research. 10.1016/j.visres.2020.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith III EL, Harwerth RS, Crawford MLJ, & Von Noorden GK (1987). Observations on the effects of form deprivation on the refractive status of the monkey. Investigative Ophthalmology and Visual Science, 28(8), 1236–1245. [PubMed] [Google Scholar]
- Smith III EL, & Hung LF (1999). The role of optical defocus in regulating refractive development in infant monkeys. Vision Research, 39(8), 1415–1435. 10.1016/S0042-6989(98)00229-6 [DOI] [PubMed] [Google Scholar]
- Smith III EL, & Hung LF (2000). Form-deprivation myopia in monkeys is a graded phenomenon. Vision Research, 40(4), 371–381. 10.1016/S0042-6989(99)00184-4 [DOI] [PubMed] [Google Scholar]
- Smith III EL, Hung LF, Arumugam B, & Huang J (2013). Negative lens-induced myopia in infant monkeys: Effects of high ambient lighting. Investigative Ophthalmology and Visual Science, 54(4), 2959–2969. 10.1167/iovs.13-11713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith III EL, Hung LF, & Huang J (2012). Protective effects of high ambient lighting on the development of form-deprivation myopia in rhesus monkeys. Investigative Ophthalmology and Visual Science, 53(1), 421–428. 10.1167/iovs.11-8652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibos LN, López-Gil N, & Bradley A (2018). What is a troland? Journal of the Optical Society of America A, 35(5), 813–816. 10.1075/pc.18.2.09rie [DOI] [PubMed] [Google Scholar]
- Tian Y, & Wildsoet CF (2006). Diurnal fluctuations and developmental changes in ocular dimensions and optical aberrations in young chicks. Investigative Ophthalmology and Visual Science. 10.1167/iovs.05-1211 [DOI] [PubMed] [Google Scholar]
- Tkatchenko TV, Shen Y, & Tkatchenko AV (2010). Mouse experimental myopia has features of primate myopia. Investigative Ophthalmology and Visual Science, 51(3), 1297–1303. 10.1167/iovs.09-4153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troilo D, Nickla DL, & Wildsoet CF (2000). Choroidal thickness changes during altered eye growth and refractive state a primate. Investigative Ophthalmology and Visual Science, 41(6), 1249–1258. [PubMed] [Google Scholar]
- Troilo D, Smith III EL, Nickla DL, Ashby RS, Tkatchenko AV, Ostrin LA, Gawne TJ, Pardue MT, Summers JA, Kee CS, Schroedl F, Wahl S, & Jones L (2019). IMI – Report on experimental models of emmetropization and myopia. Investigative Ophthalmology and Visual Science, 60(3), M31–M88. 10.1167/iovs.18-25967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troilo D, Totonelly K, & Harb E (2009). Imposed anisometropia, accommodation, and regulation of refractive state. Optometry and Vision Science, 86(1), E31–E39. 10.1097/OPX.0b013e318194072e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troland LT (1917). On the measurement of visual stimulation intensities. Journal of Experimental Psychology, 2(1), 1–33. 10.1037/h0071652 [DOI] [Google Scholar]
- Wallman J, Wildsoet CF, Xu A, Gottlieb MD, Nickla DL, Marran L, Krebs W, & Christensen AM (1995). Moving the retina: Choroidal modulation of refractive state. Vision Research, 35(1), 37–50. 10.1016/0042-6989(94)E0049-Q [DOI] [PubMed] [Google Scholar]
- Weiler R, & Akopian A (1992). Effects of background illuminations on the receptive field size of horizontal cells in the turtle retina are mediated by dopamine. Neuroscience Letters, 140(1), 121–124. 10.1016/0304-3940(92)90696-5 [DOI] [PubMed] [Google Scholar]
- Wellis DP, & Werblin FS (1995). Dopamine modulates GABA(C) receptors mediating inhibition of calcium entry into and transmitter release from bipolar cell terminals in tiger salamander retina. Journal of Neuroscience, 15(7 I), 4748–4761. 10.1523/jneurosci.15-07-04748.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whatham AR, & Judge SJ (2001). Compensatory changes in eye growth and refraction induced by daily wear of soft contact lenses in young marmosets. Vision Research. 10.1016/S0042-6989(00)00250-9 [DOI] [PubMed] [Google Scholar]
- Wildsoet CF, & Wallman J (1995). Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Research, 35(9), 1175–1194. 10.1016/0042-6989(94)00233-C [DOI] [PubMed] [Google Scholar]
- Witkovsky P, & Dearry A (1991). Functional roles of dopamine in the vertebrate retina. Progr Ret Res, 11, 11:247–292. http://gateway.webofknowledge.com/gateway/Gateway.cgi?GWVersion=2&SrcAuth=mekentosj&SrcApp=Papers&DestLinkType=FullRecord&DestApp=WOS&KeyUT=A1991HK14200010%0Apapers3://publication/uuid/DE9BD31F-80D2-42A6-A23A-4F6F81CE33D0 [Google Scholar]
- Zhang A-J, Jacoby R, & Wu SM (2011). Light- and dopamine-regulated receptive field plasticity in primate horizontal cells. The Journal of Comparative Neurology, 519(11), 2125–2134. 10.1002/cne.22604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Pardue MT, Iuvone PM, & Qu J (2017). Dopamine signaling and myopia development: What are the key challenges. Progress in Retinal and Eye Research, 61, 60–71. 10.1016/j.preteyeres.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]


