Abstract
Both left atrial and left ventricular functional parameters influence the prognosis of patients with cardiovascular diseases. This study aimed to investigate the prognostic value of a novel left atrioventricular coupling index (LACI) in a population without history of cardiovascular diseases at baseline.
Participants of the Multi-Ethnic Study of Atherosclerosis who underwent a baseline cardiovascular magnetic resonance study were analysed. LACI was defined by the ratio of the Left atrial end-diastolic volume divided by the left ventricular end-diastolic volume. Cox proportional hazard models were used to evaluate the association between LACI and atrial fibrillation, heart failure, coronary heart disease death, and hard cardiovascular disease defined by myocardial infarction, resuscitated cardiac arrest, fatal and non-fatal stroke, or coronary heart disease death.
Among the 4,124 participants (61.5±10.1 years, 47.4% men), 1,074 cardiovascular events were observed (mean follow-up 13.0±3.2 years). Greater LACI was independently associated with atrial fibrillation (HR 1.86; 95% CI [1.69-2.04]), heart failure (HR 1.50; 95% CI [1.38-1.62]), hard cardiovascular disease (1.23; 95% CI [1.13-1.34]), and coronary heart disease death (HR 1.29; 95% CI [1.15-1.45]; all p<0.0001). After adjustment for traditional cardiovascular risk factors, LACI showed significant improvement in model discrimination and reclassification compared to currently used standard models to predict outcomes.
LACI is a strong predictor for the incidence of heart failure, atrial fibrillation, hard cardiovascular disease, and coronary heart disease death. LACI has incremental prognostic value to predict cardiovascular events over traditional risk factors, and better discrimination and reclassification power compared to individual left atrial or left ventricular parameters.
Keywords: Left atrium, left ventricle, coupling, cardiovascular events, atrial fibrillation, heart failure, cardiac magnetic resonance
INTRODUCTION
Cardiovascular diseases (CVD) remain a major public health problem affecting 24.3 million people in the United States, with estimated direct and indirect costs of 351.2 billion dollars in 2015.1 Several left ventricular (LV) structural and functional parameters, such as left ventricular ejection fraction (LVEF), LV myocardial strain, LV mass to volume ratio (LVMVR), or LV global function index (LVGFI) have shown prognostic value in predicting the occurrence of CVD.2-5 However, many studies emphasize the fact that CVD does not occur exclusively because of impaired LV structure and function.6 Even with preserved LV systolic function, left atrial (LA) dysfunction may impair global heart performance and uncoupling between functional performance of the two chambers can also contribute to cardiac dysfunction and clinical heart disease.7
Atrioventricular coupling is complex because chamber filling, emptying and active contraction are not synchronous. The atria fill as the ventricles contract and eject blood into the pulmonary and systemic circulations. They empty as the ventricles relax, but, in sinus rhythm, contract at the end of ventricular diastole. Here we time simultaneous atrioventricular function in reference to ventricular systole and diastole. Indeed, deterioration in LA structure, described as a higher end-systolic LA volume index, is an independent marker of heart failure (HF).8,9 Moreover, LA end-diastolic volume index is strongly associated with CVD.10,11 LA end-diastolic volume has been reported as a better predictor of survival compared to LA end-systolic volume, given a better sensitivity for elevated LV filling pressure.10,11 Consequently, the concept of "atrial failure" has been proposed, based on the principle that even in the absence of LV disease, atrial fibrotic changes and dysfunction may trigger HF or atrial fibrillation (AF).12 Recently, LA maximum longitudinal end-systolic strain was suggested as superior to LA end-systolic volume as an index cardiac diastolic dysfunction,13 and in association with HF, AF, and death.14
Although LA and LV parameters have independent prognostic value, the close physiological relationship between LA and LV15,16 suggest that the assessment of LA/LV coupling could better reflect left atrioventricular dysfunction and be a better predictor of CVD. A single left atrioventricular parameter measuring simultaneously LA and LV could be very useful in clinical routine to improve the early prediction of CV outcomes. Indeed, LA and LV are directly connected during diastole and, in the absence of valvular heart disease, their function and filling pressures are tightly coupled.17,18 However, very few studies have ever investigated a LA/LV coupling index with very limited sample sizes.19,20 Based on such rationale, we designed an analysis to examine the association of atrioventricular coupling parameters with incident CVD in a prospective population study of individuals without history of previous heart disease. In this study, therefore, we aim to investigate the prognostic value of a new left atrioventricular coupling index (LACI), defined by the ratio between the LA end-diastolic volume and the LV end-diastolic volume assessed by cardiovascular MRI (CMR), to predict the occurrence of cardiovascular events in the Multi-Ethnic Study of Atherosclerosis (MESA).
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study population
The MESA study is a prospective, population-based multi-ethnic (White, African American, Chinese, and Hispanic) cohort study of subclinical cardiovascular disease. The study design of MESA has been described in detail previously.21 In summary, between 2000 and 2002 (Exam 1), 6,814 men and women aged from 45 to 84 years, free of clinical CVD at enrollment, were recruited from six US field centers (Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; Northern Manhattan, NY; and St Paul, MN). The methodology of baseline characteristics collection is detailed in Supplemental text 1. Blood pressure was measured 3 times using a Dinamap model Pro 100 automated oscillometric sphygmomanometer (Critikon; Tampa, FL) while the participants were resting in a seated position. The average of the last two measurements were used in the analysis. Participants with significant valvular disease at baseline were excluded. All participants provided written informed consent. All study protocols were approved by the institutional review boards of each participating field center.
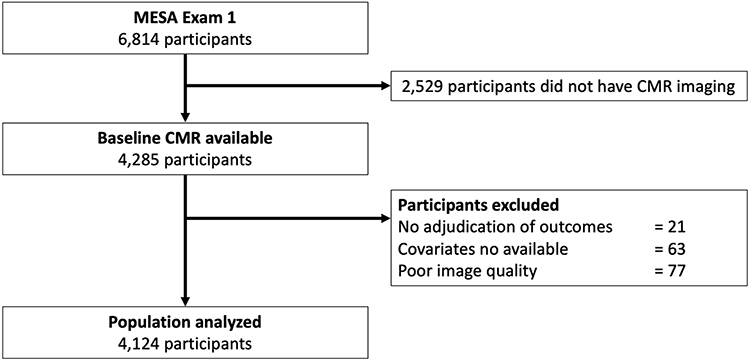
A flowchart of the MESA population investigated in this study is depicted in Figure 1. Of the 4,285 participants with baseline cardiovascular magnetic resonance (CMR) study including LA volume assessment (Exam 1), 21 individuals had no follow-up for cardiovascular events, 77 participants had missing images or a not sufficient image quality to measure LA and LV volumes, and another 63 had missing covariates, resulting in a final cohort of 4,124 subjects available for the analysis of this proposed study (Figure 1).
Figure 1. Flowchart of the study.
CMR protocol and image analysis
At baseline, CMR was performed with 1.5 T MR scanners: Signa LX or CVi (GE Medical Systems, Waukesha, WI, USA) or Symphony or Sonata (Siemens Medical Systems, Erlangen, Germany). Long-axis cine images were obtained from 2-chamber and 4-chamber views, using electrocardiogram-gated fast gradient-echo pulse sequences. A stack of short-axis cine images was acquired to encompass both ventricles and LV end-diastolic volume was measured using cardiac image modeler software (CIM version 6.0, University of Auckland, New Zealand). All cine images were acquired with a temporal resolution of ~50 ms. The complete CMR protocol, as well as details on image analysis, data quality control, calculations for LVEF, LV mass and volumes, LA volumes, and reproducibility of these measurements have been published previously.22
Multimodality tissue tracking software (MTT version 6.0, Toshiba Medical Systems Corporation, Tokyo, Japan) was used to quantify LA volume and strain from 2- and 4-chamber cine CMR images (Supplemental text 2). This method has been validated previously with good to excellent intra- and inter-reader reproducibility with intraclass correlation (ICC) of 0.88 to 0.98 (p<0.001), and pretty good inter-study reproducibility with ICC of 0.44 to 0.82 (p<0.05 to 0.001).23-25 A single experienced operator blinded to the case status of the participant defined endocardial and epicardial borders of the LA at end-systole. Using the marked points, the software creates endocardial and epicardial borders, then tracks LA tissue in subsequent frames. The endocardial and epicardial contours generated by the software are then followed by the operator during the cardiac cycle for quality control.
Left Atrioventricular Coupling Index (LACI)
LACI was defined for each participant by the ratio between the LA end-diastolic volume and the LV end-diastolic volume assessed by CMR. The LV volume was measured from the stack of short-axis cine images, while the LA volume was measured from the 2-chamber and 4-chamber views, as previously described. The LA and LV volumes were measured with a match in the same end-diastolic phase defined by the mitral valve closure (Figure 2). In addition, LACI measured in end-diastole was more accurate to predict outcomes than the assessment of a left atrioventricular coupling index performed in end-systole (Table S1). The intra- and inter-reader reproducibility of the LACI were good with ICC of 0.93 (95% CI [0.90–0.96]) and ICC of 0.81 (95% CI [0.71–0.88]), respectively.
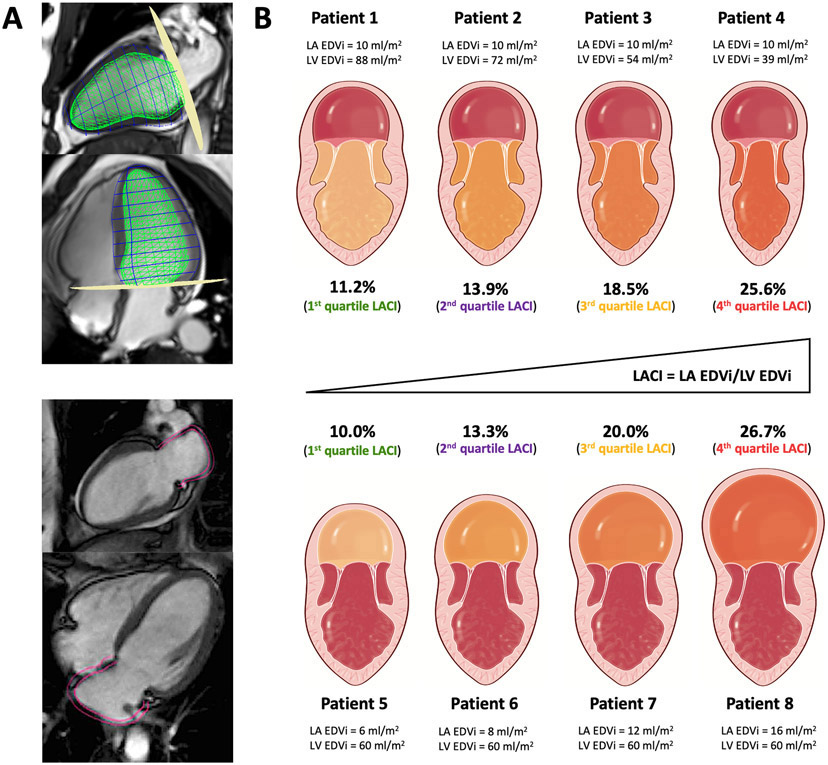
Figure 2. Schematic comparison of LACI variations in different subclinical pathophysiological settings.
Figure 2A illustrates the method to assess LACI by CMR defined by the ratio between the LA end-diastolic volume and the LV end-diastolic volume. A stack of short-axis cine images was acquired to encompass both ventricles and LV end-diastolic volume was measured using cardiac image modeler (CIM) software (green volume, top panel). LA end-diastolic volume was measured using multimodality tissue-tracking (MTT) software to track LA wall motion during the end-diastole (pink borders) in the 4-chamber and 2-chamber views (bottom panel).
In the Figure 2B, four patients from this cohort have the same normal value of LA EDVi (10 ml/m2) but different normal LV EDVi values (top panel), and four other patients with the same normal value of LV EDVi (60 ml/m2) but different normal LA EDVi values (bottom panel).
Although these values of LA EDVi or LV EDVi were identical, LACI increased significantly in all cases. These eight patients belonged to one of the LACI quartiles with significantly different risk levels of cardiovascular events which were not detected by the value of LA EDVi or LV EDVi alone. A higher LACI reflects a higher dysfunction of the left atrioventricular coupling defined by a subclinical dilation of LA compared to LV.
Abbreviations:
CMR: cardiovascular magnetic resonance; LA: left atrium; LACI: left atrioventricular coupling index; EDVi: end-diastolic volume indexed; LV: left ventricle.
The LACI value is expressed as a percentage, and a higher LACI expresses greater disproportion between the left atrial and left ventricular volumes at ventricular end diastole reflecting greater impairment of left atrioventricular coupling. The theoretical framework underlying LACI in various subclinical pathophysiological settings is illustrated in Figure 2
Outcomes
The MESA study outcome ascertainment protocols have been described in detail and are available online (www.mesa-nhlbi.org). Cardiovascular endpoints of interest were HF, AF, coronary heart disease (CHD) death, and hard CVD. In addition to MESA follow-up examinations every two years, a telephone interviewer contacted each participant (or representative) every 9-12 months to inquire about interim hospital admissions, CV outpatient diagnoses, and deaths. Two physicians reviewed all records for independent endpoint classification and assignment of event dates. Criteria for hard CVD outcomes included hard coronary events (including myocardial infarction, resuscitated cardiac arrest, and death from coronary disease), in addition to fatal and nonfatal stroke. CHD death included myocardial infarction, chest pain within the 72 hours before death, or a history of CHD and the absence of a non-cardiac cause of death. Criteria for HF as an endpoint included symptomatic HF diagnosed by a physician for a patient receiving medical treatment for HF and (1) pulmonary edema/congestion by chest X-ray and/or (2) dilated ventricle or poor LV function by echocardiography or ventriculography, or evidence of LV diastolic dysfunction. Criteria for AF as an endpoint required an AF diagnosis according to ICD-9 codes. A detailed description of the criteria used for each endpoint is provided in the Supplemental text 3. If the first cardiovascular event claim occurred before the baseline study, the participant was excluded from the analyses.
Statistical analyses
Baseline characteristics of study participants are presented as mean ± standard deviation (SD) or median (interquartile range [IQR]) for continuous variables and as counts and percentages for categorical variables in Table 1. Comparisons employed the χ2 or Fisher’s exact test for categorical variables and the Student’s t-test or Mann–Whitney–Wilcoxon test, as appropriate, for continuous variables. We used Cox regression models to study the associations between LACI and the outcomes. The proportional hazard assumption was visually tested using Schoenfeld residuals (Figure S1). The cumulative risk of cardiovascular outcomes over the follow-up years for the cohort, stratified by LACI quartiles, was determined using Kaplan–Meier curves, censored at most recent follow-up. Differences across quartiles were compared using the log-rank test. Following, the associations between LACI or all other LA and LV parameters and time-to-event were analyzed with multivariable Cox survival analyses, adjusting for traditional risk factors at baseline: age, sex, ethnicity, diabetes mellitus, systolic blood pressure, diastolic blood pressure, heart rate, current smoking, body mass index, anti-hypertensive therapy, lipid-lowering therapy, high-density lipoprotein cholesterol, total plasma cholesterol, and glomerular filtration rate.
Table 1.
Population characteristics of participants at baseline before occurrence of events by incident event categories (n=4,124)
| Parameters | No Event (n=3,426) |
HF (n=220) | AF (n=213) | CHD death (n=198) |
Hard CVD (n=443) |
|---|---|---|---|---|---|
| Age, years | 60.3 ± 9.9 | 68.2 ± 8.8 | 69.6 ± 8.0 | 70.1 ± 8.5 | 66.4 ± 9.8 |
| Male, n (%) | 1539 (44.9) | 129 (58.6) | 123 (57.7) | 119 (60.1) | 273 (61.6) |
| Ethnicity (Ca/Ch/AA/Hi), % | 39/13/26/22 | 45/11/31/19 | 55/8/19/18 | 36/12/31/20 | 40/10/24/26 |
| Hypertension, n (%) | 1328 (38.8) | 155 (70.5) | 127 (59.6) | 129 (65.2) | 269 (60.7) |
| Systolic blood pressure, mmHg | 124 ± 21 | 138 ± 22 | 134 ± 24 | 137 ± 22 | 135 ± 22 |
| Diastolic blood pressure, mmHg | 72 ± 10 | 74 ± 12 | 72.0 ± 11 | 73 ± 11 | 75 ± 11 |
| Body mass index, kg/m2 | 27.7 ± 5.0 | 29.2 ± 5.0 | 28.3 ± 5.0 | 28.2 ± 5.0 | 28.2 ± 4.6 |
| Diabetes mellitus, n (%) | 375 (10.9) | 57 (25.9) | 32 (15.0) | 51 (25.8) | 102 (23.0) |
| Smoking status, n (%) | 430 (12.6) | 28 (12.7) | 19 (8.9) | 35 (17.7) | 73 (16.5) |
| Heart rate, bpm | 63 ± 9 | 65 ± 10 | 62 ± 10 | 64 ± 10 | 64 ± 10 |
| Total cholesterol, mg/dl | 195 ± 36 | 190 ± 33 | 188 ± 31 | 190 ± 39 | 193 ± 35 |
| HDL cholesterol, mg/dl | 52 ± 15 | 49 ± 13 | 51 ± 16 | 48.1 ± 14 | 48 ± 14 |
| GFR*, ml/min/1.73m2 | 79.4 ± 15.6 | 72.5 ± 19.1 | 70.7 ± 17.1 | 72.9 ± 17.9 | 74.6 ± 18.0 |
| Chronic kidney disease†, n (%) | 239 (7.0) | 60 (27.3) | 58 (27.2) | 65 (32.8) | 87 (19.6) |
| Hypertension medication, n (%) | 1112 (32.5) | 132 (60.0) | 113 (53.1) | 109 (55.1) | 206 (46.5) |
| Lipid-lowering medication, n (%) | 517 (15.1) | 41 (18.6) | 39 (18.3) | 42 (21.2) | 91 (20.5) |
| NT-proBNP, pg/ml | 39 (12-67) | 159 (89-342) | 122 (69-256) | 118 (70-222) | 99 (64-298) |
| Agatston score, mean ± SD | 92.2 ± 283 | 390 ± 719 | 421 ± 776 | 341 ± 616 | 295 ± 544 |
| Agatston score, median (IQR) | 0 (0-78) | 81 (0-366) | 88 (0-377) | 79 (0-353) | 74 (0-332) |
| Framingham CVD risk, % | 12.8 ± 9.2 | 21.7 ± 8.7 | 20.0 ± 9.4 | 23.1 ± 7.9 | 20.6 ± 9.1 |
| CHARGE-AF score | 11.9 ± 1.2 | 12.9 ± 1.0 | 13.0 ± 1.0 | 13.0 ± 1.0 | 12.5 ± 1.1 |
| LV characteristics | |||||
| LV EDVi, ml/m2 | 68.6 ± 13.4 | 73.7 ± 19.4 | 70.5 ± 17.7 | 69.5 ± 19.3 | 68.3 ± 16.5 |
| LVEF, % | 62.6 ± 5.9 | 59.9 ± 8.1 | 63.2 ± 6.7 | 60.8 ± 7.9 | 61.5 ± 7.1 |
| LV mass index, g/m2 | 63.1 ± 12.4 | 73.1 ± 17.6 | 68.2 ± 15.6 | 69.3 ± 18.6 | 68.3 ± 15.7 |
| LVMVR, g/ml | 0.93 ± 0.17 | 1.02 ± 0.21 | 0.99 ± 0.20 | 1.02 ± 0.21 | 1.02 ± 0.19 |
| LVGFI, % | 40.3 ± 6.2 | 36.4 ± 7.2 | 39.4 ± 6.6 | 37.1 ± 7.4 | 37.5 ± 6.6 |
| LA characteristics | |||||
| LA EDVi, ml/m2 | 11.6 ± 6.1 | 17.5 ± 9.8 | 19.7 ± 11.4 | 15.4 ± 9.1 | 13.8 ± 7.6 |
| LA ESVi, ml/m2 | 29.0 ± 9.6 | 36.1 ± 13.4 | 38.8 ± 15.3 | 32.6 ± 13.0 | 31.0 ± 11.5 |
| Peak LA strain, % | 36.6 ± 10.6 | 33.4 ± 11.1 | 32.1 ± 9.9 | 33.8 ± 10.9 | 35.0 ± 11.4 |
| LACI, % | 16.9 ± 8.3 | 23.9 ± 11.9 | 27.9 ± 13.7 | 22.2 ± 11.7 | 20.4 ± 10.6 |
The comparisons with the no event population that were statistically significant with p<0.05 are shown in bold type.
Glomerular filtration rate (GFR) was calculated by chronic kidney disease epidemiology collaboration (CKD-EPI) method.
Chronic kidney disease was defined by Glomerular filtration rate < 60 ml/min/1.73m2.
Abbreviations: AA: African American; AF: atrial fibrillation; Ca: Caucasian; CHD: coronary heart disease; Ch: Chinese American; CVD: cardiovascular disease; HDL: high-density lipoprotein; HF: heart failure; Hi: Hispanic; LA: left atrium; LACI: left atrioventricular coupling index; EDVi: end-diastolic volume indexed; ESVi: end-systolic volume indexed; LV: left ventricle; LVEF: left ventricle ejection fraction; LVGFI: left ventricle global function index; LVMVR: LV mass/LV volume; NT-proBNP: N-terminal prohormone of brain natriuretic peptide.
We compared model discrimination using the C-statistic. The additional predictive value of LACI was calculated by the C-statistic increment, the categorical net reclassification improvement (NRI), and the integrative discrimination index (IDI),26 and compared to the CHARGE-AF score,27 Framingham score,28 Agatston score,29 and LA or LV parameters. NRI and IDI were computed at 10 years using the R package “survIDINRI”.30 The survival tree method was used to determine the cut-off to transform LACI into a binary variable with the best predictive value for cardiovascular event occurrences. A two-tailed p-value <0.05 was considered statistically significant. All data were analysed using R software, version 3.6.1 (R Project for Statistical Computing).
RESULTS
Study Population and Cardiovascular Events
Among the 6,814 participants of the MESA cohort, 4,124 (60.5%) underwent a baseline CMR examination with LA, LV, and outcome data available (mean age 61.5 ± 10.1 years and 47.4% male participants). Among those, 42.5% of participants had hypertension, 12.6% had diabetes mellitus, 13.0% were current smokers, and the mean body mass index was 27.8 ± 5.0 kg/m2. At baseline, 35.4% of participants were on antihypertensive therapy and 15.9% on lipid lowering medication. Population characteristics of all participants stratified by LACI quartiles are presented in Table S2. Population characteristics of all eligible participants at baseline are described in Table S3. Of note, eligible participants at baseline (n=6,814) had a higher rate of both hypertension and hypertension medication than participants of this current study (n=4,124). The baseline characteristics of the study population, divided into those who developed hard CVD (n=443, 10.7%), HF (n=220, 5.3%), AF (n=213, 5.2%), and CHD death (n=198, 4.8%) over a mean follow-up period of 13.0 ± 3.2 years are presented in Table 1. Participants were followed during a mean follow-up of 11.2 ± 3.7 years for AF and 16.2 ± 1.1 years for other outcomes. Combining all pre-specified clinical events together and reporting only the first event for each patient, 698 (16.9%) participants had a cardiovascular event. All LA and LV functional parameters were lower in participants with cardiovascular events compared to those without cardiovascular events.
LACI Distribution and Relationship to each LA and LV parameter
In the entire population, mean LACI was 17.7 ± 9.1% and the 1st–4th quartiles of LACI were ≤11.4%; 11.4–16.3%; 16.3–22.4%; >22.4%, respectively (Figure S2), without differences between females and males (17.9 ± 8.8% and 17.6 ± 9.4%, respectively). Mean LACI was 16.9 ± 8.3% in participants with no events. Regarding the definition of LACI, the LA end-diastolic volume was poorly correlated to LV end-diastolic volume (R2=0.15) (Figure S3). All LA and LV parameters are reported in Table 1 for participants with and without selected cardiovascular events. The relationship between LACI and values of other functional LV and LA parameters, and biomarkers are reported in the Figure S4. Notably, there was no relationship between LACI and systolic blood pressure at baseline (p=0.828, Figure S5).
Association of LACI with incident AF
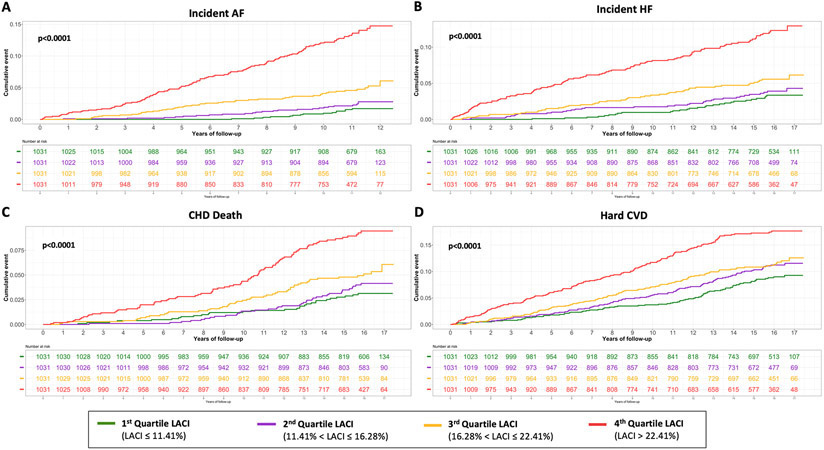
The results of unadjusted and adjusted Cox proportional hazard models for LACI and the main LA and LV parameters are indicated in Table 2 (other LA/LV parameters and biomarkers are presented in Table S4). LACI was positively associated with incident AF before and after adjustment for risk factors (adjusted hazard ratio [HR] 1.86; 95% CI [1.69–2.04] per 1 SD increment; p<0.0001). When LACI was categorized in quartiles, a LACI value >22.4% was associated with AF incidence in comparison to the 1st quartile (<11.4%) (log-rank p<0.0001) (Figure 3).
Table 2.
Univariable and multivariable analysis of CV events occurrence according to LACI and other LA or LV parameters.
| CMR Parameters | Unadjusted |
Adjusted |
||||
|---|---|---|---|---|---|---|
| HR (95%CI) | p-value | C-index (95%CI) |
HR (95%CI) | p-value | C-index (95%CI) |
|
| Incident AF | ||||||
| - LACI†, % (per 1 SD) | 2.14 (1.97-2.32) | <0.0001 | 0.76 (0.73-0.80) | 1.86 (1.69-2.04) | <0.0001 | 0.84 (0.81-0.86) |
| - LACI cut-off>25%‡ | 7.32 (5.55-9.66) | <0.0001 | 0.66 (0.63-0.69) | 4.42 (3.29-5.92) | <0.0001 | 0.81 (0.78-0.83) |
| - LA EDVi, ml/m2 (per 1 SD) | 1.83 (1.72-1.95) | <0.0001 | 0.74 (0.71-0.78) | 1.79 (1.66-1.94) | <0.0001 | 0.83 (0.81-0.85) |
| - LV EDVi, ml/m2 (per 1 SD) | 1.09 (0.95-1.25) | 0.2285 | 0.51 (0.46-0.55) | 1.20 (1.05-1.38) | 0.0084 | 0.79 (0.76-0.82) |
| - LVEF, % (per 1 SD) | 1.13 (0.98-1.30) | 0.1048 | 0.53 (0.49-0.58) | 1.07 (0.93-1.23) | 0.3640 | 0.79 (0.76-0.82) |
| - LV mass, g/m2 (per 1 SD) | 1.33 (1.19-1.50) | <0.0001 | 0.58 (0.54-0.62) | 1.26 (1.09-1.45) | 0.0012 | 0.79 (0.76-0.82) |
| - Peak LA strain, % (per 1 SD) | 0.57 (0.48-0.68) | <0.0001 | 0.63 (0.59-0.67) | 0.68 (0.57-0.81) | <0.0001 | 0.80 (0.77-0.82) |
| Incident HF | ||||||
| - LACI†, % (per 1 SD) | 1.70 (1.56-1.86) | <0.0001 | 0.69 (0.66-0.73) | 1.50 (1.38-1.62) | <0.0001 | 0.82 (0.80-0.85) |
| - LACI cut-off>25%‡ | 4.39 (3.26-5.91) | <0.0001 | 0.60 (0.57-0.63) | 2.71 (1.98-3.71) | <0.0001 | 0.80 (0.77-0.83) |
| - LA EDVi, ml/m2 (per 1 SD) | 1.57 (1.47-1.67) | <0.0001 | 0.68 (0.65-0.72) | 1.47 (1.33-1.63) | <0.0001 | 0.82 (0.80-0.85) |
| - LV EDVi, ml/m2 (per 1 SD) | 1.38 (1.21-1.57) | <0.0001 | 0.56 (0.52-0.61) | 1.48 (1.34-1.63) | <0.0001 | 0.81 (0.79-0.84) |
| - LVEF, % (per 1 SD) | 0.67 (0.60-0.75) | <0.0001 | 0.61 (0.56-0.65) | 0.66 (0.60-0.74) | <0.0001 | 0.81 (0.79-0.84) |
| - LV mass, g/m2 (per 1 SD) | 1.65 (1.52-1.79) | <0.0001 | 0.66 (0.62-0.70) | 1.63 (1.46-1.83) | <0.0001 | 0.82 (0.79-0.84) |
| - Peak LA strain, % (per 1 SD) | 0.68 (0.58-0.80) | <0.0001 | 0.61 (0.57-0.65) | 0.84 (0.72-0.98) | 0.0303 | 0.80 (0.77-0.83) |
| CHD death | ||||||
| - LACI†, % (per 1 SD) | 1.53 (1.38-1.70) | <0.0001 | 0.64 (0.60-0.68) | 1.29 (1.15-1.45) | <0.0001 | 0.84 (0.81-0.86) |
| - LACI cut-off>25%‡ | 2.86 (2.02-4.04) | <0.0001 | 0.59 (0.55-0.62) | 1.69 (1.18-2.42) | 0.0044 | 0.83 (0.80-0.85) |
| - LA EDVi, ml/m2 (per 1 SD) | 1.40 (1.28-1.53) | <0.0001 | 0.61 (0.57-0.66) | 1.29 (1.16-1.43) | <0.0001 | 0.84 (0.81-0.86) |
| - LV EDVi, ml/m2 (per 1 SD) | 1.00 (0.87-1.16) | 0.9741 | 0.47 (0.42-0.52) | 1.19 (1.04-1.37) | 0.0103 | 0.83 (0.80-0.85) |
| - LVEF, % (per 1 SD) | 0.76 (0.67-0.86) | <0.0001 | 0.55 (0.51-0.60) | 0.76 (0.67-0.86) | <0.0001 | 0.83 (0.80-0.85) |
| - LV mass, g/m2 (per 1 SD) | 1.42 (1.27-1.59) | <0.0001 | 0.57 (0.52-0.62) | 1.33 (1.15-1.53) | 0.0001 | 0.83 (0.81-0.86) |
| - Peak LA strain, % (per 1 SD) | 0.73 (0.62-0.87) | <0.0001 | 0.59 (0.55-0.63) | 0.92 (0.79-1.07) | 0.2720 | 0.83 (0.80-0.85) |
| Hard CVD | ||||||
| - LACI†, % (per 1 SD) | 1.35 (1.25-1.46) | <0.0001 | 0.59 (0.57-0.62) | 1.23 (1.13-1.34) | <0.0001 | 0.76 (0.74-0.78) |
| - LACI cut-off>25%‡ | 2.43 (1.90-3.10) | <0.0001 | 0.57 (0.55-0.59) | 1.76 (1.36-2.28) | <0.0001 | 0.75 (0.73-0.78) |
| - LA EDVi, ml/m2 (per 1 SD) | 1.24 (1.15-1.33) | <0.0001 | 0.57 (0.54-0.60) | 1.16 (1.06-1.26) | 0.0005 | 0.75 (0.73-0.77) |
| - LV EDVi, ml/m2 (per 1 SD) | 0.89 (0.81-0.99) | 0.0293 | 0.54 (0.51-0.57) | 0.98 (0.88-1.08) | 0.6375 | 0.75 (0.73-0.77) |
| - LVEF, % (per 1 SD) | 0.84 (0.77-0.92) | 0.0001 | 0.54 (0.51-0.57) | 0.88 (0.80-0.96) | 0.0039 | 0.75 (0.73-0.77) |
| - LV mass, g/m2 (per 1 SD) | 1.35 (1.25-1.46) | <0.0001 | 0.58 (0.55-0.60) | 1.18 (1.07-1.31) | 0.0014 | 0.75 (0.73-0.77) |
| - Peak LA strain, % (per 1 SD) | 0.85 (0.77-0.94) | 0.0002 | 0.55 (0.53-0.58) | 1.00 (0.91-1.10) | 0.9916 | 0.75 (0.73-0.77) |
LACI used as continuous variable
LACI used as binary variable defined by a cut-off>25%.
- All LV parameters, LA parameters and LACI values were normalized according to the following formula: (parameter–mean value)/standard deviation.
- Adjusted model included age, sex, ethnicity, diabetes mellitus, systolic blood pressure, diastolic blood pressure, heart rate, current smoking, body mass index, antihypertensive therapy, lipid-lowering therapy, HDL-cholesterol, total plasma cholesterol, glomerular filtration rate.
Abbreviations: AF: atrial fibrillation; CHD: coronary heart disease; CI: confidence interval; CVD: cardiovascular disease; HF: heart failure; HR: hazard ratio; LA: left atrium; LACI: left atrioventricular coupling index; EDVi: end-diastolic volume indexed; ESVi: end-systolic volume indexed; LV: left ventricle; LVEF: left ventricle ejection fraction.
Figure 3. Kaplan-Meier survival curves for incident AF (A), incident HF (B), CHD death (C) and hard CVD (D) by LACI quartiles.
The cumulative hazard was systematically significantly greater in the 4th quartile compared with the other quartiles for each outcome (log-rank for difference; p<0.0001).
Association of LACI with incident HF
LACI was also positively associated with incident HF before and after adjustment for risk factors (adjusted HR 1.50; 95% CI [1.38–1.62] per 1 SD increment; p<0.0001) (Table 2). The 4th quartile (>22.4%) was also associated with the HF incidence in comparison to the 1st quartile (<11.4%) (log-rank p<0.0001) (Figure 3).
Association of LACI with CHD death
LACI was positively associated with CHD death before and after adjustment for risk factors (adjusted HR 1.29; 95% CI [1.15–1.45] per 1 SD increment; p<0.0001) (Table 2). The 4th quartile (>22.4%) had a significantly higher risk of CHD death compared to the 1st quartile of LACI (<11.4%) (log rank p=0.0012) (Figure 3).
Association of LACI with hard CVD
After adjustment for risk factors, LACI was significantly associated with the hard CVD occurrence (adjusted HR 1.23; 95% CI [1.13–1.34] per 1 SD increment; p<0.0001) (Table 2). The 4th quartile (>22.4%) had a significantly higher risk of CHD death compared to the 1st quartile of LACI (<11.4%) (log rank p=0.0004) (Figure 3).
The global prognostic value of LACI was homogeneous regardless of the gender (Table S5) and of the systolic blood pressure level (Table S6).
Improvement in risk prediction with addition of LACI
The multivariable model with LACI showed significant improvement to model discrimination and reclassification compared to the multivariable model with traditional risk factors to predict incident AF (C-statistic: 0.84 vs. 0.79; NRI=0.637; IDI=0.082), incident HF (C-statistic: 0.82 vs. 0.80; NRI=0.528; IDI=0.024), CHD death (C-statistic: 0.84 vs. 0.83; NRI=0.350; IDI=0.005), and hard CVD (C-statistic: 0.77 vs. 0.76; NRI=0.227; IDI=0.009). LACI also had better a discrimination and reclassification for AF and hard CVD compared to the multivariable model with each LA or LV parameter (Table S7). Regarding the prediction of AF, LACI had better discrimination and reclassification compared to the CHARGE-AF score (C-statistic: 0.84 vs. 0.79; NRI: 0.637 vs. 0.255; IDI: 0.082 vs. 0.005). Discrimination and reclassification associated with LACI to other biomarkers and scores are presented in Table S8.
Prognostic value of binary LACI
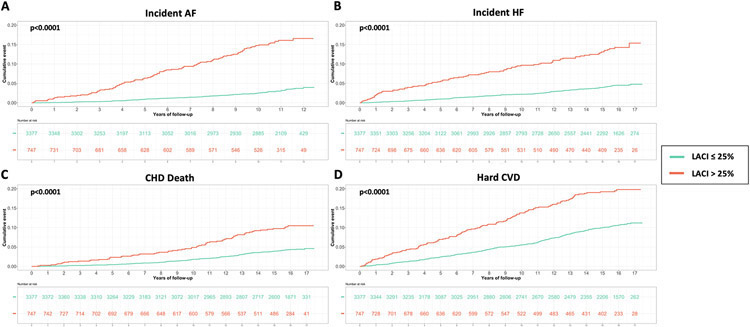
The best LACI cut-off was >29% to predict incident AF, >27%, to predict incident HF, >26% to predict CHD death, and >27% to predict hard CVD (Table S9). Using the composite outcome cut-off including incident HF, incident AF, CHD death and hard CVD, an increased LACI of >25% was independently associated with an increased occurrence of AF (adjusted HR 4.42; 95% CI [3.29–5.92]), HF (adjusted HR 2.71; 95% CI [1.98–3.71]), CHD death (adjusted HR 1.69; 95% CI [1.18–2.42]), and hard CVD (adjusted HR 1.76; 95% CI [1.36–2.28]) (p<0.0001 for all; Table 2 and Figure 4).
Figure 4. Kaplan-Meier survival curves for incident AF (A), incident HF (B), CHD death (C) and hard CVD (D) stratified by LACI >25%.
DISCUSSION
In this study, we demonstrate the predictive value of a novel left atrioventricular coupling index, LACI, for incident AF, HF, CHD death, and hard CVD in a multiethnic population, aged 45–84 years, free of clinical CVD at enrollment. Indeed, LACI showed the greatest association with those CV outcomes, improving model discrimination and reclassification of CV event risk. To our knowledge, the prognostic value of this left atrioventricular coupling index, and the incremental model discrimination of LACI over and above traditional clinical risk factors have not been previously reported.
LACI identifies an earlier stage of LA remodeling when compared to individual LA parameters, having a higher prognostic value for predicting CV events after adjustment for traditional risk factors. Therefore, relating LA to LV volume, LACI improves model discrimination and reclassification in predicting the risk of outcomes relative to such individual parameters measured separately, and saves time in clinical routine with a fast and simple measurement. Notably, the prognostic value of LACI was independent of participants' ethnicity. These results are consistent with a prior CMR study performed in 40 healthy individuals that investigated the effects of aging on left atrioventricular coupling and LV filling.20 The oldest individuals had larger LA and smaller LV volumes with larger LA/LV end-diastolic volume ratio (27 ± 6% vs. 19 ± 3%; p<0.001) and preserved LVEF. Moreover, in a canine model of early-stage hypertensive HF with preserved LVEF, left atrioventricular coupling assessed by CMR was impaired and the curvilinear LA end-reservoir pressure-volume relationship was shifted upward and leftward, indicating reduced LA compliance.31 More recently, Backhaus et al. suggested a potential prognostic value for atrioventricular mechanical coupling in predicting CV events in a clinical study of 795 patients with acute coronary syndrome.19
LA size has been conceived as a barometer of LV filling pressure and diastolic function16. Recent investigation has suggested that measuring LA end-diastolic volume10,11 or changes in LA end-diastolic volume32 may be more robust than maximal LA volume (at LV end-systole) to predict CVD. Moreover, a rise in LA end-diastolic volume has been reported as the best LA parameter to correlate with elevation of LV filling pressures.10 Such findings reflect the important interaction that exists between LA and LV performance, particularly during LV diastole, in the absence of mitral valve disease.15,16
Therefore, our study highlights the prognostic importance of atrioventricular coupling reflected by intricate hemodynamic interactions between LA and LV during LV diastole.18 At the beginning of LV diastole, passive filling begins, characterized by a rotating blood flow pattern in the LA, that gradually decreases and then stops when pressures between the two chambers are equalized. This passive filling pattern results in an early diastolic blood flow vortex formation inside the LV cavity, even stronger than the original flow rotational pattern in the LA. The resultant buildup of kinetic energy expands the LV to greater diastolic volume.17 During the diastasis period, the transmitral flow velocity is transiently reduced before the onset of active filling, which further energizes vortex formation pattern up to the end of LV diastole. Such blood flow vortex contributes to redirecting the incoming LA inflow towards the LV outflow tract, priming the LV by stretching cardiomyocytes and maximizing pre-load before the onset of LV systolic contraction.33 Such mechanisms underlie the important hemodynamic interaction of the LA and LV at end-diastole, possibly explaining the particular prognostic value of LACI, i.e. left AV coupling measured at that moment. All these reasons explain the genesis of combining these two measurements of atrial and ventricular end-diastolic volume, obtained simultaneously, in the conception of LACI, allowing through a simple measurement, to capture the combined LA and LV performance.
The prevalence of AF is estimated to be up to 2% in the U.S., with projected doubling by 2030. In this study, LACI was a stronger independent predictor of incident AF than the CHARGE-AF score and the individual LA or LV parameters, resulting in improved reclassification and discrimination. The increase in LA volume relative to that of the LV at end-diastole reflects impaired LV compliance, leading to a reduction of LA pump function, which has been proposed as an independent predictor of incident AF.18 Regarding other CV outcomes, LACI showed greater ability to predict CHD death than the Framingham and Agatston scores. We also investigated the best LACI cut-off points to predict different CV outcomes, and found slightly different best cut-off points with > 29% for incident AF, > 27% for incident HF, > 26% for CHD death and > 27% for hard CVD. This suggests different pathophysiological mechanisms involving atrioventricular coupling for different outcomes. LACI > 25% was the best cut-off for the composite of all four CV outcomes. Further studies will need to assess the clinical value of adding LACI to cardiovascular event prediction models in general population.
Limitations
Among our study limitations, the general applicability of these findings may be limited by selection and survivor biases. Indeed, participants had no known CVD at baseline and therefore, older participants undergoing CMR in this cohort represent a healthier sample than the older general population. In addition, LACI was investigated to be a primary prevention tool in the early detection of the CVD risk in asymptomatic patients without known CVD. Indeed, in a patient with CVD and who presents with both LA and LV enlargements, LACI would not be the best assessment tool. For all these reasons, the extension of these results to populations with prevalent disease would require additional investigation. Moreover, incident AF was identified based on diagnosis discharge codes, which may underestimate incident AF, as many AF cases can be asymptomatic. However, a validation sub-study on 45 MESA participants with the classification of AF based upon hospital discharge codes confirmed the diagnosis of AF in 93% of hospitalizations, implying high specificity for the adjudicated outcome.34
HF was not differentiated into HF with preserved or reduced LVEF due to the relatively low number of events. In addition, the exclusion of participants with no adjudicated outcome, with unavailable CMR data, or poor quality of images, could have introduced bias in the study. However, these excluded participants tended to be older and had more risk factors. Therefore, we hypothesize that their inclusion would more likely increase the strength of our associations. We used two instead of three dimensional methods to measure LA volumes, which may have underestimated true volumes by 11.5–20%.35 However, this method has been widely used and validated in clinical studies.23,24 Knowing that CMR is not a widely accessible test in routine, the use of LACI as a screening tool in the general population should be investigated in echocardiography, particularly with the advent of three-dimensional echocardiography. In addition, further studies should also evaluate the incremental prognostic value of LACI compared with other biomarkers such as troponin, NT-proBNP or interleukin 6, and diastolic dysfunction parameters using echocardiography. Residual confounding cannot be completely eliminated from this cross-sectional study, because only the traditional risk factors assessed at baseline were analyzed in the final models without any utilization of time-varying covariates, with incomplete accounting for traditional risk factor covariate status. Beyond left atrioventricular coupling, this study did not include analysis of left ventriculoarterial coupling. Further studies may investigate the relationship between these two couplings. Finally, while the mechanisms by which cardiovascular events are associated with left atrioventricular coupling derangements are not entirely elucidated by these observational data, the study provides important clues to the pathophysiology of cardiovascular events.
CONCLUSION
In a large multi-ethnic study population free of clinical CVD at baseline, greater LACI measured by CMR imaging was associated with high risk of incident AF, HF, CHD death, and hard CVD during a 13-year average follow-up. The addition of LACI to risk prediction models for these outcomes showed improvement to model discrimination and reclassification of cardiovascular event risks. Future studies should validate these findings to better understand the role of left atrioventricular coupling in the pathophysiology of cardiovascular events.
PERSPECTIVES
Using cardiovascular magnetic resonance data, a new left atrioventricular coupling index was identified in a large cohort of patients without cardiovascular disease at enrolment: the Multi-Ethnic Study of Atherosclerosis (MESA). This index was independently associated with the occurrence of cardiovascular events, showing an incremental long-term prognostic value over and above traditional clinical risk factors. The concept of left atrioventricular coupling is support by this coupling index which has a better prognostic value than individual LA or LV parameters measured separately.
Supplementary Material
Novelty and Significance.
What is new?
Using cardiovascular magnetic resonance data, a new left atrioventricular coupling index was identified in a large cohort of patients without cardiovascular disease at enrollment: the Multi-Ethnic Study of Atherosclerosis (MESA).
This index was independently associated with the occurrence of cardiovascular events, showing an incremental long-term prognostic value over and above traditional clinical risk factors.
What is relevant?
The concept of left atrioventricular coupling is support by this coupling index which has a better prognostic value than individual LA or LV parameters measured separately.
This left atrioventricular index could be also assessed using echocardiography allowing its worldwide spread even more easily.
Summary
The simple method to measure this new left atrioventricular index allows rapid and immediate clinical routine use as a cardiovascular risk stratification tool.
Given that the assessment of the left atrioventricular coupling has shown a more powerful and earlier stratification of cardiovascular risk than individual LA or LV parameters measured separately, further work should study this coupling more precisely.
ACKNOWLEDGMENT:
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
FUNDING:
The French Federation of Cardiology supported Dr Theo Pezel with a Post-Doctoral Research Grant at the Johns Hopkins Hospital.
The Multi-Ethnic Study of Atherosclerosis was supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS).
ABBREVIATIONS LIST
- AF
atrial fibrillation
- CHD
coronary heart disease
- CMR
cardiovascular magnetic resonance
- CVD
cardiovascular disease
- LV
left ventricle
- LVEF
left ventricular ejection fraction
- LVMVR
LV mass to volume ratio
- LVGFI
LV global function index
- LA
left atrium
- LACI
left atrioventricular coupling index
- HF
heart failure
- MESA
Multi-Ethnic Study of Atherosclerosis
- NRI
net reclassification improvement
- IDI
integrative discrimination index
Footnotes
DECLARATION OF INTEREST: Disclosure: The authors have nothing to disclose.
All authors declare that the submitted work is original and has not been published before (neither in English nor in any other language) and that the work is not under consideration for publication elsewhere.
REFERENCES
- 1.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW, American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 2.Yeboah J, Rodriguez CJ, Stacey B, Lima JA, Liu S, Carr JJ, Hundley WG, Herrington DM. Prognosis of individuals with asymptomatic left ventricular systolic dysfunction in the multi-ethnic study of atherosclerosis (MESA). Circulation. 2012;126:2713–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al Saikhan L, Park C, Hardy R, Hughes A. Prognostic implications of left ventricular strain by speckle-tracking echocardiography in population-based studies: a systematic review protocol of the published literature. BMJ Open. 2018;8:e023346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mewton N, Opdahl A, Choi E-Y, Almeida ALC, Kawel N, Wu CO, Burke GL, Liu S, Liu K, Bluemke DA, Lima JAC. Left ventricular global function index by magnetic resonance imaging--a novel marker for assessment of cardiac performance for the prediction of cardiovascular events: the multi-ethnic study of atherosclerosis. Hypertens Dallas Tex 1979. 2013;61:770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng S, Fernandes VRS, Bluemke DA, McClelland RL, Kronmal RA, Lima JAC. Age-related left ventricular remodeling and associated risk for cardiovascular outcomes: the Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging. 2009;2:191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Triposkiadis F, Pieske B, Butler J, Parissis J, Giamouzis G, Skoularigis J, Brutsaert D, Boudoulas H. Global left atrial failure in heart failure. Eur J Heart Fail. 2016;18:1307–1320. [DOI] [PubMed] [Google Scholar]
- 7.Hoit BD. Left Atrial Size and Function. J Am Coll Cardiol. 2014;63:493–505. [DOI] [PubMed] [Google Scholar]
- 8.Oliver W, Matthews G, Ayers CR, Garg S, Gupta S, Neeland IJ, Drazner MH, Berry JD, Matulevicius S, de Lemos JA. Factors Associated With Left Atrial Remodeling in the General Population. Circ Cardiovasc Imaging [Internet]. 2017. [cited 2020 Feb 11];10. Available from: 10.1161/CIRCIMAGING.116.005047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zemrak F, Ambale-Venkatesh B, Captur G, Chrispin J, Chamera E, Habibi M, Nazarian S, Mohiddin SA, Moon JC, Petersen SE, Lima JAC, Bluemke DA. Left Atrial Structure in Relationship to Age, Sex, Ethnicity, and Cardiovascular Risk Factors: MESA (Multi-Ethnic Study of Atherosclerosis). Circ Cardiovasc Imaging. 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prasad SB, Guppy-Coles K, Stanton T, Armstrong J, Krishnaswamy R, Whalley G, Atherton JJ, Thomas L. Relation of Left Atrial Volumes in Patients With Myocardial Infarction to Left Ventricular Filling Pressures and Outcomes. Am J Cardiol. 2019;124:325–333. [DOI] [PubMed] [Google Scholar]
- 11.Habibi M, Chahal H, Opdahl A, Gjesdal O, Helle-Valle TM, Heckbert SR, McClelland R, Wu C, Shea S, Hundley G, Bluemke DA, Lima JAC. Association of CMR-Measured LA Function With Heart Failure Development. JACC Cardiovasc Imaging. 2014;7:570–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bisbal F, Baranchuk A, Braunwald E, Bayés de Luna A, Bayés-Genís A. Atrial Failure as a Clinical Entity. J Am Coll Cardiol. 2020;75:222–232. [DOI] [PubMed] [Google Scholar]
- 13.Morris DA, Belyavskiy E, Aravind-Kumar R, Kropf M, Frydas A, Braunauer K, Marquez E, Krisper M, Lindhorst R, Osmanoglou E, Boldt L-H, Blaschke F, Haverkamp W, Tschöpe C, Edelmann F, Pieske B, Pieske-Kraigher E. Potential Usefulness and Clinical Relevance of Adding Left Atrial Strain to Left Atrial Volume Index in the Detection of Left Ventricular Diastolic Dysfunction. JACC Cardiovasc Imaging. 2018;11:1405–1415. [DOI] [PubMed] [Google Scholar]
- 14.Santos ABS, Roca GQ, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Fang JC, Zile MR, Pitt B, Solomon SD, Shah AM. Prognostic Relevance of Left Atrial Dysfunction in Heart Failure With Preserved Ejection Fraction. Circ Heart Fail. 2016;9:e002763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowman AW, Kovács SJ. Left atrial conduit volume is generated by deviation from the constant-volume state of the left heart: a combined MRI-echocardiographic study. Am J Physiol Heart Circ Physiol. 2004;286:H2416–2424. [DOI] [PubMed] [Google Scholar]
- 16.Barbier P, Solomon SB, Schiller NB, Glantz SA. Left atrial relaxation and left ventricular systolic function determine left atrial reservoir function. Circulation. 1999;100:427–436. [DOI] [PubMed] [Google Scholar]
- 17.Sengupta PP, Narula J. À LA mode atrioventricular mechanical coupling. JACC Cardiovasc Imaging. 2014;7:109–111. [DOI] [PubMed] [Google Scholar]
- 18.Abhayaratna WP, Fatema K, Barnes ME, Seward JB, Gersh BJ, Bailey KR, Casaclang-Verzosa G, Tsang TSM. Left Atrial Reservoir Function as a Potent Marker for First Atrial Fibrillation or Flutter in Persons ≥ 65 Years of Age. Am J Cardiol. 2008;101:1626–1629. [DOI] [PubMed] [Google Scholar]
- 19.Backhaus SJ, Kowallick JT, Stiermaier T, Lange T, Koschalka A, Navarra J-L, Uhlig J, Lotz J, Kutty S, Bigalke B, Gutberlet M, Hasenfuß G, Thiele H, Eitel I, Schuster A. Atrioventricular mechanical coupling and major adverse cardiac events in female patients following acute ST elevation myocardial infarction. Int J Cardiol. 2020;299:31–36. [DOI] [PubMed] [Google Scholar]
- 20.Germans T, Götte MJW, Nijveldt R, Spreeuwenberg MD, Beek AM, Bronzwaer JGF, Visser CA, Paulus WJ, van Rossum AC. Effects of aging on left atrioventricular coupling and left ventricular filling assessed using cardiac magnetic resonance imaging in healthy subjects. Am J Cardiol. 2007;100:122–127. [DOI] [PubMed] [Google Scholar]
- 21.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 22.Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch-Herold M, Pearson G, Sinha S, Arai A, Lima JAC, Bluemke DA. Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006;186:S357–365. [DOI] [PubMed] [Google Scholar]
- 23.Zareian M, Ciuffo L, Habibi M, Opdahl A, Chamera EH, Wu CO, Bluemke DA, Lima JAC, Venkatesh BA. Left atrial structure and functional quantitation using cardiovascular magnetic resonance and multimodality tissue tracking: validation and reproducibility assessment. J Cardiovasc Magn Reson Off J Soc Cardiovasc Magn Reson. 2015;17:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.To ACY, Flamm SD, Marwick TH, Klein AL. Clinical utility of multimodality LA imaging: assessment of size, function, and structure. JACC Cardiovasc Imaging. 2011;4:788–798. [DOI] [PubMed] [Google Scholar]
- 25.Doria de Vasconcellos H, Win TT, Chamera E, Hong SY, Venkatesh BA, Young P, Yang X, Ciuffo L, Sharma RK, Imai M, Habibi M, Wud CO, Heckbert SR, Bluemke DA, Lima JAC. References Values for Left Atrial Volumes, Emptying Fractions, Strains, and Strain Rates and Their Determinants by Age, Gender, and Ethnicity: The Multiethnic Study of Atherosclerosis (MESA). Acad Radiol. 2020; doi: 10.1016/j.acra.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pencina MJ, D’ Agostino RB, D’ Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. [DOI] [PubMed] [Google Scholar]
- 27.Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, Sinner MF, Sotoodehnia N, Fontes JD, Janssens ACJW, Kronmal RA, Magnani JW, Witteman JC, Chamberlain AM, Lubitz SA, Schnabel RB, Agarwal SK, McManus DD, Ellinor PT, Larson MG, Burke GL, Launer LJ, Hofman A, Levy D, Gottdiener JS, Kääb S, Couper D, Harris TB, Soliman EZ, Stricker BHC, Gudnason V, Heckbert SR, Benjamin EJ. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc. 2013;2:e000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. [DOI] [PubMed] [Google Scholar]
- 29.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- 30.Uno H, Tian L, Cai T, Kohane IS, Wei LJ. A unified inference procedure for a class of measures to assess improvement in risk prediction systems with survival data. Stat Med. 2013;32:2430–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zakeri R, Moulay G, Chai Q, Ogut O, Hussain S, Takahama H, Lu T, Wang X-L, Linke WA, Lee H-C, Redfield MM. Left Atrial Remodeling and Atrioventricular Coupling in a Canine Model of Early Heart Failure With Preserved Ejection Fraction. Circ Heart Fail. 2016;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Habibi M, Samiei S, Ambale Venkatesh B, Opdahl A, Helle-Valle TM, Zareian M, Almeida ALC, Choi E-Y, Wu C, Alonso A, Heckbert SR, Bluemke DA, Lima JAC. Cardiac Magnetic Resonance-Measured Left Atrial Volume and Function and Incident Atrial Fibrillation: Results From MESA (Multi-Ethnic Study of Atherosclerosis). Circ Cardiovasc Imaging. 2016;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linderer T, Chatterjee K, Parmley WW, Sievers RE, Glantz SA, Tyberg JV. Influence of atrial systole on the Frank-Starling relation and the end-diastolic pressure-diameter relation of the left ventricle. Circulation. 1983;67:1045–1053. [DOI] [PubMed] [Google Scholar]
- 34.Patton KK, Heckbert SR, Alonso A, Bahrami H, Lima JAC, Burke G, Kronmal RA. N-terminal pro-B-type natriuretic peptide as a predictor of incident atrial fibrillation in the Multi-Ethnic Study of Atherosclerosis: the effects of age, sex and ethnicity. Heart Br Card Soc. 2013;99:1832–1836. [DOI] [PubMed] [Google Scholar]
- 35.Vardoulis O, Monney P, Bermano A, Vaxman A, Gotsman C, Schwitter J, Stuber M, Stergiopulos N, Schwitter J. Single breath-hold 3D measurement of left atrial volume using compressed sensing cardiovascular magnetic resonance and a non-model-based reconstruction approach. J Cardiovasc Magn Reson Off J Soc Cardiovasc Magn Reson. 2015;17:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.