Abstract
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) are equally guideline-recommended first-line treatments for hypertension yet few head-to-head studies exist. We compared the real-world effectiveness and safety of ACE inhibitors vs. ARBs in the first-line treatment of hypertension. We implemented a retrospective, new-user comparative cohort design to estimate hazard ratios using techniques to minimize residual confounding and bias, specifically large-scale propensity score adjustment, empirical calibration, and full transparency. We included all patients with hypertension initiating monotherapy with an ACE inhibitor or ARB between 1996-2018 across eight databases from the USA, Germany, and South Korea. The primary outcomes were acute myocardial infarction (AMI), heart failure, stroke, and composite cardiovascular events (CVEs). We also studied 51 secondary and safety outcomes including angioedema, cough, syncope, and electrolyte abnormalities. Across eight databases, we identified 2,297,881 patients initiating treatment with ACE inhibitors and 673,938 patients with ARBs. We found no statistically significant difference in the primary outcomes of AMI (HR 1.11 for ACE vs. ARB; 95% CI 0.95-1.32), heart failure (HR 1.03; 0.87-1.24), stroke (HR 1.07; 0.91-1.27), or composite CVEs (HR 1.06; 0.90-1.25). Across secondary and safety outcomes, patients on ARBs had significantly lower risk of angioedema, cough, pancreatitis, and GI bleeding. In our large-scale, observational network study, ARBs do not differ statistically significantly in effectiveness at the class level compared with ACE inhibitors as first-line treatment for hypertension but present a better safety profile. These findings support preferentially prescribing ARBs over ACE inhibitors when initiating treatment for hypertension.
Keywords: angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, hypertension, real-world effectiveness, safety, cardiovascular outcomes
Introduction
Angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) effectively lower blood pressure (BP) through inhibition of the renin-angiotensin system (RAS) and are equally recommended as first-line medications in the treatment of hypertension. In the 2017 American College of Cardiology (ACC)/American Heart Association (AHA) and the 2018 European Society of Cardiology (ESC)/European Society of Hypertension (ESH) guidelines on hypertension, ACE inhibitors and ARBs both carry the strongest recommendation, class I, as first-line agents for initiation of antihypertensive therapy based on the highest level of evidence, A.1,2 There is extensive evidence that blood pressure lowering by RAS inhibition using these drug classes improves cardiovascular outcomes, as shown in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) and Heart Outcomes Prevention Evaluation (HOPE) trials as well as in meta-analyses and systematic reviews.3–10
However, there are limited head-to-head comparisons in the literature which report conflicting results. High-quality systematic reviews and meta-analyses generally conclude ARBs have similar efficacy and improved tolerability with fewer side effects as compared with ACE inhibitors, but are limited by the inclusion of no more than 4 randomized controlled trials (RCTs) for these comparisons, most of which contained fewer than 500 patients and fewer than 10 events in each cohort.3,8,11–15 Furthermore, unlike most patients initiating antihypertensive therapy in clinical practice, these RCTs were often performed in high-risk populations with preexisting vascular disease, diabetes, or in the elderly.8,13–15 In an example of conflicting data, the Reduction of Atherothrombosis for Continued Health (REACH) registry study concluded that patients on ARBs had 10% fewer cardiovascular events while another study found that ARBs instead increased the risk of myocardial infarction (MI).16,17 Accordingly, the Agency of Healthcare Research and Quality (AHRQ) called for prioritizing new comparative effectiveness studies with long-term cardiovascular outcomes in their 2011 review of ACE inhibitors and ARBs, yet scant new evidence has been generated since that time.12 Despite these limitations, both classes are recommended as first-line treatment choices, and ACE inhibitors continue to be far more commonly prescribed in the treatment of hypertension than ARBs, with lisinopril being the most commonly used antihypertensive medication worldwide.18,19
Therefore, as part of the Large-scale Evidence Generation and Evaluation across a Network of Databases for Hypertension (LEGEND-HTN) study, we sought to compare the real-world effectiveness and safety of ACE inhibitors and ARBs for the first-line treatment of hypertension across a global network of eight large observational databases.
Methods
All code, materials, and intermediate results have been made publicly available through the Observational Health Data Science and Informatics (OHDSI) network and Github and can be accessed at https://data.ohdsi.org/LegendBasicViewer/ and https://github.com/OHDSI/Legend, respectively. For commercial databases, licenses to the data are available from the owners. Patient-level data are not available for the Korean or Columbia data set.
In the open-science LEGEND-HTN study, we executed a systematic, large-scale analysis across the Observational Health Data Science and Informatics (OHDSI) distributed data network using statistical and informatics approaches to minimize confounding and bias. In total, we generated over 6 million effect estimates for 55 outcomes comparing all recommended first-line antihypertensives across 9 observational databases.20 Our approach was specifically designed to promote transparency and minimize bias and p-hacking.
Data Sources
For the comparison of ACE inhibitors and ARBs, we included all databases from the overarching set of nine LEGEND-HTN databases with at least 2,500 patients exposed to each drug class and met the inclusion/exclusion criteria below. Eight observational databases (five administrative claims and three electronic health record (EHR) databases) qualified for this study. All databases were standardized to the OMOP common data model (CDM) version 5 (https://github.com/OHDSI/CommonDataModel) and are listed as follows: 1) IBM MarketScan Commercial Claims and Encounters (CCAE, US employer-based private payer, age<=65, claims database), 2) IBM MarketScan Medicare Supplemental Beneficiaries (MDCR, US retirees, age>65, claims database), 3) IBM MarketScan Multi-state Medicaid (MDCD, US Medicaid enrollees, all ages, claims database), 4) Optum De-Identified Clinformatics Data Mart Database (Optum, US private payer, mostly age<=65, claims database), 5) Korea National Health Insurance Service/National Sample Cohort (NHIS/NSC, South Korea, all ages, claims database), 6) IMS/IQVIA Disease Analyzer Germany (German ambulatory care, all ages, EHR database), 7) Columbia University Irving Medical Center (CUMC, US academic medical center, all ages, EHR database), 8) Optum De-Identified Electronic Health Record Dataset (Optum EHR, US health systems, all ages, EHR database). All of these databases have been used extensively in prior research.20–24 All participating sites and data partners obtained prior Institutional Review Board approval or exemption to be included in this study.
Study Design and Population
We used a retrospective new-user comparative cohort design and included all patients initiating antihypertensive treatment with a single agent, either an ACE inhibitor or ARB.25 Patients were required to have at least one year of prior observation in the database before treatment initiation and a recorded diagnosis of hypertension at the time of initiation or during the one year prior. We included results across participating data sources spanning from July 1996 to March 2018. In addition, for each database, the study was restricted to the period when both treatments are observed to ensure drugs are compared when they are both on the market and available to patients. We excluded all patients who were previously exposed to any antihypertensive medication and patients who initiated another antihypertensive in the seven days after index exposure to an ACE inhibitor or ARB in order to prevent the potential inclusion of patients starting combination therapy.
Outcomes
In total, we studied the relative risk of 55 outcomes: four primary effectiveness outcomes and 51 secondary outcomes. The four primary effectiveness outcomes were: acute myocardial infarction (AMI), hospitalization for heart failure (HF), ischemic or hemorrhagic stroke (stroke), and a composite cardiovascular events (CVEs) outcome that included the previous three outcomes and sudden cardiac death. We chose these outcomes due to their clinical importance to patients and providers, which also reflects the design of previous blood pressure trials such as SPRINT.26 The 51 secondary outcomes largely represent safety outcomes or adverse effects based on known or suspected side effects from the product labels of antihypertensive medications, including angioedema, cough, hypotension, syncope, and electrolyte abnormalities. Supplemental Table S1 lists all studied outcomes. All outcome cohorts were defined by previously published algorithms, typically involving the date-stamped presence of one or more diagnosis codes, and these cohort definitions were individually reviewed and confirmed by clinical domain experts.20 For each outcome, we excluded patients who experienced that outcome prior to antihypertensive initiation.
Analysis
We defined our analysis using two different time-at-risk windows: on-treatment and intent-to-treat (ITT). For each patient, on-treatment analysis begins on the day after treatment initiation and concludes at the end of continuous treatment exposure. We define continuous exposure as consecutive drug dispensings or prescriptions in the database with less than a 30-day gap. ITT analysis includes patient observation beginning on the day after initiation and stops at the end of observation in the database. We conducted both analyses, reporting on-treatment analyses in the main paper and ITT results in the supplement.
To adjust for potential confounding and improve balance between the ACE inhibitor and ARB patient cohorts, we used propensity score (PS) models that include tens of thousands of measured baseline covariates including: demographics, diagnoses, drug exposures, drug groups, procedure occurrences, measurements (such as labs or vitals, if available), comorbidity or risk scores (including Charlson Comorbidity Index, Diabetes Complications Severity Index, CHADS2Vasc), and other observations in the database. PS models were fitted using regularized regression and used in two ways: variable-ratio matching and stratification of the two cohorts into ten strata.27–29 We then estimated hazard ratios (HR) between the ACE inhibitor and ARB cohorts using a conditional Cox proportional hazards model for the risk of each outcome in each database, accounting for time-at-risk as above and censoring. We assessed for covariate balance across these thousands of variables, defined as a standardized difference of the mean not exceeding 0.1. Finally, we aggregated HRs across data sources using a random-effects meta-analysis.30 Across these 55 outcomes, eight databases, one meta-analysis, two time-at-risk definitions and two PS adjustments, we generated a total of 1,760 individual effect estimates.
To account for residual bias even after controlling for measured confounding, we further used 76 negative control outcomes, outcomes that are unlikely to be caused or prevented by either ACE inhibitors or ARBs, and for which therefore the true hazard ratio is expected to be 1 (Supplemental Table S2). Candidate negative controls were identified through a data-rich algorithm, and manually reviewed by clinical experts.31 We supplement our negative controls with positive controls to detect biases not captured by negative controls alone, such as bias towards the null. Unlike for negative controls, we seldom know the true effect size of real positive controls, so we employ synthetic positive controls, constructed by adding simulated outcomes to real negative controls.32 These simulated outcomes are only inserted during exposure to the treatment, thus artificially increasing the effect size. By applying the same study design used to estimate the effects for the outcomes of interest, we computed hazard ratio estimates for the negative and positive control outcomes, allowing estimation of an empirical null distribution and calibration of all hazard ratio estimates, 95% confidence intervals (CIs), and p-values.33,34 To evaluate empirical equipoise, defined as the majority of patients in both cohorts having scores between 0.3 and 0.7, we used preference score distributions, a transformation of propensity scores adjusted for differences in prevalence between populations. We also report full study diagnostics for each effect estimate including power calculations, preference scores, cohort balance before and after PS-adjustment, fitted null distributions (Supplemental Figures S2-5), positive and negative calibration plots (Supplemental Figure S6), and Kaplan-Meier plots to examine proportionality assumptions of hazards over time. If any database failed these study diagnostics, we executed the meta-analysis both with and without these failed databases, presenting the meta-analysis excluding them in the main manuscript while also including the full meta-analysis with all databases in the supplement.
This study was conducted using the open-source OHDSI CohortMethod R package (https://github.com/OHDSI/CohortMethod), with large-scale analytics through the Cyclops R package (https://github.com/OHDSI/Cyclops). The prespecified LEGEND-HTN study protocols and open, executable source code are all available online (https://github.com/OHDSI/Legend).
Sensitivity Analysis
Given the potential mediatory effect of BP on cardiovascular outcomes along with the lack of such measurements in some observational databases, we also performed a sensitivity analysis which includes BP. The Optum EHR database contains records of systolic and diastolic BP for most patients within this database. We recreated the PS models to match or stratify for baseline BP, adjust for BP confounding, and recalculated effect estimates for all outcomes.
Results
Across all eight databases, we identified a total of 2,297,881 patients initiating antihypertensive treatment with an ACE inhibitor and 673,938 patients initiating treatment with an ARB. The majority of new users of ACE inhibitors received lisinopril (80%), followed by ramipril and enalapril, while the most common ARB used was losartan (45%), followed by valsartan and olmesartan. Among all patients initiating antihypertensive treatment in the overarching LEGEND-HTN study, 48% of patients initiated monotherapy with an ACE inhibitor while 15% of patients initiated treatment with an ARB. In each database, there were far more patients on ACE inhibitors than patients on ARBs except in the South Korean database NHIS/NSC.
Table 1 reports baseline patient characteristics for our largest database, CCAE, before and after PS stratification. Baseline characteristics for all other databases are available in the supplement (Supplemental Tables S2-8). In CCAE, we identified 779,041 patients initiating treatment with ACE inhibitors and 230,002 with ARBs, collectively providing over 785,000 total years of follow-up. Of these patients, over 123,000 patients on ACE inhibitors and 44,000 on ARBs had more than 500 days of follow-up. While there were significant differences in variables pre-stratification, such as comorbid diabetes or usage of diabetes drugs, all covariates in CCAE had a standardized difference of the mean of less than 0.02 after stratification, showing excellent balance across all variables. Approximately 39% of patients were female, 17% had diabetes, 37% had hyperlipidemia, and 9% had preexisting heart disease. Baseline characteristics for all databases show similarly appropriate balance after PS adjustment (Figure 1) except for NHIS/NSC, which had five variables with a standardized difference of the mean exceeding 0.1, our predefined threshold for covariate balance. Figure 2 shows the preference score distribution across all eight databases, with significant overlap demonstrating equipoise between cohorts again in all databases except NHIS/NSC, suggesting that results from this latter subpopulation may not be as generalizable. Because the NHIS/NSC database did not meet our study diagnostics, we exclude them from the main analysis results reported here in the manuscript, although analyses with all databases included remain available in the Supplement and online. All LEGEND-HTN study results are publicly accessible at https://data.ohdsi.org/LegendBasicViewer/.
Table 1: Baseline Characteristics (CCAE).
Baseline patient characteristics for angiotensin converting enzyme inhibitor (ACE) and angiotensin receptor blocker (ARB) new users in the CCAE database. We report proportion of initiators satisfying selected base-line characteristics and the standardized difference of population proportions (StdDiff) before and after stratification. Less extreme StdDiffs through stratification suggest improved balance between patient cohorts through propensity score adjustment.
| Characteristic | Before stratification | After stratification | ||||
|---|---|---|---|---|---|---|
| ACE (%) | ARB (%) | StdDiff | ACE (%) | ARB (%) | StdDiff | |
| Age Group | ||||||
| 00-04 | 0.0 | 0.0 | 0.01 | 0.0 | 0.0 | 0.01 |
| 05-09 | 0.0 | 0.0 | 0.02 | 0.0 | 0.0 | 0.02 |
| 10-14 | 0.2 | 0.0 | 0.04 | 0.1 | 0.1 | 0.02 |
| 15-19 | 0.7 | 0.3 | 0.06 | 0.6 | 0.6 | 0.01 |
| 20-24 | 1.4 | 0.9 | 0.04 | 1.3 | 1.3 | 0.00 |
| 25-29 | 2.6 | 2.0 | 0.04 | 2.4 | 2.3 | 0.01 |
| 30-34 | 5.0 | 4.4 | 0.03 | 4.9 | 4.7 | 0.01 |
| 35-39 | 8.1 | 7.5 | 0.02 | 8.0 | 7.9 | 0.00 |
| 40-44 | 12.1 | 11.9 | 0.00 | 12.0 | 12.0 | 0.00 |
| 45-49 | 16.1 | 16.4 | −0.01 | 16.1 | 16.1 | 0.00 |
| 50-54 | 18.7 | 19.3 | −0.02 | 18.9 | 18.8 | 0.00 |
| 55-59 | 18.3 | 19.3 | −0.02 | 18.6 | 18.7 | 0.00 |
| 60-64 | 15.5 | 16.5 | −0.03 | 15.7 | 16.1 | −0.01 |
| 65-69 | 1.3 | 1.4 | −0.01 | 1.3 | 1.4 | 0.00 |
| 70-74 | 0.0 | 0.0 | 0.00 | 0.0 | 0.0 | 0.00 |
| 75-79 | 0.0 | 0.0 | 0.00 | 0.0 | 0.0 | 0.00 |
| 80-84 | 0.0 | 0.0 | 0.00 | 0.0 | 0.0 | 0.00 |
| 85-89 | 0.0 | 0.0 | 0.00 | 0.0 | 0.0 | 0.00 |
| Gender: Female | 38.4 | 40.3 | −0.04 | 38.7 | 39.2 | −0.01 |
| Medical History: General | ||||||
| Acute respiratory disease | 24.5 | 26.0 | −0.03 | 24.9 | 25.0 | 0.00 |
| Attention deficit hyperactivity disorder | 1.2 | 1.1 | 0.01 | 1.1 | 1.2 | −0.01 |
| Chronic liver disease | 1.5 | 1.5 | 0.00 | 1.5 | 1.5 | 0.00 |
| Chronic obstructive lung disease | 1.8 | 2.0 | −0.02 | 1.8 | 1.9 | 0.00 |
| Crohn’s disease | 0.3 | 0.3 | 0.00 | 0.3 | 0.3 | 0.00 |
| Dementia | 0.1 | 0.1 | 0.00 | 0.1 | 0.1 | 0.00 |
| Depressive disorder | 7.4 | 6.5 | 0.04 | 7.2 | 7.5 | −0.01 |
| Diabetes mellitus | 18.3 | 13.8 | 0.12 | 17.4 | 17.5 | 0.00 |
| Gastroesophageal reflux disease | 7.8 | 8.3 | −0.02 | 7.9 | 7.9 | 0.00 |
| Gastrointestinal hemorrhage | 1.7 | 2.0 | −0.02 | 1.8 | 1.8 | 0.00 |
| Human immunodeficiency virus infection | 0.2 | 0.2 | 0.01 | 0.2 | 0.2 | 0.00 |
| Hyperlipidemia | 36.1 | 39.1 | −0.06 | 36.8 | 37.1 | −0.01 |
| Hypertensive disorder | 100.0 | 100.0 | 0.00 | 100.0 | 100.0 | 0.00 |
| Lesion of liver | 0.2 | 0.2 | 0.00 | 0.2 | 0.2 | 0.00 |
| Obesity | 8.5 | 7.3 | 0.04 | 8.3 | 8.2 | 0.00 |
| Osteoarthritis | 11.3 | 12.2 | −0.03 | 11.5 | 11.6 | 0.00 |
| Pneumonia | 1.5 | 1.5 | 0.00 | 1.5 | 1.5 | 0.00 |
| Psoriasis | 1.0 | 1.1 | −0.01 | 1.0 | 1.0 | 0.00 |
| Renal impairment | 1.1 | 1.2 | −0.01 | 1.1 | 1.1 | 0.00 |
| Rheumatoid arthritis | 0.8 | 0.9 | −0.01 | 0.8 | 0.8 | 0.00 |
| Schizophrenia | 0.1 | 0.1 | 0.01 | 0.1 | 0.1 | 0.00 |
| Ulcerative colitis | 0.3 | 0.3 | 0.00 | 0.3 | 0.3 | 0.00 |
| Urinary tract infectious disease | 5.1 | 5.6 | −0.02 | 5.2 | 5.3 | −0.01 |
| Viral hepatitis C | 0.4 | 0.4 | 0.00 | 0.4 | 0.4 | 0.01 |
| Visual system disorder | 15.5 | 16.6 | −0.03 | 15.8 | 15.8 | 0.00 |
| Medical history: Cardiovascular disease | ||||||
| Atrial fibrillation | 0.4 | 0.5 | −0.01 | 0.4 | 0.4 | 0.00 |
| Cerebrovascular disease | 1.7 | 1.5 | 0.02 | 1.7 | 1.6 | 0.00 |
| Coronary arteriosclerosis | 2.2 | 2.5 | −0.02 | 2.3 | 2.3 | 0.00 |
| Heart disease | 9.0 | 11.2 | −0.08 | 9.5 | 9.7 | −0.01 |
| Heart failure | 0.5 | 0.5 | −0.01 | 0.5 | 0.5 | 0.00 |
| Ischemic heart disease | 1.7 | 1.8 | −0.01 | 1.8 | 1.8 | 0.00 |
| Peripheral vascular disease | 4.1 | 4.5 | −0.02 | 4.2 | 4.3 | 0.00 |
| Pulmonary embolism | 0.2 | 0.2 | 0.00 | 0.2 | 0.2 | 0.00 |
| Venous thrombosis | 1.0 | 1.1 | 0.00 | 1.0 | 1.0 | 0.00 |
| Medical history: Neoplasms | ||||||
| Hematologic neoplasm | 0.5 | 0.5 | 0.00 | 0.5 | 0.6 | −0.01 |
| Malignant lymphoma | 0.2 | 0.2 | 0.00 | 0.2 | 0.2 | 0.00 |
| Malignant neoplasm of anorectum | 0.1 | 0.1 | 0.00 | 0.1 | 0.1 | 0.00 |
| Malignant neoplastic disease | 4.2 | 4.8 | −0.03 | 4.3 | 4.4 | 0.00 |
| Malignant tumor of breast | 0.7 | 0.9 | −0.02 | 0.7 | 0.8 | −0.01 |
| Malignant tumor of colon | 0.2 | 0.2 | 0.00 | 0.2 | 0.2 | 0.00 |
| Malignant tumor of lung | 0.1 | 0.1 | 0.00 | 0.1 | 0.1 | 0.00 |
| Malignant tumor of urinary bladder | 0.1 | 0.1 | 0.00 | 0.1 | 0.1 | 0.00 |
| Primary malignant neoplasm of prostate | 0.5 | 0.6 | 0.00 | 0.5 | 0.5 | 0.00 |
| Medication use | ||||||
| Antibacterials for systemic use | 48.8 | 50.1 | −0.03 | 49.0 | 49.6 | −0.01 |
| Antidepressants | 17.7 | 17.2 | 0.01 | 17.6 | 17.9 | −0.01 |
| Antiepileptics | 6.2 | 5.9 | 0.01 | 6.1 | 6.3 | −0.01 |
| Antiinflammatory and antirheumatic products | 24.0 | 23.6 | 0.01 | 24.0 | 24.2 | 0.00 |
| Antineoplastic agents | 1.4 | 1.5 | −0.01 | 1.4 | 1.5 | 0.00 |
| Antipsoriatics | 0.4 | 0.5 | −0.02 | 0.4 | 0.4 | −0.01 |
| Antithrombotic agents | 3.3 | 2.9 | 0.02 | 3.2 | 3.3 | 0.00 |
| Beta blocking agents | 0.5 | 0.5 | 0.00 | 0.5 | 0.5 | 0.00 |
| Calcium channel blockers | 0.0 | 0.0 | 0.01 | 0.0 | 0.0 | 0.00 |
| Diuretics | 0.0 | 0.0 | 0.00 | 0.0 | 0.0 | 0.00 |
| Drugs for acid related disorders | 14.1 | 15.6 | −0.04 | 14.4 | 14.6 | 0.00 |
| Drugs for obstructive airway diseases | 18.1 | 19.2 | −0.03 | 18.4 | 18.5 | 0.00 |
| Drugs used in diabetes | 15.6 | 10.3 | 0.16 | 14.5 | 14.6 | 0.00 |
| Immunosuppressants | 1.5 | 1.7 | −0.02 | 1.5 | 1.6 | −0.01 |
| Lipid modifying agents | 24.6 | 23.8 | 0.02 | 24.5 | 24.9 | −0.01 |
| Opioids | 15.2 | 14.8 | 0.01 | 15.1 | 15.4 | −0.01 |
| Psycholeptics | 17.6 | 18.2 | −0.02 | 17.6 | 18.3 | −0.02 |
| Psychostimulants, agents used for ADHD and nootropics | 2.9 | 2.9 | 0.00 | 2.9 | 3.0 | −0.01 |
Figure 1. Covariate balance before and after propensity score stratification across all databases.
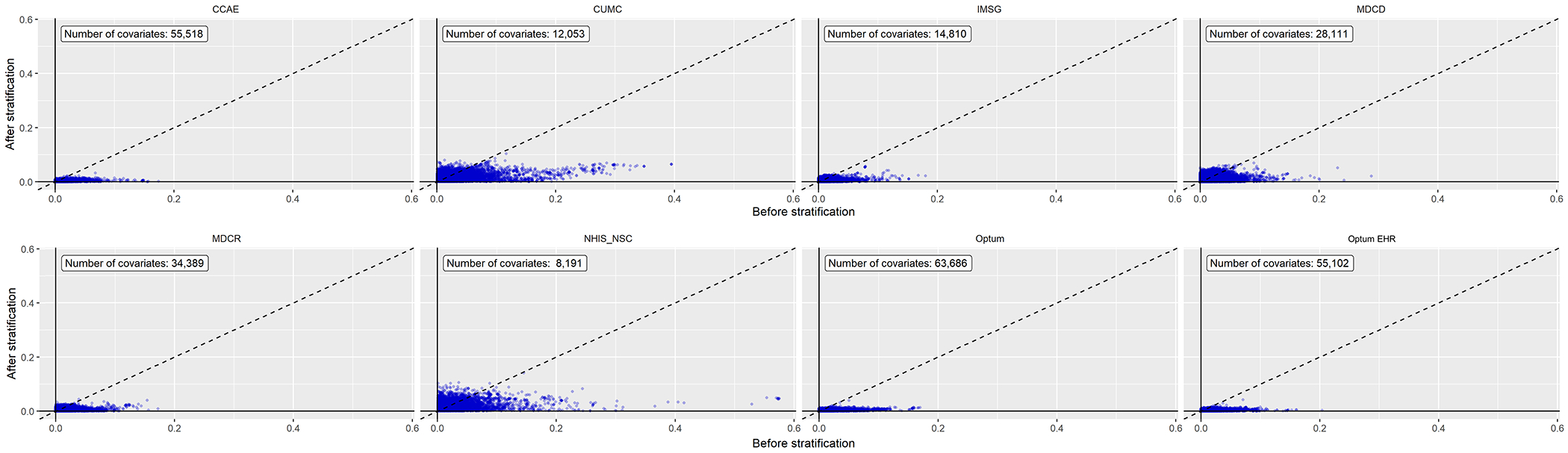
Each point represents the standardized difference of means for a single covariate before and after large-scale propensity score stratification in new users of ACE inhibitors and ARBs. All databases achieved covariate balance, defined as a standardized difference of the mean ≤ 0.1, except for the NHIS/NSC database.
Figure 2. Preference score distributions across all databases.
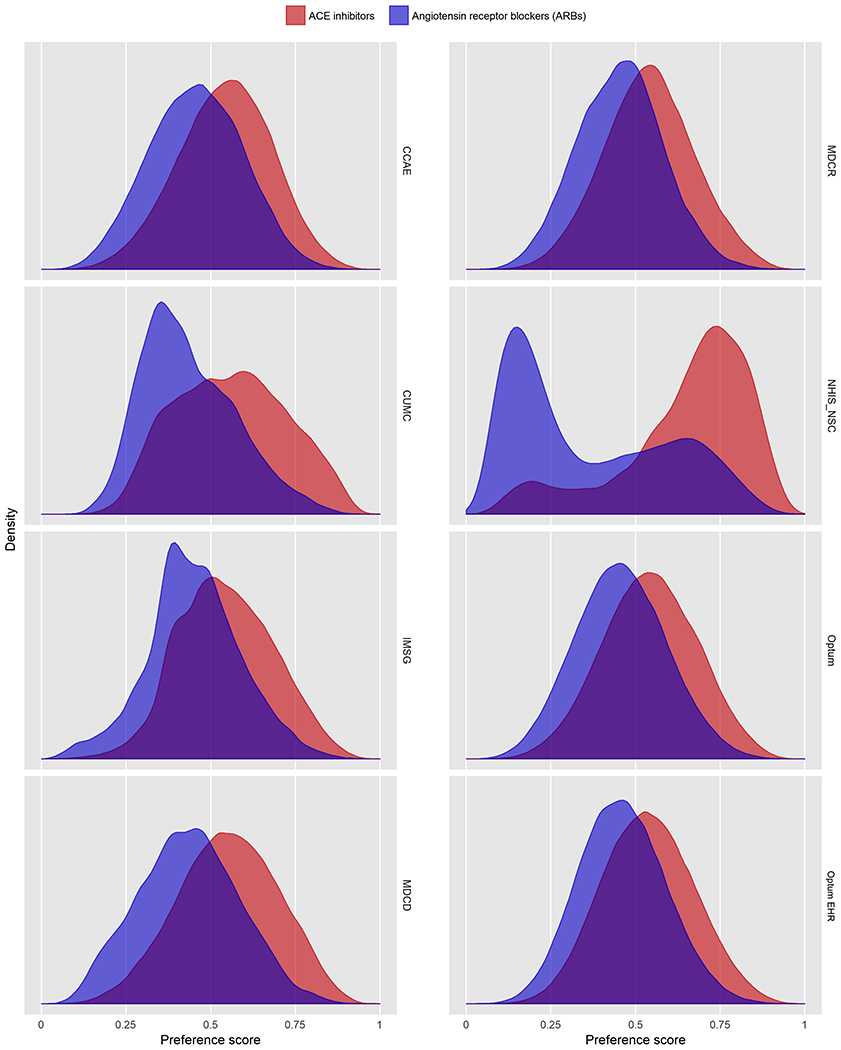
Distribution of preference scores, a transformation of propensity score adjusting for differences in prevalence, in new users of ACE inhibitors (red) as compared to ARBs (purple). Greater overlap indicates that patients in the target and comparator populations are more similar in their likelihood of receiving the target treatment. We achieve clinical empirical equipoise in all comparisons except for the NHIS/NSC database.
We found no statistically significant differences between patients on ACE inhibitors and patients on ARBs for risk of the primary outcomes of AMI (HR 1.11; 95% CI 0.95-1.32), HF (HR 1.03; 95% CI 0.87-1.24), stroke (HR 1.07; 95% CI 0.91-1.27), or composite CVEs (HR 1.06; 95% CI 0.90-1.25) (Table 2). Over the study period, patients on ACE inhibitors had a total of 5,960 AMI events, 8,165 HF events, 6,775 stroke events, and 18,213 CVEs. Patients on ARBs had a total of 1,699 AMI events, 2,468 HF events, 1,991 stroke events, and 5,463 CVEs. Before calibration, we found an increased risk of AMI for patients on ACE inhibitors using both PS stratification and PS matching along with an increased risk for composite CVE using PS matching. However, these differences were no longer statistically significant after calibration. The risk of these primary outcomes was consistent and similar, with all 95% CI’s including 1, across individual databases and meta-analyses (Figure 3).
Table 2.
Primary effectiveness outcomes for ACE inhibitors compared to ARBs (on-treatment, PS stratification, excluding NHIS/NSC)
| Outcome | HR (95% CI) | P-value | Calibrated HR (CI) | Calibrated P-value |
|---|---|---|---|---|
| Acute myocardial infarction | 1.10 (1.04-1.17) | 0.00 | 1.11 (0.95-1.32) | 0.19 |
| Composite cardiovascular events (CVEs) | 1.04 (0.99-1.10) | 0.12 | 1.06 (0.90-1.25) | 0.49 |
| Heart failure | 1.02 (0.94-1.11) | 0.64 | 1.03 (0.87-1.24) | 0.68 |
| Stroke | 1.06 (1.00-1.12) | 0.06 | 1.07 (0.91-1.27) | 0.40 |
Calibrated hazard ratios (HRs), confidence intervals (CIs) and p-values are calibrated empirically using the distributions of positive and negative control outcomes to minimize residual systematic error (see Methods for detailed description)
Figure 3: Risk of cardiovascular outcomes in ACE-inhibitors vs ARBs across all databases.
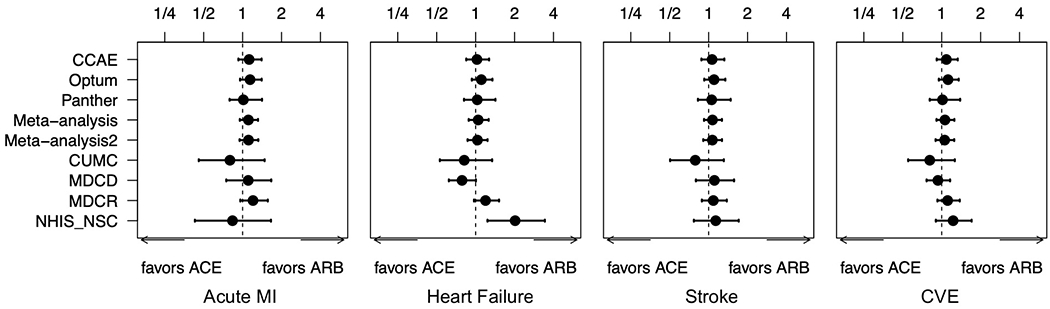
Forest plot of hazard ratios and 95% Cis for primary outcomes of acute myocardial infarction (MI), heart failure, stroke, and composite cardiovascular events (CVE) across all databases and meta-analyses. Meta-analysis 2 excludes the NHIS/NSC database while ‘Meta-analysis’ includes all databases.
Across secondary and safety outcomes, ACE inhibitors showed a significantly increased risk of acute pancreatitis (HR 1.32; 95% CI 1.04-1.70, p=0.02), angioedema (HR 3.31, 95% CI 2.55-4.51, p<0.01), cough (HR 1.32, 95% CI 1.11-1.59, p<0.01), gastrointestinal bleed (HR 1.18, 95% CI 1.01-1.41, p=0.04), and abnormal weight loss (HR 1.18, 95% CI 1.01-1.41, p=0.04), along with a corresponding decreased risk of abnormal weight gain (HR 0.84, 95% CI 0.74-0.98, p=0.04), as compared with ARBs (Table 3). There was no statistically significant difference across any of the other 49 secondary or safety outcomes including deterioration of renal function and electrolyte abnormalities. When using a conservative Bonferroni correction for 55 hypotheses, cough and angioedema remained statistically significant.
Table 3:
Secondary and Safety Outcomes for ACE inhibitors vs ARBs (on-treatment, PS stratification)
| Outcome | HR (95% CI) | P-value | Calibrated HR (95% CI) |
Calibrated P-value |
|---|---|---|---|---|
| Abdominal pain | 1.00 (0.96-1.03) | 0.87 | 1.01 (0.88-1.19) | 0.87 |
| Abnormal weight gain | 0.82 (0.79-0.86) | 0.00 | 0.84 (0.74-0.98) | 0.04 |
| Abnormal weight loss | 1.18 (1.11-1.25) | 0.00 | 1.18 (1.01-1.41) | 0.04 |
| Acute pancreatitis | 1.32 (1.09-1.60) | 0.00 | 1.32 (1.04-1.70) | 0.02 |
| Acute renal failure | 1.13 (1.08-1.18) | 0.00 | 1.14 (0.98-1.35) | 0.10 |
| Anaphylactoid reaction | 1.31 (1.00-1.72) | 0.05 | 1.31 (0.98-1.79) | 0.07 |
| Anemia | 0.96 (0.92-0.99) | 0.02 | 0.97 (0.84-1.14) | 0.76 |
| Angioedema | 3.53 (2.99-4.16) | 0.00 | 3.31 (2.55-4.51) | 0.00 |
| Anxiety | 0.98 (0.95-1.00) | 0.03 | 0.99 (0.86-1.16) | 0.91 |
| Bradycardia | 0.96 (0.86-1.08) | 0.52 | 0.98 (0.82-1.18) | 0.84 |
| Cardiac arrhythmia | 0.96 (0.91-1.02) | 0.22 | 0.98 (0.84-1.15) | 0.82 |
| Chest pain or angina | 0.99 (0.97-1.01) | 0.23 | 1.00 (0.87-1.17) | 0.92 |
| Chronic kidney disease | 1.00 (0.93-1.08) | 0.98 | 1.01 (0.87-1.20) | 0.84 |
| Cough | 1.32 (1.23-1.42) | 0.00 | 1.32 (1.11-1.59) | 0.00 |
| Decreased libido | 0.96 (0.90-1.03) | 0.29 | 0.98 (0.84-1.16) | 0.83 |
| Dementia | 1.12 (1.06-1.18) | 0.00 | 1.13 (0.97-1.34) | 0.14 |
| Depression | 1.02 (0.99-1.05) | 0.20 | 1.03 (0.90-1.21) | 0.65 |
| Diarrhea | 1.06 (1.02-1.09) | 0.00 | 1.07 (0.92-1.25) | 0.40 |
| End stage renal disease | 0.87 (0.62-1.20) | 0.39 | 0.88 (0.63-1.25) | 0.50 |
| Fall | 1.03 (0.96-1.10) | 0.46 | 1.04 (0.89-1.23) | 0.64 |
| Gastrointestinal bleed | 1.18 (1.11-1.25) | 0.00 | 1.18 (1.01-1.41) | 0.04 |
| Gout | 1.00 (0.97-1.04) | 0.83 | 1.02 (0.88-1.19) | 0.81 |
| Headache | 0.97 (0.94-1.00) | 0.04 | 0.98 (0.86-1.15) | 0.87 |
| Hepatic failure | 1.02 (0.89-1.17) | 0.74 | 1.03 (0.86-1.27) | 0.71 |
| Hospitalization with preinfarction syndrome | 1.02 (0.90-1.15) | 0.77 | 1.03 (0.86-1.25) | 0.74 |
| Hyperkalemia | 1.17 (1.04-1.30) | 0.01 | 1.17 (0.98-1.42) | 0.09 |
| Hypokalemia | 0.95 (0.89-1.03) | 0.21 | 0.97 (0.83-1.15) | 0.74 |
| Hypomagnesemia | 0.96 (0.89-1.04) | 0.36 | 0.98 (0.84-1.16) | 0.83 |
| Hyponatremia | 1.12 (1.06-1.19) | 0.00 | 1.13 (0.97-1.34) | 0.13 |
| Hypotension | 1.13 (1.09-1.17) | 0.00 | 1.14 (0.98-1.35) | 0.10 |
| Impotence | 1.06 (1.01-1.12) | 0.02 | 1.07 (0.92-1.27) | 0.37 |
| Malignant neoplasm | 0.97 (0.89-1.05) | 0.39 | 0.98 (0.84-1.16) | 0.85 |
| Measured renal dysfunction | 0.87 (0.66-1.14) | 0.31 | 0.88 (0.66-1.20) | 0.44 |
| Nausea | 1.10 (1.06-1.13) | 0.00 | 1.11 (0.95-1.30) | 0.20 |
| Neutropenia or agranulocytosis | 0.96 (0.89-1.02) | 0.18 | 0.97 (0.84-1.15) | 0.76 |
| Rash | 0.96 (0.93-1.00) | 0.04 | 0.98 (0.85-1.15) | 0.82 |
| Rhabdomyolysis | 1.10 (0.91-1.34) | 0.32 | 1.11 (0.88-1.43) | 0.37 |
| Syncope | 1.02 (0.96-1.07) | 0.56 | 1.03 (0.89-1.21) | 0.71 |
| Thrombocytopenia | 1.01 (0.96-1.06) | 0.69 | 1.02 (0.88-1.20) | 0.76 |
| Type 2 diabetes mellitus | 1.04 (0.99-1.08) | 0.12 | 1.05 (0.90-1.24) | 0.54 |
| Vasculitis | 1.01 (0.85-1.20) | 0.88 | 1.03 (0.83-1.29) | 0.80 |
| Venous thromboembolism | 0.97 (0.90-1.04) | 0.35 | 0.98 (0.84-1.16) | 0.84 |
| Vertigo | 0.95(0.92-0.99) | 0.01 | 0.97 (0.84-1.13) | 0.73 |
| Vomiting | 1.15 (1.11-1.19) | 0.00 | 1.15 (0.99-1.36) | 0.07 |
Calibrated hazard ratios (HRs), confidence intervals (CIs) and p-values are calibrated empirically using the distributions of positive and negative control outcomes to minimize residual systematic error (see Methods for detailed description)
In our sensitivity analysis, after adjusting for baseline BP in the Optum EHR database, there was no change in the results for any of primary outcomes (Supplemental Figure S1). That is, there remained no significant difference between ACE inhibitors and ARBs on the risk of AMI, HF, stroke or composite CVEs.
Discussion
In this large-scale observational study of over 3 million patients initiating antihypertensive treatment with ACE inhibitors or ARBs across eight databases worldwide, we found no statistically significant difference in the effectiveness of ACE inhibitors versus ARBs on AMI, HF, stroke, or composite CVEs, while ARBs had a better safety profile with lower risks for acute pancreatitis, angioedema, cough, and GI bleed. This study represents the largest head-to-head comparison of ACE inhibitors with ARBs and supports the preferential prescribing of ARBs over ACE inhibitors.
Our findings, which show no significant difference in effectiveness outcomes, are consistent with findings from previous systematic reviews or meta-analyses conducted from RCTs or from comparative clinical studies of other design.3,9–12,35–37 We cannot rule out a moderate difference in effectiveness (for example, the primary composite effectiveness outcome confidence interval was 0.90 to 1.25), but prior studies also demonstrated that ACE inhibitors and ARBs have similar effects on blood pressure control, along with no difference in major outcome risk including mortality, cardiovascular mortality, stroke, heart failure, MI, composite cardiovascular events, kidney disease, or diabetes.11,12,35,36 However, these studies also note the numerous limitations of existing literature: the scarcity of head-to-head studies, particularly RCTs; the lack of long-term outcomes; small sample sizes; concerns about generalizability as few studies investigated strictly hypertensive patients longitudinally; and significant heterogeneity across treatment protocols.11,12,35 In addition, there is little prior evidence and no prior head-to-head studies directly addressing first-line therapy, such as in our study here of treatment-naïve hypertensive patients.
Despite these limitations, some authors have argued there should be a preference for ACE inhibitors given the longer history and more extensive placebo-controlled RCTs which have been conducted for ACE inhibitors relative to ARBs.11 A small number of studies have reached conflicting conclusions about increased risk for AMI and CAD in patients on ARBs, as compared to ACE inhibitors, while others have suggested that ARBs actually reduce the risk for CVEs by 10%.16,17,38 However, given the length of time both drug classes have been on the market, wide availability of inexpensive generic forms, proven efficacy in hypertension, and widespread use, it is unlikely that future large RCTs will be conducted to directly compare ACE inhibitors or ARBs for the treatment of hypertension and fill any remaining evidence gaps. Therefore, real-world evidence such as the results generated by this large-scale study may be both the most appropriate and only context in which to compare the effects of these two commonly used medications.
Our findings from secondary outcomes of higher risk of side effects and worse safety profile with ACE inhibitors also build upon what has been established in the existing safety literature. The effect of ACE inhibitors on preventing degradation of bradykinin is well known, so our findings of significantly increased risk for angioedema and cough in patients on ACE inhibitors is both expected and mechanistically plausible. Given the extent of the previous literature and known associations on these side effects, confirming these same findings in our study could represent a positive litmus test for the validity of our approach. Interestingly, our study also showed a significantly increased risk of acute pancreatitis and GI bleed with ACE inhibitors. The association of ACE inhibitors with pancreatitis has been previously reported, primarily in case reports, and is thought to be precipitated by the accumulation of bradykinin leading to localized edema of the pancreatic duct and pancreas.39–41 In one case-control study, ACE inhibitors were associated with increased risk of pancreatitis with an adjusted odds ratio of 1.5 (95% CI 1.1-2.2). This effect increased with dose, and the highest risk occurred during the first 6 months of therapy.42 The association of ACE inhibitors with increased risk for GI bleeding may be a novel finding, as there is little evidence previously on this topic and no prior studies comparing the effects of both ACE inhibitors and ARBs on GI bleeding. One case-control study of patients hospitalized for GI bleed showed an unadjusted rate ratio of 1.66 with ACE inhibitors but an adjusted rate ratio of 0.95 (95% CI 0.70–1.31), while ARBs were not studied.43 These findings may also be due to chance, as they remained significant after PS adjustment and calibration, but not with conservative Bonferroni correction.
As with other observational studies, our study remains subject to the limitation of potential residual confounding and bias. However, to minimize this risk, we employed statistical methods to detect and adjust for residual bias including multiple approaches to propensity score adjustment as well as p-value calibration using positive and negative controls. We report extensive diagnostics which allow us to evaluate the validity and generalizability of our results, including covariate balance across tens of thousands of covariates to evaluate measured confounding, preference score distributions to assess equipoise, and negative controls to assess residual systematic error, which led to the exclusion of the South Korean NHIS/NSC database on the basis of its failing our predefined threshold for covariate balance or empirical equipoise. Our study was also limited to the drug class level and as such, our findings may not extend to all individual drug level comparisons within these classes. In addition, not all variables of interest may be available or recorded systematically in observational databases, such as blood pressure, social determinants of health, or cost of medications. For blood pressure, we conducted a sensitivity analysis by including BP measurements from Optum EHR to ascertain whether the results would be affected by the inclusion of BP. The study results were not substantively affected, suggesting that BP treatment effects are similar and any BP differences at baseline were likely already accounted for indirectly by other variables among the thousands of covariates currently incorporated into the models. Although we excluded patients initiating combination therapy within 7 days, patients may still receive additional therapies as escalation of treatment beyond 7 days. These criteria remain appropriate for our investigation of first-line therapy and we include both time-at-risk windows of on-treatment and intent-to-treat in our analysis. Our study was a comparison of first-line treatment only and may not be applicable to patients who are not initiating treatment such as those switching medications. Finally, lisinopril accounts for the majority ACE inhibitor use which suggests that lisinopril may have a disproportionately large effect on the drug class comparison as compared to other ACE inhibitors. However, given that this high proportion reflects real world use and we often consider ACE inhibitors equivalent as a class in terms of effectiveness and safety in both research and clinical practice, we posit that the validity and generalizability remain relevant to real-world populations.
Our large-scale, observational network study demonstrates that ARBs do not have significantly different effectiveness in long-term cardiovascular outcomes compared with ACE inhibitors and have a better safety profile. While current US and European guidelines consider ACE inhibitors and ARBs to be equally recommended first-line therapies and other international guidelines group ACE inhibitors and ARBs together as a single treatment category, these results lend further support to recent calls for the differentiation and elevation of ARBs as first-line therapy over ACE inhibitors in the treatment of hypertension.1,2,44,45 Our findings support preferentially starting ARBs rather than ACE inhibitors for patients and providers who intend to treat hypertension through RAS inhibition.
Perspectives
In this large-scale observational study of over 3 million patients worldwide, we found no statistically significant difference in the effectiveness at the class level of ACE inhibitors versus ARBs, while ARBs had a better safety profile. Despite being equally guideline-recommended first-line therapies for hypertension, these results support preferentially starting ARBs rather than ACE inhibitors when initiating treatment for hypertension for physicians and patients considering RAS inhibition. While this study compared these two classes of drugs, further study may be warranted to investigate any potential heterogeneity that may exist at the individual drug level.
Supplementary Material
Novelty and Significance.
What is New?
This large-scale propensity-matched network study represents the largest head-to-head comparison of ACE inhibitors with ARBs for the first-line treatment of hypertension
ARBs, as compared with ACE inhibitors, are not significantly different in effectiveness with regard to cardiovascular outcomes, including risk of acute myocardial infarction, heart failure, stroke, or composite cardiovascular events.
ARBs had a significantly better safety profile, with lower risk of angioedema, cough, pancreatitis, and GI bleeding
What is Relevant?
Angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) are equally recommended first-line therapies for the treatment of hypertension
ACE inhibitors are far more commonly prescribed than ARBs
Few head-to-head studies comparing ACE inhibitors with ARBs for hypertension treatment exist, some of which reach conflicting results
Summary
ARBs demonstrate no statistically significant difference in real-world effectiveness at the class level and a significantly better safety profile as compared to ACE inhibitors in the first-line treatment of hypertension. Therefore, despite their equal standing in guidelines as recommended first-line therapies, physicians and patients should consider preferentially starting ARBs rather than ACE inhibitors when initiating treatment for hypertension.
Acknowledgments
Sources of Funding:
This study was partially supported by the National Institutes of Health (grants R01 LM006910, T15 LM007079, and U19 AI135995) and National Science Foundation (grant IIS 1251151). This work was also supported by the Bio Industrial Strategic Technology Development Program (20001234) funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea) and a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea [grant number: HI16C0992]. No funding organization or sponsor was involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosures:
Dr Hripcsak reports grants from the National Library of Medicine during the conduct of the study and grants from Janssen Research outside the submitted work. Dr Suchard reports grants from the National Science Foundation and the National Institutes of Health during the conduct of the study and grants from Janssen Research and Development outside the submitted work. Dr Shea reports grants from the National Heart, Lung, and Blood Institute and the US Health Resources and Services Administration not directly relevant to the published work. Dr Madigan reports personal fees from Simon Greenstone Panatier; Williams Hart; Lanier Law Firm; Kazan, McClain, Satterley & Greenwood; Shire; and Bayer outside the submitted work. Dr Krumholz reports personal fees from UnitedHealth, IBM Watson Health, Element Science, Aetna, Facebook, Siegfried & Jenson Law Firm, Arnold & Porter Law Firm, Ben C. Martin Law Firm, and the National Center for Cardiovascular Diseases (Beijing); cofounder of Hugo Health and Refactor Health; contracts with US Centers for Medicare & Medicaid Services; and grants from Medtronic/US Food and Drug Administration, Medtronic/Johnson & Johnson, and Shenzhen Center for Health Information outside the submitted work. Dr Ryan is an employee of Janssen Research and Development and shareholder of Johnson & Johnson during the conduct of the study. Dr Schuemie is an employee and shareholder of Janssen Research and Development outside the submitted work. Dr. Pratt received grants from the Australian National Health and Medical Research Council GNT1157506. No other disclosures were reported.
Footnotes
Ethical approval: This study was approved by the Columbia University institutional review board as an OHDSI network study. The use of databases was reviewed by the New England Institution Review Board and was determined to be exempt from broad institutional review board approval because this research project did not involve human subjects research. Informed consent was also waived for this reason.
Data Sharing: All code, materials, and intermediate results have been made publicly available through the Observational Health Data Science and Informatics (OHDSI) network and Github and can be accessed at https://data.ohdsi.org/LegendBasicViewer/ and https://github.com/OHDSI/Legend, respectively. For commercial databases, licenses to the data are available from the owners. Patient-level data are not available for the Korean or Columbia data set. Requests for data can be made to the corresponding author.
References
- 1.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Himmelfarb CD, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology 2018;71:e127–e248. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 3.Reboussin David M, Allen Norrina B, Griswold Michael E, Eliseo Guallar, Yuling Hong, Lackland Daniel T., et al. Systematic Review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018;71:e116–e135. doi: 10.1161/HYP.0000000000000067. [DOI] [PubMed] [Google Scholar]
- 4.The Heart Outcomes Prevention Evaluation Study Investigators. Effects of an Angiotensin-Converting–Enzyme Inhibitor, Ramipril, on Cardiovascular Events in High-Risk Patients. New England Journal of Medicine 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 5.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major Outcomes in High-Risk Hypertensive Patients Randomized to Angiotensin-Converting Enzyme Inhibitor or Calcium Channel Blocker vs Diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 6.Dahlöf B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. The Lancet 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 7.Lithell H, Hansson L, Skoog I, Elmfeldt D, Hofman A, Olofsson B, et al. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. Journal of Hypertension 2003;21:875. [DOI] [PubMed] [Google Scholar]
- 8.The ONTARGET Investigators. Telmisartan, Ramipril, or Both in Patients at High Risk for Vascular Events. New England Journal of Medicine 2008;358:1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 9.Psaty BM, Lumley T, Furberg CD, Schellenbaum G, Pahor M, Alderman MH, et al. Health Outcomes Associated With Various Antihypertensive Therapies Used as First-Line Agents: A Network Meta-analysis. JAMA 2003;289:2534–2544. doi: 10.1001/jama.289.19.2534. [DOI] [PubMed] [Google Scholar]
- 10.van Vark LC, Bertrand M, Akkerhuis KM, Brugts JJ, Fox K, Mourad J-J, et al. Angiotensin-converting enzyme inhibitors reduce mortality in hypertension: a meta-analysis of randomized clinical trials of renin–angiotensin–aldosterone system inhibitors involving 158 998 patients. Eur Heart J 2012;33:2088–2097. doi: 10.1093/eurheartj/ehs075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li EC, Heran BS, Wright JM. Angiotensin converting enzyme (ACE) inhibitors versus angiotensin receptor blockers for primary hypertension. Cochrane Database of Systematic Reviews 2014. doi: 10.1002/14651858.CD009096.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanders GD, Coeytaux R, Dolor RJ, Hasselblad V, Patel UD, Powers B, et al. Angiotensin-Converting Enzyme Inhibitors (ACEIs), Angiotensin II Receptor Antagonists (ARBs), and Direct Renin Inhibitors for Treating Essential Hypertension: An Update. Agency for Healthcare Research and Quality (US); 2011. [PubMed] [Google Scholar]
- 13.Bremner AD, Baur M, Oddou-Stock P, Bodin F. Valsartan: long-term efficacy and tolerability compared to lisinopril in elderly patients with essential hypertension. Clin Exp Hypertens 1997;19:1263–1285. [DOI] [PubMed] [Google Scholar]
- 14.Barnett AH, Bain SC, Bouter P, Karlberg B, Madsbad S, Jervell J, et al. Angiotensin-Receptor Blockade versus Converting–Enzyme Inhibition in Type 2 Diabetes and Nephropathy. New England Journal of Medicine 2004;351:1952–1961. doi: 10.1056/NEJMoa042274. [DOI] [PubMed] [Google Scholar]
- 15.Špinar J, Vítovec J, Souček M, Dušek L, Pavlík T. CORD: COmparison of Recommended Doses of ace inhibitors and angiotensin II receptor blockers. International Journal of Cardiology 2010;144:293–294. doi: 10.1016/j.ijcard.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 16.Potier L, Roussel R, Elbez Y, Marre M, Zeymer U, Reid CM, et al. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in high vascular risk. Heart 2017;103:1339–1346. doi: 10.1136/heartjnl-2016-310705. [DOI] [PubMed] [Google Scholar]
- 17.Strauss Martin H, Hall Alistair S Angiotensin Receptor Blockers May Increase Risk of Myocardial Infarction. Circulation 2006;114:838–854. doi: 10.1161/CIRCULATIONAHA.105.594986. [DOI] [PubMed] [Google Scholar]
- 18.Hripcsak G, Ryan PB, Duke JD, Shah NH, Park RW, Huser V, et al. Characterizing treatment pathways at scale using the OHDSI network. PNAS 2016;113:7329–7336. doi: 10.1073/pnas.1510502113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiuping Gu, Burt Vicki L., Dillon Charles F., Yoon Sarah. Trends in Antihypertensive Medication Use and Blood Pressure Control Among United States Adults With Hypertension. Circulation 2012;126:2105–2114. doi: 10.1161/CIRCULATIONAHA.112.096156. [DOI] [PubMed] [Google Scholar]
- 20.Suchard MA, Schuemie MJ, Krumholz HM, You SC, Chen R, Pratt N, et al. Comprehensive comparative effectiveness and safety of first-line antihypertensive drug classes: a systematic, multinational, large-scale analysis. The Lancet 2019;394:1816–1826. doi: 10.1016/S0140-6736(19)32317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hripcsak G, Suchard MA, Shea S, Chen R, You SC, Pratt N, et al. Comparison of Cardiovascular and Safety Outcomes of Chlorthalidone vs Hydrochlorothiazide to Treat Hypertension. JAMA Intern Med 2020;180:542–551. doi: 10.1001/jamainternmed.2019.7454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duke JD, Ryan PB, Suchard MA, Hripcsak G, Jin P, Reich C, et al. Risk of angioedema associated with levetiracetam compared with phenytoin: Findings of the observational health data sciences and informatics research network. Epilepsia 2017;58:e101–e106. doi: 10.1111/epi.13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vashisht R, Jung K, Schuler A, Banda JM, Park RW, Jin S, et al. Association of Hemoglobin A1c Levels With Use of Sulfonylureas, Dipeptidyl Peptidase 4 Inhibitors, and Thiazolidinediones in Patients With Type 2 Diabetes Treated With Metformin: Analysis From the Observational Health Data Sciences and Informatics Initiative. JAMA Network Open 2018;1:e181755–e181755. doi: 10.1001/jamanetworkopen.2018.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen R, Ryan P, Natarajan K, Falconer T, Crew KD, Reich CG, et al. Treatment Patterns for Chronic Comorbid Conditions in Patients With Cancer Using a Large-Scale Observational Data Network. JCO Clinical Cancer Informatics 2020:171–183. doi: 10.1200/CCI.19.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ryan PB, Schuemie MJ, Gruber S, Zorych I, Madigan D. Empirical performance of a new user cohort method: lessons for developing a risk identification and analysis system. Drug Saf 2013;36Suppl 1:S59–72. doi: 10.1007/s40264-013-0099-6. [DOI] [PubMed] [Google Scholar]
- 26.A Randomized Trial of Intensive versus Standard Blood-Pressure Control. New England Journal of Medicine 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983;70:41–55. doi: 10.1093/biomet/70.1.41. [DOI] [Google Scholar]
- 28.Tian Y, Schuemie MJ, Suchard MA. Evaluating large-scale propensity score performance through real-world and synthetic data experiments. Int J Epidemiol 2018;47:2005–2014. doi: 10.1093/ije/dyy120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suchard MA, Simpson SE, Zorych I, Ryan P, Madigan D. Massive parallelization of serial inference algorithms for a complex generalized linear model. ACM Trans Model Comput Simul 2013;23. doi: 10.1145/2414416.2414791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 31.Voss E, Boyce R, Ryan P, van der Lei J, Rijnbeek P, Schuemie M. Accuracy of an Automated Knowledge Base for Identifying Drug Adverse Reactions. J Biomed Inform 2017;66:72–81. doi: 10.1016/j.jbi.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuemie MJ, Ryan PB, Hripcsak G, Madigan D, Suchard MA. Improving reproducibility by using high-throughput observational studies with empirical calibration. Phil Trans R Soc A 2018;376:20170356. doi: 10.1098/rsta.2017.0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schuemie MJ, Hripcsak G, Ryan PB, Madigan D, Suchard MA. Empirical confidence interval calibration for population-level effect estimation studies in observational healthcare data. PNAS 2018;115:2571–2577. doi: 10.1073/pnas.1708282114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schuemie MJ, Hripcsak G, Ryan PB, Madigan D, Suchard MA. Robust empirical calibration of p-values using observational data. Statistics in Medicine 2016;35:3883–3888. doi: 10.1002/sim.6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matchar DB, McCrory DC, Orlando LA, Patel MR, Patel UD, Patwardhan MB, et al. Systematic review: comparative effectiveness of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for treating essential hypertension. Ann Intern Med 2008;148:16–29. [DOI] [PubMed] [Google Scholar]
- 36.Ricci F, Di Castelnuovo A, Savarese G, Perrone Filardi P, De Caterina R. ACE-inhibitors versus angiotensin receptor blockers for prevention of events in cardiovascular patients without heart failure - A network meta-analysis. Int J Cardiol 2016;217:128–134. doi: 10.1016/j.ijcard.2016.04.132. [DOI] [PubMed] [Google Scholar]
- 37.Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. The Lancet 2003;362:1527–1535. doi: 10.1016/S0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 38.Bangalore S, Kumar S, Wetterslev J, Messerli FH. Angiotensin receptor blockers and risk of myocardial infarction: meta-analyses and trial sequential analyses of 147 020 patients from randomised trials. BMJ 2011;342. doi: 10.1136/bmj.d2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones MR, Hall OM, Kaye AM, Kaye AD. Drug-Induced Acute Pancreatitis: A Review. Ochsner J 2015;15:45–51. [PMC free article] [PubMed] [Google Scholar]
- 40.Kaurich T Drug-Induced Acute Pancreatitis. Baylor University Medical Center Proceedings 2008;21:77–81. doi: 10.1080/08998280.2008.11928366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Griesbacher T Kallikrein-Kinin System in Acute Pancreatitis: Potential of B2-Bradykinin Antagonists and Kallikrein Inhibitors. PHA 2000;60:113–120. doi: 10.1159/000028355. [DOI] [PubMed] [Google Scholar]
- 42.Eland IA, Sundström A, Velo GP, Andersen M, Sturkenboom MCJM, Langman MJS, et al. Antihypertensive medication and the risk of acute pancreatitis: The European case-control study on drug-induced acute pancreatitis (EDIP). Scandinavian Journal of Gastroenterology 2006;41:1484–1490. doi: 10.1080/00365520600761676. [DOI] [PubMed] [Google Scholar]
- 43.Suissa S, Bourgault C, Barkun A, Sheehy O, Ernst P. Antihypertensive drugs and the risk of gastrointestinal bleeding. The American Journal of Medicine 1998;105:230–235. doi: 10.1016/S0002-9343(98)00239-3. [DOI] [PubMed] [Google Scholar]
- 44.Messerli FH, Bangalore S, Bavishi C, Rimoldi SF. Angiotensin-Converting Enzyme Inhibitors in Hypertension: To Use or Not to Use? J Am Coll Cardiol 2018;71:1474–1482. doi: 10.1016/j.jacc.2018.01.058. [DOI] [PubMed] [Google Scholar]
- 45.Lee H- Y, Shin J, Kim G- H, Park S, Ihm S- H, Kim HC, et al. 2018 Korean Society of Hypertension Guidelines for the management of hypertension: part II-diagnosis and treatment of hypertension. Clin Hypertens 2019;25. doi: 10.1186/s40885-019-0124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


