Significance
Tuberculosis (TB) remains a global health concern despite the availability of antibiotics to treat this disease. Understanding the metabolic state of the bacteria during infection is crucial to identifying novel drug targets that are vital to Mycobacterium tuberculosis survival. Here, we demonstrate that M. tuberculosis central carbon metabolism is altered in response to acidic pH, a host-imposed stress, causing the pathogen to depend on lipids for growth. Continuous fatty acid supplementation at acidic pH is a simple and effective approach to investigate M. tuberculosis in a metabolic state that more closely reflects that of bacteria during infection and might facilitate the identification of anti-TB drugs with a greater likelihood to be active in vivo.
Keywords: Mycobacterium tuberculosis, acid stress, lipid catabolism, glyceraldehyde-3-phosphate dehydrogenase, metabolism
Abstract
Acidic pH arrests the growth of Mycobacterium tuberculosis in vitro (pH < 5.8) and is thought to significantly contribute to the ability of macrophages to control M. tuberculosis replication. However, this pathogen has been shown to survive and even slowly replicate within macrophage phagolysosomes (pH 4.5 to 5) [M. S. Gomes et al., Infect. Immun. 67, 3199–3206 (1999)] [S. Levitte et al., Cell Host Microbe 20, 250–258 (2016)]. Here, we demonstrate that M. tuberculosis can grow at acidic pH, as low as pH 4.5, in the presence of host-relevant lipids. We show that lack of phosphoenolpyruvate carboxykinase and isocitrate lyase, two enzymes necessary for lipid assimilation, is cidal to M. tuberculosis in the presence of oleic acid at acidic pH. Metabolomic analysis revealed that M. tuberculosis responds to acidic pH by altering its metabolism to preferentially assimilate lipids such as oleic acid over carbohydrates such as glycerol. We show that the activity of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is impaired in acid-exposed M. tuberculosis likely contributing to a reduction in glycolytic flux. The generation of endogenous reactive oxygen species at acidic pH is consistent with the inhibition of GAPDH, an enzyme well-known to be sensitive to oxidation. This work shows that M. tuberculosis alters its carbon diet in response to pH and provides a greater understanding of the physiology of this pathogen during acid stress.
Tuberculosis (TB) is a chronic disease mostly affecting the lungs and, despite the availability of a vaccine and antibiotic therapy, remains the leading cause of death due to a single bacterium. An estimated 2 billion people are thought to be infected with Mycobacterium tuberculosis, the causative agent of TB, with 10 million new infections each year and 1 million deaths (1). M. tuberculosis success as a pathogen can be attributed to its ability to adapt and persist within the host. This intracellular pathogen replicates within macrophages and must be able to withstand host-imposed stresses as well as gain access to nutrients to survive and proliferate. M. tuberculosis has been observed inside lipid-rich lesions during infection in humans and in animal models of TB (2, 3). Its genome contains an extensive number of redundant genes dedicated to β-oxidation necessary for lipid breakdown, indicating the importance of lipid catabolism (4). In addition to β-oxidation, lipid utilization depends on two key enzymes, isocitrate lyase (ICL) required for the glyoxylate shunt and phosphoenolpyruvate carboxykinase (PEPCK) catalyzing the first committed step of gluconeogenesis. Mutants lacking either ICL or PEPCK cannot grow with lipids as a sole carbon source and are severely attenuated in the TB mouse model (5–7). While M. tuberculosis can simultaneously use several different carbon sources to grow in vitro (8), lipids seem to be the primary source of carbon during infection (5, 7, 9–11). Whether this is because glycolytic carbon sources are scarce or inaccessible or because M. tuberculosis requires lipids to grow during infection is unknown.
Despite its ability to block phagosome–lysosome fusion, a notable fraction of phagocytosed M. tuberculosis is rapidly trafficked toward acidified compartments, and this proportion increases upon macrophage activation by T cell produced interferon-γ (IFN-γ) (12–14). Moreover, lung tissues from TB patients were found to have a median pH of pH 5.5 (15) supporting that M. tuberculosis faces acid stress during its infectious cycle in humans. M. tuberculosis can survive at a pH as low as pH 4.5 by maintaining its intracellular pH close to neutral at least in part through sustained peptidoglycan hydrolysis (16, 17). While the identification of mechanisms that enable M. tuberculosis to survive at acidic pH has taken much attention, how the pathogen adapts to an acidified environment to grow and promote disease remains ill defined. At acidity lower than pH 5.8, M. tuberculosis enters a nonreplicating state in vitro (18). This growth arrest at mildly acidic pH is surprising considering that the bacterium likely replicates in more acidic environments during infection.
The media used to culture mycobacteria contain glycerol and glucose as main carbon sources. Previous work demonstrated that growth of M. tuberculosis at acidic pH is improved by changing the carbon source (18, 19). Pyruvate promoted growth of M. tuberculosis at pH 5.7 (19) indicating that growth at acidic pH is regulated by available carbon sources. Because lipids appear to be the primary carbon source M. tuberculosis utilizes to grow in vivo (5, 7, 9–11), we hypothesized that providing host-relevant carbon sources to M. tuberculosis, such as lipids, would serve as a more physiologically relevant model to examine how M. tuberculosis responds to acidic pH. Here, we demonstrate that providing oleic acid (OA) (and other host-relevant lipids) as a carbon source to M. tuberculosis, resulted in sustained growth in acidic cultures at pH 5.5 and below. We applied metabolomics, genetic, and biochemical approaches to investigate this pH-driven use of lipids to support growth. Our work helps explain the dependence of M. tuberculosis on lipids as a primary carbon source during infection and demonstrates that M. tuberculosis is well adapted to the acidic environments it encounters during infection; in fact, it is not only able to maintain its neutral intrabacterial pH, as previously reported, but can replicate in acidic conditions similar to those within phagolysosomes.
Results
Host-Associated Lipids Support Growth of M. tuberculosis in Acidic Media.
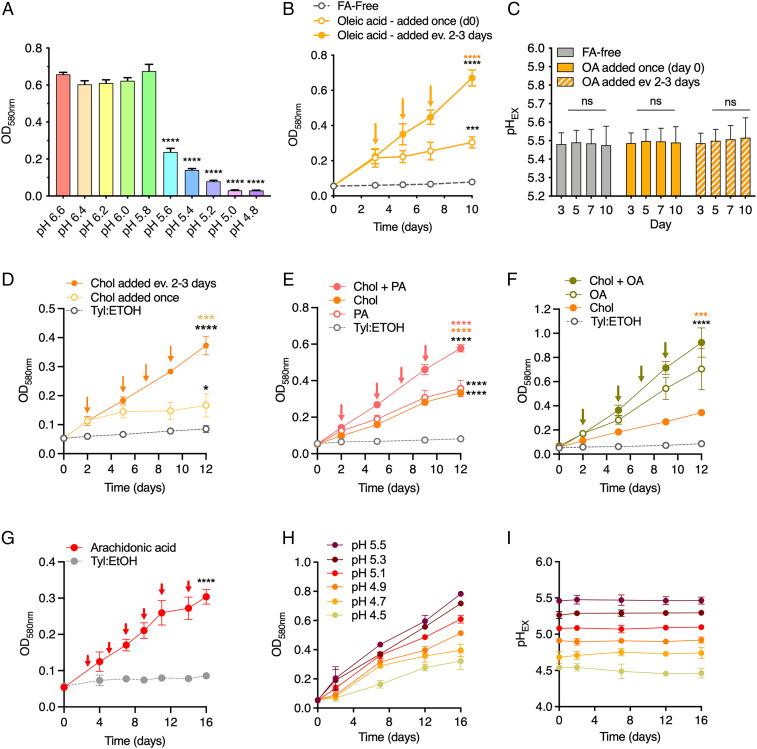
We and others have observed that growth of M. tuberculosis is greatly reduced when the pH of the culture medium is less than pH 5.8 (Fig. 1A) (18, 19). We thus speculated that this apparent sensitivity to relatively mild acidic pH in vitro could be due to the lack of a host-relevant carbon source in the culture medium. To test this hypothesis, we used OA as a model lipid, and we observed that the addition of OA (200 μM) to an acidified medium (pH 5.5) promoted growth of M. tuberculosis (Fig. 1B). Yet, after 3 d, the growth plateaued likely because of OA depletion. OA supported growth over a wide range of concentrations at pH 7.0 and pH 5.0, but concentrations above 250 μM resulted in a growth defect (SI Appendix, Fig. S1A). The concentration of 200 µM OA is comparable to that found in OADC (oleic acid-albumin-dextrose-catalase), which is commonly used to supplement culture media for M. tuberculosis. We therefore supplemented the cultures with OA (200 μM) every 2 to 3 d. This repeated supplementation allowed continued growth of M. tuberculosis at acidic pH in the presence of the buffer 2-(N-Morpholino) ethanesulfonic acid (MES), which maintained the pH of the medium (Fig. 1 B and C). These data demonstrate that OA supports growth of M. tuberculosis at pH 5.5, and replication does not require neutralization of the culture medium.
Fig. 1.
Host-associated lipids support growth of M. tuberculosis in acidic media. (A) Growth of M. tuberculosis in glycerol and glucose containing media is restricted below pH 5.8. Biomass was measured (OD580nm) after 12 d of growth in media of decreasing pH containing 0.2% glucose and 0.2% glycerol. (B) Repeated OA supplementation supports continued growth of M. tuberculosis at pH 5.5. A total of 200 μM OA was used to replenish a FA-free 7H9 medium base containing 0.2% glycerol and 0.2% glucose. (C) pH measurements of the culture media (pHEX) during the growth curve experiment in B. (D–G) Growth at pH 5.5 is supported by repeated supplementation of (D) cholesterol (100 μM), (E) palmitic acid (100 μM), (F) OA (200 μM), a combination of (E) cholesterol + palmitic acid, (F) cholesterol + OA, and (G) arachidonic acid (100 μM). In B and D through G, arrows indicate when lipid(s) were added to cultures. (H) Growth of M. tuberculosis using repeated supplementation of 200 μM OA (or 50 μM OA for pH 4.5) across a range of decreasing pH. Base media was FA-free 7H9. (I) pH measurements of the culture media (pHEX) during the growth curve experiment described in H. Tyl: ETOH is added to the cultures (D–G) as a control vehicle used as a solvent for the lipids tested (gray, dashed). Data in B–I show means ± SD of three biological replicates. Statistical significance was determined for the final time point of growth curve experiments using one-way ANOVA and Tukey’s multiple comparison test. Data in A are means ± SD of two independent experiments performed in triplicate. In A, statistical significance was determined at each pH compared to pH 6.6. In B through G, black asterisks indicates significance relative to controls (FA-free, Tyl:EtOH); orange and pink asterisks indicate significance relative to data graphed in that same color. Gly = glycerol; OA = oleic acid; FA = fatty acid; Chol = cholesterol; PA = palmitic acid; Tyl: ETOH = tyloxapol-ethanol. ***P < 0.0005; ****P < 0.0001.
To determine to what extent glycerol and glucose support M. tuberculosis growth at acidic pH when OA is present, we measured the biomass after growth with OA alone or in combination with either glycerol, glucose, or both. This revealed that most of the biomass gained at pH 5.5 was derived from OA utilization, even when glycerol and glucose were present (SI Appendix, Fig. S1B). We next sought to determine whether host-relevant lipids other than OA permit M. tuberculosis growth at acidic pH. M. tuberculosis depends on cholesterol uptake and catabolism for persistence in vivo (11). Palmitic acid may also be used as a carbon source during infection because, like OA, it is found inside lysosomes and a fluorescent analog of palmitic acid was demonstrated to be taken up by M. tuberculosis within macrophages (20, 21). Using our supplementation model, we found that cholesterol (100 μM) and palmitic acid (100 μM) supported growth at pH 5.5 (Fig. 1 D and E and SI Appendix, Fig. S1 C and D). A combination of cholesterol with either OA or palmitic acid enhanced the replication rate at pH 5.5 compared to either single carbon source, without inducing any growth inhibition (Fig. 1 E and F). This observation is consistent with the finding that fatty acids help to alleviate the metabolic cost of catabolizing cholesterol (22). Finally, we found that arachidonic acid, an omega-6 lipid, which is released from phospholipids within IFN-γ–activated infected macrophages and can translocate to M. tuberculosis–containing phagosomes (23, 24), enabled replication of M. tuberculosis at both pH 7.0 (SI Appendix, Fig. S1E) and pH 5.5 (Fig. 1G). Altogether, these data demonstrate that host-relevant lipids, but not the glycolytic carbon sources glycerol and glucose, promote M. tuberculosis growth at acidic pH.
Given the growth-promoting impact of lipids at pH 5.5, we wondered if M. tuberculosis is capable of replicating at even lower pH. Strikingly, with repeated OA supplementation, M. tuberculosis replicated even at a pH as low as pH 4.5, albeit the growth rate decreased with decreasing pH (Fig. 1H). The MES buffer included in the media ensured that the pH of the cultures (pHEX) remained stable throughout the experiment (Fig. 1I). These experiments demonstrate that M. tuberculosis can replicate in vitro at a pH similar to that observed in a phagolysosome (14).
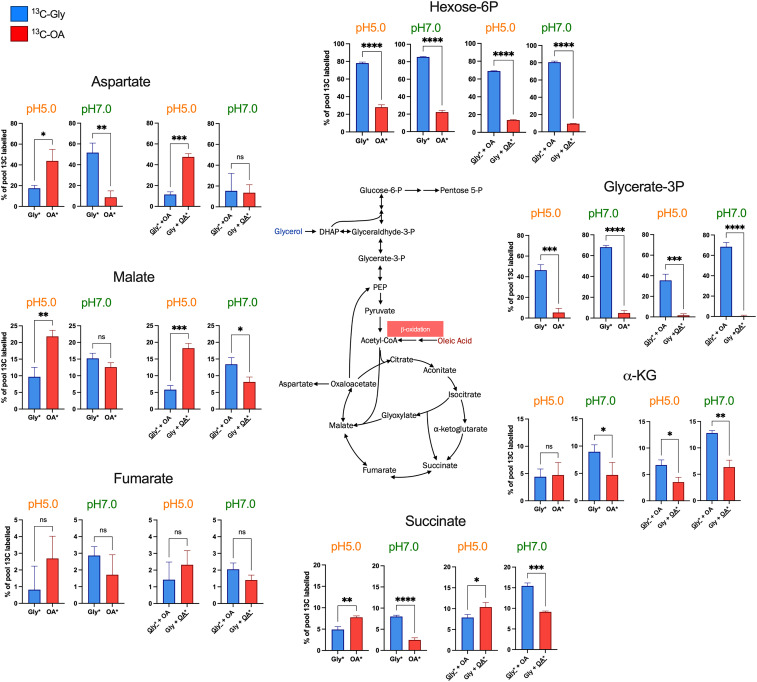
Metabolic Distribution of U-13C Isotopic-Labeled OA and Glycerol Reveals the Preferential Assimilation of OA at pH 5.0.
Unlike many other bacteria, which catabolize multiple carbon sources sequentially and exhibit diauxic growth, M. tuberculosis can cocatabolize multiple carbon sources simultaneously, each to its distinct metabolic fate (8). To investigate the impact of pH on the metabolic fate of glycerol (Gly) and OA, we performed carbon tracing experiments with uniformly (U) labeled 13C-glycerol and 13C-OA at pH 7.0 and pH 5.0 (Fig. 2). We measured the incorporation of Gly- and OA-derived carbon when provided as single carbon sources (13C-Gly, 13C-OA) or as mixtures (13C-Gly + OA and Gly + 13C-OA). These tracing experiments revealed that carbon derived from 13C-glycerol was predominantly routed to metabolites of the upper glycolysis pathway (hexose-6-phosphate and glycerate-3-phosphate) as expected and previously reported (Fig. 2) (8). The incorporation of 13C-glycerol–derived carbon into the TCA cycle intermediates succinate, fumarate, malate, and aspartate (the latter serves as proxy for the chemically labile oxaloacetate) was reduced compared to incorporation of carbon derived from 13C-OA, and this differential incorporation was reversed at pH 7.0, suggesting that assimilation of glycerol into the TCA cycle is impaired at acidic pH while OA assimilation is enhanced. The increased incorporation of OA-derived carbon occurred when 13C-OA was provided alone or in combination with unlabeled glycerol, suggesting that in acidified media OA remains a preferred carbon source even in the presence of glycerol. There was no increased incorporation of OA-derived carbon into α-ketoglutarate, indicating catabolism through the glyoxylate shunt which bypasses α-ketoglutarate. The products of the glyoxylate shunt are malate and succinate, with the intermediate glyoxylate, which is metabolized to malate by the action of malate synthase (25). We observed a substantial increase of completely 13C-OA–derived labeled succinate, fumarate, malate, and aspartate at pH 5.0 (SI Appendix, Fig. S2). The complete labeling of these metabolites requires multiple rounds of the TCA cycle, such that oxaloacetate that is 13C labeled from a previous round is used to produce citrate along with a 13C-labeled acetyl-CoA. Although during the 8-h labeling experiment 13C-glycerol–derived carbon predominantly labeled pools of hexose-6-phosphate, the 13C-OA–derived hexose-6-phosphate pool increased at pH 5.0, indicating enhanced gluconeogenic flux of 13C-OA–derived carbon (Fig. 2 and SI Appendix, Fig. S2). Considering that the TCA cycle supplies reducing equivalents for energy production and generates biosynthetic precursors, the impaired capacity of glycerol to fuel the TCA cycle likely explains why glycerol as sole carbon source failed to efficiently support growth at acidic pH. Altogether, these results reveal an altered metabolic flux toward enhanced OA-derived carbon assimilation in M. tuberculosis at acidic pH.
Fig. 2.
Metabolic distribution of U-13C isotopic-labeled OA and glycerol reveals an altered metabolic flux resulting in a preferential assimilation of OA at pH 5.0. The percentage of isotopically labeled metabolite pools were determined after 8-h incubation with 1.2 mM [U-13C]Gly, 200 μM [U-13C]OA, [U-13C]Gly + OA, and [U-13C]OA + Gly at pH 7.0 and pH 5.0. Each bar represents the percentage of the total pool that is 13C labeled. Blue bars indicate that the 13C labeling is derived from 13C-glycerol (Gly*), and red bars indicated that the labeling is derived from 13C- OA*. Data are the means ± SD of triplicates and are representative of two independent experiments. Statistical significance between the percentage of 13C-labeled pools at pH 7 and pH 5 for each metabolite was determined using unpaired t test. *P < 0.05; **P <0.005; ***P < 0.0005; ****P < 0.0001; ns = not significant. Gly = glycerol, OA = oleic acid, α-KG = α-ketoglutarate, C = carbon.
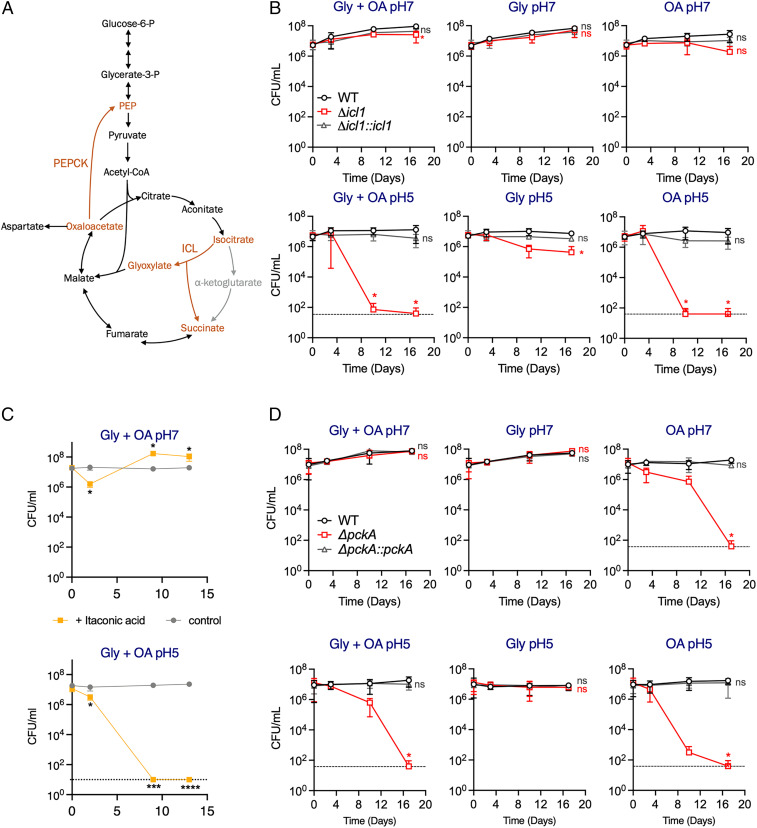
Growth and Survival of M. tuberculosis in Acidic Conditions Depend on the Glyoxylate Shunt and Gluconeogenesis.
To further investigate the importance of lipid utilization during growth at acidic pH, we determined the impact of lack of PEPCK and ICL, which are both required for lipid assimilation (Fig. 3A). Expression of both enzymes has been shown to significantly increase in response to acidification (26, 27) suggesting their probable role in the metabolic adaptation of M. tuberculosis to acidic conditions. Furthermore, both PEPCK and ICL are required for growth at pH 5.7 in pyruvate-supplemented media (18). We investigated both growth (SI Appendix, Fig. S3) and survival (Fig. 3 B and D) of M. tuberculosis lacking either PEPCK or ICL in acidic conditions in the presence of OA, or glycerol, or both. This demonstrated that M. tuberculosis lacking ICL activity (Δicl1) died rapidly at pH 5.0 when OA was provided either alone or in combination with glycerol (Fig. 3B). We observed similar kill kinetics when ICL activity was inhibited by itaconic acid in wild-type M. tuberculosis (Fig. 3C and SI Appendix, Fig. S4). M. tuberculosis lacking PEPCK activity (ΔpckA) similarly died in the presence of OA at pH 5.0 (Fig. 3D) but also at pH 7.0 when OA was the sole carbon source. Altogether, these data show that the glyoxylate shunt and gluconeogenesis are not only required for M. tuberculosis to grow with OA (SI Appendix, Fig. S3) but also to survive at acidic pH when OA is present (Fig. 3). The substantial death of M. tuberculosis lacking either ICL or PEPCK resembles the killing of both mutants during mouse infection (6, 7) and demonstrates that M. tuberculosis requires lipid assimilation to support growth at acidic pH.
Fig. 3.
Central carbon metabolic flux alterations at pH 5 depend on the glyoxylate shunt and gluconeogenesis. (A) Schematic of the reactions catalyzed by isocitrate lyase; ICL (glyoxylate shunt) and PEP carboxykinase; PEPCK (gluconeogenesis). Reactions are highlighted in orange. (B–D) CFU quantification of (B) ICL-deficient strain (H37Rv Δicl1), (C) wild-type strain treated with 4 mM itaconic acid, and (D) PEPCK-deficient (H37Rv ΔpckA) strain during incubation in neutral (pH 7.0) and acidic (pH 5.0) media supplemented with the indicated carbon sources. Dashed line indicates the limit of detection. Base medium was FAF 7H9 with no added carbon sources. OA (200 μM) was supplemented repeatedly, glycerol (0.2%) was added at day 0. The data in B and D are the means ± SD of two independent experiments. The data in C are means ± SD of triplicate and are representative of two independent experiments. Statistical significance at specific timepoints in B and D were determined using one-way ANOVA with Dunnett’s multiple comparisons test. *P < 0.05; ***P < 0.0005, ****P < 0.0001; ns = not significant. Gly = glycerol, OA = oleic acid.
GAPDH Activity Is Impaired in M. tuberculosis Cultured in Acidic Conditions.
Next, we sought to investigate why the assimilation of glycerol-derived carbon into the TCA cycle was reduced at acidic pH and examined more closely the metabolites of the lower glycolysis branch. The triose-phosphate (triose-P) pool, consisting of dihydroxyacetone phosphate (DHAP) and glyceraldehyde-3-phosphate (G3P), was significantly increased at acidic pH (Fig. 4A) consistent with reduced activity of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), which converts G3P into 1,3 bisphosphoglycerate (1,3-PG). Indeed, GAPDH activity was substantially reduced in lysates from M. tuberculosis cultured at acidic pH (Fig. 4 B and C). Compared to bacteria cultured in glycerol at pH 7.0, GAPDH activity was reduced by 72.8% in glycerol at pH 5.0 (Fig. 4C). In contrast, the activity of triose phosphate isomerase (TPI), whose inhibition could also have led to a similar metabolomic profile (28), remained unaffected by acidic pH (SI Appendix, Fig. S5). While the total pool sizes of phosphoenolpyruvate (PEP), a metabolite downstream of GAPDH, did not change (SI Appendix, Fig. S6), there was a reduction in glycerol-derived 13C in PEP pools at pH 5.0 (Fig. 4A). It is plausible that impaired GAPDH activity is responsible for the reduced flux of 13C-glycerol–derived carbon into the TCA cycle at acidic pH (Fig. 2). While GAPDH activity is necessary for glycolysis, it is also important for gluconeogenesis by catalyzing the reverse reaction necessary for the synthesis of cell wall precursors and nucleotides. Yet, the reduced GAPDH activity observed at acidic pH appears to be sufficient to support growth of M. tuberculosis with OA (Fig. 1).
Fig. 4.
Impaired GAPDH activity at acidic pH. (A) Pools sizes and 13C labeling of triose-3P (glyceraldehyde-3-phosphate [G3P] and dihydroxyacetone phosphate [DHAP]) and phosphoenolpyruvate (PEP) were determined in M. tuberculosis lysates after 8-h incubation with 1.2 mM [U-13C]Gly, 200 μM [U-13C]OA, [U-13C]Gly + OA, and [U-13C]OA + Gly at pH 7.0 or pH 5.0. Gray bars represent unlabeled fraction of the pools, and colored bars (red: OA, blue: Gly) represent the 13C- labeled fractions of the pools. (B) Measurement of NADH formation over time due to GAPDH enzyme activity in whole-cell lysates from M. tuberculosis cultured at pH 7.0 or pH 5.0 in the presence of 0.2% glycerol (Gly) or 200 μM OA. (C) Measurement of GAPDH activity at time T = 45 min from data shown in B (left panel) and percentage of GAPDH activity relative to Gly pH 7.0 (right panel). (D) Measurement of GAPDH activity at time T = 45 min in whole-cell lysates from M. tuberculosis cultured at pH 7.0 or pH 5.0 in the presence of 0.2% glycerol or 200 μM OA. When indicated, lysates were incubated 30 min at room temperature in the presence of DTT (1 mM) prior to measurements. (E) Measurements of intracellular ROS in M. tuberculosis in media at pH 5.0 or pH 7.0 in the presence of 0.2% glycerol or 200 μM OA or with no carbon source added. ROS were measured 2 h after the addition of 5 μM Cellrox Green reagent and were normalized to OD. Data in A are means ± SD of triplicates and are representative of two independent experiments. Data in B and C are means ± SD of triplicates and are representative of three independent experiments. Data in D are means ± SD of three independent experiments. Statistical significance of data (A–D) was determined using unpaired two-tailed t test. Data in E are means ± SD of duplicates and are representative of at least two independent experiments. Statistical significance in C (% GAPDH activity) and E were assessed by one-way ANOVA using the Dunnett’s multiple comparisons test. *P < 0.05; **P < 0.005; ***P < 0.0005; ****P < 0.0001; ns = not significant. Gly = glycerol; OA = oleic acid; CTRL = Control; DTT = dithiothreitol.
GAPDH is susceptible to inhibition by oxidation of its conserved catalytic cysteine residue (29, 30). Consistent with this mechanism of inhibition, addition of the reducing agent dithiothreitol (DTT) to cell lysates from M. tuberculosis cultured at pH 5.0 rescued GAPDH activity (Fig. 4D). Reactive oxygen species (ROS) production in M. tuberculosis in response to pH 5.7 has been reported (31). We thus hypothesized that reduced GAPDH activity might be caused by ROS. Indeed, we detected intracellular ROS in a pH-dependent manner (SI Appendix, Fig. S7). The amount of ROS detected in M. tuberculosis at acidic pH varied with the carbon source; growth in OA, palmitic acid, and cholesterol resulted in the least amount of ROS (Fig. 4E and SI Appendix, Fig. S8). Overall, these data suggest that ROS generation in M. tuberculosis in response to acidic pH may result in GAPDH oxidation and a corresponding decrease in its activity.
Discussion
M. tuberculosis encounters acidic conditions inside the macrophage phagosomal compartment as well as within the granuloma (12, 15). Many genes that are known to be acid induced are also induced during macrophage and/or mouse infections, including genes that are important for virulence such as icl1 and pckA (27). Thus, acidic pH is an important environmental cue that M. tuberculosis senses and responds to in order to adapt to its host niche during infection. M. tuberculosis is capable to replicate and persist within hosts, likely in acidic compartments, and can maintain its intracellular pH even when incubated at pH 4.5 (16). It was therefore surprising to us that M. tuberculosis fails to replicate in mildly acidic conditions in vitro. Other bacteria that also encounter and persist in the face of acidic pH during infection such as Salmonella typhimurium, Shigella flexneri, Listeria monocytogenes, and Escherichia coli can replicate in vitro at much lower pH (pH 4.0 to 4.2) than reported for M. tuberculosis (32–35). This led us to investigate how M. tuberculosis might be able to replicate in acidic environments. Our work reveals that M. tuberculosis carbon metabolism is altered in response to acidic pH, which likely contributes to its dependence on host-derived lipids for growth. While the importance of lipid catabolism and resistance to acidic stress for virulence of M. tuberculosis have been shown, this work reveals that these two host-relevant processes are intrinsically linked. Previous work demonstrated that pyruvate, acetate, phosphoenolpyruvate, oxaloacetate, and cholesterol support growth of M. tuberculosis at pH 5.7 with pyruvate being most effective (19). Cholesterol was used at 50 µM and not replenished, which likely explains why it did not promote strong growth. Although pyruvate was the preferred carbon source at pH 5.7, we found that it did not support growth of M. tuberculosis at pH 5.0 (SI Appendix, Fig. S1F) suggesting that lipids are favored over pyruvate at lower pH. Deletion of pckA or icl1/2 severely impaired growth with pyruvate at acidic and neutral pH consistent with their importance for gluconeogenesis (18). Nevertheless, the observed enhanced growth of ICL-deficient M. tuberculosis Erdman in glycerol at pH 5.7 is in contrast with our data and could be due to the different background strain (we used H37Rv). Notwithstanding, our findings are consistent with the reported metabolic profiling of wild-type M. tuberculosis and the pckA and icl mutants (18), which suggested an increased carbon flux through the glyoxylate shunt and decreased flux through the oxidative TCA cycle in response to acidic pH.
The pH-driven metabolic adaptations of M. tuberculosis may help explain its dependence on lipid catabolism during infection and simultaneously highlight the role of host lipids in promoting resistance to immune-driven antimicrobials such as acidic pH. We found that M. tuberculosis can replicate in acidic conditions mimicking those in phagolysosomes. In accordance, M. tuberculosis and Mycobacterium marinum have been shown to slowly replicate within acidic compartments in vivo (12, 36). To the best of our knowledge, a pH-dependent utilization of carbon sources has not been reported in other bacteria. It is however plausible that other pathogenic mycobacteria such M. marinum utilize a similar strategy and rely on lipids to grow in acidic compartments during infection. Furthermore, a similar adaptation strategy has been observed in tumor cells after long-term exposure to an acidic environment (acidosis) (37). In acid-exposed tumor cells, metabolism shifts from glycolysis to β-oxidation of fatty acids, which permits continued growth of these cells without further acidification of their niche. Despite mechanistic differences, acid-driven metabolic adaptation toward fatty acid catabolism appears to be a process that has evolved in independent biological systems.
We show that the decrease in GAPDH activity is likely contributing to the impairment of glycolytic flux of glycerol-derived carbon at low pH, but we cannot rule out that the activity of other enzymes might also be affected. The limited time frame (8 h) of our labeling experiments might not have allowed us to observe late events. For example, it has been reported that G3P can inhibit the activity of pyruvate kinase, the last enzyme of glycolysis (38). Accumulation of G3P due to GAPDH inhibition could also affect glycolysis and lead to the accumulation of other potentially growth inhibitory metabolites. Reduced GAPDH activity alone cannot explain the growth arrest at acidic pH with glycerol as a carbon source, because pyruvate, which does not depend on GAPDH activity for assimilation into the TCA cycle, failed to support growth at pH 5.0. Lipids are likely providing other benefits in acidic conditions in addition to supplying carbon. The reduced intracellular ROS levels at pH 5.0 when either OA, palmitic acid, and cholesterol are present suggest that these lipids have additional effects on M. tuberculosis physiology. Beta-oxidation may contribute to growth at acidic pH, for example, by providing additional reduced coenzymes. Finally, we cannot exclude the possibility that the import of carbon sources is altered at acidic pH and contributes to the preferential utilization of lipids for growth. Indeed, glycerol uptake by M. tuberculosis has been reported to be reduced at pH 5.7 compared to pH 7.0 (18).
The sensitivity of GAPDH to oxidation is well-documented in eukaryotes (30). The conserved cysteine residue within the active site of the enzyme is highly sensitive to ROS and reactive nitrogen species. The impact of oxidation on GAPDH activity in bacteria is less well defined, but GAPDH of Corynebacterium diphtheriae has been shown to be inactivated by oxidation (39). Here, we have shown that the impaired GAPDH activity in M. tuberculosis cultured at pH 5.0 correlates with increased levels of intracellular ROS and can be rescued by the reductive agent DTT. The generation of ROS in response to low pH is consistent with the observation that phagosomal acidification alters the redox physiology of intracellular M. tuberculosis (40). ROS are generated as a by-product of aerobic respiration, and their increased level at low pH suggests that the excess of protons might alter the function of M. tuberculosis respiratory chain. Alternatively, the increase of ROS at low pH could also result from a reduced potency of ROS detoxifying systems. How the nature of the carbon source can affect ROS levels and in particular how OA, palmitic acid, and cholesterol catabolism resulted in reduced ROS generation at low pH remains to be investigated. ROS production does not appear to be a specific response of M. tuberculosis to acidic pH as mycobacteria also generate ROS during nutrient deprivation (41).
Lipid utilization by M. tuberculosis is not only an attractive pathway for tuberculosis drug development but it also affects drug efficacy and mycobacterial drug tolerance (42, 43). Our and previous observations stress the importance of the culture medium composition, as it can have a significant impact on the physiology of M. tuberculosis and other bacteria and in some cases, may not be relevant to in vivo conditions (44, 45). This has led to the selection of carbon source-dependent inhibitors that selectively suppressed growth of M. tuberculosis in vitro but were ineffective against M. tuberculosis growing in macrophage or in mice. We propose that the fatty acid supplementation model developed in this study is a simple and effective tool to investigate M. tuberculosis in a metabolic state that more closely reflects that of bacteria during infection. Conducting screens for growth inhibitory compounds using a fatty acid carbon source at acidic pH could facilitate the identification of novel in vivo active anti-TB drugs.
Materials and Methods
Bacterial Strains and Culturing Conditions.
The wild-type M. tuberculosis strain used was H37Rv. The M. tuberculosis ΔpckA mutant was generated in H37Rv background using the same constructs and strategy as for the published Erdman mutant (7). The ΔpckA mutant was complemented by chromosomal (attL5) expression of pckA under the control of its native promoter. The Δicl1 mutant and parental H37Rv strain were gifted from Kevin Pethe (Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore) and Thomas Dick (Hackensack Meridian Health, NJ) (46). The Δicl1 mutant was complemented by chromosomal expression (attL5 site) of icl1 under the control of its native promoter (1-kb fragment upstream of ATG start codon). Mycobacteria were grown in liquid culture in Middlebrook 7H9 medium supplemented with 0.2% glycerol, 0.05% tyloxapol, and ADN (0.5 g/L bovine serum albumin [BSA], 0.2% dextrose, and 0.085% NaCl), and grown on solid media of 7H10 agar supplemented with 0.5% glycerol and 10% Middlebrook OADC enrichment (Becton Dickinson). For fatty acid free (FAF) media, FAF-BSA (fraction V Roche) was used to supplement the media. For media with no added carbon, glycerol and dextrose were not added, and FAF-BSA was used. For media supplemented with OA, a stock of 1% (40 mM) sodium oleate (1%[volume/volume] OA, 0.05N NaOH) was prepared and added to cultures at a final concentration of 200 μM when required. For regular supplementation, 200 μM OA was added to cultures every 2 to 3 d. A total of 100 mM stocks of palmitic acid, arachidonic acid, and cholesterol were prepared in tyloxapol:ethanol (1:1) warmed to 80 °C and added at the concentrations described to prewarmed media (20). pH adjustment of media was carried out by adding HCl or NaOH when appropriate. MES (2-(N-Morpholino) ethanesulfonic acid) hydrate (Sigma-Aldrich) was added to acidified media at 100 mM when specified. When specified, pyruvate was added at 10 mM final concentration. Antibiotics were added to cultures when required at the following concentrations: hygromycin 50 μg/mL, kanamycin 25 μg/mL.
Measurement of Extracellular pH.
To measure the pH of cultures (extracellular pH; pHEX), a pH indicator dye, chlorophenol red, was used. Samples of M. tuberculosis cultures (250 μL) were mixed with 0.004% chlorophenol red, and cells were pelleted by centrifugation. A total of 200 μL supernatant was transferred to wells in 96-well plate. The absorbance at 430 and 590 nm was measured, and the 430/590 nm ratio was calculated and plotted onto a calibration curve to determine the pH. For each batch of chlorophenol red, a calibration curve was generated using a set of media samples with adjusted pH values from 6.6 to 4.2.
Growth and Survival of M. tuberculosis.
Bacteria were grown to midlog phase in 7H9 media, washed twice in phosphate buffered saline with 0.05% tyloxapol (PBS-Tyl), and inoculated into 7H9 media (0.5 g/L FAF-BSA, 0.085% NaCl) at pH 7.0 or 5.0, with either glycerol (0.2%), OA (200 µM), or glycerol (0.2%) and OA (200 µM). When present, OA was added to the cultures every 2 to 3 d at a final concentration of 200 µM. The initial inoculum was 2.5 × 107 colony forming units (CFU)/mL (OD580nm: 0.05). Growth was monitored by measuring the optical density (OD580nm) over the time course of the experiment. When specified, 4 mM itaconic acid (Sigma-Aldrich, I29204) was added to the cultures. To determine the survival (CFU/mL) of the bacteria, serial dilutions of the cultures were made at indicated time points in PBS-Tyl and plated onto 7H10 solid agar plates containing glycerol and supplemented with OADC followed by incubation for 3 wk at 37 °C. For plating bacteria from cultures containing itaconic acid, 4 g/L activated charcoal (Sigma-Aldrich, C9157) was added to 7H10 agar.
Filter Culture and Metabolite Extraction.
M. tuberculosis was cultured to midlog phase and 5 × 108 CFU were seeded onto filters and placed onto 7H10 (0.2% glycerol, 0.5 g/L FAF-BSA, 0.085% NaCl, 200 μM OA) and incubated for 5 d to increase biomass. The M. tuberculosis–laden filters were transferred to “swimming pools” of 7H9 (1.2 mM glycerol, 0.5 g/L FAF-BSA, 0.085% NaCl, 200 μM OA, pH 7.0) and incubated for 24 h to allow adaptation to swimming pools before transferring to pools containing 7H9 (0.5 g/L FAF-BSA, 0.085% NaCl) with either glycerol (1.2 mM), OA (200 µM), or glycerol (1.2 mM) and OA (200 μM), at pH 7.0 or pH 5.0. After 24-h incubation, the filters were transferred to 7H9 (0.5 g/L FAF-BSA, 0.085% NaCl) with labeled carbon sources: 13C-glycerol (1.2 mM), 13C-OA (200 μM), or a combination of 13C-glycerol (1.2 mM) + 12C-OA (200 μM) and 13C-OA (200 μM) + 12C-glycerol (1.2 mM) at pH 7.0 and pH 5.0. After 4 h incubation with 13C-labeled carbon, M. tuberculosis–laden filters were transferred to prewarmed pools of new media to replenish the labeled carbon. After 8-h incubation with labeled media, the M. tuberculosis–laden filters were metabolically quenched by plunging filters into a mixture of acetonitrile/methanol/H2O (40:40:20) precooled to −40 °C, and metabolites were extracted by mechanical lysis with 0.1 mm zirconia beads in a Precellys tissue homogenizer for 3 min (6,500 rpm) three times under continuous cooling at or below 4 °C. Lysates were clarified by centrifugation and then filtered across a 0.22-μm filter.
Liquid Chromatography–Mass Spectrometry/Metabolomics.
For the detection of metabolites, an ion-pairing liquid chromatography–mass spectrometry (LC-MS) method was employed, using an Agilent 1290 Infinity LC system containing a Zorbax Rapid Resolution High Definition (RRHD) Extend C18 column (2.1 × 150 mm; Agilent) coupled to an Agilent Accurate Mass 6230 TOF. The mobile phase consisted of solvent A (5 mM tributylamine [TBA], 5.5 mM acetic acid in 97% ddH2O 3% methanol) and solvent B (5 mM TBA, 5.5 mM acetic acid in methanol) at a flow rate of 0.25 mL/min (0 to 3.5 min, 0% B; 4 to 7.5 min, 30% B; 8 to 15 min, 35% B; 20 to 24 min, 99% B; and 24.5 to 25 min, 0% B; followed by a 5 min equilibration period at 0% solvent B). Electrospray Ion Source (ESI) capillary and fragmentor voltages were set at 4,000 V and 125 V, respectively. The nebulizer pressure was set to 45 psig, and nitrogen drying gas was set to a flow rate of 8 L/min. The drying gas temperature was maintained at 325 °C. The MS acquisition rate was 1.5 spectra/sec, and m/z data ranging from 50 to 1,100 was stored. Dynamic mass axis calibration was accomplished by continuous infusion of a reference mass solution. Data were analyzed using Profinder B.08.00 software, and ions were assigned as specific metabolites based on mass accuracy within 5 parts per million and retention times within 1 min of those determined for chemical standards.
For isotopologue analysis, the percentage of metabolite isotopic labeling was determined by dividing the peak area ion intensities of each labeled isotopologue species by the summed ion intensity of all labeled and unlabeled species. Label-specific ion counts were corrected for naturally occurring 13C species. The metabolites G3P and DHAP (triose-P) cannot be differentiated by the extraction and detection techniques used in this study.
Measurements of GAPDH Activity.
Wild-type M. tuberculosis was grown in 40 mL 7H9 (0.2% glycerol, 0.5 g/L FAF-BSA, 0.085% NaCl) at pH 7.0 or pH 5.0 with glycerol (0.2%) or OA (200 μM) as main carbon source. Starting OD were adjusted depending on the growing condition to obtain OD of 0.5 for all conditions after 3 d of incubation at 37 °C with 5% CO2 (Gly pH7 OD = 0.1; OA pH7 OD = 0.2; Gly pH5 OD = 0.5; OA pH5 OD = 0.4). OA was replenished at day 2. At day 3, ODs were measured, and bacterial volumes collected adjusted to pellet a similar biomass. GAPDH measurements were done using a GAPDH activity assay kit (Sigma-Aldrich, MAK277-1KT). Bacteria were centrifuged and the pellets resuspended in GAPDH activity assay buffer before mechanical lysis with 0.1 mm zirconia beads in a Precellys tissue homogenizer for 20 s (5,000 rpm) twice under continuous cooling. In total, 50 μL lysates were tested in each condition. Lysate protein content were determined using Qubit protein assay kit (Thermofisher scientific), and results were normalized per mg of proteins (NADH/protein [nM/mg]). GAPDH activity (milliunits/mg protein) was determined using the calculation from the GAPDH activity assay kit (GAPDH activity (milliunit/mL) = NADH (nmol)/(Time (min) × Volume lysate (mL)) and divided by the quantity of proteins from each lysate (mg). To test the effect of DTT, 90 μL lysates were incubated with DTT (1 mM) for 30 min at room temperature (RT). Then, 75 μL lysates were filtered using Micro Bio-Spin 6 Columns (BIORAD) to remove traces of DTT before processing to the measurements of GAPDH activity.
Measurements of ROS.
Wild-type M. tuberculosis were grown to midlog phase in 7H9 (0.2% glycerol, 0.5 g/L FAF-BSA, 0.085% NaCl), were washed twice with PBS-Tyl, and resuspended in 4 mL tested media to an OD of 0.2 and incubated for 3 d at 37 °C with 5% CO2. Tested media were previously buffered to the tested pH and contained either no carbon source added, 0.2% glycerol, 200 μM OA, a combination of 0.2% glycerol and 200 μM OA, 0.2% glucose, 100 μM palmitic acid, 100 μM arachidonic acid, or 100 µM cholesterol. After 2 d of incubation, OA, palmitic acid, arachidonic acid, and cholesterol were supplemented to the media. At day 3, bacteria were spun down and resuspended in 1 mL of the same medium before OD measurement. OD were adjusted to OD = 0.5 with the media, and 200 μL bacteria were added to a 96-well plate (black plate/clear bottom). OD 600 nm was recorded for future normalization. To measure ROS generation, CellROX Green Reagent (Thermofisher scientific) was added to the wells at a final concentration of 5 μM. Each condition was recorded in duplicate wells, and a mean was calculated. Recording of fluorescence was done at 37 °C for 2 h using a plate reader (excitation 485 nm/emission 520 nm). Fluorescence measurements were normalized to OD (Fluo/OD) and expressed as arbitrary units (A.U.) excitation 485 nm/ emission 520 nm.
Statistical Analysis.
All experiments were performed as biologically independent experiments with technical replicates, and at least two biologically independent experiments were performed. Graphpad Prism 8 software (GraphPad Software Inc.) was used to perform statistical tests and generate graphs. Statistical significance between variables at pH 7 and pH 5 were determined using unpaired two-tailed Student’s t test. Statistical significance between ROS levels in bacteria cultured at pH 7 and pH 5 using different carbon sources was determined by one-way ANOVA using Tukey’s multiple comparison test.
Supplementary Material
Acknowledgments
We thank Dirk Schnappinger, Carl Nathan, and Tiago Beites for helpful discussions. We thank Kevin Pethe and Thomas Dick for providing the H37Rv Δicl1 mutant and parental wild-type strain. This work was supported by the Tri-Institutional TB Research Unit (NIH Grant No. U19AI111143) and NIH Grant No. P01AI143575.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2024571118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.World Health Organization , Global Tuberculosis Report 2020 (World Health Organization Geneva, Switzerland, 2020). [Google Scholar]
- 2.Kim M. J., et al., Caseation of human tuberculosis granulomas correlates with elevated host lipid metabolism. EMBO Mol. Med. 2, 258–274 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guerrini V., et al., Storage lipid studies in tuberculosis reveal that foam cell biogenesis is disease-specific. PLoS Pathog. 14, e1007223 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cole S. T., et al., Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393, 537–544 (1998). [DOI] [PubMed] [Google Scholar]
- 5.McKinney J. D., et al., Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 406, 735–738 (2000). [DOI] [PubMed] [Google Scholar]
- 6.Muñoz-Elías E. J., McKinney J. D., Mycobacterium tuberculosis isocitrate lyases 1 and 2 are jointly required for in vivo growth and virulence. Nat. Med. 11, 638–644 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marrero J., Rhee K. Y., Schnappinger D., Pethe K., Ehrt S., Gluconeogenic carbon flow of tricarboxylic acid cycle intermediates is critical for Mycobacterium tuberculosis to establish and maintain infection. Proc. Natl. Acad. Sci. U.S.A. 107, 9819–9824 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Carvalho L. P. S., et al., Metabolomics of Mycobacterium tuberculosis reveals compartmentalized co-catabolism of carbon substrates. Chem. Biol. 17, 1122–1131 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Bloch H., Segal W., Biochemical differentiation of Mycobacterium tuberculosis grown in vivo and in vitro. J. Bacteriol. 72, 132–141 (1956). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu K., Yu J., Russell D. G., pckA-deficient Mycobacterium bovis BCG shows attenuated virulence in mice and in macrophages. Microbiology (Reading) 149, 1829–1835 (2003). [DOI] [PubMed] [Google Scholar]
- 11.Pandey A. K., Sassetti C. M., Mycobacterial persistence requires the utilization of host cholesterol. Proc. Natl. Acad. Sci. U.S.A. 105, 4376–4380 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levitte S., et al., Mycobacterial acid tolerance enables phagolysosomal survival and establishment of tuberculous infection in vivo. Cell Host Microbe 20, 250–258 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaible U. E., Sturgill-Koszycki S., Schlesinger P. H., Russell D. G., Cytokine activation leads to acidification and increases maturation of Mycobacterium avium-containing phagosomes in murine macrophages. J. Immunol. 160, 1290–1296 (1998). [PubMed] [Google Scholar]
- 14.MacMicking J. D., Taylor G. A., McKinney J. D., Immune control of tuberculosis by IFN-γ-inducible LRG-47. Science 302, 654–659 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Kempker R. R., et al., Lung tissue concentrations of pyrazinamide among patients with drug-resistant pulmonary tuberculosis. Antimicrob. Agents Chemother. 61, e00226-e17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vandal O. H., Pierini L. M., Schnappinger D., Nathan C. F., Ehrt S., A membrane protein preserves intrabacterial pH in intraphagosomal Mycobacterium tuberculosis. Nat. Med. 14, 849–854 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Botella H., et al., Mycobacterium tuberculosis protease MarP activates a peptidoglycan hydrolase during acid stress. EMBO J. 36, 536–548 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker J. J., Abramovitch R. B., Genetic and metabolic regulation of Mycobacterium tuberculosis acid growth arrest. Sci. Rep. 8, 4168 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker J. J., Johnson B. K., Abramovitch R. B., Slow growth of Mycobacterium tuberculosis at acidic pH is regulated by phoPR and host-associated carbon sources. Mol. Microbiol. 94, 56–69 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nazarova E. V., et al., The genetic requirements of fatty acid import by Mycobacterium tuberculosis within macrophages. eLife 8, e43621 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kondo E., Kanai K., The lethal effect of long-chain fatty acids on mycobacteria. Jpn. J. Med. Sci. Biol. 25, 1–13 (1972). [DOI] [PubMed] [Google Scholar]
- 22.Lee W., VanderVen B. C., Fahey R. J., Russell D. G., Intracellular Mycobacterium tuberculosis exploits host-derived fatty acids to limit metabolic stress. J. Biol. Chem. 288, 6788–6800 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akaki T., Tomioka H., Shimizu T., Dekio S., Sato K., Comparative roles of free fatty acids with reactive nitrogen intermediates and reactive oxygen intermediates in expression of the anti-microbial activity of macrophages against Mycobacterium tuberculosis. Clin. Exp. Immunol. 121, 302–310 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vandal O. H., Gelb M. H., Ehrt S., Nathan C. F., Cytosolic phospholipase A2 enzymes are not required by mouse bone marrow-derived macrophages for the control of Mycobacterium tuberculosis in vitro. Infect. Immun. 74, 1751–1756 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puckett S., et al., Glyoxylate detoxification is an essential function of malate synthase required for carbon assimilation in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U.S.A. 114, E2225–E2232 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fisher M. A., Plikaytis B. B., Shinnick T. M., Microarray analysis of the Mycobacterium tuberculosis transcriptional response to the acidic conditions found in phagosomes. J. Bacteriol. 184, 4025–4032 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rohde K. H., Abramovitch R. B., Russell D. G., Mycobacterium tuberculosis invasion of macrophages: Linking bacterial gene expression to environmental cues. Cell Host Microbe 2, 352–364 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Trujillo C., et al., Triosephosphate isomerase is dispensable in vitro yet essential for Mycobacterium tuberculosis to establish infection. MBio 5, e00085-e14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hildebrandt T., Knuesting J., Berndt C., Morgan B., Scheibe R., Cytosolic thiol switches regulating basic cellular functions: GAPDH as an information hub? Biol. Chem. 396, 523–537 (2015). [DOI] [PubMed] [Google Scholar]
- 30.Mullarky E., Cantley L. C., "Diverting Glycolysis to Combat Oxidative Stress" in Innovative Medicine: Basic Research and Development [Internet], Nakao K., Minato N., Uemoto S., Eds. (Springer, Tokyo, 2015). [PubMed] [Google Scholar]
- 31.Coulson G. B., et al., Targeting Mycobacterium tuberculosis sensitivity to thiol stress at acidic pH kills the bacterium and potentiates antibiotics. Cell Chem. Biol. 24, 993–1004.e4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bagamboula C. F., Uyttendaele M., Debevere J., Acid tolerance of Shigella sonnei and Shigella flexneri. J. Appl. Microbiol. 93, 479–486 (2002). [DOI] [PubMed] [Google Scholar]
- 33.Alvarez-Ordóñez A., Fernández A., Bernardo A., López M., Acid tolerance in Salmonella typhimurium induced by culturing in the presence of organic acids at different growth temperatures. Food Microbiol. 27, 44–49 (2010). [DOI] [PubMed] [Google Scholar]
- 34.Conner D. E., Scott V. N., Bernard D. T., Growth, inhibition, and survival of Listeria monocytogenes as affected by acidic conditions. J. Food Prot. 53, 652–655 (1990). [DOI] [PubMed] [Google Scholar]
- 35.Haberbeck L. U., et al., Variability in growth/no growth boundaries of 188 different Escherichia coli strains reveals that approximately 75% have a higher growth probability under low pH conditions than E. coli O157:H7 strain ATCC 43888. Food Microbiol. 45, 222–230 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Gomes M. S., et al., Survival of Mycobacterium avium and Mycobacterium tuberculosis in acidified vacuoles of murine macrophages. Infect. Immun. 67, 3199–3206 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corbet C., et al., Acidosis drives the reprogramming of fatty acid metabolism in cancer cells through changes in mitochondrial and histone acetylation. Cell Metab. 24, 311–323 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Snášel J., Pichová I., Allosteric regulation of pyruvate kinase from Mycobacterium tuberculosis by metabolites. Biochim. Biophys. Acta Proteins Proteom. 1867, 125–139 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Hillion M., et al., The glyceraldehyde-3-phosphate dehydrogenase GapDH of Corynebacterium diphtheriae is redox-controlled by protein S-mycothiolation under oxidative stress. Sci. Rep. 7, 5020 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mishra R., et al., Targeting redox heterogeneity to counteract drug tolerance in replicating Mycobacterium tuberculosis. Sci. Transl. Med. 11, eaaw6635 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McBee M. E., et al., Production of superoxide in bacteria is stress- and cell state-dependent: A gating-optimized flow cytometry method that minimizes ROS measurement artifacts with fluorescent dyes. Front. Microbiol. 8, 459 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greenwood D. J., et al., Subcellular antibiotic visualization reveals a dynamic drug reservoir in infected macrophages. Science 364, 1279–1282 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hicks N. D., et al., Clinically prevalent mutations in Mycobacterium tuberculosis alter propionate metabolism and mediate multidrug tolerance. Nat. Microbiol. 3, 1032–1042 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pethe K., et al., A chemical genetic screen in Mycobacterium tuberculosis identifies carbon-source-dependent growth inhibitors devoid of in vivo efficacy. Nat. Commun. 1, 57 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.VanderVen B. C., et al., Novel inhibitors of cholesterol degradation in Mycobacterium tuberculosis reveal how the bacterium’s metabolism is constrained by the intracellular environment. PLoS Pathog. 11, e1004679 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gengenbacher M., Rao S. P. S., Pethe K., Dick T., Nutrient-starved, non-replicating Mycobacterium tuberculosis requires respiration, ATP synthase and isocitrate lyase for maintenance of ATP homeostasis and viability. Microbiology (Reading) 156, 81–87 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.