Abstract
As we age, sleep patterns undergo significant modifications in micro and macrostructure, worsening cognition and quality of life. These are associated with remarkable brain changes, like deterioration in synaptic plasticity, gray and white matter, and significant modifications in hormone levels. Sleep alterations are also a core component of mild cognitive impairment (MCI) and Alzheimer’s Disease (AD). AD night time is characterized by a gradual decrease in slow-wave activity and a substantial reduction of REM sleep. Sleep abnormalities can accelerate AD pathophysiology, promoting the accumulation of amyloid-β (Aβ) and phosphorylated tau. Thus, interventions that target sleep problems in elderly people and MCI patients have been suggested as a possible strategy to prevent or decelerate conversion to dementia. Although cognitive-behavioral therapy and pharmacological medications are still first-line treatments, despite being scarcely effective, new interventions have been proposed, such as auditory or light stimulation and Noninvasive Brain Stimulation (NiBS). Leveraging sleep research with NiBS possibilities is a new promising idea that may help to find AD biomarkers, address sleep symptomatology, and tackle Aβ deposition. The present review outlines the current state of the art of the relationship between sleep modifications in healthy aging and the neurobiological mechanisms underlying these age-related changes. Furthermore, we provide a critical analysis showing how sleep abnormalities influence the prognosis of AD pathology by intensifying Aβ and tau protein accumulation. We discuss potential therapeutic strategies to target sleep disruptions and conclude that there is an urgent need for testing these new therapeutic sleep interventions.
1. INTRODUCTION
Growing older is associated with remarkable sleep disruption, characterized by reduced ability to transition from wakefulness to sleep and to stay asleep without waking up. In an extensive meta-analysis, Ohayon and colleagues (2004) highlighted some of the key aging-related sleep disturbances, such as delayed circadian rhythm, a lighter and more fragmented sleep pattern, lower δ activity and less time spent in the deeper stage. Moreover, sleep features like sleep spindles and K-complexes (K-c) also dramatically drop in frequency and amplitude (Ohayon et al., 2004). The authors suggest that many factors are involved in causing sleep changes with age. As an example, the aging-associated physiological and metabolic changes, as well as the increased susceptibility to environmental factors increase the risk to suffer from primary sleep disorders, such as Insomnia and Sleep Disordered Breathing (SDB; Ohayon et al., 2004).
Furthermore, disrupted sleep patterns are also a major risk factor for developing Mild Cognitive Impairment (MCI), which may convert to Alzheimer’s Disease (AD). Sleep disruptions caused by cortical and environment modifications are often reported years before the clinical onset of the disease in elderly individuals, therefore making them a potential biomarker of the risk in AD. Then, after the onset and during the progression of MCI/AD, the sleep abnormalities undergo an even more accelerated worsening. These indicators suggest a complex bidirectional influence, for which sleep disruption may both causally contribute to AD development, and be a consequence of the onset. The current concept is that sleep quality reduction results from amyloid-beta (Aβ) aggregation that also triggers hippocampal degeneration and, ultimately, memory impairment. The hypothesis is that a reduction of slow-wave activity (SWA) during non-rapid eye movement sleep (NREM) partly arises from amyloid pathology and contributes to cognitive decline in elderly individuals (Ju et al., 2017). Because the worsening of sleep quality is among the earliest observable symptoms of MCI and AD, current theories suggest examining sleep modifications in pursuit of a biomarker to identify the greater risk of developing dementia. Within this framework, a decreased duration of rapid eye movement (REM) and the general slowing down of electroencephalogram (EEG) activity in NREM sleep has been considered as a potential biomarker. It has been argued that the promotion of slow waves during the NREM stage in the elderly population may have a protective effect on AD risk by enhancing Aβ clearance (Xie et al., 2013). On this line, it has been shown that elderly people with narcolepsy have a lower burden in amyloid deposits (Gabelle et al., 2019).
The up-regulation of SWA may even restore damage to proteins caused by oxidative stress, as demonstrated in animal models (Everson, 2014). Further, the amplitude and duration of SWA during the NREM stage are important for the long-term consolidation of newly acquired memories. The current hypothesis is that sleep improvement would slow the decline in cognitive abilities in AD patients. Within this framework, restoring slow-wave sleep (SWS) quality and duration would be a fundamental step in addressing AD symptomatology. Recently, therapeutic attempts aiming at changing sleep oscillatory activity have been proposed. Light exposure in combination with melatonin administration may be a valid way to influence the sleep-wake cycle (Dowling et al., 2008). Auditory stimulation can enhance SWA and improve memory retention (Papalambros et al., 2017). Recent evidence suggests that also noninvasive Brain Stimulation (NiBS) techniques might be able to restore the sleep quality and preserve or enhance physiologically-declining cognitive functions (Jones et al., 2018; Ketz et al., 2018; Marshall et al., 2006; Westerberg et al., 2015).
The present paper intends to review recent evidence of sleep modifications in healthy elderly individuals and AD patients along with the associated brain changes (for a comprehensive scheme see Figure 1). We aim to foster an understanding of the tight bidirectional relationship between sleep quality, normal aging, and AD pathology. Further, we discuss potential therapeutic strategies targeting sleep disruptions using NiBS and sensory stimulation to restore sleep quality and thus possibly prevent cognitive decline in healthy aging and AD.
Figure 1. Sleep and Brain changes in elderly and AD patients during the Lifespan.
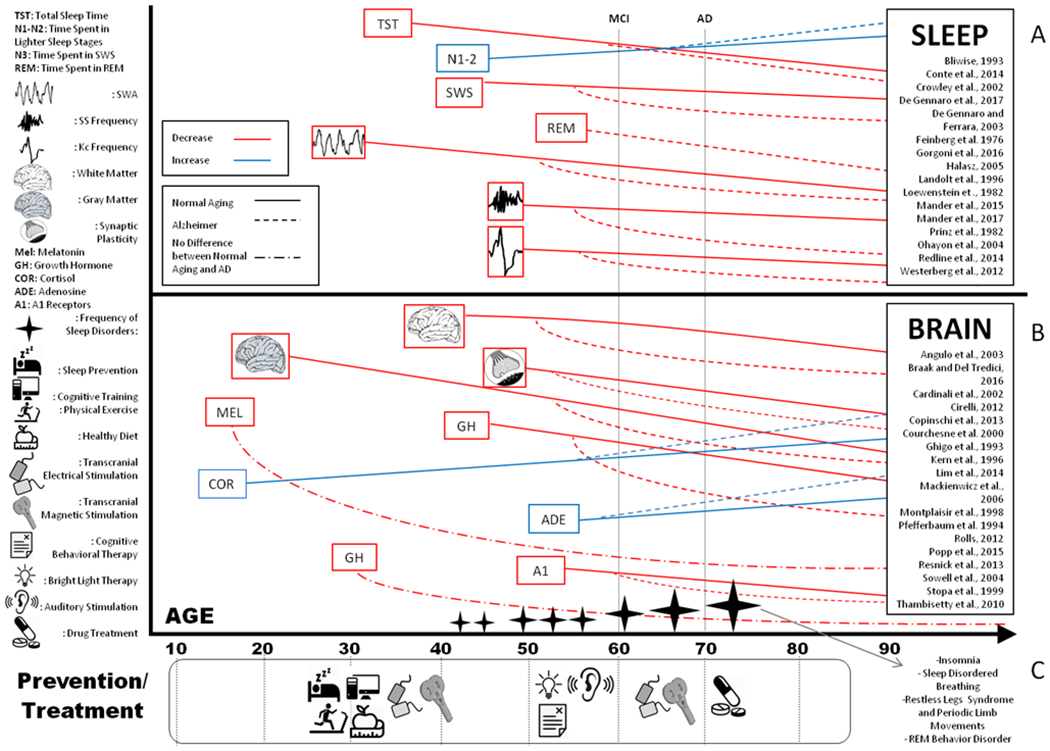
A comprehensive review of sleep modifications (panel A) and brain changes (panel B) in healthy aging individuals and AD patients. The items represent investigated and modified variables (red items show a decrease; blue items illustrate an increase). The mean age when MCI or AD is diagnosed is illustrated by vertical black dotted lines. The horizontal lines indicate the trajectories of sleep and brain changes. Sleep disruptions and cortical alterations start earlier than clinical symptoms’ appearance. Horizontal line slopes depict differences in sleep and cortical abnormalities between healthy elderly and MCI/AD. Black stars show the prevalence of sleep disorders while growing older. Panel C shows when prevention and potential treatment efficacy would be optimal. NiBS techniques (such as transcranial magnetic stimulation and transcranial electrical stimulation) are represented at different age for their applications in prevention and treatment.
2. SLEEP ALTERATIONS IN NORMAL AGING
In the next paragraph, we review age-associated sleep changes (Figure 2) in circadian rhythms, macrostructure (e.g. REM-NREM cycle), microstructure and electroencephalography (EEG) features (e.g. Slow Wave Activity), homeostatic sleep drive, and prevalence of sleep disorders. Albeit these modifications are so frequent to be an aging biomarker, individual variability, due to gender, ethnicity, race, and environment still plays a role in the age-associated sleep abnormalities. In the following chapter, we will then discuss how these changes might be related to aging-related processes in the healthy brain such as atrophy.
Figure 2. Sleep Differences in Elderly versus Young Individuals.
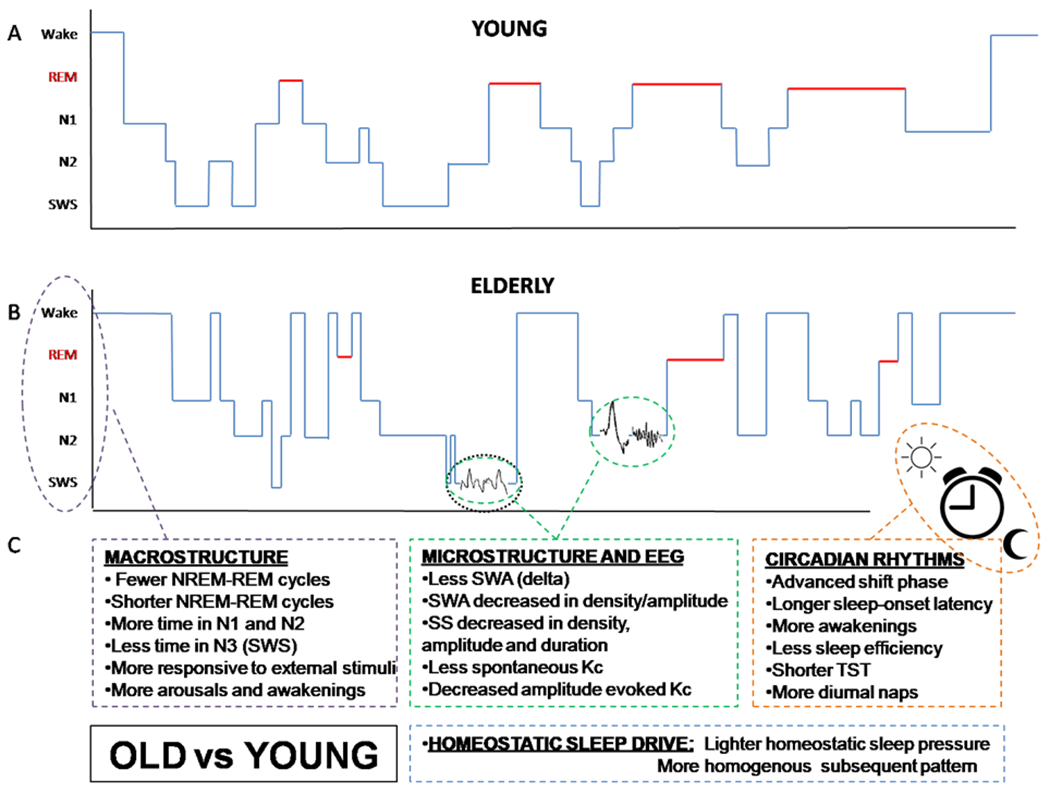
Polysomnographic (PSG) data showing sleep architecture in a young healthy adult (panel A) and an older healthy adult (panel B). The elderly show a more fragmented and less integrated sleep, shifting from deeper to lighter sleep stages multiple times during nighttime. Schematic illustration comprehensive of all sleep differences found in healthy elderly compared to young individuals (panel C). Non-rapid eye movement sleep (NREM), rapid eye movement sleep (REM), slow-wave sleep (SWS), slow waves (SW), slow-wave activity (SWA), sleep spindles (SS), K-complexes (K-c).
2.1. Circadian Rhythms
Huang and colleagues (2002) found that circadian sleep/wake rhythms (CR) were weakened and fragmented in the old and oldest groups as compared to the young and middle-aged groups (Huang et al., 2002). Macrostructure and total sleep time (TST) were also significantly altered with age. Individuals shift from a mean of 7.4h in early life to 5.6h in old age (Crowley, 2002), with some gender differences (men: 5.4h, women: 6h on average per night (Redline et al., 2004). Münch (2005) reviewed the evidence for the effects of aging on CR and found age-related modifications in terms of amplitude, earlier phase, shortened repetition time, and worsened capacity to tolerate sudden phase shifts.
CR changes start to be visible in middle-age subjects with increased sleepiness during the evening and delayed sleep-onset latency (time required to fall asleep) (Crowley, 2002). As a consequence, regular diurnal napping increases, growing 25% after the 75th year and eventually resulting in a worsened cycle shift (Foley et al., 2007). With aging, difficulties in falling asleep are present not only at the beginning but also after nighttime arousals (shifts from deeper to lighter stages). Elderly people usually spend twice as much overnight time in unwanted wakefulness (Bliwise, 1993) and this drastically drops sleep efficiency (% time in bed spent asleep). Elderly individuals are also more sensitive to the external environment, showing a lower arousal threshold to auditory stimuli (Zepelin et al., 1984). Once sleep is interrupted (spontaneously or by external causes) transition from sleep to a fully awake state occurs also more rapidly in older subjects, thereby delaying the start of sleep again (Dement et al., 1985). Further, the frequency of arousals is also remarkably higher in elderly people, with different prevalence between the various sleep stages; older subjects present significantly more shifts from SWS to N2 and from N2 to N1, while in younger subjects the typical pattern is from REM to N1 (Conte et al., 2014).
2.2. Macrostructure
Aging also influences the structure of REM-NREM cycles (Figure 2, panel B); they are shorter and fewer, with a mean of 3.46 cycles per night compared to the usual 4-5 in adults and teenagers (Conte et al., 2014). Noticeably, NREM changes are considered a reliable age-related biomarker. Elderly people spend less time in a deeper stage, N3, replaced by a greater amount in lighter phases, N1 and N2 (Bliwise, 1993; Cauter et al., 2000; Feinberg et al., 1967; Landolt et al., 1996; Ohayon et al., 2004; Redline et al., 2004). SWS (or N3) reduction begins in middle age, gradually decreasing until disappearing after the 90th year (Bliwise, 1993; Ohayon et al., 2004). The increase of time in lighter phases is greater in stage N1 than N2, but N2 microstructure undergoes more changes, with a decrement of sleep spindles and K-complexes (see below; Conte et al., 2014). Older women show a less marked difference in lighter and deeper stages, with a smaller decrement in SWS than men (Bliwise, 1993; Ohayon et al., 2004).
REM studies, on the other hand, are less developed and coherent. Although slight REM decrease has been highlighted (Ohayon et al., 2004; Redline et al., 2004), the drop is not as prominent as in NREM as seems to affect sleep macrostructure only after the 65th-70th year. Previous studies showed that, after age, 70 NREM TST differently decreases (−24 minutes per decade) as compared to REM (−10 minutes per decade; Cauter et al., 2000); while other studies do not find a difference in REM and NREM sleep (Feinberg et al., 1967; Landolt et al., 1996), leaving the discussion open.
2.3. Microstructure and EEG
Recent studies show how typical sleep oscillations are also dramatically modified on their fundamental components while growing older.
2.3.1. Slow Waves (SWA).
Evidence supports a reorganization in the N3 during aging. Slow Waves (δ waves) are oscillations with slow frequency (<2Hz) and high amplitude (>75μV) associated with a reduction of homeostatic sleep pressure (Dijk, 2009) and protective effects from awakenings and arousal (Tononi and Cirelli, 2006). Δ waves have also been suggested to be implicated in learning and memory processing (Carrier et al., 2011). A drop in total Slow Wave Activity (SWA; spectral power density from 0.5 to 4.5Hz; or δ activity), is a particularly relevant consequence of aging and is used to test sleep quality and homeostatic sleep pressure (Bliwise, 1993; Cauter et al., 2000; Feinberg et al., 1967; Landolt et al., 1996). The decrement of SWA is not spatially and temporally homogeneous across the night. There is a maximal decrease over the prefrontal cortex and in the first NREM cycle for elderly individuals (Carrier et al., 2011; Landolt et al., 1996). Both SWA amplitude and density are significantly reduced in middle-aged adults, worsening during advancing age (Carrier et al., 2011). Further, age-related decrease in power density in θ and α frequencies has been shown (Landolt et al., 1996). Another qualitative change in older individuals is a homogeneous slowing of SWA of about 0.1 Hz across all scalp sensors (Carrier et al., 2011).
2.3.2. Sleep Spindles (SS) and K-complexes (K-c).
While N2 total time is not different between young and elderly individuals, its EEG features undergo age-related modifications. Sleep Spindles (SS) expression deteriorates, indicating that sleep microstructure can change without a parallel modification in the macrostructure (Fogel et al., 2017). SS can be divided into slow (9-12Hz) and fast (13-15Hz), and these are differentially affected in aging (Clawson et al., 2016). While there is agreement about the age-related drop in fast spindles, changes in slower-frequency spindles are still not clear (Landolt et al., 1996). Total spindle frequency activity (sigma power) is significantly reduced in middle-aged and older adults compared to young participants (De Gennaro and Ferrara, 2003) with a bigger drop in the last part of the night (Landolt et al., 1996). Previous studies analyzed spindle characteristics, finding that spindles also undergo a progressive decrement in density, amplitude, and duration with aging (Carrier et al., 2011; Crowley, 2011, 2002; De Gennaro and Ferrara, 2003; Landolt et al., 1996; Martin et al., 2013; Wei et al., 1999). Spindle breakdown is most pronounced during final N2 cycles (De Gennaro and Ferrara, 2003). These results are indicative of a different propagation of spindles through the cortical network in the elderly compared to young individuals. Thus, SS alterations may be a sign of the difficulties experienced by elderly individuals in maintaining sleep during lighter NREM stages (Dang-Vu et al., 2011). The incidence of both, spontaneous and elicited K-c, decreases growing older (Halasz, 2005; Kubicki et al., 1989), with a significant drop in K-c amplitude evoked by auditory stimuli (Crowley, 2002).
2.3.3. Fast oscillations.
Faster frequencies, like β (from 15 to 25Hz) or γ range (from 30 to 120Hz), are a prominent feature of awake EEG and are typically associated with normal cognition. Although their presence in sleep has been studied, results are contradictory (Brown et al., 2012; Sprecher et al., 2016; Valderrama et al., 2012). Sleep deprivation-related EEG slowing over the frontal region during wakefulness also results in a concurrent reduction in β/γ rhythms (A. Brown et al., 2012; Li et al., 2008), thereby partially explaining the cognitive impairment associated to sleep deprivation. As aging is associated with a progressive reduction in sleep quantity and quality, several researchers investigated whether age-related cognitive decline might be related to β/γ reduction; while old studies surprisingly highlighted increased in β activity (Carrier et al., 2001; Larsen et al., 1995; Sunde et al., 1976), newer paradigms show no differences between age groups at 65 years (Sprecher et al., 2016b), leaving the question open.
Spontaneous γ rhythm mostly occurs coupled with θ rhythms during REM sleep (Canolty et al., 2006; Ferri et al., 2000), with a higher presence of low γ when eye movements are present (Gross, 1999). Importantly, γ seems to promote synaptic plasticity when coupled with θ, (Lisman and Buzsáki, 2008; Lisman and Jensen, 2013) specifically for phasic REM states, where the synchrony between θ and γ is enhanced; this suggests that tonic REM phases support offline mnemonic processing, while phasic bursts of activity may promote memory consolidation (Boyce et al., 2016; Montgomery and Woodall, 2008). As a recent study with cortical and intracortical EEG on epileptic patients under age 50 suggested a higher presence of γ activity during SWS than REM (Valderrama et al., 2012b), different roles of γ-band may be suggested in NREM and REM (memory consolidation vs. dreaming state). Sprecher and colleagues (2016) analyzed results gathered with high-density (256 channels) EEG in a cohort of 18-65 y/o participants and found greater high γ (define frequency range) power in the older group compared to the younger one. Unfortunately, this result was secondary to SWA and sigma (12-15 Hz) drop (Sprecher et al., 2016b). Moreover, because the experimental paradigm involved participants under age 65, new studies are required to find some clarity regarding high-frequency activity in older adults.
2.3.4. REM Sleep
Past studies found no changes (Ficca et al., 1999; Schwarz et al., 2017), or a decrease (Lauer et al., 1991) in REM-related eye movement patterns across the lifespan. Further, it has been shown a reduced duration of movement bursts in elderly individuals (Ficca et al., 1999) and a reduction in δ-θ range (0.25-7Hz) and low α (8.25-10Hz) during REM stages (Landolt et al., 1996).
2.4. Homeostatic Sleep Drive and Subjective Complaints
Homeostatic sleep drive is a time-awake dependent process so that the longer the time spent awake, the greater the drive to fall asleep. In healthy adult people, longer sleep deprivation and time spent in wakefulness leads to a more pressing homeostatic sleep drive, with a consequent increase in SWA and NREM during the following night (Dijk, 2009). The homeostatic pressure is reflected by SWA in the first NREM cycle during the initial part of sleep recovery and a progressive reduction of SWA during other cycles (Dijk, 2009; Landolt et al., 1996). Indeed, K-c and SWA reduction supports the idea that homeostatic sleep pressure decreases across adulthood (Schwarz et al., 2017). Current evidence supports the notion that, in elderly people, SWA is less influenced by longer-term wakefulness in comparison to younger control groups (Mander et al., 2017; Münch et al., 2005). These are indicative of a lighter homeostatic sleep drive in elderly individuals (Clawson et al., 2016).
Even though the consequences of sleep deprivation seem to be blunted in the elderly, the prevalence of subjective complaints increases significantly and steadily with advancing age (40-50% of the entire older population). The most common complaints involve overnight awake states, light sleep, less total time asleep, early waking up, and excessive daytime sleepiness (Foley et al., 1995; Vitiello, 2009). Strangely, even if sleep is qualitatively and quantitatively more disrupted in men, women complain more about subjective difficulties (Ohayon et al., 2004). Only half of healthy older adults complain of chronic sleep disruptions, albeit age-related changes occur in all “optimally aged” elderly. This means that even those who do not complain of sleep problems show poor sleep efficiency and quality (Vitiello et al., 2004).
2.5. Sleep Disorders
Elderly individuals are at increased risk of suffering from primary sleep disorders, such as insomnia and sleep-disordered breathing (SDB). Insomnia prevalence, in particular, reaches 40% in over 65 y/o individuals, with greater frequency in women (Foley et al., 1995). If untreated, insomnia aggravates depression and significantly worsen cognitive skills (Ohayon and Vecchierini, 2005). As aforementioned, adulthood arousal threshold progressively declines, resulting in more awakenings even in deeper sleep stages like N3 and REM, therefore worsening insomnia condition (Zepelin et al., 1984). SDB shows also a higher prevalence in elderly subjects. In a randomly selected cohort of 427 participants (age range 65-95) 62% of the cohort showed an SDB diagnosis (Ancoli-Israel et al., 1991).
3. AGING-RELATED NEUROBIOLOGICAL MODIFICATIONS
The aging process contributes to a great number of quantitative and qualitative modifications in the brain, starting from the reduction of synaptic density and plasticity, neuronal loss and cortical atrophy, accompanied by hormonal and extracellular changes. As a consequence of these modifications, sleep and cognition are highly disrupted. In this section, we address the main age-related brain modifications, linking them to specific age-related sleep disruptions and cognitive impairment.
3.1. Synaptic Plasticity
A reduction of cognitive activity in the elderly, and a subsequent modification in synaptic plasticity, is one of the first causative factors in SWA decline. SWA involves large populations of healthy neurons able to produce oscillations and also efficient synaptic connections among them (Esser et al., 2007). Current evidence shows that a rapid increase or decrease in synaptic strength during awake states give rise to a subsequent enhancement or decline in SWA. During sleep, slow oscillations renormalize neural activity in the N3 stage, promoting synaptic depression by low levels of norepinephrine, serotonin, and acetylcholine (for a review see Hanlon et al., 2011). As aforementioned, previous studies showed a positive correlation between SWA during sleep and the number of potentiated synapses during wakefulness. The authors argued that learning and motor tasks trigger greater SWA during sleep that might be explained by plasticity mechanisms. In line with this, an age-related drop in brain plasticity and a lack of cognitive activity would account for a reduction of SWA in the elderly.
3.2. Deterioration of Gray Matter and functional consequences on sleep-related EEG
The most characteristic biomarker of the aging brain is the so-called “shrinking brain phenomenon” (Clawson et al., 2016), a marked decrease in cortical volume (Courchesne et al., 2000; Pfefferbaum et al., 1994; Resnick et al., 2003; Thambisetty et al., 2010) that has been measured through different parameters, such as the total volume of the brain, cerebrospinal fluid (CSF), gray and white matter, cortical thickness, surface area, cell loss, and neuronal shrinkage (for a comprehensive review see Sowell et al., 2004).
The brain reaches its maximum volume in early adolescence, then it declines steadily and linearly from early through middle adulthood, with an accelerated rate after 55 years. Around age 71-80 the whole-brain volume has already dropped by 26% compared to the volume in children, while, concurrently, the total intracranial CSF volume increases (Courchesne et al., 2000). Volume decline does not occur to the same degree in all brain regions: while white matter decrease affects the whole brain, gray matter deterioration mostly affects the frontal and parietal lobes (Resnick et al., 2003; Thambisetty et al., 2010).
As SWA results from the synchronization of a large population of neurons, SWA modifications may be a result of shrinking in specific regions and not a consequence of a general whole brain atrophy (Mander et al., 2016). Several studies demonstrated that neuronal loss in the lateral and medial prefrontal cortex (PFC) predicted impairment in slow oscillations generation and propagation (Dube et al., 2015; Mander et al., 2013). It has been also shown that prefrontal regions in older individuals have lower resting metabolic activity compared to the brain of younger subjects (Kakimoto et al., 2016). These results are in line with the aforementioned maximal reduction of SW density and amplitude in frontal EEG derivations in the elderly (Carrier et al., 2011; Landolt et al., 1996). Moreover, it has been demonstrated that neuronal loss in the medial PFC and the middle frontal gyrus predicts a decrement in SWA amplitude, while density is correlated with deterioration of the areas surrounding the lateral fissure (Dube et al., 2015).
Neuronal loss might also account for the decrement in Sleep Spindles (SS) and K-complexes (K-c). Indeed, spontaneous K-c is considered an expression of slow oscillations at the level of the neural membrane potential. This link would explain the lower K-c frequency when δ oscillations are impaired (Crowley, 2011, 2002). Furthermore, neuronal loss in the hippocampus predicts the severity of reduction in SS density and duration in older individuals (Fogel et al., 2017). As regard to gender differences, a reduction of PFC volume is more pronounced in men than women (Mander et al., 2016; Redline et al., 2004). These results suggest a gender discrepancy, with reduced SWA and sleep efficiency in men compared to women (Crowley, 2002).
3.3. Hypothalamus and Brainstem Nuclei: The Sleep State Switching Process
The switch between sleep and wakefulness is regulated by a complex network within the brain stem and hypothalamic nuclei. This system is differentially affected by age on many levels (for a comprehensive review see Rolls, 2012) In particular, age-related changes in four areas are responsible for disruptions in circadian rhythms: the wake-promoting lateral hypothalamic area (LHA) and the locus coeruleus (LC) help to maintain stable periods of wakefulness, while the preoptic area (POA) modulates LHA and LC function, sending inhibitory input to initiate and maintain sleep (Saper et al., 2010). The hypothalamic suprachiasmatic nucleus (SCN) is the endogenous clock promoting wakefulness and regulating sleep (Mander et al., 2016; Wang et al., 2015). Age-related neuronal loss affects these nuclei, disrupting the balance in sleep and wakefulness. The age-related deterioration of hypothalamic and brainstem nuclei does not occur to the same extent in all nuclei involved in sleep and wake control. For example, serotoninergic neurons in the raphe nucleus undergo minimal age-related changes and do not predict sleep disruptions (Rolls, 2012).
3.3.1. Suprachiasmatic Nucleus (SCN).
The SCN coordinates hormonal and behavioral rhythms as a circadian pacemaker and is dramatically affected by the aging process. Modifications involve alterations in neuronal network functionality, membrane properties, and modifications of constituents in cellular nuclei (for a complete review see Farajnia et al., 2014). Post mortem examinations showed a decrement in SCN volume and cell number in the elderly (Swaab et al., 1985; Zhou, 1995). Specific neuronal subpopulations are particularly affected by this shrinking, including those expressing vasoactive intestinal peptide (VIP). These neurons receive direct light input from the retina and regulate synchronicity between endogenous rhythms and the external light-dark cycle (Farajnia et al., 2014). Loss of VIP-ergic neurons would lead to a lower influence from light to internal rhythms, loosing circadian control from SCN (Wang et al., 2015), therefore inducing modifications in phase shift, nighttime movements, and number of awakenings. Impairment in SCN function also reflects a desynchronized melatonin secretion cycle (see paragraph 2.6.1).
3.3.2. Hypothalamic Preoptic Area (POA).
The POA plays a major role in promoting, initiating and maintaining sleep. POA is formed by cells expressing inhibitory neuropeptide galanin (Saper et al., 2010) and undergoes a significant decline in aging. The degree of cell loss in the POA predicts the severity of sleep fragmentation in older adults (Lim et al., 2014). The POA shrinkage results in abnormalities in overnight sleep consolidation (Lim et al., 2014).
3.3.3. Lateral Hypothalamic Area (LHA) and Locus Coeruleus (LC).
The LHA contains neurons expressing hypocretin and orexin which connects to other nuclei of the brainstem ascending arousal system. LHA together with the LC collectively maintains stable wake states (Saper et al., 2010). Both undergo a reduction of their neuronal subpopulations during the aging process. Elderly individuals show a 10% loss in the number of LHA hypocretin-orexin neurons (Hunt et al., 2015) and a significant neuronal reduction in LC volume (Sasaki et al., 2006). Deterioration in these two structures and their interconnection with the POA is partially responsible for fragmentation and fragility of sleep, higher frequency arousal, and greater sleepiness during awake states in the elderly.
3.4. Deterioration of White Matter
Aging also causes white matter (WM) deterioration, although it generally is less marked compared to changes in grey matter. By age 70-80, the former is decreased by 13%, and the latter 26% compared to the average volume in children (Courchesne et al., 2000). WM does not reach its volume plateau until the 4th decade, and only after it starts declining in volume (Courchesne et al., 2000). This has been confirmed by studies using diffusion tensor imaging (DTI; Westlye et al., 2009). This deterioration can also be seen in investigations of the total length of myelinated fibers. Thinner fibers, like small collaterals, are more damaged than thicker, as main axons (Marner et al., 2003). Literature reported how the structural properties of white matter tracts affect the brain network’s ability in synchronizing themselves to produce SW (Buchmann et al., 2011). As an example, Piantoni and coworkers (2013) correlated a steeper slope of SW, suggestive with a higher axial diffusivity in major frontal regions, an indicator of better white matter integrity. SW disruptions typical of the elderly may, indeed, be partially caused by loss of white matter tracts able to maintain higher cerebral synchronicity during sleep. Furthermore, specific aging deterioration of the body and splenium of the corpus callosum predicts the severity of SS frequency decline in the elderly (Mander et al., 2016).
3.5. Adenosine
Adenosine is a metabolic byproduct that accumulates during wakefulness. It promotes homeostatic sleep drive, including SWA after sleep deprivation. Previous studies show a decrease in homeostatic drive and lower homeostatic EEG response in older subjects, suggesting lower levels of adenosine in elderly subjects. On the contrary, animal models found higher extracellular adenosine levels in older mice, with a focus on brainstem sleep and wake-promoting nuclei (Mackiewicz et al., 2006). This contradiction could be explained by reduced A1 receptor gene expression and widespread loss of adenosine A1 receptors in cortical, thalamic, and hippocampal areas occurring in advanced age (Cheng et al., 2000; Porkka-Heiskanen, 1997). This would then result in higher extracellular concentration of adenosine, but fewer receptors able to interact with it, leading to lighter homeostatic sleep drive and SWA rebound for sleep deprivation.
3.6. Hormones
The sleep process across the life span is affected by age-related endocrine metabolic alterations. In the next section, we review the role of three hormones associated with promoting and maintaining sleep and we discuss how they are modified by the aging process.
3.6.1. Melatonin.
Melatonin plays an important role in sleep regulation controlled by the SCN via a complex pathway (for a comprehensive review see Benarroch, 2008). The endogenous melatonin peak is 2 hours before habitual bedtime. The aging process attenuates overnight melatonin secretion in older individuals, while diurnal secretion is similar in elderly and young subjects (Cooke and Ancoli-Israel, 2011; Copinschi and Caufriez, 2013; Münch et al., 2005). Its overnight decrement is linked to nocturnal light exposure in the elderly. Light exposure during the night results in a dose-dependent suppression of melatonin secretion (Benarroch, 2008). By contrast, insufficient daytime light exposure, often seen in the elderly due to spending most of the time home, elicits a desynchronization of the melatonin secretion cycle (Mishima et al., 2001), concurrent with the shift in the sleep-wake cycle.
3.6.2. Growth Hormone (GH).
GH functions include control of glucose concentration and cell reproduction (Copinschi and Cauter, 1995). Normally, GH secretion follows a tight circadian schedule closely linked to SWS (Copinschi and Caufriez, 2013). In aging, a significant reduction in the GH secretor peak predicts a shorter time spent in N3 (Kern et al., 1996).
3.6.3. Cortisol.
There are age-related alterations in cortisol levels with a rising trend which is probably linked to less sleep duration and more awakenings in older individuals (Kern et al., 1996). Moreover, variations in cortisol secretion timing have been demonstrated. Specifically, there is a delayed shift in cortisol secretion in the elderly compared to younger subjects. It has been suggested that these age-related alterations could have a strong relationship with REM sleep. In this vein, a previous study demonstrates that, with aging, plasma cortisol levels progressively increase while REM sleep decreases (Copinschi and Caufriez, 2013).
4. THE RELATIONSHIP BETWEEN SLEEP AND PROTEIN CLEARANCE
Several studies showed a strong relationship between sleep quality and Aβ alterations in MCI/AD and healthy elderly subjects (Mander et al., 2015; Spira et al., 2013; Sprecher et al., 2015). It has been demonstrated that sleep disruptions are, indeed, a crucial factor affecting the severity of cortical Aβ burden and the levels of phosphorylated tau in the Cerebrospinal Fluid (CSF; Fig 6). In this paragraph, we review the causal relationship between sleep abnormalities, like the aforementioned ones explained in the last paragraph, and Aβ plaques, neurofibrillary tangles and Apolipoprotein Epsilon (ApoE), the three main hallmarks of AD pathology.
4.1. Amyloid-β and Glymphatic System
In a murine animal model-based study, Iliff and coworkers (2012) discovered that Aβ and other metabolites are cleared during sleep through a perivascular pathway. They called this system the “lymphatic pathway”. Crucially, these results suggest that at least one physiological function of sleep is the clearance of toxic substances that build up during daytime activities. Spira and colleagues (Spira et al., 2013) recruited 70 older adults in a cross-sectional study design (mean age = 76; range 53 - 91). The authors assessed the Aβ burden using the Pittsburgh Compound B (PiB) positron emission tomography and found greater Aβ burden in the individuals who self-reported shorter sleep duration and lower sleep quality. Conversely, patients with narcolepsy have a lower burden of amyloid in the brain (Gabella et al. 2019).
To better understand the relationship between sleep, Aβ and tau protein, Ju and colleagues (2017) examined 17 healthy adults (age 35 to 65) who reported no sleep or cognitive issues. Actigraphy was recorded for at least 5 successive nights, after which participants spent one night in a climate-controlled sleep room. Half of the subjects were randomly assigned to have their SWS disrupted by a series of beeps (administered through headphones). This process was repeated approximately one month later, except that those who previously had their sleep disrupted were allowed to sleep through the night uninterrupted, and those who were previously allowed to sleep through the night had their sleep disrupted. Participants underwent a spinal tap after both nights to measure the levels of Aβ and tau protein in the CSF. The authors found an approximately 10% increase in Aβ following the disrupted night’s sleep. Participants also consistently reported feeling tired after having their SWS disrupted, despite they only rarely recalled being awakened by stimuli.
The increase in Aβ levels might be due to increased production of Aβ by neurons, or decreased clearance of Aβ by the lymphatic system (see Jessen et al., 2015; Louveau et al., 2015 for an overview of brain lymphatic). In a subsequent study, Lucey and coworkers (2017) administered sodium oxybate -which causes an increase in SWA- in one group, comparing it with another group of sleep-deprived subjects and normal sleeping control participants. They found that sleep deprivation increased Aβ levels through increased overnight Aβ production and argued that, despite lymphatic clearance might contribute to the protective effects of sleep against AD, changes in Aβ production rate indeed have a major role.
Pase and coworkers (Pase et al., 2017), followed a cohort of 321 (67 +/− 5 years old) for 19 years. They found a relation between REM disruptions and dementia in 32 cases, 24 of which were likely AD. Although the mechanisms behind the relationship between Aβ and REM are still unclear, disruptions of REM sleep might coincide with the onset of cognitive impairment. A previous study showed evidence of a relation between REM sleep disruption and Aβ levels in healthy elderly and AD patients (Liguori et al., 2014). These results may be mediated by the degeneration of cholinergic transmission within the brainstem and basal forebrain, which also plays a role in the regulation of REM sleep. Indeed, in both healthy older adults and MCI/AD patients, the degree of cortical Aβ burden was correlated with the degree of basal forebrain atrophy (Kerbler et al., 2014).
4.2. Tau-associated Neurofibrillary Tangles (NFTs)
Tau-associated neurofibrillary tangles (NFTs) are formed by hyperphosphorylation and intercellular aggregation of tau protein and are the second well-known hallmark of AD (Knopman et al., 2010; Mesulam et al., 2004). Tau pathology has been suggested as the earliest neurodegenerative feature linked to AD with abnormal tau phosphorylation and aggregation in the locus coeruleus, beginning during early adulthood. It then spreads into different cortical areas such as dorsal raphe, tuberomammillary nucleus, parabrachial nucleus, and basal forebrain (for a review see Holth et al., 2017) before Aβ burden could even be detected (Braak et al., 2011; Braak and Tredici, 2011; Knopman et al., 2010; Stratmann et al., 2016). Furthermore, NFTs in the LC are found in AD patients (Dugger et al., 2011), and later on, phosphorylated tau levels in the CSF also predict cognitive decline in preclinical and clinical AD (Mattsson et al., 2009). The mediation of the LC between AD and NFTs is strongly linked to its excitatory role in the cortical ascending arousal system. In healthy individuals, noradrenergic neurons in the LC inhibits sleep (Saper et al., 2005), on the contrary, LC neurons are lost in AD. Interestingly, it has been shown that the number of LC neurons is correlated with cognitive decline in a cohort of healthy elderly (Wilson et al., 2013).
NFTs are particularly disrupting considering the hippocampus’s ability to generate ripples linked to expression of NREM SS and SWA, and how these two features have been shown to support sleep-dependent memory processing (Diekelmann et al., 2009). Studies on animals showed that hippocampal ripples are diminished and less synchronized due to the accumulation of tau in the medial temporal lobe, therefore changing neural oscillatory pattern (Witton et al., 2016). Tau has been associated with abnormally long hyperpolarized down states during SWA (Menkes-Caspi et al., 2015), explaining part of the correlation between CSF tau levels and SWS drop in patients with AD (Liguori et al., 2014). Furthermore, chronic sleep restriction, already known to be a risk factor for illness progression, impairs hippocampus-dependent memory and increases insoluble tau, helping NFTs formation (Di Meco et al., 2014; Rothman et al., 2013). Ju and colleagues (Ju et al., 2017) reported no increase in tau levels after only one night of disrupted SWS, whereas they found increased CSF tau levels in participants reporting poor sleep during several nights. Conversely, the lymphatic system during sleep promotes tau clearance, explaining why the elderly with a good sleep quality showed fewer NFTs at autopsy (Lim et al., 2013).
4.3. Apolipoprotein Epsilon (ApoE)
Apolipoprotein epsilon (ApoE) is a class of protein essential to combine fats to form lipoprotein. Lipoproteins are important to preserve and remodel neuronal membranes. ApoE is polymorphic, with three major alleles: ApoE-ε2, ApoE-ε3, and ApoE-ε4. The latter is well known to be a genetic risk factor for developing AD (Evans et al., 1997; Farrer et al., 1997; Poirier et al., 1993). ApoE-ε4 has been associated with reduced Aβ clearance with consequent pathological accumulation (Castellano et al., 2011). Normal cognitive elderly individuals with the ApoE-ε4 allele have shown a risk for developing MCI or dementia seven times higher (Burke et al., 2016). Sleep disturbances appear to be linked to ApoE-ε4 in MCI/AD patients, especially concerning REM decrease (Hita-Yañez et al., 2013) and delay in circadian rhythms (Hwang et al., 2018). It has also been proposed that increased sleep disturbances in ApoE-ε4 patients could result from alterations in melatonin production (Liu et al., 1999). Besides, ApoE-ε4 has been associated with an increased risk of SDB and cognitive impairment in patients with OSA (Gottlieb et al., 2004; Hara et al., 2005; Kadotani et al., 2001).
While some studies suggested that ε4 allele may be the major cause of sleep disruptions in elderly people at risk for dementia (Gottlieb et al., 2004; Wang and Lung, 2012), others stressed that sleep deficits and ApoE genotype may just amplify each other’s negative effects (Kaushal et al., 2012; Spira et al., 2008). The debate on ApoE-ε4 is still open given the fact that some studies showed no influence (Craig et al., 2006) or even a protective role of ApoE genotype on sleep patterns, raising the question of the true nature of this association (Yesavage et al., 2004). Even though more investigation is required, current theories propose that better sleep consolidation could attenuate the increased risk conferred by the ApoE genotype (Lim et al., 2013).
5. THE BIDIRECTIONAL LINK BETWEEN SLEEP AND ALZHEIMER’S DISEASE
As aforementioned, the current concept is that sleep disruptions accelerate AD pathogenesis by enabling Aβ and tau protein accumulation. Because sleep disruption also appears to be among the earliest observable symptoms of a wide range of neurodegenerative diseases, such as AD, Parkinson’s Disease, and Multiple Sclerosis (Kay et al., 2018; Mattis and Sehgal, 2016), its function as a biomarker to identify elderly at greater risk has been suggested (Mander et al., 2016). Below we discuss modifications in sleep patterns, macro and microstructure in MCI and AD, and how these disruptions predict clinical symptoms and cognitive performance in patients. As aforementioned, Figure 1 illustrates sleep and brain modifications in healthy and pathological aging.
5.1. Circadian Rhythms
MCI and AD patients show significantly disrupted sleep (Vitiello and Prinz, 1989) and complain about poor sleep more frequently than healthy elderly (27.6% vs 18.3%; Tractenberg et al., 2005). AD patients tend to spend more time awake during the night, due to increased sleep latency and higher frequency of nocturnal awakenings (Hatfield et al., 2004), thus resulting in more daytime sleepiness and decreased sleep efficiency (Hatfield et al., 2004; Holth et al., 2017). Severe sleep fragmentation generally corresponds with the emergence of a syndrome called sundowning, that is characterized by behavioral symptoms such as hostility, anxiety, agitation, and confusion occurring at the end of the day, and has a great impact on cognitive skills and quality of life (Ferrazzoli et al., 2013). Visual hallucinations during wakefulness are also reported by AD patients. However, hallucinations are reported in one-third of the patients during specific phases of the sleep-wake cycle. Vivid dreams are also reported, together with aggressive sleep-related and dream-related behaviors (Sinforiani et al., 2007).
Even before clinical onset, sleep disturbances have relevance to the development of cognitive dysfunctions. A previous study in community-based populations showed a link between delay and reduction of sleep-wake cycles and the probability of developing dementia (Tranah et al., 2011). Age-related sleep fragmentation is associated with a 1.5-fold increased risk of developing dementia in the following 6-year follow-up period (Lim et al., 2013). Furthermore, a longitudinal cohort study showed that sleepiness is related to twice the risk of developing dementia (Foley et al., 2001), suggesting that daytime sleepiness is also a predisposing factor for developing dementia.
It has been argued whether part of these disruptions is caused by neuronal and synaptic loss occurring in the early clinical stages of dementia. Earlier study in AD showed that alterations of sleep-wake cycles are mainly mediated by degeneration of the suprachiasmatic nucleus (SNC; Stopa et al., 1999). Stopa and colleagues determined that SNC is highly damaged in AD and that the astrocyte/neuron ratio is an accurate marker of SNC pathology (Stopa et al., 1999). Additionally, AD post-mortem studies have established that neurofibrillary tangles located in the hypothalamic preoptic area correlate with the severity of fragmented sleep (Lim et al., 2014). Tau deposition is also present in the LC and the basal forebrain of healthy older adults (Braak and Tredici, 2016). It has been suggested that tau within these regions may trigger sleep disruptions years before symptomatic onset, and this may be used as an early diagnostic biomarker (Holth et al., 2017).
Changes in CSF melatonin levels are also a major cause of sleep pattern disruptions in AD patients. It was proposed that this may be due to modification in suprachiasmatic nucleus functionality (Wu et al., 2005), or due to alteration of melatonin secretion itself (Cardinali et al., 2002). Sleep disruptions may also be linked to changes in CSF cortisol concentrations. MCI and AD patients show a significantly higher level of cortisol, strongly correlated with faster clinical worsening and cognitive decline (Popp et al., 2015). Going further, pathophysiology seems to induce hypothalamic orexin neurodegeneration seen in later AD stages with consequences on sleep-wake rhythms (Mander et al., 2015). In MCI and early AD, orexin levels are higher and predict longer sleep latency, more fragmented sleep, and shorter REM duration (Liguori et al., 2014). With disease progression, orexin levels decrease and are associated with more fragmented daytime wakefulness (Friedman et al., 2007).
5.2. REM Sleep
While REM sleep seems to be relatively preserved in normal aging, it is significantly reduced in AD patients (Prinz et al., 1982) and characterized by shorter epochs (Petit et al., 2004). AD patients also show a delayed REM sleep onset and a blunted rebound of REM sleep following selective deprivation (Prinz et al., 1982; Reynolds et al., 1990). It has been suggested that the specific degeneration of cholinergic neurons transmission in AD may constitute the basis of REM sleep changes (Montplaisir et al., 1998). The degree of basal forebrain atrophy is correlated with the degree of cortical Aβ burden, not only in MCI and AD patients but also in healthy elderly individuals (Kerbler et al., 2014). Moreover, there is a general slowing of high-frequency oscillations in AD patients (for a review Cassani et al., 2018; Huang et al., 2000). This phenomenon includes an increase in diffuse SWA and θ activity not only during wakefulness, but also during REM sleep (Hassainia et al., 1997; Peter-Derex et al., 2015; Xie et al., 2013), and this is larger in temporoparietal and frontal regions (Petit et al., 2004).
REM sleep can also predict neuropsychological impairment in older adults and AD patients (Liguori et al., 2014). It has also been showed that REM sleep has a role in emotional regulation and mood states. Thus, the manifestation of hostility, depression anxiety, emotional dysregulation and memory retention impairment (Kensinger et al., 2004) could be attributed to poor REM sleep quality.
Therefore, a therapeutic intervention aiming at improving REM sleep quality is particularly important, due to the influence of REM sleep on cognition and mood. Cholinesterase inhibitors aimed at promoting REM sleep quality and duration and their efficacy predicts the degree of memory improvement in AD patients (see below; Wdos et al., 2006).
5.3. Slow Wave Sleep (SWS)
MCI patients show a decrease in time spent in NREM related to healthy elderly controls, which already have diminished SWA during normal aging (Westerberg et al., 2012). NREM sleep progressively decreases after the onset and during illness progression (Loewenstein et al., 1982). Tau and Aβ protein levels measured in CSF predict the degree of reduced SWA time in AD patients, together with a drop in sleep efficiency and REM sleep duration (Liguori et al., 2014). SWA reduction strongly correlates with Aβ accumulation in the medial prefrontal cortex (Mander et al., 2015), which is one of the earliest regions damaged by Aβ plaques (Sepulcre et al., 2013). EEG slowing also predicts disrupted hippocampal memory consolidation (Mander et al., 2015). New studies tried to find an answer to this apparent contradiction, explaining that SWA decrease may reflect changes in K-complexes. Following this explanation, <1Hz SWA would not change significantly from healthy age-matched subjects and AD patients, as previously shown.
5.4. Sleep Spindles (SS) and K-complexes (K-c)
AD patients show a dramatic reduction, over 40%, in K-c density compared to healthy elderly controls (De Gennaro et al., 2017). Normal aging is associated with a decrease in both spontaneous and evoked K-c, while AD patients present mainly a decrease of spontaneous K-c in the frontal cortex (Montplaisir et al., 1995; Prinz et al., 1982). Lower K-c activity is also correlated with a lower Mini-Mental State Examination (MMSE) score (De Gennaro et al., 2017). AD patients showed faster mean θ frequency in both REM and SWS during post-learning sleep versus elderly controls. This significant difference was associated with better delayed episodic recall, probably enabling a compensatory mechanism to sustain memory performance (Hot et al., 2011). Similar to the reduction in K-c, various studies found a significant SS reduction in AD patients compared to healthy controls (Bliwise, 1993; Montplaisir et al., 1995; Prinz et al., 1982), mostly involving fast parietal spindle density (Westerberg et al., 2012). Results suggest that pathology-related spindle alterations start in the early stages of the disease, possibly even in preclinical stages. In support of this theory, MCI patients often show a massive decrease in SS (Gorgoni et al., 2016). This reduction comes with cognitive deterioration in patients with dementia, as with many other specific disrupted EEG features. The relationship involves mostly fast central sleep spindles intensity and impaired immediate episodic recall in AD patients (Rauchs et al., 2008). It has been suggested that thalamic damage (Bonjean et al., 2008) and disruptions in pathways of memory consolidation between the hippocampus and neocortical areas may account for spindle density drop.
5.5. Sleep Disordered Breathing and AD
Sleep disorders are often co-morbid with neurodegeneration. Over 60% of MCI/AD patients are diagnosed with at least one clinical sleep disorder, mostly SDB or insomnia, during illness progression (Guarnieri et al., 2012). Importantly, a successful treatment of sleep disorders can delay MCI onset (Osorio et al., 2015) and improves cognitive function when a patient is already in severe stages of AD (Wdos et al., 2006).
There is a complex interaction between SDB and dementia. Although previous investigations showed an increased incidence of Obstructive Sleep Apnea Syndrome (OSAS) with aging, AD patients are particularly affected, with 40-70% having five or more apneas and hypopneas per hour of sleep (Hoch et al., 1986; Kupfer et al., 1985). A diagnosis of SDB is linked with a major risk of developing dementia (Tworoger et al., 2006). Other studies found that SDB patients had an 85% increased risk to develop AD and MCI (Yaffe et al., 2011). Furthermore, individuals with sleep apnea convert to MCI and AD more frequently and also at a younger age (Osorio et al., 2015). After clinical onset, AD stage and SDB severity positively correlate, worsening together during progression (Ancoli-Israel et al., 1991).
After the clinical onset of SDB, AD patients with a diagnosis of OSAS show sleep with less REM and SWS, and more frequent awakenings compared to AD patients without OSA (Cooke et al., 2006). Frequent apneas and hypopneas during sleep are associated with deficits in memory, attention and executive tasks. Some authors suggested that cognitive deterioration may be mediated by SDB’s effect on daytime sleepiness (Sforza and Roche, 2012). Alternatively, SDB could also directly contribute to neuronal dysfunction through hypoxia, or it may be a consequence of AD-associated neurodegeneration in brainstem respiratory centers. A possible other explanations involve SDB’s relation to increase vascular risk, which is itself an independent risk factor for AD (Landry and Liu-Ambrose, 2014).
6. SLEEP TREATMENTS IN AGING AND AD
Along these lines, it has been suggested that treating sleep dysfunctions in young elderly and MCI patients could slow or prevent the progression of dementia (Roberts and Knopman, 2013). Consolidating the sleep quality, increasing total sleep time and SWA have been reported to decrease the incidence of AD onset in the elderly community (Lim et al., 2013). Current pharmacological therapies, such as cholinesterase inhibitors, hypnotic and antidepressants, can have important side effects in older individuals, for example impairing alertness, cognitive skills, and producing psychomotor effects; therefore, their use must be considered as a last resort (for a review see Bloom, 2009; Gooneratne and Vitiello, 2014). Therefore, the first line of treatment should prioritize a combination of sleep hygiene intervention and cognitive-behavioral therapy (CBT; Gooneratne and Vitiello, 2014). It has been demonstrated that both interventions are highly effective and with no negative consequences (Gooneratne and Vitiello, 2014). Other combination therapies have been proposed, for example, bright light therapy with melatonin has been shown to stabilize the sleep/wake cycle. More recently, the application of Noninvasive Brain Stimulation (NiBS), like transcranial electrical stimulation (tES) to manipulate SWS and sleep quality has been proposed as a promising intervention. In the following section, we will review evidence of innovative sleep therapies, discussing their efficacy in tackling sleep disruptions in healthy elderly and MCI/AD patients.
6.1. Pharmacological Medications
Current pharmacological treatments in sleep disruptions are effective for transient insomnia and when sleep disruption is secondary to a pathology (for a comprehensive review see Regestein et al., 1998). Chronic drug therapies can lead to serious adverse effects and tolerance development, frequently affecting daytime alertness and cognitive ability (McCurry et al., 2000). Pharmacological therapy should be considered only in situations where a definite medical condition is diagnosed, or where the use of the behavioral approach (CBT-I) has failed.
6.1.1. Cholinesterase Inhibitors.
Memory and vigilance impairments are largely caused by a disruption in the cholinergic transmission. The concentration of acetylcholine is high during wakefulness and REM sleep, while it declines during SWS (Coyle et al., 1983). It has been shown that the timing of administration of cholinesterase inhibitors plays a crucial role, morning administration causes less negative side effects (e.g. nightmares; Ancoli-Israel et al., 2005; Cooke et al., 2006; Rogers, 1998). While positive modifications in sleep patterns due to cholinesterase inhibitors are difficult to replicate, improvement in memory consolidation and clinical global functioning in AD seems to be significant (Ancoli-Israel et al., 2005; Cooke et al., 2006; Rogers, 1998).
6.1.2. Hypnotics, Antidepressants, and Antipsychotics.
Although hypnotics, whether benzodiazepines (Lorazepam) or non-benzodiazepines (Zolpidem) are effective in accelerating sleep onset and prolong overall time asleep, their side effects can be detrimental. They drastically modify sleep microarchitecture and may cause confusion and ataxia during awake states. Benzodiazepines may even cause paradoxical effects in the elderly (Peter-Derex et al., 2015). Non-benzodiazepines cause fewer negative side effects but in general, they are not generally recommended, except in cases of acute and severe insomnia (Peter-Derex et al., 2015).
Sedating antidepressants help when sleep difficulties are caused by depressive symptoms. Antidepressants result in higher overall time spent asleep and greater sleep efficiency, although side effects, such as diurnal sleepiness and dizziness, must be considered. The first choice antidepressant should be Serotonin Selective Re-uptake inhibitors (SSRIs) for their relative safety (McCurry et al., 2000), although also Trazodone or Mirtazapine proved to be effective to treat insomnia (Jaffer et al., 2017).
Antipsychotics are usually administered to treat behavioral and psychiatric manifestations of AD, but they seem to also be effective on insomnia. Atypical antipsychotics, such as risperidone, olanzapine, and quetiapine, should be preferred over typical antipsychotics though, as the latter may cause excessive diurnal sedation and increase the risk of falling (Rocca et al., 2007).
6.2. Sleep Hygiene and Cognitive-behavioral Therapy
Sleep hygiene is an intervention that manipulates daily habits to influence sleep quality by preserving a regular sleep/wake schedule (i.e. with the same rise time every day and avoiding diurnal nap) and reducing pre-sleep tension and sleep-onset latency (Gooneratne and Vitiello, 2014). Sleep hygiene might be facilitated by creating a non-disruptive sleep environment. For example, the use of earplugs and “white noise” in a dark and comfortable temperature room decreases nocturnal awakenings and arousals (Gooneratne and Vitiello, 2014). Physical exercise combined with a healthy diet is also an important contributing factor for efficient sleep hygiene. Locomotor activity in the daytime activates neuronal feedback loops in the SCN, and, as a result, the sleep/wake cycle is regulated (Hughes and Piggins, 2012).
Cognitive-behavioral therapy for insomnia (CBT-I) consists of six to ten sessions supervised by a trained therapist. CBT-I aims to change maladaptive behaviors and cognitive beliefs that perpetuate insomnia and includes relaxation techniques and tips to reduce the arousal before bedtime (e.g. avoiding watching TV, using smartphones in bed) (Gooneratne and Vitiello, 2014). Various studies in healthy elderly, MCI, and early-stage AD patients proved the high efficacy of CBT-I in improving sleep efficacy, prolonging TST, and shortening sleep-onset. Importantly, CBT-I results, unlike pharmacotherapy, last at least 6 months after the end of the treatment (Cassidy-Eagle et al., 2018; Montgomery and Dennis, 2004; Trauer et al., 2015). However, further studies need to clarify whether CBT-I is also able to modify physiological sleep oscillations (e.g. enhancing SWA) or to regulate Aβ regulation.
6.3. Melatonin
Although melatonin has no negative reactions, it is still unclear whether its administration causes significant and direct beneficial effects. Actigraphy, polysomnography, subjective reports, sleep logs, and clinical observations have been used to investigate melatonin’s effects in both healthy subjects and subjects diagnosed with AD, with varied results; some studies did not find a significant improvement, while others found a significant effect of melatonin as a hypnotic and circadian controller, mostly when in combination with Bright Light Therapy (Dowling et al., 2008; McCleery et al., 2015; Pandi-Perumal et al., 2005; Rajaratnam et al., 2004; Singer, 2003). Importantly, a significant slowing in cognitive decline has been found in AD patients when treated with prolonged-release melatonin (Wade et al., 2014). Furthermore, melatonin seems to protect against several mechanisms of neuronal death and able to prevent Aβ toxicity, an effect probably linked to its cytoprotective and antioxidant effects (for a review see Cardinali et al., 2002).
6.4. Bright Light Therapy (BLT)
Elderly people tend to spend less time exposed to daylight, aggravating sleep problems. Bright light therapy (BLT) consists of exposing healthy elderly individuals and patients to light with the aid of a full spectrum lightbox for a minimum of 30 minutes per day, preferably during the morning. It has been shown that BLT results in a reduction in overnight awakenings, increased sleep consolidation, and increased TST (Ancoli-Israel et al., 2003). Moreover, BLT also reduces daytime sleepiness and increases daytime alertness (Ancoli-Israel et al., 2003; Satlin et al., 1992). BLT efficacy is even greater when circadian rhythms are severely impaired. The combined administration of BLT and melatonin amplifies efficacy in more severely impaired subjects (Dowling et al., 2008) while showing less improvement in subjects with less severe disturbances (Dowling et al., 2005).
6.5. Auditory Stimulation
Auditory stimulation is applied overnight during the N3 stage aiming to enhance <1Hz SWA with the rationale of regulate hippocampus-dependent memory consolidation and in sleep stabilization. A promising application is auditory closed-loop stimulation, where short auditory stimuli are presented at the same frequency as endogenous slow oscillations. Ngo and colleagues (Ngo et al., 2013) used an auditory closed-loop feedback system based on an adaptive amplitude threshold method, to detect online SWA to send a brief auditory stimulation (i.e., 50ms bursts of pink noise). The authors demonstrated a significant increase of SWA, an enhancement of phase-locked spindle activity during slow oscillations up-state, and amelioration of memory performance after closed-loop auditory stimulation. These results were replicated in a subsequent study by the same group (Ngo et al., 2015) and by other groups that used auditory closed-loop systems (Leminen et al., 2017; Ong et al., 2016; Santostasi et al., 2016). All these studies increased SWA in young adults during daytime naps, in contrast, Papalambros and colleagues (2017) tested an automated and adaptive algorithm in 13 older participants (60–84yo) during one night of acoustic stimulation and one night of sham stimulation in random order. Pulses of pink noise were administered during slow-wave upstate. Promisingly, the authors found an increase in SWA and spindle activity for the active stimulation intervals compared to sham intervals. Furthermore, verbal memory was tested before and after sleep and overnight improvement in word recall was significantly greater with acoustic stimulation compared to sham and was correlated with changes in SWA (Papalambros et al., 2017).
6.6. Noninvasive Brain Stimulation (NiBS)
NiBS techniques, such as transcranial magnetic stimulation (TMS), transcranial alternating current stimulation (tACS), and oscillatory transcranial direct current stimulation (otDCS), can improve sleep quality and, in turn, cognitive functioning by promoting sleep-dependent plasticity through the modulation of SWA. The idea of targeting slow oscillations during sleep is based on the long-established relationship between sleep and memory consolidation (Diekelmann et al., 2009; Malkani and Zee, 2020). Two major evidences provide an insight into this tight connection. According to the active system consolidation model, during NREM sleep, declarative memory representations transition from a hippocampus-dependent state to a hippocampus-independent state (Diekelmann et al., 2009). As memories get consolidated in the neocortex through replay, the hippocampus frees space to encode new memories. SWS, spindles and Sharp Wave Ripples (SWRs) play a major role in this consolidation process, as shown by the disruptive effects of spindles and SWRs blockage on memory consolidation and retention (Girardeau et al., 2009).
At the synaptic level, the synaptic homeostasis hypothesis (SHY) (Tononi and Cirelli, 2012) argues that sleep is a necessary tool to maintain the brain plastic as it renormalizes the net synaptic strength potentiated by learning. By preventing the synaptic downscaling (decrease of strength), sleep deprivation would impair not only memory consolidation but even subsequent learning. Tangible indirect biological markers of the disruption of this sleep-wake dependent homeostatic regulation of synaptic strength are enhanced awake EEG θ activity, cortical excitability, and short-interval intracortical facilitation (a marker of synaptic strength). These would lead to deficient long-term potentiation (LTP)-inducibility and, ultimately, to cognitive impairment, two features that characterize AD pathology (Koch et al., 2013). Despite the different perspectives taken by these theoretical accounts, both stress the pivotal importance of sleep for memory functioning, pushing researchers to target sleep to address memory impairment both in healthy and pathological populations. NiBS techniques are particularly promising tools as they can be safely used to directly enhance/disrupt oscillatory activity during sleep (transcranial electric stimulation or tES) or to modulate the activity of one cortical area or more functionally connected regions by changing their excitability (repetitive Transcranial Magnetic Stimulation or rTMS).
Transcranial Electrical Stimulation (tES).
Two tES protocols seem to be particularly suitable for sleep modulation: tACS and otDCS. tACS delivers alternating current that continuously shifts between positive and negative voltages (Tavakoli and Yun, 2017), thus inducing periodic shifts in the transmembrane potential, alternating depolarizing and hyperpolarizing effects, therefore, enabling the entrainment of intrinsic brain oscillations due to its sinusoidal waveform (Antal and Paulus, 2013; Paulus, 2011). Differently, transcranial direct current stimulation (tDCS) sends a monophasic baseline voltage, modulating cortical tissue with depolarization and therefore promoting endogenous oscillations (anodal) or hyperpolarization, suppressing it (cathodal; Paulus, 2011). Oscillatory-tDCS (otDCS) combines the rhythmic oscillating current of tACS while still riding a directional voltage component, as tDCS creating an anodal or cathodal oscillating stimulation. In particular, these protocols drive cortical populations to oscillate at the same natural frequency as the one delivered by the stimulation itself, with a greater amplitude thanks to the resonance phenomenon. Marshall and coworkers (2006) administered anodal slow otDCS at 0.75Hz frequency over the prefrontal cortex (PFC) intending to interact with SWA during the N3 stage in young healthy participants. The author found a significant increase in δ power which was accompanied by a significant increase in declarative memory retention (Marshall et al., 2006). Some other attempts implemented innovative NiBS protocols, like the closed-loop system, to test the relationship between SWA and memory (Jones et al., 2018; Ketz et al., 2018). NiBS during sleep usually requires the experimenter to start the stimulation after 3-4 minutes of ongoing EEG typical of N2 or N3. A closed-loop algorithm can independently start the stimulation when the subject is in N3 (Jones et al., 2018; Ketz et al., 2018b; Robinson et al., 2018). This algorithm for augmentation of slow-wave sleep first detects the presence of SW oscillations, computing the mean of the power spectra of SW. The frequency of sinusoidal wave produced by tACS is then set to individualized SW frequency mean to match the stimulation frequency and phase with the natural ongoing SW activity. Further developments in transcranial stimulation protocols, and optimization and personalization of the current implemented, are necessary to precisely target SWA to study declarative memory consolidation (for a review of Barham et al., 2016).
Slow frequency otDCS mimicking slow-wave activity has been tested in older adults during afternoon naps. Westerberg targeted SWA with a subsequent improvement in word-pair performance (Westerberg et al., 2015), while Ladenbauer enhanced 1Hz oscillations and fast sleep spindles power, leading to a benefit in a visual memory task (Ladenbauer et al., 2016). Other studies with slow-wave tACS and otDCS administered overnight in the elderly failed to replicate the beneficial enhancement of SWA and memory consolidation (Eggert et al., 2013; Paßmann et al., 2016a). Authors found increased SWA and spindles activity, similar to previous results on young individuals, but no beneficial effects in the consolidation of visuospatial and verbal memories (Paßmann et al., 2016). This may be due to crucial differences in overnight memory consolidation processes between young and aged individuals or differences in protocols (waveform of electrical stimulation).
Following a different lead, Marshall and colleagues found that θ-otDCS during REM sleep, instead of NREM, did not affect consolidation, yet causes a significant increase of γ power (Marshall and Born, 2011). To date, during wakefulness, θ and γ oscillatory activity coupled during memory encoding/retrieval (Axmacher et al., 2010; Sauseng et al., 2009), while γ activity predominates during retrieval (Vaz et al., 2020) and may play a role in memory consolidation during REM. Prominent REM θ rhythm coupled with γ have been seen in rodents (Belluscio et al., 2012; Scheffzük et al., 2011) and monkeys (Takeuchi et al., 2015). γ seems to promote synaptic plasticity, supported by θ (Lisman and Buzsáki, 2008; Lisman and Jensen, 2013). Stimulating θ and γ during REM may, therefore, help consolidate mnemonic traces as well as SWS stimulation.
Nonetheless, these promising findings need further studies with larger numbers of healthy participants and follow-ups, both during NREM and REM states. Interestingly, recent evidence encourages an experimental application in neurodegenerative diseases. Ladenbauer and colleagues (2017) enrolled 16 MCI amnestic patients in a crossover design. Patients were asked to sleep for 90 minutes while electrical stimulation was applied at 0.75 Hz over the prefrontal cortex. The authors found an enhancement of overall SWA and spindle power. Moreover, participants showed a significantly improved visual declarative memory during stimulation as compared with sham (Ladenbauer et al., 2017). This study suggests that an individualized protocol may be effective to enhance the performance of visual declarative memory even in MCI patients.
Repetitive Transcranial Magnetic Stimulation (rTMS).
Compared to tES protocols, trains of TMS pulses (rTMS) can directly affect cortical neural plasticity by facilitating or preventing long-term potentiation (LTP) or long-term depression (LTD). This results in longer-lasting effects, which might help the patient comply with the treatment and enable off-line interventions. The literature on offline rTMS extensively showed its feasibility in the treatment of several sleep disorders (for a recent review see Nardone et al., 2020). Although rTMS during wakefulness does not directly elicit slow oscillations, evidence of endogenous SWA enhancement on the stimulated region during the subsequent sleep makes offline rTMS protocols a promising approach for addressing sleep disturbances in the long-term (Huber et al., 2007). This possibility is particularly exciting also considering the positive effects of rTMS in promoting cognitive functioning in the elderly (Iriarte and George, 2018; Tatti et al., 2016), and improving AD/MCI clinical outcomes (Chen et al., 2020; Weiler et al., 2020). Unfortunately, to the best of our knowledge, none of the published studies tacking cognitive impairment in AD or sleep disorder with rTMS included, respectively, an analysis of the subsequent sleep activity or a pre-post cognitive assessment. As more insights about the relationship between cognition and sleep could guide new interventions for AD, future investigations may further probe this relationship. New research on rTMS protocols in AD is also desirable to address some important safety concerns. Although rTMS is considered a safe tool (Rossi et al., 2009), the lack of extensive knowledge of the biological mechanisms of rTMS may pose a threat to the AD population. As an example, basic research evidence showed that the release of Aβ is regulated by the amount of neural activity (Mormino et al., 2011) and that low-frequency rTMS in AD-transgenic mice reduces amyloid secretion (Huang et al., 2017). This raises doubts about the applicability of high-frequency rTMS to improve sleep and cognition in AD. Vice versa, the use of low-frequency rTMS to tackle Aβ deposition (that might also affect sleep quality) is challenged by a possible cognitive burden.
Differently from tES protocols that are feasible to use during sleep, rTMS ‘online’ interventions are currently limited by the device itself. In addition to the possibility of the machine overheating with prolonged use, rTMS also requires to keep the head in position for the entire protocol to precisely target the region of stimulation. This requirement makes online rTMS protocols challenging even during a quiet night of sleep, and can, therefore, be prohibitive for both people suffering from sleep disorders (e.g. parasomnias), and the elderly population that is frequently affected by nocturia. Moreover, the noise produced by the coil (requiring earplugs/sound masking) can further disrupt sleep.
Despite these practical limitations that may hold back the researcher from studying the effects of TMS on the ongoing sleep oscillatory activity, the scant available evidence supports its online modulatory effects on SWA in the healthy population. Strong state-dependent slow oscillations can be elicited by single-pulse (Bergmann et al., 2012), paired-pulse with 100ms inter-stimulus-interval (ISI) (Stamm et al., 2015) and rTMS delivered during NREM sleep (Massimini et al., 2007). Importantly, such response is amplified when delivered on the up-state (global depolarization) of the endogenous slow-wave, when the faster rhythms (spindles, gamma, and hippocampal ripples) are concentrated and the hippocampal-neocortex transfer of memories is thought to occur (Diekelmann and Born, 2010). However, it is still unclear whether the externally triggered slow-oscillations could compensate or even replace the endogenous ones by producing the same beneficial effects on cognition.
7. CONCLUSIONS
In this review, we highlighted how sleep disturbances are a key factor in negatively impacting the quality of life and cognition in normal aging, as well as a risk factor for MCI/AD development and prognosis. Earlier studies considered sleep abnormalities as a consequence of the neurodegenerative process, while recent studies suggest that sleep degradation emerges in the prodromal phase, and is a risk factor and a collateral cause of AD exacerbation. In this framework, finding reliable biomarkers in elderly individuals at risk, such as specific micro and macro-structural sleep features may potentially slow the progression of MCI to AD. Going further, the strong link between sleep abnormalities and Aβ and Tau protein accumulation suggests that sleep research might be pivotal in addressing AD pathogenesis and promoting healthy aging. Sleep research could guide the creation of rehabilitative and/or cognitive enhancement interventions to improve the quality of life of healthy elderly, detect individuals at risk, and even slow the progression of MCI/AD.
Figure 3. Bidirectional Link between Sleep, Aβ Levels, and MCI/AD Onset.
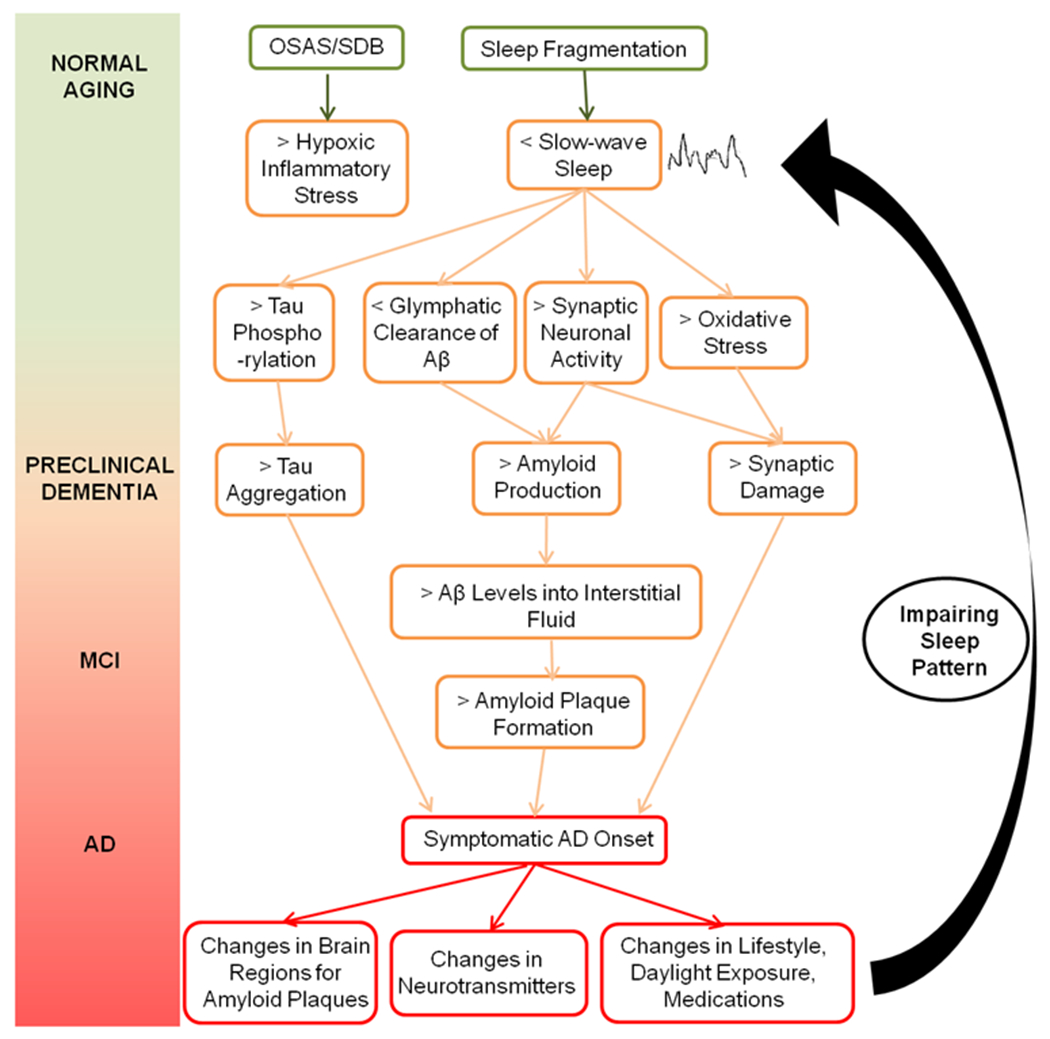
Sleep fragmentation is a key factor that triggers a cascade of pathological processes before clinical onset. Modifications in brain regions, neurotransmitter levels, and lifestyle due to AD diagnosis and prognosis will then worsen sleep patterns establishing a vicious cycle feeding itself.
ACKNOWLEDGMENTS
Emiliano Santarnecchi is supported by the BROAD Institute at Harvard-MIT (Boston, MA, USA) via 2016P000351. Emiliano Santarnecchi is supported by Defence Advanced Research Projects Agency (DARPA) Emiliano Santarnecchi HR001117S0030. Emiliano Santarnecchi is supported by the Beth Israel Deaconess Medical Center (BIDMC) via the Chief Academic Officer (CAO) grant 2017 and by NIH via R01 AG060981-01. Emiliano Santarnecchi is partially supported by R01 MH117063-01, P01 AG031720. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of Harvard University and its affiliated academic health care centers, the National Institutes of Health.
REFERENCES
- Anatomical markers of sleep slow wave activity derived from structural magnetic resonance images. - PubMed - NCBI [WWW Document], n.d. URL https://www.ncbi.nlm.nih.gov/pubmed/21435064 (accessed 3.27.20). [DOI] [PubMed]
- Ancoli-Israel S, Amatniek J, Ascher S, Sadik K, Ramaswamy K, 2005. Effects of Galantamine Versus Donepezil on Sleep in Patients With Mild to Moderate Alzheimer Disease and Their Caregivers: A Double-Blind, Head-to-Head, Randomized Pilot Study. Alzheimer Dis. Assoc. Disord 19, 240–245. 10.1097/01.wad.0000189052.48688.36 [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Ancoli-Israel S, Kripke DF, Kripke DF, Klauber MR, Mason WJ, Mason WJ, Fell R, Fell R, Kaplan O, 1991. Sleep-Disordered Breathing in Community-Dwelling Elderly. Sleep 14, 486–495. 10.1093/sleep/14.6.486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancoli-Israel S, Gehrman P, Martin JL, Shochat T, Marler M, Corey-Bloom J, Levi L, 2003. Increased Light Exposure Consolidates Sleep and Strengthens Circadian Rhythms in Severe Alzheimer’s Disease Patients. Behav. Sleep. Med 1, 22–36. 10.1207/S15402010BSM0101_4 [DOI] [PubMed] [Google Scholar]
- Antal A, Paulus W, 2013. Transcranial alternating current stimulation (tACS). Front Hum Neurosci. 3177.SRC-BaiduScholar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axmacher N, Henseler MM, Jensen O, Weinreich I, Elger CE, Fell J, 2010. Cross-frequency coupling supports multi-item working memory in the human hippocampus. Proc. Natl. Acad. Sci 107, 3228–3233. 10.1073/pnas.0911531107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barham MP, Enticott PG, Conduit R, Lum JAG, 2016. Transcranial electrical stimulation during sleep enhances declarative (but not procedural) memory consolidation: Evidence from a meta-analysis. Neurosci. Biobehav. Rev 63, 65–77. 10.1016/j.neubiorev.2016.01.009 [DOI] [PubMed] [Google Scholar]
- Belluscio MA, Mizuseki K, Schmidt R, Kempter R, Buzsáki G, 2012. Cross-frequency phase-phase coupling between θ and γ oscillations in the hippocampus. J. Neurosci. Off. J. Soc. Neurosci 32, 423–435. 10.1523/JNEUROSCI.4122-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE, 2008. Suprachiasmatic nucleus and melatonin: Reciprocal interactions and clinical correlations. Neurology 71, 594–598. 10.1212/01.wnl.0000324283.57261.37 [DOI] [PubMed] [Google Scholar]
- Bergmann TO, Mölle M, Schmidt MA, Lindner C, Marshall L, Born J, Siebner HR, 2012. EEG-guided transcranial magnetic stimulation reveals rapid shifts in motor cortical excitability during the human sleep slow oscillation. J. Neurosci. Off. J. Soc. Neurosci 32, 243–253. 10.1523/JNEUROSCI.4792-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliwise DL, 1993. Sleep in Normal Aging and Dementia. Sleep 16, 40–81. 10.1093/sleep/16.1.40 [DOI] [PubMed] [Google Scholar]
- Bloom HG, 2009. Evidence-Based Recommendations for the Assessment and Management of Sleep Disorders in Older Persons 57. [DOI] [PubMed] [Google Scholar]
- Bonjean M, Schabus M, Boly M, Darsaud A, Desseilles M, Degueldre C, Balteau E, Phillips C, Luxen A, Sejnowski T, Maquet P, de Jong L, van Veer I, Houwing J, Westendorp R, Bollen E, der Hiele K, de Bruin P, Middelkoop H, van Buchem M, Sci U, der Grond J, 2008. Dang-Vu TT1, Interplay between spontaneous and induced brain activity during human non-rapid eye movement sleep. Proc Natl Acad Sep 13 Van Strongly Reduc. Vol. Putamen Thalamus Alzheimers Dis. MRI Study Brain Dec131Pt 12327785 108, 15438–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce R, Glasgow SD, Williams S, Adamantidis A, 2016. Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science 352, 812–816. 10.1126/science.aad5252 [DOI] [PubMed] [Google Scholar]
- Braak H, Thal D, Ghebremedhin E, Tredici K, J, 2011. Del Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. Exp Neurol Nov 70, 960–9. [DOI] [PubMed] [Google Scholar]
- Braak H, Tredici K, 2016. Del Potential pathways of abnormal tau and α-synuclein dissemination in sporadic Alzheimer’s and Parkinson’s diseases. Cold Spring Harb Perspect Biol 88.SRC-BaiduScholar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Tredici K, 2011. Del The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol February121, 171–81. [DOI] [PubMed] [Google Scholar]
- Brown A, Biederman J, Valera E, Lomedico A, Aleardi M, Makris N, Seidman LJ, 2012. Working memory network alterations and associated symptoms in adults with ADHD and Bipolar Disorder. J. Psychiatr. Res 46, 476–483. 10.1016/j.jpsychires.2012.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R, Basheer R, McKenna J, Strecker R, McCarley R, 2012. Control of sleep and wakefulness. Physiol Rev July92, 1087–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke S, Maramaldi P, Cadet T, Kukull W, 2016. Associations between depression, sleep disturbance, and apolipoprotein E in the development of Alzheimer’s disease: dementia. Int Psychogeriatr ;. 28SRC-BaiduScholar, 1409–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canolty R, Edwards E, Dalal S, Soltani M, Nagarajan S, Kirsch H, Berger M, Barbaro N, Knight R, 2006. High gamma power is phase-locked to theta oscillations in human neocortex. Sci. September15313, 1626–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinali D, Brusco L, Liberczuk C, Furio A, 2002. The use of melatonin in Alzheimer’s disease. Neuro Endocrinol Lett April23Suppl 1SRC-BaiduScholar, 20–3. [PubMed] [Google Scholar]
- Carrier J, Land S, Buysse D, Kupfer D, Monk T, 2001. The effects of age and gender on sleep EEG power spectral density in the middle years of life. Psychophysiology 38, 232–242. [PubMed] [Google Scholar]
- Carrier J, Viens I, Poirier G, Robillard R, Lafortune M, Vandewalle G, Martin N, Barakat M, Paquet J, Filipini D, 2011. Sleep slow wave changes during the middle years of life: Changes in slow waves with age. Eur. J. Neurosci 33, 758–766. 10.1111/j.1460-9568.2010.07543.x [DOI] [PubMed] [Google Scholar]
- Cassani R, Estarellas M, San-Martin R, Fraga FJ, Falk TH, 2018. Systematic Review on Resting-State EEG for Alzheimer’s Disease Diagnosis and Progression Assessment. Dis. Markers 2018, 1–26. 10.1155/2018/5174815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy-Eagle E, Siebern A, Unti L, Glassman J, O’Hara R, 2018. Neuropsychological Functioning in Older Adults with Mild Cognitive Impairment and Insomnia Randomized to CBT-I or Control Group. Clin. Gerontol 41, 136–144. 10.1080/07317115.2017.1384777 [DOI] [PubMed] [Google Scholar]
- Castellano J, Kim J, Stewart F, Jiang H, DeMattos R, Patterson B, Fagan A, Morris J, Mawuenyega K, Cruchaga C, Goate A, Bales K, Paul S, Bateman R, Holtzman D, 2011. Human ApoE isoforms differentially regulate brain amyloid-β peptide clearance. Sci Transl Med June2989ra573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauter E, Leproult R, Plat L, 2000. Van Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA 284, 861–868. [DOI] [PubMed] [Google Scholar]
- Chen H-C, Hsu N-W, Chou P, 2020. Subgrouping Poor Sleep Quality in Community-Dwelling Older Adults with Latent Class Analysis - The Yilan Study, Taiwan. Sci. Rep 10, 1–9. 10.1038/s41598-020-62374-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J-T, Liu I-M, Juang S-W, Jou S-B, 2000. Decrease of adenosine A-1 receptor gene expression in cerebral cortex of aged rats. Neurosci. Lett 283, 227–229. 10.1016/S0304-3940(00)00961-7 [DOI] [PubMed] [Google Scholar]
- Clawson BC, Durkin J, Aton SJ, 2016. Form and Function of Sleep Spindles across the Lifespan. Neural Plast. 2016, 1–16. 10.1155/2016/6936381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte F, Arzilli C, Errico BM, Giganti F, Iovino D, Ficca G, 2014. Sleep Measures Expressing ‘Functional Uncertainty’’ in Elderlies’ Sleep.’ Gerontology 60, 448–457. 10.1159/000358083 [DOI] [PubMed] [Google Scholar]
- Cooke J, Liu L, Natarajan L, He F, Marler M, Loredo J, Corey-Bloom J, Palmer B, Greenfield D, Ancoli-Israel S, 2006. The effect of sleep-disordered breathing on stages of sleep in patients with Alzheimer’s disease. Behav Sleep Med 4, 219–27. [DOI] [PubMed] [Google Scholar]
- Cooke JR, Ancoli-Israel S, 2011. Normal and abnormal sleep in the elderly, in: Handbook of Clinical Neurology. Elsevier, pp. 653–665. 10.1016/B978-0-444-52006-7.00041-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copinschi G, Caufriez A, 2013. Sleep and Hormonal Changes in Aging. Endocrinol. Metab. Clin. North Am 42, 371–389. 10.1016/j.ecl.2013.02.009 [DOI] [PubMed] [Google Scholar]
- Copinschi G, Cauter EV, 1995. Effects of Ageing on Modulation of Hormonal Secretions by Sleep and Circadian Rhythmicity. Horm. Res 43, 20–24. 10.1159/000184232 [DOI] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA, 2000. Normal Brain Development and Aging: Quantitative Analysis at in Vivo MR Imaging in Healthy Volunteers. Radiology 216, 672–682. 10.1148/radiology.216.3.r00au37672 [DOI] [PubMed] [Google Scholar]
- Coyle JT, Price DL, DeLong MR, 1983. Alzheimer’s Disease: A Disorder of Cortical Cholinergic Innervation. Sci. New Ser 219, 1184–1190. [DOI] [PubMed] [Google Scholar]
- CR, Knopman D, Jagust W, Shaw L, Aisen P, Weiner M, Petersen R, Trojanowski J, 2010. Jack Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol Jan 9, 119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig D, Hart D, Passmore A, 2006. Genetically increased risk of sleep disruption in Alzheimer’s disease. Sleep 1003e7.29SRC-BaiduScholar. [DOI] [PubMed] [Google Scholar]
- Crowley K, 2011. Sleep and Sleep Disorders in Older Adults. Neuropsychol. Rev 21, 41–53. 10.1007/s11065-010-9154-6 [DOI] [PubMed] [Google Scholar]
- Crowley K, 2002. The effects of normal aging on sleep spindle and K-complex production. Clin. Neurophysiol 113, 1615–1622. 10.1016/S1388-2457(02)00237-7 [DOI] [PubMed] [Google Scholar]
- Dang-Vu TT, Bonjean M, Schabus M, Boly M, Darsaud A, Desseilles M, Degueldre C, Balteau E, Phillips C, Luxen A, Sejnowski TJ, Maquet P, 2011. Interplay between spontaneous and induced brain activity during human non-rapid eye movement sleep. Proc. Natl. Acad. Sci 108, 15438–15443. 10.1073/pnas.1112503108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gennaro L, Ferrara M, 2003. Sleep spindles: an overview. Sleep Med. Rev 7, 423–440. 10.1053/smrv.2002.0252 [DOI] [PubMed] [Google Scholar]
- De Gennaro L, Gorgoni M, Reda F, Lauri G, Truglia I, Cordone S, Scarpelli S, Mangiaruga A, D’atri A, Lacidogna G, Ferrara M, Marra C, Rossini PM, 2017. The Fall of Sleep K-Complex in Alzheimer Disease. Sci. Rep 7. 10.1038/srep39688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dement W, Richardson G, Prinz P, Carskadon M, Kripke D, Czeisler C, In C, 1985. Changes of sleep and wakefulness with age. Schneider Finch E Eds Handb. Biol. Aging Pp N. Y. Van Nostrand Reinhold Co. 692–717 SRC-BaiduScholar. [Google Scholar]
- Di Meco A, Joshi YB, Praticò D, 2014. Sleep deprivation impairs memory, tau metabolism, and synaptic integrity of a mouse model of Alzheimer’s disease with plaques and tangles. Neurobiol. Aging 35, 1813–1820. 10.1016/j.neurobiolaging.2014.02.011 [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Born J, 2010. The memory function of sleep. Nat. Rev. Neurosci 11, 114–126. 10.1038/nrn2762 [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Wilhelm I, Born J, 2009. The whats and whens of sleep-dependent memory consolidation. Sleep Med Rev Oct 13, 309–21. [DOI] [PubMed] [Google Scholar]
- Dijk D-J, 2009. Regulation and Functional Correlates of Slow Wave Sleep. J. Clin. Sleep Med 10. [PMC free article] [PubMed] [Google Scholar]
- Dowling GA, Burr RL, Van Someren EJW, Hubbard EM, Luxenberg JS, Mastick J, Cooper BA, 2008. Melatonin and Bright-Light Treatment for Rest–Activity Disruption in Institutionalized Patients with Alzheimer’s Disease: MELATONIN AND LIGHT THERAPY IN ALZHEIMER’S DISEASE. J. Am. Geriatr. Soc 56, 239–246. 10.1111/j.1532-5415.2007.01543.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling GA, Hubbard EM, Mastick J, Luxenberg JS, Burr RL, Van Someren EJW, 2005. Effect of morning bright light treatment for rest–activity disruption in institutionalized patients with severe Alzheimer’s disease. Int. Psychogeriatr 17, 221–236. 10.1017/S1041610205001584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube J, Lafortune M, Bedetti C, Bouchard M, Gagnon JF, Doyon J, Evans AC, Lina J-M, Carrier J, 2015. Cortical Thinning Explains Changes in Sleep Slow Waves during Adulthood. J. Neurosci 35, 7795–7807. 10.1523/JNEUROSCI.3956-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugger B, Tu M, Murray M, Dickson D, 2011. Disease specificity and pathologic progression of tau pathology in brainstem nuclei of Alzheimer’s disease and progressive supranuclear palsy. Neurosci Lett Mar 17491, 122–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert T, Dorn H, Sauter C, Nitsche MA, Bajbouj M, Danker-Hopfe H, 2013. No Effects of Slow Oscillatory Transcranial Direct Current Stimulation (tDCS) on Sleep-Dependent Memory Consolidation in Healthy Elderly Subjects. Brain Stimulat. 6, 938–945. 10.1016/j.brs.2013.05.006 [DOI] [PubMed] [Google Scholar]
- Esser SK, Hill SL, Tononi G, 2007. Sleep Homeostasis and Cortical Synchronization: I. Modeling the Effects of Synaptic Strength on Sleep Slow Waves. Sleep 30, 1617–1630. 10.1093/sleep/30.12.1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D, Beckett L, Field T, Feng L, Albert M, Bennett D, Tycko B, Apolipoprotein E, Mayeux R, 1997. epsilon4 and incidence of Alzheimer disease in a community population of older persons. JAMA March12277, 822–4. [PubMed] [Google Scholar]
- Everson C, 2014. Cell injury and repair resulting from sleep loss and sleep recovery in laboratory rats. Sleep 37, 1929–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farajnia S, Deboer T, Rohling JHT, Meijer JH, Michel S, 2014. Aging of the Suprachiasmatic Clock. The Neuroscientist 20, 44–55. 10.1177/1073858413498936 [DOI] [PubMed] [Google Scholar]
- Farrer L, Cupples L, Haines J, Hyman B, Kukull W, Mayeux R, Myers R, Pericak-Vance M, Risch N, van Duijn C, 1997. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. Metaanalysis APOE Alzheimer Dis. Meta Anal. Consort. JAMA October134956278, 22–29. [PubMed] [Google Scholar]
- Feinberg I, Koresko RL, Heller N, 1967. EEG sleep patterns as a function of normal and pathological aging in man. J. Psychiatr. Res 5, 107–144. 10.1016/0022-3956(67)90027-1 [DOI] [PubMed] [Google Scholar]
- Ferrazzoli D, Sica F, Sancesario G, 2013. Sundowning syndrome: a possible marker of frailty in Alzheimer’s disease? CNS Neurol. Disord. Drug Targets 12, 525–528. 10.2174/18715273113129990065 [DOI] [PubMed] [Google Scholar]
- Ferri R, Elia M, Musumeci S, Pettinato S, 2000. The time course of high-frequency bands ( Hz) in all-night spectral analysis of sleep EEG. Clin Neurophysiol July125865111, 15–45. [DOI] [PubMed] [Google Scholar]
- Ficca G, Gori S, Ktonas P, Quattrini C, Trammell J, Salzarulo P, 1999. The organization of rapid eye movement activity during rapid eye movement sleep is impaired in the elderly. Neurosci. Lett 275, 219–221. 10.1016/S0304-3940(99)00765-X [DOI] [PubMed] [Google Scholar]
- Fogel S, Vien C, Karni A, Benali H, Carrier J, Doyon J, 2017. Sleep spindles: a physiological marker of age-related changes in gray matter in brain regions supporting motor skill memory consolidation. Neurobiol. Aging 49, 154–164. 10.1016/j.neurobiolaging.2016.10.009 [DOI] [PubMed] [Google Scholar]
- Foley D, Monjan A, Masaki K, Ross W, Havlik R, White L, J, 2001. Daytime sleepiness is associated with 3-year incident dementia and cognitive decline in older Japanese-American men. Geriatr Soc 49SRC-BaiduScholar, 1628–1632. [DOI] [PubMed] [Google Scholar]
- Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG, 1995. Sleep Complaints Among Elderly Persons: An Epidemiologic Study of Three Communities. Sleep 18, 425–432. 10.1093/sleep/18.6.425 [DOI] [PubMed] [Google Scholar]
- Foley DJ, Vitiello MV, Bliwise DL, Ancoli-Israel S, Monjan AA, Walsh JK, 2007. Frequent Napping Is Associated With Excessive Daytime Sleepiness, Depression, Pain, and Nocturia in Older Adults: Findings From the National Sleep Foundation ‘2003 Sleep in America’ Poll. Am. J. Geriatr. Psychiatry 15, 344–350. 10.1097/01.JGP.0000249385.50101.67 [DOI] [PubMed] [Google Scholar]
- Friedman L, Zeitzer J, Lin L, Hoff D, Mignot E, Peskind E, Yesavage J, 2007. In Alzheimer disease, increased wake fragmentation found in those with lower hypocretin-1. Neurol. March668, 793–4. [DOI] [PubMed] [Google Scholar]
- Gabelle A, Jaussent I, Bouallègue FB, Lehmann S, Lopez R, Barateau L, Grasselli C, Pesenti C, Verbizier D. de, Béziat S, Mariano‐Goulart D, Carlander B, Dauvilliers Y, 2019. Reduced brain amyloid burden in elderly patients with narcolepsy type 1. Ann. Neurol 85, 74–83. 10.1002/ana.25373 [DOI] [PubMed] [Google Scholar]
- Girardeau G, Benchenane K, Wiener SI, Buzsáki G, Zugaro MB, 2009. Selective suppression of hippocampal ripples impairs spatial memory. Nat. Neurosci 12, 1222–1223. 10.1038/nn.2384 [DOI] [PubMed] [Google Scholar]
- Gooneratne NS, Vitiello MV, 2014. Sleep in Older Adults. Clin. Geriatr. Med 30, 591–627. 10.1016/j.cger.2014.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgoni M, Lauri G, Truglia I, Cordone S, Sarasso S, Scarpelli S, Mangiaruga A, Atri A, Tempesta D, Ferrara M, Marra C, Rossini P, Gennaro L, 2016. D’ De Parietal Fast Sleep Spindle Density Decrease in Alzheimer’s Disease and Amnesic Mild Cognitive Impairment. Neural Plast 8376108 2016SRC-BaiduScholar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb D, DeStefano A, Foley D, Mignot E, Redline S, Givelber R, Young T, 2004. APOE epsilon4 is associated with obstructive sleep apnea/hypopnea: the Sleep Heart Health Study. Neurol. August2463, 664–8. [DOI] [PubMed] [Google Scholar]
- Gross D, n.d.Correlation of high-frequency oscillations with the sleep-wake cycle and cognitive activity in humans. Neurosci. Pp 94, 1005–1018. [DOI] [PubMed] [Google Scholar]
- Guarnieri B, Adorni F, Musicco M, Appollonio I, Bonanni E, Caffarra P, Caltagirone C, Cerroni G, Concari L, Cosentino F, Ferrara S, Fermi S, Ferri R, Gelosa G, Lombardi G, Mazzei D, Mearelli S, Morrone E, Murri L, Nobili F, Passero S, Perri R, Rocchi R, Sucapane P, Tognoni G, Zabberoni S, Sorbi S, 2012. Prevalence of sleep disturbances in mild cognitive impairment and dementing disorders: a multicenter Italian clinical cross-sectional study on 431 patients. Dement Geriatr Cogn Disord 33, 50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halasz P, 2005. K-complex, a reactive EEG graphoelement of NREM sleep: an old chap in a new garment. Sleep Med Rev 9, 391–412. [DOI] [PubMed] [Google Scholar]
- Hanlon E, Vyazovskiy V, Ugo F, Tononi G, Cirelli C, 2011. Synaptic potentiation and sleep need: clues from molecular and electrophysiological studies. Curr Top Med Chem 11, 2472–2482. [DOI] [PubMed] [Google Scholar]
- Hara R, Kraemer H, Kryla N, Cao C, Miller E, Schatzberg A, Yesavage J, GM, 2005. O’ Schröder CM, Murphy Nocturnal sleep apnea/hypopnea is associated with lower memory performance in APOE epsilon4 carriers. Neurol. August2365, 642–4. [DOI] [PubMed] [Google Scholar]
- Hassainia F, Petit D, Nielsen T, Gauthier S, Montplaisir J, Quantitative E, 1997. and statistical mapping of wakefulness and REMsleep in the evaluation of mild to moderate Alzheimer’s disease. Eur Neurol 37, 219–224. [DOI] [PubMed] [Google Scholar]
- Hatfield CF, Herbert J, van Someren EJW, Hodges JR, Hastings MH, 2004. Disrupted daily activity/rest cycles in relation to daily cortisol rhythms of home-dwelling patients with early Alzheimer’s dementia. Brain J. Neurol 127, 1061–1074. 10.1093/brain/awh129 [DOI] [PubMed] [Google Scholar]
- Hita-Yañez E, Atienza M, Cantero JL, 2013. Polysomnographic and Subjective Sleep Markers of Mild Cognitive Impairment. Sleep 36, 1327–1334. 10.5665/sleep.2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch C, Kupfer D, Houck P, Berman S, Stack J, J, 1986. Reynolds CF 3rd, Sleep-disordered breathing in normal and pathologic aging. Psychiatry 499e503.47SRC-BaiduScholar. [PubMed] [Google Scholar]
- Holth JK, Patel TK, Holtzman DM, 2017. Sleep in Alzheimer’s Disease–Beyond Amyloid. Neurobiol. Sleep Circadian Rhythms 2, 4–14. 10.1016/j.nbscr.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hot P, Rauchs G, Bertran F, Denise P, Desgranges B, Clochon P, Eustache F, 2011. Changes in sleep theta rhythm are related to episodic memory impairment in early Alzheimer’s disease. Biol Psychol Jul 87, 334–9. [DOI] [PubMed] [Google Scholar]
- Huang C, Wahlund L, Dierks T, Julin P, Winblad B, Jelic V, 2000. Discrimination of Alzheimer’s disease and mild cognitive impairment by equivalent EEG sources: a cross-sectional and longitudinal study. Clin Neurophysiol 111, 1961–1967. [DOI] [PubMed] [Google Scholar]
- Huang Y-L, Liu R-Y, Wang Q-S, Van Someren EJW, Xu H, Zhou J-N, 2002. Age-associated difference in circadian sleep–wake and rest–activity rhythms. Physiol. Behav 76, 597–603. 10.1016/S0031-9384(02)00733-3 [DOI] [PubMed] [Google Scholar]
- Huang Z, Tan T, Du Y, Chen L, Fu M, Yu Y, Zhang L, Song W, Dong Z, 2017. Low-Frequency Repetitive Transcranial Magnetic Stimulation Ameliorates Cognitive Function and Synaptic Plasticity in APP23/PS45 Mouse Model of Alzheimer’s Disease. Front. Aging Neurosci 9. 10.3389/fnagi.2017.00292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R, Esser SK, Ferrarelli F, Massimini M, Peterson MJ, Tononi G, 2007. TMS-Induced Cortical Potentiation during Wakefulness Locally Increases Slow Wave Activity during Sleep. PLoS ONE 2. 10.1371/journal.pone.0000276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes ATL, Piggins HD, 2012. Feedback actions of locomotor activity to the circadian clock, in: Progress in Brain Research. Elsevier, pp. 305–336. 10.1016/B978-0-444-59427-3.00018-6 [DOI] [PubMed] [Google Scholar]
- Hunt NJ, Rodriguez ML, Waters KA, Machaalani R, 2015. Changes in orexin (hypocretin) neuronal expression with normal aging in the human hypothalamus. Neurobiol. Aging 36, 292–300. 10.1016/j.neurobiolaging.2014.08.010 [DOI] [PubMed] [Google Scholar]
- Hwang JY, Byun MS, Choe YM, Lee JH, Yi D, Choi J-W, Hwang SH, Lee YJ, Lee DY, KBASE Research Group, 2018. Moderating effect of APOE ε4 on the relationship between sleep-wake cycle and brain β-amyloid. Neurology 90, e1167–e1173. 10.1212/WNL.0000000000005193 [DOI] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M, 2012. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med 4, 147ra111. 10.1126/scitranslmed.3003748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iriarte IG, George MS, 2018. Transcranial Magnetic Stimulation (TMS) in the Elderly. Curr. Psychiatry Rep 20, 6. 10.1007/s11920-018-0866-2 [DOI] [PubMed] [Google Scholar]
- Jaffer KY, Chang T, Vanle B, Dang J, Steiner AJ, Loera N, Abdelmesseh M, Danovitch I, Ishak WW, 2017. Trazodone for Insomnia: A Systematic Review. Innov. Clin. Neurosci 14, 24–34. [PMC free article] [PubMed] [Google Scholar]
- Jessen NA, Munk ASF, Lundgaard I, Nedergaard M, 2015. The Glymphatic System: A Beginner’s Guide. Neurochem. Res 40, 2583–2599. 10.1007/s11064-015-1581-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AP, Choe J, Bryant NB, Robinson CSH, Ketz NA, Skorheim SW, Combs A, Lamphere ML, Robert B, Gill HA, Heinrich MD, Howard MD, Clark VP, Pilly PK, 2018a. Dose-Dependent Effects of Closed-Loop tACS Delivered During Slow-Wave Oscillations on Memory Consolidation. Front. Neurosci 12. 10.3389/fnins.2018.00867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AP, Choe J, Bryant NB, Robinson CSH, Ketz NA, Skorheim SW, Combs A, Lamphere ML, Robert B, Gill HA, Heinrich MD, Howard MD, Clark VP, Pilly PK, 2018b. Dose-Dependent Effects of Closed-Loop tACS Delivered During Slow-Wave Oscillations on Memory Consolidation. Front. Neurosci 12. 10.3389/fnins.2018.00867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju Y, Ooms S, Sutphen C, 2017. ES, . Slow wave sleep disruption increases cerebrospinal fluid amyloid-β levels. Brain, . 140SRC-BaiduScholar, 2104–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju YS, Ooms S, Sutphen C, Macauley S, Zangrilli M, Jerome G, Fagan A, Mignot E, Zempel J, Claassen J, Holtzman D, 2017. Slow wave sleep disruption increases cerebrospinal fluid amyloid-β levels. Brain. August1;:. 140, 2104–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadotani H, Kadotani T, Young T, Peppard P, Finn L, Colrain I, GM, Mignot E, 2001. Murphy Association between apolipoprotein E epsilon4 and sleep-disordered breathing in adults. JAMA June13285, 2888–90. [DOI] [PubMed] [Google Scholar]
- Kakimoto A, Ito S, Okada H, Nishizawa S, Minoshima S, Ouchi Y, 2016. Age-Related Sex-Specific Changes in Brain Metabolism and Morphology. J. Nucl. Med 57, 221–225. 10.2967/jnumed.115.166439 [DOI] [PubMed] [Google Scholar]
- Kaushal N, Ramesh V, Gozal D, J, 2012. Human apolipoprotein E4 targeted replacement in mice reveals increased susceptibility to sleep disruption and intermittent hypoxia. Am Regul Integr Comp Physiol July1303, R19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay DB, Tanner JJ, Bowers D, 2018. Sleep disturbances and depression severity in patients with Parkinson’s disease. Brain Behav. 8, e00967. 10.1002/brb3.967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger E, Anderson A, Growdon J, Corkin S, 2004. Effects of Alzheimer disease on memory for verbal emotional information. Neuropsychologia 42, 791–800. [DOI] [PubMed] [Google Scholar]
- Kerbler G, Fripp J, Rowe C, Villemagne V, Salvado O, Rose S, Coulson E, 2014. Alzheimer’s Disease Neuroimaging Initiative. Basal Forebrain Atrophy Correl. Amyloid Burd. Alzheimers Dis. Neuroimage Clin November277SRC-BaiduScholar, 105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern W, Dodt C, Born J, Fehm HL, 1996. Changes in Cortisol and Growth Hormone Secretion During Nocturnal Sleep in the Course of Aging. J. Gerontol. A. Biol. Sci. Med. Sci 51A, M3–M9. 10.1093/gerona/51A.1.M3 [DOI] [PubMed] [Google Scholar]
- Ketz N, Jones AP, Bryant NB, Clark VP, Pilly PK, 2018a. Closed-Loop Slow-Wave tACS Improves Sleep-Dependent Long-Term Memory Generalization by Modulating Endogenous Oscillations. J. Neurosci 38, 7314–7326. 10.1523/JNEUROSCI.0273-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketz N, Jones AP, Bryant NB, Clark VP, Pilly PK, 2018b. Closed-Loop Slow-Wave tACS Improves Sleep-Dependent Long-Term Memory Generalization by Modulating Endogenous Oscillations. J. Neurosci 38, 7314–7326. 10.1523/JNEUROSCI.0273-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketz N, Jones AP, Bryant NB, Clark VP, Pilly PK, 2018c. Closed-Loop Slow-Wave tACS Improves Sleep-Dependent Long-Term Memory Generalization by Modulating Endogenous Oscillations. J. Neurosci 38, 7314–7326. 10.1523/JNEUROSCI.0273-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Ponzo V, Di Lorenzo F, Caltagirone C, Veniero D, 2013. Hebbian and anti-Hebbian spike-timing-dependent plasticity of human cortico-cortical connections. J. Neurosci 33, 9725–9733. 10.1523/JNEUROSCI.4988-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki S, Scheuler W, Jobert M, Pastelak-Price C, G Z, 1989. The effect of age on sleep spindle and K complex density. EEG EMElektromyogr Verwandte Geb 20SRC-BaiduScholar, 59–63. [PubMed] [Google Scholar]
- Kupfer D, Taska L, Hoch C, Sewitch D, Restifo K, J, 1985. Reynolds CF 3rd, Sleep apnea in Alzheimer’s dementia: correlation with mental deterioration. Psychiatry 257e61.46SRC-BaiduScholar. [PubMed] [Google Scholar]
- Ladenbauer J, Külzow N, Passmann S, Antonenko D, Grittner U, Tamm S, Flöel A, 2016. Brain stimulation during an afternoon nap boosts slow oscillatory activity and memory consolidation in older adults. NeuroImage 142, 311–323. 10.1016/j.neuroimage.2016.06.057 [DOI] [PubMed] [Google Scholar]
- Ladenbauer Julia, Ladenbauer Josef, Külzow N, de Boor R, Avramova E, Grittner U, Flöel A, 2017. Promoting Sleep Oscillations and Their Functional Coupling by Transcranial Stimulation Enhances Memory Consolidation in Mild Cognitive Impairment. J. Neurosci 37, 7111–7124. 10.1523/JNEUROSCI.0260-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolt H-P, Dijk D-J, Achermann P, Borbély AA, 1996. Effect of age on the sleep EEG: slow-wave activity and spindle frequency activity in young and middle-aged men. Brain Res. 738, 205–212. 10.1016/S0006-8993(96)00770-6 [DOI] [PubMed] [Google Scholar]
- Landry G, Liu-Ambrose T, 2014. Buying time: a rationale for examining the use of circadian rhythm and sleep interventions to delay progression of mild cognitive impairment to Alzheimer’s disease. Front Aging Neurosci 3256.SRC-BaiduScholar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen L, Moe K, Vitiello M, Prinz P, J, 1995. Age trends in the sleep EEG of healthy older men and women. Res 4SRC-BaiduScholar, 160–72. [DOI] [PubMed] [Google Scholar]
- Lauer CJ, Riemann D, Wiegand M, Berger M, 1991. From early to late adulthood changes in EEG sleep of depressed patients and healthy volunteers. Biol. Psychiatry 29, 979–993. 10.1016/0006-3223(91)90355-P [DOI] [PubMed] [Google Scholar]
- Leminen MM, Virkkala J, Saure E, Paajanen T, Zee PC, Santostasi G, Hublin C, Müller K, Porkka-Heiskanen T, Huotilainen M, Paunio T, 2017. Enhanced Memory Consolidation Via Automatic Sound Stimulation During Non-REM Sleep. Sleep 40. 10.1093/sleep/zsx003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Wang Y, Wang M, Liu H, 2008. Effects of sleep deprivation on gamma oscillation of waking human EEG. Nat Scimater 18December10121533–1537SRC-BaiduScholar. [Google Scholar]
- Liguori C, Romigi A, Nuccetelli M, Zannino S, Sancesario G, Martorana A, Albanese M, Mercuri N, Izzi F, Bernardini S, Nitti A, Sancesario GM, Sica F, Marciani M, Placidi F, JAMA, 2014. Orexinergic system dysregulation, sleep impairment, and cognitive decline in Alzheimer disease. December71, 1498–505. [DOI] [PubMed] [Google Scholar]
- Lim ASP, Ellison BA, Wang JL, Yu L, Schneider JA, Buchman AS, Bennett DA, Saper CB, 2014. Sleep is related to neuron numbers in the ventrolateral preoptic/intermediate nucleus in older adults with and without Alzheimer’s disease. Brain 137, 2847–2861. 10.1093/brain/awu222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim ASP, Kowgier M, Yu L, Buchman AS, Bennett DA, 2013a. Sleep Fragmentation and the Risk of Incident Alzheimer’s Disease and Cognitive Decline in Older Persons. Sleep 36, 1027–1032. 10.5665/sleep.2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim ASP, Yu L, Kowgier M, Schneider JA, Buchman AS, Bennett DA, 2013b. Modification of the Relationship of the Apolipoprotein E ε4 Allele to the Risk of Alzheimer Disease and Neurofibrillary Tangle Density by Sleep. JAMA Neurol. 70, 1544. 10.1001/jamaneurol.2013.4215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Buzsáki G, 2008. A neural coding scheme formed by the combined function of gamma and theta oscillations. Schizophr. Bull 34, 974–980. 10.1093/schbul/sbn060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Jensen O, 2013. The θ-γ neural code. Neuron 77, 1002–1016. 10.1016/j.neuron.2013.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Zhou J, van Heerikhuize J, Hofman M, Swaab D, J, 1999. Decreased melatonin levels in post mortem cerebrospinal fluid in relation to aging, Alzheimer’s disease, and apolipoprotein E-epsilon4/4 genotype. Endocrinol Metab 323e7.84SRC-BaiduScholar. [DOI] [PubMed] [Google Scholar]
- Loewenstein R, Weingartner H, Gillin J, Kaye W, Ebert M, Mendelson W, 1982. Disturbances of sleep and cognitive functioning in patients with dementia. Neurobiol Aging 3SRC-BaiduScholar, 371–377. [DOI] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J, 2015. Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341. 10.1038/nature14432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucey B, Hicks T, McLeland J, Toedebusch C, Boyd J, Elbert D, Patterson B, Baty J, Morris J, Ovod V, Mawuenyega K, Bateman R, 2017. Effect of sleep on overnight CSF amyloid-β kinetics. Annals of Neurology : . 83, 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackiewicz M, Nikonova E, Zimmermann J, Romer M, Cater J, Galante R, Pack A, 2006. Age-related changes in adenosine metabolic enzymes in sleep/wake regulatory areas of the brain. Neurobiol. Aging 27, 351–360. 10.1016/j.neurobiolaging.2005.01.015 [DOI] [PubMed] [Google Scholar]
- Malkani RG, Zee PC, 2020. Brain Stimulation for Improving Sleep and Memory. Sleep Med. Clin 15, 101–115. 10.1016/j.jsmc.2019.11.002 [DOI] [PubMed] [Google Scholar]
- Mander B, Marks S, Vogel J, Rao V, Lu B, Saletin J, Ancoli-Israel S, Jagust W, Walker M, 2015. amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat Neurosci July18, 1051–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander BA, Rao V, Lu B, Saletin JM, Lindquist JR, Ancoli-Israel S, Jagust W, Walker MP, 2013. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat. Neurosci 16, 357–364. 10.1038/nn.3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander BA, Winer JR, Jagust WJ, Walker MP, 2016. Sleep: A Novel Mechanistic Pathway, Biomarker, and Treatment Target in the Pathology of Alzheimer’s Disease? Trends Neurosci. 39, 552–566. 10.1016/j.tins.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mander BA, Winer JR, Walker MP, 2017. Sleep and Human Aging. Neuron 94, 19–36. 10.1016/j.neuron.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B, 2003. Marked loss of myelinated nerve fibers in the human brain with age. J. Comp. Neurol 462, 144–152. 10.1002/cne.10714 [DOI] [PubMed] [Google Scholar]
- Marshall L, Born J, 2011. Brain Stimulation During Sleep. Sleep Med. Clin 6, 85–95. 10.1016/j.jsmc.2010.12.003 [DOI] [Google Scholar]
- Marshall L, Helgadóttir H, Mölle M, Born J, 2006. Boosting slow oscillations during sleep potentiates memory. Nature 444, 610–613. 10.1038/nature05278 [DOI] [PubMed] [Google Scholar]
- Martin N, Lafortune M, Godbout J, Barakat M, Robillard R, Poirier G, Bastien C, Carrier J, 2013. Topography of age-related changes in sleep spindles. Neurobiol. Aging 34, 468–476. 10.1016/j.neurobiolaging.2012.05.020 [DOI] [PubMed] [Google Scholar]
- Massimini M, Ferrarelli F, Esser SK, Riedner BA, Huber R, Murphy M, Peterson MJ, Tononi G, 2007. Triggering sleep slow waves by transcranial magnetic stimulation. Proc. Natl. Acad. Sci 104, 8496–8501. 10.1073/pnas.0702495104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattis J, Sehgal A, 2016. Circadian Rhythms, Sleep, and Disorders of Aging. Trends Endocrinol. Metab 27, 192–203. 10.1016/j.tem.2016.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson N, Zetterberg H, Hansson O, Andreasen N, Parnetti L, Jonsson M, Herukka S-K, van der Flier WM, Blankenstein MA, Ewers M, Rich K, Kaiser E, Verbeek M, Tsolaki M, Mulugeta E, Rosén E, Aarsland D, Visser PJ, Schröder J, Marcusson J, de Leon M, Hampel H, Scheltens P, Pirttilä T, Wallin A, Jönhagen ME, Minthon L, Winblad B, Blennow K, 2009. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA 302, 385–393. 10.1001/jama.2009.1064 [DOI] [PubMed] [Google Scholar]
- McCleery J, Cohen DA, Sharpley A, Menkes-Caspi N, Yamin H, Kellner V, Spires-Jones T, Cohen D, Stern E, 2015. Pharmacotherapies for sleep disturbances in Alzheimer’s disease. Cochrane Database Syst Rev Mar 213 Pathol. Tau Disrupts Ongoing Netw. Act. Neuron March485, 959–66. [Google Scholar]
- McCurry SM, Reynolds CF, Ancoli-Israel S, Teri L, Vitiello MV, 2000. Treatment of sleep disturbance in Alzheimer’s disease. Sleep Med. Rev 4, 603–628. 10.1053/smrv.2000.0127 [DOI] [PubMed] [Google Scholar]
- Menkes-Caspi N, Yamin HG, Kellner V, Spires-Jones TL, Cohen D, Stern EA, 2015. Pathological tau disrupts ongoing network activity. Neuron 85, 959–966. 10.1016/j.neuron.2015.01.025 [DOI] [PubMed] [Google Scholar]
- Mesulam M, Shaw P, Mash D, Weintraub S, 2004. Cholinergic nucleus basalis tauopathy emerges early in the aging-MCI-AD continuum. Ann Neurol June55, 815–28. [DOI] [PubMed] [Google Scholar]
- Mishima K, Okawa M, Shimizu T, J, 2001. Diminished melatonin secretion in the elderly caused by insufficient environmental illumination. Endocrinol Metab 12986.SRC-BaiduScholar. [DOI] [PubMed] [Google Scholar]
- Monk TH, 2005. Aging Human Circadian Rhythms: Conventional Wisdom May Not Always Be Right. J. Biol. Rhythms 20, 366–374. 10.1177/0748730405277378 [DOI] [PubMed] [Google Scholar]
- Montgomery DC, Woodall WH, 2008. An Overview of Six Sigma. Int. Stat. Rev 76, 329–346. 10.1111/j.1751-5823.2008.00061.x [DOI] [Google Scholar]
- Montgomery P, Dennis J, 2004. A systematic review of non-pharmacological therapies for sleep problems in later life. Sleep Med Rev February8, 47–62. [DOI] [PubMed] [Google Scholar]
- Montplaisir J, Petit D, Gauthier S, Gaudreau H, 1998. Décary A. Sleep Disturb. Eeg Slowing Alzheimers Dis. Sleep Res Online 1, 147–51. [PubMed] [Google Scholar]
- Montplaisir J, Petit D, Lorrain D, Gauthier S, Nielsen T, 1995. Sleep in Alzheimer’s disease: further considerations on the role of brainstem and forebrain cholinergic populations in sleep-wake mechanisms. Sleep April18, 145–8. [DOI] [PubMed] [Google Scholar]
- Mormino EC, Smiljic A, Hayenga AO, Onami SH, Greicius MD, Rabinovici GD, Janabi M, Baker SL, Yen IV, Madison CM, Miller BL, Jagust WJ, 2011. Relationships between β-amyloid and functional connectivity in different components of the default mode network in aging. Cereb. Cortex N. Y. N 1991 21, 2399–2407. 10.1093/cercor/bhr025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münch M, Knoblauch V, Blatter K, Schröder C, Schnitzler C, Kräuchi K, Wirz-Justice A, Cajochen C, 2005. Age-related attenuation of the evening circadian arousal signal in humans. Neurobiol. Aging 26, 1307–1319. 10.1016/j.neurobiolaging.2005.03.004 [DOI] [PubMed] [Google Scholar]
- Nardone R, Sebastianelli L, Versace V, Brigo F, Golaszewski S, Pucks-Faes E, Saltuari L, Trinka E, 2020. Effects of repetitive transcranial magnetic stimulation in subjects with sleep disorders. Sleep Med. 10.1016/j.sleep.2020.01.028 [DOI] [PubMed] [Google Scholar]
- Ngo H-VV, Martinetz T, Born J, Mölle M, 2013. Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron 78, 545–553. 10.1016/j.neuron.2013.03.006 [DOI] [PubMed] [Google Scholar]
- Ngo H-VV, Miedema A, Faude I, Martinetz T, Mölle M, Born J, 2015. Driving sleep slow oscillations by auditory closed-loop stimulation-a self-limiting process. J. Neurosci. Off. J. Soc. Neurosci 35, 6630–6638. 10.1523/JNEUROSCI.3133-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon M, Vecchierini M, 2005. Normative sleep data, cognitive function and daily living activities in older adults in the community. Sleep 28, 981–989. [PubMed] [Google Scholar]
- Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV, 2004a. Meta-Analysis of Quantitative Sleep Parameters From Childhood to Old Age in Healthy Individuals: Developing Normative Sleep Values Across the Human Lifespan. Sleep 27, 1255–1273. 10.1093/sleep/27.7.1255 [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV, 2004b. Meta-Analysis of Quantitative Sleep Parameters From Childhood to Old Age in Healthy Individuals: Developing Normative Sleep Values Across the Human Lifespan. Sleep 27, 1255–1273. 10.1093/sleep/27.7.1255 [DOI] [PubMed] [Google Scholar]
- Ong JL, Lo JC, Chee NIYN, Santostasi G, Paller KA, Zee PC, Chee MWL, 2016. Effects of phase-locked acoustic stimulation during a nap on EEG spectra and declarative memory consolidation. Sleep Med. 20, 88–97. 10.1016/j.sleep.2015.10.016 [DOI] [PubMed] [Google Scholar]
- Osorio R, Gumb T, Pirraglia E, Varga A, Lu S, Lim J, Wohlleber M, Ducca E, Koushyk V, Glodzik L, Mosconi L, Ayappa I, Rapoport D, de Leon M, 2015. Sleep-disordered breathing advances cognitive decline in the elderly. Neurol May1284, 1964–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandi-Perumal SR, Zisapel N, Srinivasan V, Cardinali DP, 2005. Melatonin and sleep in aging population. Exp. Gerontol 40, 911–925. 10.1016/j.exger.2005.08.009 [DOI] [PubMed] [Google Scholar]
- Papalambros NA, Santostasi G, Malkani RG, Braun R, Weintraub S, Paller KA, Zee PC, 2017. Acoustic Enhancement of Sleep Slow Oscillations and Concomitant Memory Improvement in Older Adults. Front. Hum. Neurosci 11. 10.3389/fnhum.2017.00109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pase M, Himali J, Grima N, Beiser A, Satizabal C, Aparicio H, Thomas R, Gottlieb D, Auerbach S, Seshadri S, 2017. Sleep architecture and the risk of incident dementia in the community. Neurology 89, 1244–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paßmann S, Külzow N, Ladenbauer J, Antonenko D, Grittner U, Tamm S, Flöel A, 2016a. Boosting Slow Oscillatory Activity Using tDCS during Early Nocturnal Slow Wave Sleep Does Not Improve Memory Consolidation in Healthy Older Adults. Brain Stimulat. 9, 730–739. 10.1016/j.brs.2016.04.016 [DOI] [PubMed] [Google Scholar]
- Paßmann S, Külzow N, Ladenbauer J, Antonenko D, Grittner U, Tamm S, Flöel A, 2016b. Boosting Slow Oscillatory Activity Using tDCS during Early Nocturnal Slow Wave Sleep Does Not Improve Memory Consolidation in Healthy Older Adults. Brain Stimulat. 9, 730–739. 10.1016/j.brs.2016.04.016 [DOI] [PubMed] [Google Scholar]
- Paulus W, 2011. Transcranial electrical stimulation (tES-tDCS; tRNS, tACS) methods. Neuropsychol. Rehabil . 21 SRC-BaiduScholar, 602–617. [DOI] [PubMed] [Google Scholar]
- Peter-Derex L, Yammine P, Bastuji H, Croisile B, 2015. Sleep and Alzheimer’s disease. Sleep Med. Rev 19, 29–38. 10.1016/j.smrv.2014.03.007 [DOI] [PubMed] [Google Scholar]
- Petit D, Gagnon J-F, Fantini ML, Ferini-Strambi L, Montplaisir J, 2004. Sleep and quantitative EEG in neurodegenerative disorders. J. Psychosom. Res 56, 487–496. 10.1016/j.jpsychores.2004.02.001 [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO, 1994. A Quantitative Magnetic Resonance Imaging Study of Changes in Brain Morphology From Infancy to Late Adulthood. Arch. Neurol 51, 874–887. 10.1001/archneur.1994.00540210046012 [DOI] [PubMed] [Google Scholar]
- Piantoni G, Poil S-S, Linkenkaer-Hansen K, Verweij IM, Ramautar JR, Van Someren EJW, Van Der Werf YD, 2013. Individual Differences in White Matter Diffusion Affect Sleep Oscillations. J. Neurosci 33, 227–233. 10.1523/JNEUROSCI.2030-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier J, Davignon J, Bouthillier D, Kogan S, Bertrand P, Apolipoprotein E, Gauthier S, 1993. polymorphism and Alzheimer’s disease. Lancet September18342, 697–699. [DOI] [PubMed] [Google Scholar]
- Popp J, Wolfsgruber S, Heuser I, Peters O, Schneider A, Jahn H, Luckhaus C, Perneczky R, Wagner M, Maier W, Wiltfang J, Kornhuber J, Jessen F, 2015. Hüll M, Schröder J, Möller HJ, Lewczuk P7, Frölich L, Cerebrospinal fluid cortisol and clinical disease progression in MCI and dementia of Alzheimer’s type. Neurobiol Aging February36, 601–7. [DOI] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, 1997. Adenosine: A Mediator of the Sleep-Inducing Effects of Prolonged Wakefulness. Science 276, 1265–1268. 10.1126/science.276.5316.1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz PN, Vitaliano PP, Vitiello MV, Bokan J, Raskind M, Peskind E, Gerber C, 1982. Sleep, EEG and mental function changes in senile dementia of the Alzheimer’s type. Neurobiol. Aging 3, 361–370. 10.1016/0197-4580(82)90024-0 [DOI] [PubMed] [Google Scholar]
- Rajaratnam S, Middleton B, Stone B, Arendt J, Dijk D, 2004. Melatonin advances the circadian timing of EEG sleep and directly facilitates sleep without altering its duration in extended sleep opportunities in humans. J Physiol 561, 339–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauchs G, Schabus M, Parapatics S, Bertran F, Clochon P, Hot P, Denise P, Desgranges B, Eustache F, Gruber G, Anderer P, 2008. Is there a link between sleep changes and memory in Alzheimer’s disease? Neuroreport. July16;:. 19, 1159–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redline S, Kirchner HL, Quan SF, Gottlieb DJ, Kapur V, Newman A, 2004a. The Effects of Age, Sex, Ethnicity, and Sleep-Disordered Breathing on Sleep Architecture. Arch. Intern. Med 164, 406. 10.1001/archinte.164.4.406 [DOI] [PubMed] [Google Scholar]
- Redline S, Kirchner HL, Quan SF, Gottlieb DJ, Kapur V, Newman A, 2004b. The Effects of Age, Sex, Ethnicity, and Sleep-Disordered Breathing on Sleep Architecture. Arch. Intern. Med 164, 406. 10.1001/archinte.164.4.406 [DOI] [PubMed] [Google Scholar]
- Regestein Q, Nowell P, Neylan T, Salzman C, Baltimore M, 1998. Reynolds CF 3rd, Treatment of insomnia in the elderly. [Google Scholar]
- Replay of cortical spiking sequences during human memory retrieval | Science [WWW Document], n.d. URL https://science.sciencemag.org/content/367/6482/1131.editor-summary (accessed 3.26.20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C, 2003. Longitudinal Magnetic Resonance Imaging Studies of Older Adults: A Shrinking Brain. J. Neurosci 23, 3295–3301. 10.1523/JNEUROSCI.23-08-03295.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds C, Buysse D, Kupfer D, Hoch C, Houck P, Matzzie J, George C, 1990. rd, Rapid eye movement sleep deprivation as a probe in elderly subjects. Arch Gen Psychiatry December47, 1128–36. [DOI] [PubMed] [Google Scholar]
- Roberts R, Knopman DS, 2013. Classification and epidemiology of MCI. Clin. Geriatr. Med 29, 753–772. 10.1016/j.cger.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson C, Bryant N, Maxwell J, Jones A, Robert B, Lamphere M, Combs A, Al Azzawi H, Gibson B, Sanguinetti J, Ketz N, Pilly P, Clark V, 2018. The Benefits of Closed-Loop Transcranial Alternating Current Stimulation on Subjective Sleep Quality. Brain Sci. 8, 204. 10.3390/brainsci8120204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca P, Marino F, Montemagni C, Perrone D, Bogetto F, 2007. Risperidone, olanzapine and quetiapine in the treatment of behavioral and psychological symptoms in patients with Alzheimer’s disease: Preliminary findings from a naturalistic, retrospective study: SGA and behavioral disturbances in AD. Psychiatry Clin. Neurosci 61, 622–629. 10.1111/j.1440-1819.2007.01729.x [DOI] [PubMed] [Google Scholar]
- Rogers SL, 1998. Donepezil Improves Cognition and Global Function in Alzheimer DiseaseA 15-Week, Double-blind, Placebo-Controlled Study. Arch. Intern. Med 158, 1021. 10.1001/archinte.158.9.1021 [DOI] [PubMed] [Google Scholar]
- Rolls A, 2012. Hypothalamic Control of Sleep in Aging. NeuroMolecular Med. 14, 139–153. 10.1007/s12017-012-8175-0 [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A, Safety of TMS Consensus Group, 2009. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol 120, 2008–2039. 10.1016/j.clinph.2009.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman S, Herdener N, Frankola K, Mughal M, Mattson M, 2013. Chronic mild sleep restriction accentuates contextual memory impairments, and accumulations of cortical Aβ and pTau in a mouse model of Alzheimer’s disease. Brain Res September51529SRC-BaiduScholar, 200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santostasi G, Malkani R, Riedner B, Bellesi M, Tononi G, Paller KA, Zee PC, 2016. Phase-locked loop for precisely timed acoustic stimulation during sleep. J. Neurosci. Methods 259, 101–114. 10.1016/j.jneumeth.2015.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper C, Scammell T, Lu J, 2005. Hypothalamic regulation of sleep and circadian rhythms. Nat October27437, 1257–63. [DOI] [PubMed] [Google Scholar]
- Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE, 2010. Sleep State Switching. Neuron 68, 1023–1042. 10.1016/j.neuron.2010.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Tohyama K, Kanbara Y, Otsuka K, Ehara S, Sakai A, 2006. Shibata E1, Age-related changes in locus ceruleus on neuromelanin magnetic resonance imaging at 3 Tesla. Magn Reson Med Sci December5, 197–200. [DOI] [PubMed] [Google Scholar]
- Satlin A, Volicer L, Ross V, Herz L, Campbell S, 1992. Bright light treatment of behavioral and sleep disturbances in patients with Alzheimer’s disease. Am. J. Psychiatry 149SRC-BaiduScholar, 1028–1032. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Heise KF, Gruber WR, Holz E, Karim AA, Glennon M, Gerloff C, Birbaumer N, Hummel FC, 2009. Brain oscillatory substrates of visual short-term memory capacity. Curr. Biol. CB 19, 1846–1852. 10.1016/j.cub.2009.08.062 [DOI] [PubMed] [Google Scholar]
- Scheffzük C, Kukushka VI, Vyssotski AL, Draguhn A, Tort ABL, Brankačk J, 2011. Selective Coupling between Theta Phase and Neocortical Fast Gamma Oscillations during REM-Sleep in Mice. PLOS ONE 6, e28489. 10.1371/journal.pone.0028489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JFA, Åkerstedt T, Lindberg E, Gruber G, Fischer H, Theorell-Haglöw J, 2017. Age affects sleep microstructure more than sleep macrostructure. J. Sleep Res 26, 277–287. 10.1111/jsr.12478 [DOI] [PubMed] [Google Scholar]
- Sepulcre J, Sabuncu M, Becker A, Sperling R, Johnson K, 2013. In vivo characterization of the early stages of the amyloid-beta network. Brain Jul136Pt72239–52SRC-BaiduScholar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sforza E, Roche F, 2012. Sleep apnea syndrome and cognition. Front Neurol 873.SRC-BaiduScholar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinforiani E, Terzaghi M, Pasotti C, Zucchella C, Zambrelli E, Manni R, 2007. Hallucinations and sleep-wake cycle in Alzheimer’s disease: a questionnaire-based study in 218 patients. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol 28, 96–99. 10.1007/s10072-007-0794-0 [DOI] [PubMed] [Google Scholar]
- Singer C, 2003. A multicenter, placebo-controlled trial of melatonin for sleep disturbance in Alzheimer’s disease. Sleep 26, 893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Toga AW, 2004. Mapping Changes in the Human Cortex throughout the Span of Life. The Neuroscientist 10, 372–392. 10.1177/1073858404263960 [DOI] [PubMed] [Google Scholar]
- Spira A, Blackwell T, Stone K, Redline S, Cauley J, Ancoli-Israel S, Yaffe K, J, 2008. Sleep-disordered breathing and cognition in older women. Geriatr Soc January56, 45–50. [DOI] [PubMed] [Google Scholar]
- Spira A, Gamaldo A, An Y, Wu M, Simonsick E, Bilgel M, Zhou Y, Wong D, Ferrucci L, Resnick S, JAMA, 2013. Self-reported sleep and β-amyloid deposition in community-dwelling older adults. December70, 1537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprecher K, Bendlin B, Racine A, Okonkwo O, Christian B, Koscik R, Sager M, Asthana S, Johnson S, Benca R, 2015. Amyloid burden is associated with self-reported sleep in nondemented late middle-aged adults. Neurobiol Aging September36, 2568–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprecher KE, Riedner BA, Smith RF, Tononi G, Davidson RJ, Benca RM, 2016a. High Resolution Topography of Age-Related Changes in Non-Rapid Eye Movement Sleep Electroencephalography. PLOS ONE 11, e0149770. 10.1371/journal.pone.0149770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprecher KE, Riedner BA, Smith RF, Tononi G, Davidson RJ, Benca RM, 2016b. High Resolution Topography of Age-Related Changes in Non-Rapid Eye Movement Sleep Electroencephalography. PLOS ONE 11, e0149770. 10.1371/journal.pone.0149770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm JM, Bourlas AP, Baugh CM, Fritts NG, Daneshvar DH, Martin BM, McClean MD, Tripodis Y, Stern RA, 2015. Age of first exposure to football and later-life cognitive impairment in former NFL players. Neurology 84, 1114–1120. 10.1212/WNL.0000000000001358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopa EG, Volicer L, Kuo-Leblanc V, Harper D, Lathi D, Tate B, Satlin A, 1999. Pathologic evaluation of the human suprachiasmatic nucleus in severe dementia. J. Neuropathol. Exp. Neurol 58, 29–39. [DOI] [PubMed] [Google Scholar]
- Stratmann K, Heinsen H, Korf H, Turco D, Ghebremedhin E, Seidel K, Bouzrou M, Grinberg L, Bohl J, Wharton S, Dunnen W, 2016. Del den Rüb U. Precortical Phase Alzheimers Dis. ADRelated Tau Cytoskelet. Pathol. Brain Pathol May26, 371–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunde D, Goldensohn E, The E, 1976. Hubbard 0, in centenarians. Electroencephalogr Clin NeurophysioI 40SRC-BaiduScholar, 407–17. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Fliers E, Partiman TS, 1985. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. 342, 37–44. 10.1016/0006-8993(85)91350-2 [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Mima T, Murai R, Shimazu H, Isomura Y, Tsujimoto T, 2015. Gamma Oscillations and Their Cross-frequency Coupling in the Primate Hippocampus during Sleep. Sleep 38, 1085–1091. 10.5665/sleep.4818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatti E, Rossi S, Innocenti I, Rossi A, Santarnecchi E, 2016. Non-invasive brain stimulation of the aging brain: State of the art and future perspectives. Ageing Res. Rev 29, 66–89. 10.1016/j.arr.2016.05.006 [DOI] [PubMed] [Google Scholar]
- Tavakoli AV, Yun K, 2017. Transcranial Alternating Current Stimulation (tACS) Mechanisms and Protocols. Front. Cell. Neurosci 11. 10.3389/fncel.2017.00214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thambisetty M, Wan J, Carass A, An Y, Prince JL, Resnick SM, 2010. Longitudinal changes in cortical thickness associated with normal aging. NeuroImage 52, 1215–1223. 10.1016/j.neuroimage.2010.04.258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Cirelli C, 2012. Time to Be SHY? Some Comments on Sleep and Synaptic Homeostasis [WWW Document]. Neural Plast. 10.1155/2012/415250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Cirelli C, 2006. Sleep function and synaptic homeostasis. Sleep Med Rev Feb 10, 49–62. [DOI] [PubMed] [Google Scholar]
- Tractenberg R, Singer C, Kaye J, J, 2005. Symptoms of sleep disturbance in persons with Alzheimer’s disease and normal elderly. Res 14SRC-BaiduScholar, 177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranah G, Blackwell T, Stone K, Ancoli-Israel S, Paudel M, Ensrud K, 2011. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol 70, 722–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauer J, Qian M, Doyle J, Rajaratnam S, Cunnington D, A, 2015. Cognitive Behavioral Therapy for Chronic Insomnia: Review and Meta-analysis. Ann Intern Med August4163, 191–204. [DOI] [PubMed] [Google Scholar]
- Tworoger S, Lee S, Schernhammer E, Grodstein F, 2006. The association of self-reported sleep duration, difficulty sleeping, and snoring with cognitive function in older women. Alzheimer Assoc Disord 2620 SRC-BaiduScholar, 41–48. [DOI] [PubMed] [Google Scholar]
- Valderrama M, Crépon B, Botella-Soler V, Martinerie J, Hasboun D, Alvarado-Rojas C, Baulac M, Adam C, Navarro V, Le Van Quyen M, 2012a. Human Gamma Oscillations during Slow Wave Sleep. PLoS ONE 7, e33477. 10.1371/journal.pone.0033477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valderrama M, Crépon B, Botella-Soler V, Martinerie J, Hasboun D, Alvarado-Rojas C, Baulac M, Adam C, Navarro V, Le Van Quyen M, 2012b. Human Gamma Oscillations during Slow Wave Sleep. PLoS ONE 7, e33477. 10.1371/journal.pone.0033477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello M, Larsen L, Moe K, 2004. Age-related sleep change: Gender and estrogen effects on the subjective-objective sleep quality relationships of healthy, non-complaining older men and women. J. Psychosom. Res 56, 503–510. [DOI] [PubMed] [Google Scholar]
- Vitiello M, Prinz P, 1989. Alzheimer’s disease. Sleep Sleepwake Patterns Clin Geriatr Med May5, 289–99. [PubMed] [Google Scholar]
- Vitiello MV, 2009. Recent Advances in Understanding Sleep and Sleep Disturbances in Older Adults: Growing Older Does Not Mean Sleeping Poorly. Curr. Dir. Psychol. Sci 18, 316–320. 10.1111/j.1467-8721.2009.01659.x [DOI] [Google Scholar]
- Wade A, Farmer M, Harari G, Fund N, Laudon M, Nir T, Frydman-Marom A, Zisapel N, 2014. Add-on prolonged-release melatonin for cognitive function and sleep in mild to moderate Alzheimer’s disease: a 6-month, randomized, placebo-controlled, multicenter trial. Clin Interv Aging June189 SRC-BaiduScholar, 947–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Lung F, 2012. The role of PGC-1 and ApoEpsilon4 in insomnia. Psychiatr Genet Apr 22, 82–87. [DOI] [PubMed] [Google Scholar]
- Wang JL, Lim AS, Chiang W-Y, Hsieh W-H, Lo M-T, Schneider JA, Buchman AS, Bennett DA, Hu K, Saper CB, 2015. Suprachiasmatic neuron numbers and rest-activity circadian rhythms in older humans: SCN and Rest-Activity Rhythms. Ann. Neurol 78, 317–322. 10.1002/ana.24432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wdos S, Poyares D, Guilleminault C, Ramos L, Bertolucci P, Tufik S, 2006. Moraes The effect of donepezil on sleep and REM sleep EEG in patients with Alzheimer disease: a double-blind placebo-controlled study. Sleep February29, 199–205. [DOI] [PubMed] [Google Scholar]
- Wei HG, Riel E, Czeisler CA, Dijk D-J, 1999. Attenuated amplitude of circadian and sleep-dependent modulation of electroencephalographic sleep spindle characteristics in elderly human subjects. Neurosci. Lett 260, 29–32. 10.1016/S0304-3940(98)00851-9 [DOI] [PubMed] [Google Scholar]
- Weiler M, Stieger KC, Long JM, Rapp PR, 2020. Transcranial Magnetic Stimulation in Alzheimer’s Disease: Are We Ready? eNeuro 7. 10.1523/ENEURO.0235-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerberg CE, Florczak SM, Weintraub S, Mesulam M-M, Marshall L, Zee PC, Paller KA, 2015. Memory improvement via slow-oscillatory stimulation during sleep in older adults. Neurobiol. Aging 36, 2577–2586. 10.1016/j.neurobiolaging.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerberg CE, Mander BA, Florczak SM, Weintraub S, Mesulam M-M, Zee PC, Paller KA, 2012. Concurrent Impairments in Sleep and Memory in Amnestic Mild Cognitive Impairment. J. Int. Neuropsychol. Soc 18, 490–500. 10.1017/S135561771200001X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlye L, Walhovd K, Dale A, Bjornerud A, Due-Tonnessen P, Engvig A, Grydeland H, Tamnes C, Ostby Y, Fjell A, 2009. Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cereb Cortex 20, 2055–2068. [DOI] [PubMed] [Google Scholar]
- Wilson R, Nag S, Boyle P, Hizel L, Yu L, Buchman A, Schneider J, Bennett D, 2013. Neural reserve, neuronal density in the locus ceruleus, and cognitive decline. Neurol March2680, 1202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witton J, Staniaszek L, Bartsch U, Randall A, Jones M, Brown J, J, 2016. Disrupted hippocampal sharp-wave ripple-associated spike dynamics in a transgenic mouse model of dementia. August15594, 4615–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Swaab D, J, 2005. The human pineal gland and melatonin in aging and Alzheimer’s disease. Res April38, 145–52. [DOI] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M, 2013. Sleep Drives Metabolite Clearance from the Adult Brain. Science 342, 373–377. 10.1126/science.1241224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Laffan A, Harrison S, Redline S, Spira A, Ensrud K, 2011. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 306, 613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage J, Friedman L, Kraemer H, Tinklenberg J, Salehi A, Noda A, Taylor J, Hara R, Murphy G, J, 2004. O’ Sleep/wake disruption in Alzheimer’s disease: APOE status and longitudinal course. Psychiatry Neurol March17, 20–4. [DOI] [PubMed] [Google Scholar]
- Zepelin H, McDonald C, Zammit G, J, 1984. Effects of age on auditory awakening thresholds. 39SRC-BaiduScholar, 294–300. [DOI] [PubMed] [Google Scholar]
- Zhou J, 1995. VIP neurons in the human SCN in relation to sex, age, and Alzheimer’s disease. Neurobiol. Aging 16, 571–576. 10.1016/0197-4580(95)00043-E [DOI] [PubMed] [Google Scholar]


