Abstract
Apparent treatment-resistant hypertension (ATRH) has been linked to end-stage kidney disease (ESKD) and cardiovascular disease (CVD). We tested the hypothesis that the effect of ATRH on ESKD is greater in African-Americans than in Whites and investigated the effect of ATRH on ESKD independent of APOL1 genotype. In a retrospective cohort of 139,685 hypertensive veterans (22% African-American, 5% women) in the Million Veteran Program, ATRH was defined as failure to achieve outpatient blood pressure <140/90mmHg with 3 antihypertensives including a thiazide or use of ≥4. Outcomes included incident ESKD, myocardial infarction and stroke. Poisson models were used to test effect modification by race. Over a median follow-up of 10.3 years (IQR, 5.8–11.7), 17,521 incident ATRH cases were observed. Compared to non-resistant hypertension (NRH), patients with ATRH had higher incidence rates (per 1000-person-years) of ESKD (4.7 vs. 1.6), myocardial infarction (6.7 vs. 3.4) and stroke (16.7 vs. 8.5). A greater attributable risk of ESKD due to ATRH was observed among African-Americans (44.4/1000) compared to Whites (25.5/1000). African-Americans with ATRH had a 2.3-fold higher risk of ESKD compared to African-Americans with NRH; 3-fold the risk of Whites with ATRH, and 9-fold the risk of Whites with NRH (P-interaction<0.001). Among African-Americans, ATRH remained associated with a 98% (95%CI, 1.66–2.75) higher risk of ESKD after adjustment for APOL1 genotype. ATRH patients experienced excess ESKD and CVD risk. This excess ATRH-related ESKD risk was magnified among African-Americans independently of APOL1 genotype. Targeted treatment of ATRH could curtail ESKD and CVD incidence.
Keywords: Treatment-resistant hypertension; end-stage kidney disease, race, apolipoprotein L1 variants, stroke, myocardial infarction
INTRODUCTION
African-Americans have a three-fold higher incidence of end stage kidney disease (ESKD) compared to Whites.1 Hypertension (HTN) is one of the leading causes of ESKD among African-Americans and it affects over 100 million adults in the U.S.2 Apparent-treatment resistant HTN (ATRH) is a severe form of HTN characterized by failure to respond to therapy despite the concurrent use of ≥ 3 antihypertensive agents of different classes, at maximally tolerated dose, or reaching goal with ≥4.3 ATRH is independently associated with an elevated risk of adverse renal 4, 5 and cardiovascular (CV) outcomes.6 The estimated prevalence of ATRH varies between 9 and 17% among persons with HTN, with ATRH being reportedly more common among African-Americans.7, 8 The reasons for the racial differences are unclear and data regarding variations in ATRH-related outcomes by race are scarce. One likely contributing factor is chronic kidney disease (CKD), which may exacerbate ATRH risk among African-Americans.9, 10 Identifying groups at high risk of renal and CV end-organ damage could foster targeted approaches to mitigate the adverse effects of ATRH at the population level.
Our primary aim was to investigate the interaction between ATRH and both race and baseline kidney function on the risk of ESKD. Given that apolipoprotein L1 (APOL1) risk alleles are key contributors to the elevated risk of ESKD among African-Americans11, we also investigated whether any potential excess risk of ESKD conferred by ATRH is independent of APOL1 risk alleles among African-Americans. Our secondary aim was to quantify the risk of ESKD and CV outcomes (myocardial infarction and stroke) attributable to ATRH, to inform the potential health impact among U.S. Veterans.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Design and study population
The Million Veteran Program (MVP) is a large observational cohort and mega biobank designed to investigate the genetic underpinnings of common conditions among U.S. veterans.12 Full details of the MVP design and methods have been published elsewhere.12 Briefly, participants were recruited from 63 Veterans Affairs (VA) clinics beginning in 2011. At enrollment, participants provided blood samples for genotyping and biomarker studies and completed baseline questionnaires. Participants also agreed for medical records to be accessed. The study was approved by the VA Central Institutional Review Board and patients signed informed consent.
For the current study, we assembled a retrospective cohort of 139,685 hypertensive veterans, enrolled in the MVP who were active users of the VA healthcare system, between January 1st, 2004 and December 31st, 2015 (Figure S1 in the Data Supplement). Hypertension was defined as the presence of ICD-9-CM (International Classification of Diseases, Ninth Revision, Clinical Modification) codes for HTN in the electronic health record (EHR) and the prescription of antihypertensive medication prior to cohort entry. Patients entered the cohort on the date of their first available serum creatinine in the EHR and were followed up until they experienced an event of interest, died or were censored on the date of their last VA visit. “Active VA use” was defined as having two clinic visits the year prior to cohort entry or one visit in each of the two years prior to cohort entry. This approach was used to include patients with continuity of care within the VA Healthcare System thereby mitigating ascertainment bias for patient-level outcomes. Patients who had a prior history of ESKD at baseline or an entry estimated glomerular filtration rate (eGFR) <30 mL/min/1.73m2 were excluded from this cohort.
Ascertainment of ATRH
During the 12-year follow-up period between January 1st, 2004 and December 31st, 2015, we identified 17,521 patients with ATRH using clinical data defined as: failure to achieve outpatient BP <140/90 mmHg7 with three antihypertensive drugs (AHDs), including a thiazide diuretic, or use of four or more AHDs of different classes.3, 13 We excluded BP measurements associated with a pain score > 5 or when interfering medications were prescribed. Additionally, patients with documented cocaine use or a history of secondary causes of hypertension at baseline were excluded (Table S1 in the Data Supplement). A multi-stage algorithm based on pharmacy refill data was used to ascertain ATRH (Methods in the Data Supplement). Briefly, after intensification of patients’ antihypertensive regimen with a fourth drug, refill data for all 4 drugs was required to distinguish treatment intensification versus drug switching. For patients on 3 drugs, a maximum BP > 140/90 mmHg within 15–180 days after treatment intensification with the 3rd drug was additionally required to rule-in ATRH. For patients who met ATRH criteria based on the use of 4 drugs, the date of change of exposure classification from NRH to ATRH was the date of the refill of the 4 drugs after 3–6 months of overlapping days’ supply. For patients who met ATRH criteria based on uncontrolled BP on 3 drugs, the date of change of exposure classification was the date the elevated BP (>140/90mmHg) was documented after treatment intensification with the 3rd drug. Intensification with a 3rd drug also required a refill of the 3 drugs after 3–6 months of overlapping days’ supply to confirm intensification of treatment. Participants who met the criteria for ATRH prior to cohort entry were excluded. Individuals with non-resistant hypertension (NRH) were patients who were taking 1 or 2 AHDs, regardless of BP values, or those that were controlled on 3 AHDs. Once participants met the criteria for ATRH and were classified as such, they could not revert back to NRH.
Covariates
Baseline covariates were obtained from within 730 days prior to cohort entry. Physiological covariates (e.g. baseline BP) were the closest to, and prior to, cohort entry. We defined each comorbid condition at baseline using a combination of clinical, laboratory and administrative criteria: two outpatient codes on two different dates or 1 inpatient code (Table S2 in the Data Supplement). The Chronic Kidney Disease–Epidemiology Collaboration (CKD-EPI) equation was used to calculate eGFR.14 Information on race and ethnicity were based on a combination of self-report through centralized VA data collection methods or the Observational Medical Outcomes Partnership (OMOP) data (Methods in the Data Supplement).
Outcomes
Incident ESKD, stroke and myocardial infarction (MI) were ascertained using validated algorithms based on physician administrative diagnostic codes (ICD-9-CM/CPT, Table S2 in the Data Supplement). ESKD was defined by a procedure or diagnosis code indicating dialysis, renal transplant, or an eGFR<15 mL/min/1.73m2. Except for renal transplant, a second confirmatory event (dialysis code or eGFR<15 mL/min/1.73m2), at least ≥ 90 days apart was required to confirm ESKD. Incident MI was defined as a primary discharge diagnosis for fatal or nonfatal acute MI (ICD9-CM 410.x).15 The positive predictive value (PPV) for this algorithm was up to 95%.16 The algorithm for stroke (PPV = 97%) included discharge codes for ischemic stroke (433.x1, 434, or 436), intracerebral hemorrhage (431), and subarachnoid hemorrhage (430).17 Death was ascertained using National Death Index or OMOP data.
APOL1 genotype
APOL1 variants, including rs73885319 and rs60910145, missense mutations in near absolute linkage disequilibrium, that form haplotype G1, and rs71785313 (deletion of p.N388/Y389 amino acids, denoted G2), were directly genotyped on the Affymetrix Axiom Biobank Array chip using DNA extracted from whole blood.18 Participants were defined as two risk allele carriers if they were homozygotes for G1/G1, homozygotes for G2/G2, or compound G1/G2 heterozygotes.
Statistical analysis
ATRH was modeled as a time-varying exposure with every patient being ATRH-free at baseline. Poisson regression with robust standard errors was used to estimate incidence rates (IR) and incidence rate ratios (IRR) for ATRH versus NRH. Additive interaction between ATRH and race or eGFR categories (eGFR ≥ 60 versus 30–59.9 mL/min/1.73m2) were tested using the relative excess risk due to interaction (RERI), the attributable proportion due to interaction (AP) and the synergy index (S).19, 20 For each outcome of interest, we computed the cumulative incidence, attributable risk, number needed to harm, attributable fraction in the exposed (patients with ATRH) and population attributable fraction (PAF).
Kaplan-Meier plots for each outcome were constructed for patients with ATRH and NRH, overall and stratified by race. We further examined the independent associations of ATRH with each outcome using sequential multivariable Cox proportional hazard models. Covariates in model 1 included age, sex and race. Model 2 further adjusted for baseline eGFR and calendar year of entry. Model 3 added smoking, diabetes, chronic obstructive pulmonary disease (COPD), malignancy, coronary artery disease (CAD), peripheral artery disease (PAD), stroke, body mass index (BMI), serum lipids and statin use. In sensitivity analyses we further adjusted for a) systolic and diastolic BP (at baseline and time of ATRH ascertainment); and b) time from first HTN code in the EHR to cohort entry, to examine the effect of ATRH beyond nominal BP values or duration of exposure to HTN. Further sensitivity analyses were performed by excluding ATRH ascertainment occurring within 6 months of cohort entry or incident events occurring within 1 or 2 years of cohort entry. We also performed competing-risks analysis for ESKD, MI and stroke using Fine and Gray sub-distribution hazard models with death as the competing event.21
Among African-Americans, we investigated whether the excess risk of ESKD conferred by ATRH was independent of the presence of APOL1 risk alleles by additionally adjusting for APOL1 genotype in Cox models already comprising all aforementioned covariates and 10 principal components of ancestry. We also tested for additive interaction between ATRH and APOL1 genotype using Poisson models. Interaction analyses were restricted to African-Americans with 0 or 2 APOL1 risk alleles since the presence of 1 risk allele doesn’t confer any excess risk of ESKD.
We computed the proportion of missing values for each covariate and examined missingness patterns using hierarchical cluster analysis of variables usually missing together.22 The observed patterns were suggestive of data being missing at random. Multiple imputation of missing data was performed using Harrell’s aregImpute algorithm.22 The algorithm uses different bootstrap resamples for each of the multiple imputations. Details are presented in the supplement. Five imputations were performed, creating 5 complete data sets. The regression models (containing all covariates included in the imputation model) were fitted on each complete data set, and the regression coefficients were averaged over the multiple imputations.
Statistical significance for 2-sided P values was set at 0.05. All analyses were performed using Stata v15.1 and R v3.2 in the VA informatics and computing environment.
RESULTS
Patient Characteristics
Among the 139,685 hypertensive patients included in this study, 22% were African-American; and 5% were women. The median (interquartile range [IQR]) age at baseline was 60 (54–67) years. Compared to patients with NRH, patients who developed ATRH [n = 17,521 (12.5%)] during follow-up were more likely to be male and African-American. Incident ATRH patients also had a higher baseline systolic BP and BMI as well as a higher prevalence of cardiometabolic comorbidities at baseline (Table 1). African-American patients were younger, more likely to be female, and had higher systolic BP, eGFR and higher baseline prevalence of stroke and diabetes. Conversely, they had lower baseline prevalence of CAD and PAD (Table S3 in the Data Supplement). Among patients who developed ATRH, the median number of AHDs at the time of incident ATRH was similar by race. The top four AHDs used by patients at the time of ATRH ascertainment were thiazides (100%), RAAS inhibitors (88.7%), beta-blockers (67.8%) and calcium-channel blockers (59.4%) (Table S4 in the Data Supplement).
Table 1.
Baseline characteristics of Veterans in the MVP with non-resistant hypertension and those who developed apparent treatment-resistant hypertension during follow-up from 2004 to 2015
| Baseline characteristics | Non-Resistant HTN n = 122,164 | Apparent Treatment-Resistant HTN n = 17, 521 |
|---|---|---|
| Age (IQR), years | 60 (54–67) | 59 (55–66) |
| Women, % | 5.1 | 3.9 |
| Hispanic, % | 4.4 | 4.8 |
| Race, % | ||
| Non-Hispanic Whites | 75.0 | 70.3 |
| Non-Hispanic Blacks | 21.9 | 26.6 |
| Others† | 3.1 | 3.08 |
| Body Mass Index (IQR), kg/m2 | 30.2 (27.0–34.1) | 31.0 (27.7–35.0) |
| Systolic BP (IQR), mm Hg | 136 (125–147) | 140 (130–154) |
| Diastolic BP (IQR), mm Hg | 79 (71–87) | 80 (72–89) |
| eGFR (IQR), mL/min/1.73m2 | 79.2 (65.8–93.1) | 79.0 (65.2–93.0) |
| Serum Lipids, mg/dl | ||
| Total Cholesterol | 182 (157–209) | 180 (157–208) |
| HDL Cholesterol | 41 (35–50) | 40 (34–49) |
| LDL Cholesterol | 107 (86–131) | 105 (85–129) |
| Triglycerides | 137 (93–205) | 145 (98–218) |
| Smoking history, % | ||
| Never | 24.6 | 22.7 |
| Former | 51.4 | 53.9 |
| Current | 24.1 | 23.4 |
| Comorbidities, % | ||
| Diabetes | 28.1 | 38.4 |
| Cerebrovascular disease | 3.3 | 4.0 |
| Coronary artery disease | 28.2 | 29.5 |
| Peripheral artery disease | 5.7 | 7.0 |
| COPD | 11.8 | 12.0 |
| All malignancies | 9.5 | 9.7 |
| Anti-hypertensive drugs, % | ||
| ACE-Inhibitors/ARBs | 61.4 | 68.4 |
| Beta Blockers | 37.5 | 42.4 |
| Alpha Blockers | 14.8 | 15.9 |
| Calcium Channel Blockers | 26.7 | 35.1 |
| Thiazide diuretics | 31.9 | 44.1 |
| Loop diuretics | 7.5 | 6.6 |
| Potassium-sparing diuretics | 6.9 | 6.4 |
| Vasodilators | 0.7 | 0.9 |
| Number of AHDs at cohort entry (IQR) | 2 (1–2) | 2 (1–3) |
Most between-group comparisons were statistically significant (P < 0.01 for all other baseline variables) except for age, ethnicity, baseline eGFR, COPD and malignancy.
Tabulated values for medication usage (and other variables) represent baseline values (at or prior to cohort entry) not time of ATRH ascertainment. At the time of ATRH ascertainment 100% of ATRH participants were on ≥ 3 drugs including a Thiazide (eTable 1).
Others: Asian, Pacific Islanders/Hawaiian, Native American and unspecified.
Abbreviations: ACE indicates angiotensin converting enzyme; AHDs, antihypertensive drugs; ARB, angiotensin II receptor blocker; BP, blood pressure; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; HTN, Hypertension.
Population health impact of ATRH on ESKD, MI, stroke and all-cause mortality
Over 12 years of follow-up, the cumulative incidence of ESKD, MI and stroke were 2.5%, 4.7%, 10.8% respectively. Median follow-up time for the primary outcome (ESKD) was 10.3 years (IQR, 5.8–11.7). Compared to patients with NRH, those with ATRH had higher incidence rates of ESKD (4.7 vs. 1.6/1000 person-years), MI (6.7 vs 3.4) and stroke (16.7 vs 8.5) (Table S5 in the Data Supplement). The population attributable fraction of ESKD, MI and stroke due to ATRH was 12.8, 6.8 and 7.6% respectively (Table S6 in the Data Supplement). The numbers needed-to-harm for the aforementioned outcomes were 32, 25, and 8 respectively. In stratified analyses, a greater attributable risk of ESKD due to ATRH was observed among African-Americans (44.4 per 1000) compared to Whites (25.5 per 1000) (Table S7 in the Data Supplement).
Multivariable models for primary and secondary outcomes
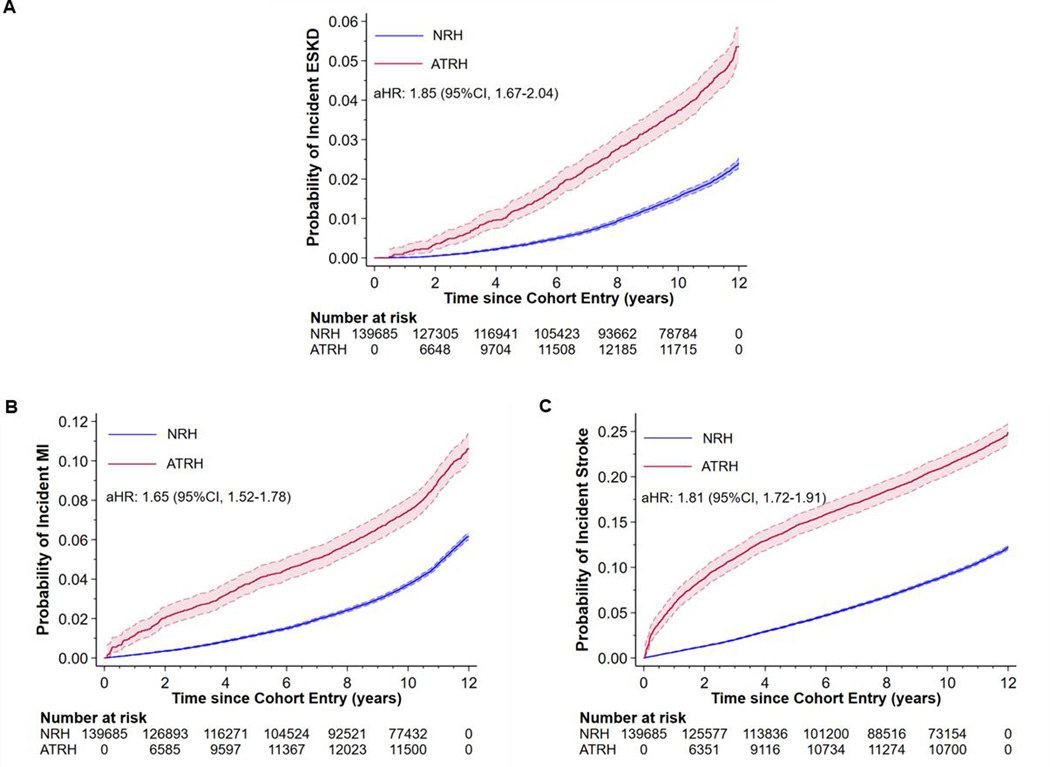
In fully adjusted Cox models, ATRH was associated with a 1.85 (95% CI, 1.67–2.04), 1.65 (95% CI, 1.52–1.78) and 1.81 (95% CI, 1.72–1.91) higher risk of incident ESKD, MI and stroke respectively, compared to patients with NRH (Figure 1). Further adjustment for systolic and diastolic BP (at baseline and time of ATRH) or duration of HTN resulted in some attenuation of the hazard ratios (Table S8 in the Data Supplement). Estimates from competing-risks analyses were similar to those obtained from the Cox models, (Table S9 in the Data Supplement) minimizing concerns about informative censoring by death.
Figure 1:
Effect of apparent treatment resistant hypertension (ATRH) on the risk of incident ESKD, MI and stroke among hypertensive Veterans in the Million Veteran Program. After full adjustment for baseline covariates, compared to NRH, ATRH was associated with an 85%, 65% and 81% higher risk of incident ESKD, MI and stroke respectively. Abbreviations: ATRH, apparent treatment resistant hypertension; ESKD, end-stage kidney disease; MI, myocardial infarction; NRH, non-resistant hypertension.
Race-stratified incidence of ESKD and effect modification by race
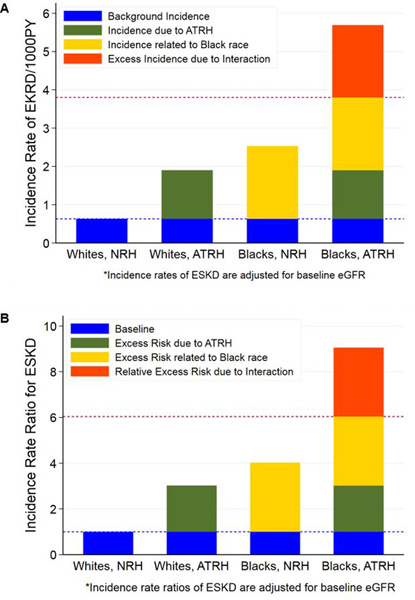
In race-stratified nonparametric survival analysis, African-Americans with ATRH had the highest probability of incident ESKD [8.4% (95%CI, 7.4–9.5)] compared to African-Americans with NRH [4.2% (95%CI, 3.9–4.5)], Whites with ATRH [4.1% (95%CI, 3.7–4.6)] and Whites with NRH [1.6% (95%CI, 1.5–1.7)] (Figure S2 in the Data Supplement). Similar patterns were observed for eGFR-adjusted incidence rates of ESKD (Table 2). Figure 2A shows the excess incidence of ESKD due to the interaction between ATRH and race. In Poisson models, African-Americans with ATRH had a 2.3-fold (95%CI, 1.96–2.59) higher risk of ESKD compared to African-Americans with NRH; 3-fold (95%CI, 2.53–3.53) the risk of Whites with ATRH, and 9-fold (95%CI, 7.88–10.38) the risk of Whites with NRH [P-interaction <0.001]. Compared to the common referent group (Whites with NRH), the eGFR-adjusted IRR were 3-fold higher in Whites with ATRH, 4-fold higher in African-Americans with NRH and over 9-fold higher in African-Americans with ATRH (Table 2). The RERI was 3.00 (95%CI, 1.79–4.21, P-interaction <0.001). Up to 33.2% (95%CI, 23.6–42.7) of the risk of ESKD among African-Americans with ATRH was attributable to the interaction between African-American race and ATRH (Figure 2B and Table 2). The additive interaction patterns remained consistent in fully adjusted Poisson models (Figure S4 in the Data Supplement). Interactions patterns for stroke were modest and there were none for incident MI (Tables S10 and S11 in the Data Supplement).
Table 2.
Additive interaction between Apparent Treatment Resistant Hypertension and both race and eGFR for the association with incident ESKD among hypertensive Veterans in the Million Veteran Program
| Interaction with race (P-interaction < 0.001) | ||||
|---|---|---|---|---|
| Parameters/Models | Whites with No ATRH (n = 86, 959) | Whites with ATRH (n = 11, 621) | Blacks with No ATRH (n = 26, 362) | Blacks with ATRH (n = 4588) |
| Incident ESKD cases | 1049 | 309 | 864 | 251 |
| Person-Years (PY) | 958, 674 | 87, 690 | 289, 066 | 33, 689 |
| Incidence rate*/1000PY (95 % CI) | 0.63 (0.58–0.68) | 1.90 (1.69–2.14) | 2.53 (2.35–2.72) | 5.69 (5.01–6.47) |
| Incidence rate ratio* (95% CI) | 1.00 (ref) | 3.02 (2.66–3.43) | 4.02 (3.67–4.40) | 9.05 (7.88–10.38) |
| Hazard ratios (95% CI) | ||||
| Model 1 | 1.00 (ref) | 2.56 (2.25–2.91) | 2.65 (2.42–2.91) | 5.17 (4.49–5.94) |
| Model 2 | 1.00 (ref) | 2.27 (2.00–2.58) | 2.77 (2.52–3.04) | 5.31 (4.26–6.11) |
| Model 3 | 1.00 (ref) | 1.98 (1.72–2.28) | 2.64 (2.37–2.94) | 4.68 (4.01–5.46) |
| Interaction with eGFR (P-interaction < 0.001) | ||||
| Parameters/Models | Patients with eGFR ≥ 60 and No ATRH (n = 102, 526) | Patients with eGFR ≥ 60 and ATRH (n = 14, 507) | Patients with eGFR < 60 but No ATRH (n = 19, 638) | Patients with eGFR < 60 and ATRH (n = 3014) |
| Incident ESKD cases | 1004 | 325 | 1134 | 288 |
| Person-Years (PY) | 1, 128, 157 | 107, 304 | 215, 177 | 23, 906 |
| Incidence rate/1000PY (95 % CI) | 0.89 (0.84–0.95) | 3.03 (2.72–3.38) | 5.27(4.97–5.59) | 12.05 (10.73–13.52) |
| Incidence rate ratio (95% CI) | 1.00 (ref) | 3.40 (3.00–3.86) | 5.92 (5.43 (6.45) | 13.54 (11.87–15.4) |
| Hazard ratios (95% CI) | ||||
| Model 1 | 1.00 (ref) | 2.55 (2.25–2.89) | 10.20 (9.31–11.57) | 17.22 (15.04–19.70) |
| Model 2 | 1.00 (ref) | 2.54 (2.24–2.88) | 10.22 (9.33–11.19) | 17.23 (15.06–19.72) |
| Model 3 | 1.00 (ref) | 2.33 (2.03–2.68) | 9.99 (9.03–11.05) | 14.63 (12.60–16.98) |
Model 1: adjusted for age, sex and race (omitted when testing the interaction with race but included for models testing eGFR interaction). Model 2: adjusted for age (restricted cubic splines with 4 knots), sex, race, calendar year of cohort entry and baseline eGFR (omitted when testing the interaction with eGFR but included for models testing interaction with race). Model 3: Model 2 + smoking + BMI (restricted cubic splines with 4 knots) + serum lipids (total cholesterol, triglycerides, LDL cholesterol and HDL cholesterol) + history of Cancer, COPD, diabetes, CAD, PAD and stroke.
Abbreviations: ATRH, apparent treatment resistant hypertension; BMI, body mass index; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; PAD, peripheral artery disease.
Incidence rates are adjusted for baseline eGFR (adjusted to the mean baseline eGFR: 80.4mL/min/1.73m2).
Overall Incidence rates of ESKD among a) patients with eGFR ≥60 = 1.08 (1.02–1.13) per 1000PY; b) patients with eGFR < 60 = 5.95 (5.64–6.27) per 1000PY.
Figure 2.
A. Excess incidence of incident ESKD due to interaction between ATRH and race. In the absence of interaction, the expected incidence rate (per 1000PY) among blacks with ATRH would be the sum of the background incidence (0.63), incidence due to ATRH alone (1.27) and incidence related to black race (1.90). However, the observed incidence (5.69) was greater than the expected (3.80); which is suggestive of synergistic additive interaction between ATRH and black race for the association with incident ESKD. The excess incidence due to interaction (dark orange bar) represents an excess of 189 incident ESKD cases per 100, 000PY among blacks with ATRH. Abbreviations: ATRH, apparent treatment resistant hypertension; ESKD, end-stage kidney disease; NRH, non-resistant hypertension; PY, person-years.
B. Relative excess risk of incident ESKD due to interaction between ATRH and race. The Y-axis represents the incidence rate ratio (IRR) for ESKD comparing whites with ATRH, blacks with NRH, blacks with ATRH to the referent group (whites with NRH). The relative excess risk due to interaction (RERI) between ATRH and race = IRR11 - IRR10 - IRR01 + 1 = 3.00 (95%CI: 1.79, 4.21); and is represented by the dark orange bar as in Figure 2A. The attributable proportion (AP) = RERI/IRR11 = 33.2 (95%CI: 23.6, 42.7) suggesting that 33.2% of the risk of incident ESKD among blacks with ATRH is due to the synergistic interaction between ATRH and race. Abbreviations: ATRH, apparent treatment resistant hypertension; ESKD, end-stage kidney disease; NRH, non-resistant hypertension.
Effect modification by baseline kidney function
Patients with reduced baseline eGFR (30–59.9 mL/min/1.73m2) and ATRH had a 2.3-fold (95%CI, 2.01–2.60) higher risk of ESKD compared to patients reduced eGFR and NRH, a 4-fold (95%CI, 3.39–4.66) higher risk compared to patients with preserved eGFR (>60 mL/min/1.73m2) and ATRH, and a 13.5-fold (95%CI, 11.87–15.43) higher risk compared to patients with preserved eGFR and NRH [P-interaction <0.001] (Table 2). The test for additive interaction remained significant in fully adjusted models.
Independent effects of ATRH and APOL1 genotype on incident ESKD among African-Americans
Among African-Americans with ATRH and NRH, we observed similar prevalence of 0 (42.2 vs. 41.2%), 1 (45.9 vs 46.1%) and 2 (11.9 vs. 12.7%) APOL1 risk allele carriers (Table S12 in the Data Supplement). Compared to African-Americans with no APOL1 risk alleles, those with 2 APOL1 risk alleles showed no significant difference in the odds of developing ATRH (odds ratio: 0.92, 95%CI, 0.81–1.02). ATRH was associated with a 97% (IRR: 1.97; 95%CI, 1.65–2.34) higher risk of incident ESKD in models adjusted for demographics and clinical variables. Further adjustment for APOL1 genotype showed no attenuation of the effect estimates (IRR: 1.98; 95%CI, 1.66–2.35) (Table 3). In addition, among patients with no APOL1 risk alleles, ATRH was associated with an adjusted 2.44-fold (95%CI: 1.74–3.14) higher risk of incident ESKD (Table 3). There was no evidence of additive interaction between ATRH and APOL1 genotype for the association with ESKD (Table 3).
Table 3a.
Effect of ATRH and APOL1 risk alleles on Incident ESKD among African-Americans with hypertension
| Risk categories | IR* per 1000 PY (95% CI) |
IRR Model 1a* (95% CI) |
IRR Model 2a** (95% CI) |
|---|---|---|---|
| 2 APOL1 risk alleles, ATRH | 9.99 (6.76–13.23) | 5.25 (3.41–7.08) | 3.18 (1.83–4.53) |
| 2 APOL1 risk alleles, NRH | 4.80 (4.11–5.50) | 2.53 (2.03–3.00) | 2.00 (1.55–2.44) |
| 0 APOL1 risk alleles, ATRH | 7.05 (5.60–8.50) | 3.71 (2.81–4.59) | 2.44 (1.74–3.14) |
| 0 APOL1 risk alleles, NRH | 1.90 (1.67–2.14) | 1.00 (ref) | 1.00 (ref) |
| P for additive interaction = 0.63 | |||
| Relative excess risk due to interaction, RERI (95% CI) = IRR11 - IRR10 - IRR01 + 1 = −0.26 (−1.69, 1.18). | |||
| Attributable proportion due to interaction, AP (95% CI) = RERI/IRR11 = 0.0 (−0.56, 0.40). | |||
Incidence rates (IR) were adjusted for age, sex and 10 PCs.
Incidence rate ratios (IRR) in model 1a are adjusted for age (restricted cubic splines with 4 knots), sex (M/F) and 10 principal components of ancestry (PCs).
Model 2a includes model 1a + baseline eGFR (restricted cubic splines with 4 knots), calendar year of cohort entry (4 categories of 3 consecutive years), smoking (never, former, current), BMI (restricted cubic splines with 4 knots), serum lipids (total cholesterol, triglycerides, LDL cholesterol and HDL cholesterol; all restricted cubic splines with 4 knots), history of Cancer, COPD, diabetes, CAD, PAD and stroke (all yes/no).
Discussion
In a large multiethnic cohort of U.S. veterans with hypertension we found that the adverse effect of incident ATRH on ESKD incidence was greater among African-Americans compared to Whites. Importantly, this effect was independent of APOL1 risk alleles. Furthermore, the population risk of ESKD, MI and stroke that was attributable to ATRH alone was substantial, highlighting the major health implications for the affected population. Additionally, reduced kidney function potentiated the ATRH effect on the risk of ESKD.
Apparent-treatment resistant hypertension is found in 10–20% of treated hypertensive patients and is an established risk factor for adverse renal and CV outcomes.23, 24 We found an appreciably high population risk of incident MI, stroke and ESKD attributable to ATRH with correspondingly small numbers-needed-to harm (ranging from 8 for stroke to 32 for ESKD) which underscores the enormous population health relevance of ATRH and our findings. Importantly our findings also suggest an early spike in the risk of incident stroke among persons with ATRH that may be related to extreme values of systolic BP observed among some patients in this group underscoring the importance of stringent BP control to curb the risk of adverse cerebrovascular events.
Prior studies – including REGARDS (REasons for Geographic And Racial Differences in Stroke), ALLHAT (Antihypertensive and Lipid-lowering Treatment to Prevent Heart Attack Trial), MASTERPLAN (Multifactorial Approach and Superior Treatment Efficacy in Renal Patients With the Aid of Nurse Practitioners) and others – have reported increased risk of ESKD in patients with ATRH.4, 5, 25 The current study further extends these findings reporting an excess incidence of ESKD in African-Americans compared to Whites among patients with ATRH – which is synonymous with a synergistic interaction between race and ATRH in the association with ESKD. In this study, we chose to assess the interaction of ATRH and race on the additive scale as this is more indicative of an underlying mechanistic interaction26–28 and provides a more useful framework to assess the potential public health benefit of an intervention (to mitigate the effect of a causal factor) across different populations, including racial groups.20, 26, 27 These observed racial disparities in the consequences of ATRH on ESKD incidence underscore the need for further research into the underlying factors that explain these findings and need for novel therapeutics to mitigate the effect of this risk factor among African-Americans. While our findings emphasize a greater severity of ATRH among African-Americans compared to Whites with respect to ESKD risk, the observed adverse effect of ATRH on kidney function remained significant after adjustment for blood pressure at time of ATRH diagnosis. Therefore, an approach emphasizing more intensive BP control may be less efficacious in the reduction of ESKD risk among hypertensive patients with reduced and preserved kidney function hence more specific interventions may be required. This interpretation is corroborated by the findings of the landmark Systolic Blood Pressure Intervention Trial (SPRINT)29 and the African American Study of Kidney Disease and Hypertension (AASK) Trial.30 In AASK, 1094 black patients with hypertensive CKD were randomized to either intensive BP-control (mean BP ~ 130/81) or standard BP-control (mean BP ~ 141/86) and followed-up for 4.6 years during the trial phase and up to 12 years during the cohort phase. In both phases, there was no significant between-group difference in the risk of the primary kidney outcome (doubling of serum creatinine, incident ESKD, or death).31 However, Wright and colleagues found that ramipril did portend a greater benefit on the composite kidney outcome than metoprolol and amlodipine suggesting ACE inhibitors may be first line treatment for patients with hypertensive CKD.30 Perhaps, for ATRH as well, more appropriate drug choices would help to mitigate adverse kidney outcomes. Recently, several studies have shown that mineralocorticoid excess or subclinical hyperaldosteronism appears to be involved as a common pathophysiological mechanism underlying ATRH.32 Spironolactone has been shown to be effective and safe in African Americans33 and CKD patients.34 Meanwhile, less than 5% of patients with ATRH in our study were on a mineralocorticoid inhibitor. Studies elucidating the molecular mechanisms involved in these physiological pathways, including among African-Americans, as well as research into novel therapeutic targets would be pertinent.
ESKD risk has been shown to cluster in families and to be partially mediated by the presence of APOL1 risk variants in African Americans.11, 35 Using data from the AASK and CRIC (Chronic Renal Insufficiency Cohort) studies, Parsa and colleagues found that African-American patients in the APOL1 high-risk group (2 APOL1 risk variants) had higher rates of ESKD and CKD progression (50% decline in eGFR or doubling of serum creatinine) compared to African-American patients in the APOL1 low-risk group (0 or 1 APOL1 risk variant) and White patients.11 In the current study, we found that while both ATRH and APOL1 high risk genotype were significant predictors of ESKD, the ATRH effect on ESKD incidence was independent of APOL1 genotype. Whether the greater ATRH-related ESKD risk observed among African-Americans compared to Whites is due to other genetic variants or environmental factors needs to be investigated further. If the genetic underpinnings underlying the occurrence of ATRH differ across racial groups, then perhaps these differential molecular mechanisms may also produce differential effects on renal outcomes. Furthermore, differential patterns of genotype-by-environment interactions among racial groups could be involved. In addition, differences in socioeconomic status (known to associate with racial disparities in ESKD incidence);36 and potential differential efficacy of anti-HTN medication across racial groups could also play a role.
Previous studies – including the Jackson Heart Study, CRIC, MASTERPLAN study and others – have reported an increased risk of ATRH in populations with CKD.9, 10, 25 In our study, we emphasize the joint effect of reduced kidney function and ATRH on ESKD incidence compared to the effect of each exposure taken singly. We observed a 13.5-fold higher risk of incident ESKD in the group with both exposures (eGFR = 30–59.9 and ATRH), which was greater than the sum of each individual effect suggesting a synergistic additive interaction between lower eGFR and ATRH on the risk of incident ESKD. This suggests that while better management of blood pressure is beneficial in ATRH patients with both preserved and reduced kidney function, a significantly greater number of incident ESKD cases could potentially be prevented by optimal and targeted interventions of ATRH among patients with reduced kidney function. The 2017 American College of Cardiology/American Heart Association clinical practice guidelines for the prevention, detection, evaluation and management of high blood pressure suggested a lower target of <130/80mmHg for all patients with CKD given that most patients with CKD die from CV complications.13 This lower target was supported by the overwhelming evidence of CV benefit in the intensive SBP lowering arm, SBP < 120 mmHg (versus the routine management arm, SBP < 140 mmHg) of the SPRINT trial – 25% reduction in the risk of the primary CV outcome comprising MI, acute coronary syndrome, stroke, congestive heart failure (CHF) and CV death.37
Our study has several limitations. Our cohort included predominantly male veterans and findings should be generalized to other populations with caution. As with any observational study that relies on administrative/physician diagnostic codes for exposure and outcome ascertainment, there is the risk of potential misclassification. The potential for survival bias is acknowledged. That said, we controlled for several known predictors of survival in the VA population including history of any malignancy, COPD, CAD, stroke and diabetes in order to mitigate this bias. We used antihypertensive medication refill data (which was similar across racial groups) as a proxy for medication adherence (Figure S4 in the Data Supplement). This may obscure cases of pseudo-resistance and result in some misclassification of ATRH. However, this approach is virtually free of recall bias and has good concordance with self-reported medication use.38 Some patients with CHF who were classified as ATRH because they were taking 4 antihypertensive drugs may actually have been misclassified. Given the overlap between treatment for CHF and HTN, even when a drug is prescribed for CHF as the primary indication, it may treat coexisting HTN. It may be infeasible to parse these with 100% accuracy, so some degree of misclassification is unavoidable in some patients with CHF. The main findings remain similar after excluding patients with concurrent CHF from the ATRH subgroup in sensitivity analyses (data not shown). We also acknowledge the potential for ascertainment bias for nonfatal outcomes in the ATRH group as these patients may have had greater interaction with the VA healthcare system which could in turn increase the likelihood of documenting data for nonfatal outcomes in their EHR. Another limitation is the potential for residual confounding related to not adjusting for individual socioeconomic status and insurance coverage, which are data we did not have access at the time of these analyses.
There are several strengths to our study. The large size and multiethnic nature of our cohort with over 20% African-American representation ensured that we had sufficient power for our interaction analyses. Our ATRH definition was constructed using pharmacy files that documented initiation of a 3rd or 4th drug, drug classes and refill data, to accurately define treatment intensification and not switching of AHDs. Among patients with incident ATRH, a long median follow-up time of 7.0 (IQR, 4.1–9.7) years post-ATRH strengthened the validity of our findings. We performed several sensitivity analyses including additional adjustment for BP and HTN duration, interaction analyses with APOL1 genotype and competing-risks regression, which were consistent with the primary results, supporting the robustness of the study findings.
In conclusion, ATRH was associated with an elevated risk of adverse kidney and CV outcomes. Population attributable risks of kidney and CV outcomes related to ATRH were considerable. The effect of ATRH on incident ESKD was magnified among patients with reduced kidney function as well as African-Americans, independently of APOL1 genotype.
Perspectives
The substantial population risk of ESKD and CV outcomes attributable to ATRH, observed in this study underscores the enormous population health relevance of ATRH. Interventions that improve reaching BP targets in patients with ATRH, including more appropriate drug choices, could have a major impact on ESKD incidence in this high-risk population. Studies deciphering the mechanisms and genetic underpinnings of this condition should be pursued in order to develop novel tools for risk stratification and identify new therapeutic targets.
Supplementary Material
Table 3b.
Effect of ATRH on incident ESKD among African-Americans with HTN in models adjusted for APOL1 risk alleles.
| Parameters/Models | Non-Resistant HTN | Apparent Treatment-resistant HTN |
|---|---|---|
| Incident ESKD cases | 675 | 199 |
| Hazard ratios (95% CI) | ||
| Model 1b | 1.00 (ref) | 2.27 (1.92–2.64) |
| Model 2b | 1.00 (ref) | 1.97 (1.65–2.34) |
| Model 3b | 1.00 (ref) | 1.98 (1.66–2.35) |
Model 1b: adjusted for age (restricted cubic splines with 4 knots), sex (M/F), 10 principal components of ancestry (PCs), baseline eGFR (restricted cubic splines with 4 knots) and calendar year of cohort entry (4 categories of 3 consecutive years).
Model 2b includes model 1b+ smoking (never, former, current) + BMI (restricted cubic splines with 4 knots) + serum lipids (total cholesterol, triglycerides, LDL cholesterol and HDL cholesterol; all restricted cubic splines with 4 knots) + history of Cancer, COPD, diabetes, CAD, PAD and stroke (all yes/no). Model 3b includes model 2b + APOL1 risk alleles (2 dummy variables for patients with 1 and 2 risk alleles; with the no risk allele group as the referent).
Abbreviations: APOL1, apolipoprotein L1; ATRH, apparent treatment resistant hypertension; BMI, body mass index; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; PAD, peripheral artery disease.
Novelty and Significance
What is New?
In this multiethnic cohort of U.S. veterans with hypertension, the adverse effect of apparent treatment-resistant hypertension on end-stage kidney disease (ESKD) risk was greater among African-Americans compared to Whites. Importantly, this effect was independent of APOL1 genotype.
What is Relevant?
Previous studies suggest apparent treatment-resistant hypertension is a strong risk factor for cardiovascular disease and ESKD.
Whether the impact of apparent-treatment resistant hypertension on the risk of ESKD differs by race and is independent of APOL1 genotype is not known.
Summary
There was a considerably high population risk of ESKD, myocardial infarction and stroke that could be attributable to apparent treatment-resistant hypertension. The excess risk of ESKD attributable to apparent treatment-resistant hypertension was greater among African-Americans and was independent of APOL1 genotype. Targeted interventions to treat apparent treatment-resistant hypertension could curtail the incidence of ESKD and adverse cardiovascular outcomes in this high-risk population.
Acknowledgements
This research is based on data from the Million Veteran Program, Office of Research and Development, Veterans Health Administration, and was supported by the CSR&D Merit award title Genetics of CKD and Hypertension-Risk Prediction and Drug Response in the MVP [#CX001897 to Adriana M. Hung]. This publication does not represent the views of the Department of Veteran Affairs or the United States Government. Acknowledgement of the MVP leadership and staff contributions can be found in the supplementary material entitled “MVP core acknowledgements.”
Sources of Funding
This work was supported by VA grant MVP Merit CX001897 (PI Hung A.M) and is based on data from the Million Veteran Program, Office of Research and Development, Veterans Health Administration. The data analysis was supported using resources and facilities of the VA Informatics and Computing Infrastructure (VINCI) and the VA Genomic Information System for Integrative Science (GenISIS). Elvis Akwo was supported by the American Heart Association Grant #20POST35210952/Akwo/2020. We would also like to acknowledge support from the “Genetics of Cardiometabolic Diseases in the VA Population” grant from the Department of Veterans Affairs I01-BX002641 (PI: Tsao, P. and Chang). This project was supported in part the by VA Clinical Science Research and Development investigator-initiated grant CX000570-06 (Roumie).
Role of Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclaimer: This publication does not represent the views of the Department of Veterans Affairs or the United States Government.
Footnotes
Disclosures
None.
References
- 1.United States Renal Data System. 2018 USRDS annual data report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2018. [Google Scholar]
- 2.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS and Muntner P. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 3.Carey RM, Calhoun DA, Bakris GL, Brook RD, Daugherty SL, Dennison-Himmelfarb CR, Egan BM, Flack JM, Gidding SS, Judd E, Lackland DT, Laffer CL, Newton-Cheh C, Smith SM, Taler SJ, Textor SC, Turan TN and White WB. Resistant Hypertension: Detection, Evaluation, and Management: A Scientific Statement From the American Heart Association. Hypertension. 2018;72:e53–e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanner RM, Calhoun DA, Bell EK, Bowling CB, Gutierrez OM, Irvin MR, Lackland DT, Oparil S, McClellan W, Warnock DG and Muntner P. Incident ESRD and treatment-resistant hypertension: the reasons for geographic and racial differences in stroke (REGARDS) study. Am J Kidney Dis. 2014;63:781–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muntner P, Davis BR, Cushman WC, Bangalore S, Calhoun DA, Pressel SL, Black HR, Kostis JB, Probstfield JL, Whelton PK, Rahman M and Group ACR. Treatment-resistant hypertension and the incidence of cardiovascular disease and end-stage renal disease: results from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Hypertension. 2014;64:1012–21. [DOI] [PubMed] [Google Scholar]
- 6.Irvin MR, Booth JN 3rd, Shimbo D, Lackland DT, Oparil S, Howard G, Safford MM, Muntner P and Calhoun DA. Apparent treatment-resistant hypertension and risk for stroke, coronary heart disease, and all-cause mortality. J Am Soc Hypertens. 2014;8:405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr., Jones DW, Materson BJ, Oparil S, Wright JT Jr. and Roccella EJ. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. [DOI] [PubMed] [Google Scholar]
- 8.Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension. 2011;57:1076–80. [DOI] [PubMed] [Google Scholar]
- 9.Tanner RM, Shimbo D, Irvin MR, Spruill TM, Bromfield SG, Seals SR, Young BA and Muntner P. Chronic kidney disease and incident apparent treatment-resistant hypertension among blacks: Data from the Jackson Heart Study. Journal of clinical hypertension (Greenwich, Conn). 2017;19:1117–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas G, Xie D, Chen HY, Anderson AH, Appel LJ, Bodana S, Brecklin CS, Drawz P, Flack JM, Miller ER 3rd, Steigerwalt SP, Townsend RR, Weir MR, Wright JT Jr., Rahman M and Investigators CS. Prevalence and Prognostic Significance of Apparent Treatment Resistant Hypertension in Chronic Kidney Disease: Report From the Chronic Renal Insufficiency Cohort Study. Hypertension. 2016;67:387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parsa A, Kao WH, Xie D, Astor BC, Li M, Hsu CY, Feldman HI, Parekh RS, Kusek JW, Greene TH, Fink JC, Anderson AH, Choi MJ, Wright JT Jr., Lash JP, Freedman BI, Ojo A, Winkler CA, Raj DS, Kopp JB, He J, Jensvold NG, Tao K, Lipkowitz MS, Appel LJ, Investigators AS and Investigators CS. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. 2013;369:2183–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaziano JM, Concato J, Brophy M, Fiore L, Pyarajan S, Breeling J, Whitbourne S, Deen J, Shannon C, Humphries D, Guarino P, Aslan M, Anderson D, LaFleur R, Hammond T, Schaa K, Moser J, Huang G, Muralidhar S, Przygodzki R and O’Leary TJ. Million Veteran Program: A mega-biobank to study genetic influences on health and disease. Journal of clinical epidemiology. 2016;70:214–23. [DOI] [PubMed] [Google Scholar]
- 13.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Williamson JD Sr. and Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2017. [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J and Ckd EPI. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roumie CL, Hung AM, Greevy RA, Grijalva CG, Liu X, Murff HJ, Elasy TA and Griffin MR. Comparative effectiveness of sulfonylurea and metformin monotherapy on cardiovascular events in type 2 diabetes mellitus: a cohort study. Annals of internal medicine. 2012;157:601–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosamond WD, Chambless LE, Sorlie PD, Bell EM, Weitzman S, Smith JC and Folsom AR. Trends in the sensitivity, positive predictive value, false-positive rate, and comparability ratio of hospital discharge diagnosis codes for acute myocardial infarction in four US communities, 1987–2000. Am J Epidemiol. 2004;160:1137–46. [DOI] [PubMed] [Google Scholar]
- 17.Roumie CL, Mitchel E, Gideon PS, Varas-Lorenzo C, Castellsague J and Griffin MR. Validation of ICD-9 codes with a high positive predictive value for incident strokes resulting in hospitalization using Medicaid health data. Pharmacoepidemiol Drug Saf. 2008;17:20–6. [DOI] [PubMed] [Google Scholar]
- 18.Bick AG, Akwo E, Robinson-Cohen C, Lee K, Lynch J, Assimes TL, DuVall S, Edwards T, Fang H, Freiberg SM, Giri A, Huffman JE, Huang J, Hull L, Kember RL, Klarin D, Lee JS, Levin M, Miller DR, Natarajan P, Saleheen D, Shao Q, Sun YV, Tang H, Wilson O, Chang KM, Cho K, Concato J, Gaziano JM, Kathiresan S, O’Donnell CJ, Rader DJ, Tsao PS, Wilson PW, Hung AM and Damrauer SM. Association of APOL1 Risk Alleles With Cardiovascular Disease in Blacks in the Million Veteran Program. Circulation. 2019;140:1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li R and Chambless L. Test for additive interaction in proportional hazards models. Annals of epidemiology. 2007;17:227–36. [DOI] [PubMed] [Google Scholar]
- 20.Rothman KJ, Greenland S and Walker AM. Concepts of interaction. American Journal of Epidemiology. 1980;112:467–70. [DOI] [PubMed] [Google Scholar]
- 21.Fine JP and Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 22.Harrell FE Jr. Regression Modelling Strategies with Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. Second ed. New York: Springer; 2015. [Google Scholar]
- 23.Sim JJ, Bhandari SK, Shi J, Reynolds K, Calhoun DA, Kalantar-Zadeh K and Jacobsen SJ. Comparative risk of renal, cardiovascular, and mortality outcomes in controlled, uncontrolled resistant, and nonresistant hypertension. Kidney international. 2015;88:622–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas G, Xie D, Chen H-Y, Anderson AH, Appel LJ, Bodana S, Brecklin CS, Drawz P, Flack JM, Miller ER 3rd, Steigerwalt SP, Townsend RR, Weir MR, Wright JT Jr., Rahman M and Investigators CS. Prevalence and Prognostic Significance of Apparent Treatment Resistant Hypertension in Chronic Kidney Disease: Report From the Chronic Renal Insufficiency Cohort Study. Hypertension (Dallas, Tex : 1979). 2016;67:387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Beus E, Bots ML, van Zuilen AD, Wetzels JF and Blankestijn PJ. Prevalence of Apparent Therapy-Resistant Hypertension and Its Effect on Outcome in Patients With Chronic Kidney Disease. Hypertension. 2015;66:998–1005. [DOI] [PubMed] [Google Scholar]
- 26.Greenland S, Lash TL and Rothman KJ. “Concepts of interaction,” chapter 5. In: Rothman KJ, Greenland S. and Lash TL, eds. Modern Epidemiology. 3rd ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2008. [Google Scholar]
- 27.VanderWeele TJ and Knol MJ. A tutorial on interaction. Epidemiol Methods. 2014;3:33–72. [Google Scholar]
- 28.Darroch J. Biologic synergism and parallelism. Am J Epidemiol. 1997;145:661–8. [DOI] [PubMed] [Google Scholar]
- 29.Cheung AK, Rahman M, Reboussin DM, Craven TE, Greene T, Kimmel PL, Cushman WC, Hawfield AT, Johnson KC, Lewis CE, Oparil S, Rocco MV, Sink KM, Whelton PK, Wright JT, Basile J, Beddhu S, Bhatt U, Chang TI, Chertow GM, Chonchol M, Freedman BI, Haley W, Ix JH, Katz LA, Killeen AA, Papademetriou V, Ricardo AC, Servilla K, Wall B, Wolfgram D and Yee J. Effects of Intensive BP Control in CKD. Journal of the American Society of Nephrology. 2017;28:2812–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright J, Jackson T, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, Hebert L, Jamerson K, Lewis J, Phillips RA, Toto RD, Middleton JP, Rostand SG, Disease ftAASoK and Group HS. Effect of Blood Pressure Lowering and Antihypertensive Drug Class on Progression of Hypertensive Kidney DiseaseResults From the AASK Trial. JAMA. 2002;288:2421–2431. [DOI] [PubMed] [Google Scholar]
- 31.Appel LJ, Wright JT, Greene T, Agodoa LY, Astor BC, Bakris GL, Cleveland WH, Charleston J, Contreras G, Faulkner ML, Gabbai FB, Gassman JJ, Hebert LA, Jamerson KA, Kopple JD, Kusek JW, Lash JP, Lea JP, Lewis JB, Lipkowitz MS, Massry SG, Miller ER, Norris K, Phillips RA, Pogue VA, Randall OS, Rostand SG, Smogorzewski MJ, Toto RD and Wang X. Intensive Blood-Pressure Control in Hypertensive Chronic Kidney Disease. New England Journal of Medicine. 2010;363:918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown JM, Robinson-Cohen C, Luque-Fernandez MA, Allison MA, Baudrand R, Ix JH, Kestenbaum B, de Boer IH and Vaidya A. The Spectrum of Subclinical Primary Aldosteronism and Incident Hypertension: A Cohort Study. Annals of internal medicine. 2017;167:630–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishizaka MK, Zaman MA and Calhoun DA. Efficacy of low-dose spironolactone in subjects with resistant hypertension. Am J Hypertens. 2003;16:925–30. [DOI] [PubMed] [Google Scholar]
- 34.Agarwal R, Rossignol P, Romero A, Garza D, Mayo MR, Warren S, Ma J, White WB and Williams B. Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet. 2019;394:1540–1550. [DOI] [PubMed] [Google Scholar]
- 35.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E and Pollak MR. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL and Stamler J. End-stage renal disease in African-American and white men. 16-year MRFIT findings. Jama. 1997;277:1293–8. [PubMed] [Google Scholar]
- 37.Wright JT Jr., Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr., Fine LJ, Cutler JA, Cushman WC, Cheung AK and Ambrosius WT. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hung AM, Roumie CL, Greevy RA, Liu X, Grijalva CG, Murff HJ, Ikizler TA and Griffin MR. Comparative effectiveness of incident oral antidiabetic drugs on kidney function. Kidney Int. 2012;81:698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.