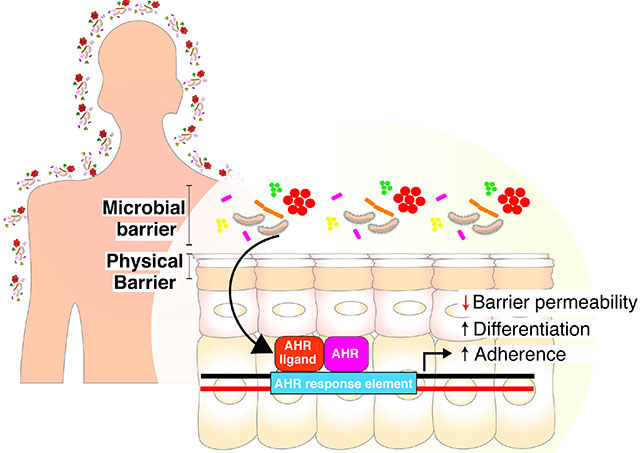
SUMMARY
The epidermis forms a barrier that defends the body from desiccation and entry of harmful substances, while also sensing and integrating environmental signals. The tightly orchestrated cellular changes required for the formation and maintenance of this epidermal barrier occur in the context of the skin microbiome. Using germ free mice, we demonstrate the microbiota is necessary for proper differentiation and repair of the epidermal barrier. These effects are mediated by microbiota signaling through the aryl hydrocarbon receptor (AHR) in keratinocytes, a xenobiotic receptor also implicated in epidermal differentiation. Mice lacking keratinocyte AHR are more susceptible to barrier damage and infection, during steady state and epicutaneous sensitization. Colonization with a defined consortium of human skin isolates restored barrier competence in an AHR-dependent manner. We reveal a fundamental mechanism whereby the microbiota regulates skin barrier formation and repair, with far-reaching implications for the numerous skin disorders characterized by epidermal barrier dysfunction.
eTOC
A self-renewing skin barrier is required for terrestrial life. Uberoi et al. demonstrate that the skin microbiota regulates barrier repair and integrity by activating keratinocyte aryl hydrocarbon receptor (AHR). This microbiota-AHR axis was targeted with a defined consortium of human skin commensals to improve barrier repair in disease models.
Graphical Abstract
INTRODUCTION
The skin is the primary barrier between the human body and the environment and functions to prevent desiccation and entry of foreign and/or harmful substances. The barrier properties of the skin reside in the epidermis, a semi-permeable stratified epithelium that is formed through keratinocyte terminal differentiation. Though continuously exposed to xenobiotic toxins, physical insults, and pathogenic microbes, the epidermis is also associated with diverse commensal microbial communities that are critical players in regulating skin physiology (Flowers and Grice, 2020). These microbial communities, collectively referred to as skin microbiota, are specialized to thrive in the unique nutrient and environmental conditions of this organ. The skin microbiome is topographically diverse, temporally complex, and distinct from other organs (Byrd et al., 2018; Grice and Segre, 2011). How the commensal microbiota influences development of skin’s barrier function is undefined, as are the molecular mechanisms that mediate these interactions.
The barrier function of the skin may be conceptualized as four intertwined “levels” consisting of microbial, immune, chemical, and physical barriers (Eyerich et al., 2018). The skin microbiome itself provides a barrier to pathogenic micro-organisms via a variety of different antimicrobial mechanisms (Parlet et al., 2019). This outermost microbial barrier also interacts with and mediates other functional levels of the cutaneous barrier. Skin microbiota play a fundamental role in the induction, training, and function of the skin immune barrier in part through the release of antimicrobial peptides, short-chain fatty acids, and polyamines (Belkaid and Segre, 2014). Neonatal colonization by microbiota has long-lasting impacts on adult immune barrier as commensal skin microbes’ prime immune cells to differentiate between commensal versus pathogenic bacterium (Leech et al., 2019). Bacterial lipases can hydrolyze lipids resulting in production of free fatty acids that impact the acidic surface pH of the skin, which dictates the chemical barrier of the skin (Elias, 2007; Schmid-Wendtner and Korting, 2006). While studies in gnotobiotic mice suggest that epidermal differentiation and barrier genes are microbially regulated (Meisel et al., 2018), mechanistic roles for the skin microbiota in development, regeneration, and function of the physical barrier are not well defined.
The epidermal permeability barrier (EPB) comprises of the stratum corneum and a complex system of tight junctions and adhesion complexes and their associated cytoskeletal networks that mediate cell-cell adhesion to create a mechanical barrier between the environment and underlying tissue (Simpson et al., 2011). In addition to congenital barrier deficiencies, EPB dysfunction is a hallmark of inflammatory skin diseases, including atopic dermatitis and psoriasis, and predisposes skin to infections (Elias, 2018; Kubo et al., 2012; Roberson and Bowcock, 2010). Epicutaneous sensitization, as a result of epidermal barrier dysfunction, may lead to atopic and allergic disease (Spergel et al., 1998).Thus, there is an urgent scientific and clinical need to define the mechanistic basis by which the commensal microbiota regulate homeostatic barrier function, as such mechanisms provide new targets for prevention and/or intervention in skin barrier deficiencies.
The sensing of xenobiotics, or compounds foreign to a living organism, is critical for barrier defense and homeostasis (Mackowiak et al., 2018). Keratinocytes function as sentinels that sense and respond to external stimuli (Nestle et al., 2009). Activation of xenobiotic receptors in keratinocytes induces expression of detoxification enzymes and membrane transporters that promote elimination of toxic compounds (Kazem et al., 2019). Roles for xenobiotic receptors extend to cellular processes beyond xenobiotic metabolism that include cellular proliferation, tissue repair, and immune responses (Mackowiak et al., 2018). The diversity of molecular signals produced by skin microbes, and how keratinocytes decipher and respond to them, remain largely unexplored.
The aryl hydrocarbon receptor (AHR) is a xenobiotic receptor that has emerged as a critical player in skin homeostasis and EPB development, function, and integrity (Esser et al., 2013; Furue et al., 2019; Furue et al., 2014). Activation of the AHR, a ligand-activated transcription factor of the basic, helix-loop-helix motif-containing Per-ARNT-Sim family (Burbach et al., 1992), induces a variety of epidermal differentiation and barrier genes, accelerates terminal differentiation, and increases stratum corneum thickness (Esser et al., 2013; Furue et al., 2014; Kennedy et al., 2013; Sutter et al., 2011; Sutter et al., 2009). The AHR can be activated by halogenated and non-halogenated aromatic hydrocarbons, including dioxins such as 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD), polychlorinated biphenyls (PCBs) and polycyclic aromatic hydrocarbons (PAHs); clinically used drugs, food-derived molecules, endobiotics, and bacterial metabolites (Esser, 2016; McIntosh et al., 2010; Nguyen and Bradfield, 2008; Stevens et al., 2009). Emerging evidence demonstrates that commensal skin microbes produce metabolites that activate the AHR and mediate various inflammatory responses (Magiatis et al., 2013; Yu et al., 2019). The efficacy of topical AHR agonists such as coal tar suggests that targeting this axis may be of therapeutic benefit in epidermal barrier and inflammatory skin disorders (Smith et al., 2017; van den Bogaard et al., 2013). In turn, AHR may sculpt skin microbial communities via upregulation of antimicrobial peptides and barrier genes (Smits et al., 2020).
Here, we investigated the role of commensal microbiota in regulation of permeability barrier homeostasis of skin. We found that commensal microbes are necessary for normal epidermal differentiation, EPB function, and repair. These effects were mediated by microbial signaling through the keratinocyte AHR. Murine skin lacking keratinocyte AHR signaling displayed increased barrier permeability, enhanced susceptibility to infection by S. aureus, and increased pathology in a model of atopic dermatitis. We show that topical colonization with a defined consortium of human skin commensals improves EPB function in murine germ-free skin and models of barrier dysfunction. Our findings reveal a fundamental role for the commensal skin microbiota in regulating the physical integrity and repair of the skin barrier, provides mechanistic insights into microbial-skin crosstalk, and uncovers therapeutic targets for improving skin barrier function.
RESULTS
Epithelial development and differentiation programs are impaired in germ free skin
To characterize microbially-mediated regulation of homeostatic epithelial gene expression programs, we performed RNA-seq on epidermal sheets isolated from dorsal skin of C57BL/6 mice of 3 different colonization states (n=8 mice each. Figure 1A): Specific pathogen free (SPF) mice that were conventionally raised in presence of microbiota, germ free (GF) mice born and raised in sterile gnotobiotic isolators, and a third group of mice that were born GF and then colonized (COL) with SPF microbiota for 2 weeks. Within 2 weeks, COL mice were colonized with similar microbiota as SPF mice as determined by 16S ribosomal RNA (rRNA) gene sequencing (Figure 1B, Figure S1A). We identified differentially expressed genes (DEGs) between colonization states by training negative-binomial linear models using DESeq2 R package and 3-way comparisons. This analysis revealed 6396, 427, and 3232 DEGs for SPF vs. GF, COL vs. GF and SPF vs. COL comparisons, respectively (Figure 1C, Table S1). We focused on the 396 shared DEGs of SPF and COL epidermis when compared to GF epidermis (Figure 1D). We reasoned that this subset of DEGs meet the criteria of being induced and sustained by microbial colonization, suggesting homeostatic control. The 396 DEGs were significantly enriched for biological functions such as skin development, keratinocyte differentiation, and epidermis development (Figure 1E). This result suggests the microbiota plays an important role in epithelial barrier formation.
Figure 1. Commensal microbiota regulates epithelial barrier genes.
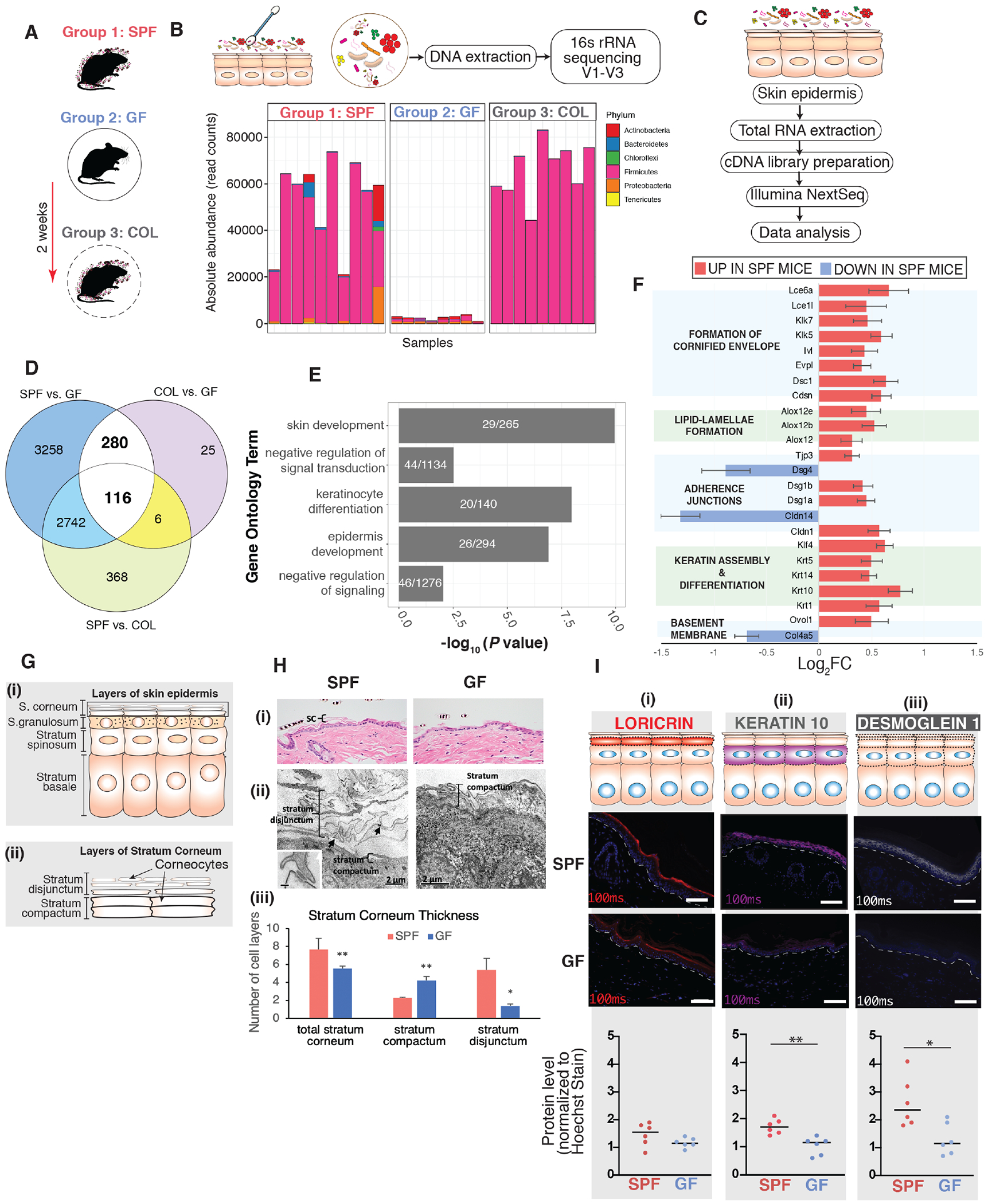
(A) Three groups of mice were employed, specific pathogen free (SPF), germ free (GF), and germ-free mice colonized (COL) with SPF microbiota for 2 weeks [n=8 mice (4 female,4 male)/group]. (B) Skin microbiota composition determined by 16S rRNA gene sequencing. Y-axis indicates absolute read counts of most abundant phylum (by relative abundance in the dataset) for each mouse (x-axis). (C) RNA-seq workflow. (D) Overlap of differentially expressed genes when comparing groups of gnotobiotic mice. (E) Shown in white are the number of genes that were further analyzed for uniquely enriched gene ontology biological process terms for aforementioned DEGs. Shown on the y-axis are the uniquely enriched terms, with p-values indicated on the x-axis. P-values are based on Fisher’s exact test and FDR-adjusted under dependency using the “BY” method. (F) Genes involved in different facets of epithelial barrier. Shown are genes that were differentially expressed in the SPF vs GF subset (p<0.001). Horizontal bars represent the Log2 fold-change comparison (genes upregulated in SPF: log2FC> 0, downregulated in SPF: log2FC < 0). Error bars represent standard error estimate for the log2 fold-change. (G) Schematic depicting layers of (i) skin epidermis and (ii) stratum corneum to aid understanding of histopathological and ultrastructural analyses in panel H. (H) Structural analysis of dorsal skin from age-matched GF and SPF mice. Light microscopy of (i) Hematoxylin and Eosin and (ii) Electron microscopy (EM) of reduced osmium tetroxide-stained tissue. Black arrows indicate connections between peripheral ends of corneocytes. Inset (scale bar=100nm) shows a corneodesmosome attaching the ends of two corneocytes. In Panels (i-ii): SC = stratum corneum; C = corneocyte; CD = corneodesmosome (iii) Quantification of the number of cell layers in the stratum corneum depicted in panel (ii) (* p<0.05; ** p< 0.01; T-test). (I) Tail-skin from SPF and GF mice. Immunofluorescence-based detection of differentiation markers (i) loricrin (red) and (ii) keratin-10 (purple) and adhesion marker (iii) desmoglein-l(grey). Nuclei are counter-stained with Hoechst stain (blue). White dashed-line indicates boundary separating epithelial-stromal compartments. Scale bar (10μm) is indicated in white (bottom-right). Images were taken (n=6 mice) at constant light exposure and integrated density of signal was normalized to Hoechst signal. Each dot corresponds to average normalized signal across 10-12 random images for each mouse. Asterisk indicates statistical significance (p<0.05, T test, two-sided). See also Table S1, S2, S3, and Figure S1.
To further examine DEGs involved in epithelial barrier function, we manually curated genes involved in different facets of epithelial barrier: keratinization, cornified envelope formation, adherence and gap junction, basement membrane function, barrier development, keratinocyte differentiation and intercellular lipid-lamellae processing (Table S2). Focusing on the SPF vs. GF subset of DEGs, multiple genes across each of these categories were expressed at lower levels in GF mice (Figure 1F, Tables S3). In particular, genes critical for cornified envelope formation [e.g. involucrin (Ivl), envoplakin (Evpl)] and its desquamation, [e.g. Kallikrien-related peptidases 5, 7(Klk5, 7)] were downregulated in GF skin. We hypothesized that such significant and widespread differences in gene expression would result in structural differences between GF and SPF skin. Consistent with prior reports (Meisel et al., 2018; Yockey et al., 2013) we did not notice any overt differences between the epithelial organization of GF vs SPF mice by traditional histopathological examination (Figure 1G, 1H, Figure S1B). On closer inspection, the GF mouse skin lacked the “basket-weave” appearance that normally characterizes the outer layers of the stratum corneum in mice and humans (Figure 1H, Panel i). Electron microscopy (EM) showed that SPF mice had a greater number of individual layers of the stratum corneum compared to GF mice (Figure 1H, Panel ii,iii). Layers of the stratum disjunctum appeared to be attached to one another almost exclusively by corneodesmosomes at the lateral edges of corneocytes. This pattern was much more prevalent in SPF versus GF mice. In contrast, the few layers of stratum corneum in GF mice were attached by corneodesmosomes dispersed throughout the intercellular space (Figure 1H, Panel ii). Other epidermal structures relevant to barrier function were similar in SPF and GF mice, including the lamellar secretory system and barrier-providing lipid lamellae (Figure S1C–E).
Immunofluorescence-based analysis of molecular biomarkers showed significantly decreased expression of cytokeratin-10 in skin of GF mice (Figure 1I), implicated in barrier integrity (Chamcheu et al., 2011; Jensen et al., 2000; Schmuth et al., 2004). Among genes that were downregulated in GF skin were tight and adherens junctions family members (Figure 1F) such as tight junction protein 3 (Tjp3), desmogleins 1a-b (Dsg1), and claudin-1 (Cldn1) that are critical players involved in skin barrier formation (Brandner et al., 2015). Remarkably, tight junction integrity appeared compromised in the suprabasal epithelium as evident by downregulated and diffused expression of Dsg1 in GF mouse epidermis (Figure 1I). Disruption of Dsg1 is associated with improper formation of desmosomes in suprabasal epithelia and skin barrier impairment (Broussard et al., 2020; Elias et al., 2001; Samuelov et al., 2013). Together, these findings suggest the hypothesis that skin barrier formation requires the commensal microbiota.
Commensal microbiota promotes skin barrier function and repair
A fully functioning stratum corneum closely controls the water concentration gradient in the skin such that passive diffusion of water occurs from inner layers towards the outside. Barrier disruption compromises the ability of the stratum corneum to maintain this water concentration gradient and results in increased transepidermal water loss (TEWL), measured using a sensor for water vapor flow density (Fluhr et al., 2006; Grubauer et al., 1989). Low TEWL values are indicative of intact skin and increased TEWL is associated with a disrupted barrier (Figure 2A, Panel i). We found that both male and female wild-type C57BL6/J mice could repair barrier that was disrupted to TEWL ~15-20 g/m2/h within 24 hours (Figure S2A–C); therefore, we chose this model for germ-free barrier disruption experiments to reduce experimental time for GF mice to maintain germ-free state. Under basal conditions, GF mice had slightly increased TEWL compared to SPF mice corroborating the findings from ultrastructure analysis (Figure S2D,E). Skin barrier of GF mice was perturbed more readily with tape stripping than SPF skin (Figure S2F), consistent with fewer layers of stratum corneum in GF mice. After comparable insults following tape-strip injury (TEWL ~15-20 g/m2/h), SPF mice more rapidly repaired their barrier compared to GF mice when measured over a period of 24 hours (Figure 2B). We observed similar delays in barrier recovery in GF Rag1−/− mice (that lack mature T and B-cells) compared to age-matched SPF Rag1−/− mice (Figure 2C, Figure S2G,H) suggesting that microbially-regulated adaptive immune responses are not responsible for the delayed barrier repair phenotype. Skin from GF Rag1−/− mice also showed decreased expression of genes involved in terminal differentiation and formation of transmembrane junctions compared to SPF Rag1−/− mice (Figure S2I) as seen in wild-type mice.
Figure 2. Commensal microbiota promotes skin barrier repair function.
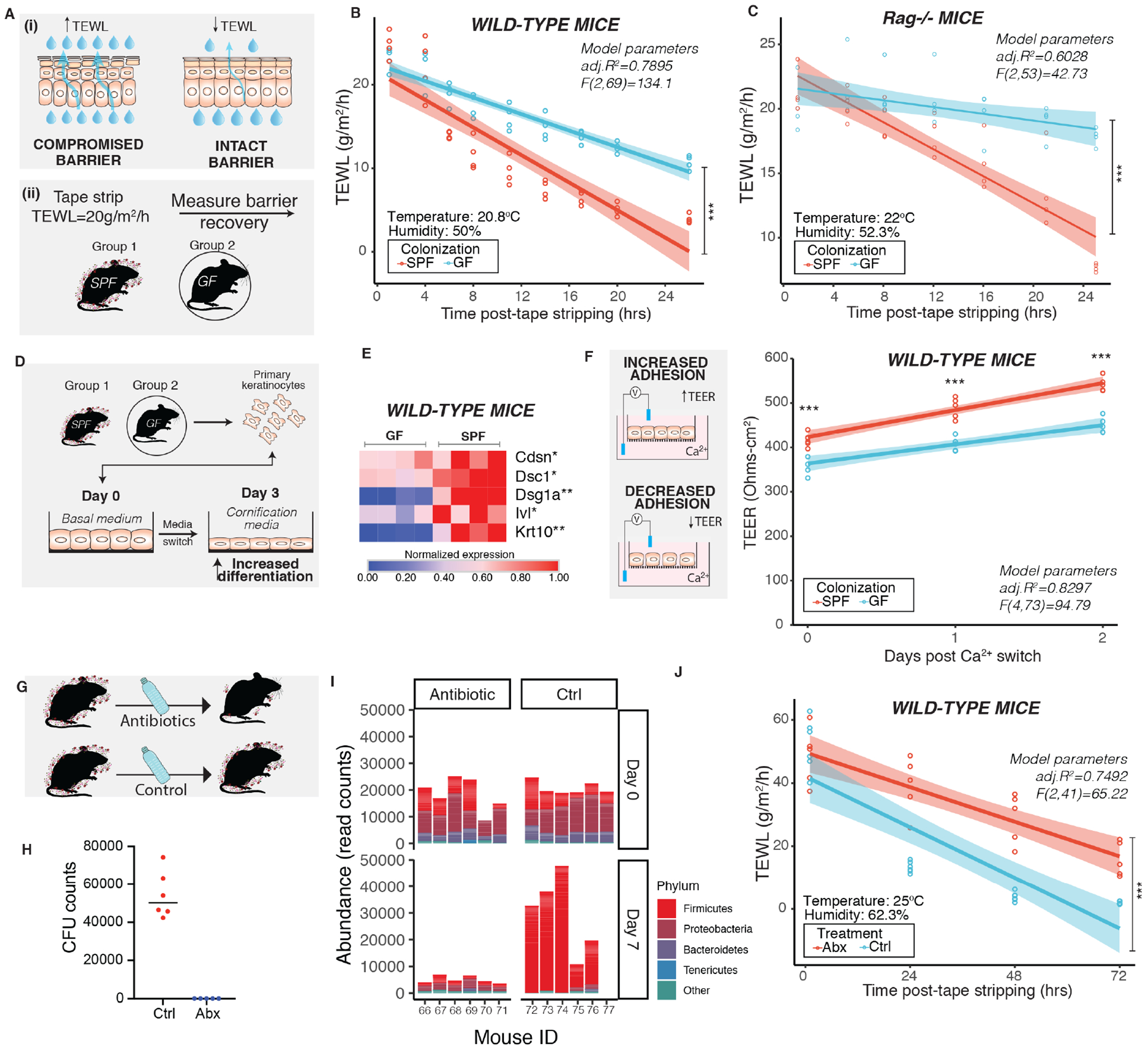
(A) Schematic depicts (i) principle of measuring transepidermal water loss (TEWL) to assess barrier repair function in adult mice (6-8 weeks old) and (ii) experimental design for assessing barrier recovery. Effect of colonization of microbes was assessed by comparing age-matched germ-free (GF) and specific pathogen-free (SPF) mice (n=4 male mice per group) in (B) wild-type C57/BL6 mice [ANCOVA, F (1,69) =50.649, ***P<0.001)] and (C) Rag1−/− mice [ANCOVA, F (1,53) =188.1, ***P<0.001)]. (D) Primary mouse keratinocytes derived from wild-type GF (n=4) and SPF (n=4) C57/BL6 mice were terminally differentiated. (E) Expression of genes involved in differentiation and adherence were assessed by qRT-PCR. Each square represents average readings from keratinocytes (n= 4 technical replicates) derived from an individual mouse (n=4 mice per group). *P<0.01, ** P<0.001 by T-test adjusted by Bonferroni correction. (F) Primary keratinocytes were grown on transwells. Epithelial adhesion was assessed by measuring transepithelial electrical resistance (TEER) at indicated time points. Data from one experiment is represented for visualization (See Figure S2). One dot represents average TEER readings from technical replicates (n=3) derived from one individual mouse (n=4 mice per group). ***P<0.001 by two-way ANOVA adjusted for multiple experiments. (G) To decrease skin microbial burden, wild-type SPF mice were treated with antibiotic cocktail (n=5 male mice/group) or vehicle (n=6 male mice/group) for two weeks. (H) Mice were swabbed 14 days after treatment and colony forming units (CFU) were determined. (I) Genomic DNA was extracted from swabs collected at baseline (Day 0) and after one week of treatment (Day 7). The V1-V3 region of the 16S rRNA gene was sequenced and analyzed. Shown is abundance of read counts classified to different phyla in each sample. Phyla with total read count <1000 are grouped into ‘Other’. (See Figure S3) (J) At the end of two weeks mice were tape stripped and TEWL was measured and plotted against time [ANCOVA, F (1,41) =26.315, ***P<0.001)]. TEWL vs time readings were fitted by linear modeling (in B, C, F and J) and significance was assessed by ANCOVA. Modeling parameters (adjusted R2 and F-statistics) are indicated on top-right for each plot. Span (shaded area) represents 95% Cl. Temperature and humidity conditions during TEWL measurement are indicated for each experiment. Also see Figures S2 and S3.
Basal keratinocytes undergo a spatiotemporal and highly controlled differentiation program dependent on intracellular calcium flux to establish and maintain barrier (Tellkamp et al., 2014). In presence of high calcium, primary epidermal keratinocytes can differentiate in vitro to express genes involved in formation of cornified envelope (Hennings et al., 1980). We derived murine primary epidermal keratinocytes from GF and SPF skin, respectively, and exposed them to high calcium containing medium (Figure 2D). We observed that keratinocytes derived from GF mice retained reduced expression of differentiation genes, compared to SPF mice, even after primary cultures were established (Figure S2). Following exposure to high calcium, expression of genes involved in terminal differentiation [cytokeratin-10 (Krt10), involucrin (Ivl)] and formation of transmembrane junctions [Corneodesmosin (Cdsn), Desmocollin-1(Dsc1), Desmoglein-1a (Dsg1a)] were reduced in GF keratinocytes compared to SPF keratinocytes (Figure 2E, Figure S2J). Additionally, GF keratinocytes showed decreased transepithelial electrical resistance (TEER) which is indicative of decreased transmembrane junction strength (Figure 2F, Figure S2K).
To examine implications of perturbing microbiota on skin barrier function we used an antibiotic depletion model (Figure 2G). Antibiotics traditionally used to disrupt gut microbiota in mice were not sufficient to disrupt skin microbiota in mice in prior studies (Naik et al., 2012). We developed a regimen consisting of antibiotics that are administered orally in hospitals and veterinary clinics to target skin bacteria (Beco et al., 2013; Bowen et al., 2017; Derrick and Reilly, 1983; Lofmark et al., 2010) and were able to inhibit prominent murine skin commensal Staphylococcus xylosus (Nagase et al., 2002; SanMiguel et al., 2017). Oral administration of antibiotics for two weeks diminished microbial burden on skin as observed by both quantitative cultures and 16S rRNA gene sequencing (Figure 2H, I) but had comparatively reduced impact on microbial burden in the gut (Figure S3). Antibiotic-treated mice were delayed in barrier repair compared to control mice that were treated with vehicle (Figure 2J). Together, these data confirm a role for commensal microbes in promoting skin barrier function and repair.
Aryl hydrocarbon receptor pathway is attenuated in germ free skin
The sensing of external physiological and chemical signals is critical for barrier defense and homeostasis in the skin (Mackowiak et al., 2018). We hypothesized that xenobiotic receptors that act as epithelial sensors and relay microbial signals would be among those genetic pathways dysregulated in GF epidermis. Previous studies have identified at least 304 xenobiotic processing genes (XPGs) in mice, which encode the enzymes, transporters, and transcription factors required to metabolize xenobiotics (Fu et al., 2016). We found that 52/304 XPGs were differentially expressed (P adj <0.1) in skin of SPF and GF mice, and the majority were upregulated in SPF mice (n=43/52; Table S4). The pregnane X receptor (PXR), constitutive androstane receptor (CAR), peroxisome proliferator-activated receptor-alpha (PPARa) and aryl hydrocarbon receptor (AHR) are key transcription factors that regulate xenobiotic processing in skin (Minzaghi et al., 2019). Of these, only the AHR gene was differentially expressed and was upregulated in SPF skin compared to GF skin. Canonically, after ligand binding, the AHR translocates to the cell nucleus and binds DNA at xenobiotic responsive elements (XRE), to regulate transcription of target genes (Wright et al., 2017). Expression of key downstream target genes, i.e. cytochrome-p450 Cyp1a1 and molecular chaperones Hsp90aa1 and Hsp90ab1 that respond to AHR activation, were either significantly downregulated or trended toward downregulation in GF murine epidermis (Figure 3A; Table S4). Primary keratinocytes derived from GF skin were also impaired in expression of these genes (Figure S2J). Overall, microbiota-mediated upregulation of AHR was consistent with changes in its canonical pathway, suggesting that regulation of xenobiotic sensing genes in the skin may be mediated through the AHR.
Figure 3. Activation of AHR signaling in skin rescues barrier dysfunction in germ free mice.
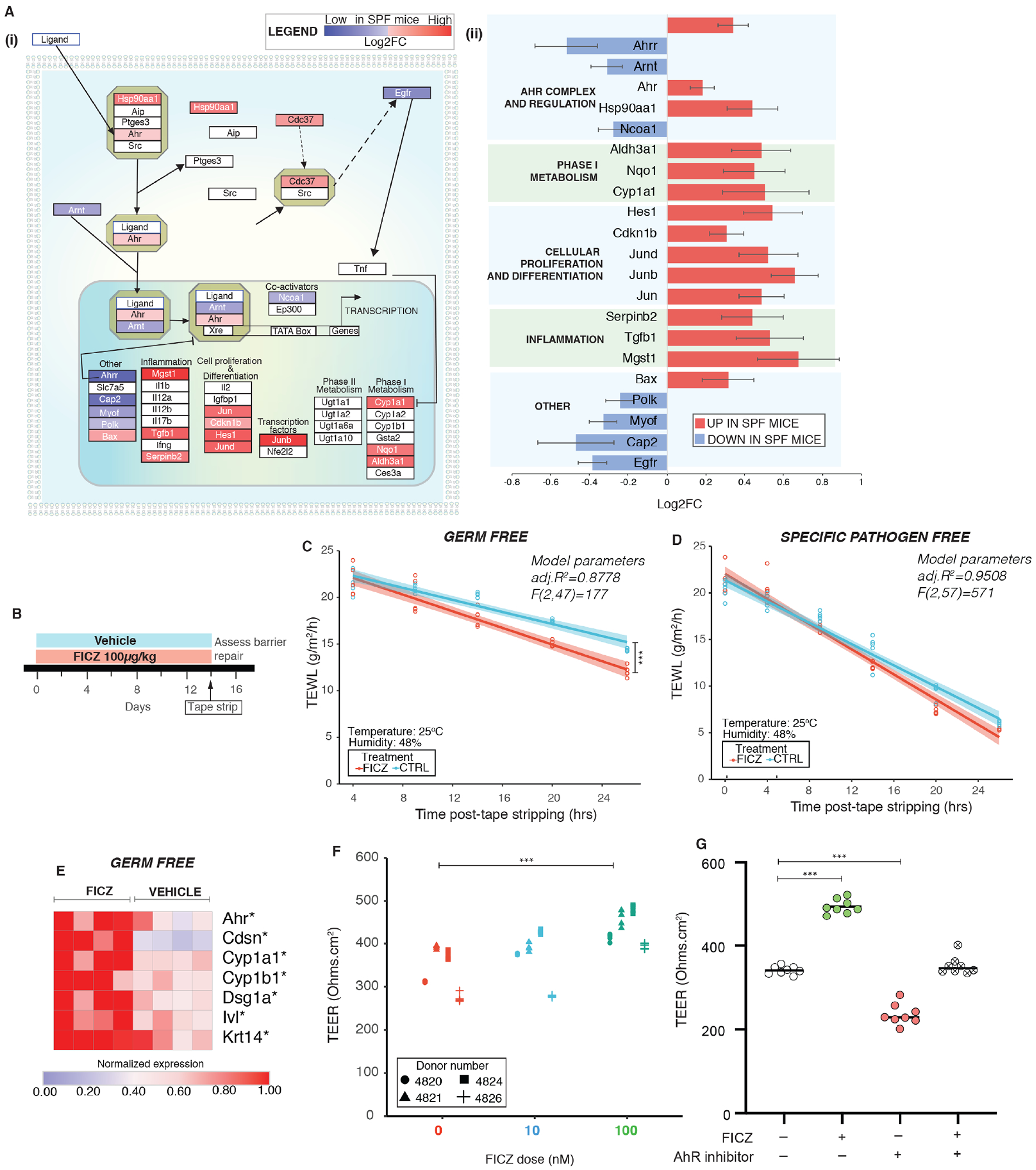
(A) Differentially expressed genes (DEGs) in SPF vs GF mice from RNAseq were (i) mapped onto the AHR pathway and (ii) Log2FC of significant DEGs (P<0.01) in SPF mice are represented. (B) Schematic illustrating experimental design. TEWL vs time readings were fitted by linear modeling and covariance was assessed by ANCOVA. Barrier recovery was compared in (C) GF mice [F (1,47) =21.9, ***P<0.001)] and (D) SPF mice [F(1,57)=2.98, *P=0.0492] that were either treated with FICZ or vehicle. (E) Expression of genes was assessed by qRT-PCR in GF skin treated with FICZ or vehicle (4 mice per group). *P<0.05, by T-test adjusted by Bonferroni correction. (F) Primary human keratinocytes grown on transwells in presence of FICZ (0nM, 10nM and 100nM) for three days and TEER was measured. Cells from different donors are represented by different symbol. See Figure S4. (G) Primary human keratinocytes were treated as indicated with FICZ and/or AHR inhibitor at 100nM doses. TEER values at the end of three days of treatment are reported. ***P<0.001 by T-test for panels F and G.
Since the AHR can activate multiple signaling pathways (Larigot et al., 2018), we explored the impact of microbial colonization on expression of genes reported to be a part of canonical and non-canonical AHR signaling networks (Figure 3A). In this context, we saw upregulation of several genes in SPF mice, including those involved in cell proliferation, differentiation, and inflammation (e.g. Hes1, Jun and Tgfb1). These findings suggest the AHR as a potential mechanism by which skin microbes modulate epithelial barrier integrity.
Treatment with an AHR agonist improves barrier function and recovery in germ free mice
We hypothesized that if the GF skin barrier phenotype we observed was due to attenuated AHR signaling, then activation via AHR ligand would improve barrier recovery. We treated adult GF mice topically with AHR ligand 6-Formylindolo[3,2-b] carbazole (FICZ), daily for 2 weeks at a low dose (100μg/kg) (Figure 3B), a regimen shown to induce expression of Cyp1a1 (Ehrlich et al., 2018). FICZ is a tryptophan photoproduct and is a well-characterized AHR ligand in skin (Fritsche et al., 2007; Syed and Mukhtar, 2015). At the end of treatment, we compared the rate of barrier recovery in tape-stripped GF mice that were either treated with FICZ or vehicle (Figure 3C). We observed that FICZ significantly accelerated barrier recovery in GF mice. In parallel, we also treated SPF mice with FICZ at the same dose and observed that FICZ accelerated early-stage barrier recovery (Figure 3D). FICZ treatment activated AHR signaling in the treated group as Cyp1a1 expression was induced in all FICZ treated mice in comparison to the untreated group (Figure 3E). Additionally, FICZ treatment increased expression of genes implicated in barrier repair (Figure 3E) in GF mice. AHR upregulation increases expression of genes involved in epidermal differentiation and formation of gap junctions (van den Bogaard et al., 2015). In primary human keratinocytes, FICZ increased epithelial resistance as measured by TEER (Figure 3F), which was diminished by co-treatment with an AHR inhibitor (Figure 3G). Overall, activation of the AHR in GF skin improves epidermal barrier recovery, supporting a mechanistic role for the commensal microbiota in homeostatic regulation of the AHR.
Mice deficient in epithelial AHR have a defective skin barrier and are more susceptible to infection
AHR is expressed in a variety of cell types in the skin, but abrogating AHR function in keratinocytes has been suggested to impact skin barrier (Haas et al., 2016). To confirm the role of keratinocyte AHR in skin barrier function, we generated mice where the floxed Ahr allele (Ahrf/f) was conditionally knocked out in the skin epithelia using a Cre driven by the keratin-14 promoter (K14CreAhrf/f). We found that topical treatment of K14CreAhrf/f skin with AHR ligand FICZ failed to induce increased expression of AHR downstream targets Ahrr, Cyp1a1, Cyp1b1 and Hsp90aa1 in the murine epidermis compared to Ahrf/f mice (Figure 4B). However, expression of downstream targets was induced in dermis and liver tissues (Figure S4A). This confirmed that Cre driven by keratin-14 promoter ablated AHR in murine epidermis. While mice that retained AHR function (Ahrf/f) repaired their barrier within 24 hours following tape-strip disruption, barrier repair was significantly diminished in K14CreAhrf/f skin (Figure 4B). Littermate controls (K14CreAhrf/+ and Ahrf/+) showed similar barrier recovery as Ahrf/f mice (Figure S4B). Further, gender did not appear to have a significant impact on barrier recovery (Figure S4C). Keratinocytes derived from K14CreAhrf/f mice showed increased TEER compared to Ahrf/f keratinocytes (Figure 4C). As seen previously in keratinocytes derived from mice lacking AHR constitutively (van den Bogaard et al., 2015), we observed that K14CreAhrf/f keratinocytes showed reduced expression of keratinocyte differentiation genes when exposed to high calcium compared to AHR-sufficient keratinocytes (Figure 4D).
Figure 4. Loss of AHR in keratinocytes impairs skin barrier in mice.
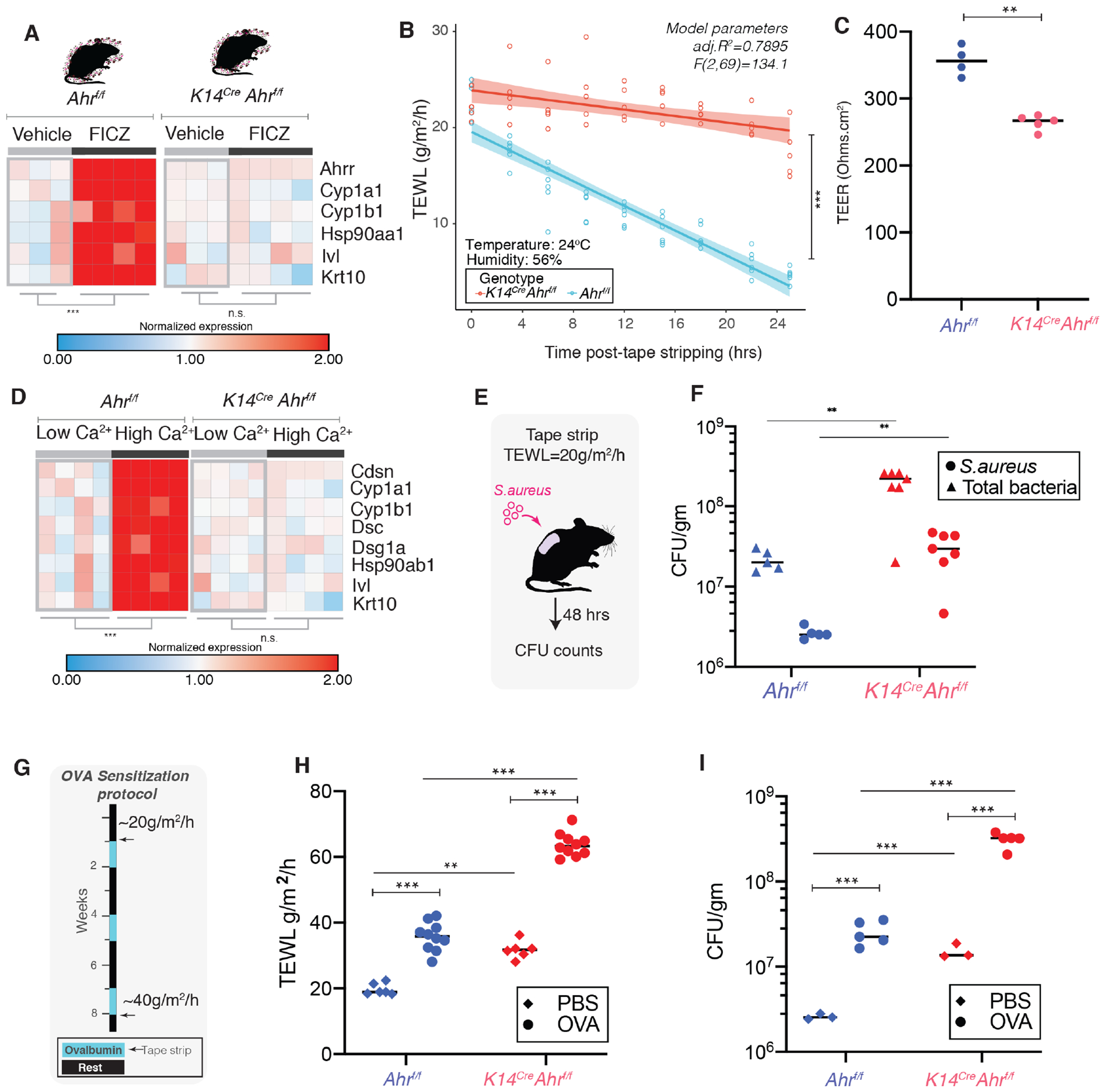
(A) Murine skin was treated with FICZ (1mg/Kg) and expression of genes involved in Ahr regulation and epithelial differentiation were assessed by qRT-PCR. **P<0.01, by T-test adjusted by Bonferroni correction. Each square represents average readings n= 3 technical replicates derived from an individual mouse (B) TEWL recovery curves were compared between Ahrf/f (n=5) and K14Cre Ahrf/f (n=6) mice [ANCOVA, F (1,96) =131.34, ***P<0.001)]. Primary mouse keratinocytes were derived from mice and polarized and (C) TEER was measured (**P<0.005, T-test). (D) Expression of genes involved in differentiation, adherence, and AHR downstream activation targets were assessed by qRT-PCR. Each square represents average readings from keratinocytes (n= 3 technical replicates) derived from an individual mouse (n=4 mice per group). ** P<0.01 by T-test adjusted by Bonferroni correction. (E) Ahrf/f (n=5) and K14Cre Ahrf/f (n=7) mice were tape-stripped (TEWL=20 g/m2/h) and 107 CFU S. aureus-tdTomato was applied to back skin. (F) 48 hours post-infection tissue was collected, weighed, homogenized and plated. S. aureus (visible as red colored colonies) and total bacterial colonies were counted (**P<0.005, T-test). (G) Ovalbumin epicutaneous sensitization model. At the end of final OVA treatment, mice were tape-stripped and 24 hours later (H) TEWL levels were assessed. (I) S. aureus was applied to back skin and CFUs were determined. Statistical significance in panels F, H and I were assessed using a 2-way ANOVA (**P<0.005, ***P<0.0005). (See Figure S4)
Patients with diseases of skin barrier impairment such as atopic dermatitis (AD) are highly susceptible to colonization and infection by pathogens including S. aureus (Alexander et al., 2020). To test if AHR-dependent barrier impairment leads to increased infection, we topically applied S. aureus to tape-stripped skin (Miller et al., 2019) and quantified bacteria following 48 hours. We observed enhanced infection of S. aureus and increased overall bacterial burden on tape stripped skin (Figure 4E) of K14CreAhrf/f mice compared to AHR sufficient controls (Figure 4F). Since barrier dysfunction is a hallmark in the development of AD (Cork et al., 2009), and mice lacking AHR are impaired in barrier repair, we hypothesized that these mice will be more prone to barrier damage, infection, and atopic disease. We adapted a mouse model where AD (Figure 4G, Figure S4D,E) is induced by repeated epicutaneous sensitization of tape-stripped skin with ovalbumin (OVA) (Spergel et al., 1998). In this model, K14CreAhrf/f skin was exacerbated in disease pathology, with enhanced TEWL (Figure 4H) and increased susceptibility to S. aureus infection compared to Ahrf/f mice (Figure 4I, Figure S4F). These data demonstrate that AHR function in keratinocytes is essential for barrier function, and loss of AHR can lead to enhanced barrier damage in the setting of inflammatory skin disease and facilitate bacterial entry.
Human skin microbial consortium restores barrier repair and function via the AHR
Given our observations that the commensal microbiota directly impacts barrier repair (Figure 1, 2) and regulates the AHR genetic pathway (Figure 3) in murine skin, we hypothesized that topical association of GF skin with human skin commensals would activate the AHR and restore skin barrier repair. To test this hypothesis, we first curated a collection of cultured skin microbes that were abundant and prevalent on healthy human skin. Referred to as Flowers’ Flora, the collection consists of members of Firmicutes phylum i.e. Staphylococcus epidermidis, S. warneri, S. hemolyticus and members of Actinobacteria phylum i.e. Micrococcus luteus, Corynebacterium aurimucosum (Figure 5A). These skin microbes activated AHR in keratinocytes as determined by way of a reporter assay consisting of the AHR reporter element conjugated with Cyp1a1 (Figure 5B). Flowers’ Flora colonized murine GF skin as determined by bacterial culture swabs and species-specific qPCR analysis (Figure 5C, D). After two weeks of colonizing with this defined consortium of human skin commensals (Figure 5E) barrier recovery function in GF skin was restored (Figure 5F). Skin of colonized mice showed elevated expression of differentiation genes as well as Cyp1a1 (Figure 5G) compared to GF skin. Upon terminal differentiation, keratinocytes derived from colonized mice were enhanced in TEER compared to GF controls (Figure 5H). Colonization of K14CreAhrf/f mice with Flowers’ Flora did not improve barrier repair (Figure 5I), further supporting an AHR-dependent mechanism. Finally, to determine if Flowers’ Flora could prevent barrier damage in AD-like disease, we pre-colonized wild-type SPF murine skin prior to short-term epicutaneous sensitization with OVA (Figure 5J, Figure S5). Pre-colonization with Flowers’ Flora significantly improved barrier recovery compared to skin that was not pre-colonized (Figure 5K). Together, these findings indicate that skin commensal microbes signal through the AHR to maintain homeostatic control of epidermal barrier integrity and suggest new targets for preventing and/or treating epidermal barrier dysfunction.
Figure 5. Commensal microbes curated from human skin restore skin barrier function via AHR activation.
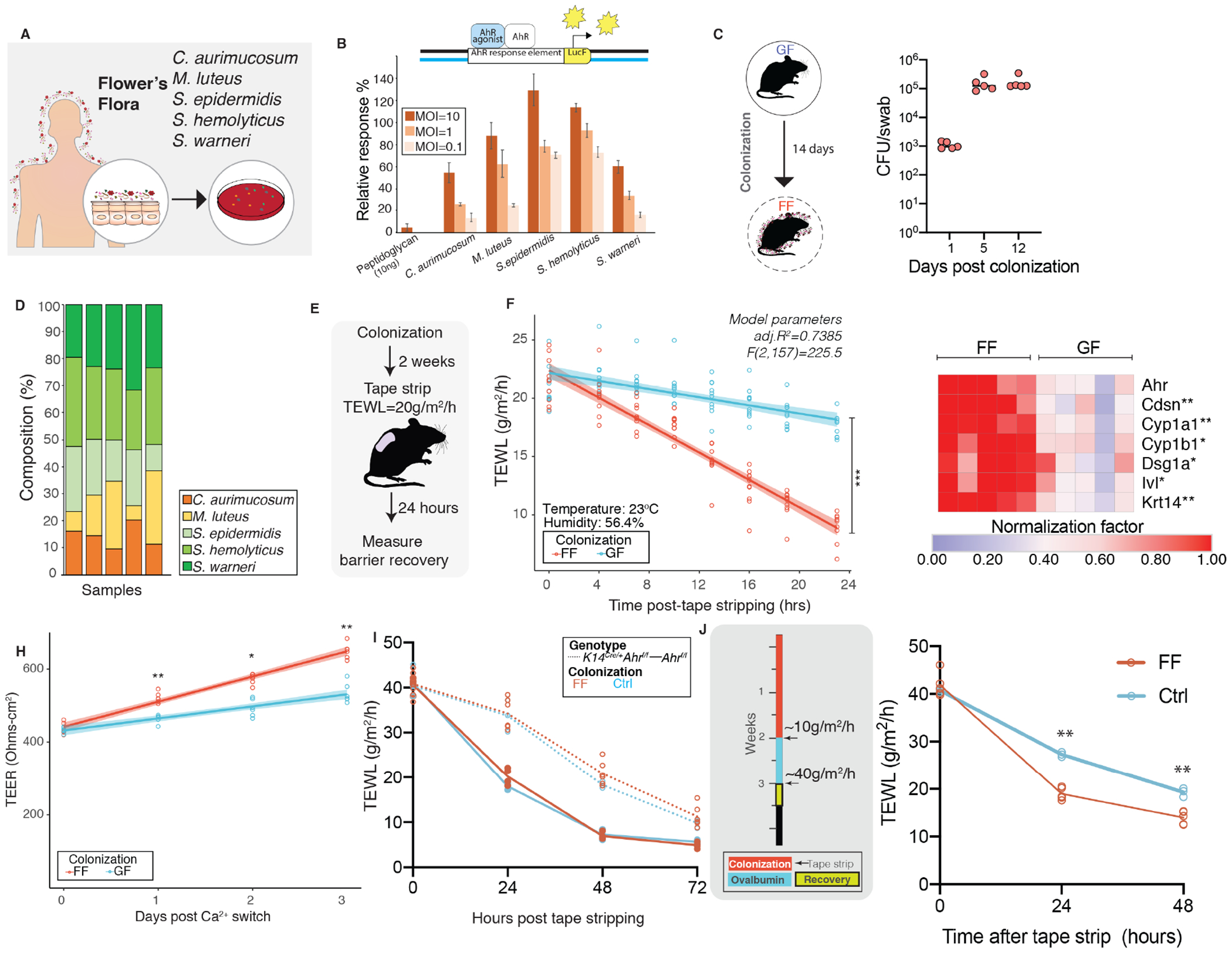
(A) Curation of bacteria for Flowers’ Flora (FF) consortium. (B) Reporter assay to assess AHR-activation in HaCaT cells using Cyp1a-luciferase reporter. Transfected cells were treated with indicated bacteria at indicated multiplicity of infection (MOI) and luminescence was measured. Relative response compared to 10nM FICZ treatment (positive control) was computed as follows: [(experimental sample ratio)-(negative control ratio)]/ [(positive control ratio)-(negative control ratio)]. (C) Germ-free mice were colonized with FF daily for two weeks. Mice were swabbed at indicated days and CFUs were enumerated. (D) To determine whether individual bacteria of FF colonized skin, qPCR analysis was conducted on genomic DNA extracted from skin swabs collected at day 14 using species-specific primers and percentage composition relative to total 16S rRNA was determined. (E) Two-weeks post colonization mice (n=5 mice/group) that were either germ-free (GF) or colonized with FF were tape stripped and (F) barrier recovery was assessed by TEWL (ANCOVA, F(1,157)=181.25, P<0.0001). (G) Expression of indicated genes was assessed by qRT-PCR in skin treated with FF or vehicle (GF). *P<0.05 and **P<0.005 by T-test, Bonferroni correction. (H) Primary mouse keratinocytes were derived, terminally differentiated and TEER was measured (**P<0.005, T-test). (I) To test if improved barrier recovery via FF is mediated through AHR, K14CreAhrf/f(n=6) were pre-colonized as shown in Fig. 4F and compared to K14CreAhrf/f (n=3) that were treated with Control (Ctrl) [ANCOVA, F(1,61)=0.1191, P=0.73115]. Additionally, Ahrf/f mice that were colonized (n=4) and untreated (n=3) were included in comparisons. (J) Experimental design to test if pre-colonization with FF could improve barrier recovery in OVA epicutaneous sensitization model. Barrier recovery kinetics were significantly improved in FF colonized versus control (non-colonized) mice [ANOVA, **P<0.01]. (See Figures S5).
DISCUSSION
The skin microbiome provides the first level of barrier defense to the human body. While commensal skin microbes have demonstrated effects on immune and chemical barriers of the skin, their regulation of the physical barrier is not well defined. Here we show that the microbiota regulates epidermal differentiation and barrier function through the AHR. Our findings parallel those in the gastrointestinal tract, where gut commensals regulate intestinal barrier formation by modulating epithelial turnover (Kaiko et al., 2016) and controlling mucus production (Johansson et al., 2015). However, unlike the simple mucosal epithelium that provides the intestinal barrier, the skin is composed of a multi-layered and terminally differentiating stratified squamous epithelium. Such complexity requires tightly orchestrated signals to balance differentiation with proliferation. Future studies will be required to address the identity of the microbial metabolites that interact spatially within the complex architecture of the skin.
The AHR is involved in many aspects of skin physiology, such as detoxification, cellular homeostasis, skin pigmentation, and skin immunity (Esser et al., 2013; Furue et al., 2019; Furue et al., 2014). Our data confirmed the findings that keratinocyte derived AHR is essential for skin barrier integrity in mouse skin (Haas et al., 2016). Mice deficient in AHR show enhanced inflammation in imiquimod-induced psoriasiform skin inflammation (Di Meglio et al., 2014). Here, we show in an OVA epicutaneous sensitization model that K14CreAhrf/f skin has exacerbated disease. Additionally, we found that keratinocytes derived from K14CreAhrf/f skin are impaired in epidermal differentiation and fail to form normal adherence junctions.
Our studies focused on the keratinocyte AHR and its role in forming the EPB, but the AHR can also be expressed by epidermal Langerhans cells, innate and adaptive immune cells, and dermal cells. We ruled out microbially-regulated adaptive immune responses, as GF Rag1−/− mice (that lack mature T and B-cells) were defective in barrier repair compared to age-matched SPF Rag1−/−' mice. Skin from GF Rag1−/− mice also showed decreased expression of genes involved in terminal differentiation and formation of transmembrane junctions compared to SPF Rag1−/− mice. Previous studies suggest that repair of the EPB is not dependent on AHR derived from Langerhans cells (Haas et al., 2016). However, during epicutaneous sensitization, Langerhans specific loss of AHR led to decreased Langerhans cells number and function and dysregulated T cell responses (Hong et al., 2020). Thus, AHR likely represents a sensor for Langerhans cell activation as part of the immunological barrier. Further studies will need to better define the cell-type specificity of AHR signaling to the different levels of barrier function.
Topical application of coal tar is one of the oldest therapies for atopic dermatitis and has been shown to activate AHR to induce epithelial differentiation (van den Bogaard et al., 2013). Recently, the natural product derived small molecule, Tapinarof, was found to bind and activate the AHR to moderate inflammatory responses in atopic dermatitis and psoriasis (Smith et al., 2017). In support of the hypothesis that AHR mediates microbial signals to promote barrier function, we found that treatment with the potent and selective AHR ligand FICZ at a low dose was able to restore epithelial barrier repair in GF mice and induced epithelial differentiation. However, the role of AHR in skin barrier regulation may be highly context dependent. In murine models, exposure to pollutants can lead to hyperactivation of AHR that results in skin barrier damage and inflammation, which mirrors the phenotype of mice that constitutively express AHR in the keratinocyte (Hidaka et al., 2017; Tauchi et al., 2005). Thus, the balance in the specificity and quantity of AHR ligand, from endogenous and environment sources, is likely a key factor in modulating downstream signaling and impact on the skin and requires further investigation.
We observed that keratinocytes derived from GF mice retained reduced expression of genes involved in differentiation and AHR pathway regulation, compared to SPF mice, even after primary cultures were established. This leads us to speculate that microbial colonization has long-term effects on the keratinocyte transcriptional program that lasts even after establishment in culture. It is possible these effects are mediated by epigenetic mechanisms (Woo and Alenghat, 2017). In the context of intestinal epithelial cells (IECs), it has been shown that 5′ CpG of Tlr4 was reduced in colonic IECs of GF mice compared to SPF mice (Takahashi et al., 2011). More recently, it has been shown that chromatin landscape in IECs is preprogrammed by the host in a region-specific manner to permit responses to microbiota (Camp et al., 2014). Whether such mechanisms exist in the context of skin remains to be discerned.
Our studies show that loss of AHR in skin led to increased susceptibility to the skin pathogen S. aureus. It remains to be determined whether increased susceptibility was due to impaired physical barrier, impaired antimicrobial barrier, or both. For example, the microbiota-induced antimicrobial protein RELMα protects against skin infection in a Vitamin A dependent manner (Harris et al., 2019). Since there is interaction between AHR and retinoic acid signaling pathways (Andreola et al., 1997; Murphy et al., 2007), this may represent a mechanism by which the skin microbiota mediates the antimicrobial barrier. The differential roles for pathogens and commensals in regulating AHR and promoting downstream effects are undefined, though are critical when considering diseases of barrier impairment or wounding, which are often complicated by S. aureus colonization and/or infection.
In summary, our findings show a role for skin microbiota in regulating epithelial differentiation and barrier function in stratified epithelia through AHR. These studies show that skin microbiome directly impacts development of the epidermal physical barrier. Future studies that address how microbial communities interact with each other to influence xenobiotic signals in homeostatic versus disease states will help leverage how personalized microbiota-based therapies can be used to improve the skin barrier.
STAR METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact and corresponding author, Elizabeth Grice (egrice@pennmedicine.upenn.edu).
Materials Availability Statement
All unique/stable reagents generated in this study are available from the Lead Contact with a complete Materials Transfer Agreement.
Data and Code Availability Statement
The datasets generated by this study are available as follows:
RNAseq data: NCBI SRA BioProject: PRJNA683863, GEO accession #: GSE162925 16S rRNA sequencing data: NCBI SRA BioProject: PRJNA684321
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animal models and husbandry conditions
All mouse experiments were conducted under protocols approved by the University of Pennsylvania Institutional Animal Care and Use Committee (Protocol #804065). Age-matched 6-8 weeks old male or female mice, as indicated, were used for all experiments. The following strains of mice were used in these studies: C57BL6/J (JAX stock #000664), Rag1 KO (JAX stock #002216)(Mombaerts et al., 1992), Ahrfx (JAX stock #006203)(Walisser et al., 2005) and K14Cre (JAX stock #018964)(Dassule et al., 2000).
Germ-free studies were conducted in the Penn Gnotobiotic Mouse facility. Indicated strains were bred and maintained as germ-free (GF) in flexible vinyl isolators at the Penn Gnotobiotic Mouse facility housed in the University of Pennsylvania, School of Veterinary Medicine. Mice were housed as 3-5 mice per cage, until they were euthanized for tissue harvest. Aggressive mice or those that showed scratching wounds were not used in the studies. SPF counterparts were purchased from Jackson Laboratories and allowed to acclimatize in the facility for one week prior to beginning any experiments. One week prior to beginning of an experiment, mice (GF or SPF) were transferred to hermetically-sealed cages with individually filtered-positive airflow. The mice were maintained in these cages for the duration of the study. This allowed similar housing conditions for both GF and SPF mice for consistent TEWL readings. All mice were given autoclaved bedding, water and irradiated chow (5021 Autoclavable Mouse Breeder Diet, LabDiet®). Mouse handling was conducted in a laminar flow cabinet through double layer protective gloves. Germ-free status of mice was confirmed by weekly bacterial checks by the germ-free facility. At the end of each experiment, mice were autopsied and germ-free status was confirmed by enlarged cecum. Additionally, skin swabs, fecal pellet, and bedding samples were cultured by on blood-agar plates. Investigators were not blinded to the colonization status of the mice.
Studies in wild-type and AHR KO mice were conducted in Clinical Research Building vivarium at the University of Pennsylvania. C57BL6/J mice were maintained and bred by lab personnel. To generate K14CreAhrf/f mice Ahrf/f mice were crossed with K14Cre mice to generate K14CreAhrf/+ F1 mice, K14CreAhrf/+ mice were backcrossed to Ahrf/f mice to obtain experimental mice (K14CreAhrf/f) and corresponding controls (Ahrf/f) lacking Cre on the same genetic background. To ensure robustness of studies, mice were randomly housed. Genotyping protocol: Ahrf/f status was determined using PCR primers OIMR6075-Reverse (5’ CAG TGG GAA TAA GGC AAG AGT GA 3’) and olMR6076-Forward (5’ GGT ACA AGT GCA CAT GCC TGC 3’) and resolving on a 5% polyacrylamide gel. Cre allele was determined using Generic Cre protocol (Protocol #22392, Jax Lab, Version 1.3) and resolved on a 2.5% Agarose gel.
Primary keratinocytes from adult mouse skin
Keratinocytes were derived from mouse tail or ear skin as described previously with slight modifications (Lichti et al., 2008). Following euthanasia mouse ears and/or tail were excised. With the help of forceps, ears were split into dorsal and ventral halves. To peel the tail skin from the bone, a scalpel was used to cut along the ventral axis from base of tail to tip. The exposed tail bone was peeled off using blunt-tip forceps. The resultant skin was cut into 0.75cm2 pieces. Skin obtained from ears and tails were incubated dermis side down in 6-well dishes and floated in ice-cold dispase (1mg/ml) in 1X PBS overnight at 4°C. Epidermal sheets were separated by lifting the epidermis using forceps. The separated epidermal sheets were cut into tiny pieces using forceps and scissors and incubated in 60mm untreated culture dish containing 2ml of 0.25% Trypsin-EDTA at 37°C, 5% CO2 for 15 minutes. At this point, 5ml suspension media (DMEM+10% FBS+ P/S) was added to the dish and the skin pieces were pipetted vigorously using a 10ml pipette to obtain a single cell suspension. Cell suspension was centrifuged at 150g for 5 minutes at 4°C and supernatant was removed. The cell pellet was suspended in 10ml suspension media and passed through 100μm cell strainer. Cell suspension was centrifuged at 150g for 15 minutes at 4°C, supernatant was removed and cells were suspended in 1ml suspension media and plated in collagen-coated 60mm dishes at 0.5 mouse equivalents (i.e. 5 million cells/ml) in plating media (low Ca2+ KSFM+ growth supplements+ 5% dialyzed FBS+4% DMEM) containing 10μΜ ROCK inhibitor (abcam120129) to prevent differentiation as described previously(Chapman et al., 2014). Typical cell counts were 2-5 x 106 cells per mouse (cell count) and viability greater than 70% (cell viability) as determined by Trypan blue exclusion assay. Dishes were collagen coated by incubating 1.5ml collagen solution (50μg/ml collagen in 0.02N acetic acid) for 1 hour at 37 °C, 5% CO2 or overnight at room temperature. After incubation plates were rinsed 3 times with 1X PBS. Cells were remained undisturbed for 48 hours and then passaged for different experiments. Cells were maintained at 37°C in an atmosphere of 5% CO2 with humidity.
Human keratinocyte cultures
Primary cultures of human keratinocytes were obtained from neonatal foreskins through the Penn Dermatology Skin Biology and Diseases Resource-based Center: Skin Translational Research Core (STaR) Core B (visit: https://dermatology.upenn.edu/sbdrc/core-b/). Each experiment was conducted with at least three donors (as indicated in text). All experiments were conducted with cells at passage number less than 4. Briefly, cell suspensions were generated using dispase and trypsin, and the cells were cultured in a keratinocyte growth media [50% Medium 154, M154500 (Life Technologies), 50% Keratinocyte SFM, 17005042 (Life Technologies), 1% HKGS supplement, S0015 (Life Technologies), 1% Antibiotic/Antimycotic, 15240062 (Invitrogen)]. For routine passaging, cells were split when they were less than 70% confluent. Cells were washed with 1X PBS and trypsinized with 0.25% Trypsin-EDTA for 5 minutes, trypsin was inactivated using trypsin inhibitor (R007100, Thermo Fisher Scientific) and cell suspension was centrifuged. Following removal of supernatant, cell pellet was suspended in culture media and seeded as per experimental design. Cells were maintained at 37°C in an atmosphere of 5% CO2 with humidity.
Immortalized human keratinocyte HaCaT cells (Boukamp et al., 1988) were used for AHR reporter assay. HaCaT cells were verified for lack of mycoplasma contamination by ATCC. Experiments were conducted on cells that were at passage numbers between 26-36. For routine cell culture, HaCaTs were maintained in DMEM high glucose (11965092, Thermo Fisher Scientific) supplemented with 1% Sodium Pyruvate, 5% FBS, 1% Antibiotic/Antimycotic, 15240062 (Invitrogen) and 1% Non-essential amino acids). For routine passaging, cells were split when they were less than 70% confluent. Cells were washed with 1X PBS and trypsinized with 0.25% Trypsin-EDTA for 5 minutes, trypsin was quenched with DMEM containing 5% FBS. Cells were maintained at 37°C in an atmosphere of 5% CO2 with humidity.
Microbial strains
The Flowers’ Flora Consortium, consisted of Staphylococcus epidermidis (EGM 2-01), Staphylococcus hemolyticus (EGM 2-08), Staphylococcus warneri (EGM 2-09), Micrococcus luteus (EGM 2-04) and Corynebacterium aurimucosum (EGM 2-02), that had been isolated from healthy human skin and maintained in the Grice lab culture repository (number in parenthesis indicates identifier code in Grice lab culture collection). The S. aureus strain (AH3926) used in these studies was generously provided by Dr. Alexander Horswill (University of Colorado, Anschutz Medical Campus). S. aureus AH3926 consists of tdTomato stably integrated into S. aureus LAC (AH1263) and its construction has been described in detail previously (Miller et al., 2019). Culturing conditions: All strains were cultured on solid blood agar plates at room temperature for 24-48 hours. For liquid cultures, all species (except M. luteus) were inoculated in tryptic soy broth and grown by shaking at 100rpm at 37°C. M. luteus was inoculated in nutrient broth.
METHOD DETAILS
RNA-sequencing of murine epithelia and analysis
Mice were shaved, and skin was collected from dorsal region. The fat layer was scraped off using a scalpel and then the skin was floated in dispase (1 mg/ml) in 1X PBS overnight at 37°C for 1 hour in order to separate the epidermis from the dermis. The epidermis was stored in RNAlater. Mouse epidermis that had been stored in RNA-Later (Thermo-Fisher) was blotted dry and approximately 20 mg of tissue was placed in a Lysing Matrix A tube (MP Bio) with 600 μl RLT buffer (Qiagen) containing 2-mercaptoethanol. The tissue was homogenized with three, 1 min bursts of bead beating in a FastPrep 24 (MP Bio). The lysate was centrifuged (14,000 x g, 3 min) and the supernatant was transferred to a new tube to which 1 volume of 70 % ethanol was added. RNA was purified using a RNeasy Tissue Kit (Qiagen), as per manufacturer’s guidelines. RNA was quantified on a Qubit and RNA-integrity was assessed using BioAnalyser according to manufacturer’s instructions. 1μg RNA was used to construct RNA-seq libraries using the stranded-TruSeq RNA Sample Prep Kit (Illumina), spiked with phiX and sequenced on the Illumina NextSeq-500 Platform in 3 runs of 1x75 reads. Three runs were used to achieve sufficient sequencing depth. The runs were aggregated and then analyzed and aligned against the mouse genome [Genome Reference Consortium Mouse Build 38 patch release 5 (GRCm38.p5)] using AlignerBoost (Zheng and Grice, 2016) and STAR 2.5.3 (Dobin et al., 2013). Gene counts were fitted into a negative binomial model where both the gnotobiotic condition (SPF, GF or COL) and sex of the mouse were included using the DESeq2 (Love et al., 2014) R package . Pairwise DEGs between conditions were obtained by setting corresponding “contrasts” for each pairwise comparison and filtering genes with FDR adjusted p-values less than 0.1. To identify enriched Gene Ontology (GO) terms, all annotated GO terms for aforementioned DEGs were retrieved using the ENSEMBL biomaRt R package (Durinck et al., 2009), and significantly enriched GO terms were identified using the topGO R package with the FDR-adjusted p-values <0.1 (Benjamini and Yekutieli, 2001). Uniquely enriched GO terms were selected by grouping similar GO terms using the online GO visualization tool REVIGO (Supek et al., 2011) with default (medium) similarity settings. Raw data are under submission and pending accession number assignment in the NCBI Gene Expression Omnibus (GEO) under accession number GSE162925.
16S rRNA Gene Sequencing
Sample collection
Mice were swabbed prior to shaving with sterile foam-tipped applicators (Puritan) as described previously (SanMiguel et al., 2017). The swabs were snap frozen and stored at −80°C immediately following collection. Bacterial DNA was extracted from swabs as described (Meisel et al., 2016). In brief, swabs were incubated for one hour at 37°C with shaking in 300μL yeast cell lysis solution (from Epicentre MasterPure Yeast DNA Purification kit) and 10,000 units of ReadyLyse Lysozyme solution (Epicentre). Samples were subjected to bead beating for ten minutes at maximum speed on a vortex mixer with 0.5 mm glass beads (MoBio), followed by a 30-minute incubation at 65°C with shaking. Protein precipitation reagent (Epicentre) was added and samples were spun at maximum speed. The supernatant was removed, mixed with isopropanol and applied to a column from the PureLink Genomic DNA Mini Kit (Invitrogen). Instructions for the Invitrogen PureLink kit were followed exactly, and DNA was eluted in 50 mL elution buffer (Invitrogen). At each sampling event, swab control samples that never came into contact with the skin were collected, prepared and sequenced exactly as the experimental samples. No significant background contamination from either reagent and/or collection procedures was recovered.
Sequencing and analysis
Amplification of the 16S rRNA gene V1–V3 region was performed as described previously (Meisel et al., 2016). Sequencing was performed at the PennCHOP microbiome core on the Illumina MiSeq using 300 bp paired-end chemistry. The mock community control (MCC; obtained from BEI Resources, NIAID, NIH as part of the Human Microbiome Project: Genomic DNA from Microbial Mock Community B (Even, Low Concentration), v5.1L, for 16S rRNA Gene Sequencing, HM-782D) was sequenced in parallel. Sequencing of the V1-V3 region was performed using 300 bp paired-end chemistry. Sequences were preprocessed and quality filtered prior to analysis, including size filtering to 460-600 nucleotides. HmmUFOtu was used for sequence alignment and phylogeny-based OTU clustering as described previously (Zheng et al., 2018).
Statistical analysis and visualization was performed using the phyloseq package (McMurdie and Holmes, 2013) in the R statistical computing environment. Raw data are under submission and pending accession number assignment in the NCBI Sequence Read Archive (SRA) under the BioProject Accession number PRJNA684321.
Barrier recovery
Barrier was assessed as described (Bradley et al., 2016; Man et al., 2015) by using noninvasive probe (Courage+Khazaka, Cologne, Germany) to measure transepidermal water loss (TEWL) by diffusion (Tewameter®, TM300) according to the manufacturer’s instructions. The dorsal flanks of mice were shaved 24 hours prior to beginning of barrier analyses. Basal epidermal permeability barrier function was assessed 24 hours after shaving. Barrier was disrupted by tape stripping (3M Scotch High Performance Packaging Tape, 2”X800”) to achieve comparable insults between experimental and control groups as indicated for each experiment. Mice were anesthetized using isoflurane during TEWL measurements. TEWL measurements were averaged at 1-second intervals for a 30 second period. Indoor ambient temperature and mean relative humidity were recorded for each experiment. For barrier recovery assessment, TEWL was measured by placing probe at the same location on the dorsal flank of the mouse each time. For consistency, the same person made all TEWL measurements.
Antibiotic treatment of mice
An antibiotic cocktail consisting of Metronidazole (1 g/L), Sulfamethoxazole (0.8g/L), Trimethoprim (0.16g/L), Cephalexin (4g/L) and Baytril (0.025g/L) dissolved in drinking water containing Splenda (1 packet/250ml) as sweetener was provided to the mice for two weeks. To ensure decreased microbial burden, cages were changed 3 times a week for antibiotic treated mice as described previously (SanMiguel et al., 2017). Control cages were changed once a week, as per conventional policies to ensure microbial biodiversity.
Differentiation assay
Confluent cells were trypsinized (0.25% Trypsin EDTA) and plated at 104 cells/well in 12 well collagen coated dishes without ROCK inhibitor. Cells were allowed to grow to confluency (typically 2-3 days) and then media was switched to cornification medium i.e. traditional E-media without EGF (3 parts DMEM+ 1 part DMEM/F12 + 5% FBS + cholera toxin + insulin + adenine + hydrocortizone + antibiotics) for three days, to induce polarization and transmembrane junction formation in keratinocytes (Simpson et al., 2010). At the end of three days cells were scraped off and processed for qPCR analyses.
Transepithelial electrical resistance (TEER) measurements
Keratinocytes were trypsinized (0.25% Trypsin EDTA) and plated at 104 cells/well on collagen coated 12mm transwells with 0.4μm pore (Sigma-Aldrich; CLS3460). Twenty-four hours post-plating cells were 100% confluent and media was switched to KSFM containing 5% FBS and 1.6mM Ca2+. Every 24 hours, transepithelial electrical resistance (TEER) was measured using an epithelial volt/ohm meter (EVOM) using a STX2 manual electrode. To measure TEER, three readings were taken per chamber to cover three different areas of the transwell membrane longitudinally over three days. The electrode was cleaned with 0.5% bleach followed by 70% ethanol between each transwell. Readings are reported in ohms-cm2.
FICZ and AHR inhibitor treatments:
For experiments described in Figure 4, primary human keratinocytes were seeded on transwells and grown in presence of 100nM each of AHR ligand 6-formylindolo[3,2-b] carbazole (FICZ) (Sigma-Aldrich, #SML1489) and/or AHR inhibitor (Sigma Aldrich, #CH-223191) throughout the course of the experiments.
Tissue preparation and immunofluorescence analysis
Murine skin tissue was collected and fixed in 4% paraformaldehyde and embedded in paraffin and sectioned at 6μm, as described previously(Meisel et al., 2018). Immunofluorescence protocols are described in detail at dx.doi.org/10.17504/protocols.io.k95cz86 (Uberoi, 2017). Briefly, tissue sections were deparaffinized with xylenes and rehydrated with graded ethanol. Heat-induced antigen retrieval was performed in 0.01M citrate buffer, pH 6.0 and blocked in 10% normal goat serum. Antibodies against the following proteins were used at indicated dilution: Cytokeratin-10 1:1000 (Biolegend, #905401), Desmoglein 1a 1:200 (Abcam, #ab124798), Loricrin 1:500 (Biolegend, #905101). Alexafluor 594 conjugated goat-anti rabbit 1:1000 (ThermoFisher Scientific, #A32740) was used as secondary antibodies. Tissue was counterstained with Hoechst stain. Wide-field fluorescent images were acquired using by means of a 20X lens objective on a Leica DM6000 Widefield Fluorescence Microscope at the University of Pennsylvania, School of Veterinary Medicine Imaging core. For purpose of quantification 10-12 random images were taken in a blinded fashion at constant light exposure of 100 miliseconds for the Alexafluor 594 channel and 10 seconds for the Hoechst Stain. Images were processed using ImageJ software version 10.2 (NIH, Bethesda, MD). Krt10, Dsg1a, and Loricrin levels of each image were calculated by the integrated density of the signal, normalized to the Hoechst stain signal from the same area. For statistical analysis, each stain was calculated by taking average levels in each corresponding to 10-12 images per mouse and a two-sided T-test was used to determine the significance of signal differences between groups.
Electron Microscopy
The ultrastructural analysis by EM was performed as described previously (Crumrine et al., 2019) at the VA Medical Center and Department of Dermatology at the University of California, San Francisco, United States. Skin samples were fixed in 2% glutaraldehyde and 2% paraformaldehyde and post-fixed in reduced ruthenium tetroxide before epoxy embedding. The samples were cut on a Leica Ultracut E microtome (Leica microsystems, Wetzlar, Germany) and imaged on a JEOL 100CX transmission electron microscope (JEOL, Tokyo, Japan) using a Gatan digital camera. For quantification, the thickness of the cornified envelope was measured in at least 25 randomly selected positions in 5 random high-powered electron micrographs of the mid stratum corneum from three mice of each colonization state. The observer recording these measurements was blinded to the groups.
AHR reporter assay
Immortalized human keratinocyte HaCaT cells (Boukamp et al., 1988) were seeded in 96 well plates at 104 cells/well in 100μl KSFM media supplemented with supplements. Twenty-four hours later, when cells were 80-90% confluent, each well was co-transfected with 20ng of Renilla luciferase DNA pGL4.74 (Promega) and 180ng of Firefly luciferase reporter plasmids: pGL4.23 which has xenobiotic response element (XRE) corresponding to Cyp1A1 activity. Construction of plasmid is described previously(Sutter et al., 2011). Transient transfections of HaCaT cells was performed with Fugene® HD (Promega, #E2311) at 3:1 Fugene transfection reagent: DNA ratio according to manufacturer instructions. Twelve hours post-transfection, media containing transfection complexes was removed and replaced with media containing either indicated bacterial strains to represent MOI=0.1, 1 and 10, peptidoglycan (10ng) or FICZ (10nM). Twenty-four hours post-infection, dual luciferase readings (Renilla and Firefly) were read using Dual-Luciferase® reporter assay system (Promega, #E1910) on the BioTek Synergy HT fluorescence plate reader. Background correction was performed by subtracting readings of empty wells from observed readings and Firefly luciferase activity was normalized to Renilla luciferase. Relative response ratio to compare CYP1A1 induction by each bacterial species to treatment with known AHR ligand FICZ (10nM) was computed as follows: RRR= [(experimental sample ratio)-(negative control ratio)]/ [(positive control ratio)-(negative control ratio)](Eggers, 2016). Experimental sample refers to cells treated with indicated bacteria or peptidoglycan; negative control refers to unstimulated cells and positive control refers to cells stimulated with 10nM FICZ.
cDNA synthesis for qPCR analyses
Mouse skin or epidermis that had been stored in RNA-Later (Thermo-Fisher) was blotted dry and approximately 20 mg of tissue was placed in a Lysing Matrix A tube (MP Bio) with 600 μl RLT buffer (Qiagen) containing 2-mercaptoethanol. The tissue was homogenized with three, 1 min bursts of bead beating in a FastPrep 24 (MP Bio). The lysate was centrifuged (14,000 x g, 3 min) and the supernatant was transferred to a new tube to which 1 volume of 70 % ethanol was added. RNA was purified using a RNeasy Tissue Kit (Qiagen). Traces of DNA were removed with DNase-1 and the RNA was stored at −80°C.RNA was quantified (Qubit) and 10 ng RNA was used as a template for Superscript III (Invitrogen) reverse transcription with random hexamer primers. Following treatment with RNase H, the cDNA was stored at −20°C.
Colonizing mice with human skin commensals
For liquid cultures of human skin commensals, S. epidermidis, S. warneri, S. hemolyticus and C. aurimucosum were inoculated in tryptic soy broth (TSB) and M. luteus was inoculated in nutrient broth media, respectively and grown by shaking at 200rpm for 16 hours at 37°C. Cultures were centrifuged, media was removed and bacterial pellets were suspended in PBS to obtain 109 CFU/ml. Equal amount of each bacteria (109 CFU) was combined in 5ml PBS and inoculated in bedding of mouse cages daily for 2 weeks. For each inoculum, a fresh batch culture was grown overnight.
Mouse treatment with AHR ligand FICZ
Mice were treated with a low dose (100μg/kg) of AHR ligand 6-Formylindolo[3,2-b] carbazole (FICZ) (Sigma-Aldrich, #SML1489), that has been shown to be sufficient to induce expression of Cyp1a1(Ehrlich et al., 2018). A stock solution (4.5mg/ml) of FICZ was made in DMSO and diluted in 50% Acetone. Mice were shaved 24 hours prior to treatment and FICZ was applied topically by directly pipetting onto shaved skin, daily for 2 weeks (Figure 3B).
S. aureus skin infection protocol
Epicutaneous infection:
Infection protocol for epicutaneous infection with S. aureus has been described previously (Wanke et al., 2013) and was implemented with slight modifications. Briefly, mice were anesthetized with isoflurane, tape stripped (TEWL=20 g/m2/h) and 24 hours later, 107 CFU S. aureus in 100μl was applied to back skin and spread using a swab. The inoculum was allowed to dry for 10 minutes and mice were returned to their cages.
CFU enumeration:
Forty-eight hours post-infection, mice were euthanized and approximately 1cm2 infected skin area was collected and weighed and transferred to tubes containing 300μl 1X PBS. Tissue was homogenized by bead beating for twenty minutes at maximum speed on a vortex mixer with 0.5 mm ceramic beads and CFUs were enumerated by serial dilution on blood agar plates after overnight incubation at 37°C. Both S. aureus and total bacterial counts were determined and normalized to weight of tissues. S. aureus colonies were visible as red colonies due to stable expression of tDTomato and could be distinguished from total bacteria.
Epicutaneous sensitization with ovalbumin
Procedures to induce barrier defects that mimic atopic dermatitis by repeated epicutaneous sensitization by ovalbumin (OVA) (Jin et al., 2009; Spergel et al., 1998) followed by infection with S. aureus (Nakatsuji et al., 2016) have been described previously. The dorsal skin of mice was shaved and TEWL was assessed to give a baseline reading. To measure and compare barrier repair between wild-type and AHR null mice, we standardized the amount of initial barrier disruption to give identical TEWL values. To achieve this, mice were tape stripped to give a reading of TEWL ~20g/m2/h. Twenty-four hours post tape-stripping, the mice were treated daily for 7 days with 100 μg OVA (Sigma Aldrich, # A5503) suspended in 100 μl PBS was applied onto backs of mice and spread using a skin swab and allowed to dry for 2 minutes. For inducing atopic dermatitis-like condition, the 7-day OVA treatment regime was repeated twice more, with 2 weeks rest between subsequent treatments. At the end of treatment mice were tape stripped to give a reading of TEWL ~40g/m2/h and S. aureus was applied as described earlier or barrier recovery was assessed.
QUANTIFICATION AND STATISTICAL ANALYSIS
Data visualization and statistics.
All statistical analysis was performed using functions built into the R statistical environment (RStudio Version 1.3.1056). Data was visualized using ggplot2 (Wickham, 2016) package and GraphPad Prism version 8.0.0 for Mac OS X, GraphPad Software, San Diego, California USA, www.graphpad.com. TEWL vs time readings were fitted by linear modeling function in R statistical package and visualized using ggplot2 package. Significance was assessed by ANCOVA analysis by controlling for covariates such as housing and gender, as necessary. Fit parameters (adjusted R2 and F-statistics) are indicated for each plot. Span indicated by shaded area represents 95% CI. TEER significance was determined by ANOVA analysis in R- statistical package. Gene expression analysis from qPCR was conducted as per guidelines described previously(Taylor et al., 2019). Cycle thresholds were normalized to housekeeping genes (Rplp2, Sptbn1 and 18s rRNA) and calibrated to quantitative cycle (Cq) values of control. Heatmaps for qPCR analysis were made using Morpheus heat map viewer from Broad Institute (https://software.broadinstitute.org/morpheus). Each square represents average normalized readings (n= 3 technical replicates). Bonferrroni correction was used to adjust for multiple comparisons. The AHR pathway was originally downloaded from wikipathways (https://www.wikipathways.org/index.php/Pathway:WP2873), then modified by highlighting DEGs identified in our analysis using a customized Perl script. The log2 fold-change values between SPF and GF mice were used to determine the color hue (red/blue: up/down in SPF vs. GF) and saturation of the highlighted DEGs.
Supplementary Material
Table S3. Skin barrier genes that are differentially expressed between SPF and GF mice, Related toFigure 1. Contains list of differentially expressed barrier genes involved in skin barrier function that were differentially expressed between SPF and GF murine skin by using genes described in Table S2 as reference.
Table S4. Differentially expressed xenobiotic processing genes, Related toFigure 3. Contains complete list of 304 xenobiotic processing genes from Fu, et al. (2016) as well as those differentially expressed in SPF vs GF mice.
Table S1. Differentially expressed genes identified by RNA-seq, Related toFigure 1. Results from differential expression analysis of RNA-seq data for genes depicted in Fig. 1F. As a result, we identified 6,396, 427, and 3,232 DEGs for SPF vs. GF, COL vs. GF and SPF vs. COL comparisons, respectively. DEGs defined as those with FDR adjusted p-values < 0.1. In Sheet SPF vs GF (upregulated in SPF: log2FC> 0, downregulated in SPF: log2FC < 0); in sheet COLvsGF (upregulated in COL: log2FC> 0, downregulated in COL: log2FC< 0); in sheet SPFvsCOL (upregulated in SPF: log2FC > 0, downregulated in SPF: log2FC < 0).
Table S2. List of genes implicated in barrier function, Related toFigure 1. Contains list of genes manually curated based on literature analyses that have been implicated in barrier function. Genes are listed under the following categories based on their functions: adherence junction formation, lipid-lamellae formation, keratin network, differentiation, skin barrier development, formation of cornified envelope and basement membrane.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Cytokeratin-10 (Dilution 1:1000) | BioLegend | BioLegend Cat# 905401, RRID:AB_2565049 |
| Desmoglein 1 (Dilution 1:200) | Abcam | Abcam Cat# ab124798, RRID:AB_10974963 |
| Loricrin (Dilution 1:500) | BioLegend | BioLegend Cat# 905101, RRID:AB_2565046 |
| Alexafluor 594 conjugated goat-anti rabbit (Dilution 1:1000) | Thermo Fisher Scientific | Thermo Fisher Scientific Cat# A32740, RRID:AB_2762824 |
| Bacterial and Virus Strains | ||
| Staphylococcus epidermidis | This paper | EGM 2-01 |
| Staphylococcus hemolyticus | This paper | EGM 2-08 |
| Staphylococcus warneri | This paper | EGM 2-09 |
| Micrococcus luteus | This paper | EGM 2-04 |
| Corynebacterium aurimucosum | This paper | EGM 2-02 |
| S. aureus strain | Gift from Dr. Alexander Horswill, University of Colorado. (Miller et al., 2019) | AH3926 |
| Biological Samples | ||
| Primary human keratinocytes | Penn Skin Biology and Disease Resource-based Center | N/A |
| Primary mouse keratinocytes | Derived from mice described in experimental models | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Keratinocyte SFM media | Life technologies | Cat#17005042 |
| Keratinocyte growth supplement | Life technologies | Cat#S0015 |
| Medium 154 | Life technologies | Cat#M154500 |
| Antibiotic-Antimycotic (100X) | Life technologies | Cat#15240062 |
| 0.25% Trypsin-EDTA | Thermo Fisher Scientific | Cat# 25200056 |
| DMEM, high glucose | Thermo Fisher Scientific | Cat# 11965084 |
| Fetal Bovine Serum | Sigma-Aldrich | Cat# F6178 |
| Dialyzed Fetal Bovine Serum | Thermo Fisher Scientific | Cat# 26400044 |
| Collagen- Rat Tail | Collaborative | Cat# 354236 |
| Keratinocyte-SFM (1X), without calcium chloride | Life technologies | Cat# 37010022 |
| Y-27632 dihydrochloride, Rho kinase inhibitor | Abcam | Cat# ab120129; CAS Number: 129830-38-2 |
| Defined Trypsin Inhibitor | Thermo Fisher Scientific | Cat# R007100 |
| Sodium Pyruvate (100X) | Thermo Fisher Scientific | Cat# 11360070 |
| MEM Amino Acids Solution (50X) | Thermo Fisher Scientific | Cat # 11130051 |
| Tryptic soy broth | Fisher Scientific | Cat # DF0370 |
| Nutrient broth | Fisher Scientific | Cat# DF0003 |
| Dispase | Sigma | Cat# D4693-1G |
| RNAlater | Ambion, Inc. | Cat# AM7020 |
| Collagenase, Type IV, powder | Life technologies | Cat# 17104019 |
| Yeast cell lysis solution | Lucigen (from MasterPure Yeast DNA Purification kit) | Cat# MPY80200 |
| Protein precipitation reagent | ||
| ReadyLyse Lysozyme solution | Lucigen | Cat# R1810M |
| Metronidazole | Sigma Aldrich | Cat# 1442009 |
| Sulfamethoxazole | Sigma Aldrich | Cat# S7507 |
| Trimethoprim | Fisher Scientific | Cat# ICN19552701 |
| Cephalexin | DOT Scientific | Cat# DSV59000 |
| Enrofloxacin (Baytril ) | Sigma - Aldrich | Cat# 17849 |
| DMEM/F-12, + GlutaMAX supplement | Life technologies | Cat # 10565018 |
| Cholera Toxin | Sigma | Cat# C8052 |
| Insulin-Transferrin-Selenium-Ethanolamine (ITS -X) 100X | Life technologies | Cat# 51500056 |
| Adenine | Sigma | Cat# A9795 |
| Hydrocortisone | Calbiochem | Cat# 386698 |
| AHR ligand 6-formylindolo[3,2-b] carbazole (FICZ) | Sigma-Aldrich | Cat# SML1489 |
| AHR inhibitor (Sigma Aldrich, #CH-223191) | Sigma Aldrich | Cat#CH-223191 |
| Fugene® HD | Promega | Cat# E2311 |
| Peptidoglycan | Sigma Aldrich | Cat # 77140 |
| Ovalbumin | Sigma Aldrich | Cat# A5503 |
| Hoechst 33342 Solution (20 mM) | Thermo Scientific | Cat# 62249 |
| Critical Commercial Assays | ||
| RNeasy Mini Kit | Qiagen | Cat # 74104 |
| Qubit RNA BR and HS Kits | Thermo Fisher Scientific | Cat # Q10210 Cat# Q32852 |
| Bioanalyser RNA 6000 Pico Kit | Agilent | Cat# 5067-1513 |
| TruSeq Strandar RNA Sample Prep Kit | Illumina | Cat # 20020595 |
| NextSeq Mid Output Kit | Illumina | Cat # 20024905 |
| PureLink Genomic DNA Mini Kit (Invitrogen). | Invitrogen | Cat # K182001 |
| Dual-Luciferase® reporter assay system | Promega | Cat #E1910 |
| Deposited Data | ||
| RNA-Sequencing data from murine epithelia (24 total RNAseq samples were collected from 3 conditions (SPF, GF and COL) each from 4 female and 4 male replicate animals.) | This paper | BioProject: PRJNA683863 SRA: SRP297369 GEO accession #: GSE162925 |
| 16S rRNA gene sequencing Datasets | This paper | BioProject: PRJNA684321 SRA |
| Experimental Models: Cell Lines | ||
| HaCaT cells | Gift from Dr. John Seykora, University of Pennsylvania (Boukamp et al., 1988) | RRID:CVCL_0038 |
| Experimental Models: Organisms/Strains | ||
| C57BL6/J | The Jackson Laboratory | stock #000664 |
| Rag1 KO | The Jackson Laboratory | stock #002216 |
| Ahrfx | Gift from Dr. Jorge Henao-Mejia, University of Pennsylvania. Available from the Jackson Laboratory | stock #006203 |
| K14cre | The Jackson Laboratory | Stock #018964 |
| Oligonucleotides | ||
| Refer to Table S5 for Taqman Probes and PCR primers used in this paper | N/A | N/A |
| Recombinant DNA | ||
| pGL4.74 Renilla luciferase | Gift from Dr. Thomas R. Sutter (Sutter et al., 2011) | pGL4.74 |
| pGL4.23 Firefly luciferase reporter plasmid with xenobiotic response element (XRE) | pGL4.23 | |
| Genomic DNA from Microbial Mock Community B (Even, Low Concentration), v5.1L, for 16S rRNA Gene Sequencing | BEI Resources | HM-782D, v5.1L |
| Software and Algorithms | ||
| GraphPad Prism version 8.0.0 for Mac OS X | GraphPad Software, San Diego, California USA | www.graphpad.com |
| Image J, Version 10.2 | National Institute of Health | https://imagej.nih.gov/ij/ |
| Morpheus heat map viewer | Broad Institute | https://software.broadinstitute.org/morpheus/ |
| AlignerBoost | (Zheng and Grice, 2016) | https://github.com/Grice-Lab/AlignerBoost |
| STAR 2.5.3 | (Dobin et al., 2013) | http://code.google.com/p/rna-star/ |
| DESeq2 | (Love et al., 2014) | http://www.bioconductor.org/packages/release/bioc/html/DESeq2.html webcite |
| biomaRt | (Durinck et al., 2009) | https://bioconductor.org/packages/release/bioc/html/biomaRt.html |
| topGO | (Alexa A, 2020) | https://bioconductor.org/packages/release/bioc/html/topGO.html |
| REVIGO | (Supek et al., 2011) | http://revigo.irb.hr/ |
| HmmUFOtu | (Zheng et al., 2018) | https://github.com/Grice-Lab/HmmUFOtu |
| PHYLOSEQ | (Desai, 1990) | https://bioconductor.org/packages/release/bioc/html/phyloseq.html |
| Other | ||
| 100μm cell strainer | Falcon | Cat# 352360 |
| Cell countess II | Invitrogen | Cat# A27977 |
| Lysing Matrix A tube | MP Biomedicals | Cat# 116910050-CF |
| FastPrep 24 | MP Biomedicals | Cat# 116004500 |
| 0.5 mm glass beads | Sigma-Aldrich | Cat# Z250465 |
| 6 mm ceramic beads | MP Biomedicals | Cat# 116540424-CF |
| Tewameter ®, TM300 | Courage+Khazaka, Cologne, Germany | https://www.courage-khazaka.de/en/16-wissenschaftliche-produkte/alle-produkte/172-tewameter-e |
| Scotch Heavy Duty Packaging Tape, 2”X800” | 3M | ID: 70005058279 |
| 12mm transwells with 0.4μm pore | Sigma-Aldrich | Cat# CLS3460 |
| EVOM2 Epithelial Voltmeter with STX2 electrode | World Precision Instruments | https://www.wpiinc.com/var-2754-epithelial-volt-ohm-teer-meter |
| Blood agar plates | Remel | Cat# R01201 |
| Tissue Culture dishes 35mm, 60mm, 100mm dia. | Cell Star | Cat#s 627160, 628160, 664160 |
Highlights.
Germ free mice have abnormal skin barrier structure and function
Keratinocyte aryl hydrocarbon receptor (AHR) mediates barrier function and repair
Human skin commensal microbes activate keratinocyte AHR
Targeting the microbiota-AHR axis promotes skin barrier repair in disease models
ACKNOWLEDGEMENTS
We thank Dr. Alex Horswill (University of Colorado) for the kind gift of the S. aureus strain; Dr. Jorge Henao-Mejia (University of Pennsylvania) for the kind gift of Ahrf/f mice; Dr. John Seykora (University of Pennsylvania) for the kind gift of HaCaT keratinocytes; Dmytro Kobuley, Gnotobiotic Mouse Facility (University of Pennsylvania); Dr. Arwa Abbas (University of Pennsylvania) for illustration design and consulting and current and former members of the Grice lab and the Department of Dermatology for critical discussion and review of the work. This work was funded by grants from the NIH, NIEHS (R56-ES-030218 to EAG/TRS, R01-ES-017014 to TRS), NIAMS (R01-AR-006663 and R00-AR-060873 to EAG), the Burroughs Wellcome Fund PATH Award (EAG), the Linda Pechenik Montague Investigator Award (EAG), and the Dermatology Foundation Sun Pharma Research Award (EAG). AU is a recipient of the Prevent Cancer Foundation Awesome Games Done Quick fellowship. This research was also supported by the Penn Skin Biology and Disease Resource-based Center (P30-AR-069589). CBM and LF were supported by the Penn Dermatology Research Training Grant, T32-AR-007465.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
The authors have no competing interests to declare.
INCLUSION AND DIVERSITY
We worked to ensure sex balance in the selection of non-human subjects. One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in science. While citing references scientifically relevant for this work, we also actively worked to promote gender balance in our reference list.
REFERENCES
- Alexander H, Paller AS, Traidl-Hoffmann C, Beck LA, De Benedetto A, Dhar S, Girolomoni G, Irvine AD, Spuls P, Su J, et al. (2020). The role of bacterial skin infections in atopic dermatitis: expert statement and review from the International Eczema Council Skin Infection Group. Br J Dermatol 182, 1331–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexa A RJ, (2020). topGO: Enrichment Analysis for Gene Ontology. In R package version 2420. [Google Scholar]
- Andreola F, Fernandez-Salguero PM, Chiantore MV, Petkovich MP, Gonzalez FJ, and De Luca LM (1997). Aryl hydrocarbon receptor knockout mice (AHR−/−) exhibit liver retinoid accumulation and reduced retinoic acid metabolism. Cancer Res 57, 2835–2838. [PubMed] [Google Scholar]
- Beco L, Guaguere E, Lorente Mendez C, Noli C, Nuttall T, and Vroom M (2013). Suggested guidelines for using systemic antimicrobials in bacterial skin infections: part 2-- antimicrobial choice, treatment regimens and compliance. Vet Rec 172, 156–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y, and Segre JA (2014). Dialogue between skin microbiota and immunity. Science 346, 954–959. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, and Yekutieli D (2001). The control of the false discovery rate in multiple testing under dependency. Ann Stat 29, 1165–1188. [Google Scholar]
- Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, and Fusenig NE (1988). Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol 106, 761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen AC, Carapetis JR, Currie BJ, Fowler V Jr., Chambers HF, and Tong SYC (2017). Sulfamethoxazole-Trimethoprim (Cotrimoxazole) for Skin and Soft Tissue Infections Including Impetigo, Cellulitis, and Abscess. Open Forum Infect Dis 4, ofx232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley CW, Morris DO, Rankin SC, Cain CL, Misic AM, Houser T, Mauldin EA, and Grice EA (2016). Longitudinal Evaluation of the Skin Microbiome and Association with Microenvironment and Treatment in Canine Atopic Dermatitis. J Invest Dermatol 136, 1182–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandner JM, Zorn-Kruppa M, Yoshida T, Moll I, Beck LA, and De Benedetto A (2015). Epidermal tight junctions in health and disease. Tissue Barriers 3, e974451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard JA, Koetsier JL, and Green KJ (2020). Desmosomes pattern cell mechanics to govern epidermal tissue form and function. bioRxiv, 2020.2001.2021.914176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbach KM, Poland A, and Bradfield CA (1992). Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. Proc Natl Acad Sci U S A 89, 8185–8189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd AL, Belkaid Y, and Segre JA (2018). The human skin microbiome. Nat Rev Microbiol 16, 143–155. [DOI] [PubMed] [Google Scholar]
- Camp JG, Frank CL, Lickwar CR, Guturu H, Rube T, Wenger AM, Chen J, Bejerano G, Crawford GE, and Rawls JF (2014). Microbiota modulate transcription in the intestinal epithelium without remodeling the accessible chromatin landscape. Genome Res 24, 1504–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamcheu JC, Siddiqui IA, Syed DN, Adhami VM, Liovic M, and Mukhtar H (2011). Keratin gene mutations in disorders of human skin and its appendages. Arch Biochem Biophys 508, 123–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman S, McDermott DH, Shen K, Jang MK, and McBride AA (2014). The effect of Rho kinase inhibition on long-term keratinocyte proliferation is rapid and conditional. Stem Cell Res Ther 5, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cork MJ, Danby SG, Vasilopoulos Y, Hadgraft J, Lane ME, Moustafa M, Guy RH, Macgowan AL, Tazi-Ahnini R, and Ward SJ (2009). Epidermal barrier dysfunction in atopic dermatitis. J Invest Dermatol 129, 1892–1908. [DOI] [PubMed] [Google Scholar]
- Crumrine D, Khnykin D, Krieg P, Man MQ, Celli A, Mauro TM, Wakefield JS, Menon G, Mauldin E, Miner JH, et al. (2019). Mutations in Recessive Congenital Ichthyoses Illuminate the Origin and Functions of the Corneocyte Lipid Envelope. J Invest Dermatol 139, 760–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassule HR, Lewis P, Bei M, Maas R, and McMahon AP (2000). Sonic hedgehog regulates growth and morphogenesis of the tooth. Development 127, 4775–4785. [DOI] [PubMed] [Google Scholar]
- Desai B (1990). Managing ecological upheavals: a Third World perspective. Soc Sci Med 30, 1065–1072. [DOI] [PubMed] [Google Scholar]
- Derrick CW Jr., and Reilly K (1983). The role of cephalexin in the treatment of skin and soft-tissue infections. Postgrad Med J 59 Suppl 5, 43–46. [PubMed] [Google Scholar]
- Di Meglio P, Duarte JH, Ahlfors H, Owens ND, Li Y, Villanova F, Tosi I, Hirota K, Nestle FO, Mrowietz U, et al. (2014). Activation of the aryl hydrocarbon receptor dampens the severity of inflammatory skin conditions. Immunity 40, 989–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, and Gingeras TR (2013). STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durinck S, Spellman PT, Birney E, and Huber W (2009). Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nature Protocols 4, 1184–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers C, H. B; Lewis S; Strayer C; Landreman A (2016). Designing a Bioluminescent Reporter Assay: Normalization Options (Promega Corporation; ). [Google Scholar]
- Ehrlich AK, Pennington JM, Bisson WH, Kolluri SK, and Kerkvliet NI (2018). TCDD, FICZ, and Other High Affinity AhR Ligands Dose-Dependently Determine the Fate of CD4+ T Cell Differentiation. Toxicol Sci 161, 310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PM (2007). The skin barrier as an innate immune element. Semin Immunopathol 29, 3–14. [DOI] [PubMed] [Google Scholar]
- Elias PM (2018). Primary role of barrier dysfunction in the pathogenesis of atopic dermatitis. Exp Dermatol 27, 847–851. [DOI] [PubMed] [Google Scholar]
- Elias PM, Matsuyoshi N, Wu H, Lin C, Wang ZH, Brown BE, and Stanley JR (2001). Desmoglein isoform distribution affects stratum corneum structure and function. J Cell Biol 153, 243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser C (2016). The Aryl Hydrocarbon Receptor in Immunity: Tools and Potential. Methods Mol Biol 1371, 239–257. [DOI] [PubMed] [Google Scholar]
- Esser C, Bargen I, Weighardt H, Haarmann-Stemmann T, and Krutmann J (2013). Functions of the aryl hydrocarbon receptor in the skin. Semin Immunopathol 35, 677–691. [DOI] [PubMed] [Google Scholar]
- Eyerich S, Eyerich K, Traidl-Hoffmann C, and Biedermann T (2018). Cutaneous Barriers and Skin Immunity: Differentiating A Connected Network. Trends Immunol 39, 315–327. [DOI] [PubMed] [Google Scholar]
- Flowers L, and Grice EA (2020). The Skin Microbiota: Balancing Risk and Reward. Cell Host Microbe 28, 190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluhr JW, Feingold KR, and Elias PM (2006). Transepidermal water loss reflects permeability barrier status: validation in human and rodent in vivo and ex vivo models. Exp Dermatol 15, 483–492. [DOI] [PubMed] [Google Scholar]
- Fritsche E, Schafer C, Calles C, Bernsmann T, Bernshausen T, Wurm M, Hubenthal U, Cline JE, Hajimiragha H, Schroeder P, et al. (2007). Lightening up the UV response by identification of the arylhydrocarbon receptor as a cytoplasmatic target for ultraviolet B radiation. Proc Natl Acad Sci U S A 104, 8851–8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu ZD, Selwyn FP, Cui JY, and Klaassen CD (2016). RNA Sequencing Quantification of Xenobiotic-Processing Genes in Various Sections of the Intestine in Comparison to the Liver of Male Mice. Drug Metab Dispos 44, 842–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furue M, Hashimoto-Hachiya A, and Tsuji G (2019). Aryl Hydrocarbon Receptor in Atopic Dermatitis and Psoriasis. Int J Mol Sci 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furue M, Takahara M, Nakahara T, and Uchi H (2014). Role of AhR/ARNT system in skin homeostasis. Arch Dermatol Res 306, 769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice EA, and Segre JA (2011). The skin microbiome. Nat Rev Microbiol 9, 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubauer G, Elias PM, and Feingold KR (1989). Transepidermal water loss: the signal for recovery of barrier structure and function. J Lipid Res 30, 323–333. [PubMed] [Google Scholar]
- Haas K, Weighardt H, Deenen R, Kohrer K, Clausen B, Zahner S, Boukamp P, Bloch W, Krutmann J, and Esser C (2016). Aryl Hydrocarbon Receptor in Keratinocytes Is Essential for Murine Skin Barrier Integrity. J Invest Dermatol 136, 2260–2269. [DOI] [PubMed] [Google Scholar]
- Harris TA, Gattu S, Propheter DC, Kuang Z, Bel S, Ruhn KA, Chara AL, Edwards M, Zhang C, Jo JH, et al. (2019). Resistin-like Molecule alpha Provides Vitamin-A-Dependent Antimicrobial Protection in the Skin. Cell Host Microbe 25, 777–788 e778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennings H, Michael D, Cheng C, Steinert P, Holbrook K, and Yuspa SH (1980). Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell 19, 245–254. [DOI] [PubMed] [Google Scholar]
- Hidaka T, Ogawa E, Kobayashi EH, Suzuki T, Funayama R, Nagashima T, Fujimura T, Aiba S, Nakayama K, Okuyama R, et al. (2017). The aryl hydrocarbon receptor AhR links atopic dermatitis and air pollution via induction of the neurotrophic factor artemin. Nat Immunol 18, 64–73. [DOI] [PubMed] [Google Scholar]
- Hong CH, Lin SH, Clausen BE, and Lee CH (2020). Selective AhR knockout in langerin-expressing cells abates Langerhans cells and polarizes Th2/Tr1 in epicutaneous protein sensitization. Proc Natl Acad Sci U S A 117, 12980–12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JM, Schutze S, Neumann C, and Proksch E (2000). Impaired cutaneous permeability barrier function, skin hydration, and sphingomyelinase activity in keratin 10 deficient mice. J Invest Dermatol 115, 708–713. [DOI] [PubMed] [Google Scholar]
- Jin H, He R, Oyoshi M, and Geha RS (2009). Animal models of atopic dermatitis. J Invest Dermatol 129, 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson ME, Jakobsson HE, Holmen-Larsson J, Schutte A, Ermund A, Rodriguez-Pineiro AM, Arike L, Wising C, Svensson F, Backhed F, et al. (2015). Normalization of Host Intestinal Mucus Layers Requires Long-Term Microbial Colonization. Cell Host Microbe 18, 582–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiko GE, Ryu SH, Koues OI, Collins PL, Solnica-Krezel L, Pearce EJ, Pearce EL, Oltz EM, and Stappenbeck TS (2016). The Colonic Crypt Protects Stem Cells from Microbiota-Derived Metabolites. Cell 165, 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazem S, Linssen EC, and Gibbs S (2019). Skin metabolism phase I and phase II enzymes in native and reconstructed human skin: a short review. Drug Discov Today 24, 1899–1910. [DOI] [PubMed] [Google Scholar]
- Kennedy LH, Sutter CH, Leon Carrion S, Tran QT, Bodreddigari S, Kensicki E, Mohney RP, and Sutter TR (2013). 2,3,7,8-Tetrachlorodibenzo-p-dioxin-mediated production of reactive oxygen species is an essential step in the mechanism of action to accelerate human keratinocyte differentiation. Toxicol Sci 132, 235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo A, Nagao K, and Amagai M (2012). Epidermal barrier dysfunction and cutaneous sensitization in atopic diseases. J Clin Invest 122, 440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larigot L, Juricek L, Dairou J, and Coumoul X (2018). AhR signaling pathways and regulatory functions. Biochim Open 7, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech JM, Dhariwala MO, Lowe MM, Chu K, Merana GR, Cornuot C, Weckel A, Ma JM, Leitner EG, Gonzalez JR, et al. (2019). Toxin-Triggered Interleukin-1 Receptor Signaling Enables Early-Life Discrimination of Pathogenic versus Commensal Skin Bacteria. Cell Host Microbe 26, 795–809 e795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichti U, Anders J, and Yuspa SH (2008). Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nat Protoc 3, 799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofmark S, Edlund C, and Nord CE (2010). Metronidazole is still the drug of choice for treatment of anaerobic infections. Clin Infect Dis 50 Suppl 1, S16–23. [DOI] [PubMed] [Google Scholar]
- Love MI, Huber W, and Anders S (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackowiak B, Hodge J, Stern S, and Wang H (2018). The Roles of Xenobiotic Receptors: Beyond Chemical Disposition. Drug Metab Dispos 46, 1361–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magiatis P, Pappas P, Gaitanis G, Mexia N, Melliou E, Galanou M, Vlachos C, Stathopoulou K, Skaltsounis AL, Marselos M, et al. (2013). Malassezia yeasts produce a collection of exceptionally potent activators of the Ah (dioxin) receptor detected in diseased human skin. J Invest Dermatol 133, 2023–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man G, Mauro TM, Zhai Y, Kim PL, Cheung C, Hupe M, Crumrine D, Elias PM, and Man MQ (2015). Topical hesperidin enhances epidermal function in an aged murine model. J Invest Dermatol 135, 1184–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh BE, Hogenesch JB, and Bradfield CA (2010). Mammalian Per-Arnt-Sim proteins in environmental adaptation. Annu Rev Physiol 72, 625–645. [DOI] [PubMed] [Google Scholar]
- McMurdie PJ, and Holmes S (2013). phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 8, e61217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel JS, Hannigan GD, Tyldsley AS, SanMiguel AJ, Hodkinson BP, Zheng Q, and Grice EA (2016). Skin Microbiome Surveys Are Strongly Influenced by Experimental Design. J Invest Dermatol 136, 947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel JS, Sfyroera G, Bartow-McKenney C, Gimblet C, Bugayev J, Horwinski J, Kim B, Brestoff JR, Tyldsley AS, Zheng Q, et al. (2018). Commensal microbiota modulate gene expression in the skin. Microbiome 6, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RJ, Crosby HA, Schilcher K, Wang Y, Ortines RV, Mazhar M, Dikeman DA, Pinsker BL, Brown ID, Joyce DP, et al. (2019). Development of a Staphylococcus aureus reporter strain with click beetle red luciferase for enhanced in vivo imaging of experimental bacteremia and mixed infections. Sci Rep 9, 16663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzaghi D, Pavel P, and Dubrac S (2019). Xenobiotic Receptors and Their Mates in Atopic Dermatitis. Int J Mol Sci 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, and Papaioannou VE (1992). RAG-1-deficient mice have no mature B and T lymphocytes. Cell 68, 869–877. [DOI] [PubMed] [Google Scholar]
- Murphy KA, Quadro L, and White LA (2007). The intersection between the aryl hydrocarbon receptor (AhR)- and retinoic acid-signaling pathways. Vitam Horm 75, 33–67. [DOI] [PubMed] [Google Scholar]
- Nagase N, Sasaki A, Yamashita K, Shimizu A, Wakita Y, Kitai S, and Kawano J (2002). Isolation and species distribution of staphylococci from animal and human skin. J Vet Med Sci 64, 245–250. [DOI] [PubMed] [Google Scholar]
- Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, Deming C, Quinones M, Koo L, Conlan S, et al. (2012). Compartmentalized control of skin immunity by resident commensals. Science 337, 1115–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuji T, Chen TH, Two AM, Chun KA, Narala S, Geha RS, Hata TR, and Gallo RL (2016). Staphylococcus aureus Exploits Epidermal Barrier Defects in Atopic Dermatitis to Trigger Cytokine Expression. J Invest Dermatol 136, 2192–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestle FO, Di Meglio P, Qin JZ, and Nickoloff BJ (2009). Skin immune sentinels in health and disease. Nat Rev Immunol 9, 679–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LP, and Bradfield CA (2008). The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol 21, 102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlet CP, Brown MM, and Horswill AR (2019). Commensal Staphylococci Influence Staphylococcus aureus Skin Colonization and Disease. Trends Microbiol 27, 497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson ED, and Bowcock AM (2010). Psoriasis genetics: breaking the barrier. Trends Genet 26, 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooks MG, and Garrett WS (2016). Gut microbiota, metabolites and host immunity. Nat Rev Immunol 16, 341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelov L, Sarig O, Harmon RM, Rapaport D, Ishida-Yamamoto A, Isakov O, Koetsier JL, Gat A, Goldberg I, Bergman R, et al. (2013). Desmoglein 1 deficiency results in severe dermatitis, multiple allergies and metabolic wasting. Nat Genet 45, 1244–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SanMiguel AJ, Meisel JS, Horwinski J, Zheng Q, and Grice EA (2017). Topical Antimicrobial Treatments Can Elicit Shifts to Resident Skin Bacterial Communities and Reduce Colonization by Staphylococcus aureus Competitors. Antimicrob Agents Chemother 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Wendtner MH, and Korting HC (2006). The pH of the skin surface and its impact on the barrier function. Skin Pharmacol Physiol 19, 296–302. [DOI] [PubMed] [Google Scholar]
- Schmuth M, Fluhr JW, Crumrine DC, Uchida Y, Hachem JP, Behne M, Moskowitz DG, Christiano AM, Feingold KR, and Elias PM (2004). Structural and functional consequences of loricrin mutations in human loricrin keratoderma (Vohwinkel syndrome with ichthyosis). J Invest Dermatol 122, 909–922. [DOI] [PubMed] [Google Scholar]
- Simpson CL, Kojima S, Cooper-Whitehair V, Getsios S, and Green KJ (2010). Plakoglobin rescues adhesive defects induced by ectodomain truncation of the desmosomal cadherin desmoglein 1: implications for exfoliative toxin-mediated skin blistering. Am J Pathol 177, 2921–2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson CL, Patel DM, and Green KJ (2011). Deconstructing the skin: cytoarchitectural determinants of epidermal morphogenesis. Nat Rev Mol Cell Biol 12, 565–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Chandrashekharappa S, Bodduluri SR, Baby BV, Hegde B, Kotla NG, Hiwale AA, Saiyed T, Patel P, Vijay-Kumar M, et al. (2019). Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat Commun 10, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SH, Jayawickreme C, Rickard DJ, Nicodeme E, Bui T, Simmons C, Coquery CM, Neil J, Pryor WM, Mayhew D, et al. (2017). Tapinarof Is a Natural AhR Agonist that Resolves Skin Inflammation in Mice and Humans. J Invest Dermatol 137, 2110–2119. [DOI] [PubMed] [Google Scholar]
- Smits JPH, Ederveen THA, Rikken G, van den Brink NJM, van Vlijmen-Willems I, Boekhorst J, Kamsteeg M, Schalkwijk J, van Hijum S, Zeeuwen P, et al. (2020). Targeting the Cutaneous Microbiota in Atopic Dermatitis by Coal Tar via AHR-Dependent Induction of Antimicrobial Peptides. J Invest Dermatol 140, 415–424 e410. [DOI] [PubMed] [Google Scholar]
- Spergel JM, Mizoguchi E, Brewer JP, Martin TR, Bhan AK, and Geha RS (1998). Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J Clin Invest 101, 1614–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens EA, Mezrich JD, and Bradfield CA (2009). The aryl hydrocarbon receptor: a perspective on potential roles in the immune system. Immunology 127, 299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supek F, Bosnjak M, Skunca N, and Smuc T (2011). REVIGO Summarizes and Visualizes Long Lists of Gene Ontology Terms. Plos One 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter CH, Bodreddigari S, Campion C, Wible RS, and Sutter TR (2011). 2,3,7,8-Tetrachlorodibenzo-p-dioxin increases the expression of genes in the human epidermal differentiation complex and accelerates epidermal barrier formation. Toxicol Sci 124, 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter CH, Yin H, Li Y, Mammen JS, Bodreddigari S, Stevens G, Cole JA, and Sutter TR (2009). EGF receptor signaling blocks aryl hydrocarbon receptor-mediated transcription and cell differentiation in human epidermal keratinocytes. Proc Natl Acad Sci U S A 106, 4266–4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed DN, and Mukhtar H (2015). FICZ: A Messenger of Light in Human Skin. J Invest Dermatol 135, 1478–1481. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Sugi Y, Nakano K, Tsuda M, Kurihara K, Hosono A, and Kaminogawa S (2011). Epigenetic control of the host gene by commensal bacteria in large intestinal epithelial cells. J Biol Chem 286, 35755–35762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauchi M, Hida A, Negishi T, Katsuoka F, Noda S, Mimura J, Hosoya T, Yanaka A, Aburatani H, Fujii-Kuriyama Y, et al. (2005). Constitutive expression of aryl hydrocarbon receptor in keratinocytes causes inflammatory skin lesions. Mol Cell Biol 25, 9360–9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SC, Nadeau K, Abbasi M, Lachance C, Nguyen M, and Fenrich J (2019). The Ultimate qPCR Experiment: Producing Publication Quality, Reproducible Data the First Time. Trends Biotechnol 37, 761–774. [DOI] [PubMed] [Google Scholar]
- Tellkamp F, Vorhagen S, and Niessen CM (2014). Epidermal polarity genes in health and disease. Cold Spring Harb Perspect Med 4, a015255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uberoi A (2017). Standard protocol for performing immunofluorescence or immunohistochemistry on paraffin embedded tissue. (protocols.io). [Google Scholar]
- van den Bogaard EH, Bergboer JG, Vonk-Bergers M, van Vlijmen-Willems IM, Hato SV, van der Valk PG, Schroder JM, Joosten I, Zeeuwen PL, and Schalkwijk J (2013). Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. J Clin Invest 123, 917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bogaard EH, Podolsky MA, Smits JP, Cui X, John C, Gowda K, Desai D, Amin SG, Schalkwijk J, Perdew GH, et al. (2015). Genetic and pharmacological analysis identifies a physiological role for the AHR in epidermal differentiation. J Invest Dermatol 135, 1320–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walisser JA, Glover E, Pande K, Liss AL, and Bradfield CA (2005). Aryl hydrocarbon receptor-dependent liver development and hepatotoxicity are mediated by different cell types. Proc Natl Acad Sci U S A 102, 17858–17863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanke I, Skabytska Y, Kraft B, Peschel A, Biedermann T, and Schittek B (2013). Staphylococcus aureus skin colonization is promoted by barrier disruption and leads to local inflammation. Exp Dermatol 22, 153–155. [DOI] [PubMed] [Google Scholar]
- Wickham H (2016). ggplot2: Elegant Graphics for Data Analysis. (New York: Springer-Verlag; ). [Google Scholar]
- Woo V, and Alenghat T (2017). Host-microbiota interactions: epigenomic regulation. Curr Opin Immunol 44, 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright EJ, De Castro KP, Joshi AD, and Elferink CJ (2017). Canonical and non-canonical aryl hydrocarbon receptor signaling pathways. Curr Opin Toxicol 2, 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yockey LJ, Demehri S, Turkoz M, Turkoz A, Ahern PP, Jassim O, Manivasagam S, Kearney JF, Gordon JI, and Kopan R (2013). The absence of a microbiota enhances TSLP expression in mice with defective skin barrier but does not affect the severity of their allergic inflammation. J Invest Dermatol 133, 2714–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Luo Y, Zhu Z, Zhou Y, Sun L, Gao J, Sun J, Wang G, Yao X, and Li W (2019). A tryptophan metabolite of the skin microbiota attenuates inflammation in patients with atopic dermatitis through the aryl hydrocarbon receptor. J Allergy Clin Immunol 143, 2108–2119 e2112. [DOI] [PubMed] [Google Scholar]
- Zheng Q, Bartow-McKenney C, Meisel JS, and Grice EA (2018). HmmUFOtu: An HMM and phylogenetic placement based ultra-fast taxonomic assignment and OTU picking tool for microbiome amplicon sequencing studies. Genome Biol 19, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q, and Grice EA (2016). AlignerBoost: A Generalized Software Toolkit for Boosting Next-Gen Sequencing Mapping Accuracy Using a Bayesian-Based Mapping Quality Framework. PLoS Comput Biol 12, e1005096. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S3. Skin barrier genes that are differentially expressed between SPF and GF mice, Related toFigure 1. Contains list of differentially expressed barrier genes involved in skin barrier function that were differentially expressed between SPF and GF murine skin by using genes described in Table S2 as reference.
Table S4. Differentially expressed xenobiotic processing genes, Related toFigure 3. Contains complete list of 304 xenobiotic processing genes from Fu, et al. (2016) as well as those differentially expressed in SPF vs GF mice.
Table S1. Differentially expressed genes identified by RNA-seq, Related toFigure 1. Results from differential expression analysis of RNA-seq data for genes depicted in Fig. 1F. As a result, we identified 6,396, 427, and 3,232 DEGs for SPF vs. GF, COL vs. GF and SPF vs. COL comparisons, respectively. DEGs defined as those with FDR adjusted p-values < 0.1. In Sheet SPF vs GF (upregulated in SPF: log2FC> 0, downregulated in SPF: log2FC < 0); in sheet COLvsGF (upregulated in COL: log2FC> 0, downregulated in COL: log2FC< 0); in sheet SPFvsCOL (upregulated in SPF: log2FC > 0, downregulated in SPF: log2FC < 0).
Table S2. List of genes implicated in barrier function, Related toFigure 1. Contains list of genes manually curated based on literature analyses that have been implicated in barrier function. Genes are listed under the following categories based on their functions: adherence junction formation, lipid-lamellae formation, keratin network, differentiation, skin barrier development, formation of cornified envelope and basement membrane.
Data Availability Statement
The datasets generated by this study are available as follows:
RNAseq data: NCBI SRA BioProject: PRJNA683863, GEO accession #: GSE162925 16S rRNA sequencing data: NCBI SRA BioProject: PRJNA684321


