Abstract
Posttraumatic stress disorder (PTSD) is a complex mental disorder afflicting approximately 7% of the population. The diverse number of traumatic events and the wide array of symptom combinations leading to PTSD diagnosis contribute substantial heterogeneity to studies of the disorder. Genomic and complimentary–omic investigations have rapidly increased our understanding of the heritable risk for PTSD. In this review, we emphasize the contributions of genome-wide association, epigenome-wide association, transcriptomic, and neuroimaging studies to our understanding of PTSD etiology. We also discuss the shared risk between PTSD and other complex traits derived from studies of causal inference, co-expression, and brain morphological similarities. The investigations completed so far converge on stark contrasts in PTSD risk between sexes, partially attributed to sex-specific prevalence of traumatic experiences with high conditional risk of PTSD. To further understand PTSD biology, future studies should focus on detecting risk for PTSD while accounting for substantial cohort-level heterogeneity (e.g., civilian versus combat-exposed PTSD cases or PTSD risk among cases exposed to specific traumas), expanding ancestral diversity among study cohorts, and remaining cognizant of how these data influence social stigma associated with certain traumatic events among underrepresented minorities and/or high-risk populations.
Keywords: posttraumatic stress disorder (PTSD), GWAS, EWAS, transcriptomics, neuroimaging
Introduction
The fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) defines a traumatic event as direct or indirect exposure to threatened death, serious injury, or sexual violence and includes a new category for trauma- and stressor-related disorders (i.e., disorders in which exposure to a traumatic or stressful event is listed explicitly as a diagnostic criterion) (American Psychiatric Association, 2013). Posttraumatic stress disorder (PTSD) is the most recognized among these diagnoses. According to the DSM-5, PTSD diagnosis includes multiple criteria (Table 1): stressor, intrusion symptoms, avoidance, negative alterations in cognitions and mood, alterations in arousal and reactivity, duration, functional significance, and exclusion. In addition to the diagnostic criteria, there are two additional specifications PTSD-affected patients are expected to experience: dissociative specification (depersonalization: being an outside observer of or detached from oneself; derealization: experience of unreality, distance, or distortion) and delayed specification (full diagnostic criteria are not met until at least six months after the trauma(s), although the onset of symptoms may occur immediately). Due to the presence of multiple diagnostic symptoms, PTSD is among the most heterogeneous psychiatric diagnoses. There are 636,120 possible PTSD diagnostic combinations (i.e., any set of symptoms for a disorder such that an individual meets criteria for that disorder if he or she exhibits that set of symptoms) and 52% of them (N=336,000) are disjoint combinations (i.e., diagnostic combinations occurring among sets of symptoms that have no overlap) (Olbert, Gala, & Tupler, 2014). Since its introduction in the DSM classification, several critiques were made with respect to PTSD diagnosis (Ball & Stein, 2012). These include: symptom overlap, high rates of comorbidity with other psychiatric disorders, the inability of PTSD diagnostic criteria to reflect the complexity of trauma response, and the variability of PTSD construct across contexts and cultures (Frueh, Elhai, & Acierno, 2010; Papa, Neria, & Litz, 2008). Although these criticisms represent valid viewpoints, DSM diagnostic criteria are used in most of the human studies of PTSD. Molecular studies of PTSD can help disentangle the complexities of PTSD diagnosis through the understanding of the biological basis linking exposure to traumatic events to psychiatric disorders and physical health outcomes. Here, we review the progress made by genomic research of PTSD from twin studies to large-scale genome-wide association studies (GWAS; Figure 1). We also describe investigations focused on other omics domains and brain imaging and their contributions to understanding the molecular changes associated with PTSD in brain and non-brain tissues. Finally, we conclude by discussing the clinical and therapeutic implications of PTSD and trauma genomic research.
Table 1.
Diagnostic and Statistical Manual of Mental Disorders (DSM-5) diagnostic criteria for posttraumatic stress disorder.
| DSM Criterion | Description | Qualifiers |
|---|---|---|
| A | Exposure to actual or threatened death, serious injury, or sexual violence |
|
| B | One or more intrusive symptom associated with the traumatic event that begins after the event occurred |
|
| C | Persistent avoidance of trauma-associated stimuli |
|
| D | Negative alterations in cognitions and mood |
|
| E | Alterations in arousal and reactivity |
|
| F | Duration of disturbance is at least one (1) month | |
| G | Clinically significant distress or impairment in social, occupational, or other important areas of functioning | |
| H | Disturbance is not attributable to the physiological effects of an illicit substance, medication, or other medical condition | |
Figure 1.
Summary of multifaceted investigations into the etiology of posttraumatic stress disorder ranging from environmental effects, genetics, multi-omics, and neuroimaging efforts.
Epidemiology
General population studies have shown that a large proportion of people in developed countries have been exposed to at least one traumatic event in their lifetime (estimates from 28 to 90%) with 82.7% prevalence of exposure to any traumatic event in the United States (Benjet et al., 2016). There are known differences among trauma types with respect to the consequent PTSD risk. In surveys from the World Health Organization (WHO), the investigators obtained representative data on trauma-specific PTSD from 24 countries (68,894 subjects) and assessed 29 lifetime traumas (Kessler et al., 2017). Trauma involving interpersonal violence had the highest risk. PTSD burden, determined by multiplying trauma prevalence by trauma-specific PTSD risk and persistence, was 77.7 person-years/100 respondents. The trauma types with the highest proportions of this burden were rape (13.1%), other sexual assault (15.1%), being stalked (9.8%), and unexpected death of a loved one (11.6%). The broad category of intimate partner sexual violence accounted for nearly 42.7% of all person-years with PTSD. Due to trauma-specific PTSD risk, the disease prevalence varies depending on population and trauma type. In the North American general adult population, lifetime PTSD prevalence ranges from 6% to 9% while the one-year prevalence is between 3.5% and 5% (Sareen, 2020). However, ~2% prevalence was reported by WHO for upper-middle income and lower-middle income countries included in their survey (Koenen, Ratanatharathorn, et al., 2017). In contrast, a recent systematic review of PTSD prevalence studies in Africa found an overall current pooled prevalence of PTSD of 25% (Ng et al., 2020).
In addition to trauma-specific PTSD risk, several pre-trauma risk factors can influence PTSD development: gender, age at trauma, education, socioeconomic status, psychiatric comorbidities, being in a confiding relationship as an adult, history of previous traumatic experience, childhood adversity and abuse, social support, and initial reaction severity to the traumatic event (Wild et al., 2016). Accordingly, the interplay between traumatic events and pre-trauma risk factors can consistently affect the frequency with which PTSD occurs. For instance, PTSD risk shows evident differences between sexes: women are four times more likely to develop PTSD when compared with men when accounting for the exposure to traumatic events. Considering specific traumas, PTSD rates between women and men are similar for accidents, natural disasters, and the sudden death of a loved one (Sareen, 2020). Differently, although women are >10-times more likely as men to be raped, PTSD incidence after rape is higher in men than that observed in women. An opposite scenario for sex-specific PTSD incidence is present for molestation and physical assault (Chivers-Wilson, 2006). PTSD is associated with several psychiatric comorbidities including depression (Dunn, Nishimi, Powers, & Bradley, 2017), substance abuse and dependence (Roberts, Roberts, Jones, & Bisson, 2015), and suicidal behaviors (Victor & Klonsky, 2014). Additionally, PTSD has also been implicated in the etiology of various physical disorders (Boscarino, 2004; Gupta, 2013; Lohr et al., 2015) such as cancer (Roberts et al., 2019; Shand, Cowlishaw, Brooker, Burney, & Ricciardelli, 2015), gastrointestinal disorders (Savas et al., 2009), and cardiovascular disease (Koenen, Sumner, et al., 2017). However, the studies regarding the physical health sequelae of PTSD are in some cases conflicting and there is still an open debate about possible explanations.
Pedigree Analyses
The study of PTSD familiarity permitted researchers to understand the genetic and environmental factors involved in the propensity to traumatic events and the vulnerability to PTSD. In particular, studies comparing monozygotic (MZ) and dizygotic (DZ) twins partitioned genetic factors into additive and non-additive effects to understand shared environmental and non-shared environmental effects (Afifi, Asmundson, Taylor, & Jang, 2010). In line with the sex difference observed in PTSD epidemiology (higher prevalence in women) (Rivollier et al., 2015), twin studies observed that, while sex-combined cohorts presented a 40–60% heritability, all-female cohorts showed higher PTSD heritability estimates than all-male cohorts (~70% vs. ~30%, respectively) (Duncan, Cooper, & Shen, 2018). As mentioned, PTSD risk is influenced by the type of trauma and several pre-trauma risk factors. Accordingly, the variation of heritability estimates observed across different cohorts is likely to be partially affected by the characteristics of the samples investigated. Additionally, exposure to certain traumatic experiences appears to present a consistent familiarity (Stein, Jang, Taylor, Vernon, & Livesley, 2002). A study of 222 monozygotic and 184 dizygotic twin pairs demonstrated that the variance of assaultive traumatic events (e.g., robbery, being held captive, being beaten up, and sexual assault) is accounted by 20% additive genetic factors, 21% shared environmental factors, and 58% non-shared environmental factors (Stein et al., 2002). Conversely, non-assaultive traumatic events (e.g., sudden death of a family member, motor vehicle accident, fire, tornado, flood, and earthquake) did not have a detectable genetic component and were accounted by shared and non-shared environmental effects (39% and 61%, respectively) (Stein et al., 2002). The environmental components of assaultive and non-assaultive traumatic events appear to be mostly independent of each other: the shared environmental correlation was 0.31 and the non-shared environmental correlation was estimated at −0.20 (Stein et al., 2002). On the other hand, the genetic components of PTSD and the exposure to certain traumatic events are highly overlapping (Smoller, 2016). They also overlap with the genetic component of resilience, i.e. the ability to maintain or regain normal psychological and physical functioning in the face of adversity (Wu et al., 2013). In 3,318 male twin pairs from the Vietnam Era Twin Registry assessed with the PTSD Checklist and the Connor‐Davidson Resilience Scale‐10, PTSD and resilience shared a single genetic factor accounting for 59% of their correlation (Wolf, Miller, et al., 2018). These shared genetic factors are not unique to trauma exposure, PTSD, and resilience, but they also overlap with other psychiatric disorders. A study conducted in 2,591 participants (996 female and 536 male twins; 625 female and 434 male nontwin siblings) reported a high genetic overlap of high-risk trauma exposure with both PTSD and major depressive disorder (MDD) (Sartor et al., 2012). Recent twin studies focused their attention on the genetic overlap of PTSD with insomnia and sleep duration (Cox, Taylor, Strachan, & Olatunji, 2020; McCall et al., 2019). A consistent phenotypic covariance of PTSD symptoms and insomnia was explained by genetic factors (36–44%) with a significant genetic correlation of insomnia with PTSD re-experiencing and avoidance symptoms (Cox et al., 2020). In a cohort including 1,865 monozygotic and 758 dizygotic twin pairs from the community-based Washington State Twin Registry, the variance in sleep duration attributable to the shared environment was moderated by PTSD severity, while the variance in PTSD symptoms attributable to additive genetics was moderated by sleep duration (McCall et al., 2019).
In addition to twin-based studies, family-based investigations contribute to characterizing the genetic vulnerability to PTSD (Skelton, Ressler, Norrholm, Jovanovic, & Bradley-Davino, 2012). For example, adult children of PTSD cases exposed to extremely severe traumatic events (e.g., Holocaust survivors and Cambodian refugees) received more frequently a PTSD diagnosis than adult children of individuals without PTSD that were exposed to the same traumatic experience (Sack, Clarke, & Seeley, 1995; Yehuda, Halligan, & Bierer, 2001). An important limitation of pedigree analyses is that PTSD can be assessed only in individuals that experience a traumatic event and we cannot determine the PTSD status of trauma-unexposed subjects.
From Candidate Genes to Genome-wide Investigations
Genetic liability to PTSD is characterized by the effect of thousands of loci across the genome. These variants present individual small effects on the overall disease risk. To identify these effects, genetic association studies test the allele frequency of genetic variants with respect to binary and quantitative traits (e.g., PTSD diagnosis and PTSD severity, respectively). Over the years, the designs of association studies were developed based on the genotyping technologies available. Early genetic association studies were based on the ability to genotype a limited number of variants in small cohorts. The genetic variants of interest were selected considering genes included in biological pathways known from the scientific literature to be related to the pathogenesis of PTSD and related psychiatric disorders. This particular approach is known as “candidate gene”. The first candidate gene study of PTSD observed a positive association of DRD2*A1 allele in two samples including a total of 37 PTSD cases and 19 controls (Comings, Muhleman, & Gysin, 1996). Because of the genotyping technology progress, larger studies reported associations of variants across multiple genes expected to play a key role in PTSD pathogenesis: serotonin transporter gene (SLC6A4) (Kilpatrick et al., 2007), dopamine transporter gene (SLC6A3) (Segman et al., 2002), catechol-O-methyltransferase gene (COMT) (Kolassa, Kolassa, Ertl, Papassotiropoulos, & De Quervain, 2010), steroid receptor chaperone FK506 binding protein 5 (FKBP5) (Zhang et al., 2020), adenylate cyclase activating polypeptide 1 (ADCYAP1) gene (Ressler et al., 2011), and brain-derived neurotrophic factor (BDNF) gene (Zhang et al., 2006). Similar to other complex traits, candidate gene studies of PTSD are often inconsistent across the different samples investigated (Sheerin et al., 2020).
With the advent of genome-wide arrays and genotype imputation based on large reference panels, genome-wide analyses permitted psychiatric geneticists to move from hypothesis-driven studies (candidate gene design) to hypothesis-generating studies (GWAS design). Genetic studies based on such wide screening can uncover loci in molecular pathways that were not previously expected to be associated with PTSD, generating novel hypotheses about disease pathogenesis. Between 2013 and 2017, several PTSD GWAS with sample size ranging from 147 to 13,690 participants identified risk alleles in several genes, including RORA (RAR Related Orphan Receptor A) (Logue et al., 2013), TLL1 (Tolloid Like 1) (Xie et al., 2013), lincRNA AC068718.1 (long intergenic non-protein coding RNA AC068718.1) (Guffanti et al., 2013), PRTFDC1 (Phosphoribosyl Transferase Domain Containing 1) (Nievergelt et al., 2015), ANKRD55 (Ankyrin Repeat Domain 55) (Stein et al., 2016), and ZNF626 (zinc finger protein 626) (Stein et al., 2016).
Although GWAS are powerful tools, their gene discovery can be affected by confounders (systematic biases affecting the analyses) (Sul, Martin, & Eskin, 2018), winner’s curse (overestimation of genetic effects) (Palmer & Pe’er, 2017), and polygenicity (thousands of variants with small effects) (Holland et al., 2020). A better understanding of the genetics of complex traits permitted investigators to establish the unreliability of results generated by candidate gene studies and relatively-small GWAS of PTSD. Indeed, similarly to other complex traits, findings of underpowered genetic association studies of PTSD independently from their design are likely to be false positive results. To conduct statistically powerful GWAS, investigators analyzed the massive cohorts via collaborative initiatives and large biobanks. The Psychiatric Genomics Consortium (PGC) is the largest collaborative experiment in the history of psychiatry, including >800 investigators from >150 institutions in >40 countries (Sullivan et al., 2018). Among PGC workgroups, PGC-PTSD investigators focus on the harmonization of genome-wide data from multiple studies to conduct powerful PTSD GWAS meta-analyses. In 2017, the first PGC-PTSD GWAS was finalized including 20,730 individuals from 11 cohorts (Duncan, Ratanatharathorn, et al., 2018). Although this was the first large PTSD GWAS meta-analysis, the sample size was too limited to identify associations surviving genome-wide multiple testing correction. However, these data were powerful enough to conduct the first analyses of PTSD polygenic inheritance. PGC-PTSD investigators reported higher PTSD SNP-heritability (i.e., the proportion of phenotypic variance attributable to the additive effects of common genetic variants) in women and significant genetic correlation (rg; i.e., the proportion of phenotypic variance that two traits share due to common genetic causes) of PTSD with schizophrenia and MDD (Duncan, Ratanatharathorn, et al., 2018). After this first GWAS meta-analysis, access to large biobanks rapidly increased the number of PTSD-informative individuals with genome-wide information. In 2019, a GWAS of PTSD reexperiencing symptoms was conducted in >165,000 participants of the US Million Veteran Program (MVP) (Gelernter et al., 2019). MVP investigators identified eight distinct risk alleles and 30 gene-based associations; reported 400 significant genetic correlations with psychiatric disorders, behavioral traits, and other complex phenotypes; and observed functional enrichments for cortex, hypothalamus, amygdala, hippocampus, basal ganglia medium, and spiny neurons in the striatum (Gelernter et al., 2019). Leveraging reexperiencing-symptom data from 117,900 UK Biobank participants of European descent, the MVP findings were replicated at a single-variant level and at a polygenic level (rg= 0.88, SE = 0.07). The same year a second PTSD GWAS meta-analysis was finalized by PGC-PTSD investigators (Nievergelt et al., 2019). This novel study included over 30,000 PTSD cases and 170,000 controls (combining UK Biobank with 60 other datasets), identifying ancestry- and sex-specific risk loci (African ancestry, European ancestry, and male sample) and confirming that PTSD SNP-based heritability varies by sex with estimates ranging around 5–20% (Nievergelt et al., 2019). To investigate the genetics of PTSD in diverse populations, PGC-PTSD investigators developed a framework for improving the inclusion of admixed individuals in large-scale association studies, using a local-ancestry informed regression model to generate ancestry-specific effect size estimates (Atkinson et al., 2020). Recently, MVP investigators completed a PTSD GWAS analyzing data from more than 250,000 MVP participants and testing a validated electronic health record-based algorithmically-defined PTSD diagnosis phenotype (48,221 cases and 217,223 controls), and PTSD quantitative symptom phenotypes (212,007 individuals) (Stein et al., 2019). Beyond the risk loci identified with respect to case-control and quantitative phenotypes, this novel MVP study showed that PTSD symptom sub-domains share most of their genetic liability (rg 0.93 – 0.98) and identified novel potential treatment from a drug repositioning analysis conducted with respect to the loci identified (CRHR1 antagonist; TCF4: darinaparsin; TCF4-PLXNA1: otenzepad; PLEKHM1: dopamine receptor antagonists, acetylcholine receptor antagonists, and angiotensin receptor antagonists) (Stein et al., 2019). Findings from PGC and MVP PTSD GWAS are summarized in Figure 2. Novel methods are being developed to conduct multivariate genome-wide investigations of complex traits, increasing the statistical power and to model the pleiotropy widespread across the human genome (Grotzinger et al., 2019). A multivariate GWAS conducted in a military cohort combining pre- and post-deployment biochemical and behavioral phenotypes identified novel loci associated with human stress response (Schijven et al., 2019). With respect to rare variants, although whole-exome sequencing (WES) is very rarely used to investigate PTSD, a study identified rare variants located in TROVE2 gene as associated with emotional memory and PTSD (Heck et al., 2017).
Figure 2.
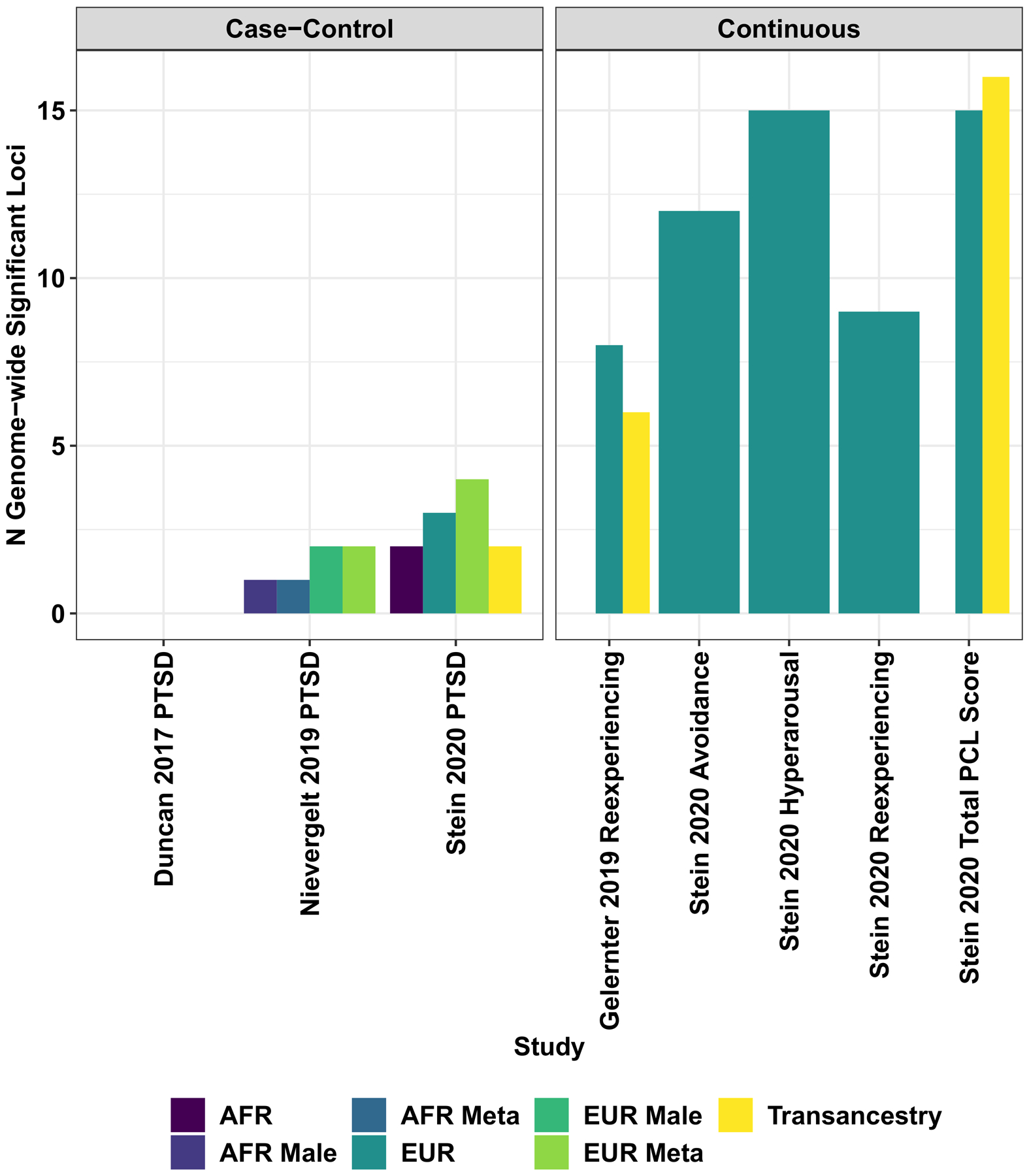
Locus discovery from genome-wide association studies of biobank and consortia case-control and continuous (i.e., symptom count) measures of posttraumatic stress disorder.
In addition to the relevant biology uncovered by genome-wide analyses, the data generated are being used as a base to conduct follow-up analyses to disentangle further the pathogenesis of PTSD. In a study focused on genetically regulated gene expression comparing 29,539 PTSD cases and 166,145 controls (Huckins et al., 2020), a substantial genetic heterogeneity based on ancestry, cohort type (military versus civilian), and sex was observed, but two significant tissue-gene associations were observed: ZNF140 (zinc finger protein 140) is predicted to be upregulated in whole blood, and SNRNP35 (small nuclear ribonucleoprotein U11/U12 subunit 35) is predicted to be downregulated in the dorsolateral prefrontal cortex.
Leveraging data from large-scale GWAS, several studies have been conducted to investigate PTSD comorbidities, applying mainly two approaches: linkage score regression to calculate genetic correlation (Bulik-Sullivan et al., 2015) and Mendelian randomization (MR) for causal inference (Smith & Ebrahim, 2003). With respect to PTSD sex differences, the polygenic component of body shape and reproductive behaviors appear to be associated with PTSD in women with potential evidence linking body shape and sexual trauma to PTSD (Polimanti et al., 2017). As shown by twin studies (Cox et al., 2020; McCall et al., 2019), there is a genetic overlap of PTSD with insomnia and sleep duration. GWAS data confirmed a moderate genetic correlation of PTSD with insomnia symptoms (rg range 0.36–0.49), oversleeping (rg range 0.32–0.44), undersleeping (rg range 0.48–0.49), but no causal effects were observed using the MR approach applied to the first PGC-PTSD GWAS (Lind et al., 2020). A causal inference analysis based on PGC-PTSD GWAS demonstrated that this genetic overlap between PTSD and educational attainment is due to a negative causal effect of socioeconomic status (measured as household income) on PTSD (Polimanti et al., 2019). Using a similar causal-inference approach, certain blood metabolites showed putative causal effects on PTSD (Carvalho et al., 2020). A more complex network of bidirectional associations was observed among PTSD, serum C-reactive protein, childhood support, and socioeconomic status (Muniz Carvalho et al., 2020). Leveraging a different GWAS-based approach, investigators also reported that the comorbidity between PTSD and late-onset Alzheimer’s disease may be due to common genetic mechanisms involved in immune response (Lutz, Luo, Williamson, & Chiba-Falek, 2020).
Gene-by-Environment Interaction
The interplay of genetic susceptibility with traumatic experiences and pre-trauma risk factors is expected to play a key role in the PTSD pathogenesis. Numerous gene-by-environment (GxE) studies of PTSD have been conducted testing candidate genes (e.g., FKBP5, BDNF, and COMT) (Jin, Jeon, Hyun, & Lee, 2019; van Rooij et al., 2016; Wang, Shelton, & Dwivedi, 2018). Similarly to candidate gene association studies, these GxE investigations present the same important limitations due to the lack of power and the potential presence of systematic bias from selection of candidate loci and environmental moderator(s) (Border et al., 2019). Some genome-wide studies explored the genetic interplay of traumatic experiences and PTSD with respect to other psychiatric traits. In a total sample of >24,000 participants, a genome-wide gene-by-trauma interaction analysis of alcohol misuse identified PRKG1 (protein kinase cGMP-dependent 1) as a risk locus modulating the effect of trauma exposure (Polimanti, Kaufman, Zhao, Kranzler, Ursano, Kessler, Gelernter, et al., 2018). The homolog of this locus in Drosophila melanogaster (foraging gene) is well-known, because its activity controls synaptic transmission tolerance to acute stress (Burns et al., 2012). Additionally, the polygenic component of bipolar disorder and schizophrenia seemed to moderate the effect of trauma exposure on alcohol abuse with high voltage-gated calcium channel activity and Beta1/Beta2 adrenergic receptor signaling as key molecular pathways (Polimanti, Kaufman, Zhao, Kranzler, Ursano, Kessler, Stein, et al., 2018). Recently, a multivariate GEWIS investigated the genetic interplay of traumatic experience and posttraumatic stress with respect to suicidality, identifying risk loci and sex-specific cell-type transcriptome enrichments related to the potential role of extracellular matrix biology and synaptic plasticity as biological mediators (Frank R. Wendt et al., 2020). A study showed enrichments for excitatory synaptic transmission and plasticity in the interaction between MVP re-experiencing PRS and attachment style with respect to PTSD symptoms assessed in the National Health and Resilience in Veterans Study (Tamman et al., 2020). Investigators have also begun to characterize the genetic architecture of traumatic experiences that appears to have a strong genetic overlap with PTSD and other psychiatric disorders and may be linked to externalizing behaviors or to a greater likelihood of reporting maltreatment (Dalvie et al., 2020). Additionally, traumatic experiences appear to affect also the genetic liability to other psychiatric disorders. A study conducted in the UK Biobank reported that MDD SNP-heritability is higher in individuals that reported trauma with a genetic overlap among trauma exposure, body composition, and MDD (Coleman et al., 2020). Further studies will be needed to understand how to disentangle the genetic and the environmental components of traumatic experiences and their effect on PTSD risk.
Epigenetics
The development of high-throughput technologies expanded the possibilities across different genomic features (Hasin, Seldin, & Lusis, 2017). Differently from genetic variation, other omics changes can be related to causative mechanisms (i.e., the molecular change is causal with respect to the trait-of-interest) or to downstream consequences (i.e., the molecular change is induced by the trait) with a consistent overrepresentation of the latter with respect to the former and accordingly often have higher effect size. With respect to PTSD research, epigenetic variation appears to be an obvious target because of its potential ability to reflect the molecular changes induced by traumatic events. Epigenome-wide association studies (EWAS) on brain specimens are expected to be informative for understanding PTSD pathogenesis, but there is limited availability of such samples and there may be also issues regarding the transferability of potential brain biomarkers to peripheral tissues of living participants. Accordingly, most EWAS are being conducted on peripheral tissues, mainly whole blood and saliva. Several candidate-gene and small PTSD EWAS have been performed (Zannas, Provencal, & Binder, 2015), but, similarly to what observed when the same designs were applied to genetic data, their results are likely to be affected by low statistical power and unaccounted confounders. Due to the well-known impact of PTSD among military personnel (Zang et al., 2017), several epigenetic studies have focused their attention on understanding whether there are specific epigenetic patterns of PTSD between individuals exposed to combat traumas and non-combat civilian traits (Hammamieh et al., 2017; Kuan, Waszczuk, Kotov, Marsit, et al., 2017; Mehta et al., 2013; Yang et al., 2013). In a cohort of military veterans (378 lifetime PTSD cases and 135 controls), an epigenome-wide significant association at cg19534438 in the gene G0S2 (G0/G1 switch 2) was observed and replicated in other military cohorts (Logue et al., 2020). A longitudinal PTSD EWAS conducted with respect pre- and post-deployment of 532 military participants showed that combat-related PTSD is associated with distinct methylation patterns mainly related to loci involved in the immune system (Snijders et al., 2020). Conversely, in civilian cohorts (545 participants), whole blood-derived DNA methylation levels at CpG sites located in HGS (hepatocyte growth factor-regulated tyrosine kinase substrate) and NRG1 (neuregulin 1) genes were associated with current PTSD (Uddin et al., 2018). Although the findings reported were replicated in some cases, they could be still due to unaccounted confounders, the limited sample size, or to differences in temporal stability of methylation signatures over time. PGC-PTSD workgroup is leading the largest collaborative effort to identify reliable epigenetic associations. Additionally, since the epigenetic variation is expected to be affected by many potential confounders, the PGC-PTSD workgroup developed a multi-site analysis pipeline to account adequately for ancestry population stratification and type I error inflation (Ratanatharathorn et al., 2017). In the PGC-PTSD EWAS meta-analysis (796 PTSD cased and 1,100 trauma-exposed controls from military and civilian cohorts) (Smith et al., 2020), ten epigenome-wide significant associations were observed in genes previously liked to other psychiatric disorders. Four signals mapped within AHRR (aryl-hydrocarbon receptor repressor) locus, which is well-known to present large methylation changes in response to tobacco smoking. The AHRR epigenetic associations observed in PGC-PTSD EWAS appeared to be independent of smoking status and were stronger in non-smokers than in smokers (Smith et al., 2020). Additionally, in a subsample with metabolomics data, AHRR methylation was associated with kynurenine level (an inflammatory marker), which was lower in PTSD subjects than in controls (Smith et al., 2020).
Epigenetic variation can also be used to assess accelerated cellular aging. Traumatic experience and posttraumatic stress are expected to have an impact on cellular regulation accelerating certain negative outcomes. In two studies conducted on US military veterans, accelerated DNA methylation aging was associated with different PTSD symptoms (avoidance, numbing, and hyperarousal) (Wolf et al., 2019; Wolf, Logue, et al., 2018). However, the pattern observed across the two studies was not completely concordant (i.e., the symptoms reported as associated were not the same). Additionally, differences were also observed across different algorithms used to estimate the accelerated DNA methylation aging. In 2018, a large PGC-PTSD meta-analysis across nine cohorts including a total of 2,186 participants from civilian and military cohorts reported that traumatic stress is associated with advanced epigenetic age and this relationship may be due to the function of immune cells (Wolf, Maniates, et al., 2018).
Growing evidence is highlighting the potential role of transgenerational effects of paternal exposure to stress vs. positive stimuli on the behavioral, affective, and cognitive characteristics of their progeny (Yeshurun & Hannan, 2019). These mechanisms appear to be related to sperm-specific epigenetic mechanisms (e.g., DNA methylation changes and variation small non-coding RNAs) (Yeshurun & Hannan, 2019). However, transgenerational epigenomics is in its infancy and further studies will be needed to understand the role of parental traumatic stress in the progeny’s physical and mental health.
Transcriptomics
Transcriptomic analyses are also contributing to understand the molecular changes associated with PTSD and traumatic experiences. A blood-based transcriptomic analysis comparing 229 PTSD and 311 controls showed co-expression networks related to specific functional modules depending on sex and modes of trauma: wound-healing module downregulated in men exposed to combat traumas; IL-12-mediated signaling module upregulated in men exposed to interpersonal-related traumas; modules associated with lipid metabolism and mitogen-activated protein kinase activity upregulated in women exposed to interpersonal-related traumas (Breen et al., 2018). Shared PTSD functional network modules were detected with respect to cytokine, innate immune, and type I interferon pathways (Breen et al., 2018). In an independent whole-blood transcriptome-wide study conducted in 324 World Trade Center responders (Kuan, Waszczuk, Kotov, Clouston, et al., 2017), a polygenic expression achieved sensitivity/specificity of 0.92/0.51, respectively for identifying current PTSD with current and past PTSD groups scoring higher than trauma-exposed controls without any history of PTSD. In a subset of the same cohort (39 World Trade Center responders) (Kuan et al., 2019), cell-specific and shared differentially expressed genes across four immune cell subpopulations (CD4T, CD8T, B cells, and monocytes) and enrichments for pathways related to mast cell activation and regulation in CD4T, interferon-beta production in CD8T, and neutrophil-related gene sets in monocytes were reported. In prefrontal cortex tissues from 22 donors with PTSD and 22 matched non-PTSD control donors, a study observed lower relative expression of TSPO and microglia-associated genes TNFRSF14 and TSPOAP1 in the female PTSD subgroup (Bhatt et al., 2020). In a recent study analyzing four prefrontal cortex subregions, a gene network of downregulated interneuron transcripts was associated with PTSD with converging evidence with MVP GWAS results related to the interneuron synaptic gene ELFN1 (Girgenti et al., 2021).
Neuroimaging
To investigate further the neurobiology of PTSD, genetic investigations can integrate information regarding brain structural and functional variation from imaging techniques. The study of brain imaging phenotypes in the context of PTSD genetics can lead to a more comprehensive understanding of the interplay between traumatic experiences and PTSD vulnerability. The PGC-PTSD workgroup joined forces with the ENIGMA (Enhancing NeuroImaging Genetics through Meta-Analysis) consortium to combine their different expertise to dissect the pleiotropic mechanisms linking PTSD and brain imaging phenotypes (Nievergelt et al., 2018). In an initial study conducted in a small sample (66 PTSD cases and 91 non-PTSD controls) (Morey et al., 2017), pleiotropic associations were observed between caudate volume and childhood trauma and between right lateral ventricular volume and lifetime alcohol use disorder. Leveraging ENIGMA and PGC-PTSD genome-wide association statistics, novel PTSD risk loci were identified when accounting for the genetic associations of putamen volume, supporting a possible involvement for the glutamatergic system (van der Merwe et al., 2019). Recently, ENIGMA-PGC-PTSD investigators investigated hippocampal markers of PTSD, depression, and the interaction of these conditions across 31 cohorts worldwide (N=3,115) (Salminen et al., 2019). Their findings highlighted that the comorbidity of PTSD and depression is strongly associated with hippocampal volumetry with the latter having a larger contribution than the former.
Future Perspectives
There are several challenges we need to overcome before translating molecular findings into PTSD clinical practice. There is still a consistent missing heritability (i.e., the difference between twin-based and SNP-based heritability estimates) with respect to PTSD genetics. Whole-genome sequencing data may be able to address this, improving our ability to investigate uncommon genetic variants in low LD regions (Wainschtein et al., 2019). Additionally, the diagnostic complexity of psychiatric disorders was associated with the predicted effect size variance for trait-associated loci (F. R. Wendt et al., 2020). Improving the ability to investigate genetic variation while addressing diagnostic heterogeneity will surely boost PTSD gene discovery, potentially leading to genetic instruments to identify high-risk individuals and characterize molecular targets to develop effective treatments. Similarly, the ongoing technological progress is helping to conduct more powerful epigenetic, transcriptomic, and brain-imaging studies that can contribute to design PTSD biomarkers. Additionally, several approaches are being proposed to integrate data generated from different high-throughput experimental data and conduct more holistic investigations of PTSD (Chakraborty, Meyerhoff, Jett, & Hammamieh, 2017; Thakur et al., 2015). In addition to these analytic challenges, like many other human diseases and traits, PTSD research has a serious diversity imbalance where underserved minorities are under-investigated (Sirugo et al., 2019). Although findings from PGC and MVP studies were generated from cohorts including individuals with diverse ancestral background, the vast majority of the participants are individuals of European descent. To avoid the widening of health disparities, molecular studies of PTSD need to increase the diversity of the cohorts analyzed to reflect adequately human variation and generate results transferrable across worldwide populations. Large-scale efforts, such as MVP and AllOfUs, are currently recruiting more diverse populations and will provide resources useful to partially address the present disparities in PTSD molecular research.
Conclusions
Our understanding of the molecular basis of PTSD is progressing rapidly, mainly because of international collaborations and large biobanks leading to an increase in statistical power. While technological and analytic progress are improving the ability to dissect PTSD pathogenesis, investigators have to continue to be particularly careful about communicating their findings to the general public to avoid that molecular insights are distorted to support a “blaming the victim” rhetoric. This is particularly important with respect to certain traumatic events like sexual assaults that are more likely to be stigmatized (Kennedy & Prock, 2018). Genetic studies of PTSD should be a further opportunity to address how to reduce the burden of traumatic experiences in human societies.
Acknowledgments
The authors thank Karestan Koenen and Caroline Nievergelt for their helpful comments.
Financial support
The authors acknowledge support from the National Institutes of Health (R21 DC018098, R21 DA047527, and F32 MH122058).
Footnotes
Conflict of interest
Dr. Polimanti is paid for their editorial work on the journal Complex Psychiatry. Dr. Wendt declares no competing interests.
References
- Afifi TO, Asmundson GJ, Taylor S, & Jang KL (2010). The role of genes and environment on trauma exposure and posttraumatic stress disorder symptoms: a review of twin studies. Clinical Psychology Review, 30(1), 101–112. doi: 10.1016/j.cpr.2009.10.002 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Trauma- and Stressor-Related DisordersDiagnostic and Statistical Manual of Mental Disorders (Fifth ed.). [Google Scholar]
- Atkinson EG, Maihofer AX, Kanai M, Martin AR, Karczewski KJ, Santoro ML, … Neale BM (2020). “Tractor”: A framework allowing for improved inclusion of admixed individuals in large-scale association studies. bioRxiv, 2020.2005.2017.100727. doi: 10.1101/2020.05.17.100727 [DOI] [Google Scholar]
- Ball TM, & Stein MB (2012). The Oxford Handbook of Traumatic Stress Disorders (Beck JG & Sloan DM Eds. 1st ed.): Oxford Handbooks Online. [Google Scholar]
- Benjet C, Bromet E, Karam EG, Kessler RC, McLaughlin KA, Ruscio AM, … Koenen KC (2016). The epidemiology of traumatic event exposure worldwide: results from the World Mental Health Survey Consortium. Psychological Medicine, 46(2), 327–343. doi: 10.1017/S0033291715001981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S, Hillmer AT, Girgenti MJ, Rusowicz A, Kapinos M, Nabulsi N, … Cosgrove KP (2020). PTSD is associated with neuroimmune suppression: evidence from PET imaging and postmortem transcriptomic studies. Nature Communications, 11(1), 2360. doi: 10.1038/s41467-020-15930-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Border R, Johnson EC, Evans LM, Smolen A, Berley N, Sullivan PF, & Keller MC (2019). No Support for Historical Candidate Gene or Candidate Gene-by-Interaction Hypotheses for Major Depression Across Multiple Large Samples. American Journal of Psychiatry, 176(5), 376–387. doi: 10.1176/appi.ajp.2018.18070881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscarino JA (2004). Posttraumatic stress disorder and physical illness: results from clinical and epidemiologic studies. Annals of the New York Academy of Sciences, 1032, 141–153. doi: 10.1196/annals.1314.011 [DOI] [PubMed] [Google Scholar]
- Breen MS, Tylee DS, Maihofer AX, Neylan TC, Mehta D, Binder EB, … Glatt SJ (2018). PTSD Blood Transcriptome Mega-Analysis: Shared Inflammatory Pathways across Biological Sex and Modes of Trauma. Neuropsychopharmacology, 43(3), 469–481. doi: 10.1038/npp.2017.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulik-Sullivan B, Finucane HK, Anttila V, Gusev A, Day FR, Loh PR, … Neale BM (2015). An atlas of genetic correlations across human diseases and traits. Nature Genetics, 47(11), 1236–1241. doi: 10.1038/ng.3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JG, Svetec N, Rowe L, Mery F, Dolan MJ, Boyce WT, & Sokolowski MB (2012). Gene-environment interplay in Drosophila melanogaster: chronic food deprivation in early life affects adult exploratory and fitness traits. Proceedings of the National Academy of Sciences of the United States of America, 109 Suppl 2, 17239–17244. doi: 10.1073/pnas.1121265109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho CM, Wendt FR, Stein DJ, Stein MB, Gelernter J, Belangero SI, & Polimanti R (2020). Investigating Causality Between Blood Metabolites and Emotional and Behavioral Responses to Traumatic Stress: a Mendelian Randomization Study. Molecular Neurobiology, 57(3), 1542–1552. doi: 10.1007/s12035-019-01823-2 [DOI] [PubMed] [Google Scholar]
- Chakraborty N, Meyerhoff J, Jett M, & Hammamieh R (2017). Genome to Phenome: A Systems Biology Approach to PTSD Using an Animal Model. Methods in Molecular Biology, 1598, 117–154. doi: 10.1007/978-1-4939-6952-4_6 [DOI] [PubMed] [Google Scholar]
- Chivers-Wilson KA (2006). Sexual assault and posttraumatic stress disorder: a review of the biological, psychological and sociological factors and treatments. McGill Journal of Medicine, 9(2), 111–118. [PMC free article] [PubMed] [Google Scholar]
- Coleman JRI, Peyrot WJ, Purves KL, Davis KAS, Rayner C, Choi SW, … Breen G (2020). Genome-wide gene-environment analyses of major depressive disorder and reported lifetime traumatic experiences in UK Biobank. Molecular Psychiatry, 25(7), 1430–1446. doi: 10.1038/s41380-019-0546-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comings DE, Muhleman D, & Gysin R (1996). Dopamine D2 receptor (DRD2) gene and susceptibility to posttraumatic stress disorder: a study and replication. Biological Psychiatry, 40(5), 368–372. doi: 10.1016/0006-3223(95)00519-6 [DOI] [PubMed] [Google Scholar]
- Cox RC, Taylor S, Strachan E, & Olatunji BO (2020). Insomnia and posttraumatic stress symptoms: Evidence of shared etiology. Psychiatry Researchearch, 286, 112548. doi: 10.1016/j.psychres.2019.112548 [DOI] [PubMed] [Google Scholar]
- Dalvie S, Maihofer AX, Coleman JRI, Bradley B, Breen G, Brick LA, … Nievergelt CM (2020). Genomic influences on self-reported childhood maltreatment. Translational Psychiatry, 10(1), 38. doi: 10.1038/s41398-020-0706-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan LE, Cooper BN, & Shen H (2018). Robust Findings From 25 Years of PTSD Genetics Research. Current Psychiatry Reports, 20(12), 115. doi: 10.1007/s11920-018-0980-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan LE, Ratanatharathorn A, Aiello AE, Almli LM, Amstadter AB, Ashley-Koch AE, … Koenen KC (2018). Largest GWAS of PTSD (N=20 070) yields genetic overlap with schizophrenia and sex differences in heritability. Molecular Psychiatry, 23(3), 666–673. doi: 10.1038/mp.2017.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn EC, Nishimi K, Powers A, & Bradley B (2017). Is developmental timing of trauma exposure associated with depressive and post-traumatic stress disorder symptoms in adulthood? Journal of Psychiatric Research, 84, 119–127. doi: 10.1016/j.jpsychires.2016.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frueh BC, Elhai JD, & Acierno R (2010). The Future of Posttraumatic Stress Disorder in the DSM. Psychological Injury and Law, 3(4), 260–270. doi: 10.1007/s12207-010-9088-6 [DOI] [Google Scholar]
- Gelernter J, Sun N, Polimanti R, Pietrzak R, Levey DF, Bryois J, … Million Veteran P (2019). Genome-wide association study of post-traumatic stress disorder reexperiencing symptoms in >165,000 US veterans. Nature Neuroscience, 22(9), 1394–1401. doi: 10.1038/s41593-019-0447-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgenti MJ, Wang J, Ji D, Cruz DA, Traumatic Stress Brain Research, G., Stein MB, … Duman RS (2021). Transcriptomic organization of the human brain in post-traumatic stress disorder. Nature Neuroscience, 24(1), 24–33. doi: 10.1038/s41593-020-00748-7 [DOI] [PubMed] [Google Scholar]
- Grotzinger AD, Rhemtulla M, de Vlaming R, Ritchie SJ, Mallard TT, Hill WD, … Tucker-Drob EM (2019). Genomic structural equation modelling provides insights into the multivariate genetic architecture of complex traits. Nature Human Behaviour, 3(5), 513–525. doi: 10.1038/s41562-019-0566-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guffanti G, Galea S, Yan L, Roberts AL, Solovieff N, Aiello AE, … Koenen KC (2013). Genome-wide association study implicates a novel RNA gene, the lincRNA AC068718.1, as a risk factor for post-traumatic stress disorder in women. Psychoneuroendocrinology, 38(12), 3029–3038. doi: 10.1016/j.psyneuen.2013.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta MA (2013). Review of somatic symptoms in post-traumatic stress disorder. International Review of Psychiatry, 25(1), 86–99. doi: 10.3109/09540261.2012.736367 [DOI] [PubMed] [Google Scholar]
- Hammamieh R, Chakraborty N, Gautam A, Muhie S, Yang R, Donohue D, … Jett M (2017). Whole-genome DNA methylation status associated with clinical PTSD measures of OIF/OEF veterans. Translational Psychiatry, 7(7), e1169. doi: 10.1038/tp.2017.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin Y, Seldin M, & Lusis A (2017). Multi-omics approaches to disease. Genome Biolology, 18(1), 83. doi: 10.1186/s13059-017-1215-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck A, Milnik A, Vukojevic V, Petrovska J, Egli T, Singer J, … Papassotiropoulos A (2017). Exome sequencing of healthy phenotypic extremes links TROVE2 to emotional memory and PTSD. Nature Human Behaviour, 1(4), 0081. doi: 10.1038/s41562-017-0081 [DOI] [Google Scholar]
- Holland D, Frei O, Desikan R, Fan CC, Shadrin AA, Smeland OB, … Dale AM (2020). Beyond SNP heritability: Polygenicity and discoverability of phenotypes estimated with a univariate Gaussian mixture model. PLOS Genetics, 16(5), e1008612. doi: 10.1371/journal.pgen.1008612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckins LM, Chatzinakos C, Breen MS, Hartmann J, Klengel T, da Silva Almeida AC, … Daskalakis NP (2020). Analysis of Genetically Regulated Gene Expression Identifies a Prefrontal PTSD Gene, SNRNP35, Specific to Military Cohorts. Cell Reports, 31(9), 107716. doi: 10.1016/j.celrep.2020.107716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin MJ, Jeon H, Hyun MH, & Lee SH (2019). Influence of childhood trauma and brain-derived neurotrophic factor Val66Met polymorphism on posttraumatic stress symptoms and cortical thickness. Scientific Reports, 9(1), 6028. doi: 10.1038/s41598-019-42563-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy AC, & Prock KA (2018). “I Still Feel Like I Am Not Normal”: A Review of the Role of Stigma and Stigmatization Among Female Survivors of Child Sexual Abuse, Sexual Assault, and Intimate Partner Violence. Trauma Violence Abuse, 19(5), 512–527. doi: 10.1177/1524838016673601 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Aguilar-Gaxiola S, Alonso J, Benjet C, Bromet EJ, Cardoso G, … Koenen KC (2017). Trauma and PTSD in the WHO World Mental Health Surveys. European Journal of Psychotraumatology, 8(sup5), 1353383. doi: 10.1080/20008198.2017.1353383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick DG, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Resnick HS, … Gelernter J (2007). The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. American Journal of Psychiatry, 164(11), 1693–1699. doi: 10.1176/appi.ajp.2007.06122007 [DOI] [PubMed] [Google Scholar]
- Koenen KC, Ratanatharathorn A, Ng L, McLaughlin KA, Bromet EJ, Stein DJ, … Kessler RC (2017). Posttraumatic stress disorder in the World Mental Health Surveys. Psychological Medicine, 47(13), 2260–2274. doi: 10.1017/S0033291717000708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen KC, Sumner JA, Gilsanz P, Glymour MM, Ratanatharathorn A, Rimm EB, … Kubzansky LD (2017). Post-traumatic stress disorder and cardiometabolic disease: improving causal inference to inform practice. Psychological Medicine, 47(2), 209–225. doi: 10.1017/S0033291716002294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolassa IT, Kolassa S, Ertl V, Papassotiropoulos A, & De Quervain DJ (2010). The risk of posttraumatic stress disorder after trauma depends on traumatic load and the catechol-o-methyltransferase Val(158)Met polymorphism. Biological Psychiatry, 67(4), 304–308. doi: 10.1016/j.biopsych.2009.10.009 [DOI] [PubMed] [Google Scholar]
- Kuan PF, Waszczuk MA, Kotov R, Clouston S, Yang X, Singh PK, … Luft BJ (2017). Gene expression associated with PTSD in World Trade Center responders: An RNA sequencing study. Translational Psychiatry, 7(12), 1297. doi: 10.1038/s41398-017-0050-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuan PF, Waszczuk MA, Kotov R, Marsit CJ, Guffanti G, Gonzalez A, … Luft BJ (2017). An epigenome-wide DNA methylation study of PTSD and depression in World Trade Center responders. Translational Psychiatry, 7(6), e1158. doi: 10.1038/tp.2017.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuan PF, Yang X, Clouston S, Ren X, Kotov R, Waszczuk M, … Luft BJ (2019). Cell type-specific gene expression patterns associated with posttraumatic stress disorder in World Trade Center responders. Translational Psychiatry, 9(1), 1. doi: 10.1038/s41398-018-0355-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind MJ, Brick LA, Gehrman PR, Duncan LE, Gelaye B, Maihofer AX, … Psychiatric Genomics Consortium Posttraumatic Stress, D. (2020). Molecular genetic overlap between posttraumatic stress disorder and sleep phenotypes. Sleep, 43(4). doi: 10.1093/sleep/zsz257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue MW, Baldwin C, Guffanti G, Melista E, Wolf EJ, Reardon AF, … Miller MW (2013). A genome-wide association study of post-traumatic stress disorder identifies the retinoid-related orphan receptor alpha (RORA) gene as a significant risk locus. Molecular Psychiatry, 18(8), 937–942. doi: 10.1038/mp.2012.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logue MW, Miller MW, Wolf EJ, Huber BR, Morrison FG, Zhou Z, … Traumatic Stress Brain Study, G. (2020). An epigenome-wide association study of posttraumatic stress disorder in US veterans implicates several new DNA methylation loci. Clininical Epigenetics, 12(1), 46. doi: 10.1186/s13148-020-0820-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr JB, Palmer BW, Eidt CA, Aailaboyina S, Mausbach BT, Wolkowitz OM, … Jeste DV (2015). Is Post-Traumatic Stress Disorder Associated with Premature Senescence? A Review of the Literature. American Journal of Geriatric Psychiatry, 23(7), 709–725. doi: 10.1016/j.jagp.2015.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz MW, Luo S, Williamson DE, & Chiba-Falek O (2020). Shared genetic etiology underlying late-onset Alzheimer’s disease and posttraumatic stress syndrome. Alzheimer’s & Dementia. doi: 10.1002/alz.12128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall CA, Turkheimer E, Tsang S, Avery A, Duncan GE, & Watson NF (2019). Sleep duration and post-traumatic stress disorder symptoms: a twin study. Sleep, 42(12). doi: 10.1093/sleep/zsz179 [DOI] [PubMed] [Google Scholar]
- Mehta D, Klengel T, Conneely KN, Smith AK, Altmann A, Pace TW, … Binder EB (2013). Childhood maltreatment is associated with distinct genomic and epigenetic profiles in posttraumatic stress disorder. Proceedings of the National Academy of Sciences of the United States of America, 110(20), 8302–8307. doi: 10.1073/pnas.1217750110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RA, Davis SL, Garrett ME, Haswell CC, Mid-Atlantic MW, Marx CE, … Ashley-Koch AE (2017). Genome-wide association study of subcortical brain volume in PTSD cases and trauma-exposed controls. Translational Psychiatry, 7(11), 1265. doi: 10.1038/s41398-017-0021-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniz Carvalho C, Wendt FR, Maihofer AX, Stein DJ, Stein MB, Sumner JA, … Polimanti R (2020). Dissecting the genetic association of C-reactive protein with PTSD, traumatic events, and social support. Neuropsychopharmacology. doi: 10.1038/s41386-020-0655-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng LC, Stevenson A, Kalapurakkel SS, Hanlon C, Seedat S, Harerimana B, … Koenen KC (2020). National and regional prevalence of posttraumatic stress disorder in sub-Saharan Africa: A systematic review and meta-analysis. PLoS Medicine, 17(5), e1003090. doi: 10.1371/journal.pmed.1003090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nievergelt CM, Ashley-Koch AE, Dalvie S, Hauser MA, Morey RA, Smith AK, & Uddin M (2018). Genomic Approaches to Posttraumatic Stress Disorder: The Psychiatric Genomic Consortium Initiative. Biological Psychiatry, 83(10), 831–839. doi: 10.1016/j.biopsych.2018.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nievergelt CM, Maihofer AX, Klengel T, Atkinson EG, Chen CY, Choi KW, … Koenen KC (2019). International meta-analysis of PTSD genome-wide association studies identifies sex- and ancestry-specific genetic risk loci. Nature Communications, 10(1), 4558. doi: 10.1038/s41467-019-12576-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nievergelt CM, Maihofer AX, Mustapic M, Yurgil KA, Schork NJ, Miller MW, … Baker DG (2015). Genomic predictors of combat stress vulnerability and resilience in U.S. Marines: A genome-wide association study across multiple ancestries implicates PRTFDC1 as a potential PTSD gene. Psychoneuroendocrinology, 51, 459–471. doi: 10.1016/j.psyneuen.2014.10.017 [DOI] [PubMed] [Google Scholar]
- Olbert CM, Gala GJ, & Tupler LA (2014). Quantifying heterogeneity attributable to polythetic diagnostic criteria: theoretical framework and empirical application. Journal of Abnormal Psychology, 123(2), 452–462. doi: 10.1037/a0036068 [DOI] [PubMed] [Google Scholar]
- Palmer C, & Pe’er I (2017). Statistical correction of the Winner’s Curse explains replication variability in quantitative trait genome-wide association studies. PLOS Genetics, 13(7), e1006916. doi: 10.1371/journal.pgen.1006916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa A, Neria Y, & Litz B (2008). Traumatic bereavement in war veterans. Psychiatric Annals, 38(10), 686–691. doi: 10.3928/00485713-20081001-07 [DOI] [Google Scholar]
- Polimanti R, Amstadter AB, Stein MB, Almli LM, Baker DG, Bierut LJ, … Psychiatric Genomics Consortium Posttraumatic Stress Disorder, W. (2017). A putative causal relationship between genetically determined female body shape and posttraumatic stress disorder. Genome Medicine, 9(1), 99. doi: 10.1186/s13073-017-0491-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polimanti R, Kaufman J, Zhao H, Kranzler HR, Ursano RJ, Kessler RC, … Stein MB (2018). A genome-wide gene-by-trauma interaction study of alcohol misuse in two independent cohorts identifies PRKG1 as a risk locus. Molecular Psychiatry, 23(1), 154–160. doi: 10.1038/mp.2017.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polimanti R, Kaufman J, Zhao H, Kranzler HR, Ursano RJ, Kessler RC, … Gelernter J (2018). Trauma exposure interacts with the genetic risk of bipolar disorder in alcohol misuse of US soldiers. Acta Psychiatrica Scandinavica, 137(2), 148–156. doi: 10.1111/acps.12843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polimanti R, Ratanatharathorn A, Maihofer AX, Choi KW, Stein MB, Morey RA, … Psychiatric Genomics Consortium Posttraumatic Stress Disorder Working, G. (2019). Association of Economic Status and Educational Attainment With Posttraumatic Stress Disorder: A Mendelian Randomization Study. JAMA Network Open, 2(5), e193447. doi: 10.1001/jamanetworkopen.2019.3447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratanatharathorn A, Boks MP, Maihofer AX, Aiello AE, Amstadter AB, Ashley-Koch AE, … Smith AK (2017). Epigenome-wide association of PTSD from heterogeneous cohorts with a common multi-site analysis pipeline. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 174(6), 619–630. doi: 10.1002/ajmg.b.32568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, … May V (2011). Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature, 470(7335), 492–497. doi: 10.1038/nature09856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivollier F, Peyre H, Hoertel N, Blanco C, Limosin F, & Delorme R (2015). Sex differences in DSM-IV posttraumatic stress disorder symptoms expression using item response theory: A population-based study. Journal of Affective Disorders, 187, 211–217. doi: 10.1016/j.jad.2015.07.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AL, Huang T, Koenen KC, Kim Y, Kubzansky LD, & Tworoger SS (2019). Posttraumatic Stress Disorder Is Associated with Increased Risk of Ovarian Cancer: A Prospective and Retrospective Longitudinal Cohort Study. Cancer Research, 79(19), 5113–5120. doi: 10.1158/0008-5472.CAN-19-1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts NP, Roberts PA, Jones N, & Bisson JI (2015). Psychological interventions for post-traumatic stress disorder and comorbid substance use disorder: A systematic review and meta-analysis. Clinical Psychology Review, 38, 25–38. doi: 10.1016/j.cpr.2015.02.007 [DOI] [PubMed] [Google Scholar]
- Sack WH, Clarke GN, & Seeley J (1995). Posttraumatic stress disorder across two generations of Cambodian refugees. Journal of the American Academy of Child and Adolescent Psychiatry, 34(9), 1160–1166. doi: 10.1097/00004583-199509000-00013 [DOI] [PubMed] [Google Scholar]
- Salminen LE, Sämann PG, Zheng Y, Dennis EL, Clarke-Rubright EK, Jahanshad N, … Logue MW (2019). Hippocampal subfield volumes are uniquely affected in PTSD and depression: International analysis of 31 cohorts from the PGC-ENIGMA PTSD Working Group. bioRxiv, 739094. doi: 10.1101/739094 [DOI] [Google Scholar]
- Sareen J (2020, January 10, 2020). Posttraumatic stress disorder in adults: Epidemiology, pathophysiology, clinical manifestations, course, assessment, and diagnosis. Retrieved September 1, 2020, from https://www.uptodate.com/contents/posttraumatic-stress-disorder-in-adults-epidemiology-pathophysiology-clinical-manifestations-course-assessment-and-diagnosis
- Sartor CE, Grant JD, Lynskey MT, McCutcheon VV, Waldron M, Statham DJ, … Nelson EC (2012). Common heritable contributions to low-risk trauma, high-risk trauma, posttraumatic stress disorder, and major depression. Archives Of General Psychiatry, 69(3), 293–299. doi: 10.1001/archgenpsychiatry.2011.1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savas LS, White DL, Wieman M, Daci K, Fitzgerald S, Laday Smith S, … El-Serag HB (2009). Irritable bowel syndrome and dyspepsia among women veterans: prevalence and association with psychological distress. Alimentary Pharmacology & Therapeutics, 29(1), 115–125. doi: 10.1111/j.1365-2036.2008.03847.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schijven D, Geuze E, Vinkers CH, Pulit SL, Schur RR, Malgaz M, … Luykx JJ (2019). Multivariate genome-wide analysis of stress-related quantitative phenotypes. European Neuropsychopharmacology, 29(12), 1354–1364. doi: 10.1016/j.euroneuro.2019.09.012 [DOI] [PubMed] [Google Scholar]
- Segman RH, Cooper-Kazaz R, Macciardi F, Goltser T, Halfon Y, Dobroborski T, & Shalev AY (2002). Association between the dopamine transporter gene and posttraumatic stress disorder. Molecular Psychiatry, 7(8), 903–907. doi: 10.1038/sj.mp.4001085 [DOI] [PubMed] [Google Scholar]
- Shand LK, Cowlishaw S, Brooker JE, Burney S, & Ricciardelli LA (2015). Correlates of post-traumatic stress symptoms and growth in cancer patients: a systematic review and meta-analysis. Psychooncology, 24(6), 624–634. doi: 10.1002/pon.3719 [DOI] [PubMed] [Google Scholar]
- Sheerin CM, Lind MJ, Bountress KE, Marraccini ME, Amstadter AB, Bacanu SA, & Nugent NR (2020). Meta-analysis of Associations Between Hypothalamic-Pituitary-Adrenal Axis Genes and Risk of Posttraumatic Stress Disorder. Journal of Traumatic Stress. doi: 10.1002/jts.22484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton K, Ressler KJ, Norrholm SD, Jovanovic T, & Bradley-Davino B (2012). PTSD and gene variants: new pathways and new thinking. Neuropharmacology, 62(2), 628–637. doi: 10.1016/j.neuropharm.2011.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AK, Ratanatharathorn A, Maihofer AX, Naviaux RK, Aiello AE, Amstadter AB, … Nievergelt CM (2020). Epigenome-wide meta-analysis of PTSD across 10 military and civilian cohorts identifies novel methylation loci. Nature Communications, In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GD, & Ebrahim S (2003). ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? International Journal of Epidemiology, 32(1), 1–22. doi: 10.1093/ije/dyg070 [DOI] [PubMed] [Google Scholar]
- Smoller JW (2016). The Genetics of Stress-Related Disorders: PTSD, Depression, and Anxiety Disorders. Neuropsychopharmacology, 41(1), 297–319. doi: 10.1038/npp.2015.266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders C, Maihofer AX, Ratanatharathorn A, Baker DG, Boks MP, Geuze E, … Nievergelt CM (2020). Longitudinal epigenome-wide association studies of three male military cohorts reveal multiple CpG sites associated with post-traumatic stress disorder. Clininical Epigenetics, 12(1), 11. doi: 10.1186/s13148-019-0798-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Chen CY, Ursano RJ, Cai T, Gelernter J, Heeringa SG, … Resilience in Servicemembers, C. (2016). Genome-wide Association Studies of Posttraumatic Stress Disorder in 2 Cohorts of US Army Soldiers. JAMA Psychiatry, 73(7), 695–704. doi: 10.1001/jamapsychiatry.2016.0350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Jang KL, Taylor S, Vernon PA, & Livesley WJ (2002). Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: a twin study. American Journal of Psychiatry, 159(10), 1675–1681. doi: 10.1176/appi.ajp.159.10.1675 [DOI] [PubMed] [Google Scholar]
- Stein MB, Levey DF, Cheng Z, Wendt FR, Harrington K, Cho K, … Gelernter J (2019). Genomic Characterization of Posttraumatic Stress Disorder in a Large US Military Veteran Sample. bioRxiv, 764001. doi: 10.1101/764001 [DOI] [Google Scholar]
- Sul JH, Martin LS, & Eskin E (2018). Population structure in genetic studies: Confounding factors and mixed models. PLOS Genetics, 14(12), e1007309. doi: 10.1371/journal.pgen.1007309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Agrawal A, Bulik CM, Andreassen OA, Borglum AD, Breen G, … Psychiatric Genomics, C. (2018). Psychiatric Genomics: An Update and an Agenda. American Journal of Psychiatry, 175(1), 15–27. doi: 10.1176/appi.ajp.2017.17030283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamman AJF, Wendt FR, Pathak GA, Krystal JH, Montalvo-Ortiz JL, Southwick SM, … Pietrzak RH (2020). Attachment Style Moderates Polygenic Risk for Posttraumatic Stress in United States Military Veterans: Results From the National Health and Resilience in Veterans Study. Biological Psychiatry. doi: 10.1016/j.biopsych.2020.09.018 [DOI] [PubMed] [Google Scholar]
- Thakur GS, Daigle BJ Jr., Dean KR, Zhang Y, Rodriguez-Fernandez M, Hammamieh R, … Doyle FJ 3rd. (2015). Systems biology approach to understanding post-traumatic stress disorder. Molecular BioSystems, 11(4), 980–993. doi: 10.1039/c4mb00404c [DOI] [PubMed] [Google Scholar]
- Uddin M, Ratanatharathorn A, Armstrong D, Kuan PF, Aiello AE, Bromet EJ, … Smith A (2018). Epigenetic meta-analysis across three civilian cohorts identifies NRG1 and HGS as blood-based biomarkers for post-traumatic stress disorder. Epigenomics, 10(12), 1585–1601. doi: 10.2217/epi-2018-0049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Merwe C, Jahanshad N, Cheung JW, Mufford M, Groenewold NA, Koen N, … Stein DJ (2019). Concordance of genetic variation that increases risk for anxiety disorders and posttraumatic stress disorders and that influences their underlying neurocircuitry. Journal of Affective Disorders, 245, 885–896. doi: 10.1016/j.jad.2018.11.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij SJ, Stevens JS, Ely TD, Fani N, Smith AK, Kerley KA, … Jovanovic T (2016). Childhood Trauma and COMT Genotype Interact to Increase Hippocampal Activation in Resilient Individuals. Frontiers in Psychiatry, 7, 156. doi: 10.3389/fpsyt.2016.00156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor SE, & Klonsky ED (2014). Correlates of suicide attempts among self-injurers: a meta-analysis. Clinical Psychology Review, 34(4), 282–297. doi: 10.1016/j.cpr.2014.03.005 [DOI] [PubMed] [Google Scholar]
- Wainschtein P, Jain DP, Yengo L, Zheng Z, Cupples LA, Shadyab AH, … Visscher PM (2019). Recovery of trait heritability from whole genome sequence data. bioRxiv, 588020. doi: 10.1101/588020 [DOI] [Google Scholar]
- Wang Q, Shelton RC, & Dwivedi Y (2018). Interaction between early-life stress and FKBP5 gene variants in major depressive disorder and post-traumatic stress disorder: A systematic review and meta-analysis. Journal of Affective Disorders, 225, 422–428. doi: 10.1016/j.jad.2017.08.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt FR, Pathak GA, Levey DF, Nunez YZ, Overstreet C, Tyrrell C, … Polimanti R (2020). Trauma and posttraumatic stress interact with sex-specific risk loci for suicidality and converge on brain extracellular matrix biology and synaptic plasticity. medRxiv, 2020.2005.2018.20105734. doi: 10.1101/2020.05.18.20105734 [DOI] [Google Scholar]
- Wendt FR, Pathak GA, Overstreet C, Tylee DS, Gelernter J, Atkinson EG, & Polimanti R (2020). Characterizing the effect of background selection on the polygenicity of brain-related traits. Genomics, 113(1 Pt 1), 111–119. doi: 10.1016/j.ygeno.2020.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild J, Smith KV, Thompson E, Bear F, Lommen MJ, & Ehlers A (2016). A prospective study of pre-trauma risk factors for post-traumatic stress disorder and depression. Psychological Medicine, 46(12), 2571–2582. doi: 10.1017/S0033291716000532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf EJ, Logue MW, Morrison FG, Wilcox ES, Stone A, Schichman SA, … Miller MW (2019). Posttraumatic psychopathology and the pace of the epigenetic clock: a longitudinal investigation. Psychological Medicine, 49(5), 791–800. doi: 10.1017/S0033291718001411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf EJ, Logue MW, Stoop TB, Schichman SA, Stone A, Sadeh N, … Miller MW (2018). Accelerated DNA Methylation Age: Associations With Posttraumatic Stress Disorder and Mortality. Psychosomatic Medicine, 80(1), 42–48. doi: 10.1097/PSY.0000000000000506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf EJ, Maniates H, Nugent N, Maihofer AX, Armstrong D, Ratanatharathorn A, … Logue MW (2018). Traumatic stress and accelerated DNA methylation age: A meta-analysis. Psychoneuroendocrinology, 92, 123–134. doi: 10.1016/j.psyneuen.2017.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf EJ, Miller MW, Sullivan DR, Amstadter AB, Mitchell KS, Goldberg J, & Magruder KM (2018). A classical twin study of PTSD symptoms and resilience: Evidence for a single spectrum of vulnerability to traumatic stress. Depression and Anxiety, 35(2), 132–139. doi: 10.1002/da.22712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Feder A, Cohen H, Kim JJ, Calderon S, Charney DS, & Mathe AA (2013). Understanding resilience. Frontiers in Behavioral Neuroscience, 7, 10. doi: 10.3389/fnbeh.2013.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie P, Kranzler HR, Yang C, Zhao H, Farrer LA, & Gelernter J (2013). Genome-wide association study identifies new susceptibility loci for posttraumatic stress disorder. Biological Psychiatry, 74(9), 656–663. doi: 10.1016/j.biopsych.2013.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang BZ, Zhang H, Ge W, Weder N, Douglas-Palumberi H, Perepletchikova F, … Kaufman J (2013). Child abuse and epigenetic mechanisms of disease risk. American Journal of Preventive Medicine, 44(2), 101–107. doi: 10.1016/j.amepre.2012.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Halligan SL, & Bierer LM (2001). Relationship of parental trauma exposure and PTSD to PTSD, depressive and anxiety disorders in offspring. Journal of Psychiatric Research, 35(5), 261–270. doi: 10.1016/s0022-3956(01)00032-2 [DOI] [PubMed] [Google Scholar]
- Yeshurun S, & Hannan AJ (2019). Transgenerational epigenetic influences of paternal environmental exposures on brain function and predisposition to psychiatric disorders. Molecular Psychiatry, 24(4), 536–548. doi: 10.1038/s41380-018-0039-z [DOI] [PubMed] [Google Scholar]
- Zang Y, Gallagher T, McLean CP, Tannahill HS, Yarvis JS, Foa EB, & Consortium, S. S. (2017). The impact of social support, unit cohesion, and trait resilience on PTSD in treatment-seeking military personnel with PTSD: The role of posttraumatic cognitions. Journal of Psychiatric Research, 86, 18–25. doi: 10.1016/j.jpsychires.2016.11.005 [DOI] [PubMed] [Google Scholar]
- Zannas AS, Provencal N, & Binder EB (2015). Epigenetics of Posttraumatic Stress Disorder: Current Evidence, Challenges, and Future Directions. Biological Psychiatry, 78(5), 327–335. doi: 10.1016/j.biopsych.2015.04.003 [DOI] [PubMed] [Google Scholar]
- Zhang H, Ozbay F, Lappalainen J, Kranzler HR, van Dyck CH, Charney DS, … Gelernter J (2006). Brain derived neurotrophic factor (BDNF) gene variants and Alzheimer’s disease, affective disorders, posttraumatic stress disorder, schizophrenia, and substance dependence. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 141B(4), 387–393. doi: 10.1002/ajmg.b.30332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Hu XZ, Yu T, Chen Z, Dohl J, Li X, … Ursano RJ (2020). Genetic association of FKBP5 with PTSD in US service members deployed to Iraq and Afghanistan. Journal of Psychiatric Research, 122, 48–53. doi: 10.1016/j.jpsychires.2019.12.014 [DOI] [PubMed] [Google Scholar]


