Abstract
Background and Purpose:
Adiponectin (APN) is an adipokine secreted from adipocytes that binds to APN receptors AdipoR1 and AdipoR2 and exerts an anti-inflammatory response through mechanisms not fully understood. There is a need to develop small molecules that activate AdipoR1 and AdipoR2 and to be used to inhibit the inflammatory response in lipopolysaccharide (LPS)-induced endotoxemia and other inflammatory disorders.
Experimental Approach:
We designed 10 new structural analogues of an AdipoR agonist, AdipoRon (APR), and assessed their anti-inflammatory properties. Bone marrow-derived macrophages (BMMs) and peritoneal macrophages (PEMs) were isolated from mice. Levels of pro-inflammatory cytokines were measured by reverse transcription and real-time quantitative polymerase chain reaction (qRT-PCR), enzyme-linked immunosorbent assay (ELISA) and microarray in LPS-induced endotoxemia mice and diet-induced obesity (DIO) mice in which systemic inflammation prevails. Western blotting, immunohistochemistry (IHC), siRNA interference and immunoprecipitation were used to detect signalling pathways.
Key Results:
A novel APN receptor agonist named adipo anti-inflammation agonist (AdipoAI) strongly suppresses inflammation in DIO and endotoxemia mice, as well as in cultured macrophages. We also found that AdipoAI attenuated the association of AdipoR1 and APPL1 via myeloid differentiation marker 88 (MyD88) signalling, thus inhibiting activation of nuclear factor kappa B (NF-κB), mitogen-activated protein kinase (MAPK) and c-Maf pathways and limiting the production of pro-inflammatory cytokines in LPS-induced macrophages.
Conclusion and Implications:
AdipoAI is a promising alternative therapeutic approach to APN and APR to suppress inflammation in LPS-induced endotoxemia and other inflammatory disorders via distinct signalling pathways.
Keywords: AdipoAI, APPL1, c-Maf, inflammation, lipopolysaccharide, MyD88
1 |. INTRODUCTION
The tissue inflammatory response caused by bacteria, trauma, toxins, heat or other factors can potentially activate the innate immune responses (Barton, 2008; Chen et al., 2018). Innate immune responses are capable of not only combating infectious microbes (Medzhitov & Horng, 2009) but also contributing to pathological situations, such as sepsis, obesity, atherosclerosis, autoimmunity and cancer (Du et al., 2017; Foster & Medzhitov, 2009; Murray & Smale, 2012). Lipopolysaccharide (LPS) is a natural adjuvant synthesized by Gram-negative bacteria that stimulates cells through Toll-like receptor 4 (TLR4) and has profound effects on inflammation and immune responses (Aderem & Ulevitch, 2000). Also, TLR4-triggered signalling depends on the adaptor proteins myeloid differentiation marker 88 (MyD88) and Toll–interleukin-1 (IL-1) receptor (TIR) domain-containing adaptor-inducing IFNβ (TRIF), which mediate MyD88-dependent and TRIF-dependent signalling pathways (Kawai & Akira, 2010; Piao et al., 2009).
In MyD88-dependent signalling pathways, the recruitment and phosphorylation of IL-1 receptor-associated kinase 1 (IRAK1) and IRAK4 are triggered by MyD88 binding to TLR4, which further facilitates oligomerization and auto-ubiquitination of tumour necrosis factor (TNF) receptor-associated factor 6 (TRAF6) (Hirotani et al., 2005; Skaug, Jiang, & Chen, 2009). Ubiquitinated TRAF6 subsequently engages other signalling proteins, such as transforming growth factor β-activated kinase (TAK1), to activate the inhibitor of κB (IκB) kinase (IKK) and mitogen-activated protein kinase (MAPK) kinase (MKK), which induce immune and inflammatory responses via transcription factors such as nuclear factor kappa B (NF-κB) and activator protein 1 (AP-1) (Kishimoto, Matsumoto, & Ninomiya-Tsuji, 2000; Lamothe et al., 2007).
As the most abundant adipokine, adiponectin (APN) exerts various biological functions, including insulin sensitization, fatty acid oxidation and glucose uptake, and plays a vital role in maintaining tissue homeostasis (Brochu-Gaudreau et al., 2010; Bruce, Mertz, Heigenhauser, & Dyck, 2005; Deepa & Dong, 2009). In addition to the critical role of APN in various metabolic diseases, its anti-inflammatory properties have also been described. For instance, APN inhibited TNF-α-stimulated IL-8 synthesis in endothelial cells by modulating NF-κB signalling (Thakur, Pritchard, McMullen, & Nagy, 2006) and normalized cytokine production in LPS-primed Kupffer cells and macrophages by suppressing ERK and p38 MAPK signalling (Kobashi et al., 2005). Furthermore, APN increased the expression of anti-inflammatory genes, such as heme oxygenase-1 (HO-1) (Mandal, Park, McMullen, Pratt, & Nagy, 2010), and the genes of autophagy (M. J. Kim et al., 2017; Pun, Subedi, Kim, & Park, 2015; Tilija Pun & Park, 2018).
AdipoR1 and AdipoR2 serve as the main receptors for APN in vivo. Both contain seven transmembrane domains (Yamauchi et al., 2003), but the structure and function are distinct from G-protein-coupled receptors (Wess, 1997). Adaptors APPL1 and APPL2 (adaptor protein-containing pleckstrin homology domain, phosphotyrosine binding [PTB] domain and leucine zipper motif) bind to APN receptors and mediate APN signalling and function in mammalian cells (X. Mao et al., 2006). AdipoRon (APR) is an orally active small molecule, which can emulate APN’s antidiabetic effects and anti-inflammatory properties through APN signalling activation (Okada-Iwabu et al., 2013).
Despite the therapeutic promise of APN, its clinical use may have significant disadvantages, including the high probability of adverse immunoreactions, the requirement of high dosage and constant intravenous (i.v.) injection to elicit beneficial effects and the challenges associated with producing APN protein on a large scale (Wu et al., 2019). In the present study, we designed 10 structural analogues of APR and focused on assessing their anti-inflammatory properties. We further identified and characterized the most potent APN receptor agonist and named it adipo anti-inflammation agonist (AdipoAI) for its ability to suppress the inflammatory response in vitro and in vivo including diet-induced obesity (DIO) and LPS-induced septic shock in mouse models. Moreover, we investigated the ability of AdipoAI to attenuate MyD88-dependent signal transduction and suppress NF-κB, MAPK and c-Maf pathways elicited by the potent pro-inflammatory stimulant LPS in macrophages.
2 |. METHODS
2.1 |. Reagents and antibodies
Mouse colony-stimulating factor-1 (MCSF-1) was purchased from PeproTech Inc (Rocky Hill, NJ, USA). APR was purchased from AdipoGen Life Sciences (San Diego, CA, USA). LPS (Escherichia coli 0111: B4) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Antibodies for β-actin (cat# ab8226, RRID: AB_306371), AdipoR1 (cat# ab126611, RRID: AB_11129655), AdipoR2 (cat# ab77613, RRID: AB_2222054), APPL2 (cat# ab154545, RRID: AB_2861345) and c-Maf (cat# ab77071, RRID: AB_1951643) were purchased from Abcam (Cambridge, MA, USA). Antibodies for APPL1 (cat# 3276, RRID: AB_2258386), MyD88 (cat# 4283, RRID: AB_10547882), phospho-NF-κB p65 (cat# 3034, RRID: AB_330561), NF-κB p65 (cat# 8242, RRID: AB_10859369), phosphor-IRAK4 (cat# 11927, RRID: AB_2797770), IRAK4 (cat# 4363, RRID: AB_2126429), phosphor-p38 MAPK (cat# 9211, RRID: AB_331614), p38 MAPK (cat# 8690, RRID: AB_10999090), phosphor-AKT (cat# 4060, RRID: AB_2315049), PI3K p85 α (cat# 13666, RRID: AB_2798288), β-catenin (cat# 8480, RRID: AB_11127855) and F4/80 (cat# 70076, RRID: AB_2799771) were purchased from Cell Signaling Technology (Danvers, MA, USA). Antibodies for Lamin B1 (cat# sc-377000, RRID: AB_2861346), TLR4 (cat# sc-293072, RRID: AB_10611320), phosphor-ERK (cat# sc-7383, RRID: AB_627545), ERK (cat# sc-514,302, RRID: AB_2571739), phosphor-JNK (cat# sc-12882, RRID: AB_654355), JNK (cat# sc-7345, RRID: AB_675864), AKT (cat# sc-81434, RRID: AB_1118808) and m-IgGκ BP-HRP antibody (cat# sc-516,102, RRID: AB_2687626) were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Normal Rabbit IgG (cat# NI01, RRID: AB_490574) was purchased from EMD Millipore (Billerica, MA, USA).
2.2 |. Cell culture of RAW 264.7 macrophages
RAW 264.7 macrophage cell line was purchased from ATCC (Manassas, VA, USA) and maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Life Technologies, Carlsbad, CA, USA) supplemented with 10% (v.v-1) fetal bovine serum (FBS) (Gibco, Life Technologies) and 1% (v.v-1) penicillin/streptomycin (Gibco, Life Technologies). The cells were routinely cultured in an incubator at 37°C in a humidified atmosphere of 5% CO2.
2.3 |. Isolation and culture of BMMs
Bone marrow-derived macrophages (BMMs) were obtained by isolating bone marrow (BM) from 4- to 8-week-old C57BL/6J mice (purchased from the Jackson Laboratory, Bar Harbor, ME, USA) and culturing in the presence of 10 mg·ml−1 of recombinant MCSF-1 (PeproTech Inc, Rocky Hill, NJ, USA) for 3 days. On Day 3, BMMs were harvested and plated in complete DMEM media containing MCSF-1. All the animal experiments were conducted under the guidelines issued by the Tufts University Institutional Animal Care and Use Committee.
2.4 |. Isolation and culture of murine PEMs
Murine peritoneal macrophages (PEMs) were isolated as described previously (Pun et al., 2015). Briefly, 6- to 7-week-old male C57BL/6J mice were treated by intraperitoneal (i.p.) injection with 1 ml of 4% (w.v-1) thioglycolate medium (Difco, Detroit, MI, USA) to induce the accumulation of macrophages in the peritoneum region. After 3 days of thioglycolate injection, PEMs were extracted with ice-cold HBSS (Sigma-Aldrich, St Louis, MO, USA) and centrifuged at 300×g for 5 min. The cell pellet was suspended in red blood cell lysis buffer (BioLegend, San Diego, CA, USA) to remove contaminated red blood cells. After centrifugation, the remaining cell pellet was mixed with RPMI 1640 medium (Gibco, Life Technologies, Carlsbad, CA, USA) containing 10% (v.v-1) FBS and 1% (v.v-1) penicillin/streptomycin. The cells were then seeded onto a culture dish (100 mm) at a density of 1 × 107 cells per dish and incubated in an incubator (37°C) under a humidified atmosphere of 5% CO2 for further experiments.
To examine the impact of AdipoAI on gene expression in vivo, 6- to 7-week-old male C57BL/6J mice were randomly divided into two groups (control and AdipoAI-treated groups with five mice per group). On Day 1, mice were injected i.p. with 1 ml of 4% thioglycolate solution. On Day 2, mice were injected i.p. with AdipoAI at a concentration of 25 mg·kg−1 of body weight. On Day 3 (after 24-h treatment with AdipoAI), PEMs were isolated and cultured for 2 h. Cells were then washed with cold phosphate-buffered saline (PBS) and used for different experiments.
2.5 |. Cytotoxicity assays
Raw264.7 cells were seeded at 2 × 103 cells/well in 96-well plates for 16 h and then serum starved for 6 hr. Cells were maintained in DMEM in the presence of different concentrations of APR (AdipoGen Life Sciences, San Diego, CA, USA) and compounds for 24 h. Cytotoxicity was determined with the Cell Counting Kit-8 (CCK-8 kit, Dojindo, Santa Clara, CA, USA) following the manufacturer’s recommendations. The detailed group assignment is shown in the individual figure legends.
2.6 |. RNA isolation and qRT-PCR
Total RNA of cells was extracted using Quick-RNA Miniprep Kit (ZYMO Research, Irvine, CA, USA), and total RNA from mouse tissues was prepared with TriZol reagent (Life Technologies) according to the manufacturer’s instructions followed by reverse transcription and real-time quantitative polymerase chain reaction (qRT-PCR) assays as we described previously (L. Zhang et al., 2014). One microgram of total RNA was used for reverse transcription using the M-MLV Reverse Transcriptase (Thermo Scientific, Waltham, MA, USA) according to the manufacturer’s protocol using PowerUp SYBR Green Master Mix (Thermo Scientific) on a Bio-Rad iQ5 thermal cycler (Bio-Rad Laboratories, Hercules, CA, USA). Differences in expression were evaluated by the comparative cycle threshold method using GAPDH or β-actin as a control. The primer sequences used for the qRT-PCR experiments are listed in Table S1.
2.7 |. mRNA microarray analysis
Raw264.7 cells were stimulated with AdipoAI for 24 h followed by incubation with 100 ng·ml−1 of LPS (E. coli 0111: B4, Sigma-Aldrich, St. Louis, MO, USA) for an additional 6 h. Detailed information of the four groups is shown in the figure legends. Total RNA of cells was extracted, and 5-μg total RNA from each cell sample was sent to Arraystar Inc. (Rockville, MD, USA) for microarray analysis. Image processing and data extraction and analysis also were performed by Arraystar Inc. using their established protocols.
2.8 |. Enzyme-linked immunosorbent assay
Cell culture media and mouse serum were collected and used for the measurement of cytokines secretion using enzyme-linked immunosorbent assay (ELISA) kits (Abcam), according to the manufacturer’s instructions.
2.9 |. Preparation of protein extracts and western blot analysis
Total cellular extracts were prepared using RIPA Lysis and Extraction Buffer containing three protease inhibitors (Thermo Scientific). Cytoplasmic and nuclear extracts were purified using NE-PER™ Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific). Experimental details of western blotting are in accordance with British Journal of Pharmacology guidelines (Alexander et al., 2018), and western blot analyses were performed as previously described (J. Zhang et al., 2017). Briefly, the total protein concentration was quantified using BCA kit (P1511; Applygen Technologies Inc., Beijing, China). The samples were separated by sodium dodecyl sulphate-polyacrylamide gel (SDS-PAGE) and electrophoretically transferred to a polyvinylidene difluoride membrane (Merck Millipore, Darmstadt, Germany). The membranes were blocked with 5% skim milk for 1–2 h at room temperature. Then, the blots were incubated with primary antibodies against AdipoR1 (1:1000), AdipoR2 (1:1000), APPL1 (1:1000), APPL2 (1:1000), MyD88 (1:1000), phospho-NF-κB p65 (1:1000), NF-κB p65 (1:1000), phosphor-IRAK4 (1:500), IRAK4 (1:1000), phosphor-p38 MAPK (1:1000), p38 MAPK (1:1000), phosphor-AKT (1:1000), AKT (1:500), phosphor-ERK (1:500), ERK (1:500), phosphor-JNK (1:500), JNK (1:500), PI3K p85 α (1:1000), β-catenin (1:1000), Lamin B1 (1:5000), TLR4 (1:500), c-Maf (1: 2000) and β-actin (1:20,000) overnight at 4°C. After washed with Tris-buffered saline with Tween (TBST) buffer three times for 5 min, the blots were incubated with HRP-conjugated anti-rabbit (1:10,000) at room temperature for 45 min. Finally, the protein bands were visualized using ECL chemiluminescence reagents from Thermo Scientific. The bands of western blotting shown are representative of three independent experiments with consistent results. All bands were quantification analysed using ImageJ (ImageJ, RRID: SCR_003070).
2.10 |. IP assays
The RAW 264.7 macrophages were seeded at a density of 6 × 106 cells per 100 mm dish. After overnight culture, the cells were stimulated with APR or AdipoAI for 24 h followed by incubation with LPS (100 ng·ml−1) for an additional 6 h. Cells were then lysed on ice with lysis buffer (25-mM Tris, 150-mM NaCl, 1-mM EDTA, 1% NP-40, 5% glycerol, 45-Mm octyl glucoside) supplemented with protease and phosphatase inhibitors cocktails. Immunoprecipitation (IP) was performed with Protein A/G Magnetic Beads (EMD Millipore) and 1 μg of specified antibodies (APPL1 or MyD88) at 4°C overnight. The immunoprecipitates were washed with lysis buffer and further analysed by polyacrylamide gel electrophoresis and Western blot analyses. The Clean-Blot IP Detection Kit (Thermo Scientific) was used as the secondary antibody. Normal Rabbit IgG (EMD Millipore) was used as a negative control in all IP experiments.
2.11 |. H&E and IHC staining
Mouse soft tissue samples were prepared for haematoxylin and eosin (H&E) staining as previously described (Lian et al., 2019). Immunohistochemistry (IHC) was performed using a Histostain-SP Kit (Life Technologies) following the manufacturer’s recommendations and British Journal of Pharmacology guidelines (Alexander et al., 2018). Primary antibodies for F4/80 (1:250) and c-Maf (1:250) were purchased from Cell Signaling Technology and Abcam, respectively. Digital images of stained tissues were taken with an Olympus BX53 microscope and quantification analysed by a blinded operator using ImageJ software (ImageJ, RRID: SCR_003070).
2.12 |. Transient transfection with siRNAs
Macrophages were seeded at a density of 5 × 105 cells/well in 12-well plates. After overnight incubation, the cells were transfected for 24 h with siRNAs targeting specific genes or scrambled control siRNAs using HiPerFect Transfection Reagent (Qiagen, Hilden, Germany) according to the manufacturer’s guidelines. Gene-silencing efficiency was monitored by qPCR or Western blot analysis. The siRNA duplexes used in this study were chemically synthesized by Thermo Scientific. The sequences of the siRNAs are listed in Table S1.
2.13 |. In vivo investigation in mouse models
The Ethics Committee on the Use and Care of Animals at Tufts University approved the study protocol (Boston, MA, USA). The animals received humane care according to the criteria outlined in the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health. All experiments involving animals or animal tissue are reported in compliance with the guidelines of ARRIVE and BJP (Kilkenny et al., 2010; McGrath & Lilley, 2015). Experimental protocols and design adhere to BJP guidelines (Curtis et al., 2015). Wild-type (WT, C57BL/6J, Jax#000664), DIO (Jax#380050) and APN KO (Jax#008195) mice were purchased from the Jackson Laboratory. The DIO mice were fed with a high-fat diet (containing 60% kcal from fat; Jackson Laboratory) at 6 weeks of age and later. The WT mice were fed with ordinary diet as normal chow (NC)-fed mice, and APN KO mice were maintained and fed with an NC diet as previously described (Tu et al., 2011). For the LPS-induced endotoxemia model, male C57BL/6 mice aged 8 weeks received oral gavage with APR (50 mg·kg−1 of body weight) or AdipoAI (25 mg·kg−1 body weight) for 24 h before by LPS (20 mg·kg−1 of body weight) treatment that was administered intraperitoneally, whereas control animals were treated with equivalent volumes of normal PBS. Mouse survival in each group was monitored and recorded up to 84 h. All mice were killed at 1 h for detection of activation of inflammatory genes, at 6 h for determination of inflammatory factors in serum and at 24 h for histological evaluation. The detailed groups and the number of mice included are shown in the figure legends. Animal data were excluded from experiments based on pre-established criteria of visible abnormal tissue structure during sample harvest or other health issues, including fighting wounds.
2.14 |. Data and statistical analysis
All studies were designed to generate groups of equal size, using randomization and blinded analysis. Group size is the number of independent values, and that statistical analysis was done using these independent values. Sample size of each protocol was determined on the basis of similar previous studies (Gu et al., 2017; Wang et al., 2017). The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2018). Data are expressed as the mean ± SEM, and GraphPad Prism software (GraphPad Software Inc., La Jolla, CA, USA) was used for statistical analyses. Statistical analysis was undertaken only for studies where each group size was at least n = 5, and not subjected to statistical analysis where each group sizes was n < 5. Statistical significance was calculated using two-tailed Student’s t-tests for comparisons between two groups and one-way ANOVA (Analysis Of Variance) with post-hoc Tukey HSD (Honestly Significant Difference) test using correction for comparisons among more than two groups. Post-hoc tests were run only if F achieved P < 0.05 and there was no significant variance inhomogeneity. P < 0.05 was considered statistically significant. To control for unwanted sources of variation, normalization of the data was carried out. The mean values of the control group were normalized to 1. In the figures, the Y-axis shows the ratio of the experimental group to that of the corresponding matched control values. When outliers are included or excluded in analysis, it is declared within the figure legend. For animal survival analysis, the Kaplan-Meier method was employed to generate graphs, and the survival curves were analyzed using log-rank analysis. Band intensity in western blot images was quantified with Image J Software.
2.15 |. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander et al., 2019).
3 |. RESULTS
3.1 |. Design of AdipoR agonists and evaluation of the anti-inflammatory properties
To develop orally active small molecule compounds with potent anti-inflammatory effects, we designed and synthesized 10 agonists of APR (Phidelis Chemical [Shanghai] Co., Ltd. China) and compared them with APR. Firstly, we investigated their ability to inhibit the mRNA expression of pro-inflammatory cytokines IL-6 and IL1-β in LPS-stimulated RAW 264.7 macrophages and found Compounds 3 and 5 (at 5 μM) to exhibit stronger inhibitory effects than APR (at 20 μM) (Figure 1a), particularly Compound 3. Therefore, we renamed it as AdipoAI.
FIGURE 1.
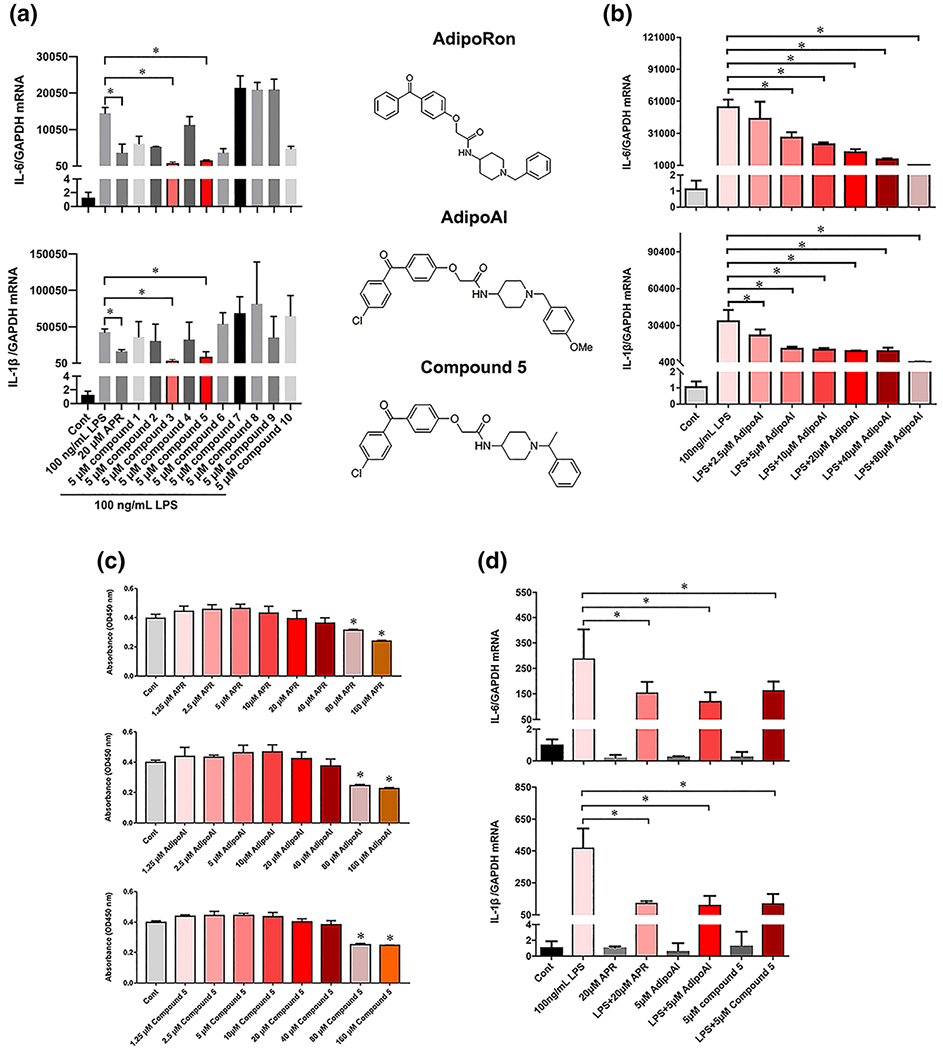
Characterization of anti-inflammatory properties of APR-like compounds. (a) Left: Raw264.7 cells were pretreated with APR (20 μM) or new compounds 1 to 10 (5 μM) for 24 h followed by incubation with LPS (100 ng ml−1) for an additional 6 h. IL-6 and IL-1β mRNA expression levels were evaluated by qPCR and normalized with GAPDH mRNA level. Right: Chemical structures of APR, AdipoAI (compound 3) and Compound 5. (b) Raw264.7 cells were pretreated with different doses of AdipoAI for 24 h followed by incubation with LPS (100 ng ml−1) for additional 6 h to evaluate dose dependency of AdipoAI in inhibiting IL6 and IL-1β mRNA expression levels by qRT-PCR and normalized with GAPDH mRNA level. (c) Evaluation of cytotoxicity of APR, AQ1321 AdipoAI and Compound 5 by CCK8 assay in Raw264.7 cells treated for 24 h. (d) BMMs were pretreated with APR (20 μM), AdipoAI (5 μM) or Compound 5 (5 μM) for 24 h followed by incubation with LPS (100 ng ml−1) for additional 6 h for the measurement of IL-6 and IL-1β mRNA expression levels by qRT-PCR and normalized with GAPDH mRNA level. Data were expressed as the mean ± SEM at five biological independent experiments. One-way ANOVA test for multiple group comparisons, *P < 0.05, significant differences between each indicated group
Treatment of RAW264.7 macrophages with increasing concentrations of AdipoAI decreased LPS-stimulated IL-6 and IL-1β mRNA expression in a dose-dependent fashion (Figure 1b). To determine whether AdipoAI, Compound 5, or APR could be mediating their anti-inflammatory effects by decreasing Raw264.7 cell number, we conducted CCK-8 cytotoxicity assay. Results revealed that neither of the three small molecules mediated significant cytotoxic effects at 40 μM or lower concentrations (Figure 1c).
Because AdipoAI exhibited the strongest inhibitory effects on LPS-stimulated IL-6 and IL-1β expression in BMMs (Figure 1d) comparing with Compound 5 and APR, we chose AdipoAI to perform the remaining experiments in this study.
3.2 |. AdipoAI inhibits LPS-stimulated inflammatory responses in macrophages
To further evaluate the anti-inflammatory impact of AdipoAI in LPS-induced macrophages, we measured the secreted production of IL-6 and IL-1β by ELISA. Results revealed that treatment with AdipoAI for 24 h followed by incubation with LPS for 6 h decreased IL-6 and IL-1β protein levels in Raw264.7 and BMMs (Figure 2a,b). We also evaluated the production of IL-6 and IL-1β by PEMs isolated from AdipoAI-treated mice (20 mg·kg−1 of body weight) and found that AdipoAI reduced IL-6 and IL-1β (Figure 2c).
FIGURE 2.

AdipoAI suppresses LPS-stimulated inflammatory responses in macrophages. (a) Raw264.7 cells or (b) BMMs were pretreated with APR (20 μM) or AdipoAI (5 μM) for 24 h followed by incubation with LPS (100 ng ml−1) for additional 6 h for the measurement of IL-6 and IL-1β in supernatants by ELISA. Data were expressed as the mean ± SEM at five biological independent experiments. One-way ANOVA test for multiple group comparisons, * vs. LPS group, *P < 0.05, significant differences between each indicated group. (c) PEMs were isolated from C57BL/6J mice (n = 5 mice/group) were injected i.p. with 1-ml 4% thioglycolate solution or AdipoAI (25 mg kg−1) for the measurement of IL-6 and IL-1β. The amounts of IL-6 and IL-1β in supernatant were measured by ELISA. Data were expressed as the mean ± SEM (n = 5). Two-tailed Student’s t test for two groups, *P < 0.05, significant differences between each indicated group. (d–f) Impact of AdipoAI on (d) basal and LPS-induced expression of an array of (e and f) proinflammatory cytokines, chemokines and receptors in Raw264.7 cells (four groups and three biological replicates/group). Data analyses were performed according to the manufacturer’s instructions with their web-based software package (www.qiagen.com/us/shop/genes-and-pathways/data-analysis-centeroverview-page/). (g) Differentiated expressed genes annotated to a gene-ontology term and example genes by gene-ontology-enrichment analysis of each component. (brown: LPS vs. control; black: Adipo AI + LPS vs. LPS). (h) Differentially expressed M1 and M2 genes are shown according to the microarray databases. No statistical analysis for n = 3
We then analysed mRNA microarray profiles and patterns in LPS-stimulated Raw264.7 cells and found that LPS stimulated the expression of an array of pro-inflammatory cytokines, chemokines and receptors (Figure 2d), whereas AdipoAI decreased the expression of the same genes to near basal levels (Figure 2e–g).
According to the bibliography, M1 macrophages are involved in host defence and pathogen removal, M2 macrophages participate in tissue repair (Xuan et al., 2016) and APN can contribute to M2 macrophage induction (Lovren et al., 2010; Mandal, Pratt, Barnes, McMullen, & Nagy, 2011; Ohashi, Shibata, Murohara, & Ouchi, 2014). However, we found that AdipoAI treatment in LPS-induced Raw264.7 cells did not alter the expression of M1 or M2 macrophage-related genes (Figure 2h).
3.3 |. AdipoAI decreases systemic inflammation in DIO mice
Low-grade systemic inflammation is a significant characteristic of obesity (Ouchi & Walsh, 2007). To investigate the impact of AdipoAI in systemic inflammation, we chose the DIO mouse model, which has been used by other investigators to study and research prediabetes and diabetes-related metabolic syndrome (Wu et al., 2019), as well as chronic systemic inflammatory response (Ohashi et al., 2014). In the present work, DIO and WT mice were treated with APR or AdipoAI via oral gavage for 14 days, and then the expression of pro-inflammatory and anti-inflammatory cytokine expression was evaluated. We found that AdipoAI inhibited mRNA expression of IL-6 (Il6) and IL-1β (Il1β) but not that of TNF-α or IL-10 in the white adipose tissue (WAT), spleen, or BM of DIO mice (Figure 3a–c). Furthermore, AdipoAI reduced IL-6 and IL-1β levels in the serum of AdipoAI-treated DIO mice (Figure 3d). In agreement with AdipoAI anti-inflammatory potential, we detected a decreased quantity of megakaryocytes and macrophages in the spleen of AdipoAI-treated DIO mice by H&E and IHC (F4/80) staining (Figures 3e,f and S6).
FIGURE 3.

AdipoAI decreases inflammation in DIO mice (n = 6). DIO mice were orally gavaged with APR (50 mg kg−1) or AdipoAI (25 mg kg−1) for 14 days, and control animals (WT or DIO mice) were administered with equivalent volumes of DMSO. mRNA expression levels of proinflammatory cytokines in (a) WAT, (b) spleen and (c) bone marrow were measured by qRT-PCR and normalized to the level of β-actin mRNA. Data are expressed as mean ± SEM. (d) Serum levels of IL-6 and IL-1β were measured by ELISA. Data are expressed as mean ± SEM. Representative images of (e) H&E and (f) IHC of F4/80 staining of spleen tissue in DIO mice (n = 6). Red arrow indicates multinucleated giant cells (megakaryocytes). One-way ANOVA test for multiple group comparisons, * vs. WT vehicle group, # vs. DIO-vehicle group. */#P < 0.05, significant differences between each indicated group. ns, not significant
3.4 |. AdipoAI protects against LPS-induced endotoxemia and organ dysfunction
Systemic inflammatory states, namely, sepsis, septic shock and endotoxemia, influence most body organs, including the lung, spleen, liver and WAT (Salomao et al., 2012). In this study, we investigated the effects of AdipoAI in WT mice with LPS-induced endotoxemia by treating mice with AdipoAI via oral gavage for 24 h before LPS challenge. Treatment with AdipoAI decreased mRNA levels of pro-inflammatory genes in various tissues, with lung exhibiting the sharpest drop, followed by the spleen, WAT and liver (Figure 4a). Whereas only 12.5% of the vehicle-treated mice survived 84 h post-LPS challenge, the survival rate of AdipoAI-treated group was 40% (Figure 4b). AdipoAI treatment also reduced serum levels of pro-inflammatory cytokines IL-6 and IL-1β at 6 h post-LPS administration (Figure 4c).
FIGURE 4.

AdipoAI protects against LPS-induced endotoxemia. Endotoxemia was induced in WT mice by intraperitoneal injection with LPS (25 mg kg−1), and control littermates were administered with equivalent volumes of PBS. APR or AdipoAI were orally gavaged into mice for 24 h before LPS challenge and control animals were administered with equivalent volumes of DMSO. (a) mRNA expression levels of pro-inflammatory cytokines in lung, spleen, liver and WAT were measured by qRT-PCR and normalized with GAPDH mRNA levels. Data are shown as mean ± SEM (n = 6). (b) Survival curve of mice with endotoxemia (n = 12). (c) Serum levels of IL-6 and IL-1β were measured by ELISA. Data are expressed as mean ± SEM (n = 6). (d) Histopathology for lung, spleen, liver and WAT tissues isolated from APR- or AdipoAI-treated mice 24 h before LPS challenge (n = 6). Upper panel: hematoxylin and eosin staining; lower panel: immunohistochemical staining for macrophage marker F4/80. Red arrow indicates F4/80 positive cells. One-way ANOVA test for multiplegroup comparisons, * vs. DMSO + PBS group, # vs. DMSO + LPS group. */#P < 0.05, significant differences between each indicated group. ns, not significant
Mice challenged with LPS have been reported to exhibit varying degrees of acute inflammation in the lung, spleen, liver and WAT tissues (Du et al., 2017). In our study, LPS-treated mice showed exacerbated lung inflammation, haemorrhage and alveolar septal thickening, whereas AdipoAI-treated mice had fewer lung lesions and decreased inflammatory cell infiltration (Figure 4d). Furthermore, the LPS-induced spleen, liver and WAT injury and activation of inflammatory responses were also alleviated in AdipoAI-treated mice (Figures 4d and S7).
3.5 |. AdipoAI inhibits NF-κB and p38 MAPK signalling pathways in LPS-induced macrophages
We then investigated the potential inhibitory effects of AdipoAI on LPS-induced NF-κB and MAPK signalling pathways in macrophages. To that end, we evaluated the phosphorylation of MAPK signalling related protein factors including IRAK4, AKT, JNK, ERK and p38 MAPK in response to LPS in the presence and absence of AdipoAI. Whereas treatment of AdipoAI in LPS-induced Raw264.7 cells decreased phosphorylation of IRAK4 and p38 by 70% (Figure 5a), it did not significantly alter phosphorylation of AKT, JNK or ERK. To assess the effects of AdipoAI on NF-κB signalling, we evaluated AdipoAI’s ability to regulate LPS-induced phosphorylation and nuclear translocation of p65 in Raw264.7 cells and found that AdipoAI suppressed LPS-induced p65 phosphorylation and nuclear translocation (Figure 5b). Quantitative analysis of bands is shown in Figure S8a,b.
FIGURE 5.

AdipoAI inhibition of NF-κB and p38 MAPK signaling pathways is dependent of AdipoR1/APPL1 axis in LPS-induced macrophages. (a) Impact of AdipoAI in the phosphorylation of related signaling proteins by western blotting. Raw264.7 cells were pretreated with APR (20 μM) or AdipoAI (5 μM) for 24 h followed by incubation with LPS (100 ng ml−1) for an additional 30 min. Representative images are shown, and quantitative analysis of protein expression was performed by densitometric analysis (see lower panel). (b) Expression of p65 protein in cytoplasmic and nuclear extracts of Raw264.7 cells. Representative images of p65 expression are presented along with Lamin B1 and β-actin expression, which were used as internal loading controls. Quantitative analysis of protein expression was performed by densitometric analysis (lower panel). (c) RAW 264.7 cells were transfected with siRNA targeting either AdipoR1, AdipoR2, APPL1, APPL2 or scrambled control siRNA for 24 h, followed by stimulation with AdipoAI or APR for 24 h, then incubation with LPS for additional 6 h. Transfection efficiency of siRNA and mRNA expression levels of IL-6 and IL-1β were measured by qPCR and normalized with GAPDH mRNA levels. Data were expressed as the mean ± SEM at five biological independent experiments. Oneway ANOVA test for multiple group comparisons, *P < 0.05, significant differences between each indicated group. ns, not significant. (d) RAW 264.7 cells were stimulated with LPS, AdipoAI or APR for different times and proteins levels determined by western blotting. Representative western blotting images are shown along with β-actin used as an internal loading control and densitometric analysis. (e) Raw264.7 cells were pretreated with APR (20 μM) or or AdipoAI (5 μM) for 24 h followed by incubation with LPS (100 ng ml−1) for additional 6 h for the measurement of related proteins by WB. Representative images are shown along with β-actin as an internal loading control and densitometric analysis. (f) Raw264.7 cells and (g) BMMs were transfected with siRNA targeting APPL1 or scrambled control siRNA for 24 h, followed by stimulation with AdipoAI for 24 h, then incubation with LPS for additional 6 h for western blotting analysis. Representative images are shown along with β-actin as an internal loading control, and densitometric analysis. No statistical analysis of western blotting for n = 3
3.6 |. AdipoR1 and APPL1 are involved in the anti-inflammatory effects of AdipoAI in macrophages
To evaluate whether the anti-inflammatory effects of AdipoAI in macrophages were dependent of AdipoRs and APPLs, we used Raw264.7 cells stably transfected with AdipoRs and APPLs siRNA or siRNA scrambled control and evaluated mRNA levels of IL-6 and IL-1β in response to AdipoAI. Results showed that AdipoAI-mediated inhibition of mRNA expression of pro-inflammatory cytokines was prevented by AdipoR1 and APPL1 suppression, but not AdipoR2 or APPL2 (Figure 5c). Moreover, detection of AdipoRs and APPLs protein expression in AdipoAI and LPS treated Raw264.7 cells revealed that AdipoAI increased APPL1 expression, whereas LPS decreased APPL1 (Figures 5d,e and S8c,d). Nevertheless, AdipoAI or LPS did not influence AdipoR1 or AdipoR2 protein levels in Raw264.7 cells in our results. To further characterize the requirement of APPL1 for the anti-inflammatory effects of AdipoAI in LPS-induced macrophages, we assessed the phosphorylation of IRAK4 and p38 MAPK in Raw264.7 cells and BMMs transfected with APPL1 siRNA or scrambled control. Preliminary results demonstrated that AdipoAI and LPS inhibited phosphorylation of IRAK4 and p38 MAPK (Figures 5f,g and S8e,f).
3.7 |. The anti-inflammatory effects of AdipoAI require APPL1/MyD88 interaction in macrophages
The activated TLR4 signalling by LPS stimulated pro-inflammatory signalling pathways that involve adapter proteins MyD88 and c-Maf. We then investigated the involvement of MyD88 and c-Maf in LPS-induced macrophage response. LPS-induced macrophages transfected with c-Maf siRNA or MyD88 siRNA were used to assess the mRNA levels of IL-6, IL-1β, IL-10 and IL-4 (Figure 6a). We found preliminarily that suppressed expression of either c-Maf or MyD88 by specific siRNAs decreased LPS-induced cytokine expression (Figure 6a) and phosphorylation of IRAK4 and p38 MAPK (Figures 6b and S8g). To explore whether the interaction between APPL1 and MyD88 was involved in the AdipoAI suppressed inflammation in LPS-induced macrophages, we conducted co-IP studies with protein extracts from control cells and cells treated with LPS, AdipoAI or both. APPL1 interacted with AdipoR1 in all groups investigated, and the interaction of APPL1 with MyD88 was increased in AdipoAI and LPS-treated cells as compared with control cells (Figure 6c). When anti-MyD88 was used as the primary antibody in co-IP studies, similar interactions of APPL1 with AdipoR1 and MyD88 were detected in AdipoAI and LPS-treated cells (Figure 6c). These preliminary data suggested that AdipoR1, APPL1 and MyD88 formed a protein complex that could potentially be critical for the anti-inflammatory effects of AdipoAI in LPS-induced macrophages.
FIGURE 6.

(a) RAW 264.7 cells were transfected with siRNA targeting MyD88, c-Maf or scrambled control siRNA for 24 h, followed by stimulation with LPS for 6 h. (b) Raw264.7 cells were transfected with siRNA targeting MyD88 or scrambled control siRNA for 24 h, followed by stimulation with LPS for 30 min for measurement of related proteins. Representative images are shown along with β-actin as an internal loading control and densitometric analysis. No statistical analysis of western blotting for n = 3. (c) Raw264.7 cells were pretreated with AdipoAI (5 μM) for 24 h followed by incubation with LPS (100 ng ml−1) for additional 6 h to analyze interactions of AdipoR1, APPL1 and MyD88 by Co-IP experiments. Proteins immunoprecipitated with the anti-APPL1 antibody were subjected to immunoblotting with antibodies to detect APPL1, AdipoR1 and MyD88. Proteins immunoprecipitated with the anti-MyD88 antibody were analyzed by immunoblotting with antibodies for MyD88, APPL1, and AdipoR1. IB, immunoblotting; IP, immunoprecipitation; Input, whole protein lysis as positive control; IgG: rabbit IgG as negative control. P < 0.05, significant differences between each indicated group. ns, not significant
3.8 |. AdipoAI decreased c-Maf activation directly in LPS-induced macrophages
Transcription factor c-Maf is the cellular homologue of the avian viral oncogene v-maf, which belongs to the AP-1 superfamily of basic region/leucine zipper factors. It can regulate disease-specific gene networks such as IL-4, IL-7 and IL-10 so that immune responses are elicited in immune cells and macrophages (Cao, Liu, Song, & Ma, 2005; Kim, Hoicfau-Grusby, & Glimcher, 1999; Xie et al., 2016).
Our mRNA microarray results revealed that c-Maf mRNA levels were induced by LPS treatment and inhibited by AdipoAI in Raw264.7 cells (Figure 7a). Using qPCR assays, we confirmed the expression pattern of c-Maf as regulated by LPS treatment and/or AdipoAI in Raw264.7 cells, BMMs and PEMs (Figure 7b). Additional qPCR and IHC staining experiments with lung, spleen, liver and WAT tissues revealed that c-Maf mRNA and protein levels were induced in LPS-challenged mice but decreased in AdipoAI-treated mice (Figures 7c,d and S9).
FIGURE 7.

(a) The cluster heat map shows mRNAs with expression change of more than twofold from microarray data (three biological replicates/group, P < 0.05). (b) Raw264.7 cells were transfected with siRNA targeting MyD88 or scrambled control siRNA for 24 h, followed by stimulation with LPS for 30 min for measurement of related proteins. Representative images are shown along with β-actin as an internal loading control and densitometric analysis. No statistical analysis of western blotting for n = 3. (c) Raw264.7 cells were pretreated with AdipoAI (5 μM) for 24 h followed by incubation with LPS (100 ng ml) for additional 6 h to analyze interactions of AdipoR1, APPL1 and MyD88 by Co-IP experiments. Proteins immunoprecipitated with the anti-APPL1 antibody were subjected to immunoblotting with antibodies to detect APPL1, AdipoR1 and MyD88. Proteins immunoprecipitated with the anti-MyD88 antibody were analyzed by immunoblotting with antibodies for MyD88, APPL1 and AdipoR1. IB, immunoblotting; IP, immunoprecipitation; Input, whole protein lysis as positive control; IgG: rabbit IgG as negative control. P < 0.05, significant differences between each indicated group. ns, not significant
4 |. DISCUSSION AND CONCLUSIONS
APN has been reported to exhibit potent anti-inflammatory properties in vitro and in vivo (Nicolas et al., 2018). Thus, APN treatment can inhibit LPS-induced expression of pro-inflammatory cytokines in macrophages from various sources (Wulster-Radcliffe, Ajuwon, Wang, Christian, & Spurlock, 2004; Yamaguchi et al., 2005; Yokota et al., 2000) and suppress BM inflammation, thereby enhancing the antibacterial activity of haematopoietic cells (Masamoto et al., 2016). Despite the therapeutic potential of APN as an anti-inflammatory and antidiabetic agent, there are potential challenges of using APN in the clinic for the treatment of inflammation and various metabolic diseases, including the high possibility of adverse immunoreactions, the need for constant i.v. injection of high doses of APN to mediate therapeutic effects, and the difficulty and cost of producing APN protein in large quantities (Wu et al., 2019). To overcome some of these issues, APR was developed, which is an orally active synthetic small-molecule AdipoR agonist with antidiabetic properties and the ability to decrease inflammation in WAT of DIO mice (Okada-Iwabu et al., 2013). In the present study, we designed 10 APR-like small-molecule compounds (Figure 1a and Fiure S1) and presented experimental evidence that AdipoAI (Compound 3) exhibited the most potent anti-inflammatory ability (Figures 1 and 2) and increased the mRNA levels of AdipoRs (Figure S2) in LPS-induced Raw264.7 cells, LPS-induced BMMs and murine PEMs. Notably, the anti-inflammatory efficacy of AdipoAI was estimated to be about 8 times higher than that of APR in macrophages.
In our study, we found that AdipoAI and APR not only inhibited expression of pro-inflammatory cytokines such as IL-6 and IL-1β but also downregulated expression of the anti-inflammatory cytokine IL-10 in LPS-induced macrophages. As previously reported, IL-10 expression was stimulated in macrophages by full-length APN to mediate the anti-inflammatory response (Kumada et al., 2004; Wolf, Wolf, Rumpold, Enrich, & Tilg, 2004). In a different report, APN inhibited pro-inflammatory signalling in human macrophages in an IL-10-independent fashion (Folco, Rocha, Lopez-Ilasaca, & Libby, 2009). In this study, treatment of microphages with AdipoAI or APR for 18 h or less increased IL-10 mRNA expression, but longer treatments resulted in decreased IL-10 expression (Figure S3). As a pro-inflammatory factor similar to IL-6 and IL-1β, the mRNA level of TNF-α in macrophages treated with AdipoAI or APR was also examined by qRT-PCR and mRNA array analysis in our study. However, the result showed that AdipoAI pretreatment did not inhibit TNF-α expression levels in LPS-induced Raw264.7 macrophage, BMMs or PEMs (data not shown). We also found that AdipoAI did not inhibit the expression of TNF-α in WAT, spleen or BM of DIO mice (Figure 3a–c) or LPS-induced endotoxemia mice (Figure 4a). In addition to the different types of macrophages and the treatment time and ways, the molecules were used by various laboratories, the complex synthetic and production pathways and metabolic mechanisms of TNF-α are all potential reasons for the different actions in different studies. Regarding the different actions between APN/APR and AdipoAI on TNF-a production, there are many underlying mechanisms yet to be further explored in our future research.
Macrophage activation presents two extremes of dynamic changing states that can be represented by classical M1 macrophages induced by LPS and alternative M2 macrophages induced by IL-4. M1-M2 polarization of macrophages is a tightly controlled process that entails a series of signalling pathways, transcriptional and post-transcriptional regulatory networks (Murray et al., 2014; Wang, Liang, & Zen, 2014). Several recent studies analysing the effect of APN on the expression of a limited number of macrophage polarization markers concluded that APN promoted the M2 phenotype in mouse PEMs, RAW264.7 cells and rat Kupffer cells (Lovren et al., 2010; Mandal et al., 2011; Ohashi et al., 2014). We completed and presented a comprehensive analysis of the relationship between AdipoAI-induced transcriptome and Raw264.7 macrophage polarization, revealing that AdipoAI could not significantly change the expression of M1 phenotype or M2 phenotype markers (Figure 2h). In agreement with our results, other investigators have reported that APN did not promote classical nor alternative activation of human macrophages (Cheng, Folco, Shimizu, & Libby, 2012). The contradictory results among different studies are probably derived from interspecies variations in the cellular mechanisms that promote macrophage polarization.
Obesity is frequently accompanied by chronic low-level inflammation over the whole body and may increase the morbidity of infections (Ouchi & Walsh, 2007). It has also been shown to cause a deficiency of APN in the BM, inflamed BM and increased proinflammation cytokines production from BM macrophages (Masamoto et al., 2016). We then conducted in vivo studies with the DIO mouse model, which we previously used for metabolic studies with APN and APR (Okada-Iwabu et al., 2013; Wu et al., 2019). Treatment with AdipoAI and APR inhibited the expression levels of IL-6 and IL-1β in WAT, spleen and BM as well as the IL-6 and IL-1β serum levels in DIO mice (Figure 3).
The function of APN in LPS-induced endotoxemia in vivo is not well known. We found APN−/− mice treated with LPS exhibited more severe tissue damage and lower survival rate than LPS-induced WT mice (Figure S4). In addition, AdipoAI played a protective role against LPS-induced endotoxemia in mice (Figure 4). Others have shown that inflammatory reaction in atherosclerosis and ethanol-induced liver injury were also influenced by APN (Folco et al., 2009; Thakur et al., 2006). These results support the notion that compounds activating APN signalling could be used in the future in the prevention of acute infections such as sepsis and septic shock.
APN mediates anti-inflammatory properties in macrophages via diverse signalling mechanisms. On one hand, treatment of APN alone was reported to drive sustained levels of phosphorylation of IκB, JNK, p38 and STAT3 but prevented further activation of these signalling molecules in the presence of LPS (Folco et al., 2009). Those findings provided a mechanistic basis for previous observations suggesting that APN could induce inflammatory activation to some extent that is likely to mediate tolerance to further treatment with pro-inflammatory stimuli (Sattar et al., 2006; Yamaguchi et al., 2005). However, we found that phosphorylation levels of JNK, Erk and Akt were not significantly different in AdipoAI-stimulated or AdipoAI and LPS-stimulated macrophages (Figure 5a). Furthermore, AdipoAI inhibited the phosphorylation of upstream kinase IRAK4 and subsequently suppressed the NF-κB and p38 MAPK signalling pathways in LPS-induced macrophages. The transcription factors NF-κB and p38 are known to contribute to LPS-stimulated inflammatory response, and IL-6 and IL-1β are their target genes in macrophages.
Activation of APN receptor AdipoR1 and AdipoR2 by APN leads to activation of AMPK and/or PPAR-α40 in vivo (Okada-Iwabu et al., 2013) as well as binding of adapter proteins APPL1 and APPL2 to APN receptors (Mao et al., 2006). In this study, our results suggested that AdipoR1 and APPL1 but not AdipoR2 and APPL2 (Figure 5c,f,g) participated in the anti-inflammatory effects of AdipoAI in Raw264.7 cells. Furthermore, APPL1 protein expression was increased by AdipoAI and APR but decreased by LPS (Figure 5d). Other researchers have reported that APPLs KO mice are more prone to endotoxic shock when challenged by LPS (Mao et al., 2014) and that TLR3/4 ligands trigger APPL1 degradation in LPS-induced murine BMMs (Chau et al., 2015). Taken together our results (Figure 5e) and prior reports (Chau et al., 2015; Mao et al., 2014), it is tempting to speculate that a crosstalk between APN and LPS signalling pathways may exist that involves APPL1 to regulate inflammatory responses in macrophages. In agreement with the involvement of APPL1 in the anti-inflammatory effects of AdipoAI in LPS-induced inflammatory response in macrophages, we found that APPL1 suppression by APPL1 siRNA affected phosphorylation of IRAK4 and p38 MAPK in response to AdipoAI and LPS (Figure 5f,g).
Adapter protein MyD88 plays a pivotal role in LPS stimulating pro-inflammatory signalling pathways (Hirotani et al., 2005; Skaug, Jiang, & Chen, 2009), and we demonstrated that MyD88 is involved in the expression of pro-inflammatory cytokines and the activation of downstream signalling pathways in LPS-stimulated macrophages (Figure 6a,b). Furthermore, we found AdipoR1, APPL1 and MyD88 formed a protein complex that is expected to contribute to the anti-inflammatory effects of AdipoAI in LPS-induced Raw264.7 cells (Figure 6c). APPL1 is recognized as a multifunctional endosomal signalling adaptor protein that is capable of binding to many interacting proteins, such as AdipoR1, APPL2, Rab5, Rab21, DCC, Akt, TBK1 and IKK, and it can activate different signalling pathways (Chau et al., 2015; Diggins & Webb, 2017). In a separate report, the p85 subunit of PI3K, but not MyD88, was shown to be associated with APPL2 and APPL1 in LPS-induced murine BMMs (Mao et al., 2014). We were not able to reproduce those results in our experimental setting (data not shown). These differences are difficult to reconcile but may be related to the different macrophage types used by various laboratories.
c-Maf was previously shown to regulate disease-specific gene networks to mediate immune responses in immune cells and macrophages (Daassi et al., 2016; van den Bosch, Palsson-Mcdermott, Johnson, & O’Neill, 2014; Xu et al., 2018). In our study, we found AdipoAI inhibited c-Maf mRNA expression in vitro and in vivo (Figures 7 and S5) and gathered experimental evidence that supported a critical role of c-Maf in the activation of the inflammatory response in LPS-induced Raw264.7 cells through c-Maf siRNA experiments (Figure 6a). Previously, investigators showed that LPS activated c-Maf transcription to promote IL-10 production in early-stage BMMs (van den Bosch, Palsson-Mcdermott, Johnson, & O’Neill, 2014). It is therefore likely that AdipoAI inhibited IL-10 production in our study partly because of the inhibited transcriptional activity of c-Maf in LPS-induced macrophages. And we also found that MyD88 appeared to regulate the mRNA expression of c-Maf in Raw264.7 cells to some degree (Figure 6a). Because c-Maf expression was inhibited in macrophages treated by AdipoAI alone, we will further investigate the possibility of a MyD88-independent mechanism in our future research.
In summary, this study characterized the strong ability of AdipoAI to attenuate inflammation in DIO and LPS-induced endotoxemia mice with low cytotoxicity. Regarding the underlying molecular mechanisms, our results suggested that AdipoR1/APPL1 and MyD88 formed a stable complex, which subsequently inhibited activation of NF-κB, MAPK and c-Maf pathways and limited production of pro-inflammatory cytokines in LPS-induced macrophages (Figure 8). Additionally, AdipoAI also directly inhibited the activation of c-Maf. Taken together, the results presented support the therapeutic potential of AdipoAI to target LPS-induced inflammatory disorders and other inflammatory diseases, particularly in diabetic patients.
FIGURE 8.

Diagram of AdipoAI-elicited molecular mechanisms to inhibit inflammatory responses in LPS-induced macrophages. AdipoAI activates AdipoR1/APPL1 pathways, and MyD88 is recruited to form a protein complex with APPL1, inhibiting activation of IRAK4, NF-κB, MAPK and c-Maf pathways and suppressing production of pro-inflammatory cytokines including IL-6 and IL-1β in LPS-induced macrophages. AdipoAI also inhibit transcriptional activation of c-Maf directly, then suppressing production of IL-6 and IL-1β in macrophages
Supplementary Material
What is already known.
Adiponectin possesses potent anti-inflammatory properties and acts as an anti-inflammatory marker.
Lipopolysaccharide (LPS) triggers MyD88-dependent signalling and NF-κB/MAPK pathways to activate inflammation.
What this study adds
The new adiponectin receptor agonist AdipoAI exerts strong anti-inflammatory actions in vitro and in vivo.
AdipoR1, APPL1 and MyD88 form a protein complex potentially critical for the anti-inflammatory impact of AdipoAI in LPS-induced macrophages.
What is the clinical significance
AdipoAI may be a useful alternative therapeutic approach to adiponectin administration to suppress inflammatory disorders.
The interaction between APPL1 and MyD88 may hold promise as a therapeutic target in inflammatory disorders.
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health (NIH) grants (R01DE21464, R01DE25681 and R01DE26507 to J.C.; R01DE25020 to TVD; R01DK102965 to L.G.D.); Tufts University Awards (Collaborates Award D740207 and Interdisciplinary Grants to Q.T.); National Natural Science Foundation of China (grant numbers 31200985 and 81400502 to Z.X.D).
Funding information
National Natural Science Foundation of China, Grant/Award Numbers: 81400502, 31200985; Tufts University Awards, Grant/Award Number: Collaborates Award D740207 and Interdisciplinary G; National Institutes of Health (NIH), Grant/Award Numbers: R01DE26507, R01DE25681, R01DE21464
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Natural Product Research, Design & Analysis, Immunoblotting and Immunochemistry, and Animal Experimentation, and as recommended by funding agencies, publishers, and other organizations engaged with supporting research.
Abbreviations:
- AdipoAI
adipo anti-inflammation agonist
- APN
adiponectin
- APR
AdipoRon
- BMMs
bone marrow-derived macrophages
- DIO
diet-induced obesity
- i.v.
intravenous
- LPS
lipopolysaccharide
- PEMs
peritoneal macrophages
- WAT
white adipose tissue
Footnotes
CONFLICT OF INTEREST
The authors declare no potential conflicts of interest concerning the authorship or publication of this article. All authors have followed the recommendations set out in the BJP editorials.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Aderem A, & Ulevitch RJ, (2000). Toll-like receptors in the induction of the innate immune response. Nature, 406(6797), 782–787. 10.1038/35021228 [DOI] [PubMed] [Google Scholar]
- Alexander SP, Christopoulos A, Davenport AP, Kelly E, Mathie A, Peters JA, … CGTP Collaborators. (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: G protein-coupled receptors. British Journal of Pharmacology, 176, S21–S141. 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Roberts RE, Broughton BRS, Sobey CG, George CH, Stanford SC, … Ahluwalia A, (2018). Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology. British Journal of Pharmacology, 175, 407–411. 10.1111/bph.14112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton GM, (2008). A calculated response: control of inflammation by the innate immune system. The Journal of Clinical Investigation, 118(2), 413–420. 10.1172/JCI34431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bosch MW, Palsson-Mcdermott E, Johnson DS, & O’Neill LA, (2014). LPS induces the degradation of programmed cell death protein 4 (PDCD4) to release Twist2, activating c-Maf transcription to promote interleukin-10 production. The Journal of Biological Chemistry, 289(33), 22980–22990. 10.1074/jbc.M114.573089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochu-Gaudreau K, Rehfeldt C, Blouin R, Bordignon V, Murphy BD, & Palin MF, (2010). Adiponectin action from head to toe. Endocrine, 37(1), 11–32. 10.1007/s12020-009-9278-8 [DOI] [PubMed] [Google Scholar]
- Bruce CR, Mertz VA, Heigenhauser GJ, & Dyck DJ, (2005). The stimulatory effect of globular adiponectin on insulin-stimulated glucose uptake and fatty acid oxidation is impaired in skeletal muscle from obese subjects. Diabetes, 54(11), 3154–3160. 10.2337/diabetes.54.11.3154 [DOI] [PubMed] [Google Scholar]
- Cao S, Liu J, Song L, & Ma X, (2005). The protooncogene c-Maf is an essential transcription factor for IL-10 gene expression in macrophages. Journal of Immunology, 174(6), 3484–3492. 10.4049/jimmunol.174.6.3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau TL, Goktuna SI, Rammal A, Casanova T, Duong HQ, Gatot JS, & Chariot A, (2015). A role for APPL1 in TLR3/4-dependent TBK1 and IKKε activation in macrophages. Journal of Immunology, 194(8), 3970–3983. 10.4049/jimmunol.1401614 [DOI] [PubMed] [Google Scholar]
- Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, & Zhao L, (2018). Inflammatory responses and inflammation-associated diseases in organs. Oncotarget, 9(6), 7204–7218. 10.18632/oncotarget.23208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Folco EJ, Shimizu K, & Libby P, (2012). Adiponectin induces pro-inflammatory programs in human macrophages and CD4+ T cells. The Journal of Biological Chemistry, 287(44), 36896–36904. 10.1074/jbc.M112.409516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Alexander S, Cirino G, Docherty JR, George CH, Giembycz MA, … Ahluwalia A, (2018). Experimental design and analysis and their reporting II: updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175, 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA, … McGrath J, (2015). Experimental design and analysis and their reporting: New guidance for publication in BJP. British Journal of Pharmacology, 172, 3461–3471. 10.1111/bph.12856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daassi D, Hamada M, Jeon H, Imamura Y, Nhu Tran MT, & Takahashi S, (2016). Differential expression patterns of MafB and c-Maf in macrophages in vivo and in vitro. Biochemical and Biophysical Research Communications, 473(1), 118–124. 10.1016/j.bbrc.2016.03.063 [DOI] [PubMed] [Google Scholar]
- Deepa SS, & Dong LQ, (2009). APPL1: Role in adiponectin signaling and beyond. American Journal of Physiology. Endocrinology and Metabolism, 296(1), E22–E36. 10.1152/ajpendo.90731.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggins NL, & Webb DJ, (2017). APPL1 is a multifunctional endosomal signaling adaptor protein. Biochemical Society Transactions, 45(3), 771–779. 10.1042/BST20160191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du M, Yuan L, Tan X, Huang D, Wang X, Zheng Z, & Huang K, (2017). The LPS-inducible lncRNA Mirt2 is a negative regulator of inflammation. Nature Communications, 8(1), 2049–2066. 10.1038/s41467-017-02229-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folco EJ, Rocha VZ, Lopez-Ilasaca M, & Libby P, (2009). Adiponectin inhibits pro-inflammatory signaling in human macrophages independent of interleukin-10. The Journal of Biological Chemistry, 284(38), 25569–25575. 10.1074/jbc.M109.019786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster SL, & Medzhitov R, (2009). Gene-specific control of the TLR-induced inflammatory response. Clinical Immunology, 130(1), 7–15. 10.1016/j.clim.2008.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu M, Zhang Y, Liu C, Wang D, Feng L, Fan S, … Huang C, (2017). Morin, a novel liver X receptor α/β dual antagonist, has potent therapeutic efficacy for nonalcoholic fatty liver diseases. British Journal of Pharmacology, 174, 3032–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S, … NC-IUPHAR. (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirotani T, Yamamoto M, Kumagai Y, Uematsu S, Kawase I, Takeuchi O, & Akira S, (2005). Regulation of lipopolysaccharide-inducible genes by MyD88 and Toll/IL-1 domain containing adaptor inducing IFN-beta. Biochemical and Biophysical Research Communications, 328(2), 383–392. 10.1016/j.bbrc.2004.12.184 [DOI] [PubMed] [Google Scholar]
- Kawai T, & Akira S, (2010). The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nature Immunology, 11 (5), 373–384. 10.1038/ni.1863 [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG, & Group NCRRGW. (2010). Animal research: Reporting in vivo experiments: the ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. 10.1111/j.1476-5381.2010.00872.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JI, Hoicfau-Grusby MJ, & Glimcher LH, (1999). The transcription factor c-Maf controls the production of interleukin-4 but not other Th2 cytokines. Immunity, 10(6), 745–751. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Kim EH, Pun NT, Chang JH, Kim JA, Jeong JH, & Park PH, (2017). Globular adiponectin inhibits lipopolysaccharide-primed inflammasomes activation in macrophages via autophagy induction: The critical role of AMPK signaling. International Journal of Molecular Sciences, 18(6), 1275–1295. 10.3390/ijms18061275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto K, Matsumoto K, & Ninomiya-Tsuji J, (2000). TAK1 mitogen-activated protein kinase kinase kinase is activated by autophosphorylation within its activation loop. The Journal of Biological Chemistry, 275(10), 7359–7364. 10.1074/jbc.275.10.7359 [DOI] [PubMed] [Google Scholar]
- Kobashi C, Urakaze M, Kishida M, Kibayashi E, Kobayashi H, Kihara S, & Kobayashi M, (2005). Adiponectin inhibits endothelial synthesis of interleukin-8. Circulation Research, 97(12), 1245–1252. 10.1161/01.RES.0000194328.57164.36 [DOI] [PubMed] [Google Scholar]
- Kumada M, Kihara S, Ouchi N, Kobayashi H, Okamoto Y, Ohashi K, & Matsuzawa Y, (2004). Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation, 109(17), 2046–2049. 10.1161/01.CIR.0000127953.98131.ED [DOI] [PubMed] [Google Scholar]
- Lamothe B, Besse A, Campos AD, Webster WK, Wu H, & Darnay BG, (2007). Site-specific Lys-63-linked tumor necrosis factor receptor-associated factor 6 auto-ubiquitination is a critical determinant of I kappa B kinase activation. The Journal of Biological Chemistry, 282(6), 4102–4112. 10.1074/jbc.M609503200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian J, Wu X, Liu Y, Qiu W, Zhu X, Wang X, … Chen J, (2019). Potential roles of miR-335-5p on pathogenesis of experimental periodontitis. Journal of Periodontal Research, 55(2), 191–198. 10.1111/jre.12701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovren F, Pan Y, Quan A, Szmitko PE, Singh KK, Shukla PC, & Verma S, (2010). Adiponectin primes human monocytes into alternative anti-inflammatory M2 macrophages. American Journal of Physiology. Heart and Circulatory Physiology, 299(3), H656–H663. 10.1152/ajpheart.00115.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal P, Park PH, McMullen MR, Pratt BT, & Nagy LE, (2010). The anti-inflammatory effects of adiponectin are mediated via a heme oxygenase-1-dependent pathway in rat Kupffer cells. Hepatology, 51 (4), 1420–1429. 10.1002/hep.23427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal P, Pratt BT, Barnes M, McMullen MR, & Nagy LE, (2011). Molecular mechanism for adiponectin-dependent M2 macrophage polarization: Link between the metabolic and innate immune activity of full-length adiponectin. The Journal of Biological Chemistry, 286(15), 13460–13469. 10.1074/jbc.M110.204644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Lin W, Nie T, Hui X, Gao X, Li K, & Wu D, (2014). Absence of Appl2 sensitizes endotoxin shock through activation of PI3K/Akt pathway. Cell & Bioscience, 4(1), 60–69. 10.1186/2045-3701-4-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X, Kikani CK, Riojas RA, Langlais P, Wang L, Ramos FJ, & Dong LQ, (2006). APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nature Cell Biology, 8(5), 516–523. 10.1038/ncb1404 [DOI] [PubMed] [Google Scholar]
- Masamoto Y, Arai S, Sato T, Yoshimi A, Kubota N, Takamoto I, & Kurokawa M, (2016). Adiponectin enhances antibacterial activity of hematopoietic cells by suppressing bone marrow inflammation. Immunity, 44(6), 1422–1433. 10.1016/j.immuni.2016.05.010 [DOI] [PubMed] [Google Scholar]
- McGrath JC, & Lilley E, (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): New requirements for publication in BJP. British Journal of Pharmacology, 172, 3189–3193. 10.1111/bph.12955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R, & Horng T, (2009). Transcriptional control of the inflammatory response. Nature Reviews. Immunology, 9(10), 692–703. 10.1038/nri2634 [DOI] [PubMed] [Google Scholar]
- Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, & Wynn TA, (2014). Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity, 41(1), 14–20. 10.1016/j.immuni.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PJ, & Smale ST, (2012). Restraint of inflammatory signaling by interdependent strata of negative regulatory pathways. Nature Immunology, 13(10), 916–924. 10.1038/ni.2391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas S, Chabry J, Guyon A, Zarif H, Heurteaux C, & Petit-Paitel A, (2018). Adiponectin: an endogenous molecule with anti-inflammatory and antidepressant properties? Medical Science (Paris), 34(5), 417–423. 10.1051/medsci/20183405014 [DOI] [PubMed] [Google Scholar]
- Ohashi K, Shibata R, Murohara T, & Ouchi N, (2014). Role of anti-inflammatory adipokines in obesity-related diseases. Trends in Endocrinology and Metabolism, 25(7), 348–355. 10.1016/j.tem.2014.03.009 [DOI] [PubMed] [Google Scholar]
- Okada-Iwabu M, Yamauchi T, Iwabu M, Honma T, Hamagami K, Matsuda K, & Kadowaki T, (2013). A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature, 503(7477), 493–499. 10.1038/nature12656 [DOI] [PubMed] [Google Scholar]
- Ouchi N, & Walsh K, (2007). Adiponectin as an anti-inflammatory factor. Clinica Chimica Acta, 380(1-2), 24–30. 10.1016/j.cca.2007.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao W, Song C, Chen H, Diaz MA, Wahl LM, Fitzgerald KA, & Medvedev AE, (2009). Endotoxin tolerance dysregulates MyD88-and Toll/IL-1R domain-containing adapter inducing IFN-beta-dependent pathways and increases expression of negative regulators of TLR signaling. Journal of Leukocyte Biology, 86(4), 863–875. 10.1189/jlb.0309189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pun NT, Subedi A, Kim MJ, & Park PH, (2015). Globular adiponectin causes tolerance to LPS-induced TNF-alpha expression via autophagy induction in RAW 264.7 macrophages: Involvement of SIRT1/FoxO3A axis. PLoS ONE, 10(5), e0124636. 10.1371/journal.pone.0124636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomao R, Brunialti MK, Rapozo MM, Baggio-Zappia GL, Galanos C, & Freudenberg M, (2012). Bacterial sensing, cell signaling, and modulation of the immune response during sepsis. Shock, 38(3), 227–242. 10.1097/SHK.0b013e318262c4b0 [DOI] [PubMed] [Google Scholar]
- Sattar N, Wannamethee G, Sarwar N, Tchernova J, Cherry L, Wallace AM, & Whincup PH, (2006). Adiponectin and coronary heart disease: A prospective study and meta-analysis. Circulation, 114 (7), 623–629. 10.1161/CIRCULATIONAHA.106.618918 [DOI] [PubMed] [Google Scholar]
- Skaug B, Jiang X, & Chen ZJ, (2009). The role of ubiquitin in NF-kappaB regulatory pathways. Annual Review of Biochemistry, 78, 769–796. 10.1146/annurev.biochem.78.070907.102750 [DOI] [PubMed] [Google Scholar]
- Thakur V, Pritchard MT, McMullen MR, & Nagy LE, (2006). Adiponectin normalizes LPS-stimulated TNF-alpha production by rat Kupffer cells after chronic ethanol feeding. American Journal of Physiology. Gastrointestinal and Liver Physiology, 290(5), G998–G1007. 10.1152/ajpgi.00553.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilija Pun N, & Park PH, (2018). Adiponectin inhibits inflammatory cytokines production by Beclin-1 phosphorylation and B-cell lymphoma 2 mRNA destabilization: Role for autophagy induction. British Journal of Pharmacology, 175(7), 1066–1084. 10.1111/bph.14144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Q, Zhang J, Dong LQ, Saunders E, Luo E, Tang J, & Chen J, (2011). Adiponectin inhibits osteoclastogenesis and bone resorption via APPL1-mediated suppression of Akt1. The Journal of Biological Chemistry, 286(14), 12542–12553. 10.1074/jbc.M110.152405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Liu W, Yao L, Zhang X, Zhang X, Ye C, … Ai D, (2017). Hydroxyeicosapentaenoic acids and epoxyeicosatetraenoic acids attenuate early occurrence of nonalcoholic fatty liver disease. British Journal of Pharmacology, 174, 2358–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Liang H, & Zen K, (2014). Molecular mechanisms that influence the macrophage M1-M2 polarization balance. Frontiers in Immunology, 5, 614–622. 10.3389/fimmu.2014.00614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wess J, (1997). G-protein-coupled receptors: Molecular mechanisms involved in receptor activation and selectivity of G-protein recognition. The FASEB Journal, 11(5), 346–354. [PubMed] [Google Scholar]
- Wolf AM, Wolf D, Rumpold H, Enrich B, & Tilg H, (2004). Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochemical and Biophysical Research Communications, 323(2), 630–635. 10.1016/j.bbrc.2004.08.145 [DOI] [PubMed] [Google Scholar]
- Wu X, Qiu W, Hu Z, Lian J, Liu Y, Zhu X, & Chen J, (2019). An adiponectin receptor agonist reduces type 2 diabetic periodontitis. Journal of Dental Research, 98(3), 313–321. 10.1177/0022034518818449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA, & Spurlock ME, (2004). Adiponectin differentially regulates cytokines in porcine macrophages. Biochemical and Biophysical Research Communications, 316(3), 924–929. 10.1016/j.bbrc.2004.02.130 [DOI] [PubMed] [Google Scholar]
- Xie Q, McGreal R, Harris R, Gao CY, Liu W, Reneker LW, & Cvekl A, (2016). Regulation of c-Maf and αA-crystallin in ocular lens by fibroblast growth factor signaling. The Journal of Biological Chemistry, 291(8), 3947–3958. 10.1074/jbc.M115.705103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Pokrovskii M, Ding Y, Yi R, Au C, Harrison OJ, & Littman DR, (2018). c-MAF-dependent regulatory T cells mediate immunological tolerance to a gut pathobiont. Nature, 554(7692), 373–377. 10.1038/nature25500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan D, Han Q, Tu Q, Zhang L, Yu L, Murry D, & Chen J, (2016). Epigenetic modulation in periodontitis: Interaction of adiponectin and JMJD3-IRF4 axis in macrophages. Journal of Cellular Physiology, 231(5), 1090–1096. 10.1002/jcp.25201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi N, Argueta JG, Masuhiro Y, Kagishita M, Nonaka K, Saito T, & Yamashita Y, (2005). Adiponectin inhibits Toll-like receptor family-induced signaling. FEBS Letters, 579(30), 6821–6826. 10.1016/j.febslet.2005.11.019 [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, & Kadowaki T, (2003). Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature, 423(6941), 762–769. 10.1038/nature01705 [DOI] [PubMed] [Google Scholar]
- Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, & Matsuzawa Y, (2000). Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood, 96(5), 1723–1732. [PubMed] [Google Scholar]
- Zhang J, Valverde P, Zhu X, Murray D, Wu Y, Yu L, & Chen J, (2017). Exercise-induced irisin in bone and systemic irisin administration reveal new regulatory mechanisms of bone metabolism. Bone Res, 5, 16056. 10.1038/boneres.2016.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Meng S, Tu Q, Yu L, Tang Y, Dard MM, & Chen J, (2014). Adiponectin ameliorates experimental periodontitis in diet-induced obesity mice. PLoS ONE, 9(5), e97824. 10.1371/journal.pone.0097824 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


