Abstract
Pelvic inflammatory disease (PID) is a clinical syndrome that has been associated with a wide range of potential causal pathogens. Three broad groups of organisms have been isolated from the genital tract of people with PID: sexually transmitted organisms such as Neisseria gonorrhoeae, Chlamydia trachomatis, Mycoplasma genitalium, and Trichomonas vaginalis; bacterial vaginosis (BV)-associated species and genera such as Atopobium vaginae, Sneathia, and Megasphaera; and genera and species usually associated with the gastrointestinal or respiratory tracts such as Bacteroides, Escherichia coli, Streptococcus, or Haemophilus influenza. Although PID is often considered to be synonymous with gonorrhea or chlamydia, these pathogens are found in only one quarter to one third of people with PID, suggesting that broader screening and diagnostic and treatment strategies need to be considered to reduce the burden of PID and its associated sequelae.
Keywords: pelvic inflammatory disease, sexually transmitted infections, gonorrhea, chlamydia, bacterial vaginosis
Pelvic inflammatory disease (PID) is a clinical syndrome associated with adverse reproductive health sequelae such as infertility, ectopic pregnancy, and chronic pelvic pain. Pelvic inflammatory disease is a clinical diagnosis, based on symptoms of pelvic or lower abdominal pain and signs of tenderness of either the cervix, adnexa, or uterus on exam [1]. The diagnosis of PID is syndromic, made in sexually active people with a uterus and cervix who have no other identifiable etiology. Pelvic inflammatory disease is a syndrome and, as such, heterogenous in its presentation, severity, and etiology. The goal of broadly inclusive diagnostic criteria is to ensure identification of all potential cases, so that they can be provided antibiotic treatment to reduce the sequelae of PID. Based on self-report of PID diagnosis, the Centers for Disease Control and Prevention estimates a lifetime prevalence of 4.4% or 2.5 million people in the United States [2]. Although diagnostic criteria have become increasingly more sensitive and less specific, over the past 2 decades the overall incidence of clinically diagnosed acute PID has decreased [3], as outlined further in this Supplement [4].
Contemporary reports of the prevalence of PID sequelae range from 3% to 7% for infertility and ectopic pregnancies in a military population [3] to 36% for chronic pelvic pain in the PID Evaluation and Clinical Health (PEACH) trial [5]. Current diagnostic and treatment guidelines focus on clinical presentation and symptom resolution, which is useful for clinicians but potentially obscures subgroups of people who are at higher risk for sequelae and/or might benefit from alternative treatments. The clinical diagnostic criteria do not grade the severity of disease, do not require evaluation of adnexa versus endometrium, nor do they require identification of the causal agent(s), all of which contribute to the heterogeneity of the syndrome. It is unclear whether evaluating the presence of upper tract disease or the etiology of infection is relevant for choosing treatment or predicting outcomes; there are few clear predictors of who has a greater likelihood of developing long-term complications from PID [6].
In the PEACH trial, chronic pelvic pain occurred in approximately 32% of participants, infertility in approximately 18%, and ectopic pregnancy in <1%, which is higher than reported rates of 6–20/1000 women in epidemiologic studies [7]. Sequelae were more common in participants who had recurrent episodes of PID, or repeat sexually transmitted infections (STIs), and in those who still had persistent symptoms 5 or 30 days after treatment [5, 6, 8]. Detection of several bacterial vaginosis (BV)-associated bacterial species or chlamydia in the cervix or endometrium by polymerase chain reaction (PCR) was also associated with an increased risk for infertility, although gonorrhea was not [9]. Trichomoniasis was associated with an increased risk for sequelae that did not reach statistical significance [10]. However, as mentioned earlier, many infertile people who have intra-abdominal adhesions and tubal occlusion do not report any history of PID, suggesting that subclinical disease may be a significant source of adverse outcomes [11].
In studies among people presenting with clinical PID, endometritis (≥5 neutrophils/400× field ± ≥1 plasma cell/120× field) is confirmed by endometrial biopsy in as few as 54% and as many as 70% [12–15]; and salpingitis evaluated by diagnostic laparoscopy is confirmed in 20%–89% [12, 15–20]. If endometritis is used as the gold standard, one study estimates the sensitivity of clinical criteria in symptomatic people at only 36% [21]. However, tubal scarring and other evidence of upper genital tract (UGT) infection and inflammation is also present in people who do not report a clinical history of PID, suggesting that symptomatic cases are only one part of the total burden of disease. Subclinical PID is defined as the presence of endometritis in the absence of clinical signs and symptoms of PID [22]. People with subclinical PID have similar demographic characteristics as those with acute PID, the diagnosis is also associated with detection of Chlamydia trachomatis, Neisseria gonorrhoeae, or BV [23], and is linked to adverse outcomes such as infertility [24]. These data suggest that even the broad, nonspecific clinical criteria for the diagnosis of PID do not capture all people at risk for sequelae.
Thus, there is a gap between people identified by the clinical diagnostic algorithm and the entire population at risk for adverse outcomes from PID. Examining the etiology of PID may allow better screening, testing, and evaluation algorithms to bridge that gap between clinically diagnosed, symptomatic cases and the full spectrum of disease. There are 3 general groups of pathogens associated with PID, which are not mutually exclusive: (1) sexually transmitted organisms (Neisseria gonorrhoeae, Chlamydia trachomatis, Mycoplasma genitalium, Trichomonas vaginalis), (2) BV-associated bacteria (eg, BVAB3, Prevotella bivia, Atopobium vaginae, Leptotrichia/Sneathia spp), and (3) gastrointestinal (GI) or respiratory bacteria (eg, anaerobes, facultative and aerobic bacteria such as Haemophilus influenzae, Escherichia coli, Bacteroides). The proportion of participants with each of these types of pathogens detected differs depending on the era in which studies were performed, how PID was defined in each study, and the sensitivity and type of testing performed (Table 1). However, detection of an organism does not necessarily mean that it is the causal agent of PID. In addition, co-occurrence of organisms from multiple groups may create a synergy that worsens the clinical course. For example, aerobic bacteria can act to create tissue necrosis and anaerobic conditions leading to the growth of anaerobes and development of a tubo-ovarian abscess [25]. A more nuanced understanding of how different pathogens and communities of pathogens contribute to PID, the severity of disease, and the risk for sequelae will help guide treatment recommendations, population-level prevention strategies, and future studies to improve care for people with PID.
Table 1.
Detection of Microbes in Cervix or Upper Genital Tract in Women With PID According to Various Definitions
| PID Definition | Organism | Proportion | References (listed in Supplemental Material) | |
|---|---|---|---|---|
| Cervix | UGT | |||
| Clinical | ||||
| Neisseria gonorrhoeae | 2%–80% | 9%–25% | [1–14] | |
| Chlamydia trachomatis (Cx) | 10%–38% | 10%–28% | [1, 3, 5, 7, 9, 12, 13] | |
| C trachomatis (NAAT) | 16%–36% | 10%–20% | [2, 4, 6, 8, 14] | |
| Mycoplasma genitalium | 13%–15% | - | [8, 15] | |
| Anaerobesa | - | 19%–64% | [2, 3, 7, 9, 11, 13, 16] | |
| Aerobes/facultativeb | - | 13%–94% | [2, 3, 7, 9, 11, 13, 16] | |
| BV-associated (Cx)c | - | 30%–60% | [2, 3] | |
| Clinical + Endometritis | ||||
| N gonorrhoeae (Cx) | 32%–44% | 13%–34% | [2, 3, 7, 17] | |
| N gonorrhoeae (NAAT) | 15% | 10% | [18] | |
| C trachomatis (Cx) | 23%–52% | 18%–39% | [3, 7, 17] | |
| C trachomatis (NAAT) | 6% | 7%–26% | [2, 18] | |
| M genitalium | 12% | 8%–12% | [18, 19] | |
| Anaerobesa | - | 32%–50% | [2, 3, 7] | |
| Aerobes/facultativeb | - | 22%–50% | [2, 3, 7] | |
| BV-associated (Cx)c | - | 30%–64% | [2, 3] | |
| BV-associated (NAAT) d | - | 74% | [20] | |
| Clinical + Salpingitis | ||||
| N gonorrhoeae (Cx) | 13%–74% | 3%–59% | [7, 9, 17, 21–28] | |
| N gonorrhoeae (NAAT) | 15% | 4%–13% | [29, 30] | |
| C trachomatis (non-NAAT) | 5%–72% | 11%–50% | [7, 9, 17, 21–24, 26–28, 31] | |
| C trachomatis (NAAT) | 6%–45% | 6%–41% | [25, 29, 30] | |
| M genitalium (NAAT) | 6%–9% | 4%–5% | [25, 29] | |
| Anaerobesa | 29% | 2%–57% | [7, 9, 21, 22, 24, 26, 27] | |
| Aerobes/facultativeb | 10% | 5%–50% | [7, 9, 21, 22, 24, 26, 27] | |
| BV-associated (NAAT)c | - | 60% | [30] | |
Abbreviations: BV, bacterial vaginosis; Cx, cultivation; NAAT, nucleic acid amplification test; PID, pelvic inflammatory disease; UGT, upper genital tract (ie, endometrium, tubal exudate, or peritoneal fluid).
aIncludes anaerobic Gram-negative rods (Porphyromonas, Prevotella) and anaerobic Gram-positive cocci (peptostreptococcus).
bIncludes Escherichia coli, Streptococcus, Staphylococcus, Enterococcus, and diphtheroids.
cIncludes Gardnerella vaginalis and Atopobium vaginae.
dIncludes G vaginalis, A vaginae, and Sneathia spp.
NEISSERIA GONORRHOEAE AND CHLAMYDIA TRACHOMATIS
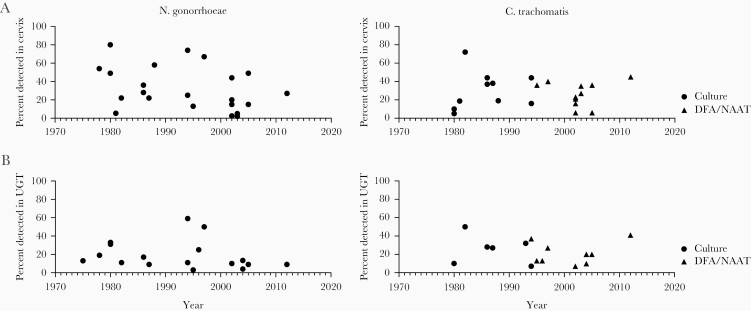
In early studies from the 1950s describing PID, the only defined causes were tuberculosis (3%) and N gonorrhoeae (1%) [26]. By 1980, 33% of cases were ascribed to N gonorrhoeae [27]. However, many of these studies were conducted before the advent of molecular testing, and they may not have identified C trachomatis simply because of poor sensitivity of the immunofluorescent, culture, or antigen-based assays used (Figure 1). In studies in which PID is diagnosed based on clinical presentation, N gonorrhoeae and/or C trachomatis are identified in the cervix or UGT in approximately one quarter to one third of participants (Table 1). When the population is restricted to people with clinical PID who also have acute histologically confirmed endometritis, a slightly higher proportion are found to have N gonorrhoeae and/or C trachomatis. However, more than half of participants with clinical PID and endometritis do not have either pathogen detected even using highly sensitive nucleic acid amplification tests (NAATs). The strictest definition of PID requires confirmation of visible salpingitis, tubal dilation, or purulent exudate at laparoscopy [1]. Overall, a slightly higher proportion of people who meet this strict definition have 1 of these 2 sexually transmitted pathogens (Table 1), but this is still fewer than half in most studies. Thus, although many consider PID as being universally associated with these 2 STIs, this does not reflect the published data.
Figure 1.
Proportion of people with pelvic inflammatory disease with Neisseria gonorrhoeae or Chlamydia trachomatis detected in the lower (vagina/cervix) vs upper (endometrium, tubes, cul-de-sac) genital tract in studies published between 1970 and 2020. References for studies included here are in Table 1 and Supplemental References. DFA, direct fluorescence antibody; NAAT, nucleic acid amplification test; UGT, upper genital tract.
MYCOPLASMA GENITALIUM
Although M genitalium is an accepted cause of male urethritis [28], its relationship with female reproductive tract disease syndromes is less clear. Mycoplasma genitalium can ascend to the fallopian tube [29], and experimental infection of fallopian tube tissue results in abnormal morphology and loss of cilia, which suggests biological plausibility. Animal studies demonstrate varying rates of ascent and inflammatory damage [30–32]. In humans, one meta-analysis of 10 studies (3 of which were prospective) found a statistically significant 2-fold increase in the risk of PID associated with M genitalium (odds ratio [OR]pooled = 2.1; 95% confidence interval [CI], 1.31–3.49) [33]. This relationship was stronger in the subset of studies that adjusted for other organisms known to cause PID (ORpooled = 2.5; 95% CI, 1.03–6.26) and in the subset of studies that used NAATs to detect M genitalium (ORpooled = 2.7; 95% CI, 1.60–4.66). In contrast, a second meta-analysis restricted to 2 studies with a prospective design reported a nonsignificant pooled risk ratio of 1.7 (95% CI, .92–3.28) for M genitalium and PID [34].
The prevalence of M genitalium and C trachomatis in people with PID is similar (6%–33% for M genitalium; 7%–39% for C trachomatis). In a direct comparison of chlamydial PID to M genitalium-associated PID, participants reported similar rates of abdominal pain, dyspareunia, intermenstrual bleeding, and cervical/adnexal tenderness, but those with M genitalium were significantly less likely to report postcoital bleeding and more likely to have lower abdominal tenderness noted on exam than people with chlamydial PID [35]. Taken together, this suggests that M genitalium may result in a less severe syndrome than does C trachomatis. However, both M genitalium and C trachomatis have a milder clinical presentation than N gonorrhoeae [36]. Recent data from a randomized trial of PID treatment comparing a regimen with versus without metronidazole reported that M genitalium was less frequently detected in the cervix and endometrium 1 month after treatment among those who received metronidazole, even though this antimicrobial agent has no activity against M genitalium [37]. This surprising finding suggests that there may be some synergy between M genitalium and vaginal dysbiosis. If so, the acquisition of M genitalium and subsequent invasion of and persistence in the upper tract may rely at least in part on the presence of BV-associated microbiota. Further investigation into interactions between M genitalium and vaginal microbiota will be needed before the independent contribution of M genitalium to PID can be determined.
TRICHOMONAS VAGINALIS
Trichomonas vaginalis can cause lesions, vaginitis, and acute inflammatory disease of the genital mucosa, but it is not a widely accepted cause of PID. Although there are only rare reports of isolation of T vaginalis in UGT specimens [38], endometrial inflammatory changes elicited by C trachomatis, N gonorrhoeae, and T vaginalis infections appear indistinguishable, suggesting an underappreciated contribution by T vaginalis in UGT inflammatory processes [39]. In the PEACH trial, participants with vaginal detection of T vaginalis had a higher odds (OR = 1.9; 95% CI, 1.0–3.3) of having endometritis, even after adjusting for the presence of N gonorrhoeae, C trachomatis, M genitalium, and BV [10]. Among African people with human immunodeficiency virus, detection of vaginal T vaginalis was association with an approximately 2-fold increase in risk for concurrent PID [40]. This association has not consistently been seen for subclinical PID [22, 41]. There are no prospective data evaluating PID or endometritis incidence in people with trichomoniasis, and this research gap must be addressed to determine the role of T vaginalis in PID.
BACTERIAL VAGINOSIS-ASSOCIATED BACTERIAL SPECIES
Bacterial vaginosis, characterized as a shift from a Lactobacillus-predominant vaginal microbiota to one with high concentrations of a diverse collection of facultative and anaerobic species [14], has been associated with PID. Bacterial vaginosis-associated organisms such as anaerobic Gram-negative rods have been isolated by culture methods from the UGT in people with endometritis and salpingitis, suggesting the potential for their involvement in the pathogenesis of PID (Table 1). People with acute endometritis are less likely to have endometrial detection of hydrogen peroxide producing Lactobacillus spp and more likely to have black-pigmented Gram-negative rods and anaerobic Gram-positive cocci, independent of detection of C trachomatis and N gonorrhoeae [13]. Among 545 participants with clinically suspected PID, those with the BV-associated genera and species Sneathia, A vaginae, and BVAB1 detected in the cervix or endometrium by PCR were significantly more likely to have histologically confirmed endometritis [9]. Kenyan people with salpingitis were more likely to have BV-associated species detected by PCR in tubal samples compared with control people without salpingitis [42].
Bacterial vaginosis diagnosed using Nugent’s criteria [43] is associated with clinical and subclinical PID [13, 22, 44, 45]. Diagnosis of BV by Amsel criteria or Nugent score was associated with an increased risk for incident PID in a longitudinal study of 2958 participants [46]. In another longitudinal study, a cluster of cultured BV-associated organisms was also associated with a 2-fold increased risk of incident PID [47]. A nested case-control study of 17 patients who developed PID versus 17 controls who did not develop PID demonstrated that cases were significantly more likely to have the BV-associated organisms A vaginae, Sneathia, BVAB-TM7, Megasphaera, Eggerthella-like bacterium, and Mobiluncus detected in vaginal samples by quantitative PCR (qPCR), with similar trends for Gardnerella vaginalis, BVAB1, BVAB2, Mageeibacillus indolicus, Prevotella timonensis, and Prevotella amnii [48]. Cases also had higher mean 16S ribosomal ribonucleic acid (rRNA) gene copies/mL compared with controls for A vaginae, Megasphaera, Eggerthella-like bacterium, and P timonensis. These data suggest that a broad range of BV-associated bacteria may increase a person’s risk of PID.
Because the majority of people with clinically diagnosed PID have neither N gonorrhoeae nor C trachomatis, some investigators have evaluated whether the presence of BV or BV-associated organisms may indicate higher risk of endometritis. In a secondary analysis of a randomized trial of outpatient PID treatment, selected BV-associated bacteria were evaluated by qPCR in vaginal samples from 169 participants. Several BV-associated species (including 3 species of Prevotella, A vaginae, G vaginalis, and Megasphaera phylotype 1) were cross-sectionally associated with histologically confirmed endometritis, whereas Lactobacillus species (Lactobacillus crispatus, Lactobacillus jensenii) were less frequent and at lower abundance among those with endometritis [49]. A combination of microbes including C trachomatis and certain BV-associated pathogens may better predict histologic endometritis than detection of individual organisms alone.
OROPHARYNGEAL, RESPIRATORY, AND GASTROINTESTINAL SPECIES
In many cases, PID is polymicrobial, with organisms from the oropharyngeal (OP), GI, and respiratory tracts identified in the endometrium, tubes, and peritoneum (Table 1). In Kenyan patients with laparoscopically confirmed acute salpingitis, tubal specimens contained 16s rRNA deoxyribonucleic acid from 3 to 10 unique phylotypes, including organisms normally found in the OP and GI tracts, as well as those associated with BV [42]. In multiple studies of participants with laparoscopically confirmed salpingitis, anaerobic organisms (Bacteroides spp, Fusobacterium spp) or facultative and aerobic organisms (E coli, Streptococcus spp, Staphylococcus spp, H influenzae) from the GI or OP tracts have been detected using cultivation methods from the tubes or peritoneum (Table 1).
It is challenging to determine whether these organisms play a causal role in the initiation of PID, or whether the alteration in the UGT environment due to infection with one of the previously discussed pathogens allows for their opportunistic growth. In cross-sectional studies comparing people with clinical PID with and without acute endometritis, diphtheroids, anaerobic Gram-negative rods, and anaerobic Gram-positive cocci were more often found in endometrial cultures of participants with confirmed endometritis versus those without [13, 14]. These organisms may, at least, be a marker of more significant upper tract disease.
HOW UNDERSTANDING ETIOLOGY INFORMS CARE OF PEOPLE WITH PELVIC INFLAMMATORY DISEASE AND PREVENTION OF DISEASE
There are many gaps in our understanding of the pathophysiology of PID and its devastating sequelae. Do differences in the etiology of PID translate into different risk of adverse outcomes? Would a more precise identification of etiologic microbes lead to personalized and more successful treatment and lower risk of sequelae? Does the presence of upper tract infection and presence of endometritis or salpingitis predict a higher risk for sequelae? There are few data to answer these questions. Larger high-quality epidemiological studies that follow participants longitudinally would help to identify microbial risk factors for PID and to evaluate the relative contribution of M genitalium, T vaginalis, the BV-associated bacteria, and other pathogens to incident PID.
The data we have reviewed suggest a significant role for pathogens other than C trachomatis and N gonorrhoeae in the etiology of PID. Thus, it is not surprising that the recent Anaerobes and Clearance of Endometritis (ACE) trial comparing an antibiotic regimen with versus without metronidazole demonstrated higher clearance of endometrial anaerobes and greater reduction in tenderness on exam in the arm treated with metronidazole [37]. As we have outlined, PID is rarely due to just C trachomatis or N gonorrhoeae, and our treatment choices should reflect that.
Conclusions
In future studies of PID, a more consistent protocol for evaluating lower and UGT microbes, as well as inflammation, would allow a more standardized comparison between populations and clinical phenotypes. Identifying biomarkers for upper tract infection and inflammation would allow noninvasive evaluation of people for endometritis and/or salpingitis, which in turn would facilitate more standardized and widespread evaluation for PID and a better understanding of the prevalence, etiology, treatment, and prevention of this disorder. Finally, long-term follow-up to assess the relationship among types of pathogens, degree of upper tract involvement, and the risk of sequelae is necessary. Understanding all of these factors will help guide prevention efforts—we cannot design interventions when we do not understand what exactly we are trying to prevent. In an age when we discuss genetic sequencing to “personalize” medicine, the syndromic management of PID and incomplete understanding of pathogenesis presents a stark example of how women’s health is undervalued and underresearched.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank Ricardo Albarran, Sagar Kumar, Katy Renfro from the Centers for Disease Control and Prevention for their support in the development of the meeting organization and notes.
Disclaimer. The views expressed in this paper are those of the authors and do not necessarily represent those of the Centers for Disease Control and Prevention.
Financial support. S. L. H.’s effort was funded by the National Institutes of Health (Grant 1U19AI144181).
Supplement sponsorship. This supplement is sponsored by the Centers for Disease Control and Prevention.
Potential conflicts of interest. C. M. M. has served as a consultant for Scynexis, Inc. and receives grant funding from Merck. C. R. C. serves as a scientific advisor to Osel, Inc. L. E. M. has received research funding from Hologic, Inc. S. L. H. is a consultant for Merck, Pfizer, Lupin, Hologic, and Daré Bioscience and receives research funding from Cepheid, Becton-Dickinson, and Curatek. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: CDC New frontiers in STD-related pelvic inflammatory disease (PID), infertility, and other sequelae meeting, November 5–7, 2019, Atlanta, GA.
References
- 1.Workowski KA, Bolan GA; Centers for Disease Control and Prevention . Sexually transmitted diseases treatment guidelines, 2015. MMWR Morb Mortal Wkly Rep 2015; 64:1–137.25590678 [Google Scholar]
- 2.Kreisel K, Torrone E, Bernstein K, Hong J, Gorwitz R. Prevalence of pelvic inflammatory disease in sexually experienced women of reproductive age - United States, 2013–2014. MMWR Morb Mortal Wkly Rep 2017; 66:80–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKee DL, Hu Z, Stahlman S. Incidence and sequelae of acute pelvic inflammatory disease among active component females, U.S. Armed Forces, 1996–2016. MMWR Morb Mortal Wkly Rep 2018; 25:2–8. [PubMed] [Google Scholar]
- 4.Kreisel K, Llata E, Haderxhanaj L, et al. . The burden of and trends in pelvic inflammatory disease 2006–2016. J Infect Dis 2021; 224:S103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haggerty CL, Peipert JF, Weitzen S, et al. ; PID Evaluation and Clinical Health (PEACH) Study Investigators . Predictors of chronic pelvic pain in an urban population of women with symptoms and signs of pelvic inflammatory disease. Sex Transm Dis 2005; 32:293–9. [DOI] [PubMed] [Google Scholar]
- 6.Trautmann GM, Kip KE, Richter HE, et al. . Do short-term markers of treatment efficacy predict long-term sequelae of pelvic inflammatory disease? Am J Obstet Gynecol 2008; 198:30 e1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ness RB, Soper DE, Holley RL, et al. . Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory disease: results from the Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) randomized trial. Am J Obstet Gynecol 2002; 186:929–37. [DOI] [PubMed] [Google Scholar]
- 8.Trent M, Bass D, Ness RB, Haggerty C. Recurrent PID, subsequent STI, and reproductive health outcomes: findings from the PID evaluation and clinical health (PEACH) study. Sex Transm Dis 2011; 38:879–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haggerty CL, Totten PA, Tang G, et al. . Identification of novel microbes associated with pelvic inflammatory disease and infertility. Sex Transm Infect 2016; 92:441–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiringa AE, Ness RB, Darville T, Beigi RH, Haggerty CL. Trichomonas vaginalis, endometritis and sequelae among women with clinically suspected pelvic inflammatory disease. Sex Transm Infect 2020; 96:436–8. [DOI] [PubMed] [Google Scholar]
- 11.Wølner-Hanssen P, Mårdh PA, Møller B, Weström L. Endometrial infection in women with Chlamydial salpingitis. Sex Transm Dis 1982; 9:84–8. [DOI] [PubMed] [Google Scholar]
- 12.Eckert LO, Hawes SE, Wölner-Hanssen PK, et al. . Endometritis: the clinical-pathologic syndrome. Am J Obstet Gynecol 2002; 186:690–5. [DOI] [PubMed] [Google Scholar]
- 13.Haggerty CL, Hillier SL, Bass DC, Ness RB; PID Evaluation and Clinical Health study investigators . Bacterial vaginosis and anaerobic bacteria are associated with endometritis. Clin Infect Dis 2004; 39:990–5. [DOI] [PubMed] [Google Scholar]
- 14.Hillier SL, Kiviat NB, Hawes SE, et al. . Role of bacterial vaginosis-associated microorganisms in endometritis. Am J Obstet Gynecol 1996; 175:435–41. [DOI] [PubMed] [Google Scholar]
- 15.Paavonen J, Aine R, Teisala K, Heinonen PK, Punnonen R. Comparison of endometrial biopsy and peritoneal fluid cytologic testing with laparoscopy in the diagnosis of acute pelvic inflammatory disease. Am J Obstet Gynecol 1985; 151:645–50. [DOI] [PubMed] [Google Scholar]
- 16.Bevan CD, Johal BJ, Mumtaz G, Ridgway GL, Siddle NC. Clinical, laparoscopic and microbiological findings in acute salpingitis: report on a United Kingdom cohort. Br J Obstet Gynaecol 1995; 102:407–14. [DOI] [PubMed] [Google Scholar]
- 17.Dan M, Samra Z, Katz A, Debby A, Gutman R, Zakut H. Etiology of acute pelvic inflammatory disease proven by laparoscopy. Sex Transm Dis 1993; 20:158–63. [DOI] [PubMed] [Google Scholar]
- 18.Eschenbach DA, Wölner-Hanssen P, Hawes SE, Pavletic A, Paavonen J, Holmes KK. Acute pelvic inflammatory disease: associations of clinical and laboratory findings with laparoscopic findings. Obstet Gynecol 1997; 89:184–92. [DOI] [PubMed] [Google Scholar]
- 19.Taylor-Robinson D, Jensen JS, Svenstrup H, Stacey CM. Difficulties experienced in defining the microbial cause of pelvic inflammatory disease. Int J STD AIDS 2012; 23:18–24. [DOI] [PubMed] [Google Scholar]
- 20.Mugo NR, Kiehlbauch JA, Nguti R, et al. . Effect of human immunodeficiency virus-1 infection on treatment outcome of acute salpingitis. Obstet Gynecol 2006; 107:807–12. [DOI] [PubMed] [Google Scholar]
- 21.Korn AP, Hessol N, Padian N, et al. . Commonly used diagnostic criteria for pelvic inflammatory disease have poor sensitivity for plasma cell endometritis. Sex Transm Dis 1995; 22:335–41. [DOI] [PubMed] [Google Scholar]
- 22.Wiesenfeld HC, Hillier SL, Krohn MA, et al. . Lower genital tract infection and endometritis: insight into subclinical pelvic inflammatory disease. Obstet Gynecol 2002; 100:456–63. [DOI] [PubMed] [Google Scholar]
- 23.Wiesenfeld HC, Sweet RL, Ness RB, Krohn MA, Amortegui AJ, Hillier SL. Comparison of acute and subclinical pelvic inflammatory disease. Sex Transm Dis 2005; 32:400–5. [DOI] [PubMed] [Google Scholar]
- 24.Wiesenfeld HC, Hillier SL, Meyn LA, Amortegui AJ, Sweet RL. Subclinical pelvic inflammatory disease and infertility. Obstet Gynecol 2012; 120:37–43. [DOI] [PubMed] [Google Scholar]
- 25.Sweet RL. Anaerobic infections of the female genital tract. Am J Obstet Gynecol 1975; 122:891–901. [DOI] [PubMed] [Google Scholar]
- 26.Mc GC Jr. Surgical management of chronic pelvic inflammatory disease; a study of 138 cases. Obstet Gynecol 1959; 13:591–7. [PubMed] [Google Scholar]
- 27.Curran JW. Economic consequences of pelvic inflammatory disease in the United States. Am J Obstet Gynecol 1980; 138:848–51. [DOI] [PubMed] [Google Scholar]
- 28.Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: from Chrysalis to multicolored butterfly. Clin Microbiol Rev 2011; 24:498–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen CR, Mugo NR, Astete SG, et al. . Detection of Mycoplasma genitalium in women with laparoscopically diagnosed acute salpingitis. Sex Transm Infect 2005; 81:463–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Møller BR, Taylor-Robinson D, Furr PM, Freundt EA. Acute upper genital-tract disease in female monkeys provoked experimentally by Mycoplasma genitalium. Br J Exp Pathol 1985; 66:417–26. [PMC free article] [PubMed] [Google Scholar]
- 31.Wood GE, Patton DL, Cummings PK, Iverson-Cabral SL, Totten PA. Experimental infection of pig-tailed Macaques (Macaca nemestrina) with Mycoplasma genitalium. Infect Immun 2017; 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGowin CL, Spagnuolo RA, Pyles RB. Mycoplasma genitalium rapidly disseminates to the upper reproductive tracts and knees of female mice following vaginal inoculation. Infect Immun 2010; 78:726–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium infection and female reproductive tract disease: a meta-analysis. Clin Infect Dis 2015; 61:418–26. [DOI] [PubMed] [Google Scholar]
- 34.Cina M, Baumann L, Egli-Gany D, et al. . Mycoplasma genitalium incidence, persistence, concordance between partners and progression: systematic review and meta-analysis. Sex Transm Infect 2019; 95:328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Latimer RL, Read TRH, Vodstrcil LA, et al. . Clinical features and therapeutic response in women meeting criteria for presumptive treatment for pelvic inflammatory disease associated with Mycoplasma genitalium. Sex Transm Dis 2019; 46:73–9. [DOI] [PubMed] [Google Scholar]
- 36.Short VL, Totten PA, Ness RB, Astete SG, Kelsey SF, Haggerty CL. Clinical presentation of Mycoplasma genitalium infection versus Neisseria gonorrhoeae infection among women with pelvic inflammatory disease. Clin Infect Dis 2009; 48:41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiesenfeld HC, Meyn LA, Darville T, Macio IS, Hillier SL. A randomized controlled trial of ceftriaxone and doxycycline, with or without metronidazole, for the treatment of acute pelvic inflammatory disease. Clin Infect Dis 2020. doi: 10.1093/cid/ciaa101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mårdh PA, Weström L. Tubal and cervical cultures in acute salpingitis with special reference to Mycoplasma hominis and T-strain mycoplasmas. Br J Vener Dis 1970; 46:179–86. [PMC free article] [PubMed] [Google Scholar]
- 39.Reighard SD, Sweet RL, Vicetti Miguel C, et al. . Endometrial leukocyte subpopulations associated with Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis genital tract infection. Am J Obstet Gynecol 2011; 205:324.e1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moodley P, Wilkinson D, Connolly C, Moodley J, Sturm AW. Trichomonas vaginalis is associated with pelvic inflammatory disease in women infected with human immunodeficiency virus. Clin Infect Dis 2002; 34:519–22. [DOI] [PubMed] [Google Scholar]
- 41.Eckert LO, Thwin SS, Hillier SL, Kiviat NB, Eschenbach DA. The antimicrobial treatment of subacute endometritis: a proof of concept study. Am J Obstet Gynecol 2004; 190:305–13. [DOI] [PubMed] [Google Scholar]
- 42.Hebb JK, Cohen CR, Astete SG, Bukusi EA, Totten PA. Detection of novel organisms associated with salpingitis, by use of 16S rDNA polymerase chain reaction. J Infect Dis 2004; 190:2109–20. [DOI] [PubMed] [Google Scholar]
- 43.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation. J Clin Microbiol 1991; 29:297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hong S, Xin C, Qianhong Y, et al. . Pelvic inflammatory disease in the People’s Republic of China: aetiology and management. Int J STD AIDS 2002; 13:568–72. [DOI] [PubMed] [Google Scholar]
- 45.Soper DE, Brockwell NJ, Dalton HP, Johnson D. Observations concerning the microbial etiology of acute salpingitis. Am J Obstet Gynecol 1994; 170:1008–14; discussion 14–7. [DOI] [PubMed] [Google Scholar]
- 46.Turpin R, Tuddenham S, Klebanoff M, Ghanem K, Brotman R. Bacterial vaginosis and behavioral factors associated with incident pelvic inflammatory disease in the longitudinal study of vaginal flora. J Infect Dis 2021;224:S137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ness RB, Kip KE, Hillier SL, et al. . A cluster analysis of bacterial vaginosis-associated microflora and pelvic inflammatory disease. Am J Epidemiol 2005; 162:585–90. [DOI] [PubMed] [Google Scholar]
- 48.Haggerty CL, Ness RB, Totten PA, et al. . Presence and concentrations of select bacterial vaginosis-associated bacteria are associated with increased risk of pelvic inflammatory disease. Sex Transm Dis 2020; 47:344–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hillier SL, Meyn LA, Avolia H, et al. Lower genital tract predictors of acute endometritis among women with signs and symptoms of pelvic inflammatory disease (PID). STI & HIV World Congress - Joint Meeting of the 23rd International Society for Sexually Transmitted Diseases Research and International Union against Sexually Transmitted Infections (Vancouver, British Columbia) 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.