Abstract
The prospect of ovarian rejuvenation offers the tantalising prospect of treating age-related declines in fertility or in pathological conditions such as premature ovarian failure. The concept of ovarian rejuvenation was invigorated by the indication of the existence of oogonial stem cells (OSCs), which have been shown experimentally to have the ability to differentiate into functional follicles and generate oocytes; however, their clinical potential remains unknown. Furthermore, there is now growing interest in performing ovarian rejuvenation in situ. One proposed approach involves injecting the ovary with platelet rich plasma (PRP).
PRP is a component of blood that remains after the in vitro removal of red and white blood cells. It contains blood platelets, tiny anucleate cells of the blood, which are responsible for forming athrombus to prevent bleeding. In addition, PRP contains an array of cytokines and growth factors, as well as a number of small molecules.The utility ofPRP has been investigatedin a range of regenerative medicine approaches and has been shown to induce differentiation of a range of cell types, presumably through the action of cytokines.
A handful ofcasereports have described the use of PRP injections into the ovaryin the human, and while these clinical data report promising results, knowledge on the mechanisms and safety of PRP injections into the ovary remain limited.In this article, we summarise some of the physiological detail of platelets and PRP, before reviewing the existing emerging literature in this area. We then propose potential mechanisms by which PRP may be eliciting any effects before reflecting on some considerations for future studies in the area. Importantly, on the basis of our existing knowledge, we suggest that immediate use of PRP in clinical applications is perhaps premature and further fundamental and clinical research on the nature of ovarian insufficiency, as well as the mechanism by which PRP may act on the ovary, is needed to fully understand this promising development.
Keywords: ovarian rejuvenation, platelet rich plasma, premature ovarian failure, oogonial stem cells, cytokines
Introduction
Female infertility is recognised by the World Health Organisation (WHO) as a global public health issue (Macaluso et al., 2010), with more than one million cycles of IVF being performed globally each year since 2005 (Zegers-Hochschild et al., 2014; Adamson et al., 2016). Female infertility can arise from a range of conditions, including endocrine dysfunction, implantation failure, endometriosis and uterine fibroids, as well as pathologies related directly to the ovary, including polycystic ovary syndrome (PCOS), primary ovarian insufficiency (POI), environmental factors and inflammatory disease. However, ‘ovarian exhaustion’ is a natural part of the ageing process. In the past 50 years, the mean age at which women have their first child in the UK has increased from 23.8 to 30.7 years (Office for National Statistics, 2020), suggesting that women are delaying childbearing. The impact of delayed childbearing means that women are moving closer to the period of climacteric for conception, and, in many cases, women are choosing not to reproduce until much later. One consequence of this has been a rise in fertility treatment and a rise in the age of women attending for medical investigation. Indeed, in the UK alone, the mean age of women attending for IVF treatment has hovered around 35 for the past 20 years (Human Fertilisation and Embryology Authority, 2020). Since the advent of clinical IVF in 1978 (Steptoe and Edwards, 1978) and associated Assisted Reproductive Techniques (ARTs), it has been possible to treat infertility in a number of cases. However, such approaches are reliant on a healthy oocyte for fertilisation and so have limited success in treating peri- or post-menopausal women without the use of donor eggs. Moreover, ARTs do little to tackle fundamental dysfunction within the ovary and in the oocytes that lead to female infertility and associated physiological adaptations.
The prospect of rejuvenating the exhausted ovary has been enticing ever since the description of oogonial stem cells (OSCs) in the ovarian cortex (White et al., 2012), hinting at a possibility of therapeutic stimulation of post-natal folliculogenesis in subfertile women. In a study by Niikura et al. (2009), transplantation of ovarian stem cells from atrophic ovaries from aged mice into young, healthy counterparts resulted in their resumption of spontaneous oogenesis, suggesting that ovarian aging or insufficiency could be reversed if OSCs are provided a healthy environment. Further work has implicated a role for mitochondria in loss of oocyte quality associated with the aged ovary (Cozzolino et al., 2019); indeed, methods to replenish mitochondria within aged oocytes are currently being explored as a means to rejuvenate them (Labarta et al., 2019). However, as with IVF, efforts to improve egg quality do not address the wider aspects of age-related ovarian dysfunction.
One recently proposed option for ovarian rejuvenation is the intraovarian injection of platelet-rich plasma (PRP) which is being used increasingly in clinical settings for a number of soft tissues, including to support wound healing and ligament and muscle repair (Suthar et al., 2017; Hurley et al., 2019; Verma et al., 2019; Zhang et al., 2020). PRP was first described for ovarian rejuvenation by Pantos et al. (2016). Their work described how PRP, which is a component of blood, could, when in injected directly into the ovary, trigger the resumption of menstrual cycles in women exhibiting signs of the climacteric. In this review, we will briefly consider the concept of ovarian rejuvenation before describing what PRP is and how it is generated and finally reflecting on the current state of knowledge of ovarian rejuvenation with PRP.
Ovarian rejuvenation
The paradigm that the mammalian ovarian reserve is fixed at birth dates back to a nineteenth century hypothesis by Waldeyer in 1870, which was reaffirmed by Zuckerman in 1951 (reviewed in Tilly et al., 2009). However, there is mounting evidence that this is only part of the story, and that it may be possible to replenish the ovarian follicle pool due to the presence of a population of oogonial stem cells (OSC) in adult ovaries (Niikura et al., 2009). It is likely that both of these explanations are true in part; there is a fixed number of follicles at birth, which declines until exhaustion (typically 40+ years of age in the human), but that a population of OSC co-exist in the ovary and may be activated under specific circumstances (Tilly and Telfer, 2009). However, spontaneous reactivation of OSCs is not yet believed to occur naturally in vivo in the adult human ovary. This is one principle that underpins the notion of ovarian rejuvenation.
As an illustration of this concept, mice rendered sterile from chemotherapeutic drugs can have fertility restored and can produce viable offspring through natural mating after undergoing an OSC transplant from neonatal or adult mouse ovaries (Zou et al., 2009). It was further demonstrated that when ovarian tissue containing premeiotic germ cells from aged mice was transplanted into young host mice, the germ cells produced NOBOX-expressing oocytes and formed follicles (Niikura et al., 2009). Combined, these studies show OSC transplantation may restore fertility and that it may be possible to produce oocytes from OSC from aged mammalian ovaries in the correct milieux.
Although data from animal models support the notion of OSCs, the presence of equivalent stem cell populations in humans remains disputed. For example, Virant-Klun et al. (2008) confirmed that ovarian stem-like cells were present on the surface epithelium of post-menopausal women and women with premature ovarian failure (POF), which aligns with the reported location of OSC in the ovaries of juvenile and young-adult mice (Tilly and Telfer, 2009). By contrast, when analysing the cell populations in the human ovarian cortex, Wagner et al. (2020) were unable to identify a population of germline stem cells. Of course, it must be acknowledged that studies on normal ovarian function in humans is rather constrained since substantial ovarian tissue from healthy, reproductive-aged women is rarely available. Furthermore, tissue from dysfunctional ovaries may not exhibit the full range of physiological function, and biopsies may not be reflective of the whole ovary as stem cells may not be uniformly spread (Horan and Williams, 2017). These factors make it challenging to determine definitively if a population of stem cells is present within the adult ovary.
If present, ovarian OSC may offer the potential for women experiencing ovarian failure as a result of menopause or POF to be treated for their infertility beyond the only current option of IVF using a donor egg. This has provided an underpinning of attempts to initiate ovarian rejuvenation in clinical settings, including investigating the utility of PRP in four pilot studies of different reproductive pathologies: POI, poor ovarian responders (POR), perimenopause and menopause (Sfakianoudis et al., 2020b).
Platelets and platelet-rich plasma
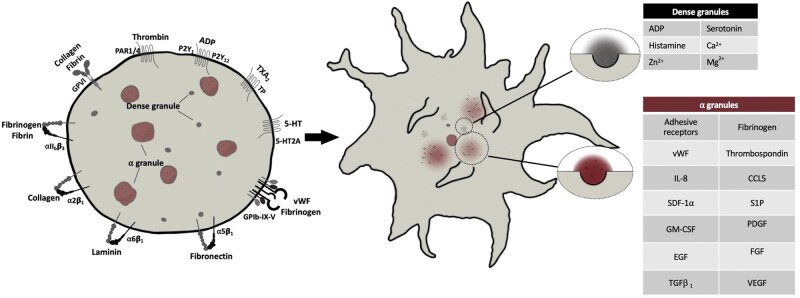
The blood platelet is a tiny, anucleate cell responsible for the initiation of formation of a thrombus (Fig. 1). Platelets are formed from a fragment of megakaryocyte membrane that is pre-packaged with a myriad of molecules and complexes necessary for its primary function, which is to sense signs of trauma within the vasculature and aggregate together to stem the loss of blood. One of the primary steps in thrombus formation is platelet activation, which is driven by ‘outside-in’ signalling, initiated through a vast repertoire of G-protein coupled receptors, integrins and glycoprotein channels on the surface of the platelet (Li et al., 2010). The activation of platelets can occur through numerous mechanisms by a seemingly endless number of agonists, including but not limited to, thrombin, collagen, adenosine diphosphate (ADP), thromboxanes, serotonin, oxidised LDL and extracellular divalent cations (Lopez-Vilchez et al., 2009; Li et al., 2010; Wraith et al., 2013; Shen et al., 2017).
Figure 1.
Granule release in activated platelets. Platelets express numerous glycoprotein, integrin and G- protein-coupled receptors that bind to a myriad of soluble and matrix proteins and molecules, resulting in tightly orchestrated intracellular signalling. This intracellular signalling significantly increases cytoplasmic calcium levels and causes drastic changes in the platelet cytoskeleton, resulting in ashape change in the platelet to an ‘echinocytic’ formation. During this process, granular storage compartments migrate inwards to the centre of the platelet and fuse with the plasma membrane and release their contents into the extracellular milleiu. PAR1/4, protease-activated receptors 1/4; GPVI, glycoprotein VI; TXA2, thromboxane A2; TP, thromboxane protstanoid receptor; 5-HT, 5-hydroxytryptomine; P2Y, purinergic receptor 2Y; vWF, von Willebrand Factor; IL-8, interleukin-8; CCL5, chemokine ligand 5; SDF-1a, stromal cell-derived factor 1 alpha; FGF, fibroblast growth factor; EGF, endothelial growth factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; TGFb1, transforming growth factor beta 1.
A core platelet response to activation is the release of the contents of intracytoplasmic granules. Platelets contain two main granule stores, the alpha and dense granules, both of which replete with factors critical for an effective platelet response to vascular damage (Fig. 1). Where alpha and dense granules are lacking, the conditions grey platelet syndrome and delta storage pool deficiency can arise. Both of these conditions are associated with an increased bleeding tendency (Bolton-Maggs et al., 2006). It is also of note that more recently, platelet secretory behaviour has been shown to extend beyond the realm of granular stores and also involves activation-dependent synthesis and release of cytokines and other bioactive molecules (Heijnen and van der Sluijs, 2015). It is, therefore, clear that the contents of platelet intracytoplasmic granules and de novo synthesis of agents are essential for the haemostatic response, and the descriptions on the functions of platelet releasate have historically focussed on its role in haemostasis (Rendu and Brohard-Bohn, 2001). However, the catalogue of bioactive proteins and molecules released by activated platelets can have multiple physiological effects which include increased angiogenesis, cell proliferation, cell differentiation and regulation or attenuation of apoptosis (Bir et al., 2011; Au et al., 2014; Golebiewska and Poole, 2015). The therapeutic role of the platelet releasate in driving tissue regeneration is of growing interest throughout modern medicine.
PRP is a term used to describe a fraction of the blood after processing. It is typically isolated from autologous whole blood retrieved by phlebotomy into a citrate-based anticoagulant. This is then subjected to differential centrifugation, resulting in the removal of red blood and immune cells, leaving behind a high concentration of platelets within plasma. Commercial sources of PRP are available, which can provide a predetermined concentration of platelets. However, in many cases, PRP is derived ‘in-house’, produced according to many subtle protocol variations. It is not uncommon for resulting PRP to retain varying concentrations of RBCs and WBCs; such contamination and absence of standardisation may result in conflicting findings regarding the effects of PRP in different applications.
In recent years, there has been significant interest in exploiting PRP in regenerative medicine. Particular attention has been paid to musculoskeletal (Scully et al., 2019, 2020), oral-maxillofacial (Xu et al., 2020) and osteoarthritis (Evans et al., 2020) applications to name but a few. For a more comprehensive account, the reader is referred to a review (Scully et al., 2018).
PRP and ovarian rejuvenation: the evidence so far
Over the past decade, there have been a growing number of studies that have reported that injection of PRP directly into the ovary can increase folliculogenesis and egg harvest. One of the earliest studies reporting this approach was from Callejo et al. (2013), who implanted cryopreserved ovarian tissue within the peritoneum. PRP was used as a pro-angiogenic and proliferative agent, and the approach supported a successful live birth. The proangiogenic effect of PRP was further highlighted in a study by Bakacak et al. (2015), who used a rat model of ovarian ischaemia induced by torsion. In that study, PRP treatment in all conditions significantly increased peritoneal vascular endothelial growth factor (VEGF) and provided protection from ROS-induced oxidative damage during reperfusion.
More recently, direct injection of PRP into ovaries has been reported. In 2016, a short communication at the ESHRE Annual Meeting indicated that infusion of PRP into the ovary of perimenopausal women led to resumption of menstrual cycles (Pantos et al., 2016). The study included only eight women but was the first reported use of PRP for rejuvenation of the perimenopausal ovary. Since then, there have been several limited investigations into the utility of PRP injection into the ovaries of perimenopausal women which are summarised in Table I. Sills et al. (2018) reported that for healthy women with a history of infertility, ovarian PRP infusion produced several MII oocytes for cryopreservation, with one individual proceeding to successful embryo transfer at time of publication. Other studies have reported similar cases; commonly ovarian PRP therapy has caused AMH to increase and FSH levels to fall in previous non-responders, leading to folliculogenesis, significant levels of oocyte retrieval, and in a handful of cases, spontaneous pregnancy (Sfakianoudis et al., 2018; Farimani et al., 2019; Pantos et al., 2019; Hsu et al., 2020).
Table I.
Summary of reports on the effect of PRP infusion in ovarian rejuvenation.
| Case reports | ||
|---|---|---|
| Author | Findings summary | Procedure and controls |
| Sfakianoudis et al. (2018) | Spontaneous resumption of menstruation 6 weeks following PRP injection, with a concomitant reduction in FSH and increase in AMH being observed. A natural IVF cycle led to the retrieval of one high-grade oocyte that, after ICSI, resulted in a grade III 6-cell cleavage stage embryo. Following implantation, confirmation of a clinical pregnancy was determined, however the pregnancy spontaneously terminated at 5 weeks of gestation. | 40-year-old woman with a history of premature menopause for 5 years and who was unable to naturally conceive for over a year. Approximately 4 ml of PRP (9 × 108/ml) was injected into each ovary. Measurement of FSH, AMH and LH pre- and 6 weeks post-injection |
| Sfakianoudis et al. (2020b) | Menstruation was restored in the pilot study for POI patients (18 out of 30), AMH, FSH and AFC also significantly improved, noting 3 spontaneous pregnancies and live births. For the Poor Ovarian Responders (POR), there was an improvement to ICSI cycle performance. The perimenopausal pilot data showed 24 out of 30 women had improved hormone levels and AFC, as well as improved menstruation regularity, noting 4 spontaneous pregnancies and 3 live births. 13 out of 30 menopausal patients were described as positively responding to PRP treatment, noting 1 spontaneous pregnancy and live birth | Recruitment of a total 120 women suffering from POI, POR or who were perimenopausal or menopausal were assigned to 4 respective pilot studies. 4 ml of calcium gluconate-activated PRP (1 × 109/ml) was injected into each ovary |
| Pantos et al. (2019) | Increased E2 and AMH and decreased FSH and LH were observed with PRP treatment. All participants resumed menstruation within 2 months post-injection and naturally conceived and carried until third trimester at time of publication | Injection of approximately 4 ml of activated PRP (concentration and agonist not reported) into the ovaries of three subfertile women who experienced >1 year of amenorrhea (2 POF, 1 menopausal). Measurement of FSH, AMH, E2, LH and AFC pre- and post-therapy |
| Sills et al. (2018) | Multiple high-grade MII oocytes obtained from all participants, resulting in at least one Day 5 embryo per round of IVF, with one participant opting for immediate embryo transfer who then developed a clinical pregnancy. PRP injection was associated with a significant reduction in FSH | 5 ml of PRP (concentration not reported) activated by calcium gluconate was injected throughout the ovaries of four women with at least one round of IVF failure or amenorrhoea for over 3 months. FSH, AMH and E2 measurements obtained pre- and post-PRP therapy. Hyperstimulation ovarian stimulation and oocyte retrieval performed from 59 days after therapy |
| Cakiroglu et al. (2020) | Spontaneous pregnancy was achieved in 23 out of 311 women diagnosed with POI, with 16 resulting in sustained implantation or livebirth. A significant increase in antral follicle count observed after PRP treatment, serum AMH increased after treatment, although serum FSH was not statistically significantly different. 201 patients developed antral follicles and attempted IVF (87 did not develop antral follicles), 57 of the 82 women who developed embryos underwent embryo transfer, 9 resulting in sustained implantation or livebirth | 311 women aged 24–40 diagnosed with POI underwent intraovarian injection (in at least one ovary) of 2–4 ml of PRP (concentration not reported). PRP injection was timed randomly in amenorrheic women, and 10 days post-menstrual bleeding in oligomenorrheic women |
| Callejo et al. (2013) | PRP-loaded ovarian tissue that was implanted into the peritoneum on the broad ligament of a woman with no ovaries and was able to spontaneously resume menstruation. A round of IVF/ICSI on two obtained oocytes was able to provide generated two embryos for transfer, resulting in a clinical pregnancy. A healthy boy child was delivered by caesarean section at 38 weeks and 6 days, weighing 3.5 kg | Implantation of thawed cryopreserved ovarian tissue in a 30-year-old woman who had a bilateral oophorectomy at 20 years of age. Tissue was impregnated in a PRP gel and surgically implanted onto the broad ligament and growth factors administered. IVF/ICSI performed on resulting oocytes and implantation was performed with two Day 2 embryos |
| Farimani et al. (2019) | PRP-treatment increased the oocyte yield and the average number of retrieved oocytes and resulting embryos was higher after PRP treatment. 3 of the 12 women that underwent therapy had live births, two of which were via spontaneous conception and one with IVF | 12 women suffering with poor ovarian reserve for more than 3 years underwent double ovarian stimulation and oocyte retrieval before and after injection of 2 ml of PRP (concentration not reported) |
| Hsu et al. (2020) | Resumption of folliculogenesis within 4 days post-PRP injection. Two rounds of hyperovulation supervovulation led to the capture of 6 oocytes, which after ICSI, led to two 8-cell and one 5-cell embryos, which were all transferred back into the uterus and resulted in a pregnancy of twins, which were delivered at 30 weeks with no documented abnormalities | 33-year-old woman, with a history of irregular periods, who had several rounds of IUI cancelled due to lack of any follicles. Injection of approximately 4 ml PRP (concentration not reported) in conjunction with 1 ml 150 IU FSH and 75 IU LH throughout the ovarian tissue |
Table I.
Continued.
| Basic research | ||
|---|---|---|
| Author | Findings summary | Procedure and controls |
| Ahmadian et al. (2020) |
|
86 rats used, 63 were IP injected with 160 mg/kg VCD to induce POI, 18 received a similar volume of normal saline. 15 POI rats injected with 10 µl low concentrated PRP (8.5 × 105/µl), 15 with 10 µl high concentrated PRP (21.6 × 105/µl), 15 injected with 10 µl normal saline (sham), 15 without interference, 15 in control group (no POI and no PRP) |
| Bakacak et al. (2015) | PRP administration significantly reduced markers of reactive oxidant damage in and decreased histopathological damage scoring in the ovary when compared to sham injections. This response, however, was incomplete and remained significantly higher with PRP treatment compared to sham controls | Induction of ovarian torsion in rats. 60 female rats used. 12 used to prepare PRP, and 8 rats per group and 12 for PRP preparation: sham operation, ischemia, ischemia/reperfusion, sham operation + PRP, ischaemia+PRP, ischemia/reperfusion+PRP. Platelet concentration of PRP was 6.9 × 105 ± 0.6 × 105/µl was used |
| Cremonesi et al. (2020) | 5 ml of PRP (1 × 109ml) injected into the left right ovary of eight cows of proven fertility. PRP injection resulted in an increase in follicle count and increased subsequent number of grade 1–2 blastocysts | 5 ml of PRP (1 × 109/ml) was injected into the left ovary of Holstein–Friesian cows, leaving the right ovary as a pseudocontrol (no injection). Superovulation induced after the 9th day of cycle following PRP injection with Gn administration in decreasing doses for 5 days. Cows were then inseminated and oestrus was induced using PGF-2a. Embryo retrieval was then performed by flushing both left and right uterine horns |
| Hosseini et al. (2017) | An increase in follicle growth was observed in response to PRP (10%) supplementation. Interestingly, a mix of both PRP (5%) and FBS (5%) did not benefit follicle growth, suggesting a dose-dependent effect of PRP on follicle maturation | Primordial follicles were isolated from ovaries donated by three healthy women after death post-mortem. PRP (concentration not reported) was activated with 20 IU/ml thrombin. Follicles were embedded in a 3D gel matrix, supplemented with either 10% PRP, 5% PRP + 5% FCS, 10% FCS or 10% HSA with a-MEM media |
|
| ||
|
Non-randomised clinical trial | ||
| Author | Findings summary | Procedure and controls |
|
| ||
| Melo et al. (2020) | There were 11 clinical pregnancies, leading to 5 total live births in those receiving PRP injection, compared to 2 clinical pregnancies and 1 live birth in the untreated group. PRP therapy was associated with an increase in AMH and a decrease in FSH levels. Total AFC were higher post-therapy versus no intervention, with those seeking IVF/ICSI resulting in higher numbers of oocytes collected. Resulting embryos were graded higher in response to PRP when compared to no injection | Non-randomised interventional study (PRP vs. no injection) involving 83 women (46 PRP vs. 37 no injection). Each arm was then subdivided into those receiving IVF versus no IVF (timed conception and IUI). PRP (citrate anticoagulant, count not reported) was activated with 10% calcium chloride and injected as a 200 µl volume into each ovary |
In the only preclinical study on the effect of PRP injection into human ovaries, Hosseini et al. (2017) obtained healthy donated ovaries from deceased donors. PRP injection led to an increase in follicle size and their viability at 10 days compared to treatment with foetal calf serum (FCS) alone. Surprisingly, a combination of FCS and PRP did induce follicular growth, which is an interesting observation worthy of further investigation.
While these case studies appear encouraging, it is important to reflect on the experimental designs. A common feature of the first studies of the effect of PRP infusion is the absence of a sham injection group. It is conceivable that the mechanical stretching and/or mild injury to the ovary resulting from the procedure is sufficient to elicit an inflammatory response leading to temporary resumption of ovarian function. For example, laparoscopic ovarian ‘drilling’ is a therapeutic option for the treatment of clomiphene-resistant PCOS (Lebbi et al., 2015) and, thus, a comparable ovarian needle stick injury may be a causative factor in the success of PRP therapy. Importantly, the recent study of Ahmadian et al. (2020) used a sham injection group, which showed no morphologically normal follicles, and the same result was observed in the ‘no injection’ group. This demonstrated that injection with saline is not sufficient to reverse the effects of premature ovarian insufficiency in this animal model, nor can it elicit a comparable response to the two groups with different concentrations of PRP, which show reduced follicular atresia and increased follicular quality. It is vital that future studies control for this component of the intervention.
An important study was published by Melo et al. (2020) who reported findings from a non-randomised interventional study involving 83 subfertile women, 46 of whom opted for several infusions of 200 µl of autologous PRP into each ovary, and 37 who opted for no treatment. These two arms were further subdivided into groups who opted for IVF, and those who continued with unassisted conception. Overall, significantly higher antral follicle counts were observed in women who received PRP infusion compared to those women who received no treatment. In addition, embryo quality was scored higher from those obtained through PRP therapy, although there was no difference in the fertilisation rate of oocytes from either group. The authors concluded that ovarian injection of PRP did lead to increased egg yield in subfertile women and prompted changes within the oocyte which may lead to increased ‘quality’ of subsequent embryos. In both the IVF and spontaneous conception groups, those receiving PRP therapy developed 13 clinical pregnancies, compared to 2 in the control group although there were insufficient data on live births to draw any definitive conclusions. Although these data are encouraging, the absence of randomisation may have led to a socioeconomic selection bias, since PRP intervention was adopted only by couples able to pay for the treatment. Examples such as this illustrate the necessity that case studies are scrutinised in detail. Ideally, a properly controlled randomised clinical trial will be necessary to confirm the efficacy of ovarian PRP therapy.
How might PRP induce ovarian rejuvenation?
Given the complexity of platelet signalling and activation, the precise details of how platelets initiate their full range of physiological effects remain unclear. However, it is well established that platelets release a range of cytokines in response to activation (Roh et al., 2016). Cytokine signalling is increasingly being shown to be involved in the interrelationship among the oocyte, granulosa and thecal cells, with dysfunction in this ecosystem resulting in deficiencies in follicle maturation, ovulation and luteinisation (Orisaka et al., 2006; Field et al., 2014). A number of the cytokines that regulate follicle development are released by platelets through secretion of their alpha and dense granule contents during platelet activation (Table II). Therefore, a working hypothesis is that PRP may provide a readily accessible, individualised, cost-effective blend of proangiogenic, proliferative and proinflammatory factors which may stimulate de-novo oogenesis and/or follicle maturation.
Table II.
Factors released by platelets with known effects in the ovary.
| Factor | Effect | Plasma | Platelet | References |
|---|---|---|---|---|
| BMPs | Essential for oocyte maturation and folliculogenesis. Involved in maintaining cumulus cell expansion. BMP2 expression associated with an increase in oocyte quality scoring | ✓ | ✓ | |
| CCL5 | Higher CCL5 levels in follicular fluid associated with increased subsequent embryo quality upon IVF | ✓ | ✓ | |
| EGF | Required for LH-mediated cumulus cell expansion | ✓ | ✓ | |
| IL-8 | Associated with higher pregnancy rates and embryo quality. Found in healthy follicular fluid | ✓ | ✓ | |
| PDGF | Expression of PDGF receptors in oocytes and granulosa cells. Inhibition of PDGFR in rat ovaries results in increased follicle atresia, reduction in primary/early and antral follicle formation and intraovarian blood vessel size. Shown to increase the stromal cell migration from the fallopian tube fimbriae towards the ovulating follicle. Involved in primordial to primary follicle transition | ✓ | ✓✓✓ | |
| PF4/CXCL4 | Strong chemoattractant for neutrophils and monocytes, inducing robust phenotypic alterations. Increased intrafollicular levels of PF4 found in those with PCOS | ✓ | ✓✓✓ | |
| P-selectin (CD62) | PSGL-1 expression in the porcine zona pellucida. Key in the recruitment of neutrophils to sites of injury | ? | ✓✓✓ | |
| SDF-1α/CXCL12 | Causes inhibition of primordial to primary follicle transition in murine neonates, resulting in smaller, more dense yet numerous oocytes. Associated with a higher preovulatory follicle size in humans. Encourages the migration of T-cells, increases granulosa cell survival and overall oocyte quality | ✓ | ✓ | |
| Serotonin | 5-HT receptors robustly expressed in human ovarian epithelium. Both Serotonin and 5-HT transporters expressed in murine cumulus-oocyte complexes. Tryptophan hydroxylase robustly expressed in cumulus cells. Shown to modulate oestradiol production in cultured rat follicles | ✓ | ✓✓✓ | |
| TGF-β1 | Strongly regulates follicle survival and apoptosis. Synergises with VEGF to regulate angiogenesis. Essential in the crosstalk between thecal cells, granulosa cells and the oocyte during folliculogenesis and maturation. Critical for transcriptional activity through Smads | ✓ | ✓✓ | |
| TSP-1 | Present in granulosa cells, follicle antra and stromal compartment. Increases migration of ovarian vascular endothelial cells in primates. Inhibition of thrombospondin diminishes follicle rupture and oocyte release. CD36 observed in both murine and human oocytes and shown to co-determine fertilisation rate with BAI1/3 | ✓ | ✓✓✓ | |
| VEGF | Regulates intraovarian vascular events, leading to increased oxygen and nutrient supply. Causes increased follicle growth and corpus luteum formation and function | ✓ | ✓✓ | |
| TIMP-4 | Complexed with MMP-2 within the cytoplasm of platelets. Platelet activation causes dissociation of the complex and efflux out of the platelet. TIMP-4 is widely expressed in the murine ovary and has been shown to regulate morphogenesis and corpus luteum longevity during pregnancy | ✓ | ✓✓ | |
| GM-CSF | Present in low levels in platelets and shown to prevent eosinophil apoptosis. High expression of α and β GM-CSF receptors in cumulus cells | ✓ | ✓ | |
| FGF | Drives folliculogenesis and follicle maturation, Increases proliferation of thecal, granulosa and stromal cells. Expression of multiple FGF receptor isoforms in oocytes and granulosa cells | ✓ | ✓✓ | |
| S1P | Abundant in follicular fluid aspirates. May increase folliculogenesis through activation of HIPPO signalling, leading to increased CCN2 expression | ✓ | ✓✓✓ |
BMP, bone morphogenic protein; CCL5/RANTES, chemokine (C-C motif) ligand 5; EGF, endothelial growth factor; IL-8, interleukin-8; PDGF, platelet-derived growth factor; PF4, platelet factor 4; CXCL4, chemokine (C-X-C motif) ligand 4; SDF-1a, stromal-cell derived factor 1 alpha; CXCL12, chemokine (C-X-C motif) ligand 12; TGF-b1, transforming growth factor beta 1; VEGF, vascular endothelial growth factor; TIMP-4, tissue inhibitor of matrix metalloprotease; TSP-1, thrombospondin-1; GM-CSF, granulocyte-monocyte colony stimulating factor; FGF, fibroblast growth factor; S1P, sphingosine-1-phosphate; CCN2, connective tissue growth factor.
One possible explanation of the observed effects of PRP on the ovary might be that it acts in a proangiogenic manner (Kakudo et al., 2014) via action of platelet-released cytokines (Table II), including, for example VEGF. Primordial follicles typically rely on stromal blood vessels, but become progressively encapsulated in a thecal capillary network during maturation, a process which is mirrored by increased VEGF expression that persists through to corpus luteum formation (Gordon et al., 1996; Barboni et al., 2000; Danforth et al., 2003; Pauli et al., 2005). Heterozygous knockdown of the hypoxia-response element within the VEGFA promoter or VEGFR antagonism in mouse ovaries leads to vascular malformation, resulting in a poor ovarian response to stimulation (Feng et al., 2017), indicative of a role for VEGF in follicle development and the overall importance of correctly regulated vascularisation in follicle development. Another major constituent of platelet releasate, platelet-derived growth factor (PDGF), has also been implicated in regulating vessel formation and maturity. This was demonstrated via intraovarian injection with an anti-PDGF antibody in rats by Pascuali et al. (2015), who consequently observed a reduction in follicle maturation paired with an increase in follicle atresia. This direct evidence for the importance of proangiogenic factors in follicle development supports the idea that PRP and/or platelet releasate can increase blood supply to the immature follicle pool and/or OSCs and encourage their maturation.
An additional potential explanation for the positive effects of PRP on the ovary is via sphingosine-1-phosphate (S1P) (Ono et al., 2013; Urtz et al., 2015). S1P has been isolated from follicular fluid at high nanomolar concentrations (Von Otte et al., 2006) and there is evidence to suggest that it can promote follicle maturation, likely through increased expression of CCN2, a connective tissue growth factor shown to drive follicle maturation (Cheng et al., 2015). Platelet alpha granules contain abundant stores of S1P, which is released upon activation and have been measured at over 300 nM per 1 × 107 platelets. If a linear relationship between S1P concentrations and platelet count exists, this would estimate that in studies that have infused activated PRP into the ovary, the amount of S1P delivered is approximately 9 µM, close to the range reported to be beneficial by Cheng et al. (2015). However, a recent study involving both murine and human follicles and human-to-murine xenotransplantation reported that although CCN2 expression was elevated in response to supraphysiological S1P doses, there was no increase in the number of follicles. By contrast, ovaries receiving S1P treatment suffered a significant reduction in follicle number compared to control counterparts (Pors et al., 2020). These findings again highlight the uncertainty of the effect of factors released by activated platelets on oocyte and follicle development and clinicians must be careful when considering such approaches.
Although it is theorised that PRP supports the development of follicles from OSCs, alternative explanations must be considered. In a study by Hosseini et al. (2017), PRP was found to improve the growth and viability in vitro of preantral follicles isolated from human ovaries post-mortem, supporting the notion that PRP may aid ovarian rejuvenation through supporting development of existing primordial follicles. However, this application relies on the patient having a supply of oocyte-containing follicles, thus, rendering the approach unsuitable for women who have experienced ovarian exhaustion. Panda et al. (2020) expressed the need for better-controlled studies to confirm the conclusions drawn by Cakiroglu et al. (2020), which found that the number of remaining follicles within the ovaries of women with POI determines their response to PRP infusion, and that women without any antral follicles are unlikely to respond to PRP.
The prospective pilot study by Sfakianoudis et al. (2020b) determined that perimenopausal women and women deemed to be POR benefitted the most from the treatment, more so than POI and menopausal patients. In an article by Sfakianoudis et al. (2020a), they describe how novel techniques (such as PRP, ovarian stem cells transplant and ovarian tissue transplant) may effectively treat ovarian insufficiency by reactivating follicular growth through restoring the microenvironment of the ovary. Therefore, it should be acknowledged that PRP infusion may only be an appropriate treatment for select ovarian disorders.
PRP: a note of caution
A primary consideration of the effect of PRP in any aspect of regenerative medicine is the ‘activation status’ of the platelet (Fig. 1). As previously discussed, platelets have the capacity to respond to agonists and release a range of molecules, creating a ‘releasate’ (Piersma et al., 2009; Parsons et al., 2018). Indeed, PRP from resting platelets differs markedly to that containing activated platelets, and the mode of activation will influence the composition of the releasate. Despite this, there is considerable variation in the activation status of platelets used in studies of ovarian rejuvenation; some studies describe using calcium (Sills et al., 2018; Hsu et al., 2020; Melo et al., 2020) or thrombin (Hosseini et al., 2017), while others inject quiescent platelets or simply do not state their activation status (Callejo et al., 2013; Farimani et al., 2019; Pantos et al., 2019). The importance of reporting the activation status and the methods therein, paired with the use of appropriate controls, is critical, given reported effects of thrombin or calcium alone in the regulation of ovarian function. For example, thrombin has been shown to regulate progesterone synthesis in the preovulatory ovary homogenates, with multiple cell types within the ovary readily expressing PAR1 and PAR4 receptors (Cheng et al., 2012) through which thrombin elicits biological function directly. In addition, there is good evidence of an interaction between calcium signalling and ovarian steroidogenesis (reviewed in Kouba et al., 2019).
To date, it appears that efforts to investigate the role of platelet activation in the context of ovarian rejuvenation remain limited. For example, platelets possess CD40 and αIIbβ3 on their surface in a resting state (Inwald et al., 2003; Li et al., 2010). Thus, it is conceivable that these adhesive receptors and ligands are sufficient to elicit folliculogenesis or to recruit immune cells to the ovary without the need for platelets within the PRP to have become activated prior to injection. By contrast, activation and subsequent degranulation may be the critical function required for PRP to elicit an effect and quiescent PRP may become activated through exposure to platelet-activating matrices within the ovarian stroma. Differentiating the effects of stimulated versus unstimulated PRP should be a focus of future investigations and may help isolate the most effective agents that cause the reported regenerative effect in the ovary, paving the way for more defined interventions.
The contents of platelet granules may not all be beneficial for re-establishing female fertility among all disease settings. As a theoretical example, thrombospondin-1 has been implicated in follicle development (Kõks et al., 2010; Bender et al., 2019), yet it inhibits the proangiogenic action of VEGF (Greenaway et al., 2007) which may be undesirable where perfusion of the ovaries is limited. In addition, increased intraovarian VEGF and blood flow is thought to play a role in the pathogenesis of PCOS (Chan et al., 2003; Carmina et al., 2005; Peitsidis and Agrawal, 2010). Conversely, Anvari et al. (2019) recently reported that PRP therapy partially re-established hormonal balance in a rat model of PCOS. Here, PRP treatment increased the expression of oestrogen receptors α and β and of superoxide dismutase and glutathione peroxidase in ovarian homogenates. PRP-treated ovaries had significantly more pre-antral and antral follicles up to 30 days after treatment, suggesting that PRP may be a viable option for driving folliculogenesis in females with PCOS. In addition, platelets also release significant quantities of IL-15 when activated (de Miguel-Gómez et al., 2020). Increased IL-15 concentrations in follicular fluid have been negatively correlated with pregnancy outcomes via IVF, indicating that this cytokine may be detrimental to follicle maturation (Spanou et al., 2018). Interestingly, it is highly expressed in immature follicles, and falls during their maturation, which raises the potential importance of IL-15 in the activation of germline stem cells, as IL-15 is a potent regulator of other stem cell types (Huntington et al., 2009; Gómez-Nicola et al., 2011). This interplay and opposing effects of PRP constituents in different contexts serve to illustrate the importance of detailed studies of the mechanisms of how PRP might act on the ovary, and much additional work is required before any conclusions can safely be drawn.
Conclusions and future prospects
Even though there are other biological derivatives, such as human umbilical cord plasma, which may provide additional benefits in the sphere of ART (de Miguel-Gómez et al., 2020), the appeal of PRP lies in its balance among therapeutic effect, cost effectiveness, ease of isolation and autologous nature. However, with the increased interest of ovarian PRP injection inconclusive efficacy and lack of understanding of mechanism of action, fundamental research into the effect of this therapy on the cellular level is required. Dysregulation of early processes in oocyte maturation and subsequent embryo development can lead to drastic changes in the growing foetus, possibly leading to increased risk of disease in early years and onwards. Indeed, the long-term safety of new treatments in ART must be robustly assessed (Harper et al., 2012), especially given the context that there is still concern that ART itself may increase the risk of birth defects (Luke et al., 2020). Perhaps most of most relevance in the context of PRP, the precise physiological causes of premature ovarian failure or primary ovarian insufficiency outside the natural ageing process remain poorly understood, and further work to understand the role of putative OSCs is required.
With ovarian PRP therapy in its infancy, understandably, there is poor standardisation among research groups and clinics, however, this must soon be addressed to form a consensus as to the efficacy of this treatment. To assist this, we would propose that authors carrying out research in this area commit to reporting of key basic information regarding PRP. At a minimum, we suggest that such studies should include platelet count, activation status, activation agent (if any), platelet function testing, origin of PRP, volume infused, anticoagulant used, clinical account of menstrual status based on AMH level, and a detailed reporting of the participant’s fertility history.
Additionally, while there are encouraging data supporting the notion that PRP treatment might have some future use in the ART setting, it is paramount that we undertake robust and detailed basic studies to understand mechanism of action and to try to identify unintended outcomes, before moving into whole animal studies. These precursors would be an important bedrock on which to carry out well-designed clinical studies, allowing us to investigate this new technology with all rigour currently available. There are, at the time of writing, 13 registered clinical trials investigating the effect of PRP on ovarian rejuvenation either recruiting or underway. Strikingly, few of these trials describe the inclusion of appropriate PRP controls. It is only from well-controlled trials, built on detailed mechanistic understanding that we can clarify the platelet-mediated effects of PRP therapy in ovarian rejuvenation and folliculogenesis.
Data availability
No new data were generated or analysed in support of this research.
Acknowledgements
The authors wish to express their gratitude to the two anonymous reviewers who made supportive and insightful comments during the peer review process.
Authors’ roles
L.A. and R.G.S. conceived the manuscript. L.A., F.M. and R.G.S. wrote the manuscript. L.A. and F.M. prepared the tables and L.A. prepared the figure. All authors reviewed and agreed the final text.
Funding
The authors are grateful to the Hull IVF Charitable Trust for financial support for research costs.
Conflict of interest
The authors declare no conflict of interest regarding this work.
References
- Adamson G, de Mouzon J, Chambers G, Zegers-Hochschild F, Mansour R, Ishihara O, Banker M, Dyer S, Kupka M. World Report: ART 2016 (preliminary). International Committee for Monitoring Assisted Reproductive Technology; 2016. https://www.icmartivf.org/reports-publications/.
- Ahmadian S, Sheshpari S, Pazhang M, Bedate AM, Beheshti R, Abbasi MM, Nouri M, Rahbarghazi R, Mahdipour M.. Intra-ovarian injection of platelet-rich plasma into ovarian tissue promoted rejuvenation in the rat model of premature ovarian insufficiency and restored ovulation rate via angiogenesis modulation. Reprod Biol Endocrinol 2020;18:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amireault P, Dubé F.. Serotonin and its antidepressant-sensitive transport in mouse cumulus-oocyte complexes and early embryos. Biol Reprod 2005;73:358–365. [DOI] [PubMed] [Google Scholar]
- Anvari SS, Dehgan G, Razi M.. Preliminary findings of platelet-rich plasma-induced ameliorative effect on polycystic ovarian syndrome. Cell J 2019;21:243–252. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arici A.Interleukin-8 expression and modulation in human preovulatory follicles and ovarian cells. Endocrinology 1996;137:3762–3769. [DOI] [PubMed] [Google Scholar]
- Assoian RK, Komoriya A, Meyers CA, Miller DM, Sporn MB.. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization – PubMed. J Biol Chem 1983;258:7155–7160. [PubMed] [Google Scholar]
- Au AEL, Sashindranath M, Borg RJ, Kleifeld O, Andrews RK, Gardiner EE, Medcalf RL, Samson AL.. Activated platelets rescue apoptotic cells via paracrine activation of EGFR and DNA-dependent protein kinase. Cell Death Dis 2014;5:e1410–e1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakacak M, Bostanci MS, İnanc F, Yaylali A, Serin S, Attar R, Yildirim G, Yildirim OK.. Protective effect of platelet rich plasma on experimental ischemia/reperfusion injury in rat ovary. Gynecol Obstet Invest 2015;81:225–231. [DOI] [PubMed] [Google Scholar]
- Barboni B, Turriani M, Galeati G, Spinaci M, Bacci ML, Forni M, Mattioli M.. Vascular endothelial growth factor production in growing pig antral follicles. Biol Reprod 2000;63:858–864. [DOI] [PubMed] [Google Scholar]
- Ben-Ezra J, Sheibani K, Hwang DL, Lev-Ran A.. Megakaryocyte synthesis is the source of epidermal growth factor in human platelets – PubMed. Am J Pathol 1990;137:755–759. [PMC free article] [PubMed] [Google Scholar]
- Ben-Haroush A, Abir R, Ao A, Jin S, Kessler-Icekson G, Feldberg D, Fisch B.. Expression of basic fibroblast growth factor and its receptors in human ovarian follicles from adults and fetuses. Fertil Steril 2005;84:1257–1268. [DOI] [PubMed] [Google Scholar]
- Bender HR, Campbell GE, Aytoda P, Mathiesen AH, Duffy DM.. Thrombospondin 1 (THBS1) promotes follicular angiogenesis, luteinization, and ovulation in primates. Front Endocrinol (Lausanne) 2019;10:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bir SC, Esaki J, Marui A, Sakaguchi H, Kevil CG, Ikeda T, Komeda M, Tabata Y, Sakata R.. Therapeutic treatment with sustained-release platelet-rich plasma restores blood perfusion by augmenting ischemia-induced angiogenesis and arteriogenesis in diabetic mice. J Vasc Res 2011;48:195–205. [DOI] [PubMed] [Google Scholar]
- Bolton-Maggs PHB, Chalmers EA, Collins PW, Harrison P, Kitchen S, Liesner RJ, Minford A, Mumford AD, Parapia LA, Perry DJ.. et al. A review of inherited platelet disorders with guidelines for their management on behalf of the UKHCDO. Br J Haematol 2006;135:603–633. [DOI] [PubMed] [Google Scholar]
- Brenner B, Harney JT, Ahmed BA, Jeffus BC, Unal R, Mehta JL, Kilic F.. Plasma serotonin levels and the platelet serotonin transporter. J Neurochem 2007;102:206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu S, Cao C, Yang Y, Miao C, Hu Z, Cao Y, Sang Q, Duan E.. Localization and temporal regulation of tissue inhibitor of metalloproteinases-4 in mouse ovary. Reproduction 2006;131:1099–1107. [DOI] [PubMed] [Google Scholar]
- Cakiroglu Y, Saltik A, Yuceturk A, Karaosmanoglu O, Kopuk SY, Scott RT, Tiras B, Seli E.. Effects of intraovarian injection of autologous platelet rich plasma on ovarian reserve and IVF outcome parameters in women with primary ovarian insufficiency. Aging (Albany NY) 2020;12:10211–10222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callejo J, Salvador C, González-Nuñez S, Almeida L, Rodriguez L, Marqués L, Valls A, Lailla JM.. Live birth in a woman without ovaries after autograft of frozen-thawed ovarian tissue combined with growth factors. J Ovarian Res 2013;6:33–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmina E, Orio F, Palomba S, Longo RA, Lombardi G, Lobo RA.. Ovarian size and blood flow in women with polycystic ovary syndrome and their correlations with endocrine parameters. Fertil Steril 2005;84:413–419. [DOI] [PubMed] [Google Scholar]
- Chan CCW, Ng EHY, Li CF, Ho PC.. Impaired ovarian blood flow and reduced antral follicle count following laparoscopic salpingectomy for ectopic pregnancy. Hum Reprod 2003;18:2175–2180. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Feng Y, Jansson L, Sato Y, Deguchi M, Kawamura K, Hsueh AJ.. Actin polymerization-enhancing drugs promote ovarian follicle growth mediated by the Hippo signaling effector YAP. FASEB J 2015;29:2423–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Kawamura K, Deguchi M, Takae S, Mulders SM, Hsueh AJW.. Intraovarian thrombin and activated protein C signaling system regulates steroidogenesis during the periovulatory period. Mol Endocrinol 2012;26:331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutier N, Allaeys I, Marcoux G, Machlus KR, Mailhot B, Zufferey A, Levesque T, Becker Y, Tessandier N, Melki I.. et al. Platelets release pathogenic serotonin and return to circulation after immune complex-mediated sequestration. Proc Natl Acad Sci U S A 2018;115:E1550–E1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzolino M, Marin D, Sisti G.. New frontiers in IVF: MtDNA and autologous germline mitochondrial energy transfer. Reprod Biol Endocrinol 2019;17:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremonesi F, Bonfanti S, Idda A, Anna LC.. Improvement of embryo recovery in Holstein cows treated by intra-ovarian platelet rise plasma before superovulation. Vet Sci 2020;7:16. doi: 10.3390/vetsci7010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danforth DR, Arbogast LK, Ghosh S, Dickerman A, Rofagha R, Friedman CI.. Vascular endothelial growth factor stimulates preantral follicle growth in the rat ovary1. Biol Reprod 2003;68:1736–1741. [DOI] [PubMed] [Google Scholar]
- Demiray S, Yilmaz O, Goker ET, Tavmergen E, Calimlioglu N, Sezerman U, Soykam H, Oktem G.. Expression of the bone morphogenetic protein-2 (BMP2) in the human cumulus cells as a biomarker of oocytes and embryo quality. J Hum Reprod Sci 2017;10:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuel TF, Seniort RM, Chang D, Griffint GL, Heinriksont RL, Kaiser ET.. Platelet factor 4 is chemotactic for neutrophils and monocytes (inflammation/blood vessels/thrombosis/C5-derived chemotactic activity). Med Sci 1981;78: 4584–4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disdier M, Legrand C, Bouillot C, Dubernard V, Pidard D, Nurden AT.. Quantitation of platelet fibrinogen and thrombospondin in Glanzmann’s thrombasthenia by electroimmunoassay. Thromb Res 1989;53:521–533. [DOI] [PubMed] [Google Scholar]
- Dragovic RA, Ritter LJ, Schulz SJ, Amato F, Thompson JG, Armstrong DT, Gilchrist RB.. Oocyte-secreted factor activation of SMAD 2/3 signaling enables initiation of mouse cumulus cell expansion. Biol Reprod 2007;76:848–857. [DOI] [PubMed] [Google Scholar]
- Duncan WC, S Van Den D, Fraser HM.. Inhibition of vascular endothelial growth factor in the primate ovary up-regulates hypoxia-inducible factor-1α in the follicle and corpus luteum. Endocrinology 2008;149:3313–3320. [DOI] [PubMed] [Google Scholar]
- Evans A, Ibrahim M, Pope R, Mwangi J, Botros M, Johnson SP, Kassis SA.. Treating hand and foot osteoarthritis using a patient’s own blood: a systematic review and meta-analysis of platelet-rich plasma: PRP for small joint osteoarthritis. J Orthop 2020;18:226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farimani M, Heshmati S, Poorolajal J, Bahmanzadeh M.. A report on three live births in women with poor ovarian response following intra-ovarian injection of platelet-rich plasma (PRP). Mol Biol Rep 2019;46:1611–1616. [DOI] [PubMed] [Google Scholar]
- Feng Y, Cui P, Lu X, Hsueh B, Möller Billig F, Zarnescu Yanez L, Tomer R, Boerboom D, Carmeliet P, Deisseroth K.. et al. CLARITY reveals dynamics of ovarian follicular architecture and vasculature in three-dimensions. Sci Rep 2017;7:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field SL, Dasgupta T, Cummings M, Orsi NM.. Cytokines in ovarian folliculogenesis, oocyte maturation and luteinisation. Mol Reprod Dev 2014;81:284–314. [DOI] [PubMed] [Google Scholar]
- Geng JG, Raub TJ, Baker CA, Sawada GA, Ma L, Elhammer ÅP.. Expression of a P-selectin ligand in zona pellucida of porcine oocytes and P-selectin on acrosomal membrane of porcine sperm cells. Potential implications for their involvement in sperm-egg interactions. J Cell Biol 1997;137:743–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golebiewska EM, Poole AW.. Platelet secretion: from haemostasis to wound healing and beyond. Blood Rev 2015;29:153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Nicola D, Valle-Argos B, Pallas-Bazarra N, Nieto-Sampedro M. Interleukin-15 regulates proliferation and self-renewal of adult neural stem cells; 2011. http://www.molbiolcell.org/cgi/. [DOI] [PMC free article] [PubMed]
- Gordon JD, Mesiano S, Zaloudek CJ, Jaffe RB.. Vascular endothelial growth factor localization in human ovary and fallopian tubes: possible role in reproductive function and ovarian cyst formation. J Clin Endocrinol Metab 1996;81:353–359. [DOI] [PubMed] [Google Scholar]
- Greenaway J, Lawler J, Moorehead R, Bornstein P, Lamarre J, Petrik J.. Thrombospondin-1 inhibits VEGF levels in the ovary directly by binding and internalization via the low density lipoprotein receptor-related protein-1 (LRP-1). J Cell Physiol 2007;210:807–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J, Cristina Magli M, Lundin K, Barratt CLR, Brison D.. When and how should new technology be introduced into the IVF laboratory? Hum Reprod 2012;27:303–313. [DOI] [PubMed] [Google Scholar]
- Hart CE, Bailey M, Curtis DA, Osborn S, Raines E, Ross R, Forstrom JW.. Purification of PDGF-AB and PDGF-BB from human platelet extracts and identification of all three PDGF dimers in human platelets. Biochemistry 1990;29:166–172. [DOI] [PubMed] [Google Scholar]
- Heijnen H, van der Sluijs P.. Platelet secretory behaviour: as diverse as the granules … or not? J Thromb Haemost 2015;13:2141–2151. [DOI] [PubMed] [Google Scholar]
- Henriksen R, Dizeyi N, Abrahamsson P.. Expression of serotonin receptors 5-HT1A, 5-HT1B, 5-HT2B and 5-HT4 in ovary and in ovarian tumours – PubMed. Anticancer Res 2012;32:1361–1366. [PubMed] [Google Scholar]
- Holt JE, Jackson A, Roman SD, Aitken RJ, Koopman P, McLaughlin EA.. CXCR4/SDF1 interaction inhibits the primordial to primary follicle transition in the neonatal mouse ovary. Dev Biol 2006;293:449–460. [DOI] [PubMed] [Google Scholar]
- Horan CJ, Williams SA.. Oocyte stem cells: fact or fantasy? Reproduction 2017;154:R23–R35. [DOI] [PubMed] [Google Scholar]
- Hosseini L, Shirazi A, Naderi MM, Shams-Esfandabadi N, Borjian Boroujeni S, Sarvari A, Sadeghnia S, Behzadi B, Akhondi MM.. Platelet-rich plasma promotes the development of isolated human primordial and primary follicles to the preantral stage. Reprod Biomed Online 2017;35:343–350. [DOI] [PubMed] [Google Scholar]
- Hsu CC, Hsu L, Hsu I, Chiu YJ, Dorjee S.. Live birth in woman with premature ovarian insufficiency receiving ovarian administration of platelet-rich plasma (PRP) in combination with gonadotropin: a case report. Front Endocrinol (Lausanne) 2020;11:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Chou C, Chen M, Chen S, Ho H, Yang Y.. Platelet factor 4 – an antiangiogenic chemokine that is first identified to be possibly associated with the aberrant folliculogenesis in polycystic ovarian syndrome. Fertil Steril 2016;106:e251. [Google Scholar]
- Huang G, Zhou C, Wei C, Ju Zhao S, Sun F, Zhou H, Xu W, Liu J, Yang C, Wu L.. et al. Evaluation of in vitro fertilization outcomes using interleukin-8 in culture medium of human preimplantation embryos. Fertil Steril 2017;107:649–656. [DOI] [PubMed] [Google Scholar]
- Human Fertilisation & Embryology Authority. Fertility treatment 2018: trends and figures; 2020. https://www.hfea.gov.uk/about-us/publications/research-and-data/fertility-treatment-2018-trends-and-figures/.
- Huntington ND, Legrand N, Alves NL, Jaron B, Weijer K, Plet A, Corcuff E, Mortier E, Jacques Y, Spits H.. et al. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J Exp Med 2009;206:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley ET, Lim Fat D, Moran CJ, Mullett H.. The efficacy of platelet-rich plasma and platelet-rich fibrin in arthroscopic rotator cuff repair: a meta-analysis of randomized controlled trials. Am J Sports Med 2019;47:753–761. [DOI] [PubMed] [Google Scholar]
- Hussein TS, Froiland DA, Amato F, Thompson JG, Gilchrist RB.. Oocytes prevent cumulus cell apoptosis by maintaining a morphogenic paracrine gradient of bone morphogenetic proteins. J Cell Sci 2005;118:5257–5268. [DOI] [PubMed] [Google Scholar]
- Inwald DP, McDowall A, Peters MJ, Callard RE, Klein NJ.. CD40 is constitutively expressed on platelets and provides a novel mechanism for platelet activation. Circ Res 2003;92:1041–1048. [DOI] [PubMed] [Google Scholar]
- Italiano JE, Richardson JL, Patel-Hett S, Battinelli E, Zaslavsky A, Short S, Ryeom S, Folkman J, Klement GL.. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet α granules and differentially released. Blood 2008;111:1227–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe EA, Leung LLK, Nachman RL, Levin RI, Mosher DF.. Thrombospondin is the endogenous lectin of human platelets. Nature 1982;295:246–248. [DOI] [PubMed] [Google Scholar]
- Kakudo N, Morimoto N, Kushida S, Ogawa T, Kusumoto K.. Platelet-rich plasma releasate promotes angiogenesis in vitro and in vivo. Med Mol Morphol 2014;47:83–89. [DOI] [PubMed] [Google Scholar]
- Kalén A, Wahlström O, Linder CH, Magnusson P.. The content of bone morphogenetic proteins in platelets varies greatly between different platelet donors. Biochem Biophys Res Commun 2008;375:261–264. [DOI] [PubMed] [Google Scholar]
- Kõks S, Velthut A, Sarapik A, Altmäe S, Reinmaa E, Schalkwyk LC, Fernandes C, Lad HV, Soomets U, Jaakma Ü.. et al. The differential transcriptome and ontology profiles of floating and cumulus granulosa cells in stimulated human antral follicles. Mol Hum Reprod 2010;16:229–240. [DOI] [PubMed] [Google Scholar]
- Kouba S, Ouldamer L, Garcia C, Fontaine D, Chantome A, Vandier C, Goupille C, Potier-Cartereau M.. Lipid metabolism and calcium signaling in epithelial ovarian cancer. Cell Calcium 2019;81:38–50. [DOI] [PubMed] [Google Scholar]
- Kryczek I, Frydman N, Gaudin F, Krzysiek R, Fanchin R, Emilie D, Chouaib S, Zou W, Machelon V.. The chemokine SDF-1/CXCL12 contributes to T lymphocyte recruitment in human pre-ovulatory follicles and coordinates with lymphocytes to increase granulosa cell survival and embryo quality. Am J Reprod Immunol 2005;54:270–283. [DOI] [PubMed] [Google Scholar]
- Labarta E, de los Santos MJ, Escribá MJ, Pellicer A, Herraiz S.. Mitochondria as a tool for oocyte rejuvenation. Fertil Steril 2019;111:219–226. [DOI] [PubMed] [Google Scholar]
- Lebbi I, Ben TR, Fadhlaoui A, Feki A.. Ovarian drilling in PCOS: Is it really useful? Front Surg 2015;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledee N, Lombroso R, Lombardelli L, Selva J, Dubanchet S, Chaouat G, Frankenne F, Foidart JM, Maggi E, Romagnani S.. et al. Cytokines and chemokines in follicular fluids and potential of the corresponding embryo: the role of granulocyte colony-stimulating factor. Hum Reprod 2008;23:2001–2009. [DOI] [PubMed] [Google Scholar]
- Lee J, Kim Y, Lim J, Kim M, Han K.. G-CSF and GM-CSF concentrations and receptor expression in peripheral blood leukemic cells from patients with chronic myelogenous leukemia – PubMed. Ann Clin Lab Sci 2008;38:331–337. [PubMed] [Google Scholar]
- Li Z, Delaney MK, O'Brien KA, Du X.. Signaling during platelet adhesion and activation. ATVB 2010;30:2341–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Vilchez I, Diaz-Ricart M, White JG, Escolar G, Galan AM.. Serotonin enhances platelet procoagulant properties and their activation induced during platelet tissue factor uptake. Eur Soc Cardiol 2009;84:309–316. [DOI] [PubMed] [Google Scholar]
- Luke B, Brown MB, Wantman E, Forestieri NE, Browne ML, Fisher SC, Yazdy MM, Ethen MK, Canfield MA, Watkins S.. The risk of birth defects with conception by ART. Hum Reprod 2020;36:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaluso M, Wright-Schnapp TJ, Chandra A, Johnson R, Satterwhite CL, Pulver A, Berman SM, Wang RY, Farr SL, Pollack LA.. A public health focus on infertility prevention, detection, and management. Fertil Steril 2010;93:16.e1–16.e10. [DOI] [PubMed] [Google Scholar]
- Machlus KR, Johnson KE, Kulenthirarajan R, Forward JA, Tippy MD, Soussou TS, El-Husayni SH, Wu SK, Wang S, Watnick RS.. et al. CCL5 derived from platelets increases megakaryocyte proplatelet formation. Blood 2016;127:921–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massberg S, Konrad I, Schürzinger K, Lorenz M, Schneider S, Zohlnhoefer D, Hoppe K, Schiemann M, Kennerknecht E, Sauer S.. et al. Platelets secrete stromal cell-derived factor 1α and recruit bone marrow-derived progenitor cells to arterial thrombi in vivo. J Exp Med 2006;203:1221–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo P, Navarro C, Jones C, Coward K, Coleman L.. The use of autologous platelet-rich plasma (PRP) versus no intervention in women with low ovarian reserve undergoing fertility treatment: a non-randomized interventional study. J Assist Reprod Genet 2020;37:855–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merten M, Thiagarajan P.. P-selectin expression on platelets determines size and stability of platelet aggregates. Circulation 2000;102:1931–1936. [DOI] [PubMed] [Google Scholar]
- Meyer A, Wang W, Qu J, Croft L, Degen JL, Coller BS, Ahamed J.. Platelet TGF-β1 contributions to plasma TGF-β1, cardiac fibrosis, and systolic dysfunction in a mouse model of pressure overload. Blood 2012;119:1064–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Miguel-Gómez L, López-Martínez S, Campo H, Francés-Herrero E, Faus A, Díaz A, Pellicer A, Domínguez F, Cervelló I.. Comparison of different sources of platelet-rich plasma as treatment option for infertility-causing endometrial pathologies. Fertil Steril 2020;115:490–500. [DOI] [PubMed] [Google Scholar]
- Niikura Y, Niikura T, Tilly JL.. Aged mouse ovaries possess rare premeiotic germ cells that can generate oocytes following transplantation into a young host environment. Aging (Albany NY) 2009;1:971–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson E, Parrott JA, Skinner MK.. Basic fibroblast growth factor induces primordial follicle development and initiates folliculogenesis. Mol Cell Endocrinol 2001;175:123–130. [DOI] [PubMed] [Google Scholar]
- Nilsson EE, Detzel C, Skinner MK.. Platelet-derived growth factor modulates the primordial to primary follicle transition. Reproduction 2006;131:1007–1015. [DOI] [PubMed] [Google Scholar]
- Nishigaki A, Okada H, Okamoto R, Sugiyama S, Miyazaki K, Yasuda K, Kanzaki H.. Concentrations of stromal cell-derived factor-1 and vascular endothelial growth factor in relation to the diameter of human follicles. Fertil Steril 2011;95:742–746. [DOI] [PubMed] [Google Scholar]
- Office for National Statistics. Birth characteristics in England and Wales: 2019; 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/bulletins/birthcharacteristicsinenglandandwales/2019#age-of-parents.
- Ono Y, Kurano M, Ohkawa R, Yokota H, Igarashi K, Aoki J, Tozuka M, Yatomi Y.. Sphingosine 1-phosphate release from platelets during clot formation: close correlation between platelet count and serum sphingosine 1-phosphate concentration. Lipids Health Dis 2013;12:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orisaka M, Mizutani T, Tajima K, Orisaka S, Shukunami KI, Miyamoto K, Kotsuji F.. Effects of ovarian theca cells on granulosa cell differentiation during gonadotropin-independent follicular growth in cattle. Mol Reprod Dev 2006;73:737–744. [DOI] [PubMed] [Google Scholar]
- Von Otte S, Paletta JRJ, Becker S, König S, Fobker M, Greb RR, Kiesel L, Assmann G, Diedrich K, Nofer JR.. Follicular fluid high density lipoprotein-associated sphingosine 1-phosphate is a novel mediator of ovarian angiogenesis. J Biol Chem 2006;281:5398–5405. [DOI] [PubMed] [Google Scholar]
- Panda SR, Sachan S, Hota S.. A systematic review evaluating the efficacy of intra-ovarian infusion of autologous platelet-rich plasma in patients with poor ovarian reserve or ovarian insufficiency. Cureus 2020;12:e12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantos K, Nitsos N, Kokkali G, Vaxevanoglou T, Markomichali C, Pantou A, Grammatis M, Lazaros L, Sfakianoudis K.. P-401 ovarian rejuvenation and folliculogenesis reactivation in peri-menopausal women after autologous platelet-rich plasma treatment. Hum Reprod 2016;31:310–311. [Google Scholar]
- Pantos K, Simopoulou M, Pantou A, Rapani A, Tsioulou P, Nitsos N, Syrkos S, Pappas A, Koutsilieris M, Sfakianoudis KA.. Case series on natural conceptions resulting in ongoing pregnancies in menopausal and prematurely menopausal women following platelet-rich plasma treatment. Cell Transplant 2019;28:1333–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons MEM, Szklanna PB, Guerrero JA, Wynne K, Dervin F, O'Connell K, Allen S, Egan K, Bennett C, McGuigan C.. et al. Platelet releasate proteome profiling reveals a core set of proteins with low variance between healthy adults. Proteomics 2018;18:1870131. [DOI] [PubMed] [Google Scholar]
- Pascuali N, Scotti L, Abramovich D, Irusta G, Di PM, Bas D, Tesone M, Parborell F.. Inhibition of platelet-derived growth factor (PDGF) receptor affects follicular development and ovarian proliferation, apoptosis and angiogenesis in prepubertal eCG-treated rats. Mol Cell Endocrinol 2015;412:148–158. [DOI] [PubMed] [Google Scholar]
- Pauli SA, Tang H, Wang J, Bohlen P, Posser R, Hartman T, Sauer MV, Kitajewski J, Zimmermann RC.. The vascular endothelial growth factor (VEGF)/VEGF receptor 2 pathway is critical for blood vessel survival in corpora lutea of pregnancy in the rodent. Endocrinology 2005;146:1301–1311. [DOI] [PubMed] [Google Scholar]
- Peitsidis P, Agrawal R.. Role of vascular endothelial growth factor in women with PCO and PCOS: a systematic review. Reprod Biomed Online 2010;20:444–452. [DOI] [PubMed] [Google Scholar]
- Peralta OA, Bucher D, Fernandez A, Berland M, Strobel P, Ramirez A, Ratto MH, Concha I.. Granulocyte-macrophage colony stimulating factor (GM-CSF) enhances cumulus cell expansion in bovine oocytes. Reprod Biol Endocrinol 2013;11:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervushina O, Scheuerer B, Reiling N, Behnke L, Schröder J-M, Kasper B, Brandt E, Bulfone-Paus S, Petersen F.. Platelet factor 4/CXCL4 induces phagocytosis and the generation of reactive oxygen metabolites in mononuclear phagocytes independently of Gi protein activation or intracellular calcium transients. J Immunol 2004;173:2060–2067. [DOI] [PubMed] [Google Scholar]
- Piersma SR, Broxterman HJ, Kapci M, Rr de H, Hoekman K, Verheul HMW, Jiménez CR.. Proteomics of the TRAP-induced platelet releasate. J Proteomics 2009;72:91–109. [DOI] [PubMed] [Google Scholar]
- Pinkas H, Fisch B, Rozansky G, Felz C, Kessler-Icekson G, Krissi H, Nitke S, Ao A, Abir R.. Platelet-derived growth factors (PDGF-A and -B) and their receptors in human fetal and adult ovaries. Mol Hum Reprod 2008;14:199–206. [DOI] [PubMed] [Google Scholar]
- Pintucci G, Froum S, Pinnell J, Mignatti P, Rafii S, Green D.. Trophic effects of platelets on cultured endothelial cells are mediated by platelet-associated fibroblast growth factor-2 (FGF-2) and vascular endothelial growth factor (VEGF). Thromb Haemost 2002;88:834–842. [PubMed] [Google Scholar]
- Pors S, Hardardottir L, Olesen H, Riis M, Jensen L, Andersen A, Cadenas J, Gronning A, Colmorn L, Dueholm M.. et al. Effect of sphingosine-1-phosphate on activation of dormant follicles in murine and human ovarian tissue. Mol Hum Reprod 2020;26:301–311. [DOI] [PubMed] [Google Scholar]
- Radomski A, Jurasz P, Sanders EJ, Overall CM, Bigg HF, Edwards DR, Radomski MW.. Identification, regulation and role of tissue inhibitor of metalloproteinases-4 (TIMP-4) in human platelets. Br J Pharmacol 2002;137:1330–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raidem S, Schnettini J, Salamone G, Trevani A, Vermeulen M, Gamberale R, Giordano M, Geffner J.. Human platelets produce granulocyte-macrophage colony-stimulating factor and delay eosinophil apoptosis. Lab Invest 2003;83:589–598. [DOI] [PubMed] [Google Scholar]
- Reizel Y, Elbaz J, Dekel N.. Sustained activity of the EGF receptor is an absolute requisite for LH-induced oocyte maturation and cumulus expansion. Mol Endocrinol 2010;24:402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendu F, Brohard-Bohn B.. The platelet release reaction: granules’ constituents, secretion and functions. Platelets 2001;12:261–273. [DOI] [PubMed] [Google Scholar]
- Rival CM, Xu W, Shankman LS, Morioka S, Arandjelovic S, Lee CS, Wheeler KM, Smith RP, Haney LB, Isakson BE.. et al. Phosphatidylserine on viable sperm and phagocytic machinery in oocytes regulate mammalian fertilization. Nat Commun 2019;10:4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh YH, Kim W, Park KU, Oh JH.. Cytokine-release kinetics of platelet-rich plasma according to various activation protocols. Bone Joint Res 2016;5:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully D, Naseem KM, Matsakas A.. Platelet biology in regenerative medicine of skeletal muscle. Acta Physiol 2018;223:e13071. [DOI] [PubMed] [Google Scholar]
- Scully D, Sfyri P, Verpoorten S, Papadopoulos P, Muñoz-Turrillas MC, Mitchell R, Aburima A, Patel K, Gutiérrez L, Naseem KM.. et al. Platelet releasate promotes skeletal myogenesis by increasing muscle stem cell commitment to differentiation and accelerates muscle regeneration following acute injury. Acta Physiol 2019;225:e13207. [DOI] [PubMed] [Google Scholar]
- Scully D, Sfyri P, Wilkinson HN, Acebes-Huerta A, Verpoorten S, Muñoz-Turrillas MC, Parnell A, Patel K, Hardman MJ, Gutiérrez L.. et al. Optimising platelet secretomes to deliver robust tissue-specific regeneration. J Tissue Eng Regen Med 2020;14:82–98. [DOI] [PubMed] [Google Scholar]
- Sfakianoudis K, Rapani A, Grigoriadis S, Retsina D, Maziotis E, Tsioulou P, Giannelou P, Pantos K, Koutsilieris M, Vlahos N.. et al. Novel approaches in addressing ovarian insufficiency in 2019: Are we there yet? Cell Transplant 2020a;29:1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfakianoudis K, Simopoulou M, Grigoriadis S, Pantou A, Tsioulou P, Maziotis E, Rapani A, Giannelou P, Nitsos N, Kokkali G.. et al. Reactivating ovarian function through autologous platelet-rich plasma intraovarian infusion: pilot data on premature ovarian insufficiency, perimenopausal, menopausal, and poor responder women. JCM 2020b;9:1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfakianoudis K, Simopoulou M, Nitsos N, Rapani A, Pappas A, Pantou A, Chronopoulou M, Deligeoroglou E, Koutsilieris M, Pantos K.. Autologous platelet-rich plasma treatment enables pregnancy for a woman in premature menopause. JCM 2018;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Sampietro S, Wu J, Tang J, Gupta S, Matzko CN, Tang C, Yu Y, Brass LF, Zhu L.. et al. Coordination of platelet agonist signaling during the hemostatic response in vivo. Blood Adv 2017;1:2767–2775. [ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shweiki D, Itin A, Neufeld G, Gitay-Goren H, Keshet E.. Patterns of expression of vascular endothelial growth factor (VEGF) and VEGF receptors in mice suggest a role in hormonally regulated angiogenesis. J Clin Invest 1993;91:2235–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sills ES, Rickers NS, Li X, Palermo GD.. First data on in vitro fertilization and blastocyst formation after intraovarian injection of calcium gluconate-activated autologous platelet rich plasma. Gynecol Endocrinol 2018;34:756–760. [DOI] [PubMed] [Google Scholar]
- Spanou S, Kalogiannis D, Zapanti E, Gazouli M, Sfontouris IA, Siristatidis C, Mastorakos G.. Interleukin 15 concentrations in follicular fluid and their effect on oocyte maturation in subfertile women undergoing intracytoplasmic sperm injection. J Assist Reprod Genet 2018;35:1019–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe PC, Edwards RG.. Birth after reimplantation of a human embryo. Lancet 1978;312:366. [DOI] [PubMed] [Google Scholar]
- Suthar M, Gupta S, Bukhari S, Ponemone V.. Treatment of chronic non-healing ulcers using autologous platelet rich plasma: a case series. J Biomed Sci 2017;24:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly JL, Niikura Y, Rueda BR.. The current status of evidence for and against postnatal oogenesis in mammals: a case of ovarian optimism versus pessimism? Biol Reprod 2009;80:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly JL, Telfer EE.. Purification of germline stem cells from adult mammalian ovaries: a step closer towards control of the female biological clock? Mol Hum Reprod 2009;15:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urtz N, Gaertner F, von Bruehl M-L, Chandraratne S, Rahimi F, Zhang L, Orban M, Barocke V, Beil J, Schubert I.. et al. Sphingosine 1-phosphate produced by sphingosine kinase 2 intrinsically controls platelet aggregation in vitro and in vivo. Circ Res 2015;117:376–387. [DOI] [PubMed] [Google Scholar]
- Valeri CR, Saleem B, Ragno G.. Release of platelet-derived growth factors and proliferation of fibroblasts in the releasates from platelets stored in the liquid state at 22°C after stimulation with agonists. Transfusion 2006;46:225–229. [DOI] [PubMed] [Google Scholar]
- Verma R, Negi G, Kandwal A, Chandra H, Gaur DS, Harsh M.. Effect of autologous PRP on wound healing in dental regenerative surgeries and its correlation with PDGF levels. Asian J Transfus Sci 2019;13:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virant-Klun I, Zech N, Rzǒman P, Vogler A, Cvjetičanin B, Klemenc P, Maličev E, Meden-Vrtovec H.. Putative stem cells with an embryonic character isolated from the ovarian surface epithelium of women with no naturally present follicles and oocytes. Differentiation 2008;76:843–856. [DOI] [PubMed] [Google Scholar]
- Wagner M, Yoshihara M, Douagi I, Damdimopoulos A, Panula S, Petropoulos S, Lu H, Pettersson K, Palm K, Katayama S.. et al. Single-cell analysis of human ovarian cortex identifies distinct cell populations but no oogonial stem cells. Nat Commun 2020;11:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White YAR, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL.. Ovaries of reproductive age women. Nat Med 2012;18:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wraith KS, Magwenzi S, Aburima A, Wen Y, Leake D, Naseem KM.. Oxidized low-density lipoproteins induce rapid platelet activation and shape change through tyrosine kinase and Rho kinase-signaling pathways. Blood 2013;122:580–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynendaele W, Derua R, Hoylaerts MF, Pawinski A, Waelkens E, De Bruijn EA, Paridaens R, Merlevede W, Van Oosterom AT.. Vascular endothelial growth factor measured in platelet poor plasma allows optimal separation between cancer patients and volunteers: a key to study an angiogenic marker in vivo? Ann Oncol 1999;10:965–971. [DOI] [PubMed] [Google Scholar]
- Xu J, Gou L, Zhang P, Li H, Qiu S.. Platelet-rich plasma and regenerative dentistry. Aust Dent J 2020;65:131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh C-H, Chen P-C, Chen C-H, Hsu C-F, Huang R-L, Ding D-C, Chu T-Y.. Platelet-derived growth factor in the ovarian follicle attracts the stromal cells of the fallopian tube fimbriae. PLoS One 2016;11:e0158266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaslavsky A, Baek KH, Lynch RC, Short S, Grillo J, Folkman J, Italiano JE, Ryeom S.. Platelet-derived thrombospondin-1 is a critical negative regulator and potential biomarker of angiogenesis. Blood 2010;115:4605–4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegers-Hochschild F, Mansour R, Ishihara O, Adamson GD, De Mouzon J, Nygren KG, Sullivan EA.. International committee for monitoring assisted reproductive technology: world report on assisted reproductive technology, 2005. Fertil Steril 2014;101:366–378. [DOI] [PubMed] [Google Scholar]
- Zhang J, Muri J, Fitzgerald G, Gorski T, Gianni-Barrera R, Masschelein E, D’Hulst G, Gilardoni P, Turiel G, Fan Z.. et al. Endothelial lactate controls muscle regeneration from ischemia by inducing M2-like macrophage polarization. Cell Metab 2020;31:1136–1153. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou K, Yuan Z, Yang Z, Luo H, Sun K, Zhou L, Xiang J, Shi L, Yu Q, Zhang Y.. et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol 2009;11:631–636. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.