Abstract
Background and Purpose-
The incidences of intracranial aneurysm and aneurysmal subarachnoid hemorrhage are high in post-menopausal women. While population-based studies suggest that hormone replacement therapy is beneficial for post-menopausal women with intracranial aneurysms, estrogen replacement may no longer be recommended for the prevention of chronic diseases given its association with adverse outcomes, such as cancer and ischemic stroke. The isoflavone daidzein and its intestinal metabolite equol are bioactive phytoestrogens and potent agonists of estrogen receptors. Given their estrogenic properties, we investigated whether the isoflavones daidzein and equol are protective against the formation and rupture of intracranial aneurysms in a mouse model of the post-menopausal state.
Methods-
We induced intracranial aneurysms in ovariectomized adult female mice using a combination of induced systemic hypertension and a single injection of elastase into the cerebrospinal fluid. We fed the mice with an isoflavone-free diet with/without daidzein supplementation, or in a combination of intraperitoneal equol, and/or oral vancomycin treatment. We also used estrogen receptor beta knockout mice.
Results-
Both dietary daidzein and supplementation with its metabolite, equol, were protective against aneurysm formation in ovariectomized mice. The protective effects of daidzein and equol required estrogen-receptor-β. The disruption of the intestinal microbial conversion of daidzein to equol abolished daidzein’s protective effect against aneurysm formation. Mice treated with equol had lower inflammatory cytokines in the cerebral arteries, suggesting that phytoestrogens modulate inflammatory processes important to intracranial aneurysm pathogenesis.
Conclusions-
Our study establishes that both dietary daidzein and its metabolite, equol, protect against aneurysm formation in ovariectomized female mice through the activation of estrogen-receptor-β and subsequent suppression of inflammation. Dietary daidzein’s protective effect required the intestinal conversion to equol. Our results indicate the potential therapeutic value of dietary daidzein and its metabolite, equol, for the prevention of the formation of intracranial aneurysms and related subarachnoid hemorrhage.
Keywords: Intracranial aneurysm, subarachnoid hemorrhage, animal model
Subject terms: Cerebral aneurysm, cerebrovascular disease/stroke
Graphical Abstract
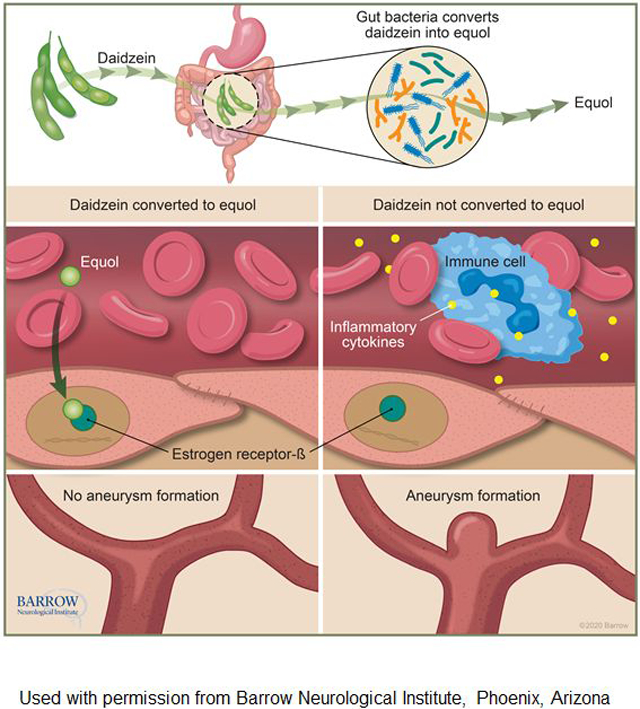
Introduction
The pathogenesis of intracranial aneurysms is poorly understood. The incidences of intracranial aneurysm and aneurysmal subarachnoid hemorrhage (SAH) are high in post-menopausal women, suggesting estrogen may be protective against aneurysm formation or rupture.1–4 Moreover, population-based studies suggest an association between estrogen-containing hormone replacement therapy and a reduced incidence of aneurysmal SAH.5–9 In animal models of intracranial aneurysms, estrogen has been shown to prevent aneurysm formation and rupture, primarily through activation of estrogen receptor-β (ER-β) and modulation of inflammatory processes.10–12 While these findings suggest the potentially protective effect of estrogen hormone replacement therapy against the formation and rupture of intracranial aneurysm in post-menopausal women, hormone replacement therapy is associated with an increased risk of other significant adverse outcomes, including cancer and ischemic stroke.13–16 Therefore, hormone replacement therapy is contraindicated for the prevention of chronic diseases.17
Isoflavones are plant-based, diet-derived compounds that structurally resemble estradiol and exert estrogenic activities with tissue and receptor specificity.18–20 Regular consumption of isoflavones has been shown to alleviate the vasomotor symptoms of estrogen-deficiency and associated with the reduced incidence of estrogen-dependent diseases in post-menopausal women.21–23 Daidzein is one of the common isoflavones in diets – found in legumes, peas, and beans – and is a potent phytoestrogen that acts as an estrogen receptor (ER) agonist.20, 24, 25 Daidzein is converted to equol by gut microbiota.20, 26 After being absorbed from the gut, equol exerts estrogenic activity on various tissues. Equol is more bioactive and estrogenic than its precursor daidzein.25 More importantly, equol preferentially binds to ER-β,20 a receptor-subtype responsible for the protective effects of estrogen against the formation and rupture of intracranial aneurysm in ovariectomized female mice, a model of the post-menopausal state.
Given their estrogenic properties, we hypothesized that phytoestrogens may prevent intracranial aneurysm formation and SAH through activation of ER-β and modulation of inflammatory processes in post-menopausal women who are at increased risk for aneurysmal formation and rupture. As a first step, we investigated whether the phytoestrogens daidzein and equol are protective against the formation and rupture of intracranial aneurysms in ovariectomized female mice.
Methods
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Mouse model of intracranial aneurysm
Experiments were conducted following guidelines approved by the Institutional Animal Care and Use Committee. Details of the intracranial aneurysm mouse model were previously described.11, 27–31 We used 8–10-week-old C57BL/6J and ER-β knockout mice (Jackson Laboratory, Bar Harbor, Maine). All mice used in the experiments are summarized in Table I (please see https://www.ahajournals.org/journal/str).
Intracranial aneurysms were induced by combining systemic hypertension and a single injection of elastase (35.0 milli-units) into the cerebrospinal fluid at the right basal cistern.11, 27–31 To induce systemic hypertension, we used the deoxycorticosterone acetate-salt hypertension method.11, 28–31 Bilateral ovariectomy and left nephrectomy were performed three weeks before elastase injection.
Drug treatment and dietary supplement
We replaced standard chow with an isoflavone-free diet (AIN-93G, no soybean oil, D10012G, Research Diets Inc.) at the same time as ovariectomy/nephrectomy. Systemic treatment with equol (0.5 mg/kg/day, in 20% dimethyl sulfoxide in polyethylene glycol-300) was delivered via an implanted mini-osmotic pump (Model 1004, Alzet) for four weeks starting one week before elastase injection. For the vehicle-treated group, mini-osmotic pumps were filled with 20% dimethyl sulfoxide in polyethylene glycol-300. In dietary daidzein treatment experiments, the isoflavone-free diet was supplemented with daidzein (0.1%) for four weeks starting one week before elastase injection. Oral vancomycin (50 mg/kg/day) started at the same time as dietary daidzein was used to block the conversion of daidzein to equol by the gut microbiota following the protocol established by Blair et al.32
Evaluation of aneurysm formation and rupture
Two observers who were blinded to the treatments performed neurological examinations daily to detect aneurysmal rupture using a neurological scoring system as previously described.10, 28, 30, 31 Mice were euthanized when they developed neurological symptoms (score 1–5, for details, see Supplemental Material). Asymptomatic mice were euthanized 21 days after aneurysm induction as previously described.10, 28, 30, 31
Plasma equol measurement
We used a Sciex (Foster City, CA) QTRAP 6500+ LC–MS/MS system, which consists of the Sciex Exion ultra-high performance liquid chromatography coupled with a hybrid triple quadrupole/linear ion trap mass spectrometer (please see https://www.ahajournals.org/journal/str).
Real-time PCR detection of cytokines
We collected total RNA samples from cerebral arteries (Circle of Willis and its major branches) 3 or 5 days after aneurysm induction, as previously described.31, 33 We measured mRNA expression levels of inflammation-related cytokines (IL-1β [interleukin-1β], IL-6 [interleukin-6], MCP-1 [monocyte chemoattractant protein-1], MMP-9 [Matrix metallopeptidase 9], and TNF-α [tumor necrosis factor-α]) (please see https://www.ahajournals.org/journal/str).
Statistical analysis
Fisher’s exact test was used to analyze the incidences of aneurysm formation and subarachnoid hemorrhage. The survival rate was evaluated by Log-rank (Mantel-Cox) test. We used multiple t-test with post-hoc Holm-Sidak method for the analysis of real-time PCR data. P-values < 0.05 were considered statistically significant. Data are expressed as means ± standard deviation.
Results
Equol reduced the formation of aneurysms in ovariectomized female mice.
Figure 1 shows representative unruptured and ruptured aneurysms from the mouse model used in the current study. To assess the potential role of phytoestrogens in the pathophysiology of intracranial aneurysms, we tested the effect of equol, the intestinal metabolite of daidzein, on the formation and rupture of intracranial aneurysm. Three weeks prior to aneurysm induction, female mice underwent bilateral ovariectomy and were started on an isoflavone-free diet. Systemic treatment with equol (0.5 mg/kg/day) or vehicle began one week before aneurysm induction and was continued for four weeks (Figure 2A).
Figure 1.
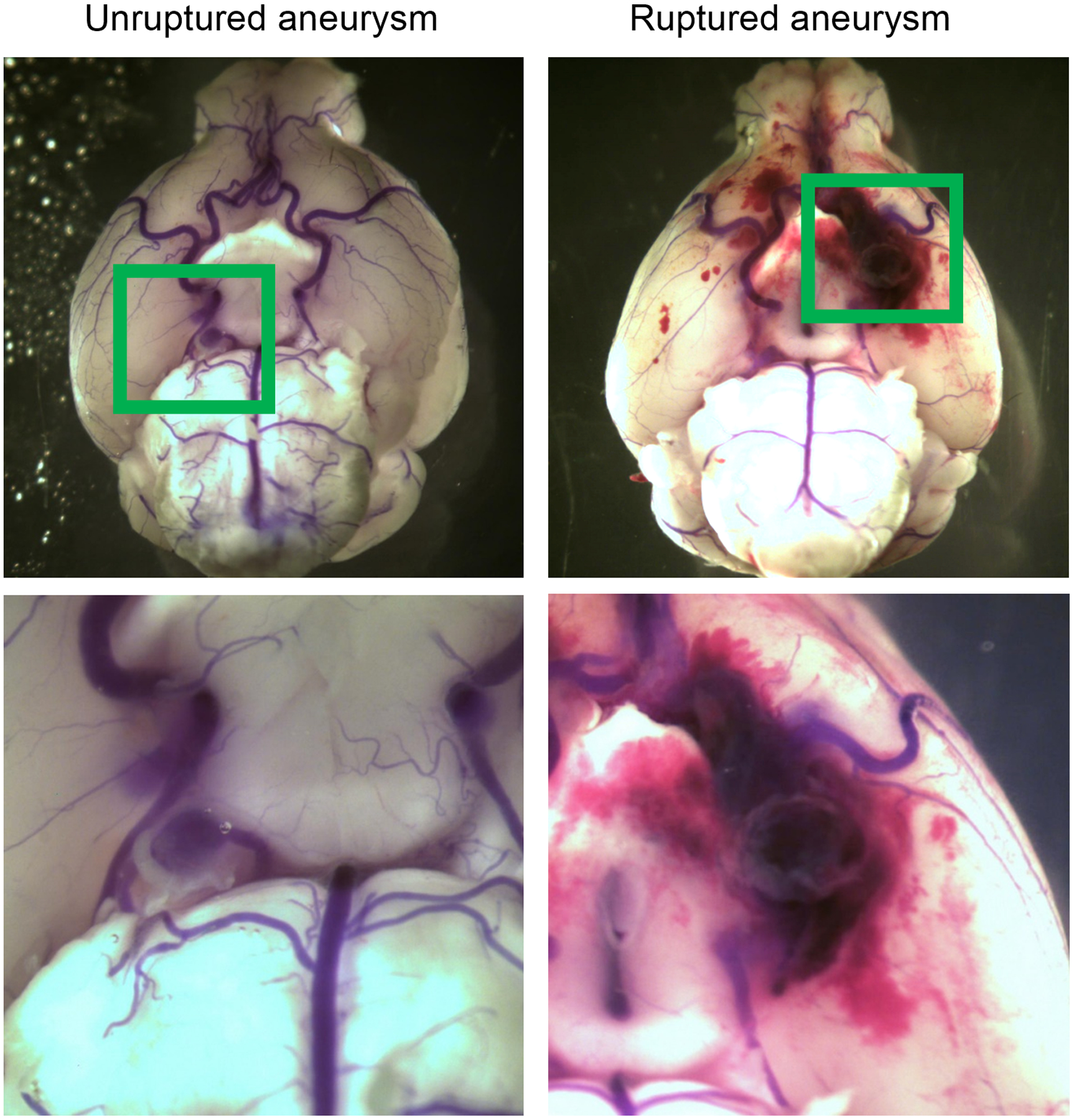
Representative images of unruptured and ruptured aneurysms.
Figure 2. Equol reduced the formation of intracranial aneurysms in ovariectomized female mice.
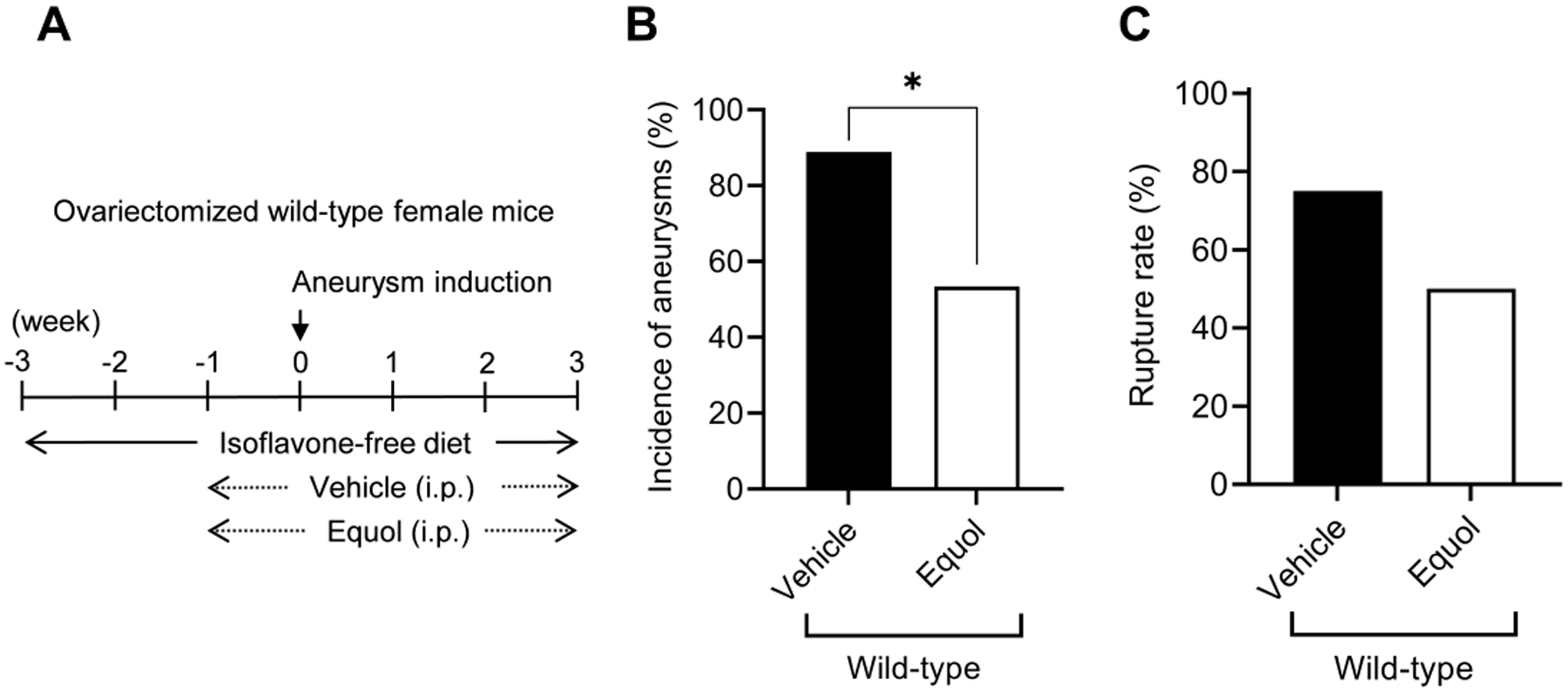
A. Schematic diagram of experimental protocols for ovariectomized wild-type female mice. B and C, equol treatment significantly reduced the incidence of aneurysms (B, * P < 0.05).
Equol treatment significantly reduced the incidence of aneurysm formation compared to vehicle (vehicle vs. equol; 89% vs. 53%; n = 16/18 vs. n = 8/15. P < 0.05) (Figure 2B). There was a trend for equol-treated mice to have lower incidence of aneurysmal rupture than vehicle-treated mice (vehicle vs. equol; 75% vs. 50%; n = 12/16 vs. n = 4/8. P = 0.2) (Figure 2C). There was no difference in the blood pressure between the two groups (Figure I, please see https://www.ahajournals.org/journal/str).
Equol’s protective effect against aneurysm formation required estrogen receptor-β.
We previously found that ER-β activation is necessary for estrogen-mediated prevention of intracranial aneurysm formation in ovariectomized female mice.10 To determine if equol’s protective effect also requires ER-β, we treated ovariectomized ER-β knockout mice with equol and assessed for incidence of aneurysm formation and rupture (Figure 3A).
Figure 3. Equol’s protective effect against aneurysm formation required estrogen receptor-β.
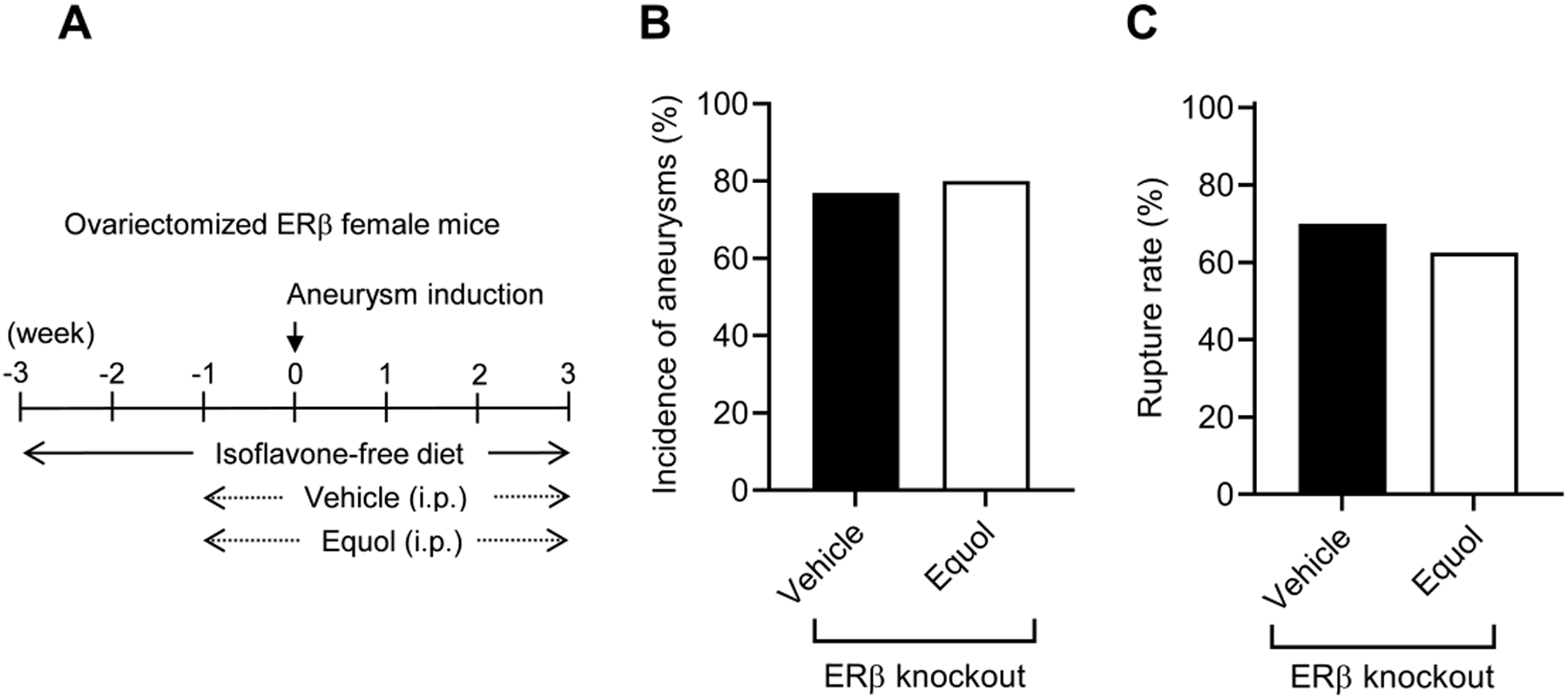
A. Schematic diagram of experimental protocols for ovariectomized ER-β knockout female mice. B and C: The protective effect of equol was lost in ovariectomized ER-β knockout female mice.
Unlike our results for ovariectomized wild-type mice, we found no significant difference in the incidence of aneurysm formation between vehicle and equol-treated groups in ovariectomized ER-β knockout mice (vehicle vs. equol; 77% vs. 80%; n = 10/13 vs. n = 8/10. P = 1, Figure 3B). Likewise, there was no difference in the incidence of rupture between vehicle and equol-treated groups (vehicle vs. equol; 70% vs. 63%; n = 7/10 vs. 5/8. P = 0.4, Figure 3C). Taken together, these data demonstrate that equol’s protective effect against aneurysm formation requires ER-β activation. There was no difference in the blood pressure between the groups at any time point (Figure I).
Equol decreased mRNA expression of pro-inflammatory cytokines in cerebral arteries.
We previously found that the anti-inflammatory effects of ER-β activation mediate estrogen’s protection against aneurysm formation.10 Therefore, we investigated whether systemic treatment reduces the expression of anti-inflammatory cytokines in cerebral arteries.
There was a trend for IL-1β and IL-6 levels 3 days after aneurysm induction in the equol-treated mice to be lower than those in controls (upper panel, Figure 4). At 5 days of post-aneurysm induction, mRNA levels of IL-6 were significantly lower in equol-treated mice compared to vehicle-treated mice (adjusted P-value, P < 0.05, multiple t-test). There was a trend for reduced IL-1β (P = 0.17) and TNFα (P = 0.15) levels in the equol-treated mice compared to vehicle-treated mice (lower panel, Figure 4).
Figure 4. Equol decreased mRNA expression of pro-inflammatory cytokines in ovariectomized female mice.
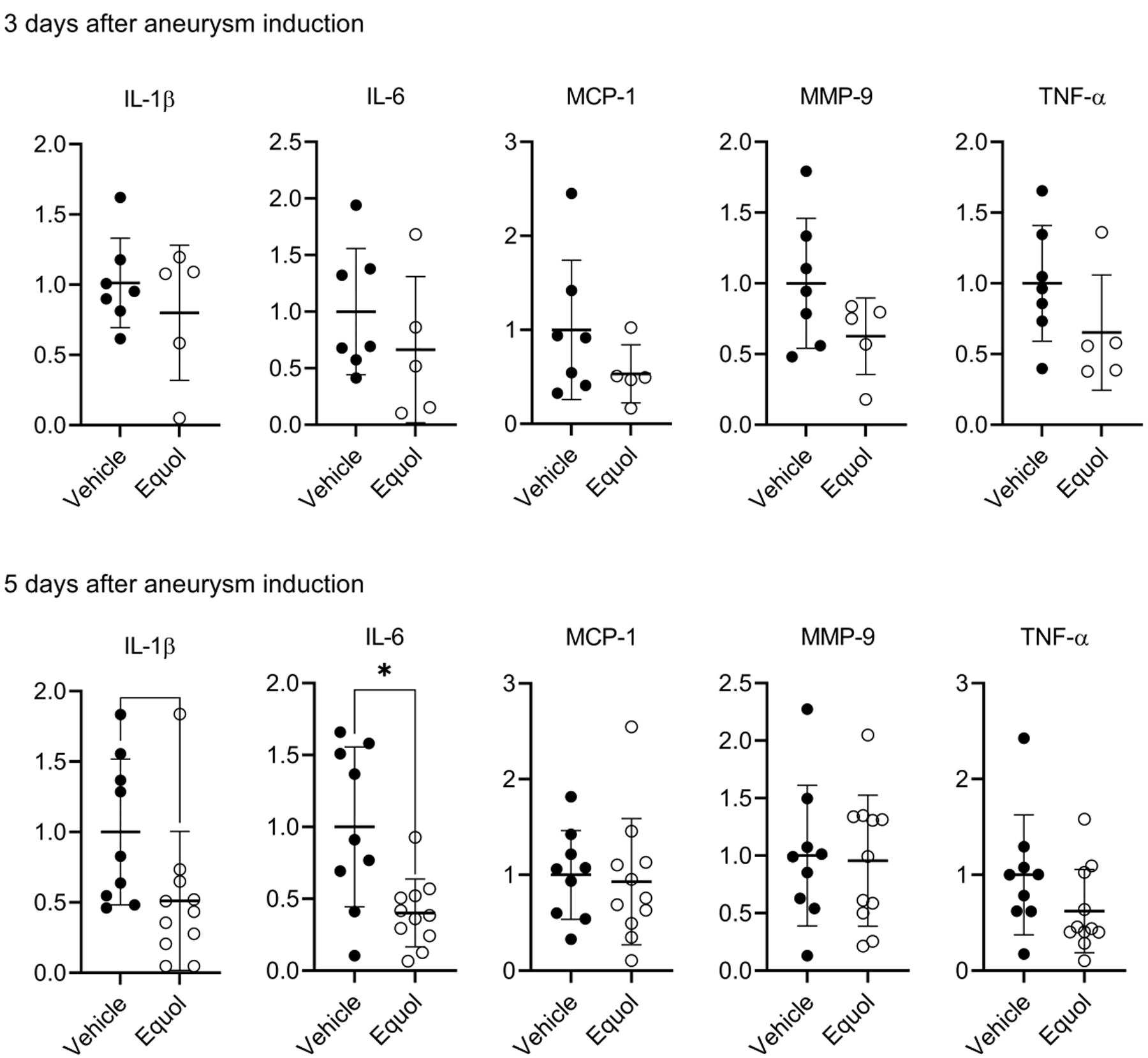
There was a trend of reduction in IL-1β and IL-6 expression levels 3 days after aneurysm induction in the equol-treated mice relative to controls (upper panel). At 5 days of post-aneurysm induction, mRNA level of IL-6 was significantly lower in equol-treated mice compared to vehicle (lower panel, * P < 0.05). There was a tendency for reduced IL-1β and TNFα levels in the equol-treated mice compared to vehicle-treated animals, though this difference did not achieve statistical significance.
Dietary daidzein reduced aneurysm formation in ovariectomized female mice.
Next, we tested whether equol’s dietary precursor, daidzein, also protects against the formation of aneurysms in ovariectomized female mice. An isoflavone-free diet was started three weeks before aneurysm induction. In the experimental group, daidzein was added to the diet one week prior to aneurysm induction; the control group received the same amount of isoflavone-free chow (Figure 5A).
Figure 5. Daidzein reduced aneurysm formation in ovariectomized female mice.
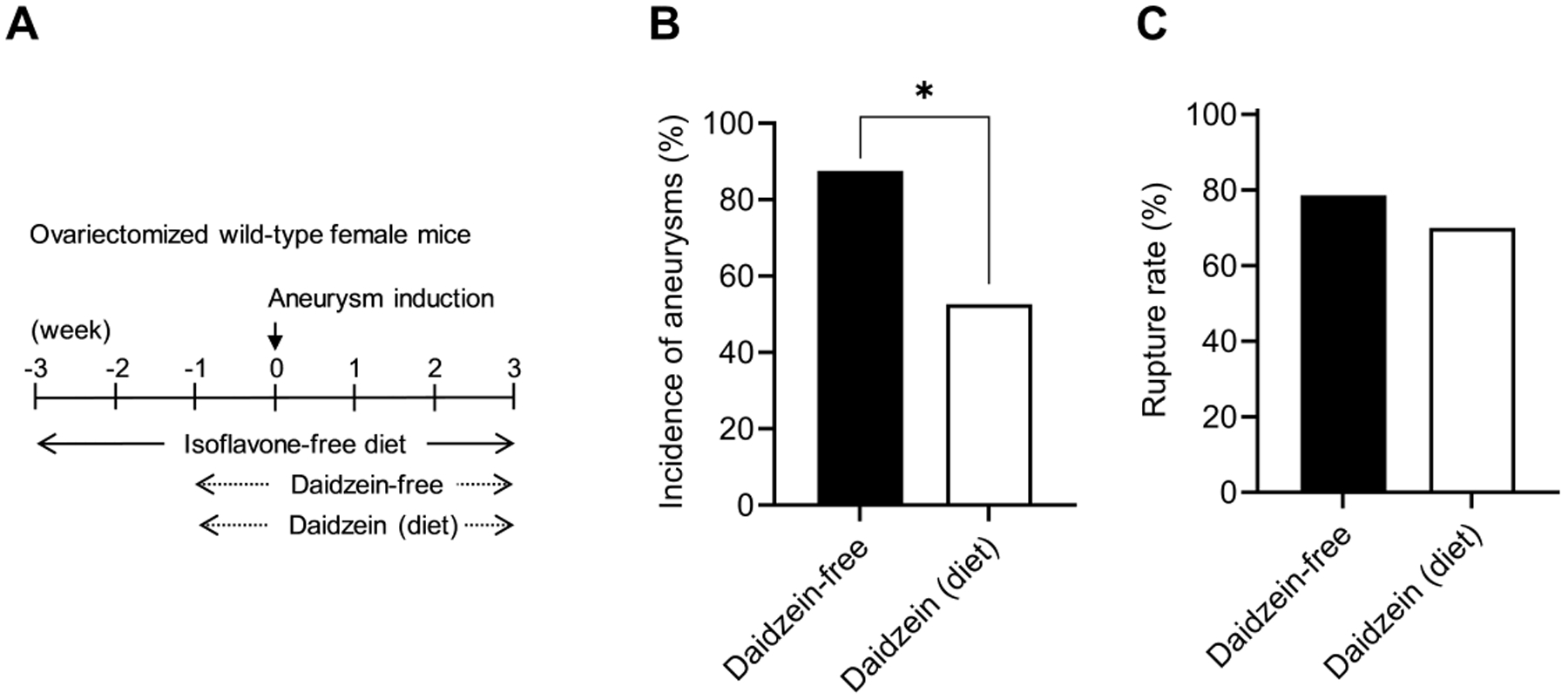
A. Schematic diagram of experimental protocols for dietary daidzein treatment in ovariectomized wild-type female mice. B and C, there is a significant difference between daidzein-containing diet and daidzein-free diet-fed groups in the incidence of aneurysms (B, * P < 0.05).
Similarly to the systemic equol treatment, dietary daidzein reduced the incidence of aneurysm formation (daidzein-containing diet vs. daidzein-free diet: 53% vs. 88%; n = 10/19 vs. n = 14/16. P < 0.05) (Figure 5B). There was no difference in aneurysmal rupture between the two groups (daidzein-containing diet vs. daidzein-free diet: 70% vs. 79%; n = 7/10 vs. n = 11/14 (P = 0.5, Figure 5C).
Daidzein’s protective effect against aneurysm formation was dependent on intestinal conversion to equol by gut microbiota.
Equol is produced by gut microbiota from its dietary precursor, daidzein.34–37 Oral vancomycin treatment reduces the conversion of daidzein to equol by 99% by disrupting the composition of gut microbiota,32 while systemic absorption of oral vancomycin is negligible.38, 39 Therefore, we used oral vancomycin to assess whether the intestinal conversion to equol is required for the protective effect of daidzein.
An isoflavone-free diet was started three weeks before aneurysm induction. One week prior to aneurysm induction, mice were concurrently given a daidzein supplemented diet and started on oral vancomycin. As an additional control, another group of mice received oral vancomycin without daidzein to evaluate for a potential confounding effect of vancomycin. Another group received a combination of daidzein, oral vancomycin, and systemic equol to confirm equol protected against aneurysm formation, despite blocking the intestinal metabolism of daidzein (Figure 6A).
Figure 6. Daidzein’s protective effect against aneurysm formation was dependent on intestinal metabolism to equol by gut microbiota.
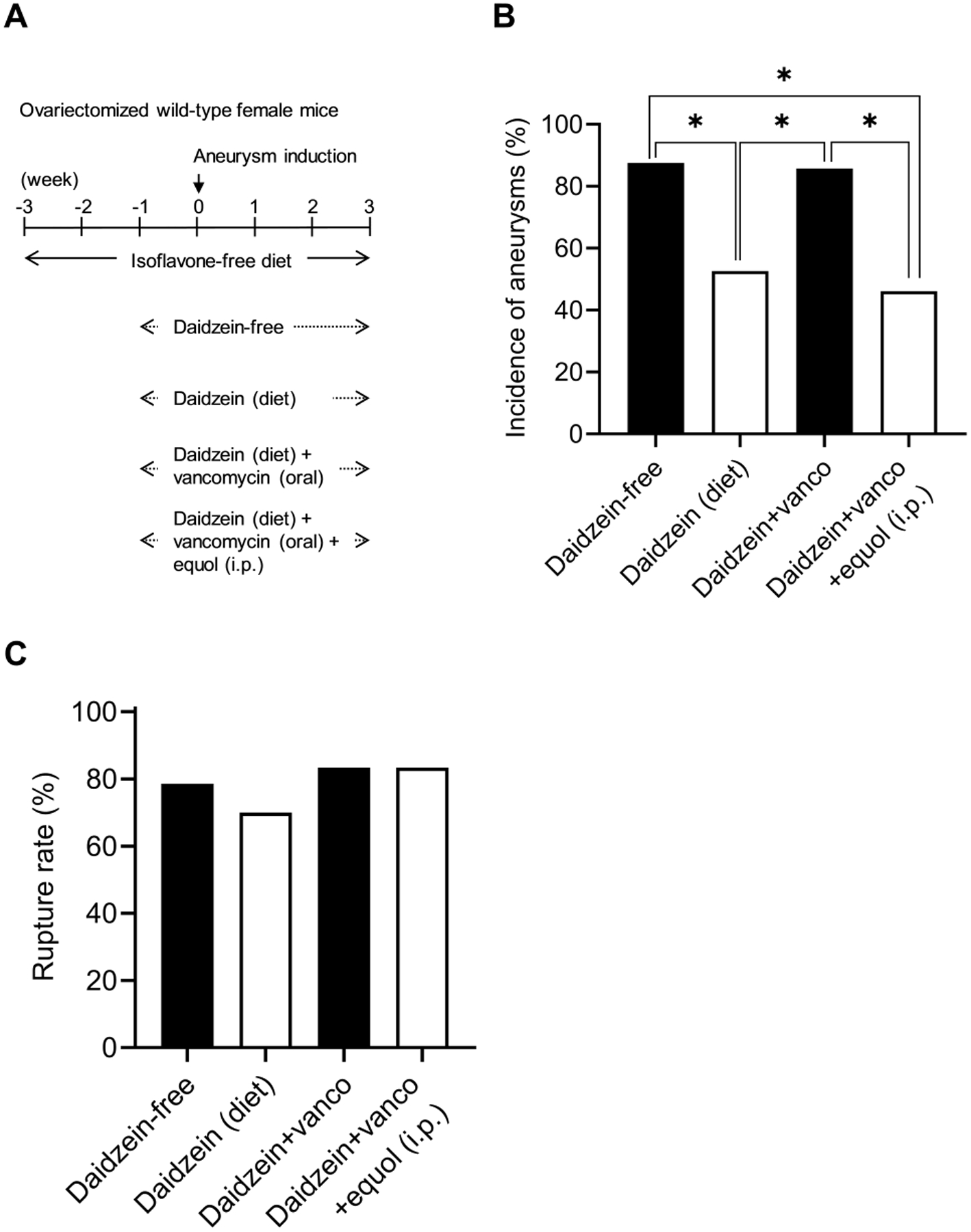
A. Schematic diagram of experimental protocols for dietary daidzein, oral vancomycin, and intraperitoneal administration of equol in ovariectomized wild-type female mice. B. Oral vancomycin treatment abolished the protective effect of dietary daidzein on the incidence of aneurysm formation; systemic equol administration rescued the protective effects of dietary daidzein from oral vancomycin treatment (* P < 0.05). C. There is no significant effect of the drugs on the incidence of SAH.
We found that oral vancomycin abolished the protective effect of daidzein on the incidence of aneurysm formation (daidzein diet vs. daidzein diet with vancomycin: 53% vs. 86%; n = 10/19 vs. n = 12/14. P < 0.05) (Figure 6B), confirming daidzein’s protective effect requires the intestinal conversion of daidzein to equol. When systemic equol was administered to mice receiving daidzein and vancomycin, the protective effects were rescued (daidzein diet+vancomycin vs. daidzein diet+vancomycin+equol: 86% vs. 46%; n = 12/14 vs. n = 6/13. P < 0.05) (Figure 6B). Vancomycin treatment alone did not affect the formation of aneurysms (data not shown). There was no significant difference among any of the groups in aneurysm rupture rate for any treatments, including vancomycin (P = 0.88, Fisher’s exact test, Figure 6C). This non-statistical difference observed in this graph is within the margin of error for this model. There was no difference in the blood pressure between the groups at any time point (Figure I).
To assess the equol production from dietary daidzein, we measured the plasma equol concentration in mice that received daidzein-free diet, daidzein-containing diet, and daidzein-containing diet and vancomycin treatment. We measured the plasma equol concentration after two weeks of the diet and vancomycin treatments. As shown in Supplemental Figure II, feeding with the daidzein-containing diet for two weeks resulted in the plasma concentration of equol at 7.72 ± 2.98 (nM), while the plasma equol concentration in the daidzein-free diet group was under the detection level. More importantly, oral vancomycin treatment significantly reduced the plasma equol concentration, suggesting the effective blocking of the intestinal conversion of daidzein to equol (please see https://www.ahajournals.org/journal/str). As a comparison, plasma equol concentration reached 103 ± 70 (nM) in mice treated with equol.
Discussion
Both clinical and pre-clinical studies have shown the potentially protective effect of estrogen against the formation and rupture of intracranial aneurysms in post-menopausal women.1–4, 10–12 However, estrogen replacement is associated with adverse outcomes such as cancer and ischemic stroke, partly due to lack of tissue or receptor subtype specificities of estradiol.5, 6, 14–16 Thus, a treatment strategy that targets the estrogen receptor with the subtype specificity while not causing estrogen’s adverse outcomes may be viable for the prevention of aneurysm formation and rupture in post-menopausal women.
Isoflavones, including genistein, daidzein, and glycitein, resemble estradiol and are potent estrogen receptor agonists. The dietary isoflavone, daidzein, and its metabolite equol have more potent estrogenic activity than any other isoflavone or isoflavone-derived metabolite.20, 40 Diets rich in isoflavones are reported to be effective in alleviating vasomotor symptoms of menopause and may be protective against estrogen-dependent diseases.21 Therefore, the use of phytoestrogens has the potential as a therapeutic against aneurysm formation and rupture in post-menopausal women.
Here, we have shown the isoflavone daidzein and its metabolite, equol, are protective against aneurysm formation in ovariectomized female mice. Consistent with our previous findings on estrogen deficiency,10, 11 equol’s protective effect against aneurysm formation was dependent on the activation of ER-β.
We found that daidzein’s protective effect against aneurysm formation is primarily exerted by its bio-active, bacterially-produced metabolite equol.20 Similar to systemically-administered equol, daidzein was protective against aneurysm formation. Several factors influence the bioavailability of isoflavones, including gut microbiota, bowel disease, age, and sex.41 We found systemically-administered equol resulted in higher plasma concentration than daidzein ingestion and gut absorption alone, thus providing a higher degree of protection against the formation of aneurysms. Daidzein is a natural compound, found primarily in legumes and beans and is metabolized to equol by gut microbiota.20 We found that blocking the intestinal conversion of dietary daidzein to equol abolished daidzein’s protective effect, suggesting the importance of the intestinal conversion of phytoestrogens for their protective effects.
In our previous studies, estrogen was found to be protective against aneurysm formation in ovariectomized female mice.10–12 While the exact pathogenesis of aneurysm formation and rupture remains fully elucidated, numerous studies have demonstrated that inflammation is likely to play a pivotal role.42–46 It is, therefore, possible that the protection estrogen confers may be through modulation of inflammatory processes. We previously found estrogen’s protective effect required activation of ER-β and the production of nitric oxide.10 Nitric oxide is involved in acute and chronic inflammation, processes integral to the pathophysiology of intracranial aneurysm. Hoh et al. also reported a link between estrogen deficiency and inflammation, suggesting estrogen deficiency promotes aneurysm rupture by upregulating IL-17, leading to the downregulation of E-cadherin and macrophage infiltration in the aneurysm wall.12 Consistent with these findings, we found treatment with equol significantly reduced pro-inflammatory cytokines, suggesting a link between estrogen and inflammation in the pathogenesis of intracranial aneurysm.
While both equol and daidzein were protective against the formation of aneurysms, we observed only a trend for their protective effect against the development of aneurysmal rupture. It is possible that our studies were underpowered to detect the potentially protective effect of phytoestrogens against the development of aneurysmal rupture. Also, the duration of treatments may have been too short to exert protective effects. Alternatively, these findings simply reflect the weaker estrogenic potency of phytoestrogens compared to estrogens. Future studies with longer treatment duration and higher doses may be needed.
There are other limitations to our study. While our mouse model recapitulates key features of human intracranial aneurysms, it may not completely replicate the pathogenesis of aneurysm formation and rupture in humans.33, 47, 48 Additionally, ovariectomy in pre-menopausal young mice may not completely replicate physiologic menopause.49, 50 Female mice reach reproductive maturity at approximately 7 weeks, and menopause occurs at approximately 12–14 months.51 While older rodents may be used to model menopause, only 25–40% naturally model the human menopausal transition.52 In contrast, ovariectomy in young, female mice has been widely used as a model for menopause in pre-clinical translational research due to its simplicity and consistency.52–54 Further studies using older post-menopausal mice are needed to confirm our findings. In addition, it needs to be tested whether the protections offered by phytoestrogen can be extended to males and pre-menopausal females. We recognize different lines of ER-β knockout mice have slightly different phenotypes.55–57 Some of the key differences are noted in reproductive organs such as the prostate.56, 57 However, there is no report suggesting apparent differences in the vascular or inflammatory cells in these lines of ER-β knockout mice. While it is unlikely that the subtle differences in reproductive organs represent significant confounding factors for our study, we cannot completely exclude such possibility. Another limitation of this study is that we did not determine the effect of vancomycin on the inflammatory cytokines. Given its poor absorption, the effect of oral vancomycin may be restricted to the gut, and a systemic effect of oral vancomycin is not expected.38, 39, 58, 59 With expansive experiments, we may be able to examine the effect of vancomycin on the inflammatory cytokines and the rupture rate (on which vancomycin treatment showed a slight trend of increase). However, such expansive experiments are beyond the scope of this manuscript.
While laboratory mice can consistently produce equol in response to soy or daidzein ingestion, not all humans are equol producers. Only 25 to 30% of Caucasians are host to microbiota capable of converting daidzein to equol, compared to 50 to 60% of Asians.21, 60 Ultimately, human studies are needed to confirm the contribution of isoflavone consumption to the pathogenesis of intracranial aneurysm.
Finally, apart from their estrogenic activity, isoflavones are potent antioxidants, and as such, they may exert protective effects against aneurysm formation or SAH independent of estrogen receptor activation.61 However, our ER-β knockout mice experiment suggests their protective effects on the formation of intracranial aneurysm are primarily mediated by estrogen receptor activation.
Conclusions
Our study firmly established that both dietary daidzein and supplementation with its metabolite, equol, were protective against aneurysm formation in ovariectomized female mice. These protective effects of daidzein and equol required ER-β activation. In addition, our results illustrate the potential role of gut microbiota in the pathophysiology of intracranial aneurysms since the metabolism of the isoflavone daidzein to the biologically active phytoestrogen equol is dependent on gut microbiota.33 Our results indicate the potential therapeutic value of dietary daidzein and its metabolite, equol, for the prevention of the formation of intracranial aneurysms.
Supplementary Material
Acknowledgment
We thank Dr. Wonsuk Yoo (Ivy Brain Tumor Center and Department of Neurobiology, Barrow Neurological Institute) for his advice on the statistical analysis of the data and Ms. Cindy Giljames and Barrow Neurological Institute Neuroscience Publications for the production of visual abstract.
Funding source
The project was supported by grant number R01NS109382 (TH, SE), and R01NS109584 (TH), R01 HL128324 (SE), R01 HL133248, and R01DK111042 (SE) from the National Institutes of Health; L. Nelson “Nick” Hopkins / Neurosurgery Research & Education Foundation Research Fellowship Grant (JFB); Cami Clark Chair of Research and Fight Like Frank Chair of Research (HS), Fight Like Frank Chair of Research and Team Cindy Escape from Alcatraz Chair of Research (CR), and Shirley Dudek Demmer Chair of Research and Taylor Richelsen Chair of Research (JA) from Brain Aneurysm Foundation; Robert J. Dempsey Joint Cerebrovascular Section Award (RR) from American Association of Neurological Surgeons/Congress of Neurological Surgeons; Barrow Neurological Foundation (TH and JA). Ben and Catherine Ivy Foundation (AT and TM). The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Non-standard Abbreviations and Acronyms
- CT
cycle value
- ER
estrogen receptor
- ER-β
estrogen receptor-β
- IL-1β
interleukin-1β
- IL-6
interleukin-6
- IL-17
interleukin-17
- MCP-1
monocyte chemoattractant protein-1
- MMP-9
Matrix metallopeptidase 9
- SAH
subarachnoid hemorrhage
- TNFα
tumor necrosis factor-α
Footnotes
References
- 1.Kongable GL, Lanzino G, Germanson TP, Truskowski LL, Alves WM, Torner JC, Kassell NF. Gender-related differences in aneurysmal subarachnoid hemorrhage. J Neurosurg. 1996;84:43–48 [DOI] [PubMed] [Google Scholar]
- 2.Menghini VV, Brown RD Jr., Sicks JD, O’Fallon WM, Wiebers DO. Incidence and prevalence of intracranial aneurysms and hemorrhage in olmsted county, minnesota, 1965 to 1995. Neurology. 1998;51:405–411 [DOI] [PubMed] [Google Scholar]
- 3.Bonita R, Thomson S. Subarachnoid hemorrhage: Epidemiology, diagnosis, management, and outcome. Stroke. 1985;16:591–594 [DOI] [PubMed] [Google Scholar]
- 4.de Rooij NK, Linn FH, van der Plas JA, Algra A, Rinkel GJ. Incidence of subarachnoid haemorrhage: A systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry. 2007;78:1365–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Longstreth WT, Nelson LM, Koepsell TD, van Belle G. Subarachnoid hemorrhage and hormonal factors in women. A population-based case-control study. Ann Intern Med. 1994;121:168–173 [DOI] [PubMed] [Google Scholar]
- 6.Mhurchu CN, Anderson C, Jamrozik K, Hankey G, Dunbabin D, Australasian Cooperative Research on Subarachnoid Hemorrhage Study G. Hormonal factors and risk of aneurysmal subarachnoid hemorrhage: An international population-based, case-control study. Stroke. 2001;32:606–612 [DOI] [PubMed] [Google Scholar]
- 7.Pedersen AT, Lidegaard O, Kreiner S, Ottesen B. Hormone replacement therapy and risk of non-fatal stroke. Lancet. 1997;350:1277–1283 [DOI] [PubMed] [Google Scholar]
- 8.Falkeborn M, Persson I, Terent A, Adami HO, Lithell H, Bergstrom R. Hormone replacement therapy and the risk of stroke. Follow-up of a population-based cohort in sweden. Arch Intern Med. 1993;153:1201–1209 [PubMed] [Google Scholar]
- 9.Stampfer MJ, Colditz GA, Willett WC, Manson JE, Rosner B, Speizer FE, Hennekens CH. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the nurses’ health study. N Engl J Med. 1991;325:756–762 [DOI] [PubMed] [Google Scholar]
- 10.Tada Y, Makino H, Furukawa H, Shimada K, Wada K, Liang EI, Murakami S, Kudo M, Kung DK, Hasan DM, et al. Roles of estrogen in the formation of intracranial aneurysms in ovariectomized female mice. Neurosurgery. 2014;75:690–695; discussion 695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tada Y, Wada K, Shimada K, Makino H, Liang EI, Murakami S, Kudo M, Shikata F, Pena Silva RA, Kitazato KT, et al. Estrogen protects against intracranial aneurysm rupture in ovariectomized mice. Hypertension. 2014;63:1339–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoh BL, Rojas K, Lin L, Fazal HZ, Hourani S, Nowicki KW, Schneider MB, Hosaka K. Estrogen deficiency promotes cerebral aneurysm rupture by upregulation of th17 cells and interleukin-17a which downregulates e-cadherin. Journal of the American Heart Association. 2018;7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson CS, Feigin V, Bennett D, Lin RB, Hankey G, Jamrozik K, Australasian Cooperative Research on Subarachnoid Hemorrhage Study G. Active and passive smoking and the risk of subarachnoid hemorrhage: An international population-based case-control study. Stroke. 2004;35:633–637 [DOI] [PubMed] [Google Scholar]
- 14.Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, Anderson G, Howard BV, Thomson CA, LaCroix AZ, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the women’s health initiative randomized trials. JAMA. 2013;310:1353–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and estrogen/progestin replacement study (hers) research group. JAMA. 1998;280:605–613 [DOI] [PubMed] [Google Scholar]
- 16.Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL, Stefanick ML. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–1477 [DOI] [PubMed] [Google Scholar]
- 17.Force USPST, Grossman DC, Curry SJ, Owens DK, Barry MJ, Davidson KW, Doubeni CA, Epling JW Jr., Kemper AR, Krist AH, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: Us preventive services task force recommendation statement. JAMA. 2017;318:2224–2233 [DOI] [PubMed] [Google Scholar]
- 18.Jackson RL, Greiwe JS, Schwen RJ. Emerging evidence of the health benefits of s-equol, an estrogen receptor beta agonist. Nutr Rev. 2011;69:432–448 [DOI] [PubMed] [Google Scholar]
- 19.Mayo B, Vazquez L, Florez AB. Equol: A bacterial metabolite from the daidzein isoflavone and its presumed beneficial health effects. Nutrients. 2019;11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Setchell KD, Clerici C, Lephart ED, Cole SJ, Heenan C, Castellani D, Wolfe BE, Nechemias-Zimmer L, Brown NM, Lund TD, et al. S-equol, a potent ligand for estrogen receptor beta, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. The American journal of clinical nutrition. 2005;81:1072–1079 [DOI] [PubMed] [Google Scholar]
- 21.Bolanos R, Del Castillo A, Francia J. Soy isoflavones versus placebo in the treatment of climacteric vasomotor symptoms: Systematic review and meta-analysis. Menopause. 2010;17:660–666 [PubMed] [Google Scholar]
- 22.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263 [DOI] [PubMed] [Google Scholar]
- 23.Frankenfeld CL. Cardiometabolic risk and gut microbial phytoestrogen metabolite phenotypes. Mol Nutr Food Res. 2017;61 [DOI] [PubMed] [Google Scholar]
- 24.Vitale C, Mendelsohn ME, Rosano GM. Gender differences in the cardiovascular effect of sex hormones. Nat Rev Cardiol. 2009;6:532–542 [DOI] [PubMed] [Google Scholar]
- 25.Muthyala RS, Ju YH, Sheng S, Williams LD, Doerge DR, Katzenellenbogen BS, Helferich WG, Katzenellenbogen JA. Equol, a natural estrogenic metabolite from soy isoflavones: Convenient preparation and resolution of r- and s-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg Med Chem. 2004;12:1559–1567 [DOI] [PubMed] [Google Scholar]
- 26.Setchell KD, Brown NM, Summer S, King EC, Heubi JE, Cole S, Guy T, Hokin B. Dietary factors influence production of the soy isoflavone metabolite s-(-)equol in healthy adults. The Journal of nutrition. 2013;143:1950–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nuki Y, Tsou TL, Kurihara C, Kanematsu M, Kanematsu Y, Hashimoto T. Elastase-induced intracranial aneurysms in hypertensive mice. Hypertension. 2009;54:1337–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanematsu Y, Kanematsu M, Kurihara C, Tada Y, Tsou TL, van Rooijen N, Lawton MT, Young WL, Liang EI, Nuki Y, et al. Critical roles of macrophages in the formation of intracranial aneurysm. Stroke. 2011;42:173–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makino H, Tada Y, Wada K, Liang EI, Chang M, Mobashery S, Kanematsu Y, Kurihara C, Palova E, Kanematsu M, et al. Pharmacological stabilization of intracranial aneurysms in mice: A feasibility study. Stroke. 2012;43:2450–2456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimada K, Furukawa H, Wada K, Korai M, Wei Y, Tada Y, Kuwabara A, Shikata F, Kitazato KT, Nagahiro S, et al. Protective role of peroxisome proliferator-activated receptor-gamma in the development of intracranial aneurysm rupture. Stroke. 2015;46:1664–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimada K, Furukawa H, Wada K, Wei Y, Tada Y, Kuwabara A, Shikata F, Kanematsu Y, Lawton MT, Kitazato KT, et al. Angiotensin-(1–7) protects against the development of aneurysmal subarachnoid hemorrhage in mice. J Cereb Blood Flow Metab. 2015;35:1163–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blair RM, Appt SE, Franke AA, Clarkson TB. Treatment with antibiotics reduces plasma equol concentration in cynomolgus monkeys (macaca fascicularis). The Journal of nutrition. 2003;133:2262–2267 [DOI] [PubMed] [Google Scholar]
- 33.Shikata F, Shimada K, Sato H, Ikedo T, Kuwabara A, Furukawa H, Korai M, Kotoda M, Yokosuka K, Makino H, et al. Potential influences of gut microbiota on the formation of intracranial aneurysm. Hypertension. 2019;73:491–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iino C, Shimoyama T, Iino K, Yokoyama Y, Chinda D, Sakuraba H, Fukuda S, Nakaji S. Daidzein intake is associated with equol producing status through an increase in the intestinal bacteria responsible for equol production. Nutrients. 2019;11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopes DB, de Avila ARA, de Queiros LD, Macedo JA, Macedo GA. Bioconversion of isoflavones into bioactive equol: State of the art. Recent Pat Food Nutr Agric. 2016;8:91–98 [DOI] [PubMed] [Google Scholar]
- 36.Matthies A, Loh G, Blaut M, Braune A. Daidzein and genistein are converted to equol and 5-hydroxy-equol by human intestinal slackia isoflavoniconvertens in gnotobiotic rats. The Journal of nutrition. 2012;142:40–46 [DOI] [PubMed] [Google Scholar]
- 37.Tamura M, Hori S, Nakagawa H, Yamauchi S, Sugahara T. Effects of an equol-producing bacterium isolated from human faeces on isoflavone and lignan metabolism in mice. J Sci Food Agric. 2016;96:3126–3132 [DOI] [PubMed] [Google Scholar]
- 38.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koeth RA, Levison BS, Culley MK, Buffa JA, Wang Z, Gregory JC, Org E, Wu Y, Li L, Smith JD, et al. Gamma-butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of l-carnitine to tmao. Cell Metab. 2014;20:799–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jackson RL, Greiwe JS, Desai PB, Schwen RJ. Single-dose and steady-state pharmacokinetic studies of s-equol, a potent nonhormonal, estrogen receptor beta-agonist being developed for the treatment of menopausal symptoms. Menopause. 2011;18:185–193 [PubMed] [Google Scholar]
- 41.Soukup ST, Helppi J, Muller DR, Zierau O, Watzl B, Vollmer G, Diel P, Bub A, Kulling SE. Phase ii metabolism of the soy isoflavones genistein and daidzein in humans, rats and mice: A cross-species and sex comparison. Archives of toxicology. 2016;90:1335–1347 [DOI] [PubMed] [Google Scholar]
- 42.Starke RM, Chalouhi N, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, Wada K, Shimada K, Hasan DM, Greig NH, et al. Critical role of tnf-alpha in cerebral aneurysm formation and progression to rupture. J Neuroinflammation. 2014;11:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ali MS, Starke RM, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, Owens GK, Koch WJ, Greig NH, Dumont AS. Tnf-alpha induces phenotypic modulation in cerebral vascular smooth muscle cells: Implications for cerebral aneurysm pathology. J Cereb Blood Flow Metab. 2013;33:1564–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gounis MJ, Vedantham S, Weaver JP, Puri AS, Brooks CS, Wakhloo AK, Bogdanov AA, Jr. Myeloperoxidase in human intracranial aneurysms: Preliminary evidence. Stroke. 2014;45:1474–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hasan DM, Mahaney KB, Brown RD Jr., Meissner I, Piepgras DG, Huston J, Capuano AW, Torner JC, International Study of Unruptured Intracranial Aneurysms I. Aspirin as a promising agent for decreasing incidence of cerebral aneurysm rupture. Stroke. 2011;42:3156–3162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sawyer DM, Pace LA, Pascale CL, Kutchin AC, O’Neill BE, Starke RM, Dumont AS. Lymphocytes influence intracranial aneurysm formation and rupture: Role of extracellular matrix remodeling and phenotypic modulation of vascular smooth muscle cells. J Neuroinflammation. 2016;13:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mitsui K, Ikedo T, Kamio Y, Furukawa H, Lawton MT, Hashimoto T. Tlr4 (toll-like receptor 4) mediates the development of intracranial aneurysm rupture. Hypertension. 2019:HYPERTENSIONAHA11812595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kamio Y, Miyamoto T, Kimura T, Mitsui K, Furukawa H, Zhang D, Yokosuka K, Korai M, Kudo D, Lukas RJ, et al. Roles of nicotine in the development of intracranial aneurysm rupture. Stroke. 2018;49:2445–2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williams JK. A mouse model of the perimenopausal transition: Importance for cardiovascular research. Arterioscler Thromb Vasc Biol. 2005;25:1765–1766 [DOI] [PubMed] [Google Scholar]
- 50.Mayer LP, Dyer CA, Eastgard RL, Hoyer PB, Banka CL. Atherosclerotic lesion development in a novel ovary-intact mouse model of perimenopause. Arterioscler Thromb Vasc Biol. 2005;25:1910–1916 [DOI] [PubMed] [Google Scholar]
- 51.Silver LM. Mouse genetics : Concepts and applications. New York: Oxford University Press; 1995. [Google Scholar]
- 52.Diaz Brinton R Minireview: Translational animal models of human menopause: Challenges and emerging opportunities. Endocrinology. 2012;153:3571–3578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Kempen TA, Milner TA, Waters EM. Accelerated ovarian failure: A novel, chemically induced animal model of menopause. Brain Res. 2011;1379:176–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brinton RD. Investigative models for determining hormone therapy-induced outcomes in brain: Evidence in support of a healthy cell bias of estrogen action. Ann N Y Acad Sci. 2005;1052:57–74 [DOI] [PubMed] [Google Scholar]
- 55.Antal MC, Krust A, Chambon P, Mark M. Sterility and absence of histopathological defects in nonreproductive organs of a mouse erbeta-null mutant. Proc Natl Acad Sci U S A. 2008;105:2433–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci U S A. 1998;95:15677–15682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maneix L, Antonson P, Humire P, Rochel-Maia S, Castaneda J, Omoto Y, Kim HJ, Warner M, Gustafsson JA. Estrogen receptor beta exon 3-deleted mouse: The importance of non-ere pathways in erbeta signaling. Proc Natl Acad Sci U S A. 2015;112:5135–5140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moellering RC Jr. Pharmacokinetics of vancomycin. J Antimicrob Chemother. 1984;14Suppl D:43–52 [DOI] [PubMed] [Google Scholar]
- 59.Fagarasan S, Muramatsu M, Suzuki K, Nagaoka H, Hiai H, Honjo T. Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science. 2002;298:1424–1427 [DOI] [PubMed] [Google Scholar]
- 60.Peeters PH, Slimani N, van der Schouw YT, Grace PB, Navarro C, Tjonneland A, Olsen A, Clavel-Chapelon F, Touillaud M, Boutron-Ruault MC, et al. Variations in plasma phytoestrogen concentrations in european adults. The Journal of nutrition. 2007;137:1294–1300 [DOI] [PubMed] [Google Scholar]
- 61.Moosmann B, Behl C. The antioxidant neuroprotective effects of estrogens and phenolic compounds are independent from their estrogenic properties. Proc Natl Acad Sci U S A. 1999;96:8867–8872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tada Y, Kanematsu Y, Kanematsu M, Nuki Y, Liang EI, Wada K, Makino H, Hashimoto T. A mouse model of intracranial aneurysm: Technical considerations. Acta Neurochir Suppl. 2011;111:31–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kanematsu Y, Kanematsu M, Kurihara C, Tsou TL, Nuki Y, Liang EI, Makino H, Hashimoto T. Pharmacologically induced thoracic and abdominal aortic aneurysms in mice. Hypertension. 2010;55:1267–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


