Abstract
Background
The effects of childhood adversities on cognitive function in later life are well reported. However, few studies have examined the cumulative mechanism, especially in Chinese population. This study aims to explore this cumulative effects of childhood adversities on mid to late cognitive decline in China.
Methods
Data were drawn from the second and third wave of the China Health and Retirement Longitudinal Study (CHARLS). We included 9,942 respondents aged 45 and above and retrospectively collected information on childhood adversities. Cognitive function was measured in three dimensions: orientation and calculation, immediate memory, and delayed memory. A structural equation model was employed for analysis.
Results
Age (β = -0.155, P<0.001) and mid to late depressive symptoms (β = -0.041, P<0.001) showed direct effects on cognitive decline. Low mid to late life socioeconomic status (SES) showed a direct effect on mid-late cognitive impairment (β = 0.603, P<0.001) and an indirect effect through depression (β = 0.007, P<0.001). Low childhood SES (β = 0.310, P<0.001), lack of friends (β = 0.208, P<0.001), parental mental health problems (β = 0.008, P<0.001), and poor relationship with parents (β = 0.001, P<0.001) had an indirect effect on cognitive impairment.
Conclusions
Childhood adversities had negative effects on cognitive function among middle aged and elderly population in China. The findings suggest that early counter measures on childhood adversities may lead to an effective reduction of cognitive impairment.
Introduction
With the escalation of the aging population worldwide, cognitive impairment has emerged as a major public health concern [1,2]. Alzheimer’s Disease International (ADI) [3] estimated that by 2050 the number of people with Alzheimer’s disease (AD) globally would increase to 132 million from 47 million in 2015. China accounted for 25% of the world’s elderly patients with dementia in 2016, which has brought an immense socioeconomic burden [4]. It has been demonstrated that the annual cost of AD in China was over US $167 billion in 2015 and is projected to reach US $1.89 trillion by 2050, emphasizing the importance of dementia as a public health priority [5].
A sizable body of research have emphasized the importance of identifying the biological, psychological, and social factors for maintaining or improving cognitive function [6]. Age, educational attainment, family history, chronic diseases such as diabetes, mental health factors like depression, and repeated stress were major risk factors for cognitive decline [7–9]. Additionally, some studies indicated that adversities in early life such as an absent parent, bad early child–parent relationship quality, and inadequate social support were all negatively associated with cognitive capability in later life [10–14]. However, few studies have explored the mediating effects of childhood adversities on cognitive function in later life.
Life course theory was introduced to estimate the contribution of early life experiences to later life outcomes over the whole life process, which has provided a widely used framework for psychological and biological research [15]. Moreover, it provides significant insight into the study of aging and the accumulation of inequality.
Therefore, this study aimed to examine the cumulative effects of childhood adversities on cognitive impairment among middle aged and elderly Chinese people using the structural equation model (SEM).
Theoretical framework for constructing the SEM
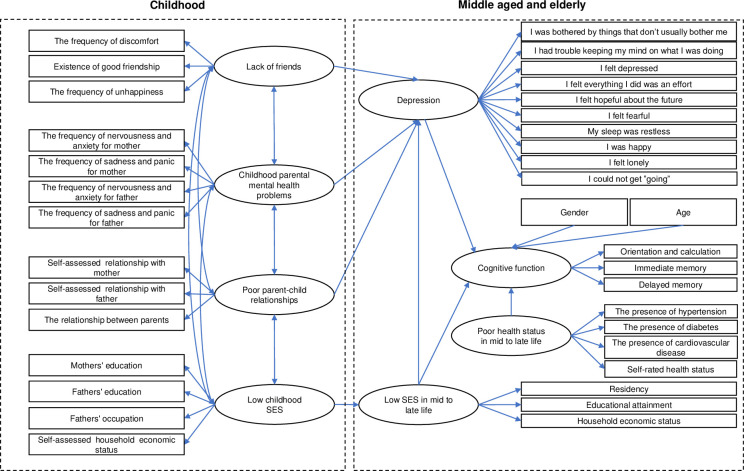
Based on the framework of life course, we propose the following theoretical SEM model (Fig 1), which hypothesizes that four childhood adversities and other potential risk factors might act on mid to late life cognitive function in China.
Fig 1. Initial structural equation model of the influence of childhood adversities on mid-late cognitive function.
Age, gender, and cognitive function
Age is regarded as the greatest risk factor for cognitive decline in mid to late life [16–19]. The percentage of people with Alzheimer’s increases dramatically with age: 3% for people aged 65–74, 17% for people aged 75–84, and 32% for people aged 85 or above [17]. In contrast, findings of a gender difference in cognitive deficit among middle aged and elderly populations remain controversial [20]. Some previous studies suggested gender differences in cognitive performance among Chinese older adults [21,22]. By contrast, other studies indicated non-significant direct association between gender and cognitive performance [23]. In our study, we intended to test the direct effects of age and gender on cognitive function in mid to late life.
Health status and cognitive function
Health status in middle aged and elderly people, such as chronic diseases, can also affect cognitive abilities. For example, several systematic reviews have shown an increased risk of cognitive decline among individuals with diabetes [24,25]. So, in our study we hypothesized that poor health status could directly affect cognitive function in mid to late life among the Chinese population.
Depression and cognitive function
Many studies have found that depression can adversely affect cognitive functioning in late life [26–30]. A recent longitudinal study showed that depressive symptoms increased cognitive decline in older adults [31], which is consistent with the findings of Chinese studies in which depression was generally associated with a certain degree of cognitive impairment [32,33]. Thus, we aimed to examine the direct effect of mid to late life depressive symptoms on cognitive impairment.
Socioeconomic status and cognitive function
A large number of studies have documented the impact of low SES in childhood on cognitive deficit in later life [34–36]. Lower SES has been found to be associated with a host of negative outcomes, including poorer general health, inequitable access to health services and increased risk for mental illness such as depression and anxiety [37–40]. Aartsen’s study further asserted that advantaged childhood SES was connected with higher cognitive functioning but stronger cognitive decline in older age [41]. A 5-year period cohort study also found that childhood SES is closely related to late-life baseline cognition [42]. Therefore, we intend to verify the direct and indirect association between low SES in mid to late life (associating it with low SES in childhood) and cognitive deficit mediated by depressive symptoms.
Childhood relationships with parents and cognitive function
Several studies reported the effect of suboptimal parent–child relationships on cognitive decline in mid to late life [14,43,44]. Adverse experiences from early life had a significant impact on individual outcomes in late life [45]. People who experienced childhood abuse were more likely to have trauma-associated symptoms such as personality disorders, substance abuse, posttraumatic stress disorder, chronic physical conditions, depression, and suicidal ideations [46–48]. Additionally, child neglect and domestic violence between parents have been shown to be associated with depression and health impairments [47,49,50]. Therefore, it is essential to test the potential connection between poor parent–child relationships and cognitive function indirectly through depression in our study.
Childhood parental mental health and cognitive function
Parental mental health problems were reported as important risk factors for cognitive functioning issues in children [51–53]. Familial factors in childhood such as shared genetic factors and a shared living environment had profound effects on children’s health outcomes [45]. For instance, Bennett’s study argued that the severity of mental health conditions for children aged 2–17 was positively related to parental mental illness [54]. Moreover, a 30-year-follow-up study found that children of parents with depressive symptoms were linked to a higher morbidity and mortality rate related to depression [55]. As such, we aimed to examine the indirect relationship between parental mental health issues and cognitive decline in their offspring through the mediation effect of late-life depressive symptoms.
Childhood friendship and cognitive function
Previous studies asserted that friendship support was a positive predictor for cognitive development [56–58]. Friendship is closely related to social adaptability, subjective well-being, and mental health. People with fewer friends were at a higher risk of suicide ideation, which was largely explained by self-assessed depression [59]. Teo’s research confirmed that high-quality social relationships were protective against depression [60]. An 18-year follow-up study also demonstrated that individuals with no friends were approximately twice as likely to experience internalizing symptoms (e.g. depression, anxiety, psychosomatic complaints) compared to those who had at least one friend in childhood [61]. Therefore, it is reasonable to test the association between lack of friends in childhood and mid to late life cognitive decline through depression in this study.
Materials and methods
Ethical approvals
The Ethics Review Committee of Peking University approved the study and informed consent was obtained from all participants. All methods were carried out in accordance with the relevant guidelines and regulations.
Respondents
The data were derived from the second and third wave of the China Health and Retirement Longitudinal Study (CHARLS) (data and documentation are available at http://charls.pku.edu.cn/), which is a nationally representative longitudinal survey. CHARLS employed multistage probability sampling to recruit 150 counties of 28 provinces of mainland China except Hainan, Ningxia and Tibet. At the household level, CHARLS conducted mapping and operations within each village-level unit to create the sample frame. Therefore, households with a member 39 years of age or older were included. Then, randomly sampling was employed to recruit one Individual aged 39 years and over in the household. Selected individuals aged 45 years or older and their spouses were interviewed in the first wave of 2011, and those who were between 39 and 45 years of age were not interviewed and designated for inclusion in a future refreshment sample. More detailed information of the study design and sampling procedure can be found in the cohort profile of CHARLS [62].
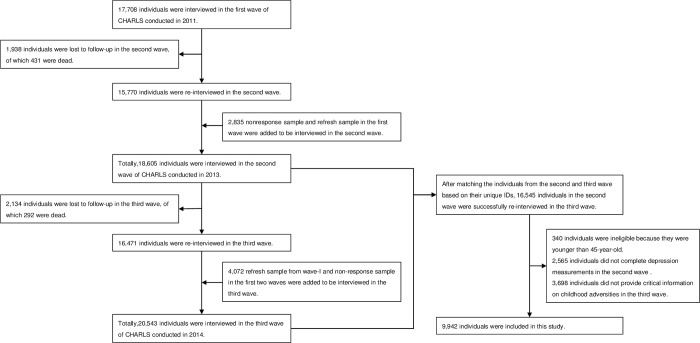
The interviewers were trained at Peking University by CHARLS staff members. Data was collected in respondents’ homes by well-trained clinicians in a face-to-face, computer-aided personal interview (CAPI) manner. A total of 17,708 individuals agreed to participate in the baseline survey. The second wave of CHARLS, conducted in 2013, was a regular follow-up survey, which included demographic information, family structure, health status, income, and expenditures. In the second wave, 1,938 individuals were lost to follow-up, of which 431 were dead. Besides, 2,835 nonresponse sample and refresh sample in wave 1 were added for interview in wave 2. Totally, 18,605 individuals were surveyed in the second wave. The third wave survey, performed in 2014, was a special survey that retrospectively collected life history information of all longitudinal responsive samples. This wave included information regarding childhood SES, childhood history, health and health care history, and so forth. In the third wave, 2,134 individuals were lost to follow-up, of which 292 individuals were dead. And 4,072 refresh sample from wave 1 and non-response sample in wave 1 and wave 2 were included for interview in wave 3. Overall, 20,543 individuals were interviewed in the third wave.
We matched the individuals from Waves 2 and 3 based on their unique IDs in order to trace childhood adversities. The wave 3 survey successfully re-interviewed 16,545 individuals among the respondents in wave 2. To be eligible for the study, respondents had to satisfy three inclusion criteria: 1) they must have been aged 45 or older; 2) they fully provided critical information on childhood adversities and other potential risk factors; and 3) they were interviewed in both Waves 2 and 3. In the current analyses, 340 individuals were younger than 45-year-old, 2,565 individuals did not complete depression measurements in wave 2, and 3,698 individuals did not provide critical information on childhood adversities in wave 3. They were excluded. Thus, there were 9,942 individuals included in the final sample (Fig 2).
Fig 2. Flow chart of participants in the study.
Measures
Assessments of cognitive function
Cognitive function was measured in three dimensions (ten items for each dimension, 30 items in total), including orientation and calculation, immediate memory, and delayed memory (S1 Table). A 10-item questionnaire was adapted to assess orientation and calculation. It required respondents to tell exactly the current year, month, date, week, and season, and subtract 7 from 100 serially for five iterations. As for immediate memory assessment, participants were asked to immediately repeat in any order ten Chinese nouns just read to them. For assessment of delayed memory, respondents were required to recall the 10 words that had been read before by the investigators. A wrong answer for each item received a score of 0, and a correct answer got a score of 1. Adding up scores for each item generated a valid range from 0–30, with lower scores indicating a higher severity of cognitive impairment.
Assessments of depression
The 10-item Center for Epidemiological Studies Depression Scale (CESD-10) was deployed for the measurement of depressive symptoms [63,64]. It had 10 self-reported items, and responses to each item were rated on a 4-point Likert scale ranging from 0 (rare) to 3 (most or all of the time). As for the frequency of negative emotions, the answers were rated from 0 (rarely or none of the time) to 3 (most or all of the time). Regarding the frequency of positive emotions such as “I was happy”, the score was reversely rated from 0 (most or all of the time) to 3 (rarely or none of the time). The sum of these 10 items (range: 0 to 30) reflected individuals’ depression. Thus, higher scores prompted worse depressive symptoms, and the cut-off point for depression was equal to or greater than 10 [64]. The Cronbach alpha coefficient of CESD-10 in this study was 0.797, consistently indicating comparable reliability with previous studies on depression among Chinese middle aged and older adults [65,66].
Assessments of socioeconomic status in mid to late life
SES in mid to late life was assessed by three indicators: residency, educational attainment, and household economic status. Residency was dichotomized into either an urban or a rural area. Educational attainment was categorized into six groups from illiteracy to bachelor’s degree or above. In most cases, household economic status refers to an income index such as family income. However, due to inaccurate income reporting in China, there are potential limitations when evaluating household economic status using income indicators. Thus, researchers proposed using an asset-based method in which they constructed an asset index to assess the household economic status [67,68]. In this study, we divided all households into five levels of “household economic status” based on the asset index we generated using principal component analysis on a scale from 1 (very poor) to 5 (very good) (S1 Table).
Assessments of poor health status in mid to late life
Health status in mid to late life was evaluated in four aspects: self-rated health status, the presence of hypertension, the presence of diabetes, and the presence of cardiovascular disease. The self-reported health status was assessed using a 5-point Likert scale from 1 (very good) to 5 (very poor). The remaining three items were dichotomous (S1 Table). People who self-reported having hypertension or diabetes, and who had objective measured values higher than the diagnostic standard were defined as having hypertension or diabetes. Cardiovascular diseases were self-rated by the participants.
Assessments of childhood adversities
Low childhood socioeconomic status. We measured childhood SES using parents’ education, father’s occupation, and self-assessed household economic status. Education level was categorized into six groups from illiteracy to bachelor’s degree or above. Father’s occupation was divided into nonagricultural, farming, and unemployment. In addition, a 5-point scale from 1 (very poor) to 5 (very good) was applied to estimate household economic status (S1 Table).
Lack of friends. Lack of friends was measured in three dimensions: the frequency of discomfort, the frequency of unhappiness, and existence of good friendship. The first two indicators used a 4-point Likert scale ranging from 1 (never), 2 (not very often), 3 (sometimes), to 4 (often). The last item was dichotomous (S1 Table).
Childhood parental mental health problems. We assessed parental mental health problems using the frequency of nervousness, the frequency of anxiety, the frequency of sadness, and panic for parents. All four indicators used a 4-point scale ranging from (most of the time) to 4 (a little of the time) (S1 Table).
Poor parent–child relationships. Parent–child relationships were evaluated by three indicators: a self-assessment of the mother–child relationship, a self-assessment of the father–child relationship, and the relationship between parents. All three indicators followed a 5-point Likert scale ranging from 1 (poor) to 5 (excellent) (S1 Table).
Model construction and statistical method
Based on the theoretical framework, we constructed SEM to analyze the effect of childhood adversities on cognitive function among middle-aged and elderly Chinese individuals. Latent variables and observed variables in the model are shown in S1 Table. The initial structural equation model is shown in Fig 1.
Structural equation modelling analyses were performed with SPSS version 19.0 software (SPSS Inc., Chicago, IL, USA), using the weighted least squares means and variance adjusted estimation (WLSMV). The model was considered to have a good fit when root mean square error of approximation (RMSEA)<0.05 [69], comparative fit index (CFI)>0.90, the goodness of fit index (GFI)>0.90, and the normed fit index (NFI)>0.90 [70]. In the present study, the chi-square value was excluded when determining whether a structural equation model had a good fit or not as it is sensitive to sample size, and the chi-square value increased with a larger sample size. Adjusting or deleting the path between the two variables with lager modification index (MI) will be more conducive to the adjustment and optimization of the model. According to the model results, we reconstructed the model by removing non-significant associations and re-assessing the model fitness. Standardized regression coefficients (equivalent to path coefficients) among endogenous and exogenous latent variables were shown in the final model.
Results
Demographic characteristics
The description of respondents’ demographic characteristics is presented in Table 1. Of the 9,942 respondents, 52.9% were female. The average age was 59.93 (SD = 8.34), and only 1.86% of the respondents had an education level of some college or above. For location, 63.92% of participants lived in a rural area, and 81.78% reported fair or worse health status. Respondents with hypertension, diabetes, and cardiovascular diseases accounted for 38.74%, 8.32%, and 15.74% respectively. The score of depressive symptoms followed a skewed distribution: the median score was 6.00 (interquartile range, 4–11). The average cognitive function score was 13.53 (SD = 5.57). Other descriptive information about childhood adversities is shown in S2 Table. The differences of demographic characteristics between the excluded (n = 6,603) and included (n = 9,942) individuals were reported in S3 Table.
Table 1. Demographic characteristics of respondents (weighted).
| Overall (N = 9,942) | |
|---|---|
| Variable | ± SD/n(%) |
| Age, Mean | 59.93±8.34 |
| Age | |
| Aged 45–60 | 5543 (55.75) |
| Aged 60–75 | 3800 (38.22) |
| Aged 75–90 | 593 (5.96) |
| Aged 90+ | 6 (0.06) |
| Gender | |
| Male | 4680 (47.07) |
| Female | 5262 (52.93) |
| Educational attainment | |
| Illiterate | 2488 (25.03) |
| Primary school | 4058 (40.82) |
| Junior high school | 2182 (21.95) |
| High school (secondary specialized school) | 1029 (10.35) |
| Some college | 133 (1.34) |
| Bachelor degree or above | 52 (0.52) |
| Residency | |
| Rural | 6355 (63.92) |
| Urban | 3587 (36.08) |
| Household economic status | |
| Very poor | 1821 (18.32) |
| Poor | 1959 (19.70) |
| Fair | 2035 (20.47) |
| Good | 2051 (20.63) |
| Very good | 2076 (20.88) |
| The presence of hypertension | |
| Yes | 3852 (38.74) |
| No | 6090 (61.26) |
| The presence of diabetes | |
| Yes | 827 (8.32) |
| No | 9115 (91.68) |
| The presence of cardiovascular disease | |
| Yes | 1565 (15.74) |
| No | 8377 (84.26) |
| Self-rated health status | |
| Very poor | 1281 (12.88) |
| Poor | 3571 (35.92) |
| Fair | 3279 (32.98) |
| Good | 1178 (11.85) |
| Very good | 633 (6.37) |
| Cognitive function, Mean | 13.53±5.57 |
Structural equation modeling
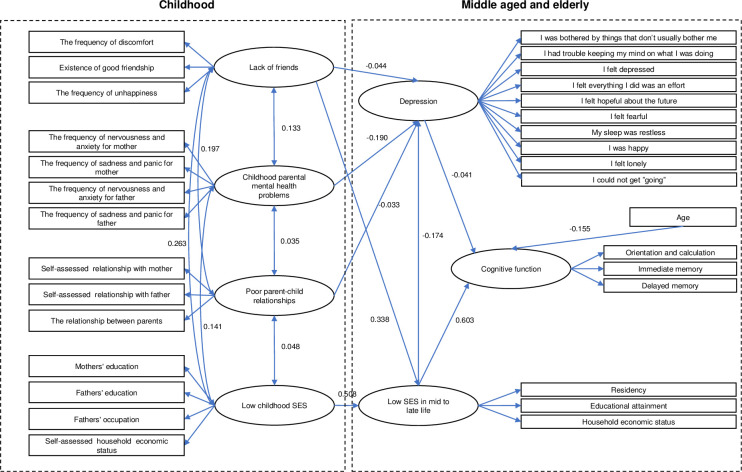
The confirmatory factor analysis for the measuring model based on the theoretical framework indicated an appropriate factor structure with a good model fit: RMSEA = 0.056, GFI = 0.904, TLI = 0.781, CFI = 0.799. All factor loadings from observed to latent variables were significant. Two hypothesized paths failed to reach significance: 1) from health status in mid to late life to cognitive function in mid to late life (P = 0.418) and 2) from gender to cognitive function in mid to late life (P = 0.062). Therefore, we deleted the insignificant pathways and reassessed each model. Then, new results indicated that the modification index (MI) between "lack of friends" and "socioeconomic status in mid to late life" was large (MI = 1417.749, parameter change = 0.569). After adding the pathway from "lack of friends" to" socioeconomic status in mid to late life", the final model was ascertained (Fig 3). A more satisfactory model was attained with a good model fit: RMSEA = 0.041, GFI = 0.952, TLI = 0.908, CFI = 0.918.
Fig 3. Final structural equation model of the influence of childhood adversities on mid to late cognitive function.
All path coefficients in the final model were standardized and significant (Table 2). Within the final model, lack of friends significantly indicated low SES in mid to late life (β = 0.338, SE = 0.014) and more severe depressive symptoms (β = -0.044, SE = 0.012). Low SES in mid to late life was strongly associated with higher severity of cognitive impairment (β = 0.603, SE = 0.051) and significantly predicted more severe depressive symptoms (β = -0.174, SE = 0.017). In addition, depression in mid to late life was significantly associated with cognitive impairment (β = -0.041 SE = 0.031). Parental mental health problems during childhood and bad parent–child relationships both had a significant influence on cognitive decline (β = -0.190, SE = 0.011; β = −0.033, SE = 0.012). We also found that increased age predicted a lower level of cognitive function (β = -0.155, SE = 0.028), and SES in childhood was strongly associated with SES in mid to late life (β = 0.508, SE = 0.081).
Table 2. Path coefficients and standard errors for the final SEM model (N = 9,942).
| Independent variable | Dependent variable | β a | SE | CR | P |
|---|---|---|---|---|---|
| Low childhood SES | Low SES in mid to late life | 0.508 | 0.081 | 16.477 | <0.001 |
| Lack of friends | Low SES in mid to late life | 0.338 | 0.014 | 18.799 | <0.001 |
| Lack of friends | Depression | -0.044 | 0.012 | -2.719 | 0.007 |
| Childhood parental mental health problems | Depression | -0.19 | 0.011 | -15.765 | <0.001 |
| Low SES in mid to late life | Depression | -0.174 | 0.017 | -9.954 | <0.001 |
| Poor parent–child relationships | Depression | -0.033 | 0.012 | -2.789 | 0.005 |
| Depression | Cognitive function | -0.041 | 0.031 | -3.472 | <0.001 |
| Age | Cognitive function | -0.155 | 0.028 | -15.107 | <0.001 |
| Low SES in mid to late life | Cognitive function | 0.603 | 0.051 | 29.918 | <0.001 |
SES, socioeconomic status; β, path coefficient; SE, standard error; C.R., critical ratio.
a, standardized parameter.
Standardized direct and indirect effects of childhood adversities on cognitive function in mid to late life are summarized in Table 3. It has been reported that SES in mid to late life has the largest effect on cognitive function (β = 0.610, P<0.001). Low SES in mid to late life had a positive effect on the cognitive impairment directly and indirectly, with the indirect effects mediated by depression. SES in childhood had the second largest total effect on mid to late life cognitive decline (β = 0.310, P<0.001) before the total effect of lack of friends during childhood (β = 0.208, P<0.001). Low SES in childhood had an indirect effect on poor cognitive performance mediated by low SES in mid to late life (β = 0.310, P<0.001). Lack of friends affected cognitive impairment through SES in mid to late life and depressive symptoms (β = 0.208, P<0.001). Parental mental health problems during childhood and poor parent–child relationships presented no direct effect on cognitive decline but contributed to depression (β = 0.008, P<0.001; β = 0.001, P<0.001). Additionally, age and depressive symptoms both indicated direct effects on cognitive function (β = -0.155, P<0.001; β = -0.041, P<0.001). The correlations of four latent variables of childhood adversities are demonstrated in Table 4.
Table 3. The standardized direct effects, indirect and total effects of the childhood adversities on cognitive function in mid to late life (N = 9,942).
| Variable | Standardized direct effects | Standardized indirect effects | Standardized total effects |
|---|---|---|---|
| Age | -0.155⁎⁎⁎ | - | -0.155⁎⁎⁎ |
| Low Childhood SES | - | 0.310⁎⁎⁎ | 0.310⁎⁎⁎ |
| Lack of friends | - | 0.208⁎⁎⁎ | 0.208⁎⁎⁎ |
| Childhood parental mental health problems | - | 0.008⁎⁎⁎ | 0.008⁎⁎⁎ |
| Poor parent–child relationships | - | 0.001⁎⁎⁎ | 0.001⁎⁎⁎ |
| Depression | -0.041⁎⁎⁎ | - | -0.041⁎⁎⁎ |
| Low SES in mid to late life | 0.603⁎⁎⁎ | 0.007⁎⁎⁎ | 0.610⁎⁎⁎ |
SES, socio-economic status.
⁎⁎⁎P<0.001.
Table 4. Correlations of four latent variables of childhood adversities (N = 9942).
| Latent variables | r a | S.E. | C.R. | P | |
|---|---|---|---|---|---|
| Lack of friends | Childhood parental mental health problems | 0.133 | 0.007 | 10.452 | <0.001 |
| Lack of friends | Poor parent–child relationships | 0.197 | 0.007 | 14.845 | <0.001 |
| Lack of friends | Low childhood SES | 0.263 | 0.005 | 12.24 | <0.001 |
| Childhood parental mental health problems | Poor parent–child relationships | 0.035 | 0.005 | 3.075 | 0.002 |
| Childhood parental mental health problems | Low childhood SES | 0.141 | 0.003 | 8.558 | <0.001 |
| Poor parent–child relationships | Low childhood SES | 0.048 | 0.002 | 3.399 | <0.001 |
SES, socio-economic status; r, correlation coefficient; SE, standard error; C.R., critical ratio.
a, standardized parameter.
Discussion
In our study, we intended to unpack the mechanism explanation between childhood adversities and cognitive impairment among middle aged and elderly Chinese people using different mediators. Consistent with our hypotheses and previous studies, age, depressive symptoms, and SES in mid to late life were directly associated with cognitive deficit [28,71,72]. Low SES in mid to late life showed the largest total effect on cognitive function among all the direct relationships, which is possibly due to reasons such as less access to health services, limited social support, fewer opportunities for success, and a higher probability of exposure to life adversities [40,73]. However, we did not find a significant direct association between gender and cognitive performance, which is consistent with the findings of a previous study in China [23].
It was shown that lack of friends in childhood indirectly affected cognitive decline in mid to late life mediated by depressive symptoms and low SES in mid to late life. Social support and social networks were protective factors for people’s mental health outcomes including cognitive ability in later life [74,75]. We argued that satisfying social relationships with others could enhance children’s psychological well-being by providing strong emotional support and strengthening coping abilities when children encounter life adversities. Additionally, friendship is closely related to parental educational level and occupation, which indirectly reflects SES [13]. Therefore, our results indicate that lack of friends was associated with an increasing risk of depressive symptoms and low SES in mid to late life, which subsequently increased the likelihood of low cognitive ability in mid to late life.
In accord with our hypothesis, it was shown that children whose parents had poor mental health were more likely to experience cognitive decline in later life, indirectly mediated through depressive symptoms in mid to late life. A recent study among older Chinese adults demonstrated that children who were exposed to parents with mental health problems were more likely to suffer depression in mid to late life [50]. Similarly, a significant association between depression and cognitive impairment in mid to late life was also found in our model, which is consistent with other findings [8,76,77]. A likely explanation could be that children living with parents who have poor mental health tend to receive inadequate emotional care and be influenced by substance abuse, anxiety, depression, suicide ideation, maltreatment, and violence from parents. These risk factors predict children’s mental health outcomes from childhood and have a cumulative impact on their depression and cognitive decline in mid to late life.
Our study also found a significant positive indirect association between poor parent–child relationships in childhood and a deficiency in cognitive function in mid to late life, which was mediated by depressive symptoms. A systematic review illustrated that children with secure attachments to parents tend to develop more positive health and mental health outcomes including better cognitive functioning [43]. Korkeila also found that good parent–child interactions were associated with increasing optimism, which could serve as a partial buffer when confronting adversities [78]. The indirect effects can be explained through the long-term impact of poor parent–child relationships developing over time on depression in mid to late life, which also significantly predicts cognitive impairment.
In addition, the results showed that experiencing low SES in childhood predicted a higher probability of cognitive decline, through the mediation effect of low SES in mid to late life as shown in previous studies [79,80]. Children growing up with low SES are less likely to gain adequate financial backups, higher educational attainment, and supportive social networks, which are crucial factors for future success. They have a higher risk of low SES in mid to late life, which in turn may increase their likelihood of experiencing poor nutrition and decrease their ability to afford health services such as prevention and treatments for depression.
Although this study benefitted from the use of life course theory to explore cumulative effects of childhood adversities on cognitive deficit in later life, we recognize several limitations as well. First, the CHARLS does not possess data from three provinces in mainland China (Hainan, Ningxia and Tibet), hence it is not entirely representative of the nation. Second, childhood information was collected with a retrospective method, which may cause recall bias. Third, as all the data were collected by questionnaire and were self-reported, it may have reporting bias. Fourth, individuals who had adverse events earlier in life may have died before reaching 45 years old, which may led to survival bias. Fifth, although the CHARLS survey was collected following a well-administered process with a low lost-to-follow-up rate, attrition bias and non-response bias may exist. Last, adverse events and other unobserved confounding factors were not available from the CHARLS, so we could not further test their effects on depression and cognitive impairment.
Conclusions
This study examined the potential paths from four aspects of childhood adversities including lack of friends, poor parental mental health status, poor parent–child relationships, and low SES to cognitive impairment in middle-aged and elderly Chinese populations. The results demonstrated that all four variables were associated with mid to late life cognitive decline through indirect processes. From the life course perspective, the negative impacts of adverse experiences in childhood on people’s mental health are not isolated. Instead, these experiences cumulatively influence cognitive deficit in mid to late life. These important findings suggest the urgent need to invest available resources to prevent childhood adversities, subsequently reducing the prevalence of cognitive decline.
Supporting information
(PDF)
(PDF)
(PDF)
Acknowledgments
We would like to acknowledge the China Health and Retirement Longitudinal Study (CHARLS) team for providing high quality, nationally representative data.
Data Availability
The data was derived from the second and third wave of the China Health and Retirement Longitudinal Study (CHARLS) (data and documentation are available at http://charls.pku.edu.cn/).
Funding Statement
PQ received a grant from China Medical Board (grant number CMB-14-198). The funders initiated the idea, developed the plan for analysis, and revised the paper.
References
- 1.WHO A. Dementia: a public health priority. Geneva: World Health Organization. 2012. [Google Scholar]
- 2.Sepanlou SG, Parsaeian M, Krohn KJ, Afshin A, Farzadfar F, Roshandel G, et al. Disability-Adjusted Life-Years (DALYs) for 315 diseases and injuries and Healthy Life Expectancy (HALE) in Iran and its neighboring countries, 1990–2015: findings from Global Burden of Disease Study 2015. Arch. Iran. Med. 2017;20(7):403–18. [PubMed] [Google Scholar]
- 3.Prince MJ. World Alzheimer Report 2015: the global impact of dementia: an analysis of prevalence, incidence, cost and trends: Alzheimer’s Disease International; 2015.
- 4.Nichols E, Szoeke CEI, Vollset SE, Abbasi N, FoadAbd-Allah, Abdela J, et al. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(1):88–106. doi: 10.1016/S1474-4422(18)30403-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jia J, Wei C, Chen S, Li F, Tang Y, Qin W, et al. The cost of Alzheimer’s disease in China and re-estimation of costs worldwide. Alzheimers Dement. 2018;14(4):483–91. doi: 10.1016/j.jalz.2017.12.006 [DOI] [PubMed] [Google Scholar]
- 6.Hendrie HC, Albert MS, Butters MA, Gao S, Knopman DS, Launer LJ, et al. The NIH cognitive and emotional health project: report of the critical evaluation study committee. Alzheimers Dement. 2006;2:12–32. doi: 10.1016/j.jalz.2005.11.004 [DOI] [PubMed] [Google Scholar]
- 7.Elias MF, Elias PK, Sullivan LM, Wolf PA, D’Agostino RB. Obesity, diabetes and cognitive deficit: the Framingham Heart Study. Neurobiol. Aging. 2005;26(1):11–6. doi: 10.1016/j.neurobiolaging.2005.08.019 [DOI] [PubMed] [Google Scholar]
- 8.Panza F, Frisardi V, Capurso C, D’Introno A, Colacicco AM, Imbimbo BP, et al. Late-life depression, mild cognitive impairment, and dementia: possible continuum? Am. J. Geriatr. Psychiatry. 2010;18(2):98–116. doi: 10.1097/JGP.0b013e3181b0fa13 [DOI] [PubMed] [Google Scholar]
- 9.Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron. 2012;73:962–77. doi: 10.1016/j.neuron.2011.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santos DN, Assis AMO, Bastos CSA, Santos LM, Santos CAS, Strina A, et al. Determinants of cognitive function in childhood: A cohort study in a middle income context. BMC Public Health. 2008;8:202. doi: 10.1186/1471-2458-8-202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Majer M, Nater UM, Lin J-MS, Capuron L, Reeves WC. Association of childhood trauma with cognitive function in healthy adults: a pilot study. BMC Neurol. 2010;10(1):61. doi: 10.1186/1471-2377-10-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burri A, Maercker A, Krammer S, Simmen-Janevska K. Childhood Trauma and PTSD Symptoms Increase the Risk of Cognitive Impairment in a Sample of Former Indentured Child Laborers in Old Age. PLoS One. 2013;8(2):e57826. doi: 10.1371/journal.pone.0057826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin Z, Chen X. Adverse Childhood Circumstances and Cognitive Function in Middle-aged and Older Chinese Adults: Lower Level or Faster Decline? SSM-Popul. Hlth. 2021;14:100767. doi: 10.1016/j.ssmph.2021.100767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng C, Burr JA, Yang D, Nan L. Early Child–Parent Relationship Quality and Cognitive Function in Older Rural Chinese Adults: The Mediating Role of Educational Attainment. J. Aging Health. 2021;33(2):089826432199656. doi: 10.1177/0898264321996562 [DOI] [PubMed] [Google Scholar]
- 15.Elder GH. The life course as developmental theory. Child Dev. 1998;69:1–12. [PubMed] [Google Scholar]
- 16.Barnes JN. Exercise, cognitive function, and aging. Adv Physiol Educ. 2015;39(2):55–62. doi: 10.1152/advan.00101.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaugler J, James B, Johnson T, Marin A, Weuve J. 2019 Alzheimer’s disease facts and figures. Alzheimers Dement. 2019;15(3):321–87. [Google Scholar]
- 18.Gaugler JE, Duval S, Anderson KA, Kane RL. Predicting nursing home admission in the US: a meta-analysis. BMC Geriatr. 2007;7(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tabbarah M, Crimmins EM, Seeman TE. The relationship between cognitive and physical performance: MacArthur Studies of Successful Aging. J. Gerontol. 2002;57(4):M228–35. doi: 10.1093/gerona/57.4.m228 [DOI] [PubMed] [Google Scholar]
- 20.Han M, Huang X-F, Xiu MH, Hui L, Liu H, Kosten TR, et al. Gender differences in cognitive function of patients with chronic schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2012;39(2):358–63. doi: 10.1016/j.pnpbp.2012.07.010 [DOI] [PubMed] [Google Scholar]
- 21.Lei X, Smith JP, Sun X, Zhao Y. Gender differences in cognition in China and reasons for change over time: evidence from CHARLS. J. Econ. Ageing. 2014;4:46–55. doi: 10.1016/j.jeoa.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lei X, Hu Y, McArdle JJ, Smith JP, Zhao Y. Gender Differences in Cognition among Older Adults in China. J. Hum. Resour. 2012;47(4):951–71. doi: 10.3368/jhr.47.4.951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kesavayuth D, Liang Y, Zikos V. An active lifestyle and cognitive function: Evidence from China. J. Econ. Aging. 2018;12:183–91. [Google Scholar]
- 24.Gudala K, Bansal D, Schifano F, Bhansali A. Diabetes mellitus and risk of dementia: A meta‐analysis of prospective observational studies. J. Diabetes Investig. 2013;4(6):640–50. doi: 10.1111/jdi.12087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta‐analysis of longitudinal studies. Intern. Med. J. 2012;42(5):484–91. doi: 10.1111/j.1445-5994.2012.02758.x [DOI] [PubMed] [Google Scholar]
- 26.Biringer E, Mykletun A, Dahl AA, Smith AD, Engedal K, Nygaard HA, et al. The association between depression, anxiety, and cognitive function in the elderly general population—the Hordaland Health Study. Int. J. Geriatr. Psychiatry. 2005;20:989–97. doi: 10.1002/gps.1390 [DOI] [PubMed] [Google Scholar]
- 27.Donovan NJ, Wu Q, Rentz DM, Sperling RA, Marshall GA, Glymour MM. Loneliness, Depression and Cognitive Function in Older U.S. Adults. Int. J. Geriatr. Psychiatry. 2017;32:564–73. doi: 10.1002/gps.4495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDermott LM, Ebmeier KP. A meta-analysis of depression severity and cognitive function. J. Affect. Disord. 2009;119:1–8. doi: 10.1016/j.jad.2009.04.022 [DOI] [PubMed] [Google Scholar]
- 29.Vinkers DJ, Gussekloo J, Stek ML, Westendorp RGJ, van der Mast RC. Temporal relation between depression and cognitive impairment in old age: prospective population based study. BMJ. 2004;329(7471):881. doi: 10.1136/bmj.38216.604664.DE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuring JK, Mathias JL, Ward L. Risk of Dementia in persons who have previously experienced clinicallysignificant Depression, Anxiety, or PTSD: A Systematic Review and Meta-Analysis. J. Affect. Disord. 2020;274(2020):247–61. doi: 10.1016/j.jad.2020.05.020 [DOI] [PubMed] [Google Scholar]
- 31.Holmquist S, Nordström A, Nordström P. The association of depression with subsequent dementia diagnosis: A Swedish nationwide cohort study from 1964 to 2016. PLoS Med. 2020;17(1):e1003016. doi: 10.1371/journal.pmed.1003016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chi I, Chou KL. Depression predicts cognitive decline in Hong Kong Chinese older adults. Aging Ment Health. 2000;4(2):148–57. [Google Scholar]
- 33.Giri M, Chen T, Yu W, Lü Y. Prevalence and correlates of cognitive impairment and depression among elderly people in the world’s fastest growing city, Chongqing, People’s Republic of China. Clin. Interv. Aging. 2016;11:1091–8. doi: 10.2147/CIA.S113668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karlamangla AS, Miller-Martinez D, Aneshensel CS, Seeman TE, Wight RG, Chodosh J. Trajectories of cognitive function in late life in the United States: demographic and socioeconomic predictors. Am. J. Epidemiol. 2009;170:331–42. doi: 10.1093/aje/kwp154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee Y, Back JH, Kim J, Byeon H. Multiple socioeconomic risks and cognitive impairment in older adults. Dement. Geriatr. Cogn. Disord. 2010;29:523–9. doi: 10.1159/000315507 [DOI] [PubMed] [Google Scholar]
- 36.Hackman DA, Farah MJ. Socioeconomic status and the developing brain. Trends Cogn. Sci. 2009;13(2):65–73. doi: 10.1016/j.tics.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goodman E, Slap GB, Huang B. The public health impact of socioeconomic status on adolescent depression and obesity. Am. J. Public Health. 2003;93(11):1844–50. doi: 10.2105/ajph.93.11.1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fryers T, Melzer D, Jenkins R. Social inequalities and the common mental disorders. Soc. Psychiatry Psychiatr. Epidemiol. 2003;38(5):229–37. doi: 10.1007/s00127-003-0627-2 [DOI] [PubMed] [Google Scholar]
- 39.Barbeau EM, Krieger N, Soobader M-J. Working Class Matters: Socioeconomic Disadvantage, Race/Ethnicity, Gender, and Smoking in NHIS 2000. Am. J. Public Health. 2004;94(2):269–78. doi: 10.2105/ajph.94.2.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang C, Lei X, Strauss J, Zhao Y. Health Insurance and Health Care among the Mid-Aged and Older Chinese: Evidence from the National Baseline Survey of CHARLS. Health Econ. 2017;26:431–49. doi: 10.1002/hec.3322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aartsen MJ, Cheval B, Sieber S, Van der Linden BW, Gabriel R, Courvoisier DS, et al. Advantaged socioeconomic conditions in childhood are associated with higher cognitive functioning but stronger cognitive decline in older age. Proc. Natl. Acad. Sci. U. S. A. 2019;116:5478–86. doi: 10.1073/pnas.1807679116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sha T, Yan Y, Cheng W. Associations of childhood socioeconomic status with mid-life and late-life cognition in Chinese middle-aged and older population based on a 5-year period cohort study. Int. J. Geriatr. Psych. 2018;33(10):1335–45. doi: 10.1002/gps.4930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ranson KE, Urichuk LJ. The effect of parent-child attachment relationships on child biopsychosocial outcomes: a review. Early Child Development and Care. 2008;178(2):129–52. [Google Scholar]
- 44.Thompson RA. Early attachment and later development: Familiar questions, new answers. New York: NY: The Guilford Press; 2008. [Google Scholar]
- 45.Ferraro KF, Shippee TP. Aging and cumulative inequality: How does inequality get under the skin? The Gerontologist. 2009;49(3):333–43. doi: 10.1093/geront/gnp034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levitan RD, Rector NA, Sheldon T, Goering P. Childhood adversities associated with major depression and/or anxiety disorders in a community sample of Ontario: Issues of co‐morbidity and specificity. Depress. Anxiety. 2003;17(1):34–42. doi: 10.1002/da.10077 [DOI] [PubMed] [Google Scholar]
- 47.Herrenkohl TI, Hong S, Klika JB, Herrenkohl RC, Russo MJ. Developmental impacts of child abuse and neglect related to adult mental health, substance use, and physical health. J. Fam. Violence. 2013;28(2):191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gonzalez A, Boyle MH, Kyu HH, Georgiades K, Duncan L, MacMillan HL. Childhood and family influences on depression, chronic physical conditions, and their comorbidity: findings from the Ontario Child Health Study. J. Psychiatr. Res. 2012;46(11):1475–82. doi: 10.1016/j.jpsychires.2012.08.004 [DOI] [PubMed] [Google Scholar]
- 49.Hovens JG, Wiersma JE, Giltay EJ, Van Oppen P, Spinhoven P, Penninx BW, et al. Childhood life events and childhood trauma in adult patients with depressive, anxiety and comorbid disorders vs. controls. Acta Psychiatr. Scand. 2010;122(1):66–74. doi: 10.1111/j.1600-0447.2009.01491.x [DOI] [PubMed] [Google Scholar]
- 50.Tian F, Meng SS, Qiu P. Childhood adversities and mid-late depressive symptoms over the life course: Evidence from the China health and retirement longitudinal study. J. Affect. Disord. 2019;245:668–78. doi: 10.1016/j.jad.2018.11.028 [DOI] [PubMed] [Google Scholar]
- 51.McManus BM, Poehlmann J. Parent-child interaction, maternal depressive symptoms and preterm infant cognitive function. Infant Behav. Dev. 2012;35(3):489–98. doi: 10.1016/j.infbeh.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Slomian J, Honvo G, Emonts P, Reginster J-Y, Bruyère O. Consequences of maternal postpartum depression: A systematic review of maternal and infant outcomes. Womens Health. 2019;15(1780):174550651984404. doi: 10.1177/1745506519844044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stein A, Pearson RM, Goodman SH, Rapa E, Rahman A, McCallum M, et al. Effects of perinatal mental disorders on the fetus and child. The Lancet. 2014;384(9956):1800–19. doi: 10.1016/S0140-6736(14)61277-0 [DOI] [PubMed] [Google Scholar]
- 54.Bennett AC, Brewer KC, Rankin KM. The association of child mental health conditions and parent mental health status among US children, 2007. Matern. Child Hlth. J. 2012;16(6):1266–75. [DOI] [PubMed] [Google Scholar]
- 55.Weissman MM, Berry OO, Warner V, Gameroff MJ, Skipper J, Talati A, et al. A 30-year study of 3 generations at high risk and low risk for depression. JAMA Psychiatry. 2016;73(9):970–7. doi: 10.1001/jamapsychiatry.2016.1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mazurek MO, Kanne SM. Friendship and internalizing symptoms among children and adolescents with ASD. J. Autism Dev. Disord. 2010;40:1512–20. doi: 10.1007/s10803-010-1014-y [DOI] [PubMed] [Google Scholar]
- 57.Mikami AY. The importance of friendship for youth with attention-deficit/hyperactivity disorder. Clin. Child Fam. Psychol. Rev. 2010;13:181–98. doi: 10.1007/s10567-010-0067-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Harmelen AL, Kievit RA, Ioannidis K, Neufeld S, Jones PB, Bullmore E, et al. Adolescent friendships predict later resilient functioning across psychosocial domains in a healthy community cohort. Psychol. Med. 2017;47:2312–22. doi: 10.1017/S0033291717000836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marver JE, Galfalvy HC, Burke AK, Sublette ME, Oquendo MA, Mann JJ, et al. Friendship, Depression, and Suicide Attempts in Adults: Exploratory Analysis of a Longitudinal Follow‐Up Study. Suicide Life‐Threat. 2017;47(6):660–71. [DOI] [PubMed] [Google Scholar]
- 60.Teo AR, Choi H, Valenstein M. Social relationships and depression: ten-year follow-up from a nationally representative study. PLoS One. 2013;8(4):e62396. doi: 10.1371/journal.pone.0062396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sakyi KS, Surkan PJ, Fombonne E, Chollet A, Melchior M. Childhood friendships and psychological difficulties in young adulthood: an 18-year follow-up study. Eur. Child Adolesc. Psychiatry. 2015;24(7):815–26. doi: 10.1007/s00787-014-0626-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China health and retirement longitudinal study (CHARLS). Int. J. Epidemiol. 2014;43:61–8. doi: 10.1093/ije/dys203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang X, Wang X, Ma H. Psychological assessment scale manual. Beijing, P.R.China: Chinese Mental Health Journal press. 1999. [Google Scholar]
- 64.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am. J. Prev. Med. 1994;10(2):77–84. [PubMed] [Google Scholar]
- 65.Huang Q, Wang X, Chen G. Reliability and validity of 10-item CES-D among middle aged and older adults in China. China Journal of Health Psychology. 2015(7):24. [Google Scholar]
- 66.Yang L, Jia C-X, Qin P. Reliability and validity of the Center for Epidemiologic Studies Depression Scale (CES-D) among suicide attempters and comparison residents in rural China. BMC Psychiatry. 2015;15(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Filmer D, Pritchett LH. Estimating wealth effects without expenditure data—or tears: an application to educational enrollments in states of India. Demography. 2001;38(1):115–32. doi: 10.1353/dem.2001.0003 [DOI] [PubMed] [Google Scholar]
- 68.Morris SS, Carletto C, Hoddinott J, Christiaensen LJJJoE, Health C. Validity of rapid estimates of household wealth and income for health surveys in rural Africa. J. Epidemiol. Community Health. 2000;54(5):381–7. doi: 10.1136/jech.54.5.381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Browne MW, Cudeck R. Alternative ways of assessing model fit. Sociol. Method Res. 1992;21(2):230–58. [Google Scholar]
- 70.Bentler PM. Comparative fit indexes in structural models. Psychol. Bull. 1990;107(2):238. doi: 10.1037/0033-2909.107.2.238 [DOI] [PubMed] [Google Scholar]
- 71.Deary IJ, Corley J, Gow AJ, Harris SE, Houlihan LM, Marioni RE, et al. Age-associated cognitive decline. Br. Med. Bull. 2009;92(1):135–52. doi: 10.1093/bmb/ldp033 [DOI] [PubMed] [Google Scholar]
- 72.Lyu J, Burr JA. Socioeconomic status across the life course and cognitive function among older adults: An examination of the latency, pathways, and accumulation hypotheses. J. Aging Health. 2016;28(1):40–67. doi: 10.1177/0898264315585504 [DOI] [PubMed] [Google Scholar]
- 73.Nurius PS, Fleming CM, Brindle E. Life Course Pathways From Adverse Childhood Experiences to Adult Physical Health: A Structural Equation Model. J. Aging Health. 2019;31:211–30. doi: 10.1177/0898264317726448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fratiglioni L, Wang H-X, Ericsson K, Maytan M, Winblad B. Influence of social network on occurrence of dementia: a community-based longitudinal study. The Lancet. 2000;355(9212):1315–9. doi: 10.1016/S0140-6736(00)02113-9 [DOI] [PubMed] [Google Scholar]
- 75.Gow AJ, Pattie A, Whiteman MC, Whalley LJ, Deary IJ. Social support and successful aging: Investigating the relationships between lifetime cognitive change and life satisfaction. J. Indiv. Differ. 2007;28(3):103–15. [Google Scholar]
- 76.Richard E, Reitz C, Honig LH, Schupf N, Tang MX, Manly JJ, et al. Late-life depression, mild cognitive impairment, and dementia. JAMA Neurol. 2013;70(3):374–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Steffens DC, Otey E, Alexopoulos GS, Butters MA, Cuthbert B, Ganguli M, et al. Perspectives on depression, mild cognitive impairment, and cognitive decline. Arch. Gen. Psychiatry. 2006;63(2):130–8. doi: 10.1001/archpsyc.63.2.130 [DOI] [PubMed] [Google Scholar]
- 78.Korkeila K, Kivelä S-L, Suominen S, Vahtera J, Kivimäki M, Sundell J, et al. Childhood adversities, parent-child relationships and dispositional optimism in adulthood. Soc. Psychiatry Psychiatr. Epidemiol. 2004;39(4):286–92. doi: 10.1007/s00127-004-0740-x [DOI] [PubMed] [Google Scholar]
- 79.Everson-Rose SA, Mendes de Leon CF, Bienias JL, Wilson RS, Evans DA. Early life conditions and cognitive functioning in later life. Am. J. Epidemiol. 2003;158(11):1083–9. doi: 10.1093/aje/kwg263 [DOI] [PubMed] [Google Scholar]
- 80.Kaplan GA, Turrell G, Lynch JW, Everson SA, Helkala E-L, Salonen JT. Childhood socioeconomic position and cognitive function in adulthood. Int. J. Epidemiol. 2001;30(2):256–63. doi: 10.1093/ije/30.2.256 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
Data Availability Statement
The data was derived from the second and third wave of the China Health and Retirement Longitudinal Study (CHARLS) (data and documentation are available at http://charls.pku.edu.cn/).