Abstract
Aims:
Therapies that recapitulate the health benefits of caloric restriction in older adults are needed. Phosphodiesterase 4 inhibitors demonstrate such promise. We examined their effects on body weight and composition, physical and cognitive function in aged mice using Compound D159687 (D159687).
Methods:
Nineteen 18 months old mice were randomized to receive either control (DMSO) or D159687 for seven weeks. We assessed food intake, body weight, and body composition over time and performed once the following tests: treadmill, inverted grip strength, rotarod, spontaneous Y maze tests and skeletal muscle mitochondrial biogenesis.
Results:
Four of the D159687 treated mice died in the first week. Necropsy suggests acute lung injury. D159687 treated mice weighed more than control mice at baseline. After controlling for baseline weight, D159687 treated mice lost 4.2grams more weight than control mice, mainly from fat mass loss (p value < 0.001). Muscle mass was unchanged between the two mice groups. D159587 mice ate significantly more food than the control mice. We found no difference between the two groups in the results of treadmill, rotarod and spontaneous Y maze tests and in mitochondrial biogenesis.
Conclusion:
Compound D159687 induced weight loss, predominantly fat mass loss and increased food intake in aged mice. The caloric restriction and lean mass preservation potential of PDE4D inhibitors deserve further verification. Findings may have major therapeutic implications when translated to the older adult population. Although physical and cognitive parameters were unchanged in this study, further studies would be needed to verify these results. The high death rate in the D159687 treated mice may have been due to the technical aspects of oral gavage.
Keywords: Obesity, aging, calorie restriction, AMPK, phosphodiesterase
I). INTRODUCTION
In addition to the increased mortality risk from aging with obesity [1], obesity leads to a progressively increased risk of disability over time [2]. Intentional weight loss through caloric restriction and physical exercise can be challenging for older adults and can be limited by the interaction of various diseases in these patients. Sarcopenia, or lean mass lost with aging, is hard to regain [3], especially without exercise [3, 4]. Yet, the addition of exercise to weight loss regimen is critical to preserving lean tissue and increasing physical function in older adults [5]. Weight loss, and in particular fat mass loss [6] is an essential process to improving physical function in older adults with obesity [7]. Unfortunately, few medications for weight loss exist and those in the market have side effect profiles and contra-indications which limit their use in older adults [8]. Laparoscopic bariatric surgery, though with low absolute perioperative risk, is riskier in older adults than in younger adults with obesity [9] and has unproven long-term benefit to risk ratios for the older population [10]. Thus, clinicians have very limited treatment options to offer older adults with obesity. A need, therefore, exists to find therapies that replicate the metabolic benefits of caloric restriction and physical exercise for use in older adults.
In both obesity and in aging, caloric restriction has metabolic and longevity benefits [11] [12, 13]. These benefits are predominantly attributed to downstream actions of the cell’s determinant of energy status, cyclic AMP [14]. Cellular levels of cyclic AMP can be increased through inhibition of phosphodiesterase, the enzyme that degrades cyclic AMP. Our previous research elucidates that the caloric restriction effects of resveratrol is partly due to inhibition of phosphodiesterase 4 (PDE4) enzyme [15]. Clinical studies of the FDA-approved PDE4 inhibitor, roflumilast, demonstrate similar metabolic benefits [16, 17]. The largest of the eleven known classes of phosphodiesterases [18], PDE4 exists in four known isoforms-- PDE4A, PDE4B, PDE4C and PDE4D [18]. Pan-PDE4 inhibitors’ use in human and animal studies has, however, been hindered because of their emetic side effects [19, 20] and PDE4B and PDE4D enzyme isoforms have been implicated in the central regulation of nausea [21]. In translational studies of obesity and energy homeostasis, emesis side effect can have profound implications on the study results.
Compound D159687 (D159687), a PDE4D inhibitor, is unique from all other PDE inhibitors in that it inhibits PDE enzyme activity, not through direct competitive inhibition as is with most PDE inhibitors but through modulation of the PDE4 enzyme structure [22]. As such structural alteration by D159687 results in approximately 80% inhibition of PDE4D enzyme activity [22], a nausea side effect ought to be expected. However, several studies had demonstrated that a marker of the side effect of nausea is significantly reduced with the use of the PDE4D inhibitor [22-24]. Hence, to further evaluate the potential of a PDE4 inhibitor as a caloric restriction mimetic, we set out to determine the degree to which PDE4D inhibitor recapitulates the calorie restriction benefits of nonspecific PDE4 inhibitors. We investigated the effects of seven weeks administration of D159687 on body weight and composition and on mitochondrial biogenesis. As PDE4 inhibitors can improve physical performance [15] and PDE4D inhibitors can improve cognitive function [23, 24], we also evaluated physical and cognitive function in these aged mice. We hypothesize that D159687 would cause weight loss, improve physical and cognitive function and increase skeletal muscle mitochondrial biogenesis without decreasing food intake.
II). MATERIALS AND METHODS:
Twenty mice, which were donated by the National Institute of Aging, were obtained in a set of 10 mice per month. Mice were fed standard NIH31 diet with ad libidum access to food and water before and during the study. One mouse died before the beginning of the study. Hence, nineteen C57B6J male mice (aged 18 months old at the onset of the study) were randomized, by the cages in which they dwelled, to either control and to D159687 (study) groups. The mice were kept on a 12 hours light/dark cycle. Dimethylsulfate (DMSO) was used as the control drug whereas D159687 was administered at 3 mg/kg of body weight on every weekday. These substances were administered by oral gavage by a laboratory technician who was blinded to the identity of the substance administered. This Institutional Animal Care and Use Committee (ACUC) approved this study.
A). Assessment of weight and metabolic status:
Food intake was assessed by measuring the amount of chow provided to each cage and the amount left after pre-determined intervals. Body weights were obtained weekly and used to determine the dose of D159687 to administer for the week. Body composition was obtained using EchoMRI NMR machine. Body composition were performed at baseline and after 4 and 7 weeks of exposure to the assigned therapy.
B). Assessment of Physical Function
i). Treadmill endurance test:
Treadmill endurance test was performed after 6 weeks of assigned therapy as previously described [15] with the exception that mice in this study had two, instead of three days of pre-training.
ii). Rotarod test:
We assessed coordination using rotarod test (Rotarod Rotamex 5, from Columbus Instruments) after 6 weeks on assigned therapy. Training session was performed one day before the rotarod test to condition the mice to the rotarod at a constant speed of 4 rpm. Each mouse received a single test on the accelerating rotarod at a speed of 4–40 rpm with a cut off period of five minutes.
iii). Inverted grip strength:
We assessed muscle strength by performing the inverted grip test after 4 weeks on the assigned therapy. Mice were placed on top of the center of a metal grid held above a padded surface. The experimenter slowly inverted the grid 180 degrees. The natural tendency for the mice is to hold on to the grid with all four feet to avoid falling. With the grid in reverse horizontal position and the mice firmly griping the grid, the experimenter measured the time (in seconds) that it took for the mice to release from the grid due to fatigue. If a mouse stayed on the grid for 180seconds, the experimenter stopped the test for that mouse. Each mouse underwent a total of three repetitions of the test and an average of the performance was used. A fourth trial was performed only on those mice with highly variable results during the first three trials.
C). Assessment of Cognitive Function
We assessed working memory by performing the Spontaneous Y Maze test (instrument from MedAssociates) after 5 weeks on the assigned therapy. Mice were placed in a three identical-armed maze shaped in the form of a “Y” and allowed to explore the maze freely for 5 minutes. Mice were expected to alternate visits to the arms of the Y maze while exploring the maze; this required the mice to remember the previously visited arm of the maze. Arm visits were recorded for the duration of the test, and the alteration rate was calculated as the number of alternations divided by the total number of possible alternations. Mice movements were tracked using Anymaze behavioral tracking software (from Stoelting).
D). Assessment of mitochondrial biogenesis:
Skeletal muscle mitochondrial biogenesis was assessed as previously described [25].
E). Statistical analysis:
Changes in body weight, body composition and food intake were assessed using linear mixed model. Group differences in treadmill, rotarod, spontaneous Y maze, hand grip strength and mitochondrial biogenesis were assessed with a t test. Statistical analysis was performed using SPSS Statistics version 23 for all tests except for food intake, treadmill and rotarod tests. Food intake was assessed with SPSS version 25 and rotarod and treadmill tests analysis were performed with Excel. Figures, correlation and linear regression were performed using Graph Pad Prism version 7.02. A two sided p value of less than 0.05 was considered to be statistically significant.
III). RESULTS:
Four of the nineteen mice died during the study. All four deaths occurred within the first week of the study and occurred in the D159687 treated mice groups. Three of the four mice that died were found dead in the cage the next morning after apparent state of well-being. One mouse in the D159687 treated group was sacrificed for health reasons. We performed necropsy on two of these mice and found mottled lungs (suspicious for congestion and/or pneumonia and/or hemorrhage) and pale livers in both mouse but no other apparent abnormalities. Given that four of the nine mice randomized to the D159687 treated group died early in the study leaving only five mice in this group, we randomly selected a cage with two mice from the control group to join the D159687 treatment group in the second week of the study. This mice redistribution resulted in eight mice for the control group, which were housed in two cages and seven D159687 mice group which were in four cages. We were limited to this number of mice for this study due to an unanticipated interruption in the supply of appropriately aged mice to our laboratory shortly prior to the study’s onset. Thus, data presented here were on these 15 mice that were alive throughout the remaining course of the study and included results from the first week of the study. Data from the first week for the two mice which started in the control group were analyzed under the assumption that they started out in the D159687 group.
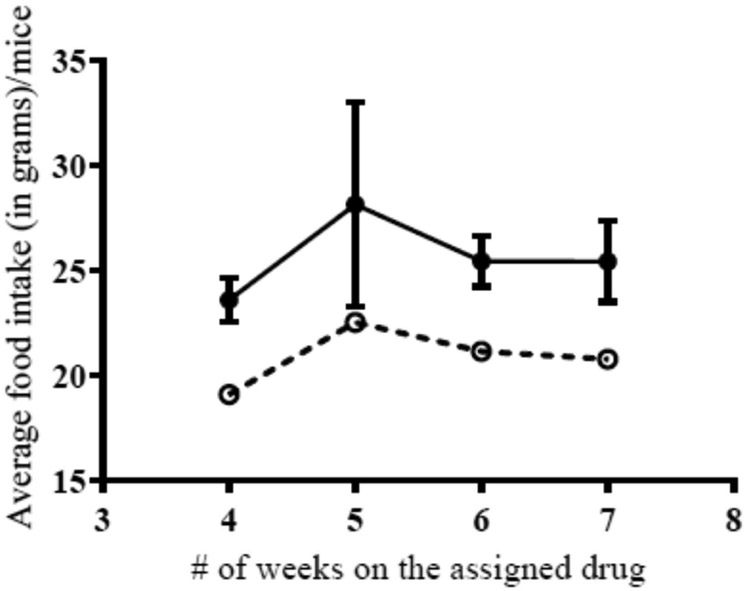
We had incomplete food intake data due to initial uncertainties about whether or not to continue the study after the initial deaths and also due to inadvertent refilling of chow by the general veterinary technician, instead of by our assigned technician. For the weeks that we were able to obtain reliable food data, we observed a significant group difference in food intake (Figure 1). D159687 treated mice ate significantly more food than control group mice.
Figure 1:
Graph of mean food intake per mice and standard error for each week on the assigned drug therapy. Closed circles are data for D159687 mice which resided in 4 cages and open circles indicate data for control group mice which resided in 2 cages. Linear mixed model demonstrate significant group and time differences (p value=0.034 and 0.001, respectively), but not group by time interaction (p value=0.881). Analysis did not control for the number of cages.
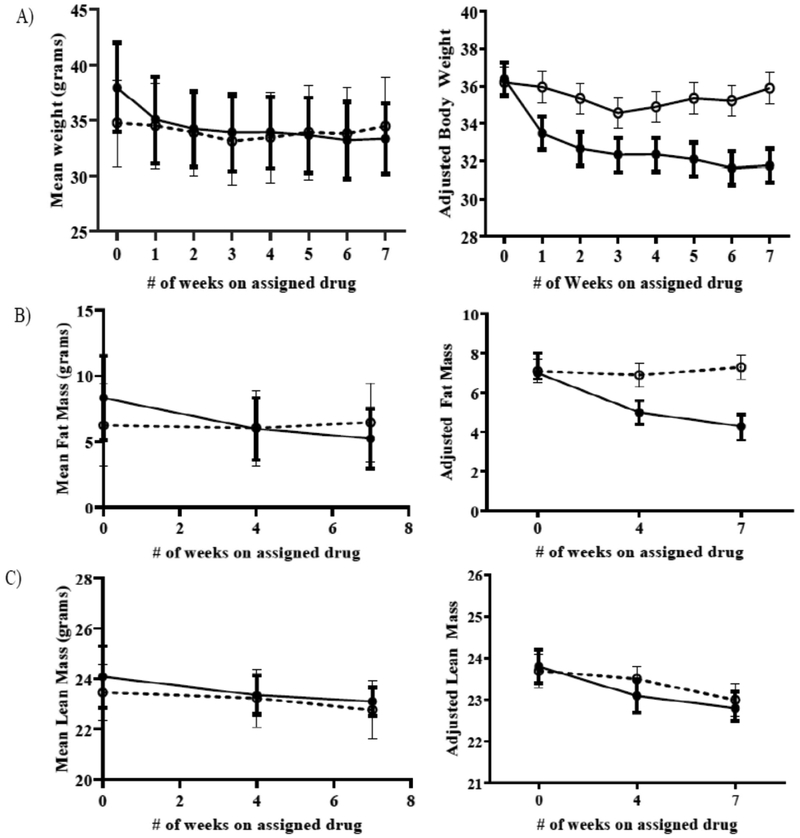
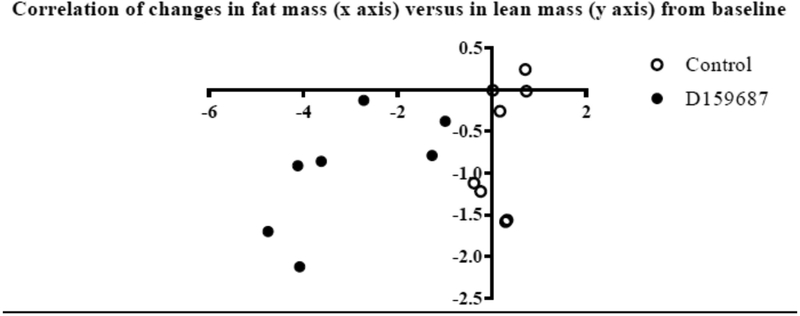
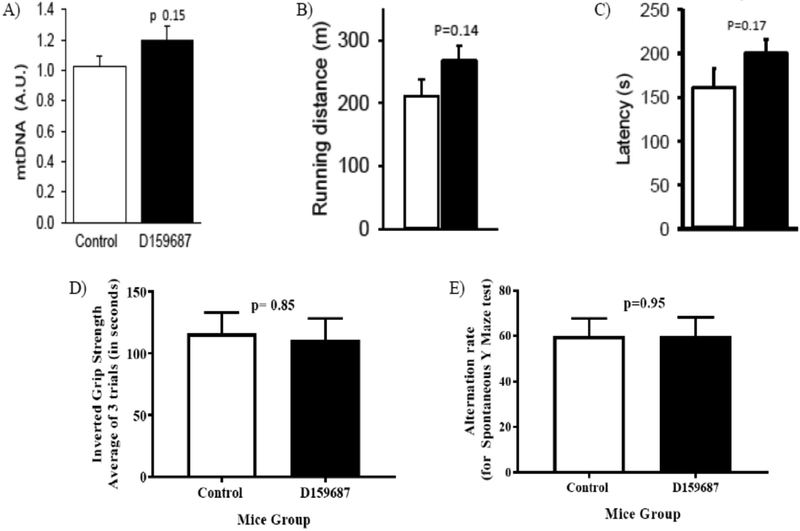
D159687 treated mice weighed more at baseline than control mice (mean weight of 38grams versus 34grams in control). In our linear mixed model analysis, after controlling for baseline weight, D159687 treated mice lost 4.2 grams more weight than the control mice. We found a statistically significant effect of time and mice group on the final weight (p value < 0.001 for both variables, Table 1) as well as an interaction between these variables (p-value < 0.001, Table 1). In other words, the D159687 treated mice lost progressively more weight over time than the control mice, whose weight were largely stable. We also observed that D159687 treated mice lost significantly more fat mass over time than the control group (Figure 2b). Lean mass decreased over time in both groups—a finding that would be expected of age-associated loss of muscle mass. Spearman correlation highlights a greater loss of fat mass than lean mass in the D159687 treated group (Figure 3) not seen in control group. We found no group differences in mitochondrial biogenesis (p value=0.15, Figure 4A). We also found no differences between the two mice groups in physical function as assessed using the inverted grip strength, rotarod and treadmill tests (Figure 4D, 4C, 4B respectively). We also did not observe any statistically significant difference between the two mice groups in their performance on the Spontaneous Y maze (Figure 4E)
Table 1:
Predictors of weight, fat mass and lean mass.
| Predictors of weight | Baseline weight | Group | Time | Group by time interaction | |
| F statistics | 269 | 29 | 15.8 | 9.1 | |
| P value | <0.001 | <0.001 | <0.001 | < 0.001 | |
| Predictors of Fat mass | Baseline Fat mass | Group | Time | Group by time interaction | |
| F statistics | 276 | 22 | 21.7 | 22 | |
| P value | <0.001 | <.001 | <0.001 | <0.001 | |
| Baseline Lean mass | Group | Time | Group by time interaction | ||
| Predictors of lean mass | F statistics | 75 | 0.432 | 16.5 | 2.4 |
| P value | <0.001 | 0.532 | <0.001 | 0.114 | |
Figure 2:
Mean Body weight (row A), Fat mass (row B) and lean mass (row C), unadjusted (column 1) and adjusted (column 2) for baseline weight, baseline fat mass and baseline lean mass respectively for the control and D159687 groups. Results are depicted with 95% confidence intervals for each time point.
Figure 3:
Correlation of changes, from baseline, in fat mass (x axis) with changes in lean mass (y axis). Changes are in grams units. Spearman correlation coefficient is 0.24 for the control (p value=0.58) and 0.79 for the D159687 treated groups (p value=0.048).
Figure 4:
Results from the following tests: Mitochondrial biogenesis (A), Treadmill (B) and rota rod (C), inverted grip strength (D) and Spontaneous Y Maze test ( E). P values for each test are reported above the bar graphs
IV). DISCUSSION:
One of the central features of aging is a change in body composition marked by an increase in body fat and a decrease in lean mass [3]. To prevent further lean mass loss and improve physical function, exercise is highly recommended in older adults on an intentional weight loss program. However, the predilection towards fat mass accretion with aging and the increasing prevalence of aging with obesity predispose a large population of individuals to physical disability [2] which would make losing weight through exercise difficult. Hence, a need exists for medications that recapitulate the benefits of caloric restriction and physical activity for use in older adults. In this study, we evaluated the potential impact of D159687, a phosphodiesterase 4D inhibitor, on body weight and composition and on physical function. To our knowledge, no previously published studies exist on the effect of PDE4D inhibitor on body weight and composition in rodents. We also evaluated this inhibitor’s effect on cognitive function. Our main finding is that D159687 induces significant weight loss over time and in particular, causes a selective reduction in fat mass while preserving lean mass. This finding of greater fat mass than lean mass loss, if proven with additional studies, could have major clinical implications. Presently, medications are yet to accomplish a selective reduction of fat mass and avoidance of worsening sarcopenia in middle and older aged adults. We also found that the weight loss induced with D159687 occurred despite significantly increased food intake in the D159687 mice group. Hence, the finding of weight loss despite increased food intake suggests that PDE4D inhibitor exerts caloric restriction effects and recapitulates some of the benefits found in the non-specific PDE4 inhibitor, rolipram [15].
We observed that the D159687 mice ate more food than the control mice. This increased food intake may be due to the fact that more D159687 treated mice were housed singly in cages than the control mice and hence, had less competition to food supply than the control mice. Nevertheless, such finding of increased food intake ought to have predisposed the D159687 mice to weight gain during the study. Instead,.D159687 caused a significant 4.2 grams weight loss from baseline in the treated mice when compared to the controls. While it is possible that the observed weight loss is due to the stress of the oral gavage, progressive weight loss in both mice groups should have been observed if this was the case. Instead, we found progressive weight and fat mass loss in the D159687 treated group only. Possible reasons for the observed fat mass loss0 include improved mitochondria function and increased fatty acid oxidation [15, 26, 27]. Our analysis did not reveal that increased mitochondrial biogenesis contributed to the observed body composition results. Furthermore, published studies demonstrate improved physical and cognitive functions with PDE4 inhibitors’ use in rodents [15, 24]. In our study, however, we found no difference in physical and cognitive functions between control and D159687 treated mice.
Our study has various strengths and weaknesses. We assess parameters—body weight and composition, physical and cognitive function—which are clinically relevant for both the obese and older adult population. The distribution of mice from the control group to the D159687 treated group likely attenuated the magnitude of the observed group differences. Despite this, these group differences remained significant, adding to the strength of the study. Thirdly, we strived to assess for changes in body composition over time in response to a potential weight loss therapy. This assessment over time is needed in preclinical studies in order to gain a full picture of which anti-obesity medications translate well in older adults. Fourth, we reported the unanticipated mortality difference between the two mice groups. Extrapolating from this, such report from preclinical studies is important as candidate therapies for age-related diseases must have minimal risk to benefits ratio.
Despite our study strengths, our study has various weaknesses. First, the high mortality rate in the D159687 treated mice limited our sample size and cast a dark cloud over the drug’s potential benefits. However, our necropsy result suggestive of acute lung injury implicates technical error--due to accidental administration of D159687 into the airways—instead of medication side effect in the observed mortality difference. A second weakness of our study was that we did not subject these aged mice to thorough baseline health assessment with a veterinarian in order to detect conditions that can lead to increased mortality during the study. Thirdly, our limited food intake measurements limited our ability to fully assess the impact of food intake on body weight. Fourth, we did not collect data on baseline physical and cognitive function and hence cannot determine whether our negative findings in these domains were due to worsened baseline function in one mice group.
In conclusion, we found that the PDE4D inhibitor, Compound D159687, show promise in inducing weight loss in aged rodents without causing lean mass loss or limiting their food intake. The weight loss induced with D159687 is predominantly fat mass loss. This finding is of interest clinically, given the natural tendency for muscle mass loss and fat mass gain to occur with aging. We did not observe any effect of Compound D159687 on cognitive and physical functions. The limitations of our study likely contributed to these negative results. Longer and larger studies are needed to re-evaluate these findings.
Supplementary Material
Study Highlights:
Seven weeks of Compound D159687 (D159687) induces weight loss in aged mice.
D159687 causes a selective loss of fat mass while preserving lean mass.
The weight loss in D159687 treated mice occurred despite increased food intake.
Acknowledgements:
We wish to thank Dr. Paul Wakim and his staff at the NIH’s Biostatistics and Clinical Epidemiology Service for their statistical help and mentorship.
Funding: This work was supported by the Intramural Research Program, National Heart Lung and Blood Institute at National Institute of Health. The institute provided funding, research personnel, facility and equipment for the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ijeoma M Muo, Email: ijeoma.muo@nih.gov.
Sung Jung Park, Email: parksj2@nhlbi.nih.gov.
Antoine Smith, Email: smitha@nhlbi.nih.gov.
Danielle Springer, Email: springerd@nhlbi.nih.gov.
Michele Allen, Email: allenm@nhlbi.nih.gov.
Timothy J Hagen, Email: thagen@niu.edu.
Jay Chung, Email: chungj@nhlbi.nih.gov.
REFERENCES:
- 1.Adams KF, et al. , Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med, 2006. 355(8): p. 763–78. [DOI] [PubMed] [Google Scholar]
- 2.Wong E, et al. , The role of obesity duration on the association between obesity and risk of physical disability. Obesity (Silver Spring), 2015. 23(2): p. 443–7. [DOI] [PubMed] [Google Scholar]
- 3.Newman AB, et al. , Weight change and the conservation of lean mass in old age: the Health, Aging and Body Composition Study. Am J Clin Nutr, 2005. 82(4): p. 872–8; quiz 915-6. [DOI] [PubMed] [Google Scholar]
- 4.Beavers KM, et al. , Effect of an 18-month physical activity and weight loss intervention on body composition in overweight and obese older adults. Obesity (Silver Spring), 2014. 22(2): p. 325–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villareal DT, et al. , Aerobic or Resistance Exercise, or Both, in Dieting Obese Older Adults. N Engl J Med, 2017. 376(20): p. 1943–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beavers KM, et al. , Fat mass loss predicts gain in physical function with intentional weight loss in older adults. J Gerontol A Biol Sci Med Sci, 2013. 68(1): p. 80–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rejeski WJ, et al. , Lifestyle change and mobility in obese adults with type 2 diabetes. N Engl J Med, 2012. 366(13): p. 1209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saunders KH, et al. , Pharmacotherapy for Obesity. Endocrinol Metab Clin North Am, 2016. 45(3): p. 521–38. [DOI] [PubMed] [Google Scholar]
- 9.Ritz P, et al. , Benefits and risks of bariatric surgery in patients aged more than 60 years. Surg Obes Relat Dis, 2014. [DOI] [PubMed] [Google Scholar]
- 10.Panagiotou OA, et al. , Comparative Effectiveness and Safety of Bariatric Procedures in Medicare-Eligible Patients: A Systematic Review. JAMA Surg, 2018: p. e183326. [DOI] [PubMed] [Google Scholar]
- 11.Ravussin E, et al. , A 2-Year Randomized Controlled Trial of Human Caloric Restriction: Feasibility and Effects on Predictors of Health Span and Longevity. J Gerontol A Biol Sci Med Sci, 2015. 70(9): p. 1097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heilbronn LK, et al. , Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA, 2006. 295(13): p. 1539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mercken EM, et al. , Calorie restriction in humans inhibits the PI3K/AKT pathway and induces a younger transcription profile. Aging Cell, 2013. 12(4): p. 645–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burkewitz K, Zhang Y, and Mair WB, AMPK at the nexus of energetics and aging. Cell Metab, 2014. 20(1): p. 10–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park SJ, et al. , Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell, 2012. 148(3): p. 421–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensterle M, Kocjan T, and Janez A, Phosphodiesterase 4 inhibition as a potential new therapeutic target in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab, 2014. 99(8): p. E1476–81. [DOI] [PubMed] [Google Scholar]
- 17.Jensterle M, et al. , Short term monotherapy with GLP-1 receptor agonist liraglutide or PDE 4 inhibitor roflumilast is superior to metformin in weight loss in obese PCOS women: a pilot randomized study. J Ovarian Res, 2015. 8: p. 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azevedo MF, et al. , Clinical and molecular genetics of the phosphodiesterases (PDEs). Endocr Rev, 2014. 35(2): p. 195–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hebenstreit GF, et al. , Rolipram in major depressive disorder: results of a double-blind comparative study with imipramine. Pharmacopsychiatry, 1989. 22(4): p. 156–60. [DOI] [PubMed] [Google Scholar]
- 20.Heaslip RJ and Evans DY, Emetic, central nervous system, and pulmonary activities of rolipram in the dog. Eur J Pharmacol, 1995. 286(3): p. 281–90. [DOI] [PubMed] [Google Scholar]
- 21.Mori F, et al. , The human area postrema and other nuclei related to the emetic reflex express cAMP phosphodiesterases 4B and 4D. J Chem Neuroanat, 2010. 40(1): p. 36–42. [DOI] [PubMed] [Google Scholar]
- 22.Burgin AB, et al. , Design of phosphodiesterase 4D (PDE4D) allosteric modulators for enhancing cognition with improved safety. Nat Biotechnol, 2010. 28(1): p. 63–70. [DOI] [PubMed] [Google Scholar]
- 23.Zhang C, et al. , Comparison of the Pharmacological Profiles of Selective PDE4B and PDE4D Inhibitors in the Central Nervous System. Sci Rep, 2017. 7: p. 40115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruno O, et al. , GEBR-7b, a novel PDE4D selective inhibitor that improves memory in rodents at non-emetic doses. Br J Pharmacol, 2011. 164(8): p. 2054–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park SJ, et al. , DNA-PK Promotes the Mitochondrial, Metabolic, and Physical Decline that Occurs During Aging. Cell Metab, 2017. 25(5): p. 1135–1146 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kraynik SM, Miyaoka RS, and Beavo JA, PDE3 and PDE4 isozyme-selective inhibitors are both required for synergistic activation of brown adipose tissue. Mol Pharmacol, 2013. 83(6): p. 1155–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oknianska A, et al. , Long-term regulation of cyclic nucleotide phosphodiesterase type 3B and 4 in 3T3-L1 adipocytes. Biochem Biophys Res Commun, 2007. 353(4): p. 1080–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.