Abstract
Management of lung disease in patients with alpha-1 antitrypsin deficiency (AATD) includes both non-pharmacological and pharmacological approaches. Lifestyle changes with avoidance of environmental pollutants, including tobacco smoke, improving exercise levels and nutritional status, all encompassed under a disease management program, are crucial pillars of AATD management. Non-pharmacological therapies follow conventional treatment guidelines for chronic obstructive pulmonary disease. Specific pharmacological treatment consists of administering exogenous alpha-1 antitrypsin (AAT) protein intravenously (augmentation therapy). This intervention raises AAT levels in serum and lung epithelial lining fluid, increases anti-elastase capacity, and decreases several inflammatory mediators in the lung. Radiologically, augmentation therapy reduces lung density loss over time, thus delaying disease progression. The effect of augmentation therapy on other lung-related outcomes, such as exacerbation frequency/length, quality of life, lung function decline, and mortality, are less clear and questions regarding dose optimization or route of administration are still debatable. This review discusses the rationale and available evidence for these interventions in AATD.
Keywords: alpha-1 antitrypsin, alpha-1 antitrypsin deficiency, augmentation therapy, disease management programs, exacerbations, inflammation, lung volume reduction, pulmonary rehabilitation, quality of life
Introduction
Lung disease is a characteristic pathological feature of alpha-1 antitrypsin deficiency (AATD). Although some patients present with bronchiectasis of variable severity, the most common lung manifestation is emphysema and clinical chronic obstructive pulmonary disease (COPD). Therefore, the treatment of lung disease in AATD encompasses therapies recommended by the Global Initiative for Chronic Obstructive Lung Disease (GOLD).1 In addition, an approved and specific treatment for emphysema due to AATD is the intravenous (IV) administration of alpha-1 antitrypsin (AAT) therapy (referred to as augmentation therapy). Augmentation therapy has been shown to slow the progression of lung density loss;2,3 however, more solid evidence of effect in other clinical outcomes is needed.
We briefly review the currently indicated non-pharmacological and pharmacological options for the management of lung disease in patients with AATD, placing special emphasis on the existing evidence for these therapies in this specific population, highlighting knowledge gaps and future directions of care.
Non-pharmacological interventions in the management of AATD-associated lung disease
Non-pharmacological interventions are an essential part of therapy for respiratory diseases and are not specific for AATD. They provide symptomatic improvement and better quality of life; some strategies can also prolong survival.4
What we know
Smoking cessation and lifestyle modification
Exposure to environmental and tobacco smoke is the most important risk factor for the development of lung disease in AATD. Patients who smoke are at a far higher risk of developing lung disease compared with those who do not, and smoking is known to accelerate the decline in lung function in this condition.5,6 Patients with AATD who have never smoked are usually asymptomatic, typically identified through family testing, and often have a normal lifespan.7 Although no interventional clinical trials of smoking cessation in AATD are available, these observations strongly indicate that smoking cessation is a disease-modifying lifestyle intervention that can improve outcomes in patients with AATD.8 In addition, it has been shown that smoking oxidizes AAT and diminishes its anti-elastase function,9 a compulsory reason to mandate that patients with AATD stop smoking prior to the initiation of augmentation therapy.10 Exposure to pollutants also produces an effect similar to tobacco smoke. Occupational exposure to mineral dust, smoke, and fumes is related to poorer lung-related outcomes in patients with AATD.11 Therefore, patients in high-risk occupations (e.g. farmers and construction workers, etc.) should consider ways to reduce their exposure to these agents.
Disease management
Disease management programs such as the Alpha-1 Disease Management and Prevention Program (ADMAPP) developed by AlphaNet, a non-profit organization, encompass many patient-focused initiatives, including patient education, with the goal of optimizing patient outcomes. One of the key objectives of ADMAPP is to empower patients by having access to detailed educational material relevant to their care. In addition, AlphaNet provide a telephone monitoring service that allows patients to interact with coordinators on a monthly basis, thereby providing close follow-up of patients enrolled in the program.12 Patients enrolled in ADMAPP were shown to be more likely to take preventive measures, such as smoking cessation and exercise, and felt more informed about their condition.12 This program has also been shown to improve medication usage and was associated with decreased exacerbations, improvements in quality of life, and decreased healthcare utilization.13 Moreover, disease management programs may be useful tools for informing and improving preventive measures taken by patients with AATD.12
What we do not know
Surgical and interventional approaches
Lung transplantation is the only treatment option available for patients with AATD-associated end-stage lung disease, and is associated with favorable outcomes.14,15 This is discussed in more detail within the transplantation chapter of this series of reviews by Zamora and Ataya.16 However, the role of lung volume reduction techniques in AATD is less clear. Lung volume reduction surgery (LVRS), a successful therapy for palliative care for carefully selected patients with end-stage emphysema, has been shown to improve lung function,17,18 exercise capacity,19 and quality of life.20 In the National Emphysema Treatment Trial, which investigated the effects of LVRS in 1218 patients with emphysema, only 16 (1.3%) had severe AATD, and, of these, 10 were randomized to undergo LVRS.21 In this group of 10 patients, trends were observed toward shorter-lived improvements in forced expiratory volume in 1 s (FEV1) after treatment compared with patients with non-AATD-related emphysema.21 There was also higher mortality in AAT-deficient individuals randomized to surgery compared with medical treatment, suggesting caution in recommending LVRS in patients with AATD.21
In AATD specifically, LVRS can produce significant improvements in pulmonary function tests (PFTs) and exercise capacity in the short term but levels return to baseline between 6 and 12 months post surgery.22 PFTs also show further deterioration after 24 months.22 These short-term results are unlikely to be due to inadequate lung resection and may be due to a more rapid progression in the remaining tissue, and/or impairment of the diaphragm induced by the surgery.22 In AATD, lung function improvements last up to 3.5 years after LVRS,23 while in non-AATD associated emphysema, benefits of LVRS can last up to 5 years.24 Endobronchial lung volume reduction (ELVR) is a less invasive form of LVRS, but most studies have excluded patients with AATD as they were previously deemed unsuitable for the procedure based on LVRS criteria (AATD-associated emphysema typically affects lower lung lobes in contrast to smoking-induced emphysema, which more often affects upper lobes). In 12 patients homozygous for AATD (genotype not specified), it was demonstrated that ELVR can increase FEV1 (mean: 54%) and improve quality of life.25 Two patients were even taken off oxygen therapy completely,25 suggesting there is no reason to exclude patients with AATD from ELVR surgery. Nevertheless, this study was only conducted in a small group of carefully selected patients and the use of ELVR in patients with AATD, therefore, requires further investigation.
Endobronchial coil treatment (ECT) is another less invasive procedure for emphysema patients but there are few data for ECT in AATD, as AATD is classically an exclusion criterion in randomized controlled trials (RCTs). However, in a post hoc analysis of the REVOLENS study,26 in which 50 patients were randomized to receive ECT, there were significant decreases in hyperinflation and improvements in quality of life for the six patients with AATD.27 Results also suggested that patients with AATD had similar efficacy and safety outcomes at 1 year to patients with non-AATD-related severe emphysema.27 As with ELVR, appropriately powered studies are required to assess the true effects of ECT in AATD.
Pulmonary rehabilitation and exercise
Pulmonary rehabilitation is defined by the American Thoracic Society as a multidisciplinary program of care for patients with chronic respiratory impairment that is individually tailored and designed to optimize physical and social performance and autonomy.28 The program encompasses exercise training (e.g. aerobic exercise and strength training, as well as specific respiratory muscle training), education (e.g. breathing strategies, energy conservation, and work simplification), and facilitating beneficial psychosocial and behavioral interventions.28 In COPD, pulmonary rehabilitation has been shown to relieve dyspnea and fatigue, improve emotional function and enhance the sense of control that individuals have over their condition. It is also beneficial in improving health-related quality of life, exercise capacity and inspiratory capacity.29,30 Patients with COPD undergoing an individualized, structured, high-intensity training program improved their exercise capacity, gained muscle mass, and improved their quality of life.31 These proven benefits of pulmonary rehabilitation are likely applicable to patients with AATD. A study comparing the effects of pulmonary rehabilitation in patients with AATD-related COPD and general COPD concluded that the benefits in terms of physical performance increase were similar in both groups of patients.32 However, data on emphysema distribution, comorbidities, and individual pharmacological therapy were not available in the database used for this study and so it cannot be excluded that patient comorbidities, which can have profound effects on quality of life, may have influenced results. The effects of exercise on specific clinical outcomes in AATD are likely positive but require further investigation.
Nutrition
Weight loss and malnutrition, characterized by cachexia and muscle wasting, are commonly reported in patients with COPD and emphysema, and weight loss has been recognized as a poor prognostic factor in these patients.5,33 In AATD, concentrations of total body protein and plasma transthyretin (a biomarker of lean body mass and catabolism) are reduced and indicate early signs of malnutrition.34 Nutritional supplement therapy is effective for maintaining and improving muscle strength and exercise tolerance in patients with COPD who are poorly nourished, thereby decreasing morbidity and mortality.33
The relationship between obesity and risk of respiratory disease is controversial.35,36 While it is established that obesity increases the risk of respiratory disorders, including COPD,37 it has also been observed that obesity in COPD may be associated with improved survival and lung function.38 In obese patients with COPD, it is recommended that patients adhere to dietary restrictions alongside resistance strength training regimens.39 In addition to respiratory disorders, obesity has also been linked to AATD-associated liver disease.40 In a study of the epigenetic features of liver biopsies, a marked overlap was found between samples from patients with AATD liver cirrhosis and those with obesity/fatty liver-driven disease.40
Overall, it is clear that appropriate nutrition is important in the management of patients with COPD, but it is currently unclear whether requirements differ for patients with AATD, with a possible link between obesity and liver disease warranting further investigation. In the absence of more specific guidance, general COPD recommendations should be followed in relation to nutrition and AATD.
Vaccinations
There is little specific evidence for the use of influenza and pneumococcal immunization in AATD-associated COPD. One observational study has shown no significant impact of influenza vaccination on exacerbations in a cohort of patients with AATD during one flu season.41 However, given the inherent limitations of this observational study, and the limited evidence in this area overall, there is no rationale to deviate from COPD guidelines in this regard. Until further evidence is available, practice should follow national influenza and pneumococcal vaccination protocols for COPD.42,43 The role of hepatitis in the promotion of AATD-related liver disease has been suggested, but there are few supporting data.10 Nevertheless, hepatitis A and B vaccinations may be beneficial in patients with established liver disease, especially in pediatric patients.44
Pharmacological management of AATD-associated lung disease
Pharmacological therapies are instrumental in the management of patients with lung disease and AATD. Recommendations for management of chronic symptoms and acute exacerbations in patients with AATD generally follow those for general COPD, as described in the guidelines from GOLD,1 and include the use of bronchodilators and inhaled corticosteroids (ICSs).45 Key changes in the most recent GOLD guidelines are summarized in Table 1.
Table 1.
Key changes to recommendations in the most recent GOLD guidelines update.1
| Domain | Change |
|---|---|
| Assessment methods | • Assess disease severity based on symptoms and exacerbations using mMRC and AECOPD guidelines, whilst also taking risk factors and spirometry measurements into account |
| Devices | • The importance of inhaler device training and education cannot
be over-emphasized • Inhaler technique should be regularly assessed • Effectiveness and safety of e-cigarettes as a smoking cessation aid is uncertain at present |
| Pharmacological treatment | • Pharmacological therapy can reduce symptoms, reduce
exacerbation frequency and severity, and improve health status
and exercise tolerance • Each pharmacological treatment regimen should be individualized and guided by severity of symptoms, exacerbation risk, side effects, comorbidities, drug availability and cost, and the patient’s response, preference, and ability to use various drug delivery devices • Influenza and pneumococcal vaccinations decrease the incidence of lower respiratory tract infections |
| Non-pharmacological treatment | • Long-term oxygen therapy improves survival in patients with
severe resting chronic hypoxemia but should not be prescribed
routinely for stable COPD and resting/exercise-induced moderate
desaturation – individual patient factors must be considered
when evaluating the patient’s need for oxygen
supplementation • Long-term non-invasive ventilation may decrease mortality and prevent re-hospitalization in patients with severe chronic hypercapnia and a history of hospitalization for acute respiratory failure • Pulmonary rehabilitation improves symptoms, quality of life, and physical and emotional participation in everyday activities • Surgical or bronchoscopic interventional treatments may be beneficial in select patients with advanced emphysema* |
Note that lung volume reduction is generally considered less suitable for patients with alpha-1 antitrypsin deficiency.10
AECOPD, acute exacerbations of chronic obstructive pulmonary disease; COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; mMRC, modified Medical Research Council.
What we know
Patients with AATD have generally been excluded from RCTs studying pharmacological therapies in COPD. The only trial of an inhaled therapy in AATD is a small ICS trial in eight patients with the most severe form of AATD (PI*ZZ).46 The trial showed that the addition of ICS to long-acting beta-agonists decreased airway narrowing and reduced dynamic hyperinflation, with marked improvements in exercise tolerance (as measured via a shuttle walking test), and dyspnea.46
In the acute management of disease exacerbations, early antibiotic use has been shown to decrease exacerbation length in patients with AATD.47
What we do not know
Although no specific RCT exists for patients with AATD and COPD, all other beneficial studies in COPD are widely used to guide recommendations for the management of AATD. These interventions include the use of long-acting muscarinic antagonists, the continuous or intermittent use of antibiotics to prevent or reduce COPD exacerbations,48,49 mucolytics to reduce exacerbations,50 the use of supplemental oxygen in subjects with severe hypoxemia (partial pressure of oxygen <55 mmHg),51 and the use of ICS in patients with frequent exacerbations and eosinophilia.52–54 It is clear that studies are needed to confirm the effects of these therapies for patients with AATD and whether these may differ from general COPD but these are unlikely to be performed. The benefits of other pharmacologic agents recommended by the GOLD report, such as roflumilast, azithromycin, or N-acetyl cysteine,1 have not yet been proven in RCTs for individuals with AATD. Nevertheless, these medications represent a reasonable step-up when escalation of therapy beyond inhalers is required.1
Specific pharmacological treatment of AATD-associated lung disease: augmentation therapy
Augmentation therapy consists of scheduled (usually weekly) IV infusion of exogenous AAT protein, derived from the plasma of healthy individuals. The aim is to increase AAT levels in the blood and lungs of patients with AATD in order to restore the balance of unopposed protease activity [by neutrophil elastase (NE) and other proteases]. Augmentation therapy is indicated for patients with severe AAT deficiency (with serum AAT level <11 µM) and evidence of lung disease, namely emphysema with decline in lung function. Once started, treatment is lifelong.
What we know
Biochemical efficacy
The biochemical efficacy of augmentation therapy was first shown in 1987, where weekly IV AAT therapy significantly increased AAT levels in both serum and lung epithelial-lining fluid in 21 patients with AATD given AAT at 60 mg/kg per week.55 On this basis, augmentation therapy was approved by the United States (US) Food and Drug Administration with 60 mg/kg per week as the standard dose. The biochemical efficacy of AAT therapy has been well established in several studies, where this standard dose has been shown to raise serum AAT levels and increase NE inhibition.56,57 NE inhibition leads to mitigation of elastin degradation and hence progression of emphysema. Desmosine and isodesmosine are unique amino acid cross-links in mature elastin fibers and can serve as biomarkers of elastin degradation.58 Augmentation therapy is associated with significant reductions of both amino acids in plasma, bronchoalveolar lavage fluid, and urine.59,60
Lung density
Changes in lung density measured by computed tomography are now used as a surrogate to measure changes in emphysema progression.61 The RAPID trial, which was a 2-year randomized, double-blind, placebo controlled trial to assess clinical efficacy of augmentation therapy, compared outcomes for 92 patients with AATD lung disease receiving augmentation therapy and 85 AATD patients receiving placebo treatment.2 The trial demonstrated that patients treated with augmentation therapy exhibited a significantly lower annual rate of lung density loss at total lung capacity (−1.45 g/L) compared with patients receiving placebo (−2.19 g/L; p = 0.03).2 In a 2-year open-label extension study (RAPID-OLE) in which 140 patients from the RAPID trial were all treated with augmentation therapy, patients who were previously assigned to the augmentation therapy group showed a significantly reduced decline in lung density loss compared with patients initially assigned to placebo.3 Notably, during the extension study, annual lung density loss in patients who had not received IV AAT in the initial RAPID study improved significantly (from −2.26 g/L to −1.26 g/L; p = 0.02), while lung density loss in patients who had previously received AAT showed little change (from −1.51 g/L to −1.63 g/L; p = 0.823),3 demonstrating that the efficacy of AAT was maintained throughout the RAPID study and into the extension study. Overall, these data support a disease-modifying effect of augmentation therapy, that is, change the course of disease progression.8 It is also worthy to mention that a post hoc analysis showed that baseline lung function had no major impact on the efficacy of augmentation therapy on lung density decline.62
Results from the RAPID study were in line with those from earlier pilot studies evaluating augmentation therapy; the Danish–Dutch study and the EXACTLE trials.63–65 A combined analysis of data obtained from these studies, which enrolled a total of 119 patients, showed a significant reduction in the decline in lung density loss in AAT-treated patients compared with control patients (−4.082 g/L versus −6.379 g/L; p = 0.006) over the 2.5-year study period, corresponding to annual declines of −1.73 g/L and −2.74 g/L, respectively.65
FEV1 decline
Observational evidence indicates that augmentation therapy also slows the decline in FEV1, at least in a subset of patients.66 The Alpha-1 Antitrypsin Deficiency Registry Study Group found no overall difference in observed FEV1 decline between patients who received augmentation therapy and those who did not (not a randomized study), but noted that treated patients with an FEV1 35–49% of predicted at baseline, had a significant slowing of FEV1 decline of 27 mL/year compared with untreated patients with a similar range of lung function (p = 0.03).66 A meta-analysis of five observational trials, enrolling a total of 1509 patients, demonstrated that patients treated with AAT had a 23% lower rate of FEV1 decline (corresponding to an absolute difference of 13.4 mL/year) compared with patients not receiving augmentation therapy.67 Importantly, this effect was primarily mediated by a subset of patients with a FEV1 30–65% of predicted at baseline, which showed a 26% reduction in FEV1 decline (corresponding to an absolute difference of 17.9 mL/year); reductions in patients with an FEV1 percentage of predicted <30% or >65% were not significant.67 This may be due to the variability of FEV1 decline as an outcome measure; patients with moderate impairment in FEV1 show less variability in readings.66
On the other hand, none of the existing RCTs evaluating augmentation therapy (RAPID, EXACTLE, Danish–Dutch study), either alone or in combination, showed an overall effect of augmentation therapy on FEV1 (RAPID: −3.1% and −2.3% compared with baseline for AAT and placebo, respectively; EXACTLE/Danish–Dutch study combined analysis: FEV1% predicted was 48.0% versus 47.9% at end of study for AAT and placebo, respectively).2,65 It is important to highlight that none of these studies were designed to assess FEV1 differences, as such a study requires enrollment of hundreds of patients with many years of follow-up.68
Effect on inflammation
In addition to its anti-elastase properties, AAT has important immunomodulatory and anti-inflammatory functions.69,70 For example, AAT upregulates the expression of the anti-inflammatory cytokine interleukin (IL)-1 receptor antagonist and blocks the release of IL-1 and tumor necrosis factor (TNF)-α in stimulated peripheral blood mononuclear cells.71,72 Within a week, augmentation therapy can lower serum IL-8 levels.73 Within a month, the current standard dose of 60 mg/kg per week has been shown to significantly reduce sputum elastase activity and sputum levels of the chemoattractant leukotriene B4, and a trend towards decreased sputum myeloperoxidase and IL-8 has been observed.74 A state of neutrophil “hyperactivity” has been described in AATD, which can be mitigated in vivo with the administration of augmentation therapy.75,76 AAT also acts on the lung endothelium, an active participant in the inflammatory response, by assisting in the resolution of chronic inflammation.77 Some individuals still exhibit residual systemic and pulmonary inflammation that can be further improved with increased dosing of AAT (120 mg/kg per week).78
The anti-inflammatory properties of AAT have led to its evaluation in other non-AATD inflammatory and autoimmune conditions, such as diabetes type I, rheumatoid arthritis, solid organ transplants, and graft-versus-host disease (GVHD).79–81 Investigations are beginning to shed light on the specific signaling pathways involved in AAT’s anti-inflammatory mode of action. In a mouse model of acute myocardial infarction, treatment with AAT reduced caspase-1 activity in the ischemic myocardium by >90%.82 In the same study, AAT treatment preserved viable myocardium by reducing cell death by >50% and prevented adverse cardiac remodeling.82 Treatment with AAT has also been shown to suppress several inflammatory mediators in models of GVHD. AAT treatment suppresses CD8+ cell proliferation and suppresses increased expression of the IL-32, TNF-α, IL-6, and IL-8 inflammatory cytokines in mixed lymphocyte cultures from patients with chronic GVHD and in a murine transplant model.81
Safety
Augmentation therapy is generally very well tolerated and of the few side effects that have been reported the majority have been mild and rarely required intervention and therapy interruption.83 In a German study between 1989 and 1995, 443 patients received 58,000 infusions of 60 mg/kg per week IV AAT for the treatment of severe AATD and only 124 adverse reactions were reported in 65 patients.84 In the majority of these patients, the adverse reactions were typical for IV infusion of proteins, such as fever/chills, urticaria, nausea/vomiting, and fatigue.84 Only three patients terminated treatment due to repeated episodes of fever and chills immediately following AAT infusion.84 Five severe adverse reactions were reported in five patients, all of which made complete recovery: anaphylactic reactions in four patients and worsened congestive heart failure with concomitant respiratory failure in one patient.84 There were no deaths or viral transmission related to AAT infusion.84
The frequency of adverse reactions following AAT augmentation was also very low in a US study, with 0.019–0.023 events per patient-month reported.85 The most common adverse reactions were headache, dizziness, and dyspnea, which was classified as a severe reaction.85 Patients who received weekly IV AAT did, however, report a higher rate of total adverse reactions compared with those who received treatment every 2–3 weeks, or monthly (0.030, 0.024, and 0.005 events per patient-month, respectively).85 Furthermore, higher rates of severe and moderate adverse events were reported in those treated weekly compared with those who received treatment every 2–3 weeks, who generally reported milder adverse reactions.85 Nevertheless, AAT augmentation was generally considered as well tolerated.
AAT augmentation was also well tolerated within the RAPID and RAPID-OLE studies described above. In the RAPID study, reported adverse events were similar between the treatment and the placebo groups.2 Only one treatment-emergent adverse event in one patient within the treatment group led to withdrawal from the study; there were 10 treatment-emergent adverse events in four patients that led to study withdrawal from the placebo group.2 This higher number of withdrawals from the placebo group was unexplained but was consistent with the greater loss of lung density within this group of patients.2 In the RAPID-OLE study, there were no safety concerns associated with treatment; most treatment-emergent adverse events were related to the underlying disease, or events that commonly occur in the general population, and were similar to those in the RAPID study.3
In a small pilot study, the effects of increased dosing were investigated. After 4 weeks of receiving a double dose of AAT (120 mg/kg per week), there were no adverse events reported as related to augmentation therapy.78 There were, however, three minor adverse events that were assessed as probably/likely related to study procedures.78 Whilst these safety results suggest that dosing higher than the current standard dose is well tolerated, the pilot study was underpowered (n = 8 patients) and administered for only 4 weeks. In a slightly longer study, the safety of increased dosing was assessed in 30 patients with AATD over two treatment periods of 8 weeks, where patients were on either 60 or 120 mg/kg per week in first treatment period before being administered the alternate dose in the second treatment period.86 In this crossover study, 120 mg/kg per week dose was considered to be as well tolerated as the standard 60 mg/kg per week dose, with just one treatment-related reaction associated with the 120 mg/kg per week dose and five reactions associated with the standard dose.86 All adverse events were of mild intensity, of which COPD exacerbation was the most common; no severe reactions, study withdrawals, or deaths were reported.86 These studies suggest that higher AAT dosing is well tolerated and should be further investigated.
What we do not know
Effect on exacerbation frequency
As with FEV1 decline, there are no RCTs designed to test the effect of augmentation therapy on exacerbations and the evidence of observational studies is less compelling. In a US survey of 96 adult patients receiving AAT and 47 patients not receiving therapy, patients on augmentation therapy subjectively reported a markedly lower frequency and severity of lung infection compared with patients not receiving AAT.87 Results of a questionnaire assessing exacerbation rates in an Austrian–German survey suggested lower exacerbations in patients on augmentation therapy (2.13 ± 1.2) compared with those on no therapy (3.47 ± 2.8).88 A retrospective record review of exacerbation frequency in 127 patients showed a marked reduction in exacerbations after augmentation therapy was started, in particular in prior exacerbators.89 In the RAPID and EXACTLE RCTs, although not statistically significant, patients that received augmentation therapy experienced more exacerbations than patients who received placebo.90 With the studies combined in a meta-analysis, this difference reached significance (a 0.29/year higher incidence; p = 0.02).90 It is clear that further research needs to be done on this topic. Augmentation therapy may not change the incidence of exacerbations but may modify its severity. Indeed, in a post hoc analysis of the EXACTLE RCT, a decrease in severe exacerbations (6.7% versus 13.5%; p = 0.013) and hospitalizations due to exacerbations (15.8% versus 28.2%) was observed in the AAT-treated patients compared with controls, although the effect on hospitalizations was not statistically significant.64
Survival/mortality
A comparison study of patients from the UK (AAT-naive) and US (treated with AAT), matched by age and lung function, showed an overall positive effect of AAT treatment on survival and time to lung transplantation.91 A survival advantage was also suggested in the Alpha-1 Antitrypsin Deficiency Registry Study Group study, which reported that patients who received AAT had a significantly lower risk of mortality than patients who did not receive AAT (risk ratio = 0.64; p = 0.02).66 Although this effect has not been confirmed in a RCT, a post hoc analysis of the RAPID program demonstrated that augmentation therapy was associated with a projected 5.6 life-years gained compared with patients not receiving treatment, based on an extrapolation to the time to reach a terminal lung density threshold of approximately 20 g/L (the average lung density of seven patients who exited the program due to respiratory failure, death, or lung transplantation).3,92
Quality of life
AATD has a severe impact on the quality of life of affected individuals.93,94 One major goal of the management of patients with AATD is to enhance quality of life, but due to a lack of data, there is no definitive evidence on how quality of life is impacted by augmentation therapy.95 A meta-analysis of data from RCTs of augmentation therapy (EXACTLE and RAPID) indicates a small non-significant difference in favor of treatment.90 For studies to determine the impact on quality of life, longer evaluations on larger sample sizes are required, which can be difficult to achieve in studies on rare diseases.96 Patient registries may be the answer to obtaining data on quality of life as they can recruit much larger numbers of patients and follow them for many years.96
What is the ideal route or dose of therapy with AAT?
There is growing interest in the inhaled route of AAT administration as initial studies showed that more AAT reaches the lungs via inhalation than through the IV route (14.6% versus 2%, respectively).97 Advantages of inhaled AAT include convenience for patients to self-administer, lower cost, and avoiding risks associated with IV administration such as thrombosis and infection.98 The safety and efficacy of inhaled AAT was recently investigated in a large study in which 168 patients were randomized to receive twice-daily inhalations of 80 mg AAT or placebo for a total period of 50 weeks.99 The study reported no significant difference between treatment groups for time to the first event-based exacerbation or mean rate of annual exacerbations (3.12 in treated patients and 2.67 in controls; p = 0.31).99 However, a post hoc analysis revealed that patients treated with inhaled AAT experienced a significantly lower decline in FEV1 compared with controls. Over the 50-week study period, the difference in FEV1 decline between the two groups was 48 mL/year (unpublished data).100 However, due to the route of administration, it is not clear whether the observed difference in FEV1 decline is due to a bronchodilator effect. In terms of safety, while more patients treated with inhaled AAT experienced adverse events (AEs) (57.5% versus 46.9%) and serious AEs (6.9% versus 0%) during the entire study compared with placebo, an improvement in the safety profile was observed after changes were made to the handling of the nebulizer device.99 Although the results from this study are mixed, they point toward a potential beneficial effect on lung function that warrants verification in a carefully designed follow-up study.
In recent years there has been a focus on other ways of improving patient quality of life, largely through patient-centric initiatives to improve the convenience of IV augmentation therapy infusions through self-administration and extended-interval dosing.96 The standard dose of 60 mg/kg AAT per week aims to achieve a target serum AAT concentration of >11 µM;56 the putative target threshold. However, variations to the typical weekly dosing schedule appear to be common; for example, some patients in the RAPID study received 120 mg/kg every other week and dosing in the Danish–Dutch trial was 250 mg/kg every 4 weeks.65,101 These biweekly and monthly dosing intervals have been shown to result in trough serum AAT concentrations slightly below the 11 µM target threshold for augmentation therapy,102,103 and therefore, fixed weekly dosing should be recommended unless other dosing scenarios are absolutely necessary.
Whether the standard recommended dose of 60 mg/kg is most adequate remains unclear, as some patients on the standard dose can still exhibit residual inflammation, protease activity, and elastin degradation which can be further mitigated by increasing the treatment dose.78 Pharmacometric analysis of data from the RAPID trials has suggested that increasing serum levels above the 11 µM threshold is associated with greater clinical effect;56,104 a finding that was supported by a recent pilot study, which observed significant improvements in multiple markers of disease activity in patients up-titrated from 60 to 120 mg/kg AAT per week.78 Increasing the dose to 120 mg/kg per week was also well tolerated and restored serum AAT levels to be more in line with the normal range (>25 µM). However, the long-term benefit of increased dosing requires further investigation in a larger cohort of patients; the clinical efficacy of higher dosing will be addressed by post-marketing studies of augmentation therapy as requested by regulatory agencies, which will investigate the efficacy of 60 versus 120 mg/kg weekly dosing.56,105
Summary of augmentation therapy
In summary, the available data support the conclusion that augmentation therapy improves anti-protease balance, reduces elastin degradation products, and reduces lung density decline in patients with AATD. Moreover, augmentation therapy appears to show some survival benefit and slow FEV1 decline in patients presenting with an FEV1 in the range 30−60% predicted, although this effect may be related to limitations of FEV1 as an outcome measure. Further beneficial effects may be noted with different administration routes or dosing aimed at normalizing serum AAT levels. Although the treatment does not appear to have a clear effect on frequency of exacerbations, it may help reduce the severity of exacerbations. AAT also appears to have anti-inflammatory effects that are applicable to AATD in addition to a wider range of clinical areas; however, this has yet to be demonstrated in a large-scale human study. With current evidence, augmentation therapy is indicated only in patients with severe AAT deficiency (serum AAT <11 µM) with evidence of lung impairments (emphysema and FEV1 ⩽65%).
Who benefits from augmentation therapy?
Augmentation therapy is currently recommended only for patients with severe AATD genotypes, for example, PI*ZZ (European and US guidelines) and PI*SZ (US guidelines only), or those with an FEV1 ⩽65%, as there is well-documented benefit in these patients.2,3,10,106–108 For patients with mild AATD genotypes (e.g. PI*MZ and those with a FEV1 >65%), augmentation therapy is not recommended as there is a lack of evidence for benefit in these patients.10,109,110 Augmentation therapy is also not recommended for patients that continue to smoke, or in patients that have had a liver transplantation.10 Clinical guidelines are less clear for individuals where the clinical significance of their AATD genotype is unclear or variable (e.g. PI*SS). For patients with AATD that do not meet the criteria for augmentation therapy, there are many new therapeutic technologies being investigated, which are discussed in more detail within the chapter on research and emerging treatment strategies.111,112
Conclusions
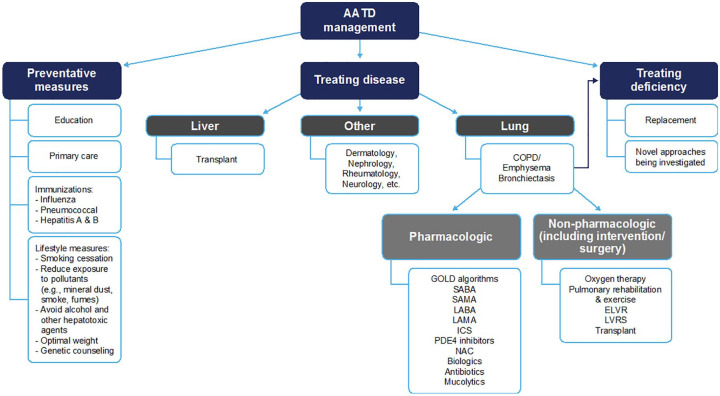
Most pharmacological and non-pharmacological management methods for COPD do not have direct evidence for efficacy in AATD, but this should not necessarily preclude use of these interventions. Only smoking cessation and disease-specific disease management programs are supported by good evidence for benefit in AATD specifically. AAT augmentation therapy is currently the only treatment with an established disease-modifying effect in patients with AATD, as evidenced in clinical studies with robust data and outcomes. Based upon the information provided within this chapter, an algorithm for the treatment of AATD has been proposed (Figure 1).
Figure 1.
AATD treatment algorithm.
AATD, alpha-1 antitrypsin deficiency; COPD, chronic obstructive pulmonary disease; ELVR, endobronchial lung volume reduction; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICS, inhaled corticosteroids; LABA, long-acting beta-2 agonists; LAMA, long-acting muscarinic antagonists; LVRS, lung volume reduction surgery; NAC, N-acetylcysteine; PDE4, phosphodiesterase type 4; SABA, short-acting beta-2 agonists; SAMA, short-acting muscarinic antagonists.
Acknowledgments
Medical writing assistance was provided by Ben McDermott and Steven Foster of Meridian HealthComms Ltd., Plumley, UK, in accordance with good publication practice (GPP3), funded by CSL Behring.
Footnotes
Author contributions: Both authors contributed to the writing of the manuscript, reviewed the manuscript, and approved the manuscript for submission.
Conflict of interest statement: IB reports grants from NHLBI, COPD Foundation, AMGEN, GE Healthcare, Theravance and Mylan, and reports consultancy fees from GSK, Astra Zeneca, Boehringer Ingelheim, Verona Pharma, Grifols, CSL Behring, GE Healthcare, Theravance and Mylan. MC reports grants from Grifols, CSL Behring, and Alpha-1 Foundation.
Contributor Information
Igor Barjaktarevic, Division of Pulmonary and Critical Care Medicine, David Geffen School of Medicine at University of California Los Angeles, 10833 Le Conte Avenue, Los Angeles, CA 90095, USA.
Michael Campos, Division of Pulmonary, Allergy, Critical Care and Sleep Medicine, University of Miami School of Medicine, Miami, FL, USA.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease 2020. report, https://goldcopd.org/wp-content/uploads/2019/12/GOLD-2020-FINAL-ver1.2-03Dec19_WMV.pdf (2020, accessed 10 March 2020).
- 2.Chapman KR, Burdon JGW, Piitulainen E, et al. Intravenous augmentation treatment and lung density in severe α1 antitrypsin deficiency (RAPID): a randomised, double-blind, placebo-controlled trial. Lancet 2015; 386: 360–368. [DOI] [PubMed] [Google Scholar]
- 3.McElvaney NG, Burdon J, Holmes M, et al. Long-term efficacy and safety of α1 proteinase inhibitor treatment for emphysema caused by severe α1 antitrypsin deficiency: an open-label extension trial (RAPID-OLE). Lancet Respir Med 2017; 5: 51–60. [DOI] [PubMed] [Google Scholar]
- 4.Safka KA, McIvor RA.Non-pharmacological management of chronic obstructive pulmonary disease. Ulster Med J 2015; 84: 13–21. [PMC free article] [PubMed] [Google Scholar]
- 5.Hutchison DC, Cooper D; British Thoracic Society. Alpha-1-antitrypsin deficiency: smoking, decline in lung function and implications for therapeutic trials. Respir Med 2002; 96: 872–880. [DOI] [PubMed] [Google Scholar]
- 6.Seersholm N, Kok-Jensen A.Clinical features and prognosis of life time non-smokers with severe α1-antitrypsin deficiency. Thorax 1998; 53: 265–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanash HA, Nilsson PM, Nilsson JA, et al. Survival in severe alpha-1-antitrypsin deficiency (PiZZ). Respir Res 2010; 11: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chorostowska-Wynimko J.Disease modification in emphysema related to alpha-1 antitrypsin deficiency. COPD 2016; 13: 807–815. [DOI] [PubMed] [Google Scholar]
- 9.Taggart C, Cervantes-Laurean D, Kim G, et al. Oxidation of either methionine 351 or methionine 358 in alpha 1-antitrypsin causes loss of anti-neutrophil elastase activity. J Biol Chem 2000; 275: 27258–27265. [DOI] [PubMed] [Google Scholar]
- 10.Sandhaus RA, Turino G, Brantly ML, et al. The diagnosis and management of alpha-1 antitrypsin deficiency in the adult. Chronic Obstr Pulm Dis 2016; 3: 668–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayer AS, Stoller JK, Bucher Bartelson B, et al. Occupational exposure risks in individuals with PI*Z alpha(1)-antitrypsin deficiency. Am J Respir Crit Care Med 2000; 162: 553–558. [DOI] [PubMed] [Google Scholar]
- 12.Perkins JT, Choate R, Mannino DM, et al. Benefits among patients with alpha-1 antitrypsin deficiency enrolled in a disease management and prevention program. Chronic Obstr Pulm Dis 2016; 4: 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campos MA, Alazemi S, Zhang G, et al. Effects of a disease management program in individuals with alpha-1 antitrypsin deficiency. COPD 2009; 6: 31–40. [DOI] [PubMed] [Google Scholar]
- 14.Gulack BC, Mulvihill MS, Ganapathi AM, et al. Survival after lung transplantation in recipients with alpha-1 antitrypsin deficiency compared to other forms of chronic obstructive pulmonary disease: a national cohort study. Transpl Int 2018; 31: 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stone HM, Edgar RG, Thompson RD, et al. Lung transplantation in alpha-1-antitrypsin deficiency. COPD 2016; 13: 146–152. [DOI] [PubMed] [Google Scholar]
- 16.Zamora M, Ataya A.Lung and liver transplantation in patients with alpha-1 antitrypsin deficiency. Ther Adv Chronic Dis 2021; 12_suppl(2): 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujimoto T, Teschler H, Hillejan L, et al. Long-term results of lung volume reduction surgery. Eur J Cardiothorac Surg 2002; 21: 483–488. [DOI] [PubMed] [Google Scholar]
- 18.Gelb AF, McKenna RJ, Jr, Brenner M, et al. Lung function 5 yr after lung volume reduction surgery for emphysema. Am J Respir Crit Care Med 2001; 163: 1562–1566. [DOI] [PubMed] [Google Scholar]
- 19.Dolmage TE, Waddell TK, Maltais F, et al. The influence of lung volume reduction surgery on exercise in patients with COPD. Eur Respir J 2004; 23: 269–274. [DOI] [PubMed] [Google Scholar]
- 20.Hamacher J, Büchi S, Georgescu CL, et al. Improved quality of life after lung volume reduction surgery. Eur Respir J 2002; 19: 54–60. [DOI] [PubMed] [Google Scholar]
- 21.Stoller JK, Gildea TR, Ries AL, et al. Lung volume reduction surgery in patients with emphysema and alpha-1 antitrypsin deficiency. Ann Thorac Surg 2007; 83: 241–251. [DOI] [PubMed] [Google Scholar]
- 22.Cassina P, Teschler H, Konietzko N, et al. Two-year results after lung volume reduction surgery in alpha1-antitrypsin deficiency versus smoker’s emphysema. Eur Respir J 1998; 12_suppl: 1028–1032. [DOI] [PubMed] [Google Scholar]
- 23.Tutic M, Bloch KE, Lardinois D, et al. Long-term results after lung volume reduction surgery in patients with α1-antitrypsin deficiency. J Thorac Cardiovasc Surg 2004; 128: 408–413. [DOI] [PubMed] [Google Scholar]
- 24.Ciccone AM, Meyers BF, Guthrie TJ, et al. Long-term outcome of bilateral lung volume reduction in 250 consecutive patients with emphysema. J Thorac Cardiovasc Surg 2003; 125: 513–525. [DOI] [PubMed] [Google Scholar]
- 25.Hillerdal G, Mindus S.One- to four-year follow-up of endobronchial lung volume reduction in alpha-1-antitrypsin deficiency patients: a case series. Respiration 2014; 88: 320–328. [DOI] [PubMed] [Google Scholar]
- 26.Deslée G, Mal H, Dutau H, et al. Lung volume reduction coil treatment vs usual care in patients with severe emphysema: the REVOLENS randomized clinical trial. JAMA 2016; 315: 175–184. [DOI] [PubMed] [Google Scholar]
- 27.Perotin JM, Leroy S, Marquette CH, et al. Endobronchial coil treatment in severe emphysema patients with alpha-1 antitrypsin deficiency. Int J Chron Obstruct Pulmon Dis 2018; 13: 3645–3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American Thoracic Society. Pulmonary rehabilitation-1999. Am J Respir Crit Care Med 1999; 159: 1666–1682. [DOI] [PubMed] [Google Scholar]
- 29.McCarthy B, Casey D, Devane D, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015; 2: CD003793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spielmanns M, Boeselt T, Nell C, et al. Effect of pulmonary rehabilitation on inspiratory capacity during 6-min walk test in patients with COPD: a prospective controlled study. J Cardiopulm Rehabil Prev 2018; 38: 264–268. [DOI] [PubMed] [Google Scholar]
- 31.Boeselt T, Nell C, Lütteken L, et al. Benefits of high-intensity exercise training to patients with chronic obstructive pulmonary disease: a controlled study. Respiration 2017; 93: 301–310. [DOI] [PubMed] [Google Scholar]
- 32.Jarosch I, Hitzl W, Koczulla AR, et al. Comparison of exercise training responses in COPD patients with and without alpha-1 antitrypsin deficiency. Respir Med 2017; 130: 98–101. [DOI] [PubMed] [Google Scholar]
- 33.Rawal G, Yadav S.Nutrition in chronic obstructive pulmonary disease: a review. J Transl Int Med 2015; 3: 151–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piitulainen E, Areberg J, Lindén M, et al. Nutritional status and muscle strength in patients with emphysema and severe alpha1-antitrypsin deficiency. Chest 2002; 122: 1240–1246. [DOI] [PubMed] [Google Scholar]
- 35.Iyer AS, Dransfield MT.The “obesity paradox” in chronic obstructive pulmonary disease: can it be resolved? Ann Am Thorac Soc 2018; 15: 158–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanson C, Rutten EP, Wouters EFM, et al. Influence of diet and obesity on COPD development and outcomes. Int J Chron Obstruct Pulmon Dis 2014; 9: 723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fuller-Thomson E, Howden KEN, Fuller-Thomson LR, et al. A strong graded relationship between level of obesity and COPD: findings from a national population-based study of lifelong nonsmokers. J Obes 2018; 2018: 6149263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galesanu RG, Bernard S, Marquis K, et al. Obesity in chronic obstructive pulmonary disease: is fatter really better? Can Respir J 2014; 21: 297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McDonald V, Gibson P, Scott H, et al. Obesity in COPD, how should it be managed? - the effect of weight loss and resistance training in obese COPD patients. Eur Respir J 2014; 44: P3035. [Google Scholar]
- 40.Wang L, Marek GW, III, Hlady RA, et al. Alpha-1 antitrypsin deficiency liver disease, mutational homogeneity modulated by epigenetic heterogeneity with links to obesity. Hepatology 2019; 70: 51–66. [DOI] [PubMed] [Google Scholar]
- 41.Campos MA, Alazemi S, Zhang G, et al. Influenza vaccination in subjects with alpha1-antitrypsin deficiency. Chest 2008; 133: 49–55. [DOI] [PubMed] [Google Scholar]
- 42.Sehatzadeh S.Influenza and pneumococcal vaccinations for patients with chronic obstructive pulmonary disease (COPD): an evidence-based review. Ont Health Technol Assess Ser 2012; 12_suppl: 1–64. [PMC free article] [PubMed] [Google Scholar]
- 43.Froes F, Roche N, Blasi F.Pneumococcal vaccination and chronic respiratory diseases. Int J Chron Obstruct Pulmon Dis 2017; 12_suppl: 3457–3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Torres-Durán M, Lopez-Campos JL, Barrecheguren M, et al. Alpha-1 antitrypsin deficiency: outstanding questions and future directions. Orphanet J Rare Dis 2018; 13: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh D, Agusti A, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J 2019; 53: 1900164. [DOI] [PubMed] [Google Scholar]
- 46.Corda L, Bertella E, La Piana GE, et al. Inhaled corticosteroids as additional treatment in alpha-1-antitrypsin deficiency-related COPD. Respiration 2008; 76: 61–68. [DOI] [PubMed] [Google Scholar]
- 47.Vijayasaratha K, Stockley RA.Relationship between frequency, length, and treatment outcome of exacerbations to baseline lung function and lung density in alpha-1 antitrypsin-deficient COPD. Int J Chron Obstruct Pulmon Dis 2012; 7: 789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mammen MJ, Sethi S.Macrolide therapy for the prevention of acute exacerbations in chronic obstructive pulmonary disease. Pol Arch Med Wewn 2012; 122: 54–59. [PubMed] [Google Scholar]
- 49.Herath SC, Normansell R, Maisey S, et al. Prophylactic antibiotic therapy for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev 2018; 10: CD009764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poole P, Chong J, Cates CJ.Mucolytic agents versus placebo for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2015; 7: CD001287. [DOI] [PubMed] [Google Scholar]
- 51.COPD Working Group. Long-term oxygen therapy for patients with chronic obstructive pulmonary disease (COPD): an evidence-based analysis. Ont Health Technol Assess Ser 2012; 12_suppl: 1–64. [PMC free article] [PubMed] [Google Scholar]
- 52.Calzetta L, Rogliani P, Matera MG, et al. A systematic review with meta-analysis of dual bronchodilation with LAMA/LABA for the treatment of stable COPD. Chest 2016; 149: 1181–1196. [DOI] [PubMed] [Google Scholar]
- 53.Gartlehner G, Hansen RA, Carson SS, et al. Efficacy and safety of inhaled corticosteroids in patients with COPD: a systematic review and meta-analysis of health outcomes. Ann Fam Med 2006; 4: 253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miravitlles M, Anzueto A, Jardim JR.Optimizing bronchodilation in the prevention of COPD exacerbations. Respir Res 2017; 18: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wewers MD, Casolaro MA, Sellers SE, et al. Replacement therapy for alpha 1-antitrypsin deficiency associated with emphysema. N Engl J Med 1987; 316: 1055–1062. [DOI] [PubMed] [Google Scholar]
- 56.Brantly ML, Lascano JE, Shahmohammadi A.Intravenous alpha-1 antitrypsin therapy for alpha-1 antitrypsin deficiency: the current state of the evidence. Chronic Obstr Pulm Dis 2019; 6: 100–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stocks JM, Brantly M, Pollock D, et al. Multi-center study: the biochemical efficacy, safety and tolerability of a new alpha1-proteinase inhibitor, Zemaira. COPD 2006; 3: 17–23. [DOI] [PubMed] [Google Scholar]
- 58.Turino GM, Ma S, Cantor JO, et al. Biomarkers in alpha-1 antitrypsin deficiency chronic obstructive pulmonary disease. Ann Am Thorac Soc 2016; 13(Suppl. 4): S336–S340. [DOI] [PubMed] [Google Scholar]
- 59.Ma S, Lin YY, Cantor JO, et al. The effect of alpha-1 protease inhibitor (A1PI) on biomarkers of elastin degradation in alpha-1 antitrypsin deficiency: an analysis of the RAPID/RAPID extension trials. Chronic Obstr Pulm Dis 2016; 4: 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ma S, Lin YY, He J, et al. Alpha-1 antitrypsin augmentation therapy and biomarkers of elastin degradation. COPD 2013; 10: 473–481. [DOI] [PubMed] [Google Scholar]
- 61.Campos MA, Diaz AA.The role of computed tomography for the evaluation of lung disease in alpha-1 antitrypsin deficiency. Chest 2018; 153: 1240–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ficker J, Chapman KR, Turner A, et al. Alpha-1 antitrypsin (A1-PI) treatment slows emphysema progression independent of baseline FEV1. Eur Respir J 2017; 50(Suppl. 61): OA3416. [Google Scholar]
- 63.Dirksen A, Dijkman JH, Madsen F, et al. A randomized clinical trial of alpha1-antitrypsin augmentation therapy. Am J Respir Crit Care Med 1999; 160: 1468–1472. [DOI] [PubMed] [Google Scholar]
- 64.Dirksen A, Piitulainen E, Parr DG, et al. Exploring the role of CT densitometry: a randomised study of augmentation therapy in alpha1-antitrypsin deficiency. Eur Respir J 2009; 33: 1345–1353. [DOI] [PubMed] [Google Scholar]
- 65.Stockley RA, Parr DG, Piitulainen E, et al. Therapeutic efficacy of α-1 antitrypsin augmentation therapy on the loss of lung tissue: an integrated analysis of 2 randomised clinical trials using computed tomography densitometry. Respir Res 2010; 11: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.The Alpha-1 Antitrypsin Deficiency Registry Study Group. Survival and FEV1 decline in individuals with severe deficiency of alpha-1-antitrypsin. Am J Respir Crit Care Med 1998; 158: 49–59. [DOI] [PubMed] [Google Scholar]
- 67.Chapman KR, Stockley RA, Dawkins C, et al. Augmentation therapy for alpha1 antitrypsin deficiency: a meta-analysis. COPD 2009; 6: 177–184. [DOI] [PubMed] [Google Scholar]
- 68.Schluchter MD, Stoller JK, Barker AF, et al. Feasibility of a clinical trial of augmentation therapy for alpha1-antitrypsin deficiency. The Alpha 1-Antitrypsin Deficiency Registry Study Group. Am J Respir Crit Care Med 2000; 161: 796–801. [DOI] [PubMed] [Google Scholar]
- 69.Janciauskiene S, Larsson S, Larsson P, et al. Inhibition of lipopolysaccharide-mediated human monocyte activation, in vitro, by alpha1-antitrypsin. Biochem Biophys Res Commun 2004; 321: 592–600. [DOI] [PubMed] [Google Scholar]
- 70.Petrache I, Fijalkowska I, Medler TR, et al. Alpha-1 antitrypsin inhibits caspase-3 activity, preventing lung endothelial cell apoptosis. Am J Pathol 2006; 169: 1155–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aldonyte R, Jansson L, Janciauskiene S.Concentration-dependent effects of native and polymerised alpha1-antitrypsin on primary human monocytes, in vitro. BMC Cell Biol 2004; 5: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tilg H, Vannier E, Vachino G, et al. Antiinflammatory properties of hepatic acute phase proteins: preferential induction of Interleukin 1 (IL-1) receptor antagonist over IL-1 beta synthesis by human peripheral blood mononuclear cells. J Exp Med 1993; 178: 1629–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schmid ST, Koepke J, Dresel M, et al. The effects of weekly augmentation therapy in patients with PiZZ α1-antitrypsin deficiency. Int J Chron Obstruct Pulmon Dis 2012; 7: 687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stockley RA, Bayley DL, Unsal I, et al. The effect of augmentation therapy on bronchial inflammation in alpha1-antitrypsin deficiency. Am J Respir Crit Care Med 2002; 165: 1494–1498. [DOI] [PubMed] [Google Scholar]
- 75.Bergin DA, Reeves EP, Hurley K, et al. The circulating proteinase inhibitor α-1 antitrypsin regulates neutrophil degranulation and autoimmunity. Sci Transl Med 2014; 6: 217ra211. [DOI] [PubMed] [Google Scholar]
- 76.Bergin DA, Reeves EP, Meleady P, et al. α-1 antitrypsin regulates human neutrophil chemotaxis induced by soluble immune complexes and IL-8. J Clin Invest 2010; 120: 4236–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lockett AD, Kimani S, Ddungu G, et al. α1-antitrypsin modulates lung endothelial cell inflammatory responses to TNF-α. Am J Resp Cell Mol 2013; 49: 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Campos MA, Geraghty P, Holt G, et al. The biological effects of double-dose alpha-1 antitrypsin augmentation therapy. A pilot clinical trial. Am J Respir Crit Care Med 2019; 200: 318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim M, Cai Q, Oh Y.Therapeutic potential of alpha-1 antitrypsin in human disease. Ann Pediatr Endocrinol Metab 2018; 23: 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bergin DA, Hurley K, McElvaney NG, et al. Alpha-1 antitrypsin: a potent anti-inflammatory and potential novel therapeutic agent. Arch Immunol Ther Exp (Warsz) 2012; 60: 81–97. [DOI] [PubMed] [Google Scholar]
- 81.Marcondes AM, Li X, Tabellini L, et al. Inhibition of IL-32 activation by α-1 antitrypsin suppresses alloreactivity and increases survival in an allogeneic murine marrow transplantation model. Blood 2011; 118: 5031–5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Toldo S, Seropian IM, Mezzaroma E, et al. Alpha-1 antitrypsin inhibits caspase-1 and protects from acute myocardial ischemia-reperfusion injury. J Mol Cell Cardiol 2011; 51: 244–251. [DOI] [PubMed] [Google Scholar]
- 83.Petrache I, Hajjar J, Campos M.Safety and efficacy of alpha-1-antitrypsin augmentation therapy in the treatment of patients with alpha-1-antitrypsin deficiency. Biologics 2009; 3: 193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wencker M, Banik N, Buhl R, et al. Long-term treatment of alpha1-antitrypsin deficiency-related pulmonary emphysema with human alpha1-antitrypsin. Wissenschaftliche Arbeitsgemeinschaft zur Therapie von Lungenerkrankungen (WATL)-alpha1-AT-study group. Eur Respir J 1998; 11: 428–433. [DOI] [PubMed] [Google Scholar]
- 85.Stoller JK, Fallat R, Schluchter MD, et al. Augmentation therapy with alpha1-antitrypsin: patterns of use and adverse events. Chest 2003; 123: 1425–1434. [DOI] [PubMed] [Google Scholar]
- 86.Campos MA, Kueppers F, Stocks JM, et al. Safety and pharmacokinetics of 120 mg/kg versus 60 mg/kg weekly intravenous infusions of alpha-1 proteinase inhibitor in alpha-1 antitrypsin deficiency: a multicenter, randomized, double-blind, crossover study (SPARK). COPD 2013; 10: 687–695. [DOI] [PubMed] [Google Scholar]
- 87.Lieberman J.Augmentation therapy reduces frequency of lung infections in antitrypsin deficiency: a new hypothesis with supporting data. Chest 2000; 118: 1480–1485. [DOI] [PubMed] [Google Scholar]
- 88.Köhnlein T, Janciauskiene S, Welte T.Diagnostic delay and clinical modifiers in alpha-1 antitrypsin deficiency. Ther Adv Respir Dis 2010; 4: 279–287. [DOI] [PubMed] [Google Scholar]
- 89.Barros-Tizon JC, Torres ML, Blanco I, et al. Reduction of severe exacerbations and hospitalization-derived costs in alpha-1-antitrypsin-deficient patients treated with alpha-1-antitrypsin augmentation therapy. Ther Adv Respir Dis 2012; 6: 67–78. [DOI] [PubMed] [Google Scholar]
- 90.Edgar RG, Patel M, Bayliss S, et al. Treatment of lung disease in alpha-1 antitrypsin deficiency: a systematic review. Int J Chron Obstruct Pulmon Dis 2017; 12_suppl: 1295–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ellis P, Holm K, Choate R, et al. Comparison of outcomes in augmentation naïve and augmented patients with alpha-1 antitrypsin deficiency related lung disease. Eur Respir J 2019; 54(Suppl. 63): PA3383. [Google Scholar]
- 92.Rahaghi FF, Miravitlles M.Long-term clinical outcomes following treatment with alpha 1-proteinase inhibitor for COPD associated with alpha-1 antitrypsin deficiency: a look at the evidence. Respir Res 2017; 18: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Campos MA, Alazemi S, Zhang G, et al. Clinical characteristics of subjects with symptoms of alpha1-antitrypsin deficiency older than 60 years. Chest 2009; 135: 600–608. [DOI] [PubMed] [Google Scholar]
- 94.Stockley RA.Alpha-1 antitrypsin deficiency: phenotypes and quality of life. Ann Am Thorac Soc 2016; 13(Suppl. 4): S332–S335. [DOI] [PubMed] [Google Scholar]
- 95.Gotzsche PC, Johansen HK.Intravenous alpha-1 antitrypsin augmentation therapy for treating patients with alpha-1 antitrypsin deficiency and lung disease. Cochrane Database Syst Rev 2016; 9: CD007851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chorostowska-Wynimko J, Barrecheguren M, Ferrarotti I, et al. New patient-centric approaches to the management of alpha-1 antitrypsin deficiency. Int J Chronic Obstruct Pulmon Dis 2020; 15: 345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kropp K, Wencker M, Hotze A, et al. Inhalation of [123I]alpha1-protease inhibitor: toward a new therapeutic concept of alpha1-protease inhibitor deficiency? J Nucl Med 2001; 42: 744–751. [PubMed] [Google Scholar]
- 98.Griese M, Scheuch G.Delivery of alpha-1 antitrypsin to airways. Ann Am Thorac Soc 2016; 13(Suppl. 4): S346–S351. [DOI] [PubMed] [Google Scholar]
- 99.Stolk J, Tov N, Chapman KR, et al. Efficacy and safety of inhaled α1-antitrypsin in patients with severe α1-antitrypsin deficiency and frequent exacerbations of COPD. Eur Respir J 2019; 54: 1900673. [DOI] [PubMed] [Google Scholar]
- 100.KAMADA Investor Presentation. Inhaled AAT phase II/III trial: summary of results, https://www.kamada.com/files/files/Kamada%20Presentation%20October%20%202016.pdf (2016, accessed 3 June 2020).
- 101.Seersholm N, Sandhaus R, Chapman KR, et al. Safety of bi-weekly infusion of A1PI augmentation therapy in RAPID. Eur Respir J 2015; 46: PA999. [Google Scholar]
- 102.Barker AF, Iwata-Morgan I, Oveson L, et al. Pharmacokinetic study of alpha1-antitrypsin infusion in alpha1-antitrypsin deficiency. Chest 1997; 112: 607–613. [DOI] [PubMed] [Google Scholar]
- 103.Hubbard RC, Sellers S, Czerski D, et al. Biochemical efficacy and safety of monthly augmentation therapy for alpha 1-antitrypsin deficiency. JAMA 1988; 260: 1259–1264. [PubMed] [Google Scholar]
- 104.Tortorici MA, Rogers JA, Vit O, et al. Quantitative disease progression model of α-1 proteinase inhibitor therapy on computed tomography lung density in patients with α-1 antitrypsin deficiency. Br J Clin Pharmacol 2017; 83: 2386–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sorrells S, Camprubi S, Griffin R, et al. SPARTA clinical trial design: exploring the efficacy and safety of two dose regimens of alpha1-proteinase inhibitor augmentation therapy in alpha1-antitrypsin deficiency. Respir Med 2015; 109: 490–499. [DOI] [PubMed] [Google Scholar]
- 106.American Thoracic Society and European Respiratory Society. American Thoracic Society/European Respiratory Society statement: standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency. Am J Respir Crit Care Med 2003; 168: 818–900. [DOI] [PubMed] [Google Scholar]
- 107.Miravitlles M, Dirksen A, Ferrarotti I, et al. European Respiratory Society statement: diagnosis and treatment of pulmonary disease in alpha1-antitrypsin deficiency. Eur Respir J 2017; 50: 1700610. [DOI] [PubMed] [Google Scholar]
- 108.Stoller JK, Tomashefski J, Jr, Crystal RG, et al. Mortality in individuals with severe deficiency of alpha1-antitrypsin: findings from the national heart, lung, and blood institute registry. Chest 2005; 127: 1196–1204. [DOI] [PubMed] [Google Scholar]
- 109.Molloy K, Hersh CP, Morris VB, et al. Clarification of the risk of chronic obstructive pulmonary disease in α1-antitrypsin deficiency PiMZ heterozygotes. Am J Respir Crit Care Med 2014; 189: 419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sandhaus RA, Turino G, Stocks J, et al. Alpha-1 antitrypsin augmentation therapy for PI*MZ heterozygotes: a cautionary note. Chest 2008; 134: 831–834. [DOI] [PubMed] [Google Scholar]
- 111.Cortes-Lopez R, Barjaktarevic I.Alpha-1 antitrypsin deficiency: a rare disease? Curr Allergy Asthma Rep 2020; 20: 51. [DOI] [PubMed] [Google Scholar]
- 112.Rahaghi FF.Alpha-1 antitrypsin deficiency research and emerging treatment strategies: what’s down the road? Ther Adv Chronic Dis 2021; 12_suppl(2): 77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]