Abstract
Coronavirus disease 2019 (COVID-19) has become a global pandemic since its discovery in December 2019, and as the disease continues to evolve, varying complications associated with it continue to arise. In this regard, computed tomography has played an extremely important role in the diagnosis and evaluation of COVID-19 pneumonia and its complications. We encountered a case of a male patient with neurofibromatosis (type I) who developed concurrent pneumothorax and pleural effusion during his recovery period from severe COVID-19 pneumonia. Pulmonary fibrosis and emphysema were also confirmed. Furthermore, an eosinophil pleural effusion appeared and was prolonged during the healing process of COVID-19. This clinical presentation suggests that fibrosis and emphysema formation due to neurofibromatosis may have caused pneumothorax and pleural effusion.
Keywords: Coronavirus disease 2019 (COVID-19), neurofibromatosis, pneumothorax, eosinophilic pleural effusion, computed tomography (CT)
Introduction
Coronavirus disease 2019 (COVID-19) continues to spread worldwide and the number of COVID-19 cases globally remains at the highest levels at the time of writing since its discovery in Wuhan, China in December 2019 [1]. Currently, several mutant strains of COVID-19 have emerged and become prevalent, and further studies are required to verify their characteristics. Chest computed tomography (CT) is useful for diagnosing and evaluating COVID-19 pneumonia [2]. The complication rate of COVID-19 has been reported to be less than 10% [3]. Although the CT features of severe COVID-19 vary, pneumothorax and pleural effusion are uncommon. However, the possibility of spontaneous pneumothorax has been reported after COVID-19 in the presence of emphysematous lesions in the lungs [4]. Patients with neurofibromatosis are at an increased risk of developing spontaneous pneumothorax due to the complications of cysts and interstitial diseases [5]. On the other hand, pleural effusion is not typically seen on the CT images of patients with neurofibromatosis [6]. Here, we demonstrate the pathogenesis of pneumothorax and pleural effusion in patients with COVID-19 and neurofibromatosis on CT images.
Case report
A male patient in his 60s, previously diagnosed with neurofibromatosis type I, had no history of smoking and had complications of benign prostatic hyperplasia. In early April 2020, the patient presented with a persistent fever of 37°C, and subsequently tested positive for COVID-19 using the PCR pharyngeal swab test. Fever and hypoxemia were observed upon admission and administration of favipiravir was started 10 days after onset. Later, he developed bacterial pneumonia and his respiratory condition worsened. He was transferred to our hospital 14 days after the onset of COVID-19.
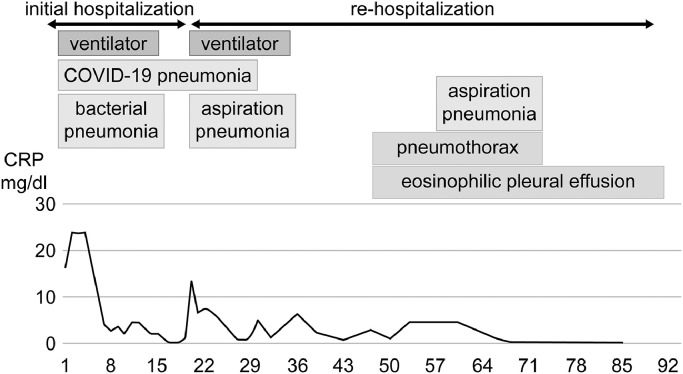
At the time of transfer, his body temperature was 36.9°C, blood pressure was 83/60 mmHg, and pulse rate was 62/min. There were several café-au-lait spots on the trunk. Pertinent laboratory findings include the following: hemoglobin, 16.6/dl; white blood cell count, 12080/µl (neutrophils 94.3%, eosinophils 0%, lymphocytes: 2.6%, monocytes: 3.0%); platelet count, 27.9 × 104/µl; D-dimer, 2.5 µg/ml; C-reactive protein, 11.4 mg/dl. His chest CT showed extensive ground-glass opacities and infiltration shadows predominantly in the right lung. As shown in (Fig. 1), he was brought to the intensive care unit (ICU) and placed on ventilator management on the first day. He was treated with several medications, including antibiotics and favipiravir, which gradually improved his respiratory condition, as confirmed by a decrease in C-reactive protein (CRP). The patient was then transferred to their former doctor on the 20th day of admission after weaning off the ventilator on the 16th day. However, the patient developed aspiration pneumonia and his respiratory condition worsened, prompting readmission to our hospital again on the 21st day after the initial admission, and was managed with a ventilator. Chest CT on the 21st day showed contractile changes in his bilateral lungs, considered part of the healing process after COVID-19 pneumonia infection. He was taken off the ventilator on the 25th day and moved to the general ward from the ICU on the 30th day because nasopharynx testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV2 PCR) was negative. The aspiration pneumonia improved and administration of antibiotics had was on the 34th day. In addition to the onset of pneumothorax, the formation of pleural effusion was observed at the bottom of the right lung on CT imaging on the 47th day. Furthermore, thoracentesis revealed an eosinophil ratio of 46%, in contrast to 3.3% in the peripheral blood, indicating an exudative pleural effusion. The SARS- CoV2 PCR test result for the pleural effusion was negative as was the bacterial culture. Since he had mild pneumothorax, he was followed up with rest management. On the 56th day, he developed a second aspiration pneumonia and was treated with antibiotics. On the 72nd day, his pneumothorax improved, but his right pleural effusion persisted. Since his respiratory condition had stabilized, he was referred to another hospital for rehabilitation on the 91st day as the pleural effusion remained unchanged.
Fig. 1.
Clinical time course of the patient during admission to our hospital. On admission, C-reactive protein (CRP) markedly increased due to COVID-19 pneumonia and bacterial infection. On the 20th day, CRP decreased and his respiratory condition improved. On the 21st day, immediately after transfer to the former hospital, he developed aspiration pneumonia and was returned to our hospital. He was treated with antibiotics and the aspiration pneumonia improved by the 34th day. On the 47th day, pneumothorax and pleural effusion were observed. On the 56th day, he once again suffered from aspiration pneumonia but subsequently improved. On the 72nd day, the pneumothorax healed spontaneously, in contrast to the pleural effusion that persisted until discharge from our hospital.
Discussion
Neurofibromatosis type 1 is an autosomal dominant disorder that affects all organ systems due to the proliferation of neural crest cells. In this case, pneumothorax and pleural effusion were observed during the recovery period of severe COVID-19 pneumonia.
The first cause of pneumothorax in this patient is the small emphysematous lesions in the right lung associated with neurofibromatosis, as confirmed by chest CT at the time of COVID-19 infection (Fig. 2). It has also been reported that the typical CT pattern of neurofibromatosis-related diffuse lung disease (NF-DLD) includes bras in the upper lobe, interstitial shadows at the bases of both lungs, and emphysematous changes [7]. Changes in the CT findings during the hospitalization period are shown in (Fig. 3). It is speculated that SARS- CoV2 infects lungs with emphysematous changes, develops pneumothorax due to contractile changes during the healing process, and develops pleural effusion. Second, the use of ventilators has been reported to be associated with pneumothorax that develops during the convalescent period of COVID-19 pneumonia [8]. The formation of emphysematous cysts could be confirmed by changes over time in CT findings (Fig. 4). During the recovery phase of COVID-19 pneumonia, neurofibromatosis-affected lungs were shown to develop fibrosis and emphysematous cysts. It was speculated that the emphysematous cyst is formed by the check-valve mechanism, suggesting that these emphysematous lesions developed pneumothorax due to pleural rupture caused by swelling and coughing [9].
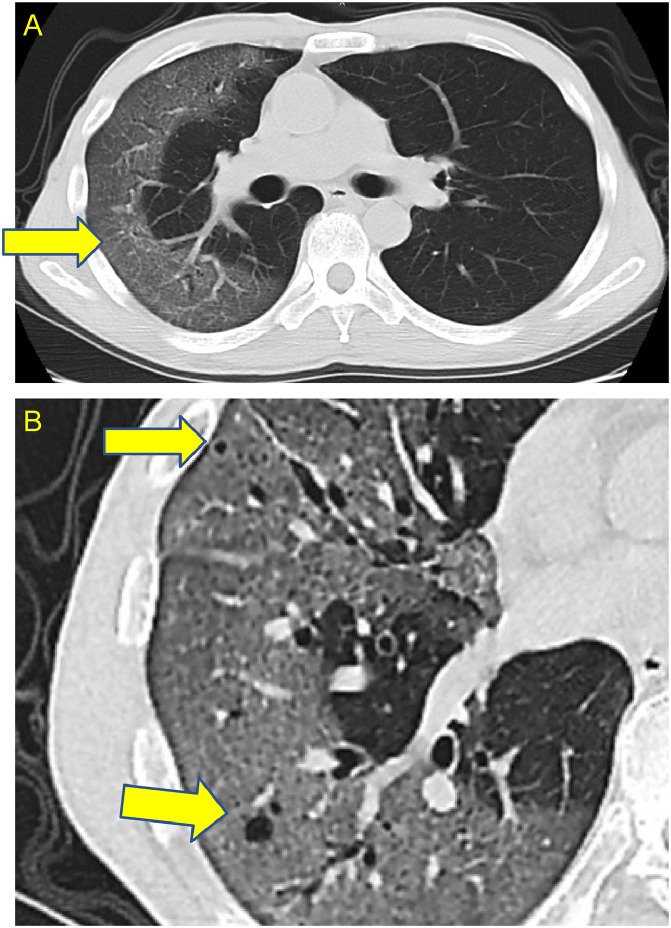
Fig. 2.
(A) Computed tomography (CT) image in the former hospital before admission to our hospital showed a non-segmented ground-glass shadow (yellow arrow) on the peripheral side of the right lung. (B) High-resolution CT findings showed small emphysematous lesions (yellow arrows) in the lung with neurofibromatosis. (Color version of figure is available online).
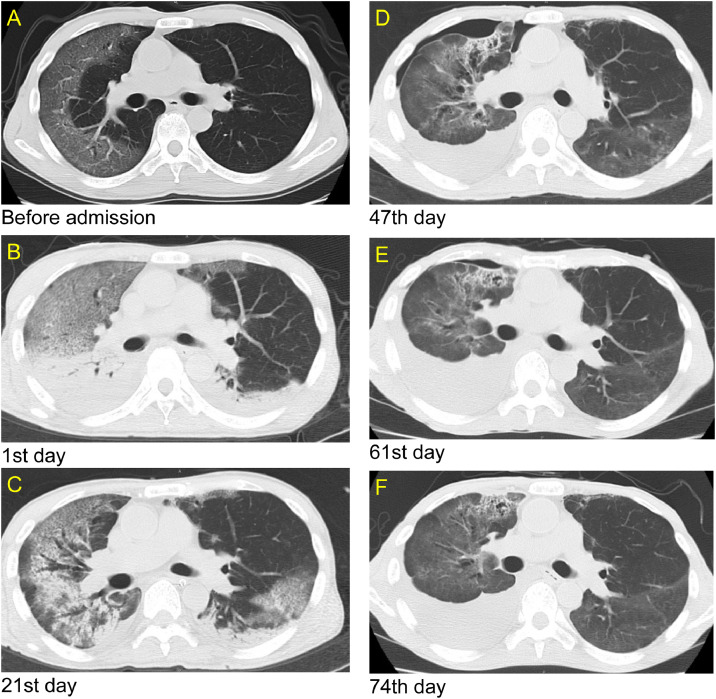
Fig. 3.
(A) Computed tomography (CT) image before admission, (B) CT image at admission to our hospital when COVID-19 pneumonia exacerbation was observed. (C) CT image on the 21st day after admission when aspiration pneumonia developed. (D) CT image showed a pneumothorax and pleural effusion 47 days after admission. (E) CT image showed that pneumothorax improved 61 days after admission, but pleural effusion did not resolve. (F) CT image showing pleural effusion on the 74th day before hospital transfer.
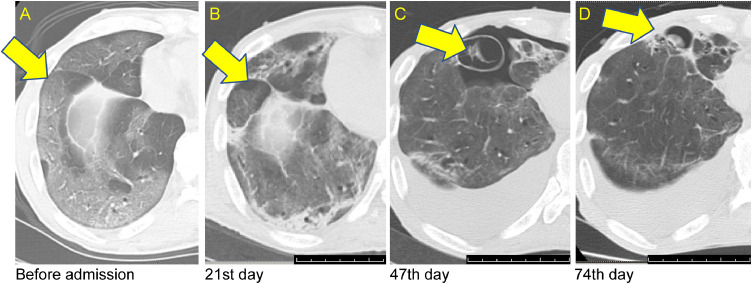
Fig. 4.
(A) Computed tomography (CT) image before admission shows ground glass shadows (yellow arrow) of COVID-19 pneumonia at the peripheral site of the lower lobe of the right lung. (B) CT image on the 21st day after admission revealed fibrosis and emphysematous changes (yellow arrow) at the same site. (C) CT image on 47 days after admission showed enlarged emphysema at the same site, as well as pneumothorax (yellow arrow).( D) CT image on 74 days after admission showed smaller emphysema (yellow arrow) at the same site and a resolved pneumothorax. (Color version of figure is available online).
In addition, the onset of pleural effusion is also interesting to note. Most patients with pneumothorax do not have findings of pleural effusion on chest radiography because the increase in pleural pressure caused by the pneumothorax inhibits the transfer of interstitial liquid into the pleural space [10]. In this case, pleural effusion appeared almost at the same time as the pneumothorax. Thoracentesis was performed and the eosinophil ratio in the pleural effusion was as high as 46%, which met the criteria of eosinophilic pleural effusion of 10% or more. Moreover, the PCR test result for SARS-CoV2 in the pleural effusion was negative, suggesting that the association between pleural effusion and the activity of COVID-19 was weak. A previous report has shown that the number of eosinophils in the peripheral blood increases in neurofibromatosis [11]. However, to the best of our knowledge, this is the first case report to describe an eosinophilic pleural effusion after COVID-19 pneumonia. Eosinophilic pleural effusion has been reported to be most often associated with air previously introduced into the pleural space [12]. In this case, as shown in (Fig. 1), pneumothorax and pleural effusion developed after improvement of aspiration pneumonia. This indicates that the air present in the thoracic cavity due to the pneumothorax may have increased the number of eosinophils in the pleural effusion, and the lung with neurofibromatosis may have led to prolonged pleural effusion due to its contractile changes.
Conclusion
In conclusion, during the recovery period after COVID-19 pneumonia, pulmonary fibrosis in a patient with neurofibromatosis type I progressed, leading to contractile changes. Subsequently, pneumothorax and an eosinophilic pleural effusion were observed. This is a valuable case in which the onset of pneumothorax could be confirmed from the changes in CT findings over time.
Acknowledgments
We would like to thank Dr. Yuki Shin, Kentaro Hara, Aya Yamaguchi, Mari Sato, Masaki Aikawa, and Shunichi Kono, from the Department of Respiratory Medicine, Gunma University Graduate School of Medicine for their technical assistance and critical advice. We would like to thank Editage (www.editage.com) for English language editing.
Footnotes
Competing interests: The authors declare no conflicts of interest.
References
- 1.World Health Organization . World Health Organization; Geneva: May 4, 2021. COVID-19 weekly epidemiological, update 38. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19_4-may-2021 (accessed May 23, 2021) [Google Scholar]
- 2.Li K, Wu J, Wu F, Guo D, Chen L, Fang Z. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol. 2020;55(6):327–331. doi: 10.1097/RLI.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou S, Wang Y, Zhu T, Xia L. CT features of coronavirus disease 2019 (COVID-19) pneumonia in 62 patients in Wuhan, China. AJR Am J Roentgenol. 2020;214(6):1287–1294. doi: 10.2214/AJR.20.22975. [DOI] [PubMed] [Google Scholar]
- 4.Dennison J, Carlson S, Faehling S, Lieb M, Mubarik A. Case report: Spontaneous pneumothorax in resolved, uncomplicated COVID-19 Pneumonia-A literature review. Respir Med Case Rep. 2020;31 doi: 10.1016/j.rmcr.2020.101291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lorentzen T, Madsen H, Lausten-Thomsen MJZ, Bygum A. Spontaneous pneumothorax as a clinical manifestation of neurofibromatosis type 1. BMJ Case Rep. 2021;14(3) doi: 10.1136/bcr-2020-238694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bommart S, Bourdin A, Solovei L, Canaud L, Kovacsik H. Chest wall vasculopathy in a patient with type 1 neurofibromatosis. Am J Respir Crit Care Med. 2015;192(3):e42–e43. doi: 10.1164/rccm.201502-0229IM. [DOI] [PubMed] [Google Scholar]
- 7.Alves Júnior SF, Zanetti G, Alves de Melo AS, Souza AS, Jr, Souza LS, de Souza Portes Meirelles G. Neurofibromatosis type 1: State-of-the-art review with emphasis on pulmonary involvement. Respir Med. 2019;149:9–15. doi: 10.1016/j.rmed.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Xu W, Luo X, Wang H, Shen C, Song Y, Sun T. Pulmonary emphysema, bullae, and pneumothorax in COVID-19 pneumonia. Radiol Case Rep. 2021;16(5):995–998. doi: 10.1016/j.radcr.2021.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kadota T, Shimizu K, Tsurushige C, Kawaishi M, Araya J, Nakayama K. Organizing pneumonia complicated by cyst and pneumothorax formation. Intern Med. 2012;51(22):3155–3158. doi: 10.2169/internalmedicine.51.8319. [DOI] [PubMed] [Google Scholar]
- 10.Sahn SA, Heffner JE. Spontaneous Pneumothorax. N Engl J Med. 2000;342(12):868–874. doi: 10.1056/NEJM200003233421207. [DOI] [PubMed] [Google Scholar]
- 11.Katsenos S, Nikolopoulou M, Rallis E, Constantopoulos SH. Chronic eosinophilic pneumonia associated with neurofibromatosis type 1: an unusual complication. J Med Invest. 2009;56(1-2):64–69. doi: 10.2152/jmi.56.64. [DOI] [PubMed] [Google Scholar]
- 12.Adelman M, Albelda SM, Gottlieb J, Haponik EF. Diagnostic utility of pleural fluid eosinophilia. Am J Med. 1984;77(5):915–920. doi: 10.1016/0002-9343(84)90542-4. / [DOI] [PubMed] [Google Scholar]