Abstract
The major pathway of brain cholesterol turnover relies on its hydroxylation into 24S-hydroxycholesterol (24S-HC) using brain-specific cytochrome P450 46A1 (CYP46A1). 24S-HC produced exclusively in the brain normally traverses the blood-brain barrier to enter the circulation to the liver for excretion; therefore, the serum 24S-HC level is an indication of cholesterol metabolism in the brain. We recently reported an upregulation of CYP46A1 following hypoxia-ischemia (HI) in the neonatal mouse brain and a correlation between serum 24S-HC levels and acute brain damage. Here, we performed a longitudinal study to investigate whether the serum 24S-HC concentrations predict long-term brain structural and functional outcomes. In postnatal day 9 mice subjected to HI, the serum 24S-HC levels increased at 6 h and 24 h after HI and correlated with the infarct volumes measured histologically or by T2-weighted MRI. The 24 h levels were associated with white matter volume loss quantified by MBP immunostaining and luxol fast blue staining. The animals with higher serum 24S-HC at 6 h and 24 h corresponded to those with more severe motor and cognitive deficits at 35-40 days after HI. These data suggest that 24S-HC could be a novel and early blood biomarker for severity of neonatal HI brain damage and associated functional impairments.
Keywords: Biomarkers, brain development, cholesterol metabolism, hypoxia-ischemia, neonatal brain injury
Introduction
Neonatal hypoxic-ischemic encephalopathy (HIE), which occurs in 3 per 1000 live births, remains a leading cause of perinatal death and lifelong neurological deficits with few tools for timely diagnosis and intervention.1–3 Specific and reliable markers that are associated with brain damage and long-term neurological impairments in newborns with encephalopathy at birth are currently unavailable. We use a murine model of neonatal hypoxia-ischemia (HI) at postnatal day 9 (p9) to mimic HIE in term infants. Our recent work suggests that the brain-derived cholesterol metabolite 24S-hydroxycholesterol (24S-HC) might be a promising candidate to serve as a novel lipid biomarker for the extent of HI brain injury.4 24S-HC is produced exclusively in the brain by neuron-specific cytochrome P450 46A1 (CYP46A1 in human, cholesterol 24-hydroxylase, Cyp46a1 in mouse). This oxysterol is capable of crossing the blood–brain barrier (BBB) into circulation by concentration gradient (>100-fold) and is eventually excreted in bile in the liver. This is the primary pathway for brain cholesterol elimination.5–9 The hypothesis of using this oxysterol as an HI brain injury marker is made for several reasons: (1) 24S-HC is a cholesterol metabolite specific to the brain;5 (2) HI-induced upregulation of Cyp46a1 mediates the increased formation of brain 24S-HC, leading to its concurrent elevation in the blood at 6 h and 24 h after HI;4 (3) there is a co-localization of enhanced Cyp46a1 with apoptotic cell death as seen by the expression of cleaved-caspase 3 at 24 h after HI;4 and (4) serum 24S-HC levels correlate well with HI-induced acute brain damage at 6 h and 24 h as evaluated by the buildup of spectrin breakdown products and cleaved-caspase 3, which indicate activation of calpain and caspase.4 Therefore, higher serum 24S-HC reflects enhanced brain cholesterol turnover and probably ongoing neuronal death after neonatal HI.
Alterations in the levels of 24S-HC in the circulation have been reported in the conditions involving aberrant cholesterol homeostasis in the brain. For example, children with autism spectrum disorders have a significantly higher 24S-HC plasma level,10 while those with the Smith–Lemli–Opitz syndrome have reduced concentrations.11 This oxysterol is considered as a likely biomarker for disease activity and progression in various neurodegenerative disorders.12,13 Clinical studies showed an increase or a tendency for an increase of serum 24S-HC in early stages but decreased levels in later stages as a consequence of extensive loss of metabolically active neurons, the brain cells that express abundant CYP46A1,7,9,14 and brain atrophy in multiple sclerosis (MS)15 and Alzheimer’s disease (AD) patients.16 Elevated levels of 24S-HC in the cerebrospinal fluid (CSF) were found in young MS patients with positive brain lesions on magnetic resonance imaging (MRI) scan.15 CSF levels of 24S-HC also correlate with the disease duration in Parkinson’s disease.17 It is speculated that the temporarily increased 24S-HC is released from neuronal death-associated plasma membrane breakdown or through demyelination at active stages in neurodegeneration, which have not been demonstrated with animal data. Our finding of the upregulation of Cyp46a1 following neonatal HI may explain the corresponding increase of 24S-HC in the ipsilateral hemisphere and in the blood.4
In this study, we extended our previous work to investigate the relationship between the serum 24S-HC levels and brain structural integrity and long-term functional outcomes to evaluate its value as a diagnostic and prognostic blood marker for the severity of neonatal HI brain injury. We specifically examined the association between this oxysterol and the longitudinal development of histological outcome and motor and cognitive impairments.
Materials and methods
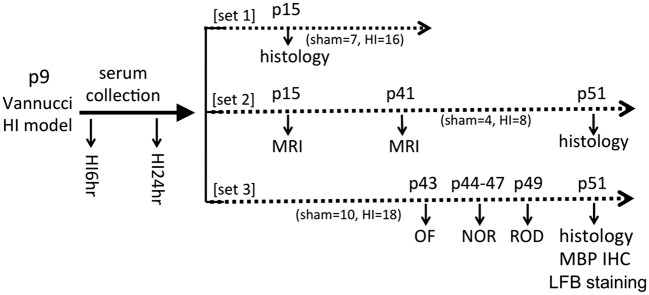
All animal procedures were performed in accordance with the recommendations outlined in the Guide for the Care and Use of Laboratory Animals of National Institutes of Health, approved by the University of California San Francisco Institutional Animal Care and Use Committee, and reported in compliance with the Animal Research: Reporting of In Vivo Experiments guidelines. C57BL/6 mice (Charles River Laboratory, Hollister, CA) with litters were allowed food and water ad libitum. Both sexes were used on postnatal day 9 (p9). Three sets of animals were used for histology, brain imaging, and behavioral testing. The experimental time line was shown in Figure 1.
Figure 1.
The experimental time line of the study. Three sets of animals were used for this study. C57BL/6 mice received HI procedure using the Vannucci model on postnatal day 9 (p9). The serum samples were collected at 6 h and 24 h after HI for 24S-HC measurement. Histology, brain MRI, and the behavioral testing were performed at indicated postnatal (p) day (s) (OF: open field; NOR: novel object recognition; ROD: rotarod; MBP: myelin basic protein; IHC: immunohistochemistry; LFB: luxol fast blue). The animal numbers for sham and HI were listed for each set of experiment. HI: hypoxia-ischemia; MRI: magnetic resonance imaging.
Neonatal brain HI
Neonatal HI was performed using the Vannucci model.18 On p9, the pups underwent left common carotid artery coagulation through a vertical midline neck incision under isoflurane anesthesia (2%–3% isoflurane, balanced oxygen). The animals were allowed to recover for 1 h with their dam and then exposed to 60 min of hypoxia in a humidified chamber at 37°C with 10% oxygen/balanced nitrogen. Sham-operated control animals received isoflurane anesthesia and exposure of the left common carotid artery without coagulation and hypoxia.
Blood sample collection and measurement of serum 24S-HC
Blood was collected from the pups using a BD SST microtainer capillary blood collection tube with serum separator (#365967; BD Diagnostics, Franklin Lakes, NJ) after tail snip. The samples were placed on ice for 30 min to allow blood to clot and then centrifuged at 10,000 r/min for 3 min. Serum was pipetted and kept at −80°C until use. A competitive ELISA kit (ab204530; Abcam, Cambridge, MA) was used to measure the serum levels of 24S-HC as described previously.4 The concentration of serum 24S-HC was normalized to serum protein concentration (ng/mg protein).
Evaluation of HI brain injury with histology methods
Six days (p15) or six weeks (42 days post-HI or on p51, after the second MRI scan or the behavioral tests) following the HI procedure, the animals were euthanized for histology study to evaluate brain injury. Animals were anesthetized and perfused intracardiac with cold 4% paraformaldehyde (PFA) in 0.1 mol/l phosphate buffer. Brains were dissected and post-fixed in 4% PFA overnight followed by cryoprotection with 30% sucrose for three days. Serial brain sections at 50 μm thickness were obtained using a vibratome and stained with cresyl violet (CV) and Perl’s iron. Volumetric injury analysis was performed on a series of 8 CV-stained sections using Image J software. Brain total infarct volume (%) was calculated and presented as [(volume of contralateral hemisphere − volume of ipsilateral hemisphere)/volume of contralateral hemisphere] × 100%.19
Myelin basic protein and Cyp46a1 immunohistochemistry staining
Myelin basic protein (MBP) immunohistochemistry was carried out to assess the amount of myelin and white matter injury after HI. We also stained Cyp46a1 to study its regional and cellular expression in the developing mouse (p9) brain. After washing with phosphate-buffered saline (PBS), the brain cryo-sections were blocked with blocking buffer (10% goat serum and 0.1% Triton X-100 in PBS) for 1 h and then incubated overnight at 4°C with rabbit monoclonal MBP antibody (1:200, #78896; Cell Signaling Technology, Danvers, MA) or mouse anti-Cyp46a1 antibody (1:100, MAB2259; Millipore Sigma, St. Louis, MO). Biotinylated goat anti-rabbit IgG (#BA-1000; Vector Laboratories, Inc., Burlingame, CA) or goat anti-mouse IgG (#BA-9200, Vector Laboratories) were used as the secondary antibody, and Vectastain ABC (Avidin/Biotin Complex) HRP kit (#PK-4000, Vector Laboratories) with 3,3′Diaminobenzidine as the chromogen was employed to visualize the positive signals. Injury to the ipsilateral white matter was analyzed by measuring MBP-positive area with seven serial sections. The MBP-positive area was measured by thresholding method in Image J for both hemispheres and the percentage (%) of ipsilateral MBP volume loss was calculated as (1 − ipsi/contra MBP volume) × 100%.
Luxol fast blue staining
Luxol fast blue (LFB) staining was performed on vibratome brain sections to visualize myelin tracts in the white matter. After rehydration in 100% and 95% ethanol, the sections were stained with 0.1% LFB solution for 2.5 h at 60°C followed by differentiation in 0.05% lithium carbonate solution and 70% ethanol for 45 s to 2 min until the gray matter was colorless while the white matter remained blue and sharply definedThe sections were washed in H2O, dehydrated, cleared, and coverslipped. Similar to quantification of MBP by thresholding technique, injury to the ipsilateral white matter was analyzed by measuring LFB-positive area with seven serial sections. The percentage (%) of ipsilateral LFB volume loss was calculated as (1 − ipsilateral/contralateral LFB volume) × 100%.
Brain MRI and analysis
T2-weighted (T2W) and diffusion tensor imaging (DTI) were performed at 6 days (p15) and 32 days (p41) post-HI. Images were acquired in anesthetized pups using a Bruker 3T small animal imaging system with a 1H transmit-receive volume coil (inner diameter, 40 mm). The mice were anesthetized with isoflurane (1.5%–2% mixed into 100% oxygen) delivered through a facemask with bite bar and head restraint and wrapped in a water-recirculating warming pad to maintain core temperature. We obtained T2-weighted conventional spin-echo multi-slice images (repetition time/echo time = 4750/60 ms) covering the entire brain with consecutive 0.5-mm axial sections with field of view = 2 × 2 cm2, and data matrix of 160 × 160 points (0.125-mm in-plane resolution). A set of seven coronal sections that spanned the entire damaged tissue with hyper-intense signals (located between the appearance of the lateral ventricle and the posterior hippocampus) was selected for assessment of injury. Diffusion imaging was performed using the same geometry, a lower resolution (field of view = 1.8 × 1.5 cm2, matrix size = 96 × 80, i.e., 0.1875-mm in-plane resolution), repetition time/echo time = 2000/30 ms and 30 diffusion sensitizing directions, allowing for rotationally invariant diffusion coefficient (Dav) and fractional anisotropy to be quantified. Images were analyzed using custom-built software. The area of hyper-intense regions on T2-weighted images as well as the entire ipsilateral and contralateral hemispheres of the seven sections was measured. The infarct volume (mm3) was calculated as (the sum of the infarct area on seven sections) × 0.5 mm (thickness). The percentage (%) of total brain infarct volume was presented as (Vc−Vi)/Vc × 100%, where Vc = volume of the contralateral hemisphere; Vi = volume of the remaining ipsilateral hemisphere = ipsilateral hemisphere volume − the infarct volume.
Behavioral testing
The behavioral tests were performed between 34 and 40 days (p43–p49) after HI in the following sequence: open-field (OF) test on day 34 (p43), novel object recognition (NOR) test from day 35 to 38 (p44–p47), and rotarod (ROD) test on day 40 (p49).
ROD test
The ROD test provides a rapid and simple examination for the function of motor coordination and balance. This test can accurately measure sensorimotor impairments in multiple animal models including neonatal HI. After a 1-min adaptation period on the rod (15 cm above the base) with a slow speed (4 r/min), the rod was accelerated by 5 r every 15 s, and the maximum speed was 32 r/min. The length of time that the mouse remained on the rod (fall latency or retention time) was individually recorded.
Open field
The test was used here to evaluate anxiety-related behavior. The mouse was placed in a 40 × 40 cm plastic chamber divided into central (20 × 20 cm) and peripheral zones and was allowed to explore freely in the entire arena for 5 min while being recorded by an overhead camera. The percentage of time each mouse spent in the center zone of the chamber was recorded to represent the level of anxiety.
2-object NOR
NOR is used to evaluate hippocampus- and cortex-dependent non-spatial learning and memory. This test is based on the intrinsic exploratory drive of rodents to spend more time exploring a novel object than a familiar one. The time exploring the novel object reflects the use of learning and recognition memory. On two consecutive days, the mouse was habituated to an open field arena for 5 min. On the third day, the mouse was exposed to the familiar arena with two identical objects and allowed to explore for 5 min. In the 3-min test trial performed 24 h following the familiarization phase, one of the familiar objects was replaced by a novel object of the same size. The time the mouse spent exploring each object was recorded, and the percentage of time spent with the novel object was calculated as [time with novel object/(time with old + with novel object)] × 100%.
Statistical analysis
Analyses were performed using Prism 5 (GraphPad Software, Inc). The medians were presented and compared with Wilcoxon–Mann–Whitney test. Spearman nonparametric correlation coefficient analysis was used to evaluate the relationship between the serum 24S-HC concentrations and the outcome measurements. Bonferroni correction was conducted for multiple comparisons to adjust the levels of statistical significance (α value).
Results
The serum levels of 24S-HC increased at 6 h and 24 h after HI
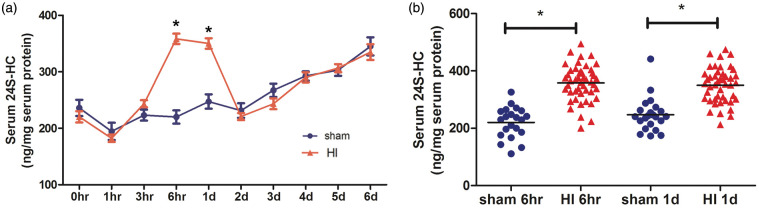
An initial experiment was performed in which the blood samples were taken repeatedly at multiple time points to establish the time course of the changes in the serum levels of 24S-HC. Unlike what we published previously,4 blood was collected from mousetails instead of by decapitation. Figure 2 shows that this oxysterol increased significantly at 6 h and 24 h (one day) post-HI (Figure 2(a), n = 7 for sham, n = 17 for HI, *p < 0.001 vs. the sham values at the same time point), which is consistent with our previous data.4 This result was further confirmed in the three sets of animals (Figure 1) where blood was collected only at two points: at 6 h, and again at 24 h after HI (Figure 2(b), n = 21 for sham, n = 42 for HI, *p < 0.001 vs. the sham values at the same time points). There was no gender difference in the levels of serum 24S-HC, so their values were combined in all analyses.
Figure 2.
The serum levels of 24S-HC increased at 6 h and 24 h after HI. (a) Serum was collected immediately after HI (0 h) and repeatedly at multiple time points over six days. The 24S-HC levels were significantly higher in the HI animals than those in the sham at 6 h and 24 h (1d) (n = 7 for sham, n = 17 for HI, *p < 0.001 vs. the sham values at the same time point). (b) The results were confirmed in mice where serum was collected at 6 h and 24 h (1d) only (i.e., all three sets of animals in Figure 1, n = 21 for sham, n = 42 for HI, *p < 0.001 vs. the sham values at the same time points). 24S-HC: 24S-hydroxycholesterol; HI: hypoxia-ischemia.
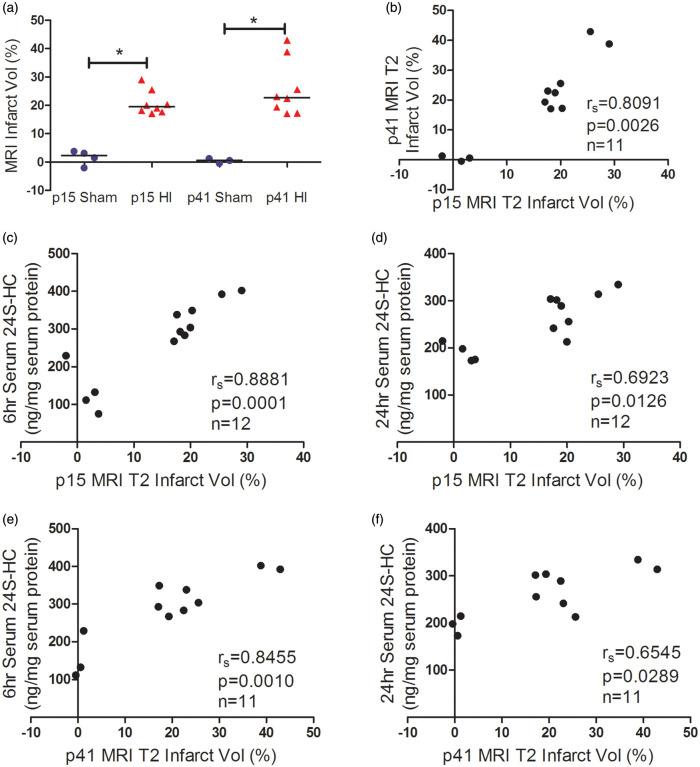
The serum 24S-HC levels were correlated with HI brain histological injury
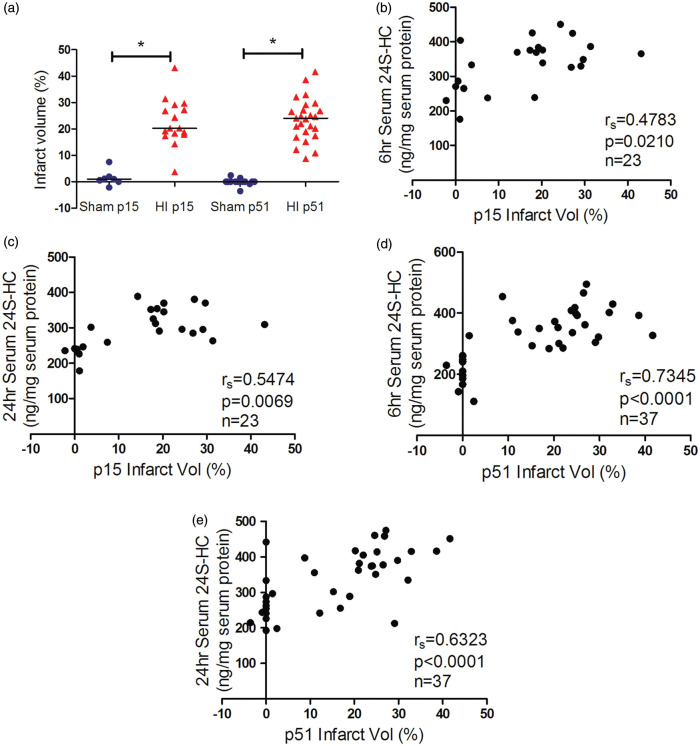
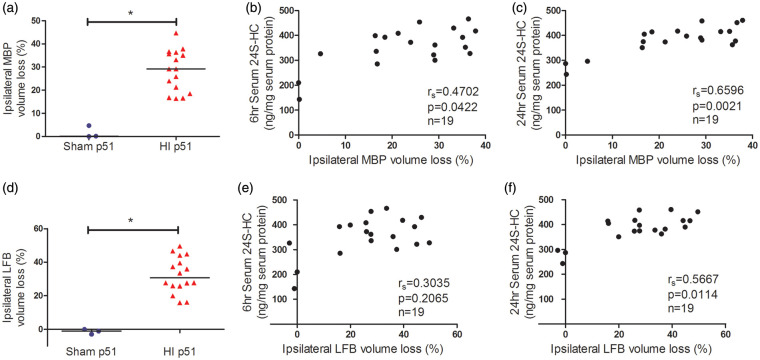
HI-induced brain damage evolves over time from hours to days and is mediated by pathways of oxidative stress, excitotoxicity, programmed cell death, and inflammation in the early phase.1–3 We have previously shown a positive correlation of serum 24S-HC levels and activation of calpain and caspase-3 acutely after HI (6 h and 24 h).4 In this study, we chose to evaluate brain injury at two stages: 6 days (on p15) after the insult when cell death is complete and full extent of injury is developed while endogenous plasticity and neural repair is subtle and six weeks (42 days after HI, on p51) corresponding to young human adults and the age when mice are capable of conducting the behavioral tasks. As shown in Figure 3, the average infarct volumes observed on six days and six weeks after HI were similar (Figure 3(a)). There was a strong positive correlation between the serum levels of 24S-HC (6 and 24 h) and the infarct volumes on p15 and p51 (Figure 3(b) to (e), the animal numbers, spearman rs and p values were listed in the individual panels). White matter injury was assessed by immunohistochemistry with the oligodendrocyte-specific protein MBP and LFB staining on p51, which consistently showed an average of 30% loss of ipsilateral white matter (Figure 4(a) and (d)). The serum 24S-HC concentrations at 24 h, but not at 6 h, were highly correlated with the ipsilateral MBP and LFB volume loss (Figure 4(b), (c), (e), and (f), for each comparison, the significant level α was specified at 0.05/2 = 0.025 after Bonferroni correction). There were no gender differences in the infarct volumes and white matter volume loss.
Figure 3.
The serum 24S-HC levels were correlated with HI histological infarct volumes. (a) The total infarct volumes (%) at six days (p15) and six weeks (p51) after HI. There was a significant positive correlation between the levels of serum 24S-HC at 6 h (b and d) or 24 h (c and e) and the infarct volumes evaluated at p15 (b and c) and p51 (d and e). The animal numbers, spearman rs, and p values were listed in the individual panels. 24S-HC: 24S-hydroxycholesterol; HI: hypoxia-ischemia. *p < 0.0001.
Figure 4.
The serum 24S-HC levels at 24 h after HI were associated with the ipsilateral MBP and LFB volume loss at p51. MBP immunohistochemistry and LFB staining were used to assess brain white matter injury and showed significant volume loss at six weeks (p51) after HI (a and d). The serum 24S-HC concentrations at 24 h (c and f), but not at 6 h (b and e), were highly correlated with the ipsilateral MBP/LFB volume loss. The animal numbers, spearman rs, and p values were listed in the individual panels. For each comparison, the significant level α was specified at 0.05/2 = 0.025 after Bonferroni correction. 24S-HC: 24S-hydroxycholesterol; HI: hypoxia-ischemia; MBP: myelin basic protein; LFB: luxol fast blue. *p < 0.0001.
The serum 24S-HC levels were associated with MRI-defined brain infarcts
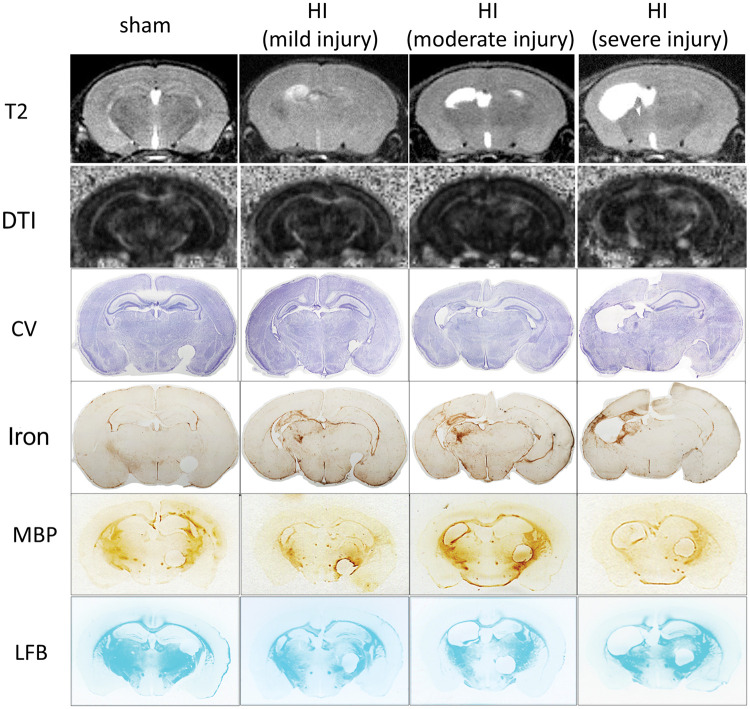
MRI is increasingly used as a quantitative tool for the assessment of injury location, severity, evolution, and prognostication in neonatal encephalopathy (NE) or HIE. The pattern of injury shown on the conventional structural T2 weighted images was consistent with that on the brain sections stained by CV or iron (Figure 5) with the hyper-intense regions in the ipsilateral hippocampus; the lower layers of cortex and thalamus were most damaged. Figure 6 shows that the MRI-defined infarct volumes on p41 (32 days after HI) were slightly higher than those on p15 (6 days after HI) (Figure 6 (a)), and their values were correlated (Figure 6(b)). The serum 24S-HC levels at 6 h (Figure 6(c) and (e)) and 24 h (Figure 6(d) and (f)) were associated with the infarct volumes assessed by T2W-MRI at both early (Figure 6(c) and (d)) and late (Figure 6(e) and (f)) time points (for each comparison, the significant level α was specified at 0.05/2 = 0.025 after Bonferroni correction).
Figure 5.
The representative images of MRI (T2W and DTI-fractional anisotropy map), histology staining (CV and Iron, LFB), and MBP immunohistochemistry. In examples of sham, mild, moderate, and severe HI injury, the injury patterns were similar using the imaging and histology methodology with the damage mainly located in ipsilateral hippocampus, cortex, and thalamus. MBP- and LFB-positive areas are mainly corpus callosum, external capsule, caudate, thalamus, hippocampal fimbria, and internal capsule (note that the circles on the right side of the MBP/LFB sections were punched to label the contralateral hemisphere, not the actual injury). HI: hypoxia-ischemia; MBP: myelin basic protein; LFB: luxol fast blue; CV: cresyl violet; DTI: diffusion tensor imaging.
Figure 6.
Correlation of serum 24S-HC levels with brain HI infarct volumes defined by T2-weighted MRI. The infarct volumes assessed at 6 days (p15) and 32 days (p41) after HI using MRI were correlated (a and b). The serum 24S-HC levels at 6 h (c and e) and 24 h (d and f) were associated with the infarct volumes assessed at both early (c and d) and late (e and f) time points after HI. The animal numbers, spearman rs, and p values were listed in the individual panels. For each comparison, the significant level α was specified at 0.05/2 = 0.025 after Bonferroni correction. Please note that one animal died after the first MRI scan on p15. MRI: magnetic resonance imaging; 24S-HC: 24S-hydroxycholesterol; HI: hypoxia-ischemia. *p < 0.001.
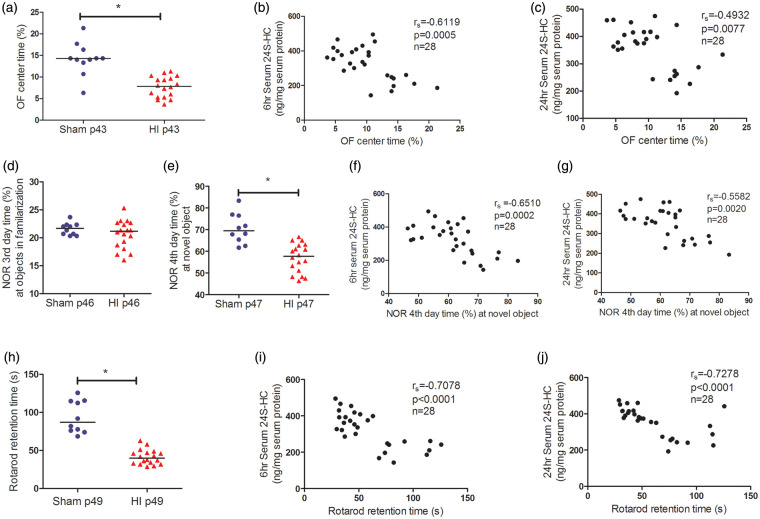
The serum 24S-HC levels predicted brain long-term functional outcomes after HI
In the OF test, the injured mice stayed less time in the center than the sham animals, indicating increased anxiety (Figure 7(a), p < 0.0001). The serum levels of 24S-HC negatively correlated with the percentage of time the mice spent in the center of the arena, i.e., higher 24S-HC was associated with higher level of anxiety (Figure 7(b) and (c)).
Figure 7.
The high levels of serum 24S-HC predicted poor behavioral outcomes. The HI-injured mice showed increased anxiety in OF test (a, n = 10 for sham, n = 18 for HI, *p < 0.0001), worse NOR memory (e, *p < 0.0001), and deficits in motor coordination function measured by rotarod test (h, *p < 0.0001). The learning ability was not altered in the HI animals (d). The serum levels of 24S-HC at 6 h (b, f, and i) and 24 h (c, g, and j) were negatively correlated with the performance in the three behavioral tests. The animal numbers, spearman rs, and p values were listed in the individual panels. For each comparison, the significant level α was specified at 0.05/3 = 0.017 after Bonferroni correction. 24S-HC: 24S-hydroxycholesterol; HI: hypoxia-ischemia; OF: open field; NOR: novel object recognition.
In the NOR test, there was no difference in time spent with the two objects between the sham and HI animals in day 3 familiarization phase, suggesting that the learning ability was not adversely impacted by HI (Figure 7(d)). However, in day 4 test, the injured mice spent significantly less time exploring the novel object compared to the sham animals indicating a deficit in NOR memory (Figure 7(e), p < 0.0001). The higher serum 24S-HC levels were associated with poorer NOR outcomes (Figure 7(f) and (g)).
In the ROD test, on 40 days after HI (p49), the injured mice performed poorly on the rod with a gradually increased speed (significantly reduced retention time compared to the sham animals, Figure 7(h), p < 0.0001). The animals with higher serum 24S-HC levels at 6 h (Figure 7(i)) and 24 h (Figure 7(j)) corresponded to those with less retention time indicating worse motor coordination function. For the comparisons between 24S-HC levels and the performance in the three behavioral tests, the significant level α was specified at 0.05/3 = 0.017 after Bonferroni correction.
Discussion
With its unique cerebral origin and ability to diffuse directly cross the BBB, the circulating 24S-HC is an ideal dynamic indicator for brain cholesterol metabolism. In the present study, we found a significant increase in the levels of serum 24S-HC at 6 h and 24 h after neonatal HI. Their levels correlated with the extent of brain injury, as well as the long-term functional outcomes, suggesting its additional role as a potential diagnostic and prognostic marker for neonatal HI brain damage, which may be translated to clinical use.
Expression of Cyp46a1, the enzyme responsible for the formation of 24S-HC, in normal p9 mouse brain showed a similar pattern to that in the adult mouse brain.7,9,14 It is mainly localized in the large pyramidal neurons in cortical layers V and VI, hippocampus and thalamus (Supplementary Figure), namely, the most metabolically active neurons and also the ones that are most vulnerable to HI and energy failure. Activation of Cyp46a1 leading to an increase in 24S-HC in our HI model could be a result of excitotoxicity, given that glutamate20 and oxidative stress21,22 have been reported to enhance Cyp46a1 promoter activity. Based on the speculation from human studies of neurodegenerative diseases, 24S-HC may be released from dying neurons or during acute demyelination. This is because that excess free cholesterol, from either disrupted plasma membranes or myelin breakdown at the site of injury, needs to be cleared out by hydroxylation to 24S-HC, which readily crosses the BBB and is then excreted in the liver. This may explain, in part, the association between 24S-HC levels and the degree of gray or white matter damage. In our model, the co-localization of Cyp46a1 with cleaved-caspase 3 in the dead or dying neurons4 seems to support the above inference. This hypothesis can be tested by using an apoptosis inhibitor to see whether 24S-HC amount is diminished accordingly. In this case, increased 24S-HC can be considered to be a by-product of neuronal cell death, with levels that change with the severity of brain injury.
Besides neurons, 24S-HC can be additionally derived from glial cells under pathological conditions. For example, glial-localized Cyp46a1 (in astrocytes, microglia or macrophages) has been shown in traumatic brain injury models23,24 and in AD patients.25 We observed Cyp46a1 expression in neurons and oligodendrocytes in p9 naive brain and at 24 h after HI;4 however, their relative contribution to 24S-HC production is unknown. Extended time points should be investigated to determine the cellular sources of the increased 24S-HC following neonatal HI. Conditional Cyp46a1 activation in glial cells can facilitate the conversion of the excess cholesterol to 24S-HC in the brain and thus abnormally higher flux into the circulation.
Another explanation for the association of 24S-HC with HI brain damage is that the toxic levels of 24S-HC produced by upregulation of Cyp46a1 may cause cell death and directly contribute to brain injury. 24S-HC could promote N-methyl-D-aspartate receptor (NMDAR)-mediated excitotoxic neuronal death under HI because it is a potent allosteric modulator in enhancing the function of NMDAR.26,27 Exogenous 24S-HC or Cyp46a1 overexpression exacerbate oxygen-glucose deprivation-induced neurotoxicity through NMDAR in primary hippocampal neurons.28 In addition, 24S-HC is cytotoxic at supraphysiologic concentrations by inducing apoptosis or necroptosis.29,30 The Noguchi laboratory reported a series of studies and reviews describing the role of 24S-HC in modulating neuronal cell death.29–33 However, the related mechanistic studies were performed using human neuroblastoma SH-SY5Y and Jurkat lymphocyte cell lines, which were informative but need to be validated in vivo in the brain. Again, without utilizing a Cyp46a1 inhibitor, the relationship between 24S-HC production and brain damage remains ambiguous.
Our work has translational potential to clinical studies as the human brain contains about 80% of the 24S-HC in the whole body,34 and more than 90% of 24S-HC circulating in the blood can be attributed to the central nervous system (CNS), a higher percentage than that in rodents.35,36 Serum 24S-HC levels in humans are markedly higher in infants and children in the first decade of life compared to adults.34 Term babies have higher serum concentrations of 24S-HC than preterm infants but lower serum cholesterol amounts.37 Therefore, the serum level of 24S-HC is a rational marker to inform the dynamics of cholesterol metabolism in the human brain, especially in the developing brain.
The continuous efforts of identifying plasma biomarkers for HIE or NE with multicenter randomized trials have proposed many candidates for brain injury, including brain-specific proteins released from damaged CNS cells (UCH-L1, NSE, GFAP, S100β, total Tau, etc.), oxidative stress-related products, metabolic markers, microRNAs, inflammatory cytokines and chemokines, neural exosomes, etc.38–41 The results from evaluating these markers have been inconsistent and thus limited their validity due to the complexity of HIE etiology, variability of injury responses, the timing of the insult and blood sampling, as well as the approaches to assess clinical grade of brain damage and outcomes. An ideal biomarker not only informs the condition at the time of the measurement for a timely treatment but also predicts disease progression in order to provide patients with more information on future clinical outcomes. It is elusive to find a single molecule to achieve this goal and a panel of several blood biomarkers may be required. Based on the brain specificity, and the correlation with brain injury evaluated either early or late after HI, circulating levels of 24S-HC may be added to this panel as an earlier lipid marker with the capabilities for both diagnostic and prognostic application for HIE infants.
Our study demonstrated that serum 24S-HC could be an acute marker of HI brain injury if measured within 24 h after the insult, when secondary energy failure and cell death responses peak, but is of limited value if it is measured beyond this time window. It is difficult to pinpoint exactly when HI occurs in HIE infants, especially when it happens before birth. But in the cases when the patients show evidence of acute peripartum or intrapartum hypoxia or interruption of placental blood flow, serum 24S-HC can be measured within 24 h to evaluate brain injury and provide early prognostic information. As the 6 h time window is currently the standard for initiating therapeutic hypothermia in term infants, the 6 h 24S-HC levels can be used in conjunction with other biochemical and imaging criteria for selecting babies eligible for cooling. The trajectory of 24S-HC production may be different in HIE infants and needs to be validated for possible clinical use for these newborns. Future studies are also required to compare the predictive ability of 24S-HC with other markers of HI brain injury. Although the exact role of Cyp46a1 and 24S-HC in the pathophysiology of neonatal HI brain injury is not clear yet, our preclinical mouse data suggest that 24S-HC in the blood is a plausible, novel, and promising biomarker for monitoring HIE injury that is worth further exploring in future clinical trials.
Supplemental Material
Supplemental material, JCB911910 Supplemental Material for Serum 24S-hydroxycholesterol predicts long-term brain structural and functional outcomes after hypoxia-ischemia in neonatal mice by Fuxin Lu, Shujuan Fan, Andrea R Romo, Duan Xu, Donna M Ferriero and Xiangning Jiang in Journal of Cerebral Blood Flow & Metabolism
Acknowledgements
The authors would like to thank Dr Zinaida S. Vexler, University of California San Francisco, for her critical and helpful comments on the manuscript.
Footnotes
Authors’ contribution: Fuxin Lu performed the HI animal experiments, carried out the behavioral testing, measured serum 24S-HC, collected data, performed statistical analyses, prepared the figures, and revised the section of material and methods. Shujuan Fan performed MRI brain imaging and related analyses. Andrea R Romo assisted in the histology and immunohistochemistry experiments. Duan Xu participated in the design of the brain imaging study. Donna M Ferriero provided funding, reviewed, and revised the manuscript. Xiangning Jiang has contributed toward concept and design, obtained funding, interpreted data, drafted, and revised the manuscript. All authors have approved the final version of the manuscript. None of the material included in this manuscript has been published or is under consideration elsewhere, including the Internet.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute of Neurological Disorders and Stroke (RO1NS084057 to Dr. Jiang, R35NS097299 to Dr. Ferriero).
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplemental material: Supplemental material for this is available online.
References
- 1.Douglas-Escobar M, Weiss MD.Hypoxic-ischemic encephalopathy: a review for the clinician. JAMA Pediatr 2015; 169: 397–403. [DOI] [PubMed] [Google Scholar]
- 2.Ferriero DM.Neonatal brain injury. N Engl J Med 2004; 351: 1985–1995. [DOI] [PubMed] [Google Scholar]
- 3.Millar LJ, Shi L, Hoerder-Suabedissen A, et al. Neonatal hypoxia ischaemia: mechanisms, models, and therapeutic challenges. Front Cell Neurosci 2017; 11: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu F, Zhu J, Guo S, et al. Upregulation of cholesterol 24-hydroxylase following hypoxia-ischemia in neonatal mouse brain. Pediatr Res 2018; 83: 1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjorkhem I, Lutjohann D, Diczfalusy U, et al. Cholesterol homeostasis in human brain: turnover of 24S-hydroxycholesterol and evidence for a cerebral origin of most of this oxysterol in the circulation. J Lipid Res 1998; 39: 1594–1600. [PubMed] [Google Scholar]
- 6.Dietschy JM.Central nervous system: cholesterol turnover, brain development and neurodegeneration. Biol Chem 2009; 390: 287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lund EG, Guileyardo JM, Russell DW.cDNA cloning of cholesterol 24-hydroxylase, a mediator of cholesterol homeostasis in the brain. Proc Natl Acad Sci U S A 1999; 96: 7238–7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lutjohann D, von Bergmann K.24S-hydroxycholesterol: a marker of brain cholesterol metabolism. Pharmacopsychiatry 2003; 36: S102–S106. [DOI] [PubMed] [Google Scholar]
- 9.Russell DW, Halford RW, Ramirez DM, et al. Cholesterol 24-hydroxylase: an enzyme of cholesterol turnover in the brain. Annu Rev Biochem 2009; 78: 1017–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grayaa S, Zerbinati C, Messedi M, et al. Plasma oxysterol profiling in children reveals 24-hydroxycholesterol as a potential marker for autism spectrum disorders. Biochimie 2018; 153: 80–85. [DOI] [PubMed] [Google Scholar]
- 11.Bjorkhem I, Starck L, Andersson U, et al. Oxysterols in the circulation of patients with the Smith–Lemli–Opitz syndrome: abnormal levels of 24S- and 27-hydroxycholesterol. J Lipid Res 2001; 42: 366–371. [PubMed] [Google Scholar]
- 12.Leoni V, Caccia C.24S-hydroxycholesterol in plasma: a marker of cholesterol turnover in neurodegenerative diseases. Biochimie 2013; 95: 595–612. [DOI] [PubMed] [Google Scholar]
- 13.Leoni V, Caccia C.Potential diagnostic applications of side chain oxysterols analysis in plasma and cerebrospinal fluid. Biochem Pharmacol 2013; 86: 26–36. [DOI] [PubMed] [Google Scholar]
- 14.Ramirez DM, Andersson S, Russell DW.Neuronal expression and subcellular localization of cholesterol 24-hydroxylase in the mouse brain. J Comp Neurol 2008; 507: 1676–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leoni V, Masterman T, Diczfalusy U, et al. Changes in human plasma levels of the brain specific oxysterol 24S-hydroxycholesterol during progression of multiple sclerosis. Neurosci Lett 2002; 331: 163–166. [DOI] [PubMed] [Google Scholar]
- 16.Hughes TM, Rosano C, Evans RW, et al. Brain cholesterol metabolism, oxysterols, and dementia. J Alzheimers Dis 2013; 33: 891–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bjorkhem I, Lovgren-Sandblom A, Leoni V, et al. Oxysterols and Parkinson’s disease: evidence that levels of 24S-hydroxycholesterol in cerebrospinal fluid correlates with the duration of the disease. Neurosci Lett 2013; 555: 102–105. [DOI] [PubMed] [Google Scholar]
- 18.Rice JE, III, Vannucci RC, Brierley JB.The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol 1981; 9: 131–141. [DOI] [PubMed] [Google Scholar]
- 19.Swanson RA, Morton MT, Tsao-Wu G, et al. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab 1990; 10: 290–293. [DOI] [PubMed] [Google Scholar]
- 20.Mast N, Anderson KW, Johnson KM, et al. In vitro cytochrome P450 46A1 (CYP46A1) activation by neuroactive compounds. J Biol Chem 2017; 292: 12934–12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohyama Y, Meaney S, Heverin M, et al. Studies on the transcriptional regulation of cholesterol 24-hydroxylase (CYP46A1): marked insensitivity toward different regulatory axes. J Biol Chem 2006; 281: 3810–3820. [DOI] [PubMed] [Google Scholar]
- 22.Sodero AO, Weissmann C, Ledesma MD, et al. Cellular stress from excitatory neurotransmission contributes to cholesterol loss in hippocampal neurons aging in vitro. Neurobiol Aging 2011; 32: 1043–1053. [DOI] [PubMed] [Google Scholar]
- 23.Cartagena CM, Ahmed F, Burns MP, et al. Cortical injury increases cholesterol 24S hydroxylase (Cyp46) levels in the rat brain. J Neurotrauma 2008; 25: 1087–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smiljanic K, Lavrnja I, Mladenovic Djordjevic A, et al. Brain injury induces cholesterol 24-hydroxylase (Cyp46) expression in glial cells in a time-dependent manner. Histochem Cell Biol 2010; 134: 159–169. [DOI] [PubMed] [Google Scholar]
- 25.Brown J, III, Theisler C, Silberman S, et al. Differential expression of cholesterol hydroxylases in Alzheimer’s disease. J Biol Chem 2004; 279: 34674–34681. [DOI] [PubMed] [Google Scholar]
- 26.Linsenbardt AJ, Taylor A, Emnett CM, et al. Different oxysterols have opposing actions at N-methyl-D-aspartate receptors. Neuropharmacology 2014; 85: 232–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paul SM, Doherty JJ, Robichaud AJ, et al. The major brain cholesterol metabolite 24(S)-hydroxycholesterol is a potent allosteric modulator of N-methyl-D-aspartate receptors. J Neurosci 2013; 33: 17290–17300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun MY, Taylor A, Zorumski CF, et al. 24S-hydroxycholesterol and 25-hydroxycholesterol differentially impact hippocampal neuronal survival following oxygen-glucose deprivation. PLoS One 2017; 12: e0174416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noguchi N, Urano Y, Takabe W, et al. New aspects of 24(S)-hydroxycholesterol in modulating neuronal cell death. Free Radic Biol Med 2015; 87: 366–372. [DOI] [PubMed] [Google Scholar]
- 30.Yamanaka K, Saito Y, Yamamori T, et al. 24(S)-hydroxycholesterol induces neuronal cell death through necroptosis, a form of programmed necrosis. J Biol Chem 2011; 286: 24666–24673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shibuya K, Watanabe T, Urano Y, et al. Synthesis of 24(S)-hydroxycholesterol esters responsible for the induction of neuronal cell death. Bioorg Med Chem 2016; 24: 2559–2566. [DOI] [PubMed] [Google Scholar]
- 32.Takabe W, Urano Y, Vo DH, et al. Esterification of 24S-OHC induces formation of atypical lipid droplet-like structures, leading to neuronal cell death. J Lipid Res 2016; 57: 2005–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vo DK, Urano Y, Takabe W, et al. 24(S)-hydroxycholesterol induces RIPK1-dependent but MLKL-independent cell death in the absence of caspase-8. Steroids 2015; 99: 230–237. [DOI] [PubMed] [Google Scholar]
- 34.Lutjohann D, Breuer O, Ahlborg G, et al. Cholesterol homeostasis in human brain: evidence for an age-dependent flux of 24S-hydroxycholesterol from the brain into the circulation. Proc Natl Acad Sci U S A 1996; 93: 9799–9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lund EG, Xie C, Kotti T, et al. Knockout of the cholesterol 24-hydroxylase gene in mice reveals a brain-specific mechanism of cholesterol turnover. J Biol Chem 2003; 278: 22980–22988. [DOI] [PubMed] [Google Scholar]
- 36.Meaney S, Lutjohann D, Diczfalusy U, et al. Formation of oxysterols from different pools of cholesterol as studied by stable isotope technique: cerebral origin of most circulating 24S-hydroxycholesterol in rats, but not in mice. Biochim Biophys Acta 2000; 1486: 293–298. [DOI] [PubMed] [Google Scholar]
- 37.Lutjohann D, Bjorkhem I, Locatelli S, et al. Cholesterol dynamics in the foetal and neonatal brain as reflected by circulatory levels of 24S-hydroxycholesterol. Acta Paediatr 2001; 90: 652–657. [DOI] [PubMed] [Google Scholar]
- 38.Graham EM, Burd I, Everett AD, et al. Blood biomarkers for evaluation of perinatal encephalopathy. Front Pharmacol 2016; 7: 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graham EM, Everett AD, Delpech JC, et al. Blood biomarkers for evaluation of perinatal encephalopathy: state of the art. Curr Opin Pediatr 2018; 30: 199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lv H, Wang Q, Wu S, et al. Neonatal hypoxic ischemic encephalopathy-related biomarkers in serum and cerebrospinal fluid. Clin Chim Acta 2015; 450: 282–297. [DOI] [PubMed] [Google Scholar]
- 41.Massaro AN, Wu YW, Bammler TK, et al. Plasma biomarkers of brain injury in neonatal hypoxic-ischemic encephalopathy. J Pediatr 2018; 194: 67.e1–75.e1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, JCB911910 Supplemental Material for Serum 24S-hydroxycholesterol predicts long-term brain structural and functional outcomes after hypoxia-ischemia in neonatal mice by Fuxin Lu, Shujuan Fan, Andrea R Romo, Duan Xu, Donna M Ferriero and Xiangning Jiang in Journal of Cerebral Blood Flow & Metabolism