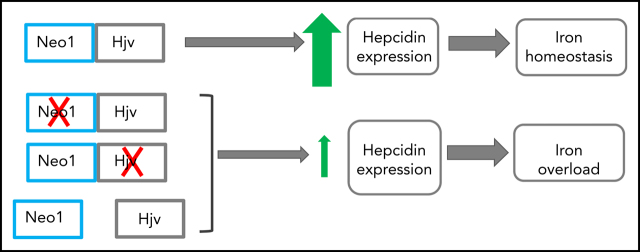
Neogenin (NEO1) is a transmembrane receptor responsible for crucial functions in diverse cellular processes ranging from cell motility and adhesion to survival and differentiation. To mediate these functions, neogenin binds different ligands, one of which is hemojuvelin (HJV), the protein mutated in juvenile hemochromatosis. But its role in iron homeostasis has been controversial. Enns et al resolve this by reporting compelling evidence in murine models that hepatocyte NEO1 is essential for systemic iron homeostasis, upregulating hepcidin expression via its association with HJV.
Key Points
Ablation of hepatocyte neogenin in mice reduces hepcidin expression and causes iron overload with no obvious developmental defects.
Neogenin induction of hepcidin depends on its interaction with hemojuvelin, which leads to neogenin accumulation on the plasma membrane.
Visual Abstract
Abstract
Neogenin (NEO1) is a ubiquitously expressed multifunctional transmembrane protein. It interacts with hemojuvelin (HJV), a BMP coreceptor that plays a pivotal role in hepatic hepcidin expression. Earlier studies suggest that the function of HJV relies on its interaction with NEO1. However, the role of NEO1 in iron homeostasis remains controversial because of the lack of an appropriate animal model. Here, we generated a hepatocyte-specific Neo1 knockout (Neo1fl/fl;Alb-Cre+) mouse model that circumvented the developmental and lethality issues of the global Neo1 mutant. Results show that ablation of hepatocyte Neo1 decreased hepcidin expression and caused iron overload. This iron overload did not result from altered iron utilization by erythropoiesis. Replacement studies revealed that expression of the Neo1L1046E mutant that does not interact with Hjv, was unable to correct the decreased hepcidin expression and high serum iron in Neo1fl/fl;Alb-Cre+ mice. In Hjv−/− mice, expression of HjvA183R mutant that has reduced interaction with Neo1, also displayed a blunted induction of hepcidin expression. These observations indicate that Neo1-Hjv interaction is essential for hepcidin expression. Further analyses suggest that the Hjv binding triggered the cleavage of the Neo1 cytoplasmic domain by a protease, which resulted in accumulation of truncated Neo1 on the plasma membrane. Additional studies did not support that Neo1 functions by inhibiting Hjv shedding as previously proposed. Together, our data favor a model in which Neo1 interaction with Hjv leads to accumulation of cleaved Neo1 on the plasma membrane, where Neo1 acts as a scaffold to induce the Bmp signaling and hepcidin expression.
Introduction
Neogenin (NEO1) is a transmembrane protein that is expressed in most tissues. It consists of 4 immunoglobulin-like domains, 6 fibronectin III (FNIII) repeats, a single transmembrane domain, and a cytoplasmic region (Figure 1A). NEO1 is a multifunctional receptor that binds the repulsive guidance molecules a and b (RGMa and RGMb) and hemojuvelin (HJV; also known as RGMc) through its FNIII 5-6 domains.1-4 RGMa, RGMb, and HJV belong to the highly conserved RGM family; however, their functions differ. RGMa and RGMb are mainly restricted to the developing nervous system, where they are necessary for neural axon guidance.5 By contrast, HJV is expressed in hepatocytes, skeletal muscle cells, and cardiomyocytes. It is critical in hepcidin expression and iron homeostasis.6 Clinical studies show that mutations in the HJV gene diminish hepatic hepcidin expression and lead to juvenile hemochromatosis, a severe iron overload disease.6 Animal studies also show that hepatic Hjv is critical in the maintenance of normal iron levels.7-10
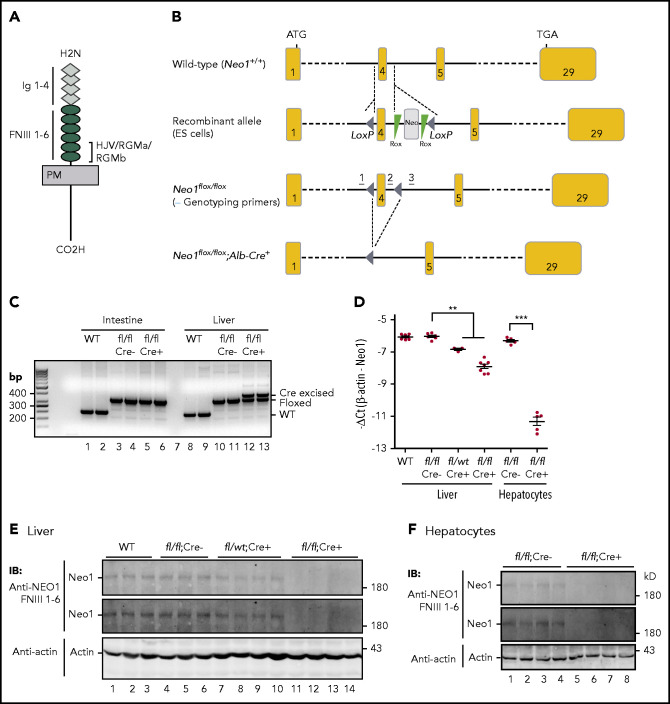
Figure 1.
Generation of hepatocyte-specific conditional Neo1 knockout (Neo1fl/fl;Alb-Cre+) mice. (A) Diagram of NEO1 and the domains that HJV/RGMa/RGMb bind. FNIII, fibronectin III; Ig, immunoglobulin; PM, plasma membrane. (B) Schematic depictions of loxP-flanked (floxed) Neo1 allele and the allele after Cre recombinase-mediated excision. Genotyping primer positions are indicated. (C) PCR analysis of genomic DNA extracted from the liver and intestine of wild-type (WT), Neo1fl/fl;Alb-Cre−, and Neo1fl/fl;Alb-Cre+ mice. (D) qRT-PCR analysis of Neo1 mRNA levels in the liver and isolated hepatocytes from 8-week-old WT, Neo1fl/fl;Alb-Cre−, Neo1fl/fl;Alb-Cre+, or Neo1fl/wt;Alb-Cr+ mice. Each group consists of at least 3 animals. Data shown are means ± SD. **P < .01 and ***P < .001 relative to the respective Neo1fl/fl;Alb-Cre− controls. (E-F) Representative images of western blot analysis for Neo1 and β-actin in the liver (E) and isolated hepatocytes (F). Two images of Neo1 with different exposure times were presented. IB, immunoblotting.
Hepcidin is peptide hormone that is secreted mainly by hepatocytes. It regulates iron efflux from duodenal epithelial cells, macrophages, and hepatocytes into the circulation by directly occluding the iron pore of the plasma membrane iron exporter, ferroportin, as well as by inducing ferroportin internalization and degradation.11,12 The BMP-signaling pathway induces hepcidin expression. A selective set of BMP ligands (BMP2 and BMP6), BMP receptors (ALK2, ALK3, ActRIIA, and Bmpr2), and cytoplasmic SMADs (SMAD1/5/8) are used to stimulate HJV-mediated hepcidin expression.13-24 Additionally, hemochromatosis protein (HFE) and transferrin receptor-2 (TfR2) play a less defined role in hepcidin expression.25
Several lines of evidence suggest that the function of HJV relies on its interaction with NEO1. Hjv and Neo1 are coexpressed in murine hepatocytes.26,27 HJV-NEO1 interactions have been demonstrated by different approaches.1-4,28,29 The most common juvenile hemochromatosis-causing mutation in HJV, G320V, disrupts its interaction with NEO1 in human.1,3 In animal studies, Hjv mutants that have decreased interaction with Neo1 display attenuated ability to induce Bmp-signaling and hepcidin expression.30 Importantly, global Neo1 mutant mice have reduced hepcidin expression and severe iron overload that is indistinguishable from Hjv-/- mice.26 However, interpretation of these data is limited by the severe developmental defects and perinatal death.26,30,31 In hepatoma cells, HJV was reported to induce hepcidin expression independently of NEO1.32 Thus, the role of NEO1 in iron homeostasis remains controversial.
In this study, we generated a hepatocyte-specific conditional Neo1 knockout (Neo1fl/fl;Alb-Cre+) mouse model that circumvented the developmental defects of global Neo1 ablation. Results demonstrated a pivotal role of Neo1-Hjv interaction in hepcidin expression and iron homeostasis.
Materials and methods
Complementary DNA constructs
Mouse Neo1 ORF (NM_008684) with a C-terminal FLAG/MYC epitope in pCMV6 vector (pCMV6-fNeo1) was obtained from Origene (#MR226235). pCMV6-fNeo1L1046E mutant was generated as described in the supplemental Information on the Blood Web site. All sequences were verified by DNA sequencing.
AAV8-fNeo1 and AAV8-fNeo1L1046E constructs were generated by subcloning into an AAV8 construct containing a strong liver-specific promoter as described in our previous study.33 AAV8 constructs containing a N-terminal 3xFLAG-tagged Hjv (fHjv) or fHjvA183R mutant were previously generated.30 AAV8 viral vectors were produced at the Molecular Virology Support Core, Oregon Health & Science University (OHSU).
Animal studies
All animal procedures were approved by OHSU/DCM. Mice carrying a Neo1 conditional allele (Neo1fl/wt) on a C57BL/6J background were generated by flanking exon-4 of the Neo1 gene with loxP sites (Cyagen) (Figure 1B). Homozygous hepatocyte-specific conditional Neo1 knockout (Neo1fl/fl;Alb-Cre+) mice, heterozygous knockout (Neo1fllwt;Alb-Cre+) mice, and littermate Cre– controls were generated by crossing Neo1fl/wt mice with mice expressing a Cre recombinase transgene driven by a hepatocyte-specific albumin (Alb) promoter on a C57BL/6J background (The Jackson Laboratory). Genotyping was performed by using mouse tail snipping and polymerase chain reaction (PCR). The mice of both genders were euthanized at about 5, 8, and 15 weeks of age for analysis as described in the following section and in the supplemental Information.
Eight-week-old Neo1fl/fl;Alb-Cre+ male mice were intraperitoneally injected with AAV8-fNeo1 or fNeo1L1046E viral vectors at ∼3.5 × 1010 viral genome particles per mouse. The AAV8 vector alone had no effect on iron homeostasis in mice.30,31 Injection of phosphate-buffered saline (PBS) was included as a control. Mice were euthanized at 3 weeks after injection for analysis. Age, gender, and background-matched Cre− littermates were included as additional controls.
We obtained the Hjv−/− mice on 129/SvEvTac background from Nancy Andrews (Duke University, Durham, NC). The detailed procedures for studies of Hjv−/− mice were described in our earlier studies30 and in the supplemental Information. All mice were fed a PicoLab Laboratory Rodent Diet-5L0D containing 240 ppm iron (LabDiet).
qRT-PCR
Quantitative reverse transcription PCR (qRT-PCR) analyses of Neo1, hepcidin, inhibitor of DNA binding-1 (Id1), Bmp6, Bmp2, Hjv, Hfe, Tfr2, Alk2, Alk3, and β-actin transcripts in the liver were conducted as described in supplemental Information. Results are expressed as −ΔCt ± the standard deviation (SD) (ie, the cycle threshold differences between reference [β-actin] and target genes within each group). For some analyses, the amounts relative to that of β-actin (2–ΔΔCt) were also presented.
Immunodetection
fNeo1 and fHjv in the liver were probed directly by using a horseradish peroxidase–coupled mouse anti-FLAG M2 IgG (Sigma). Endogenous Neo1, Tfr2, Hjv, and β-actin were detected by using rabbit anti-NEO1 FNIII 1-6,32 rabbit anti-Tfr2,34 mouse anti-HJV,35 and mouse anti-β-actin (Sigma) antibodies. All detailed procedures are described in the supplemental Information.
Cell lines and transfection
Hep3B cells (ATCC) were transiently transfected to determine the processing of fNeo1, fNeo1L1046E, fHjv, and fHjvA183R and their induction of hepcidin expression. HepG2 cells stably expressing HJV (HepG2-HJV) were previously generated.27,32,36 All detailed procedures are described in the supplemental Information.
Statistical analysis
A 2-tailed Student t test was used to compare 2 sets of data. One-way analysis of variance and Tukey’s posttests were used to compare 3 or more sets of data.
Results
Hepatocyte-specific Neo1 knockout circumvents the developmental defects of global Neo1 ablation in mice
We generated a hepatocyte-specific conditional Neo1 knock-out (Neo1fl/fl;Alb-Cre+) mouse model on a C57BL/6J background. Excision of LoxP-flanked Neo1 was confirmed by PCR of total liver genomic DNA from Neo1fl/fl;Alb-Cre+ mice (Figure 1C, lanes 12-13). The lack of complete excision was due to the expression of Neo1 in nonparenchymal cells.27 There was no excision in the duodenum (Figure 1C, lanes 5-6). Compared with wild-type C57BL/6J and Neo1fl/fl;Alb-Cre− littermate controls, ∼80% and 98% decreases in Neo1 messenger RNA (mRNA) were detected in the liver and isolated hepatocytes of Neo1fl/fl;Alb-Cre+ mice, respectively (Figure 1D; supplemental Figure 1A). The Neo1 protein levels in the liver and isolated hepatocytes of Neo1fllfl;Alb-Cre+ mice fell below the limits of detection by western blot (Figure 1E-F). As predicted, ∼50% decrease in Neo1 mRNA and protein was found in the liver of heterozygous Neo1fllwt;Alb-Cre+ animals (Figure 1D-E). These results indicate that Neo1fl/fl;Alb-Cre+ mice had a nearly complete deletion of hepatocyte Neo1.
Distinct from global Neo1 mutant mice,26,37,38 Neo1fl/fl;Alb-Cre+ mice were born in the predicted Mendelian ratio. They displayed no perinatal death and were not different in appearance when compared with wild-type Neo1fl/fl;Alb-Cre− littermates. Both Neo1fl/fl;Alb-Cre+ and Neo1fl/fl;Alb-Cre− littermates had similar gender-dependent body weights at 5, 8, and 15 weeks old (supplemental Figure 1B-C). Both male and female Neo1fl/fl;Alb-Cre+ mice were fertile. These data indicate that depletion of hepatocyte Neo1 has no evident effect on development or fertility.
Depletion of hepatocyte Neo1 causes iron overload
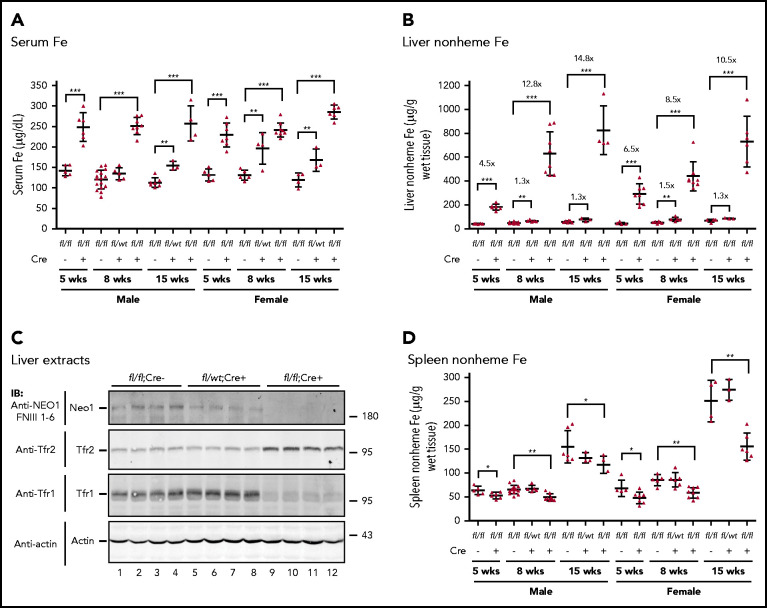
We used Neo1fl/fl;Alb-Cre+ mice to study the roles of hepatocyte Neo1 in iron homeostasis. As shown in Figure 2A, Neo1fl/fl;Alb-Cre+ mice displayed significantly elevated serum iron concentrations at the age of 5, 8, and 15 weeks for both sexes, when compared with their corresponding Neo1fl/fl;Alb-Cre− littermate controls. Consistently, a significant increase in liver nonheme iron levels was detected in Neo1fl/fl;Alb-Cre+ mice at 5 weeks, and greater increases at 8 and 15 weeks (Figure 2B). The liver nonheme iron is widely used as an indicator of body iron storage. In agreement with the elevation of iron in serum and liver, western blot revealed an increase of Tfr2 and a decrease of Tfr1 in the liver extracts of Neo1fl/fl;Alb-Cre+ mice (Figure 2C, lanes 9-12). Tfr2 is stabilized by increased transferrin saturation, and the Tfr1 mRNA is unstable when cellular iron is replete.25 Similar to global Neo1 mutant mice26 and Hjv−/− mice,7,8 Neo1fl/fl;Alb-Cre+ mice had decreased spleen nonheme iron levels (Figure 2D). Together, these data indicate that depletion of hepatocyte Neo1 causes iron overload.
Figure 2.
Neo1fl/fl;Alb-Cre+mice have iron overload. (A) Serum iron (Fe) assay for Neo1fl/fl;Alb-Cre−, Neo1fl/wt;Alb-Cr+, and Neo1fl/fl;Alb-Cre+ male and female mice at 5, 8, and 15 weeks old. (B) Liver nonheme iron assay. Iron concentration is expressed as micrograms of iron per gram of wet tissue. (C) Representative images of western blot analysis for Neo1, Tfr2, Tfr1, and β-actin in the liver extracts of 8-week-old Neo1fl/fl;Alb-Cre−, Neo1fl/wt;Alb-Cr+, and Neo1fl/fl;Alb-Cre+ mice. (D) Spleen nonheme iron assay. Each group consists of at least 3 animals. The means ± SD are presented. A 2-tailed Student t test was used to analyze the data for 5-week-old mice, and 1-way analysis of variance (ANOVA) and Tukey’s posttests were used to analyze the data for 8- and 15-week-old mice. *P < .05; **P < .01; ***P < .001.
Analysis of blood parameters ruled out the possibility that the iron overload resulted from decreased iron utilization by erythropoiesis. Neo1fl/fl;Alb-Cre+ mice had significantly increased levels of hemoglobin, hematocrit, mean cell volume, and mean corpuscular hemoglobin in 5-, 8-, and 15-week-old males and in 5- and 8-week-old females with no change of red blood cell counts (Tables 1 and 2). These increases were likely caused by increased serum iron supply (Figure 2A).
Table 1.
Hematologic parameters for Neo1fl/fl;Alb-Cre−, Neo1fl/wt;Alb-Cre+, and Neo1fl/fl;Alb-Cr+ male mice
| Neo1fl/fl;Alb-Cre− (5 wk) (n = 5) | Neo1fl/fl;Alb-Cre+ (5 wk) (n = 6) | Neo1fl/fl;Alb-Cre− (8 wk) (n = 15) | Neo1fllwt;Alb-Cre+ (8 wk) (n = 5) | Neo1fl/fl;Alb-Cre+ (8 wk) (n = 8) | Neo1fl/fl;Alb-Cre− (15 wk) (n = 7) | Neo1fl/fl;Alb-Cre+ (15 wk) (n = 4) | |
|---|---|---|---|---|---|---|---|
| RBC | 8.34 ± 0.36 | 8.42 ± 0.22 | 8.75 ± 0.35 | 8.53 ± 0.50 | 8.87 ± 0.29 | 8.85 ± 0.42 | 8.91 ± 0.15 |
| Hb | 12.40 ± 0.16 | 12.78 ± 0.27* | 12.54 ± 0.49 | 12.29 ± 0.53 | 13.33 ± 0.29† | 13.03 ± 0.41 | 13.77 ± 0.59‡ |
| HCT | 42.70 ± 2.28 | 43.86 ± 0.86* | 39.64 ± 1.86 | 39.48 ± 1.97 | 42.51 ± 1.38‡ | 39.43 ± 0.90 | 43.22 ± 1.89‡ |
| MCV | 48.02 ± 1.73 | 50.11 ± 0.89* | 45.34 ± 0.89 | 45.96 ± 1.53 | 47.90 ± 0.79† | 44.58 ± 1.99 | 48.47 ± 1.77* |
| MCH | 14.57 ± 0.24 | 15.01 ± 0.26* | 14.38 ± 0.44 | 14.54 ± 0.22 | 15.02 ± 0.35‡ | 14.74 ± 0.63 | 15.45 ± 0.55* |
Data are expressed as means ± SD. A 2-tailed Student t test was used for statistical analysis between Neo1fl/wt;Alb-Cr+ mice and the age-matched Neo1fl/fl;Alb-Cre− control group at 5 and 15 wk old. One-way ANOVA and Tukey’s posttests were used to compare 8-wk-old mice.
Hb, hemoglobin (g/dL); HCT, hematocrit (%); MCV, mean cell volume (fL); MCH, mean corpuscular hemoglobin (pg); RBC, red blood cell (×106/µL).
P < .05.
P < .001.
P < .01.
Table 2.
Hematologic parameters for Neo1fl/fl;Alb-Cre−, Neo1fl/wt;Alb-Cre+, and Neo1fl/fl;Alb-Cr+ female mice
| Neo1fl/fl;Alb-Cre− (5 wk) (n = 4) | Neo1fl/fl;Alb-Cre+ (5 wk) (n = 7) | Neo1fl/fl;Alb-Cre− (8 wk) (n = 6) | Neo1fllwt;Alb-Cre+ (8 wk) (n = 5) | Neo1fl/fl;Alb-Cre+ (8 wk) (n = 8) | Neo1fl/fl;Alb-Cre− (15 wk) (n = 4) | Neo1fl/fl;Alb-Cre+ (15 wk) (n = 5) | |
|---|---|---|---|---|---|---|---|
| RBC | 8.57 ± 0.17 | 8.48 ± 0.25 | 8.83 ± 0.40 | 8.98 ± 0.33 | 8.83 ± 0.41 | 9.19 ± 0.51 | 9.15 ± 0.48 |
| Hb | 12.65 ± 0.24 | 12.95 ± 0.15* | 12.76 ± 0.51 | 12.90 ± 0.61 | 13.81 ± 0.36† | 13.57 ± 0.35 | 13.90 ± 0.36 |
| HCT | 42.72 ± 0.55 | 44.18 ± 0.81* | 40.06 ± 1.49 | 40.86 ± 3.03 | 43.93 ± 1.47† | 42.35 ± 2.26 | 44.60 ± 2.47 |
| MCV | 48.40 ± 0.39 | 50.40 ± 1.66* | 45.36 ± 1.16 | 45.86 ± 0.28 | 49.81 ± 1.64† | 46.10 ± 1.48 | 48.70 ± 0.63* |
| MCH | 14.40 ± 0.28 | 14.85 ± 0.29* | 14.45 ± 0.58 | 14.42 ± 0.20 | 15.67 ± 0.87* | 14.92 ± 0.29 | 15.24 ± 0.52 |
Data are expressed as means ± SD. Two-tailed Student t test was used for statistical analysis between Neo1fl/wt;Alb-Cr+ mice and the age-matched Neo1fl/fl;Alb-Cre− control group at 5 and 15 wk old. One-way ANOVA and Tukey’s posttest were used to compare 8-wk-old mice.
P < .05.
P < .001.
Additionally, we also found mild increases in serum iron and liver nonheme iron levels in heterozygous Neo1fllwt;Alb-Cre+ mice (Figure 2A-B). These results suggest that Neo1 is likely a limiting factor for iron homeostasis.
Depletion of hepatocyte Neo1 reduces hepatic hepcidin expression
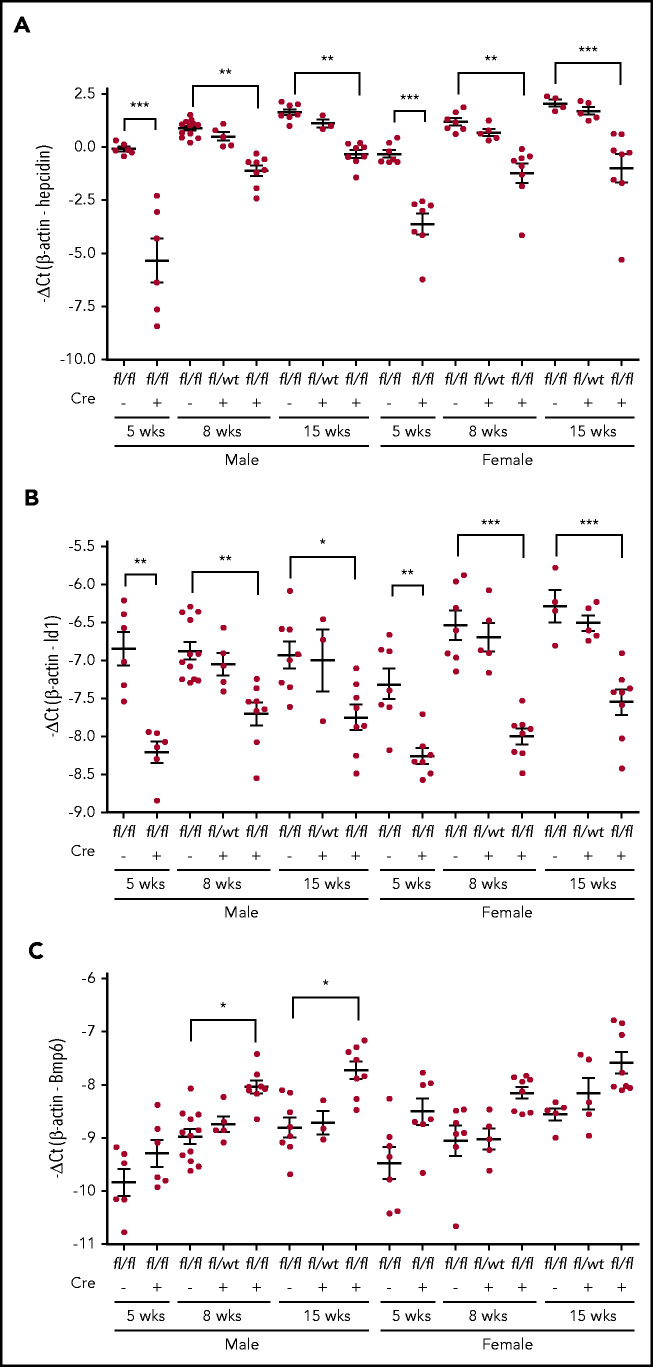
We next determined whether iron overload in Neo1fl/fl;Alb-Cre+ mice results from reduced hepatic hepcidin expression. As shown in Figure 3A and supplemental Figure 2A, we observed an age-dependent increase in hepcidin mRNA in Neo1fl/fl;Alb-Cre− controls. Compared with age- and sex-matched Neo1fl/fl;Alb-Cre− littermates, Neo1fl/fl;Alb-Cre+ mice displayed significant decreases in hepcidin mRNA levels by both −ΔCt and 2–ΔΔCt analyses. Notably, the most robust reduction was observed in 5-week-old groups with about 14.4- and eightfold decrease for males and females, respectively. About fourfold decrease was found in 8- and 15-week-old Neo1fl/fl;Alb-Cre+ groups. A trend decrease of hepcidin mRNA was also detected in heterozygous Neo1fllwt;Alb-Cre+ mice (supplemental Figure 2A). These results indicate that lack of hepatocyte Neo1 reduces hepcidin expression, which is consistent with the iron overload.
Figure 3.
qRT-PCR analysis. (A) Hepatic hepcidin. (B), Id1. (C) Bmp6 mRNA. Each group consists of at least 3 animals. Data shown are means ± SD. A 2-tailed Student t test was used to analyze the data for 5-week-old mice, and 1-way ANOVA and Tukey’s posttests were used to analyze the data for 8- and 15-week-old mice. *P < .05; **P < .01; ***P < .001.
To test whether the reduced hepcidin expression in Neo1fl/fl;Alb-Cre+ groups is caused by decreased Bmp-signaling, we examined Id1 mRNA levels in the liver. Id1 is a direct downstream target of the Bmp signaling. Results showed parallel decreases of Id1 mRNA with hepcidin mRNA (Figure 3A-B; supplemental Figure 2A-B). These data suggest that the reduced hepcidin expression in Neo1fl/fl;Alb-Cre+ mice resulted from decreased Bmp signaling.
Previous studies show that hepatic Bmp6 expression is induced by iron overload.13,39 We detected increases in Bmp6 mRNA in Neo1fl/fl;Alb-Cre+ mice (Figure 3C; supplemental Figure 2C) with no significant change of Bmp2 mRNA levels (data not shown). Additional analysis revealed no significant reduction of Hjv, Alk2, Alk3, Hfe, and Tfr2 mRNA in the liver of 8-week-old Neo1fl/fl;Alb-Cre+ mice (supplemental Figure 3). The unchanged Tfr2 mRNA was not in conflict with increased Tfr2 protein in the liver of Neo1fl/fl;Alb-Cre+ mice (Figure 2C), because Tfr2 is stabilized by increased transferrin saturation.40,41 These results suggest that the reduction of hepcidin expression in Neo1fl/fl;Alb-Cre+ mice did not result from decreases in the mRNA levels of the key components of the Bmp-signaling pathway. Rather the downstream signaling response was impaired.
Induction of hepcidin expression by hepatocyte Neo1 depends on its interaction with Hjv
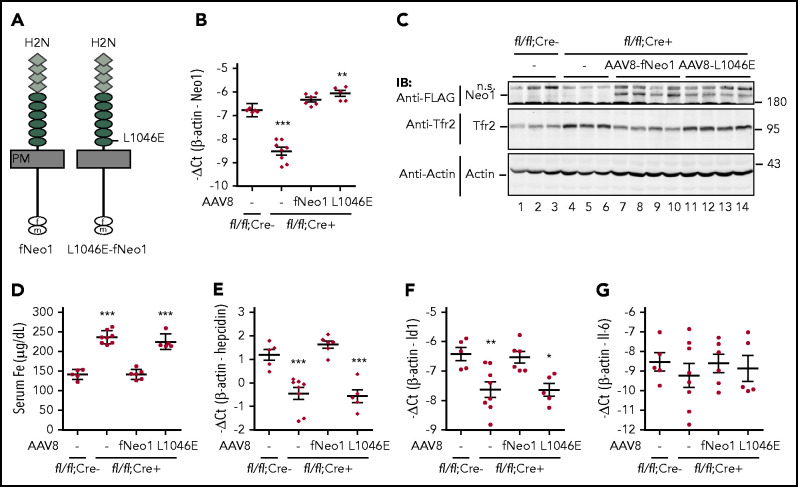
We previously showed that Hjv induction of hepcidin expression depends on its interaction with Neo1.32 To determine whether hepatocyte Neo1 induction of hepcidin expression also requires the interaction with Hjv, we conducted replacement studies by transducing fNeo1L1046E mutant into the liver of Neo1fl/fl;Alb-Cre+ mice using AAV8 vectors. The Leu1046Glu substitution (Neo1L1046E; Figure 4A) abolishes the Neo1-Hjv interaction.3 Transduction of fNeo1 was used as a positive control, and injection of PBS vehicle served as a negative control. A C-terminal FLAG/MYC epitope was added to both constructs for the convenience of immunodetection. Previous studies demonstrated a hepatocyte-specific expression of transduced proteins with an even distribution throughout the liver by AAV8 vector,42 similar to the homogenous distribution of native Neo1 and Hjv mRNA.26,27 Because AAV8-transduced complementary DNA rarely integrates into the genome, animals were euthanized for analysis at 3 weeks after viral administration. The expression levels of Neo1 in the transduced livers were analyzed by qRT-PCR and western blot. The Neo1 mRNA in fNeo1 and fNeo1L1046E groups were modestly higher than those in Neo1fl/fl;Alb-Cre− controls (Figure 4B; supplemental Figure 4A). The expressed proteins in liver extracts were confirmed by using an anti-FLAG antibody (Figure 4C). Both fNeo1 and fNeo1L1046E migrated at the predicted molecular weights.
Figure 4.
Induction of hepcidin expression by hepatocyte Neo1 depends on its interaction with Hjv. (A) Diagrams of fNeo1 and fNeo1L1046E constructs with C-terminal FLAG/MYC tag. f, FLAG; m, MYC. (B) qRT-PCR analysis of hepatic Neo1 mRNA from Neo1fl/fl;Alb-Cre− mice, PBS-injected Neo1fl/fl;Alb-Cre+ mice (–), and Neo1fl/fl;Alb-Cre+ mice transduced with AAV8-fNeo1 and fNeo1L1046E. (C) Representative images of western blot analysis for fNeo1/fNeo1L1046E, Tfr2, and β-actin in the liver extracts (250 µg protein) from mice described in panel B using anti-FLAG, Tfr2, and β-actin antibodies. (D) Serum iron (Fe) assay. (E-G) qRT-PCR analysis of hepatic hepcidin, Id1, and IL-6 mRNA. Each group consists of at least 5 animals. Data shown are means ± SD. One-way ANOVA and Tukey’s posttests were used to analyze the data relative to Neo1fl/fl;Alb-Cre− mice. *P < .05; **P < .01; ***P < .001. ns, nonspecific band.
Functional analysis revealed that expression of exogenous fNeo1 in Neo1fl/fl;Alb-Cre+ mice was able to fully correct the high serum iron and low hepcidin mRNA status (Figure 4D-E; supplemental Figure 4B). The parallel increase in Id1 mRNA (Figure 4F; supplemental Figure 4C) indicates that these changes were accomplished by fNeo1 induction of the Bmp signaling. Consistent with our earlier studies,33 there was no increase of Il-6 mRNA (Figure 4G; supplemental Figure 4D), suggesting that the increased hepcidin expression did not result from inflammation.43 In agreement with the decrease in serum iron by fNeo1, the elevated Tfr2 returned to the comparable levels of Neo1fl/fl;Alb-Cre− controls (Figure 4C, lanes 7-10). These results indicate that the transgenic fNeo1 acts similarly to native Neo1 and that addition of a C-terminal FLAG/MYC epitope does not affect the function of Neo1.
In contrast to fNeo1, expression of fNeo1L1046E in Neo1fl/fl;Alb-Cre+ mice displayed no significant effects on serum iron, hepcidin mRNA, Id1 mRNA, and Tfr2 levels when compared with PBS-injected negative controls (Figure 4B-F; supplemental Figure 4A-C). These results indicate that fNeo1L1046E was not functional. To determine whether this was due to the lack of fNeo1L1046E trafficking to the plasma membrane, we compared the levels of cell surface fNeo1 and fNeo1L1046E in transfected Hep3B cells, a human hepatoma cell line. No evident difference was observed (supplemental Figure 5). These results indicate that the Leu1046Glu substitution does not affect the folding and trafficking of fNeo1 in the cells and suggest that the lack of hepcidin induction by fNeo1L1046E likely resulted from its inability to interact with Hjv.
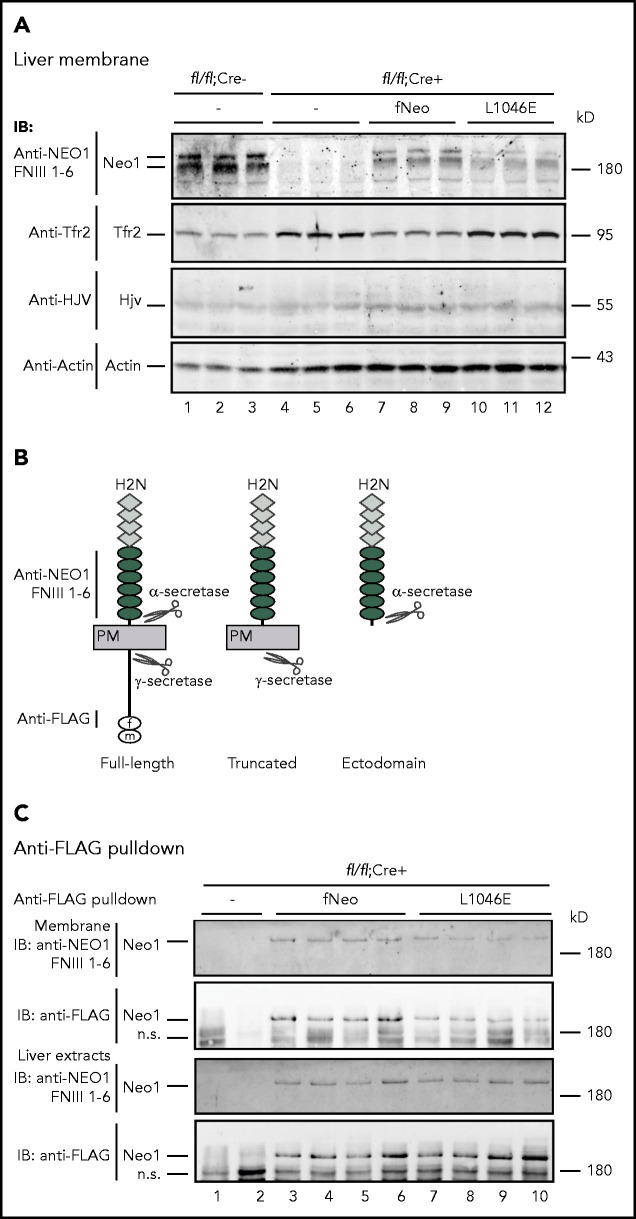
Neo1-Hjv interaction increases the level of membrane-associated Neo1 but not Hjv in the liver
To determine whether Neo1 induces hepcidin expression by increasing Hjv, we examined its level in liver membrane preparations by western blot. No significant difference was observed between Neo1fl/fl;Alb-Cre− and Neo1fl/fl;Alb-Cre+ mice (Figure 5A, lanes 1-6; supplemental Figure 6), which is consistent with the qRT-PCR analysis of Hjv mRNA (supplemental Figure 3A). Similarly, induction of hepcidin expression by fNeo1 did not alter Hjv levels in Neo1fl/fl;Alb-Cre+ mice (Figure 5A, lanes 7-9; supplemental Figure 6). In contrast, elevated Tfr2 was detected in Neo1fl/fl;Alb-Cre+ mice with and without fNeo1L1046E expression, consistent with whole liver extracts (Figures 4C and 5A). These results suggest that Neo1 induction of hepcidin expression is not mediated by increasing Hjv.
Figure 5.
Lack of Hjv interaction decreases the level of membrane-associated Neo1, but not Hjv in the liver. (A) Representative images of western blot analysis for endogenously expressed Neo1, Tfr2, Hjv, and β-actin, as well as the introduced fNeo1 and fNeo1L1046E, in the liver membrane preparation (250 µg protein) of Neo1fl/fl;Alb-Cre− mice, PBS-injected Neo1fl/fl;Alb-Cre+ mice (–), and Neo1fl/fl;Alb-Cre+ mice transduced with AAV8-fNeo1 and fNeo1L1046E. (B) Diagrams of the predicted cleavage sites by α- and γ-secretases in fNeo1, as well as the antibodies used for western blot analysis in panels A and C. (C) Representative images of western blot analysis for concentrated fNeo1 and fNeo1L1046E from the liver membrane preparation (membrane) and the whole liver extracts (liver extracts) of Neo1fl/fl;Alb-Cre+ mice transduced with AAV8-fNeo1 and fNeo1L1046E. fNeo1 and fNeo1L1046E from ∼2 mg extract proteins was pulled down by using anti-FLAG affinity gel (A2220; Sigma), followed by elution using the 3xFLAG peptide at ∼200 µg/mL (F4799; Sigma) and immunodetection using anti-NEO1 FNIII 1-6 and anti-FLAG antibody.
We next tested whether interaction with Hjv leads to increases in Neo1 itself. In the liver membrane preparations of Neo1fl/fl;Alb-Cre− control mice, we detected 2 distinct endogenously-expressed Neo1 bands by an anti-NEO1-FNIII 1-6 antibody (Figure 5A, lanes 1-3). Neo1 is an Asn-linked glycoprotein with 8 potential Asn-linked glycosylation sites.44 Digestion with PNGase-F, which cleaves both high mannose and Golgi-modified complex oligosaccharides, revealed a parallel downshift, suggesting that the multiple bands were not caused by differential Asn-glycosylation (supplemental Figure 7). In the transduced Neo1fl/fl;Alb-Cre+ mice, we also observed 2 fNeo1 or fNeo1L1046E bands. In comparison, the intensities for fNeo1L1046E were much weaker (Figure 5A, lanes 7-12; supplemental Figure 8A). These results suggest that the interaction with Hjv may stabilize Neo1 because the mRNA levels of fNeo1 and fNeo1L1046E were comparable (Figure 4B).
Hjv increases a γ-secretase-like protease-cleaved Neo1 in the membrane
NEO1 can be cleaved by α- and γ-secretases (Figure 5B).32,45-47 In transfected Hep3B cells that endogenously express these secretases,48,49 both the truncated fNeo1 produced by a γ-secretase-like protease cleavage and the Neo1 ectodomain produced by an α-secretase-like protease cleavage were detectable on cell surface and in conditioned medium, respectively, by an anti-NEO1 FNIII 1-6 antibody (supplemental Figure 5; Figure 5B). To distinguish whether the lower Neo1 band seen in Figure 5A is derived from the γ-secretase-like protease cleavage, membrane extracts from Neo1 and fNeo1L1046E-transduced Neo1fl/fl;Alb-Cre+ mice were subjected to anti-FLAG bead pulldown, followed by immunodetection with anti-FLAG and anti-NEO1-FNIII 1-6 antibodies. As shown in Figure 5C, anti-FLAG antibody was only able to pulldown the upper Neo1 band that corresponds to the full-length form (lanes 3-10). As a result, the lower Neo1 bands in the membrane extracts (Figure 5A) likely represent the γ-secretase-like protease-cleaved products. Consistently, pulldown analysis also revealed lower levels of full-length fNeo1L1046E than fNeo1 (Figure 5C; supplemental Figure 8B), which supports the findings in Figure 5A that Neo1-Hjv interaction increases the levels of membrane-associated Neo1.
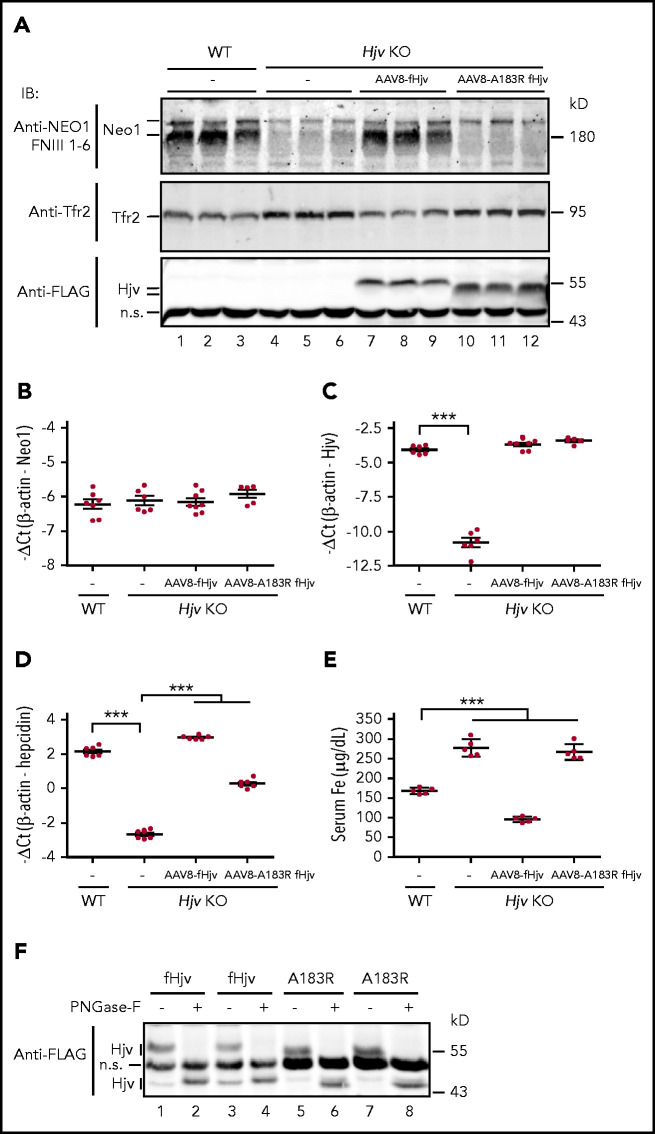
We next compared the Neo1 levels in the liver membrane preparations of wild-type and Hjv−/− mice to seek insights into the mechanism by which Hjv increases Neo1. Similar to Neo1fl/fl;Alb-Cre− controls (Figure 5A), 2 distinct Neo1 bands were detected in wild-type 129S mice with the γ-secretase-like protease-cleaved lower Neo1 band as the predominant form (Figure 6A, lanes 1-3). γ-secretase is expressed in the liver.50 Intriguingly, lack of Hjv in Hjv−/− mice resulted in a marked decrease of both Neo1 forms (Figure 6A, lanes 4-6). These decreases did not result from reduced transcription of Neo1 because there was no significant change of hepatic Neo1 mRNA levels in Hjv−/− mice (Figure 6B; supplemental Figure 9A). When fHjv was expressed in the liver of Hjv−/− mice at a comparable mRNA level to wild-type counterparts, we detected a significant increase in the γ-secretase-like protease-cleaved lower Neo1 band (Figure 6A, lanes 7-9) with no evident change in Neo1 mRNA (Figure 6B). In contrast, expression of similar level of fHjvA183R displayed no evident effect (Figure 6A, lanes 10-12). Ala183Arg mutation in fHjv disrupts its interaction with Neo1 but has no effects on the folding and trafficking to the plasma membrane.3,30 These results indicate that interaction with Hjv leads to increases in γ-secretase-like protease-cleaved Neo1 in the membranes. Consistent with our previous studies,30 expression of transduced fHjv, but not fHjvA183R, was able to fully correct the low hepcidin and high serum iron status in Hjv−/− mice (Figure 6C-E; supplemental Figure 9B-C). fHjvA183R was estimated to retain about 15% of the ability to upregulate hepcidin expression.
Figure 6.
Hjv increases γ-secretase-like protease-cleaved Neo1 in the membrane. (A) Representative images of western blot analysis for Neo1, Tfr2, fHjv, and fHjvA183R in the liver membrane preparation (250 µg protein) of WT mice, PBS-injected Hjv−/− mice (–), and Hjv−/− mice transduced with AAV8-fHjv or fHjvA183R. qRT-PCR analysis of hepatic Neo1 (B), Hjv (C), and hepcidin (D) mRNA from mice in panel A. Data shown are means ± SD. (E) Serum iron (Fe) assay for mice in panel A. Each group consists of at least 5 animals. The means ± SD are presented. One-way ANOVA and Tukey’s posttests were used to analyze the data. ***P < .001. (F) PNGase F digestion. Liver membrane extracts (∼250 µg protein) from Hjv−/− mice transduced with AAV8-fHjv or fHjvA183R were subjected to PNGase-F digestion, followed by immunodetection with a horseradish peroxidase–conjugated anti-FLAG antibody. An image with 2 animals for each group was presented.
In agreement with the lack of regulation of Hjv by Neo1 in Neo1fl/fl;Alb-Cre+ mice (Figure 5A; supplemental Figure 6A), we detected similar levels of fHjv and fHjvA183R in the liver membrane extracts (Figure 6A, lanes 7-12). The relatively faster migration of fHjvA183R in immunoblots (Figure 6A, lanes 10-12) was not caused by changes of Asn-linked glycosylation, because PNGase-F digestion led to parallel downshifts of fHjvA183R and fHjv bands (Figure 6F). Similar results were also observed in transfected Hep3B cells (supplemental Figure 9D). Thus, it is likely that the faster migration of fHjvA183R is due to changes of the charge by the amino acid substitution as previously reported for TfR1T104D mutant.51
To determine whether Hjv-mediated accumulation of Neo1 could be recapitulated in hepatoma cells, Hep3B cells were transfected with fHjv, fHjvA183R, fNeo1, and fNeo1L1046E in various combinations. Our clone of Hep3B cells endogenously expresses low levels of NEO1 and HJV mRNA at about 20% and <1% of those in mouse liver tissues, respectively (supplemental Figures 3A and 10; Figure 1D). Unlike the previously discussed in vivo studies, expression of fHjv failed to specifically increase the γ-secretase-like protease-cleaved Neo1 (supplemental Figure 11A-B). Hep3B cells also endogenously express a low level of hepcidin that can be markedly induced by BMP6 (supplemental Figure 12A). Expression of fHjv, fNeo1, or fHjv/fNeo1 did not significantly increase hepcidin mRNA (supplemental Figure 12B-G). However, expression of HJV in HepG2 cells indeed increased hepcidin mRNA (supplemental Figure 13A-B). HepG2 cells are a relatively more differentiated human hepatoma cell line that endogenously expresses low levels of TfR2, NEO1 (supplemental Figures 10 and 13C), as well as α/γ-secretases.48,49 In contrast to in vivo studies, a decrease of NEO1 was detected by HJV expression (supplemental Figure 13C). Neither NEO1 protein nor hepcidin mRNA level was altered by incubation with the γ-secretase inhibitor N-[N-(3,5-difluorophenylacetyl)-l-alanyl]-(S)-phenylglycine t-butyl ester46 (supplemental Figure 13). Together, these observations suggest that hepatoma cell lines are not appropriate models to study Neo1/Hjv induction of hepcidin expression.
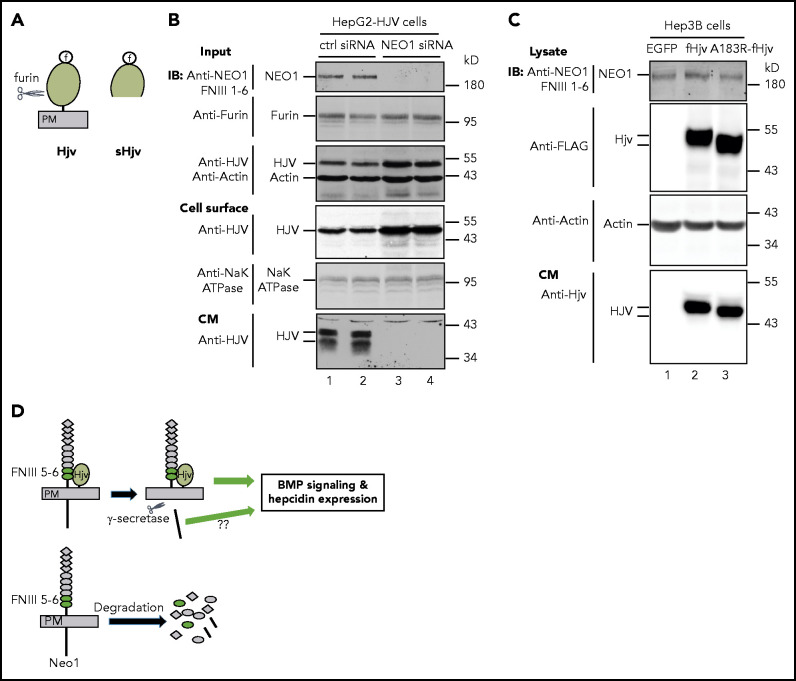
NEO1 does not inhibit HJV shedding in hepatoma cells
HJV can be cleaved by the ubiquitously expressed furin proprotein convertase (Figure 7A) and matriptase-2 in the cells.30,32,52-55 An earlier study suggests that NEO1 enhances the BMP signaling and hepcidin expression by inhibiting the shedding of furin-cleaved HJV.26 We tested the roles of NEO1 in HJV secretion in HepG2-HJV cells that endogenously express furin (Figure 7B) and a detectable matriptase-2 activity.36 There were 2 forms of soluble HJV in the conditioned medium of HepG2-HJV cells, of which the top band (∼40 kD) was the furin-cleaved products and the lower band (∼36 kD) represented the matriptase-2-cleaved products (Figure 7B, lanes 1-2). Knockdown of endogenous NEO1 by specific small interfering RNA abolished the secretion of HJV and increased cellular HJV (Figure 7B, lanes 3-4). These increases in cellular HJV by NEO1 knockdown are unlikely an artifact because similar results were also observed when HepG2-HJV cells were incubated with soluble NEO1 ectodomain in our earlier studies.32 Additionally, we compared the shedding and cell surface localization of fHjv and fHjvA183R in Hep3B cells. No significant difference was detected (Figure 7C; supplemental Figures 9D and 11A). Consistently, our earlier in vivo studies revealed comparable levels of soluble fHjv and fHjvA183R in the serum of transduced Hjv−/− mice.30 These in vitro and in vivo observations indicate that NEO1 does not inhibit HJV shedding.
Figure 7.
NEO1 is required for furin-mediated cleavage of HJV in HepG2 cells. (A) Diagram of HJV and furin-cleaved soluble HJV (sHJV). PM, plasma membrane. (B) Knockdown of endogenous NEO1 by small interfering RNA (siRNA) abolishes HJV shedding from HepG2-HJV cells. HepG2-HJV cells were transfected with control or NEO1-specific siRNA. Cell surface proteins were biotinylated at 4°C, followed by pull-down of the biotinylated proteins using streptavidin agarose beads. The eluted cell surface proteins, ∼10% of input lysate, and a fraction of concentrated conditioned medium (CM) were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and immunodetection by using anti-NEO1 FNIII 1-6, furin, HJV, Na+K+ ATPase, and β-actin antibodies. Experiments were repeated 3 times with consistent results. (C) fHjv and fHjvA183R shedding from Hep3B cells. Hep3B cells were transiently transfected with pCMV9-fHjv or pCMV9-fHjvA183R construct. Total cell extracts and a fraction of concentrated CM were subjected to SDS-PAGE and immunodetection by using anti-NEO1 FNIII 1-6, FLAG, β-actin, and HJV antibodies. Experiments were repeated three times with consistent results. (D) A model for the induction of hepcidin expression by hepatocyte Neo1 via its interaction with Hjv.
Discussion
In this study, we demonstrated an essential role of hepatocyte Neo1 in hepcidin expression and iron homeostasis by taking advantage of the Neo1fl/fl;Alb-Cre+ mice. This animal model circumvented the developmental issues of global Neo1 ablation. Results show that lack of hepatocyte-Neo1 reduced Bmp signaling, decreased hepcidin expression, and caused iron overload with no effects on iron utilization by erythropoiesis. Further studies indicate that Neo1-mediated hepcidin expression depends on its interaction with Hjv, and that interaction with Hjv allows the accumulation of a γ-secretase-like protease-cleaved Neo1 on the membrane. Additional studies failed to support the idea that Neo1 functions by inhibiting Hjv shedding as previously suggested.26
Hepatocytes are critical in the maintenance of iron homeostasis. Here, we showed that hepatocyte-Neo1 plays an essential role in this process. Earlier studies report that global Neo1-ablated mice display a massive liver iron accumulation at postnatal day 25 and severe defects of neural, bone, and skeletal muscle development.26,37,38 They die before postnatal day 30.26 Whether the iron accumulation resulted from increased absorption, tissue iron redistribution, or lack of iron utilization by erythropoiesis was not clear. Contrary to global Neo1 ablation, hepatocyte-specific depletion of Neo1 circumvented the developmental defects. Similar to Hjv−/− mice,7,8 Neo1fl/fl;Alb-Cre+ mice exhibited high serum iron and an age-dependent iron accumulation in the liver. Increased hemoglobin levels ruled out the possibility that the iron overload in Neo1fl/fl;Alb-Cre+ mice was caused by reduced iron utilization by erythropoiesis. These results demonstrate that hepatocyte Neo1 is indeed required for iron homeostasis.
Our data indicate that hepatocyte Neo1 regulates iron homeostasis by facilitating hepcidin expression via the Bmp-signaling pathway. In agreement with global Neo1 mutant mice,26 the iron overload in Neo1fl/fl;Alb-Cre+ mice was associated with decreases in Bmp signaling and hepcidin expression. These findings were validated by correction studies. Expression of transgenic fNeo1 in the liver of Neo1fl/fl;Alb-Cre+ mice was able to fully correct the low Bmp signaling, low hepcidin expression, and high serum iron status. Our studies ruled out the possibilities that the decreased hepcidin expression in Neo1fl/fl;Alb-Cre+ mice was caused by reduced expression of the key components in the hepcidin-induction pathway, including Hjv, Hfe, Tfr2, Alk2, Alk3, Bmp6, or Bmp2.
We obtained additional evidence in support of the pivotal role of Hjv/Neo1 interaction in the induction of hepcidin-expression. Hjv and Neo1 are coexpressed in hepatocytes.26,27 Our earlier studies suggest that Hjv induction of hepcidin expression depends on its interaction with Neo1.30 In Hjv−/− mice, the low Bmp signaling and low hepcidin expression are fully corrected by expression of transduced wild-type Hjv, but not by expression of Hjv mutants with disrupted interaction with Neo1.30 In this study, we found that expression of fNeo1, but not fNeo1L1046E mutant that lacks a key interaction with Hjv,3 was able to correct the high serum iron and low hepcidin expression in Neo1fl/fl;Alb-Cre+ mice. These observations strongly indicate that normal hepcidin expression requires an interaction between Neo1 and Hjv. The correction studies in Hjv−/− mice with fHjvA183R predict that about 85% of the ability of Hjv to upregulate hepcidin relies on its interaction with Neo1. Consistently, increased fHjvA183R expression in Hjv−/− mice at about 25-fold higher than that of wild-type mice was able to correct the low hepcidin and high serum iron status in our earlier studies.30 When compared with Hjv−/− mice on 129/SvEvTac background,30,33 the Neo1fl/fl;Alb-Cr+ mice on a C57BL/6J background displayed a less reduction of hepcidin expression and a less extent of liver nonheme iron overload. This discrepancy could, to some extent, result from their different genetic background.
Mechanistic studies indicate that the induction of hepcidin expression by Hjv/Neo1 interaction is associated with the increases of a γ-secretase-like protease-cleaved Neo1. Our earlier studies indicate that HJV and NEO1 traffic separately to the plasma membrane, and that they interact after reaching the plasma membrane.56 Consistent with this idea, the Hjv level in membrane extracts was not altered by disrupting its interaction with Neo1 in transfected cells and the liver.30 However, the Neo1 levels in membrane were elevated by Hjv interaction. In Hjv−/− mice, the marked decrease in a γ-secretase-like protease-cleaved Neo1 was largely restored by expressing fHjv, but not fHjvA183R that failed to interact with Neo1.3,30 These observations are consistent with the finding that interaction with RGMa induces Neo1 proteolysis in zebrafish.47
Our studies also reconcile the controversy as to whether the Neo1/Hjv interaction is involved in hepcidin induction.57 Consistent with an earlier NEO1 knockout study,57 overexpression of Neo1 in Hep3B cells does not increase hepcidin expression. Different from the previous observations that Hjv induces hepcidin expression in Hep3B cells,57-59 we did not detect any significant effect by Hjv overexpression. This is likely because of the possibility that different Hep3B clones were used for studies. In the more differentiated HepG2 cells, however, overexpression of HJV indeed increases hepcidin expression, and knockdown of endogenous NEO1 abolishes HJV induction of hepcidin as shown in our earlier studies.27 In contrast to the in vivo studies, no evident Hjv-induced increase of a γ-secretase-like protease-cleaved Neo1 or full-length Neo1 was observed in either cell line. These observations suggest that hepatoma cells are not appropriate models to study the HJV/NEO1 regulation of hepcidin expression, and imply that the Hjv-induced γ-secretase-like protease cleavage of Neo1 requires other factors that are lacking in these cell lines.
Additional studies in hepatoma cells do not support the idea that Neo1 regulates iron homeostasis by inhibiting Hjv shedding as previously proposed.26 Knockdown studies in HepG2 cells indicate that NEO1 is required for HJV shedding. In Hep3B cells, there was no evident difference between the shedding of fHjv and fHjvA183R. Consistently, similar levels of fHjv and fHjvA183R were detected in the serum of transduced Hjv−/− mice.30 Importantly, in vivo studies indicate that the furin-cleaved Hjv from the liver does not alter hepcidin expression.30 Together, these observations favor the idea that HJV shedding constitutes the major pathway of cellular HJV turnover,56 rather than the control of hepcidin expression.
On the basis of the data from this and earlier studies,30,46,47 we propose a model for the induction of hepcidin expression by hepatocyte Neo1 (Figure 7D). Upon Hjv binding to Neo1 on the plasma membrane, it triggers the γ-secretase-like protease cleavage of Neo1 to delete its cytoplasmic domain. This cleavage prevents the degradation of truncated Neo1, leading to its accumulation on plasma membrane. This truncated form of Neo1 acts as a scaffold to facilitate the Bmp signaling and the transcription of hepcidin gene. Alternatively, the cleaved Neo1 cytoplasmic domain could act as a signal to the nucleus, as suggested by other systems.46,47 Future studies will examine this possibility and explore whether Neo1 regulates iron homeostasis through other signaling pathways. Conversely, in the absence of Hjv association, Neo1 undergoes a rapid internalization and degradation. In summary, this study provided compelling evidence to support that hepatocyte Neo1 is essential for iron homeostasis and that Neo1 induction of hepcidin expression requires its association with Hjv.
Acknowledgments
The authors thank Aaron Wortham for technical assistance and the Molecular Virology Core of Oregon Health & Science University (OHSU) for the generation of AAV8 vectors.
This work was supported by grants from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases R37DK054488 (C.A.E.) and R01DK102791 (A.S.Z.).
Footnotes
For original data, contact the corresponding author.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: C.A.E. designed the studies, analyzed the data, and wrote the manuscript; S.J. performed experiments and analyzed the data; A.-S.Z. designed the studies, performed experiments, analyzed the data, and wrote the manuscript; and all authors contributed to the editing of the final manuscript.
Conflict-of-interest disclosure: C.A.E and A.-S.Z. have acted as consultants to Disc Medicine.
Correspondence: An-Sheng Zhang, Department of Cell, Developmental, and Cancer Biology L215, OHSU, 3181 SW Sam Jackson Park Rd, Portland, OR 97239; e-mail: zhanga@ohsu.edu.
REFERENCES
- 1.Zhang AS, West AP Jr., Wyman AE, Bjorkman PJ, Enns CA. Interaction of hemojuvelin with neogenin results in iron accumulation in human embryonic kidney 293 cells. J Biol Chem. 2005;280(40):33885-33894. [DOI] [PubMed] [Google Scholar]
- 2.Yang F, West AP Jr., Allendorph GP, Choe S, Bjorkman PJ. Neogenin interacts with hemojuvelin through its two membrane-proximal fibronectin type III domains. Biochemistry. 2008;47(14):4237-4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell CH, Healey E, van Erp S, et al. Structure of the repulsive guidance molecule (RGM)-neogenin signaling hub. Science. 2013;341(6141): 77-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Healey EG, Bishop B, Elegheert J, Bell CH, Padilla-Parra S, Siebold C. Repulsive guidance molecule is a structural bridge between neogenin and bone morphogenetic protein. Nat Struct Mol Biol. 2015;22(6):458-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsunaga E, Chédotal A. Repulsive guidance molecule/neogenin: a novel ligand-receptor system playing multiple roles in neural development. Dev Growth Differ. 2004;46(6):481-486. [DOI] [PubMed] [Google Scholar]
- 6.Papanikolaou G, Samuels ME, Ludwig EH, et al. Mutations in HFE2 cause iron overload in chromosome 1q-linked juvenile hemochromatosis. Nat Genet. 2004;36(1):77-82. [DOI] [PubMed] [Google Scholar]
- 7.Niederkofler V, Salie R, Arber S. Hemojuvelin is essential for dietary iron sensing, and its mutation leads to severe iron overload. J Clin Invest. 2005;115(8):2180-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang FW, Pinkus JL, Pinkus GS, Fleming MD, Andrews NC. A mouse model of juvenile hemochromatosis. J Clin Invest. 2005;115(8):2187-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen W, Huang FW, de Renshaw TB, Andrews NC. Skeletal muscle hemojuvelin is dispensable for systemic iron homeostasis. Blood. 2011;117(23):6319-6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gkouvatsos K, Wagner J, Papanikolaou G, Sebastiani G, Pantopoulos K. Conditional disruption of mouse HFE2 gene: maintenance of systemic iron homeostasis requires hepatic but not skeletal muscle hemojuvelin. Hepatology. 2011;54(5):1800-1807. [DOI] [PubMed] [Google Scholar]
- 11.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090-2093. [DOI] [PubMed] [Google Scholar]
- 12.Aschemeyer S, Qiao B, Stefanova D, et al. Structure-function analysis of ferroportin defines the binding site and an alternative mechanism of action of hepcidin. Blood. 2018;131(8):899-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kautz L, Meynard D, Monnier A, et al. Iron regulates phosphorylation of Smad1/5/8 and gene expression of Bmp6, Smad7, Id1, and Atoh8 in the mouse liver. Blood. 2008;112(4):1503-1509. [DOI] [PubMed] [Google Scholar]
- 14.Babitt JL, Huang FW, Wrighting DM, et al. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet. 2006;38(5):531-539. [DOI] [PubMed] [Google Scholar]
- 15.Wang RH, Li C, Xu X, et al. A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab. 2005;2(6):399-409. [DOI] [PubMed] [Google Scholar]
- 16.Steinbicker AU, Bartnikas TB, Lohmeyer LK, et al. Perturbation of hepcidin expression by BMP type I receptor deletion induces iron overload in mice. Blood. 2011;118(15):4224-4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayeur C, Leyton PA, Kolodziej SA, Yu B, Bloch KD. BMP type II receptors have redundant roles in the regulation of hepatic hepcidin gene expression and iron metabolism. Blood. 2014;124(13):2116-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang CY, Core AB, Canali S, et al. Smad1/5 is required for erythropoietin-mediated suppression of hepcidin in mice. Blood. 2017;130(1):73-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang AS, Anderson SA, Wang J, et al. Suppression of hepatic hepcidin expression in response to acute iron deprivation is associated with an increase of matriptase-2 protein. Blood. 2011;117(5):1687-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canali S, Zumbrennen-Bullough KB, Core AB, et al. Endothelial cells produce bone morphogenetic protein 6 required for iron homeostasis in mice. Blood. 2017;129(4): 405-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koch PS, Olsavszky V, Ulbrich F, et al. Angiocrine Bmp2 signaling in murine liver controls normal iron homeostasis. Blood. 2017;129(4):415-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andriopoulos B Jr., Corradini E, Xia Y, et al. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet. 2009;41(4):482-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meynard D, Kautz L, Darnaud V, Canonne-Hergaux F, Coppin H, Roth MP. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat Genet. 2009;41(4):478-481. [DOI] [PubMed] [Google Scholar]
- 24.Wang CY, Xiao X, Bayer A, et al. Ablation of hepatocyte Smad1, Smad5, and Smad8 causes severe tissue iron loading and liver fibrosis in mice. Hepatology. 2019;70(6):1986-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muckenthaler MU, Rivella S, Hentze MW, Galy B. A red carpet for iron metabolism. Cell. 2017;168(3):344-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee DH, Zhou LJ, Zhou Z, et al. Neogenin inhibits HJV secretion and regulates BMP-induced hepcidin expression and iron homeostasis [published correction appears in Blood. 2010;116(1):151]. Blood. 2010;115(15):3136-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang AS, Yang F, Wang J, Tsukamoto H, Enns CA. Hemojuvelin-neogenin interaction is required for bone morphogenic protein-4-induced hepcidin expression. J Biol Chem. 2009;284(34):22580-22589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuns-Hashimoto R, Kuninger D, Nili M, Rotwein P. Selective binding of RGMc/hemojuvelin, a key protein in systemic iron metabolism, to BMP-2 and neogenin. Am J Physiol Cell Physiol. 2008;294(4):C994-C1003. [DOI] [PubMed] [Google Scholar]
- 29.Yang F, West AP Jr., Bjorkman PJ. Crystal structure of a hemojuvelin-binding fragment of neogenin at 1.8Å. J Struct Biol. 2011;174(1):239-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao N, Maxson JE, Zhang RH, Wahedi M, Enns CA, Zhang AS. Neogenin facilitates the induction of hepcidin expression by hemojuvelin in the liver. J Biol Chem. 2016;291(23):12322-12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao J, Chen J, De Domenico I, et al. Hepatocyte-targeted HFE and TFR2 control hepcidin expression in mice. Blood. 2010;115(16):3374-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Enns CA, Ahmed R, Zhang AS. Neogenin interacts with matriptase-2 to facilitate hemojuvelin cleavage. J Biol Chem. 2012;287(42):35104-35117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang AS, Gao J, Koeberl DD, Enns CA. The role of hepatocyte hemojuvelin in the regulation of bone morphogenic protein-6 and hepcidin expression in vivo. J Biol Chem. 2010;285(22):16416-16423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wortham AM, Goldman DC, Chen J, Fleming WH, Zhang AS, Enns CA. Extrahepatic deficiency of transferrin receptor 2 is associated with increased erythropoiesis independent of iron overload. J Biol Chem. 2020;295(12):3906-3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Enns CA, Jue S, Zhang AS. The ectodomain of matriptase-2 plays an important nonproteolytic role in suppressing hepcidin expression in mice. Blood. 2020;136(8):989-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maxson JE, Chen J, Enns CA, Zhang AS. Matriptase-2- and proprotein convertase-cleaved forms of hemojuvelin have different roles in the down-regulation of hepcidin expression. J Biol Chem. 2010;285(50):39021-39028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Z, Xie J, Lee D, et al. Neogenin regulation of BMP-induced canonical Smad signaling and endochondral bone formation. Dev Cell. 2010;19(1):90-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bae GU, Yang YJ, Jiang G, et al. Neogenin regulates skeletal myofiber size and focal adhesion kinase and extracellular signal-regulated kinase activities in vivo and in vitro. Mol Biol Cell. 2009;20(23):4920-4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim PJ, Duarte TL, Arezes J, et al. Nrf2 controls iron homeostasis in haemochromatosis and thalassaemia via Bmp6 and hepcidin. Nat Metab. 2019;1(5):519-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson MB, Enns CA. Diferric transferrin regulates transferrin receptor 2 protein stability. Blood. 2004;104(13):4287-4293. [DOI] [PubMed] [Google Scholar]
- 41.Robb A, Wessling-Resnick M. Regulation of transferrin receptor 2 protein levels by transferrin. Blood. 2004;104(13):4294-4299. [DOI] [PubMed] [Google Scholar]
- 42.Wang L, Takabe K, Bidlingmaier SM, Ill CR, Verma IM. Sustained correction of bleeding disorder in hemophilia B mice by gene therapy. Proc Natl Acad Sci USA. 1999;96(7):3906-3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee P, Peng H, Gelbart T, Beutler E. The IL-6- and lipopolysaccharide-induced transcription of hepcidin in HFE-, transferrin receptor 2-, and beta 2-microglobulin-deficient hepatocytes. Proc Natl Acad Sci USA. 2004;101(25):9263-9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keeling SL, Gad JM, Cooper HM. Mouse neogenin, a DCC-like molecule, has four splice variants and is expressed widely in the adult mouse and during embryogenesis. Oncogene. 1997;15(6):691-700. [DOI] [PubMed] [Google Scholar]
- 45.Okamura Y, Kohmura E, Yamashita T. TACE cleaves neogenin to desensitize cortical neurons to the repulsive guidance molecule. Neurosci Res. 2011;71(1):63-70. [DOI] [PubMed] [Google Scholar]
- 46.Goldschneider D, Rama N, Guix C, Mehlen P. The neogenin intracellular domain regulates gene transcription via nuclear translocation. Mol Cell Biol. 2008;28(12):4068-4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown S, Jayachandran P, Negesse M, Olmo V, Vital E, Brewster R. Rgma-induced Neo1 proteolysis promotes neural tube morphogenesis. J Neurosci. 2019;39(38):7465-7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang XJ, Feng CW, Li M. ADAM17 mediates hypoxia-induced drug resistance in hepatocellular carcinoma cells through activation of EGFR/PI3K/Akt pathway. Mol Cell Biochem. 2013;380(1-2):57-66. [DOI] [PubMed] [Google Scholar]
- 49.Li H, Lan T, Xu L, et al. NCSTN promotes hepatocellular carcinoma cell growth and metastasis via β-catenin activation in a Notch1/AKT dependent manner. J Exp Clin Cancer Res. 2020;39(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hébert SS, Serneels L, Dejaegere T, et al. Coordinated and widespread expression of gamma-secretase in vivo: evidence for size and molecular heterogeneity. Neurobiol Dis. 2004;17(2):260-272. [DOI] [PubMed] [Google Scholar]
- 51.Rutledge EA, Root BJ, Lucas JJ, Enns CA. Elimination of the O-linked glycosylation site at Thr 104 results in the generation of a soluble human-transferrin receptor. Blood. 1994;83(2):580-586. [PubMed] [Google Scholar]
- 52.Zhang AS, Yang F, Meyer K, et al. Neogenin-mediated hemojuvelin shedding occurs after hemojuvelin traffics to the plasma membrane. J Biol Chem. 2008;283(25):17494-17502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Silvestri L, Pagani A, Camaschella C. Furin-mediated release of soluble hemojuvelin: a new link between hypoxia and iron homeostasis. Blood. 2008;111(2):924-931. [DOI] [PubMed] [Google Scholar]
- 54.Silvestri L, Pagani A, Nai A, De Domenico I, Kaplan J, Camaschella C. The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin. Cell Metab. 2008;8(6):502-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin L, Nemeth E, Goodnough JB, Thapa DR, Gabayan V, Ganz T. Soluble hemojuvelin is released by proprotein convertase-mediated cleavage at a conserved polybasic RNRR site. Blood Cells Mol Dis. 2008;40(1):122-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maxson JE, Enns CA, Zhang AS. Processing of hemojuvelin requires retrograde trafficking to the Golgi in HepG2 cells. Blood. 2009;113(8):1786-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xia Y, Babitt JL, Sidis Y, Chung RT, Lin HY. Hemojuvelin regulates hepcidin expression via a selective subset of BMP ligands and receptors independently of neogenin. Blood. 2008;111(10):5195-5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin L, Goldberg YP, Ganz T. Competitive regulation of hepcidin mRNA by soluble and cell-associated hemojuvelin. Blood. 2005;106(8):2884-2889. [DOI] [PubMed] [Google Scholar]
- 59.Canali S, Core AB, Zumbrennen-Bullough KB, et al. Activin B induces noncanonical SMAD1/5/8 signaling via BMP type I receptors in hepatocytes: evidence for a role in hepcidin induction by inflammation in male mice. Endocrinology. 2016;157(3):1146-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]