Abstract
Plant secondary metabolites (PSMs) are plant products that are discontinuously distributed throughout the plant kingdom. These secondary compounds have various chemical groups and are named according to their chemical constituents. For their ability to defend biotic and abiotic stresses they are considered as plants' defensive compounds. These metabolites take part in plant protection from insects, herbivores, and extreme environmental conditions. They are indirectly involved in plants’ growth and development. Secondary metabolites are also used by people in the form of medicines, pharmaceuticals, agrochemicals, colors, fragrances, flavorings, food additives, biopesticides, and drugs development. However, the increase in atmospheric temperature by several anthropogenic activities majorly by the combustion of hydrocarbons is a great issue now. On the other hand, climate change leaves an impact on the quality and quantity of plant secondary metabolites. It is measured that several greenhouse gases (GHGs) are present in the atmosphere, like Chlorofluorocarbons (CFCs), nitrous oxides (NOx), Carbon dioxide (CO2), Methane (CH4) and Ozone (O3), etc. CO2, the major greenhouse gas is essential for photosynthesis. On the other hand, CO2 plays a significant role in the up-regulation of atmospheric temperature. Plants produce various types of primary metabolites such as carbohydrates, proteins, fats, membrane lipids, nucleic acids, and chlorophyll as well as a variety of secondary metabolites from photosynthesis. The high temperature in the atmosphere creates heat stress for plants. As a matter of fact many morphological, physiological and biochemical changes occur in the plant. The high temperature invariably elicits the production of several secondary metabolites within plants. Various strategies have been universally documented to improve the production of PSMs. With this objective, the focus of the current review is to further investigate and discuss futuristic scenarios the effect of elevated CO2 and high temperature on PSMs production which may perhaps beneficial for pharmaceutical industries, biotechnology industries, and also in climate change researches.
Keywords: Climate change, Greenhouse gases, Photosynthesis, Heat stress, Secondary metabolites, Elicitation
Climate change; Greenhouse gases; Photosynthesis; Heat stress; Secondary metabolites; Elicitation.
1. Introduction
Changes in temperature and precipitation patterns due to increased atmospheric CO2 concentrations can profoundly affect terrestrial plant growth and productivity (Marchi et al., 2004; Reddy et al., 2010; Kim and Kang, 2011). Earth is getting warmer day by day by changing atmospheric temperature. Climate change refers to changes in weather conditions that persist for an extended period, usually decades or longer. It refers to any change in climate over time, whether due to natural variability or as a result of human activity and it can be identified by using several statistical tools (IPCC, 2019). Global environmental changes act as a stringent stimulus on the physiological processes of plants. By absorbing heat from solar radiation, greenhouse gasses (GHGs) make the atmosphere warmer. Scientists postulated that the earth would get warmer around 2–4 °C higher by the end of the twenty-first (21st) century. Climate change is highly correlated with elevated CO2 concentration in the air. CO2 as a greenhouse gas increases the Earth's temperature (IPCC, 2014). During the last 50 years, the quantity of atmospheric CO2 has amplified exponentially, which has touched the level of 409.8 (μmol mol−1) in 2019, increasing the amount of CO2 in the atmosphere at a rate of 2.5 ppm (i.e., part per million) per year (Lindsey, 2020).It is projected that by 2050 its level will upsurge to 550 (μmol mol−1) and will rise to 700 (μmol mol−1) by the end of the current century (Collins et al., 2013).
Increasing CO2 concentrations in the atmosphere are due to the burning of fossil fuels, deforestation and the construction of high-rise buildings, and chiefly the combustion of hydrocarbons, leading to a steady increase in the temperature of the atmosphere. The presence of pollutants and experienced house fumes in the air triggers the heat-trapping mechanisms of the atmosphere. The level of CO2 concentrations in the atmosphere is accelerating at an exponential rate and this acceleration behaves like a natural phenomenon because of the human's poor activities towards the environment. Earlier studies reported that initially, concentration of CO2 was 280 ppm at the time of the industrial revolution in the year 1750, now it is 410 ppm in September 2019 (IPCC, 2019). It is predicted that the level of CO2 will be doubled at the end of this century. At the regional parameters, global warming mediated climate change strongly affects arctic and boreal areas. It changes the precipitation rate, drought, and cloudiness of the atmosphere. It also takes part in changing temperature-mediated environmental processes in the forest such as macro and micronutrient availability (IPCC, 2019). The increase in concentrations of several GHGs in the atmosphere has resulted in an average global temperature increase of 0.85 °C over the period 1880–2012, It is estimated that it will increase to 0.4–2.6 °C in 2046–2065 and 0.3–4.8 °C in 2081–2100, respectively in comparison to the period of 1986–2005 (IPCC, 2014). An elevated level of atmospheric CO2 concentration is not only responsible for climate warming, but also hampering the primary productivity of ecosystems. e [CO2] is expected to affect the defenses of plants through its effect on plant primary metabolisms (Marchi et al., 2004; Kim and Kang, 2011). CO2 is utilized by plants for the processes of photosynthesis mechanism. The photosynthetic carbon sequestration phenomenon is the main mechanism for creating secondary metabolites from primary metabolites. e [CO2] improves the rate of photosynthesis in plants, thus affecting PSMs productions (Jia et al., 2014), but a very high concentration of CO2 may prove toxic and the rate of photosynthesis will go down. Elevated atmospheric CO2 changes total phenolic, flavonoid, and condensed tannin content in different plant species (Levine et al., 2008; Novriyanti et al., 2012; Jia et al., 2014). By alternating metabolites content in the plants, atmospheric warming leads to benefit for the quantitative and qualitative production of PSMs. Major global climate change factors like increased CO2 and heat conditions have foremost distinctive and infrequently different effects on PSMs. Plants were grown at high CO2 levels display noteworthy variations in their chemical composition. So, along with PSMs production chemo diversity happens in plants (Moore et al., 2014). Generally, the quantity of carbohydrates in plants increases with higher atmospheric CO2, as the increase in CO2 induces a higher photosynthesis rate in plants (Long et al., 2006; Reddy et al., 2010; Kimball, 2016). Considering the current and future upsurge in CO2 concentration in the atmosphere, it can be concluded that it has a putative effect on the chemical composition of PSMs (Zavala et al., 2017). Chen et al. (2005) reported that under e [CO2], the contents of gossypol and, tannin is increased and Bt protein synthesis decreased in Gossypium barbadense L. and total phenolics content increases in Brassica napus L. (Reddy et al., 2004). Xu et al. (2019) found in their study that under e [CO2] there was an increase of 5.13 % in the total phenolics content of Zea mays L. Li et al. (2017a, b) found that e [CO2] meaningfully amplified the concentrations of total catechin and other polyphenols as well as theanine and free amino acids. In conifers e [CO2] mainly influenced an increase in concentrations of few individual monoterpenes (Williams et al., 1994), as well as an increase in the concentration of total phenolics, have been reported (Idso and Idso, 2000). Exposing plants to high levels of CO2 increases the number of secondary metabolites and antioxidant activity (Ibrahim and Jaafar, 2012). Total phenolics content of Elaeis guineensis seedling increased by 52 %–91 % under e [CO2] as related to ambient CO2 condition (Ibrahim and Jaafar, 2012). Apart from that, in vitro generated somatic embryos of Eleutherococcus senticosus showed significant variation in PSMs production in exposure to heat stress (Peak et al., 2005). Exposure to high temperatures along with light enhanced the ginsenosides production in Panax ginseng hairy root culture (Paek et al., 2005). Naghiloo et al. (2012) stated that secondary metabolite phenolics belonging to the phenols metabolite group increase in high temperature in the root, leaves, and flowers of Astracantha compacta.
The plants with optimal CO2 concentration may get benefitted by getting well plant growth, plant morphology, and plant physiological aspects from the CO2 fertigation experiments like Open Top Chamber (OTC), Free Air CO2 Enrichment (FACE), and Controlled Environmental Chamber (CEC). On the other hand, Scientists developed a Free Air Temperature Increase (FATI) system for the study of plant responses to high temperature at a particular ecosystem level (Harte et al., 1995). Climate changes may involve in alteration of plant fitness (Galen and Stanton, 1993) also the reproductive successes of plants and plant-plant interactions by affecting the flowering phenology (Beattie et al., 1973; Bishop and Schemske, 1998; Lacey and Pace, 1983; English-Loeb and Karban, 1992; Schmitt, 1983; Peterson, 1997). Climate change controls the various abiotic factors of forest trees and their physiology, growth, and fighting together with elicited substance production (Metlen et al., 2009). Wang and Taub (2010) reported a rise of the below-ground biomass allocation of genus Betula by 59 % in doubled CO2 concentration from 350 ppm to 700 ppm e [CO2] increased leaves, stem, and root biomass in Gmelina arborea seedlings as compared to ambient CO2 (Rasineni et al., 2011).Dijkstra et al. (2002) reported that e [CO2] significantly increases the above ground biomass of Quercus myrtifolia and Quercus chapmanii. Sharma et al. (2020) reported that e [CO2] increased higher dry root biomass in Hypericum perforatum L. at 280 days as compared to ambient and FACE + FATI experiment. Korner et al. (2005) documented that; five deciduous plants were affected very little by 530 ppm concentrations of CO2 in free-air CO2 concentration (FACE) experiments performed for four years. Experiments performed by different workers suggest that different plants require different levels of optimal CO2 concentrations for biological processes. The optimal requirement of CO2 concentrations has an impact on PSMs production. This review article highlights the effect of e [CO2] and e [CO2] mediated heat stress in PSMs production.
1.1. Types and uses of secondary metabolites
Despite the dysfunctional role in various metabolic processes found in plants, PSMs are the key machinery for plants that help them to interact with their environment including blocking ultraviolet radiation, enzyme inhibition, antioxidant activity, and pigment development (Bourgaud et al., 2001; Dixon, 2001; Kennedy and Wightman, 2011). Plants devote more energy to the synthesis of PSMs than primary metabolites, which specifies the important nature of PSMs (Gershenzon, 1994). Plant secondary metabolites have huge diversities in chemical and structural patterns. They also appear as either non-volatile compounds or volatile compounds. PSMs are also part of plants’ innate immunity (Dixon and Strack, 2003). Alkaloids (approximately 21,000 compounds) (Wink, 2010), Terpenoids (near about 30,000 compounds) (Lamke and Unsicker, 2018), and phenolic compounds (approximately 8,000 compounds) (Munne-Bosch, 2012) are the most important known secondary compounds of plants.
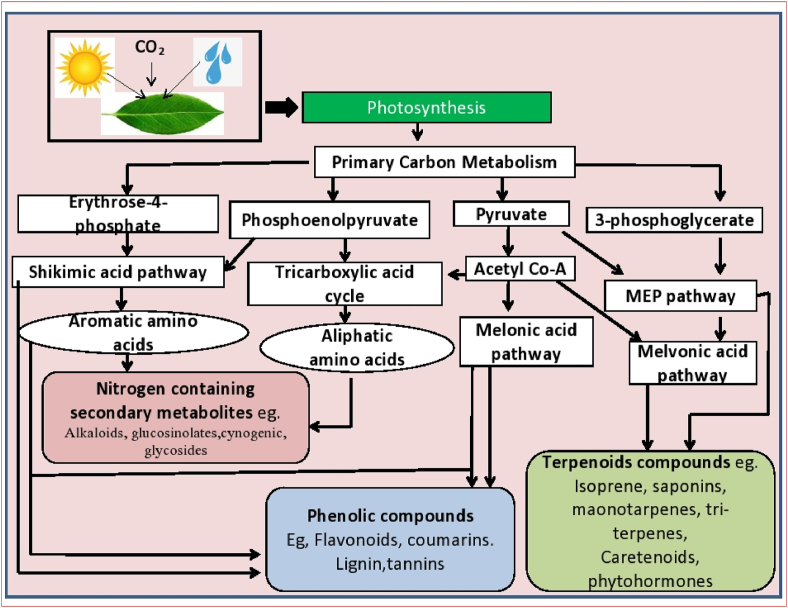
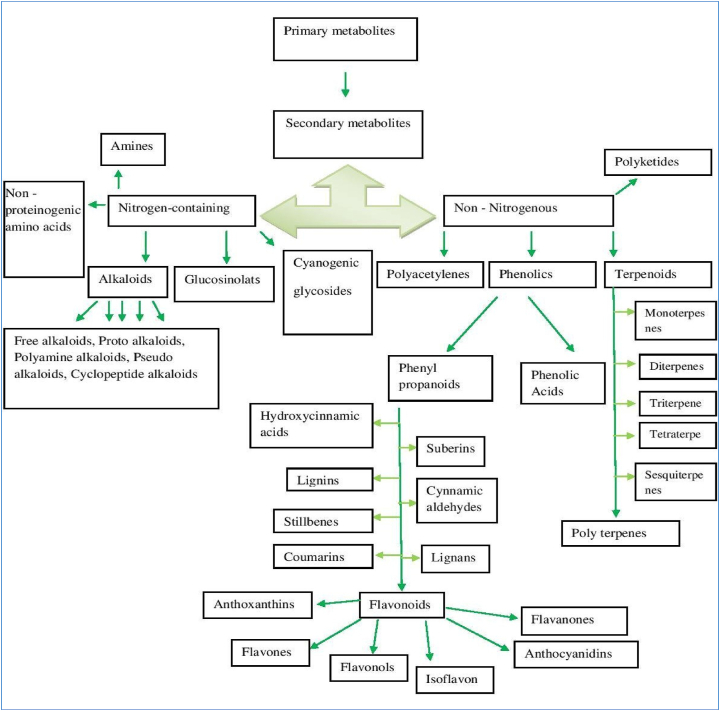
Secondary metabolites are derived from primary metabolites by different biosynthetic pathways [Figure 1]. These metabolites fall into two groups: nitrogenous and non-nitrogenous compounds. Nitrogenous compounds are those compounds that have nitrogen as their chemical constituent. Non – nitrogenous compounds do not have nitrogen. Nitrogenous compounds are further classified in amines, non-proteinogenic amino acids, glucosinolates, alkaloids (like, cyanogenic glycosides. Alkaloids are also classified into several types like free alkaloids, proto alkaloids, polyamine alkaloids, pseudo alkaloids, cyclopeptide alkaloids. The alkaloids are primarily derived from precursors of several amino acids (Wink, 2010). Only coniferous alkaloids are derived through the polyketide biosynthetic pathway (Seigler, 1998). On the other hand, non – nitrogenous compounds are divided into four main types such as polyacetylenes, phenolics (like arylpyrones and styrylpyrones) (Fang et al., 2011), terpenoids, polyketides. Phenolics compounds are extremely diverse in chemical structure. The synthesis of total known phenolic compounds occurs either by the shikimic acid pathway or by the malonate/acetate pathway (Figure 1) (Rodney et al., 2000). Phenolics are divided into phenylpropanoids and phenolic acids. Phenyl propanoids are mainly of eight types i.e., hydroxyl – cinnamic acid, lignin, suberin, flavonoids, lignans, coumarins, stillbenes, and cinnamic aldehydes. Flavonoids are one of the largest classes of plant phenolics, which are found as polyphenolic compounds in nature (Jamwal et al., 2018) and are often abundant in fruits, vegetables, and beverages such as fruit drinks, tea, and coffee (Pridham, 1960). Flavonoids are mainly anthocyanins, anthoxanthins, flavones, flavonols, isoflavones, and flavanones [Figure 2]. Terpenoids are the major group of PSMs derived from the five-carbon precursor isopentenyl diphosphate (IPP) (Thirumurugan et al., 2018), synthesized by either the mevalonate pathway or the methyl erythritol phosphate (MEP) pathway (Bartramet al., 2006). Terpenes are divided into monoterpenes, sesquiterpenes (such as ABA), diterpenes (such as Gibberellins); triterpenes (like sterols), and tetraterpenes (such as Carotenoids) (Ashraf et al., 2018). Terpenoids complete protein synthesis than alkaloids and phenolics (Koricheva, 2002).
Figure 1.
A general sketch of biosynthetic pathways of secondary metabolites. Photosynthesis stimulates primary metabolism. Primary metabolism leads to the formation of secondary metabolites like nitrogen containing secondary metabolites, phenolic compounds, and terpenoids compounds.
Figure 2.
Types of secondary metabolites. Secondary metabolites derive from primary metabolites. It finally leads to the formation of versatile secondary metabolites.
PSMs are also responsible for creating specific, tastes, odors, and colors in plants (Ravishankar and Venkataraman, 1990). Untold applications are found for these molecules in medicative, alimental, and cosmetic industries besides importance in stress biology and plant signalling. Although it's not involved in plant growth and developmental phases; however, they play a role in plant defense against predators, pathogens and herbivores, and nematodes. Some of these metabolites are also known to attract animals for pollination and seed dispersal. Recently, several secondary metabolites are urged to possess vital ecological functions in plants. They shield the plants against being eaten up by herbivores by making bitter tastes. Some secondary metabolites like flavonoids protect plants against UV radiation damage (Jamwal et al., 2018). Several types of PSMs like alkaloids, phenylpropanoids, and terpenoids, are being considered for drug development (Sanchita and Sharma, 2018).
Many alkaloids are used as salts in the treatment of various diseases (Thirumurugan et al., 2018). Some examples include vinblastine which has antitumor properties (Jordan and Leslie, 2004) which is used in the treatment of cancer, quinine has anti-malarial and has antipyretic properties (Reyburn et al., 2009) and reserpine is used to control high blood pressure. Polyphenols, which are found in abundance in plants, are used for the treatment of Tuberculosis (TB) disease (Mazlun et al., 2019).
Genistein, an isoflavone binds to estrogens hormone receptor β and it has been reported to improve modifications in the skin. According to In vitro data, at low concentrations, the soy isoflavones-genistein and daidzein are capable of stimulating the proliferation of estrogen receptor alpha positive (ERα+) breast cancer cells (Mohiuddin, 2019). Camptotheca acuminate has an indole alkaloid named 10-Hydroxycamptothecin (10-HCPT), which hinders the topoisomerase I activity and has a wide-ranging spectrum of anticancer activities both in“in vitro” and “in vivo”. Isoprene is synthesized through the 2-C-methylerythritol-5-phosphate (MEP) pathway. That pathway also produces abscisic acid (ABA). Pelargonidin chloride is an anthocyanidin chloride that has pelargonidin as the cationic portion. It has a role as a phytoestrogen for containing pelargonidin (Mohiuddin 2019).
Our goal is to review all earlier studies which explain the role of e [CO2]and high temperature on the production of PSMs. Both e [CO2] and high temperature are known to bring a dramatic change in PSMs production. Some previous studies have shown how e [CO2] and high temperatures affect the formation of secondary metabolites but only a few points have been discussed in earlier studies. The review highlights the Increasing interests in mining and formation of bioactive compounds in presence of e [CO2] and high temperature.
2. Material and methods
2.1. Methodology design for an extensive review
For a comprehensive and effective review, which contains complete information, published literature was analyzed from electronic-based journal articles, grey literature, and books. A total of seven publication databases: Web of Science (https://clarivate.com/webofsciencegroup/solutions/web-of-science/), PubMed (https://pubmed.ncbi.nlm.nih.gov/), Scopus (http://scopus.co.in/), EBSCO Green FILE (https://www.ebsco.com/products/research-databases/greenfile), Google Scholar (https://scholar.google.com/), and Research gate (https://www.researchgate.net/), were searched. The reason behind the selection of these databases was that they are generally used for searching various articles which are related to the current topic. To make the study novel and significant, various previous researches have been thoroughly included, as well as various images have been applied for making an effective review article. Apart from that, website search and concept development from IPCC (https://www.ipcc.ch/), BBC News (https://www.bbc.com/news), and YouTube (https://www.youtube.com/) was also performed and incorporated.
2.2. Literature search strategy
To acquire relevant information for the review, Web of Science Core Collection was chosen as the main search database; similarly, Google Scholar was found helpful. Firstly, various research papers, case studies, review papers, short communications related to this topic which have been published before January 2021 were searched; to search the research papers, case studies, review papers, short communications; some main keywords related to this topic such as (climate change, heat stress, secondary metabolites and e [CO2]) were used. Apart from this, the entire thesis related to this topic was also studied which was found in the library of HNBGU Srinagar Garhwal Uttarakhand, India.
We also used various other electronic means, such as Pubmed, SCI Hub, and Research gate to identify the literature related to our study for how the rising temperature and high concentrations of CO2 affect the formation of secondary metabolites in plants. For this, we analyzed various literature related to our study from 1960 to 2020. After searching and studying the research and review papers, issues related to this topic were investigated and for further processing, these papers were classified into the following categories (1) Plant responses with changing climate (2) Production of PSMs under various abiotic stresses (3) Effect of heat stress on PSMs production (4) Effect of CO2 enrichment on the synthesis of PSMs. A total of 405 research papers, case studies, review papers, short communications were searched for this study, out of which 156 were finalized for the study. In our study, we have searched many journals, newspapers, news channels. Among them, Web of Science Core Collection, Google Scholar, Pubmed, SCI Hub, and Research gate, Scopus, EBSCO Green FILE, IPCC, BBC News, and YouTube were found useful for us for gaining concepts and better understanding. The final set of articles was critically evaluated for their design, focusing on the effect of e [CO2] and high temperature on the production of PSMs.
3. Literature survey analyses
3.1. Climate change and plant secondary metabolites
Futuristic scenarios in climate are going to affect the physiological performance of overall world vegetation (Austen et al., 2019). The accumulation of PSMs is often subject to changes in environmental conditions, including e [CO2], high temperature, heavy metal toxicity, light intensity, viral and fungal infection, and the allelopathic effects of other plants (Bourgaud et al., 2001). Resisting stress in the environment is one of the major challenges facing plants (Austen et al., 2019). In normal conditions, the synthesis of PSMs is comparatively low. Plants accumulate a high quantity of secondary metabolites during stress conditions. Therefore, stress boosts the production of secondary compounds and acts as a potent elicitor in the accumulation of secondary metabolites. Thus, various environmental factors are important determinants for biosynthesis, regulation, and fluctuations in PSMs (Verma and Shukla, 2015).
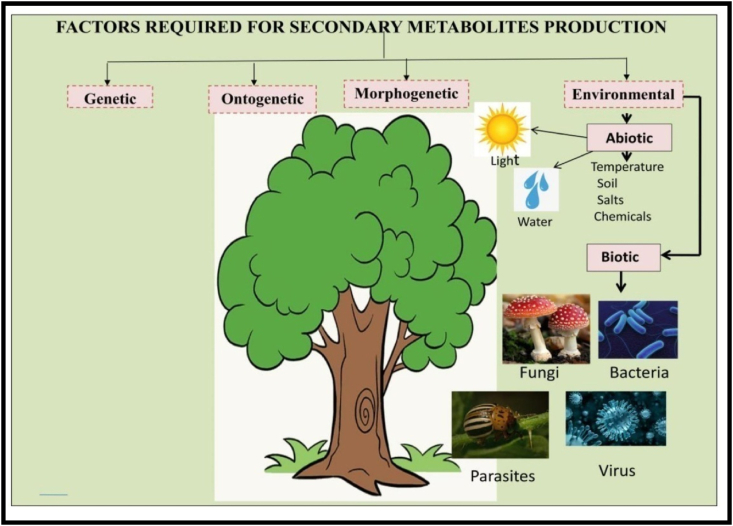
Both biotic and abiotic stress factors change plants’ metabolism [Figure 3]. Figure 3 depicts the factors required for the development of plant secondary metabolites. Genetic, Ontogenetic, environmental, and morphogenetic factors are responsible for the development of secondary compounds in plants. Different negative abiotic factors present in the environment, such as low and high temperatures, high light intensity, drought, flood, and the various toxic chemicals present in the soil produce secondary stresses in plants that act as catalysts in the biosynthesis of PSMs (Verma and Shukla, 2015). Experiment results show that the biosynthesis of PSMs triggers in the presence of temperature stress.
Figure 3.
Factors involved in secondary metabolite production. Among factors like genetic ontogenetic, morphogenetic and environmental factors; abiotic and biotic environmental factors are involved for secondary metabolites production. They are the elicitors for secondary metabolites production.
The role of climate change in secondary metabolite production in medicinal plants is still under research. The standard of chemical compounds derived from secondary metabolites in the forest system is stricken by high heat (Kundu et al., 2012). Several of the volatile compounds derived from secondary organic compounds, influence cloud formation in the atmosphere by manufacturing secondary organic aerosols (Rose et al., 2018; Zhao et al., 2017; Scott et al., 2018). In this manner, secondary metabolites facilitate plants to adapt to global climate change (Niinemets, 2018). Usually, when plants facing stress conditions secondary substances production might increase resulting in restrained growth due to chemical change, and also the carbon fixation is not allotted to growth, instead directed to secondary compounds production (Mooney et al., 1991). Many experiments have postulated that the result of high temperature on metabolites production of the plants is positive in most cases however a number of these have negative results too (Jochum et al., 2007). Some experiments show that secondary compounds increase with relevant elevated temperature (Litvak et al., 2002), some workers hypothesized that there is down regulation in the production of phytoconstituents (Snow et al., 2003). Intrinsically the responses of secondary metabolites to increased temperature are under research. An increased amount of volatile organic compounds has been usually detected (Loreto et al., 2006).
3.2. Role of high temperature in development of secondary metabolites
Plant growth and development are directly linked to a certain range of temperature (Li et al., 2020) at which all the physiological processes of a plant are completed. The rate of photosynthesis increased or decreased with an increase in temperature, depending on whether the current temperature has a profound effect on respiration (Turnbull et al., 2002). The biosynthesis of secondary metabolites is also correlated with the high temperature in plants (Verma and Shukla, 2015). Secondary metabolites elicit along with the stress factors up-regulation. High temperature down-regulates or up-regulates the responding genes and affects the growth and development of plants (Li et al., 2016). Temperature strongly influences the metabolic activities of the plants and high temperatures cause premature leaf senescence (Morison, 1999).
Plant enzymes act in optimal temperature zone and any deviation from the range result in denaturation and inactivation of the enzymatic machinery which disturbs plant physiological as well as biochemical activities. It must be concluded that growth temperature is always species-specific in the plant kingdom. The plant kingdom is stretched in diverse areas from tropical forests to alpine areas. So, the different optimum temperature is required for plant physiological growth and survival. Above this temperature, plants suffer from heat-mediated oxidative stress which is the reason for damage to plant cells (Chaitanya et al., 2002). Many earlier studies reported that only a few plant's secondary metabolites increase with elevated temperature in vitro conditions. Temperature stress in plants is mostly legendary to induce the active scavenging enzymes like superoxide dismutase enzyme (SOD), catalase, peroxidise, and few antioxidants, superoxide anionas a primary scavenger of radicals, provides the first defense strategy of the antioxidant system (Asada, 1999; Zhu et al., 2009). Temperature stress is the reason for membrane and lipid denaturation, inactivation of enzymes. The temperature has antagonistic effects in stabilizing membrane integrity. These stress factors will alter the secondary metabolites concentrations and chemical compositions within the plant. So, it might be used as the indicator of stress-connected injury in plants.
High temperature leads to changing root secondary metabolites concentrations in Panax quinquefolius (Jochum et al., 2007). Increasing temperature by 5 °C alter all the chemical action and biomass production of the herb Panax quinquefolius, on the other hand, the storage of ginsenoside is reported to be increased. The root ginsenosides contents enhance with the elevated temperature in Panax quinquefolius plants (Jochum et al., 2007). Cawood et al. (2018) considered the effect of temperature on the production of secondary metabolites in the Amaranthus cruentus plant. For this, they grown A. cruentus at three different temperatures and found that plants grown at higher temperatures had the maximum composition of major secondary metabolite compounds than low and ambient temperatures, where its level in the ambient temperature was 75.69 %, while at the higher temperature it was 91.89 %. As per reports of Zobayed et al. (2005), it is postulated that concentration of the secondary metabolites like hypericin, hyperforin, and pseudo hypericin decreases with the down-regulation of temperature within the shoot tissue of Hypericum perforetuma a highly valuable medicinal plant. The Hypericin concentration in Free Air Temperature Increase (FATI) was 13.76 % higher as compared to the ambient temperature in Hypericum perforatum L. (Sharma et al., 2020). The composition of some secondary compounds such as α-guaiene and globulol content in Valeriana jatamansi increased under the FATI experiment as compared to ambient and FACE conditions (Kaundal et al., 2018).Certain plant secondary metabolites like flavonoids (Lavola et al., 2013) and monoterpenes (Semiz et al., 2007) are beneath strong genotypic management. This may elevate the potentiality for forest tree chemical defenses to evolutionary adaption to gradual environment changes (Lavola et al., 2013). Julkunen- Tiitto et al. (2015) explained the reduction in leaf phenolics at high temperatures by a dilution impact. A lot of carbon sequestration processes trigger growth along with slow phenolics storage (Kosonen et al., 2012). Several earlier studies reported that high temperature reduces flavonoids production in plants (Zhang et al., 2018; Nissinen et al., 2016). Phenolic and Salicylic acids (Sivadasan et al., 2018) in deciduous trees and total phenolics content in Gymnosperms reduce with high temperature (Zhang et al., 2018) (Table 2). Warm condition is needed for increasing the foliar terpenes concentrations (Julkunen-Tiitto et al., 2015; Valkama et al., 2007). Sallas et al. (2003) explained improved plant growth and larger storage space for stored terpenes such as monoterpenes and resin acids in Pinus sylvestris. Recent studies showed higher monoterpenes concentration in xylem resin (Ferrenberg et al., 2017) an increased number of needle resin canals (Kivimaenpaa et al., 2016) in Pinus sp. An increase in temperature of 2 °C may increase the concentration of Picea abies needle alkaloids (Virjamo et al., 2014) and temperature range attached with conifer alkaloids concentration (Virjamo and Julkunen-Tiitto, 2016; Gerson et al., 2009). High temperature reduces the total concentration of phenolic compounds (Julkune-Tiitto et al., 2015). The combined effect of high temperature and elevated UVB radiation has confirmed that high temperature has a more positive effect on foliage phenolics (Nissinen et al., 2017) and terpenoids emission (Maja et al., 2016) than increasing exposure to UVB. Many earlier studies reported that the high temperature significantly reduced the concentration of most phenolic compounds and thus total phenolic acid, flavonoids, and plant glycosides concentration but the opposite was found for salicin, tremulacin, and neochlorogenic acid. Randriamanana et al. (2015) reported that the concentration of Gallo catechin, HCH salicortin, and diglucoside of salicyl alcohol elevated in response to up-regulated temperature (Randriamanana et al., 2015). Sobuj et al. (2018) reported that under elevated temperature the total amount of salicylates gets lowered by 22 % in the bark of Populus tremula stem compared to the control treatment. Salicyl alcohol obtained through phenolic metabolites is dominantly present in Populus sp. (Tsai et al., 2006; Randriamanana et al., 2015; Keefover-Ring et al., 2014). The total flavonoids concentration was decreased in response to the elevated temperature. Warming reduces the total concentration of phenolic compounds by 55 % in the stem bark of Populus tremulawhen compared with control plants (Sobuj et al., 2018). Alhaithloul et al. (2019) reported that the number of few plants’ secondary compounds such as flavonoid, phenols, and saponin contents decreases in response to drought and heat stress; however, the quantity of other secondary compounds such as alkaloids, tannins, and terpenoids regulate under heat and drought stress in Mentha pipertita and Catharanthus roseus.
Table 2.
Role of plant secondary compounds in high temperature in forest trees.
| S. No. | Plant Volatile Compounds | Increase/Decrease | References |
|---|---|---|---|
| 1 | Volatile terpenoids | Strongly increased | Penuelas and Staudt (2010) |
| A | Isoprenoids + MBO | Strongly increased | Penuelas and Staudt (2010) |
| B | MT + SQT (Monotarpenes + Sesquitarpenes) | Strongly increased | Penuelas and Staudt (2010) |
| C | GLVs + HIPV | Strongly increased | Penuelas and Staudt (2010) |
| 2 | Phenolics | Decreased | Zvereva and Kozlov (2006); Julkunen-Tiitto et al. (2015) |
| A | Phenolic acids | Decreased | Julkunen-Tiitto et al. (2015) |
| B | Flavonoids | Decreased | Julkunen-Tiitto et al. (2015) |
| C | Salicylates | Decreased | Julkunen-Tiitto et al. (2015) |
| D | Tannins | Slightly decreased | Julkunen-Tiitto et al. (2015) |
| 3 | Terpenoids | Slightly increased | Zvereva and Kozlov (2006) |
3.3. Reduction in phenolics compounds with elevated temperature
Temperature strongly influences the production and sequestration of secondary compounds in plants. Phenolics compounds represent a vital category of aromatic secondary metabolites (Malocovska et al., 2014) with the phenyl ring having one or more attached acidic hydroxyl groups (Ali et al., 2013; Achakzai et al., 2009). The synthesis of phenolic compounds in plants is measured byvarious enzymes like phenylalanine ammonia-lyase (PAL), UDP glucose flavonoid glucosyltransferase (UFGT), and chalcone isomerase (CHI) (Cohen and Kennedy, 2010). It has been observed that the concentration of some phenolic compounds in plants decreases with elevated temperatures. According to the scientist, the plant phenolics are biosynthesized in plants from a common biosynthetic intermediate, phenylalanine or its close precursor shikimic acid through the shikimic acid pathway and the growth of plants depends on the common precursor L-phenylalanine ammonia-lyase (PAL) enzyme (McDonald et al., 1999; Matsuki, 1996). It was observed that the higher growth in plants reduces the activity of phenylalanine ammonia-lyase resulting in decreases in the formation of plant secondary compounds (McDonald et al., 1999; Matsuki, 1996). High temperature decreases the concentration of phenolics compounds. However, several alternative earlier studies supported that the concentration of phenolic compounds increases with elevated temperature. Rivero et al. (2001) reported that elevated temperature may increase the PAL enzymatic activity in tomatoes, and the total concentration of phenolic compounds is elevated with heat stress. Wang and Zheng (2001) observed that the quantity of total phenolic compounds in Fragaria × ananassa plant up-regulated under elevated temperature treatment, whereas high temperatures considerably reduce total phenolic compounds in leaves of Ipomoea batatas. A decrease in the total amount of some secondary compounds, such as stillbenes and acetophenones has been observed during high-temperature stress (Zhang et al., 2018). The number of total phenolics content in seedling stems of Picea abies from all sources was significantly lower at a higher temperature (5–18 %) (Zhang et al., 2018). Higher temperature significantly decreases the concentration of total acetophenones, lignans, flavonoids, and stilbenes decreased in phenolics content caused by increasing temperature have been reported in many other tree species, like Pinus sylvestris (Sallus et al., 2001), Betula pendula (Lavola et al., 2013), Salix myrsinifolia (Nybakken and Julkanen-Tiitto, 2013) and Populus tremula (Randriamanana et al., 2015).
3.4. Emission of volatile organic compounds under high temperature
Warming has been most intensively studied among all the factors studied in volatile plant secondary metabolites (Penuelas and Staudt, 2010) [Figure 4]. The emission of volatile organic compounds is strongly upregulated by light and temperature and some of them are not stored in leaves (Sharkey and Yeh, 2001). Isoprene creates approximately 50 % of the estimated biogenic volatile organic compounds emission.Near about, 10 % of mounted carbon is used to create volatile organic compounds (VOCs) in plants with temperature stress (Penuelas and Staudt, 2010). Emissions of monoterpenes and sesquiterpenes are usually around 15 and 3% respectively (Guenther et al., 2012). The elevated temperature increased the release of volatile terpenoids most consistently (Penuelas and Staudt, 2010) by enhancing biosynthesis (Fini et al., 2017; Loreto and Schnitzler, 2010) and emission from leaf storage structures (Penuelas and Staudt, 2010; Grote and Niinemets, 2008). Recent studies showed that the volatile organic compounds involved in emission are high in conifers. The emission rate is 40 % higher in Pinus sp., and a little concentration in deciduous plants (Ghirardo et al., 2010). Differences in release responses of monoterpenes and sesquiterpenes to temperature might be due to primary storage of fewer volatile sesquiterpenes in leaf coat waxes (Joensuu et al., 2016), and their release (Himanen et al., 2015). Some other studies also reported the isoprene-emitting and monoterpene emitting rate in Quercus plant has increased with warming. An increase in temperature of about 3 °C increased 70 % release of isoprene and monoterpenes in Quercus sp. (Staudt et al., 2017). Long-term warming of 1 °C along with ambient increased monoterpenes (fivefold), sesquiterpenes (fourfold), and leaf volatile (40 %) emission of Betula pendula (Hartikainen et al., 2012). Few specific monoterpenes of forest trees, like b-ocimene, are temperature-stress indicators (Jardine et al., 2017).
Figure 4.
Pictorial presentation of emission of volatile secondary compounds in presence of high temperature.
3.5. Role of secondary metabolites under heat stress
The rise in atmospheric temperature because of e [CO2] leads to heat stress in plants. Heat stress changes morphological as well as physiological parameters in plants. Heat stress also plays a critical role in the formation of many plants’ secondary metabolites. Heat stress alters the quantity of PSMs (Mooreet al., 2014). Experiments performed in greenhouses under three different temperatures (i.e., 10 °C, 20 °C, and 30 °C) showed that higher temperatures created the formation of more alkaloid content (Jansen et al., 2009). 35 °C temperature for 15 days up-regulates the hypericin and hyperforin composition in the shoot tissue of Hypericum perforatum (Zobayed and Afreen et al., 2005). The experiments on temperature affecting alkaloid concentration in sixty-day-old seedling of Catharanthus roseus depicted that under short-term heat shock, the concentration of several secondary compounds such as catharanthine, vinblastine, and vindoline in the seedling leaves was higher at 40 °C than those at 30 °C. To be precise, catharanthine was elevated by 40 % after incubation at 40 °C for 2 h, while it elevated very little at 30 °C and raised the highest value at 6 h, and in a long-term experiment at 35 °C, the concentrations of alkaloids, catharanthine, monomeric and vindoline resulted in a precise increase (Guo et al., 2007). Elevated temperature incubation results in an immediate increase of 10- hydroxycamptothecin (HCPT) so that six-fold sequestration of 10-hydroxycamptothecin in leaves of Cyanea acuminata seedlings occurred after incubation at 40 °C for 2 h (Zu et al., 2003). Few examples are given below (Tables 1, 2), (Figures 5, 6, 7, 8, 9, 10, 11). Moreover, the contents of tannin in tea leaves also get modified by climatic changes. It is found in surveys that the catechin content of tea gets elevated at high temperatures. It is also founded that in the warmer seasons the catechin content of tea leaves gets higher than in the cooler seasons (Yao et al., 2005).
Table 1.
High heat mediated increase of secondary metabolites in various plant species.
| SN | Metabolite Class | Metabolite Name | Abiotic factors | Concentration Change | Plant species | References |
|---|---|---|---|---|---|---|
| 1 | Alkaloids | 10-hydroxy Camptothecin |
40 °C for 2 h only | Concentration Increased |
Cyanea acuminata | Wang et al., 2003 |
| 2 | Alkaloids | Vindoline | Short-term heat exposure | Concentration Increased |
Catheranthus Roseus |
Guo et al., 2007 |
| 3 | Alkaloids | Catharanthine | Long-term heat exposure | Concentration Increased |
Catheranthus Roseus |
Guo et al., 2007 |
| 4 | Terpenes | Isoprene | High temperature | Concentration Increased |
Quercus Rubra |
Hanson and Sharkey (2001) |
| 5 | Terpenes | α-farnesene | High temperature | Concentration Increased |
Daucus Carota |
Helmig et al. (2007) |
| 6 | Flavones | a. Quercetin | High temperature | Concentration Increased |
Brassica Oleracea |
Molmann et al. (2015) |
Figure 5.
Pictorial presentation of Cyanea acuminata plant producing 10-hydroxy Camptothecin compound.
Figure 6.
Pictorial presentation of Catheranthus roseus plant producing Catharanthine compound.
Figure 7.
Pictorial presentation of Catheranthus roseus plant producing Vindoline compound.
Figure 8.
Pictorial presentation of Quercus rubra plant producing Isoprene compound.
Figure 9.
Pictorial presentation of Daucus carota plant producing α-farnesene compound.
Figure 10.
Pictorial presentation of Brassica oleracea plant producing Kaempferol compound.
Figure 11.
Pictorial presentation of Brassica oleracea plant producing Quercetin compound.
3.6. Relevance of e [CO2] in plant secondary metabolite formation
High levels, of CO2 concentration, increase phenolic content in foliage and reduce the terpenoid compounds each in leaf and emission. Ibrahim and Jaafar (2012) observed that PAL enzyme activity increased with e [CO2] in Eleais guineensis Jacq seedlings. e [CO2] showed a positive relation with phenolic and flavonoids content, which may indicate up-regulation of PSMs production with increased PAL enzyme activity. Warming conditions decreased the number of phenolics in leaves and increase terpenoid content in foliage and emission (Penuelas and Staudt, 2010; Zevereva and Kozlov, 2006) but CO2 with warming conditions increase foliage phenolics content, however, lower the phenolics content in woody tissues (Zevereva and Kozlov, 2006). Alkaloids, flavonoids, and saponins were accrued in Robnia pseudoacacia mature in the heated OTC having high CO2 concentration, compared to seedlings grown in an open area (Zhao et al., 2016). Increasing the level of CO2 is related to a decrease in the concentration of terpenoid compounds in conifer needles was mitigated by high temperature and CO2 induced increase in phenolics was intensified by high temperature (Zvereva and Kozlov, 2006). Total phenolics and total flavonoids concentration increase significantly in Zingiber officinale rhizomes, stems, and leaves (Ghasemzadeh and Jaafar, 2011).
e [CO2] increases the composition of secondary compounds in Valeriana jatamansi oil (Kaundal et al., 2018), similar results have been found in Thymus hyemalis (Biel at al., 2005) and Thymus vulgaris (Vuro et al., 2009). This may be because e [CO2] increases the rate of photosynthesis in plants which has a positive effect on the production of primary metabolites as well as PSMs and indicates that e [CO2] in the future may be beneficial for the production of PSMs.
Other greenhouse gases (GHGs) such as Methane (CH4), Nitrous oxide (N2O), Chlorofluorocarbons (CFC), and Ozone (O3) are also present in the atmosphere. All these GHGs absorb and emit radiation in the thermal infrared range (IPCC, 2014).
Methane (CH4) is the second major greenhouse gas after CO2. CH4 is mainly emitted from the burning of fossil fuels, cattle farming, and agriculture. Currently, the concentration of CH4 is 1.8 μmol mol−1 (IPCC, 2014). CH4 in the form of GHGs is 30 times more potent than CO2 (IPCC, 2014). Excess methane emissions are harmful to plants because methane increases the concentration of ozone (O3) in the troposphere, which causes harmful chlorosis and yellowing of leaves in plants. About 93 % of crop losses in the upcoming century will be due to non–carbon dioxide emissions, the most harmful of which is methane (Branscombe, 2016).
In the lower atmosphere (troposphere) Ozone (O3) acts as a greenhouse gas and is phototoxic to plants (Vapaavuori et al., 2009; Lindroth, 2010). O3 acts as a pollutant near the troposphere and is harmful to both plants and animals. Due to the high concentration of O3 in the atmosphere, the stomata of the plants are closed, due to which the rate of plant growth and photosynthesis slows down (Bueker et al., 2015; Li et al., 2017a, b). A high concentration of O3 causes oxidative stress in plants due to which the plant cells and tissues are damaged (Vainonen and Kangasjärvi, 2015). Several earlier studies reported that total phenolics compounds (Gao et al., 2016), flavonoids (Cotrozzi et al., 2018), and condensed tannins (Couture et al., 2017) were increased in response to O3 treatment in the foliage of deciduous trees, but this was not similar in the needles of the coniferous trees (Riikonen et al., 2012).
Nitrous oxide (N2O) and nitric oxide (NO) both act as greenhouse gas (GHGs) (Cassia et al., 2018). Soil contains different types of microbes that are mainly responsible for the emission of (N2O) and (NO) through the process of nitrification and denitrification. In the last century, their global emissions have increased significantly, mainly due to human intervention (IPCC, 2014). This is mainly due to using more nitrogen fertilizer in the soil toincrease agriculture production which causes high nitrogen-rich soil and increases microbial activity thereby affecting the N cycle.Nitrous oxide (N2O) is a strong greenhouse gas (GHGs), while nitric oxide (NO) indirectly contributes to ozone (O3) synthesis (Cassia et al., 2018). As GHG, N2O is potentially 300 times stronger than CO2 (Cassia et al., 2018).In the troposphere, NO quickly oxidizes to nitrogen dioxide (NO2). NO, and NO2 (termed as NOx) may react with volatile organic compounds (VOCs) and hydroxyl, resulting in organic nitrates and nitric acid, respectively. On the other hand, nitrogen deposition in the soil in several ways such as wet (with rainfall and snow) and dry (as gaseous compounds) influences the C/N ratio, increases soil acidity, decreases species richness and composition.
Previous studies show that nitrogen (N) deposition into the soil promotes the synthesis of total phenolic compounds in such as Satureja hortensis L. (Alizadeh et al., 2010), Daucus carota L. (Smoleń and Sady, 2009), and Stevia rebaudiana (Tavarini et al., 2015), but opposite effects were also observed in many plants. Themetabolism patterns of the phenolic compound with N deposition can also be linked with root, stem, and leaves (Malik et al., 2012; Tavarini et al., 2015) or phenolic subclasses (e.g., flavonoid) (Mokgehle et al., 2017).
4. Conclusion and future strategies
This review article illustrated the role of elevated CO2 and high temperature in secondary metabolite production. From the present overview, it is postulated that plant secondary metabolites are responsible for defending plants in adverse environmental conditions. Plant secondary metabolites help the plant kingdom to survive against oxidative stress-mediated damages which causes cell death and damage to cell membranes. How PSMs are involved in the defense mechanisms is still under research. Based on several earlier studies, it is proved that plants’ first line of defense is created by producing secondary metabolites. The role of various greenhouse gases (GHGs) (CO2, CH4, N2O, and O3) is to elicit the atmospheric temperature, on the other hand, e [CO2] and heat stress lead to the generation of a higher number of few secondary metabolites; however, climate change and the warmer environment are harmful it is quite beneficial for the plant kingdom. Several other greenhouse gases (GHGs) also affect plant growth and development as well as their primary productivity. There are only a few studies conducted on the effects of these GHGs on PSMs production. Elicitation of secondary metabolites by e [CO2] mediated heat stress and creates α-chemo diversity (Chemical diversity among plants). This α-chemo diversity leads to the development of β-chemo diversity among plants (chemical diversity among groups). In plants, PSMs help plants in uptaking nutrients. They are helpful in hormone up-regulation. Secondary metabolites are also important for human beings in socio-economic aspects as these are the main constituents of several recreational drugs, perfumes, pharmaceuticals, etc. Secondary metabolites are highly essential for combating several human diseases. They are anti-fungal, anti-microbial. They have cytotoxic roles and antagonistic effects against cancer. Secondary metabolites help humans to fight against cancer, HIV, brain dysfunction, inflammation, RNA virus-mediated diseases, etc. But it is postulated that all the secondary metabolites have cytotoxic potentiality apart from their therapeutic potentiality. The cytotoxicity is also helpful in controlling cancer cell proliferation. In metastatic cells, the cytotoxicity will result in triggering apoptosis. If cytotoxic potentiality increases along with high temperatures, it may not be useful for society. Secondary metabolites are also useful in the cosmetic industry as essential oil. But in the high concentration, they are cytotoxic. Secondary Metabolites have high market value. Moreover, many PSMs have no biological functions, but they are the precursors of several important PSMs. In this regard, the production of secondary metabolites is very much important to maintain many socioeconomic purposes. After screening many reviews and research articles, we can say that the industrial production of PSMs can be possible by using heat as the catalyzing factor. That idea will enrich socio-economic conditions.
After reviewing earlier studies, we found that the effect of e [CO2] and high temperature is different on secondary metabolites found in plants, as well as the effect is also species-specific. Different plants react differently to e [CO2] and high temperatures, while an increase in temperature increases the number of secondary metabolites in some plants while in some it reduces the number of secondary metabolites. In this regard, e [CO2] and high temperature can be used in controlled environment conditions, the concentration of secondary metabolites in plants can be increased so that they can be used for the welfare of mankind. It is observed that plant secondary metabolites and volatile compounds are mostly accumulated in the plant system with the up-regulation of temperature. Research on temperature-regulated MT (Monoterpenes) SQT (Sesquiterpenes) resin acids production is also under research. It is evident that climate change influences growth and PSMs production in higher plants. Plant productivity also depends on the changing ecosystem. Most of the C3 plants i.e., whose first stable product after carbon assimilation is a three-carbon-containing product; favor a warmer environment. So, a higher amount of secondary metabolite production is possible in C3 plants.
It is observed that high temperature will increase secondary metabolite production might be helpful to produce transgenic plants with high metabolite yielding capacity and new drugs. Several biotechnological tools are employed for the enhanced production of secondary metabolites. So, a warming environment may lead to the innovation of a new industry and help in economic progress. It will also deplete the environmental food web and the survival of the lower group of plants and animals.
Secondary metabolites are not abundant in all plants like primary metabolites; they are limited to only a few plants. Currently, the demand for these secondary metabolites is increasing day by day to keep the world's growing population healthy and safe. Therefore, keeping in mind the importance of metabolites maximum emphasis should be focussed on their production. For this, the emphasis should be on the use of OTC, CEC, FACE, and FATI systems. On the other hand, there is also a need to study the combined effect of e [CO2] and high temperature on the production of these secondary metabolites.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
No data was used for the research described in the article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We are thankful to the laboratory and library staff for their kind cooperation. We are thankful to PubChem software (https://pubchem.ncbi.nlm.nih.gov/) for several secondary metabolites' structures.
References
- Achakzai A.K.K., Achakzai P., Masood A., Kayani S.A., Tareen R.B. Response of plant parts and age on the distribution of secondary metabolites on plants found in Quetta. Pakistan J. Bot. 2009;41(5):2129–2135. [Google Scholar]
- Alhaithloul H.A., Soliman M.H., Ameta K.L., El-Esawi M.A., Elkelish A. Changes in ecophysiology, osmolytes, and secondary metabolites of the medicinal plants of Mentha piperita and Catharanthus roseus subjected to drought and heat stress. Biomolecules. 2019;10:43. doi: 10.3390/biom10010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M., Abbasi B.H., Ihsan-ul-haq Production of commercially important secondary metabolites and antioxidant activity in cell suspension cultures of Artemisia absinthiumL. Ind. Crop. Prod. 2013;49:400–406. [Google Scholar]
- Alizadeh A., Khoshkhui M., Javidnia K., Firuzi O., Tafazoli E., Khalighi A. Effects of fertilizer on yield, essential oil composition, total phenolic content and antioxidant activity in Satureja hortensis L. (Lamiaceae) cultivated in Iran. J. Med. Plants Res. 2010;4:33–40. [Google Scholar]
- Asada K. The water–water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999;50:601–639. doi: 10.1146/annurev.arplant.50.1.601. [DOI] [PubMed] [Google Scholar]
- Ashraf M.A., Iqbal M., Rasheed R., Hussain I., Riaz M., Arif M.S. Environmental stress and secondary metabolites in plants: an overview. In: Ahmad P., Ahanger M.A., Singh V.P., Tripathi D.K., Alam P., Alyemeni M.N., editors. Plant Metabolites and Regulation Under Environmental Stress. Academic Press; London: 2018. pp. 153–167. [Google Scholar]
- Austen N., Walker H.J., Lake J.A., Phoenix G.K., Cameron D.D. The regulation of plant secondary metabolism in response to abiotic stress: interactions between heat shock and elevated CO2. Front. Plant Sci. 2019;10:1463. doi: 10.3389/fpls.2019.01463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartram S., Jux A., Gleixner G., Boland W. Dynamic pathway allocation in early terpenoid biosynthesis of stress-induced lima bean leaves. Phytochemistry. 2006;67:1661–1672. doi: 10.1016/j.phytochem.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Beattie A.J., Breedlove D.E., Ehrlich P.R. The ecology of pollinators and predators of Frasera speciosa. Ecol. 1973;54:81–91. [Google Scholar]
- Biel C., Save R., Cristobal R., Cases M.A. Effects of atmospheric carbon dioxide concentrations on Thymus vulgaris, Thymus zygisandThymus hyemalis. Acta Hortic. 2005;676:61–65. [Google Scholar]
- Bourgaud F., Gravot A., Milesi S., Gontier E. Production of plant secondary metabolites: a historical perspective. Plant Sci. 2001;161:839e851. [Google Scholar]
- Bishop J.G., Schemske D.W. Variation in flowering phenology and its consequences for lupines colonizing Mount St. Helens. Ecol. 1998;79:534–546. [Google Scholar]
- Branscombe A. Which greenhouse gas does the most damage to crops? Eos. 2016;97 [Google Scholar]
- Bueker P., Feng Z., Uddling J., Briolat A., Alonso R., Braun S. New flux based dose-response relationships for ozone for European forest tree species. Environ. Pollut. 2015;206:163–174. doi: 10.1016/j.envpol.2015.06.033. [DOI] [PubMed] [Google Scholar]
- Cassia R., Nocioni M., Correa-Aragunde N., Lamattina L. Climate change and the impact of greenhouse gasses: CO2 and NO, friends and foes of plant oxidative stress. Front. Plant Sci. 2018;9:273. doi: 10.3389/fpls.2018.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawood M.E., Allemann I., Allemann J. Impact of temperature stress on secondary metabolite profile and phytotoxicity of Amaranthus cruentus L. leaf extracts. Acta Agric. Slov. 2018;111:609–620. [Google Scholar]
- Chaitanya K., Sundar D., Masilamani S., Ramachandra R.A. Variation in heat stress-induced antioxidant enzyme activities among three mulberry cultivars. Plant Growth Regul. 2002;36:175–180. [Google Scholar]
- Chen F.J., Wu G., Ge F., Parajulee M.N., Shrestha R.B. Effects of elevated CO2 and transgenic Bt cotton on plant chemistry, performance, and feeding of an insect herbivore, the cotton bollworm. Entomol. Exp. Appl. 2005;115:341–350. [Google Scholar]
- Cohen S.D., Kennedy J.A. Plant metabolism and the environment: implications for managing phenolics. Crit Rev. Food Sci. 2010;50:620–643. doi: 10.1080/10408390802603441. [DOI] [PubMed] [Google Scholar]
- Collins M.R., Knutti R., Arblaster J., Dufresne J.L., Fichefet T., Friedlingstein P., Gao X., Gutowski W.J., Johns T., Krinner G., Shongwe M., Tebaldi C., Weaver A.J., Wehner M. Long-term climate change: projections, commitments and irreversibility. In: Stocker T.F., Qin D., Plattner K., Tignor M., Allen S.K., Boschung J., Nauels A., Xia Y., Bex V., Midgley P.M., editors. Climate Change: the Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press; Cambridge, United Kingdom and New York, USA: 2013. pp. 1029–1136. [Google Scholar]
- Cotrozzi L., Campanella A., Pellegrini E., Lorenzini G., Nali C., Paoletti E. Phenylpropanoids are key players in the antioxidant defense to ozone of European ash, Fraxinus excelsior. Environ. Sci. Pollut. Res. 2018;25:8137–8147. doi: 10.1007/s11356-016-8194-8. [DOI] [PubMed] [Google Scholar]
- Couture J.J., Meehan T.D., Rubert-Nason K.F., Lindroth R.L. Effects of elevated atmospheric carbon dioxide and tropospheric ozone on phytochemical composition of trembling aspen (Populus tremuloides) and paper birch (Betula papyrifera) J. Chem. Ecol. 2017;43:26–38. doi: 10.1007/s10886-016-0798-4. [DOI] [PubMed] [Google Scholar]
- Dijkstra P., Hymus G., Colavito D., Vieglais D.A., Cundari C.M., Johnson D.P. Elevated atmospheric CO2 stimulates aboveground biomass in a fire-regenerated scruboak ecosystem. Global Change Biol. 2002;8:90–103. [Google Scholar]
- Dixon R.A. Natural products and plant disease resistance. Nature. 2001;411:843e847. doi: 10.1038/35081178. [DOI] [PubMed] [Google Scholar]
- Dixon R., Strack D. Phytochemistry meets genome analysis, andbeyond. Phytochemistry. 2003;62:815–816. doi: 10.1016/s0031-9422(02)00712-4. [DOI] [PubMed] [Google Scholar]
- English-Loeb G.M., Karban R. Consequences of variation in flowering phenology for seed head herbivory and reproductive success in Erigeron glaucus (Compositae) Oecologia (Berl.) 1992;89:588–595. doi: 10.1007/BF00317168. [DOI] [PubMed] [Google Scholar]
- Fang X., Yang C.-Q., Wei Y.-K., Ma Q.-X., Yang L., Chen X.-Y. Genomics grand for diversified plant secondary metabolites. Plant Div. Res. 2011;33(1):53–64. [Google Scholar]
- Ferrenberg S., Langenhan J.M., Loskot S.A., Rozal L.M., Mitton J.B. Resin monoterpene defenses decline within three widespread species of pine (Pinus) along a 1530-m elevational gradient. Ecosphere. 2017;8 [Google Scholar]
- Fini A., Brunetti C., Loreto F., Centritto M., Ferrini F., Tattini M. Isoprene responses and functions in plants challenged by environmental pressures associated to climate change. Front. Plant Sci. 2017;8:1281. doi: 10.3389/fpls.2017.01281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galen C., Stanton M.L. Short-term responses of alpine buttercups to experimental manipulations of growing season length. Ecol. 1993;74:1052–1058. [Google Scholar]
- Gao F., Calatayud V., Garcia-Breijo F., Reig-Arminana J., Feng Z. Effects of elevated ozone on physiological, anatomical and ultrastructural characteristics of four common urban tree species in China. Ecol. Indicat. 2016;67:367–379. [Google Scholar]
- Gershenzon J. The cost of plant chemical defense against herbivory: a biochemical perspective. In: Bernays E.A., editor. Insect-plant Interactions. CRC Press; Boca Raton: 1994. p. 105e173. [Google Scholar]
- Gerson E.A., Kelsey R.G., St Clair J.B. Genetic variation of piperidine alkaloids in Pinus ponderosa: a common garden study. Ann. Bot. 2009;103:447–457. doi: 10.1093/aob/mcn228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemzadeh A., Jaafar H.Z. Effect of CO2 enrichment on synthesis of some primary and secondary metabolites in ginger (Zingiber officinale Roscoe) Int. J. Mol. Sci. 2011;12(2):1101–1114. doi: 10.3390/ijms12021101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghirardo A., Koch K., Taipale R., Zimmer I., Schnitzler J., Rinne J. Determination of de novo and pool emissions of terpenes from four common boreal/alpine trees by 13 CO2 labelling and PTR-MS analysis. Plant Cell Environ. 2010;33:781–792. doi: 10.1111/j.1365-3040.2009.02104.x. [DOI] [PubMed] [Google Scholar]
- Grote R., Niinemets U. Modeling volatile isoprenoid emissions – a story with split ends. Plant Biol. 2008;10:8–28. doi: 10.1055/s-2007-964975. [DOI] [PubMed] [Google Scholar]
- Guenther A.B., Jiang X., Heald C.L., Sakulyanontvittaya T., Duhl T., Emmons L.K. The model of emissions of gases and aerosols from nature version 2.1 (MEGAN2.1): an extended and updated framework for modeling biogenic emissions. Geosci. Model Dev. (GMD) 2012;5:1471–1492. [Google Scholar]
- Guo X.R., Yang L., Yu J.H., Tang Z.H., Zu Y.G. Alkaloid variations in Catharanthus roseus seedlings treated by different temperatures in short term and long term. J. For. Res. 2007;18:313–315. [Google Scholar]
- Hanson D.T., Sharkey T.D. Effect of growth conditions on isoprene emission and other thermotolerance-enhancing compounds. Plant Cell Environ. 2001;24:929–936. [Google Scholar]
- Harte J., Torn M.S., Chang F.R., Feifarek B., Kinzig A.P., Shaw R., Shen K. Global warming and soil microclimate: results from a meadow-warming experiment. Ecol. Appl. 1995;5(1):132–150. [Google Scholar]
- Hartikainen K., Riikonen J., Nerg A., Kivimäenpää M., Ahonen V.A. Impact of elevated temperature and ozone on theemission of volatile organic compounds and gas exchange of silver birch (Betula pendula Roth) Environ. Exp. Bot. 2012;84:33–43. [Google Scholar]
- Helmig D., Ortega J., Duhl T., Tanner D., Guenther A., Harley P., Wiedinmyer C., Milford J., Sakulyanontvittaya T. Sesquiterpene emissions from pine trees-identifications, emission rates and flux estimates for the contiguous United States. Environ. Sci. Technol. 2007;41:1545–1553. doi: 10.1021/es0618907. [DOI] [PubMed] [Google Scholar]
- Himanen S.J., Bui T.N.T., Maja M.M., Holopainen J.K. Utilizing associational resistance for biocontrol: impacted by temperature, supported by indirect defence. BMC Ecol. 2015;15:16. doi: 10.1186/s12898-015-0048-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibraham M.H., Jaafar H.Z.E. Impact of elevated carbon dioxide on primary, secondary metabolites and antioxidant responses of Eleais guineensis Jacq. (Oil Palm) seedlings. Molecules. 2012;17:5195–5211. doi: 10.3390/molecules17055195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idso C.D., Idso K.E. Forecasting world food supplies: the impact of rising atmospheric CO2 concentration. Technology. 2000;7:33–55. [Google Scholar]
- IPCC . Climate change. synthesis report, In: Core Writing Team, Pachauri R.K., Meyer L.A., editors. Proceedings of the Contributionof Working Groups I, II and III to the Fifth Assessment Report of theIntergovernmentalPanelon Climate Change. 2014. p. 151. (Geneva: IPCC) [Google Scholar]
- IPCC . 2019. Intergovernmental Panel on Climate Change. Report “Choice Made Now Is Critical for the Future of Our Ocean.https://www.ipcc.ch/srocc [Google Scholar]
- Jamwal K., Bhattacharya S., Puri S. Plant growth regulator mediated consequences of secondary metabolites in medicinal plants. J. Appl. Res. Med. Aromatic Plants. 2018;9:26–38. [Google Scholar]
- Jansen G., Jürgens H.U., Ordon F. Effects of temperature on the alkaloid content of seeds of Lupinus angustifolius cultivars. J. Agric. Crop Sci. 2009;195:172–177. [Google Scholar]
- Jardine K.J., Jardine A.B., Holm J.A., Lombardozzi D.L., Negron-Juarez R.I., Martin S.T. Monoterpene "thermometer’ of tropical forestatmosphere response to climate warming. Plant Cell Environ. 2017;40:441–452. doi: 10.1111/pce.12879. [DOI] [PubMed] [Google Scholar]
- Jia X., Wang W.K., Chen Z.H., He Y.H., Liu J.X. Concentrations of secondary metabolites in tissues and root exudates of wheat seedlings changes under atmospheric CO2 and cadmium-contaminated soils. Environ. Exp. Bot. 2014;107 134e143. [Google Scholar]
- Jochum G.M., Mudge K.W., Thomas R.B. Elevated temperatures increase leaf senescence and root secondary metabolite concentration in the understory herb Panax quinquefolius (Araliaceae) Am. J. Bot. 2007;94:819–826. doi: 10.3732/ajb.94.5.819. [DOI] [PubMed] [Google Scholar]
- Joensuu J., Altimir N., Hakola H., Rostas M., Raivonen M., Vestenius M. Role of needle surface waxes in dynamic exchange of monoand sesquiterpenes. Atmos. Chem. Phys. 2016;16:7813–7823. [Google Scholar]
- Jordan M.A., Leslie W. Microtubules as a target for antic cancer drugs. Nat. Rev. Canc. 2004;4(4):253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- Julkunen-Tiitto R., Nybakken L., Randriamanana T., Virjamo V. Boreal woody species resistance affected by climate change. In: Björkman C., Niemelä P., editors. CAB International); Wallingford: 2015. pp. 54–73. (In Climate Change and Insect Pests). [Google Scholar]
- Keefover-Ring K., Ahnlund M., Abreu I.N., Jansson S., Moritz T., Albrectsen B.R. No evidence of geographical structure of salicinoid chemotypes within Populus tremula. PloS One. 2014;9 doi: 10.1371/journal.pone.0107189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy D.O., Wightman E.L. Herbal extracts and phytochemicals: plant secondary metabolites and the enhancement of human brain function. Adv. Nutr. 2011;2:32e50. doi: 10.3945/an.110.000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Kang H. Effects of elevated CO2 and Pb on phytoextraction and enzyme activity. Water. Air Soil Pollut. 2011;219:365e375. [Google Scholar]
- Kimball B.A. Crop responses to elevated CO2 and interactions with H2O, N and temperature. Curr. Opin. Plant Biol. 2016;31:36–43. doi: 10.1016/j.pbi.2016.03.006. [DOI] [PubMed] [Google Scholar]
- Kivimäenpää M., Ghimire R.P., Sutinen S., Haikio E., Kasurinen A., Holopainen T. Increases in volatile organic compound emissions of Scots pine in response to elevated ozone and warming are modified by herbivory and soil nitrogen availability. Eur. J. For. Res. 2016;135:343–360. [Google Scholar]
- Koricheva J. Meta-analysis of sources of variation in fitness costs of plant antiherbivore defenses. Ecology. 2002;83:176–190. [Google Scholar]
- Korner C., Asshoff R., Bignucolo O., Hättenschwiler S., Keel S.G., Peláez-Riedl S., Pepin S., Siegwolf R.T.W., Zotz G. Carbon flux and growth in mature deciduous forest trees exposed to elevated CO2. Science. 2005;309:1360–1362. doi: 10.1126/science.1113977. [DOI] [PubMed] [Google Scholar]
- Kosonen M., Keski-Saari S., Ruuhola T., Constabel C.P., Julkunen- Tiitto R. Effects of overproduction of condensed tannins and elevated temperature on chemical and ecological traits of genetically modified hybrid aspens (Populus tremula x P. tremuloides) J. Chem. Ecol. 2012;38:1235–1246. doi: 10.1007/s10886-012-0193-8. [DOI] [PubMed] [Google Scholar]
- Kaundal M., Bhatt V., Kumar R. Elevated CO2 and temperature effect on essential oil content and composition of Valeriana jatamansi jones. With organic manure application in a western himalayan region. J. Ess. Oil Bear. Plants. 2018;21(4):1041–1050. [Google Scholar]
- Kundu S., Fisseha R., Putman A.L., Rahn T.A., Mazzoleni L.R. High molecular weight SOA formation during limonene ozonolysis: insights from ultrahigh-resolution FT-ICR mass spectrometry characterization. Atmos. Chem. Phys. 2012;12:5523–5536. [Google Scholar]
- Lacey E., Pace R. Effect of flowering and dispersal times on offspring fate in Daucus carota(Apiaceae) Oecologia. 1983;60:274–278. doi: 10.1007/BF00379533. [DOI] [PubMed] [Google Scholar]
- Lämke J.S., Unsicker S.B. Phytochemical variation in treetops: causes and consequences for tree-insect herbivore interactions. Oecologia. 2018;187:377–388. doi: 10.1007/s00442-018-4087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavola A., Nybakken L., Rousi M., Pusenius J., Petrelius M., Kellomaki S. Combination treatment of elevated UVB radiation, CO2 and temperature has little effect on silver birch (Betula pendula) growth and phytochemistry. Physiol. Plantarum. 2013;149:499–514. doi: 10.1111/ppl.12051. [DOI] [PubMed] [Google Scholar]
- Levine L.H., Kasahara H., Kopka J., Erban A., Fehrl I., Kaplan F., Zhao W., Littell R.C., Guy C., Wheeler R., Sager J., Mills A., Levine H.G. Physiologic and metabolic responses of wheat seedlings to elevated and super-elevated carbon dioxide. Adv. Space Res. 2008;42:1917e1928. [Google Scholar]
- Li P., Feng Z., Catalayud V., Yuan X., Xu Y., Paoletti E. A meta-analysis on growth, physiological, and biochemical responses of woody species to ground-level ozone highlights the role of plant functional types. Plant Cell Environ. 2017;40:2369–2380. doi: 10.1111/pce.13043. [DOI] [PubMed] [Google Scholar]
- Li X., Zhang L., Ahammed G.J., Li Z.X., Wei J.P., Shen C. Stimulation in primary and secondary metabolism by elevated carbon dioxide alters green tea quality in Camellia sinensis L. Sci. Rep. 2017;7:7937. doi: 10.1038/s41598-017-08465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K.H., Huang W., Wang G.L., Wu Z.J., Zhuang J. Expression profile analysis of ascorbic acid-related genes in response to temperature stress in the tea plant, Camellia sinensis (L.) O. Kuntze Genet. Mol. Res. 2016;15:1–10. doi: 10.4238/gmr.15048756. [DOI] [PubMed] [Google Scholar]
- Li Y., Kong D., Fu Y., Sussman M.R., Wu H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020;148:80–89. doi: 10.1016/j.plaphy.2020.01.006. [DOI] [PubMed] [Google Scholar]
- Lindroth R.L. Impacts of elevated atmospheric CO2 and O3 on forests: phytochemistry, trophic interactions, and ecosystem dynamics. J. Chem. Ecol. 2010;36:2–21. doi: 10.1007/s10886-009-9731-4. [DOI] [PubMed] [Google Scholar]
- Lindsey R. 2020. Climate Change: Atmospheric Carbon Dioxide.https://www.climate.gov/news-features/understanding-climate/climate-change-atmospheric-carbon-dioxide [Google Scholar]
- Litvak M.E., Constable J.V.H., Monson R.K. Supply and demand processes as controls over needle mononterpene synthesis and concentration in Douglas fir [Pseudotsugamenziesii(Mirb.) Franco] Oecologia. 2002;132:382–391. doi: 10.1007/s00442-002-0964-y. [DOI] [PubMed] [Google Scholar]
- Long S.P., Ainsworth E.A., Leakey A.D.B., Nosberger J., Ort D.R. Food for thought: lower-than-expected crop yield stimulation with rising CO2 concentrations. Science. 2006;312:1918–1921. doi: 10.1126/science.1114722. [DOI] [PubMed] [Google Scholar]
- Loreto F., Schnitzler J.P. Abiotic stresses and induced BVOCs. Trends Plant Sci. 2010;15:154–166. doi: 10.1016/j.tplants.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Loreto F., Barta C., Brilli F., Nogues I. On the induction of volatile organic compound emissions by plants as consequence of wounding or fluctuations of light and temperature. Plant Cell Environ. 2006;29:1820–1828. doi: 10.1111/j.1365-3040.2006.01561.x. [DOI] [PubMed] [Google Scholar]
- Maja M.M., Kasurinen A., Holopainen T., Julkunen-Tiitto R., Holopainen J.K. The effect of warming and enhanced ultraviolet radiation on gender-specific emissions of volatile organic compounds from European aspen. Sci. Total Environ. 2016;547:39–47. doi: 10.1016/j.scitotenv.2015.12.114. [DOI] [PubMed] [Google Scholar]
- Malcovska S.M., Ducaiova Z., Maslaáková I., Backor M. Effect of silicon on growth, photosynthesis, oxidative status and phenolic compounds of maize (Zea mays L.) grown in cadmium excess. Water. Air Soil Pollut. 2014;225(8):1. [Google Scholar]
- Malik A.A., Ahmad J., Suryapani S., Abdin M.Z., Ali M. Effect of inorganic and biological fertilizer treatments on essential oil composition of Ruta graveolens L. J. Herbs, Spices, Med. Plants. 2012;18:191–202. [Google Scholar]
- Marchi S., Tognetti R., Vaccari F.P., Lanini M., Kaligaric M., Miglietta F., Raschi A. Physiological and morphological responses of grassland species to elevated atmospheric CO2 concentrations in FACE-systems and natural CO2 springs. Funct. Plant Biol. 2004;31:181e194. doi: 10.1071/FP03140. [DOI] [PubMed] [Google Scholar]
- Matsuki M. Regulation of plant phenolic synthesis: from biochemistry to ecology and evolution. Aust. J. Bot. 1996;44:613–634. [Google Scholar]
- Mazlun M.H., Sabran S.F., Mohamed M., Abu, Bakar M.F., Abdullah Z. Phenolic compounds as promising drug candidates in Tuberculosis therapy. Molecules. 2019;24(13):2449. doi: 10.3390/molecules24132449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald E.P., Agrell J., Lindroth R.L. CO2 and light effects on deciduous trees: growth, foliar chemistry, and insect performance. Oecologia. 1999;119:389–399. doi: 10.1007/PL00008822. [DOI] [PubMed] [Google Scholar]
- Metlen K.L., Aschehoug E.T., Callaway R.M. Plant behavioural ecology: dynamic plasticity in secondary metabolites. Plant Cell Environ. 2009;32:641–653. doi: 10.1111/j.1365-3040.2008.01910.x. [DOI] [PubMed] [Google Scholar]
- Mohiuddin A.K. Impact of various environmental factors on secondary metabolism of medicinal plants. J of Pharmacol& Clin Res. 2019;7(1):555704. [Google Scholar]
- Mokgehle S.N., Tesfay S.Z., Araya H.T., du Plooy C.P. Antioxidant activity and soluble sugars of African ginger (Siphonochilusaethiopicus) in response to irrigation regimen and nitrogen levels. Acta Agr. Scandi. B — S. P. 2017;67:425–434. [Google Scholar]
- Molmann J.A.B., Steindal A.L.H., Bengtsson G.B., Seljasen R., Lea P. Effects of temperature and photoperiod on sensory quality and contents of glucosinolates, flavonols and vitamin C in broccoli florets. Food Chem. 2015;172:47–55. doi: 10.1016/j.foodchem.2014.09.015. [DOI] [PubMed] [Google Scholar]
- Mooney H.A., Winner W.E., Pell E.J. Academic Press; San Diego, California, USA: 1991. Response of Plants to Multiple Stresses. [Google Scholar]
- Moore B.D., Andrew R.L., Külheim C., Foley W.J. Explaining intraspecific diversity in plant secondary metabolites in an ecological context. New Phytol. 2014;201(3):733–750. doi: 10.1111/nph.12526. [DOI] [PubMed] [Google Scholar]
- Morison J.I.L., Lawlor D.W. Interactions between increasing CO2 concentration and temperature on plant growth. Plant Cell Environ. 1999;22:659–682. [Google Scholar]
- Munné-Bosch S. Nova Science Publishers, Inc; New York, NY: 2012. Phenolic Acids: Composition, Applications and Health Benefits. [Google Scholar]
- Naghiloo S., Movafeghi A., Delazar A., Nazemiyeh H., Asnaashari S., Dadpour M.R. Ontogenetic variation of total phenolics and antioxidant activity in roots: leaves and flowers of Astragalus compactus Lam. (Fabaceae) Bioimpacts. 2012;2(2):105–109. doi: 10.5681/bi.2012.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets U. What are plant-released biogenic volatiles and how they participate in landscape- to global-level processes? In: Perera A.H., Peterson U., Martínez Pastur G., Iverson L.R., editors. In Ecosystem Services from Forest Landscapes. Springer; Cham: 2018. pp. 29–56. [Google Scholar]
- Nissinen K., Nybakken L., Virjamo V., Julkunen-Tiitto R. Slow-growing Salix repens (Salicaceae) benefits from changing climate. Environ. Exp. Bot. 2016;128:59–68. [Google Scholar]
- Nissinen K., Virjamo V., Randriamanana T., Sobuj N., Sivadasan U., Mehtatalo Responses of growth and leaf phenolics in European aspen (Populus tremula) to climate change during juvenile phase change. Can.J. For. Res. 2017;47:1350–1363. [Google Scholar]
- Novriyanti E., Watanabe M., Kitao M., Utsugi H., Uemura A., Koike T. High nitrogen and elevated [CO2] effects on the growth, defense and photosynthetic performance of two eucalypt species. Environ. Pollut. 2012;170:124e136. doi: 10.1016/j.envpol.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Nybakken L., Julkunen-Tiitto R. Gender differences in Salix myrsinifolia at the pre reproductive stage are little affected by simulated climatic change. Physiol. Plantarum. 2013;147:465–476. doi: 10.1111/j.1399-3054.2012.01675.x. [DOI] [PubMed] [Google Scholar]
- Peñuelas J., Staudt M. BVOCs and global change. Trends Plant Sci. 2010;15:133–144. doi: 10.1016/j.tplants.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Peterson M.A. Host plant phenology and butterfly dispersal: causes and consequences of uphill movement. Ecol. 1997;78:167–180. [Google Scholar]
- Peak K.Y., Yu K.W., Murthy H.N., Hahn E.J. Ginsenoside production by hairy root cultures of Panax ginseng: infuence of temperature and light quality. Biochem. Eng. J. 2005;23(1):53–56. [Google Scholar]
- Pridham J.B. Pergamon Press; New York: 1960. Phenolics in Plants in Health and Disease; pp. 34–35. [Google Scholar]
- Randriamanana T.R., Lavola A., Julkunen-Tiitto R. Interactive effects of supplemental UV-B radiation and temperature in European aspen seedlings: implications for growth, leaf traits, phenolic defense and associated organisms. Plant Physiol. Biochem. 2015;93:84–93. doi: 10.1016/j.plaphy.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Rasineni G.K., Guha A., Reddy A.R. Responses of Gmelina arborea, a tropical deciduous tree species, to elevated atmospheric CO2: growth, biomass productivity and carbon sequestration efficacy. Plant Sci. 2011;181(4):428–438. doi: 10.1016/j.plantsci.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Ravishankar G.A., Venkataraman L.V. Food applications of plant cell cultures. Curr. Sci. 1990;59:914–920. [Google Scholar]
- Reddy G.V.P., Tossavainen P., Anne-Marja N., Holopainen J.K. Elevated atmospheric CO2 affects the chemical quality of Brassica plants and the growth rate of the specialist, Plutellaxylostella, but not the generalist, Spodoptera littoralis. J. Agric. Food Chem. 2004;52:4185–4191. doi: 10.1021/jf049358v. [DOI] [PubMed] [Google Scholar]
- Reddy A.R., Rasineni G.K., Raghavendra A.S. The impact of global elevated CO2 concentration on photosynthesis and plant productivity. Curr. Sci. 2010;99:46–57. [Google Scholar]
- Reyburn H., Mtove G., Hendriksen I., von Seidlein L. Oral qui-nine for the treatment of uncomplicated malaria. Br. Med. J. 2009;339:b2066. doi: 10.1136/bmj.b2066. [DOI] [PubMed] [Google Scholar]
- Riikonen J., Kontunen-Soppela S., Ossipov V., Tervahauta A., Tuomainen M., Oksanen E. Needle metabolome, freezing tolerance and gas exchange in Norway spruce seedlings exposed to elevated temperature and ozone concentration. Tree Physiol. 2012;32:1102–1112. doi: 10.1093/treephys/tps072. [DOI] [PubMed] [Google Scholar]
- Rivero R.M. Resistance to cold and heat stress: accumulation of phenolic compounds in tomato and watermelon plants. Plant Sci. 2001;160:315–321. doi: 10.1016/s0168-9452(00)00395-2. [DOI] [PubMed] [Google Scholar]
- Rodney C., Toni M., Kutchan N., Lewis G. Biochemistry and molecular biology of plants. In: Buchanan B., Gruissem W., Jones R., editors. Natural Products. Wiley; Rockville, MD., USA: 2000. pp. 1253–1348. [Google Scholar]
- Rose C., Zha Q., Dada L., Yan C., Lehtipalo K., Junninen H. Observations of biogenic ion-induced cluster formation in the atmosphere. Sci. Adv. 2018;4:eaar5218. doi: 10.1126/sciadv.aar5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallas L., Kainulainen P., Utriainen J., Holopainen T., Holopainen J.K. The influence of elevated O3 and CO2 concentrations on secondary metabolites of Scots pine (Pinus sylvestris L.) seedlings. Global Change Biol. 2001;7:303–311. [Google Scholar]
- Sallas L., Luomala E.M., Utriainen J., Kainulainen P., Holopainen J.K. Contrasting effects of elevated carbon dioxide concentration and temperature on Rubisco activity, chlorophyll fluorescence, needle ultrastructure and secondary metabolites in conifer seedlings. Tree Physiol. 2003;23:97–108. doi: 10.1093/treephys/23.2.97. [DOI] [PubMed] [Google Scholar]
- Sanchita, Sharma A. 2018. Gene Expression Analysis in Medicinal Plants under Abiotic Stress Conditions. Plant Metabolites and Regulation under Environmental Stress; pp. 407–414. [Google Scholar]
- Schmitt J. Density dependent pollinator foraging flowering phenology and temporal pollen dispersal patterns in Linanthusbicolor. Evolution. 1983;37:1247–1257. doi: 10.1111/j.1558-5646.1983.tb00241.x. [DOI] [PubMed] [Google Scholar]
- Scott C.E., Monks S.A., Spracklen D.V., Arnold S.R., Forster P.M., Rap A. Impact on short-lived climate forcers increases projected warming due to deforestation. Nat. Commun. 2018;9:157. doi: 10.1038/s41467-017-02412-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigler D.S. Kluwer Academic Publishers; Dordrecht: 1998. Plant Secondary Metabolism. [Google Scholar]
- Semiz G., Heijari J., Isik K., Holopainen J.K. Variation in needle terpenoids among Pinus sylvestris L. (Pinaceae) provenances from Turkey. Biochem. Systemat. Ecol. 2007;35:652–661. [Google Scholar]
- Sharkey T., Yeh S. Isoprene emission from plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001;52:407–436. doi: 10.1146/annurev.arplant.52.1.407. [DOI] [PubMed] [Google Scholar]
- Sharma S., Walia S., Rathore S., Kumar P., Kumar R. Combined effect of elevated CO2 and temperature on growth, biomass and secondary metabolite of Hypericum perforatum L. in a western Himalayan region. J. Appl. Res. Med. Aromat. Plants. 2020;16:100239. [Google Scholar]
- Sivadasan U., Chenhao C., Nissinen K., Randriamanana T., Nybakken L., Julkunen-Tiitto R. Growth and defence of aspen (Populus tremula) after three seasons under elevated temperature and ultraviolet-B radiation. Can. J. For. Res. 2018;48:629–641. [Google Scholar]
- Smoleń S., Sady W. The effect of various nitrogen fertilization and foliar nutrition regimes on the concentrations of sugars, carotenoids and phenolic compounds in carrot (Daucus carota L.) Sci. Hortic. 2009;120:315–324. [Google Scholar]
- Snow M.D., Bard R.R., Olszyk D.M., Minster L.M., Hager A.N., Tingey D.T. Monoterpene levels in needles of Douglas fir exposed to elevated CO2 and temperature. Physiol. Plantarum. 2003;117:352–358. doi: 10.1034/j.1399-3054.2003.00035.x. [DOI] [PubMed] [Google Scholar]
- Sobuj N., Virjamo V., Zhang Y., Nybakken L., Julkunen-Tiitto R. Impacts of elevated temperature and CO2 concentration on growth and phenolics in the sexually dimorphic Populus tremula (L.) Environ. Exp. Bot. 2018;146:34–44. [Google Scholar]
- Staudt M., Morin X., Chuine I. Contrasting direct and indirect effects of warming and drought on isoprenoid emissions from Mediterranean oaks. Reg. Environ. Change. 2017;17:2121–2133. [Google Scholar]
- Tavarini S., Sgherri C., Ranieri A.M., Angelini L.G. Effect of nitrogen fertilization and harvest time on steviol glycosides, flavonoid composition, and antioxidant properties in Stevia rebaudianaBertoni. J. Agric. Food Chem. 2015;63:7041–7050. doi: 10.1021/acs.jafc.5b02147. [DOI] [PubMed] [Google Scholar]
- Tsai C.J., Harding S.A., Tschaplinski T.J., Lindroth R.L., Yuan Y.N. Genome wide analysis of the structural genes regulating defense phenylpropanoid metabolism in Populus. New Phytol. 2006;172:47–62. doi: 10.1111/j.1469-8137.2006.01798.x. [DOI] [PubMed] [Google Scholar]
- Thirumurugan D., Cholarajan A., Raja S.S., Vijayakumar R. An introductory chapter: secondary metabolites. Second metab—sources Appl. 2018:1–21. [Google Scholar]
- Turnbull M.H., Murthy R., Griffin K.L. The relative impacts of daytime and nighttime warming on photosynthetic capacity in Populus deltoids. Plant Cell Environ. 2002;25:1729–1737. [Google Scholar]
- Vainonen J.P., Kangasjärvi J. Plant signalling in acute ozone exposure. Plant Cell Environ. 2015;38:240–252. doi: 10.1111/pce.12273. [DOI] [PubMed] [Google Scholar]
- Valkama E., Koricheva J., Oksanen E. Effects of elevated O3, alone and in combination with elevated CO2, on tree leaf chemistry and insect herbivore performance: a meta-analysis. Global Change Biol. 2007;13:184–201. [Google Scholar]
- Vapaavuori E., Holopainen J.K., Holopainen T., Julkunen-Tiitto R., Kaakinen S., Kasurinen A. Rising atmospheric CO2 concentration partially masks the negative effects of elevated O3 in silver birch (Betula pendula Roth) Ambio. 2009;38:418–424. doi: 10.1579/0044-7447-38.8.418. [DOI] [PubMed] [Google Scholar]
- Verma N., Shukla S. Impact of various factors responsible for fluctuation in plant secondary metabolites. J. Appl. Res. Med. Aromat. Plants. 2015;2(4):105–113. [Google Scholar]
- Virjamo V., Julkunen-Tiitto R. Variation in piperidine alkaloid chemistry of Norway spruce (Piceaabies) foliage in diverse geographic origins grown in the same area. Can. J. For. Res. 2016;46:456–460. [Google Scholar]
- Virjamo V., Sutinen S., Julkunen-Tiitto R. Combined effect of elevated UVB, elevated temperature and fertilization on growth, needle structure and phytochemistry of young Norway spruce (Picea abies) seedlings. Global Change Biol. 2014;20:2252–2260. doi: 10.1111/gcb.12464. [DOI] [PubMed] [Google Scholar]
- Vurro E., Bruni R., Bianchi A., di Toppi L.S. Elevated atmospheric CO2 decreases oxidative stress and increases essential oil yield in leaves of Thymus vulgaris grown in a mini- FACE system. Environ. Exp. Bot. 2009;65(2):99–106. [Google Scholar]
- Wang S.Y., Zheng W. Effect of plant growth temperature on antioxidant capacity in strawberry. J. Agric. Food Chem. 2001;49:4977–4982. doi: 10.1021/jf0106244. [DOI] [PubMed] [Google Scholar]
- Wang X., Taub D.R. Interactive effects of elevated carbon dioxide and environmental stress on root mass fraction in plants: a meta-analytical synthesis using Pairwise Techniques. Oecologia. 2010;163:1–11. doi: 10.1007/s00442-010-1572-x. [DOI] [PubMed] [Google Scholar]
- Williams R.S., Lincoln D.E., Thomas R.B. Loblolly pine grown under elevated CO2 affects early instarpine sawfly performance. Oecologia. 1994;98:64–71. doi: 10.1007/BF00326091. [DOI] [PubMed] [Google Scholar]
- Wink M. Introduction: biochemistry, physiology and ecological functions of secondary metabolites. Biochemistry of Plant Secondary Metabolism. 2010:1–19. Oxford Wiley-Blackwell. [Google Scholar]
- Xu H., Xie H., Wu S., Wang Z., He K. Effects of elevated CO2 and increased N fertilization on plant secondary metabolites and chewing insect fitness. Front. Plant Sci. 2019;10:739. doi: 10.3389/fpls.2019.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L., Caffin N., Darcy B., Jiang Y., Shi J., Singanusong R. Seasonal variations of phenolic compounds in Australia-grown tea (Camellia sinensis) J. Agric. Food Chem. 2005;53:6477–6483. doi: 10.1021/jf050382y. [DOI] [PubMed] [Google Scholar]
- Zavala J.A., Gog L., Giacometti R. Anthropogenic increase in carbon dioxide modifies plant-insect interactions. Ann. Appl. Biol. 2017;170:68–77. [Google Scholar]
- Zhang Y., Virjamo V., Du W., Yin Y., Nissinen K., Nybakken L., Guo H., Julkunen-Tiitto R. Effects of soil pyrene contamination on growth and phenolics in Norway spruce (Piceaabies) are modified by elevated temperature and CO2. Environ. Sci. Pollut. Res. 2018;25:12788–12799. doi: 10.1007/s11356-018-1564-7. [DOI] [PubMed] [Google Scholar]
- Zhao D.F., Buchholz A., Tillmann R., Kleist E., Wu C., Rubach F. Environmental conditions regulate the impact of plants on cloud formation. Nat. Commun. 2017;8:14067. doi: 10.1038/ncomms14067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.H., Jia X., Wang W.K., Liu T., Huang S.P., Yang M.Y. Growth under elevated air temperature alters secondary metabolites in Robiniapseudoacacia L. seedlings in Cd- and Pb-contaminated soils. Sci. Total Environ. 2016;565:586–594. doi: 10.1016/j.scitotenv.2016.05.058. [DOI] [PubMed] [Google Scholar]
- Zobayed S.M.A., Afreen F., Kozai T. Temperature stress can alter photosynthetic efficiency and secondary metabolite concentrations in St. John’s wort. Plant Physiol. Biochem. 2005;43:977–984. doi: 10.1016/j.plaphy.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Zu Y.G., Tang Z.H., Yu J.H., Liu S.G., Wang W., Guo X.R. Different responses of camptothecin and 10-hydroxycamptothecin to heat shock in Camptotheca acuminata seedlings. Acta Bot. Sin. 2003;45:809–814. [Google Scholar]
- Zhu J.J., Zhang J.L., Liu H.C., Cao K.F. Photosynthesis, non-photochemical pathways and activities of antioxidant enzymes in a resilient evergreen oak under different climatic conditions from a valley-savanna in Southwest China. Physiol. Plantarum. 2009;135:62–72. doi: 10.1111/j.1399-3054.2008.01171.x. [DOI] [PubMed] [Google Scholar]
- Zvereva E., Kozlov M. Consequences of simultaneous elevation of carbon dioxide and temperature for plant-herbivore interactions: a metaanalysis. Global Change Biol. 2006;12:27–41. [Google Scholar]
- Wang W., Vinocur B., Altman A. Plant responses to drought, salinity and extreme temperature towards genetic engineering for stress tolerance. Planta. 2003;218:1–14. doi: 10.1007/s00425-003-1105-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.