Abstract
The term NADome refers to the intricate network of intracellular and extracellular enzymes that regulate the synthesis or degradation of nicotinamide adenine dinucleotide (NAD) and to the receptors that engage it. Traditionally, NAD was linked to intracellular energy production through shuffling electrons between oxidized and reduced forms. However, recent data indicate that NAD, along with its biosynthetic and degrading enzymes, has a life outside of cells, possibly linked to immuno-modulating non-enzymatic activities. Extracellular NAD can engage puriginergic receptors triggering an inflammatory response, similar - to a certain extent – to what described for adenosine triphosphate (ATP). Likewise, NAD biosynthetic and degrading enzymes have been amply reported in the extracellular space, where they possess both enzymatic and non-enzymatic functions. Modulation of these enzymes has been described in several acute and chronic conditions, including obesity, cancer, inflammatory bowel diseases and sepsis. In this review, the role of the extracellular NADome will be discussed, focusing on its proposed role in immunomodulation, together with the different strategies for its targeting and their potential therapeutic impact.
Keywords: nucleotides, NAD, signaling, DAMPs, immune cell regulation, immunometabolism, tumor microenvironment
Introduction: The Many Faces of NAD, From Energetic Factor to Danger Signal
NAD is an essential intracellular metabolite with key roles in energy metabolism and electron transfer (1–7). In addition, NAD is a cofactor for different families of enzymes, including sirtuins and poly-ADP-ribosyl polymerases (PARPs). NAD can be present outside of cells, with levels fluctuating widely in response to extracellular signals (8–11). A firm observation is that under steady state extracellular (e)NAD levels are thousands of times lower (nM) compared to the intracellular ones (µM-mM) (7, 12–16).
However, during conditions of cellular stress, such as those observed in an inflamed microenvironment, or during hypoxia, or in conditions of shear stress due to physical distortion, plasma membrane damage, stress elicited by cytotoxic agents, NAD concentrations may rapidly spike. This observation, together with the finding that some purinergic receptors are activated by NAD suggested that eNAD serves as a “danger signal” that alerts the immune system to tissue damage (8–10, 12, 17–20). According to this view, eNAD could be considered as damage-associated molecular pattern molecule (DAMP), able to activate the innate immune system, like what has been shown for pathogen-associated molecular patterns (PAMPs) (18, 21–24). For example, released eNAD from active neuronal cells can serve as neurotransmitter and neuromodulator (25–27); or in a mouse model of inflammation, induced by injection of polyacrylamide beads, eNAD reached a concentration of 10mM acting as danger signal (28).
NAD release may occur by several mechanisms involving active exocytosis, or diffusion through transmembrane transporters (e.g., pannexin, connexin) in living cells, or passive leakage across the membrane from necrotic or injured cells (15, 29–32).
Homeostasis is rapidly restored through a scavenging circuit operated by nucleotide-catabolizing enzymes that produce the immunosuppressant adenosine (ADO) and inosine, which can re-enter the cell, reconstituting the nucleotide pool (5, 33–36). All these mechanisms of nucleotide/nucleoside release to alert or switch off the immune system, respectively, are enhanced during acute and chronic inflammation, including cancer (29, 37, 38). Even though very unlikely, eNAD synthesis has not been conclusively ruled out, also in consideration of the presence of several key NAD biosynthetic enzymes (NBEs) (16, 39).
Intracellular and Extracellular NAD-Metabolizing Machinery
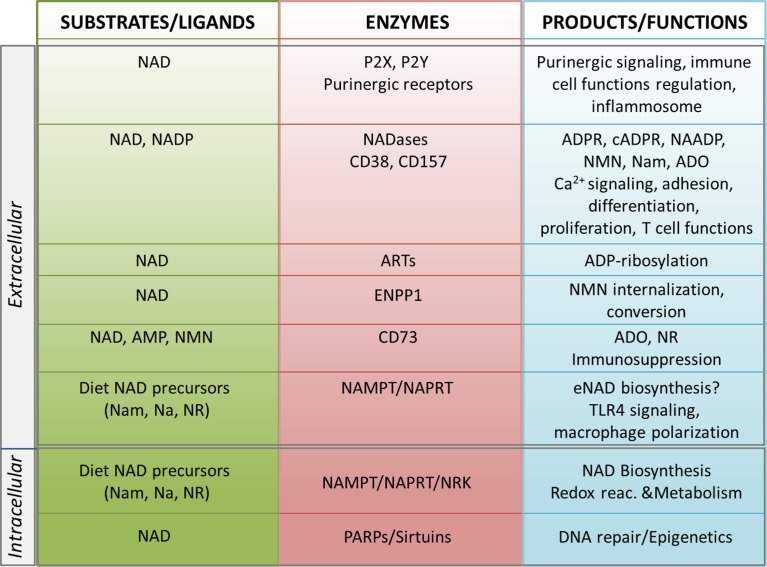
The biosynthesis of NAD takes place in different locations in the cell, through one de novo pathway starting from the catabolism of tryptophan, and via degradation of vitamin B3 precursors. The latter is considered a salvage pathway that occurs through the metabolism of three precursors [i.e. nicotinic acid (Na), nicotinamide (Nam) and nicotinamide riboside (NR)]. In the majority of tissues, intracellular NAD is generated mostly from Nam, which is the degradation product of all NAD-consuming signaling reactions (6, 40–42). Under normal conditions >70% of the cellular NAD content is stored and is utilized in the mitochondria primarily for metabolic purposes (16, 43). The cytosolic and nuclear NAD pools serve primarily to sustain activity of PARPs and sirtuins, which are NAD-dependent enzymes with key roles in regulating DNA repair and epigenetic controlling of gene transcription, respectively (Figure 1) (7, 44, 45). NAD levels can therefore restrict the activity of these two classes of NAD-metabolizing enzymes. Intriguingly, NAD can rapidly shuttle between different cellular compartments to reconstitute the pool that allows enzyme activation, as has recently been shown (46). When in the extracellular space, eNAD functions are linked to the modulation of cell surface P2X and P2Y purinergic receptor families, thereby acting in an apparently enzyme-independent way and eliciting pro-inflammatory immune responses (Figure 1). In addition, within the extracellular space, a complete network of different ectonucleotidases can rapidly hydrolyze eNAD generating intermediates that modulate signaling, cell metabolism, adhesion, migration and activate immunoregulatory circuits (14, 39, 47), as summarized in Figure 1. eNAD is degraded by different classes of ectoenzymes: the NADases CD38 and CD157 (48–50), the ADP-ribosyltransferases (ARTs) (51), the Ectonucleotide Pyrophosphatase/Phosphodiesterase 1 (ENPP1) and the ecto-5′-nucleotidase CD73 (34, 52, 53). NADase, ENPP1 and CD73 can lead to the formation of ADO, a potent natural immunosuppressive factor mediating the activation of the inhibitory P1 purinergic receptors (34, 54, 55). In addition, eNAD can be cleaved to nicotinamide mononucleotide (NMN) and subsequently dephosphorylated to NR by CD38 and CD73 (53, 56, 57). All these intermediates can enter the cell as NAD precursors and can be used by NBEs, reconstituting the intracellular pool (57, 58) (Figure 1).
Figure 1.
Schematic representation of the NADome. Schematic representation of the network of substrates/ligands, NAD-metabolizing cell surface and intracellular enzymes and their products in the extracellular and intracellular space. Biological functions regulated by NAD-related enzymes and products are listed. NAD, nicotinamide adenine dinucleotide; NADP, NAD phosphate; eNAD, extracellular NAD; Nam, nicotinamide; NR, nicotinamide riboside; Na, nicotinic acid; NAMPT, nicotinamide phosphoribosyltransferase; NAPRT, nicotinate phosphoribosyltransferase; NRK, nicotinamide riboside kinase; ARTs, mono adenosine diphosphate (ADP)-ribose transferases; PARPs, poly ADP-ribose polymerases; ADPR, ADP ribose; cADPR, cyclic ADP ribose; NAADP, nicotinic acid adenine dinucleotide phosphate; Ca2+, calcium; NMN, nicotinamide mononucleotide; ADO, adenosine; AMP, adenosine monophosphate; ENPP1; ectonucleotide pyrophosphatase/phosphodiesterases; TLR4, toll-like receptor 4.
In the next sections of this review, we will summarize the role of eNAD, its derived-metabolites and a set of NAD-dependent enzymes, giving examples of their role in the regulation of specific immune responses.
eNAD and Purinergic Receptors
The idea of purinergic signaling, i.e., of nucleotides acting as extracellular signaling molecules, was initially put forward by the seminal work of Geoff Burnstock in 1972 (59, 60).
Since then, this complex network of receptors has progressively been unveiled to reveal seven evolutionarily conserved subtypes of the P2X ion channel receptors and eight subtypes of the P2Y G protein-coupled receptor, all with roles in immune cell activation (5, 24, 61). On the contrary, four subtypes of the ADO P1 receptors on effector T cells have immunosuppressive effects. Shifting the balance from pro-inflammatory P2R signaling to anti-inflammatory P1R signaling or vice versa, the purinergic signaling system fine-tunes immune cell functions (5). eNAD can bind different subtypes of purinergic P2 receptors as summarized in Figure 2.
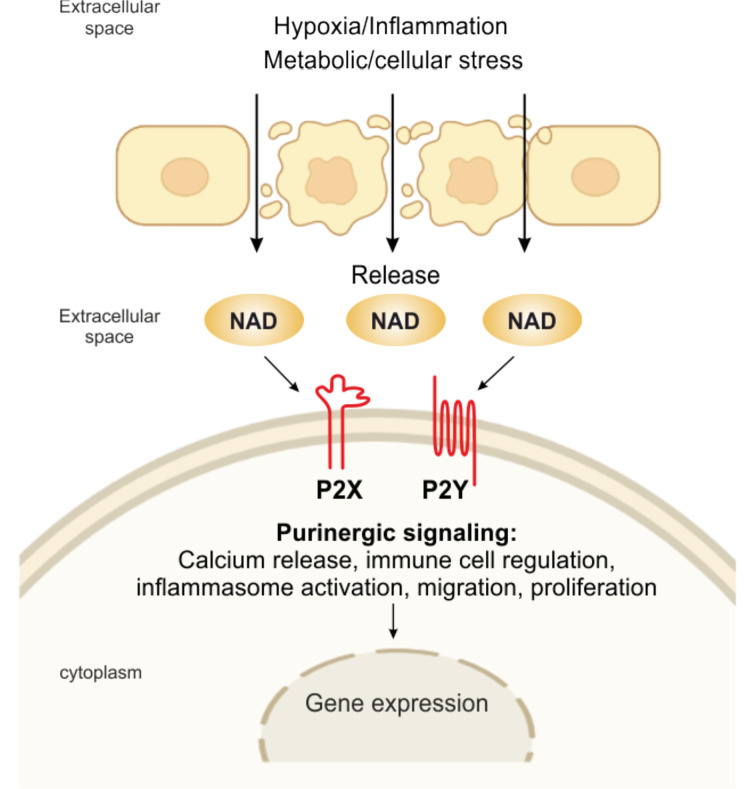
Figure 2.
eNAD and purinergic signaling. Pathological or physiological stimuli, including hypoxic and inflammatory conditions, metabolic and cellular stress, promote the release of NAD from the cell. NAD can then bind to P2X (isoforms P2X1, P2X4, P2X7) and P2Y (isoforms P2Y1, P2Y11), activating several intracellular signaling and modulating immune responses.
For example, eNAD activates human granulocytes by binding P2Y11 and triggering: (i) overproduction of cyclic (c)AMP, (ii) activation of protein kinase A, (iii) stimulation of ADP-ribosyl cyclase and overproduction of cyclic ADP-ribose (cADPR), a universal calcium (Ca2+) mobilizer, and (iv) influx of extracellular Ca2+, ultimately causing increased proliferation and migration (62). eNAD can bind P2Y1 and P2Y11 in human monocytes activated with lipopolysaccharide (LPS), triggering a transient rise in intracellular Ca2+, which is caused by a release of Ca2+ from IP (3)-responsive intracellular stores and an influx of extracellular Ca2+ (63). eNAD has also been identified as an agonist at P2Y1 receptors in human embryonic kidney (HEK) cells and mouse colonic muscle (27, 64). Moreover, binding to postsynaptic P2Y1 receptors, like ATP, eNAD also acts as a neurotransmitter, released by stimulated terminals of mammalian central nervous system and peripheral nervous system neurons (65). In addition, it has been shown that purinoceptors, including P2X1, P2X4, and P2X7, are engaged in eNAD-mediated signaling (27, 63, 66). However, more experimental data should be published to confirm this direct binding of NAD per se.
eNAD may also engage P2X7R receptors, the main eATP receptor, extensively studied in the context of inflammation and immunity (24). P2X7R signaling is a major regulator of the intensity and duration of inflammatory responses (24, 67, 68). The receptor/channel is prominently expressed on all cells of innate and adaptive immunity and aberrant signaling has been linked to diverse inflammatory and autoimmune diseases, as recently reviewed in (5, 24). P2X7R signaling mediates NLR family pyrin domain containing 3 (NLRP3) inflammasome activation, cytokine, and chemokine release [i.e., interleukin (IL)-1β, tumor necrosis factor (TNF), IL-6, monocyte chemoattractant protein-1 (MCP-1/CCL2)], T lymphocyte survival and differentiation, transcription factor activation, and cell death (24, 69, 70). At inflammatory sites, P2X7R could also be bound directly by alternative ligands, including eNAD that accumulates at sites of inflammation and tissue damage (28). In murine T lymphocytes, eNAD serves as an ADP-ribose donor to ADP-ribosylate the P2X7R at arginine 125, close to the ATP-binding pocket (71). This reaction, catalyzed by the plasma membrane enzyme ART2.2, causes long-lasting activation of mouse P2X7R, negatively affecting T-regulatory (Treg) and natural killer T (NKT) cell survival and arguing in favor of a direct role of eNAD in the pathophysiological mechanism of P2X7R activation. The reduction of Treg function via NAD-induced gating of P2X7 can be employed in vivo as a strategy to promote the antitumor response of effector T cells. Systemic injection of NAD results in the selective depletion of Tregs via NAD-mediated activation of P2X7, which enhances anticancer immune responses in several mouse tumor models (20, 72). While highly interesting and potentially relevant for human immune responses, this mechanism of P2X7R activation via eNAD is demonstrated only in mouse models: additional research is needed to determine whether it is relevant for human immune responses too.
eNAD Degradation-Signaling System in Regulating Immune Responses
One of the reasons why eNAD levels are generally low is that there are several extracellular enzymes that rapidly transform it, guaranteeing recycling of a high energy molecule through the generation of products that can be easily up-taken by cells. The intermediates, however, have a life of their own as signaling molecules, thereby modulating activity of immune cells. The best-known NAD-degrading/signaling systems rely on the activity of CD38, an immunomodulatory enzyme (Figure 3).
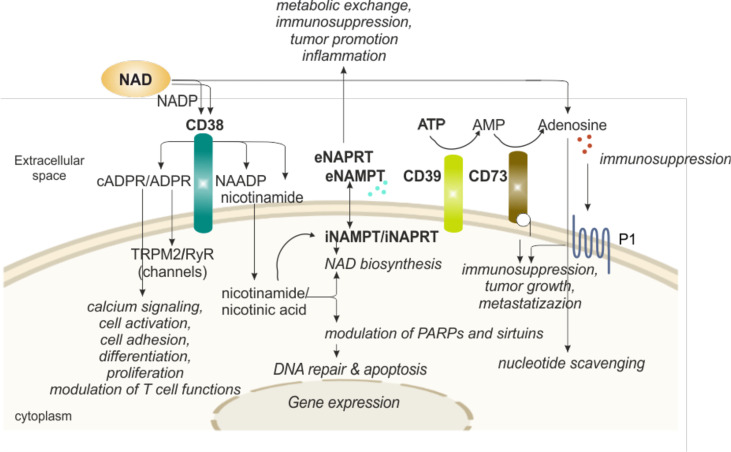
Figure 3.
eNAD and enzymatic/functional extracellular machinery in regulating immune responses. Extracellular NAD can also be metabolized by a series of enzymes of the cell surface that are involved in scavenging of nucleotides. The end product of the reaction, adenosine, can then be internalized and reconverted to related nucleosides (e.g., ATP or NAD). In particular, CD38 hydrolyzes NAD to generate intermediates (cADPR and ADPR), potent intracellular Ca2+-mobilizing agents, through binding RyR or TRPM2 receptors. CD38 activation induces migration, proliferation, and modulation of immune responses, specifically T cell functions, as detailed in the text. In addition, CD38 activity releases nicotinamide (the main NAD precursor) that can be internalized into the cell and, together with a second precursor nicotinic acid, converted in NAD by NAMPT and NAPRT activities, respectively, increasing intracellular NAD levels and affecting sirtuins and PARPs (NAD-consuming enzymes) functions. NAMPT and NAPRT can be secreted/released in the extracellular space acting as cytokine-like proteins. Finally, NAD and ATP can be converted in adenosine. ATP is metabolized by CD39 to AMP that is further hydrolyzed by ecto-5’-nucleotidase/CD73 which promotes the formation of adenosine. Adenosine then activates adenosine receptors (purinergic receptor P1). The final outcome depends on the relative concentrations of substrates and products and on the expression of nucleotide-metabolizing ecto-enzymes and NAD-biosynthetic enzymes. NAD, nicotinamide adenine dinucleotide; NADP, NAD phosphate; NAMPT, nicotinamide phosphoribosyltransferase; NAPRT, nicotinate phosphoribosyltransferase; PARPs, poly ADP-ribose polymerases; ADPR, ADP ribose; cADPR, cyclic ADP ribose; NAADP, nicotinic acid adenine dinucleotide phosphate; ATP, adenosine triphosphate; AMP, adenosine monophosphate; P1, adenosine purinergic receptor; RYR, ryanodine receptors; TRPM2, transient receptor potential melastatin-related 2; i, intracellular; e, extracellular.
Human CD38, the main member of the NADase/ADPR cyclase family that includes also CD157/BST-1, is a surface glycoprotein characterized by a relatively large extracellular domain that contains the catalytic site, a single transmembrane pass, and a short cytoplasmic tail (73, 74). CD38 is a multifunctional ectoenzyme, involved in the catabolism and degradation of eNAD (under normal pH) and NAD phosphate (NADP, under acidic pH), producing ADP ribose (ADPR) together with signaling metabolites involved in intracellular Ca2+ mobilization. The main catalytic activity is the NAD glycohydrolase that generates Nam and ADPR. CD38 can also act as NAD cyclase, producing cADPR, which is then hydrolyzed to ADPR. Lastly, in the presence of NADP and Na, under acidic pH levels, CD38 can generate nicotinic acid adenine dinucleotide phosphate (NAADP) (49). The finding of an extracellular enzymatic activity of CD38 leading to the generation of messengers that enter cells to induce intracellular Ca2+ fluxes remains an unsolved “topological paradox” (49, 75). More recent data have enriched the picture by showing that CD38 can also be found in the nucleus and mitochondrial membrane and that a soluble form of CD38 is most likely present in the cytoplasm, leading to the hypothesis of a compartmentalized generation of NAD-derived signaling metabolites (49, 76–78). ADPR, cADPR and NAADP share the ability to mobilize Ca2+ ions from intracellular stores: cADPR binds to ryanodine receptors (RyR) expressed on the endoplasmic reticulum, ADPR binds to membrane melastatin related transient receptor potential cation channels TRPM2 (49, 79) and NAADP binds to receptors expressed by acidic organelles, such as lysosomes, suggesting a role as Ca2+ messenger in the endocytic pathway (80). It is therefore likely that during an immune response, NAD, released outside of cells due to local conditions of inflammation and cellular damage is converted into Ca2+-active metabolites through the action of CD38 expressed by activated lymphocytes, which in turn contribute to lymphocyte activation through Ca2+ signaling (80–82).
There is a second alternative possibility that is gaining momentum in the context of tumor immunosuppression. According to this hypothesis, ADPR could also be short-circuited to ADO via the action of CD203, which generates AMP from ADPR and CD73 (53, 83, 84), which cleaves the last phosphate, generating ADO (49). In this way, CD38 could contribute to the generation of a tumor-favorable environment, as recently demonstrated in tumors characterized by a large T cell infiltrate (85). Therefore, it seems that according to the environment, CD38 can generate both immune-boosting and immune-suppressive metabolites, thereby activating or suppressing immune responses.
These at times opposing roles of CD38 in defining immune responses are in part confirmed by studies on CD38-deficient mice. Interestingly, when the animals are kept in clean facilities without infectious challenges, they grow and develop normally, without major defects (86). On the other hand, during infections they show impaired lymphocyte activation and homing and are ultimately more susceptible to death due to sepsis (87, 88). CD38-deficient animals also show reduced tumor formation, attributed to the lack CD38-mediated immunosuppression.
In the human system, CD38 is widely expressed on the surface of immune cells, particularly in conditions of cellular activation. On the cell surface, CD38 is part of the immunological synapse, forming lateral associations with critical receptors on T, B, and myeloid cells, thereby positioning itself at the center of action (48, 49). In fact, it was reported that CD38 localizes in close contact with T cell receptor (TCR), the B cell receptor (BCR), and key chemokine receptors, among other molecules (89). Perhaps the best understood function of CD38 is in the regulation of T lymphocyte functions, where the enzyme works again different ways (90–93).
First, CD38-dependent-Ca2+ signaling directly contributes to T cell activation, likely providing an essential second signal that drives gene expression and consequently differentiation, development, and cytotoxicity (93–95).
As a second level of T cell regulation, the NAD/CD38 axis was proposed to control T cell metabolic reprogramming needed for full T lymphocyte activation through the modulation of sirtuin activity (90, 96). Several studies are shedding light on this molecular circuit as an important metabolic checkpoint contributing to several aspects of cellular energy metabolisms, including glycolysis, oxidative phosphorylation (OXPHOS), glutaminolysis, which are strictly associated with T cell functional fate (90, 93, 97, 98). According to the models proposed, expression of CD38 on the cell surface would limit intracellular NAD levels, negatively impacting on the activities of the NAD-dependent enzymes SIRT1 and SIRT3, which are deacetylases with fundamental roles in epigenetic regulation (93). Lastly, recent data indicate that CD38 is highly expressed by specific subsets of immunosuppressive tumor infiltrating lymphocytes, including regulatory T cells and T helper 17 cells (90, 99–101). Expression of the molecule occurs often in association with exhaustion markers, such as programmed cell death protein 1 (PD-1), pointing to an active role of CD38 in modulating T cell fate toward the generation of an immune tolerant landscape in tumors, likely through the generation of ADO (90) (Figure 3).
What remains unclear so far is what are the factors that tip the balance in favor of Ca2+-active metabolites and hence immune activation or in favor of ADO and hence immunosuppression (93, 94, 102–104). Therefore, inhibition of CD38 is a valid therapeutic strategy to reestablish a functional immune surveillance (105), open the way to combination therapies with immune checkpoint inhibitors, as discussed in a separate paragraph.
eNAD Biosynthetic-Signaling System in Regulating Immune Responses
Beside NAD-consuming, also NBEs were reported in the extracellular compartment. The best known and characterized among them is nicotidamide phosphoribosyltransferase (NAMPT), which catalyzes the conversion of Nam to NMN in the presence of phosphoribosyl pyrophosphate (PRPP) and ATP (7, 44).
The presence of NAMPT in biological fluids is now well established: however, several years were needed before realizing that a cytokine promoting B cell differentiation and originally described in mid-nineties (106), and an extracellular adipokine called visfatin were in fact the same protein as NAMPT (11, 107, 108). Of note, different cell types, including neutrophils, monocytes, macrophages, and cancer cells secrete eNAMPT in the extracellular space in response to inflammation, cellular stress, infections, and hypoxic conditions, among others. In human plasma eNAMPT normal levels are in the low nanomolar range (2-4 ng/ml), but it is over-expressed in several inflammatory and metabolic disorders, including cancer, where concentrations can increase 10-20 times (11, 108).
The second NBE dosed in biological fluids is nicotinic acid phosphoribosyltransferase (NAPRT), which controls the NAD generation pathway starting from Na. While the NAMPT pathway is probably the predominant one in most cells and tissues, considering that all NAD-consuming enzymes generate Nam, the activity of NAPRT is believed to boost NAD levels in stress conditions (44, 109–111). Information on eNAPRT is far more limited, even though concentration data indicate again a physiological level in low nanomolar range (1-2 ng/ml), raising sometimes dramatically, particularly during sepsis (112).
Whether these enzymes are active in the extracellular compartment remains uncertain, mainly because of the absence of detectable PRPP levels, an essential co-factor to produce NMN and nicotinic acid mononucleotide (NaMN). In addition, the rest of the enzymatic cascade producing NAD has never been reported in the extracellular space (109). From data present in the literature, we can exclude a direct eNAD synthesis in physiological conditions, but we cannot exclude a site-specific and transient eNAD synthesis in inflammatory conditions, due to release of intracellular molecules (ATP, PRPP) and enzymes. In favor of a compartment-specific function, the active forms of these enzymes are in a dimeric conformation, but within the extracellular compartment they should be in a monomeric, and hence inactive, form (113). Lastly, functional studies have shown that eNAMPT and eNAPRT, genetically modified to be enzymatically inactive, retain their pro-inflammatory properties (112) (Figure 3).
A second area of investigation concerns the mechanisms of trafficking of these enzymes from the intracellular to the extracellular space, which appear “non-classical”, as secretion is unaffected by monensin and brefeldin A, two inhibitors of the classical endoplasmatic reticulum (ER)–Golgi secretory pathway (114–117). An interesting finding indicates that NAMPT secretion could be regulated through SIRT1- and SIRT6-deacetylation, thereby linking NAD-biosynthetic and -consuming enzymes, and potentially suggesting eNAMPT secretion as regulatory mechanism to decrease its intracellular concentrations (118, 119). Recent evidence showed that eNAMPT is carried in extracellular vesicles (EVs) through systemic circulation in mice and humans. EV-contained-eNAMPT is internalized into cells, enhancing NMN and hence NAD synthesis (120). eNAMPT is actively secreted via exosomes also from microglia during neuroinflammation due to ischemic injury (121). These findings support the possibility of metabolic exchange between tumor/inflammatory and immune cells and vice versa within the site of inflammation or the tumor microenvironment (TME), as previously described for other cytokines and metabolic molecules (122, 123).
The conclusion from these data is that outside of cells it is unlikely that NAMPT and NAPRT function as NAD-producing enzymes, raising the alternative possibility that they possess different functions. In fact, eNAMPT can directly bind Toll-like receptor 4 (TLR4) (112, 124) and C–C chemokine receptor type 5 (CCR5) (125), which might explain how the protein is involved in the activation of an inflammatory signature. The binding with TLR4 was demonstrated in different cellular models, leading to activation of specific intracellular signaling pathways (e.g., STAT3, NF-κB, Akt, P38) within minutes, and activation of inflammasome in few hours (112, 124).
Less recently, it was reported that eNAMPT can selectively inhibit infection of monocytes by human immunodeficiency virus (HIV) and this activity was linked to a direct interaction with CCR5, shown using surface plasmon resonance (SPR) (126). More recently, Torretta et al. suggested that eNAMPT acts as a natural antagonist of CCR5 in cancer cells (125). Within the cancer microenvironment, eNAMPT seems to contribute to shape an immunotolerant environment, mostly acting on the myeloid component. We described a role for eNAMPT in the differentiation of circulating monocytes from chronic lymphocytic leukemia (CLL) patients toward tumor-supporting M2 macrophages (127). Recently, it was demonstrated that iNAMPT acts also on myeloid-derived suppressor cells (MDSCs) via a SIRT1/hypoxia-inducible factor (HIF)-1α axis, promoting their mobilization (128). The activation of these circuits creates an immunosuppressive and tumor-promoting microenvironment (Figure 3).
Much less is known on eNAPRT, even though from early information it seems to possess properties similar to NAMPT when in extracellular fluids. Managò et al. demonstrated that eNAPRT binds TLR4 on macrophages triggering NF-kB activation and pro-inflammatory cytokines secretion (112). Moreover, eNAPRT shares with eNAMPT the activation of a transcriptional program, maybe mediated by the induction of macrophage colony-stimulating factor (M-CFS), to force monocyte differentiation into macrophages. In turn, macrophages are a source of eNAMPT and eNAPRT in vivo (112). Even if several issues remain to be investigated, a functional role of these enzymes in primary innate immunity responses is clearly emerging, opening the way to target these enzymes to modulate inflammation.
Is the NADome a Therapeutic Target?
Alterations in the NADome have been described in several human diseases, including inflammatory conditions (gastric and intestinal inflammatory diseases, graft-versus-host disease, sepsis and multiple organ failure, allergies particularly in the lungs, atherosclerosis, age-associated insulin resistance, neuroinflammation/degeneration), autoimmune diseases (multiple sclerosis, psoriasis, systemic lupus erithematosous), cardiovascular diseases and cancer (7, 55, 129).
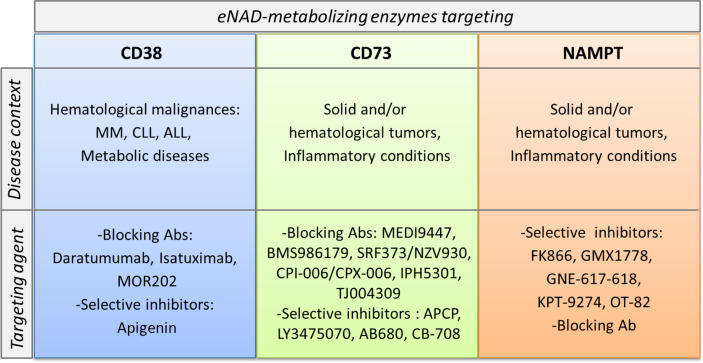
In addition to their role in shaping the immune system and in creating immunosuppressive conditions, in some instances NAD-metabolizing enzymes are considered biological prognostic markers and therapeutic targets. Among them, the most promising are CD38, CD73 and NAMPT and the disease setting is cancer (Figure 4).
Figure 4.
CD38, CD73 and NAMPT as markers and therapeutic targets in pathological conditions. CD38, CD73 and NAMPT expression levels increase in several pathological conditions. These molecules become markers of aggressive disease. In the lower part of the cartoon specific pharmacological inhibitors and/or blocking antibodies, currently in preclinical or clinical trials for each target are listed.
CD38 is expressed in hematological malignancies, including acute B lymphoblastic leukemia (B-ALL), acute myeloid leukemia (AML), mantle cell lymphoma (MCL), CLL, multiple myeloma (MM) and NK/T cell leukemia (T-ALL) (55, 94, 102, 105, 130–132). The role of CD38 has been widely explored and defined in CLL and in MM. On CLL B lymphocytes, CD38 associates with the BCR complex [BCR/CD81/CD19/CD21] and cooperates to amplify the signal transduction driving cell proliferation (55, 133, 134).
Patients with CLL with a higher proportion of leukemic cells expressing CD38 ≥30% experience a shorter time to first treatment and a more aggressive clinical course with inferior overall survival compared to patients who have <30% of CD38+ CLL cells, thus establishing surface CD38 as a marker of poor prognosis (135–137).
MM is a plasma cell neoplastic aggressive disease with a median overall survival of 4.4–7.1 years (138). CD38 is highly and ubiquitously expressed on MM cells and at low levels on normal lymphoid and myeloid cells (49, 139). Daratumumab is a first-in-class anti-CD38 therapeutic monoclonal antibody (mAb) approved in 2015 for the treatment of relapsed/refractory MM (140). The documented mechanisms of action include antibody-dependent cell cytotoxicity (ADCC), complement dependent cytotoxicity (CDC), antibody-dependent cellular phagocytosis (ADCP), and inhibition of CD38 enzymatic activities and induction of apoptosis in a caspase-dependent manner (132, 141, 142). This Ab is now used in combination with other drugs; however, the density of CD38 molecules on MM cells is a predictive factor to the efficacy and durability of daratumumab treatment (143). In CLL, CD38 engagement by daratumumab modulates BCR signaling and enhances the anti-CLL activity of ibrutinib, an inhibitor of BCR signaling (144). In addition, CD38 is highly expressed in different solid tumors (i.e., gliomas, pancreatic cancer, non-small cell lung cancer, melanoma, hepatocellular carcinoma), generally associated to increased aggressiveness and creating a tumor-supporting microenvironment (145), providing a rationale for the expansion of daratumumab’s field of action.
Targeting CD73 to interfere with the degradation of AMP into ADO, reducing the generation of an immunosuppressed and pro-angiogenic niche that promotes the onset and progression of cancer, is an attractive therapeutic option (146). CD73 expression is higher in the majority of human solid tumors. Its expression and activity are closely associated with tumor invasiveness and metastasis (147, 148).
Inhibition of CD73 using either mAb or small molecule inhibitors such as a,b-methylene-ADP (APCP) have demonstrated antitumor activities in preclinical tumor mouse models (148, 149). Furthermore, a number of anti-CD73 mAbs (MEDI9447, BMS986179, SRF373/NZV930, CPI-006/CPX-006, IPH5301, TJ004309) and selective inhibitors (LY3475070, AB680, CB-708) are being tested in early phase clinical trials, as recently reviewed in (147, 150).
Therefore, combination therapies with CD73 blocking Abs or small molecule inhibitors and other therapeutic strategies including immune checkpoint blockade, adoptive T cell therapy, agonistic immunotherapy, chemotherapy, and radiation therapy, could have synergic effects in various cancers boosting immune response to keep the tumor cells in control, as emerged by recent studies (148, 151).
The first NAMPT inhibitor FK866 (also known as APO866) was described in 2003 by Hasmann et al. (152) Since that, several specific NAMPT inhibitors were developed as recently reviewed in in (7, 153, 154). The rationale was mainly supported by the over-expression of NAMPT in cancer cells, as extensively described by us and by several research groups (11, 108, 117, 127, 155–158).
This led to a first wave of molecules that entered clinical trials for cancer therapy; however, no molecules reported to have progressed to later stages [www.clinicaltrials.gov (7, 153)].
Toxicity of old inhibitors and rescue mechanisms by the activation of other NBEs following NAMPT block, have limited the use of NAMPT inhibitors as single agents. However, increasing evidence suggests that a better selection of tumor subtype rely exclusively on NAMPT activity to generate NAD, as well as novel drugs less toxic, could open a second life for NAMPT inhibition strategy. Moreover, a combination between NAMPT inhibitors and selective inhibitors of oncogenic signaling driving cancer progression could be therapeutically exploited as suggested (11, 117, 159).
An unknown notion is whether these inhibitors could also affect eNAMPT activity, even if, as mentioned before, the enzymatic activity of eNAMPT is controversial. Travelli et al. developed novel inhibitors that can’t cross the plasma membrane and have more activity to block eNAMPT form, demonstrating reduced growth of triple negative mammary carcinoma in mice (160). On the other hand, there is also intense research to develop a blocking antibody to neutralize eNAMPT and reduce its “cytokine-like activity” within the TME. The group of Garcia firstly has devised a polyclonal eNAMPT neutralizing antibody (pAb) (161). They used this Ab in different models of inflammation and cancer, including lung injury and prostate cancer. Recently, in acute respiratory distress syndrome (ARDS) they demonstrated the highly significant contribution of endothelial cell (EC)-derived NAMPT to the severity of inflammatory lung injury in preclinical ARDS models. Intravenous delivery of either eNAMPT-neutralizing pAb/mAb significantly attenuated inflammatory lung injury in mouse model. In vitro studies on EC demonstrated that eNAMPT-neutralizing antibodies strongly abrogate eNAMPT-induced TLR4 pathway activation (162). In invasive prostate cancer (PCa) Sun et al. proved the activity of eNAMPT in supporting the invasive features of PCa, and the tumor blocking activity of the anti-eNAMPT neutralizing antibody in a pre-clinical in vivo model of PCa invasion (163). In parallel, the group of Prof. Genazzani in Italy is developing a novel monoclonal antibody (C269) that neutralizes in vitro the cytokine-like action of eNAMPT and that reduces its serum levels in rodents. This Ab is able to significantly reduce acute and chronic colitis in two models of induced-colitis (164), suggesting a role of eNAMPT in the pathogenesis of inflammatory bowel disease (IBD) and the therapeutic potential of its neutralization in this pathology. The general idea of targeting eNAMPT in tumors and in inflammatory diseases is increasing to counteract the extracellular functions of this protein, mainly linked to the activation of TLR4 and modulation of immune responses. The best option could be to combine i/eNAMPT targeting with immunomodulatory agents to obtain a tumor growth regression and a concomitant reversion of immunosuppressive conditions, acting on the immune system. In support of this, two papers demonstrated that NAMPT inhibitors enhance the anti-tumor efficacy of immune checkpoint inhibitors, i.e. antibody against PD-1 (128, 165).
Conclusions and Future Perspectives
Since the discovery of the presence of extracellular nucleotides such as ATP and NAD released from intracellular stores in conditions of cell stress or inflammation, they are considered “danger signals” to alert the immune system, participating in the recruitment, activation, and differentiation of immune cells, and promoting the production and release of pro-inflammatory cytokines. Within the TME, extracellular nucleotides create pro-tumor conditions acting directly on tumor aggressive features but also on immune cells promoting a general immunosuppression.
The extracellular machinery that regulates eNAD functions is complex, as we summarized in this review several eNAD-metabolizing enzymes rapidly degrade it into metabolites that in turn can function as signaling messengers or can be internalized and used to reconstitute the intracellular NAD pool. Directly, eNAD can bind purinergic receptors and activate signaling. The effects of eNAD are therefore dependent on the presence of receptors, metabolizing enzymes and cellular stress conditions within the microenvironment. Understanding this intricate machinery remains the most important challenge to develop therapeutic strategies to modulate expression of these extracellular nucleotides, relative enzymes, and receptors to re-educate the immune system in different diseases, including cancer.
Author Contributions
SD designed and reviewed the work, which was assembled by VA, with contribution of VM and LB. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Ministry of Education University and Research-MIUR, PRIN Project 2017CBNCYT and Progetto strategico di Eccellenza Dipartimentale #D15D18000410001 (the latter awarded to the Dept. of Medical Sciences, University of Turin) and ITN INTEGRATA program (grant agreement 813284), and by Associazione Italiana Ricerca sul Cancro (AIRC), Investigator Grant –IG 2019 #23095.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science (2009) 324(5930):1029–33. 10.1126/science.1160809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonora M, Patergnani S, Rimessi A, De Marchi E, Suski JM, Bononi A, et al. ATP Synthesis and Storage. Purinergic Signal (2012) 8(3):343–57. 10.1007/s11302-012-9305-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiao W, Wang RS, Handy DE, Loscalzo J. NAD(H) and NADP(H) Redox Couples and Cellular Energy Metabolism. Antioxid Redox Signal (2018) 28(3):251–72. 10.1089/ars.2017.7216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yaku K, Okabe K, Nakagawa T. NAD Metabolism: Implications in Aging and Longevity. Ageing Res Rev (2018) 47:1–17. 10.1016/j.arr.2018.05.006 [DOI] [PubMed] [Google Scholar]
- 5.Linden J, Koch-Nolte F, Dahl G. Purine Release, Metabolism, and Signaling in the Inflammatory Response. Annu Rev Immunol (2019) 37:325–47. 10.1146/annurev-immunol-051116-052406 [DOI] [PubMed] [Google Scholar]
- 6.Amjad S, Nisar S, Bhat AA, Shah AR, Frenneaux MP, Fakhro K, et al. Role of NAD(+) in Regulating Cellular and Metabolic Signaling Pathways. Mol Metab (2021) 49:101195. 10.1016/j.molmet.2021.101195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie N, Zhang L, Gao W, Huang C, Huber PE, Zhou X, et al. NAD(+) Metabolism: Pathophysiologic Mechanisms and Therapeutic Potential. Signal Transduct Target Ther (2020) 5(1):227. 10.1038/s41392-020-00311-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Virgilio F, Sarti AC, Falzoni S, De Marchi E, Adinolfi E. Extracellular ATP and P2 Purinergic Signalling in the Tumour Microenvironment. Nat Rev Cancer (2018) 18(10):601–18. 10.1038/s41568-018-0037-0 [DOI] [PubMed] [Google Scholar]
- 9.Haag F, Adriouch S, Brass A, Jung C, Moller S, Scheuplein F, et al. Extracellular NAD and ATP: Partners in Immune Cell Modulation. Purinergic Signal (2007) 3(1-2):71–81. 10.1007/s11302-006-9038-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheuplein F, Schwarz N, Adriouch S, Krebs C, Bannas P, Rissiek B, et al. NAD+ and ATP Released From Injured Cells Induce P2X7-Dependent Shedding of CD62L and Externalization of Phosphatidylserine by Murine T Cells. J Immunol (2009) 182(5):2898–908. 10.4049/jimmunol.0801711 [DOI] [PubMed] [Google Scholar]
- 11.Audrito V, Messana VG, Deaglio S. NAMPT and NAPRT: Two Metabolic Enzymes With Key Roles in Inflammation. Front Oncol (2020) 10:358. 10.3389/fonc.2020.00358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pellegatti P, Raffaghello L, Bianchi G, Piccardi F, Pistoia V, Di Virgilio F. Increased Level of Extracellular ATP at Tumor Sites: In Vivo Imaging With Plasma Membrane Luciferase. PloS One (2008) 3(7):e2599. 10.1371/journal.pone.0002599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilhelm K, Ganesan J, Muller T, Durr C, Grimm M, Beilhack A, et al. Graft-Versus-Host Disease Is Enhanced by Extracellular ATP Activating P2X7R. Nat Med (2010) 16(12):1434–8. 10.1038/nm.2242 [DOI] [PubMed] [Google Scholar]
- 14.O’Reilly T, Niven DF. Levels of Nicotinamide Adenine Dinucleotide in Extracellular Body Fluids of Pigs may be Growth-Limiting for Actinobacillus Pleuropneumoniae and Haemophilus Parasuis. Can J Vet Res (2003) 67(3):229–31. [PMC free article] [PubMed] [Google Scholar]
- 15.Billington RA, Bruzzone S, De Flora A, Genazzani AA, Koch-Nolte F, Ziegler M, et al. Emerging Functions of Extracellular Pyridine Nucleotides. Mol Med (2006) 12(11-12):324–7. 10.2119/2006-00075.Billington [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Stefano M, Conforti L. Diversification of NAD Biological Role: The Importance of Location. FEBS J (2013) 280(19):4711–28. 10.1111/febs.12433 [DOI] [PubMed] [Google Scholar]
- 17.Trautmann A. Extracellular ATP in the Immune System: More Than Just a “Danger Signal”. Sci Signal (2009) 2(56):pe6. 10.1126/scisignal.256pe6 [DOI] [PubMed] [Google Scholar]
- 18.Corriden R, Insel PA. Basal Release of ATP: An Autocrine-Paracrine Mechanism for Cell Regulation. Sci Signal (2010) 3(104):re1. 10.1126/scisignal.3104re1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schilling E, Hauschildt S. Extracellular ATP Induces P2X7-Dependent Nicotinamide Phosphoribosyltransferase Release in LPS-Activated Human Monocytes. Innate Immun (2012) 18(5):738–44. 10.1177/1753425912439614 [DOI] [PubMed] [Google Scholar]
- 20.Adriouch S, Haag F, Boyer O, Seman M, Koch-Nolte F. Extracellular NAD(+): A Danger Signal Hindering Regulatory T Cells. Microbes Infect (2012) 14(14):1284–92. 10.1016/j.micinf.2012.05.011 [DOI] [PubMed] [Google Scholar]
- 21.Bianchi ME. DAMPs, PAMPs and Alarmins: All We Need to Know About Danger. J Leukoc Biol (2007) 81(1):1–5. 10.1189/jlb.0306164 [DOI] [PubMed] [Google Scholar]
- 22.Venereau E, Ceriotti C, Bianchi ME. DAMPs From Cell Death to New Life. Front Immunol (2015) 6:422. 10.3389/fimmu.2015.00422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka K, Choi J, Cao Y, Stacey G. Extracellular ATP Acts as a Damage-Associated Molecular Pattern (DAMP) Signal in Plants. Front Plant Sci (2014) 5:446. 10.3389/fpls.2014.00446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S. The P2X7 Receptor in Infection and Inflammation. Immunity (2017) 47(1):15–31. 10.1016/j.immuni.2017.06.020 [DOI] [PubMed] [Google Scholar]
- 25.Smyth LM, Bobalova J, Mendoza MG, Lew C, Mutafova-Yambolieva VN. Release of Beta-Nicotinamide Adenine Dinucleotide Upon Stimulation of Postganglionic Nerve Terminals in Blood Vessels and Urinary Bladder. J Biol Chem (2004) 279(47):48893–903. 10.1074/jbc.M407266200M407266200[pii [DOI] [PubMed] [Google Scholar]
- 26.Breen LT, Smyth LM, Yamboliev IA, Mutafova-Yambolieva VN. Beta-NAD Is a Novel Nucleotide Released on Stimulation of Nerve Terminals in Human Urinary Bladder Detrusor Muscle. Am J Physiol Renal Physiol (2006) 290(2):F486–95. 10.1152/ajprenal.00314.2005 [DOI] [PubMed] [Google Scholar]
- 27.Mutafova-Yambolieva VN, Hwang SJ, Hao X, Chen H, Zhu MX, Wood JD, et al. Beta-Nicotinamide Adenine Dinucleotide Is an Inhibitory Neurotransmitter in Visceral Smooth Muscle. Proc Natl Acad Sci USA (2007) 104(41):16359–64. 10.1073/pnas.0705510104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adriouch S, Hubert S, Pechberty S, Koch-Nolte F, Haag F, Seman M. NAD(+) Released During Inflammation Participates in T Cell Homeostasis by Inducing ART2-Mediated Death of Naive T Cells In Vivo . J Immunol (2007) 179(1):186–94. 10.4049/jimmunol.179.1.186 [DOI] [PubMed] [Google Scholar]
- 29.Cekic C, Linden J. Purinergic Regulation of the Immune System. Nat Rev Immunol (2016) 16(3):177–92. 10.1038/nri.2016.4 [DOI] [PubMed] [Google Scholar]
- 30.Bruzzone S, Guida L, Zocchi E, Franco L, De Flora A. Connexin 43 Hemi Channels Mediate Ca2+-Regulated Transmembrane NAD+ Fluxes in Intact Cells. FASEB J (2001) 15(1):10–2. 10.1096/fj.00-0566fje [DOI] [PubMed] [Google Scholar]
- 31.Hwang SJ, Durnin L, Dwyer L, Rhee PL, Ward SM, Koh SD, et al. Beta-Nicotinamide Adenine Dinucleotide Is an Enteric Inhibitory Neurotransmitter in Human and Nonhuman Primate Colons. Gastroenterology (2011) 140(2):608–17 e6. 10.1053/j.gastro.2010.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mottahedeh J, Haffner MC, Grogan TR, Hashimoto T, Crowell PD, Beltran H, et al. CD38 is Methylated in Prostate Cancer and Regulates Extracellular NAD(). Cancer Metab (2018) 6:13. 10.1186/s40170-018-0186-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sitkovsky MV, Ohta A. The ‘Danger’ Sensors That STOP the Immune Response: The A2 Adenosine Receptors? Trends Immunol (2005) 26(6):299–304. 10.1016/j.it.2005.04.004 [DOI] [PubMed] [Google Scholar]
- 34.Deaglio S, Robson SC. Ectonucleotidases as Regulators of Purinergic Signaling in Thrombosis, Inflammation, and Immunity. Adv Pharmacol (2011) 61:301–32. 10.1016/B978-0-12-385526-8.00010-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaisitti T, Audrito V, Serra S, Bologna C, Brusa D, Malavasi F, et al. NAD+-Metabolizing Ecto-Enzymes Shape Tumor-Host Interactions: The Chronic Lymphocytic Leukemia Model. FEBS Lett (2011) 585(11):1514–20. 10.1016/j.febslet.2011.04.036 [DOI] [PubMed] [Google Scholar]
- 36.Kazemi MH, Raoofi Mohseni S, Hojjat-Farsangi M, Anvari E, Ghalamfarsa G, Mohammadi H, et al. Adenosine and Adenosine Receptors in the Immunopathogenesis and Treatment of Cancer. J Cell Physiol (2018) 233(3):2032–57. 10.1002/jcp.25873 [DOI] [PubMed] [Google Scholar]
- 37.Di Virgilio F. Purines, Purinergic Receptors, and Cancer. Cancer Res (2012) 72(21):5441–7. 10.1158/0008-5472.CAN-12-16000008-5472.CAN-12-1600[pii [DOI] [PubMed] [Google Scholar]
- 38.Di Virgilio F, Sarti AC, Coutinho-Silva R. Purinergic Signaling, DAMPs, and Inflammation. Am J Physiol Cell Physiol (2020) 318(5):C832–C5. 10.1152/ajpcell.00053.2020 [DOI] [PubMed] [Google Scholar]
- 39.Gasparrini M, Sorci L, Raffaelli N. Enzymology of Extracellular NAD Metabolism. Cell Mol Life Sci (2021) 78(7):3317–31. 10.1007/s00018-020-03742-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Audrito V, Messana VG, Moiso E, Vitale N, Arruga F, Brandimarte L, et al. NAMPT Over-Expression Recapitulates the BRAF Inhibitor Resistant Phenotype Plasticity in Melanoma. Cancers (Basel) (2020) 12(12):3855–77. 10.3390/cancers12123855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Houtkooper RH, Canto C, Wanders RJ, Auwerx J. The Secret Life of NAD+: An Old Metabolite Controlling New Metabolic Signaling Pathways. Endocr Rev (2010) 31(2):194–223. 10.1210/er.2009-0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hassinen IE. Signaling and Regulation Through the NAD(+) and NADP(+) Networks. Antioxid Redox Signal (2019) 30(6):857–74. 10.1089/ars.2017.7479 [DOI] [PubMed] [Google Scholar]
- 43.Nikiforov A, Dolle C, Niere M, Ziegler M. Pathways and Subcellular Compartmentation of NAD Biosynthesis in Human Cells: From Entry of Extracellular Precursors to Mitochondrial NAD Generation. J Biol Chem (2011) 286(24):21767–78. 10.1074/jbc.M110.213298M110.213298[pii [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruggieri S, Orsomando G, Sorci L, Raffaelli N. Regulation of NAD Biosynthetic Enzymes Modulates NAD-Sensing Processes to Shape Mammalian Cell Physiology Under Varying Biological Cues. Biochim Biophys Acta (2015) 1854(9):1138–49. 10.1016/j.bbapap.2015.02.021 [DOI] [PubMed] [Google Scholar]
- 45.Canto C, Menzies KJ, Auwerx J. NAD(+) Metabolism and the Control of Energy Homeostasis: A Balancing Act Between Mitochondria and the Nucleus. Cell Metab (2015) 22(1):31–53. 10.1016/j.cmet.2015.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grolla AA, Miggiano R, Di Marino D, Bianchi M, Gori A, Orsomando G, et al. A Nicotinamide Phosphoribosyltransferase-GAPDH Interaction Sustains the Stress-Induced NMN/NAD(+) Salvage Pathway in the Nucleus. J Biol Chem (2020) 295(11):3635–51. 10.1074/jbc.RA119.010571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nikiforov A, Kulikova V, Ziegler M. The Human NAD Metabolome: Functions, Metabolism and Compartmentalization. Crit Rev Biochem Mol Biol (2015) 50(4):284–97. 10.3109/10409238.2015.1028612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chini EN. CD38 as a Regulator of Cellular NAD: A Novel Potential Pharmacological Target for Metabolic Conditions. Curr Pharm Des (2009) 15(1):57–63. 10.2174/138161209787185788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malavasi F, Deaglio S, Funaro A, Ferrero E, Horenstein AL, Ortolan E, et al. Evolution and Function of the ADP Ribosyl Cyclase/CD38 Gene Family in Physiology and Pathology. Physiol Rev (2008) 88(3):841–86. 10.1152/physrev.00035.2007 [DOI] [PubMed] [Google Scholar]
- 50.Deaglio S, Malavasi F. The CD38/CD157 Mammalian Gene Family: An Evolutionary Paradigm for Other Leukocyte Surface Enzymes. Purinergic Signaling (2006) 2:431–41. 10.1007/s11302-006-9002-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seman M, Adriouch S, Haag F, Koch-Nolte F. Ecto-ADP-Ribosyltransferases (ARTs): Emerging Actors in Cell Communication and Signaling. Curr Med Chem (2004) 11(7):857–72. 10.2174/0929867043455611 [DOI] [PubMed] [Google Scholar]
- 52.Katada T, Kontani K, Wada T, Hosoda N, Hoshino S, Nishina H. Enzymic and Signal Transduction Properties of CD38/NADase and PC-1/Phosphodiesterase. Chem Immunol (2000) 75:60–78. 10.1159/000058762 [DOI] [PubMed] [Google Scholar]
- 53.Garavaglia S, Bruzzone S, Cassani C, Canella L, Allegrone G, Sturla L, et al. The High-Resolution Crystal Structure of Periplasmic Haemophilus Influenzae NAD Nucleotidase Reveals a Novel Enzymatic Function of Human CD73 Related to NAD Metabolism. Biochem J (2012) 441(1):131–41. 10.1042/BJ20111263 [DOI] [PubMed] [Google Scholar]
- 54.Horenstein AL, Chillemi A, Zaccarello G, Bruzzone S, Quarona V, Zito A, et al. A CD38/CD203a/CD73 Ectoenzymatic Pathway Independent of CD39 Drives a Novel Adenosinergic Loop in Human T Lymphocytes. Oncoimmunology (2013) 2(9):e26246. 10.4161/onci.26246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vaisitti T, Arruga F, Guerra G, Deaglio S. Ectonucleotidases in Blood Malignancies: A Tale of Surface Markers and Therapeutic Targets. Front Immunol (2019) 10:2301. 10.3389/fimmu.2019.02301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kemmer G, Reilly TJ, Schmidt-Brauns J, Zlotnik GW, Green BA, Fiske MJ, et al. NadN and E (P4) are Essential for Utilization of NAD and Nicotinamide Mononucleotide But Not Nicotinamide Riboside in Haemophilus Influenzae. J Bacteriol (2001) 183(13):3974–81. 10.1128/JB.183.13.3974-3981.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grozio A, Sociali G, Sturla L, Caffa I, Soncini D, Salis A, et al. CD73 Protein as a Source of Extracellular Precursors for Sustained NAD+ Biosynthesis in FK866-Treated Tumor Cells. J Biol Chem (2013) 288(36):25938–49. 10.1074/jbc.M113.470435M113.470435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sociali G, Raffaghello L, Magnone M, Zamporlini F, Emionite L, Sturla L, et al. Antitumor Effect of Combined NAMPT and CD73 Inhibition in an Ovarian Cancer Model. Oncotarget (2015) 7(3):2968–84. 10.18632/oncotarget.65026502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burnstock G. Purinergic Nerves. Pharmacol Rev (1972) 24(3):509–81. [PubMed] [Google Scholar]
- 60.Burnstock G, Verkhratsky A. Evolutionary Origins of the Purinergic Signalling System. Acta Physiol (Oxf) (2009) 195(4):415–47. 10.1111/j.1748-1716.2009.01957.x [DOI] [PubMed] [Google Scholar]
- 61.Burnstock G. Purine and Purinergic Receptors. Brain Neurosci Adv (2018) 2:2398212818817494. 10.1177/2398212818817494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moreschi I, Bruzzone S, Nicholas RA, Fruscione F, Sturla L, Benvenuto F, et al. Extracellular NAD+ Is an Agonist of the Human P2Y11 Purinergic Receptor in Human Granulocytes. J Biol Chem (2006) 281(42):31419–29. 10.1074/jbc.M606625200 [DOI] [PubMed] [Google Scholar]
- 63.Klein C, Grahnert A, Abdelrahman A, Muller CE, Hauschildt S. Extracellular NAD(+) Induces a Rise in [Ca(2+)](i) in Activated Human Monocytes via Engagement of P2Y(1) and P2Y(11) Receptors. Cell Calcium (2009) 46(4):263–72. 10.1016/j.ceca.2009.08.004 [DOI] [PubMed] [Google Scholar]
- 64.Alefishat E, Alexander SP, Ralevic V. Effects of NAD at Purine Receptors in Isolated Blood Vessels. Purinergic Signal (2015) 11(1):47–57. 10.1007/s11302-014-9428-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Durnin L, Hwang SJ, Ward SM, Sanders KM, Mutafova-Yambolieva VN. Adenosine 5-Diphosphate-Ribose Is a Neural Regulator in Primate and Murine Large Intestine Along With Beta-NAD(+). J Physiol (2012) 590(Pt 8):1921–41. 10.1113/jphysiol.2011.222414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grahnert A, Klein C, Hauschildt S. Involvement of P2X Receptors in the NAD(+)-Induced Rise in [Ca (2+)] (I) in Human Monocytes. Purinergic Signal (2009) 5(3):309–19. 10.1007/s11302-009-9144-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khakh BS, North RA. P2X Receptors as Cell-Surface ATP Sensors in Health and Disease. Nature (2006) 442(7102):527–32. 10.1038/nature04886 [DOI] [PubMed] [Google Scholar]
- 68.Dubyak GR. P2X7 Receptor Regulation of Non-Classical Secretion From Immune Effector Cells. Cell Microbiol (2012) 14(11):1697–706. 10.1111/cmi.12001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adinolfi E, Giuliani AL, De Marchi E, Pegoraro A, Orioli E, Di Virgilio F. The P2X7 Receptor: A Main Player in Inflammation. Biochem Pharmacol (2018) 151:234–44. 10.1016/j.bcp.2017.12.021 [DOI] [PubMed] [Google Scholar]
- 70.Giuliani AL, Sarti AC, Falzoni S, Di Virgilio F. The P2X7 Receptor-Interleukin-1 Liaison. Front Pharmacol (2017) 8:123. 10.3389/fphar.2017.00123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Seman M, Adriouch S, Scheuplein F, Krebs C, Freese D, Glowacki G, et al. NAD-Induced T Cell Death: ADP-Ribosylation of Cell Surface Proteins by ART2 Activates the Cytolytic P2X7 Purinoceptor. Immunity (2003) 19(4):571–82. 10.1016/S1074-7613(03)00266-8 [DOI] [PubMed] [Google Scholar]
- 72.Hubert S, Rissiek B, Klages K, Huehn J, Sparwasser T, Haag F, et al. Extracellular NAD+ Shapes the Foxp3+ Regulatory T Cell Compartment Through the ART2-P2X7 Pathway. J Exp Med (2010) 207(12):2561–8. 10.1084/jem.20091154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alessio M, Roggero S, Funaro A, De Monte LB, Peruzzi L, Geuna M, et al. CD38 Molecule: Structural and Biochemical Analysis on Human T Lymphocytes, Thymocytes, and Plasma Cells. J Immunol (1990) 145(3):878–84. [PubMed] [Google Scholar]
- 74.Malavasi F, Deaglio S, Ferrero E, Funaro A, Sancho J, Ausiello CM, et al. CD38 and CD157 as Receptors of the Immune System: A Bridge Between Innate and Adaptive Immunity. Mol Med (2006) 12(11-12):334–41. 10.2119/2006-00094.Malavasi [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Flora A, Guida L, Franco L, Zocchi E. The CD38/cyclic ADP-Ribose System: A Topological Paradox. Int J Biochem Cell Biol (1997) 29(10):1149–66. 10.1016/s1357-2725(97)00062-9 [DOI] [PubMed] [Google Scholar]
- 76.Zhao YJ, Lam CM, Lee HC. The Membrane-Bound Enzyme CD38 Exists in Two Opposing Orientations. Sci Signal (2012) 5(241):ra67. 10.1126/scisignal.2002700 [DOI] [PubMed] [Google Scholar]
- 77.Shrimp JH, Hu J, Dong M, Wang BS, MacDonald R, Jiang H, et al. Revealing CD38 Cellular Localization Using a Cell Permeable, Mechanism-Based Fluorescent Small-Molecule Probe. J Am Chem Soc (2014) 136(15):5656–63. 10.1021/ja411046j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu J, Zhao YJ, Li WH, Hou YN, Li T, Zhao ZY, et al. Cytosolic Interaction of Type III Human CD38 With CIB1 Modulates Cellular Cyclic ADP-Ribose Levels. Proc Natl Acad Sci U.S.A. (2017) 114(31):8283–8. 10.1073/pnas.1703718114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sumoza-Toledo A, Penner R. TRPM2: A Multifunctional Ion Channel for Calcium Signalling. J Physiol (2011) 589(Pt 7):1515–25. 10.1113/jphysiol.2010.201855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee HC. Structure and Enzymatic Functions of Human CD38. Mol Med (2006) 12(11-12):317–23. 10.2119/2006-00086.Lee [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Adebanjo OA, Anandatheerthavarada HK, Koval AP, Moonga BS, Biswas G, Sun L, et al. A New Function for CD38/ADP-Ribosyl Cyclase in Nuclear Ca2+ Homeostasis. Nat Cell Biol (1999) 1(7):409–14. 10.1038/15640 [DOI] [PubMed] [Google Scholar]
- 82.Guse AH. Cyclic ADP-Ribose: A Novel Ca2+-Mobilising Second Messenger. Cell Signal (1999) 11(5):309–16. 10.1016/S0898-6568(99)00004-2 [DOI] [PubMed] [Google Scholar]
- 83.Colgan SP, Eltzschig HK, Eckle T, Thompson LF. Physiological Roles for Ecto-5’-Nucleotidase (CD73). Purinergic Signal (2006) 2(2):351–60. 10.1007/s11302-005-5302-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Allard D, Allard B, Gaudreau PO, Chrobak P, Stagg J. CD73-Adenosine: A Next-Generation Target in Immuno-Oncology. Immunotherapy (2016) 8(2):145–63. 10.2217/imt.15.106 [DOI] [PubMed] [Google Scholar]
- 85.Chen Z, Han ZC. STAT3: A Critical Transcription Activator in Angiogenesis. Med Res Rev (2008) 28(2):185–200. 10.1002/med.20101 [DOI] [PubMed] [Google Scholar]
- 86.Fukushi Y, Kato I, Takasawa S, Sasaki T, Ong BH, Sato M, et al. Identification of Cyclic ADP-Ribose-Dependent Mechanisms in Pancreatic Muscarinic Ca2+ Signaling Using CD38 Knockout Mice. J Biol Chem (2001) 276(1):649–55. 10.1074/jbc.M004469200 [DOI] [PubMed] [Google Scholar]
- 87.Partida-Sanchez S, Cockayne DA, Monard S, Jacobson EL, Oppenheimer N, Garvy B, et al. Cyclic ADP-Ribose Production by CD38 Regulates Intracellular Calcium Release, Extracellular Calcium Influx and Chemotaxis in Neutrophils and Is Required for Bacterial Clearance In Vivo . Nat Med (2001) 7(11):1209–16. 10.1038/nm1101-1209 [DOI] [PubMed] [Google Scholar]
- 88.Partida-Sanchez S, Goodrich S, Kusser K, Oppenheimer N, Randall TD, Lund FE. Regulation of Dendritic Cell Trafficking by the ADP-Ribosyl Cyclase CD38: Impact on the Development of Humoral Immunity. Immunity (2004) 20(3):279–91. 10.1016/S1074-7613(04)00048-2 [DOI] [PubMed] [Google Scholar]
- 89.Munoz P, Mittelbrunn M, de la Fuente H, Perez-Martinez M, Garcia-Perez A, Ariza-Veguillas A, et al. Antigen-Induced Clustering of Surface CD38 and Recruitment of Intracellular CD38 to the Immunologic Synapse. Blood (2008) 111(7):3653–64. 10.1182/blood-2007-07-101600 [DOI] [PubMed] [Google Scholar]
- 90.Chatterjee S, Daenthanasanmak A, Chakraborty P, Wyatt MW, Dhar P, Selvam SP, et al. CD38-NAD(+)Axis Regulates Immunotherapeutic Anti-Tumor T Cell Response. Cell Metab (2018) 27(1):85–100.e8. 10.1016/j.cmet.2017.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Krejcik J, Casneuf T, Nijhof IS, Verbist B, Bald J, Plesner T, et al. Daratumumab Depletes CD38+ Immune Regulatory Cells, Promotes T-Cell Expansion, and Skews T-Cell Repertoire in Multiple Myeloma. Blood (2016) 128(3):384–94. 10.1182/blood-2015-12-687749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sharif T, Martell E, Dai C, Ghassemi-Rad MS, Kennedy BE, Lee PWK, et al. Regulation of Cancer and Cancer-Related Genes via NAD. Antioxid Redox Signal (2018) 30(6):906–23. 10.1089/ars.2017.7478 [DOI] [PubMed] [Google Scholar]
- 93.Kar A, Mehrotra S, Chatterjee S. CD38: T Cell Immuno-Metabolic Modulator. Cells (2020) 9(7):1716–36. 10.3390/cells9071716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hogan KA, Chini CCS, Chini EN. The Multi-Faceted Ecto-Enzyme CD38: Roles in Immunomodulation, Cancer, Aging, and Metabolic Diseases. Front Immunol (2019) 10:1187. 10.3389/fimmu.2019.01187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Feske S. Calcium Signalling in Lymphocyte Activation and Disease. Nat Rev Immunol (2007) 7(9):690–702. 10.1038/nri2152 [DOI] [PubMed] [Google Scholar]
- 96.Jeng MY, Hull PA, Fei M, Kwon HS, Tsou CL, Kasler H, et al. Metabolic Reprogramming of Human CD8(+) Memory T Cells Through Loss of SIRT1. J Exp Med (2018) 215(1):51–62. 10.1084/jem.20161066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Geltink RIK, Kyle RL, Pearce EL. Unraveling the Complex Interplay Between T Cell Metabolism and Function. Annu Rev Immunol (2018) 36:461–88. 10.1146/annurev-immunol-042617-053019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chang HC, Guarente L. SIRT1 and Other Sirtuins in Metabolism. Trends Endocrinol Metab (2014) 25(3):138–45. 10.1016/j.tem.2013.12.001S1043-2760(13)00206-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Feng X, Zhang L, Acharya C, An G, Wen K, Qiu L, et al. Targeting CD38 Suppresses Induction and Function of T Regulatory Cells to Mitigate Immunosuppression in Multiple Myeloma. Clin Cancer Res (2017) 23(15):4290–300. 10.1158/1078-0432.CCR-16-3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Newton R, Priyadharshini B, Turka LA. Immunometabolism of Regulatory T Cells. Nat Immunol (2016) 17(6):618–25. 10.1038/ni.3466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang L, Xu H, Peng G. TLR-Mediated Metabolic Reprogramming in the Tumor Microenvironment: Potential Novel Strategies for Cancer Immunotherapy. Cell Mol Immunol (2018) 15(5):428–37. 10.1038/cmi.2018.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Morandi F, Airoldi I, Marimpietri D, Bracci C, Faini AC, Gramignoli R. CD38, A Receptor With Multifunctional Activities: From Modulatory Functions on Regulatory Cell Subsets and Extracellular Vesicles, to a Target for Therapeutic Strategies. Cells (2019) 8(12):1527–44. 10.3390/cells8121527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hartman WR, Pelleymounter LL, Moon I, Kalari K, Liu M, Wu TY, et al. CD38 Expression, Function, and Gene Resequencing in a Human Lymphoblastoid Cell Line-Based Model System. Leuk Lymphoma (2010) 51(7):1315–25. 10.3109/10428194.2010.483299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Glaria E, Valledor AF. Roles of CD38 in the Immune Response to Infection. Cells (2020) 9(1):228–44. 10.3390/cells9010228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chini EN, Chini CCS, Espindola Netto JM, de Oliveira GC, van Schooten W. The Pharmacology of CD38/NADase: An Emerging Target in Cancer and Diseases of Aging. Trends Pharmacol Sci (2018) 39(4):424–36. 10.1016/j.tips.2018.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I. Cloning and Characterization of the cDNA Encoding a Novel Human Pre-B-Cell Colony-Enhancing Factor. Mol Cell Biol (1994) 14(2):1431–7. 10.1128/mcb.14.2.1431-1437.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rongvaux A, Shea RJ, Mulks MH, Gigot D, Urbain J, Leo O, et al. Pre-B-Cell Colony-Enhancing Factor, Whose Expression Is Up-Regulated in Activated Lymphocytes, is a Nicotinamide Phosphoribosyltransferase, a Cytosolic Enzyme Involved in NAD Biosynthesis. Eur J Immunol (2002) 32(11):3225–34. [DOI] [PubMed] [Google Scholar]
- 108.Heske CM. Beyond Energy Metabolism: Exploiting the Additional Roles of NAMPT for Cancer Therapy. Front Oncol (2019) 9:1514. 10.3389/fonc.2019.01514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hara N, Yamada K, Shibata T, Osago H, Tsuchiya M. Nicotinamide Phosphoribosyltransferase/Visfatin Does Not Catalyze Nicotinamide Mononucleotide Formation in Blood Plasma. PloS One (2011) 6(8):e22781. 10.1371/journal.pone.0022781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Galassi L, Di Stefano M, Brunetti L, Orsomando G, Amici A, Ruggieri S, et al. Characterization of Human Nicotinate Phosphoribosyltransferase: Kinetic Studies, Structure Prediction and Functional Analysis by Site-Directed Mutagenesis. Biochimie (2012) 94(2):300–9. 10.1016/j.biochi.2011.06.033 [DOI] [PubMed] [Google Scholar]
- 111.Duarte-Pereira S, Pereira-Castro I, Silva SS, Correia MG, Neto C, da Costa LT, et al. Extensive Regulation of Nicotinate Phosphoribosyltransferase (NAPRT) Expression in Human Tissues and Tumors. Oncotarget (2016) 7(2):1973–83. 10.18632/oncotarget.6538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Manago A, Audrito V, Mazzola F, Sorci L, Gaudino F, Gizzi K, et al. Extracellular Nicotinate Phosphoribosyltransferase Binds Toll Like Receptor 4 and Mediates Inflammation. Nat Commun (2019) 10(1):4116. 10.1038/s41467-019-12055-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sayers SR, Beavil RL, Fine NHF, Huang GC, Choudhary P, Pacholarz KJ, et al. Structure-Functional Changes in eNAMPT at High Concentrations Mediate Mouse and Human Beta Cell Dysfunction in Type 2 Diabetes. Diabetologia (2020) 63(2):313–23. 10.1007/s00125-019-05029-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Revollo JR, Korner A, Mills KF, Satoh A, Wang T, Garten A, et al. Nampt/PBEF/Visfatin Regulates Insulin Secretion in Beta Cells as a Systemic NAD Biosynthetic Enzyme. Cell Metab (2007) 6(5):363–75. 10.1016/j.cmet.2007.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tanaka M, Nozaki M, Fukuhara A, Segawa K, Aoki N, Matsuda M, et al. Visfatin is Released From 3T3-L1 Adipocytes via a Non-Classical Pathway. Biochem Biophys Res Commun (2007) 359(2):194–201. 10.1016/j.bbrc.2007.05.096 [DOI] [PubMed] [Google Scholar]
- 116.Grolla AA, Torretta S, Gnemmi I, Amoruso A, Orsomando G, Gatti M, et al. Nicotinamide Phosphoribosyltransferase (NAMPT/PBEF/visfatin) is a Tumoural Cytokine Released From Melanoma. Pigment Cell Melanoma Res (2015) 28(6):718–29. 10.1111/pcmr.12420 [DOI] [PubMed] [Google Scholar]
- 117.Audrito V, Manago A, La Vecchia S, Zamporlini F, Vitale N, Baroni G, et al. Nicotinamide Phosphoribosyltransferase (NAMPT) as a Therapeutic Target in BRAF-Mutated Metastatic Melanoma. J Natl Cancer Inst (2018) 110(3):290–303. 10.1093/jnci/djx198 [DOI] [PubMed] [Google Scholar]
- 118.Yoon MJ, Yoshida M, Johnson S, Takikawa A, Usui I, Tobe K, et al. SIRT1-Mediated eNAMPT Secretion From Adipose Tissue Regulates Hypothalamic NAD+ and Function in Mice. Cell Metab (2015) 21(5):706–17. 10.1016/j.cmet.2015.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sociali G, Grozio A, Caffa I, Schuster S, Becherini P, Damonte P, et al. SIRT6 Deacetylase Activity Regulates NAMPT Activity and NAD(P)(H) Pools in Cancer Cells. FASEB J (2019) 33(3):3704–17. 10.1096/fj.201800321R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yoshida M, Satoh A, Lin JB, Mills KF, Sasaki Y, Rensing N, et al. Extracellular Vesicle-Contained eNAMPT Delays Aging and Extends Lifespan in Mice. Cell Metab (2019) 30(2):329–42 e5. 10.1016/j.cmet.2019.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lu YB, Chen CX, Huang J, Tian YX, Xie X, Yang P, et al. Nicotinamide Phosphoribosyltransferase Secreted From Microglia via exosome during ischemic injury. J Neurochem (2019) 150(6):723–37. 10.1111/jnc.14811 [DOI] [PubMed] [Google Scholar]
- 122.Chiarugi P, Cirri P. Metabolic Exchanges Within Tumor Microenvironment. Cancer Lett (2015) 380(1):272–80. 10.1016/j.canlet.2015.10.027 [DOI] [PubMed] [Google Scholar]
- 123.Kaymak I, Williams KS, Cantor JR, Jones RG. Immunometabolic Interplay in the Tumor Microenvironment. Cancer Cell (2021) 39(1):28–37. 10.1016/j.ccell.2020.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Camp SM, Ceco E, Evenoski CL, Danilov SM, Zhou T, Chiang ET, et al. Unique Toll-Like Receptor 4 Activation by NAMPT/PBEF Induces NFkappaB Signaling and Inflammatory Lung Injury. Sci Rep (2015) 5:13135. 10.1038/srep13135srep13135[pii [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Torretta S, Colombo G, Travelli C, Boumya S, Lim D, Genazzani AA, et al. The Cytokine Nicotinamide Phosphoribosyltransferase (eNAMPT; PBEF; Visfatin) Acts as a Natural Antagonist of C-C Chemokine Receptor Type 5 (CCR5). Cells (2020) 9(2):495–509. 10.3390/cells9020496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Van den Bergh R, Morin S, Sass HJ, Grzesiek S, Vekemans M, Florence E, et al. Monocytes Contribute to Differential Immune Pressure on R5 Versus X4 HIV Through the Adipocytokine Visfatin/NAMPT. PloS One (2012) 7(4):e35074. 10.1371/journal.pone.0035074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Audrito V, Serra S, Brusa D, Mazzola F, Arruga F, Vaisitti T, et al. Extracellular Nicotinamide Phosphoribosyltransferase (NAMPT) Promotes M2 Macrophage Polarization in Chronic Lymphocytic Leukemia. Blood (2015) 125(1):111–23. 10.1182/blood-2014-07-589069blood-2014-07-589069[pii [DOI] [PubMed] [Google Scholar]
- 128.Travelli C, Consonni FM, Sangaletti S, Storto M, Morlacchi S, Grolla AA, et al. Nicotinamide Phosphoribosyltransferase (NAMPT) Acts as a Metabolic Gate for Mobilization of Myeloid-Derived Suppressor Cells. Cancer Res (2019) 79(8):1938–51. 10.1158/0008-5472.CAN-18-1544 [DOI] [PubMed] [Google Scholar]
- 129.Audrito V, Manago A, Gaudino F, Sorci L, Messana VG, Raffaelli N, et al. NAD-Biosynthetic and Consuming Enzymes as Central Players of Metabolic Regulation of Innate and Adaptive Immune Responses in Cancer. Front Immunol (2019) 10:1720. 10.3389/fimmu.2019.01720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Naik J, Themeli M, de Jong-Korlaar R, Ruiter RWJ, Poddighe PJ, Yuan H, et al. CD38 as a Therapeutic Target for Adult Acute Myeloid Leukemia and T-Cell Acute Lymphoblastic Leukemia. Haematologica (2019) 104(3):e100–e3. 10.3324/haematol.2018.192757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zeijlemaker W, Grob T, Meijer R, Hanekamp D, Kelder A, Carbaat-Ham JC, et al. CD34(+)CD38(-) Leukemic Stem Cell Frequency to Predict Outcome in Acute Myeloid Leukemia. Leukemia (2019) 33(5):1102–12. 10.1038/s41375-018-0326-3 [DOI] [PubMed] [Google Scholar]
- 132.van de Donk N, Richardson PG, Malavasi F. CD38 Antibodies in Multiple Myeloma: Back to the Future. Blood (2018) 131(1):13–29. 10.1182/blood-2017-06-740944 [DOI] [PubMed] [Google Scholar]
- 133.Damle RN, Temburni S, Calissano C, Yancopoulos S, Banapour T, Sison C, et al. CD38 Expression Labels an Activated Subset Within Chronic Lymphocytic Leukemia Clones Enriched in Proliferating B Cells. Blood (2007) 110(9):3352–9. 10.1182/blood-2007-04-083832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Malavasi F, Deaglio S, Damle R, Cutrona G, Ferrarini M, Chiorazzi N. CD38 and Chronic Lymphocytic Leukemia: A Decade Later. Blood (2011) 118(13):3470–8. 10.1182/blood-2011-06-275610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Damle RN, Wasil T, Fais F, Ghiotto F, Valetto A, Allen SL, et al. Ig V Gene Mutation Status and CD38 Expression as Novel Prognostic Indicators in Chronic Lymphocytic Leukemia. Blood (1999) 94(6):1840–7. 10.1182/blood.V94.6.1840 [DOI] [PubMed] [Google Scholar]
- 136.Durig J, Naschar M, Schmucker U, Renzing-Kohler K, Holter T, Huttmann A, et al. CD38 Expression Is an Important Prognostic Marker in Chronic Lymphocytic Leukaemia. Leukemia: Off J Leukemia Soc America Leukemia Res Fund UK (2002) 16(1):30–5. 10.1038/sj.leu.2402339 [DOI] [PubMed] [Google Scholar]
- 137.Deaglio S, Vaisitti T, Zucchetto A, Gattei V, Malavasi F. CD38 as a Molecular Compass Guiding Topographical Decisions of Chronic Lymphocytic Leukemia Cells. Semin Cancer Biol (2010) 20(6):416–23. 10.1016/j.semcancer.2010.08.003 [DOI] [PubMed] [Google Scholar]
- 138.Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al. Improved Survival in Multiple Myeloma and the Impact of Novel Therapies. Blood (2008) 111(5):2516–20. 10.1182/blood-2007-10-116129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kawano Y, Moschetta M, Manier S, Glavey S, Gorgun GT, Roccaro AM, et al. Targeting the Bone Marrow Microenvironment in Multiple Myeloma. Immunol Rev (2015) 263(1):160–72. 10.1111/imr.12233 [DOI] [PubMed] [Google Scholar]
- 140.Lonial S, Weiss BM, Usmani SZ, Singhal S, Chari A, Bahlis NJ, et al. Daratumumab Monotherapy in Patients With Treatment-Refractory Multiple Myeloma (SIRIUS): An Open-Label, Randomised, Phase 2 Trial. Lancet (2016) 387(10027):1551–60. 10.1016/S0140-6736(15)01120-4 [DOI] [PubMed] [Google Scholar]
- 141.van der Veer MS, de Weers M, van Kessel B, Bakker JM, Wittebol S, Parren PW, et al. The Therapeutic Human CD38 Antibody Daratumumab Improves the Anti-Myeloma Effect of Newly Emerging Multi-Drug Therapies. Blood Cancer J (2011) 1(10):e41. 10.1038/bcj.2011.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Khagi Y, Mark TM. Potential Role of Daratumumab in the Treatment of Multiple Myeloma. Onco Targets Ther (2014) 7:1095–100. 10.2147/OTT.S49480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Garcia-Guerrero E, Gotz R, Doose S, Sauer M, Rodriguez-Gil A, Nerreter T, et al. Upregulation of CD38 Expression on Multiple Myeloma Cells by Novel HDAC6 Inhibitors Is a Class Effect and Augments the Efficacy of Daratumumab. Leukemia (2021) 35(1):201–14. 10.1038/s41375-020-0840-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Manna A, Aulakh S, Jani P, Ahmed S, Akhtar S, Coignet M, et al. Targeting CD38 Enhances the Antileukemic Activity of Ibrutinib in Chronic Lymphocytic Leukemia. Clin Cancer Res (2019) 25(13):3974–85. 10.1158/1078-0432.CCR-18-3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wo YJ, Gan ASP, Lim X, Tay ISY, Lim S, Lim JCT, et al. The Roles of CD38 and CD157 in the Solid Tumor Microenvironment and Cancer Immunotherapy. Cells (2019) 9(1):26–44. 10.3390/cells9010026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Antonioli L, Yegutkin GG, Pacher P, Blandizzi C, Hasko G. Anti-CD73 in Cancer Immunotherapy: Awakening New Opportunities. Trends Cancer (2016) 2(2):95–109. 10.1016/j.trecan.2016.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Roh M, Wainwright DA, Wu JD, Wan Y, Zhang B. Targeting CD73 to Augment Cancer Immunotherapy. Curr Opin Pharmacol (2020) 53:66–76. 10.1016/j.coph.2020.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chen S, Wainwright DA, Wu JD, Wan Y, Matei DE, Zhang Y, et al. CD73: An Emerging Checkpoint for Cancer Immunotherapy. Immunotherapy (2019) 11(11):983–97. 10.2217/imt-2018-0200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Allard D, Chrobak P, Allard B, Messaoudi N, Stagg J. Targeting the CD73-Adenosine Axis in Immuno-Oncology. Immunol Lett (2019) 205:31–9. 10.1016/j.imlet.2018.05.001 [DOI] [PubMed] [Google Scholar]
- 150.Perrot I, Michaud HA, Giraudon-Paoli M, Augier S, Docquier A, Gros L, et al. Blocking Antibodies Targeting the CD39/CD73 Immunosuppressive Pathway Unleash Immune Responses in Combination Cancer Therapies. Cell Rep (2019) 27(8):2411. 10.1016/j.celrep.2019.04.091 [DOI] [PubMed] [Google Scholar]
- 151.Allard B, Allard D, Buisseret L, Stagg J. The Adenosine Pathway in Immuno-Oncology. Nat Rev Clin Oncol (2020) 17(10):611–29. 10.1038/s41571-020-0382-2 [DOI] [PubMed] [Google Scholar]
- 152.Hasmann M, Schemainda I. FK866, a Highly Specific Noncompetitive Inhibitor of Nicotinamide Phosphoribosyltransferase, Represents a Novel Mechanism for Induction of Tumor Cell Apoptosis. Cancer Res (2003) 63(21):7436–42. [PubMed] [Google Scholar]
- 153.Galli U, Colombo G, Travelli C, Tron GC, Genazzani AA, Grolla AA. Recent Advances in NAMPT Inhibitors: A Novel Immunotherapic Strategy. Front Pharmacol (2020) 11:656. 10.3389/fphar.2020.00656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dalamaga M, Christodoulatos GS, Mantzoros CS. The Role of Extracellular and Intracellular Nicotinamide Phosphoribosyl-Transferase in Cancer: Diagnostic and Therapeutic Perspectives and Challenges. Metabolism (2018) 82:72–87. 10.1016/j.metabol.2018.01.001 [DOI] [PubMed] [Google Scholar]
- 155.Sampath D, Zabka TS, Misner DL, O’Brien T, Dragovich PS. Inhibition of Nicotinamide Phosphoribosyltransferase (NAMPT) as a Therapeutic Strategy in Cancer. Pharmacol Ther (2015) 151:16–31. 10.1016/j.pharmthera.2015.02.004 [DOI] [PubMed] [Google Scholar]
- 156.Lucena-Cacace A, Otero-Albiol D, Jimenez-Garcia MP, Peinado-Serrano J, Carnero A. NAMPT Overexpression Induces Cancer Stemness and Defines a Novel Tumor Signature for Glioma Prognosis. Oncotarget (2017) 8(59):99514–30. 10.18632/oncotarget.20577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhu Y, Liu J, Park J, Rai P, Zhai RG. Subcellular Compartmentalization of NAD(+) and Its Role in Cancer: A sereNADe of Metabolic Melodies. Pharmacol Ther (2019) 200:27–41. 10.1016/j.pharmthera.2019.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chowdhry S, Zanca C, Rajkumar U, Koga T, Diao Y, Raviram R, et al. NAD Metabolic Dependency in Cancer is Shaped by Gene Amplification and Enhancer Remodelling. Nature (2019) 569(7757):570–5. 10.1038/s41586-019-1150-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tateishi K, Wakimoto H, Iafrate AJ, Tanaka S, Loebel F, Lelic N, et al. Extreme Vulnerability of IDH1 Mutant Cancers to NAD+ Depletion. Cancer Cell (2015) 28(6):773–84. 10.1016/j.ccell.2015.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Travelli C, Aprile S, Mattoteia D, Colombo G, Clemente N, Scanziani E, et al. Identification of Potent Triazolylpyridine Nicotinamide Phosphoribosyltransferase (NAMPT) Inhibitors Bearing a 1,2,3-Triazole Tail Group. Eur J Med Chem (2019) 181:111576. 10.1016/j.ejmech.2019.111576 [DOI] [PubMed] [Google Scholar]
- 161.Oita RC, Camp SM, Ma W, Ceco E, Harbeck M, Singleton P, et al. Novel Mechanism for Nicotinamide Phosphoribosyltransferase Inhibition of TNF-Alpha-Mediated Apoptosis in Human Lung Endothelial Cells. Am J Respir Cell Mol Biol (2018) 59(1):36–44. 10.1165/rcmb.2017-0155OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Quijada H, Bermudez T, Kempf CL, Valera DG, Garcia AN, Camp SM, et al. Endothelial eNAMPT Amplifies Preclinical Acute Lung Injury: Efficacy of an eNAMPT-Neutralising mAb. Eur Respir J (2021) 57(5):2002536. 10.1183/13993003.02536-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sun X, Sun BL, Babicheva A, Vanderpool R, Oita RC, Casanova N, et al. Direct Extracellular NAMPT Involvement in Pulmonary Hypertension and Vascular Remodeling. Transcriptional Regulation by SOX and HIF-2alpha. Am J Respir Cell Mol Biol (2020) 63(1):92–103. 10.1165/rcmb.2019-0164OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Colombo G, Clemente N, Zito A, Bracci C, Colombo FS, Sangaletti S, et al. Neutralization of Extracellular NAMPT (Nicotinamide Phosphoribosyltransferase) Ameliorates Experimental Murine Colitis. J Mol Med (Berl) (2020) 98(4):595–612. 10.1007/s00109-020-01892-0 [DOI] [PubMed] [Google Scholar]
- 165.Soncini D, Caffa I, Zoppoli G, Cea M, Cagnetta A, Passalacqua M, et al. Nicotinamide Phosphoribosyltransferase Promotes Epithelial-to-Mesenchymal Transition as a Soluble Factor Independent of Its Enzymatic Activity. J Biol Chem (2014) 289(49):34189–204. 10.1074/jbc.M114.594721 [DOI] [PMC free article] [PubMed] [Google Scholar]