Abstract
IMPORTANCE
To test potential treatments for age-related macular degeneration (AMD), clinical trials need standardized outcome measures that are valid for predicting AMD progression in different study populations.
OBJECTIVE
To evaluate the validity of the Age-Related Eye Disease Study (AREDS) detailed and simple AMD severity scales by comparing rates of development of late AMD (neovascular AMD and/or central geographic atrophy) between AREDS and AREDS2 participants.
DESIGN, SETTING, AND PARTICIPANTS
Both AREDS (1992–2001) and AREDS2 (2006–2012) enrolled patients from academic and community-based retinal practices across the United States. In AREDS (n = 4519), participants with varying severity of AMD—from no AMD to late AMD in 1 eye—were enrolled. In AREDS2 (n = 4203), participants with bilateral large drusen or large drusen in the study eye and late AMD in the fellow eye were enrolled.
MAIN OUTCOMES AND MEASURES
Five-year incidence of late AMD, assessed by annual masked centralized fundus photograph grading.
RESULTS
In AREDS, the mean (SD) age of the patients was 69.3 (5.7) years, and 2519 (55.7%) were female. In AREDS2, the mean (SD) age of the patients was 73.1 (7.7) years, and 2388 (56.8%) were female. The 5-year rates of late AMD did not differ between AREDS2 and AREDS participants within nearly all baseline AMD detailed severity scale levels: levels 1 to 3: 2.4% vs 0.5% (difference, 1.9%; 95% CI, −0.2% to 4.0%; P < .001); level 4: 6.5% vs 4.9% (difference, 1.6%; 95% CI, −1.7% to 4.8%; P = .34); level 5: 8.0% vs 5.6% (difference, 2.4%; 95% CI, −1.2% to 5.9%; P = .22); level 6: 12.8% vs 13.7% (difference, −0.9%; 95% CI, −4.8% to 3.1%; P = .66); level 7: 26.2% vs 27.8% (difference, −1.5%; 95% CI, −6.6% to 3.5%; P = .54); and level 8: 46.4% vs 44.7% (difference, 1.7%; 95% CI, −7.5% to 10.9%; P = .72). Within simple scale levels, AREDS2 and AREDS 5-year rates did not differ significantly except for level 1 (9.4% vs 3.1%, P = .02; level 2: 12.8% vs 11.8%, P = .65; level 3: 26.3% vs 25.9%, P = .90; and level 4: 45.6% vs 47.3%, P = .57).
CONCLUSIONS AND RELEVANCE
The AREDS detailed and simple AMD severity scales were useful measures for assessing the risk of developing late AMD in the AREDS2 population; these data suggest that they should be useful tools for clinical trials of AMD treatments.
Age-related macular degeneration (AMD) is one of the leading causes of visual impairment and blindness in older populations in the United States and other countries.1,2 Although available treatments result in vision preservation or even improvement in persons with neovascular AMD, they are costly in terms of both economics3 and burden to patients,4 who must return to the ophthalmologist for additional intraocular injections. No effective therapies are available for the atrophic form of AMD. Furthermore, prevention of late AMD is likely to be more cost-effective and less burdensome to the patient than treatment of established disease.5
Strategies to slow progression of AMD were evaluated in the Age-Related Eye Disease Study (AREDS),6 which found that a combination of antioxidant vitamins and zinc significantly reduced the risk of developing late AMD in patients at high risk of progression (those with intermediate AMD [bilateral large drusen] or with late AMD in 1 eye). AREDS2 was conducted to determine whether adding ω−3 fatty acids and/or a combination of lutein and zeaxanthin to the AREDS formulation might further reduce the risk of late AMD (neovascular AMD or central geographic atrophy [GA]) in high-risk eyes.7 Although AREDS2 found no evidence of further benefits from adding ω−3 fatty acids, it demonstrated that a combination of lutein and zeaxanthin should be substituted for β-carotene in the original AREDS formulation for incremental beneficial effects and improved safety. AREDS2 recruited participants with at least intermediate AMD, a group at high risk for progression to late AMD.
Because potential therapies for AMD are increasingly targeted at specific risk groups, often characterized by particular AMD lesion types and severities, a standard grading system to capture this information is necessary; the system must also provide similar prognostic information in different study populations. As with the Early Treatment Diabetic Retinopathy Study scale for diabetic retinopathy, a scale for evaluating the progression of AMD along a detailed grading scale may be a useful measure for assessing the risk of developing late AMD in future studies of therapies for AMD. We performed an external validation study of the AREDS detailed and simple severity scales for AMD by assessing incidence rates of late AMD within baseline severity score categories in AREDS2 and comparing these rates with those found in AREDS.
Methods
We used data from AREDS2 to compute baseline and follow-up AMD detailed and simple severity scale scores for each eye. AREDS2 (2006–2012) enrolled 4203 participants (aged 50–82 years from 82 clinical sites across the United States) who were at risk for progression to late AMD (bilateral large drusen or large drusen in the study eye and late AMD in the fellow eye). AREDS (1992–2001) enrolled 4757 participants (aged 55–80 years from 11 clinical sites across the United States) into 4 AMD categories based on their risk of progression (category 1, free of AMD in both eyes; category 2, early AMD; category 3, intermediate; and category 4, late AMD in one eye). Both AREDS and AREDS2 were approved by the appropriate institutional review boards or ethics committees, and written informed consent was obtained from all participants.6,7 Our aim was to validate the AREDS AMD detailed and simple severity scales by comparing the rates of progression in AREDS2 with the rates of progression in AREDS that were used for the development of the AREDS AMD detailed severity scale.8
The study designs for AREDS and AREDS2 were previously described in detail.6,7 Briefly, both AREDS and AREDS2 obtained fundus photographs at baseline and annual visits. Interim study visits in AREDS and interim telephone contacts in AREDS2 were conducted to obtain a history of treatment for late AMD. The procedures for fundus photography and grading in AREDS2, which were similar to those of AREDS, have been described previously.9 Images were graded at a central reading center (University of Wisconsin) by masked and certified graders (A.D. and R.P.D.) using a standardized protocol. Features graded included 5 signs of neovascularization: (1) subretinal fluid; (2) intraretinal, subretinal, or subretinal pigment epithelium blood associated with neovascular AMD; (3) intraretinal lipid exudates; (4) subretinal fibrin or fibrosis; and (5) fibrovascular or serous pigment epithelial detachment. The presence of 2 or more of these 5 features categorized an eye as having neovascular AMD in AREDS2. A similar protocol was used to define neovascular AMD in AREDS except that hard exudates were not included in the signs and 1 or more of the remaining 4 signs needed to be present to classify the eye as having neovascular AMD. In addition to signs of neovascular AMD, photographs were graded for increased or decreased retinal pigmentation, area, size and predominance of drusen, and GA. The presence and area of drusen were cross-tabulated by the presence and extent of retinal pigmentary changes according to the AREDS detailed severity scale,8 which is a detailed, 9-level, eye-specific scale that reflects the risk of progression to late AMD in the AREDS population. Late AMD was defined as photographic grading of neovascular AMD, history of treatment for neovascular AMD, or photographic grading of central GA. Central GA was defined as GA in the central subfield with at least questionable involvement of the center of the macula.
We also evaluated the validity of the AREDS simplified scale,10 a clinically useful version of the detailed scale that was developed from detailed fundus photograph gradings better suited for research. Briefly, the simplified scale is assigned to a person. To compute the person-level simple score, each eye is scored separately for the presence or absence of large drusen (diameter ≥125 μm) and retinal pigmentary changes (hyperpigmentation or hypopigmentation). The presence of each factor in each eye would be counted as 1 risk factor, with the maximum count being 2 for each eye. The presence of late AMD, either neovascular AMD or central GA, would give the eye a score of 2. The score for the person is the sum of the scores for the 2 eyes. The resultant score is associated with the risk of progression to late AMD for that person in at least 1 eye. The score can range from 0 to 4, and the 5-year risks for late AMD are roughly 0.5%, 3%, 12%, 25%, and 50%, respectively.
The AREDS2 color fundus photograph grading results were analyzed with reference to the detailed AREDS severity scale8 by assessing the association of baseline ocular status with the risk of developing late AMD (neovascular AMD or central GA) by using 5-year rates based on the number at risk at baseline.11 In addition, analyses were performed for subgroups of study participants who were randomized to the original AREDS formulation as part of the AREDS2 second-tier randomization or elected to take the original AREDS formulation.7 Crude 5-year percentages were estimated as the number of events from study baseline through the 5-year visit divided by the number at risk at baseline. Comparisons between percentages were performed by using z tests.11 Statistical significance was defined as P < .05. SAS statistical software, version 9.3 (SAS Institute Inc) was used for all analyses.
Results
Baseline characteristics of participants in AREDS and AREDS2 who were free of late AMD in both eyes at baseline are given in Table 1 and eTable 1 in the Supplement. In AREDS, the mean (SD) age of the patients was 69.3 (5.7) years, and 2519 (55.7%) were female. In AREDS2, the mean (SD) age of the patients was 73.1 (7.7) years, and 2388 (56.8%) were female. As expected, because of the different enrollment criteria between the 2 studies, AREDS2 participants had significantly more severe AMD at baseline for all individual signs of AMD and were significantly older than AREDS participants.
Table 1.
Severity Scale and Simple Scale Scores of AREDS and AREDS2 Participants Free of Late Age-Related Macular Degeneration in Both Eyes at Baseline
| Score | AREDS | AREDS2 | P Value |
|---|---|---|---|
|
| |||
| AREDS detailed severity scale score for all eyes (n = 6426 in AREDS and 5440 in AREDS2, respectively) | |||
|
| |||
| 1 | 2893 (45.0) | 41 (0.8) | |
|
| |||
| 2 | 899 (14.0) | 73 (1.3) | |
|
| |||
| 3 | 378 (5.9) | 92 (1.7) | |
|
| |||
| 4 | 653 (10.2) | 324 (6.0) | |
|
| |||
| 5 | 380 (5.9) | 595 (10.9) | <.001 |
|
| |||
| 6 | 483 (7.5) | 1365 (25.1) | |
|
| |||
| 7 | 488 (7.6) | 2163 (39.8) | |
|
| |||
| 8 | 190 (3.0) | 561 (10.3) | |
|
| |||
| 9 | 62 (1.0) | 226 (4.1) | |
|
| |||
| AREDS simple scale score for study participants (n = 3211 and 2719 in AREDS and AREDS2, respectively) | |||
|
| |||
| 0 | 1466 (45.6) | 9(0.3) | |
|
| |||
| 1 | 635 (19.8) | 53 (1.9) | |
|
| |||
| 2 | 465 (14.5) | 532 (19,6) | <.001 |
|
| |||
| 3 | 328 (10.2) | 681 (25.0) | |
|
| |||
| 4 | 317 (9.9) | 1444 (53.1) | |
Abbreviation: AREDS, Age-Related Eye Disease Study.
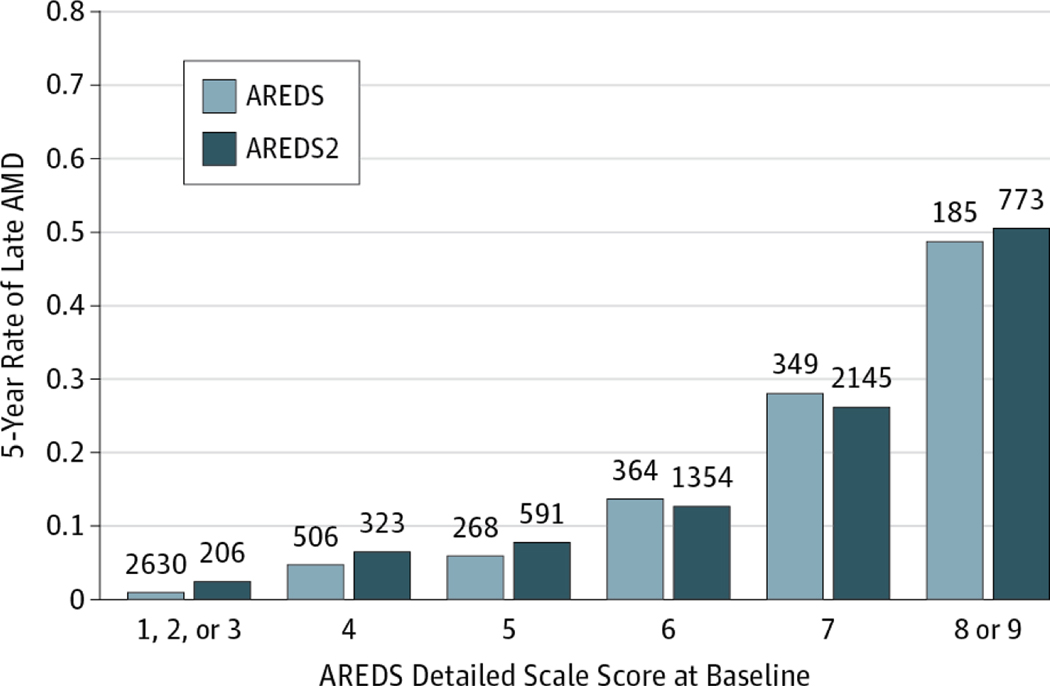
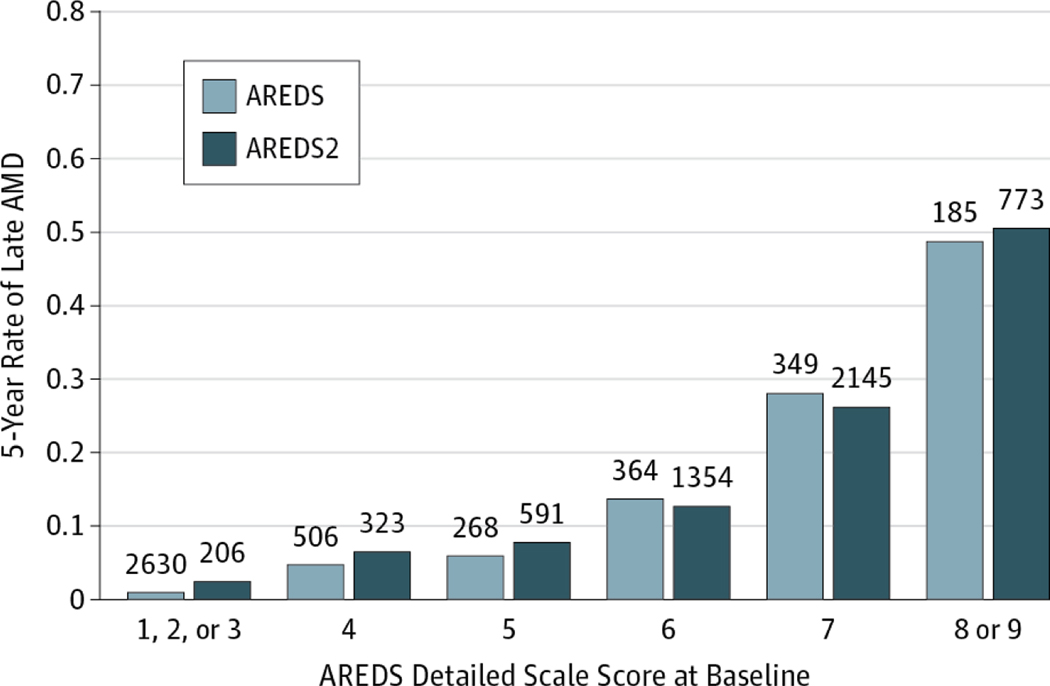
We compared the percentage of eyes that developed late AMD between AREDS2 and AREDS in participants free of late AMD in both eyes at baseline, stratifying by baseline AMD detailed scale score (Figure 1 and Table 2) and by each AMD characteristic at baseline (eTable 2 in the Supplement; shown for right eyes for comparability with the AREDS results8). Table 2 gives the percentage of eyes developing late AMD in 5 years by levels of the detailed AREDS severity scale for right and left eyes combined. No individual score differed significantly between the 2 studies6,7 except for the combined category 1 to 3, which contained patients whose AMD status was not eligible for AREDS2. Because all participants in AREDS2 received a version of the AREDS original formulation, with or without lutein and zeaxanthin and/or ω−3 fatty acids, we compared AREDS2 rates with the AREDS rates of those participants receiving the AREDS supplement (ie, excluding those in the placebo group).
Figure 1.
Comparing Rates of Progression to Late Age-Related Macular Degeneration (AMD) (Neovascular AMD, Central Geographic Atrophy, or Both) in the Age-Related Eye Disease Study (AREDS) and AREDS2
The numbers above each bar are the numbers at risk in that category.
Table 2.
Percentage of Participants Developing Late AMD in 5 Years With Both Eyes Free of Late AMD at Baseline
| No. (%)/Total No. of Participants | |||
|---|---|---|---|
|
| |||
| Scare | ARED5a | AREDS2 | P Value |
|
| |||
| AREDS detailed severity scale score for ail eyes (n = 4302 and 5440 in AREDS and AREDS2, respectively) | |||
|
| |||
| Total | 285/4302 (6.6) | 1232/5440 (22.6) | NA |
|
| |||
| 1, 2, or 3 | 13/2630 (0.5) | 5/206 (2.4) | <.001 |
|
| |||
| 4 | 25/506 (4.9) | 21/323 (6.5) | .34 |
|
| |||
| 5 | 15/268 (5.6) | 47/591 (8.0) | .22 |
|
| |||
| 6 | 50/364 (13.7) | 174/1354 (12.8) | .66 |
|
| |||
| 7 | 97/349 (27.8) | 563/2145 (26.2) | .54 |
|
| |||
| 8 | 63/141 (44.7) | 257/554 (46.4) | .72 |
|
| |||
| 9 (Noncentral GA) | 22/44 (50.0) | 134/219 (61.2) | .17 |
|
| |||
| AREDS simple scale score for all participants (n = 3211 and 2719 in AREDS and AREDS2, respectively) | |||
|
| |||
| Total | 316/3211 (9.8) | 910/2719(33.5) | NA |
|
| |||
| 0 | 6/1466 (0.4) | 0/9 (0) | .85 |
|
| |||
| 1 | 20/635 (3.1) | 5/53 (9.4) | .02 |
|
| |||
| 2 | 55/465 (11.8) | 68/532 (12,8) | .65 |
|
| |||
| 3 | 85/328 (25.9) | 179/681 (26.3) | .90 |
|
| |||
| 4 | 150/317 (47.3) | 658/1444 (45.6) | .57 |
Abbreviations: AMD, age-related macular degeneration; AREDS, Age-Related Eye Disease Study; GA, geographic atrophy; NA, not applicable.
Sample size of 4302 includes treated eyes only (excludes placebo).
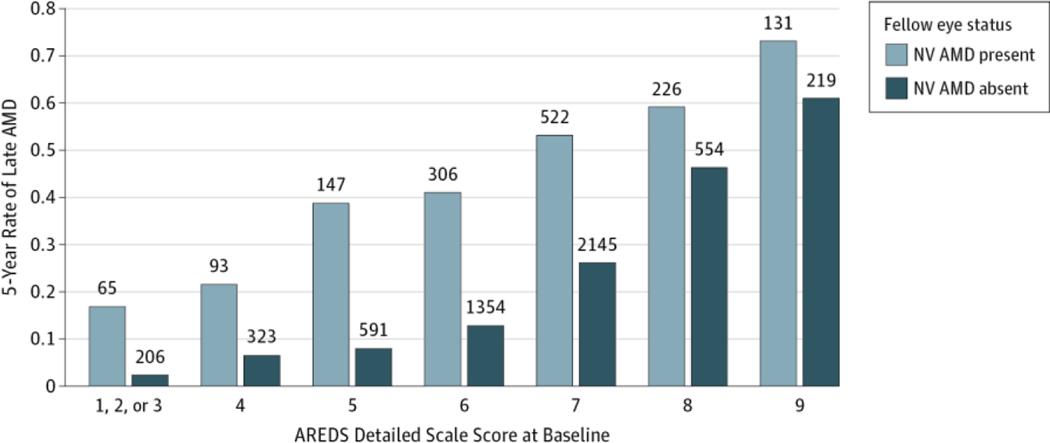
For drusen area (eTable 2 in the Supplement), although the percentage of eyes developing late AMD within 5 years was similar between AREDS2 and AREDS for larger areas of drusen, the percentage was substantially higher in AREDS2 than in AREDS for all categories less than area O-2 (644 μm2). Similarly, for maximum drusen size (eTable2 in the Supplement), the 5-year percentage was higher in AREDS2 than in AREDS except for the largest category (C-2 or higher [250 μm]). When we assessed the effects of increased pigmentation, we observed that AREDS2 percentages were higher in all categories except questionable (eTable 2 in the Supplement). It is possible that the higher rates in AREDS2 may be the result of these eyes having some what more severe baseline lesions within subgroups for drusen and pigmentary changes, both factors important in the development of late AMD. We also compared the 5-year rate of participants in AREDS2 who developed late AMD, stratifying by baseline AMD detailed severity score and by who had late AMD in 1 eye at baseline. The 5-year rates of late AMD did not differ significantly between the 2 studies within baseline AMD detailed severity scale levels (Table 2) (level 4:6.5% vs 4.9% [difference: 1.6%; 95% CI:−1.7% to 4.8%; P = .34]; level 5: 8.0% vs 5.6% [difference: 2.4%; 95% CI: −1.2% to 5.9%; P = .22]; level 6: 12.8% vs 13.7% [difference: −0.9%; 95% CI: −4.8% to 3.1%; P = .66]; level 7: 26.2% vs 27.8% [difference: −1.5%; 95% CI: −6.6% to 3.5%; P = .54]; level 8:46.4 vs 44.7% [difference: 1.7%; 95% CI: −7.5% to 10.9%; P = .72]) except for the combined level 1 to 3 (2.4% vs 0.5%; difference, 1.9%; 95% CI, −0.2% to 4.0%; P < .001). As anticipated, rates of late AMD were higher when the fellow eye had late AMD at baseline for all steps on the detailed severity scale (Figure 2).
Figure 2.
Five-Year Rates of Progression to Late Neovascular (NV) Age-Related Macular Degeneration (AMD) in the Age-Related Eye Disease Study 2 (AREDS2) Stratified by Status of Fellow Eye at Baseline
The numbers above each bar are the numbers at risk in that category.
We compared the 5-year rate of participants developing late AMD by levels of the AREDS simple scale score (Table 2) (category 0 was not included because persons in category 0 were not eligible for AREDS2). The rates were similar between AREDS2 and AREDS for simple scores in all categories but category 1, where the rate for AREDS2 was higher than for AREDS (9.4% vs 3.1%, P = .02). We also compared participants in AREDS and AREDS2 who had late AMD in 1 eye at baseline, stratifying by simple score. Rates of progression were nearly identical for persons with scores 3 and 4 (level 3: 35.4% vs 36.0%, P = .90; level 4: 53.1 % vs 53.6%,P = .09 for AREDS and AREDS2, respectively).
We examined additional outcome measures describing 5-year progression of AMD in participants free of late AMD in both eyes at baseline (Figure 3). Rates of progression to late AMD increased with each increasing step on the detailed scale and were similar in AREDS and AREDS2 (Figure 3; eTable 3 in the Supplement, where rates are given separately for neovascular AMD and central GA). We also examined these outcomes in eyes from participants in whom the fellow eye had neovascular AMD at baseline (eTable 4 in the Supplement). Finally, we pooled the information from AREDS and AREDS2 for each level of the detailed severity scale to provide more robust estimates of 5-year risk of late AMD (eTable 5 in the Supplement).
Figure 3.
Five-Year Rates of Age-Related Macular Degeneration (AMD) Progression in Persons Free of Late AMD at Baseline for the Age-Related Eye Disease Study (AREDS) and AREDS2
Five-year progression rates to stages 7 to 9 and to late AMD and 5-year progression rates to neovascular (NV) AMD only and to central geographic atrophy (GA) only are shown. The numbers above each bar are the numbers at risk in that category.
Discussion
The AREDS detailed severity scale was designed to provide baseline risk categories that would allow quantification of the risk of developing late AMD.8 In AREDS, the 5-year risk of developing late AMD increased for each increasing step on the detailed scale from less than 1% to approximately 50%, although not in a strictly linear fashion. As for any grading scale, it is important to evaluate its performance in independent populations.12
The AREDS detailed AMD severity scale was developed from rigorous and detailed examination of the associations of different retinal signs and their prognostic value for 5-year progression of AMD. Because AREDS2 recruited participants with more severe levels of AMD than did AREDS, we needed to compare rates within levels of potentially confounding characteristics. The identical grading systems used in AREDS and AREDS2 allowed us to compare rates of 5-year progression to late AMD between the studies within strata of baseline AMD characteristics. We observed that rates were similar within baseline levels of the AMD detailed severity scale.
The performance of the AREDS detailed severity scale has been evaluated in several previous studies.13–15 Ying et al13 applied the AREDS detailed severity scale to the untreated eye of participants in the Complication of Age-Related Macular Degeneration Prevention Trial (CAPT). In the CAPT, all participants had large drusen in both eyes at baseline. Consistent with our findings, they reported that the detailed scale was predictive of development of late AMD, with similar risk estimates for CAPT and AREDS participants for each step on the scale. However, they also noted fluctuation in the detailed scale steps overtime, particularly decreases of scale score, in part attributable to disappearance of drusen or pigment. They also observed that eyes progressing to central GA might have a different scale pattern over time than eyes progressing to choroidal neovascularization (CNV). We limited our comparisons of AREDS2 and AREDS data to the combined outcome of CNV orcentral GA, as in the original AREDS report describing the detailed severity scale.8
In a study by the AREDS investigators,14 phenotypic (AMD simple scale score, presence of very large drusen), demographic (age, body mass index, and educational level), environmental (current smoking), and genetic (family history, CFH Y402 Halleles, and ARMS2 A69 Salleles) risk factors were valuated as predictors of 5-year development of late AMD. They reported that a model that incorporated environmental or demographic and phenotypic predictors performed best, with genetic factors not contributing significantly to performance not because they are unimportant but because they are highly correlated with the phenotypic predictors. They also performed an external validation study using CAPT data and found no significant differences in model performance in that population.
McCarthy et al15 developed predictive models for development of CNV and GA by using AREDS data, focusing on 3-year incidence of CNV and GA (separately). They found that adding clinical, demographic, and environmental information to a model with the AREDS simple score yielded slightly better predictive ability than the AREDS simple score alone and that genetic information did not notably improve the predictive performance of the model. A potential short coming of this study was that the validation sample (participants without genetic information) was not comparable to the development sample (participants with genetic information), although the 3-year incidence rates appear similar to those previously reported.8 The purpose of the study by McCarthy et al15 was to identify models to allow selection of various risk thresholds based on AMD and patient characteristics rather than to evaluate whether the AREDS simple grading scale performed similarly in a separate study.
A limitation of our study is that AREDS2 recruited people with more severe levels of AMD than did AREDS. We accounted for this difference in part by comparing within strata of the detailed and simple grading scales. However, it is possible that additional differences in the 2 patient populations might have introduced unknown biases into the estimation procedure. For example, eyes in the earlier detailed or simple scale levels may be biased in the direction of more severe AMD because these eyes were clinically selected to have the more severe eligibility levels for AREDS2.
Another limitation of our study is that we have only evaluated the validity of the AREDS detailed and simple grading scales for AMD in the AREDS2, a clinical trial evaluating dietary treatments for AMD. It is possible that participants in this type of clinical trial differ from participants in trials of diverse treatments (eg, surgical, injection) for AMD as well as differ from the general population with AMD. Evaluation of the AREDS grading scales in different populations will help establish their validity in varied settings.
Conclusions
We found that rates of late AMD are similar between AREDS2 and AREDS when stratified by baseline AREDS detailed or simple scale severity scores for AMD. These data provide further evidence that the AREDS detailed severity scale and simplified severity scale for AMD are useful measures for assessing the risk of developing late AMD in the AREDS2 population; these data suggest that the scales should be useful tools for future clinical trials of treatments for AMD.
Supplementary Material
Key Points.
Question Are the Age-Related Eye Disease Study (AREDS) detailed and simple severity scales for age-related macular degeneration (AMD) generalizable to studies other than the original AREDS?
Findings In this comparison of the 5-year incidence rates of late AMD between AREDS and AREDS2, stratifying by baseline AMD scale score, the rates did not differ.
Meaning This study suggests that the AMD severity scales are generalizable to studies other than the original AREDS for which they were developed.
Acknowledgments
Funding/Support: This study was supported by the intramural program funds and contract HHS-N-260–2005-00007-C and ABD contract N01-EY-5–00007 from the National Eye Institute (NEI), National Institutes of Health (NIH), Department of Health and Human Services, Bethesda, Maryland (design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication), and in part by an unrestricted grant from Research to Prevent Blindness to the Department of Ophthalmology and Visual Sciences, University of Wisconsin, Madison (Drs Domalpally and Danis) (collection, management, analysis, and interpretation of the data).
Role of the Funder/Sponsor: The staff of the NEI and their contractors, led by Dr Chew, had complete control of the design and conduct of the study; the collection, management, analysis, and interpretation of the data; and the preparation, review, or approval of the manuscript. The contributing institutes from the NIH were consulted for various aspects of the study, including its design and conduct. The analyses were completed at the NEI/NIH. The sponsors who donated the study medications had no role in the design or conduct of the study and had no access to the data during the study.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Ferris reported holding a patent for the AREDS formulation with Bausch & Lomb. No other disclosures were reported.
Group Information: The Age-Related Eye Disease Study 2 (AREDS2) Research Group members are listed in the eAppendix in the Supplement.
REFERENCES
- 1.Congdon N, O’Colmain B, Klaver CC, et al. ; Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4): 477–485. [DOI] [PubMed] [Google Scholar]
- 2.Friedman DS, O’Colmain BJ, Muñoz B, et al. ; Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564–572. [DOI] [PubMed] [Google Scholar]
- 3.Stein JD, Newman-Casey PA, Mrinalini T, Lee PP, Hutton DW. Cost-effectiveness of bevacizumab and ranibizumab for newly diagnosed neovascular macular degeneration. Ophthalmology. 2014;121(4): 936–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haller JA. Current anti-vascular endothelial growth factor dosing regimens: benefits and burden. Ophthalmology. 2013;120(5)(suppl):S3–S7. [DOI] [PubMed] [Google Scholar]
- 5.Brown GC, Brown MM, Kertes P, Lieske H, Lieske PA, Brown K. Value-based medicine comparative effectiveness and cost-effectiveness analyses: oral vitamin, and zinc supplements for the treatment of atrophic age-related macular degeneration. Evid Based Ophthalmol. 2011;12(3):160–167. [Google Scholar]
- 6.Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119(10):1417–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309(19): 2005–2015. [DOI] [PubMed] [Google Scholar]
- 8.Davis MD, Gangnon RE, Lee LY, et al. ; Age-Related Eye Disease Study Group. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS report No. 17. Arch Ophthalmol. 2005;123(11):1484–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danis RP, Domalpally A, Chew EY, et al. ; AREDS2 Study Group. Methods and reproducibility of grading optimized digital color fundus photographs in the Age-Related Eye Disease Study 2 (AREDS2 Report Number 2). Invest Ophthalmol Vis Sci. 2013; 54(7):4548–4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferris FL, Davis MD, Clemons TE, et al. ; Age-Related Eye Disease Study (AREDS) Research Group. A simplified severity scale for age-related macular degeneration: AREDS report No. 18. Arch Ophthalmol. 2005;123(11):1570–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armitage P, Berry G. Statistical Methods in Medical Research. 3rd ed. Oxford, England: Blackwell Scientific Publications; 1994. [Google Scholar]
- 12.Altman DG, Vergouwe Y, Royston P, Moons KGM. Prognosis and prognostic research: validating a prognostic model. BMJ. 2009;338:b605. [DOI] [PubMed] [Google Scholar]
- 13.Ying S, Maguire MG, G-Alexander J, Martin RW, Antoszyk AN; Complications of Age-related Macular Degeneration Prevention Trial Research Group. Description of the Age-Related Eye Disease Study 9-step severity scale applied to participants in the Complications of Age-related Macular Degeneration Prevention Trial. Arch Ophthalmol. 2009;127(9): 1147–1151. [DOI] [PubMed] [Google Scholar]
- 14.Klein ML, Francis PJ, Ferris FLIII III, Hamon SC, Clemons TE. Risk assessment model for development of advanced age-related macular degeneration. Arch Ophthalmol. 2011;129(12):1543–1550. [DOI] [PubMed] [Google Scholar]
- 15.McCarthy LC, Newcombe PJ, Whittaker JC, et al. Predictive models of choroidal neovascularization and geographic atrophy incidence applied to clinical trial design. Am J Ophthalmol. 2012;154(3):568–578.e12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.