Abstract
Research has shown that sleep is beneficial for the long-term retention of memories. According to theories of memory consolidation, memories are gradually reorganized, becoming supported by widespread, distributed cortical networks, particularly during postencoding periods of sleep. However, the effects of sleep on the organization of memories in the hippocampus itself remains less clear. In a 3-d study, participants encoded separate lists of word–image pairs differing in their opportunity for sleep-dependent consolidation. Pairs were initially studied either before or after an overnight sleep period, and were then restudied in a functional magnetic resonance imaging (fMRI) scan session. We used multivariate pattern similarity analyses to examine fine-grained effects of consolidation on memory representations in the hippocampus. We provide evidence for a dissociation along the long axis of the hippocampus that emerges with consolidation, such that representational patterns for object–word memories initially formed prior to sleep become differentiated in anterior hippocampus and more similar, or overlapping, in posterior hippocampus. Differentiation in anterior hippocampal representations correlated with subsequent behavioral performance. Furthermore, representational overlap in posterior hippocampus correlated with the duration of intervening slow wave sleep. Together, these results demonstrate that sleep-dependent consolidation promotes the reorganization of memory traces along the long axis of the hippocampus.
The hippocampus has long been considered critical for encoding new memories; however, the effects of consolidation on hippocampal memory traces has remained an area of active research. Memories are thought to be stabilized for the long term as they become distributed across neocortical networks (Buzsáki 1989; Alvarez and Squire 1994; McClelland et al. 1995), a process supported by mechanisms during sleep (Diekelmann and Born 2010; Rasch and Born 2013). Whereas much research has been devoted to understanding the hippocampal contributions to the long-term retention of memories, open questions remain in considering how sleep-dependent consolidation affects the organization of hippocampal traces.
The hippocampus has previously been shown to be critical for binding disparate elements of an experience together (Cohen and Eichenbaum 1993; Davachi 2006). Theories suggest that the hippocampus quickly encodes new experiences, while the cortex, with a slower learning rate, gradually comes to represent the central features from this hippocampal trace, resulting in abstracted memories that can be integrated into long-term cortical stores (McClelland et al. 1995). Prior research has demonstrated evidence for a coordinated hippocampal–cortical dialogue during sleep (Andrade et al. 2011; Bergmann et al. 2012; Ngo et al. 2020) as well as enhanced hippocampal–cortical functional connectivity after learning, facilitating the retention of memories (Tambini et al. 2010; Tompary et al. 2015; Murty et al. 2017; Cowan et al. 2021). Reports suggest consolidation results in more integrated cortical memory traces in the cortex (Richards et al. 2014; Tompary and Davachi 2017; Cowan et al. 2020); however, it remains an open question whether the active consolidation processes that support memory reorganization across hippocampal–cortical networks also transform hippocampal memory traces.
Research on the fate of the hippocampal trace with consolidation has often focused on questions about the permanence of memories in the hippocampus. Theories of systems consolidation have classically debated whether the hippocampal trace is time-limited (Alvarez and Squire 1994), or, rather, whether the hippocampus continues to represent memories in perpetuity (Nadel and Moscovitch 1997; Winocur and Moscovitch 2011; Moscovitch et al. 2016; Sekeres et al. 2018a). Another theory posits that while the original hippocampal trace is transient, during retrieval the hippocampus reconstructs details of an experience from cortical traces (Barry and Maguire 2019). Much research in this vein has focused on investigating changes in hippocampal blood-oxygenation level-dependent (BOLD) univariate activation with time (Bosshardt et al. 2005a,b; Takashima et al. 2006, 2009; Gais et al. 2007; Sterpenich et al. 2007, 2009; Yamashita et al. 2009; Milton et al. 2011; Watanabe et al. 2012; Ritchey et al. 2015; Baran et al. 2016; Dandolo and Schwabe 2018) and the effects of hippocampal lesions in animals and humans (Winocur et al. 2001; Frankland and Bontempi 2005; Winocur and Moscovitch 2011; Moscovitch et al. 2016) with mixed results. Interestingly, pinpointing these effects along the long axis of the hippocampus has also proven unclear. Some reports have found that only the anterior hippocampus exhibits time-dependent changes in retrieval-related univariate activation, with evidence of decreases with delay (Takashima et al. 2006; Milton et al. 2011; Dandolo and Schwabe 2018), but also evidence of greater activation for more remote, compared with recent, memories (Bosshardt et al. 2005a,b). At the same time, other studies have found decreases in univariate activation only in the posterior hippocampus (Bosshardt et al. 2005b; Takashima et al. 2009; Yamashita et al. 2009; Milton et al. 2011; Watanabe et al. 2012; Ritchey et al. 2015; Sekeres et al. 2018b).
Because of these conflicting findings, instead of asking just about dependence or overall changes in activation in the hippocampus, theories and empirical research have instead increasingly considered the organization of memory representations in the hippocampus (Robin and Moscovitch 2017; Sekeres et al. 2018a). Broadly, using representational similarity analyses, several studies have shown that hippocampal memory representations tend to become differentiated over learning, particularly for memories with overlapping content (LaRocque et al. 2013; Schlichting et al. 2015; Chanales et al. 2017; Brunec et al. 2020). Furthermore, it has been suggested that information is represented at different scales or “granularity” along the long axis of the hippocampus, in line with place field size differences (Kjelstrup et al. 2008; Komorowski et al. 2013), with anterior hippocampus representing more similar, coarse-grained, or gist-like information, while the posterior hippocampus represents fine-grained, detail-oriented representations (Evensmoen et al. 2013; Poppenk et al. 2013; Robin and Moscovitch 2017; Brunec et al. 2018, 2020). However, limited work has investigated whether this representational organization is altered with consolidation. Reports have shown that memory representations sharing overlapping content become more similar over a delay (Tompary and Davachi 2017; Audrain and McAndrews 2020), yet other work has found that hippocampal representations were not modulated by time (Ritchey et al. 2015; Ezzyat et al. 2018). Intriguingly, reports indicating greater differentiation in memories in anterior compared with posterior hippocampus with consolidation (Tompary and Davachi 2017; Dandolo and Schwabe 2018; Ezzyat et al. 2018) raise the possibility that the representational granularity along the anteroposterior axis may be transformed with consolidation. Thus, more work is needed to understand how consolidation influences the representational structure of memories in the hippocampus. In particular, despite much research connecting sleep to consolidation (Diekelmann and Born 2010; Rasch and Born 2013), it remains unknown whether sleep-dependent processes facilitate such delay-dependent transformations to the hippocampus.
Active processes in the sleeping brain seem to be optimized for systems consolidation. Currently, the best mechanistic evidence for sleep-dependent consolidation comes from studies on hippocampal replay showing the repeated reactivation of encoding-related patterns of hippocampal activity (Buzsáki 1989; Wilson and McNaughton 1994; Girardeau and Zugaro 2011), which seems to be coordinated with replay in areas of the cortex (Ji and Wilson 2007; Peyrache et al. 2009; Wierzynski et al. 2009). It is thought that the coupling between oscillations during non-REM sleep stages (particularly slow wave sleep [SWS])—including sharp wave ripples that support replay, thalamocortical spindles, and slow oscillations—facilitates the hippocampal–cortical dialogue and information transfer to the cortex (Buzsáki 1996; Sirota et al. 2003; Steriade 2006; Clemens et al. 2011; Mölle and Born 2011; Staresina et al. 2015). Indeed, our previously published work from the present study provided supporting evidence that the density of thalamocortical sleep spindles (11–16 Hz) during overnight sleep is related to enhanced hippocampal–cortical functional connectivity measures, and increased similarity, or greater representational overlap, among memories in the ventromedial prefrontal cortex (vmPFC) (Cowan et al. 2020). Yet, while some prior work has shown that features of sleep, including spindle density and the duration of non-REM SWS, are related to decreased retrieval-related hippocampal activation for memoranda learned prior to sleep (Takashima et al. 2006; Baran et al. 2016; Hennies et al. 2016), it remains unclear how the reactivation of hippocampal traces during replay may impact the way memories are organized along the long axis of the hippocampus.
To examine the effects of sleep-dependent consolidation on the neural representation of memories in the hippocampus, we designed a within-participant 3-d study using overnight polysomnography (PSG), functional magnetic resonance imaging (fMRI), and behavioral measures of memory (Fig. 1). In this study, aspects of which have been previously published (Cowan et al. 2020), participants first studied a list of word–image pairs before sleeping overnight (Sleep List), during which PSG was recorded. Upon waking in the morning, participants studied a new list of pairs (Morning List). The word–image pairs from these two lists were then restudied while undergoing an fMRI scan, intermixed with a third, novel list of pairs (Single Study List). Associative memory was tested immediately after the scan and again 24 h later. We compared measures of multivariate pattern similarity and univariate BOLD signal for the lists learned prior to, or after, sleep to probe how modulating the opportunity for sleep-dependent consolidation impacts the way memories are organized across the long axis of the hippocampus. Furthermore, our design allowed us to examine how features of overnight sleep are related to the representational organization of memories learned prior to the sleep period, as well as the behavioral benefit of changes to the organization of these memories. Thus, our study provides a novel examination of the effects of sleep-dependent consolidation on the representation of memories along the long axis of the hippocampus.
Figure 1.
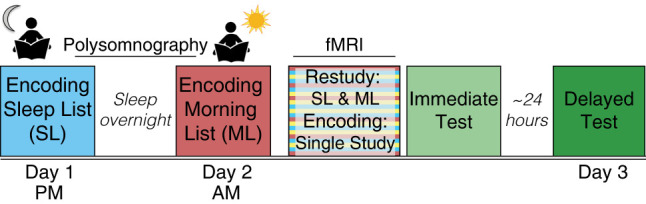
Study design. For all encoding and restudy sessions, participants were asked to form an association between a word and an image. Participants first encoded the Sleep List (blue) before sleeping overnight while polysomnography was recorded. The next morning (day 2), participants encoded a second set of novel word–image pairs (Morning List). After a short delay (∼2 h), participants restudied these two sets of pairs, intermixed with novel pairs (Single Study List) in the functional magnetic resonance imaging (fMRI) scanner. Source memory was tested immediately after the scan and after a 24-h delay (day 3).
Results
Behavioral results
The results from the behavioral memory tests have been reported previously (Cowan et al. 2020). In brief, according to a 3(Encoding List: Sleep, Morning, Single Study) × 2(Test: Immediate, Delayed) × 2(Category: scene–word, object–word) repeated measures analysis of variance (ANOVA), associative memory performance was significantly better for pairs from the Sleep and Morning Lists compared with the Single Study List; however, performance did not significantly differ between the Sleep and Morning Lists (Encoding List main effect: F(2,36) = 57.44, P < 0.0001), consistent with the twice-presented pairs from the Sleep and Morning Lists being better remembered compared to the Single Study List pairs, which were presented only once, and for the first time, during the scan. Additionally, a significant main effect of test (F(1,18) = 136, P < 0.001) was indicative that performance was better for the Immediate test than Delayed test, consistent with forgetting over time.
Response times during restudy
Another way to assess memory in this task is to look at response times (RTs) during the scan session, while participants rated their associations between each word–image pair. Unlike the two subsequent memory tests, which follow the second exposure, RT data provides an implicit measure of memory accessibility: if participants remember the previously studied Sleep and Morning Lists, then we might expect faster RTs for pairs on these lists compared with trials for the completely novel Single Study List when these pairs are presented.
As shown in Figure 2, consistent with better implicit memory for previously studied pairs, a 3(Encoding List: Sleep List, Morning List, Single Study List) × 2(Category: object–word, scene–word) repeated measures ANOVA demonstrated that participants made significantly faster responses for the Sleep List and Morning List pairs compared with the novel Single Study List (main effect of List: F(2,36) = 84.64, P < 0.0001; SL < SS t(37) = −8.74, P < 0.001; ML < SS t(37) = −10.02, P < 0.001). Interestingly, participants were also slightly faster to respond to the Morning List pairs than the Sleep List pairs, perhaps because the former were more recently learned (t(37) = 2.18, P = 0.04). The ANOVA also yielded a significant main effect of Category (F(1,18) = 30.01, P < 0.001) and a significant List × Category interaction effect (F(2,36) = 28.02, P < 0.0001), indicating that RTs were not equated for the two categories of word–image pairs. Participants responded faster to the object–word pairs compared with the scene–word pairs for both the Sleep List (t(18) = −6.4, P < 0.001) and Morning List (t(18) = −6.73, P < 0.001). There was not a significant difference between the categories for the Single Study List (t(18) = −0.79, P = 0.44), indicating the effect observed in the other Lists is not driven only by differential perceptual processing, but instead may indicate that the scene- and object-associated stimuli were unevenly remembered at the time of the reexposure in the scanner. There may therefore be a potential learning difference between the category of word–image pairs, whereby object–word pairs may be easier or faster to learn and recall compared with scene–word pairs.
Figure 2.
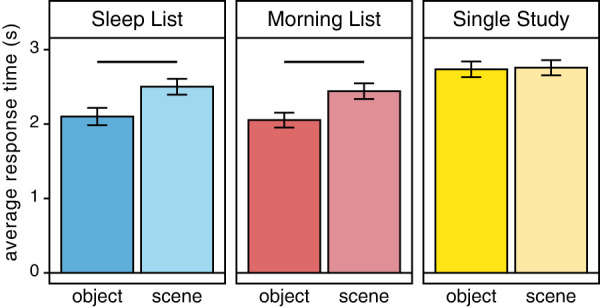
Response time during a functional magnetic resonance imaging (fMRI) scan session. Average response time for judgments made during the restudy scan were faster for pairs that had been previously studied, the Sleep List (blues, left) and Morning List (reds, middle), compared with the novel Single Study List pairs (yellows, right), providing an implicit measure of memory retention. However, this benefit was uneven across categories: for both the Sleep and Morning List pairs, response times were faster for the object–word pairs (darker shades) than scene–word pairs (lighter shades).
Neuroimaging analysis: approach
To understand how sleep-dependent consolidation affects memory representations along the long axis of the hippocampus, we use a multivariate pattern similarity analysis approach. We measure the pattern of activity on each trial and compare it with patterns elicited by all other trials learned during the same Encoding List, to examine changes in the organization of memories depending on delay from initial encoding (see the Materials and Methods). We first calculate a measure of “all trial” interitem pattern similarity, calculated across trials regardless of later memory success, separately for each Encoding List. Since the scan session consisted of a restudy and first encoding session, the all trial similarity measure adjudicates overall delay-dependent differences in the patterns evoked in response to the reactivation (or first presentation) of the pairs. We can then examine the behavioral benefits of changes to the representational organization. We test the relationship to subsequent memory success on the memory tests both across subjects, as the correlation between the all trial representational pattern similarity and subsequent memory, and by calculating a “subsequent memory interitem pattern similarity” metric examining the organization of trials when sorted by later memory status. Together, these measures test how consolidation contributes to transformations in memory traces that facilitate memory retention and maintenance. Finally, leveraging the design of this experiment (see Fig. 1), we examine whether features of the intervening sleep period are related to the representational pattern for information initially learned prior to sleep. Together, this approach allows us to assess changes to the organization of memories and its behavioral relevance, as well as the role of sleep in such transformations.
Interitem pattern similarity in the hippocampus
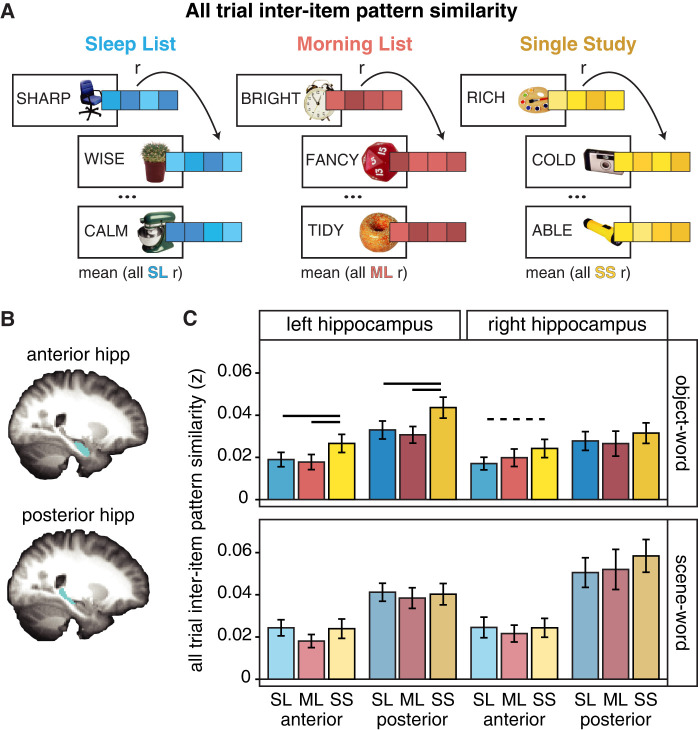
We first computed an “all trial interitem pattern similarity” measure across all trials regardless of later memory success, within each Encoding List (Fig. 3A; see the Materials and Methods). This more general similarity measure tests how the representations of multiple events (trials) initially learned during the same session change depending on the opportunity for sleep-dependent consolidation. In this analysis, higher values of interitem pattern similarity for a given memory could be interpreted as more overlap in the representational patterns with other memories learned at the same time (e.g., on the same list), while lower interitem pattern similarity values can be interpreted as differentiation among the representations for memories that had been learned at the same time.
Figure 3.
All trial interitem pattern analysis in anterior and posterior hippocampus. (A) To compute the all trial interitem pattern analysis, patterns of activation across voxels within regions of interest (ROIs) were extracted for each trial and sorted by the Encoding List during which it was first learned. All trials within a List were correlated across runs, separated by category, and averaged, resulting in a similarity metric for each List. (B) Representative images of anterior and posterior hippocampus ROIs, defined for each participant in each hemisphere (see the Materials and Methods). (C) Overall there was greater differentiation for object–word pairs in the Sleep and Morning Lists compared with the Single Study List. All trial interitem pattern similarity differed by Encoding List for the object–word pairs (top), but not the scene–word pairs (bottom).
As shown in Figure 3C, a repeated measures ANOVA with Encoding List (SL, ML, SS), Category (object–word, scene–word), Axis region (anterior, posterior), and Hemisphere (right, left) as factors resulted in a main effect of List (F(2,36) = 16.59, P < 0.0001). On average, the pairs encoded the night before (Sleep List) were more differentiated from each other compared with the Single Study List trials (SL < SS: t(151) = −3.4, P = 0.0008). This same pattern was also evident for the Morning List pairs, with lower pattern similarity compared with the Single Study List trials (ML < SS: t(151) = −5.0, P < 0.0001). In other words, the pattern similarity among twice-studied pairs (SL, ML) was lower than the novel pairs (SS). There was no significant difference between the Sleep and Morning List pairs (t(151) = 1.32, P = 0.19). Therefore, time or repetition seems to lead to reductions in the overlap in the evoked representational patterns across voxels.
Furthermore, the ANOVA yielded results suggesting anterior and posterior hippocampus exhibited differential representational patterns by category and hemisphere. In addition to main effects of Axis (F(1,18) = 36.01, P < 0.0001) and Category (F(1,18) = 10.38, P = 0.005), there were significant interaction effects between Category × Axis (F(1,18) = 25.46, P < 0.0001), Category × Hemisphere (F(1,18) = 17.48, P = 0.0006), and Category × Hemisphere × Axis (F(1,18) = 10.86, P = 0.004). These interactions seemed to be driven by a significant difference between the representations of scene–word and object–word pairs in the posterior but not the anterior hippocampus (posterior: t(113) = 6.64, P < 0.0001; anterior: t(113) = 1.26, P = 0.21), and significant category differences in the right hemisphere, but not in the left hemisphere (right: t(113) = 6.46, P < 0.00001; left: t(113) = 1.54, P = 0.13). Thus, the object–word and scene–word pairs seem to be particularly differentiable in the right posterior hippocampus.
These results provide evidence for greater differentiation in the representation pattern of information that is reexposed after a delay compared with novel information, raising the possibility that consolidation may contribute to the transformation in the organization of memories, although these results cannot differentiate between the effect of time versus repetition on the representations. The all trial interitem pattern similarity results also suggest that category information may be differentially represented across hemispheres and the long axis of the hippocampus.
Relationship between interitem pattern similarity and memory
While the all trial interitem pattern similarity results are suggestive of changes in how memories are represented, we next queried the behavioral significance of this representational organization. We assessed whether the extent of overlap or differentiation in the representations for memories encoded at the same time confers a benefit on the subsequent memory tests by examining the relationship between the all trial interitem pattern similarity and the behavioral measure of associative memory (collapsed across the Immediate and Delayed tests). For this analysis, we used the Morning List as a within-participant control for both the brain and behavioral measures. Each participant's Morning List interitem pattern similarity and memory performance was subtracted from these corresponding measures from the Sleep List. As many features overlap for the Sleep and Morning Lists, including that both were seen twice with the second presentation during the restudy scan, this subtraction analysis allows us to better isolate effects due to the sleep-filled delay that is specific to the Sleep List. We therefore refer to this subtraction measure as a “sleep-specific” similarity. For all across-subjects analysis, we used robust regression to downweight the influence of potential outliers (see the Materials and Methods).
As shown in Figure 4, for the object–word pairs, all trial interitem pattern similarity in the right anterior hippocampus showed a significant negative correlation with successful associative memory (r = −0.46, robust regression: β = −5.54, P = 0.002). Thus, lower similarity, or greater differentiation, among the sleep-related anterior hippocampal representations was related to better associative memory for those pairs. The correlation between all trial interitem pattern similarity and associative memory was not significant in the right posterior hippocampus (r = 0.19, robust: β = 0.89, P = 0.45). The correlations between similarity and subsequent memory for the right anterior and posterior hippocampus showed a marginally significant difference according to a Williams’ test for dependent correlations (t = −1.96, P = 0.067). Additionally, correlations between interitem pattern similarity and correct associative memory were not significant in any region of the left hippocampus (left anterior: r = 0.11, posterior: r = −0.03, all Ps > 0.05).
Figure 4.
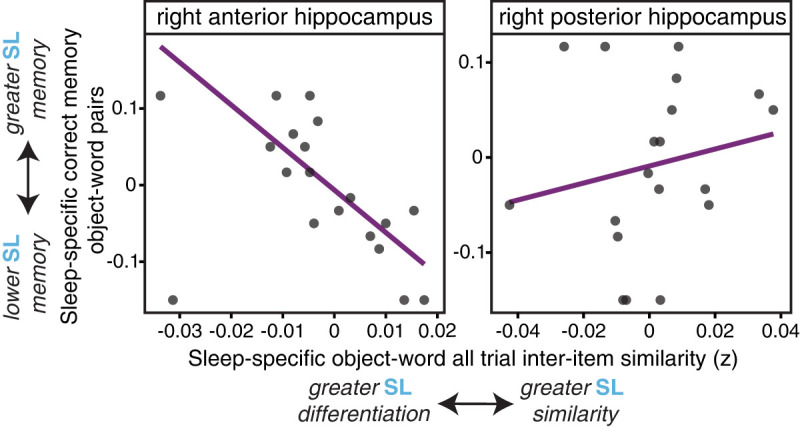
Anterior hippocampal all trial interitem pattern similarity correlates with behavioral memory success. (Left) The all trial sleep-specific (SL-ML) interitem pattern similarity in the right anterior hippocampus among object–word pairs negatively correlates with sleep-specific (SL-ML) associative memory performance (source correct), such that greater differentiation in the representations is related to better associative memory, indicative that this pattern may be beneficial for memory retention. (Right) The same relationship was not significant in the right posterior hippocampus.
For scene–word pairs, the all trial interitem pattern similarity did not significantly correlate with correct associative memory in any region of the hippocampus in the right hemisphere (anterior: r = −0.26, posterior: r = 0.02) or the left hemisphere (anterior: r = −0.08, posterior: r = −0.16), perhaps in line with the RT category differences presented above (Fig. 2). Therefore, differentiation in the anterior hippocampal representational patterns for object–word pairs learned prior to the sleep period seems to confer a behavioral benefit for the retention of these memories.
Subsequent memory interitem pattern analyses
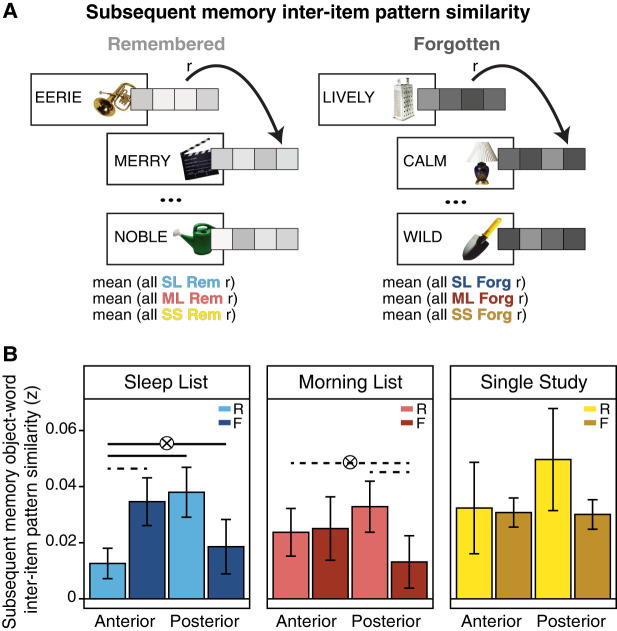
While we found that differentiation in the all trial interitem pattern similarity in the right anterior hippocampus correlated with memory across subjects, this analysis treated all the trials identically regardless of their memory status. We were therefore interested in examining the organization of anterior and posterior hippocampal representations using a within-subjects analysis by specifically sorting the trials based on subsequent memory status. We therefore computed a “subsequent memory” interitem pattern similarity metric, separately examining the trials that were later remembered or later forgotten, for each Encoding List and category (see schematic in Fig. 5A; Materials and Methods). As we found significant category differences in the all trial interitem pattern similarity, we separately analyzed the results for the object– and scene–word pairs with 3(Encoding List: SL, ML, SS) × 2(Axis region: anterior, posterior) × 2(Hemisphere: right, left) × 2(Memory: remembered, forgotten) repeated measures ANOVAs.
Figure 5.
Subsequent memory interitem pattern analysis. (A) For the subsequent memory interitem pattern analysis, patterns of activity for each trial were extracted and sorted by Encoding List. However, only those trials that were subsequently remembered (correct source memory on either memory test for each Encoding List) were correlated for the “remembered” similarity metric, while only those that were subsequently forgotten were correlated for the “forgotten” similarity metric. (B) In the right anterior and posterior hippocampus, only the Sleep List interitem pattern similarity object–word pairs showed a significant interaction between memory outcome and axis region. Representations for remembered pairs were significantly more differentiated in the anterior than posterior hippocampus (light blue bars), while the representational patterns did not differ for forgotten trials in these regions (dark blue). (Middle panel) In contrast, the Morning List showed only a marginal interaction between memory and region of the hippocampus, with a marginal difference between the interitem pattern similarity in posterior hippocampus (remembered > forgotten). (Right panel) There were no significant effects in the Single Study List.
For the object–word pairs, the ANOVA yielded a main effect of Encoding List (F(2,36) = 3.44, P = 0.04), and a significant Hemisphere × Axis × Memory interaction (F(1,18) = 7.17, P = 0.015) suggesting differences in representations based on memory success, region along the long axis, and by hemisphere. Furthermore, a marginally significant List × Axis × Memory interaction effect (F(2,36) = 3.03, P = 0.06), indicated that, depending on when the memories were initially learned, pattern similarity also tended to differ by subsequent memory success. To unpack these interaction effects and probe our main hypothesis about the effects of sleep-dependent consolidation on memory representations across the long axis of the hippocampus, we examined the interitem pattern similarity within each hemisphere and Encoding List separately for the two categories using ANOVAs with Axis (anterior, posterior) and Memory (remembered, forgotten) as factors.
For the Sleep List object–word pairs in the right hippocampus, there was a significant interaction indicating subsequent memory interitem pattern similarity differed depending on memory status and axis region (Memory × Axis interaction: F(1,18) = 9.71, P = 0.006; main effect of Memory: F(1,18) = 0.02). This interaction effect was driven by a significant difference in the pattern similarity in the right anterior and posterior hippocampal regions for the subsequently remembered pairs (t(18) = −2.55, P = 0.02). Representations for object–word pairs that were later remembered were more differentiated in the anterior hippocampus compared with the posterior hippocampus (Fig. 5B). There was not a significant difference in the anterior and posterior hippocampal representations for Sleep List pairs that were later forgotten (t(18) = 1.37, P = 0.19).
Anterior right hippocampal representations for the Sleep List object–word pairs were also modulated by later memory success, as remembered pairs were marginally more differentiated compared with forgotten pairs (t(18) = −2.04, P = 0.057). The representations in the posterior hippocampus did not show this effect, and, in fact, the Sleep List pairs that were remembered were nonsignificantly, but numerically, more similar (i.e., greater representational overlap) than pairs that were forgotten (t(18) = 1.66, P = 0.11) (Fig. 5B). These results indicate that within participants, memories learned prior to overnight sleep that became more differentiated in the anterior hippocampus were retained on subsequent memory tests, consistent with the across-subjects effects presented above (Fig. 4).
Critically, these effects were not seen for the Morning List pairs. An ANOVA did result in a marginally significant Memory × Axis interaction effect for the right hippocampus (F(1,18) = 3.3, P = 0.09), with no significant main effects (Memory: F(1,18) = 1.45, Axis: F(1,18) = 0.02). However, the representations did not differ between the right anterior and posterior hippocampal regions for either memory outcome (remembered: t(18) = −0.77, P = 0.45; forgotten: t(18) = 1.22, P = 0.24) (Fig. 6B). There was also not a significant difference in the anterior hippocampal representations for remembered and forgotten Morning List object–word pairs (t(18) = −0.16, P = 0.87), although in the posterior hippocampus, there was marginally greater overlap in the representations among remembered pairs compared with forgotten pairs (t(18) = −1.87, P = 0.08). Likewise, the same analyses on the Single Study List pairs did not yield a significant Memory × Region interaction effect (F(1,18) = 0.76, P = 0.40), or any significant main effects on subsequent memory interitem pattern similarity (Memory: F(1,18) = 0.60, P = 0.45; Region: F(1,18) = 0.583, P = 0.46).
Figure 6.
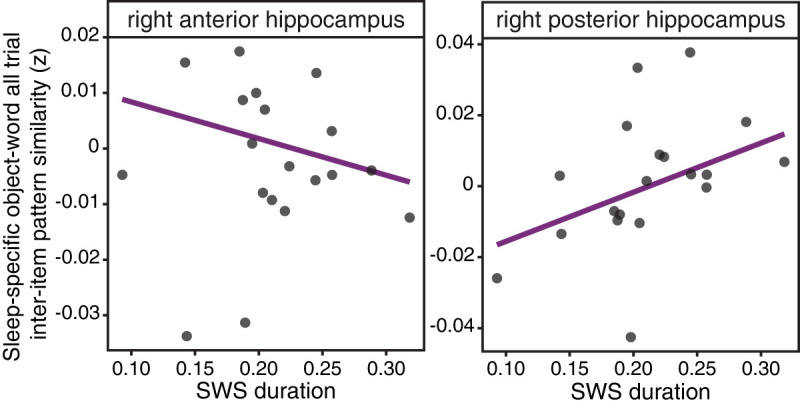
Slow-wave sleep (SWS) duration correlates with all trial interitem pattern similarity in the right posterior hippocampus. In the right posterior hippocampus (right), the all trial sleep-specific (SL-ML) object–word pairs that were later correctly remembered showed a significant, positive correlation with the duration of SWS (as a proportion of total sleep time); however, this relationship was not significant for the sleep-specific all trial similarity in right anterior hippocampus (left).
Taken together, these results highlight that only those object–word pairs learned prior to sleep showed differences in the mnemonic representations along the long axis of the hippocampus, with greater differentiation among memory representations in the right anterior hippocampus, compared with more overlapping representations in the posterior hippocampus. This modulation by memory and region was only observed for the right hemisphere; interitem pattern similarity did not differ by Encoding List or Memory in left hemisphere regions (see Supplemental Information S1), and scene–word pairs were not modulated by memory (see Supplemental Information S2).
Relationship between interitem pattern similarity relationship and overnight sleep measures
Thus far, we have determined that the organization of the memory representations for information initially learned prior to sleep differed by the region along the long axis of the hippocampus. The specificity of these effects to the Sleep List raises the possibility that such changes are modulated by aspects of the intervening sleep period. Therefore, we next examined the relationship between features of sleep, particularly the duration of SWS, measured as the proportion of total sleep time (TST) (see the Materials and Methods), in facilitating changes in the representational organization of memories in the right anterior and posterior hippocampus.
To examine whether sleep features correlate with the representational organization of these pairs learned prior to sleep, we first examined the relationship with all trial interitem pattern similarity. We used the same “sleep-specific” metric of all trial interitem pattern similarity that was found to correlate with memory accuracy in the right anterior hippocampus (Fig. 4). All trial sleep-specific interitem pattern similarity in the right posterior hippocampus positively correlated with the duration of SWS during overnight sleep (r = 0.47, robust: β = 0.14, P = 0.01), such that the duration of SWS was associated with more overlapping pattern representations in the right posterior hippocampus for the Sleep List object–word pairs, relative to the Morning List (Fig. 6). Although the correlation between the sleep-specific interitem pattern similarity in the right posterior hippocampus and the duration of stage 2 sleep was significant, it did not survive robust regression (r = −0.58, robust: β = −0.15, P = 0.17). The all trial sleep-specific similarity in the right anterior hippocampus did not correlate with either SWS (r = 0.006) or stage 2 sleep (r = 0.04). Thus, it seems that the duration of SWS in the intervening overnight sleep period is related to the representation of object–word pairs initially learned prior to the sleep period.
We next tested whether the duration of SWS correlated with the representation in the right posterior hippocampus when considering the pattern similarity for object–word pairs sorted by subsequent memory status. The sleep-specific interitem pattern similarity for subsequently remembered object–word pairs in the right posterior hippocampus did not significantly correlate with the duration of SWS (r = 0.33, P = 0.16), although there was a marginally significant negative correlation between this similarity measure and the duration of stage 2 sleep (r = −0.52, β = −0.22, P = 0.09). In the right anterior hippocampus, the sleep-specific similarity for object–word pairs that were later remembered did not correlate with either SWS (r = 0.26, β = 0.03) or stage 2 sleep (r = −0.13, β = −0.02).
In line with prior work, we next considered the relationship between features of sleep and a more broad measure of the univariate hippocampal BOLD signal (Supplemental Information S3). This analysis yielded a similar pattern as the all trial interitem pattern similarity: sleep-specific univariate activation in the right posterior hippocampus for object–word pairs that were subsequently remembered positively correlated with the duration of SWS (r = 0.51, β = 110.4, P = 0.036) (Supplemental Fig. S1). In other words, a longer duration of SWS during overnight sleep was related to greater activation in the right posterior hippocampus. Univariate activation in right anterior hippocampus did not correlate with measures of sleep (Supplemental Information S3). Thus, while hippocampal univariate activation did not seem to be modulated by later memory success (Supplemental Information S3), individual differences in overnight sleep measures, namely, SWS duration, is related to the univariate activation in the right posterior hippocampus.
Together, these results highlight that the duration of time spent in SWS during the intervening overnight period is related to the right posterior hippocampal representation of object–word information initially learned prior to sleep.
Univariate control analysis
Finally, while we did not find main effects of Encoding Lists for univariate BOLD activation (see Supplemental Information S3), it is still possible that the results from multivariate pattern analyses were influenced by differences in univariate responses in these ROIs. Thus, we next conducted separate control analyses for all the aforementioned analyses, examining whether the significant effects were maintained when accounting for differences in univariate activation. We conducted mixed effects linear regressions on a trial-level basis, including the interitem pattern similarity and activation in models predicting similarity differences. We compared these models with models without activation as a predictor, and created a contrast to confirm the effects remained significant when the model accounted for univariate activation. All previously reported significant comparisons remained statistically significant when accounting for univariate activation differences (see Supplemental Table S2).
Discussion
This study used a multimodal approach to demonstrate that consolidation impacts the organization of memories in the hippocampus. Using multivariate pattern analyses, we identified a time-dependent transformation in hippocampal representations for memories learned prior to a period of overnight sleep. When provided the opportunity for consolidation, memories for object–word pairs showed more differentiated representations in the right anterior hippocampus but greater representational overlap in the right posterior hippocampus. This pattern of results was not seen for the stimuli learned after the sleep period, demonstrating a dissociation along the long axis of the hippocampus that emerges with consolidation. Additionally, differentiation in the right anterior hippocampus among the pairs learned prior to sleep correlated with subsequent memory accuracy, indicating such representational organization may facilitate memory retention or maintenance. Furthermore, both the extent of representational overlap and univariate activation in the right posterior hippocampus for object–word pairs positively correlated with the duration of SWS, suggesting that sleep in particular may facilitate the organization of memories in this region. Together, these results indicate that consolidation changes the way memories initially formed prior to overnight sleep are represented the next day, and highlights the heterogeneity in the representational organization along the hippocampal long axis.
Much work has suggested that the anterior and posterior regions of the hippocampus support different functions. The rodent model, drawing primarily on findings of spatial coding in place cells, involves a gradient in place field size along the long axis of the hippocampus, with larger place fields in the ventral (akin to anterior) hippocampus, and smaller in the dorsal (akin to posterior) hippocampus (Kjelstrup et al. 2008; Komorowski et al. 2013). Drawing on these findings as a general marker of representational organization, it has been suggested that such differences in place field “granularity” map onto the computations processed in these regions, such that the anterior hippocampus supports broader contextual representations, and the posterior hippocampus represents more fine-grained, spatial details (Poppenk et al. 2013; Robin and Moscovitch 2017; Sekeres et al. 2018a; Brunec et al. 2020). Recent work using multivariate pattern analysis has been consistent with this proposed dichotomy (Collin et al. 2015; Ritchey et al. 2015; Schlichting et al. 2015; Brunec et al. 2018; Sekeres et al. 2018b). However, our results do not clearly support this model. In the present study, memories initially learned prior to sleep were more differentiated in the anterior hippocampus, and more overlapping in the posterior hippocampus. Thus, this suggests more coarse representations in the posterior hippocampus and more fine-grained representations in the anterior hippocampus in contrast to prior work.
One intriguing possible explanation for this difference is that our work focused on how memory traces are represented after a delay. Indeed, of the few studies that have examined the effect of consolidation on hippocampal memory representations, several have reported findings in line with those reported here, with differentiated memory representations in the anterior hippocampus (Bonnici et al. 2012; Dandolo and Schwabe 2018; Ezzyat et al. 2018), and overlapping representations in the posterior hippocampus (Tompary and Davachi 2017; Dandolo and Schwabe 2018; Ezzyat et al. 2018; Audrain and McAndrews 2020). Interestingly, while several studies have shown more overlapping representations (e.g., greater similarity) among memories with shared content than without shared content (Ritchey et al. 2015; Tompary and Davachi 2017; Audrain and McAndrews 2020), Tompary and colleagues found that in the anterior hippocampus, this representational shift across a week delay was driven by increased differentiation among the representations for memories without shared content. The present findings provide complementary evidence for this effect, as we tested the pattern similarity among individual memories learned at the same time (e.g., within an encoding list), but without any directly shared content. Thus, it is possible that the anterior hippocampus may both integrate memories with explicitly shared content, while also differentiating the memories that do not have similar information. Future work will need to modulate the degree of similarity among the memoranda to determine the effect on the representations along the long axis of the hippocampus. Additionally, while in our design the Single Study List was learned for the first time during the scan, we did not find evidence of a difference in granularity across the long axis; it is possible that the lower accuracy in memory for these pairs precluded clear results, potentially driven by the large memory load with the Sleep and Morning Lists, a potential buildup of proactive interference, and/or the single presentation of these pairs. Thus, future work that matches memory success but examines patterns during encoding and after a period of consolidation will be important.
Active processes during sleep may underlie the observed changes in the organization of memories. Much prior work has shown that memory retention is benefitted by features of sleep, including the duration of SWS (Takashima et al. 2006; Diekelmann and Born 2010; Wilhelm et al. 2011; Alger et al. 2012; Rasch and Born 2013; Baran et al. 2016). However, despite work linking sleep to consolidation-related processes, very little research has examined how sleep contributes to changes in the representational organization of memories. We previously published evidence that the density of thalamocortical sleep spindles correlates with increased representational overlap in the ventromedial prefrontal cortex for object–word pairs learned prior to sleep (Cowan et al. 2020), in line with the purported role of spindles in facilitating the hippocampal–cortical dialogue (Siapas and Wilson 1998; Sirota et al. 2003; Diekelmann and Born 2010; Rasch and Born 2013; Staresina et al. 2015). In the present study, focusing on the results of sleep-dependent consolidation on hippocampal representations, we found that the duration of SWS positively correlated with the more general all trial measure of representational overlap in the posterior hippocampus for the object–word pairs learned prior to the sleep period. This is consistent with our findings indicating greater similarity, or representational overlap, in posterior hippocampus for the subsequently remembered Sleep List pairs.
Interestingly, despite the observed interaction between the long-axis region (anterior vs. posterior) and memory as well as the correlation with sleep, posterior hippocampal similarity did not correlate with behavioral measures of memory across subjects. It is possible that the source memory test used here, which probed for specific associative memories, was ill-suited to capture the contributions of the posterior hippocampal representations, which the increased representational overlap suggests may become more gist-based or integrated across memories. This may also explain why we did not see a correlation between SWS and the similarity for subsequently remembered pairs in the right posterior hippocampus in particular. Intriguingly, data from Audrain and McAndrews (2020) suggests that consolidation-dependent increases in similarity in posterior hippocampal representations could also reflect specificity in memory, rather than coarser or gist-like memories, reporting greater similarity for objects paired with the same scene than those paired with similar scenes from the same schema, or a difference scene. Therefore, a critical avenue for future work will therefore be to systematically probe whether transformations to anterior and posterior representations with consolidation facilitate dissociable measures of gist and specific memory.
In contrast to the effects in the right posterior hippocampus, differentiation in the right anterior hippocampal representations did correlate with subsequent memory, indicating that such representational organization may facilitate the retention or maintenance of memories. However, we did not find a significant correlation between differentiation in the right anterior hippocampus and measures of overnight sleep. This null effect raises the question of whether the shift in the right anterior hippocampus is driven by processes dependent on sleep or via consolidation-dependent mechanisms that generally unfold over time. There has been considerable work demonstrating the particular beneficial effects of sleep on memory retention (Diekelmann and Born 2010; Rasch and Born 2013); however, there is likewise burgeoning evidence that consolidation can also occur during periods of wake rest (Tambini and Davachi 2019). Thus, with little work connecting sleep with changes in neural representational organization, the distinction between time-dependent and sleep-dependent consolidation remains an open question.
The duration of sleep stage measure used here is relatively coarse, and our results raise the question of whether more fine-grained metrics of hippocampal replay might be a better measure to understand changes in the representations along the long axis. Replay is a local phenomenon in which ensembles of neurons that had been active during the initial learning experience are repeatedly reactivated during subsequent offline periods of sleep (Buzsáki 1989; Wilson and McNaughton 1994; Girardeau and Zugaro 2011). It is possible such repeated reactivations could also underlie the shift in the representational patterns for recently learned information—particularly altering the representation of pairs that will be later remembered. Thus, future work examining the incidence of sharp wave ripples in the hippocampus may be better equipped to demonstrate how active processes during sleep impact representations along the long axis of the hippocampus.
The duration of SWS also positively correlated with the univariate BOLD activation in the right posterior hippocampus for pairs learned prior to sleep, relative to pairs learned after sleep. This result suggests that the duration of intervening SWS facilitates greater activation in the posterior hippocampus. On the surface, this is in contrast to prior work that found the duration of SWS during a nap was associated with decreased hippocampal univariate activation when retrieving memories learned before the sleep period (Takashima et al. 2006; Baran et al. 2016). However, it is important to consider that in our design, unlike these other studies, scanning occurred during a restudy session in which the pairs learned prior to sleep were reexposed rather than explicitly retrieved. While our analysis of the RT data did show that memory was intact for these sleep-consolidated pairs, it is possible that the activation elicited by the restudy period was due to further processing or encoding.
Finally, the reported results consistently pointed to category differences in the changes in the representations for object– and scene–word pairs. One possible explanation is that the object–word pairs are better learned after the initial exposure compared with the scene–word pairs. The RT data, an implicit measure of recognition memory, seems to support this explanation. For previously studied pairs, the Sleep List and Morning List, participants responded faster to object–word pairs than scene–word pairs. As there were no significant category differences for the entirely novel Single Study List pairs, this effect does not seem to be driven by perceptual differences between the categories alone. However, an interesting area for future work will be to disentangle how category differences and memory strength interact with the effects of sleep-dependent consolidation.
While theories have provided differing views about consolidation's effects on the hippocampus, most studies have only queried whether retrieval of a memory continues to evoke the hippocampus over time. Here, we provided evidence that the relationship between consolidation and the hippocampus is more complex, suggesting that hippocampal memory traces also be transformed along the structure's long axis with consolidation, facilitating memory retention for the long term.
Materials and Methods
Participants
Other results from this data set have been reported previously (Cowan et al. 2020). A total of 22 participants were recruited for this institutional review board (IRB)-approved experiment (all methods approved by the NYU School of Medicine's IRB). Two participants were excluded due to problems with the MRI scanner, and one participant was excluded as an outlier on TST (>2.5 standard deviations below the group average). All participants were between 18–35 years of age (mean = 25.3), fluent in English, did not have any diagnoses of neurologic, psychiatric or sleep disorders, and were not using any psychoactive medications. Participants had not traveled across time zones or completed night-shift work in the month preceding the first study session. All participants had a maximum body-mass index (BMI) of 30 and did not have any contraindications for the MRI. All participants provided informed consent, and were compensated for their time. The sample size was based on similar studies examining sleep effects on neural activation (Takashima et al. 2006; Gais et al. 2007; Sterpenich et al. 2007; Hennies et al. 2016) and studies examining multivariate pattern similarity (LaRocque et al. 2013; Tompary and Davachi 2017).
Procedure
The design of the experiment is outlined in Figure 1. For the 48-h prior to the beginning of session 1 (day 1), participants were asked to log their sleep patterns, refrain from any alcohol and drug use, and reduce caffeine intake to one cup per day. Participants reported to the NYU Langone Comprehensive Epilepsy Center/Sleep Center for the first session of the experiment at 8:00 p.m. They first were asked to complete questionnaires to assess eligibility for study participation and query sleep habits, including the MoCA, Insomnia Symptom Questionnaire, Morningness–Eveningness Questionnaire, STOP-BANG Questionnaire, and Epworth Sleepiness Scale. Participants then prepared for bed and the technicians completed the PSG setup.
In all encoding sessions, participants viewed word–image pairs (word–scene or word–object pairs) and were instructed to form a vivid mental association between the word and image on screen, and then rate how well the association could be formed, responding either “very well,” “somewhat well,” or “not well.” All stimuli were presented on an Apple MacBook laptop, and responses were made using the computer's keyboard. Word–image pairs were each presented for 4500 msec. Trials involved the presentation of a central red fixation cross for 500 msec, the word–image pair for 4500 msec, followed by a black fixation cross for 500 msec. Following the black fixation cross for each trial, participants completed an active baseline task (Stark and Squire 2001). Numeric digits ranging between 1 and 9 were presented, and participants were instructed to respond “even” or “odd.” The ITI active baseline task lasted a total of 8.5 sec: each digit was presented for a maximum of 2000 msec or until a response was entered and was followed by a black fixation cross for 250 msec (e.g., between each digit a fixation cross was shown). Sixty trials were presented, with a 30-sec break halfway through. The order of word–image pairs was randomized for each participant and intermixed between the categories.
We heretofore refer to the first encoding session as the “Sleep List,” as participants slept overnight after completing the encoding task. Participants got into bed, the PSG setup was completed, and the lights were turned off. Participants were not allowed reading material or access to cell phones while in bed. The subsequent morning, they were woken up by 7:30 a.m. and provided time to eat breakfast. Participants then encoded the second Encoding List, referred to as the “Morning List,” which was followed by a short phonemic fluency task and a second administration of the Epworth Sleepiness Questionnaire to assess level of arousal. Technicians disconnected and removed all EEG electrodes.
Participants then traveled to the Center for Brain Imaging at New York University for the fMRI session (scan began at ∼10:00 a.m.). In the scanner, participants were represented with all previously studied word–image pairs from both the Sleep and Morning Lists, as well as a third set of novel pairs (the “Single Study List”); during the scanning session, the 120 word–image pairs from each Encoding List were randomly intermixed, and divided into six separate runs, with 60 trials per run. Participants performed the same task as in the previous two encoding sessions (forming an association between the word and image, rating how well they could do so). All responses were made using an MRI-compatible button box. After the encoding task, participants completed a localizer task in which they viewed novel objects, scenes, and scrambled objects, which was used in a separate line of inquiry. A high-resolution anatomical image of each participant's brain was acquired at the end of the scan.
Immediately after participants were removed from the scanner, we probed participants’ memory for the word–image associations by testing source memory, using the word as a cue. Participants also returned to the laboratory 24 h later for a second, “Delayed” memory test. Participants were asked to try to remember whether they had seen the word before and whether it had been paired with a scene or object image. The possible responses included: “old–scene,” “old–object,” “word only,” or “new.” The legend for response options was presented under the cue word on screen. Participants were instructed to use the “scene” and “object” responses only if they could remember the specific image with which the word had been paired, while the “word only” key was to be used if they recognized the test word but could not remember the associated category of the image it had been paired with. The “new” key was to be used if participants believed the word to be novel, or if they were unsure whether it had been studied. Half of the words from each Encoding List were included on each of the two tests (Immediate and Delayed) to avoid retesting stimuli, along with novel foil words. Each test trial consisted of a red fixation cross presented for 500 msec, followed by a centrally presented word cue, which was on screen for a maximum of 12 sec, or until a response was made, and was followed by a black fixation cross for 200 msec.
Stimuli
Outdoor scene stimuli were randomly selected from an online database (at http://cvcl.mit.edu/database.html; Oliva and Torralba 2001). Object stimuli were selected from the MIT Massive Memory set (Brady et al. 2008) and word stimuli were adjectives from the MRC psycholinguistics database (http://websites.psychology.uwa.edu.au/school/MRCDatabase/uwa_mrc.htm). Words were presented in size 36 black Helvetica font on a white background. Each of the three Encoding Lists consisted of 120 word–image pairs (60 object–word and 60 scene–word pairs). Half of the words from each list (30 object–word, 30 scene–word), and 60 novel foil words were included on each memory test. Word–image pairs were counterbalanced across Encoding Lists and across participants, and presented in a randomized order. During the localizer task, an additional set of 144 object stimuli (72 per run), and 144 scene stimuli were included, sourced from the aforementioned databases.
Statistical analyses
All statistical analyses are two-tailed and P < 0.05 was considered significant for all statistical tests. Repeated measures ANOVAs were performed and followed up with two-tailed paired sample t tests where applicable. Williams’ tests were also used to test the difference between dependent correlations sharing a variable. Statistics were performed with RStudio (RStudio, version 0.99.903) and Matlab (MathWorks) using both built-in and custom functions. The R “robust” package was used to calculate robust regression to downweight potential outliers while retaining the most data possible.
Polysomnography
Overnight PSG was conducted at the NYU Langone Sleep Center using the Xltek data acquisition system (Natus Medical). PSG measurements included standard electroencephalography (International 10/20 Electrode Placement; Fp1, Fp2, F7, F3, F8, F4, Fz, Cz, T3, C3, C4, T4, T5, P3, Pz, P4, T6, O1, O2, and A1, A2 mastoid references), Electrooculogram (Left/Right EOG), Chin Electromyogram (EMG), as well as Chest and Leg movements, Respiratory monitoring, and Blood Oxygenation (SpO2). EEG data were digitized at a sampling rate of 256 Hz.
Each participant's night of sleep was scored by a sleep technician and checked over by a board-certified sleep physician, according to the American Academy of Sleep Medicine manual (Berry et al. 2012). The data was staged in 30-sec epochs and categorized as stage 1, 2, SWS, or REM sleep. Together, the first three stages were defined as non-REM sleep. The total duration of each sleep stage was normalized by each participant's TST before being correlated with imaging results. The average duration of all sleep stages are presented in Supplemental Table S1. In the present work, we were particularly interested in SWS as a broader measure as a stage of sleep thought to be relevant for replay (Diekelmann and Born 2010; Rasch and Born 2013) and which has been previously related to both benefits in memory (Takashima et al. 2006; Diekelmann and Born 2010; Wilhelm et al. 2011; Alger et al. 2012; Rasch and Born 2013; Baran et al. 2016) as well as changes in hippocampal univariate signal (Takashima et al. 2006; Baran et al. 2016).
Behavioral analyses
Correct associative memory was defined as accurate source memory judgments (“remembered”), while previously studied pairs judged as “new” were considered “forgotten.” For all analyses, memory was collapsed across the Immediate and Delayed tests to increase statistical power.
MRI acquisition and preprocessing
Scanning was completed on a 3T Siemens Allegra head-only scanner. Functional imaging data was collected using an echo-planar imaging (EPI) pulse sequence (TR = 2000 msec, TE = 15 msec, 34 interleaved slices oriented parallel to the AC–PC axis, flip angle = 82°, voxel size = 3 × 3 × 3 mm). The first six volumes were discarded to allow for T1 stabilization. A high-resolution T1 weighted anatomical scan (magnetization-prepared rapid-acquisition gradient echo sequence, voxel size = 1 × 1 × 1 mm) was acquired to aid in functional image coregistration.
Preprocessing on functional data was performed using the FSL (version 5.0.2.2) fMRI Expert Analysis Tool version 6 (FSL: http://fsl.fmrib.ox.ac.uk/fsl). Functional images were brain-extracted, high-pass filtered (110-sec cutoff), realigned to correct for interleaved acquisition, and MCFLIRT was applied for motion correction. For univariate analyses, data was spatially smoothed with a 5-mm FWHM kernel. FSL's motion outliers tool was used to identify outlier time points (using DVARS), which were included as an additional regressor in all subsequent general linear models (GLMs). Functional data was registered to the high-resolution anatomical scans with FSL's FLIRT tool (12 degrees of freedom [DOF], nonlinear registration 10-mm warp resolution), then to standard Montreal Neurological Institute (MNI) space using FNIRT's nonlinear registration with a 10-mm warp resolution.
Interitem pattern similarity analyses
For the interitem pattern similarity analyses, functional data was preprocessed as outlined above with a few exceptions: the data was smoothed using a 3-mm FWHM kernel and kept in native space for each participant. After preprocessing, each run was aligned to the first run. For each run, trial-level GLMs were constructed by modeling one regressor per trial (60 regressors in total), with additional regressors for extreme head motion and temporal derivative (Mumford et al. 2014; Cowan et al. 2020). Resulting t-stat maps were imported into Matlab, where t-stat activation maps were extracted for each ROI in native space.
A linear vector containing the BOLD activation in each voxel in each ROI was extracted for each trial in the three Encoding Lists (Sleep List, Morning List, and Single Study List), separated by the category condition (e.g., word–scene vs. word–object pairings), and the Pearson's correlation coefficient (r) was computed between each pairwise vector. This allowed assessment of the extent to which the activation patterns for each trial correlated with all other trials from the same list across scanning runs, thereby avoiding confounds from temporal autocorrelation (Mumford et al. 2014). The averaged trial-level correlations were then normalized using a Fisher r-to-z transformation and averaged, resulting in similarity estimates for each Encoding List. Correlations were performed in two ways: with all other trials from the same Encoding List, irrespective of later memory outcome (“all trial”) (Fig. 3A), and separately only among later remembered or forgotten trials from the same Encoding List (“subsequent memory”) (Fig. 5A). Akin to earlier studies, this analysis allowed for the examination of representations among memories learned at the same time (LaRocque et al. 2013; Tompary and Davachi 2017; Ezzyat et al. 2018). Of note, one participant did not have sufficient “forgotten” trials for the Morning List scene pairs to complete the across-run subsequent memory similarity analysis; thus, the analysis for scene pairs by subsequent memory interitem pattern similarity analysis included 18 participants.
Univariate analyses
Two different types of univariate analyses were performed: the first examined “all trial” activation for all trials on the Encoding Lists, and the second was broken down by subsequent memory status (e.g., remembered vs. forgotten). For the “all trial” GLMs, regressors were created for each Encoding List and image category (object or scene), for a total of six regressors (SL-object, SL-scene, ML-object, ML-scene, etc.). Planned contrasts between the Encoding Lists were modeled, in addition to the temporal derivative and temporal filtering. For “subsequent memory” GLMs, 18 regressors were included to model the possible memory outcomes: remembered (source correct), item (source incorrect or word only responses), or forgotten (miss), broken down by Encoding List and the category of paired image, collapsed across memory test.
Region of interest (ROI) definition
Anatomical participant-specific hippocampal ROIs were defined using FSL's automatic segmentation tool, FIRST. For each participant, we generated left and right hippocampal masks, which were then divided lengthwise into anterior and posterior halves using custom code in MATLAB. Averaging across the subject-specific ROIs, we found that the number of voxels in each region was as follows: right anterior hippocampus contained 86 voxels, right posterior contained 57 voxels, left anterior contained 84 voxels, and left posterior contained 60 voxels. See Figure 3B for an example of hippocampal anterior and posterior ROIs.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant R01-MH-076492 to L.D., Dart Neuroscience Grant to L.D., NYU Clinical Translational Science Initiative Grant to A.A.L., and a National Science Foundation Graduate Research Fellowship to E.T.C. We thank Alexa Tompary and Vishnu Murty for helpful comments on an earlier draft of this manuscript.
Footnotes
[Supplemental material is available for this article.]
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.053438.121.
References
- Alger SE, Lau H, Fishbein W. 2012. Slow wave sleep during a daytime nap is necessary for protection from subsequent interference and long-term retention. Neurobiol Learn Mem 98: 188–196. 10.1016/j.nlm.2012.06.003 [DOI] [PubMed] [Google Scholar]
- Alvarez P, Squire LR. 1994. Memory consolidation and the medial temporal lobe: a simple network model. Proc Natl Acad Sci 91: 7041–7045. 10.1073/pnas.91.15.7041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade KC, Spoormaker VI, Dresler M, Wehrle R, Holsboer F, Samann PG, Czisch M. 2011. Sleep spindles and hippocampal functional connectivity in human NREM sleep. J Neurosci 31: 10331–10339. 10.1523/JNEUROSCI.5660-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain S, McAndrews MP. 2020. Schemas provide a scaffold for neocortical integration at the cost of memory specificity over time. bioRxiv 10.1101/2020.10.11.335166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran B, Mantua J, Spencer RMC. 2016. Age-related changes in the sleep-dependent reorganization of declarative memories. J Cogn Neurosci 28: 792–802. 10.1162/jocn_a_00938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry DN, Maguire EA. 2019. Remote memory and the hippocampus: a constructive critique. Trends Cogn Sci 23: 128–142. 10.1016/j.tics.2018.11.005 [DOI] [PubMed] [Google Scholar]
- Bergmann TO, Mölle M, Diedrichs J, Born J, Siebner HR. 2012. Sleep spindle-related reactivation of category-specific cortical regions after learning face-scene associations. Neuroimage 59: 2733–2742. 10.1016/j.neuroimage.2011.10.036 [DOI] [PubMed] [Google Scholar]
- Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF, et al. 2012. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. J Clin Sleep Med 8: 597–619. 10.5664/jcsm.2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnici HM, Chadwick MJ, Lutti A, Hassabis D, Weiskopf N, Maguire EA. 2012. Detecting representations of recent and remote autobiographical memories in vmPFC and hippocampus. J Neurosci 32: 16982–16991. 10.1523/JNEUROSCI.2475-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosshardt S, Degonda N, Schmidt CF, Boesiger P, Nitsch RM, Hock C, Henke K. 2005a. One month of human memory consolidation enhances retrieval-related hippocampal activity. Hippocampus 15: 1026–1040. 10.1002/hipo.20105 [DOI] [PubMed] [Google Scholar]
- Bosshardt S, Schmidt CF, Jaermann T, Degonda N, Boesiger P, Nitsch RM, Hock C, Henke K. 2005b. Effects of memory consolidation on human hippocampal activity during retrieval. Cortex 41: 486–498. 10.1016/S0010-9452(08)70189-8 [DOI] [PubMed] [Google Scholar]
- Brady TF, Konkle T, Alvarez GA, Oliva A. 2008. Visual long-term memory has a massive storage capacity for object details. Proc Natl Acad Sci 105: 14325–14329. 10.1073/pnas.0803390105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunec IK, Bellana B, Ozubko JD, Man V, Robin J, Liu ZX, Grady C, Rosenbaum RS, Winocur G, Barense MD, et al. 2018. Multiple scales of representation along the hippocampal anteroposterior axis in humans. Curr Biol 28: 2129–2135.e6. 10.1016/j.cub.2018.05.016 [DOI] [PubMed] [Google Scholar]
- Brunec IK, Robin J, Olsen RK, Moscovitch M, Barense MD. 2020. Integration and differentiation of hippocampal memory traces. Neurosci Biobehav Rev 118: 196–208. 10.1016/j.neubiorev.2020.07.024 [DOI] [PubMed] [Google Scholar]
- Buzsáki G. 1989. Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience 31: 551–570. 10.1016/0306-4522(89)90423-5 [DOI] [PubMed] [Google Scholar]
- Buzsáki G. 1996. The hippocampo-neocortical dialogue. Cereb Cortex 6: 81–92. 10.1093/cercor/6.2.81 [DOI] [PubMed] [Google Scholar]
- Chanales AJH, Oza A, Favila SE, Kuhl BA. 2017. Overlap among spatial memories triggers repulsion of hippocampal representations. Curr Biol 27: 2307–2317.e5. 10.1016/j.cub.2017.06.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens Z, Mölle M, Eross L, Jakus R, Rásonyi G, Halász P, Born J. 2011. Fine-tuned coupling between human parahippocampal ripples and sleep spindles. Eur J Neurosci 33: 511–520. 10.1111/j.1460-9568.2010.07505.x [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H. 1993. Memory, amnesia, and the hippocampal system. The MIT Press, Cambridge, MA. [Google Scholar]
- Collin SHP, Milivojevic B, Doeller CF. 2015. Memory hierarchies map onto the hippocampal long axis in humans. Nat Neurosci 18: 1562–1564. 10.1038/nn.4138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan E, Liu A, Henin S, Kothare S, Devinsky O, Davachi L. 2020. Sleep spindles promote the restructuring of memory representations in ventromedial prefrontal cortex through enhanced hippocampal–cortical functional connectivity. J Neurosci 40: 1909–1919. 10.1523/JNEUROSCI.1946-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan ET, Fain MR, O'Shea I, Ellman LM, Murty VP. 2021. VTA and anterior hippocampus target dissociable neocortical networks for post-novelty enhancements. J Neurosci (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandolo LC, Schwabe L. 2018. Time-dependent memory transformation along the hippocampal anterior–posterior axis. Nat Commun 9: 1205. 10.1038/s41467-018-03661-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L. 2006. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol 16: 693–700. 10.1016/j.conb.2006.10.012 [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Born J. 2010. The memory function of sleep. Nat Rev Neurosci 11: 114–126. 10.1038/nrn2762 [DOI] [PubMed] [Google Scholar]
- Evensmoen HR, Lehn H, Xu J, Witter MP, Nadel L, Håberg AK. 2013. The anterior hippocampus supports a coarse, global environmental representation and the posterior hippocampus supports fine-grained, local environmental representations. J Cogn Neurosci 25: 1908–1925. 10.1162/jocn_a_00436 [DOI] [PubMed] [Google Scholar]
- Ezzyat Y, Inhoff M, Davachi L. 2018. Differentiation of human medial prefrontal cortex activity underlies long-term resistance to forgetting in memory. J Neurosci 38: 2290–2217. 10.1523/JNEUROSCI.2290-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. 2005. The organization of recent and remote memories. Nat Rev Neurosci 6: 119–130. 10.1038/nrn1607 [DOI] [PubMed] [Google Scholar]
- Gais S, Albouy G, Boly M, Dang-Vu TT, Darsaud A, Desseilles M, Rauchs G, Schabus M, Sterpenich V, Vandewalle G, et al. 2007. Sleep transforms the cerebral trace of declarative memories. Proc Natl Acad Sci 104: 18778–18783. 10.1073/pnas.0705454104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardeau G, Zugaro M. 2011. Hippocampal ripples and memory consolidation. Curr Opin Neurobiol 21: 452–459. 10.1016/j.conb.2011.02.005 [DOI] [PubMed] [Google Scholar]
- Hennies N, Lambon Ralph MA, Kempkes M, Cousins JN, Lewis PA. 2016. Sleep spindle density predicts the effect of prior knowledge on memory consolidation. J Neurosci 36: 3799–3810. 10.1523/JNEUROSCI.3162-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D, Wilson MA. 2007. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci 10: 100–107. 10.1038/nn1825 [DOI] [PubMed] [Google Scholar]
- Kjelstrup KB, Solstad T, Brun VH, Hafting T, Leutgeb S, Witter MP, Moser EI, Moser M-B. 2008. Finite scale of spatial representation in the hippocampus. Science 321: 140–143. 10.1126/science.1157086 [DOI] [PubMed] [Google Scholar]
- Komorowski RW, Garcia CG, Wilson A, Hattori S, Howard MW, Eichenbaum H. 2013. Ventral hippocampal neurons are shaped by experience to represent behaviorally relevant contexts. J Neurosci 33: 8079–8087. 10.1523/JNEUROSCI.5458-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRocque KF, Smith ME, Carr VA, Witthoft N, Grill-Spector K, Wagner AD. 2013. Global similarity and pattern separation in the human medial temporal lobe predict subsequent memory. J Neurosci 33: 5466–5474. 10.1523/JNEUROSCI.4293-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O'Reilly RC. 1995. Why there are complementary learning systems in the hippocampus and neo-cortex: insights from the successes and failures of connectionists models of learning and memory. Psychol Rev 102: 419–457. 10.1037/0033-295X.102.3.419 [DOI] [PubMed] [Google Scholar]
- Milton F, Muhlert N, Butler CR, Smith A, Benattayallah A, Zeman AZ. 2011. An fMRI study of long-term everyday memory using SenseCam. Memory 19: 733–744. 10.1080/09658211.2011.552185 [DOI] [PubMed] [Google Scholar]
- Mölle M, Born J. 2011. Slow oscillations orchestrating fast oscillations and memory consolidation. Prog Brain Res 193: 93–110. 10.1016/B978-0-444-53839-0.00007-7 [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Cabeza R, Winocur G, Nadel L. 2016. Episodic memory and beyond: the hippocampus and neocortex in transformation. Annu Rev Psychol 67: 105–134. 10.1146/annurev-psych-113011-143733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford JA, Davis T, Poldrack RA. 2014. The impact of study design on pattern estimation for single-trial multivariate pattern analysis. Neuroimage 103: 130–138. 10.1016/j.neuroimage.2014.09.026 [DOI] [PubMed] [Google Scholar]
- Murty VP, Tompary A, Adcock RA, Davachi L. 2017. Selectivity in postencoding connectivity with high-level visual cortex is associated with reward-motivated memory. J Neurosci 37: 537–545. 10.1523/JNEUROSCI.4032-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel L, Moscovitch M. 1997. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr Opin Neurobiol 7: 217–227. 10.1016/S0959-4388(97)80010-4 [DOI] [PubMed] [Google Scholar]
- Ngo HV, Fell J, Staresina B. 2020. Sleep spindles mediate hippocampal-neocortical coupling during long-duration ripples. eLife 9: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva A, Torralba A. 2001. Modeling the shape of the scene: a holistic representation of the spatial envelope. Int J Comput Vis 42: 145–175. 10.1023/A:1011139631724 [DOI] [Google Scholar]
- Peyrache A, Khamassi M, Benchenane K, Wiener SI, Battaglia FP. 2009. Replay of rule-learning related neural patterns in the prefrontal cortex during sleep. Nat Neurosci 12: 919–926. 10.1038/nn.2337 [DOI] [PubMed] [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. 2013. Long-axis specialization of the human hippocampus. Trends Cogn Sci 17: 230–240. 10.1016/j.tics.2013.03.005 [DOI] [PubMed] [Google Scholar]
- Rasch B, Born J. 2013. About sleep's role in memory. Physiol Rev 93: 681–766. 10.1152/physrev.00032.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards BA, Xia F, Santoro A, Husse J, Woodin MA, Josselyn SA, Frankland PW. 2014. Patterns across multiple memories are identified over time. Nat Neurosci 17: 981–986. 10.1038/nn.3736 [DOI] [PubMed] [Google Scholar]
- Ritchey M, Montchal ME, Yonelinas AP, Ranganath C. 2015. Delay-dependent contributions of medial temporal lobe regions to episodic memory retrieval. eLife 4: e05025. 10.7554/eLife.05025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin J, Moscovitch M. 2017. Details, gist and schema: hippocampal–neocortical interactions underlying recent and remote episodic and spatial memory. Curr Opin Behav Sci 17: 114–123. 10.1016/j.cobeha.2017.07.016 [DOI] [Google Scholar]
- Schlichting ML, Mumford JA, Preston AR. 2015. Learning-related representational changes reveal dissociable integration and separation signatures in the hippocampus and prefrontal cortex. Nat Commun 6: 8151. 10.1038/ncomms9151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekeres MJ, Winocur G, Moscovitch M. 2018a. The hippocampus and related neocortical structures in memory transformation. Neurosci Lett 680: 39–53. 10.1016/j.neulet.2018.05.006 [DOI] [PubMed] [Google Scholar]
- Sekeres MJ, Winocur G, Moscovitch M, Anderson JAE, Pishdadian S, Martin Wojtowicz J, St-Laurent M, McAndrews MP, Grady CL. 2018b. Changes in patterns of neural activity underlie a time-dependent transformation of memory in rats and humans. Hippocampus 28: 745–764. 10.1002/hipo.23009 [DOI] [PubMed] [Google Scholar]
- Siapas AG, Wilson MA. 1998. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron 21: 1123–1128. 10.1016/S0896-6273(00)80629-7 [DOI] [PubMed] [Google Scholar]
- Sirota A, Csicsvari J, Buhl D, Buzsaki G. 2003. Communication between neocortex and hippocampus during sleep in rodents. Proc Natl Acad Sci 100: 2065–2069. 10.1073/pnas.0437938100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Bergmann TO, Bonnefond M, van der Meij R, Jensen O, Deuker L, Elger CE, Axmacher N, Fell J. 2015. Hierarchical nesting of slow oscillations, spindles and ripples in the human hippocampus during sleep. Nat Neurosci 18: 1679–1686. 10.1038/nn.4119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CEL, Squire LR. 2001. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci 98: 12760–12766. 10.1073/pnas.221462998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M. 2006. Grouping of brain rhythms in corticothalamic systems. Neuroscience 137: 1087–1106. 10.1016/j.neuroscience.2005.10.029 [DOI] [PubMed] [Google Scholar]
- Sterpenich V, Albouy G, Boly M, Vandewalle G, Darsaud A, Balteau E, Dang-Vu TT, Desseilles M, D'Argembeau A, Gais S, et al. 2007. Sleep-related hippocampo-cortical interplay during emotional memory recollection. PLoS Biol 5: 2709–2722. 10.1371/journal.pbio.0050282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterpenich V, Albouy G, Darsaud A, Schmidt C, Vandewalle G, Dang-Vu TT, Desseilles M, Phillips C, Degueldre C, Balteau E, et al. 2009. Sleep promotes the neural reorganization of remote emotional memory. J Neurosci 29: 5143–5152. 10.1523/JNEUROSCI.0561-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima A, Petersson KM, Rutters F, Tendolkar I, Jensen O, Zwarts MJ, McNaughton BL, Fernández G. 2006. Declarative memory consolidation in humans: a prospective functional magnetic resonance imaging study. Proc Natl Acad Sci 103: 756–761. 10.1073/pnas.0507774103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima A, Nieuwenhuis ILC, Jensen O, Talamini LM, Rijpkema M, Fernandez G. 2009. Shift from hippocampal to neocortical centered retrieval network with consolidation. J Neurosci 29: 10087–10093. 10.1523/JNEUROSCI.0799-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambini A, Davachi L. 2019. Awake reactivation of prior experiences consolidates memories and biases cognition. Trends Cogn Sci 23: 876–890. 10.1016/j.tics.2019.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambini A, Ketz N, Davachi L. 2010. Enhanced brain correlations during rest are related to memory for recent experiences. Neuron 65: 280–290. 10.1016/j.neuron.2010.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompary A, Davachi L. 2017. Consolidation promotes the emergence of representational overlap in the hippocampus and medial prefrontal cortex. Neuron 96: 228–241.e5. 10.1016/j.neuron.2017.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompary A, Duncan K, Davachi L. 2015. Consolidation of associative and item memory is related to post-encoding functional connectivity between the ventral tegmental area and different medial temporal lobe subregions during an unrelated task. J Neurosci 35: 7326–7331. 10.1523/JNEUROSCI.4816-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Kimura HM, Hirose S, Wada H, Imai Y, Machida T, Shirouzu I, Miyashita Y, Konishi S. 2012. Functional dissociation between anterior and posterior temporal cortical regions during retrieval of remote memory. J Neurosci 32: 9659–9670. 10.1523/JNEUROSCI.5553-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzynski CM, Lubenov E V, Gu M, Siapas AG. 2009. State-dependent spike-timing relationships between hippocampal and prefrontal circuits during sleep. Neuron 61: 587–596. 10.1016/j.neuron.2009.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm I, Diekelmann S, Molzow I, Ayoub A, Molle M, Born J. 2011. Sleep selectively enhances memory expected to be of future relevance. J Neurosci 31: 1563–1569. 10.1523/JNEUROSCI.3575-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M, McNaughton B. 1994. Reactivation of hippocampal ensemble memories during sleep. Science 265: 676–679. 10.1126/science.8036517 [DOI] [PubMed] [Google Scholar]
- Winocur G, Moscovitch M. 2011. Memory transformation and systems consolidation. J Int Neuropsychol Soc 17: 766–780. 10.1017/S1355617711000683 [DOI] [PubMed] [Google Scholar]
- Winocur G, McDonald RM, Moscovitch M. 2001. Anterograde and retrograde amnesia in rats with large hippocampal lesions. Hippocampus 11: 18–26. [DOI] [PubMed] [Google Scholar]
- Yamashita K, Hirose S, Kunimatsu A, Aoki S, Chikazoe J, Jimura K, Masutani Y, Abe O, Ohtomo K, Miyashita Y, et al. 2009. Formation of long-term memory representation in human temporal cortex related to pictorial paired associates. J Neurosci 29: 10335–10340. 10.1523/JNEUROSCI.1328-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.