Abstract
Context
While age-related changes in menstrual cycle length are well known, it is unclear whether anti-Müllerian (AMH) or other ovarian reserve biomarkers have a direct association with cycle length.
Objective
To determine the association between biomarkers of ovarian reserve and menstrual cycle length.
Methods
Secondary analysis using data from time to conceive (TTC), a prospective time to pregnancy cohort study. The age-independent association between cycle length and biomarkers of ovarian reserve was analyzed using linear mixed and marginal models. Study participants were TTC-enrolled women aged 30-44 years with no history of infertility who were attempting to conceive for <3 months were enrolled. Serum AMH, follicle-stimulating hormone, and inhibin B levels were measured on cycle day 2, 3, or 4. Participants recorded daily menstrual cycle data for ≤4 months. The primary outcome was menstrual cycle length; follicular and luteal phase lengths were secondary outcomes.
Results
Multivariable analysis included 1880 cycles from 632 women. Compared with AMH levels of 1.6 to 3.4 ng/mL, women with AMH <1.6 ng/mL had cycles and follicular phases that were 0.98 (95% CI –1.46, –0.50) and 1.58 days shorter (95% CI –2.53, –0.63), respectively, while women with AMH >8 ng/mL had cycles that were 2.15 days longer (95% CI 1.46, 2.83), follicular phases that were 2 days longer (95% CI 0.77, 3.24), and luteal phases that were 1.80 days longer (95% CI 0.71, 2.88).
Conclusion
Increasing AMH levels are associated with longer menstrual cycles due to both a lengthening of the follicular and the luteal phase independent of age.
Keywords: anti-Müllerian hormone (AMH, menstrual cycle length, ovarian reserve
Ovarian reserve, the number of oocytes remaining in the ovary, can be indirectly measured using hormones such as early follicular phase inhibin B, follicle-stimulating hormone (FSH), estradiol (E2), and anti-Müllerian hormone (AMH) (1). AMH is a dimeric glycoprotein and member of the transforming growth factor beta family that is produced by the granulosa cells of secondary, pre-antral, and early antral follicles (≤6 mm) (2). AMH levels are inversely correlated with age due to a gradual decline in the primordial follicle pool as women approach menopause (3, 4). Despite the known age-related decline in ovarian reserve, studies have demonstrated high interindividual variability in AMH levels among similarly aged women (5).
Abnormalities in menstrual cycle length may have implications for natural fertility, with studies suggesting a role for menstrual cycle length as a surrogate for reproductive health (6). Long (>35 days) or irregular menstrual cycles are more likely to be anovulatory (7), and are associated with decreased fecundability (8). Furthermore, long menstrual cycles and/or oligomenorrhea (defined as <9 menstrual cycles annually) represent diagnostic criteria for polycystic ovarian syndrome (PCOS) (9). In contrast, short menstrual cycles (<21 days) may be a sign of ovarian aging, as they occur more frequently as menopause approaches (10), and are associated with poor response to ovarian stimulation among women with infertility (11). Age-related changes in cycle length are well known, and, at the same time, AMH declines with age, making it unclear whether AMH or other ovarian reserve biomarkers have a direct association with cycle length. There are few studies evaluating the relationship between AMH and menstrual cycle length among women of reproductive age. Our primary objective was to evaluate the association between AMH levels and menstrual cycle length in a prospective cohort of women of reproductive age attempting to conceive.
Materials and Methods
Study Design
We performed a secondary analysis of data collected from the time to conceive (TTC) study, a time to pregnancy cohort study designed to examine ovarian reserve biomarkers and fecundability (2008-2015) (12). The cohort included English-speaking women aged 30-44 years who were living with a male partner and had been attempting to conceive for 3 months or less at study entry. Study participants were screened for eligibility using a telephone questionnaire. Women were excluded if they had a history of fertility problems (eg, prior diagnosis of polycystic ovarian syndrome, surgically diagnosed endometriosis, prior sterilization, tubal blockage, previous or current use of fertility treatment) or a partner with a history of infertility. Women who were currently breastfeeding or had used injectable hormonal contraception in the past year were also excluded. The original study was approved by the institutional review board at the University of North Carolina at Chapel Hill, and this analysis was approved by the Duke University institutional review board. All participants provided written informed consent.
Women completed a questionnaire at enrollment that solicited information on demographics, hormonal contraceptive use, smoking, and alcohol use. Women were scheduled a study visit on menstrual cycle day 2, 3, or 4, and blood was collected. Women completed daily menstrual diaries for up to 4 months, documenting information on vaginal bleeding or spotting, medication and supplement use, sexual intercourse, and pregnancy test results. Women were not required to use ovulation predictor kits (OPKs) but if they did, they were asked to record the results of the tests in their diary. Starting in 2013, OPKs were distributed to participants as a part of the study. Women were withdrawn from the study if they initiated fertility treatment or if they no longer wished to participate.
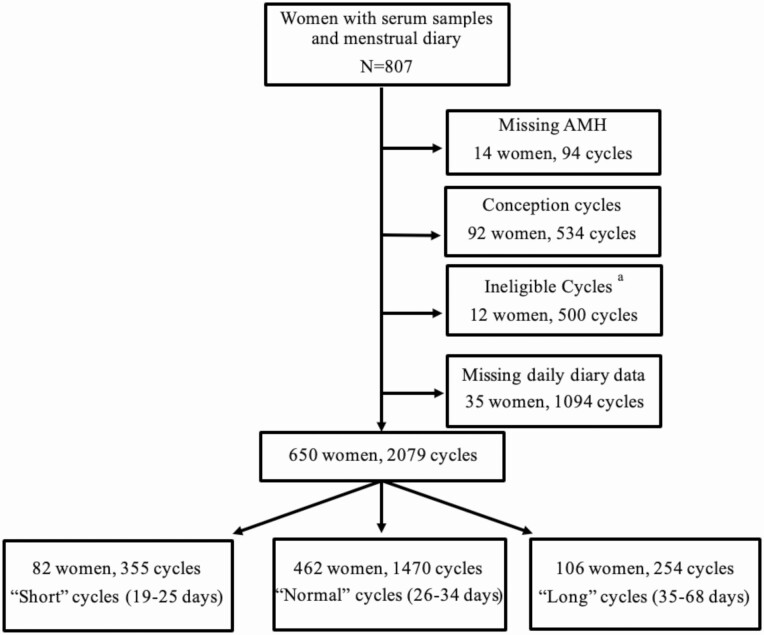
A total of 1042 women were enrolled in the TTC study. Of these, 807 women had a serum sample available (Fig. 1).
Figure 1.
Flow diagram of study enrollment and cohort selection. aIneligible cycles among women who stopped trying to conceive or began fertility treatment.
Biomarkers of Ovarian Reserve
Serum samples from the in-person visit were stored at –30°C and shipped frozen in a single batch to the University of Southern California Reproductive Endocrinology Laboratory. The samples were assayed for AMH using an ultrasensitive enzyme-linked immunosorbent assay (Ansh Labs) with a lower limit of detection 0.078 ng/mL; FSH and inhibin B were also measured by enzyme-linked immunosorbent assay (Ansh Labs) with limits of detection of 0.016 mIU/mL and 1.6 pg/mL, respectively. Interassay coefficients of variation ranged from 9% to 11% for AMH, 4% to 5% for FSH, and 5% to 8% for inhibin B (12). Women missing ovarian reserve measures were excluded (N = 94 cycles, 14 women; Fig. 1).
Menstrual Cycle Length
The onset of menses was identified by 2 consecutive days of vaginal bleeding (not spotting) (13). The first day of bleeding during menses was the first day of the menstrual cycle. Menstrual cycle length was the number of days from the first day of menses up to, but not including, the first day of the next menses. Three dichotomous variables were also created: “long cycles” of 35 days or more, “short cycles” of 25 days or less, and “normal” cycles. After limiting to daily diary cycles and excluding cycles ending in pregnancy (N = 534; because they have an undefined cycle length) and ineligible cycles (N = 500), the unadjusted analysis dataset contained 650 women and 2079 cycles (Fig. 1). The fully adjusted analysis dataset contained 1880 menstrual cycles from 632 women.
Phase Length
Ovulation was defined using the participants’ daily diary recording of ovulation test results, including cervical mucus monitoring or basal body temperature. OPKs were distributed to participants starting in 2013. Prior to this year, if participants voluntarily purchased kits they were able to record their test results, but this was uncommon, and ovulation information was often missing for these earlier cycles (N = 1084 cycles missing ovulation data) (14). Ovulation was estimated to have occurred 24 hours after a positive OPK. If a positive OPK was not available, then ovulation was defined as the last reported day of type 4 cervical mucus or based on the rise in recorded basal body temperature (14). Follicular and luteal phase analyses only included cycles with documented ovulation via the aforementioned methods. Follicular phase was defined as the number of days from the first day of menses to the day before ovulation (15). For the follicular phase analysis (N = 1127 phase lengths), we included conception cycles. Follicular phase length was analyzed both continuously and with 2 dichotomous variables: “long” (≥ 18 days) and “short” (≤10 days), based on the 90th and 10th percentiles, respectively. We defined luteal phase as the number of days from the day following ovulation to the day prior to the onset of the subsequent menses (15). For the luteal phase analysis (N = 882 cycles), we excluded conception cycles. Luteal phase length was analyzed continuously and with 1 dichotomous variable: “long” (≥18 days) with short luteal phase cycles excluded (16). Analyses of short luteal phase (eg, luteal phase defects) were not performed, as they were reported in a prior publication using this cohort (17).
Covariates
Variables examined as potential confounders were based on the literature (13) and included self-reported age, race, body mass index (BMI), and education. Additional covariates of interest were smoking, alcohol, and caffeine consumption, and recent hormonal contraceptive use. Age was examined as a continuous variable and divided into 3 categories: age <35, 35-37, and >37. Race was divided into 3 categories: White, Black, and Other (Asian, Native American, Pacific Islander). BMI was divided into 4 categories: underweight (<18.5 kg/m2), normal (18.5-24.9 kg/m2), overweight 25-29.9 kg/m2), and obese (≥30 kg/m2). Education level was categorized as less than high school or some college, 4-year degree, or graduate degree (Masters, Doctorate, and Professional). Women were categorized by use of hormonal contraceptives within 3 months of the cycle start date. Participants completed a baseline questionnaire that queried their alcohol, cigarette smoking, and caffeine consumption. Alcohol consumption (weekly number of alcoholic beverages multiplied by the number of alcoholic drinks per episode when drinking) was collected and divided into 2 categories (≤7 or >7 drinks per week) to characterize unhealthy alcohol use (18). Cigarette smoking was classified as current, former, or none. Caffeinated beverage consumption (number of cups of caffeinated coffee + number of other caffeinated drinks per day) was divided into 4 categories (<0.5, >0.5-<2, 2 to <3, and ≥3).
Statistical Analysis
Study participant characteristics were described with frequencies and percentages, and stratified by categories of AMH (specified below).
Continuous menstrual cycle length (in days) was analyzed with a linear mixed model with a random intercept for each woman. To ensure normality of the residuals, the model was limited to cycles between 22 and 36 days (N = 1880 cycles), excluding cycles resulting in pregnancy. Similarly, to ensure normality of plotted residuals for follicular phase (N = 1127 cycles) and luteal phase (N = 882 cycles) analyses, the continuous analyses were limited to phase lengths between 5 and 45 days, including conception cycles.
We did not have any a priori assumptions regarding the shape of the associations between AMH and cycle or phase lengths. We determined the appropriate parameterization by modeling deciles of AMH and cycle length, adjusting for age, and then plotting the estimated associations. After visual inspection of the plotted associations, 4 AMH categories were selected (<1.6, 1.6-3.4, >3.4-8, and >8 ng/mL). AMH of the 1.6 to 3.4 category was used as the reference group, given that it was the largest group (N = 606 cycles). In addition, AMH was evaluated continuously as a linear variable. We also investigated AMH as a dichotomous variable: diminished ovarian reserve (DOR), defined as AMH <0.7 ng/mL (12). An a priori clinical cutoff of 10 mIU/mL was used for serum FSH (12). For the purposes of analysis, serum FSH <10 mIU/mL was the reference group. In addition, FSH was evaluated continuously as a linear variable. Inhibin B (pg/mL) was modeled linearly (per 10 pg/mL increase) as there were no available clinical cut points. For the cycle length analysis, we presented 2 models: 1 minimally adjusted (age only) and 1 fully adjusted (age, race, education, BMI, time since oral contraceptive use, alcohol, smoking, and caffeine consumption). For all phase length analyses, we performed a sensitivity analysis limited to cycles in which ovulation was detected by OPK (N = 592 and N = 443 for follicular and luteal phases, respectively).
The associations between ovarian reserve biomarkers and long or short cycles (compared with normal cycles) were analyzed with separate marginal logistic regression models estimated with generalized estimated equations with an exchangeable working correlation matrix. For example, long cycles were compared with normal cycles and short cycles were excluded from that specific regression model. There is the possibility of bias due to informed cluster size, as women who are more fertile will have shorter time to pregnancy, resulting in a smaller number of observed cycles. To address this bias in our menstrual cycle analysis, we employed mixed models. For our long cycle analysis using generalized estimated equations, we conducted a sensitivity analysis by weighting each cycle by the inverse of the number of contributed cycles (19). Stata (version 16; StataCorp; College Station, TX) was used for statistical analysis.
Results
Most women in this analysis were between 30 and 35 years of age (69%) (N = 448), reported their race as white (77%), had a normal BMI (61%), and were well educated with an earned graduate degree (62%). Most women had never smoked (77%) and had not used hormonal contraception in the last 3 months (82%) (Table 1). The median AMH level was 2.63 ng/mL (interquartile range [IQR] 3.4). The median FSH and inhibin B levels were 6.7 mIU/mL (IQR 2.9) and 74.4 pg/mL (IQR 56.7), respectively. Most women in the cohort (71%) had cycles ranging from 26 to 34 days. The mean cycle length for the cohort was 28.6 days (standard deviation 3.13) (Table 1).
Table 1.
Study participant characteristics stratified by AMH categories (N = 2079 cycles, 650 women)
| No. of womena | ≤1.6 N cycles (%) | >1.6 to 3.4 N cycles (%) | >3.4 to 8 N cycles (%) | >8 N cycles (%) | |
|---|---|---|---|---|---|
| 650 | 602 (28.9) | 658 (31.6) | 620 (29.7) | 205 (9.8) | |
| Menstrual cycle length | |||||
| Short (19-25) | 82 (12.5) | 170 (28.2) | 121 (18.4) | 50 (8.1) | 14 (6.8) |
| Normal (26-34) | 462 (70.6) | 402 (66.8) | 476 (72.3) | 471 (76.0) | 121 (59.0) |
| Long (35-68) | 106 (16.2) | 30 (5.0) | 59 (9.0) | 95 (15.3) | 70 (34.1) |
| Missing | 4 (0.6) | 0 (0.0) | 2 (0.3) | 4 (0.6) | 0 (0.0) |
| Age (y) | |||||
| <35 | 448 (68.5) | 282 (46.8) | 426 (64.7) | 470 (75.8) | 169 (82.4) |
| 35-37 | 127 (19.4) | 144 (23.9) | 141 (21.4) | 104 (16.8) | 30 (14.6) |
| >37 | 78 (11.9) | 176 (29.2) | 90 (13.7) | 46 (7.4) | 6 (2.9) |
| Missing | 1 (0.2) | 0 (0.0) | 1 (0.2) | 0 (0.0) | 0 (0.0) |
| Race | |||||
| White | 504 (77.1) | 472 (78.4) | 459 (69.8) | 470 (75.8) | 161 (78.5) |
| Black | 61 (9.3) | 65 (10.8) | 94 (14.3) | 69 (11.1) | 12 (5.9) |
| Otherb | 89 (13.6) | 65 (10.8) | 105 (16.0) | 81 (13.1) | 32 (15.6) |
| Education | |||||
| ≤HS or some college | 53 (8.1) | 42 (7.0) | 67 (10.2) | 57 (9.2) | 19 (9.3) |
| 4-year degree | 195 (29.8) | 201 (33.4) | 211 (32.1) | 170 (27.4) | 65 (31.7) |
| Graduate degree | 406 (62.1) | 359 (59.6) | 380 (57.8) | 393 (63.4) | 121 (59.0) |
| Body mass index (kg/m 2 ) at baseline | |||||
| <18.5 | 20 (3.1) | 4 (0.7) | 28 (4.3) | 23 (3.7) | 1 (0.5) |
| 18.5-24.9 | 398 (60.9) | 353 (58.6) | 381 (57.9) | 377 (60.8) | 121 (59.0) |
| 25 - 30 | 130 (19.9) | 121 (20.1) | 108 (16.4) | 144 (23.2) | 55 (26.8) |
| ≥30 | 105 (16.1) | 123 (20.4) | 141 (21.4) | 76 (12.3) | 28 (13.7) |
| Missing | 1 (0.2) | 1 (0.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Time since oral contraceptive use | |||||
| >3 months | 534 (81.7) | 542 (90.0) | 587 (89.2) | 550 (88.7) | 186 (90.7) |
| 1 month or less | 67 (10.2) | 23 (3.8) | 22 (3.3) | 30 (4.8) | 5 (2.4) |
| 2 months | 22 (3.4) | 12 (2.0) | 16 (2.4) | 12 (1.9) | 3 (1.5) |
| 3 months | 31 (4.7) | 25 (4.2) | 33 (5.0) | 28 (4.5) | 11 (5.4) |
| Mean alcoholic drinks per week at baseline | |||||
| None | 226 (34.6) | 192 (31.9) | 246 (37.4) | 201 (32.4) | 75 (36.6) |
| ≤7 | 368 (56.3) | 350 (58.1) | 356 (54.1) | 360 (58.1) | 105 (51.2) |
| >7 | 58 (8.9) | 56 (9.3) | 53 (8.1) | 59 (9.5) | 25 (12.2) |
| Missing | 2 (0.3) | 4 (0.7) | 3 (0.5) | 0 (0.0) | 0 (0.0) |
| Smoking history | |||||
| Never | 500 (76.5) | 439 (72.9) | 525 (79.8) | 471 (76.0) | 160 (78.0) |
| Current | 11 (1.7) | 24 (4.0) | 4 (0.6) | 4 (0.6) | 1 (0.5) |
| Past | 143 (21.9) | 139 (23.1) | 129 (19.6) | 145 (23.4) | 44 (21.5) |
| Mean caffeinated drinks per day at baseline | |||||
| ≤0.5 | 138 (21.1) | 111 (18.4) | 141 (21.4) | 135 (21.8) | 50 (24.4) |
| >0.5-<2 | 233 (35.6) | 211 (35.0) | 260 (39.5) | 205 (33.1) | 79 (38.9) |
| 2-<3 | 189 (28.9) | 188 (31.2) | 164 (24.9) | 187 (30.2) | 48 (23.4) |
| ≥3 | 94 (14.4) | 92 (15.3) | 93 (14.1) | 93 (15.0) | 28 (13.7) |
a In the “No. of women” column, each woman is categorized by her mean menstrual cycle length during the study.
b “Other” included American Indian/Alaska Native, Asian/Pacific Islander, Hispanic, and mixed or unknown race.
Menstrual Cycle Length
Anti-Müllerian hormone
For all analyses, where minimal differences were observed between the age-only adjusted models versus the fully adjusted models, we describe only the fully adjusted model results. In the fully adjusted model, compared with women with AMH of 1.6 to 3.4 ng/mL, women with AMH < 1.6 ng/mL had cycles that were shorter by 0.98 days (95% CI –1.46, –0.50; P < .001) (Table 2). Women with AMH 3.4 to 8 ng/mL had cycles that were longer by 1.35 days (95% CI 0.88, 1.82; P < .001). Those with AMH > 8 ng/mL had cycles that were 2.15 days longer (95% CI 1.46, 2.83; P < .001). For each 1 ng/mL unit increase in AMH, menstrual cycle length increased by 0.30 days (95% CI 0.24, 0.36; P < .001).
Table 2.
Association AMH with difference in menstrual cycle lengtha, long menstrual cycles, and short menstrual cycles
| AMH (ng/mL) | No. of cycles | Adjustedb Δ in cycle length (days) (95% CI)e | Adjustedc Δ in cycle length (days) (95% CI)e | |
|---|---|---|---|---|
| Linear AMH, per 1 ng/mL increase | 1880 | 0.30 (0.24, 0.36) | 0.30 (0.24, 0.36) | |
| <1.6 | 568 | –1.00 (–1.48, –0.53) | –0.98 (–1.46, –0.50) | |
| 1.6-3.4 | 606 | Reference | ||
| >3.4-8 | 558 | 1.32 (0.86, 1.78) | 1.35 (0.88, 1.82) | |
| >8 | 148 | 2.08 (1.40, 2.77) | 2.15 (1.46, 2.83) | |
| Long cycles (>35 days) | aOR (95% CI) b | aOR (95% CI) c | aOR (95% CI) d | |
| <1.6 | 430 | 0.67 (0.39, 1.16) | 0.64 (0.36, 1.14) | 0.93 (0.46, 1.86) |
| 1.6-3.4 | 534 | Reference | ||
| >3.4-8 | 566 | 1.68 (1.10, 2.59) | 1.69 (1.08, 2.65) | 1.79 (1.06, 2.99) |
| >8 | 191 | 4.73 (2.86, 7.84) | 4.92 (2.89, 8.36) | 4.41 (2.41, 8.06) |
| Short cycles (<25 days) | aOR (95% CI) b | aOR (95% CI) c | aOR (95% CI) d | |
| <1.6 | 567 | 1.65 (1.19, 2.28) | 1.61 (1.14, 2.26) | 1.96 (1.32, 2.89) |
| 1.6-3.4 | 596 | Reference | ||
| >3.4-8 | 521 | 0.46 (0.30, 0.70) | 0.44 (0.28, 0.68) | 0.59 (0.36, 0.96) |
| >8 | 135 | 0.46 (0.23, 0.90) | 0.42 (0.21, 0.85) | 0.24 (0.11, 0.53) |
a To achieve normality of the model residuals, the continuous analysis was limited to cycles between 22 and 36 days long.
b Adjusted for age.
c Adjusted for age, race, education, BMI, time since oral contraceptive use, alcohol, smoking, and caffeine consumption.
d Sensitivity analysis of the association of categorical AMH with long and short cycles weighted by the inverse of cycles contributed by each woman (fully adjusted).
e All comparison P values were <.001.
Women with AMH >8 ng/mL had 4.92 (95% CI 2.89, 8.36) times the odds of having long cycles after full adjustment (Table 2). In a sensitivity analysis weighted by the inverse of cycles contributed by each woman, these results were unchanged.
Women with AMH <1.6 ng/mL had 1.61 (95% CI 1.14, 2.26) times the odds of having short cycles after full adjustment (Table 2). In a sensitivity analysis weighted by the inverse of cycles contributed by each woman, the association was unchanged. As AMH categories increased, the odds of short cycles gradually decreased in all 3 models (age adjusted, fully adjusted, and weighted analysis).
Follicle-stimulating hormone
In the fully adjusted model, women with FSH ≥10 mIU/mL had menstrual cycles that were shorter by 1.10 days (95% CI –1.76, –0.44; P < .001) than women with FSH <10 mIU/mL (Table 3) (20). For each 1 mIU/mL increase in FSH, menstrual cycle length decreased by 0.15 days (95% CI –0.23, –0.08; P < .001).
Table 3.
Association FSH with difference in menstrual cycle lengtha, long menstrual cycles, and short menstrual cycles
| FSH (mIU/mL) | No. cycles | Adjustedb Δ in cycle length days (95% CI) | Adjustedc Δ in cycle length days (95% CI) | |
|---|---|---|---|---|
| Linear FSH, per 1 mIU/mL increase | 1773 | –0.15 (–0.22, –0.08) | –0.15 (–0.23, –0.08) | |
| <10 | 1563 | Reference | ||
| ≥10 | 210 | –1.04 (–1.70, –0.39) | –1.10 (–1.76, –0.44) | |
| Long cycles (>35 days) | aOR (95% CI) b | aOR (95% CI) c | aOR (95% CI) d | |
| <10 | 1435 | Reference | ||
| ≥10 | 158 | 0.31 (0.15, 0.65) | 0.32 (0.15, 0.71) | 0.24 (0.10, 0.60) |
| Short cycles (<25 days) | aOR (95% CI) b | aOR (95% CI) c | aOR (95% CI) d | |
| <10 | 1506 | Reference | ||
| ≥10 | 214 | 1.46 (0.97, 2.19) | 1.52 (1.01, 2.30) | 1.43 (0.88, 2.32) |
Results are displayed as change in cycle length in days for a given FSH category, compared to the reference group.
a To achieve normality of the model residuals, the continuous analysis was limited to cycles between 22 and 36 days long.
b Adjusted for age.
c Adjusted for age, race, education, BMI, time since oral contraceptive use, alcohol, smoking, and caffeine consumption.
d Sensitivity analysis the association of categorical FSH with long and short cycles weighted by the inverse of cycles contributed by each woman.
Women with FSH ≥10 mIU/mL had 0.32 (95% CI 0.15, 0.71) times the odds of having long menstrual cycles in the fully adjusted model. In a sensitivity analysis weighted by the inverse of cycles contributed by each woman, the results were similar (Table 3) (20). Despite having slightly shorter cycles than the reference group, FSH ≥10 mIU/mL was not associated with short menstrual cycles (defined as ≤10 days) in any of the models (age adjusted, fully adjusted, or weighted analysis).
Inhibin B
There was no statistically significant association between inhibin B levels and continuous menstrual cycle length. In addition, there was no association between inhibin B levels and long or short menstrual cycles (Table 1 (20)).
Follicular Phase Length
Anti-Müllerian hormone
After full adjustment, women with AMH >8 ng/mL had follicular phases that were longer by 2 days (95% CI 0.77, 3.24; P = .001) (Table 4). In contrast, women with AMH <1.6 ng/mL had follicular phases that were approximately 1.6 days shorter (95% CI –2.53, –0.63; P = .001). In a sensitivity analysis limited to ovulation prediction with OPKs, the results were similar.
Table 4.
Association of categorical AMH with follicular phase lengtha, long follicular phase, and short follicular phase
| AMH (ng/mL) | No. of cycles | Adjustedb Δ follicular phase length, days (95% CI) | P value | Adjustedc Δ in follicular phase length, days (95% CI) | P value | Adjustedd Δ in follicular phase length, days (95% CI) | P value | |
|---|---|---|---|---|---|---|---|---|
| <1.6 | 326 | –1.59 (–2.52, –0.65) | .001 | –1.58 (–2.53, –0.63) | 0.001 | –1.45 (–2.66, –0.24) | .019 | |
| 1.6-3.4 | 343 | Reference | ||||||
| >3.4-8 | 338 | 0.43 (–0.47, 1.32) | .354 | 0.47 (–0.44, 1.37) | 0.311 | 0.53 (–0.62, 1.68) | .367 | |
| >8 | 120 | 2.02 (0.78, 3.27) | .001 | 2.00 (0.77, 3.24) | 0.001 | 0.63 (–1.08, 2.34) | .471 | |
| Long follicular phase (≥18 days) | aOR (95% CI) b | aOR (95% CI) c | aOR (95% CI) d | aOR (95% CI) e | ||||
| <1.6 | 284 | 0.48 (0.28, 0.83) | — | 0.44 (0.25, 0.79) | — | 0.45 (0.19, 1.09) | — | 0.33 (0.16, 0.69) |
| 1.6-3.4 | 314 | Reference | ||||||
| >3.4-8 | 320 | 1.41 (0.90, 2.20) | — | 1.43 (0.89, 2.30) | — | 2.30 (1.10, 4.82) | — | 0.87 (0.47, 1.61) |
| >8 | 117 | 2.28 (1.37, 3.79) | — | 2.52 (1.48, 4.28) | — | 3.65 (1.54, 8.67) | — | 1.39 (0.73, 2.62) |
| Short follicular phase (≤ 10 days) | aOR (95% CI) b | aOR (95% CI) c | aOR (95% CI) d | aOR (95% CI) e | ||||
| <1.6 | 287 | 1.20 (0.69, 2.08) | — | 1.28 (0.74, 2.24) | — | 1.68 (0.67, 4.26) | — | 1.58 (0.74, 3.39) |
| 1.6-3.4 | 261 | Reference | ||||||
| >3.4-8 | 236 | 0.72 (0.37, 1.38) | — | 0.72 (0.36, 1.43) | — | 1.05 (0.31, 3.58) | — | 0.67 (0.27, 1.64) |
| >8 | 72 | 0.36 (0.11, 1.19) | — | 0.33 (0.10, 1.11) | — | 0.71 (0.08, 6.68) | — | 0.46 (0.12, 1.79) |
Results are displayed as change in phase in days for a given AMH category, compared to the reference group.
a To achieve normality of the model residuals, the continuous analysis was limited to phases between 5 and 45 days long.
b Adjusted for age.
c Adjusted for age, race, education, BMI, time since oral contraceptive use, alcohol, smoking, and caffeine consumption.
d Sensitivity analysis of follicular phase length with ovulation determined by OPK (cycle N = 592).
e Sensitivity analysis the association of categorical AMH with long and short cycles weighted by the inverse of cycles contributed by each woman.
Women with AMH >8 ng/mL had 2.52 (95% CI 1.48, 4.28) times the odds of having a long follicular phase (Table 4). In the sensitivity analysis limited to ovulation prediction with OPKs, the results were similar. In contrast, AMH was not associated with short follicular phase.
Follicle-stimulating hormone
Women with FSH ≥10 mIU/mL had follicular phases that were shorter by 1.42 days (95% CI –2.38, –0.45; P = .004) in the fully adjusted model (Table 2 (20)). Women with FSH ≥10 mIU/mL had 0.28 (95% CI 0.13, 0.57) times the odds of having a long follicular phase in the fully adjusted model. In sensitivity analyses limited to ovulation prediction with OPKs, the results were similar.
Inhibin B
An increase of 10 pg/mL in inhibin B was associated with a 0.12 day (95% CI –0.21, –0.03) shorter follicular phase in the fully adjusted model (Supplemental Table 3 (20)). In sensitivity analyses limited to ovulation prediction with OPKs, the results were similar. Inhibin B was not associated with long or short follicular phases.
Luteal Phase Length
Anti-Müllerian hormone
Women with AMH >8 ng/mL had luteal phases that were longer by approximately 1.8 days in both the minimally and fully adjusted models (Table 5). They also had 2.89 (95% CI 1.25, 6.67) and 2.86 (95% CI 1.26, 6.46) times the odds of having a long luteal phase in the minimally and fully adjusted models, respectively. In a sensitivity analysis limited to ovulation prediction with OPKs, the results were unchanged.
Table 5.
Association of categorical AMH with luteal phase lengtha and long luteal phase
| AMH (ng/mL) | No. of cycles | Adjusted Δ in luteal phase length, days (95% CI)b | P value | Adjusted a Δ in luteal phase length, days (95% CI)c | P value | Adjusted a Δ in luteal phase length, days (95% CI)d | P value | |
|---|---|---|---|---|---|---|---|---|
| <1.6 | 267 | –0.19 (–1.03, 0.63) | .639 | –0.06 (–0.92, 0.80) | .897 | 0.26 (–0.81, 1.33) | .631 | |
| 1.6-3.4 | 262 | Reference | ||||||
| >3.4-8 | 254 | 0.46 (–0.36, 1.29) | .268 | 0.34 (–0.50, 1.18) | .424 | 0.49 (–0.57, 1.56) | .364 | |
| >8 | 99 | 1.87 (0.78, 2.95) | .001 | 1.80 (0.71, 2.88) | .001 | 2.04 (0.54, 3.55) | .008 | |
| Long luteal phase (≥18 days) | aOR (95% CI) b | aOR (95% CI) c | aOR (95% CI) d | aOR (95% CI) e | ||||
| <1.6 | 213 | 0.55 (0.23, 1.28) | — | 0.57 (0.25, 1.31) | — | 0.86 (0.26, 2.82) | — | 0.87 (0.33, 2.28) |
| 1.6-3.4 | 206 | Reference | ||||||
| >3.4-8 | 203 | 1.73 (0.81, 3.71) | — | 1.52 (0.70, 3.32) | — | 3.34 (0.90, 12.46) | — | 1.93 (0.83, 4.51) |
| >8 | 82 | 2.89 (1.25, 6.67) | — | 2.86 (1.26, 6.46) | — | 5.08 (1.10, 23.38) | — | 2.66 (1.01, 7.03) |
Results are displayed as change in phase in days for a given AMH category, compared with the reference group.
a To achieve normality of the model residuals, the continuous analysis was limited to phases between 5 and 45 days long.
b Adjusted for age.
c Adjusted for age, race, education, BMI, time since oral contraceptive use, alcohol, smoking, and caffeine consumption.
d Sensitivity analysis of luteal phase length with ovulation determined by OPK (cycle N = 443).
e Sensitivity analysis the association of categorical AMH with long luteal phase weighted by the inverse of cycles contributed by each woman.
Follicle-stimulating hormone
There was no statistically significant association between FSH and linear luteal phase length in either the minimally adjusted (–0.52 days, 95% CI –1.67, 0.62) or fully adjusted (–0.22 days, 95% CI –1.39, 0.95) models (Table 2 (20)). A sensitivity analysis showed similar results.
Inhibin B
There was no statistically significant association between inhibin B levels and luteal phase length (Table 3 (20)).
Diminished Ovarian Reserve
Anti-Müllerian hormone
Compared with the reference group (AMH >0.7 ng/mL), women with DOR (defined as AMH <0.7 ng/mL) had cycle lengths that were approximately 1.7 days shorter in both the minimally and fully adjusted models (Table 4 (20)). As expected, women with DOR had follicular phase length that was 1.92 (95% CI –3.24, –0.60) days and 1.65 (95% CI –2.98, –0.32) days shorter in the minimally and fully adjusted models, respectively, than in the reference group. In a sensitivity analysis, a statistically significant association between DOR and follicular phase length is no longer present (Table 4 (20)).
Discussion
This is the first study to examine the possible association between AMH and menstrual patterns determined from prospectively recorded data on menses and ovulation. In our cohort of women aged 30-44 attempting to conceive naturally, lower AMH was associated with shorter menstrual cycles and a shorter follicular phase, while increasing levels of AMH were associated with longer menstrual cycles, a longer follicular phase, and a longer luteal phase. Consistent with these associations, high levels of FSH were also associated with shorter menstrual cycle and follicular phase lengths, respectively. Inhibin B levels were not significantly associated with changes in menstrual cycle length. However, there was a small association with follicular phase length and inhibin B levels, though likely not clinically significant.
There is considerable interindividual variation in AMH levels among similarly aged women (21-23), which can account for variations in menstrual cycle physiology. In murine models, AMH produced by pre-antral and small antral follicles has been shown to inhibits further follicle recruitment through negative feedback, with AMH-deficient mice showing a rapid decline of primordial follicles early in life (24-27). This inhibitory effect of AMH was also demonstrated in in vitro human ovarian cells (28), but data for humans, in vivo, is lacking. AMH also inhibits FSH-induced aromatase (CYP19a1) activity which results in decreased intrafollicular E2 levels (29). As a result, AMH functions early in the menstrual cycle to regulate small antral follicle (<8 mm) E2 production prior to selection (30-32). Increasing follicle size has been shown to be associated with decreasing intrafollicular AMH levels, with a sharp decline in follicles larger than 8 mm (31), reflective of dominant follicle selection and rapidly increasing E2 production. The association between increasing circulating AMH and menstrual cycle length could be explained by the locally suppressive effect of AMH on E2 production by antral follicles thereby increasing follicular phase length. Alternatively, a decreasing primordial follicle pool and AMH level as women approach menopause results in decreased luteal phase inhibition of FSH-induced aromatase activity, early dominant follicle recruitment, and shorter menstrual cycles (29, 33).
In PCOS, significantly more AMH is produced per granulosa cell in nonovulatory PCOS than ovulatory PCOS, both of which produce significantly higher AMH levels than non-PCOS ovaries (34). Given that AMH decreases FSH-induced aromatase expression in granulosa cells (35), high AMH levels seen in PCOS may inhibit FSH-induced aromatase production, decrease E2, and prevent the inhibitory effects of E2 on further AMH production. Without the emergence of a dominant follicle and accompanying rapid increase in E2, high AMH may contribute to anovulation and therefore prolonged cycles (29).
Variability in menstrual cycle length is predominantly due to variability in follicular phase length, while luteal phase length is relatively constant (36). Long menstrual cycles in humans are associated with longer follicular phases secondary to delayed ovulation (37-39). Potential mechanisms for delayed ovulation could include extended periods of low follicular estrogen (40) or diminished response to gonadotropin stimulation (37). Long menstrual cycles may also be associated with delayed intrafollicular estrogen rise (7, 41), or in some cases, normal early follicular phase rises in estrogen followed by variations in estrogen rise or fall (41). Our findings of a relationship between cycle length (and specifically follicular phase length) and AMH are consistent with the important role of AMH in menstrual cycle physiology through its effects on E2 production and follicle recruitment.
Few other studies have examined a relationship between cycle length and markers of ovarian reserve; however, the 3 existing studies are consistent with our findings. First, in a cross-sectional study of 200 healthy women aged 21-45, increasing AMH was associated with increasing average menstrual cycle length. Specifically, after adjusting for age, women who reported long (>35 days) and normal (25-34 days) menstrual cycles had serum AMH levels that were >5- and >2-fold higher, respectively, compared with those with short cycles (42). Second, in a population-based, cross-sectional study of 366 women increasing AMH levels were associated with increased self-reported menstrual cycle length (33). These studies were limited by sample size and, particularly by menstrual cycle data being obtained from participant recall, which may be misclassified due to digit preference or inaccurate recall, compared with prospective collection (43). In addition, the latter study was limited to healthcare workers, which may limit generalizability. Finally, in a meta-analysis of 11 studies and >12 000 women undergoing fertility treatment, short menstrual cycles (21-27 days) were associated with lower ovarian reserve testing values (AMH or antral follicle counts) compared with normal cycles (28-31 days) and long cycles (32-35 days) (44). These findings were independent of age, consistent with our study findings; albeit in a different study population of women with history of infertility.
Our findings of an association between high AMH and higher odds of long luteal phase are in contrast to prior literature demonstrating a role for higher AMH contributing to luteal phase deficiency, particularly among women with PCOS (45). Potential mechanisms for luteal phase defects (ie, short luteal phase) in the setting of high AMH include inhibition of proliferation and progesterone function of human granulosa-derived lutein cells (46), reduced luteinizing hormone (LH) receptor expression (47), and decreased aromatase activity in granulosa-derived lutein cells (48).
Inhibin B is secreted by granulosa cells in developing follicles and is thought to play a major role in selection of a dominant follicle. FSH produced by granulosa cells stimulates production of inhibin B, which peaks in the midfollicular phase and inhibits further FSH secretion through a negative feedback loop. Inhibin B also contributes to theca cell androgen production, which acts as a substrate for E2 synthesis and increases FSH and LH receptor expression. The follicle that makes the most inhibin B will thus have the highest androgen production and the most FSH and LH receptors and be the most able to survive amidst declining FSH levels. This leads to the selection of a single dominant follicle for ovulation. Inhibin B levels have been shown to decline with age, and the attenuated negative feedback on FSH leads to an increase in FSH and E2 levels (49).
In our analysis of the relationship between inhibin B and follicular phase length, we would therefore expect physiologically decreasing inhibin B levels (and thus increasing FSH levels) to be associated with shorter follicular phases (50, 51). These expected physiologic findings would be in agreement with a statistically significant inverse relationship between inhibin B and FSH levels in our study samples (Pearson coefficient –0.1316; P = .0224). However, we found the opposite association, with increasing inhibin B levels related to slightly shorter follicular phases. While the association was small and likely not clinically relevant, we are unable to explain these results based on the biology of inhibin B. Regarding the luteal phase, a prior study using this same cohort has evaluated the association between biomarkers of ovarian reserve and luteal phase defects (17). Specifically, after adjusting for age, race, previous miscarriages, and prior pregnancies, inhibin B was the only biomarker of ovarian reserve associated with a short luteal phase, with the risk of short luteal phase decreasing with increasing inhibin B (17). This result was also unexplained by the biology of inhibin B. More studies are needed to elucidate the role of inhibin B in follicular and luteal phases lengths.
Our study has several strengths. The cohort was prospective and included menstrual cycle length with ovulation data, facilitating calculation of follicular and luteal phase lengths, respectively. The cohort was recruited from the community and therefore representative of women in the Triangle area of North Carolina (52).
This study has several limitations. Most of the women in this study were white and well educated, which may limit generalizability of the results. Also, it is possible that some women in the study had undiagnosed PCOS, which could underlie the relationship between AMH and cycle length, although we saw associations between AMH and cycle length across the full spectrum of both AMH values and cycle lengths. Lastly, we acknowledge role of AMH as a potential marker for PCOS. Multiple studies have shown an association between elevated AMH and PCOS, though an appropriate AMH cut-off value for diagnosis of PCOS is debated (53, 54). Therefore, a specific AMH cut-off for potential PCOS was not utilized in this analysis.
These findings suggest that biomarkers of ovarian reserve, AMH and FSH, are each associated with menstrual cycle length and follicular and luteal phase lengths. Inhibin B is not associated with overall cycle length but may affect follicular and/or luteal phase length. These findings would suggest that lower ovarian reserve is associated with shorter menstrual cycles and early ovulation, even after adjustment for age. These findings contribute to our understanding of ovarian reserve and the role of biomarkers of ovarian reserve in reproductive physiology.
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences under project number Z01ES103333. We would also like to acknowledge Dr. Quaker Harmon and Dr. Alyson Gregoire for comments on an earlier version of this manuscript. In addition, we would like to acknowledge Dr. Anita Subramanian for her code review.
Financial Support: NIH/NICHD (R21 HD060229-01 and R01 HD067683-01) and Intramural Research Program of the National Institute of Environmental Health Sciences (Z01ES103333).
Glossary
Abbreviations
- AMH
anti-Müllerian
- BMI
body mass index
- DOR
diminished ovarian reserve
- E2
estradiol
- FSH
follicle-stimulating hormone
- IQR
interquartile range
- LH
luteinizing hormone
- OPK
ovulation predictor kit
- PCOS
polycystic ovarian syndrome
- TTC
time to conceive
Additional Information
Disclosures: B.H. has nothing to declare. A.Z.S. had financial support from NICHD/NIH for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work. A.Z.S. has previously consulted for Prima-Temp and Seikagaku Corporation outside the submitted work. A.M.J. received vitamin D supplements from Therologix, Inc. for a study outside the submitted work.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril. 2015;103(3):e9-e17. [DOI] [PubMed] [Google Scholar]
- 2. Broekmans FJ, Visser JA, Laven JS, Broer SL, Themmen AP, Fauser BC. Anti-Müllerian hormone and ovarian dysfunction. Trends Endocrinol Metab. 2008;19(9):340-347. [DOI] [PubMed] [Google Scholar]
- 3. Lie Fong S, Visser JA, Welt CK, et al. Serum anti-müllerian hormone levels in healthy females: a nomogram ranging from infancy to adulthood. J Clin Endocrinol Metab. 2012;97(12):4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. La Marca A, Broekmans FJ, Volpe A, Fauser BC, Macklon NS; ESHRE Special Interest Group for Reproductive Endocrinology–AMH Round Table . Anti-Mullerian hormone (AMH): what do we still need to know? Hum Reprod. 2009;24(9):2264-2275. [DOI] [PubMed] [Google Scholar]
- 5. La Marca A, Grisendi V, Griesinger G. How much does AMH really vary in normal women? Int J Endocrinol. 2013;2013:959487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gizzo S, Andrisani A, Noventa M, et al. Menstrual cycle length: a surrogate measure of reproductive health capable of improving the accuracy of biochemical/sonographical ovarian reserve test in estimating the reproductive chances of women referred to ART. Reprod Biol Endocrinol. 2015;13:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mumford SL, Steiner AZ, Pollack AZ, et al. The utility of menstrual cycle length as an indicator of cumulative hormonal exposure. J Clin Endocrinol Metab. 2012;97(10):E1871-E1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jensen TK, Scheike T, Keiding N, Schaumburg I, Grandjean P. Fecundability in relation to body mass and menstrual cycle patterns. Epidemiology. 1999;10(4):422-428. [DOI] [PubMed] [Google Scholar]
- 9. Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370(9588):685-697. [DOI] [PubMed] [Google Scholar]
- 10. Burger HG, Hale GE, Dennerstein L, Robertson DM. Cycle and hormone changes during perimenopause: the key role of ovarian function. Menopause. 2008;15(4 Pt 1):603-612. [DOI] [PubMed] [Google Scholar]
- 11. Beckers NG, Macklon NS, Eijkemans MJ, Fauser BC. Women with regular menstrual cycles and a poor response to ovarian hyperstimulation for in vitro fertilization exhibit follicular phase characteristics suggestive of ovarian aging. Fertil Steril. 2002;78(2):291-297. [DOI] [PubMed] [Google Scholar]
- 12. Steiner AZ, Pritchard D, Stanczyk FZ, et al. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. Jama. 2017;318(14):1367-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jukic AMZ, Wilcox AJ, McConnaughey DR, Weinberg CR, Steiner AZ. 25-Hydroxyvitamin D and long menstrual cycles in a prospective cohort study. Epidemiology. 2018;29(3):388-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jukic AMZ, Padiyara P, Bracken MB, McConnaughey DR, Steiner AZ. Analgesic use at ovulation and implantation and human fertility. Am J Obstet Gynecol. 2020;222(5):476.e1-476.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lenton EA, Landgren BM, Sexton L, Harper R. Normal variation in the length of the follicular phase of the menstrual cycle: effect of chronological age. Br J Obstet Gynaecol. 1984;91(7):681-684. [DOI] [PubMed] [Google Scholar]
- 16. Lenton EA, Landgren BM, Sexton L. Normal variation in the length of the luteal phase of the menstrual cycle: identification of the short luteal phase. Br J Obstet Gynaecol. 1984;91(7):685-689. [DOI] [PubMed] [Google Scholar]
- 17. Pfister A, Crawford NM, Steiner AZ. Association between diminished ovarian reserve and luteal phase deficiency. Fertil Steril. 2019;112(2):378-386. [DOI] [PubMed] [Google Scholar]
- 18. Curry SJ, Krist AH, Owens DK, et al. ; US Preventive Services Task Force . Screening and behavioral counseling interventions to reduce unhealthy alcohol use in adolescents and adults: US preventive services task force recommendation statement. JAMA. 2018;320(18):1899-1909. [DOI] [PubMed] [Google Scholar]
- 19. Williamson JM, Datta S, Satten GA. Marginal analyses of clustered data when cluster size is informative. Biometrics. 2003;59(1):36-42. [DOI] [PubMed] [Google Scholar]
- 20. Harris BS, Steiner AZ, Jukic AM. Data from: ovarian reserve biomarkers and menstrual cycle length in a prospective cohort study. Figshare 2020. Deposited January 12, 2020. Private Link: https://figshare.com/s/e0885ff9851fd36c1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gougeon A. Ovarian follicular growth in humans: ovarian ageing and population of growing follicles. Maturitas. 1998;30(2):137-142. [DOI] [PubMed] [Google Scholar]
- 22. Almog B, Shehata F, Suissa S, et al. Age-related normograms of serum antimullerian hormone levels in a population of infertile women: a multicenter study. Fertil Steril. 2011;95(7):2359-e2351. [DOI] [PubMed] [Google Scholar]
- 23. La Marca A, Spada E, Sighinolfi G, et al. Age-specific nomogram for the decline in antral follicle count throughout the reproductive period. Fertil Steril. 2011;95(2):684-688. [DOI] [PubMed] [Google Scholar]
- 24. Behringer RR, Finegold MJ, Cate RL. Müllerian-inhibiting substance function during mammalian sexual development. Cell. 1994;79(3):415-425. [DOI] [PubMed] [Google Scholar]
- 25. Durlinger AL, Visser JA, Themmen AP. Regulation of ovarian function: the role of anti-Müllerian hormone. Reproduction. 2002;124(5):601-609. [DOI] [PubMed] [Google Scholar]
- 26. Gigli I, Cushman RA, Wahl CM, Fortune JE. Evidence for a role for anti-Mullerian hormone in the suppression of follicle activation in mouse ovaries and bovine ovarian cortex grafted beneath the chick chorioallantoic membrane. Mol Reprod Dev. 2005;71(4):480-488. [DOI] [PubMed] [Google Scholar]
- 27. Nilsson E, Rogers N, Skinner MK. Actions of anti-Mullerian hormone on the ovarian transcriptome to inhibit primordial to primary follicle transition. Reproduction. 2007;134(2):209-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carlsson IB, Scott JE, Visser JA, Ritvos O, Themmen AP, Hovatta O. Anti-Müllerian hormone inhibits initiation of growth of human primordial ovarian follicles in vitro. Hum Reprod. 2006;21(9):2223-2227. [DOI] [PubMed] [Google Scholar]
- 29. Dewailly D, Andersen CY, Balen A, et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update. 2014;20(3):370-385. [DOI] [PubMed] [Google Scholar]
- 30. Weenen C, Laven JS, Von Bergh AR, et al. Anti-Müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10(2):77-83. [DOI] [PubMed] [Google Scholar]
- 31. Andersen CY, Schmidt KT, Kristensen SG, Rosendahl M, Byskov AG, Ernst E. Concentrations of AMH and inhibin-B in relation to follicular diameter in normal human small antral follicles. Hum Reprod. 2010;25(5):1282-1287. [DOI] [PubMed] [Google Scholar]
- 32. Jeppesen JV, Anderson RA, Kelsey TW, et al. Which follicles make the most anti-Mullerian hormone in humans? Evidence for an abrupt decline in AMH production at the time of follicle selection. Mol Hum Reprod. 2013;19(8):519-527. [DOI] [PubMed] [Google Scholar]
- 33. Bentzen JG, Forman JL, Johannsen TH, Pinborg A, Larsen EC, Andersen AN. Ovarian antral follicle subclasses and anti-mullerian hormone during normal reproductive aging. J Clin Endocrinol Metab. 2013;98(4):1602-1611. [DOI] [PubMed] [Google Scholar]
- 34. Pellatt L, Hanna L, Brincat M, et al. Granulosa cell production of anti-Müllerian hormone is increased in polycystic ovaries. J Clin Endocrinol Metab. 2007;92(1):240-245. [DOI] [PubMed] [Google Scholar]
- 35. Pellatt L, Rice S, Dilaver N, et al. Anti-Müllerian hormone reduces follicle sensitivity to follicle-stimulating hormone in human granulosa cells. Fertil Steril. 2011;96(5):1246-51.e1. [DOI] [PubMed] [Google Scholar]
- 36. Johnson SR, Miro F, Barrett S, Ellis JE. Levels of urinary human chorionic gonadotrophin (hCG) following conception and variability of menstrual cycle length in a cohort of women attempting to conceive. Curr Med Res Opin. 2009;25(3):741-748. [DOI] [PubMed] [Google Scholar]
- 37. Barrett ES, Thune I, Lipson SF, Furberg AS, Ellison PT. A factor analysis approach to examining relationships among ovarian steroid concentrations, gonadotrophin concentrations and menstrual cycle length characteristics in healthy, cycling women. Hum Reprod. 2013;28(3):801-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fehring RJ, Schneider M, Raviele K. Variability in the phases of the menstrual cycle. J Obstet Gynecol Neonatal Nurs. 2006;35(3):376-384. [DOI] [PubMed] [Google Scholar]
- 39. Waller K, Swan SH, Windham GC, Fenster L, Elkin EP, Lasley BL. Use of urine biomarkers to evaluate menstrual function in healthy premenopausal women. Am J Epidemiol. 1998;147(11):1071-1080. [DOI] [PubMed] [Google Scholar]
- 40. Ferrell RJ, Rodríguez G, Holman D, O’Connor K, Wood JW, Weinstein M. Hypoestrogenic “inactive phases” at the start of the menstrual cycle: changes with age and reproductive stage, and relationship to follicular depletion. Fertil Steril. 2012;98(5):1246-53.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Harlow SD, Baird DD, Weinberg CR, Wilcox AJ. Urinary oestrogen patterns in long follicular phases. Hum Reprod. 2000;15(1):11-16. [DOI] [PubMed] [Google Scholar]
- 42. Zhu R, Lee BH, Huang Z, et al. Antimüllerian hormone, antral follicle count and ovarian volume predict menstrual cycle length in healthy women. Clin Endocrinol (Oxf). 2016;84(6):870-877. [DOI] [PubMed] [Google Scholar]
- 43. Jukic AM, Weinberg CR, Wilcox AJ, McConnaughey DR, Hornsby P, Baird DD. Accuracy of reporting of menstrual cycle length. Am J Epidemiol. 2008;167(1):25-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Younis JS, Iskander R, Fauser BCJM, Izhaki I. Does an association exist between menstrual cycle length within the normal range and ovarian reserve biomarkers during the reproductive years? A systematic review and meta-analysis. Hum Reprod Update. 2020;26(6):904-928. [DOI] [PubMed] [Google Scholar]
- 45. Boutzios G, Karalaki M, Zapanti E. Common pathophysiological mechanisms involved in luteal phase deficiency and polycystic ovary syndrome. Impact on fertility. Endocrine. 2013;43(2):314-317. [DOI] [PubMed] [Google Scholar]
- 46. Kim JH, Seibel MM, MacLaughlin DT, et al. The inhibitory effects of müllerian-inhibiting substance on epidermal growth factor induced proliferation and progesterone production of human granulosa-luteal cells. J Clin Endocrinol Metab. 1992;75(3):911-917. [DOI] [PubMed] [Google Scholar]
- 47. Pellatt L, Rice S, Mason HD. Anti-Müllerian hormone and polycystic ovary syndrome: a mountain too high? Reproduction. 2010;139(5):825-833. [DOI] [PubMed] [Google Scholar]
- 48. Grossman MP, Nakajima ST, Fallat ME, Siow Y. Müllerian-inhibiting substance inhibits cytochrome P450 aromatase activity in human granulosa lutein cell culture. Fertil Steril. 2008;89(5 Suppl):1364-1370. [DOI] [PubMed] [Google Scholar]
- 49. Yding Andersen C. Inhibin-B secretion and FSH isoform distribution may play an integral part of follicular selection in the natural menstrual cycle. Mol Hum Reprod. 2017;23(1):16-24. [DOI] [PubMed] [Google Scholar]
- 50. Sowers M, McConnell D, Gast K, et al. Anti-Müllerian hormone and inhibin B variability during normal menstrual cycles. Fertil Steril. 2010;94(4):1482-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Klein NA, Illingworth PJ, Groome NP, McNeilly AS, Battaglia DE, Soules MR. Decreased inhibin B secretion is associated with the monotropic FSH rise in older, ovulatory women: a study of serum and follicular fluid levels of dimeric inhibin A and B in spontaneous menstrual cycles. J Clin Endocrinol Metab. 1996;81(7):2742-2745. [DOI] [PubMed] [Google Scholar]
- 52. Steiner AZ, Long DL, Herring AH, Kesner JS, Meadows JW, Baird DD. Urinary follicle-stimulating hormone as a measure of natural fertility in a community cohort. Reprod Sci. 2013;20(5):549-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li HW, Wong BP, Ip WK, Yeung WS, Ho PC, Ng EH. Comparative evaluation of three new commercial immunoassays for anti-Müllerian hormone measurement. Hum Reprod. 2016;31(12):2796-2802. [DOI] [PubMed] [Google Scholar]
- 54. Pigny P, Gorisse E, Ghulam A, et al. Comparative assessment of five serum antimüllerian hormone assays for the diagnosis of polycystic ovary syndrome. Fertil Steril. 2016;105(4):1063-1069.e3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.