Abstract
Context
Vertical sleeve gastrectomy (VSG) is becoming a prioritized surgical intervention for obese individuals; however, the brain circuits that mediate its effective control of food intake and predict surgical outcome remain largely unclear.
Objective
We investigated VSG-correlated alterations of the gut-brain axis.
Methods
In this observational cohort study, 80 patients with obesity were screened. A total of 36 patients together with 26 normal-weight subjects were enrolled and evaluated using the 21-item Three-Factor Eating Questionnaire (TFEQ), MRI scanning, plasma intestinal hormone analysis, and fecal sample sequencing. Thirty-two patients underwent VSG treatment and 19 subjects completed an average of 4-month follow-up evaluation. Data-driven regional homogeneity (ReHo) coupled with seed-based connectivity analysis were used to quantify VSG-related brain activity. Longitudinal alterations of body weight, eating behavior, brain activity, gastrointestinal hormones, and gut microbiota were detected and subjected to repeated measures correlation analysis.
Results
VSG induced significant functional changes in the right putamen (PUT.R) and left supplementary motor area, both of which correlated with weight loss and TFEQ scores. Moreover, postprandial levels of active glucagon-like peptide-1 (aGLP-1) and Ghrelin were associated with ReHo of PUT.R; meanwhile, relative abundance of Clostridia increased by VSG was associated with improvements in aGLP-1 secretion, PUT.R activity, and weight loss. Importantly, VSG normalized excessive functional connectivities with PUT.R, among which baseline connectivity between PUT.R and right orbitofrontal cortex was related to postoperative weight loss.
Conclusion
VSG causes correlated alterations of gut-brain axis, including Clostridia, postprandial aGLP-1, PUT.R activity, and eating habits. Preoperative connectivity of PUT.R may represent a potential predictive marker of surgical outcome in patients with obesity.
Keywords: vertical sleeve gastrectomy, fMRI, eating habits, postprandial aGLP-1, ghrelin, metagenomic sequencing, Clostridia
Obesity, defined as an excess of whole-body adipose tissue, has become a global health problem and a leading cause of several chronic complications, including type 2 diabetes and cardiovascular diseases (1). Bariatric surgical procedures such as Roux-en-Y gastric bypass (RYGB) and vertical sleeve gastrectomy (VSG) are potent, effective interventions that can produce substantial and similar reductions in body weight and clinical improvements in obesity-related comorbidities (2, 3). More than 80% of patients experience an approximately 20% to 35% total body weight loss following surgery and maintain reduced levels of body fat for many years (4); however, ~20% of patients start to regain weight 2 to 3 years later (5), and such varied clinical responses to bariatric procedures have not yet been fully elucidated (6).
Despite being designed with the aim of restricting food intake and energy absorption, it has been long recognized that these procedures profoundly impact brain activity and eating behaviors (7). Some evidence acquired through the use of task functional magnetic resonance imaging (fMRI) experiments supports a model of a distributed reward-driven brain circuit, in which RYGB mainly mediates food wanting and caloric intake, thereby resulting in reduction of body weight (8, 9). However, it remains unclear whether VSG intervention, which has become a dominant bariatric procedure due to its safety and effectiveness, shapes brain circuits in a similar manner to RYGB, as comparable weight loss and hormone changes are achieved after the 2 bariatric surgeries (10, 11). Emerging evidence indicates that VSG therapy not only reduces response to reward value in the striatum, but also leads to improvement in both reward-related and executive performance in adolescent patients with obesity at 4 months after surgery (12). Beyond changes in focal brain activity, VSG surgery also causes brain-wide alterations of functional connectivity with ventromedial and dorsomedial subdivisions of the prefrontal cortex, dorsal anterior cingulate cortex, precuneus, and insula as measured by resting-state fMRI (13), a technique that measures synchronous spontaneous fluctuations of the blood oxygen level–dependent (BOLD) signal between brain regions (14). Given the dietary cultures or patterns of Chinese adults who are predominantly insensitive to calories during daily food selection (15), we hypothesize that apart from brain reward circuit, the habit circuit that mediates daily eating routines may also actively contribute to the therapeutic effect of VSG.
Moreover, both RYGB and VSG surgeries cause substantial alterations of a vast array of gastrointestinal signals in patients with obesity (4, 5, 10), including increases in levels of postprandial active glucagon-like peptide-1 (aGLP-1) (16), and decreases in levels of Ghrelin (17). In fasted healthy volunteers, administration of GLP-17–36 amide led to reductions in energy intake accompanied by altered brain activity in appetite centers, including the caudate, nucleus accumbens, amygdala, insula, and orbitofrontal cortex (OFC) (18). By contrast, intravenous administration of Ghrelin caused increased neural response to food pictures in the striatum, amygdala, anterior insula, and OFC in healthy volunteers (19), which is in line with the imaging findings showing decreased activity in these areas of patients with obesity after RYGB (20), but limited imaging study after VSG precludes the comparison. Of note, despite the large difference in stomach anatomy as a result of the 2 procedures, RYGB and VSG surgery similarly improved postprandial aGLP-1 secretion and weight control (10). These data suggest that intestinal hormones, such as aGLP-1 and Ghrelin could function as important mediators modulating specific brain circuits that control postsurgery food intake. However, to date, there is little evidence showing the association of intestinal hormones and brain circuits after VSG.
Previous studies have reported that gut microbiota exerts an important role on body weight homeostasis by affecting energy balance, serum metabolome, and metabolic inflammation, which are intimately associated with benefits of bariatric surgeries (21, 22). Our recent work demonstrated that weight loss intervention by VSG in Chinese patients with obesity particularly increased abundance of Bacteroides thetaiotaomicron at 1 and 3 months after the surgery, which was involved in partially reversed obesity-associated metabolic alterations (23). Long-term changes in gut microbiota induced by RYGB may even contribute to stable fat mass maintenance 9 years after intervention (24). In-depth examination of the VSG-triggered event chain, including intestinal microbiota alterations, intestinal hormones, brain activity, behavioral adjustment, and weight loss, may facilitate the development of effective, nonsurgical therapies for obesity by targeting gut microbiota (23).
As such, our additional specific aim is to identify a preoperative neuroimaging marker with appropriate biological underpinnings which may be applied to predict clinical response to VSG surgery. To this end, we used a holistic approach combining anthropometric and behavioral measurements, biochemistry profiling, deep metagenomic sequencing, and resting-state fMRI in a cohort of normal-weight volunteers and obese young Han Chinese before and after VSG surgery. We hypothesized that VSG intervention preferentially impacts the brain-wide habit circuit and remodels eating habitual behavior, which is potentially modulated by surgery-driven alterations in gut microbiota species and intestinal hormones such as aGLP-1.
Methods
Participants
Patients with obesity (body mass index [BMI] ≥ 30 kg/m2) were evaluated and recruited continuously from the specialized obesity outpatient clinic of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine (SJTUSM). Briefly, patients with obesity were recruited into the Genetics of Obesity in Chinese Young (GOCY) study established by Ruijin Hospital and registered at ClinicalTrials.gov, and individuals with secondary or syndromic obesity were excluded (Clinical trial reg. no. NCT01084967 and NCT02653430, http://www.clinicaltrials.gov/) (23).
In this study, we evaluated 80 subjects with obesity for gastric surgery between September 2013 and December 2016, of whom 36 subjects (PreOB) who met the following inclusion criteria were finally recruited: (1) 40 years ≥ age ≥ 16 years; (2) BMI ≥ 35 kg/m2, or BMI ≥ 32 kg/m2 in the presence of at least one metabolic comorbidity, such as type 2 diabetes, hypertension, and/or obstructive sleep apnea hypopnea syndrome, etc.; (3) right-handed; and (4) willing to undertake extensive anthropometric, questionnaire, blood biochemical, fecal, and imaging examinations. Exclusion criteria for obese subjects were: (1) history of brain injury or diseases impacting the central nervous system; (2) history of anorexia nervosa, depression, or psychosis; (3) the use of weight loss medications or any agents known to affect appetite or GLP-1 metabolism (eg, metformin, GLP-1 receptor agonists, and dipeptidyl peptidase 4 inhibitors); (4) MRI contraindication (metal implants, pregnancy or claustrophobia); (5) history of gastrointestinal tract surgery; and (6) history of usage of any antibiotic treatment within 3 months before surgery and follow-up time point, or intake of any food containing probiotics such as yogurt within 7 days before sample collection. In parallel, 26 normal-weight subjects (NW, 18.0 kg/m2 < BMI < 23.0 kg/m2) matched for age and sex with PreOB were recruited from the local community by advertisements in social media. The main exclusion criteria for NW were history of obesity, hypertension, impaired glucose regulation, diabetes mellitus, dyslipidemia, abnormal liver and kidney function, gastrointestinal disease, and gastrointestinal surgery within 5 years before recruitment. We collected a set of anthropometric measurements including body weight, height, waist and hip circumference (23), Three-Factor Eating Questionnaire (TFEQ) (25), blood and fecal samples, and MRI data for each patient before the surgery (within 1 week) and NW. Four subjects with obesity later withdrew from the surgical treatment for personal reasons. The remaining 32 subjects with obesity received VSG treatment, and 19 postoperative patients (PostOB) were scheduled for follow-up visit at 4.3 ± 1.7 (mean ± SD) months after the operation, collecting imaging data and blood and fecal samples on the same day (Fig. 1A). This study was approved by the Institutional Review Board of the Ruijin Hospital, SJTUSM and a written informed consent was obtained from each participant.
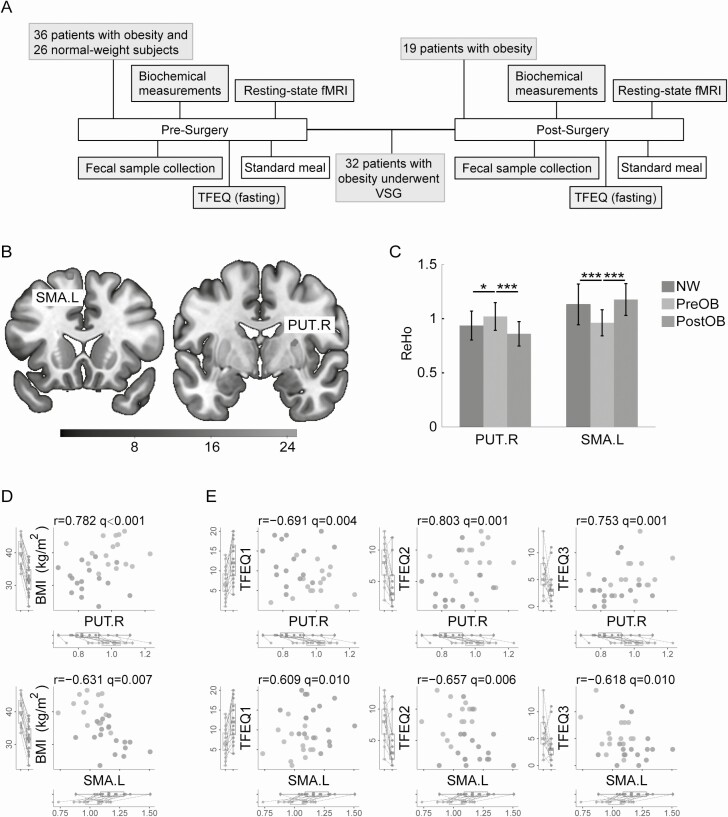
Figure 1.
Study protocol and associations between regional brain activity, weight loss, and eating behaviors. (A) Diagram of the experimental design. (B) Brain regions showing significant functional changes (indexed by regional homogeneity, ReHo) in patients with obesity before and after VSG surgery (FDR corrected). (C) Comparisons of ReHo in PUT.R and SMA.L across 3 groups (PreOB, n = 33; PostOB, n = 16; NW, n = 25; *P < 0.05; ***P < 0.001). Data are presented as mean ± standard error of the mean. (D) Scatter plots of ReHo in PUT.R/SMA.L against BMI before and after VSG surgery (PreOB, n = 16; PostOB, n = 16). (E) Scatter plots of ReHo in PUT.R/SMA.L against TFEQ subscores before and after VSG surgery (PreOB, n = 16; PostOB, n = 16). Abbreviations: BMI, body mass index; fMRI, functional magnetic resonance imaging; NW, normal-weight subjects; OGTT, oral glucose tolerance test; PostOB, postoperative obese group; PreOB, preoperative obese group; PUT.R, right putamen; SMA.L, left supplementary motor area; TFEQ, Three-Factor Eating Questionnaire; VSG, vertical sleeve gastrectomy.
Evaluation of Eating Behavior
All participants, including NW, completed the eating questionnaire after a 12- to 14-hour fast. The TFEQ is used to assesses 3 cognitive and behavioral aspects of eating, and it demonstrates high internal consistency, stability, and construct validity (25). A Chinese, 21-item version of the Three-Factor Eating Questionnaire (TFEQ-R21) was used with permission from the author of the scale (26). All item responses are grouped into 3 scales: scale I involves cognitive control over food intake to influence body weight and body shape, with higher scores indicating more conscious dietary restraint; scale II indicates disinhibition of control, with higher scores representing more episodes of loss of control over eating; and scale III evaluates the subjective sensitivity and response to hunger.
MRI Data Acquisition
Fifteen minutes before MRI scanning, subjects were requested to eat a 100-kcal standard meal. Seven-minute resting-state fMRI and high-resolution 3D T1-weighted datasets were collected with an 8-channel head coil on a 3.0 Tesla Signa HDxT (GE, Milwaukee, WI, USA) scanner in Ruijin Hospital, SJTUSM. Subjects were instructed to lie still with earplugs and eyes closed, and to try not to think about anything in particular. Whole-brain blood oxygen level–dependent fMRI images were acquired with the following parameters: repetition time = 2000 ms, echo time = 30 ms, flip angle = 90°, field of view = 240 mm × 240 mm, in-plane resolution = 3.75 × 3.75 mm2, 4 mm slice thickness with no gap, 33 slices and 210 volumes. For spatial normalization, T1-weighted anatomical images were acquired using a 3D magnetization-prepared rapid acquisition gradient-echo sequence with an axial fast spoiled gradient-echo sequence with the following parameters: repetition time = 5.98 ms, echo time = 1.87 ms, inversion time = 450 ms, flip angle = 12°, and spatial resolution = 1 × 1 × 1 mm3.
MRI Data Analysis
Functional imaging data were preprocessed using the Data Processing Assistant for Resting-State fMRI (DPARSF, http://rfmri.org/DPARSF) (27). Preprocessing steps included slice timing correction, motion correction, spatial normalization into Montreal Neurological Institute (MNI) space, and reslicing to 3 × 3 × 3 mm3 voxels. Friston-24 parameters of head motion, white matter, and ventricle signals were regressed out, followed by linear drift correction and temporal filtering (0.01-0.1 Hz). A brain mask was applied to exclude the cerebellum and cerebrospinal fluid components. Three PreOB subjects, 1 NW subject, and 3 PostOB subjects were excluded from further data analysis due to excessive head motion (> 3.0 mm or 3.0 degree). Twenty-five NW (BMI, 20.07 ± 1.10 kg/m2, mean ± SD), 33 PreOB subjects (BMI, 39.28 ± 4.86 kg/m2), and 16 PostOB subjects (BMI, 31.67 ± 4.37 kg/m2) were ultimately included in the subsequent statistical analysis.
After preprocessing, we deployed data-driven, voxel-wise regional homogeneity (ReHo) analysis in tandem with seed-based connectivity analysis using DPARSF to detect longitudinal functional changes in core brain regions and brain-wide connectivity caused by VSG surgery. The ReHo method is used to evaluate regional brain activity during rest by examining the degree of regional synchrony of fMRI time courses and has been applied to detect functional brain changes of metabolism diseases, such as type 2 diabetes mellitus and anorexia nervosa (28, 29). ReHo maps were generated in a voxel-wise analysis by calculating Kendall’s coefficient of concordance (KCC) of the time series of a given voxel with those of its nearest neighbors (26 voxels). Each individual ReHo map was normalized through dividing the KCC among each voxel by whole-brain mean KCC values (30) and then subject to spatial smoothing with a Gaussian kernel (full width at half maximum [FWHM] = 6 mm) along all directions.
The brain regions identified with abnormal ReHo values were determined as seeds to examine changes in functional connectivity in obese subjects before and after VSG. The correlation coefficient between the mean time course of each seed and the time course of every voxel in the brain was calculated to generate a correlation map. The resulting correlation map was normalized using Fisher’s r to z transformation and spatially smoothed (a Gaussian kernel with FWHM = 6 mm) before group comparison.
Biochemical and Gastrointestinal Hormone Measurements
Evaluation of blood hormones was conducted after an overnight fast of 12 to 14 hours. An oral glucose tolerance test (OGTT) was performed approximately 1 week before MRI scanning. Participants ingested a 75 g glucose load, with blood sampling obtained via an arterialized hand vein at 0, 30, 60, 120, and 180 minutes. Plasma levels of Ghrelin, aGLP-1, total peptide tyrosine-tyrosine (PYY) and Leptin were measured using a human metabolic hormone magnetic bead panel (Cat. No. HMHEMAG-34K, Millipore) following the manufacturer’s instructions.
Fecal Sample Collection and Sequencing
Fecal sample collection and DNA extraction were conducted following procedures described in our previous work (23), which are briefly summarized as follows. Participants did not receive antibiotic treatment for at least 3 months before sample collection. Subjects were instructed not to take any drugs or food products containing probiotics, such as probiotic yogurt, for at least 1 week prior to sampling. Each sample was either frozen immediately at −80 °C, or briefly stored in −20 °C freezers in the participants’ home units before transport to −80 °C refrigerator within 24 hours. All samples were sequenced on the Illumina platform (paired-end; insert size, 350 bp; read length, 150 bp). Adaptor and low-quality reads were discarded from the raw reads, and the remaining reads were filtered in order to eliminate human host DNA based on the human genome reference (https://www.ncbi.nlm.nih.gov/assembly/GCF_000001405.26/, GRCh38).
Metagenomics Taxonomic and Functional Profiling
MetaPhlAn2 (31) was performed for quantitative profiling of the taxonomic composition of the microbial communities at species level, which uses a library of clade-specific markers. To obtain the functional profile, high-quality reads were aligned against the integrated gene catalog (IGC) containing 9.9 million nonredundant microbial genes using the SOAP2 (an improved version of Short Oligonucleotide Alignment Program) with the criterion of identity ≥ 95%, and sequence-based gene abundance profiling was performed as previously described (23). The relative abundance of each Kyoto Encyclopedia of Genes and Genomes (KEGG) orthology group was estimated by summing up the relative abundances of genes annotated belonging to the same KEGG ortholog group using KEGG annotations. Pathway-enrichment analyses were conducted based on KEGG annotations and reporter scores from the Z-scores of individual KEGG orthologs (32).
Analysis of gut metabolic modules (GMMs) was applied to assess the short-chain fatty acids production potential of the species. GMMs reflect bacterial and archaeal metabolism specific to the human gut environment, with a focus on anaerobic fermentation processes. Representative genomes of the species were downloaded from img/hmp v4.0. The coverage and abundance of the GMMs in each species were analyzed in the original article (33).
Statistical Analysis
Group differences in age and sex were analyzed using Student’s t-test and chi-square test, respectively. To minimize potential confounding effects, both age and sex were treated as covariates in all group analyses. Two-sample t-tests were then performed to examine group differences in anthropometric measures and TFEQ scores between obese groups and NW. P < 0.05 was considered the threshold for statistical significance.
Linear mixed-effects modeling (LMEM) analysis was conducted on anthropometric measures, TFEQ scores, blood hormones, ReHo, and seed-based functional connectivity measurements in obese subjects before and after surgery. The LMEM was used to deal with longitudinal missing data and take within-subject variability into account in fMRI group analysis (34, 35). The LMEM analysis was completed using R and lme4 package (36). We treated age (centered) and sex (without interaction term) as fixed effects of the model and had intercepts for subjects as random effects [ReHo/functional connectivity~ group + sex + age + (1 | subject)]. P values were obtained by likelihood ratio tests of the full model with the effect of surgery group against the model without the effect of surgery group. To control for multiple comparisons in ReHo and seed-based functional connectivity analyses, a region was considered to have a statistically significant surgery effect after masking outside of brain if the P value associated with its test was less than a designated critical value [individual voxel threshold P < 0.001 and cluster size ≥ 10, to yield a false-positive discovery rate (FDR) corrected P < 0.05].
Once VSG-affected brain areas and connnectivities were determined, post hoc t-tests were performed to identify differences between groups with obesity and normal-weight healthy volunteers. We extracted ReHo/functional connectivity of the identified brain areas in LMEM for PreOB and PostOB subjects, as well as for NW, and performed group-wise comparisons. These altered functional connectivity with the seeds were further categorized based on their potential involvements in different habit circuits including cognitive control (associative) network, sensorimotor network, and limbic network (37, 38).
Since the data of gut microbiota that did not follow a normal distribution were not suitable for LMEM analysis, we performed 2-tailed Wilcoxon matched-pairs signed rank test for pair-wise comparison in the PreOB and PostOB groups (P < 0.05, FDR corrected, hereafter q-values). Repeated measures correlation (39) (R software, rmcorr package) was used to explore pair-wise associations between changes in BMI, eating behavior, brain activity, intestinal hormones, and relative abundance of gut microbiota in patients with obesity. Partial correlation analyses were conducted to evaluate the relationships between preoperative regional brain activity and functional connectivity with the right putamen and weight loss, after controlling for age and sex.
Results
Demographic and Clinical Characteristics
Demographic and clinical data of patients with obesity and NW are summarized in Table 1. There were no significant differences in age and sex between obese groups and NW. After VSG, the PostOB group showed a significant decrease in body weight, BMI, waist circumference, hip circumference, and waist-to-hip ratio (P < 0.001). These parameters were significantly higher in PreOB than in NW. To quantitatively characterize eating habits, all participants were administered the TFEQ-R21. We found that cognitive restraint score (TFEQ1) was significantly increased in the PostOB compared with PreOB (P < 0.001), whereas it did not differ initially between PreOB and NW (P = 0.311). Moreover, disinhibition score (TFEQ2) in obese cases was robustly reduced by surgery (P < 0.001), to the comparable level of that in NW (PostOB vs NW, P = 0.996). The PostOB hunger score (TFEQ3) showed a decreasing trend in comparison to PreOB (P = 0.053), close to the level of NW (PostOB vs NW, P = 0.980) (Table 1). These results indicate that VSG treatment imposed significant influence mainly on 2 dimensions of eating habits: cognitive control ability and disinhibition to feeding.
Table 1.
Anthropometry, eating questionnaires, and clinical characteristics in all participants
| NW | PreOB | PostOB | PreOB vs PostOB | NW vs PreOB | NW vs PostOB | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Num. | Mean ± SD | Num. | Mean ± SD | Num. | P valuea | P valueb | P valueb | |
| Age (years) | 26.92 ± 4.46 | 25 | 28.06 ± 4.95 | 33 | 28.56 ± 4.10 | 16 | - | 0.369 | 0.243 |
| Gender (M/F) | 7/18 | 25 | 10/23 | 33 | 4/12 | 16 | 0.700c | 0.849c | 0.833c |
| BW (kg) | 55.2 ± 5.50 | 25 | 110.26 ± 16.94 | 33 | 88.07 ± 16.55 | 16 | < 0.001 | < 0.001 | < 0.001 |
| BMI (kg/m2) | 20.07 ± 1.10 | 25 | 39.28 ± 4.86 | 33 | 31.67 ± 4.37 | 16 | < 0.001 | < 0.001 | < 0.001 |
| WC (cm) | 73.50 ± 4.79 | 25 | 117.13 ± 12.22 | 33 | 100.41 ± 10.53 | 16 | < 0.001 | < 0.001 | < 0.001 |
| HC (cm) | 90.80 ± 3.90 | 25 | 121.62 ± 12.60 | 33 | 107.38 ± 9.70 | 16 | < 0.001 | < 0.001 | < 0.001 |
| WHR | 0.81 ± 0.04 | 25 | 0.97 ± 0.07 | 33 | 0.94 ± 0.06 | 16 | 0.041 | < 0.001 | < 0.001 |
| TFEQ1 | 5.76 ± 4.48 | 25 | 6.93 ± 3.83 | 28 | 12.44 ± 5.12 | 16 | < 0.001 | 0.311 | < 0.001 |
| TFEQ2 | 4.12 ± 3.22 | 25 | 7.68 ± 3.33 | 28 | 4.13 ± 3.24 | 16 | < 0.001 | < 0.001 | 0.996 |
| TFEQ3 | 3.40 ± 3.00 | 25 | 5.36 ± 3.35 | 28 | 3.38 ± 3.07 | 16 | 0.053 | 0.030 | 0.980 |
Abbreviations: BMI, body mass index; BW, body weight; HC, hip circumference; NW, normal-weight subjects; PostOB, postoperative obese group; PreOB, preoperative obese group; TFEQ, Three-Factor Eating Questionnaire; WC, waist circumference; WHR, waist-to-hip ratio.
Statistical tests performed: a, linear mixed-effects model; b, two-sample t-tests; c, chi-square test.
Associations Between Regional Brain Activity, Body Weight, and Eating Behavior
We next performed whole-brain voxel-wise analysis and found a significant decrease in ReHo in the right putamen (PUT.R) (P < 0.001) and an increase in ReHo in the left supplementary motor area (SMA.L) (P < 0.001) in obese subjects after surgery (Fig. 1B). Furthermore, post hoc analysis revealed that PreOB cases exhibited higher ReHo in the PUT.R (P = 0.019) and lower ReHo in the SMA.L (P < 0.001) than NW. After VSG surgery, no significant differences in ReHo in the PUT.R and SMA.L were observed between obese and NW subjects (P = 0.065 and P = 0.421, respectively) (Fig. 1C).
Correlation analyses showed a positive correlation between ReHo of the PUT.R and BMI (r = 0.782, q < 0.001) and a negative correlation between ReHo of the SMA.L and BMI (r = −0.631, q = 0.007) in patients with obesity before and after surgery (Fig. 1D). Moreover, we observed that ReHo in the PUT.R was negatively correlated with cognitive restraint score (r = −0.691, q = 0.004), and positively correlated with disinhibition score (r = 0.803, q = 0.001) and hunger score (r = 0.753, q = 0.001). Meanwhile, we found a positive correlation between ReHo in the SMA.L and cognitive restraint score (r = 0.609, q = 0.010), whereas it was negatively correlated with disinhibition score (r = −0.657, q = 0.006) and hunger score (r = −0.618, q = 0.010) (Fig. 1E).
Brain-Wide Functional Connectivity With Right Putamen Is Restored After VSG
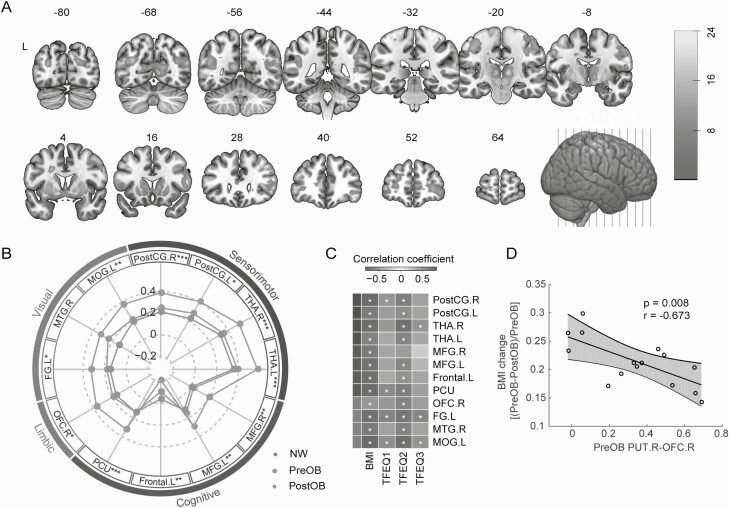
Seed-based connectivity analysis showed that connectivity with the PUT.R was significantly altered in patients with obesity by VSG in the areas including visual (left fusiform gyrus, right middle temporal gyrus, and left middle occipital gyrus), cognitive control regions (bilateral middle frontal gyrus [MFG], precuneus, and white matter area in left frontal lobe), sensorimotor areas (bilateral postcentral gyrus and thalamus), and a limbic area (right OFC) (Fig. 2A and 2B). Post hoc analysis of PUT.R connections showed significant differences between PreOB and NW, with the exception of the right middle temporal gyrus (Fig. 2B). After VSG surgery, most of the PUT.R connections in PostOB were comparable to the level of NW. In contrast, there were no significant differences in functional connectivity with the SMA.L between PreOB and PostOB.
Figure 2.
Brain-wide functional connectivity with right putamen is restored after VSG. (A) Seed-based analysis of the right putamen revealed brain-wide alterations of functional connectivity (FDR corrected). Color bar indicates t values. (B) Radar plot shown the comparisons of altered functional connectivity with the right putamen shown in (A) among obese groups and NW (PreOB, n = 33; PostOB, n = 16; NW, n = 25). Involved brain regions are grouped according to 4 putative neural circuits (color-coded) that may regulate eating habits. Asterisks denote significance levels between NW and PreOB (*P < 0.05; **P < 0.01; ***P < 0.001). (C) Correlation heatmap of changes in the right putamen-centered connectivity with BMI and TFEQ subscores (PreOB, n = 16; PostOB, n = 16). Red and blue colors indicate positive and negative correlation coefficients. Symbols denote significance levels of the correlations (+q < 0.05; *q < 0.01). The reported q-values here were FDR corrected for multiple comparisons. (D) Preoperative functional connectivity between PUT.R and OFC.R was significantly correlated with changes in BMI scores in patients with obesity (n = 16). Shaded areas indicate 95% CI. Abbreviations: FG.L, left fusiform gyrus; Frontal.L, white matter area in left frontal lobe; MFG.L, left middle frontal gyrus; MFG.R, right middle frontal gyrus; MOG.L, left middle occipital gyrus; MTG.R, right middle temporal gyrus; OFC.R, right orbitofrontal cortex; PCU, Precuneus; PostCG.L, left postcentral gyrus; PostCG.R, right postcentral gyrus; THA.L, left thalamus; THA.R, right thalamus.
Functional connectivities of the PUT.R in patients with obesity were also significantly correlated with BMI and disinhibition score (Fig. 2C). Specifically, we found that preoperative connectivity between the PUT.R and OFC.R in patients with obesity was negatively correlated with BMI change by surgery (r = −0.673, P = 0.008) (Fig. 2D).
Associations Between Gastrointestinal Hormones, Regional Brain Activity and Weight Loss
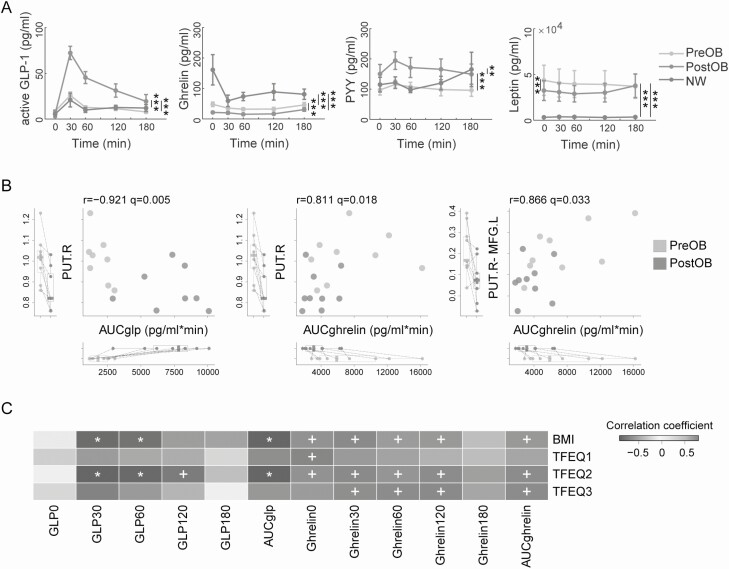
We next measured changes in gastrointestinal hormones, including aGLP-1, Ghrelin, and PYY, in response to oral glucose intake with an OGTT test in all subjects. LMEM analysis showed that levels of postprandial aGLP-1 and PYY were increased in PostOB compared with PreOB (P < 0.001), whereas no significant differences in these 2 hormones were found between PreOB and NW (P = 0.688 and P = 0.064, respectively). Postprandial Ghrelin was decreased in PostOB relative to PreOB (P < 0.001) and exhibited a robust decrease in PreOB compared with NW (P < 0.001). We further examined the concentrations of Leptin, an adipokine inhibiting appetite, and found that the level of Leptin in PostOB showed a significant decrease relative to PreOB (P < 0.001) but was much higher in PreOB than NW (P < 0.001) (Fig. 3A).
Figure 3.
Associations between intestinal hormones and regional brain activity. (A) Hormone plots of the oral glucose tolerance test (OGTT) in patients with obesity before (n = 19 for aGLP-1, Ghrelin, and Leptin; n = 18 for PYY) and after (n = 10 for 4 hormones) surgery and in normal-weight subjects (n = 10 for aGLP-1, Ghrelin, and Leptin; n = 9 for PYY). (B) Repeated measures correlations between brain activity and changes of intestinal hormones. Left panel (n = 16) and middle panel (n = 18) are scatter plots of AUC of aGLP-1 and Ghrelin, respectively, in the OGTT test against functional activity of the right putamen (ReHo). Right panel (n = 18) shows a scatter plot of AUC of Ghrelin in the OGTT test against functional connectivity of the PUT.R-MFG.L. (C) Correlation heatmap of changes in aGLP-1 and Ghrelin in the OGTT test with BMI and TFEQ subscores (n = 16 or 18). Red and blue colors indicate positive and negative correlations. Symbols denote significant levels of the correlations (+q < 0.05; *q < 0.01). Abbreviations: aGLP-1, active glucagon-like peptide-1; AUC, area under the curve; PYY, peptide tyrosine-tyrosine.
Correlation analyses showed that areas under the curves (AUCs) of postprandial aGLP-1 were negatively correlated with ReHo in the PUT.R (r = −0.921, q = 0.005), while fasting Ghrelin and AUCs of postprandial Ghrelin were positively correlated with ReHo in the PUT.R (r = 0.883, q = 0.004; and r = 0.811, q = 0.018, respectively) in obese subjects before and after surgery. In addition, AUCs of postprandial Ghrelin were positively correlated with functional connectivity between the PUT.R and MFG.L (r = 0.866, q = 0.033) (Fig. 3B). Importantly, AUCs of postprandial aGLP-1 were negatively correlated with BMI (r = −0.896, q = 0.006) and disinhibition scores (r = −0.896, q = 0.006) in patients with obesity before and after surgery, whereas AUCs of postprandial Ghrelin were positively correlated with BMI (r = 0.692, q = 0.036), disinhibition (r = 0.761, q = 0.035), and hunger scores (r = 0.757, q = 0.035). Of note, increased postprandial aGLP-1 at 30 and 60 min were correlated with BMI (r = −0.876, q = 0.006, and r = −0.830, q = 0.006, respectively) and disinhibition (r = −0.913, q = 0.006, and r = −0.880, q = 0.006, respectively) (Fig. 3C). There were no significant correlations between PYY or Leptin with BMI or TFEQ scores.
Gut Microbiota Alterations Associated With Gastrointestinal Hormones, Brain Activity, and Weight Loss
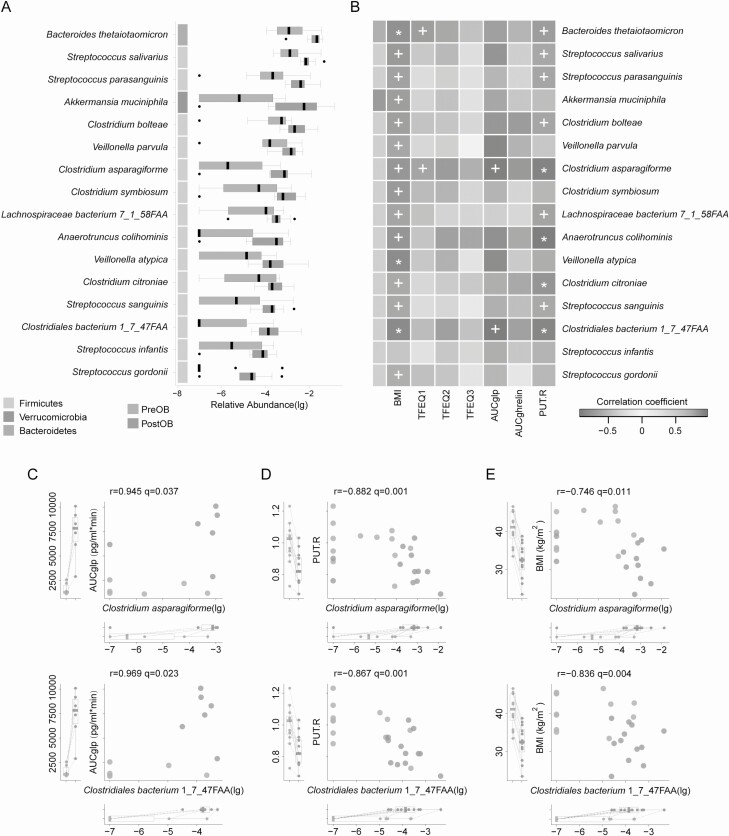
Metagenomic analysis showed that the relative abundances of multiple gut microbial species were significantly changed in PostOB compared with PreOB, including the increase in B. thetaiotaomicron and Akkermansia muciniphila in PostOB (Fig. 4A), consistent with our previous report (23). Notably, we observed species from Clostridium and Streptococcus were also significantly increased after surgery. More importantly, the relative abundances of Clostridium asparagiforme and Clostridiales bacterium 1_7_47FAA were positively correlated with AUCs of postprandial aGLP-1 (r = 0.945, q = 0.037; and r = 0.969, q = 0.023, respectively), and negatively correlated with ReHo in the PUT.R (r = −0.882, q = 0.001; and r = −0.867, q = 0.001, respectively) and BMI (r = −0.746, q = 0.011; and r = −0.836, q = 0.004, respectively) in patients with obesity before and after surgery (Fig. 4B-4E). Moreover, Clostridium asparagiforme was positively correlated with cognitive restraint (r = 0.777, q = 0.043) (Fig. 4B). No significant correlation was found between changes in gut microbiota composition and Ghrelin, PYY, or Leptin. Besides, relative abundance of B. thetaiotaomicron was negatively correlated with ReHo in the PUT.R (r = −0.701, q = 0.020) and BMI (r = −0.788, q = 0.007), and positively correlated with cognitive restraint (r = 0.804, q = 0.043). All species of bacteria except Streptococcus infantis with relatively increased abundance after surgery were correlated with BMI (Fig. 4B).
Figure 4.
Associations between alterations in gut microbiota, intestinal hormones, and brain activity. (A) Increased species of gut microbe in patients with obesity after VSG surgery (PreOB, n = 12; PostOB, n = 12; q < 0.05). The x-axis represents the relative abundance of each species. (B) Correlation heatmap of changes in gut microbiota with changes of BMI, TFEQ subscores, gastrointestinal hormones, and regional activity in the right putamen (n = 12 or 24). Red and blue colors represent positive and negative correlations, respectively. Symbols denote significance levels of the correlations (+q < 0.05; *q < 0.01). (C-E) Scatter plots of the relative abundance of 2 bacteria against aGLP-1 (n = 12) (C), ReHo in the right putamen (n = 24) (D), and BMI (n = 24) (E) before and after the surgery. Abbreviation: lg, log-transformed.
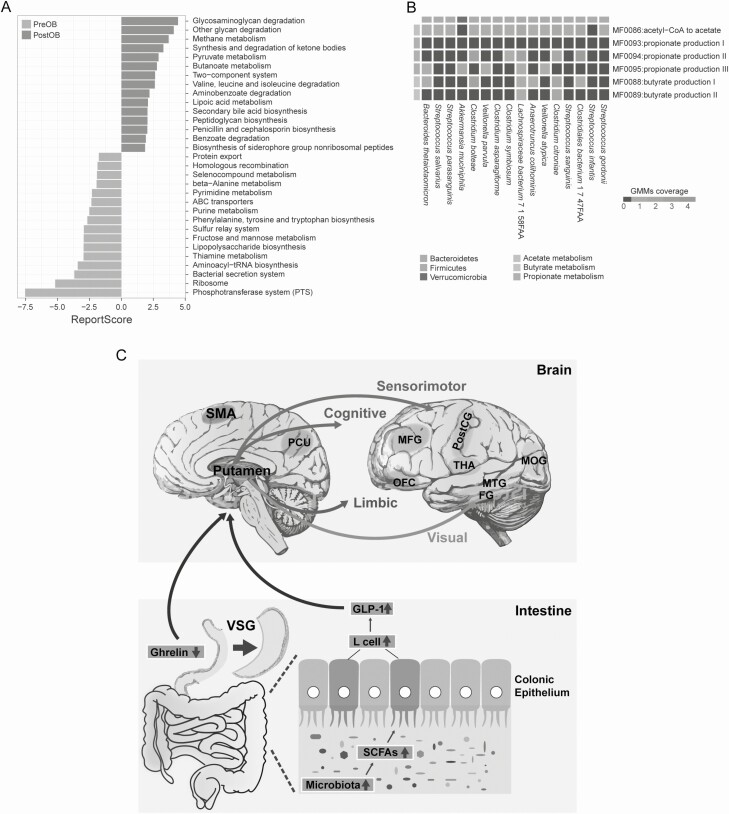
To characterize changes in the functional potential of microbes adapted to gut remodeling, we explored overall gut microbial function in patients with obesity according to the KEGG database. We found that several increased KEGG modules in PostOB were closely associated with pyruvate, butanoate, and methane metabolism whereas the main modules with decreased KEGG function were largely involved in the phosphotransferase system, lipopolysaccharide biosynthesis, and metabolism of amino acids (phenylalanine, tyrosine, tryptophan, purine, pyrimidine, and beta-alanine) (Fig. 5A). Analysis of short-chain fatty acids (SCFAs) production potential revealed that microbial species altered in the PostOB gut microbiome including B. thetaiotaomicron, A. muciniphila, and microbial species in Clostridia were involved in the metabolism of SCFAs, such as acetate, butyrate, and propionate (Fig. 5B).
Figure 5.
Functional pathway analysis of compositional changes in gut microbiota. (A) Overall microbial functional enrichment analysis of the gut microbiota before and after surgery based on KEGG pathways (PreOB, n = 12; PostOB, n = 12). KEGG with reporter score > 1.65 or < −1.65 is shown. Blue and red colors represent after- and before-surgery enriched pathways, respectively. (B) Heatmap showing the SCFAs related GMM coverage of the species shown in Fig. 4. (C) Diagram illustrating the indicated gut-brain interaction that may mediate the surgical weight loss effects by VSG. Abbreviations: GMM, gut metabolic module; SCFAs, short-chain fatty acids.
Discussion
VSG Restores Brain Habit Circuit Centering Right Putamen Predicts Weight Loss
The present imaging analysis identified 2 core regions, the right putamen and left supplementary motor area with abnormal ReHo activity changed in young, severely obese cases, which was restored by VSG treatment back to the equivalent levels in lean subjects, paralleled by eating behavioral changes and weight loss. Both the putamen and SMA have been implicated as important nodes of habit-related circuits in the cortico-basal ganglia loop (37, 38). It has been reported that RYGB reduced brain responses to high-calorie food cues in the putamen and resting-state activity in left SMA was altered during the 1-year postoperative period in patients with obesity (20, 40). Given involvement of the putamen in habit-related motor circuit and distinct surgical procedures and potential common mechanisms underlying for RYGB and VSG (4, 10), we postulate that VSG reverses the over-strengthened activity of the putamen, thereby inhibiting abnormal habitual eating actions in the obese state. A recent study in healthy subjects demonstrates that post-ingestive dopamine release in the putamen is inversely correlated to the desire to eat (41). The role of the putamen actively involved in control of food intake has also been manifested as its robust activation during actual food consumption and has direct relevance to individual BMI (42). Interestingly, studies in which either diet or physical exercise therapy lead to successful weight loss in patients with obesity support the association between functional activity in the putamen with cognitive control of dietary intake (43) and weight loss maintenance (44).
Furthermore, VSG therapy has restored elevated functional connectivities with the right putamen, whereby most brain regions have been reported as having reduced activations in patients after RYGB (20, 45), including the precuneus, OFC, occipital and somatosensory cortices, which are central to the putative habit formation circuitry, involving cognitive control, limbic, visual, sensorimotor networks (37). This large-scale functional remodeling with the putamen seems to be plausible to explain the global eating behavioral adaptation after VSG, especially improved loss of control for overeating. More importantly, enhanced preoperative connectivity of the right putamen with the right OFC predicted a less favorable outcome of VSG treatment. The OFC is well known to relate to reward processing and incentive valuation, widely connected with brain regions including the thalamus, amygdala, cingulate cortex, insula/operculum, hypothalamus, hippocampus, striatum, and dorsolateral prefrontal cortex (46), but the functional role of its direct connectivity with the putamen remains to be elucidated. We posit that hyperconnectivity between the putamen and OFC identified here may indicate a stronger predisposition to erratic eating habits in obese individuals, the extent to which it was normalized by surgical intervention was predictive of surgical outcome. To gain the mechanistic understanding of this pathway merits future investigation.
Association of VSG-Induced Secretion of Intestinal Hormones and Brain Activation
Unlike RYGB surgery, the intestine was not anatomically disturbed in VSG procedure; however, VSG treatment induced a series of concordant changes in meal-stimulated intestinal hormone secretion including aGLP-1, Ghrelin, and PYY. Among them, a postprandial increase in aGLP-1 was correlated to decreased activation in the right putamen and weight loss, which is complementary to prior findings in RYGB treatment (20). Considering that endogenous aGLP-1 is capable of crossing the blood-brain barrier and GLP-1 receptors have been confirmed distributed in parietal cortex, hypothalamus and medulla of the human brain (47), it is possible for GLP-1 to directly modulate neural activity in the putamen. Note that there exists alternative or compensatory signaling pathways in addition to GLP-1 that may mediate brain activity in humans, because the results obtained from genetic rodent models of Glp-1 receptor deficiency indicate that Glp-1 receptor activity seems not to be necessary for the metabolic improvements induced by VSG surgery in mice (48). Identification of the putamen activity of subjects carrying loss-of-function mutations in GLP-1 receptor will be valuable for illustrating the potential discrepancy between species. In parallel, we observed that changes in fasting Ghrelin before and after VSG surgery were associated with reduced activity in the right putamen and weight loss, extending the modulatory effect of Ghrelin previously observed in hippocampus and ventral tegmental area (49). Yet the results from genetic rodent models showed that VSG procedure was equally effective in Ghrelin-deficient and Ghrelin-intact mice (50), indicating Ghrelin is neither sufficient nor critical to surgical outcome at least in mice. Unlike GLP-1, the association between changes in Ghrelin and activity in the putamen was independent of the feeding condition (before and after meal), which may implicate the role of Ghrelin in the long-term regulation of body weight (17). Nonetheless, there may exist other circulating gastrointestinal hormones and adipokines that are likely to participate in the therapeutic process of bariatric surgery (51). The present study measured PYY and Leptin which did not result in associations with brain activity or weight loss. These results may suggest co-existence of multiple intestinal hormones signaling pathways triggered by VSG procedure attributing to the adjustment of eating behavior and the loss of body weight.
Adaptation of Human Gut Microbiota to Bariatric Surgery
An emerging theory postulates that metabolic regulation starts from the gut and then sends signals to endocrine organs and the brain after the bariatric surgery (22). In addition to our recent findings (23), we identified that stable increased abundances of B. thetaiotaomicron, A. muciniphila, and microbial species in Firmicutes in the postoperative group were associated with weight loss in patients with obesity. It seems that these changes of the microbiota were not specific to VSG or Chinese subjects. The level of Bacteroides was observed to be increased at 3 and 6 months after RYGB in French patients with obesity (52). Rapid and stable increase in the relative abundance of Verrucomicrobia (A. muciniphila) was also observed in American subjects and in mice after RYGB (53, 54). Moreover, the abundance of the Akkermansia genus of individuals with a successful weight loss after RYGB was significantly higher in comparison to individuals which presented late weight regain (55). Alterations of 2 species in Clostridia, Clostridium asparagiforme and Clostridiales bacterium 1_7_47FAA, were in accord with changes in aGLP-1 (but not Ghrelin), brain activity, and body weight in patients with obesity. Obesity causes a shift in the abundance of Bacteroidetes and Firmicutes (56). It has been shown in animal studies that decreased abundance of Bacteroidetes and Firmicutes resulted in augmentation of GLP-1 secretion (57). Dietary manipulation of the gut microbiota using nutrients or specific microbes also enables to stimulate GLP-1 secretion and hence contributes to the regulation of glucose, lipid, and energy homeostasis (58). Moreover, subsequent GMM analysis revealed that microbial species altered by VSG, particularly species in Clostridia, were involved in the metabolism of SCFAs, such as acetate, butyrate, and propionate, pointing to a conceivable pathway that links gut microbiota and intestinal hormones. These 3 SCFAs can be produced by many commensal bacteria in the gut and have been demonstrated to reduce the risk of gastrointestinal disorders. Butyrate was proposed to stimulate the release of GLP-1 from intestinal L-cells (59), while acetate and propionate both actively participate in lipid and glucose metabolism (60). We provide further evidence to support the view that loss of Clostridia of Firmicutes was recently found as one of key features associated with obesity, and Clostridia treatment has been applied to control lipid absorption and reduce adiposity (61). Future studies on animal models and humans are required to examine the underlying mechanism of the candidate species/metabolites that regulate functional brain activity.
In conclusion, we identified 2 core brain regions and their associated functional connectivities altered by VSG therapy that may mediate positive adjustment of eating behaviors and consequent body weight in patients with obesity, which may be helpful to stratify patients who are more likely to experience better surgical benefits (Fig. 5C). These longitudinal alterations of the brain habit-related circuit were in tight correlation with adaptation of postsurgery levels of aGLP-1 and Ghrelin and the remodeling of gut microbiota composition, which offers novel insights into developing nonsurgical interventions for obesity. Meanwhile, caution should be taken in the interpretation of these correlations, and further clinical and basic work is needed to validate the causality and the gut-brain connection.
Acknowledgments
We thank Drs. Xiaohong Xu and Valerie Voon for their stimulating discussions and suggestions during the preparation of this study, and we thank all participants for their involvements in this study.
Financial Support: This work was supported by the National Key R&D Program of China (2018YFC1313803, 2017YFC1310400), grants from National Natural Science Foundation (91957124, 91857205, 81870584, 31771174, 81822009, 81930021, 81730023, 81870585, 82070863), the Strategic Priority Research Program of Chinese Academy of Science (XDB32030000), Shanghai Municipal Science and Technology Major Project (2018SHZDZX05), the Outstanding Academic Leader Project of Shanghai Municipal Health Commission (2018BR01), and Key-Area Research and Development Program of Guangdong Province (2019B030335001).
Clinical Trial Information: ClinicalTrials.gov registration no. NCT01084967 and NCT02653430.
Author Contributions: G.N., Z.W., and J.W. were responsible for the concept and the design of the study; J.H., T.B., L.X., and N.H. managed the study; J.H., J.W., W.G., and W.W. made the clinical diagnosis and recruited subjects; L.X., W.L., P.L., Y.C., J.S., and D.L. performed intervention, collected samples, and determinedclinical phenotypes; N.H., Y.Z., and F.Y. collected MRI data; T.B., Y.Z., and Z.W. performed MRI data analysis; X.X. and R.L. performed metagenomics sequence and data analysis; Z.W., J.W., and T.B. wrote the manuscript; X.X., R.L., and G.N. contributed to text revision and discussion.
Glossary
Abbreviations
- aGLP-1
active glucagon-like peptide-1
- AUC
area under the curve
- BMI
body mass index
- DPARSF
Data Processing Assistant for Resting-State fMRI
- FDR
false discovery rate
- fMRI
functional magnetic resonance imaging
- GLP-1
glucagon-like peptide-1
- GMM
gut metabolic module
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- LMEM
linear mixed-effects model
- MRI
magnetic resonance imaging
- NW
normal-weight subjects
- OFC
orbitofrontal cortex
- OGTT
oral glucose tolerance test
- PostOB
postoperative obese subjects
- PreOB
preoperative obese subjects
- PUT.R
right putamen
- PYY
peptide tyrosine-tyrosine
- ReHo
regional homogeneity
- RYGB
Roux-en-Y gastric bypass
- SCFA
short-chain fatty acid
- SJTUSM
Shanghai Jiao Tong University School of Medicine
- SMA.L
left supplementary motor area
- TFEQ
Three-Factor Eating Questionnaire
- VSG
vertical sleeve gastrectomy
Additional Information
Disclosures: All authors declare no conflict of interest.
Data Availability
All scripts and routines used for analyzing data are available from the corresponding author upon request.
References
- 1. Ng M, Fleming T, Robinson M, et al. . Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arterburn DE, Telem DA, Kushner RF, Courcoulas AP. Benefits and risks of bariatric surgery in adults: a review. JAMA. 2020;324(9):879-887. [DOI] [PubMed] [Google Scholar]
- 3. Casimiro I, Sam S, Brady MJ. Endocrine implications of bariatric surgery: a review on the intersection between incretins, bone, and sex hormones. Physiol Rep. 2019;7(10):e14111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miras AD, le Roux CW. Mechanisms underlying weight loss after bariatric surgery. Nat Rev Gastroenterol Hepatol. 2013;10(10):575-584. [DOI] [PubMed] [Google Scholar]
- 5. Madsbad S, Holst JJ. GLP-1 as a mediator in the remission of type 2 diabetes after gastric bypass and sleeve gastrectomy surgery. Diabetes. 2014;63(10):3172-3174. [DOI] [PubMed] [Google Scholar]
- 6. Al-Najim W, Docherty NG, le Roux CW. Food intake and eating behavior after bariatric surgery. Physiol Rev. 2018;98(3):1113-1141. [DOI] [PubMed] [Google Scholar]
- 7. Gautron L, Elmquist JK, Williams KW. Neural control of energy balance: translating circuits to therapies. Cell. 2015;161(1):133-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ten Kulve JS, Veltman DJ, Gerdes VEA, et al. . Elevated postoperative endogenous GLP-1 levels mediate effects of roux-en-Y gastric bypass on neural responsivity to food cues. Diabetes Care. 2017;40(11):1522-1529. [DOI] [PubMed] [Google Scholar]
- 9. Frank S, Heinze JM, Fritsche A, et al. . Neuronal food reward activity in patients with type 2 diabetes with improved glycemic control after bariatric surgery. Diabetes Care. 2016;39(8):1311-1317. [DOI] [PubMed] [Google Scholar]
- 10. Stefater MA, Wilson-Pérez HE, Chambers AP, Sandoval DA, Seeley RJ. All bariatric surgeries are not created equal: insights from mechanistic comparisons. Endocr Rev. 2012;33(4): 595-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dang JT, Karmali S. Is RYGB more effective than sleeve gastrectomy? Nat Rev Endocrinol. 2019;15(3):134-135. [DOI] [PubMed] [Google Scholar]
- 12. Pearce AL, Mackey E, Cherry JBC, et al. . Effect of adolescent bariatric surgery on the brain and cognition: a pilot study. Obesity (Silver Spring). 2017;25(11):1852-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li G, Ji G, Hu Y, et al. . Bariatric surgery in obese patients reduced resting connectivity of brain regions involved with self-referential processing. Hum Brain Mapp. 2018;39(12): 4755-4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537-541. [DOI] [PubMed] [Google Scholar]
- 15. He Y, Ma G, Zhai F, et al. . Dietary patterns and glucose tolerance abnormalities in Chinese adults. Diabetes Care. 2009;32(11):1972-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Larraufie P, Roberts GP, McGavigan AK, et al. . Important role of the GLP-1 axis for glucose homeostasis after bariatric surgery. Cell Rep. 2019;26(6):1399-1408.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cummings DE, Weigle DS, Frayo RS, et al. . Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346(21):1623-1630. [DOI] [PubMed] [Google Scholar]
- 18. De Silva A, Salem V, Long CJ, et al. . The gut hormones PYY 3-36 and GLP-1 7-36 amide reduce food intake and modulate brain activity in appetite centers in humans. Cell Metab. 2011;14(5):700-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Malik S, McGlone F, Bedrossian D, Dagher A. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab. 2008;7(5):400-409. [DOI] [PubMed] [Google Scholar]
- 20. Ochner CN, Kwok Y, Conceição E, et al. . Selective reduction in neural responses to high calorie foods following gastric bypass surgery. Ann Surg. 2011;253(3):502-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kong LC, Tap J, Aron-Wisnewsky J, et al. . Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am J Clin Nutr. 2013;98(1):16-24. [DOI] [PubMed] [Google Scholar]
- 22. Sinclair P, Brennan DJ, le Roux CW. Gut adaptation after metabolic surgery and its influences on the brain, liver and cancer. Nat Rev Gastroenterol Hepatol. 2018;15(10):606-624. [DOI] [PubMed] [Google Scholar]
- 23. Liu R, Hong J, Xu X, et al. . Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat Med. 2017;23(7):859-868. [DOI] [PubMed] [Google Scholar]
- 24. Tremaroli V, Karlsson F, Werling M, et al. . Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab. 2015;22(2):228-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29(1):71-83. [DOI] [PubMed] [Google Scholar]
- 26. Cappelleri JC, Bushmakin AG, Gerber RA, et al. . Psychometric analysis of the Three-Factor Eating Questionnaire-R21: results from a large diverse sample of obese and non-obese participants. Int J Obes (Lond). 2009;33(6):611-620. [DOI] [PubMed] [Google Scholar]
- 27. Chao-Gan Y, Yu-Feng Z. DPARSF: a MATLAB toolbox for “Pipeline” data analysis of resting-state fMRI. Front Syst Neurosci. 2010;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cui Y, Jiao Y, Chen YC, et al. . Altered spontaneous brain activity in type 2 diabetes: a resting-state functional MRI study. Diabetes. 2014;63(2):749-760. [DOI] [PubMed] [Google Scholar]
- 29. Ehrlich S, Borchardt V, Walter M, Seidel M. F63. Abnormal spontaneous regional brain activity in acutely underweight patients with anorexia nervosa. Biol Psychiatry. 2019;85(10):S237. [DOI] [PubMed] [Google Scholar]
- 30. Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22(1):394-400. [DOI] [PubMed] [Google Scholar]
- 31. Truong DT, Franzosa EA, Tickle TL, et al. . MetaPhlAn2 for enhanced metagenomic taxonomic profiling. Nat Methods. 2015;12(10):902-903. [DOI] [PubMed] [Google Scholar]
- 32. Feng Q, Liang S, Jia H, et al. . Gut microbiome development along the colorectal adenoma-carcinoma sequence. Nat Commun. 2015;6:6528. [DOI] [PubMed] [Google Scholar]
- 33. Vieira-Silva S, Falony G, Darzi Y, et al. . Species-function relationships shape ecological properties of the human gut microbiome. Nat Microbiol. 2016;1(8):16088. [DOI] [PubMed] [Google Scholar]
- 34. Chen G, Saad ZS, Britton JC, Pine DS, Cox RW. Linear mixed-effects modeling approach to FMRI group analysis. Neuroimage. 2013;73:176-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Flannery JS, Riedel MC, Poudel R, et al. . Habenular and striatal activity during performance feedback are differentially linked with state-like and trait-like aspects of tobacco use disorder. Sci Adv. 2019;5(10):eaax2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bates D, Machler M, Bolker BM, Walker SC. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1):1-48. [Google Scholar]
- 37. Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7(6):464-476. [DOI] [PubMed] [Google Scholar]
- 38. Jahanshahi M, Obeso I, Rothwell JC, Obeso JA. A fronto-striato-subthalamic-pallidal network for goal-directed and habitual inhibition. Nat Rev Neurosci. 2015;16(12):719-732. [DOI] [PubMed] [Google Scholar]
- 39. Bakdash JZ, Marusich LR. Repeated measures correlation. Front Psychol. 2017;8:456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Olivo G, Zhou W, Sundbom M, et al. . Resting-state brain connectivity changes in obese women after Roux-en-Y gastric bypass surgery: a longitudinal study. Sci Rep. 2017;7(1):6616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thanarajah SE, Backes H, DiFeliceantonio AG, et al. . Food intake recruits orosensory and post-ingestive dopaminergic circuits to affect eating desire in humans. Cell Metab. 2019;29(3): 695-706.e4. [DOI] [PubMed] [Google Scholar]
- 42. van Bloemendaal L, Veltman DJ, Ten Kulve JS, et al. . Brain reward-system activation in response to anticipation and consumption of palatable food is altered by glucagon-like peptide-1 receptor activation in humans. Diabetes Obes Metab. 2015;17(9):878-886. [DOI] [PubMed] [Google Scholar]
- 43. Sweet LH, Hassenstab JJ, McCaffery JM, et al. . Brain response to food stimulation in obese, normal weight, and successful weight loss maintainers. Obesity (Silver Spring). 2012;20(11):2220-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Murdaugh DL, Cox JE, Cook EW 3rd, Weller RE. fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. Neuroimage. 2012;59(3):2709-2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Scholtz S, Miras AD, Chhina N, et al. . Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding. Gut. 2014;63(6):891-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6(9):691-702. [DOI] [PubMed] [Google Scholar]
- 47. Farr OM, Sofopoulos M, Tsoukas MA, et al. . GLP-1 receptors exist in the parietal cortex, hypothalamus and medulla of human brains and the GLP-1 analogue liraglutide alters brain activity related to highly desirable food cues in individuals with diabetes: a crossover, randomised, placebo-controlled trial. Diabetologia. 2016;59(5):954-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wilson-Pérez HE, Chambers AP, Ryan KK, et al. . Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like Peptide 1 receptor deficiency. Diabetes. 2013;62(7):2380-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Faulconbridge LF, Ruparel K, Loughead J, et al. . Changes in neural responsivity to highly palatable foods following roux-en-Y gastric bypass, sleeve gastrectomy, or weight stability: An fMRI study. Obesity (Silver Spring). 2016;24(5):1054-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chambers AP, Kirchner H, Wilson-Perez HE, et al. . The effects of vertical sleeve gastrectomy in rodents are ghrelin independent. Gastroenterology. 2013;144(1):50-52.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Perakakis N, Kokkinos A, Peradze N, et al. . Circulating levels of gastrointestinal hormones in response to the most common types of bariatric surgery and predictive value for weight loss over one year: Evidence from two independent trials. Metabolism. 2019;101:153997. [DOI] [PubMed] [Google Scholar]
- 52. Furet JP, Kong LC, Tap J, et al. . Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59(12):3049-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Liou AP, Paziuk M, Luevano JM Jr, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5(178):178ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhang H, DiBaise JK, Zuccolo A, et al. . Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A. 2009;106(7):2365-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Faria SL, Santos A, Magro DO, et al. . Gut microbiota modifications and weight regain in morbidly obese women after roux-en-Y gastric bypass. Obes Surg. 2020;30(12):4958-4966. [DOI] [PubMed] [Google Scholar]
- 56. Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102(31):11070-11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hwang I, Park YJ, Kim YR, et al. . Alteration of gut microbiota by vancomycin and bacitracin improves insulin resistance via glucagon-like peptide 1 in diet-induced obesity. Faseb J. 2015;29(6):2397-2411. [DOI] [PubMed] [Google Scholar]
- 58. Everard A, Cani PD. Gut microbiota and GLP-1. Rev Endocr Metab Disord. 2014;15(3):189-196. [DOI] [PubMed] [Google Scholar]
- 59. Yadav H, Lee JH, Lloyd J, Walter P, Rane SG. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J Biol Chem. 2013;288(35):25088-25097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Blaut M. Gut microbiota and energy balance: role in obesity. Proc Nutr Soc. 2015;74(3):227-234. [DOI] [PubMed] [Google Scholar]
- 61. Petersen C, Bell R, Klag KA, et al. . T cell-mediated regulation of the microbiota protects against obesity. Science. 2019;365(6451):eaat9351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All scripts and routines used for analyzing data are available from the corresponding author upon request.