Abstract
We report on a wearable tear bioelectronic platform, integrating a microfluidic electrochemical detector into an eyeglasses nose-bridge pad, for non-invasive monitoring of key tear biomarkers. The alcohol-oxidase (AOx) biosensing fluidic system allowed real-time tear collection and direct alcohol measurements in stimulated tears, leading to the first wearable platform for tear alcohol monitoring. Placed outside the eye region this fully wearable tear-sensing platform addresses drawbacks of sensor systems involving direct contact with the eye as the contact lenses platform. Integrating the wireless electronic circuitry into the eyeglasses frame thus yielded a fully portable, convenient-to-use fashionable sensing device. The tear alcohol sensing concept was demonstrated for monitoring of alcohol intake in human subjects over multiple drinking courses, displaying good correlation to parallel BAC measurements. We also demonstrate for the first time the ability to monitor tear glucose outside the eye and the utility of wearable devices for monitoring vitamin nutrients in connection to enzymatic flow detector and rapid voltammetric scanning, respectively. These developments pave the way to build an effective eyeglasses system capable of chemical tear analysis.
Keywords: Tears; wearable sensor; alcohol, glucose; vitamins; electrochemical sensor
Graphical Abstract
Introduction
Wearable electronics have become commonplace in our daily life and have generated a tremendous commercial interest. Such interest has stimulated considerable efforts towards the development of wearable and mobile sensing systems. (Bandodkar et al., 2016; Kim et al., 2018a; Liu et al., 2017). While early attention was given primarily to wearable mobility and physical sensors, recent efforts have shifted to the development of systems capable of non-invasive monitoring of (bio)chemical markers. (Bariya et al., 2018; Heikenfeld et al., 2018; Yang and Gao, 2018). Wearable (bio)chemical sensors have thus been designed to be conveniently incorporated into a wearer’s daily routine, providing useful insights into the wearer’s health and fitness levels, or its surroundings. (Sempionatto et al., 2017b). Non-invasive wearable chemical sensing platforms, operating in readily sampled biofluids, such as sweat, saliva or tears, have thus garnered considerable interest due to their potential to provide useful real-time insights into changes in biomarkers concentrations without necessitating blood sampling. Wearable chemical sensors have thus been integrated into a variety of body conformable platforms ranging from wristbands (Gao et al., 2016) and temporary tattoos (Bandodkar et al., 2015) to textiles (Jeerapan et al., 2016) and from mouthguard (Kim et al., 2015) to contact lenses (Park et al., 2018), with target analytes including key metabolites (such as glucose, lactate or alcohol) and electrolytes (e.g., Na+, Cl−, K+, etc.) (Campbell et al., 2018; Roh et al., 2016). As this exciting field and the available technologies advance rapidly, it is necessary to develop new, easy-to-use and fashionable platforms that enhance the user’s comfort and acceptance, such as eyeglasses, and to explore new target biomarkers in different non-invasive biofluids, such as tears, towards fostering even greater acceptance, versatility and scope of wearable chemical sensors.
Tears, also known as lachrymal fluid, are generated through the lachrymal gland to coat and protect the eyes. Tears are less complex than blood, but they contain a variety of biomarkers present through either intracellular biomolecule secretion or via passive leakage of low-weight compounds from blood plasma (Farandos et al., 2015; Pankratov et al., 2016; Thaysen and Thorn, 1954). In the latter case, the concentrations of various metabolites in tears reflect concurrent blood levels, making the tears an attractive medium for non-invasive monitoring of important physiological parameters. Wearable sensing systems based on the tear biofluid have been reported for several analytes, particularly glucose and lactate (Senior, 2014; Taormina et al., 2007; Thomas et al., 2012; Yao et al., 2012). These systems have focused primarily on the incorporation of electrochemical sensors into contact lens-based platforms with integrated electronic components for direct measurements in basal tears. (Falk et al., 2013; Senior, 2014; Thomas et al., 2012; Yao et al., 2012). Efforts for improving such tear sensing systems have continued toward integration of appropriate power sources and wireless data transmission, along with advanced sensor designs (Kim et al., 2017; Kownacka et al., 2018; Park et al., 2018). Nevertheless, major challenges remain for reliable operation of fully integrated wireless tears-based wearable chemical sensing platforms.
Here we describe, for the first time, a non-invasive wearable tear biosensor system mounted on eyeglasses and demonstrate its robust and attractive analytical performance for real-time monitoring of different target analytes such as alcohol, vitamins and glucose in tears. Recently, we described an eyeglasses-based sensing platform for monitoring sweat metabolites and electrolytes using nose-bridge pad electrodes contacting the skin. (Sempionatto et al., 2017b). In the present work, we mounted an on-line fluidic device onto the eyeglasses nose-bridge pad to allow direct collection of stimulated tears (Figure 1), along with the flow of the sampled tears over an alcohol oxidase (AOx)-based electrochemical detector and rapid fluid replenishment from the device. Eyeglasses represent a commonly used lifestyle accessory with close proximity to tear fluid, hence providing convenient access to the nearby stimulated tears while addressing drawbacks associated with contact-lens based sensing platforms (Badugu et al., 2018; Jiang et al., 2018; Park et al., 2018). These drawbacks relate to placing the lenses directly on the eye, limited user compliance (particularly in younger subjects) and potential vision impairment due to the embedded sensor system (Farandos et al., 2015). To address these crucial issues, the present eyeglasses platform relies on placing the electrochemical detection system and its wireless electronic backbone outside the eye area, while collecting stimulated tears on the external miniaturized flow detector mounted on the eyeglasses pad. Integration of wireless electronic circuitry into the eyeglasses frame (for the amperometric and voltammetric operations and data transmission) thus leads to a fully portable, convenient-to-use, yet fashionable wearable sensing platform.
Figure 1. Eyeglasses-based Fluidic Device.
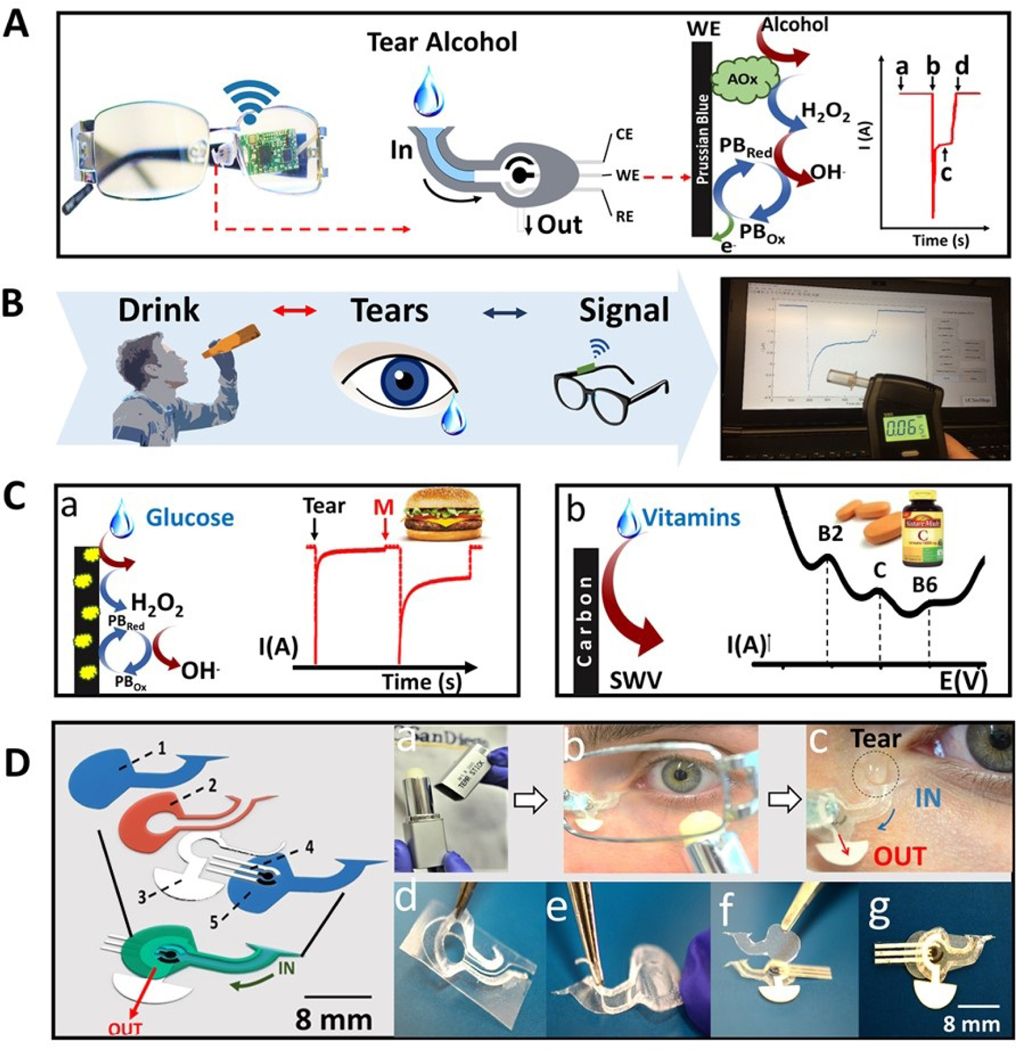
A) Photographic depiction and schematics of the fluidic device and wireless electronics integrated into the eyeglasses platform along with a representation of enzymatic alcohol detection and signal transduction, where, (a) corresponds to the baseline; (b) current change due the captured tear; (c) the measured alcohol signal and (d) drying of the device. B) Steps for tear alcohol detection consisting of ingestion of an alcoholic drink followed by tear stimulation and amperometric measurement. C) Representation of glucose (a) and vitamin detection (b). For the enzymatic glucose detection, the enzymatic reaction is presented showing the oxidation of tear glucose and hydrogen peroxide as byproduct along with its detection by the Prussian blue electrode. For the vitamin detection a typical SWV response is showed indicating the peak for vitamins B2, C and B6. D) Exploded view of fluidic device: (1) is the top polycarbonate membrane, (2) is the double adhesive spacer, (3) is the paper outlet, (4) the electrochemical (bio)sensor and (5) the bottom polycarbonate membrane. Tears stimulation: (a) Menthol tear stick (b) Volunteer applying the tear stick under the left eye. (c) Tear entering the inlet of the device. Fluidic device fabrication: (d) Adhesive spacer removed from PET substrate. (e) Spacer is placed on the bottom membrane. (f) Electrode and outlet are placed on top of spacer followed by top membrane. (g) Final device on eyeglasses nose-bridge pad.
Alcohol represents an extremely important target opportunity for wearable sensing devices as it is the most widely used substance of abuse worldwide which leads annually to hundreds of billions of dollars in related costs (associated with lost productivity, crime, health care, etc.) in the United States alone (Grant et al., 2017). Thus, the continuous real-time monitoring of alcohol intake and assessment of an individual’s level of intoxication have been the subject of tremendous research efforts (Campbell et al., 2018; Thungon et al., 2017). However, the reported devices have downsides, such as limited specificity and time delay after the alcohol intake (Campbell et al., 2018; Karns-Wright et al., 2017). Recent reports have demonstrated wearable alcohol biosensors capable of greater specificity and near real-time monitoring through amperometric detection in liquid sweat (Gamella et al., 2014; Hauke et al., 2018; Kim et al., 2016) and interstitial fluid (Mohan et al., 2017). Despite of these advances, new and improved non-invasive alcohol monitoring platforms are greatly desired. The detection of alcohol in tears has been reported first by Giles et al. in the 1980s (Giles et al., 1987, 1988) with measurements carried out using a thermal resistivity sensor in vapors above the eyes. Since then, no wearable sensing system has focused on the detection of alcohol in tears.
The tear alcohol monitoring method described in this paper utilizes a wearable eyeglasses-based platform with an alcohol biosensor flow detector mounted onto the nose-bridge pad for alcohol measurements in stimulated tears (Figure 1A). Such wearable non-invasive alcohol bioelectronic platform is shown to be extremely useful for measuring tear alcohol levels in real-life scenarios with good correlation to concurrent blood alcohol concentration (BAC). We demonstrate that the wearable tear alcohol biosensor, along with the integrated on-board wireless electronics, reliably detects alcohol intake in human subjects, as validated by parallel BAC monitoring. By placing the sensing system outside the eye region, the new system obviates problems associated with wearable sensors placed directly in the eye. The microfluidic design of this system, along with the chemical tear stimulation method, minimizes common errors associated with tear sampling, such as low sample volume, tear evaporation, and changing tear composition upon mechanical stimulation (Mishima et al., 1966; Stuchell et al., 1984; Yan et al., 2011). We also demonstrate utility of the eyeglasses-based sensing platform for non-invasive monitoring of tear glucose and vitamins. To the best of our knowledge, this represents the first example of non-invasive monitoring of vitamins toward potential personal nutrition applications. Such multi-vitamin sensing has been carried out using rapid square-wave voltammetry (SWV). Despite of its multi-analyte capability, sensitivity and speed, SWV has rarely been explored for wearable sensing applications. Considering the importance of alcohol, glucose and vitamins target analytes, and the versatility and comfort of the eyeglasses platform, the new tear bioelectronic system represents a major step toward the non-invasive monitoring of biomarkers.
Materials and Methods
Materials and chemicals
Ag/AgCl ink (E2414) and carbon-Prussian blue (PB) ink (C2070424P2) were obtained from Gwent Inc. (Torfaen, UK). Carbon graphite ink (E3449) was obtained from Ercon Inc (Wareham, MA, USA). All inks were used as received. Polyethylene terephthalate (PET) was used as the substrate for fabrication of screen-printed electrodes. Analytical grade potassium phosphate dibasic, potassium phosphate monobasic, potassium chloride, ethanol, D-glucose, lactate, uric acid, ascorbic acid, glucose oxidase from Aspergillus Niger type X-S (EC 1.1.3.4), alcohol oxidase from Pichia pastoris and analytical standards of vitamins were obtained from Sigma-Aldrich (St. Louis, MO, USA).. Vitamin tablets were purchased from a local supermarket. Vitamin pills include; 100 mg of vitamin B2, 100 mg of vitamin B6 and 1000 mg of vitamin C. All reagents were used without further purification. Isopore™ Polycarbonate filters membranes (25 mm diameter and 10 μm pore diameter) were purchased from EMD Millipore (Massachusetts, USA). For in vitro studies, a Potentiostat/Galvanostat (PalmSens4 Instrument BV, Houten, Netherlands) was used. For on-body measurements a commercial Breathalyzer (BACtrack(R) s80) was used to assess blood alcohol concentrations and a commercial glucometer (Accu-Chek Aviva Plus) was used to evaluate blood glucose concentrations. A menthol tear stick (Art. #3005, Kryolan, CA, USA) was used to stimulate tears.
Screen-printed electrodes fabrication process
Screen printing was carried out using a semiautomatic MMP-SPM screen printer (Speedline Technologies, Franklin, MA, USA) and custom stencils designed using AutoCAD software (Autodesk, San Rafael, CA) and produced by Metal Etch Services (San Marcos, CA, USA) using stainless steel stencils with dimensions of 12 in × 12 in. Alcohol biosensors were screen-printed on a flexible PET sheet through multiple steps (Figure S1). First, Ag/AgCl ink was used to prepare the reference electrode and the conductive trails used for the electrical connections. The printout was then cured at 85 °C for 15 min. Next, Carbon Prussian Blue (PB) ink was used to print the counter and working electrodes and the layer was cured at 85 °C for 15 min. Transparent insulator (Dupont 5036, Wilmington, DE) was used to define the electrode area. For the glucose biosensor, a mixture of PB ink and GOx (15.3 mg GOx/250 mg ink, vigorously shaken for 10 minutes) was used to print the working electrode (Figure S1) and the layer was cured at 50 °C for 10 min. The reference electrode and electrode connections were printed using Ag/AgCl ink (Figure S1). Bare carbon electrodes were used for the tear vitamin monitoring.
Microfluidic device assembly
A cutting machine (Cricut Explore Air 2) was used to cut the different parts of the device before assembly. The paper outlets were laser cut using an Orion Motor CO2 Laser with a 40 W CO2 laser tube and scanning speed range of 0–400 mm/s. The device assembly consisted of several steps shown in Figure 1D. First, a double-sided adhesive layer (Scotch-brite MMM136: 1/2” x 250”) was transferred to a PET substrate and cut to be used as a spacer (Figure 1D-d). Subsequently, two polycarbonate membranes were cut with our specific design and the spacer was placed over the bottom membrane (Figure 1D-e). Then, the electrodes and the paper outlet were also placed over the bottom membrane (Figure 1D-f). Finally, the device was closed by another layer of polycarbonate membrane on the top (Figure 1D-g) and integrated with the eyeglasses pad for further use (Figure 1A). The schematic view of the full device assembly is shown on the left side of Figure 1D.
Electrochemical assays
A screen-printed three-electrode system was used for the amperometric sensor design. For alcohol and glucose analysis, the counter and working electrodes were printed using PB ink, with the working electrode being further modified either by drop casting or ink composition. For the alcohol biosensor, 3 μL of enzyme mixture (9:1 ratio of stock AOx to 10 mg/mL BSA) was applied to the working electrode and allowed to dry at 4 °C for 2 h. Next, 2 μL of 0.5 % chitosan was applied over the dried enzyme layer, followed by 0.5 μL of 2% glutaraldehyde, and the biosensor was dried at 4 °C overnight. For the glucose biosensor, a mixture of 15.3 mg GOx/250 mg PB ink was vigorously shaken for 10 min and used to print the working electrode. All solutions for the in vitro measurements were prepared in 0.1 M PBS (pH 7.4). For tear vitamin analysis, the counter and working electrodes were printed using carbon ink. After printing, the bare carbon working electrode was electrochemically pretreated in saturated Na2CO3 solution by applying 1.2 V vs Ag/AgCl for 5 min before assembling with the eyeglasses platform. Chronoamperometric detection of alcohol and glucose was carried out by applying potentials of−0.1 V and −0.2 V (vs Ag/AgCl), respectively, at room temperature. Square wave voltammetry (SWV) with a potential range of −1.0 V to +1.0 V, amplitude of 25 mV, and frequency of 10 Hz was used for tear vitamin analysis. Tears were stimulated using a menthol tear stick (Art. #3005, Kryolan, CA, USA) applied on the volunteer’s skin at ~1 cm under the eye. For the in vitro tear analysis, the teardrop was collected and a volume of 10 μL was used for electrochemical measurements. For the flow injection analysis (FIA), a wall-jet flow cell configuration was used with a peristaltic pump Watson Marlow sci-Q 400 (Massachusetts, USA) and a flow rate of 200 μL/min. All in vitro studies were performed using a Potentiostat/Galvanostat (PalmSens4 Instrument BV, Houten, Netherlands).
Wireless electronics
All on-body experiments were conducted using our customized printed circuit board (PCB) capable of performing amperometric and square wave voltammetric(SWV) measurements along with wireless data transmission as in our previous work (Sempionatto et al., 2017a, 2017b). The PCB contained a controller CC2640 from Texas Instruments (TI, Dallas, TX), which had an integrated Bluetooth Low Energy (BLE) function to enable wireless communication between the sensor and a host device. The PCB was placed on the arm of the eyeglasses frame and used to control the electrochemical operations.. A reference waveform was generated by a digital-to-analog converter (DAC) DAC8563 from TI. A feedback loop compares the output of the DAC with the buffered potential of the reference electrode and controls the potential with a driver circuit. The DAC had 16-bits resolution to enable precise voltage sweep and was controlled by the controller via serial peripheral interface (SPI). A transimpedance amplifier (TIA) was used to convert the forward and reverse currents at the working electrode into voltages. The output of the trans-impedance amplifier was sampled and digitized by an analog-to-digital converter (ADC) integrated in the controller. The final current was calculated as a difference between the forward and reverse currents. The sampling timing of the ADC, as well as the trigger for changing the output of the DAC, was controlled by a timer function in the controller. For CA measurements, the output of the DAC was set to a constant value to apply a constant potential (−0.1 V or −0.2 V) between working and reference electrodes. The current at working electrode was measured by the trans-impedance amplifier and digitized by the ADC. For both SWV and CA, the sensor current signals were first stored in the controller, and then transmitted to the host device as BLE packets. The data was displayed using a custom-made graphic interface in the host laptop. The PCB electronic board was powered by a rechargeable Li-ion 3.6 V battery.
On-body measurements with human subjects
Three healthy volunteers were asked to participate in the on-body studies. The volunteers were given the eyeglasses tear flow device fully assembled with the wireless electronic transducer. For the alcohol analysis, before every tear stimulation, the individual’s blood alcohol level was checked using a commercial breathalyzer (BACtrack(R) s80). In the case of glucose experiments, the individual’s blood glucose level was checked using a commercial glucometer (Accu-Chek Aviva Plus). A menthol tear stick (Art. #3005, Kryolan, CA, USA) was applied on the volunteer’s skin at ~1 cm under the eye to stimulate a teardrop. Tear stimulation was performed as instructed in the supplier safety data sheet, which ensured no eye damage. Potential skin irritation after prolonged contact of the menthol stick with the skin can be avoided with the use of protective facial-cream layer that prevent such direct contact. Detailed protocol for the alcohol, glucose and vitamins on-body experiments is given in the Supporting Information.
Results and discussion
Rationale for non-invasive blood alcohol monitoring in tears
We report the first example of a tear alcohol biosensor integrated on a wearable eyeglasses-based platform for non-invasive monitoring of BAC. When alcohol is consumed, it proceeds through ethanol adsorption, distribution, metabolism, and elimination processes (Campbell et al., 2018). Most (~90%) of the consumed alcohol is metabolized in the liver, while the rest is excreted through breath, sweat, urine, and tears. Such alcohol can be represented as a function of BAC. Additionally, ethanol concentration in collected human tears were shown to reflect blood alcohol levels and relate to tear film disturbances (Kim et al., 2012).
Methods of tear collection include mechanical stimulation via capillary tube or filter paper extraction at the eye conjunctiva and chemically stimulated the tear generation by an external agent, such as menthol or onion vapor (Baca et al., 2007; La Belle et al., 2016). These methods can provide external tear analysis without necessitating an on-eye platform but are limited by small sample volumes of the extracted tears, ease of sample evaporation during collection (particularly in sampling ethanol), potential composition variations between individuals and dependence on the sampling method, in comparison to basal tears (Daum and Hill, 1984; Stuchell et al., 1984; Yan et al., 2011). Therefore, the successful realization of continuous real-time monitoring of alcohol in tears requires expansion of the measurement strategies for improved reliability and convenience, with consistent sampling methodology and rapid analysis.
Integration of wearable tear alcohol biosensor on eyeglasses platform
The eyeglasses-based alcohol biosensor system consisted of four major components: a fluidic device for collecting the tears, an AOx-modified electrochemical flow detector, wireless electronics, and the supporting eyeglasses platform (Figure 1A). The fluidic device includes a super-hydrophilic polycarbonate membrane as a capillary absorbent for effective sample intake with an inlet for tears collection, a reservoir with the enclosure biosensor and a passive paper outlet for tears continuous replenishment. The fluidic device is discussed in more detail in the next section. Tears were stimulated by rubbing a commercial menthol stick on the skin at ~1 cm under the eye. Once applied, the warmth from the skin releases menthol vapors to the eyes, stimulating tears production. The strong capillary force in the inlet, generated by the super-hydrophilic membranes, captures the low volume of generated tears, which easily enters the fluidic device located on the nose pad of eyeglasses (Figure 1A). When the fluidic device reservoir is filled with tears, the incorporated biosensor with immobilized AOx detects the alcohol levels in the tears. AOx catalyzes the oxidation of ethanol molecules to produce acetaldehyde and hydrogen peroxide. The generated hydrogen peroxide is selectively reduced at the screen printed PB transducer, and the integrated wireless electronics transmits the reduction current to a laptop via Bluetooth communication (Movie S1). Finally, after the measurement, the sensor surface is renewed by complete removal of the tears through a paper outlet. The drying time can vary from 10–30 min (passive drying time, no external interference) to 2 min (using auxiliary tissue paper contacted with the paper outlet). The performance of the device was demonstrated with human subjects for practical non-invasive alcohol monitoring in tears along with the consumption of alcoholic drinks. The biosensor tear alcohol response was compared between the sober state and after alcoholic drink along with validation through blood alcohol measurements using a commercial breathalyzer (Figure 1B). Tears glucose detection was performed in a similar fashion as the tear alcohol. The glucose detection involved biocatalytic conversion of the target glucose to gluconic acid and hydrogen peroxide using immobilized glucose oxidase (GOx) (Figure 1C-a). In contrast, the detection of vitamin in tears was performed using SWV, using the bare carbon electrode detector for signal transduction, with the oxidation peak current proportional to the vitamins level (Figure 1C-b).
Design and integration of fluidic device
Although previous studies have demonstrated correlations between concurrent blood and tear alcohol levels, accurate monitoring of alcohol in tears is complicated by several issues stemming from the different methodologies used for sample collection (Giles et al., 1988; Kim et al., 2012). Accurate real-time measurements of tear alcohol require advanced sampling methods that address the small sample volumes of stimulated tears, ease of tears evaporation, and temporal variations in tear composition. These issues are addressed in the present work by incorporating a fluidic device that collects the generated tears and contains an amperometric AOx alcohol flow-through biosensor (Figure 1Da-c). The fluidic device includes a super-hydrophilic polycarbonate membrane as a capillary absorbent for effective sample intake placed at the top and bottom layer of the fluidics. A spacer (Figure 1D-d) was used to conform the two polycarbonate membranes into an inlet and a reservoir (Figure 1D-e). A screen-printed electrochemical biosensor was inserted inside the reservoir, and a paper-based outlet completed the flow-through system for rapid tears sampling and replenishment (Figure 1D-f). The super- hydrophilic material ensured the collection of enough tears in a short time period, while the tears are flowing on the face toward the inlet (~2 sec) (Figure 1D-c). The time needed for complete filling of the reservoir depends primarily on the device geometry. The thickness of the inlet, determined by the spacer, has a profound effect upon achieving a large capillary pressure. Further discussion and optimization of the channel dimensions are given in Supporting Information and Figure S2. The shape of the inlet is also important to define the volume of collected tears. A funnel-like entrance was chosen to collect the maximum possible tears volume in the short time while the tears are rolling down from the eyes. The optimization of the shape of the inlet is shown in Table S1. A filter paper was used as an outlet to ensure complete replenishment of the tears. A portion of the paper outlet was in contact with the tear inside the reservoir, and once in contact with the fluid, the paper was soaked, and the tears began to dry by evaporation from the paper. Such complete replenishment is essential for eliminating carry-over effects, by replacing old tears with new tears, which may compromise the accuracy.
In vitro characterization of electrochemical tear alcohol biosensors
The analytical performance of the alcohol biosensor flow detector was evaluated in in-vitro FIA measurements and static measurements (Figure S3). The detailed experiment and results are shown in the Supporting Information. In addition, the correlation between tears alcohol and BAC, was performed with real tears collected from three volunteers before and after alcohol intake, along with background signal testing for real tears. Detailed results are also shown in the Supporting Information (Figure S4 and S5, respectively). The results of the in-vitro studies indicate that the alcohol content in tears correlates strongly to the blood alcohol levels (BAC) with Pearson’s r=0.852, when correlating the electrical current values and BAC levels of the three volunteers (Figure S6).
Continuous on-body tear alcohol monitoring with human subjects using integrated eyeglasses biosensor
To evaluate the applicability of our wearable eyeglasses-based alcohol tear sensing platform under realistic scenarios, we examined its performance using three healthy human subjects. All human trials were carried out in strict compliance with a protocol approved by the Institutional Review Board (IRB) at the University of California San Diego. For these on-body experiments, each subject recorded baseline readings in induced tears at an initial measured BAC of 0.000 %, followed by alcohol intake and subsequent periodic tear generation and alcohol measurements, in a manner displayed in (Figure 2A-a).
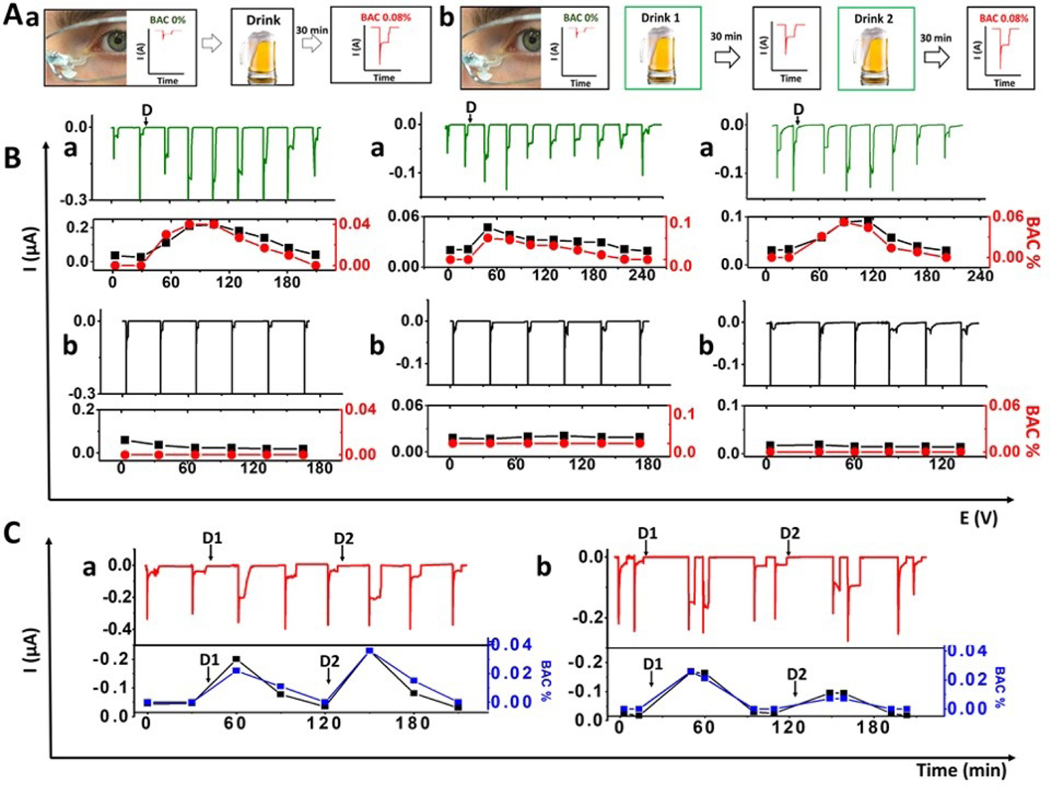
Figure 2. Real-time monitoring of alcohol intake in human subjects.
A) Timeline used for tears alcohol detection during on-body experiments in human subjects with alcohol consumption. First, tears were stimulated and measured before the alcohol consumption. Then, the subject consumed a drink and after 30 min the tears were stimulated again, and the corresponding chronoamperogram was recorded. For the first part the process (B(a)) was repeated until BAC 0.000% (a), and in the second part (C,a), a second drink was taken after BAC 0.000% and the cycle was repeated (b). B (a) Monitoring of tears alcohol concentrations prior to and upon the alcohol consumption until measured BAC reached 0.000 % for three subjects. Time of alcohol intake indicated by “D” in each plot. (b) Control experiment using the AOx-modified sensor, without alcohol consumption. In the bottom section black curves corresponds to amperometric current response and the red curve to BAC measured by breathalyzer. C) Chronoamperograms obtained for subjects a and b following consumption of two alcoholic drinks (D1 and D2). Below each amperogram is the BA- current/signal correlation for each volunteer, relating the observed tears alcohol current response (black) to the concurrent measured BAC (blue).
Tears were collected at the detector inlet, attached to the nose-bridge pad of the eyeglasses without any user involvement (e.g., head tilting or adjustments). The sampled tears moved through the fluidic system to the biosensor compartment where the alcohol level was measured. Upon obtaining a stable amperometric signal of the peroxide product of the AOx reaction the fluidic outlet was dried (using tissue paper), resulting in rapid return to the flat current baseline. The eyeglasses-based alcohol sensor thus offered exhibited real-time detection of changing alcohol tears concentrations, with rapid response to new stimulated tear samples (Figure 2B-a). The observed current changes correlate well to concurrent BAC levels associated with the alcohol intake for the three subjects participated in this study. The average direct correlation (R2) of tear alcohol response and BAC levels of the same individual was 0.912. In contrast, no apparent current signals or BAC response, are observed for the three subjects in a similar control experiment using the AOx biosensor but without alcohol consumption (Figure 2B-b). Variations in the temporal tear alcohol concentration profiles between the individual subjects (Figure 2B-a) reflect differences in their alcohol metabolism. The ability of the wearable alcohol monitoring platform to detect dynamic changes in alcohol concentrations in real-time, along with correlation to concurrent BAC measurements, illustrate the potential of the eyeglasses platform for convenient non-invasive BAC sensing.
We further examined the stability, carry-over effects and overall performance of the eyeglasses alcohol sensing platform during extended on-body operation. The tear alcohol concentrations of two subjects were monitored over such long-time periods, including two separate alcohol intakes (Figure 2C-a, b). The fluidic nature of the alcohol sensor allows convenient monitoring of repeated fluctuations in alcohol concentrations for both subjects, along with concurrent BAC measurements, while ensuring no mixing with older tear sample. In each case, the observed current response increased upon alcohol intake along with concurrent BAC levels, followed by a return to a flat baseline after each drink, confirming the absence of carry-over effects from previously sampled tears. During this entire biosensing operation, the device was kept on the body, displaying high stability over testing periods as long as 9 hours. The changes observed in the tear alcohol readings were found to correlate closely with simultaneous changes in the BAC response (Figure 2B-a and C-a, b, bottom part). The ability to monitor alcohol concentrations non-invasively, with real-time correlation to concurrent BAC, provides vital information on the current status of intoxication and eliminates time-lag issues common to existing alcohol sensing systems (Karns-Wright et al., 2017). Such attractive performance in practical scenarios and during extended operation supports the potential of the wearable eyeglasses system for convenient non-invasive BAC monitoring.
Continuous non-invasive tear glucose monitoring with human subjects using integrated eyeglasses biosensor
The presence of glucose in tears was reported since the 1950s (Giardini et al., 1950; Lewis, 1957). Challenges related to tears collection methodologies and the use of contact lens sensors have been one of the main barriers for implementing non-invasive or in-vitro tear glucose analysis (Posa et al., 2013). Most of the existing collection protocols involve complex and uncomfortable procedures, such as placing a small capillary device inside the eye (Andoralov et al., 2013; Peng et al., 2013), applying filter paper in direct contact to the eye for tears flow (Posa et al., 2013) and the use of functional and integrated contact lenses systems (Farandos et al., 2015; Park et al., 2018; Yao et al., 2012). To address these challenges and drawbacks, we extended our eyeglasses-based tear monitoring platform to glucose-based measurements. The attractive analytical performance of the glucose biosensor flow detector was evaluated first using in-vitro FIA measurements (Figure S7). These data indicate a well-defined current response, with fast linear response (A), high selectivity in presence of common interferences (B) and good stability over 10 hours (C).
On-body tear glucose monitoring was performed before and after each meal, analogous to the alcohol sensing trials involving alcohol intake. A waiting time of 15 min after completing the meal is essential for measuring tear glucose levels with the highest accuracy. The average equilibration time for consumed glucose within the bloodstream is ~10 min (Stout et al., 2004) and the time-lag between blood and tear glucose concentrations is ~5 min (Zhang et al., 2011); hence, a tear glucose detection after 15 min waiting period allows the measurement of the blood glucose at its peak.
The on-body monitoring of changing tear glucose levels is illustrated in Figure 3, including amperograms obtained before and after each meal (Figure 3B-a and Figure 3C). The glucose response increases rapidly with the arrival of the stimulated tear sample to the GOx detector. A close correlation between the tear glucose current response Δi (Black curve;) and the measured blood glucose levels (Red curve) is observed (C, bottom) for each of the measured signals. The same experiment was repeated with a second volunteer using three meals over the entire day (Figure S8). The observed current changes follow closely the blood glucose levels, with the current increasing around 0.2 μA after each meal (where blood glucose increases ~40 mg/dL). Note also that no carryover or surface fouling effects are observed between successive glucose measurements, as the signal decreases and returns to the expected values even after measuring high glucose concentrations. Such absence of carry over effects indicates good promise for routine non-invasive glucose monitoring.
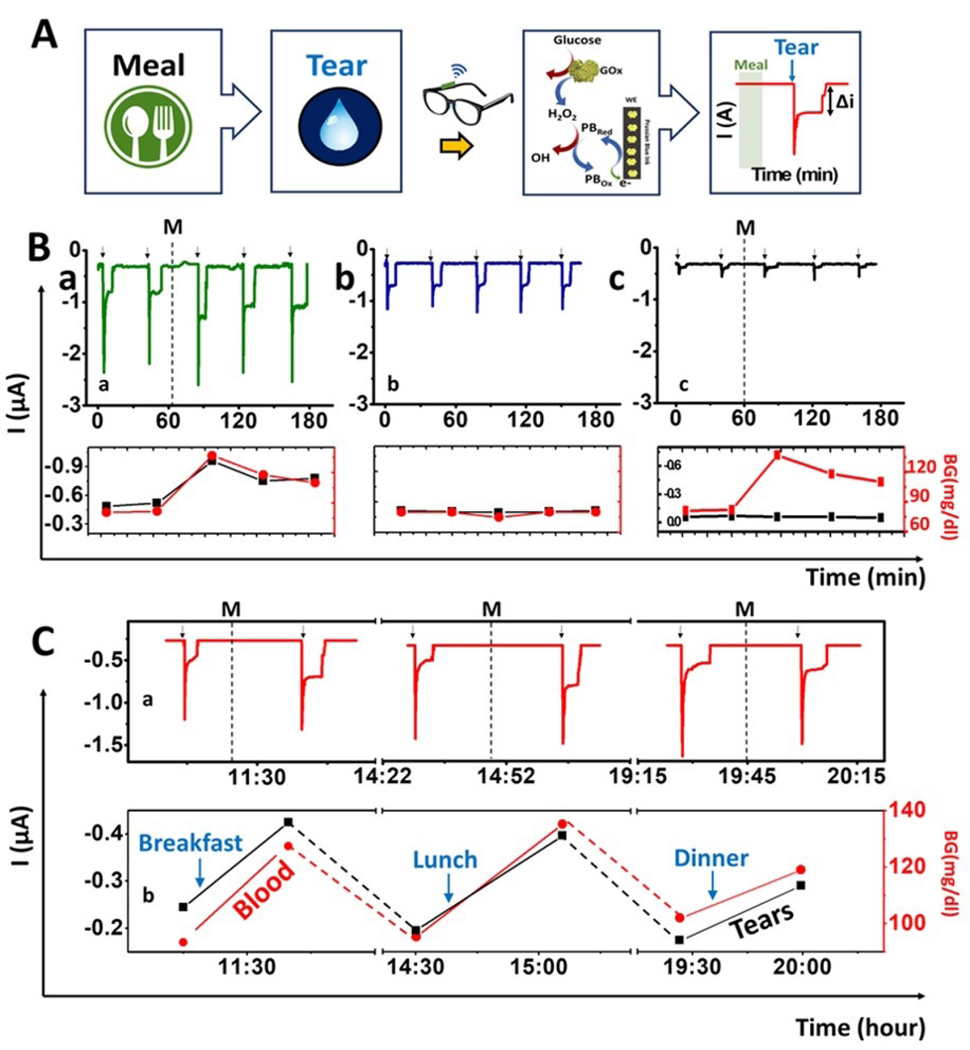
Figure 3. On-body evaluation of tear glucose biosensor.
(A) Scheme of the procedure used for on-body glucose sensing experiments: Tears are stimulated ~15 min after a meal to measure the glucose levels using the GOx-modified PB detector using chronoamperometry. When the tears reach the biosensor, a current change is observed which is proportional to the tear glucose levels. Amperograms showing non-invasive measurement of glucose in tears during 3 h (B) using: a) GOx sensor having meal (M) b) GOx sensor without a meal and c) Prussian blue sensor without enzyme following a meal (M). Bottom: Correlation between current tear signal and the glucose blood level. Red: Blood glucose concentration measured with a glucometer; Black: current intensity obtained with the glasses wearable sensor. (C) Monitoring of glucose levels during the entire day: breakfast, lunch and dinner. Note: Tear stimulation (arrows) and meal (M). Bottom, correlation between blood glucose measurements with a glucometer (red) and the current response of the wearable tear sensor (black). Applied potential, −0.2 V.
To confirm that observed differences in signals obtained before and after meal were truly due to the increment of glucose levels only, and that GOx was the primary provider for the signal, two control experiments were carried out. For the first control, a regular enzyme modified electrode was used, but the volunteers kept a fasting state (no meal) during the entire experiment (~3h) (Figure 3B-b). For the second control, sensors were screen printed with PB ink only, without GOx (Figure 3B-c). All control experiments were conducted under the same conditions as previous trials, the blood glucose levels were measured before every tear stimulation, only one sensor was used, no washing steps were applied, and the device stayed at room temperature for the duration of all experiments. The corresponding glucose levels (red curve) and resulting electrochemical signal (black curve) are shown in Figure 3 (bottom part of B and C) for each experiment. For direct visual comparison, regular on-body experiments were also conducted in the same time frame as the controls (Figure 3B-a). As expected, the results were similar to those related to alcohol monitoring. The current increased around 0.2 μA after the meal with good correlation to the blood glucose levels provided by the glucometer. For the control experiment performed without meal (Figure 3B-b), a decrease in current was also observed, due to the blood glucose level of the volunteers. However, no significant variations of the current intensity or of blood glucose levels were observed during this control experiment. This result strongly suggests that the current change after having a meal is solely due to the increased tear glucose levels. In the second control (without GOx) (Figure 3B-c), a considerable increment in the blood glucose was observed after the meal (Figure 3B-c bottom section), but no response was measured by the sensor. These analyses demonstrate that the signals measured on the regular experiments were solely due to the GOx enzymatic reaction of the glucose in tears. Additionally, the later control experiment without the enzyme modification (Figure 3B-c) confirmed that electroactive tears constituents (e.g., ascorbic acid) have a negligible effect upon the response of the Prussian-blue transducer (as expected from its low detection potential). Overall, the response of the non-invasive tear glucose flow system sensor responds linearly to glucose levels on tears, with high selectivity and high stability with no significant biofouling (during this operating period). In addition, we verified the general correlation obtained with all the on-body experiments performed by all volunteers, yielding an R Pearson’s value of ~ 0.7 (Figure S9). This result indicates good correlation between blood glucose levels and the current response of our sensor. These preliminary findings indicate considerable promise toward real-time non-invasive monitoring of glucose levels in tears. Yet, extensive large-scale validation trials are required for confirming the clinical significance of these tear-based glucose assays.
Non-Invasive tear vitamin monitoring with human subjects using integrated eyeglasses biosensor
In addition to alcohol and glucose, we examined the utility of the wearable eyeglasses sensing platform for non-invasive screening of vitamin constituents of tears. Tears are an attractive biofluid for implementing vitamin monitoring as a measure of body’s nutrient levels owing to their rich composition of amino acids, vitamins and nutrients supplied to the corneal surface (Kaufman et al., 1998). The detection of vitamins in tear fluids by liquid chromatography–mass spectrometry (LC-MS) technique was reported recently (Khaksari et al., 2017) and the tear-serum vitamin D concentrations was demonstrated using enzyme-linked immunoassay (ELISA) (Sethu et al., 2016). Although these analytical methods are accurate, sensitive and selective, they require time consuming sampling and pretreatment steps in connection to sophisticated instruments available only in centralized laboratories. To the best of our knowledge, a wearable sensor for on-body measurements of vitamins in tears have not been reported.
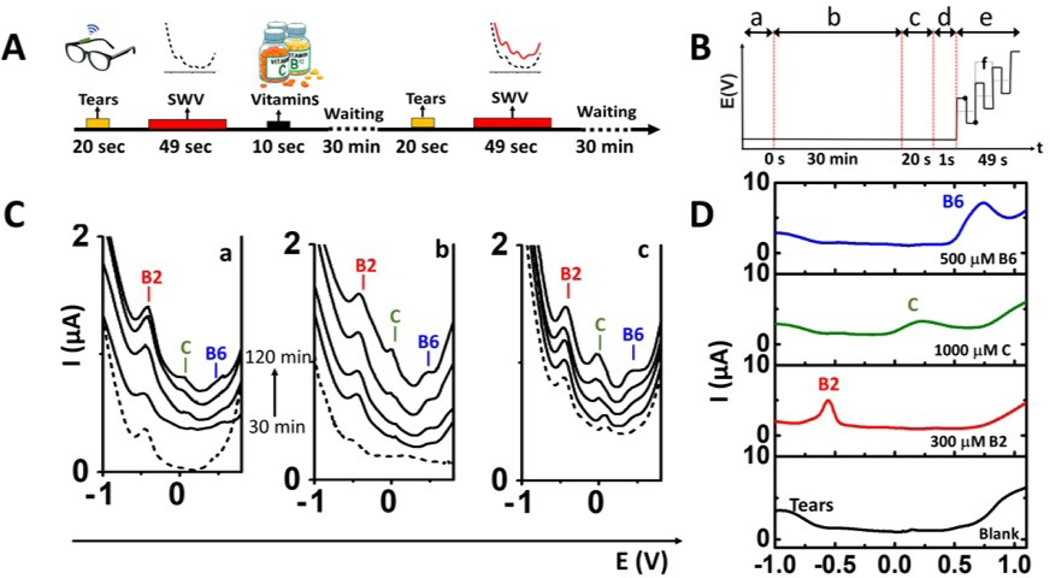
Herein, we demonstrate an efficient non-invasive tear vitamin monitoring approach based on the eyeglasses-biosensor platform. As most vitamins are electroactive (Mohan et al., 2015), their levels in tears can be monitored voltammetrically using the current signals associated with their redox reactions. Highly sensitive and rapid square wave voltammetry (SWV) was used here for the simultaneous on-body monitoring of three different vitamin constituents of multivitamin pills in connection to three human volunteers. The tear vitamin levels were monitored before and after taking the commercial multivitamin tablets. The square-wave voltammogram was recorded when the fluidic device was filled with the stimulated tears (Figure 4A and B). Figure 4-A illustrates the time-line sequence used for such on-body tear vitamin voltammetric analysis while Figure 4-B displays the time-line of applying the SWV scan during the experiment, showing the typical potential-time waveform that couples high speed with effective discrimination against the charging current. While offering high sensitivity and speed along with multi-analyte capability, SWV has rarely been explored for wearable sensing applications. The current integration of miniaturized SWV electronics board onto the eyeglasses frame enables on-body multi-analyte detection capability towards such tear multivitamin analysis. The SWV scan (49 sec duration), initiated during the passage of the stimulated tears over the detector, resulted in several well-defined peaks at specific potentials for the corresponding vitamins. On-body monitoring of vitamins in tears were performed as follows. Initially, the tears were stimulated and monitored before taking any vitamin, so that the SWV baseline could be acquired. Subsequently, the volunteers took a multivitamin tablet containing 200 mg, 1000 mg and 200 mg of vitamins B2, C and B6 respectively. Next, tears were stimulated and the SWV response was recorded every 30 minutes to measure changes in the peak currents associated with dynamic vitamin concentration changes. The tear stimulation process and data acquisition took ~1 min to complete (Figure 4B). The eyeglasses flow system was worn throughout the experiment and the device was dried completely before each measurement.
Figure 4. On-body evaluation of tears vitamins sensor.
(A) Scheme of the procedure used for vitamin sensing: Tears are stimulated before taking the vitamins and the device is filled with tears. Once filled, SWV is performed and the baseline is obtained. Then, the device is dried, and the volunteer consumes tables of different vitamins (B2, C and B6). Finally, tears vitamins are monitored every 30 min by SWV. (B) Time-line for the applied potential during the on-body experiments where (a) represents the final of vitamin intake, (b) the waiting time for vitamin absorption by the body, (c) the tears stimulation process, (d) the time to fill the tears fluidic device with the stimulated teardrop and (e) the typical SWV waveform for the multi-vitamin analysis. Where the dots represent the amplitude and “f” the frequency applied. (C) On-body measurements of tears vitamins by SWVs using the eyeglasses flow system for 3 subjects after 30 minutes intervals (30, 60, 90, 120 minutes) of taking vitamin tablets, along with control experiments before taking vitamin (dotted line). (D) Collected tears spiked with standard solutions of vitamins B6 (500 μM, blue plot), C (1000 μM, green plot) and B2 (300 μM, red plot), compared with SWV of collected tears without taking of any vitamin (black plot). SWV Conditions: potential range: −1.0 V- +1.0 V, amplitude: 25mV, frequency: 10Hz.
Figure 4-C shows the SWV response for each volunteer during such experiment (a-c). These voltammograms display well defined oxidation peaks, at peak potentials of −0.55 V, +0.20 V and +0.60 V (vs Ag/AgCl), corresponding to the electro-oxidation of vitamin B2, C and B6, respectively, with peak currents increasing over time (2h). These vitamins undergo electrochemical redox processes and display a well-established voltammetric behavior in aqueous solutions (Mohan et al., 2015). The vitamin peak potentials were verified by spiking standard solutions of vitamins B2, C and B6 to collected tears (Figure 4-D). SWVs of individual vitamins in PBS (pH 7.4) and of tears spiked with different concentrations of vitamin standard solutions are shown in Figure S10, along with their corresponding calibration curves. The baseline measurements (before taking vitamins) showed different profiles for each volunteer, reflecting their individual metabolism and diet. Note also that the baseline tear response of these subjects shows a small peak at −0.55 V corresponding to natural levels of vitamin B2 in the body, while no apparent response is observed for vitamins C and B6 (Fig 4C, dotted lines). Vitamin B2 concentration in tears have been reported to be in the range of 0.011– 0.08 μM (Khaksari et al., 2017). After the oral administration of the corresponding vitamin pills, the SWV peaks corresponding to the oxidation of vitamins B2, C and B6 were detected simultaneously. These oxidation signals increased gradually with time for voltammograms recorded at 30 min intervals over the 2-hour experiment (Fig. 4C). These experiments demonstrate the first proof-of-concept of continuous non-invasive monitoring vitamin levels in tears. Future studies will be focused on obtaining quantitative vitamin levels in tears and understanding better the correlation of tear-blood vitamin levels.
Conclusions
We demonstrated, for the first time, a non-invasive wearable tear alcohol biosensor mounted on eyeglasses. Placed outside the eye region this new tear-sensing platform addresses the drawbacks of contact lenses systems, including potential infections and impaired vision. The new device relies on enclosing the electrochemical biosensor within a microfluidic chamber, with the supporting electronics embedded onto the eyeglasses’ inner frame. This new platform was evaluated by continuously monitoring the tear alcohol levels of human subjects after alcohol consumption. The measured tear alcohol levels of three volunteers were successfully measured on-body and validated by comparing to concurrent BAC values, indicating tremendous potential of monitoring tear alcohol concentrations for determining blood alcohol concentrations. In addition, the analysis of alcohol in tears can overcome some limitations of the commonly used breathalyzers, such as high BAC measurements, due to alcohol remaining in the oral cavity. Larger-scale studies are needed for assessing the possibility of tear alcohol monitoring with wearable eyeglasses-based sensors and for better understating the influence of the tear fluid collection method on the measured tear analyte concentrations.
We also presented an evaluation of the eyeglasses platform for monitoring tear glucose and vitamins, with a favorable response and no apparent carry-over effects over repetitive measurements. These results clearly indicate that blood alcohol and glucose levels can be monitored effectively through their tear concentrations. We have also demonstrated a first example of non-invasive vitamins detection in tears by following their intakes. Such capability holds a considerable promise toward personal nutrition applications. Further analyses are still required for enhancing the accuracy and precision of the system and for gaining further insights into the effect of the tear stimulation and collection upon the analyte concentration and of the detector geometry upon the tear dilution. These studies could lead to enhanced non-invasive tear analysis while addressing the drawbacks of contact-lenses sensing platform. These new capabilities of the eyeglasses-based tear bioelectronic concept indicate considerable potential for non-invasive detection of diverse biomarkers, paving the way for widespread application of these tear-based non-invasive wearable biosensing diagnostic platforms. Extensive validation studies and correlation to blood concentrations are essential for establishing the clinical and forensic utility of the new wearable tear-sensor system prior to widespread applications. Appropriate algorithms will be required to report the corresponding concentration values. Overall, these encouraging early results clearly indicate the tremendous potential and versatility of the eyeglasses biosensing platform for non-invasive tear biomarker monitoring.
Supplementary Material
| Term | Definition | Author’s name |
|---|---|---|
| Conceptualization | Ideas; formulation or evolution of overarching research goals and aims | Joseph Wang and Juliane R. Sempionatto |
| Methodology | Development or design of methodology; creation of models | Juliane R. Sempionatto, Laís Canniatti Brazaca, Laura García- Carmona, Aida Martin and Guangda Tang |
| Validation | Verification, whether as a part of the activity or separate, of the overall replication/ reproducibility of results/experiments and other research outputs | Juliane R. Sempionatto, Laís Canniatti Brazaca, Laura GarcíaCarmona and Gulcin Bolat |
| Formal Analysis | Application of statistical, mathematical, computational, or other formal techniques to analyze or synthesize study data | Juliane R. Sempionatto and Laís Canniatti Brazaca |
| Investigation | Conducting a research and investigation process, specifically performing the experiments, or data/evidence collection | Juliane R. Sempionatto, Laís Canniatti Brazaca, and Laura GarcíaCarmona, Gulcin Bolat, Alan S. Campbell, Guangda Tang, Rushabh Shah, Rupesh K. Mishra |
| Resources | Provision of study materials, reagents, materials, patients, laboratory samples, animals, instrumentation, computing resources, or other analysis tools | Valtencir Zucolotto, Alberto Escarpa and Joseph Wang |
| Writing – Original Draft | Preparation, creation and/or presentation of the published work, specifically writing the initial draft (including substantive translation) | Juliane R. Sempionatto, Laís Canniatti Brazaca, and Laura GarcíaCarmona, Gulcin Bolat, Alan S. Campbell, Jayoung Kim and Joseph Wang |
| Writing – Review & Editing | Preparation, creation and/or presentation of the published work by those from the original research group, specifically critical review, commentary or revision – including pre-or postpublication stages | Juliane R. Sempionatto, Laís Canniatti Brazaca, Rupesh K. Mishra and Joseph Wang |
| Visualization | Preparation, creation and/or presentation of the published work, specifically visualization/ data presentation | |
| Supervision | Oversight and leadership responsibility for the research | Joseph Wang and |
| activity planning and execution, including mentorship external to the core team | Juliane R. Sempionatto | |
| Project Administration |
Management and coordination responsibility for the research activity planning and execution | Joseph Wang |
| Funding Acquisition |
Acquisition of the financial support for the project leading to this publication | Joseph Wang |
Highlights.
Wearable fluidic tears biosensor and wireless electronic circuitry integrated into the eyeglasses for fast, non-invasive monitoring of tear biomarkers.
Placed outside the eye region this fully wearable tear-sensing platform addresses drawbacks of sensor systems involving direct contact with the eye as the contact lenses platform.
Real-time tear collection and direct alcohol measurements in stimulated tears, leading to the first wearable platform for tear alcohol monitoring.
Acknowledgement
Funding: This work was supported by the Defense Threat Reduction Agency Joint Science and Technology Office for Chemical and Biological Defense (grant number: HDTRA 1–16-1–0013). J. R. S., L.C.B., L.G.C. and G.B. acknowledge fellowships from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (grant number: 216981/2014–0), Sao Paulo Research Foundation (FAPESP) (grant number: 2017/03779–0), University of Alcala, and the Scientific and Technological Research Council of Turkey (grant number: TUBITAK 2219), respectively. A.S.C. acknowledges funding through the NIH NIAAA T32 (Training Grant AA013525).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supporting Information
Supplementary data associated with this article can be found in the online version at doi:https://doi.org/10.1016/j.bios.2019.04.058
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andoralov V, Shleev S, Arnebrant T, Ruzgas T, 2013. Anal. Bioanal. Chem 405, 3871–3879. [DOI] [PubMed] [Google Scholar]
- Baca JT, Finegold DN, Asher SA, 2007.. Ocul. Surf 5, 280–93. [DOI] [PubMed] [Google Scholar]
- Badugu R, Reece EA, Lakowicz JR, 2018.. J. Biomed. Opt 23, 057005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandodkar AJ, Jeerapan I, Wang J, 2016.. ACS Sensors 1, 464–482. [Google Scholar]
- Bandodkar AJ, Jia W, Wang J, 2015. Electroanalysis 27, 562–572. [Google Scholar]
- Bariya M, Nyein HYY, Javey A, 2018.. Curr. Opin. Electrochem 10, 126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum KM, Hill RM, 1984.. Acta Ophthalmol. 62, 472–478. [DOI] [PubMed] [Google Scholar]
- Falk M, Andoralov V, Silow M, Toscano MD, Shleev S, 2013. Anal. Chem 85, 6342–6348. [DOI] [PubMed] [Google Scholar]
- Farandos NM, Yetisen AK, Monteiro MJ, Lowe CR, Yun SH, 2015.. Adv. Healthc. Mater 4, 792–810. [DOI] [PubMed] [Google Scholar]
- Gamella M, Campuzano S, Manso J, Rivera G.G. de, López-Colino F, Reviejo AJ, Pingarrón JM, 2014.. Anal. Chim. Acta 806, 1–7. [DOI] [PubMed] [Google Scholar]
- Gao W, Emaminejad S, Nyein HYY, Challa S, Chen K, Peck A, Fahad HM, Ota H, Shiraki H, Kiriya D, Lien D-H, Brooks GA, Davis RW, Javey A, 2016.. Nature 529, 509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardini A, Roberts JRE, 1950. Br. J. Ophthalmol 34, 737–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles HG, Sandrin S, Saldivia V, Israel Y, 1988. Clin. Exp. Res 12, 255–258. [DOI] [PubMed] [Google Scholar]
- Giles HG, Sandrin S, Kapur BM, Thiessen JJ, 1987. Can J Physiol Pharmacol 65, 2491–2493. [DOI] [PubMed] [Google Scholar]
- Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, Huang B, Jung J, Zhang H, Fan A, Hasin DS, 2017.. JAMA Psychiatry 74, 911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauke A, Simmers P, Ojha YR, Cameron BD, Ballweg R, Zhang T, Twine N, Brothers M, Gomez E, Heikenfeld J, 2018. Lab Chip. 18, 3750–3759 [DOI] [PubMed] [Google Scholar]
- Heikenfeld J, Jajack A, Rogers J, Gutruf P, Tian L, Pan T, Li R, Khine M, Kim J, Wang J, Kim J, 2018. Lab Chip 18, 217–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeerapan I, Sempionatto JR, Pavinatto A, You J-M, Wang J, 2016. J. Mater. Chem. A 4, 18342–18353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang N, Montelongo Y, Butt H, Yetisen AK, 2018. Small 14, 1704363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karns-Wright TE, Roache JD, Hill-Kapturczak N, Liang Y, Mullen J, Dougherty DM, 2017. Alcohol Alcohol. 52, 35–41. 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman HE, Barron BA, McDonald MB, 1998. The cornea, 2 nd. ed. Butterworth-Heinemann, Boston. [Google Scholar]
- Khaksari M, Mazzoleni LR, Ruan C, Kennedy RT, Minerick AR, 2017.. Exp. Eye Res 155, 54–63. [DOI] [PubMed] [Google Scholar]
- Kim J, Campbell AS, Wang J, 2018a.. Talanta 177, 163–170. [DOI] [PubMed] [Google Scholar]
- Kim J, Imani S, de Araujo WR, Warchall J, Valdés-Ramírez G, Paixão TRLC, Mercier PP, Wang J, 2015. Biosens. Bioelectron 74, 1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Jeerapan I, Imani S, Cho TN, Bandodkar A, Cinti S, Mercier PP, Wang J, 2016. ACS Sensors 1, 1011–1019. [Google Scholar]
- Kim J, Kim M, Lee M-S, Kim K, Ji S, Kim Y-T, Park J, Na K, Bae K-H, Kyun Kim H, Bien F, Young Lee C, Park J-U, 2017. Nat. Commun 8, 14997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kim JH, Nam WH, Yi K, Choi DG, Hyon JY, Wee WR, Shin YJ, 2012. Ophthalmology 119, 965–971. [DOI] [PubMed] [Google Scholar]
- Kownacka AE, Vegelyte D, Joosse M, Anton N, Toebes BJ, Lauko J, Buzzacchera I, Lipinska K, Wilson DA, Geelhoed-Duijvestijn N, Wilson CJ, 2018. Biomacromolecules. 19, 4504–4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Belle JT, Adams A, Lin C-E, Engelschall E, Pratt B, Cook CB, 2016. Chem. Commun 52, 9197–9204. [DOI] [PubMed] [Google Scholar]
- Lewis JG, 1957. Br. Med. J 1, 585. [Google Scholar]
- Liu Y, Pharr M, Salvatore GA, 2017. ACS Nano. 11, 9614–9635. [DOI] [PubMed] [Google Scholar]
- Mishima S, Gasset A, Klyce SD, 1966. Ophthalmol. Vis. Sci 5, 264–276. [PubMed] [Google Scholar]
- Mohan AMV, Brunetti B, Bulbarello A, Wang J, 2015. Analyst 140, 7522–7526. [DOI] [PubMed] [Google Scholar]
- Mohan AMV, Windmiller JR, Mishra RK, Wang J, 2017. Biosens. Bioelectron 91, 574–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankratov D, González-Arribas E, Blum Z, Shleev S, 2016. Electroanalysis 28, 1250–1266. [Google Scholar]
- Park J, Kim J, Kim S-Y, Cheong WH, Jang J, Park Y-G, Na K, Kim Y-T, Heo JH, Lee CY, Lee JH, Bien F, Park J-U, 2018. Sci. Adv 4, eaap9841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng B, Lu J, Balijepalli AS, Major TC, Cohan BE, Meyerhoff ME, 2013. Biosens. Bioelectron 49, 204–209. [DOI] [PubMed] [Google Scholar]
- Posa A, Bräuer L, Schicht M, Garreis F, Beileke S, Paulsen F, 2013. Ann. Anat. - Anat. Anzeiger 195, 137–142. [DOI] [PubMed] [Google Scholar]
- Roh C, Lee J, Kang C, 2016. Molecules. 21, 798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sempionatto JR, Mishra RK, Martín A, Tang G, Nakagawa T, Lu X, Campbell AS, Lyu KM, Wang J, 2017a. ACS Sensors 2, 1531–1538. [DOI] [PubMed] [Google Scholar]
- Sempionatto JR, Nakagawa T, Pavinatto A, Mensah ST, Imani S, Mercier P, Wang J, 2017b. Lab Chip 17, 1834–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior M, 2014. Nat. Biotechnol 32, 856–856. [DOI] [PubMed] [Google Scholar]
- Sethu S, Shetty R, Deshpande K, Pahuja N, Chinnappaiah N, Agarwal A, Sharma A, Ghosh A, 2016. Eye Vis. 3, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout PJ, Racchini JR, Hilgers ME, 2004. Diabetes Technol. Ther 6, 635–644. [DOI] [PubMed] [Google Scholar]
- Stuchell RN, Feldman JJ, Farris RL, Mandel ID, 1984. Investig. Ophthalmol. Vis. Sci 25, 374–377. [PubMed] [Google Scholar]
- Taormina CR, Baca JT, Asher SA, Grabowski JJ, Finegold DN, 2007. J. Am. Soc. Mass Spectrom 18, 332–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaysen JH, Thorn NA, 1954. Am. J. Physiol 178, 160–164. [DOI] [PubMed] [Google Scholar]
- Thomas N, Lähdesmäki I, Parviz BA, 2012. Sensors Actuators B Chem. 162, 128–134. [Google Scholar]
- Thungon PD, Kakoti A, Ngashangva L, Goswami P, 2017. Biosens. Bioelectron 97, 83–99. [DOI] [PubMed] [Google Scholar]
- Yan Q, Peng B, Su G, Cohan BE, Major TC, Meyerhoff ME, 2011. Anal. Chem 83, 8341–8346. [DOI] [PubMed] [Google Scholar]
- Yang Y, Gao W, 2018. Chem. Soc. Rev doi. 10.1039/C7CS00730B [DOI] [Google Scholar]
- Yao H, Liao Y, Lingley AR, Afanasiev A, Lähdesmäki I, Otis BP, Parviz BA, 2012. J. Micromechanics Microengineering 22, 075007. [Google Scholar]
- Zhang J, Hodge W, Hutnick C, Wang X, 2011. J. Diabetes Sci. Technol 5, 166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.