Abstract
Objective
To describe the discrimination and calibration of clinical prediction models, identify characteristics that contribute to better predictions and investigate predictors that are associated with unplanned hospital readmissions.
Design
Systematic review and meta-analysis.
Data source
Medline, EMBASE, ICTPR (for study protocols) and Web of Science (for conference proceedings) were searched up to 25 August 2020.
Eligibility criteria for selecting studies
Studies were eligible if they reported on (1) hospitalised adult patients with acute heart disease; (2) a clinical presentation of prediction models with c-statistic; (3) unplanned hospital readmission within 6 months.
Primary and secondary outcome measures
Model discrimination for unplanned hospital readmission within 6 months measured using concordance (c) statistics and model calibration. Meta-regression and subgroup analyses were performed to investigate predefined sources of heterogeneity. Outcome measures from models reported in multiple independent cohorts and similarly defined risk predictors were pooled.
Results
Sixty studies describing 81 models were included: 43 models were newly developed, and 38 were externally validated. Included populations were mainly patients with heart failure (HF) (n=29). The average age ranged between 56.5 and 84 years. The incidence of readmission ranged from 3% to 43%. Risk of bias (RoB) was high in almost all studies. The c-statistic was <0.7 in 72 models, between 0.7 and 0.8 in 16 models and >0.8 in 5 models. The study population, data source and number of predictors were significant moderators for the discrimination. Calibration was reported for 27 models. Only the GRACE (Global Registration of Acute Coronary Events) score had adequate discrimination in independent cohorts (0.78, 95% CI 0.63 to 0.86). Eighteen predictors were pooled.
Conclusion
Some promising models require updating and validation before use in clinical practice. The lack of independent validation studies, high RoB and low consistency in measured predictors limit their applicability.
PROSPERO registration number
CRD42020159839.
Keywords: cardiology, adverse events, risk management
Strengths and limitations of this study.
Largest investigation of unplanned hospital readmission risk to date, including 81 unique prediction models in the systematic review.
Independent and standardised procedures for study selection, data collection and risk of bias (RoB) assessment.
High RoB in current prediction models and unexplained heterogeneity between models limit recommendations for using prediction model in clinical practice.
Introduction
Hospital readmissions in patients with acute heart disease are associated with a high burden on patients, healthcare and costs.1 The identification of high-risk hospitalised patients is important to provide timely interventions. Prediction models guide healthcare providers in daily practice to assess patients’ probability of readmission within a certain time frame and include candidate variables identified by clinical perspectives, literature or data-driven approaches, for example, using machine learning techniques.2 Data are often collected from observational cohorts of intervention studies and subsequently analysed to examine what set of predictors best predict the risk of readmission. The clinical applicability of risk prediction models in daily practice is currently limited. Statistical models are often not presented in a clinically useful way or models based on administrative data are considered.3 These models therefore cannot be readily used in daily practice. In addition, prediction models are often developed for a very specific population, which asks from clinicians to be familiar with several models. Furthermore, patients may belong to multiple populations because of cardiac comorbidities. Numerous systematic reviews have previously investigated the prediction of unplanned hospital readmissions in several populations.3–12 While some have included hospitalised patients in general,11 12 others have focused specifically on patients with heart failure (HF)4–8 10 or acute myocardial infarction (AMI).3 9 The conclusion is generally the same, the discrimination is poor to adequate, and there is little consistency in the type of predictors included in the models.
We believe that the state of the art on risk prediction can be improved if more knowledge is available on the performance of clinical risk prediction models and risk predictors across different populations of patients with heart disease. Although heterogeneity in models and predictors is often considered as a limitation, it can inform effect moderators on how predictions can be improved.13 For example, perhaps we can identify predictors who demonstrate a consistent association with hospital readmission regardless of the underlying disease. If this can be identified, a more general prediction model could be developed that is relevant for the heterogeneous group of patients on cardiac care units. This might contribute to the early recognition and onset of preventive interventions in patients with heart disease at risk of readmission.
We therefore performed a systematic review and meta-analysis on clinical risk prediction models for the outcome unplanned hospital readmission in patients hospitalised for acute heart disease. Our aims were to describe the discrimination and calibration of clinical prediction models, to identify characteristics that contribute to better predictions, and to investigate predictors that are consistently associated with hospital readmissions.
Methods
A protocol was registered in PROSPERO (registration number: CRD42020159839). The results are reported following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement.14
Eligibility criteria
Studies were eligible if (1) the study population included hospitalised adult patients with (symptoms of) heart disease; (2) a prediction model with c-statistic was reported; (3) a clinically useful presentation of the model with risk factors was reported; (4) the outcome was unplanned hospital readmissions within 6 months; (5) the study design was appropriate, that is, (nested) case–control study (prospective and retrospective) cohort study, database and registry study, or secondary analysis of a trial; (6) they were reported in English.
Information sources
A search strategy was designed with an information specialist (PROSPERO protocol and online supplemental text 1). We searched the Medline, EMBASE, WHO ICTPR search portal (for study protocols) and Web of Science (for conference proceedings) databases up to 25 August 2020 without any restrictions for eligible studies. We searched for full-text manuscripts of the identified protocols. After selecting the full-text manuscripts, we screened references lists and prospective citations (using Google Scholar) for additional eligible studies.
bmjopen-2020-047576supp001.pdf (932KB, pdf)
Study selection
Three reviewers were involved in the study selection process. Each reviewer independently screened two-thirds of the titles, abstracts and full-text articles of potentially relevant references identified in the literature search. Disagreements were resolved through consensus. Sixteen authors were contacted and six delivered data for readmission when a composite outcome was used. Two authors were also contacted when data were reported combining multiple patient populations. However, no additional data were provided for the population with heart disease and these studies were excluded.
Data extraction
Data extraction was performed based on the ‘Critical Appraisal and Data Extraction for Systematic Reviews’ of prediction modelling studies checklist using standardised forms in the Distiller Systematic Review Software (see online supplemental text 2 for the data items).15 The checklist includes items on 11 relevant domains, including source of data, participants, outcomes, candidate predictors, sample size, missing data, model development, model performance, model evaluation, results and interpretation. One reviewer collected the data and the second reviewer verified the extracted data. Disagreements were resolved through consensus. Eight authors were contacted and two delivered data to resolve uncertainties or missing data.
Risk of bias
The Prediction model Risk Of Bias ASsessment Tool (PROBAST) tool16 was used to assess the risk of bias (RoB) for four ‘quality’ domains, that is, the participants, predictors, outcome and analysis for each model. One author assessed the RoB as low, high or unclear, and the second author verified the extracted data and RoB conclusion. Disagreements were resolved through consensus. In addition, the applicability of the included studies based on our research question was assessed for three domains, that is, participants, predictors and outcome domains and rated as low concerns, high concerns or uncertain concerns regarding applicability.
Summary measures
The discrimination of the prediction models was described using the concordance (c)-statistic. Missing SEs were derived from the sample data.17 The calibration was described using the number of observed and expected events, the calibration slope, calibration in large or the Hosmer-Lemeshow test. A definition of the commonly used measures is described in box 1.
Box 1. Definitions of commonly used measures.
Discrimination:
Refers to the ability of a prediction model to discriminate between a patient with and without the outcome, for example, readmission.
C-statistic:
Is a measure of discrimination. For binary outcomes, the c-statistic is equivalent to the area under the curve: 1 indicates perfect discrimination, and 0.5 indicates that the models does not perform better than chance. Harrell’s c-statistic is often used in survival models.
Calibration:
Refers to the agreement between the predicted and the observed probability (or the outcome value for linear models). Calibration is expressed using different measures, for example, calibration slope, calibration in large, Hosmer-Lemeshow test.
Calibration slope:
The slope should be 1, a value <1 indicates extreme predictions, and a value of >1 indicates to moderate predictions.
Calibration in large:
The value should be 0, a negative value indicates overestimation of the prediction, and a positive value indicates underestimation of the prediction.
Hosmer-Lemeshow test:
This is a goodness-of-fit test for binary outcomes. A significant p value, usually <0.05, indicates poor goodness-of-fit.
Derivation/development cohort:
A cohort of patients that is used to estimate the predictor values that are used in a prediction model to estimate a patient’s probability for an outcome.
Validation cohort:
A cohort of patients that is used to evaluate how well the developed model performs (in terms of discrimination and calibration).
Internal validation:
Estimates how well the performance of a model will be reproduced in the target population. Several techniques can be used, for example, random-split sample, cross-validation and bootstrapping techniques.
External validation:
Evaluates how well a model performs in a new sample and can consist of temporal validation (sample contains more recently treated patients), geographical validation (sample is from a different centre) of a fully independent validation (validation by an independent team).
The association between risk predictors and hospital readmission was described using regression coefficients. Missing SEs for the coefficients were considered missing completely at random and were not imputed. A complete case analysis was performed.
Synthesis of results and analyses
Meta-analyses using random-effects models, with the Hartung-Knapp modification, were performed to describe the distribution of the between-study variance of the different prediction models and their predictors. Because we considered that there would be substantial heterogeneity, conclusions were not based on the precision of the pooled estimates.
The c-statistic from each model was pooled and a meta-regression was performed to investigate the moderation effect of age and the number of predictors on the discrimination. A subgroup analysis was performed to investigate the moderation effect of the different patient populations, design, outcome definition and endpoint. The c-statistic of the validated model was used if available; otherwise, the c-statistic from the development phase was used.
The c-statistics of specific prediction models that were evaluated in multiple studies were pooled for the endpoint 30-day follow-up.
Coefficients of predictors that were similarly defined in at least five studies were pooled for the endpoint 30-day follow-up. The patient populations were defined as subgroups to explore consistency and heterogeneity (I2, tau) in the effect estimates.
Analyses were performed using the ‘metan’ package in STATA V.15 IC and the ‘metamisc package’ in Rstudio.
Public and patient involvement
Because of the design of the study and because we did not collect primary date, we did not involve patients or the public in the design, conduct or reporting of our research.
Results
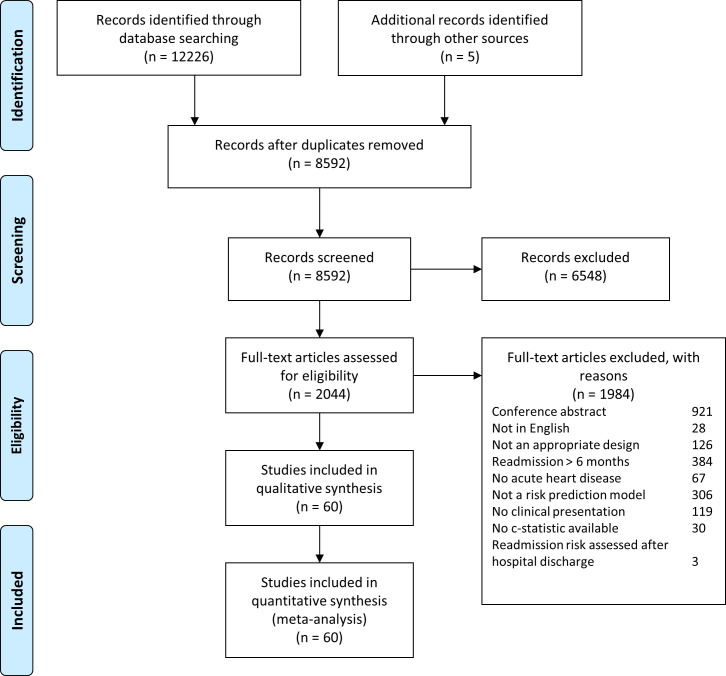
A total of 8588 abstracts were reviewed and 60 studies describing 81 separate models were included (figure 1). Table 1 provides an overview of the included studies and models, which were published between 2001 and 2020. The majority of the studies (n=40) was performed in the USA. The data sources used were mostly retrospective cohort studies (n=15), hospital databases (n=13) and registries (n=13). Included populations were mainly patients with HF(n=29), surgical patients (n=14) and patients with an AMI or acute coronary syndrome (n=10). The average age was between 56.5 and 84 years. The sample size of development cohorts ranged from 182 to 193 899 patients and of the validation cohorts between 104 and 321 088 patients. The outcome of interest was mostly all-cause readmission (n=41) and measured on 30 days (n=55). The incidence of readmission per study ranged from 3% to 43%.
Figure 1.
Flowchart. In total, 8592 records were screened and 60 studies with 81 prediction models were included.
Table 1.
Study characteristics
| Study | Model | Data source | Development | Validation | Sample size | Population | Average age | Outcome | Readmission (%) | ||
| Dev | Val | Dev | Val | ||||||||
| Moretti et al57 | EuroHeart PCI score | Hospital database | NA | Ext | – | 1192 | ACS | 71 (7) | 30d | 4.7 | |
| Asche et al46 | NR | Retrospective cohort | Yes | Split | 2446 | 612 | AMI | 65 (15) | 30d | 8.9 | |
| Cediel et al58 | TARRACO Risk score | Retrospective cohort | Yes | No | 611 | 401 | AMI type 2, ischaemia | D: 78 (17) V: 60 (21) |
30d | 2.6 | |
| Retrospective cohort | Yes | No | 611 | 401 | AMI type 2, ischaemia | D: 78 (17) V: 60 (21) |
180d | 7.9 | |||
| Chotechuang et al36 | GRACE | Retrospective cohort | NA | Ext | – | 152 | AMI | 60.5 (6.3) | 30d | 5.3 | |
| GRACE | Retrospective cohort | NA | Ext | – | 152 | AMI | 60.5 (6.3) | 180d | 9.2 | ||
| Hilbert et al59 | AMI decision tree | Registry | Yes | Ext | 10 848 | 10 701 | AMI | NR | 30d | 20.6 | 19.7 |
| Dodson et al18 | SILVER-AMI 30-day readmission calculator | Prospective cohort | Yes | Split | 2004 | 1002 | AMI | 81.5 (5.0) | 30d | 18.2 | |
| Kini et al60 | NR | Registry | Yes | Split | 60 742 | 26 107 | AMI | 76.5 (8.0) | 90d | 27.5 | |
| Nguyen et al19 | AMI READMITS score | Retrospective cohort | Yes | Split | 661 | 165 | AMI | 65.5 (12.8) | 30d | 13 | |
| Full-stay AMI model | Retrospective cohort | Yes | Split | 661 | 165 | AMI | 65.5 (12.8) | 30d | 13 | ||
| CMS AMI administrative model | Retrospective cohort | NA | Ext | – | 826 | AMI | 65.5 (12.8) | 30d | 13 | ||
| Krumholz et al20 | CMS AMI administrative model | Registry | Yes | Split, Ext | 100 465 | 321 088 | AMI | 78.7 (8.0) | 30d | 18.9 | 20.0 (Ext) NR (split) |
| CMS AMI medical model | Registry | Yes | Split | 130 944 | 130 944 | AMI | 76.2 (7.3) | 30d | 20 | ||
| Rana et al33 | Elixhauser index | Hospital database | NA | Ext | – | 1660 | AMI | 67.9 | 30d | 6.3 | |
| HOSPITAL score | Hospital database | NA | Ext | – | 1660 | AMI | 67.9 | 30d | 6.3 | ||
| Atzema et al47 | AFTER Part 2 scoring system | Retrospective cohort | Yes | Split | 2343 | 1167 | Arrhythmia, AF | D: 68.6 (14.7) V: 68.3 (15.1) |
30d | 7 | 7.6 |
| Lahewala et al40 | CHADS2 | Administrative | NA | Ext | – | 116 450 | Arrhythmia, AF | <75 | 30d | 15.8 | |
| CHADS2 | Administrative | NA | Ext | – | 116 450 | Arrhythmia, AF | <75 | 90d | 25.1 | ||
| CHA2DS-VASc | Administrative | NA | Ext | – | 116 450 | Arrhythmia, AF | 65–74 | 30d | 15.8 | ||
| CHA2DS-VASc | Administrative | NA | Ext | – | 116 450 | Arrhythmia, AF | 65–74 | 90d | 25.1 | ||
| Benuzillo et al61 | CRSS | Hospital database | Yes | Boot, Ext | 2589 | 896 (Ext) 500 (Boot) |
CABG | 66.7 (9.9) | 30d | 9.1 | 8.2 (Ext) 9.1 (Boot) |
| Deo et al62 | 30-day CABG readmission calculator | Administrative | Yes | Boot | 155 054 | 1000 | CABG | 65.4 (10.4) | 30d | 12.5 | |
| Engoren et al55 | NR | Hospital database | Yes | Split | 2644 | 2711 | CABG | NR | 30d | 7.6 | 8 |
| Lancey et al63 | NR | Registry | Yes | Split | 2341 | 2520 | CABG | 64.5 (10.5) | 30d | 8.8 | 9.5 |
| Rosenblum et al41 | The STS PROM score | Hospital database | NA | Ext | – | 21 719 | CABG | 63.5 (10.7) | 30d | 9.3 | |
| Zitser-Gurevich et al64 | NR | Prospective cohort | Yes | Split | 2266.5 | 2266.5 | CABG | 65–74 | 30d | 13.3 | |
| NR | Prospective cohort | Yes | Split | 2266.5 | 2266.5 | CABG | 65–74 | 100d | 24.1 | ||
| Zywot et al42 | CABG risk scale | Administrative | Yes | Ext | 126 519 | 94 318 | CABG | D: 70–74 V: 70–74 |
30d | 23 | 21 |
| Ahmad et al21 | CMS HF administrative model | Prospective cohort | NA | Ext | – | 183 | HF | 61 (18) | 30d | 22.4 | |
| Amarasingham et al22 | ADHERE | Hospital database | NA | Ext | – | 1372 | HF | 56.5 | 30d | 24.1 | |
| CMS HF administrative model | Hospital database | NA | Ext | – | 1372 | HF | 56.5 | 30d | 24.1 | ||
| Tabak mortality score | Hospital database | NA | Ext | – | 1372 | HF | 56.5 | 30d | 24.1 | ||
| Au et al23 | Administrative claims model: HF 30-day mortality | Administrative | NA | Ext | 59 652 | 59 652 | HF | 75.8 (12.7) | 30d | 15.9 | |
| Charlson Comorbidity Score | Administrative | NA | Ext | 59 652 | 59 652 | HF | 75.8 (12.7) | 30d | 15.9 | ||
| CMS HF administrative model | Administrative | NA | Ext | 59 652 | 59 652 | HF | 75.8 (12.7) | 30d | 15.9 | ||
| LACE | Administrative | NA | Ext | 59 652 | 59 652 | HF | 75.8 (12.7) | 30d | 15.9 | ||
| Bardhan et al65 | NR | Hospital database | Yes | No | 40 983 | – | HF | 69.2 (15.7) | 30d | 7 | |
| Betihavas et al66 | NR | RCT secondary analysis | Yes | Boot | 280 | 200 | HF | 74 (64–81) | 28d | 18 | |
| Cox et al24 | CMS HF administrative model | Hospital database | No | Ext | – | 1454 | HF | 75 (12) | 30d | 21.5 | |
| CMS HF medical model | Hospital database | No | Ext | – | 1454 | HF | 75 (12) | 30d | 21.5 | ||
| Delgado et al67 | 15-day CV readmission risk score | Prospective cohort | Yes | Boot | 1831 | 500 | HF | 72.4 (12.1) | 15d | 7.1 | |
| 30-day CV readmission risk score | Prospective cohort | Yes | Boot | 1831 | 500 | HF | 72.4 (12.1) | 30d | 13.9 | ||
| Formiga et al30 | CMS HF medical model | Hospital database | NA | Ext | – | 719 | HF | 78.1 (9) | 30d | 7.6 | |
| CMS HF medical model | Hospital database | NA | Ext | – | 719 | HF | 78.1 (9) | 90d | 14.4 | ||
| Frizzell et al25 | CMS HF administrative model | Registry | NA | External | – | 56 477 | HF | 80 (2) | 30d | 21.2 | |
| Hammill et al26 | CMS HF administrative model | Registry | NA | Ext | – | 24 163 | HF | 81 | 30d | 21.9 | |
| Hilbert et al59 | HF decision tree | Registry | Yes | Ext | 39 682 | 38 409 | HF | NR | 30d | 25.5 | 25.2 |
| Hummel et al31 | CMS HF medical model | Prospective cohort | NA | Ext | – | 1807 | HF | 79.8 (7.6) | 30d | 27 | |
| Huynh et al48 | NR | Prospective cohort | Yes | Ext | 430 | 1046 | HF | D: 75 (19) V: 67 (17) |
30d | 21 | 24 |
| NR | Prospective cohort | Yes | Ext | 430 | 1046 | HF | D: 75 (19) V: 67 (17) |
90d | 43 | 42 | |
| Ibrahim et al34 | HOSPITAL score | Retrospective cohort | NA | Ext | – | 692 | HfpEF | 68.3 (11.8) | 30d | 27.3 | |
| LACE/LACE+ index | Retrospective cohort | NA | Ext | – | 692 | HfpEF | 68.3 (11.8) | 30d | 27.3 | ||
| Keenan et al27 | CMS HF administrative model | Registry | Yes | Split, Ext. | 28 319 | 845 291 | HF | 79.9 (7.8) | 30d | 23.6 | 23.7 (Ext) NR (Split) |
| CMS HF medical model | Registry | Yes | Split, Ext. | 64 329 | 64 329 | HF | 75–84 | 30d | 23.7 | ||
| Kitamura et al53 | FIM | Retrospective cohort | NA | Ext | – | 113 | HF | 80.5 (6.7) | 90d | 20.4 | |
| Leong et al68 | 30-day HF readmission risk score | Retrospective cohort | Yes | Split | 888 | 587 | HF | D: 70.0 (12.7) V: 69.1 (12.8) |
30d | 9.9 | |
| Li et al49 | NR | Retrospective cohort | Yes | Split | 51 783 | 25 887 | HF | D: 84 (12) V: 84 (11) |
30d | 24.2 | |
| Lim et al69 | NR | Registry | Yes | No | 4566 | – | HF | 70.5 (12.0) | 30d | 6.6 (car) 13 (all) | |
| Reed et al28 | AH model | Administrative | Yes | Split | NR | NR | HF | NR | 30d | NR | |
| CMS HF administrative model | Administrative | NA | Split | – | NR | HF | NR | 30d | NR | ||
| Hasan | Administrative | NA | Split | – | NR | HF | NR | 30d | NR | ||
| LACE | Administrative | NA | Split | – | NR | HF | NR | 30d | NR | ||
| PARR-30 | Administrative | NA | Split | – | NR | HF | NR | 30d | NR | ||
| Salah et al70 | ELAN-HF score | Prospective cohort secondary analysis | Yes | No | 1301 | – | HF | 74 (16) | 180d | 36.1 | |
| Sudhakar et al32 | CMS HF medical model | Hospital database | NA | Ext | – | 1046 | HF | 65.2 (16.6) | 30d | 35.3 | |
| Tan et al71 | NR | Hospital database | Yes | Split | 246 | 104 | HF | D: 67.7 (12.3) V: 69.0 (12.9) |
90d | 24.5 | 11.7 |
| Wang et al72 | NR | Hospital database | Yes | No | 4548 | – | HF | 68.5 (27.6) | 30d | 25.1 | |
| Wang et al38 | LACE | Retrospective cohort | NA | Ext | – | 253 | HF | 56.6 (11.5) | 30d | 24.5 | |
| Yazdan-Ashoori et al29 | CMS HF administrative model | Prospective cohort | NA | Ext | – | 378 | HF | 73.1 (13.1) | 30d | 26 | |
| LACE | Prospective cohort | NA | Ext | – | 378 | HF | 73.1 (13.1) | 30d | 26 | ||
| Disdier Moulder et al73 | NR | Prospective cohort | Yes | No | 258 | HF, ACS, NR | 70.5 (23) | 30d | 17 | ||
| NR | Prospective cohort | Yes | No | 258 | HF, ACS, NR | 70.5 (23) | 180d | 38 | |||
| Raposeiras-Roubín et al37 | GRACE | Retrospective cohort | NA | Ext | – | 4229 | HF, ACS | 68.2 (18.7) | 30d | 2.6 | |
| Burke et al35 | HOSPITAL score | Retrospective cohort | NA | Ext | – | HF: 3189 AMI: 767 |
HF, AMI | 65.8 (16.8) | 30d | HF: 18.2 AMI: 17.4 | |
| Minges et al74 | NR | Registry | Yes | Split | 193 899 | 194 179 | HF, PCI | 65+ | 30d | 11.4 | |
| Pack et al75 | NR | Administrative | Yes | Split | 30 826 | 7706 | HVD | 64.9 (12.2) | 90d | 12.8 | |
| Oliver-McNeil et al76 | ICD readmission-risk score | Registry | Update | Ext | 182 | – | ICD | 69 (11) | 30d | 17.6 | |
| Wasfy et al52 | Pre-PCI model | Registry | Yes | Split | 24 052 | 12 008 | NR | 64.8 (12.5) | 30d | 10.4 | |
| Barnett et al77 | NR | Registry | Update | Ext | 19 964 | 19 964 | Surgical | 65.3 (12.4) | 30d | 11.4 | |
| Brown et al43 | STS augmented clinical model | Prospective cohort | Update | Boot | 1046 | NR | Surgical | 65.4 (9.8) | 30d | NR | |
| STS 30-day readmission model | Prospective cohort | NA | Ext | – | 1194 | Surgical | 73.3 (10.1) | 30d | NR | ||
| Espinoza et al78 | 30-day readmission score after cardiac surgery | Retrospective cohort | Yes | Split | 2529 | 2567 | Surgical | 65.1 (11.5) | 30d | 11.9 | |
| Ferraris et al54 | READMIT | Prospective cohort | Yes | 2574 | Surgical | 63 (11) | 30d | 9.8 | |||
| Kilic et al79 | NR | Retrospective cohort | Yes | Split | 3898 | 1295 | Surgical | D:61.9 (14.7) V: 61.6 (15.1) |
30d | 10 | 11 |
| Stuebe et al80 | NR | Hospital database | Yes | No | 4800 | Surgical | 60–69 | 30d | 12 | ||
| Tam et al44 | NR | Retrospective cohort | Yes | Boot | 63 336 | NR | Surgical | 66.2 (10.7) | 30d | 11.3 | |
| Khera et al45 | TAVR 30-Day readmission risk model | Administrative | Yes | Boots, Ext | 39 305 | 40 (Boot) 885 (Ext) | TAVR | D: 81.3 V: 81.7 |
30d | 16.2 | 16.2 (Boot) 18.9 (Ext) |
| Sanchez et al50 | NR | Registry | Yes | Split | 6903 | 3442 | TAVR | D: 81.1 (7.9) V: 81.3 (7.9) |
30d | 9.8 | 10.7 |
Age is reported as mean (SD); median (IQR) or average age as reported in the study.
ACS, acute coronary syndrome; ADHERE, Acute Decompensated Heart Failure Registry; AF, atrial fibrillation; AH, Adventist Health Off-the-shelf model; AMI, acute myocardial infarction; Boot, bootstrapping; CABG, coronary artery bypass grafting; Car, cardiac-related; CHA2DS2-VASc, congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, stroke or transient ischemic attack (TIA), vascular disease, age 65 to 74 years, sex category; CMS, Centers for Medicare and Medicaid Services; CRSS, CABG Readmission Risk Score; d, days; Dev, development; ELAN-HF, European Collaboration on Acute Decompensated Heart Failure; Ext, external validation; FIM, motor and cognitive Functional Independence Measure; GRACE, Global Registration of Acute Coronary Events; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HVD, heart valve disease; ICD, implantable cardioverter defibrillator; LACE, Length of stay, acuity of the Admission, Comorbidity of the patient and Emergency department use in the duration of 6 months before admission; NA, not applicable; NR, not reported; PARR-30, Patients at Risk of Re-admission within 30 days; PCI, percutaneous coronary intervention; READMITS, Renal Function, Elevated Brain Natriuretic Peptide, Age, Diabetes Mellitus, Nonmale Sex, Intervention with Timely Percutaneous Coronary Intervention, and Low Systolic Blood Pressure; SILVER-AMI, Comprehensive Evaluation of Risk Factors in Older Patients with AMI; Split, random split; STS PROM, Society of Thoracic Surgeons Predicted Risk of Mortality; TARRACO, Troponin Assessment for Risk stRatification of patients without Acute COronary atherothrombosis; TAVR, transcatheter aortic valve replacement; Val, validation.
Risk of bias
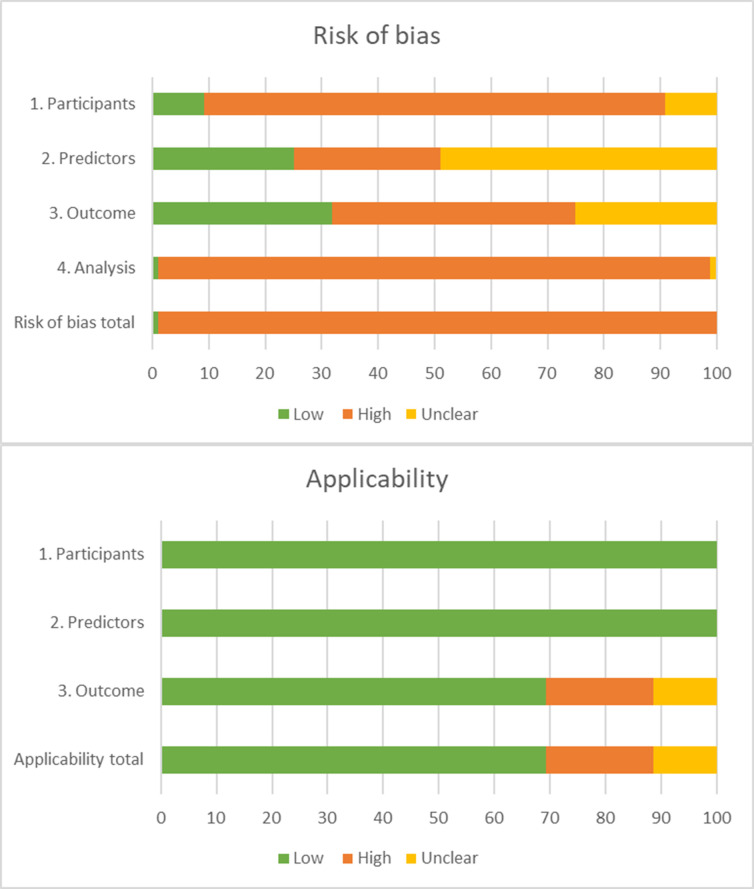
Figure 2 summarises the RoB and applicability assessment (online supplemental table 1A). The overall RoB was high in 98.9% of the models and only one study18 showed low RoB in all four domains.
Figure 2.
PROBAST (Prediction model Risk Of Bias ASsessment Tool) risk of bias and applicability. The PROBAST tool16 was used to assess the risk of bias for the participants, predictors, outcome and analysis for each model. Only one study demonstrated low risk of bias on all domains.
For the domain participants, 82.4% of studies was assessed as high RoB because most studies performed retrospective data analyses or used data from existing sources with large number of candidate predictors that were originally developed for other purposes, for example, administrative databases or registries. The domain predictors were assessed as high RoB in 27.5% of the models, 24.2% as low RoB and 48.4% as unclear RoB. For the domain outcome, 41.8%, 34.1% and 24.2% were assessed as high, low and unclear RoB, respectively.
The domain analysis was assessed as high RoB in 97.8%. Most studies did not use appropriate statistics for the development or validation of prediction models. For example, a description on how complexities in data were handled (eg, competing risk of death) was often missing and relevant performance measures were incomplete (eg, calibration).
The domain participants and predictors were assessed as low concerns regarding applicability in all studies. For the domain outcome, 70.3% of studies used all-cause readmission as the outcome of interest and were therefore assessed as low concerns regarding applicability.
Prediction models
A total of 43 new models were developed for patients with HF (n=15), undergoing surgical procedures (n=12), AMI (n=9), transcatheter aortic valve replacement (TAVR) (n=2), a mixed sample with HF and coronary syndromes (n=2), arrhythmias (n=1), valvular disease (n=1), while one study did not specify the sample (table 1). The c-statistic was lower than 0.6 in 5 models, between 0.6 and 0.7 in 24 models, between 0.7 and 0.8 in 6 models, and between 0.8 and 0.9 in 2 models. In six models, the c-statistic was only reported for a validation cohort (table 2).
Table 2.
Model discrimination and calibration
| Study | Model | Setting | Predictors; n | Cohort | Discrimination | Type calibration | Calibration |
| Moretti et al57 | EuroHeart PCI score | ACS | 16 | External | 0.59 (0.48–0.71) | NA | |
| Asche et al46 | NR | AMI | 19 | Development; random split | 0.74; NR | NA | |
| Cediel et al58 | TARRACO risk score | AMI type 2; ischaemia | 7 | Development (30d) | 0.71 (0.61–0.82) | NA | |
| AMI type 2; ischaemia | 7 | Development (180d) | 0.71 (0.64–0.78) | NA | |||
| Burke et al35 | HOSPITAL score | AMI | 7 | External | 0.66 (0.61–0.71) | HLT | p=0.49 |
| Chotechuang et al36 | GRACE | AMI | 9 | External (30d) | 0.77 (0.65–0.88) | NA | |
| GRACE | AMI | 9 | External (180d) | 0.63 (0.49–0.77) | NA | ||
| Hilbert et al59 | AMI decision tree | AMI | 44 | Development; External | 0.65 (0.64–0.66) 0.61 (0.61–0.62) |
NA | |
| Dodson et al18 | SILVER-AMI 30-day readmission calculator | AMI | 10 | Development; random split | 0.65; 0.63 | HLT | p>0.05; p=0.05 |
| Kini et al60 | NR | AMI | 12 | Development; random split | NR; 0.66 | Slope; in large; plot | 0.973 (p=0.330); −0.038 (p=0.221) |
| Nguyen et al19 | AMI READMITS score | AMI | 7 | Development; random split | 0.75 (0.70–0.80) 0.73 (0.71–0.74) |
Plot; plot | |
| Full-stay AMI model | AMI | 10 | Development; random split | 0.78 (0.74–0.83) 0.75 (0.74–0.76) |
Plot | ||
| CMS AMI administrative model | AMI | 32 | External | 0.74 (0.69–0.74) | Plot | ||
| Krumholz et al20 | CMS AMI administrative model | AMI | 32 | Development; external; random split | 0.63; 0.63; 0.62 | In large; slope | |
| CMS AMI medical model | AMI | 45 | Development; random split | 0.58; 0.59 | NA | 0, 1/0.015; 0.997/0.015; 0.983 | |
| Rana et al33 | Elixhauser index | AMI | 30 | External | 0.53 (0.42–0.65) | NA | |
| HOSPITAL core | AMI | 7 | External | 0.60 (0.47–0.73) | NA | ||
| Atzema et al47 | AFTER Part 2 scoring system | Arrhythmia; AF | 12 | Development | 0.69; NR | NA | |
| Lahewala et al40 | CHADS2 | Arrhythmia; AF | 5 | External (30d) | 0.64 | NA | |
| CHADS2 | Arrhythmia; AF | 5 | External (90d) | 0.63 | NA | ||
| CHA2DS-VASc | Arrhythmia; AF | 9 | External (30d) | 0.65 | NA | ||
| CHA2DS-VASc | Arrhythmia; AF | 9 | External (90d) | 0.63 | NA | ||
| Benuzillo et al61 | CRSS | CABG | 5 | Development; bootstrapping | 0.63; 0.63 | HLT | 7.13 (p=0.52); 9.31 (p=0.32) |
| Deo et al62 | 30-days CABG readmission calculator | CABG | 20 | Development | 0.65 | NA | |
| Engoren et al55 | NR | CABG | 6 | Development; random split | 0.68 (0.64–0.72) 0.68 (0.64–0.68) | NA | |
| Lancey et al63 | NR | CABG | 8 | Development; random split | 0.64; 0.57 | NA | |
| Rosenblum et al41 | The STS PROM score | CABG | 40 | External | 0.59 (0.57–0.60) | NA | |
| Zitser-Gurevich et al64 | NR | CABG | 17 | Development; external (30d) | 0.63; 0.66/0.63 | HLT | 7.91 (p=0.44) |
| NR | CABG | 13 | Development (100d) | 0.65 | HLT | 6.76 (p=0.56) | |
| Zywot et al42 | CABG risk scale | CABG | 27 | Development; external | NR; 0.70 | Plot | |
| Ahmad et al21 | CMS HF administrative model | HF | 37 | External | 0.66 (0.57–0.76) | HLT | p=0.19 |
| Amarasingham et al22 | ADHERE | HF | 3 | External | 0.56 (0.54–0.59) | NA | |
| CMS HF administrative model | HF | 37 | External | 0.66 (0.63–0.68) | NA | ||
| Tabak mortality score | HF | 18 | External | 0.61 (0.59–0.64) | NA | ||
| Au et al23 | Administrative claims model, HF 30-day mortality | HF | 17 | External | 0.58 (0.58–0.59) | NA | |
| Charlson Comorbidity Score | HF | 32 | External | 0.55 (0.55–0 56) | NA | ||
| CMS HF administrative model | HF | 37 | External | 0.59 (0.59–0.60) | NA | ||
| LACE | HF | 18 | External | 0.58 (0.58–0.59) | NA | ||
| Bardhan et al65 | NR | HF | 30 | Development | 0.56 | NA | |
| Betihavas et al66 | NR | HF | 7 | Development; bootstrapping | NR; 0.80 | NA | |
| Burke et al35 | HOSPITAL score | HF | 7 | External | 0.67 (0.65–0.70) | HLT | p=0.10 |
| Cox et al24 | CMS HF administrative model | HF | 37 | External | 0.61 | NA | |
| CMS HF medical model | HF | 20 | External | 0.60 | NA | ||
| Delgado et al67 | 15-day CV readmission risk score | HF | 5 | Development; bootstrapping | 0.65; 0.63 | Plot | |
| 30-day CV readmission risk score | HF | 11 | Development; bootstrapping | 0.66; 0.64 | Plot | ||
| Formiga et al30 | CMS HF medical model | HF | 19 | External (30d) | 0.65 (0.57–0.72) | NA | |
| CMS HF medical model | HF | 19 | External (90d) | 0.62 (0.56–0.68) | NA | ||
| Frizzell et al25 | CMS HF administrative model | HF | 37 | External | 0.60 | NA | |
| Hammill et al26 | CMS HF administrative model | HF | 37 | External | 0.59 | Plot | |
| Hilbert et al59 | HF decision tree | HF | 44 | Development; External | 0.59 (0.58–0.60) 0.58 (0.58–0.59) |
NA | |
| Hummel et al31 | CMS HF medical model | HF | 28 | External | 0.61 | NA | |
| Huynh et al48 | NR | HF | 12 | Development; external (30d) | 0.82 (0.76–0.87) 0.73 (0.69–0.77) |
NA | |
| NR | HF | 12 | Development; external (90d) | NR; 0.65 | NA | ||
| Ibrahim et al34 | HOSPITAL score | HfpEF | 7 | External | 0.60 (0.55–0.64) | NA | |
| LACE | HfpEF | 18 | External | 0.55 (0.50–0.60) | NA | ||
| LACE+ index | HfpEF | 24 | External | 0.57 (0.52–0.62) | NA | ||
| Keenan et al27 | CMS HF administrative model | HF | 37 | Development; external; random split | 0.60; 0.60; 0.61 | In large; slope | 0, 1/0.02; 1.01/ 0.09; 1.05 |
| CMS HF medical model | HF | 30 | Development; random split | 0.58; 0.61 | In large; slope | 0, 1/0, 1 | |
| Kitamura et al53 | FIM | HF | 13 | External | 0.78 | NA | |
| Leong et al68 | 30-day HF readmission risk score | HF | 7 | Development; random split | 0.76; 0.76 | NA | |
| Li et al49 | NR | HF | 10 | Development; random split | 0.63 (0.62–0.63) 0.63 (0.62–0.63) |
HLT; plot | 0.15 (p>0.005) |
| Lim et al69 | NR | HF | 13 | Development | 0.68 (car); 0.62 (all) | HLT | 27.5 (p=0.001) (car) 8.0 (p=0.429) (all) |
| Reed et al28 | AH model | HF | 14 | Development; random split | 0.86 (0.85–0.86) 0.85 (0.84–0.86) | NA | |
| CMS HF administrative model | HF | 37 | Random split | 0.55 (0.54–0.56) 0.55 (0.54–0.57) |
NA | ||
| Hasan | HF | 9 | Random split | 0.80 (0.79–0.81) 0.80 (0.80–0.82) |
NA | ||
| LACE | HF | 18 | Random split | 0.75 (0.74–0.81) 0.74 (0.73–0.76) |
NA | ||
| Reed et al (continued)28 | PARR-30 | HF | 10 | Random split | 0.82 (0.81–0.83) 0.81 (0.80–0.82) |
NA | |
| Salah et al70 | ELAN-HF Score | HF | 10 | Development | 0.60 (0.56–0.64) | NA | |
| Sudhakar et al32 | CMS HF medical model | HF | 20 | External | 0.61 (0.57–0.64) ≥65 y, 0.59 (0.53–0.64) Random patient-level, 0.58 (0.50–0.65) |
NA | |
| Tan et al71 | NR | HF | 3 | Random split | 0.73 | HLT; plot | p=0.62 |
| Wang et al72 | NR | HF | 12 | Development | 0.65 | NA | |
| Wang et al38 | LACE | HF | 18 | External | 0.56 (0.48–0.64) | NA | |
| Yazdan-Ashoori et al29 | CMS HF administrative model | HF | 37 | External | 0.61 (0.55–0.67) | NA | |
| LACE | HF | 18 | External | 0.59 (0.52–0.65) | HLT | p=0.73 | |
| Disdier Moulder et al73 | NR | HF; ACS; NR | 4 | Development (30d) | 0.68 | NA | |
| NR | HF; ACS; NR | 5 | Development (180d) | 0.69 | NA | ||
| Raposeiras-Roubín et al37 | GRACE | HF; ACS | 9 | External | 0.74 (0.73–0.80) | HLT | p=0.14 |
| Minges et al74 | NR | HF; PCI | 35 | Development; random split | 0.67; 0.66 | NA | |
| Pack et al75 | NR | HVD | 28 | Development; random split | 0.67 (full dev)/ 0.65 (nomogram); 0.67 (full val) |
Harrell’s E; O, E; Harrell’s E; plot | 0.1%; 1.9%; 1.6% |
| Oliver-McNeil et al76 | ICD readmission-risk score | ICD | 4 | Update; External | 0.69 (0.58–0.79) | HLT; plot | 3.44 (p=0.49) |
| Wasfy et al52 | Pre-PCI model | NR | 23 | Development; random split | 0.68; 0.67 | HLT; plot | p=0.59 |
| Barnett et al77 | NR validation | Surgical | 15 | External | 0.59 | NA | |
| NR update | Surgical | 18 | Update | 0.60 (0.59–0.62) | NA | ||
| Brown et al43 | STS augmented clinical model | Surgical | 27 | Update (bootstrap); random split; external (bootstrap) | 0.66 (0.61–0.72); 0.56; 0.47 (0.42–0.53) |
HLT | p=1.0 |
| STS 30-day readmission model | Surgical | 21 | Update (bootstrap); random split; external (bootstrap) | 0.66 (0.62–0.71), 0.58, 0.47 (0.41–0.52) |
HLT | p=0.492 | |
| Espinoza et al78 | 30-day readmission score after cardiac surgery | Surgical | 5 | Development; random split | 0.66 (0.63–0.70) 0.64 (0.61–0.67) |
NA | |
| Ferraris et al54 | READMIT | Surgical | 9 | Development | 0.70 | HLT | 5.966 (p=0.651) |
| Kilic et al79 | NR | Surgical | 15 | Development; random split | NR; 0.64 | HLT; plot | p=0.45; p=0.57 |
| Stuebe et al80 | NR | Surgical | 7 | Development | 0.63 | NA | |
| Tam et al44 | NR | Surgical | 29 | Development; bootstrapping | 0.63; 0.65 | Plot | |
| Khera et al45 | TAVR 30-Day readmission risk model | TAVR | 11 | Development; random split; external | NR; 0.63; 0.69 | HLT; RMSE; RMSE; plot | p=0.33; 0.978; 0.928 |
| Sanchez et al50 | NR | TAVR | 10 | Development; random split | 0.61; 0.60 | HLT | p=0.749; p=0.403 |
ACS, acute coronary syndrome; ADHERE, Acute Decompensated Heart Failure Registry; AF, atrial fibrillation; AH, Adventist Health Off-the-shelf model; AMI, acute myocardial infarction; CABG, coronary artery bypass grafting; Car, cardiac-related; CHADS2, Congestive heart failure, Hypertension, Age, Diabetes, previous Stroke/transient ischemic attack; CHADS2-VASc, congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, stroke or transient ischemic attack (TIA), vascular disease, age 65 to 74 years, sex category; CMS, Centers for Medicare and Medicaid Services; CRSS, CABG Readmission Risk Score; d, days; dev, development; FIM, motor and cognitive Functional Independence Measure; GRACE, Global Registry of Acute Coronary Events; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HLT, Hosmer-Lemeshow test; HOSPITAL, Hemoglobin level, discharged from Oncology, Sodium level, Procedure during admission, Index admission Type, Admission, Length of stay; HVD, heart valve disease; ICD, implantable cardioverter defibrillator; LACE, Length of stay, acuity of the Admission, Comorbidity of the patient and Emergency department use in the duration of 6 months before admission; NA, not applicable; NR, not reported; O, E, observed, expected; PARR-30, Patients at Risk of Re-admission within 30 days; PCI, percutaneous coronary intervention; plot, calibration plot; READMITS, Renal Function, Elevated Brain Natriuretic Peptide, Age, Diabetes Mellitus, Nonmale Sex, Intervention with Timely Percutaneous Coronary Intervention, and Low Systolic Blood Pressure; SILVER-AMI, Comprehensive Evaluation of Risk Factors in Older Patients with AMI; STS, Society of Thoracic; STS, Society of Thoracic; STS PROM, Society of Thoracic Surgeons Predicted Risk of Mortality; TAVR, transcatheter aortic valve replacement; val, validation.
A total of 38 separate models were externally validated for patients with HF (n=26), AMI (n=4), surgical patients (n=3), acute coronary syndrome (n=2), arrhythmias (n=2), mixed sample with HF and coronary syndromes (n=1). The discrimination was lower than 0.6 in 16 models, between 0.6 and 0.7 in 15 models, between 0.7 and 0.8 in 5 models, and between 0.8 and 0.9 in 2 models (table 2).
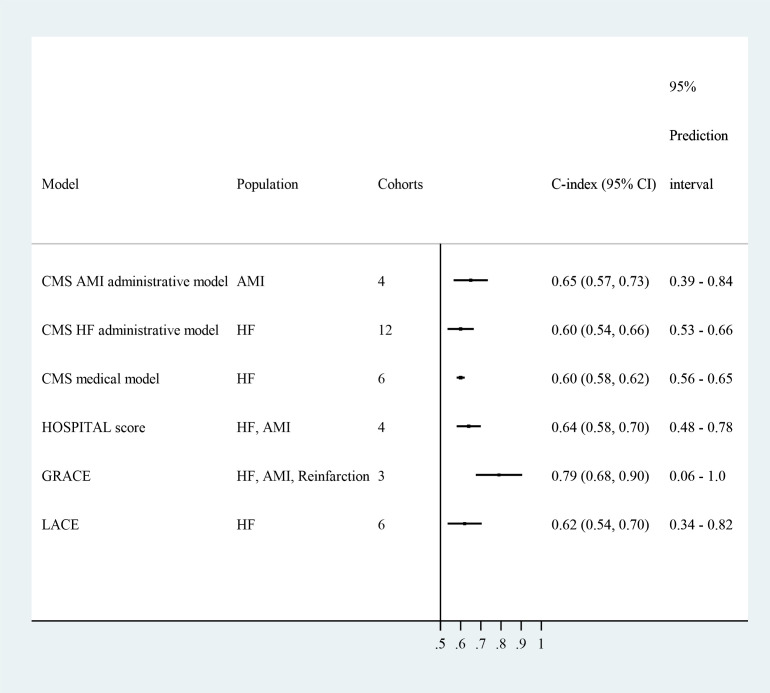
The discrimination of six models was evaluated in multiple independent cohorts and was pooled in meta-analyses (figure 3, online supplemental figures 1–6): the CMS AMI (Centers for Medicare and Medicaid Services Acute Myocardial Infarction) administrative model19 20 (0.65, 95% CI 0.56 to 0.73); the CMS HF (Heart Failure) administrative model21–29 (0.60, 95% CI 0.58 to 0.62); the CMS HF medical model24 27 30–32 (0.60, 95% CI 0.58 to 0.62); the HOSPITAL (Hemoglobin level, discharged from Oncology, Sodium level, Procedure during admission, Index admission Type, Admission, Length of stay) score33–35 (0.64, 95% CI 0.58 to 0.70); the GRACE (Global Registration of Acute Coronary Events) score36 37 (0.78, 95% CI 0.63 to 0.86); and the LACE (Length of stay, acuity of the Admission, Comorbidity of the patient and Emergency department use in the duration of 6 months before admission) score23 28 29 34 38 (0.62, 95% CI 0.53 to 0.70).
Figure 3.
Meta-analysis of prediction models. Random-effect models were used to pool similar models reported in independent cohorts. For the HOSPITAL score, the discriminations for the HF and AMI samples were similar (0.65 and 0.64). For GRACE, the discriminations for the AMI and reinfarction samples were similar (0.77 and 0.74), and was higher for the HF sample (0.83). Only GRACE demonstrated adequate discrimination in external cohorts. AMI, acute myocardial infarction; CMS, Centers for Medicare and Medicaid Services; CF, heart failure. Abbreviations: CMS = Centers for Medicare and Medicaid Services; AMI = Acute Myocardial Infarction; HF = Heart Failure; HOSPITAL = Hemoglobin level, discharged from Oncology, Sodium level, Procedure during admission, Index admission Type, Admission, Length of stay; GRACE = Global Registry of Acute Coronary Events; LACE = length of stay (L), acuity of the admission (A), comorbidity of the patient (C) and emergency department use in the duration of 6 months before admission.
On average, models for patients with AMI had the best discrimination (0.67, n=16), followed by patients with TAVR (0.65, n=2), patients with HF (0.64, n=45) and surgical patients (0.63, n=17). The discrimination was highest in studies using secondary analysis (0.70, n=2) and retrospective cohort studies (0.69, n=23), and was lowest in studies using registries (0.61, n=17) and hospital databases (0.61, n=18). The discrimination decreased when the number of predictors increased (beta −0.002, n=90). There were no moderation effects based on the average age of the sample, outcome definition and endpoint of the prediction (online supplemental figures 7–8 and online supplemental table 1B).
The calibration was reported for 27 models using multiple measures and could not be pooled (table 2).
Predictors
A total of 766 predictor values were estimated in the included models. The median number of predictors per model was 15 (IQR=9–28). The predictors were mostly situated in the domains medical comorbidities (n=211), disease and hospital characteristics (n=128), demographic data (n=128), laboratory values (n=97) and medical history characteristics (n=51). Age (n=47), presence of diabetes (n=26), insurance status (n=24), length of stay (n=28) and gender (n=23) were the most prevalent predictors. There was little consistency in the definition of predictors, and most studies did not report how they were measured.
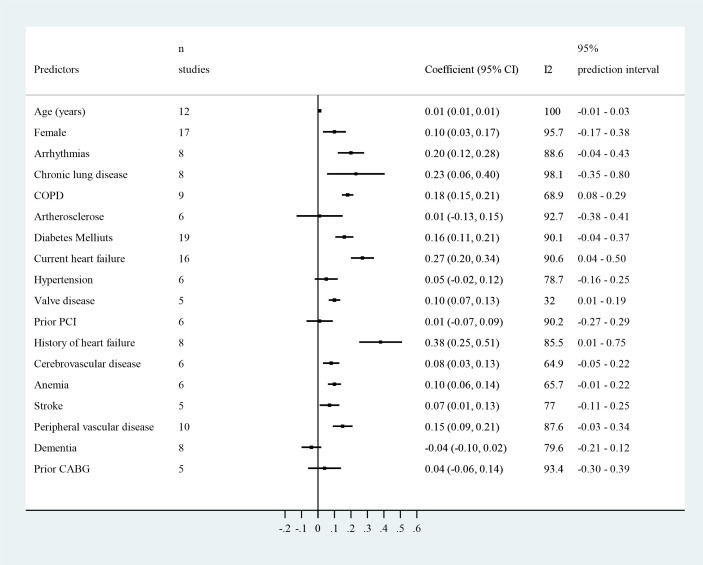
Only 18 predictors were similarly defined in multiple studies and could be pooled for the outcome readmission at 30 days (figure 4, online supplemental table 2A and online supplemental figures 9–26). The coefficients of four predictors demonstrated a consistent and significant association across the different samples: chronic obstructive pulmonary disease (COPD), HF or history of HF, and valvular disease. The coefficients of 11 predictors demonstrated an overall significant association, that is, age, female gender, arrhythmias, chronic lung disease, diabetes mellitus, cerebrovascular disease, cardiovascular accident, anaemia, peripheral vascular disease, urgent admission and infection, but this was not consistent across the samples and the prediction intervals were not significant. The effect of these predictors was mostly smaller in the HF samples.
Figure 4.
Predictors of unplanned hospital readmission. The plot provides an overview of the random-effects meta-analyses that were performed for predictors who were similarly defined for the outcome unplanned hospital readmission at 30-day follow-up. See online supplemental table 2A and online supplemental figures 9–26 for more details. CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; PCI, percutaneous coronary intervention.
The coefficients for most predictors could not be pooled because they had different definitions, cut-off values or reference categories. However, renal disease, including dialysis, a longer length of stay, creatinine, NT-proBNP (N-Terminal-PRO hormone Brain Natriuretic Peptide) and previous hospital admissions demonstrated a consistent association with readmissions.
Discussion
In this systematic review, we included 60 studies that reported the results from 81 separate clinical risk prediction models and 766 risk predictors for unplanned readmission in patients with acute heart disease. We found some promising prediction models, however, no clinical model demonstrated good discrimination (ie, c-statistic >0.8) in independently externally validated cohorts, regardless of the underlying patient populations. GRACE was the only model that demonstrated adequate discrimination in multiple cohorts in patients with acute coronary syndromes36 37 and HF.37 There was little consistency in the measurement of risk predictors.
The results of our review are in line with previous systematic reviews which have mainly focused on samples of patients with HF, AMI or focused on generic prediction models. All reviews confirm that the discrimination is generally low. Our review confirms the importance of previous HF5 6 and previous hospital admissions6 8 as consistent predictors of the risk of readmission. In addition, two prevalent comorbidities, COPD and valve disease, were also consistent predictors across the different populations. Other reviews also identified the importance of age, gender, comorbidities and certain laboratory values. These were also significant in our review but the association was not always consistent across the different populations or heterogeneously measured making comparisons difficult. As a result, no clinical risk prediction model or set of predictors that is relevant for different populations of heart disease could be identified.
Our review focused specifically on prediction models with a clinical presentation that can be used in daily practice, for example, risk scores or nomograms. These simple models do not consider interactions between predictor values or non-linear link functions in their predictions. This may partially explain the poor discrimination.39 Using web applications or electronic patient records to run more complex prediction algorithms can likely offer a solution for future models. A recent systematic review observed an average c-statistic of 0.74 for models using electronic patient records and machine learning algorithms.11 Our review included 11 studies18 29 32 36 37 40–45 that developed or validated electronic tools for risk prediction and their discrimination ranged between 0.59 and 0.77. However, these electronic tools were mostly derived from score charts and nomograms.
There are also concerns about the generalisability of the prediction models. The median age of patients included in the samples was 68 years (IQR=65–75). However, older and frail patients suffer more multimorbidity and geriatric syndromes, and the distribution of predictor and outcome values will also be different than in younger samples. It is therefore unlikely that the majority of the current models will hold their value in daily clinical practice where there is a high prevalence of older patients. Only eight studies18 20 27 46–50 included one or more geriatric risk factors (eg, physical performance, dementia) as predictors for readmission. The performance of models including geriatric conditions was similar to models without these conditions. This might be explained by the relative young mean age of the samples in our review. Mahmoudi et al11 reported that functional and frailty status are important predictors, but were only included in a small number of studies. Frailty was not identified in any of the models in our review. It might be valuable to examine the additive value of these predictors in prediction models for patients with heart disease.
We observed high RoB in almost all clinical risk prediction models (98.8%). This was mainly because the calibration was lacking or not fully reported (eg, only p value of Hosmer-Lemeshow test). Furthermore, most studies performed retrospective data analyses or used data from existing sources. However, our results demonstrate that studies using these data sources had the lowest c-statistic, and that the c-statistic decreased when more predictors were tested. Databases often have missing data, misclassification bias and random measurement error, which likely explains their average poor performance.51 Only the SILVER-AMI (Comprehensive Evaluation of Risk Factors in Older Patients with AMI) study18 demonstrated low RoB on all domains. However, their readmission risk calculator for older patients with AMI only discriminated modestly (c-statistic=0.65).
Our review shows the current state-of-the art of risk prediction in patients with acute heart disease. The timely identification of patients with acute heart disease at risk of readmission remains challenging with the prediction models identified in this systematic review. Therefore, further research in risk prediction remains important and some recommendations for further research can be derived from this review. First, consistency is needed in the definition and measurement of predictors. More homogeneity might improve the identification of important predictors and their effect on readmission. Based on our insights, we believe that models could be improved by incorporating some key predictors, that is, age, gender, comorbidity scores (or at least heart failure, COPD, cardiovascular disease, diabetes mellitus), admission status, readmission history and the geriatric profile (eg, functional status, cognitive status). Because there are a still a large number of potential predictors, a large sample size is needed to estimate the coefficients with sufficient precision, and to prevent against overfitting the models. Some selection of predictors may still be warranted, and penalised techniques (eg, lasso regression) should be preferred over traditional selection based on p values. Second, the results suggest that multiple predictors are associated with readmissions regardless of the underlying population. Therefore, attention might be shifted from developing new risk prediction models to updating and externally validating existing prediction models in different populations with heart disease. For example, the Adventist Health Off-the-shelf model28 showed high discrimination rates in both the development (0.86) and the validation cohorts (0.85). External validation is recommended to examine the generalisability of this model in other settings. In addition, the AMI READMITS (Acute Myocardial Infarction Renal Function, Elevated Brain Natriuretic Peptide, Age, Diabetes Mellitus, Nonmale Sex, Intervention with Timely Percutaneous Coronary Intervention, and Low Systolic Blood Pressure) score,19 full-stay AMI readmission model,19 pre-PCI model,52 motor and cognitive Functional Independence Measure (FIM),53 READMIT,54 30-day readmission model of Huynh et al,48 and the model of Engoren et al55 were examined in one study and showed reasonable c-statistics in the development (0.68–0.82) and validation cohorts (0.64–0.78). For these studies, model updating recalibration and external validation is recommended to improve the predictive performance and generalisability of these prediction models. Third, the applicability of current prediction models in daily practice is an important concern as most models had poor performance, were not replicated and had high RoB. More high-quality studies are needed that evaluate the discrimination, calibration and clinical usefulness. To limit the RoB as much as possible, future studies should adhere to the relevant reporting guidelines56 and could use PROBAST16 as a guidance to plan their study. Fourth, more complex models integrated in electronic patient records may results in better predictions.
Limitations
Although we performed an extensive literature search, we might have missed some eligible studies, particularly those published in non-English languages. We were able to perform meta-analysis for predictors that were often (≥5 models) reported. However, it might be possible that some less frequently mentioned predictors (eg, geriatric predictors) are a valuable addition in clinical practice. The review included a large number of results and statistical tests which may result in an inflated alpha error. The meta-regression identified that models with less predictors had a better discrimination, but this could also be explained by overfitting models; this could not be tested.
Conclusion
A large number of clinical models have recently been developed. Although some models are promising as they demonstrated adequate to good discrimination, no model can currently be recommended for clinical practice. The lack of independently validated studies, high RoB and low consistency in measured predictors limit their applicability. Model updating and external validation is urgently needed.
Supplementary Material
Footnotes
Twitter: @Patricia_Jepma, @mieke_deschodt
Contributors: BVG and PJ had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. BVG and PJ contributed equally as first authors. Concept and design: All authors. Acquisition, analysis or interpretation of data: BVG, PJ, CR, ML, JD. Drafting the manuscript: BVG, PJ. Critical revision of the manuscript: All authors. Analysis: BVG, PJ. Supervision: BB.
Funding: This work was partly supported by the Research Foundation Flanders (FWO) fellowship grant (grant number 1165518N (BVG)), and by the Dutch Research Council (NWO) (grant number 023.009.036 (PJ)). The funders had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics-2020 update: a report from the American heart association. Circulation 2020;141:e139–596. 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 2.Shipe ME, Deppen SA, Farjah F, et al. Developing prediction models for clinical use using logistic regression: an overview. J Thorac Dis 2019;11:S574–84. 10.21037/jtd.2019.01.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith LN, Makam AN, Darden D, et al. Acute myocardial infarction readmission risk prediction models: a systematic review of model performance. Circ Cardiovasc Qual Outcomes 2018;11:e003885. 10.1161/CIRCOUTCOMES.117.003885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Tanna GL, Wirtz H, Burrows KL, et al. Evaluating risk prediction models for adults with heart failure: a systematic literature review. PLoS One 2020;15:e0224135. 10.1371/journal.pone.0224135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahajan SM, Heidenreich P, Abbott B, et al. Predictive models for identifying risk of readmission after index hospitalization for heart failure: a systematic review. Eur J Cardiovasc Nurs 2018;17:675–89. 10.1177/1474515118799059 [DOI] [PubMed] [Google Scholar]
- 6.O'Connor M, Murtaugh CM, Shah S, et al. Patient characteristics predicting readmission among individuals hospitalized for heart failure. Med Care Res Rev 2016;73:3–40. 10.1177/1077558715595156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rahimi K, Bennett D, Conrad N, et al. Risk prediction in patients with heart failure: a systematic review and analysis. JACC Heart Fail 2014;2:440–6. 10.1016/j.jchf.2014.04.008 [DOI] [PubMed] [Google Scholar]
- 8.Betihavas V, Davidson PM, Newton PJ, et al. What are the factors in risk prediction models for rehospitalisation for adults with chronic heart failure? Aust Crit Care 2012;25:31–40. 10.1016/j.aucc.2011.07.004 [DOI] [PubMed] [Google Scholar]
- 9.Desai MM, Stauffer BD, Feringa HHH, et al. Statistical models and patient predictors of readmission for acute myocardial infarction: a systematic review. Circ Cardiovasc Qual Outcomes 2009;2:500–7. 10.1161/CIRCOUTCOMES.108.832949 [DOI] [PubMed] [Google Scholar]
- 10.Ross JS, Mulvey GK, Stauffer B, et al. Statistical models and patient predictors of readmission for heart failure: a systematic review. Arch Intern Med 2008;168:1371–86. 10.1001/archinte.168.13.1371 [DOI] [PubMed] [Google Scholar]
- 11.Mahmoudi E, Kamdar N, Kim N, et al. Use of electronic medical records in development and validation of risk prediction models of hospital readmission: systematic review. BMJ 2020;369:m958. 10.1136/bmj.m958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou H, Della PR, Roberts P, et al. Utility of models to predict 28-day or 30-day unplanned spital readmissions: an updated systematic review. BMJ Open 2016;6:e011060. 10.1136/bmjopen-2016-011060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song F, Sheldon TA, Sutton AJ, et al. Methods for exploring heterogeneity in meta-analysis. Eval Health Prof 2001;24:126–51. 10.1177/016327870102400203 [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Distiller . Distiller systematic review software. Available: https://www.evidencepartners.com/products/distillersr-systematic-review-software/ [Accessed 25 Aug 2020].
- 16.Wolff RF, Moons KGM, Riley RD, et al. PROBAST: a tool to assess the risk of bias and applicability of prediction model studies. Ann Intern Med 2019;170:51–8. 10.7326/M18-1376 [DOI] [PubMed] [Google Scholar]
- 17.Newcombe RG. Confidence intervals for an effect size measure based on the mann-whitney statistic. part 2: asymptotic methods and evaluation. Stat Med 2006;25:559–73. 10.1002/sim.2324 [DOI] [PubMed] [Google Scholar]
- 18.Dodson JA, Hajduk AM, Murphy TE, et al. Thirty-day readmission risk model for older adults hospitalized with acute myocardial infarction. Circ Cardiovasc Qual Outcomes 2019;12:e005320. 10.1161/CIRCOUTCOMES.118.005320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen OK, Makam AN, Clark C, et al. Predicting 30-Day Myocardial Infarction: The AMI "READMITS" (Renal Function, elevated brain natriuretic peptide, age, diabetes mellitus, nonmale sex, intervention with timely percutaneous coronary intervention, and low systolic blood pressure) score. J Am Heart Assoc 2018;7:e008882. 10.1161/JAHA.118.008882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krumholz HM, Lin Z, Drye EE, et al. An administrative claims measure suitable for profiling hospital performance based on 30-day all-cause readmission rates among patients with acute myocardial infarction. Circ Cardiovasc Qual Outcomes 2011;4:243–52. 10.1161/CIRCOUTCOMES.110.957498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmad FS, French B, Bowles KH, et al. Incorporating patient-centered factors into heart failure readmission risk prediction: a mixed-methods study. Am Heart J 2018;200:75–82. 10.1016/j.ahj.2018.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amarasingham R, Moore BJ, Tabak YP, et al. An automated model to identify heart failure patients at risk for 30-day readmission or death using electronic medical record data. Med Care 2010;48:981–8. 10.1097/MLR.0b013e3181ef60d9 [DOI] [PubMed] [Google Scholar]
- 23.Au AG, McAlister FA, Bakal JA, et al. Predicting the risk of unplanned readmission or death within 30 days of discharge after a heart failure hospitalization. Am Heart J 2012;164:365–72. 10.1016/j.ahj.2012.06.010 [DOI] [PubMed] [Google Scholar]
- 24.Cox ZL, Lai P, Lewis CM, et al. Customizing national models for a medical center's population to rapidly identify patients at high risk of 30-day all-cause Hospital readmission following a heart failure hospitalization. Heart Lung 2018;47:290–6. 10.1016/j.hrtlng.2018.05.012 [DOI] [PubMed] [Google Scholar]
- 25.Frizzell JD, Liang L, Schulte PJ, et al. Prediction of 30-day all-cause readmissions in patients hospitalized for heart failure: comparison of machine learning and other statistical approaches. JAMA Cardiol 2017;2:204–9. 10.1001/jamacardio.2016.3956 [DOI] [PubMed] [Google Scholar]
- 26.Hammill BG, Curtis LH, Fonarow GC, et al. Incremental value of clinical data beyond claims data in predicting 30-day outcomes after heart failure hospitalization. Circ Cardiovasc Qual Outcomes 2011;4:60–7. 10.1161/CIRCOUTCOMES.110.954693 [DOI] [PubMed] [Google Scholar]
- 27.Keenan PS, Normand S-LT, Lin Z, et al. An administrative claims measure suitable for profiling hospital performance on the basis of 30-day all-cause readmission rates among patients with heart failure. Circ Cardiovasc Qual Outcomes 2008;1:29–37. 10.1161/CIRCOUTCOMES.108.802686 [DOI] [PubMed] [Google Scholar]
- 28.Reed J, Bokovoy J, Doram K. Unplanned readmissions after hospital discharge among heart failure patients at risk for 30-day readmission using an administrative dataset and “off the shelf” readmission models. Internet J Cardiovasc Res 2014;9:07–15. [Google Scholar]
- 29.Yazdan-Ashoori P, Lee SF, Ibrahim Q, et al. Utility of the lace index at the bedside in predicting 30-day readmission or death in patients hospitalized with heart failure. Am Heart J 2016;179:51–8. 10.1016/j.ahj.2016.06.007 [DOI] [PubMed] [Google Scholar]
- 30.Formiga F, Masip J, Chivite D, et al. Applicability of the heart failure readmission risk score: a first European study. Int J Cardiol 2017;236:304–9. 10.1016/j.ijcard.2017.02.024 [DOI] [PubMed] [Google Scholar]
- 31.Hummel SL, Katrapati P, Gillespie BW, et al. Impact of prior admissions on 30-day readmissions in medicare heart failure inpatients. Mayo Clin Proc 2014;89:623–30. 10.1016/j.mayocp.2013.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sudhakar S, Zhang W, Kuo Y-F, et al. Validation of the readmission risk score in heart failure patients at a tertiary hospital. J Card Fail 2015;21:885–91. 10.1016/j.cardfail.2015.07.010 [DOI] [PubMed] [Google Scholar]
- 33.Rana S, Tran T, Luo W, et al. Predicting unplanned readmission after myocardial infarction from routinely collected administrative hospital data. Aust Health Rev 2014;38:377–82. 10.1071/AH14059 [DOI] [PubMed] [Google Scholar]
- 34.Ibrahim AM, Koester C, Al-Akchar M, et al. Hospital score, lace index and lace+ index as predictors of 30-day readmission in patients with heart failure. BMJ Evid Based Med 2020;25:166-167. 10.1136/bmjebm-2019-111271 [DOI] [PubMed] [Google Scholar]
- 35.Burke RE, Schnipper JL, Williams MV, et al. The hospital score predicts potentially preventable 30-day readmissions in conditions targeted by the hospital readmissions reduction program. Med Care 2017;55:285–90. 10.1097/MLR.0000000000000665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chotechuang Y, Phrommintikul A, Muenpa R, et al. The prognostic utility of grace risk score in predictive cardiovascular event rate in STEMI patients with successful fibrinolysis and delay intervention in non PCI-capable Hospital: a retrospective cohort study. BMC Cardiovasc Disord 2016;16:212. 10.1186/s12872-016-0383-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raposeiras-Roubín S, Abu-Assi E, Cambeiro-González C, et al. Mortality and cardiovascular morbidity within 30 days of discharge following acute coronary syndrome in a contemporary European cohort of patients: how can early risk prediction be improved? the six-month grace risk score. Rev Port Cardiol 2015;34:383–91. 10.1016/j.repc.2014.11.020 [DOI] [PubMed] [Google Scholar]
- 38.Wang H, Robinson RD, Johnson C, et al. Using the lace index to predict hospital readmissions in congestive heart failure patients. BMC Cardiovasc Disord 2014;14:97. 10.1186/1471-2261-14-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kerr KF, Pepe MS. Joint modeling, covariate adjustment, and interaction: contrasting notions in risk prediction models and risk prediction performance. Epidemiology 2011;22:805–12. 10.1097/EDE.0b013e31823035fb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lahewala S, Arora S, Patel P, et al. Atrial fibrillation: Utility of CHADS2 and CHA2DS2-VASc scores as predictors of readmission, mortality and resource utilization. Int J Cardiol 2017;245:162–7. 10.1016/j.ijcard.2017.06.090 [DOI] [PubMed] [Google Scholar]
- 41.Rosenblum JM, Lovasik BP, Hunting JC, et al. Predicted risk of mortality score predicts 30-day readmission after coronary artery bypass grafting. Gen Thorac Cardiovasc Surg 2019;67:661–8. 10.1007/s11748-019-01079-6 [DOI] [PubMed] [Google Scholar]
- 42.Zywot A, Lau CSM, Glass N, et al. Preoperative scale to determine all-cause readmission after coronary artery bypass operations. Ann Thorac Surg 2018;105:1086–93. 10.1016/j.athoracsur.2017.11.062 [DOI] [PubMed] [Google Scholar]
- 43.Brown JR, Jacobs JP, Alam SS, et al. Utility of biomarkers to improve prediction of readmission or mortality after cardiac surgery. Ann Thorac Surg 2018;106:1294–301. 10.1016/j.athoracsur.2018.06.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tam DY, Fang J, Tran A, et al. A clinical risk scoring tool to predict readmission after cardiac surgery: an Ontario administrative and clinical population database study. Can J Cardiol 2018;34:1655–64. 10.1016/j.cjca.2018.09.004 [DOI] [PubMed] [Google Scholar]
- 45.Khera S, Kolte D, Deo S, et al. Derivation and external validation of a simple risk tool to predict 30-day hospital readmissions after transcatheter aortic valve replacement. EuroIntervention 2019;15:155–63. 10.4244/EIJ-D-18-00954 [DOI] [PubMed] [Google Scholar]
- 46.Asche CV, Ren J, Kirkness CS. A prediction model to identify acute myocardial infarction (AMI) patients at risk for 30-day readmission. SCSC 2016;1:1–8https://dl.acm.org/doi/ 10.5555/3015574.3015575 [DOI] [Google Scholar]
- 47.Atzema CL, Dorian P, Fang J, et al. A clinical decision instrument to predict 30-day death and cardiovascular hospitalizations after an emergency department visit for atrial fibrillation: the atrial fibrillation in the emergency room, part 2 (AFTER2) study. Am Heart J 2018;203:85–92. 10.1016/j.ahj.2018.06.005 [DOI] [PubMed] [Google Scholar]
- 48.Huynh Q, Negishi K, De Pasquale CG, et al. Validation of predictive score of 30-day hospital readmission or death in patients with heart failure. Am J Cardiol 2018;121:322–9. 10.1016/j.amjcard.2017.10.031 [DOI] [PubMed] [Google Scholar]
- 49.Li L, Baek J, Jesdale BM, et al. Predicting 30-day mortality and 30-day re-hospitalization risks in Medicare patients with heart failure discharged to skilled nursing facilities: development and validation of models using administrative data. J Nurs Home Res Sci 2019;5:60–7. [PMC free article] [PubMed] [Google Scholar]
- 50.Sanchez CE, Hermiller JB, Pinto DS, Jr PDS, et al. Predictors and risk calculator of early unplanned hospital readmission following contemporary self-expanding transcatheter aortic valve replacement from the STS/ACC TVT registry. Cardiovasc Revasc Med 2020;21:263–70. 10.1016/j.carrev.2019.05.032 [DOI] [PubMed] [Google Scholar]
- 51.Jordan K, Moons K. Electronic healthcare records and prognosis research. In: Prognosis research in healthcare. concepts, methods and impact. Oxford: Oxford University press, 2019. [Google Scholar]
- 52.Wasfy JH, Rosenfield K, Zelevinsky K, et al. A prediction model to identify patients at high risk for 30-day readmission after percutaneous coronary intervention. Circ Cardiovasc Qual Outcomes 2013;6:429–35. 10.1161/CIRCOUTCOMES.111.000093 [DOI] [PubMed] [Google Scholar]
- 53.Kitamura M, Izawa KP, Taniue H, et al. Relationship between activities of daily living and readmission within 90 days in hospitalized elderly patients with heart failure. Biomed Res Int 2017;2017:7420738. 10.1155/2017/7420738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferraris VA, Ferraris SP, Harmon RC, et al. Risk factors for early Hospital readmission after cardiac operations. J Thorac Cardiovasc Surg 2001;122:278–86. 10.1067/mtc.2001.114776 [DOI] [PubMed] [Google Scholar]
- 55.Engoren M, Habib RH, Dooner JJ, et al. Use of genetic programming, logistic regression, and artificial neural nets to predict readmission after coronary artery bypass surgery. J Clin Monit Comput 2013;27:455–64. 10.1007/s10877-013-9444-7 [DOI] [PubMed] [Google Scholar]
- 56.et alCollins G, Reitsma J, Altman D. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement, 2020. Available: https://www.equator-network.org/reporting-guidelines/tripod-statement/ [Accessed 20 Aug 2020]. [DOI] [PubMed]
- 57.Moretti C, D'Ascenzo F, Omedè P, et al. Thirty-day readmission rates after PCI in a metropolitan center in Europe: incidence and impact on prognosis. J Cardiovasc Med 2015;16:238–45. 10.2459/JCM.0000000000000136 [DOI] [PubMed] [Google Scholar]
- 58.Cediel G, Sandoval Y, Sexter A, et al. Risk estimation in type 2 myocardial infarction and myocardial injury: the tarraco risk score. Am J Med 2019;132:217–26. 10.1016/j.amjmed.2018.10.022 [DOI] [PubMed] [Google Scholar]
- 59.Hilbert JP, Zasadil S, Keyser DJ, et al. Using decision trees to manage Hospital readmission risk for acute myocardial infarction, heart failure, and pneumonia. Appl Health Econ Health Policy 2014;12:573–85. 10.1007/s40258-014-0124-7 [DOI] [PubMed] [Google Scholar]
- 60.Kini V, Peterson PN, Spertus JA, et al. Clinical model to predict 90-day risk of readmission after acute myocardial infarction. Circ Cardiovasc Qual Outcomes 2018;11:e004788. 10.1161/CIRCOUTCOMES.118.004788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Benuzillo J, Caine W, Evans RS, et al. Predicting readmission risk shortly after admission for CABG surgery. J Card Surg 2018;33:163–70. 10.1111/jocs.13565 [DOI] [PubMed] [Google Scholar]
- 62.Deo SV, Raza S, Altarabsheh SE, et al. Risk calculator to predict 30-day readmission after coronary artery bypass: a strategic decision support tool. Heart Lung Circ 2019;28:1896–903. 10.1016/j.hlc.2018.11.007 [DOI] [PubMed] [Google Scholar]
- 63.Lancey R, Kurlansky P, Argenziano M, et al. Uniform standards do not apply to readmission following coronary artery bypass surgery: a multi-institutional study. J Thorac Cardiovasc Surg 2015;149:e1:850–7. 10.1016/j.jtcvs.2014.08.059 [DOI] [PubMed] [Google Scholar]
- 64.Zitser-Gurevich Y, Simchen E, Galai N, et al. Prediction of readmissions after CABG using detailed follow-up data: the Israeli CABG study (ISCAB). Med Care 1999;37:625–36. 10.1097/00005650-199907000-00002 [DOI] [PubMed] [Google Scholar]
- 65.Bardhan I, Oh J, Zhiqiang Z, et al. Predictive analytics for readmission of patients with congestive heart failure. Information Systems Research 2015;26:19–39. [Google Scholar]
- 66.Betihavas V, Frost SA, Newton PJ, et al. An absolute risk prediction model to determine unplanned cardiovascular readmissions for adults with chronic heart failure. Heart Lung Circ 2015;24:1068–73. 10.1016/j.hlc.2015.04.168 [DOI] [PubMed] [Google Scholar]
- 67.Delgado JF, Ferrero Gregori A, Fernández LM, et al. Patient-associated predictors of 15- and 30-day readmission after hospitalization for acute heart failure. Curr Heart Fail Rep 2019;16:304–14. 10.1007/s11897-019-00442-1 [DOI] [PubMed] [Google Scholar]
- 68.Leong KTG, Wong LY, Aung KCY, et al. Risk stratification model for 30-day heart failure readmission in a multiethnic South East Asian community. Am J Cardiol 2017;119:1428–32. 10.1016/j.amjcard.2017.01.026 [DOI] [PubMed] [Google Scholar]
- 69.Lim N-K, Lee SE, Lee H-Y, et al. Risk prediction for 30-day heart failure-specific readmission or death after discharge: data from the Korean acute heart failure (KorAHF) registry. J Cardiol 2019;73:108–13. 10.1016/j.jjcc.2018.07.009 [DOI] [PubMed] [Google Scholar]
- 70.Salah K, Kok WE, Eurlings LW, et al. A novel discharge risk model for patients hospitalised for acute decompeNsated heart failure incorporating N-terminal pro-B-type natriuretic peptide levels: a European coLlaboration on acute decompensated heart failure: ELAN-HF score. Heart 2014;100:115–25. 10.1136/heartjnl-2013-303632 [DOI] [PubMed] [Google Scholar]
- 71.Tan B-Y, Gu J-Y, Wei H-Y, et al. Electronic medical record-based model to predict the risk of 90-day readmission for patients with heart failure. BMC Med Inform Decis Mak 2019;19:193. 10.1186/s12911-019-0915-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang LE, Shaw PA, Mathelier HM, et al. Evaluating risk-prediction models using data from electronic health records. Ann Appl Stat 2016;10:286–304. 10.1214/15-AOAS891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Disdier Moulder MP, Larock JM, Garofoli A, et al. Family help with medication management: a predictive marker for early readmission. Mayo Clin Proc Innov Qual Outcomes 2017;1:211–8. 10.1016/j.mayocpiqo.2017.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Minges KE, Herrin J, Fiorilli PN, et al. Development and validation of a simple risk score to predict 30-day readmission after percutaneous coronary intervention in a cohort of medicare patients. Catheter Cardiovasc Interv 2017;89:955–63. 10.1002/ccd.26701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pack QR, Priya A, Lagu T, et al. Development and validation of a predictive model for short- and medium-term hospital readmission following heart valve surgery. J Am Heart Assoc 2016;5:e003544. 10.1161/JAHA.116.003544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oliver-McNeil S, Templin TN, Haines DE. Preoperative ICD risk score variables predict 30-day readmission after implantable cardioverter defibrillator implantation in patients with heart failure. Heart Lung 2016;45:29–33. 10.1016/j.hrtlng.2015.09.006 [DOI] [PubMed] [Google Scholar]
- 77.Barnett SD, Sarin E, Kiser AC, et al. Examination of a proposed 30-day readmission risk score on discharge location and cost. Ann Thorac Surg 2020;109:1797–803. 10.1016/j.athoracsur.2019.09.048 [DOI] [PubMed] [Google Scholar]
- 78.Espinoza J, Camporrontondo M, Vrancic M, et al. 30-day readmission score after cardiac surgery. Clin Trials Regul Sci Cardiol 2016;20:1–5. 10.1016/j.ctrsc.2016.05.006 [DOI] [Google Scholar]
- 79.Kilic A, Magruder JT, Grimm JC, et al. Development and validation of a score to predict the risk of readmission after adult cardiac operations. Ann Thorac Surg 2017;103:66–73. 10.1016/j.athoracsur.2016.05.107 [DOI] [PubMed] [Google Scholar]
- 80.Stuebe J, Rydingsward J, Lander H, et al. A pragmatic preoperative prediction score for nonhome discharge after cardiac operations. Ann Thorac Surg 2018;105:1384–91. 10.1016/j.athoracsur.2017.11.060 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-047576supp001.pdf (932KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.