Abstract
Background
Small and nutritionally at-risk infants under 6 months (<6m) are a vulnerable group at increased risk of mortality, morbidity, poor growth and sub-optimal development. Current national and international (World Health Organization) management guidelines focus mainly on infants’ needs, yet growing evidence suggests that maternal factors also influence infant outcomes. We aimed to inform future guidelines by exploring the impacts of maternal-focused interventions on infant feeding and growth.
Methods
We conducted a systematic review of reviews published since 2008 (PROSPERO, register number CRD 42019141724). We explored five databases and a wide variety of maternal-focused interventions based in low- and middle-income countries. Infant outcomes of interest included anthropometric status, birthweight, infant mortality, breastfeeding and complementary feeding practices. Given heterogenous interventions, we present a narrative synthesis of the extracted data.
Results
We included a total of 55 systematic reviews. Numerous maternal interventions were effective in improving infant growth or feeding outcomes. These included breastfeeding promotion, education, support and counselling interventions. Maternal mental health, while under-researched, showed potential to positively impact infant growth. There was also some evidence for a positive impact of: women’s empowerment, m-health technologies, conditional cash transfers, water, sanitation and hygiene and agricultural interventions. Effectiveness was increased when implemented as part of a multi-sectoral program. Antenatal supplementation with macronutrient, multiple micronutrients, Vitamin D, zinc, iron folic acid and possibly calcium, iodine and B12 in deficient women, improved birth outcomes. In contrast, evidence for postnatal supplementation was limited as was evidence directly focusing on small and nutritionally at-risk infants; most reviews focused on the prevention of growth faltering.
Conclusion
Our findings suggest sufficient evidence to justify greater inclusion of mothers in more holistic packages of care for small and nutritionally at-risk infants aged <6m. Context specific approaches are likely needed to support mother-infant dyads and ensure infants survive and thrive.
Introduction
Malnutrition remains a major global health problem, with approximately 47 million children under five years wasted, and 149 million children still stunted in 2020 [1]. Undernutrition is an underlying cause of 45% of childhood mortality [2]. Recently, there has been an increasing awareness that infants aged under six months (<6m) make up a sizable proportion of wasted children [3], with some 3.8 million infants <6m severely wasted and 4.7 million moderately wasted [4]. An even greater number of infants are “small or nutritionally at-risk” [5]. This term includes infants who are at increased risk of morbidity, mortality or suboptimal development due to any of the following: low birth weight (LBW) due to intra-uterine growth retardation and/or prematurity; anthropometric deficit as used in standard case definitions of undernutrition: low weight-for-age, weight-for-length or length-for-age; postnatal growth faltering even if above the usual -2 Z-score criteria for undernutrition.
Short term risks include infection and mortality which are higher among this age group than in older children [6, 7]. Poor early-life nutrition has also been linked with adverse long-term outcomes, such as impaired cognitive development and higher risk of non-communicable diseases later in life [8–12].
Despite being so vulnerable, the management of small and nutritionally at-risk infants <6m represents a critical care gap, as available evidence often does not address them directly [13]. In 2013, the World Health Organization Guidelines for the Management of Severe Acute Malnutrition included, for the first time, a chapter focused just on infants <6m, however recommendations were based on limited and low quality evidence [14]. Many of the recommended management interventions focused on the clinical and nutritional needs of the infant, yet growing evidence shows a wide range of underlying causes of nutritional vulnerability [15], many of which could be better addressed through maternal-focused interventions, and management of a mother-infant dyad [16–20].
Maternal factors including poor antenatal and postnatal nutrition are well documented as being associated with adverse neonatal and infant outcomes [2, 21–23]. Evidence also suggests that maternal social, environmental, and other physical and mental health factors all also contribute to infant health and growth [16, 18–20, 24]. What is missing is a collation of this evidence to help understand what might best improve future care for small and nutritionally at-risk infants <6m. In this review we thus aim to collate available evidence from existing literature reviews on the impacts of maternal-focused interventions on infant feeding and growth in low- and middle-income countries (LMICs). This will help to inform future research and policymaking.
Methods
To capture as wide as possible a range of interventions, we conducted a systematic review of reviews. We focused on interventions that impact the immediate and underlying causes of undernutrition as depicted by the UNICEF Conceptual Framework on the Determinants of Maternal and Child Nutrition [25]. We registered our work on the International prospective register of systematic reviews (PROSPERO, register number CRD 42019141724), which we later expanded to also include a search of maternal macro- and micronutrient supplementation (MEDLINE and Cochrane databases only).
Search strategy
We searched five different databases: MEDLINE, EMBASE, Global Health, Cochrane Library and CINALH plus. We considered reviews published since 2008 (the last 10 years since this project was originally started). The most recent search took place on 29th of October 2020. Reference lists of eligible reviews and personal communications with relevant authors were additional search sources.
Search concepts based around the below PICO were developed for each database. Eligible studies were systematic reviews that included the following:
Population (P): Studies targeting mothers, pregnant women, or women of reproductive age.
Intervention (I): A wide range of intervention types was considered, broadly categorised as: education and counselling; hydration; water, sanitation and hygiene; women’s empowerment; relaxation therapies; multi-sectoral interventions; m-health (i.e. those that use mobile or wireless technologies); conditional cash transfers; agriculture; maternal supplementation during pregnancy; and maternal supplementation during lactation.
Control (C): Though reviews of randomised controlled trials were ideal, we also included reviews which included observational and uncontrolled original studies.
Outcomes (O): We included any review documenting infant growth or nutritional status in the first six months of life, including those aimed at preventing deterioration.
Search terms included: "pregnant women” "mothers", "nutrition- sensitive interventions” "nutrition-specific interventions", "dietary supplementation", "nutritional status", "malnutrition", "feeding practices", "infant mortality", "infant growth", "breastfeeding promotion” "relaxation therapy", and "mental health" (S1 Table).
Search results were limited to human studies from LMICs as defined by the World Bank [26], published since 2008, and with full texts available in English language.
Search results were exported to Endnote software X9 (Clarivate Analytics, Philadelphia, USA) where duplicates were removed. Two researchers independently screening titles and abstracts followed by full texts. Where an updated version of an existing review was available, only the most recent version was included.
Data extraction and quality assessment
We used a structured form to extract information from each article, including the author, type of publication, type of intervention, setting of the intervention, outcome measures and the reported effectiveness of the interventions. A meta-analysis was not performed due to the diverse nature of the interventions and outcomes reported, instead data was synthesised in a narrative form. Two methods were used to assess the quality of the evidence: 1) the quality of the review methodology and reporting, and 2) the quality of the evidence available, as assessed by the review authors. In order to rank the quality of the review methodology (1), we used the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) Working Group approach according to British Medical Journal Best Practice [27].
Results
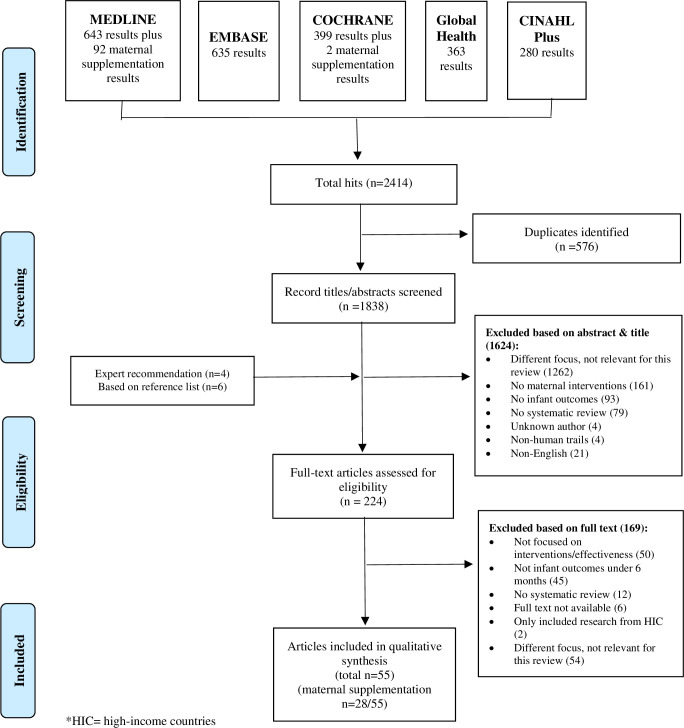
The initial search revealed a total of 2320 reviews, with an additional 94 returned by our search specifically on maternal supplementation (Fig 1). We reviewed 224 full texts (214 from the database searches, four recommended by experts via personal communication, and six identified via reference lists) and ultimately identified 55 relevant systematic reviews for inclusion. While all reviews captured studies from LMICs, as per our inclusion criteria, 34 (61%) reviews also incorporated studies from high-income countries (HICs). The studies within the reviews took place across Africa, Asia, The Americas, Europe and Australasia; Asia was the region with the most studies represented. The majority of the evidence related to the prevention of growth faltering, rather than management of existing growth faltering or existing infant undernutrition. Related to this, most studies were aimed at pregnant women rather than during the postnatal period. Many of the reviews also included infants up to 24 months, not just infants <6m. Interventions took place at household-, community- and medical facility level. Three reviews focused their intervention only on adolescent girls [28–30].
Fig 1. PRISMA flow diagram of search results.
We categorised the reviews into the following types of interventions: education and counselling (n = 7); water, sanitation and hygiene (n = 3); women’s empowerment (n = 2); relaxation therapies (n = 1); multi-sectoral interventions (n = 8); m-health (i.e. those that use mobile or wireless technologies) (n = 3); conditional cash transfers (n = 2); agriculture (n = 1); maternal supplementation during pregnancy (n = 23); maternal supplementation during lactation (n = 4); and maternal supplementation during either pregnancy or lactation (n = 1).
Quality of evidence
The overall quality of the reviews was rated high according to the GRADE evaluation for the majority of reviews (S2 and S3 Tables); only eight (15%) reviews were rated moderate or low. Forty-one (75%) reviews included a meta-analysis. The quality of the studies included in the reviews, however, varied greatly. Most, 48(87%) of the reviews incorporated a structured quality assessment of the primary studies. Low quality evidence was mainly attributed to small sample size, high heterogeneity among study participants, inadequate randomisation, and insufficient blinding in the study set up.
Maternal-focused interventions
Education, counselling and peer support
Breastfeeding education, counselling and peer support. Three reviews considered the impact of breastfeeding education and counselling interventions. Given the known benefits of breastfeeding for infant health and survival [31], providing breastfeeding support should be a key management and prevention strategy for small and nutritionally at-risk infants <6m. However, Shakya et al. (2017) did not find a sufficient number of eligible studies to assess the effect of community-based peer support on infant nutritional status [32]. Giugliani et al. (2015) and Lassie et al. (2020) also did not find an impact on infant growth outcomes [33, 34]. However, studies largely consisted of healthy infants in HICs, whereas some of the trials that focused on providing breastfeeding education for mothers of LBW infants did find an increase in weight and length [32–34]. Besides growth outcomes, several studies found counselling increased exclusive breastfeeding (EBF) and early initiation of breastfeeding [32–34].
Other education, counselling and peer support. Twelve reviews evaluated nutritional interventions containing an educational component focusing on topics beyond breastfeeding practices for pregnant women and/or breastfeeding women [28, 34–44]. Five reviews analysed just counselling and education interventions, while seven included counselling/education alongside other interventions e.g. m-health, micronutrient powders, and kangaroo mother care. Most education interventions focusing on the appropriate introduction of complementary feeding were associated with an increase in infants’ weight and length [34, 38] (Table 1). A Cochrane review concluded that complementary feeding education may reduce the risk of growth faltering for term-born infants, but modest effects may not be clinically significant, and long-term effects are uncertain [38].
Table 1. Summary of maternal non-supplementation interventions during lactation and pregnancy-reviews.
| Study | Intervention | Outcome measures | Summary of findings (separated into prevention and management) | Review quality | Reported quality of primary evidence** |
|---|---|---|---|---|---|
| Education and counselling and support groups | |||||
| Giugliani et al. (2015) [33]. | Breastfeeding promotion interventions | Weight, length/height z scores, body mass index, WHZ | Prevention**: Non-significant increase in mean weight z score (MD 0.03, -0.06 to 0.12), and mean length/height z scores (MD 0.03, 95% CI: -0.02 to 0.08); mean weight z score, negative effect in low income countries (MD -0.11, CI -0.20 to -0.02), but non-significant positive effect in middle income countries (MD 0.11, CI -0.01 to 0.22). | High-moderate | High-quality evidence (26%); very low/low risk of bias (46%); high risk of bias (29%) |
| Management***: RCT for mothers with LBW infants–Increase weight (3620±229 g vs 3315±301 g, P<0.001) and length (50.2±1.3 cm vs 48.7±1.6 cm, P<0.001) at 30-, 45- and 60-days vs control. | |||||
| Shakya et al. (2017) [32] | Breastfeeding support: Community based one-on-one and/or group peer support | Underweight, stunting, wasting, child feeding practices (birth-6 months) | Prevention: Insufficient number of studies to analyse impact on child nutritional status or feeding outcomes; focus on EBF initiation, EBF at 3 months. | High | 41 RCTs/quasi-experimental: high risk of bias for blinding participants, personal and outcome assessment |
| Management: An RCT found low-weight babies and malnourished children (under 5 years) who were receiving home visits by mentor mothers were rehabilitated in shorter periods of time compared to those in the control group. | |||||
| 6 observational studies: 30% of studies had high risk for incomplete data, blinding of assessment and measurement of exposure and selective reporting | |||||
| Lassi et al. (2020) [34] | Breastfeeding and complementary feeding education | EIBF, EBF, HAZ, WAZ, WHZ, neonatal mortality, infant mortality | Prevention: Breastfeeding education—Positive impact on early initiation (RR 1.20, CI 1.12 to 1.28); no impact on HAZ (MD 0.10, CI -0.04 to 0.25), WAZ (MD -0.04, CI -0.12 to 0.05), WHZ (MD 0.01, CI -0.07 to 0.09), neonatal mortality (RR 1.10, CI 0.90 to 1.24) or infant mortality (RR 0.86, CI 0.73 to 1.02). | High | Overall: very low (13.3%), low (50%), moderate (23.3%) and high (13.3%) quality |
| Breastfeeding: very low-low quality | |||||
| Complementary feeding education–Food secure settings impact on WAZ (MD 0.41, CI 0.07 to 0.75) and HAZ (MD 0.29, CI 0.04 to 0.54) and in food insecure impact on WAZ (MD 0.47, CI 0.35 to 0.59), HAZ (MD 0.25, CI 0.09 to 0.41) and WHZ (MD 0.50, CI 0.35 to 0.65). | |||||
| Management: Breastfeeding education– 2 studies reported on LBW infant outcomes, one study found an increase in weight (3620±229 g vs 3315±301 g, P<0.001) and length after 2 months (50.2±1.3 cm vs 48.7±1.6 cm, P<0.001), the other found no effect on infant weight, length, head circumference at 6 months. | |||||
| Jonmahamed et al. (2020) [35] | Community home visits and mother/peer groups promoting child health, nutrition and feeding practices. | EIBF, EBF, minimum dietary diversity, minimum meal frequency, stunting, underweight and wasting | Prevention: Meta-analysis of RCTs of home visits increased odds of EIBF (OR 1.5, CI 1.12 to 1.99) and EBF (OR 4.42, CI 2.28 to 8.56). Mother/peer groups had no significant effect on EIBF and EBF. Combined intervention increased odds of EIBF (OR 2.13, CI 1.12 to 4.05) EBF (OR 2.43, CI 1.70 to 3.46) and wasting (OR 0.77, CI 0.67 to 0.89), but no effect on stunting and underweight. | High | Overall: very low (17.6%), low (58.8%), moderate (17.6%) and high (5.9%) quality |
| Arikpo.et al. (2018) [36] | Educational intervention on the introduction of complementary feeding | Weight, height/length, MUAC, WAZ, height/length age z score, weight for height/length z score, morbidity, mortality | Prevention: Little evidence for growth patterns—at 6 months non-significant increased weight 0.03 kg (CI -0.10 to 0.17 higher) and mean height/length 0.16 cm (CI: -0.21 to 0.52); no data on infant mortality; Improvement of complementary feeding; only community intervention increased duration of EBF (RR 2.32, CI 1.45 to 3.73). | High | Overall: moderate to very low quality |
| Lassi et al. (2019) [37] | Community-based health education care packages | Neonatal mortality, perinatal mortality, neonatal infections, and health behaviours. | Prevention: Reduced overall neonatal mortality (RR 0.87, CI 0.78 to 0.96), early neonatal mortality (RR 0.74, CI 0.66–0.84), late neonatal mortality (RR 0.54, CI 0.40 to 0.74) and perinatal mortality (RR 0.83, CI 0.75 to 0.91); positive effect timely initiation of breastfeeding (RR 1.56, CI 1.37 to 1.77). | High | Overall: very low-low |
| Ojha et al. (2020) [38] | Nutrition education interventions for infant weaning | WAZ, HAZ, child growth and neuro-development | Increased WAZ (MD 0.15, CI 0.07 to 0.22) and HAZ (MD 0.12, CI 0.05 to 0.19) by 12 months of age. Uncertain effects on long-term growth and neuro-development outcomes. | High | Overall: low-moderate |
| WASH | |||||
| Dangour et al. (2013) [45] | Water intervention, coverage / use sanitation facilities, handwashing w/soap | WHZ, WAZ, or HAZ | Prevention: No effect on WAZ (MD 0.05, 95% CI -0.01 to 0.12), WHZ (MD 0.02, 95% CI -0.07 to 0.11). Small effect HAZ (MD 0.08, 95% CI 0.00 to 0.16). | High | Overall: low quality |
| Gera et al. (2018) [46] | Hygiene promotion, education, water intervention, sanitation improvement | Weight, height, WHZ, MUAC, prevalence malnutrition, non-diarrheal morbidity, infant mortality | Prevention: Overall—Lower risk of underweight (RR 0.81, 95% CI 0.69 to 0.96), stunting (RR 0.77, 95% CI 0.68 to 0.86), wasting (RR 0.12, CI 0.02 to 0.85). Reduced mortality (RR 0.65, 95% CI 0.25 to 1.70). | Moderate | Majority very low quality |
| Improvement in water supply/quality—decreased risk of stunting by 13% (RR 0.87, 95% CI 0.81 to 0.94), no effect WAZ (MD 0.03, 95% CI 0.00 to 0.06), reduced mortality (RR 0.45, 95% CI 0.25 to 0.81). | |||||
| combined WASH—improved HAZ (MD 0.22, 95% CI 0.12 to 0.32). | |||||
| Gizaw et al. (2019) [47] | Water treatment, hand washing, hygiene education, home based water treatment, latrine construction/promotion | Child nutritional status, HAZ | Prevention: Increased mean HAZ (SMD 0.14, 95% CI = 0.09 to 0.19), better effect of combined interventions on child growth (SMD 0 0.15, 95% CI = 0.09 to 0.20), pooled effect mean HAZ (SMD 0.19, CI 0.15 to 0.23). | High | Most studies had low risk of bias |
| Women’s empowerment | |||||
| Kraft et al. (2014) [29] | Gender accommodating-/ transformative, Behaviour Change Communication | Infant mortality, stunting | Prevention: Reduction stunting among children > 24 months, nutrition and empowerment combination most effective together. | Moderate-low | Not formally assessed, only included studies with moderate-to-strong evaluation designs |
| Taukobong et al. (2016) [48] | Gender empowerment, family planning, health, nutrition, agriculture, WASH, financial services | Stunting, wasting, underweight, body mass index, MUAC, length/age weight/age, weight/length | Prevention: Higher levels of decision-making power improved nutritional status in children + reduced risk of stunting. | Moderate | Not formally assessed, reported that strength of design and analyses varied; limited RCT’s and quasi-experimental studies |
| Relaxation therapy | |||||
| Shukri et al. (2018) [49] | Relaxation therapy | Infant dietary intake, weight gain, body mass index, breastfeeding (breast milk volume/ milk yield) | Prevention: No studies reported on infant growth/behaviours; reduction on maternal stress, evidence for increasing milk yield and fat content. | High-moderate | All studies had limitations regarding design or sample collection procedures–(2/5) not randomised, studies unable to blind participates, many had small sample sizes, did not consider all potential confounders |
| Management: Strongest evidence from increasing milk yields, and possible fat content in mothers of preterm infants. | |||||
| Multisectoral interventions | |||||
| Bhutta et al. (2013) [28] | MMN, folic acid, iron/iron folic acid, calcium, iodine, vitamin D, zinc and balanced energy and protein supplementation, nutrition education, WASH interventions, deworming, IPTp/ITN for malaria in pregnancy, mental health support. | Growth, stunting wasting, mortality, breastfeeding, complementary feeding practices (6–24 months) | Prevention: MMN—significant effects on LBW (RR 0.88, 95% CI 0.85 to 0.91), SGA (RR 0.89, 95% CI 0.83 to 0.96), preterm birth (RR 0.97, 95% CI 0.94 to 0.99). | High-moderate | Not reported, however, only captured reviews with good quality methods for all interventions. |
| Folic acid—mean birth weight (MD 135.75g, CI 47.85–223.68g). | |||||
| Iron/iron folic acid—reduction in LBW (RR 0.81, CI 0.68 to 0.97). | |||||
| Calcium—population with low intake, increase birth weight (85g, CI 37 to 133g) and reduction in preterm birth (RR 0.97, CI 0.94 to 0.99). | |||||
| Iodine—insufficient data to draw conclusions on mortality, children physical and mental development. | |||||
| Vit D -Lack of evidence for functional pregnancy outcomes. | |||||
| Zinc—Evidence for reduction in preterm birth, but insufficient evidence for routine supplementation. | |||||
| Balanced energy/protein supplementation—reduction in SGA (RR 0.66, CI 0.49 to 0.89), still births (RR 0.62, CI 0.40 to 0.98) and small increase in birth weight (MD 73g, CI 116 to 130g). | |||||
| Nutrition education food secure populations—height gain (SMD 0.35, 95% CI 0.08 to 0.62), HAZ (SMD 0.22, 95% CI 0.01 to 0.43). Complementary feeding w/without education HAZ (SMD 0.39, 95% CI 0.05 to 0.73), education food insecure populations: HAZ (SMD 0.25, 95% CI 0.09 to 0.42), stunting (RR: 0.68, 95% CI 0.60 to 0.76). | |||||
| WASH—20% reduction open defecation—0.1‐SD increase height. | |||||
| IPTp/INT—reduction in low birth weight. | |||||
| Mental health—promising effect of cognitive behaviour theory during pregnancy on depression post-partum, no evidence effect on infant weight or linear growth. | |||||
| Goudet et al. (2019) [39] | Zinc, micronutrient and macro nutrition supplementation and nutrition education. | length-for-age/HAZ birth weight, LBW | Prevention: Zinc–no effect on new-born length (MD -0.13, CI -0.36 to 0.10), LBW (MD -36.2, CI -83.61 to 11.35). | High | Overall: Low-moderate quality |
| Micro or macro nutrient supplementation- unclear effect for length-for-age/HAZ. | |||||
| Education- Positive impact on LBW (MD 478.4g, 95% CI 423.55 to 533.32g). | |||||
| Ruel et al. (2013) [30] | Agriculture, Social safety nets, Early child development | Maternal and child nutritional status | Prevention: Community-based peer support is effective in increasing EBF duration among mothers. Targeted agriculture interventions have an important role in supporting healthy diets, but inconclusive evidence for the impact on children’s nutritional status, with the possible exception of Vit A intake and status. | Moderate | Not formally assessed, however, reported that studies often suffered from poor quality impact evaluations |
| Victora et al. (2012) [40] | Large scale iron folic acid, food supplementation/ nutrition education, counselling/ + conditional cash transfers programmes | Birth weight, maternal weight gain, caregiver knowledge, behavioural change, dietary habits, anaemia | Prevention: iron folic acid: Positive impact on the prevalence of maternal anaemia. | Low | Not formally assessed, only large scale programs that were adequately evaluated were utilised, high risk of publication bias for grey lit |
| Food supplementation/ nutrition education + counselling: improved caregiver knowledge and behaviour change and improving utilisation of antenatal care, dietary habits, no effect on maternal weight gain, effect on birth weight unclear. | |||||
| + conditional cash transfers: some evidence for positive impact on birth weight. | |||||
| Park et al. (2019) [41] | Micronutrient supplementation, lipid-based supplementation or food supplementation, deworming, maternal education, and WASH. | Preterm birth, birth weight, length-for-age z score | Prevention: MMN reduced preterm birth (OR 0.54, CI 0.27 to 0.97), while iron, iron plus calcium, calcium plus vitamin D, zinc, iron folic acid plus vitamin A, MMN, a single dose of deworming plus iron and 118 kcal of Lipid-based nutrient supplementation, showed modest improvements in birth weight. | High | Most studies had low/unclear risk of bias, apart from performance bias, where 23.1% of studies had a high risk of bias due participants not being blinded to intervention group |
| No intervention for mothers with infants 0-6months led to statistically significant improvements in length-for-age z score. | |||||
| Park et al. (2020) [42] | MMN, iron folic acid, Vit D lipid based nutrient supplements, deworming, WASH, food provision and maternal education | Length-for-age z score | Prevention: No statistically significant effect of MMN, Vit D, iron folic acid supplementation for lactating mothers, food supplements, maternal education, deworming or WASH on length-for-age z score. | High | Majority of studies had low/unclear risk of bias, but high risk of performance bias (34% high risk) due to lack of blinding participants |
| Ota et al. (2015) [43] | Nutrition education and balanced energy and protein supplementation | Preterm, birth weight, neonatal death, child growth, still birth, neurological development | Prevention: Nutritional education—Decrease risk preterm (RR 0.46, 95% CI 0.21 to 0.98), LBW (RR 0.04, CI 0.01 to 0.14), increased birth weight undernourished women (MD 489.76 g, CI 427.9- to 551.6). | High | Majority low-moderate quality |
| Balanced energy and protein supplementation–positive impact on stillbirths (RR 0.60, CI 0.39 to 0.94), mean birth weight (MD 40g, CI 4.66 to 77.26), and SGA (RR 0.79, CI 0.69 to 0.90), no effect on preterm birth. | |||||
| High protein supplementation—increased risk of SGA (RR 1.58, CI 1.03 to 2.41). | |||||
| East et al. (2019) [44] | Social support via home visits, antenatal clinic visits, telephone or combination for women at increased risk of LBW babies | Birth weight, stillbirth/neonatal death, gestational age, infant morbidity, postnatal depression | Prevention: Overall slight positive impact on postnatal depression, may have a slight impact on birth weight (RR 0.94, CI 0.86–1.04) and gestational age (RR 0.92, CI 0.84 to 1.01); no impact on stillbirth/neonatal death; positive impact on caesarean sections (RR 0.90, CI 0.83–0.97) and number of antenatal hospital admissions (RR 0.78, CI 0.68 to 0.91). | High | Overall: moderate quality |
| m-Health | |||||
| Saronga et al. (2019) [50] | Interventions using mobile Health technologies | Birth weight, growth, breastfeeding initiation/duration; pregnancy weight gain, maternal knowledge | Prevention: LBW vs control group (30% vs. 35%); No other infant nutritional outcomes reported. Maternal weight gain improved. | High-moderate | Only 4 studies captured but all studies were positive/neutral quality |
| Palmer et al. (2020) [51] | Targeted client communication via mobile phones. | Health behaviour change, service utilisation e.g. antenatal care, maternal morbidity/mortality, maternal morbidity (mental), neonatal health, partner violence, well-being, acceptability. | Prevention: In settings where breastfeeding is less common, women receiving targeted client communication via mobile may breastfeed more, when compared to standard care. | High | Overall: very low-low quality |
| Targeted client communication via mobile devices effect on women’s and infant’s morbidity and mortality when compared to standard care is unclear as evidence is of very low certainty. | |||||
| Sondaal et al. (2016) [52] | Unidirectional text/voice messaging, direct two-way communication, multidirectional text messages, unidirectional telephone counselling | Maternal and neonatal service utilisation, maternal health, mortality and morbidity, perinatal mortality, maternal knowledge. | Prevention: No consistent effects of mHealth interventions on maternal and neonatal health outcomes were observed; however, single studies found positive trends in reducing perinatal mortality and improving breastfeeding practices. | High-moderate | Overall: moderate quality |
| Inconclusive findings were observed for mHealth effects on maternal knowledge but positive effects on service utilisation. | |||||
| Conditional cash transfers | |||||
| Leroy et al. (2009) [53] | Conditional cash transfers with or without micronutrient- fortified food, health nutritional education | Height, HAZ, weight, weight-for length, WAZ, child micronutrient status | Prevention: Small effect on the elimination of linear growth retardation, small impact on micronutrient nutrition status. | Moderate-low | Not formally assessed, however reported low publication bias |
| Lagarde et al. (2009) [54] | Conditional cash transfers, to households +/- nutrition supplementation | Weight, growth, height, HAZ, WAZ children <24 months, stunting, underweight | Prevention: Increase in height of 1cm amongst children < 4 y; impact on HAZ mixed results (significant increase while another one found a negative impact); Decrease probability of being stunted, underweight or chronically malnourished. | High | Moderate quality for all outcome apart from health service utilisation (low quality) |
| Agriculture | |||||
| Masset et al. (2012) [55] | Bio-fortification, home gardens, small scale fisheries/ aquaculture, dairy development | Height-for-age, weight-for-age, weight-for-height, prevalence stunting, wasting, underweight, micronutrient intake | Prevention: Out of 8 studies, only one found a small but statistically significant effect on stunting; however, out of seven studies, three and two studies found a positive statistically significant effect on underweight and wasting, respectively. | Moderate | Studies were not of high quality, high overall risk of bias; bias introduced due to the low number of RCT’s, high selection bias, small samples sizes, lack of power calculations and outcomes measurements. |
Height-for-age z score (HAZ); weight-for-age z score (WAZ); mid-upper arm circumference (MUAC); small for gestational age (SGA); Mean difference (MD); Relative risk (RR); low birth weight (LBW); Randomised control trials (RCTs); multiple micronutrients (MMN); Water sanitation and hygiene (WASH); intermittent prevention treatment in pregnancy/insecticide treated bed nets (IPTp/ITN); weight-for-height z scores (WHZ); Early initiation of breastfeeding (EIBF); Exclusive breastfeeding (EBF): Odds ratio (OR); Standard mean difference (SMD).
*Quality reported in the respective reviews
** Prevention of growth faltering in infants <6m
***Management of small and nutritionally at-risk infants <6m.
Community health worker home visits combined with mother/peer support groups showed a positive effect on reducing the risk of wasting, but no effect on stunting or underweight [35, 42]. Reviews of antenatal nutrition education programs have found mixed effects, with some suggesting they can improve infant birth weight and reduce risk of preterm birth, but others showed no or limited effect [39–44]. Community health education programmes were also shown to reduce neonatal mortality, especially when provided during both antenatal and postnatal periods, and when additional family members are incorporated [37]. However, a Cochrane review of social support programs (home visit, regular antenatal care) for mothers at increased risk of LBW babies concluded that they are unlikely to have a large impact on the proportion of LBW and preterm births [44].
Water, sanitation and hygiene (WASH)
The effectiveness of WASH interventions on infant outcomes are mixed [28, 41, 42, 45–47]. A Cochrane review including 14 studies found that an increase in height was more responsive to WASH interventions in children under 24 months of age, whereas increase in weight was more responsive in children 25‐60 months of age [45]. Overall, combined WASH interventions were found to be more effective than single interventions in improving infant outcomes [45–47]. Only a small selection of possible WASH interventions was applied within the studies, with poor quality assessment and relatively limited duration [45–47].
Women’s empowerment
Women’s empowerment has been hypothesised to improve children’s nutritional outcomes by enabling women to use and realign resources and household practices to enhance their children’s nutritional well-being. Related interventions were assessed in two different reviews [29, 48]. The three most important gender-related levers were identified as being: control over income/assets/resources, decision-making power and education [48]. Higher levels of decision-making power were associated with improved nutritional status in children and a reduced risk of stunting [48]. Interventions targeting both men and women, however, showed mixed results on effectiveness, while adolescent- specific interventions had no effect on infant health outcomes [29].
Relaxation therapy
There are a small but growing number of studies on relaxation therapy, the core idea being that relaxation (via a range of options including music and guided breathing exercises) could be an easy, quick and deliverable intervention with a clear and direct biological pathway to improved infant growth via increased breastmilk supply. One systematic review was identified. Though it found no studies reporting on infant outcomes, it did find that maternal relaxation was associated with reduced maternal stress, increased milk yield and milk fat levels, especially in mothers with preterm infants [49].
m-Health
Three reviews focused on m-health interventions [50–52]. One found that LBW was less common among women in the intervention group (short message service, audio voice messages or a combination) compared with the control group [50]. M-Health was also found to be useful in encouraging consumption of micronutrient supplements in pregnancy [50]. Targeted messaging via mobile devices was found to marginally improve breastfeeding rates, but had little or no effect when compared to non-digital strategies [51]. Few studies within the reviews presented growth and mortality outcomes, but one study, which combined educational text messages and two-way voice calls found a significant reduction in perinatal mortality [52].
Conditional cash transfer programs
Three reviews assessed the impact of conditional cash transfers on infant outcomes. However, these reviews were mostly based on programs implemented in Latin and Central America and referred to the same study populations [40, 53, 54]. The programs generally combined basic cash transfer with health and nutrition education [53]. An overall positive effect of conditional cash transfers programs on the risk of stunting and underweight was reported [54] alongside a possible impact on birth weight [40]. There was a greater impact of conditional cash transfers on younger children (infants <6m vs children 6–48 months), children from lower socio-economic backgrounds and children with longer exposure to the intervention [53, 54]. Studies also found improvements in maternal micronutrient consumption but also consumption of high-fat foods [53, 54].
Maternal mental health
While there are strong associations between maternal mental health and infant outcomes in observational work, intervention studies are still lacking. One review included maternal mental health interventions alongside other interventions. They report that cognitive behaviour therapy during pregnancy had no impact on infant weight or growth, but a positive impact on depression post-partum [28]. A Cochrane review of social support programs for mothers at increased risk of LBW babies found a slight positive impact on postnatal depression [44].
Agriculture interventions
Agricultural interventions such as bio-fortification, home gardens, small scale fisheries and aquaculture, dairy development, animal husbandry and poultry development rarely reported the effect on child nutritional outcomes [55]. A limited amount of studies reported a positive impact on Vitamin A status, stunting, underweight and wasting [30, 55]; however, studies often lacked high-quality impact evaluations. The effect of agricultural interventions appeared to be bigger when targeting women within empowerment or educational activities to foster their knowledge and skills and increase control over income [30].
Multi-sector interventions
Given that the determinants of infant growth are multi-faceted, eight reviews summarised the evidence base for multiple interventions across sectors [28, 30, 39–44]. Many of them combined micronutrient supplementation with antenatal education or counselling, however WASH interventions, mental health and social support, agriculture and social safety nets were also captured. Scaling up of nutrition packages that include optimising infant feeding and increasing maternal dietary diversity during pregnancy may successfully reduce the risk for stunting and wasting in children under five years [28]. Implementing a range of multisectoral actions/interventions rather than focusing on one particular intervention appears to increase program effectiveness [28, 30]. However, more research is needed as interventions are still often implemented and evaluated in isolation [41]. Furthermore, more comprehensive evaluation tools are needed, to avoid dismissing an effective program due to a lack of effect on a small set of outcomes [40].
Maternal supplementation during pregnancy
Of the 28 reviews focused on maternal supplementation and infant outcomes, most, 23 (82%) review the evidence of supplementation in pregnancy (Table 2). Overall, the reviews found that food fortification and macronutrient interventions had a positive effect on birth weight and length, risk of stillbirth and small for gestational age (SGA) [28, 41, 43, 56–60] though there was no evidence of longer-term benefits for child growth and development [57]. A recent Cochrane review on lipid-based nutrient supplements in pregnancy found positive impacts on birth weight, length and SGA when compared to iron folic acid supplementation, but no benefit compared to multiple micronutrient (MMN) supplementation [61].
Table 2. Summary of maternal supplementation interventions, during lactation and pregnancy–reviews.
| Study | Intervention | Outcome measures | Outcomes article | Review quality | Reported quality of primary evidence* |
|---|---|---|---|---|---|
| Pregnancy | |||||
| Gresham et al. (2014) [56] | Food and fortified food product, macronutrient interventions, counselling plus food | Birth weight, LBW, weight, SGA, head circumference macrosomia, large for gestational age, perinatal mortality | Overall: Dietary interventions targeted at-risk populations positive impact birth weight (SMD 0.26, CI 0.09 to 0.41) and LBW (SMD -0.19, CI -0.37 to -0.02). | High | Positive (61%) and neutral (39%) quality |
| Food and fortified products: impacted birth weight (SMD 0.27 CI 0.14 to 0.40), the incidence of LBW (SMD -0.22, CI -0.37 to -0.06). | |||||
| Macronutrient interventions: impacted length (SMD 0.07, CI 0.01 to 0.14) and birth weight (SMD 0.23, CI 0.07 to 0.38) and incidence LBW (SMD -0.19, CI -0.34 to -0.04). | |||||
| Visser et al. (2018) [57] | Food distribution and supplementary feeding programmes | SGA, stillbirth, birth weight, birth length, height gain, weight gain, neonatal death | Impacted stillbirth (RR 0.60, CI 0.39 to 0.94), infant birth weight (MD 40.96, CI 4.66 to 77.26), SGA, birth length (MD 0.16 CI 0.10 to 0.31). No long-term benefits for the child in terms of growth and neurocognitive development. | High | Very low-moderate quality |
| High protein supplementation was associated with increased risk of SGA. | |||||
| Stevens et al. (2015) [58] | Balanced protein energy supplementation in undernourished women | Birth weight, birth length, head circumference, physical growth | Impacted birth weight (MD 0.20g, CI 0.03 to 0.38g). | High | Low (28.2%), moderate (43.6%) and high (28.2%) quality |
| No significant effect on birth length and head circumference. | |||||
| Impact on long-term growth is inconclusive–One RCT which showed a significant difference in height till 60 months and weight until 24 months. | |||||
| Imdad et al. (2011) [59] | Balanced protein energy supplementation | SGA, neonatal mortality, birth weight | Impact SGA (RR 0.69, CI 0.56 to 0.85), birth weight (MD 59.89g, CI 33.09 to 86.86g), effect was more pronounced in malnourished women. | High | Overall moderate quality |
| No impact on neonatal mortality. | |||||
| Pimpin et al. (2019) [60] | Protein from animal-sourced foods | Birth weight, LBW, SGA, height, and length | Impacted birth weight (MD 0.06kg, CI 0.02 to 0.11kg) and maternal weight gain. No impact LBW, SGA, height or weight in later childhood. | High | Low (34%), moderate (255) and high (41%) quality |
| Ramakrishnan et al. (2012) [62] | MMN supplementation | LWB, birth weight, SGA, gestational age, preterm birth, stillbirth, neonatal death, maternal mortality | Impacted LBW (RR 0.86, CI 0.81 to 0.91), birthweight (MD 52.6g, CI 43.18 to 62.03g), SGA RR 0.83, CI 0.73 to 0.95), gestational age (WMD 0.08 weeks, CI 0.01 to 0.14 weeks). | High | Overall moderate-high quality |
| No impact preterm, stillbirth, neonatal death, maternal mortality; increased risk of neonatal death when compared with iron–folate in the subgroup of five trials that began after the first trimester (RR 1.38, CI 1.05 to 1.81). No impact maternal mortality (RR 0.96, CI 0.64 to 1.45). | |||||
| Thorne-Lyman et al. (2012) [63] | Vitamin A and carotenoids | LBW, SGA, birth weight, weight gain in pregnancy, preterm birth, gestational age, neonatal mortality, morbidity, infant and child growth, mortality and morbidity, maternal mortality, pre-eclampsia, maternal anaemia | No impact on SGA and birth weight, preterm, stillbirth foetal death, maternal mortality, morbidity or birth complications, infants and neonatal mortality; no impact of mother-to-child HIV transmission in a pooled analysis, although some evidence suggests that it may increase transmission. | High-moderate | Overall low-moderate quality; very low (10%), low (45%), moderate (21%) and high (24%) quality |
| Impact on LBW for HIV+ populations (RR 0.79, CI 0.64 to 0.99). | |||||
| Little evidence of an effect on WAZ and HAZ. | |||||
| Improved haemoglobin levels and risk of anaemia (RR 0.81, CI 0.69 to 0.94) | |||||
| Das et al. (2018) [61] | Lipid-based nutrient supplement | Maternal anthropometric status, maternal mortality, adverse maternal outcomes, LBW, birth weight, birth length, SGA, preterm, child development, duration of gestation, stillbirths, head circumference, infant mortality, neonatal mortality, MUAC, stunting, wasting and underweight in the first 6 months. | compared iron folic acid: impacted birth weight (MD 53.28g, CI 28.22 to 78.33), birth length (MD 0.24cm, CI 0.11 to 0.36), SGA (RR 0.94, CI 0.89 to 0.99), length of gestation (MD 0.18weeks, CI 0.04 to 0.32), stunting (RR 0.82, CI 0.71 to 0.94); Insufficient data of child development and no data on wasting and infant mortality | High | Overall moderate quality |
| compared to MMN: No data stillbirth, wasting and infant mortality, no impact maternal, infant and birth outcomes. | |||||
| Middleton et al. (2018) [64] | Omega-3 fatty acids | Preterm, gestation, perinatal deaths, LBW, Large for gestational age, SGA, intrauterine growth restrictions, adverse maternal effects, child neurodevelopment and growth, adult body mass index | Positive impact on preterm birth (RR 0.89, CI 0.81 to 0.97), perinatal deaths (RR 0.75, CI 0.54 to 1.03) and LBW (RR 0.90, CI 0.82 to 0.99); Small increase large for gestational age (RR 1.15, CI 0.97 to 1.36), little/no difference in SGA or intrauterine growth restrictions. | High | Very low-moderate quality |
| Little difference in child neurodevelopment and growth outcomes | |||||
| insufficient evidence to determine effects on maternal adverse events and postnatal depression | |||||
| Harding et al. (2017) [65] | Iodine | Perinatal mortality, LBW, preterm birth, SGA, infant/child physical and mental development, mortality, Impacted postpartum hyperthyroidism and digestive intolerance in pregnancy | Positive impact perinatal mortality (RR 0.66, CI 0.42 to 1.03), congenital anomalies (RR 0.27, CI 0.12 to 0.60), uncertain data on neonatal mental/motor development but higher child mental development score (MD 11.21, CI 7.97 to 14.46) | High | Overall very low-low quality |
| Impacted postpartum hyperthyroidism (RR 0.32, CI 0.11 to 0.91) and digestive intolerance in pregnancy (RR 15.33, CI 2.07 to 113.7) | |||||
| No trials reported on infant/child growth or infant death; No impact LBW, preterm or SGA; All data from regions with mild to moderate iodine deficiency. | |||||
| Farebrother at al. (2018) [66] | Iodine | Birth length, weight, and head circumference | Overall: no impact on birth length, weight or head circumference: severely deficient women: positive impact on head circumference and birth weight (MD 200g, CI 183 to 217g). | High | Overall very low quality |
| Zhou et al. (2013) [67] | Iodine | Child development and growth | Regions severe deficiency: reduced risk of cretinism, but no impact on childhood intelligence, gross development, growth, or pregnancy outcomes, although evidence of improvement in some motor functions. | High-moderate | None of the studies reported adequate random-sequence generation, high risk of bias due to incomplete data, unclear risk for blinding due to inadequate reporting |
| Regions mild-moderate deficiency: No data on child growth and development. | |||||
| Rumbold et al. (2015) [68] | Vitamin E | Preeclampsia, preterm, stillbirth, neonatal death, perinatal death, intrauterine growth restrictions, maternal death, maternal morbidity, infant death, gestational age, child growth, congenital malformations, Apgar score | No impact stillbirth, neonatal death, infant death, preterm, pre-eclampsia, intrauterine growth restrictions, birth weight, maternal death, gestational age, congenital malformations, Apgar score, maternal morbidity/adverse effects. | High | Majority moderate-high quality but low for preterm PROM |
| Increased risk self‐reported abdominal pain (RR 1.66, CI 1.16 to 2.37) and term prelabour rupture of membranes (PROM) (RR 1.77, CI 1.37 to 2.28); no corresponding increased risk for preterm PROM. No data on child growth. | |||||
| Rumbold et al. (2015) [69] | Vitamin C | Stillbirth, neonatal death, perinatal death, intrauterine growth retardation, preterm, maternal death, infant death, gestational age, birth weight, congenital malformations, poor childhood growth, PROM, eclampsia | No impact on stillbirth, neonatal death, intrauterine growth restriction, perinatal death, preterm birth, preterm PROM, term PROM, maternal death, eclampsia, infant death congenital malformations, birth weight; no reported adverse effects. | High | Majority moderate-high quality but low for preterm PROM |
| Impacted gestational age (MD 0.31, CI 0.01 to 0.61) and the risk of self-reported abdominal pain. | |||||
| Risk of term and preterm PROM differed by type of supplementation–reduced risk for term and preterm PROM when supplemented alone, but increased risk when supplementation included vitamin C and E (RR 1.73, CI 1.34 to 2.23) | |||||
| Rogne et al. (2017) [70] | B12 | Birth weight, LBW, SGA, preterm | No associations between B12 levels and birth weight but B12-deficiency was associated with an increased risk of LBW (RR1.15, CI 1.01 to 1.31) and higher B12 was associated with higher birth weight in LMIC. Linear association between maternal levels of B12 and risk of preterm birth (0.89 per one SD increase (CI 0.82 to 0.97). | High-moderate | Overall moderate-high quality |
| Makrides et al. (2014) [71] | Magnesium | Perinatal mortality, maternal mortality, pre-eclampsia, stillbirths, neonatal death prior to discharge, preterm, birth weight, LBW, gestational age | No impact perinatal mortality, SGA, stillbirth, LBW, pre-eclampsia; No data on maternal death; Increase in neonatal death (RR 2.21, CI 1.02–4.75); fewer babies with an Apgar score less (RR 0.34, CI 0.15 to 0.80). | High | Lack of high-quality evidence; selective reporting unclear for all trials; 20% of trials high risk due to rack of blinding of participants |
| Chaffee et al. (2012) [72] | Zinc | Preterm, LBW, SGA, birth weight, gestational age, head circumference | Impacted preterm (RR 0.86, CI 0.75 to 0.99); no effect on LBW, SGA, birth weight, gestational age, head circumference. | High | Overall very low-low |
| Buppasiri et al. (2015) [73] | Calcium | Preterm birth, LBW, maternal death, maternal weight, perinatal mortality, neonatal death, birth length, head circumference, intrauterine growth restrictions, mineral bone density | Impacted birth weight (MD 56.40g, CI 13.55 to 99.25g), and infant total and tibial bone mineral density. | High | Overall moderate quality |
| No impact LBW, or preterm birth, maternal death, perinatal mortality, birth length, head circumference, intrauterine growth restrictions, maternal bone mineral density. No data on neonatal death. | |||||
| Lassi et al. (2013) [74] | Folic Acid | Preterm birth, stillbirths/neonatal death, pre-eclampsia, LBW, birth weight, maternal haemoglobin | No impact preterm, no impact on stillbirth/neonatal deaths, LBW and mean birth weight, maternal haemoglobin. | High | Included studies old (30 to 45 years ago); outcome measurement varied, poor compliance with random allocation, concealment and blinding. Bias and confounding seemed likely explanations for findings |
| Vitamin D | |||||
| Bi et al. (2018) [75] | Vitamin D | Infant growth, birth weight, LBW, morbidity, and mortality | Positive impact SGA (RR 0.72, CI 0.52 to 0.99), risk of foetal/neonatal mortality (RR 0.72, CI 0.47 to 1.11) or cognitive abnormalities (RR 0.94, CI 0.61 to 1.43), birth weight (MD, 75.38g, CI 22.88 to 127.88 g), and weight at 3, 6, 9, and 12 months. No significant difference in LBW or preterm birth. | High | Majority low/unclear risk of bias |
| Perez-Lopez et al. (2015) [76] | Vitamin D | Pre-eclampsia, SGA, LBW, preterm, birth weight, birth length | Impact on birth weight (MD 107.6g, CI 59.9 to 155.3g), and birth length (MD 0.3cm, CI 0.1 to 0.41cm). No impact on pre-eclampsia, SGA, LBW and preterm birth. | High | Majority unclear/low risk of bias |
| Low risk of bias for all studies for incomplete data, selective reporting and other sources | |||||
| Gallo et al. (2020) [77] | Vitamin D | Preeclampsia, SGA, birth weight and length | Increase in infant 25 (OH)D concentration, and birth weight (MD 114.2g, CI 63.4 to 165.1g). No impact on SGA and birth length or pre-eclampsia. | High | Overall fair quality |
| Maugeri et al. (2019) [78] | Vitamin D | LBW, SGA, birth weight, length, and head circumference | Positive impact birth weight (MD 103.17g, CI 62.29 to 122.04g), birth length (MD 0.50 cm, CI 0.08 to 0.92cm), head circumference (MD 0.19cm, CI 0.1 to 0.24cm), LBW (RR 0.40, CI 0.22 to 0.74), SGA (RR 0.69, CI 0.51 to 0.92); all outcomes impacted with Vit D supplemented alone, no impact when combined with other micronutrients. | High | Ranged very low (head circumference) to moderate quality (birth weight, birth length, LBW, and SGA) |
| Lactation | |||||
| Ndikom, et al. (2014) [79] | Extra fluids for breastfeeding mothers for increasing milk production. | Weight gain; EBF duration; Breastmilk production | No data on LMIC found despite inclusion in the search. Only one study met the inclusion criteria: reported no impact on breastmilk production (measured using test weighting) following advice to mothers to drink extra fluids. No data on other primary or secondary infant outcomes. | High-moderate | Low-quality |
| Foong et al. (2020) [80] | Oral galactagogues | Continued breastfeeding, infant weight, milk volume | Pharmacological: one study that reported on infant weight, little/no difference; possible effect on milk volume (MD 63.82 ml, CI 25.91 to 101.72ml). | High | Very low-low quality |
| Natural: Moringa and mixed botanical tea may increase infant weight; little/no difference in milk volume. | |||||
| Uncertain of adverse effect for mothers, those reported were minor complaints. | |||||
| Campion-Smith et al. (2020) [7] | MMN and polyunsaturated fatty acid supplementation. | Infant and child mortality, morbidity, and growth: weight, length, head circumference, WAZ, length-for-age z score | Overall: no impact infant and child mortality and morbidity | Moderate-low | Not available for all outcomes: Wide range very low-high |
| Polyunsaturated fatty acid supplementation vs placebo: no impact infant length, weight and head circumference | |||||
| Polyunsaturated fatty acid supplementation during gestation and lactation: no significant growth effects, some evidence for impact on child attention beyond 24 months of age. | |||||
| An RCT of maternal calorie supplementation + breastfeeding support: no impact WAZ or length for age z score; Increased infant breast milk intake and EBF. | |||||
| Abe et al. (2016) [81] | MMN | Infant mortality, morbidity, adverse effects within three days of supplementation | No evidence to quantitatively assess the effectiveness of MMN supplementation in improving health outcomes in mother and baby. | High-moderate | Poorly reported among original studies; unclear risk of bias |
| Impact on maternal anaemia (1 study) and vitamin B12 and folic acid milk concentration, but no significant dereferences were reported in serum concentrations (1 study). | |||||
| Pregnancy and lactation | |||||
| Petry et al. (2016) [82] | Low does iron and zinc via supplementation or fortification | Birth weight, LBW, HAZ, WAZ, WHZ, mental and motor development, morbidity. | Zinc, pregnancy: No impact on LBW, birth weight, one study found a beneficial effect of HAZ at 6months, no impact on other growth parameters. Zinc, lactation: One study (20mg, 40mg and control), no effect on breastmilk, growth or serum zinc concentration in exclusively breastfed infants. | High-moderate | Majority low-moderate quality |
| Iron, pregnancy: No impact on birth weight, LBW, mental development scores; no data on child growth. Iron, lactation: No impact on birth weight, LBW, mental development scores; no data on child growth. | |||||
Standard mean difference (SMD); Height-for-age z score (HAZ); Weight-for-age z score (WAZ); Mid-upper arm circumference (MUAC); Weight-for-height z scores (WHZ); Small for gestational age (SGA); Mean difference (MD); Relative risk (RR); Low birth weight (LBW); Randomised control trails (RCTs); Multiple micronutrients (MMN); Premature rupture of membranes (PROM); Low middle-income countries (LMIC).
*Quality reported in the respective review.
Reviews of MMN supplementation found that compared to no supplementation or supplementation with three or fewer micronutrients, they positively impacted risk of LBW and SGA [41, 62]. There was no statistically significant difference between supplements that contained at least 60 mg compared to supplement containing 30mg of iron and there was no difference in the timing of the intervention [62]. There was an increased risk of neonatal death in MMN intervention beginning after the first trimester when compared to iron folic acid supplementation, though this finding was based on low-quality evidence [62].
Vitamin D supplementation is a growing area of research; four reviews found a positive impact on birth weight, particularly if given after 20 weeks’ gestation, with potentially continued effect at 3, 6, 9 and 12 months [75–78]. Deficient women may benefit more [77]. Zinc supplementation has a positive effect on risk of preterm birth but no evidence for other infant outcomes [28, 72, 82]. Iron folic acid supplementation was associated with increased birth weight [28, 41]. Iodine supplementation may improve growth outcomes in severely deficient women [28, 65–67]. When stratified by country income there was an association between vitamin B12 and birth weight in LMICs, but not in HICs [70]. There is mixed evidence of the effect of omega-three fatty acid supplementation on birth outcomes [64]. There was also mixed evidence for calcium supplementation on birth outcomes [28, 73]. It may be beneficial in low intake populations, or if given after 20 weeks of gestation [28, 73]. Cochrane reviews of vitamin A, E and C supplementation showed no clinically significant impact on infant outcomes [63, 68, 69]. There is no quality evidence on effect of magnesium supplementation on birth outcomes [71].
Maternal supplementation during lactation
We found only five reviews examining the role of supplementation during lactation [7, 79–82]. There was no conclusive evidence that zinc supplementation, polyunsaturated fatty acid supplementation nor MMN provided to breastfeeding mothers impacted infant health and growth outcomes [82]. A review of galactagogues found some impact of Moringa and mixed botanical galactagogues on infant weight [7, 80] (Table 2). One Cochrane review explored extra fluids for breastfeeding mothers, with the rationale that this might increase breastmilk production [79]. Authors concluded that there was “Not enough evidence to increase fluid intake beyond what likely to require for comfort” [79].
Discussion
Our review found a large number of good quality reviews which explore maternal-focused interventions to prevent or address growth faltering in infants <6m. Evidence from these can inform future management strategies for small and nutritionally at-risk infants <6m in LMICs. The options for maternal interventions include a wide range of interventions as reflected in our results categorisation: breastfeeding promotion interventions; education and counselling; WASH; women’s empowerment; relaxation therapies; maternal mental health; m-health; conditional cash transfers; agriculture, maternal supplementation in pregnancy; maternal supplementation during lactation; and multi-sectoral interventions.
Breastfeeding promotion interventions during pregnancy and promotion and counselling during lactation are effective at improving infant feeding practices however evidence is more limited on anthropometric impacts. Outcomes relating to infant feeding practices are generally self-reported and not objectively measured [83]. More research is needed on community-based breastfeeding education and counselling that effectively improve growth outcomes in small or nutritionally at-risk infants <6m. Most evidence is for prevention of growth faltering, however there is evidence that lactation support can be used to successfully manage growth faltering at birth [84–86], and in older infants [87, 88]. In the documented experiences of implementing a “MAMI” approach (Management of small and nutritionally at-risk infants under six months) in an inpatient setting, most infants presenting with growth faltering had the possibility to breastfeed and tailored, structured lactation counselling was successfully used to establish EBF and secure weight gain in most infants [87–89]. However, there were considerable challenges in achieving this for some higher risk infants and sustaining progress post-discharge in the absence of community follow-up [87]. Educating family members, such as fathers and mothers-in-laws, has also shown a positive effect on neonatal mortality and feeding practices, emphasizing the importance of an inclusive approach and considering the wider family dynamics that influence feeding practices [37, 90]. Breastfeeding support packages in the workplace have shown positive impacts on breastfeeding initiation and duration. In high income settings they have also been found to be feasible to implement, presenting an opportunity to further target and support mothers and their infants [91]. However, this is much less evidenced in LMICs that also need to consider the informal work settings that so many mothers worldwide engage in. Education and counselling interventions, such as those focused on complementary feeding or care practices, generally have a positive but modest effect on infant growth outcomes; as with any education intervention, the effectiveness is likely to be proportional to the quality, content and uptake of the education provided [92]. Education interventions in combination with food supplementation or conditional cash transfers appear to be more impactful. Guidance on what constitutes effective education and counselling interventions for improving infant growth would be valuable for programmers faced with managing small and nutritionally at-risk infants <6m.
WASH interventions had some positive effects on linear growth, although less so for infants <6m. Women’s empowerment and m-health interventions were also potentially effective when combined with agriculture interventions or nutrition-specific interventions such as micronutrient supplementation. One possible mechanism behind these interventions is that maternal diet is improved, either during pregnancy or lactation, which in turn supports better in utero/infant growth and nutrition. The rationale behind conditional cash transfer programmes is similar and there is evidence of some positive impact on infant growth outcomes, however the evidence notes an important potential side-effect, which is the increased maternal consumption of high-fat or high-sugar foods in middle income settings. While low maternal body mass index and micronutrient deficiencies in pregnancy and during lactation are associated with infant growth faltering, so is high body mass index [2, 93–95].
Maternal mental health is perhaps one of the most promising but under-researched areas for post-natal intervention. Evidence for the association between maternal mental health and infant growth is strong; a systematic review found postnatal depression was associated with underweight in infants <6m in two studies and poor weight and height in a third, and is linked to poor breastfeeding practice and increased illnesses in other studies [96]. Alongside maternal depression, increased stress biomarkers are associated with intrauterine growth restrictions and poor growth through the post-natal period [97]. A meta-analysis of cohort studies found maternal anxiety during pregnancy was associated with significant increased risk of pre-term birth and LBW [98]. A meta-analysis estimated that 23% to 29% fewer children would be underweight or stunted if the infant population were unexposed to maternal depressive symptoms [99]. Despite strong associations, we found few reviews on maternal mental health interventions for infant growth and nutrition. One review found that cognitive behavioural therapy during pregnancy had no impact on infant weight or growth, but a positive impact on depression post-partum. However, Fotiou et al (2017) found that cognitive behavioural counselling had a positive impact on breastfeeding initiation and the duration of EBF [100]. In addition, there is promising evidence from relaxation therapy while breastfeeding, which has been found to reduce maternal stress levels, increased milk yield and milk fat levels, especially in mothers of preterm infants. Since that review was published, a randomised controlled trial (RCT) found greater weight gain and body mass index in infants in the relaxation intervention group but no effect on head circumference and length [101]. They also found a significant effect on the duration of sleep at 6–8 weeks and reduced cortisol concentrations in breastmilk [101]. Relaxation therapy is a fast-evolving area of research [102, 103]; other context-specific interventions that relieve maternal stress while breastfeeding could have similar effects.
Lastly, interventions to supplement maternal diets with macronutrients, MMN, vitamin D, zinc, iron folic acid, and possibly calcium, iodine and B12 in deficient women, in pregnancy, can improve birth outcomes. However, a recent RCT, not captured by the reviews, found that ready-to-use supplementary food plus daily MMN antenatal supplementation for moderately malnourished pregnant women was only modestly effective at improving length at birth, 6 weeks and 12 weeks, despite the large quantity of calories provided; this suggests that in high-risk settings stunting in utero is unlikely to be reduced by supplementation alone [104]. While there is evidence that low postnatal maternal body mass index is a risk factor for infant growth faltering, there is currently little evidence for the effect of maternal supplementation during lactation to improve infant growth. There were also few intervention studies for improving maternal dietary diversity during pregnancy and lactation, a known risk factor for poor infant growth outcomes [105]. Interventions seeking to address maternal dietary diversity could also influence infant diets during complementary feeding after six months age, another known risk factor for poor later child growth [106].
Limitations
We acknowledge several limitations to our review. First, limiting our search to reviews means that some primary evidence may have been missed. On the other hand, this approach also enabled us to capture a broader range of interventions and set the stage for important future research.
We also acknowledge that we only included reviews published since 2008. This is unlikely however to have altered our overall conclusions since individual reviews still include earlier original studies. Another limitation is that the same primary studies could have been included in several reviews; this may have given unwarranted weighting of the evidence from one study included across multiple reviews. Furthermore, while all reviews included studies from LMIC, several reviews (61%) also included studies from HIC; the inclusion of HIC may affect the applicability of our results to LMIC settings.
We were constrained in our ability to disaggregate data depending on how data was reported. Many reviews did not disaggregate data on infants <6m from older children; recognising infants <6m as an important distinct age category is vital to build evidence for best practice. Similarly, some reviews included studies that combined the exposure of interest with supplementation or included studies with special populations, such as those at increased risk due to underlying conditions. This may have affected the comparability of results but due to the small number of these studies, we do not believe this significantly affected our study’s findings. Variability across the infant outcomes measured made comparing and quantifying the effects of different interventions difficult. Whilst the quality of the reviews was generally high, the quality of the respective primary studies was sometimes low. GRADE criteria were used to assess the quality of the included reviews; while it is a standardised and widely recognised method, it does not account for the inclusion of grey literature, which can increase the quality of reviews. There were also several interventions with only very limited evidence, such as a small number of studies from a limited number of contexts, not RCTs, and small sample sizes.
Most evidence we found relates to the prevention of growth faltering, rather than the management of already small and nutritionally compromised infants <6m. This highlights the need for future studies to focus specifically on this group; whilst it is plausible and likely that interventions which work for prevention also work for management, it is not certain and effect sizes may differ. Most studies were also timed around the antenatal rather than the postnatal period. Future programmes should support mothers throughout (and before) pregnancy but better understanding of the relative impact of different interventions at different times is still important to help prioritise and target. Whilst this review focused only on outcomes for the infant, outcomes for the mother are equally important and also warrant attention and action.
Policy implications
The heterogeneity across the zero to six month period, transitioning from the umbilical cord to solid feeding, and the contextual dynamic and needs of the individual mother-infant dyad, will likely warrant an assortment of both maternal and infant-focused interventions, working together in synergy. While a coordinated strategy is required, one approach or set of interventions alone is unlikely to suit all contexts and all infants <6m. Addressing maternal as well as infant needs reflects a recent WHO report on Essential Nutrition Actions and the 2021 Lancet Maternal and Child Undernutrition series, recognising the importance of addressing nutrition throughout the life course [17, 107]. The UN ‘Global Action Plan’ (GAP) on Child Wasting: Framework for Action also stresses the importance of maternal nutrition interventions during the preconception, pregnancy and postnatal period, to improve infant outcomes, specifically for the reduction of LBW [108]. The need for essential nutrition packages to be integrated into national health systems and policies is also highlighted [108]; essential nutrition packages must include multifaced maternal interventions for managing small and nutritionally at-risk infant <6m. This could be achieved though strengthening referral pathways between services that are already available (e.g. maternal mental health, infant and young child feeding) or through the adoption of adaptable community-based interventions that combine maternal and infant nutrition to fill service gaps. One example of such an approach is a recently released integrated care pathway for managing small and nutritionally at-risk infants <6m and their mothers [109].
Conclusion
Our review of reviews identifies a variety of interventions aimed at mothers in the antenatal and postnatal period in LMIC that could be used to also improve infant nutrition outcomes. Several interventions proved to be effective in preventing growth faltering in infants <6m, including: breastfeeding, education and peer support, counselling interventions, antenatal supplementation combined with women’s empowerment, m-health technologies, WASH, conditional cash transfers, and/or agriculture interventions. Relaxation therapies and mental health interventions also have potential–but more evidence is needed. Besides galactagogues, evidence on post-natal maternal supplementation for managing infant growth faltering has shown limited impact, although the number of studies is limited. There is limited evidence that directly considers impact of interventions on small and nutritionally at-risk infants <6m, highlighting an important research gap. Based on this collated evidence, more holistic packages of care that include maternal interventions are justified; contextualised support of the mother-infant dyad could help small and nutritionally vulnerable infants aged <6m survive and thrive.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
This paper is made possible by the support from Irish Aid and the Eleanor Crook Foundation.
List of abbreviations
- <6m
Less than 6 months
- EBF
Exclusive breastfeeding
- GRADE
Grading of Recommendations, Assessment, Development and Evaluations
- HAZ
Height-for-age z‐score
- LBW
Low birth weight
- LMIC
Low -and middle- income countries
- MD
Mean difference
- MMN
Multiple micronutrient
- MUAC
Middle-upper-arm-circumference
- RCT
Randomised control trial
- RR
Relative Risk
- SGA
Small for gestational age
- WASH
Water Sanitation and Hygiene
- WAZ
Weight‐for‐age z-score
- WHZ
Weight‐for‐height z‐score
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
Time contributed to authoring this paper was supported by the Eleanor Crook Foundation and Ireland’s Department for Foreign Affairs (Irish Aid) for EB, NL and MM. MK was supported by the Eleanor Crook Foundation. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The ideas, opinions and comments therein are entirely the responsibility of its author(s) and do not necessarily represent or reflect Irish Aid policy.
References
- 1.FAO, IFAD, UNICEF, WFP, WHO. The State of Food Security and Nutrition in the World. FAO; 2020. [Google Scholar]
- 2.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382(9890):427–51. doi: 10.1016/S0140-6736(13)60937-X [DOI] [PubMed] [Google Scholar]
- 3.Kerac M, Mwangome M, McGrath M, Haider R, Berkley JA. Management of acute malnutrition in infants aged under 6 months (MAMI): current issues and future directions in policy and research. Food Nutr Bull. 2015;36(1 Suppl):S30–4. doi: 10.1177/15648265150361S105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kerac M, Blencowe H, Grijalva-Eternod C, McGrath M, Shoham J, Cole TJ, et al. Prevalence of wasting among under 6-month-old infants in developing countries and implications of new case definitions using WHO growth standards: a secondary data analysis. Archives of disease in childhood. 2011;96(11). doi: 10.1136/adc.2010.191882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Management of At Risk Mothers and Infants (MAMI)—main website 2021 [Available from: https://www.ennonline.net/ourwork/research/mami].
- 6.Grijalva‐Eternod CS, Kerac M, McGrath M, Wilkinson C, Hirsch JC, Delchevalerie P, et al. Admission profile and discharge outcomes for infants aged less than 6 months admitted to inpatient therapeutic care in 10 countries. A secondary data analysis. Matern Child Nutr. 2017;12(3). doi: 10.1111/mcn.12345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campion-Smith TJ, Kerac M, McGrath M, Berkley JA. Antimicrobial and micronutrient interventions for the management of infants under 6 months of age identified with severe malnutrition: a literature review. PeerJ. 2020;8:e9175. doi: 10.7717/peerj.9175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanson MA, Low FM, Gluckman PD. Epigenetic epidemiology: the rebirth of soft inheritance. Annals of Nutrition and Metabolism. 2011;58(Suppl. 2):8–15. doi: 10.1159/000328033 [DOI] [PubMed] [Google Scholar]
- 9.Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35(7):595–601. doi: 10.1007/BF00400248 [DOI] [PubMed] [Google Scholar]
- 10.Lelijveld N, Seal A, Wells JC, Kirkby J, Opondo C, Chimwezi E, et al. Chronic disease outcomes after severe acute malnutrition in Malawian children (ChroSAM): a cohort study. The Lancet Global Health. 2016. doi: 10.1016/S2214-109X(16)30133-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grantham‐McGregor S, Powell C, Walker S, Chang S, Fletcher P. The Long‐Term Follow‐up of Severely Malnourished Children Who Participated in an Intervention Program. Child development. 1994;65(2):428–39. [PubMed] [Google Scholar]
- 12.Grey K, Gonzales GB, Abera M, Lelijveld N, Thompson D, Berhane M, et al. Severe malnutrition or famine exposure in childhood and cardiometabolic non-communicable disease later in life: a systematic review. BMJ Global Health. 2021;6(3):e003161. doi: 10.1136/bmjgh-2020-003161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerac M, McGrath M. Management of Acute Malnutrition in Infants under 6 Months of Age. The Biology of the First 1,000 Days.Oxidative Stress and Disease: Routledge; 2017. p. 207–20. [Google Scholar]
- 14.WHO. Updates on the management of severe acute malnutrition in infants and children (Guideline) 2013 [Available from: http://www.who.int/nutrition/publications/guidelines/updates_management_SAM_infantandchildren/en/index.html]. [PubMed]
- 15.Kerac M, Frison S, Connell N, Page B, McGrath M. Informing the management of acute malnutrition in infants aged under 6 months (MAMI): risk factor analysis using nationally-representative demographic & health survey secondary data. PeerJ. 2019;6:e5848. doi: 10.7717/peerj.5848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mertens A, Benjamin-Chung J, Colford JM, Coyle J, Laan vdM, Hubbard AE, et al. Causes and consequences of child growth failure in low- and middle-income countries. medRxiv. 2020:2020.06.09.20127100. [Google Scholar]
- 17.Victora CG, Christian P, Vidalettie LP, Gatica-Dominguez G, Menon P, Black RE. Revisiting maternal and child undernutrition in low-income and middle-income countries: variable progress towards an unfinished agenda. Lanct. 2021(Maternal and Child Undernutrion Progess). 2021: 397(10282):1388–1399. doi: 10.1016/S0140-6736(21)00394-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dadi AF, Miller ER, Mwanri L. Postnatal depression and its association with adverse infant health outcomes in low-and middle-income countries: A systematic review and meta-analysis. BMC Pregnancy and Childbirth. 2020;20(1). doi: 10.1186/s12884-020-03092-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wachs T. Multiple influences on children’s nutritional deficiencies: a systems perspective. Physiol Behav. 2008;94(1):48–60. doi: 10.1016/j.physbeh.2007.11.018 [DOI] [PubMed] [Google Scholar]
- 20.Manda SOM. Birth intervals, breastfeeding and determinants of childhood mortality in Malawi. Social Science & Medicine. 1999;48(3):301–12. doi: 10.1016/s0277-9536(98)00359-1 [DOI] [PubMed] [Google Scholar]
- 21.Heidkamp R, Clermont A, Phillips E. Modeling the Impact of Nutrition Interventions on Birth Outcomes in the Lives Saved Tool (LiST). Journal of Nutrition. 2017;147(11):2188S–93S. doi: 10.3945/jn.116.243667 [DOI] [PubMed] [Google Scholar]
- 22.WHO. Malnutrition key facts 2018, February 16 [Available from: https://www.who.int/news-room/fact-sheets/detail/malnutrition].
- 23.Christian P, Mullany LC, Hurley KM, Katz J, Black RE. Nutrition and maternal, neonatal, and child health. Seminars in Perinatology. 2015;39(5):361–72. doi: 10.1053/j.semperi.2015.06.009 [DOI] [PubMed] [Google Scholar]
- 24.Bazzano AN, Kaji A, Felker-Kantor E, Bazzano LA, Potts KS. Qualitative Studies of Infant and Young Child Feeding in Lower-Income Countries: A Systematic Review and Synthesis of Dietary Patterns. Nutrients. 2017;9(10). doi: 10.3390/nu9101140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.UNICEF. Nutrition, for Every Child: UNICEF Nutrition Strategy 2020–2030. UNICEF, New York: United Nations Children’s Fund; 2020. [Google Scholar]
- 26.The World Bank. World Bank Country and Lending Groups 2019 [Available from: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519].
- 27.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhutta ZA, Das JK, Rizvi A, Gaffey MF, Walker N, Horton S, et al. Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost? Lancet. 2013;382(9890):452–77. doi: 10.1016/S0140-6736(13)60996-4 [DOI] [PubMed] [Google Scholar]
- 29.Kraft JM, Wilkins KG, Morales GJ, Widyono M, Middlestadt SE. An evidence review of gender-integrated interventions in reproductive and maternal-child health. (Special Issue: Population-level behavior change to enhance child survival and development in low- and middle-income countries: a review of the evidence.). Journal of Health Communication: International Perspectives. 2014;19(Suppl. 1):122–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruel MT, Alderman H. Nutrition-sensitive interventions and programmes: how can they help to accelerate progress in improving maternal and child nutrition? Lancet. 2013;382(9891):536–51. doi: 10.1016/S0140-6736(13)60843-0 [DOI] [PubMed] [Google Scholar]
- 31.Victora CG, Bahl R, Barros AJ, Franca GV, Horton S, Krasevec J, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387(10017):475–90. doi: 10.1016/S0140-6736(15)01024-7 [DOI] [PubMed] [Google Scholar]
- 32.Shakya P, Kunieda MK, Koyama M, Rai SS, Miyaguchi M, Dhakal S, et al. Effectiveness of community-based peer support for mothers to improve their breastfeeding practices: A systematic review and meta-analysis. PLoS ONE [Electronic Resource]. 2017;12(5):e0177434. doi: 10.1371/journal.pone.0177434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giugliani ERJ, Horta BL, Loret de Mola C, Lisboa BO, Victora CG. Effect of breastfeeding promotion interventions on child growth: a systematic review and meta-analysis. Acta Paediatrica. 2015;104(S467):20–9. doi: 10.1111/apa.13160 [DOI] [PubMed] [Google Scholar]
- 34.Lassi ZS, Rind F, Irfan O, Hadi R, Das JK, Bhutta ZA. Impact of infant and young child feeding (Iycf) nutrition interventions on breastfeeding practices, growth and mortality in low-and middle-income countries: Systematic review. Nutrients. 2020;12(3). doi: 10.3390/nu12030722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janmohamed A, Sohani N, Lassi ZS, Bhutta ZA. The Effects of Community Home Visit and Peer Group Nutrition Intervention Delivery Platforms on Nutrition Outcomes in Low and Middle-Income Countries: A Systematic Review and Meta-Analysis. Nutrients. 2020;12(2):440. doi: 10.3390/nu12020440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arikpo D, Edet ES, Chibuzor MT, Odey F, Caldwell DM. Educational interventions for improving primary caregiver complementary feeding practices for children aged 24 months and under. Cochrane Database Syst Rev. 2018;5(5). doi: 10.1002/14651858.CD011768.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lassi ZS, Kedzior SGE, Bhutta ZA. Community-based maternal and newborn educational care packages for improving neonatal health and survival in low- and middle-income countries. Cochrane Database Syst Rev. 2019;11:11. doi: 10.1002/14651858.CD007647.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ojha S, Elfzzani Z, Kwok TC, Dorling J. Education of family members to support weaning to solids and nutrition in later infancy in term-born infants. Cochrane Database Syst Rev. 2020;7(7):CD012241. doi: 10.1002/14651858.CD012241.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goudet SM, Bogin BA, Madise NJ, Griffiths PL. Nutritional interventions for preventing stunting in children (birth to 59 months) living in urban slums in low‐ and middle‐income countries (LMIC). Cochrane Database Syst Rev. 2019;6(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Victora CG, Barros FC, Assuncao MC, Restrepo-Mendez MC, Matijasevich A, Martorell R. Scaling up maternal nutrition programs to improve birth outcomes: a review of implementation issues. Food Nutr Bull. 2012;33(2 Suppl):S6–26. doi: 10.1177/15648265120332S102 [DOI] [PubMed] [Google Scholar]
- 41.Park JJH, Fang ML, Harari O, Dron L, Siden EG, Majzoub R, et al. Association of Early Interventions With Birth Outcomes and Child Linear Growth in Low-Income and Middle-Income Countries: Bayesian Network Meta-analyses of Randomized Clinical Trials. JAMA Network Open. 2019;2(7):e197871–e. doi: 10.1001/jamanetworkopen.2019.7871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park JJH, Siden E, Harari O, Dron L, Mazoub R, Jeziorska V, et al. Interventions to improve linear growth during exclusive breastfeeding life-stage for children aged 0–6 months living in low- and middle-income countries: a systematic review with network and pairwise meta-analyses. Gates Open Res. 2019;3:1720. doi: 10.12688/gatesopenres.13082.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ota E, Hori H, Mori R, Tobe‐Gai R, Farrar D. Antenatal dietary education and supplementation to increase energy and protein intake. Cochrane Database Syst Rev. 2015(6). doi: 10.1002/14651858.CD000032.pub3 [DOI] [PubMed] [Google Scholar]
- 44.East CE, Ma B, Fredericks S, Lau R. Support during pregnancy for women at increased risk of low birthweight babies. Cochrane Database Syst Rev. 2019(4). doi: 10.1002/14651858.CD000198.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dangour AD, Watson L, Cumming O, Boisson S, Che Y, Velleman Y, et al. Interventions to improve water quality and supply, sanitation and hygiene practices, and their effects on the nutritional status of children. Cochrane Database Syst Rev. 2013(8):Cd009382. doi: 10.1002/14651858.CD009382.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gera T, Shah D, Sachdev HS. Impact of Water, Sanitation and Hygiene Interventions on Growth, Non-diarrheal Morbidity and Mortality in Children Residing in Low- and Middle-income Countries: A Systematic Review. Indian Pediatr. 2018;55(5):381–93. [PubMed] [Google Scholar]
- 47.Gizaw Z, Worku A. Effects of single and combined water, sanitation and hygiene (WASH) interventions on nutritional status of children: a systematic review and meta-analysis. Italian journal of pediatrics. 2019;45(1):77. doi: 10.1186/s13052-019-0666-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taukobong HF, Kincaid MM, Levy JK, Bloom SS, Platt JL, Henry SK, et al. Does addressing gender inequalities and empowering women and girls improve health and development programme outcomes? Health Policy Plan. 2016;31(10):1492–514. doi: 10.1093/heapol/czw074 [DOI] [PubMed] [Google Scholar]
- 49.Shukri NHM, Wells JCK, Fewtrell M. The effectiveness of interventions using relaxation therapy to improve breastfeeding outcomes: a systematic review. Matern Child Nutr. 2018;14(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saronga NJ, Burrows T, Collins CE, Ashman AM, Rollo ME. mHealth interventions targeting pregnancy intakes in low and lower-middle income countries: Systematic review. Matern Child Nutr. 2019;15(2):e12777. doi: 10.1111/mcn.12777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palmer MJ, Henschke N, Bergman H, Villanueva G, Maayan N, Tamrat T, et al. Targeted client communication via mobile devices for improving maternal, neonatal, and child health. Cochrane Database Syst Rev. 2020(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sondaal SF, Browne JL, Amoakoh-Coleman M, Borgstein A, Miltenburg AS, Verwijs M, et al. Assessing the Effect of mHealth Interventions in Improving Maternal and Neonatal Care in Low- and Middle-Income Countries: A Systematic Review. PLoS One. 2016;11(5):e0154664. doi: 10.1371/journal.pone.0154664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leroy JL, Ruel M, Verhofstadt E. The impact of conditional cash transfer programmes on child nutrition: a review of evidence using a programme theory framework. Journal of Development Effectiveness. 2009;1(2):103–29. [Google Scholar]
- 54.Lagarde M, Haines A, Palmer N. The impact of conditional cash transfers on health outcomes and use of health services in low and middle income countries. Cochrane Database Syst Rev. 2009(4). doi: 10.1002/14651858.CD008137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Masset E, Haddad L, Cornelius A, Isaza-Castro J. Effectiveness of agricultural interventions that aim to improve nutritional status of children: systematic review. BMJ. 2012;344:d8222. doi: 10.1136/bmj.d8222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gresham E, Byles JE, Bisquera A, Hure AJ. Effects of dietary interventions on neonatal and infant outcomes: a systematic review and meta-analysis. Am Clin Nutr. 2014;100(5). doi: 10.3945/ajcn.113.080655 [DOI] [PubMed] [Google Scholar]
- 57.Visser J, McLachlan MH, Maayan N, Garner P. Community-based supplementary feeding for food insecure, vulnerable and malnourished populations—an overview of systematic reviews. Cochrane Database Syst Rev. 2018;11(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stevens B, Buettner P, Watt K, Clough A, Brimblecombe J, Judd J. The effect of balanced protein energy supplementation in undernourished pregnant women and child physical growth in low- and middle-income countries: a systematic review and meta-analysis. Matern Child Nutr. 2015;11(4). doi: 10.1111/mcn.12183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Imdad A, Bhutta ZA. Effect of balanced protein energy supplementation during pregnancy on birth outcomes. BMC public health. 2011;11Suppl 3(Suppl 3). doi: 10.1186/1471-2458-11-S3-S17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pimpin L, Kranz S, Liu E, Shulkin M, Karageorgou D, Miller V et al. Effects of animal protein supplementation of mothers, preterm infants, and term infants on growth outcomes in childhood: a systematic review and meta-analysis of randomized trials. Am Clinical Nutrition. 2019;110(2). doi: 10.1093/ajcn/nqy348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Das JK, Hoodbhoy Z, Salam RA, Bhutta AZ, Valenzuela-Rubio NG, Prinzo WZ, et al. Lipid-based nutrient supplements for maternal, birth, and infant developmental outcomes. Cochrane Database Syst Rev. 2018;8(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramakrishnan U, Grant FK, Goldenberg T, Bui V, Imdad A, Bhutta AZ. Effect of Multiple Micronutrient Supplementation on Pregnancy and Infant Outcomes: A Systematic Review. Paediatric and Perinatal Epidemiology. 2012;26(1):153–167. doi: 10.1111/j.1365-3016.2012.01276.x [DOI] [PubMed] [Google Scholar]
- 63.Thorne-Lyman AL, Fawzi WW. Vitamin A and carotenoids during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta-analysis. Paediatric and perinatal epidemiology. 2012;26Suppl 1(0 1). doi: 10.1111/j.1365-3016.2012.01284.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Middleton P, Gomersall J, Gould JF, Shepherd E, Olsen SF, Makrides M. Omega-3 fatty acid addition during pregnancy. Cochrane Database Syst Rev. 2018;11(11). doi: 10.1002/14651858.CD003402.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harding KB, Pena-Rosas JP, Webster AC, Yap CM, Payne BA, Ota E, et al. Iodine supplementation for women during the preconception, pregnancy and postpartum period. Cochrane Database Syst Rev. 2017;3(3). doi: 10.1002/14651858.CD011761.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Farebrother J, Nuade CE, Nicol L, Sang Z, Yang Z, Jooste PL, et al. Effects of Iodized Salt and Iodine Supplements on Prenatal and Postnatal Growth: A Systematic Review. Advances in nutrition. 2018;9(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou SJ, Anderson AJ, Gibson RA, Makrides M. Effect of iodine supplementation in pregnancy on child development and other clinical outcomes: a systematic review of randomized controlled trials. Am Clin Nutr. 2013;98(5). doi: 10.3945/ajcn.113.065854 [DOI] [PubMed] [Google Scholar]
- 68.Rumbold A, Ota E, Hori H, Miyazaki C, Crowther CA. Vitamin E supplementation in pregnancy. Cochrane Database Syst Rev. 2015(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rumbold A, Ota E, Nagata C, Shahrook S, Crowther CA. Vitamin C supplementation in pregnancy. Cochrane Database Syst Rev. 2015(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rogne T, Tielemans MJ, Chong MF, Yajnik CS, Krishnaveni GV, Poston L, et al. Associations of Maternal Vitamin B12 Concentration in Pregnancy With the Risks of Preterm Birth and Low Birth Weight: A Systematic Review and Meta-Analysis of Individual Participant Data. American journal of epidemiology. 2017;185(3). doi: 10.1093/aje/kww212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Makrides M, Crosby DD, Bain E, Crowther CA. Magnesium supplementation in pregnancy. Cochrane Database Syst Rev. 2014;2014(4). doi: 10.1002/14651858.CD000937.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chaffee BW, King JC. Effect of zinc supplementation on pregnancy and infant outcomes: a systematic review. Paediatric and perinatal epidemiology. 2012;26Suppl 1(0 1). doi: 10.1111/j.1365-3016.2012.01289.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Buppasiri P, Lumbiganon P, Thinkhamrop J, Ngamjarus C, Laopaiboon M, Medley N. Calcium supplementation (other than for preventing or treating hypertension) for improving pregnancy and infant outcomes. Cochrane Database Syst Rev. 2015(10). doi: 10.1002/14651858.CD007079.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lassi ZS, Kedzior SGE, Tariq W, Jadoon Y, Das JK, Bhutta ZA. Effects of Preconception Care and Periconception Interventions on Maternal Nutritional Status and Birth Outcomes in Low- and Middle-Income Countries: A Systematic Review. Nutrients. 2020;12(3):606. doi: 10.3390/nu12030606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bi WG, Nuyt AM, Weiler H, Leduc L, Santamaria C, Wei SQ. Association Between Vitamin D Supplementation During Pregnancy and Offspring Growth, Morbidity, and Mortality: A Systematic Review and Meta-analysis. JAMA pediatrics. 2018;172(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pérez-López FR, Pasupuleti V, Mezones-Holguin E, Benites-Zapata VA, Thota P, Deshpande A, et al. Effect of vitamin D supplementation during pregnancy on maternal and neonatal outcomes: a systematic review and meta-analysis of randomized controlled trials. Fertility and sterility. 2015;103(5). [DOI] [PubMed] [Google Scholar]
- 77.Gallo S, Mc Dermid JM, Al-Nimr RI, Hakeem R, Moreschi JM, Pari-Keener M, et al. Vitamin D Supplementation during Pregnancy: An Evidence Analysis Center Systematic Review and Meta-Analysis. Journal of the Academy of Nutrition and Dietetics. 2020;120(5). doi: 10.1016/j.jand.2019.07.002 [DOI] [PubMed] [Google Scholar]
- 78.Maugeri A, Barchitta M, Blanco I, Agodi A. Effects of Vitamin D Supplementation During Pregnancy on Birth Size: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2019;11(2). doi: 10.3390/nu11020442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ndikom CM, Fawole B, Ilesanmi RE. Extra fluids for breastfeeding mothers for increasing milk production. Cochrane Database Syst Rev. 2014(6). doi: 10.1002/14651858.CD008758.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Foong SC, Tan ML, Foong WC, Marasco LA, Ho JJ, Ong JH. Oral galactagogues (natural therapies or drugs) for increasing breast milk production in mothers of non‐hospitalised term infants. Cochrane Database Syst Rev. 2020(5). doi: 10.1002/14651858.CD011505.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abe SK, Balogun OO, Ota E, Takahashi K, Mori R. Supplementation with multiple micronutrients for breastfeeding women for improving outcomes for the mother and baby. Cochrane Database Syst Rev. 2016;18(2). doi: 10.1002/14651858.CD010647.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Petry N, Olofin I, Boy E, Angel MD, Rohner F. The Effect of Low Dose Iron and Zinc Intake on Child Micronutrient Status and Development during the First 1000 Days of Life: A Systematic Review and Meta-Analysis. Nutrients. 2016;8(12). doi: 10.3390/nu8120773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Owino V, Moleah T. World Breastfeeding Week 2017: Understanding Breastfeeding Patterns Through Nuclear Science 2017. [Available from: https://www.iaea.org/newscenter/news/world-breastfeeding-week-2017-understanding-breastfeeding-patterns-through-nuclear-science]. [Google Scholar]
- 84.Ahmed AH. Breastfeeding preterm infants: an educational program to support mothers of preterm infants in Cairo, Egypt. Pediatric Nursing. 2008;34(2):125. [PubMed] [Google Scholar]
- 85.Ahmadi S, Kazemi F, Masoumi SZ, Parsa P, Roshanaei G. Intervention based on BASNEF model increases exclusive breastfeeding in preterm infants in Iran: a randomized controlled trial. International BreastfeedJ. 2016;11(1):30. doi: 10.1186/s13006-016-0089-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Agrasada GV, Gustafsson J, Kylberg E, Ewald U. Postnatal peer counselling on exclusive breastfeeding of low‐birthweight infants: A randomized, controlled trial. Acta Paediatrica. 2005;94(8):1109–15. doi: 10.1111/j.1651-2227.2005.tb02053.x [DOI] [PubMed] [Google Scholar]
- 87.Mwangome M, Murunga S, Kahindi J, Gwiyo P, Mwasho G, Talbert A, et al. Individualized breastfeeding support for acutely ill, malnourished infants under 6 months old. Matern Child Nutr. 2020;16(1):e12868. doi: 10.1111/mcn.12868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Burrell A, Barthorp H. GOAL’s experiences of management of at-risk mothers and infants (MAMI) programming in Ethiopia. Field Exchange 62. 2020:30. [Google Scholar]
- 89.Kueter AM, Burrell A, Butler S, Sarwar M, Rahaman H. Piloting the C-MAMI approach in the Rohingya response in Bangladesh. Field Exchange 58. 2018:51. [Google Scholar]
- 90.Rana R, McGrath M, Sharma E, Gupta P, Kerac M. Effectiveness of Breastfeeding Support Packages in Lowand Middle-Income Countries for Infants under Six Months: A Systematic Review. Nutrients. 2021;13(2):681. doi: 10.3390/nu13020681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dinout LM, Szaro JM. Employer-Based Programs to Support Breastfeeding Among Working Mothers: A Systematic Review. Breastfeeding medicine. 2017;12. [DOI] [PubMed] [Google Scholar]
- 92.Ashworth A, Ferguson E. Dietary counseling in the management of moderate malnourishment in children. Food Nutr Bull. 2009;30(3_suppl3):S405–S33. doi: 10.1177/15648265090303S304 [DOI] [PubMed] [Google Scholar]
- 93.Kuzawa CW, Hallal PC, Adair L, Bhargava SK, Fall CH, Lee N, et al. Birth weight, postnatal weight gain, and adult body composition in five low and middle income countries. American Journal of Human Biology. 2012;24(1):5–13. doi: 10.1002/ajhb.21227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Han Z, Lutsiv O, Mulla S, McDonald SD. Maternal height and the risk of preterm birth and low birth weight: a systematic review and meta-analyses. J Obstet Gynaecol Can. 2012;34(8):721–46. doi: 10.1016/S1701-2163(16)35337-3 [DOI] [PubMed] [Google Scholar]
- 95.Widen EM, Bentley ME, Kayira D, Chasela CS, Jamieson DJ, Tembo M, et al. Maternal Weight Loss during Exclusive Breastfeeding Is Associated with Reduced Weight and Length Gain in Daughters of HIV-Infected Malawian Women123. The Journal of Nutrition. 2013;143(7):1168–75. doi: 10.3945/jn.112.171751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dadi AF, Miller ER, Mwanri L. Postnatal depression and its association with adverse infant health outcomes in low- and middle-income countries: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2020;20(1):416. doi: 10.1186/s12884-020-03092-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Surkan PJ, Patel SA, Rahman A. Preventing infant and child morbidity and mortality due to maternal depression. Best Pract Res Clin Obstet Gynaecol. 2016;36:156–68. doi: 10.1016/j.bpobgyn.2016.05.007 [DOI] [PubMed] [Google Scholar]
- 98.Ding X-X, Wu Y-L, Xu S-J, Zhu R-P, Jia X-M, Zhang S-F, et al. Maternal anxiety during pregnancy and adverse birth outcomes: a systematic review and meta-analysis of prospective cohort studies. J Affect Disord. 2014;159:103–10. doi: 10.1016/j.jad.2014.02.027 [DOI] [PubMed] [Google Scholar]
- 99.Surkan PJ, Kennedy CE, Hurley KM, Black MM. Maternal depression and early childhood growth in developing countries: systematic review and meta-analysis. Bull World Health Organ. 2011;89(8):608–15. doi: 10.2471/BLT.11.088187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fotiou C, Siahanidou T, Vlastarakos PV, Tavoulari EF, Chrousos G. The effect of body and mind stress-releasing techniques on the breastfeeding of full-term babies: a critical analysis of published interventional studies. The Journal of Maternal-Fetal & Neontal Medicine. 2017;31(1):98–105. [DOI] [PubMed] [Google Scholar]
- 101.Shukri NHM, Wells J, Eaton S, Mukhtar F, Petelin A, Jenko-Praznikar Z, et al. Randomized controlled trial investigating the effects of a breastfeeding relaxation intervention on maternal psychological state, breast milk outcomes, and infant behavior and growth. Am J Clin Nutr. 2019;1(110):121–30. [DOI] [PubMed] [Google Scholar]
- 102.Yu J, Wells J, Wei Z, Fewtrell M. Effects of relaxation therapy on maternal psychological state, infant growth and gut microbiome: protocol for a randomised controlled trial investigating mother-infant signalling during lactation following late preterm and early term delivery. Int Breastfeed J. 2019;14:50. doi: 10.1186/s13006-019-0246-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dib S, Wells JCK, Fewtrell M. Mother And late Preterm Lactation Study (MAPLeS): a randomised controlled trial testing the use of a breastfeeding meditation by mothers of late preterm infants on maternal psychological state, breast milk composition and volume, and infant behaviour and growth. Trials. 2020;21(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Callaghan-Gillespie M, Schaffner AA, Garcia P, Fry J, Eckert R, Malek S, et al. Trial of ready-to-use supplemental food and corn-soy blend in pregnant Malawian women with moderate malnutrition: a randomized controlled clinical trial. Am Clin Nutr. 2020;106(4):1062–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Madzorera I, Ghosh S, Wang M, Fawzi W, Isanaka S, Hertzmark E, et al. Prenatal dietary diversity may influence underweight in infants in a Ugandan birth‐cohort. Maternl Child Nutr. 2021:e13127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Marriott BP, White A, Hadden L, Davies JC, Wallingford JC. World Health Organization (WHO) infant and young child feeding indicators: associations with growth measures in 14 low‐income countries. Matern Child Nutr. 2012;8(3):354–70. doi: 10.1111/j.1740-8709.2011.00380.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.WHO. Essential Nutrition Actions: mainstreaming nutrition throughout the life course 2019 [Available from: https://www.who.int/nutrition/publications/essential-nutrition-actions-2019/en/].
- 108.WHO UNHCR, UNICEF WFP. Global action plan on child wasting: A framework for action to accelerate progress in preventing and managing child wasting and the achievement of the Sustainable Development Goals. 2020. [Google Scholar]
- 109.ENN. MAMI Care Pathway Package, Version 3: ENN; 2021 [Available from: https://www.ennonline.net/mamicarepathway].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.