Abstract
Background
Fretting and corrosion in metal-on-polyethylene total hip arthoplasty (THA) modular junctions can cause adverse tissue reactions that are responsible for 2% to 5% of revision surgeries. Damage within cobalt-chromium-molybdenum (CoCrMo) alloy femoral heads can progress chemically and mechanically, leading to damage modes such as column damage, imprinting, and uniform fretting damage. At present, it is unclear which of these damage modes are most detrimental and how they may be linked to implant alloy metallurgy. The alloy microstructure exhibits microstructural features such as grain boundaries, hard phases, and segregation bands, which may enable different damage modes, higher material loss, and the potential risk of adverse local tissue reactions.
Questions/purposes
In this study, we asked: (1) How prevalent is chemically dominated column damage compared with mechanically dominated damage modes in severely damaged metal-on-polyethylene THA femoral heads made from wrought CoCrMo alloy? (2) Is material loss greater in femoral heads that underwent column damage? (3) Do material loss and the presence of column damage depend on alloy microstructure as characterized by grain size, hard phase content, and/or banding?
Methods
Surgically retrieved wrought CoCrMo modular femoral heads removed between June 2004 and June 2019 were scored using a modified version of the Goldberg visually based scoring system. Of the total 1002 heads retrieved over this period, 19% (190 of 1002) were identified as severely damaged, exhibiting large areas of fretting scars, black debris, pits, and/or etch marks. Of these, 43% (81 of 190) were excluded for metal-on-metal articulations, alternate designs (such as bipolar, dual-mobility, hemiarthroplasty, metal adaptor sleeves), or previous sectioning of the implant for past studies. One sample was excluded retroactively as metallurgical analysis revealed that it was made of cast alloy, yielding a total of 108 for further analysis. Information on patient age (57 ± 11 years) and sex (56% [61 of 108] were males), reason for removal, implant time in situ (99 ± 78 months), implant manufacturer, head size, and the CoCrMo or titanium-based stem alloy pairing were collected. Damage modes and volumetric material loss within the head tapers were identified using an optical coordinate measuring machine. Samples were categorized by damage mode groups by column damage, imprinting, a combination of column damage and imprinting, or uniform fretting. Metallurgical samples were processed to identify microstructural characteristics of grain size, hard phase content, and banding. Nonparametric Mann-Whitney U and Kruskal-Wallis statistical tests were used to examine volumetric material loss compared with damage mode and microstructural features, and linear regression was performed to correlate patient- and manufacturer-specific factors with volumetric material loss.
Results
Chemically driven column damage was seen in 48% (52 of 108) of femoral heads, with 34% (37 of 108) exhibiting a combination of column damage and imprinting, 12% (13 of 108) of heads displaying column damage and uniform fretting, and 2% (2 of 108) exhibiting such widespread column damage that potentially underlying mechanical damage modes could not be verified. Implants with column damage showed greater material loss than those with mechanically driven damage alone, with median (range) values of 1.2 mm3 (0.2 to 11.7) versus 0.6 mm3 (0 to 20.7; p = 0.03). Median (range) volume loss across all femoral heads was 0.9 mm3 (0 to 20.7). Time in situ, contact area, patient age, sex, head size, manufacturer, and stem alloy type were not associated with volumetric material loss. Banding of the alloy microstructure, with a median (range) material loss of 1.1 mm3 (0 to 20.7), was associated with five times higher material loss compared with those with a homogeneous microstructure, which had a volume loss of 0.2 mm3 (0 to 4.1; p = 0.02). Hard phase content and grain size showed no correlation with material loss.
Conclusion
Chemically dominated column damage was a clear indicator of greater volume loss in this study sample of 108 severely damaged heads. Volumetric material loss strongly depended on banding (microstructural segregations) within the alloy. Banding of the wrought CoCrMo microstructure should be avoided during the manufacturing process to reduce volumetric material loss and the release of corrosion products to the periprosthetic tissue.
Clinical Relevance
Approximately 30% of THAs rely on wrought CoCrMo femoral heads. Most femoral heads in this study exhibited a banded microstructure that was associated with larger material loss and the occurrence of chemically dominated column damage. This study suggests that elimination of banding from the alloy could substantially reduce the release of implant debris in vivo, which could potentially also reduce the risk of adverse local tissue reactions to implant debris.
Introduction
Although total hip arthroplasty (THA) remains one of the most successful surgical interventions available today, implant failure due to fretting and corrosion damage is still a major clinical concern [4, 17, 25]. Metal-on-metal THA failure due to adverse local tissue reactions (ALTR) to cobalt-chromium-molybdenum (CoCrMo) debris is well documented [10, 11, 34, 39]. However, less is known about damage processes within modular taper junctions of metal-on-polyethylene THAs and why they too can lead to ALTR with severity similar to metal-on-metal implants [15, 16]. One recent single-center study reported a 5% incidence of fretting corrosion–related revisions in THA [28], while others attributed up to 2% of THA revisions to ALTR [4, 14], which is within the same magnitude as implant failure due to periprosthetic infection (1% to 3%) [9, 41]. Furthermore, patients undergoing revision of metal-on-polyethylene THA exhibit a high risk of early major complications [7, 52]. Thus, ALTRs caused by debris generated from CoCrMo alloy within modular taper junctions have evident clinical repercussions.
In general, damage within the taper junctions can be categorized as mechanically assisted crevice corrosion or fretting corrosion [20, 26, 27]. Although both terms apply to certain aspects of the damage seen, they are simplifications of the damage processes, as damage modes observed in head-stem modular junctions of failed metal-on-polyethylene implants can vary by type and severity [26] and cannot be fully classified by two categories. It has been reported that the onset of micromotion and subsequent fretting and corrosion damage to the femoral head is considerably affected by various factors such as taper surface topography, flexural rigidity, and taper angle [2, 22, 32, 36, 44, 49]. Once damage within the modular junction begins with the onset of micromotion, other distinct damage modes may progress that can be mechanically dominated, chemically dominated, or a combination of both. Prominent examples of dominant and easily identifiable damage modes on femoral head tapers are imprinting and column damage, which can usually be seen on the implant macroscopically [23]. Imprinting is a mechanically dominated fretting process known to occur in heads coupled with a rough stem taper topography that drives local contact mechanics at the machining line peaks, leading to localized fretting and penetration of the machining line peaks into the head taper surface [6]. Femoral heads that are coupled with a smooth stem taper would usually not exhibit imprinting but can still undergo fretting, as evidenced by areas of uniform fretting damage [26]. Column damage is not fretting driven, but rather a chemically dominated etching process that is dictated by banding within the microstructure of many wrought CoCrMo alloys. This banding is suspected to result from the formation of segregations in the alloy during thermomechanical processing, resulting in bands that are depleted of molybdenum content [23]. Considering the importance of molybdenum in the corrosion behavior of the alloy [19, 38, 45], areas with low molybdenum content are considered preferential corrosion sites, which can be visualized by etching or by in vivo corrosion processes resulting in the column damage pattern [5, 23, 29, 48].
Although imprinting and fretting can occur in any type of metal femoral head, column damage is a unique feature to wrought alloy heads and would not be expected in the cast alloys that are commonly used in femoral heads of large metal-on-metal bearings [23, 46, 48]. It is important to note that currently more than 33% of femoral heads used in the United States are made from CoCrMo alloy. Additionally, 88% of heads have a diameter of either 36, 32, or 28 mm and are predominantly made from wrought CoCrMo alloy [1]. Therefore, it is of clinical importance to ensure that the microstructure of this alloy is best equipped to withstand an in vivo corrosive attack.
Besides the aforementioned banding, additional microstructural characteristics may affect the corrosion behavior of wrought CoCrMo alloy, specifically the grain size and hard phase content. Grain size is an important factor for the strength of a metallic alloy, but there is little knowledge regarding its impact on the in vivo electrochemical behavior of CoCrMo alloys used in THA. Hard phases—which may include carbides and intermetallic phases—provide resistance to deformation and abrasion, yet in vitro studies also suggest that hard phases provide preferential corrosion sites in wrought alloys [35, 50]. It is not known how great an effect these microstructural features may have on volumetric material loss in vivo.
After damage modes begin to proliferate through the modular junction, corrosion products from the implant are released into the surrounding tissues and synovial space. Previous studies have shown ALTR development that includes widespread necrosis in the surrounding hip capsule tissue [10, 11, 15, 24, 43]. A more in-depth classification of the damage modes and alloy characteristics occurring in CoCrMo femoral heads associated with large volumes of corrosion product release may lead to better identification of risk factors for implant failure and adverse patient outcomes. Therefore, we sought to characterize the damage patterns and to quantify material loss of femoral head tapers exhibiting severe mechanically and chemically dominated damage modes, and to examine the role of alloy microstructure, patient factors, and implant design features in the damage process.
We asked: (1) How prevalent is chemically dominated column damage compared with mechanically dominated damage modes in severely damaged metal-on-polyethylene THA femoral heads made from wrought CoCrMo alloy? (2) Is material loss greater in femoral heads that underwent column damage? (3) Do material loss and the presence of column damage depend on alloy microstructure as characterized by grain size, hard phase content, and/or banding?
Materials and Methods
Overview
To answer our research questions, all available retrieved femoral heads from metal-on-polyethylene THAs with severe damage received between June 2004 and June 2019 were identified. We examined the head taper junctions and classified the dominant damage modes based on the visible damage features. We distinguished groups with either mechanically or chemically dominated damage and those that exhibited both. The volumetric material loss was determined for each femoral head and compared between different damage groups. Finally, we sectioned all femoral heads for a metallographic assessment of the alloy microstructure with respect to grain size, hard phase volume fraction, and the presence of banding. Microstructural metrics were correlated with the occurrence of chemically dominant damage—specifically column damage—and volumetric material loss.
Retrieval Sample Cohort
All surgically retrieved CoCrMo femoral heads collected from June 2004 to June 2019 (n = 1002) in the Biocompatibility and Implant Pathology Laboratory at Rush University Medical Center in Chicago, IL, USA, were evaluated (Fig. 1). Patient records were reviewed to identify patient and implant characteristics that may influence the mechanically assisted crevice corrosion or fretting corrosion processes. Patient characteristics included patient age at the time of implantation, sex, reason for removal, and implant time in situ (Table 1). Implant characteristics included manufacturer, head size, and stem alloy type (CoCrMo or titanium alloy [Ti-alloy]) (Table 2). For this study, we only considered femoral heads from metal-on-polyethylene THAs with severe damage (damage score 4, see next section), and excluded metal-on-metal THAs, bipolar and dual mobility implants, and implants with a metal adaptor sleeve. We also excluded 22 femoral heads that were sectioned before the availability of an assessment tool of volumetric material loss.
Fig. 1.

STROBE diagram of cohort selection from the Rush University Medical Center implant repository.
Table 1.
Patient demographics, reason for revision, and femoral head time in situ (n = 108)
| Age in years | 57 ± 11 |
| Sex | |
| Male | 56 (61) |
| Female | 44 (47) |
| Time in situ in months | 99 ± 78 |
| Reason for revision | |
| Aseptic loosening | 25 (27) |
| Dislocation/instability | 18 (19) |
| Infection | 16 (17) |
| ALTR | 9 (10) |
| Pain | 8 (9) |
| PE liner wear | 8 (9) |
| Corrosion | 2 (2) |
| Periprosthetic fracture | 2 (2) |
| Femoral stem fracture | 1 (1) |
| Iliopsoas impingement | 1 (1) |
| Not available | 10 (11) |
Data presented as mean ± SD or % (n).
Table 2.
Femoral head manufacturer, size, and paired stem alloy
| Variable | Number of heads |
| Head manufacturer | |
| Zimmer Biomet | 58 |
| DePuy Synthes | 18 |
| Stryker | 13 |
| Richards (now Smith & Nephew) | 4 |
| WMT (now Stryker) | 3 |
| Biomet (now Zimmer Biomet) | 4 |
| Howmedica (now Stryker) | 3 |
| Smith & Nephew | 2 |
| Johnson & Johnson Orthopaedics (now DePuy Synthes) | 1 |
| Exactech | 1 |
| PLO (now Smith & Nephew) | 1 |
| Head size | |
| 26 mm | 1 |
| 28 mm | 34 |
| 32 mm | 35 |
| 36 mm | 29 |
| 40 mm | 7 |
| 44 mm | 2 |
| Femoral stem alloy | |
| CoCrMo | 45 |
| Ti-alloy | 32 |
| Unknown | 31 |
Assessment of Damage Severity and Type
Damage severity of CoCrMo femoral heads was conducted by two independent observers (DJH, RP), using a modified version of the Goldberg visually based scoring system after examination under a stereo light microscope [21, 26]. The modified Goldberg method used in this study examined both fretting and corrosion damage severity. Damage was scored from least to most severe as (1) none/minimal; (2) mild damage: single band or bands of fretting scars involving at most three machine lines and/or less than 30% of taper surface discolored or dull; (3) moderate: several bands of fretting scars or single bands involving more than three machine lines and/or more than 30% of taper surface discolored or dull and/or less than 10% of the taper surface containing black debris, pits, or etch marks; or (4) severe: several bands of fretting scars or single bands involving several adjacent machine lines and/or flattened areas with nearby fretting scars and/or more than 10% of taper surface containing black debris, pits, or etch marks [21, 26]. Observers were blinded to background data of the implant before scoring. Heads with a score of 4 were further examined to identify the damage modes present (Fig. 1). Our focus was on global damage features. Besides areas with uniform fretting damage, we studied two types of global damage modes that we have previously reported on [23]: imprinting (defined as areas exhibiting an imprint of the stem taper topography into the head taper) and column damage (defined as areas covered by distal-to-proximal running columns that are primarily associated with etching and to a lesser degree may also exhibit fine pitting). These damage modes are labeled as “global” because the associated damage features cover large areas on head taper surfaces and can be spotted by the unaided eye. However, these damage features were additionally confirmed using light microscopic inspection and review of photo realistic images of taper surfaces by three observers (SMM, DJH, RP) so that even femoral heads with early onset of either damage process could be accurately identified. The photo realistic images of the inner head taper surface were obtained from high-precision molds (Microset Blue, Microset) that were measured using an optical coordinate measuring machine (CMM) (Ortholux CMM, Redlux Ltd) with a white light chromatic confocal sensor. To achieve a sufficient amount of data points for an accurate three-dimensional (3D) reconstruction of the taper surface, molds were scanned with two data points per degree of taper circumference and 70 rotations per millimeter of taper length. Light intensity maps of the generated data provided photo realistic images. For better visualization of the entire surface, the 3D data files were unwrapped to a two-dimensional (2D), flat representation of the taper surfaces (Fig. 2) [23]. Based on the visual examination and review of the intensity maps, we differentiated the following groups of femoral heads: Group A, column damage with and without visible areas of uniform fretting damage (Fig. 2A); Group B, imprinting only (Fig. 2B); Group C, column damage and imprinting (Fig. 2C); and Group D, uniform fretting only (Fig. 2D). Additionally, head tapers exhibiting shape deviation from the initial taper geometry were seen across these groups (Fig. 2E). Femoral heads with severe damage encompassing the entire taper surface were also observed across groups (Fig. 2F). We further distinguished femoral heads as (1) implants with chemically dominated damage, that is, any column damage (Groups A and C), versus (2) implants with only mechanically dominated damage, that is, only imprinting or fretting (Groups B and D). We also conducted a scanning electron microscopic (SEM) (JSM-IT500HR/LV, JEOL) analysis of representative examples of each group to provide high-magnification images of damage features associated with the different damage mode groups (Fig. 3).
Fig. 2.
A-F Redlux coordinate measuring machine scans of femoral head tapers. Heat maps, in color, represent variations in surface height, with red representing higher points and purple indicating lower. Sensor intensity maps (black and gray) are photo realistic images of the stem taper surface. Red boxes indicate areas with scanning electron microscopy images included in Fig. 3. (A) Chemically driven column damage (Group A) can be seen in and around areas with the most material loss. (B) Mechanically driven imprinting (Group B) covering almost the entire contact area of the femoral head. The original topography can still be seen distally where the stem taper was not in contact. (C) Combination of column damage and imprinting (Group C) as shown here was observed in 37 implants. (D) Mechanically driven uniform surface fretting (Group D) covering the entire surface of the head taper. (E) Severe column damage (Group A) can be seen in this implant covering almost the entire contact area of the head taper. The distal area of the head taper was not in contact with the stem. It can be seen that the head exhibited a radial shape deviation, possibly caused during the manufacturing process. (F) This head taper is an example of excessive damage (in this case, mostly column damage) covering the entire head taper area. Thus, fitting and reconstruction of the initial taper geometry and volume loss calculation were impossible.
Fig. 3.

A-F SEM micrographs oriented from proximal to distal (top of the image to bottom) of femoral head tapers representing various damage modes: (A) imprinted (large horizontal bands) and (B) original head taper topography (small horizontal bands) of the imprinted implant illustrated in Fig. 2B and marked by red squares. (C) Overlapping imprinting (arrows) and column damage (vertical bands) features indicated in Fig. 2C. (D) Low and (E) high magnification of typical fretting/ fretting corrosion features observed in a head taper with widespread fretting damage as indicated in Fig. 2D. (F) An example of severe column damage as illustrated in Fig. 2E.
Final Sample Selection
Based on the described exclusion criteria and the results of the damage scoring, we excluded 812 heads for lack of severe (score 4) damage. We excluded an additional 81 heads because they were determined to be from metal-on-metal, bipolar, or dual-mobility designs; had metal adaptor sleeves; or had been previously sectioned. Of the remaining 109 heads, one was removed from the study retroactively after metallographic analysis revealed it to be made from cast alloy rather than wrought alloy. This resulted in a final count of 108 femoral heads (Fig. 1) from 11 manufacturers. Head sizes ranged from 26 to 44 mm (Table 2). Matched femoral stems were available for 31 of the 108 femoral heads.
Assessment of Volumetric Material Loss from Femoral Head Taper Surfaces
Total volume loss of head taper surfaces and head taper contact area were quantified from CMM data using RedLux Studio Version 4.0 software (Redlux Ltd) by fitting a perfect cone geometry to undamaged areas of the taper. This method is specified by ASTM standard F3129 and was previously validated for the optical CMM and software we used [13]. Volume loss was determined in areas deviating from the ideal cone shape, and heat maps were generated to visualize areas of material loss (Fig. 2) [12]. Additionally, heat maps were also used to identify contact area between the stem and head tapers. Areas not in contact with the stem showed completely unaltered original head taper topography. The distance from the distal to the proximal end of the contact area was measured to calculate the nominal contact area between head and stem. Total material loss could be calculated for 75 implants, whereas in 33 implants, no material loss could be computed because severe damage covering the entire contact area made it impossible to reconstruct the original taper geometry for reference (Fig. 2F). Heads for which material loss could not be computed were distributed between groups as follows: Group A = 5, Group B = 8, Group C = 18, and Group D = 2, constituting 33%, 22%, 49%, and 11% of each group, respectively (Table 3).
Table 3.
Damage mode groups
| Group | Observed damage modes | Mechanically or chemically driven | Number of femoral heads in group | Number with no volume measurement calculable |
| Group A | Column damage (with/without fretting) | Chemical (+ mechanical) | 15 | 5 |
| Group B | Imprinting | Mechanical | 37 | 8 |
| Group C | Imprinting and column damage | Chemical + mechanical | 37 | 18 |
| Group D | Uniform surface fretting | Mechanical | 19 | 2 |
Metallographic Assessment of Femoral Head Alloy Microstructure
Femoral heads were processed for metallographic analysis to identify microstructural characteristics. Two samples were made per head. The first sample was used to analyze banding of the microstructure and to determine the mean grain size of the alloy. Both characteristics can be visualized using light microscopy after a chemical etching treatment. The second sample was used to determine the hard phase content and to classify the alloy subtypes, where a low hard phase fraction of less than 0.5% was classified as a low carbon alloy and alloys with a considerable hard phase fraction greater than 2% were classified as high carbon alloy [46, 48]. Heads were sectioned in the longitudinal direction, corresponding to the machining direction of bar stock material. Struers equipment (Secotom-50, CitoPress-1, Tegramin-30, Struers Inc) was used for sectioning, processing, and polishing samples to a 1-µm mirror finish for etching. Chemical etching was conducted using prior protocols for the visualization of metallurgical characteristics [23]. Banding was visualized using a Nikon Eclipse LV150N (Nikon Inc), and mean grain size and hard phase area fraction were computed using NIS-Elements 64-bit software (Nikon Inc) (Fig. 4).
Fig. 4.
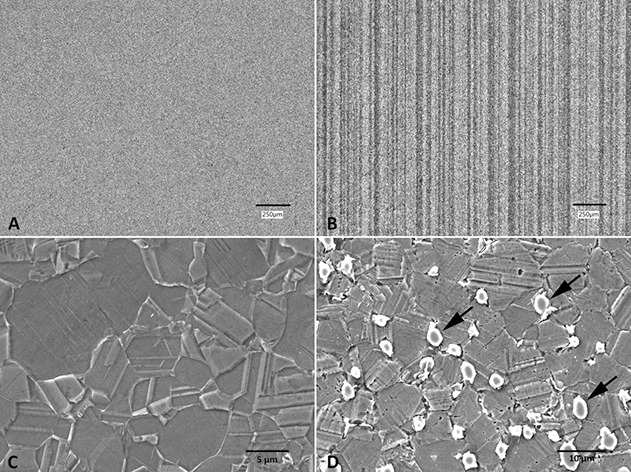
A-D Polished and etched cross sections of retrieved wrought CoCrMo alloy femoral heads, demonstrating metallurgical characteristics. (A) Nonbanded microstructure showing homogenous alloy. (B) Banded microstructure showing vertical alloy segregations depleted in molybdenum content. (C) Low carbon alloy. (D) High carbon alloy with carbides and intermetallic phases (arrows) (x 2500).
Primary and Secondary Study Outcomes
The primary outcomes of this study were the prevalence of global damage modes (column damage, imprinting, uniform fretting) and their combinations, volumetric material loss due to the generation of corrosion products in vivo depending on the acting global damage modes, as well as microstructural metrics of wrought CoCrMo alloy (grain size, hard phase volume fraction, and banding) and how they relate to material loss. Secondary outcomes included the effect of time in situ, patient age, sex, contact area, femoral head size, and femoral stem material on the material loss from wrought CoCrMo femoral heads.
Ethical Approval
Ethical approval for this study was obtained from the institutional review board of Rush University Medical Center (ORA number: 10070701-IRB03-CR09).
Statistical Analysis
Statistical analysis was conducted using SPSS Premium 22 software (IBM Corp). Volume loss was not normally distributed; therefore, we conducted comparisons between different groups with nonparametric methods using the Kruskal-Wallis and Mann-Whitney U tests. Separate linear regression models were conducted to correlate time in situ, patient age, and stem-head contact area with volumetric material loss. Statistical significance was set as p ≤ 0.05.
Results
Prevalence of Chemically Dominated Column Damage
The results highlight that chemically driven column damage was seen in just under half, 48% (52 of 108), of the severely damaged heads in this study. Column damage occurred in combination with imprinting in 34% (37 of 108) of femoral heads, and 12% (13 of 108) of heads exhibited column damage with at least some areas of uniform fretting. The remaining two femoral heads with column damage exhibited no clearly identifiable areas of mechanically dominated damage, largely because excessive column damage scars made identification of previous fretting damage features too difficult (Fig. 2F). Mechanically dominated modes of damage, manifesting as either imprinting or uniform fretting damage, were the sole damage mode observed in 34% (37 of 108) and 18% (19 of 108), respectively.
Volumetric Material Loss
Heads with chemically dominated damage exhibited greater median (range) volumetric material loss than those without (1.2 mm3 [0.2 to 11.7] versus 0.6 mm3 [0 to 20.7]; p = 0.03) (Fig. 5). Across all femoral heads, the median material loss was 0.9 mm3 (0 to 20.7). Linear correlation revealed that material loss did not correlate with time in situ (R2 = 0; p = 0.89), patient age (R2 = 0.01; p = 0.38), and contact area between head and stem taper (R2 = 0.002; p = 0.72). Nonparametric tests showed independence of material loss from implant- and patient-specific characteristics. Mann-Whitney U tests considering patient sex showed a median material loss of 0.7 mm3 (0.4 to 10.9) in male patients and 0.7 mm3 (0.4 to 10.9) in female patients (p = 0.59). Heads paired with CoCrMo femoral stems had a median material loss of 0.7 mm3 (0 to 5.3), and Ti-alloy stems had 1.2 mm3 (0 to 20.7; p = 0.32). Material loss by manufacturer and femoral head size showed no differences (see Table 1; Supplemental Digital Content 1, http://links.lww.com/CORR/A570)
Fig. 5.

The presence of chemically dominated damage (column damage) is associated with larger material loss. Femoral heads (n = 108) were categorized based on macroscopic and CMM observation of chemically driven column damage type damage mode. There was more material loss in the column damage group (p = 0.03) as compared with nonchemically driven damage types.
With respect to the damage type Groups A through D, the median material loss was 1.3 mm3 (0.2 to 11.7), 0.7 mm3 (0 to 20.7), 1.1 mm3 (0.3 to 7.4), and 0.2 mm3 (0 to 13.3), respectively. A difference between groups was observed with the Kruskal-Wallis test (p = 0.02). Upon further examination, both Group A and Group C exhibited higher material loss compared with Group D (p = 0.02 and p = 0.01, respectively) (Fig. 6). No difference was noted between Groups B and D, which exhibited the mechanically dominated damage modes. No other group comparisons exhibited a difference.
Fig. 6.

Damage mode groups versus material loss. Femoral heads (n = 108) were categorized into four groups based on the macroscopic and CMM observation of damage mode type: Group A, column damage with and without visible areas of uniform fretting damage; Group B, imprinting only; Group C, column damage and imprinting; and Group D, uniform fretting only. A difference was found between Groups A and D (p = 0.02) and between Groups C and D (p = 0.01). Visual representations of these damage modes are provided. (Fig. 2).
Microstructural Effects on Damage Mode and Volumetric Material Loss
The presence of banding was the only microstructural feature that affected material loss and was observed in all femoral heads with column damage. Femoral heads with banding had larger volumetric material loss than those with a homogenous microstructure (1.1 mm3 [range 0 to 20.7] versus 0.2 mm3 [0 to 4.1]; p = 0.02) (Fig. 7). Surface etching revealed that 81% (88 of 108) of implants had a banded microstructure (Fig. 4) including all heads removed for ALTR or corrosion based on operative reports (Table 1). Only one head with banding had a high carbon content. The mean ± SD grain size across all femoral heads was 4.9 ± 1.7 µm, and linear regression revealed that grain size was not correlated with material loss (R2= 0.007; p = 0.49). In all, 93% (100 of 108) of heads were made from low carbon alloy, and only 7% (8 of 108) from high carbon alloy (Fig. 4). The mean hard phase volume fraction in high carbon alloys was 5.7% ± 1.2%. Comparison of median (range) material loss from high and low carbon alloy heads demonstrated no difference (p = 0.2) with 0.9 mm3 (0 to 20.7) and 0.2 mm3 (0 to 4.1), respectively.
Fig. 7.
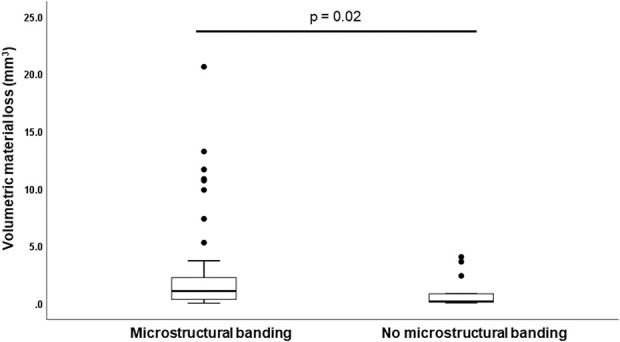
Banded microstructure versus material loss. Femoral heads (n = 108) were categorized based on banding of the microstructure visualized through etching processes. The banded group had more material loss (p = 0.02) compared with alloys with no banding.
Other Findings
It was an unexpected secondary finding that 24% (26 of 108) of femoral heads exhibited circumferential out-of-roundness visible outside of the contact area, with a local diameter differential ranging from 4 to 12 μm (Fig. 2E).
Discussion
Approximately one-third of femoral heads used in metal-on-polyethylene THAs are made from wrought CoCrMo alloy and are a major source of implant debris—generated under fretting and corrosion within modular taper junctions—eliciting adverse local tissue reactions and implant failure in some patients. Banding within the wrought alloy microstructure can occur and appears to offer preferential corrosion sites that enable a chemically dominated damage mode known as column damage in vivo. Yet, it is not known how prevalent or damaging to the femoral head column damage is, and how other microstructural features may relate to the release of implant debris from CoCrMo femoral heads. We sought to answer the following research questions: (1) How prevalent is column damage compared with purely fretting-driven damage modes in severely damaged femoral heads? (2) Is material loss greater from femoral heads that developed column damage? (3) Do material loss and column damage depend on alloy microstructure as characterized by grain size, hard phase content, and banding? We found that column damage occurred in close to 50% of the included femoral heads and was indeed associated with double the material loss compared with heads that only underwent fretting damage. Furthermore, all femoral heads with column damage exhibited banding of the microstructure, and femoral heads with banding exhibited five times larger material loss compared with femoral heads with a homogenous microstructure, independent of the occurrence of column damage.
Limitations
There are several limitations to this study. First, the frequency of certain implant designs was higher than others, yielding an uneven distribution of femoral heads due to the fact that implants were retrieved at a single medical center. Retrievals in this study overwhelmingly reflect devices typically used by surgeons at Rush University Medical Center, with only 6% (7 of 108) of the retrieved implants resulting from a primary surgery outside of this institution. Despite the uneven manufacturer groups, we did not observe any difference between material loss or any microstructural features—including the incidence of banding—among manufacturers. It is our understanding that, predominantly, manufacturers use the same suppliers for wrought CoCrMo bar stock, which may explain why the manufacturer is not a determining factor for material loss. Secondly, other important variables such as angular mismatch and the corresponding stem taper topography could not be identified for 68% of the retrieved implants because only the femoral head was replaced during revision surgery to conserve femoral bone stock, so no additional data on the stem tapers could be collected. For the same reason, the damage mode and material loss on the stem taper side could not be assessed for most of the retrieved implants in this study. Material loss for 31 stem tapers was available (see Table 2; Supplemental Digital Content 2, http://links.lww.com/CORR/A571). We found less material loss in stem tapers made from Ti-alloy compared with those made from CoCrMo alloy and overall lower material loss in either type of stem compared with femoral heads, consistent with previous studies [18, 31]. Based on our earlier work, we expected that mechanically dominated fretting damage would occur independent of stem material, whereas chemically dominated damage modes such as intergranular corrosion and phase boundary corrosion would mainly occur on CoCrMo stems [26]. We also learned previously that pitting corrosion—another chemically dominated damage process—can occur within femoral heads but would only be detected at high magnifications in an SEM [26]; such analysis was beyond the scope of the current study. Other chemically dominated damage modes such as intergranular corrosion and phase boundary corrosion were not detected to a noticeable extent. These were not expected, however, as those damage modes are more common in cast CoCrMo alloys, mostly occurring on the stem taper side of metal-on-polyethylene components [26, 48] and within larger heads used in metal-on-metal THAs [8, 46]. Finally, we were not able to compute volumetric material loss for all samples due to damage of the entire head taper area in 31% of implants.
Another limitation is that surgical factors could not be considered in this study. The load applied by the surgeon during head/stem assembly and intraoperative cleaning of the taper have been shown to be important factors that enable fretting and corrosion [22, 30, 33, 37]. However, these factors would likely only impact the onset of micromotion and subsequent fretting but not the progression. By focusing only on femoral heads with severe damage in this study, it can be argued that factors driving the start of the damage process may not play a role in the later stage progression of the damage.
Prevalence of Chemically Dominated Column Damage
Chemically dominated column damage was the second most frequent observation in femoral head tapers with severe damage, although it also coincided with areas of mechanically dominated damage in most implants. The only femoral heads without any type of visible features associated with mechanically dominated damage modes were excessively scarred by column damage, so it was not possible to determine whether other damage modes may have taken place at an earlier stage. It therefore appears possible that column damage occurs secondary to mechanically dominated damage modes. Imprinting was the damage mode that occurred most frequently among all heads with severe damage, whereas uniform fretting occurred in only 16% of implants. Both damage modes can be categorized as mechanically dominated fretting processes with underlying fretting corrosion [23, 48]. Whether one or the other occurs appears to depend on the surface topography of the stem taper, where a microgrooved topography results in imprinting and a smooth topography results in less predictable contact mechanics, more evenly distributed contact points within the head/stem taper junction, and subsequently areas with uniform damage features [3, 36]. Based on our previous research, we have seen that the onset of micromotion is more likely, and that the resulting damage scores are higher with a smooth stem taper topography [49]. Yet, if micromotion is initiated, modular junctions will undergo fretting and damage will manifest in different ways depending on the topography-driven contact mechanics.
Volumetric Material Loss
We found larger volumetric material loss in femoral heads that exhibited column damage compared with those that exhibited only mechanically dominated damage modes, especially uniform fretting. The reason for this difference is likely the combined effect of chemically induced etching of preferential corrosion sites and the wear under micromotion. The average material loss found across groups was larger than that of a previous study examining material loss of CoCrMo femoral heads, which was 0.4 mm3 (0 to 4.3) [31]. This difference is mostly attributed to the fact that the previous study evaluated across all damage scores, whereas this study focused only on severely damaged head tapers. It is important to note that the overall material loss was independent of time in situ. Therefore, no material loss rate—analogous to volumetric wear rates for articulating surfaces—was established. Moreover, material loss was also independent of the nominal contact area between head and stem taper, and of patient age, which is a common surrogate for patient activity level [47]. The lack of these relationships highlights that the damage process is erratic, which may partially be explained by the complex chemical environment within the modular junction that is variable over time.
Microstructural Effects on Damage Mode and Volumetric Material Loss
In this study, banding of the wrought CoCrMo microstructure was associated with greater material loss and occurred in all femoral heads exhibiting column damage. Therefore, based on a group of 108 wrought CoCrMo alloy femoral heads from different manufacturers, a banded alloy microstructure appeared to adversely affect material loss by providing preferential corrosion sites often resulting in column damage. The in vivo conditions resulting in a chemical attack on such corrosion sites remain unknown. These findings also align with previous electrochemical tests demonstrating inferior corrosion behavior for CoCrMo alloy surfaces with a banded microstructure [48]. We have also previously shown that banding of the alloy is characterized by alternating areas with different molybdenum content and, to a lesser degree, chromium content [23]. Such segregation is possibly the result of an insufficient homogenization applied during the thermomechanical alloy processing [53]. Its occurrence is impactful because both molybdenum and chromium are essential elements for the formation and stability of the protective passive film that instantaneously forms on the surface of CoCrMo alloy [45]. Banding likely enables a gradient of the electrochemical potential, leading to local galvanic effects that promote higher corrosion rates in areas depleted of molybdenum and chromium. However, we could not confirm previous reports of implant-wide galvanic corrosion leading to larger material loss on CoCrMo heads coupled with Ti-alloy stems compared with those with CoCrMo alloy stems [40, 42]. However, these previous studies compared only the average damage scores of femoral heads coupled with different stems, whereas another recent study of the maximum linear corrosion depth within femoral head tapers also found no difference depending on stem type [51]. Interestingly, femoral heads with a banded microstructure that did not exhibit column damage also had higher material loss (Fig. 7); in fact, all outliers with excessive material loss fell within the banded group. This observation suggests that the local depletion of molybdenum and chromium may negatively affect the formation and stability of the passive film promoting cyclic abrasion and reformation under micromotion (fretting corrosion).
Clinical Relevance
More than 30% of femoral heads in metal-on-polyethylene THA implanted today are made from wrought CoCrMo alloy [1]. In this study, more than 80% of the subset of severely damaged CoCrMo alloy heads examined exhibited banding of the microstructure, and this was associated with five times higher material loss compared with the 20% with a homogeneous microstructure. This banded microstructure appears to be related to the occurrence of column damage. Although to date the dose dependence between material loss from the modular taper junction and the severity of ALTR in metal-on-polyethylene THA has not been established, it may be speculated that reduced material loss may also lower the risk of an adverse local tissue reaction to corrosion products and subsequent implant failure. This speculation is supported by the fact that all 10 femoral heads in this study removed for ALTR or corrosion (based on operative diagnosis codes) (Table 1) exhibited banding.
Conclusion
We determined that chemically dominated column damage occurred in 48% of metal-on-polyethylene THA femoral heads made from wrought CoCrMo alloy in our study of 108 retrievals selected for severe damage. Column damage was associated with larger material loss and appeared to be enabled by banding of the alloy microstructure that occurred in 80% of wrought alloy heads in this study. A banded microstructure provides preferential corrosion sites and also seems to exhibit inferior fretting behavior in this cohort. It is an important finding that the 20% of implants that did not exhibit banding of the microstructure had five times lower material loss in the form of corrosion products. The reduction of corrosion products may potentially reduce the risk of implant failure due to an adverse local tissue reaction, which is a current clinical concern. Both types of microstructure—banded and homogenous—are currently not differentiated in material standards and are therefore permissible. Thus, we suggest that banding be eliminated from alloys used for implant components that constitute a modular junction. Further research is needed to determine whether there is a dose threshold as well as the type of corrosion products and their potential relationships to ALTR in metal-on-polyethylene THA, and therefore, how advantageous a homogenous wrought CoCrMo alloy microstructure would be clinically.
Supplementary Material
Acknowledgments
We thank Mozart Queiroz Neto PhD, Jennifer Wright, and Mable Je for their support in conducting the metallographic analysis for this study, and we thank Robert Urban for his contributions to the establishment and management of the retrieval repository.
Footnotes
One of the authors certifies that he (JJJ), or a member of his immediate family, has received or may receive payments or benefits, during the study period, in an amount of less than USD 10,000 from Implant Protection; in an amount of less than USD 10,000 from Medtronic; in an amount of less than USD 10,000 from Nuvasive; and in an amount of less than USD 10,000 from Zimmer Biomet.
One of the authors certifies that she (HJL), or a member of her immediate family, has received or may receive payments or benefits, during the study period, in an amount of less than USD 10,000 from Zimmer Biomet.
One of the authors certifies that he (RP), or a member of his immediate family, has received or may receive payments or benefits, during the study period, in an amount of less than USD 10,000 from Zimmer Biomet.
The institutions of one or more of the authors (MTM, HJL, RP) have received, during the study period, funding from the NIH/NIAMS (R01 AR070181).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Ethical approval for this study was obtained from the institutional review board of Rush University Medical Center (ORA number: 10070701-IRB03-CR09).
Contributor Information
Deborah J. Hall, Email: deborah_hall@rush.edu.
Mathew T. Mathew, Email: mtmathew@uic.edu.
Joshua J. Jacobs, Email: Joshua.Jacobs@rushortho.com.
Hannah J. Lundberg, Email: hannah_lundberg@rush.edu.
Robin Pourzal, Email: robin_pourzal@rush.edu.
References
- 1.American Joint Replacement Registry. Fourth AJJR Annual Report on Hip and Knee Arthroplasty Data. Available at: http://connect.ajrr.net/2019-ajrr-annual-report. Accessed April 13, 2021.
- 2.Arnholt CM, MacDonald DW, Underwood RJ, et al. Do stem taper microgrooves influence taper corrosion in total hip arthroplasty? A matched cohort retrieval study. J Arthroplasty. 2017;32:1363-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bechstedt M, Gustafson JA, Mell SP, et al. Contact conditions for total hip head-neck modular taper junctions with microgrooved stem tapers. J Biomech. 2020;103:109689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berstock JR, Whitehouse MR, Duncan CP. Trunnion corrosion: what surgeons need to know in 2018. Bone Joint J. 2018;100-B:44-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bijukumar DR, Salunkhe S, Morris D, et al. In vitro evidence for cell-accelerated corrosion within modular junctions of total hip replacements. J Orthop Res. 2020;38:393-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bishop N, Witt F, Pourzal R, et al. Wear patterns of taper connections in retrieved large diameter metal-on-metal bearings. J Orthop Res. 2013;31:1116-1122. [DOI] [PubMed] [Google Scholar]
- 7.Bonner B, Arauz P, Klemt C, et al. Outcome of re-revision surgery for adverse local tissue reaction in metal-on-polyethylene and metal-on-metal total hip arthroplasty. J Arthroplasty. 2020;35:S284-S288. [DOI] [PubMed] [Google Scholar]
- 8.Bowsher JG, Nevelos J, Williams PA, Shelton JC. ‘Severe’ wear challenge to ‘as-cast’ and ‘double heat-treated’ large-diameter metal-on-metal hip bearings. Proc Inst Mech Eng H. 2006;220:135-143. [DOI] [PubMed] [Google Scholar]
- 9.Bozic KJ, Kurtz S, Lau E, et al. The epidemiology of bearing surface usage in total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91:1614-1620. [DOI] [PubMed] [Google Scholar]
- 10.Campbell P, Beaulé PE, Ebramzadeh E, et al. The John Charnley Award: A study of implant failure in metal-on-metal surface arthroplasties. Clin Orthop Relat Res. 2006;453:35-46. [DOI] [PubMed] [Google Scholar]
- 11.Campbell P, Ebramzadeh E, Nelson S, et al. Histological features of pseudotumor-like tissues from metal-on-metal hips. Clin Orthop Relat Res. 2010;468:2321-2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook RB, Bolland BJRF, Wharton JA, et al. Pseudotumour formation due to tribocorrosion at the taper interface of large diameter metal on polymer modular total hip replacements. J Arthroplasty. 2013;28:1430-1436. [DOI] [PubMed] [Google Scholar]
- 13.Cook RB, Maul C, Strickland AM. Validation of an optical coordinate measuring machine for the measurement of wear at the taper interface in total hip replacement. In: Greenwald AS, Kurtz SM, Lemons JE, Mihalko WM, eds. Modularity and Tapers in Total Joint Replacement Devices. ASTM International; 2015:362-378. [Google Scholar]
- 14.Cooper HJ, Della Valle CJ, Berger RA, et al. Corrosion at the head-neck taper as a cause for adverse local tissue reactions after total hip arthroplasty. J Bone Joint Surg Am. 2012;94:1655-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper HJ, Urban RM, Wixson RL, Meneghini RM, Jacobs JJ. Adverse local tissue reaction arising from corrosion at the femoral neck-body junction in a dual-taper stem with a cobalt-chromium modular neck. J Bone Joint Surg Am. 2013;95:865-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Villiers D, Traynor A, Collins SN, Shelton JC. The increase in cobalt release in metal-on-polyethylene hip bearings in tests with third body abrasives. Proc Inst Mech Eng H. 2015;229:611-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Della Valle CJ, Calkins TE, Jacobs JJ. Diagnosing taper corrosion: when is it the taper and when is it something else? J Arthroplasty. 2018;33:2712-2715. [DOI] [PubMed] [Google Scholar]
- 18.Di Laura A, Hothi H, Henckel J, et al. Retrieval analysis of metal and ceramic femoral heads on a single CoCr stem design. Bone Joint Res. 2017;6:345-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer A, Janssen D, Wimmer MA. The influence of molybdenum on the fretting corrosion behavior of CoCr/TiAlV couples. Biotribology. Available at: http://www.sciencedirect.com/science/article/pii/S2352573816300555. Accessed May 30, 2021.
- 20.Gilbert JL, Buckley CA, Jacobs JJ. In vivo corrosion of modular hip prosthesis components in mixed and similar metal combinations. The effect of crevice, stress, motion, and alloy coupling. J Biomed Mater Res. 1993;27:1533-1544. [DOI] [PubMed] [Google Scholar]
- 21.Goldberg JR, Gilbert JL, Jacobs JJ, Bauer TW, Paprosky W, Leurgans S. A multicenter retrieval study of the taper interfaces of modular hip prostheses. Clin Orthop Relat Res. 2002;401:149-161. [DOI] [PubMed] [Google Scholar]
- 22.Gustafson JA, Levine BR, Jacobs JJ, Lundberg HJ. Modeling changes in modular taper micromechanics due to surgeon assembly technique in total hip arthroplasty. Bone Joint J. 2020;102:33-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall DJ, McCarthy SM, Ehrich J, et al. Imprinting and column damage on CoCrMo head taper surfaces in total hip replacements. In: Mihalko WM, Lemons JE, Greenwald AS, Kurtz SM, eds. Symposium on Beyond the Implant: Retrieval Analysis Methods for Implant Surveillance. STP1606. ASTM International; 2018:131-155. [Google Scholar]
- 24.Hall DJ, Pourzal R, Della Valle CJ, Galante JO, Jacobs JJ, Urban RM. Corrosion of modular junctions in femoral and acetabular components for hip arthroplasty and its local and systemic effects. In: Greenwald AS, Kurtz SM, Lemons JE, Mihalko W, eds. Modularity and Tapers in Total Joint Replacement Devices. ASTM STP1591. ASTM International; 2015:410-427. [Google Scholar]
- 25.Hall DJ, Pourzal R, Jacobs JJ. What surgeons need to know about adverse local tissue reaction in total hip arthroplasty. J Arthroplasty. 2020;35:S55-S59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall DJ, Pourzal R, Lundberg HJ, Mathew MT, Jacobs JJ, Urban RM. Mechanical, chemical and biological damage modes within head-neck tapers of CoCrMo and Ti6Al4V contemporary hip replacements. J Biomed Mater Res B Appl Biomater. 2018;106:1672-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hannon CP, Cotter EJ, Cooper HJ, et al. Adverse local tissue reaction due to mechanically assisted crevice corrosion presenting as late instability following metal-on-polyethylene total hip arthroplasty. J Arthroplasty. 2020;35:2666-2670. [DOI] [PubMed] [Google Scholar]
- 28.Hussey DK, McGrory BJ. Ten-year cross-sectional study of mechanically assisted crevice corrosion in 1352 consecutive patients with metal-on-polyethylene total hip arthroplasty. J Arthroplasty. 2017;32:2546-2551. [DOI] [PubMed] [Google Scholar]
- 29.Igual Munoz A, Schwiesau J, Jolles BM, Mischler S. In vivo electrochemical corrosion study of a CoCrMo biomedical alloy in human synovial fluids. Acta Biomater. 2015;21:228-236. [DOI] [PubMed] [Google Scholar]
- 30.Knutsen A, Park S-H, Ebramzadeh E, Campbell P. The importance of cleaning modular parts on visual scores of taper damage. In: Greenwald AS, Kurtz SM, Lemons JE, Mihalko W, eds. Modularity and Tapers in Total Joint Replacement Devices. ASTM International; 2015:351-361. [Google Scholar]
- 31.Kocagoz SB, Underwood RJ, MacDonald DW, Gilbert JL, Kurtz SM. Ceramic heads decrease metal release caused by head-taper fretting and corrosion. Clin Orthop Relat Res. 2016;474:985-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kocagöz SB, Underwood RJ, Sivan S, et al. Does taper angle clearance influence fretting and corrosion damage at the head-stem interface? A matched cohort retrieval study. Semin Arthroplasty. 2013;24:246-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krull A, Morlock MM, Bishop NE. The influence of contamination and cleaning on the strength of modular head taper fixation in total hip arthroplasty. J Arthroplasty. 2017;32:3200-3205. [DOI] [PubMed] [Google Scholar]
- 34.Langton DJ, Jameson SS, Joyce TJ, Hallab NJ, Natu S, Nargol AVF. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: a consequence of excess wear. J Bone Joint Surg Br. 2010;92:38-46. [DOI] [PubMed] [Google Scholar]
- 35.Liao Y, Pourzal R, Stemmer P, et al. New insights into hard phases of CoCrMo metal-on-metal hip replacements. J Mech Behav Biomed Mater. 2012;12:39-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lundberg HJ, Ha NQ, Hall DJ, Urban RM, Levine BR, Pourzal R. Contact mechanics and plastic deformation at the local surface topography level after assembly of modular head-neck junctions in modern total hip replacement devices. In: Greenwald AS, Kurtz SM, Lemons JE, Mihalko W, eds. Modularity and Tapers in Total Joint Replacement Devices. ASTM International; 2015:59-82. [Google Scholar]
- 37.Marinier M, Edmiston TA, Kearns S, Hannon CP, Levine BR. A survey of the prevalence of and techniques to prevent trunnionosis. Orthopedics. 2018;41:e557-e562. [DOI] [PubMed] [Google Scholar]
- 38.Martin EJ, Pourzal R, Mathew MT, Shull KR. Dominant role of molybdenum in the electrochemical deposition of biological macromolecules on metallic surfaces. Langmuir. 2013;29:4813-4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matthies A, Underwood R, Cann P, et al. Retrieval analysis of 240 metal-on-metal hip components, comparing modular total hip replacement with hip resurfacing. J Bone Joint Surg Br. 2011;93:307-314. [DOI] [PubMed] [Google Scholar]
- 40.Meyer H, Mueller T, Goldau G, Chamaon K, Ruetschi M, Lohmann CH. Corrosion at the cone/taper interface leads to failure of large-diameter metal-on-metal total hip arthroplasties. Clin Orthop Relat Res. 2012;470:3101-3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ong KL, Kurtz SM, Lau E, Bozic KJ, Berry DJ, Parvizi J. Prosthetic joint infection risk after total hip arthroplasty in the Medicare population. J Arthroplasty. 2009;24:105-109. [DOI] [PubMed] [Google Scholar]
- 42.Osman K, Panagiotidou AP, Khan M, Blunn G, Haddad FS. Corrosion at the head-neck interface of current designs of modular femoral components: essential questions and answers relating to corrosion in modular head-neck junctions. Bone Joint J. 2016;98:579-584. [DOI] [PubMed] [Google Scholar]
- 43.Perino G, Ricciardi BF, Jerabek SA, et al. Implant based differences in adverse local tissue reaction in failed total hip arthroplasties: a morphological and immunohistochemical study. BMC Clin Pathol. 2014;14:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Porter DA, Urban RM, Jacobs JJ, Gilbert JL, Rodriguez JA, Cooper HJ. Modern trunnions are more flexible: a mechanical analysis of THA taper designs. Clin Orthop Relat Res. 2014;472:3963-3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pourbaix M. Electrochemical corrosion of metallic biomaterials. Biomaterials. 1984;5:122-134. [DOI] [PubMed] [Google Scholar]
- 46.Pourzal R. Possible Pathways of Particle Formation in CoCrMo Sliding Wear. Fortschritts-Berichte VDI. VDI-Verlag; 2012. [Google Scholar]
- 47.Pourzal R, Cip J, Rad E, et al. Joint line elevation and tibial slope are associated with increased polyethylene wear in cruciate-retaining total knee replacement. J Orthop Res. 2020;38:1596-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pourzal R, Hall DJ, Ehrich J, et al. Alloy microstructure dictates corrosion modes in THA modular junctions. Clin Orthop Relat Res. 2017;475:3026-3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pourzal R, Hall DJ, Ha NQ, et al. Does surface topography play a role in taper damage in head-neck modular junctions? Clin Orthop Relat Res. 2016;474:2232-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stemmer P, Pourzal R, Liao Y, et al. Microstructure of retrievals made from standard cast HC-CoCrMo alloys. In: Kurtz SM, Greenwald AS, Mihalko WH, Lemons J, eds. Metal-On-Metal Total Hip Replacement Devices. ASTM International; 2013:251-267. [Google Scholar]
- 51.Valente G, Lanting B, MacDonald S, Teeter MG, Van Citters D, Howard J. Femoral head material loss at the head-neck junction in total hip arthroplasty: the effect of head size, stem material and stem offset. Hip Int. 2019;29:647-651. [DOI] [PubMed] [Google Scholar]
- 52.Waterson HB, Whitehouse MR, Greidanus NV, Garbuz DS, Masri BA, Duncan CP. Revision for adverse local tissue reaction following metal-on-polyethylene total hip arthroplasty is associated with a high risk of early major complications. Bone Joint J. 2018;100:720-724. [DOI] [PubMed] [Google Scholar]
- 53.Zachariah Z, Balachandran S, Liu Z, et al. On the formation mechanism of column damage within modular taper junctions. J Arthroplasty. Published online March 4, 2021. DOI: 10.1016/j.arth.2021.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



