Abstract
Background
Lung cancer is one of the most commonly diagnosed cancers and is the leading cause of cancer-related deaths. Metastatic bone disease occurs in 20% to 40% of patients with lung cancer, and these patients often present with pain or skeletal-related events (SREs) that are associated with decreased survival. Bone-modifying agents such as denosumab or bisphosphonates are routinely used; however, to our knowledge, there has been no quantitative synthesis of randomized controlled trial data to determine the most effective pharmacologic treatment of metastatic bone disease because of lung cancer.
Questions/purposes
We aimed to perform a network meta-analysis of randomized trials to identify the bone-modifying agent that is associated with the (1) highest overall survival, (2) longest time to SRE, (3) lowest SRE incidence, and (4) greatest likelihood of pain resolution.
Methods
We conducted our study according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses protocol and pre-registered the analysis on PROSPERO (ID: CRD42019124364). We performed a librarian-assisted search of MEDLINE, PubMed, EMBASE, Cochrane Library, and Chinese databases including China National Knowledge Infrastructure and Wanfang Data. We included randomized controlled trials reporting outcomes specifically for patients with lung cancer treated with a bisphosphonate or denosumab. SREs included pathologic fractures, spinal cord compression, hypercalcemia of malignancy, or pain resulting in surgical intervention or radiation therapy. We excluded trials exclusively reporting surrogate outcomes such as changes in bone turnover markers. Screening, data extraction, risk of bias evaluation, and Grading of Recommendations Assessment, Development, and Evaluation evaluations were performed in duplicate. We included 131 randomized controlled trials that evaluated 11,105 patients with skeletal metastases from lung cancer. The network meta-analysis was performed using a frequentist model and the R statistical software. Results are reported as relative risks or mean differences, and the I2 value is reported for heterogeneity. The P-score, a measure of ranking certainty that accounts for standard error, is reported for each outcome. Heterogeneity in the network was considered moderate for overall survival and time to SRE, mild for the incidence of SRE, and low for pain resolution.
Results
For overall survival, denosumab was ranked above zoledronic acid and estimated to confer a mean of 3.3 months (95% CI 0.3-6.3) of increased overall survival compared with untreated patients (P-score = 89%). For the time to SRE, denosumab was ranked first with a mean of 9.1 additional SRE-free months (95% CI 6.7-11.5) compared with untreated patients (P-score = 99%), while zoledronic acid conferred an additional 4.8 SRE-free months (95% CI 3.6-6.1). Reduction in the incidence of SREs was not different between patients treated with denosumab (relative risk 0.54; 95% CI 0.33-0.87) and those treated with zoledronic acid (relative risk 0.56; 95% CI 0.46-0.67). Patients treated with the combination of ibandronate and systemic therapy were more likely to experience successful pain resolution than untreated patients (relative risk 2.4; 95% CI 1.8-3.2).
Conclusion
In this comprehensive synthesis of all available randomized controlled trial evidence guiding the pharmacologic treatment of bone metastases from lung cancer, denosumab was ranked above zoledronic acid for overall survival and time to SRE and was not different for reducing the incidence of SRE. Both were superior to no treatment for each of these outcomes. Given this, we encourage physicians to consider the use of denosumab or zoledronic acid in treating this patient population. The combination of ibandronate and systemic therapy was the most effective at reducing pain because of metastases. No cost-effectiveness analysis has yet been performed for denosumab and zoledronic acid on patients with metastatic lung cancer, and this represents an avenue for future research.
Level of Evidence
Level I, therapeutic study.
Introduction
Lung cancer is one of the most commonly diagnosed cancer types worldwide and is the leading cause of all cancer-related deaths [3]. Although the prognosis for patients with lung cancer remains poor, it is improving, and metastatic bone disease is present in 20% to 40% of all patients with lung cancer [27, 43]. Patients with bone metastases may present symptomatically with pain and skeletal-related events (SREs) such as pathologic fractures or spinal cord compression, which often result in intervention by an orthopaedic surgeon [43]. Metastatic bone disease affects the quality of life of patients with lung cancer, and SREs have been independently linked to decreased overall survival [21]. Thus, decreasing the incidence of SREs has become an important clinical goal in the care of patients with metastatic lung cancer.
Although the metastasis microenvironment promotes bone turnover and resorption leading to SREs, bisphosphonates function by directly inhibiting osteoclast function and inducing osteoclast apoptosis [28]. Denosumab, a monoclonal antibody to the receptor activator of nuclear factor kappa-B ligand, functions by inhibiting osteoclast differentiation from progenitor cells as well as reducing osteoclast activity and survival [12, 28]. Currently, there is strong evidence from several randomized controlled trials (RCTs) supporting the use of bone-modifying agents such as bisphosphonates or denosumab for reducing SREs in patients with metastatic bone disease because of breast and prostate cancer [1, 15, 19]. Recently, smaller RCTs have specifically enrolled only patients with lung cancer and evaluated denosumab and various bisphosphonates [30, 41, 47]. However, to our knowledge, no systematic review to date has quantitatively synthesized all of the available RCT data in order to determine the best therapy specifically for patients with metastatic lung cancer. Three previous meta-analyses excluded denosumab, used mainly observational data, or included patients without lung cancer [14, 22, 26]. Furthermore, no network meta-analysis has been performed on this patient population. Network meta-analyses allow quantitative comparisons and relative rankings of all interventions studied in RCTs, even in the absence of trials directly comparing each treatment. A regular systematic review would only be able to include trials evaluating the same interventions. Given the multitude of interventions evaluated for this patient population, only a network meta-analysis can use all published RCT data to derive the most accurate estimates of treatment effects and provide rankings of all treatments, even those that have not been directly compared with one another. Although the American Board of Orthopaedic Surgeons lists bisphosphonates and denosumab among topics that orthopaedic surgeons should be familiar with [2], the ranking of these treatments for patients with metastatic lung cancer of bone is not yet established.
We therefore sought to perform a network meta-analysis of randomized trials to identify the bone-modifying agent that is associated with the (1) highest overall survival, (2) longest time to SRE, (3) lowest SRE incidence, and (4) greatest likelihood of pain resolution.
Patients and Methods
We performed a systematic review and network meta-analysis in accordance with the Cochrane Handbook for Systematic Reviews of Interventions and the Preferred Reporting Items for Systematic Reviews and Meta-analyses protocol [29]. This network meta-analysis was prospectively registered on PROSPERO (ID: CRD42019124364).
Search Strategy
We conducted a librarian-assisted (SS) search of MEDLINE, PubMed, EMBASE, and the Cochrane Library up to January 2019. Once it became apparent that many studies meeting our inclusion criteria were published in Chinese, but not in any other foreign language, several major Chinese databases, including Wanfang Data, Wanfang Med Online, China National Knowledge Infrastructure, and Chongqing VIP Information were searched using a similar strategy adapted by a local translator (JD). We also thoroughly reviewed the reference sections of previous meta-analyses to identify relevant studies, and included so-called “gray literature” such as research meeting abstracts in our search. Our inclusion and exclusion criteria were developed a priori. We included RCTs reporting outcomes specifically for patients with lung cancer treated with any bisphosphonate or denosumab. We excluded studies that did not involve bone-modifying agents and studies that reported surrogate outcomes such as changes in markers of bone turnover but not the outcomes of interest.
Trial Selection
We screened titles and abstracts in duplicate, and all conflicts were resolved by consultation with a senior author (AB). We included RCTs evaluating the effect of bone-modifying agents on metastatic bone disease in patients with a diagnosis of lung cancer, regardless of the subtype. Studies were included if they enrolled only patients with lung cancer or if they reported outcomes for the subgroup of patients with lung cancer.
All types of bisphosphonates were included in the study regardless of dosage, route (intravenous or oral), frequency, or treatment duration. For the outcome of pain resolution, systemic treatments such as chemotherapy, radiation therapy, or radionuclide therapy were sometimes offered concurrently with bisphosphonates. The large number of trials for this outcome (121) allowed for combinations of treatments to be included and evaluated. We included articles written in English or Chinese.
Papers written in Chinese were translated into English by a local translator (JD) before screening. If a study included patients with lung cancer but did not report outcomes specifically for this subgroup, we contacted the authors or data holders to request aggregate-level outcome data.
Included Trials
We included 131 RCTs that evaluated 11,105 patients with skeletal metastases from lung cancer (see Supplementary Table 1; Supplemental Digital Content 1, http://links.lww.com/CORR/A544). Specific reported subtypes included adenocarcinoma in 2218 patients, squamous cell carcinoma in 1669, adenosquamous carcinoma in 262, and small-cell lung cancer in 216. The remaining lung cancer subtypes were not specified. The age of the included patients ranged from 18 to 90 years, and 62% of patients in studies that reported sex were male. The included studies were published between 1999 and 2018 (Fig. 1).
Fig. 1.
This Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart shows the included RCTs.
Data Abstraction
We extracted data from all included trials in duplicate into spreadsheets for analysis (Microsoft Excel version 16.2). Discrepancies in data were resolved by consensus by referring to the original trial. Data abstracted included the year of the study, demographic data of the patients, overall survival, SRE incidence, time to SRE, and pain remission efficacy. Overall survival and the time to SRE were measured in months since patient enrollment. SREs included pathologic fractures, spinal cord compression, hypercalcemia of malignancy, or pain resulting in surgical intervention or radiation therapy [7]. Pain remission efficacy was measured as the number of patients who experienced substantial pain relief. We included all reported pain scales (such as numerical rating scale, verbal rating scale, and VAS), and we performed a meta-regression analysis to test for the correlation between the scale used and the outcome. In studies where information on pain remission efficacy was not provided, patients who experienced complete or partial pain remission were categorized as having effective relief, while patients who had not experienced any change in pain levels were deemed to have ineffective relief.
For RCTs that did not report the SD for continuous outcomes, the 95% CI was used to derive the SD, based on methods recommended by the Cochrane Handbook [17]. If an article published neither the SD nor the CI, a pooled SD value was derived from the SD values of other studies in the network, using methods described by Hedges [13]. Furukawa et al. [10] have shown that using pooled SD values in meta-analyses yields adequate concordance with actual SD values.
Risk of Bias in Individual Studies
The quality of the included studies was evaluated by two reviewers (RB and MD) in duplicate using the Cochrane Collaboration’s tool for assessing the risk of bias [16]. We used low, unclear, and high to evaluate the effectiveness of the methodologies used for random-sequence generation, allocation concealment, blinding of participants and personnel, and blinding of outcome assessments, as well as for the completeness of outcome data, the presence of selective reporting, and other biases for each study. Unless a study specifically mentioned blinding, a high risk of bias was given. The same standards were applied to studies published in English and Chinese.
Most of the included trials (98% [128 of 131]) used appropriate randomization methods. However, fewer than 1% (one of 131) of the studies explicitly mentioned their method for concealing allocation. Blinding of participants was observed in 6% (eight of 131) of the included trials, and outcome assessors were blinded in 1.5% (two of 131). Although Chinese-language studies were not formatted for the risk of bias tool, there were no large, observable differences in the risk of bias scores between Chinese and Western studies (see Supplementary Table 2; Supplemental Digital Content 2, http://links.lww.com/CORR/A545).
Quality of Evidence
The overall quality of evidence was assessed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework by two reviewers (AB and JD) in duplicate for each outcome [11]. In this network meta-analysis, we used Confidence in Network Meta-Analysis, an online network quality appraisal tool developed in accordance with the GRADE framework [11], to evaluate and synthesize the quality of direct and indirect evidence for each outcome network, according to the latest methodologic guidelines. Confidence in Network Meta-Analysis evaluates a network’s quality based on six criteria: within-study bias (risk of bias), across-study bias, indirectness, imprecision, heterogeneity, and incoherence [31]. We reported the overall GRADE of the quality of evidence for each outcome. The GRADE for overall survival was moderate because of suspected across-study bias and concerns about incoherence between direct and indirect network estimates of effect. The GRADE for time to SRE was moderate because of suspected across-study bias and concerns about incoherence. The overall GRADE for the quality of evidence was high for SRE incidence and pain remission.
Ethical Approval
Because this was a network meta-analysis, ethical approval of this study was waived by the Hamilton Integrated Research Ethics Board.
Statistical Analysis
We used a frequentist framework and a fixed-effects model network meta-analysis to assess direct and indirect evidence of the effect of bone-modifying agents on four primary outcomes: overall survival, time to SRE, SRE incidence, and successful pain remission. We created a network diagram of all interventions (nodes) and trials (edges) for each outcome and report the results of the network meta-analysis as relative risks or mean differences. We present forest plots, ranks, and a P-score for the treatments evaluated for each outcome. The P-score, based on point estimates and standard error, represents the likelihood that a given treatment will rank first in a specific category; a score closer to100% represents a greater chance that treatment is best [37]. The P-score is equivalent to the surface under the cumulative ranking curve score in Bayesian network meta-analyses [37]. Global inconsistency across the network model is described using the I2 value, which represents the percentage of variation across studies because of study heterogeneity [45]. Network heterogeneity was 12% for pain remission, 41% for the incidence of SRE, 62% for the time to SRE, and 77% for overall survival.
For all outcomes where some included studies administered concurrent systemic therapies such as radiation therapy or chemotherapy to both the treated and untreated groups, Bayesian meta-regression analyses were performed to determine whether the observed treatment effects were similar to those of studies without concurrent systemic therapies. For the outcome of pain remission, a meta-regression analysis was performed to determine any interaction between reported successful pain remission and the length of follow-up and/or the pain scale that was used. All statistical analyses were performed using R version 3.4.2 [42].
Results
Overall Survival
Denosumab was ranked first, conferring a mean survival benefit of 3.3 months compared with untreated patients (95% CI 0.3-6.3). Zoledronic acid (ZA) was ranked second and estimated to confer a mean of 2.1 months (95% CI 1.0-3.2) of increased survival compared with untreated patients. The P-score was 89% for denosumab and 60% for ZA (Fig. 2A). We performed meta-regressions to assess for any interaction between overall survival and concurrent systemic treatment with chemotherapy or radionuclides. Only the use of concurrent radionuclide therapy was associated with higher overall survival (see Supplementary Fig. 1; Supplemental Digital Content 3, http://links.lww.com/CORR/A546). Nine RCTs comprising 1911 patients and comparing three treatment arms contributed to this outcome (Fig. 2B).
Fig. 2.
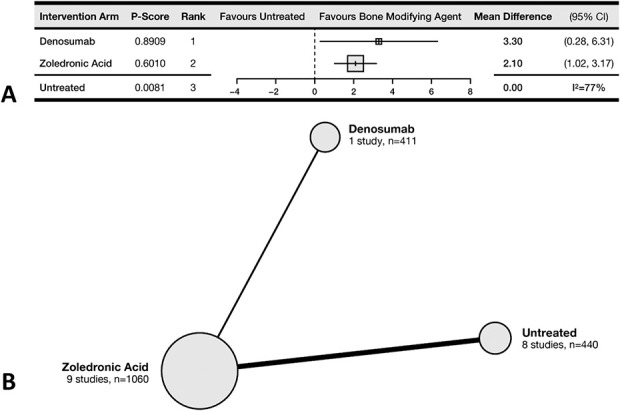
This (A) forest plot and (B) network diagram represent overall survival.
Time to SRE
The most effective therapy for delaying SREs was denosumab, conferring a mean of 9.1 additional SRE-free months (95% CI 6.7-11.5) compared with untreated patients (P-score = 99%). Treatment with ZA was ranked second and estimated to confer a mean of 4.8 additional SRE-free months (95% CI 3.6-6.1) compared with untreated patients (P-score = 57%). Ibandronate, another bisphosphonate, was estimated to confer an additional 4.0 SRE-free months (95% CI 0.9-7.1) (Fig. 3A). The meta-regression analysis showed no demonstrable effect of concurrent treatment with radionuclides. Five RCTs comprising 1201 patients and comparing four treatment arms contributed to this outcome (Fig. 3B).
Fig. 3.
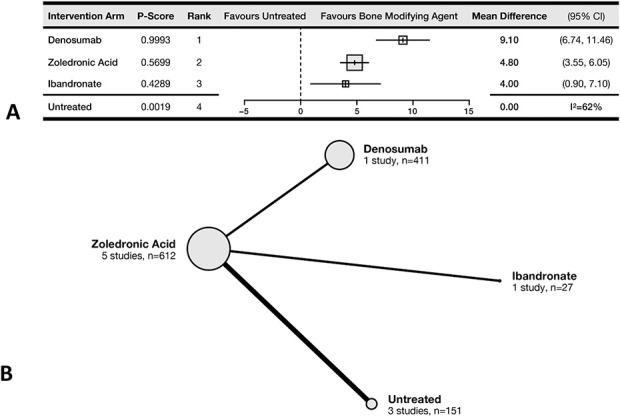
This (A) forest plot and (B) network diagram represent time to SRE.
SRE Incidence
The effectiveness of denosumab and ZA for decreasing the incidence of SRE was not different (P-score = 77% and 74%, respectively). Patients treated with ZA had 0.56 the risk of SRE (95% CI 0.46-0.67) compared with untreated patients, while those treated with denosumab had 0.54 the risk of SRE (95% CI 0.33-0.87) (Fig. 4A). Patients treated with ibandronate (relative risk 0.57; 95% CI 0.30-1.10) or pamidronate (relative risk 1.18; 95% CI 0.58-2.38) did not have a different risk of SRE than untreated patients did. In the meta-regression analysis, concurrent treatment with chemotherapy or radionuclides did not affect the odds of SRE, nor was the length of follow-up of the included studies associated with the observed outcomes. Seventeen RCTs comprising 2398 patients and comparing denosumab with four bisphosphonates contributed to this outcome (Fig. 4B).
Fig. 4.
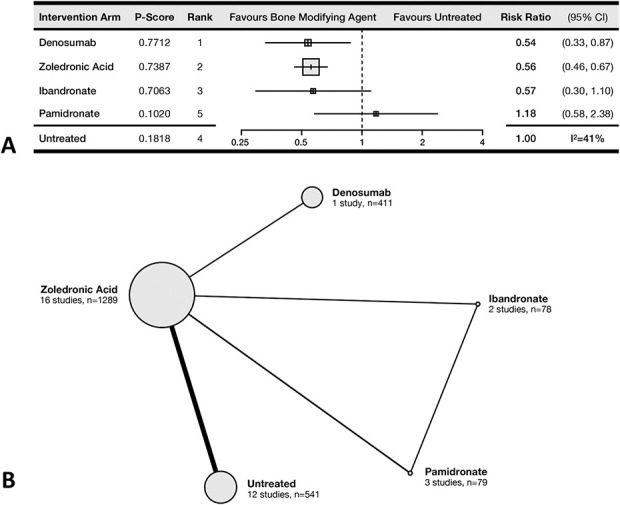
This (A) forest plot and (B) network diagram represent the incidence of SRE.
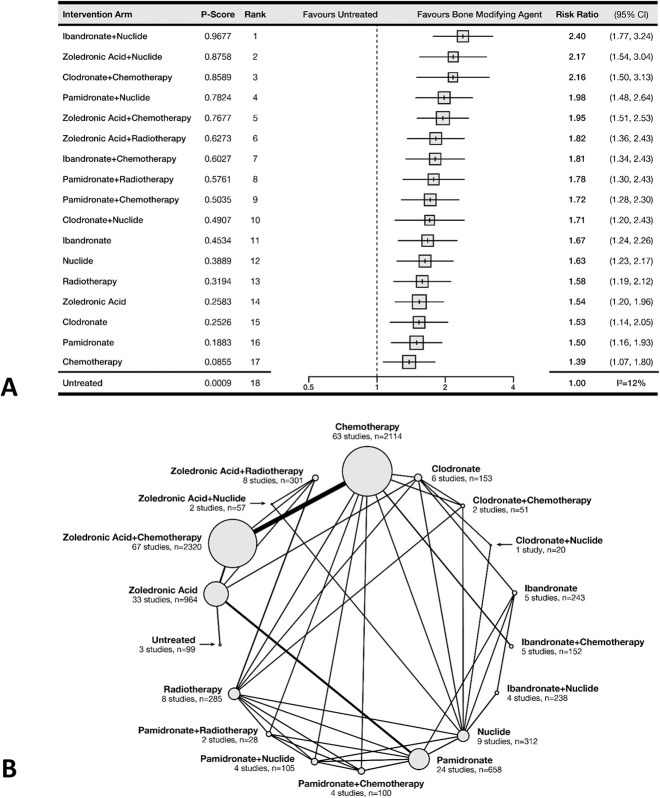
Pain Remission
The most effective therapy for successful pain remission was the combination of ibandronate and radionuclide (P-score = 97%). Patients given this treatment were more likely to experience successful pain resolution than untreated patients (relative risk 2.4; 95% CI 1.8-3.2). In general, the combination of bisphosphonates and systemic therapy provided high odds of successful pain remission (Fig. 5A). No trial evaluated denosumab for this outcome. The meta-regression analysis showed no interaction between the length of follow-up (see Supplementary Fig. 2; Supplemental Digital Content 4, http://links.lww.com/CORR/A547) or between the pain scale used in the studies and the observed treatment effects (see Supplementary Fig. 3; Supplemental Digital Content 5, http://links.lww.com/CORR/A548). A total of 121 RCTs comprising 8200 patients and comparing 18 treatment arms contributed to this outcome. This outcome was supported by the densest network (many direct estimates between treatments), which permitted the analysis of bone-modifying agents and concurrent systemic therapies as distinct treatment arms (Fig. 5B).
Fig. 5.
This (A) forest plot and (B) network diagram represent pain remission.
We provide a summary of findings table for all outcomes (Table 1).
Table 1.
Summary of findings
| Bone-modifying agent | Overall survival | Time to SRE | SRE incidence | Pain remission | ||||
| Months (95% CI) | P-score | Months (95% CI) | P-score | RR (95% CI) | P-score | RR (95% CI) | P-score | |
| Denosumab | 3.3 (0.3-6.3) | 89% | 9.1 (6.7-11.5) | 99% | 0.54 (0.33-0.87) | 77% | ||
| Zoledronic acid | 2.1 (1.0-3.2) | 60% | 4.8 (3.6-6.1) | 57% | 0.56 (0.46-0.67) | 74% | 1.55 (1.2-2.0) | 26% |
| Ibandronate | 4.0 (0.9-7.1) | 43% | 0.57 (0.30-1.1) | 71% | 1.7 (1.2-2.3) | 45% | ||
| Pamidronate | 1.18 (0.58-2.38) | 10% | 1.5 (1.1-1.9) | 19% | ||||
Discussion
Although an increasing number of patients who have cancer are living with metastatic bone disease, a comprehensive, quantitative synthesis of all available RCT data to quantify and rank the treatment effects of bone-modifying agents on metastatic bone disease from lung cancer has, to our knowledge, not been performed. Our network meta-analysis ranked denosumab first for overall survival, conferring a mean survival benefit of 3.3 months compared with untreated patients. ZA ranked second with a survival benefit of 2.1 months. Similarly, denosumab was ranked first for prolonging the time to SRE by a mean of 9.1 months, while ZA ranked second, conferring a mean of 4.8 additional SRE-free months compared with untreated patients. Regarding the incidence of SRE, denosumab conferred a 46% relative risk reduction of SRE, and ZA was ranked first among all bisphosphonates, conferring a 44% relative risk reduction in the odds of SRE compared with untreated patients. The network for successful pain remission, based on 121 RCTs, ranked ibandronate and concurrent radionuclide therapy first, with a relative risk of 2.4 in terms of pain remission compared with untreated patients. In general, bisphosphonates and systemic therapies performed better for pain remission than no treatment.
Limitations
This study has several limitations. Despite our rigorous librarian-assisted search strategy, we may have missed some studies with relevant data. However, we took several steps (described earlier) to gather as much eligible data as possible, including acquiring unpublished subgroup outcome data from a large trial. Another potential limitation is that the networks for our outcomes are relatively sparse. Whereas an analysis of 450 published network meta-analyses found a median of seven (interquartile range 5-9) interventions evaluated per outcome network and a median of 21 studies per outcome network (interquartile range 13-40) [44], three of our four outcomes fall short of those medians. The GRADE working group has stated that because of insufficient data in sparse networks to reliably estimate variances, “the result, however, may be spuriously wide confidence intervals … and inappropriately low ratings of the certainty of the evidence through rating down for serious imprecision” [4]. Therefore, we followed the GRADE working group recommendation to use a frequentist fixed-effects model, as opposed to a Bayesian random-effects model, to avoid spuriously widening CIs in sparser networks [4]. Finally, despite performing several meta-regression analyses showing no interaction of the length of follow-up or the pain scale used, overall network heterogeneity was moderate (50%-75%) for two outcomes, mild (25%-49%) for one, and low (< 25%) for one. Heterogeneity in our outcomes was inversely correlated with the number of trials included in each outcome.
Overall Survival, SRE Incidence, and Time to SRE
Current guidelines regarding the use of bone-modifying agents for metastatic bone disease from lung cancer are either non-specific or based on data from single trials. The National Comprehensive Cancer Network 2017 update on metastatic lung cancer broadly states that “Patients with widespread metastatic disease (Stage IV) are usually candidates for systemic therapy (consisting of chemotherapy, targeted therapy, or immunotherapy,” without specific guidance for the treatment of bony metastases [8]. The 2017 European Society of Medical Oncology guidelines stated that “denosumab is not inferior to and shows a trend towards superiority to ZA in lung cancer in terms of SRE prevention” [32] based on a 2011 RCT by Henry et al. [15]. Our findings support the European Society of Medical Oncology’s statement and are based on pooled evidence from an additional four trials [9, 20, 23, 46]. Furthermore, nine trials provided evidence that denosumab may be superior to ZA in terms of overall survival and time to SRE [5, 18, 23, 24, 30, 33, 38, 46, 48]. Given that the median life expectancy for patients with metastatic lung cancer is 5 months, prolongation of even 1 to 2 months is likely to be important to patients [36]. Overall, this study provides a rationale for updating guidelines regarding the pharmacologic treatment of metastatic lung cancer of bone. Because the care of patients with metastatic bone disease is usually multidisciplinary, orthopaedic surgeons should be aware of the ranking and efficacy of bone-modifying agents in order to help mitigate the risk of SREs by ensuring that their patients are receiving the therapy with the best supporting evidence.
Pain Remission
Regarding successful pain remission, the highest-ranked treatments were combinations of a bone-modifying agent and systemic therapy. This reflects current practice, which often includes a multidisciplinary and multimodal approach to patient care. However, the systemic therapy that contributed the most to pain remission, intravenous radionuclides, is not commonly used at our center or generally across North America. For the treatment of bone metastases, strontium-89 is most often used, although other compounds with different half-lives and depth of tissue penetration are available [25]. Strontium is in the same column of the periodic table as calcium and replaces calcium in hydroxyapatite in sites of osteoblastic activity [25]. Studies of strontium-89 monotherapy for bone pain due to metastases have shown response rates of 65% to 90%, with complete pain remission and cessation of opioid analgesia in up to 20% of patients [40]. Pain relief may occur as early as 3 days but is more commonly seen at around 3 weeks and can last for 3 to 6 months. Radionuclide therapy has also been shown to delay the time to SRE in studies in Canada and the United Kingdom [34, 35], but there are mixed and inconclusive effects of radionuclide therapy for prolonging survival [40]. However, our meta-regression analysis indicated that concurrent radionuclide therapy may increase overall survival. The main adverse event of radionuclide therapy is marrow suppression, with a 30% to 40% reduction in platelets and leukocytes occurring in 30% to 50% of patients by 5 to 8 weeks and resolving by 10 to 16 weeks [40]. Possible barriers to increased use of radionuclide therapy in North America include cost, availability, and familiarity [6, 39].
Conclusion
In this comprehensive synthesis of all available RCT evidence guiding the pharmacologic treatment of bone metastases from lung cancer, denosumab was ranked above ZA for overall survival and time to SRE. Both treatments were superior to no treatment for all outcomes. Physicians and guideline committees can use our findings about the efficacy and ranking of available bone-modifying agents to inform their treatment decisions for patients with metastatic bone disease from lung cancer. No cost-effectiveness analysis has yet been done for denosumab and ZA in patients with metastatic lung cancer, and this represents an avenue for future research.
Supplementary Material
Acknowledgments
We thank the STEM Fellowship at McMaster University for providing the opportunity to undertake this project.
Footnotes
Each author certifies that neither he or she, nor any member of his or her immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Ethical approval for this study was waived by the Hamilton Integrated Research Ethics Board, Hamilton, Ontario, Canada.
This work was performed at McMaster University, Hamilton, Ontario, Canada.
Contributor Information
Jiawen Deng, Email: dengj35@mcmaster.ca.
Umaima Abbas, Email: umaimaabbas25@gmail.com.
Richa Bhasin, Email: richabhasin16@gmail.com.
Marisa Deodat, Email: deodatm@mcmaster.ca.
Sajid Wariach, Email: wariachs@mcmaster.ca.
Stephanie Sanger, Email: sangers@mcmaster.ca.
Daniel Axelrod, Email: daniel.axelrod@medportal.ca.
Karim Masrouha, Email: karim.masrouha@medportal.ca.
Robert Turcotte, Email: robert.turcotte@muhc.mcgill.ca.
David Wilson, Email: wilsondaj@gmail.com.
Michelle Ghert, Email: ghertm@mcmaster.ca.
References
- 1.Amadori D, Aglietta M, Alessi B, et al. Efficacy and safety of 12-weekly versus 4-weekly zoledronic acid for prolonged treatment of patients with bone metastases from breast cancer (ZOOM): a phase 3, open-label, randomised, non-inferiority trial. Lancet Oncol. 2013;14:663-670. [DOI] [PubMed] [Google Scholar]
- 2.American Board of Orthopaedic Surgery. ABOS part I certification examination blueprint. Available at: https://www.abos.org/wp-content/uploads/2020/09/blueprint_partI.pdf. Accessed September 1, 2020.
- 3.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [DOI] [PubMed] [Google Scholar]
- 4.Brignardello-Petersen R, Murad MH, Walter SD, et al. GRADE approach to rate the certainty from a network meta-analysis: avoiding spurious judgments of imprecision in sparse networks. J Clin Epidemiol. 2019;105:60-67. [DOI] [PubMed] [Google Scholar]
- 5.Chen S, Li T, Ge HB. Study on zoledronic acid combined with pemetrexed in the treatment of advanced non-small cell lung cancer with bone metastasis. J Clin Pulm Med. 2010;15:1528-1530. [Google Scholar]
- 6.Chow E, Danjoux C, Wong R, et al. Palliation of bone metastases: a survey of patterns of practice among Canadian radiation oncologists. Radiother Oncol. 2000;56:305-314. [DOI] [PubMed] [Google Scholar]
- 7.Delea T, Langer C, McKiernan J, et al. The cost of treatment of skeletal-related events in patients with bone metastases from lung cancer. Oncology. 2004;67:390-396. [DOI] [PubMed] [Google Scholar]
- 8.Ettinger DS, Wood DE, Aisner DL, et al. Non–small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Can Netw. 2017;15:504-535. [DOI] [PubMed] [Google Scholar]
- 9.Francini F, Pascucci A, Bargagli G, et al. Effects of intravenous zoledronic acid and oral ibandronate on early changes in markers of bone turnover in patients with bone metastases from non-small cell lung cancer. Int J Clin Oncol. 2011;16:264-269. [DOI] [PubMed] [Google Scholar]
- 10.Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol. 2006;59:7-10. [DOI] [PubMed] [Google Scholar]
- 11.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanley D, Adachi J, Bell A, Brown V. Denosumab: mechanism of action and clinical outcomes. Int J Clin Pract. 2012;66:1139-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hedges LV. Distribution theory for Glass's estimator of effect size and related estimators. Journal of Educational Statistics. 1981;6:107-128. [Google Scholar]
- 14.Hendriks LEL, Hermans BCM, van den Beuken-van Everdingen MH, Hochstenbag MMH, Dingemans A-MC. Effect of bisphosphonates, denosumab, and radioisotopes on bone pain and quality of life in patients with non–small cell lung cancer and bone metastases: a systematic review. J Thorac Oncol. 2016;11:155-173. [DOI] [PubMed] [Google Scholar]
- 15.Henry DH, Costa L, Goldwasser F, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29:1125-1132. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions .Vol 5. Wiley Online Library; 2008. [Google Scholar]
- 18.Hirsch V, Major P, Lipton A. Zoledronic acid and survival in patients with metastatic bone disease from lung cancer and elevated markers of osteoclasts activity. J Thorac Oncol. 2008;3:228-236. [DOI] [PubMed] [Google Scholar]
- 19.Hortobagyi GN, Van Poznak C, Harker WG, et al. Continued treatment effect of zoledronic acid dosing every 12 vs 4 weeks in women with breast cancer metastatic to bone: the OPTIMIZE-2 randomized clinical trial. JAMA Oncol. 2017;3:906-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kritikos K, Heras P, Hatzopoulos A, Androutsos N. Efficacy and safety of intravenous zoledronic acid (ZA) 4 MG infused over 15 minutes: results from a 2-year study of lung cancer patients (LCP) with metastatic bone disease. Paper presented at: Annals of Oncology Meeting; September16, 2008; Stockholm, Sweden. [Google Scholar]
- 21.Kuchuk M, Addison CL, Clemons M, Kuchuk I, Wheatley-Price P. Incidence and consequences of bone metastases in lung cancer patients. J Bone Oncol. 2013;2:22-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LeVasseur N, Clemons M, Hutton B, Shorr R, Jacobs C. Bone-targeted therapy use in patients with bone metastases from lung cancer: a systematic review of randomized controlled trials. Cancer Treat Rev. 2016;50:183-193. [DOI] [PubMed] [Google Scholar]
- 23.Li NY, Chai H, Yao Z-Q, et al. Clinical research of zoledronic acid and strontium-89 in treatment of patients with asymptomatic bone metastases from non-small cell lung cancer. Chinese Journal of Cancer Prevention and Treatment. 2018;25:962-967. [Google Scholar]
- 24.Li T, Ge H-B, Zhang J-R. Zoledronic acid combined with GP chemotherapy for advanced non-small cell lung cancer with bone metastases. Medical Journal of West China. 2010;4. [Google Scholar]
- 25.Liberal FDG, Tavares AAS, Tavares JMR. Palliative treatment of metastatic bone pain with radiopharmaceuticals: a perspective beyond strontium-89 and samarium-153. Appl Radiat Isot. 2016;110:87-99. [DOI] [PubMed] [Google Scholar]
- 26.Lopez-Olivo MA, Shah NA, Pratt G, Risser JM, Symanski E, Suarez-Almazor ME. Bisphosphonates in the treatment of patients with lung cancer and metastatic bone disease: a systematic review and meta-analysis. Support Care Cancer. 2012;20:2985-2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macedo F, Ladeira K, Pinho F, et al. Bone metastases: an overview. Oncol Rev. 2017;11:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maurizi A, Rucci N. The osteoclast in bone metastasis: player and target. Cancers (Basel). 2018;10:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264-269. [DOI] [PubMed] [Google Scholar]
- 30.Murakami H, Yamanaka T, Seto T, et al. Phase II study of zoledronic acid combined with docetaxel for non‐small‐cell lung cancer: West Japan Oncology Group. Cancer Sci. 2014;105:989-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nikolakopoulou A, Higgins JP, Papakonstantinou T, et al. CINeMA: an approach for assessing confidence in the results of metwork meta-analysis. PLoS Med. 2020;17:e1003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Novello S, Barlesi F, Califano R, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v1-v27. [DOI] [PubMed] [Google Scholar]
- 33.Pandya KJ, Gajra A, Warsi GM, Argonza-Aviles E, Ericson SG, Wozniak AJ. Multicenter, randomized, phase 2 study of zoledronic acid in combination with docetaxel and carboplatin in patients with unresectable stage IIIB or stage IV non-small cell lung cancer. Lung Cancer. 2010;67:330-338. [DOI] [PubMed] [Google Scholar]
- 34.Porter A, McEwan A, Powe J, et al. Results of a randomized phase-III trial to evaluate the efficacy of strontium-89 adjuvant to local field external beam irradiation in the management of endocrine resistant metastatic prostate cancer. Int J Radiat Oncol Biol Phys. 1993;25:805-813. [DOI] [PubMed] [Google Scholar]
- 35.Quilty PM, Kirk D, Bolger JJ, et al. A comparison of the palliative effects of strontium-89 and external beam radiotherapy in metastatic prostate cancer. Radiother Oncol. 1994;31:33-40. [DOI] [PubMed] [Google Scholar]
- 36.Riihimäki M, Hemminki A, Fallah M, et al. Metastatic sites and survival in lung cancer. Lung Cancer. 2014;86:78-84. [DOI] [PubMed] [Google Scholar]
- 37.Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methdol. 2015;15:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scagliotti GV, Hirsh V, Siena S, et al. Overall survival improvement in patients with lung cancer and bone metastases treated with denosumab versus zoledronic acid: subgroup analysis from a randomized phase 3 study. J Thorac Oncol. 2012;7:1823-1829. [DOI] [PubMed] [Google Scholar]
- 39.Shi C, Tian L, Hai-Bo G. Study on zoledronic acid combined with pemetrexed in the treatment of advanced non-small cell lung cancer with bone metastasis. Journal of Clinical Pulmonary Medicine. 2010;11. [Google Scholar]
- 40.Silberstein EB. Teletherapy and radiopharmaceutical therapy of painful bone metastases. Paper presented at: Seminars in Nuclear Medicine; June 18-22, 2005; Toronto, Canada. [DOI] [PubMed] [Google Scholar]
- 41.Team RC. R: A language and environment for statistical computing. 2015. [Google Scholar]
- 42.Tsuya A, Kurata T, Tamura K, Fukuoka M. Skeletal metastases in non-small cell lung cancer: a retrospective study. Lung Cancer. 2007;57:229-232. [DOI] [PubMed] [Google Scholar]
- 43.Veroniki AA, Straus SE, Rücker G, Tricco AC. Is providing uncertainty intervals in treatment ranking helpful in a network meta-analysis? J Clin Epidemiol. 2018;100:122-129. [DOI] [PubMed] [Google Scholar]
- 44.Veroniki AA, Vasiliadis HS, Higgins JP, Salanti G. Evaluation of inconsistency in networks of interventions. Int J Epidemiol. 2013;42:332-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Tao H, Yu X, Wang Z, Wang M. Clinical significance of zoledronic acid and strontium-89 in patients with asymptomatic bone metastases from non-small-cell lung cancer. Clin Lung Cancer. 2013;14:254-260. [DOI] [PubMed] [Google Scholar]
- 46.Zarogoulidis K, Boutsikou E, Zarogoulidis P, et al. The impact of zoledronic acid therapy in survival of lung cancer patients with bone metastasis. Int J Cancer. 2009;125:1705-1709. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Q, Wengui X, Dai D, Song X. Safety and efficiency evaluation of the combined therapy of 89Sr plus zoledronic acid in patients with painful bone metastases. Chinese Journal of Clinical Oncology. 2015:1138-1142. [Google Scholar]
- 48.Zhao J. The effect of zoledronic acid combined with chemotherapy in the treatment of bone metastases from non-small cell lung cancer. Modern Journal of Integrated Traditional Chinese and Western Medicine. 2015;24:962-964. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.