Abstract
Background:
Pulmonary hypertension (PH) is a common complication in patients with Alveolar capillary dysplasia with misalignment of pulmonary veins (ACDMPV), a severe congenital disorder associated with mutations in the FOXF1 gene. While the loss of alveolar microvasculature causes PH in ACDMPV patients, it is unknown whether increasing neonatal lung angiogenesis could prevent PH and right ventricular (RV) hypertrophy.
Methods:
We used echocardiography, RV catheterization, immunostaining and biochemical methods to examine lung and heart remodeling and RV output in Foxf1WT/S52F mice carrying the S52F Foxf1 mutation (identified in ACDMPV patients). The ability of Foxf1WT/S52F mutant embryonic stem cells (ESCs) to differentiate into respiratory cell lineages in vivo was examined using blastocyst complementation. Intravascular delivery of nanoparticles with a non-integrating Stat3 expression vector was used to improve neonatal pulmonary angiogenesis in Foxf1WT/S52F mice and determine its effects on PH and RV hypertrophy.
Results:
Foxf1WT/S52F mice developed PH and RV hypertrophy after birth. The severity of PH in Foxf1WT/S52F mice directly correlated with mortality, low body weight, pulmonary artery muscularization and increased collagen deposition in the lung tissue. Increased fibrotic remodeling was found in human ACDMPV lungs. Mouse ESCs carrying the S52F Foxf1 mutation were used to produce chimeras via blastocyst complementation and to demonstrate that Foxf1WT/S52F ESCs have a propensity to differentiate into pulmonary myofibroblasts. Intravascular delivery of nanoparticles carrying Stat3 cDNA protected Foxf1WT/S52F mice from RV hypertrophy and PH, improved survival and decreased fibrotic lung remodeling.
Conclusions:
Nanoparticle therapies increasing neonatal pulmonary angiogenesis may be considered to prevent PH in ACDMPV.
Keywords: Pulmonary hypertension, Alveolar capillary dysplasia, FOXF1, STAT3, Nanoparticle delivery system
INTRODUCTION
Alveolar capillary dysplasia with misalignment of pulmonary veins (ACDMPV) is a rare congenital disorder which requires lung transplantation early in life 1, 2. Early onset of ACDMPV, rapidly progressing respiratory insufficiency, and poor availability of pediatric donor lungs greatly complicate clinical care for ACDMPV patients, leading to mortality before lung transplantation. There is an urgent clinical need for new therapeutic approaches for ACDMPV patients. ACDMPV is characterized by reduced capillary density in alveolar regions, hypertrophy of small pulmonary arteries, and misaligned and congested pulmonary veins, the latter of which represent abnormal anastomoses between pulmonary and bronchial circulations 1. Pulmonary hypertension (PH) occurs in a vast majority of ACDMPV cases, exacerbating respiratory insufficiency and edema and leading to pulmonary hemorrhage and extensive lung remodeling 1, 3. While the pathophysiology of PH in ACDMPV is not entirely clear, reduced capillary density, severe lung tissue hypoxia and aberrant arterial-venal anastomoses appear to be the major contributing factors to PH. Recent studies reported a subset of rare ACDMPV cases with a late onset of PH, prolonged survival and a mosaic pattern of vascular abnormalities in the lung tissue 4, 5, supporting a link between the loss of alveolar microvasculature and PH. Based on histological assessment of ACDMPV fetuses and lung biopsies, a paucity of pulmonary capillaries and misalignment of pulmonary veins occur before birth as a result of abnormal lung vascular development, whereas PH and RV hypertrophy occur postnatally 1. While the loss of alveolar capillaries precedes PH in ACDMPV, it remains unknown whether neonatal proangiogenic therapies can prevent or delay PH in ACDMPV patients.
Heterozygous copy-number variant deletions and point mutations in the Forkhead Box F1 (FOXF1) gene locus have been recently linked to ACDMPV 2, 3. FOXF1, a transcription factor expressed in pulmonary endothelial cells, fibroblasts and their mesenchymal progenitors 6-9 , is essential for lung vascular development 10, 11 and lung repair after injury 12-15. During lung development, FOXF1 stimulates formation of alveolar capillaries by regulating genes critical for VEGF, PDGF and NOTCH signaling pathways 6, 16. Foxf1−/− mice exhibit embryonic mortality before the initiation of lung development due to vascular abnormalities in the yolk sac and allantois 17. Mice heterozygous for the Foxf1 null allele (Foxf1+/−) or the S52F Foxf1 mutant allele (Foxf1WT//S52F) develop the alveolar capillary dysplasia before birth 2, 18. However, it is unknown whether these mice have PH.
Recently developed nanoparticle carriers have been used to deliver small molecule compounds, DNA expression vectors, and stabilized mRNAs into cells in vivo. Nanoparticle carriers have shown minimal toxicity and accelerated the development of novel therapies for human cancers, diabetes and chronic inflammatory disorders. We have recently developed polyethylenimine-(5) myristic acid/ poly (ethylene glycol)-oleic acid/ cholesterol (PEI600-MA5/PEG-OA/Cho) nanoparticles to deliver non-integrating cDNA expression vectors into endothelial cells for the purpose of improving neonatal lung angiogenesis 2, 19. Delivery of Foxf1 cDNA increased lung angiogenesis after neonatal hyperoxic injury 20, whereas delivery of Stat3, a direct transcriptional target of FOXF1, increased neonatal angiogenesis in Foxf1WT//S52F mutant mice 2. While the nanoparticle treatment stimulated the development of alveolar capillaries by transiently activating endothelial proliferation 2, 20, it is unclear whether restoring the pulmonary microvascular network will be sufficient to prevent or decrease PH and RV hypertrophy in ACDMPV mice.
In this study, we used nanoparticle delivery of Stat3 cDNA to demonstrate that increasing neonatal lung angiogenesis prevents PH and RV hypertrophy, increases arterial oxygenation, reduces lung remodeling and improves survival in Foxf1WT//S52F mutant mice that contain the S52F Foxf1 ACDMPV mutation. Nanoparticle-based proangiogenic therapy may be considered for treatment of human ACDMPV.
MATERIALS AND METHODS
Ethics Statement
The data, analytic methods, and study materials will be made available upon request from the corresponding author of this manuscript to other researchers for purposes of reproducing the results or replicating the procedures 2, 20. All animal studies were based on AAALAC guidelines and approved by the Institutional Animal Care and Use Committee of the Cincinnati Children’s Hospital.
Mice
C57BL/6 mice and CD1 mice for generating chimeras were purchased from Jackson laboratory. The Foxf1WT//S52F mouse line was generated and maintained in C57BL/6 x 129/J genetic background as described 2. Foxf1WT//S52F mice were genotyped by PCR (Figure IA-B in the Supplement). The Primers for genotyping were: Foxf1 +77/+94, GGCGGCCAGGCCATGGAC; Foxf1 +112/+133, CACCAAGGCCAAGAAGACCAAC; Foxf1 +334/+313, GATGAAGCACTCGTTGAGCGAC. Postnatal day 2 (P2) mice were i.v. injected (25μl) with nanoparticles carrying a CMV expression plasmid containing Stat3 cDNA or an empty CMV vector (5μg) through the facial vein. Mice were examined at 1, 4 and 6 months of age. Measurements of arterial oxygenation were recorded for 10 min using MouseOx Plus (STARR Life Sciences) as described 21.
Transthoracic echocardiography and right heart catheterization
Echocardiography was performed in a blinded manner by the Cincinnati Childre´s Hospital Medical Center echocardiography core. Echocardiographic measurements were taken under spontaneous respiration using Visualsonics Vevo 2100 ultrasonography (Visual Sonics, Toronto, Canada) with 40-MHz transducer as previously described 22. Diastolic and systolic dimensions, tricuspid annular plane systolic excursion (TAPSE), S wave, stroke volume (SV) and cardiac output (CO) were recorded on M-mode, B-mode and tissue-Doppler images. The pulmonary artery peak velocity was measured to calculate the ratio of pulmonary acceleration time (PAT) to pulmonary ejection time (PAT/PET). Right heart catheterization and measurements of right ventricular systolic pressure (RVSP) were performed using FTH-1211B-0018 catheter as described 23.
Histology and immunostaining
Paraffin sections (5μm) or cryosections (7μm) were used for histological staining with hematoxylin and eosin (H&E), Sirius red, Masson’s trichrome, wheat germ agglutinin (WGA) conjugated with Alexa Fluor 488 (Sigma) or immunostaining with various antibodies as described 24-27. Antibodies are listed in Table I in the Supplement. Heath-Edwards classification was used to evaluate the grade of pulmonary vascular remodeling 28. Relative vascular wall thickness was determined as (do–di)/do x 100% (do, external diameter; di, internal diameter) as described 29. De-identified human lung sections from ACDMPV patients were obtained from the Baylor College of Medicine 2, 3. Whole mount immunostaining was described in 21. For co-localization experiments, secondary antibodies conjugated with Alexa Fluor 488, Alexa Fluor 594 and Alexa Fluor 647 (all from Invitrogen) were used as described 30, 31. Fluorescence images were captured by a Nikon Ti-E inverted confocal microscope. Brightfield images were captured by a Zeiss Axioplan2 microscope. Mean linear intercept (MLI) length was quantified by Image J software as previously described 20, 32.
Measurements of collagen content
The collagen concentration in lung tissue (left lobe) was determined by Sircol Soluble Collagen Assay (S1000, Biocolor) as previously described 7. Collagen content (mg) was calculated according to a standard curve and was normalized to the weight (g) of each lung tissue sample.
Quantitative real-time PCR (qRT-PCR) and microarray analysis
RNA was isolated from lung or heart tissues using RNeasy micro kit (Qiagen). RNA samples were reverse-transcribed into cDNA by iScript Reverse Transcription Reagents (BioRad) 33, 34. Samples were amplified using inventoried TaqMan primers (Applied Biosystems) listed in Table II in the Supplement. qRT-PCR was performed as previously described 35 using StepOnePlus Real-Time PCR system (Applied Biosystems). mRNAs were normalized to β-actin mRNA. The microarray data from donor and ACDMPV lungs (accession number GSE54780) were retrieved from the GEO database using GEOquery R package and analyzed by the R package limma software.
FACS analysis and Western blot
The single-cell suspension for flow cytometry was generated from enzyme-digested lung tissues as previously described 36, 37. Viability of cells was > 90% as determined with 7-AAD or zombie-UV (eBioscience). Antibodies are listed in Table I in the Supplement. Cell fixation and permeabilization protocol for intracellular staining was described previously 38. Isotype IgG were used as controls for intracellular staining. The data were acquired and analyzed using 5-laser LSRII (BD Biosciences). Western blotting of total lung protein extract was performed as described 2, 39.
Blastocyst complementation and generation of chimeras
Mouse embryonic stem cells (ESCs) containing the S52F Foxf1 mutation and the tdTomato transgene (tdT-S52F Foxf1 ESCs) were generated using blastocysts from Foxf1WT//S52F; tdTomatotg/+ mice as described 21, 40. tdT-S52F Foxf1 ESCs and WT ESCs expressing GFP (GFP-WT) 21 were maintained in vitro for chimera generation. Male and female C57BL/6 mice were bred to produce blastocysts. Each blastocyst was injected with 16 donor ESCs (8 tdT-S52F Foxf1 + 8 GFP-WT) using a piezo-assisted micromanipulator (Eppendorf). Chimeric blastocysts were transferred into the uteri of pseudopregnant recipient mice to continue normal embryonic development in utero. The chimera mice were sacrificed and analyzed at P7.
Nanoparticles
Polyethylenimine-(5) myristic acid/ poly (ethylene glycol)-oleic acid/ cholesterol (PEI600-MA5/PEG-OA/Cho) nanoparticles were synthesized as previously described 2, 19. A mass ratio of 1:24 was used to encapsulate DNA (CMV-Stat3 or CMV-empty plasmids) into polymers. Nanoparticle-DNA complexes were injected intravenously (i.v.) into P2 pups via the facial vein (25 μl total volume). The injection mixture contained 5 μg of plasmid DNA encapsulated into PEI600-MA5/PEG-OA/Cho nanoparticles (120 μg) as described 20.
Statistical analysis
Student’s T-test and one-way ANOVA were used to determine statistical significance. The right skewed measurements were log-transformed to meet normality assumption prior to analysis. Multiple means were compared using one-way analysis of variance with the post-hoc Tukey test. P values less than 0.05 were considered significant. For datasets with n<5, non-parametric Mann-Whitney U test was used to determine statistical significance. Values for all data were showed as the mean ± standard error of mean (SEM).
RESULTS
Foxf1WT/S52F mice exhibit right ventricular hypertrophy and pulmonary hypertension.
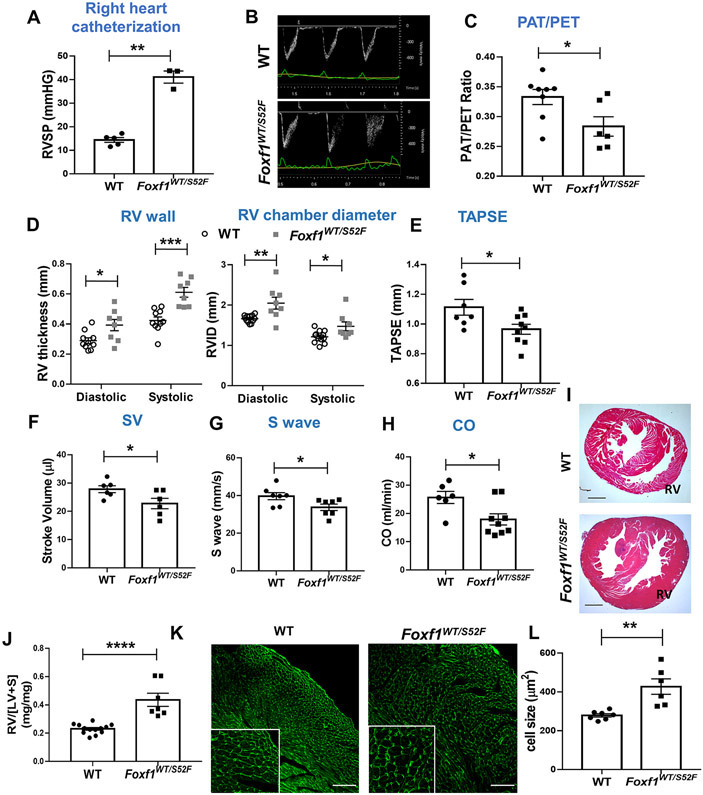
Since human ACDMPV is associated with PH 1, we examined whether Foxf1WT/S52F knock-in mice (containing the heterozygous S52F Foxf1 mutation which is identical to those found in ACDMPV patients 2) have PH. Right ventricular (RV) systolic pressure (RVSP) was increased in adult Foxf1WT/S52F mice compared to wild type (WT) littermates as shown by RV catheterization (Figure 1A). To compare the pulmonary vascular resistance, we used transthoracic echocardiography to measure the pulmonary acceleration time (PAT) and the pulmonary ejection time (PET) and calculated the PAT/PET ratio, a reliable indicator of pulmonary artery (PA) blood flow and RV function 22. The PAT/PET ratio was decreased in adult Foxf1WT/S52F mice (Figure 1B-C), indicating higher PA pressure and worse RV function compared to WT littermates. Diastolic and systolic RV wall thickness and the RV chamber area were increased in Foxf1WT/S52F mice (Figure 1D and Figure IC in the Supplement). TAPSE, stroke volume, S wave and cardiac output were reduced (Figure 1E-H), consistent with impaired RV function in Foxf1WT/S52F mice. While interventricular septum (IVS), left ventricular (LV) mass and LV chamber dimensions were mostly similar between WT and Foxf1WT/S52F mice, diastolic LV internal diameter and diastolic LV volume were increased (Figure ID in the Supplement).
Figure 1. Foxf1WT/S52F mice develop right ventricular hypertrophy and pulmonary hypertension.
(A) Right heart catheterization shows increased RVSP in adult Foxf1WT/S52F mice compared to WT controls (n=3-5 mice per group). (B-C) Echocardiography shows a sharpened pulmonary artery velocity curve and a decreased ratio of pulmonary acceleration time (PAT) to pulmonary ejection time (PAT/PET) in adult Foxf1WT/S52F mice (n=6-8 mice per group). (D-H) Foxf1WT/S52F mice exhibit increased diastolic and systolic RV wall thickness and right ventricular internal diameter (RVID), whereas TAPSE, stroke volume (SV), S wave and cardiac output (CO) are decreased as determined by echocardiography (n=6-10 mice per group). (I) H&E staining of heart transverse sections shows increased thickness of RV walls in Foxf1WT/S52F mice. Scale bars are 2mm. (J) The ratio of right ventricle weight to the combined weight of left ventricle and interventricular septum (RV/[LV+S]) was determined after autopsy. RV/[LV+S] ratio is increased in Foxf1WT/S52F adult mice (n=7-13 mice per group). (K-L) Wheat germ agglutinin (WGA) staining of heart sections shows enlarged RV cardiomyocytes in Foxf1WT/S52F mice (n=6-7 mice per group). Scale bars are 100μm. * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001, **** indicates p < 0.0001.
Foxf1WT/S52F hearts were enlarged and the heart weight to body weight ratio was increased compared to WT controls (Figure IIA-C in the Supplement). Histological examination of Foxf1WT/S52F hearts showed an increased thickness of RV walls (Figure 1I), and an increased ratio of RV weight to the combined weight of LV and interventricular septum (RV/[LV+S]) (Figure 1J), findings consistent with RV hypertrophy. Expression of cardiac hypertrophy genes Nppa, Nppb and Myh7, and the Myh7/Myh6 ratio were increased in total RNA samples prepared from right ventricles of Foxf1WT/S52F mice (Figure IID-H in the Supplement). Based on wheat germ agglutinin (WGA) staining, the size of RV cardiomyocytes was increased in Foxf1WT/S52F hearts (Figure 1K-L), whereas the size of LV cardiomyocytes was unchanged (Figure II I-J in the Supplement). There was no fibrosis in Foxf1WT/S52F hearts as demonstrated by Sirius red staining (Figure IIK in the Supplement). Thus, the S52F Foxf1 mutation causes right ventricular hypertrophy and pulmonary arterial hypertension in adult mice. Interestingly, FOXF1 is expressed in the lung but not in the heart tissue (Figure IIIA in the Supplement), suggesting that lung vascular abnormalities cause RV hypertrophy in Foxf1WT/S52F mice.
Foxf1WT/S52F mice exhibit alveolar remodeling and thickening of small pulmonary arteries.
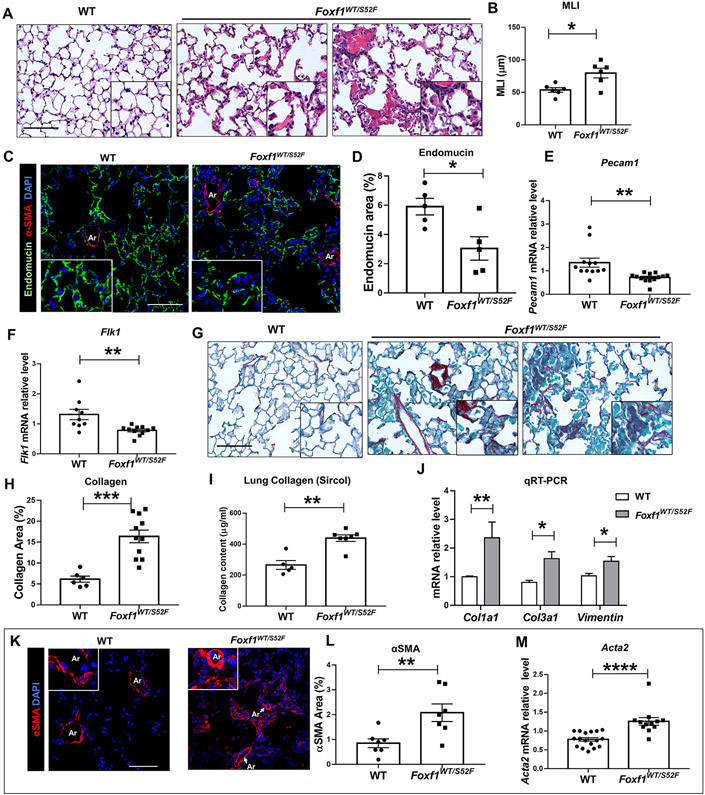
PH is frequently associated with lung remodeling, including alveolar simplification, capillary dysplasia, small artery muscularization and excessive collagen depositions into perivascular and alveolar regions 1. Since FOXF1 is expressed in pulmonary endothelial cells and fibroblasts 2, 7, we examined lung remodeling in adult Foxf1WT/S52F mice with PH. Histological examination of Foxf1WT/S52F lungs showed diffuse pulmonary inflammation and alveolar simplification (Figure 2A), the latter of which was confirmed by measurements of the mean linear intercept (Figure 2B). Capillary density and expression of endothelial Pecam1 and Flk1 mRNAs were decreased in Foxf1WT/S52F lungs (Figure 2C-F). STAT3 and p-STAT3 proteins were also decreased as shown by Western blot (Figure IVA-D in the Supplement) and immunostaining (Figure IVE-F in the Supplement), findings consistent with transcriptional activation of the Stat3 gene by FOXF1 2. Compared to WT controls, increased fibrotic deposition was evident in Foxf1WT/S52F mice as determined by Sirius red staining (Figure 2G-H), Sircol assay measurements of collagen content (Figure 2I), and qRT-PCR for Col1a1, Col3a1 and Vim mRNAs (Figure 2J). Consistent with PH, pulmonary arteries were remodeled in Foxf1WT/S52F mice, showing thick smooth muscle layers and increased expression of αSMA protein and mRNA (Figure 2K-M). While pulmonary arteries of all sizes were remodeled (Figure IIIB-D in the Supplement), small arteries exhibited the highest degree of remodeling (Figure IIIE in the Supplement). Vegfa mRNA was increased in Foxf1WT/S52F lungs, whereas Hif1a mRNA was unaltered (Figure VA-F in the Supplement). Neither VEGFα nor HIF-1α were changed in the arterial wall (Figure VG-H in the Supplement). Thus, in addition to RV hypertrophy and PH, Foxf1WT/S52F mice exhibit alveolar simplification, decreased capillary density, fibrotic lung remodeling and thickening of pulmonary arteries.
Figure 2. Adult Foxf1WT/S52F mice exhibit lung remodeling and vascular abnormalities.
(A-B) H&E staining of lung sections shows diffuse pulmonary inflammation and alveolar simplification in Foxf1WT/S52F adult mice. Mean linear intercept (MLI) was determined using 5 random images from each of 3 H&E-stained lung sections per mouse (n=6 mice). Scale bars are 50μm. (C-D) Immunostaining for Endomucin (green) and αSMA (red) shows reduced capillary density in lungs from Foxf1WT/S52F mice. Lung sections were counterstained with DAPI (blue). Five random lung images per mouse (n=5 mice per group) were used for quantification. Scale bars are 25μm. (E-F) qRT-PCR shows that Pecam1 and Flk1 mRNAs are decreased in Foxf1WT/S52F lungs. mRNAs were normalized using β-actin mRNA (n=9-13 mice per group). (G-H) Sirius red staining shows increased collagen deposition in Foxf1WT/S52F lungs. Scale bars are 50μm. Data were quantified using 5 random lung images per mouse (n=6-11 mice per group). (I) Sircol assay shows increased collagen content in the left lobe of Foxf1WT/S52F lungs (n=5-7 mice per group). (J) qRT-PCR shows that Col1a1, Col3a1 and Vimentin mRNAs are increased in Foxf1WT/S52F lungs (n=6-9 mice per group). (K-L) Images show increased αSMA staining (red) with small artery muscularization (inserts) in lungs from Foxf1WT/S52F mice. Lung sections were counterstained with DAPI (blue). Data were quantified using 5 random lung images per mouse (n=7 mice per group). Scale bars are 50μm. (M) qRT-PCR shows that Acta2 mRNA is increased in lungs from Foxf1WT/S52F mice (n=12-17 mice per group). * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001, **** indicates p < 0.0001. Abbreviations: MLI, Mean linear intercept; Ar, artery; V, vein.
Pulmonary vascular abnormalities precede right ventricular hypertrophy in Foxf1WT/S52F mice.
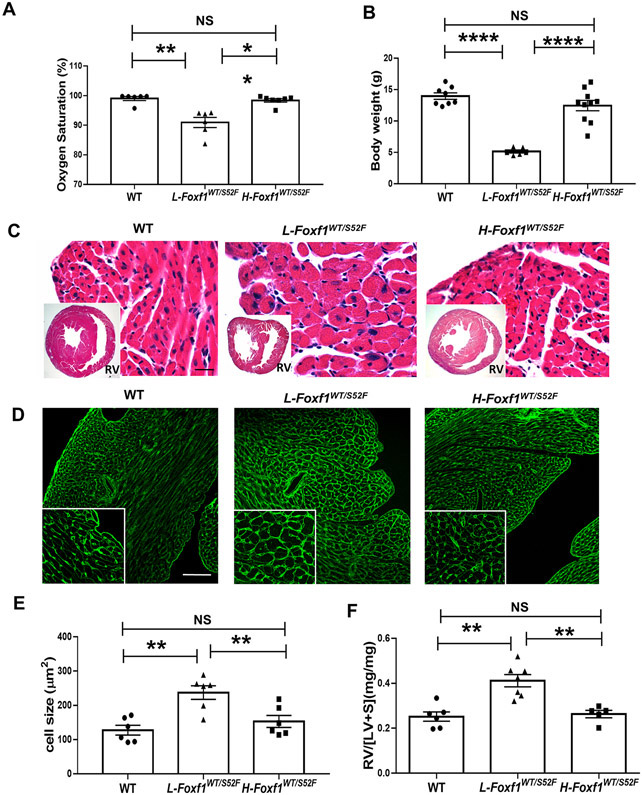
Published studies showed that Foxf1WT/S52F embryos exhibited alveolar capillary dysplasia and misalignment of pulmonary veins prior to birth, whereas the pulmonary artery thickening occurred postnatally 2. To determine the timing of RV hypertrophy in Foxf1WT/S52F mice, we examined hearts of Foxf1WT/S52F embryos and juvenile mice. At embryonic day 17.5 (E17.5), histological structure, IVS and myocardial thickness in the right ventricle were normal in Foxf1WT/S52F hearts (Figure VI in the Supplement). Thus, the S52F Foxf1 mutation does not cause RV hypertrophy before birth. After birth, Foxf1WT/S52F mice had low body weights compared to WT littermates and often died before weaning (Figure VIIA-B in the Supplement). The mortality rate in Foxf1WT/S52F mice progressively increased after birth, reaching 69.2% at P28 (Figure VIIA in the Supplement). Autopsy of the dead Foxf1WT/S52F pups showed RV hypertrophy (Figure VIIC-D in the Supplement). Based on body weights and arterial oxygenation at 4 weeks of age, Foxf1WT/S52F mice were subdivided into two groups: L-Foxf1WT/S52F and H-Foxf1WT/S52F (Figure 3A-B and Figure VIIIA in the Supplement). The L-Foxf1WT/S52F group included Foxf1 mutant mice with decreased arterial oxygenation and body weights lower than 6 grams, whereas H-Foxf1WT/S52F mice had normal arterial oxygenation and body weights higher than 6 grams (Figure 3A-B).
Figure 3. Low body weight in juvenile Foxf1WT/S52F mice is associated with right ventricular hypertrophy.
(A) Measurements of arterial oxygenation show decreased pO2 in L-Foxf1WT/S52F mice compared to either WT or H-Foxf1WT/S52F littermates (n=6-7 mice per group). (B) Body weights are smaller in L-Foxf1WT/S52F mice compared to other groups (n=6-10 mice per group). (C) H&E staining of transverse heart sections shows histology and thickness of RV walls in WT, L-Foxf1WT/S52F and H-Foxf1WT/S52F littermates. Scale bars are 25μm. (D-E) Wheat germ agglutinin (WGA) staining of heart sections shows enlarged RV cardiomyocytes in L-Foxf1WT/S52F mice. Average cell size was determined using 5 random heart images per mouse (n=6 mice per group). Scale bars are 100μm. (D) Ratio of RV weight to the combined weight of LV and interventricular septum (RV/[LV+S]) is increased in L-Foxf1WT/S52F mice (n=5-7 mice per group). NS indicates no significance, ** indicates p < 0.01, **** indicates p < 0.0001.
Morphological examination revealed that L-Foxf1WT/S52F hearts were lighter compared to hearts of WT and H-Foxf1WT/S52F littermates (Figure VIIIB-C in the Supplement). However, the ratio of heart weight to body weight (HW/BW) in L-Foxf1WT/S52F mice was increased (Figure VIIID in the Supplement). L-Foxf1WT/S52F hearts had a thicker myocardium and enlarged cardiomyocyte sizes in the right ventricle compared to hearts of WT and H-Foxf1WT/S52F littermates (Figure 3C-E). The RV/[LV+S] ratio in L-Foxf1WT/S52F mice was higher, whereas the RV/[LV+S] ratio in H-Foxf1WT/S52F mice was similar to WT controls (Figure 3F). Consistent with RV hypertrophy, Nppa, Nppb and Myh7 mRNAs, H19 lncRNA, and the Myh7/Myh6 mRNA ratio were selectively increased in the right ventricles of L-Foxf1WT/S52F but not H-Foxf1WT/S52F mice (Figure IXA-F in the Supplement). Capillary density in the right ventricle was similar in WT and Foxf1 mutant hearts as shown by immunostaining for endomucin (Figure XA-B in the Supplement). There was no evidence for cardiac fibrosis (Figure XC in the Supplement). In contrast to the right ventricle, the cardiomyocyte size in the left ventricle was unchanged among all groups of mice (Figure XD-E in the Supplement). Altogether, RV hypertrophy in Foxf1WT/S52F mice occurs postnatally and directly correlates with mortality, low body weight and decreased arterial oxygenation.
Right ventricular hypertrophy in Foxf1WT/S52F mice directly correlates with reduced density of alveolar capillaries and arterial wall muscularization.
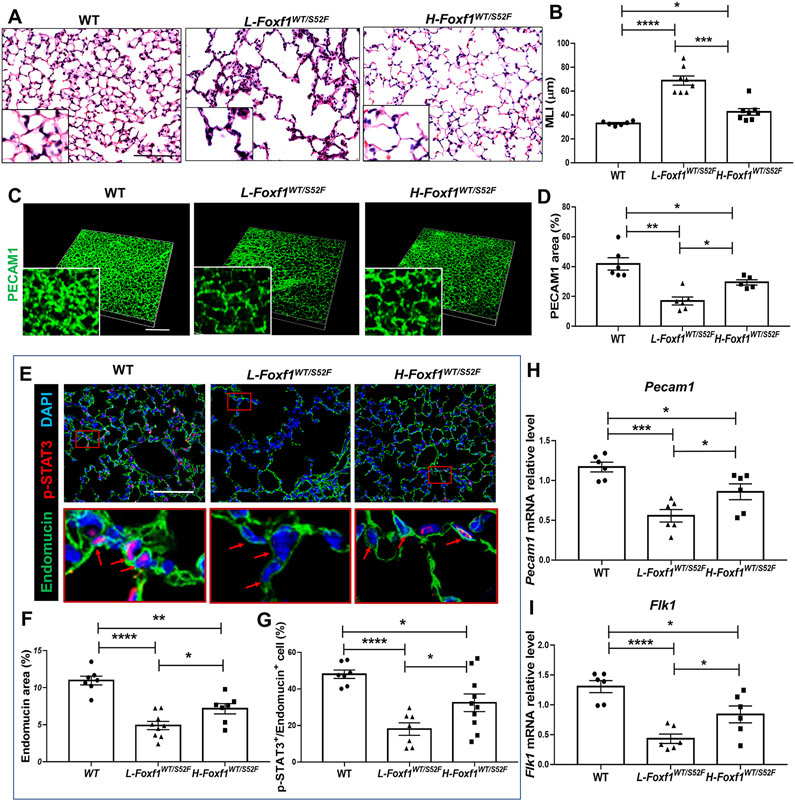
Histological examination revealed alveolar simplification and emphysema in Foxf1WT/S52F mice at 4 weeks of age, which was more severe in the L-Foxf1WT/S52F compared to the H-Foxf1WT/S52F group (Figure 4A-B). Immunostaining for PECAM1 and Endomucin revealed a paucity of alveolar capillary networks in both groups of Foxf1WT/S52F mutant mice, with the decrease of capillary density being more severe in L-Foxf1WT/S52F mice (Figure 4C-F). L-Foxf1WT/S52F lungs exhibited the lowest numbers of endothelial cells with nuclear staining for p-STAT3 (Figures 4E and 4G), a critical transcriptional regulator of endothelial proliferation and a direct target of FOXF1 2. Pecam1 and Flk1 mRNAs were lower in L-Foxf1WT/S52F lungs compared to H-Foxf1WT/S52F and WT lungs (Figure 4H-I). Consistent with decreased alveolar capillary density and reduced arterial oxygenation, L-Foxf1WT/S52F lungs exhibited increased expression of hypoxia-associated genes 41, including Vegfa, Binp3, Slc2a1 and Epas1, the latter of which encodes HIF-2α transcription factor (Figure XI in the Supplement). Interestingly, HIF-1α staining and Hif1a mRNA were unchanged in L-Foxf1WT/S52F lungs (Figures XIA and XIIA-C in the Supplement). Consistent with reduced capillary density, L-Foxf1WT/S52F lungs exhibited decreased endothelial cell proliferation in the early postnatal period as shown by FACS analysis of P7 lungs for CD31 (PECAM1) and Ki-67 (Figure XIII in the Supplement).
Figure 4. Low body weight in juvenile Foxf1WT/S52F mice is associated with alveolar capillary dysplasia and decreased p-STAT3.
(A-B) H&E staining of lung sections shows severe alveolar simplification in L-Foxf1WT/S52F mice. Mean linear intercept (MLI) was determined using 5 random images from each of 3 lung sections per mouse (n=6-8 mice per group). Scale bars are 50μm. (C-D) Whole mount PECAM1 immunostaining of lung slices shows decreased capillary density in L-Foxf1WT/S52F lungs. Data were quantified using 5 random lung images per mouse (n=5-6 mice per group). Scale bars are 100μm. (E-G) Immunostaining for Endomucin (green) and p-STAT3 (red) shows decreased capillary density and a decreased number of endothelial cells expressing p-STAT3 in L-Foxf1WT/S52F lungs. Bottom images show the area in red box. Arrows point to nuclei of endothelial cells. Slides were counterstained with DAPI (blue). Five random lung images per mouse were used to quantify the data (n=7-10 mice per group). Scale bars are 100μm. (H-I) qRT-PCR shows that Pecam1 and Flk1 mRNAs are decreased in total lung RNA from L-Foxf1WT/S52F mice. mRNAs were normalized using β-actin mRNA (n=6 mice per group). NS indicates no significance, * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001, **** indicates p < 0.0001.
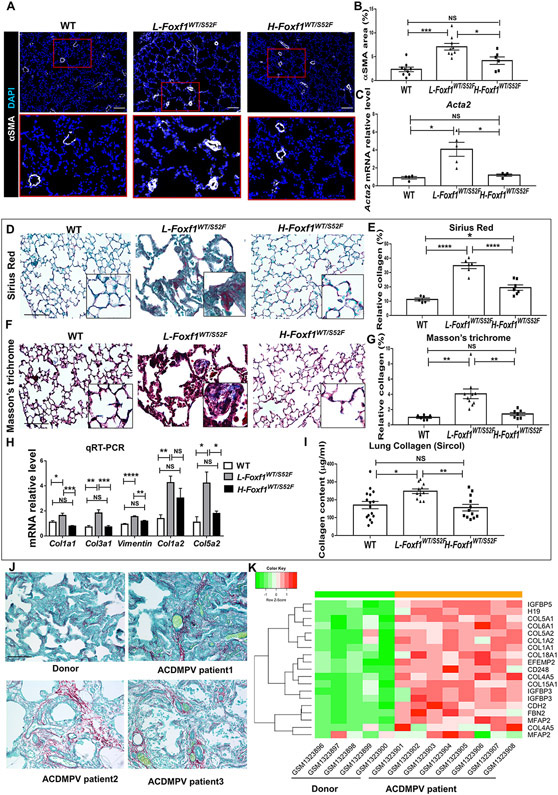
Alveolar capillary dysplasia leads to increased blood pressure in the pulmonary circulation, causing hyperextension of pulmonary arteries and subsequent muscularization of the arterial wall 1. Consistent with the most severe decreases in alveolar capillary density, muscularization of pulmonary arteries occurred in L-Foxf1WT/S52F but not in H-Foxf1WT/S52F lungs (Figure 5A-B) and was associated with increased Acta2 mRNA (Figure 5C). Pulmonary arteries of all sizes were remodeled in L-Foxf1WT/S52F mice but their lumens were not occluded (Figure XIVA-D in the Supplement). Proliferation of αSMA+ arterial smooth muscle cells and PDGFRα+ fibroblasts was increased in Foxf1WT/S52F lungs at P7 (Figure XVA-C in the Supplement), whereas proliferation of endothelial cells lining pulmonary arteries was unchanged (Figure XVD-E in the Supplement). Interestingly, FOXF1 was not expressed in arterial smooth muscle cells at either P7 or P28 (Figure XVIA-B in the Supplement), supporting indirect effects of the S52F Foxf1 mutation on arterial remodeling. Altogether, reduced capillary density and muscularization of pulmonary arteries directly correlate with decreased arterial oxygenation, RV hypertrophy, low body weight and mortality in Foxf1WT/S52F juvenile mice.
Figure 5. Human ACDMPV and mouse L-Foxf1WT/S52F lungs have increased collagen deposition.
(A-B) Immunostaining for αSMA shows muscularization of small lung arteries in L-Foxf1WT/S52F mice. Bottom images show the area in red box. Sections were counterstained with DAPI (blue). Data were quantified using 5 random lung images per mouse (n=6-9 mice per group). Scale bars are 100μm. (C) qRT-PCR shows that Acta2 mRNA is increased in L-Foxf1WT/S52F lungs. Acta2 mRNA was normalized using β-actin mRNA (n=4-5 mice per group). (D-E) Sirius red staining shows increased collagen deposition in L-Foxf1WT/S52F lungs (n=5-7 mice per group). Five random lung images per mouse were used to quantify collagen deposition. Scale bars are 50μm. (F-G) Masson’s trichrome staining shows increased collagen deposition in L-Foxf1WT/S52F mice (n=6-10 mice per group). Scale bars are 50μm. (H) qRT-PCR shows that Col1a1, Col3a1, Vimentin, Col1a2 and Col5a2 mRNAs are increased in L-Foxf1WT/S52F lungs (n=6-13 mice per group). (I) Sircol assay shows increased collagen content in the left lobe of L-Foxf1WT/S52F lungs (n=11-16 mice per group). (J) Sirius red staining of lung biopsies shows increased collagen deposition in alveolar and perivascular regions of ACDMPV patients compared to donor lungs (n=3). Scale bars are 50μm. (K) Microarray analysis of whole lung RNA from ACDMPV patients (n=8) shows increased expression of ECM genes compared to age-matched donor lungs (n=5). NS indicates no significance, * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001, **** indicates p < 0.0001.
Human and mouse ACDMPV lungs exhibit fibrotic lung remodeling.
Excessive collagen depositions were observed in L-Foxf1WT/S52F lungs by Sirius red (Figure 5D-E) and Masson’s trichrome staining (Figure 5F-G). Increased collagen amounts and elevated Col1a1, Col1a2, Col5a2, Col3a1 and Vim mRNAs were found in L-Foxf1WT/S52F lungs by Sircol assay and qRT-PCR (Figure 5H-I). There was no lung fibrosis in WT and H-Foxf1WT/S52F littermates (Figure 5D-I). Consistent with the murine ACDMPV model, lung biopsies from ACDMPV patients showed increased collagen depositions in alveolar and perivascular regions (Figure 5J and Figure XVII in the Supplement). Compared to donor lungs, microarray analysis of whole lung RNA from human ACDMPV lungs (n=8) revealed increased expression of multiple ECM genes, including COL1A1, COL1A2, COL5A2, COL6A1 and FBN2 (Figure 5K and Figure XVIII in the Supplement), a finding consistent with fibrotic lung remodeling. Both human ACDMPV and mouse L-Foxf1WT/S52F lungs exhibited increased expression of H19 lncRNA (Figure 5K and Figures XI and XVIII in the Supplement), which is associated with PH and RV dysfunction 42. Thus, human and mouse ACDMPV lungs exhibited increased fibrotic remodeling.
The S52F Foxf1 mutation promotes differentiation of lung myofibroblasts from embryonic stem cells.
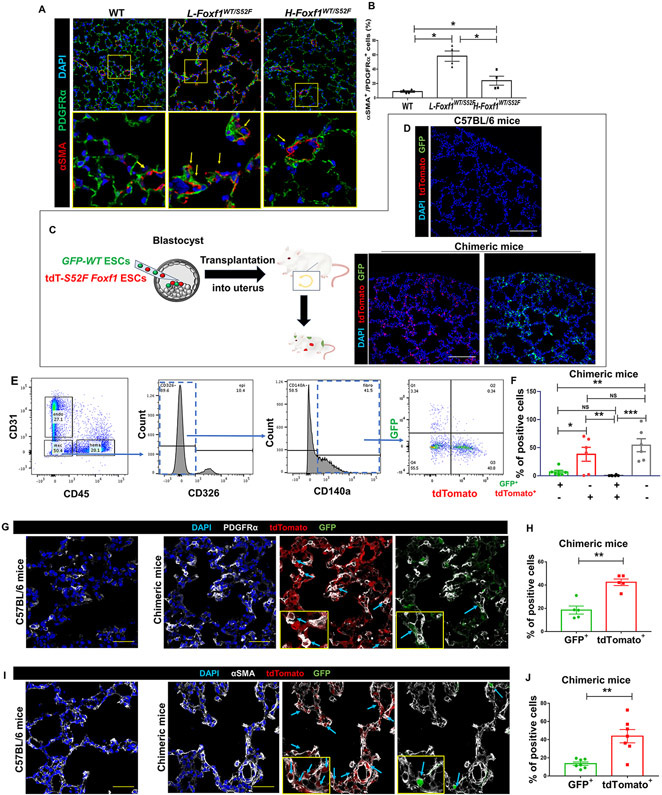
Excessive collagen depositions in ACDMPV lungs can occur due to aberrant differentiation of PDGFRα+ fibroblasts into myofibroblasts. Therefore, we used immunostaining for αSMA and PDGFRα (CD140a) to identify myofibroblasts in Foxf1WT/S52F and control lungs. The percentage of αSMA+PDGFRα+ myofibroblasts among PDGFRα+ fibroblasts was increased in alveolar regions of L-Foxf1WT/S52F mice as determined by immunostaining of tissue sections (Figure 6A-B) and FACS analysis of enzyme-digested lung tissue (Figure XIX in the Supplement).
Figure 6. Embryonic stem cells with S52F Foxf1 mutation have a propensity to differentiate into pulmonary myofibroblasts.
(A-B) Immunostaining shows an increased number of myofibroblasts co-expressing αSMA and PDGFRα in L-Foxf1WT/S52F lungs. Bottom images show the area in the yellow box. Sections were counterstained with DAPI (blue). Arrows point to αSMA+PDGFRα+ myofibroblasts. The percentage of αSMA+PDGFRα+ cells among PDGFRα+ fibroblasts in the alveolar region was counted using 5 random lung images per mouse (n=4-6 mice per group). Scale bars are 100μm. (C) Schematic diagram showing the injection of donor ESCs into mouse blastocysts and generation of chimeric mice. (D) Confocal images show contributions of donor S52F Foxf1 ESCs expressing tdTomato (tdT-S52F Foxf1 ESCs, red) and WT ESCs expressing GFP (GFP-WT ESCs) to the lung tissue of chimeric mice. Donor-derived cells are detected in DAPI-stained lung tissue sections. Scale bars are 100μm. (E-F) FACS analysis of enzymatically-digested lung tissue from chimeras shows higher contribution of tdT-S52F Foxf1 ESCs to the myofibroblast cell lineage (CD140a+CD31−CD45−CD326−) compared to GFP-WT ESCs. Lungs were harvested at P7 (n=6). (G-J) Immunostaining of frozen lung sections shows higher contribution of tdT-S52F Foxf1 ESCs (red) to αSMA+ and PDGFRα+ cells compared to GFP-WT ESCs (green). Sections were counterstained with DAPI (blue). Arrows point to ESC-derived myofibroblasts. The percentage of ESC-derived myofibroblasts in the alveolar region was calculated using 5 random lung images per mouse (n=5-7 mice). Scale bars are 40μm. NS indicates no significance, * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001.
Next, we tested whether the S52F Foxf1 mutation accelerates differentiation of lung myofibroblasts from pluripotent embryonic stem cells (ESCs) in vivo. ESCs containing the S52F Foxf1 mutation and the tdTomato lineage marker (tdT-S52F Foxf1 ESCs) were generated from Foxf1WT/S52F; tdTomato transgenic mice. tdT-S52F Foxf1 ESCs formed typical ECS cell colonies in vitro (Figure XXA in the Supplement), which were similar to previously established GFP-labeled WT ESCs (GFP-WT ESCs) 21. To compare the ability of tdT-S52F Foxf1 and GFP-WT ESCs to differentiate into respiratory cell lineages in vivo, we used a blastocyst complementation method. Equal amounts of tdT-S52F Foxf1 and GFP-WT ESCs were injected into the same WT blastocysts and the chimeric embryos were transplanted into the uterus of surrogate WT females to undergo normal embryonic development (Figure 6C). Mouse chimeras were harvested at P7 and examined for donor-derived cells using tdTomato and GFP fluorescence. Donor ECS-derived GFP+ and tdTomato+ cells were abundant in the lung tissue of chimeric mice (Figure 6D and Figure XXB in the Supplement). Consistent with pluripotency of donor ESCs, FACS analysis showed that both tdT-S52F Foxf1 and GFP-WT ESCs efficiently contributed to multiple respiratory cell types (Figure 6E-F and Figure XXIA-D in the Supplement). Cells formed by the fusion of GFP+ and tdTomato+ progenies in the lung tissue were rare (Figure 6E-F and Figure XXIA-D in the Supplement). The contribution of tdT-S52F Foxf1 ESCs to the myofibroblast cell lineage was higher compared to GFP-WT ESCs as shown by FACS analysis (Figure 6E-F) and immunostaining of chimeric lungs for αSMA and PDGFRα (Figure 6G-J). Contributions of tdT-S52F Foxf1 and GFP-WT ESCs to pericytes, endothelial, epithelial and hematopoietic cell lineages in chimeric lungs were similar (Figure XXI in the Supplement). Thus, S52F Foxf1 ESCs have a propensity to differentiate into pulmonary myofibroblasts in the neonatal lung. The S52F Foxf1 mutation does not influence ESC differentiation to other respiratory cell types.
Nanoparticle delivery of Stat3 cDNA prevents RV hypertrophy and decreases lung remodeling in Foxf1WT/S52F mice.
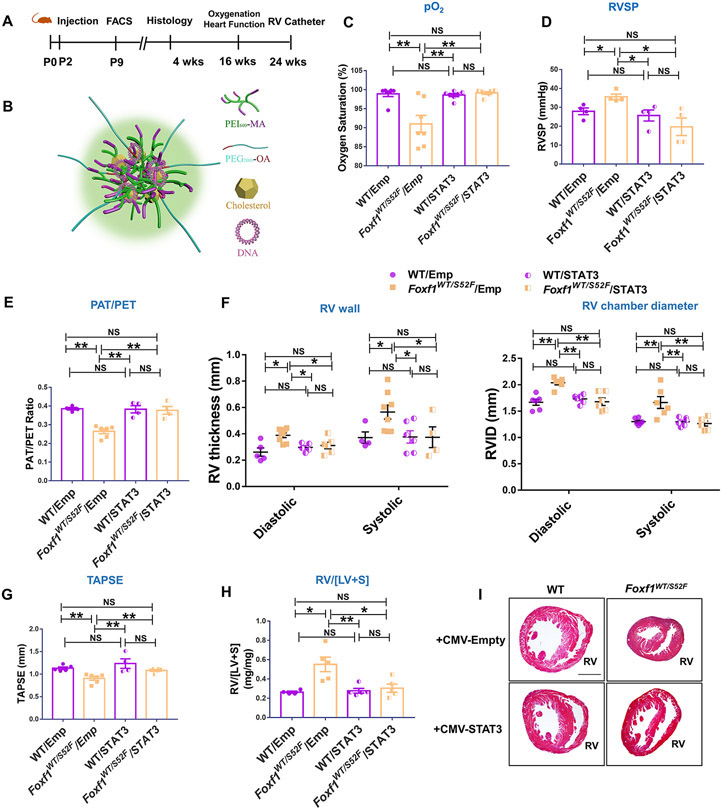
Published studies demonstrated that nanoparticle delivery of the FOXF1 downstream target gene, STAT3, directly into endothelial cells increased alveolar capillary density in Foxf1WT/S52F lungs during the early neonatal period 2. To determine whether increased capillary density is sufficient to protect the L-Foxf1WT/S52F mice from PH later in life, we injected the PEI600-MA5/PEG-OA/Cho nanoparticles carrying either a non-integrating CMV-Stat3 expression vector or a CMV empty vector into the facial vein of P2 pups and harvested the mice at P28 (Figure 7A-B). Consistent with published studies 2, nanoparticle delivery of STAT3 improved the capillary density (Figure XXIIA-B in the Supplement), increased the number of p-STAT3+ endothelial cells (Figure XXIIC in the Supplement), and increased Pecam1 and Flk1 mRNAs in Foxf1WT/S52F lungs (Figure XXIID-E in the Supplement). Increased capillary density in STAT3-treated Foxf1WT/S52F lungs was associated with increased arterial oxygenation (Figure 7C), supporting an improvement in alveolar gas exchange. Since the PEI600-MA5/PEG-OA/Cho nanoparticles do not target smooth muscle cells 19, neither STAT3 nor p-STAT3 were changed in pulmonary artery smooth muscle cells after the nanoparticle treatment (Figures XXIII-XXIV in the Supplement).
Figure 7. Nanoparticle delivery of Stat3 cDNA prevents RV hypertrophy and increases survival of Foxf1WT/S52F mice.
(A) Schematic representation of nanoparticle treatment of Foxf1WT/S52F mice. Nanoparticle containing either CMV-Stat3 (STAT3) or a CMV empty plasmid (Emp) were injected at P2 via the facial vein. FACS analysis was performed at P9. Histology and immunostaining were carried out at P28. Echocardiography and measurements of arterial oxygenation were performed at 4 months of age, whereas RV catheterization was performed at 6 months of age. (B) Schematic representation of nanoparticle structure shows PEI and PEG polymers, cholesterol and plasmid DNA. (C) Measurements of arterial oxygenation show that nanoparticle delivery of Stat3 DNA increases pO2 in Foxf1WT/S52F mice (n=7 mice per group). (D) STAT3 treatment decreases RVSP in Foxf1WT/S52F mice (n=4 mice per group). (E-G) Echocardiography shows improved ratio of pulmonary acceleration time (PAT) to pulmonary ejection time (PAT/PET) in Foxf1WT/S52F mice injected with STAT3 nanoparticles compared to CMV empty controls. Nanoparticle delivery of Stat3 cDNA increases TAPSE but decreases diastolic and systolic RV wall thickness and the right ventricular internal diameter (RVID) in Foxf1WT/S52F mice (n=4-8 mice per group). (H) Nanoparticle delivery of Stat3 cDNA decreases RV/[LV+S] in P28 Foxf1WT/S52F mice (n=4-5 mice per group). (I) H&E staining shows transverse heart sections of WT and Foxf1WT/S52F mice that were either treated with STAT3 or control nanoparticles. Scale bars are 0.8mm. NS indicates no significance, * indicates p < 0.05, ** indicates p < 0.01. Abbreviations: RV/[LV+S], ratio of right ventricle weight to the combined weight of left ventricle and interventricular septum; RV, right ventricle; TAPSE, Tricuspid annular plane systolic excursion.
Delivery of STAT3 was sufficient to prevent PH and RV hypertrophy in Foxf1WT/S52F mice as shown by RV catheterization (Figure 7D) and transthoracic echocardiography to determine the PAT/PET ratio (Figure 7E and Figure XXVA in the Supplement), RV thickness and internal diameter (Figure 7F), TAPSE (Figure 7G), stroke volume, S wave and cardiac output (Figure XXVB-D in the Supplement). Decreased RV hypertrophy after STAT3 delivery was also supported by measurements of RV/[LV+S] (Figure 7H-I), and WGA staining to determine the size of cardiomyocytes in the right ventricle (Figure XXVIA-B in the Supplement). Although the nanoparticles targeted endothelial cells of several organs with equal efficiency, capillary density in the right ventricle was unchanged (Figure XXVIIA-D in the Supplement). Nanoparticle delivery of STAT3 did not alter p-STAT3 staining and the percentage of STAT3+ endothelial cells in the heart and liver tissues (Figure XXVIIIA-C in the Supplement). In the lung tissue of Foxf1WT/S52F mice, alveolarization and pulmonary artery remodeling were improved after nanoparticle delivery of STAT3 compared to an empty vector (Figures XXIXA-B and XXXA-C in the Supplement). Nanoparticle delivery of STAT3 did not change expression of epithelial markers pro-SPC and T1α (Figure XXXI in the Supplement) but decreased fibrotic depositions in Foxf1WT/S52F lungs as demonstrated by Sirius red staining (Figure XXXIIA-B in the Supplement) and expression of pro-fibrotic genes in whole lung RNA (Figure XXXIIC in the Supplement). Vegfa mRNA was decreased to normal levels, whereas Hif1a mRNA was unchanged (Figure XXXIIIA-B in the Supplement). Body weights and lung sizes of STAT3-treated Foxf1WT/S52F mice were higher compared to L-Foxf1WT/S52F mice injected with nanoparticles containing an empty vector (Figures XXXIV and XXXVA in the Supplement). Finally, a single nanoparticle delivery of STAT3 at P2 decreased 4-week mortality in Foxf1WT/S52F mice compared to untreated mice or Foxf1WT/S52F mice treated with nanoparticles containing an empty vector (Figure XXXVB in the Supplement). Altogether, increasing alveolar capillary density at the early neonatal period through nanoparticle delivery of STAT3 was sufficient to increase arterial oxygenation, prevent PH and RV hypertrophy, decrease lung remodeling and improve survival in Foxf1WT/S52F mice.
DISCUSSION
Pathological findings in ACDMPV lungs include the paucity of the alveolar capillary network, abnormal positioning (misalignment) of pulmonary veins and global lung hypoplasia 1. Altogether, these congenital lung abnormalities result in severe PH and fulminant respiratory failure shortly after birth 1. While PH exacerbates this syndrome and contributes to high mortality rate, the pathophysiological mechanisms causing PH in ACDMPV remain unclear. In the present study, we demonstrated that Foxf1WT//S52F mice not only develop the salient features of ACDMPV but also pulmonary hypertension, as evidenced by increased RVSP, decreased pulmonary artery acceleration time, pulmonary artery muscularization, and RV hypertrophy and dilation. We also demonstrated that pulmonary vascular remodeling precedes the development of RV hypertrophy, and this change in RV weight was not secondary to increased cardiac fibrosis but occurred mostly due to an increase in cardiomyocyte mass. Our studies suggest the PH phenotype in the setting of ACDMPV can be prevented using a systemic administration of nanoparticles containing the STAT3 expression vector, which increases alveolar capillary density via activation of endothelial proliferation 2. It is unlikely that the nanoparticle STAT3 therapy can correct the misalignment of pulmonary veins and lung hypoplasia since these defects occur early during lung development. Despite the expression vector delivered by the nanoparticles is active only for 7 days in the neonatal lung 20, increasing alveolar capillary density at the early neonatal period was sufficient to prevent PH, decrease RV hypertrophy and improve survival in Foxf1WT//S52F mice. Our studies raise a possibility of using proangiogenic pulmonary therapies in human ACDMPV either caused by the S52F FOXF1 mutation or associated with decreased STAT3 signaling.
The pulmonary circulation is a low-pressure circulatory system both at rest and during exercise 43. Up to 45% of the total pulmonary vascular resistance results from the alveolar wall capillaries. Therefore, the PH phenotype seen in Foxf1WT//S52F mice could be a consequence of abnormal development of alveolar microvasculature, similarly to other forms of PH that are associated with abnormal or incomplete lung development in bronchopulmonary dysplasia (BPD) and congenital diaphragmatic hernia (CDH). Foxf1WT//S52F lungs did not have arterial luminal occlusions or plexiform-like lesions that characterize other forms of pulmonary hypertension in mice or rat models. Although the loss of the FOXF1 enhancer can cause capillary hemangiomatosis/pulmonary veno-occlusive disease 44, we did not observe histological changes consistent with pulmonary capillary hemangiomatosis in Foxf1WT//S52F mice. Decreased alveolar capillary density in Foxf1 mutant mice can lead to reduced arterial oxygenation and lung tissue hypoxia, directly contributing to pulmonary fibrosis, arterial wall remodeling and PH. Decreased PAT/PET ratio, significant RV hypertrophy, increased RVSP and RV chamber dilation are all consistent with chronically elevated RV afterload in Foxf1 mutant mice. One limitation of our study is that we were unable to predict which Foxf1 mutant mice will develop L-Foxf1WT//S52F or H-Foxf1WT//S52F phenotype at birth. It is possible that the nanoparticle treatment outcome may be influenced by skewed inclusion of mice destined to become L-Foxf1WT//S52F or H-Foxf1WT//S52F mice in the treatment groups. Heterogeneity of the phenotypes in Foxf1 mutant mice can be a consequence of the hybrid C57BL/6 x 129/J genetic background, in which the Foxf1WT//S52F mouse line is maintained to avoid uniform mortality after birth. To better understand ACDMPV pathogenesis, it will be interesting to identify genetic modifiers influencing the ratio between L-Foxf1WT//S52F and H-Foxf1WT//S52F phenotypes in Foxf1 mutant mice.
Despite decreased arterial oxygenation and increased hypoxia-associated Vegfa, Binp3, Epas1 and Slc2a1 transcripts in the lung tissue, expression of HIF-1α was unchanged in Foxf1 mutant mice. Since multiple FOXF1-binding sites are present in the Hif1a promoter, it is possible that FOXF1 transcriptionally activates the Hif1a gene and that FOXF1 deficiency prevents the increased expression of HIF-1α during hypoxia. STAT3 is known to promote arterial remodeling and PH when upregulated in pulmonary artery smooth muscle cells 45. However, we did not observe increased STAT3 and p-STAT3 in vascular smooth muscle cells. These results are consistent with published studies 19 demonstrating that the PEI600-MA5/PEG-OA/Cho nanoparticles do not target arterial smooth muscle cells after i.v. administration.
Several members of the Forkhead box (FOX) family of transcription factors have been implicated in the development of PH 46. Expression of FOXM1 was increased in the lungs of patients with idiopathic pulmonary arterial hypertension 47, and FOXM1 stimulated smooth muscle cell proliferation and arterial remodeling in rodents 48. Inhibition of FOXO1 in smooth muscle cells caused pulmonary vascular remodeling and spontaneous PH 49. FOXF1, a causative gene for ACDMPV 3, is expressed in pulmonary endothelial cells and fibroblasts but is absent in the adult heart. Here we demonstrate that the S52F Foxf1 mutation increases the number of pulmonary myofibroblasts in the alveolar walls and perivascular regions. Fibrotic lung remodeling in Foxf1WT//S52F mice can be explained by hypoxia due to decreased microvascular density. Alternatively, FOXF1 may influence lung myofibroblasts via cell autonomous mechanisms. Consistent with this hypothesis, myofibroblast-specific inactivation of Foxf1 exacerbated lung fibrosis in a bleomycin-induced mouse model 7. Since the S52F Foxf1 mutation encodes transcriptionally inactive S52F FOXF1 protein 2 and the number of myofibroblasts is increased in Foxf1WT//S52F lungs, our studies suggest that FOXF1 inhibits myofibroblasts differentiation. Blastocyst complementation of WT embryos with S52F Foxf1 mutant embryonic stem cells was performed in these studies, directly supporting the increased ability of S52F Foxf1 mutant cells to differentiate into myofibroblasts, even in the WT lung environment. In addition to inhibiting myofibroblast differentiation, FOXF1 may decrease production of collagens by pulmonary myofibroblasts. FOXF1 protein directly bound to active repressors of Col1a2 and Col5a2 genes 50, providing direct support for this concept.
In summary, nanoparticle-mediated Stat3 gene therapy (performed after birth) ameliorated PH, decreased RV hypertrophy and increased survival of Foxf1WT//S52F mice. Improvements in pulmonary hemodynamics and RV remodeling were the result of reduction in vascular resistance due to an increase in alveolar capillary density after nanoparticle treatment. Our findings suggest that nanoparticle STAT3 gene therapy could be considered for the treatment of fulminant PH in patients with ACDMPV.
Supplementary Material
CLINICAL PERSPECTIVE.
What is new?
Foxf1WT/S52F mice carrying the inactivating S52F Foxf1 mutation (identified in ACDMPV patients) develop pulmonary hypertension and right ventricular hypertrophy which directly correlate with severity of lung remodeling in these mice.
Pulmonary hypertension in Foxf1WT/S52F mice is secondary to alveolar capillary dysplasia, fibrotic lung remodeling and decreased endothelial expression of STAT3, a key downstream target of FOXF1 transcription actor.
Nanoparticle delivery of STAT3 into the neonatal circulation prevents pulmonary hypertension and increases survival of Foxf1WT/S52F mice by improving alveolar microvascular networks and arterial oxygenation.
What are the clinical implications?
Nanoparticle delivery of STAT3 may be considered for treatment of newborns and infants with ACDMPV associated with inactivating FOXF1 mutations.
Increasing neonatal lung angiogenesis may be considered for prevention of severe pulmonary hypertension and fulminant respiratory failure in patients with congenital lung diseases associated with abnormal or incomplete lung development.
ACKNOWLEDGMENTS
We thank Erika Smith for excellent editorial assistance.
SOURCES OF FUNDING
This work was supported by NIH Grants HL141174 (to V.V.K.), HL149631 (to V.V.K.), HL152973 (to V.V.K. and T.V.K.), HL132849 (to T.V.K.) and HL152094 (to R.S.H.).
Non-standard Abbreviations and Acronyms
- ACDMPV
alveolar capillary dysplasia with misalignment of pulmonary veins
- Acta2
actin alpha 2
- αSMA
alpha smooth muscle actin
- Binp3
BCL2 Interacting protein 3
- BPD
bronchopulmonary dysplasia
- CDH
congenital diaphragmatic hernia
- CMV
cytomegalovirus
- Col1a1
collagen type 1 alpha 1 chain
- Col1a2
collagen type 1 alpha 2 chain
- Col3a1
collagen type 3 alpha 1 chain
- Col5a2
collagen type 5 alpha 2 chain
- Col6a1
collagen type 6 alpha 1 chain
- di
internal diameter
- do
external diameter
- Epas1
endothelial PAS domain protein 1
- ESCs
embryonic stem cells
- FBN2
fibrillin 2
- Flk1
fetal liver kinase 1
- FOXF1
forkhead Box F1
- GFP
green fluorescent protein
- H&E
hematoxylin and eosin
- Hif1a
hypoxia inducible factor 1 subunit alpha
- i.v.
intravenous
- IVS
interventricular septum
- IVS;d
interventricular septal thickness at diastole
- IVS;s
interventricular septal thickness at systole
- LA
left atrium
- LV
left ventricular
- LVID;d
left ventricular internal diameter at the end of diastole
- LVID;s
left ventricular internal diameter at the end of systole
- LV Mass
left ventricular mass
- LVPW;d
left ventricular posterior wall thickness at the end of diastole
- LVPW;s
left ventricular posterior wall thickness at the end of systole
- LV Vol;d
left ventricular volume at the end of diastole
- LV Vol;s
left ventricular volume at the end of systole
- MLI
mean linear intercept
- Myh6
myosin heavy chain 6
- Myh7
myosin heavy chain 7
- Nppa
natriuretic peptide A
- Nppb
natriuretic peptide B
- PA
pulmonary artery
- PAT
pulmonary acceleration time
- PDGFRα
platelet-derived growth factor Alpha
- Pecam1
platelet and endothelial cell adhesion molecule 1
- PEI600-MA5/PEG-OA/Cho
polyethylenimine-(5) myristic acid/ poly (ethylene glycol)-oleic acid/ cholesterol
- PET
pulmonary ejection time
- PH
pulmonary hypertension
- RV
right ventricular
- RVID
right ventricular internal diameter
- RVSP
right ventricular systolic pressure
- RV/[LV+S]
ratio of RV weight to the combined weight of LV and interventricular septum
- Slc2a1
solute carrier family 2 member 1
- Stat3
signal transducer and activator of transcription 3
- SV
stroke volume
- TAPSE
tricuspid annular plane systolic excursion
- tdT
tdTomato
- Vegfa
vascular endothelial growth factor A
- Vim
vimentin
- WGA
wheat germ agglutinin
- WT
wild type
Footnotes
DISCLOSURES
Authors of this manuscript have no conflicts of interest.
REFERENCES
- 1.Bishop NB, Stankiewicz P and Steinhorn RH. Alveolar capillary dysplasia. Am J Respir Crit Care Med. 2011;184:172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pradhan A, Dunn A, Ustiyan V, Bolte C, Wang G, Whitsett JA, Zhang Y, Porollo A, Hu YC, Xiao R, et al. The S52F FOXF1 Mutation Inhibits STAT3 Signaling and Causes Alveolar Capillary Dysplasia. Am J Respir Crit Care Med. 2019;200:1045–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dharmadhikari AV, Szafranski P, Kalinichenko VV and Stankiewicz P. Genomic and Epigenetic Complexity of the FOXF1 Locus in 16q24.1: Implications for Development and Disease. Curr Genomics. 2015;16:107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards JJ, Murali C, Pogoriler J, Frank DB, Handler SS, Deardorff MA and Hopper RK. Histopathologic and Genetic Features of Alveolar Capillary Dysplasia with Atypical Late Presentation and Prolonged Survival. J Pediatr. 2019;210:214–219 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Towe CT, White FV, Grady RM, Sweet SC, Eghtesady P, Wegner DJ, Sen P, Szafranski P, Stankiewicz P, Hamvas A, et al. Infants with Atypical Presentations of Alveolar Capillary Dysplasia with Misalignment of the Pulmonary Veins Who Underwent Bilateral Lung Transplantation. J Pediatr. 2018;194:158–164 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whitsett JA, Kalin TV, Xu Y and Kalinichenko VV. Building and Regenerating the Lung Cell by Cell. Physiol Rev. 2019;99:513–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Black M, Milewski D, Le T, Ren X, Xu Y, Kalinichenko VV and Kalin TV. FOXF1 Inhibits Pulmonary Fibrosis by Preventing CDH2-CDH11 Cadherin Switch in Myofibroblasts. Cell Rep. 2018;23:442–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim IM, Zhou Y, Ramakrishna S, Hughes DE, Solway J, Costa RH and Kalinichenko VV. Functional characterization of evolutionary conserved DNA regions in forkhead box f1 gene locus. J Biol Chem. 2005;280:37908–37916. [DOI] [PubMed] [Google Scholar]
- 9.Kalinichenko VV, Gusarova GA, Shin B and Costa R. The Forkhead Box F1 Transcription Factor is Expressed in Brain and Head Mesenchyme during Mouse Embryonic Development. Gene Expr Patterns. 2003;3:153–158. [DOI] [PubMed] [Google Scholar]
- 10.Bolte C, Whitsett JA, Kalin TV and Kalinichenko VV. Transcription Factors Regulating Embryonic Development of Pulmonary Vasculature. Adv Anat Embryol Cell Biol. 2018;228:1–20. [DOI] [PubMed] [Google Scholar]
- 11.Ustiyan V, Bolte C, Zhang Y, Han L, Xu Y, Yutzey KE, Zorn AM, Kalin TV, Shannon JM and Kalinichenko VV. FOXF1 transcription factor promotes lung morphogenesis by inducing cellular proliferation in fetal lung mesenchyme. Dev Biol. 2018;443:50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolte C, Flood HM, Ren X, Jagannathan S, Barski A, Kalin TV and Kalinichenko VV. FOXF1 transcription factor promotes lung regeneration after partial pneumonectomy. Sci Rep. 2017;7:10690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai Y, Bolte C, Le T, Goda C, Xu Y, Kalin TV and Kalinichenko VV. FOXF1 maintains endothelial barrier function and prevents edema after lung injury. Sci Signal. 2016;9:ra40. [DOI] [PubMed] [Google Scholar]
- 14.Kalinichenko VV, Zhou Y, Shin B, Beer-Stoltz D, Watkins SC, Whitsett JA and Costa RH. Wild Type Levels of the Mouse Forkhead Box f1 Gene are Essential for Lung Repair. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1253–L1265. [DOI] [PubMed] [Google Scholar]
- 15.Kalin TV, Meliton L, Meliton AY, Zhu X, Whitsett JA and Kalinichenko VV. Pulmonary mastocytosis and enhanced lung inflammation in mice heterozygous null for the Foxf1 gene. Am J Respir Cell Mol Biol. 2008;39:390–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bolte C, Ren X, Tomley T, Ustiyan V, Pradhan A, Hoggatt A, Kalin TV, Herring BP and Kalinichenko VV. Forkhead box F2 regulation of platelet-derived growth factor and myocardin/serum response factor signaling is essential for intestinal development. J Biol Chem. 2015;290:7563–7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahlapuu M, Ormestad M, Enerback S and Carlsson P. The forkhead transcription factor Foxf1 is required for differentiation of extra-embryonic and lateral plate mesoderm. Development. 2001;128:155–166. [DOI] [PubMed] [Google Scholar]
- 18.Kalinichenko VV, Lim L, Beer-Stoltz D, Shin B, Rausa FM, Clark J, Whitsett JA, Watkins SC and Costa RH. Defects in Pulmonary Vasculature and Perinatal Lung Hemorrhage in Mice Heterozygous Null for the Forkhead Box f1 transcription factor. Dev Biol. 2001;235:489–506. [DOI] [PubMed] [Google Scholar]
- 19.Dunn AW, Kalinichenko VV and Shi D. Highly Efficient In Vivo Targeting of the Pulmonary Endothelium Using Novel Modifications of Polyethylenimine: An Importance of Charge. Adv Healthc Mater. 2018;7:e1800876. [DOI] [PubMed] [Google Scholar]
- 20.Bolte C, Ustiyan V, Ren X, Dunn AW, Pradhan A, Wang G, Kolesnichenko OA, Deng Z, Zhang Y, Shi D, et al. Nanoparticle Delivery of Proangiogenic Transcription Factors into the Neonatal Circulation Inhibits Alveolar Simplification Caused by Hyperoxia. Am J Respir Crit Care Med. 2020;202:100–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang G, Wen B, Ren X, Li E, Zhang Y, Guo M, Xu Y, Whitsett JA, Kalin TV and Kalinichenko VV. Generation of Pulmonary Endothelial Progenitor Cells for Cell-Based Therapy Using Interspecies Mouse-Rat Chimeras. Am J Respir Crit Care Med. 2021; Online ahead of print, doi: 10.1164/rccm.202003-0758OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCulley DJ, Wienhold MD, Hines EA, Hacker TA, Rogers A, Pewowaruk RJ, Zewdu R, Chesler NC, Selleri L and Sun X. PBX transcription factors drive pulmonary vascular adaptation to birth. J Clin Invest. 2018;128:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Pandey RN, York AJ, Mallela J, Nichols WC, Hu YC, Molkentin JD, Wikenheiser-Brokamp KA and Hegde RS. The EYA3 tyrosine phosphatase activity promotes pulmonary vascular remodeling in pulmonary arterial hypertension. Nat Commun. 2019;10:4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang IC, Meliton L, Ren X, Zhang Y, Balli D, Snyder J, Whitsett JA, Kalinichenko VV and Kalin TV. Deletion of Forkhead Box M1 transcription factor from respiratory epithelial cells inhibits pulmonary tumorigenesis. PloS One. 2009;4:e6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Bhattacharyya D, Dennewitz MB, Zhou Y, Kalinichenko VV, Lepe R and Costa RH. Rapid Hepatocyte Nuclear Translocation of the Forkhead Box M1B (FoxM1B) Transcription factor Causes a Transient Increase in Size of Regenerating Transgenic Hepatocytes. Gene Expr. 2003;11:149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bolte C, Zhang Y, Wang IC, Kalin TV, Molkentin JD and Kalinichenko VV. Expression of Foxm1 transcription factor in cardiomyocytes is required for myocardial development. PloS One. 2011;6:e22217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramakrishna S, Kim IM, Petrovic V, Malin D, Wang IC, Kalin TV, Meliton L, Zhao YY, Ackerson T, Qin Y, et al. Myocardium defects and ventricular hypoplasia in mice homozygous null for the Forkhead Box M1 transcription factor. Dev Dyn. 2007;236:1000–1013. [DOI] [PubMed] [Google Scholar]
- 28.Heath D and Edwards JE. The pathology of hypertensive pulmonary vascular disease; a description of six grades of structural changes in the pulmonary arteries with special reference to congenital cardiac septal defects. Circulation. 1958;18:533–547. [DOI] [PubMed] [Google Scholar]
- 29.Rol N, Timmer EM, Faes TJ, Vonk Noordegraaf A, Grunberg K, Bogaard HJ and Westerhof N. Vascular narrowing in pulmonary arterial hypertension is heterogeneous: rethinking resistance. Physiol Rep. 2017;5:e13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ustiyan V, Wert SE, Ikegami M, Wang IC, Kalin TV, Whitsett JA and Kalinichenko VV. Foxm1 transcription factor is critical for proliferation and differentiation of Clara cells during development of conducting airways. Dev Biol. 2012;370:198–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ustiyan V, Zhang Y, Perl AK, Whitsett JA, Kalin TV and Kalinichenko VV. beta-catenin and Kras/Foxm1 signaling pathway are critical to restrict Sox9 in basal cells during pulmonary branching morphogenesis. Dev Dyn. 2016;245:590–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xia H, Ren X, Bolte CS, Ustiyan V, Zhang Y, Shah TA, Kalin TV, Whitsett JA and Kalinichenko VV. Foxm1 regulates resolution of hyperoxic lung injury in newborns. Am J Respir Cell Mol Biol. 2015;52:611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milewski D, Pradhan A, Wang X, Cai Y, Le T, Turpin B, Kalinichenko VV and Kalin TV. FoxF1 and FoxF2 transcription factors synergistically promote rhabdomyosarcoma carcinogenesis by repressing transcription of p21Cip1 CDK inhibitor. Oncogene. 2017;36:850–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Milewski D, Balli D, Ustiyan V, Le T, Dienemann H, Warth A, Breuhahn K, Whitsett JA, Kalinichenko VV and Kalin TV. FOXM1 activates AGR2 and causes progression of lung adenomas into invasive mucinous adenocarcinomas. PLoS Genet. 2017;13:e1007097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoggatt AM, Kim JR, Ustiyan V, Ren X, Kalin TV, Kalinichenko VV and Herring BP. The transcription factor Foxf1 binds to serum response factor and myocardin to regulate gene transcription in visceral smooth muscle cells. J Biol Chem. 2013;288:28477–28487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun L, Ren X, Wang IC, Pradhan A, Zhang Y, Flood HM, Han B, Whitsett JA, Kalin TV and Kalinichenko VV. The FOXM1 inhibitor RCM-1 suppresses goblet cell metaplasia and prevents IL-13 and STAT6 signaling in allergen-exposed mice. Sci Signal. 2017;10: eaai8583. [DOI] [PubMed] [Google Scholar]
- 37.Ren X, Zhang Y, Snyder J, Cross ER, Shah TA, Kalin TV and Kalinichenko VV. Forkhead box M1 transcription factor is required for macrophage recruitment during liver repair. Mol Cell Biol. 2010;30:5381–5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ren X, Ustiyan V, Guo M, Wang G, Bolte C, Zhang Y, Xu Y, Whitsett JA, Kalin TV and Kalinichenko VV. Postnatal Alveologenesis Depends on FOXF1 Signaling in c-KIT(+) Endothelial Progenitor Cells. Am J Respir Crit Care Med. 2019;200:1164–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pradhan A, Ustiyan V, Zhang Y, Kalin TV and Kalinichenko VV. Forkhead transcription factor FoxF1 interacts with Fanconi anemia protein complexes to promote DNA damage response. Oncotarget. 2016;7:1912–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wen B, Li E, Ustiyan V, Wang G, Guo M, Na CL, Kalin GT, Galvan V, Xu Y, Weaver TE, et al. In Vivo Generation of Lung and Thyroid Tissues from Embryonic Stem Cells Using Blastocyst Complementation. Am J Respir Crit Care Med. 2021;203:471–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Voelkel NF, Mizuno S and Bogaard HJ. The role of hypoxia in pulmonary vascular diseases: a perspective. Am J Physiol. 2013;304:L457–L465. [DOI] [PubMed] [Google Scholar]
- 42.Omura J, Habbout K, Shimauchi T, Wu WH, Breuils-Bonnet S, Tremblay E, Martineau S, Nadeau V, Gagnon K, Mazoyer F, et al. Identification of Long Noncoding RNA H19 as a New Biomarker and Therapeutic Target in Right Ventricular Failure in Pulmonary Arterial Hypertension. Circulation. 2020;142:1464–1484. [DOI] [PubMed] [Google Scholar]
- 43.Naeije R and Chesler N. Pulmonary circulation at exercise. Compr Physiol. 2012;2:711–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dello Russo P, Franzoni A, Baldan F, Puppin C, De Maglio G, Pittini C, Cattarossi L, Pizzolitto S and Damante G. A 16q deletion involving FOXF1 enhancer is associated to pulmonary capillary hemangiomatosis. BMC Med Genet. 2015;16:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paulin R, Meloche J and Bonnet S. STAT3 signaling in pulmonary arterial hypertension. JAKSTAT. 2012;1:223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stenmark KR, Hu CJ and Pullamsetti SS. How Many FOXs Are There on The Road to Pulmonary Hypertension? Am J Respir Crit Care Med. 2018;198:704–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dai Z, Zhu MM, Peng Y, Jin H, Machireddy N, Qian Z, Zhang X and Zhao YY. Endothelial and Smooth Muscle Cell Interaction via FoxM1 Signaling Mediates Vascular Remodeling and Pulmonary Hypertension. Am J Respir Crit Care Med. 2018;198:788–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bourgeois A, Lambert C, Habbout K, Ranchoux B, Paquet-Marceau S, Trinh I, Breuils-Bonnet S, Paradis R, Nadeau V, Paulin R, et al. FOXM1 promotes pulmonary artery smooth muscle cell expansion in pulmonary arterial hypertension. J Mol Med. 2018;96:223–235. [DOI] [PubMed] [Google Scholar]
- 49.Savai R, Al-Tamari HM, Sedding D, Kojonazarov B, Muecke C, Teske R, Capecchi MR, Weissmann N, Grimminger F, Seeger W, et al. Pro-proliferative and inflammatory signaling converge on FoxO1 transcription factor in pulmonary hypertension. Nat Med. 2014;20:1289–1300. [DOI] [PubMed] [Google Scholar]
- 50.Flood HM, Bolte C, Dasgupta N, Sharma A, Zhang Y, Gandhi CR, Kalin TV and Kalinichenko VV. The Forkhead box F1 transcription factor inhibits collagen deposition and accumulation of myofibroblasts during liver fibrosis. Biol Open. 2019;8:bio039800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.