Abstract
Current literature regarding systemic autoimmune diseases in X‐chromosome aneuploidies is scarce and limited to case reports. Our aim was to evaluate the frequency of anti‐nuclear (ANAs), extractable nuclear (ENA), anti‐double‐stranded DNA (dsDNAs), anti‐smooth muscle (ASMAs) and anti‐mitochondrial (AMAs) antibodies in a large cohort of adults with Klinefelter’s syndrome (KS, 47,XXY) and rare higher‐grade sex chromosome aneuploidies (HGAs) for the first time. Sera from 138 X‐chromosome aneuploid patients [124 adult patients with 47,XXY KS and 14 patients with HGA (six children, eight adults)] and 50 age‐matched 46,XY controls were recruited from the Sapienza University of Rome (2007–17) and tested for ANAs, ENAs, anti‐dsDNAs, ASMAs and AMAs. Non‐organ‐specific immunoreactivity was found to be significantly higher in patients with 47,XXY KS (14%) than in the controls (2%, p = 0.002). Among all the antibodies investigated, only ANAs were observed significantly more frequently in patients with 47,XXY KS (12.1%) than in the controls (2%, p = 0.004). No anti‐dsDNA immunoreactivity was found. Stratifying by testosterone replacement therapy (TRT), non‐organ‐specific autoantibody frequencies were higher in TRT‐naive (p = 0.01) and TRT‐treated groups than in controls. No patients with HGA were found positive for the various autoantibodies. Non‐organ‐specific autoantibodies were significantly present in 47,XXY adult patients. Conversely, HGAs did not appear to be target of non‐organ‐specific immunoreactivity, suggesting that KS and HGAs should be considered as two distinct conditions. The classification and diagnosis of systemic autoimmune diseases is frequently difficult. To support a correct clinical evaluation of KS disease and to prevent eventual secondary irreversible immune‐mediated damages, we highlight the importance of screening for non‐organ‐specific autoimmunity in Klinefelter’s syndrome.
Keywords: AMA, ANA, anti‐DNA, ASMA, ENA, Klinefelter’s syndrome, non‐organ‐specific autoimmunity, X‐chromosome aneuploidies
Aim of our study was to evaluate, for the first time, the frequency of anti‐nuclear‐, extractable nuclear‐, anti‐double‐strandedDNA‐, anti‐smooth muscle‐, and anti‐mitochondrial‐antibodies in a large cohort of adults with Klinefelter’s syndrome (KS, 47,XXY) and rare higher‐grade sex chromosome aneuploidies (HGAs). Non‐organ–specific autoantibodies were significantly present in 47,XXY adult patients. Conversely, HGAs did not appear to be target of non‐organ–specific immunoreactivity. We highlight the importance of screening for non‐organ–specific autoimmunity in Klinefelter’s syndrome to support a correct clinical evaluation of KS disease and to prevent eventual secondary irreversible immune‐mediated damages of systemic autoimmune diseases.

INTRODUCTION
Klinefelter’s syndrome (KS) is the most frequent sex chromosomal disorder in men, with the 47,XXY karyotype occurring in approximately 150 in 100 000 men [1]. More rare and more severe tetrasomy and pentasomy have also been described [2], with 48,XXYY, 48,XXXY and 49,XXXXY occurring in 1:18 000–1:40 000 [3], 1:50 000 [4] and 1:85 000–1:100 000 men [5, 6], respectively, whereas the prevalence of 49,XXXYY remains unknown. Due to significant differences between all these variants, no exact definition of X‐chromosome aneuploids in the scientific community exists. A recent paper suggested considering KS and higher‐grade aneuploidies (HGA) of the sexual chromosomes (chromosomes 48 and 49) as two distinct medical conditions and acknowledging them as more severe X‐chromosome aneuploids [7]. A direct relationship between the number of additional sex chromosomes and the severity of the phenotype is generally assumed. All X‐chromosome aneuploidies present with testicular dysgenesis and are associated with hypergonadotropic hypogonadism. Additionally, they have their own distinct features, such as dysmorphic facial features, skeletal deformities, hypotonic musculature, tremors, genital anomalies and neurological and cognitive impairment [4, 7]. It has long been recognized that most autoimmune diseases exhibit considerable sex dimorphism with a higher incidence in women [8]. Two major factors are thought to contribute to this dimorphism: gonadal hormones and direct X chromosome effects [8, 9, 10, 11, 12]. As early as the 1960s and 1970s, several reports have described the concomitant occurrence of X‐chromosome aneuploidies with organ‐specific autoimmune diseases [13, 14, 15, 16]. More recently, we demonstrated a comprehensive pattern of humoral endocrine organ‐specific immunoreactivity in a large cohort of children and adult Caucasians with 47,XXY KS as well as in patients with rare HGA [17, 18]. To date, limited information is available from sparse case reports and retrospective studies on autoimmune diseases associated with systemic autoantigens in patients with 47,XXY KS and HGA [19, 20]. It is extremely difficult to precisely diagnose non‐organ‐specific autoimmune diseases in clinical practice. A correct diagnosis for these diseases relies heavily upon adequate history‐taking and physical examination. Laboratory tests can also be performed to predict the onset or confirm the diagnosis of non‐organ‐specific autoimmune diseases.
ANA detection is usually the first autoantibody test prescribed to patients with suspected systemic autoimmune disorders such as systemic lupus erythematosus (SLE), mixed connective tissue disease (MCTD), Sjögren’s syndrome (SS), progressive systemic sclerosis (PSS), juvenile idiopathic arthritis, dermatomyositis/polymyositis (DM/PM) and autoimmune hepatitis [21, 22, 23, 24].
AMAs are found in more than 90% of primary biliary cirrhosis cases and 3–11% of chronic active hepatitis cases, but not in patients with extrahepatic biliary obstruction and other liver diseases. High AMA titers are the most sensitive and specific primary biliary cirrhosis immune‐serological markers, with a specificity close to 100% [25, 26]. ASMA is found in the majority of chronic active hepatitis and acute viral hepatitis cases, as well as occasionally in primary biliary cirrhosis cases. They are routinely tested for the diagnosis of autoimmune hepatitis type I [27, 28, 29].
Anti‐DNA antibodies are a heterogeneous group of immunoglobulins with different degrees of specificity and avidity. In our study, we analyzed antibodies that were directed against double‐helix DNA (dsDNA). They recognize epitopes located along the deoxyribose–phosphate backbone with a binding site that comprises approximately six nucleotides. These autoantibodies have high avidity and specificity and are widely used in the diagnosis and monitoring of SLE [30, 31, 32]. ENAs represent a large family of non‐organ‐ and non‐species‐specific autoantibodies, the detection of which is of great importance in the laboratory diagnosis of systemic rheumatic autoimmune diseases [33, 34, 35, 36].
The aim of this study was to evaluate the frequency of a spectrum of non‐organ‐specific autoantibodies, such as anti‐nuclear antibodies (ANAs), anti‐mitochondrial antibodies (AMAs), anti‐smooth muscle antibodies (ASMAs), anti‐double‐stranded DNA (dsDNA) and extractable nuclear antigens (ENAs), in a large cohort of adult patients with 47,XXY KS and patients with HGA.
SUBJECTS AND METHODS
Patients
A total of 138 Caucasian patients with X‐chromosome aneuploidies were recruited from the Center of Rare Diseases, Department of Experimental Endocrinology, La Sapienza University of Rome, from 2007 to 2017 (Table 1). The diagnosis was based on clinical features and high serum follicle‐stimulating hormone and luteinizing hormone and low total testosterone concentrations. The diagnosis was confirmed by karyotype analysis from cultured peripheral blood lymphocytes. Serum follicle‐stimulating hormone, luteinizing hormone, total testosterone, estradiol and sex hormone‐binding globulin concentrations were measured using chemiluminescent microparticle immunoassay technology (Architect System; Abbott Diagnostics Division, Chicago, Illinois, USA). The mean serum follicle‐stimulating hormone, luteinizing hormone, total testosterone, estradiol and sex hormone‐binding globulin concentrations are reported in Table 1. Overall, 124 patients had 47,XXY KS and were aged more than 18 years (median = 35 ± 11 years; range = 18–63 years), where 14 patients had the HGA karyotype (median = 19 ± 10 years; range = 5–34 years).
TABLE 1.
Characteristics of adult 47,XXY KS patients and HGA patients
| 47,XXY KS patients | HGA patients | |
|---|---|---|
| n = 124 | n = 14 | |
| Age (years) ± SD range | 35 ± 11 (18–63) | 19 ± 10 (5–34) |
| FSH (mIU/ml) ± SD | 23.5 ± 15 | 31 ± 17 |
| LH (mIU/ml) ± SD | 12.6 ± 8 | 16.9 ± 10 |
| T (nmol/l) ± SD | 10.5 ± 8 | 11.1 ± 8 |
| E2 (pg/ml) ± SD | 31.9 ± 15 | 27.4 ± 14 |
| SHBG (nmol/l) ± SD | 42.2 ± 25 | 59.1 ± 54 |
Abbreviations: E2, estradiol; FSH, follicle‐stimulating hormone; HGA, higher‐grade aneuploid patients; KS, Klinefelter’s syndrome; LH, luteinizing hormone; SD, standard deviation; SHBG, sex hormone binding globulin; T, total testosterone.
Adult patients with 47,XXY KS were further divided according to testosterone replacement therapy (TRT) into a TRT‐naive group that consisted of 70 patients with KS who had never undergone TRT (median age = 34.5 ± 10.9 years; range = 18–63 years) and a TRT treatment group that consisted of 54 patients with KS who underwent TRT (median age = 34.7 ± 11.3 years; range = 19–61 years) (Table 2).
TABLE 2.
Characteristics of TRT‐naive and TRT‐treated adult KS patients and age‐matched 46,XY control group
| TRT‐naive | TRT‐treated | Control group | |
|---|---|---|---|
| KS patients ≥ 18 years | n = 70 | n = 54 | n = 50 |
| Age (years) ± SD range | 35 ± 11 (18–63) | 35 ± 11 (19–61) | 43 ± 12 (21–64) |
| FSH (mIU/ml) ± SD | 27.9 ± 13.2 | 17.5 ± 14.4 | 4.3 ± 1.8 |
| LH (mIU/ml) ± SD | 14.5 ± 6.1 | 10.5 ± 8.7 | 3.2 ± 1.3 |
| T (nmol/l) ± SD | 10.5 ± 8.4 | 10.4 ± 8.4 | 5.9 ± 1.6 |
| E2 (pg/ml) ± SD | 33.0 ± 13.4 | 30.7 ± 16.7 | 29.3 ± 12.6 |
| SHBG (nmol/l) ± SD | 45.4 ± 24.8 | 37.8 ± 23.3 | 34.2 ± 14.1 |
Abbreviations: E2, estradiol; FSH, follicle‐stimulating hormone; LH, luteinizing hormone; SD, standard deviation; SHBG, sex hormone‐binding globulin; T, total testosterone; TRT, testosterone replacement therapy.
The 14 patients with HGA (48,XXXY, 48,XXYY, 49,XXXXY) included five patients who were aged less than 18 years (median = 9 ± 5 years; range = 5–17 years) and nine patients who were aged 18 or more years (median age = 25 ± 6 years; range = 18–34 years).
46,XY controls
Data from the aneuploid patients were compared with those from 50 serum samples collected from 46,XY men [median age = 43 years ± standard deviation (SD) = 12; range = 21–64 years] (Table 2).
All adult controls had a history of spontaneous puberty with gonadotropin levels and physical parameters within the normal range (Table 2) and no clinical evidence of autoimmune diseases.
Autoantibody detection
All subjects were investigated for the presence of autoantibodies specific to non‐organ‐specific autoimmune diseases. In detail, we evaluated ANA, AMA, ASMA, dsDNA and ENA. ANAs were tested by indirect immunofluorescence test (IF) on HEp‐2 cells (ZENIT RA ANA ref. 37805; A. Menarini Diagnostics Ltd, Florence, Italy). AMAs and ASMAs were detected using an indirect immunofluorescence on the stomach/liver (ZENIT RA ref. 37787; A. Menarini Diagnostics Ltd). Screening for anti‐dsDNA was performed by chemiluminescent immunoassay (ZENIT RA dsDNA IgG ref. 41413; A. Menarini Diagnostics Ltd). ENA were measured using chemiluminescent immunoassay (ZENIT RA ENA screen, ref. 41412; A. Menarini Diagnostics Ltd) for qualitative determination of the specific immunoglobulin (Ig)G antibodies directed against SS‐A/Ro (60 and 52 kDa), SS‐B/La, Sm, U1‐snRNP (70 kDa, A and C), Scl‐70 and Jo‐1 antigens. Anti‐thyroid peroxidase (anti‐TPO) antibodies were measured using Architect® chemiluminescent microparticle immunoassay (ARCHITECT® Anti‐TPO, ref. 2K47 Architect System; Abbott Diagnostics Division).
Statistical analysis
SAS version 2 software was used for statistical analysis. Data were expressed as frequencies or mean ± standard deviation or median values. Differences in frequency were calculated using the Fisher’s exact test. A p‐value of ˂ 0.05 was considered significant.
RESULTS
Non‐organ‐specific humoral autoimmunity in adult patients with 47,XXY KS and patients with HGA
Overall, immunoreactivity was found in 12.8% (17 of 133; 95% CI = 7.1–18.5) of adult X‐chromosome aneuploid patients (KS and HGA). Seventeen adult patients with 47,XXY KS were positive for at least one of the non‐organ‐specific autoantibodies investigated (Table 3) with a frequency (17 of 124, 13.7%; 95% CI = 7.7–19.8) significantly higher than in controls (one of 50, 2.0%; 95% CI = −1.9 to 5.9) (p = 0.02). The most representative immunoreactivity in patients with 47,XXY KS was against ANA (12.1%, 15 of 124; 95% CI = 6.4–17.8) with a frequency significantly higher than that in the control group (one of 50, 2.0%; 95% CI = 1.2–6.2) (p = 0.04). Lower frequencies were detected for ENA (1.6%, two of 124; 95% CI = −0.6 to 3.8), AMA (0.8%, one of 124; 95% CI = −0.8 to 2.4) and ASMA (0.8%, one of 124; 95% CI = −0.8 to 2.4), whereas no samples were positive for anti‐dsDNA antibodies. Overall, only two patients with KS were positive for both ANA and ENA, whereas the remaining patients were positive for just one of the autoantibodies investigated. None of the patients with HGA were positive for the non‐organ‐specific autoantibodies investigated. The clinical and autoimmune characteristics of autoantibody‐positive patients are shown in Table 4.
TABLE 3.
Frequency of non‐organ‐specific autoantibodies in adult 47,XXY KS patients and age‐matched control group
| Autoantibodies | 47,XXY KS patients, n = 124 | Control group, n = 50 | p |
|---|---|---|---|
| ≥ 1 antibody | 17 (13.7%) | 1 | 0.02 |
| ANA | 15 (12.1%) | 1 | 0.04 |
| AMA | 1 (0.8%) | 0 | NS |
| ASMA | 1 (0.8%) | 0 | NS |
| dsDNA | 0 | 0 | NS |
| ENA | 1 (0.8%) | 0 | NS |
Abbreviations: AMA, anti‐mitochondrial antibodies; ANA, anti‐nuclear antibodies; ASMA, anti‐smooth muscle antibodies; dsDNA, anti‐double‐stranded DNA; ENA, anti‐extractable nuclear antigens; NS, not significant; TRT, testosterone replacement therapy.
TABLE 4.
Immunoreactivity patterns of autoantibody‐positive X‐chromosome aneuploid patients
| Patient number | Age (years) | Karyotype | TRT | Duration therapy (years) | ANA nuclear pattern (dilution) | AMA | ASMA | dsDNA | ENA | Autoimmune disease development |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 18 | 47,XXY | – | 0 | – | + (1:40) | – | – | – | – |
| 2 | 19 | 47,XXY | – | 0 | + homogeneous (1:160) | – | – | – | – | – |
| 3 | 20 | 47,XXY | – | 0 | – | – | + (1:40) | – | – | – |
| 4 | 23 | 47,XXY | – | 0 | + homogeneous (1:80) | – | – | – | – | – |
| 5 | 30 | 47,XXY | + | 1 | + homogeneous (1:80) | – | – | – | – | – |
| 6 | 31 | 47,XXY | + | 14 | + homogeneous (1:160) | – | – | – | – | – |
| 7 | 33 | 47,XXY | – | 0 | + homogeneous (1:160) | – | – | – | – | – |
| 8 | 37 | 47,XXY | – | 0 | + speckled (1:160) | – | – | – | + (1:40) anti–RNP | – |
| 9 | 37 | 47,XXY | – | 0 | + speckled (1:160) | – | – | – | + (1:40) SS–A/Ro | – |
| 10 | 37 | 47,XXY | – | 0 | + homogeneous (1:80) | – | – | – | – | – |
| 11 | 38 | 47,XXY | – | 0 | + homogeneous (1:640) | – | – | – | – | – |
| 12 | 40 | 47,XXY | + | 2 | + homogeneous (1:80) | – | – | – | – | – |
| 13 | 40 | 47,XXY | + | 1 | + homogeneous (1:80) | – | – | – | – | – |
| 14 | 40 | 47,XXY | – | 0 | + homogeneous (1:160) | – | – | – | – | – |
| 15 | 44 | 47,XXY | + | 1 | + homogeneous (1:80) | – | – | – | – | – |
| 16 | 44 | 47,XXY | + | 2 | + centromere (1:1280) | – | – | – | – | – |
| 17 | 45 | 47,XXY | – | 0 | + homogeneous (1:160) | – | – | – | – | – |
Abbreviations: AMA, anti‐mitochondrial antibodies; ANA, antinuclear antibodies; ASMA, anti‐smooth muscle antibodies; dsDNA, anti‐double strained DNA; ENA, anti‐extractable nuclear antigens; TRT, testosterone replacement therapy.
Humoral autoimmunity in adult patients with 47,XXY KS subdivided by TRT
Autoantibody frequencies in the TRT‐naive group, TRT treatment (mean therapy duration = 10 years; range = 1–33 years) group and controls are reported in Figure 1. Serum samples from 11 (15.7%; 95% CI = 7.2–24.2) of the 70 TRT‐naive patients with KS and six of the 54 TRT‐treated patients (11.1%; 95% CI, = 2.7–19.5) were positive for at least one of the non‐organ‐specific autoantibodies investigated. The immunoreactivities of these two groups were comparable and both were higher than those in the controls (2%, one of 50; 95% CI = 1.2–6.2), although this difference was only statistically significant (p = 0.01) between TRT‐naive patients and controls (Figure 1). Nine TRT‐naive (nine of 70, 12.8%; 95% CI = 5.0–20.7) and six TRT‐treated patients (six of 54, 11.1%; 95% CI= 2.7–19.5) were positive for ANA. The ANA frequency in TRT‐naive patients was significantly higher than that in the controls (p = 0.04). In the TRT‐naive group, lower frequencies were detected for ENA (2.9%, two of 70; 95% CI = −1.0 to 6.8), AMA (1.4%, one of 70; 95% CI = 1.4–4.2) and ASMA (1.4%, one of 70; 95% CI = −1.4 to 4.2), and no samples were positive for anti‐dsDNA antibodies. Meanwhile, the TRT treatment patient group was negative for ENA, AMA, ASMA and anti‐dsDNA antibodies.
FIGURE 1.
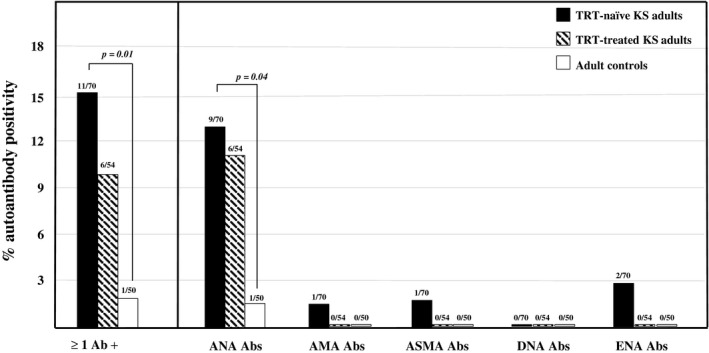
Frequency of non‐organ‐specific autoantibodies in TRT‐naive and TRT‐treated adult patients with 47,XXY Klinefelter’s syndrome and age‐matched controls. TRT, testosterone replacement therapy; ANA, anti‐nuclear antibodies; AMA, anti‐mitochondrial antibodies; ASMA, anti‐smooth muscle antibodies; dsDNA, anti‐double‐stranded DNA; ENA, anti‐extractable nuclear antigens
Autoantibody‐positive patients
The clinical and autoimmune profiles of the autoantibody‐positive patients (17 of 124, 13.7%) are shown in Table 3. Eleven of these patients had no history of TRT, whereas six were undergoing TRT at the time of blood sample collection (mean therapy duration = 6 years; range = 1–14 years). Almost 90% (15 of 17) of autoantibody‐positive patients screened were positive for ANA (Table 4). Four of the ANA‐positive X‐chromosome aneuploid patients were immunoreactive to anti‐thyroglobulin antibodies. Serum samples from 15 immunoreactive patients showed immunoreactivity against only one non‐organ‐specific autoantibody: 13 were positive for ANA, one for AMA and one for ASMA. Only two samples were positive for both ANA and ENA. Of these two patients, one was found positive for anti‐RNP antibodies, whereas the other was immunoreactive for SS‐A/Ro antibodies. None of the investigated samples were found positive for anti‐dsDNA antibodies.
DISCUSSION
In the present study, we evaluated the frequency of ANA, ENA, anti‐ dsDNA, ASMA and AMA in a large cohort of adult patients with 47,XXY KS and patients with HGA for the first time. Apart from sparse case reports and retrospective studies, limited information exists on these specific forms of non‐organ‐specific autoimmunity in X‐chromosome aneuploidies in the current literature [13, 14, 15, 16]. Data regarding non‐organ‐specific autoimmunity, in addition to those obtained in our previous studies [17, 18] on the frequency of organ‐specific autoimmunity in the same cohort of adult patients with 47,XXY KS and patients with HGA, help in describing and more clearly understanding the multiple pathways of humoral autoimmunity that occur in these patients.
Overall, 14% of the adult patients with 47,XXY KS analyzed were positive for at least one of the non‐organ‐specific autoantibodies investigated. This frequency was significantly higher than that in the age‐matched control group and was comparable to those previously published for endocrine organ‐specific humoral autoimmunity by our research group [17, 18]. The main immunoreactivity detected in patients with 47,XXY KS was directed against anti‐nuclear antigens. The disease that is most closely linked to ANA positivity is SLE. Previous case reports have documented the co‐existence of KS and SLE [20]; however, only a few studies investigated the prevalence of KS in a large population of patients with SLE [37, 38]. Scofield et al. demonstrated that the frequency of KS is increased 14‐fold in men with SLE compared with men without SLE [38]. In the same paper, they estimated that there was only one patient with SLE among every 960 men with KS. Similarly, KS was found in excess among men with SS [39]. Harris et al. demonstrated that the frequency of SS is increased 38‐fold in men with KS compared with men without SLE. Detection of traditional serum autoantibodies for disease classification plays a central role even in the diagnosis and classification of SS [40]. In our study, ANAs were found in 12.1% of patients with KS. However, these autoantibodies are known to be highly frequent in the general population, in both female and male individuals. For this reason, as well as to avoid a possible relevant bias in the study, we also analyzed other non‐organ‐specific autoantibodies in the same cohort of adult controls. It has been well documented that autoimmune diseases may occur years after the presence of their associated autoantibodies. Thus, detection of these autoantibodies may be used to predict the development of these autoimmune diseases and/or their rate of progression in healthy subjects [41]. Nevertheless, ANA positivity alone in healthy individuals cannot be regarded as a real predictor of developing connective tissue disease. A different consideration must be performed for patients with KS because, as recently underlined by Li et al., the predicting value of ANA positivity increases with the presence of either one or more X‐chromosomes or with organ‐specific autoimmunity [41]. Our results demonstrate that the frequency of ANA positivity in adult patients with 47,XXY KS was significantly higher than that in the controls, thus differentiating ‘benign’ ANA positivity in healthy individuals from that which puts patients with KS at high risk of autoimmune diseases. To our knowledge, the present study is the first to analyze the frequency of the main risk serological biomarker of SLE and systemic autoimmune rheumatic diseases development in a large cohort of patients with KS. To measure ANA, we used the widely used indirect immunofluorescence assay on HEp2 cells (HEp2‐IFA), which has long been viewed as the gold standard [42, 43]. Interestingly, large‐scale controlled studies have demonstrated an increased incidence of Hashimoto’s thyroiditis and Graves’ disease in patients with SLE compared with that in the general population [44]. In our previous reports, all KS and HGA sera were investigated for TPO antibodies [17, 18]. None of the ANA‐positive patients were positive for these autoantibodies. We extended this evaluation to the anti‐thyroglobulin antibodies and thyroid hormones. Four of the ANA‐positive X‐chromosome aneuploid patients were immunoreactive for this autoantigen (data not shown) and none had hypothyroidism.
Lower frequencies were found in adult patients with 47,XXY KS for AMA, ASMA and EN7A. By investigating the role of anti‐dsDNA autoantibodies in the progression of SLE, Arbuckle et al. demonstrated that these autoantibodies are typically produced alongside the characteristic clinical exacerbation of the disease [45]. As Dillon et al. reported that SLE among X‐chromosome aneuploid patients is not as severe as that in patients with 46,XY SLE [37], we decided to screen all X‐chromosome aneuploid patients regardless of clinical examination to establish the real frequency of anti‐dsDNA autoantibodies. No immunoreactivity was detected against dsDNA. Interestingly, the majority of adult patients with 47,XXY KS showed immunoreactivity against just one non‐organ‐specific autoantibody, whereas only two patients were positive for two of the autoantibodies investigated. Both these patients were positive for ANA and ENA. After stratifying by therapy, the frequency of positivity for at least one of the non‐organ‐specific autoantibodies investigated in adult TRT‐naive patients with 47,XXY KS was higher than that in TRT‐treated patients (11.1%). This interesting difference, which was not statistically significant, was probably due to the limited number of patients investigated. This needs to be further investigated, as it may be related, at least in part, to the immunosuppressive effects of TRT on humoral immunoreactivity, as also hypothesized by Kocar et al. and Olsen et al. [19, 46]. The TRT treatment group started therapy before being enrolled, thus making the comparison of serum autoantibody titers before and after beginning TRT impossible. Interestingly, only one of 124 patients with KS was immunoreactive for both organ‐ and non‐organ‐specific autoantibodies, whereas in the remaining patients no overlapping of the immunoreactivities was detected. This may suggest that the two pathways of autoimmunity occur independently in patients with KS. Of note, none of patients with HGA were positive for the systemic autoantibodies investigated. In our previous study, we found that almost 20% of patients with HGA (a total of 16 patients: eight children, eight adults) were positive for organ‐specific autoimmunity [18]. Despite the small number of patients investigated, due essentially to the extremely low prevalence of these patients, our novel result represents an important difference in respect to organ‐specific autoimmunity that deserves to be further investigated. We recommend that future studies aim to evaluate these preliminary interesting results in an increased number of patients with HGA. These results suggest a potentially different pattern of autoimmunity in the rarest higher‐grade X‐chromosome aneuploidy patients in comparison to the 47,XXY karyotype, as also hypothesized by Spaziani et al. [7]. In this regard, KS and HGAs should be considered as two distinct conditions.
To date, the 17 autoantibody‐positive patients did not show clinical or biochemical signs of the related systemic autoimmune diseases. A possible aim of our future studies includes the follow‐up of all the autoantibody‐positive patients to evaluate the potential development of the related autoimmune diseases.
In conclusion, non‐organ‐specific humoral autoimmunity is significantly present in adult patients with 47,XXY, but not in patients with HGA. Because it is always clinically difficult to precisely diagnose a systemic autoimmune disease [22, 23, 24, 31, 33, 35] and some autoimmune diseases, such as SLE, are more severe in these patients than in 46,XY men [37], the additional information from the presence of non‐organ‐specific autoantibodies is important, as it may strongly support the analysis of the clinical presentation and physical examination of each patient. As reported in the literature, the occurrence of these autoantibodies precedes the possible development and progression of most autoimmune disorders [47, 48], which suggests the importance of screening non‐organ‐specific autoimmunity in X‐chromosome aneuploidies in clinical practice to identify patients at risk of developing non‐organ‐specific autoimmune diseases. It also shows the importance in monitoring the pre‐clinical and clinical phases of the disease to prevent morbidity that is related to unrecognized or undiagnosed disease. Therapeutic approaches may involve both pharmacological and non‐pharmacological methods to normalize the ratios of gonadal sex hormones that modulate the progression of autoimmune responses to quickly prevent irreversible immunomediated damage [49, 50, 51, 52, 53].
Furthermore, considering the results of our present and previous studies [17, 18], almost 25% of adult patients with 47,XXY KS are observed as targets of autoimmunity against a particular organ or systemic autoantigen. Two major triggers may contribute to the development of organ and non‐organ‐specific autoimmunity in KS and patients with HGA: the long‐term consequences of hypergonadotropic hypogonadism and the direct X‐chromosome dosage. Sex hormones seem to play an important role as modulators of the onset and/or perpetuation of autoimmune disease with estrogens as enhancers of autoimmunity and androgens and progesterone as immune suppressors [8, 9, 11, 54, 55, 56, 57]. In KS, hypogonadism is usually not evident during puberty, but as the patients progress to adulthood hypogonadism tends to become more prevalent [58]. Serum total T levels drop into the low–normal range and an increase in estrogen levels may similarly contribute to the development of autoimmunity in women. Other studies have underlined the role of genes on the X chromosome in triggering and maintaining the autoimmune process [12, 59, 60, 61, 62, 63]. Previous studies have focused upon the parent‐of‐origin effects, DNA demethylation of genes on the silenced X chromosome and differential expression of the versatile epigenetic regulator O‐linked N‐acetylglucosamine transferase from the X chromosome [12]. As described by Sawalha et al., the risk of development of SLE is related to a gene–dose effect mediated by abnormal inactivation of genes on the X chromosome, such as CD40L, or by genetic polymorphism, as has been demonstrated for Xq28 [61]. Finally, Baldassarri et al. recently suggested a role for polyQ polymorphism of the androgen receptor (Xq12) as being involved in testosterone immune‐modulation [64]. Furthermore, we suggested in our previous study that the risk of organ‐specific autoimmunity progressively increases with the severity of X‐chromosome polisomy [18]. In contrast, our present results describe a decrease of non‐organ‐specific autoimmunity in HGA relative to patients with KS. It is also important to underline that several other intrinsic and extrinsic etiological factors, such as genetic predisposition and environmental factors, may contribute to autoimmune disease progression and pathogenesis. Further studies should be undertaken to more clearly assess the multiple mechanisms that lead to the development of both pathways of autoimmunity in X‐chromosome aneuploidies.
INSTITUTION ETHICS APPROVAL AND PATIENT CONSENT
All subjects were followed under a Region Centre of Rare Diseases’ protocol approved as an integrated care pathway by both Policlinico Umberto I and the Lazio Region. Written informed consent was obtained from all the participants.
CONFLICT OF INTERESTS
No potential conflicts of interest relevant to this article were reported.
AUTHOR CONTRIBUTIONS
Francesca Panimolle contributed to data collection, statistical analysis and data interpretation and wrote the manuscript; Claudio Tiberti contributed to data interpretation and revised the manuscript; Matteo Spaziani, Antonella Anzuini, Gloria Riitano and Giuseppe Lucania contributed to data collection; Andrea Lenzi and Daniele Gianfrilli revised the manuscript. Antonio F. Radicioni is the guarantor of this work and, as such, had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Francesca Panimolle assures that for the manuscript the following was fulfilled: (1) this material is the authors’ own original work, which has not been previously published elsewhere; (2) the paper is not currently being considered for publication elsewhere; (3) the paper reflects the authors’ own research and analysis in a truthful and complete manner; (4) the paper properly credits the meaningful contributions of co‐authors and co‐researchers; (5) all authors have been personally and actively involved in substantial work leading to the paper and will take public responsibility for its content.
ACKNOWLEDGEMENT
This work was partially funded by the MIUR research project number 2017N8CK4K.
Panimolle F, Tiberti C, Spaziani M, Riitano G, Lucania G, Anzuini A, et al. Non‐organ‐specific autoimmunity in adult 47,XXY Klinefelter patients and higher‐grade X‐chromosome aneuploidies. Clin Exp Immunol. 2021;205:316–325. 10.1111/cei.13616
DATA AVAILABILITY STATEMENT
The data for this study are available from the corresponding author.
REFERENCES
- 1.Lanfranco F, Kamischke A, Zitzmann M, Nieschlag E. Klinefelter’s syndrome. Lancet. 2004;364:273–83. [DOI] [PubMed] [Google Scholar]
- 2.Linden MG, Bender BG, Robinson A. Sex chromosome tetrasomy and pentasomy. Pediatrics 1995;96:672–82. [PubMed] [Google Scholar]
- 3.Visootsak J, Graham JM. Klinefelter syndrome and other sex chromosomal aneuploidies. Orphanet J Rare Dis. 2006;1:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Visootsak J, Graham JM Jr. Social function in multiple X and Y chromosome disorders: XXY, XYY, XXYY, XXXY. Dev Disabil Res Rev. 2009;15:328–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Visootsak J, Aylstock M, Graham JM. Klinefelter syndrome and its variants: an update and review for the primary pediatrician. Clin Pediatr. 2001;40:639–51. [DOI] [PubMed] [Google Scholar]
- 6.Tartaglia N, Ayari N, Howell S, D’Epagnier C, Zeitler P. 48, XXYY, 48, XXXY and 49, XXXXY syndromes: not just variants of Klinefelter syndrome. Acta Paediatr. 2011;100:851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spaziani M, Mileno B, Rossi FM, Granato S, Tahani N, Anzuini A, et al. Endocrine and metabolic evaluation of classic Klinefelter syndrome and high grade aneuploidies of sexual chromosome with male phenotype: are they different clinical conditions? Eur J Endocrinol. 2018;178:343–52. [DOI] [PubMed] [Google Scholar]
- 8.Rubtsova K, Marrack P, Rubtsov AV. Sexual dimorphism in autoimmunity. J Clin Invest. 2015;125:2187–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Markle JG, Fish EN. SeXX matters in immunity. Trends Immunol. 2014;35:97–104. [DOI] [PubMed] [Google Scholar]
- 10.Invernizzi P, Pasini S, Selmi C, Miozzo M, Podda M. Skewing of X chromosome inactivation in autoimmunity. Autoimmunity 2008;41:272–7. [DOI] [PubMed] [Google Scholar]
- 11.Kivity S, Ehrenfeld M. Can we explain the higher prevalence of autoimmune disease in women? Exp Rev Clin Immunol. 2010;6:691–4. [DOI] [PubMed] [Google Scholar]
- 12.Abramowitz LA, Olivier‐Van Stichelen S, Hanover JA. Chromosome imbalance as a driver of sex disparity in disease. J Genomics. 2014;2:77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oktenli C, Yesilova Z, Kocar IH, Musabak U, Ozata M, Inal A, et al. Study of autoimmunity in Klinefelter’s and idiopathic hypogonadotropic hypogonadism. J Clin Immunol. 2002;22:137–43. [DOI] [PubMed] [Google Scholar]
- 14.Seminog OO, Seminog AB, Yeates D, Goldacre MJ. Associations between Klinefelter's syndrome and autoimmune diseases: English national record linkage studies. Autoimmunity 2015;48:125–8. [DOI] [PubMed] [Google Scholar]
- 15.Cai XP, Zhao L, Mao M. A case of Klinefelter’s syndrome with type 1 diabetes mellitus. Chin Med J. 2012;125:937–40. [PubMed] [Google Scholar]
- 16.French MAH, Hughes P. Systemic lupus erythematosus and Klinefelter’s syndrome. Ann Rheum Dis. 1983;42:471–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panimolle F, Tiberti C, Granato S, Semeraro A, Gianfrilli D, Anzuini A, et al. Screening of endocrine organ‐specific humoral autoimmunity in 47,XXY Klinefelter’s syndrome reveals a significant increase in diabetes‐specific immunoreactivity in comparison with healthy control men. Endocrine 2016;52:157–64. [DOI] [PubMed] [Google Scholar]
- 18.Panimolle F, Tiberti C, Granato S, Anzuini A, Pozza C, Lenzi A, et al. Evidence of increased humoral endocrine organ‐specific autoimmunity in severe and classic X‐chromosome aneuploidies in comparison with 46, XY control subjects. Autoimmunity 2018;28:1–8. [DOI] [PubMed] [Google Scholar]
- 19.Olsen NJ, Kovacs WJ. Case report: Testosterone treatment of systemic lupus erythematosus in a patient with Klinefelter’s syndrome. Am J Med Sci. 1995;310:158–60. [DOI] [PubMed] [Google Scholar]
- 20.Lv J, Feng Y, Qian Y, Chen JJ. Klinefelter’s syndrome with systemic lupus erythematosus and atrial fibrillation. Lupus 2019;28:1477–9. [DOI] [PubMed] [Google Scholar]
- 21.Dinse GE, Parks CG, Weinberg CR, Meier H, Co CA, Chan E, et al. Antinuclear antibodies and mortality in the National Health and Nutrition Examination Survey (1999–2004). PLOS ONE. 2017;12:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos H, Alexander E, Carsons S, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American–European Consensus Group. Ann Rheum Dis. 2002;61:554–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller FW, Rider LG, Plotz PH, Isenberg DA, Odds CV. Diagnostic criteria for polymyositis and dermatomyositis. Lancet. 2003;362:1763. [DOI] [PubMed] [Google Scholar]
- 24.Walker JG, Pope J, Bron M, LeClercq S, Hudson M, Taillefer S, et al. The development of systemic sclerosis classification criteria. Clin Rheumatol. 2007;26:1401–9. [DOI] [PubMed] [Google Scholar]
- 25.Hirschfield GM, Heathcote EJ. Antimitochondrial antibody‐negative primary biliary cirrhosis. Clin Liv Dis. 2008;12:323–31. [DOI] [PubMed] [Google Scholar]
- 26.Liu B, Shi XH, Zhang FC, Zhang W, Gao LX. Antimitochondrial antibody‐negative primary biliary cirrhosis: a subset of primary biliary cirrhosis. Liver Int. 2008;28:233–9. [DOI] [PubMed] [Google Scholar]
- 27.Whittingham S, Irwin J, Mackay IR, Smalley M. Smooth muscle autoantibody in ‘autoimmune’ hepatitis. Gastroenterology 1966;51:499–505. [PubMed] [Google Scholar]
- 28.Muratori L, Deleonardi G, Lalanne C, Barbato E, Tovoli A, Libra A, et al. Autoantibodies in autoimmune hepatitis. Dig Dis. 2015;33:65–9. [DOI] [PubMed] [Google Scholar]
- 29.Manns MP, Czaja AJ, Gorham JD, Krawitt EL, Mieli‐Vergani G, Vergani D, et al. Diagnosis and management of autoimmune hepatitis. Hepatology 2010;51:2193–213. [DOI] [PubMed] [Google Scholar]
- 30.Sherer Y, Shoenfeld Y. The idiotypic network in antinuclear‐antibody‐associated diseases. Int Arch Allergy Immunol. 2000;123:10–5. [DOI] [PubMed] [Google Scholar]
- 31.Hochberg M. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 32.Smeenk RJT. Detection of antibodies to dsDNA: current insights into its relevance. Clin Exp Rheumatol. 2002;20:294–300. [PubMed] [Google Scholar]
- 33.Litwin CM, Rourk AR. Anti‐ENA antibody profiles in patients with hepatitis C virus infection. J Clin Lab Anal. 2018;32:e22279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emad Y, Gheita T, Darweesh H, Klooster P, Gamal R, Fathi H, et al. Antibodies to extractable nuclear antigens (ENAS) in systemic lupus erythematosus patients: correlations with clinical manifestations and disease activity. Reumatismo. 2018;70:85–91. [DOI] [PubMed] [Google Scholar]
- 35.Petri M. Review of classification criteria for systemic lupus erythematosus. Rheum Dis Clin North Am. 2005;31:245–55. [DOI] [PubMed] [Google Scholar]
- 36.Alsubki R, Tabassum H, Alfawaz H, Alaqil R, Aljaser F, Ansar S, et al. Association between antinuclear antibodies (ANA) patterns and extractable nuclear antigens (ENA) in HEp‐2 cells in patients with autoimmune diseases in Riyadh, Saudi Arabia. Intract Rare Dis Res. 2020;9:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dillon S, Aggarwal R, Harding JW, Li L‐J, Weissman MH, Li S, et al. Klinefelter's syndrome (47, XXY) among men with systemic lupus erythematosus. Acta Paediatr. 2011;100:819–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scofield RH, Bruner GR, Namjou B, Kimberly RP, Ramsey‐Goldman R, Petri M, et al. Klinefelter’s syndrome (47, XXY) in male systemic lupus erythematosus patients: support for the notion of a gene–dose effect from the X chromosome. Arthritis Rheum. 2008;58:2511–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harris VM, Sharma R, Cavett J, Kurien BT, Liu K, Koelsch KA, et al. Corrigendum to ‘Klinefelter’s syndrome (47, XXY) is in excess among men with Sjögren’s syndrome’. Clin Immunol. 2018;187:137–8. [DOI] [PubMed] [Google Scholar]
- 40.Shen L, Suresh L. Autoantibodies, detection methods and panels for diagnosis of Sjogren’s syndrome. Clin Immunol. 2017;182:24–9. [DOI] [PubMed] [Google Scholar]
- 41.Li QZ, Karp DR, Quan J, Branch VK, Zhou J, Lian Y, et al. Risk factors for ANA positivity in healthy persons. Arthritis Res Ther. 2011;13:R38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pisetsky DS, Bossuyt X, Meroni PL. ANA as an entry criterion for the classification of SLE. Autoimmun Rev. 2019;18:102400. [DOI] [PubMed] [Google Scholar]
- 43.Olsen NJ, Choi MY, Fritzler MJ. Emerging technologies in autoantibody testing for rheumatic diseases. Arthritis Res Ther. 2017;19:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Biró E, Szekanecz Z, Czirják L, Dankó K, Kiss E, Szabó NA, et al. Association of systemic and thyroid autoimmune diseases. Clin Rheumatol. 2006;25:240–5. [DOI] [PubMed] [Google Scholar]
- 45.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–33. [DOI] [PubMed] [Google Scholar]
- 46.Koçar IH, Yesilova Z, Özata M, Turan M, Sengül A, Özdemir IÇ, et al. The effect of testosterone replacement treatment on immunological features of patients with Klinefelter’s syndrome. Clin Exp Immunol. 2000;121:448–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma WT, Chang C, Gershwin ME, Lian ZX. Development of autoantibodies precedes clinical manifestations of autoimmune diseases: A comprehensive review. J Autoimmun. 2017;83:95–112. [DOI] [PubMed] [Google Scholar]
- 48.Suurmond J, Diamond B. Autoantibodies in systemic autoimmune diseases: specificity and pathogenicity. J Clin Invest. 2015;125:2194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verthelyi D. Sex hormones as immunomodulators in health and disease. Int Immunopharmacol. 2001;1:983–93. [DOI] [PubMed] [Google Scholar]
- 50.Vidaver R. Molecular and clinical evidence of the role of estrogen in lupus. Trends Immunol. 2002;23:229–30. [DOI] [PubMed] [Google Scholar]
- 51.Van Vollenhoven RF. Dehydroepiandrosterone for the treatment of systemic lupus erythematosus. Expert Opin Pharmacother. 2002;3:23–31. [DOI] [PubMed] [Google Scholar]
- 52.Van Vollenhoven RF, Morabito LM, Engleman EG, McGuire JL. Treatment of SLE with DHEA: 50 patients treated up to 12 months. J Rheumatol. 1998;25:285–9. [PubMed] [Google Scholar]
- 53.Olsen NJ, Kovacks WJ. Case report: Testosterone treatment of SLE in a patient with Klinefelter’s syndrome. Am J Med Sci. 1995;310:158–60. [DOI] [PubMed] [Google Scholar]
- 54.Ackerman LS. Sex hormones and the genesis of autoimmunity. Arch Dermatol. 2006;142:371–6. [DOI] [PubMed] [Google Scholar]
- 55.Cutolo M, Sulli A, Capellino S, Villaggio B, Montagna P, Seriolo B, et al. Sex hormones influence on the immune system: basic and clinical aspects in autoimmunity. Lupus 2004;13:653–8. [DOI] [PubMed] [Google Scholar]
- 56.Olsen NJ, Kovacs WJ. Gonadal steroids and immunity. Endocr Rev. 1996;17:369–84. [DOI] [PubMed] [Google Scholar]
- 57.Crescioli C, Sottili M, Bonini P, Cosmi L, Chiarugi P, Romagnani P, et al. Inflammatory response in human skeletal muscle cells: CXCL10 as a potential therapeutic target. Eur J Cell Biol. 2012;91:139–49. [DOI] [PubMed] [Google Scholar]
- 58.Radicioni AF, De Marco E, Gianfrilli D, Granato S, Gandini L, Isidori AM, et al. Strategies and advantages of early diagnosis in Klinefelter’s syndrome. Mol Hum Reprod. 2010;16:434–40. [DOI] [PubMed] [Google Scholar]
- 59.Huang Q, Parfitt A, Grennan DM, Manolios N. X‐chromosome inactivation in monozygotic twins with systemic lupus erythematosus. Autoimmunity 1997;26:85–93. [DOI] [PubMed] [Google Scholar]
- 60.Gleicher N, Barad DH. Gender as risk factor for autoimmune diseases. J Autoimmun. 2007;28:1–6. [DOI] [PubMed] [Google Scholar]
- 61.Sawalha AH, Harley JB, Hal SR. Autoimmunity and Klinefelter’s syndrome: when men have two X chromosomes. J Autoimmun. 2009;33:31–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bianchi I, Lleo A, Gershwin ME, Invernizzi P. The X chromosome and immune associated genes. J Autoimmun. 2012;38:187–92. [DOI] [PubMed] [Google Scholar]
- 63.Hewagama A, Gorelik G, Patel D, Liyanarachchi P, McCune WJ, Somers E, et al. Overexpression of X‐linked genes in T cells from women with lupus. J Autoimmun. 2013;41:60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baldassarri M, Picchiotti N, Fava F, Fallerini C, Benetti E, Daga S, et al. Shorter androgen receptor polyQ alleles protect against life‐threatening COVID‐19 disease in European males. EBioMedicine 2021;26:103246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data for this study are available from the corresponding author.


