Highlights
-
•
All the analytical methods have been successfully applied to acid oils and fatty acid distillates.
-
•
A detailed description of the sample preparation for analysis and applied analytical methods is provided as a compendium of methods in the supplementary material.
-
•
These methods will be extremely useful to improve the quality control of these heterogeneous feed ingredients.
Keywords: Analytical methods, Quality control, Fat by-products, Feed ingredients
Abbreviations: AO, acids oils; p-AnV, p-anisidine value; FA, fatty acids; FAD, fatty acid distillates; FAME, fatty acid methyl esters; FFA, free fatty acids; HPLC, high performance liquid chromatograph; I, insoluble impurities; M, moisture; MIU, sum of moisture, insoluble impurities and unsaponifiable matter; RSD, relative standard deviation; T, tocopherols; T3, tocotrienols; U, unsaponifiable matter; U/S ratio, unsaturated/saturated fatty acid ratio
Abstract
Acid oils and fatty acid distillates are by-products from the refining of edible oils and fats. They are used as feed ingredients, but their highly variable composition sometimes affects the productive parameters of the animals. Thus, their quality control and standardization are necessary. The official methods recommended for crude and refined fats and oils must be modified to give reliable results when applied to acid oils and fatty acid distillates. This article summarizes the drawbacks that were encountered during the setup of the analytical methods and how were they overcome by adapting the methods to these type of fat samples. Some methods such as the determinations of fatty acid composition, tocopherol and tocotrienol content, unsaponifiable matter, acidity and peroxide value had to be minimally adapted. However, others such as the determinations of moisture and volatile matter, insoluble impurities, lipid classes and p-anisidine value showed important drawbacks that required a more significant adaptation.
-
•
All the analytical methods have been successfully applied to acid oils and fatty acid distillates.
-
•
A detailed description of the sample preparation for analysis and applied analytical methods is provided as a compendium of methods in the supplementary material.
-
•
These methods will be extremely useful to improve the quality control of these heterogeneous feed ingredients.
Graphical abstract
Specifications table
| Subject Area: | Chemistry |
| More specific subject area: | Quality control of the feed fat ingredients |
| Method name: | Analytical methods to determine the quality of fat by-products used in animal feeding |
| Name and reference of original method: | SeeTable 2for this information |
| Resource availability: | N/A |
Introduction
The acid oils (AO) and fatty acid distillates (FAD) are fat by-products coming from refining of edible oils and fats. The AO come from the acidification of the soapstocks obtained during the neutralization step of the chemical refining; while the FAD come from the deodorization step of the physical refining, where the free fatty acids (FFA) and other volatile compounds are removed subjecting the oil to steam stripping at high temperatures under vacuum [1]. Thus, both by-products have a high content in FFA and they have been included in feeds to increase the dietary energy. Indeed, both AO and FAD are included in the European catalogue of feed materials [2]. However, the composition of AO and FAD has been reported to be very variable [3]. This is one of the reasons why nowadays, many feed producers and farmers are reluctant of using them routinely. In many cases, they even encounter differences in the productive parameters between batches from the same producer. Therefore, there is a need for quality control and standardization of these by-products before their use in animal feeding. This is important because an increased use of these by-products in animal feeding will lead to a more sustainable food chain.
In the quality control of the fatty ingredients of feeds is very important to determine the amount of compounds that can dilute their energy content, such as moisture (M), insoluble impurities (I) and unsaponifiable matter (U) (globally known as MIU value) or the non-elutable material that includes MIU plus oxidized and polymerized fatty acids and acylglycerols [4]. In addition, it is important to determine the FFA content and the U/S ratio (the ratio unsaturated fatty acids to saturated fatty acids) because it is widely recognized that these parameters affect the digestible and apparent metabolizable energy of the fatty ingredients [4,5] However, the fatty ingredients also provide liposoluble vitamins, such as vitamin E, and other essential lipid nutrients, such as linoleic and linolenic acids that can be easily oxidized.
An accurate determination of these parameters would contribute to obtain adequate conclusions on the nutritive value and stability of AO and FAD, which is essential to revalorize these by-products as animal feed ingredients. Nevertheless, analytical methods that are available to determine these parameters are intended for the analysis of crude or refined fats and oils. Compared to AO and FAD, crude and refined fats have a more homogenous composition, usually characterized by lower M, I, U and FFA contents, and higher triacylglycerol content. This sometimes makes it difficult to obtain accurate results when these analytical methods are applied to AO and FAD. Therefore, the objective of this article is to provide analytical protocols specifically adapted to the analysis of AO and FAD from different botanical origins. When available, the analytical methods selected were official methods for the analysis of crude or refined oils, but that were setup and sometimes modified to be adapted to these type of fat samples. The parameters for which no official methods were available were analyzed following in-house validated methods available in the scientific literature. These methods were applied to 92 samples, 79 AO and 13 FAD, coming from the refining of vegetable fats and oils and intended for animal feeding (Table 1). As these samples were very heterogeneous [5], the application of these methods presented several drawbacks that were overcome to obtain reliable results for all types of samples. This article summarizes the drawbacks that were found during the setup of the analytical methods (Table 2) and how were they overcome by adapting the methods. In addition, a detailed description of the sample preparation for analysis and of the customized analytical methods is provided as a compendium of methods in the supplementary material. This compendium will be extremely useful to control the quality of these very particular and variable feed ingredients.
Table 1.
Samples classified according to the refining process and the botanical origin.
| Refining process | Botanical origin | Subgroup: different mixtures | n | Total |
|---|---|---|---|---|
| Chemical refining (Acid oils, AO) | Blends of AO from seed oils, cocoa butter and palm oilsa | Cocoa butter, rapeseed, soybean and palm oils (40/30/20/10) | 2 | 12 |
| Cocoa butter, palm and seed oils | 10 | |||
| Blends of AO from seed and palm oilsa | Soybean, rapeseed and palm oils (40/40/20) | 2 | 5 | |
| Sunflower, soybean, palm, corn and rapeseed oils | 3 | |||
| AO from olive pomace oil and blends of AO from olive pomace and olive oils | Olive pomace oil | 13 | 18 | |
| Olive pomace and olive oils (90/10) | 5 | |||
| Blends of AO from seed oilsab | Sunflower (80-90), rapeseed (20-10) and traces of palm and palm kernel oils and palm stearin | 1 | 9 | |
| Sunflower, corn and grapeseed oils (40/30/30) | 3 | |||
| Sunflower, soybean and corn oils | 3 | |||
| Sunflower, high oleic sunflower, soybean, corn and olive pomace oils | 2 | |||
| AO from sunflower oil | Sunflower oil | 18 | 18 | |
| Blends of AO from sunflower and soybean oilsa | Sunflower and soybean oils | 4 | 15 | |
| Sunflower and soybean oils (10/90) | 7 | |||
| Sunflower and soybean oils (80/20) | 2 | |||
| Sunflower and soybean oils (90/10) | 2 | |||
| AO from soybean oil | Soybean oil | 2 | 2 | |
| Physical refining (Fatty acid distillates, FAD) | FAD from coconut oil and blends of FAD from coconut and palm kernel oils (lauric FAD)a | Coconut and palm kernel oils | 3 | 5 |
| Coconut oil | 2 | |||
| FAD from palm oil | Palm oil | 6 | 6 | |
| FAD from olive pomace and olive oils | Olive pomace oil | 1 | 2 | |
| Olive oil | 1 |
For some blends the proportions were unknown.
Some blends have traces of fruit oils.
Table 2.
Analytical methods that were adapted and used to control the quality of acid oils and fatty acid distillates.
| Analytical method | Main referencea |
|---|---|
| Preparation of test sample | |
| Fatty acid composition | Guardiola et al. [6] |
| Tocopherol and tocotrienol content | Aleman et al. [7] |
| Moisture and volatile matter | AOCS official method Ca 2d-25 [8] |
| Insoluble impurities | ISO 663:2017 [12] |
| Unsaponifiable matter | AOCS official method Ca 6b-53 [13] |
| Acidity (free fatty acid content) and acid value | ISO 660:2009 [14] |
| Lipid classes (%): polymeric compounds, acylglycerols and free fatty acids | IUPAC method 2508 [15] |
| Peroxide value | Commission regulation (EEC) 2568/91 and its amendments [17] |
| p-Anisidine value | AOCS official method Cd 18-90 [18] |
| Oxidative stability by Rancimat instrument | AOCS official method Cd 12b-92 [19] |
For some methods other references have been used, see the supplementary materials for the complete list of references. No particular reference was followed for the preparation of the test sample.
Setup and adaptation of the methods to the samples
The main difficulty found during the preparation of the sample for the analysis was to achieve the complete homogenization of the sample. The samples had very different nature and they had to be homogenized at temperatures that range from room temperature to 65 °C. The heating temperatures and times were always the minimum necessary to avoid any alteration of the samples by oxidation or polymerization (method S1, supplementary materials).
The fatty acid composition was determined after a double methylation in methanolic medium, first with sodium methoxide and later with boron-trifluoride, to ensure that FFA were completely methylated. Then, fatty acid methyl esters (FAME) were separated and identified by gas chromatography-flame ionization detector. The FAME were quantified by peak area normalization (the quantitative results are obtained by expressing the area of a given peak as a percentage of the sum of the areas of all the identified peaks). This method is based on the method described by Guardiola et al. [6] and it was adapted to the samples without any problem. To evaluate the intralaboratory repeatability, six determinations of two samples (one AO and one FAD) were carried out by the same analyst within a day, using the same reagents, equipment and instruments and the relative standard deviation (RSD, %) ranged from 0.13–1.31% for different fatty acids (method S2, supplementary materials).
The content in tocopherols (T) and tocotrienols (T3) was determined using a high performance liquid chromatograph (HPLC) equipped with a silica column and a fluorescence detector. In this procedure, prior to injection into the HPLC system, the sample was subjected to saponification and the unsaponifiable matter was extracted with petroleum ether, filtered, evaporated, and dissolved in n-hexane. The eight homologs of vitamin E: α-, β-, γ-, and δ-T and their corresponding T3 were quantified by external standard calibration and the results were expressed in mg/kg. This method is based on the method described by Aleman et al. [7] and it was adapted to the samples without any problem showing good intralaboratory repeatability for AO and FAD. To evaluate the repeatability, eight determinations were carried out for two samples (one AO and one FAD) and the RSD ranged from 4.5–13.5% for the four T. The recovery of the method was also good for all T, because even the recovery for δ-T in AO was quite low, in all cases the variability of the recovery was extremely low (method S3, supplementary materials).
The moisture, including other volatile matter at the conditions of the test, was determined by a vacuum oven method in which the sample was subjected to heat (58 °C) and vacuum (66 mbar, approximately 50 mm Hg) and the % loss in weight was reported as moisture and volatile matter. The method is based on the AOCS official method Ca 2d-25 [8], which showed some drawbacks when applied to these samples. Some AO samples can rise problems due to an excess of moisture, so they can explode producing splattering inside the vacuum oven. To avoid that, once the sample was weighed into the tared dish, the uncovered dish was kept overnight (16 h) into the desiccator applying vacuum progressively until a pressure of 10 mm Hg was reached to remove part of the moisture and volatiles from the sample. After this operation, the determination followed the procedure described in method S4 (supplementary materials). Another problem occurred with lauric FAD, which contain a significant amount of C6:0-C12:0 fatty acids. In the case of the lauric FAD coming from the physical refining of coconut or palm kernel oils, most of the fatty acids (65–82%) are in free form. It has been reported that some of these FFA, mainly C6:0 (boiling point at 50 mm Hg = 135 °C), can be volatilized in the vacuum oven. This volatilization under our working conditions (constant weight achievement) conducted to an overestimation of the moisture and volatile content. This overestimation was proven in several lauric FAD samples by comparing the results obtained as described above and following the one-component reagent volumetric Karl Fischer method (ISO 8534:2017) [9]. As it can be observed in Table 3, if constant weight was attained, the overestimation was considerably high; however, if only one 1 h-drying period was applied, the difference with the Karl Fischer results was minimum. These results agree with the fact that during the first drying period, water (boiling point at 50 mm Hg = 37.5 °C) and the most volatile compounds are lost. Thus, for lauric FAD only one drying period of 1 h was applied to obtain the results of moisture and volatile compounds. When other methods to determine moisture, such as the hot plate methods, AOCS official methods Ca 2b-38 [10] and Tb 1a-64 [11], were applied to lauric FAD, erroneous results were obtained due to the presence of a high amount of C6:0-C12:0 FFA. Once these drawbacks were solved, the intralaboratory repeatability of the method for AO and FAD was improved. To evaluate the repeatability, eight determinations were carried and the RSD obtained for an AO was 0.96% (mean of moisture and volatile matter = 0.98%), for a non-lauric FAD, 12.80% (mean = 0.07%) and for a lauric FAD, 8.43% (mean = 0.21%).
Table 3.
Moisture (%) content in lauric fatty acid distillates determined by different methods (determinations in duplicate).
| Karl Fischer (ISO 8534:2017) [9] | Vacuum oven (method S4, supplementary materials) |
||
|---|---|---|---|
| One drying period (1 h) | Constant weight (5 drying periods of 1h) | ||
| Sample 1 | 0.28 | 0.32 | 0.47 |
| Sample 2 | 0.16 | 0.14 | 0.36 |
| Sample 3 | 0.18 | 0.21 | 0.57 |
The insoluble impurities were determined by a procedure that implies dissolving the dried samples with an excess of light petroleum ether. Then, the obtained solution was filtered, and the filter and residue were washed with the same solvent and then dried at 103 °C in an air oven and weighed to determine the percentage of insoluble impurities retained in the filter. The results can be expressed on wet weight or on dry weight. The method determines dirt and other foreign substances insoluble in light petroleum ether and it is based on the ISO 663:2017 method [12]. The application of this method to the samples showed some drawbacks. For most samples, about 5 g of AO or FAD were accurately weighed into a tared moisture dish and the moisture and volatile matter were determined according method S4 (supplementary materials). Once the sample was dried, it was dissolved in petroleum ether and filtered to determine impurities (method S5, supplementary materials). However, samples with a high percentage of insoluble impurities may be difficult to filter. For such samples, 2 g can be weighted without significantly affecting the precision of the method. According to our results, it can be concluded that in AO samples with impurities percentages higher than 10% it is necessary to reduce the sample weight to 2 g instead of 5 g, in order to carry out the method without any problem in the filtration step nor in transferring the entire sample from the dish to the filter. In addition, in the case that some remains did not dissolve in the petroleum ether and remained in the moisture dish, they had to be weighed and this value must be added to the total weight for the calculation of the impurities. Once these drawbacks were overcome, the intralaboratory repeatability of the method for AO and FAD improved. To evaluate the repeatability, six determinations were carried for five different samples (four AO and one FAD) using different weights of sample and the RSD ranged from 7.72–12.75%.
The unsaponifiable matter content was determined by saponifying the sample with an ethanolic potassium hydroxide solution. In this method, the unsaponifiable matter was extracted from the soap solution with diethyl ether. The solvent was then evaporated, and the residue was weighed after drying and reported as percentage of unsaponifiable matter. The method is based on the AOCS official method Ca 6b-53 [13], which is a method recommended for samples having an unsaponifiable content higher than that usually found in fats and oils. Although the AOCS does not recommend this method for feed-grade fats, the method was successfully applied to AO and FAD (method S6, supplementary materials). The method showed a good intralaboratory repeatability for AO and FAD. To evaluate the repeatability, six determinations were carried for two samples (one AO and one FAD) and the RSD obtained for the AO was 5.35% (mean unsaponifiable matter = 5.02%) and for the FAD, 3.46% (mean = 1.22%). In fact, the repeatability RSD reported for a crude rapeseed oil having an unsaponifiable content of 1.43% in an interlaboratory study organized by IUPAC was quite high, 24.7% [13].
The acidity (or FFA content) and the acid value were determined by a volumetric titration in which the sample is dissolved in a mixture of ethanol 96% and toluene (1:1, v/v), and the acids present in the solution are then titrated with an 0.1 M ethanolic solution of potassium hydroxide. While the acidity or free fatty acid content is expressed as mass percent of lauric, palmitic, oleic or erucic acids, according to the fatty acid composition of the sample, the acid value is expressed as the number of milligrams of potassium hydroxide required to neutralize the free fatty acids present in 1 g of fat. This method is based on the ISO 660:2009 method [14] and it was adapted to the samples without significant problems. As all samples were very rich in FFA and some of them were quite dark, to clearly observe the endpoint, only 0.5 g of sample was weighed. In these conditions, the method showed a good intralaboratory repeatability for AO and FAD (method S7, supplementary materials). To evaluate the repeatability, six determinations were carried for two samples (one AO and one FAD) and the RSD obtained for the AO was 4.04% (mean = 61.98 g oleic acid/100 g or 123.30 mg KOH/g of fat) and for the FAD, 1.06% (mean = 74.77 g lauric acid/100 g or 209.74 mg KOH/g of fat).
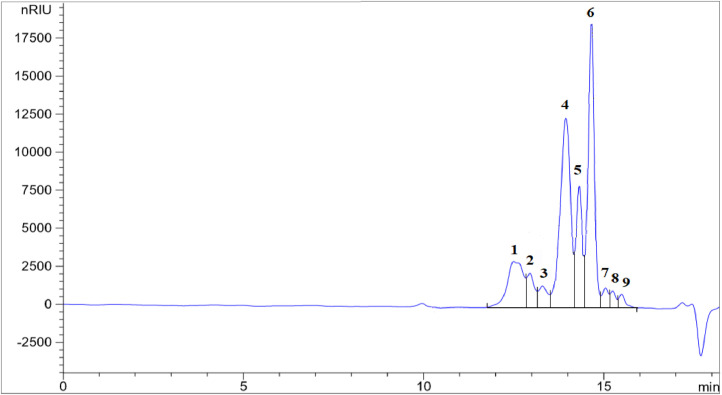
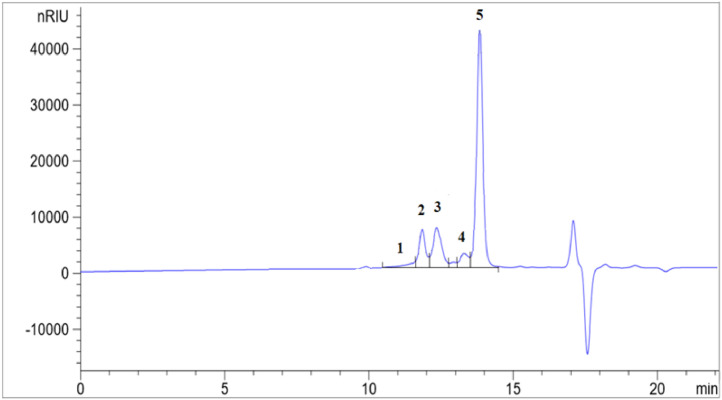
The lipid classes (polymeric compounds, triacylglycerols, diacylglycerols, monoacylglycerols and FFA) present in the samples were separated by size exclusion chromatography by means of two HPLC columns connected in series and determined through a refractive index detector. The lipid classes were quantified by peak area normalization (the quantitative results are obtained by expressing the area of a given peak as a percentage of the sum of the areas of all the peaks corresponding to the different lipid classes). The method is based on the IUPAC method 2508 [15] and is not applicable to fats and oils rich in medium- and/or short-chain fatty acids, such as lauric FAD, as their lipid classes have a wide range of molecular weights, which disables the size exclusion columns from separating them. We noted this problem using several triacylglycerol, monoacylglycerol and FFA standards with different molecular weights. For instance, monolaurin is included in the peak corresponding to C16 and C18 FFA (Fig. 1). This is because the molecular weights of monolaurin, palmitic and oleic acids are 274.4, 256.4 and 282.5 g/mol, respectively. For the same reason, tricaprin elutes together with the diacylglycerols in peak 2 (Fig. 1). Therefore, we discarded this method for lauric FAD since the separation between lipid classes cannot be achieved as the ranges of molecular weights of different lipid classes are clearly overlapped. On the contrary, the method separates well the lipid classes in fats and oils mainly composed of fatty acids with 16 and 18 carbons (Fig. 2). However, in some AO with low content of polymeric compounds, the peak corresponding to these compounds (peak 1, Fig. 2) appears as a shoulder before the peak corresponding to the triacylglycerols (peak 2). The method showed a good intralaboratory repeatability for AO and non-lauric FAD. To evaluate the repeatability, six determinations were carried for two samples (one AO and one non-lauric FAD). The results obtained for the AO sample were: 2.51% polymeric compounds (RSD = 11.38%); 21.39% triacylglycerols (RSD = 0.79%); 17.98% diacylglycerols (RSD = 1.13%); 4.05% monoacylglycerols (RSD = 5.04%); and 54.07% FFA (RSD = 0.73%). For the non-lauric FAD the results were: 6.03% triacylglycerols (RSD = 6.79%); 5.88% diacylglycerols (RSD = 9.83%); 88.09% FFA (RSD = 0.93%); the polymeric compounds and the monoacylglycerols were not detected in this sample (method S8, supplementary materials). Moreover, to determine the lipid classes (sums of triacylglycerols, diacylglycerols, monoacylglycerols and free fatty acids) in lauric FAD it is necessary to apply much more selective methods such as high-temperature gas chromatography-mass spectrometry [16]. With these highly selective methods, the different triacylglycerols, diacylglycerols, monoacylglycerols and free fatty acids can be determined individually at once and then added together in the different lipid classes. Of course, these selective methods will provide additional information, but they have not yet been applied to determine lipid classes in lauric fats and oils. This complex analytical problem should be addressed in the future since the relative quantity of triacylglycerols, diacylglycerols, monoacylglycerols and FFA affects the digestibility of fats in different animal species and therefore the dietary energy provided by these by-products, which is a crucial information to formulate feeds [5].
Fig. 1.
Separation of the lipid classes from a lauric fatty acid distillate. Main compounds corresponding to peaks: 1, triacylglycerols; 2, diacylglycerols; 3, monoacylglycerols; 4, C16 and C18 free fatty acids; 5, myristic acid; 6, lauric acid; 7, capric acid; and 8 and 9, free fatty acids with less than 10 carbons.
Fig. 2.
Separation of the lipid classes from an acid oil coming from the refining of olive pomace oil. Main compounds corresponding to peaks: 1, polymeric compounds; 2, triacylglycerols; 3, diacylglycerols; 4, monoacylglycerols; and 5, C16 and C18 free fatty acids.
The peroxide value was determined by a volumetric titration in which the sample is dissolved in chloroform-acetic acid and treated with a solution of potassium iodide. The iodine liberated by oxidation of potassium iodide is then titrated with a standard volumetric sodium thiosulfate solution. The oxidant substances under these conditions are generally assumed to be peroxides. The peroxide value is the quantity of peroxides (expressed in milliequivalents of active oxygen per kg of fat) in the sample. This method is based on the method adopted by the European Union for olive oils [17] and it was adapted to the samples without significant problems. Since some AO samples were very dark and this made it difficult to observe the endpoint of the iodometric titration, no more than 2.5 g were weighed, even if the expected peroxide value of the sample was much lower than 10 (the 92 samples analyzed had peroxide values lower than 10 and the 95% had peroxide values between 0 and 3). The method applied in the conditions described in the supplementary material (method S9), paying special attention to the observation of the titration endpoint, showed a good intralaboratory repeatability. To evaluate the repeatability, six determinations were carried and the RSD obtained for a dark AO sample was 2.14% (mean = 2.63 milliequivalents of active oxygen per kg of fat, sample weight = 2.5 g) and for a non-dark FAD sample, 3.05% (mean = 1.27 milliequivalents of active oxygen per kg of fat, sample weight = 2.5 g).
The p-anisidine value (p-AnV) was determined by spectrophotometry. The method determines the amount of aldehydes (principally 2-alkenals and 2,4-dienals), which react with p-anisidine in an isooctane/acetic acid solution of the sample. The reaction products formed are measured at 350 nm. The intensity of the absorption depends not only on the amount of aldehydic compounds but also on their structure. A double bond in the carbon chain conjugated with the carbonyl double bond increases the molar absorbance at 350 nm four to five times, so especially 2-alkenals and 2,4-dienals will contribute substantially to the value found. The p-AnV is defined by convention as 100 times the optical density measured at 350 nm in a 1 cm cuvette of a solution containing 1 g of the sample in 100 mL of a mixture of solvents and p-anisidine reagent. The method is based on the AOCS official method Cd 18-90 [18] and when applied to these samples, it showed some drawbacks. Most AO samples showed suspended particles after dissolution in isooctane and a filtration step was necessary to obtain a limpid solution before measuring its absorbance. Depending on the turbidity observed the filtration was carried using filters of 5 µm or 0.45 µm of pore and the sample weight was reduced (method S10, supplementary materials). On the contrary, limpid solutions were obtained for FAD samples and thus, the filtration step was not necessary. For all samples, the weight used depended on the linearity of the method. To check the linearity of the response, the p-AnV of various AO and FAD samples was determined in duplicate using different weights between 0.5 and 1.5 g. The linearity of the response was confirmed up to an absorbance after reaction with p-anisidine (As value) of 3.2, since until this As value the p-AnV of several samples was independent of the weight. Thus, in all cases the weight of the samples was adjusted in order to obtain As values equal or lower than 3.0. The method applied in the conditions described in the supplementary material (method S10) showed an improved intralaboratory repeatability for AO and FAD. To evaluate the repeatability, six determinations were carried for two samples (one AO and one FAD) and the RSD obtained for the AO sample was 2.96% (mean = 13.47, sample weight = 0.8 g) and for a the FAD sample, 6.98% (mean = 12.62, sample weight = 1.5 g).
The oxidative stability of the samples (induction time in hours) was measured by the Rancimat instrument from Metrohm (Herisau, Switzerland). In the Rancimat instrument, the sample is subjected to an air flow and a constant temperature which produce a deterioration of the sample. The air stream sweeps the highly volatile oxidation products such as alcohols, aldehydes, ketones and carboxylic acids, among others, which are transferred into the measuring vessel containing Milli-QⓇ water and the electrode that measures the conductivity. The conductivity is continuously registered and increases slowly to a sudden jump, at which point the oxidation accelerates and becomes high very rapid. The time elapsed until this rapid acceleration of the oxidation is a measure of the resistance to oxidation and is referred to as the induction time or induction period, which allows a reliable estimate of the oxidative stability of the sample. The computer software determines the break point of the conductivity curve by using the maximum of the second derivative of the curve and gives the induction time expressed in hours. The longer is this time, the higher is the oxidative stability of the sample. This method is based on the AOCS official method Cd 12b-92 [19] and its application to the samples required only slight modifications. However, in 10 AO samples the conductivity curve did not show a clear jump and the induction time could not be determined. The method applied in the conditions described in the supplementary material (method S11) performed as usual for the rest of samples (69 AO and 13 FAD) since the RSD was calculated for the duplicates of the 82 samples determined and the median of the RSD was 2.33%, similar to other types of samples.
Conclusion
Some methods such as the determinations of fatty acid composition, tocopherol and tocotrienol content, unsaponifiable matter, acidity and peroxide value, had to be minimally adapted from methods recommended for crude and refined fats and oils. However, others such as the determinations of moisture and volatile matter, insoluble impurities, lipid classes and p-AnV showed important drawbacks that required a more significant adaptation. Indeed, in the case of the determination of moisture and volatile matter, the method proposed varies depending on the botanical origin of the FAD, and in the case of the determination of the lipid classes, the method can only be applied to AO and FAD from non-lauric origin. After adaptations, in general, all the analytical methods have been successfully applied to AO and FAD. A detailed description of all the methods is included in the supplementary material. These methods will be extremely useful to improve the quality control of these heterogeneous feed ingredients. In fact, the animal feed sector needs this information because the official methods recommended for the analysis of crude or refined fats and oils must be modified to give reliable results for these samples, which will help to accurately evaluate the energy and nutritive values of these feed ingredients.
Acknowledgments
This work was funded by the Spanish Ministry of Economy and Competitiveness through the project AGL2015-64431-C2-2-R (MINECO/FEDER, UE). The study was also supported through a pre-doctoral contract within the FPI program (BES-2016-077486) granted to Elisa Varona and post-doctoral contract within the RyC program (RYC-2017-23601) granted to Alba Tres.
We also thank all the suppliers of the samples analyzed in this study: edible oil refineries, AO producers and feed producers.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.mex.2021.101334.
Appendix. Supplementary materials
References
- 1.FEDIOL, the EU vegetable oil and protein meal industry association, represents the interest of the European oilseed crushers, vegetable oil refiners. (http://www.fediol.eu, accessed 14 February 2021).
- 2.Commission regulation (EU) 68/2013 and its ammendments on the Catalogue of feed materials. Off. J. Eur. Union. 2013;L29:1–64. [Google Scholar]
- 3.Nuchi C., Guardiola F., Bou R., Bondioli P., Bella L.Della, Codony R. Assessment of the levels of degradation in fat co-and byproducts for feed uses and their relationships with some lipid composition parameters. J. Agric. Food Chem. 2009;57:1952–1959. doi: 10.1021/jf803369h. [DOI] [PubMed] [Google Scholar]
- 4.Ravindran V., Tancharoenrat P., Zaefarian F., Ravindran G. Fats in poultry nutrition: digestive physiology and factors influencing their utilisation. Anim. Feed Sci. Technol. 2016;213:1–21. doi: 10.1016/j.anifeedsci.2016.01.012. [DOI] [Google Scholar]
- 5.Varona E., Tres A., Rafecas M., Vichi S., Barroeta A.C., Guardiola F. Composition and nutritional value of acid oils and fatty acid distillates used in animal feeding. Animals. 2021;11:196. doi: 10.3390/ani11010196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guardiola F., Codony R., Rafecas M., Boatella J., López A. Fatty acid composition and nutritional value of fresh eggs, from large- and small-scale farms. J. Food Comp. Anal. 1994;7:171–188. doi: 10.1006/jfca.1994.1017. [DOI] [Google Scholar]
- 7.Aleman M., Nuchi C.D., Bou R., Tres A., Polo J., Guardiola F., Codony R. Effectiveness of antioxidants in preventing oxidation of palm oil enriched with heme iron: A model for iron fortification in baked products. Eur. J. Lipid Sci. Technol. 2010;112:761–769. doi: 10.1002/ejlt.200900220. [DOI] [Google Scholar]
- 8.AOCS official method Ca 2d-25, 7th ed. AOCS Press; Champaign, IL: 2017. Moisture and Volatile Matter, in Fats and Oils, Vacuum Oven Method, Official Methods and Recommended Practices of the American Oil Chemists’ Society. [Google Scholar]
- 9.International Standard ISO 8534:2017, Animal and vegetable fats and oils, determination of water content, Karl Fischer method (pyridine free). 3rd ed. 2017.
- 10.AOCS official method Ca 2b-38, 7th ed. AOCS Press; Champaign, IL: 2017. Moisture and Volatile Matter in Butter, Fats, Margarines, and Oils, Hot Plate Method. Official Methods and Recommended Practices of the American Oil Chemists’ Society. [Google Scholar]
- 11.AOCS official method Tb 1a-64 . Official Methods and Recommended Practices of the American Oil Chemists’ Society. 7th ed. AOCS Press; Champaign, IL: 2017. Moisture and Volatile Matter in Fatty Acids, Hot Plate Method. [Google Scholar]
- 12.International Standard ISO 663:2017, Animal and vegetable fats and oils, determination of insoluble impurities content. 5th ed. 2017.
- 13.AOCS official method Ca 6b-53 . Official Methods and recommended practices of the American Oil Chemists’ Society. 7th ed. AOCS Press; Champaign, IL: 2017. Unsaponifiable Matter, High Level Method. [Google Scholar]
- 14.International Standard ISO 660:2009, Animal and vegetable fat and oils, determination of acid value and acidity. 3rd ed. 2009, reviewed and confirmed in 2014.
- 15.International Union of Pure and Applied Chemistry (IUPAC) 7th ed. IUPAC, Blackwell Scientific Publications; Oxford, UK: 1991. Determination of Polymerized Triglycerides in Oils and Fats by High Performance Liquid Chromatography (method 2508). In Standard Methods for the Analysis of Oils, Fats and Derivatives. [Google Scholar]
- 16.Michael-Jubeli R., Bleton J., Baillet-Guffroy A. High-temperature gas chromatography-mass spectrometry for skin surface lipids profiling. J. Lipid Res. 2011;52:143–151. doi: 10.1194/jlr.d008094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Commission regulation (EEC) 2568/91 and its amendments on the Characteristics of olive oil and olive-residue oil and on the relevant methods of analysis. Annex III - Determination of the peroxide value. Off. J. Eur. Communities. 1991;L248:1–83. [Google Scholar]
- 18.AOCS official method Cd 18-90, 7th ed. AOCS Press; Champaign, IL: 2017. p-Anisidine Value. Official Methods and Recommended Practices of the American Oil Chemists’ Society. [Google Scholar]
- 19.AOCS official method Cd 12b-92, 7th ed. AOCS Press; Champaign, IL: 2017. Oil Stability Index. Official Methods and Recommended Practices of the American Oil Chemists’ Society. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.