Abstract
Due to its role in brain development, the DYRK1A kinase (dual-specificity tyrosine phosphorylation-regulated kinase 1a) has been proposed as a drug target for Down syndrome, and diseases associated with neurodegeneration including Alzheimer's and Parkinson's. Other diseases in which DYRK1A is implicated include cancer and diabetes. Hence, there is need for potent and selective DYRK1A inhibitors. To screen large diversity compound libraries versus DYRK1A requires the development of a cost-effective high-throughput screen. In this study, we have taken a commercial time-resolved fluorescence energy transfer (TR-FRET)-based assay for DYRK1A and optimized for smaller volumes and homogenous format at room temperature. Tracer and enzyme concentrations were determined. DYRK1A-GST, anti-GST Ab and tracer were pre-combined and total assay volume reduced 2-fold. The assay was validated using whole plate minimum and maximum signal wells with a Z’ of 0.7-0.8 determined. Overall, this method:
-
•
Results in an optimized low volume, homogenous and validated assay for DYRK1A.
-
•
Delivers a cost effective high-throughput assay format for DYRK1A inhibitor screening
Keywords: High-throughput screening, DYRK1A, TR-FRET
Graphical abstract
Graphical abstract
Specifications table
| Subject Area: | Pharmacology, Toxicology and Pharmaceutical Science |
| More specific subject area: | High-throughput kinase assay |
| Method name: | DYRK1A HTS assay |
| Name and reference of original method: | NA |
| Resource availability: | NA |
Method details
Assay reagents and chemicals
The assay to measure DYRK1A kinase activity utilizes components from a LanthaScreen™ Eu Kinase Binding Assay (Thermo Fisher Scientific). Assay components DYRK1A-GST protein (catalog #PR7189B), Kinase Tracer 236 and Europium-anti GST antibody were from Life Technologies (Thermo Fisher Scientific). 384-well low volume black round bottom polystyrene non-binding surface microplates were from Corning, NY (catalog # 4514). Unless otherwise stated, all reagents and compounds were purchased from Thermo Fisher Scientific (Waltham, MA) or Sigma Aldrich (St. Louis, MO) at the highest level of purity possible.
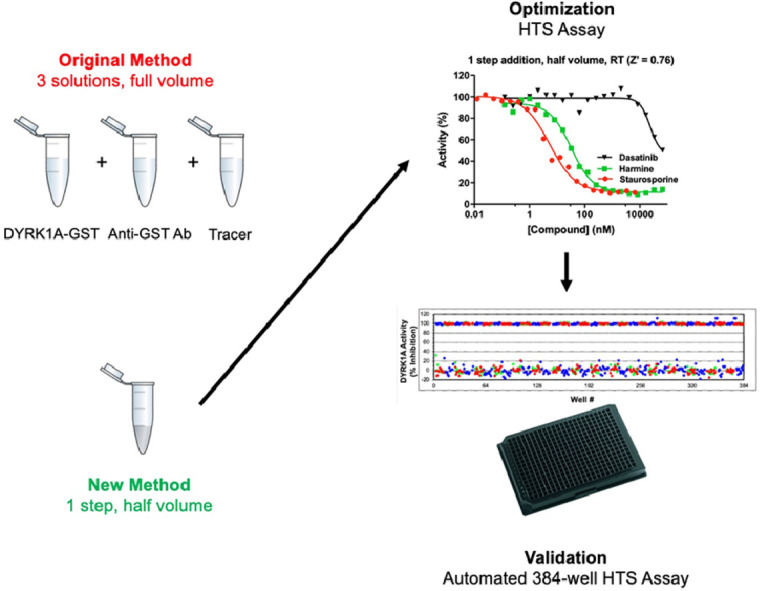
Assay optimization for reduced volume and one-step addition
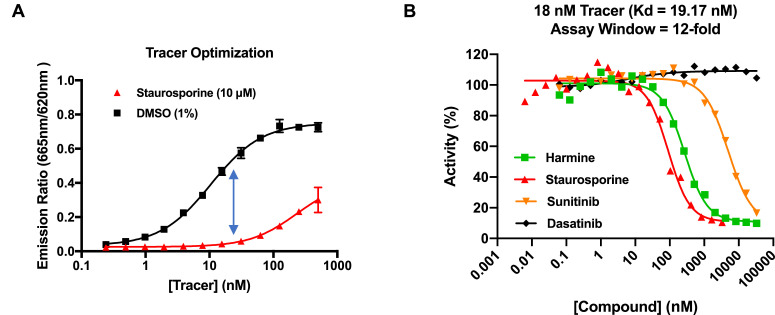
The development of the TR-FRET based kinase assay platform is described in [1]. Components from a commercially available TR-FRET based-assay for DYRK1A (LanthaScreen, Life Technologies; Carlsbad, CA, USA, https://assets.thermofisher.com/TFS-Assets/LSG/manuals/DYRK1A_LanthaScreen_Binding.pdf) were employed for this study with modifications made to improve the workflow by reducing assay steps, utilizing automation and minimizing reagent use. For the assay, the concentration of the competitive binding tracer (Kinase Tracer 236) was determined to be optimal at 18 nM, with 2 nM of Europium-anti GST antibody and 5 nM of DYRK1A-GST kinase (Fig. 1A), and provided an assay window of 12-fold as assessed using the known DYRK1A inhibitor harmine [2], [3], [4] and a non-selective kinase inhibitor staurosporine (Fig. 1B).
Fig. 1.
DYRK1A TR-FRET tracer optimization and determination of assay window. A. Tracer optimization: optimal assay window (11.5) with Tracer concentration at 18 nM. B. Dose response curves to determine assay window. Dasatinib included as negative control.
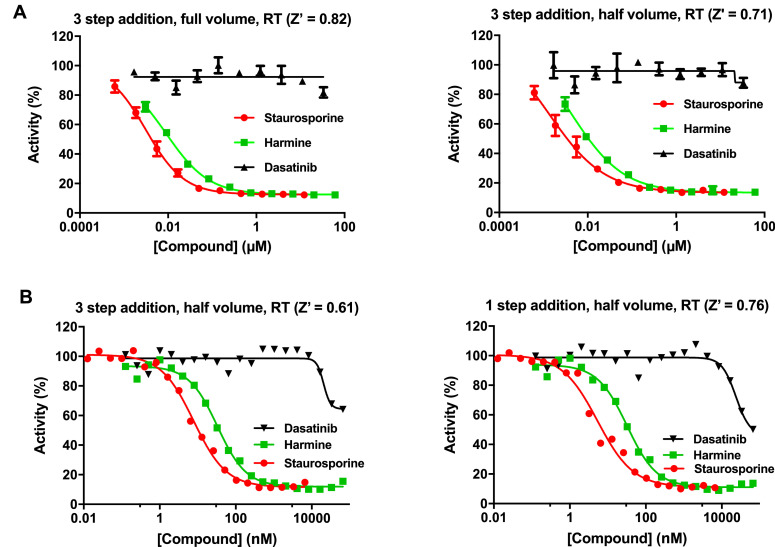
Departing from the published protocol (e.g. as used in [5]), a validated method for half-volume assay (Figs. 2A) and one-step reagent addition (Figs. 2B) was developed wherein a 3 × kinase + antibody solution and a tracer solution were made in 1 × Kinase buffer A (Life Technologies) just prior to the experiment. These two solutions were combined at a ratio of 2:1 (kinase-antibody:tracer) and immediately dispensed at 7.5 µL per well into 384-well low volume black round bottom polystyrene plates using a NanoScreen NSX-1536 equipped with a 384 head (Beckman Coulter; Brea, CA, USA). Compounds were added to into the final assay volume of 7.5 µL as 10- or 20-point dose response titrations using a Biomek NX workstation (Beckman-Coulter) equipped with a Pintool array (VP Scientific; San Diego CA). Plates were briefly centrifuged and then incubated covered in the dark at room temperature (RT) for 1 h. Plates were then read using a PHERAstar dual-emission HTRF module (665 nm / 620 nm) plate reader (BMG Labtech; Cary, NC, USA). The final automatic protocol is shown in Table 1.
Fig. 2.
DYRK1A TR-FRET assay optimization. A. Comparative dose response curves at full versus half volume. B. Comparative dose response curves for three addition steps versus one addition step.
Table 1.
Automation protocol for DYRK1A TR-FRET HTS assay optimized and validated for ½ reagent volume & one-step reagent addition.
| Step | Event | Parameter | Description |
|---|---|---|---|
| 1 | Reagent preparation | 36 ml for ten 384 well plates | Separately prepare reagents in Kinase Buffer A and combine to give 3x concentrations • DYRK1A-GST kinase (15 nM) • Eu-tagged anti-GST (6 nM) • Tracer 236 (54 nM) |
| 2 | Dispense | 7.5 µL | Add assay reagents to a set of ten plates using Nanoscreen |
| 3 | Dispense | 50 nL | Pin-tool addition of 1 mM test compounds (Columns 3-22) and DMSO (Columns 1,2,23,24) using Biomek NX |
| 4 | Incubate | 60 min | Centrifuge assay plates and incubate in dark at RT |
| 6 | Read | Ex620/Em665 | PheraStar |
Assay validation for HTS
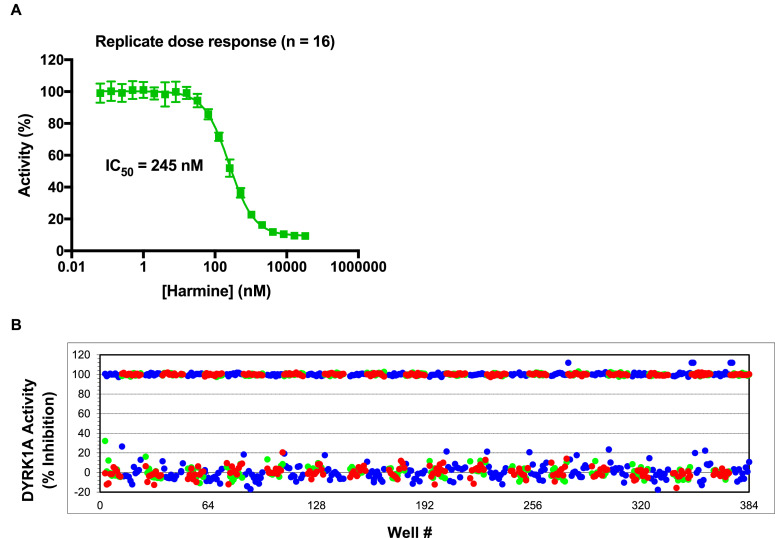
Replicate dose response curves for harmine generated an IC50 value of 245 nM (Fig. 3A). For HTS validation, the DYRK1A assay was run in full automation mode with whole 384-well plates of minimum signal (DYRK1A+Ab+tracer+harmine, upper trace) and maximum signal (DYRK1A+Ab+tracer+DMSO, lower trace) (Fig. 3B). Harmine was used at 6.7 µM (~25-fold its determined IC50 value in this assay). The Z’-factor was determined as a measure of both assay quality and performance [6]. Z’-factor calculations were performed in Excel. The validation was run three times generating Z’ values of 0.7, 0.8 and 0.8.
Fig. 3.
DYRK1A TR-FRET low volume one step assay validation for HTS. A. Dose response analysis of the DYRK1A inhibitor harmine using the optimized homogenous DYRK1A TR-FRET assay. Normalized dose response inhibition curves generated using Graphpad Prism 7.0. B. DYRK1A assay variability was assessed at minimum signal (DYRK1A+Ab+tracer+harmine, upper trace) and at maximum signal (DYRK1A+Ab+tracer+DMSO, lower trace).
Significance
To facilitate cost effective and robust HTS, the TR-FRET DYRK1A assay was simplified into a reduced volume one step addition and validated.
Acknowledgements
In memoriam Thomas Caligan. The authors would like to thank Ginger Smith at BRITE for technical advice and funding from NIH grants R15CA208651, P20CA202924, U54MD012392 and U54CA137844.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Lebakken C.S., Riddle S.M., Singh U., Frazee W.J., Eliason H.C., Gao Y., Reichling L.J., Marks B.D., Vogel K.W. Development and applications of a broad-coverage, TR-FRET-based kinase binding assay platform. J. Biomol. Screen. 2009;14:924–935. doi: 10.1177/1087057109339207. [DOI] [PubMed] [Google Scholar]
- 2.Adayev T., Wegiel J., Hwang Y.W. Harmine is an ATP-competitive inhibitor for dual-specificity tyrosine phosphorylation-regulated kinase 1A (Dyrk1A) Arch Biochem. Biophys. 2011;507:212–218. doi: 10.1016/j.abb.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bain J., Plater L., Elliott M., Shpiro N., Hastie C.J., McLauchlan H., Klevernic I., Arthur J.S., Alessi D.R., Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem. J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Göckler N., Jofre G., Papadopoulos C., Soppa U., Tejedor F.J., Becker W. Harmine specifically inhibits protein kinase DYRK1A and interferes with neurite formation. FEBS J. 2009;276:6324–6337. doi: 10.1111/j.1742-4658.2009.07346.x. [DOI] [PubMed] [Google Scholar]
- 5.Kumar K., Wang P., Sanchez R., Swartz E.A., Stewart A.F., DeVita R.J. Development of kinase-selective, harmine-based DYRK1A inhibitors that induce pancreatic human β-cell proliferation. J. Med. Chem. 2018;61:7687–7699. doi: 10.1021/acs.jmedchem.8b00658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang J.-H., Chung T.D., Oldenburg K.R. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]