Abstract
Previous reports suggested that non-dioxin-like (NDL) PCB153 effects on cytochrome P450 3A (Cyp3a) expression in Atlantic killifish (Fundulus heteroclitus) gills differed between F0 generation fish from a PCB site (New Bedford Harbor; NBH) and a reference site (Scorton Creek; SC). Here, we examined effects of PCB153, dioxin-like (DL) PCB126, or a mixture, on Cyp3a56 mRNA in killifish generations removed from the wild, without environmental PCB exposures. PCB126 effects in liver and gills differed between populations, as expected. Gill Cyp3a56 was not affected by either congener in NBH F2 generation fish, but was induced by PCB153 in SC F1 fish, with females showing a greater response. PCB153 did not affect Cyp3a56 in liver of either population. Results suggest a heritable resistance to NDL-PCBs in killifish from NBH, in addition to that reported for DL PCBs. Induction of Cyp3a56 in gills may be a biomarker of exposure to NDL PCBs in fish populations that are not resistant to PCBs.
Keywords: PCB153, Fish, Gills, Cyp3a, Resistance
1. Introduction
Polychlorinated biphenyls (PCBs) are persistent legacy pollutants abundant in many parts of the world. The PCB congeners with meta- and para- but no ortho-chlorine substituents, known as dioxin-like PCBs (DL PCBs), are avid ligands for the aryl hydrocarbon receptor (Ahr) and are acutely toxic, eliciting molecular effects and toxicity through activation of the Ahr. Prominent molecular effects of the DL PCBs include induction of drug metabolizing enzymes, especially cytochrome P450 1A (Cyp1a) and other members of the Cyp1 gene family. Induction of Cyp1a has long been used as a biomarker of exposure to DL PCBs and other Ahr agonists in the aquatic environment (Stegeman and Lech, 1991). In contrast to the DL PCBs, the molecular effects of non-dioxin-like PCBs (NDL PCBs) in fish are less well known, despite the fact that they are vastly more abundant in the environment. This study addresses the molecular effects of the NDL PCBs including an exposure biomarker, and a possible molecular adaptation to high levels of NDL PCBs, in the estuarine fish model, killifish (Fundulus heteroclitus).
Some fish populations inhabiting areas heavily contaminated by PCBs show impaired CYP1A biomarker responses, rather than robust induction (Elskus et al., 1999; Förlin and Celander, 1995; Wirgin and Waldman, 2004). A well-studied example is the killifish population inhabiting the New Bedford Harbor (NBH) in Massachusetts (MA; USA), a site severely contaminated by PCBs for more than 70 years (Nacci et al., 2010). Killifish in NBH have evolved a heritable resistance to the effects of DL PCBs, including little or no induction of CYP1A by DL PCBs or other Ahr agonists (Bello et al., 2001; Nacci et al., 1999). In addition to the DL PCBs, killifish in NBH are also exposed to extremely high levels of NDL PCBs (Gräns et al., 2015; Lake et al., 1995), among the highest in the world (Martinez et al., 2017).
Despite severely high body-burdens of PCBs, the NBH killifish population survive and reproduce, which implies that they have developed a resistance to the adverse effects of NDL PCBs in addition to DL PCBs. However, the molecular mechanisms involved in PCB resistance are not known.
In some mammals, NDL PCBs activate the pregnane-X-receptor (Pxr) and/or the constitutive androstane receptor (Car) and induce the expression of Cyp3a and Cyp2 genes (Al-Salman and Plant, 2012; Jacobs et al., 2005; Kopec et al., 2010). Some mammalian Pxr agonists can activate zebrafish Pxr in vitro (Bainy et al., 2013), but whether NDL PCBs act through similar pathways in killifish is unclear. In a previous study, Pxr and Cyp3a mRNA levels in liver of killifish from either NBH or Scorton Creek (SC) in MA (a reference site), showed either slight or no response to laboratory exposures to the NDL congener PCB153 (Gräns et al., 2015). In contrast, gills of killifish from SC treated with PCB153 showed elevated levels of both Pxr and Cyp3a mRNAs, while Pxr and Cyp3a mRNA levels in gills of fish from NBH did not show an effect of PCB153 (Gräns et al., 2015). As expected, the DL PCB126 did elicit strong induction of Cyp1a mRNA, in both gills and livers of SC fish but not in NBH fish.
In the previous study (Gräns et al. 2015), the fish had been collected from the wild, and while they had been held for a few months in the laboratory before being exposed to PCB153 or PCB126. Previous work indicated that the fish from NBH would still retain extremely high levels of PCBs, including PCB153 (Nacci, D., unpublished). Those PCBs already present in the NBH fish weakened inferences about the effects of added PCB153, and could obscure actual differences in effect between these two populations. In the present study, we examined PCB153 effects on Pxr and Cyp3a in NBH and SC killifish one or two generations removed from the wild, with no residual body-burdens of PCBs. The aim of this study was to examine the effects of PCB153 on Cyp3a56 expression in lab-reared killifish from the NBH and SC populations. We focused on gills based on previous results, where the response in SC fish pointed to a molecular response to PCB153. The absence of such a response in NBH fish suggested an adaptation to NDL PCBs in that population (Gräns et al., 2015). A response to PCB153 in gills also could suggest a biomarker for exposure to NDL PCBs in fish populations that are not resistant to PCBs.
2. Materials and Methods
2.1. Chemicals
PCB126 (3,3’,4,4’,5-pentachlorobiphenyl) and PCB153 (2,2’,4,4’,5,5’-hexachlorobiphenyl) were purchased from Ultra Scientific, Kingston, RI, USA.
2.2. Fish collection and breeding
Adult killifish were collected in the summer of 2010 from NBH. These fish (denoted NBH F0 generation) were transported to the Atlantic Coastal Environmental Science Division of the United States Environmental Protection Agency (US-EPA), Narragansett in Rhode Island (RI; USA), and were maintained in the lab until 2012. Embryos (F1 generation) were collected from adult NBH F0 generation fish during free spawning events in the summer of 2012 and raised to maturity. Embryos (F2 generation) were collected from those F1 fish during free spawning events in the summer of 2015 and raised to maturity. In December 2017, a subset of these F2 fish was selected based on size similarity and used in this study. Adult killifish were collected in the summer of 2015 from the reference site SC and maintained in the US-EPA lab (denoted SC F0 generation). Embryos (F1 generation) were collected from those F0 fish during free spawning events in the summer of 2015 and raised to maturity. In December 2017, a subset of these F1 fish was selected based on size similarity and used in this study. All adult fish were held in ambient temperature flowing seawater and fed Tetramin® flakes food ad libitum. The average body weights ± standard deviations (SD) were 5.7 ± 0.8 g for the F2 generation from NBH, and 5.3 ± 0.8 g for the F1 generation from SC. All fish were acclimated up to test temperature of 23 °C at the rate of 2 °C twice per week and held at the test temperature for two weeks prior to the initiation of this experiment (December 4, 2017).
2.3. Fish PCB exposure experiment
Fish were treated by intraperitoneal injections of PCB126 (1.38 ng/μL), PCB153 (100 ng/μL) or a mixture of both (1.38 ng/μL PCB126 + 100 ng/μL PCB153) dissolved in corn oil. Corn oil alone was used as vehicle control. The injection volume was 21.20 μl/g fish. In the SC population, there were 8 fish with a male (M) to female (F) ratio of 5:3 in vehicle treated group, 9 fish (M:F-ratio 5:4) in the PCB126 treated group, 11 fish (M:F-ratio 6:5) in the PCB153 group and 9 fish (M:F-ratio 5:4) in the mix PCB126/153 group. In the NBH population, there were 10 fish male (M):female (F) ratio 5:5 in vehicle treated group, 8 fish (M:F-ratio 4:4) in the PCB126 treated group, 10 fish (M:F-ratio 5:5) in the PCB153 group and 8 fish (M:F-ratio 5:3) in the mix PCB126/153 group. The goal of these exposures was to achieve doses of these congeners similar to those measured in livers of wild-caught fish from NBH (nominal targets of 29 ng PCB126/g fish, 2120 ng PCB153/g fish, and the mixture of both) (Gräns et al., 2015). Fish were injected December 4 and sampled three days later December 7, 2017. Fish were killed by cervical transection. Gills (gill arches and filaments) and livers were immediately dissected out. Livers were cut in three parts, one part for PCB concentration analyses and two parts for RNA analyses. Gills and liver tissues for RNA analyses were snap-frozen in liquid nitrogen and transported to the Woods Hole Oceanographic Institution and stored in −80 °C upon arrival. Procedures used in the studies were conducted under the Woods Hole Oceanographic Institution IACUC permit number BI21981.01 and US-EPA IACUC Eco23–03-002.
2.4. Isolation of total RNA, cDNA synthesis and quantitative PCR (qPCR)
Total RNA was isolated from gills and liver using the TRI Reagent and the Direct-zol RNA Mini Preps Plus kit from Zymo Research, Irvine, CA, USA. Total RNA concentrations were analyzed using a NanoDrop 2000 spectrophotometer (ThermoFisher Scientific). For cDNA synthesis, 1 μg total RNA was reverse transcribed in a 20 μl reaction volume performed using the iScript Reverse Transcription Supermix for RT-PCR from BioRad, Hercules, CA, USA. The cDNA samples were diluted with nuclease-free water to a final volume of 100 μl and stored in −80 °C prior analysis. For qPCR analysis, 2 μl of the diluted cDNA sample was transferred to a 96-well PCR-plate, and real-time PCR was carried out in a total reaction volume of 10 μl using the SsoFast EvaGreen Supermix from BioRad, and a CFX96 Real-Time System PCR machine from BioRad, Hercules, CA, USA. Two technical replicates were assayed for each fish sample and gene analyzed. Three target genes were analyzed, Cyp3a56, Pxr and Cyp1a. Three housekeeping genes were analyzed, elongation factor 1α (ef1α), β-actin and Arnt2. The annealing temperature for Pxr, Cyp3a56, ef1α, and Arnt2 was 58 °C and 55 °C for Cyp1a and /β-actin. The standard qPCR protocol was used followed by melting curve analysis to confirm single PCR products for each primer pair. The pattern of target gene expressions between the different treatment groups appeared independent of the housekeeping gene used. The PCR threshold-levels for ef1α were in the same range as that for Cyp3a56. The data presented are therefore normalized to ef1α. The primer efficiencies for Cyp3a56 and ef1α in gills were 1.00 and 0.99 respectively. The qPCR data are presented as 2-ΔCt (target gene-ef1α) ± standard deviation (SD). Although there are four different Cyp3a genes present in the killifish genome, the principal gene expressed in killifish adults is Cyp3a56 (Hegelund and Celander,2003; Goldstone, J.V., unpublished) The Cyp3a56 primers used here are specific, and do not match and will not amplify the other, minor Cyp3a genes. The qPCR primer sequences were (5’to 3’):
Cyp3a56 forward: CATGGATGTGGTAACCAGCACGG
Cyp3a56 reverse: GAAAGAAAGCGACGGCGAGGAAG
Cyp1a forward: GCCTTTCACAATCCCACACTGCTC
Cyp1a reverse: TGGGTCGTGGTTTATCTGCCACTG
Pxr forward: AAGCCTTGAGGACTTCCGGTCTC
Pxr reverse: CCCAGTTGCTGGTTGTGGAGTTG
Ef1α forward: CTCAGAGCTTCAACGCTCAGGTC
Ef1α reverse: ATCAGCTCGTTGAACTTGCAGGC
β-actin forward: TGGAGAAGAGCTACGAGCTCC
β-actin reverse: CTCGGAATGGAGTCCTGCGG
Arnt2 forward: TTCCAGGCGCTCCTTTATTTGCC
Arnt2 reverse: TAGACGGTCCCAAGCCATTCCTG
2.5. PCB analyses in livers
Individual livers from control and PCB-treated fish were analyzed for residues of PCB126 and PCB153, using established methods (Gutjahr-Gobell et al., 1999; Jayaraman et al., 2001). Calibration curves ranged from 50 to 500 ng/mL for PCB153 and 1.5 to 48 ng/mL for PCB126. Livers from four vehicle treated fish, one of each sex and population and 61 PCB treated fish of each sex and population were analyzed for PCB126 and PCB153. Two matrix spikes were prepared. The first one was spiked with 500 ng/g PCB153 and 7.50 ng/g PCB126. The recoveries were 101 and 109 for PCB153 and PCB126, respectively. The second one was spiked with 250 ng/g PCB153 and 15 ng/g PCB126. The recoveries were 92.1 and 111 for PCB153 and PCB126, respectively.
2.6. Statistics
All statistical analyses were conducted using Graph Pad Prism 8.4. The data were tested for deviation from the Gaussian ideal using the Shapiro-Wilk normality test. The qPCR data were log-transformed to achieve approximately normal distributed residuals. A three-way ANOVA was conducted to compare the factors of treatment, population, and sex and their interaction effects on gene expression. A two-way ANOVA was conducted to test for statistically significant interactions between treatments and populations. ANOVA comparisons were performed followed by a multiple comparison using Tukey correction to adjust the critical values. Statistical significance was accepted at the p < 0.05 level for two-way interactions.
3. Results
Comparisons of levels of Cyp1a, Pxr and Cyp3a56 mRNA between the two killifish populations to various PCB-treatments were performed. We focused on these few genes as they are the ones that the prior study suggested (Gräns et al., 2015), and because of data from other vertebrate species (e.g. Kopec et al., 2010). Comparisons between the two populations on Cyp1a, Pxr, and Cyp3a56 mRNA levels in gills from pooled sexes are listed in Table 1. The statistics comparing responses to treatments within each population for each sex and pooled sexes are provided as supplemental material in Table S1. The statistics comparing responses to treatment between the two populations for each sex and pooled sexes are provided as supplemental material in Tables S2.
Table 1.
Gill mRNA levels from pooled sexes of F1/F2 generation killifish.
| Treatment |
Cyp1a Mean adj. p-value |
±SD |
Pxr Mean adj. p-value |
±SD |
Cyp3a56 Mean adj. p-value |
±SD |
|---|---|---|---|---|---|---|
| Scorton Creek (SC) population – F1 Generation | ||||||
| Vehicle (n=8; M:F ratio 5:3) |
0.050 | 0.033 | 0.003 | 0.001 | 0.030 | 0.019 |
| PCB126 (n=9; M:F-ratio 5:4) |
0.275 | 0.219 | 0.002 | 0.001 | 0.052 | 0.044 |
| PCB153 (n=11; M:F-ratio 6:5) |
0.057 | 0.029 | 0.004 | 0.001 | 0.105 | 0.089 |
| Mix PCB126/153 (n=9; M:F-ratio 5:4) |
0.571 | 0.479 | 0.002 | 0.001 | 0.069 | 0.053 |
| New Bedford Harbor (NBH) population – F2 Generation | ||||||
| Vehicle (n=10; M:F-ratio 5:5) |
0.014 adj. p = 0.003 ** |
0.016 | 0.006 adj. p = 0.580 |
0.004 | 0.099 adj. p > 0.999 |
0.197 |
| PCB126 (n=8; M:F-ratio 4:4) |
0.005 adj. p < 0.001 *** |
0.003 | 0.002 adj. p = 0.997 |
0.001 | 0.025 adj. p = 0.850 |
0.021 |
| PCB153 (n=10; M:F-ratio 5:5) |
0.006 adj. p < 0.001 *** |
0.006 | 0.003 adj. p = 0.843 |
0.002 | 0.014 adj. p < 0.001 *** |
0.016 |
| Mix PCB126/153 (n=8; M:F-ratio 5:3) |
0.011 adj. p < 0.001 *** |
0.016 | 0.003 adj. p = 0.708 |
0.001 | 0.026 adj. p = 0.473 |
0.021 |
Each value represents the mean ±standard deviation (SD). The number of fish (n) are presented in parenthesis. The male (M) and female (F) ratios are provided for each treatment group. The mRNA data are presented as 2−ΔCt(target gene–ef1α) ±SD, followed by gene-specific two-way ANOVA Tukey adjusted (adj.) p-value comparing the two populations of each treatment.
p < 0.01
p < 0.001.
One female SC vehicle sample, one female SC PCB126 sample and one male NBH PCB126 sample were classified as outliers based on house keeping gene correlation analyses (ef1α vs. Arnt2 and ef1α vs. β-actin, respectively). These three fish are excluded.
As expected, when compared to vehicle control, Cyp1a mRNA levels were significantly induced in gills in F1 generation fish from SC treated with the DL congener PCB126 (F(3, 65) = 6.92; adjusted p = 0.017) as well as in fish treated with the PCB126/153 mixture (adjusted p < 0.001). These treatments did not affect Cyp1a mRNA levels in gills in F2 generation fish from NBH. No changes in Pxr or Cyp3a56 mRNA levels were observed in gills in F2 generation NBH killifish treated with the either NDL PCB153, the DL PCB126, or a mixture of the two congeners compared to vehicle control. In the F1 generation SC killifish, the mean Cyp3a56 mRNA level was more than three-times higher upon treatment with PCB153 compared to vehicle control fish, yet not significantly different (adjusted p = 0.475) due to intragroup variation (Table S1).
Next, mRNA levels in gills from pooled sexes were compared between populations that received the same treatment. The Cyp1a mRNA levels differed between SC and NBH (F(1,65) = 205; p < 0.001) for all exposure groups: Vehicle (adjusted p =0.003), PCB126 (adjusted p < 0.001), PCB153 (adjusted p < 0.001), Mix PCB126/PCB153 (adjusted p < 0.001). Despite a large variation in Cyp3a56 mRNA levels in each group, the two-way ANOVA revealed a statistically significant difference between populations (F(1,65) = 16,70; adjusted p < 0.001). In fact, Cyp3a56 mRNA levels were more than seven times in gills in SC fish treated with PCB153 compared to that in NBH fish treated with PCB153. A post-hoc analysis using Tukey adjusted alpha levels of 0.05 was performed, indicating a significant difference in Cyp3a56 mRNA levels between PCB 153-treated SC and NBH fish, adjusted p < 0.001. In contrast to Cyp3a56, levels of Pxr mRNA in gills were constant in all treatment groups in both populations (Table 1).
Intrapopulation sex differences in response to PCB153 in gills on Cyp3a56 mRNA levels were observed. Fish from NBH, treated with PCB153, had eight times lower Cyp3a56 mRNA levels in males (adjusted p = 0.724) and two times lower Cyp3a56 mRNA levels in females (adjusted p = 0.479) compared to vehicle-treated fish of the respective sex. Fish from SC, treated with PCB153, had 1.6 times higher Cyp3a56 mRNA levels in males (p > 0.999) and 5 times higher CYP3A mRNA levels in females (p = 0.012) compared to vehicle-treated fish of the respective sex (Table 2).
Table 2.
Cyp3a56 mRNA levels in gills in F1/F2 generation killifish, separated by sexes.
| Treatment |
Cyp3a56 – Males Mean (n) adj. p-value |
±SD |
Cyp3a56 – Females Mean (n) adj. p-value |
±SD |
|---|---|---|---|---|
| Scorton Creek population – F1 Generation | ||||
| Vehicle | 0.028 (n=5) | 0.005 | 0.033 (n=3) | 0.034 |
| PCB126 | 0.068 (n=5) adj. p = 0.955 |
0.053 | 0.033 (n=4) adj. p > 0.999 |
0.023 |
| PCB153 | 0.045 (n=6) adj. p > 0.999 |
0.040 | 0.177 (n=5) adj. p = 0.012 * |
0.077 |
| Mix PCB126/153 | 0.052 (n=5) adj. p = 0.999 |
0.040 | 0.090 (n=4) adj. p > 0.435 |
0.065 |
| New Bedford Harbor population – F2 Generation | ||||
| Vehicle | 0.188 (n=5) | 0.259 | 0.010 (n=5) | 0.005 |
| PCB126 | 0.013 (n=4) adj. p = 0.381 |
0.006 | 0.037 (n=4) adj. p = 0.143 |
0.024 |
| PCB153 | 0.023 (n=5) adj. p = 0.724 |
0.019 | 0.005 (n=5) adj. p = 0.479 |
0.003 |
| Mix PCB126/153 | 0.033 (n=5) adj. p = 0.857 |
0.024 | 0.014 (n=3) adj. p > 0.990 |
0.002 |
Each value represents the mean and the mRNA data are presented as 2−ΔCt(Cyp3a56–ef1α) ±standard deviation (SD). The number of fish (n) are presented in parenthesis followed by sex-specific two-way ANOVA Tukey adjusted (adj.) p-value comparing PCB treatment to vehicle control.
p < 0.05.
When comparing the two PCB153 treated populations, SC females had 35 times higher (adjusted p < 0.001) and males had two times higher (adjusted p = 0.979) Cyp3a56 mRNA levels compared to that in NBH females and males, respectively (Table S2). Levels of Cyp1a and Pxr mRNA in gills, separated by sexes, are provided in Table S3. A sex-difference was observed on Cyp1a mRNA levels, with males from SC having 6 to 13 times higher Cyp1a mRNA levels upon treatment with the DL PCB126 alone or mixed with PCB153. Elevated Cyp1a mRNA levels, albeit not statistically significant, were seen in female fish from SC treated with PCB126 alone or mixed with PCB153 (Table S3).
In addition, a three-way ANOVA was conducted to examine the effect of 1) population; 2) sex and 3) treatment on Cyp3a56 mRNA levels, showing a statistically significant three-way interaction (F(3, 57) = 6.088; adjusted p = 0.001). The population factor was most significant, adjusted p < 0.001. The differences in responses to PCB treatment between the two populations in Cyp3a56 mRNA levels and CYP1A mRNA levels in female fish are illustrated in a histobar diagram in Figure 1, and as a scatterplot in Figure S1.
Figure 1.
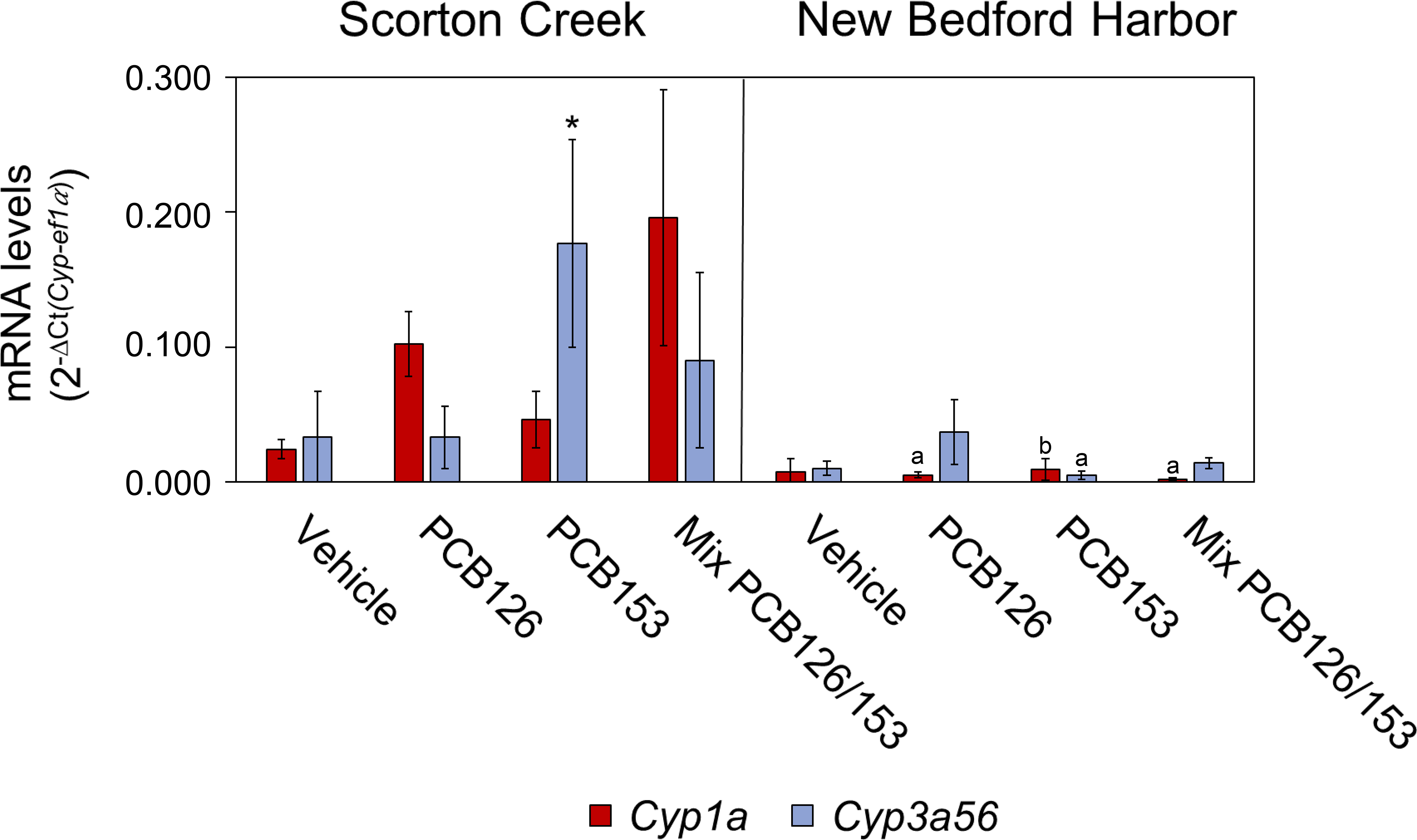
Cyp1a and Cyp3a56 mRNA levels in gills in F1 generation female killifish from Scorton Creek population and in F2 generation female fish from the New Bedford Harbor population raised and exposed in the laboratory with PCB126, PCB153 or a mixture of both congeners. Each bar represents the mean of 3 to 5 fish ± standard deviation; * p < 0.05 compared to vehicle control in the Scorton Creek population; a p < 0.001 and b p < 0.05 population comparisons to the same treatment. The statistics for exposure comparisons within each population are provided in Table S1 and for population comparisons in Table S2. A scatterplot showing Cyp3a56 mRNA versus Cyp1a mRNA in female fish from both populations and the different treatments is provided in Figure S1.
For comparison, Pxr, Cyp3a56 and Cyp1a mRNA levels also were analyzed in livers from these fish. Similar to that in gills, hepatic levels of Pxr mRNA were the same in all treatment groups in both populations. In contrast to the response in gills, the Cyp3a56 were not induced by PCB153 in livers of fish from SC or NBH. As expected, and similar to the effect in gill, CYP1A mRNA levels were induced in livers of F1 SC fish exposed to PCB126, whereas in F2 NBH fish, no increased Cyp1a expression was seen in liver (Table 3). The results with PCB126 thus confirm that the F1/F2 generations fish used in these experiments had the expected resistance to effects of DL PCBs in the NBH population.
Table 3.
Liver mRNA levels from pooled sexes of F1/F2 generation killifish.
| Treatment |
Cyp1a Mean adj. p-value |
±SD |
Pxr Mean adj. p-value |
±SD |
Cyp3a56 Mean adj. p-value |
±SD |
|---|---|---|---|---|---|---|
| Scorton Creek population – F1 Generation | ||||||
| Vehicle (n=8; M:F-ratio 4:4) |
0.047 | 0.026 | 0.003 | 0.001 | 0.142 | 0.105 |
| PCB126 (n=8; M:F-ratio 4:4) |
0.660 adj. p = 0.046 * |
0.370 | 0.004 adj. p > 0.999 |
0.002 | 0.113 adj. p = 0.993 |
0.108 |
| PCB153 (n=7; M:F-ratio 4:3) |
0.060 adj. p > 0.999 |
0.081 | 0.003 adj. p > 0.999 |
0.002 | 0.120 adj. p > 0.999 |
0.060 |
| Mix PCB126/153 (n=8; M:F-ratio 5:3) |
0.123 adj. p = 0.837 |
0.131 | 0.005 adj. p > 0.999 |
0.008 | 0.097 adj. p = 0.988 |
0.060 |
| New Bedford Harbor population – F2 Generation | ||||||
| Vehicle (n=7; M:F-ratio 3:4) |
0.170 | 0.155 | 0.014 | 0.026 | 0.239 | 0.192 |
| PCB126 (n=7; M:F-ratio 4:3) |
0.074 adj. p = 0.842 |
0.059 | 0.003 adj. p = 0.924 |
0.002 | 0.193 adj. p = 0.908 |
0.128 |
| PCB153 (n=8; M:F-ratio 4:4) |
0.082 adj. p = 0.834 |
0.090 | 0.004 adj. p > 0.999 |
0.003 | 0.196 adj. p = 0.992 |
0.113 |
| Mix PCB126/153 (n=8; M:F-ratio 5:3) |
0.071 adj. p = 0.631 |
0.065 | 0.004 adj. p = 0.956 |
0.005 | 0.200 adj. p > 0.999 |
0.088 |
Each value represents the mean ±standard deviation (SD). The number of fish (n) are presented in parenthesis. The male (M) and female (F) ratios are provided for each treatment group. Fish with non-detectable mRNA levels are excluded. The mRNA data are presented as 2−ΔCt(target gene–ef1α) ±SD, followed by gene-specific two-way ANOVA Tukey adjusted (adj.) p-value comparing PCB treatments with vehicle control.
p < 0.05.
Levels of PCB153 and PCB126 were measured in 61 of these fish, including both PCB-treated and vehicle-treated fish. A small amount of PCB153 was detected in the liver of one a F1 female fish from SC. The remaining three vehicle-treated liver samples were free of PCB153 and PCB126 (Table 4). The targeted concentration of 29 ng PCB126/g wet weight (ww) was not achieved in most fish; within treatment variation was high and many samples were below detection limit. However, average concentrations were similar between populations, ~20 ng PCB126/g ww. Similarly, within treatment variation was high for PCB153 in most fish, but the average was lower than the targeted concentration of 2120 ng PCB153/g ww in both the single congener and the mixture treatments.
Table 4.
Liver PCB126 and PCB153 concentrations in F1/F2 generation killifish.
| Treatment | PCB126 Mean |
±SD | PCB153 Mean |
±SD |
|---|---|---|---|---|
| Scorton Creek population – F1 Generation | ||||
| Vehicle – Males (n=1*) | 2.5 | 2.5 | ||
| Vehicle – Females (n=1) | 2.5 | 52.0 | ||
| PCB126 – Males (n=5; 3*) | 19.1 | 23.5 | ||
| PCB126 – Females (n=5; 2*) | 15.4 | 12.6 | ||
| PCB153 – Males (n=6) | 1384.4 | 749.5 | ||
| PCB153 – Females (n=5) | 939.7 | 344.3 | ||
| Mix PCB126/153 – Males (n=5; 2*) | 17.6 | 15.8 | 881.3 | 318.3 |
| Mix PCB126/153 – Females (n=4) | 15.6 | 3.5 | 539.4 | 201.7 |
| New Bedford Harbor population – F2 Generation | ||||
| Vehicle – Males (n=1*) | 2.5 | 2.5 | ||
| Vehicle – Females (n=1*) | 2.5 | 2.5 | ||
| PCB126 – Males (n=5; 2*) | 21.9 | 23.6 | ||
| PCB126 – Females (n=4; 3*) | 8.3 | 11.6 | ||
| PCB153 – Males (n=5) | 653.8 | 77.6 | ||
| PCB153 – Females (n=5) | 688.0 | 252.7 | ||
| Mix PCB126/153 – Males (n=5; 2*) | 12.2 | 9.8 | 615.4 | 202.8 |
| Mix PCB126/153 – Females (n=3) | 30.7 | 19.8 | 1188.4 | 775.7 |
Each value represents the mean ±standard deviation (SD) for the number of samples measured (n). The PCB concentrations are presented as ng PCB/g wet liver weight ±SD. For the sake of this calculation, samples below detection limit (n*) were assigned the values of ½ detection limit equal to 2.5 ng/g.
4. Discussion
Ortho-substituted NDL PCB congeners are legacy pollutants ubiquitous in the environment, with incompletely understood effects in humans and animals, especially in non-mammalian animals such as fish. In the present study, we focused on Cyp3a56 responses in gills in killifish exposed to the NDL PCB153. In a preceding study, F0 generation fish collected directly from the SC reference site showed an increase in expression of Pxr and Cyp3a56 in gills of fish treated with additional PCB153, an effect not seen in F0 fish collected from the PCB-polluted NBH site (Gräns et al., 2015). The environmental levels of NDL PCBs in the SC F0 generation fish are extremely low, and would not be expected to interfere with effects of additional laboratory PCB153 exposures in those fish. In contrast, F0 fish collected from NBH have extremely high levels of environmental PCB153 and other NDL PCB congeners in their tissues (Gräns et al., 2015), which could have interfered with any effect of added PCB153 in the laboratory. Hence, the body burdens of PCBs in the NBH fish reported in the preceding study draw into question interpretations about differences in response to added PCB153 between these two populations.
In the present study, we focused on killifish that were raised in the laboratory and which were one or two generations removed from the wild. The response to PCB153 observed in the F1 SC killifish gills thus confirm that this NDL PCB can indeed induce Cyp3a56 gene expression in this species. By comparison, the lack an effect of PCB153 on Cyp3a56 expression in the gills of the F2 NBH fish, without a confounding pre-existing body burden of PCBs in the experimental fish, suggests that the killifish in NBH are resistant to effects of PCB153 and possible other NDL PCBs. As these fish are two generations removed from the wild, these observations further suggest that this resistance is heritable. This appears to be much like the well-known heritable lack of induction of Cyp1a by the DL PCBs in the NBH killifish population (Bello et al., 2001; Nacci et al., 1999; Gräns et al. 2015).
The mechanism of resistance of killifish even to the DL PCBs is still not completely known, although there are significant genomic differences between resistant and non-resistant populations (Nacci et al., 2016). Laboratory and field studies have identified several genes as probable selective agents for the inherited differences observed in responses to PCBs between NBH and reference killifish. Specifically, genes associated with the Ahr signaling response pathway have been correlated with tolerance to DL PCBs in a Quantitative Trait Locus study (Nacci et al., 2016). Population genetics studies have confirmed genetic variation at these loci when NBH (and other tolerant killifish populations) are compared with reference killifish populations (Hahn et al., 2004: Proestou et al., 2014; Reitzel et al., 2014; Reid et al., 2016). In addition, Cyp3a was identified as a variant in a population genetic study that included NBH killifish (Proestou et al., 2014). However, it is currently unknown whether these concurrent genetic variations in the Ahr pathway components and Cyp3a56 in NBH killifish reflect related, unrelated, or compensatory mechanisms at play in their ability to survive in extremely PCB-contaminated environment.
In another fish species, tomcod (Microgadus tomcod), resistance to DL PCB effects involves sequence variation in the Ahr (Wirgin et al., 2011). At some level, DL PCB resistance in killifish is certain to involve the Ahr signaling pathway. Although the exact molecular mechanism is still hidden (Aluru et al., 2011; Reitzel et al., 2014), it appears to be the result of complex interactions between different components of the stress response network (Nacci et al., 2016).
The lack of an effect of PCB153 on Cyp3a56 expression in liver of F1 SC fish was somewhat surprising, given the Cyp3a56 induction in gills. However, a lack of effect of PCB153 on Cyp3a56 mRNA in liver was observed also in the SC reference population fish in the previous study in the F0 generation (Gräns et al., 2015). In addition, immunohistochemical study in killifish showed greater staining of CYP3a proteins in gills compared to that in livers (Hegelund and Celander, 2003). Such a difference could involve different abundances of some responsive cell types in the two organs, or selective expression of a receptor that may be involved. Although intriguing, the difference is not understood, but may be resolved when the mechanism of PCB153 effect on Cyp3a56 expression is identified. Increased Cyp1a induction in gills relative to liver has been observed for different Ahr ligands, suggesting that gills might be an important bio-indicator tissue (Gao et al., 2011).
In mammals, the effects of NDL PCBs on expression of Cyp genes have been linked to the Pxr, and to the related constitutive androstane receptor (Car; NR113) (Gahrs et al., 2013). Teleost fish do not possess a gene for Car(e.g. Handschin et al., 2004). In zebrafish Pxr has been implicated in regulation of the expression of Cyp3a genes and Pxr itself, by a known mammalian Pxr agonist, pregnenolone (Kubota et al., 2015; Salanga et al., 2020). Also in zebrafish, both the Ahr and Pxr are involved in regulation of Cyp3a65 (Kubota et al., 2015), the ortholog of killifish Cyp3a56. The lack of effect of PCB126 on Cyp3a56 in SC fish gill suggests that Ahr is not involved in Cyp3a56 regulation in killifish, at least not in in gills. This hypothesis is supported by the lack of any xenobiotic (dioxin) response elements (XRE/DRE) within 2kb of the start site of Cyp3a56 gene, and only 2 DRE sequences in the 5kb cis regulatory region, in distinct contrast to zebrafish (Zeruth and Pollenz, 2007). The lack of a clear-cut effect of PCB153 on Pxr expression in gills further suggests that the regulation is different from the action of pregnenolone in zebrafish. Additional unpublished data also suggest that Pxr is not the primary regulator of transcriptional responses to PCB153 in zebrafish (Goldstone et al., 2019).
As with the organ differences in response to PCB153, the basis for the sex difference showing a stronger PCB effect in females in the F1 SC fish is unknown. However, both PCB153 and PCB126 have been shown to affect hormonal function in other systems (e.g. Sechman et al., 2016), and the NBH environment itself has been reported to be estrogenic (Graytak and Callard, 2007) Thus, some hormonal pathway also may be involved in the effect of PCB153 on Cyp3a56 expression.
There may be other molecular adaptions to the NDL PCBs in NBH fish. Unlike the DL PCBs, the NDL PCBs are not acutely toxic. However, the levels of NDL PCBs in F0 NBH fish (up to 118 000 μg/g dry weight (Fritsch et al., 2015) are as high as those that can activate and severely disrupt ryanodine receptor (RyR) (intracellular calcium channel) function (Holland et al., 2017a) causing toxicity in animals. The fact that the NBH fish are a reproducing population implies that there is adaptation in a variety of other pathways, including some involving the RyRs. Although there are some NBH-SC population differences in RyR pathways (Fritsch et al., 2015; Holland et al., 2017b), we do not expect these to be related to the response in Cyp3a56 seen in the present study. Identifying the receptors involved in ortho-PCB-induced effects and the roles of target genes is essential to a full understanding of ortho-PCB toxicity, and the diversity of systems that may be involved.
Biomarker implications
For many years the induction of Cyp1a gene expression has been used to indicate exposure to biologically significant levels of Ahr agonists, including the DL PCB congeners. Simple gene expression biomarkers of exposure to NDL PCBs have been lacking. The expression of Cyp3a56 in gills may be such a biomarker in fish populations that have not developed resistance to PCBs exposures on Ahr-Cyp1a signaling. Induction of Cyp1a in gills has long been known (Miller et al., 1989), and the gill tissue has been suggested as a relevant organ for biomarker analysis (Husøy et al., 1994; Jönsson et al., 2004). The gills are good targets for direct exposures in the water as well as exposure by the diet, or even experimentally via injection, as in this study. There could be different cell types involved, although pillar cells (endothelial cells) are likely to be involved, as is the case with Cyp1a (Jönsson et al., 2004; Miller et al., 1989).
There are still unanswered questions about the results here, regarding the sex difference in the responses to PCBs, and the difference between gills and liver. Until the mechanism of Cyp3a56 induction by the NDL PCB153 is identified, the mechanism of resistance to that effect in killifish from NBH will remain unknown. Further specifics are needed on the regulation of Cyp3a56 in killifish, addressable in part through genomic comparisons of the wild-type and PCB-resistant populations. Such questions are to be addressed in further studies.
Supplementary Material
Acknowledgments
This study was supported by the sabbatical program from the Faculty of Science at the University of Gothenburg (MC), and by the Swiss National Science Foundation P2EZP2–165200 (NRB). The study was supported in part by the Superfund Hazardous Substances Research Program at Boston University NIH P42ES007381 (JVG, JJS). This research was also funded partly by the US Environmental Protection Agency (SJ, DN), including an appointment (BC) with the Postdoctoral Research Program at the US Environmental Protection (US-EPA) Office of Research and Development administered by the Oak Ridge Institute for Science and Education (ORISE), through Interagency Agreement No. DW92429801 between the US Department of Energy and the US-EPA. The contents do not reflect the views of the US-EPA, nor does mention of trade names or commercial products constitute endorsement or recommendation for use by the US-EPA. We thank Rene Francolini at the Woods Hole Oceanographic Institutions for excellent technical assistance and Dr. Sibel Karchner and Dr. Mark Hahn at the Woods Hole Oceanographic Institutions for valuable discussions and comments on the manuscript.
References
- Al-Salman F, Plant N, 2012. Non-coplanar polychlorinated biphenyls (PCBs) are direct agonists for the human pregnane-X receptor and constitutive androstane receptor, and activate target gene expression in a tissue-specific manner. Toxicol. Appl. Pharmacol 263, 7–13. 10.1016/j.taap.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Aluru N, Karchner SI, Hahn ME, 2011. Role of DNA methylation of AHR1 and AHR2 promoters in differential sensitivity to PCBs in Atlantic Killifish, Fundulus heteroclitus. Aquat. Toxicol 101,288–294. 10.1016/j.aquatox.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainy AC, Kubota A, Goldstone JV, Lille-Langøy R, Karchner SI, Celander MC, Hahn ME, Goksøyr A, Stegeman JJ, 2013. Functional characterization of a full length pregnane X receptor, expression in vivo, and identification of PXR alleles, in zebrafish (Danio rerio). Aquat. Toxicol 142–143, 447–457. 10.1016/j.aquatox.2013.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello SM, Franks DG, Stegeman JJ, Hahn ME, 2001. Acquired resistance to Ah receptor agonists in a population of Atlantic killifish (Fundulus heteroclitus) inhabiting a marine superfund site: in vivo and in vitro studies on the inducibility of xenobiotic metabolizing enzymes. Toxicol. Sci 60, 77–91. 10.1093/toxsci/60.1.77. [DOI] [PubMed] [Google Scholar]
- Elskus AA, Monosson E, McElroy AE, Stegeman JJ, Woltering DS, 1999. Altered CYP1A expression in Fundulus heteroclitus adults and larvae: a sign of pollutant resistance? Aquat. Toxicol 45, 99–113. 10.1016/S0166-445X(98)00102-7. [DOI] [Google Scholar]
- Förlin L, Celander M, 1995. Studies of the inducibility of P450 1A in perch from the PCB-contaminated Lake Järnsjön in Sweden. Mar. Environ. Res 85–88. 10.1016/0141-1136(94)00029-O. [DOI] [Google Scholar]
- Fritsch EB, Stegeman JJ, Goldstone JV, Nacci DE, Champlin D, Jayaraman S, Connon RE, Pessah IN, 2015. Expression and function of ryanodine receptor related pathways in PCB tolerant Atlantic killifish (Fundulus heteroclitus) from New Bedford Harbor, MA, USA. Aquat. Toxicol 159, 156–166. 10.1016/j.aquatox.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahrs M, Roos R, Andersson PL, Schrenk D, 2013. Role of the nuclear xenobiotic receptors CAR and PXR in induction of cytochromes P450 by non-dioxinlike polychlorinated biphenyls in cultured rat hepatocytes. Toxicol. Appl. Pharmacol 272, 77–85. 10.1016/j.taap.2013.05.034 [DOI] [PubMed] [Google Scholar]
- Gao K, Brandt I, Goldstone JV, Jönsson M 2011. Cytochrome P450 1A, 1B, and 1C mRNA induction patterns in three-spined stickleback exposed to a transient and a persistent inducer. Comp. Biochem. Physiol. Part C 154, 42–55. 10.1016/j.cbpc.2011.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstone JV, Brun NR, Lemaire B, Stegeman JJ, 2019. Transcriptomic analyses of ortho-PCB exposures in larval zebrafish. Toxicological Sciences 168, Abstract 1201.https://www.toxicology.org/pubs/docs/Tox/2019Tox.pdf
- Gräns J, Wassmur B, Fernandez-Santoscoy M, Zanette J, Woodin BR, Karchner SI, Nacci DE, Champlin D, Jayaraman S, Hahn ME, Stegeman JJ, Celander MC, 2015. Regulation of pregnane-X-receptor, CYP3A and P-glycoprotein genes in the PCB-resistant killifish (Fundulus heteroclitus) population from New Bedford Harbor. Aquat. Toxicol 159, 198–207. https://doi.org/10.1016Zj.aquatox.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graytak SR, Callard GV, 2007. Cloning of three estrogen receptors (ER) from killifish (Fundulus heteroclitus): Differences in populations from polluted and reference environments. Gen. Comp. Endocrinol 150, 174–188. 10.1016/j.ygcen.2006.07.017 [DOI] [PubMed] [Google Scholar]
- Gutjahr-Gobell RE, Black DE, Mills LJ, Pruell RJ, Taplin BK, Jayaraman S, 1999. Feeding the mummichog (Fundulus heteroclitus ) a diet spiked with non-ortho- and mono-ortho-substituted Polychlorinated biphenyls: Accumulation and effects. Environ. Toxicol. Chem. 18, 699–707. 10.1002/etc.5620180416. [DOI] [Google Scholar]
- Hahn ME, Karchner SI, Franks DG, Merson RR 2004. Aryl hydrocarbon receptor polymorphisms and dioxin resistance in Atlantic killifish (Fundulus heteroclitus). Pharmacogenetics 14, 131–143. 10.1097/00008571-200402000-00007 [DOI] [PubMed] [Google Scholar]
- Handschin C, Blättler S, Roth A, Looser R, Oscarson M, Kaufmann MR, Podvinec M, Gnerre C, Meyer UA 2004. The evolution of drug-activated nuclear receptors: one ancestral gene diverged into two xenosensor genes in mammals. Nucl. Recept,2, 7. https://doi: 10.1186/1478-1336-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegelund T, Celander MC, 2003. Hepatic versus extrahepatic expression of CYP3A30 and CYP3A56 in adult killifish (Fundulus heteroclitus). Aquat. Toxicol 64, 277–291. 10.1016/S0166-445X(03)00057-2 [DOI] [PubMed] [Google Scholar]
- Holland EB, Feng W, Zheng J, Dong Y, Li X, Lehmler HJ, Pessah IN, 2017a. An Extended Structure-Activity Relationship of Nondioxin-Like PCBs Evaluates and Supports Modeling Predictions and Identifies Picomolar Potency of PCB 202 Towards Ryanodine Receptors. Toxicol. Sci 155, 170–181. 10.1093/toxsci/kfw189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland EB, Goldstone JV, Pessah IN, Whitehead A, Reid NM, Karchner SI, Hahn ME, Nacci DE, Clark BW, Stegeman JJ, 2017b. Ryanodine receptor and FK506 binding protein 1 in the Atlantic killifish (Fundulus heteroclitus): A phylogenetic and population-based comparison. Aquat. Toxicol 192, 105–115. 10.1016/j.aquatox.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husøy AM, Myers MS, Willis ML, Collier TK, Celander M, Goksøyr A, 1994. Immunohistochemical localization of CYP1A and CYP3A-like isozymes in hepatic and extrahepatic tissues of Atlantic cod (Gadus morhua L.), a marine fish. Toxicol. Appl. Pharmacol 129, 294–308. 10.1006/taap.1994.1254. [DOI] [PubMed] [Google Scholar]
- Jacobs MN, Nolan GT, Hood SR, 2005. Lignans, bacteriocides and organochlorine compounds activate the human pregnane X receptor (PXR). Toxicol. Appl. Pharmacol 209, 123–133. 10.1016/j.taap.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Jayaraman S, Pruell RJ, McKinney R, 2001. Extraction of organic contaminants from marine sediments and tissues using microwave energy. Chemosphere 44, 181–191. 10.1016/S0045-6535(00)00201-0. [DOI] [PubMed] [Google Scholar]
- Jonsson ME, Brunström B, Ingebrigtsen K, Brandt I, 2004. Cell-specific CYP1A expression and benzo[a]pyrene adduct formation in gills of rainbow trout (Oncorhynchus mykiss) following CYP1A induction in the laboratory and in the field. Environ. Toxicol. Chem 23, 874–882. 10.1897/03-211. [DOI] [PubMed] [Google Scholar]
- Kopec AK, Burgoon LD, Ibrahim-Aibo D, Mets BD, Tashiro C, Potter D, Sharratt B, Harkema JR, Zacharewski TR, 2010. PCB153-elicited hepatic responses in the immature, ovariectomized C57BL/6 mice: comparative toxicogenomic effects of dioxin and non-dioxin-like ligands. Toxicol. Appl. Pharmacol 243, 359–371. 10.1016/j.taap.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota A, Goldstone JV, Lemaire B, Takata M, Woodin BR, Stegeman JJ, 2015. Role of pregnane X receptor and aryl hydrocarbon receptor in transcriptional regulation of pxr, CYP2, and CYP3 genes in developing zebrafish. Toxicol. Sci 143, 398–407. 10.1093/toxsci/kfu240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake JL, McKinney R, Lake CA, Osterman FA, Heltshe J, 1995. Comparisons of patterns of polychlorinated biphenyl congeners in water, sediment, and indigenous organisms from New Bedford Harbor, Massachusetts.. Arch. Environ. Contam. Toxicol 29, 207–220. 10.1007/BF00212972. [DOI] [Google Scholar]
- Martinez A, Hadnott BN, Awad AM, Herkert NJ, Tomsho K, Basra K, Scammell MK, Heiger-Bernays W, Hornbuckle KC, 2017. Release of Airborne Polychlorinated Biphenyls from New Bedford Harbor Results in Elevated Concentrations in the Surrounding Air. Environ. Sci. Technol. Lett 4, 127–131. 10.1021/acs.estlett.7b00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MM, Hinton DE, Stegeman JJ, 1989. Cytochrome P450E induction and localization in gill pillar (endothelial) cells of scup and rainbow trout. Aquat. Toxicol 14, 307–322. 10.1016/0166-445X(89)90029-5 [DOI] [Google Scholar]
- Nacci D, Coiro L, Champlin D, Jayaraman S, McKinney R, Gleason TR, W.R.;, Specker MJ, Cooper JL, K.R., 1999. Adaptations of wild populations of the estuarine fish Fundulus heteroclitus to persistent environmental contaminants. Mar. Biol 134, 9–17. 10.1007/s002270050520. [DOI] [Google Scholar]
- Nacci D, Proestou D, Champlin D, Martinson J, Waits ER, 2016. Genetic basis for rapidly evolved tolerance in the wild: adaptation to toxic pollutants by an estuarine fish species. Mol. Ecol 25, 5467–5482. 10.1111/mec.13848 [DOI] [PubMed] [Google Scholar]
- Nacci DE, Champlin D, Jayaraman S, 2010. Adaptation of the Estuarine Fish Fundulus heteroclitus (Atlantic Killifish) to Polychlorinated Biphenyls (PCBs). Estuaries and Coasts 33, 853–864. https://doi.org/10.1007Zs12237-009-9257-6. [Google Scholar]
- Proestou DA, Flight P, Champlin D, Nacci D, 2014. Targeted approach to identify genetic loci associated with evolved dioxin tolerance in Atlantic killifish (Fundulus heteroclitus). BMC Evol. Biol 14, 7. 10.1186/1471-2148-14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid NM, Proestou DA, Clark BW, Warren WC, Colbourne JK, Shaw JR, Karchner SI, Hahn ME, Nacci D, Oleksiak MF, Crawford DL, Whitehead A, 2016. The genomic landscape of rapid repeated evolutionary adaptation to toxic pollution in wild fish. Science. 354, 1305–1308. 10.1126/science.aah4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitzel AM, Karchner SI, Franks DG, Evans BR, Nacci D, Champlin D, Vieira VM, Hahn ME, 2014. Genetic variation at aryl hydrocarbon receptor (AHR) loci in populations of Atlantic killifish (Fundulus heteroclitus) inhabiting polluted and reference habitats. BMC Evol. Biol 14, 6. 10.1186/1471-2148-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanga MC, Brun NR, Francolini RD, Stegeman JJ, Goldstone JV, 2020. CRISPR-Cas9-mutated pregnane X receptor (pxr) retains pregnenolone-induced expression of cyp3a65 in zebrafish (Danio rerio) larvae. Toxicol Sci 174, 51–62. 10.1093/toxsci/kfz246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sechman A, Batoryna M, Antos PA, Hrabia A, 2016. Effects of PCB 126 and PCB 153 on secretion of steroid hormones and mRNA expression of steroidogenic genes (STAR, HSD3B, CYP19A1) and estrogen receptors (ERα, ERβ) in prehierarchical chicken ovarian follicles. Toxicol. Letters 264, 29–37. 10.1016/j.toxlet.2016.11.001 [DOI] [PubMed] [Google Scholar]
- Stegeman JJ, Lech JJ, 1991. Cytochrome P-450 monooxygenase systems in aquatic species: carcinogen metabolism and biomarkers for carcinogen and pollutant exposure. Environ Health Perspect 90, 101–109. 10.1289/ehp.90-1519513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirgin I, Roy NK, Loftus M, Chambers RC, Franks DG, Hahn ME, 2011. Mechanistic basis of resistance to PCBs in Atlantic tomcod from the Hudson River. Science. 331, 1322–1325. 10.1126/science.1197296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirgin I, Waldman JR, 2004. Resistance to contaminants in North American fish populations. Mutat. Res 552, 73–100. 10.1016/j.mrfmmm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Zeruth G, Pollenz RS, 2007, Functional analysis of cis-regulatory regions within the dioxin-inducible CYP1A promoter/enhancer region from zebrafish (Danio rerio). Chem. Biol. Interact 170, 100–113. 10.1016/j.cbi.2007.07.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


