(Cell 184, 3086–3108; June 10, 2021)
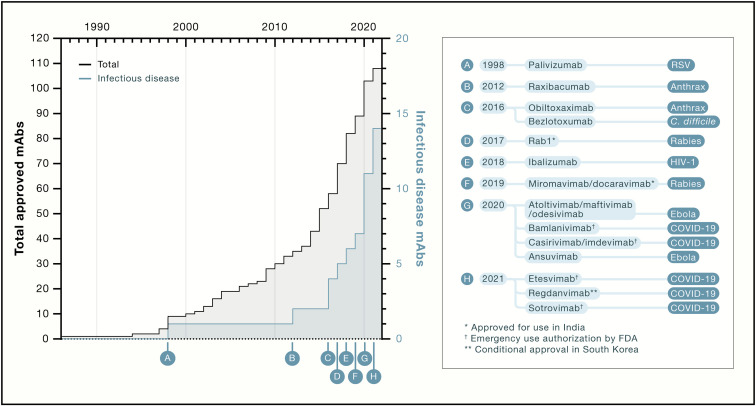
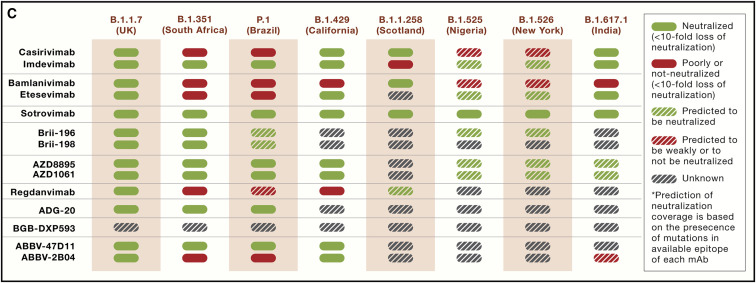
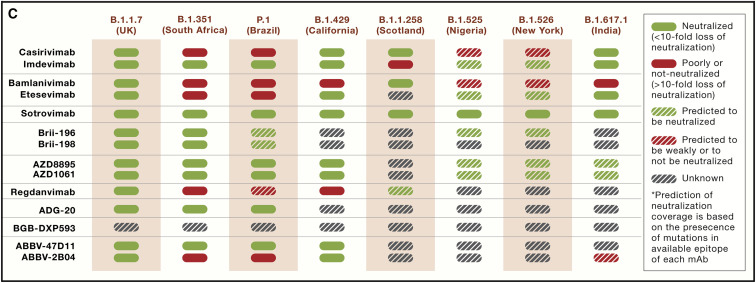
During the preparation of the figures for this review, “etesevimab” was mistakenly written as “etesvimab” in Figure 1, and Figure 5C inadvertently indicated the solid red box meant <10-fold loss of neutralization when it should have indicated >10-fold loss. In addition, in Table 1, EUA for VIR-7831/VIR-7832 was indicated for trial NCT04501978 (late treatment), but it should have been indicated for trial NCT04545060 (early treatment). These errors do not affect any results or conclusions described in the review and have been corrected online, and we apologize for any confusion they may have caused.
Figure 1.
Timeline of approval of mAbs for all indications (black) and for infectious disease (blue) (original)
Figure 5C.
Mutations on the SARS-CoV-2 S in VOCs and resistance profile of clinical mAbs (original)
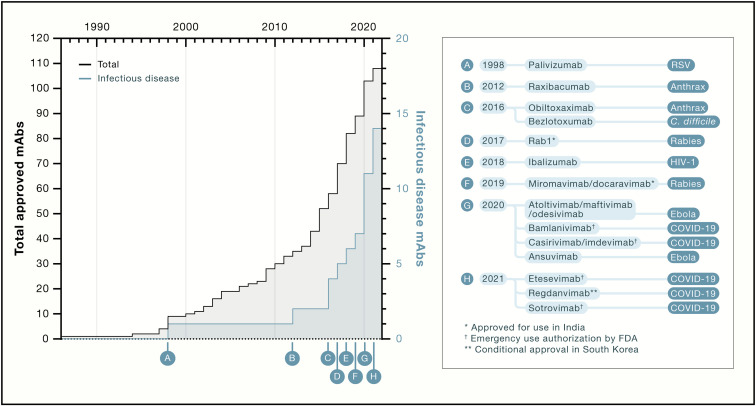
Figure 1.
Timeline of approval of mAbs for all indications (black) and for infectious disease (blue) (corrected)
Figure 5C.
Mutations on the SARS-CoV-2 S in VOCs and resistance profile of clinical mAbs (corrected)