Abstract
Objective:
Comprehensive neuropsychological criteria (NP criteria) for mild cognitive impairment (MCI) has reduced diagnostic errors and better predicted progression to dementia than conventional MCI criteria that rely on a single impaired score and/or subjective report. This study aimed to implement an actuarial approach to classifying MCI in the Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) study.
Method:
ACTIVE study participants (N=2755) were classified as cognitively normal (CN) or as having MCI using NP criteria. Estimated proportion of MCI participants and reversion rates were examined as well as baseline characteristics by MCI subtype. Mixed effect models examined associations of MCI subtype with 10-year trajectories of self-reported independence and difficulty performing instrumental activities of daily living (IADLs).
Results:
The proportion of MCI participants was estimated to be 18.8%. Of those with MCI at baseline, 19.2% reverted to CN status for all subsequent visits. At baseline, the multidomain-amnestic MCI group generally had the greatest breadth and depth of cognitive impairment and reported the most IADL difficulty. Longitudinally, MCI participants showed faster IADL decline than CN participants (multidomain-amnestic MCI > single domain-amnestic MCI > nonamnestic MCI).
Conclusions:
NP criteria identified a proportion of MCI and reversion rate within ACTIVE that is consistent with prior studies involving community-dwelling samples. The pattern of everyday functioning change suggests that being classified as MCI, particularly amnestic MCI, is predictive of future loss of independence. Future work will apply these classifications in ACTIVE to better understand the relationships between MCI and health, social, and cognitive intervention-related factors.
Keywords: mild cognitive impairment, diagnostic criteria, everyday functioning, longitudinal trajectories, cognitive aging
Introduction
Mild Cognitive Impairment (MCI) is a construct thought to represent the prodromal stage of dementia (Petersen et al., 1999; Winblad et al., 2004). While the field is moving toward earlier identification of those at risk for Alzheimer’s disease (AD) prior to showing clinical symptoms of cognitive impairment, the need for sensitive yet reliable diagnostic criteria for the clinical syndrome of MCI remains a priority. Recent statistics show that if all individuals alive in 2018 who will develop Alzheimer’s disease were diagnosed in the MCI stage, rather than after progression to dementia, there could be a cost savings of up to $7.9 trillion (Alzheimer’s Association, 2019). This savings would likely be due to lower medical/long-term care costs and lower costs immediately before and after the diagnosis if it is made during the MCI stage compared to spike in costs surrounding a diagnosis first made during the dementia phase. Although the advent of AD biomarkers, such as amyloid and tau PET and biofluid markers, has contributed to significant gain in terms of research on AD pathogenesis, these approaches are often expensive, invasive, inaccessible due to location (e.g., rural settings), cost, or medical contraindications (e.g., on blood thinners), and many are still in experimental phases in regard to understanding the possible implications of “positive” findings, particularly in a clinical context (Glymour et al., 2018). Therefore, there is a significant need to for early and accurate characterization of cognitive changes and a reliable method for MCI diagnosis.
Prevalence rates of MCI vary widely across samples and method of diagnosis (Ganguli et al., 2010; Jak et al., 2009; Manly et al., 2008; Petersen et al., 2010). Sachdev and colleagues (Sachdev et al., 2015) examined the prevalence of MCI across 11 studies from Australia, Asia, Europe, and USA and found published MCI prevalence estimates that ranged from 5.0%−36.7%. Jak and colleagues (Jak et al., 2009) demonstrated that comprehensive neuropsychological criteria (NP criteria) offer an optimal balance of sensitivity and reliability by pairing a more liberal >1 standard deviation (SD) cut-off for impairment (rather than a 1.5–2SD cut-off) with the need for at least two impaired test scores within one cognitive domain. These criteria have now been applied to large, aging cohorts including the Alzheimer’s Disease Neuroimaging Initiative (Bondi et al., 2014; Edmonds et al., 2015; Thomas et al., 2019a) and the Framingham Heart study (Jak et al., 2016; Wong et al., 2018).
Application of the NP criteria method to MCI classification in ADNI and Framingham have demonstrated that, relative to conventional MCI criteria that require only one test (often only memory) to be >1.5 SD below the mean, the NP criteria approach improves the accuracy of MCI classification, particularly in regard to reducing the number of “false positive” MCI diagnoses. Within ADNI, approximately one-third of participants diagnosed as MCI using criteria similar to conventional criteria (Petersen et al., 2010) were found to be cognitively normal based on NP criteria (Bondi et al., 2014; Edmonds et al., 2015). In addition to normal cognitive profiles despite their MCI diagnosis, these participants also demonstrated MRI (Edmonds et al., 2016), amyloid PET (Bangen et al., 2016), CSF biomarker profiles (Edmonds et al., 2015; Eppig et al., 2017), and everyday functioning trajectories (Thomas et al., 2017) more consistent with a cognitively normal classification than an MCI classification.
The Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) study is a randomized control trial of cognitive training in older adults in which participants were randomized into one of four groups (memory-trained, reasoning-trained, speed-trained, no-contact control) and followed longitudinally for 10 years to assess cognitive, functional, and health-related outcomes (Ball et al., 2002; Jobe et al., 2001; Rebok et al., 2014; Willis et al., 2006). Participants in ACTIVE were community-dwelling and 26% of the sample identified as Black/African American. ACTIVE was not designed initially to determine clinical neurocognitive diagnoses, so there is need for a reliable, algorithmic approach to classify prevalent and incident cases of MCI. Prior work has examined different algorithmic methods for classifying cognitive impairment within ACTIVE, including focusing only on those participants with memory impairment on a word-list learning test (Unverzagt et al., 2007) or use of cognitive domain composite scores to determine MCI classification (Cook et al., 2013). However, this is the first time the well-validated NP criteria (Bondi et al., 2014; Jak et al., 2009) have been applied in ACTIVE and the first time any MCI criteria have been applied through the Year 10 ACTIVE study visit. The intent of this study is to test the application of the NP criteria in ACTIVE and assess the ten-year trajectories by MCI classification. Establishment of a widely-accepted MCI classification criteria in ACTIVE will facilitate future studies to improve our understanding of ACTIVE’s cognitive training effects among participants classified as MCI or whether cognitive training reduces risk of future MCI classification.
The present study has three goals. First, we will apply NP criteria to classify participants as cognitively normal (CN) or as having MCI and examine stability, progression, and reversion of the classifications over time. Second, we will describe demographic, health, and functional characteristics by cognitive status (CN, MCI subtypes). Third, we will investigate differences in longitudinal everyday functioning trajectories between persons classified as having MCI relative to CN participants at baseline to test the hypothesis that those we classified as having MCI would experience faster decline in everyday functioning.
Methods
Participants
Participants from the ACTIVE study at baseline who had sufficient cognitive test data for a determination of CN or MCI status were included in analyses (N=2755). Detailed inclusion criteria for ACTIVE has been described elsewhere (Jobe et al., 2001). Briefly, participants were included if they had a score of >23 on the Mini-Mental State Exam (MMSE), no prior diagnosis of dementia, and intact basic activities of daily living such as bathing, dressing, etc. Participants in ACTIVE were recruited from six sites throughout the United States (University of Alabama at Birmingham, Hebrew Rehabilitation Center for Aged in Boston, Indiana University, Johns Hopkins University, Wayne State University, and Pennsylvania State University); all procedures were approved by the Institutional Review Boards, in compliance with the Helsinki Declaration, and informed consent was obtained prior to participation. Each participant was randomized to receive one of three 10-week cognitive training interventions (memory, reasoning, or speed) or to a no-contact control group. Participants had assessments at baseline, immediately post-intervention, and 1-, 2-, 3-, 5, and 10-years after the initial visit. Consistent with other ACTIVE papers (e.g., Marsiske et al., 2013) and given the very small number of participants who identified as a race other than White or Black/African American (n=20), the current analyses excluded these 20 participants. The number of participants at each occasion was determined based on the number of individuals with a baseline classification of either CN or MCI and with instrumental activities of daily living (IADL) data, as this was the longitudinal outcome of interest (baseline N=2755; Year 1 N=2249; Year 2 N=2168; Year 3 N=2050; Year 5 N=1831; Year 10 N=1194).
Materials
Cognitive Measures.
Seven cognitive total test scores were used to determine MCI status in this study and included three memory measures: Hopkins Verbal Learning Test (HVLT) immediate free recall, Rey Auditory Verbal Learning Test (AVLT) immediate free recall and AVLT delayed recognition (hits - false positives + 35); two reasoning measures: Word Series and Letter Sets total correct; and two speed of processing measures: Digit Symbol Substitution total correct and Useful Field of View (UFOV) Task 2. In ACTIVE, the delayed recall trials of the memory tests were not administered; however, the AVLT recognition trial was administered after a delay. Additionally, written responses, rather than verbal responses were used for the memory tests, as this allowed some of the tests to be administered in a group setting. The inductive reasoning measures required the participant to identify patterns and determine what answer would come next in the series. UFOV Task 2 requires the participant to identify a target of either a truck or a car that is presented at a central fixation point on the screen and to identify the location of a peripheral car that appeared in one of eight locations on the screen. Scores were recorded based on the minimum stimulus duration in which the participant responded correctly 75% of the time.
Health and Clinical Variables.
Additional measures were used to characterize the MCI subtypes and compare group differences at baseline. Self-reported health measures included presence or absence of: hypertension, diabetes, number of current medications, and self-reported physical functioning from the 36-Item Short-Form Health Survey (SF-36; Ware & Sherbourne, 1992); objectively-measured health measures included: systolic and diastolic blood pressure and body mass index (BMI). The MMSE measured global cognition (Folstein et al., 1975) and the Center for Epidemiological Studies-Depression-12 scale (CES-D) assessed depressive symptom severity (Radloff, 1977).
Everyday Functioning.
The self-reported Minimum Data Set (MDS; (Morris et al., 1997) IADL Performance (i.e., how much of the activity did you do on your own?) and Difficulty (i.e., how difficult was it for you to do the activity on your own? Or, if someone else did the activity for you, how difficult would it have been for you to do the activity on your own?) subscales were examined at each study visit. The two subscales were the sums of the respective ratings for 19 daily tasks spanning meal preparation, housework, finances, health care, telephone, shopping, travel, and need for assistance in dressing, personal hygiene, and bathing. Higher scores represent less independence (Performance subscale) and more difficulty (Difficulty subscale).
MCI Classification
At baseline, participants were classified as either CN or as having MCI (since dementia was excluded at baseline). MCI classification was determined based on the comprehensive NP criteria (Bondi et al., 2014; Jak et al., 2009, 2016), which required performance of >1 SD below an age-, education-, sex-, and race-adjusted predicted score on at least two cognitive measures within the same cognitive domain (memory, reasoning, or speed). Race was coded as Black/African American or White. There were only 15 Hispanic participants (3 Black/African American and 12 White), so ethnicity was not explicitly examined.
Consistent with our previous work (Edmonds et al., 2015; Thomas et al., 2019a) and due to evidence of increased sensitivity when using a “robust” normative control group (e.g., Kramer et al., 2020), in combination with the majority of tests in ACTIVE either non having published norms and not using standardized test administration procedures for the memory tests (e.g., written responses instead of verbal), we first identified a “robust” normative control group to derive the demographically-adjusted z-scores. This normative control group was determined based on participants from the no-contact untrained group who were in the study for at least two years and, for the duration of their time in the study, maintained an MMSE score of >26 for white participants (Markwick, Zamboni, & de Jager, 2012) or >24 for Black/African American participants. Recent work suggested that a traditional cognitive screening cut-score may lack specificity in predicting cognitively normal and MCI status in African American older adults, so these cut-scores were used to reduce the likelihood of misclassifying African American participants as impaired (Rossetti et al., 2019) as well as to ensure that Black/African American participants were proportionally represented in the normative control group since this robust norming approach is only appropriate when the participants closely match the demographic profile of those in the normative group (Kramer et al., 2020). These criteria resulted in a normative control group of 253 participants (mean age=72.78, range=65–88 years; mean education=14.04, range=6–20 years; 69.6% female; 28.5% Black/African American). Second, within the normative control group, each cognitive test score was regressed on the demographic variables (age, education, sex, and race) at each occasion. The resulting regression weights were then used to produce predicted scores for each participant had they remained cognitively stable and been untrained. Third, participant z-scores were calculated based on the discrepancy between the observed and predicted scores and divided by the test-specific regression model’s standard error of the estimate. This method is largely consistent with how z-scores were determined in prior studies applying these NP criteria in ADNI (Edmonds et al., 2016; Thomas et al., 2019a).
The z-score distributions of some of the cognitive measures were non-normal, so a cutoff of <16th percentile (comparable to a z-score of < −1 for a normally distributed measure) was used to determine impairment for each test. Participants who did not meet NP criteria for MCI were considered CN.
Participants who met criteria for MCI were then classified as one of four possible subtypes (Petersen, 2004; Berndt Winblad et al., 2004), including: single domain amnestic MCI (single-aMCI) if they were impaired (i.e., 2 scores > 1 SD below age-, education-, sex-, and race-adjusted predicted score) in memory only; single domain non-amnestic MCI (single-naMCI) if they were impaired in either reasoning or speed only; multi-domain amnestic MCI (multi-aMCI) if they were impaired in memory plus reasoning and/or speed; or multi-domain non-amnestic MCI (multi-naMCI) if they were impaired in both reasoning and speed, but not memory.
Statistical Analyses
Stability and Reversion.
The stability and reversion of MCI status was examined from baseline-to-Year 2, baseline-to-Year 5, and baseline-to-Year 10. Since training booster sessions were provided to some participants just prior to Years 1 and Years 3, Year 2 was chosen as the first follow-up to examine since this would have allowed for at least a year to have elapsed since the first booster training. Year 5 was chosen as the second follow-up to examine since over two years would have elapsed since the last booster training. Year 10 stability and reversion are reported; however, by Year 10, a significant portion of those with MCI at baseline were missing data (i.e., of 518 baseline MCI participants, 142 had Year 10 IADL data), and those who remained were disproportionally the least impaired at baseline (i.e., single-naMCI). Reversion rates by cognitive intervention group were also examined.
Baseline characteristics.
Baseline demographic, health, cognitive, and everyday functioning variables for each group were examined using one-way analyses of variance (ANOVAs; with independent post-hoc t-tests), Kruskal-Wallis tests (with post-hoc Mann-Whitney U), or chi-squared tests for categorical variables. Given the large number of tests conducted and the large sample, alpha for all analyses was set to .005 (Ioannidis, 2018). An exception to this rule was for the attrition analysis (see below); to ensure a strong, well-specified model, predictors were considered for inclusion/retention if they had a p-value of <.05.
Attrition.
A forward stepwise logistic regression was conducted to determine the unique predictors of those who had available IADL data at Year 10 (Retained; N=1194) compared to those participants with missing data at Year 10 (Missing; N=1561); the Missing group included both participants who dropped out of the study and who were missing the MDS IADL measure at Year 10. Demographic variables (age, education, sex, race), intervention group (memory training, reasoning training, speed training, no-contact), whether or not they were assigned to receive booster training sessions, physical functioning, depressive symptoms, and MCI status were all considered in the model. The variables that uniquely predicted Retained status were included as covariates in the longitudinal models. Supplemental material Table 1 shows the predictors of attrition and their effects. The attrition-related covariates explained a large proportion of group differences in attrition (C=.716, p<.001). Given their relationship with attrition, age, education, sex, race/ethnicity, physical functioning, depressive symptoms, and booster training were included as covariates in the mixed effects models.
Functional Trajectories.
Ten-year longitudinal trajectories of everyday functioning (IADL Performance, IADL Difficulty) by baseline cognitive status were examined using mixed-effects models. For these analyses, the single-naMCI and multi-naMCI participants were combined into a non-amnestic (naMCI) group due to the small number of multi-naMCI participants (n=22). Variables associated with missing Year 10 data, as well as study site and replicate/cohort (consistent with other ACTIVE manuscripts to adjust for geographic and seasonal variation across participants; (Willis et al., 2006) were included as covariates in the models. Since our goal was to use these models to validate the MCI classification rather than to examine intervention effects on IADL trajectories (Rebok et al., 2014), we adjusted for both the main effect of intervention group and intervention group x time interactions in the models. Full information maximum likelihood allowed for use of all available data (Singer & Willett, 2003). The time variable included six occasion time points over 10 years (baseline, Year 1, Year 2, Year 3, Year 5, and Year 10) and was modeled as a continuous parameter. Both the linear and quadratic effects of time were examined given the potential for accelerated functional decline with worsening cognition. The random effects of intercept and linear slope were included in the model.
Results
Prevalence
At baseline, of the 2755 participants with sufficient data to be classified, 518 (18.8%) met NP criteria for MCI. Within the MCI group, 225 (43.4%) were single-aMCI, 164 (31.7%) were single-naMCI, 107 (20.7%) were multi-aMCI, and 22 (4.2%) were multi-naMCI. Within the single-naMCI group, 89 (54.3%) were classified based on impairment in the reasoning domain and 75 (45.7%) were classified based on impairment in the speed domain. Within the multi-aMCI group, in addition to being impaired in the memory domain, 35 (32.7%) participants were impaired in reasoning only, 46 (43.0%) were impaired in speed only, and 26 (24.3%) were impaired in both reasoning and speed.
Stability and Reversion
Figure 1 shows the stability and reversion of participants’ classification at 2, 5, and 10-year intervals. There was no clinical dementia diagnostic evaluation within ACTIVE at follow-up visits; thus, the term generic ‘cognitively impaired’ was used to identify those that met NP criteria for MCI at follow-up visits since it is uncertain whether participants had MCI or would be more appropriately classified as probable dementia. There were 1928 participants with classifications at both baseline and Year 2 (CN n=1634, MCI n=294). Of the 1634 participants who were unimpaired at baseline, 164 (10.0%) progressed to cognitive impairment at Year 2. Of the 294 participants classified as MCI at baseline, 198 (67.3%) remained cognitively impaired at Year 2 and 96 (32.7%) reverted to CN status. At Year 2, participants who received cognitive training did not revert to CN at a higher rate (31.0%) than untrained participants (36.9%; χ2=0.97, df=1, p=.326) and none of the individual training groups had a higher rate of reversion than the untrained group (χ2=1.145, df=3, p=.766).
Figure 1.
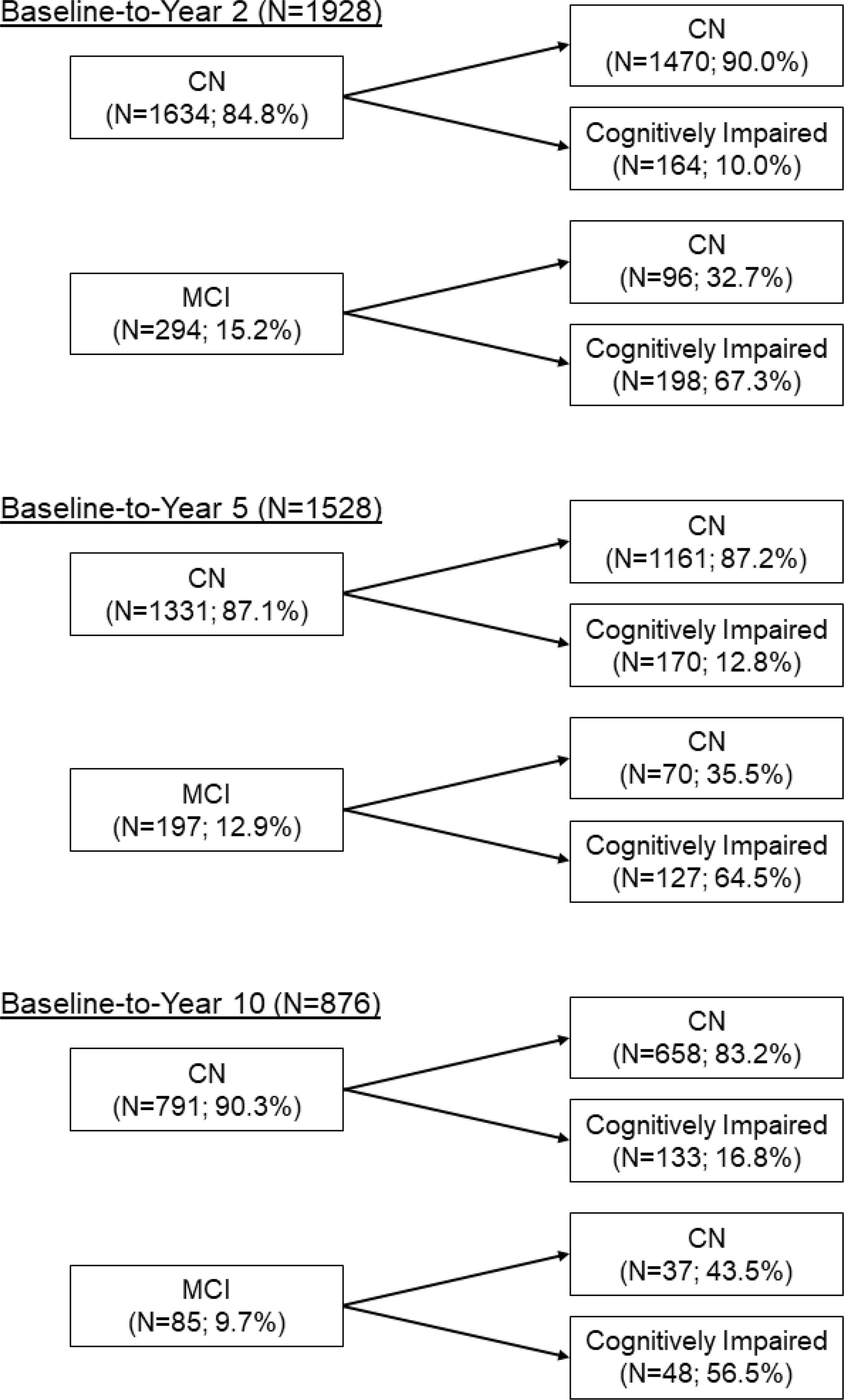
Stability and reversion of baseline classifications to Year 2, Year 5, and Year 10. CN=cognitively normal; MCI=mild cognitive impairment.
There were 1528 participants with classifications at both baseline and Year 5 (CN n=1331, MCI n=197). Of the 1331 participants who were unimpaired at baseline, 170 (12.8%) progressed to cognitively impaired at Year 5. Of the 197 participants who were classified as MCI at baseline, 127 (64.5%) remained cognitively impaired at Year 5 and 70 (35.5%) reverted to CN status. At Year 5, participants who received cognitive training did not revert to CN at a higher rate (36.6%) than untrained participants (32.7%; χ2=0.26, df=1, p=.609) and none of the individual training groups had a higher rate of reversion than the untrained group (χ2=0.29, df=3, p=.962).
There were 876 participants with classifications at both baseline and Year 10 (CN n=791, MCI n=85). Of the 791 participants who were unimpaired at baseline, 133 (16.8%) progressed to cognitively impaired at Year 10. Of the 85 participants who were classified as MCI at baseline, 48 (56.5%) remained cognitively impaired at Year 10 and 37 (43.5%) reverted to CN status. At Year 10, participants who received cognitive training did not revert to CN at a higher rate (43.3%) than untrained participants (44.0%; χ2=0.003, df=1, p=.955) and none of the individual training groups had a higher rate of reversion than the untrained group (χ2=7.20, df=3, p=.066).
Since individuals on the border of CN and MCI may fluctuate between classifications (Roberts et al., 2014), we also examined the reversion rate when considering all three of the follow-up visits described (Year 2, Year 5, Year 10). Of the initial 518 participants classified as MCI at baseline, 317 had at least one follow-up visit. Of these 317 participants initially classified as MCI, 61 (19.2%) participants reverted to CN at all follow-up visits, 76 (23.9%) fluctuated between CN and MCI, and 180 (56.8%) remained stable MCI across all occasions. Those baseline MCI participants who reverted to CN and remained CN across all remaining visits were not disproportionally in a cognitive training group (23.6%) relative to the untrained group (17.5%; χ2=1.51, df=1, p=.219) and none of the individual training groups had a higher rate of reversion than the untrained group (χ2=2.67, df=3, p=.446).
Baseline Characteristics
Table 1 shows the baseline demographic, health, cognitive and everyday functioning characteristics by cognitive group. Regarding demographic variables, there was a small association with age such that the single-aMCI group was the oldest group while the multi-naMCI group was the youngest. Participants in the CN group had higher education than the single-aMCI participants. There were no significant sex or race differences.
Table 1.
Baseline characteristics by MCI subtype.
| CN (N=2237) | Single-aMCI (N=225) | Single-naMCI (N=164) | Multi-aMCI (N=107) | Multi-naMCI (N=22) | F, H, or χ2 | ηp2, η2, or V | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
|
| ||||||||||||
| Age | 73.45d | 5.73 | 76.02abce | 6.81 | 72.97d | 5.77 | 73.22d | 5.89 | 73.61d | 5.87 | F=11.35* | ηp2=.016 |
| Education | 13.62d | 2.68 | 12.96e | 2.67 | 13.04 | 2.74 | 13.03 | 2.92 | 13.73 | 2.55 | F=5.38* | ηp2=.008 |
| Female, % | 75.2% | - | 77.3% | - | 81.7% | - | 73.8% | - | 90.9% | - | χ2=6.83 | V=.050 |
| Black/African American, % | 25.2% | - | 28.9% | - | 31.7% | - | 33.6% | - | 22.7% | - | χ2=7.86 | V=.053 |
| Hypertension, % | 50.8% | - | 46.4% | - | 61.1% | - | 51.4% | - | 45.5% | - | χ2=8.82 | V=.057 |
| Diabetes, % | 11.6%bd | - | 18.8%e | - | 17.1% | - | 21.5%e | - | 18.2% | - | χ2=20.32* | V=.086 |
| # of medications | 4.44 | 2.89 | 4.86 | 3.36 | 4.36 | 2.67 | 5.26 | 3.48 | 4.33 | 2.67 | H=6.94 | η2=.001 |
| SF-36 Physical Functioning | 70.13bd | 23.35 | 62.77e | 27.31 | 65.44 | 24.39 | 59.32e | 26.24 | 68.54 | 27.62 | F=10.07* | ηp2=.015 |
| Systolic blood pressure | 136.91 | 20.24 | 136.41 | 22.65 | 136.54 | 20.19 | 136.83 | 22.70 | 133.67 | 21.82 | F=0.16 | ηp2=.000 |
| Diastolic blood pressure | 76.40 | 11.27 | 75.06 | 12.04 | 76.14 | 11.61 | 75.82 | 10.78 | 74.88 | 12.92 | F=0.81 | ηp2=.001 |
| BMI | 28.56 | 5.47 | 28.32 | 5.93 | 28.87 | 5.61 | 28.19 | 5.94 | 27.97 | 7.35 | F=0.38 | ηp2=.001 |
| CES-D | 4.92bcd | 5.08 | 6.37be | 5.30 | 5.76be | 479 | 8.43acde | 5.35 | 4.35b | 3.87 | H=76.10* | η2=.027 |
| MMSE | 27.61abcd | 1.89 | 25.98e | 1.95 | 26.50be | 1.98 | 25.63ce | 2.02 | 25.32e | 1.91 | H=252.07* | η2=.090 |
| Vocabulary | 12.86abcd | 3.70 | 10.83be | 4.10 | 10.49be | 4.08 | 8.69cde | 4.40 | 9.91e | 4.36 | F=56.09* | ηp2=.076 |
| HVLT Immediate Recall | 27.37abcd | 4.54 | 18.15ace | 5.13 | 24.66bde | 4.94 | 18.00ace | 4.28 | 24.41bde | 3.79 | F=298.55* | ηp2=.303 |
| AVLT Immediate Recall | 50.87bcd | 8.88 | 33.52ace | 8.50 | 47.15bde | 8.89 | 34.75ace | 7.23 | 48.77bd | 8.14 | F=269.47* | ηp2=.282 |
| AVLT Recognition | 42.44bcd | 7.58 | 29.40ace | 7.88 | 38.64bde | 9.17 | 28.88ace | 8.17 | 39.32bd | 7.40 | H=518.47* | η2=.187 |
| Word Series | 10.34abcd | 4.75 | 6.97bce | 3.99 | 5.18de | 2.72 | 4.14de | 2.32 | 4.18e | 1.94 | F=120.25* | ηp2=.149 |
| Letter Sets | 6.20abcd | 2.69 | 4.83abce | 2.34 | 3.15de | 2.07 | 2.76de | 2.02 | 2.32de | 1.09 | F=109.24* | ηp2=.138 |
| Digit Symbol Substitution | 42.30abcd | 10.47 | 35.89abce | 9.01 | 31.23bde | 10.66 | 26.79cde | 8.92 | 26.09de | 5.23 | F=119.93* | ηp2=.149 |
| UFOV Task 2 | 110.13abcd | 104.00 | 185.83abe | 135.06 | 238.13abe | 157.88 | 314.92cde | 150.75 | 343.38cde | 118.30 | H=377.11* | η2=.136 |
| IADL Performance | 4.22b | 4.88 | 5.03 | 5.34 | 3.74b | 4.71 | 5.67ce | 5.54 | 4.95 | 5.31 | H=18.69* | η2=.005 |
| IADL Difficulty | 1.24bd | 2.22 | 2.11e | 3.10 | 1.54b | 2.50 | 2.66ce | 3.41 | 2.41 | 3.10 | H=55.37* | η2=.019 |
p<.005; CN=cognitively normal; Single-aMCI=single-domain amnestic MCI; Single-naMCI=single-domain non-amnestic MCI; Multi-aMCI=multidomain amnestic MCI; Multi-naMCI=multidomain non-amnestic MCI; SF-36=36-Item Short-Form Health Survey; BMI=Body Mass Index; CES-D=Center for Epidemiological Studies-Depression-12; MMSE=Mini-Mental State Exam; HVLT=Hopkins Verbal Learning Test; AVLT=Rey Auditory Verbal Learning Test; UFOV=Useful Field of View; IADL=Instrumental Activities of Daily Living.
represents significant difference from Multi-naMCI
represents significant difference from Multi-aMCI
represents significant difference from Single-naMCI
represents significant difference from Single-aMCI
represents significant difference from CN.
Partial eta-squared and eta-squared are reported for ANOVA and Kruskal-Wallis tests, respectively, and Cramer’s V is reported for chi-squared tests.
Regarding health variables, none of the groups differed on self-reported hypertension or objectively-measured systolic or diastolic blood pressures. Participants in the single-aMCI and multi-aMCI groups had higher proportions of individuals with diabetes than the CN group, and while not statistically significant, the single- and multi-naMCI had higher rates of diabetes than the CN group. There were no group differences in BMI or in the number of medications participants were taking. Participants in the single-aMCI and multi-aMCI group reported worse physical functioning than the CN group. Participants in the multi-aMCI group reported the most depressive symptoms, followed by the single-aMCI.
Regarding everyday functioning, the multi-aMCI group reported poorer functioning than the CN and single-naMCI groups on the IADL Performance scale. On the IADL Difficulty scale, both the single- and multi-aMCI groups reported greater difficulty than the CN group; the multi-aMCI group also reported greater difficulty than the single-naMCI group.
Everyday Functioning Trajectories
Mixed-effects models examined the longitudinal change of everyday functioning by MCI status. Due to the low number of multi-naMCI participants classified at baseline, the single-naMCI and multi-naMCI participants were combined into an overall naMCI group; thus, the longitudinal analyses compared trajectories of CN, single-aMCI, multi-aMCI, and naMCI. Key parameter estimates are shown in Table 2 and the trajectories are depicted in Figure 2. For clearer presentation, the categorical variables of replicate, site, booster, intervention group, intervention group x linear time, and intervention x quadratic time are not included in Table 2, but were included as covariates in each model (see supplemental material for description of these effects).
Table 2.
Parameter estimates for mixed effects models of longitudinal everyday functioning trajectories by cognitive group
| IADL Performance | IADL Difficulty | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| b | S.E. | t | p | r | b | S.E. | t | p | r | |
|
|
||||||||||
| Intercept | 8.154 | 1.201 | 6.790 | <.001 | .130 | 0.081 | 0.625 | 0.125 | .897 | .003 |
| Age | 0.013 | 0.013 | 0.933 | .351 | .018 | 0.029 | 0.007 | 4.097 | <.001 | .081 |
| Education | 0.053 | 0.030 | 1.774 | .076 | .034 | 0.031 | 0.016 | 1.993 | .046 | .040 |
| Female | −5.340 | 0.178 | −30.049 | <.001 | −.503 | −0.393 | 0.092 | −4.243 | <.001 | −.085 |
| Black/African American | −0.932 | 0.205 | −4.556 | <.001 | −.088 | −0.069 | 0.106 | −0.651 | .516 | −.013 |
| SF-36 Physical Functioning | −0.024 | 0.003 | −7.382 | <.001 | −.142 | −0.033 | 0.002 | −19.165 | <.001 | −.359 |
| CES-D | −0.028 | 0.015 | −1.864 | .062 | −.036 | 0.056 | 0.008 | 7.014 | <.001 | .139 |
| Time | 0.350 | 0.049 | 7.206 | <.001 | .175 | 0.434 | 0.042 | 10.436 | <.001 | .252 |
| Time2 | 0.058 | 0.007 | 7.925 | <.001 | .103 | 0.047 | 0.005 | 8.708 | <.001 | .116 |
| Cognitive Group | ||||||||||
| CN (ref) | - | - | - | - | - | - | - | - | - | |
| naMCI | 0.145 | 0.326 | 0.445 | .656 | .008 | 0.383 | 0.179 | 2.134 | .033 | .040 |
| single-aMCI | 0.833 | 0.310 | 2.689 | .007 | .047 | 0.256 | 0.174 | 1.472 | .142 | .026 |
| multi-aMCI | 0.263 | 0.447 | 0.588 | .557 | .010 | 0.104 | 0.256 | 0.286 | .685 | .007 |
| Time x Cognitive Group | ||||||||||
| Time x CN (ref) | - | - | - | - | - | - | - | - | - | |
| Time x naMCI | 0.160 | 0.103 | 1.563 | .118 | .038 | 0.250 | 0.087 | 2.866 | .004 | .070 |
| Time x single-aMCI | 0.614 | 0.103 | 5.982 | <.001 | .132 | 0.666 | 0.086 | 7.759 | <.001 | .170 |
| Time x multi-aMCI | 0.932 | 0.153 | 6.088 | <.001 | .129 | 0.820 | 0.127 | 6.575 | <.001 | .136 |
| Time2 x Cognitive Group | ||||||||||
| Time2 x CN (ref) | - | - | - | - | - | - | - | - | - | |
| Time2 x naMCI | −0.018 | 0.015 | −1.175 | .240 | −.015 | 0.009 | 0.011 | 0.780 | .435 | .010 |
| Time2 x aMCI | −0.017 | 0.016 | −1.092 | .275 | −.013 | 0.009 | 0.011 | 0.822 | .409 | .010 |
| Time2 x multi-aMCI | 0.043 | 0.023 | 1.815 | .070 | .022 | 0.044 | 0.017 | 2.668 | .011 | .033 |
Bold values represent p<.005. CN=cognitively normal; single-aMCI=single-domain amnestic MCI; multi-aMCI=multi-domain aMCI; naMCI=nonamnestic MCI; CES-D=Center for Epidemiological Studies-Depression-12; SF-36=36-Item Short-Form Health Survey; IADL=Instrumental Activities of Daily Living. Site, replicate, booster group, intervention group, Time x intervention, and Time2 x intervention group were also adjusted for in the models (see supplemental material).
Figure 2.
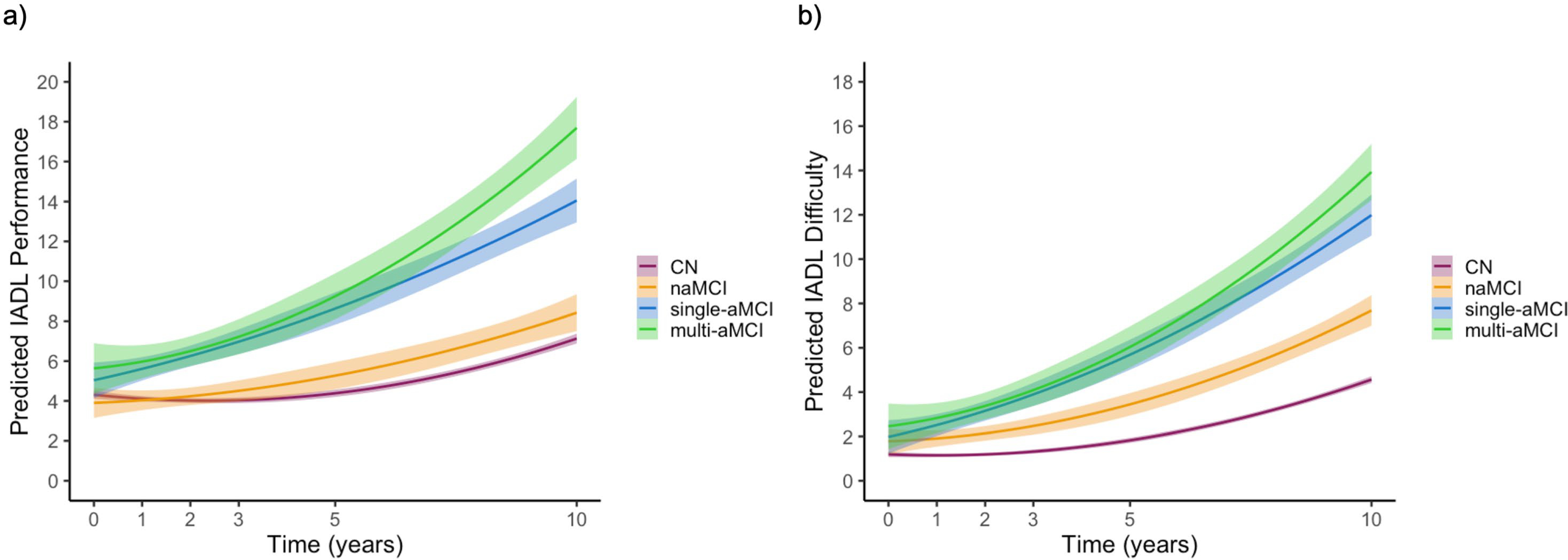
Everyday functioning trajectories of a.) IADL Performance and b) IADL Difficulty subscales by cognitive group status.
CN=cognitively normal; single-aMCI=single-domain amnestic MCI; multi-aMCI=multi-domain amnestic MCI; naMCI=nonamnestic MCI. Shaded area represents the 99.5% confidence interval.
Change in self-reported IADL Performance over 10 years was examined. The cognitive group x linear time interaction was significant such that both the single-aMCI [t(2011.06)=5.98, p<.001, r=.132] and multi-aMCI [t(2200.95)=6.09, p<.001, r=.129] groups had greater decline over time (i.e., greater endorsement of being unable to perform task independently) compared to the CN group. The naMCI group trajectories did not significantly differ from the CN group (p=.118). The cognitive group x quadratic time interaction was not suggestive of accelerated decline in the MCI subtypes relative to the CN group (ps>.05).
When comparing the IADL Performance trajectories of the MCI subtypes with multi-aMCI as the reference group, the cognitive group x linear time interaction showed that the multi-aMCI group had faster linear decline than the naMCI group [t(2045.53)=−4.27, p<.001, r=−.083], but not the single-aMCI (p=.079). Qualitatively, there was a pattern of accelerated decline in the multi-aMCI group relative to the naMCI group [t(6806.80)=−2.20, p=.028, r=−.027] and the single-aMCI group [t(6902.56)=−2.15, p=.031, r=−.026], but this interaction did not reach the strict statistical significance criterion.
Examination of the self-reported IADL Difficulty measure over 10 years demonstrated a significant cognitive group x linear time interaction such that the naMCI [t(1684.29)=2.87, p=.004, r=.069], single-aMCI [t(2015.32)=7.76, p<.001, r=.170], and multi-aMCI [t(2190.25)=6.44, p<.001, r=.136] groups all reported a faster rate of decline over time (i.e., greater endorsement of difficulty performing the tasks) compared to the CN group. For the cognitive group x quadratic time interaction, the multi-aMCI group appeared to show a pattern of accelerated functioning decline [t(6435.08)=2.53, p=.011; r=.033], but this interaction did not reach the strict statistical significance criterion relative to the CN group.
When comparing the MCI subtype IADL Difficulty trajectories with multi-aMCI as the reference group, the cognitive group x linear time interaction showed that the multi-aMCI group had faster linear decline than the naMCI group [t(2031.45)=−3.77, p<.001, r=−.083], but not the single-aMCI group (p=.308). There were no significant cognitive group x quadradic time interactions (ps>.05).
Discussion
The current study classified participants as MCI by applying NP criteria (Bondi et al., 2014; Jak et al., 2009) in the ACTIVE study. Application of these actuarial criteria resulted in 18.8% of the ACTIVE sample to be classified as MCI. Consistent with the field’s general understanding of MCI (Albert et al., 2011), there were only small effects of cognitive group on IADL subscales at baseline, with the multi-aMCI group reporting the least independence and most difficulty performing IADLs. The longitudinal trajectories of the self-reported IADL subscales demonstrated that, on average, individuals in the single- and multi-aMCI subtypes became less independent and had more difficulty performing IADLs over time compared to the CN participants, with the multi-aMCI group showing the steepest rate of change. The naMCI group showed a pattern of faster decline relative to the CN group for the IADL Difficulty subscale.
The proportion of 18.8% classified as MCI at baseline is consistent with other studies of community-dwelling older adults (12.05% amnestic MCI, 6.75% nonamestic MCI), despite the use of some experimental tasks in this battery. Evaluation of multiple studies has suggested that MCI prevalence estimates generally range from 16%−20%, but that there are higher estimates for some studies conducted with multiethnic cohorts or clinic-based samples (Roberts & Knopman, 2013). Specifically, a random sample of predominantly-white residents from Olmstead County, MN, were found to have a prevalence estimate of 16% MCI (11.1% amnestic MCI, 4.9% nonamnestic MCI; Petersen et al., 2010) and the Cardiovascular Health Study reported an overall MCI prevalence estimate of 18.8% (Lopez et al., 2003). The Aging, Demographics, and Memory Study (ADAMS), a nationally representative sample drawn from the Health and Retirement Study, yielded cognitive impairment without dementia prevalence estimates of 16% for those 71–79 years old, 29.2% for those 80–89 years old, and 39.0% for those 90+ years old, for an overall prevalence estimate of 22.2% (Plassman et al., 2008). A study that included multi-ethnic residents of northern Manhattan determined that 26.9% of the 1992 cohort and 21.8% of the 1999 cohort met criteria for MCI (Manly et al., 2008); similarly, the Indianapolis-Ibadan Dementia Project, which included only African American participants, estimated a prevalence of cognitive impairment, no dementia (CIND) among community-dwelling participants to be 23.4% (Unverzagt et al., 2001). When looking at the rate of progression from CN status at baseline to cognitively impaired status at Year 2, the estimated annual progression rate of about 5% in this study (10% over 2 years) is consistent with several other studies that report an annual MCI incidence rate of about 5% (Gao et al., 2014; Manly et al., 2008; Plassman et al., 2011).
The rate of reversion from being classified as MCI at baseline to CN at a follow-up visit varied depending on the duration of follow-up (32.7% at Year 2; 35.5% at Year 5; 43.5% at Year 10), and the higher reversion rate at Year 10 was very likely influenced by selective attrition with the most impaired individuals dropping out and, therefore, not being counted as stably cognitively impaired. Previous work has demonstrated that participants who have reverted from MCI-to-CN are still at greater risk for progression to dementia compared to those who did not have an initial MCI classification (Aerts et al., 2017; Roberts et al., 2014). Therefore, we examined the proportion of participants who were MCI at baseline and had at least one follow-up visit (Year 2, Year 5, and/or Year 10), but then reverted to CN for each of the subsequent visits examined. This approach resulted in a reversion rate of 19.2%. These rates of reversion are higher than what was found using the comprehensive neuropsychological approach to MCI classification in the ADNI study, which has shown a one-year reversion rate of 15.8% (Thomas et al., 2019a). Classifying participants at each occasion using the normative control group at the corresponding occasion likely helped to adjust for the mean practice effects of the untrained normative control group. Notably, those participants who reverted were not significantly more likely to have been in one of the three cognitive training groups than the untrained group. This pattern is somewhat inconsistent with prior ACTIVE study results showing the interventions, especially speed and reasoning, had long-term effects on cognition (Rebok et al., 2014). However, the fact that there was not significantly higher rates of reversion in the intervention groups suggests that the NP criteria were robust to exposure to material similar to the cognitive tests used for the MCI classifications as well as mood, self-efficacy, or quality of life benefits of being in the training group (Wolinsky et al., 2006, 2009, 2010). The current study used cognitive/experimental tasks for classification, particularly in the non-memory domains, that may have been more challenging and led to a higher rate of non-amnestic MCI than has been observed in previous studies using these NP criteria. Non-amnestic MCI participants tend to revert to CN at a higher rate (Pandya, et al., 2017; Thomas et al., 2019a), so the greater proportion of non-amnestic MCI participants in this current study may also result in a higher reversion rate.
Although the reversion rate is higher than prior studies using the NP criteria (Jak et al., 2016; Thomas et al., 2019a; Wong et al., 2018), the ACTIVE study reversion rate is in-line with other reversion rates in community-dwelling cohorts. For example, in a northern Manhattan multi-ethnic study, the reversion rate was found to be 45.2% when looking only at baseline and the first visit; it was 30.2% when reversion was defined as not having MCI or dementia at any follow-up visits (Manly et al., 2008). The Mayo Clinic Study of Aging reported a reversion rate of 38% over a median follow up period of 5.1 years (Roberts et al., 2014). One meta-analysis of MCI to cognitively normal reversion rates reported a reversion rate of 31% in community-based studies (Malek-Ahmadi, 2016) and another meta-analysis reported a reversion rate of 25% in population-based studies (Canevelli et al., 2016). Furthermore, the prior MCI criteria used in ACTIVE that implemented cognitive composite scores had a BL-to-Year 2 reversion rate of 42.6%, BL-to-Year 5 reversion rate of 54.8%, and did not examine BL-to-Year 10 reversion (Cook et al., 2013). Thus, the current study is not only the first to extend an MCI classification out to the full 10 years of data collection in ACTIVE, but it also improved upon the stability of classification despite many fewer people being classified as MCI (18.8%) using this current NP criteria approach relative to the previously published MCI criteria that classified 33% of the ACTIVE sample as MCI at baseline. This improvement in stability and reliability of MCI classification is likely due to the requirement of two test scores to be impaired within a domain to establish reliable cognitive impairment within the domain, rather than impairment on a single composite score, which can be heavily driven by only one score in the composite. Additionally, the lower proportion of non-amnestic participants identified using the current NP criteria (6.8%) relative to the prior ACTIVE MCI classifications from 2013 (25%) may have also improved stability given the known higher rate of reversion to CN status in those with non-amnestic MCI (Pandya, et al., 2017; Thomas et al., 2019a).
Despite only very small differences in the baseline IADL Performance scale between CN and MCI groups, both the single-aMCI and multi-aMCI groups reported a faster decline in IADL Performance (i.e., higher scores) than the CN group over time, with the multi-aMCI group showing the fastest rate of decline. This pattern was similar for the IADL Difficulty subscale; however, all of the MCI subtypes, including the naMCI group showed faster decline in IADL Difficulty. These findings with the aMCI groups showing the fastest declines are consistent with the literature showing that older adults with aMCI are more likely to progress to AD than naMCI (Manly et al., 2008; Roberts et al., 2014). Additionally, previous work has shown that impairment in memory plus another domain, particularly executive functioning, is associated with faster progression to both AD and non-AD dementia (Roberts et al., 2014) and is associated with faster decline in everyday functioning relative to those with memory impairment alone (Thomas et al., 2017). In addition to the aMCI groups showing the fastest rate of functional declines, at baseline, the single-aMCI and multi-aMCI groups differed from the CN group in proportion of participant who reported having diabetes, though all of the MCI subtypes showed higher proportions of participants with diabetes relative to the CN group. The higher rate of diabetes in the MCI groups, especially the aMCI groups, is in-line with evidence that diabetes is a risk factor for MCI (Luchsinger et al., 2007) and both AD and vascular dementias (Cheng et al., 2012).
The current study has several strengths including the large sample that is more representative than many of the large aging cohorts in which NP criteria has previously been applied. Additionally, the 10 years of follow-up data allowed for longitudinal validation of the MCI criteria via changes in IADLs. The ACTIVE study, however, did not collect AD biomarkers, which would have been another independent validation of the MCI criteria. While ACTIVE did exclude individuals thought to have dementia at baseline, it is possible that a small proportion of the MCI group, particularly the multi-aMCI group would be better characterized as very early dementia. Also, ACTIVE was not a clinical study so incident clinical dementia was not identified; however, ongoing research in ACTIVE will allow for the examination of Medicare/Medicaid records, thus allowing for an independent validation of the MCI classifications in the future.
The use of a sample-specific normative group for this study is similar to the identification of a robust normative control group, defined as those who have stable “normal” performance over time, used in previous work to get the regression weights for the demographically-adjusted z-scores (Edmonds et al., 2015; Thomas et al., 2019a). Although published normative studies do not often have the luxury of longitudinal data from which to determine a normative group that does not show significant decline over time, robust normative data have been shown to be more sensitive to early detection of cognitive impairment (Kramer et al., 2020; Pedraza et al., 2010). The use of the MMSE for identifying this robust normative control group in the current study allowed for a way of identifying individuals without significant cognitive decline over time that was independent of the cognitive measures used to later classify CN and MCI status. Our previous work has used a very similar approach to determine a robust control group in the ADNI cohort; however, rather than using the MMSE, we used the ADNI-based cognitively normal classifications, which is heavily driven by the Clinical Dementia Rating (CDR; e.g., Edmonds et al., 2015, Thomas et al., 2019b). While we would have preferred to use this approach over an MMSE cutoff, neither the CDR nor clinical diagnostic classifications were available in ACTIVE. While the MMSE is certainly limited as a cognitive screener and may be weighted to memory-type items, this approach also allowed for the same normative control participants to be used at each occasion, which helped to factor in practice effects of the no-contact control group without also washing out the potential training effects that were tested.
Although the comprehensive neuropsychological approach to classifying MCI has previously been applied to multiple aging cohorts that have traditional neuropsychological measures (e.g., Framingham, ADNI; (Bondi et al., 2014; Jak et al., 2016; Wong et al., 2018), it had not previously been applied to a large, aging study that includes more traditional clinical neuropsychological measures (e.g., AVLT, HVLT, Digit Symbol), some of which were administered in a non-traditional way (e.g., written responses and no delayed free recall on AVLT or HVLT), and experimental cognitive aging measures (e.g., Word Series, Letter Sets, UFOV) that were not developed to determine cognitive impairment. Furthermore, since neither a confrontation naming test nor a semantic fluency test were included in the ACTIVE cognitive battery, it was not possible to create a meaningful language domain to assess for a dysnomic subtype of MCI. Despite the limitations of the cognitive test battery that may have been the biggest test of the flexibility of these NP criteria to date, the application of the NP criteria produced MCI classification rates and stability estimates consistent with other community-dwelling samples and the MCI classification appeared to perform as expected on measures of everyday functioning, both cross-sectionally and longitudinally. This highlights the flexible nature of these criteria, as they were designed to be applicable across different studies and for use with different neuropsychological measures; this study extends even this definition to show the utility of the NP criteria for implementation in studies that include non-traditional cognitive measures.
The current classifications and findings open up a number of opportunities for future research, including improving the understanding of cross-sectional and longitudinal associations between mild cognitive difficulties and health factors, social forces, and everyday functioning. Further, the ACTIVE study allows for the unique opportunity to examine whether mild cognitive difficulties impact the magnitude or breadth of benefit from the cognitive interventions as well as whether the cognitive interventions reduce the rates of progression to MCI over time.
Supplementary Material
Key Points.
Question:
This paper tests the use of neuropsychological criteria for the classification of MCI in the ACTIVE study.
Findings:
The proportion of ACTIVE study participants classified as MCI (18.8%) and the rates of reversion from MCI to cognitively normal using the neuropsychological criteria were consistent with other studies, and MCI participants, particularly those with a memory impairment, had the fastest decline in everyday functioning.
Importance:
Results show that this approach of classifying MCI is extremely flexible and produced meaningful MCI classifications in a study that did initially intend to identify cognitive impairment.
Next Steps:
Future research will use these MCI classifications in ACTIVE to examine cognitive training effects among participants classified as MCI or whether cognitive training reduces risk of future MCI classification.
Acknowledgements
This work was supported by grants from the National Institute on Aging (U01 AG14260, U01 AG14282, U01 AG14263, U01 AG14289, U01 AG014276, R01 AG056486, T32 AG020499, R01 AG049810, K24 AG026431) and the National Institute of Nursing Research (U01 NR04508, U01 NR04507). Dr. Thomas received salary support from Career Development Award 1IK2 CX001865 from the U.S. Department of Veterans Affairs Clinical Science Research and Development Service and the Alzheimer’s Association (AARF-17-528918). Dr. Gross was supported by NIA K01AG050699.
References
- Aerts L, Heffernan M, Kochan NA, Crawford JD, Draper B, Trollor JN, Sachdev PS, & Brodaty H (2017). Effects of MCI subtype and reversion on progression to dementia in a community sample. Neurology, 88(23), 2225–2232. 10.1212/WNL.0000000000004015 [DOI] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, & Phelps CH (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia, 7(3), 270–279. 10.1016/j.jalz.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s Association. (2019). 2019 Facts and Figures. Alzheimer’s & Dementia, 15(3), 321–387. 10.1016/j.jalz.2019.01.010 [DOI] [Google Scholar]
- Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, Morris JN, Rebok GW, Smith DM, Tennstedt SL, Unverzagt FW, Willis SL, & for the ACTIVE Study Group, for the A. S. (2002). Effects of Cognitive Training Interventions With Older Adults. JAMA, 288(18), 2271. 10.1001/jama.288.18.2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangen KJ, Clark AL, Werhane M, Edmonds EC, Nation DA, Evangelista N, Libon DJ, Bondi MW, & Delano-Wood L (2016). Cortical Amyloid Burden Differences Across Empirically-Derived Mild Cognitive Impairment Subtypes and Interaction with APOE ɛ4 Genotype. Journal of Alzheimer’s Disease, 52(3), 849–861. 10.3233/JAD-150900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi MW, Edmonds EC, Jak AJ, Clark LR, Delano-Wood L, McDonald CR, Nation DA, Libon DJ, Au R, Galasko D, & Salmon DP (2014). Neuropsychological Criteria for Mild Cognitive Impairment Improves Diagnostic Precision, Biomarker Associations, and Progression Rates. Journal of Alzheimer’s Disease, 42(1), 275–289. 10.3233/JAD-140276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canevelli M, Grande G, Lacorte E, Quarchioni E, Cesari M, Mariani C, Bruno G, & Vanacore N (2016). Spontaneous Reversion of Mild Cognitive Impairment to Normal Cognition: A Systematic Review of Literature and Meta-Analysis. Journal of the American Medical Directors Association, 17(10), 943–948. 10.1016/J.JAMDA.2016.06.020 [DOI] [PubMed] [Google Scholar]
- Cheng G, Huang C, Deng H, & Wang H (2012). Diabetes as a risk factor for dementia and mild cognitive impairment: a meta‐analysis of longitudinal studies. Internal medicine journal, 42(5), 484–491. [DOI] [PubMed] [Google Scholar]
- Cook SE, Marsiske M, Thomas KR, Unverzagt FW, Wadley VG, Langbaum JBS, & Crowe M (2013). Identification of Mild Cognitive Impairment in ACTIVE: Algorithmic Classification and Stability. Journal of the International Neuropsychological Society, 19(01), 73–87. 10.1017/S1355617712000938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds EC, Delano-Wood L, Clark LR, Jak AJ, Nation DA, McDonald CR, Libon DJ, Au R, Galasko D, Salmon DP, & Bondi MW (2015). Susceptibility of the conventional criteria for mild cognitive impairment to false-positive diagnostic errors. Alzheimer’s & Dementia, 11(4), 415–424. 10.1016/j.jalz.2014.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds EC, Eppig J, Bondi MW, Leyden KM, Goodwin B, Delano-Wood L, McDonald CR, & Alzheimer’s Disease Neuroimaging Initiative, F. the A. D. N. (2016). Heterogeneous cortical atrophy patterns in MCI not captured by conventional diagnostic criteria. Neurology, 87(20), 2108–2116. 10.1212/WNL.0000000000003326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppig JS, Edmonds EC, Campbell L, Sanderson-Cimino M, Delano-Wood L, Bondi MW, & Initiative, for the A. D. N. (2017). Statistically Derived Subtypes and Associations with Cerebrospinal Fluid and Genetic Biomarkers in Mild Cognitive Impairment: A Latent Profile Analysis. Journal of the International Neuropsychological Society, 23(07), 564–576. 10.1017/S135561771700039X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Ganguli M, Chang C-CH, Snitz BE, Saxton JA, Vanderbilt J, & Lee C-W (2010). Prevalence of Mild Cognitive Impairment by Multiple Classifications: The Monongahela-Youghiogheny Healthy Aging Team (MYHAT) Project. The American Journal of Geriatric Psychiatry, 18(8), 674–683. 10.1097/JGP.0B013E3181CDEE4F [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Unverzagt FW, Hall KS, Lane KA, Murrell JR, Hake AM, … & Hendrie HC (2014). Mild cognitive impairment, incidence, progression, and reversion: findings from a community-based cohort of elderly African Americans. The American Journal of Geriatric Psychiatry, 22(7), 670–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glymour MM, Brickman AM, Kivimaki M, Mayeda ER, Chêne G, Dufouil C, & Manly JJ (2018). Will biomarker-based diagnosis of Alzheimer’s disease maximize scientific progress? Evaluating proposed diagnostic criteria. European Journal of Epidemiology, 33(7), 607–612. 10.1007/s10654-018-0418-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis JPA (2018). The Proposal to Lower P Value Thresholds to .005. JAMA, 319(14), 1429. 10.1001/jama.2018.1536 [DOI] [PubMed] [Google Scholar]
- Jak AJ, Bondi MW, Delano-Wood L, Wierenga C, Corey-Bloom J, Salmon DP, & Delis DC (2009). Quantification of Five Neuropsychological Approaches to Defining Mild Cognitive Impairment. The American Journal of Geriatric Psychiatry, 17(5), 368–375. 10.1097/JGP.0b013e31819431d5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jak AJ, Preis SR, Beiser AS, Seshadri S, Wolf PA, Bondi MW, & Au R (2016). Neuropsychological Criteria for Mild Cognitive Impairment and Dementia Risk in the Framingham Heart Study. Journal of the International Neuropsychological Society, 22(09), 937–943. 10.1017/S1355617716000199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobe JB, Smith DM, Ball K, Tennstedt SL, Marsiske M, Willis SL, Rebok GW, Morris JN, Helmers KF, Leveck MD, & Kleinman K (2001). Active: A cognitive intervention trial to promote independence in older adults. Controlled Clinical Trials, 22(4), 453–479. 10.1016/S0197-2456(01)00139-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer AO, Casaletto KB, Umlauf A, Staffaroni AM, Fox E, You M, & Kramer JH (2020). Robust normative standards for the California Verbal Learning Test (CVLT) ages 60–89: A tool for early detection of memory impairment. The Clinical Neuropsychologist, 34(2), 384–405. 10.1080/13854046.2019.1619838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez OL, Jagust WJ, DeKosky ST, Becker JT, Fitzpatrick A, Dulberg C, Breitner J, Lyketsos C, Jones B, Kawas C, Carlson M, & Kuller LH (2003). Prevalence and Classification of Mild Cognitive Impairment in the Cardiovascular Health Study Cognition Study. Archives of Neurology, 60(10), 1385. 10.1001/archneur.60.10.1385 [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Reitz C, Patel B, Tang MX, Manly JJ, & Mayeux R (2007). Relation of diabetes to mild cognitive impairment. Archives of neurology, 64(4), 570–575. [DOI] [PubMed] [Google Scholar]
- Malek-Ahmadi M (2016). Reversion From Mild Cognitive Impairment to Normal Cognition. Alzheimer Disease & Associated Disorders, 30(4), 324–330. 10.1097/WAD.0000000000000145 [DOI] [PubMed] [Google Scholar]
- Manly JJ, Tang M-X, Schupf N, Stern Y, Vonsattel J-PG, & Mayeux R (2008). Frequency and course of mild cognitive impairment in a multiethnic community. Annals of Neurology, 63(4), 494–506. 10.1002/ana.21326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwick A, Zamboni G, & De Jager CA (2012). Profiles of cognitive subtest impairment in the Montreal Cognitive Assessment (MoCA) in a research cohort with normal Mini-Mental State Examination (MMSE) scores. Journal of clinical and experimental neuropsychology, 34(7), 750–757. [DOI] [PubMed] [Google Scholar]
- Marsiske M, Dzierzewski JM, Thomas KR, Kasten L, Jones RN, Johnson KE, … & Rebok GW (2013). Race-related disparities in 5-year cognitive level and change in untrained ACTIVE participants. Journal of aging and health, 25(8_suppl), 103S–127S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JN, Fries BE, Steel K, Ikegami N, Bernabei R, Carpenter GI, Gilgen R, Hirdes JP, & Topinková E (1997). Comprehensive Clinical Assessment in Community Setting: Applicability of the MDS-HC. Journal of the American Geriatrics Society, 45(8), 1017–1024. 10.1111/j.1532-5415.1997.tb02975.x [DOI] [PubMed] [Google Scholar]
- Pedraza O, Lucas JA, Smith GE, Petersen RC, Graff-Radford NR, & Ivnik RJ (2010). Robust and Expanded Norms for the Dementia Rating Scale. Archives of Clinical Neuropsychology, 25(5), 347–358. 10.1093/arclin/acq030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC (2004). Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine, 256(3), 183–194. 10.1111/j.1365-2796.2004.01388.x [DOI] [PubMed] [Google Scholar]
- Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, Jack CR, Jagust WJ, Shaw LM, Toga AW, Trojanowski JQ, & Weiner MW (2010). Alzheimer’s Disease Neuroimaging Initiative (ADNI): Clinical characterization. Neurology, 74(3), 201–209. 10.1212/WNL.0b013e3181cb3e25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Roberts RO, Knopman DS, Geda YE, Cha RH, Pankratz VS, Boeve BF, Tangalos EG, Ivnik RJ, & Rocca WA (2010). Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology, 75(10), 889–897. 10.1212/WNL.0b013e3181f11d85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, & Kokmen E (1999). Mild Cognitive Impairment. Archives of Neurology, 56(3), 303. 10.1001/archneur.56.3.303 [DOI] [PubMed] [Google Scholar]
- Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, Burke JR, Hurd MD, Potter GG, Rodgers WL, Steffens DC, McArdle JJ, Willis RJ, & Wallace RB (2008). Prevalence of cognitive impairment without dementia in the United States. Annals of Internal Medicine, 148(6), 427–434. 10.7326/0003-4819-148-6-200803180-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassman BL, Langa KM, McCammon RJ, Fisher GG, Potter GG, Burke JR, Steffens DC, Foster NL, Giordani B, Unverzagt FW, Welsh-Bohmer KA, Heeringa SG, Weir DR, & Wallace RB (2011). Incidence of dementia and cognitive impairment, not dementia in the United States. Annals of neurology, 70(3), 418–426. 10.1002/ana.22362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D Scale. Applied Psychological Measurement, 1(3), 385–401. 10.1177/014662167700100306 [DOI] [Google Scholar]
- Rebok GW, Ball K, Guey LT, Jones RN, Kim H-Y, King JW, Marsiske M, Morris JN, Tennstedt SL, Unverzagt FW, & Willis SL (2014). Ten-Year Effects of the Advanced Cognitive Training for Independent and Vital Elderly Cognitive Training Trial on Cognition and Everyday Functioning in Older Adults. Journal of the American Geriatrics Society, 62(1), 16–24. 10.1111/jgs.12607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R, & Knopman DS (2013). Classification and epidemiology of MCI. Clinics in Geriatric Medicine, 29(4), 753–772. 10.1016/j.cger.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RO, Knopman DS, Mielke MM, Cha RH, Pankratz VS, Christianson TJH, Geda YE, Boeve BF, Ivnik RJ, Tangalos EG, Rocca WA, & Petersen RC (2014). Higher risk of progression to dementia in mild cognitive impairment cases who revert to normal. Neurology, 82(4), 317–325. 10.1212/WNL.0000000000000055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti HC, Smith EE, Hynan LS, Lacritz LH, Cullum CM, Van Wright A, & Weiner MF (2019). Detection of Mild Cognitive Impairment Among Community-Dwelling African Americans Using the Montreal Cognitive Assessment. Archives of Clinical Neuropsychology, 34(6), 809–813. 10.1093/arclin/acy091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev PS, Lipnicki DM, Kochan NA, Crawford JD, Thalamuthu A, Andrews G, Brayne C, Matthews FE, Stephan BCM, Lipton RB, Katz MJ, Ritchie K, Carrière I, Ancelin M-L, Lam LCW, Wong CHY, Fung AWT, Guaita A, Vaccaro R, … Cohort Studies of Memory in an International Consortium (COSMIC), C. S. of M. in an I. C. (2015). The Prevalence of Mild Cognitive Impairment in Diverse Geographical and Ethnocultural Regions: The COSMIC Collaboration. PloS One, 10(11), e0142388. 10.1371/journal.pone.0142388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JD, & Willett JB (2003). Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence—Judith D. Singer, John B. Willett, Charles William Eliot Professor John B Willett, John B.. Willett—Google Books. Oxford University Press. [Google Scholar]
- Thomas KR, Edmonds EC, Delano-Wood L, & Bondi MW (2017). Longitudinal Trajectories of Informant-Reported Daily Functioning in Empirically Defined Subtypes of Mild Cognitive Impairment. Journal of the International Neuropsychological Society, 23(06), 521–527. 10.1017/S1355617717000285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KR, Edmonds EC, Eppig JS, Wong CG, Weigand AJ, Jak AJ, … Bondi MW (2019a). MCI-to-normal reversion using neuropsychological criteria in the Alzheimer’s Disease Neuroimaging Initiative. Alzheimer’s & Dementia, 15(10), 1322–1332. 10.1016/j.jalz.2019.06.4948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KR, Eppig JS, Weigand AJ, Edmonds EC, Wong CG, Jak AJ, … & Bondi MW (2019b). Artificially low mild cognitive impairment to normal reversion rate in the Alzheimer’s Disease Neuroimaging Initiative. Alzheimer’s & Dementia, 15(4), 561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unverzagt FW, Gao S, Baiyewu O, Ogunniyi AO, Gureje O, Perkins A, Emsley CL, Dickens J, Evans R, Musick B, Hall KS, Hui SL, & Hendrie HC (2001). Prevalence of cognitive impairment: Data from the Indianapolis Study of Health and Aging. Neurology, 57(9), 1655–1662. 10.1212/WNL.57.9.1655 [DOI] [PubMed] [Google Scholar]
- Unverzagt FW, Kasten L, Johnson KE, Rebok GW, Marsiske M, Koepke KM, … Tennstedt SL (2007). Effect of memory impairment on training outcomes in ACTIVE. Journal of the International Neuropsychological Society, 13(6), 953–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JE, & Sherbourne CD (1992). The MOS 36-Item Short-Form Health Survey (SF-36). Medical Care, 30(6). [PubMed] [Google Scholar]
- Willis SL, Tennstedt SL, Marsiske M, Ball K, Elias J, Koepke KM, Morris JN, Rebok GW, Unverzagt FW, Stoddard AM, Wright E, & ACTIVE Study Group, for the. (2006). Long-term Effects of Cognitive Training on Everyday Functional Outcomes in Older Adults. JAMA, 296(23), 2805. 10.1001/jama.296.23.2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund L-O, Nordberg A, Backman L, Albert M, Almkvist O, Arai H, Basun H, Blennow K, de Leon M, DeCarli C, Erkinjuntti T, Giacobini E, Graff C, Hardy J, … Petersen RC (2004). Mild cognitive impairment—Beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. Journal of Internal Medicine, 256(3), 240–246. 10.1111/j.1365-2796.2004.01380.x [DOI] [PubMed] [Google Scholar]
- Winblad Berndt, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund L-O, Nordberg A, & et al. (2004). Mild cognitive impairment–beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. Journal of Internal Medicine, 256(3), 240–246. [DOI] [PubMed] [Google Scholar]
- Wong CG, Thomas KR, Edmonds EC, Weigand AJ, Bangen KJ, Eppig JS, Jak AJ, Devine SA, Delano-Wood L, Libon DJ, Edland SD, Au R, & Bondi MW (2018). Neuropsychological Criteria for Mild Cognitive Impairment in the Framingham Heart Study’s Old-Old. Dementia and Geriatric Cognitive Disorders, 46(5–6), 253–265. 10.1159/000493541 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


