Abstract
For many individuals with end-stage liver disease, the only treatment option is liver transplantation. However, liver transplant rejection is observed in 24%–80% of transplant patients and lifelong drug regimens that follow the transplant procedure lead to serious side effects. Furthermore, the pool of donor livers available for transplantation is far less than the demand. Well-characterized and physiologically relevant models of liver transplantation are crucial to a deeper understanding of the cellular processes governing the outcomes of liver transplantation and serve as a platform for testing new therapeutic strategies to enhance graft acceptance. Such a model has been found in the rat transplant model, which has an advantageous size for surgical procedures, similar postoperative immunological progression, and high genome match to the human liver. From rat liver transplant studies published in the last 5 years, it is clear that the rat model serves as a strong platform to elucidate transplant immunological mechanisms. Using the model, we have begun to uncover potential players and possible therapeutic targets to restore liver tolerance and preserve host immunocompetence. Here, we present an overview of recent literature for rat liver transplant models, with an aim to highlight the value of the models and to provide future perspectives on how these models could be further characterized to enhance the overall value of rat models to the field of liver transplantation.
INTRODUCTION
The liver is an astonishing, unique organ capable of versatile immune responses and responsible for critical functions including detoxification, metabolism, and protein anabolism.1 Today, prevalence of liver diseases is on the rise and liver transplantation (LT) remains the only treatment for end-stage liver disease, resulting in an increasing number of annual transplant operations of the liver.2 Despite the immunologically privileged status of liver3 and advances in LT surgical techniques and postoperative care since the first human LT in 1963, leading to an overall 5-year graft survival of 76% in adults,4,5 complications prevail. Long-term immunosuppression is part of the standard of care for LT patients with common immunosuppressive (IS) therapy including corticosteroids, antimetabolite, and specific inhibitors which block calcineurin and T-cell activation and proliferation.6,7 However, lifelong drug regimens significantly impact an individual’s quality of life and lead to higher risk of carcinomas, diabetes, viral infections, and opportunistic disease development.6-8 A serious LT complication, postoperative acute cellular rejection (ACR), develops in 24%–80% of patients, presents within days to weeks after surgery, and is a major contributor to graft failure leading to nearly 4% of posttransplant deaths.4 Currently, the management of ACR relies mainly on fine-tuned immunosuppression.5 By dissecting the mechanism of post-LT ACR and key players linked to immune tolerance, we may not only improve LT recipient’s survival but also quality of life.
An appropriate and relevant animal model that is able to recapitulate posttransplant liver immune microenvironment, delineate mechanisms involved in cellular rejection, and explore promising therapeutics can help advance the field faster and in a more physiologically relevant way. The laboratory rat (Rattus norvegicus) model is the gold-standard animal model in orthotopic liver transplantation (OLT) for reasons such as optimal size for surgical operation and immunological similarity to the human liver.9,10 Many studies have explored the immunobiology and immunopathology of the liver or have tested for potential immunotargets using the rat model, as human liver samples are scarce. In this review, we will discuss how rat models are helping to advance the standard of care of LT patients, summarize the recent findings in the field, address the limitations of the models, and discuss the most promising way forward.
THE LANDSCAPE OF A HEALTHY LIVER
In the healthy state, parenchymal and nonparenchymal hepatic cells work cooperatively to establish the tolerogenic environment of the liver. Hepatocytes, liver sinusoidal endothelial cells (LSECs), hepatic stellate cells, liver resident macrophages (Kupffer cells [KCs]), regulatory T cells (Treg), and dendritic cells (DCs) among other hepatic cells work in synergy to create an IS microenvironment enriched in interleukin (IL)-10, transforming growth factor β (TGF-β), hepatocyte growth factor, retinoic acid, and prostaglandin E2 (PGE2).11-20 Professional antigen-presenting cells (APCs), such as DCs and KCs, and nonprofessional APCs, such as LSECs and hepatic stellate cells, not only favor the development of Treg in detriment of cytotoxic T lymphocytes but also promote clonal anergy and deletion of effector T and B cells.11,21-26 This response can be attributed mainly to the upregulation of inhibitory signals, such as programmed death ligand 1 (PD-L1) and cytotoxic T-lymphocyte–associated protein 4 (CTLA-4), and the low levels of the major histocompatibility complex (MHC) class I and of costimulatory molecules in the liver.21,22,24
The inherent tolerogenic state of the liver reflects in less stringent criteria for matching LT donors and recipients when compared with other solid organs.4 As mentioned here, however, this perceived tolerogenic environment is not sufficient to control alloimmune responses, with most LT recipients requiring lifelong IS therapy. Interestingly, a small fraction of stable LT recipients have exhibited persistent graft tolerance following complete weaning of IS and are known as operationally tolerants.27,28 Identifying biomarkers that can guide IS therapy withdrawal and understanding how this tolerogenic state can be achieved post-LT are key to develop drug-free, permanent tolerance that preserves host immunocompetence in LT patients. As part of this effort to identify reliable biomarkers that can determine whether LT patients are fit for IS withdrawal, Pérez-Sanz et al29 examined the predictive capacity of 20 biomarkers previously associated with LT tolerance and identified SENP6, FEM1C, miR31, and miR95 as promising candidates worthy of further investigation for their biological relevance and value in clinical practice.
OF RATS AND MEN
Rat Liver Transplant Models
A number of animal models have been used in LT research including the first animal OLT performed in canine species, pigs, mice, and rats.9,30,31 Of note, the rat model is especially valuable as it is small enough for easy handling and large enough to encompass complex microsurgical procedures, all the while presenting up to 90% genome match and post-LT immune progression similarity to humans.32 The rat OLT surgical procedure was first established in 1973 by Lee et al33 and has since served as a valuable experimental tool in rat LT research. Hindered by limitations of excessive bleeding and lengthy surgical time, the procedure was later modified by Kamada and Calne34 to include the cuff technique that significantly reduced the anhepatic phase and improved surgery outcomes. Besides instrumental when improving surgical procedures, organ preservation, and IS drug therapy in LT, as we will further discuss here, the rat model has been serving as a platform for the investigation of rejection and tolerance posttransplantation. Interestingly, liver allograft rejection is observed to have less severe manifestations or the graft is accepted without rejection when OLT involves donor-recipient pairs from certain rat strains,31 while it leads to fatal ACR in others.31,35 Table 1 shows the main combinations of rat strains used when studying tolerance or rejection post-LT. Analyzing 42 studies published within the past 5 years, the preferred methods for the investigation of ACR are (1) Lewis (LEW) as donor and Brown Norway (BN) as recipient (LEW→BN) (53%), and (2) Dark Agouti (DA) as donor and LEW as recipient (DA→LEW) (33%). It is important to highlight that by inverting the strains serving as donor and recipient, that is, BN→LEW and LEW→DA, we obtain instead a model for tolerance in LT studies (Table 1). Further understanding the mechanisms of rejection and tolerance in these models and their parallel/relevance to humans is critical for the design and testing of novel therapeutic strategies aimed at improving survival and health of LT recipients.
TABLE 1.
Combinations of rat strains as orthotopic liver transplantation donor and recipient to model postoperative acute cellular rejection and tolerance
| Donor strain (MHC) | Recipient strain (MHC) | References |
|---|---|---|
| Acute cellular rejection | ||
| LEW (RT1l) | BN (RT1n) | 36-57 |
| DA (RT1av1) | LEW (RT1l) | 58-71 |
| LEW (RT11) | ACI (RT1a) | 72 |
| ACI (RT1a) | LEW (RT1l) | 73,74 |
| Wistar (RT1a/RT1u) | SD (RT1u/RT1b) | 75,76 |
| SD (RT1u/RT1b) | Wistar (RT1a/RT1u) | 51 |
| Tolerance | ||
| BN (RT1n) | LEW (RT1l) | 50 |
| LEW (RT1l) | DA (RT1av1) | 77-80 |
| DA (RT1av1) | PVG (RT1c) | 67 |
| Wistar (RT1a/RT1u) | August (RT1c) | 81 |
ACI, AxC 9935 Irish; BN, Brown Norway; DA, Dark Agouti; LEW, Lewis; MHC, major histocompatibility complex; PVG, piebald virol glaxo; SD, Sprague Dawley.
Similar to rats, select strain combinations in mice can also model both rejection and tolerance without IS treatment. For example, LT in the C3H→B10 mouse model presents rejection, while reversing the donor-recipient pair shows high tolerance rates despite mismatched MHC.82 Although mouse models have been helping to uncover molecular mechanisms and potential biomarkers of tolerance after LT (reviewed in Thomson et al83), 1 major limitation of the model is its small size. LT surgeries comprise complex microvasculature reconnections involving sutures, cuff techniques, and lengthy operation times that are made difficult in small animals.9,84 Not only is anastomosis important in improving animal survival posttransplant, but the overall operation time also plays a large role in postsurgery complications and animal mortality.85 The establishment of surgical protocols in rats, which are 10 times the size of mice, allows for a more direct comparison with human LT by facilitating intricate microsurgery that mimics the human LT procedure, including hepatic artery reconstruction, which yields improved survival rates and reduced secondary complications from operational challenges.9,85,86 Higher precision in arterial and duct reconnections are especially important when trying to recapitulate the human physiological conditions in long-term tolerance modeling.86
Assessment of Liver Allograft Acceptance
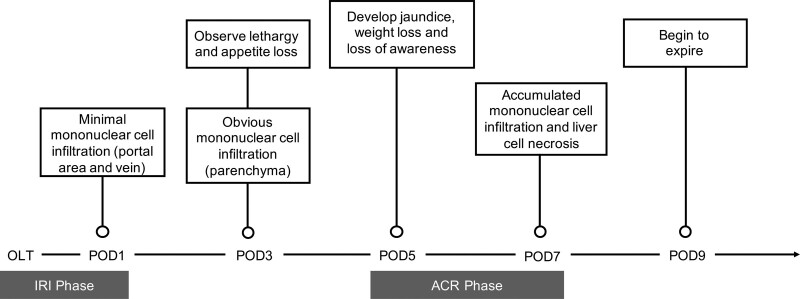
The use of rat models to explore ACR and tolerance in LT has provided valuable insights into postoperative rejection progression and served as a platform to test ACR treatments. Depending on the rat donor-recipient strain combinations used, specific levels and lengths of rejection vary. The degree of ACR can be determined with the help of laboratory-based and symptom-based tests. In laboratory-based histology, the Banff pattern is used to calculate Rejection Activity Index (RAI) based on changes in venous endothelial inflammation (E score), bile duct damage (B score), and portal inflammation (P score).87 In rejection rat combinations such as LEW→BN and DA→LEW, higher RAI values in tissue histology are indicative of increased rejection severity, hepatocyte necrosis, and worsened graft outcomes. In human LT, ACR normally presents in the first month after surgery88 and biopsies are taken to assess histology according to the Banff criteria.89 Additional measures of liver graft function in both human and rat LT involve characterization of liver enzyme activity, where higher serum values of aspartate aminotransferase, alanine transaminase, and total bilirubin correlate to lower liver function. Serum levels of biomarkers as well as soluble proinflammatory and anti-inflammatory factors may also inform rejection. Symptom-based indicators of graft rejection in rats assess rat activity, appetite, weight, and other physical factors such as jaundice or ascites development. Mortality as a result of graft rejection is tracked through survival analyses. LEW→BN rat OLT models typically survive for a median survival time (MST) of average 13.66 days with minimum and maximum MST reported at 4 and 33 days from 14 studies,36-48,90 while DA→LEW models survive on average 11.22 days with minimum and maximum MST of 8 and 15.5 days from 9 studies.58-66 As shown in Figure 1, rats in both LEW→BN and DA→LEW OLT models of rejection similarly begin to show poor appetite at postoperative day (POD) 3 and symptoms worsen to jaundice development and loss of awareness by POD7.37,46,48 These observations are accompanied by diminished liver function indicated by progressively increasing liver enzyme measures posttransplantation.46,48,58,64 At a cellular level, RAI values in LEW→BN and DA→LEW rat models increase significantly at POD5 and POD7 and hepatocyte structural damage assessed by TdT-mediated dUTP-X nick-end labeling staining is notable at POD7.46,48,59,64
FIGURE 1.
Development of ACR symptoms common to LEW→BN and DA→LEW rat OLT models. OLT rat recipients in alloimmune strain combinations that present ACR typically show lethargy and loss of appetite by POD3, and symptoms progressively worsen to development of severe jaundice and ascites from POD5. At the cellular level, assessment of RAI shows mononuclear cell infiltration as early as POD1, which continues to expand to liver parenchyma, with indication of severe ACR at POD7. LEW→BN rat OLT recipients typically survive for an average MST of 13.66 d, while DA→LEW models survive on average 11.22 d. ACR, acute cellular rejection; BN, Brown Norway; DA, Dark Agouti; IRI, ischemia-reperfusion injury; LEW, Lewis; MST, median survival time; OLT, orthotopic liver transplantation; POD, postoperative day; RAI, Rejection Activity Index.
THE INTERCONNECTION BETWEEN THE LIVER AND THE BODY
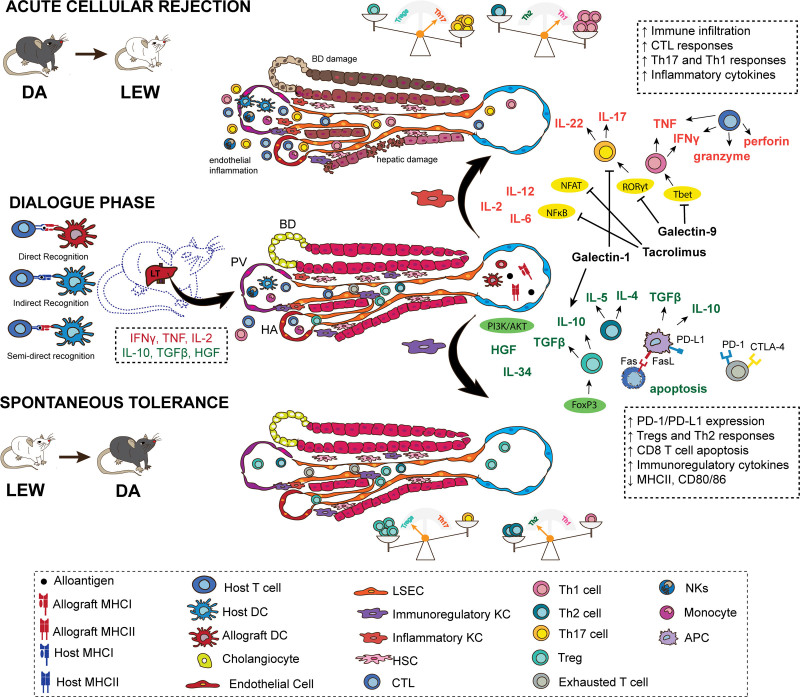
The multifunctional, tolerogenic, and complex nature of the liver and the central position that it occupies in the physiology of the body must be taken into consideration when trying to decipher the phenomena of tolerance and rejection after LT. Further examining how the liver is seen once transplanted into another body and how it interacts and communicates with the body that it is within are the first steps toward a better understanding of LT. The first days following LT are marked by upregulation of IL-2, interferon γ (IFN-γ), tumor necrosis factor (TNF), adhesion molecules such as vascular cell adhesion molecule-1 (VCAM-1), and intragraft leukocytes infiltration73 (Figure 2). This initial response can be attributed to (1) direct alloantigen recognition, where allograft APCs present intact alloantigen directly to host T cells, and (2) indirect alloantigen recognition, where host APCs process and present the allograft alloantigen to host T cells (Figure 2).91 The so-called passenger leukocytes (graft-derived immune cells) can also reach host lymphoid tissue and contribute to direct recognition of the alloantigen.92 The architecture of the liver combining a unique sinusoidal microvasculature marked by fenestrated endothelium, low blood pressure, and pattern of adhesion molecules facilitate the interaction between circulating immune cells and both parenchymal and nonparenchymal hepatic cells. Interestingly, in a mouse model of liver tolerance, a reduction in intragraft donor DCs was followed by an increase in host APCs expressing graft MHC molecule (also known as cross-dressing), which can present intact alloantigens to host T cells without further processing.93 Examining the cross-talk between donor hepatic cells, including parenchymal and immune cells, and the host immune cells is critical to determine the mechanisms associated with rejection and tolerance post-LT. Two aspects of LT that are especially intriguing are (1) the operational tolerance observed in a parcel of the LT recipients, and (2) the development of “infectious tolerance” post-LT. As mentioned, operational tolerant individuals can sustain transplant tolerance in the absence of IS therapy.27,28,94 Although the mechanisms associated with this state are poorly understood, there is evidence suggesting that increased frequency of Treg and hyporesponsive or exhausted T cells are important for sustaining tolerance in these individuals.95-99 “Infectious tolerance” on the other hand speaks to the extended tolerogenicity offered by liver allografts to other solid grafts from the same donor and has also been observed in rat models.31,100,101 Understanding the underlining mechanisms associated with spontaneous acceptance of graft is of great interest to medicine, and having animal models able to recapitulate the human LT procedures and outcomes can help advance the field in a more assertive way. Here, we review key mediators of rejection and tolerance, including recent findings uncovered by rat OLT models detailed in Table 2.
FIGURE 2.
The days that follow an LT are marked by a dialogue between the liver graft and the host, through direct, indirect, and semidirect allorecognition. In this process of getting acquainted, the communication between different cells and the signals in the microenvironment of the liver will determine whether tolerance or rejection will follow. An initial inflammatory response, marked by IL-2, IFN-γ, TNF, and VCAM-1, leads to intragraft leukocyte infiltration independent of the final outcome and, in the presence of hepatic signals, is key to prime the response seen afterward. For instance, IFN-γ secretion is key to upregulate PD-L1 expression by LSECs, hepatocytes, and KCs, which subsequently contributes to the establishment of a tolerogenic environment. If, however, the alloantigen load or inflammatory signals, such as IL-2, are overexpressed, then the response is skewed toward acute cellular rejection. KCs are found in a spectrum from anti-inflammatory to proinflammatory phenotypes and are important contributors to the final outcome following LT. Activation of transcription factors, such as NF-κB, NFAT, RORγt, T-bet, leads to an environment enriched in proinflammatory cytokines and guide T-cell differentiation toward Th17 and Th1 responses. On the other hand, the engagement of PIK3/AKT and the upregulation of coinhibitory molecules, such as PD-L1, lead to a tolerogenic environment, with predominance of Treg and Th2 responses. APC, antigen-presenting cell; BD, bile duct; CTL, cytotoxic T lymphocyte; CTLA-4, cytotoxic T-lymphocyte–associated protein 4; DA, Dark Agouti; DC, dendritic cell; FasL, Fas ligand; FGL2, higher fibrinogen protein 2; FOXP3, forkhead box P3, HA, hepatic artery; HGF, hepatic growth factor; HSC, hepatic stellate cell; IFN-γ, interferon γ; KC, Kupffer cell; LEW, Lewis; LSEC, liver sinusoidal endothelial cell; LT, liver transplant; MHC, major histocompatibility complex; NF-κB, kappa-light-chain-enhancer of activated B cells; NFAT, nuclear factor of activated T cells; NK, natural killer; PD-1, programmed cell death protein 1; PD-L1, PD-1 ligand; PV, portal vein; RORγt, retinoic acid receptor (RAR)–related orphan receptor (ROR)γt transcription factor; T-bet, T-box transcription factor; TGF-β, tumor growth factor β; Th, T helper cell; TNF, tumor necrosis factor; Treg, regulatory T cells; VCAM-1, vascular cell adhesion molecule-1.
TABLE 2.
Mechanisms of liver transplantation ACR and tolerance uncovered by rat OLT models
| Cell type | Mechanisms | Effect | Outcome | References |
|---|---|---|---|---|
| Cytotoxic T cells | Recognize alloantigen presented by APCs through direct or indirect recognition | Initiate downstream cell death pathways, inflammatory cytokine production, and immune cell recruitment to the liver graft | ACR | 36,49,73 |
| Th cells | Th1 and Th17 cells secrete IFN-γ, TNF, IL-2, IL-17, IL-22 | Promote proinflammatory microenvironment | ACR | 39,46,48,50,60,63,64,75 |
| Th2 cells produce IL-10, TGF-β, IL-4, IL-13 | Promote anti-inflammatory microenvironment | Tolerance | 39,46,48,60 | |
| Treg | Produce IL-10 and TGF-β | Modulate and maintain immature DC phenotype | Tolerance | 58 |
| Secrete exosomes | Inhibit CD8+ T-cell levels and function | 51 | ||
| DCs | Express FasL | Induce immunosuppressive T-cell (Treg, Th2) differentiation, reduce T-cell proliferation, induce antigen-specific T-cell apoptosis/anergy, reduce MHC and costimulatory molecule expression | Tolerance | 45,58,60,62,90 |
| Secrete IL-10 and TGF-β | ||||
| Macrophages, KCs | Express costimulatory molecules | Activate lymphocytes and inflammatory cytokine secretion | ACR | 55 |
| Produce IL-10 and TGF-β | Downregulate MHC and costimulatory molecule, upregulate MGAT5, which decreases T-cell activation and induces Treg differentiation | Tolerance | 41 | |
| Express PD-L1 | Inhibit T-cell proliferation, induce apoptosis, and suppress inflammatory cytokines | Tolerance | 37 | |
| MSCs | Express TGF-β | Induce Treg expansion and reduce Th17 frequency | Tolerance | 43,63,72 |
| Boost IL-10 produced by Treg | Promote anti-inflammatory microenvironment | 63 | ||
| Express PD-L1 | Bind PD-1 to induce immunoregulatory signals | 42,76 | ||
| Express HO-1 | Expand Treg population, maintain ACR attenuation past POD7 | 48 | ||
| Molecule/pathway | Role in ACR | ACR/tolerance | ACR rat model | References |
| Gal-1/NF-κB/RelB | Gal-1 transfusion maintains tolerant DC phenotype and alleviates ACR | Tolerance | DA→LEW | 60,65 |
| Limits DC-mediated CD4+ proliferation and increased Treg/Th17 ratio through NF-κB/RelB-IL-27 pathway | Tolerance | |||
| Reduces NF-κB/RelB to maintain tolerant DC | Tolerance | |||
| Gal-9 | Perfusion with recombinant Ad-Gal-9 suppressed Th1 and Th17 differentiation | Tolerance | LEW→BN | 39 |
| Inflammasome activation pathway | Blocking ASC-mediated caspase-1 activation of IL-1β reduces ACR inflammation | Tolerance | DA→LEW | 68 |
| Ceruloplasmin/Nrf-2/ROS | Knock-down of ceruloplasmin diminished hepatocyte survival and lost protection from oxidative damage | Tolerance | DA→LEW | 67 |
| IL-22/STAT3 | Neutralization of IL-22 early in IRI stage worsened graft function | ACR | LEW→BN | 50 |
| Neutralization of IL-22 late in ACR stage improved function | Tolerance | |||
| NFAT-BATF/JUN/IRF4-IL-21 | Inhibition of BATF/JUN/IRF4 complex attenuated rejection injury and decreased Bcl-6 and IL-21 in Tfh | ACR | DA→LEW | 66 |
| Fas/FasL | Mediates T-cell apoptosis to promote spontaneous acceptance of graft | Tolerance | DA→LEW | 62,90 |
| ERS-related IRE-1α/TRAF2/NF-κB | Suppression by gastrodin resulted in ACR attenuation and promotion of tolerogenic macrophage phenotype | ACR | LEW→BN | 40 |
| OX40/OX40L | Blocking OX40/OX40L resulted in less hepatic damage and longer survival | ACR | LEW→BN | 44 |
| HO-1 | Overexpression of HO-1 expanded Treg to maintain tolerance | Tolerance | LEW→BN | 48 |
| CTLA-4/B7 | Blocking CD28-B7 results in improved liver function and increased levels of immunomodulatory cytokines | Tolerance | LEW→BN | 45,55 |
| IDO | Overexpression of IDO led to fewer ACR symptoms and better survival | Tolerance | LEW→BN | 55 |
| Autophagy pathway | Blocking autophagy decreased CD8+ T function, prolonging graft survival | ACR | LEW→BN | 36 |
| FGL/STAT1/NF-κB | Overexpression of sFGL2 ameliorates ACR through anti-inflammatory cytokines and inhibit STAT1, NF-κB signaling, and induces immunoregulatory macrophage polarization | Tolerance | LEW→BN | 52 |
| IL-34/PI3K/Akt | Overexpression of IL-34 polarized immunoregulatory macrophages and inhibited ACR | Tolerance | LEW→BN | 53 |
| β-actin | Stabilizing β-actin increases CD8+ T-cell apoptosis and predicts ACR episodes | ACR | LEW→BN | 49 |
| PD-L1 | MSCs upregulate PD-L1 expression to attenuate ACR | Tolerance | LEW→BN | 42 |
| PD-L1 expression on KCs downregulated in ACR | Tolerance | 37 | ||
| Silencing PD-L1 increases inflammatory cytokines and decreases tolerogenic cytokines | 37 | |||
| MSCs modified to express PD-L1Ig inhibited lymphocyte activity and attenuated ACR | Tolerance | Wistar→SD | 76 |
ACR, acute cellular rejection; ASC, apoptosis-associated speck-like protein containing a caspase recruitment domain; BN, Brown Norway; CTLA-4, cytotoxic T-lymphocyte–associated protein 4; DA, Dark Agouti; DC, dendritic cell; ERS, endoplasmic reticulum stress; FasL, Fas ligand; Gal, galectin; HO-1, heme oxygenase-1; IDO, indoleamine 2,3-dioxygenase; IL, interleukin; IRI, ischemia-reperfusion injury; KC, Kupffer cell; LEW, Lewis; MGAT5, anti-N-acetylglucosaminyltransferase V; MSC, mesenchymal stem cell; NFAT, nuclear factor of activated T cell; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; OLT, orthotopic liver transplantation; PD-1, programmed cell death protein 1; PD-L1, programmed death ligand 1; ROS, reactive oxygen species; SD, Sprague Dawley.
Mechanisms Associated With Rejection of a Liver Graft
As previously noted, the initial immune response post-LT is mediated through direct and indirect alloantigen presentation by APCs to host T cells, however little is known of the specific interactions within the graft immune microenvironment which dictate graft rejection versus tolerance. The experimental rat model with its high parallels to human LT has been utilized to facilitate generation of insights into ACR mechanisms and potential therapeutic targets.
CD8+ T cells are known to recognize allograft antigens and differentiate into effector cells that target the graft for destruction through initiating cell death pathways, inflammatory cytokine production, and immune cell recruitment.102,103 Similar to human liver ACR, studies have shown the role of T-cell–mediated rejection in rat graft survival, wherein CD8+ T-cell infiltration into the graft is observed early after LT.36,49,73 In the LEW→BN ACR rat model, CD8+ T-cell infiltration gradually increases post-LT and peaks on POD9,49 while in the AxC 9935 Irish (ACI)→LEW ACR rat model, immunoelectron microscopic analysis depicted T-cell transmigration across portal vein endothelial cells and observed MHC I cell appearance at POD2, which later peaked at POD4.73 The LEW→BN rat model studied by Chen et al49, apart from reporting lymphocyte migration in the ACR rat group, also showed that upregulating β-actin expression with jasplakinolide (actin-stabilizing drug) in allogeneic rats correlated with higher CD8+ apoptosis, leading to prolonged survival. β-actin is a component of the cytoskeleton and is important in cellular functions, such as the regulation of immune cell apoptosis, infiltration and migration, and functions through the dynamic assembly and disassembly of its filament building blocks.104 It is capable of binding to TNF receptor 2 (TNFR2) and augment activation-induced cell death apoptosis through the death receptor pathway.105 A separate study of the LEW→BN model suggested an association between autophagy of CD8+ T cells and increased CD8+ T-cell activation and proliferation, where higher levels of autophagy correlated with more severe rejection.36 Inhibiting the autophagy pathway with 3-methyladenine increased T-cell apoptosis and effectively enhanced rat survival while minimizing ischemia-reperfusion hepatocyte damage.36 Interestingly, a subset of CD8+ T cells expressing low levels of CD45RC was recognized in a study exploring rapamycin effects in DA→LEW rats to cultivate an IS environment.61 Along with myeloid-derived suppressor cells, these CD8+CD45RClow T cells were upregulated in the rapamycin-treated rat group, induced graft acceptance, and prolonged rat survival.61
Two subsets of helper T cells, Th1 and Th17, are involved in promoting the proinflammatory condition in ACR106 (Figure 2), and these observations are mirrored in the rat model. Closely linked with cellular responses, the liver microenvironment consists of a network of cytokines both secreted by and acting upon aforementioned immune cells such as helper T cells, Treg, DCs, and macrophages, which also play a role in directing graft toward rejection or tolerant states.91 Cytokine factors released by Th1 cells, including IFN-γ, TNF, IL-2, and IL-12, are reported to promote ACR progression in multiple rat studies.39,46,48,60 Groups that induced ACR in LEW→BN rat LT saw significant increases in IFN-γ, TNF, and IL-2 beginning on POD1 and peaking on POD7.36,37,46,48,49,59 Th17-related cytokines, such as IL-17, IL-6, IL-22, and IL-27, have likewise been implicated in rat models.39,46,48,50,63,64,75 Serum IL-17 and IL-6 levels are consistently reported to be higher in a number of ACR rat combinations including Wistar→Sprague Dawley (SD),75 DA→LEW,60,64 and LEW→BN.46,48 A study by Zhou et al75 using Wistar→SD rats, on top of observing high levels of IL-17 and IL-6, injected IL-17-neutralizing antibodies in the ACR group starting from POD1 to POD3 and saw increased levels of Treg in the graft with improved liver function and survival (MST, 8.0 ± 1.2 d to MST, 32.0 ± 4.8 d). IL-22 has more recently been studied as an inflammatory cytokine associated with Th17 proliferation that acts through STAT3 and seemingly plays a temporal and dual role. Zhang et al50 found in a LEW→BN ACR model that neutralizing IL-22 early in the ischemia-reperfusion injury phase (POD1) led to decreased liver function but resulted in improved graft acceptance in the latter ACR stage (POD7), suggesting that timing is key in targeting IL-22 as a potential therapeutic. IL-27 is a proinflammatory cytokine, member of the IL-12 cytokine family, which has been shown to promote inflammatory helper T-cell differentiation.107,108 Lu et al65 showed in the DA→LEW model that downregulating IL-27 through the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)/RelB pathway by galectin-1 (Gal-1) administration limited Th17 cell proliferation, leading to improved liver function and survival. Expanding on galectin treatments, a study by Liu et al39 explored the role of galectin-9 (Gal-9) in LT by perfusing Ad-Gal-9 in a LEW→BN ACR LT during the cold ischemia time of the operation. In assessing ACR, levels of T-box transcription factor, retinoic acid receptor-related orphan receptor gamma t (RORγt), GATA-binding protein 3 and forkhead box P3 (FOXP3) mRNA, and protein expression of IFN-γ, IL-17, and IL-10 were measured. T-box transcription factor and RORγt are the key transcription factors involved in Th1 and Th17 cell differentiation, respectively109 and levels of both transcription factors were revealed to be lower in the Gal-9-treated group showing ACR attenuation. This observation correlated with lower IFN-γ and IL-17 levels, suggesting that the ACR attenuation was a result of Th1 and Th17 reduction and diminished proinflammatory environment.39 Further mechanistic exploration of tacrolimus-mediated graft acceptance by Tang et al64 identified a downregulation of interferon regulatory factor 4 (IRF4) expression in DA→LEW LT recipients after tacrolimus administration, where IRF4 expression specified by RORγt is important in mediating Th17 differentiation, suggesting that the nuclear factor of activated T cells–IRF4 signal pathway may be important in modulating the proinflammatory environment post-LT. An additional DA→LEW study examining the impact of tacrolimus also revealed that inhibition of BATF/JUN/IRF4 complex downstream of nuclear factor of activated T cells promotes tolerance and downregulation of inflammatory IL-21 secretion.66
In understanding the T-cell role in ACR, recent discovery showed that T-cell costimulatory molecule OX40 interacts with OX40L (CD252) on APCs to expand CD4+ T cells, influence Th1/Th2 differentiation, and increase effector and memory functions through intracellular signaling,110 providing a potential route for tolerance induction. A rat LEW→BN study experimentally reduced OX40/OX40L signaling through postoperative Ad-OX40Ig injection and observed less hepatic damage in the treatment groups compared with control with longer survival time that matched tacrolimus-treated results in terms of liver function, rejection, and apoptosis at POD7.44
Mechanisms Associated With Acceptance of a Liver Graft
The genome similarity between rat and human, physiological relevance of rat OLT procedures, and spontaneous presentation of tolerance in select strain combinations of rat LT donors and recipients can help uncover the underlying mechanisms of graft tolerance.
As mentioned, an inflammatory response (marked by IL-2 and IFN-γ) is present early after human LT and is common to both rejecting and tolerant rat models. In particular, IFN-γ secretion seems to upregulate PD-L1 expression in LSECs and hepatocytes111 and was shown as necessary for inducing tolerance.112 The tolerogenic environment of the liver, marked by increased expression of IL-10 and TGF-β, weakens T-cell activation leading to short-lived T-cell populations incapable of assembling an effective immune response.113 Specifically, liver APCs show low expression of costimulatory molecules (CD80/CD86) and elevated levels of inhibitory markers (PD-L1, CTLA-4) that impair T-cell activation (Figure 2). In addition, the liver microenvironment lacks stimulatory cytokines that act as the third signal in the events leading to T-cell activation, which include T-cell receptor (TCR) and CD28 interaction to MHC and B7 (also known as CD80/CD86) molecules on APCs as signals 1 and 2, respectively. The ability to induce Treg and mediate T-cell apoptosis is critical to liver graft acceptance.114,115 Blockade of PD-L1-PD-1 or B7-CTLA-4 in mice prevented apoptosis of alloreactive cells and converted spontaneous liver allograft tolerance into ACR.116,117 Meanwhile, Fas ligand (FasL) on APCs interact with Fas on T cells to induce specific T-cell apoptosis and promote immune tolerance.90
Very much like in humans, the tolerance-promoting immune microenvironment in rats consists of primarily IS cytokines TGF-β, and IL-10, as depicted in Figure 2. In LEW→BN and DA→LEW rat combinations within the 42 rat model-based articles, TGF-β and IL-10 levels were observably diminished post-LT, correlating with higher rejection and shorter survival times. In assessing the role of TGF-β in tolerance promotion, Qiu et al62 cotransfected immature DCs (imDCs) with TGF-β and FasL and injected the modified imDCs into DA→LEW ACR recipients 5 days before LT. Post-LT, groups treated with single and cotransfections showed reduction in costimulatory molecules, diminished T-cell proliferation, and increased liver function and survival.62 Similarly, another study, through overexpression of TGF-β on mesenchymal stem cells (MSCs) in DA→LEW rats, saw ACR attenuation, improved mortality, and increased Treg/Th17 ratio within the graft.63 In contrary to Th1 cells, the main helper T cells population driving tolerogenic states in the rat liver are Th2 cells that secrete IL-4 and IL-13 along with TGF-β and IL-10, and evaluation of these IS cytokine expressions are often reported in parallel to Th1-associated cytokines in the literature.45
Other soluble factors such as fibrinogen-like protein 2 (FGL2), discussed below, and acute-phase protein ceruloplasmin can also inform a tolerogenic microenvironment in the liver. Ceruloplasmin is produced by hepatocytes to mediate attenuation of reactive oxygen species damage and has been implicated to enhance hepatocyte survival posttransplantation.67 Ceruloplasmin levels are significantly higher in tolerant DA→Piebald Virol Glaxo rat models at POD63 than in DA→LEW models that expire at POD14, where systematic profiling showed that ceruloplasmin may be induced by IL-1β through ERK1/2-, JNK1-, and p39 MAPK activation of the AP-1 pathway which may contribute to operational tolerance.67
LSECs and KCs are key cellular mediators of liver tolerance. They hold potent endocytic capacity and are able to present alloantigens to both CD8+ and CD4+ T cells. However, the low expression of costimulatory molecules (CD80/CD86) and high expression of the coinhibitory molecule PD-L1 promote alloreactive T-cell apoptosis, IL-10 and TGF-β secretion and Treg differentiation. The latter is one of the most accepted agents in liver acceptance. Treg (CD4+CD25+FOXP3+) direct the immune microenvironment toward a more tolerant state and are known to inhibit DC maturation through IL-10, TGF-β, PD-L1, and IDO induction.58 The bidirectional interaction between DCs and Treg is important in orchestrating graft tolerance,58 and the balance between Treg and Th17 populations has also been suggested to maintain tolerance post-LT for individuals with liver disease.118 Furthermore, Treg have been associated with the mechanism of “infectious tolerance,” mentioned previously and are currently being assessed as a therapy in clinical trials, with most trials testing Treg infusion near the time of transplantation, as a way to induce tolerance (reviewed in Tang et al119 and Tanimine et al120).
In the context of rat OLT, Treg have shown similar importance in inducing IS environments. In a study where Treg isolated from LEW rat spleens were infused into DA→LEW recipients 5 days before LT, rejection characterized by RAI values were significantly lower on POD3 and POD7 compared with control. Additionally, IS cytokines TGF-β and IL-10 were heightened, while proinflammatory IL-12 levels were inhibited.58 This immunotolerance effects of Treg can be moreover combined with the administration of immature DCs, further described below, to produce synergistic ACR attenuation effects.58 Furthermore, exosomes from Treg have also been implicated in tolerance induction. Exosomes were collected from CD4+CD25+ Treg culture medium and injected into SD→Wistar LT recipients on select days post-LT and resulted in an increased survival time from 28 to 90 days.51 In vitro, CD8+ T-cell proliferation was inhibited by both Treg and Treg-derived exosomes, for which the effect could be reversed with exosome inhibitor administration.51 One of the challenges involving adoptive transfer of Treg as cell therapy, however, is clinical-grade manufacturing, especially for ex vivo expansion Treg.119 Pharmacological and immunological treatments that favor in vivo expansion of this population are also being considered.119,121-123
Myeloid-derived DCs (mDCs) in the liver microenvironment differentiate into a more tolerogenic phenotype. The presence of hepatocyte growth factor upregulates IL-10 expression by DCs monocytes16 and the strong interaction established between Ag-specific Treg and DCs can result in the removal of the MHC-antigen complex from the surface of the DCs, compromising their ability to present antigens.124 DCs have been extensively explored in rat models of LT. More specifically, DCs in the immature state (imDCs) or tolerogenic DCs, which express lower levels of MHC-II and costimulatory surface molecules, have been explored as local immunosuppression inducers in the liver posttransplantation.90 As previously mentioned, imDCs confer synergistic IS effects with Treg through upregulating tolerance-promoting cytokines, reducing T-cell proliferation, and inducing antigen-specific T-cell apoptosis.58 When constructed to overexpress IL-10 and FasL and injected into LEW→BN recipients 5 days before LT, DCs were able to reduce expression of MHC-II and costimulatory molecules (CD80/CD86), decrease T-cell expansion, and induce an IS environment.90 In particular, cotransduction with both IL-10 and FasL led to better results than single transductions alone.90 Tolerogenic DCs can be generated through induction by Gal-1 and other agents such as dexamethasone, VitD3, and rapamycin. Gal-1 regulates activated T-cell apoptosis, increases Treg, and suppresses direct DC recognition.125 A previous study by Peng et al60 explored treating DA→LEW recipients with Gal-1-induced DCs (DCgal-1) and apoptotic lymphocytes, both individually and synergistically. Proinflammatory cytokines declined on POD7 and an increase in tolerant cytokines was observed in the long term, while the transfusion promoted recipient T-cell hyporesponsiveness and Treg expansion. Treatment with both Gal-1 and apoptotic lymphocyte rendered better results compared with individual treatments alone and increased MST from 10 to 43.5 days.60
Liver resident macrophages (KCs) have emerged as a critical determinant of posttransplant outcomes126,127 and are traditionally classified as anti-inflammatory or proinflammatory where polarization is dynamically influenced by cells such as Treg that moderate IS microenvironments.128,129 Activated KCs in the early ischemia-reperfusion injury stage release inflammatory IL-1, IL-6, and TNF cytokines and promote Th1 subtype differentiation, while anti-inflammatory KCs regulate Th2 differentiation and increase T-cell apoptosis.52,53 Previous rat studies, described below, show that proinflammatory phenotype can be directed through the NF-κB and MAPK pathway,54 while regulatory phenotype is promoted by higher FGL2 and IL-34 exposure and acts through the critical mechanistic target of rapamycin intermediate step,52,53 which informed current IS therapies of rapamycin inhibitors (eg, mechanistic target of rapamycin-Is).7
To explore the role macrophages play in immune tolerance induction post-LT and assess whether polarized macrophages direct tolerance, Yang et al41 performed an adoptive transfer experiment of CD163+, TGF-β- and IL-10-secreting anti-inflammatory macrophages into LEW→BN ACR recipients through the portal vein during OLT. The study resulted in diminished CD8+ T-cell infiltration, increased cellular apoptosis, and improved graft function and survival (25.0 ± 12.2 and 26.0 ± 12.4 d in BN- and LEW-derived anti-inflammatory macrophage-treated group, compared with 16.6 ± 2.3 d in PBS-treated group).41 The anti-inflammatory macrophage-treated rat group also saw a decreased expression of MHC-II+ cells and increased anti-N-acetylglucosaminyltransferase V expression, which has been previously implicated as a downstream target of IL-10 in limiting T-cell activation.41 Analogous to human observations of increased coinhibitory molecule PD-L1 on APCs to induce tolerance, an increase in PD-L1 expression on LEW→BN donor rat liver KCs likewise led to reduced T-cell proliferation and function, which in turn promotes tolerance in the graft.37 To further characterize the role of PD-L1 in the same study, Gong et al37 cocultured KCs that were silenced by PD-L1-shRNA interference plasmids with T cells and observed significantly higher levels of inflammatory IL-2, IFN-γ, and TNF cytokines.37 A separate in vitro study by Li et al55 cocultured isolated donor LEW KCs, transfected for RNA interference vector of costimulatory B7 molecules, with donor BN lymphocytes and saw that decreased B7 expression prevented lymphocyte activation and proliferation, encouraging tolerance.55 These results of coinhibitory and costimulatory molecule expression in rat macrophages reflect observations in human LT, once again demonstrating the rat model’s relevance and high resemblance to the human model.
To elucidate the mechanism of macrophage-induced tolerance, Karpova et al81 studied the course of immunoproteasome and macrophage changes post-LT in the Wistar→August ACR rat model. They proposed that tolerance induction occurs in 2 phases: (1) early phase 1 (POD 1–5), where recipient immune cells infiltrate the graft, KCs and LSECs process and present antigens, and T-cell activation takes place, and (2) phase 2 (POD 5–14), where KC polarization and composition of graft immune cells dictate ACR versus tolerance progression. Specifically, low levels of immunoproteasome-expressing immune cells were observed on POD3 and the characters of immune cells filling the niche thereafter are presumed to drive ACR or tolerance. Through monitoring changes in the LMP2 and LMP7 subunits of the immunoproteasome responsible for alloantigen recognition and processing, it was suggested that subunit levels may associate with anti-inflammatory macrophage activation through regulating antigen presentation and promote tolerance induction in phase 2.81
In the search for ways to regulate macrophage polarization toward anti-inflammatory character, FGL2, which has previously been shown to possess immunoregulatory function130 and ability to promote tolerance in mouse and rat cardiac transplant models,131,132 was examined in the rat OLT model. Pan et al52 first observed upregulation of FGL2 mRNA and protein levels in the tolerant BN→LEW model and found that in vitro, FGL2 levels correlated with decreased STAT1 and NF-κB phosphorylation levels important for macrophage polarization. In vivo, overexpression of soluble FGL2 through injection of adeno-associated virus expressing FGL2 (AAV-FGL2) into recipient rats of the ACR LEW→BN model before LT, induced polarization toward tolerogenic KC, promoted an IL-10 and TGF-β-rich immunotolerant microenvironment, and led to improved graft function and survival.52
Another recently explored cytokine associated with IS ability is IL-34. IL-34 is secreted by human and rodent Treg and has previously been associated with transplant tolerance in a rat cardiac transplant model.133 To determine IL-34 effects in the rat liver model, Zhao et al53 first noticed lower levels of IL-34 in LEW→BN ACR rat LT. Then through AAV-mediated overexpression of IL-34 and administration 30 days before LT in LEW→BN recipients, they observed attenuation of ACR in the treatment group. In vitro, IL-34 induced a phenotype switch from inflammatory KCs to noninflammatory KCs through activation of the PI3K/Akt pathway. Further adoptive transfer of lipopolysaccharide-stimulated and unstimulated KCs into AAV-IL-34-treated rats showed improved graft acceptance compared with no-KC transfer and control LEW→BN groups, suggesting that the ACR attenuation by IL-34 was mediated through noninflammatory KCs.53
Additionally, an increase of NK T-cell population was associated with acceptance of liver graft contributing to downregulating inflammatory responses and promoting a tolerogenic environment in mice.134 Further studies exploring the role of hepatic NK T cells in the induction of peripheral tolerance are also warranted.134
As discussed here, different strategies able to induce tolerance in solid organ transplantation, including chimerism-based tolerance, and the main obstacles in the field are topic of great interest.135,136 Chimerism marks the establishment of donor hematopoietic cells within the recipient after transplantation. This state can be either transient or permanent and encompass >1% of hematopoietic cell lineages from donor origin (macrochimerism) or <1% (microchimerism).135 Several chimerism tolerance protocols based on bone marrow transplantation or hematopoietic stem cell transplantation have been tested both in preclinical and clinical settings and focuses specially on living-related renal transplantation.135 These protocols include different conditioning regimens of irradiation, IS, and T-cell depletion (such as anti-CD8 monoclonal antibodies), which often start days before transplantation.135 Despite promising, chimerism-based protocols are still faced with several constraints, including safety, feasibility, and applicability to deceased donor grafts, HLA-mismatched patients, and limited exploration in solid organs other than kidney.135 Chaudhry et al137 tested whether the induction of transient donor chimerism would lead to liver tolerance posttransplant using cynomolgus macaques as a model. Liver rejection was observed shortly after IS withdrawal (POD28) and suggested that interventions able to act during the inflammatory phase post-LT, such as depletion of CD8+ memory T cells, may also be required.137
Bone marrow cells have the capability of differentiating into liver KCs, endothelial cells, and hepatocytes and have been frequently explored as a way to induce tolerance in rat ACR models. In the DA→LEW model, replacing donor KCs with recipient bone marrow cells after total body irradiation pre-LT led to a decrease in IL-2 and IFN-γ levels at POD7 and attenuation of ACR.59 Multiple studies in LEW→BN, DA→LEW, and LEW→ACI rat models have also shown that MSCs, a subpopulation of cells within the BM, specifically function to attenuate ACR and improve survival rates in allogeneic transplants, with clear reductions in both liver enzymes and RAI at POD3 and POD7.38,42,46,48,72,76 MSCs originate from the bone marrow, are capable of self-renewal and pluripotency, and are speculated to attenuate ACR either through soluble immune modulators or through cell to cell contact mechanisms.42,76,136,138 Treatment with MSCs in some cases may effectively hinder symptom progression by weeks or altogether.46,136,138 In particular, MSCs seem to mediate tolerance mechanisms through upregulating PD-L1 coinhibitory molecule expression to mitigate ACR progression.42,136 Assessment of helper T cells in MSC-treated conditions indicated that infusion of MSCs prefers anti-inflammatory-associated Th2 and Treg differentiation over Th1 and Th17 cells.46 Aligning with these observations, proinflammatory cytokine levels were reduced, while anti-inflammatory cytokine levels of IL-10 and TGF-β increased.46,48,72,136,138 MSCs were also associated with an upregulation of splenocyte Treg and these IS effects were enhanced when MSCs were transduced with heme oxygenase-1, an immunoregulatory player previously implicated in tolerance (Figure 2).48
Limitations
Despite the key benefits of employing rat models of OLT, including optimal size for microsurgery, similarity in postoperative immunological progression, and high genome match of up to 90% resemblance to the human liver,32 the model is not without limitations. For one, although resembling human genetics,10,139 the MHC of the rat, RT1, and the rat genome is not as thoroughly characterized compared with the mouse model.9 Recent efforts, however, have advanced genome characterization in the rat model.139 As expected, differences also exist between rat and human genomes where certain genes map to different chromosomes and chromosomal locations, such as with the RT1 MHC class I gene.140 Moreover, although bigger in size compared with mouse models,9 rats are comparably smaller animals to humans, which render microsurgical techniques demanding for surgeons.141 Often, physiological relevance for long-term studies obtained from anastomoses that mimic human transplantation need to be balanced with simpler procedures and shortened surgical time.141
UNDERSTANDING THE LIVER IMMUNE MICROENVIRONMENT—CHALLENGES AND FUTURE DIRECTIONS
Understanding the dynamic interaction between host and graft during rejection and tolerance post-LT and how different cell populations adapt to the evolving microenvironment and shape this environment is critical to the development of novel therapies. The systemic events that follow a liver transplant, in addition to events within the liver graft, are strong evidence of the ability of the liver to not only neutralize circulatory donor-specific antibodies but also to reshape itself and immune responses in different organs. Strategies able to instruct the recipient’s immune system to recognize donor antigens as self, eliminating the need for IS and maintaining their immune competence, are the ultimate goal. Grasping this complex network can certainly shed light on how posttransplant immunotolerance can be achieved and perhaps how to make more organs suitable for transplantation. The combination of single-cell transcriptomic techniques and functional assays can provide a more accurate description of the complex network of the liver, its interaction with the body, and key molecular pathways associated with rejection/acceptance. The use of single-cell RNA sequencing is revolutionizing the way we look at the cellular composition and function of different organs142 and has provided in-depth characterization of the liver landscape.128 This framework should be applied to the study of LT.
LT offers a unique opportunity to study mechanisms of immunosuppression, specially aimed at reprogramming the recipient immune system to establish and sustain tolerance in the absence of IS therapy. The characterization of rat OLT models helps to fully explore their potential as platforms to perform mechanistic studies of rejection and tolerance during transplantation. This may also contribute to new therapies that can help recover or improve outcomes with marginal organs, making them suitable for transplantation.
CONCLUSION
Through elegant rat models that recapitulate aspects of ACR and tolerance and can be modified to promote or treat either, we have begun to understand the underlying mechanisms of ACR and spontaneously developed operational tolerance, with hopes to inform therapeutics that will restore liver tolerance, obliterate IS therapy, and improve quality of postoperative life. However, the role of specific cell populations (donor and recipient) in transplant rejection remains unknown, which is limiting the ability to specifically target hepatic populations as part of IS strategies. Employing more specific strategies rather than systemic immunosuppression would limit off-target effects in patients. The overall goal of a deep characterization of immunological changes posttransplantation in rejection and tolerant individuals is to enable uncovering of the mechanistic basis that will guide the development of new therapeutic strategies to restore liver tolerance and preserve host immunocompetence.
Footnotes
We would like to acknowledge the Toronto General and Western Hospital Foundation, and the Natural Sciences and Engineering Research Council Discovery Grant program RGPIN-2018-05958 and the Canadian Institutes of Health Research PJT 162098 to S.A.M. C.T.P has received postdoctoral funds from the Canadian Network on Hepatitis C and PSC Partners Canada. Canadian Network on Hepatitis C is funded by a joint initiative of the Canadian Institutes of Health Research (NHC-142832) and the Public Health Agency of Canada.
The authors declare no conflicts of interest.
S.A.M. and C.T.P. conceived, designed, and coordinated the review. X.W. conducted the search, interpreted the relevant literature, and drafted the article. All authors contributed to the writing and reviewing of the article and approved the final version for submission.
REFERENCES
- 1.Kubes P, Jenne C. Immune responses in the liver. Annu Rev Immunol. 2018;36:247–277. [DOI] [PubMed] [Google Scholar]
- 2.Guillot A, Tacke F. Liver macrophages: old dogmas and new insights. Hepatol Commun. 2019;3:730–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horst AK, Neumann K, Diehl L, et al. Modulation of liver tolerance by conventional and nonconventional antigen-presenting cells and regulatory immune cells. Cell Mol Immunol. 2016;13:277–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choudhary NS, Saigal S, Bansal RK, et al. Acute and chronic rejection after liver transplantation: what a clinician needs to know. J Clin Exp Hepatol. 2017;7:358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song ATW, Avelino-Silva VI, Pecora RAA, et al. Liver transplantation: fifty years of experience. World J Gastroenterol. 2014;20:5363–5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neuberger J. An update on liver transplantation: a critical review. J Autoimmun. 2016;66:51–59. [DOI] [PubMed] [Google Scholar]
- 7.Levitsky J, Burrell BE, Kanaparthi S, et al. Immunosuppression withdrawal in liver transplant recipients on sirolimus. Hepatology. 2020;72:569–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lucey MR, Terrault N, Ojo L, et al. Long-term management of the successful adult liver transplant: 2012 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl. 2013;19:3–26. [DOI] [PubMed] [Google Scholar]
- 9.Qian S, Fung JJ, Demetris AJ, et al. Orthotopic liver transplantation in the mouse. Transplantation. 1991;52:562–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doorschodt BM, Teubner A, Kobayashi E, et al. Promising future for the transgenic rat in transplantation research. Transplant Rev (Orlando). 2014;28:155–162. [DOI] [PubMed] [Google Scholar]
- 11.Crispe IN. Immune tolerance in liver disease. Hepatology. 2014;60:2109–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heymann F, Tacke F. Immunology in the liver—from homeostasis to disease. Nat Rev Gastroenterol Hepatol. 2016;13:88–110. [DOI] [PubMed] [Google Scholar]
- 13.Mehrfeld C, Zenner S, Kornek M, et al. The contribution of non-professional antigen-presenting cells to immunity and tolerance in the liver. Front Immunol. 2018;9:635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dou L, Ono Y, Chen YF, et al. Hepatic dendritic cells, the tolerogenic liver environment, and liver disease. Semin Liver Dis. 2018;38:170–180. [DOI] [PubMed] [Google Scholar]
- 15.Dunham RM, Thapa M, Velazquez VM, et al. Hepatic stellate cells preferentially induce Foxp3+ regulatory T cells by production of retinoic acid. J Immunol. 2013;190:2009–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rutella S, Bonanno G, Procoli A, et al. Hepatocyte growth factor favors monocyte differentiation into regulatory interleukin (IL)-10++IL-12low/neg accessory cells with dendritic-cell features. Blood. 2006;108:218–227. [DOI] [PubMed] [Google Scholar]
- 17.Weiskirchen R, Tacke F. Cellular and molecular functions of hepatic stellate cells in inflammatory responses and liver immunology. Hepatobiliary Surg Nutr. 2014;3:344–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carambia A, Frenzel C, Bruns OT, et al. Inhibition of inflammatory CD4 T cell activity by murine liver sinusoidal endothelial cells. J Hepatol. 2013;58:112–118. [DOI] [PubMed] [Google Scholar]
- 19.Carambia A, Freund B, Schwinge D, et al. TGF-β-dependent induction of CD4+CD25+Foxp3+ Tregs by liver sinusoidal endothelial cells. J Hepatol. 2014;61:594–599. [DOI] [PubMed] [Google Scholar]
- 20.Erhardt A, Biburger M, Papadopoulos T, et al. IL-10, regulatory T cells, and Kupffer cells mediate tolerance in concanavalin A-induced liver injury in mice. Hepatology. 2007;45:475–485. [DOI] [PubMed] [Google Scholar]
- 21.Thomson AW, Knolle PA. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol. 2010;10:753–766. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Lu L, Qian S, et al. Hepatic stellate cells directly inhibit B cells via programmed death-ligand 1. J Immunol. 2016;196:1617–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breous E, Somanathan S, Vandenberghe LH, et al. Hepatic regulatory T cells and Kupffer cells are crucial mediators of systemic T cell tolerance to antigens targeting murine liver. Hepatology. 2009;50:612–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diehl L, Schurich A, Grochtmann R, et al. Tolerogenic maturation of liver sinusoidal endothelial cells promotes B7-homolog 1-dependent CD8+ T cell tolerance. Hepatology. 2008;47:296–305. [DOI] [PubMed] [Google Scholar]
- 25.Schurich A, Berg M, Stabenow D, et al. Dynamic regulation of CD8 T cell tolerance induction by liver sinusoidal endothelial cells. J Immunol. 2010;184:4107–4114. [DOI] [PubMed] [Google Scholar]
- 26.Limmer A, Ohl J, Kurts C, et al. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nat Med. 2000;6:1348–1354. [DOI] [PubMed] [Google Scholar]
- 27.Takatsuki M, Uemoto S, Inomata Y, et al. Weaning of immunosuppression in living donor liver transplant recipients. Transplantation. 2001;72:449–454. [DOI] [PubMed] [Google Scholar]
- 28.Orlando G, Soker S, Wood K. Operational tolerance after liver transplantation. J Hepatol. 2009;50:1247–1257. [DOI] [PubMed] [Google Scholar]
- 29.Pérez-Sanz F, Revilla-Nuin B, Martínez-Alarcón L, et al. Tolerance biomarkers in liver transplantation: independent external validation of the predictive strength of SENP6 and FEM1C gene expression. Transplantation. 2019;103:1887–1892. [DOI] [PubMed] [Google Scholar]
- 30.Busuttil RW, De Carlis LG, Mihaylov PV, et al. The first report of orthotopic liver transplantation in the Western world. Am J Transplant. 2012;12:1385–1387. [DOI] [PubMed] [Google Scholar]
- 31.Kamada N. The immunology of experimental liver transplantation in the rat. Immunology. 1985;55:369–389. [PMC free article] [PubMed] [Google Scholar]
- 32.Gibbs RA, Weinstock GM, Metzker ML, et al. ; Rat Genome Sequencing Project Consortium. Genome sequence of the Brown Norway rat yields insights into mammalian evolution. Nature. 2004;428:493–521. [DOI] [PubMed] [Google Scholar]
- 33.Lee S, Charters AC, Chandler JG, et al. A technique for orthotopic liver transplantation in the rat. Transplantation. 1973;16:664–669. [DOI] [PubMed] [Google Scholar]
- 34.Kamada N, Calne RY. A surgical experience with five hundred thirty liver transplants in the rat. Surgery. 1983;93(Pt 1):64–69. [PubMed] [Google Scholar]
- 35.Settaf A, Milton AD, Spencer SC, et al. Donor class I and class II major histocompatibility complex antigen expression following liver allografting in rejecting and nonrejecting rat strain combinations. Transplantation. 1988;46:32–40. [DOI] [PubMed] [Google Scholar]
- 36.Chen X, Wang L, Deng Y, et al. Inhibition of autophagy prolongs recipient survival through promoting CD8+ T cell apoptosis in a rat liver transplantation model. Front Immunol. 2019;10:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gong J, Cao D, Chen Y, et al. Role of programmed death ligand 1 and Kupffer cell in immune regulation after orthotopic liver transplantation in rats. Int Immunopharmacol. 2017;48:8–16. [DOI] [PubMed] [Google Scholar]
- 38.Sun Z, Li T, Wen H, et al. Immunological effect induced by mesenchymal stem cells in a rat liver transplantation model. Exp Ther Med. 2015;10:401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu YM, Chen Y, Li JZ, et al. Up-regulation of Galectin-9 in vivo results in immunosuppressive effects and prolongs survival of liver allograft in rats. Immunol Lett. 2014;162(Pt A):217–222. [DOI] [PubMed] [Google Scholar]
- 40.Yuan F, Xu X, Wu Y, et al. Gastrodin ameliorates acute rejection via IRE1α/TRAF2/NF-κB in rats receiving liver allografts. Biomed Res Int. 2019;2019:9276831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang P, Zhang X, Lin Z, et al. Adoptive transfer of polarized M2c macrophages ameliorates acute rejection in rat liver transplantation. Am J Transl Res. 2020;12:2614–2626. [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Q, Zhou R, Zhang Y, et al. Bone marrow mesenchymal stromal cells attenuate liver allograft rejection may via upregulation PD-L1 expression through downregulation of miR-17-5p. Transpl Immunol. 2018;51:21–29. [DOI] [PubMed] [Google Scholar]
- 43.Gao W, Zhang L, Zhang Y, et al. Adipose-derived mesenchymal stem cells promote liver regeneration and suppress rejection in small-for-size liver allograft. Transpl Immunol. 2017;45:1–7. [DOI] [PubMed] [Google Scholar]
- 44.Chen ZH, Wang C, Wei FX, et al. Adenovirus-mediated OX40Ig gene transfer induces long-term survival of orthotopic liver allograft in rats. Transpl Immunol. 2018;48:32–38. [DOI] [PubMed] [Google Scholar]
- 45.Wu Y, Yu Z, Gong J, et al. Effects of combined genes of CTLA4Ig and IDO in post-liver transplantation immune tolerance of rats. Ann Hepatol. 2016;15:729–737. [DOI] [PubMed] [Google Scholar]
- 46.Yang Y, Shen ZY, Wu B, et al. Mesenchymal stem cells improve the outcomes of liver recipients via regulating CD4+ T helper cytokines in rats. Hepatobiliary Pancreat Dis Int. 2016;15:257–265. [DOI] [PubMed] [Google Scholar]
- 47.Chen Y, Liang S, Long F, et al. Augmenter of liver regeneration attenuates acute rejection after rat liver transplantation. Am J Surg. 2016;212:128–137. [DOI] [PubMed] [Google Scholar]
- 48.Wu B, Song HL, Yang Y, et al. Improvement of liver transplantation outcome by heme oxygenase-1-transduced bone marrow mesenchymal stem cells in rats. Stem Cells Int. 2016;2016:9235073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen X, Zheng J, Cai J, et al. The cytoskeleton protein β-actin may mediate T cell apoptosis during acute rejection reaction after liver transplantation in a rat model. Am J Transl Res. 2017;9:4888–4901. [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y, Wang X, Mao L, et al. Dual roles of IL-22 at ischemia-reperfusion injury and acute rejection stages of rat allograft liver transplantation. Oncotarget. 2017;8:115384–115397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen L, Huang H, Zhang W, et al. Exosomes derived from T regulatory cells suppress CD8+ cytotoxic T lymphocyte proliferation and prolong liver allograft survival. Med Sci Monit. 2019;25:4877–4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pan G, Zhao Z, Tang C, et al. Soluble fibrinogen-like protein 2 ameliorates acute rejection of liver transplantation in rat via inducing Kupffer cells M2 polarization. Cancer Med. 2018;7:3168–3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao Z, Pan G, Tang C, et al. IL-34 inhibits acute rejection of rat liver transplantation by inducing Kupffer cell M2 polarization. Transplantation. 2018;102:e265–e274. [DOI] [PubMed] [Google Scholar]
- 54.Wu Y, Wang Y, Li M, et al. Gadolinium chloride suppresses acute rejection and induces tolerance following rat liver transplantation by inhibiting Kupffer-cell activation. Exp Ther Med. 2014;8:1777–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li T, Zhu JY, Wang FS, et al. Down-regulation of donor Kupffer cell B7 expression reduced recipient lymphocyte activation and secretion of interleukin-2 in vitro. Transplant Proc. 2015;47:2985–2990. [DOI] [PubMed] [Google Scholar]
- 56.Feng Y, Han Z, Feng Z, et al. Approaching treatment for immunological rejection of living-donor liver transplantation in rats. BMC Gastroenterol. 2020;20:7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Lin ZB, Yang PJ, Zhang X, et al. Translationally controlled tumor protein exerts a proinflammatory role in acute rejection after liver transplantation. Hepatobiliary Pancreat Dis Int. 2020;19:235–243. [DOI] [PubMed] [Google Scholar]
- 58.He W, Chen L, Zheng L, et al. Prolonged survival effects induced by immature dendritic cells and regulatory T cells in a rat liver transplantation model. Mol Immunol. 2016;79:92–97. [DOI] [PubMed] [Google Scholar]
- 59.Endo K, Hori T, Jobara K, et al. Pretransplant replacement of donor liver grafts with recipient Kupffer cells attenuates liver graft rejection in rats. J Gastroenterol Hepatol. 2015;30:944–951. [DOI] [PubMed] [Google Scholar]
- 60.Peng Y, Ye Y, Jia J, et al. Galectin-1-induced tolerogenic dendritic cells combined with apoptotic lymphocytes prolong liver allograft survival. Int Immunopharmacol. 2018;65:470–482. [DOI] [PubMed] [Google Scholar]
- 61.Hamdani S, Thiolat A, Naserian S, et al. Delayed and short course of rapamycin prevents organ rejection after allogeneic liver transplantation in rats. World J Gastroenterol. 2017;23:6962–6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qiu M, Chen Y, Chen L, et al. Transforming growth factor β1 and Fas ligand synergistically enhance immune tolerance in dendritic cells in liver transplantation. J Surg Res. 2017;218:180–193. [DOI] [PubMed] [Google Scholar]
- 63.Tang J, Yang R, Lv L, et al. Transforming growth factor-β-expressing mesenchymal stem cells induce local tolerance in a rat liver transplantation model of acute rejection. Stem Cells. 2016;34:2681–2692. [DOI] [PubMed] [Google Scholar]
- 64.Tang T, Lu Q, Yang X, et al. Roles of the tacrolimus-dependent transcription factor IRF4 in acute rejection after liver transplantation. Int Immunopharmacol. 2015;28:257–263. [DOI] [PubMed] [Google Scholar]
- 65.Lu H, Dai X, Li X, et al. Gal-1 regulates dendritic cells-induced Treg/Th17 balance through NF-κB/RelB-IL-27 pathway. Ann Transl Med. 2019;7:628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tang T, Xu T, Liu X, et al. Roles of BATF/JUN/IRF4 complex in tacrolimus mediated immunosuppression on Tfh cells in acute rejection after liver transplantation. J Cell Physiol. 2020;236:1776–1786. [DOI] [PubMed] [Google Scholar]
- 67.Wang PW, Wu TH, Pan TL, et al. Integrated proteome and cytokine profiles reveal ceruloplasmin eliciting liver allograft tolerance via antioxidant cascades. Front Immunol. 2018;9:2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hong BJ, Liu H, Wang ZH, et al. Inflammasome activation involved in early inflammation reaction after liver transplantation. Immunol Lett. 2017;190:265–271. [DOI] [PubMed] [Google Scholar]
- 69.Vitalone MJ, Wei L, Fujiki M, et al. Liver microRNA profile of induced allograft tolerance. Transplantation. 2016;100:781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wei W, Huang XH, Liang D, et al. A proteomic analysis of transplanted liver in a rat model of chronic rejection. Clin Res Hepatol Gastroenterol. 2015;39:340–350. [DOI] [PubMed] [Google Scholar]
- 71.Xie H, Zhu H, Zhou K, et al. Target-oriented delivery of self-assembled immunosuppressant cocktails prolongs allogeneic orthotopic liver transplant survival. J Control Release. 2020;328:237–250. [DOI] [PubMed] [Google Scholar]
- 72.Niu J, Wang Y, Liu B, et al. Mesenchymal stem cells prolong the survival of orthotopic liver transplants by regulating the expression of TGF-β1. Turk J Gastroenterol. 2018;29:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kariya T, Ueta H, Xu XD, et al. Direct evidence for activated CD8+ T cell transmigration across portal vein endothelial cells in liver graft rejection. J Gastroenterol. 2016;51:985–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xie Y, Wu Y, Xin K, et al. Delayed donor bone marrow infusion induces liver transplant tolerance. Transplantation. 2017;101:1056–1066. [DOI] [PubMed] [Google Scholar]
- 75.Zhou Y, Yang X, Zhang H, et al. The roles of T helper type 17/regulatory T cells in acute rejection after liver transplantation in rats. Transplantation. 2015;99:1126–1131. [DOI] [PubMed] [Google Scholar]
- 76.Li P, Zhang YY, Deng J. PDL1Ig gene-loaded BMSCs Induce liver transplantation immune tolerance. Eur Rev Med Pharmacol Sci. 2018;22:3214–3223. [DOI] [PubMed] [Google Scholar]
- 77.Kataoka M, Margenthaler JA, Ku G, et al. “Infectious tolerance” develops after the spontaneous acceptance of Lewis-to-Dark Agouti rat liver transplants. Surgery. 2003;134:227–234. [DOI] [PubMed] [Google Scholar]
- 78.Asakura H, Takayashiki T, Ku G, et al. The persistence of regulatory cells developing after rat spontaneous liver acceptance. Surgery. 2005;138:329–334. [DOI] [PubMed] [Google Scholar]
- 79.Jiang Z, Chen Y, Feng X, et al. Hepatic stellate cells promote immunotolerance following orthotopic liver transplantation in rats via induction of T cell apoptosis and regulation of Th2/Th3-like cell cytokine production. Exp Ther Med. 2013;5:165–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhuo M, Fujiki M, Wang M, et al. Identification of the rat NKG2D ligands, RAE1L and RRLT, and their role in allograft rejection. Eur J Immunol. 2010;40:1748–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Karpova YD, Ustichenko VD, Alabedal’karim NM, et al. Change in the content of immunoproteasomes and macrophages in rat liver at the induction of donor-specific tolerance. Acta Naturae. 2017;9:71–80. [PMC free article] [PubMed] [Google Scholar]
- 82.Qian S, Demetris AJ, Murase N, et al. Murine liver allograft transplantation: tolerance and donor cell chimerism. Hepatology. 1994;19:916–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thomson AW, Vionnet J, Sanchez-Fueyo A. Understanding, predicting and achieving liver transplant tolerance: from bench to bedside. Nat Rev Gastroenterol Hepatol. 2020;17:719–739. [DOI] [PubMed] [Google Scholar]
- 84.Czigány Z, Iwasaki J, Yagi S, et al. Improving research practice in rat orthotopic and partial orthotopic liver transplantation: a review, recommendation, and publication guide. Eur Surg Res. 2015;55:119–138. [DOI] [PubMed] [Google Scholar]
- 85.Kamada N, Calne RY. Orthotopic liver transplantation in the rat. Technique using cuff for portal vein anastomosis and biliary drainage. Transplantation. 1979;28:47–50. [PubMed] [Google Scholar]
- 86.Chen X, Sekhon M, Ma X, et al. Reduced complications after arterial reconnection in a rat model of orthotopic liver transplantation. J Vis Exp. 2020. doi: 10.3791/60628. [DOI] [PubMed] [Google Scholar]
- 87.Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997;25:658–663. [DOI] [PubMed] [Google Scholar]
- 88.Boyd A, Cain O, Chauhan A, et al. Medical liver biopsy: background, indications, procedure and histopathology. Frontline Gastroenterol. 2020;11:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dogan N, Hüsing-Kabar A, Schmidt HH, et al. Acute allograft rejection in liver transplant recipients: incidence, risk factors, treatment success, and impact on graft failure. J Int Med Res. 2018;46:3979–3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen L, Zhang L, Zhu Z, et al. Effects of IL-10- and FasL-overexpressing dendritic cells on liver transplantation tolerance in a heterotopic liver transplantation rat model. Immunol Cell Biol. 2019;97:714–725. [DOI] [PubMed] [Google Scholar]
- 91.Lei H, Reinke P, Volk HD, et al. Mechanisms of immune tolerance in liver transplantation-crosstalk between alloreactive T cells and liver cells with therapeutic prospects. Front Immunol. 2019;10:2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brunetta DM, Albuquerque LM, Batista AHM, et al. Passenger lymphocyte syndrome in liver transplantation. Rev Bras Hematol Hemoter. 2017;39:364–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ono Y, Perez-Gutierrez A, Nakao T, et al. Graft-infiltrating PD-L1hi cross-dressed dendritic cells regulate antidonor T cell responses in mouse liver transplant tolerance. Hepatology. 2018;67:1499–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Levitsky J, Feng S. Tolerance in clinical liver transplantation. Hum Immunol. 2018;79:283–287. [DOI] [PubMed] [Google Scholar]
- 95.Li Y, Koshiba T, Yoshizawa A, et al. Analyses of peripheral blood mononuclear cells in operational tolerance after pediatric living donor liver transplantation. Am J Transplant. 2004;4:2118–2125. [DOI] [PubMed] [Google Scholar]
- 96.Martínez-Llordella M, Puig-Pey I, Orlando G, et al. Multiparameter immune profiling of operational tolerance in liver transplantation. Am J Transplant. 2007;7:309–319. [DOI] [PubMed] [Google Scholar]
- 97.Pons JA, Revilla-Nuin B, Baroja-Mazo A, et al. FoxP3 in peripheral blood is associated with operational tolerance in liver transplant patients during immunosuppression withdrawal. Transplantation. 2008;86:1370–1378. [DOI] [PubMed] [Google Scholar]
- 98.Bohne F, Londoño MC, Benítez C, et al. HCV-induced immune responses influence the development of operational tolerance after liver transplantation in humans. Sci Transl Med. 2014;6:242ra81. [DOI] [PubMed] [Google Scholar]
- 99.Taubert R, Danger R, Londoño MC, et al. Hepatic infiltrates in operational tolerant patients after liver transplantation show enrichment of regulatory T cells before proinflammatory genes are downregulated. Am J Transplant. 2016;16:1285–1293. [DOI] [PubMed] [Google Scholar]
- 100.Kobayashi E, Kamada N, Enosawa S, et al. Prevention by liver transplantation of the graft-versus-host reaction and allograft rejection in a rat model of small bowel transplantation. Transplantation. 1994;57:177–181. [DOI] [PubMed] [Google Scholar]
- 101.Miller DG. What is early diagnosis doing? Cancer. 1976;37(suppl 1):426–432. [DOI] [PubMed] [Google Scholar]
- 102.Taner T. Liver transplantation: rejection and tolerance. Liver Transpl. 2017;23(S1):S85–S88. [DOI] [PubMed] [Google Scholar]
- 103.Strom TB. Transplant rejection and paradigms lost. J Clin Invest. 2013;123:2360–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pollard TD, Cooper JA. Actin, a central player in cell shape and movement. Science. 2009;326:1208–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Krammer PH, Arnold R, Lavrik IN. Life and death in peripheral T cells. Nat Rev Immunol. 2007;7:532–542. [DOI] [PubMed] [Google Scholar]
- 106.Wang K, Song ZL, Wu B, et al. The T-helper cells 17 instead of Tregs play the key role in acute rejection after pediatric liver transplantation. Pediatr Transplant. 2019;23:e13363. [DOI] [PubMed] [Google Scholar]
- 107.Lucas S, Ghilardi N, Li J, et al. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc Natl Acad Sci U S A. 2003;100:15047–15052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Takeda A, Hamano S, Yamanaka A, et al. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-Bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170:4886–4890. [DOI] [PubMed] [Google Scholar]
- 109.Liu Z, Fan H, Jiang S. CD4(+) T-cell subsets in transplantation. Immunol Rev. 2013;252:183–191. [DOI] [PubMed] [Google Scholar]
- 110.Wu Q, Tang Y, Hu X, et al. Regulation of Th1/Th2 balance through OX40/OX40L signalling by glycyrrhizic acid in a murine model of asthma. Respirology. 2016;21:102–111. [DOI] [PubMed] [Google Scholar]
- 111.Morita M, Joyce D, Miller C, et al. Rejection triggers liver transplant tolerance: Involvement of mesenchyme-mediated immune control mechanisms in mice. Hepatology. 2015;62:915–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bishop GA, Sun J, DeCruz DJ, et al. Tolerance to rat liver allografts: III. Donor cell migration and tolerance-associated cytokine production in peripheral lymphoid tissues. J Immunol. 1996;156:4925–4931. [PubMed] [Google Scholar]
- 113.Bowen DG, Zen M, Holz L, et al. The site of primary T cell activation is a determinant of the balance between intrahepatic tolerance and immunity. J Clin Invest. 2004;114:701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Qian S, Lu L, Fu F, et al. Apoptosis within spontaneously accepted mouse liver allografts: evidence for deletion of cytotoxic T cells and implications for tolerance induction. J Immunol. 1997;158:4654–4661. [PMC free article] [PubMed] [Google Scholar]
- 115.Meyer D, Thorwarth M, Otto C, et al. Apoptosis of alloreactive T cells in liver allografts during tolerance induction. Transplant Proc. 1999;31:474. [DOI] [PubMed] [Google Scholar]
- 116.Li W, Zheng XX, Kuhr CS, et al. CTLA4 engagement is required for induction of murine liver transplant spontaneous tolerance. Am J Transplant. 2005;5:978–986. [DOI] [PubMed] [Google Scholar]
- 117.Sandner SE, Clarkson MR, Salama AD, et al. Role of the programmed death-1 pathway in regulation of alloimmune responses in vivo. J Immunol. 2005;174:3408–3415. [DOI] [PubMed] [Google Scholar]
- 118.Jhun JY, Lee SH, Lee SK, et al. Serial monitoring of immune markers being represented regulatory T cell/T helper 17 cell ratio: indicating tolerance for tapering immunosuppression after liver transplantation. Front Immunol. 2018;9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tang Q, Vincenti F. Transplant trials with Tregs: perils and promises. J Clin Invest. 2017;127:2505–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tanimine N, Ohira M, Tahara H, et al. Strategies for deliberate induction of immune tolerance in liver transplantation: from preclinical models to clinical application. Front Immunol. 2020;11:1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sánchez-Fueyo A, Whitehouse G, Grageda N, et al. Applicability, safety, and biological activity of regulatory T cell therapy in liver transplantation. Am J Transplant. 2020;20:1125–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Todo S, Yamashita K, Goto R, et al. A pilot study of operational tolerance with a regulatory T-cell-based cell therapy in living donor liver transplantation. Hepatology. 2016;64:632–643. [DOI] [PubMed] [Google Scholar]
- 123.Shams K, Kalantar K, Karimi MH, et al. Fas, FasL and Foxp3 gene expression in post-liver transplant autoimmune hepatitis patients with and without acute rejection. Clin Exp Hepatol. 2019;5:103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Akkaya B, Oya Y, Akkaya M, et al. Regulatory T cells mediate specific suppression by depleting peptide-MHC class II from dendritic cells. Nat Immunol. 2019;20:218–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Obregon C, Kumar R, Pascual MA, et al. Update on dendritic cell-induced immunological and clinical tolerance. Front Immunol. 2017;8:1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hidaka M, Eguchi S, Takatsuki M, et al. The Kupffer cell number affects the outcome of living donor liver transplantation from elderly donors. Transplant Direct. 2016;2:e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dai H, Zheng Y, Thomson AW, et al. Transplant tolerance induction: insights from the liver. Front Immunol. 2020;11:1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.MacParland SA, Liu JC, Ma XZ, et al. Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nat Commun. 2018;9:4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lin J, Li M, Wang Z, et al. The role of CD4+CD25+ regulatory T cells in macrophage-derived foam-cell formation. J Lipid Res. 2010;51:1208–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang L, Yang C, Xu M, et al. The role of soluble fibrinogen-like protein 2 in transplantation: protection or damage. Transplantation. 2014;97:1201–1206. [DOI] [PubMed] [Google Scholar]
- 131.Xie L, Ichimaru N, Morita M, et al. Identification of a novel biomarker gene set with sensitivity and specificity for distinguishing between allograft rejection and tolerance. Liver Transpl. 2012;18:444–454. [DOI] [PubMed] [Google Scholar]
- 132.Bézie S, Picarda E, Tesson L, et al. Fibrinogen-like protein 2/fibroleukin induces long-term allograft survival in a rat model through regulatory B cells. PLoS One. 2015;10:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bézie S, Picarda E, Ossart J, et al. IL-34 is a Treg-specific cytokine and mediates transplant tolerance. J Clin Invest. 2015;125:3952–3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li W, Li Z, Wang Z, et al. NKT cells in the liver are important for peripheral tolerance induction. Transplantation. 2018;102:S694. [Google Scholar]
- 135.Messner F, Etra JW, Dodd-O JM, et al. Chimerism, transplant tolerance, and beyond. Transplantation. 2019;103:1556–1567. [DOI] [PubMed] [Google Scholar]
- 136.Ezekian B, Schroder PM, Freischlag K, et al. Contemporary strategies and barriers to transplantation tolerance. Transplantation. 2018;102:1213–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chaudhry S, Kato Y, Weiner J, et al. Transient-mixed chimerism with nonmyeloablative conditioning does not induce liver allograft tolerance in nonhuman primates. Transplantation. 2020;104:1580–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shao L, Pan S, Zhang QP, et al. Emerging role of myeloid-derived suppressor cells in the biology of transplantation tolerance. Transplantation. 2020;104:467–475. [DOI] [PubMed] [Google Scholar]
- 139.Walter L. Nomenclature report on the major histocompatibility complex genes and alleles of the laboratory rat (Rattus norvegicus). Immunogenetics. 2020;72:5–8. [DOI] [PubMed] [Google Scholar]
- 140.Günther E, Walter L. The major histocompatibility complex of the rat (Rattus norvegicus). Immunogenetics. 2001;53:520–542. [DOI] [PubMed] [Google Scholar]
- 141.Kashfi A, Mehrabi A, Pahlavan PS, et al. A review of various techniques of orthotopic liver transplantation in the rat. Transplant Proc. 2005;37:185–188. [DOI] [PubMed] [Google Scholar]
- 142.Regev A, Teichmann SA, Lander ES, et al. The human cell atlas. Tissues and organs. Preprint. Posted online October 11, 2018. arXiv:1810.05192.